2
Components of a National Health Information Infrastructure
CHAPTER SUMMARY
A comprehensive approach to patient safety requires the ability to anticipate and protect against circumstances that might lead to adverse events and implement corrective actions. Both adverse events and near misses require standard collection/reporting processes, datasets, definitions, and analytic approaches that can be achieved only by integrating patient safety reporting systems into the context of health information systems in both large institutions and office practices. These systems employ multiple detection methods and multiple reporting channels and involve a broad array of data elements. Establishing a national health information infrastructure is necessary to provide the backbone for such systems.
This chapter is divided into three sections: the first provides a general overview of the national health information infrastructure and a conceptual model of standards-based integrated data systems to support patient safety in institutional and office practice settings for all audiences; the second presents a technical review of the informatics components that support an information infrastructure for the technical reader; and the third provides a discussion of how standards-based clinical systems can be and have been implemented to support this endeavor for both audiences.
GENERAL OVERVIEW
Improving patient safety requires much more than systems for reporting and analyzing events; errors must be prevented from occurring in the first place. Several effective tools are available that can assist in the prevention of adverse events. Clinical decision support systems (CDSSs), such as those for medication order entry, can prevent many errors from occurring (Bates et al., 1997, 1998, 1999). Computer-based reminder systems can facilitate adherence to care protocols (Balas et al., 2000); computer-assisted diagnosis and management programs can improve clinical decision making at the point of care (Durieux et al., 2000; Evans et al., 1998); and immediate access to clinical information, such as results of laboratory and radiology tests, can reduce redundancy, allowing for more efficient decision making. Incorporation of new research findings into clinical practice is also important for improving patient safety. Balas and Boren found that it takes an average of 17 years for research to reach clinical practice, whereas newer technological innovations take an average of 4 to 6 years. Actionable knowledge representation through the use of information systems holds promise for better connecting clinical research and patient care practices (Balas and Boren, 2000). In addition, the Internet can be used for customized health education for patients, thereby promoting more effective self-management of chronic and other medical conditions (Cain et al., 2000; Goldsmith, 2002). The Internet can be used as well for communication among all authorized members of the care team (e.g., primary care providers, specialists, nurses, pharmacists, home health aides, the patient, and lay caregivers), a capability that is especially important for the chronically ill. The capabilities provided by these clinical information systems cannot be achieved, however, without standards-based interoperability founded on the national health information infrastructure (NHII).
The NHII is defined as a set of technologies, standards, applications, systems, values, and laws that support all facets of individual health, health care, and public health (National Committee on Vital and Health Statistics, 2001). It encompasses an information network based on Internet protocols, common standards, timely knowledge transfer, and transparent government processes with the capability for information flows across three dimensions: (1) personal health, to support individuals in their own wellness and health care decision making; (2) health care providers, to ensure access to complete and accurate patient data around the clock and to clinical decision support systems; and (3) public health, to address and track public health concerns and health education campaigns (National Committee on Vital and Health
Statistics, 2001). As shown in Figure 2-1, there are significant areas of overlap among these three dimensions in terms of functionality and applications.
With the NHII, information systems will be able to provide the right information, at the right time, and to the right individuals, enabling safe care and supporting robust safety reporting systems for cases in which adverse events and near misses do occur. The NHII also will yield many other benefits in terms of new opportunities for care access, efficiency, and effectiveness; public health; homeland security; and clinical and health services research. For example, electronic health records (EHRs), in conjunction with secure data exchange, may allow for early detection of and rapid response to infectious diseases. The NHII will also facilitate the organization and execution of large-scale inoculation programs, as well as the dissemination to clinicians and patients of up-to-date information and practice guidelines on the presentation and treatment of morbidity due to chemical and biological threats.
Standards-based information systems built on the foundation of the NHII will permit cross-organizational data sharing. Several promising
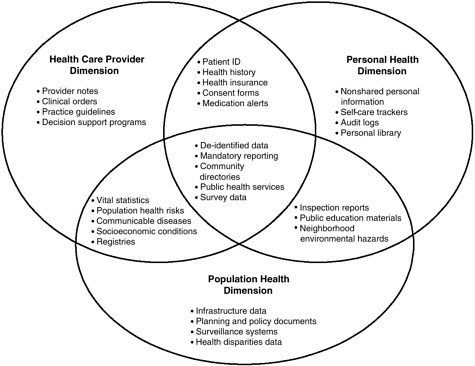
FIGURE 2-1 Examples of content for the three NHII dimensions and their overlap.
SOURCE: National Committee on Vital and Health Statistics, 2001.
public–private information technology demonstrations currently under way nationwide are exchanging data outside traditional organizational boundaries. One such project is the New England Healthcare Electronic Data Interchange (EDI) Network (NEHEN)—a consortium initiated in 1998 and led by Computer Science Corporation (New England Healthcare EDI Network, 2002). Membership is open to providers, health plans, and payers in Massachusetts and Rhode Island; there are currently 14 members, including most of the region’s largest insurers and health plans. NEHEN provides its members, who pay a flat monthly fee, with access to a secure high-speed network for sending and receiving transactions. Members can either integrate NEHEN functions directly into their own management systems or access the NEHEN network using NEHENLite, a Web-based application.
A second promising project is the Indiana Network for Patient Care (INPC), initiated 10 years ago in Indianapolis by the Regenstrief Institute for Health Care. Currently, all 13 acute care hospitals in the city and approximately 20 percent of the metropolitan area’s outpatient physician practices are participating (Overhage, 2003). Participating institutions pay a monthly fee for access to selected electronic information that forms the basis for an “operational community-wide electronic medical record” that includes reports from emergency room visits, laboratory results, admission notes/discharge summaries, operative reports, radiology reports, surgical pathology reports, inpatient medications, immunizations, and a tumor registry (Overhage, 2003). Each health care provider retains its patients’ information in its organization’s database; however, selected information in those datasets can be shared among organizations through use of a Global Patient Index (Overhage, 2003). INPC not only allows for the secure storage and exchange of clinical information but also provides clinical decision support and public health surveillance and reporting.
A third example of a regional data sharing network is the Santa Barbara County Care Data Exchange, initiated in 1998 through a partnership between CareScience and the California Healthcare Foundation (CareScience, 2003). More than 75 percent of the health care providers in Santa Barbara County are participating, including medical groups, hospitals, clinics, laboratories, pharmacies, and payers. The Care Data Exchange allows for rapid and secure delivery of patient data to authorized users who have informed consent.
While the above projects are all extremely promising, they remain isolated examples. Such efforts are unlikely to be replicated on a larger scale until the major technical, organizational, and financial impediments to the development of the NHII are addressed.
From a technical perspective, the NHII will require the construction of an information and communications infrastructure in much the same way as one builds an electrical power grid. The “materials” for constructing the infrastructure are the core informatics components required to generate data flows: data acquisition methods and user interfaces, health care data standards, data repositories and clinical event monitors, data mining techniques, digital sources of evidence or knowledge, communication technologies, and clinical information systems (each discussed in detail later in this chapter). To facilitate the development of the NHII, the Institute of Medicine (IOM) recently proposed several demonstration projects aimed at establishing state-of-the-art health care information and communications infrastructure at the community, state, and regional levels (Institute of Medicine, 2002a). That report suggests that information and communications infrastructure can contribute to improvements in four areas of relevance to patient safety: communication, access to patient information, knowledge management, and decision support.
At the organizational level, moving forward with a health information infrastructure requires the development of comprehensive, standards-based systems necessary for delivering clinical information at the point of care, facilitating communication for care coordination, and supporting patient safety systems for detection and prevention of adverse events and for detection and recovery from near misses. The first section of this chapter presents a conceptual model of a standards-based data system that draws on the above core informatics components of a national health information infrastructure; the second section provides a brief overview of each of those components. The results of a demonstration project to assess the current state of vendor information systems in attaining the conceptual model are then summarized. The next section presents several practical approaches to moving forward with integrated health data systems. Finally, we discuss how challenges to overcoming the implementation of information technology in the national health information infrastructure can be overcome.
CONCEPTUAL MODEL OF STANDARDS-BASED, INTEGRATED DATA SYSTEMS TO SUPPORT PATIENT SAFETY
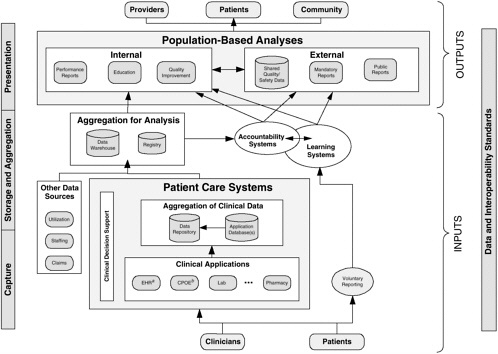
A conceptual model for standards-based, integrated data systems to support patient safety is presented in Figure 2-2. This conceptual model encompasses several key principles of such systems:
-
Reuse of data
-
Aggregation of data for learning and accountability
-
Feedback from learning that results in improvement and system changes
-
Interoperability standards as essential glue
-
Parallel reporting pathway outside patient care systems
-
Usable by both providers and patients
Integrated Systems and Large Institutions
Under this model, patient data are captured in a variety of clinical applications, such as EHRs and computerized physician order entry systems, in a variety of inpatient and outpatient settings as part of the health care delivery process. Patients may also enter such data as symptoms and self-care behaviors directly into clinical systems and review aspects of their record, such as laboratory results.
In some organizations, patient data from different clinical applications are integrated in a clinical data repository; in other organizations, the EHR can be utilized for data integration. For patient safety purposes, data about adverse events and near misses also can be integrated and fed into the repository through CDSSs. Evidence-based care is enhanced over time with a constant infusion of new medical knowledge from the biomedical literature into decision support systems so that significant aspects of care are supported for such purposes as delivering preventive care reminders to clinicians.
