D
Adoption of Drug Abuse Treatment Technology in Specialty and Primary Care Settings
Cindy Parks Thomas
Brandeis University
Dennis McCarty
Oregon Health and Science University
OVERVIEW
Investments in neuroscience and the development of pharmacotherapies for drug abuse treatment seem to be near fruition. Changes in federal legislation coupled with the approval of Subutex (buprenorphine hydrochloride) and Suboxone (buprenorphine hydrochloride in combination with naltrexone) for the treatment of opioid dependence offer an opportunity to engage primary care physicians directly in the treatment of dependence on heroin and other drugs. Advances in immunotherapy and depot medications are also promising. New pharmacotherapies, however, will only be effective to the extent they are accepted by clinicians and their use is facilitated through adequate financing and organizational and community support.
Newly approved pharmacotherapies are usually rapidly and widely adopted in general medicine. For substance abuse treatment, however, diffusion of medications has been a slower and less predictable process. Naltrexone for alcoholism treatment, for example, reached only a fraction of its expected market. Differences in the structure of the substance abuse treatment environment (less often built around a physician delivery model and commonly in specialty treatment settings) and differences in financing of substance abuse treatment have contributed to slower adoption of naltrexone and other such therapies. With the development of additional new pharmacological-based treatments for addictions, more individuals may be drawn to receive treatment in primary care settings. These patients often have different needs than most patients typically found in primary
care and family medicine settings. Difficulties in developing linkages between primary care and the ancillary services used in addiction treatments may pose barriers to the adoption of new treatment technologies. Specialty treatment settings may also be limited in their ability or interest in adopting new pharmacotherapies due to philosophical resistance and lack of training and/or resources.
This appendix applies a framework from health services research on technology diffusion to identify elements that may be important in understanding the adoption of treatment technologies in the substance abuse field. Literature on the adoption of substance abuse treatment technologies is reviewed, and particular challenges and opportunities are outlined—including the organization, financing, and delivery of specialty addiction treatments that may inhibit rapid adoption. Implications for primary care and other treatment settings are discussed relative to the availability of new pharmacology-based interventions. Finally, strategies for making these medications available and encouraging their appropriate use are examined.
ADOPTION OF INNOVATIONS IN MEDICAL CARE
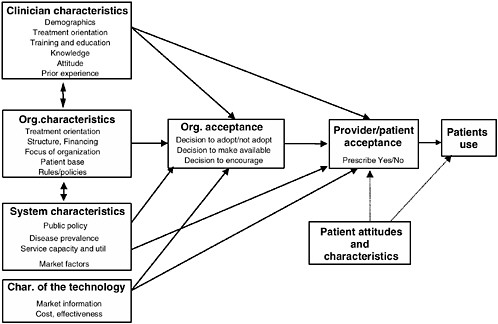
Classical diffusion theory suggests the nature of the technology, the organizational structures and associated financial influences in which the technology is disseminated, characteristics of the providers and patients, and the communication methods (by whom and through what channels) affect the rate and direction of the adoption pattern (Banta and Luce, 1993; Office of Technology Assessment, 1994; Rogers, 1995). Figure D-1 shows a conceptual model of the factors contributing to technology adoption, described below.
Technology Attributes
Adoption depends in part on the attributes of the innovation and how practitioners perceive them (Meyer and Goes, 1988; Rogers, 1995). Characteristics affecting an innovation’s adoption include the relative advantages over existing technologies, whether in economic, clinical, or social terms; compatibility with values, experiences, and needs of potential users; complexity or simplicity of use; “trialability,” or the potential to try on a limited basis without significant risk; and the extent to which results are observable (Rogers, 1995). After a new technology is introduced, uncertainty often remains regarding its use. Emerging technologies are commonly used in ways other than initially intended (Gelijns and Rosenberg, 1994). Modification of the technology occurs after initial adoption (Greer, 1988),
and uneven use occurs at a high rate early in the diffusion process (Wennberg, 1988).
While similar diffusion patterns exist for medications, devices, and surgical procedures, medications may have a lower adoption “threshold”—it is easier to write a prescription than learn a new procedure or approach (Fendrick and Schwartz, 1994). While medications and devices must first gain approval from the Food and Drug Administration for use in general clinical practice, different combinations and uses of pharmacotherapies in practice are not well evaluated (Sisk and Glied, 1994), and uses by physicians of medications on the market for indications or in combinations other than that for which they received FDA approval (so-called off-label prescribing) is thought to be common.
Although physicians are able to prescribe medications upon FDA approval, a number of factors may inhibit adoption. Innovations that depart from existing practices and are counter to prevailing attitudes are much less likely to be adopted (Office of Technology Assessment, 1994). Physicians may reject new medication therapy because of what it might do to the physician’s case mix, because other practice costs will rise, or because of inadequate time for patient visits. They may also reject a new therapy if there is inadequate evidence of cost-effectiveness (particularly in comparison to existing approaches).
In summary, immunotherapy and depot medications can present promising new strategies for treating drug dependence and abuse if they have potential relative advantage over existing treatments, compatibility with current drug treatment practices, and both providers and patients find them easy to use. But it is likely that ways in which clinicians and treatment settings perceive the new interventions will affect adoption.
Provider Attributes
Members of the social system and professional networks are an important element in the diffusion process (Rogers, 1995). Typically in general health care, exponential growth in the use of new treatments often ensues in an “epidemic” pattern throughout the larger provider community, as information disseminates regarding the new technology through professional networks, media, and advertising and following positive reports from initial users and demand from patients. However, at the same time, if incentives, education, and resources are not in place to adopt new treatment strategies, physicians can be somewhat resistant (Eisenberg, 1993).
Research has examined the characteristics of individuals associated with innovation as independent practitioners (Kimberly and Evanisko, 1981) and as leaders of organizational policy (D’Aunno, Vaughn, and McElroy, 1999; Friedmann, Alexander, and D’Aunno, 1999b). Variables
including younger age (Alexander et al., 1997; Counte and Kimberly, 1974), more education (Rogers, 1995), more urban practice, higher certification, specialization (Alexander et al., 1997; Counte and Kimberly, 1974; Rogers, 1995), academic affiliation, and group rather than individual practice were associated with earlier adoption of new technologies (Coleman, Katz, and Menzel, 1966; Fendrick and Schwartz, 1994; Freiman, 1985). Decisions to adopt have also been linked to factors such as authority roles among peers and the relationships of providers within an organization (Posner, Gild, and Winans, 1995).
Studies of physicians’ prescribing behavior suggest that the decision to write a prescription is a complex process, influenced by organizational rules, training, treatment philosophy, experience, information, and opinion leaders. Physician characteristics (Dybwad et al., 1997), provider assessments of the need for a prescription, likelihood of patient compliance, and the likely outcome of treatment (Brown et al., 1997; Denig, Haaijer-Ruskamp, and Zijsling, 1988; Lambert et al., 1997; Turk and Okifuji, 1997) do not fully explain variations in prescribing patterns. Advertising, education, and patient demand also affect prescribing patterns (Hemminki, 1975).
Organizational Structures and Financing of Treatment
The organization, its internal structure, and its response to external influences, such as competition or reimbursement on rates of technology acquisition and use affect adoption (Escarce et al., 1995; Hodgkin and McGuire, 1994; Romeo, Wagner, and Lee, 1984; Teplensky et al., 1995). Factors that are positively associated with earlier and more thorough adoption of innovation include size (Moch and Morse, 1977), resources (D’Aunno et al., 1999; Nohria and Gulati, 1995), academic network, leader behavior (Becker, 1970; Chilingerian and Glavin, 1994), system openness, organizational slack, a more competitive marketplace, and favorable reimbursement for the innovation. Thus, having resources available to explore and adopt a new innovation and having leadership with interest in and commitment to innovation are important factors in technology adoption.
The treatment setting’s environment—that is, “the specific collection of organizations providing the critical sources of inputs, and markets for outputs, required for an organization’s survival” (Scott, 1993, p. 292)—includes its competitors, state and federal regulators, parent organizations, managed care organizations, pharmaceutical companies, and potential clients. Each group exerts different demands or poses particular threats or incentives to the focal treatment setting that may drive it to adopt and encourage or reject and discourage new pharmacotherapies for drug de-
pendence (see Strang and Soule, 1998). Thus, managed care organizations and insurers may promote adoption of new pharmacotherapies by including them in the formulary or by issuing treatment guidelines that advocate their use. States can encourage the use of particular medications by covering treatment under Medicaid benefits. Regional location may influence decisions to accept and encourage pharmacotherapy in addiction treatments. Methadone treatment units in the northeastern United States used more effective treatment practices than those in other regions (D’Aunno and Vaughn, 1992). This may be due to the high concentration of top-tier medical schools and academic health centers in the area and the resulting exposure to and competition to remain at the cutting edge of medical science. Other treatment organizations may influence the decision to adopt particular medications, particularly if competitors have done so (Abrahamson, 1991; DiMaggio and Powell, 1988; Tolbert and Zucker, 1983; Westphal, Gulati, and Shortell, 1997). Professional organizations (e.g., American Society of Addiction Medicine) may improve an organization’s appraisal of immunotherapy and depot medications through official endorsements and dissemination of information about treatments. Pharmaceutical companies may also encourage adoption through marketing campaigns, particularly direct and repeated marketing to the focal organization (Van den Bulte and Lilien, 2001).
