PART TWO IN BRIEF: STRATEGIES FOR CONTROLLING POWER PLANT EMISSIONS
RELATION OF EMISSIONS TO AMBIENT AIR QUALITY AND CHEMISTRY OF PRECIPITATION
Man-made emissions of sulfur oxides in the United States have been increasing at about 4 percent per year and amounted to about 34 million tons in 1970 (see Table 6–2). The most important sources are electric power plants (57 percent), industrial processes (16 percent), and other stationary sources (22 percent) (see Table 6–2). The greatest density of emissions is in the northeastern states (east of the Mississippi and north of Alabama-South Carolina (see Figures 6–2; 13–10). There are also substantial emissions (3–4 million tons per year) in parts of southeast Canada adjacent to the U.S. (see Table 7–2). At least in this region, man-made emissions greatly exceed natural emissions (see Table 7–2).
Most emissions are in the form of sulfur dioxide. After emission, sulfur dioxide mixes with the ambient air by diffusing both vertically and horizontally, and is transported (generally eastwards or northeastwards) by the wind. Some of the sulfur dioxide is oxidized to form sulfates, which may in turn form aerosols and travel long distances with the wind (see Chapter 6). The estimated rate of oxidation of sulfur dioxide to sulfates in the atmosphere varies considerably, from as low as 0.1 percent per hour to as high as 30 percent or more per hour, depending on local conditions such as the humidity and the relative concentrations of other air pollutants. This rate of conversion is typically more rapid in urban air than in rural air (see Chapters 6 and 7).
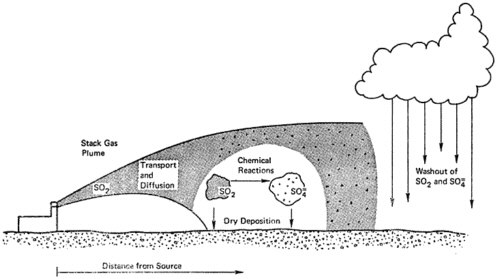
The principal means of removal of sulfur oxides from the atmosphere include absorption of gaseous sulfur dioxide by the ground or by vegetation, and deposition of sulfates in rain and snow (see Chapter 7). Surveys conducted in the northeastern United States suggest that roughly 33 percent of the sulfur oxides are eventually returned to earth as sulfates in precipitation (see Table 7–2). Figure 6–1 pictures the way sulfur oxides are transported after emission, transformed into sulfates, and ultimately returned to ground.
Because sulfur dioxide is absorbed fairly rapidly by the ground, emissions from stacks are probably more important than low level emissions as a source of sulfate aerosols downwind (see Chapters 6, 7). For the same reason, ambient concentrations of sulfur dioxide measured at ground level are determined primarily by sources nearby and a short distance upwind; in contrast, ambient concentrations of sulfates are determined by sources further upwind (see Chapter 6). Accordingly, ambient concentrations of sulfur dioxide are generally greater than those of sulfates at urban stations, but are lower at some rural stations (see Chapter 6). Measurements of suspended sulfate aerosols (see Figure 6–4) and of sulfates in precipitation indicate that high levels of sulfate are dispersed very widely throughout the northeastern United States and eastern Canada (see Figure 7–1). The pattern of deposition suggests transport over distances of several hundred km downwind from the principal source areas (see Chapter 7, and Figure 7–1).
The acidity of the suspended sulfate aerosols has not been measured directly, but can be determined indirectly by measuring the acidity of precipitation (see Chapter 7). Acid precipitation is a regional phenomenon in the northeastern United States and eastern Canada, and its distribution covers roughly the same area as experiences the highest sulfate levels (see Figures 7–2, 7–4). The fraction of sulfates falling out as acid sulfates in precipitation is in some areas as much as 80 percent; its regional average is about 24 percent (see Table 7–3). About three-quarters
of the acidity of precipitation is in the form of sulfuric acid and is attributable to sulfur oxide emissions; most of the remainder consists of nitric acid and is attributable to nitrogen oxide emissions (see Chapter 7, and Table 7–3).
Between 1960 and 1970 total emissions of sulfur oxides in the United States increased by about 45 percent, primarily due to a near-doubling in emissions from electric power plants (see Table 6–2). However, as a result of limitations imposed on the amount of sulfur permitted in fuels, sulfur oxide emissions in urban areas were reduced substantially during that decade (while those in non-urban areas increased disproportionately: see Table 6–2). In consequence ambient concentrations of sulfur dioxide in urban areas decreased significantly. However, ambient concentrations of suspended sulfates in urban areas remained approximately constant throughout the decade and there is some evidence that ambient sulfate levels in non-urban areas have increased (see Chapter 6). This probably reflects the long-range dispersal of air-borne sulfates, and indicates that ambient sulfate concentrations are determined primarily by total regional emissions. The acidity of precipitation has increased more rapidly than total emissions, perhaps reflecting a depletion of neutralizing materials in the air (see Table 7–3). The area affected by acid precipitation has also expanded to include most of eastern North America (see Figure 7–1).
In the absence of emission controls, emissions of sulfur oxides from power plants are projected to double again in the decade 1970–80, and a small increase in SOx emissions from other sources is considered likely (see Chapter 6). Extrapolating from past trends and considering the relative importance of urban and non-urban sources, this growth in SOx emissions is projected to cause only a small increase (0 to 20 percent) in average urban sulfur dioxide concentrations, but a larger increase (18 to 42 percent) in average urban sulfate concentrations (see Chapter 6). The total acidity of precipitation is expected to increase by much larger factors (perhaps as much as threefold) and its distribution may extend over a wider
area than previously was the case (see Table 7–4). Part of the increase in acidity of precipitation would be attributable to nitric acid and would not be averted by sulfur oxide emission controls (see Table 7–4, Chapter 7).
A highly simplified model is presented in Chapter 13 to predict the likely impact of a single source of sulfur dioxide on rural and urban air quality downwind of it. Applying reasonable average values for rates of diffusion, absorption and oxidation of sulfur dioxide, it is demonstrated that a single power plant of 600 MW burning 3 percent sulfur coal could cause an increase of the order of 0.15 ug/m3 in the annual average level of suspended sulfates in an urban area approximately five hundred km (300 miles) downwind. (The range of uncertainty in this calcuation is wide: 0.03 to 0.3 ugm/m3.) The figure of 0.15 ugm/m3 is consistent with the analysis of the regional distribution of emissions and ambient air quality summarized in Chapter 6. The impact of a power plant on sulfate air quality far downwind is quite strongly dependent on the rate of oxidation of sulfur dioxide to sulfate, and hence, among other factors, on the amounts and distributions of other pollutants in the atmosphere.
EFFICIENT PRICING AND CONSERVATION
One important means of achieving the broad goals of our national energy policy is to promote conservation. Limiting the consumption of electricity or reducing its rate of growth would reduce emissions of sulfur oxides and other pollutants and thus the damages to health and the environment from the utilization of coal to produce electric power.
One essential device in limiting wasteful consumption of energy is to see to it that its price is equated to its marginal social cost of production. In fact, our pricing of electricity falls far short of this requirement in many ways.
