1
Introduction
The goal of protecting and enhancing air quality to protect and promote human health and public welfare1 has been consistently set forward in the United States during the latter part of the twentieth century. To accomplish this goal, numerous regulations and standards, a broad suite of management tools, and several monitoring networks to track progress have been established. All of these components depend on robust and up-to-date scientific and technical input, which includes an understanding of relationships between air pollutant levels and impacts on human health, ecosystems, atmospheric visibility, and materials. The National Research Council Committee on Air Quality Management in the United States was asked to evaluate the effectiveness of the nation’s air quality management (AQM) system and the extent to which it is informed by the most advanced science and technology. This chapter begins with a brief summary of the current scientific and technical understanding of air pollution and its impacts, as well as an overview of the AQM system in the United States and the federal legislation that has motivated and driven much of its development in the latter part of the twentieth century. This overview discussion is intended to provide an introduction to aspects of the AQM system that are described in more detail and critiqued in later chapters of this report. The report is not intended to provide a comprehensive description of AQM activities in the
United States; pertinent references are provided for those who desire more background information on general concepts of scientific and technical understanding of air pollution and its impacts.
AIR POLLUTION SCIENCE
The atmosphere is composed of a mixture of gases and particles. An air pollutant is generally defined as any substance in air that, in high enough concentrations, harms humans, ecosystems (other animals and vegetation), or materials (such as buildings and monuments) and reduces visibility. In this report, the committee uses the term air pollutant to denote the subset of harmful atmospheric substances that are present, at least in part, because of human activities rather than natural production and whose principal deleterious effects occur as a result of exposure at ground level. Greenhouse gases, as well as pollutants that cause depletion of ozone (O3) in the stratosphere, the layer of atmosphere extending from about 10 to 16 kilometers (km) up to 50 km altitude, are addressed only in the context of managing ground-level air quality.2
The science of air pollution is primarily concerned with quantitatively understanding the so-called “source-receptor relationships” that link specific pollutant emissions to the pollutant concentrations and deposition observed in the environment as a function of space and time. This quantitative understanding is developed through extensive field and laboratory measurements and analysis and is then tested and documented in air quality models that use mathematical and numerical techniques to simulate the physical and chemical processes that affect air pollutants as they disperse and react in the atmosphere. As illustrated in Figure 1-1, the pollutants at a particular time and place depend on the proximity to sources that emit pollutants or their precursors; the chemical reactions that pollutants or their precursors undergo once in the atmosphere; and the impact of mixing, dilution, transport, and removal or deposition processes (Seinfeld and Pandis 1998). The areas of air quality science and air quality management are closely coupled, because the tools developed by scientists and engineers to carry out the tasks described above are also widely used by the agencies tasked with controlling air pollution. For example, the instrumentation used by scientists in field experiments is also used by regulatory agencies to monitor air pollution exposures, trends, and compliance. Similarly, the models developed by scientists to simulate and better understand air pollution are used in AQM to help design effective strategies for air pollution mitigation.
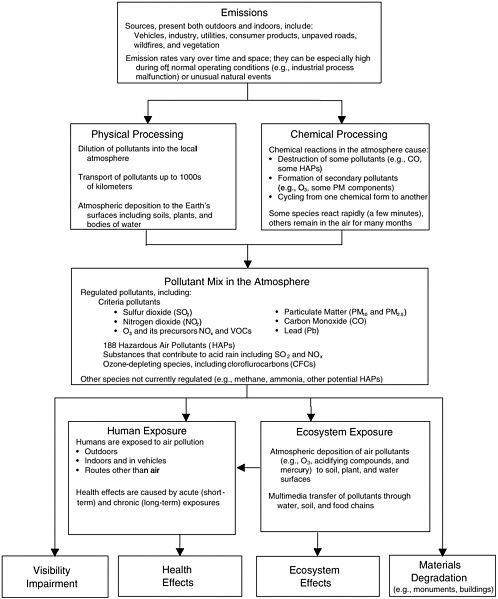
FIGURE 1-1 Schematic of the factors influencing the pollutant mix in the atmosphere and the resultant impacts of pollution. Greenhouse gases and climate change impacts are not included because they fall outside the committee’s charge.
Air pollutants are often characterized by how they originate: pollutants emitted directly into the atmosphere are called primary pollutants; those formed as a result of chemical reactions within the atmosphere are called secondary pollutants. Control of secondary pollutants is generally more problematic than that of primary pollutants, because mitigation of second-
ary pollutants requires identification of the precursor compounds and their sources as well as an understanding of the specific chemical reactions that result in the formation of the secondary pollutants. Control can be further complicated when the chemical reactions resulting in secondary-pollutant formation involve complex, nonlinear interactions among the precursors. Under those conditions, a 1:1 relationship might not exist between a reduction in precursor emissions and reductions in secondary-pollutant concentrations. Ground-level O3 is an example of such a secondary pollutant; it is formed by reactions of nitrogen oxides (NOx) and volatile organic compound (VOC) species3 in the presence of sunlight. In some circumstances, O3 concentrations are most effectively controlled by lowering both VOC and NOx emissions. For other circumstances, lowering VOC or NOx emissions may be most effective (NRC 1991).
