9
Standardized Reporting
CHAPTER SUMMARY
Effective and efficient patient safety reporting systems within the context of an integrated health information infrastructure are essential to the creation of a new standard of care for evidence-based medicine and the ongoing improvement of clinical practice. However, many of the existing reporting systems vary in a number of design features (Institute of Medicine, 2000) and approaches to patient safety (e.g., voluntary, mandatory, internal, external). The data standards used within these systems also vary widely, rendering the data incomparable across systems for more extensive research and analysis.
This chapter develops a framework for the standardized collection and codification of those report data most important to detecting, analyzing, understanding, and learning from patient safety-related events. The first section emphasizes the need for a standardized report format and outlines its essential elements. The second section discusses a common set of data standards for patient safety reporting that can enable the aggregation of data from voluntary and state reporting systems, as well as support the establishment of a national patient safety database first called for in To Err Is Human (Institute of Medicine, 2000). Next, the chapter reviews factors affecting the implementation of the report format and issues of deidentification and data protection. Lastly, the chapter provides examples of primary and secondary uses of the report data.
THE NEED FOR A STANDARDIZED REPORT FORMAT
At present, there is no agreement on a common set of data elements for representing patient safety information, much less specification of allowable values for those elements. Each entity self-determines the content of its reports; some even develop their own terminology to represent the information. At the state level, for example, New York and Florida are 2 of 21 states with mandatory requirements for reporting adverse events. The data elements they collect for the most serious adverse events have some areas of commonality:
-
Similar patient information is collected.
-
Similar information is collected on the time/location of the incident.
-
Each requires a description of the occurrence and analysis of its root causes.
-
Each requires a description of the corrective actions taken.
-
Only one health care data standard, the International Classification of Diseases, Ninth Edition (ICD-9), Clinical Modification (CM), is used by each to identify the diagnosis and procedures associated with the event (Rosenthal et al., 2000).
However, each state has developed its own taxonomy for classifying actual events. The New York Patient Occurrence Reporting and Tracking System (NYPORTS) works within the broad categories of statutorily defined reportable incidents. The system makes use of a detailed list of 54 reportable codes with “includes” and “excludes,” organized by type of event, to promote greater consistency among state hospitals (Rosenthal et al., 2001). Florida, on the other hand, divides the events into two categories—those that must be reported within 15 days and on an annual basis (Rosenthal et al., 2001). Each category includes four or five broad types of events to be reported. Another important difference is that the New York system has a set of questions designed specifically for medication errors, a known major adverse event category; the Florida system does not. The differences among the 15 state patient safety systems are even more pronounced.
The following “real world” example further illustrates the problem with numerous disparate data elements for documenting an adverse event. If an individual suffered a serious adverse drug event (ADE) while in a New York hospital, the clinician would first file a report internally for review by the designated hospital representative. A second report would be filed with the New York State Department of Health through NYPORTS. Another third
report could be voluntarily submitted to the Food and Drug Administration (FDA), either through the FDA MedWatch reporting system or through private-sector organizations such as the United States Pharmacopia (USP), to inform the FDA of potential serious problems with the drug. Adding further to the burden of disparate and multiple methods for representing an ADE are the voluntary reporting requirements of the hospitals’ accrediting organization, the Joint Commission for Accreditation of Healthcare Organizations (JCAHO), whose proposed taxonomy provides yet another dataset for classifying and reporting such events. Already this example involves four different reports with varying data elements for the same ADE.
In the case of the FDA, reports are submitted to support the agency’s regulatory obligations for postmarket surveillance of drugs marketed in the United States, particularly those associated with ADEs. To this end, the organization needs the capability to analyze and compare the ADEs occurring during the clinical trial process with those experienced in clinical practice. However, the FDA uses one terminology, MedDRA, for representation of ADEs experienced by patients during clinical trials and documentation in the manufacturer’s dossier for regulatory approval, and another in its MedWatch reporting system. The agency also accepts data from private-sector organizations using different data standards. Thus, for the FDA alone, the data related to one particular ADE is represented by three different data sources. More importantly, the data from clinical trials and postmarket surveillance cannot be compared without costly mapping of the terms among the different taxonomies.
An additional consideration relates to the ability to share and compare data in integrated systems. For example, a clinician who wanted to conduct an analysis of or research on ADE reports compared with events detected and/or prevented with various decision support systems (e.g., pharmacy systems, computerized physician order entry, bar-code medication administration) could not do so without common methods for representing the most basic ADE data (e.g., drug involved, type of event, route of administration, dosage). The ability to compare the factors contributing to an ADE among systems would add to the knowledge and understanding of events. It would also provide a common reference point for classifying event data derived from other sources (e.g., malpractice claims, complaints, claims attachments) and different health care settings (e.g., primary care, inpatient, nursing home). However, such analysis cannot be undertaken without a common language.
The remedy for the disparate scenario described above is the development of a common reporting format of domain areas, data elements, and
terminologies that would serve as a common language for reporting, research, and analysis on patient safety. The format would be able to accommodate the rich text of narrative reports that will likely remain the mainstay of patient safety reporting. Common data standards would be used to make the information comparable to the patient safety data extracted from clinical information systems and electronic health record systems (EHRs) such as that from automatic trigger systems. With the common format, health care organizations would experience less burden in fulfilling both data capture and reporting requirements.
New knowledge obtained from the reports could be fed back into clinical information systems and care processes in a standardized manner and thereby be applied for preventing and detecting future adverse events. The standard report format could be employed for a number of purposes: populating a national patient safety database; meeting the functional requirements for the establishment of patient safety organizations in the private sector as proposed in pending legislation; providing a format easily implemented by those states that have not yet established patient safety reporting systems; and serving as a common format for mapping data across established state reporting systems, as well as to a national database. The data protection and legal considerations that arise in the generation of numerous reports from shared data elements are addressed later in this chapter.
During the development process to establish a high-functioning model for the aviation industry, Farrier (1997) voiced many concerns about standardized reporting. Although there will always be discussions about how and why the present state of affairs came to pass and which entities hold the key to correcting any problems, the obstacles, complications, and objections to broad-based information flow can be distilled down to essentially four principal issues: disagreement over the proper form and content of databases, ambiguities in the terminology used in reports, proper versus improper involvement of investigative models in the data collection process, and competition among end users of safety information. The incident reporting systems of the aviation industry have been in operation for quite some time, yet the limitations of coded data remain in several areas: data retrieval is only as good as the original coding; databases are often incomparable because of proprietary language, architecture, or other features; and judgments about what data should be captured and how they should be indexed are subjective and change over time, constraining the study of different events in the same code-based system (Farrier, 1997). The chief barriers to coding event data have been a lack of standardization and the ambigu-
ity of descriptive terms among investigators, as well as the perceived requirement that investigators draw conclusions about an event instead of limiting themselves to informed assessments for learning and improvement purposes (Farrier 1997).
The committee took these concerns into consideration in developing its recommendations on data standards to support patient safety. Data standards, including the standardized report format, should be dynamic and respond to new knowledge in an evolutionary manner while adhering to the basic principles and purposes of standardization. As stated in To Err Is Human, a standardized report format can (1) permit data to be combined and tracked over time, (2) lessen the burden on health care organizations that operate in multiple states or are subject to the reporting requirements of multiple agencies and/or private oversight processes and group purchasers, and (3) facilitate communication with consumers and purchasers about patient safety (Institute of Medicine, 2000).
A common report format could also augment the recent effort by the federal government to integrate its numerous patient safety reporting systems. Specifically, the Department of Health and Human Services (DHHS) has planned a three-phased integration of the federal systems at the Agency for Healthcare Research and Quality (AHRQ), the Centers for Disease Control and Prevention (CDC), the Centers for Medicare and Medicaid Services (CMS), and the Food and Drug Administration (FDA). Phase I, currently being carried out by the Kevric Company, involves designing a common database, incorporating a common user interface, and linking the six primary FDA and CDC reporting systems. Phase II involves integrating the database and the remaining DHHS reporting systems, while Phase III encompasses integrating the reporting systems of other government agencies (i.e., Department of Defense [DOD], Veterans Health Administration [VHA]), the states that have reporting systems, and data provided by other countries. Appendix C provides detailed examples of selected federal, state, and private sector reporting systems. Use of a standardized report format among these patient safety systems would facilitate its dissemination and widespread adoption across the health sector.