If a pharmacotherapy is perceived as a cost-effective treatment or a highly effective treatment, superior to other methods, organizations will experience substantial pressure to adopt it in order to enhance their performance and compete with other treatment organizations for individual and group clients (i.e., managed care contracts, state contracts). Cultural attitudes toward new pharmaceuticals in treatment will drive the pressures from the institutional environment. A shared view that pharmacotherapy represents the cutting edge of treatment for drug dependence may encourage physicians and organizations to adopt new pharmacotherapies in order to enhance their reputation or increase their market. Alternatively, if professional organizations and treatment organizations begin to accept these innovations, others may follow and come to view accepting particular new pharmacotherapies as a necessary move.
Channels of Communication
Channels of communication influence what information is transferred to potential users and its credibility. Professional information regarding a technological innovation is generally transferred in several ways, both formal (scientific literature, meetings, training) and informal (opinion leaders, colleagues, advertising, and press reports). However, while scientific evidence is an important factor, most adoption decisions depend
on the transfer of subjective information regarding the treatment from one member of a group who has already tried the innovation to another person in the group (Rogers, 1995). The change agent tends to be most effective when it is someone much like the potential adopter.
A major method of communicating information regarding new pharmaceuticals that has been particularly effective is marketing by drug manufacturers. In recent years the medications with the greatest growth in sales have been those that have been heavily marketed (National Institute of Health Care Management, 2002). However, marketing of prescription drugs can be a double-edged sword: While the message regarding the availability of new pharmacotherapies has been effectively communicated and can reach new potential audiences, information provided by manufacturers may be biased and must be complemented by additional objective sources. Further, there is some concern that extensive marketing efforts in the case of new medications in general medicine can lead to overprescribing and inappropriate use (Altman and Thomas, 2002). However, in the case of new drug treatment pharmacotherapies, marketing efforts, which are known to be a powerful driver of adoption of new medications, may be limited by how manufacturers perceive the profitability of new treatments.
The transtheoretical stages-of-change model (Prochaska and DiClemente, 1983) provides an additional framework for assessing behavioral changes and communication strategies. Adoption of innovations is viewed as a multistep process, integrating the practice setting and an ability to move through a continuum of five steps: precontemplation (no knowledge yet regarding the action), contemplation (awareness of the new behavior and motivation to adopt), action (development of a strategy to use the technique), implementation of the technique, and maintenance. Rather than examine the structural characteristics of a health care system, studies assess organizational and individual readiness to accept new treatment strategies (Backer, 1995). Investigators focus on the dynamics of the change process in order to understand differences between early- and late-adopter individuals and organizations and to improve technology transfer. Studies of cancer screenings and treatments (Johnson, Warnecke, and Aitken, 1996; Kaluzny et al., 1990) and cessation of addictive behaviors (Prochaska et al., 1994) illustrate the model’s broad base of support and the value of matching interventions with readiness to change.
Summary
Rogers’s (1995) framework for the diffusion of technology and the transtheoretical model of change provide structures for disaggregating the process of diffusion and analyzing critical components. The adoption
of pharmacotherapies for the treatment of drug abuse disorders, particularly in primary care, may be particularly sensitive to the characteristics of the medication (compatibility, complexity, and observability), the use of people in recovery as change agents, analyses of the persuasion process, and the nature of the social and organizational systems found in drug treatment programs. While the historical pattern in the United States is that of relatively rapid adoption of new pharmacotherapies, there are reasons to believe that the adoption of medications to treat drug and alcohol dependence will be more reserved. The use of medications as complementary interventions with behavioral therapies represents a vast change in the nature of treatment of drug abuse challenging current practitioner and provider structures. Multiple professional and social obstacles may offset the easy “trialability” of pharmacotherapy. Furthermore, to the extent that substance abuse treatment medications are used to enhance the efficacy of existing therapies, they may significantly contribute to increased costs of addiction treatment. The literature on adoption of technology in alcohol and drug abuse treatment may be informative.
ADOPTION OF TECHNOLOGIES IN THE TREATMENT OF ALCOHOL AND DRUG ABUSE
The peer-reviewed literature on the adoption of new technologies in alcohol and drug abuse treatment settings is surprisingly limited; systematic empirical investigations are uncommon. A review of the literature finds one randomized trial of dissemination methods, a few analyses of the adoption of naltrexone for the treatment of alcohol dependence, and a handful of essays reflecting on barriers to adoption and strategies to address the barriers.
Randomized Trial
A randomized trial tested dissemination strategies to promote an evidence-based practice to improve employment among patients in drug treatment (Job Seekers Workshop; Sorensen et al., 1988). Drug treatment programs (n = 172) were randomized to four levels of information about the employment training intervention: (1) training materials only (i.e., a 20-page summary of the workshop and effectiveness data plus a manual on conducting the workshop), (2) the training materials plus one day of on-site technical assistance, (3) the training materials plus an expenses-paid 2-day training, and (4) a nonintervention comparison where training materials were provided after the follow-up period. A questionnaire mailed three months after the interventions assessed the extent to which the training materials had been used and the number of workshops con-
ducted. Adoption was higher among programs that received a technical assistance site visit (28 percent) or participated in the 2-day training (19 percent) than at the sites that received only printed materials (4 percent) and among programs in the nonintervention group (0 percent). Hands-on, in-person demonstrations appear to be an important element in the adoption of new drug abuse treatment interventions. Despite the strength of the finding, dissemination efforts continue to emphasize distribution of brochures and manuals.
The Sorensen et al. study remains the only randomized trial that tested interventions to promote the adoption of an empirically supported drug abuse treatment technology. Subsequent investigations examined differences between practitioners who adopted or did not adopt new technologies and provide useful insights into variables associated with adoption. But in the absence of random assignment, multiple factors may contribute to the observed differences in adoptions.
Adoption of Naltrexone
Using naltrexone for the treatment of alcohol dependence remains an intriguing example of limited adoption of a medication for addiction treatment. A mail survey conducted in Massachusetts, Tennessee, and Washington state among physicians with a substance abuse specialization (135 responses, 63 percent response rate) and certified addiction counselors (1,116 responses, 65 percent response rate) found limited use of naltrexone (Thomas, 2000; Thomas et al., 2003). Most (80 percent) of the physicians reported current or prior use of naltrexone, but only 15 percent prescribed it often (11 percent) or for almost all patients (4 percent). A majority (54 percent) of counselors, in contrast, had never suggested use of naltrexone to patients, and few recommended it often (4 percent) or for almost all of their patients (1 percent). Logistic regression models suggested that adoption was more likely among physicians involved in research (odds ratio = 19.7) and physicians located in organizations that promoted the use of naltrexone (odds ration = 11.6). Physicians in recovery (odds ratio = 0.2) and physicians with multiple degrees (odds ratio = 0.1) were less likely to prescribe naltrexone. Organizational support to use naltrexone was the strongest influence on counselors recommending it to patients (odds ratio = 7.9). Counselors who reported receiving marketing information on naltrexone were also more likely to recommend its use (odds ratio = 3.2).
Patient access to insurance that covered naltrexone also affected counselor behavior. Counselors with a higher proportion of Medicaid patients were more likely to prescribe naltrexone, and those with more patients funded through block grant and self-pay were less likely. (Medicaid in all
three states covered naltrexone prescriptions, while block grant funding did not pay for it.) Washington state actively encouraged counselors to support the use of naltrexone, and counselors in Washington (compared to Massachusetts and Tennessee) were more likely (odds ratio = 1.5) to recommend that their patients use naltrexone. Recovery status did not have a significant influence on counselor use of naltrexone. Overall, these results suggest that organizational support, financing mechanisms, and state policies may influence the adoption of medications to treat alcohol and drug abuse.
Roman and Johnson (2002) examined organizational influences on the adoption of naltrexone. In a sample of 400 alcoholism treatment centers, 44 percent reported current use of naltrexone. Levels of use among patients, however, were low among both alcohol- (13 percent of the caseload) and opiate-dependent (11 percent of the caseload) patients. Logistic regression suggested that any naltrexone use was greatest in centers where counselors were more likely to have master’s degrees (odds ratio = 1.7) and with more patients in commercial health maintenance organization and preferred provider organization health plans (odds ratio = 1.02). Centers that were older and those with higher caseloads of patients with a history of relapse also were more likely to use naltrexone. Importantly, structural characteristics of the organization (e.g., hospital setting, larger corporation, physician availability) were not significant influences when tested in multivariate models. The investigators suggest that addiction treatment programs have not encountered rapid change in technology, so older, more experienced programs and administrators are more willing to assume the risk of adoption. They also noted that levels of education among clinical staff are a key factor in the adoption of naltrexone but that the overall magnitude of use is still minimal (Roman and Johnson, 2002).
In a Researcher in Residence Program piloted in New York state, nationally recognized investigators provided hands-on technical assistance to facilitate adoption of research-based technologies for alcoholism treatment (Hilton, 2001). Investigators provided one to three days of on-site assistance and at three sites either a reconnaissance visit or a booster session. Participating programs requested assistance with the use of naltrexone (two sites), clinical assessment (two sites), motivational interviewing (one site), and services for patients with comorbidities (one site). Interviews with program directors and clinical staff were conducted three to six months after visits to assess impacts and adoption. Case studies were prepared for each of the six sites, and commonalities were abstracted. Hilton (2001) concluded that the site visits fostered adoption but that organizational change is difficult, takes time, and requires sustained leadership. The Researchers in Residence Program provided clinical staff with opportunities to have personal experience with the new technolo-
gies, and that experience seemed to promote adoption and use. Staff turnover, however, inhibited follow-through and adoption was observed in some but not all of the clinical settings. When adoption required more change in practice style, change was slower and less likely to be observed in a short follow-up. Surprisingly, limited reimbursement for prescription medications and negative staff attitudes toward the use of medications did not inhibit the use of naltrexone (Hilton, 2001). The results of the Researcher in Residence Program echo the findings from Sorensen et al. (1988)—hands-on technical assistance is often an essential aspect of adopting a new treatment technology.