Similar complications arise in the mitigation of suspended particulate matter (PM), which refers to a heterogeneous collection of solid and liquid particles that include ultrafine particles (diameters of less than 0.1 micrometers [μm]); fine particles (diameters of 0.1 to a few micrometers), which are commonly dominated by sulfate, nitrate, organic, and metal components; and relatively coarse particles (diameters of a few micrometers or more), which are often dominated by dust and sea salt. PM can be a primary or secondary pollutant. As a primary pollutant, PM is emitted directly to the atmosphere, for instance, as a result of fossil fuel combustion. As a secondary pollutant, PM is formed in the atmosphere as a result of such processes as oxidation of sulfur dioxide (SO2) gas to form sulfate particles. Because the reactions that result in the formation of secondary PM often depend on the concentration and composition of preexisting airborne PM, control strategies that lower the emissions of one chemical constituent of airborne PM might not affect or might in some cases increase the concentrations of other components of PM. Even though pollutants have been typically treated independently in many of the air quality regulations in the United States, pollutants are often closely coupled. For example, most pollutants are emitted into the atmosphere by the same source types (see Figure 1-2). They also often share similar precursors and similar chemical interactions once in the atmosphere. For example, many of the VOCs that react to form O3 are also identified as hazardous air pollutants
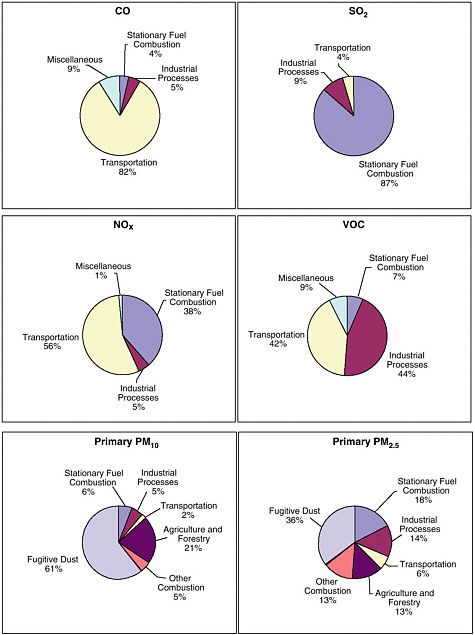
FIGURE 1-2 National average emission categories for carbon monoxide (CO), sulfur dioxide (SO2), nitric oxide and nitrogen dioxide (NOx), volatile organic compounds (VOCs), particulate matter less than 10 micrometers in diameter (PM10), and particulate matter less than 2.5 micrometers in diameter (PM2.5) for 2001. For primary PM10 and PM2.5, a significant fraction of the PM fugitive dust emissions return to the ground after a few minutes. Biogenic emissions (such as VOC emissions from vegetation and NOx emissions from soil microbial processes) are not included. SOURCE: Adapted from EPA 2003a.
(HAPs). VOCs and HAPs can also be precursors to or components of PM. In a similar manner, NOx has several significant environmental effects that warrant its control. In addition to being an O3 precursor, it has a direct impact on human health, is a precursor for acid rain and the formation of inhalable fine particles, decreases atmospheric visibility, and contributes to the eutrophication of water bodies.
AIR POLLUTION IMPACTS
The primary objective of most air quality standards in the United States is the protection of human health. Humans are exposed to air pollution outdoors and indoors, including during transit in vehicles. Indoor air pollution comprises a mixture of contaminants penetrating from outdoors and those generated indoors. Especially high exposure to air pollution can also occur in various microenvironments, referred to here as “hot spots,” which include highway toll plazas; truck stops; airport aprons; and areas adjacent to industrial facilities, busy roadways, and idling vehicles. Some studies have suggested that disproportionate exposures may be found in low-income and predominantly minority communities and have raised concerns about environmental justice (see discussion on environmental justice in Chapter 2 and references cited therein).
Many types of health effects have been attributed to air pollution, including pulmonary, cardiac, vascular, and neurological impairments—all of which can lead directly to mortality. In addition, a number of regulated air pollutants are known or probable carcinogens. Some health effects, such as an increase in asthma attacks, have been observed in conjunction with episodes of high pollution concentrations lasting 1 or 2 days. Such effects are considered acute, because they are associated with short-term exposures to a pollutant. Other health effects, particularly increased risk of cancer, are associated with long-term exposure (EPA 2002b).
