8
Research Issues
The first attempts to ascertain the prevalence of late effects of childhood cancer were made in the mid 1970s by Anna Meadows and her colleagues at the Children’s Hospital of Philadelphia (Meadows and D’Angio, 1974; Meadows et al., 1975; Meadows and Evans, 1976). Nearly three decades later, much remains to be known. This chapter reviews types of research of relevance to cancer survivorship, describes major ongoing survivorship research initiatives, and summarizes the state of survivorship research and levels of support. The chapter concludes with a listing of prioritized areas of research identified by the Board.
SURVIVORSHIP RESEARCH
Research of relevance to survivors of cancer generally aims to: (1) identify late effects and their implications for health and well-being; (2) modify and improve cancer treatment to minimize late effects; and (3) develop post-treatment interventions to reduce the consequences of late effects for individuals and their families. Research to date has focused primarily in the first two areas. There has been limited research on interventions to prevent late effects among high-risk individuals or on strategies to ameliorate the consequences of late effects among individuals who have developed them. Also lacking are studies of survivors and their families regarding the psychosocial burden and economic costs associated with late effects.
Research to Identify Late Effects and Their Implications
Most of what has been learned about the late effects of cancer treatment has been the result of longitudinal or cross-sectional studies of survivors treated according to standard protocols within single institutions. These investigations have provided important insights into the frequency and potential risk factors for late effects occurring at relatively high frequency. Such studies have, for example, helped to identify impaired fertility following alkylating agent treatments, hearing impairment following cisplatin-based chemotherapy regimens, and restrictive lung disease following pulmonary radiation. Because these studies were conducted in a single institution, included few subjects, and involved treatment that did not vary markedly across subjects, estimates of risk associated with variations in treatment could usually not be well quantified.
Some studies of long-term survivors have been carried out within established pediatric cooperative groups (e.g., Children’s Cancer Group [CCG], Pediatric Oncology Group [POG], National Wilms Tumor Study Group [NWTSG], Intergroup Rhabdomyosascoma Study Group [IRSG]).1 The primary objective of these groups is to conduct therapeutic clinical trials; and while questions of health-related outcomes are of interest, the resources necessary to support such studies have generally not been available. The potential to conduct research on late effects in these settings is substantial because as many as 50 to 60 percent of U.S. children with cancer are treated at Children’s Oncology Group (COG) member institutions (Shochat et al., 2001). A Late Effects Committee established in the mid 1980s promotes and facilitates research on adverse health-related outcomes after childhood cancer (Bhatia, 2002). Research efforts in this area have, however, been limited (Box 8.1). Some of the early findings on cognitive late effects among children with leukemia emerged from follow-up studies of children enrolled in clinical trials of the Cancer and Leukemia Group B Cooperative Group (CALGB). Investigators of the National Wilms’ Tumor Study Group (NWTSG) were instrumental in recognizing the late effects of therapies among survivors of Wilms’ tumor and adjusting treatment (e.g., omission of radiotherapy) in an effort to avoid these late side effects.
COG has plans for a long- term follow-up center to provide a cost-effective mechanism for tracking and maintaining contact with patients and their families and for collecting long-term data on health and health-related quality of life. This system complements a web-based remote data entry
|
1 |
The National Cancer Institute-sponsored pediatric cooperative groups, now merged into the Children’s Oncology Group, are also discussed in Chapter 5. |
|
Box 8.1 Past, Current, and Planned Research Activities of COG’s Late Effects Committee Past studies: 8 publications on second malignant neoplasms, neuropsychological, cardiac and other health-related outcomes (cancers studied included acute lymphoblastic leukemia (ALL), Wilms tumor, Rhabdomyosarcoma, osteosarcoma, Ewing’s sarcoma) Current studies:
Proposed studies:
SOURCE: Bhatia, 2002. |
system for all patients entering COG studies at member institutions. Member institutions report each individual diagnosed with cancer who is seen at the institutions to the COG Research Data Center Registry of New Malignancies in Children and Adolescents (Children’s Oncology Group, 2001). Patient responses to therapy are centrally collected, monitored, and analyzed. With these mechanisms in place, patients treated within COG institutions could potentially be followed prospectively and their outcomes linked to their primary treatments.
For research encompassing extended intervals from the original cancer diagnosis (e.g., two or more decades), well-designed cohort studies are needed. Organized consortia have been able to successfully address selected topics relating to childhood cancer survivorship. The Late Effects Study Group (LESG), an international consortium of institutions, has provided important insights into the risk of second malignancies, particularly among Hodgkin’s disease survivors (Bhatia et al., 1996).