Electric utilities are in most jurisdictions regulated on an original, or historic, cost
basis. This means that their capital costs are measured by, and as a return on, the cost historically incurred. But the only measure of marginal cost that has any economic significance is current costs, or, as one sets rates for the future, the cost that will be incurred or saved during the period when those rates are in effect. In times of rapid inflation, those marginal costs tend, naturally, to rise relative to average company revenue requirements, when the latter are based heavily on historic costs. Basing all electricity rates on incremental costs in these circumstances would produce excessive revenues, by traditional regulatory standards. The requirements of economic efficiency could, however, be approximated by setting rates at incremental cost for those categories of demand that are particularly responsive to price. Alternatively, excess revenues could be rebated equally (or in some other manner not related to individual consumption) to all customers.
Second, and operating in the same direction as the use of average rather than marginal cost pricing, is the failure of electricity rates typically to reflect peak responsibility. The required level of investment in generating and transmitting capacity depends specifically on the level of demand at the times of system peak consumption. If the economically proper amount of capacity is to be constructed, therefore, it is that particular consumption, i.e., consumption at the time of peak utilization, that must be charged the full marginal costs at that time of making that capacity available. This could be done, for example, by installation of time-of-day meters. In contrast, consumption truly and inalterably off peak should not pay any capacity costs. It is clear, generally, that the failure of most electric utility pricing to reflect these peak responsibility principles, as well as to measure capacity costs in current rather than historical terms, gives rise to a greater demand for electricity and a consequent greater construction of plant than would otherwise occur.
While efficient pricing of energy is one very important means for reducing wasteful use
of electricity and, therefore, for reducing sulfur dioxide emissions, it is by no means the only device, nor is it necessarily sufficient. Improved effectiveness of fuel utilization typically requires capital outlays either by individuals or businesses. Capital shortages and high interest rates both inhibit investment in energy-conserving fixed assets. These considerations argue for government assistance to individuals and businesses in such forms as tax rebates or low-interest loans for energy-conserving investments. At the minimum, non-economic institutional barriers which make it difficult for many to obtain financing, even at market rates of interest, should be overcome.
MODIFICATION OF DEMAND FOR ELECTRIC POWER
Modifications of demand for electricity, and therefore of emissions of sulfur oxides, can be brought about by improvements in effectiveness of fuel utilization in end-uses and by shifting from oil and gas to coal as primary fuel for space heating.
Demand for fuels for electricity generation can be reduced by increasing end-use effectiveness in two general ways: (1) generation of electricity as byproduct of certain industrial processes; and (2) improvement of efficiency of electrical apparatus used in the residential, commercial, and industrial sectors.
Generation of Byproduct Electricity in Industry
Process steam raising and direct combustion heating account for 74 percent of total industrial fuel consumption. If all process steam were to be raised in combination with electricity generation as much as 1270 billion kw-hrs of electricity could be generated at a net fuel saving of about 6.2 quads or 258 million tons of coal per year in 1985.
Similarly, if waste heat from industrial heating processes were to be recovered by bottoming cycle electric generators, as much as 182 billion kw-hrs of electricity could be
generated at a net fuel saving of 1.82 quad or 76 million tons of coal per year in 1985.
Not all of the potential by-product electricity can be generated because some industrial plants are too small in scale or operate too few hours per year to justify the expense for the installation of the fuel saving equipment. The rate at which fuel-saving equipment will be installed will depend on fuel and electricity prices. If the price of electricity sold by a utility is determined by average capital costs of supplies rather than capital costs of new supplies, then an individual firm would likely prefer to purchase electricity rather than generate by-product electricity.
To illustrate the effect of pricing electricity based upon average rather than incremental costs, a comparison was made between on-site power generators and purchased electricity. This analysis showed that with present prices for electricity, the overall costs to an industrial user were about equal for purchased electricity vs. a bottoming cycle to generate by-product power from waste heat. However, the incremental power demand placed on electric utilities for purchased power results in the expenditure of almost 50 percent more of the nation’s capital than would be required if the bottoming cycle were installed. These comparisons are made on the assumption that the industrial demands in question fall no less than the average of all other demands on the system peak, and so would be incrementally responsible for a propertionate share of the costs of providing the requisite capacity.
Improved Efficiency of Equipment and Processes
Significant opportunities exist for fuel saving through improvements of effectiveness of electrical equipment used in various applications and through re-optimization of electricity consuming industrial processes. Several examples are included here to illustrate the potential for modifications of demand for electricity.
Air-conditioning and refrigeration equipment accounts for over 20 percent of all U.S. electricity and 5.4 percent of fuel consumed for all purposes. The performance (Btu cooling per watt hour of electricity) of such equipment can readily be improved by 30 percent using known heat-transfer technology. The potential fuel saving would be 1.8 quads, or 75 million tons of coal per year in 1985.
A comprehensive study of all electricity consuming processes in industry is needed in order to establish the potential fuel saving. The aluminum electrolysis process serves as an illustration of what might be obtained. By operating the Hall-Process electrolysis cells at lower current density, which is equivalent to increasing capital cost by 22 percent, the electricity required per ton of primary aluminum can be decreased by 16 percent, namely by 2,500 kw-hrs per ton. The potential fuel saving would be 0.25 quads, or 10 million tons of coal per year in 1985. Still further savings in aluminum production may be realized through changes in the process itself such as that being investigated by Alcoa.
Applying recently published FEA guidelines on lighting, it it possible to reduce demand by 133 billion kw-hr in 1985. The fuel saving by electric power plants would be 75 million tons of coal per year.
Shift of Space Heating Load from Oil and Gas to Coal as Primary Fuel
The use of electricity, and the overall consumption of fuel, could increase enormously if a large-scale shift from the direct use of oil and gas to electrical resistance for space and water heating were to take place. Little, if any, increase in overall fuel consumption, and a more moderate increase in electricity demand would occur if the shift were to electrically-powered heat pumps. The heat pump also appears to require a lower overall capital investment for the combined fuel supply and heating equipment than does electrical resistance heating; this would clearly be so if widespread resort to
electric heating results in the emergence of a winter peak, because the heat pump would, in that event, produce major savings in the required amount of generating capacity. The alternative of producing gas from coal to serve the heating market also appears to require substantially less capital and less total fuel consumption than electric resistance heating. Relative fuel requirements and capital investment needs, including equipment for both fuel supply and end-use, were estimated for each option to be as follows:
Comparison of Fuel and Capital Requirements for Alternatives to Electric Resistance Space Heating
|
|
Fuel Consumption |
Capital Requirements |
|
Electric Resistance |
1.0 |
1.0 |
|
Electric Heat Pump |
0.55 |
0.78 |
|
Coal Gasification |
0.78 |
0.57 |
Of course, other options are available for meeting the nation’s space-heating requirements, for example, using solar energy or new oil and gas resources, and each of these should also be assessed.
FLUE GAS DESULFURIZATION (FGD)
Introduction
Are flue gas desulfurization systems reliable and operable for scrubbing stack gas effluents from the combustion of high sulfur coal of the eastern United States?
We have considered this question in light of the definition of industrial-scale reliability set forth by the National Academy of Engineering’s panel in 1970—viz., (a) satisfactory operation on a 100-Mw or larger unit for more than 1 year and (b) availability
of adequate technical and economic data for confident projection of commercial designs for specific local and regional conditions.
