CHAPTER 11
FLUE GAS DESULFURIZATION
1.0 INTRODUCTION
Are flue gas desulfurization (FGD) systems reliable and operable for scrubbing stack gas effluents from the combustion of high sulfur coal of the eastern United States?
It is important to consider this question both in light of the recent large increase in knowledge of FGD technologies and also with sober regard to the disappointments anad failures that have contributed to the new knowledge.
In 1970, a panel of the National Academy of Engineering (NRC 1970) advised that “…there is an urgent need for commercial demonstration of the more promising processes, to make reliable engineering and economic data available to engineers who are designing full-scale facilities to meet specific local and regional conditions. [Emphasis in orginal.] The panel’s definition of proven industrial-scale reliablility is satisfactory operation on a 100-Mw or larger unit for more than 1 year. Also, technical and economic data developed must be adequate for confident projection to full commercial scale. Pilot scale refers to investigation using flue gas in the capacity range of 10 to 25 Mw. Smaller sizes and studies
using synthetic gas mixtures are considered to be bench scale.”
Spokesmen who affirm that industrial-scale reliability is now available, as well as spokesmen who deny it, often quote the NAE panel’s requirement of 1 year of operation at the 100-Mw scale. They argue whether or not this has been acheived in a particular unit, and whether or not the experience in this unit is generally applicable.
Not much attention has been paid to the other important ingredient by the panel as necessary to insure industrial process availability: the requirement that technical and economic data must be available to permit design of full-scale units to meet specific local and regional conditions.
The NAE panel did its work at a time when the chemistry of sulfur oxides scrubbing appeared far simpler than it does today. The panel considered 16 stack gas control procedures. A reflection of the subsequent advance in knowledge is the fact that 10 of the 16 were not represented by presentations at a meeting that EPA held in Atlanta in early November of 1974 to review the status of control technology. The Atlanta meeting considered 13 processes, of which 7 were not on the list of the 1970 NAE panel.
It should also be remembered that there have been expensive large-scale development failures in sulfur oxide emission control (see Table 11–1). One process, limestone injection into a boiler followed by a scrubber, that EPA urged upon utilities as late as early 1972 (Walsh 1972), is no longer being offered for sale.
The record would stand as an indictment of the engineering profession were it not for the fact, now evidient, that the engineer was compelled to press forward into design and construction of scrubbing equipment of unprecedented size in absence of adequate chemical knowledge. Never before had the chemical engineer been asked to treat such a large flow of gas even for a chemistry that was well understood. It is not surprising, therefore, that many of the early disappointments involved failure of large
TABLE 11–1
Large-Scale Development Failures in Sulfur Oxides Emission Control
|
Dry limestone injection processes |
Estimated cost |
|
175-MW E.P.A. test at Shawnee Station of T.V.A. |
$4–5 million |
|
80-MW test at Dairyland Power Co-op’s Alma Station |
? |
|
Limestone injection followed by lime scrubbing |
|
|
125-MW test at Meramec Station of Union Electric |
$10–15 million |
|
125- and 400-MW units at Lawrence Station of Kansas Power & Light (the former is badly corroded, operating poorly, and will be replaced by a new scrubber of a different process; the latter will be converted to a limestone scrubbing unit) |
? |
|
100-MW unit at Kansas City Power & Light’s Hawthorn Station (has been converted to limestone scrubbing) |
? |
|
[This system is no longer being offered for sale.] |
|
|
Potassium solution scrubbing |
|
|
25-MW test at Baltimore Gas & Electric’s Crane Station |
$3.5 million |
|
Sulfoxyl process |
|
|
22-MW test at Commonwealth Edison’s State Line Station |
$8.5 million |
|
Molten carbonate process |
|
|
10-MW test (oil fired) at Consolidated Edison’s Arthur Kill Station |
$4 million |
|
Rheinluft process |
|
|
10- and 15-MW tests in Germany |
? |
|
Manganese oxide process (DAP-Mn process) |
|
|
110-MW test in Japan |
? |
scrubbing equipment to perform properly either because poor materials of construction had been chosen or because the design failed to provide adequate contacting of gas and scrubbing medium. For some FGD processes under development (notably limestone injection followed by a scrubber), it was only after these mechanical problems began to come under control that the chemical problems began to be appreciated.
Much progress can now be acknowledged. Table 11–2 provides a list of commercial scrubbing units believed to be successful. It should be noted that little time has been available to make the critical judgments needed for compiling Table 11–2, and there may well be important omissions.
A feature of Table 11–2 is that many of the successful scrubbers operate on oil-fired boilers. Another feature is that many operate with an “open water loop”, a manner of operation to be explained shortly.
Two broad changes in the outlook for scrubber technology have occurred since 1970 NAE panel’s study:
-
It is now appreciated that a process successful for oil firing cannot in general be transferred wholesale to coal firing without process refinement and a new commercial demonstration on a coal-fired boiler. The trend of thinking for wet scrubbing processes has been toward providing an electrostatic precipitator for removal of most of the fly ash ahead of the scrubber, instead of relying upon the scrubber for particulate control. This is because fly ash can interfere with both scrubber chemistry (see section 2.05) and mechanical operation. The 1970 NAE panel did not distinguish between scrubbing and the products of combustion of oil and coal, nor did most of the literature on scrubbing of that time. (It should be assumed that scrubbing flue gas from oil firing is necessarily always the easier task. See Appendix 11-A for a discussion of the difficulty of removing fume particles that are produced in an oil-fired boiler.)
-
It is also now better appreciated that new difficulties arise in the chemistry of a wet scrubbing process if it must be operated
TABLE 11–2
Partial List of Commercial Scrubbers Handling Boiler Flue Gas and Believed To Be Succsssful
Note: The criterion for listing a unit here has been a belief that it has been continuously available for commercial service for a period of at least several months. This is not necessarily a complete list.
|
Carbide Lime |
Fuel |
Equivalent Electricity Capacity |
Inlet SO2, ppm |
Water Loop |
|
Paddy’s Run (see 2.01) |
Coal |
100-MW |
1800–2000 |
Closed |
|
Mitsui Miike (see 2.02) |
Coal |
160-MW |
2000–2350 |
Open |
|
Lime |
||||
|
Mohave (see 2.12) |
Coal |
170-MW |
200 |
Closed* |
|
Kansai Electric, Amagasaki |
Oil |
120-MW |
1100 |
Open |
|
Kansai Electric, Kainan |
Oil |
120-MW |
500 |
Open |
|
Tohoku Electric, Hachinohe |
Oil |
115-MW |
820 |
Open |
|
Limestone |
||||
|
Cholla (see 3.10) |
Coal |
115-MW |
420 |
Closed* |
|
Will County (see 3.02) |
Coal |
84-MW |
1200** |
Open |
|
LaCygne (see 3.03) |
Coal |
700-MW |
4500*** |
Open |
|
Tokyo Electric, Hokosuka |
Oil |
130-MW |
250 |
Open |
|
Chugoku Electric, Mizuchima |
Oil |
104-MW |
300 |
Open |
|
Ishihara Chemical, Yokkaichi |
Oil |
77-MW |
2000 |
Open |
|
Sodium-Lime Double Alkali |
||||
|
Showa Denko, Chiba |
Oil |
150-MW |
1200–1500 |
Open |
|
Tohoku Electric, Shinsendai |
Oil |
150-MW |
600–800 |
Open |
|
*Mohave and Cholla experience little rainfall, and water losses due to evaporation from their sludge ponds are significant. **Earlier operation at higher inlet SO2 levels was plagued by formation of deposits. ***Cleanout of deposits is necessary about every 5 days, but the operator deems the installation to be successful. |
||||
|
Chiyoda (sulfuric acid-limestone double alkali) |
Fuel |
Equivalent Electricity Capacity |
Inlet SO2 ppm |
Water Loop |
|
Fuji Kosan Co., Kainan |
Oil |
50-MW |
? |
Open |
|
Daicel Co., Aboshi |
Oil |
30-MW |
1500 |
Open |
|
[Three units larger than 100-MW were scheduled to begin operating on oil-fired boil rs in Japan during 1974.] |
||||
|
Wellman-Lord (sodium salts) |
||||
|
Japan Synthetic Rubber, Chiba |
Oil |
70-MW |
1800 |
Open |
|
Chubu Electric, Nishi Nagoya |
Oil |
220-MW |
1500 |
Open |
|
Sumitomo Chiba Chemical Co., Chiba |
Oil |
120-MW |
1300 |
Open |
|
[Seven units larger than 100-MW were scheduled to begin operating on oil-fired boilders in Japan during 1974.] |
||||
-
subatantially without discharge of salty water to the environment. That is to say, it is more difficult to operate if all water leaving the scrubber must be returned to the scrubber except the water that is discharged along the wet solid waste or sludge. Operation without discharge of salty water is termed “closed loop”, and an operation that discards salty water is said to have an “open water loop”. Although operation with a closed water loop is not a recent concept, the 1970 NAE panel did not mention its special problems.
These developments reinforce the 1970 NAE panel’s judgment that an adequate technical data base must be available on which to rest a commercial design for each given specific situation.
An ideal base in support of a new commercial design for a sulfur oxides scrubbing process would include:
-
complete and detailed knowledge of the scrubber chemistry selected,
-
understanding of the mechanical and process performance of the scrubbing hardware selected as well as the proper materials of construction,
-
adequate correlations between performance of bench scale, pilot scale, and commercial scale scrubbers of the selected hardware and chemistry, and
-
adequate numbers of chemists who share and agree upon the relevant chemical knowledge, as well as adequate numbers of chemical engineers who understand the scrubbing hardware, in the employ of engineering firms that supply scrubbing systems.
As Table 11–2 shows, only lime and limestone scrubbers have yet operated successfully on coal at the commercial scale for extended periods of time. The question of scrubber reliability and operability must be addressed here in detail only for these alternatives. The status of other FGD processes is discussed briefly in Section 4.0.
Lime and limestone scrubber experience will be discribed in Sections 2.0 and 3.0 with
emphasis upon the question, is a design support basis available to allow engineering firms to build large-scale scrubbers for medium and high sulfur coal with confidence? It may be noted that “medium sulfur coal”, containing between 1 and 3 percent sulfur, accounted for 33 percent of all deliveries of coal in the United States in 1973. “High sulfur coal”, with more than 3 percent sulfur, constituted 29 percent. The remaining “low sulfur coal” delivered, containing less than 1 percent sulfur, were mostly taken by the steel industry.
The discussion in Sections 2.0 and 3.0 is written on the assumption that most locations in the eastern United States are such as to require operation of a scrubber in the closed loop mode. The discussion also emphasizes scrubbers of the vertical design characteristic of the great majority of scrubbing installations now undergoing commercial trials or under construction (however, see Sections 2.12, 2.15, and 3.12).
In reference to the foregoing ideal base, item (b) need be considered only briefly. Although there is room for improvement and especially need for wider dissemination of the available knowledge, there have been major advances during the past five years in knowledge of scrubber performance and of materials of construction. The chemical engineer judges a scrubber’s performance in terms of its efficiency in the contacting of gas and liquor. In a large scrubber, the engineer can expect to see some local variation in performance, since it is a practicable impossibility to effect an absolutely uniform distribution of gas and liquor moving through the scrubber, so that each small quantity of liquid would come into contact with exactly the same small quantity of gas. Often, one of the points to be settled by a large trial is a determination whether or not the efficiency of contacting that is afforded by the practicable scrubber is adequate for the inherent requirements of the chemistry of the process under trial. As a result of recent advances, a failure in a large-scale test will probably not result from a design failure that
causes the test scrubber to fall far short of the best for the scrubber’s type.
Item (d) of the ideal technical base will be treated in Section 8.0.
Sections 2.0 and 3.0 will concentrate upon (a), availability of chemical knowledge, and (c), the adequacy of performance comparisons among bench, pilot, and commerical scrubbers.
A technical data base falling somewhat below the ideal may be adequate, to the extent that a good empirical knowledge in respect to (c) may be used to offset some ignorance in respect to (a). However, (c) is a sine qua non, and the comparisons of commercial experience with bench and pilot units should cover the range of variables important for meeting the desired range of specific local and regional conditions.
The discussion to follow might seem to imply criticism of some industrial operators who may not have sufficiently appreciated the experimental nature of their scrubbers. The discussion might also seem to imply criticism of some designers who may not have appreciated problems that now seem obvious. Further, the discussion might sometimes seem to imply criticism of experimentalists, whom only the naive critic might expect to have mounted an earlier attack on the unobvious problems that are only now coming clearly into view. No criticism is intended here. Hindsight is easy, and the questioning of motives, cheap.
Progress in a complex technological art is often crabwise. The need today is for a keener appreciation of the difficulties and the fastest possible dissemination of information, bad as well as good. Fortunately, the power industry is geared for rapid exchange of information. It is accustomed to attacking its problems through industrial committees. Historically, it found need to hire relatively few chemical engineers, and so it has been far better prepared for exchange of information concerning electrical or mechanical arts than chemical. The industry has recently begun to hire more chemical engineers, and this fact along with the advent of the Electric Power Research Institute should greatly improve the transfer of scrubbing experience.
The large number of scrubbers now on order (see Appendix B) is often cited as proof of commerical availability. It is of course no such thing. Their owners must regard the units as experimental, needing to be staffed in expectation of discoveries and of need for revision.
In view of the record, it will be remarkable if at least a few of the installations do not experience serious difficulties.
The record also justifies an optimistic view of the future of scrubbing technologies. The issue of scrubber availability on power plants burning high sulfur coals can be resolved in the near future by a program of experimentation that can now be specified with reasonable confidence. There is a reasonable expectation that scrubbers will become available for routine purchase for a wide range of specific conditions, if an analysis of cost versus benefit shows a purchase to be justified.
It may also be noted that both lime and limestone scrubbers appear to be reliable for application on power plants burning low-sulfur western coals (See Sections 2.12 and 3.10).
2.0 LIME SCRUBBING FOR MEDIUM AND HIGH SULFUR COAL
The chemistry of lime scrubbing is too complex (Hollinden 1974, Borgwardt 1974) to summarize briefly. It will be sufficient here to understand that the alkalinity needed to scrub sulfur dioxide from the flue gas stream is supplied by the dissolving of calcium sulfite particles in the scrubbing liquor as it passes through the scrubber. Although the solubility of calcium sulfite in water is relatively small, the liquor is unsaturated in respect to this species. As the calcium sulfite enters solution, sulfite ions react with sulfur dioxide to form bisulfite ions:
(1)
(2)
Removal of sulfite ions by reaction (2) tends to cause additional sulfite ions to enter the solution by reation (1), and the ions further react with Sulfur dioxide. Typically, about 3 percent of the entering calcium sulfite particles might be expected to dissolve as the liquor flows through the scrubber.
The spent liquor that leaves the scrubber is rich in bisulfite ion, and is conducted to a tank where it is mixed with a slurry of lime. In a rapid reaction, bisulfite ions in the liquor are neutralized by the lime to form calcium sulfite:
(3)
(4)
The calcium sulfite precipitates to form small crystals of CaSo3·0.5 H2O. The greater part of the scrubbing liquor, carrying a burden of these crystals, is returned to the scrubber. A small part is sent to a step for clarifying the liquor to provide a concentrated sludge of calcium sulfite particles for discard. The clarifier returnes a clear stream to the scrubber.
Early problems of lime scrubbing relating to corrosion of materials of construction are now largely solved, provided the process is controlled to maintain the pH of the scrubbing liquor within the proper range.
Problems of formation of scale and deposits remain a major concern in scrubbers of designs typical of most existing and pending installations. These problems are related to the degree of oxidation of sulfite to sulfate in the scrubber, a subject that will be treated more fully below.
Critical points are the passages in spray nozzles for introducing the liquor into the scrubber and the passages in the mist eliminator that must be provided beyond the active scrubbing zone in order to prevent droplets of scrubbing liquor from leaving the system. The danger at these critical points is that they will become plugged by either soft mud-like
deposits, or blocked by a scale consisting of dense crystalline deposits of gypsum.
Essential to continous operation is that the passages of the mist eliminator be washed to keep them clear. Water is lost from the scrubbing system both in the sludge waste and in the form of water vapor in the stack gas that did not arise from combustion of hydrogen in the coal. Relatively small amounts of fresh water must be added to the scrubber system to make good these losses, and this water may be used to wash the mist eliminator. Alternatively, or in addition, the passages of the mist eliminator may be washed with some of the clear stream returned to the scrubber from the clarifier. If these procedures succeed, it is possible to operate the system as a closed loop. If the washing procedures do not succeed, the mist eliminator must either be cleaned out periodically during a shutdown or washed with additional fresh water to keep its passages open. Additional fresh water would force the operation to discard at least some of the clear liquor from the clarifier, opening the water loop.
