G
Costs and Benefits of Immunotherapies or Depot Medications for the Treatment of Drug Abuse
Mark A.R. Kleiman
University of California, Los Angeles
SUMMARY
Two related but distinct cost-benefit questions could be asked about a proposed immunotherapy or depot medication designed to prevent a given drug of abuse from crossing the blood-brain barrier. One is whether the application of such a treatment technique to some particular patient or class of patients would be cost-justified, once it had been developed, approved, and marketed. For a treatment with high efficacy and acceptable side effects, answering that question will turn out to be trivially easy as applied to patients with severe and chronic substance abuse disorders because the benefits per application will be very large multiples of the marginal cost of production and administration.1
An efficacious immunotherapy or depot medication administered to a chronic heavy user of a low-recovery-rate drug (such as tobacco, heroin, alcohol, or cocaine) might easily cut years from the otherwise expected length of that patient’s active addiction career. A very rough calculation (given below under “Example: Cigarette Smoking”) suggests that the excess of costs over benefits for a month of active heavy cigarette smoking is on the order of $500. The comparable figures for active cocaine or heroin use might exceed that by an order of magnitude. Thus the expected gross
benefits of administering an effective antismoking treatment to a long-term smoker would be in the range of thousands of dollars per patient. The amount would be substantially higher for a chronic alcoholic and higher still—in the tens of thousands of dollars—for someone addicted to heroin or cocaine.
It is hard to imagine that the financial costs of making an immunotherapy agent, administering it to a patient, and doing the necessary follow-up could even approach such levels. Current estimates are that the treatments will cost on the order of a thousand dollars per administration and that each administration will be efficacious for a few months. So if a highly efficacious, low-side-effect immunotherapy were developed for any of the major drugs of abuse, its application to anyone with an established chronic problem with that drug would almost certainly be cost-justified.
If the efficacy were only partial, if side effects were substantial, or if substitution of other drugs turned out to be a major problem, the calculation would become more challenging. An immunotherapy that prevented three-quarters of an abusable drug from getting to the brain might have much less than three-quarters of the benefits of a completely effective immunotherapy, or it might have virtually the same benefits, depending on behavioral responses that as yet can only be guessed at. (Partial interception would be equivalent in some ways to a price increase, and the behavioral response would reflect an analog of the price elasticity of demand. The more elastic [sensitive] consumption of a drug is to its price, the greater the benefit of a partially effective immunotherapy.)
Use in patients with less chronic conditions, or prophylactically in those without established drug problems but engaged in drug-taking patterns that threaten to escalate, would be less beneficial per case but might still be cost-justified in some instances (National Research Council, 2001).
The second kind of cost-benefit question that might be raised involves expenditures on the development of such therapies. That development analysis uses the patient-by-patient analysis as its starting point, but the relevant part of the patient-by-patient analysis is not the part that deals with the interesting close questions such as the possibility of prophylactic use or use in cases of a relatively mild abuse disorder or a disorder not yet shown to be chronic. Instead it is the benefits in the cases that are most obvious in the patient-by-patient analysis—patients with severe, chronic disorders—that need to be summed and then measured against the costs of a development effort and its probability of success. This appendix will pass over the questionable cases to concentrate on the clear ones. (It would be somewhat perverse to oppose the development of a medication on the grounds that, if developed, it might then be used badly in some instances, though far from perverse to try to anticipate and forestall such usages.)
In considering whether to attempt to develop an immunotherapy or depot medication, the relevant comparison is between, on the one hand, the aggregate amount by which the benefits of use would exceed the costs, summing over total applications and, on the other hand, the development costs, appropriately adjusted both for the risks of failure—failure to develop a safe and efficacious medication, failure to secure regulatory approval, failure of adoption by providers and patients—and for the time value of money (Hubbard and French, 1991).
In addition to the benefits that accrue to patients who use the new therapy in place of other treatments, there would be another, potentially much larger, flow of benefits from patients attracted to try desistance from heavy use by the availability of a treatment that might be less effortful as well as more likely to succeed.
Against those benefits must be set the costs, including the opportunity cost of the treatment dollars that would pay for administration of a new therapy. But that sort of opportunity-cost analysis implicitly assumes that the overall level of funding is invariant to the range of therapies available, and that assumption may not be valid in this case. There are reasons to expect that an effective immunotherapy or depot medication might turn out to have characteristics more appealing to those who make decisions about drug treatment than its current competitors. The most demonstrably effective drug treatments in use today are the opiate substitution therapies, which are highly acceptable to many, though far from all, persons suffering from opiate dependency but which remain controversial politically because they do not promise a “cure” for the underlying addiction. Other treatments, while no one doubts their utility for some patients, face lower success rates and more resistance among potential clients, as reflected in both reluctance to enter treatment and high rates of dropout and treatment recidivism. These facts constitute part of the political background against which funding decisions are made and also of the professional background against which medical providers make treatment decisions, insurers make coverage decisions, and medical schools and other educators of health care professionals design curricula. It is not at all far-fetched to imagine the development of effective immunotherapies as a catalyst for changes in attitudes that would lead to changes in funding.
The sheer magnitude of the social costs of substance abuse means that even development programs with modest probabilities of success will be cost-justified. A treatment for smoking that had net benefits per patient measured in thousands of dollars, and a potential patient base measured in tens of millions, would have development benefits that might rise into the tens of billions. The potential patient base for treatment of cocaine addiction is more than an order of magnitude smaller, but the potential gains per patient are in the range of an order of magnitude greater, sug-
gesting comparable potential for aggregate social gain (see Office of National Drug Control Policy, 2001).
That suggests that a $50 million development effort with a 1 percent chance of a “home run” success against cocaine or nicotine would easily be worth the investment. In practice, development efforts are not decided on all at once. Funding is allocated sequentially, with several opportunities to put a losing project out of its misery. (Formally, this could be modeled using decision analysis or dynamic programming; practically, the gains in understanding from doing so now would be modest at best.) In addition, it suggests that pursuing more than one approach per drug might be justified, both because that would increase the probability of developing at least one successful therapy and because the marginal benefit of having more than one therapy available for a given drug of abuse might still be very substantial, if different therapies turn out to appeal to only partially overlapping populations of potential treatment clients.
In the case of alcohol the benefits would be greater still, perhaps not great enough to justify making substantial investments now in the face of apparently discouraging technical facts, but great enough to justify some continued basic studies. The social damage from heroin is currently probably comparable to that from cocaine, especially considering its role in the spread of infectious disease, but the existence of a set of efficacious substitution pharmacotherapies somewhat lowers the potential benefits of developing a new treatment, and the wide variety of closely substitutable opiates and opioids would tend to reduce the value of an immunotherapy targeted at only a single molecule. The social gain from developing a treatment for methamphetamine (high damage per month but a small and largely transient population of heavy users) and cannabis (more problem users at any one time but lower damage per month and moderate chronicity under current conditions) would be smaller than the others but still in the billions.
