3
Geochemistry and Petrology
PRESENT STATE OF KNOWLEDGE
Most of what is known about the composition of Mars comes from three types of measurements:
-
In situ analysis of the rocks and regolitha by landers;
-
Obital observations carried out by emission and reflectance spectroscopy; and
-
Studies of meteorites that are inferred to have come from Mars.
These sources provide some zero-order compositional data on elemental concentrations in rocks and regolith at a few discrete sites on the planet; limited basic characterization of the global distribution of rock types; and some very detailed knowledge of rocks presumed to come from Mars (the meteorites), which have no geologic context. Much of the first-order information needed to understand the origin and evolution of Mars is still missing.
Elemental Compositions
This subsection discusses only remote and in situ analyses. Far more is known about the compositions of the few dozen meteorites presumed to have come from Mars, but using this information to better understand Mars is difficult because of the lack of geologic context. (The subsection “Knowledge Based on Martian Meteorites” in this chapter focuses on the subject.)
The two Viking landers in the 1970s each carried an x-ray fluorescence spectrometer. These instruments returned data on the major-element composition of the regolith fines at their respective sites in the northern hemisphere.1 Mars Pathfinder (in 1996) had an Alpha-Proton-X-ray Spectrometer (APXS), which analyzed rock and regolith samples via alpha backscatter, proton-induced x-ray emission, and x-ray fluorescence.2 The Pathfinder lander returned data on both rocks and regolith fines. The latter were similar in composition to the fines at the two Viking sites (see Figure 3.1a), but the former were richer in silica than the rocks at the Viking sites, and were described as being andesitic in composition (see Figure 3.1b). The normativeb mineralogies of the samples
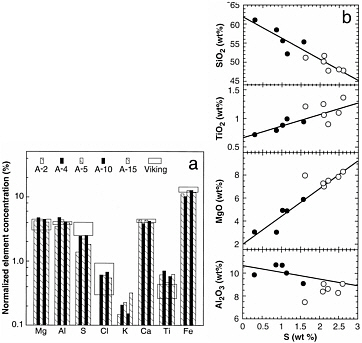
FIGURE 3.1 Comparison of the chemical composition of martian soils as measured on Viking and Pathfinder missions. (a) Comparison of five measured Pathfinder soils (bars) and Viking soil data (boxes). (b) Linear regression lines for several elements versus S in Pathfinder rocks (filled symbols). Soils (open symbols) were not included in the regression but plot on the regression line, indicating that the zero-S values represent a soil-free rock composition. This composition is higher in SiO2than are the soils and is indicative of an andesitic rock type. SOURCE: Reprinted with permission from R.T. Rieder, T. Economou, H. Wänke, A. Turkevich, J. Crisp, J. Brückner, G. Dreibus, and H.Y. McSween, Jr., “The Chemical Composition of Martian Soil and Rocks Returned by the Mobile Alpha Proton X-ray Spectrometer: Preliminary Results from the X-ray Mode,” Science 278:1771–1774, 1997. Copyright 1997 by the American Association for the Advancement of Science.
are dominated by feldspars, orthopyroxenes, and quartz, but because only a chemical analysis was performed, the mineralogy remains uncertain. The rocks may not even be igneous.
The rocks at the Pathfinder landing site are much more silica-richc than any of the martian meteorites. The regolith fines at all three sites are much higher than the rocks in Mg and Fe, and are similar to the meteorites, but with the addition of S and Cl. The fines at the Pathfinder landing site require the addition of Fe and Mg in some form, perhaps from basalts such as the shergottite meteorites, for their compositions to be explained if they are derived from the Pathfinder rocks. The enhanced abundances of S and Cl in the fines suggest that there may have been mobility of water-soluble compounds in the near surface (see Chapter 5).
