|
Summary of Findings: Reducing Maternal Mortality and Morbidity in Developing Countries
|
2
Reducing Maternal Mortality and Morbidity
According to recent estimates, each year more than 500,000 women between the ages of 15 and 49 die of causes related to pregnancy and childbirth—a leading cause of death among women in that age group (Hill et al., 2001; World Health Organization, 1999; Murray and Lopez, 1997; Weil and Fernandez, 1999). Almost all maternal deaths (99 percent) occur in the developing world (World Health Organization and United Nations Children’s Fund, 1996; AbouZahr et al., 1996), and more than half occur in Africa (Hill et al., 2001). The vast majority of these deaths are preventable. Researchers also estimate that more than 40 percent of pregnant women experience obstetric disorders that are not immediately fatal (Weil and Fernandez, 1999). Approximately 15 percent of all births are complicated by a potentially fatal condition that requires emergency care (World Health Organization, 1999).
When mothers are malnourished or ill, or when they receive inadequate maternity care, their children also face high risks of disease and death (Tinker, 2000). Tinker (1997) estimates that 30 to 40 percent of infant deaths (1.5-2.5 million) could be averted by maternal interventions alone. This burden of death and illness is borne not only by women and their children, but also by the families and communities that depend upon them (Royston and Armstrong, 1989). For women of child-bearing age (15-44), maternal disorders are the leading causes of death, accounting for almost 16 percent of deaths in this age group (Murray and Lopez, 1997).
According to the International Classification of Diseases (ICD)-10 definition, maternal death is “the death of a woman while pregnant or within
42 days of termination of pregnancy, irrespective of the duration and site of the pregnancy, from any cause related to or aggravated by the pregnancy or its management but not from accidental or incidental causes” (World Health Organization, 1999). The most frequently cited measure of maternal mortality, known as the maternal mortality ratio (sometimes mistakenly referred to as a “rate”), is the number of maternal deaths in a population that occur during a given year per 100,000 live births. This number, which represents the risk associated with a single pregnancy, differs by a factor of more than 100 between the highest- and lowest-mortality settings and varies widely among developing countries (see Tables 2-1a and 2-1b) (World Health Organization, United Nations Children’s Fund, United Nations Population Fund, 2001).
Another useful measure of maternal mortality is lifetime risk—the odds that a woman in a given population will die as a result of pregnancy. In Eastern Africa, as many as 1 woman in 11 dies of pregnancy-related causes, as compared with as few as 1 in 4000 in Western Europe and 1 in 3,500 in North America (World Health Organization, United Nations Children’s Fund, United Nations Population Fund, 2001). Table 2-1a lists regional and global estimates of the maternal mortality ratio, total annual maternal deaths, and lifetime risk of maternal death. It is important to note that these numbers represent crude estimates at best, since in the regions where the problem of maternal mortality is most acute, it is least likely to be measured accurately (World Health Organization, United Nations Children’s Fund, United Nations Population Fund, 2001; World Health Organization, 1999). The same caveats apply to estimates of maternal morbidity, which has been reported to occur in up to 30 women for every 1 woman who dies from maternal conditions (Donnay, 2000). In the developing world, one in four women suffers from acute or chronic disability related to pregnancy (Donnay, 2000; World Bank, 1999). Surveillance of maternal mortality, along with other pregnancy and birth outcomes, is discussed in detail in Chapter 5.
CAUSES OF MATERNAL MORBIDITY AND MORTALITY
The five most important direct causes of maternal mortality in developing countries are hemorrhage, sepsis, unsafe abortion, eclampsia, and obstructed labor (Figure 2-1). Together these causes account for more than two-thirds of maternal mortality in the world. Indirect causes of maternal death, which are responsible for approximately 20 percent of maternal mortality worldwide, include preexisting conditions such as malaria and viral hepatitis that are exacerbated by pregnancy or its management (World Health Organization, 1999).
TABLE 2-1a Maternal Mortality Ratio, Maternal Deaths, and Lifetime Risk of Maternal Death: World and Regional Estimates
(UNICEF classification of countries used [see Table 2-1b].)
|
|
Maternal Mortality Ratio1 |
Maternal Deaths Annually |
Lifetime Risk of Maternal Death 1 in: |
|
World Total |
400 |
515,000 |
75 |
|
More Developed Countries |
21 |
2,800 |
2,500 |
|
Less Developed Countries |
440 |
512,000 |
60 |
|
Least Developed Countries2 |
1,000 |
230,000 |
16 |
|
Africa |
1,000 |
273,000 |
16 |
|
Eastern |
1,300 |
122,000 |
11 |
|
Middle |
1,000 |
39,000 |
13 |
|
Northern |
450 |
20,000 |
49 |
|
Southern |
360 |
4,500 |
65 |
|
Western |
1,100 |
87,000 |
13 |
|
Asia* |
280 |
217,000 |
110 |
|
Eastern |
55 |
13,000 |
840 |
|
South-central |
410 |
158,000 |
55 |
|
Southeastern |
300 |
35,000 |
95 |
|
Western |
230 |
11,000 |
95 |
|
Europe |
28 |
2,200 |
2,000 |
|
Eastern |
50 |
1,600 |
1,100 |
|
Northern |
12 |
140 |
3,900 |
|
Southern |
12 |
170 |
5,000 |
|
Western |
14 |
280 |
4,000 |
|
Latin America and Caribbean |
190 |
22,000 |
160 |
|
Caribbean |
400 |
3,100 |
85 |
|
Central America |
110 |
3,800 |
240 |
|
South America |
200 |
15,000 |
150 |
|
Northern America |
11 |
490 |
3,500 |
|
Oceania* |
260 |
560 |
260 |
|
Australia and New Zealand |
8 |
25 |
5,500 |
|
Melanesia |
310 |
560 |
60 |
|
Micronesia |
— |
— |
— |
|
Polynesia |
33 |
5 |
700 |
|
1Maternal deaths per 100,000 live births. 2Subset of less developed countries. *Australia/New Zealand and Japan have been excluded from the regional totals but are included in the total for developed countries. SOURCE: World Health Organization, United Nations Children’s Fund, United Nations Population Fund, 2001. |
|||
TABLE 2-1b Countries Grouped by UNICEF Regions
|
Industrialized countries Andorra; Australia; Austria; Belgium; Canada; Denmark; Finland; France; Germany; Greece; Holy See; Iceland; Ireland; Israel; Italy; Japan; Liechtenstein; Luxembourg; Malta; Monaco; Netherlands; New Zealand; Norway; Portugal; San Marino; Slovenia; Spain; Sweden; Switzerland; United Kingdom; United States of America. |
|
Developing countries Afghanistan; Algeria; Angola; Antigua and Barbuda; Argentina; Armenia; Azerbaijan; Bahamas; Bahrain; Bangladesh; Barbados; Belize; Benin; Bhutan; Bolivia; Botswana; Brazil; Brunei Darussalam; Burkina Faso; Burundi; Cambodia; Cameroon; Cape Verde, Central African Republic; Chad; Chile; China; Colombia; Comoros; Congo; Congo, Democratic Republic of; Cook Islands; Costa Rica; Côte d’Ivoire; Cuba; Cyprus; Djibouti; Dominica; Dominican Republic; Ecuador; Egypt; El Salvador; Equatorial Guinea; Eritrea; Ethiopia; Fiji; Gabon; Gambia; Georgia; Ghana; Grenada; Guatemala; Guinea; Guinea-Bissau; Guyana; Haiti; Honduras; India; Indonesia; Iran; Iraq; Israel; Jamaica; Jordan; Kazakhstan; Kenya; Kiribati; Korea, Democratic People’s Republic; Korea, Republic of; Kuwait; Kyrgyzstan; Lao People’s Democratic Republic; Lebanon; Lesotho; Liberia; Libyan Arab Jamahiriya; Madagascar; Malawi; Malaysia; Maldives; Mali; Marshall Islands; Mauritania; Mauritius; Mexico; Micronesia, Federated States of; Mongolia; Morocco; Mozambique; Myanmar; Namibia; Nauru; Nepal; Nicaragua; Niger; Nigeria; Niue; Oman; Pakistan; Palau; Panama; Papua New Guinea; Paraguay; Peru; Philippines; Qatar; Rwanda; Saint Kitts and Nevis; Saint Lucia; Saint Vincent/ Grenadines; Samoa; Sao Tome and Principe; Saudi Arabia; Senegal; Seychelles; Sierra Leone; Singapore; Solomon Islands; Somalia; South Africa; Sri Lanka; Sudan; Suriname; Swaziland; Syria; Tajikistan; Thailand; Togo; Tonga; Trinidad and Tobago; Tunisia; Turkey; Turkmenistan; Tuvalu; Uganda; United Arab Emirates; United Republic of Tanzania; Uruguay; Uzbekistan; Vanuatu; Venezuela; Viet Nam; Yemen; Zambia; Zimbabwe. |
|
Least developed countries Afghanistan; Angola; Bangladesh; Benin; Bhutan; Burkina Faso; Burundi; Cambodia; Cape Verde; Central African Republic; Chad; Comoros; Congo, Democratic Republic of; Djibouti; Equatorial Guinea; Eritrea; Ethiopia; Gambia; Guinea; Guinea-Bissau; Haiti; Kiribati; Lao People’s Democratic Republic; Lesotho; Liberia; Madagascar; Malawi; Maldives; Mali; Mauritania; Mozambique; Myanmar; Nepal; Niger; Rwanda; Samoa; Sao Tome and Principe; Sierra Leone; Solomon Islands; Somalia; Sudan; Togo; Tuvalu; Uganda; United Republic of Tanzania; Vanuatu; Yemen; Zambia. |
|
SOURCE: World Health Organization, 2001. |
Hemorrhage
Hemorrhage—primarily postpartum hemorrhage (PPH)—is the leading contributor to maternal mortality worldwide, causing about 24 percent of all maternal deaths (World Health Organization, 1999). In some regions, such as certain Chinese provinces, hemorrhage is reported to account for nearly half of all maternal deaths (Kwast, 1991a). In Indonesia, excessive postpartum bleeding (self-reported) occurs in 7 percent of live births (Central Bureau of Statistics et al., 1995).
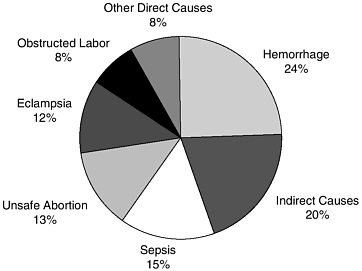
FIGURE 2-1 Global estimates of the causes of maternal deaths.
SOURCE: World Health Organization, 1999.
PPH is the excessive loss—usually of 500 milliliters or more—of blood from the genital tract within 24 hours of delivery (World Health Organization, 1998). If uncontrolled, hemorrhage can quickly lead to shock and death, which generally occurs within 7 days of childbirth. Because of the difficulty of measuring blood loss, a more practical definition of PPH is any blood loss that causes a physiological change such as low blood pressure that threatens a woman’s life (McCormick et al., 2002). Immediate PPH is most commonly due to uterine atony, inadequate contraction of the uterus, and a retained placenta or placental fragments (McCormick et al, 2002). Other causes include damage to the genital tract such as cervical tears, perineal lacerations, and episiotomy. Even relatively mild PPH can aggravate existing anemia caused by poor nutritional intake of iron and folate, hookworm infestation, malaria, or repeated short birth intervals. Women who survive hemorrhage frequently suffer from chronic anemia.
Severe anemia, common in developing countries, contributes to high mortality from postpartum hemorrhage. Delivery at home without a skilled birth attendant can result in long delays in obtaining emergency treatment. When the first measures such as use of drugs to stop the bleeding or bimanual compression of the uterus are not taken or are not effective, uterine artery ligation or hysterectomy may be needed, both of which require access to comprehensive essential care services that may involve significant expense and travel. When blood transfusions are required, women are
exposed to the risk of infection with HIV, hepatitis B, C, and D, malaria, syphilis, cytomegalovirus, and other agents if blood supplies are unscreened and unsafe.
INFECTIONS1
Sepsis
The second leading cause of maternal mortality, sepsis, is estimated to cause 15 percent of all maternal deaths worldwide (World Health Organization, 1999). Puerperal infections are caused by transfer of an infectious agent from the cervix or vagina to the uterus during labor or pelvic examination or by transfer of bacteria from skin, nostrils, and perineum by contaminated fingers or instruments (AbouZahr et al., 1998). The risk of puerperal sepsis is higher for women with sexually transmitted and other infections, premature rupture of membranes, retained products of conception, diabetes, cesarean or other operation, postpartum hemorrhage, anemia, poor nutritional status, history of previous complications of labor, and poor infection control.
