5
Delivering Survivorship Care
Increased recognition of cancer’s late effects has meant that some childhood cancer survivors have joined the ranks of the relatively large group of children with chronic conditions and ongoing health problems. This chapter first reviews the current status of pediatric cancer care—both initial treatment and follow-up care—in terms of where care is provided, who provides care, and how care is paid for. Next, similarities and differences are drawn between the long-term health care needs of cancer survivors and other children with chronic illness or disabilities. Finally, the components of an ideal care system designed to meet the unique continuing health care needs of childhood cancer survivors are described and the relative strengths and limitations of alternate delivery models for follow-up care are outlined.
CURRENT STATUS OF PEDIATRIC CANCER CARE
Childhood cancer is rare and therefore accounts for a relatively small share of health care. An estimated 3 per 1,000 pediatric ambulatory visits and an equal share of pediatric hospitalizations are for the care of patients under age 20 with cancer (Table 5.1). Each year there are an estimated 605,600 cancer-related ambulatory care visits and 20,590 hospital discharges among children (Table 5.1). These estimates from large national surveys and administrative data sets pertain to the entire spectrum of cancer care, from diagnosis and treatment to end-of-life care. Pediatric cancer care, once offered predominantly in hospitals, has increasingly been provided on an outpatient basis (Mullen et al., 1999; Wolfe, 1993; Wollnik, 1976).
Pediatric cancer care also appears to be concentrated in specialty settings— nearly half (46 percent) of cancer-related ambulatory care is provided in hospital-based outpatient clinics and 58 percent of cancer-related hospital care takes place in urban, teaching hospitals (Table 5.1).
The implications of uninsuredness for children with cancer are dire given the complexity of care and its associated costs. An estimated 7 percent of cancer-related ambulatory care visits made from 1995 to 1999 by children were not covered by insurance, and 3 percent of cancer-related hospital discharges in 1997 lacked coverage (Table 5.1). Coverage of cancer-related ambulatory care visits is primarily through private insurance (62 percent) and to a lesser extent the Medicaid program (6 percent) (Table 5.1). Cancer-related hospital care is more heavily dependent on public programs—31 percent of hospitalizations were paid for by the Medicaid program and 60 percent were paid for by private insurance in 1997 (Table 5.1). Some low-income individuals and families who lack health insurance, but who are not eligible for Medicaid, “spend down” to become eligible for Medicaid to help pay for expensive hospitalizations. Pediatric cancer care tends to be intensive, lengthy, and costly. An estimated 18 percent of cancer-related hospitalizations had length of stays of 14 or more days (National Cancer Policy Board [NCPB] special tabulations). Total charges associated with cancer-related hospital care are very high; 22 percent of discharges had total charges of $40,000 and above in 1997 (NCPB special tabulations).
There have been relatively few studies of the costs associated with caring for children with cancer, but one study conducted in the early 1980s suggests that family out-of-pocket expenses add about 50 percent to the total cost of disease-related care and consumed 38 percent of gross annual family income (Bloom et al., 1985). Not measured are the broader costs incurred by the family, including lost wages and opportunity costs (e.g., lack of job advancement).
Initial Treatment of Childhood Cancer: The Intersection of Cancer Care and Research
It is generally recognized that children undergoing their initial treatment for cancer and their families have special needs that can best be met by specialized children’s cancer centers. Such centers use a team approach involving a variety of specialists— pediatric oncologists, surgeons, radiation oncologists, pediatric oncology nurses, nurse practitioners, psychologists, social workers, child life specialists, nutritionists, rehabilitation and physical therapists, and educators—who can support and educate the entire family. In recognition of improved outcomes associated with such specialized care, the American Academy of Pediatrics (AAP) recommends that
TABLE 5.1 Estimates of the Number and Distribution of Cancer-Related Pediatric Ambulatory Care Visits and Hospital Discharges, by Age, Sex, Race/Ethnicity, Payment, and Site of Care, NAMCS and NHAMCS, 1995-1999, HCUP NIS, 1997
|
|
Ambulatory Visits |
Hospital Discharges |
||
|
Characteristic |
Annual population estimatea |
% (se) |
Annual population estimatea |
% (se) |
|
Total |
605,600 |
100.0 |
20,590 |
100.0 |
|
Age |
||||
|
0 |
44,600 |
7.4 (2.0) |
930 |
4.5 (0.5) |
|
1-4 |
161,500 |
26.7 (3.4) |
5,170 |
25.1 (1.1) |
|
5-9 |
126,600 |
20.9 (3.1) |
5,190 |
25.2 (1.0) |
|
10-14 |
154,900 |
25.6 (3.4) |
4,210 |
20.4 (0.9) |
|
15-19 |
117,900 |
19.5 (3.1) |
5,090 |
24.7 (1.8) |
|
Sex |
||||
|
Male |
377,000 |
62.3 (3.7) |
11,690 |
56.8 (1.1) |
|
Female |
228,500 |
37.7 (3.7) |
8,890 |
43.2 (1.1) |
|
Race/ethnicityb |
||||
|
White, non-Hispanic |
437,400 |
72.2 (3.5) |
10,100 |
65.7 (3.6) |
|
White, Hispanic |
98,700 |
16.3 (2.8) |
2,300 |
15.0 (2.9) |
|
African American |
34,300 |
5.7 (1.8) |
1,920 |
12.5 (1.6) |
|
Other |
35,200 |
5.8 (1.8) |
1,040 |
6.8 (1.7) |
children and adolescents with newly diagnosed or recurrent malignancies receive their treatment in a pediatric cancer center (American Academy of Pediatrics, 1997). The AAP also recommends that the oncologic care of a child or adolescent with cancer be coordinated by a pediatric hematologist/ oncologist who is board-certified or board-eligible in the subspecialty of pediatric hematology and oncology by the American Board of Pediatrics. By 2002, 1,740 pediatric hematology/oncology physician specialists had been board certified (http://www.abp.org/STATS/numdips.htm, accessed March 25, 2003) (roughly 80 percent of these physicians were in practice). The AAP also recognizes many other professionals as essential members of the cancer care health care team, including nurses, social workers, and psychologists. In 2003, there were an estimated 2,000 active members of the Association of Pediatric Oncology Nurses (APON) (Louise S. Miller, Executive Director, APON, personal communication to Maria Hewitt, March 24, 2003) and roughly 250 members of the Association of Pediatric
|
|
Ambulatory Visits |
Hospital Discharges |
||
|
Characteristic |
Annual population estimatea |
% (se) |
Annual population estimatea |
% (se) |
|
Main payment source |
||||
|
Private |
373,300 |
61.7 (3.7) |
12,300 |
59.7 (2.0) |
|
Medicaid |
38,800 |
6.4 (1.8) |
6,300 |
30.6 (1.6) |
|
Uninsured |
39,800 |
6.6 (1.9) |
550 |
2.7 (0.5) |
|
Other/unknownc |
153,700 |
25.3 (3.4) |
1,430 |
6.9 (1.7) |
|
Site of cared |
||||
|
Specialty setting |
278,900 |
46.1 (3.8) |
12,040 |
58.5 (7.2) |
|
Non-specialty setting |
326,700 |
53.9 (3.8) |
8,530 |
41.4 (7.3) |
|
NOTE: n = sample size; % = percent distribution; se = standard error. aAnnual estimates for the number of ambulatory care visits are based on a 5-year average (1995-1999). A total of 528 cases from NAMCS and NHAMCS were weighted to obtain population estimates. A total of 4,430 cases from HCUP, 1997, were weighted to obtain an annual estimate for hospital care. Numbers may not add to total because of rounding errors. bValues are missing for 22.6% of cases for hospital discharges. cAn estimated 7% of the “other/unknown” category are insured by Medicare. Other sources of insurance include the military. dFor ambulatory care, specialty setting is a hospital outpatient department, and non specialty setting is a physician’s office. For hospital care, a specialty setting is an urban teaching hospital and non-specialty setting is a rural or urban non teaching hospital. SOURCES: National Ambulatory Medical Care Survey (NAMCS) and National Hospital Ambulatory Medical Care Survey (NHAMCS), 1995-1999; Healthcare Cost and Utilization Project (HCUP), 1997; special tabulations, NCPB staff. |
||||
Oncology Social Workers (APOSW) (June McAtee, APOSW Membership Chair, personal communication to Maria Hewitt, March 21, 2003).