It could reasonably be suggested that the data on which to perform such calculations with anything approaching precision do not exist. The cost of developing a therapy, its costs in use, its efficacy in a technical sense (what proportion of the population would derive benefit from it, the proportion of the abusable drug the new therapy would trap before it reached the brain), its clinical utility (depending on the drug-taking behavior of actual patient populations, which may be different from the reactions of participants in clinical trials, in the face of an imperfect barrier between drug-taking and enjoying the desired psychological effects of the drug), the effect of immunization against the effect of one drug on consumption of other drugs, the side effects profile of the new medication, its acceptability among different categories of potential clients, difficulties in achieving regulatory approval, and adoption by treatment
providers are all matters of speculation. Moreover, the probability of success is not a single number. Any actual research program might produce a range of results from a “home run” to a medication capable of gaining regulatory approval but of only marginal clinical utility. Even those factors in the calculation that relate to current rather than hypothetical facts—the number of persons suffering from a severe and chronic substance abuse disorder for any given drug, the rate of turnover in that population, and the cost (to the affected individual, to his or her intimates, to other individuals such as potential crime victims, and to the budgets and functioning of institutions such as police and health care providers) associated with active abuse that would be averted by successful treatment—are not nearly as well measured as they ought to be (National Research Council, 2001).
To undertake a formal sensitivity analysis around such poorly grounded calculations would itself suggest more certainty than the data will actually support. But simple critical value calculations are enough to support the idea that, if development seems technically plausible, the risk of funds is likely to be thoroughly cost-justified. As long as the probability of a highly successful development is at least a few percent, elaborate calculations are probably superfluous. Moreover, the extremely discouraging histories of pharmacotherapies for substance abuse other than the opiate maintenance agents give some reassurance that the opportunity cost of funds taken from other parts of the National Institute of Drug Abuse’s medication development effort to support work on immunotherapies and depot medications is unlikely to be very high (see, for example, Tai, Chiang, and Bridge, 1997).
As is always the case in thinking about the social benefits to be derived from pharmaceutical development, the mechanics of pricing create a potential problem. Pricing near marginal cost will not recoup the investment in development efforts; pricing designed to recoup that investment will inefficiently squeeze some patients out of the market.
The fact that patent protection permits pricing well above marginal cost, in principle, ought to be ignored in a full cost-benefit analysis of the decision to administer a drug; the producer’s surplus from supra-marginal-cost pricing is a mere transfer from whoever pays for the treatment to whoever holds the patent. From a cost-benefit perspective, the relevant comparison is between the marginal social cost of producing, distributing, and administering an additional unit of the medication and the benefit that could be derived from that treatment, over and above the benefits, minus the costs, of whatever treatment is the next-best. Of course, if high price will lead to low utilization, that reduction in volume is a fact about the world that ought to be incorporated into the analysis of the development decision.
If the proposed therapies came to represent anything approaching a reliable “cure” for drug addiction, the possibility exists that introducing them will have unwanted effects on the rates of initiation to the drugs whose abuse syndromes they treat. That issue presents both conceptual and empirical challenges that probably put it outside the reach of any numerical cost-benefit analysis. Those risks lurk in the background of any decision about development. Depending on the extent of the effect and the long-term harm from nonchronic bouts of substance abuse, the losses on the prevention side might (or might not) substantially cut into the benefits on the treatment side; it is conceivable that the prevention losses might even exceed the treatment benefits.2
Whether and how to consider such risks in deciding on the development of treatments for a life-threatening group of diseases pose tricky problems in bioethics. It might plausibly be argued, as it has in the partially analogous case of medication development for HIV/AIDS,3 that it would be wrong to deny treatment to those currently suffering from some disorder out of concern that treating them might, through one mechanism or another, increase the rate of incidence of that disorder. Fortunately for the author, those issues are beyond the scope of this appendix.
A CONCEPTUAL MODEL
Assume the introduction of a new treatment, T, for abuse and dependency related to drug D. In particular, let T be a depot medication or immunotherapy designed to reduce or eliminate, for a period of months, the bioavailability of D to a patient given T.
The relevant direct costs are the costs of T itself, the effort required to induce clients to accept it, and the ancillary treatment required to make it effective, plus whatever negative value is assigned to the side effects. Insofar as T competes for resources or clients with other forms of drug treatment, the benefits of whatever other treatment is foregone are an opportunity cost of T, and the costs associated with those foregone treatment episodes are a benefit of T. Thus it will matter greatly whether the clients treated with T would otherwise have pursued other forms of treatment.
Treatment cost is also influenced by the extent of treatment recidivism (a somewhat unfortunate but now established term for repeated rounds of treatment and relapse [see, e.g., McKay et al., 1996]). A treatment that is expensive per treatment episode but has a high rate of long-
term success may be less costly in the long run than one that is cheaper per episode but that generates multiple episodes. Whether to treat these savings as adjustments to the cost side of the calculation or to include them as benefits is partly an arbitrary choice of analytic conventions, but the choice ought to depend in part on the impacts of various sorts of savings on the treatment system. Nothing guarantees that the opportunity cost of a treatment dollar expended or saved will be exactly or even nearly $1. It might be much more than $1 if existing treatment is highly cost- beneficial and resource-constrained and less than $1 for ineffective treatments.
In some cases the alternative to T will not be some other form of substance abuse therapy but rather jail or prison. That situation requires a different analysis; the resource savings if T is used instead of incarceration are likely to be large, but those savings may not accrue in a way that makes it possible to recycle them into other treatment efforts.
The benefit picture is much more complicated, and estimating it numerically will require constructing a number of counterfactual hypotheticals concerning what would have happened had T not been available or not been used. One place to start is with a single representative individual, A, at risk of a drug abuse disorder involving D, in a world without T. Moore (1990) has described a quasi-Markov process4 that provides a basis for estimating the damage done to and by A as a result of D (Figure G-1).
Starting as a nonuser of D, in each period (say, arbitrarily, each month) A has some probability of starting to use D. Assume that all initiations are, in the first instance, to occasional, casual, or use not meeting diagnostic criteria for abuse or dependency. Still, A might suffer and/or impose on others, on a probabilistic basis, some monthly flow of harm (net of whatever benefit A receives from use of D).
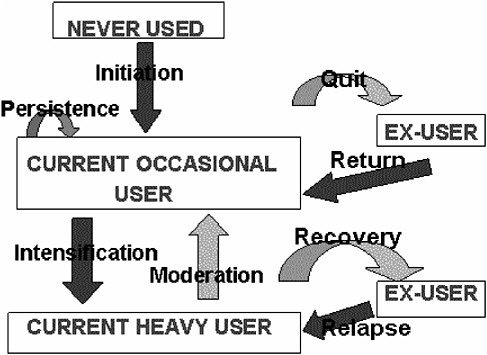
FIGURE G-1 Drug taking as a system of states and transition probabilities.
In every month in which A uses D on a casual basis, A has some probability of desisting from use and some probability of intensifying to heavy or problematic use amounting to diagnosable substance abuse disorder. (Obviously, this treats as a set of discrete states what in fact is a continuum; a more adequate model would have to be more complex. But for purposes of exposition this simplified model displays most of the relevant features of the situation.)
