Nonhuman Primates in Preclinical Research: The EU Situation
Gerhard Hunsmann, MD
In March 2002, a survey was conducted among 34 institutions involved in nonhuman primate (NHP) research located in eight European Union (EU) countries and Switzerland to identify the numbers of NHPs imported, bred, and used for biomedical research. The questionnaire (Table 1) was sent to the scientists responsible for NHP studies in public and industrial facilities. The response rate in the public sector was 79% (15/19) and from industry, 67% (10/15). While among institutes in the smaller countries using fewer NHPs in biomedical research the response to the survey was 100% (except Belgium), the return from NHP users in the larger countries with larger numbers of NHPs used for preclinical work in academia and industry ranged between 61 and 83%.
BREEDING OF NHPS IN EU COUNTRIES
The overall number of NHPs bred for biomedical research in European institutions in 2001 was 1120:518 common marmosets (Callithrix jacchus); 564 animals of the two macaque species—rhesus monkey (Macaca mulatta) (n = 311) and long-tailed or crab-eating monkey (cynomolgus, Macaca fascicularis) (n = 253). Smaller numbers were also bred of the common squirrel monkey (Saimiri sciureus) (n = 19) and the cotton-top tama
German Primate Center, Goettingen, Germany
TABLE 1 Questions to the Nonhuman Primate (NHP) Users
|
Do you breed NHPs (species, annual production)? Do you produce/require specific pathogen-free (SPF) and/or genetically characterized NHPs? Do you import NHPs (species, number of animals per year)? From which country do you import NHPs? What type of work is conducted with these NHPs? Which are the financial resources for this work? Should the number of NHPs bred for biomedical research be increased in Europe? Are you aware of any further NHP user not yet included in our mailing list? |
rin (Saguinus oedipus) (n = 19). Although Germany breeds the majority of marmosets (n = 320), the United Kingdom produces most of the crabeating monkeys (n = 200) (Figure 1). With respect to production and usage of common marmosets, Europe is self-sufficient.
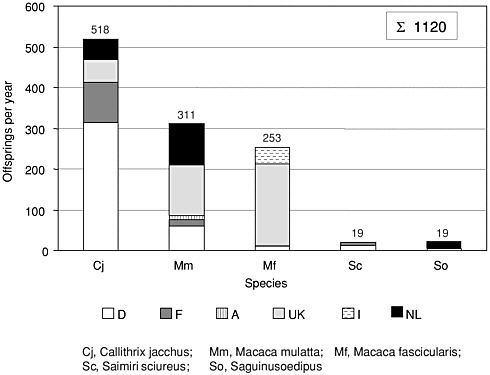
FIGURE 1 EU-bred NHPs. Species codes: Cj, Callithrix jacchus (common marmoset); Mm, Macaca mulatta (rhesus monkey); Mf, Macaca fascicularis (long-tailed or crab-eating monkey); Sc, Saimiri sciureus (common squirrel monkey); So, Saguinus oedipus (cotton-top tamarin). Country codes: D, Germany; F, France; A, Austria; UK, United Kingdom; I, Italy; NL, The Netherlands.
IMPORT OF NHPs
Last year, 1866 NHPs were imported into the EU belonging to the two macaque species (Figure 2a). A total of 74% originated from commercial breeders in China, 12.5% from Israel, 10% from Mauritius, 3% from La Réunion, and 0.5% from the United States (Figure 2b). These numbers are certainly slightly underestimated since the return of the questionnaire was not 100%, and one institute’s response did not communicate the number of animals used annually. The percentage of imported NHPs differs significantly in individual European countries from 100 to 36%. The overall number of NHPs currently imported into the EU slightly more than 2000 annually. This total is up to 70% of the NHPs used for biomedical research in both academic and industrial institutions; 74% of macaques imported originate from a single country.
THE NHP BREEDING GAP
Figure 3 indicates the difference in numbers of NHPs used and bred in each individual country. Although some countries depend totally on NHP importation, others have substantial breeding facilities. The overall number of imported NHPs was about 2000 in the year 2001. Consequently, probably up to 70% of NHPs required for biomedical research in the EU countries and Switzerland must be imported.
SPECIFIC REQUIREMENTS FOR NHPs
There is an increasing need for specific pathogen-free NHPs, as well as for those typed for major histocompatibility complex (MHC), which have a known pedigree or specific blood groups. More than half of the institutes replying to the questionnaire would require such animals, but sufficient numbers are unavailable as yet. Only four breeding colonies producing a limited number of animals free of at least some unwanted pathogens exist in Europe. Likewise, only three institutes are able to provide a few MHC-typed macaques.
EXPERIMENTAL PROTOCOLS AND FUNDING
In institutions of the pharmaceutical industry, most animals are used for pharmacokinetics and toxicology studies. However, university laboratories and public research centers require NHPs for a broad spectrum of research in the fields of neurosciences, reproduction and fertility control, cardiovascular and metabolic research, gene therapy, infectious disease models and vaccine studies, and immunological studies on allo- and xenotransplantation, as well as on allergy and autoimmunity.
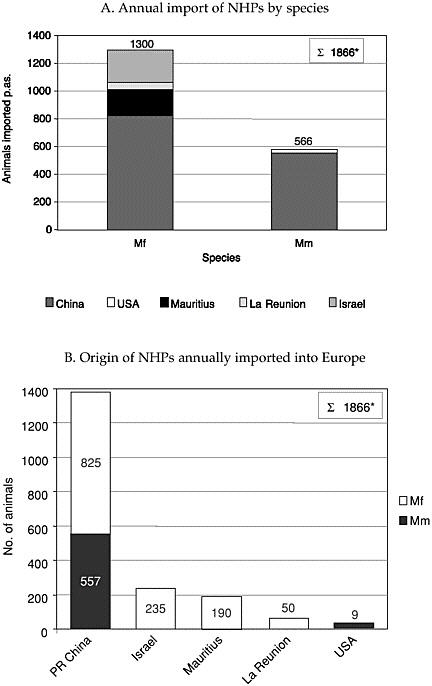
FIGURE 2 Importation of NHPs into Europe. (A and B) For country and species codes, see Figure 1 legend. (B) white column indicates Mf; and black column Mm. *These census numbers are incomplete due to missing information from one responder. The percentage refers to the overall number of imported NHPs.
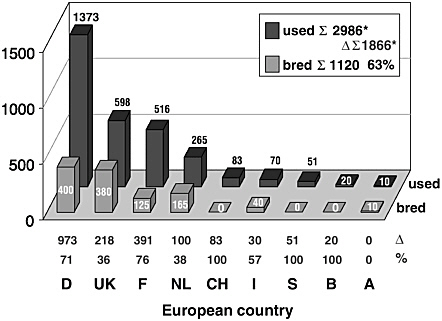
FIGURE 3 Annual number of NHPs bred and required. For country codes, see Figure 1 legend; CH, Switzerland; S, Sweden; B, Belgium. Δ indicates the number of NHPs imported by each country, the percentage gives the rate of imported animals. *Refer to Figure 2 legend. % is the percentage of animals that are imported.