Our point of view has been that an operation at most locations in the eastern United States would not be able to discharge salty water. That is, the operation must work in the closed loop mode.
Only lime and limestone scrubbers have yet operated successfully on coal at the commercial scale for extended periods of time, and we have considered the foregoing question in detail only for these systems.
Although there is room for improvement, advances in knowledge of the mechanical design of scrubbers and of the selection of materials for their constructions are sufficient to provide reasonable assurance that a failure in a large-scale test will probably not be the result of a design failure that causes the test scrubber to fall far short of the best for the scrubber’s type.
Accordingly, our analysis has concentrated upon the availability of chemical knowledge and the adequacy of performance comparisons among bench, pilot, and commercial scrubbers. Although an ideal technical base for scrubber design would include complete and detailed knowledge of the chemistry, we recognize that the base can fall somewhat short of this ideal if the performance comparisions at various sizes of equipment provide a good empirical knowledge to offset some ignorance in respect to scrubber chemistry. However, commercial experience at scrubber conditions that realistically represent those to be expected for a given design is a sine qua non. This is especially true for a design that is recognized to be difficult. We accept the NAE 1970 study’s criterion of substantially 100-Mw capacity as necessary to provide commercial experience.
Until just a little more than 1–1/2 years ago, it was believed that a lime or limestone scrubber in the closed loop mode inevitably operated with a liquor that was supersaturated in respect to gypsum, and that an operation free of troubles from plugging and scaling would depend upon keeping the degree of
supersaturation below a critical level beyond which scaling by deposits of gypsum become intolerable. It was recognized that the danger from scaling increased as the degree of oxidation of sulfur to the sulfate form became greater.
It has been learned recently that lime scrubbers can advantageously operate with a liquor that is unsaturated in respect to gypsum. The availability of this option depends, among other things, upon putting some limit upon the degree of oxidation of sulfur to sulfate.
Two lime scrubbers frequently cited as examples of outstanding commercial success are known to operate in the unsaturated mode. Performance comparisons among bench, pilot, and commercial scrubbers are available only for this mode. Accordingly, this mode must be considered the furthest along in its development and the nearest to readiness for wide application. We will treat this option first and in the greatest detail. We will follow with briefer treatments of lime scrubbing in the supersaturated mode, and of the limestone scrubbing in both supersaturated and unsaturated modes.
Lime Scrubbing in the Unsaturated Mode
The unsaturated mode of operation has been observed in the followin lime scrubbing systems:
-
Paddys Run Station of Louisville Gas & Electric. This is a 65-Mw coal-fired peaking unit. The longest operation has been 45 days, during which the unit followed typical load variations for a utility boiler. The unit uses about 80 percent excess air (an unusually high amount), and so the flue gas flow rate is roughly equivalent to 100-Mw; on this basis, the flue gas is equivalent to that from coal at 2 percent sulfur. The operation is closed loop.
-
Mitsui Miike industrial boiler in Japan. The flue gas flow is equivalent to about 160-MW electrical capacity. The coal is 2 percent sulfur. All scrubbing operations in Japan are open loop, including the Mitsui Miike unit. The operation is steady around the clock,
-
and the unit operated more than 2 years before its recent conversion to limestone scrubbing.
-
EPA’s pilot lime scrubber at TVA’s Shawnee Station. The flue gas flow is for about 10-Mw electrical capacity. The coal is 3.5 percent sulfur. The operation in the unsaturated mode lasted 17 days, and was shut down voluntarily. Heavy scale formed on the mist eliminators early in the run. The operation did not simulate load following.
-
TVA’s bench lime scrubber at TVA’s coal-fired Colbert Station. The flue gas flow is for about 1-MW electrical capacity. The coal is 3 percent sulfur. The operation in the unsaturated mode lasted three months. It did not simulate load following.
-
EPA’s bench lime scrubber at Research Triangle Park, handling flue gas equivalent to 0.1-MW. The fuel is low-sulfur oil or gas, and sulfur dioxide is added. Sometimes fly ash is added to the scrubbing liquor together with lime. An operation at a given set of conditions is typically for five days.
Scrubbers 3, 4, and 5 operated in the closed loop mode.
If we could regard the operation at Paddys Run as typical of conditions to be encountered by designers of lime scrubbers for wide distribution in the eastern United States, the performance comparisions among the above-named scrubbers might provide an adequate technical base for design. Unfortunately, Paddys Run is not typical. The coal at Paddys Run is unusually low in chlorine content, and the lime used is a by-product of acetylene manufacture from calcium carbide.
By an unfortunate coincidence, Mitsui Miike’s scrubber is unusual in just these same two respects.
Data obtained from operation of scrubber 5 has recently cast important new light upon the chemistry of lime scrubbing, especially in explaining some of the difficulties that scrubbing experiments have encountered. The data reveal that presence of chloride ion in the liquor makes operation in the unsaturated mode more difficult, while presence of magnesium ion makes such operation easier.
Although scrubbers 3 and 4 operated with liquors containing substantial quantities of both chloride and magnesium ions, we regard the absence of commercial-scale experience with a coal of moderate to high chlorine content to represent a serious deficiency in the technical base now available to the designer of a scrubber for medium to high sulfur coal. This judgment is reinforced by the fact that the degree to which sulfur is oxidized to the sulfate condition in the Paddys Run scrubber is remarkably low, between about 1.5 to 3 percent. Oxidation in the other scrubbers is higher, and sometimes markedly so. As noted earlier, operation in the unsaturated mode becomes more difficult the higher the degree of oxidation.
Experience can and should be obtained quickly for a commercial lime scrubber using liquor that contains chloride ion at a high level. For example, the Paddys Run scrubber could be operated with admixture of hydrogen chloride gas to the flue gas entering the scrubber. Any test of an operation with such a liquor should provide a realistic assessment of the problems of maintaining a strictly closed water loop.
Confidence in the technical base could be much enhanced by operating the Paddys Run scrubber with ordinary lime in place of the carbide lime that has been used in operations to date.
Confidence in the technical base could also be much enhanced if further experimentation at the bench or pilot scale can reveal what variables are important in promoting or minimizing oxidation of sulfur to sulfate in the scrubbing liquor. Quantitative relations connecting the variables to the oxidation level would be of great value. At present, we cannot be sure that we can write down a complete list of the important variables, and quantitative relationships are completely lacking.
We reiterate, however, that lime scrubbing in the unsaturated mode is furthest along in its development. A resolution of the question of its commercial viability can be acquired soon, through easily obtained commercial experience that will allow adequate performance comparisons
among bench, pilot, and commercial units. The probability that the question will be answered favorably is a matter for engineering judgment in light of the now available chemical knowledge and performance comparisons. Some of us judge the probability to be 90 percent, while one member judges it at 70 percent.
Lime Scrubbing in the Supersaturated Mode
A large scrubber (of a novel, horizontal design) has operated in the supersaturated mode at Mohave Station of Southern California Edison Co. The coal is of unusually low sulfur content, and the system is yet untried on medium or high sulfur coals, for which design is more difficult.
EPA’s 10-Mw lime scrubber at TVA’s Shawnee Station has usually operated in the supersaturated mode, and the operators regard its performance as successful, although there has been trouble at the mist eliminators.