Pediatric cancer care is usually delivered through academic centers involved in research (Wittes, 2003). Roughly 50 to 60 percent of all children and adolescents newly diagnosed with cancer in the United States are enrolled on clinical trials (Murphy, 2002; Shochat et al., 2001). This is quite remarkable, given that fewer than 5 percent of adults newly diagnosed with cancer are enrolled in trials. Clinical trials establish standards for an appropriate diagnostic workup, review of pathology, surgical approach, radiotherapy, and chemotherapy administration, as well as therapeutic efficacy and toxicity. Peer-reviewed treatment plans have provided standards of
care that have diffused into community practice and benefited all patients, whether participating in clinical trials or not (Simone and Lyons, 1998) (clinical trials are discussed further in Chapter 8).
Beginning in the 1960s, evaluations of cancer treatment were organized through major pediatric centers (e.g., St Jude Children’s Research Hospital, Dana Farber Cancer Institute) and national cooperative groups. The major pediatric clinical trials groups based in North America—the Children’s Cancer Group (CCG), the Pediatric Oncology Group (POG), the Intergroup Rhabdomyosarcoma Study Group (IRS) and the National Wilms’ Tumor Study Group (NWTSG)—merged in 2001 to form a single, nationwide group, the Children’s Oncology Group (COG). The Children’s Oncology Group is a National Cancer Institute-supported clinical trials cooperative group devoted exclusively to childhood and adolescent cancer research (See Chapter 8 for a discussion of COG-sponsored research). It develops and coordinates cancer clinical trials conducted within its 235 member institutions, which include cancer centers of all major universities and teaching hospitals throughout the United States and Canada, as well as sites in Europe and Australia (http://www.nccf.org/COG/index.asp, accessed March 15, 2003). Member institutions also conduct research that is independent of COG. The location of the 213 participating U.S. institutions is shown in Figure 5.1. Three states—Montana, Wyoming, and Alaska—do not have a COG-affiliated institution and geographic access to these institutions is limited in certain areas of the West and Midwest. Despite the geographic dispersion of specialized centers, as many as 94 percent of pediatric cancer cases (under age 15) diagnosed from 1989 to 1991 were seen at an institution that was a member of the cooperative clinical trials groups (i.e., POG or CCG) (Ross et al., 1993, 1996).
Follow-Up Care
Despite the growth in the population of childhood cancer survivors, no established guidelines outline appropriate components of follow-up care or provide models of how to deliver such care. The AAP recommends that centers providing care for children and adolescents with cancer have a “mechanism for ensuring long-term follow-up of successfully treated patients, either at the original treatment center, or by a specialist who is familiar with the potential adverse effects of treatment for childhood cancer” (American Academy of Pediatrics, 1997). Some professional organizations and advocacy groups have called for assurance of specialized long-term follow-up care for survivors of childhood cancer (Alliance for Childhood Cancer, 2002; Arceci et al., 1998). Having an on-site, long term follow-up service for survivors of pediatric cancer is a requirement for COG membership. The COG membership criteria state that the “outpa-
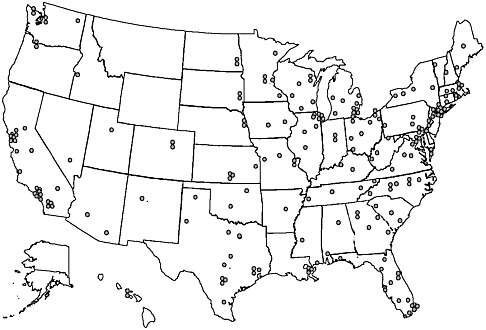
FIGURE 5.1 Map of Children’s Oncology Group (COG) member institutions in the United States.
SOURCE: COG, Public Presentation Graphic, 2002.
tient clinic should ensure the long-term follow-up of successfully treated patients and those with lifelong chronic disorders” (Children’s Oncology Group, 2001). The COG membership requirements are drawn from the general criteria and guidelines for pediatric cancer centers established by the Section on Hematology/Oncology of the American Academy of Pediatrics and the American Society of Pediatric Hematology/Oncology (Children’s Oncology Group, 2001) (Box 5.1, sections pertaining to follow-up care are underlined).
Although many late effects of childhood cancer have been recognized, there is no clear agreement on what constitutes appropriate care. Defining the specific components of long-term services that are needed is difficult because of the variable nature of long-term outcomes associated with childhood cancer. The focus of long-term follow-up of survivors of childhood cancer should be on conditions for which effective clinical interventions are available that improve survival and/or quality of life. There are many areas for which the evidence regarding the effectiveness of follow-up care is incomplete, and for which additional research is needed. There are, however, examples of follow-up care falling into the area of prevention and psychosocial support that are supported by principles of public health and compassionate care:
|
Box 5.1 Requirements for Institutional Membership, Children’s Oncology Group Required On-Site Personnel
Required On-Site Services
|
-
Educating and counseling survivors regarding the specific risks to which they are susceptible and guidance on self-monitoring for signs of late effects.
-
Applying preventive approaches known to be effective for the general population, including encouragement of abstinence from tobacco, limited exposure to alcohol, sun protection, physical activity, maintenance of a healthy weight, consumption of fruits and vegetables. At a minimum, the surveillance techniques for detecting cancer in the general population should
Personnel and Services That Must Be Available and Readily Accessible Personnel
Services
In addition to these requirements, Comprehensive Pediatric Hematology/Oncology Programs should have regularly scheduled multidisciplinary tumor boards as well as case conferences designed to discuss children and adolescents with serious hematologic problems. The outpatient clinic should ensure the long-term follow-up of successfully treated patients and those with lifelong chronic disorders. SOURCE: COG, Institutional Membership Application Procedures, 1/14/01. |
-
be performed as recommended (e.g., screening for cancers of the breast, cervix, and colorectum).
-
Providing psychosocial support services to survivors and their families.
-
Providing reproductive and sexuality counseling.
-
Providing genetic counseling for individuals with a hereditary cancer and their family members.