Patient care data, along with other useful data sources, are aggregated for analysis in registries, analytic databases, and data warehouses. They can be used for analysis and reporting to support learning and accountability both within and outside individual health care organizations. These aggregated data resources can be used to generate insights into patient care processes and to monitor performance. Finally, while the majority of data for learning and accountability are reused from clinical care, it is essential that voluntary reports from patients and clinicians also feed into these systems—represented in Figure 2-2 as the “voluntary reporting” pathway.
While Figure 2-2 is the overall objective for integrated systems in the NHII, technology is currently at varying degrees of implementation across different health care settings. Thus, a strategy is needed to progressively increase the informatics capabilities, interoperability, and utilization of clinical systems and decision support applications. To begin the integration and data sharing process, local systems can establish interoperability by incorpo-
rating common standards for messaging formats, a generic information model, and terminology standards where appropriate. The primary systems that support most decision support applications—pharmacy, laboratory, radiology, and administrative databases—can also be linked so that computerized physician order entry, alert/reminder, and other such systems can be implemented. For patient safety, triggers can be implemented to identify potential adverse events or patient contraindications in laboratory and pharmacy systems, integrated with systems that can accept narrative patient safety reports.
Integrated Systems and Office Practice
A key result of the NHII will be to permit information exchange across institutional boundaries, providing more complete patient information and enabling better coordination of care. Traditionally, most data exchange has occurred within the boundaries of larger institutions or health systems. However, since most providers practice, at least in part, outside of large institutions, much of the anticipated benefit of the NHII may result from improved data linkages with and among smaller, office-based practices.
While large institutions and office practices require somewhat different information technology architectures, the informatics requirements to support systems integration and clinical decision support tools are the same. Instead of linking with internal departmental systems within larger organizations that account for the majority of patient data (e.g., pharmacy, laboratory, radiology), office practices will be able to use data exchange standards to send and receive important patient data (e.g., results of a laboratory test, a discharge summary) to/from external systems and retrieve information from knowledge sources (e.g., a medical literature database, disease registries).
Instead of the information technology architecture of distributed systems connected through a central data repository that would characterize a large institution, small office practices will use a simpler architecture, with the EHR and/or practice management system as the principal repository for information on their patients and general office operations. These systems will still link to external systems using common message and data standards. Patient safety systems will be connected to office practices by one of several means: (1) direct integration with the internal database of a practice as part of a quality improvement program, (2) linkage to an external patient safety organization, or (3) voluntary or mandatory participation in external public repositories. Common standards will allow the systems to exchange data that can be integrated into patient records and support tools in a manner
that retains data comparability. Additionally, integrated systems and common data standards in clinical practice will yield the benefits of data reuse, lessening the burden of clinicians’ regulatory obligations for reporting on quality measures, patient safety, accreditation, and the like.
Under this model, office practices will utilize the wide range of clinical information systems that make up the totality of the EHR. Information technology systems may include computers, personal digital assistants (PDAs), and/or voice recognition devices. These systems will be available in every examining room and clinician’s office, offering the promise of greater convenience, accessibility, integration, and accuracy in information about patients and their health conditions (Bodenheimer and Grumbach, 2003). Electronic communications will enhance efficiency in patient–physician and physician–physician communications. For example, it will be possible to handle many interactions—such as reporting test results, arranging specialty referrals, receiving data on home glucose levels, and adjusting medication doses—by e-mail (Bodenheimer and Grumbach, 2003). Using electronic devices or computers, physicians will be able to store or electronically access vital knowledge bases, such as directories of pharmacies and specialists, descriptions of medications and drug interactions, reference texts, practice guidelines, and evidence-based abstracts (Bodenheimer and Grumbach, 2003).
To date, much discussion related to the use of technology in office practices has focused on administrative and financial transactions defined under the Health Insurance Portability and Accountability Act (HIPAA) and on the incorporation of the EHR. By 2002, however, only 17 percent of U.S. primary care physicians were using an EHR system, compared with 58 percent in the United Kingdom and 90 percent in Sweden. The lag in U.S. adoption of the EHR has been the result of several factors that are now being addressed: the cost of investing in health information technologies, inertia and a lack of incentives for change, the quality of medical information available on the Web, incompatibility of software programs, privacy concerns, lack of reimbursement, and concern about compromising the personal interaction between physician and patient (Bodenheimer and Grumbach, 2003). To move forward with the implementation of clinical information technology systems, a central focus of initiatives to implement the NHII must be on providing small practices with support comparable to that extended to large health care institutions. Public–private partnerships will be required that provide opportunities for financial incentives, technical assistance, and the development of a migration strategy that addresses the special needs of small practice physicians.
The process for creating integrated systems requires consideration and incorporation of the functionalities associated with the primary informatics components that support an information infrastructure. A more technical discussion of these components is provided in the next section.
TECHNICAL CONSIDERATIONS: INFORMATICS COMPONENTS OF THE INFORMATION INFRASTRUCTURE
The informatics components of the NHII provide a foundation for a comprehensive standards-based system and a migration strategy for its implementation. This section briefly describes the key components of a health information infrastructure that supports patient safety.
Data Acquisition Methods and User Interfaces
Data become available to learning or accountability systems by various means, including abstraction from paper records; direct entry into a computer system (keyboard entry, voice, touch screen, pen); and reuse of data collected by other systems, such as those used for clinical care or administrative purposes. Information capture per se takes many forms, including speech, free text, document imaging, clinical imaging (e.g., x-rays), motion video, binary electronic data representation (e.g., laboratory values, device settings, operational status, measurements), waveforms (e.g., electrocardiograms), graphical codes (e.g., digital ink), and indexing/clinical encoding (e.g., extensible markup language [XML], International Classification of Diseases [ICD]) (Waegemann et al., 2002). Regardless of entry mode, data that are captured in standardized terminologies are more accessible for reuse than narrative text. As discussed later in this chapter, however, significant advances have been made in the use of natural language processing of narrative text for the detection and prevention of adverse events.
Methods of acquiring data may also vary by domain. For example, speech input works well in radiology, where the reporting is structured. On the other hand, pen-based data entry using a wireless tablet computer suits the task of documentation associated with home health care nursing. Laboratory and pharmacy data that are essential to the detection and prevention of adverse events and near misses are typically available from department-level information systems and can be reused for patient safety and quality management purposes. Given variations in levels of technology adoption and the needs of different clinical domains, organizations should maintain
the ability to accommodate various methods of data acquisition and styles of documentation in progressing toward fully automated learning and accountability systems.
Waegemann et al. (2002) have developed a set of essential overall principles for optimal information capture and report generation with information technologies. According to these principles, such technologies should provide for unique identification of the patient, accuracy of information capture through the use of standards-based terminologies, completeness of information and minimization of duplication, timeliness such that data can be captured at the point of care, interoperability with any clinical information system, retrievability so that information can be found efficiently, authentication and accountability so that all data can be attributed to its source, auditability for ongoing assessments of quality, and confidentiality and security features to protect the data.
An intuitive and efficient user interface, that part of the computer system that communicates with the user, is utilized for interactive data entry, and controls the execution and flow of data (van Bemmel and Musen, 1997); it is another key component of clinical information systems (Shortliffe et al., 2001). User interface tools to facilitate data acquisition are still in the early stages of development, and a number of research projects are now under way to resolve associated impediments to the widespread implementation of clinical information systems. Much is being learned from the ubiquity of Web interfaces (Shortliffe et al., 2001). Current research integrates a number of methodologies from both engineering and cognitive science to evaluate and design systems from the perspective of terminology use (e.g., coded data entry) and navigation (Cimino et al., 2001); customization for the intended users and their unique requirements related to data structure, collection, and display (e.g., physician, nurse, patient) (Kinzie et al., 2002); and integration with emerging advanced technologies, such as speech recognition, multimedia, hypermedia (documents that contain links to various media), and virtual reality (van Bemmel and Musen, 1997). Core guidelines for the successful design of user interfaces identify several approaches to facilitate usability, including grouping of information, minimization of information overload, consistent and standards-based information display, information highlighting relative to importance, use of graphics, optimal text presentation, and use of icons (van Bemmel and Musen, 1997). Additional information on standards for user interfaces is provided in Chapter 4.
Health Care Data Standards
Health care data standards are the foundation for any learning and accountability system, regardless of whether the system involves abstraction of paper charts with entry into a stand-alone database or is part of an integrated information system. As shown in Table 2-1, the types of interchange, content, and measurement standards discussed elsewhere in this report are necessary regardless of the technology base of the system. The representation of terms related to patient safety in computer-based systems in a manner that renders them machine processible and available for reuse for patient safety accountability and learning systems is essential for both stand-alone databases and integrated systems.
Data exchange standards, such as reference information models, message definition frameworks, and clinical document architectures, support semantic interoperability (i.e., the ability to receive and understand data from another system) among the heterogeneous computer-based systems that form an integrated information system. Data exchange standards provide the technical specifications for the functioning of application programs, equipment and media systems, decision support systems, and other technologies. The status of various types of health care data standards and the gaps that need to be addressed are described in detail in Chapter 4.