No recent commercial experience is available for comparison with the 10-Mw results. Although operation of the Fulham Station lime scrubbers in England in the late 1930s was reportedly successful, information on this operation is not sufficient to provide a basis for modern scrubber design.
Some workers take the view that operation far into the supersaturated mode, at a high degree of oxidation, will be possible even in the closed loop mode if the scrubber is designed physically to operate without trouble from gypsum deposits. Such a mode of operation would require a new path of development that might be said to have only barely been begun.
Limestone Scrubbing in the Supersaturated Mode
The sucessful operation of a large limestone scrubber at the Cholla Station of Arizona Public Service contributes relatively little to the technical base for design of scrubbers for medium or high sulfur coals, because of the low sulfur in the coal burned at Cholla Station.
The operation is closed loop, but experiences water loss by evaporation from the sludge pond.
Large limestone scrubbers (of similar design) are operating at Commonwealth Edison’s Will County Station and at Kansas City Power & Light’s LaCygne Station. Both systems operate in the open loop mode. Availability of the Will County scrubber has recently been good, but the operation now blends Western low sulfur coal into high sulfur Illinois coal, to provide a fuel at an average sulfur level of about 1.5 percent. The LaCygne operation is on coal containing 5.5 percent sulfur, and is subject to fouling and plugging that is dealt with by cleaning out each of seven scrubbing modules about once every five nights.
EPA’s nominally 10-Mw pilot limestone scrubbing system (at TVA’s Shawnee Station) has operated in the closed loop, supersaturated mode. The ratio of liquor flow to gas flow is considerably higher than in the Will County LaCygne design. There is a higher content of solids in the liquor. A longer time is allowed for reaction of limestone with the spent liquor. The gas velocity in the scrubber is reduced, thereby derating the unit to about 7-Mw. These conditions are closely comparable to conditions reported to have been successful in operating limestone scrubbers in the closed loop mode at Fulham Station in England in the late 1930s.
No recent commercial experience is available for comparison with the 10-Mw results.
TVA’s 1-Mw bench limestone scrubber at TVA’s Colbert Station has operated successfully with a novel mist eliminator whose design prevents wash liquor (used to keep the mist eliminator clear) from entering the main flow of scrubbing liquor. Commercial experience for comparison with the 1-Mw results will be obtained at TVA’s Widows Creek Station.
Limestone scrubbers in Japan commonly operate with high degrees of oxidation of sulfur to sulfate and high supersaturation of the scrubbing liquor. In these units, the objective is to produce gypsum for wallboard manufacture. The operations are open loop. The Mitsui Miike unit cited earlier has recently converted to limestone. Several other coal-fired units have
recently begun to operate, or are about to operate, with limestone scrubbing systems.
As mentioned earlier in connection with lime scrubbing, some workers take the view that operation far into the supersaturated mode, at a high degree of oxidation, will be possible if the scrubber is designed to accomodate deposition of gypsum. The operation would be closed loop, unlike the systems in Japan. A new path of development would be required to provide the proposed mode of operation.
Limestone Scrubbing in the Unsaturated Mode
Limestone scrubbing in the unsaturated mode has been demonstrated in EPA’s tiny bench unit at Research Triangle Park. There is general agreement that operation in the unsaturated mode will be much more difficult for limestone scrubbing than for a lime system. This option could not be taken seriously until it has been demonstrated on a scale larger than the 0.1-Mw bench unit.
Availability of Lime and Limestone Scrubber Technology
Commercial lime scrubbers for an Eastern United States utility coal medium in sulfur (1 to 3 percent) and low in chlorine (less than 0.04 percent) can be ordered today with reasonable confidence.
No commercial experience is available for a lime scrubber on commonly occurring coal both medium or high in sulfur and higher than 0.04 percent in chlorine. Chlorine is known to interfere with the lime scrubber chemistry and to make operation more difficult. Experience can and should be obtained quickly for medium and high sulfur coals containing chlorine beyond 0.04 percent. The experience would resolve the issue of commercial availability of lime scrubbers for these coals. The probability that the issue will be resolved favorably is a matter for engineering judgment in light of data now available from 1.0 and 10 Mw lime scrubbers.
Some engineer members of the Committee felt the probability to be 90 percent, while one member judged it at 70 percent.
No other scrubbing technology is available for order today with confidence for Eastern United States coals medium or high in sulfur. Other scrubbing techniques await commercial demonstration on such coals.
Regenerative Flue Gas Desulfurization Processes
The Wellman-Lord and magnesium oxide processes, both of which recover a useful by-product, are now being installed in high sulfur coal plants. Successful operation of these plants would represent a major advance in FGD technology, in that a proven regenerable process would now be available. In addition, there is a non-regenerable process, sodium carbonate, operating on low sulfur western coal.
There is substantial successful FGD experience in Japan, but it is not directly applicable because the installations are not coal fired and operate in an open loop mode which permits discharge of untreated water to the environment. Within the U.S. there are several Wellman-Lord processes installed in the petroleum/petrochemical industry for sulfur recovery. These are operating successfully.
Cost of Flue Gas Desulfurization
The installed cost of a lime scrubbing FGD system ordered today for a new coal fired plant will be about $100/kw. The installation cost could be as low as $60/kw or as high as $130/kw. The estimated operating costs for lime FGD systems are 3 to 6 mills/kwh, including ponding of sludge, energy loss, and capacity derating. About one half this operating cost is capital charges. If expensive pond facilities (e.g., plastic lined) are required, as opposed to a clay lined pond, an additional charge of up to 0.7 mills/kwh would result. Chemical fixation, if used or needed, could raise the 3 to 6 mills/kwh by 0 to 1.0 mills/kwh. There is
considerable uncertainty in fixation costs, because of limited experience with the processes. Other FGD processes, limestone, Wellman Lord, etc. will require about the same capital cost as lime. If the cost of sludge disposal becomes large enough, say about 2 mills/kwh, a regenerable process with by-product credit would have a potentially significant economic advantage.
Residuals Produced from Flue Gas Desulfurization
Particulate concentrations leaving a FGD system are typically .01 to .02 grains/SCF which is adequate to meet air quality criteria. Some very limited data suggests that about 40 percent of the particulates leaving a scrubber are sulfates (calcium, magnesium) and not fly ash.
A typical 1000 MW power plant will generate about 1,185,000 tons/year of fly ash and sludge for disposal (wet basis). This requires, over the lifetime of the plant, about 377 acres over the life of the plant for disposal compared with 108 acres for disposal of fly ash only. The 377 acres is of the same order of magnitude as that required for the power generation facilities. Chemical fixation is now being developed to produce a stabilized material with low permeability and leachability. Tests on commercial scale modules are now underway in several locations to further evaluate fixation processes and potential secondary pollution problems. Results are reported to be encouraging.
Capability of Vendors and Utility Companies
There are about 3 to 5 vendors who have substantial commercial experience in FGD installations and another 10 or so who have capabilities in this area. Estimates have been made that each vendor can design and install 3 to 5 FGD systems per year. The EPA has estimated that 90,000 MW of FGD systems are needed by 1980 to control sulfur dioxide emissions from coal fired plants. There are now
17 systems under construction and 63 planned—a total of 37,000 MW of generating capacity. It will be difficult to meet the target of 90,000 MW by 1980.