The most common sign of puerperal infection is fever, but a small percentage of women with postpartum fever may have an infection at another site or no infection. Coupled with the unavailability and inappropriate use of effective antibiotics, relatively minor puerperal infections can rapidly become life-threatening. Women who survive puerperal sepsis are frequently left to cope with chronic ill health due to pelvic pain, dysmenorrhoea, menorrhagia, and/or infertility (AbouZahr et al., 1998). Information on the incidence and outcome of puerperal sepsis is limited because the majority of women in developing countries deliver at home or are in a clinic or hospital only briefly.
Malaria
More than 40 percent of the world’s population lives in malarious areas, and 90 percent of the estimated 300 to 500 million malaria cases occur in sub-Saharan Africa (United Nations Children’s Fund, 2000). Malaria in pregnancy has serious health consequences for the newborn, as well as for the mother (see Chapters 3 and 6). Women are more susceptible to malaria infection during pregnancy, but this susceptibility decreases with
|
1 |
Perinatal transmission of HIV/AIDS is addressed in Chapter 8. Primary infection of women with HIV/AIDS and infection with tuberculosis, while important, are outside the scope of this report on birth outcomes. |
successive pregnancies (Duffy and Fried, 1999; Miller and Smith, 1998; Brabin, 1983). Where malaria is endemic, adults rarely experience severe illness; however, pregnant women in these populations are at increased risk for high parasitemias and anemia (Miller and Smith, 1998; Diagne et al., 1997). In areas of low malarial transmission, immunity is low, and infection during pregnancy can cause severe disease, including fever and central nervous system complications (Steketee et al., 1996a). HIV infection appears to interfere with the maintenance of pregnancy-specific immunity acquired during first and second pregnancies, placing HIV-positive multigravidae in endemic areas at increased risk for the clinical consequences of malaria (Steketee et al., 1996b; Verhoeff et al., 1999).
Viral hepatitis
Viral hepatitis is the most common cause of liver disease during pregnancy (Pastorek, 1993). The disease, which is caused by several diverse types of virus, is endemic in many regions of Asia, Africa, the Middle East, and Central America where sanitation practices are inadequate (Michielsen and Van Damme, 1999). One form of the disease, hepatitis E, is of greatest concern during pregnancy because of its reported mortality rate of up to 25 percent among pregnant women, compared with a rate of less than 1 percent among the general population (Skidmore, 1997; Aggarwal and Krawczynski, 2000). Pregnant women who contract hepatitis E during the third trimester appear highly susceptible to developing a fulminant infection. Even when the mother escapes liver failure, this infection often causes a fetal death (Michielsen and Van Damme, 1999).
Unsafe Abortion
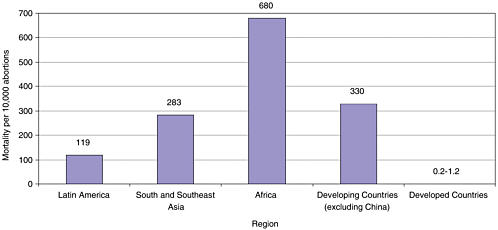
WHO estimates that about one-quarter of all pregnancies end in abortion, a total of 50 million per year. Of these abortions, an estimated 20 million are performed with unsafe methods, by untrained providers, or by the woman herself (Berer, 2000). About 90 percent of unsafe abortions worldwide occur in developing countries (World Health Organization, 1994a), but there is substantial regional variation in abortion-related mortality, as shown in Figure 2-2. In some areas of Africa, where unsafe abortion exacts the highest death toll, it has been found to contribute to between 20 and 50 percent of maternal mortality (Rogo et al., 1999; Benson et al., 1996; Okonofua, 1997).
Unsafe abortion can lead to a variety of complications, including sepsis, hemorrhage, genital and abdominal trauma, tetanus, perforated uterus, and poisoning from abortifacient medicines (Maine et al., 1994; Bernstein and Rosenfield, 1998; Brabin et al., 2000; Rochat and Akhter, 1999). These
complications have been estimated to result in at least 70,000 maternal deaths per year, accounting for at least 13 percent of all maternal mortality (Bernstein and Rosenfield, 1998; Maine et al., 1994). Moreover, the treatment of abortion complications consumes a disproportionate share of limited health care resources in developing countries (AbouZahr and Ahman, 1998). For example, in Bolivia in the late 1980s, treatment of abortion complications was reported to consume 60 percent of national spending for obstetric and gynecological care (Maine et al., 1994).
Hypertensive Disease of Pregnancy
Eclampsia is estimated to cause approximately 12 percent of all deaths due to pregnancy-related causes in developing countries (World Health Organization, 1999). A review of hospital-based studies on maternal mortality associated with hypertensive disorders in Africa, Asia, Latin America, and the Caribbean revealed similar rates—between 10 and 15 percent of all maternal deaths—among all regions. In Pakistan, where maternal mortality due to eclampsia has reached an estimated 500 deaths per 100,000 live births, a hospital-based study showed eclampsia to occur in 1 of every 60 deliveries (Jamelle, 1997). Several studies suggest that mortality associated with hypertensive disease of pregnancy is more difficult to prevent than deaths due to other pregnancy-related causes (Duley, 1992; Moodley, 1990; Loudon, 1991).
Obstructed Labor
Obstructed labor is estimated to cause 8 percent of all maternal deaths and also presents serious risks for the fetus and neonate (World Health Organization, 1999). Its incidence varies widely and is particularly high where levels of nutrition are poor and early marriage is common (Kwast, 1992; Konje and Ladipo, 2000). Obstructed labor can often be anticipated, as it is caused by mechanical factors. Women whose growth has been stunted by malnutrition or untreated infection or who bear children before pelvic growth is complete are at greatest risk for cephalopelvic disproportion, disproportion between the size of the infant’s head and the bony birth canal, which is the main cause of obstructed labor; fetal malpresentation is another, less common cause (Kwast, 1992).
Prolonged obstructed labor may produce injuries to multiple organ systems, such as vesico-vaginal or recto-vaginal fistulae, and is associated with increased risk of sepsis, hemorrhage, and uterine rupture (Arrowsmith et al., 1996; Konje et al., 1992). In the developing world, women who suffer physical injuries with long-term sequelae resulting from prolonged obstructed labor may also face serious social problems, such as divorce;
exclusion from religious and other social activities; and ultimately, worsening poverty and malnutrition (Arrowsmith et al., 1996).
INTERVENTIONS
The Safe Motherhood Initiative was launched in 1987 as an inter-agency, international partnership intended to raise awareness of the scope and consequences of poor maternal health in developing countries and provoke action to address the issue of maternal mortality. Through these efforts, access to safe pregnancy and childbirth is beginning to be viewed not just as a public health concern, but as a human right (Thompson, 1999). Yet after more than a decade of increased attention to maternal deaths in the developing world, maternal mortality ratios are essentially unchanged (World Health Organization, United Nation’s Children’s Fund, 1996). This outcome, which stands in stark contrast to the success of the Child Survival Initiative, resulted in part from a lack of strategic focus in the Safe Motherhood Initiative (Maine and Rosenfield, 1999; Weil and Fernandez, 1999). See Chapter 1 for a discussion of the history of the Safe Motherhood and Child Survival Initiatives.
Maternal health has yet to be perceived as a global priority (Graham, 2002). It is estimated that maternal health services account for 5 to11 percent of total donor contributions to the health sector in developing countries, and 4 to 12 percent of domestic health expenditure (Borghi, 2001). Today the challenge is to provide essential maternal care, consisting of interventions that are most likely to reduce maternal deaths and promote maternal health. Many such interventions, described in the remainder of this chapter, are known to improve fetal and neonatal survival as well, as depicted in Figure 2-3.
Interventions Involving Behavioral Change
Reducing risks for maternal, neonatal, and fetal mortality frequently involves behavioral changes for women. While such changes are often difficult to achieve, they can be facilitated with information about pregnancy, risks, and healthy behaviors (Harrison, 1997). Some examples of behavioral changes in women that are discussed in this report include not reproducing after age 35; eating a healthy diet; limiting or avoiding alcohol consumption; stopping smoking; using a bednet to protect against malaria; arranging for a skilled birth attendant at labor and delivery; and recognizing and acting promptly on signs of a complicated delivery.
Strategies that improve birth outcomes in monitored clinical trials may fail when introduced into large, unmonitored populations if compliance with the intervention is inadequate. As a result, the recommendations in
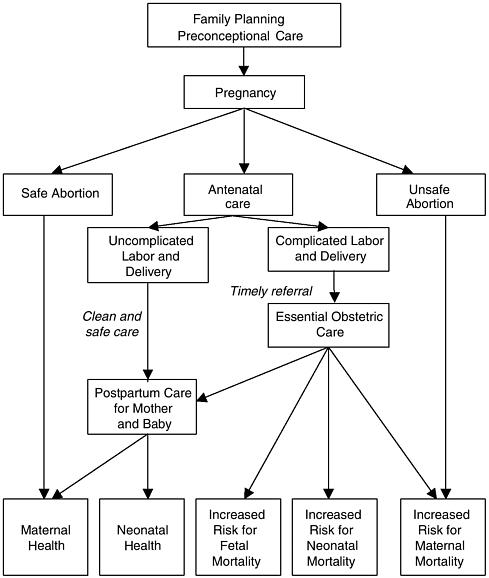
FIGURE 2-3 Health care decisions for improved birth outcomes.
this report focus on strategies that have proven effective in both clinical trials and in large comparable populations. Research that identifies additional strategies for encouraging healthy behaviors can contribute significantly to the success of health interventions that rely on patient compliance over time. Such efforts might involve education of women through campaigns and advice or counseling during antenatal care. They might also involve the development and showing of movies that initiate changes in social behaviors.
Antenatal Care
Many antenatal interventions have been shown to reduce neonatal morbidity and mortality (Bergsjo and Villar, 1997); however, evidence for the effectiveness of antenatal care in reducing maternal mortality (and to a lesser extent, morbidity) is less compelling (McDonagh, 1996). Therefore the main discussion of antenatal care will be presented in Chapter 3 of this report, which concerns the neonate.
It is widely accepted that screening pregnant women to identify those at risk for obstetric complications is not a replacement for skilled care during labor and delivery. More maternal deaths occur in the much larger group of low-risk women. As a result, antenatal care will not necessarily prevent complications from occurring (Maine and Rosenfield, 1999). This was demonstrated in a study in Gambia in the early 1980s in which a relatively high standard of antenatal care was not able to identify the specific risk factors that could predict which women were more likely to experience fatal complications (Greenwood et al., 1987). In addition, those who did experience complications were often located too far from a competent medical facility to receive treatment. As a result, maternal mortality remained extremely high at 2,000 deaths per 100,000 live births (Greenwood et al., 1987).
Where adequate medical care is available, however, certain antenatal interventions appear to be effective in reducing adverse maternal outcomes (Carroli et al., 2001; Villar and Bergsjo, 1997). These include the recognition and treatment of hypertensive disease of pregnancy, detection and treatment of asymptomatic bacteriuria, and external cephalic version at term (to prevent obstructed labor) (Carroli et al., 2001; Villar and Bergsjo, 1997); more controversial are antenatal interventions to prevent maternal anemia and other forms of nutritional supplementation. In addition to the potential for reducing specific causes of maternal morbidity and mortality, antenatal care can also encourage birth preparedness and the use of skilled assistance in labor and delivery.
Recognition of hypertensive disease in pregnancy
A variety of symptoms may occur in women with pre-eclampsia, including headache, edema, visual disturbance, abdominal pain, and nausea. Any of these symptoms warrants a blood pressure check and screening for proteinuria (Walker, 2000). Recording of blood pressure at every antenatal visit is recommended so that hypertensive disease in pregnancy can be recognized and treated before symptoms develop and to prevent eclampsia. A dipstick test for proteinuria is also recommended at the first visit for all women and at subsequent visits for all nulliparous women and those with previous preeclampsia or hypertension (Villar and Bergsjo, 1997). Antihypertensive treatment for women with mild to moderate hypertension during
pregnancy remains controversial (Magee and Duley, 2001). The management of hypertensive disease in pregnancy is addressed in a subsequent section.
Detection and treatment of asymptomatic bacteriuria
Routine screening for and treatment of asymptomatic bacteriuria has been shown to be cost-effective (Rouse et al., 1995). Antibiotic treatment prevents pyelonephritis and may also reduce the risk of preterm delivery (Smaill, 2001).
