5
Comprehensive Patient Safety Programs in Health Care Settings
CHAPTER SUMMARY
Based on the premise that patient safety is an integral part of the delivery of quality care, health care settings should establish comprehensive patient safety programs. The committee sets forth in this chapter a complete program for improving patient safety within a culture of safety. This program needs to be pilot tested, with the results discussed widely and supported by a research program.
The key elements of a culture of safety include (1) a shared belief that although health care is a high-risk undertaking, delivery processes can be designed to prevent failures and harm to participants; (2) an organizational commitment to detecting and analyzing patient injuries and near misses; and (3) an environment that balances the need for reporting of events and the need to take disciplinary action. Improving patient safety requires a multiphased process beginning with the detection of injuries and near misses and ending with a mechanism for ensuring that improvements in patient safety are maintained. A model for the introduction of safer care is presented. The application of these ideas is illustrated through two case studies—one relating to adverse drug events and the other to postoperative deep wound and organ space infections.
A key aspect of a patient safety program is the involvement of patients and their families in the process. Finally, to foster the development and implementation of comprehensive patient safety programs, a research agenda for knowledge generation, tool development, and dissemination is needed.
A CULTURE OF SAFETY
Improvements in patient safety are best achieved when health care delivery organizations adopt a culture of safety. A culture of safety can be defined as an integrated pattern of individual and organizational behavior, based upon shared beliefs and values, that continuously seeks to minimize patient harm that may result from the processes of care delivery (Kizer, 1999).
A measurement strategy based on a culture of safety is sometimes called a just (i.e., fair) system. Such a strategy implements two complementary ideas. First, it describes a system within which health professionals can report injuries and near misses safe from blame, humiliation, and retaliation (O’Leary, 2003). Second, such open and complete reporting is key in creating an environment that reliably avoids injuries and near misses—that is, a care delivery system that is safe for patients.
A culture of safety encompasses the following elements (adapted from Kizer, 1999): shared beliefs and values about the health care delivery system; recruitment and training with patient safety in mind; organizational commitment to detecting and analyzing patient injuries and near misses; open communication regarding patient injury results, both within and outside the organization; and the establishment of a just culture. Aspects of organizational leadership relating to the implementation of information technology systems were addressed in Chapter 2.
Systemic improvements in the way health care is delivered should not be made at the expense of a weakening of the sense of professional responsibility. Health care professionals still need to be adequately prepared both mentally and physically to carry out their responsibilities. They also need to be aware of the environment in which they practice and seek to eliminate distractions that can be avoided. In addition, they need to be vigilant in identifying hazardous situations and able to respond to these situations when they occur.
Shared Beliefs and Values
A culture of safety requires a shared recognition among all members of a health care delivery organization, reinforced regularly and rigorously by professional and organizational leaders, that health care is a highly complex, error-prone, and thus high-risk undertaking. Failures are inevitable when dealing with humans and complex systems, regardless of how hard the humans involved try to avoid errors. However, hazards and errors can be anticipated, and processes can be designed both to avoid failures and to prevent patient harm when a failure occurs.
Recruitment and Training with Patient Safety in Mind
A culture of safety requires organizational understanding that knowledge and skills are an essential foundation for safe practices. Also required is a recognition that such competence is ephemeral and must be actively maintained. At present, health professions education does not address many subjects critical to a safe care delivery environment.
Organizational Commitment to Detecting Patient Injuries and Near Misses
As part of a culture of safety, organizations need to commit to detecting as many patient injuries and near misses as possible through the following means:
-
Active surveillance based on case finding through real-time, interventional, prospective data-based clinical trigger systems, as well as retrospective chart review driven by code-based trigger systems.
-
Routine self-assessments to identify error-prone or high-risk processes, systems, or settings that could jeopardize patient safety (see Box 5-1).
-
Standardized, widely understood, and easily accessible mechanisms for voluntary reporting, with an independent team completing all the paperwork. These mechanisms could include a simple computerized reporting system allowing front-line care professionals to mark possible injuries for independent review; telephone and e-mail tip lines enabling front-line professionals, patients, and family members to report potential adverse events or near misses; and a system for asking front-line health professionals, as they leave work, whether they experienced any unsafe conditions or observed any injuries or near misses during their just-completed workday.
|
BOX 5-1
|
-
These procedures could be augmented by internal safety experts and organizational leaders conducting regular “walk-around” reviews to identify potential weaknesses in patient safety.
-
Appropriate protections and rewards for individuals who report injuries and near misses. The most potent reward for front-line health professionals may be seeing their reports lead to real changes in systems that result in a safer care environment.
Organizational Commitment to Analyzing Patient Injuries and Near Misses
In parallel with a commitment to detecting as many patient injuries and near misses as possible, there should be an organizational commitment to developing a management structure for tracking and rigorously analyzing injury-related events. There should also be a commitment to monitoring proven solutions from outside the organization that may address sources of injury the organization has yet to encounter. In addition, there should be a commitment to identifying and prioritizing possible actions to reduce injury rates; verifying actions taken, their effectiveness, and whether there were untoward secondary effects; and ensuring leadership involvement in and coordination of all these activities.
Open Communication
Another key element of a culture of safety is an organizational commitment to open communication. This commitment begins with leadership setting clear expectations regarding patient safety through publicized organizational goals. It also includes open sharing of patient injury results, both within and outside the organization (i.e., with front-line professionals, boards of directors or trustees, patients and patient representatives, and health care overseers) as part of a transparent care delivery system.