TABLE 2-1 Data Standards of Relevance to the Structure of Patient Safety Systems
|
Standard |
Stand-alone Database |
Integrated Information System |
|
Data element definitions, including standardized measures |
• |
• |
|
Datasets representing clinical practice measures |
• |
• |
|
Standardized terminology |
• |
• |
|
Knowledge representation (concepts, guidelines) |
|
• |
|
Health identifiers |
|
• |
|
Reference information model |
|
• |
|
Message structure |
|
• |
|
Clinical document architecture |
|
• |
The ability to utilize, process, analyze, and reuse information, whether for patient safety or clinical care purposes, depends directly on the ability to organize the information into meaningful domains with hierarchies of specificity. Structured terminologies support information management for all levels of technology integration. Standardized terminologies exist for the core phenomena of clinical practice: (1) patient problems (e.g., medical diagnoses, nursing diagnoses, signs and symptoms; (2) interventions, including those focused on prevention and health promotion; and (3) health outcomes (e.g., disability, functional status, symptom status, quality of life). Several authors have identified characteristics of a computer-processible terminology that would define health care concepts nonamibiguously and promote data reuse (Campbell, 1998; Chute et al., 1998; Cimino, 1998). These criteria form the basis for the recommendations of the National Committee on Vital and Health Statistics (NCVHS) regarding core terminologies to support the EHR. Terminologies are discussed in greater detail in Chapter 4.
Knowledge representation standards address the acquisition and maintenance of medical knowledge in databases (called knowledge bases) that systematically organize the information collected to facilitate decision making or help solve problems. Because the information collected combines both scientific knowledge from the medical literature and systemic reviews based on experiential knowledge from patient databases or validated clinical guidelines (van Bemmel and Musen, 1997), modeling of cognitive deductive and inductive processes requires standards for such matters as logic, decision trees, rule-based reasoning, frames (concepts and their defining attributes), and semantic networks, as well as the capability to represent the data at multiple levels of granularity. Knowledge representation is the foundation for the standardized data utilized in clinical decision support systems and other digital sources of evidence.
Data Repositories and Clinical Event Monitors
A clinical data repository is a database that collects and stores patient care information from diverse data sources. It is typically optimized for storage and retrieval of information on individual patients and used to support health care delivery, surveillance, and clinical decision support (e.g., drug–drug interactions at the time of order entry, reminders about preventive care). Further, such broad repositories are essential to patient safety because the clinical context usually cuts across multiple data sources. For example, determining a drug–laboratory value interaction requires information from the pharmacy and the clinical laboratory. Currently, most inpatient and out-
patient specialty care organizations use “departmental” systems for limited functions to serve administrative, research, archiving, pharmacy, physiological function laboratory (e.g., electrocardiogram), clinical laboratory, radiology, and other purposes (van Bemmel and Musen, 1997). However, most such systems operate as silos as a result of the nature of older legacy systems and past nonuse of interoperability standards. Lack of information access and integration across the enterprise frequently results in issues of quality of care and safety.
Clinical event monitors work together with clinical data repositories, supporting real-time error prevention. They are usually triggered by clinical events (e.g., patient visit, medication order, new laboratory result), either when data representing the event enter a repository or when a provider uses a clinical information system. The event monitor uses clinical rules, the triggering event, and information present in the repository to generate alerts, reminders, and other messages of prime importance in preventing errors of both commission and omission. These messages are routed to the appropriate provider(s) using a variety of communication technologies and are also stored in the repository. A recent comprehensive review of studies in which information technology was used to detect adverse events documented the utility of clinical event monitors for preventing adverse drug events, nosocomial infections, and injurious falls (Bates et al., 2003). Another type of data repository that is useful for patient safety and quality management is a clinical data warehouse that contains information similar to that in the clinical data repository but optimized for long-term storage, retrieval, and analysis of records aggregated across patient populations. Consequently, the data warehouse is a core resource for data mining (discussed below), benchmarking, and other types of safety- and quality-related analysis. Data warehouses may be institution specific, regional, national, or even international. The systems implementation section of this chapter describes warehousing activities in greater detail and provides an example of a proprietary data warehouse to which more than 500 institutions subscribe.
Data Mining Techniques
Data mining is a method for obtaining useful information from large databases and includes data collection, extraction, manipulation, and summarization, as well as analysis (Berson, 1997; Fayyad et al., 1996; Mitchell, 1999). Data mining techniques have been used primarily with abstracted clinical data and less frequently with narrative clinical data. Uses of data mining relevant to learning and accountability systems for patient safety in-
clude surveillance (Brossette, 2000; Brossette et al., 1998), case-based reasoning (Aha et al., 2001), and rule induction for expert systems (Goodwin, 1997; Tsumoto and Tanaka, 1997).
Because health care data are often narrative, natural language processing (NLP) is another important technique for mining data for quality improvement and patient safety purposes (Bates et al., 2003). Sophisticated NLP techniques can extract information and structure from machine-readable narrative text. Consequently, the structured data are available for such purposes as triggering alerts and reminders (e.g., preventive care guidelines) and detecting potential adverse events. However, natural language extraction is currently a difficult and knowledge-intensive task.
To date, only a few NLP systems have been integrated with clinical information systems and used for improved quality of care and patient safety, including error detection and prevention, but the results of such efforts are encouraging (Fiszman and Haug, 2000; Friedman et al., 1995; Haug et al., 1990). One such system, MedLEE, a rule-based NLP system (Friedman et al., 1995), resulted in a significant decrease in respiratory isolation errors for patients with tuberculosis (Knirsch et al., 1998). In another study, NLP was performed on 889,921 radiological reports (Hripscak et al., 2002), and correlations between findings and changes over time were computed. Results showed that the NLP encoded output was more accurate than ICD-9 codes. A lexically based system for NLP has shown promise as a means of detecting adverse events in outpatient visit notes (Honigman et al., 2001).
Although the potential uses of NLP to promote quality and safety are broad, its wider implementation is hampered by a lack of standards. Of prime importance are standards related to the clinical document architecture (CDA), markup language, and a comprehensive standardized clinical vocabulary. A CDA is a critical step in the standardization of clinical reports and is essential to pave the way for widespread deployment of NLP systems. A standard CDA would make it possible to write simple NLP routines that could be based on regularities in the structure of the reports. For example, if all discharge summaries had a diagnosis section with the same tags and the same structure, it would be possible to write a relatively simple program to extract the diagnoses automatically from the reports. Increased functionality would be possible if the naming of clinical domains were standardized. Efforts are currently under way to establish a standardized ontology for documents through the Document Ontology Task Force at Health Level Seven. Subsequent efforts would be invaluable if the CDA were integrated with standardized clinical terminology and a standard way of expressing complex clinical conditions.
Data mining techniques, including NLP, are essential to both learning and accountability systems; however, many health care institutions lack the infrastructure, tools, and expertise to take advantage of those techniques. In addition, there is a need for studies that compare informatics approaches and develop methods for deploying such approaches more widely, particularly to rural and community hospitals (Bates et al., 2003).
Digital Sources of Evidence or Knowledge
Digital sources of evidence (i.e., health care knowledge) are another key component of a health information infrastructure and are essential for evidence-based practice. Sources of evidence, including bibliographic references, evidence-based clinical guidelines, and comparative databases, must be integrated with clinical expertise as practitioners make decisions (Bakken, 2001). Table 2-2 provides examples of digital sources of evidence. To sup-
TABLE 2-2 Digital Sources of Evidence or Knowledge: Examples
|
Type |
Sources |
|
Bibliographic |
|
|
Primary literature |
|
|
Traditional |
MEDLINE, Cumulative Index of Nursing and Allied Literature |
|
Full Text |
OVID database; individual journals (e.g., British Medical Journal) |
|
Structured reporting |
Trial Bank Project (clinical trials) |
|
Synthesized |
|
|
Electronic textbooks |
Harrison’s Principles of Medicine |
|
Systematic reviews |
Cochrane Collaboration |
|
Practice parameters |
|
|
Standards of care |
American Association of Critical Care Nurses |
|
Practice guidelines |
National Guideline Clearinghouse |
|
Disease management plans |
American Diabetes Association |
|
Comparative databases |
Health Plan Employer Data and Information Set (HEDIS) |
|
Knowledge bases |
|
|
Diagnostic decision support |
DXplain, Iliad |
|
Pharmacy |
National Drug File, Micromedex |
|
Genomic |
Genbank, Molecular Modeling database |
|
SOURCE: Bakken, 2001. |
|
port the redesign of care processes, the health information infrastructure must also facilitate the incorporation of new evidence derived from clinical practice (Bakken, 2001).
Many digital sources of evidence have applicability to management systems for patient safety and quality of care and play an important role in detection, analysis, recovery, and prevention for adverse events and near misses, as well as in quality management (Balas, 1998; Balas et al., 1998b). For example, within the context of a system to support patient safety and quality of care that is integrated with clinical care processes, digital sources of evidence would include, among others, guidelines related to the 20 priority health areas identified by the IOM, access to context-specific bibliographic retrieval, diagnostic decision support systems to assist with difficult diagnoses, and alerts and reminders of relevance to errors of omission and commission. Informatics techniques have the potential to decrease the amount of time from discovery to application of evidence in practice, as well as to deliver the evidence in a context-specific manner (Balas et al., 1998a).
A key challenge that is amenable to standards development is translating clinical practice guidelines into a format that can be shared across applications and organizations. This capability has significant potential to impact safety and quality care and will be a major contributor to the functionality of the EHR. Presenting guidelines on a computer monitor is the first stage in digitizing; the next level of automation occurs when the computer is able to make use of the patient’s clinical data, follow its own algorithm internally, and present only information relevant to the current state (Maviglia et al., 2003). Models and tools for extracting and organizing knowledge, representation models for publishing and sharing guidelines, and computational models for implementing guidelines are in various stages of development (Maviglia et al., 2003). The range of possible applications for computer-based guidelines is very broad and includes disease management, encounter workflow facilitation, reminders/alerts, design and conduct of clinical trials, care plan/critical path support, appropriateness determination, risk assessment, demand management, education and training, and reference (Greenes et al., 2001).
