3
Federal Leadership and Public–Private Partnerships
CHAPTER SUMMARY
Common clinical data standards are critical to establishing a national health information infrastructure that can support patient safety. While progress is being made in selecting core groups of these clinical data standards for national adoption, issues remain with the different standards development, approval, and maintenance processes currently employed by the multitude of organizations that produce data interchange and terminology standards. Delays in the ability of health care organizations to implement data standards will likely slow investment in information technology and necessitate sizable reworking of the information technology systems that currently exist to enable connectivity. Successful transition to and operation of the national health information infrastructure will require a more efficient, streamlined mechanism for standards development and implementation processes that can be achieved only through strong federal leadership and effective public–private partnerships. This chapter reviews current standards development and implementation processes and presents the committee’s recommendations for leveraging existing organizations and standards initiatives to build and sustain the informatics infrastructure envisioned in this report.
Development and implementation of data standards for the national health information infrastructure (NHII) will require the participation of
both industry and government to create an optimal set of specifications that meet compatibility and interoperability needs, enable regulatory requirements, and allow for continued innovation and technology advancement by a variety of vendors. The organizations associated with the development of the three types of standards required—data interchange, terminologies, and knowledge representation—have differing methods for developing and implementing those standards. Both standards and methods have remained rather uncoordinated to date, resulting in overlaps and gaps in the comprehensive set of data standards needed for full operation of the national health information infrastructure. This chapter describes current processes for setting each type of standard; reviews current standards activities in the federal and private sectors; and presents the committee’s recommendations for how the standards development, implementation, and dissemination process can be streamlined and coordinated for greater usefulness and efficiency.
CURRENT STANDARDS-SETTING PROCESSING
Data Interchange Standards
Data interchange standards are developed by three means—federal mandate by legislation or regulation, voluntary consensus through balloting of an industry professional group or sector, or de facto as the result of dominance in the commercial marketplace (see Figure 3-1). Once standards have been developed and approved, an integral part of their utilization is the conformity assessment process used to evaluate the compliance of products and processes with particular standards.
Most technical standards in the health sector and other industries are developed at the national and international levels through the voluntary consensus process, with the participation of industry members of standards development organizations (SDOs) and government representatives having an interest in the use of the standard. More recently, these three pathways have converged, primarily as a result of the administrative simplification provisions of the Health Insurance Portability and Accountability Act (HIPAA), which require that standards for transactions be selected from those developed through the voluntary consensus process and/or those available because of marketplace dominance, rather than from government-unique standards.
The American National Standards Institute (ANSI) bears the responsibility for endorsing consensus standards in the United States and for representing U.S. interests internationally in the International Organization for
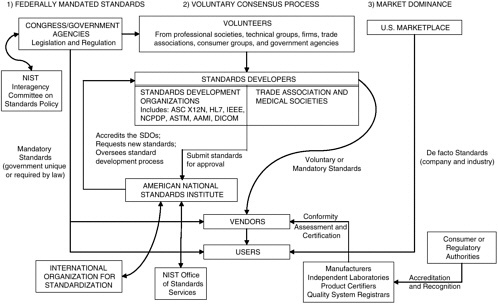
FIGURE 3-1 Overview of processes used to set standards for the exchange of health care data in the United States.
NOTE: AAMI = American Association of Medical Instrumentation; ASC = Accredited Standards Committee; ASTM = American Society for Testing and Materials; DICOM = Digital Imaging and Communication in Medicine; HL7 = Health Level Seven; IEEE = Institute for Electrical and Electronics Engineers; NCPDP = National Council for Prescription Drug Programs; NIST = National Institute of Standards and Technology.
Standardization (ISO). ANSI does not produce standards itself but functions as an accreditor of SDOs through its Accredited Standards Committee (ASC). ANSI primarily ensures that SDOs adhere to the principles of openness, balance of interests, due process, and an appeals process and approves standards that could become U.S. national standards. ANSI does not perform quality checks on the data interchange standards developed or distribute standards interpretation and implementation guides for consistency. Several primary SDOs in the United States develop the various types of data interchange standards required for health care data (see Box 3-1).
Other independent groups are involved indirectly. They include the National Uniform Billing Committee, which develops a single billing form and standard dataset to be used nationwide by institutional providers and payers for handling health care claims, and the National Uniform Claims Committee, which develops a standardized dataset for use by the noninstitutional health care community in transmitting claim and encounter information to and from all third-party payers.
|
BOX 3-1 ASC X12N—the ANSI committee responsible for developing health care–related electronic data interchange (EDI) standards for administrative and financial transactions. Health Level Seven (HL7)—chief developer of clinical data exchange, vocabulary, and document architecture standards. Institute for Electrical and Electronics Engineers (IEEE)—developer of medical device transmission and vocabulary specifications. American Society for Testing and Materials (ASTM)—developer of standards for medical and surgical materials and devices, emergency medical services, and health information systems. Digital Imaging and Communication in Medicine (DICOM)—developer of transmission and vocabulary standards for radiological images; created jointly by the American College of Radiology and the National Equipment Manufacturers Association and now is an international organization. American Dental Association (ADA)—developer of all standards relating to dentistry. National Council for Prescription Drug Programs (NCPDP)—developer of transmission standards for documents related to prescription drugs. |
As the chief endorser of U.S. standards, ANSI recognizes SDOs and their standards through three methods:
-
ANSI determines that a U.S. national standard is needed, but there is no work in this area. An organization to develop the needed standard is either created or contracted from existing sources. ASC then recognizes and oversees the standards development process.
-
An SDO independently develops standards following ANSI-prescribed rules and processes (i.e., balloting) for consensus standards.
-
An SDO submits an independently developed standard to ANSI for endorsement as a national standard, after canvassing the sector for objections and comments.
In the consensus balloting process (used by Health Level Seven [HL7], the Institute of Electrical and Electronics Engineers [IEEE], and the National Council for Prescription Drug Programs [NCPDP]), a draft standard is circulated to the SDO members and other interested parties for comment over a 6-week period. Care is taken to ensure balance among voters, with no more than 50 percent being vendors. All comments are considered, and negative ballots must be specifically addressed. If a negative ballot is persuasive, the standard is modified and must be reballoted, particularly if the changes are significant. If a negative ballot is not persuasive, the SDO requests its removal. If neither of these situations occurs, comments are sent to the entire balloting group for consideration. The resulting vote determines the content of the standard; for health care data interchange standards, over 90 percent agreement among the parties is usually required for a standard to be approved (American National Standards Institute, 2002). In fact, some SDOs, such as HL7, also have a preliminary approval process by a technical committee before a new or revised standard is presented for open balloting by ANSI rules (Health Level Seven, 2002).
For the canvassing process (used by the American Society for Testing and Materials [ASTM] and Digital Imaging and Communication in Medicine [DICOM]), a notice of a proposed standard is published in an official registry, and interested persons may provide objections and/or comments. In the absence of negative comments, the standard is approved. This process is generally not preferred over the consensus process because public notices are often ignored as the result of a lack of immediate need for interoperability, use of other data standards, or other reasons, resulting in a much less open or scientifically rigid process for achieving standardization.