A Just Culture
A “just” culture is a key element of a safe culture (Reason and Hobbs, 2003). If data to support a learning environment are to be collected, employees must be willing to report adverse events and near misses without threat of retribution. On the other hand, a totally blame-free environment, sometimes referred to as a “bungler’s charter,” is not acceptable. A just culture seeks to balance the need to learn from mistakes and the need to take disciplinary action (Marx, 2001). Processes for differentiating between blameless and blameworthy acts have been proposed (Reason and Hobbs, 2003).
On the basis of experience from other industries and with some important exceptions given later, the committee believes protection from disciplinary action should be afforded to front-line workers when they report injuries, errors, and near misses even if they were personally involved. This belief derives from proven performance in other endeavors, such as airline transportation, nuclear power, safe manufacturing environments, and high-reliability military operations (e.g., aircraft carrier operations). Without such protections, injury reporting rates drop drastically, and with them the ability to prevent future injuries. Such protections reflect an acknowledgment that errors are nearly never intentional, nor are they caused by simple human failures alone. Health care delivery organizations should be held accountable for designing and implementing safe processes, which in turn make it possible for front-line health professionals to deliver safe care.
Three important exceptions apply, however. Protection is not granted for criminal behavior (e.g., a physician treating a patient while inebriated), for active malfeasance (e.g., a nurse who purposely violates safety policies or short-circuits built-in protections), or cases in which an injury is not reported in a timely manner (usually within 1 to 2 days).
A MODEL FOR INTRODUCING SAFER CARE
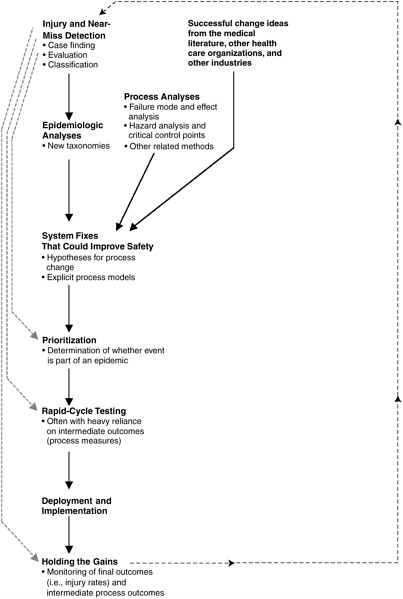
The ultimate aim of standardized patient safety data is safer care. While not all change produces improvement, safer care requires change. Data standards and data collection have no utility unless they lead to change that produces safer care. The process for positive change has the following elements (see Figure 5-1):
-
Injury and near-miss detection
-
Epidemiological analyses
-
Generation of hypotheses for change—develop a list of system fixes that could potentially improve safety results
-
Prioritization of improvement opportunities
-
Rapid-cycle testing
-
Deployment and implementation
-
Holding the gains
Each of these elements is discussed in turn below.
Injury and Near-Miss Detection
Detection of injuries and near misses contributes to safety improvement at four key points by:
-
Providing information for epidemiological analyses.
-
Using relative failure rates to help formulate research priorities.
-
Demonstrating what changes work in practice.
-
Checking to see whether the expected improvements are sustained over time.
Traditional reporting systems can grossly underdetect injuries, significantly impeding the ability to improve. A balanced detection system necessarily relies on case finding through surveillance, working together with voluntary incident reporting systems. Injury surveillance uses data-based clinical trigger systems that lead to prospective expert review, as well as retrospective review of patient records identified by International Classification of Diseases (ICD)-9, Clinical Modification (CM) discharge codes, and External Causes of Injury Codes (E-Codes) (Xu et al., 2003). Until research efforts (discussed later in this chapter) make more such tools available, data-
based clinical trigger systems would initially focus on adverse drug events (ADEs) and hospital-acquired infections, then move on to other common causes of injuries. This injury detection and tracking effort should start with hospitals and then be introduced into other care delivery settings, such as nursing homes, surgical centers, and outpatient offices.
In the future, adverse event detection should become much more a part of the routine fabric of care. Systems for adverse event detection and prevention should be embedded within the broader proactive hazard analysis framework—an approach to identifying and minimizing or eliminating hazards.
Epidemiologic Analyses, Hypotheses for Change Generation, and Prioritization
Effective, standardized injury detection and reporting plays a key role in patient safety by providing the information with which patient safety officers and other researchers can conduct epidemiologic analyses of injury data. Improvement teams often must try several different ways of organizing and analyzing injury data before finding an approach that leads to successful change and improvement. Good patient safety data systems need to be capable of supporting new, innovative classification approaches, or taxonomies, as health professionals seek system solutions that can prevent future failures.
Other proven sources of change hypotheses include failure mode and effect analysis (FMEA) and hazard analysis and critical control points (HACCP) (McDonough, 2002) (see also Appendix D), as well as process changes that have been demonstrated to work in other settings within and outside health care delivery. Failure mode and effect analysis and hazard analysis and critical control points are used to analyze process work flow, with the aim of identifying likely failure points, rather than relying upon epidemiologic analysis of actual injuries and near misses. They thus offer the possibility of preventing failures even before the first patient has been injured.
Human beings cannot think about a problem—for example, how to deliver patient care or collect and analyze data—without an underlying mental model (Smith, 1998). When measuring, managing, and improving care delivery processes, it is highly useful to make underlying models visible by writing them down. Written models help produce consensus and enable critical examination, leading to improvement of the underlying mental models themselves (James, 2003). Just as important, a written model helps iden-
tify key measurement factors and grounds those measurements within the care delivery context. Successful process change often relies as much on data about the performance of process steps—intermediate outcomes—as on final near-miss and injury rates.