The scientific techniques for assessing health impacts of air pollution include air pollutant monitoring, exposure assessment, dosimetry, toxicology, and epidemiology (NRC 1998b, 1999a). Because most of the health effects attributable to air pollutants can also be attributable to a wide variety of other risk factors, the impact of air pollution on human health can be difficult to distinguish and quantify. Determining the impact of air pollution on human health is further complicated by human exposure to a mixture of substances at various concentrations present in the air. Also, a number of subgroups within the human population at large are considered more susceptible to the effects of air pollution. They include people who have coronary disease, asthma, or chronic pulmonary diseases; the elderly; and infants. Fetuses are also possibly susceptible (Wilhelm and Ritz 2003).
In addition to air pollution effects on human health, impacts on ecosystem form and function are also a serious concern. Moreover, because ecosystems often supply society with valuable services (such as cleaning and purifying water), damage to ecosystems from air pollution can exact a significant economic as well as an environmental cost (Daily 1997). Terrestrial, aquatic, and coastal ecosystems are exposed to air pollution via atmospheric substances (such as O3), or by deposition of substances (such as acids, nutrients, and metals). In terrestrial ecosystems, air pollution deposition affects plant physiology; microbial processes; biogeochemical cycles of substances, such as nitrogen; and plant community dynamics. In aquatic ecosystems, acidic deposition results in acidification of waterways, the mobilization of trace metals in surface waters, and ultimately, the loss of aquatic biodiversity (Driscoll et al. 2001a). Atmospheric deposition is also a major source of mercury to some aquatic ecosystems in North America. When mercury is present as methylmercury in sufficient quantities in the food chain, this contaminant is toxic to humans and animals (NRC 2000a). In addition, atmospheric deposition of nitrate and ammonium might be an important source of nitrogen in coastal regions, contributing to eutrophication, increased or harmful algal blooms, hypoxic and anoxic bottom waters, loss of sea grasses, and reduced fish stocks (Fisher and Oppenheimer 1991; D’Elia et al. 1992; Boynton et al. 1995; Paerl 1997; Castro and Driscoll 2002).
Protection of visibility in national parks and wilderness areas has traditionally played a smaller but nonetheless important role in driving air quality regulation. Scenic vistas in most U.S. parklands are often diminished by haze that reduces contrast, washes out colors, and renders distant landscape features indistinct or invisible. Haze degrades visibility primarily through scattering or absorption of light by fine atmospheric particles (NRC 1993a; Watson 2002).
Air pollution can discolor or damage commonly used building materials and works of art. In addition, such pollutants as sulfate can accelerate the natural weathering process of materials, including metals, painted surfaces, stone, and concrete.
AIR QUALITY MANAGEMENT IN THE UNITED STATES
As the health, ecological, and economic impacts of air pollution in the United States have become increasingly evident through more sophisticated scientific approaches, the nation has endeavored to protect air quality through increasingly complex and ambitious legislation (Table 1-1). The federal government’s first major efforts in this regard began in 1955 with the Air Pollution Control Act. These efforts were enhanced over the next 15 years through a series of enactments, including the Clean Air Act (CAA)
TABLE 1-1 Federal Air Quality Management Legislation
|
Date |
Legislation |
Authorization |
|
1955 |
Air Pollution Control Act |
Provided funds to local and state agencies for research and training |
|
1959 |
Air Pollution Control Act Extension |
Extended the 1955 act |
|
1960 |
Motor Vehicle Exhaust Study |
Authorized the Public Health Service (PHS) to study automotive emissions and health |
|
1962 |
Air Pollution Control Act Extension |
Extended 1955 act and required PHS to include auto emissions in their program |
|
1963 |
Clean Air Act |
Research at the federal level Aid to states for training Federal authority to abate interstate pollution Matching grants to local/state agencies for air pollution control |
|
1965 |
Motor Vehicle Air Pollution Control Act |
National standards for auto emissions Coordinated pollution control between United States, Canada, and Mexico Research into SO2 and auto emissions |
|
1967 |
Air Quality Act |
Air quality control regions (AQCRs) Air quality criteria Control technology documents State implementation plans (SIPs) Separate automotive emission standards for California |
|
President Nixon (1970) created the Environmental Protection Agency (EPA) by Executive Order |
||
|
1970 |
Clean Air Act Amendments |
National ambient air quality standards (NAAQS) SIPs to achieve NAAQS by 1975 New source performance standards (NSPS) National emission standards for hazardous air pollutants (NESHAP) Aircraft emission standards to be developed by EPA Automotive emission standards for hydrocarbons and CO for 1975 models and for NOx for 1976 models States allowed to adopt air quality standards more stringent than federal standards Motor vehicle emissions inspection and maintenance (I/M) program Citizens allowed to sue for air pollution violations |
|
1977 |
Clean Air Act Amendments |