Trouble may arise from deposits of both calcium sulfite and calcium sulfate:
-
If the pH of the scrubbing liquor is too high, because lime is present in excess, lime will react directly with sulfur dioxide and carbon dioxide in the flue gas undergoing treatment to form a hard scale consisting of calcium sulfite and calcium carbonate.
-
Some oxidation of sulfite to sulfate, both within the scrubber and in the reaction tank, appears to be inevitable. This leads to danger of precipitation of gypsum crystals, CaSO4·2 H2, from the solution.
The tendency for deposits to prove troublesome is greater for a coal of higher sulfur content, that leads to a flue gas from which more sulfur dioxide must be removed.
Until a little more than 1–1/2 years ago, it was believed that a lime scrubber in the closed loop mode inevitably operated with a liquor that was supersaturated in respect to gypsum, and that successful operation depended upon keeping the degree of supersaturation below a critical
level beyond which scaling by deposits of gypsum became intolerable.
It is now recognized that a lime scrubber can preferably operate with a liquor that is unsaturated in respect to gypsum, thereby obviating difficulties from deposits of this species (Borgwardt 1974). Indeed, the only recent successful operation of commercial-scale scrubbers on medium sulfur coal has been in the unsaturated mode (see Sections 2.01 and 2.02 below). Accordingly, the discussion which follows concentrates upon operation in the unsaturated mode. Operation in the saturated mode will be considered in section 2.14.
Whether or not a given scrubber can operate in the unsaturated mode depends upon the degree of oxidation of sulfite to sulfate in the scrubber. At low levels of oxidation, the calcium sulfate in the solution, although unsaturated in respect to gypsum, nevertheless coprecipitates with the calcium sulfite crystals that form in the reaction tank. The crystals that are produced have the geometry of crystals of pure CaSO3·0.5 H2O, but contain some calcium sulfate as if it were in a “solid solution” (Borgwardt 1974). There is an upper limit to the concentration of the coprecipitated calcium sulfate (to be discussed below). If the degree of oxidation exceeds this upper limit, the solution becomes supersaturated in respect to gypsum, and danger from deposits of this material arises.
Of all aspects of lime scrubbing chemistry, least well understood are the factors that cause or prevent oxidation of sulfite to sulfate. Lowering of the pH of the scrubbing liquor promotes oxidation by increasing the concentration of bisulfite ions, making more of these ions available for reaction with oxygen in the flue gas. A greater amount of excess air, leading to higher oxygen level, is believed to promote oxidation. Presence of fly ash is known sometimes to promote oxidation (see Section 2.05), and it is reasonable to expect that the effects may be greater for some ash compositions than for others. A lengthening of residence time of liquor in the reaction tank is believed to promote oxidation (Borgwardt 1974). Some
researchers suspect that nitrogen dioxide in the flue gas may tend to oxidize sulfite to sulfate, and that a flue gas with an unusually high concentration of nitrogen dioxide may cause difficulties in respect to oxidation level (Rochelle 1975).
An aspect of scrubber operation that emerges from the foregoing discussion is that control of pH is important for success: to prevent scaling by coprecipitated calcium sulfite and calcium carbonate when pH is too high, and to prevent oxidation of sulfite to sulfate when pH is too low.
Control of pH depends, among other things, upon providing a scrubber that effectively promotes excellent and uniform contacting of all of the flue gas with all of the scrubbing liquor. If a part of the liquor, for example, sees too much flue gas, its pH will drop too low. In a scrubber of poor design, with maldistributed gas and liquor flows, the operator will tend to run at higher liquor rate in an effort to assure that all of the flue gas sees an adequate amount of liquor.
In equipment of the general types exemplified by the scrubber and reaction tank, chemical engineers are not surprised to see poorer contacting in equipment of larger size. Often, much of the development task involves learning how to live with a deterioration in contacting efficiency that has accompanied scale-up. It will be important to bear this in mind in considering differences between bench, pilot, and commercial scrubbers.
Presence of magnesium ions can help the performance of a lime scrubber (Borgwardt 1974). The amount of calcium sulfate that can be purged in the solids without supersaturation is increased. The solubility of calcium sulfate is enhanced, reducing risk of scaling. The solubility of calcium sulfite is also increased, tending to promote greater efficiency of sulfur dioxide removal, but also creating a higher level of sulfite ions in the liquor, a factor tending to promote oxidation.
It is probable that sodium ions would affect performance in similar ways (Weir 1975).
Some workers have appreciated these effects for a number of years: for example, the Tennessee Valley Authority experimented with additions of magnesium several years ago, and both M.W.Kellogg and Dravo have promoted use of magnesium. It appears, however, that an appreciation of the potential os such additions has become general only recently2. There may have been some resistance to the idea because presence of magnesium puts a greater premium upon maintaining a strict closure of the water loop, in order to prevent all possibility that substantial amounts of water containing magnesium sulfate in solution will be discharged.
Also, it has become generally appreciated only recently that presence of chloride ions can make it more difficult to operate with a liquor unsaturated in respect to gypsum (Borgwardt 1974).
-
The solubility of calcium sulfate is less at high chloride ion levels, increasing danger from deposits of gypsum.
-
In presence of chloride ions, less calcium sulfate can coprecipitate with CaSO3·O.5 H2O. This lowers the degree of oxidation of sulfite to sulfate that is allowable before the liquor becomes supersaturated toward gypsum.
-
Chloride ions cause a greater drop to occur in pH of liquor flowing through the scrubber. This is apparently the result of a lowered solubility of calcium sulfite, whose dissolution is important to the capability of the liquor to absorb sulfur dioxide. As a result of this effect, it is advisable to operate with a greater rate of flow of scrubbing liquor if chloride ions are present (Borgwardt 1974).
The harmful effect of chloride ions can be offset by addition of magnesium, which suppresses calcium ion, so that the chloride ions are in effect tied up with magnesium rather than calcium. In addition, as noted above, magnesium ions enhance the amount of calcium
sulfate that may coprecipitate with calcium sulfite.
For a solution that is low in both chloride and magnesium ions, the limiting amount of calcium sulfate that can coprecipitate with calcium sulfite is about 18 percent. Laboratory data (Borqwardt 1974) suggest that the limiting amount is primarily a function of the activity of sulfate ion in the solution. This decreases with increase of chloride ion, and increases with increase of magnesium ion. A coprecipitate containing as much as 30 percent sulfate has been observed for a laboratory lime scrubber (Borgwardt 1974), although at such a high level of magnesium ion as to make the sustaining of the operation in the closed loop mode almost imperative.
It follows from effect (3) above that a scrubber design of marginal contacting efficiency, that might be adequate for a liquor low in chloride ion content, may lead to trouble at a high chloride ion level, because maldistribution of gas and liquor flows could lead to a region of excessively low pH, where the local flow of flue gas exceeds the capability of the local flow of liquor to remove all of the sulfur dioxide in the gas.
The Tennessee Valley Authority has measured hydrogen chloride content of flue gas from a wide variety of the coals that TVA burns, to find that most of its coals produce a flue gas containing in the vicinity of 50 to 75 ppm of HC1 (Hollinden 1975). This concentration of HC1 would correspond, very roughly, to about 0.6 to 0.9 percent chlorine in the coal. Such a chlorine content would not ordinarily be considered to be particularly troublesome from standpoint of boiler fouling and corrosion, and generally speaking, the fuels technologist would term the TVA coals to be low in chlorine. Some “high chlorine” coals, in the usual meaning of this expression, occur in Illinois, where chlorine contents as high as 0.65 percent are known (Simon 1975). Analysis of 82 coals of the Illinois Basin (Illinois, Indiana, and Western Kentucky) gave values distributed as follows (Ruch et al. 1974):
19 coals between 0.00 and 0.2 percent chlorine
|
13 |
0.03–0.05% |
|
8 |
0.06–0.11% |
|
22 |
0.12–0.23% |
|
11 |
0.24–0.35% |
9 coals greater than 0.36 percent chlorine Analysis of 9 coals from Ohio, Pennsylvania, and West Virginia gave 2, 3, 3, 0, 1, and 0 coals in the respective categories listed above.
All HC1 present in flue gas entering a lime scrubber is absorbed (an advantage of the process), in general with formation of calcium chloride. This salt is highly soluble in water. Although it is believed that up to about one-half of the chloride present in a lime scrubbing liquor can coprecipitate with calcium sulfite (Borgwardt 1975), much of the chloride ion can leave the system only in water solution, either in water that accompanies the waste sludge, or in an undesirable water discard to the environment. The level to which the chloride ion concentration builds in the scrubbing liquor, before input of HC1 balances the discharge of chloride salts, is greatest for closed loop operation. Since the quantity of water discharged in the sludge depends directly upon the quantity of sludge, the chloride ion level is inversely proportional to the sulfur content of the coal. If the scrubber handles fly ash, the quantity of sludge will be greater and the chloride levle of the liquor will be lower.
Before considering experience gained in the several lime scrubbers now in operation, it is important to recall that the chemistry of the operation is more complex than their brief review might make it appear. It is also sobering to remember that much of the chemistry became generally recognized only in November of 1974, and even now this knowledge does not appear to be widely disseminated, even among operators of lime scrubbing equipment.
2.01 Paddys Run
A lime scrubbing system (Louisville Gas & Electric’s Paddys Run Station) has been demonstrated to be reliable in intermittent
service on a 65-megawatt peaking unit fired with a coal of 3 to 3.5 percent sulfur. The level of Sulfur dioxide in the flue gas is about 1800 to 2000 ppm, reflecting a greater than usual degree of excess air, viz., about 80 percent excess air in contrast to the usual 20 to 30 percent. Accordingly, the scrubber operation is best regarded as simulating the cleaning of gas from combustion of coal of roughly 2 to 2.5 percent sulfur in a 100-MW unit.
The operation is unusual in two respects:
-
The coal is low in chlorine content, about 0.03 to 0.04 percent. Chloride ion concentration in the scrubbing liquor runs between about 300 and 500 ppm, with a highest measured value of 600 ppm.
-
The lime is a waste carbide lime sludge accumulated from manufacture of acetylene.
The operators believe the unit to function without discharges of salty water to the environment (i.e., closed loop). Residence time of liquor in the reaction tank is about 25 minutes.
The system receives flue gas from an electrostatic precipitator of about 95 percent efficiency, fly ash enters the system at a loading between about 0.2 and 0.4 grains per standard cubic foot.
Turndown of the unit is accomplished by reducing both liquor flow and gas speed, although when the load drops below about 80 percent of capacity, flue gas is recirculated to maintain gas velocity. During a period of 45 days of service, the operation was typically at 55 to 70-MW in the day, about 30 to 35 at night, and about 3-MW over the weekend.
The degree of oxidation is only about 1.5 to 3 percent, the lowest value reported for any lime scrubber, in spite of the large amount of excess air. The system operates with liquor less than 50 percent of saturation in respect to calcium sulfate, this species being coprecipitated with calcium sulfite.
A first set of bench-scale tests by Combustion Engineering with use of the carbide lime in a small scrubber suggested that the lime
contained an oxidation inhibitor, but later tests failed to confirm this finding (Martin et al. 1974).
The operator believes that the low oxidation experienced in this unit may be a result of a relatively short time during which the flue gas remains in contact with scrubbing liquor.
2.02 Mitsui Miike Industrial Boiler
A second lime scrubbing system (a Mitsui Miike Industrial boiler in Japan, discharging combustion products roughly equivalent to a 160-MW utility boiler) has also operated successfully, and in continuous service.
By an unfortunate coincidence, this system, too, is unusual in exactly the same two respects noted for the Paddys Run system above.
The unit operates steadily, and its operators have not had the problem of following changes in load.
Water management is such as to guarantee discharges of water to the environment from time to time, thereby rendering the operation open loop. Many visitors to the unit have now seen water overflowing from the sludge pond during rainfall.
The oxidation level is 10 percent, the sludge containing 10 percent calcium sulfate coprecipitated with calcium sulfite. The liquor is probably unsaturated in respect to gypsum.
2.03 Phillips Station
A lime scrubbing system (Duquesne Light’s Phillips Station) operating on a part of a 387-MW station’s stack gas is still in trouble. Mechanical problems appear to be coming under control, but process control of the pH of the scrubbing liquor is poor. The pH varies with load on the station, tending to fall at high flue gas throughout. The capability to supply lime is not adequate, and pH control is poor lacking automatic features and timely response The operators believe that the chloride ion content of the liquor would be beyond 1100 ppm
if the operation were closed loop, but recent operation has been at around 400 to 600 ppm, reflecting the extent to which the operation is taking in and discharging extra water. The need for the extra fresh water reflects in large part the need to use fresh water to wash mist eliminators and induced-draft fans, as well as to reduce deposits in other parts of the equipment. The operators in the past have expected to shut down and enter the equipment for cleaning about once or twice a month, and there have also been forced shutdowns due to plugging of the demisters and bleed lines, as well as to accumulation of deposits at dampers. It is possible that performance of the unit could be greatly improved by better pH control. Tests with a lime high in magnesium showed better sulfur dioxide removal.
2.04 EPA’s 10-MW Pilot Lime Scrubber
Operation in the unsaturated mode has been observed in EPA’s 10-MW pilot scale time scrubbing system (at TVA’s Shawnee Station) for 392 hours with a closed water loop (Princiotta 1975). Chloride ion was 2000 to 3000 ppm during the latter two-thirds of the run, while magnesium ion was about 3000 ppm. Oxidations ranged from 15 to 28 percent, and the liquor was 45 percent saturated. The liquid-to-gas ratio, L/G, was 90. (L/G is expressed in terms of gallons per minute of liquor supplied to the scrubber per one thousand actual cubic feet per minute of stack gas treated by the scrubber.) This is a much higher liquor rate than that used in the Paddys Run scrubber, where L/G is between 32 and 40. The EPA pilot scrubber takes gas upstream of the Shawnee Station precipitator, the gas containing about 3 grains of fly ash per cubic foot.
The 10-MW scrubber has always operated steadily, and has not been subjected to an experiment simulating the following of load variations that might typically be expected in operation of a commercial utility boiler (Moore 1975).
The run in the unsaturated mode was shut: down voluntarily, but scale had formed on the mist eliminator early in the run. This has chronically given trouble in the 10-MW operation, scaling having appeared even in trials of fresh water for washing the mist eliminator. The original design of the mist eliminator appears to have provided a sort of “mini-scrubber”, where residual sulfur dioxide in the flue gas can react with calcium ions in the wash liquor to produce dense gypsum scale. After the shutdown of the run in the unsaturated mode, emphasis of the work returned to attempts to solve the problem of the mist eliminator in tests conducted in the supersaturated mode. A new mist eliminator of design similar to that used at Paddys Run was installed, but this also scaled, and has now been removed.
It should be noted that even a base-loaded commercial operation might be willing to shut down to clean out mist eliminators every few months. However, a non-scaling design is greatly to be desired.
2.05 TVA’s 1-MW Bench Lime Scrubber
The Tennessee Valley Authority has a 1-MW bench scale scrubber at TVA’s Colbert Station that is normally used for test operations looking toward design of the limestone scrubber for TVA’s Widows Creek Station. The 1-MW scrubber was operated continuously for a month as a lime scrubber well in the unsaturated mode (Hollinden 1975). Flue gas was supplied to the scrubber from a point downstream from the electrostatic precipitator of the coal-fired Colbert Station. The flue gas averaged 0.022 grains per standard cubic foot and 2430 ppm sulfur dioxide. The operation was effectively closed loop in respect to build-up of chloride and magnesium ion levels, which ran about 5000 ppm and 2300 ppm respectively. (The mist eliminator of this scrubber is arranged so that it can be washed with fresh water that does not combine with the primary flow of scrubbing liquor. During the first two of three months of lime operation, the wash water was discarded;
during the final month, the wash system operated with a closed water loop.) The test was intended to simulate opertion of the Paddys Run system, insofar as possible. However, in addition to the higher levels of chloride and magnesium ions, L/G was 60 versus 32 to 40 at Paddys Run. Also, the Colbert Station operates at far less excess air. Oxidation level was 5 to 7 percent, appreciably higher than at Paddys Run. Since both chloride and magnesium ions tend to promote oxidation, not much can be said concerning the comparison of the oxidation levels in the 1-MW bench scale scrubber and at Paddys Run. Approach to saturation was 20 to 30 percent.