Once data interchange standards have been approved by ANSI, they become American national standards, and the SDOs and ANSI can sell them to vendors. The standards must then be reviewed at least every 5 years for currency and appropriateness.
Implementing the standards is another challenge. A process is required for ensuring that vendor products comply with the standards. Conformity assessment is the comprehensive term for measures taken by manufacturers, their customers, regulatory authorities, and independent third parties to evaluate and determine whether products and processes conform to particular standards (National Research Council, 1995). Conformity is critical in ensuring standards compliance and quality of products for buyers, users, and regulators. In addition, the organizations that conduct conformity assessments are themselves accredited by consumer and/or regulatory authorities. Accreditors can be either public (e.g., the National Voluntary Laboratory Accreditation Program operated by the National Institute of Standards and Technology [NIST]) or private (e.g., the American Association for Laboratory Accreditation). Accreditation may involve the review of technical procedures used, staff qualifications, product sampling, test equipment calibration and maintenance, quality control, independence, and financial stability (National Research Council, 1995). The government is then involved in assessing the competence of programs that accredit conformity assessment organizations. Government acceptance or recognition has the effect of confirming official approval of the methods used by the accreditation organizations (National Research Council, 1995).
Most primary SDOs have signed memoranda of understanding with ANSI and with each other to define their areas of responsibility. Also, there is a strong drive toward harmonization of standards at the international level with the Comité Européen de Normalisation (CEN—the chief SDO for Europe, similar to ANSI) and ISO. Despite these efforts, significant overlap in the standards being developed by the SDOs still contributes to problems in their interpretation and implementation (see Table 3-1). Often the standards are further modified at the local level to accommodate personal preferences, which can directly affect vendors’ ability to compete effectively in the marketplace. The consequence has been a continued lack of interoperability.
ANSI’s Healthcare Informatics Standards Board (HISB) has recently made some efforts to encourage cooperation across the various standards-setting bodies. Two major issues have impeded the implementation of standards to support increased data comparability and system interoperability: (1) wide variation in the maturity and ease of implementation of ANSI voluntary consensus standards, and (2) the fact that even where mature, usable
TABLE 3-1 Overlap of Work by the Major Standards Development Organizations
|
Category |
ASTM |
DICOM |
HL7 |
NCPDP |
X12N |
IEEE |
CEN |
ISOa |
|
Clinical laboratory |
X |
|
X |
|
|
|
X |
X |
|
Data interchange |
X |
X |
X |
X |
X |
X |
|
X |
|
Vocabulary |
X |
X |
X |
X |
X |
X |
X |
X |
|
Object modeling |
X |
X |
X |
|
X |
|
X |
X |
|
Security |
X |
X |
X |
X |
X |
|
X |
|
|
Patient information |
X |
X |
X |
X |
X |
|
X |
X |
|
Accounting |
|
|
X |
X |
X |
|
|
|
|
XMLb |
X |
|
X |
|
X |
|
X |
X |
|
Electronic health records (EHR) |
X |
|
X |
|
|
|
X |
X |
|
Medical devices |
|
|
|
|
|
X |
|
X |
|
Templates |
X |
X |
X |
|
|
|
X |
|
|
aISO documents are generally derived from standards originally developed by CEN, DICOM, HL7, and/or IEEE. bExtensible markup language. |
||||||||
standards exist, terminologies have not been agreed upon for all the important domain areas (e.g., laboratory, devices). The committee believes that stronger leadership and coordination can position the ANSI HISB to address these issues.
Another important issue in standards development and implementation is financial support. Producing a standard is expensive in terms of both time and money. In the United States, vendors and users must be willing to support the hours of work involved (usually on “company time”), the travel expenses, and the costs of documentation and distribution. In contrast, most European and Asian (e.g., Japanese, Australian, and Korean) standards are developed and entirely funded by a government agency, then designated for widespread adoption.
Terminologies
The process for developing and updating terminologies is highly variable. Currently, there are well over 150 terminology systems in use to describe various medical domains. Other than the HIPAA-mandated standards, there has been little agreement on or implementation of common clinical terminologies across institutions and settings. Many of the terminologies that are used are either locally developed and maintained and exchangeable with other entities only at great effort; developed by proprietary
vendors for specific system implementations; or broad international terminologies (e.g., the International Classification of Diseases [ICD]) that are often modified at the local level. Thus, one laboratory system has not been able to communicate with another without great difficulty. This “custom” approach incurs high costs and inhibits efforts to reuse clinical data to understand and prevent patient safety events. For computer interoperability, data must be recorded in a terminology that is recognized and understood by the receiving computer; local terms are not interoperable. Adoption of a set of core terminologies for use in clinical information systems will accomplish this task, and such terminologies are in the process of being selected through public–private partnerships. Vendor compliance with the standards will ensure the interoperability of products for buyers, users, and regulators. Box 3-2 and the following subsections provide a brief overview of the processes used by these organizations to develop their terminology data standards.
International Organizations
Some international organizations (i.e., World Health Organization [WHO] for ICD codes and International Classification of Functioning, Disability and Health [ICF], and the World Organization of National Colleges, Academies and Academic General Practice and Family Physicians for the International Classification of Primary Care [ICPC]) engage in terminology development through a relatively formalized consensus process that requires approval by a governing oversight or steering committee (Institute of Electrical and Electronics Engineers, 2001; World Health Organization, 1999). For WHO terminologies, submissions for changes are accepted by the heads of the WHO collaborating centers established around the world. If accepted by the WHO regional office, they are then circulated to the other centers no later than 6 months in advance of the official WHO annual meeting, where they may receive final approval upon consensus of the group.
Federal Government
Development of clinical modifications for the ICD codes is accomplished through the National Center for Health Statistics at the Centers for Disease Control and Prevention (CDC) using an open and formal process that accepts suggestions by the public and private sectors and is managed by the Coordination and Maintenance Committee. Proposals for a new code must include a description of the code, why it is needed, and supporting
|
BOX 3-2
|
references and literature. Final decisions are made by the director of the National Committee on Health Statistics and the administrator of the Coordination and Maintenance Committee (National Center for Health Statistics and Centers for Disease Control and Prevention, 2003).
The Food and Drug Administration’s (FDA) process for incorporating new National Drug Codes (NDCs) has been highly informal; new codes are
|
submitted by the manufacturer, reviewed by the FDA for redundancy or overlap, and added to the set of codes. However, this procedure has been associated with several issues. One such issue is that drug codes for pharmaceuticals that are considered obsolete are often reused by the manufacturer, a practice that contributes to problems in tracking medication usage and potential drug interactions or contraindications. A second issue is the lack
of a single standard for chemical names, ingredient listings, and dosage sizes associated with NDCs, resulting in variation from one organization to another.