Mineralogical Compositions and Rock Types
As noted above, the Viking and Pathfinder missions provided no direct determination of the mineralogy of the rocks at any of the martian landing sites. However, Mars Global Surveyor carried a Thermal Emission Spectrometer (TES), which returned many spectra of the martian surface.3 The spectra over most of the planet seem to fall into two broad categories, one indicative of basalt and the other indicative of andesite, consistent with the Viking and Pathfinder analyses. The andesitic composition is more prominent in the northern lowlands, and the basaltic in the older southern highlands. Analysis of the silica-rich rocks at the Pathfinder site indicates that their composition is similar to the average composition of Earth’s crust4 except for their higher Fe content, which may simply reflect the higher Fe content of the martian mantle compared with Earth’s.5
The TES spectra conclusively identified one major mineral, a coarsely crystalline, gray hematite (a-Fe2O3), which is concentrated in a small region near the equator, Sinus Meridiani.6 This mineral is significant in that it may be diagnostic of large-scale water interactions. TES is sensitive to a wide range of silicate minerals, and analysis of the TES data suggests a significant component of feldspar, as well as high-calcium pyroxene, in both the basaltic and andesitic lithologies.7 There also appears to be a large component of high-silica glass in the andesite regions, and definitive observations of olivine have been made in the basaltic region of Syrtis Major.8,9
Visible/near-infrared observations of Mars have revealed properties of the iron minerals at the surface. Bands at 1 and 2 µm observed in telescopic reflectance spectra indicate the presence of pyroxene in basaltic rocks,10,11 and orbital observations indicate the presence of both low- and high-calcium pyroxene, consistent with mineralogy of the basaltic SNC meteorites.12 The red color of Mars is due to the presence of ferric oxide minerals. Bell and colleagues demonstrated that crystalline hematite is present in abundances of up to 4 percent in some bright regions,13 while much of the ferric mineralogy is best explained by nanophase hematite.14 These forms of hematite are distinct from the gray hematite observed by TES for a restricted region of the surface. The overall spectral properties across the visible/near-infrared can be fit with palagonite,d which suggests that much of the surface is composed of poorly crystallized alteration products. Although carbonates, sulfates, or hydrated minerals have not been definitively observed by any spacecraft measurements to date, the observations have been hampered by poor spatial resolution and/or poor spectral resolution.
Knowledge Based on Martian Meteorites
Members of the SNC category of meteorites described in Chapter 1, comprising the shergottites, nakhlites, and chassignites, plus the unique meteorite ALH84001, are thought to have come from Mars. A variety of data has been used to make this inference, but the strongest case has been made by comparison between the composition of the martian atmosphere as measured by the Viking missions, and the composition of gas trapped by shock in a glass component of the shergottite EETA79001.15 Because of the importance of these meteorites, they have been studied in great detail. Relating all that is known from these meteorites to what they can tell us about Mars is beyond the scope of this report.16
Five different rock types are known in the SNC collection. They are basalts and lherzolites (shergottites), clinopyroxenites (nakhlites), a dunite (Chassigny), and an orthopyroxenite (ALH84001). Most appear to be igneous cumulates. Interestingly, none of these rocks matches the composition of the andesites found at the Mars Pathfinder landing site. The basaltic meteorites have a high Fe/Mg ratio and low Al content relative to terrestrial basalts, and all of the SNC meteorites have oxide minerals consistent with formation under highly oxidizing conditions.
The compositions of these meteorites have been used by two different groups to estimate the bulk composition of Mars.17,18,19 Though the models disagree on the extent of fractionation among the refractory elements, both models agree that the composition of the martian mantle is similar to that of Earth but with some important differences: Mars is richer in Fe3+ and in abundances of moderately volatile elements. Estimates of the size and composition of the core have been made by Treiman and colleagues by accounting for the calculated abundances
of siderophile and chalcophile elements in the mantle.20 These estimates are within the ranges allowed by the mean density and moment of inertia of Mars (see Chapter 2).
McSween has discussed various estimates of the major-element compositions of the melts from which the SNC meteorites formed.21 There are significant difficulties in estimating the melt composition from rocks that are igneous cumulates, because they contain cumulus minerals in abundance greater than what would crystallize from the melt. Nevertheless, it seems clear that all of the magmas have low Al contents, which yield small amounts of plagioclase that forms late in the crystallization sequence of minerals from the melt, and they are all high in Fe.
Trace-element compositions of parent magmas are a little easier to calculate than are major-element compositions, as one can measure the trace-element composition of a cumulus mineral grain and calculate the composition of the coexisting liquid using mineral/liquid partition coefficients. Though the absolute values of the partition coefficients for a given mineral vary with temperature and melt composition, ratios of partition coefficients are not nearly as variable. Thus, the pattern of trace-element abundances can be calculated with some confidence, even if the absolute abundances cannot. McSween has reviewed these calculations.22 All shergottite parent magmas, as well as the parent of the unique ALH84001, are depleted in light rare-earth elements (LREEs), but the parent magmas of Nakhla and Chassigny are LREE-enriched. Longhi has estimated the abundances of incompatible elements other than the rare-earth elements and found that, like the rare-earth elements, the patterns of these elements in the parent magmas of Shergotty and Nakhla were complementary; that is, the elements most enriched in the Nakhla liquid are most depleted in the Shergotty liquid.23 This observation suggests that both parent magmas could have come from the same source at different times.