Essays on Adoption
The most common, but still infrequent, papers on the adoption of technologies in addiction treatments are personal reflections on variables that contributed to or inhibited adoption of evidence-based drug abuse treatment technologies. Brown’s thoughtful essays review linkages between research and practice, lament the lack of strategies to foster technology transfer, and encourage adoption of research findings (Brown, 1987, 1995, 1997, 1998, 2000; Brown and Flynn, 2002). Backer summarizes the technology transfer and dissemination literature and generalizes from classic work on technology diffusion to the adoption and use of drug abuse prevention and treatment technologies (Backer, 1991, 1995; Backer and David, 1995; Backer, Rogers, and Sopory, 1992). Naranjo and Bremner describe their efforts and frustrations implementing the use of a clinical tool (the Clinical Institute Withdrawal Assessment for Alcohol) to improve detoxification services in rural areas of Canada (Naranjo and Bremner, 1996). Similarly, Morgenstern (2000) reflects on his experiences promoting the use of cognitive behavioral therapies in traditional 12-step treatment settings.
Most recently, the focus has shifted toward viewing technology transfer as a process of organizational change. The Addiction Technology Transfer Centers promote an organizational change model to support the adoption of evidence-based practices in alcohol and drug abuse treatment centers. The Change Book offers a 10-step structure to foster organizational change and support the adoption and use of new drug abuse treatment technologies (Addiction Technology Transfer Centers, 2000). Finally, in a promising development, Simpson (2002) reviews the literature on technology transfer and drafts a model of the factors that contribute to organizational change and the adoption of new technologies for drug abuse treatment; early results are encouraging. It is critical, therefore, to have an overview of the financing and organization of specialty drug and alcohol treatment programs.
SPECIALTY DRUG AND ALCOHOL TREATMENT SERVICES
The specialty clinics that constitute much of the nation’s alcohol and drug abuse treatment system trace their roots to the narcotics hospitals in Lexington, Kentucky, and Fort Worth, Texas (which opened in 1932 and 1938, respectively) and the lack of access to medical and psychiatric facilities in the 1960s and 1970s (Institute of Medicine, 1990a, 1990b, 1997, 1998). As a result, the financing and structure of the services developed idiosyncratically and are relatively autonomous from the nation’s primary care system.
Financing of Services
The nation’s expenditures for treating alcohol and drug disorders were estimated as $11.9 billion in 1997, or about 1 percent of total expenditures on health care ($1,057 billion) and 14 percent of expenditures on behavioral health ($82.2 billion; Coffey et al., 2001; Mark et al., 2000). The distribution of expenditures by provider type begins to illustrate the idiosyncratic nature of the treatment system for alcohol and drug abuse. Hospitals and specialty treatment centers account for nearly three-quarters of the expenditures for chemical dependency treatment services. Alcohol and drug treatment services primarily occur in hospitals (40 percent of total expenditures) for inpatient detoxification and in specialty clinics (33 percent of total expenditures) for outpatient and residential counseling services.
Hospitals also account for the largest portion of expenditures for total health care (35 percent) and for mental health treatment (30 percent), but specialty substance abuse treatment services make invisible contributions (less than one percent) to expenditures for mental health and health care services. Independent practitioners, mental health centers, and prescription drugs account for little of the expenditures in alcohol and drug abuse treatment but for substantially greater proportions of mental health and general health care: independent practitioners (health care = 26.5 percent; mental health = 28.5 percent; substance abuse = 11.1 percent); mental health centers (health care = less than 1 percent; mental health = 15 percent; substance abuse = 9 percent); prescription drugs (health care = 7.5 percent; mental health = 12.3 percent; substance abuse = 0.3 percent) (Coffey et al., 2001; Mark et al., 2000).
Expenditure analyses also show that payers differ (Coffey et al., 2001; Mark et al., 2000). Alcohol and drug treatment services rely more on federal funding other than Medicaid and Medicare (16 percent of expenditures) compared to mental health and total health care (4 percent each). This reflects the role of the federal Substance Abuse Prevention and Treat-
ment Block Grant. Medicare makes less of a contribution to funding for alcohol and drug treatment (8 percent of expenditures) compared to treatment for mental health (12.3 percent) and general health care (20.3 percent). Patients also contribute proportionately less out-of-pocket revenues for substance abuse treatment (9.2 percent) than for mental health services (16.9 percent) and general health care (17.7 percent). State and local revenues make up more of the funding for mental health and substance abuse services (20 percent each) than for general health care (6.6 percent), but general health care receives more support from private insurance (33 percent versus 24 percent for mental health and substance abuse treatment).
The summary of expenditures for alcohol and drug abuse treatment suggests that integration of alcohol and drug treatment with primary care and general health care services is inhibited by differences in financing and differences in treatment settings and practitioners. The presence of a large and autonomous system of specialty chemical dependency treatment settings reflects a legacy of poor service for alcohol and drug disorders in health care and mental health care settings, limited coverage in insurance plans, and the resulting divergence in payer sources and regulatory mechanisms.
Specialty Chemical Dependency Treatment Services
The most current source of data on facilities that offer drug and alcohol treatment is the 2000 National Survey of Substance Abuse Treatment Services (N-SSATS; previously called the Uniform Facilities Data Set—UFDS; Substance Abuse and Mental Health Services Administration, 2002). The report is available through the SAMHSA Website at http://www.samhsa.gov/oas/dasis.htm#nssats2. N-SSATS is an annual census and point prevalence recording of program and patient characteristics. The 2000 N-SSATS found 13,428 facilities offering treatment for alcohol and drug dependence that served slightly more than one million patients as of October 1, 2000. Six of 10 (60 percent) treatment centers are nonprofit and about one in four (26 percent) operate as for-profit organizations; the remainder are operated by state and local governments (11 percent), federal agencies (2 percent), and tribal governments (1 percent; Substance Abuse and Mental Health Services Administration, 2002).
Most (61 percent) designate themselves as substance abuse treatment settings rather than combined substance abuse and mental health organizations (25 percent), mental health care organizations (9 percent), or health care settings (3 percent). Facilities are most likely to offer outpatient treatment (78 percent), 26 percent offer residential rehabilitation, and about 8 percent provide inpatient detoxification; 9 percent of facilities reported
using methadone. Treatment services vary, but more than two of three reported offering assessment (94 percent); individual therapy (95 percent); group therapy (88 percent); discharge planning (80 percent); urine screens for drug use (79 percent); relapse prevention, family counseling, and aftercare (77 percent); and case management (68 percent). Medical services were provided less routinely: pharmacotherapy and prescription medications (42 percent), tuberculosis screening (38 percent), testing for HIV (33 percent), hepatitis (25 percent), and sexually transmitted diseases (25 percent). Programs tend to be small—45 percent reported an active caseload of less than 30 patients, and 78 percent served fewer than 100 patients. Thus, the picture that emerges from the N-SSATS census is of a treatment system composed of small specialty outpatient clinics that provide limited medical services and have little overlap with the larger general medical system of care. Current financing systems, however, do not encourage greater integration of substance abuse and primary care services. What steps have been taken to encourage more integration with primary care?
INTEGRATION OF ADDICTION TREATMENT WITH PRIMARY CARE
Similarities between drug and alcohol dependence and chronic illnesses like diabetes, asthma, and heart disease (e.g., diagnosis, genetic heritability, etiology, pathophysiology, treatment response, rates of retreatment) suggest that addiction could be viewed as a chronic disorder (McLellan, Lewis, O’Brien, and Kleber, 2000). Primary care settings with linkages to support and counseling services, therefore, may be appropriate environments for treating alcohol and drug dependence. Editorials in the Journal of the American Medical Association (Stein and Friedmann, 2001) and the Journal of General and Internal Medicine (O’Connor and Samet, 2002), in fact, encourage expanded roles for primary care clinicians because abuse of alcohol, tobacco, and other drugs is common among patients, it co-occurs with HIV/AIDS and psychiatric disorders, and it is a chronic health problem.
Two models have been described for integrating primary care and addiction treatment: centralized and distributed (Samet, Friedmann, and Saitz, 2001). Centralized models offer primary care and behavioral health services (substance abuse and/or mental health care) at a single location. Delivering both services at the same location eliminates geographic distance and travel time as barriers to linkage and facilitates access for patients who may have limited motivation to seek care and whose lives are often disorganized. Distributive models recognize that reimbursement mechanisms and licensing requirements inhibit co-location of services and seek to optimize the existing pattern of independent service settings for
primary care and behavioral health services. Strong referral mechanisms are required, and practitioners in both settings need to recognize and acknowledge problems that require referral. Case management can facilitate appointments and transitions between service settings.
Barriers to a fuller integration of treatment systems include provider education, financing mechanisms and disincentives, confidentiality requirements and concerns, and the persistent presence of stigma (Samet et al., 2001). The distributive and centralized models of integrated care implicitly recognize that primary care clinicians are unlikely to assume full responsibility for caring for alcohol and drug disorders. Stein and Friedmann (2001) acknowledge that only a small portion of primary care clinicians will choose to specialize in patients with alcohol and drug disorders, but they recommend that all physicians should be able to screen for potential alcohol and drug abuse problems and to make appropriate interventions and referrals.