Industrial studies are funded through the respective pharmaceutical companies. However, a substantial fraction of NHPs kept in Europe are being maintained through public resources originating from ministries of health and research and defense, as well as public funding agencies such as the Max-Planck-Gesellschaft, Deutsche Forschungsgemeinschaft, Agence Nationale de Recherche sur le SIDA, Medical Research Council, and the Wellcome Trust.
SUMMARY AND CONCLUSION
The overall response rate to the questionnaire was 73% (25/34), with an unexplained country-specific variability of 25 to 100%. Academic institutions responded more frequently (79%) than industrial facilities (67%).
The number of NHPs used for biomedical research in the European countries surveyed adds up to slightly more than 3000. Approximately two thirds of those must be imported from breeding facilities outside Europe. Of these animals, 70% originate from the Peoples’ Republic of China. Most of the researchers contacted believe that NHP breeding
within Europe should be increased to reduce the overall number of imported primates and the dependence on a single importing country. Moreover, NHPs with certain microbiological and genetic specifications are required in increasing numbers.
ACKNOWLEDGMENT
I am indebted to colleagues of 25 institutions from academia and industry in nine European countries for providing critical information to this survey.
Providing Investigators and Vaccine Producers with Laboratory Primates in the Russian Federation
Boris A. Lapin, PhD, MD
The use of laboratory primates in Russia has decreased sharply during the last 10 to 12 years due to economic conditions, the limited possibility of purchasing monkeys in their natural habitat, the difficulties of their transportation, and, quite often, complex veterinary conditions in the countries where the monkeys naturally live, which hampers obtaining veterinary permit for importation. Today the number of countries exporting monkeys from their natural habitat has also decreased sharply. The variety of species offered by the exporters is as a rule limited to Papio anubis and African green monkeys. The above-mentioned difficulties are aggravated by the refusal of many air companies to ship monkeys in passenger flights, necessitating charter flights and ordering more animals than are needed at the time, both of which increase expenses.
The Russian Federation provisions for fauna do not include the primate species. The supply of primates for experiments has been covered either by importing primates from their natural habitat or by breeding them in the pedigree monkey colony of the Sukhumi Institute of Experimental Pathology and Therapy of the USSR Academy of Medical Sciences (IEPaT AMS). The main colony and several of its branches in the vicinity of Sukhumi numbered about 7500 monkeys, mainly Papio hamadryas and
Institute of Medical Primatology of the Russian Academy of Medical Sciences, SochiAdler, Russia
Macaca mulatta of Indian origin. In addition, after the ban of export macaques from India, a small number of macaques of Vietnamese and Chinese origin were imported to increase the monkey livestock. Besides rhesus monkeys, the Sukhumi monkey colony maintained M. fascicularis and small groups of M. nemestrina and M. arctoides. P. hamadryas numbered 2000 animals. There were also small numbers of Papio anubis, hybrids P. anubis × P. hamadryas, and African green monkeys.
As mentioned above, the total number of monkeys in the Sukhumi monkey colony was 7500 and the annual number of newborns was between 800 and 900. Monkey harems were housed in special monkey houses in large cages with floor areas of 10 to 12 m2 and large open compounds with floor areas of several hundred square meters. Many monkeys were kept in mountains and forest reserves in conditions similar to those existing in their natural habitat. To train monkeys not to go far away, they were fed every day in a definite place at a definite time. It was very important to feed them when it was snowing in the mountains. Despite the considerable decrease in the surrounding temperature, the monkeys acclimated very well to the cold weather; they did not catch cold, and not a single case of death from supercooling was registered. Each monkey from the colony or game reserve has its life history documented with information about how the animal moves from area to area, from cage to another cage, or to the open compound; the laboratory analyses (once a year); the pregnancy and delivery of babies, and so forth.
The number of monkeys including newborns in the Sukhumi monkey colony has met the need of all Russian institutions using monkeys in experiments except for the polio vaccine producers. It was necessary for them to import monkeys from India, Vietnam, or China. At first, mainly M. mulatta were imported; however, due to the subsequent high rate of macaques infected with SV-40, African green monkeys were substituted according to advice from the World Health Organization. I did not agree with this decision because African green monkeys are often infected with STLV-1 and EBV-like viruses. However, it was necessary to import about 3000 of African green monkeys to obtain cell cultures and control residual neurovirulence.
The method of growing vaccinia virus has now been improved, and the bulk of the monkeys have been used to control the residual neurovirulence of the polio vaccine. Taking into consideration the considerable reduction of primates in their natural habitat, which I believe will affect African green monkeys in the near future, the Commission on Medico-biological investigations on monkeys under the RAMS Presidium has now recommended that polio vaccine producers develop measures for sharply reducing their use of African green monkeys in polio vaccine production.
After the military conflict between Abkhazia and Georgia had arisen, it became impossible to continue working in the Sukhumi Institute, and the main staff had to move to Sochi-Adler. There, on the basis of an existing branch of IEPaT AMS, a new Institute of Medical Primatology was founded with a large monkey colony affiliated with the Russian Academy of Medical Sciences (IMP RAMS). The Adler monkey colony currently has 2500 monkeys, including 1,000 M. mulatta, 600 M. fascicularis, 500 P. hamadryas, 100 P. anubis, 100 African green monkeys, and a few animals of other species. Every year the Adler monkey colony is reinforced with 500 newborns of different species. The IMP RAMS in Adler carries out its own wide research program, and the Institute also provides visiting researchers from the Institutes of RAMS and the Health Ministry of the Russian Federation with the opportunity to carry out experiments on monkeys. Some researchers perform their investigations as collaborators according to the signed agreement between IMP RAMS and the other Institutes concerning testing of vaccines and biopreparations.
To meet the national need for monkeys independently from importation, IMP RAMS plans to increase the livestock of monkeys up to a total of 4000. To fulfill this plan, we intend to construct more monkey houses and corrals during the next 3 years. Only the Institute of Poliomyelitis and Virus Encephalitis is still an exception and must still import African green monkeys annually to produce polio vaccine and to test residual neurovirulence. Taking into consideration the above-mentioned situation that limits the importation of monkeys, it becomes clear that we can rely only on monkeys bred in colonies in captivity. This approach ensures that we will diminish the risk of infection imported from the monkeys’ natural habitat, which can be dangerous for the monkey colonies, the monkey handlers, and the experimenters alike.
One can predict that in the near future, the only way to obtain monkeys for experiments will be to develop monkey colonies and breed them in captivity. The sooner we use monkeys bred in colonies, the more effectively we will be able to protect and save endangered species of monkeys, even though the main reason for reducing the natural monkey population is the economic activity of humans.
Nonhuman Primate Resource Needs: A Moving Target
Jerry Robinson, PhD,* and Greg Beattie, MSc, D.A.B.T.†
Thank you, Mr. Chairman. I also wish to thank the organizing committee for convening such an extremely important meeting to address the growing needs for nonhuman primate resources for biomedical research. This is a joint presentation by Dr. Greg Beattie of the Sierra Biomedical Research Division of Charles River Laboratories, Inc., and myself. The reason for our collaboration is that Dr. Beattie is involved in the commercial enterprise of providing nonhuman primates for biomedical research. I, however, am more involved with National Institutes of Health (NIH) grants that support biomedical research efforts predominately at academic institutions. It is hoped that the two of us will cover two distinct aspects of nonhuman primate research resources.