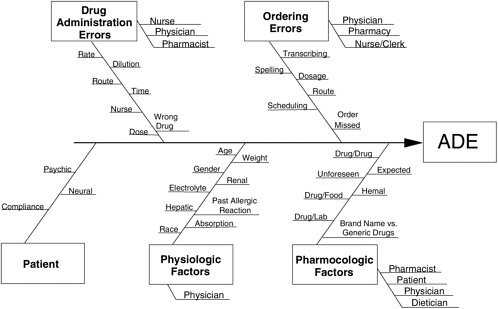
A cause-and-effect diagram such as that shown in Figure 5-2 is one relatively unstructured way of making a mental model explicit (Sholtes et al., 2003). Other, more highly organized methods for displaying models include conceptual flow diagrams (a form of flow charting, leading to traditional decision flow charts) and outcome chains.
All sources of injury are not created equal. A Pareto chart (see Table 5-1) ranks causes or possible solutions from most to least frequent, with the aim of targeting improvement activities to those areas that will achieve the most benefit for patients (Sholtes et al., 2003).
Other factors beyond injury rates may be important for setting improvement priorities. For example, existing leadership, available measurement systems, the local culture of health professionals, readiness for change at the front-line level, and sound theory identifying likely process improvements can all greatly affect the likelihood of success within a particular area. However, actual failure rates always play an essential part in the choice of points of attack for safety improvement—the second key role for an effective, standardized patient safety data system. The same principle applies at a larger scale: some sources of injury, such as ADEs, hospital-acquired infections, and decubitus ulcers—are orders of magnitude more common than some other, more sensational, sources of injury, such as wrong-side surgery.
Rapid-Cycle Testing
Having helped choose an aim for safety improvement and generate a list of potential changes that might lead to that goal, effective, standardized patient injury detection and reporting plays a third critical role: it allows an improvement team to determine whether a change is an improvement (Langley et al., 1996). As noted earlier, an improvement team often must try a series of change ideas before finding a combination that results in demonstrated better performance. Often, some elements of the final, successful change strategy are unique to the particular local environment within which the improvement effort occurred. Local circumstances, such as available data systems, clinical culture, the nature of patient populations being served, and organizational readiness for change, can make a large difference in what works. Local injury and near-miss tracking can therefore play an important role as a team discovers what works in its particular circumstances.
TABLE 5-1 Frequency of Adverse Drug Events by Cause
Deployment and Implementation
“Pilot and deploy” is an approach to implementing improvements that has been successful in a number of care delivery organizations. The idea is simple: choose an important systemwide safety problem; then determine methods for achieving demonstrated performance within a small group using rapid-cycle improvement tools. A small group often avoids larger organizational change issues and thus can discover effective process steps more rapidly. Once the necessary process steps are known, they can be implemented in other parts of the organization. Demonstrated success in the pilot
group makes the discovered changes concrete and often provides a potent incentive to other teams. Pilot team members frequently become natural advocates and consultants, with high credibility at the peer-to-peer level.
Deployment and implementation methods, when used as part of an improvement strategy, differ from traditional clinical research in two ways. First, improvement often focuses more on clinical work flow and operational process than on patients’ clinical response to treatment. It aims to make the process do the right thing, the right way, the first time, every time (James, 1989) to achieve demonstrated excellent performance. Often, this means carefully designing care delivery systems so that health professionals find it easy to do it right (James, 2001). It involves building best care into standard work processes, with publication of new biomedical science as a secondary goal. Second, even though the pilot project may have identified key process factors that play important roles in implementation in other settings, most delivery settings, as noted above, include unique local factors. Therefore, successful deployment requires the ability to try change ideas locally and determine whether they do in fact produce better results in the particular setting. In other words, effective, standardized detection and reporting of injuries and near misses is a key part of deployment. Under a pilot and deploy strategy, ideas tried during the deployment phase have the advantage of having shown success in at least one previous setting. When such ideas are implemented with local testing in other settings, the rate of successful change accelerates.
Holding the Gains
The aim of improvement is to establish a new baseline, but achieving this aim often requires new work processes, support systems, and professional habits. These requirements feed back to the need for new case finding methods, evaluation procedures, and classification systems. An effective, standardized injury detection and reporting system therefore plays a fourth key role: once successful change has been implemented, it helps the care delivery team maintain the gains (Juran, 1989). Otherwise, processes and performance can drift back to their old baselines as attention shifts.
TWO CASE STUDIES
The application of the above ideas is illustrated through two case studies. One concerns ADEs and the other postoperative deep wound and organ space infections. Throughout these case studies, the key elements of the
model for introducing safer care detailed in the preceding section are highlighted in bold print.