Knowledge of the composition of the SNC parent magmas is important, as it allows one to estimate the composition of the source regions of the magmas. Jones24 and Longhi,25 using estimated magma compositions and constraints provided by radiogenic isotopic composition, concluded that the sources for the SNC magmas had time-integrated LREE depletions significantly greater than those of terrestrial magmas. Small degrees of melting of this source could produce the parent magmas of the nakhlites and Chassigny. The shergottite parent magmas could then have formed later from this same source, allowing for the removal of the nakhlite parent liquid, thus accounting for the complementarity described above. An interesting aspect of this model is that all the SNC meteorites could have been derived from a similar source.
NEAR-TERM OPPORTUNITIES
Mars Odyssey
The Mars Odyssey spacecraft was launched in April 2001 and entered orbit about Mars 6 months later. It carries the Thermal Emission Imaging System (THEMIS) and the Gamma-Ray Spectrometer (GRS), both of which will address issues related to geochemistry and petrology.
THEMIS images the martian surface using multispectral, thermal-infrared spectral bands. These images have a spatial resolution of 100 m/pixel, which permits features to be observed at much smaller scale than with the 3-km resolution of TES on Mars Global Surveyor. The high spatial resolution comes at the expense of spectral resolution, which is poorer than that of TES. The diminished spectral resolution may be offset by an ability to discover rock outcrops that are too small to be seen by TES; such outcrops might be of a pure rock type and not a mixture of different rocks or rocks and dust. The poorer spectral resolution may not allow for the determination of the mineralogy, but it should permit mapping of the rock types and other spectral features found by TES. In addition, perhaps other minerals associated with hydrous alteration may be found in areas that are much smaller than the hematite region found by TES.
The GRS will determine the elemental abundances of the surface materials to an accuracy of about 10 percent with a spatial resolution that varies for different elements but is no better than the order of several hundred kilometers.26 This is not a fine enough spatial scale to sample many of the smaller geologic features, but it will permit global and regional geologic features to be analyzed. It should, for example, be able to determine the chemical differences between the ancient southern highlands and the younger northern lowlands. The chemical composition of the volcanic provinces can also be determined, to see how they differ from the surrounding
highlands. Interpretation of the data may be complicated by the ever-present dust component, but the analyses of pure regolith fines by both Viking and Mars Pathfinder may allow a correction for this component on the basis of an element that is enriched in the fines, for example, chlorine. It may be possible to calculate the normative mineralogy from the chemical composition, as was done for the rocks analyzed by Pathfinder; but in an environment where significant amounts of weathering may have occurred, such a calculated mineralogy could be very misleading.
Mars Exploration Rovers—2003
The Mars Exploration Rovers (MERs), scheduled for launch in late May and early June 2003, will each deliver a lander with a rover that is much larger and more scientifically capable than was the Mars Pathfinder rover. The twin MERs will be delivered to sites thought to be good candidates for a future Mars sample-return mission. They will contain a panoramic camera (Pancam); an Alpha Particle X-ray Spectrometer (APXS), similar to that on Pathfinder, for chemical analyses; a Mini-TES for mineralogical analyses; and a microscopic imager for textural analyses to support the mineralogical and elemental analyses. They will also employ a coring device to permit the study of rocks free from weathering products, and a Mössbauer spectrometer for the study of specific iron-bearing phases. This investigation will increase understanding by obtaining data from new sites; additionally, the data will be more comprehensive than that of earlier missions.
RECOMMENDED SCIENTIFIC PRIORITIES
Earlier COMPLEX recommendations of strategies for the exploration of Mars placed strong emphasis on chemical and petrological studies of the planet. Studies of this type provide key information for understanding the processes responsible for the evolution of Mars and its potential as an abode for life. For example, the committee’s 1978 report, Strategy for Exploration of the Inner Planets: 1977–1987, gives the highest priority to the following objective: “to establish the chemical, mineralogical, and petrological character of different components of the surface material, representative of the known diversity of the planet” (Appendix B: [1.3]).27 This includes the following (Appendix B: [1.4]):
-
Gross chemical analysis (all principal chemical elements with a sensitivity of 0.1 percent by atom and an accuracy of at least 0.5 atom percent for the major constituents).