TABLE 5.2 Suggested Evaluation for Suspected Late Effects
|
Late effecta |
Screening test |
Recommendations if screening results abnormal |
|
Short stature |
Growth curve |
Bone age, growth hormone tests |
|
|
Sitting height |
Thyroid function testsa |
|
|
Parental heights |
Endocrinologist consultation |
|
Obesity or weight loss |
Growth curve |
Thyroid function testsa |
|
|
Diet history |
Nutritionist, endocrinologist consultation |
|
Scoliosis |
Physical examination |
Spine radiography; evaluate again during adolescent growth spurt Orthopedist consultation |
|
Bone asymmetries (hypoplasia, atrophy) |
Bone lengths, circumference |
Orthopedist consultation; bone radiography; plastic surgeon consultation |
|
Avascular necrosis or osteoporosis |
History of pain, fractures |
Bone scan |
|
|
Bone radiography |
Serum estradiol level; Ca, P Orthopedist consultation; physical therapist consultation |
|
Soft tissue hypoplasia, contractures, edema |
Physical examination |
Plastic surgeon consultation |
|
Dental abnormalities |
Physical examination |
Dentist, oral surgeon consultation |
|
Learning disabilities |
Communication with school, family; psychological testing |
CT or MRI scan of head; special education classes |
|
Leukoencephalopathy |
CT or MRI (See also Learning Disabilities, above) |
Cerebrospinal fluid basic myelin protein; neurologist consultation |
|
Neuropathy |
Physical examination |
Neurologist consultation |
|
Hearing loss |
Audiogram |
Otorhinolaryngologist consultation; audiologist consultation |
|
Infertility |
History (primary versus secondary dysfunction) |
Endocrinologist consultation |
|
|
Gonadal function testingb |
Obstetrician or gynecologist consultation |
|
Thyroid dysfunction |
Thyroid function testinga |
Endocrinologist consultation |
|
Cardiomyopathy or pericarditis |
Electrocardiogram; echocardiogram; radio-nuclide angiography |
Cardiologist consultation |
|
Vasoocclusive disease |
Angiography; Doppler pulses |
Vascular surgeon |
|
Pneumonitis or pulmonary fibrosis |
Chest radiography |
Lung biopsy |
|
|
Pulmonary function tests |
Pulmonologist consultation |
|
Late effecta |
Screening test |
Recommendations if screening results abnormal |
|
Chronic enteritis |
Growth curves |
Serum folate, carotene |
|
|
Nutritional assessment |
Small-bowel studies; barium enema; gastroenterologist consultation |
|
Hepatitis or cirrhosis |
Liver function tests |
Liver biopsy, hepatitis screen; liver scan; gastroenterologist consultation |
|
Nephritis, rickets (tublar defects) |
Urinalysis; BUN, creatinine, serum electrolytes, CO2, Ca, P, alkaline phosphatase; wrist radiographs |
24-h creatinine clearance or glomerular filtration rate; intravenous urogram or sonogram; nephrologist consultation |
|
Hemorrhagic cystitis |
Urinalysis |
Cytoscopy; urologist consultation |
|
Thrombotic thrombocytopenic purpura |
CBC/platelets, BUN, creatinine; peripheral blood smear |
|
|
Sepsis |
Compliance with prophylactic antibiotics |
|
|
Second malignancy |
Studies on an individual basis |
Oncologist consultation |
|
NOTE: BUN, blood urea nitrogen; CBC, complete blood cell count; CT, computed tomogra-phy; MRI, magnetic resonance imaging. aThyroid function tests include thyroxine (T4), thyrotropin, free T4. bGonadal function tests: Tanner staging for boys older than 14 years at the time of evaluation or girls not yet menstruating by age 12 years or if menses become irregular; follicle-stimulating hormone, luteinizing hormone, and testosterone (semen analysis) or estradiol, as appropriate. SOURCE: Dreyer et al., 2002. Reprinted with permission. |
||
For many other areas of concern to survivors, there is a sufficient body of evidence to support general guidance on screening and evaluating late effects of childhood cancer. Recently published recommendations are shown in Table 5.2 (Dreyer et al., 2002). More extensive practical advice to physicians on providing follow-up care for childhood cancer survivors is available (Schwartz et al., 1994) and efforts are underway by the COG Late Effects Committee to create practice guidelines for follow-up care (Bhatia, 2002). Information on childhood cancer late effects and advice on follow-up care are also available for survivors and their families (Keene et al., 2000).
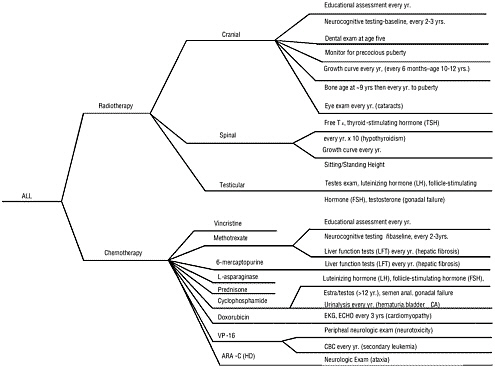
While general guidance is available, there is no consensus regarding the specifics of appropriate follow-up care. Specialized long-term follow-up programs have been established, but each has tended to define its own follow-up protocols. Figure 5.2 illustrates the complexity of follow-up care showing an algorithm developed by Schwartz and her colleagues to guide follow-up care of children treated for acute lymphoblastic leukemia (ALL) (Schwartz et al., 1994). As is clear from this algorithm, practitioners providing follow-up care need to have precise information regarding the cancer diagnosis and treatments. Lacking, however, are rigorous studies to assess the cost and benefits of screening and follow-up procedures, in terms of both reduced morbidity or mortality and improved quality of life.
Assessments of late effects or the future risk of late effects may take place at the completion of primary therapy. Other assessments, including growth monitoring, could conceivably be easily integrated into routine primary care. Whether or not a distinct follow-up program is necessary to achieve appropriate levels of follow-up is uncertain and depends in large part on the level of risk faced by the cancer survivor. In the absence of specialized follow-up care, a high level of communication and support from oncology providers to the primary care provider engaged in follow-up is necessary. Despite some uncertainly regarding the details of follow-up, it is generally agreed that all survivors of childhood cancer should maintain regular contact with a health care provider who is familiar with the potential long-term health risks to which survivors are susceptible (American Academy of Pediatrics, 1997; Arceci et al., 1998; Bleyer et al., 1993; Hollen and Hobbie, 1995; Masera et al., 1996; Wallace et al., 2001).
While available guidelines support the need for a follow-up program for survivors of childhood cancer, a survey conducted in 1997 of the 219 members of the Children’s Cancer Group and the Pediatric Oncology Group (groups that have since been merged into COG) indicated that only about half of them had long-term follow-up clinics (96 of 182 respondents) (Oeffinger et al., 1998). The website of the Association of Cancer Online Resources (http://www.acor.org/ped-onc/treatment/surclinics.html, accessed August 17, 2001) listed 28 follow-up programs that were considered comprehensive.1 To learn more about these programs, in June and July 2001, Board staff interviewed program coordinators associated with 16 of the 28 programs listed on the website.2
|
1 |
Comprehensive programs were those that had a dedicated time and place for the clinic, met at least twice a month, were staffed by a doctor with experience in the late effects after treatment for childhood cancer, had a nurse coordinator, provided state-of-the-art screening for individual’s risk of late effects, provided referrals to appropriate specialists, and provided wellness education (www.acor.org/ped-onc/treatment/surclinics.html, accessed March 25, 2003). |
|
2 |
For more details of the interviews, see the background paper prepared by Eric Trabert (www.IOM.edu/ncpb). |
All of the specialized, comprehensive, multidisciplinary follow-up programs that were contacted were located within major cancer centers and most had been developed within the past decade. The clinics function to diagnose and manage treatment-related sequelae; provide education and counseling; develop surveillance recommendations; address issues related to insurance, education, and employment; and conduct research on late effects. Pediatric nurse practitioners trained in oncology generally manage the clinics in collaboration with one or more pediatric oncologists. Additional personnel involved, usually on a referral basis, include social workers, psychologists and other specialists (e.g., cardiologists, fertility specialists, genetic counselors). Well-established programs typically assessed between 300 and 400 survivors annually, while newer programs or those serving smaller patient populations reported seeing only 50 or 60 patients each year. Most programs picked up patients after they had completed their care from their treating oncologist, generally when they were two years removed from the completion of therapy and/or three to five years from diagnosis, and disease-free. Treating oncologists generally provide follow-up for a few years following treatment to monitor for disease recurrence.
Survivors of ALL and Hodgkin’s disease tended to be overrepresented in follow-up clinics, because of their relatively high risk of late effects. Another high-risk group, survivors of brain/CNS tumors, however, were not generally seen in these clinics, but were instead followed by neurooncology specialists. The schedule for follow-up varied depending upon factors such as patient age, initial diagnosis, and type of treatment received, but almost all programs encouraged patients to return annually for evaluation and possible diagnostic workup during the first 10 years of follow-up, or until after completion of puberty, whichever occurs later. Since puberty is a time of dramatic physical and psychological development, many late effects first present during this period and may require immediate intervention. Few follow-up programs had transition clinics that specifically targeted the needs of young adult survivors.