Prevention and treatment of malaria
Pregnant women who live in malaria-endemic areas need access to prevention and/or treatment of malaria and associated anemia. The Cochrane Library has reviewed trials on the effectiveness of prompt treatment of malaria infection, prophylaxis with antimalarial drugs to prevent parasitemia, and reduced exposure to infection by using insecticide-impregnated bednets (Garner and Gülmezoglu, 2000). Prophylaxis with antimalarials is clearly associated with a reduced frequency of disease—lower antenatal parasitemia, lower malarial infection, less anemia, and fewer episodes of fever, and fewer low birth weight infants and preterm births. A recent study in the Gambia (Okoko et al., 2002) adds to the Cochrane review, findings in Ghana published 20 years earlier (McGregor et al., 1983), and findings in other African settings (Garner and Brabin, 1994; Steketee et al., 2001) that intermittent preventive treatment with antimalarials confirms these findings. Intermittent preventive treatment has several advantages: it is easier to sustain over time, more cost-effective, and less likely to cause resistance to antimalarial drugs. The 20th WHO Expert Committee Report recommends an effective one-dose regimen for women in malaria-endemic areas who are in their first and second pregnancies (Steketee, 2002). Sulfadoxine-pyrimethamine is effective in a single dose to semi-immune women, is not bitter, and is relatively well tolerated. In non-African settings where malaria transmission is lower and Plasmodium vivax and multidrug resistant P. falciparum coexist, finding an appropriate drug regimen is more difficult (Steketee, 2002).
Although insecticide-impregnated bednets have been shown to reduce malaria infection and death among children (Binka et al., 1997; Lengeler, 2000), and are provided free of charge to pregnant women in Kenya (Guyatt et al., 2002), their effectiveness in preventing malaria among pregnant women has not been established. Further studies of bednets are warranted as bednet use requires considerable effort to maintain good adherence and requires resources, yet has significant potential for pregnant women.
Prevention of anemia
Based on data from 1988, WHO estimates that 55 percent of all pregnant women living in developing countries and 18 percent of those in developed countries have anemia, defined as a blood hemoglobin concentration of less than 11 grams per deciliter (World Health Organization, 1992). The effects of anemia on maternal mortality are less well understood. Reports from India, Kenya, Nigeria, and Malawi identify anemia as the underlying cause of 8 to 16 percent of maternal deaths (AbouZahr and Royston, 1991). WHO estimates that one-tenth of maternal mortality in developing countries is atttributable to iron deficiency (World Health Organization, 2002a). Another study concludes that a significant body of causal evidence exists to suggest maternal mortality from severe anemia (Stoltzfus, 2001).
On the other hand, a critical review of existing research in this area concluded that the data available are inadequate for determining the contribution of maternal anemia to maternal mortality (Allen, 2000).
Many factors may predispose a pregnant woman to become anemic, and their relative importance varies by geographic area and by season (van den Broek and Letsky, 2000). The common causes include nutritional deficiencies—iron, folate, and less often vitamin B12; blood loss (childbirth, hookworm infestation); infections (malaria, HIV/AIDS); and genetic defects (sickle cell, α- and β-thalassemias, and some metabolic disorders). In the developing world, nutritional iron deficiency appears to be the predominant cause of anemia; other important causes include malaria and hookworm infection, as well as other micronutrient deficiencies (Guidotti, 2000; van den Broek and Letsky, 2000).
Guidelines for developing countries compiled by WHO, UNICEF, and the International Nutritional Anemia Consultative Group recommend that all pregnant women receive 60 mg of elemental iron and 400 micrograms of folic acid daily to reduce the prevalence of severe maternal anemia (van den Broek, 1998). The guidelines also advise prophylaxis against malaria and hookworm for anemic women in areas where these infections are common. Although iron supplementation can prevent low hemoglobin at birth and at 6 weeks postpartum, there is inconclusive evidence of a beneficial effect on pregnancy outcomes for either mother or child (Mahomed, 2002; Sloan et al., 2002). A multicenter, double-blind, randomized trial in Mexico observed a greater increase in hemoglobin levels in women receiving a daily supplement of both iron (80 milligrams) and folate (370 milligrams) than of iron alone, but did not measure an impact on birth outcomes (Juarez-Vazquez et al., 2002). Rigorous trials need to examine more successful strategies for supplementing iron consumption in communities where iron deficiency is common and anemia is a serious health problem (Mahomed, 2002).
Vitamin A supplementation
Studies conducted in Nepal indicate that vitamin A or β-carotene supplementation may reduce morbidity and mortality in pregnant women related to night blindness, nausea, and length of labor (Christian et al., 2000a, 2000b; West et al., 1999).
Nutritional interventions
Widespread maternal malnutrition in developing countries has created a demand for nutritional interventions. As noted in Chapter 1, malnourished mothers are at increased risk for complications and death during pregnancy and childbirth. In addition, their children tend to have low birth weight, fail to grow at a normal rate, and have higher rates of disease and early death (Tinker, 2000; United Nations Administrative Committee on Coordination/ Sub-Committee on Nutrition, 1994). Evidence is insufficient, however, with regard to the clinical efficacy and cost-effectiveness of nutritional supplementation designed specifically to prevent maternal morbidity and mortality, particularly in comparison with other interventions (Ladipo, 2000; Kulier et al., 1998; Rush, 2000). There is some concern that nutritional programs divert resources from interventions that could be more effective in reducing maternal mortality and morbidity (Rush, 2000). While it has been noted that past improvements in nutrition in Western Europe had little effect on maternal mortality, these women were not as malnourished as the target populations for contemporary nutritional programs (Loudon, 2000).
Prenatal counseling to recognize signs of complications
Since every pregnant, delivering, or postpartum woman is at risk for serious, life-threatening complications, an important goal of antenatal care in developing countries should be to teach women and their families to recognize signs of obstetric complications and respond promptly (Akalin and Maine, 1995). Signs and symptoms of pregnancy and labor complications are not always recognized as causes for concern. In rural West African communities, for example, symptoms such as swelling of the feet (a possible sign of pre-eclampsia), late-term spotting or bleeding (a sign of antepartum hemorrhage), and long labors are not viewed as potential medical emergencies (The Prevention of Maternal Mortality Network, 1992).
Prenatal counseling to use a skilled birth attendant
Antenatal care can also contribute to successful pregnancy outcomes by encouraging women to obtain skilled care for labor and delivery. According to WHO estimates, more than half of all women give birth without the
assistance and supervision of a skilled birth attendant (World Health Organization, 1997). A study of 300 women from low- and middle-income families in urban India showed that those who received a relatively high level of antenatal care were four times more likely than those who had little or no antenatal care to deliver with a skilled attendant (Bloom et al., 1999). Antenatal care providers can help women and their families find a place to give birth, a skilled attendant, and the essential items necessary for a clean delivery. Planning for delivery should also anticipate complications and the need for referral to an appropriate medical facility with the appropriate level of good quality essential obstetric care. It may involve transport arrangements, emergency funds, a family member to accompany the woman and assist in decisionmaking.
Skilled Attendance at Childbirth
There are two important challenges to achieving a significant reduction in maternal mortality: obtaining skillful services from the birth attendant at labor and delivery and access to higher level obstetric care in the event of complications (Weil and Fernandez, 1999; Koblinsky et al, 1999). Meeting these challenges requires competent health professionals as well as an environment in which they can perform effectively (Graham et al, 2001). This section discusses the evidence for the use of a skilled birth attendant during childbirth.
According to a comprehensive definition of the “skilled birth attendant” given in a 1999 joint statement by WHO, the United Nations Fund for Population Activities (UNFPA), UNICEF, and the World Bank (World Health Organization, 1999), a skilled birth attendant is a person with midwifery skills, such as a midwife, nurse, or physician, who has been trained to proficiency in the skills necessary to manage normal labor and delivery. A skilled attendant recognizes the onset of complications, performs essential interventions, starts treatment, and supervises the referral of mother and baby for interventions that are beyond their competence or not possible in the particular setting. More detailed information on the essential competencies of a skilled birth attendant is given in Appendix C.
There are major differences worldwide and among developing countries in the proportion of deliveries with skilled attendance, the quality of that attendance, the proportion of deliveries that take place in health facilities, and the quality of services in these facilities. There are also important differences in the risks for maternal and neonatal mortality in different settings. In some urban areas of developing countries and in all developed countries, most childbirth takes place in a hospital attended by a physician or midwife. In developing-country urban areas, childbirth may also take
place in the home with or without medically trained attendants or in a health clinic with a nurse or physician. In rural areas of the developing world, most childbirth takes place at home, generally without skilled birth attendance, and often with poor access to medical care.
What is the evidence that skilled attendance at childbirth reduces mortality?
For an issue as important as the role of skilled attendance, it might be assumed that randomized, controlled trials would have been undertaken in a range of low- and middle-income settings. Such rigorous trials are particularly challenging (Safe Motherhood Inter-Agency Group, 2000), however, and have not been done. The appropriate outcomes—maternal, neonatal, and fetal mortality—are able to be measured, but since maternal mortality is a relatively rare event, obtaining an accurate estimate of the effectiveness of skilled attendance at childbirth on reducing maternal mortality would require a very large population study. The individual follow-up of each pregnancy adds an additional complication to a very large trial (compared with simpler interventions such as mass vaccination). Such a trial may also have ethical issues involving the withholding of skilled birth attendance from a population of women who are serving as controls in the trial. The result of the cost and complexity of conducting a rigorous trial is that only now—in 2003—is the first discussion of rigorous studies, possibly randomized, controlled trials (RCTs), underway by Initiative for Maternal Mortality Programme Assessment (IMMPACT). It is anticipated that this program will address measurements of maternal, neonatal, and fetal mortality, will undertake rigorous trials on the effectiveness of different strategies to reduce mortality and severe morbidities during childbirth, and that these assessments will include the impact of skilled attendance. There are serious difficulties to be addressed, such as how to randomize the women who are delivering to trained and untrained attendants. Although rigorous cause and effect data are not available, the committee has reviewed the wide range of less rigorous data that are currently available in order to address this important issue. In the committee’s judgment, skilled birth attendance has the best evidence so far for reducing maternal and neonatal mortality.
Historical trends
Maternal mortality in 1870 in much of what is now the developed world exceeded 600 per 100,000 live births, a figure comparable with current maternal mortality ratios in many developing countries (Safe Motherhood Inter-Agency Group, 2000). Significant reductions in maternal mor-
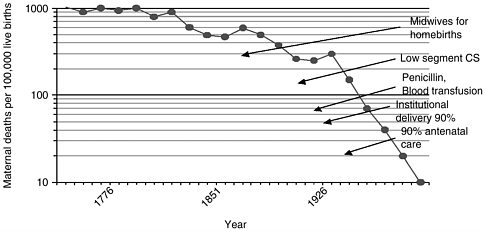
FIGURE 2-4 Maternal mortality in Sweden 1751-1980.
NOTES: CS is cesarean section.
SOURCE: Högberg et al., 1986.
tality were accomplished first in northwestern Europe (Sweden, Norway, Denmark, and the Netherlands) in the mid- to late-19th century, and several decades later in Britain and the United States (Loudon, 2000). In the mid-18th century, policy-makers in Sweden concluded—on the basis of newly collected vital statistics—that maternal mortality could be greatly reduced if all births were attended by qualified midwives (Högberg et al, 1986). The country actively recruited and trained midwives, and, over the course of more than a century, developed a cadre of largely autonomous midwives who worked under the supervision of local physicians (Van Lerberghe and De Brouwere, 2001). Between 1860 and 1900, the percentage of deliveries in Sweden attended by certified midwives increased from 40 to 78 percent, while the maternal mortality rate declined by more than 40 percent (Van Lerberghe and De Brouwere, 2001). This was in marked contrast to the United States, where skilled birth attendance was not promoted and maternal mortality remained at 800 per 100,000 live births (Van Lerberghe and De Brouwere, 2001). Figure 2-4 shows the decrease in maternal mortality in Sweden between 1870 and 1900 considered (but not proven) to be due to the effectiveness of skilled attendance at childbirth. It also shows a second phase of decreasing maternal mortality for about 30 years beginning in 1937, which is considered to be the result of a series of medical advances—cesarean section, penicillin, blood transfusion, institutional delivery, and antenatal care.