If A desists from using D, A faces some monthly risk of resuming use. If A progresses to heavy use, the monthly flow of harms increases compared to continuing casual use. A then has monthly probabilities of moderating his or her use—going back to being a casual user—or quitting altogether and/or going into recovery. (Ex-casual users and ex-heavy users may continue to suffer harm due to their past use, but for these purposes it is better to attribute damage on an “accrual” rather than a “cash” basis, charging each month with the future as well as current consequences of that month’s use.)
Thus we have identified, in the abstract, a small number of rates that, among them, determine total expected harm to A due to drug D: the initiation rate, the quit and intensification rates from casual use, the rate of
harm from casual use, the return rate from former occasional use, the rates of recovery and moderation from heavy use, the rate of harm from heavy use, and the relapse rate from recovery. Persistence in casual use is the reciprocal of the sum of the quit and intensification rates. The chronicity of heavy use depends on the recovery, moderation, and relapse rates. With estimates of these we could in principle solve the model for A’s expected lifetime damage from D. Moreover, we ought to be able to understand the impact of any proposed intervention in terms of its impact on initiation, persistence, return, intensification, moderation, recovery, relapse, and the two harm rates.
The sources of harm, both to the person suffering from a substance abuse disorder and to others, are multifarious and will vary from drug to drug. A partial list might include:5
Physical toxicity
Direct (to user)
Indirect (e.g., environmental tobacco smoke)
Behavioral toxicity (crimes and accidents due to intoxication)
Damage to victims
Damage to community
Damage to intoxicated person (including risks of punishment)
Psychological toxicity (and associated health care costs)
Infectious disease risks (and associated health care costs)
User’s infection risk
Risk of re-transmission
Expenditures of drugs
Costs to users
Costs to users’ family members
Support for illicit markets
Increasing supply to other current and future users of the same drug
Generating illicit-market side-effects
Violence
Disorder
Corruption
Damage to juveniles employed in illicit trade
Enforcement costs
Budget costs
Losses to dealers and their families due to incarceration
Again simplifying for concreteness, harm can be identified with the use rate itself. That will be more appropriate for cigarette use, for example, than for cocaine or heroin use, but even for the “hard drugs” total damage is likely to track, albeit imperfectly, total consumption.
In this model a treatment technology appears as something that increases the rates of recovery and moderation, decreases the relapse rate, or decreases the flow of harm from heavy use. The greater the chronicity of heavy D use in the absence of some new treatment, and the greater the harms associated with continued heavy use, the greater the potential benefit of a new treatment. The net outflow, after adjusting for relapse, among individuals with long-established tobacco or heroin problems appears to be on the order of 3 percent per year,6 though other individuals pass through the heavy-use state relatively quickly and remain out of it once they leave (see Goldstein, 2001, pp. 261-263; Trosclair et al., 2002). Recent aggregate-level data seem to suggest that heavy cocaine use, especially cocaine smoking, may create a condition of comparable chronicity.7
The fact that heavy users of a given drug are likely to be heterogeneous with respect to the length of the “addiction careers” they face (even evaluated ex ante, on an expected-value basis) will greatly complicate the task of assigning a value to any new treatment technology because the group that volunteers to be treated with it may not be a random draw from the population suffering from the substance abuse disorder to which the treatment applies.
The rate of recovery—quitting from heavy use—can be decomposed into a monthly probability, P(a), that someone with a D problem will attempt to recover in that month and another probability, P(s), that a given recovery attempt will be successful. Any treatment that influences P(s) may also influence P(a), since the risk of failure is known to be one deterrent to attempting to desist from problem drug use (Institute of Medicine, 1990).
The hypothetical new treatment, T, can change these rates in several ways. Obviously, it can increase the success probability conditional on
|
6 |
Note that, although some 70 percent of smokers express a desire to quit (a figure undoubtedly higher than comparable proportions of heavy users of heroin and cocaine), only 4.7 percent of daily smokers were able to quit for more than 3 months in any given year, according to a recent report (see Trosclair et al., 2002). Kleber has estimated that, with 40 million Americans having quit smoking cigarettes over a period of 20 years, the actual cessation rate (net of relapse) may be closer to 2 percent (http://www.nationalfamilies.org/update/dau-111001.html). Heroin addiction may be even more intractable; see Hser et al., 2001). This study showed “remarkably stable use patterns” in a cohort of heroin users over at least 11 years since a previous survey of the same group of addicts. |
|
7 |
Rydell and Everingham (1994:17-19) discuss the differences in consumption patterns between “light” and “heavy” users and a “Two-State Markovian Model” of cocaine consumption (“demand”). For further details, see Chapter 2 in Everingham and Rydell (1994). |
attempting to quit, P(s). More subtly it can also decrease the perceived costs to the sufferer from attempting to quit; reportedly, much of the unpleasantness associated with quitting is the constant struggle with temptation and the constant fear of backsliding, and patients who arrange to physically isolate themselves from any possibility of acquiring their drug of abuse appear to have a much easier time of quitting than those for whom a decision to backslide could be executed within minutes (DeLeon, 2000).
(In this regard, some empirical work could be done on opiate-dependent physicians and other health care professionals required to take narcotic antagonists daily on a “Directly Observed Medication” basis as a condition of maintaining their licenses. The reported high success rates in such attempts are often attributed to the subjects, having a great deal to lose and an unusual amount of self-discipline, but it may be the case that the temptation-reduction benefits of a daily dose of an antagonist in fact make quitting easier for this group than for other detoxified opiate-dependent individuals who do not take an antagonist.8 A vaccine or depot medication would have this advantage to an even greater degree, since there would not even be a potential daily inner struggle over whether to take the medication, attempt to fake taking it, or leave the program entirely.)
Reduced stress associated with the recovery attempt and increased probability of succeeding will tend to increase the rate at which patients undertake recovery attempts if T is present, compared to its being absent. Thus so far there are three classes of benefit from T: increased success probability, P(s), due to the efficacy of T; increased attempt probability, P(a), due to increased perceived benefits from attempting to recover; and further increased attempt probability due to decreased perceived costs (in the economist’s generalized sense of that term) of attempting to recover. (For some patients the irreversibility of T will appear as a disadvantage and a source of discouragement to attempt T, but that will not reduce P(a) compared to what it would have been, since alternative technologies, including unassisted quitting, would still be available.)
Finally, an immunotherapy or depot medication might reduce the relapse rate, especially in the early months of recovery when that risk is typically at its highest. That would seem to be among the strongest advantages of an immunotherapy or depot medication over, for example, traditional detoxification. The opportunity to extend the period of protection by readministering T would accentuate this advantage.
If we were to imagine a repeatable vaccine or depot medication that provided complete and non-dose-overridable protection, the cost to the patient of the substance abuse disorder in question would then be effectively capped at the cost of the treatment itself. If, say, four injections per year costing $1,000 each could entirely prevent a cocaine abuser from getting any psychoactive effect from cocaine, then that person could ensure against any risk of relapse (at least any relapse to cocaine) at an annual cost of $4,000. Because the decision not to use cocaine could be made only four times a year rather than having to be made again and again whenever the temptation presented itself, the risk of relapse through weakness of will would be greatly reduced, along with the stress of the struggle to maintain abstinence.