|
CASE STUDY 1 In 1988, researchers working within a 520-bed, tertiary teaching hospital’s departments of clinical epidemiology, pharmacy, and medical informatics (the improvement team) questioned whether the hospital’s existing nurse incidence reporting system adequately detected ADEs. They compared three different ADE detection systems in a parallel trial: (1) traditional nurse incidence reporting; (2) enhanced reporting; and (3) prospective expert case review, driven by a data-based clinical trigger system. Enhanced reporting allowed nurses to simply flag a patient through the computerized charting system, avoiding the time and effort of filling out an incident report. A representative from the improvement team reviewed the patient’s chart, determined whether an ADE had occurred, and completed the documentation. The clinical trigger system involved a series of treatment markers for ADEs, such as the use of antidote drugs (e.g., naloxone to counteract an opiate), abnormal values on specific laboratory tests (e.g., a twofold increase in blood creatinine), or other clinical indicators (e.g., reports of rash or itching in nursing notes). A positive clinical trigger led to prospective review by a clinical pharmacist within 24 hours, using explicit criteria. A clinical pharmacist from the improvement team also used explicit criteria to review all cases detected by traditional nurse incidence reporting to confirm whether an actual ADE had occurred. During the review, all ADEs were staged as mild, moderate, or severe, and their causes and patient outcomes were documented. Over 18 months (May 1, 1989, through October 31, 1990), covering 36,653 hospitalizations, standard nurse incidence reporting, enhanced reporting, and prospective expert review driven by data-based clinical triggers found, respectively, 9, 92, and 731 confirmed ADEs (Classen et al., 1991). While enhanced reporting increased ADE detection rates by an order of magnitude, prospective expert review driven by data-based clinical triggers increased detection 80-fold. Three members of the improvement team, expert in ADEs, reviewed more than 200 charts to identify ADE causes. Early analyses that classified ADEs by hospital location (e.g., emergency department versus operating room versus nursing unit) and by drug type (i.e., narcotics versus antibiotics) were not as useful as those that classified failures by process mechanism (epidemiological analyses and hypotheses for change generation). The team organized its findings as a cause-and-effect diagram (see Figure 5-2), then tallied actual ADEs to generate a Pareto chart of prioritized causes |
|
(Evans et al., 1994). Table 5-2 lists the most common sources of detected ADEs. On the basis of its ADE causal analysis, the improvement team began to devise, test, and implement changes to drug ordering, delivery, and review systems within the hospital (rapid-cycle testing) (Classen et al., 1992; Evans et al., 1994). Figure 5-3 shows ADE rates at the hospital as the detection system was enhanced (1988–1990) and then as system fixes were implemented to prevent or reduce the consequences of ADEs (1993–1999). Several changes produced better performance. Under the data-based clinical trigger system, rapid case review by a pharmacist led to more rapid recognition of an event with immediate clinical reaction, averting some ADEs in earlier, less severe stages. The hospital’s electronic pharmacy system was programmed to recommend safer alternatives when a physician ordered highly allergenic medications. The electronic pharmacy system was also programmed to calculate ideal medication doses for each dose delivered, based on patient age, gender, and body mass; estimates of kidney function; estimates of liver function; and other blood chemistry values. It was demonstrated that similar results can be obtained without an electronic medication decision support system by having a pharmacist join physicians and nurses as they conduct patient rounds each day or by having pharmacists conduct their own independent patient rounds (Leape et al., 1999). The same ADE prevention system was later deployed to sister hospitals in the region (deployment and implementation). The hospital system continued to monitor ADE rates to ensure that its investment in safer patient care did not deteriorate as organizational attention was shifted to other major sources of injury (holding the gains). In March 2000, a visiting clinical researcher analyzed almost 10 years of data on ADEs detected by the hospital’s data-based clinical trigger system (Henz, 2000). As Table 5-2 shows, among more than 70 clinical triggers in active use during the trial, 14 accounted for more than 95 percent of all ADEs detected. A number of groups have used the resulting list of high-yield clinical triggers to build manual and automated ADE detection systems, with the aim of delivering safer care. More recent internal investigation has suggested that the data-based clinical triggers could be improved even further through examination of interactions among triggers on the list (Kim, 2003). Other researchers have investigated enhanced case finding based on ICD-9 CM discharge abstract codes and E-Codes, followed by retrospective chart review using explicit criteria to detect ADEs. Initial results suggest that such methods can roughly double the total number of ADEs detected relative to those found by the data-based clinical trigger system (Xu et al., 2003). Such activities represent the start of a second major improvement cycle, which if successful, could lead to a further decline in the single largest source of care-related injuries Americans face when hospitalized. |
TABLE 5-2 Major Causes of Adverse Drug Events
|
ADE |
Alert |
Location |
True Positive Rate (%) |
% of All ADEs Detected |
Cumulative % Detected |
|
1. |
Use of naloxone |
Pharmacy |
21.9 |
28.3 |
28.3 |
|
2. |
Use of benadryl |
Pharmacy |
21.0 |
20.8 |
49.1 |
|
3. |
Use of inapsine |
Pharmacy |
39.2 |
20.4 |
69.5 |
|
4. |
Use of lomotil |
Pharmacy |
26.8 |
7.5 |
77.0 |
|
5. |
Nurse reports of rash/itching |
Nurse reporting |
17.9 |
5.1 |
82.1 |
|
6. |
Use of loperamide |
Pharmacy |
22.3 |
3.4 |
85.5 |
|
7. |
Test for c.difficile toxin |
Clinical laboratory |
24.3 |
3.1 |
88.6 |
|
8. |
Digoxin level > 2 |
Clinical laboratory |
2.3 |
2.2 |
90.8 |
|
9. |
Abrupt med. stop/reduction |
Pharmacy |
48.0 |
1.0 |
91.8 |
|
10. |
Use of vitamin K |
Pharmacy |
4.8 |
0.9 |
92.7 |
|
11. |
Doubling of blood creatinine |
Clinical laboratory |
0.4 |
0.8 |
93.5 |
|
12. |
Use of kaopectate |
Pharmacy |
21.8 |
0.7 |
94.2 |
|
13. |
Use of paregoric |
Pharmacy |
9.8 |
0.7 |
95.0 |
|
14. |
Use of flumazenil |
Pharmacy |
77.3 |
0.7 |
95.7 |
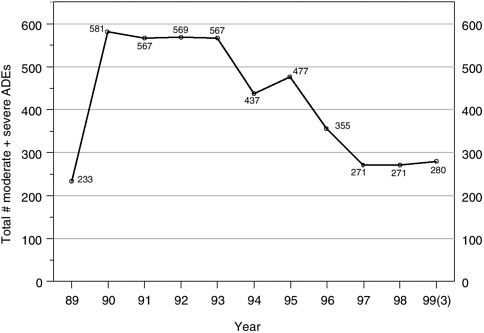
FIGURE 5-3 Detected ADE rates at a large teaching hospital, as a more effective detection system was put in place (1988–1990) and as a series of medication ordering, delivery, and follow-up systems were implemented (1994–1999). Comparing 1990–1993 (preintervention period) with 1997–1999 (postintervention period), the detected ADE rate fell from 571 to 274 ADEs per year on average—a 52 percent decline.