Professional Associations and Academic Institutions
Many terminologies in use are developed by professional associations and academic institutions. The processes for terminology development vary, generally depending on the size of the organization and the purpose of the standard. Organizations such as the College of American Pathologists (which produces the Systemized Nomenclature of Human and Veterinary Medicine [SNOMED]) and the American Medical Association (which produces Current Procedural Terminology [CPT]) have relatively formal sets of processes for developing and refining terminology concepts that rely on an editorial board for final approval. SNOMED technical subgroups evaluate and model terminology and then advise the editorial board regarding scope of coverage, creation of hierarchies, semantic definitions, and scientific accuracy (SNOMED International, 2000). Because CPT codes are used for reimbursement, new codes are not based on technical criteria but on the need to code new medical procedures. These updates are incorporated quarterly once approved by the American Medical Association editorial board. Processes used by other terminology development organizations, such as nursing groups, are very informal, with general group approval occurring annually.
Standards Development Organizations and Other Private Entities
IEEE has a highly formal approach to developing standards based on due process, openness, consensus, balance, and right of appeal. The membership of working groups is defined. Three-quarters of the members of a group must vote on ballots of official documents, and of those who vote, three-quarters must support the document for it to be accepted.
The HL7 process for obtaining consensus on terminologies is not as formal as that for data interchange standards since HL7 is not actually a terminology developer per se but maps registered terminologies for encoding in its messaging formats. Participants in the HL7 terminology special interest group agree to the additions, mappings, and technical specifications. However, sign-off by the HL7 steering committee is still required.
Private companies developing terminologies, such as Medicomp, create new terms at will without a formal or open process and as required by cli-
ents for the integration of clinical information systems. Vendors of laboratory systems also have historically developed and maintained terminologies that are modified locally for use within their systems.
Standardized Mappings of Terminologies
In addition to the terminology developers, two organizations have undertaken the development of standardized mappings among the different terminologies to facilitate data interchange—the National Library of Medicine (NLM) and HL7. While not a terminology developer, NLM has created a single database of the various terminologies—the Unified Medical Language System (UMLS). NLM has played a unique role in the development of the standardized mappings and cross-references from one terminology to another, and the UMLS is now considered the global reference database for linking disparate terminologies (National Library of Medicine, 2002). The UMLS cross-referencing function unifies terminologies that may have different content, structure, or semantics (National Library of Medicine, 2002), making it the key database for the development and maintenance of terminology extensions and/or new terminologies to represent medical concepts.
The UMLS is the result of a major collaboration among terminology developers and NLM (National Library of Medicine, 2002), which will be even more important to the evolution and maintenance of the National Committee on Vital and Health Statistics (NCVHS)–Consolidated Health Informatics (CHI) core terminology group for the electronic health records (EHR). To date, NLM has also collaborated with HL7 and pharmacy knowledge vendors, among others, to develop a common representation for clinical drugs as well as a comprehensive clinical drug reference terminology (Nelson et al., 2002). For example, NLM and several other federal agencies (i.e., Veterans Health Administration [VHA], CDC, FDA, National Aeronautics and Space Administration [NASA], and National Cancer Institute [NCI]) have contracted with Apelon, a private software and informatics company, to develop a set of integrated terminologies for clinical information (Apelon, 2003). In addition, terminology developers themselves are engaging in more collaborative relationships to establish terminology coverage for all medical domain areas and the multiplicity of uses defined for the NHII (e.g., College of American Pathologists and the United Kingdom’s National Health Service collaborated to create SNOMED Clinical Terms [CT]) (Bakken et al., 2000; Ozbolt, 2000; Wang et al., 2002). The College of American Pathologists also recently licensed SNOMED CT to the public sector through the UMLS, as announced in July 2003 by the secretary of the
Department of Health and Human Services (DHHS) (Department of Health and Human Services, 2003).
Because the UMLS has the informatics infrastructure to cross-reference many different terminology systems and provides its services in the public domain, NLM is ideally positioned to lead the oversight and maintenance of the core terminology group to be determined by the CHI initiative and associated patient safety data standards. Thus it is highly important that the UMLS be capable of adapting and constantly evolving to reflect current thinking in medicine and informatics (Campbell et al., 1998). To support the data standards initiatives of the NHII, NLM should establish a more formalized process for working in collaboration with terminology developers on the evolution and maintenance of the necessary data standards.
Knowledge Representation Standards
The establishment and maintenance of data standards are integrally linked to the advancement of clinical knowledge. The discovery of new knowledge leads to the redefinition of what constitutes best practices in a specific clinical area. Changes in best practices have implications for the design of care processes specifically for the clinical data requirements to support care delivery. For example, in 1981 new scientific evidence became available indicating that early diagnosis of certain eye conditions leads to improved outcomes in diabetes care (Diabetic Retinopathy Study Research Group, 1981). To ensure that this new evidence is applied consistently in practice, it must be translated into a practice guideline, and in 1988 the American Diabetes Association published eye care guidelines for patients with diabetes mellitus that included a recommended annual eye exam (American Diabetes Association, 1988).
Once a guideline has been issued, hospitals, physicians, and other providers must modify their care processes to be consistent with the new best practice. Likewise, information systems must be modified to capture the new information. However, the current health care delivery system lacks well-defined processes for translating new knowledge into consistent practice. For example, according to a 1997 report by the National Committee on Quality Assurance, the national rate for an annual diabetic eye exam (38.4 percent) was still below the recommended level (National Committee for Quality Assurance, 1997). Similar examples can be found in virtually every area of clinical practice (Balas and Boren, 2000; Chassin, 1997). Indeed, another recent study found that patients receive only about half (55 percent) of the recommended care interventions (McGlynn et al., 2003). Overall, the
toll in terms of lost lives, pain and suffering, and wasted resources is staggering. The Agency for Healthcare Research and Quality (AHRQ) has established a track record in funding the synthesis and dissemination of clinical knowledge and best practices through its work with the evidence-based practice centers (EPCs), primary care practice-based research networks (PBRNs), and the Integrated Delivery System Research Network (IDSRN). There are currently 13 EPCs developing evidence-based reports and technology assessments based on rigorous analysis of the scientific literature on clinical, social science/behavioral, economic, and other health care and delivery issues (Agency for Healthcare Research and Quality, 2003a). The EPCs and their partners, including federal and state agencies, private-sector professional societies, health delivery systems, providers, payers, and others, are expected to translate the findings into practice guidelines or other implementation tools to improve the quality of care within their organizations. To date, most of the EPCs have focused on selected chronic conditions, such as diabetes, epilepsy, stroke, congestive heart failure, and cancer.
Putting these evidence-based guidelines into a computer-readable format that can be used with decision support systems during clinical encounters is the objective of the informatics community. Many groups have been undertaking applied research on various approaches to modeling the guidelines (Peleg et al., 2003); however, none of these approaches are providing optimum performance in clinical practice (Maviglia et al., 2003). Another issue that impacts the development of computer-readable guidelines is the need for interoperability with a number of information systems operating in the context of the NHII. Therefore, several of the research groups are banding together to use the best from research to date and develop a generic model intended to serve as the baseline standard guideline format (Peleg et al., 2003). Chapter 9 provides a detailed discussion of these efforts.