Comparative databases (e.g., health plan utilization, disease registries, quality indicator databases) and knowledge bases (e.g., for pharmacy) are useful for benchmarking. Such databases associated with the priority areas identified by the IOM have the potential to provide a valuable source of evidence to support the attainment of national goals for quality improvement and patient safety. Many health care–related comparative databases are associated with specific quality measures for regulatory purposes, such
as those of the Diabetes Quality Improvement Project (DQIP) developed through the Centers for Medicare and Medicaid Services. The addition of data related to errors of omission and commission associated with the DQIP measures would further facilitate redesign of care processes and improve safety and quality.
With the exception of guideline knowledge integrated into CDSSs, most digital sources of evidence operate as stand-alone systems, lacking true integration with clinical information systems. One approach to providing for retrieval of context-specific information during use of clinical information systems is the “infobutton” at New York Presbyterian Hospital. Infobuttons link digital sources of evidence to a particular section in the clinical information system. For example, a laboratory test for a drug level could be mapped to a National Drug Code or a drug trade name to search Micromedex for prescribing information (Cimino, 2000).
Leveraging the vast quantities of health care data and enterprise-wide knowledge requires the development of health information resource networks at the regional or national level. These networks must have the functionality and standards to acquire, share, and operationalize the various modalities of knowledge that exist in the health care domain (Abidi and Yu-N, 2000).
Communication Technologies
A number of authors have documented the importance of excellent communication in ensuring patient safety and providing quality care (Coiera, 2000; Covell et al., 1985; McKnight et al., 2001). More recently, investigators have turned their attention to the use of technologies that can enhance communication among members of multidisciplinary health care teams and between clinicians and patients (Coiera, 2000; McKnight et al., 2001; van Bemmel and Musen, 1997). Within the NHII, the primary mode of data exchange between organizations will be through the Internet and e-mail, while that within an organization will be through an Intranet or virtual private networks. Browser software developed for the Internet has made it easy to connect to, search, browse, and download information from anywhere on the network as if it were located on the user’s personal computer (Institute of Medicine, 1997). In addition, this software has graphical, intuitive, and common interfaces to functions that locate and interact with remote data on the broader Internet without requiring the user to have technical knowledge (Institute of Medicine, 1997). Such features and their ease of use should contribute significantly to the facility of information transfer envisioned with
the NHII. Whether the Internet will be adopted more widely for this purpose given the potential benefits will depend on the technical capabilities it can provide compared with other networking alternatives (National Research Council, 2000) and the development of clearly defined and enforced parameters for online health care communications.
Five technical factors have been identified that need to be considered when planning for the implementation of communication technologies: bandwidth needed and available, latency in transmission across the network, availability of the network on a continuous basis, confidentiality and security of data, and ubiquity of access to the network (National Research Council, 2000). From the communications perspective, it is necessary to resolve a number of parameters related to the primary types of health care–related data exchange:
-
physician–physician communications,
-
physician–patient communications,
-
patient–patient support communications,
-
interactive media and communication campaigns, and
-
public availability of medical literature.
The credibility of online health information must also be addressed (Rice and Katz, 2001). Now that organizations are in the process of implementing the security protocols mandated in the Final HIPAA Security Rule, it is expected that integration of clinical information systems and use of the Internet will gain momentum.
Despite the flurry of interest in utilizing the Internet among many in the health sector, the incorporation of potential applications has yet to be fully realized. Of those organizations that do utilize communications technologies, many continue to rely on private networks (National Research Council, 2000). Table 2-3 provides examples of network-based applications currently in use.
In addition to the Internet and private networks, mobile communication technologies, such as cellular telephones, digital pagers, and personal digital assistants, are increasingly being used to support safety and quality in point-of-care applications. These technologies have the potential for widespread adoption because of their greater flexibility, convenience, and mobility relative to wired network communication systems. Many physicians and other health care providers are already incorporating handheld devices into their day-to-day functioning to better manage the care of their patients while at the same time reduce medical errors, administrative burdens, and overall
TABLE 2-3 Representative Applications Conducted over the Internet and Private Networks
|
Functions Commonly Performed Today over the Internet |
Functions Performed Today over Private Networks |
Functions Not Commonly Performed Today over Either the Internet or Private Networks |
|
|
|
|
SOURCE: National Research Council, 2000. |
||
health care expenditures. Handheld devices have been developed to serve such purposes as physician documentation in an EHR, results review, alert notification, bedside registration, e-prescribing, case management, pharmacy, and materials management. For handheld applications, data are exchanged through “synching” directly with land-based or wireless networks. With increased security features and integration with land-based information technologies, wireless local area networks will further transform hospital and clinical communication networks, allowing for radio wave transmission of important data from handheld devices and portable personal computers. Along with handheld devices, paging and telecommunication systems are vital to patient safety. However, use of such technologies will depend on data standards and other components of the NHII so that compatibility and interoperability within the health information system can be established.
Clinical Information Systems
The components of the informatics infrastructure are linked through clinical information systems that provide the mechanism for sharing data collected from the various systems, reducing or eliminating redundancies in data collection/documentation and increasing the reliability and comprehensiveness of patient data available to the clinician. Within the context of a comprehensive integrated system, clinical information systems can support patient safety and quality management through the use of decision support tools for the prevention and detection of adverse events and near misses.
Ideally, the NHII will rely on the EHR as the central integrating component for data acquisition, analysis, and storage. Key capabilities of an EHR system include core health information, results management, order management, decision support, communication, patient support, and reporting (Institute of Medicine, 2003). Technical issues related to the EHR structure, function, and data standards are being resolved by NCVHS and by private-sector standards development organizations (e.g., Health Level Seven).
Decision support systems are the key tools enabling clinicians to access health care knowledge at the point of care as they progress through the care continuum. For example, encoded medical knowledge about the meaning and significance of changing laboratory test results would allow a system to provide alerts, an active function, in addition to the passive data retrieval function (Institute of Medicine, 1997). Methodologies for decision support can take many forms—reminders and alerts, embedded controls, decision assistance, and/or risk prediction (Institute of Medicine, 1992)—all of which have significant potential to improve patient safety. CDSSs can be only as effective as the strength of the underlying evidence base (Sim et al., 2001). Therefore, CDSSs must be designed to be evidence adaptive such that the clinical knowledge base is derived from and continually reflects the most up-to-date evidence from the research literature and practice-based resources (Sim et al., 2001).
IMPLEMENTING THE SYSTEMS
The IOM–Health Level Seven Demonstration Project: Where Are We Now?
The committee participated in the Health Level Seven (HL7) Interoperability Demonstration project at the Annual Conference of the Health Information Management Systems Society (HIMSS), held February 10–13,
2003, in San Diego, California, as an assessment of where the majority of the industry stands in relation to information systems and associated data standards for data interchange. The Interoperability Demonstration Project was a series of live technology demonstrations, conducted in partnership with the Centers for Disease Control and Prevention (CDC), the Food and Drug Administration (FDA), the Markle Foundation/Connecting for Health initiative, and 19 participating member organizations. The project presented real-world scenarios and clinical cases focused on the prevention and reporting of potential adverse drug events (ADEs), public health reporting of notifiable diseases, and continuity of care. It was intended to highlight several HL7 standards and show how they currently address these critical health care issues, as well as to explore the gaps between what is available today and what is needed to meet a set of increasingly complex demands on the health care system.
The basis for one of the interoperability demonstrations was a patient scenario describing an ADE as well as a near miss. The events in this scenario were characterized by the need to coordinate care across multiple providers in different settings; thus the demonstration was focused in particular on exploring the potential for using interoperability standards to improve care coordination. From the committee’s perspective, the goals of participating in this demonstration were to:
-
Highlight how interoperability standards can improve communication and coordination of clinical data among care settings (inpatient, outpatient, pharmacy).
-
Identify limitations of existing systems and emerging standards with regard to patient safety.
The participants in the demonstration worked together over several months to define what functions would be demonstrated and how they would be implemented. The scenario was modified to reflect the available vendor participants and corresponding system functionality (see Box 2-1).
Participants learned several lessons in preparing for the demonstration. They spent considerable time making decisions, compromising, and agreeing on how to capture and share data, in part because there were several potential approaches and no clearly established standard for implementing particular functions. In the scenario, for example, the clinicians treating the patient in the emergency department and in the hospital must discover what medications she is taking; as it turns out, medication recently prescribed for hypertension may have resulted in an ADE. This information may be re-
quested from and retrieved from the family physician’s electronic records in several ways: as an order or prescription, from a medication list, or from a progress note. In this case, the demonstration participants chose to use the HL7 CDA to retrieve the prescription information, which was then used to drive clinical decision support (drug–laboratory interaction) and to inform the clinicians of the potential ADE.
During the demonstration, it became clear that while an ADE can be recorded as an allergy or as a problem on a problem list, current data standards do not support the documentation of an ADE as such. An ADE is not an allergy, although some individuals do have allergies to certain medications. This observation prompted HL7 to define a specification for representing ADEs. Finally, patients should be able to authorize release/transmission of their data. The demonstration showed that this functionality is not possible with the current systems and standards. Box 2-2 presents the results of the demonstration in terms of a report card summarizing progress to date on interoperability standards in health information management systems.
From the data standards perspective, the assessment of systems functionality was based on whether a standard exists and was/was not used in the demonstration, whether there are nonstandard methods for executing the task, or whether no technology exists to solve this interoperability issue.
|
BOX 2-1
|
|
BOX 2-2 As part of the demonstration process, HL7 presented a report card delineating progress with regard to interoperability standards. Key findings in this assessment include the following:
More information about the Interoperability Demonstration project can be found at http://www.hl7.org/library/himss/2003SanDiego/HL72003DemoPressOverview.zip. |
Table 2-4 provides an outline of the functions executed for the scenario and the standards utilized. The scenario demonstrated that even with the current state of relatively disparate data standards and interaction of multiple vendors, use of available data standards1 allowed for a level of interoperability to support cross-organizational data flows and care coordination.