ESSENTIAL ELEMENTS OF A STANDARDIZED REPORT FORMAT
Several reporting formats currently in use or in development can serve as a foundation and reference point for the establishment of a common for-
mat and taxonomy for patient safety events. Systems of interest include AHRQ’s proposed taxonomy for the integration of all DHHS patient safety reporting systems, the VHA system, and the Australian Patient Safety Foundation’s (APSF) Advanced Incident Monitoring System (AIMS). Other reporting systems—such as that of United States Pharmacopeial Convention, Inc., the Medical Event Reporting System for Transfusion Medicine (MERS TM), and the systems used by medical specialties (e.g., anesthesia, emergency room)—have many characteristics that should also be incorporated into certain domain areas of the common reporting format.
The committee believes the format should be designed to support the full range of reporting systems (e.g., federal, state, internal/external institutional, paper, electronic) and multiple detection methods (e.g., chart review, electronic surveillance, administrative reports). It should also be flexible enough to meet local data collection needs and accommodate evolving views of what constitutes the appropriate data to collect. The quality of adverse event reporting itself is contingent on many factors, each influenced by the cognitive and social characteristics of the participants (Cook, 2003). Formulation of a classification and assessment process for the reporting format must be accomplished in a manner that supports feedback from the reporting system, to research and analysis on patient safety, and back to the organizational system, providing new knowledge for learning and improvement. Research on and analysis of patient safety events require that reporting systems supply a steady flow of the data needed for continuous quality improvement, in much the same way that other industries utilize data for hazard analysis (Cook, 2003). The reporting format must also be capable of providing information that leads to a greater understanding of the nature of adverse events—for example, why such events occur, how they are recognized, what the critical control points are along the care continuum, what types of recovery or corrective actions are taken, and the effect on patient outcomes (Cook, 2003).
In an effort to develop a prototype for reporting events related to transfusion medicine, Battles et al. undertook a study in 1997 to assess the ideal attributes of a medical event reporting system. The study tapped a group of experts in the fields of aviation safety, nuclear power, cognitive psychology, industrial engineering, artificial intelligence, education and training, and transfusion medicine from the United States, the United Kingdom, and Australia (Battles et al., 1998). The result was a set of system design parameters for overall features and for system input, data collection, the analytical process, and interventions (see Box 9-1). The VHA chartered its National Cen-
|
BOX 9-1 Overall
System input
Data collection
Analytical process
Interventions
SOURCE: Battles et al., 1998. |
ter for Patient Safety (NCPS) in 1998 and began operations in 1999, building the system according to many of these basic principles. However, the VHA system, like others, does not include “nonreprisal” treatment for intentionally unsafe acts. The parameters shown in Box 9-1 are directly applicable to the development of a generic reporting format for the wide range of adverse events, medical errors, and near misses. The committee has incorporated these principles into its recommended design for a standard reporting format.
Basic Domains
At the time of the MERS TM study, most existing incident reporting systems described only what happened and paid little attention to why the event occurred; root-cause analyses were often cursory and incomplete (Battles et al., 1998). Historically, all reporting systems contained the most basic elements of a reporting format, regardless of the type of report—itemized, computerized, or narrative text. The reports typically included the following:
-
Who discovered the incident, classified according to role in the health system (e.g., physician, nurse)
-
How the incident was discovered
-
What happened—the type of event
-
Where in the care process the incident was discovered or occurred
-
When the incident occurred
-
Why—preliminary delineation by the most dominant cause, with the seriousness of the event determining whether a detailed investigation would be conducted.
The inability to produce patient safety data that were meaningful enough to create change in the care process can be attributed mainly to a failure to recognize that causal analysis is the most important element of the report. The best source of data for the causal analysis is the report’s narrative text. While the what, where, when, who, and why of an event are necessary components of any report, detailed causal analysis gets to the heart of the circumstances that led to the event and informs the process for system improvement.
The Australian system incorporates an innovative approach to address this need. AIMS is structured to differentiate between two levels of detail: (1) a minimum dataset of initial information about the event reflective of the
basic domain areas—where, when, and to whom the event occurred, what happened, and the severity of the incident (with automatic generation of a risk matrix), and (2) a detailed dataset of comprehensive information for events that caused harm to the patient or that pose a major future risk (Australian Patient Safety Foundation, 2003). The detailed dataset expands the data collection beyond the minimum dataset to include information on the mechanism of the incident, root causes, contributing factors, actions taken, outcome, and consequences to the organization (Australian Patient Safety Foundation, 2003).
Event-Type Taxonomy
Event types are used to classify events in a taxonomy (e.g., medication or surgical event). Such taxonomies are generally hierarchical to distinguish events within a category. For example, a medication event might be further differentiated in the taxonomy as a “right medication/wrong dose” event or a “wrong medication” event. It is also important to note that the terms “event,” “occurrence,” and “incident” are synonymous and used according to preference in different patient safety systems. For example, NYPORTS uses “occurrence,” AIMS uses “incident,” and most other systems (and the IOM) use “event.” However, the terms all represent the same concept—that an adverse event or near miss has happened. All three terms also represent the totality of the mishaps that occurred along the chain of clinical processes leading to the outcome, rather than a single mishap.
Currently, no single taxonomy comprehensively represents all event types. Instead, over the past decade, several different taxonomies have been developed by health care organizations, medical specialties (e.g., anesthesia, trauma), and state and federal regulatory agencies to accommodate their particular interests in patient safety. Although organizations and regulatory agencies accept reports on all types of events, their structured taxonomies were developed as distinct and independent sets to reflect the most common types of serious events of importance to them, rather than a comprehensive set embodying the universe of events and injuries that can occur. Most terms represented are errors of commission, excluding errors of omission and near misses. If an event occurs that is not represented in the taxonomy, it is placed in an ambiguous “other” or “miscellaneous” category. One example of an overall approach is the National Quality Forum’s (NQF) “short list” of 27 reportable events that result in serious harm to or death of the patient, such as wrong-site surgery, wrong drug administration, or falls. The NQF list reflects the states’ efforts to standardize the types of events that are most
often reported to the state government with a narrow focus on events in which serious harm or death occurred. Standardization of even a “short list” can be considered progress. However, from an organizational perspective, it is necessary to evolve from a primary focus on sentinel events to the broader issues of adverse events and near misses. For this purpose, a safety event that would have a high impact on quality improvement needs to be captured, classified, and coded.
Because the inherent rules or conditions for classifying events differ for each patient safety taxonomy or organization, the taxonomies lack the capacity for comparison. The 21 state-based reporting systems vary significantly in how events are defined, classified, and coded. The Massachusetts Department of Health uses 17 broad categories (e.g., falls, medication, neglect) to organize 137 different types of incidents (e.g., fall-fracture, fall-laceration) (New York Patient Occurrence Reporting and Tracking System, 2001). NYPORTS has 8 major categories for 54 types of incidents, differentiated according to “includes” and “excludes” (New York Patient Occurrence Reporting and Tracking System, 2002). Tennessee’s taxonomy is very similar to that of NYPORTS, yet it uses different code numbers and has modified some of the term definitions (Tennessee Department of Health, 2003). The end result is a number of disparate, incomplete taxonomies that are beset by inconsistencies and vagueness (Nebeker et al., 2002).
The National Academy for State Health Policy has attempted to address this issue among the states by facilitating the establishment of a workgroup, the State Alliance for Error Reporting, to review and seek clarity on the NQF list of serious reportable events, identify similarities and differences between this list and existing state reporting systems, and discuss strategies for ensuring consistent implementation of the NQF list. After conducting its assessment, the State Alliance developed a crosswalk of the NQF event list and the systems of the states that had mandatory reporting requirements at the time, supplemented by a user’s guide to assist with implementation. This effort represents a significant step toward a common language among state reporting systems; however, it remains far too limited, and maintaining the operational crosswalks will prove costly in both time and resources, particularly when more events are added to the list.