-
Identification of the principal mineral phases present (i.e., those making up at least 90 percent of the material in soils and rocks).
-
Establishment of a classification of rocks (igneous, sedimentary, and metamorphic) and fines that define martian petrogenetic processes.
-
Characterization of the state of oxidation, particularly of the fine material and rock surfaces.
-
Characterization of the content of volatiles or volatile-producing species (H2O, SO3, CO2, NO2).
-
Determination of the selected minor and trace-element contents. (a) Primordial radionuclides: K with a sensitivity of at least 0.05 percent; U and Th with a sensitivity of at least 1 ppm. (b) Selected minor and trace elements (e.g., C, N, F, P, S, Cl, Ti, Ni, As, and rare-earth elements Bi, Cu, Rb, Sr).
-
Measurement of physical properties (magnetic, and in the case of fines, density and size distribution, and rheological properties).
In addition, in its 1990 report The Search for Life’s Origins, the Committee on Planetary Biology and Chemical Evolution recommended similar measurements (Appendix B: [2.1]) but added “sedimentological and paleontological studies” and called out specific types of sites “where there is evidence of hydrologic activity in any early clement epoch. . . .”28 In subsequent reports, COMPLEX added other objectives to its strategy for the exploration of Mars and made specific recommendations for developments to aid in the implementation of the strategy, but it has not changed the level of priority assigned in the original 1978 strategy. For example, in the 1995 NASA publication An Exobiological Strategy for Mars Exploration a recommendation was made (Appendix B: [5.2]) for “a sequence of landed missions, beginning with development of a geochemically oriented payload
capable of regional chemical and mineralogical analyses, oxidant identification, and volatile element detection.”29 The fact that the original COMPLEX priority has not changed is a testament both to how well conceived the strategy was and to how little Mars has been studied until recently.
ASSESSMENT OF PRIORITIES IN THE MARS EXPLORATION PROGRAM
The Mars Exploration Program has already addressed some of the scientific priorities referred to in the preceding section. Mars Pathfinder’s APXS investigation, as outlined above, has satisfied the objective for a gross chemical analysis, but only of one rock type at one locality. Mars Global Surveyor’s TES instrument has returned global spectral thermal emission data, from which a mineralogic assessment of low-albedo, basaltic, and basaltic-andesite terrains has been derived.30,31 However, it has been more problematic to determine the modal mineralogy of other regions, probably because of the confounding effects of the poorly crystalline martian dust, and thus it seems unlikely that detailed quantitative mineralogical data of the type recommended by COMPLEX will come out of that investigation. The Mars Odyssey mission (see Table A.1 in Appendix A of this report) has delivered another thermal emission instrument to Mars—THEMIS—with far better spatial, but poorer spectral, resolution. It is too early to say with certainty now, but it is unlikely that this instrument will enable determination of the mineralogy of the surface to the extent recommended by COMPLEX. The gamma-ray spectrometer will return elemental analyses of global-scale regions, but its data will not satisfy the need to sample the diversity of rock types.
The two MERs planned for launch in 2003 should be able to satisfy the need for a gross chemical analysis, just as the Mars Pathfinder APXS did, and it can be hoped that they will find rock types different from those analyzed previously. The Mössbauer spectrometer, Pancam, and Mini-TES instruments will provide insight into the mineralogy of the different rocks and surfaces analyzed, but the detailed petrographic nature of the samples may be hidden by surface alteration and coatings. The Mars Reconnaissance Orbiter (MRO) to be launched in 2005 will look for signs of water, with very high spatial resolution, and the expected high-spatial-resolution visible/near-infrared spectrometer will provide new information relevant to mineralogy and petrology. Nevertheless, the spatial coverage will not be global, and the wavelength range is not sensitive to the full range of minerals expected in the crust and on the surface. As with the synergism between TES and THEMIS, the OMEGA experiment on the European Space Agency’s Mars Express, scheduled for launch in 2003, will provide global coverage at a typical spatial resolution of 1 km/pixel, the opposite of the MRO high-resolution spectrometer with its smaller degree of coverage.