|
CASE STUDY 2 The second most common source of significant inpatient injuries is postoperative deep wound infection (Gawande et al., 1999; Leape et al., 1991). The same hospital-based improvement team as that in case study 1 recognized that infection detection within its hospital, based upon recommendations developed and widely distributed by the Centers for Disease Control and Prevention (CDC), represented a data-based clinical trigger system. The team enhanced the hospital’s ability to detect postoperative deep wound infections by implementing careful patient follow-up after hospital discharge through calls to attending physicians’ offices and, occasionally, directly to patients (injury and near-miss detection). The improvement team also created a working model for infection prevention (hypotheses for change generation), then used expert opinion to focus its model on the timing of delivery of prophylactic antibiotics for clean or clean-contaminated surgery cases (prioritization). For most case types, postoperative infection rates were significantly lower if the antibiotics were started within 2 hours before the initial surgical incision was made, which produced high antibiotic levels in the patient’s blood and tissue at the time the surgery started (Classen et al., 1992). Having established a strong link between a key intermediate outcome (timing of antibiotic prophylaxis) and the primary outcome of interest (postoperative deep wound and organ space infection rates), the improvement team was able to use a process factor (whether the antibiotic prophylaxis was started within the ideal 2-hour time window) to drive change. Failure to deliver antibiotic prophylaxis within the ideal time window is usually a near miss; only in a minority of cases does the process failure produce an outcome failure. However, use of a process step as a primary performance measure greatly increased the sample size (compared with infection rates) and enhanced the improvement team’s ability to tell when a change had resulted in improvement. The improvement team then devised (hypotheses for change generation) and tested (rapid-cycle testing) a series of process change hypotheses to bring the hospital closer to the established clinical ideal. Table 5-3 shows on-time antibiotic prophylaxis rates and associated postoperative deep wound and organ space infection rates over time as the hospital’s process improved. The process change that finally worked best in this hospital’s care delivery environment involved fully preparing the intravenous prophylactic antibiotic, then having the anesthesiologist start the medicine immediately after initial induction of surgical anesthesia. |
|
TABLE 5-3 Postoperative Deep Wound and Organ Space Infection Rates, as Process Changes Were Implemented to Improve Timing of Delivery of Antibiotic Prophylaxis The care delivery group of which the improvement team was a part deployed its proven process steps to all other sister hospitals within its system (deployment and implementation). Other groups achieved similar success in the timing of antibiotic prophylaxis and subsequent infection rates through changes that involved other members of the operating room staff. Figure 5-4 shows rates of deep wound and organ space infections for the system as a whole as the pilot was deployed. Since initial deployment, the improvement team has shifted its attention to other aspects of the infection prevention process. Current efforts are focused on applying published national guidelines to ensure that all the right patients, and only the right patients, receive antibiotic prophylaxis; that all patients receive the recommended antibiotics; and that antibiotic prophylaxis is discontinued at the appropriate time. Other infectious disease specialists, surgeons, and infection control nurses have examined the conceptual model used by the team and suggested improvements. For example, recent research indicates that process changes addressing blood sugar control, tissue oxygen tension, and tissue temperature could make further contributions to lowering infection rates. The injury and near-miss detection system continues to play a vital role in helping to maintain the gains already achieved (holding the gains) and in driving and supporting further improvement in the future. |
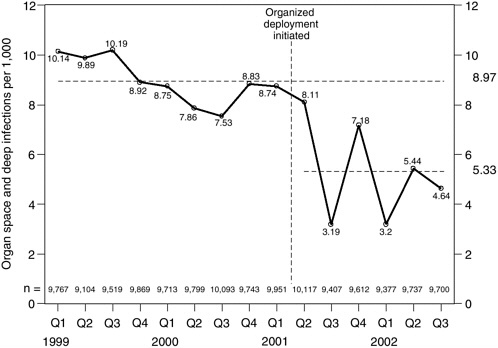
FIGURE 5-4 Postoperative deep wound and organ space infections per 1,000 clean and clean-contaminated elective surgical cases, as process changes to improve timing of antibiotic prophylaxis were deployed across a hospital system.
Engaging Patients and Their Families More in Patient Safety
Patients generally assume a basic level of quality in health care—though recent media reports on adverse events have raised questions and concerns among the public. Assuring safety and quality in health care requires an integrated effort that includes a new role for patients. With regard to adverse events and near misses, patients are possibly the last point at which event detection and prevention can occur.
Qualitative research conducted through focus groups has contributed to an understanding of the patient’s role in assuring safe and high-quality care. Focus groups conducted by Voluntary Hospitals of America, Inc., revealed that for the most part, consumers perceived quality in terms of service issues (Voluntary Hospitals of America, 2000). However, it was also found that specific information about clinical quality and reports (i.e., evidence-based guidelines and system design approaches to reduce medical
error) generated participant interest and changed attitudes about the ability to differentiate hospitals on the basis of quality. These findings led to a recommendation that initial education efforts target information about the role of hospitals in monitoring and controlling quality and the definition and dissemination of information on clinical quality that can be used by consumers in monitoring their care.
Other focus groups conducted by the Centers for Medicare and Medicaid Services revealed that patient safety messages receiving the highest rankings tended to be those that indicated specific ways for patients to inform their health professionals and themselves about what the health professionals were doing. Messages that stressed keeping one’s doctor informed and informing oneself were better received than those seen as embarrassing or rude (e.g., asking health providers whether they had washed their hands). The conclusion of this research was that consumer messages on reducing medical errors work best if they:
-
Advocate a collaborative doctor–patient relationship.