Geographic regions (Classes I, II, III) to preserve air quality EPA-sanctioned emission offsets and emission banking within nonattainment regions State permits that require prevention of significant deterioration (PSD) studies |
|
Date |
Legislation |
Authorization |
|
|
|
|
Lowest-achievable emission rate (LAER) in nonattainment regions Delayed auto emission standards set in 1970 Clean Air Act Amendments Section 169A declared a national goal of preventing and remedying visibility impairment due to anthropogenic pollution in mandatory Class I areas |
|
|
1990 |
Clean Air Act Amendments |
Title I: Nonattainment regions Nonattainment regions for ozone are ranked in terms of pollution severity; each has a deadline to achieve NAAQS. New and amended NAAQS must be attained in 5 years with a possible extension for another 5 years. |
|
|
|
|
Classification (applicable only to ozone nonattainment areas) |
Years to achieve NAAQS |
|
|
|
Marginal |
3 |
|
|
|
Moderate |
6 |
|
|
|
Serious |
9 |
|
|
|
Severe |
15 (17 with a 1988 design value between 0.190 and 0.280 ppm) |
|
|
|
Extreme (Los Angeles) |
20 |
|
|
|
Title II: Mobile sources Gasoline reformulation toward lower toxic and VOC generation by 1997 Reduction in 1990 NOx emissions standards for light-duty vehicles (LDVs) by 60% beginning in 1994 Reduction in 1990 hydrocarbons emissions standards for LDVs by 40% beginning in 1994 Introduction of cold temperature (20°F) CO emissions standards set at 10 g/mile beginning in 1994 “Clean car” (ZEV, electric car) pilot program in California 150,000 vehicles by model year 1996 300,000 vehicles by model year 1999 Title III: Toxics Emissions of 189 hazardous air pollutants (HAPs) controlleda Mass ≥ 10 tons/yr for a specific HAP Mass ≥ 25 tons/yr for a combination of HAPs EPA-approved maximum achievable control technology (MACT) mandated After 8 years, EPA must promulgate more stringent standards to address residual risks where necessary. |
|
of 1963. In 1970, two landmark events took place that helped to establish the basic framework by which air quality is managed in the United States. These events were the creation of the U.S. Environmental Protection Agency (EPA) and the passage of the CAA Amendments of 1970. This framework was further developed and refined with the passage of the CAA Amendments of 1977 and 1990.
Five major goals for protecting and promoting human health and public welfare are identified in the CAA as amended:
-
Mitigating potentially harmful human and ecosystem exposure to six criteria pollutants: CO, NO2, SO2, O3, PM, and lead (Pb).4
-
Limiting the sources of and risks from exposure to HAPs, which are also called air toxics.
|
4 |
The term “criteria pollutants” derives from the requirement that EPA must describe the characteristics and potential health and welfare effects of these pollutants (see Chapter 2). |
-
Protecting and improving visibility impairment in wilderness areas and national parks.
-
Reducing the emissions of species that cause acid rain, specifically SO2 and NOx.
-
Curbing the use of chemicals that have the potential to deplete the stratospheric O3 layer.
The CAA prescribes a complicated set of responsibilities and relationships among federal, state, tribal,5,6 and local agencies. This matrix is referred to in this report as the nation’s air quality management (AQM) system. The federal government’s role is coordinated by EPA and is intended in part to provide a degree of national uniformity in air quality standards and approaches to pollution mitigation so that all individuals in America are assured a basic level of environmental protection. State and local governments are given much of the responsibility for implementing and enforcing the federally mandated rules and regulations within their jurisdictional domains, including developing and implementing specific strategies and control measures to meet national air quality standards and goals. Although many aspects of the AQM system assume a collaborative relationship between the federal, state, and local agencies, the CAA empowers EPA to oversee the activities carried out by those agencies. This oversight includes the power to impose federal sanctions and federally devised pollution-control plans on delinquent areas in some cases.
The federal courts also have a role in AQM. Final agency rules promulgated under the CAA are subject to judicial review, usually in the Court of Appeals for the District of Columbia Circuit, and the reviewing court may set aside any portion of a regulation found to be “arbitrary and capricious.” Under this standard, the courts take a “hard look” at the agency’s reasoning and the support in the rule-making record for critical factual conclusions, but the court is not supposed to substitute its judgment for that of the agency (Motor Vehicle Manufacturer’s Association v. State Farm Mutual Automobile Insurance Co., 463 U.S. 29, 43, 1983). A court will set aside an agency rule only if it finds that the decision was not based on a consideration of the relevant factors or that the agency committed a clear error of judgment. In addition, any citizen may file a civil action in district court against EPA that challenges the agency’s failure to perform any nondiscretionary act or duty, and the courts have the authority to order EPA to perform that act or duty and to compel agency action that is
“unreasonably delayed” (CAA § 304(a)(2), 42 U.S.C. § 7604(a)(2)). The courts have vigorously implemented this authority in the past to mandate, for example, a schedule for EPA action (Melnick 1983).