The lime scrubbing operation was continued in the 1-MW unit for a second and third month with flue gas supplied from a point upstream of the precipitator. Fly ash in gas entering the scrubber averaged 4 grains per standard cubic foot. The oxidation level rose to 10 to 12 percent, with two peaks as high as 17 percent. Approach to saturation rose to 60 to 90 percent. Because of the greater amount of sludge, chloride ion and magnesium ion levels fell to about 4000 and 1500 ppm respectively. Reaction tank residence time was 18 minutes early in the run, and was reduced to 5 minutes with no deterioration in performance.
The unit was not subjected to a test simulating load variation during the lime run.
2.06 EPA’s 0.1-MW Bench Lime Scrubber
EPA’s 0.1-MW oil-fired bench scale lime scrubbing system (300 ft3/min) at Research Triangle Park has recently provided valuable new chemical information, that has cast light upon the operation in the unsaturated mode that characterizes the successful scrubbing system cited in 2.01 above, and probably also the system cited in 2.02 (Borgwardt 1974). Broadly, results from the bench scale scrubber may be summarized as follows:
-
A test simulating the operation conditions in the Paddys Run system provided an oxidation level of 4
-
percent, only a little higher than that experienced in the larger unit. The liquor was at 30 percent of saturation in respect to gypsum. The test differed from Paddys Run in several respects:
-
Ordinary lime was used instead of carbide lime.
-
The liquor-to-gas ratio, L/G, was 50 versus 32 to 40 at Paddys Run. The higher L/G would be expected to hold down oxidation.
-
The residence time of liquor in the reaction tank was 2 minutes versus 25 minutes at Paddys Run. The shorter residence time would be expected to tend to hold down oxidation.
-
The excess air was appreciably less, so that the gas to be scrubbed contained about 4.5 percent oxygen versus typically 9 percent at Paddys Run.
-
-
Most of the remaining test data, usually obtained in 5-day runs, were at an L/G of 77, roughly double that at Paddys Run.
-
Tests at low level of chloride ion (between 600 ppm) were in the unsaturated mode (between 50 and 70 percent of saturation). Runs with high magnesium ion (about 1000 ppm) tended to be higher in oxidation but no higher in degree of saturation.
-
Most tests at low levels of magnesium ion and at 3000 to 8300 ppm of chloride ion were substantially at the borderline between the unsaturated mode and supersaturation. Most oxidation levels were markedly higher, between 7 and 18 percent.
-
Tests at chloride ion levels between 2400 and 5100 ppm and at magnesium ion
-
levels of about 1100 ppm were in the unsaturated mode (67 to 85 percent).
Oxidation levels tended to scatter, and replication of oxidation levels in similar runs appeared to be difficult. The oxidation levels could not be readily interpreted in terms of the relevant variables thought to affect oxidation. No general correlation of oxidation with these variables has yet emerged from the work.
Some tests were made with carbide lime instead of ordinary lime, and some tests were made with addition of coal fly ash to the reaction tank along with the lime. (Most workers would regard adding fly ash to the reaction tank to provide a less satisfactory test of the effect of fly ash than taking flue gas from a point upstream from an electrostatic precipitator of a coal-fired boiler, because of the possibility that “young” fly ash has properties not preserved during storage.) The operators believed (Borgwardt 1974) that these factors did not affect oxidation level, but the unexplained scatter in oxidation values from all of the runs renders this judgment tentative.
Although the work has cast new light upon lime scrubber chemistry, it is hard to escape the impression that much work remains before the chemistry is well understood. This is not to depreciate the value of the new information, especially in allowing a better understanding of the troubles that scrubbers have experienced.
2.07 Conclusion for Medium Sulfur Coal of Low Chlorine Content
In light of the above facts, the probability would appear to be greater than 90 percent that a lime scrubbing unit could be ordered with a reasonable prospect for reliable performance in a closed loop unsaturated mode for use in a power plant burning a medium sulfur coal (1 to 3 percent sulfur) where the chlorine content is below about 0.04 percent. The design should provide an electrostatic precipitator for removal of fly ash at high efficiency, preferably beyond 99 percent. A prudent design
would provide the possibility of operating at an L/G least as high as 77.
The probability for this prospect would be enhanced (to around 99 percent, say) if the Paddys Run unit were operated successfully with ordinary lime instead of carbide lime to provide assurance that Paddys Run’s success has not depended upon some unknown factor peculiar to carbide lime.
2.08 Conclusion for Scrubbing without Electrostatic Precipitator
The chemical knowledge base is not yet adequate to permit confident design of a commercial lime scrubber for a coal of medium (1 to 3 percent) or high (beyond 3 percent) sulfur content without provision of an electrostatic precipitator. Fly ash has been convincingly demonstrated to promote oxidation in the 1-MW Colbert Station scrubber. Some workers suspect that the fly ash of some coals may contain metal species that are catalytic toward oxidation of sulfite to sulfate, and this is a reasonable suspicion. It is plausible that the catalytic activity might be a function of the “freshness” of the fly ash. Much more research in respect to these possibilities would seem to be required.
2.09 Conclusion in Respect to Chemical Knowledge Base
The chemical knowledge base is not yet adequate to permit confident design of a commercial lime scrubber for a coal of medium or high sulfur content at a chlorine level beyond about 0.04 percent. No generalized correlation of oxidation is yet available in respect to the relevant scrubber variables:
Inlet sulfur dioxide level
Degree of excess air
Overall efficiency of the scrubber
Dispersion of efficiencies for local regions of the scrubber, created by less than
perfect distribution of gas and liquor flows
Ratio of liquor to gas rate (L/G)
Chloride ion concentration in liquor
Magnesium ion concentration
Time of contact of scrubbing liquor with flue gas
Reaction tank residence time
Presence or absence of fly ash
Kind of fly ash
One cannot even be certain that this list of variables is complete. Is nitrogen oxide level in the flue gas important? Is the level of iron ions in the scrubbing liquor important? The effects of the variables known to be important are understood only qualitatively.
The known or suspected interactions among the variables are such that the chemical knowledge base would have to be very good indeed in absence of strong assurance, provided by a relatively large number of comparisons of performance among bench, pilot, and commercial scale scrubbers, that performance of the latter can be expected to duplicate performance of the former with high probability.
2.10 Conclusion in Respect to Adequacy of Performance Comparisons
The comparisons between bench scale scrubbers at 0.1-MW and 1-MW, the pilot scale scrubber at 10-MW, and Paddys Run are insufficiently exact to provide a basis for confident design, even if the chemical knowledge base were adequate.
Table 11–3 summarizes performance comparisons for scrubbers treating flue gas from coal combustion. The higher L/G that was apparently required for the spray tower of the 10-MW EPA scrubber may reflect this unit’s poorer distribution of gas and liquor flows through its contacting region.
Although the oil-fired 0.1-MW bench unit at Research Triangle Park observed an oxidation level of only 4 percent in one five-day test simulating Paddys Run conditions, the scatter in
TABLE 11–3
Comparisons among Bench, Pilot, and Commercial Lime Scrubbers Operating in Closed Loop, Unsaturated Mode on Flue Gas from Coal-Fired Boilers
|
|
Paddy’s Run |
EPA Scrubber at Shawnee |
TVA Scrubber at Colbert Station |
|
|
Scrubber type |
Marble bed |
Spray tower |
T.C.A.* |
|
|
Kind of lime |
Carbide |
ordinary |
||
|
Size |
100-MW equivalent |
10-MW |
1-MW |
|
|
Fly ash present or not |
No** |
Yes |
No*** |
Yes |
|
Length of run |
45 days |
17 days |
30 days |
60 days |
|
Load following |
Yes |
No |
No |
No |
|
L/G (gpm/Macfm) |
32 to 40 |
90 |
60 |
60 |
|
Chloride, ppm |
300 to 500 |
2000 to 3000 |
5000 |
4000 |
|
Magnesium, ppm |
low |
3000 |
2300 |
1500 |
|
Oxygen in flue |
9% |
about 4.5% |
||
|
Residence time of liquor in reaction tank, minutes |
25 |
10 |
18 |
5 to 18 |
|
Oxidation of sulfite to sulfate |
1.5 to 3% |
15 to 28% |
3 to 7% |
10 to 12% |
|
Degree of saturation in respect to gypsum |
50% |
45% |
20 to 30% |
60 to 90% |
|
*“Turbulent Contact Absorber”, in which mobile, hollow polyethylene spheres are retained by horizontal grids. *Precipitator at 95% efficiency. *Precipitator at 99% efficiency. |
||||
oxidation results on the 0.1-MW unit greatly reduces the significance of this comparison.
The low oxidation level at Paddys Run remains, in the eyes of most workers in the field, an unresolved mystery.
2.11 Conclusion in Respect to Confidence in Early Resolution of Question of Availability of Lime Scrubbers
In spite of the reservations stated in 2.09 and 2.10, the comparisons of Table 11–3 as well as the recent advance in chemical understanding warrant considerable confidence that a well-planned program, put into effect promptly, could provide a sufficient number of performance comparisons as well as a marked improvement in chemical knowledge.
It is possible to be reasonably confident that the program can resolve the question of availability of lime scrubbers for wide application in the affirmative.
Without an intent to specify a program, and purely by way of illustration, it might be suggested that a program could include:
-
Statistically planned experiments in bench scale scrubbers to obtain a better understanding of the effects of variables listed in 2.09 on oxidation and upon approach to saturation. Such experiments could include studies of effects of carbide lime and addition of fly ash (preferably by taking gas from a coal-fired power station ahead of a precipitator).
-
Similar experiments in 10-MW pilot scale scrubbers, that might reasonably be less extensive in covering the range of variables, if work under (1) provides adequate direction. The experiments should include simulations of load following. The present APA 10-MW lime scrubber at Shawnee, a venturi followed by a spray tower, does not provide much built-in positive control
-
over distribution of liquor and gas flows to assure that the rates of both of these flows are well matched in each small zone of the scrubbing equipment. It is tempting to associate the relatively high rate of oxidation in the recent experiement in the unsaturated mode (15 to 28 percent) with the scrubber design, and one might wish to see data for lime scrubbing at the 10-MW scale in a design that provides trays or grids, with or without packing. Resolution of the problem of the mist eliminator scaling is an urgent matter.
-
As insurance against the possibility that factors affecting oxidation may remain obscure, it might be advisable to establish a pilot scale scrubber (larger than 10-MW) with its own captive coal-burning equipment. Such an installation could be used for pilot scale tests on a variety of coals, and especially on a specific coal projected for use with a specific proposed commercial scrubber.
-
In interests of speed, an alternative would be to supply flue gas to a pilot scale scrubber from a boiler fitted with a precipitator working at high efficiency, and then to spike the flue gas with HC1 and/or fly ash from coal intended for a specific proposed commercial scrubber.
-
Consideration might be given to trails at Paddys Run with spiking of flue gas with HC1 and/or fly ash from other power stations. Such tests should bear in mind that the L/G at Paddys Run are markedly lower than the L/G’s of the bench scale scrubbers, and it might be found necessary to increase the L/G at Paddys Run, if this should be possible. The trials should include load following. If magnesium is added to
-
offset the adverse effects of chloride ion, the operation should continue long enough to provide a realistic assessment of the problems of operating with a strictly closed water loop at high levels of chloride and magnesium ions.
-
Since the Phillips Station scrubber (see 2.03) will operate in any event, consideration might be given to a study looking toward its revision for an attempt to operate in the closed loop, unsaturated or borderline mode. The revision ought to bring pH under firm control, to separate the main scrubber loop from loops serving venturi scrubbers whose job is to remove fly ash, and no doubt to effect other improvements as well.
-
The Phillips Station scrubber is a retrofit, and a more advantageous alternative might be found among the 7 lime scrubbing installations (totalling 3,312-M) now under construction or on firm order for high-sulfur coals.
In connection with (6) and (7), it should be remembered that research on lime scrubbing chemistry on the 100+-MW scale must be regarded, under normal circumstances, as imprudent. Certainly, (6) would be costly, and Duquesne Light might well feel that it would need financial help if the task is to be done quickly, and perhaps a loan of personnel as well.
2.12 Horizontal Scrubber at Mohave Station
A novel horizontal scrubber has been in successful use of the 170-M scale at Southern California Edison’s coal-fired Mohave Station. Most of the operation of this unit has been with an inlet sulfur dioxide concentration of only about 200 ppm. Cloride ion levels are extremely high. Susbtantially all of the absorbed sulfur
is oxidized to the sulfate form. The effective L/G is 80, but liquor arrives to the scrubber at an L/G at 20: there are four stages of scrubbing with repeated use of the same liquor in countercurrent flow in respect to the gas. The pH may be adjusted separately in each stage, although this has not been done to date. The unit is fitted with a mist eliminator arranged so that it can be washed with fresh water that does not combine with the primary flow of scrubbing liquor.
The Mohave installation experiences loss of water by evaporation from the sludge pond, and there is ordinarily no return of water from the pond to the scrubber.
The horizontal design has also been tested at the 10-M scale in intermittent service (10 hours a day, 4 days a week) where chloride ion levels are negligibly small. Tests at the 10-M scale have extended to inlet sulfur dioxide concentrations of 3000 ppm.
The horizontal scrubber may prove to be a valuable “what then?” if vertical scrubbers of the more common designs are proved not to provide sufficiently uniform distribution of gas and liquor flows for operation at high chloride ion levels or at other conditions promoting oxidation.
Advocates of the horizontal scrubber also feel that it could operate well into the supersaturated mode without trouble from deposits.
2.13 Open versus Closed Water Loop
As noted earlier, the point of view here has been that substantially all locations in the eastern United States where a lime scrubber might be situated are such as to require operation of the scrubber in the closed loop mode. In a few locations, operation of an open loop system may be allowable, and this will be easier. No commercial system, however, can yet be said to be operting with complete success on a medium or high sulfur coal in the open loop mode, except for the unusual system cited in 2.02 above.
2.14 Lime Scrubbing in the Supersaturated Mode
The path of development emphasized here has been one toward an operation in the unsaturated or borderline mode. In view of the success of the Paddys Run scrubber, this path seems closest to achieving commercial results for medium or high sulfur coals.
The Fulham Station scrubber in England (see 3.01 below) is reported to have operated with lime in a supersaturated mode in the late 1930s.
Operators of EPA’s 10-M lime scrubber believe their work to have demonstrated a viable practice in a supersaturated mode, although the operation was marred by trouble at the mist eliminator and has not yet simulated load variations. One might also worry about the sensitivity of the degree of supersaturation in some of the runs to the sulfur oxide level in the flue gas, small variations in the latter producing large changes in the former (Epstein et al. 1974). It is not necessary here to examine this option in depth, for no recent commercial practice is available for a performance comparison. It is possible that some of the 7 lime scrubbing installations now under construction may intend to operate in the supersaturated mode, and if so, the necessary performance comparisons may be forthcoming.
2.15 Operation at a High Degree of Oxidation
Some workers take the view that operation far into the supersaturated mode, and at a high degree of oxidation of sulfite to sulfate, will be possible if the scrubber is designed physically to operate without trouble from gypsum deposits, even if gypsum forms freely within the scrubber.
Such an operation would enjoy the advantage that gypsum settles from a water suspension more quickly than crystals of calcium sulfite, and the settled material occupies less volume. These properties of gypsum would ease the preparation of a concentrated sludge of waste solid for disposal, as well as reduce the volume of the sludge deposit.
These points of view seem valid, but there is little experience to back them up. They require a new path of development that might be said to have only barely been begun.
3.0 LIMESTONE SCRUBBING FOR MEDIUM AND HIGH SULFUR COAL
The chemistry of limestone scrubbing is also complex (Hollinden 1974, Borgwardt 1974), and a brief summary can touch only highlights. The alkalinity needed to scrub sulfur dioxide from the flue gas stream is supplied by the dissolving of calcium carbonate particles in the scrubbing liquor as it passes through the scrubber. Although the solubility of calcium carbonate in water is small—indeed, far smaller than the solubility of calcium sulfite, which provides alkalinity to the lime scrubber—nevertheless, the liquor is unsaturated in respect to this species. As the calcium carbonate enters solution, carbonate ions react with sulfur dioxide to form bisulfite ions and to release carbon dioxide gas:
(1)
(2)
Typically, about 1.5 percent of the entering limestone might be expected to disolve as the liquor flows through the scrubber.