Plans exist for a long-range long-duration advanced rover mission—the Mars Science Laboratory (MSL)e — in 2007f to pave the way for sample return (see Table A.1). The claim is made that such a mission will help develop technologies that should improve the ability to find and collect high-quality samples for Earth return. NASA is wise to take the time necessary for development of the technologies to improve the ability to select interesting sites for sample-return missions; however, there is little justification on scientific grounds for further reconnaissance, following the missions planned through 2005, before the return of the first samples, in a series of sample-return missions. Based on past recommendations of COMPLEX, which are reemphasized here, it is clear that no major strides toward satisfying COMPLEX’s highest-priority science objectives in the area of geochemistry and petrology will be made until samples are returned.
REFERENCES
1. P. Toulmin III, A.K. Baird, B.C. Clark, K. Keil, H.J. Rose, R.P. Christian, H.P. Evans, and W.C. Kelliher, “Geochemi-cal and Mineralogical Interpretation of the Viking Inorganic Chemical Results,”Journal of Geophysical Research82: 4625–4633, 1977.
2. R.T. Rieder, T. Economou, H. Wänke, A. Turkevich, J. Crisp, J. Brückner, G. Dreibus, and H.Y. McSween, Jr., “The Chemical Composition of Martian Soil and Rocks Returned by the Mobile Alpha Proton X-ray Spectrometer: Prelimi-nary Results from the X-ray Mode,”Science278: 1771–1774, 1997.
3. P.R. Christensen, “Introduction to the Special Section: Mars Global Surveyor Thermal Emission Spectrometer,”Journal of Geophysical Research, Planets105: 9507, 2000, and 13 subsequent papers onpp. 9509–9739.
4. R.T. Rieder, T. Economou, H. Wänke, A. Turkevich, J. Crisp, J. Brückner, G. Dreibus, and H.Y. McSween, Jr., “The Chemical Composition of Martian Soil and Rocks Returned by the Mobile Alpha Proton X-ray Spectrometer: Prelimi-nary Results from the X-ray Mode,”Science278: 1771–1774, 1997.
5. H. Wänke and G. Driebus, “Chemical Composition and Accretional History of Terrestrial Planets,”PhilosophicalTransactions of the Royal Society of LondonA235: 545–557, 1988.
6. P.R. Christensen, J.L. Bandfield, R.N. Clark, K.S. Edgett, V.E. Hamilton, T. Hoefen, H.H. Kieffer, R.O. Kuzmin, M.D. Lane, M.C. Malin, R.V. Morris, J.C. Pearl, R. Pearson, T.L. Roush, S.W. Ruff, and M.D. Smith,“Detection of Crystalline Hematite Mineralization on Mars by the Thermal Emission Spectrometer: Evidence for Near-Surface Water,”Journal of Geophysical Research105: 9623–9642, 2000.
7. J.L. Bandfield, V.E. Hamilton, and P.R. Christensen, “A Global View of Martian Surface Compositions from MGS-TES,”Science287: 1626–1630, 2000.
8. V.E. Hamilton, R.V. Morris, and P.R. Christensen, “Determining the Composition of Martian Dust and Soils Using MGS TES: Mid-infrared Emission Spectra of Variable-Composition Palagonites,”Lunar and Planetary Science Con-ference 32, Abstract #2123, 2001.
9. T.M. Hoefen and R.N. Clark, “Compositional Variability of Martian Olivines Using Mars Global Surveyor Thermal Emission Spectra,”Lunar and Planetary Science Conference 32, Abstract #2049, 2001.
10. T.B. McCord and J.B. Adams, “Spectral Reflectivity of Mars,”Science163: 1058–1060, 1969.
11. R.B. Singer and H.Y. McSween, Jr., “The Igneous Crust of Mars: Compositional Evidence from Remote Sensing and the SNC Meteorites,”pp. 709–736 in Resources of Near-Earth Space, J.S. Lewis, M.S. Matthews, and M.L. Guerrieri (eds.), University of Arizona Press, Tucson, 1993.
12. J.F. Mustard and J.M. Sunshine, “Seeing Through the Dust: Martian Crustal Heterogeneity and Links to the SNC Meteorites,”Science267: 1623–1626, 1995.
13. J.F. Bell, T.B. McCord, and P.D. Owensby, “Observational Evidence of Crystalline Iron Oxides on Mars,”Journal ofGeophysical Research95: 14447–14461, 1990.