There is also a potential scarcity of engineering manpower to design and construct the FGD systems. The utility companies for the most part do not have on their plant staffs skilled process engineers who can provide the technical service so necessary to make an FGD system, once installed, operate successfully.
CONTROL OF AMBIENT SULFUR DIOXIDE CONCENTRATIONS WITH TALL STACKS AND/OR INTERMITTENT CONTROL SYSTEMS
Temporal emission controls for reducing ambient sulfur dioxide levels, otherwise known as intermittent control systems (ICS), have been recognized as a viable air pollution control technique in this country only for certain limited situations, and have been the focus of a lively debate between various regulatory agencies and some parts of the electric utility industry. The term “ICS” refers to a system of control whereby the rate of emissions from a pollutant source is curtailed when meteorological conditions conducive to ground-level pollutant concentrations in excess of the ambient standard exist or are anticipated to occur. For power plants, two intermittent control strategies are potentially available: (1) fuel switching, i.e., burning a temporary supply of low sulfur fuel; or (b) load switching, i.e., switching a portion of the electrical load to an interconnected generating station with available capacity in excess of demand.
Tall stacks are inherently an integral part of ICS programs, since increased stack height can yield a decreased need for intermittent emission reductions. For example, at its Kingston Steam Plant, TVA suggests that anticipated average yearly requirements for ICS measures will drop from 55 days per year (with a 7-hour average duration) for the plant’s current stack configuration (four 250-foot stacks and
five 300-foot stacks) to zero days per year for two 1000-foot stacks.
Legal Background
The application of tall stack/ICS measures for dispersal of SOx pollutants can be effective in reducing ground-level sulfur dioxide concentrations in the vicinity of power plants burning high sulfur coals and oil. However, on the basis of long-term averages, such measures provide generally negligible reductions in the amount of pollutants emitted. It is principally for this reason that the Environmental Protection Agency considers constant emission reduction techniques far superior to dispersion strategies. EPA claims the dispersion concept is not compatible with the Clean Air Act requirement that constant emission limitations be enforced whenever possible. In the most significant legal decision to date on this subject. Natural Resources Defense Council v. EPA, the 5th Circuit Court of Appeals upheld this contention, ruling that there is an express Congressional mandate in the 1970 Clean Air Act amendments that emission reduction is the preferred method of meeting ambient pollutant standards. A case addressing this same issue (Tennessee Valley Authority v. EPA) is currently in the 6th Circuit Court of Appeals. More recent legislation, the Energy Supply and Environmental Coordination Act of 1974 (ESECA), provides additional insight into congressional intent regarding implementation of tall stack-ICS technology. According to language contained in ESECA, the application of ICS technology is permitted only under certain circumstances; for example, these systems can sometimes be used to comply with interim control requirements when extensions of time are granted to utilities to meet emission standards.
Although recent court decisions, EPA regulations, and ESECA suggest otherwise, this evaluation of tall stack-ICS technology was undertaken under the assumption that this technique could become a legally permissible
method of controlling sulfur dioxide concentrations. This is because:
-
ICS measures could provide an alternate means of attaining and maintaining sulfur dioxide ambient air quality standards, independent of reliance on flue gas desulfurization (FGD) technology or use of low sulfur fuels.
-
Implementation of these systems could result in attainment of existing ambient standards in a shorter period of time than would otherwise be possible.
-
These measures could be important interim techniques, given limitations on availability of FGD systems and low sulfur fuels over the next few years, given the difficulty in retrofitting some facilities with FGD systems, and given distribution and allocation problems associated with low sulfur fuels.
Assessment of Intermittent Control Systems (ICS)
Assessments of four aspects of ICS technology are offered, including an analysis of system performance, potential implementation, cost, and secondary environmental impacts.
Performance
A number of different types of potential ICS applications exist, with individual situations requiring (a) various degrees of difficulty in air quality modeling and forecasting, (b) different problems in monitoring actual air quality, and (c) assorted frequencies and severities of required emission reductions. Operating tall stack-ICS systems have generally proven effective in reducing the number and extent of excess concentrations of sulfur dioxide in the vicinity of single SOx sources. However, for operating systems for which data are currently available, only the TVA Paradise Steam Plant ICS installation appears to be meeting all air quality objectives for the time period considered. This is due to the fact that the Paradise plant, according to indicators which measure the degree of difficulty associated with
a given application of ICS technology, is located where relatively few problems would be anticipated in implementing ICS control.
Constraints on ICS Control Implementation
Availability of control by tall stack-ICS methods is a function of stack and emission parameters, as well as local meteorology and terrain. For nine TVA power plants under different sets of conditions, reduced emissions are called for between 0.1 to 4.4 percent of the time, on an hours per year basis. These decreases in emissions must be supplied either by load shifting or fuel shifting; a number of constraints apply concerning each of these techniques which limit the potential for emission reductions by these methods at various plants.
Costs
Costs for implementing the tall stack-ICS approach are significantly less than the costs involved in installing FGD systems, both in terms of capital and annual cost requirements. Based on TVA operating experience, with an expanded cost range to allow for the different conditions that may be confronted, costs for ICS control can be expected to be as follows:
|
Capital costs |
=$4−10/kw |
|
Operating costs, including annualized capital charges |
=0.15−0.4 mills/kwh |
Agency costs for monitoring and enforcement of ICS systems represent a secondary cost of control, not included in the estimates above.
Secondary Environmental Impact
Much of the controversy surrounding implementation of the tall stack-ICS approach is associated with the impact of this technology on acid-sulfate particulate matter formation, and
effects of these sulfates on health, welfare, and aesthetics. These matters are discussed elsewhere in this report. It is important to note here that Application of tall stack-ICS technology does not significantly reduce total emissions of sulfur oxides; hence, this strategy does not decrease ambient sulfate concentrations, so ambient sulfate concentrations will not be reduced.
Enforcement
An important issue regarding implementation of ICS technology concerns the legal enforceability of these systems. Recently proposed EPA regulations for ICS control place particular emphasis on enforcement of violations of emission limitations included within an approved operational manual for each system, as well as enforcement based on violations of ambient sulfur dioxide standards. Enforcement based on this dual concept, which was not done previously, should be more effective than enforcement based on violations of ambient standards alone. Many elements of the enforcement procedure can be established prior to approval of the ICS approach by the control agency.
Public Policy Toward Tall Stack-ICS Control
A potential compromise position regarding public policy toward tall stack-ICS technology is as follows: the technology should not yet be accepted and permanent control technique because of the probability of substantial potential risks due to increased sulfur dioxide atmospheric loadings and hence of sulfates downwind. At the same time, the technology could be implemented as an interim control measure in carefully defined situations because it permits ambient standards to be met while continuous controls are implemented, and because it would help reduce the current clean fuels deficit.
OTHER TECHNIQUES FOR REDUCTION OF SULFUR IN THE ATMOSPHERE
The options immediately available to the utility industry for greatly reducing sulfur emissions to the atmosphere are severely limited. They are restricted to stack scrubbing and shifting to low sulfur fuels. A number of advanced technologies that will bear significantly on the problem are now under investigation, but they will not have reached the stage of commercial deployment until the early to mid 1980s. Several elements of our national energy position will tend to increase the amount of sulfur emitted to the atmosphere.