Since 1993, AHRQ has supported important research through PBRNs. A PBRN is a group of ambulatory practices devoted to the primary care of patients, formed to investigate research questions related to community-based clinical practice (Agency for Healthcare Research and Quality, 2001). Typically, PBRNs draw on the experience and insight of practicing clinicians to identify and frame research questions that can be investigated with rigorous research methods to produce findings that can improve primary care practice (Agency for Healthcare Research and Quality, 2001). To date, the PBRNs have studied such topics as the role of antibiotics in improving outcomes in children with acute otitis media, the referral process in pediatric care, and primary and secondary prevention of coronary artery disease and stroke (Agency for Healthcare Research and Quality, 2001). Nineteen net-
works are established across the United States, providing an outstanding resource on which AHRQ can draw to assist in the implementation of the clinical information systems, data standards, patient safety reporting systems, and other components of the NHII.
The IDSRN was initiated by AHRQ in September 2000 with the purpose of linking researchers and large health care delivery systems. It is made up of nine partners, selected because they provide health care services to large populations in a variety of organizational settings. Each partner is working with several collaborators, including other health care systems, research institutions, and managed care organizations, to conduct research within their integrated delivery systems and then disseminate the scientific evidence obtained (i.e., organizational best practices related to care delivery) to the entire network. The ISDRN also includes researchers and sites that are testing ways to adapt and apply existing knowledge to care delivery. To date, 44 IDSRN projects have been funded covering a wide range of topics, including quality measurement and improvement, bioterrorism, information technology, organization and financing, and disparities in access to care. The efforts of the EPCs, IDSRN, and others, including the Cochrane Collaboration (Cochrane Collaboration, 2003) and the ACP Journal Club (American College of Physicians, 2003), are excellent models and provide the building blocks for a more comprehensive effort to address the 20 priority areas identified by the Institute of Medicine (IOM) that account for the bulk of health care services (Institute of Medicine, 2003).
AHRQ’s Centers for Education and Research on Therapeutics (CERTs) program is a national initiative to conduct research and provide education on the benefits and risks of new, existing, or combined uses of therapeutics (drugs, medical devices, biologics) (Agency for Healthcare Research and Quality, 2002). The CERTs program has three primary aims: (1) to increase awareness of both the uses and risks of new drugs and drug combinations, biological products, and devices, as well as of mechanisms to improve their safe and effective use; (2) to provide clinical information to patients and consumers, health care providers, pharmacists and pharmacy benefit managers and purchasers, health maintenance organizations and health care delivery systems, insurers, and government agencies; and (3) to improve quality while reducing cost of care by increasing the appropriate use of drugs, medical devices, and biological products and by preventing their adverse effects and the consequences of those effects (such as unnecessary hospitalizations) (Agency for Healthcare Research and Quality, 2002). Because adverse drug events (ADEs) are one of the most common types of patient safety event, use of AHRQ’s expertise in evaluating information and dis-
seminating it to the public should prove very helpful and may contribute greatly to the number of ADE reports that are submitted.
Another recent promising initiative in this area of linking the evidence base to care is MedBiquitous, a consortium of professional medical associations and related organizations that is creating an extensible markup language (XML) framework for professional medicine, with a focus on medical education and credentialing (MedBiquitous Consortium, 2003). The XML standards allow providers to search the literature more easily to locate specific types of content related to particular medical conditions and also permit professional societies to verify board certifications of providers automatically.
Reporting Standards
The health care sector also currently lacks standardized measurement and reporting mechanisms for routinely monitoring the extent to which health care is safe and effective. In designing and building information technology systems, it is helpful to know in advance the reporting specifications that must be satisfied. As noted earlier in this report, many safety and quality measurement and improvement efforts are sponsored by health care providers, public and private purchasers, federal and state agencies, and accreditors. Some focus on near misses or adverse events, while others assess compliance with best practices through medical care performance and outcome measures. However, as noted by a previous IOM committee, too many resources are spent on health care measures that are either duplicative or ineffective, and little comparative quality information is made available in the public domain for use by beneficiaries, health professionals, or other stakeholders (Institute of Medicine, 2002). In addition, users of the available measures are hindered by the lack of reporting standards and consistent methodologies (Eddy, 1998; Rhew et al., 2001).
Standardized measurement and reporting mechanisms not only will facilitate the building of effective information technology systems and reduce confusion over reporting requirements but also could drive quality improvement in other ways, such as assisting efforts to reward quality care through payment or other means. For example, the Centers for Medicare and Medicaid Services (CMS), in partnership with Premier, Inc., a private alliance of more than 200 hospitals and health care systems, is currently conducting a demonstration project to evaluate the linking of standard performance measurements to differential hospital reimbursement (McGinley, 2003; Premier, 2003). Over the course of this 3-year project, those hospitals identified as
top performers will receive an additional 1 to 2 percent reimbursement over current diagnosis-related group levels for a subset of conditions. Another program linking payment to quality has been proposed in House Resolution 2033, the Medicare Equity and Access Act (Government Printing Office, 2003). This proposal would award financial incentives to Medicare+Choice organizations that demonstrate superior-quality health care, based on their Health Plan Employer Data and Information Set (HEDIS) and Consumer Assessment of Health Plans data. Other ways in which standardized reporting and measurement could drive improvement include aiding in the development of national benchmarks that could be used to identify regional differences, enabling the research community to identify the factors that promote or hinder quality health care, and assisting in linking patient outcomes with those responsible for those outcomes (Institute of Medicine, 2002).
Recently, some noteworthy efforts have been made to encourage standardization of reporting requirements. In terms of event reporting, the Patient Safety Task Force is beginning to standardize the reporting mechanisms among DHHS agencies. This task force, which began functioning in 2000 and was rechartered in 2001 by the secretary of DHHS, includes representatives from AHRQ, CDC, FDA, and CMS. Their primary activity has been a project to integrate the patient safety reporting systems within DHHS through a contract awarded to the Kevric Corporation. This integration will result in user-friendly reporting formats, cross-matching and electronic analysis of data, and more rapid responses to patient safety problems. The identification of standards for coding the content of reports made to these systems is a primary task of the project. While the project is initially integrating six of the DHHS systems, all patient safety reporting systems under the jurisdiction of the department will eventually be incorporated. (For further information on federal patient safety reporting systems, see Appendix C.)
In the area of best practices, the Quality Interagency Coordination Task Force (QuIC) was established in 1998 with representation from all of the federal agencies involved in purchasing, providing, studying, or regulating health care services.2 QuIC has worked to address tasks that are key to the use of quality performance measures, such as developing an inventory of all
the measures and risk adjustment methods being used by federal agencies; documenting their uses, strengths, and weaknesses; and examining how to institute appropriate risk adjustment methods (Quality Interagency Coordination Task Force, 2001). QuIC has also made considerable progress in establishing standardized safety and quality measures and tools, such as the Diabetes Quality Improvement Project measures, which have been incorporated into multiple government health care programs.
Several private-sector groups are also working in this area. For example, the National Quality Forum (NQF), an organization created to develop and implement a national strategy for health care quality measurement and reporting, has established standardized reporting requirements for a set of 27 preventable adverse events called “never” or serious reportable adverse events (National Quality Forum, 2002).