Childhood Cancer Survivor Study (CCSS)
The Childhood Cancer Survivor Study (CCSS) is a large, National Cancer Institute-sponsored, multi-institutional initiative to organize a cohort of survivors for long-term follow-up. Included in the cohort are 20,276 childhood cancer survivors diagnosed between 1970 and 1986 who have survived 5 or more years after treatment (Robison et al., 2002); http://www.cancer.umn.edu/ltfu, accessed March 25, 2002). The study also includes approximately 3,500 siblings of survivors, who serve as control subjects for the study. The CCSS cohort has been assembled through the efforts of 27 participating centers in the United States and Canada and is coordinated by investigators at the University of Minnesota. Initiated in 1993, the study was recently funded by the National Cancer Institute for continuation through 2004.
Selected demographic and cancer-related characteristics of the CCSS cohort are shown in Table 8.1.
To date, baseline information has been collected, including demographic data and treatment history of study participants as well as data regarding the occurrence of cancer and certain hereditary conditions in their first-degree relatives. In addition to completing the baseline questionnaire, survivors agreed to release medical records of their cancer treatment. Participating centers have provided detailed abstracts of treatment records for all consenting survivors enrolled in the cohort from their site. Treatment information includes cumulative dose-specific exposure to chemotherapy, surgical procedures, and radiation therapy. Follow-up data (information from medical charts and self-reported information on health status and behaviors) has been collected from participants, and investigators have begun collecting biologic materials, including tumor specimens from participants who develop subsequent cancers, buccal (cheek) cells from all participants—including siblings—as a source of genomic DNA, and peripheral blood samples from a subset of survivors and sibling controls to establish cell lines as a source of genomic DNA and RNA. These materials will be used to evaluate the role of genetics in the occurrence of cancer and long-term adverse outcomes among survivors.
A likely outcome of a large cohort study such as the CCSS is the early identification of emerging or changing patterns of late effects. With the increasing age of the population of childhood cancer survivors, it is anticipated that new adverse health-related outcomes are likely to emerge. With continued surveillance, these new and/or unexpected events can be ascertained and characterized. Cohorts like CCSS can provide an early warning system for the emergence of unrecognized late effects of treatment. An example is the relatively recent appreciation of the magnitude of excess risk for breast cancer in patients treated for Hodgkin’s disease during child-
TABLE 8.1 Characteristics of the CCSS Participants (n = 14,054)
|
Characteristic |
Percent |
|
Sex |
|
|
Male |
54 |
|
Female |
46 |
|
Race |
|
|
White |
87 |
|
Black |
2 |
|
Hispanic |
5 |
|
Asian/Pacific Islander |
1 |
|
Other |
5 |
|
Diagnosis |
|
|
Leukemia |
34 |
|
Brain/CNS |
13 |
|
Hodgkin’s |
14 |
|
Non-Hodgkin’s |
7 |
|
Kidney |
9 |
|
Neuroblastoma |
7 |
|
Soft-tissue sarcoma |
9 |
|
Bone |
8 |
|
Age at diagnosis |
|
|
<1 year |
7 |
|
1 to 3 years |
25 |
|
4 to 7 years |
22 |
|
8 to 10 years |
11 |
|
11 to 14 years |
17 |
|
15 to 20 years |
18 |
|
Age at entry on CCSS |
|
|
<20 years |
32 |
|
20 to 29 years |
42 |
|
30 to 39 years |
22 |
|
40+ years |
3 |
|
SOURCE: Robinson et al., 2002. |
|
hood. This observed association was identified through the LESG cohort of Hodgkin’s disease survivors. Adverse events recognized in smaller studies can be confirmed and further defined in existing cohort studies and the information used to develop new protocols that seek to minimize such complications. Some late effects may be observed among long-term survivors following exposures to therapies that are no longer in use. Studies of more recent cohorts exposed to newer treatments are needed to ascertain declines in treatment-related late effects and the emergence of new late effects.
Ongoing contact and interaction offers an opportunity to provide survivors with information regarding cancer survivorship, and allows survivors to make their concerns and ideas known.
Current and proposed research based on the CCSS cohort is shown in Box 8.2.
While cohort studies such as the CCSS provide invaluable information pertaining to late effects, such studies are difficult to conduct and have some inherent limitations. The greatest challenge in these studies is enrolling and maintaining a cohort of patients that represents the population of patients eligible for the study. A nonrepresentative cohort could result if only certain patients volunteered for the study, or if patients who did enroll were lost to follow up on the basis of an important study variable. If, for example, patients who entered the study and then became very ill were more difficult to follow up, their absence in the follow-up period could contribute to the failure to associate an exposure during treatment to subsequent morbidity or mortality.
Gathering longitudinal information on children is especially difficult because of the problem of frequent moves. Participants may also be lost to follow-up when they turn age 18 and must provide consent for study participation (prior to that age, parents provide consent). Interest in participating in research may wane among adolescents and young adults. Obtaining medical records regarding care is complicated when children’s providers change and when they transition from pediatric to adult providers. Obtaining accurate information regarding treatment-related exposures and health-related outcomes is key to the success of a cohort study, but the inaccessibility and sometimes incomplete nature of medical records can compromise the ability to obtain such information.