The committee found participation in the demonstration to be a useful experience. This project revealed some of the potential of interoperability standards. It also highlighted current gaps in standards supporting the communication of patient information between systems and revealed areas in which additional standards—e.g., for documenting ADEs—are needed. Based on these capabilities, and with effort in linking vendors through exist-
TABLE 2-4 IOM–HL7 Demonstration Project—Patient Scenario Data Standards
|
Scenario Event |
Location |
|
78-year-old woman sees her family doctor at a small office practice. She is diagnosed with hypertension. |
Doctor’s office |
|
A blood pressure medication is prescribed. |
Doctor’s office |
|
Prescription is sent to the pharmacy. |
Doctor’s office |
|
The patient calls her physician and is referred to a hospital emergency department. She is admitted with symptoms of diarrhea, disorientation, and rash. |
Emergency Department |
|
The patient is admitted. The admitting physician views the outpatient progress note. |
Hospital |
|
Physician orders complete blood count and blood chemistries. |
Hospital |
|
Lab results indicate low sodium (hyponatremia). The patient is treated. A review of the patient’s inpatient chart verifies an ADE due to a preadmission-prescribed diuretic. An ADE report is sent to the FDA. |
Hospital |
|
The physician prescribes a second drug to treat the patient’s hypertension. The EHR alerts the physician that this drug may also cause an adverse reaction. An alternative drug is recommended and prescribed. |
Hospital |
|
The patient is discharged back to the care of her family physician. The family physician wants the hospitalization records and discharge medications. |
Doctor’s office |
|
Practice manager can track the patient’s status, but confidentiality rules prevent access to the body of clinical documents. |
Doctor’s office |
|
The family physician and hospital submit claims but are asked for further information. |
Hospital and doctor’s office (billing) |
|
Standards-Based Actions |
Applications |
|
CDA progress note created. Findings are SNOMED encoded through a call to a remote vocabulary server. |
XML forms editor, terminology server |
|
Medication is documented in the “plan” portion of the CDA progress note. |
XML forms editor, terminology server |
|
RxNorm code is inserted by call to a remote server. An HL7 Version 3 prescription order is created automatically from the information in the CDA note and presented to the physician for review. |
|
|
HL7 Java parser. Java Refined Message Information Model graph. |
HL7 RIM application programming interface |
|
HL7 Version 2 ADE message created from CDA header. Admission diagnosis, signs/symptoms coded with SNOMED and ICD9CM and sent to hospital. |
Interface engine, EHR |
|
Outpatient CDA note is retrieved via Web services call to repository. |
Clinical document repository (CDR), EHR |
|
HL7 Version 3 lab order is sent and translated by router from Version 3 to Version 2. Version 2 order is received by lab. Results are translated back to Version 3. LOINC codes are used for orders and test results. |
HL7 toolkit and server |
|
Using CDA as the data source, a draft HL7 Version 3 ADE report message is created and sent to the FDA. Report uses RxNorm and FDA-specified codes and terms. |
EHR |
|
Drug–allergy interaction checking between the EHR and decision support system is performed via HL7 CCOW. Data are communicated via RxNORM. Alert is sent via HL7 Version 2 OBX. |
EHR, decision support |
|
Repository is queried. ADE message, medications, and CDA discharge summary are retrieved. Discharge summary is human readable but not coded for automated decision support (it does not encode symptom data or diagnosis). |
Portal, CDR |
|
CDA confidentiality codes (user-defined) indicate which portions should be accessible. |
Portal |
|
Information is supplied by hybrid X12/HL7 electronic claims attachment. ICD9CM and LOINC codes used for clinical data. |
Claim attachments, EHR, server |
ing data standards, health care organizations can immediately begin to aggregate information on patient safety events using several well-developed terminologies that are currently in use or are planned for implementation, including the HIPAA-mandated code sets and the NCVHS core terminology group.
PRACTICAL APPROACHES TO MOVING FORWARD WITH STANDARDS-BASED DATA SYSTEMS
The development of integrated, standards-based data systems to improve patient safety can be perceived as a daunting task. Fortunately, many paths can lead to the optimal automation environment encompassed by the conceptual model presented earlier. This section describes several different practical approaches and effective interim solutions for moving forward with the integration of standards-based systems.
The committee believes the optimum means of implementing the systems that make up the conceptual model is to pursue a progressive migration plan for the implementation of EHRs, with appropriate adaptation to the various health care settings. Specific patient safety systems and data requirements are part of the overall strategy for institution of the NHII. Several health care organizations in the public and private sectors have already started to integrate the informatics components discussed above and can serve as successful models for progressing toward the envisioned infrastructure. Despite the operating differences that exist among large institutions and small office practices, a well-designed organizational strategy that aligns business and information technology goals can ease the transition and overcome challenges to implementation of the many applications that make up the EHR.
The committee’s letter report on key capabilities of an EHR provides guidance on such a strategy, including considerations for inpatient care, ambulatory care, nursing homes, and personal health/self-care (Institute of Medicine, 2003) (see Appendix E). The committee’s recommendations in that report encompass those CDSSs of high value to patient safety, as well as reporting formats. Building comprehensive systems to support both EHRs and patient safety systems must begin with a solid infrastructure based on the essential informatics components discussed in this chapter. Early adopters of EHRs are already using many of the data standards recommended in this report. As standards continually evolve and the integration of clinical systems gains momentum, early adopters can be expected to be prepared for a full transition to the data standards identified by NCVHS. In addition, the
implementation guides to be developed and made publicly available will address issues associated with both transitioning from other local standards and adopting information technology for the first time.
To date, a number of organizations have successfully integrated the EHR employed by the Nicholas E. Davies award winners listed in Box 2-3. This award is given to those organizations that have demonstrated a favorable impact on health care quality, costs, and access to care through the use of computerized patient records (CPRs). Among the institutions that have received the award, some have implemented commercial software offerings, and some have developed their own systems. Some of these institutions have
|
BOX 2-3
SOURCE: Wise, 2003. |
been at the forefront of the clinical information systems movement, and some have quietly assembled highly effective infrastructure systems and services in relative obscurity. To illustrate the variety of effective systems possible, we provide below a review of some very different but successful approaches utilizing commercially available CPR software.
In the history of the Davies award, several institutions have been recognized for CPR achievements utilizing commercially available software. Such software is capable of supplying many of the basic components necessary to begin the process of building integrated data systems to support patient safety. We provide detailed overviews of two Davies Award winners—Kaiser Permanente of Ohio and North Mississippi Health Services. Both have employed relatively low-technology approaches that have yielded high value. The approach of Kaiser Permanente (see Figure 2-3 and Box 2-4) illustrates how a network of ambulatory care sites associated with a single provider organization can use computer systems to simplify office practice and support the physician with important clinical reminders. The Kaiser Permanente system allows clinicians to use both paper and technology without requiring a complete shift to a paperless system. North Mississippi Health Services exemplifies how providers that work in rural environments or areas of low resources can develop basic computer systems for administrative and clinical information (see Box 2-5). This achievement is particularly impressive given that the technology integration involved was accomplished during the early stages of implementation of information technology, before its incorporation into the mainstream of daily life.
In the public sector, the Veteran’s Health Administration (VHA) and the Military Health System (MHS) have developed and implemented models for quality improvement through the integration of comprehensive health information systems. In general terms, their information systems evolved from automated systems for administrative and financial transactions to gradually incorporate modified off-the-shelf technology and specially designed middleware for integrating disparate and legacy systems (Institute of Medicine, 2002b). As integration of clinical systems progressed, a foundation for the EHR was established that enables electronic documentation of health data, real-time access to important clinical information at the point of care (e.g., radiological images and laboratory test results), and linkages to facilitate administrative and financial processing. VHA and MHS also have implemented a consumer-oriented, Internet-based e-health model to support their patient population’s communication and information needs (Institute of Medicine, 2002b). Other applications, such as those for reporting adverse events, are spearheading the use of health information systems to
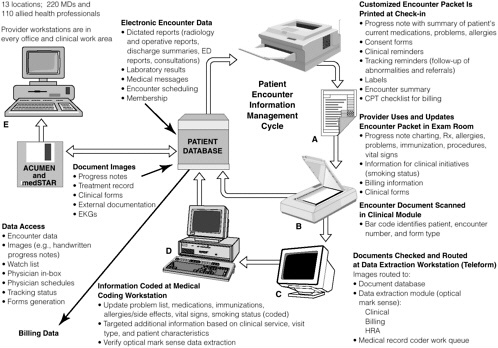
FIGURE 2-3 Information management in the medical automated record system of Kaiser Permanente of Ohio.
NOTE: ACUMEN = Ambulatory Clinical User Meaningful Enterprise Navigation; CPT = common procedure terminology; ED = emergency department; EKG = electrocardiogram; HRA = health risk assessment; Rx = prescription.