Existing and projected shortages of natural gas will reduce the amount of clean fuel (low in sulfur, low in particulates) available for electric utility use. Domestic petroleum production has not been able to meet domestic demand for a number of years and new national policies are aimed at further reducing the imports that have made up the deficit. As a result the electric utility industry will have even less petroleum to use in the future than it has in the past. These factors will tend to increase total sulfur emissions from power plants.
Nuclear capacity, which had been projected to rise sharply between now and 1985, will, as a result of recent decisions delaying its installation made by utility companies, fall far short of early projections. Estimates by the Atomic Energy Commission in 1972 placed 1985 nuclear capacity at approximately 250,000 Mwe. It now appears likely that actual capacity in that year will not exceed 120,000 Mwe. If the growth in total electrical demand does not decrease proportionately, this will throw an increased burden on coal to the degree that the decrease in nuclear capacity exceeds the decrease in electrical demand from earlier forecasts. Much of the delayed capacity is in the eastern part of the United States where low sulfur fuels are not readily available.
A range of actions are available to state and federal governments that could result in reversing the downward trend in expected
additions to nuclear capacity and recapturing a substantial portion of the capacity that is now being delayed. This could result in a reduction in coal consumption of 75 million tons in 1980 and very substantial additional tonnage by 1985. If these actions are not taken, sulfur emissions may be expected to increase.
Eastern low-sulfur coal reserves are large but much of the reserves are held for metallurgical use, and are not available to the electric industry. Existing production capacity for these low-sulfur coals is inadequate and is likely to remain so. By shifting available low-sulfur coal to plants not meeting primary standards from plants which could burn higher sulfur coal and still meet the primary standards, some reduction in violations of existing ambient air quality standards could be achieved. It is estimated that a shift of about 36 million tons could be made to reduce the tonnage in violation by about 15 percent. Low sulfur western coals will be usable in new coal fired plants designed to burn them if transportation capacity is increased but their use in retrofitted plants will be limited.
A number of technologies may be available in future years that could favorably impact on sulfur emissions.
A low sulfur-low ash product can be produced from coal; commercial (solvent refining) plants to do so may be in operation by 1982–83. The products made from a coal with costs of 80 cents per million BTU are estimated to cost in the range of $1.75 to $2.00 per million BTU. Alternatively, a low BTU-low sulfur gas can be made from coal (80 cents per million BTU) with costs in approximately the same range. Commercial plants making gas may be operational in 1981 or 1982.
High sulfur coals may be burned directly in an environmentally acceptable way in a fluidized bed operated either at atmospheric or elevated pressure. A full scale unit designed for atmospheric pressure could be in operation by 1980 or 1981 and a pressurized unit approximately two years later. If fluidized bed combustion can be successfully developed, it should produce a clean fuel for boiler use that
is less costly than either a low sulfur-low ash coal or low BTU-low sulfur gas produced from coal.
Proven conventional coal cleaning methods can reduce the sulfur content of coal significantly. However, unless the original sulfur content is already low enough to nearly meet the sulfur oxide emission standards, conventional coal cleaning methods will not bring most coals into compliance. Advanced coal cleaning methods may be able to increase the amount of sulfur and ash removed but all of the processes are in their early stages of development and many may be high cost for the extra sulfur removal that is accomplished.
Methods of generating electricity at increased efficiencies (in order to reduce the pollution load per unit of electricity generated) are not expected to come into widespread use until 1985 or later. As important as it is to continue R&D on these advanced power cycles, they offer no solution for reducing sulfur oxide or particulate emissions in the period between 1975 and 1985.
For many uses, particularly in new installations, either electricity or a pipeline gas made from coal could be used to supply energy requirements. The choice of which route to select should be based on supplying energy at the lowest marginal cost to the user.
Pipeline gas (of heat content of approximately 1000BTU/cu ft) from coal can be produced at the gasification plant by the early 1980s for approximately $2.50 to $3.00 per million BTU. Electricity produced at a new coal-fired base load plant (equipped with air and water pollution controls) would cost about 2.6 cents per kwh or $8.10 per million BTU. With resistance heating (100 percent conversion of electricity to useful heat) electricity costs would be approximately twice that of gas from coal (excluding transmission and distribution costs for both fuels). Under the same assumption, with a heat pump with a seasonal performance factor of 2, electricity would cost 30 to 60 percent more than gas.
Total costs to the consumer for electricity (after adjusting transmission and distribution
costs upward by 50 percent over the 1968 costs) using resistance heating (at 100 percent efficiency) would be about $12.80 per million BTU, or from 100 percent to 120 percent more than gas made from coal. If a heat pump were used (with a seasonal performance factor of 2) the cost would be about $6.40 per million BTU, or about the same, for the same usable BTUs, as gas made from coal.
Solar heating of domestic hot water is technologically feasible and probably economic in many areas of the country now. If institutional barriers to its widespread deployment are overcome, it would reduce the use of coal for generation of electricity and release natural gas for other purposes and thus make a measurable contribution to air quality.
ANALYSIS OF ALTERNATIVE STRATEGIES
The basic question addressed in analyzing alternatives is whether the benefits from sulfur oxide removal by such means as flue gas desulfurization or switching to low-sulfur coal justify the additional increment of cost in electricity generation. This question is addressed by examining representative values of the economic and emissions-related characteristics for different generic classes of power plants. The increase in cost of electricity is compared with the social benefit acheived through reduction in sulfur oxide emissions to find the value at which a switch in abatement techniques is advisable.
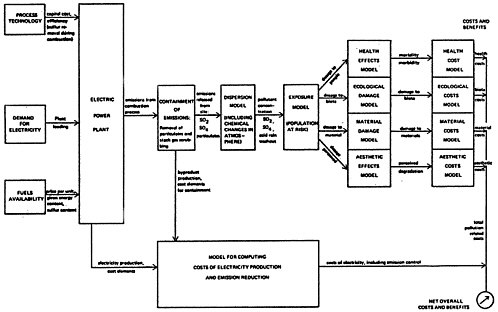
In this report a comprehensive cost-benefit formulation is proposed, illustrated by the diagram of Figure 13–1. The intention of this formulation is to provide a methodology in which the influence of the various factors on the decision is made readily apparent. This methodology is available for analyzing the decision among abatement strategies on a case by case basis in cost-benefit terms. The marginal increase in the cost of electricity is compared to the marginal benefit per pound of sulfur oxide emission reduction achieved, for each alternative strategy. This methodology can be
used to determine priorities for implementation of abatement technologies.
The accuracy of model studies depends on inclusion and accurate description of all important cause-and-effect relationships. The limited information presently available makes decisions among strategy alternatives difficult. The use of models is more to enhance understanding of the relative importances of the various factors that affect the decision than to determine with precision what is the optimum strategy. If changing a factor in the analysis within its range of uncertainty leads to a change in the preferred strategy alternative, then that uncertainty would clearly be worth something to resolve before a commitment to a particular strategy is taken. Likewise, it may be that despite uncertainties in many factors, one alternative appears sufficiently better than the others that a commitment now appears preferable to the costs incurred by deferring the decision. The purpose of the analysis is to enhance understanding of a complex problem, including the effects of the uncertainty upon the decision. While specific numbers are used to illustrate the calculations, it should be appreciated that many of the numbers and relationships in the model are uncertain, and subject to considerable change as additional information becomes available. The analysis should be taken as a method for organizing and trying to place in perspective the information presently available to serve as a basis for decision making on sulfur oxide emissions control.