Resources can be marshaled to overcome many of the practical difficulties of tracking cohort members and keeping them engaged in the study. A well-conducted cohort study can provide estimates of the prevalence of late effects and the degree of excess risk. It can also provide invaluable information on quality-of-life outcomes such as achievement and progress in school, employment, and use and satisfaction with health care. Such information can help guide program development and the design of interventions.
Research to Modify or Improve Cancer Treatment to Minimize Late Effects
Once late effects have been recognized, clinical trials can be designed to test modifications of treatments that are likely to reduce their occurrence. A clinical trial involving children with localized lymphomas demonstrated that radiation therapy can be safely omitted without jeopardizing high cure rates (Link et al., 1997). More recently, researchers have conducted trials
|
Box 8.2 Current and Proposed CCSS Analyses Mortality
Physical late effects
Reproductive consequences
Genetics
Psychosocial late effects
Behavioral risk factors
Economic and health care consequences
Methods
SOURCE: http://www.cancer.umn.edu/ltfu, accessed March 25, 2002. |
of agents designed to protect normal tissues from the cytotoxic effects of chemotherapy and radiation (amifostine or dexrazoxane [Zinecard®]) to evaluate their promise of fewer adverse effects in survivors of cancer treatment. Another strategy to minimize late effects while maintaining high rates of survival involves tailoring treatments to patients’ risk status, and assigning less intense treatment for patients with a lower risk of relapse, with the expectation that this group would have less frequent and severe acute toxicity, fewer and milder late effects, and a better quality of long-term survival. Risk strata are determined using a combination of clinical and biologic prognostic indicators. There are many examples of evaluations of risk-adapted treatments, including those for childhood acute lymphoblastic leukemia (Smith et al., 1996), rhabdomyosarcoma (Raney et al., 2001), and neuroblastoma (Castleberry, 1997). Long-term follow-up is needed as part of these studies to assure safety and efficacy.
Research on Interventions to Reduce the Consequences of Late Effects
Interventional research designed to prevent the emergence of late effects and to reverse or forestall the progression of already-established complications of cancer treatment is less advanced, but urgently needed. Approaches in this area include the use of hormone replacement therapy or chemoprevention for reduction of the risk of second malignant neoplasms among high-risk populations, and after-load reduction therapy with ACE inhibitors such as enalapril in patients with asymptomatic left ventricular dysfunction following anthracycline treatment. Secondary prevention approaches also need to be tested in efforts to forestall or ameliorate late effects. Lifestyle counseling, family interventions to address psychosocial issues, and educational interventions to improve cognitive function are examples of such areas of needed research.
STATUS OF CHILDHOOD SURVIVORSHIP RESEARCH
This section first describes publication trends in childhood cancer survivorship and then summarizes federal and private support for such research.
Publication Trends
The evaluation of trends in research publications is one way to assess the level of activity within a discipline. One resource that can be used to track such studies is the National Library of Medicine’s PubMed database, which stores information about individual citations including index terms used to characterize each article (articles are indexed according to a dictio-
nary of medical subject headings called MESH terms). The PubMed database includes citations from MedLine, HealthStar, and other bibliographic databases.
There have been few English-language articles on childhood cancer survivorship in recent years; however, the volume of articles appears to have increased somewhat from 1993 to 2001, from 101 to 153 (Figure 8.1). In 2001, these articles accounted for less than 3 percent of all pediatric cancer-related citations indexed in the medical literature (Figure 8.2). While there are relatively few published articles regarding childhood survivorship, they represent a relatively large share of citations on survivorship, nearly a third (31.9 percent) of such citations in 2001 (Figure 8.3). These trends reflect articles written in the English language, but not necessarily by U.S. investigators. Figures 8.1, 8.2, and 8.3 therefore reflect trends in the general medical literature, and not necessarily trends in the United States. These trends must be interpreted with caution because they may reflect changes in the ways in which MESH headings were applied to index the literature rather than real increases in cancer-related research. The term “survivors,” the MESH heading used to identify citations, refers to “per-
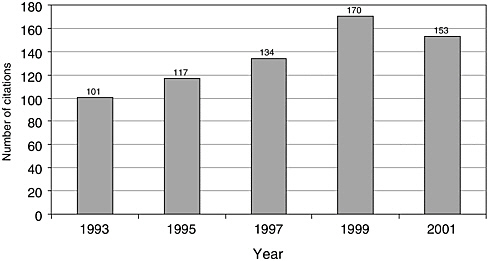
FIGURE 8.1 PubMed citations for childhood cancer survivorship research, 1993-2001.
NOTE: A wildcard allows any ending to follow the base word in replace of the asterisk. For instance, survivors and survivorship would be included in a keyword search of survivor*.
SOURCE: National Library of Medicine’s PubMed database.