The spent liquor that leaves the scrubber is rich in bisulfite ion, and is mixed with a slurry of limestone in a reaction tank. The reaction of the bisulfite ions is relatively slow, to produce calcium sulfite:
(1)
(3)
(4)
Calcium sulfite precipitates to form crystalline CaSO3·0.5 H2O. Liquor leaving the reaction tank contains unreacted limestone, as well as calcium sulfite crystals. The greater part of the scrubbing liquor is returned to the scrubber. A small part is sent to a step for clarifying the liquor to provide a concentrated sludge for discard. It will be appreciated that inherently the sludge must contain some limestone. Generally, the supply of stone must amount to about 150 percent of the stoichiometric requirement for conversion of sulfur dioxide in the flue gas. In contrast, a lime scrubber requires but 100 percent of stoichiometric. Lime, however, is much more expensive than limestone.
Early problems of limestone scrubbing relating to materials of construction are now largely solved, provided large pH excursions are avoided.
Oxidation of sulfite to sulfate is inherently a more serious problem for a closed loop limestone scrubber than for the lime system. This is because the pH in the limestone system is buffered by presence of limestone at a pH in the vicinity of 5.5. Thus, the liquor might enter a limestone scrubber typically at a pH of about 5.8 and leave the scrubber at 5.4. In contrast, liquor entering a lime scrubber might be at about 8 to 8.5 pH, and the liquor would typically leave at about 5.6, since crystals of CaSO3 in this liquor provide no buffering action. As a consequence, the bisulfite ion level is generally higher throughout the limestone scrubber, providing more opportunity for oxidation.
All commercial limestone scrubbers, whether open or closed loop, appear to have operated with a liquor that was supersaturated in respect to gypsum. In scrubbers of the general type employed in most tests underway or projected in the United States, successful operation depends upon keeping the degree of supersaturation below a critical level, generally believed to be about 130 percent, beyond which scaling by deposits of gypsum become intolerable. [However, see Section 3.12.]
Operation of a limestone scrubbing system has been observed in a closed loop, unsaturated mode in one tiny bench scale scrubber. There is general agreement that operation in such a mode will be considerably more difficult in a large limestone system than in a large lime system.
Accordingly, the emphasis here will be upon operation in the supersaturated mode.
Although the limestone system is buffered in respect to pH, nevertheless, excursions of pH in the downward direction can occur and are to be feared. This is because the dissolution of limestone is so slow that a sudden downward excursion of pH of the total scrubbing liquor flow can be corrected only relatively slowly by adding more limestone to the reaction tank. This characteristic of the system may make load following more difficult for the limestone system.
Local downward excursions of pH are possible in the scrubber itself in a zone oversupplied with gas and undersupplied with liquor.
If a pH excursion dips below about 4.8, experience at both a bench scale scrubber (TVA’s 1-M unit at Colbert Station) and a large unit (Will County) has shown that extreme difficulty may arise from the blinding of the limestone particles by a “candy coating” of calcium sulfite when stone is added in an atempt to restore the pH. When this happens, experience has shown that the operator’s only resort is to dump the liquor system and begin again with a new inventory of limestone. Blinding is difficult to duplicate, and hence difficult to study, and so the kinetics are unknown, and it cannot be said whether this is a problem only for a mishap that affects the overall liquor system, or is also a problem for a scrubber with bad internal distribution of gas and liquor.
3.01 Fulham Station
Full scale limestone scrubbers began to be used in England in 1933, and experience was acquired there for both open and closed loop operation. The coal-fired Fulham Station was fitted with a closed loop system in about 1938,
and this system operated until 1940, when it was shut down because it was believed that the plume attracted enemy aircraft. The system employed four scrubbers each 45-M in capacity. As a result of pilot tests in the closed loop mode, developers of the Fulham Station system provided higher rate of flow of scrubbing liquor relative to flow of flue gas (higher L/G) and a larger burden of circulating limestone than the levels of these variables that have been used in commercial limestone scrubbers built recently in the United States. The designers also provided a scrubbing tower of a larger size, so that the speed of the gas was appreciably lower, and a longer residence time was provided in the reaction tank. Reports on the operation at Fulham Station contain remarks suggesting presence of all of the problems that scrubbers have experienced here in respect to materials of construction, scaling, fouling by deposits, and corrosion, although operations were deemed successful. It is understood, however, that the Fulham Station scrubber was so badly corroded when it was shut down that it would have had to be rebuilt if further operation had been required after the War.
3.02 Will County Station
A limestone scrubber for 84-M capacity at Commonwealth Edison’s Will County Station has operated in the open loop mode. Much truble was experienced with fouling by deposits, among other problems, as long as attempts were made to operate on coal containing 3 percent sulfur. Since April of 1974, availability of the scrubber has been good (still in open loop mode), but the station has fired mixtures of low sulfur Western coal (about 1 percent suflur) and the Illinois coal. The sulfur level has averaged about 1.5 percent, and it might be suspected that this represents an approximate upper limit for operation of a system of the Will County design in the open loop mode without difficulty from gypsum deposits. It should be noted, however, that the design uses an appreciably lower liquor rate, higher gas
velocity, lower solids burden, and shorter reaction tank residence time than the Fulham Station design that was reported to be successful in closed loop operation (see 3.01). It is doubtful that the Will County unit could be modified to approximate the Fulham Station experience in view of space limitations. In any event, such modification would be expensive.
3.03 LaCygne Station
A limestone scrubbing installation for 820-MW at Kansas City Power & Light’s LaCygne Station is mechanically similar to the installation just described at Will County. The LaCygne Station burns coal containing 5.5 percent sulfur. The coal also has a high ash content. Experience in operating the scrubbers at LaCygne has been generally similar to the experience at Will County for 3 percent sulfur coal.
The operation is open loop, although it should be pointed out that the system does not discharge water beyond the station’s property line having a salt content in violation of Missouri or Kansas regulations in respect to quality of industrial waste water. This is because the property includes a 2600 acre lake to receive scrubber effluent and hold it for dilution by rainfall before it eventually crosses the property line.
The original design was recognized to be experimental. Because of factors not fully appreciated when the design was executed, the LaCygne Station has had to be derated to 700 megawatts. The derating can be overcome by installing one scrubber module in addition to the seven modules already present, and by installing another forced draft fan.
The operator expects to shut down one or two scrubber modules each night on the graveyard shift, and to enter them to clean out deposits. Each scrubber is entered for cleaning about once every five days. Although the operator is not yet completely satisfied with the system availability, he regards the installation as a viable one from the standpoint of his particular
needs, since he is content to reduce the production of electricity during the early morning hours. It should be remarked that such a reduction might not be congenial to most utility operators of new baseload power units, whose good fuel efficiency depends upon operating them steadily around the clock for long periods at a time. It may also be noted that the State of Kansas Public Service Commission at first took the position that the operation was not satisfactory, and delayed until recently its approval of the entering of the investment in the scrubbing system into its owner’s rate base.
Alternation of the system to approximate the Fulham Station experience would be costly, if not infeasible.
3.04 EPA’s 10-M Pilot Limestone Scrubber
The operators of EPA’s 10-M pilot limestone scrubbing system (at TVA’s Shawnee Station) have developed a mode of operation on flue gas high in sulfur dioxide and HC1 in the closed loop mode. The liquor is supersaturated in respect to calcium sulfate and high in chloride ion content. The success appears to depend upon keeping the degree of supersaturation below about 120 percent, and this has been done by
-
maintaining a high liquor-to-gas ratio in the scrubber (L/G=about 80 gallons per minute per thousand actual cubic feet per minute of gas entering the scrubber),
-
maintaining a high solids content in the liquor (about 15 percent by weight),
-
providing for a residence time of liquor in the reaction tank of about 20 minutes, and
-
reducing the gas velocity from 12.8 to 9 feet per second in the scrubber, thereby derating the unit to about 7 megawatts.
These conditions are closely comparable to the reportedly successful Fulham Station design.
A recent run lasted for 2000 hours under steady load. The unit has not been tested with simulated variations of load.
Attempts to operate the EPA pilot unit in the unsaturated, closed loop mode have failed to date, but the failure may be explained by the long liquor residence time in the reaction tank of the pilot scrubber.
3.05 EPA’s 0.1-M Bench Limestone Scrubber
EPA’s 0.1-M oil-fired bench scale limestone scrubbing system (300 ft3/min) at Research Triangle Park has recently demonstrated operation in a closed loop unsaturated mode at 7000 ppm chloride ion and high magnesium ion levels (2400 ppm and beyond). Work with this unit has only very recently revealed that operation in an unsaturated mode is a possibility. The factors promoting or hindering such operation are much the same as those discussed in Section 2.0 for lime scrubbing, except the effect of chloride ion is greater for limestone scrubbing, and the ameliorating effect of magnesium ion is less. Operation in an unsaturated mode without presence of a substantial quantity of magnesium does not appear possible. Operation in the unsaturated mode requires that oxidation of sulfite to sulfate remain below some critical level, and no generalized correlation of oxidation is yet available.
3.06 TVA’s 1-MW Bench Limestone Scrubber
The Tennessee Valley Authority’s 1-MW bench scale scrubber at TVA’s Colbert Station has been used to study design variables looking toward construction of a large limestone scrubber (550-MW) at TVA’s Widows Creek Station.
In general, it appears that the planners of the large scrubber have not been willing to incur the extra expense entailed by the high liquor rate, low gas rate, and long reaction
tank residence time suggested by the Fulham Station practice. The residence time in the bench scale scrubber is 5 minutes.
A major innovation in respect to the mist eliminator, howeve, gives the planners hope that a design somewhat closer to that used at Will County and LaCygne can be made to function satisfactorily, although with a somewhat higher liquor rate than these existing commercial scrubbers provided. The mist eliminator is arranged so that it can be washed with fresh water that does not combine with the primary flow of scrubbing liquor. At first, it was expected that the water discard from the mist eliminator could be discharged from the system, and it may still turn out that this is the case. However, in absence of definitive regulations in respect to the quality of the water that may be discharged, strenuous efforts have been made over the past three years to close the mist eliminator wash water loop, by recirculating this water and bleeding water make-up for the scrubbing system proper from the mist eliminator loop. These efforts had been unsuccessful until recent encouraging experiments in which a relatively small quantity of sodium carbonate has been added to the wash water loop to precipitate calcium carbonate and raise the alkalinity of the water.
The 1-M limestone scrubber has been subjected successfully to tests simulating variations in load.
3.07 Paddys Run Operated as a Limestone Scrubber
A six-week test of the scrubber at Lousville Gas & Electric’s Paddys Run Station (see Section 2.01) with limestone was deemed not successful by the operators. The scrubbing liquor was supersaturated in respect to calcium sulfate and low in chloride ion. The plant’s operators are aware of the possibility of operating in an unsaturated mode, and the unit normally operates successfully in this mode as a lime scrubber. It should be remarked that the residence time of the liquor in the reaction tank when in use for
lime scrubbing is 25 minutes, and the experience with the 0.1-M bench scale scrubber suggests that shortening this time appreciably might be conducive, if not indeed necessary, to achieving operation in the unsaturated mode. It is not known whether or not the operators reduced the residence time for the limestone test.
3.08 Conclusion in Resepct to Commercial Availability of Three Modes of Operation
It is not possible at this time to select a certain winner among three possible modes of closed loop operation revealed by the foregoing experience:
-
The Fulham Station mode, tested at 10-Mw
-
The Widows Creek mode, projected for TVA’s Widows Creek Station and demonstrated at 1-Mw
-
The unsaturated mode with high magnesium ion level, observed at 0.1-Mw
It would appear that the earliest comparison of bench scale and commercial performance will be provided by TVA’s Widows Creek scrubber, which TVA rightly regards as an experimental unit.
The nature of the designs provided by Peabody Engineering for Detroit Edison’s St. Clair Station (180-M), by Riley Stoker/Environeering for Central Illinois Light Co.’s Duck Creek Station (100-M), and by U.O.P. for Springfield Utility Board’s Southwest Station (200-M) is not known. Perhaps further experiments might be arranged in these units on order or under construction to provide trials for the first or third options.
The third option, however, could hardly be taken seriously until it has been observed in something larger than the 0.1-M bench scale unit at Research Triangle Park.
3.09 Conclusion in Respect to Technical Base for Design
The chemical knowledge base is not yet adequate to permit confident design of a commercial limestone scrubber for a medium or high sulfur coal to operate in a closed loop mode.
Performance comparisons among bench, pilot, and commercial scale scrubbers operating in such a mode are not available.
3.10 Cholla Station
Information acquired from the successful operation of the 115-M limestone scrubber at the Cholla Station of Arizona Public Service has limited value for judging the operability and reliability of limestone scrubbing in the Northeastern United States, because of the low sulfur content of the coal burned at this station. The inlet sulfur dioxide concentration is typically 420 ppm.
The Cholla scrubber system loses water by evaporation from its sludge pond, and there is ordinarily no return of water from the pond to the scrubber.
3.11 Open versus Closed Water Loop
As noted in Section 2.13, the point of view here has been that substantially all locations in the Eastern United States would require operation of a limestone scrubber in the closed loop mode. Open loop systems have come onstream in Japan recently, or are about to come onstream, for coal-fired boilers, the objective being to produce gypsum for wallboard manufacture. With this end in view, the systems are tailored to promote oxidation of sulfite ion to sulfate. One of the systems in the Mitsui Miike scrubber described in Section 2.02, which has recently begun to operate as a limestone scrubber.
3.12 Operation with Free Formation of Gypsum in Scrubber
The path of development emphasized here has been one toward operation in scrubbers of the general vertical design that appears in substantially all of the commercial systems now under test, under construction, or on order.
Some workers take the point of view that scrubbers of significantly different design would be immune from troubles caused by scaling and deposits, even if gypsum were to form freely within the scrubber. (See Sections 2.12 and 2.15.) This would require a new path of development that at present is at an early stage.
4.0 ENVIRONMENTAL CONSIDERATIONS
4.01 Sludge Disposal
One of the most troublesome problems associated with lime/limestone processes is the difficulty of sludge disposal. The EPA has estimated the following rates of waste production rates for a typical 1000-Mw coal fired power plant (3 percent sulfur, 12 percent ash, 6400 hours/year operation, limestone scrubbing).
|
|
Production Rate, 103 Tons/Year |
||
|
|
Dry |
Wet, Separate Collection and Disposal |
Wet, Common Collection and Disposal |
|
Scrubber Sludge |
381 |
763–50% Solids |
|
|
Coal Ash |
338 |
422–80% Solids |
|
|
|
719 |
1185 |
1439 |
The EPA has also estimated the land requirements at 377 acres for sludge and fly ash disposal and 108 acres for fly ash disposal only. (EPA estimates that 90,000 MW of FGD systems will be installed in the eastern U.S. Not all of these will necessarily be lime/limestone.) If 90,000
NW of lime/limestone scrubbing capacity were installed, 130,000,000 tons per year of wet sludge including ash would be produced (50 percent solids basis).
EPA has summarized the following qualitative comparison between scrubber sludge and fly ash.
-
The sludges typically contain calcium sulfite, calcium sulfate, calcium carbonate and fly ash in varying compositions. Fly ashes typically contain silica, alumina and hematite. The compounds in the scrubber sludge are more soluble than those in the fly ash. Table 11–3 shows the characteristics of sludges obtained from operating sulfur dioxide scrubbers.
-
Both sludge solids and ash will contain trace elements and other species originating in the coal, lime/limestone or water. The primary source of trace metals is the coal (ash).
-
Sludge and ash liquors will both contain dissolved species. Untreated sludge liquors normally have a lower pH than fly ash liquors, hence trace metal solubility is generally greater.
-
Sludge liquors may contain significant quantities of chlorides.
-
Untreated sludge settles to about 50 percent solids, and will require more storage volume per unit weight than ash which settles to about 80 percent.
At this time disposal of scrubbers sludges by ponding and land fill appears to be the only important near term alternative. Table 11–4 presents the current sludge disposal practices of several utilities. Nationwide ponding represents about 60 percent of the disposal methods and landfill 40 percent.
