Below is the uncorrected machine-read text of this chapter, intended to provide our own search engines and external engines with highly rich, chapter-representative searchable text of each book. Because it is UNCORRECTED material, please consider the following text as a useful but insufficient proxy for the authoritative book pages.
3 Bioconfinement of Plants METHODS OF BIOCONFINEMENT Many approaches have been proposed for the biological confinement of plant transgenes (Table 3-1; Daniell, 2002). Some are based on pre- existing agronomic or horticultural methods, others are newly developed, and some are hypothetical. In a few cases, there are data that illustrate the efficacy of those approaches; in other cases, the approaches are untested. This chapter reviews and analyzes as many bioconfinement methods for genetically engineered plants as the committee could identify, although the survey is incomplete because new methods are proposed constantly. The discussion begins with strategies for blocking sexual and vegetative repro- duction. Other techniques that reduce the spread and persistence of transgenes in wild and cultivated populations of plants are reviewed. The chapter also considers--as best as possible, given the limited data available--the efficacy of those methods at various spatial scales. There is a discussion of whether the methods could affect the populations and ecosystems in which they are deployed. Given that bioconfinement methods are expected to be less than 100% effective, the chapter also asks how to monitor for escape of plant transgenes and whether detection and subsequent culling would be an effec- tive backup to a primary bioconfinement method. Case studies are provided to highlight the bioconfinement issues specific to transgenic trees, turfgrasses and algae. The chapter concludes by asking what consequences might accrue and what mitigation might be necessary if bioconfinement and monitoring of genetically engineered organisms (GEOs) fail. 65
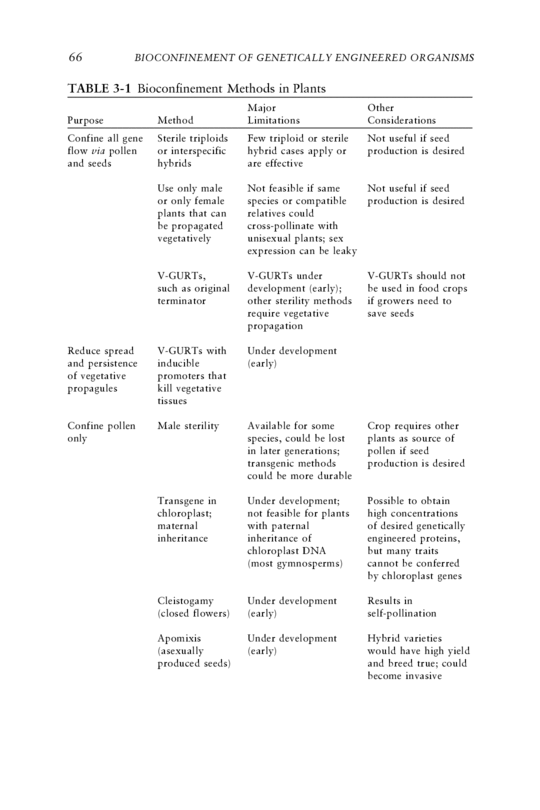
66 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS TABLE 3-1 Bioconfinement Methods in Plants Major Other Purpose Method Limitations Considerations Confine all gene Sterile triploids Few triploid or sterile Not useful if seed flow via pollen or interspecific hybrid cases apply or production is desired and seeds hybrids are effective Use only male Not feasible if same Not useful if seed or only female species or compatible production is desired plants that can relatives could be propagated cross-pollinate with vegetatively unisexual plants; sex expression can be leaky V-GURTs, V-GURTs under V-GURTs should not such as original development (early); be used in food crops terminator other sterility methods if growers need to require vegetative save seeds propagation Reduce spread V-GURTs with Under development and persistence inducible (early) of vegetative promoters that propagules kill vegetative tissues Confine pollen Male sterility Available for some Crop requires other only species, could be lost plants as source of in later generations; pollen if seed transgenic methods production is desired could be more durable Transgene in Under development; Possible to obtain chloroplast; not feasible for plants high concentrations maternal with paternal of desired genetically inheritance inheritance of engineered proteins, chloroplast DNA but many traits (most gymnosperms) cannot be conferred by chloroplast genes Cleistogamy Under development Results in (closed flowers) (early) self-pollination Apomixis Under development Hybrid varieties (asexually (early) would have high yield produced seeds) and breed true; could become invasive
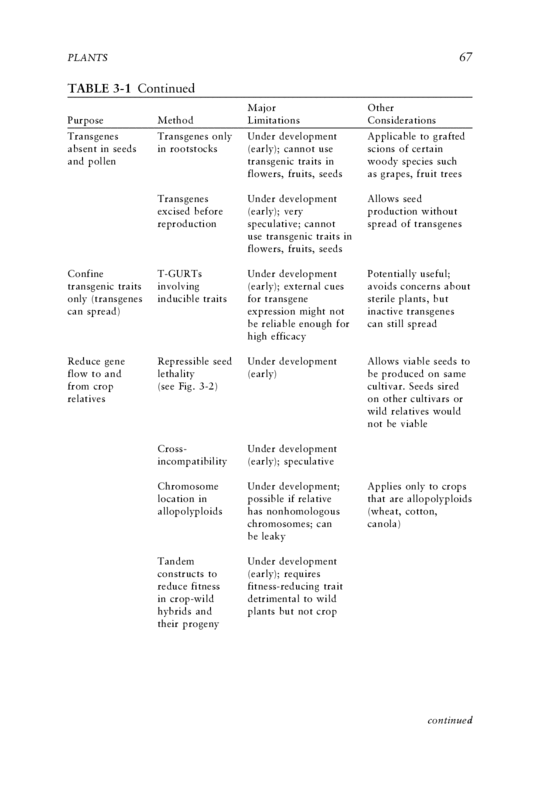