SOURCE: Khoury, 1998.
|
BOX 2-4 Kaiser Permanente of Ohio has developed a Medical Automated Record System that has been fully implemented in 13 ambulatory care locations in Cleveland and surrounding communities, linking 220 physicians and 110 allied health professionals. The system was designed to meet the needs of providers—require minimal training and minor changes to physician documentation (e.g., physician’s record of diagnoses, medications, allergies, and immunizations), capture information from external sources (e.g., reports, consults), and be implementable without affecting physician productivity. The approach selected utilizes personal computers that provide access to patient information in all physician offices and work areas, a paper intermediary to provide patient information and document clinical encounter information, and document imaging to capture nonelectronic information. Figure 2-3 provides a conceptual view of the system. In the ambulatory setting, when a patient checks in, a customized information packet is printed that includes a summary of current problems, medications, allergies, etc.; a summary of diagnoses and vital signs from past visits; laboratory test results from the previous month; patient-specific clinical reminders generated from the organization’s quality initiatives; standardized forms to collect coded information (e.g., billing, clinical interventions); and other forms (e.g., consent). The packet also serves as a charting document for physician notes. The use of clinical reminders in targeted clinical areas has led to increased physician compliance with guidelines and substantial improvement of the health care provided to Kaiser patients in the treatment of coronary artery disease, congestive heart failure, asthma, diabetes, and hypertension. Reminders also address other clinical areas, such as mammography and smoking cessation. The reminders have made a significant contribution to Kaiser’s quality improvement initiatives and could do the same for patient safety. Patient safety reminders, such as those related to drug–drug interactions and comormidities, could easily be added to Kaiser’s information system and generated for the patient’s clinical information packet. Further, several spaces on the clinician documentation sheet could be added for documentation of any adverse events or near misses that might occur during the treatment process. More comprehensive documentation of the event could follow, whereby the clinician could provide details either through direct use of the electronic system or on paper for input by a designated patient safety officer. SOURCE: Khoury, 1998. |
improve patient safety. The success of the VHA and MHS systems is rooted in a commitment to standardizing the information processing of the organization’s architecture with off-the-shelf technology, specially developed applications, and medical vocabulary for describing clinical phenomena. In addition, the success of these systems is rooted in a philosophy that is patient centered and strives to achieve ongoing quality improvement. The system architectures are built around these important concepts.
Those organizations not planning to build large data repositories in the short term but seeking to improve patient safety reporting capabilities can participate in external comparative clinical performance offerings. One such offering, which is also an example of a public–private partnership to test the
|
BOX 2-5 North Mississippi Health Services (NMHS) is a rural-based health system that serves patients in a 22-county region with five acute care hospitals, six dialysis centers, three nursing homes, and 12 offices for home care services. Over a 20-year period, NMHS progressively developed its integrated information systems, producing an EHR with automated input of the following information: medications, intake/output, vital signs, nurses’ notes, histories and physicals, operative reports, consult reports, and cardiology results. The online records can be accessed on an as-needed basis in 100 different buildings in a two-state area. NMHS understood that building an EHR was a complex process requiring a significant level of planning. In assessing the technology capabilities of all the sites to be linked in the network, NMHS found that many of the ancillary systems were at different stages of automation, requiring equipment upgrades and investment to establish the backbone of the network for interconnectivity. NMHS first initiated its system integration by automating and integrating the financial systems as the foundation for expansion to clinical and operational areas. Services and capabilities were added to the information systems on an annual basis as the need arose or as the technology became available. Given technology innovations and advances over the past decades, a migration strategy to an integrated health network is attainable in an accelerated time frame by 2010. Of particular note, the NMHS EHR incorporates real-time decision support tools to screen patients at risk for adverse events and provide caregivers with individualized clinical information. NMHS’s long-standing programs encompass drug–drug and drug–food interactions and drug allergy checks. If a problem exists, a notice is printed at the nursing unit and pharmacy. In addition, the adverse drug reaction monitoring program is designed so that each day the computer searches for the use of certain drugs. The pharmacist reviews this information daily and, based on guidelines for determining whether an ADE has occurred, contacts the physician. Another highly effective patient safety program is the dosage screening program for two sets of high-risk patients—pediatric and chemotherapy patients. A pediatric dosage screen was established that checks a patient’s dose against preestablished dosing guidelines for milligrams per kilograms. Regardless of the way the patient’s weight is entered, the computer converts it to kilograms and then performs a calculation to determine the dosage range for that patient. The same method of dosage calculation is applied to those receiving chemotherapy. As with the Kaiser Permanente of Ohio system (see Box 2-4), an application for generating patient safety reports could simply be added to the existing clinical information systems. SOURCE: Bozeman et al., 1997. |
effectiveness of a Medicare reimbursement premium for quality, is Perspective Online from Premier, Inc., a clinical data program used to illustrate the functions and processes associated with such systems. Premier collects and aggregates the data elements, subjecting each facility’s raw data to various data management procedures that result in reliable comparative information. Premier currently manages clinical and administrative data for more than 500 facilities using several data management methodologies: data cleaning and editing procedures, patient de-identification methods, and procedures for capturing missing or incomplete data. Specific procedures are summarized in Box 2-6. Premier uses software controls to block access to specific data elements. For its hospital customers, the tool is used to limit access to patient-identifiable information from facilities other than their own, thus preventing unauthorized access to the data and downloading of any data elements. The extensive data management procedures employed suggest not only that there is ample opportunity to simplify data collection and submission for hospitals but also that there is considerable room for streamlining data management if data standards are adopted.
Other approaches taken by some organizations employ systems that directly target patient safety. Two systems in particular are gaining popularity in efforts to minimize medication events—computerized physician order entry systems and barcode medication administration systems. The order entry systems utilize data from pharmacy, laboratory, radiology, and patient monitoring systems to relay the physician’s or nurse practitioner’s diagnostic and therapeutic plans and alert the provider to any allergy or contraindication the patient may have so that the order can be revised immediately at the point of entry (Metzger et al., 2003) before being forwarded electronically for the targeted medical action. This is a critical step in the care process, a point at which intervention through the use of clinical information systems can have a high impact on preventing adverse events and improving adherence to care guidelines (Metzger et al., 2003). In fact, one study found that 50 percent of all ADEs originate with errors during medication ordering (Bates et al., 1995). Because the essence of computerized provider order entry is managing orders, these systems impact not only the physician or nurse practitioner but also their decision making and care planning, the pharmacist’s decision making and work flow, the nurse’s work flow and documentation, and communication with ancillary services (e.g., laboratory, radiology) (Metzger et al., 2003). While such a system requires computer workstations and/or wireless devices to function, a fast, highly responsive ordering interface is necessary to win the clinician’s acceptance (Metzger et al., 2003). Also, because computerized provider order entry systems are de-
|
BOX 2-6
|
pendent on data from departmental systems, they necessitate upgrading of legacy systems for interoperability and the use of common data standards to allow sharing of data.
VHA has implemented a bar-code medication administration system for inpatient care, in which all products in the pharmacy are bar coded in single dosage units. The patient also is provided with a bar-coded wristband upon admission to the hospital. The VHA system links such data as demographic data, medical history, medication history, drug terminology, drug reference
data, drug interaction data, and drug–laboratory correlations. The system is used at the point of care to validate that the medication ordered, timing of administration, and dosage are correct and to maintain a medication administration history (Department of Veterans Affairs, 2001). For patient safety and quality research, reports can be generated for medication log, missed medications by patient or ward, missing dose request, follow-up and report, medication due list, medication administration history, drug inquiry, and other information (Department of Veterans Affairs, 2001).
OVERCOMING CHALLENGES TO IMPLEMENTATION OF INFORMATION TECHNOLOGY FOR THE NATIONAL HEALTH INFORMATION INFRASTRUCTURE
Organizational Leadership
Traditionally, a lack of organizational commitment to information technology and organizational culture have been significant barriers to the development of an informatics infrastructure within health care organizations. Leaders of health care organizations struggle with their organizations’ use of and commitment to information technology (Glaser, 2002), and the health sector as a whole continues to lag significantly behind other industries in this regard. Achieving the vision described in this chapter requires commitment, leadership, and strategy.
Aligning information technology strategy with business strategy requires adjustment of the organizational structure to provide strong leadership and strategic support at the highest levels of management, adequate resources and incentives to support the required cultural change, and front-line decision making and feedback regarding the development and maintenance of patient safety and quality improvement systems. While structures, strategies, and approaches vary among organizations, certain fundamental principles correlate directly with successful integration of information technology and business strategies:
-
A high-level, long-term commitment to information technology that starts at the level of the board of directors and senior management
-
An integrated vision for the building of an information technology infrastructure
-
Direct linkage between the information technology division and users, creating a feedback loop that provides for input and adjustment of the system to ensure that its functionality meets user needs
-
Implementation of an adoption strategy for information technology systems and active support by senior medical staff for the cultural change necessary for effective adoption
-
Systematic implementation (within an integrated vision) to build experience and confidence, to uncover unexpected problems, and to spread the cost out over time
-
Continual adaptation and modification of systems and processes to reflect current medical science and technological advancements
The cultural change that is inherent in the deployment of information technology is dependent on organizational drivers from both the top down and the bottom up. An example to illustrate this point is offered by the success of the Latter Day Saints (LDS) Hospital in Salt Lake City, Utah, in creating a culture for both innovative clinical systems automation and quality improvement. Top management made its support known through planning, providing the necessary resources, and encouraging an attitude of willingness to change and experiment. Simultaneously, clinical department leadership undertook with zeal the effort to achieve continuous improvement. When the clinical information system and clinical improvement processes were transferred from LDS Hospital to other institutions, one of the greatest challenges was to transfer the continuous improvement mind-set (e.g., emerging deficiencies in information technology systems were often viewed as “works in progress” rather than failures). Careful attention to both the product being developed, whether information technology systems or patient safety reporting, and the culture in which they reside is essential to success.
Comprehensive systems such as the NHII develop over time. Because of the dynamic nature of medical practice and information systems, one of the most important principles for organizational leadership to embrace is the need for constant adaptation and modifications to reflect science and technological innovation and advancement. Strategic planning with fore-thought to incorporate this need for continued evolution can assist organizations in achieving greater business value for their information technology investments as the horizon continually shifts (Glaser, 2002). The organization’s culture and leadership should also encourage innovation through creativity and experimentation in addressing business problems, crises, and opportunities for better meeting the needs of those interacting with the systems (Glaser, 2002). For example, an area of expected high-growth opportunity that would require system expansion could include personal health records for consumers. The personal health record includes a subset of the
data in the individual’s EHR and information recorded by them to support their care, disease management, and clinical communication (Markle Foundation, 2003). The personal health record could be made available to consumers through a link to a health care organization’s secure Web-based portal that utilized HIPAA standards for authentication.