Because of their public availability, the current most commonly used terminologies for representing adverse events are the ICD-9/10 CM External Causes and Injury Codes (E-codes) for diagnoses and procedures and Logical Observation Identifiers, Names and Codes (LOINC) for representing laboratory and other types of data that support surveillance for infectious diseases and biological threats. Most adverse events today are detected
through multiple sources and generally documented according to the ICD–9/10 CM E-Codes (Bates et al., 2003). Those codes provide direct and indirect evidence of the clinical state of the patient, comormid conditions, and the patient’s progress during hospitalization or a visit (Bates et al., 2003). However, the codes lack temporal information and clinical content (Campbell, 2002), cannot differentiate events that occurred prior to hospitalization from those that occurred during hospitalization, lack the ability to categorize degree of harm, are unable to capture near misses (Williams, 2003), and cannot be linked to a complication or E-Code from the ICD–9/10 CM procedure codes. Despite their limitations, the ICD–9/10 CM E-Codes are useful as one mechanism for detecting adverse events. The committee recommends additional investment to expand them and to enhance their capacity to capture patient safety information. Doing so would also facilitate international collaboration, given the work being done by the World Health Organization in this area.
For example, the Utah Department of Health has been studying the potential value of the E-Codes for patient safety event reporting and has recommended several improvements to enable the terminology to function as part of a statewide patient safety tracking information network that would feed into a national database: (1) add yes/no fields or an additional digit associated with each code to indicate whether a condition was present on admission (note that some states have developed a method in their taxonomies or discharge data requirements for this purpose); (2) improve the codes for degree of harm with additional fields delineating temporary harm, permanent harm, or death; (3) improve the codes for intentionality; (4) allow codes for multiple similar adverse events; and (5) improve the ability to code events documented only by nonphysicians (e.g., notes from pharmacists or nurses) (Williams, 2003). In addition, population-specific extensions should be made available for more accurate representation of patient data.
While the ICD codes have been applied in several studies for successful identification of ADEs involving chart reviews, pharmacy and laboratory data can also provide direct evidence of adverse events, such as dosing errors, antidotes, and clinical values out of range (Bates et al., 2003), and are important to automated surveillance systems. LOINC is the primary terminology for laboratory databases, providing data designed to identify and name test results (Laboratory LOINC) and/or clinical observations (Clinical LOINC), including information about the amount, route, and timing of physiologic challenges (e.g., oral glucose tolerance test) (Regenstreif Institute, 2001). Laboratory LOINC is also vital to CDC’s public health surveillance activities aimed at identifying the presence of nosocomial infections,
biological threats, infectious diseases, and the like within the American population. Clinical LOINC’s extensiveness and flexibility for multiple purposes render significant potential for representing adverse events. Columbia University, which has developed and maintained the MERS TM terminology for blood transfusion–related events, has undertaken research to extend the terminology for application in general therapeutic areas (MERS TH) and to create LOINC codes for aspects of root-cause analysis (e.g., antecedent events, contributing factors, and outcomes) (Kaplan, 2003).
Medical specialty groups have developed event-type taxonomies that are more specific to their particular domain area and often have more granularity in term definitions. For example, MERS TM divides events into two primary sections—those related to the blood center (15 categories, such as initial donor suitability, testing, and labeling) and those related to the transfusion service (13 categories, such as order entry, sample handling, and unit issue). Transfusion service events are specified in 97 event codes (e.g., for sample testing, computer warning overridden, and sample tubes mixed up) (Westat, 2001). The greater specificity of the event-type definitions allows for a more directed analysis and comparison of the critical control points related to a particular event. Key event types in the expanded version, MERS TH, have been assigned codes in the LOINC system. The complexity of drug and medical device events also requires a more comprehensive terminology, for example, when utilizing the more sophisticated decision support tools.
As with the problems posed by the disparate taxonomies used by state systems, anesthesia specialists have long been frustrated by the inability to collect and analyze uniform data elements across departments and institutions. Disparate data has been a barrier to improving anesthesia care, reducing errors, and identifying opportunities to reduce health care costs (Anesthesia Patient Safety Foundation, 2003). Adding to the complexity of the anesthesia domain are the technological demands associated with real-time monitoring of patients both electronically and physically using any number of devices simultaneously, such as those for electrocardiography, pulse oximetry, and capnography and infusion pumps (Gaba, 2000), each employing different data standards depending on the organization and vendor. In 2000, the Anesthesia Patient Safety Foundation (APSF) established an overall effort to create a common data dictionary and a Distributed Anesthesia Terms and Mapping System so that the different systems would have a common reference point. As in other domains, having common terms allows for the automated collection and comparison of large volumes of clinical data from
multiple institutions for outcome research and benchmarking (Anesthesia Patient Safety Foundation, 2003). To facilitate the use of common anesthesia terms in clinical information systems, patient safety terms in the data dictionary have been incorporated into Systemized Nomenclature of Human and Veterinary Medicine, Clinical Terms (SNOMED CT). This example emphasizes the need for an integrated approach to patient safety encompassng all clinical care.
Patient safety terminology should be incorporated into the National Committee on Vital and Health Statistics (NCVHS) core terminology group. In particular, a terminology and taxonomy for patient safety events should be developed and included in SNOMED CT (e.g., the anesthesia model). As stated in Chapter 4, the committee supports incorporating the Universal Medical Device Nomenclature System (UMDNS) as the medical device terminology and normalized notations for clinical drugs (RxNORM) as the clinical drug terminology in the core group. Patient safety terms in the core terminology group should be mapped through aggregation logic to important supplemental terminologies, such as MedDRA and the Global Medical Device Nomenclature (GMDN), to facilitate automated report generation. Finally, efforts should be undertaken by the National Library of Medicine (NLM) to create and maintain the patient safety terminology mappings and disseminate them publicly for widespread adoption.
To further efforts toward data standardization, the committee believes that the best method of satisfying the terminology requirements for Phase I of the federal patient safety data integration project is the use of the designated NCVHS core terminology group with mappings to supplemental terminologies. This approach would support research and analysis of data from the national patient safety database and federal reporting systems.
Australia’s AIMS takes a similar approach by leveraging the term/concept capabilities of a relational database that relies on a Generic Reference Model (GRM). The Health Incident Type taxonomy of event categories (e.g., falls, pressure ulcers, medication), plus a number of specialty areas (e.g., anesthesia, intensive care, surgery), were created as the entry point into the system. Once an incident type has been determined, the GRM is used to elicit more detail about the factors contributing to the incident (Australian Patient Safety Foundation, 2002). For example, while the Health Incident Types are at the highest level (e.g., medical devices), when one clicks on that domain, the information system shifts to the highly detailed clinical taxonomy/terminology for devices—UMDNS. The system then can request from the reporter more clinical detail in terms appropriate to that type of
medical device event. Appendix G provides a listing of the health incident types (Australian Patient Safety Foundation, 2002).
Once the basic domain areas for an event have been documented, the next important step is assessment of the event’s seriousness (i.e., severity) to determine what further action should be taken.
Risk Assessment Index
As the study of patient safety systems has progressed, more sophisticated systems have begun to emphasize the collection of more detailed information for the causal analysis and investigation of serious incidents. To improve the systems’ organization, most have incorporated a risk assessment index to help gauge whether a full investigation of the incident is warranted. Risk assessment can be used to estimate the effect of a particular disease or patient safety incident on the physiological integrity of the patient (Iezzoni, 1997) and can include an evaluation of functional status. For patient safety, explicit criteria for assessing the degree of risk can be expressed as a risk matrix that enables the severity of the outcome of an incident to be plotted against the likelihood of the incident recurring (Australian Patient Safety Foundation, 2003). The risk assessment is used as a tool to set priorities and identify which areas require root-cause analysis or further attention (Australian Patient Safety Foundation, 2003).
In its simplest form, a risk matrix can be a one-dimensional scale for determining the range of severity from near miss to death. USP’s MedMARx system uses a nine-tier approach1 to rank medication events, see Table 9-1. Definitions of severity are clearly set to minimize confusion by clinicians using the system.