The Need to Consider Decisions on a Case by Case Basis
For a specific power plant, differences in fuel availability and price, plant loading, plant age, and the economics of abatement strategies may differ considerably from the representative values used in the analysis. Hence the marginal cost of emissions reduction per pound of sulfur removed may differ appreciably from the values used in the
calculations of Chapter 13. The calculation of marginal costs of sulfur oxide abatement strategies should be based on the particular economic and technical factors for each individual power plant.
The consequences of emissions may vary considerably from one power plant location to another, and these differences should also be taken into account in the decision among alternative strategies. The relation between emissions of sulfur oxides and ambient levels of sulfur dioxide and sulfates, and the potential for damage to human health, vegetation and other ecological systems, material property, and aesthetic values will in general differ as a result of regional and local factors. As a result, the value of reducing emissions should be assessed separately for different power plants. At some locations the value per pound of emissions reduction may be judged much higher than at other locations.
Another reason for examining the emissions control decision on a regional and local basis is that limited resources are available to implement quickly the widespread use of low sulfur coal or stack gas scrubbing. Scrubbing equipment should be installed first in those situations where the benefits of emissions abatement exceed the costs by the greatest amount, and thereafter should be installed on other plants where the benefits are judged to exceed the costs, as the equipment becomes available.
Assessing the Value of Sulfur Oxide Emissions Reduction
The assessment of the deleterious consequences of sulfur oxide emissions to human health, vegetation and living systems, material property and aesthetic values is difficult. Decisions on emissions control strategy require a trade-off between eliminating emissions and increasing the cost of producing electricity. It is better to have explicit judgments stated for these trade-offs so that emissions control decisions can be made on a consistent basis that
takes into account variations in local and regional situations, and so that the affected parties (utilities, consumers of electricity, those impacted by the consequence of the emissions) are aware of the basis on which emissions control priorities are being set. Hence, it is highly advisable to carry out an assessment of pollution consequences in monetary terms, that is, per pound of sulfur oxides emitted. These assessments should reflect the price that society is willing to pay in increased electricity costs to reduce sulfur emissions.
For the analysis described in Chapter 13 three generic cases were used to illustrate how this methodology might be put into effect: a representative existing plant in a remote rural location (e.g., the Eastern Ohio-Western Pennsylvania-West Virginia area), a new plant to be constructed in the remote rural location, and an oil fired plant located in the vicinity of a major metropolitan area such as New York that might be converted back to coal. To the extent permitted by the limited time and resources allowed for the study, available literature and expert judgement were used to estimate human health, ecological, and materials damage, and aesthetic consequences of sulfur oxide ambient levels, and to assign monetary costs to these effects. To relate ambient levels to sulfur oxide emissions, a model for oxidation and dispersion was constructed. Together these steps allowed a pollution cost to be computed per pound of sulfur emitted from the power plant. Assessment of pollution consequences in terms of cost per pound of pollutant emitted permits the comparison of costs and removal efficiencies on an economic basis and helps to identify the most important factors that affect the choice among the alternative strategies.
The available information for carrying through the analysis is extremely limited, and the decisions among alternative strategies appear quite close. For the case of a plant in or near an urban area, some alternative for reducing total emissions (e.g., low sulfur eastern coal or flue gas desulfurization)
appears clearly preferable to burning of high sulfur coal.
The main result of the analysis is to characterize of the uncertainties. The most important areas of uncertainty appear to be the sulfur oxide emissions to ambient sulfate relationship and the health effects of ambient sulfate. A simple model was used for the relationship between sulfur oxide emissions from a power plant and the incremental increase in ambient sulfate in urban areas several hundred miles downwind. The chemistry of the oxidation process for transforming sulfur dioxide into sulfate was identified as the most crucial area of uncertainty in determining the emission to ambient relationship. Variations in oxidation rate over a reasonable range of values could lead to changes in the increase of ambient sulfate levels from given sulfur emissions over the range of a factor of ten. Health effects were modeled using a very crude dose-response relation drawn mainly from the limited data of the CHESS studies. Chronic respiratory disease and aggravation of heart-lung disease symptoms appear to be the most important health effects. The range of uncertainty for the incidence of each health effect for a given change in ambient sulfate was judged to be a factor of twenty.
These uncertainties were roughly characterized in terms of probability distributions, and an overall probability distribution calculated for the pollution cost per pound of sulfur emitted, for the representative plant in a remote rural location and the representative plant in an urban region. These distributions indicate considerable uncertainty; new information on the emissions to ambient relationship or on health effects could easily change the estimated pollution cost by at least a factor of two or more in either direction from the nominal values.
The Marginal Cost of Reducing Sulfur Oxide Emissions
Analysis of the representative cases yields the following results. With 0.9 percent sulfur eastern coal priced at $32/ton ($1.33 MMBTU), 33
percent above a high (3 percent) sulfur coal available for $24/ton ($1.00 MMBTU), a switch from the high sulfur to low sulfur coal is advisable if sulfur emissions abatement is worth at least 19 cents per pound of sulfur removed from the stack gases. Under the assumption for a new plant of $100/kw capital cost for a lime scrubber, 17 percent amortization, 0.8 mills/kwh operating costs (including 0.3 mills/kwh for sludge disposal), and a 6 percent energy loss and capacity derating, flue gas desulfurization using lime scrubbing adds 4.5 mills/kwh to the cost of producing electricity. Flue gas desulfurization becomes preferred to the premium priced low-sulfur coal if sulfur removal is worth at least 37 cents per pound. If low-sulfur coal is not available, flue gas desulfurization is preferred to burning high sulfur coal if the value of emissions reduction is at least 23 cents per pound.
For an existing plant the cross-over value of sulfur removal for switching from high-sulfur coal to low-sulfur coal remains the same, 19 cents per pound of sulfur removed. With the assumptions for a retrofit installation of $125/kw capital cost, 17 percent amortization for the scrubber, 1.1 mills/kwh operating costs (including 0.5 mills/kwh for sludge disposal), and 6 percent energy loss and capacity derating, flue gas desulfurization using lime scrubbing adds 6.1 mills/kwh to the cost of producing electricity. It becomes preferred to premium priced low-sulfur coal only when sulfur removal is worth at least 53 cents per pound. If low-sulfur coal is not available, flue gas desulfurization is preferred to coal preparation if the value of emissions reduction exceeds 26 cents per pound of sulfur removed. In urban or near-urban areas where disposal of sludge is difficult, costs of sludge disposal may be of the order of 0.9 mills/kwh. Lime scrubbing would then not be preferred to low-sulfur coal unless sulfur abatement was worth 59 cents per pound. If low-sulfur coal is not available, lime scrubbing is advisable when sulfur removal is worth at least 28 cents per pound.