-
Specify action to be taken.
-
Clearly indicate how that action can be taken.
A number of organizations have sponsored educational activities to assist patients and their families in becoming more involved in their care. The National Patient Safety Foundation (www.npsf.org) has produced a number of publications that emphasize what patients can do to make health care safer. The Agency for Healthcare Research and Quality (AHRQ) also has developed patient materials setting forth ways to help prevent medical errors. One document in particular is designed for low-literacy patients and is presented in a comic strip format (Agency for Healthcare Research and Quality, 2001). The Institute for Safe Medication Practice sponsors a series of newsletters designed to help patients protect themselves from medication errors (www.ismp.org). Several state-based patient safety coalitions have developed and disseminated patient education materials. Finally, in March 2002 the Joint Commission on Accreditation of Healthcare Organizations launched the SPEAK UP campaign to help patients get involved in their care (Joint Commission on Accreditation of Healthcare Organizations, 2002).
Similar activities have taken place in Australia. Following consumer pressure in the late 1980s, legislation was passed in 1992 to ensure that consumer information on medicines would be available for new and existing drugs by 2004. In addition, the Medicine Information Persons project be-
gan in the early 1990s. This project trains older people as volunteer peer educators and aims to reduce the inappropriate use of medications among older people (Pharmaceutical Health and Rational Use of Medicines Committee, 2001).
Evaluating the Approach
To achieve an acceptable standard of patient safety, the committee recommends that all health care settings establish comprehensive patient safety programs operated by trained personnel within a culture of safety and involving adverse event and near-miss detection and analysis. The program put forward in this chapter is innovative and needs to be pilot tested to determine which levels of investment will bring the best returns. The results of these rigorous evaluations should then be widely circulated and discussed by all the key stakeholders.
APPLIED RESEARCH AGENDA
To foster the implementation of comprehensive patient safety systems, a robust applied research agenda for knowledge generation, tool development, and dissemination is needed. As noted earlier, near-miss analysis in health care is a much less mature discipline than adverse event analysis. As a consequence, fundamental research is needed on a number of topics related to near misses to improve analysis of these events and thereby enhance patient safety. Research is also needed in a number of areas to improve analysis of adverse events.
Knowledge Generation
High-Risk Patients
A greater focus is needed in adverse event systems on enhancing knowledge about risks and about how to identify patients at risk for medication errors, nosocomial infections, falls, and other high-frequency adverse events. Such knowledge is necessary to implement better prevention strategies.
Testing a Fundamental Assumption of Near-Miss Analysis
Near-miss analysis is predicated on the “causal continuum” assumption—that the causal factors of consequential accidents (adverse events) are
similar to those of nonconsequential incidents (near misses) (Wright, 2002). This vital assumption, according to which the causes of near misses can be used predictively in preventing actual adverse events, needs to be examined for every major medical domain to optimize the cost/benefit ratio for investments in patient safety. Equally important is the strong motivation provided to potential near-miss reporters if they are aware that their contributions to achieving better insight into small, relatively trivial events indeed help in foreseeing and preventing real harm to patients.
Developing and Testing a Suitable Recovery Taxonomy
Prevention of failure factors has been the traditional approach to improving safety and will continue to play a vital role. However, when insight into recovery factors derived from near-miss analysis rounds out our understanding of what jeopardizes patient safety, a potentially powerful alternative means of improving patient safety becomes available: strengthening the (in)formal barriers and defenses between (partially unavoidable) errors/failures and their adverse consequences. Ideally, the active components of the recovery factors can be linked to the static components of the organizational structure that are positively associated with reliability and safety.
Integrating Individual Human Error/Recovery Models with Team-Based Error/Recovery Models
In most cases, health care is delivered by teams, not isolated individuals. However, the current models of individual human error and recovery have not yet been integrated with those of group and team processes as necessary to achieve the better understanding required to improve the safety performance of health care teams. In particular, there are advantages to developing specific crisis management algorithms in response to certain constellations of clinical signs or signals from monitors of vital signs. In addition, simulators have been demonstrated to have a role in training teams to respond to common crises (Jha et al., 2001).
Integration of Retrospective and Prospective Techniques
Retrospective risk analysis techniques (such as incident analysis) and prospective ones (such as hazard analysis and critical control points and failure mode and effect analysis) should be integrated for mutual validation and increased efficiency. Both techniques aim at insight into weaknesses (and
strengths) at the system level: retrospective approaches, such as analysis of reported incidents, achieve such insight on the basis of actual deviations in an operational system, while prospective/predictive approaches, such as hazard analysis and critical control points and failure mode and effect analysis attempt to predict such deviations in the design/operational phases. Combining the two approaches may make it possible to validate predictions, feed failure scenarios with real data, and check reporting systems for biases.
Cost/Benefit Analysis of Patient Safety Programs
The qualitative changes resulting from the introduction of patient safety programs, including adverse event and near-miss analysis, must be documented. Comparing various introduction strategies across different types of health care organizations may make it possible to achieve continuing improvements in best practices. In the longer term, it would be desirable to quantify the benefits of patient safety programs—the reduction of adverse events in terms of frequency and/or severity—and the resources used to achieve this reduction. It would also be desirable to quantify the reduction of negative consequences in other areas, such as equipment failure, environmental releases, and logistic and operational costs, as they would be expected to stem from the same underlying organizational characteristics.