Another area for organizational expansion is the establishment of global health networks of population-based information. Organizational leadership should also pursue a more global perspective in aligning the organization’s evolutionary adaptations and modifications with accepted international standards and technologies that can support the development of and linkage to a global health network. Global health networks based on common data standards can facilitate information access for health care providers on important concerns related to public health, such as infectious disease surveillance and the effects of bioterrorism that may directly affect their patients. The recent emergence of severe acute respiratory syndrome–SARS–is a prime example of this critical need. At the time the disease presented, each country was utilizing different standards to define and store salient information. It was not possible to share electronically vital information that could have eased the burden of tracking and monitoring the spread of the disease or facilitated a global research database.
Financial Incentives
The committee recognizes that building the NHII is an enormous undertaking with sizable costs in terms of human, organizational, financial, and governmental resources. The committee also understands that the majority of the effort for developing and implementing the information systems and data standards of the NHII will fall to the private sector. Estimating the costs to build the NHII is a major endeavor and one that was outside the scope of this study. However, the primary areas where costs are expected to arise include those related to the NHII (e.g., architecture, consumer health, homeland security, research, and population health), data standards (e.g., data interchange, terminologies, and knowledge representation), and patient safety reporting systems (e.g., organizational, state, national). These areas of significant cost can best be evaluated by those organizations that are directly involved in their respective areas. Those organizations should initiate large-scale studies of the costs and resources required to fulfill the goal of building the NHII.
To date, there has also been some broader-scale progress in addressing the challenges to the development of the NHII. In particular, the NHII
Conference, convened in July 2003 by the Department of Health and Human Services, brought stakeholders together to develop a consensus on a national action agenda to guide the development of the NHII (Department of Health and Human Services, 2003). The conference focused on several key topics, including data standards and vocabulary, as well as financial incentives.
Private-sector investments, such as those discussed earlier, will likely account for a good deal of the capital required to build the NHII, but the federal government also has an important role in providing financial support. To achieve the greatest gains, federal financial support should be targeted toward three areas. First, federal resources should support the development of critical building blocks of the NHII that are unlikely to receive adequate support through the combined investments of individual private-sector stakeholders. On the regional level, this support should come in the form of start-up funds to public–private partnership organizations to develop secure platforms for exchanging patient and other data and for carrying out transactions, along with the necessary technical assistance to enable providers to begin using the platform. Nationally, the federal government should provide the financial support and leadership needed for the establishment and ongoing maintenance of national data standards.
Second, the federal government should provide financial incentives to stimulate private-sector investments in the necessary information technology. Multiple approaches should be taken to this end and then evaluated to identify those that are most effective. These approaches might include revolving loans, differential payments to providers with certain information technology capabilities (e.g., inclusion of fees in the Medicare fee schedule for the provision of information technology services to patients, such as e-mail communications), or one-time-only payments to small physician offices to offset the costs and loss of productivity associated with the transition from paper to computer-based records.
Third, in the absence of considerable help from the federal government, safety net providers will likely fall behind in the transition to a safer health care delivery system. The federal government should provide grants, in-kind contributions, and technical assistance to such providers. This support might include offering one-time-only grants through the Health Resources and Services Administration to federally qualified health centers or VHA making its Veterans Health Integration System and Technology Architecture—VISTA available in the public domain and facilitating its use by safety net providers.
The federal government should thoroughly evaluate the various possibilities for providing incentives and investment support to facilitate the
adoption of information technology in health care, with a special focus on office practice providers. Specific criteria for participation in each incentive and/or investment support program should be determined, along with parameters for analysis of program effectiveness.
Technical Assistance
A significant amount of technical assistance will be needed to support those implementing the clinical systems and EHRs associated with the NHII. Because the United States has a private-sector–based health care delivery system, many companies have already been established specifically for the purpose of providing technical assistance and support for the implementation of information technology systems in health care organizations. These companies will play an important role in bringing the conceptual model presented earlier to fruition. Likewise, there is an important role for the Agency for Healthcare Research and Quality (AHRQ) in providing assistance to safety net providers and in leveraging and/or expanding existing educational programs for small group providers in office practice settings.
Enforcement of Privacy and Security
Enforcement of privacy provisions and security protocols will be essential to build the confidence of providers and patients utilizing the networks and information systems of the NHII. Consequently, the federal government will play a particularly important leadership role in the enforcement of HIPAA standards for privacy and security. Penalties for violations must be strongly enforced. Likewise, from a technology perspective, the federal government, through AHRQ, should develop strong application certification requirements for health-sector technologies to minimize potential threats to information systems that compose the NHII. For example, a requirement could be established that all application programs used in health care be certified as defect-free such that all known “holes” in software programming that could be exploited have been appropriately corrected. Given recent events and the current climate, moreover, the federal government may have an interest in extending its scope as a resource in handling sensitive situations and Internet-related problems that may affect health information systems.
CONCLUSIONS
Because patient safety is an integral part of delivering quality health care, the committee believes that ensuring patient safety requires multiple measures throughout the continuum of care that can be accomplished through the establishment of an NHII. The key to the development of the NHII is threefold: (1) the implementation of a strategic plan for progressive migration from the current state to the comprehensive integrated network incorporating the principal informatics components outlined earlier in this chapter, (2) the provision of financial incentives by the federal government to support investments in information technologies, and (3) the implementation of common data standards for interoperability and comparability of health information.
Although a health information infrastructure that supports learning and accountability systems for patient safety has not been implemented in most organizations to date, the barriers involved are not primarily technological. The technologies needed for building the integrated systems described in this chapter exist today. Rather, the lack of technology implementation and the failure to use common data standards have been the principal barriers. This chapter has explicitly highlighted the need for standards for (1) a concept-oriented terminology that supports nonambiguous definitions of concepts and data reuse for safety and quality purposes; (2) a CDA that will improve the utility of using NLP techniques for extracting the data required for learning and accountability systems from textual documents, such as clinical notes and voluntary reporting systems; (3) messaging standards that enable data integration across disparate computer-based systems, including those that cut cross organizations; and (4) knowledge representation standards that support the development of computable guidelines for evidence-based practice and decision support rules that are shared among organizations. The accelerated adoption of such standards in turn requires public–private partnerships and sufficient incentives, rather than technical innovation, as discussed in the next chapter.
REFERENCES
Abidi, S. S. R., and C. Yu-N. 2000. A Convergence of Knowledge Management and Data Mining: Towards ‘Knowledge-Driven’ Strategic Services. Proceedings from the Third International Conference on the Practical Applications of Knowledge Management, Manchester.
Aha, D. W., L. A. Breslow, and H. Munoz-Avila. 2001. Conversational case-based reasoning. Appl Intel 14 (1):9–32.
Bakken, S. 2001. An informatics infrastructure is essential for evidence-based practice. J Am Med Inform Assoc 8 (3):199–201.
Balas, E. A. 1998. From appropriate care to evidence-based medicine. Pediatr Ann 27 (9):581–584.
Balas, E. A., and S. A. Boren. 2000. Managing clinical knowledge for health care improvement. Yearbook of Medical Informatics 65–70.
Balas, E. A., S. A. Boren, L. L. Hicks, A. M. Chonko, and K. Stephenson. 1998a. Effect of linking practice data to published evidence: A randomized controlled trial of clinical direct reports. Med Care 36 (1):79–87.
Balas, E. A., K. C. Solem, J. F. Su, Z. R. Li, and G. Brown. 1998b. Upgrading clinical decision support with published evidence: What can make the biggest difference? Medinfo 9 (2):845–848.
Balas, E. A., S. Weingarten, C. T. Garb, D. Blumenthal, S. A. Boren, and G. D. Brown. 2000. Improving preventive care by prompting physicians. Arch Intern Med 160 (3):301–308.
Bates, D. W., D. J. Cullen, N. Laird, L. A. Petersen, S. D. Small, D. Servi, G. Laffel, B. J. Sweitzer, B. F. Shea, R. Hallisey, M. Vander Vliet, R. Nemeskal, and L. L. Leape. 1995. Incidence of adverse drug events and potential adverse drug events: Implications for prevention. JAMA 274 (1):29–34.
Bates, D. W., N. Spell, D. J. Cullen, E. Burdick, N. Laird, L. A. Petersen, S. D. Small, B. J. Sweitzer, and L. L. Leape. 1997. The costs of adverse drug events in hospitalized patients: Adverse drug events prevention study group. JAMA 277 (4):307–311.
Bates, D. W., L. L. Leape, D. J. Cullen, N. Laird, L. A. Petersen, J. M. Teich, E. Burdick, M. Hickey, S. Kleefield, B. Shea, M. Vander Vliet, and D. L. Seger. 1998. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA 280 (15):1311–1316.
Bates, D. W., J. M. Teich, J. Lee, D. Seger, G. J. Kuperman, N. Ma’Luf, D. Boyle, and L. Leape. 1999. The impact of computerized physician order entry on medication error prevention. J Am Med Inform Assoc 6 (4):313–321.
Bates, D. W., S. Murff, H. Evans, P. D. Stetson, L. Pizziferri, and G. Hripcsak. 2003. Policy and the future of adverse event detection using information technology. J Am Med Inform Assoc 10 (2):226–228.
Berson, A. 1997. Data Warehousing, Data Mining, and OLAP. New York, NY: McGraw-Hill.
Bodenheimer, T., and K. Grumbach. 2003. Electronic technology: A spark to revitalize primary care. JAMA 290 (2):259–264.