14. R.V. Morris, D.G. Agresti, H.F. Laver, J.A. Newcomb, T.D. Shelfer, and A.V. Murali, “Evidence for Pigmentary Hematite on Mars Based on Optical, Magnetic, and Mössbauer Studies of Superparamagnetic (Nanocrystalline Hema-tite),”Journal of Geophysical Research94: 2760–2778, 1989.
15. For a discussion see, for example, R.O. Pepin, “On the Origin and Evolution of Terrestrial Planet Atmospheres and Meteoritic Volatiles,”Icarus92: 2–79, 1991.
16. For a comprehensive review of what martian meteorites can tell us about Mars see, for example, H.Y. McSween, “What We Have Learned About Mars from SNC Meteorites,”Meteoritics29: 757–779, 1994.
17. G. Dreibus and H. Wänke, “Mars, a Volatile-Rich Planet,” (abstract), Meteoritics20: 367–381, 1985.
18. G. Dreibus and H. Wänke, “Volatiles on Earth and Mars: A Comparison,”Icarus71: 221–245, 1987.
19. A.H. Treiman, M.J. Drake, M.-J. Janssens, R. Wolf, and M. Ebihara, “Core Formation in the Earth and Shergottite Parent Body (SPB): Chemical Evidence from Basalts,”Geochimica et Cosmochimica Acta50: 1071–1091, 1986.
20. A.H. Treiman, J.H. Jones, and M.J. Drake, “Core Formation in the Shergottite Parent Body and Comparison with Earth,”Journal of Geophysical Research92 (supplement): E627–E632, 1987.
21. H.Y. McSween, “What We Have Learned About Mars from SNC Meteorites,”Meteoritics29: 757–779, 1994.
22. H.Y. McSween, “What We Have Learned About Mars from SNC Meteorites,”Meteoritics29: 757–779, 1994.
23. J. Longhi, “Complex Magmatic Processes on Mars: Inferences from the SNC Meteorites,”pp. 695–709 in Proceed-ings of Lunar and Planetary Science, Volume 21, G. Rhyder and V.L. Sharpton (eds.), Lunar and Planetary Institute, Houston, Texas, 1991.
24. J.H. Jones, “Isotopic Relationships Among the Shergottites, the Nakhlites, and Chassigny,”pp. 465–474 in Proceed-ings of the 19th Lunar and Planetary Science Conference, G. Rhyder and V.L. Sharpton (eds.), Cambridge University Press, Cambridge, England, 1989.
25. J. Longhi, “Complex Magmatic Processes on Mars: Inferences from the SNC Meteorites,”pp. 695–709 in Proceed-ings of Lunar and Planetary Science, Volume 21, G. Rhyder and V.L. Sharpton (eds.), Lunar and Planetary Institute, Houston, Texas, 1991.
26. W.V. Boynton, J.I., Trombka, W.C. Feldman, J.R. Arnold, P.A.J. Englert, A.E. Metzger, R.C. Reedy, S.W. Squyres, H. Wänke, S.H. Bailey, J. Brückner, J.L. Callas, D.M. Drake, P. Duke, L.G. Evans, E.L. Haines, F.C. McCloskey, H. Mills, C. Shinohara, and R. Starr,“Science Applications of the Mars Observer Gamma-Ray Spectrometer,”Journal ofGeophysical Research97: 7681–7698, 1992.
27. Space Studies Board, National Research Council, Strategy for Exploration of the Inner Planets: 1977-1987, National Academy of Sciences, Washington, D.C., 1978.
28. Space Studies Board, National Research Council, The Search for Life’s Origins: Progress and Future Directions inPlanetary Biology and Chemical Evolution, National Academy Press, Washington, D.C., 1990.
29. Exobiology Program Office, NASA, An Exobiological Strategy for Mars Exploration, J. Kerridge (ed.), NASA SP-530, NASA, Washington, D.C., 1995.
30. P.R. Christensen, J.L. Bandfield, M.D. Smith, V.E. Hamilton, and R.N. Clark, “Identification of a Basaltic Compo-nent on the Martian Surface from Thermal Emission Spectrometer Data,”Journal of Geophysical Research105: 9609–9622, 2000.
31. J.L. Bandfield, V.E. Hamilton, and P.R. Christensen, “A Global View of Martian Surface Compositions from MGS-TES,”Science287: 1626–1630, 2000.