The nation’s AQM system may be conceptualized to operate in a variety of ways to meet the goals and requirements set forth in the CAA. The committee chose a model that describes the system’s operation in terms of four broadly defined sequential activities (Figure 1-3). The first three are the following:
-
Setting air quality standards and objectives (either in the CAA or by EPA).
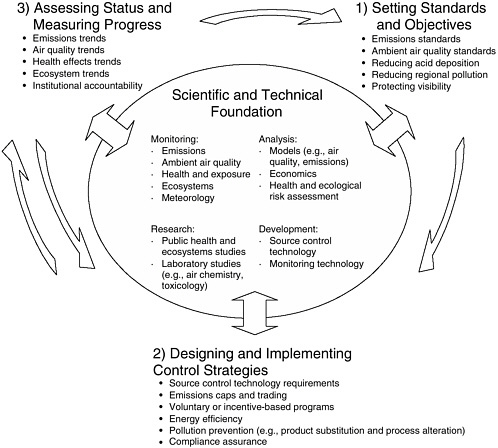
FIGURE 1-3 Idealized schematic showing three of the four sequential activities carried out by the nation’s air quality management system. The fourth iterative activity involves a return to activities 1 and/or 2 to account for new information to correct deficiencies identified in step 3. Bullets under each heading provide examples; listings are not necessarily comprehensive.
-
Designing and implementing control strategies to meet those standards and objectives.
-
Assessing status and progress.
Because the AQM system typically functions with substantial scientific, technological, and societal uncertainties, there is a need for a fourth iterative activity: to revisit the first and second activities, taking advantage of new information and any deficiencies identified in the third. Examples of the four activities are provided in Figure 1-3. A more-detailed discussion of how each of these activities is carried out in the United States is provided in subsequent chapters.
THE ROLE OF SCIENCE
Although an understanding of the causes and remedies of air pollution is not yet complete, it is now well-established that the vast majority of the air pollutants addressed by the CAA arise from the burning of fossil fuels and the emission of the myriad of materials and chemicals produced and used in the commerce of this country. However, for a number of broader societal and technical reasons, a total termination of the nation’s dependence on fossil fuels and the products and industrial processes that result in pollutant emissions is not a viable option. Indeed, the substantial disruption likely to result could conceivably cause greater damage to human health and welfare than that caused by air pollution in the United States. A more viable option, and the one our society uses, is to control air pollutants at concentrations that pose a minimal or acceptable level of risk to human health and welfare without unduly disrupting the technological infrastructure and economic engine that underpins the nation’s economy. To accomplish such control, science and technology are required. Their roles include the following:
-
Quantifying risks to human health and public welfare (such as ecosystems) associated with varying concentrations, mixtures, and rates of deposition of air pollutants to establish air quality standards and goals.
-
Quantifying the source-receptor relationships that relate pollutant emission rates to ambient pollutant concentrations and deposition rates in order to develop air pollution mitigation strategies to maximize benefits and minimize costs.
-
Quantifying the expected demographic and economic trends with and without air pollution control strategies to better account for growth in activity that might offset pollution control measures and to better design control strategies that are compatible with the economic incentives of those who must implement them.
-
Designing and implementing air quality monitoring technologies and methods for documenting pollutant exposures to identify risks and set priorities.
-
Designing, testing, and implementing technologies and systems for efficiently preventing or reducing air pollutant emissions.
-
Designing and implementing methods and technologies for tracking changes in pollutant emissions, pollutant concentrations, and human health and welfare outcomes to document and ultimately improve the effectiveness of air pollution mitigation activities.
As indicated in Figure 1-3, the aforementioned contributions of science and technology are made through monitoring, analysis, research, and development. Monitoring provides the data necessary to determine trends in emissions, air quality, and various health and ecosystem outcomes. Such observational data are essential for determining the effectiveness of regulations and assuring compliance, providing valuable input to air quality models, and supporting long-term health and ecosystem assessments. In addition, the data are used by the scientific research community. Analysis activities also provide critical information to air quality managers who use model results, risk assessments, and economic and other analyses to better characterize their air quality problems and the impacts of various control strategies. Finally, research and development efforts furnish advances in the fundamental understanding of the science and impacts of air pollution, the instruments needed for monitoring, and the technology available for controlling emissions. Thus, at each stage of CAA implementation, science and technology provide a fundamental basis for sound decisions; at the same time, the requirements of the CAA to continually improve air quality and the understanding of it serves as an important incentive to promote scientific and technological advances.