If ponding is used, water pollution problems can be prevented by proper pond design, installation of a pond liner and operating in a closed loop mode (do not discharge water from the pond). Costs for pond liners vary from $1 per square yard for clay and thin plastic to $9 per square yard for 30 mil rubber coated fabrics. A cost of $1 per square yard is
TABLE 11–4
Current Sludge Disposal Practices in the Utility Industry in the United States
|
|
|
|
Pretreatment Method |
|
|||||||
|
Facility |
Location |
Efficient Management |
Oxidation |
Clarifier |
Vacuum Filter |
Centifuge |
Pond |
Chemical Fixation |
Ultimate Disposal Method |
||
|
Ponding |
Landfill |
Utilization |
|||||||||
|
Lawrence 4 |
Lawrence, Kansas |
Closed loop |
|
|
|
|
X |
|
X |
|
|
|
Lawrence 5 |
Lawrence, Kansas |
Closed loop |
|
|
|
|
X |
|
X |
|
|
|
Hawthorn 3 |
Kansas City, Missouri |
Open loop(a) |
|
X |
|
|
X |
|
X |
|
|
|
Hawthorn 4 |
Kansas City, Missouri |
Open loop(a) |
|
X |
|
|
X |
|
X |
|
|
|
Will County 1 |
Lockport, Illinois |
Closed loop |
|
X |
|
|
|
X(d) |
|
X |
|
|
Stock Island |
Key West, Florida |
Open loop |
|
|
|
|
X |
|
X |
|
|
|
La Cygne |
La Cygne, Kansas |
Closed loop |
|
|
|
|
X |
|
X |
|
|
|
Cholla |
Joseph City, Arizona |
Open loop(b) |
|
|
|
|
X |
|
X |
|
|
|
Paddy’s Run |
Louisville, Kentucky |
Closed loop |
|
X |
X |
|
|
|
|
X |
|
|
Phillips |
South Heights, Pa. |
Closed loop |
|
X |
|
|
X |
X(e) |
|
X |
|
|
Mohave 2 |
South Point, Nevada |
Closed loop(c) |
|
X |
|
|
X |
X(c) |
X |
X |
|
|
Parma |
Parma, Ohio |
Closed loop |
|
X |
X |
|
|
|
|
X |
|
|
(a) Closed loop with respect to clarifier and open loop with respect to pond. (b) Solar evaporation. (c) Aided by solar evaporation. (d) Chicago Fly Ash method. (e) Dravo method. |
|||||||||||
roughly equivalent to $5/kw capital and 0.15 mills/kwh annualized operation.
If landfilling is used, chemical fixation will most likely eliminate land deterioration problems. Fixation if successfully applied will avoid water pollution problems in unlined landfill sites, but this has not been demonstrated. However, Dravo has received a permit in Pennsylvania for disposal of fixed sludge at the Bruce Mansfield plant. As is shown on Table 11–5, at least three utilities are using chemical fixation methods, but there do not seem to be any reliable published measurements of leaching, ground water runoff etc., that will determine the commercial effectiveness of fixation. Dravo and Will County are both currently conducting leachate tests.
Fixation methods have been developed by three companies (Chenfix, Dravo, and IUCS). In general, they are a means of increasing the sludge strength and shear properties. Selmeczi and Elnaggar have published the screen and sub sieve analysis for sludges from eastern coals:
|
Diameter, microns |
50 |
40 |
30 |
20 |
15 |
10 |
7 |
5 |
2 |
|
Cumulative weight % |
1 |
2 |
5 |
12 |
21 |
39 |
51 |
68 |
68 |
This indicates that the particle sizes fall in the range of silt with minor percentages in the fine sand and clay size particles. The water content of settled sludge from the thickener may vary from 250 percent to 150 percent of the dry weight (160 percent is most typical) or 35–40 percent solids. The specific gravity is 2.5.
The settled sludge has a very soft consistency and its structure is unstable in that it liquefies when disturbed.
Fixation (stabilizing) is the process of adding chemicals to the sludge which will strengthen and harden the sludge. Each of the three companies have different additives but they are basically intended for the same purpose. Costs of chemical fixation are reported to be $15/ton on a dry solids basis (50 percent solids stream to be treated).
TABLE 11–5
Sludge Treatment/Disposal Techniques for Four Selected Utility FGD Systems Nearing Completion.
|
|
|
Dewatering |
Final Disposition |
|
Detroit Edison |
Limestone Eastern Coal |
Pond |
Landfill—no fixation |
|
TVA Widows Creek |
Limestone Eastern Coal |
Pond |
Ponding—lined |
|
Ohio Edison Bruce Mansfield |
Limestone Eastern Coal |
Clarifier |
Landfill—fixation |
|
Northern States Power Sherburne |
Limestone—flyash Eastern coal |
Clarifier |
Pond—clay lined |
Because of the uncertainties regarding sludge stabilization (performance and economics), further study will be done in an advanced program at the Shawnee test facility under the direction of the Aerospace Corporation. The three different proprietary processes will be studied: Davo, IU Conversion Systems and Chemfix. There will be two large ponds for untreated sludge (one lime, one limestone) and three small ponds for treated sludge (one for each process). Each pond will have a leachate well and a ground water well to monitor any ground water contamination.
Utilization of scrubber sludges as a building material is technically feasible, and the process of gypsum production for wall board is practiced in Japan. The first large scale application of sludge utilization in the U.S. will be at the limestone scrubber installation at Mohave Unit No. 1, where IU Conversion Systems will convert the sludge into a building material.
One of the difficulties in sludge disposal is that a potentially useful product (sulfur) is being thrown away. EPA estimates have shown lime/limestone FGD systems are probably not competitive with regenerable FGD systems (where the sulfur is recovered) if the sludge disposal costs exceed about $4–6 per wet ton. (However sulfur recovery (not H2SO4) in an integrated FGD unit on a utility boiler will not be demonstrated until 1975–76 at the NIPSCO plant, Wellman-Lord process.) The magnesium oxide process, to be installed in two locations, will also recover a useful byproduct.
Table 11–5 summarizes the sludge disposal chosen by four utilities who have FGD systems that are fairly close to completion.
5.0 WATER POLLUTION
As pointed out above there are potentially serious water pollution problems from leaching and/or runoff from unlined ponds. There appears to be no problem for a lined and well engineered pond. Experimental work is now underway to
improve the technology and further define the costs of sludge disposal.
The Wellman-Lord Process, currently under installation at NIPSCO does not produce a sludge. This process does require a bleed stream from the scrubber circulating liquid. Roughly 5 to 10 percent of the incoming sulfur (sulfur dioxide) is oxidized to sulfate and may be discharged as sodium sulfate (dissolved in water), or as dry sodium sulfate. There is well known technology available to treat the problem, but its application will be relatively expensive. Davy Powergas (licensor for the Wellman-Lord Process) has an active research program underway to find methods of minimizing or eliminating the sulfur oxidation. Until actual operating data becomes available, the magnitude and cost of this problem in a high sulfur eastern coal utility is difficult to assess with reliability.
6.0 PARTICULATE REMOVAL EFFICIENCIES
Almost all of the FGD systems report fly ash removal efficiencies in terms of grains/SCF. These data indicate that outlet particulates concentrations of 0.01 to 0.03 grains/SCF are very typical for a wide range of inlet loadings. These outlet concentrations are adequate to meet air quality criteria.
Two important parameters have not, in general, been measured. These are the removal efficiencies as a function of size range, and analysis of the outlet particulates for fly ash and sulfates, if any.
There are two sets of published data on particulates removal efficiencies as a function of size range.
Weir et al. (1974) have recently presented the results of particulate removal analysis at the Southern California Edison plant. (This is a horizontal scrubber designed by Weir, so interpretation of data for vertical scrubbing may be difficult). Figure 11–3 shows the total outlet particulate loading (grains/SCF) as a function of total inlet particulate loading (grains/SCF) for the 170 MW scrubber. The
particulate removal varies from 70 percent at 0.01 grains/SCF inlet to 98 percent at 1.00 grains/SCF inlet.
The particle size in the flue gas at Mohave is usually small—90 cumulative wt. percent less than 4 microns, 70 wt. percent less than 2 microns, 40 wt. percent less than 1 micron and 15 wt. percent less than 0.5 microns. The plant does have an electrostatic precipitator, and these measurements are downstream from it.
Fractional size collection efficiencies for both the pilot plant and the commercial plant are shown below:
|
Particle Size Microns |
% Pilot Plant (.02 GR/SCF inlet) |
% Commercial Unit (.08 GR/SCF inlet) |
|
>1.5 |
97 |
92 |
|
1.0–1.5 |
96 |
91 |
|
0.5–1.0 |
87 |
85 |
|
0.3–0.5 |
75 |
76 |
Epstein et al. (1974) and Bechtel (for EPA) have published particulate removal data for TVA Shawnee operations, for limestone and lime scrubbing. The Bechtel report contains data on overall particulate removal efficiencies for several different scrubbers—venturi and spray scrubbers, TCA scrubber with five grids and a marbel bed scrubber. All removal efficiencies appear to be in the 98–99+ percent range, but the inlet gas particulate loadings are also high.
Figure 11–4 shows inlet and outlet particle size distributions for the TCA scrubber. Figure 11–5 shows the TCA particulate removal efficiency as a function of particle size. (The data are given as percent penetration, which is 100 minus percent removal). As would be expected, the removal efficiency drops rapidly with decreasing particle size, especially at low pressure drops. Because of the limited number of tests (shown on these figures), firm conclusions regarding collection efficiency
should await additional testing to be carried out.
Very few data are available on the fly ash content of the particulates leaving the FGD system. What data are available suggest that the fly ash represents about 60 percent of the total and the remainder (40 percent) comes from entrainment from the slurry liquor. This is apparently calcium and magnesium salts but very little definitive work has been done in this area.
7.0 COSTS OF FLUE GAS DESULFURIZATION SYSTEMS
McGlamery and Torstrick (1974) have presented results of an EPA sponsored cost appraisal of the most advanced FGD systems, using as a base case a new 500 MW unit burning a 3.5 percent sulfur coal. The unit costs were estimated at $45/kw for lime slurry and ranged up to about $80–90/kw for catalytic oxidation. Most utility companies believe that these costs are in error and much too low. In addition these are for new units; retrofit installations will cost more. The results of studies on retrofit plants are discussed later. Also, the costs from McGlamery’s study are based on early 1974 data, and do not include the rapid construction cost escalation experienced in recent months. The major cost factors used are summarized below:
-
Project schedule and location. Project assumed to start in mid-1972 with 3-year construction period ending mid-1975. Midpoint of construction costs mid-1974; Chemical Engineering Cost Index—160.2. Startup—mid-1975. A midwestern plant location is assumed.
-
Power unit size. Costs for three unit sizes—200, 500, and 1000 MW—are projected.
-
Fuel type. Systems for both coal- and oil-fired units are costed—coal 12,000 Btu/lb, 12 percent ash, oil—18,500 Btu/lb, 0.1 percent ash.
-
Sulfur content of fuel. Costs for three sulfur levels are evaluated for each fuel—
-
2.0, 3.5, and 5.0 percent for coal; 1.0, 2.5, and 4.0 percent for oil.
-
Plant status. Although systems for both new and existing power units are evaluated, only a simple, moderately difficult (scrubbing system installed on vacant space beyond the stack) retrofit is estimated since such systems can vary over such a wide range of configurations and restrictions. New units are designed for a 30-year life, 127,500 hours of operation. Costs for new and existing systems are not directly comparable.
Clearly the costs of a new plant ordered today will be higher. However the relative costs, i.e. differences between cases, is probably valid.
Table 11–6 is a summary of the total capital investments as presented by McGlamery. The effects of power plant size and sulfur content of the coal on unit investments are shown in Figures 11–6 and 11–7 respectively. Table 11–7 is McGlamery’s summary of the total operating costs for the same cases as shown on Table 11–6 for capital investments. Again these numbers 2.0–2.5 mills/kwh. are probably too low, because the capital charges (resulting from the low investment costs) will be too low. Also note that investment and operating costs for disposal of fly ash and byproduct credit have been excluded. The effect of excluding product credits from annual operating cost estimates is shown below.
|
Process |
Total Average Annual operating cost, $ |
Annual credit for byproducts, $a |
Net annual operating cost, $ |
|
Limestone |
7,702,700 |
- |
7,702,700 |
|
Lime |
8,101,900 |
- |
8,101,900 |
|
Magnesia |
9,210,800 |
883,200 |
8,327,600 |
|
Sodium |
11,601,500 |
1,077,500 |
10,524,000 |
|
Cat-Ox |
8,873,900 |
659,400 |
8,214,500 |
|
aCorresponds to credit fo $8/ton 100% H2SO4 as 98% H2SO4 for magnesia slurry process; $25/short ton sulfur, $20/ton Na2SO4 for the sodium solution process; and $6/ton 100% H2SO4 as 80% H2SO4 for the catalytic oxidation process. |
|||
TABLE 11–6. Summarya—Total Capital Investment Requirements For Flue Gas Desulfurization Processes (McGlamery and Torstrick 1974
|
Magnesia process |
Sodium process |
Cat-Ox processb |
|||
|
$ |
$/kw |
$ |
$/kw |
$ |
$/kw |
|
14,139,000 |
70.7 |
16,198,000 |
81.0 |
19,537,000 |
97.7 |
|
14,372,000 |
71.9 |
17,149,000 |
85.7 |
17,735,000 |
88.7 |
|
26,026,000 |
52.1 |
31,208,000 |
62.4 |
37,907,000 |
75.8 |
|
22,958,000 |
45.9 |
26,706,000 |
53.4 |
42,520,000 |
85.0 |
|
26,406,000 |
52.8 |
30,491,000 |
61.0 |
42,736,000 |
85.5 |
|
29,355,000 |
58.7 |
33,709,000 |
67.4 |
42,928,000 |
85.9 |
|
38,717,000 |
38.7 |
47,721,000 |
47.7 |
62,913,000 |
62.9 |
|
38,865,000 |
38.9 |
45,832,000 |
45.8 |
69,889,000 |
69.9 |
|
25,568,000 |
51.1 |
29,127,000 |
58.3 |
- |
- |
|
- |
- |
- |
- |
- |
- |
|
32,213,000 |
64.4 |
37,957,000 |
75.9 |
43,816,000 |
87.6 |
|
8,861,000 |
44.3 |
10,324,000 |
51.6 |
13,069,000 |
65.3 |
|
12,695,000 |
25.4 |
15,198,000 |
30.4 |
28,067,000 |
56.1 |
|
16,080,000 |
32.2 |
18,945,000 |
37.9 |
28,277,000 |
56.6 |
|
18,765,000 |
37.5 |
21,893,000 |
43.8 |
28,449,000 |
56.9 |
|
20,376,000 |
40.8 |
24,445,000 |
48.9 |
32,824,000 |
65.6 |
|
23,656,000 |
23.7 |
28,765,000 |
28.8 |
46,356,000 |
46.4 |
|
b All Cat-Ox installations require particulate removal to 0.005 gr/scf prior to entering converter. Because existing units are assumed to already meet EPA standards (0.1 lb particulate/MM BTU of heat input). Only incremental additional precipitator is required. |
|||||
Most utilities and vendors today will estimate FGD costs of $60–100/kw, with the majority believing that the costs will be closer to $100/kw.
The latest investment (December 1974) figure for the FGD system being installed at the Ohio Edison Bruce Mansfield plant is $133/kw. These are two-800 MW coal fired boilers. The breakdown of the costs is given in Table 11–8. Ash removal and disposal costs are included in the $133/kw.
McGlamery has also published cost estimates of “limestone slurry process investment with modified scope,” which is shown on Table 11–9. These costs are intended to reflect possible additional capital charges associated with FGD systems, and include such items as overtime to accelerate the project, replacement of energy consumed by the FGD system, retrofit difficulty etc. The total of these potential charges is about $60/kw. Obviously not all charges are applicable to any given case, but the point is that some of them may be, and the capital charges cited above of $60–100/kw may not include all necessary charges. For example, provisions for redundancy (item D of Table 11–9) may be needed to provide adequate assurance of reliable operation.
A major component (about one-half of the operating costs) is the charge to reheat the stack gases before discharge to the atmosphere. Experimental data now becoming available indicates that addition of magnesium to the scrubber liquor can improve sulfur dioxide removal efficiency substantially (e.g. from 90 to 95 percent). This would allow bypassing a small amount of hot gas to provide at least some of the reheat needed for the scrubbed gas and therefore reduce operating costs.
Further the data available indicates that 5 to 7 percent of a utility plant’s power output will be required by an FGD system. Aside from energy costs, this can have important implications in estimating reserve capacity.