There are relatively few follow-up programs for survivors of adult cancer according to an informal survey of academic centers conducted for the Board (Winn, 2002). Adult survivors of childhood cancer could potentially be referred to one of these programs. Box 5.2 describes some of the programs designed to assess and manage survivorship-related concerns. A few other programs are available to address specific late effects or concerns of survivors of adult cancers (e.g., lymphedema among breast cancer survivors, sexuality, fatigue).
Although some comprehensive, specialized follow-up programs have emerged to address the concerns of cancer survivors and their families, there have been no evaluations of their effectiveness or value. As a consequence, a referral to a long-term follow-up program is often initially met by
|
Box 5.2 Characteristics of Selected Programs Serving Adult Survivors Life After Cancer Care University of Texas, M.D. Anderson Cancer Center This clinic accepts individuals who have completed primary therapy and the first 1-2 years of surveillance. Staffed by an endocrinologist and nurse practitioner, the clinic emphasizes the management of endocrine dysfunction, especially premature menopause and thyroid dysfunction. Living Well After Cancer Program University of Pennsylvania This program, established in 2001 with support from the Lance Armstrong Foundation, is staffed by an oncologist/epidemiologist, senior oncology nurse, social worker, and cardiology and primary care providers. Referrals are made for consultation on issues related to sexuality and genetic counseling. Post-Treatment Resource Center Memorial Sloan Kettering This program is staffed by social workers and provides education, counseling, and advocacy support to cancer survivors. Lectures on survivorship, support groups, and one-on-one counseling are provided. Information and counseling on legal and discrimination issues are available. SOURCE: Winn, 2002. |
denial from health insurers who contend that such care is not medically necessary. Efforts to overturn these denials usually succeed in securing authorization for follow-up care, but insurers often stipulate that all lab and diagnostic tests be performed within network (Trabert, 2001). This may present logistical problems to patients who must travel extended distances to access follow-up care. In addition, reimbursement for services provided in long-term follow-up typically falls far short of compensation for the time and effort required to evaluate and manage these patients. In fact, many services garner no reimbursement for surveillance programs, including those provided by social workers, education specialists, genetic counselors, nutritionists, or dentists. Consequently, hospitals often rely on grant support or philanthropic donations to partially subsidize the costs of providing long-term follow-up care.
To what extent are survivors of childhood cancer receiving follow-up care, either at an organized clinic or through other providers? In a recent
study of 635 5-year survivors of childhood cancer (members of the CCSS cohort), 44 percent stated that they had attended a clinic expressly for follow-up of their cancer (Kadan-Lottick et al., 2002). In another study of 9,434 adult survivors of childhood cancer (members of the CCSS cohort), 87 percent of survivors had had some general contact with the medical system within the previous 2 years, but only 19 percent had been seen at an oncology center or clinic, and nearly a third (30 percent) had not had a general physical examination in the past 2 years. Older age and longer time interval from cancer diagnosis were associated with lower rates of physical examination, cancer-related visits, and visits to a cancer center (Oeffinger et al., in press). Evidence suggests that once primary therapy is completed, contact with the treating institution diminishes. In the absence of formal follow-up programs, communication with patients and their families regarding the need for future care is essential. Patients and families must be able to make available to their subsequent health care providers diagnostic and treatment information needed to assess late effects.
A prerequisite to appropriate follow-up care is knowledge of what treatments were administered during primary treatment. At the conclusion of therapy, patients and their families should be provided with a summary of their treatment and informed of possible late effects and the need for follow-up care. Some evidence suggests that effective communication is not taking place. When asked in a recent study of 635 5-year survivors of childhood cancer (members of the CCSS cohort) if past therapies could cause a serious health problem with the passage of time, 35 percent responded affirmatively; 46 responded negatively; and 19 percent did not know (Kadan-Lottick et al., 2002). Only 15 percent reported that they had ever received a written statement of their disease diagnoses and treatments to keep as a reference in the future. This is consistent with preliminary results of a Robert Wood Johnson study of barriers to care among childhood cancer survivors where only 19 percent of 441 5-year survivors reported that they had been given a written summary of their treatment and could easily find it (Kevin Oeffinger, personal communication to Maria Hewitt, January 8, 2002). Many survivors and families have anecdotally reported that they were not informed of late effects or of the need for follow-up (Keene, 2002).
A sizable proportion of childhood cancer survivors lack specific knowledge of their prior diagnosis and treatment, which is information necessary to plan for future care. In the recent study of Kadan-Lottick and colleagues, 72 percent of 5-year cancer survivors could accurately identify their diagnosis. The accuracy of reporting their treatment history varied by type of treatment; accurate recall occurred among 94 percent of those who had had chemotherapy, 89 percent for radiation, and 93 percent for splenectomy.
Among those who received radiotherapy, 70 percent recalled the site of radiotherapy (Kadan-Lottick et al., 2002).
A “Cancer Patient’s Treatment Record” has been developed so that survivors can print it out and request information from their treating institution (http://patientcenters.com/survivors/, last accessed March 15, 2003). As systems of care move toward uniform electronic medical records, individuals might be issued a “smartcard” or other electronic record of their medical history and treatment. Ideally, at the conclusion of their treatments, cancer survivors would have information about their cancer and its treatment and an individualized plan for follow-up and guidance on preventive health practices.
Long-Term Care Needs of Children with Chronic Illness and Disability
Long-term care services may be needed by survivors of childhood cancer who have late effects that lead to functional limitations and disability. These survivors are not alone in their need for planned and coordinated follow-up care. In some cases, the clinical, psychological, educational, and other supportive interventions needed by child and adult survivors of HIV/ AIDS, brain injury, hemophilia, and other conditions may also be appropriate for childhood cancer survivors. Children with special health care needs are those “who have, or are at increased risk for, chronic physical, developmental, behavioral, or emotional conditions and who also require health and related services of a type or amount beyond that required by children generally” according to the U.S. Department of Health and Human Services, Health Resources and Services Administration’s Maternal and Child Health Bureau (www.mchb.hrsa.gov, last accessed on March 15, 2003). Figure 5.3 shows estimates made by Newacheck and colleagues of the percentage of children in the United States with chronic physical conditions, children with special health care needs (excluding at-risk children), and disability (e.g., limitations in the activities of daily living) (Newacheck et al., 1998). Nearly one in five (18 percent) of U.S. children under age 18 (13 million children) has special health care needs, excluding those at-risk children (Newacheck et al., 1998). The estimated 95,000 children and adolescents with a history of cancer would be counted among this group if they experienced late effects of treatment or if they required follow-up care or surveillance beyond that expected within the context of routine pediatric care. The additional 175,000 adult survivors of childhood cancer would require a similar designation among adults.
It may well be the case that there are common services needed by the relatively large, heterogeneous group of children with chronic conditions, special needs, and disabilities. If so, there may be opportunities to reach a large group of individuals and families who have common needs with
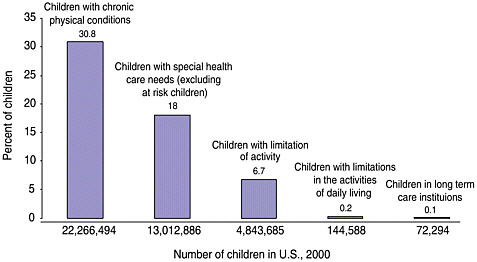
FIGURE 5.3 Prevalence of chronic conditions, special needs, and disability among children under age 18.