Patient Roles
Applied research is needed on how patients and their families can help with the prevention, early detection, and mitigation of harm due to errors. Health care organizations should implement policies and procedures designed to assist patients and their families in understanding their role in assuring the safety of patients while in a health care institution. Specific strategies should be designed to meet the needs of vulnerable populations, such as those with limited English, low literacy, and cognitive impairment, as well as others whose ability to understand and take action on health care information may be compromised. In particular, patient safety systems should be designed to elicit and receive information on adverse events and near misses from patients, their families, and their designees. Mechanisms should be in place to provide feedback to patients on the disposition of this information.
Evaluating the Impact of New Technologies for Detecting Near Misses
Technologies such as smart pumps, intensive care unit monitoring systems, and computerized physician order entry can be used to identify near misses. There is a need to investigate these systems and how the near misses they identify can be used to improve patient safety.
Tool Development
Early Detection
Automated triggers already allow for the detection of some types of adverse events, such as nosocomial infections (Evans et al., 1986) and ADEs (Classen et al., 1991; Jha et al., 1998), and it appears likely that this general approach could be extended to other types of adverse events (Bates et al., 2003). The approach works through detection of a signal, such as a high serum drug level, use of an antidote, or a laboratory abnormality in the context of use of a specific medication. A program called an event monitor is integrated with the clinical database to detect the presence of such a signal. Once a signal has been identified, it can be sent to the appropriate person or written to a file for later action. Currently, such detection approaches have high false-positive rates (Bates et al., 2003; Jha et al., 1998). Further research is needed to reduce false-positive rates for ADEs and nosocomial infections and to develop and validate computerized clinical trigger detection systems for other high-frequency sources of injury, such as decubitus ulcers, patient falls, complications of blood product transfusions, and complications of central and peripheral venous lines.
Prevention Capabilities
Tools such as computerized physician order entry incorporate capabilities to prevent adverse events, for example, by checking to see whether drug interactions with negative side effects could occur. Further research is needed to convert the growing knowledge base on patient safety risks into existing and new point-of-care decision support tools.
Verifying Adverse Events
Verification of adverse events can be problematic, and issues regarding the reliability of such assessments have been raised (Sanazaro and Mills, 1991; Thomas et al., 2002). For some types of adverse events, such as ADEs, scales having high interrater reliability, such as the Naranjo algorithm, have been developed (Naranjo et al., 1981). Overall, reliability in identifying adverse events can be expected to be higher with the use of triggers than with chart review because the evaluation relates to a discrete event. Nonetheless, greater standardization in the verification of adverse events is important—for example, using highly structured definitions of events, as is the case for nosocomial infections, or tools similar to the Naranjo algorithm.
Developing Data Mining Techniques for Large Patient Safety Databases
The size of patient safety databases at the state and regional levels will quickly become far too great for any individual to oversee their contents. Data mining will therefore be necessary to uncover patterns, test hypotheses, and even recognize whether individual new reports have been seen before.
Natural Language Processing
Much clinical information is contained in clinical notes and incident reports. Natural language processing can be used to analyze such data. Research is needed to develop natural language processing tools for patient safety applications.
Dissemination
Knowledge Dissemination
New methods are needed for promoting and speeding up the dissemination of knowledge and tools related to patient safety to aid and support health care administrators, care providers, and patients.
Audit Procedures
Existing knowledge and tools regarding patient safety need to be incorporated into audit criteria used to determine whether a health care organiza-
tion is detecting most adverse events that occur. The term “audit” can describe a series of activities ranging from unstructured self-assessments (National Quality Forum) to comprehensive reviews of structure, process, and outcomes (Joint Commission on Accreditation of Healthcare Organizations). For patient safety data standards, audit means independent review of injury case finding, evaluation, and classification using explicit criteria for the structure and function of the data systems and for the review process itself. The aim of a data system audit should be to provide assurance that the numbers reported are reasonably complete, accurate, and reproducible and thus useful for shared analysis and comparison. By design, such an audit does not address how a health care organization responds to the injury data obtained or produce judgments about safety performance. In other industries, such audit assurance is an essential element of transparency and a potent antidote to misrepresentation, cheating, and corruption. Research is needed to develop fully functional quality-of-care audit criteria and to determine how such systems might be administered.
REFERENCES
Agency for Healthcare Research and Quality. 2001. Ways You Can Help Your Family Prevent Medical Errors. Online. Available: http://www.ahcpr.gov/consumer/5tipseng/5tips.pdf [accessed July 30, 2003].
Bates, D. W., R. S. Evans, H. Murff, P. D. Stetson, L. Pizziferri, and G. Hripcsak. 2003. Detecting adverse events using information technology. J Am Med Inform Assoc 10 (2):115–128.
Classen, D. C., S. L. Pestotnik, R. S. Evans, and J. P. Burke. 1991. Computerized surveillance of adverse drug events in hospital patients. JAMA 266 (20):2847–2851.
Classen, D. C., R. S. Evans, S. L. Pestotnik, S. D. Horn, R. L. Menlove, and J. P. Burke. 1992. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med 326 (5):337–339.
Evans, R. S., R. A. Larsen, J. P. Burke, R. M. Gardner, F. A. Meier, J. A. Jacobson, M. T. Conti, J. T. Jacobson, and R. K. Hulse. 1986. Computer surveillance of hospital-acquired infections and antibiotic use. JAMA 256 (8):1007–1011.
Evans, R. S., S. L. Pestotnik, D. C. Classen, S. D. Horne, S. B. Bass, and J. P. Burke. 1994. Preventing adverse drug events in hospitalized patients. Ann Pharmacother 28 (4):523–527.
Gawande, A. A., E. J. Thomas, M. J. Zinner, and T. A. Brennan. 1999. The incidence and nature of surgical adverse events in Colorado and Utah in 1992. Surgery 126 (1):66–75.