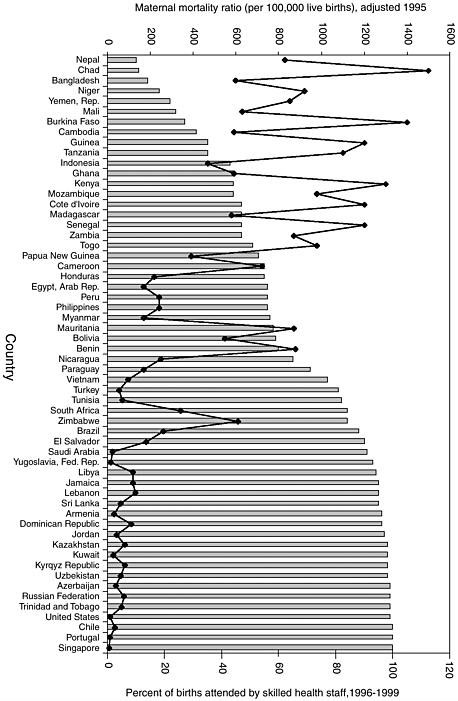
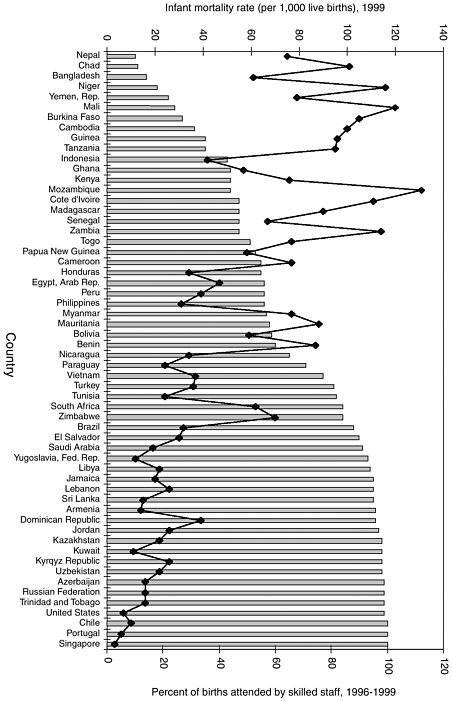
Epidemiological trends
National percentages of childbirths assisted by a skilled birth attendant are shown with corresponding maternal mortality ratio (MMR) and infant mortality rate2 (IMR) data in Figures 2-5 and 2-6 respectively. Similar data are given by region in Table 2-2. Both MMRs and IMRs tend to be lowest where most women give birth with a skilled attendant. In settings where a typical birth takes place at home, not attended by a skilled birth attendant (World Health Organization, 1997), MMRs and IMRs tend to be highest. The associations of skilled care with reduced maternal and infant mortality appear to be strong. Again caution is appropriate in drawing inferences about causality from these associations as other factors could also be involved. In assessing the reliability of the data, it is necessary to consider the problems involved in estimating maternal and neonatal mortality and coverage by skilled attendants. The definition of skilled attendant may vary with country and setting, the effectiveness of attendants varies with their support in terms of supplies and equipment, access to strong referral facilities, their abilities at convincing patients to be referred and accomplishing that in time to influence the outcome, and their oversight and continuing training. The data available aggregates skilled care provided by physicians, nurses, and midwives, which may distort the results that would be observed for midwives alone. Finally, measurement of the association of skilled attendance with neonatal, not infant mortality—almost two-thirds of which is neonatal—would be more specific and therefore more accurate.
Provision of clinical strategies during childbirth
Clinical strategies to address childbirth and its complications are identified in Chapters 2 to 4 of this report. Providing a skilled birth attendant during childbirth who has the knowledge and experience to use certain strategies when they are needed is a key step to reducing mortality and severe disability in childbirth. The second key strategy is provision of good-quality obstetric care for complicated deliveries. For many, childbirth proceeds normally and attendants can focus on the provision of safe and hygienic care and guidance to new mothers on their care and the care of their infants. However, most complications of childbirth cannot be predicted and, when they occur, having a skilled attendant at the delivery is generally the only safe way to provide life-saving clinical strategies. An alternative strategy is to provide broad access to basic or comprehensive essential obstetric care. This is more realistic in urban than rural areas. However, even when higher-level care is
TABLE 2-2 Maternal and Neonatal Mortality Compared with Rates of Skilled Care and Use of Health Facilities for Childbirth
|
Region |
Maternal Deaths per 100,000 live births (1990) |
Neonatal Deaths per 1,000 live births (2001) |
Deliveries with Skilled Attendant at Delivery (%) (1996) |
Deliveries in Health Facilities (%) (1996) |
|
World |
430 |
31 |
57 |
46 |
|
Developed |
27 |
5 |
99 |
98 |
|
Less developed |
480 |
34 |
53 |
40 |
|
Africa |
870 |
42 |
42 |
36 |
|
Eastern |
1,060 |
41 |
34 |
32 |
|
Middle |
950 |
39 |
42 |
41 |
|
Northern |
340 |
32 |
63 |
39 |
|
Southern |
260 |
18 |
79 |
76 |
|
Western |
1,020 |
54 |
34 |
32 |
|
Asia |
390 |
34 |
53 |
37 |
|
Eastern |
95 |
20 |
86 |
54 |
|
South-central |
560 |
46 |
34 |
26 |
|
Southeastern |
440 |
24 |
53 |
33 |
|
Western |
320 |
22 |
68 |
57 |
|
Europe |
36 |
6 |
98 |
97 |
|
Eastern |
62 |
9 |
NA |
NA |
|
Northern |
11 |
4 |
NA |
NA |
|
Southern |
14 |
5 |
NA |
NA |
|
Western |
17 |
3 |
NA |
NA |
|
Latin America/ Caribbean |
190 |
17 |
75 |
71 |
|
Caribbean |
400 |
19 |
71 |
70 |
|
Central America |
140 |
13 |
65 |
58 |
|
South America |
200 |
18 |
80 |
78 |
|
North America |
11 |
4 |
99 |
99 |
|
NA: Data not available. SOURCES: World Health Organization, 1996, 1997; Save the Children, 2001. |
||||
very readily accessible, the birth attendant must first recognize a complication and arrange an effective referral.
Although rigorous trials are not available at this time, the committee views the overall association of skilled care with reduced mortality at childbirth, coupled with the need for a skilled birth attendant who can apply the clinical strategies identified in this report when they are needed as sufficient grounds for recommending that a skilled attendant assist at every birth. Providing every delivery with an attendant who has certification in the
essential skills, and also the necessary supplies and equipment, access to essential obstetric and neonatal care for complications, an effective referral system, a regular caseload, and appropriate accountability and oversight have been found to be effective in the countries with lower maternal and neonatal mortality. Training a cadre of midwives to provide life-saving care for women during childbirth and developing a strong network of essential care for referral of complicated deliveries is a challenge that must eventually be addressed by all countries.
In most settings, traditional birth attendants (TBAs) are guided by traditional, often untested practices, rather than medical experience. They generally do not carry a regular caseload and do not therefore have the opportunity to build the experience of a nurse or midwife. Many TBAs have received training on safer birth practices, including clean delivery and avoidance of harmful practices. However, they have not been effective in reducing mortality during childbirth. Managing normal deliveries, recognizing complications, and managing and referring patients with complications requires more knowledge, training, and oversight, as well as the ongoing experience that is gained from a regular caseload. Since TBAs are trusted and respected in their community, they can provide comfort for the mother and family during labor and delivery and introduce and facilitate the work of a midwife in the community, but they should not be seen as a substitute for a skilled birth attendant.
In some settings, auxiliary nurse/midwives, community midwives, village midwives, and health visitors have received some training in childbirth skills. These workers may have more education, training, and supervision than TBAs, and (unlike some midwives) live in and know the community, and be less expensive in both their training and continuing compensation. Despite the attractiveness of an apparently less expensive option, the ability of attendants without the skills and experience of a skilled birth attendant to reduce maternal, neonatal, and fetal mortality must be established in trials in similar settings before being adopted for a wider population.3
Management of Childbirth
The first stage of labor
In order to prevent maternal mortality and morbidity associated with prolonged labor, the progress of labor should be monitored. Simple and effective monitoring of labor was first used in Zimbabwe in the 1970s (Philpott and Castle, 1972a; Philpott and Castle, 1972b). This involved
|
3 |
This issue is also discussed in Appendix E, Dissenting Note by Dr. Abhay Bang. |
|
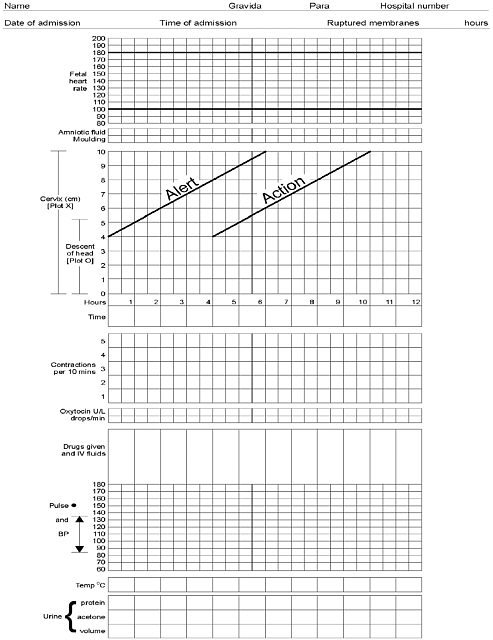
BOX 2-1 The partograph is intended for use by health workers trained in childbirth who can observe and conduct normal labor and delivery, perform vaginal examinations, and assess and accurately plot cervical dilation on a graph against time. (WHO Partograph: Figures 2-7a and 2-7b; see following pages). During first-stage labor, before the cervix has dilated to 3 centimeters, progress may be slow and irregular. This latent phase normally lasts 8 hours or fewer. In active, second-stage labor, cervical dilatation normally progresses at the rate of at least 1 centimeter per hour, indicated as the alert line. Slower rates would be recorded to the right of the alert line, signaling that the woman should be referred to a central facility. There, medical personnel must decide, based on their determination of maternal and fetal condition and the effectiveness of contractions, whether labor should be augmented with oxytocin or delivery should proceed by cesarean section. The WHO multicenter trial found that using the partograph along with an agreed labor-management protocol produced several significant benefits. Labors lasting more than 18 hours were reduced from 8.3 percent to 4.5 percent in nulliparous women, and from 6.4 percent to 3.4 percent in all women. The proportion of labors requiring augmentation with oxytocin declined from 20.7 percent to 9.1 percent, and the rates of forceps deliveries and postpartum sepsis in both nulliparous and multiparous women reduced to a significant extent. Intrapartum late fetal deaths declined as well from 0.5 percent to 0.3 percent (World Health Organization, 1994b). |
graphically tracking cervical dilation over time. A refined version of the initial device, known as the partograph, is now widely used to reduce maternal and fetal morbidity due to prolonged or obstructed labor. The central feature of the partograph is a graphical representation of the progress of labor—cervical dilatation, descent of presenting part, and duration and frequency of contractions—and its relationship to maternal and fetal condition (see Box 2-1). The pattern of cervical dilatation in normal labor among different ethnic groups is so similar that a partograph is useful throughout the world (Lennox and Kwast, 1995).
In the early 1990s, a partograph produced and promoted by WHO was tested in a multicenter trial in Southeast Asia involving 35,484 women (World Health Organization, 1994b). Based on the encouraging results of this trial, WHO recommends widespread use of the partograph. The partograph was revised in 2002 (Figures 2-7a and 2-7b). When used at a health center or maternity center, the device provides an early warning that labor is likely to be prolonged, and the woman should be transferred to a hospital. In the hospital, it can provide a warning that extra vigilance or an
emergency procedure, such as cesarean section, is needed. Use of the partograph has been reviewed by the Cochrane Database of Systematic Reviews (Buchmann et al., 2002). It has been found to assist labor management by clearly indicating departures in the progress of labor and anticipating interventions before complications occur. Interventions that may prevent mortality or serious morbidity for mother or fetus include labor augmentation, cesarean section, or transfer to a more sophisticated facility. The Cochrane review does caution against assuming that all women will progress through labor at the same rate. This assumption could have adverse effects such as increased rates of artificial rupture of the membranes, oxytocin augmentation, and use of analgesia. Despite its effectiveness, however, the partograph has not been universally adopted. A recent survey of 420 physicians and midwives in Enugu, Nigeria, revealed that although about 90 percent of respondents had heard of the partograph, only about 25 percent used it (Umezulike et al., 1999). Introduction of the partograph needs to be accompanied by training in its use with appropriate supervision and follow-up.
The second stage of labor
A recent review of clinical trials concludes that an inadequate number of methodologically stringent comparisons of labor positions have been conducted to allow recommending one position over another; thus women should be encouraged to give birth in the position they find most comfortable (Gupta and Nikodem, 2000). Sustained, early bearing down may slightly decrease the duration of the second stage of labor, but can result in maternal exhaustion and compromised maternal-fetal gas exchange (Mayberry et al., 1999/2000). Several brief periods of breath holding and bearing down during each contraction, which tend to occur spontaneously, appear to be safer for the fetus than Valsalva-type extended pushing with sustained breath holding (Sleep et al., 1989; Mayberry et al., 1999/2000).
Episiotomy is used in many deliveries for first-time mothers despite the fact that there is little evidence to support the frequent use of this technique (Sleep et al., 1989). In fact, episiotomies have been shown in some cases to cause an increase in the rate of perineal trauma (Moller Bek and Laurberg, 1992).