For the representative cases considered, low-sulfur eastern coal or flue gas
desulfurization appears to be somewhat more cost effective per pound of sulfur removed than use of low-sulfur western coal and coal preparation. However, these latter alternatives may be cost effective in some situations. For new plants in the mideastern region low-sulfur western coal may be an attractive alternative, especially if its cost relative to eastern coal is reduced. Use of low-sulfur western coal in existing plants will generally not be economic because derating or expensive retrofitting will be required. Coal preparation appears barely competitive with flue gas desulfurization if low-sulfur coal is not available: sulfur removal can be accomplished by coal washing at a cost of about 25 cents per pound for an existing plant. Even with sludge disposal assumed to cost 0.5 mills/kwh, a lime scrubber retrofit will be preferred if sulfur removal is worth at least 26 cents per pound of sulfur. Lime scrubbing, moreover, reduces net emissions by about 90 percent compared to about 33 percent for coal washing. The costs and effectiveness of coal washing vary considerably depending on the type of coal. In some situations coal preparation may be an attractive strategy, especially as an interim measure.
The value of the cross-over points are sensitive to the emissions levels and costs of electricity given in Table 13–22. If different values are used the cross over points will change. The cross-over between low sulfur coal and flue gas desulfurization is particularly sensitive: A change of 1 mill per kilowatt-hour in the cost of flue gas desulfurization changes the cross over point by 23.2 cents for a new plant, and 19.8 cents for a retrofit installation. If the comparison is between high sulfur coal and flue gas desulfurization, the sensitivity is rather low: A change of 1 mill per kilowatt-hour in the cost of flue gas desulfurization causes a change in the cross-over point by 5.0 cents for a new plant and 4.3 cents for a retrofit installation. The cross-over point between high sulfur and low sulfur coal changes by 6.3 cents for a 1 mill increase in the cost for low sulfur coal for a new plant, and 5.5 cents for an existing plant. These are
both equivalent to 0.57 cents increase in the cross-over point for a 1 cent per million Btu change in the price differential of low sulfur coal over high sulfur coal.
Results of the Analysis for Representative Plants
For both the new and existing plant in a remote rural location the nominal pollution cost is computed to be about 20 cents per pound of sulfur emitted. The range of uncertainty is at least 8 to 40 cents per pound. The decision for both new and existing plants is very close between the alternatives of burning high-sulfur coal without abatement measures and switching to low-sulfur eastern coal. The nominal value of the emissions reduction is just above the marginal increase in cost of electricity incurred by switching to the low-sulfur coal. This marginal cost was assessed as 19 cents per pound of sulfur removed. For the representative urban case, an existing urban plant to be reconverted to coal, the nominal pollution cost is computed to be about 55 cents per pound of sulfur, with a range of uncertainty of at least 19 to 110 cents per pound. For this case the decision very close for an existing plant between eastern low sulfur coal and flue gas desulfurization; the alternative of buring high-sulfur coal appears poor by comparison. If low-sulfur eastern coal is not available, flue gas desulfurization appears to be the best decision. The marginal cost of lime scrubbing (compared to burning high sulfur coal) is 28 cents per pound of sulfur removed with a sludge disposal cost of 0.9 mills/kwh.
An assumption of considerable importance is the value associated with health effects. Willingness by the individuals potentially affected to pay to avoid sickness may not be an adequate standard to judge the value of morbidity caused by air pollution. It is quite possible that health values considerably higher than those used in this analysis ($250 per case of chronic respiratory disease, $20 per day of aggravated heart-lung disease symptoms) will be
judged appropriate as the basis for setting public policy on sulfur emissions control. If, for example, values four times those of our nominal assignments were used for the health effects, sensitivity analysis shows that the pollution cost is shifted up to a range where for both the representative rural and urban plants the best decision would be flue gas desulfurization.
The Value of Resolving Uncertainty
The decisions on control strategy depend on the adequacy of the information available at the time the decsion must be made. There is great value to improving our information about certain aspects of sulfur oxide pollution. The value of resolving uncertainty derives from the idea that better information might show, for example, that pollution costs are lower than was estimated, and costly abatement methods are not warranted. With some probability then, their extra cost might be saved. In particular, a better understanding of the health effects of sulfates and of the chemistry of the conversion of sulfur dioxide to atmospheric sulfates could have a significant effect on future decisions on control of sulfur oxides. A rough calculation of the value of resolving these uncertainties gives a value of about $2 million per year for the representative 600 MW plant in the remote rural location. If low-sulfur coal is not available, the value of resolving uncertainty drops to a little over $1 million per year. For the urban location, the value of resolving uncertainty on the sulfur oxide emission to ambient sulfate relationship and on the magnitude of the health effects is in the range of $1 million a year.
Extrapolating these values to the collection of eastern power plants that now or in the near future might burn high sulfur coal yields an estimate of the order of a quarter of a billion dollars per year. This is roughly 25 times the annual cost estimated by EPA for a research program to resolve these uncertainties.
Other areas of our knowledge on the effects of sulfate emissions should be greatly refined. Health effects not included in our analysis might prove far more serious than those identified so far: for example, sulfate might prove to have a causative role in chronic lung diseases such as emphysema, or in lung cancer. In addition to health effects, acid rain, decreases in visibility, materials damage, adverse effects on ecological systems, and possible climatic effects of sulfur emissions all deserve much more extensive investigation than has heretofore been undertaken.
Decisions to be made on sulfur oxide emission from power plants will involve tens of billions of dollars in electrical generation costs in the next decade and massive effects on human health and welfare. Greatly expanded efforts should be made to develop improved models and data for use on a case by case basis to improve decisionmaking on emission control strategy alternatives.
Emissions Charges as an Instrument of Policy
Considerations of strategy with respect to sulfur compounds emission pertain not only to the application of alternative technologies to the problem but to the selection of policy instruments and administrative practices. In this connection an emissions charge appears to be a well suited policy instrument for inducing efficient sulfur emissions control. The application of an emission charge on SOx, perhaps at the level of the estimated incremental cost of the pollution consequences from the average power plant, would provide a strong, immediate, and across the board incentive to undertake emissions controls activities. At the same time, in view of the still existing disagreements about the applicability of particular technologies, the special circumstances of particular plants, supply constraints, and other complexities of the situation, it would permit flexibility of response. This flexible response would be achieved in a decentralized manner without the
necessity of administrative agencies and the courts trying to decide every individual case in an adversary atmosphere. The latter approach invites delays and frequently arbitrary decisions, and establishes the incentive to hire lawyers rather than to proceed with emissions control. Emissions charges exert a persistent incentive to act whereas variances and delays in imposing requirements allow the emitter free use of environmental resources, with no incentive to act as long as these can be obtained. Moreover, a charges policy would have desirable efficiency characteristics. It would tend toward an application of controls first at those locations where costs per unit of SOx reduction are lowest. In the longer run it would provide a powerful spur for the development of more efficient technologies.
When first suggested the idea of emissions charges was greeted with some skepticism by many policy makers and environmentally concerned persons. For various reason industry was also opposed. In recent years this policy option has gained increasing acceptance among conservationists, environmentalists, policy makers here and abroad, and even industry as indicated by a recent Committee on Economic Development report. The sulfur emissions problem is a highly suitable one for the applications of emissions charges as a policy instrument.