My major role at the NIH National Center for Research Resources (NCRR) is the administrative oversight of our National (formerly Regional) Primate Research Centers program (NPRC). In addition to the eight National Centers across the country, NCRR supports additional nonhuman primate resource centers at other sites in the country. I am also
the program administrator of the NCRR grants that support these, thus, I am fairly familiar with the nonhuman primate resources that are supported by NCRR. In short, NCRR:
-
Serves as a catalyst for discovery for NIH-supported investigators throughout the nation;
-
Creates, develops, and provides a comprehensive range of human, animal, technological, and other resources to enable biomedical research advances; and
-
Seeks scientific knowledge that will lead to better health and reduced illness and disability for our nation’s citizens.
NCRR is rather unique among the Institutes that make up the NIH. NCRR is not a categorical disease institute like Cancer, Infectious Diseases, or Mental Health. However, NCRR, through grants, supports the basic infrastructures on which the biomedical research community depends to accomplish their biomedical research goals. We believe that we are the catalyst for biomedical research discoveries.
One of the major programs within NCRR is the NPRC Program. Each NPRC is affiliated with a major academic institution. The NCRR grants that support these Centers provide funding to support the basic infrastructure, which includes specialized animal housing facilities, animal care staff, veterinary care, and clinical laboratories, as well as the other specialized equipment and personnel necessary for the conduct of biomedical research. These eight Centers are a unique network that provides access to more than 20,000 nonhuman primates and provides the infrastructure support for more than 1200 scientists. Obviously, these National Resource Centers play a critical role in the conduct of biomedical research using nonhuman primate models.
One of the key questions that speakers were charged with addressing was to identify “the species and numbers of monkeys that are maintained in your country or region for biomedical research.” NCRR recently completed a survey of 1999 NIH grantees who use nonhuman primates for research. Approximately 13,000 nonhuman primates were used in 1999 by NIH grantees. Of those 13,000 animals, nearly two thirds were rhesus macaques.
According to the US Department of Agriculture (USDA) annual survey of the number of animals used in 2000 (2001 figures are not yet available), more than 57,000 nonhuman primates were used in biomedical research that year. Unfortunately, the USDA does not report a breakdown by species, so we have no way of knowing which species are utilized in the greatest numbers. However, macaques, especially rhesus, are the most likely used as a research animal model.
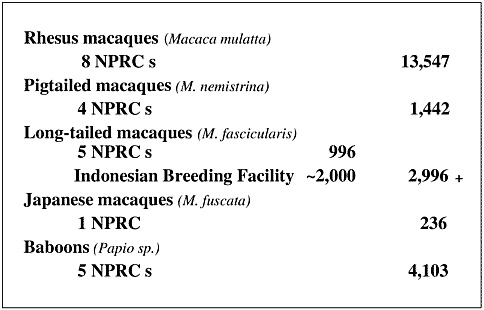
So where do we get animals for biomedical research? Dr. Doug Bowden, Washington NPRC, gave a talk in Japan earlier this year and has shared some of the data he compiled. As can be seen in Table 1, overseas breeding colonies provide the majority of our nonhuman primate resource needs. US primate centers and other primate breeding facilities do provide significant numbers of animals. We are also aware of US commercial breeding operations, but we do not know how many animals are produced or the numbers used in the private sector.
Figure 1 provides a breakdown by species of the animals imported from 1995 to 2000. The long-tailed macaque was imported to the greatest extent. Although importation of rhesus is second, the majority of these macaques are imported mainly from China. Exportation of rhesus monkeys from India has been banned since the mid-1970s. Later in the program, Mr. Tom DeMarcus from the CDC will perhaps provide us with more up-to-date statistics in his presentation on the importation of nonhuman primates.
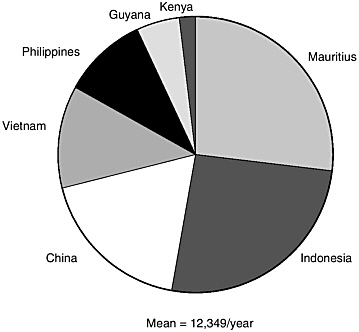
In Figure 2, the major countries appear from which nonhuman primates are derived. Again, the majority of the animals are long-tailed macaques and more than half are derived from Indonesia and Mauritius.
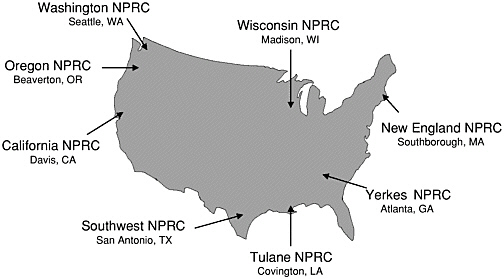
As I mentioned earlier, NCRR has a major program that supports the National Primate Research Centers. As can be seen in Figure 3, the majority of these Centers are located in the south and on the west coast. NCRR, through Center base grants, provides support for specialized facilities, personnel, equipment, and nonhuman primate resources for the conduct of biomedical research. These Centers provide infrastructure support for several hundred scientists doing research in neurosciences, infectious diseases, cardiovascular diseases, and other related human health problems that require nonhuman primates as the animal model. Such research efforts are supported by more than 500 NIH grants as well as other funding sources.
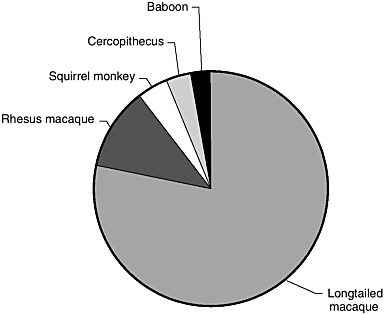
The rhesus macaque is the predominant species maintained at the National Primate Research Centers (Figure 4). However, significant numbers of baboons and long-tailed and pig-tailed macaques can be found at half of these Centers as well. Some of the Centers do maintain colonies of
TABLE 1 Sources of US Research Primates: 2002
|
Overseas Breeding Colonies |
11,400 |
|
US Primate Center Breeding Colonies |
2,000 |
|
Other Research Breeding Colonies |
4,000 |
|
Primate Supply Info Clearinghouse |
3,000 |
|
US Commercial Breeding Colonies |
??? |
new world species, marmosets, tamarins, and squirrel monkeys. In addition, NCRR supports the following: large colonies of rhesus macaques (n=2029) at the University of Puerto Rico’s Caribbean Primate Research Center; a squirrel monkey colony (New World species; n=439) at the University of South Alabama; a baboon resource colony (n=167) at the University of Oklahoma; and two chimpanzee colonies located in Bastrop, Texas (n=180), and New Iberia, Louisiana (n=130). The squirrel monkey colony is our only Center dedicated solely to New World species. This animal model is excellent for malaria studies as well as other infectious diseases such as Chagas’ disease. There are also increasing demands for baboons, particularly in the area of organ transplantation and diabetes. The chimpanzee colonies were established initially as potential models for AIDS research. Although the animals could be infected with HIV, they never developed the disease. These animals, however, are still good models for hepatitis and respiratory syncytial viral diseases.