As pointed out in Section 5.01 ponding increases operating costs from 0.15 mills/kwh to over one mill/kwh. Sludge fixation, instead of ponding, will further increase the operting costs
TABLE 11–7. Summarya/—Total Average Annual Operating Costs Flue Gas Desulfurization Processes (McGlamery and Torstrick 1974)
|
Magnesia process |
Sodium process |
Cat-Ox process |
|||
|
Total annual operating cost, $ |
Mills/ kWh |
Total annual operating cost, $ |
Mills/ kWh |
Total annual operating cost, $ |
Mills/ kWh |
|
4,776,800 |
3.41 |
5,971,700 |
4.27 |
4,232,700 |
3.02 |
|
5,091,200 |
3.64 |
7,377,700 |
5.27 |
5,849,400 |
4.18 |
|
9,607,900 |
2.75 |
14,658,000 |
4.19 |
12,399,600 |
3.54 |
|
7,523,400 |
2.15 |
9,101,700 |
2.60 |
8,801,200 |
2.51 |
|
9,210,800 |
2.63 |
11,601,500 |
3.31 |
8,873,900 |
2.54 |
|
10,768,500 |
3.08 |
13,983,300 |
4.00 |
8,940,500 |
2.55 |
|
15,481,900 |
2.21 |
25,118,500 |
3.59 |
21,460,800 |
3.07 |
|
14,347,000 |
2.05 |
18,391,300 |
2.63 |
13,957,600 |
1.99 |
|
8,789,700 |
2.51 |
10,834,300 |
3.10 |
- |
- |
|
- |
- |
- |
- |
- |
- |
|
11,227,300 |
3.21 |
16,389,200 |
4.68 |
13,598,300 |
3.89 |
|
3,204,400 |
2.29 |
4,269,200 |
3.05 |
2,750,100 |
1.96 |
|
4,633,100 |
1.32 |
5,854,700 |
1.67 |
5,743,600 |
1.64 |
|
6,092,700 |
1.74 |
8,305,100 |
2.37 |
5,677,500 |
1.62 |
|
7,393,500 |
2.11 |
10,640,500 |
3.04 |
5,565,100 |
1.59 |
|
7,308,700 |
2.09 |
10,261,600 |
2.93 |
11,126,100 |
3.18 |
|
9,715,900 |
1.59 |
13,686,200 |
1.96 |
8,911,900 |
1.27 |
|
Investment and operating cost for disposal of fly ash excluded. |
|||||
TABLE 11–8
Installation Cost, Bruce Mansfield Plant, Flue Gas Desulfurization System
|
Scrubber System |
|
|
Chemico Contract |
$32,000,000 |
|
Foundations, Electrical, I&C, Linings, Oil Storage, Control Room, Erection |
32,200,000 |
|
Sludge Ponds with Lining |
2,500,000 |
|
Lime Dock, Handling and Storage Facilities |
11,700,000 |
|
Chimney with LIning |
7,600,000 |
|
Subtotal |
$86,000,000 |
|
Distributables |
18,300,000 |
|
Contingency |
3,000,000 |
|
Escalation |
5,000,000 |
|
Engineering |
1,500,000 |
|
Owner’s Cost Including Alleviance for Funds During Construction |
15,100,000 |
|
Total Scrubber System |
$128,500,000 |
|
Off-site Waste Disposal |
|
|
Dravo Contract |
$59,500,000 |
|
Land, Right-of-way, Electrical, Calcilox Harbor, Grits Conveyor, Pipe Rack |
2,800,000 |
|
Subtotal |
$62,300,000 |
|
Contingency |
6,000,000 |
|
Escalation |
5,300,000 |
|
Dravo Engineering |
4,000,000 |
|
Owner’s Costs Including Allowances for Funds During Construction |
6,700,000 |
|
Total Off-site Waste Disposal |
$84,300,000 |
TABLE 11–9
Limestone Slurry Process Investment with Modified Project Scope
|
|
|
Investment, $/kw |
|
BASE INVESTMENT—LIMESTONE SLURRY PROCESS (Including Fly Ash Removal But Not Disposal) |
|
|
|
500-MW new coal-fired unit burning coal with 3.5% S, 12% ash, 90% SO2 removal, 30-year life 127,500 hours operation, onsite solids disposal, proven system, only pumps spared, no bypass ducts, experienced design and construction team, no overtime, 3-year program, 5% per year escalation, mid-1974 cost basis for scaling |
50.30 |
|
|
A. |
Overtime to accelerate project or cover local demand requirements (50% of construction labor requirements) |
3.20 |
|
B. |
Research and development costs for first of a kind process technology (as allowed by FPC accounting practice) |
5.00 |
|
C. |
Power generation capital for lost capacity (normally covered by appropriate operating costs for power used in process) |
4.50 |
|
D. |
Reliability provisions with added redundancy of scrubbers, other equipment, ducts and dampers, instrumentation for change over (assumes no permission to run power plant without meeting SO2 removal emission standards at all times) |
7.00 |
|
E. |
Additional bypass ducts and dampers |
1.00 |
|
F. |
Retrofit difficulty—moderate, space available beyond stack, less than three shutdowns required for tie-ins, field fabrication feasible |
10.00 |
|
G. |
Fly ash pond including closed-loop provisions |
5.50 |
|
H. |
500-ft stack added to project cost |
6.00 |
|
I. |
Air quality monitoring system, 2–15 mile radius, 10 stations |
0.70 |
|
J. |
Cost escalation of 10% per year instead of 5% |
4.80 |
|
K. |
Possible delay of up to 2 years in equipment and material deliveries (1977 completion instead of 1975) |
15.00 |
|
|
Total |
113.00 |
(of a non-regenerable FGD system.) For example, a cost of $4.50 per ton (wet basis) is roughly equal to 0.6 mills/kwh. As a rough guide EPA believes that if the cost of sludge disposal exceeds $4 to $6 per ton (wet basis), a regenerable process which recovers a useful byproduct, will be more economic than a non-regenerable process. (This assumes byproduct credit.)
7.1 Retrofit Flue Gas Desulfurization Systems
Cost estimation for retrofit FGD systems is extremely difficult because of the wide variation in specific details for each power plant. The most recent studies have been completed by Radian Corporation, which extended and complemented a previous study by M.W. Kellogg, and a second study by Kellogg. Radian’s study was completed in December 1973 and was primarily addressed to the utility industry in Ohio. However, the conclusions reached are probably applicable to the eastern U.S. Radian studied retrofitting of lime/limestone processes, the magnesium oxide process and the Wellman-Lord process. They concluded that the space requirements and ease of retrofit should not vary significantly with the process selected (of those considered).
Space required for the scrubbing section of the process is the major concern in retrofit since this equipment must be placed adjacent to the stack. Process equipment outside of the scrubber area is of less concern to the retrofit problem since it can usually be located on the peripheral areas of the plant. In some cases vertical space limitations may cause problems, but this should not occur in most plants where retrofit is possible.
Radian estimated that if both hold tanks and scrubbers are placed adjacent to the stack, a plot area of 45 square feet/MW will be required. If the hold tanks can be placed on the periphery of the powerhouse (or placed directly under the scrubbers), Radian estimates a requirement of 23.8 square feet/MW. This estimate was made for a 550 MW power plant, but the unit space
requirements (sq. ft./MW) are probably fairly independent of power plant size.
Radian then studied in some detail the power plants in the state of Ohio, using as a criterion that any unit with an available area less than 20 square feet/MW can be retrofitted only with great difficulty (if at all). The data on utility plant size and plot availability was taken from the first Kellogg study. A high percentage of large, new boilers have sufficient space for flue gas cleaning systems. More than 87 percent of the surveyed units ten years old or less may be retrofitted. About 85 percent of capacity in existing units larger than 500 MW have sufficient space.
In terms of total capacity, about 70 percent can be retrofitted by including only boilers less than 20 years old or greater than 100 MW capacity. About 74 percent of the total capacity surveyed had available area equal to or greater than 20 square feet/MW. The results of the study are shown graphically in Figure 11–8.
Two fairly detailed cost estimates were made in 1973 comparing costs for new and retrofit FGD. One, done for EPA, by Catalytic Incorporated (Jain 1972) estimated a 30 percent greater price for a retrofit. Radian compared two designs for FGD systems done by the same vendor (Babcock and Wilcox)—Commonwealth Edison’s Will County and Kansas City Power and Light La Cygne Station. After adjusting for size difference (using curves developed by Radian), they concluded that the actual cost increase due to retrofit was 23 percent. McGlamery’s and Torstrick’s (1974) data and estimates suggest that a 25–30 percent cost increase would seem to be typical.
Radian also presented estimates of the incremental cost trends for flue gas cleaning in the State of Ohio. The combination of higher unit investment costs, low load factor and short operating life results in a dramatic increase in “incremental control costs” associated with the degree of retrofit. Incremental costs are those associated with moving from larger new units to smaller old units. Since most of the capacity is less than 20 years old or greater than 100 MW in size, the incremental present cost of flue
gas cleaning remains constant for most units. As smaller, older units are included in further retrofit capacity, incremental control costs escalate rapidly.
Figure 11–9 illustrates this concept. The data was developed for the State of Ohio, but is probably in line with the Eastern U.S., since Ohio boilers account for about 14 percent of the total U.S. sulfur emissions from coal-fired generating capacity.
The second Kellogg study investigated retrofitting a limestone process at eight specific plants. They estimated installed costs of $40 to $100/kw, excluding the cost of the pond ($7 to $12/kw) and the price of the land. The report also states that the overall accuracy of the estimates is 30–35 percent with very little probability of underrun. Further no space scrubbing capacity was installed. Consideration of escalation factors, and the comments above would suggest that the Kellogg estimates would provide a comparable retrofit cost as suggested by McGlamery and Radian, i.e. a 30 percent increase from new plant costs which, in turn are $60–$100/kw with $100/kw being most likely.
8.0 INSTITUTIONAL BARRIERS TO THE APPLICATION OF SULFUR OXIDE CONTROL SYSTEMS
There have been two recent studies discussing institutional barriers to the installation of FGD systems. These have been done by EPA (SOCTAP 1973) and by Radian Corporation. The SOCTAP report is much more detailed than the Radian report but both reach essentially the same conclusions, which are summarized below.
Typically utilities required about a 20 percent reserve capacity within a power region. In 1972 the actual reserves were between 7.8 and 24.3 percent, and about 15 percent on the east coast. Installation of FGD systems requires about 5 to 7 percent of the utility plant output and this will, of course, eventually decrease the reserve capacity.
In existing plants, scrubbers would have to be installed during a shutdown and take into
account the reserves available. It has been estimated that (because of these scheduling difficulties) no more than 10 to 20 percent of the capacity can be retrofitted each year.
Most utilities do not yet feel that they will be directly involved in chemical processing, (either SOx control technology or chemical cleaning of fuels), and their engineering staffs have remained relatively unchanged. There is a general feeling in the utilities that they can ultimately rely on the vendors; yet there is general skepticism of the vendors’ claims at this time. If a decision is made at the management level that the utilities must turn to stack gas scrubbing as an abatement strategy, there will be heavy demand for in-house engineering talent to prepare specifications, review bids, provide liaison during the construction and shakedown phases, and assume responsibility for reliable operation of the scrubbers. That type of manpower will probably be in a very short supply.
Similarly, most utility companies do not have available on their staffs a supply of chemical process engineers. This manpower must be available to provide technical service capabilities and maintenance capabilities which are absolutely essential to keeping FGD systems operating once they are installed.
Currently there are about 15 vendors that have some established expertise in flue gas desulfurization and of these three or four have substantial experience and the capability to expand their services rapidly. This would suggest that the data given in Appendix 11, showing new scrubber installations, provides a reasonable estimate of the possible rate of installation—some 20 or so installations per year. Radian has estimated that vendors can produce 3–5 systems per year each. Radian’s report also states that three specific vendors felt they could only have a combined total of 30 systems on line within three years of the contract award.
ACKNOWLEDGMENTS
The authors of this chapter gratefully acknowledge assistance of many individuals, especially Messrs. Frank Princiotta, Gary Rochelle, Robert Borgwardt, Richard Engdahl, Harvey Rosenberg, Gerald A.Hollinden, and R.P. Van Ness, who gave freely of their time in numerous telephone conversations. Also acknowledged with gratitude is the privilege of seeing while on visits to a number of scrubbing installations, unpublished reports prepared by a team at Battelle Memorial Institute under contract to the Electric Power Research Institute, as well as permission to use the data contained therein.
FOOTNOTES
APPENDIX 11-A
1.0 CONTROL OF EMISSIONS OF SULFURIC ACID VAPOR AND MIST
Why have sulfate particulates in New York City air remained substantially constant over the past decade, while sulfur oxide emissions have decreased more than 85 percent? Any examination of this question ought to consider the hypothesis (Heller 1975) that emissions of sulfuric acid vapor and mist in New York City have declined nowhere nearly in proportion to the decline in sulfur content of the fuel.
The data that it was possible to gather in the time available tend to confirm the hypothesis, and point to need for experimental studies to determine sulfur dioxide emissions from representative space heating equipment in New York City while it burns oil containing 0.3 per cent sulfur, the level presently in use. Measurements would have to extend over a period of time, to recognize the rather wide variations in Sulfur dioxide production that might be expected at different firing rates and with upsets in the firing conditions.
Additional measurements at the higher sulfur levels typical of oils fired 10 years ago would be needed for comparison.
It would appear that emissions of sulfuric acid vapor and mist are typically much less for coal firing than for oil firing, even when coal at high sulfur level is compared with oil at
much lower sulfur level. This raises the question whether or not the incidence of acid rains in Sweden late in the 1950’s (Eriksson 1971) might have not have been associated with the massive conversion from coal to oil that took place in Sweden at about that time. A similar question would be raised concerning the acid rains of the northeastern United States in recent years.
1.01 Production of Sulfur Trioxide during Combustion
The production of sulfur trioxide in a furnace or boiler can vary widely, as data for oil and coal firing, to be presented in Section 4.03 and 4.05, will illustrate. This section will introduce the subject briefly.
Sulfur trioxide can be a marked function of the age of a combustion device, its design, and the method of firing. For a given device, it is a function of sulfur level in the fuel when a fuel of a given type is fired at varying sulfur content. In comparing different combustion devices, the sulfur content of the fuel can be far outweighed in importance as a factor affecting sulfur trioxide production by such things as the degree of excess oxygen and by the method of mixing of fuel with the oxygen (Jackson et al. 1969, Krause 1959).
One mechanism for producing sulfur trioxide operates at the temperature of the flame (Krause 1959, Widersum 1967, Brown 1966, Widell 1953, Williams 1964, Barrett et al. 1966). Sulfur dioxide reacts with atomic oxygen to yield concentrations of sulfur trioxide that paradoxically exceed the thermodynamic equilibrium yield for reaction of sulfur dioxide with elemental oxygen at the temperature of the flame.
The equilibrium conversion of Sulfur dioxide to Sulfur trioxide increases as the temperature in the flue gas drops. For the amounts of excess combustion air that are normally supplied, the equilibrium conversion closely approaches 100 percent at normal stack temperatures. Catalytic conversion becomes
important at lower temperatures, especially, it would appear in the general temperature range between about 1200 degrees and 700 degrees F (Krause 1959, Widersum 1967, Barrett et al. 1966).
1.02 Conversion of Sulfur Trioxide to Sulfuric Acid Vapor
Below about 750 degrees F, sulfur trioxide combines with water vapor in the flue gas to form sulfuric acid vapor (Gundry et al. 1964).
If the flue gas were to be cooled indefinitely, a temperature would be reached at which a mist of sulfuric acid particles would begin to appear in the gas. This temperature is termed the dew-point of the flue gas, and is a function of both the concentration of sulfuric acid vapor in the gas and also the concentration of water vapor. Muller’s relation for dew-point versus sulfur trioxide of flue gas appears to have been confirmed by the best available data and to have general support among workers on the subject (Lisle and Sensenbaugh 1965, Dismukes 1975). Another view (Gimitro and Vermeulen 1964) reflects the complexity of the thermodynamic situation, and there is room for further research.
Small combustion devices, such as equipment for space heating and small industrial boilers, generally operate at stack temperatures far above the dew-point, so that substantially all emissions of sulfur trioxide from small devices are in form of sulfuric acid vapor. This would be true for coal or oil firing.
Large utility boilers fired with oil frequently operate at a stack temperature just above the dew-point. If the temperature were allowed to fall below the dew-point, serious corrosion of heat transfer surface in the air heater would occur. The dew-point is lower if the combustion uses less excess air (see Section 1.04).