SOURCE: Adapted from Newacheck et al., 1998.
services delivered outside of a disease-specific context. There is some rationale for this insofar as therapeutic goals for chronically ill children and their families are different from those of children with acute illness. Some common goals include integration into community life, enhancing child and family responsibility for self-care, and optimal social and educational development (Perrin and Starr, 1993). An array of health and family support services are often needed to meet these therapeutic goals, and coordination of services becomes central to the provision of care. Care coordination is often complicated because there is no single entry point to multiple systems of care, and complex criteria determine the availability of funding and services among public and private payers of care (Ziring et al., 1999). Families themselves usually assume most of the care coordination responsibilities for children with cancer, but health care providers can play a vital role in concert with families. The concept of a “medical home” has been developed to ensure that medical care is accessible, continuous, comprehensive, family centered, coordinated, compassionate, and culturally effective (American Academy of Pediatrics, 2002a). The medical home is a source of ongoing community-based routine health care whereby providers and families work as partners to meet the needs of children and family (http://www.mchb.hrsa.gov/programs/specialneeds/measuresuccess.htm, accessed March 15, 2003). The medical home assists in the early identification of special health care needs; provides ongoing primary care; and coordinates with a broad range of other specialty, ancillary, and related services. The
need of a medical home is especially important for children with special health care needs and the U.S. Department of Health and Human Services Healthy People 2010 goals and objectives state that “all children with special health care needs will receive regular ongoing comprehensive care within a medical home” (http://www.mchb.hrsa.gov/programs/specialneeds/measuresuccess.htm, accessed March 15, 2003).
Acute care insurance policies often cover a limited number of supportive services such as home care, physical therapy, or occupational therapy services following an acute episode of illness, but generally this coverage does not extend beyond certain time limits. Supportive services needed to maintain function after an acute episode has resolved are usually excluded from health insurance policies. A number of public and private programs are available to fill in the gaps, but parents often complain of a patchwork of available services that are often hard to learn about and access.
Several federal programs help to finance or deliver home- and community-based long-term supportive services. The Supplemental Security Income (SSI) program of the Social Security Administration (SSA), for example, offers cash assistance to poor families whose children meet SSA’s medical disability eligibility criteria. Once determined eligible for SSI, a child becomes eligible for Medicaid, an important source of acute and long-term care services. The Medicare program covers Americans living with disabilities and those with end-stage renal disease (Medicaid, SSI, and Medicare programs are reviewed in Chapter 7). Long-term care services are also often available through federally supported, state-run Children with Special Health Care Needs programs (these Title V programs are described in Chapter 7). The largest federal program serving children with disabilities is delivered through the public school system. Young children with disabilities are eligible for the “Part H” program, and older children can benefit from special education and “related services” that can include supportive long-term care services such as occupational and physical therapy (educational programs are described in Chapter 6).
Selected private organizations involved in advocacy and the provision of supportive services for families of children with cancer are described in Box 5.3. These programs provide information, peer support, special services such as camping programs, and opportunities for advocacy.
Alternate Models of Delivery of Follow-Up Care
While there is consensus that the primary treatment of childhood cancer requires specialized care, and that a plan for follow-up should be in place for all survivors of childhood cancer, it is unclear whether oncology-based specialized follow-up care is the only appropriate model of care to meet the long-terms needs of survivors of childhood cancer. Are oncology-
|
Box 5.3 Services for Children with Cancer and Their Families Offered by Selected Voluntary Organizations SUPPORT GROUPS/PROGRAMS
|
based follow-up programs always advisable? Are alternative models of care needed that are based in primary care? In part, the answer to these questions depends on an understanding of the unique characteristics of the pediatric cancer care system and the distinct follow-up care needs among survivors of childhood cancer.
An argument for specialized follow-up programs within cancer centers can be made on the basis of the multidiciplinary expertise represented in such institutions and their familiarity with late effects. It is likely that persistent concerns regarding cancer recurrence can best be addressed in these settings. The fact that the majority of children treated for cancer are cared for in specialized centers also points to the advantage of continuity should follow-up care be assumed by the institutions initially treating the cancer. The primary treating oncologist is already providing a considerable amount of follow-up care. Oncologists and their colleagues typically fol-
|
ONLINE SUPPORT
CAMPS Several organizations provide camping opportunities for children with cancer.
|
low their patients for 2 to 3 years after the conclusion of treatment. Having pediatric oncologists provide longer-term follow-up care could effectively familiarize these providers with late effects and provide them with insights as to how best modify initial treatments to minimize adverse late sequelae.
When follow-up care is provided at a cancer center, is it necessary to have a separate clinic apart from the one where children come to be treated for their cancer? Specialized clinics were, in fact, first developed to address the limitations of follow-up within the oncology outpatient clinic. Typically, clinicians in such clinics would try to evaluate the medical and psychosocial needs of survivors during the brief time usually allotted for acute care visits. Consequently, follow-up was limited to checking for symptoms of disease recurrence or second malignant neoplasms at the expense of treating the complete physical and psychological needs of survivors. The inadequacy of this approach led some to instead develop nurse-led,
multidisciplinary clinics with a more holistic approach as described earlier (Hobbie and Hollen, 1993). Such programs also facilitate the collection of extensive data for medical research.
Another reason why separate follow-up clinics were developed was in response to cancer survivors’ reluctance to return to the clinic where they received treatment. A return to the clinic, amidst patients who are receiving active therapy, can stir up unpleasant and sometimes painful memories. Some programs, however, favor holding follow-up in the acute care setting because it facilitates the establishment of mentoring programs between survivors and newly diagnosed patients.
Since late effects may not emerge until several years after treatment is completed, it is important to maintain active follow-up of childhood cancer survivors as they transition through adolescence and into adulthood (MacLean et al., 1996; Oeffinger et al., 1998). A distinct disadvantage of having follow-up clinics in pediatric settings is the difficulty of involving clinicians who understand how therapy given during childhood manifests as health problems in adults. Some speculate that many adolescent and young adult survivors are lost to follow-up simply because they do not want to receive care in a pediatric institution, perhaps feeling that they have outgrown the type of care normally provided in this setting. The American Academy of Pediatrics issued a policy statement in 1996 and a consensus statement in 2002 to guide pediatricians as they assist adolescents with special health care needs to positively adapt within an adult-focused system of health care (American Academy of Pediatrics, 1996; 2002).3
Potential advantages to having follow-up take place in the primary care setting include gaining a sense of a return to normalcy; getting care within the context of total health needs and with one’s familiar practitioner; and avoidance of trips to a cancer center that may be far from home. Importantly, a child’s pediatrician can play a pivotal role as a support to, and community advocate for, the child and family (Pizzo, 1990). The schedule of visits for preventive pediatric health care recommended by the American Academy of Pediatrics is shown in Box 5.4 (http://www.aap.org/policy/re9939.html, accessed March 15, 2003). Some cancer-related follow-up care might be provided during routinely scheduled visits. Other follow-up care might involve additional visits or referrals to other providers.
Since many late effects will not show up immediately after treatment, the need for follow-up care may first be recognized during adulthood when individuals have established ties to adult primary care providers. Survivors may wish to remain under the care of their adult primary care provider for the management of any late effects.
|
3 |
The American Academy of Pediatrics consensus statement is summarized in chapter 9. |
Potential disadvantages to having follow-up take place in the primary care setting could include the primary care provider lacking knowledge about late effects and their management, and a lack of expertise in certain aspects of the physical exam important in detecting cancer recurrence. Some of these disadvantages can be overcome with appropriate levels of communication between oncology and primary care providers and with the provision of education and training opportunities.
With improved survival among children who have had cancer, primary care physicians will increasingly become involved in their long-term follow-up. But despite the growing numbers of survivors, visits made by such children and young adults will be rare events for the typical pediatrician, family practitioner, or internist. Innovative methods are needed to alert these providers to the unique needs of these patients. There have been recent calls for research on the organization of care for children with disabilities, and in particular, on ways to enhance communication and collaboration between primary and subspecialty care providers (Perrin, 2002).