Although the inputs of science and technology are important, they are not the sole determinants of the success of an AQM system. Effective AQM decisions are made and implemented by elected and appointed leaders in the context of diverse social, economic, and political considerations. Successful AQM requires that the input from the scientific and technological communities is utilized by those leaders to produce adequate and cost-effective pollutant emission reductions for which a variety of societal considerations, including environmental justice, are taken into account. The U.S. AQM system entails the promulgation of rules and regulations on specific types of emissions, the institution of programs that provide incentives for the creative development of new technologies, and the use of emission control technologies and systems that reduce air pollution to a sufficient degree to protect public health. However, the effectiveness of AQM can be undermined by a breakdown in any of these components.
This report’s recommendations are aimed at improving the nation’s AQM system to better integrate the tools and methods of the scientific and technological communities, and to provide an improved mechanism for assuring that the system and its components are achieving the intended public benefits.
ESTIMATING THE COSTS AND BENEFITS OF THE FEDERALLY MANDATED AIR QUALITY MANAGEMENT SYSTEM
The management of the nation’s air quality is a major and complex undertaking. AQM in the United States involves the work of tens of thousands of people who monitor the concentrations of various air pollutants at over a thousand sites, regulate thousands of different emission sources, and maintain a multimillion dollar research and development program to better understand the sources, fate, and effects of air pollutants. The CAA requires regulatory control of air pollutants that have widely varying properties. Some pollutants are rapidly removed from the atmosphere so that effects are largely limited to the immediate source area. Other pollutants (such as O3 and PM) can be transported in significant amounts for hundreds to thousands of miles; therefore, their control requires cooperation between states and, in some instances, nations.
Implementation of the CAA has clearly contributed to the reduction in pollutant emissions in the United States. For example, over the past 30 years, the nation’s gross domestic product and total vehicle miles traveled increased by more than 2-fold, and its energy consumption increased by a factor of about 1.5. However, over the same period, EPA (2002a) reported that the total aggregate of emissions that directly affect the ambient concentrations of six criteria pollutants has decreased by 25% (see Figure 1-4). This trend suggests that the nation has been able to decouple, to some extent, pollutant emission rates from economic activity. EPA argues that the CAA played a major role in bringing about this decoupling. In the absence of the CAA, EPA estimated that emissions of CO, SO2, NOx, and PM in 1990 would have been larger by factors of about 2, 1.6, 1.4, and 3, respectively (EPA 1997). Others argued that factors other than environmental regulation (for example, increased income and technological advances) might be the main causes of the decrease in pollutant emissions (for example, Lomborg 2001; Pacala et al. 2003). However, Pacala et al. (2003) concluded that although a variety of factors contribute to observed benefits, regulation plays a prominent role. Although it is not possible to know precisely what the levels of pollutant emissions in the United States would be in the absence of a federally mandated AQM system, it is reasonable to conclude that this system has had an important role in controlling and lowering these emissions over the past 30 years.
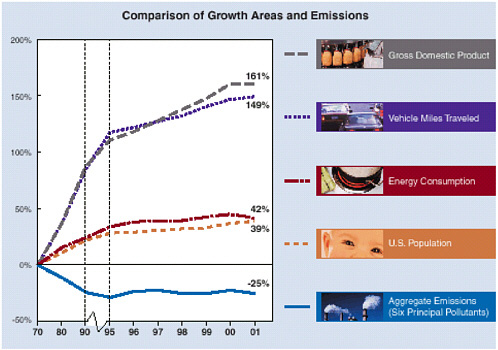
FIGURE 1-4 Comparison of growth areas and emission trends. Note that the trends in the graph (except for aggregate emissions) did not change substantially in 1995; only the scale of the graph changed. SOURCE: EPA 2002a.
EPA (1997) estimated that the benefits that have accrued in human health and welfare as a result of the aforementioned decreases in pollutant emissions have been substantial. The estimated benefits include about 100,000 to 300,000 fewer premature deaths per year and 30,000 to 60,000 fewer children each year with intelligence quotients below 70. However, these estimates are highly uncertain (OMB 1997). They require quantification of the air quality responses to pollutant emission changes and quantification of the human health and welfare responses to those air quality changes. Because it is difficult to isolate the effects of air pollution exposures from those of other risk factors that humans face daily, little direct empirical evidence is available to carry out the latter quantification. As a result, assessments of the benefits of pollution control often rely on complex models instead of direct empirical evidence.7 These models, in turn, tend to depend on a variety of estimated input parameters and assumptions.