Large utility boilers fired with coal are able to operate at lower stack temperatures, without serious air heater corrosion, than are generally possible for oil-fired units, except
for units that use low excess air. The lower stack temperatures possible in coal-fired units at usual amounts of excess air may in part reflect a smaller production of sulfur trioxide, but also, they seem to reflect an uptake of sulfuric acid vapor by adsorption upon fly ash or reaction with alkaline material in fly ash. Emissions of sulfuric acid vapor or mist are generally lower for coal-fired boilers, unless the comparison is with an oil-fired unit at low excess air. Total sulfate emissions, of course, are significantly lower for a coal-fired unit only if an efficient electrostatic precipitator or an efficient scrubber is provided to remove fly ash.
1.03 Levels of Sulfur Trioxide in Flue Gas from Oil Firing
The wide variation in production of sulfur trioxide in oil-fired furnaces can best be appreciated by discussing in turn the several experiments for which data are summarized in Figures App. 11-A1 and 2.
The hour glass symbol ![]() in Figure App. 11-A1 indicates the range of data for firing natural gas spiked with H2S, to simulate fuel at 1, 2.5, and 6 percent sulfur, in a small laboratory combustor (Barrett et al. 1966). The unit had stainless steel walls cooled to 500 F, and the stainless steel surface at this temperature was demonstrated to lack any catalytic virtue for conversion of Sulfur dioxide and sulfur trioxide. The tests were conducted at 15 Percent excess air, and the data represent the production of Sulfur trioxide in the flame itself, unaided by catalytic effects, for the specified experimental conditions.
in Figure App. 11-A1 indicates the range of data for firing natural gas spiked with H2S, to simulate fuel at 1, 2.5, and 6 percent sulfur, in a small laboratory combustor (Barrett et al. 1966). The unit had stainless steel walls cooled to 500 F, and the stainless steel surface at this temperature was demonstrated to lack any catalytic virtue for conversion of Sulfur dioxide and sulfur trioxide. The tests were conducted at 15 Percent excess air, and the data represent the production of Sulfur trioxide in the flame itself, unaided by catalytic effects, for the specified experimental conditions.
The open and closed triangles, Δ and, ▲ Figure App. 11-A1 are data for combustion of oil in a small laboratory furnace with a refractory lining (Crumbley and Fletcher) which undoubtedly contributed some catalytic effect (Barrett et al. 1966). The open triangles are for operation at a furnace-wall temperature of 2300 F. The closed triangles are for 2010 F, illustrating the pronounced effect of firing procedure upon
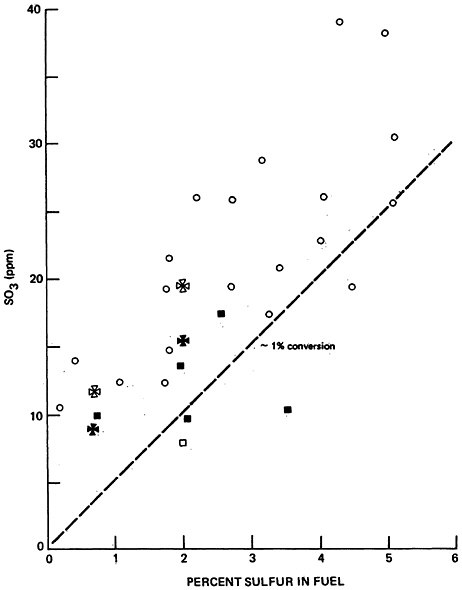
FIGURE App. 11-A2: Sulfur Trioxide Levels in Flue Gas from Oil Combustion in
(a) Laboratory Furnace Tests (the Circles) More Nearly Representative of Practical Small-Scale Combustion Devices, and
(b) Small, Industrial Boilers (the Remaining Points), between 40 and 175 Thousand Pounds per Hour of Steam.
(See text for explanation of test arrangements).
sulfur trioxide production in the same furnace. The series of experiments at 2300 F gave 67 ppm Sulfur trioxide at 3 percent sulfur in oil.
If each of the three carefully done laboratory tests for which data are presented in Figures is considered in isolation, a smooth curve can be drawn through each set of data that includes the origin.
The open circle ○ in Figure App. 11-A2 give data for a small laboratory furnace with a refractory lining (Rendle and Wildsdon 1956). The combustion chamber was stated to be at 1830 F. Each point plotted for this furnace in Figure App. 11-A2 is the average of Sulfur trioxide readings for a sample of gas taken from the combustion chamber and a sample taken from a “cool” exit chamber at about 650 F. The tests used 25 percent excess air. Fuels were a variety of oils, both oils inherently containing sulfur, and oils to which sulfur was added in form of carbon disulfide. The data shown by the circles in Figure App. 11-A2 are probably more nearly representative of the performance of practical small-scale oil-fired combustion devices than the data of the more carefully done laboratory tests given in Figure App. 11-A1.
The closed squares ■ in Figure App. 11-A2 are data for an industrial boiler able to supply about 50,000 lbs/hr of steam (Corbett 1953). Each of the four points at the higher sulfur levels in the oil is an average of 15 to 20 readings for samples taken at several temperature levels in the boiler and at several steaming rates. The point at 0.75 percent sulfur is deduced from an average of dew-point readings for several steaming rates, judging from the average dew-points for the operations at the higher sulfur levels. The excess air ranged upward from about 12 percent.
The open square □ is for a 40,000 lbs/hr industrial boiler burning 2 percent sulfur oil at about 25 percent excess air (personal communications from confidential source 1975).
The open and closed Maltese crosses, ![]() and
and ![]() are for two larger industrial boilers, for 175,000 and 130,000 lbs/hr of steam respectively, at about 10 percent excess air
are for two larger industrial boilers, for 175,000 and 130,000 lbs/hr of steam respectively, at about 10 percent excess air
(Dichl and Luksch 1964). (These are boilers B and C respectively in Figure App. 11-A3).
A feature of the data is that the production of sulfur trioxide remains above about 8 ppm of sulfur trioxide in the flue gas from the lowest sulfur level in oil that was reported.
Although the careful laboratory tests yield data consistent with gentle curves that included the origin, the rougher tests illustrated in Figure 2 App. 11-A2 suggest that a high percentage of the sulfur in a low-sulfur oil, even an oil at 0.3 percent sulfur, can sometimes be converted to sulfur trioxide. Notice that the dashed lines in Figures App. 11-A1 and 2 represent very approximately 1 percent conversion of sulfur dioxide in flue gas to sulfur trioxide.
It is possible to suppose that the situation in respect to space heating equipment and other small furnaces in New York City may be worse, possibly even much worse, than the data of Figure App. 11-A2 might suggest.
An old oil-fired boiler is reported to convert typically 5 percent of the sulfur to sulfur trioxide when using oil at 2 percent sulfur (Weir 1975). After a thorough cleaning, this boiler characteristically does not make much sulfur trioxide for a week or so, but conversion to sulfur trioxide is renewed thereafter. The boiler burned oil containing vanadium. Similar effects of cleaning and time of operation upon sulfur trioxide levels have been reported by others (Crumley and Fletcher), as well as excursions upward in sulfur trioxide production with changes in load (Jackson et al. 1969).
Iron rust is catalytic toward the conversion of sulfur dioxide to sulfur trioxide (Krause 1959, Barrett et al. 1966). Vanadium fly ash has also been shown to be catalytic. Small-scale tests have shown conversions as high as 20 percent of the sulfur in a Venezuelan residual oil when residence time of about 4 seconds was provided in the 600 to 1000 F temperature range (Kapo et al. 1973), and conversions beyond 90 even for oils containing very little vanadium percent were observed at a residence time of about 1 minute (Kapo 1975).
Old combustion equipment in New York City may well be characterized by vanadium fly ash deposits and rust. Space heating equipment generally operates at efficiencies no better than about 70 to 75 percent, so that stack temperature can be expected often to be in a range where catalytic effects can operate. Tall buildings mean tall flues, and, often, a relatively long residence time for conversion to occur.
1.04 Control of Sulfur Trioxide Emissions from Oil Firing
1.041 Low Excess Air
A control measure accessible to the operator of a large utility boiler, and not to the user of small combustion equipment, is the limitation of excess air. Figure App. 11-A3 illustrates the sharp decline in Sulfur trioxide when the excess air is reduced below about 10 percent.
Two curves are shown in Figure App. 11-A3 for boiler A (Jackson et al. 1969). The lower Sulfur trioxide values were for a fairly usual firing arrangement. The upper values were observed when special means were introduced to obtain intimate mixing of oil and air, in order to acheive “better” combustion conditions. A sister boiler produced over 100 ppm Sulfur trioxide at 1.5 percent oxygen in flue gas (Jackson et al. 1969).
Two sets of curves are given in Figure App. 11-A3 for boilers B and C (Diehl and Luksch 1964). The decrease in sulfur trioxide when 0.72 percent sulfur oil was substitutes for oil at 2.05 percent sulfur was not in proportion to the sulfur level in the two oils. As before, in connection with Figure App. 11-A2, one is led to the conclusion that sulfur trioxide emissions fron the firing of oil at 0.3 percent sulfur will not be negligibly small at the levels of excess air usual in small combustion equipment.
Boiler D had a “standard” firing arrangement (Jackson et al. 1969). The low emissions of sulfur trioxide from this boiler, as well as the
comparison of emissions from boiler A with “standard” and “improved” combustion, lead to the impression that sulfuric acid vapor emissions might be reduced by appropriate adjustments, as yet not well understood, in the firing practice.
That emissions from large utility boilers can be kept small be proper firing at low levels of excess air is illustrated by the following data (personal communication from a confidential source 1975):
|
Boiler Size |
% S in Oil |
% O2 in Flue Gas |
ppm SO3 |
|
100-MW |
2.5% |
1.6% |
2 |
|
|
|
2.5% |
3 |
|
|
1.5% |
1.7% |
0.8 |
|
|
|
2.3% |
1.7 |
|
200-MW |
0.8% |
1.5% |
2.6 |
|
400-MW |
0.9% |
2.5% |
3.5 |
The first four sets of data were from tests on the same 100-MW boiler.
It should be appreciated that operation at low excess air requires extreme care and good control procedures. There is risk that failure of the air rate to track changes in fuel rate will lead to a fuel-rich mixture and development of an explosive gas mixture in the boiler. The difficulties become serious below about 5 percent excess air, and routine operation at 3 percent excess air would demand the utmost caution.
1.042 Scrubbing
A 10-MW horizonal scrubber (see Chapter 11, Section 2.12) on an old oil-fired boiler sometimes receives sulfuric acid mist at 50 ppm when oil at 2 percent sulfur is used. The efficiency of removal is typically 50 percent (Weir 1975), although the efficiency has been varied by changes in scrubber operating parameters. Presumably higher removal efficiencies can be acheived by operating the scrubber at greater drop in pressure in the flue gas being treated.
If the primary object of a scrubber on an oil-fired boiler is to achieve a plume of low opacity, rather than to remove sulfur dioxide, then scrubbing may be more difficult for oil combustion than for coal. The particle size of ash resulting from oil firing is typically 0.1 tp 0.3 micron, much smaller than the fly ash from coal combustion. As the gas is cooled for scrubbing, the oil ash serves as condensation nuclei for sulfuric acid vapor (Weir 1975). A removal efficiency for the oil ash beyond about 50 percent may be difficult to acheive.
1.043 Electrostatic Precipitation
Tests by Consolidated Edison Company of New York (Ramsdell 1975) have shown that electrostatic precipitators working at about 300 F on flue gas from firing of oil at 1 percent sulfur can provide a collection efficiency of about 50 percent. The collected material comprised oil ash particles, typically 0.1 to 0.3 micron in size, on which some sulfuric acid vapor had condensed. The amount of sulfuric acid removed in this way is not known.
Operation of the precipitator at this temperature level is difficult, and Con Edison does not normally keep the precipitators in service. The difficulty arises from the hygroscopic character of the ash particles collected and their tendency to form a gummy mass.
It may be noted that Con Edison’s precipitators are unusually large, having been increased in size during the latter 1960’s to afford 99+percent collection efficiency for fly ash from combustion of low-sulfur coal.
One of Con Edison’s precipitators, on its Ravenswood No. 3 boiler, was installed to work at about 700 F. It afforded test efficiencies between 99.2 and 99.6 percent when working some years ago on coal containing 1 percent sulfur, the results averaging at 99.4 percent.
A test of this precipitator on ash from oil of 1 percent sulfur gave an efficiency of 75 percent. A fire in an ash hopper has temporarily put this precipitator out of
service, and illustrates another difficulty in the handling of oil ash.
A precipitator operating at 700 F does not prevent sulfuric acid vapor from reaching the environment, for either coal or oil firing.
1.044 Additives
For many years, Long Island Lighting Co. has injected finely divided magnesium oxide into boilers fired with high-sulfur Venezuelan residual oil. The effect is to suppress sulfur trioxide emissions and to capture vanadium in form of magnesium vanadate. The Company enjoys a considerable revenue from sale of the vanadium-rich fly ash, and indeed, supplies a significant fraction of the nation’s requirement for vanadium.
1.045 Control of Small Combustion Equipment
Control of sulfuric acid vapor emissions from small oil-fired combustion devices may not be easy. In any case, measurements are needed before too much thought is given to control procedures and appropriate levels of control.
It is possible that a good measure of control may be obtained simply by subjecting such devices and their flues to a thorough cleaning.
It might be added that space heating equipment accounted for about 57 percent of sulfur oxide emissions in 1973 in New York City. Electricity generation accounted for about 37 percent.
1.05 Control of Sulfate Particulate Emissions from Coal Firing
1.051 Precipitation
A summary of data for a large number of boilers (Rosohl 1956) suggests that dew-points for flue gases from coal firing are generally
far below those of oil firing at usual levels of excess air.
The differences may arise not so much from a lower production of Sulfur diovide in a coal flame as from a tendency for the vapor pressure of sulfuric acid to be considerably lower in the presence of fly ash. Data suggesting such a tendency have been reported for a boiler fitted with an electrostatic precipitator working at about 95 percent efficiency (Archbold 1961). The level of sulfur trioxide at 750 to 700 F, just ahead of the air heater, was 22.9 ppm. The temperature at the outlet of the air heater was about 280 to 285 F, and the sulfur trioxide level was 7.1 ppm, far below the equilibrium content of sulfuric acid vapor in coal combustion gas at 280 to 285 F. The gas entered the precipitator at 7.1 ppm of Sulfor trioxide. The outlet of the precipitator showed 2.0 ppm, suggesting that the uptake of Sulfur trioxide by fly ash, whether by adsorption or by chemical reaction with alkalinity in the ash, had continued during the approximately 1.6 seconds of gas residence in the precipitator. It does not seem probable that the sulfur trioxide level would have been reduced much further if the gas residence had been longer, as is characteristic of modern precipitators working at 99+percent efficiency.
Tests on at least one coal-fired power station showed fly ash collected by a precipitator at 95 percent efficiency had a pH of 7 or even a bit above, while the fines passing through this precipitator has a pH of 2.5 to 3 (Grob 1961). This observation is consistent, it might be noted, with a finding that the greatest accumulation of sulfur in California urban aerosels is on particles in the 0.1 to 0.65 micron range of size (Flocchini et al. 1974).
These facts suggest that fly ash particles act as condensation nuclei for sulfuric acid vapor in flue gas from coal combustion. Inherently, it would appear that the content of sulfuric acid vapor in coal flue gas from a utility boiler operating at high efficiency is usually small. It is plausible to suppose that the vapor condenses more or less uniformly over
all of the particulate surface that is available, so that smaller particles will take up more of the acid vapor, unless alkalinity in the ash directs acid to the larger particles.
This view is consistent with the fact that fly ash from a coal of low sulfur content is difficult to collect by electrostatic precipitation of around 300 F, but can be made more collectible by addition of sulfur trioxide to the flue gas. If done properly, the conditioning of fly ash by addition of sulfur trioxide does not lead to significant quantities of sulfuric acid vapor in the stack effluent (personal communication from two confidential sources 1975).
It would appear that an electrostatic precipitator working at high efficiency should prevent most sulfate particulate matter, as well as sulfuric acid vapor, from reaching the atmosphere. Of course, there seems to be general agreement that larger particles of such matter are not injurious to health, and a chemical examination of the ultra-fine particles that escape the precipitator would seem to be in order.
1.052 Scrubbing
The level of sulfuric acid mist typically reaching the 10-MW limestone scrubber at Shawnee Station (see Chapter Section 3.04) is about 8 to 10 ppm (Moore 1975). This is a representative level for T.V.A.’s coal-fired stations, that generally produce a flue gas containing between about 5 and 10 ppm of sulfur trioxide. Although the 10-MW limestone scrubber displays excellent efficiency for removing fly ash, the efficiency for removing sulfuric acid mist is poor (Princiotta 1975), the effluent usually containing about 5 ppm of sulfuric acid mist (Moore 1975).