A recent clinical trial demonstrated the value of integrating primary care physicians into an addiction treatment setting (Weisner et al., 2001). Patients who entered treatment for chemical dependency in a large health maintenance organization were randomly assigned to receive primary care in the addiction treatment setting or continue with their usual primary care clinician located in a separate clinic. Six months after randomization the rates of abstinence did not differ significantly among the patients who received integrated care (68 percent) compared to the treatment as usual—independent primary care (63 percent). Patients with a substance abuse-related medical condition, however, were significantly more likely to achieve abstinence when treated in an integrated setting (69 percent) rather than when primary care was provided in a different setting (55 percent). Costs were not significantly higher in the integrated setting and, consequently, the cost-effectiveness ratio was substantially better for integrated care (Weisner et al., 2001).
Studies of drug abuse treatment services, however, find that most do not provide on-site primary care. A 1995 survey of outpatient drug abuse treatment programs, for example, reported that 48 percent provided on-site physical examinations, and 40 percent offered routine medical care on-site (Friedmann et al., 1999a). The outpatient programs that were most likely to provide on-site primary care were certified by the Joint Commission on Accreditation of Healthcare Organizations and offered methadone treatment. Similarly, an analysis of the 96 programs participating in the Drug Abuse Treatment Outcome Study reported that 15 percent offered complete medical care on-site and 34 percent used a combination of on-site services and referrals (Friedmann, McCullough, and Saitz, 2001). Use of medical services during the first month of drug abuse treatment was generally low (30 to 40 percent of patients). When all services were pro-
vided on-site, however, 27 percent of patients received at least three medical visits compared to 10 to 14 percent in all other programs (Friedmann et al., 2001).
Despite the apparent value of integrating primary care and interventions for alcohol and drug use disorders, adoption in primary care settings has been relatively limited. Studies of screening and brief intervention and the adoption of buprenorphine to treat opioid dependence suggest that there is great opportunity for higher levels of impact and adoption.
Screening and Brief Interventions
Research suggests that individuals with high-risk patterns of alcohol and drug use can be identified in health care settings. Moreover, relatively brief interventions by physicians and other health care professionals lead to significant reductions in levels of alcohol use (Fleming et al., 1997; Ockene et al., 1999). Despite the strength of these findings, physicians often neglect to screen for alcohol and drug use. A survey of physician screening practices (57 percent response rate) reported that 88 percent screen new patients for alcohol use but that only 13 percent use formal screening tools (Friedmann et al., 2000) and 68 percent inquire about drug use (Friedmann et al., 2001). Psychiatrists were more likely to screen than primary care clinicians and more likely to intervene (Friedmann et al., 2000, 2001). A minority but still substantial number of primary care physicians miss the opportunity to examine their patients’ use of alcohol and other drugs (Friedmann et al., 2000).
Saitz et al. (2000, 2003) identified two types of barriers that inhibit adoption of screening and intervention tools: clinician-specific barriers (negative attitudes toward addicted patients, limited knowledge and experience regarding treatments, lower professional satisfaction, lack of perceived responsibility for treatment of addictions) and resource-related barriers (limited time, inadequate reimbursement mechanisms, limited office support for such services, and inadequate linkages with referrals).
Screening and interventions for smoking cessation are becoming more widely implemented in primary care as well. Availability of medications in treatment has changed how physicians approach smoking. While significant evidence indicates the importance of smoking cessation for personal health and the overall health care system, screening and intervention have still not been universally adopted in primary care (Cornuz et al., 2000; Fiore, 2000; Fiore et al., 2000; Jaen et al., 2001). Similar to other addiction disorder treatments, barriers to successful adoption have included lack of medical education in this area (Spangler et al., 2002), low provider expectations for success, and little office support (Gottlieb et al., 2001; McIlvain et al., 2002). However, tobacco cessation guidelines now exist
(Fiore, 2000; Fiore et al., 2000), evidence regarding their cost effectiveness in primary care has been published (Cromwell et al., 1997; U.S. Veterans Administration, 1999a), and screening is now recommended as part of standard healthcare systems and health plan evaluation criteria (U.S. Veterans Administration, 1999b; U.S. Public Health Service, 2000).
Adherence to guidelines is improved with organizational support and policies for implementation (including screening systems and prompting), better physician familiarity with the guidelines, improved counseling skills, and greater belief on the part of physicians in the effectiveness of treatment (Fiore, 2000; Fiore et al., 2000; Stone, et al., 2002; Vaughan et al., 2002). Successes in tobacco cessation treatment have also likely been encouraged in part by pharmaceutical manufacturers’ marketing to both clinicians and patients in the presence of a vast potential market, in combination with the development of accepted guidelines for treatment.
An emerging and more challenging frontier is office-based treatment of opioid dependence. Recent approval of buprenorphine for the treatment of opioid dependence offers opportunities for primary care physicians to become more directly involved in the treatment of drug use disorders.
Adoption of Buprenorphine
In the United States, policy makers and advocates see potential for primary care and specialist physicians to take leadership roles in the treatment of patients dependent on opioids because of increased options for opioid maintenance and detoxification medications (Fiellin and O’Connor, 2002; Merrill, 2002). The Food and Drug Administration (FDA) approved the use of Subutex (buprenorphine hydrochloride) and Suboxone (buprenorphine hydrocholoride plus naltrexone) for the treatment of opioid dependence in October 2002. Within eight months, SAMHSA’s Buprenorhine Physician Locator Web page (http://buprenorphine.samhsa.gov/bwns_locator/dr_search.htm) listed 1,028 physicians as qualified to write prescriptions (this reflects only the physicians who chose to be listed and is an undercount of the number with waiver approval). The relatively small number of listed practitioners suggests that the challenge of promoting adoption among physicians is substantial.
Office-based dispensing and prescribing of maintenance medications are expected to increase access to treatment, reduce the stigma associated with seeking drug treatment, and provide better patient care (Fiellin and O’Connor, 2002). Randomized clinical trials suggest that physicians can treat opioid-dependent patients effectively in office-based practices. In one study, opioid-dependent patients were randomly assigned to receive buprenorphine three times a week in either a primary care clinic or a
traditional narcotics treatment program (a methadone maintenance center; O’Connor et al., 1998). Patients treated in the primary care clinic had a higher 12-week retention rate (78 versus 52 percent), had lower rates of urine positive for opioids (63 versus 85 percent), and were more likely to achieve at least three weeks of abstinence from opioids (43 versus 13 percent; O’Connor et al., 1998). It is important to note that the primary care clinic was affiliated with a drug abuse treatment service and patients participated in a weekly group counseling session at the clinic—drug abuse treatment services were integrated into the primary care clinic. A 6-month trial of office-based methadone maintenance also found that maintenance medication could be provided safely and effectively in a primary care setting (Fiellin et al., 2001).
Given the brief time since FDA approval and the requirements for receiving a waiver, information is limited on the adoption of buprenorphine in the United States. France, however, approved its use for the treatment of opiate dependence in 1995. Within a year, 25,000 French citizens were receiving prescriptions from general practitioners (Moatti et al., 1998). An April 1996 telephone survey of nearly 1,200 randomly selected and eligible general practitioners in France (70 percent response rate) found that one in four (24 percent) reported caring for patients who injected drugs (Moatti et al., 1998). Physicians with experience caring for injection drug users were more willing to prescribe buprenorphine (31 versus 7.5 percent) (Moatti et al., 1998). A second assessment found that 27 percent of French physicians prescribed and 52 percent of pharmacists dispensed buprenorphine at least once in the first two years of availability (Vignau et al., 2001). Mean dosage levels (6 mg per day), however, suggested that doses for many patients were below recommended therapeutic levels (6 to 16 mg per day) and may indicate “a lack of experience and training” in the treatment of opiate dependence. Another study showed that the mean daily dose among French general practitioners was higher (11.5 mg), accompanied by high levels of concurrent benzodiazepine use by some patients (Thirion et al., 2002). Variations in dosing and concurrent pharmacotherapy suggest that practitioner training is a critical element in promoting adoption, diffusion, and effective use of buprenophine.
Primary Care and Addiction Interventions
Options and models for integrating primary care and drug abuse treatment services are emerging. Because alcohol use and abuse are more common than drug abuse, research has tended to emphasize patients with alcohol-related problems. There has been much less work on integrating services for drug-dependent patients (Samet et al., 2001). It may be more challenging to develop effective integration for drug patients because of
less experience with drug-dependent individuals and more suspicion of their motives for seeking care. An ethnographic study of the treatment of opiate-dependent patients in a teaching hospital, for example, concluded that attending and resident physicians were inexperienced and unskilled in working with addicted patients and the lack of skill inhibited better care (Merrill et al., 2002). Patients, moreover, perceived inconsistent and hesitant care and concluded that they were being treated poorly because of their drug use. Most physicians are untrained in the treatment of alcohol and drug disorders and are unlikely to seek greater skill. Physician training and education, however, are key to effective integration of primary care and services for alcohol and drug dependence.
INTEGRATION OF IMMUNOTHERAPIES AND DEPOT MEDICATIONS INTO TREATMENT SETTINGS
The reviews of technology adoption in alcohol and drug services, specialty treatment programs, and treatment of alcohol and drug disorders in primary care settings suggest general implications for dissemination of immunotherapies and depot medications. There are also implications for specialty and primary care settings.