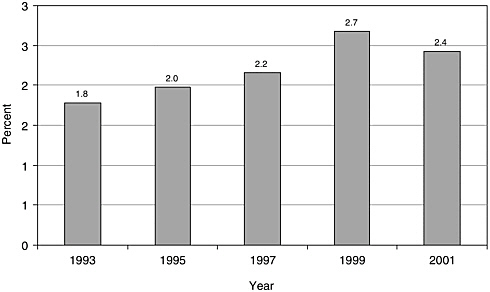
FIGURE 8.2 PubMed citations for childhood cancer survivorship research as a percentage of all pediatric cancer-related citations, 1993-2001. Percentages were calculated as the numbre of childhood cancer survivorship-related citations (as described in Figure 8.1) divided by the total number of citations categorized under the MESH terms “neoplasms” and either “pediatrics” or “child.” Only articles published in English are counted.
SOURCE: National Library of Medicine’s PubMed database.
sons who have experienced a prolonged survival after serious disease or who continue to live with a usually life-threatening condition as well as family members, significant others, or individuals surviving traumatic life events” (http://www.ncbi.nlm.nih.gov:80/entrez/meshbrowser.cgi, accessed March 25, 2002). Both keywords and MESH headings were used to identify citations. There would be an underestimate of survivorship- related citations if the “Survivors” MESH term was not applied by abstractors to the citations or if the title and abstracts of articles varied in their inclusion of keywords (e.g., survivors, survivorship, late effect, long-term effects).
SUPPORT FOR SURVIVORSHIP RESEARCH
A more direct way to assess the status of U.S.-based research on childhood cancer survivors is to describe topics of investigation and levels of research spending. There is no one comprehensive source of information on research support, and as part of its review, the National Cancer Policy Board relied on the following sources:
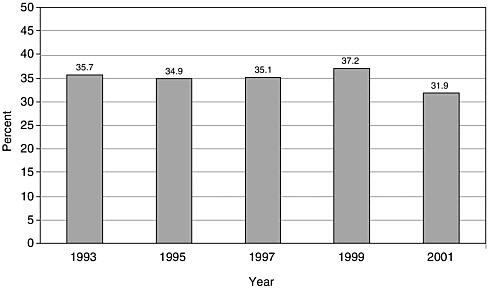
FIGURE 8.3 PubMed citations for childhood cancer survivorship research as a percentage of all cancer survivorship-related research, 1993-2001. Percentages were calculated as the numbre of childhood cancer survivorship-related citations (as described in Figure 8.1) divided by the total number of citations categorized under the MESH terms “neoplasms” and either “pediatrics” or “child.” Only articles published in English are counted.
NOTE: A wildcard allows any ending to follow the base word in replace of the asterisk. For instance, survivors and survivorship would be included in a keyword search of survivor*.
SOURCE: National Library of Medicine’s PubMed database.
-
Listings of research projects in the CRISP (Computer Retrieval of Information on Scientific Projects), a searchable database of federally funded biomedical research projects conducted at universities, hospitals, and other research institutions,2
-
Review of organizations’ web sites,
-
Presentations to the Board by agency representatives (e.g., NCI, Office of Cancer Survivorship), and
|
2 |
The database, maintained by the Office of Extramural Research at the National Institutes of Health, includes projects funded by the National Institutes of Health (NIH), Substance Abuse and Mental Health Services (SAMHSA), Health Resources and Services Administration (HRSA), Food and Drug Administration (FDA), Centers for Disease Control and Prevention (CDCP), Agency for Health Care Research and Quality (AHRQ), and Office of Assistant Secretary of Health (OASH) (https://www-commons.cit.nih.gov/crisp/, accessed March 25, 2002). |
-
Contacts with organization representatives (e.g., American Cancer Society, Lance Armstrong Foundation, Centers for Disease Control and Prevention).
The Board’s review of research support is limited to federal agencies, primarily the National Institutes of Health and selected private organizations and foundations (i.e., American Cancer Society, Lance Armstrong Foundation). Although these organizations are not the only sponsors of research on cancer survivorship, they represent the major funding sources for such research. Excluded from this review is research supported by health plans, insurers, pharmaceutical companies, and other private organizations. Much of the research done in those settings is proprietary.