Active management of third-stage labor
Delivery of the placenta and membranes is a particularly hazardous part of childbirth for mothers, because of the risk of postpartum hemorrhage (PPH). Active management involves three steps to augment uterine contractions and prevent PPH due to uterine atony: (1) give a uterotonic
drug within one minute of birth; (2) clamp and cut the umbilical cord soon after birth; and (3) deliver the placenta by controlled cord traction and counter pressure on the uterus through the abdomen (McCormick et al., 2002).
Three large RCTs have compared postpartum hemorrhage and other outcomes in deliveries with active management of the third stage of labor and those with physiologic management. These trials—undertaken in Bristol (Prendiville et al., 1988), Hinchingbrooke (Rogers et al., 1998), and Abu Dhabi (Khan et al., 1997)—have been reviewed by the Cochrane Library (Prendiville et al., 2001) and McCormick et al. (2002). The components of active management in the three trials included a prophylactic oxytocic drug during or after delivery of the anterior shoulder, immediate clamping of the umbilical cord, and delivery of the placenta by controlled cord traction or maternal effort. Mothers in the physiologic management group received no oxytocic drug in two trials and only after the delivery of the placenta in the third; no cord clamping until the placenta was delivered, pulsation ceased, or the baby was delivered; and delivered the placenta without assistance. In these RCTs, there was a significantly higher rate of postpartum hemorrhage in the physiologic management group than in the active management group (17.9 vs. 5.9 percent, 16.5 vs. 6.8 percent, and 11 vs. 5.8 percent). Active management thus reduced the need for blood transfusion, the occurrence of a prolonged third stage, and the need for additional uterotonic drugs. There was no difference between the two groups in blood pressure or need for manual removal of retained placenta. The maternal position (upright or supine) did not influence PPH in either group, and neonatal outcomes were not affected by the management of delivery. Giving a uterotonic drug immediately after birth rather than after the placenta is delivered was shown in the Abu Dhabi trial to provide the greatest reduction in PPH. When the placenta was delivered by maternal effort, use of oxytocin reduced the risk of PPH, shortened the time for the third stage of labor, raised hemoglobin levels at 48 hours postpartum, and did not increase the risk of retained placenta.
Currently, oxytocin or syntometrine (oxytocin and ergometrine) are the prophylactic drugs of choice to prevent postpartum hemorrhage (Rogers et al., 1998). The Cochrane Library (McDonald et al., 2000) and McCormick et al (2002) have reviewed several RCTs comparing oxytocin and syntometrine (oxytocin and ergometrine) for their effectiveness in the active management of third-stage labor. While one study showed syntometrine to produce a small but significant reduction in PPH compared with oxytocin (McDonald et al., 2000), the latter was the preferred drug because ergometrine can raise blood pressure and is, therefore, contraindicated for women with hypertensive disease of pregnancy, and it frequently causes nausea and vomiting (El-Refaey et al., 2000). Both drugs require
refrigeration and protection from light to maintain their potency, and must be injected, which reduces their practicality in low-resource settings. Increasing evidence indicates that oral or rectal misoprostol, an inexpensive, stable drug, shows promise for reducing postpartum hemorrhage (Ng et al., 2001; Goldberg et al., 2001; Hofmeyr et al., 1998; Surbek et al., 1999; Walley et al., 2000; Bamigboye et al., 1998; O’Brien et al., 1998). Misoprostol4 is particularly suitable in developing-country settings where oxytocin and ergometrine are unavailable.
An essential package of interventions for care during labor and delivery
In conclusion, an essential package of interventions for care during labor and delivery should include the following:
-
Monitoring the progress of labor using a partograph
-
Using aseptic practices
-
Supporting the birthing position of the mother’s choice
-
Avoiding medical episiotomy unless specifically indicated
-
Preventing postpartum hemorrhage through active management of the third stage of labor
Complications of labor and delivery and provision of essential obstetric care
Even when women receive the highest-quality antenatal care and have skilled providers at the delivery, complications can arise and cause maternal, neonatal, or fetal death. The major causes of maternal mortality described earlier in the chapter (hemorrhage, sepsis, unsafe abortion, hypertensive disease of pregnancy, and obstructed labor) can be addressed by essential obstetric care (EOC) in developing county settings (United Nations Children’s Fund, World Health Organization, United Nations Population Fund, 1997). See Table 2-3.
Basic EOC involves six signal services: antibiotic, oxytocic, and anticonvulsant drugs; manual removal of the placenta; removal of retained products of conception (by manual vacuum aspiration with a large syringe); and performance of assisted vaginal delivery (manual assistance, vacuum extraction, or forceps delivery). Other functions are also important, but for
TABLE 2-3 Essential Obstetric Care Services to Address Major Causes of Maternal Mortality
|
EOC services |
|
Basic
|
|
Comprehensive All basic EOC services
|
|
aParenteral administration of drugs is administration by injection or intravenous infusion (“drip”). SOURCE: United Nations Children’s Fund, World Health Organization, United Nations Population Fund, 1997. |
the purposes of monitoring, the six functions are considered sufficient for most EOC activities.
These six services can prevent a large portion of obstetric deaths and can be carried out by a skilled attendant in a clinic or community health center prepared with medication and intravenous fluid—if the skilled attendant is trained and focused on the frequent direct causes of obstetric deaths. For some cases of postpartum hemorrhage these services would be sufficient. Such a clinic can function as a referral site for patients with these complications who have delivered at home or it can serve as a delivery site. Massive hemorrhage or true obstructed labor will require a hospital facility with blood transfusion, anesthesia, and the capacity for major surgery (cesarean delivery or hysterectomy.) Even then, basic EOC can save women’s lives by stabilizing them before referral and a journey that may take many hours (United Nations Children’s Fund, World Health Organization, United Nations Population Fund, 1997).
Comprehensive EOC involves the six basic services and two additional ones: the ability to perform surgery, including administering anesthesia, and provision of blood transfusion.
In developing countries, women with complicated labors face many barriers to receiving timely and appropriate medical care. These obstacles can be summarized as the following four delays (Lawn et al., 2001), which
have been adapted from the original three delays described by Thaddeus and Maine (1994) and Maine (1997):
-
Delay in recognizing complications
-
Delay in deciding to seek care
-
Delay in reaching a health facility because of a lack of transportation or resources
-
Delay in receiving appropriate care at the facility
Significant reductions in maternal—as well as neonatal and fetal—mortality can be achieved if complications are anticipated and addressed promptly. For example, a major reduction in maternal mortality achieved over a 15-20 year period in a rural area of the Gambia has been attributed to a combination of increased availability of emergency obstetric care, improved transport, and increased communication (Walraven et al., 2000). These and other interventions to strengthen health care delivery, which are critical to the success of any strategy to improve birth outcomes in developing countries, are discussed in Chapter 5. The following section describes specific interventions to address major complications of labor and delivery.
Management of postpartum hemorrhage requires vigilance to prevent and detect this frequently fatal condition, as well as rapid response to address it when it arises. While the use of blood transfusions may be limited to hospital deliveries, other interventions can be performed at peripheral health centers. These include manual removal of the placenta; bimanual uterine compression; repair of cervical, vaginal, or perineal lacerations; administration of parenteral oxytocics; and uterine massage.
The medications used to control postpartum hemorrhage in the United States include oxytocin (pitocin), methylergonovine (Methergine), 15-methyl PGF (Hemabate), and Dinoprostone (Prostin E2). Unfortunately, most of these require parenteral administration and/or refrigeration,5 conditions that make them unsuitable for use in many rural areas of developing countries. Misoprostol, discussed above as a possible means of preventing postpartum hemorrhage as part of active management of third-stage labor, also appears promising as a means of controlling hemorrhage, particularly in low-resource settings. Unlike other oxytocic agents, misoprostol does not require refrigeration, an important advantage (O’Brien et al., 1998).
Management of hypertensive disease in pregnancy aims to prevent the occurrence or recurrence of convulsions, which can be life threatening. Magnesium sulfate has been used extensively in the United States for the management and prevention of eclamptic seizures. Several studies, including
|
BOX 2-2 In 1995, the results of an international multicenter randomized trial enrolling nearly 1700 women offered the most compelling evidence to date in favor of magnesium sulfate over diazempam or phenytoin for the treatment of eclampsia (Eclampsia Trial Collaborative Group, 1995). The risk of recurrent convulsions was 52 percent lower for women allocated magnesium sulfate than among those treated with diazepam, and 67 percent lower than among women who were given phenytoin. Although no significant difference in maternal mortality was found among the treatment groups, fewer women allocated magnesium sulfate were ventilated, treated for pneumonia, or admitted to intensive care facilities than women who received phenytoin. Similarly, no significant differences were found between the groups for major outcomes of perinatal morbidity and mortality. However, infants of women receiving magnesium sulfate were less likely to be incubated at the place of delivery, or to be admitted to a special care nursery, than babies of mothers who had been allocated phenytoin. Other studies have also suggested that the use of magnesium sulfate may reduce the risk of cerebral palsy and possibly mental retardation for very low birth weight infants (Nelson and Grether, 1995; Schendel et al., 1996). A 1996 quantitative overview of nine randomized, controlled trials of magnesium sulfate for the treatment of preeclampsia and eclampsia—that is, to prevent recurrence of seizures in eclampsia and for seizure prophylaxis in pre-eclampsia—also found the drug to be superior to phenytoin and diazepam (Chien et al., 1996). A 1999 analysis of five trials (involving more than 1200 women) comparing the ability of magnesium sulfate and diazepam to control eclamptic seizures and prevent further seizures concluded the drug was “substantially more effective than diazepam for the treatment of eclampsia” (Duley and Henderson-Smart, 2000). A more recent study, the Magpie Trial, showed that in comparison with placebo, magnesium sulfate halved the risk of eclampsia among 10,000 women worldwide. Magnesium sulfate also reduced the risk of maternal death by half, but this result did not reach statistical significance (Magpie Trial Collaborative Group, 2002; Greene, 2003; Belfort et al., 2003). |
a large collaborative trial, have shown the drug to be superior to phenytoin and diazepam for seizure prevention (See Box 2-2). Results of the collaborative trial suggest that magnesium sulfate confers additional advantages for both mother and neonate (Eclampsia Trial Collaborative Group, 1995). While magnesium sulfate has been viewed as a promising drug for low-resource settings because it is inexpensive and relatively easy to produce, its delivery by intravenous drip or intramuscular injection restricts its use.
A recent review of randomized trials concluded that there is not enough evidence to establish the benefits and hazards of anticonvulsants for women with pre-eclampsia (Duley et al., 2000). A trial involving 14,000 women is currently under way in the United Kingdom to further evaluate the benefits
and risks of treating pre-eclampsia with magnesium sulfate (Duley and Neilson, 1999). The Magpie Trial, a recent international study involving 10,000 women, found that magnesium sulfate halved the risk of eclampsia (Magpie Trial Collaborative Group, 2002). A review of the use of magnesium sulfate for pre-eclampsia concludes that, “There is now international consensus that magnesium is the treatment of choice for preeclampsia and eclampsia, but the mechanism underlying its salutary effect remains debatable” (Greene, 2003).
Management of obstructed labor involves timely interventions, including vacuum extraction, forceps, and cesarean section. These procedures, traditionally the domain of physicians, have been performed successfully by trained medical assistants and nurses in Mozambique (Vaz et al., 1999) and Zaire (Duale, 1992), allowing such services to be maintained in rural areas. Mortality and complication rates for cesarean sections performed by these workers were reported to be comparable to those performed by physicians. This is a topic for further studies.
Prevention and management of maternal infection. Infection during labor and in the postpartum period can be reduced through aseptic delivery practices and careful attention to risk factors for infection, including excessive vaginal examinations, premature rupture of membranes, and prolonged labor. Induction of labor in cases of uncomplicated prelabor rupture of membranes has been shown to reduce maternal and neonatal infection (Tan and Hannah, 2000). The early detection of infection and the timely use of antibiotics also reduce maternal morbidity and mortality (AbouZahr et al., 1998; Kwast, 1991b).
Prevention of abortion-related morbidity and mortality. Of the five major causes of maternal morbidity and mortality discussed in this chapter, complications of abortion are the most amenable to reduction through prevention—that is, through improved access to and use of contraception (Maine et al., 1994; AbouZahr and Ahman, 1998). Better contraception would not only decrease the number of unwanted pregnancies, but also reduce neonatal and fetal mortality and morbidity associated with closely spaced births, multiparity, and maternal age. Unfortunately, many barriers restrict women’s access to family planning information and services. Even where family planning resources are readily available, unwanted pregnancies occur as a result of failure to use contraception and contraceptive failure (AbouZahr and Ahman, 1998; Henshaw and Kost, 1996).