For the rest of my talk, I wish to focus on the species that is in the greatest demand, the rhesus macaque. From the NCRR survey of 1999 NIH grantees, we learned that the rhesus macaque comprised approximately 65% of the nonhuman primates used for biomedical research. In addition, we learned from examining the animal census at the NPRCs that 60% of the animals being maintained at these Centers are rhesus.
So what are these animals being used for? One of the greatest demands these days is for AIDS research, particularly for testing potential vaccine candidates. Simian immunodeficiency virus infection in macaques almost exactly parallels HIV progression and pathogenesis in humans. At present, it is the only real animal model for studying this disease. The recent anthrax scare has created additional demands for the rhesus macaque. Initial testing of vaccines in the 1960s by the Department of Defense used rhesus as the animal model. To test new vaccines and therapies, they obviously want to use this species again.
There have also been significant developments in the neurosciences using nonhuman primates. There are nonhuman primate models for Parkinson’s and Alzheimer’s diseases. Scientists at the California NPRC have developed gene therapy techniques for inserting genes to produce nerve growth factor as a treatment method for overcoming neurological deficiencies in macaques. Similar treatments may be used in the treatment of Parkinson’s. The nonhuman primate has also played a key role in the reproductive sciences: cloning, transgenic monkeys, and the establishment of embryonic stem cell lines. Rhesus macaques are also in demand for studies examining organ/tissue transplantation; they are the animal models used for kidney and pancreatic islet cells experimentation.
It is obvious that there is an ever-increasing need for nonhuman primates. To help meet the needs of the biomedical research community, the
NPRCs have increased their breeding colonies (Table 2). From 1996 to 2000, the number of animals in the breeding colonies has increased from 6200 to more than 7600. Although this increase is significant, not all of these animals are capable of reproducing at the present time. The rhesus female does not reach menarche until 2.5 to 3.0 years of age, and male puberty does not occur until 3.5 to 4.0 years of age. Thus, many of the animals in the breeding colonies are just getting to their reproductive age (Table 3).
Part of the problem is that the greatest demand is for animals 2 to 5 years of age. However, to build up the breeding, these are the animals, particularly the females, that are needed to increase the breeding stock (Table 4). Given the length of time needed for the monkeys to reach sexual maturity, we are looking at a 5 to 10 year period to increase production capabilities at the eight NPRCs significantly.
Other complicating factors that are putting additional strains on rhesus supplies come from the demands for specialized resource needs. The AIDS research community as well as those investigators looking at transplantation tolerance need animals free of specific pathogens. Animals not free of certain retroviruses can compromise the experimental findings. There is also a need for genetically defined animals; for example, certain types of AIDS research require animals with a known major histocompatibility complex. Such demands further limit availability of these precious resources.
Rhesus macaques of Chinese origin have become available and were thought to be a possible solution to the limited supply of rhesus. However, the different immunological and genetic makeup of these animals has led to some research complications. The pathogen status of some of these animals is also problematic. Nonetheless, these animals can be utilized in certain types of experimentation.
TABLE 2 National Primate Research Centers, Rhesus Macaque Colony Statistics
|
|
Breeding Colony Statistics |
||
|
Year |
Animal Numbers |
No. Animals |
% Total |
|
1996 |
11,706 |
6,271 |
53.4 |
|
1997 |
11,641 |
5,580 |
50.3 |
|
1998 |
11,828 |
6,028 |
51.0 |
|
1999 |
12,546 |
6,023 |
48.3 |
|
2000 |
13,584 |
7,620 |
56.1 |
TABLE 3 Rhesus Demographics at 8 NPRCs: January 2001
|
Total Number of Animals: |
13,584 |
|
|||
|
Number of Animals—Asian Indian Origin |
12,038 |
88.7% |
|||
|
Number of Animals—Chinese or Hybrid |
1,529 |
11.3% |
|||
|
Age Distribution: |
|||||
|
0-5 |
years of age |
7,929 |
58.4% |
|
|
|
6-10 |
years of age |
3,278 |
24.1% |
||
|
11-15 |
years of age |
1,323 |
9.7% |
||
|
16-10 |
years of age |
582 |
4.3% |
||
|
>20 |
years of age |
482 |
3.5% |
||
Another problem confronting investigators is a limitation of specific types of housing. Many research protocols, particularly vaccine challenge studies that study infectious diseases, required specialized caging (biosafety level 2, 3, and even 4 in some cases). Although the NPRCs are trying to increase their holding facilities, their current capacity is very limited and is being utilized to the maximum. Studies of infectious organisms such as anthrax will place even greater demands on specialized housing needs.
So what is NCRR doing to try to help meet these needs? In September 2000, NCRR awarded five grants to establish rhesus macaque colonies that are free of specific pathogens (SPF colonies) and then added a sixth colony in 2001 (Table 5). These colonies were established in coordination with the Office of AIDS Research. Thus, the offspring generated will be dedicated to the AIDS research community. It is projected that these colonies will produce more than 2300 animals in 5 years.
Because the projected needs from the NCRR survey include more than 7000 animals and we are aware that an increasing number of vaccine candidates must be tested, NCRR reissued the announcement soliciting the establishment of additional colonies in 2002. It is anticipated that five
TABLE 4 National Primate Research Centers: Rhesus Monkey Statistics May 2001
|
Total Numbers |
|
|
13,547 |
|
Asian Indian Origin |
12,016 |
88.7% |
|
|
Chinese Origin |
1,531 |
11.3% |
|
|
No. of Animals in Breeding Colonies |
|
56.1% |
7,604 |
|
No. of Females Capable of Conceiving |
|
2,921 |
|
|
No. of Offspring Produced 2000 |
1,828 |
||
TABLE 5 NCRR-Supported SPF Rhesus Colonies
|
Site |
Host Institution |
5-year Projections |
|
1. California NPRC |
University of California-Davis |
215 |
|
2. Caribbean PRC |
University of Puerto Rico |
350 |
|
3. New England NPRC |
Harvard University |
335 |
|
4. Oregon NPRC |
Oregon Health Sciences University |
700 |
|
5. Southwest NPRC |
Southwest Foundation for Biomedical Research |
365 |
|
6. Tulane NPRC |
Tulane University |
400 |
|
|
Projected Totals |
2,365 |
or six more SPF colonies will be supported to help meet these growing needs. Again, we are talking about a 5- to 10-year period to establish these colonies to the point where they begin producing significant numbers of monkeys.
Obviously, NCRR cannot provide all of the nonhuman primate resources needed by the biomedical research community. We are trying to facilitate importation of more macaques from international sources to help meet some of these needs. However, some investigators may have to turn to the private sector to meet their animal needs (Table 6). Alternatively, other investigators may want to consider other nonhuman primate species as alternative models for their studies of human diseases. Other nonhuman primate species such as the long-tailed macaque are more readily available.