Federal Research Support
National Cancer Institute
NCI supports research through a variety of mechanisms, including cooperative agreements (referred to as U01 or U19 cooperative agreements), and investigator-initiated grants (referred to as R01 and P01 grants). The largest investment in childhood cancer research is through support of pediatric clinical trials cooperative groups and other research consortia. Each year about 4,000 children enter one of approximately 100 ongoing clinical trials sponsored by NCI (National Cancer Institute, 2002a). And as part of the Childhood Cancer Survivor Study, a cohort of more than 20,000 5-year survivors are being followed. In fiscal year 2001, NCI spent $128 million to support studies of the biology, causes, and effective treatment of pediatric cancer, and to support research on the health status and well-being of children surviving cancer (National Cancer Institute, 2002a). Much of this research is conducted through the following organized pediatric cooperative groups and research consortia (National Cancer Institute, 2002a):
-
Children’s Oncology Group involves 238 member institutions in the conduct of clinical trials for children and adolescents with cancer (see map showing U.S. member institutions in Chapter 5). The group was formed in 2000 to merge the Children’s Cancer Group (CCG), Intergroup Rhabdomyosarcoma Study Group (IRSG), National Wilm’s Tumor Study Group (NWTSG), and the Pediatric Oncology Group (POG),
-
Pediatric Brain Tumor Consortium involves nine academic institutions that conduct phase 1 and 2 clinical evaluations of new therapeutic drugs, intrathecal agents, delivery technologies, biological therapies, and radiation treatment strategies in children and young adults (up to age 21) with primary CNS tumors,
-
New Approaches to Neuroblastoma Therapy Consortium involves eight university and children’s hospitals to test promising new therapies for neuroblastoma, and
-
Childhood Cancer Survivor Study involves 27 participating centers in the United States and Canada in following a cohort of over 20,000 5-year survivors of childhood cancer (see description above). The study was initiated in 1993 and is funded through 2004.
Office of Cancer Survivorship The locus of cancer survivorship research at the federal level is the NCI’s Office of Cancer Survivorship (OCS), established in 1996 to support research on the physical, psychosocial, and economic consequences of cancer among survivors (of all ages), their families, and caregivers (National Cancer Institute, 1999; National Cancer Institute, 2002b). The OCS supports research related to:
-
the identification, prevention, and amelioration of the late effects of cancer and its treatment,
-
follow-up care and surveillance of cancer survivors, and
-
communication to cancer survivors and their families and public education regarding survivorship issues.
OCS awarded its first research grants in 1997, totaling $4 million over 2 years (National Cancer Institute, 1999). In 1998, OCS awarded another $15 million over 5 years. OCS awarded $1 million in 2000 to support supplements to comprehensive cancer centers (P30s) to conduct pilot or exploratory research on issues related to the functioning of family members of survivors. In 2001, using a similar mechanism, OCS awarded an additional $1.06 million to support pilot research on issues faced by minority and underserved cancer survivors. The OCS has also participated in two trans-NIH Requests for Applications (RFAs), which led to funding of a mind-body center in cancer at a cost of $10 million over 5 years, and research on adherence to post-treatment interventions budgeted at $2 million over 4 years. While OCS does not have any initiatives with set-aside funds designated for 2002, the office actively encourages investigators to utilize a number of existing program announcements (PAs). These include s the R03 and R21 Behavioral PAs, the Epidemiologic Cohort PA, and a number of trans-NIH collaborative initiatives (such as those on sleep, palliative care, physical activity), all of which contain language relevant to cancer survivorship research, designed to stimulate and support research (Julia Rowland, NCI Office of Cancer Survivorship, personal communication to Maria Hewitt, June 19, 2002).
NCI-supported research in childhood survivorship is shown in Box 8.3 along with other NIH-supported survivorship research.
In its budget proposal for Fiscal Year 2004, the NCI has identified cancer survivorship as an “Extraordinary Opportunity for Investment.” Extraordinary Opportunities for Investment are identified with formal input from members of the research community, advisory groups, and advocacy organizations and represent areas of discovery that hold promise for making significant progress against all cancers. The budget proposal includes a request for $46 million to support the following six objectives (National Cancer Institute, 2002c):
-
expand research efforts to understand the biological, physical, psychological, and social mechanisms and their interactions, that affect a cancer patient’s response to disease, treatment, and recovery,
-
accelerate the pace of intervention research in order to reduce cancer-related chronic and late morbidity and mortality,
-
develop tools to assess the quality-of- life and care of post-treatment cancer survivors and their family members,
-
enhance NCI’s capacity to track outcomes for cancer survivors,
-
ensure the development and dissemination of new interventions and best practices, in collaboration with other federal and health- or cancer-related professional and non-profit organizations, and
-
expand the scientific base for understanding the biologic and physiologic mechanisms in the adverse late effects of current and new cancer treatments.
Specific initiatives identified within each of these objectives relate to cancer survivors of all ages, including survivors of childhood cancer. One initiative—the establishment of a separate registry for pediatric cancer survivors seen within the pediatric clinical trials network—is specific to survivors of childhood cancer.
Health Resources and Services Administration
In 1981 Congress authorized a federal set-aside for Special Projects of Regional and National Significance (SPRANS) as part of the MCH Block Grant (Health Resources and Services Administration, 2000b). Fifteen percent of MCH Block Grant funds support research, training, and demonstration programs (Table 8.2).