Abortion tends to be vastly safer in countries where it is legal than where it is prohibited. Legalization of abortion does not appear to increase abortion rates, but does reduce morbidity and mortality (Serbanescu et al., 1995). Yet even where the procedure is legal, safe abortion may still be unavailable to many women because of expense, distance, or social barriers (AbouZahr and Ahman, 1998).
Overused or Inappropriate Interventions
Interventions such as cesarean sections, episiotomies, and use of oxytocics in the early stages of labor tend to be overused in some developing country settings, while they are not always available when needed in other settings (Buekens, 2001). More than 15 percent of deliveries involve a cesarean section in a majority of Latin American countries (Belizan et al., 1999) and in some regions of Asia (Buekens, 2001; Cai et al., 1998). Cesarean sections are less common in Africa, although they are used in more than 5 percent of deliveries in many urban areas of East and Southern Africa and in Ghana. In the poorest rural areas of Africa, the problem is a lack of access to cesarean sections, which are done in less than 1 percent of deliveries.
Episiotomies have become increasingly common, but a Cochrane review has found this increased use to be without scientific justification (Carrolli and Belizan, 2000). More selective use of episiotomy causes less posterior perineal trauma, reduced need for suturing, and fewer healing complications, but there is an increased risk of anterior perineal trauma. Several studies have shown episiotomy rates in African hospitals as high as 46 percent of all deliveries and 87 percent of primiparae deliveries. Similar high rates have been reported in some Latin American countries (Buekens, 2001).
As discussed earlier in this chapter, the routine use of oxytocics during third stage labor has been found beneficial (Prendiville et al., 2001). Use of oxytocin during the first and second stages of labor is, however, controversial, especially in developing countries where it is may be administered intramuscularly or with less control of the speed of infusion, which may hyperstimulate or cause uterine contracture. Use of oxytocin during labor has been associated, in studies in West Africa and Nepal, with increased risk of fetal distress and neonatal morbidity (Dujardin et al.,1995; Ellis et al., 2000).
Inappropriate interventions include pubic shaving, enema, and vacuum and forceps extraction. In settings where the level of hygiene is less strong, vaginal examination is not appropriate, while in other settings these should not be more frequent than necessary because of the risk of infection. Reducing both overuse and inappropriate use of interventions during labor and delivery is best addressed by basing clinical practice on a strong evidence base. This requires continuing evaluation of practices through randomized controlled trials and comprehensive education of birth attendants through influential health leaders, provision of educational materials, and audit and feedback (Buekens, 2001).
RECOMMENDATIONS
A formidable barrier to improving birth outcomes in many developing countries is the social status of women. Achievement of gender equity, and with it increased resources for primary health care, is a certain but long-term means to improving women’s reproductive health. More immediate reductions in maternal mortality can be accomplished by addressing its most frequent causes: hemorrhage, hypertensive disease of pregnancy, obstructed labor, sepsis, and unsafe abortion. Significant reductions in maternal—as well as fetal and neonatal—mortality depend on broad access to essential life-saving services during labor and delivery and immediately thereafter. This requires (1) a skilled birth attendant, and (2) access to good-quality essential obstetric care in the event of complications.
Recommendation 1. Every delivery, including those that take place in the home, should be assisted by a skilled birth attendant (a midwife, physician, or nurse) who has been trained to proficiency in basic techniques for a clean and safe delivery, and recognition and management of prolonged labor, infection, and hemorrhage. Where necessary, the birth attendant should also be prepared to stabilize and swiftly refer the mother to a facility providing essential obstetric care. 6 (See Chapter 3 for the neonatal component to this recommendation.)
Recommendation 2. Essential obstetric care should be accessible to address complications of childbirth that cannot be managed by a skilled birth attendant. This requires a network of good-quality essential care facilities that provide basic essential obstetric care: administration of antibiotic, oxytocic, and anticonvulsant drugs; manual removal of the placenta; removal of retained products of conception; and assisted vaginal delivery. Comprehensive essential obstetric care facilities have the capacity to perform these basic services and also surgery and blood transfusion. Access for the majority of a population to the appropriate level of care also requires strong referral systems that include communication with, and transportation to, referral facilities. (See Chapters 3 and 5 for additional components to this recommendation.)
These two interventions, which represent the highest priority for reducing maternal mortality, should be extended where possible by a program of postpartum maternal care addressing major causes of maternal mortality and morbidity during the first month after childbirth.
|
6 |
This issue is also discussed in Appendix E, Dissenting Note by Dr. Abhay Bang. |
Recommendation 3. Postpartum care is critical during the first hours after birth and important throughout the first month. For the mother, such care should emphasize the prevention, timely recognition, and treatment of infection; postpartum hemorrhage; and complications of hypertensive disease of pregnancy. (See Chapter 3 for a neonatal component to this recommendation.)
While many of the benefits of antenatal care accrue to the fetus and neonate, certain preconceptional and antenatal interventions can significantly reduce maternal mortality and morbidity.
Recommendation 4. The following strategies are recommended for incorporation into preconceptional and antenatal care:
-
Greater access for women and men of reproductive age to family planning services that provide effective contraception along with counseling on the risks for adverse birth outcomes.
-
Early detection and timely management of hypertensive disease of pregnancy.
-
Intermittent preventive and early treatment of malaria, especially for primiparae.
RESEARCH NEEDS
The challenge for research in the 21st century is to identify interventions that can reduce maternal, neonatal, and fetal mortality in the developing world and thus make childbirth a safe event. This will require particular attention to the obstetric and neonatal problems of populations with high mortality. Promising interventions must be tested with trials that are both rigorous and practical. Successful interventions must be monitored and adjusted for optimal effectiveness. A wide range of basic and applied research will need to be encouraged and funded through partnerships of ministries of health, international organizations and development agencies, nongovernmental organizations, and philanthropic foundations.
Each country will determine its research agenda according to local priorities and the resources that can be made available. Setting priorities for health-related research involves consideration of several factors: the magnitude of a health problem in the local population, the likelihood of identifying a successful intervention, the interests and capabilities of researchers, and the public perception of the importance of the health problem. It is important to consider all factors in each setting and to balance them for the best interests of the population in question (Brown, 1977).
The following areas of research have been identified by the committee as key to the continued improvement of maternal and overall birth outcomes. Research priorities that target other topics appear in the corresponding chapters.
-
Studies are needed to determine the burden of disease caused by maternal and neonatal bacterial infections in different settings. Research should include the identification of etiologic agents and their antibiotic susceptibility. Strategies for prevention and treatment should be informed by community-based data, including laboratory evaluations. Simple methods to identify mothers and neonates with presumed bacterial infection (such as algorithms based on patient history and physical findings) are also needed.
-
For areas of the world with limited laboratory capacity, there is a need to develop simple, cost-effective diagnostic tests that can be used in a field setting. Diagnostic capabilities at health centers and referral hospitals must also be strengthened.
-
Large, multicenter trials are needed to examine the cost-effectiveness of food and micronutrient supplementation in relation to maternal and neonatal health and fetal survival, particularly in areas where undernutrition is common. Local studies can determine the most effective means of supplementation to improve the nutritional status of the population, and thus of women who become pregnant.
-
Studies are needed to determine whether the level of antibiotic resistance in rural communities is significantly lower than in urban hospitals.
-
Studies are needed to evaluate the targeted use of antibiotics for those women at risk for infection during delivery who cannot be transferred to a hospital or who refuse hospital care.
-
Strategies to prevent malaria during pregnancy are needed, including ways to reduce exposure (e.g., insecticide-impregnated bednets). Antimalarial drug resistance is widespread. Research on the safety and efficacy of new drugs and drug combinations should target pregnant women.
-
Trials are needed to compare the effectiveness of intermittent prophylactic antimalarials with early treatment of malaria for women having their first or second baby. These strategies need to be tested in populations where malaria is endemic and women have some acquired immunity and in those where malaria is not endemic and there is less acquired immunity.
-
Trials are needed to identify more effective approaches to accomplishing behavior changes that reduce risks for adverse birth outcomes. The behavioral changes sought include stopping smoking, avoiding pregnancy over the age of 35 years, and recognizing the need for skilled care in pregnancy.
CONCLUSION
The wide gap between MMRs in developed countries and developing countries, where the vast majority of maternal deaths occur, suggests that much can be done to improve maternal survival. The two central, interdependent elements of any strategy to improve maternal health are the provision of skilled assistance for every delivery and access to essential obstetric care for complicated cases. Efforts to improve maternal outcomes could be greatly strengthened through programs of antenatal and postpartum care focused on the prevention and recognition of complications of pregnancy and childbirth. Substantial reduction of maternal mortality and morbidity will require long-term investment in community education and family planning and, ultimately, the empowerment of women.
Many measures that can be taken to improve maternal health—from specific medical interventions, to research, to the strengthening of women’s socioeconomic status—are likely to benefit the fetus and neonate as well. The interventions recommended in this chapter can work in conjunction with interventions that address neonatal and fetal mortality.
REFERENCES
AbouZahr C, Ahman E. 1998. Unsafe Abortion and Ectopic Pregnancy. In: Murray CJL, Lopez AD (eds). Health Dimensions of Sex and Reproduction: The Global Burden of Sexually Transmitted Diseases, HIV, Maternal Conditions, Perinatal Disorders, Perinatal Disorders, and Congenital Anomalies. Boston: Harvard School of Public Health. Pp. 266–296.
AbouZahr C, Royston E. 1991. Maternal Mortality: A Global Factbook. Geneva: WHO.
AbouZahr C, Wardlaw T, Stanton C, Hill K. 1996. Maternal mortality. World Health Statistics Quarterly 49(2):77–87.
AbouZahr C, Ahman E, Guidotti R. 1998. Puerperal Sepsis and Other Puerperal Infections. In: Murray CJL, Lopez AD (eds). Health Dimensions of Sex and Reproduction: The Global Burden of Sexually Transmitted Diseases, HIV, Maternal Conditions, Perinatal Disorders, and Congenital Anomalies. Geneva: WHO. Pp. 191–218.
Aggarwal R, Krawczynski K. 2000. Hepatitis E: an overview and recent advances in clinical and laboratory research. Journal of Gastroenterology and Hepatology 15(1):9–20.
Akalin MZ, Maine D. 1995. Strategy of risk approach in antenatal care: evaluation of the referral compliance. Social Science and Medicine 41(4):595–596.
Allen LH. 2000. Anemia and iron deficiency: effects on pregnancy outcome. American Journal of Clinical Nutrition 71(5 suppl):1280S–1284S.
Arrowsmith S, Hamlin EC, Wall LL. 1996. Obstructed labor injury complex: obstetric fistula formation and the multifaceted morbidity of maternal birth trauma in the developing world. Obstetrics and Gynecology Survey 51(9):568–574.
Bamigboye AA, Merrell DA, Hofmeyr GJ, Mitchell R. 1998. Randomized comparison of rectal misoprostol with syntometrine for management of third stage of labor. Acta Obstetricia et Gynecologica Scandinavica 77(2):178–181.
Belfort, MA, Anthony J, Saade GR, Allen JC Jr. Nimodipine Study Group. 2003. A comparison of magnesium sulfate and nimodipine for the prevention of eclampsia. New England Journal of Medicine 23(4)304-311.
Belizan JM, Althabe F, Barros FC, Alexander S. 1999. Rates and implications of caesarean sections in Latin America: ecological study. British Medical Journal 319(7222):1397–1400.
Benson J, Nicholson LA, Gaffikin L, Kinoti SN. 1996. Complications of unsafe abortion in sub-Saharan Africa: a review. Health Policy and Planning 11(2):117–131.
Berer M. 2000. Making abortions safe: a matter of good public health policy and practice. Bulletin of the World Health Organization 78(5):580–592.
Bergsjo P, Villar J. 1997. Scientific basis for the content of routine antenatal care: II. Power to eliminate or alleviate adverse newborn outcomes: some special conditions and examinations. Acta Obstetricia et Gynecologica Scandinavica 76(1):15–25.
Bernstein PS, Rosenfield A. 1998. Abortion and maternal health. International Journal of Gynaecology and Obstetrics 63(suppl 1):S115–S122.
Binka FN, Mensha OA, Mills A. 1997. The cost-effectiveness of permethrin impregnated bednets in preventing child mortality in Kassena-Nankana district of northern Ghana. Health Policy 41(3):229–239.
Bloom SS, Lippeveld T, Wypij D. 1999. Does antenatal care make a difference to safe delivery? A study in urban Uttar Pradesh, India. Health Policy and Planning 14(1):38–48.
Borghi J. 2001. What is the cost of maternal health care and how can it be financed? Studies in Health Services and Organization Policy 17:247–296.