TABLE 6 NPRC—Commercial Relationships
|
• Some NPRCs obtain up to 50% of their animals from commercial sources • Research by companies can be accomplished at the NPRCs, but only accounts for 5%-20%; |
|
|
|
– NIH supports about 80% of biomedical research in the US – Most commercial use of primates is routine toxicology; not a common focus of university researchers’ interest – NIH does not subsidize company research, so costs are high – Intellectual property considerations: universities encourage publication, which can be counterproductive for companies |
|
• Most US pharmaceutical and biotech companies either have their own primate research facilities or contract the work to private research companies. |
|
Center for the Breeding and Conservation of Primates of the Peruvian Primatology Project
Enrique Montoya, DVM
BACKGROUND
There is worldwide concern about preserving the environment and its natural resources since current and future generations have the right to enjoy an environment that is healthy, balanced, and suited for life, in harmony with the landscape and nature. Significant trends have had an impact on natural resources in the Peruvian Amazon. The 19th century and the first half of the 20th century were characterized by the exploitation of plant resources. Then in the 1960s, depredatory trade in wild fauna and their by-products proliferated, exporting live specimens such as mammals, psittacidae, and ornamental fish.
In 1974, Peru ratified the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) and promulgated the Wild Flora and Fauna Act (decree law no. 21147), which regulated the hunting or capture of species of wild fauna and their use for scientific and cultural purposes. In 1972, the Study on Nonhuman Primate Populations in Peru was promoted, with the involvement of the Veterinary Institute of Tropical and High Altitude Research (IVITA) of the School of Veterinary Medicine of the San Marcos National University (UNMSM) and technical cooperation from the Pan American Health Organization/World Health Organization (PAHO/WHO). In 1975, the First International Conference on Conservation of Nonhuman Primates in the New World and their Utilization in Biomedical Research was held, which recommended the development of a Primatology Project.
A Letter of Agreement between the government of Peru and PAHO/ WHO was signed in 1975, laying the foundations for cooperation in implementing a biological research project on nonhuman primates—the Peru
Peruvian Primatology Project
vian Primatology Project (PPP)—with a view to meeting the needs of the biomedical scientific community and at the same time using appropriate techniques to ensure the sustainable management of species in their natural habitat. Within this framework, the Center for the Breeding and Conservation of Primates of Iquitos (CRCP-PPP) was created, which is affiliated with the IVITA Experimental Station.
OBJECTIVES
The objectives of the CRCP-PPP are to generate knowledge and apply techniques for managing neotropical primate species in captivity, contributing to biomedical research and improvements in the quality of life of the population as well as to the sustainable management of Amazon biodiversity. These objectives will be achieved by (1) generating techniques and knowledge for efficient management of primates in captivity, and (2) developing techniques for evaluating and managing primate populations in controlled natural areas (islands).
INFRASTRUCTURE
The CRCP-PPP is located in the Department of Loreto near the city of Iquitos in the IVITA Experimental Station, which has 8 ha of land. It has offices, sheds, a library, a laboratory, storerooms, and electricity and potable water services. It also has a field, a laboratory, and computer materials and equipment, as well as land and water vehicles.
The Padre Island Biological Station comprises an area of 8.3 km2 and 96 m2 of buildings with reinforced concrete walls and a corrugated metal roof. The Station has demonstration agroforest plots. The Muyuy Island Biological Station is located 18 km from Iquitos, along the Amazon River. In 1989, a 150 m2 building was constructed of wood with a sheet metal roof.
ACTIVITIES AND STUDIES
Basically, activities and studies at the CRCP-PPP are for the purposes of breeding and health in captivity and management in controlled natural areas (islands). In this presentation, I will describe the progress and results of activities conducted in recent years.
BREEDING, HEALTH, AND HUSBANDRY IN CAPTIVITY
Most of the research on breeding and health is conducted at the CRCP. In addition to the study on population dynamics of colonies and health
management and monitoring, research has been conducted in the areas of biometrics, breeding, diagnostic criteria, and disease prevalence.
POPULATION DYNAMICS OF AOTUS AND SAIMIRI COLONIES
After 20 years of operation, we have reached the fifth generation (F5) of Aotus nancymae and the third generation (F3) of A. vociferans. The breeding activity of the Aotus colonies is currently at very satisfactory levels because the colonies consist chiefly of specimens born in captivity, and replacement breeders are selected on the basis of the clinical and breeding history of their parents. Breeding outcomes are also satisfactory in the Saimiri colony.
The origin of the breeders in the A. nancymae colony has been analyzed. Currently, 75% have been born in the colony, and 25% have been taken from natural areas; the breakdown for the A. vociferans colony is 59 and 41%, respectively. Similarly, the origin of the Saimiri sciureus colony has been established: 65% have been born in the colonies and 35% have been captured; the composition of the S. boliviensis colony is 41 and 59%, respectively.
FEEDING
The colonies consume 45 to 50 g of a wafer-like concentrate and fruit per animal (Aotus) or 50 to 60 g per animal (Saimiri), per day. The concentrate consists of soybean, wheat, and rice meal, sugar, eggs, vegetable oil, and peanuts obtained locally; premixes of vitamins and specific minerals for nonhuman primates are also included, which are imported from the United States. The nutritional content of the wafer is 24% crude protein, 10% fat, and 4% ash.
HEALTH MONITORING AND CONTROL OF COLONIES
Health monitoring and control are carried out with support from the CRCP Laboratory, especially for clinical analysis of specimens and microbiological monitoring of food and water intake to keep species in captivity in good health. The laboratory also checks the Center’s colonies for parasites every 3 months.
Ivermectin and mebendazole are the parasiticides used to fight Strongyloides and Trypanoxiurus, and metronidazole is used to fight flagellate protozoans. The parasites observed most frequently have been Strongyloides, Hymenolepis, Trypanoxiurus, and flagellate protozoans. In Saguinus, Prosthenorchis have also been observed.
Necropsies indicate that the leading causes of death in adult Aotus have been pneumonia and heart disease, as well as liver degeneration in animals that have been in captivity for more than 10 years. In offspring, the leading causes have been respiratory and enteric infections.
Adult and elderly Saimiri present with enteric and respiratory infections (pneumonias). In offspring, the deaths were caused by rejection, cannibalism, and pneumonias in neonates.
GROWTH AND DEVELOPMENT DURING THE FIRST 6 MONTHS OF LIFE
Growth and development parameters for the first 6 months of life were established as basic knowledge for managing A. nancymae offspring, which have an average birthweight of 91 g. At the end of the study, the specimens reached an average weight of 494 g (500 g for males and 488 g for females). In Figure 1, the evolution of the weight and length of A. nancymae are shown.
In S. boliviensis, birthweight does not differ by gender (average weight 99 g). However, when they are 180 days old, males weigh more than females (413 vs. 365 g).
STUDIES TO IMPROVE BREEDING
In the breeding study to evaluate options for lowering the weaning and breeding ages in A. nancymae, weaning ages of 5, 6, 7, 8, 9, and 10 months were evaluated. In Figure 2, the changes in weight at 12 months of
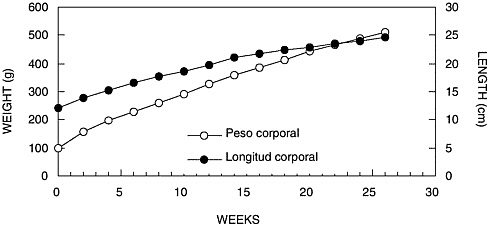
FIGURE 1 Weight and length of Aotus nancymae, up to 26 weeks of age: 1998.