An open question—the answer to which will probably vary from treatment to treatment, drug to drug, and patient to patient—is the level of craving and the relapse probability after the immunological (or other pharmacological) effect has dissipated. While the option of readministration to extend the treatment’s active life makes this question less crucial than it would otherwise be, it remains an important one and would be more important if diminishing efficacy or accumulating side effects made long-term application unattractive.
Competing considerations make it unclear whether the post-direct efficacy relapse rates would be higher or lower for remissions secured through immunotherapies or depot medications than for remissions occurring as a result of other treatment approaches, through group self-help, or “spontaneously.” On the one hand, a period of months of abstinence with no, or reduced, cravings due to the effective unavailability of the drug of abuse might make long-term success more likely. On the other hand, if many who would have relapsed quickly under other treatment regimens succeed using T, that population may be selected to be less relapse-resistant than those who managed to abstain for a period in the face of active temptation.
Thus a depot medication or immunotherapy can reduce the average length of the combined active phases of an addiction career in three ways. It can do so directly by increasing the probability that a given quit attempt will succeed and by decreasing the relapse rate. (Call these effects “efficacy improvements.”) Efficacy improvements, especially if combined with decreased discomfort through reduced cravings, will make attempts to quit more attractive, thus increasing their number (“treatment demand” effects). If such therapies are actually more cost-effective than conventional therapies, and if the resulting cost savings are available to be recycled into the treatment effort itself, the result could be an effective expansion of the capacity of the treatment system, which might be called the “treatment supply effect.” (The importance of these two latter classes
of effects will depend in part on external conditions. The treatment supply effect will be of more importance when funded treatment slots are scarce compared to volunteers; the treatment demand effect will be more important when volunteers are scarce compared to slots.)
Efficacy, treatment demand, and treatment supply effects will all contribute to a reduction in the average number of months of heavy drug use in a typical addiction career. The benefits of such reductions will depend on the costs of addiction careers of different lengths, which costs are likely to vary with characteristics of the underlying drug, existing therapies, the client, and the context, in particular the nature and extent of pressures on clients to participate.
Obviously, highly toxic, illegal, expensive drugs with highly socially disruptive markets, high chronicity, and poor alternative treatment options offer greater potential savings per month of active heavy use avoided than drugs with the opposite characteristics. Drugs with close and comparably harmful pharmacological substitutes not affected by the proposed therapy will be less attractive candidates for treatment insofar as some users make the substitution and wind up comparably dependent on the substitute (e.g., see Fairbank, Dunteman, and Condelli, 1993).9 On the other hand, treating dependency on drugs that are frequently used in combination (e.g., cocaine with alcohol) will tend to have carry-over benefits in reducing abuse of the complementary drugs.
Examples, even with made-up numbers, may be more illuminating here than the mere exposition of principles. Tobacco and cocaine present such different pictures that they may nearly bracket the range of variation among target drugs.
EXAMPLE: CIGARETTE SMOKING
Assume an immunotherapy for nicotine of such high efficacy that 90 percent or more of patients report no subjective effect of smoking a cigarette in the 3 months following immunization. Also, assume low side effects of the therapy (apart from those of quitting itself, such as weight gain, depression, and reduced productivity).
Imagine a person now suffering from nicotine dependency in the form of cigarette smoking who expresses a desire to quit (as about 90 percent of smokers do). Each additional pack of cigarettes smoked does some amount of expected damage to his or her health, wallet, and other people (e.g., family), net of whatever value the smoker places on the pleasure,
comfort, or capacity for concentration, or relaxation provided by smoking. That net marginal cost of smoking a pack of cigarettes is presumably a declining function of cumulative packs smoked and of the smoker’s age and presumably varies with other factors as well, but for concreteness and simplicity assume that it is $10.10
Again simplifying, assume that the person, a male, smokes a little more than a pack and a half a day, or 50 packs per month. Thus his smoking generates a net loss of $500 per month. That person also has some probability, P(q), of trying to quit in any given time period (say a month); the probability certainly varies from person to person and may vary with the availability and efficacy of various treatment options as perceived by the smoker. If he tries to quit, he has some probability, P(s), of succeeding, where success means (say) going a whole month without smoking (at all or over some low threshold). The product P(q)P(s) is his monthly probability of a successful quit. Once he quits, he faces some (probably declining) monthly probability, P(r), of relapsing. From assumptions about those probabilities, his expected lifetime months of smoking could be computed. (That calculation would be complicated by the impact of his smoking on his life expectancy and by the time-value of money, but those problems can be ignored for now.)
In particular, one could calculate the reduction in expected cumulative months of smoking that will result if there is a successful quit attempt in the current month. Again for concreteness, assume that a successful quit reduces the expected cumulative lifetime periods of smoking—the length of the active addiction career—by 20 months, a fairly modest estimate given that smoking careers are typically measured in pack-years and that the median successful cigarette quitter succeeds in quitting and not relapsing on about the sixth try. That would put a value on successful quitting of $500 × 20 = $10,000.
Against this must be offset the costs of quitting, such as weight gain and psychological distress. For most smokers those effects will be tolerable, but not for all. Smoking is such a major health risk that those who treat it tend to ignore its benefits. Since relapse is always an option, those patients who really cannot function without nicotine presumably usually
do relapse. An immunotherapy, assuming it is irreversible during its term, might actually pose some risks—directly in the form of reduced productivity, bad behavior, or psychiatric disorder and indirectly through substitution of other drugs or other bad habits (overeating, for example) for the unavailable cigarettes. This might be considered a side-effect risk of immunotherapy absent from, for example, the nicotine-substitution therapies. Part of the clinical development of any nicotine immunotherapy ought to be exploration of the size of the population that cannot function well without nicotine and the means of determining whether a given candidate for immunotherapy is part of that subpopulation.
Assume that P(s/T), the conditional probability of success in any given quit attempt in which the smoker uses T, is higher than P(s/~T)). The smoker has a better chance of success if he uses T than if he does not. Then the gain in success probability from using T is P(s/T)−P(s/~T). Again for concreteness, assume that P(s/~T) is 20 percent and P(s/T) is 90 percent.11 Then the value of T is an additional 70 percent chance of success; if a success is worth $10,000, the gross value of T (before reckoning financial costs and side effects) would be $7,000. (Where T substitutes not for an alternative quit attempt but for no quit attempt, the benefit is $9,000.)
So far we have considered T merely as a means of increasing the probability that a quit attempt will succeed rather than fail. If T were sufficiently low in side effects so that it could be repeated prophylactically to prevent relapse, a successful quit using T will in fact be much more valuable (much longer lasting on average) than the average successful quit. Relatively few ex-smokers report deciding to go back to smoking, as opposed to succumbing to temptation (Office on Smoking and Health, 1989). Thus (again assuming low side effects) the renewal rate might be high and the net relapse rate low. The value of T might then be a multiple of the $7,000 figure, though of course repeated use would also increase cost.