Several SPRANS-supported projects and projects supported by another set-aside program authorized by Congress in 1989 (Community Integrated Service Systems or CISS) relate to the integration of services for children with special health needs. Some of these programs attempt to facilitate the development of comprehensive systems of care for individuals with complex chronic conditions such as hemophilia, sickle cell disease, and trau-
|
Box 8.3 Childhood Survivorship Research Supported by the National Institutes of Health (Extramural Grants) NCI, Office of Cancer Survivorship
Other NCI
Other NIH National Center for Research Resources
|
TABLE 8.2 Special Projects of Regional and National Significance (SPRANS) Grant Funding Levels, Fiscal Year 2000 (in Millions), by Category of SPRANS Grant
|
Category |
Funding level ($ in millions) |
|
|
Total |
$109.14 |
|
|
• |
MCH research |
8.53 |
|
• |
MCH training |
41.83 |
|
• |
Genetic disease testing, counseling, and information dissemination |
9.20 |
|
• |
Hemophilia diagnostic and treatment centers |
5.35 |
|
• |
Other special projects to improve maternal and child health |
44.24 |
|
SOURCE: Health Resources and Services Administration, 2000b. |
||
National Institute for Nursing Research
National Institute for Child Health and Human Development
National Institute for Deafness and Other Communication
National Institute of Mental Health
NOTE: This list was compiled using the OCS current research portfolio and the following four searches of the Computer Retrieval of Information on Scientificc Projects (CRISP) database: (1) “pediatric” and “cancer” and “survivors”; (2) “child” and “cancer” and “survivors”; (3) “child” and “cancer” and “late” and “effects;” and 4) “pediatric” and “cancer” and “late” and “effects.” SOURCE: CRISP, accessed on June 6, 2002; OCS, 2002; Julia Rowland, Director, NCI Office of Cancer Survivorship, personal communication to Maria Hewitt, June 20, 2002. http://www.crisp.cit.nih.gov/. |
matic brain injury. Individuals with these conditions share some similarities with childhood cancer survivors in terms of their continuing care needs (Table 8.3).
Private Research Support
This section of the report describes the research activities of two private national organizations that support cancer research relevant to survivors of childhood cancer, the American Cancer Society and the Lance Armstrong Foundation. Many other private organizations provide support for education, advocacy, and service programs (e.g., Candelighters Childhood Cancer Foundation; the Starbright Foundation) or provide research support to a local hospital or university (e.g., Children’s Cancer Research Fund of the University of Minnesota). Some disease-specific foundations also have small research portfolios devoted to children’s cancer (e.g., Children’s Brain Tumor Foundation; the Leukemia and Lymphoma Society), but this re
TABLE 8.3 Selected State Projects Supported Through Special Projects of Regional and National Significance (SPRANS) and Community Integrated Service Systems (CISS) Grants
|
State |
Description of project |
|
|
Children with special health needs projects |
||
|
Alaska |
• |
Developing systems of specialty care in rural and remote Alaska |
|
|
• |
Integrating pathways between the medical home and early intervention system using a parent navigation system |
|
California |
• |
Developing of a seamless, integrated system of care |
|
Kansas |
• |
Building systems for children with special health care needs in child care settings |
|
Minnesota |
• |
Developing transition from school to work services for youth with disabilities |
|
New York |
• |
Developing guidelines and outcome indicators for asthma, spina bifida, and sickle cell disease for managed care providers |
|
Oklahoma |
• |
Developing systems for children with special health needs and their families |
|
Oregon |
• |
Promoting partnerships between families of children with special health care needs and managed care plans |
|
Vermont |
• |
Implementing changes in the education and practice of families and professionals |
|
Hemophilia and sickle cell disease projects |
||
|
Alabama |
• |
Development of a statewide regionalized pediatric care system for individuals with hemoglobinopathies |
|
Iowa |
• |
Ensuring that a full range of services exist for adolescent and young adult patients with congenital bleeding disorders |
|
Louisiana |
• |
Providing for transition to adult care for adolescent sickle cell patients |
|
North Carolina |
• |
Providing comprehensive services for individuals with hemophilia and their families |
|
Texas |
• |
Providing comprehensive services, including medical and psychosocial services, for persons with hemophilia and related bleeding disorders, and for all complications of the disease |
|
Traumatic brain injury (TBI) projects |
||
|
Arizona |
• |
Developing a continuum of care for children with TBI and their families |
|
Florida |
• |
Enhancing the continuum of care and long-term supports for individuals with TBI through the development of interagency linkages and partnerships |
|
Missouri |
• |
Developing a comprehensive community-based program to meet the needs of individuals with TBI |
|
Nevada |
• |
Updating and implementing a statewide action plan to ensure a comprehensive community-based system of care |
|
SOURCE: Health Resources and Services Administration, 2000a. |
||
search is devoted almost exclusively to basic and clinical research that does not have a focus on survivorship. St. Jude Children’s Research Hospital, located in Memphis, Tennessee, sponsors research and treatment of pediatric cancer, but does not have an extramural research program (see a description of its services in Chapter 5). The National Childhood Cancer Foundation supports research of the Children’s Oncology Group and awards fellowships to outstanding young physicians to encourage their research work in the field of pediatric oncology (www.nccf.org/foundation/fellowships.asp, accessed March 25, 2003). Only national organizations with a research grant program were included in the Board’s review.