On the performance and outcome measurement side, a large part of the work of the National Committee for Quality Assurance (NCQA), the accreditation program for managed care organizations, is reporting on a set of performance measurements in selected areas using HEDIS (National Committee for Quality Assurance, 2003). The set is updated annually and allows for comparison of the quality of commercial, Medicaid, and Medicare managed care plans. It measures the quality of care for many common health conditions and incorporates other established measure sets, including the Consumer Assessment of Health Plans, the Diabetes Quality Improvement Project, and the Health Outcomes Survey. Another private-sector group working in the area of standardization of performance measures is the Joint Commission on Accreditation of Healthcare Organizations (JCAHO), which accredits hospitals, ambulatory clinics and surgical centers, clinical laboratories, home health agencies, assisted living and long-term care facilities, behavioral health services, hospices, integrated delivery systems, health maintenance organizations, and preferred provider organizations. JCAHO initiated the ORYX initiative in 1997, under which accredited hospitals must contract with listed performance measurement vendors, who work to aggregate the organizations’ patient-level data and report on a set of core performance measures in a standardized manner to JCAHO (Joint Commission on Accreditation of Healthcare Organizations, 2003). The Foundation for Accountability (FACCT), an organization of health care purchasers and consumer groups, has also been working toward more standardized performance measurement. Beginning in 1997, under a project initially funded by the Health Care Financing Administration (now CMS) for Medicare beneficiaries, FACCT created a “consumer information framework” that consists of a set of standard measures in five major categories:
the basics, staying healthy, getting better, living with illness, and changing needs (Foundation for Accountability, 1999). FACCT selected measures from multiple sources and is continuing to develop new measures to fill perceived gaps.
Despite all of these efforts, work on standardizing event reporting and performance measurement activities has been slow. In 2002, the IOM recommended that QuIC be given the statutory authority and adequate resources to coordinate and standardize the government’s activities in the area of quality performance reporting (Institute of Medicine, 2002). This committee endorses that recommendation and agrees with the previous IOM committee that QuIC should coordinate its efforts with private-sector groups—including NQF, NCQA, JCAHO, and FACCT—involved in the promulgation of standardized event reporting and performance and outcome measures.
CURRENT STANDARDS ACTIVITIES IN THE FEDERAL AND PRIVATE SECTORS
As noted earlier, the efforts of both the public and private sectors to invest in information technology are hampered by the lack of national standards for the collection, coding, classification, and exchange of clinical, administrative, and reporting and quality assurance data. The role of the federal government in the promulgation of standards is one that is well developed in other sectors of the economy. For example, the Securities and Exchange Commission has statutory authority to establish financial accounting and reporting standards for all publicly held companies under the Securities and Exchange Act of 1934 (University of Cincinnati College of Law, 2003). Despite some well-publicized recent failures, these standards are meant to require credible, transparent, and comparable financial information that can be used by investors, creditors, and auditors. The commission often authorizes private-sector entities, such as the Financial Accounting Standards Board, to conduct this work and then officially recognizes the standards these entities develop as authoritative. In the health care sector, however, there has been a historical lack of federal coordination in the establishment of national standards. With the exception of morbidity and mortality codes for public health reporting (i.e., ICD-9 codes) and code sets for reimbursement (i.e., ICD-9, CPT, and the Healthcare Financing Administration Common Procedure Coding System), each agency has determined any additional data interchange, reporting, and terminology standards for its own system.
This trend is changing, and efforts are currently under way that constitute important building blocks toward a national infrastructure for the promulgation of health care reporting requirements and standards. These efforts include AHRQ’s EPCs and the work of QuIC, discussed earlier in this chapter, as well as the partnership between NCVHS and the CHI initiative.
National Committee on Vital and Health Statistics
A significant effort to establish common data standards is in progress under the leadership of NCVHS (National Committee on Vital and Health Statistics, 2000). NCVHS, first established in 1949 as a federal advisory committee on heath statistics issues, is made up of 18 members from the private sector. Its role was broadened by HIPAA to include identifying and recommending standards for administrative simplification and for the privacy and security of health care information, as well as to “study the issues related to the adoption of uniform data standards for patient medical record information (PMRI) and the electronic exchange of such information.” While NCVHS has the lead in identifying and recommending clinical data standards, it has only an advisory role to DHHS and has not been empowered to designate or mandate standards.
Specifically, the HIPAA standards accomplished the following:
-
Designated specific SDOs for the development and maintenance of HIPAA standards.
-
Approved the ANSI ASC X12N standard as the EDI messaging format standard for eight administrative/financial transactions and NCPDP as the messaging standard for pharmacy billing transactions.
-
Selected code sets for diagnoses and procedures for administrative/ financial transactions, i.e., ICD-9 Clinical Modification (CM); Healthcare Financing Administration Common Procedure Coding System (HCPCS); CPT-4; NDCs; and Common Dental Terminology, Second Edition (CDT-2).
-
Mandated identifiers for employers.
-
Established privacy rules and security safeguards for the protection of personal health information.
Compliance deadlines have been established for all of the above HIPAA standards. In addition, the use of unique identifiers for health care providers has undergone the Notice of Proposed Rulemaking process and is awaiting announcement of the final rule. The HIPAA standard for claims attachments is being developed.
Consolidated Health Informatics Initiative
NCVHS is also serving as the primary advisory body to the CHI initiative, established in October 2001 as the first of the 24 Office of Management and Budget eGOV initiatives to streamline and consolidate government programs among like sectors (Office of Management and Budget, 2003). DHHS was designated as the managing partner for the CHI initiative, with CMS in the lead. Other members of CHI include the VHA, the Department of Defense, the Indian Health Service, the U.S. Department of Agriculture, Environmental Protection Agency, and the FDA. CHI’s mission is to articulate and execute a coherent vision and strategy for the adoption of federal interoperability standards for health care information while providing technical support to selected projects. As noted earlier in this report, in March 2003 the secretary of DHHS announced that DHHS, the Department of Defense, and VHA would be adopting an initial set of clinical data interchange standards recommended to the secretary by NCVHS, including HL7, NCPDP, IEEE 1073, DICOM, and the Logical Observation Identifiers, Names, and Codes (LOINC) for laboratory tests and results (usually referred to as Laboratory LOINC). This announcement was based directly on the recommendations of CHI for standards that should be adopted government-wide. CHI is also continuing to identify standardized terminologies for the clinical domain areas associated with the EHR for use among federal agencies. The organizations are expected to announce their selection of the core group of standard terminologies in fall 2003. Given the sizable purchasing power (more than 40 percent of health care expenditures) and regulatory authority of the federal government, incorporation of these data standards into government programs is a powerful and effective approach to establishing national standards. However, CHI still lacks a clear mandate to establish standards that will be applied by all government programs. The CHI initiative also would benefit from greater participation of private-sector stakeholders and standards-setting bodies and from more clearly defined linkages with AHRQ’s evidence synthesis program and QuIC’s performance measurements standardization program.