Moreover, since the discomfort of attempting to quit and the fear of failure are important barriers to quitting, and since it has been reported that the subjective discomfort of being deprived of nicotine is dramatically less if cigarettes are simply unavailable than if the temptation to smoke must be battled moment-to-moment, there could be a significant treatment demand effect from T, especially if T-assisted quitting proved more successful, more durable, and more comfortable than quitting using other means. A therapy T as assumed might in fact convert nicotine
dependency into a reliably treatable disorder, which would in turn further increase P(q) by increasing the social pressures on smokers to quit.
The social value of having T available would be the value of the total additional reduction in expected cumulative lifetime smoking generated by T treatment compared to the next-best treatment, plus the additional reduction generated by increased quit attempts (T treatment as opposed to no treatment), plus the value of reduced discomfort from T-assisted quit attempts compared to non-T-assisted quit attempts, plus the saved financial costs of non-T-assisted quitting.
That would have to be compared with the costs of T, both the capital cost of developing it and the costs of T-assisted quitting itself. But thousands of dollars in gross benefit per treatment, minus costs probably measured in the hundreds, times tens of millions of long-term-dependent cigarette smokers suggests total gains in the range of tens of billions of dollars.
Assuming that 30 million of the roughly 37 million current smokers are nicotine-dependent, that one in six of them would try T, that trying T increased the probability of a successful quit that month by 70 percent, that a successful quit cuts 20 months off the active smoking career, and that the net cost of an active month is $500, the total gross benefits would come to $35 billion and total costs, after development, to about a seventh of that ($1,000 per treatment times 5 million treatments is $5 billion), leaving nearly $30 billion in gross social surplus (an analog to “profit”) from having developed the treatment.
Even adjusting that figure down for the time lag between research and development expenditures and having the treatment available, not adjusting it upward for the annual flow of new potential treatment candidates, and assigning no value to the development of a treatment with less attractive characteristics than hypothesized or to the possibility that more than one-sixth of today’s dependent smokers decided to try T, a development effort with a price tag of $50 million would be cost-justified even if its chance of producing such a successful result were even one-half of 1 percent.
This estimate is most sensitive to reductions in the assumed length of remission. If the therapy costs $1,000 but needs to be repeated every 3 months, two-thirds of its benefit disappears. If remission from a single treatment is as long-lasting as assumed, even doubling the estimated cost of treatment has very little effect on that answer directly (net benefit per treatment falls only from $6,000 to $5,000) because the benefits of treatment so far outstrip the costs. However, a higher price would be expected to reduce benefits by reducing the rate of uptake of the new therapy. The price of the treatment would be much more significant a factor if it turned out that maintaining recovery required frequent readministration.
The calculation is also sensitive to reductions in the assumed probability of success and reductions in the assumed uptake rate. However, even if figures given above for market penetration, efficacy, and duration of remission are halved, the breakeven value of the success probability for a $50 million effort remains below 5 percent. That being the case, any approach that seems technically plausible is probably worth pursuing. Moreover, the sensitivity of the calculation to cost and duration of action suggests the value of achieving a longer-lasting and/or lower-cost treatment, even at the expense of greater development cost.
EXAMPLE: COCAINE
Now assume a treatment with the same high efficacy but for cocaine rather than nicotine. The same basic framework of analysis can be used, but all the other facts will be different. The costs of active heavy cocaine use are much higher, both to the user and to the people around him or her. The drug is more toxic and much more likely to lead to dangerous behavior. Unlike cigarette smoking, heavy cocaine use tends to be inconsistent with good performance in work or family roles. It is also illegal and therefore very expensive. A typical member of the population of 2 million or so heavy cocaine users in this country is estimated to spend $10,000 to $15,000 per year on the drug (Office of National Drug Control Policy, 2001). Since only a small proportion of heavy cocaine users have access to that much extra cash from licit sources, much of the money involved is the product of illicit activities—theft, prostitution, cocaine dealing. The portion derived from theft has a social cost that is some multiple of the base amount, both because stolen property typically yields far less to the thief than its loss cost the owner and because of the costs of the precautions that potential victims take against theft. Cocaine dealing, in addition to its contribution to the spread of cocaine abuse and dependency, is associated with neighborhood disruption and violence.
Moreover, all of these illegal activities are likely to force the cocaine-dependent individual into the arms of the criminal justice system. It has been estimated that three-quarters of heavy cocaine users are arrested in the course of any given year. Arrest, conviction, and incarceration generate costs for the public and perhaps even greater costs for the individual involved. In particular, a criminal record greatly complicates the problem of reentry into the workforce. In addition, even users who do not participate in the cocaine market as sellers still participate as buyers and thus as contributors to the revenue base that keeps the market turning, with the resultant costs in violence, disruption, and the recruitment of new dealers (especially juveniles).
Any attempt to sum all of the losses (evaluated in willingness-to-pay
terms) involved in a month of heavy cocaine use by a criminally active cocaine user, while it would run into very substantial problems of both data and conceptualization—particularly regarding the benefit that should be counted for the pleasures of cocaine use itself—could hardly reach an answer that was not some multiple of the dollar cost of the cocaine itself, thus putting it in the range of thousands of dollars.
Heavy cocaine users who are not criminally active (other than as cocaine buyers) almost certainly generate less in the way of external costs (at least extra family costs) than their criminally active counterparts, but they are on average wealthier, which would be expected to increase their own willingness-to-pay to be shed of their destructive habit. Moreover, their family members are presumably wealthier than the family members of criminally active cocaine users; the family members’ willingness-to-pay will also be correspondingly greater. Again, it would be foolish to pretend that the arithmetic could be done with anything approaching precision, but a reasonable estimate would probably put total monthly net social cost in the same thousands-of-dollars range as the costs of cocaine abuse among the criminally active.12
An alternative calculation reaches an answer of the same order of magnitude. If the external financial costs of substance abuse actually totaled $150 billion per year (Office of National Drug Control Policy, 2001), if the nonfinancial external costs and the net costs to the substance abusers themselves came to an equal amount, if half the total were attributable to cocaine, and if 80 percent of the cocaine-related damage is due to 2 million heavy cocaine users, then the damage per person per year is $60,000, or $5,000 per month.
With a cocaine-dependent population about one-fifteenth the size of the nicotine-dependent population, and the benefits of a month’s remission from cocaine about 10 times those of a month’s nicotine remission, the total potential gain from a “cure” for cocaine abuse would therefore be of the same order of magnitude as the total potential gain from a “cure” for cigarette smoking, assuming that the two problems turn out to be comparably chronic in the absence of such a breakthrough.13 (The apparent stabilization in aggregate national consumption of cocaine suggests that the outflow from the heavy-cocaine-using population is slower than was
once hoped, so comparable chronicity may be a reasonable guess; only time will tell.)