Even more uncertainty is added when the benefits are compared with the costs of regulatory compliance by using a cost-benefit analysis, which
requires a monetization of the benefits—a typically controversial and difficult process (Croote 1999). Despite such difficulties, cost-benefit analysis is often used in government to evaluate the merit of environmental regulations. In fact, the 1990 CAA Amendments specifically require EPA to carry out periodic evaluations of the costs and benefits of the implementation of the act. Despite arguments by others identified above, most comprehensive cost-benefit analyses of the nation’s AQM system have suggested that the benefits of air pollution control have been equivalent to or exceeded the costs, albeit with significant uncertainties. For example, EPA estimated that the benefits of implementation of the CAA between 1970 and 1990 were $5–50 trillion greater than the costs (EPA 1997). Although others (Lutter and Belzer 2000; Brown et al. 2001) argue over whether EPA’s analysis overstates likely benefits and understates costs (also see Chapter 6), the White House Office of Management and Budget (OMB) recently reported that benefits of environmental regulations far outweigh the costs. OMB found monetary benefits over the years of regulation from 1992 to 2002 by EPA to range from roughly $121 billion to $193 billion and costs to range from $23 billion to $27 billion (OMB 2003a). A large fraction of aggregate benefits found by OMB pertain to rules limiting PM, NOx, and SO2. The SO2 provisions of the 1990 CAA Amendments alone account for $80 billion of the aggregate benefit estimate.
THE FUTURE
Despite the nation’s significant progress in improving air quality, the problems posed by pollutant emissions in the United States are by no means solved. For example, it is estimated that the demand for electrical power in the United States will increase by 40% over the next 20 years (DOE 2003), and a substantial amount of the increased demand will be met by the burning of fossil fuels (see Figure 1-5). Increases of over 50% in total vehicle miles traveled by light-duty vehicles on the nation’s roads and highways, as well as increases of off-road vehicular use, are also projected (DOE 2003). Thus, substantial improvements in cleaner power-generating and automotive technologies will be needed if the nation is to maintain the current level of air quality. Some of these improvements are already under way (for example, response to Tier II emission standards for automobiles [see Chapter 4]), and others are being considered (for example, multipollutant emission caps for power plants [see Chapter 5]).
However, even as additional emission reductions and new technologies are needed in the coming years just to maintain the current level of air quality, additional effort is likely to be deemed necessary to adequately protect human health and welfare. A number of major goals and requirements of the CAA Amendments of 1990 have yet to be met (Figure 1-6);
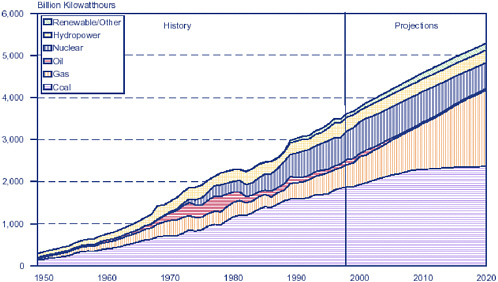
FIGURE 1-5 Electricity generation by fuel in billion kilowatt hours, 1949–1999, and projections for the Reference Case, 2000–2020. Projections: National Energy Modeling System, run M2BASE.D060801A. SOURCE: EIA 2000.
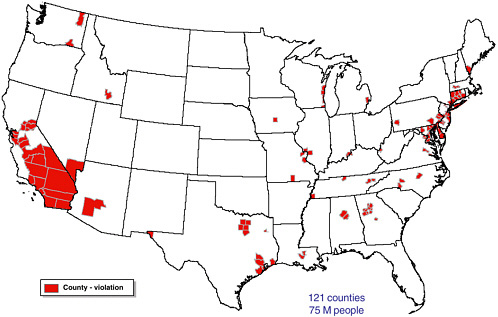
FIGURE 1-6 Counties in the continental United States where any NAAQS were violated in 1999. SOURCE: EPA 2002c.
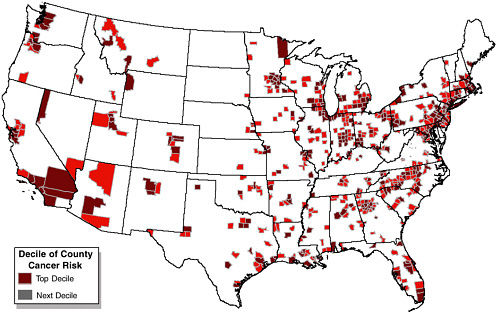
FIGURE 1-7 High cancer risk counties for urban air toxics in 1996. SOURCE: EPA 2002c.
those include total compliance with the NAAQS for O3 and PM and the establishment of a technology-based regulatory program to reduce the emissions of all 188 HAPs (see Figure 1-7). Perhaps even more important, new data on the health effects of O3 and PM led to the promulgation in 1997 of stricter NAAQS for O3 and new NAAQS for PM2.5 that will require even greater reductions in pollutant emissions than had been envisioned at the time the CAA Amendments of 1990 were enacted (see Figure 1-8). EPA (1999a) estimated that complete implementation of the 1990 CAA Amendments costs the nation about $27 billion annually. Most of the costs are directly borne by industry and consumers. Approximately $600 million is expended annually in federal funds, and a large amount is expended by states, tribes, and localities. Of the federal funds, approximately $200 million is dedicated to air quality research and monitoring (OMB 2003b).