Public Health Data Standards Consortium
In the public sector, in addition to CHI’s activities, an organization that is involved in the promotion of national data standards is the Public Health Data Standards Consortium, which is coordinated by the National Center for Health Statistics. It was developed to organize the public health and
health services research communities on data standards issues. This consortium serves as a mechanism for ongoing representation of public health and health services research interests in HIPAA implementation and other data standards–setting processes. Its membership comprises a variety of state-based public health data organizations, health services research organizations, federal public health representatives (e.g., CDC, AHRQ, and CMS), managed care organizations, business coalitions, and consumer groups. The consortium’s tasks have included an ad hoc work group on External Causes of Injury Codes (E-Codes), which will evaluate current practices in E-Codes collection and propose next steps to improve E-Codes reporting in discharge data systems and electronic reporting standards. Two other consortium work groups recently developed a standardized format as a guide for reporting health care service data. This guide is compatible with the health claim transaction set standards identified by HIPAA. It is intended to provide assistance in developing and executing the electronic transfer of health care systems data for reporting purposes to local, state, and federal agencies that use the data for monitoring utilization rates, assessing patterns of health care quality and access, and other purposes required by legislative and regulatory mandates. Given the past difficulties and financial constraints of state-based public health organizations, the consortium could serve as one of the key facilitators helping states to implement national data standards for the NHII and guiding implementation of the common format for reporting to the AHRQ national patient safety database.
In addition to the efforts of NCVHS, other activities to promotion and implement data standards are being conducted in the private sector. The Markle Foundation’s Connecting for Health initiative is focusing on building consensus around and accelerating the development of clinical data standards. In June 2003, this initiative released the results of a 9-month collaborative effort focused on key aspects of the adoption of clinical data standards, which included identifying strategies and solutions for the secure and private transmission of medical information and actively working to understand what consumers will need and expect from an interconnected electronic health system.
National Alliance for Health Information Technology
Another recent private-sector effort in the area of promotion and implementation of data standards is the National Alliance for Health Information Technology, founded in June 2002 by the American Hospital Associa-
tion and other interested organizations (The National Alliance for Health Information Technology, 2002). Its membership currently includes provider organizations, such as hospitals, health systems, medical groups, and professional associations; technology companies and vendors; and other stakeholders, such as standards groups, the government, and payers. The purpose of the alliance is to develop and promote interoperability standards for health care information technology systems in order to improve patient outcomes and increase patient safety. In the near term, the alliance is focusing on standardized bar codes for products used by health care organizations. Plans for the longer term are to focus on connectivity and communications technology, automated order entry, electronic medical records, and standardized nomenclatures.
Integrating the Healthcare Enterprise
A third effort in the private sector was initiated by the Healthcare Information and Management Systems Society and the Radiological Society of North America in November 1998. The Integrating the Healthcare Enterprise initiative is working to facilitate the adoption of existing standards for communicating clinical and operational data by specifying how to apply such standards to real-world scenarios and integration problems (HIMSS, 2003). The initiative process consists of four steps: (1) identification of common integration problems and needs by clinicians and information technology experts, represented by their professional societies; (2) specification of the existing health care or other general information technology standards that address the identified needs; (3) participation by vendors in an open testing process; and (4) publication of Initiative Integration Statements documenting the integration profiles, which can be accessed by users to assist in the vendor selection process (HIMSS, 2003). To date, Integrating the Healthcare Enterprise has introduced several integration profiles—12 for radiology and 5 for information technology infrastructure—which were published in August 2003 (HIMSS, 2003). Despite these activities, the absence of national leadership in the establishment of standards for the collection, coding, and classification of data means that the information and communications systems built over the coming decade will provide inadequate support for the delivery of safe and effective care, will be unable to share information among all of a patient’s caregivers, and will require costly rework to respond to external reporting requirements.
NEED FOR MORE FORMALIZED LEADERSHIP
If the NHII is to be realized, more formalized leadership in the establishment of data standards will be required. The committee’s recommendations to this end center on a principal partnership among CHI, NCVHS, and NLM; overarching coordination; and strengthened leadership on the part of DHHS.
Principal Partnership: Consolidated Health Informatics, National Committee on Vital and Health Statistics, and National Library of Medicine
It is critical that Congress provide clear direction, enabling authority, and financial support for the establishment of national data standards to support patient safety. DHHS should be given the lead role in establishing and maintaining a public–private partnership for the promulgation of national standards for data interchange, terminologies, knowledge representation, and reporting (see Figure 3-2). Central to this public–private partnership is the CHI initiative, which should work collaboratively with NCVHS to identify data standards appropriate for national adoption as well as gaps in existing standards that need to be filled. Although NCVHS has already become a primary advisory body to CHI, this relationship needs to be formalized to ensure adequate representation of all stakeholders in the decision-making process. As a federal body that is subject to the Federal Advisory Committee Act, CHI cannot have private-sector members, but the viewpoints of many interested parties outside of the federal government should be considered. NCVHS is in a strong position to provide that input, as it is already designated as a private-sector advisory body to DHHS. The current membership of NCVHS includes individuals from health plans, universities, and other private organizations and associations with backgrounds in health statistics, electronic interchange of health care information, privacy and security of electronic information, population-based public health, purchasing or financing of health care services, integrated computerized health information systems, health services research, consumer interests in health information, health data standards, epidemiology, and the provision of health services (National Committee on Vital and Health Statistics, 2001). The membership of NCVHS should continue to be broad and diverse with adequate representation of all stakeholders, including consumers, state governments, professional groups, and standards-setting bodies.
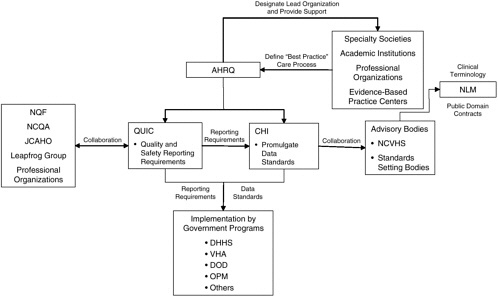
FIGURE 3-2 Proposed public-private partnership to establish national standards.
NOTE: AHRQ = Agency for Healthcare Research and Quality; CHI = Consolidated Health Informatics; DHHS = Department of Health and Human Services; DOD = Department of Defense; JCAHO = Joint Commission on Accreditation of Healthcare Organizations; NCQA = National Committee for Quality Assurance; NCVHS = National Committee on Vital and Health Statistics; NLM = National Library of Medicine; NQF = National Quality Forum; OPM = Office of Personnel Management; QuIC = Quality Interagency Coordinating Task Force; VHA = Veterans Health Administration.
One of the most powerful roles played by the federal government in the U.S. health care sector is that of regulator, and the government has historically used this role to address quality and patient safety concerns. Regulatory requirements (e.g., Medicare conditions of participation) generally focus on institutional providers, clinicians, and health plans that seek to receive payment from or deliver care under an identified program; however, these responsibilities can also be exercised by state governments that administer the programs. Therefore, once CHI and NCVHS have identified national data standards, those standards should be incorporated into the contractual and regulatory requirements of the major federal government health care programs, including those operated or sponsored by DHHS, VHA, and the Department of Defense. Broad stakeholder input into the data standards selection process should facilitate such incorporation, which will aid in rapid adoption of the identified data standards nationwide.