The transfer of new technologies into treatment for alcohol and drug abuse may be challenging. Brown (1995) enumerates factors important to support technology transfer and noted that the absence of any one feature can inhibit dissemination: the relevance and timeliness of the innovation, style of communication regarding the innovation, credibility of the source as well as the message, availability of resources to adopt the innovation, acceptability of the innovation within current treatment orientations, and consistency of the innovation with current organizational mandates. In many programs, staff training relies on an apprenticeship (experiential training) emphasizing traditional approaches rather than the more theoretical and academic perspective found in graduate education. Diffusion studies consistently report that early adopters of new technologies tend to be more highly educated (Rogers, 1995). Counselors with formal post-graduate training, therefore, may respond differently than those without graduate training. The heterogeneous structure of the substance abuse workforce may require different change messages and change agents for different subgroups of counselors. The innovation decision process must be examined for both groups.
Progress in the development of medications for the treatment of drug dependence will lead to little application of pharmacotherapy if drug abuse treatment practitioners and programs are not ready, willing, and able to embrace medication technologies. Six broad sets of barriers to the diffusion and adoption of emerging technologies in drug abuse treatment
settings were identified in the Institute of Medicine’s (1998) analysis of the linkages between research and practice:
-
Structure—small programs with limited resources may be unable to afford the medical staff and training required to fully utilize medications.
-
Financing—the multiple funding streams that support drug treatment may have unique rules and may not provide coverage for new therapies, including medications.
-
Education and training—in many programs training for staff relies more heavily on an apprenticeship (experiential training) emphasizing traditional approaches rather than the more theoretical and cosmopolitan perspective found in graduate education.
-
Stigma—ignorance and prejudice about drug abuse contribute to inadequate training in graduate programs and medical schools, inhibit the construction and location of facilities, and reduce investments in technology development.
-
Lack of knowledge about technology transfer—a lack of systematic research on technology adoption in drug abuse treatment settings slows the development of more effective dissemination strategies.
-
Policy—local, state, and federal policies sometimes restrict the types of services available and the individuals who receive those services.
Individuals seeking treatment and their families are the most direct beneficiaries of effective pharmacotherapy. Their attitudes toward medications and their beliefs about the efficacy and effects of medications will be critical in the adoption and diffusion of new pharmacotherapies. Moreover, because of the value of group support to recovery, there is a whole social system of individuals in recovery whose attitudes and beliefs could have substantial impact on the acceptability of medications to the field. Similarly, counselors communicate their beliefs and opinions to clients and as authority figures can potentially facilitate or inhibit the use of medications. Social and normative influences must be considered when assessing the cognitive factors that contribute to behavioral decisions, because much of what clients know about treatment comes from interactions with counselors and other clients.
Specialty Settings
Despite the potential of new and emerging medication therapies for substance abuse treatment, the drug and alcohol treatment field appears reticent to embrace them. The winter 2002 issue of Hazelden Voice, for ex-
ample, includes a commentary on “the pros and cons of addiction medications” (available on the Web at http://www.hazelden.org/newsletter_detail.dbm?id=1345; Owen, 2002). (Hazelden is one of the nation’s most recognized specialty programs for the treatment of chemical dependency.) The essay suggests that adoption of medications will be inhibited in many specialty alcohol and drug abuse treatment centers because of experience with recovery without the use of medications, concern about unanticipated side effects and addiction potential, discomfort with the research supporting the use of medications, and perceived incompatibilities with traditional treatment approaches. The potential value of medications is acknowledged, but there is a strong sense of resistance and skepticism. Four negatives associated with the potential use of medications were noted:
-
“We are puzzled why some providers are so enthusiastic about medications, when we see, for our patients, that recovery is possible without them.”
-
“We worry that some medications … may prove to be mood-altering.”
-
“Research findings … are often framed in non-familiar and in fact sometimes non-desirable outcomes (e.g., ‘reduced alcohol use,’ ‘fewer drinking days’ or ‘fewer drinks per drinking day’). For abstinence-based programs these are not necessarily impressive outcomes.”
-
“It is possible that addicts/alcoholics may believe the medication will help them control their substance use rather than focusing on the goal of abstinence” (pp. 3, 12).
At the same time, the essay identified four reasons for considering medications:
-
“We know that not everyone is helped by our treatment approach; maybe other methods would help.”
-
“As a disease, alcohol/drug dependence has a biological basis. Could a medication be part of the multidimensional approach?”
-
“Medications are used as part of a treatment regimen for other diseases that have a behavioral component, such as heart disease or diabetes.”
-
“Incorporation of new ideas is part of the ‘Minnesota Model’” (p. 12).
The essay concludes that medications may eventually prove to be an effective facet of a comprehensive addiction treatment program but that
medications alone are unlikely to be sufficient to ensure a stable recovery. Hazelden, therefore, “will watch the research … [and] when it becomes clear that other approaches have something significant to offer that fits with our model of care, Hazelden will incorporate them” (Owen, 2002, p. 12).
The Hazelden commentary provides insight and perspective on the challenges that await efforts to foster adoption of new medication technologies in the programs that treat alcohol and drug dependence. A treatment organization’s internal structure, staffing, and other resources have a large influence on the adoption of new technologies. For instance, staff expertise, availability of physicians, and adequate training are essential for adoption of innovation. This has particular significance for specialty treatment organizations, which are not centered around physicians and thus do not have the clinical expertise and a medical approach to the management of addictions, nor do nonphysicians have the ability to prescribe medications. Adoption of new pharmacotherapies, especially in these settings, requires significant physician involvement in the management of patients.
Management structures, norms, and expectations about appropriate and expected behaviors, reimbursement mechanisms, and state and federal policies also affect the flow of information and the response to emerging medications and immunotherapies. In treatment organizations, decisions about innovation may be optional (each clinician and patient chooses), collective (choices are made as a group), or authoritative (policy is set by management). Thus training must include the counselors and a recognition that staff turnover is high in many treatment programs. Finally, financing for medications is not usually included in the reimbursement provided for most specialty drug abuse treatments. New financing mechanisms must be developed before rapid adoption is likely in publicly funded treatment centers.
Primary Care Settings
Implementation challenges are also apparent for medical settings. Primary care settings (physicians’ offices and clinics) are typically organized around procedural services and medications as the focus of treatment. While a portion of primary care has always been devoted to the management of conditions that require ongoing psychosocial therapy, the linkages with psychosocial support systems have for the most part been secondary to medical therapy. Primary care physicians who are willing to address problems of addiction have not yet done so due to several factors: lack of training or skills specific to substance abuse screening or treatment, lack of linkages between service systems, limited provider time and
financial resources to address problems of substance abuse, and the stigma often associated with patients who have addiction problems. Additional challenges in promoting linkages between primary and specialty services include difficulties communicating across settings, confidentiality standards for the treatment of alcohol and drug disorders that often inhibit sharing medical and psychosocial information, and concerns regarding coerced treatment.
Confidentiality Regulations
Alcohol and drug abuse treatment records have a unique level of federal protection. In most cases, information in the clinical record may not be shared without the specific consent of the patient. Authority for confidentiality standards for alcohol dependence treatment records was included in the Comprehensive Alcohol Abuse and Alcoholism Prevention, Treatment, and Rehabilitation Act of 1970 (Hughes Act, P.L. 91-616) and extended to drug abuse treatment records in the Drug Abuse Prevention, Treatment, and Rehabilitation Act of 1972 (P.L. 92-255; Lopez, 1994). The regulations were designed to protect the privacy of individuals entering care (Legal Action Center, 1991). The strict confidentiality requirements prohibit disclosure of information from a “federally assisted” treatment program unless the patient provides a valid consent to the release or specific conditions are met for a court-ordered release (Legal Action Center, 1991). “Federally assisted” is broadly defined to include any form of federal funds, a grant of tax-exempt status, an authorization to conduct business, or an agency of federal, state, or local government. As a result, the rules apply to all facilities that are licensed or authorized by state regulations.
State regulations may be more restrictive but cannot permit disclosures that are prohibited by the federal regulations. The strict limits on disclosure are unique to alcohol and drug abuse treatment programs. Medical records and mental health records do not enjoy the same level of protection. As a result, primary health care practitioners may be unaware that their patients are simultaneously receiving treatment for alcohol and drug disorders. The confidentiality regulations complicate efforts to integrate care. The recent implementation of stricter confidentiality standards for medical records (Health Insurance Portability and Accountability Act) does not obviate the stricter standards applied to alcohol and drug abuse treatment records but may foster consistent strategies for releasing and sharing information, including treatment for alcohol and drug disorders in health care settings.
Financing
Differences in financing between general medical care and mental health/substance abuse treatment will also challenge adoption of new treatments. First, many insurance programs limit funding for counseling and recovery support. Second, in cases in which care is fully or partially capitated (either all services or carved out to specialty substance abuse programs), new medications and treatments may need to prove they are cost effective in order to be adopted onto formularies and incorporated into treatment.