Brabin BJ. 1983. An analysis of malaria in pregnancy in Africa. Bulletin of the World Health Organization 61(6):1005–1016.
Brabin L, Fazio-Tirrozzo G, Shahid S, Agbaje O, Maxwell S, Broadhead R, Briggs N, Brabin B. 2000. Tetanus antibody levels among adolescent girls in developing countries. Transactions of the Royal Society of Tropical Medicine and Hygeine 94(4):455–459.
Brown S. 1977. Staff Paper. Policy Issues in the Health Sciences. Washington, DC: Institute of Medicine.
Buekens P. 2001. Over-medicalisation of Maternal Care in Developing Countries. In: De Brouwere V, Van Lerberghe W (eds). Safe Motherhood Strategies: A Review of the Evidence. Studies in Health Services Organization and Policy, 17. Antwerp: ITG Press. Pp. 195–206.
Buchmann EJ, Gülmezoglu AM, Nikodem VC. 2002. Partogram for assessing the progress of labour (Cochrane Review). The Cochrane Database of Systematic Reviews, Issue 2.
Cai WW, Marks JS, Chen CH, Zhuang YX, Morris L, Harris JR. 1998. Increased cesarean section rates and emerging patterns of health insurance in Shanghai, China. American Journal of Public Health 88(5):777–780.
Carroli G, Belizan J. 2000. Episiotomy for vaginal birth. Cochrane Database of Systematic Reviews (2):CD000081.
Carroli G, Rooney C, Villar J. 2001. WHO programme to map the best reproductive health practices: how effective is antenatal care in preventing maternal mortality and serious morbidity? Paediatric and Perinatal Epidmiology 15(suppl 1):1–42.
Central Bureau of Statistics, State Ministry of Population/National Family Planning Coordination Board, Ministry of Health, Macro International Inc. 1995. Indonesia Demographic and Health Survey 1994. Calverton, MD: Central Bureau of Statistics and Macro International.
Chien PF, Khan KS, Arnott N. 1996. Magnesium sulphate in the treatment of eclampsia and pre-eclampsia: an overview of the evidence from randomised trials. British Journal of Obstetrics and Gynaecology 103(11):1085–1091.
Christian P, West KP, Khatry SK, Katz J, LeClerq SC, Kimbrough-Pradhan E, Dali SM, Shrestha SR. 2000a. Vitamin A or beta-carotene supplementation reduces symptoms of illness in pregnant and lactating nepali women. Journal of Nutrition 130(11):2675–2682.
Christian P, West KP, Khatry SK, Kimbrough-Pradhan E, LeClerq SC, Katz J, Shrestha SR, Dali SM, Sommer A. 2000b. Night blindness during pregnancy and subsequent mortality among women in Nepal: effects of vitamin A and beta-carotene supplementation. American Journal of Epidemiology 152(6):542–547.
Diagne N, Rogier C, Cisse B, Trape JF. 1997. Incidence of clinical malaria in pregnant women exposed to intense perennial transmission. Transactions of the Royal Society of Tropical Medicine and Hygiene 91(2):166–170.
Donnay F. 2000. Maternal survival in developing countries: what has been done, what can be achieved in the next decade. International Journal of Gynaecology and Obstetrics 70(1):89–97.
Duale S. 1992. Delegation of responsibility in maternity care in Karawa rural health zone, Zaire. International Journal of Gynaecology and Obstetrics 38(suppl):S33–S35.
Duffy PE, Fried M. 1999. Malaria during pregnancy: parasites, antibodies and chondroitin sulphate A. Biochemical Society Transactions 27(4):478–482.
Dujardin B, Boutsen M, De Schampheleire I, Kulker R, Manshande JP, Bailey J, Wollast E, Buekens P. 1995. Oxytocics in developing countries. International Journal of Gynaecology and Obstetrics 50(3):243–251.
Duley L. 1992. Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the Caribbean. British Journal of Obstetrics and Gynaecology 99(7):547–553.
Duley L, Henderson-Smart D. 2000. Magnesium sulphate versus diazepam for eclampsia. Cochrane Database of Systematic Review (2):CD000127.
Duley L, Neilson JP. 1999. Magnesium sulphate and pre-eclampsia. Trial needed to see whether it’s as valuable in pre-eclampsia as in eclampsia. British Medical Journal 319(7201):3–4.
Duley L, Gülmezoglu AM, Henderson-Smart DJ. 2000. Anticonvulsants for women with pre-eclampsia. Cochrane Database of Systematic Review (2):CD000025.
Eclampsia Trial Collaberative Group. 1995. Which anticonvulsant for women with eclampsia? Evidence from the Collaborative Eclampsia Trial. Lancet 345(8963):1455–1463.
Ellis M, Manandhar N, Manandhar DS, Costello AM. 2000. Risk factors for neonatal encephalopathy in Kathmandu, Nepal, a developing country: unmatched case-control study. British Medical Journal 320(7244):1229–1236.
El-Refaey H, Nooh R, O’Brien P, Abdalla M, Geary M, Walder J, Rodeck C. 2000. The misoprostol third stage of labour study: a randomised controlled comparison between orally administered misoprostol and standard management. BJOG: An International Journal of Obstetrics and Gynaecology 107(9):1104–1010.
Garner P, Brabin B. 1994. A review of randomized controlled trials of routine antimalarial drug prophylaxis during pregnancy in endemic malarious areas. Bulletin of the World Health Organization 72(1):89–99.
Garner P, Gülmezoglu AM. 2000. Prevention versus treatment for malaria in pregnant women (Cochrane Review). The Cochrane Library, Issue 2.
Goldberg AB, Greenberg MB, Darney PD. 2001. Misoprostol and pregnancy. New England Journal of Medicine 344(1):38–47.
Graham W. 2002. Now or never—the case for measuring maternal mortality. Lancet 359:701–704.
Graham WJ, Bell JS, Bullough C. 2001. Can skilled attendance at delivery reduce maternal mortality in developing countries? In: De Brouwere V, Van Lerberghe W (eds). Safe Motherhood Strategies: A Review of the Evidence. Studies in Health Services Organization and Policy, 17. Antwerp: ITG Press. Pp. 97–130.
Greene MF. 2003. Magnesium sulfate for preeclampsia. New England Journal of Medicine 348(4):275–276.
Greenwood AM, Greenwood BM, Bradley AK, Williams K, Shenton FC, Tulloch S, Bypass P, Oldfield FS. 1987. A prospective survey of the outcome of pregnancy in a rural area of the Gambia. Bulletin of the World Health Organization 65:635–643.
Guidotti RJ. 2000. Anaemia in pregnancy in developing countries. BJOG: An International Journal of Obstetrics and Gynaecology 107(4):437–438.
Gupta JK, Nikodem VC. 2000. Woman’s position during second stage of labour. Cochrane Database of Systematic Review (2):CD002006.
Guyatt HL, Gotink MH, Ochola SA, Snow RW. 2002. Free bednets to pregnant women through antenatal clincs. I. Kenya: a cheap, simple and equitable approach to delivery. Tropical Medicine and International Health 7(5):409–420.
Harrison, KA. 1997. The importance of the educated healthy woman in Africa. Lancet 349(9052):644–647.
Henshaw SK, Kost K. 1996. Abortion patients in 1994–95: characteristics and contraceptive use. Family Planning Perspectives 28(4):140–147, 158.
Hill K, AbouZhar C, Wardlaw T. 2001. Estimates of maternal mortality for 1995. Bulletin of the World Health Organization 79(3):182–193.
Hofmeyr GJ, Nikodem VC, de Jager M, Gelbart BR, 1998. A randomised placebo controlled trial of oral misoprostol in the third stage of labor. British Journal of Obstetrics and Gynaecology 105(9):971–975.
Högberg U, Wall S, Brostrom G. 1986. The impact of early medical technology on maternal mortality in late 19th century Sweden. International Journal of Gynaecology and Obstetrics 24(4):251–261.
Jamelle RN. 1997. Eclampsia—a taxing situation in the Third World. International Journal of Gynaecology and Obstetrics 58(3):311–312.
Juarez-Vazquez J, Bonizzoni E, Scotti A. 2002. Iron plus folate is more effective than iron alone in the treatment of iron deficiency anaemia in pregnancy: a randomised, double blind clinical trial. British Journal of Obstetrics and Gynaecology 109(9):1009–1014.
Khan GQ, John LS, Wani, S, Doherty T, Sibai BM. 1997. Controlled cord traction versus minimal intervention techniques in delivery of the placenta: a randomized controlled trial. American Journal of Obstetrics and Gynecology 177:770-774.
Koblinsky MA, Campbell O, Heichelheim J. 1999. Organizing delivery care: what works for safe motherhood? Bulletin of the World Health Organization 77(5):399–406.
Konje JC, Ladipo OA. 2000. Nutrition and obstructed labor. American Journal of Clinical Nutrition 72(1 suppl):291S–297S.
Konje JC, Obisesan KA, Ladipo OA. 1992. Obstructed labor in Ibadan. International Journal of Gynaecology and Obstetrics 39(1):17–21.
Kulier R, de Onis M, Gülmezoglu AM, Villar J. 1998. Nutritional interventions for the prevention of maternal morbidity. International Journal of Gynaecology and Obstetrics 63(3):231–246.
Kwast BE. 1991a. Postpartum haemorrhage: its contribution to maternal mortality. Midwifery 7(2):64–70.
Kwast BE. 1991b. Puerperal sepsis: its contribution to maternal mortality. Midwifery 7(3):102–106.
Kwast BE. 1992. Obstructed labour: its contribution to maternal mortality. Midwifery 8(1):3–7.
Ladipo OA. 2000. Nutrition in pregnancy: mineral and vitamin supplements. American Journal of Clinical Nutrition 72(1 suppl):280S–290S.
Lawn J, McCarthy BJ, Ross S. 2001. The Healthy Newborn: A Reference Manual for Program Managers. Atlanta, GA: CDC, CARE.
Lengeler C. 2000. Insecticide-treated bednets and curtains for preventing malaria. Cochrane Database of Systematic Reviews (2):CD000363.
Lennox CE, Kwast BE. 1995. The partograph in community obstetrics. Tropical Doctor 25(2):56–63.
Loudon I. 1991. Some historical aspects of toxaemia of pregnancy. A review. British Journal of Obstetrics and Gynaecology 98(9):853–858.
Loudon I. 2000. Maternal mortality in the past and its relevance to developing countries today. American Journal of Clinical Nutrition 72(1 suppl):241S–246S.
Magee LA, Duley L. 2000. Oral beta-blockers for mild to moderate hypertension during pregnancy (Cochrane Review). Cochrane Database of Systematic Reviews (4):CD002863.
Magpie Trial Collaborative Group. 2002. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet 359(9321):1877–1890.
Mahomed K. 2002. Iron supplementation in pregnancy (Cochrane Review). The Cochrane Library, Issue 1.
Maine D. 1997. About the PMM Network. International Journal of Gynaecology and Obstetrics 59(suppl 2):S3–S6.
Maine D, Rosenfield A. 1999. The Safe Motherhood Initiative: why has it stalled? American Journal of Public Health 89(4):480–482.
Maine D, Karkazis K, Bolan N. 1994. The bad old days are still here: abortion mortality in developing countries. Journal of the American Medical Womens’ Association 49(5):137–142.
Mayberry LJ, Wood SH, Strange LB, Lee L, Heisler DR, Neilson-Smith K. 1999/2000. Managing second-stage labor. Association of Women’s Health, Obstetric and Neonatal Nurses Lifelines 3(6):28–34.
McCormick ML, Sanghvi HC, Kinzie B, McIntosh N. 2002. Preventing postpartum hemorrhage in low-resource settings. International Journal of Gynaecology and Obstetrics 77(3):267–275.
McDonagh M. 1996. Is antenatal care effective in reducing maternal morbidity and mortality? Health Policy Planning 11(1):1–15.
McDonald S, Prendiville WJ, Elbourne D. 2000. Prophylactic syntometrine versus oxytocin for delivery of the placenta. Cochrane Database of Systematic Reviews (2):CD000201.
McGregor IA, Wilson ME, Billewicz WZ. 1983. Malaria infection of the placenta in The Gambia, West Africa: its incidence and relationship to stillbirth, birthweight and placental weight. Transactions of the Royal Society of Tropical Medicine and Hygiene 77(2):232–244.
Michielsen PP, Van Damme P. 1999. Viral hepatitis and pregnancy. Acta Gastro-enterologica Belgica 62(1):21–29.
Miller LH, Smith JD. 1998. Motherhood and malaria. Nature Medicine 4(11):1244–1245.
Moller Bek K, Laurberg S. 1992. Intervention during labor: risk factors associated with complete tear of the anal sphincter. Acta Obstetricia et Gynecologica Scandinavica 71(7):520–524.