PLANTS 67 TABLE 3-1 Continued Major Other Purpose Method Limitations Considerations Transgenes Transgenes only Under development Applicable to grafted absent in seeds in rootstocks (early); cannot use scions of certain and pollen transgenic traits in woody species such flowers, fruits, seeds as grapes, fruit trees Transgenes Under development Allows seed excised before (early); very production without reproduction speculative; cannot spread of transgenes use transgenic traits in flowers, fruits, seeds Confine T-GURTs Under development Potentially useful; transgenic traits involving (early); external cues avoids concerns about only (transgenes inducible traits for transgene sterile plants, but can spread) expression might not inactive transgenes be reliable enough for can still spread high efficacy Reduce gene Repressible seed Under development Allows viable seeds to flow to and lethality (early) be produced on same from crop (see Fig. 3-2) cultivar. Seeds sired relatives on other cultivars or wild relatives would not be viable Cross- Under development incompatibility (early); speculative Chromosome Under development; Applies only to crops location in possible if relative that are allopolyploids allopolyploids has nonhomologous (wheat, cotton, chromosomes; can canola) be leaky Tandem Under development constructs to (early); requires reduce fitness fitness-reducing trait in crop-wild detrimental to wild hybrids and plants but not crop their progeny continued
68 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS TABLE 3-1 Continued Major Other Purpose Method Limitations Considerations Phenotypic and Domestication Under development; fitness handicaps phenotypes does not prevent to reduce need gene flow for confinement Auxotrophy Under development; (dependence on does not prevent specific nutrients gene flow or growing conditions) Reduce exposure Tissue- and Promoters available, Could alleviate to transgenic organ-specific but greater efficacy the need for products in promoters that needed in many cases; bioconfinement plants limit expression confines transgenic in some cases of transgene traits but not the transgenes; transgenes can spread Minimize or Choice of Economic costs can Often feasible and eliminate alternative be high, especially if highly recommended need for organisms; decision to change when appropriate; bioconfinement choice not to course is made after alternative choices release in field; economic investment should be examined choice not to before GEO is proceed with developed GEO For thorough confinement, pollen dispersal, seed dispersal, and vegetative persistence must be considered. V-GURT, variety genetic use restriction technology; T-GURT, trait genetic use restriction technology. Sterility Because transgene escape by pollen or seeds is not possible for plants that do not produce fertile pollen or seeds, the task of bioconfinement is simplified because it is necessary only to keep track of vegetative dispersal units, such as tillers, rhizomes, and stolons. Bananas and seedless grapes are among the sterile food crops that are propagated vegetatively. Many non- sterile cultivated plants are sold as cloned vegetative material, including some varieties of potato, turfgrass, and ornamental plants and poplar trees. Several mechanical, chemical, and genetic methods can be used to block the production of fertile pollen or seeds in those plants. This section reviews genetic approaches that achieve sterility. They include nontransgenic methods
PLANTS 69 (triploids); transgenic sterility that is nonreversible; and transgenic approaches that allow for reversible sexual sterility that permits further breeding. The sections that follow discuss options for blocking vegetative spread and for obtaining male sterility. Interspecific Hybrids Interspecific hybrids often exhibit partial or full sterility (e.g., Grant, 1981; Stace, 1975). The sterility of the mule, a horse and donkey hybrid, is well known. In some cases, interspecific hybrids have almost complete male and female sterility. However, most interspecific plant hybrids are not fully sterile (e.g., Stace, 1975). In a surprising number of cases, hybrid fitness has been shown to be as high as or higher than that of the parental genotypes (Arnold, 1997; Arnold and Hodges, 1995). For example, Arriola and Ellstrand (1997) compared the fitness of hybrids of Sorghum bicolor (the crop, grain sorghum) and S. halepense (the weed, johnsongrass) and genetically pure S. halepense siblings under field conditions. They report that the hybrids