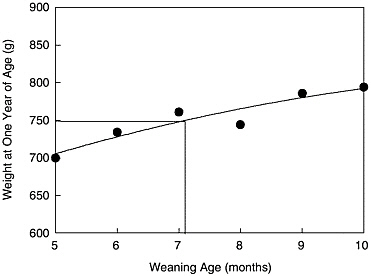
FIGURE 2 Weight at 1 year of age of Aotus nancymae in relation to weaning age: 2000.
age in relation to weaning age are shown. We observed that it is possible to obtain specimens weighing approximately 750 g that are appropriate for selection as breeders with a weaning age of 7 months.
A test was conducted with 20 A. nancymae females weaned before age 1 and paired at 14 months of age on average, to evaluate the possibility of lowering the breeding age. Of these females, 70% had their first reproductive event after an average interval of 14 months and obtained their first offspring at 28 months of age on average.
STUDIES ON THE PREVALENCE OF ANTIBODIES
The study on prevalence of antibodies against T. gondii revealed the following: 13.6% in A. nancymae, 12.3% in S. boliviensis, and 18.7% in S. sciureus in captivity. Recorded prevalence was higher in females and, in relation to age, was higher in juveniles than in adults and subadults. However, there were no clinical manifestations of natural infection.
Various arboviruses cause febrile illnesses in humans in the tropics. To determine the importance of neotropical primates in the transmission of these agents, serum samples were collected from recently captured A. vociferans, A. nancymae, and S. boliviensis. Using the enzyme-linked immunosorbent assay technique, the samples are being analyzed against an arbovirus panel (dengue, yellow fever, mayaro, oropuche, and Venezuelan equine encephalitis).
PHYSIOLOGICAL PROFILES FOR DIAGNOSIS
Under the handling conditions described, it has been possible to establish normal physiological profiles for A. nancymae with respect to hematology, blood biochemistry, and creatinine and liver enzyme serum levels. Hematology profiles have also been established for A. vociferans, Saguinus mystax, and S. labiatus; and urea, creatinine, and liver enzyme serum profiles for S. boliviensis.
EFFECTIVENESS OF IVERMECTIN 0.1% AND FENBENDAZOLE AGAINST GASTROINTESTINAL PARASITES
Doses of fenbendazole and ivermectin were given to S. mystax and S. fuscicollis specimens found positive for Strongyloides spp., Trichostrongylus spp., and Prostenorchis elegans from Padre Island and natural areas. The doses effectively controlled Strongyloides spp. and Trichostrongylus spp. within 72 hours; however, the prevalence of P. elegans did not show any variation.
INFLUENCE OF HUMAN COMMUNITIES ON IQUITOS ISLAND ON THE PARASITE LOAD IN CEBIDAE
The sample took persons (n=223) from hamlets on Iquitos and Padre Islands; 97% tested positive for parasitic infections (Ascaris lumbricoides, 67%; Trichuris trichura, 31%; and Uncinaria, 2%). At the same time, in A. nancymae (n=132), 91% tested positive for parasitic infections; a Strongyloides sp. prevalence of 26% was recorded in simple infections; in mixed infections, the association between flagellate protozoans and Strongyloides was 15%. The differences in the parasite profiles suggest that there is no human influence on the parasite load in A. nancymae.
CONTROLLED NATURAL AREAS (ISLANDS)
The research on the islands includes the following: (1) evaluating the population dynamics of primates, especially Saguinus spp., to estimate the size of the colony it can support and the optimal rate of collection; and (2) establishing alternative land use systems to reduce the pressure of local populations on the primates’ habitat.
STUDIES ON POPULATION DYNAMICS
Saguinus mystax on Padre Island. Three consecutive introductions of specimens took place between 1977 and 1980, for a total of 87. Since 1980
the number, size, and structure of family groups have been evaluated, as well as increases in population. In Figure 3, the evolution of the population, which rose consistently except in the collection years, is shown. Even so, the increase resumes after the collection. According to the intrinsic growth rate (without collections), the support capacity was estimated at 428 individuals, which would be reached in 2005; and from that time, growth would begin to stabilize and fall. For the population to maintain its rate of growth, 35 specimens should be removed every 3.5 years.
Saguinus labiatus on Muyuy Island. In December 1989, 31 specimens were introduced on the island, and family groups are evaluated annually to determine population dynamics. In 1996, 54 specimens were surveyed, and the size and structure of three family groups were determined as well as the plant species that make up their diet and the behavior of this species in the presence of human activities. In 1999, of the 20 troops identified, 14 were fully counted and 100 individuals were recorded. The number of pregnant females indicates satisfactory breeding of the species on this island.
Cebidae census on Iquitos Island. In 1998, Cebidae population censuses were conducted on Iquitos Island (A. nancymae and S. boliviensis) in
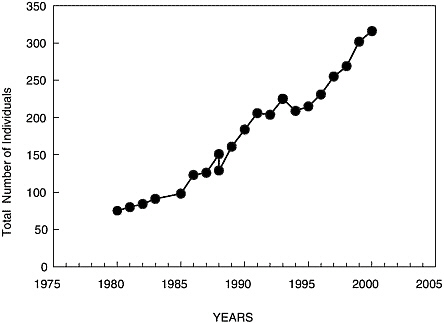
FIGURE 3 Evolution of the Saguinus mystax population on Padre Island.
the vicinity of the hamlet of San Pedro de Huashalado. The respective population densities were 35 and 92.3 individuals per km2.
Breeding behavior and breeding efficiencies of these colonies may be seen in Tables 1 and 2, respectively.
ALTERNATIVE LAND USE SYSTEMS
The populations introduced onto islands must deal with pressure from human groups settled in the environs. One way to reduce the pressure from the inhabitants is by educating communities about the value of primates, in addition to offering options for more intensive land use.
DEMONSTRATION AGROFOREST PLOTS
Since 1993, 7 ha of demonstration agroforest plots of arazá, carambola, camu camu, rice, and yucca on Padre Island have been evaluated. This activity extends to Iquitos Island and the right bank of the Amazon River through the cultivation of seedbeds of fruit and forest species. In 1996, a 0.5-ha demonstration plot was installed on Muyuy Island with the same species.
The plots are maintained and periodically evaluated, and seedlings are transferred to the communities. Currently, the agroforest plots are systematically compared with traditional island production systems.
INSTITUTIONAL IMPACT
The impact of the CRCP-PPP is not limited to its institutional objectives; instead, through academic and social support, it has reached out to
TABLE 1 Breeding Behavior of the Aotus and Saimiri Colonies: 2001a
|
Species |
Initial Population |
Breeding Groups |
Births |
Type of Breeding |
Final Population |
Breeding Groups |
|
A. nancymae |
284 |
95 |
87 |
Pairs |
338 |
106 |
|
A. vociferans |
165 |
53 |
44 |
Pairs |
203 |
61 |
|
S. boliviensis |
154 |
15:76 |
41 |
Harem |
164 |
21:83 |
|
S. sciureus |
39 |
4:21 |
5 |
Harem |
38 |
6:23 |
|
TOTAL |
642 |
743 |
|
|||
|
aBreeding efficiency has been consolidated (number of offspring born/number of breeding females × 100) (Table 2) through the maintenance of breeding records, gradual renewal of the breeding group, adequate diet, the rehabilitation of infrastructure, and disease surveillance and control. |
||||||
TABLE 2 Breeding Efficiency in Captivity: 2001
|
Species |
Breeding Groups |
Reproductive Eventa |
Breeding Efficiency |
||
|
LB |
SB |
MC |
|||
|
Aotus nancymae |
95 |
87 |
2 |
6 |
91% |
|
Aotus vociferans |
53 |
44 |
4 |
5 |
83% |
|
Saimiri boliviensis |
17:76 |
41 |
5 |
54 |
|
|
Saimiri sciureus |
6:21 |
5 |
24 |
|
|
|
aLB, live births; SB, stillbirths; MC, miscarriages. |
|||||
the community. It regularly hosts academic events such as courses and training for students from the UNMSM Schools of Veterinary Medicine and Biology, as well as from other national and international universities and local technical institutes.