Chronic Care Model
A valuable approach to the management of addiction treatment in primary care settings would be to apply principles of optimal chronic disease management. A recently demonstrated approach to managing chronic illness was applied to tobacco addiction (Bodenheimer, Wagner, and Grumbach, 2002a, 2002b). This model recognizes and operationalizes linkages across the systems in which chronic care takes place—community resources and health care, financing, and provider organizations. Proactive teams address six essential elements of care: community resources and policies, health care organization, self-management support, delivery system design, decision support, and clinical information systems. The chronic care model improved outcomes of care and in some cases reduced costs for certain conditions. However, payment incentives are not always in alignment with the chronic care model approach and can provide obstacles to coordination of care.
Emergency Medicine
Finally, the emergency medical setting must be considered a potential setting for adoption and implementation of immunotherapies or depot medications. The prevalence of substance abuse in emergency room patients is estimated at 15 to 24 percent (Teplin, Abram, and Michaels, 1989; Cherpitel, 1996). Emergency personnel, however, detect and refer only a small proportion of substance abuse problems (Fortney and Booth, 2001). As treatment options for overdose and relapse prevention increase, physicians and hospitals will have to make decisions to adopt interventions that may require better detection of drug dependence. Protocols will have to be developed and individualized to the particular setting. Some issues in emergency care may be the same as those of primary care, in particular lack of training specific to addiction problems and inadequate
linkages or follow-up for individuals treated in emergency rooms. Additional barriers are specific to emergency departments:
-
A high proportion of emergency care is uninsured, so reimbursement for expensive interventions will be difficult to obtain.
-
Many individuals treated in emergency departments are lost to follow-up, so linkages to care will be critical.
-
Prioritizing and triaging patients are important components of emergency care, but some individuals being treated for addictions may receive lower priority than others needing urgent care.
Thus, the adoption of immunotherapies and depot medications will be challenging whether in specialty settings, primary care, or emergency medicine.
CONCLUSIONS
Extensive literature indicates that adoption of innovations is the result of characteristics of the provider, treatment setting, financing strategies, the technology itself, and the manner in which information is communicated. Several characteristics of the substance abuse treatment system have in the past worked to diminish the speed and extent to which innovations have been adopted in addiction treatment. Addiction treatment technologies have achieved less than anticipated success in the market, most recently in the case of naltrexone, where financing, education, and questions regarding effectiveness have played a large part in the lack of adoption. Studies suggest that many of the barriers to adoption of new substance abuse treatments may be amenable to policy interventions, including appropriate education, adequate financing, and improved linkages between primary care and specialty treatment. Specific approaches to technology transfer can promote new therapies for drug abuse treatment and may have particular significance for the successful diffusion of depot medications and immunotherapies. These innovations have the potential to reach a wide population at need and bring primary care settings to play a greater role in addiction treatment. However, in order to do so, policy makers and providers must influence financing strategies, organizational structures, and educational approaches that will facilitate use of these innovations. See Figure D-1 for a summary of the health care system components that must be addressed to promote appropriate adoption of immunotherapies and depot medications for the treatment of drug dependency disorders.
Integration of treatment of substance abuse disorders is not universally implemented in primary care. However, research suggests that
several factors can facilitate appropriate and informed use of new medications. These strategies can be considered prior to widespread availability of immunotherapies and depot medications.
A necessary step prior to making immunotherapy and depot medications available is to develop professional standards that guide the application of the therapies to specific patient groups, including adolescents. Guidelines for prophylaxis are also needed. Several areas have made progress in the development of practical guidelines for screening and treatment. Particularly effective are alcohol screening tools and smoking cessation programs. These approaches, however, must be applied regularly in practice in order to be effective. Therefore, an accompanying approach is provider education. It is clear from the literature that multi-faceted education efforts for physicians and other providers must be in place to inform them about all aspects of the use of these therapies. As has been shown with naltrexone, a lack of information supported a host of other questions surrounding the drug’s effectiveness, and adoption in primary care has been negligible.
On the other hand, in the case of buprenorphine, a multipronged approach is taking place in which guidelines are being developed by the federal government, providers are being certified through professional societies to treat patients in office settings, it is being incorporated on formularies, and patient education materials are being developed. The importance of linkages between primary care and related support services is being addressed, although it presents a continuing challenge. How this pharmacotherapy is addressed in primary care, and how this innovation may affect the treatment of substance abuse disorders, will be important to document.
Education directed toward providers must be complemented by efforts to educate the public regarding both the chronic disease nature of addiction disorders and the importance of screening and treatment. With regard to immunotherapies and depot medications that may be available for prophylaxis, particular problems may arise regarding appropriate use and public perceptions surrounding this approach to management.
Additionally, insurance and financing are necessary components of successful adoption of any therapy into practice. It is essential to understand the structure of the market for immunotherapies and depot medications, so that manufacturers’ efforts to promote these medications can be balanced by objective information from other sources. It is important to note that financing for substance abuse treatments occurs through various avenues in the public and private sectors. While inclusion on insurers’ formularies is important for the private sector, funding through public programs at the federal and state levels is essential after a medication becomes available.
Finally, managing the use of immunotherapies and depot medications will require strong linkages between primary care and a spectrum of services. As noted, an important approach to promote is the chronic care model, which incorporates both medical and psychosocial treatments. As this type of care is still implemented on only a limited basis, demonstrations and evaluations of such care models will be essential to identify the most effective implementation approaches for various populations.
In conclusion, immunotherapies and depot medications have great potential to improve access to treatment for alcohol and drug dependence. Before the medications can be used most effectively, however, policy makers and practitioners must prepare the field. Strategies to improve linkages with primary care, to train primary care practitioners, and to educate drug abuse treatment programs are essential to the long-term adoption of these emerging technologies.
REFERENCES
Abrahamson, E. (1991). Managerial fads and fashions: The diffusion and rejection of innovation. Academy of Management Review, 16, 586-612.
Addiction Technology Transfer Centers. (2000). The change book: A blueprint for technology transfer. Kansas City, MO: Addiction Technology Transfer Center National Office.
Alexander, J.A., Lichtenstein, R., D’Aunno, T., McCormick, R., Muramatsu, N., and Ullman, E. (1997). Determinants of mental health providers’ expectations of patients’ improvements. Psychiatric Services, 48(5), 671-677.
Altman, S.A., and Thomas, C.P. (2002). Controlling spending for prescription drugs [Editorial]. New England Journal of Medicine, 346(11), 855-856.
Backer, T. E. (1991). Drug abuse technology transfer. Rockville, MD: National Institute on Drug Abuse.
Backer, T.E. (1995). Assessing and enhancing readiness for change: Implications for behavior change. In T.E. Backer, S.L. David, and G. Soucy (Eds.), Reviewing the behavioral science knowledge base on technology transfer. Rockville, MD: National Institute on Drug Abuse.
Backer, T.E., and David, S.L. (1995). Synthesis of behavioral science learnings about technology transfer. In T.E. Backer, S.L. David, and G. Soucy (Eds.), Reviewing the behavioral science knowledge base on technology transfer (pp. 262-279). Rockville, MD: National Institute on Drug Abuse.
Backer, T.E., Rogers, E.M., and Sopory, P. (1992). Designing health communication campaigns: What works? Thousand Oaks, CA: Sage.
Banta, H.D., and Luce, B.R. (1993). Health care technology and its assessment. Oxford, England: Oxford University Press.
Becker, M.H. (1970). Sociometric location and innovativeness: Reformulation and extension of the diffusion model. American Sociological Review, 35, 262-282.
Bodenheimer, T., Wagner, E.H., and Grumbach, K. (2002a). Improving primary care for patients with chronic illness. Journal of the American Medical Association, 288(14), 1775-1779.
Bodenheimer, T., Wagner, E.H., and Grumbach, K. (2002b). Improving primary care for patients with chronic illness: The chronic care model, part 2. Journal of the American Medical Association, 288(15), 1909-1914.
Brown, B.S. (1987). Networking between research and service delivery. International Journal of the Addictions, 22(4), 301-317.
Brown, B.S. (1995). Reducing impediments to technology transfer in drug abuse programming. In T.E. Backer, S.L. David, and G. Soucy (Eds.), Reviewing the behavioral science knowledge base on technology transfer. Rockville, MD: National Institute on Drug Abuse.
Brown, B.S. (1997). Staffing pattern and services for the war on drugs. In J.A. Egertson, D.M. Fox, and A.I. Leshner (Eds.), Treating drug abusers effectively (pp. 99-124). Malden, MA: Blackwell Publishers and the Milbank Memorial Fund.
Brown, B.S. (1998). Making a difference: Is journal publication enough? Journal of Substance Abuse Treatment, 15(2), 87-88.
Brown, B.S. (2000). From research to practice: The bridge is out and the water’s rising. Advances in Medical Sociology, 7, 345-365.
Brown, B.S., and Flynn, P.M. (2002). The federal role in drug abuse technology transfer: A history and perspective. Journal of Substance Abuse Treatment, 22(4), 245-257.
Brown, R.L., Saunders, L.A., Castelaz, C.A., and Papasouliotis, O. (1997). Physicians’ decisions to prescribe benzodiazepines for nervousness and insomnia. Journal of General Internal Medicine, 12(1), 44-52.
Cherpitel, C.J. (1996). Drinking patterns and problems and drinking in the event: An analysis of injury by cause among casualty patients. Alcohol: Clinical and Experimental Research, 20(6), 1130-1137.
Chilingerian, J.A., and Glavin, M.P. (1994). Temporary firms in community hospitals: Elements of a managerial theory of clinical efficiency. Medical Care Review, 51(3), 289-335.