did not significantly differ from the weeds in terms of biomass, tiller number, seed set, or pollen viability. Furthermore, in many species, relatively or fully sterile hybrids reproduce and spread by vegetative reproduction, sometimes even more vigorously than do their sexually fertile relatives (e.g., Ellstrand et al., 1996). It is well known that the fitness of hybrids varies tremendously in different environments (Anderson, 1949; Arnold, 1997). Thus, housing transgenes in interspecific hybrids might afford some moderate bioconfine- ment relative to nonhybrids, but for any given hybrid genotype, male fertility, female fertility, and vegetative reproduction (if appropriate) must be measured in a range of potential field environments to allow an estimate of what amount of bioconfinement might be expected. Strengths In cases where there is complete or near-complete sterility, interspecific hybridity could yield a reasonably easy way to obtain bioconfinement in plants, as in the case of triploid hybrids. As long as sterility is maintained in a variety of environments, the genes of those plants are unlikely to spread through pollen or seed. Weaknesses Sterile interspecific plant hybrids will not be a general solution for plant bioconfinement. Specific hybrids might prove to be very sterile, but it is more likely that interspecific plant hybrids would offer moderate bio- confinement at best and no bioconfinement at all in some cases.
70 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS Sterile Triploids Breeding methods that disrupt chromosomal pairing during sexual reproduction have been used to create sterile plants. Most plants are chro- mosomally diploid (characterized as 2n). That is, they have two sets of matching homologous chromosomes in their somatic cells. The two sets pair up and separate during the process of gamete formation, and the number of chromosomes is halved for each pollen grain or ovule (those gametes have n chromosomes). The diploid number is restored when the gametes fuse to create a zygote. Organisms with three sets of chromosomes are called triploids (3n). In humans, triploidy is lethal, and it is a rare condition in wild organisms (Chapter 4). It is not uncommon in cultivated plants (Grant, 1981), how- ever, many commercial banana cultivars are triploid and thus seedless (Simmonds, 1995). Spontaneous triploids primarily appear to result from the fusion of a normal gamete (n) with an aberrant unreduced (diploid, 2n) gamete. Spontaneous triploids also can occur from the fusion of a gamete from a diploid species with one from a related tetraploid (4n) species (which produces gametes that bear 2n chromosomes). For example, if a 2n plant is crossed with a 4n plant, all of their progeny would be 3n and would be expected to be sterile. Triploid plants found in the wild typically are par- tially or fully sterile with respect to pollen and seed production. Those that are fully sterile persist only if they are capable of asexual seed production (apomixis) or vegetative reproduction. Triploidy in cultivated plants is main- tained mostly through vegetative propagation. Thus, induction of triploidy (and other odd-numbered chromosome counts) represents a possible option for bioconfinement. Chromosomal situations other than odd ploidy--extra or missing indi- vidual chromosomes (aneuploidy) and translocation heterozygosity--also disrupt gamete formation during meiosis. Although they can cause reduced fertility, they apparently have not been examined for use in bioconfinement. More information on chromosomal variation in plants and its consequences for plant fertility is found in Burnham (1962) and Levin (2002). Strengths If triploidy results in pollen and seed sterility, and if the degree of sterility does not vary from one environment to another, induction of triploidy could be an effective method of bioconfinement. Triploidy induc- tion will be most effective for organisms that do not reproduce asexually, although that complicates options for further breeding and multiplication. Triploidy also can be induced in other transgenic organisms such as fish (Chapter 4).