The VHA requires prioritization scoring for both close calls and adverse events for (1) the actual or potential severity of the event and (2) the probability of occurrence according to specific definitions (Eldridge, 2001). For severity, the scale is catastrophic, major, moderate, and minor; for probability, it is frequent, occasional, uncommon, and remote (Department of Veterans Affairs, 2001). The parameters are organized in a 4 × 4 matrix. Once these two parameters have been established, the prioritization score is available from the Safety Assessment Code (SAC) Matrix of 3 = highest risk, 2 = intermediate risk, and 1 = lowest risk (Eldridge, 2001). The SAC is then
TABLE 9-1 USP MedMARx Error Outcome Categories (severity scale)
|
Category |
Definition |
|
No error |
|
|
Category A |
Circumstances or events that have the capacity to cause error |
|
Error, No Harm |
|
|
Category B |
An error occurred, but the error did not reach the patient |
|
Category C |
An error occurred that reached the patient but did not cause the patient harm |
|
Category D |
An error occurred that reached the patient and required monitoring to confirm that it resulted in no harm to the patient and/or required intervention to preclude harm |
|
Error, Harm |
|
|
Category E |
An error occurred that may have contributed to or resulted in temporary harm to the patient and required intervention |
|
Category F |
An error occurred that may have contributed to or resulted in temporary harm to the patient and required initial or prolonged hospitalization |
|
Category G |
An error occurred that may have contributed to or resulted in permanent patient harm |
|
Category H |
An error occurred that required intervention necessary to sustain life |
|
Error, Death |
|
|
Category I |
An error occurred that may have contributed to or resulted in the patient’s death |
|
SOURCE: U.S. Pharmacopeia, 2003. |
|
used to determine what action must be taken (Department of Veterans Affairs, 2001). The AIMS risk assessment index is based on the VHA model but extends the matrix to 5 × 5, as seen in Table 9-2.
MERS TM has developed an even more sophisticated risk assessment index (RAI), outlined in Table 9-3 for comparison, using four axes for classification of events—severity and probability as in the VHA and AIMS models, but on a 5 × 6 axis, with two adjustment factors applied to the severity/ probability calculation—product issued and unplanned recovery. In contrast with the other systems, MER TM assigns numerical values to the matrix panels, and the RAI is calculated as a product of severity multiplied by probability. If the blood product was issued, 0.2 is added to the RAI, and/or if there was an unplanned recovery, 0.1 is added to the RAI (Westat, 2001). A root-cause analysis is recommended if the RAI is greater than or equal to
TABLE 9-2 AIMS Risk Assessment Index
|
Likelihood |
Insignificant |
Minor |
Moderate |
Major |
Catastrophic |
|
Almost Certain |
Yellow |
Orange |
Orange |
Red |
Red |
|
Likely |
Yellow |
Yellow |
Orange |
Red |
Red |
|
Possible |
Green |
Yellow |
Yellow |
Orange |
Red |
|
Unlikely |
Green |
Green |
Yellow |
Orange |
Orange |
|
Rare |
Green |
Green |
Yellow |
Orange |
Orange |
|
SOURCE: Australian Patient Safety Foundation, 2003. |
|||||
0.5, or if it is less than 0.5 but the risk is high for the organization (Westat, 2001). Organizational risk is considered an effect that may result in financial loss or damaged reputation (Westat, 2001).
AHRQ has specifically stated that a risk assessment scale will be included in its DHHS integration project. The committee believes a risk assessment scale should be included in the common patient safety report format. In addition to an RAI, the committee believes that differentiating between probability and preventability is important to the analysis of events. Therefore, a method for assessing and a taxonomy for representing preventability should also be agreed upon and implemented. For example, if the event in question is a medication error that resulted from a mixup of medi-
TABLE 9-3 MERS TM Risk Assessment Index
|
|
|
Quantified Estimate of Probability of Recurrence |
|||||
|
Qualified Estimate of Severity of Patient Harm |
Extremely High |
Very High |
High |
Medium |
Low |
Very Low |
|
|
|
|
0.99 |
0.90 |
0.75 |
0.50 |
0.25 |
0.10 |
|
Extremely High |
0.99 |
1.0 |
0.90 |
0.70 |
0.50 |
0.2 |
0.1 |
|
Very High |
0.90 |
0.9 |
0.80 |
0.70 |
0.40 |
0.2 |
0.1 |
|
High |
0.75 |
0.7 |
0.70 |
0.60 |
0.40 |
0.2 |
0.1 |
|
Medium |
0.50 |
0.5 |
0.40 |
0.40 |
0.20 |
0.1 |
0.05 |
|
Low |
0.25 |
0.2 |
0.20 |
0.20 |
0.10 |
0.1 |
0.02 |
|
Very Low |
0.10 |
0.1 |
0.10 |
0.10 |
0.05 |
0.02 |
0.01 |
|
SOURCE: Westat, 2001. |
|||||||
cations spelled and pronounced similarly, there is a definite way to prevent recurrences: change the name of one of the medications. This type of event would be rated highly preventable. From another perspective, the probability of recurrence would be high if the name of a medication is not changed. The preventability is based on the anticipated frequency of one medication again being accidentally substituted for the other. In this case, the cause might relate to poor handwriting and other similarities between the medications (e.g., dosing regimen).
Causal Analysis
Once it has been determined that an event is or could have been serious, a root cause analysis (RCA) should be performed. An RCA is considered mandatory for serious events by JCAHO and state regulatory agencies. The VHA, AIMS, MERS TM, several states, and other organizations have well-developed models for root cause analysis. To date, methodologies for RCA have been guided by the pioneering work of Jens Rasmussen in assessing system, environmental, and human aspects of errors and James Reason in understanding the dynamics of human factors and latent conditions that lead to error (Reason, 1990).
The VHA’s NCPS uses narrative text for causal analysis based on the principles surrounding human, organizational, and technical factors identified by Rasmussen and Reason, but employing an interpretation for VHA facilities focused on six areas (Bagian et al., 2001):
-
Human factors communication
-
Human factors training
-
Human factors fatigue/scheduling
-
Environment and equipment
-
Rules, policies, and procedures
-
Barriers (safeguards)
Each area has a series of specific questions to guide reporters in their documentation (Bagian et al., 2001). The RCA occurs at each hospital and includes the narrative, the analysis, proposed remedies, and a plan for implementation and follow-up (Gosbee, 2003). Information is extracted from the report of the analysis (usually in narrative form) and recorded in a relational database. The goal of NCPS is to analyze system vulnerabilities in hospitals, find and implement solutions, and provide support to the facilities (McKnight and Gosbee, 2002). Maintaining the information in a relational
database facilitates the discovery of commonalities or trends among RCA reports that can lead to the identification of similar system failures/issues (McKnight and Gosbee, 2002). In fact, NCPS has instituted the Primary Analysis and Categorization project to synthesize information from each RCA narrative text report into coded keywords with definitions (i.e., a glossary) to facilitate the identification of commonalities among events (McKnight and Gosbee, 2002). The initial scope of the project involves categorizing each RCA into five domain areas (McKnight and Gosbee, 2002):
-
Location of event
-
Activities or processes surrounding the event
-
Activity or process outcomes characterizing the event
-
Actions taken to prevent a similar event from happening in the future
-
Outcomes that measure whether the actions were effective
At VHA, each director signs the RCA plans for change and system improvements resulting from the root cause analysis. If the patient safety manager believes that learning from the RCA would have VHA-wide or worldwide application, a secondary analysis is performed that results in a clearly defined nationwide alert or advisory, a newsletter or monthly meeting item, a change to national policy (such as VHA’s correct-surgery directive), and/ or work with medical device companies on a product redesign (Gosbee, 2003).
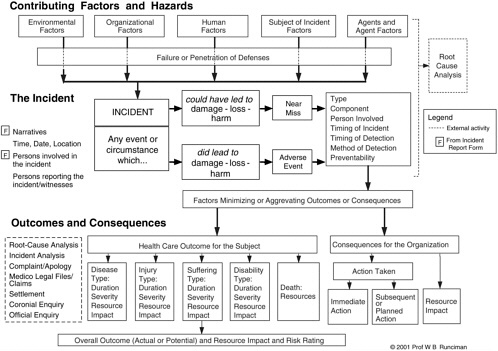
AIMS has incorporated into its GRM (with permission) an adaptation of the model developed by VHA. The GRM encompasses a system that represents the contributing factors of an event as determined in the causal analysis. In AIMS, these contributing factors are called component factors. The GRM provides a framework that defines relationships among the component factors of the classification system and a set of terms describing the attributes of each component. These definitions vary by type of incident and classification, with permutations currently exceeding 500,000 in number (Australian Patient Safety Foundation, 2003). Figure 9-1 is a diagram of the GRM.