Defining Quality Survivorship Care
Irrespective of who provides survivorship care or where it is provided, an ideal system of survivorship care would:
-
provide a range of direct services to survivors to identify, prevent, treat, and ameliorate late effects,
-
bridge the realms of primary and specialty health care with education and outreach,
-
coordinate medical care with educational and occupational services, and
-
conduct research to better understand late effects and their prevention.
These functions of an ideal follow-up system of care for survivors of childhood cancer are described in Box 5.5. Implicit in the “ideal” is the goal to improve outcomes of survivors of childhood cancer. The system described includes evaluations of effectiveness of interventions to ameliorate late effects and measure the prevalence of late effects. These two activities would likely yield results that would inform the success of the proposed follow-up system.
There are different models of care through which appropriate follow-up care might be attained (Harvey et al., 1999; Hollen and Hobbie, 1995; Oeffinger, 2002; Oeffinger et al., 1998; Wallace et al., 2001). The one described most frequently in the literature relies on the development of specialized long-term follow-up clinics, usually at a cancer center.
|
Box 5.4 Recommendations for Preventive Pediatric Health Care (RE9939) Committee on Practice and Ambulatory Medicine Each child and family is unique; therefore, these Recommendations for Preventive Pediatric Health Care are designed for the care of children who are receiving competent parenting, have no manifestations of any important health problems,
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
and are growing and developing in satisfactory fashion. Additional visits may become necessary if circumstances suggest variations from normal. These guidelines represent a consensus by the Committee on Practice and Ambulatory Medicine in consultation with national committees and sections of the American Academy of Pediatrics. The Committee emphasize the great importance of continuity of care in comprehensive health supervision and the need to avoid fragmentation of care.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SOURCE: www.aap.org/policy/re9939.html. |
||||||||||||||||||||||||||||||
|
Box 5.5 Functions of an Ideal Follow-Up System for Survivors of Childhood Cancer Provide services
Educate and train professionals
Conduct research
|
The Late Effects Committee of the United Kingdom Children’s Cancer Study Group has recently suggested a tiered approach with postal or telephone contacts made with those at lowest risk of late effects, follow-up by a nurse or primary care doctor for those at moderate risk, and a medically supervised late effects clinic for those with high risk of late effects (Wallace et al., 2001) (Table 5.3).
TABLE 5.3 Possible Levels of Follow-Up More Than 5 Years from Completion of Treatment
|
Level |
Treatment |
Method of follow-up |
Frequency |
Examples of tumors |
|
1 |
• Surgery alone • Low risk chemotherapy |
Mail or telephone |
1-2 years |
• Wilms’ tumor stage I or II • Langerhans cell histiocytosis (single system disease) • Germ cell tumors (surgery only) |
|
2 |
• Chemotherapy • Low dose cranial irradiation (<24 Gy) |
Led by nurse or primary care doctor |
1-2 years |
• Most patients (e.g., ALL in first remission) |
|
3 |
• Radiotherapy, except low dose cranial irradiation • Megatherapy |
Medically supervised late effects clinic |
Annual |
• Brain tumors • After bone marrow transplant • Patients with stage IV tumors (any tumor type) |
|
SOURCE:Wallace et al., 2001. |
||||
A novel strategy for long-term follow-up has been proposed that relies extensively on distance networking through the Internet and telecommunication technologies (Oeffinger, 2002). This model would link the survivor to a nationally supported center that would be responsible for facilitating health care needs. The center would have four components: a national cancer registry, care coordinators, a repository of information, and a decision-making board. Upon diagnosis of cancer, children would be entered in the registry. Upon completion of primary therapy, the treating cancer center would provide the national center with a summary of treatment and complications. Care coordinators would develop a survivor-specific plan of action, assess health care resources in the survivor’s environment, and orchestrate care with appropriate health care providers located near the survivor. The repository would include guidelines for screening and surveillance, current literature about survivor-related health care problems and needs, and patient and physician education materials. The board, including health care providers and survivors, would garner necessary resources to facilitate and enhance the process.
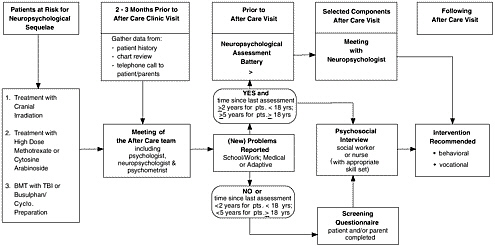
A comprehensive regional approach to providing follow-up care to survivors of childhood cancer has been adopted by the Canadian province of Ontario (Greenberg, 2002). A network of after-care programs is being set up by the Pediatric Oncology Group of Ontario (POGO) to extend follow-up care through adulthood. The program integrates research and health care, with a focus on clinical care, surveillance, and health promotion and disease prevention. A set of consensus algorithms and guidelines is available to providers. Figure 5.4 illustrates one of their algorithms, a strategy for providing neuropsychological testing for long-term survivors of childhood cancer.
Aftercare through the POGO program generally begins two years after completion of all therapy (or four years from diagnosis). Visits are annual for up to 10 years following diagnosis and biannual thereafter, although in some specified circumstances, visits may be made more frequently (e.g., when growth failure is being monitored). An essential component of the program is a “Passport to Health,” which is a credit card sized, portable, comprehensive abbreviated summary of diagnosis, treatment, complications, potential adverse effects, hepatitis status, and other relevant information necessary for the survivor and his or her health care practitioners. In the area of health education, counseling is provided to minimize risk, written materials are provided on prevention and aftercare, and links to sources of information (e.g., books, websites) are given. A centralized database will link childhood treatment data to outcomes using standardized data collection procedures. Some sites of care will be in a pediatric setting, while others will be incorporated in internal medicine programs or adult-based cancer centers. Services to residents of remote areas will be provided by a traveling team of oncology experts. This system, while still in its development, appears to incorporate many of the ideal components outlined at the beginning of this chapter.
An interesting model of health care delivery and research outside of the area of cancer is a program of the Cystic Fibrosis Foundation (CFF) that accredits a network of more than 115 care centers across the United States.4 CFF-accredited centers must meet criteria for personnel, facilities, services, and research (www.cff.org/chapters_and_care_centers/, last accessed March 15, 2003). Care is provided according to CFF clinical practice guidelines developed by an advisory group of CF experts. Consensus conferences are held to update guidelines. A central patient registry tracks the health of
|
4 |
Cystic fibrosis is a genetic disease caused by a single gene defect that results in the faulty transport of salt in organs such as the lungs and the pancreas. The defective gene causes the body to produce thick, sticky mucus that blocks the ducts in these organs, disrupting their normal functions (CFF, 2002). |
patients enrolled in CFF clinics and analyses of these data have provided new insights into the consequences of the disease (e.g., growth and development, reproduction), treatment, and preventive health strategies (Cystic Fibrosis Foundation, 2002). The dual focus on guideline-driven standardized care and research is an attractive feature of this program and such an initiative should be considered for its applicability to survivors of childhood cancer.
To date, there have been no demonstration projects to assess alternative models of delivery and no evaluations of existing programs of follow-up care to survivors of childhood cancer. It is likely that multiple models of care will be needed to accommodate the varied circumstances and preferences of survivors and families. For some survivors, long-term follow-up clinics will serve survivors’ needs best. For other survivors, primary care providers may be able to provide the most appropriate follow-up care, especially as other chronic illnesses of age develop. We do not yet know what will work best. Demonstration and research programs conducted under the discretionary grant programs of the Maternal and Child Health Block Grant Program may inform the development of delivery systems appropriate for cancer survivors. These programs have included initiatives aimed at improving care for individuals with hemophilia, sickle cell anemia, and traumatic brain injury (see a description of selected grants in Chapter 7).