Coffey, R.M., Mark, T., King, E., Harwood, H., McKusick, D., Genuardi, J., Dilonardo, J., and Chalk, M. (2001). National estimates of expenditures for substance abuse treatment, 1997. Rockville, MD: Center for Substance Abuse Treatment.
Coleman, J.S., Katz, E., and Menzel, H. (1966). Medical innovation: A diffusion study. Indianapolis, IN: Bobbs-Merrill.
Counte, M.A., and Kimberly, J.R. (1974). Organizational innovation in a professionally dominated system: Responses of physicians to a new program in medical education. Journal of Health and Social Behavior, 15(3), 188-198.
Cromwell, J., Bartosch, W.J., Fiore, M.C., Hasselblad, V., and Baker, T. (1997). Cost-effectiveness of the clinical practice recommendations in the AHCPR guideline for smoking cessation. Journal of the American Medical Association, 278(21), 1759-1766.
Cornuz, J., Ghali, W.A., DiCarlantonio, D., Pecoud, A., and Paccaud, F. (2000). Physicians’ attitudes toward prevention: Importance of intervention-specific barriers and physicians’ health habits. Family Practice, 17(6), 535-540.
D’Aunno, T., Vaughn, M., and McElroy, P. (1999). An institutional analysis of HIV prevention efforts by the nation’s outpatient drug abuse treatment units. Journal of Health and Social Behavior, 40(2), 175-192.
D’Aunno, T.A., and Vaughn, T.E. (1992). Variations in methadone treatment practices: Results from a national study. Journal of the American Medical Association, 267(2), 253-258.
Denig, P., Haaijer-Ruskamp, F.M., and Zijsling, D.H. (1988). How physicians choose drugs. Social Science and Medicine, 27(12), 1381-1386.
DiMaggio, P.J., and Powell, W.W. (1988). The iron cage revisited: Institutional isomorphism and collective rationality in organizational fields. American Sociological Review, 48, 147-160.
Dybwad, T.B., Kjolsrod, L., Eskerud, J., and Laerum, E. (1997). Why are some doctors high-prescribers of benzodiazepines and minor opiates? Family Practice, 14(5), 361-368.
Eisenberg, J.M. (1993). Economics. Journal of the American Medical Association, 270(2), 198-200.
Escarce, J.J., Bloom, B.S., Hillman, A.L., Shea, J.A., and Schwartz, J.S. (1995). Diffusion of laparoscopic cholecystectomy among general surgeons in the United States. Medical Care, 33(3), 256-271.
Fendrick, M.A., and Schwartz, J.S. (1994). Physicians’ decisions regarding the acquisition of new technology. In A.C. Gelijns and H. Dawkins (Eds.), Adopting new medical technology (pp. 71-84). Committee on Technological Innovation in Medicine, Institute of Medicine. Washington, DC: National Academy Press.
Fiellin, D.A., and O’Connor, P.G. (2002). Office-based treatment of opioid-dependent patients. New England Journal of Medicine, 347(11), 817-823.
Fiellin, D.A., O’Connor, P.G., Chawarski, M., Pakes, J.P., Pantalon, M.V., and Schottenfeld, R.S. (2001). Methadone maintenance in primary care: A randomized controlled trial. Journal of the American Medical Association, 286(14), 1724-1731.
Fiore, M.C. (2000). U.S. Public Health Service clinical practice guidelines: Treating tobacco use and dependence. Respiratory Care, 45(10), 1200-1262.
Fiore, M.C., Bailey, W.C., Cohen, S.J., Dorfman, S.F., Goldstein, M.G., Gritz, E.R., Heyman, R.B., Jaen, C.R., Kottke, T.E., Lando, H.A., Mecklenburg, R.E., Mullen, P.D., Nett, L.M., Robinson, L., Stitzer, M.L., Tommasello, A.C., Villejo, L., and Wewers, M.E. (2000). Treating tobacco use and dependence: Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services.
Fleming, M.F., Barry, K.L., Manwell, L.B., Johnson, K., and London, R. (1997). Brief physician advice for problem alcohol drinkers: A randomized controlled trial in community-based primary care practices. Journal of the American Medical Association, 277(13), 1039-1045.
Fortney, J., and Booth, B.M. (2001). Access to substance abuse services in rural areas. In M. Galanter (Ed.), Recent developments in alcoholism, Vol. 15, Services research in the era of managed care (pp.177-208). New York: Plenum Press.
Freiman, M.P. (1985). The rate of adoption of new procedures among physicians: The impact of specialty and practice characteristics. Medical Care, 23(8), 939-945.
Friedmann, P.D., Alexander, J., and D’Aunno, T. (1999). Organizational correlates of access to primary care and mental health services in drug abuse treatment units. Journal of Substance Abuse Treatment, 16(1), 71-80.
Friedmann, P.D., Alexander, J.A., Jin, L., and D’Aunno, T.A. (1999). On-site primary care and mental health services in outpatient drug abuse treatment units. Journal of Behavioral Health Services and Research, 26(1), 80-94.
Friedmann, P.D., McCullough, D., Chin, M.H., and Saitz, R. (2000). Screening and intervention for alcohol problems: A national survey of primary care physicians and psychiatrists. Journal of General Internal Medicine, 15(2), 84-91.
Friedmann, P.D., McCullough, D., and Saitz, R. (2001). Screening and intervention for illicit drug abuse: A national survey of primary care physicians and psychiatrists. Archives of Internal Medicine, 161(2), 248-251.
Gelijns, A., and Rosenberg, N. (1994). The dynamics of technological change in medicine. Health Affairs, 13(3), 28-46.
Gottlieb, N.H., Guo, J.L., Blozis, S.A., and Huang, P.P. (2001). Individual and contextual factors related to family practice residents’ assessment and counseling for tobacco cessation. Journal of the American Board of Family Practice, 14(5), 343-351.
Greer, A.L. (1988). The state of the art versus the state of the science: The diffusion of new medical technologies into practice. International Journal of Technology Assessment in Health Care, 4(1), 5-26.
Hemminki, E. (1975). Review of literature on factors affecting drug prescribing. Social Science and Medicine, 9(2), 111-116.
Hilton, M.E. (2001). Researcher in residence program: Experiences from New York state. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism.
Hodgkin, D., and McGuire, T. (1994). Payment levels and hospital response to prospective payment. Journal of Health Economics, 13(1), 1-29.
Institute of Medicine. (1990a). Broadening the base of treatment for alcohol problems. Committee on Treatment of Alcohol Problems. Washington, DC: National Academy Press.
Institute of Medicine. (1990b). Treating drug problems, volume 1. Committee for the Substance Abuse Coverage Study. D.R. Gerstein and H.J. Harwood (Eds.). Washington, DC: National Academy Press.
Institute of Medicine. (1997). Managing managed care: Quality improvement in behavioral health. Committee on Quality Assurance and Accreditation Guidelines for Managed Behavioral Health Care. M. Edmunds, R. Frank, M. Hogan, D. McCarty, R. Robinson-Beale, and C. Weisner (Eds.). Washington, DC: National Academy Press.
Institute of Medicine. (1998). Bridging the gap between practice and research: Forging partnerships with community-based drug and alcohol treatment. Committee on Community-Based Drug Treatment. S. Lamb, M.R. Greenlick, and D. McCarty (Eds.). Washington, DC: National Academy Press.
Jaen, C.R., McIlvain, H.E., Pol, L., Phillps, R.L., Flocke, S., and Crabtree, B.F. (2001). Tailoring tobacco counseling to the competing demands in the clinical encounter. Journal of Family Practice, 50(10), 859-863.
Johnson, T.P., Warnecke, R.B., and Aitken, M.J. (1996). Changing practice patterns. In A.D. Kaluzny and R.B. Warnecke (Eds.), Managing a health care alliance: Improving community cancer care (pp. 105-128). San Francisco: Jossey-Bass.
Kaluzny, A.D., Morrissey, J.P., and McKinney, M.M. (1990). Emerging organizational networks: The case of the Community Clinical Oncology Program. In S.S. Mick (Ed.), Innovations in health care delivery. San Francisco: Jossey-Bass.
Kimberly, J.R., and Evanisko, M.J. (1981). Organizational innovations: The influence of individual, organizational, and contextual factors on hospital adoption of technological and administrative innovations. Academy of Management Journal, 24(4), 689-713.
Lambert, B.L., Salmon, W.J., Stubbings, J., Gilomen-Study, G., Valuck, R.J., and Kezlarian, K. (1997). Factors associated with antibiotic prescribing in a managed care setting: An exploratory investigation. Social Science and Medicine, 45(12), 1767-1779.
Legal Action Center. (1991). Confidentiality: A guide to the federal laws and regulations. New York: Author.
Lopez, F. (1994). Confidentiality of patient records for alcohol and other drug treatment. Rockville, MD: Center for Substance Abuse Treatment.
Mark, T.L., Coffey, R.M., King, E., Harwood, H., McKusick, D., Genuardi, J., Dilonardo, J., and Buck, J.A. (2000). Spending on mental health and substance abuse treatment, 1987-1997. Health Affairs, 19(4), 108-120.
McIlvain, H.E., Backer, E.L., Crabtree, B.F., and Lacy, N. (2002). Physician attitudes and the use of office-based activities for tobacco control. Family Medicine, 34(2), 114-119.
McLellan, A.T., Lewis, D.C., O’Brien, C.P., and Kleber, H.D. (2000). Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. Journal of the American Medical Association, 284(13), 1689-1695.
Merrill, J.O. (2002). Policy progress for physician treatment of opiate addiction. Journal of General Internal Medicine, 17(5), 361-368.