The effect on treatment demand among heavy cocaine users from the introduction of a therapy with a high probability of success and free from the moment-to-moment struggle with temptation is an open question. Given the extreme misery and social dislocation created by heavy cocaine use, especially cocaine smoking and especially among the criminally active population, a strong motivation to quit, or at least to have quit, should surely be present. However, cravings are by no means the only source of discomfort for heavy users trying to stop. Anhedonia is widely reported, and a cocaine immunotherapy would likely do little if anything to ease it. (The depression that can accompany nicotine withdrawal seems to be more treatable.)
Moreover, many heavy cocaine users would be quite miserable even if they were free of their drug dependency; both personal distress and social distress are often among the causes of taking up cocaine in the first place and among the sequelae of heavy use itself. It seems plausible that the proportion of heavy cocaine users who will find themselves unable to live without cocaine (or some substitute, not necessarily another stimulant) will be higher than the proportion of heavy smokers who find themselves unable to live without nicotine and that enough of the current heavy users would fear that they fell into that class to limit demand for such a therapy were it introduced.
On the other hand, while virtually all attempts at tobacco cessation are more or less voluntary (made, perhaps, under family or social pressure, but not legal compulsion), a significant number of heavy cocaine users today find themselves facing legal demands that they quit or at least accept treatment. Abstinence from illegal drug use is a routine condition of probation, though probation departments tend to be lax in enforcing that requirement. Drug treatment in lieu of punishment is already fairly standard in the criminal justice system. A major limitation of the approach is the difficulty in getting those who are ordered into treatment, or who “volunteer” for treatment when the alternative is prison, to actually carry through on their end of the bargain.
In a typical diversion program, as many as half of the offenders referred never show up even for a first treatment appointment, and in most places the capacity of the probation system to chase absconders is not high enough to be an effective deterrent. Observing treatment attendance, treatment compliance, and desistance from drug use are difficult in part because every day is a new day, and the criminal justice system has proven largely incapable of administering programs that deliver consistent low-intensity sanctions for deviating from its orders. Thus the legal demands that criminally active heavy cocaine users desist from cocaine
use are so imperfectly enforced as to be of only limited use in reducing the cocaine-dependent population.
By contrast, whether a probationer has shown up at the clinic to receive a cocaine vaccination is easy to determine, and, if the person has and the vaccination is highly effective, there is much less need to attempt to observe whether the person continues to take cocaine. (Testing might still be needed to deter, or detect, substitution of other drugs.)
Thus an immunotherapy or depot medication would greatly simplify the challenge faced by criminal justice agencies and the courts in converting their legal hold over criminally active cocaine users into effective pressure on them to quit. A judge might reasonably require an offender offering to undergo vaccination as part of a sentence bargain to actually receive the vaccine before the judge formally enters the sentence. While attendance at and compliance with treatment are matters of more and less and to some extent matters of opinion, receiving a vaccination is an observable, yes-or-no phenomenon. That might make enforcement considerably easier.
Since, as noted, most of the population of heavy cocaine users comes to the attention of the criminal justice system in the course of any given year, the combination of a new therapy with the power of the state might lead to a far more dramatic increase in the exit rate from heavy cocaine use than could be achieved for cigarette smoking.
The ethical question of mandating a pharmacological treatment with potential side effects (as opposed to attendance at counseling sessions) is outside the scope of this analysis, except to note that both courts and treatment providers will have to wrestle with the question (National Research Council, 2001, Chapters 6 and 8, Appendix E). But the operational issues are also substantial and likely to reduce the benefits and increase the costs of administering immunotherapies or depot medications. The criminal justice system, not being fundamentally a diagnostic enterprise, may well mandate such therapies for individuals suffering from transient, rather than chronic, cocaine abuse or from no diagnosable substance abuse disorder. That is already an issue with the various drug diversion programs, including drug courts, and an immunotherapy is exactly the sort of “magic bullet” likely to catch the imagination of some judges and other officials. If the costs are modest and the side effects mild, administration of such a therapy to some people not really in need of it may be a tolerable price to pay. If the side effects are significant, a therapy that would still be a blessing for someone with no other way out of chronic cocaine abuse may be a very poor idea for someone merely arrested for cocaine possession.
The benefits of such a therapy would also be lower, and the costs higher, if many of those who receive it involuntarily or semivoluntarily under criminal justice pressure found life without cocaine intolerable.
They might well substitute other drugs, not necessarily stimulants. There is no way to guess in advance how the damage done as a result of the use of those substitutes might compare to the damage avoided from cocaine. Nor is there any good basis for estimating what proportion of court-mandated cocaine immunotherapy patients would in fact be unable to function without cocaine or would attempt substitution from mere disinclination to attempt a nonintoxicated life-style.
Still, the potential aggregate benefits from developing an immunotherapy or depot medication for treating cocaine dependency would be enormous. Assume that one-third of the roughly 1.5 million criminally active heavy cocaine users in this country could be induced to accept such a therapy and that the result of that therapy was, as assumed for tobacco, a 70 percent increase in the chance of a successful quit attempt, where a success would cut 20 months, valued at $5,000 per month, off the expected length of the active addiction career (net of substitution with other drugs). That gives gross benefits of 500,000 treatments × 0.7 × 20 × $5000, or about $35 billion, or roughly the same figure (given the error bands) as the estimate given earlier for nicotine. Per-patient costs dealing with an involuntary criminally active population would be far higher than in the case of nicotine, but the number of patients treated would be much smaller, leaving comparable net benefits as well.
While the nicotine calculation was sensitive to the assumed duration of remission after a single administration and, if that duration proved to be short, to the cost of the treatment itself, the very high cost of a month of cocaine use makes the calculation for cocaine robust in that regard. Even if the treatments cost $2,500 each and need to be repeated every 3 months, the cost of a treatment would still be only one-sixth of its benefits. The value of an immunotherapy for cocaine depends almost entirely on its efficacy, the number of heavy users who can be induced to accept it, and the rate of substitution of other drugs. That suggests that, insofar as there are tradeoffs to be made, the development effort should focus on improving efficacy rather than reducing cost or extending duration.
ADDITIONAL ISSUES
Other Drugs
Other than the efficacy, costs, and side effects of a treatment, all of which are hard to gauge in advance, the key factors in determining the benefits of developing an immunotherapy or depot medication for a given drug are the size of the population of long-term heavy users, the chronicity of the disorder in that population, and the social cost per month of heavy use.
While alcohol generates no illicit market and thus no illicit-market
crime, and while its users typically do not engage in income-producing crime in order to buy it, the aggregate costs associated with long-term heavy alcohol use probably exceed those associated with any other drug because of the very high prevalence of alcohol use and its moderate “capture rate” to abuse (estimated at 17 percent of all drinkers on a lifetime basis), the extreme chronicity of heavy drinking among the minority of problem drinkers whose problem recurs, and the physical and behavioral toxicity of the drug itself, in particular its relationship to both accidents and violent crimes. The potential benefits of developing an efficacious immunotherapy or depot medication are therefore extremely high, even compared to the benefits of developing such treatments for cocaine or nicotine. However, even a very high reward for success cannot justify a major development effort unless and until a technically plausible approach is invented.