Moodley J. 1990. Treatment of eclampsia. British Journal of Obstetrics and Gynaecology 97(2):99–101.
Murray CJ, Lopez AD. 1997. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 349(9061):1269–1276.
Nelson KB, Grether JK. 1995. Can magnesium sulphate reduce the risk of cerebral palsy in very low birthweight infants? Pediatrics 95:263–269.
Ng PS, Chan AS, Sin WK, Tang LC, Cheung KB, Yuen PM. 2001. A multicentre randomized controlled trial of oral misoprostol and i.m. syntometrine in the management of the third stage of labor. Human Reproduction 16(1):31–35.
O’Brien P, El-Refaey H, Gordon A, Geary M, Rodeck CH. 1998. Rectally administered misoprostol for the treatment of postpartum hemorrhage unresponsive to oxytocin and ergometrine: a descriptive study. Obstetrics and Gynecology 92(2):212–214.
Okoko BJ, Ota MO, Yamuah LK, Idiong D, Mkpanam SN, Avieka A, Banya WA, Osinusi K. 2002. Influence of placental malaria infection on foetal outcome in the Gambia: twenty years after Ian Mcgregor. Journal of Health, Population, and Nutrition 20(1):4–11
Okonofua F. 1997. Preventing unsafe abortion in Nigeria. African Journal of Reproductive Health 1(1):25–36.
Pastorek JG. 1993. The ABCs of hepatitis in pregnancy. Clinical Obstetrics and Gynecology 36(4):843–854.
Philpott RH, Castle WM. 1972a. Cervicographs in the management of labour in primigravidae. I. The alert line for detecting abnormal labour. The Journal of Obstetrics and Gynaecology of the British Commonwealth 79(7):592–598.
Philpott RH, Castle WM. 1972b. Cervicographs in the management of labour in primigravidae. II. The action line and treatment of abnormal labour. The Journal of Obstetrics and Gynaecology of the British Commonwealth 79(7):599–602.
Prendiville WJ, Harding JE, Elbourne DR, Stirrat GM. 1988. The Bristol third stage trial: active versus physiological management of third stage of labour. British Medical Journal 297(6659):1295–300.
Prendiville WJ, Elbourne D, McDonald S. 2001. Active versus expectant management in the third stage of labour. Cochrane Pregnancy and Childbirth Group. Cochrane Database of Systematic Reviews (1):CD000007.
Rochat R, Akhter HH. 1999. Tetanus and pregnancy-related mortality in Bangladesh. Lancet 354(9178):565.
Rogers J, Wood J, McCandlish R, Ayers S, Truesdale A, Elbourne D. 1998. Active versus expectant management of third stage of labour: the Hinchingbrooke randomised controlled trial. Lancet 351(9104):693–699.
Rogo KO, Bohmer L, Ombaka C. 1999. Developing community-based strategies to decrease maternal morbidity and mortality due to unsafe abortion: pre-intervention report. East African Medical Journal 76(11 suppl):S1–S71.
Rouse DJ, Andrews WW, Goldenberg RW, Owen J. 1995. Screening and treatment of asymptomatic bacteriuria of pregnancy to prevent pyelonephritis: a cost-effectiveness and cost-beneficial analysis. Obstetrics and Gynecology 86:119–123.
Royston E, Armstrong S. 1989. Preventing Maternal Deaths. Geneva: WHO.
Rush D. 2000. Nutrition and maternal mortality in the developing world. American Journal of Clinical Nutrition 72(1 suppl):212S–240S.
Safe Motherhood Inter-Agency Group. 2000. Skilled Care During Childbirth: A Review of the Evidence. New York: Family Care International.
Save the Children. 2001. Making the Case. In: State of the World’s Newborns: a Report from Saving Newborn Lives. Available online at www.savethechildren.org/mothers/newborns.
Schendel DE, Berg CJ, Yeargin-Allsopp M, Boyle CA, Decoufle P. 1996. Prenatal magnesium sulfate exposure and the risk for cerebral palsy or mental retardation among very low-birth-weight children aged 3 to 5 years. Journal of the American Medical Association 276(22):1805–1810.
Serbanescu F, Morris L, Stupp P, Stanescu A. 1995. The impact of recent policy changes on fertility, abortion, and contraceptive use in Romania. Studies in Family Planning 26(2):76–87.
Skidmore SJ. 1997. Tropical aspects of viral hepatitis. Hepatitis E. Transactions of the Royal Society of Tropical Medicine and Hygiene 91(2):125–126.
Sleep J, Roberts J, Chalmers I, 1989. Care during the second stage of labor. In: Chalmers I, Enkin M, Keirse MJNC (eds). Effective Care in Pregnancy and Childbirth. Oxford: Oxford University Press.
Sloan NL, Jordan E, Winikoff B. 2002. Effects of iron supplementation on maternal hematologic status in pregnancy. American Journal of Public Health 92(2):288–293.
Smaill F. 2001. Antibiotics for asymptomatic bacteriuria in pregnancy (Cochrane Review). Cochrane Library, Issue 2.
Steketee RW. 2002. Malaria prevention in pregnancy: when will the prevention programme respond to the science. Journal of Health, Population, and Nutrition 20(1):1–3.
Steketee RW, Wirima JJ, Slutsker L, Heymann DL, Breman JG. 1996a. The problem of malaria and malaria control in pregnancy in sub-Saharan Africa. American Journal of Tropical Medicine and Hygiene 55(1 suppl):2–7.
Steketee RW, Wirima JJ, Bloland PB, Chilima B, Mermin JH, Chitsulo L, Breman JG. 1996b. Impairment of a pregnant woman’s acquired ability to limit Plasmodium falciparum by infection with human immunodeficiency virus type-1. American Journal of Tropical Medicine and Hygiene 55(1 suppl):42–49.
Steketee RW, Nahlen BL, Parise ME, Menendez C. 2001. The burden of malaria in pregnancy in malaria-endemic areas. American Journal of Tropical Medicine and Hygiene 64(1-2 Suppl):28–35.
Stoltzfus RA. 2001. Iron-deficiency anemia: reexamining the nature and magnitude of the public health problem. Journal of Nutrition 131:697S–701S.
Surbek DV, Fehr PM, Hosli I, Holzgreve W. 1999. Oral misoprostol for third stage of labor: a randomized placebo-controlled trial. Obstetrics and Gynecology 94(2):255–258.
Tan BP, Hannah ME. 2000. Oxytocin for prelabour rupture of membranes at or near term. Cochrane Library, Issue 2.
Thaddeus S, Maine D. 1994. Too far to walk: maternal mortality in context. Social Science and Medicine 38(8):1091–110.
The Prevention of Maternal Mortality Network. 1992. Barriers to treatment of obstetric emergencies in rural communities of West Africa. Studies in Family Planning 23(5):279–291.
Thompson A. 1999. Poor and pregnant in Africa: safe motherhood and human rights. Midwifery 15(3):146–153.
Tinker A. 1997. Safe motherhood as a social and economic investment. Presentation at Safe Motherhood Technical Consultation, Inter-Agency Group for Safe Motherhood, October 18–23, Colombo, Sri Lanka.
Tinker A. 2000. Women’s health: the unfinished agenda. International Journal of Gynaecology and Obstetrics 70(1):149–158.
Umezulike AC, Onah HE, Okaro JM. 1999. Use of the partograph among medical personnel in Enugu, Nigeria. International Journal of Gynaecology and Obstetrics 65(2):203–205.
United Nations Administrative Committee on Coordination/Sub-Committee on Nutrition (UNACC/SCN). 1994. Fourth Report on the World Nutrition Situation. Global and Regional Results. Geneva: UNACC/SCN.
United Nations Children’s Fund (UNICEF). 2000. The global malaria burden. The Prescriber. Number 18.
United Nations Children’s Fund (UNICEF), World Health Organization (WHO), United Nations Population Fund (UNFPA). 1997. Guidelines for Monitoring the Availability and Use of Obstetric Services. New York: UNICEF
van den Broek N. 1998. Anaemia in pregnancy in developing countries. British Journal of Obstetrics and Gynaecology 105(4):385–390.
van den Broek NR, Letsky EA. 2000. Etiology of anemia in pregnancy in south Malawi. American Journal of Clinical Nutrition 72(1 suppl):247S–256S.
Van Lerberghe W, De Brouwere. 2001. Of blind alleys and things that have worked: history’s lessons on reducing maternal mortality. In: De Brouwere V, Van Lerberghe W (eds). Safe Motherhood Strategies: A Review of the Evidence. Studies in Health Services Organization and Policy, 17. Antwerp: ITG Press.
Vaz F, Bergstrom S, Vaz Md, Langa J, Bugalho A. 1999. Training medical assistants for surgery. Bulletin of the World Health Organization 77(8):688–691.
Verhoeff FH, Brabin BJ, Hart CA, Chimsuku L, Kazembe P, Broadhead RL. 1999. Increased prevalence of malaria in HIV-infected pregnant women and its implications for malaria control. Tropical Medicine and International Health 4(1):5–12.
Villar J, Bergsjo P. 1997. Scientific basis for the content of routine antenatal care. I. Philosophy, recent studies, and power to eliminate or alleviate adverse maternal outcomes. Acta Obstetricia et Gynecologica Scandinavica 76(1):1–14.
Walker, JJ. 2000. Severe pre-eclampsia and eclampsia. Baillière’s Obstetrics and Gynaecology 14(1):55–71.
Walley RL, Wilson JB, Crane JM, Matthews K, Sawyer E, Hutchens D. 2000. A double-blind placebo controlled randomised trial of misoprostol and oxytocin in the management of the third stage of labour. BJOG: An International Journal of Obstetrics and Gynaecology 107(9):1111–1115.
Walraven G, Telfer M, Rowley J, Ronsmans C. 2000. Maternal mortality in rural Gambia: levels, causes and contributing factors. Bulletin of the World Health Organization 78(5):603–613.
Weil O, Fernandez H. 1999. Is safe motherhood an orphan initiative? Lancet 354(9182):940–943.
West KP, Katz J, Khatry SK, LeClerq SC, Pradhan EK, Shrestha SR, Connor PB, Dali SM, Christian P, Pokhrel RP, Sommer A. 1999. Double blind, cluster randomised trial of low dose supplementation with vitamin A or beta-carotene on mortality related to pregnancy in Nepal. The NNIPS-2 Study Group. British Medical Journal 318(7183):570–575.
World Bank. 1999. Safe Motherhood and the World Bank: Lessons From Ten Years Of Experience. Washington, DC: World Bank.
World Bank. 2002. World Development Indicators. Washington, DC: The World Bank.
World Health Organization (WHO), Maternal Health and Safe Motherhood Programme, Nutrition Programme. 1992. The Prevalence of Anemia in Women. 2nd edition. Geneva: WHO.
World Health Organization (WHO). 1994a. Abortion: A Tabulation of Available Data on the Frequency and Mortality of Unsafe Abortion. Geneva: WHO.
World Health Organization (WHO). 1994b. World Health Organization partograph in the management of labour. Lancet 343(8910):1399–1404.
World Health Organization (WHO). 1996. Revised 1990 Estimates of Maternal Mortality: A New Approach by WHO and UNICEF. Geneva: WHO.
World Health Organization (WHO). 1997. Coverage of Maternity Care: A Listing of Available Information. 4th edition. Geneva: WHO.
World Health Organization (WHO). 1998. WHO Mother-Baby Package: Implementing safe motherhood in countries. Geneva: WHO. Available online at http://www.who.int/reproductive-health/publications/MSM_94_11/MSM_94_11_table_of_contents.en.html.
World Health Organization (WHO). 1999. Reduction of Maternal Mortality: A Joint WHO/ UNFPA/UNICEF/World Bank Statement. Geneva: WHO.
World Health Organization (WHO). 2001. Maternal mortality in 1995: Estimates developed by WHO, UNICEF, UNFPA. Geneva: WHO.
World Health Organization (WHO). 2002a. The World Health Report 2002: Reducing Risks, Promoting Healthy Life. Geneva: WHO.
World Health Organization (WHO). 2002b. Managing Complications in Pregnancy and Childbirth: A Guide for Midwives and Doctors. 1st edition, 2nd Printing. Geneva: WHO.
World Health Organization (WHO), United Nation’s Children’s Fund. 1996. Revised 1990 Estimates of Maternal Mortality: A New Approach. Geneva: WHO and UNICEF.
World Health Organization (WHO), United Nations Children’s Fund (UNICEF), United Nations Population Fund (UNFPA). 2001. Maternal Mortality in 1995: Estimates Developed by WHO, UNICEF, UNFPA. Geneva: WHO
Wulf, D. 1999. Sharing Responsibility: Women, Society and Abortion Worldwide. New York: The Alan Guttmacher Institute.