American Cancer Society
The American Cancer Society (ACS) is a nationwide, community-based, voluntary health organization involved in research, education, advocacy, and service (http://www.cancer.org/docroot/AA/AA_0.asp, accessed March 25, 2003). The ACS’s overall annual expenditure in research was more than $130 million in Fiscal Year 2001, which includes extramural grants, intramural epidemiology and surveillance research, and an intramural behavioral research center. The extramural research program focuses primarily on peer-reviewed projects initiated by beginning investigators working in leading medical and scientific institutions.
A review of the ACS portfolio of extramural grants suggests that a total of $1.5 million was spent on four research projects related to pediatric cancer survivorship (Cheri Richard, Research Program Analyst, American Cancer Society, personal communication to Maria Hewitt, April 5, 2002):
-
The Impact of Medical Treatment on the Quality of Life of Childhood Cancer Survivors: A Case Controlled Study
-
Validation Testing of a New Brain-specific Measure for Pediatric Cancer Patients and Survivors
-
Quality of Life in Children Who Survived Neuroblastoma
-
Health Profiles in Adolescent Childhood Cancer Survivors
Lance Armstrong Foundation
Founded in 1997 by Lance Armstrong, the cancer survivor and four-time winner of the Tour de France bicycle race, the foundation bearing his name focuses on enhancing the quality of survival through support of survivor resources and support, groundbreaking survivorship programs, national advocacy initiatives, and scientific and clinical research grants (www.laf.org, accessed June 12, 2002). In 2000 and 2001 the foundation supported the following research activities:
-
Promoting Health Behaviors Among Pediatric Cancer Survivors,
-
Psychosocial, Behavioral and Pain Outcomes in Long-term Survivors of Childhood Cancer,
-
A Pilot Intervention to Enhance Psychosexual Development in Adolescents and Young Adults with Cancer,
-
Developing a Standardized Psychological Screening Tool for Childhood Cancer Survivors, and
-
Pilot Test of an Intervention to Reduce Post-traumatic Stress in Young Adult Survivors of Childhood Cancer.
The foundations program and community care grants included:
-
Life After Cancer Program at Cook Children’s Medical Center,
-
Living Well After Cancer Program at the University of Pennsylvania, and
-
Wonders and Worries, Inc., a non-profit organization dedicated to providing psychological support for children, youth, and families coping with chronic life threatening illness.
RESEARCH PRIORITIES
Several priority areas for research emerged from the Board’s review of childhood cancer survivorship research activities:
Assess the prevalence and etiology of late effects
-
Support prospective longitudinal studies
-
Develop a national childhood cancer registry from which cohorts could be selected (e.g., through the cooperation of existing population-based registries)
-
Invest in infrastructure to improve capacity for long-term follow-up (e.g., systems to maintain current addresses)
-
Advance methods to ensure maintenance of representative cohorts (e.g., minimize loss to follow-up)
-
Standardize exposure and outcome measures (late effects, quality of life)
Test the potential for the reduction of late effects during treatment
-
Expand support for clinical trials specifically designed to test the reduction of late effects with modifications of existing therapies
-
Expand support for research on the substitution of current therapies with those likely to cause far less toxicity3
Develop interventions to prevent or reduce late effects after treatment
-
Assess counseling and other interventions to mitigate long-term psychosocial effects of cancer and its treatment on individuals and their families
-
Develop screening tests to identify those at high risk (e.g., early detection of impending ovarian failure)
-
Evaluate the effectiveness of preventive health interventions (e.g., smoking cessation counseling, cancer screening regimens)
-
Test chemoprevention strategies in this unique high-risk population.
Further improve quality of care to ameliorate the consequences of late effects on individuals and families
-
Identify appropriate components of follow-up care through systematic evidence reviews, health services research, and consultation with patients and their families
-
Identify optimal methods of delivering follow-up care through demonstration projects
-
Develop screening tools to help determine who might benefit from psychosocial and other support services. Determine the optimal timing, frequency, and duration of interventions
-
Identify models to facilitate reintegration into school or work following cancer treatment
-
Assess the unique needs of medically underserved groups (minority populations) or populations (geographic areas)
-
Evaluate methods to improve the education and training of health care providers
Investments in education and training are needed to further research. Examples of actions that could help expand the body of research in this area include: increased support for targeted fellowship programs in survivorship research, training and career development awards, center grants, visiting professorships, and the creation of special interest groups at academic meetings.
|
3 |
The National Cancer Policy Board has a study in progress that will recommend ways to improve the process for developing better treatments for cancer, including a special focus on pediatric cancers (www.IOM.edu/ncpb). |
SUMMARY AND CONCLUSIONS
There is a growing recognition that only through continued, systematic follow-up of large cohorts of survivors will the full extent of late effects be known. Amelioration of these late effects will require investments in intervention research. Ultimately, clinical research to find targeted therapies that maximize survival while minimizing late effects will likely improve the outlook for future generations of childhood cancer survivors. In the meantime, research is needed to optimize the recovery of cancer survivors and to test ways of delivering appropriate clinical and supportive care services.