CHARGE TO THE COMMITTEE ON AIR QUALITY MANAGEMENT IN THE UNITED STATES
Given the sizable investment in air quality management envisioned for the nation over the next decade and the role science and technology can have in optimizing the effectiveness of this investment, the following ques-
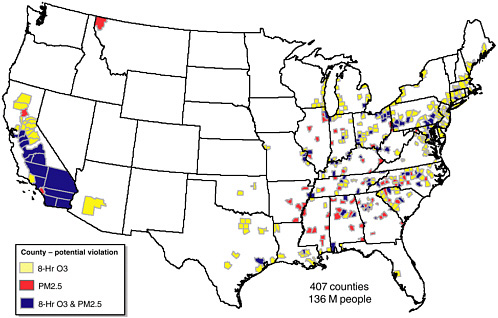
FIGURE 1-8 Potential violations of the PM2.5 (1999–2000 data) and 8-hr O3 (1997–1999 data) NAAQS by county. SOURCE: EPA 2002c.
tions arise: (1) how well are scientific and technological advances incorporated into the current AQM system? and (2) to what extent could the AQM system be improved by changing the way scientific understanding and approaches and new technologies are used for air quality management in the United States? These questions are the basis for this report.
The committee has been charged to develop scientific and technical recommendations for strengthening the nation’s AQM system with respect to the way it identifies and incorporates important sources of exposure to humans and ecosystems and integrates new understanding of human and ecosystem risks.8
In carrying out its charge, the committee has evaluated the effectiveness of the major air quality provisions of the CAA and their implementation by federal, state, tribal, and local government agencies. It also reviewed scientific and technical aspects of the policies and programs intended to manage important air pollutants, including but not limited to criteria pollutants and HAPs. In addition, the committee evaluated scientific and technical aspects of current approaches for health and environmental problem identification, regulatory standards development, AQM plan development, plan imple-
|
8 |
The committee’s full Statement of Task is included in Appendix B. |
mentation, compliance assurance, and progress evaluation. Stratospheric O3 protection and greenhouse gas emission control were not included in the scope of the study except in regard to strategies in tropospheric air quality control programs to control emissions.
A wide range of external factors beyond the scientific and technical aspects of air quality can drive the character and effectiveness of an AQM system and are relevant to our review. Governmental policies on economic growth, energy production and use, transportation, and land use, for example, affect pollutant emissions and can therefore reinforce or frustrate AQM policies. Decisions and practices of consumers concerning the technologies and products they purchase and use also affect pollutant emissions.
Legal and institutional factors also affect the implementation of AQM. Most important, the nation’s federal system of government, with specific authorities assigned to the federal government and others to the states, limits the kinds of regulatory structures that can be used to administer the AQM system in the United States. For example, although air pollution issues often demand regional controls, all such controls can only be enforced at the state or federal levels.
It is beyond the scope of this report to comprehensively analyze these external factors and assess how they could be changed to enhance the effectiveness of the nation’s AQM system. However, at each stage in considering implementation of the CAA, the committee attempted to take into account the degree to which these larger factors could reduce the effectiveness of specific control measures (for example, the growth in travel and its relation to automobile emission standards). The committee found that considerable progress in air quality improvement has been accomplished in the United States over the past 2-3 decades even in the face of these confounding external factors. Thus, the recommendations advanced here tend to be evolutionary in nature and do not involve a major overhaul of the AQM system.
REPORT STRUCTURE
To provide a basic foundation for conclusions and recommendations, the committee reviewed and critiqued the key elements of the CAA and the concomitant methods and approaches to manage air quality in the United States. The discussion in Chapter 2 focuses on how standards and goals are set. Chapter 3, 4, and 5 describe the design and implementation of control strategies adopted by federal, regional, state, and local governments. Chapter 6 discusses how progress in meeting the AQM goals is measured, particularly with respect to health and ecosystem outcomes. After consideration of the major air quality challenges facing the nation in the coming
decades, Chapter 7 provides a series of recommendations. In formulating these recommendations, the committee endeavored to look beyond the statutorily mandated constraints, methods, and approaches currently imposed on the nation’s AQM system by the CAA and other relevant acts. Thus, pursuit of many of the recommendations will require broad acceptance within the policy-making communities and perhaps legislative action.






