NLM also will need to assume new responsibilities for ensuring the establishment of national data standards for patient safety. As noted earlier in this chapter, NLM has worked to develop standardized mappings from one terminology to another through the UMLS and is therefore ideally positioned to become the primary oversight body for maintenance of the core terminology group to be established by CHI and associated patient safety data standards. The committee also recommends that NLM be designated as the responsible entity for the distribution of all national clinical terminologies related to patient safety and for assuring the quality of terminology mappings. NLM should work closely with the terminology developers to establish a more formalized development process and serve as a primary information source for CHI–NCVHS regarding available terminologies and areas in which terminologies are still needed.
Overarching Coordination
In addition to the need to strengthen the partnership and leadership of CHI, NCVHS, and NLM, the committee believes that AHRQ is an agency positioned to provide overarching coordination among all public- and private-sector organizations involved in the development, implementation, and dissemination of data standards, evidence-based guidelines, and patient safety and quality improvement programs. AHRQ is already the chief agency leading efforts in two of the three areas—evidence-based guidelines and patient safety and quality improvement programs—and as such has well-established core competencies in these areas, public–private networks, and relationships with the provider community that are critical to the suc-
cessful development of the NHII. The committee believes it is important to build on AHRQ’s expertise and capabilities by extending that agency’s role in overarching coordination for the implementation and dissemination of data standards that support the agency’s programs in clinical guidelines, patient safety data, and quality measures, as well as public health and the NHII. Additionally, AHRQ is playing a key role with providers and public health entities in addressing the challenges of building the NHII and has allocated $50 million of its $84 million fiscal year 2004 budget to support health information technology initiatives ($24 million for safety- and quality-related projects and $26 million for community and rural projects); $10 million of the budget has been allocated for standards adoption projects (Agency for Healthcare Research and Quality, 2003b). AHRQ also has the breadth and depth of resources to support the overarching coordination needed for widespread development of the NHII. The committee recognizes that the costs to support the corresponding programs and initiatives that would fall within the scope of AHRQ’s coordinating activities would be significant. Because of AHRQ’s historical, extensive experience in critical areas that directly relate to the NHII, it is best placed to provide a detailed cost estimate for development, implementation, and dissemination activities.
AHRQ can support the work of CHI–NCVHS in several ways. AHRQ should:
-
Provide financial support for standards development activities as necessary to fill the standards gaps identified by CHI–NCVHS.
-
Work with SDOs to ensure the development of implementation guides (which provide the specifications and instructions for how to implement a standard (Federal Register, 2000), certification procedures, and conformance testing for all data standards to facilitate their adoption by vendors and users. Additional information on the standards implementation process is presented in Chapter 4.
-
Coordinate activities and maintain a clearinghouse of information in support of national data standards and their implementation to improve patient safety.
-
Move forward with the establishment of a national patient safety database that utilizes a common report format and associated data standards as recommended by the IOM (2000).
-
Take on a chief role in the development of a robust agenda for applied research in patient safety, focused on enhancing knowledge, developing information technology tools, and disseminating research findings as outlined in Chapter 5.
-
Build on its track record in funding the synthesis and dissemination of clinical knowledge and assume a lead coordinating role in working with health professions leadership, specialty societies, academic institutions, and others to translate clinical knowledge into best-practice care processes, which in turn will inform the CHI–NCVHS decision-making process regarding national knowledge representation standards.
Strengthened Department of Health and Human Services Leadership for the National Health Information Infrastructure
The NCVHS report Information for Health provides a high-level strategy for building the NHII (National Committee on Vital and Health Statistics, 2001). It includes as one of its chief recommendations the establishment of a new and separate office to handle policy, coordination, and strategic oversight for NHII initiatives and projects, led by a senior officer reporting directly to the secretary of DHHS. Since the report was issued, the NHII office has been established in the Office of Science and Data Policy within the Office of the Assistant Secretary for Planning and Evaluation. The NCVHS recommendations empowered the Office of Science and Data Policy with broad responsibilities, including coordination with all relevant stakeholders, the strategic planning for NHII development, management of the NHII budget, promotion of effective training methods in health informatics, and assurance that all population groups will share in the activities and benefits of information technology integration. The office is now beginning to exercise leadership and held its first NHII conference to develop specific goals and a strategic plan for the key areas of the infrastructure’s operation: architecture, standards and vocabulary, safety and quality, financial incentives, consumer health, homeland security, privacy and security, and research and population health. The committee fully supports the NCVHS recommendations and the NHII activities conducted to date. At the same time, the committee believes that the NHII office must step forward more aggressively with stronger leadership and an accelerated approach to the integration of information technology into the health care delivery system.
REFERENCES
Agency for Healthcare Research and Quality. 2001. Primary Care Practice-Based Research Networks: Fact Sheet. Online. Available: http://www.ahcpr.gov/research/pbrnfact.htm [accessed September 5, 2003].
———. 2002. Centers for Education and Research on Therapeutics: Overview. Online. Available: http://www.ahcpr.gov/clinic/certsovr.htm [accessed September 15, 2003].
———. 2003a. AHRQ—Evidence-Based Practice. Online. Available: http://www.ahrq.gov/clinic/epcix.htm [accessed April 25, 2003a].
———. 2003b. U.S. Department of Health and Human Services FY 2004 Budget in Brief. Online. Available: http://www.hhs.gov/budget/04budget/fy2004bib.pdf [accessed November 10, 2003].
American College of Physicians. 2003. About ACP Journal Club. Online. Available: http://www.acpjc.org/shared/purpose_and_procedure.htm [accessed July 14, 2003].
American Diabetes Association. 1988. Eye care guidelines for patients with diabetes mellitus. Diabetes Care 11 (9):745–746.
American National Standards Institute. 2002. ANSI Procedures for the Development and Coordination of American National Standards. Online. Available: http://public.ansi.org/ansionline/Documents/Standards%20Activities/American%20National%20Standards/Procedures,%20Guides,%20and%20Forms/anspro2002r.doc [accessed June 1, 2003].
Apelon. 2003. Apelon Awarded $4.7 Million Contract to Assist VA with Enterprise Terminology Development. Online. Available: http://www.apelon.com/news/press_040803.htm [accessed July 28, 2003].
Bakken, S., K. E. Campbell, J. J. Cimino, S. M. Huff, and W. E. Hammond. 2000. Toward vocabulary domain specifications for Health Level 7-coded data elements. J Am Med Inform Assoc 7 (4):333–342.
Balas, E. A., and S. A. Boren. 2000. Managing clinical knowledge for health care improvement. Yearbook of Medical Informatics 65–70.
Campbell, K. E., D. E. Oliver, and E. H. Shortliffe. 1998. The unified medical language system: Toward a collaborative approach for solving terminologic problems. J Am Med Inform Assoc 5 (1):12–16.
Chassin, M. R. 1997. Assessing strategies for quality improvement. Health Aff (Millwood) 16 (3):151–161.