One effective social outreach focus is the dissemination of information on alternative island land use systems when talks are given on eco-development, agroforestry, wildlife management, human health, and the use of primates in research, as well as technical assistance in community farming areas. During these activities, the creation of family agroforest plots is supported. In addition, since 1996, 10,080 grafts of cedar, huito, arazá, carambola, and camu have been distributed to 60 beneficiary families in nine communities on Padre and Iquitos Islands.
The publication of research findings has been abundant. There are articles in science journals, abstracts at scientific congresses on this specialty, and institutional reports. As part of this effort, the publication of La Primatología en el Perú (Primatology in Peru) Volumes I and II is noteworthy.
Finally, there have been periodic transfers of neotropical primates of biomedical interest to scientific institutions through PAHO/WHO. The numbers are estimated at 250 specimens per year in the past decade. Biological samples were also sent for specific studies.
OUTLOOK
The research and outreach activities during the period make the CRCP-PPP a sort of precursor in science and biomedical culture in the area of influence. Its performance has appreciated the value of nonhuman primates for medical research and has raised awareness in some interest
groups about the value of the country’s biodiversity. The progress and results must be consolidated through the following initiatives:
-
Validation of techniques for captive and semicaptive (island) breeding of species of scientific and cultural interest and for recognition and treatment of spontaneous diseases.
-
Contributions to the knowledge of captive state physiology, nutrition, breeding, pathology, and behavior of native species.
-
Generation of semicaptive state (islands) population management techniques, which include agroforestry and enrichment of the forest for its rational use. This experiment, with the participation of local communities, is likely to extend to other species of Amazon fauna and flora, including research on and management of medicinal plants. The CRCP-PPP is a pioneering experiment in the management of nonhuman primates of biomedical interest.
-
Sustainable use of nonhuman primate populations of biomedical and cultural interest through the development of management techniques, together with studies on biodiversity and forest productivity. Our experience indicates that the periodic collection or capture of species, such as A. nancymae, A. vociferans, Saguinus mystax, S. fuscicollis, S. boliviensis, and S. sciureus, is feasible. Collection expeditions should also be used to study the biology of the species in natural areas and their importance in maintaining biodiversity and forest productivity.
-
Inventory and identification of primate species in Peru, as well as their geographic distribution and population dynamics. This strategy would protect endangered species and would make it possible to determine their geographic distribution and population dynamics (density and group composition). Authorities must step up environmental education efforts and support for control officials to achieve effective protection and conservation.
Session 2:
Panel Discussion
Participants:
John G. Vandenbergh—Session Chair, North Carolina State University, USA
Gerhard Hunsmann—Deutsches Primatenzentrum, Germany
Boris A. Lapin—Institute of Medical Primatology, Russia
Jerry Robinson—NIH/NCRR, USA
Greg Beattie—Sierra Biomedical, USA
Enrique Montoya—Peruvian Primatology Project, Peru
QUESTIONS AND ANSWERS
DR. VANDENBERGH (John G. Vandenbergh, North Carolina State University): One of the concerns that occurred to me as I was sitting here is that we are talking about a resource, the nonhuman primate, that is becoming more and more limited and more and more difficult to work with in many ways. Dr. Lapin and some of the other speakers referred to the idea that we need to have highly justified pieces of research on this. We have heard our opening speaker today, Dr. Hearn, talk about strategic research versus basic research.
What concerns me, as the nonhuman primate becomes a more limited resource, is whether we will focus almost entirely on the strategic and the applied and will dry up that well of basic information that we need. I wonder whether the panel would be interested in responding to that
concern: whether we will have continued opportunities for basic research as well as important drug testing and other very applied things that we need.
DR. ROBINSON (Jerry Robinson, National Center for Research Resources): You had addressed the important issue with respect to the primate centers, and that part of the original charge of the primate centers was to do conservation and field studies. However, with the cost of biomedical research skyrocketing as it is, the ability is very limited to provide resources for those people to do those important studies in the wild as well as maintain the animals in captivity.
DR. VANDENBERGH: And not just the wild as we all understand. I am also concerned about the laboratory aspect.
DR. HUNSMANN (Gerhard Hunsmann, Deutsches Primatenzentrum): We at the German Primate Center have a substantial amount of our overall funds being used for field studies. That amount is increasing, actually. The new philosophy now is that biomedical research must carry its own weight meaning that money for it must come from outside— either grants or contracts. We have studies in the field (e.g., a field station in Madagascar and the University in Iquitos), where studies are supported by the core grant of the primate center. It is a little different from what we hear is going on in the United States.
DR. ROBINSON: I would like to add that NCRR, through their small instrumentation grants and so forth, serves the primate facilities that engage in imaging small animals. A great deal of physiological basic understanding is being derived from such studies.
PARTICIPANT: It is my perception that at least with regard to rhesus, baboons, and chimpanzees, there has been a sort of waxing and waning of the needs and the demands for those species over time. Whenever the demand has built up, there has been a response from NIH to produce more animals and make them available for research. When there have been enough animals, or demand has decreased because scientific needs change every 5, 10, or 15 years, there has been the sense that these animals have been in excess and therefore should be reduced to keep the costs down. If that perception is correct, it is my sense that perhaps it would be more economical to maintain a surplus of these critical species as insurance so that when demand picks up again (and it always seems to because it always has as long as I have been working with nonhuman primates), it is actually possible to meet that demand quickly. That approach might actually be less expense than allowing the numbers to decrease to exactly what the demand is during a period of low demand.
So, I have two questions. First, is that your perception also or do you see the situation somewhat differently? Second, if your perception is the same, can you envision any way of changing the priorities not to have this
waxing and waning supply that never seems to be in synchrony with demand?
DR. ROBINSON: I totally agree with your perception, which I tried to express in the table I showed with the primate center breeding colonies. Yes, it does appear to come and go. In primate centers in the past (e.g., when I was at Wisconsin), it seemed as if we maintained animals for long periods of time. However, with regard to the long-term strategy, perhaps this latest crunch is a wakeup call for the government to maintain certain levels of nonhuman primates so that they are available. I mean, bioterrorism issue is a key example because they suddenly need to retest all of these vaccines and so forth, and the animals are not available.