AIMS also includes data on outcomes and consequences of events, which the committee believes to be an important part of the causal analysis. Two sets of data are collected: (1) patient outcome as related to the duration, severity, and resource impact of the disease type, injury type, suffering, disability, or death; and (2) consequences for the organization, including immediate and subsequent actions taken, impact in terms of cost, and legal liability (Australian Patient Safety Foundation, 2003). Outcome data in relation
to actions taken can help clinicians better understand the recovery aspect of adverse events.
Other health care organizations have been involved in further research on causal analysis methodologies employed successfully by other sectors and have adapted those models to meet their needs. The Eindhoven Classification Model, developed at Eindhoven University of Technology in the Netherlands, is often used in the chemicals sector and other high-risk industries and was chosen as the RCA model for the TM study; it incorporates the earlier work of Rasmussen and Reason (Battles et al., 1998). Using the Eindhoven Classification Model: Medical Version, investigators examine the root causes of an incident from three perspectives (Battles et al., 1998):
-
Technical factors—equipment, software, forms
-
Organizational factors—policies, procedures, and protocols
-
Human factors—knowledge-based (familiar procedures applied to frequent decision-making situations), rule-based (routine tasks requiring little conscious effort), and skill-based (problem solving activities often in new situations) (Battles et al., 1998)
For ease of application, the model incorporates a causal tree that is useful for displaying critical activities and decisions in both logical and chronological order (Battles et al., 1998) as the investigation is undertaken. The event is diagrammed using all possible causes and recoveries gathered during the investigation, revealing the event’s underlying root causes (see Figure 9-2); the codes used in the model are defined in Table 9-4.
Of particular importance in the RCA is the ability to discover points of recovery and prevention (i.e., critical control points) to minimize future events. Recovery is the distinguishing factor between an accident and a near miss. Van der Schaff defines human recovery as the feature of the human system component to detect, localize, and correct earlier component failures (Van der Schaaf, 1992). These component failures may be either one’s own previous errors, those of a colleague, or technical factors. Use of the Eindhoven model to assess near misses is vital to the identification of causal and recovery factors, providing the new knowledge that can be integrated at the front line of care to develop a more highly reliable system. AHRQ has designated the Eindhoven model as the causal analysis taxonomy for the federal patient safety integration project. The committee supports the adoption of the Eindhoven model, with the following additions to the taxonomy:
-
Classification for recovery factors associated with near-miss events, as stated in Chapter 4
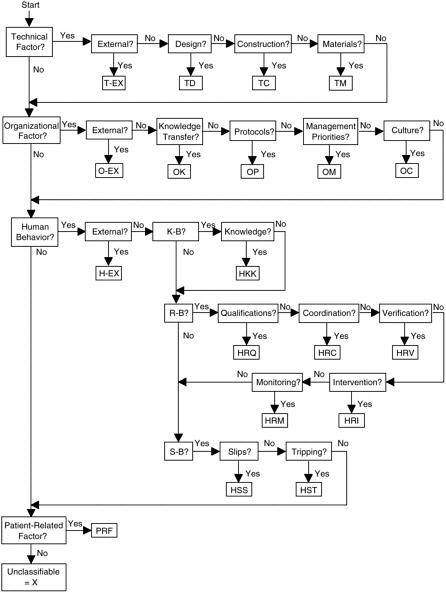
FIGURE 9-2 Eindhoven Classification Model: Medical Version.
SOURCE: Eindhoven Safety Management Group, 1997.
-
Representation of corrective actions that were taken to recover from actual incidents, as stated in the functional requirements set forth in Chapter 3
-
Representation of patient outcome/functional status (outcome) as a result of corrective actions taken
TABLE 9-4 Codes Used in Eindhoven Classification Model, Medical Version
|
Category |
Description |
Code |
|
Latent errors |
Errors that result from underlying system failures |
|
|
Technical |
Refers to physical items, such as equipment, physical installations, software, materials, labels, and forms |
|
|
External |
Technical failures beyond the control and responsibility of the investigating organization |
TEX |
|
Design |
Failures due to poor design of equipment, software, labels, or forms |
TD |
|
Construction |
Correct design was not followed accurately during construction |
TC |
|
Materials |
Material defects not classified under TD or TC |
TM |
|
Organizational |
|
|
|
External |
Failures at an organizational level beyond the control and responsibility of the investigating organization |
OEX |
|
Transfer of knowledge |
Failures resulting from inadequate measures taken to ensure that situational or domain-specific knowledge or information in transferred to all new or inexperienced staff |
OK |
|
Protocols/procedures |
Failures related to the quality and availability of the protocols within the department (too complicated, inaccurate, unrealistic, absent, or poorly presented) |
OP |
|
Management priorities |
Internal management decisions in which safety is relegated to an inferior position in the face of conflicting demands or objectives. This is a conflict between production needs and safety (e.g., decisions about staffing levels) |
OM |
|
Culture |
Failures resulting from collective approach to risk and attendant modes of behavior in the investigating organization |
OC |
|
Category |
Description |
Code |
|
Active errors (human) |
Errors or failures resulting from human behavior |
|
|
External |
Human failures originating beyond the control and responsibility of the investigating organization |
HEX |
|
Knowledge-based behaviors |
|
|
|
Knowledge-based errors |
The inability of an individual to apply existing knowledge to a novel situation |
HKK |
|
Rule-based behaviors |
|
|
|
Qualifications |
Incorrect fit between an individual’s qualifications, training, or education and a particular task |
HRQ |
|
Coordination |
Lack of task coordination within a health care team in an organization |
HRC |
|
Verification |
Failures in the correct and complete assessment of a situation, including relevant conditions of the patient and materials to be sued, before starting the intervention |
HRV |
|
Intervention |
Failures that result from faulty task planning (selecting the wrong protocol) and/or execution (selecting the right protocol but carrying it out incorrectly) |
HRI |
|
Monitoring |
Failures during monitoring of process or patient status during or after intervention |
HRM |
|
Skill-based behaviors |
|
|
|
Slips |
Failures in performance of fine motor skills |
HSS |
|
Tripping |
Failures in whole-body movements |
HST |
|
Other |
|
|
|
Patient-related factor |
Failures related to patient characteristics or conditions that influence treatment and are beyond the control of staff |
PRF |
|
Unclassifiable |
Failures that cannot be classified in any other category |
X |
|
SOURCE: Battles et al., 1998. |
||
Lessons Learned
Evaluation of the RCA should seek to document the lessons learned from the event and a process for eliminating or controlling its causes. Documentation of lessons learned has been a key reason for the success of other high-risk industries in identifying and maintaining a record of actions that can prevent events or help in recovering from them.
Summary of Domain Areas for a Common Report Format
This section has described those elements of a common report format that the committee believes would be most productive in the aggregation and evaluation of adverse events and near misses within the context of a partially or fully integrated health information system. The information to be input into this report format can be derived from the original narratives provided by the reporters of the events and supplemental information from causal analysts. The report format consists of core domain areas for which appropriate taxonomies need to be developed where none exist or refined where they do exist. Given the concept of data reuse and the interconnectedness of the NHII, health information systems should be able to capture common data elements for the generation of multiple reports without redundant data entry. Likewise, all report generation should meet the privacy and security requirements outlined in the Health Insurance Portability and Accountability Act of 1996 (HIPAA). Box 9-2 summarizes the domain areas that the committee believes to be most important to the establishment of standards for more comprehensive reporting of patient safety events.
The patient safety report can be made available electronically using the Health Level Seven (HL7) Clinical Document Architecture (CDA) standard. The form can be printed for those who prefer documentation on paper or completed electronically by those comfortable with doing so. At this early stage, reporting should focus on the collection of narrative free text from the reporter. Taxonomies for the common report format can be developed and employed by the patient safety representative to classify the report. A more structured report format can be available that utilizes both designated domains for areas for reporting and narrative text. The taxonomies to classify the report data should evolve over time as clinicians and researchers gain new knowledge and a better understanding of the nature, causes, and recovery aspects of events.
|
BOX 9-2 The discovery
The event itself
Narrative of the event—includes contributing factors Ancillary information
Detailed causal analysis—On the basis of the above information, a decision should be made on whether a formal root-cause analysis should be carried out. The analysis should include examination of the following:
Lessons learned |
As stated earlier in this chapter, there is no comprehensive patient safety taxonomy for representing adverse event and near-miss data in the United States, although JCAHO has expressed interest in this concept and has been working on the development of a taxonomy framework. Because growing international awareness of the importance of patient safety to quality care, the World Health Organization (WHO) has contracted with JCAHO to evaluate the methods used in countries around the world to define and clas-
sify patient safety data; develop a framework for analyzing the strengths and weaknesses of different patient safety classification and reporting systems; and develop a common dictionary of patient safety terms and a taxonomy for patient safety reporting that could be used for cross-country, cross-organizational comparisons. JCAHO will guide the development of the international taxonomy in consultation with WHO and its partners, including the Australian Patient Safety Foundation.