Cochrane Collaboration. 2003. Cochrane Collaboration Brochure. Online. Available: http://www.cochrane.org/software/docs/newbroch.pdf [accessed July 14, 2003].
Department of Health and Human Services. 2003. HHS Launches New Efforts to Promote Paperless Health Care System. Online. Available: http://www.hhs.gov/news/press/2003pres/20030701.html [accessed July 7, 2003].
Diabetic Retinopathy Study Research Group. 1981. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS report number 8: The diabetic retinopathy study research group. Ophthalmology 88 (7):583–600.
Eddy, D. M. 1998. Performance measurement: Problems and solutions. Health Aff (Millwood) 17 (4):7–25.
Federal Register. 2000. Rules and regulations—Part 160: General administrative requirements. Fed Regist 65 (160):50365–50367.
Foundation for Accountability. 1999. The FACCT Consumer Information Framework. Online. Available: http://www.facct.org/facct/doclibFiles/documentFile_203.doc [accessed July 30, 2003].
Government Printing Office. 2003. H.R. 2033: Medicare Equity and Access Act. Online. Available: http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=108_cong_bills&docid=f:h2033ih.txt.pdf [accessed July 30, 2003].
Health Level Seven. 2002. Bylaws of Health Level Seven, Inc. Online. Available: http://www.hl7.org/about/bylaw.htm [accessed June 1, 2003].
HIMSS. 2003. Integrating the Healthcare Enterprise (IHE) Fact Sheet. Online. Available: http://www.himss.org/content/files/IHE_FactSheet.pdf [accessed July 30, 2003].
Institute of Electrical and Electronics Engineers. 2001. About IEEE 1073. Online. Available: http://www.ieee1073.org/info/about-contacts.html [accessed February 12, 2002].
Institute of Medicine. 2000. To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press.
———. 2002. Leadership by Example: Coordinating Government Roles in Improving Health Care Quality. Washington, DC: The National Academies Press.
———. 2003. Priority Areas for National Action: Transforming Health Care Quality. Washington, DC: The National Academies Press.
Joint Commission on Accreditation of Healthcare Organizations. 2003. Performance Measurement. Online. Available: http://www.jcaho.org/pms/index.htm [accessed July 30, 2003].
Maviglia, S. M., R. D. Zielstorff, M. Paterno, J. M. Teich, D. W. Bates, and G. J. Kuperman. 2003. Automating complex guidelines for chronic disease: Lessons learned. J Am Med Inform Assoc 10:154–165.
McGinley, L. 2003, May 27. Medicare plan would give bonuses for superior care. The Wall Street Journal. Online. Available: http://online.wsj.com/article/0,,SB105397331989909600,00.html.
McGlynn, E. A., S. M. Asch, J. Adams, J. Keesey, J. Hicks, A. DeCristofaro, and E. A. Kerr. 2003. The quality of health care delivered to adults in the United States. N Engl J Med 348 (26):2635–2645.
MedBiquitous Consortium. 2003. MedBiquitous Homepage. Online. Available: http://www.medbiq.org [accessed May 13, 2003].
National Center for Health Statistics and CDC. 2003. ICD 9 CM Coordination and Maintenance Committee. Online. Available: http://www.cdc.gov/nchs/about/otheract/icd9/maint/maint.htm [accessed August 1, 2003].
National Committee for Quality Assurance. 1997. Quality Compass 1997. Annapolis Junction, MD: NCQA Publications Center.
———. 2003. NCQA Overview. Online. Available: http://www.ncqa.org/Communications/Publications/overviewncqa.pdf [accessed July 28, 2003].
National Committee on Vital and Health Statistics. 2000. Uniform Data Standards for Patient Medical Record Information. Online. Available: http://ncvhs.hhs.gov/hipaa000706.pdf [accessed April 15, 2002].
———. 2001. Information for Health: A Strategy for Building the National Health Information Infrastructure. Online. Available: http://ncvhs.hhs.gov/nhiilayo.pdf [accessed April 18, 2002].
National Library of Medicine. 2002. UMLS Knowledge Sources. January Release—13th Edition. Washington, DC: U.S. Department of Health and Human Services.
National Quality Forum. 2002. Serious Reportable Events in Patient Safety: A National Quality Forum Consensus Report. Washington, DC: National Quality Forum.
National Research Council. 1995. Standards, Conformity Assessment, and Trade: Into the 21st Century. Washington, DC: National Academy Press.
Nelson, S. J., S. H. Brown, M. S. Erlbaum, N. Olson, T. Powell, B. Carlsen, J. Carter, M. S. Hole, and W. T. Tuttle. 2002. A semantic normal form for clinical drugs in the UMLS: Early experiences with the VANDF. J Am Med Inform Assoc 557–561.
Office of Management and Budget. 2003. Consolidated Health Informatics. Online. Available: http://www.whitehouse.gov/omb/egov/gtob/health_informatics.htm [accessed April 21, 2003].
Ozbolt, J. 2000. Terminology standards for nursing: Collaboration at the summit. J Am Med Inform Assoc 7 (6):517–522.
Peleg, M., S. Tu, J. Bury, P. Ciccarese, J. Fox, R. Greenes, R. Hall, P. Johnson, N. Jones, A. Kumar, S. Miksch, S. Quaglini, A. Seyfang, E. Shortliffe, and M. Stefanelli. 2003. Comparing computer-interpretable guideline models: A case study approach. J Am Med Inform Assoc 10(1):52–68.
Premier, Inc. 2003. HHS, Premier Announce New Initiative to Improve, Reward Healthcare Quality. Online. Available: http://www.premierinc.com/all/newsroom/press-releases/03-jul/premier-cms.htm [accessed July 14, 2003].
Quality Interagency Coordination Task Force. 2001. QuIC Fact Sheet. Online. Available: http://www.quic.org/about/quicfact.htm [accessed April 28, 2003].
Rhew, D. C., M. B. Goetz, and P. G. Shekelle. 2001. Evaluating quality indicators for patients with community-acquired pneumonia. Jt Comm J Qual Improv 27 (11):575–590.
SNOMED International. 2000. About SNOMED. Online. Available: http://www.snomed.org/governance_txt.html [accessed August 1, 2003].
The National Alliance for Health Information Technology. 2002. The National Alliance for Health Information Technology Prospectus. Online. Available: http://www.hospitalconnect.com/nahit/content/prospectus.pdf [accessed May 30, 2003].
University of Cincinnati College of Law. 2003. The Securities Lawyer’s Deskbook: Securities Exchange Act of 1934. Online. Available: http://www.law.uc.edu/CCL/34Act/index.html [accessed August 5, 2003].
Wang, A. Y., J. H. Sable, and K. A. Spackman. 2002. The SNOMED clinical terms development process: Refinement and analysis of content. Proc AMIA Symp 845–849.
World Health Organization. 1999. Procedures for Updating ICD-10. Online. Available: http://www.who.int/whosis/icd10/update.htm [accessed August 1, 2003].