The costs of heroin addiction are second only to those of cocaine addiction among the illicit drugs because of its high chronicity and its links to income-producing crime and the spread of infectious diseases. However, by contrast with cocaine, opiate addiction can be managed with substitution therapies (methadone, and more recently buprenorphine and LAAM [levo-alpha acetyl methadol]). That somewhat reduces the urgency of developing a heroin immunotherapy, and the sheer variety of opiates and opioids that are relatively closely intersubstitutable (in addition to diacetylmorphine [heroin], morphine itself, oxycodone [the active agent in Percodan and Oxycontin], hydrocodone [Vicodin], hydromorphone [Dilaudid], meperidine [Demerol], and the fentanil compounds) would tend to reduce the value of an immunotherapy targeted at only a single molecule.
Methamphetamine has costs per month of heavy use that are comparable to those of cocaine, perhaps higher if the long-term physical and psychological sequelae of heavy use are considered, but a far smaller number of heavy users (perhaps one-quarter as many) and probably significantly higher “natural” turnover among that population because of the drug’s punishing side effects (see National Household Survey on Drug Use, 2001). Lower chronicity reduces the benefit of treating any given patient and thus the aggregate benefits unless a mechanism were developed to identify heavy methamphetamine users relatively early in their use careers and induce them to undergo treatment quickly. The sheer population size difference suggests that methamphetamine is only about one-quarter as attractive a target as cocaine, and the higher turnover rate would reduce that even further. Still, the aggregate gross benefits of successful development would surely be in the billions of dollars.
Cannabis has more heavy users than any other illicit drug. No firm estimate exists, but 3 million, or about half again as large as the cocaine
population, seems to be a rough consensus figure. How many of them want to quit is an open question. Historically, demand for cannabis treatment among adults has been small, though there is some evidence this is changing, perhaps due to the falling age of onset to heavy cannabis use. Early onset has also increased the number of teenagers in need of treatment. The damage from a month of heavy use is greater than that associated with cigarette smoking but far less than that associated with any other intoxicant. The median duration of the first period of heavy use (daily use over a period of months) has been estimated at nearly 4 years. The proportion of heavy users who have recurrent spells of heavy use has not been estimated, so overall chronicity cannot be known with anything like certainty. The value of developing an immunotherapy for cannabis would clearly be smaller, perhaps by as much as an order of magnitude, than the value of developing such a treatment for nicotine or cocaine, but more precision than that is not possible given the paucity of data.
The Pricing Problem
The actual marginal cost of an immunotherapy or depot medication—the cost of administering it to an additional patient once it is available—is likely to be so far below the benefit of that administration in cases of well-established heavy use of any of the intoxicants as to make a cost-benefit analysis superfluous. (That might not be true for cigarette smoking if the treatment has to be repeated frequently.) But the price of any such therapy, if it is developed along conventional pharmaceutical company lines, will be much greater than its marginal cost.
That must be true as long as the costs of drug development, including the costs and risks of the regulatory process, are borne by private entities under the incentive provided by the promise of patent-protected monopoly. The owner of the patent on an efficacious immunotherapy for cocaine (if there were only one on the market) might well want to price it near its perceived expected benefit to high-income cocaine users, as the producers of the nicotine patch have priced their product near the price of the cigarettes it displaces.
That sort of pricing would greatly reduce the social benefit available from the original drug development by pricing out of the market large numbers of potential patients whose willingness or ability to pay for treatment is lower. Even if the potential benefits from reducing cocaine use in the criminally active population are measured in the tens of billions of dollars, the agencies involved are highly unlikely to come up with billions of dollars to pay for it.
Thus the cost-benefit analysis is not invariant to the pricing structure, and if public or charitable money is to go into the development of these drugs, there ought to be agreements in advance with the potential patent holders or licensees regarding the pricing issue.
Imperfect Efficacy
The discussion to this point has been about high-efficacy therapies. That need not mean therapies that are efficacious for all, or almost all, of the population. A therapy that worked well for half the population and completely failed for the other half would have about half the benefits of a therapy that worked for everyone.
Unfortunately, however, it would not be the case that a therapy that reduced bioavailability by 75 percent in all patients would be 75 percent as beneficial as a therapy that reduced bioavailability effectively to zero. If three out of four molecules are put out of action before they reach the brain, a user who can acquire four times his or her normal dose can overcome the effects of the vaccine.14 The result resembles a fourfold price increase for the drug.
Fortunately, the old opinion that drug demand among dependent individuals is highly inelastic (i.e., unresponsive) to changes in effective price is no longer in vogue. Current opinion holds that demand is fairly elastic to price. That makes it unlikely that many users will make a habit of “shooting over” the vaccines. The animal data are also reassuring on this point.
But it is not at all unlikely, if it is known that a large dose can reproduce something like the old drug effect, that a substantial number of users will make the attempt occasionally. The combined uncertainties about the quantity and purity of drugs acquired on the illicit market, how effective the therapy is, and how much a user’s tolerance declines because of a period of abstinence might create significant overdose risk with respect to cocaine, heroin, or methamphetamine.
In the case of cigarette smoking, overdose seems unlikely to be a risk, but if the word were to spread among smokers that, say, smoking two cigarettes in quick succession, and doing so with attention to maximizing nicotine absorption, would get enough nicotine through the blockade to do its job, the temptation-reducing aspect of the immunotherapy or depot medication would be noticeably reduced, at least for some patients.
Substitution of Immunotherapy or Depot Medication for Noncriminal Justice Social Sanctions
Physicians and other health care professionals who use drugs illegally risk losing their licenses and when caught are frequently put on a kind of professional probation (sometimes in the case of opiate or opioid users, involving a requirement to take, under observation, a daily dose of a narcotic antagonist). Workers in the transportation industry also can lose their jobs if drugs are found in their systems. Members of the armed services are subject to dismissal. Mothers can lose custody of their children. Immunotherapies and depot medications, if developed, might be used in any of these circumstances, and the benefits and costs in those cases would be different from either the truly voluntary case or the case of coercion from the criminal justice system.
Prophylactic Administration
Vaccination is usually a prophylactic rather than a therapeutic procedure. The discussion up to now has assumed that (except for possibly overenthusiastic application to offenders) immunotherapies and depot medications would be used only in the treatment of persons with diagnosed substance abuse disorders. But the level of fear among parents about illicit drug use by their children is such that some parents would want their children “immunized” against, for example, cocaine, if such a treatment were available. Some might want “immunization” against cigarette smoking. Clearly, the cost-benefit ratio in such applications would be far lower than in the cases assumed above.
An intermediate case would involve children found to be using one or another drug but who are not yet diagnosably dependent on it. Here the parental demand would be more insistent and the justification at least somewhat more plausible. In each case, parent-child conflict is a possibility, and health care providers might find themselves caught in the middle.
It is also possible that some drug users not (yet) diagnosably abusing or dependent might find their own use of some drug so worrisome, and their confidence in their self-command so shaky, that they would want to undergo immunotherapy or depot medication. The benefits of such prophylactic administration, while far lower than the benefits of therapeutic administration, might still exceed the costs.