Henz, S. 2000. Improved ADR Detection Without Using a Computer-Assisted Alert System. Salt Lake City, UT: Institute of Healthcare Delivery Research.
James, B. C. 1989. Quality Management for Health Care Delivery. Chicago, IL: Hospital Research and Education Trust (American Hospital Association).
———. 2001. Making it easy to do it right. NEJM 345 (13):991–992.
———. 2003. Information system concepts for quality measurement. Med Care 41 (1):supplement I-71–I-79.
Jha, A. K., G. J. Kuperman, J. M. Teich, L. Leape, B. Shea, E. Rittenberg, E. Burdick, D. L. Seger, M. Vander Vliet, and D. W. Bates. 1998. Identifying adverse drug events: Development of a computer-based monitor and comparison with chart review and stimulated voluntary report. J Am Med Inform Assoc 5 (3):305–314.
Jha, A. K., B. W. Duncan, and D. W. Bates. 2001. Chapter 45: Simulator-based training and patient safety. In: Making Health Care Safer: A Critical Analysis of Patient Safety Practices: Evidence Report/Technology Assessment No. 43. Rockville, MD: Agency for Health Care Research and Quality.
Joint Commission on Accreditation of Healthcare Organizations. 2002. SPEAK UP: National Campaign Urges Patients to Join Safety Efforts. Online. Available: http://www.jcaho.org/news+room/press+kits/speak+up+national+campaign+urges+patients+to+join+safety+efforts+.htm [accessed August 5, 2003].
Juran, J. M. 1989. Juran on Leadership for Quality. New York, NY: Free Press.
Kim, Y. 2003. Interaction Among Data-Based Clinical Triggers. Personal communication to Institute of Medicine’s Committee on Data Standards for Patient Safety.
Kizer, K. W. 1999. Large system change and a culture of safety. In: Enhancing Patient Safety and Reducing Errors in Health Care. Chicago, IL: National Patient Safety Foundation.
Langley, G. J., K. M. Nolan, C. L. Norman, L. P. Provost, and T. W. Nolan. 1996. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. San Francisco, CA: Jossey-Bass.
Larsen, R. A., R. S. Evans, J. P. Burke, S. L. Pestotnick, R. M. Gardner, and D. C. Classen. 1989. Improved perioperative antibiotic use and reduced surgical wound infections through the use of computer decision analysis. Infect Control Hosp Epidemiol 10 (7):316–320.
Leape, L. L., T. A. Brennan, N. M. Laird, A. G. Lawthers, A. R. Localio, B. A. Barnes, H. L. Newhouse, P. C. Weiler, and H. Hiatt. 1991. Incidence of adverse events and negligence in hospitalized patients: Results of the Harvard Medical Practice Study II. N Engl J Med 324:377–384.
Leape, L. L., D. J. Cullen, M. D. Clapp, E. Burdick, H. J. Demonaco, J. I. Erickson, and D. W. Bates. 1999. Pharmacist participation on physician rounds and adverse drug events in intensive care unit. JAMA 282 (3):267–270.
Marx, D. 2001. Patient Safety and the “Just Culture”: A Primer for Health Care Executives. Funded by a grant from the National Heart, Lung, and Blood Institute, National Institutes of Health (Grant RO1 HL53772, Harold S. Kaplan, M.D., Principal Investigator). New York, NY: Trustees of Columbia University.
McDonough, J. E. 2002. Proactive Hazard Analysis and Health Care Policy. New York, NY: MilBank Memorial Fund/ECRI.
Naranjo, C. A., U. Busto, E. M. Sellers, P. Sandor, I. Ruiz, E. A. Roberts, E. Janecek, C. Domecq, and D. J. Greenblatt. 1981. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 30 (2):239–245.
O’Leary, D. S. 2003. Perspectives and Insights. Council on Teaching Hospitals, Spring Meeting. Phoenix, AZ: Joint Commission on Accreditation of Healthcare Organizations.
Pharmaceutical Health and Rational Use of Medicines Committee, Australian Pharmaceutical Advisory Council. 2001. Quality Use of Medicines: A Decade of Research, Development and Service Activity 1991–2001. Canberra: Commonwealth Department of Health and Aged Care.
Reason, J., and A. Hobbs. 2003. Managing Maintenance Error: A Practical Guide. Burlington, VT: Ashgate.
Sanazaro, P. J., and D. H. Mills. 1991. A Critique of the Use of Generic Screening in Quality Assessment. JAMA 265 (15):1977–1981.
Sholtes, P. R., B. L. Joiner, and B. J. Streibel. 2003. Team Handbook, Third Edition. Madison, WI: Oriel.
Smith, E. R. 1998. Mental representation and memory. In: D. T. Gilbert, S. T. Fiske, and G. Lindzey, eds. The Handbook of Social Psychology. Boston, MA: McGraw-Hill. Pp. 391–445.
Thomas, E. J., S. R. Lipsitz, D. M. Studdert, and T. A. Brennan. 2002. The reliability of medical record review for estimating adverse event rates. Ann Intern Med 136 (11):812–816.
Voluntary Hospitals of America. 2000. Consumer Demand for Clinical Quality: The Giant Awakes: VHA’s 2000 Research Series—Volume 3. Irving, TX: Voluntary Hospitals of America, Inc.
Wright, L. B. 2002. The Analysis of UK Railway Accidents and Incidents: A Comparison of Their Causal Patterns. Glasgow: University of Strathclyde.
Xu, W., P. Hougland, S. Pickard, C. Masheter, G. Petratos, and S. D. Williams. 2003. Detecting Adverse Drug Events Using ICD-9-CM Codes. Arlington, VA: AHRQ Second Annual Patient Safety Research Conference.