The committee believes this is an important step toward more comprehensive and standardized reporting on patient safety; however, we also emphasize that it is just as important that JCAHO’s work on a taxonomy framework meet the requirements for comprehensive reporting in each domain area outlined throughout this report, and particularly in this chapter. Ultimately, the methods JCAHO employs to develop its taxonomy framework should also reflect the work being undertaken by AHRQ in its project to integrate all federal safety-related reporting systems. The committee believes AHRQ should commission a group of stakeholders to fully develop the patient safety event taxonomy.
IMPLEMENTATION OF THE REPORT FORMAT
This chapter has provided a detailed set of recommendations on the elements of a patient safety report format that are important to the establishment of high-functioning patient safety systems and the generation of meaningful data to support quality improvement. Initially, a preliminary short-term pilot study should be undertaken to evaluate the cost and accuracy of the proposed report format. Adjustments to the committee’s recommendations can be made based on the results of this research. Next, a sound process is necessary for the format’s implementation.
Commitment to use of the report format is essential. AHRQ should play a leadership role in the implementation of the standardized format. To facilitate its adoption, AHRQ could require that the report format and associated data standards be utilized in the agency’s ongoing patient safety programs and for population of the national patient safety database. All forthcoming legislation related to the establishment of voluntary patient safety organizations could require use of the standardized format as a condition for certification of compliance with federal regulations. Population of the national patient safety database will be accomplished using submissions from both voluntary patient safety organizations and state regulatory agencies; therefore, state organizations that do not have established reporting systems can implement and use the standardized format for dual pur-
poses (with de-identification of the data forwarded to the national database). States that do have reporting systems in place can initially map their current format to the standardized format, with plans to migrate to the latter over time for simplification. From the perspective of health care providers, particularly for physicians who operate independently out of small group offices, use of a common report format should ease the incorporation of a safety program for documenting adverse events and near misses. Likewise, clinicians who operate in various settings with different reporting formats (e.g., nursing homes and home care) will be able to map their data to the standardized format for analysis and comparison of their event detection and prevention with the efforts of other settings. Finally, larger health care organizations will have a reliable method for assessing patient safety and quality with other organizations inside and outside their network.
The second requirement for adoption of the common report format is the provision of adequate support for its implementation in the form of tools, guides, and technical assistance. State agencies may also require financial assistance. From a technical standpoint, HL7 can play an important role. At HL7’s May 2003 meeting, formation of a Patient Safety Special Interest Group was proposed to the Technical Steering Committee; sponsorship of the group would fall under the Regulated Clinical Research Information Management Technical Committee. Formation of this group was approved at HL7’s September 2003 meeting.
The group’s mission is to create the message components required for the exchange of patient safety information and clinical documents for the reporting of adverse events. Specific immediate tasks include the following:
-
Create scenarios to define the requirements for identifying and reporting medical errors, adverse events, and near misses.
-
Identify the set of trigger events to initiate the transmission of such data.
-
Develop the messages, message segments, and data fields necessary to support the reporting of medical errors.
-
Create clinical documents for reporting medical errors.
-
Identify and promote the required terminology to support the reporting of medical errors.
-
Coordinate and cooperate with other groups interested in using these messages and documents.
-
Enable and promote the use of these standards and make them as widely available as possible.
Along with definition of the technical specifications for implementing the report format, other tools, guides, and assistance are needed. For example, as part of its project to integrate federal patient safety reporting systems, AHRQ has required that the contractor develop a user version of the front-end data entry system and the prototype data warehouse that can serve as a local institution’s own event reporting system and database (Agency for Healthcare Research and Quality, 2002). Having a software program available to users at nominal or no cost is an important component of AHRQ’s implementation plan. Training materials will be developed and workshops provided to enable hospital staff and other users to utilize the system (Agency for Healthcare Research and Quality, 2002).
The committee believes that AHRQ should also extend a request for proposals for the development of a generic software application of the report format employing the terminologies and data standards presented in this chapter. A built-in reporting capacity for physicians’ offices and small hospitals would ease the burden of their migration to information systems. Further, it is unlikely that these entities will be able to begin that migration without financial and technical assistance. The software application for reporting will help generate standardized reports as a baseline for patient safety systems. Providing the software to these small entities will help address two of the main barriers to adoption of the report format: (1) per physician cost and operating costs and (2) culture and readiness.
The Australian AIMS provides a suite of software tools to facilitate use of the reporting system; similar tools should be developed and included in AHRQ’s package for standardized patient safety reporting. The high functionality of the AIMS database is a direct result of this software suite, which facilitates the implementation and use of the reporting system. The AIMS interface is downloadable to the health care provider’s computer, along with the suite of tools (Australian Patient Safety Foundation, 2003):
-
Data Manager, to manage incident reports
-
Analyzer, to generate standard and user-defined reports from the database
-
Administrator, to set up and maintain the AIMS software and generate audit trails
-
Database Administrator, for downloading updates to AIMS and applying them to the database; for importing unclassified data from third-party systems; for archiving audit trails; and for uploading de-identified, classified data
-
Workflow Manager, for informing staff when incidents are reported and for managing action plans (including multiple timelines and persons responsible) and allowing administration to access the status of each project
-
Risk Register, for managing ongoing risks either linked to or independent of particular incidents or events
DE-IDENTIFICATION AND DATA PROTECTION
Internal and external patient safety reports serve different yet overlapping purposes. Whereas internal reports must contain highly detailed and often identifiable information, most external reports need contain only deidentified data. For both internal and external reports, standardized report formats would facilitate reuse of the data contained in clinical care systems, use of standardized analysis techniques, and comparative analysis of aggregate data. Although internal and external patient safety reports will not be identical, they will share many common elements, some of which must be de-identified for external reporting.
The key challenge to patient safety reporting system development is determining how to design the systems in a manner that addresses the needs of all stakeholders while encouraging reporting of medical errors and providing public access to reports of preventable errors. Consumers, payers, trial attorneys, and other groups demand open and public disclosure of adverse events that result in death or serious harm. Health care providers and malpractice carriers insist on confidentiality and legal protection for reported errors. Fear of legal liability in a punitive environment can dampen providers’ willingness to generate information about errors and thus limit what can be learned about how, when, and where medical mistakes occur. Underreporting of medical errors due to provider anxieties about legal exposure can undermine the effectiveness of patient safety reporting systems.
Generally, disclosures to external organizations should not include identifiable information, with the exception of specific public health activities required by law. However, concern about potential discoverability and fear of retaliation against those who report safety incidents are major impediments to effective reporting programs. Traditionally, the proceedings of quality management reviews are protected from discoverability by state laws regarding peer review protections and reporting system authorizing statutes. Many states are evaluating whether data from patient safety reports should be conclusively protected under these statutes or publicly available to afford transparency and accountability to the public. Current legislative proposals address solely the protection of voluntary reporting systems by strengthen-
ing peer review statutes at the national level. Enhanced protection could facilitate the establishment of voluntary reporting systems and willingness to populate a national patient safety database of de-identified data. The committee believes that adequate legal protection of voluntarily reported data is essential for the integrity and effectiveness of patient safety learning systems. The committee also believes that further study is needed to define the appropriate conditions for disclosure and protection of data from patient safety reports in all systems.
Under these conditions, clinical information can be reused to generate reports on an ADE to government regulatory agencies (e.g., FDA) and state public health regulatory agencies (e.g., NYPORTS). If patient safety initiatives are explicitly defined to be a part of quality initiatives, a hospital may choose to participate with USP in a program to monitor medication errors under the terms of a business associate agreement. In accordance with terms of that agreement, USP could accept patient safety reports with identifiable information and create de-identified reports to the national patient safety database. To meet the requirements for JCAHO’s sentinel event policy, the hospital could submit de-identified data containing an RCA of the event and an action plan of corrective measures. Integrated health information systems would automatically screen the data elements for each report requirement in accordance with the established legal protections. Further research is needed on how information systems can be leveraged in this manner.