Several ongoing research activities will answer many outstanding questions about late effects among childhood cancer survivors. The Childhood Cancer Survivor Study, in particular, will provide many opportunities for researchers. Relatively little multi-institutional survivorship research has taken place within the member institutions of the Children’s Oncology Group, even though the majority of children with cancer receive their care in these settings. A renewed commitment to such research, along with investments in infrastructure to improve the ability to systematically identify and follow patients, would greatly improve the capacity and opportunities for survivorship research. While clinical, epidemiologic, and behavioral research in childhood survivorship has emerged to provide insights into childhood cancer survivorship, there appears to have been relatively little health services research to understand the health care experience and needs of childhood cancer survivors and their families.
REFERENCES
Bhatia S, Robison LL, Oberlin O, Greenberg M, Bunin G, Fossati-Bellani F, Meadows AT. 1996. Breast cancer and other second neoplasms after childhood Hodgkin’s disease. N Engl J Med 334(12):745-51.
Bhatia S. 2002. Children’s Oncology Group Late Effects Committtee. National Cancer Policy Board Meeting. Washington, DC.
Castleberry RP. 1997. Biology and treatment of neuroblastoma. Pediatr Clin North Am 44(4):919-37.
Children’s Oncology Group. 2001. Requirements for Institutional Membership. Arcadia, CA : COG.
Health Resources and Services Administration. 2000a. Title V: A Snapshot of Maternal and Child Health, 2000. Rockville, MD: Health Resources and Services Administration.
Health Resources and Services Administration. 2000b. Understanding Title V of the Social Security Act. Rockville, MD: Health Resources and Services Aministration, The Maternal and Child Health Bureau.
Link, MP, Shuster JJ, Donaldson SS, Berard CW, Murphy SB. 1997. Treatment of children and young adults with early-stage non-Hodgkin’s lymphoma. N Engl J Med. Oct 30; 337(18):1259-66.
Meadows AT, D’Angio GJ. 1974. Late effects of cancer treatment: methods and techniques for detection. Semin Oncol 1(1):87-90.
Meadows AT, Evans AE. 1976. Effects of chemotherapy on the central nervous system. A study of parenteral methotrexate in long-term survivors of leukemia and lymphoma in childhood. Cancer 37(2 Suppl):1079-85.
Meadows AT, D’Angio GJ, Evans AE, Harris CC, Miller RW, Mike V. 1975. Oncogenesis and other late effects of cancer treatment in children. Radiology 114(1):175-80.
National Cancer Institute. 1999. NCI Press Release: Cancer Survivorship. National Cancer Institute. Bethesda, MD: National Institutes of Health.
National Cancer Institute. National Cancer Institute. 2002a. News from the NCI. National Cancer Institute Research on Childhood Cancers. Bethesda, MD: National Institutes of Health (January 10, 2002).
National Cancer Institute. 2002b. Office of Cancer Survivorship Factsheet. Bethesda, MD: National Institutes of Health.
National Cancer Institute. 2002c. The Nation’s Investment in Cancer Research: A Plan and Budget Proposal for Fiscal Year 2004. Bethesda, MD: National Institutes of Health.
Raney RB, Anderson JR, Barr FG, Donaldson SS, Pappo AS, Qualman SJ, Wiener ES, Maurer HM, Crist WM. 2001. Rhabdomyosarcoma and undifferentiated sarcoma in the first two decades of life: a selective review of intergroup rhabdomyosarcoma study group experience and rationale for Intergroup Rhabdomyosarcoma Study V. J Pediatr Hematol Oncol 23(4):215-20.
Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SS, Green DM, Li FP, Meadows AT, Mulvihill JJ, Neglia JP, Nesbit ME, Packer RJ, Potter JD, Sklar CA, Smith MA, Stovall M, Strong LC, Yasui Y, Zeltzer LK. 2002. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol 38(4):229-39.
Shochat SJ, Fremgen AM, Murphy SB, Hutchison C, Donaldson SS, Haase GM, Provisor AJ, Clive-Bumpus RE, Winchester DP. 2001. Childhood cancer: patterns of protocol participation in a national survey. CA Cancer J Clin 51(2):119-30.
Smith M, Arthur D, Camitta B, Carroll AJ, Crist W, Gaynon P, Gelber R, Heerema N, Korn EL, Link M, Murphy S, Pui CH, Pullen J, Reamon G, Sallan SE, Sather H, Shuster J, Simon R, Trigg M, Tubergen D, Uckun F, Ungerleider R. 1996. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol 14(1):18-24.






