Bozeman, T. E., K. Harvey, I. Jarrell, W. Jones, K. Kock, L. Morgan, G. Parada, F. Perry, K. Robinson, and G. Wages. 1997. The Development and Implementation of a Computer-Based Patient Record in a Rural Integrated Health System. Proceedings from the Third Annual Nicholas E. Davies CPR Recognition Symposium. Chicago, IL: Health Information Management Systems Society.
Brossette, S. E. 2000. A data mining system for infection control surveillance. Meth Inform Med 39 (4):303–310.
Brossette, S. E., A. P. Sprague, J. M. Hardin, K. B. Waites, W. T. Jones, and S. A. Moser. 1998. Association rules and data mining in hospital infection control and public health surveillance. J Am Med Inform Assoc 5 (4):373–381.
Cain, M. M., R. Mittman, J. Sarasohn-Kahn, and J. C. Wayne. 2000. Health E-People: The Online Consumer Experience. Oakland, CA: Institute for the Future, California Health Care Foundation.
Campbell, K. E., D. E. Oliver, K. A. Spackman, and E. H. Shortliffe. 1998. Representing thoughts, words, and things in the UMLS. J Am Med Inform Assoc 5 (5):421–431.
CareScience. 2003. Santa Barbara County Care Data Exchange. Online. Available: http://www.carescience.com/healthcare_providers/cde/care_data_exchange_santabarbara_cde.shtml [accessed May 30, 2003].
Chute, C. G., S. P. Cohn, and J. R. Campbell. 1998. A framework for comprehensive terminology systems in the United States: Developmental guidelines, criteria for selection, and public policy. J Am Med Inform Assoc 5 (6):503–510.
Cimino, J. J. 1998. Desiderata for controlled medical vocabularies in the twenty-first century. Methods Inf Med 37 (4–5):394–403.
———. 2000. From data to knowledge through concept-oriented terminologies. J Am Med Inform Assoc 7 (3):288–297.
Cimino, J. J., V. L. Patel, and A. W. Kushniruk. 2001. Studying the human-computer-terminology interface. J Am Med Inform Assoc 8 (2):163–173.
Coiera, E. 2000. When conversation is better than computation. J Am Med Inform Assoc 7 (3):277–286.
Covell, D., G. Uman, and P. Manning. 1985. Information needs in office practice: Are they being met? Ann Intern Med 103:596–599.
Department of Health and Human Services. 2003. Federal Government Announces First Federal EGov Health Information Exchange Standards. Online. Available: http://www.dhhs.gov/news/press/2003pres/20030321a.html [accessed May 29, 2003].
Department of Veterans Affairs. 2001. VISTA Monograph. Washington, DC: Department of Veterans Affairs.
Durieux, P., R. Nizard, P. Ravaud, N. Mounier, and E. Lepage. 2000. A clinical decision support system for prevention of venous thromboembolism: Effect on physician behavior. JAMA 283 (21):2816–2821.
Evans, R. S., S. L. Pestotnik, D. C. Classen, T. P. Clemmer, L. K. Weaver, J. F. Orme Jr., J. F. Lloyd, and J. P. Burke. 1998. A computer-assisted management program for antibiotics and other anti-infective agents. N Engl J Med 338 (4):232–238.
Fayyad, U. M., G. Piatetsky-Shapiro, P. Smyth, and R. Uthurusamy. 1996. Advances in Knowledge Discovery and Data Mining. Menlo Park, CA: AAAI Press.
Fiszman, M., and P. J. Haug. 2000. Using Medical Language Processing to Support Real-Time Evaluation of Pneumonia Guidelines. Proceedings of the Annual American Medical Informatics Association Symposium. Philadelphia, PA: Hanley and Belfus, 235–239.
Friedman, C., G. Hripcsak, W. DuMouchel, S. B. Johnson, and P. D. Clayton. 1995. Natural language processing in an operational clinical information system. Natural Language Engineering 1:83–108.
Glaser, J. P. 2002. The Strategic Application of Information Technology in Health Care Organizations (Second Edition). San Francisco, CA: Jossey-Bass.
Goldsmith, J. 2002. The Internet and managed care: A new wave of innovation. Health Aff (Millwood) 19 (6):42–56.
Goodwin, L. 1997. Data mining issues for improved birth outcomes. Biomed Sci Instrumentation 34:291–296.
Greenes, R. A., M. Peleg, A. T. S. Boxwala, V. Patel, and E. H. Shortliffe. 2001. Sharable computer-based clinical practice guidelines: Rationale, obstacles, approaches, and prospects. Medinfo 10 (Pt 1):201–205.
Haug, P., D. Ranum, and P. Frederick. 1990. Computerized extraction of coded findings from free-text radiologic reports (work in progress). Radiology 174 (2):543–548.
Honigman, B., P. Light, R. M. Pulling, and D. W. Bates. 2001. A computerized method for identifying incidents associated with adverse drug events in outpatients. Int J Med Inf 61 (1):21–32.
Hripscak, G., J. H. Austin, P. O. Alderson, and C. Friedman. 2002. Use of natural language processing to translate clinical information from a database of 889,921 chest radiographic reports. Radiology 224:157–163.
Institute of Medicine. 1992. Guidelines for Clinical Practice: From Development to Use. Washington, DC: National Academy Press.
———. 1997. The Computer-Based Patient Record: An Essential Technology for Health Care. Washington, DC: National Academy Press.
———. 2002a. Fostering Rapid Advances in Health Care: Learning from System Demonstrations. Washington, DC.: The National Academies Press.
———. 2002b. Leadership by Example: Coordinating Government Roles in Improving Health Care Quality. Washington, DC: The National Academies Press.
———. 2003. Key Capabilities of an Electronic Health Record System: Letter Report. Washington, DC: The National Academies Press.
Khoury, A. T. 1998. Support of quality and business goals by an ambulatory automated medical record system in Kaiser Permanente of Ohio. American College of Physicians: Effective Clinical Practice 1:73–82.
Kinzie, M. B., W. F. Cohn, M. F. Julian, and W. A. Knaus. 2002. A user-centered model for Web site design. J Am Med Inform Assoc 9 (4):320–330.
Knirsch, C. A., N. Jain, A. Pablos-Mendez, C. Friedman, and G. Hripcsak. 1998. Respiratory isolation of tuberculosis patients using clinical guidelines and an automated clinical decision support system. Infect Cont Hosp Epi 19 (2):94–100.
Markle Foundation. 2003. Connecting for Health—A Public–Private Collaborative: Key Themes and Guiding Principles. New York, NY: Markle Foundation.
Maviglia, S. M., R. D. Zielstorff, M. Paterno, J. M. Teich, D. W. Bates, and G. J. Kuperman. 2003. Automating complex guidelines for chronic disease: Lessons learned. J Am Med Inform Assoc 10:154–165.
McKnight, L. K., P. D. Stetson, S. Bakken, C. Curran, and J. J. Cimino. 2001. Perceived Information Needs and Communication Difficulties of Inpatient Physicians and Nurses. S. Bakken, ed. Proceedings of the Annual American Medical Informatics Association. Philadelphia, PA: Hanley and Belfus, 596–599.
Metzger, J., J. Fortin, and California Health Care Foundation. 2003. Computerized Physician Order Entry in Community Hospitals. Online. Available at http://www.fcg.com/healthcare/ps-cpoe-research-and-publications.asp [accessed Apr., 2003].
Mitchell, T. 1999. Machine learning and data mining. Com ACM 42 (11):31–36.
National Committee on Vital and Health Statistics. 2001. Information for Health: A Strategy for Building the National Health Information Infrastructure. Online. Available: http://ncvhs.hhs.gov/nhiilayo.pdf [accessed April 18, 2002].
National Research Council. 2000. Networking Health: Prescriptions for the Internet. Washington, DC: National Academy Press.
New England Healthcare EDI Network. 2002. NEHEN About Us. Online. Available: http://www.nehen.net/ [accessed May 30, 2003].
Overhage, M. June 7, 2003. Enhancing Public Health, Healthcare System, and Clinician Preparedness: Strategies to Promote Coordination and Communication. The Indiana Network for Patient Care. PowerPoint presentation to the Institute of Medicine Committee on Data Standards for Patient Safety.
Rice, R. E., and J. E. Katz. 2001. The Internet and Health Communication: Experiences and Expectations. Thousand Oaks, CA: Sage Publications, Inc.
Shortliffe, E. H., L. E. Perreault, G. Wiederhold, and L. M. Fagan. 2001. Medical Informatics: Computer Applications in Healthcare and Biomedicine. New York, NY: Springer-Verlag.
Sim, I., P. Gorman, R. A. Greenes, R. B. Haynes, B. Kaplan, H. Lehmann, and P. C. Tang. 2001. Clinical decision support systems for the practice of evidence-based medicine. J Am Med Inform Assoc 8 (6):527–534.
Tsumoto, S., and H. Tanaka. 1997. Incremental Learning of Probabilistic Rules from Clinical Databases Based on Rough Set Theory. Proceedings of the Annual American Medical Informatics Association 198–202. Bethesda, MD: American Medical Informatics Association.
van Bemmel, J. H., and M. A. Musen. 1997. Handbook of Medical Informatics. Heidelberg, Germany: Springer-Verlag.
Waegemann, C. P., C. Tessier, A. Barbash, B. H. Blumenfeld, J. Borden, R. M. Brinson, T. Cooper, E. L. Elkin, J. M. Fitzmaurice, S. Helbig, K. M. Hunter, B. Hurley, B. Jackson, J. M. Maisel, D. Mohr, K. Rockel, J. H. Schenieder, T. Sullivan, and J. Weber. 2002. Healthcare Documentation: A Report on Information Capture and Report Generation. Newton, MA: Medical Records Institute.
Wise, P. July 2, 2003. Healthcare Information and Management Systems Society List of Davies Winners. Personal communication to Institute of Medicine’s Committee on Data Standards for Patient Safety.