Statewide Comprehensive Cancer Control
Opportunities in the United States to develop regional approaches to care for childhood cancer survivors could be facilitated by the Centers for Disease Control and Prevention (CDC) efforts to build the capacities of states—and, in turn, their local partners—to both develop and implement comprehensive cancer control plans. As part of CDC’s National Comprehensive Cancer Control Program, such plans have been defined as those with an integrated and coordinated approach to reducing the incidence and the rates of morbidity and mortality from cancer through prevention, early detection, treatment, rehabilitation, and palliation (www.cdc.gov/cancer/ncccp/index.htm, accessed March 15, 2003).
CDC has identified a useful framework for the establishment of a state cancer control program and has provided various models for comprehensive planning and evaluation. Essential elements of a comprehensive plan include (Abed et al., 2000a; Abed et al., 2000b) the following:
-
strategies and mechanisms for developing and maintaining partnerships,
-
assessments and surveillance,
-
infrastructure development,
-
public education,
-
professional education,
-
policy and legislative activities, and
-
evaluation and monitoring.
Phases of implementation of a comprehensive state plan include setting optimal objectives that are data-driven, determining optimal strategies that are science-driven, establishing feasible priorities given the capacity, and implementing effective strategies that are assessed by evaluations of outcomes (Abed et al., 2000a; Abed et al., 2000b). Many states have in place some of the essential elements of a comprehensive program. Nearly half of the states, for example, have cancer registries that achieve standards of completeness, timeliness, and coverage to provide accurate cancer incidence data for planning and evaluation. According to a recent CDC assessment, however, only 13 states have comprehensive state plans that are being implemented (or that are ready to be implemented), 14 states and the District of Columbia are creating a new plan (or are updating an old plan), and 23 states have no plan or one that is outdated (Figure 5.5).
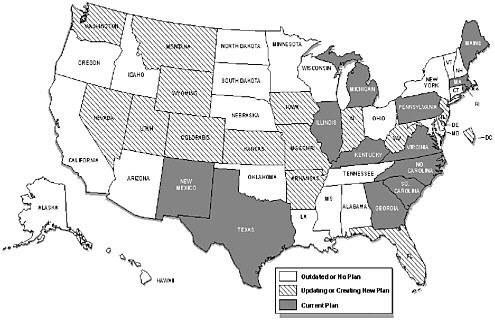
FIGURE 5.5 Comprehensive cancer control plans, 2001.
SOURCE: L. Given, CDC, Division of Cancer Prevention and Control, personal communication to Maria hewitt, July 10, 2001.
Although considerable variations in state capacities have been observed and certain barriers to implementation have been identified, it is unclear what levels and types of investment are needed to build state and local capacities and how these needs may vary across the nation. CDC’s Division of Cancer Prevention and Control spends an estimated $250 million on cancer control and prevention annually, but much of the money is categorically targeted to specific activities (e.g., cancer registries), populations, or cancer sites. Since 1998, 19 states and 1 tribal organization have received grant support totalling approximately $37 million from CDC to develop and implement a comprehensive cancer control (CCC) plan. In addition, states and tribal organizations have been provided technical assistance regarding CCC plans with $1 million from the CDC (Leslie Given, Division of Cancer Prevention and Control, CDC, personal communication to Maria Hewitt, September 9, 2002). The CDC-funded states are developing a variety of programs, depending on the needs and organizational preferences of each state. The key to each program is, however, the same—fostering collaborative efforts among many sectors within the states to increase individual and organizational awareness of the state’s cancer burden and to achieve objectives that will lead to future reductions in that burden (Tim Byers, University of Colorado School of Medicine, unpublished). Resources appear to be inadequate to meet the need for CCC plan development and implementation. In 2002, for example, CDC had resources to support only half of the requests for assistance from states, territories, and Indian tribes in response to its National Cancer Prevention and Control Program Announcement (Leslie Given, Division of Cancer Prevention and Control, CDC, personal communication to Maria Hewitt, IOM, August 26, 2002). The CDC estimates that $30 million per year would be needed before states would have plans developed and implementation in progress by 2005 (Leslie Given, Division of Cancer Prevention and Control, CDC, personal communication to Maria Hewitt, IOM, August 26, 2002).
A bill recently introduced in Congress, the Cancer Survivorship Research and Quality of Life Act of 2002 (HR 4963), calls for expansion of CDC comprehensive cancer programs to improve cancer survivorship. Among its provisions is support of innovative post-treatment programs, services, and demonstrations designed to support and advance cancer survivorship. Comprehensive state plans have potential, but to date, very few have addressed issues related to pediatric cancer or to survivorship issues.
SUMMARY AND CONCLUSIONS
Fifty to sixty percent of children with cancer are initially treated in specialized cancer centers, but somewhat fewer—an estimated 40-45 percent—are receiving follow-up care in specialized clinics. A disturbing find-
ing from recent research is that the majority of cancer survivors appear to be unaware of their level of risk and need for follow-up care, and to lack the specific information regarding their disease history and treatment that would be needed by a clinician to provide appropriate care.
The need for a plan for survivorship follow-up care is widely acknowledged and general recommendations for such care are available to clinicians, survivors, and their families. An active research program is needed to address the many outstanding questions regarding the necessary components of follow-up care in the identification, prevention, and amelioration of specific late effects. Needed also are evaluations of models of care to assess which of them confer benefits in terms of preventing or ameliorating late effects and improving quality of life, and which survivors might prefer. Cancer survivors, while having some unique needs, have similarities with survivors of other chronic illness. There are likely opportunities to develop efficient systems of care to address at least some of the needs of individuals with a broad range of chronic illnesses and conditions.
REFERENCES
Abed J, Reilley B, Butler MO, Kean T, Wong F, Hohman K. 2000a. Comprehensive cancer control initiative of the Centers for Disease Control and Prevention: an example of participatory innovation diffusion. J Public Health Manag Pract 6(2):79-92.
Abed J, Reilley B, Butler MO, Kean T, Wong F, Hohman K. 2000b. Developing a framework for comprehensive cancer prevention and control in the United States: an initiative of the Centers for Disease Control and Prevention. J Public Health Manag Pract 6(2):67-78.
Alliance for Childhood Cancer. 2002. Core Principles for Comprehensive Quality Cancer Care for Children and Adolescents. Washington, DC: Alliance for Childhood Cancer Care.
American Academy of Pediatrics. 1996. Transition of care provided for adolescents with special health care needs. American Academy of Pediatrics Committee on Children with Disabilities and Committee on Adolescence. Pediatrics 98(6 Pt 1):1203-6.
American Academy of Pediatrics. 1997. Guidelines for the pediatric cancer center and role of such centers in diagnosis and treatment. American Academy of Pediatrics Section Statement Section on Hematology/Oncology. Pediatrics 99(1):139-41.
American Academy of Pediatrics. 2002a. The medical home. Pediatrics 110(1 Pt 1):184-6.
American Academy of Pediatrics. 2002b. A consensus statement on health care transitions for young adults with special health care needs. Pediatrics 110(6 Pt 2):1304-6.
Arceci RJ, Reaman GH, Cohen AR, Lampkin BC. 1998. Position statement for the need to define pediatric hematology/oncology programs: a model of subspecialty care for chronic childhood diseases. Health Care Policy and Public Issues Committee of the American Society of Pediatric Hematology/Oncology. J Pediatr Hematol Oncol 20(2):98-103.
Bhatia S. 2002. Children’s Oncology Group Late Effects Committtee. National Cancer Policy Board Meeting. Washington, DC.