Merrill, J.O., Rhodes, L.A., Deyo, R.A., Marlatt, G.A., and Bradley, K.A. (2002). Mutual mistrust in the medical care of drug users: The keys to the “narc” cabinet. Journal of General Internal Medicine, 17(5), 327-333.
Meyer, A.D., and Goes, J.B. (1988). Organizational assimilation of innovations: A multilevel contextual analysis. Academy of Management Journal, 31, 897-923.
Moatti, J.P., Souville, M., Escaffre, N., and Obadia, Y. (1998). French general practitioners’ attitudes toward maintenance drug abuse treatment with buprenoprhine. Addiction, 93(10), 1567-1575.
Moch, M.K., and Morse, E.V. (1977). Size, centralization and organizational adoption of innovation. American Sociological Review, 42, 716-725.
Morgenstern, J. (2000). Effective technology transfer in alcoholism treatment. Substance Use and Misuse, 35(12-14), 1659-1678.
Naranjo, C.A., and Bremner, K.E. (1996). Dissemination of research results regarding the pharmacotherapy of substance abuse: Case examples and critical review. Substance Abuse, 17, 39-50.
National Institute of Health Care Management. (2002). Prescription drug expenditures in 2000: Another year of escalating costs. Available: www.nihcm.org/spending2001.pdf [December 24, 2003].
Nohria, N., and Gulati, R. (1995). Is slack good or bad for innovation? Academy of Management Journal, 39, 716-725.
O’Connor, P.G., and Samet, J.M. (2002). Substance abuse: The expanding role of general internal medicine. Journal of General Internal Medicine, 17(5), 398-399.
O’Connor, P.G., Oliveto, A.H., Shi, J.M., Triffleman, E.G., Carroll, K.M., Kosten, T.R., Rounsaville, B.J., Pakes, J.A., and Schottenfeld, R.S. (1998). A randomized trial of buprenorphine maintenance for heroin dependence in a primary care clinic for substance users versus a methadone clinic. American Journal of Medicine, 105(2), 100-105.
Ockene, J.K., Adams, A., Hurley, T.G., Wheeler, E.V., and Hebert, J.R. (1999). Brief physician- and nurse practitioner-delivered counseling for high-risk drinkers: Does it work? Archives of Internal Medicine, 159(18), 2198-2205.
Office of Technology Assessment. (1994). Identifying health technologies that work: Searching for evidence. Washington, DC: U.S. Congress, Office of Technology Assessment.
Owen, P. (2002). The pros and cons of addiction medications. Hazelden Voice, 7, 3-12.
Posner, K.L., Gild, W.M., and Winans, E.V. (1995). Changes in clinical practice in response to reductions in reimbursement. Medical Anthropology Quarterly, 9(4), 476-492.
Prochaska, J.O., and DiClemente, C.C. (1983). Stages and processes of self-change in smoking: Towards an integrative model of change. Journal of Consulting and Clinical Psychology, 51(3), 390-395.
Prochaska, J.O., Velicer, W.F., Rossi, J.S., Goldstein, M.G., Marcus, B.H., Rakowski, W., Fiore, C., Harlow, L.L., Redding, C.A., Rosenbloom, D., and Rossi, S.R. (1994). Stages of change and decisional balance for 12 problem behaviors. Health Psychology, 13(1), 39-46.
Rogers, E.M. (1995). Diffusion of innovations, 4th ed. New York: The Free Press.
Roman, P.M., and Johnson, J.A. (2002). Adoption and implementation of new technologies in substance abuse treatment. Journal of Substance Abuse Treatment, 22(4), 211-218.
Romeo, A.A., Wagner, J.L., and Lee, R.H. (1984). Prospective reimbursement and the diffusion of new technologies in hospitals. Journal of Health Economics, 3(1), 1-24.
Saitz, R., Sullivan, L.M., and Samet, J. (2000). Training community-based clinicians in screening and brief intervention for substance abuse problems. Substance Abuse, 21(1), 21-31.
Saitz, R., Horton, N., Sullivan, L., Moskowitz, M., and Samet, J. (2003). Addressing alcohol problems in primary care: A cluster randomized controlled trial of a systems intervention. Annals of Internal Medicine, 138(5), 372-382.
Samet, J.M., Friedmann, P.D., and Saitz, R. (2001). Benefits of linking primary medical care and substance abuse services. Archives of Internal Medicine, 161(1), 85-91.
Scott, W.R. (1993). The organization of medical care services: Toward an integrated theoretical model. Medical Care Review, 50(3), 271-303.
Simpson, D.D. (2002). A conceptual framework for transferring research to practice. Journal of Substance Abuse Treatment, 22(4), 171-182.
Sisk, J.E., and Glied, S.A. (1994). Innovation under federal health care reform. Health Affairs, 13(3), 82-97.
Sorensen, J.L., Hall, S.M., Loeb, P., Allen, T., Glaser, E.M., and Greenberg, P.D. (1988). Dissemination of a job seekers’ workshop to drug treatment programs. Behavior Therapy, 19, 143-155.
Spangler, J.G., Goerge, G., Foley, K.L., and Cradell, S.K. (2002). Tobacco intervention training: Current efforts and gaps in U.S. medical schools. Journal of the American Medical Association, 288(9), 1102-1109.
Stein, M.D., and Friedmann, P.D. (2001). Generalist physicians and addiction care: From turfing to sharing the turf. Journal of the American Medical Association, 286(14), 1764-1765.
Stone, T.T., Longo, D.R., Phillps, R.L., Hewett, E., and Riley, S.L. (2002). Health care system and insurer support for smoking cessation guideline implementation. Journal of Health Care Finance, 29(2), 78-86.
Strang, D., and Soule, S.A. (1998). Diffusion in organizations and social movements: From hybrid corn to poison pills. Annual Review of Sociology, 24, 265-290.
Substance Abuse and Mental Health Services Administration. (2002). National Survey of Substance Abuse Treatment Services (N-SSATS): 2000. Data on substance abuse treatment facilities. Rockville, MD: Author.
Teplensky, J.D., Pauly, M.V., Kimberly, J.R., Hillman, A.L., and Schwartz, J.S. (1995). Hospital adoption of medical technology: An empirical test of alternative models. Health Services Research, 30(3), 437-465.
Teplin, L.A., Abram, K.M., and Michaels, S.K. (1989). Blood alcohol level among emergency room patients: A multivariate analysis. Journal of Studies on Alcohol, 50(5), 441-447.
Thirion, X., Lapierre, V., Micallef, J., Ronfle, E., Masut, A., Pradel, V., Coudert, C., Mabriez, J.C., and Sanmarco, J.L. (2002). Buprenorphine presription by general practitioners in a French region. Drug and Alcohol Dependence, 65(2), 197-204.
Thomas, C.P. (2000). No magic bullet: Adoption of naltrexone by clinical providers. Dissertation, Brandeis University.
Thomas, C.P., Wallack, S., Lee, S.S., McCarty, D., and Swift, R. (2003). Research to practice: Factors affecting the adoption of naltrexone in alcoholism treatment. Journal of Substance Abuse Treatment, 24(1), 1-11.
Tolbert, P.S., and Zucker, L.G. (1983). Institutional sources of change in the formal structure of organizations: The diffusion of civil service reform. Administrative Science Quarterly, 28, 22-39.
Turk, D.C., and Okifuji, A. (1997). What factors affect physicians’ decisions to prescribe opioids for chronic noncancer pain patients? The Clinical Journal of Pain, 13(4), 330-336.
U.S. Public Health Service. (2000). Treating tobacco use and dependence—A systems approach. A guide for health care administrators, insurers, managed care organizations, and purchasers. Available: http://www.surgeongeneral.gov/tobacco/systems.htm [December 24, 2003].
U.S. Veterans Administration. (1999a). Relative cost effectiveness of tobacco use cessation pharmacotherapies. Pharmacoeconomic Center Update, 8.
U.S. Veterans Administration. (1999b). Tobacco use cessation in the primary care setting. Pharmacoeconomic Center Update, 1.
Van den Bulte, C., and Lilien, G.L. (2001). Medical innovation revisited: Social contagion versus marketing effort. American Journal of Sociology, 106, 1409-1435.
Vaughan, T.E., Ward, M.M., Doebbing, B.N., Uden-Holman, T., Clarke, W.T., and Woolson, R.F. (2002). Organizational and provider characteristics fostering smoking cessation practice guideline adherence: An empirical look. Journal of Ambulatory Care Management, 25(2), 17-31.
Vignau, J., Duhamel, A., Catteau, J., Legal, G., Pho, A.H., Grailles, I., Beauvillain, J., Petit, P., Beauvillain, P., and Parquet, P.J. (2001). Practice-based buprenorphine maintenance treatment (BMT): How do French healthcare providers manage the opiate-addicted patients? Journal of Substance Abuse Treatment, 21(3), 135-144.
Weisner, C., Mertens, J., Parthasarathy, S., Moore, C., and Lu, Y. (2001). Integrating primary medical care with addiction treatment: A randomized controlled trial. Journal of the American Medical Association, 286(14), 1715-1723.
Wennberg, J. (1988). Improving the medical decision-making process. Health Affairs, 7(1), 99-106.
Westphal, J.D., Gulati, R., and Shortell, S.M. (1997). Customization or conformity? An institutional and network perspective on the content and consequences of TQM adoption. Administrative Science Quarterly, 42, 366-394.