PRIMARY AND SECONDARY USES OF REPORT DATA
Information stemming from the proposed patient safety reports, particularly the causal analysis, has both primary and secondary applications in improving the care processes. Most primary uses relate to the feedback loop that supplies data for refining care processes and enhancing decision support tools, while secondary uses include database research into the epidemiology of occurrences, public health surveillance, drug safety surveillance, and other studies (Lowrance, 2002). Several organizations that currently have patient safety reporting systems are using the data for safety and quality improvement purposes. This section provides brief descriptions of selected programs implemented by these organizations.
VHA has instituted a solid flow of information for primary applications involving both short- and long-term system improvements. The data flow from the reporter to the patient safety manager, from the manager to the RCA team, and again between the reporter and the RCA team to provide feedback and add to the findings. The feedback to the reporter is relatively
immediate to demonstrate the meaningfulness of the reporter’s efforts, as well as to ensure reinforcement for learning. For example, VHA’s directive Ensuring Correct Surgery relies on patient safety data as primary feedback to address surgical events. The directive provides specific information on what steps must be taken to ensure that a surgical procedure is performed on the right patient, at the correct site, and if applicable with the correct implant (Department of Veterans Affairs, 2002). Review and analysis of patient safety reports on surgical events have led to the development of a specific set of minimum preoperative procedures to be completed days to 1 hour before surgery (i.e., consent form validating the site, laterality, name, and reason for the procedure, plus marking of the site soon before the surgery); a set of minimum procedures to be carried out just before or when the patient enters the operating room, 1 hour or less before surgery (i.e., staff must ask the patient to state his or her name, social security number, and location on body of procedure); and a set of minimum operating room procedures for completion minutes to seconds before surgery (i.e., a designee is responsible for ensuring that all procedures are in place that require verification—patient, procedure, site, implant—by operating room personnel) (Department of Veterans Affairs, 2002). Procedures are listed on posters and placed at strategic points to assist VHA staff in remembering them at each stage and are also included in brochures provided to patients who register for surgery. From January through June 2003, VHA experienced no reported cases of an incorrect surgery when the procedures required by the Ensuring Correct Surgery directive were followed (Eldridge, 2003).
Another example, related to the needs of special populations, is associated with the Child Health Accountability Initiative (CHAI) undertaken by the Child Health Corporation of America. CHAI has established a three-track approach to quality: Track I—clinical improvement in the areas of medication safety, patient safety, pain management, and clinical research; Track II—building bridges among key organizations to establish national priorities and share vital data on pediatric quality and safety improvement measures; and Track III—informing the field by disseminating information to policy makers and the public (Payne and Throop, 2002). The initiative’s work on medication safety began with an intense investigation of all medication orders written at participating hospitals, followed by an analysis of the types and patterns of events and the creation of site-specific improvement plans (Payne and Throop, 2002). The first phase of this effort laid the groundwork for the subsequent phases of study and resulted in a 24.7 percent decrease in prescribing errors, a 73.9 percent increase in intercepted errors, and a 49.2 percent reduction in prescribing errors not intercepted
(Payne and Throop, 2002). For the second phase, the hospitals focused on a specific type of adverse event related to two high-risk areas for pediatric patients—sedation and analgesia—and one hospital in particular developed and tested a trigger system methodology for identifying and resolving ADEs. Several CHAI hospitals achieved a 75 percent reduction in medication errors (Payne and Throop, 2002). Using the modified trigger tools first developed by David Classen, ADE identification per 100 hospital days was 40 percent higher than with hospital-wide reporting mechanisms (Payne and Throop, 2002). Table 9-5 provides an overview of the CHAI medication safety project.
The most common secondary use of patient data is to satisfy needs for public health surveillance. Epidemiological methods for scientific data analysis are necessary to identify and track health threats, assess population health, create and monitor programs and services, and conduct research, particularly given the new threat of bioterrorism. Incident reports and surveillance with trigger tools enable public health authorities to screen efficiently for emerging diseases or epidemics (National Committee on Vital and Health Statistics, 2001). Common data standards related to patient safety and public health events are vitally important to the cross-dimensional communications across local, regional, and national entities of the NHII.
Other secondary uses of data are being employed by payers. For example, Blue Cross of California, owned by Wellpoint, Inc., has initiated an award of bonuses to physicians who meet certain quality-of-care measures, including childhood immunization rates; screening rates for breast, cervical, and colorectal cancers; and quality indicators related to asthma, diabetes, and depression (Desmarais, 2002). Another program in development by employers and health plans will provide financial incentives to physicians who establish the following: clinical information programs, such as an EHR, in their offices; a system for regularly following up on the care of chronically ill patients; or patient education programs (Desmarais, 2002). All programs are designed according to evidence-based medicine and evaluate patient outcomes as part of the criteria for the award. At present, however, many providers lack the technologies and data standards needed to engage in these programs. The ability to reuse patient data, including safety data, for quality improvement purposes would ease providers’ transition to electronic systems while at the same producing immediate gains and reinforcement for collection of the data and follow-up on patient care. Wellpoint recently joined with the RAND Corporation on a grant from the Robert Wood Johnson Foundation to assess payer-oriented incentive programs.
CONCLUSIONS
Improving patient safety requires the determination and dissemination of best safety practices and systemic improvements derived through rigorous scientific analysis of data collected from numerous sources on the wide range of adverse events and near misses that can occur within a health care organization. The analysis should encompass multiple comparability studies of abundant patient safety report data available at the organizational, state, and/or federal level, as well as other valuable sources of patient safety data within the context of an integrated health information infrastructure (e.g., chart reviews, malpractice data, surveillance of trigger data, patient safety reports, quality measures, disease registries). To this end, safety reporting systems should incorporate commonly defined data elements that can meet the needs of multiple agencies and purposes within the context of integrated systems and the NHII.
The purpose of this chapter has been to develop a framework, summarized in Box 9-2, for the collection and codification of the report data most important to the discovery, analysis, and understanding of and learning from patient safety events. This framework should produce a strong evidence base of specific safety measures that can lead to safer care, the prevention of events from occurring in the first place, and facilitated recovery when they do occur. Maintaining the value of rich narrative data from free-text reports, the framework provides a model from which to initiate codification of these narrative data to resolve conflicts and relieve burdens resulting from the current state of overly complex and disparate reporting requirements. The framework includes domains for basic event data (who, what, when, where, why, how), ancillary product and patient information, a risk assessment model, a causal analysis model, recovery factors, outcome, and lessons learned. The appropriate taxonomies need to be developed or refined to fully represent each domain area. These taxonomies should encompass the core terminologies identified by NCVHS as central to the NHII and those identified for the AHRQ sponsored project to integrate federal patient safety reporting systems. As organizations migrate to fully integrated health information systems, it should be possible to generate multiple reports automatically using common data elements compatible with the NHII.
The committee believes the achievement of a safety culture is a national imperative. To this end, health care organizations, state and federal regulatory agencies, and research organizations must move forward in the near term to adopt the common patient safety data standards proposed in this
TABLE 9-5 Overview of Child Health Accountability Initiative (CHAI) Medication Safety Project
|
Year |
1999 |
2000 |
|
Area of emphasis |
Pediatric Intensive Care Unit |
Sedation and Analgesia |
|
Description of CHAI projects |
Formed multidisciplinary teams centered on rapid improvement in medication errors Developed new medication error tracking tools Investigated all medication orders written at participating hospitals Analyzed types and patterns of errors Developed and implemented site-specific improvement plans |
Implemented new reporting form Developed standardized data dictionary Created medication usage process maps Completed (IHI) Breakthrough Series for ADEs Participated in IHI Idealized Design for the Medication System |
|
Result |
24.7 percent decrease in the pediatric ICU medical prescribing error rate 73.9 percent increase in intercepted errors 49.2 percent reduction in prescribing errors that were not intercepted |
75 percent reduction in medication errors in sedation and analgesia Systems that are safer by a factor of 10 New clinical processes shown to reduce errors Identification of automation as essential in reducing errors Trigger system concept for identifying and resolving ADEs in pediatrics |
|
SOURCE: Payne and Throop, 2002. |
||
|
2001 |
2002 |
|
|
Trigger System Methodology |
Trigger System and Technology |
|
|
Adapted and tested a trigger chart review tool for the pediatric population Implemented the trigger system across CHAI hospitals |
Identified and defined a collection of medication rule sets for the top 100 drugs used for pediatrics Implemented personal digital assistant (PDA) technology for data collection using nine pediatric triggers Tested five to seven new pediatric-specific triggers Compared methodology with other methods of ADE reporting |
The Child Health Accountability Initiative (CHAI) medication safety project has created the foundation for national safety measures in pediatrics. CHAI is working toward a goal of national adoption of the CHAI pediatric trigger methodology and integration of the triggers into computerized physician order entry as the industry standard. |
|
A highly effective, new method of reducing errors; triggers had a higher rate of ADE identification than any other method Identification of nine triggers that have the greatest number of ADEs in pediatric hospital settings Realization that most trigger- and nontrigger-identified ADEs are attributable to prescribing/ordering errors |
|
Through its Informing the Field and Building Bridges tracks, CHAI is collaborating with health care providers and national quality and safety organizations to reduce medication errors for the youngest health care consumers. |
report. Doing so will make it possible to streamline and simplify the mechanisms for research, analysis, and learning with regard to adverse events in our health care system, as has been done in the aviation industry and other high-risk sectors. Standardization of data will enable the evolution of a new knowledge base of patient safety information and system improvements that can be readily incorporated into the practice of evidence-based medicine.