Bleyer WA, Smith RA, Green DM, DeLaat CA, Lampkin BC, Coltman CA, Brady AM, Simon M, Krischer JP, Menck HR. 1993. American Cancer Society Workshop on Adolescents and Young Adults with Cancer. Workgroup #1: Long-term care and lifetime follow-up. Cancer 71(7):2413.
Bloom BS, Knorr RS, Evans AE. 1985. The epidemiology of disease expenses. The costs of caring for children with cancer. JAMA 253(16):2393-7.
Children’s Oncology Group. 2001. Requirements for Institutional Membership. Arcadia, CA: COG.
Children’s Oncology Group. 2002. Public Presentation Graphic. Bethesda, MD. Cystic Fibrosis Foundation. Patient Registry 2001 Annual Report. Bethesda, MD: 2002.
Dreyer ZE, Blatt J, Bleyer A. 2002. Late Effects of Childhood Cancer and Its Treatment. Pizzo PA, Poplack DG, Eds. Principles and Practice of Pediatric Oncology. 4th ed. Philadelphia: Lippincott Williams & Wilkins.
Erenberg A, Lemons J, Sia C, Trunkel D, Ziring P. 1999. Newborn and infant hearing loss: detection and intervention. American Academy of Pediatrics, task Force on newborn and Infant hearing, 1998-1999. Pediatrics, Feb, 103(2):527-30.
Ettinger A. 2002. Long-Term Survivor Programs: A Paradigm for the Advanced Practice Nurse. NCPB Commissioned Paper (www.iom.edu/ncpb).
Greenberg M. 2002. The Ontario Aftercare Program: Dawn of a New Era. National Cancer Policy Board Meeting. Washington, DC.
Harvey J, Hobbie WL, Shaw S, Bottomley S. 1999. Providing quality care in childhood cancer survivorship: learning from the past, looking to the future. J Pediatr Oncol Nurs 16(3):117-25.
Hobbie WL, Hollen PJ. 1993. Pediatric nurse practitioners specializing with survivors of childhood cancer. J Pediatr Health Care 7(1):24-30.
Hollen PJ, Hobbie WL. 1995. Establishing comprehensive specialty follow-up clinics for long-term survivors of cancer. Providing systematic physiological and psychosocial support. Support Care Cancer 3(1):40-4.
Kadan-Lottick NS, Robison LL, Gurney JG, Neglia JP, Yasui Y, Hayashi R, Hudson M, Greenberg M, Mertens AC. 2002. Childhood cancer survivors’ knowledge about their past diagnosis and treatment: Childhood Cancer Survivor Study. JAMA 287(14):1832-9.
Keene N. 2002. Solicitation of concerns of cancer survivors and their family through survivorship listserv and provided to the National Cancer Policy Board.
Keene N, Hobbie W, Ruccione K. 2000. Childhood Cancer Survivors: A Practical Guide to Your Future. Sebastopol, CA: O’Reilly and Associates, Inc.
MacLean WE Jr, Foley GV, Ruccione K, Sklar C. 1996. Transitions in the care of adolescent and young adult survivors of childhood cancer. Cancer 78(6):1340-4.
Masera G, Chesler M, Jankovic M, Eden T, Nesbit ME, Van Dongen-Melman J, Epelman C, Ben Arush MW, Schuler D, Mulhern R. 1996. SIOP Working Committee on Psychosocial issues in pediatric oncology: guidelines for care of long-term survivors. Med Pediatr Oncol 27(1):1-2.
Mullen CA, Petropoulos D, Roberts WM, Rytting M, Zipf T, Chan KW, Culbert SJ, Danielson M, Jeha SS, Kuttesch JF, Rolston KV. 1999. Outpatient treatment of fever and neutropenia for low risk pediatric cancer patients. Cancer 86(1):126-34.
Murphy S. 2002. Clinical Trials: Issues Impacting Design and Conduct of Clinical Trials, NCPB Commissioned Paper. (www.iom.edu/ncpb).
National Ambulatory Medical Care Survey and National Hospital Ambulatory Medical Care Survey, 1995-1999; Healthcare Cost and Utilization Project, 1997; special tabulations NCPB staff, 2003.
Newacheck PW, Strickland B, Shonkoff JP, Perrin JM, McPherson M, McManus M, Lauver C, Fox H, Arango P. 1998. An epidemiologic profile of children with special health care needs. Pediatrics 102(1 Pt 1):117-23.
Oeffinger KC. 2002. Longitudinal Cancer-related Health Care for Adult Survivors of Childhood Cancer (IOM commissioned background paper) (www.iom.edu/ncpb).
Oeffinger KC, Eshelman DA, Tomlinson GE, Buchanan GR. 1998. Programs for adult survivors of childhood cancer. J Clin Oncol 16(8):2864-7.
Oeffinger KC, Mertens AC, Hudson MM, Gurney JG, Casillas J, Chen H, Yeazel M, Whitton J, Yasui Y, Robison LL. 2003. Health care of young adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Annals of Family Medicine.
Perrin EC, Starr MC. 1993. Pediatric chronic illness. J Learn Disabil 26(7):426-7.
Perrin JM. 2002. Health services research for children with disabilities. Milbank Q 80(2):303-24.
Pizzo PA. 1990. Cancer and the pediatrician: an evolving partnership. Pediatr Rev 12(1):5-6.
Ross JA, Severson RK, Robison LL, Pollock BH, Neglia JP, Woods WG, Hammond GD. 1993. Pediatric cancer in the United States. A preliminary report of a collaborative study of the Childrens Cancer Group and the Pediatric Oncology Group. Cancer 71(10 Suppl):3415-21.
Ross JA, Severson RK, Pollock BH, Robison LL. 1996. Childhood cancer in the United States. A geographical analysis of cases from the Pediatric Cooperative Clinical Trials groups. Cancer 77(1):201-7.
Schwartz C, Hobbie W, Constine L, Ruccione K. 1994. Survivors of Childhood Cancer: Assessment and Management. St. Louis, MO: Mosby.
Shochat SJ, Fremgen AM, Murphy SB, Hutchison C, Donaldson SS, Haase GM, Provisor AJ, Clive-Bumpus RE, Winchester DP. 2001. Childhood cancer: patterns of protocol participation in a national survey. CA Cancer J Clin 51(2):119-30.
Simone JV, Lyons J. 1998. The evolution of cancer care for children and adults. J Clin Oncol 16(9):2904-5.
Trabert, E. 2001. Long-term Follow-up Programs for Survivors of Childhood Cancer. National Cancer Policy Board Background Paper (www.iom.edu/ncpb).
Wallace WH, Blacklay A, Eiser C, Davies H, Hawkins M, Levitt GA, Jenney ME. 2001. Developing strategies for long term follow up of survivors of childhood cancer. BMJ 323(7307):271-4.
Winn R.J. 2002. Care of the Cancer Survivor: Role and Availability of Specialized Clinic Services: National Cancer Policy Board Commissioned Paper (www.iom.edu/ncpb).
Wittes, R.E. 2003. Therapies for cancer in children—past success, future challenges. N Engl J Med Feb 20; 348(8):747-9.
Wolfe LC. 1993. A model system. Integration of services for cancer treatment. Cancer 72(11 Suppl):3525-30.
Wollnik L. 1976. Management of the child with cancer on an outpatient basis. Nurs Clin North Am 11(1):33-48.
Ziring PR, Brazdziunas D, Cooley WC, Kastner TA, Kummer ME, Gonzalez de Pijem L, Quint RD, Ruppert ES, Sandler AD, Anderson WC, Arango P, Burgan P, Garner C, McPherson M, Michaud L, Yeargin-Allsopp M, Johnson CP, Wheeler LS, Nackashi J, Perrin JM. 1999. American Academy of Pediatrics. Committee on Children with Disabilities. Care coordination: integrating health and related systems of care for children with special health care needs. Pediatrics 104(4 Pt 1):978-81.