1.06 Conclusion in Respect to Emissions of Sulfuric Acid Vapor
The hypothesis (Heller 1975) that sulfate particulate levels in the air of New York City and other urban communities have remained high because of significant emissions of sulfuric acid vapor even from the firing of oil at 0.3 percent sulfur is sufficiently credible to warrant an experimental test.
Measurements are needed to compare emissions today and ten years ago from typical oil-fired devices, especially small furnaces and space heating equipment. The tests should reflect typical operating practices.
The evidence that it has been possible to find in the time available supports a hypothesis that emissions of sulfuric acid vapor have not declined in New York City in proportion to decline in sulfur content of fuel. It might be further hypothesized that the limiting factor that has determined the level of sulfate particulate matter in New York City has been the surface area of the total particulate matter that is available for adsorption of sulfuric acid vapor. Under the latter hypothesis, contours of sulfate particulate matter and of the acidity of rainfall around New York City might be expected to reveal the City as a source of sulfuric acid vapor that was nor adsorbed by particulates within the city. Such contours would also, of course, shed a sharp light upon the hypothesis that sulfate particulate matter in New York City has originated from sulfur dioxide emitted by power stations at a distance, this sulfur dioxide having been converted to sulfate as it moved toward the city. In other words, is New York City a “hot spot”, or does its atmosphere represent a region extending westward, say, to Ohio?
TABLE App. 11–1
Summary of Electric Utility Flue Gas Desulfurization Facilities in the United States
|
MW Capacity (No. of Plants) |
||||
|
PROCESS |
CURRENTLY INSTALLED(1) |
UNDER CONSTRUCTION |
PLANNED |
TOTALS |
|
Limestone Scrubbing |
1,904(8) |
2,950(7) |
8,897(20) |
13,351(35) |
|
Lime Scrubbing |
715(4) |
2,944(6) |
4,651(17) |
8,310(27) |
|
Limestone or Lime |
30(1) |
650(1) |
6,100(10) |
6,780(12) |
|
Magnesium Oxide Scrubbing |
370(3) |
— |
576(2) |
946(5) |
|
Catalytic Oxidation |
110(1) |
— |
— |
110(1) |
|
Wellman-Lord |
— |
115(1) |
715(2) |
830(3) |
|
Aqueous Sodium Base Scrubbing |
250(2) |
125(1) |
125(1) |
500(4) |
|
Double Alkali |
32(1) |
20(1) |
— |
52(2) |
|
Process Not Selected |
— |
— |
6,140(11) |
6,140(11) |
|
TOTAL |
3,411(20) |
6,404(17) |
27,204(63) |
31,019(100) |
|
(1) Not necesaarily in operation. Some are plants which have recently been shut down. |
||||
TABLE App. 11–2
Summary of Installed Electric Utility Flue Gas Desulfurization Systems in the United States
|
FGD PROCESS/POWER STATION |
NEW OR RETROFIT |
SIZE MW |
FUEL |
||
|
TYPE |
% ASH |
% S |
|||
|
LIMESTONE SCRUBBING |
|
||||
|
Arizona Public Service Cholla No. 1 |
R |
115 |
Coal |
5–15 |
0.4–1.0 |
|
City of Key West |
N |
37 |
Oil |
- |
2.75 |
|
Commonwealth Edison Will County No. 1 |
R |
167 |
Coal |
10 |
2.0(1) |
|
Kansas City Power & Light |
|
||||
|
La Cygne No. 1 |
N |
820 |
Coal |
10–20 |
4.0 |
|
Hawthorne 3 & 4 |
R |
240 |
Coal |
- |
0.6–3.0 |
|
Lawrence 4 & 5 |
R/N |
525 |
Coal |
- |
3.5 |
|
LIME SCRUBBING |
|
||||
|
DuQuesne Light Phillips |
R |
410 |
Coal |
10 |
2.3 |
|
Louisville Gas & Electric Paddy’s Run No. 6 |
R |
65 |
Coal |
14 |
3.7 |
|
Southern California Edison Mohave No. 2 |
R |
160 |
Coal |
|
0.5–0.8 |
|
LIME/LIMESTONE SCRUBBING |
|
||||
|
TVA Shawnee No. 10(2) |
R |
30 |
Coal |
12–15 |
3.0 |
|
MAGNESIUM OXIDE SCRUBBING |
|
||||
|
Boston Edison Mystic No. 6 |
R |
150 |
Oil |
- |
2.8 |
|
Potomac Electric, Dickerson 3 |
R |
95 |
Coal |
10–20 |
2.0 |
TABLE App. 11–3
Flue Gas Desulfurization Systems Under Construction in the United States
|
Size, MW |
New or Retrofit |
Fuel |
Process |
Contractor |
Location |
|
|
|
%S |
Type |
|
|||
|
250 |
N |
0.44 |
Coal |
Limestone |
Research Cottrell |
Arizona Public Service Cholla No. 2 |
|
100 |
N |
2.5–3 |
Coal |
Limestone |
Riley Stoker |
Central Illinois Light Co., Duck Creek No. 1 |
|
375 |
N |
|
Coal |
Lime |
UOP |
Columbus and Southern Ohio, Conesville 5 |
|
375 |
N |
|
Coal |
Lime |
UOP |
Conesville 6 |
|
180 |
R |
3.7 |
Coal |
Limestone |
Peabody |
Detroit Edison St. Clair No. 6 |
|
510 |
R |
|
Coal |
Lime |
Chemico |
Duquesne Light Elrama |
|
64 |
R |
3.8 |
Coal |
Lime |
American Air Filter |
Kentucky Utilities Green River 1, 2, 3 |
|
178 |
R |
3.5–4.0 |
Coal |
Lime |
American Air Filter |
Louisville Gas & Elect. Cane Run 4 |
|
425 |
R |
3.5–4.0 |
Coal |
Lime |
American Air Filter |
Mill Creek 3 |
|
360 |
N |
0.8 |
Coal |
Lime |
C.E.A. |
Montana Power Colstrip 1 |
|
360 |
N |
0.8 |
Coal |
Lime |
C.E.A. |
Colstrip 2 |
|
125 |
R |
0.5–1.0 |
Coal |
Sodium Carbonate |
C.E.A. |
Nevada Power Reid Gardner 3 |
|
115 |
R |
3.2–3.5 |
Coal |
Wellman Lord |
Davy Powergas |
NIPSCO Mitchell 11 |
|
Size, MW |
New or Retrofit |
Fuel |
Process |
Contractor |
Location |
|
|
|
%S |
Type |
|
|||
|
680 |
N |
1.0 |
Coal |
Limestone |
Combustion Engineering |
Northern States Power Sherburne 1 |
|
680 |
N |
1.0 |
Coal |
Limestone |
Combustion Engineering |
Sherburne 2 |
|
880 |
N |
4.3 |
Coal |
Lime |
Chemico |
Pennsylvania Power Co. Bruce Mansfield 1 |
|
800 |
N |
4.3 |
Coal |
Lime |
Chemico |
Bruce Mansfield 2 |
|
120 |
R |
2.5 |
Coal |
Magnesium Oxide |
United Engineering |
Philadelphia Electric Eddystone 1 (now in startup phase) |
|
160 |
R |
0.5–0.8 |
Coal |
Limestone |
UOP |
Southern Calif. Edison Mohave 1 |
|
345 |
N |
0.5 |
Coal |
Lime/Limestone |
Combustion Engineering |
Southwest Public Serv. Harrington 1 |
|
200 |
N |
|
Coal |
Limestone |
UOP |
Springfield Utility Bd. Southwest 1 |
|
550 |
R |
3.7 |
Coal |
Limestone |
TVA |
TVA Widows Creek 8 |
|
793 |
N |
0.4 |
Coal |
Limestone |
Research Cottrell |
Texas Utilities Martin Lake 1 |
|
793 |
N |
0.4 |
Coal |
Limestone |
Research Cottrell |
Martin Lake 2 |
|
650 |
N |
1.5 |
Coal |
Lime/Limestone |
Combustion Engineering |
Pub. Serv. Co. of Ind. (Gibson #2) |
LITERATURE CITED
Archbold, M.J. (1961) Combustion, May, pp. 22–32.
Barrett, R.E., J.D.Hummell, and W.T.Reid (1966) Trans. ASME, J. Eng. Power, vol. 88, Series A, no. 2, April, pp. 165–172.
Bell, B.A., T.A.LiPuma, and K.Allison Lime/Limestone Scrubbing in a Pilot Dustraxtor, EPA Report 65012–74–077.
Borgwardt, Robert H. (1974) EPA/RTP pilot studies related to unsaturated operation of lime and limestone scrubbers, paper presented at EPA symposium on flue gas desulfurization, Atlanta, Georgia, November 4–7.
Borgwardt, Robert H. (1975) Environmental Protection Agency, Research Triangle Park, North Carolina, personal communication, February.
Brown, T.D. (1966) Combustion, April, pp. 40–45.
Corbett, P.F. (1953) J. Inst. Fuel, vol. 26, pp. 92–106.
Crumley, P.H. and A.W.Fletcher J. Inst. Fuel, vol. 29, pp. 322–327.
Diehl, H. and F.Luksch (1964) Mitteilungen der Vereinigung der Grosskesselbesitzer, No. 92, October, pp. 366–373.
Dismukes, E.B. (1975) Southern Research Institute, Birmingham, Alabama, personal communication, February 1975.
Epstein, M., L.Sybert, S.C.Wang, and C.C.Leivo. EPA Alkali Scrubbing Test Facility: Limestone Wet Scrubbing Test Results, EPA-650/2–74–010.
Epstein, M., L.Sybert, S.C.Wang, C.C.Leivo, and R.G.Rhudy (1974) Limestone and lime test results at the EPA alkali scrubbing test facility at the TVA Shawnee power plant, paper presented at EPA Symposium on Flue Gas Desulfurization, Atlanta, Georgia, November 4–7.
Eriksson, Erik (1971) The Fate of Sulfur Dioxide and NOx in the Atmosphere, chapter in Power Generation and Environmental Change, David A.Berkowitz and Arthur M.Squires, editors. The MIT Press, Cambridge, Massachusetts, pp. 289–301.
Flocchini, R.G., T.A.Cahill, D.J.Shadoan, S.Lange, R.A.Eldred, P.J.Feeney, G.Wolfe, D.Simmeroth, and J.Suder (1974) Monitoring California’s Aerosols by size and Elemental Composition, Part I: Analytical Techniques, paper submitted to Environmental Science and Technology.
Gimitro, J.I. and T.Vermeulen (1964) AIChE Journal, vol. 10, pp. 740–746.
Grob, John J. (1961) Consolidated Edison company of New York, personal communication, January.
Gundry, J.T.S., B.Lees, L.K.Rendle, and E.J.Wicks (1964) Combustion, October, pp. 39–47.
Heller, Austin (1975) State of New York Council of Environmental Advisers, personal communication, February.
Hesketh, H.E. (1974) Sulfur Dioxide Scrubbing Technology, testimony presented before Colorado Air Pollution Control Commission, November 19.
Hollinden, Gerald A. (1974). Chemistry of lime/limestone scrubbing liquor from power plant stack gases, paper presented at 35th annual meeting of International Water Conference, Pittsburgh, Pennsylvania, October 30–November 1.
Hollinden, Gerald A. (1975) Tennessee Valley Authority, Chattanooga, Tennessee, personal communication, January.
Jackson, P.J., W.E.Langdon, and P.J.Reynolds (1969) Automatic Continuous Measurement of Sulfur Trioxide in Flue Gases, American
Jain, L.K. (1972) Preliminary Problem Definition SO2 Control Process Utilization, EPA Contract 68–02–0241. Catalytic Inc., Charlotte, N.C.
Kapo, G., L.Gomez, F.Pena, E.Torres, J. Bilbao, and K.Mazeika (1973) The Vanox Process for Stack Desulfurization and Vanadium Recovery, paper presented at International Symposium on Vanadium and Other Metals in Petroleum, University of Zulia, Maracaibo, Venezuela, August 19–22.
Kapo, George (1975) Caracas, Venezuela, personal communication, February.
Kellogg, M.W., Applicability of Sulfur Dioxide Control Processes to Power Plants, EPA R2–72–100.
Kellogg, M.W., Evaluation of the Controllability of power Plants Having a Significant Impact on Air Quality Standards, EPA 450/3–74–002.
Krause, H.H. (1959) Oxides of Sulfur in Boilers and Gas Turbines, chapter in Corrosion and Deposits in Boilers and Gas Turbines, report of ASME Research Committee on Corrosion and Deposits from Combustion Gases, prepared by Battelle Memorial Institute, ASME, New York, pp. 44–77.
Lisle, E.S. and J.D.Sensenbaugh (1965) Combustion, pp. 12–16.
Martin, J.R., A.L.Plumley, and B.M.Minor (1974) The C.E. Lime Wet Scrubbing Process from Concept to Commercial Operation, paper presented at National Coal Association Symposium on Coal and the Environment, Louisville, Kentucky, October 23.
McGlamery, C.G. and R.L.Torstrick (1974) Cost Comparisons of Flue Gas Desulfurization Systems, paper presented at FGD Symposium, Atlanta, Georgia, November.
Moore, Neal (1975) Tennessee Valley Authority, Chattanooga, Tennessee, personal communication, February.
National Research Council (1970) Ad Hoc Panel on Control of Sulfur Dioxide from Stationary Combustion Sources, Committee on Air Quality Management, Committees on Pollution Abatement and Control, Division of Engineering, Abatement of sulfur oxide emissions from stationary combustion sources, COPAC-2, PB 192887, Washington, D.C.
Princiotta, Frank T. (1975) Environmental Protection Agency, Washington, D.C., personal communication, January.
Radian Corporation, Factors Affecting Ability to Retrofit Flue Gas Desulfurization Systems, EPA-450/3–74–015; NTIS PB-232376.
Ramsdell, Roger (1975) Consolidated Edison Company of New York, personal communication, February.
Rendle, L.K. and R.D.Wildsdon (1956) J. Inst. Fuel, vol. 29, pp. 372–380.
Rochelle, Gary (1975) Department of Chemical Engineering, University of California at Berkeley, personal communication, January.
Rosohl, O. (1956) Mitteilungen VIK. (Vereinigung Industrielle Kraftwirtschaft) Essen, No. 4, pp. 53–61; see Krause (1959), p. 66.
Ruch, R.R., H.J.Gluskoter, and N.F.Shimp (1974) Occurrence and distribution of potentially volatile trace elements in coal: a final report, Environmental Geology Notes, No. 72, Illinois State Geological Survey, Urbana, Illinois, August.
Selmeczi, J.G. and H.A.Elnagger, Properties and Stabilization of Sulfur Dioxide Scrubbing Sludges, paper presented at Coal and the Environment Meeting—National Coal Association, October 22–24, Louisville, Kentucky.
Simon, Jack (1975) Illinois State Geological Survey, Urbana, Illinois, personal communication, January.
Society of Mechanical Engineers Paper 69-WA/APC-2 (ASME Winter Annual Meeting, November, Los Angeles, California).
SOCTAP (Sulfur Oxide Control Technology Assessment Panel) (1973) Projected Utilization of Stack Gas Cleaning Systems by Steam-Electric Plants, Final Report, April.
Van Mersbergen, Ronald (1972) testimony for Illinois Pollution Control Board, Chicago, Illinois, January 5.
Walsh, Robert T. (1971) Chief of Source Control Branch, EPA, testimony for West Virginia Air Pollution Control Commission, Charleston, West Virginia, December 15.
Walsh, Robert T. (1972) testimony for Illinois Pollution Control Board, Chicago, Illinois, January 26.
Weir, A., J.M.Johnson, D.G.Jones, and S.T.Carlisle (1974) The Horizontal Cross Flow Scrubber, paper presented at FGD Symposium, Atlanta, Georgia, November 4.
Weir, Alexander, Jr. (1975) Southern California Edison Co., Rosemead, California, personal communication, February.
Widell, Torsten (1953) Combustion, June, pp. 53–55.
Widersum, G.C. (1967) Corrosion and Deposits from Combustion Gases—A Review, American Society of Mechanical Engineers Paper 67-PWR-8 (ASME-IEEE Joint Power Generation Conference, Detroit, Michigan, September.
Williams, D.J. (1964) Oxidation of Sulfur Dioxide in Combustion Processes, Coal Research in CSIRO, No. 23, July, pp. 7–14