Risk Compensation
The concern that improved access to drug treatment will have a perverse impact on initiation and escalation rates has long been dismissed by
those familiar with the phenomenon of drug abuse. After all, few people who start to use abusable drugs expect to become addicted, and drug treatment to date has been sufficiently unreliable and unpleasant that its availability does not offer much comfort to someone contemplating the risk of addiction even if they do think about it. But that does not mean that the fear of addiction—thought of as a mysterious, incurable, relapsing condition—does not play an important role in reducing initiation and making most users of most drugs watchful over their own use patterns or that changing the meaning of “addiction” by making dependency curable might not substantially change the initiation rate.
A “home run” immunotherapy of the kind imagined above would substantially change the risk analysis, from the user’s viewpoint, of “experimenting” with the drug whose addiction it treats. It seems hard to deny that the increased curability of some sexually transmitted diseases certainly contributed to a rise in risky sexual activity, and this case might be similar. An immunotherapy sounds enough like a “magic bullet” treatment that the problem of “risk compensation”—increased participation in a risky activity as a result of a reduction in the risk—needs to be considered.
If the dependency syndrome around any of the major drugs of abuse became a curable illness in the same sense that tuberculosis or syphilis is curable, the long-term effects on people’s opinion of the drug might be profound. It is not obvious that the net result would be undesirable, but it might be very much so. It might be found that lowering the chronicity of the substance abuse disorder increased its incidence substantially, with unknown impacts on steady-state prevalence. That risk would be especially severe if the efficacy of the new therapy as perceived by potential drug users, especially young people, exceeded its efficacy in practice. In addition, even if aggregate problems from addiction went down, problems associated with casual use, which in the case of alcohol constitute a nontrivial fraction of the total social cost, might go up.
The risks of an upsurge in drug initiation as a result of the promise of an effective and relatively low-stress treatment are virtually impossible to quantify, even by the loose standards of quantification used elsewhere in this paper. But that does not mean that those risks ought to be ignored in planning for a world in which such therapies become available.
REFERENCES
Blower, S.M., Schwartz, E.J., and Mills, J. (2003). Forecasting the future of HIV epidemics: The impact of antiretroviral therapies and imperfect vaccines. AIDS Reviews, 5(2), 113-125.
DeLeon, G. (2000). The therapeutic community: Theory, model, and method. New York: Springer-Verlag.
Everingham, S.S., and Rydell, C.P. (1994). Modeling the demand for cocaine. Santa Monica, CA: RAND Corporation.
Fairbank, J.A., Dunteman, G.H., and Condelli, W.S. (1993). Do methadone patients substitute other drugs for heroin? Predicting substance use at 1-year follow-up. American Journal of Drug and Alcohol Abuse, 19(4), 465-474.
Goldberg, J., and Fischoff, B. (2000). The long-term risks in the short-term benefits: Perceptions of potentially addictive activities. Health Psychology, 19(3), 299-303.
Goldstein, A. (2001). Addiction: From biology to drug policy (Second Edition). New York: Oxford University Press.
Hser, Y., Hoffman, V., Grella, C.E., and Anglin, D. (2001). A 33-year follow-up of narcotics addicts. Archives of General Psychiatry, 58(5), 503-508.
Hubbard, R.L., and French, M.T. (1991). New perspectives on the benefit-cost and cost-effectiveness of drug abuse treatment. In W.S. Cartwright and J.M. Kaple (Eds.), Economic costs, cost-effectiveness, financing, and community-based drug treatment (pp. 94-113). Rockville, MD: National Institute on Drug Abuse.
Institute of Medicine. (1990). Treating drug problems, volume 1. Committee for the Substance Abuse Coverage Study, D.R. Gerstein and H.J. Harwood (Eds.). Washington, DC: National Academy Press.
Johnson, B.D., Rosenblum, A., and Kleber, H. (2003). A new opportunity to expand treatment for heroin users in New York City: Public policy challenges for bringing buprenorphine into drug treatment programs and general medical practice. White paper for New York City Department of Health and Mental Hygiene. Available: http://www.nyc.gov/html/doh/pdf/public/dmh/whitepaper.pdf [August 21, 2003].
Kleiman, M.A.R. (1999). Economic cost measurements, damage minimization and drug abuse control policy. Addiction, 94(5), 638-641.
MacCoun, R.J. (1993). Drugs and the law: A psychological analysis of drug prohibition. Psychological Bulletin, 113(3), 497-512.
MacCoun, R.J., Reuter, P., and Schelling, T. (1996). Assessing alternative drug control regimes. Journal of Policy Analysis and Management, 15(3), 330-352.
McKay, J.R., Rutherford, M.J., Cacciola, J.S., Kabasakalian-McKay, R., and Alterman, A.I. (1996). Gender differences in the relapse experiences of cocaine patients. Journal of Nervous and Mental Disease, 184(10), 616-622.
Moore, M.H. (1990). Supply reduction and drug law enforcement. In M. Tonry and J.Q. Wilson (Eds.), Drugs and crime (pp. 109-158). Chicago: University of Chicago Press.
National Household Survey on Drug Use. (2001). Methamphetamine usage data. Available: http://www.samhsa.gov/oas/NHSDA/2k1NHSDA/vol2/appendixh_1.htm [August 21, 2003].
National Research Council. (2001). Informing America’s policy on illegal drugs: What we don’t know keeps hurting us. Committee on Data and Research for Policy on Illegal Drugs. C.F. Mansiki, J.V. Pepper, and C.V. Petrie (Eds.). Committee on Law and Justice and Committee on National Statistics. Commission on Behavioral and Social Sciences and Education. Washington, DC: National Academy Press.
Office of National Drug Control Policy. (2001). The economic costs of drug abuse in the United States, 1992-1998. Washington, DC: Executive Office of the President. Available: http://www.whitehousedrugpolicy.gov/publications/pdf/economic_costs98.pdf [August 21, 2003].
Office on Smoking and Health. (1989). Reducing the health consequences of smoking: 25 years of progress: A report to the Surgeon General. Washington, DC: U.S. Department of Health and Human Services.
Rydell, C.P., and Everingham, S.S. (1994). Controlling cocaine: Supply versus demand programs. Santa Monica, CA: RAND Corporation.
Tai, B., Chiang, N., and Bridge, P. (Eds.). (1997). Medication development for the treatment of cocaine dependence: Issues in clinical efficacy trials. Rockville, MD: National Institute on Drug Abuse. Available: http://www.drugabuse.gov/pdf/monographs/monograph175/download175.html [August 21, 2003].
Trosclair, A., Husten, C., Pederson, L., and Dhillon, I. (2002). Cigarette smoking among adults—United States, 2000. Morbidity and Mortality Weekly Report, 51(29), 642-645. Available: http://cisat.isciii.es/mmwr/preview/mmwrhtml/mm5129a3.htm [August 21, 2003].




