DR. ERVIN (Frank Ervin, McGill University): Having sat on NIH review committees for well over 25 years, I would like to question Dr. Robinson’s statement that of the 13,000 NHP used by NIH investigators every year, 65% of which are rhesus, that a significant number of rhesus were required for experiments. In fact, most of them did not need a rhesus macaque. They just thought they would like to have a rhesus. For some of them, that was the only macaque they could spell. For some of them, it was the one that was available in the local colony. For some of them, their major competitor in the field had used a rhesus, but there was no biological justification for using a rhesus. If you would like to increase your rhesus availability, Jean Baulu in Barbados and Frank Ervin in St. Kitts can cover half of your use of wasted rhesus and use them for something else.
DR. ROBINSON: I totally agree, and I think one of the things NCRR is trying to do is to make investigators more aware of other nonhuman primate species to make them consider the most appropriate biological model.
DR. ERVIN: It seems to me that concept is terribly important. Not only do they have to use a monkey, but do they have to use a particular kind of monkey? Some people only need a monkey.
DR. ROBINSON: I would also like to say that part of an experimental design must go through their IACUC. That is the very first thing IACUC members look at—whether a monkey is really required to do an experiment. Then, given the availability of other potential nonhuman primates at that particular center, if they can direct an investigator to use those other nonhuman primates species, they do so.
DR. BELOTTO (Albino Belotto, Pan American Health Organization): First, I would like to add that at the Peruvian Primatology Project, we have worked together with the Pan American Health Organization and the Bolivian government for the last 25 years. We consider it a very successful project. In the beginning, we were very fortunate to have strategic support from NIH.
We have had the transfer of animals that Dr. Montoya mentioned, through our very successful association with NIH and Dr. Taylor. We, of course, would like to continue this work. We follow the legal process of the Peruvian government. Now that I hear about NIH providing support not only for research itself but also for breeding and primate development, I would like to know how to obtain financial support from Peru as well as from NIH.
DR. ROBINSON: Let me understand the question correctly. You are asking how a foreign country applies for NIH funding. Unfortunately, NCRR, which is the only institute I can speak for in this regard, does not directly support primate facilities through a grant mechanism like that. However, if there is an affiliation with a US institution (e.g., Indonesia with the Washington Primate Center), we provide support through the Washington Primate Center grant that supports that breeding facility in Indonesia. That is one example.
PARTICIPANT: I have one question and one comment. Dr. Beattie, I saw the data you mentioned presented at the Association of Primate Veterinarians in the context of lymphocyte subpopulations between different geographic origin cynomolgus. I would like to know whether any of that information has been published because I think it is critical in understanding the variability of populations and giving us better insight, not regarding whether one population is preferable over another as much as to characterize the populations we are working with and understanding the relevance to particular experimental designs. Could you comment on that?
DR. BEATTIE (Greg Beattie, Sierra Biomedical): The data have been presented at various meetings including the Society of Toxicological Pathologists as well as the Society of Toxicology meeting just this past year. They have not been formally published as a journal article yet; however, we anticipate publishing it soon. People such as yourself, and even people internally, ask regularly. We are using the data currently to select the species in a geographic region for which we will continue a program.
We are seeing that clients who have started a program have different responses based on their studies up to a certain point. We may not know exactly where the animals came from, but we end up with a 3-month study with different responses that we cannot interpret.
PARTICIPANT (CONTINUED): The University of California at Davis was originally the national center for primate biology, and now it has gone “full circle” and is the national/regional center for primates. I was struck by the original opening comments about availability of opportunities for research on the basic biology of species and that there will be more focus on drug development, which particularly affects the primate centers program. When they revised the base grant format, they elimi
nated the RO1-type research, but they introduced the mechanism of resource research projects. We took advantage of that revision at Davis, looking at basic biological aspects. One project we are considering is biobehavioral characterization of very young animals. I think when you go back to that basic concept, looking at the biology of these species and understanding more about population variability, you go back to NCRR and the primate center’s program, which is a real core value that has again been emphasized in the primate center’s program.
DR. STEWART (V. Ann Stewart, Walter Reed Army Medical Center): I would like to point out that one of the statistics that was relatively hidden in that USDA figure is that there are two kinds of nonhuman primate protocol. There are protocols that use primates, and there are protocols that use primates up. Certainly, there are good scientific justifications for continuing with the species once you have started with it in a program. One of the reasons for the current rhesus shortage is the fact that the share of the research became a one-way trip for a lot of these animals. Clearly many of the toxicology studies are also a one-way trip for a lot of these animals. So I am wondering whether it is perhaps time to develop some sort of policy about categorizing or prioritizing research needs based on whether they are protocols that use rhesus or use rhesus up—whether we can try to create some sort of program for getting these animals into a couple of shoot and bleed types of studies and then have them go to SHF or toxicology studies.
PARTICIPANT: I would like to comment that at the primate centers, this practice is in place. Although I do not like using the term, animals are “recycled,” if you will. In the chimpanzee program, for example, the National Institute of Allergy and Infectious Diseases program, they begin with young chimpanzees and study respiratory virus. Then the study progresses to other types of experimentation, and then it may end up with, for example, hepatitis. I would like to return to what Dr. Hearn said this morning about the development of the technique to recover embryos from rhesus macaque, which enables those animals to continue to be reproductively active. Although there are old methods of doing multiple surgeries on an animal to recover embryos like that, there are limitations on the things that can be done. So that is another example.
HANS-ERIK CARLSSON (Hans-Erik Carlsson, Uppsala University): I would like to hear the panel’s comment on the number of animals used. We are currently conducting a survey and looking at all of the published studies on primates used during 2001. We have found that approximately 3500 studies have been done. The number of animals used in the United States appears to be around 17,000, which agrees with Dr. Robinson’s presentation, except for the USDA’s figures, which totaled about 60,000 animals in the United States, I believe. Do you have a comment on that?
DR. BEATTIE: The USDA’s number was about 56,000, as I remember, for 2000, and yours was much less than that. However, I was dealing only with NIH grantees. The USDA covers the pharmaceutical companies. They are required to report how many nonhuman primates they use as well. Whereas the survey I was dealing with was just slightly over 1000 NIH grantees, actually only 641 of them reported back.
HANS-ERIK CARLSSON: So that means that a large number of animals used in studies are not published?
DR. ROBINSON: Not necessarily. They are reported to the USDA, but that does not mean that they are reported to the NIH, because the NIH does not support all of those studies. In other words, of the large number of studies done, only a fraction is reported through the NIH mechanism because the people hold grants.
DR. BEATTIE: It is my understanding that the pharmaceutical companies are required to report the number of animals they use through the USDA, but the USDA does not require a breakdown other than to identify them as nonhuman primates.
DR. BAUDOIN (Mario Baudoin, Ministry of Sustainable Development and Planning): Before I announce the appointment of Dr. Ervin as my marketing director (laughter), I have a question for Dr. Hunsmann from the German Primate Center. You mentioned the total number of primates imported in the European Union, and you should add to that the 800 green monkeys we have sent each year to those countries for at least the last 10 years. The important thing is that you may have considered only the macaques. Alternatively, if you did ask for other species, I understand very well why you did not get the answer because most private companies, pharmaceutical companies, are very reluctant to report how many monkeys they use when they are producing vaccines. This reluctance is simply to protect themselves or at least keep proprietary information or whatever.
I also would like to ask why in Iquitos the breeding efficiency is only 5% with saimiri, whereas there is much greater success with marmosets.
DR. ROBINSON: There were only five animals—a very small number.