REFERENCES
Agency for Healthcare Research and Quality. 2002. Patient Safety Database: Request for Proposal No. AHRQ-02-0015. Online. Available: http://www.ahcpr.gov/fund/rfp02015.htm [accessed June 2002].
Anesthesia Patient Safety Foundation. 2003. Data Dictionary Task Force Unveils DATAMS. Online. Available: http://www.apsf.org/dictionary/DATAMS/datams.html [accessed August, 2003].
Australian Patient Safety Foundation. June 3, 2002. Briefing Book: Australian Incident Monitoring System. Adelaide, South Australia: Australian Patient Safety Foundation.
———. 2003. Australian Incident Monitoring System: Collect, Classify, Analyze, Learn. Adelaide, South Australia: Australian Patient Safety Foundation.
Bagian, J. P., C. Lee, J. Gosbee, J. DeRosier, E. Stalhandske, N. Eldridge, R. Williams, and M. Burkhardt. 2001. Developing and deploying a patient safety program in a large health care delivery system: You can’t fix what you don’t know about. Jt Comm J Qual Improv 27 (October) (10):522–532.
Bates D. W., R. S. Evans, H. Murff, P. D. Stetson, and G. Hripcsak. 2003. Detecting Adverse Events Using Information Technology. Boston, MA: Division of General Medicine and Primary Care, Brigham and Women’s Hospital.
Battles, J. B., H. S. Kaplan, T. W. Van der Schaaf, and C. E. Shea. 1998. The attributes of medical event-reporting systems: Experience with a prototype medical event-reporting system for transfusion medicine. Arch Pathol Lab Med 122 (3):231–238.
Campbell, J. R. August 22, 2002. Presentation and Testimony on Converging the Clinical Care Model. National Committee on Vital and Health Statistics, Subcommittee on Standards and Security. Washington, DC.
Cook, R. I. 2003. Prospects for Using Adverse Event Reporting to Improve Patient Safety. Commissioned paper for the Institute of Medicine Committee on Data Standards for Patient Safety.
Department of Veterans Affairs. 2001. National Center for Patient Safety Triage Cards for Root Cause Analysis. Ann Arbor, MI: Veterans Health Administration.
______. 2002. National Center for Patient Safety: Ensuring Correct Surgery Directive. Ann Arbor, MI: Veteran Health Administration.
Desmarais, H. November 25, 2002. Presentation to the IOM Committee on Data Standards for Patient Safety. Health Insurance Association of America on Patient Safety and Quality Initiatives. Washington, DC.
Eindhoven Safety Management Group. 1997. The Development of an Incident Analysis Tool for the Medical Field. Report EUT/BDK/85. Netherlands: Eindhoven University of Technology.
Eldridge, N. 2001. Presentation on the Veterans Health Administration National Center for Patient Safety to the National Committee on Vital and Health Statistics, Subcommittee on Populations, Workgroup on Quality. Washington, DC.
______. 2003. Veterans Health Administration National Center for Patient Safety. Personal communication to Institute of Medicine’s Committee on Data Standards for Patient Safety.
Farrier, T. A. 1997. Breaking the Codes: Barriers to Effective Collection and Retrieval of Safety Data. Proceedings at the International Society of Air Safety Investigators.
Gaba, D. M. 2000. Anaesthesiology as a model for patient safety in health care. BMJ 320 (7237):785–788.
Gosbee, J. 2003. Veterans Health Administration National Center for Patient Safety. Personal communication to Institute of Medicine’s Committee on Data Standards for Patient Safety.
Iezzoni, L. I., ed. 1997. Risk Adjustment for Measuring Healthcare Outcomes. Chicago, IL: Foundation of the American College of Healthcare Executives.
Institute of Medicine. 2000. To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press.
Kaplan, H. 2003. Meeting with the Institute of Medicine Staff to Committee on Data Standards for Patient Safety Regarding MERS TH.
Lowrance, W. W. 2002. Learning from Experience: Privacy and the Second Use of Data in Health Research. London, UK: The Nuffield Trust for Research and Policy Studies in Health Services.
McKnight, S., and J. Gosbee. 2002. Improved Searching and Analysis of RCS Data. Veteran Health Administration National Center for Patient Safety. White paper.
National Committee on Vital and Health Statistics. 2001. Information for Health: A Strategy for Building the National Health Information Infrastructure. Online. Available: http://ncvhs.hhs.gov/nhiilayo.pdf [accessed April 18, 2002].
Nebeker, J. R., J. F. Hurdle, J. M. Hoffman, B. Roth, C. R. Weir, and M. H. Samore. 2002. Developing a taxonomy for research in adverse drug events: Potholes and signposts. J Am Med Inform Assoc 9:80–85.
New York Patient Occurrence Reporting and Tracking System. 2001. NYPORTS User’s Manual. Version 2.1. Albany, NY: New York State Department of Health.
———. 2002. NYPORTS: Includes/Excludes Occurrence List. Albany, NY: New York State Department of Health.
Payne, D., and C. Throop. November 25, 2002. The Patient Safety and Quality Initiatives of Child Health Corporation of America: A Briefing for the Institute of Medicine. Presentation to the Institute of Medicine Committee on Data Standards for Patient Safety. Washington, DC.
Reason, J. 1990. Human Error. Cambridge, UK: Cambridge University Press.
Regenstreif Institute. 2001. Logical Observation Identifiers, Names and Codes: User’s Guide. Indianapolis, IN: Regenstreif Institute.
Rosenthal, J., T. Riley, and M. Booth. 2000. State Reporting of Medical Errors and Adverse Events: Results of a 50-State Survey. Portland, ME: National Academy for State Health Policy.
Rosenthal, J., M. Booth, L. Flowers, and T. Riley. 2001. Current State Programs Addressing Medical Errors: An Analysis of Mandatory Reporting and Other Initiatives. Portland, ME: National Academy for State Health Policy.
Tennessee Department of Health. 2003. Hospital Fax Reporting of Incidents and Abuse. Provided by J. Rosenthal to Institute of Medicine Staff to Committee on Data Standards for Patient Safety.
U.S. Pharmacopeia. 2003. Summary of Information Submitted to MedMARx in the Year 2002: The Quest for Quality. Rockville, MD: The United States Pharmacopeial Convention, Inc. Provided by Diane Cousins on December 2003.
Van der Schaaf, T. W. 1992. Near Miss Reporting in the Chemical Process Industry. Eindhoven, Netherlands: Technische Universiteit Eindhoven.
Westat. 2001. MERS-TM: Medical Event Reporting System for Transfusion Medicine Reference Manual. In support of Columbia University under a grant from the National Heart, Lung, and Blood Institute, National Institutes of Health (Grant RO1 HL53772, Harold S. Kaplan, MD., Principal Investigator). Version 3.0. New York, NY: Trustees of Columbia University.
Williams, S. D. January 8, 2003. Comments on Using ICD-9-CM and ICD-10-CM Codes to Identify Medical Errors or Adverse Events. Letter to the Institute of Medicine Committee on Data Standards for Patient Safety.