PLANTS 71 Weaknesses Much like interspecific hybridity, the efficacy of triploidy induction varies by genotype and environment. Unisexual Plants Lacking Mates Many dioecious (unisexual) plants can be propagated vegetatively, among them holly, kiwi, gingko, avocado and asparagus, such that only one sex is used for genetic engineering. Sex-specific molecular markers can be used to identify male or female plants before massive propagation (e.g., Khadka et al., 2002; Reamon-Buttner, 1998). In fields, bioconfinement could be achieved if such plants are grown in unisexual stands far from conspecifics or wild relatives with which there could be cross-pollination. For example, all-female cultivars of ornamental nonnative plants could be used in this context. However, this method of bioconfinement is unlikely to be practical in most cases. First, the number of species for which the condi- tions would be met (along with sufficient economic advantages) is small. Second, dioecy is known to be quite leaky (Krohne et al., 1980; Poppendieck and Petersen, 1999); seeds could be produced in low frequency by "male" plants, especially in large-scale plantings. Finally, human error could result in mix-ups that allow both sexes to occur in the same population, resulting in a breakdown of bioconfinement. Strengths This method might be desirable if it is used in combination with other confinement approaches in small-scale plantings. Weaknesses This method is unlikely to be reliable, and it applies only to a narrow range of species. Transgenic Sterility Transgenic methods are available for developing plants that abort young flower buds and thus become sterile through ablation. The resulting plants cannot be used for breeding or for multiplication by seed, but this method has been considered for some clonally propagated plants, such as poplar trees. Strauss and colleagues (1995) reviewed the rationale for at- tempting to engineer nonreversible sterility in forest trees. One strategy for creating sterility-causing transgenes that is particularly attractive for peren-
72 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS nial plants is to ablate floral tissues by the expression of cytotoxin genes that are fused to developmentally induced promoters expressed in flowers. Promoters from floral-specific genes tend to work well across species. Thus, ablation methods based on these genes probably will not require cloning of new gene homologues from each new transgenic species and genotype. Practical constraints include the requirement for vegetative propagation if complete sterility is engineered and the need for sterility to be highly stable in long-lived species such as trees and perennial grasses. Strauss and col- leagues (1995) suggest that long-term stability could require suppression of more than one floral gene or use of more than one genetic mechanism for sterility. A shortcoming of nonreversible sterility is that it precludes options for further breeding and seed production within the genetically engineered line that could be needed in the future. For trees or other perennials that do not flower in the first 510 years--the breeding period is longer than the generation of new transformants--that limitation might not be a major concern because new transformants could be made within the same period. The engineering of sterility by ablation can be conducted as the last step in the improvement process after breeding or genetic engineering for other traits has been accomplished. The preablation, fertile versions of the lines would still be available for use in breeding or seed production. Reversible Transgenic Sterility Plants that are permanently sterile, such as those described above, constitute an evolutionary dead-end. Researchers have proposed various transgenic methods by which sterility can be gained or lost by design (Fig- ure 3-1; Daniell, 2002). One type of reversible sterility blocks gene flow through pollen and seeds, thereby, for example, preserving a seed company's ownership of transgenic germplasm. With this method, transgenes that confer desirable traits are linked to transgenes that cause sterility, and the two are inherited together. Because this strategy restricts access to fertile plants, it is known as variety genetic use restriction technology (V-GURT). Trait genetic use restriction technologies (T-GURTs) induce transgenic traits in fertile plants by means of a specific stimulus, such as a chemical spray. The term GURT has gained wide use in scientific and policy discussions (e.g., FAO, 2002), but this report focuses on bioconfinement uses of GURTs and related techniques, keeping in mind that incentives for developing those methods are often based on proprietary commercial goals. One of the first V-GURTs was the so-called terminator technology protection system in which transgenic plants produced dead seeds. V-GURTs have not yet been used in any deregulated or commercialized crops, but, the terminator technology patent application was extremely
PLANTS 73 Fertile V-GURTs: Dead seeds; plants original "Terminator" Sterile Fertile seeds; plants but only during breeding; dead seeds on field plants T-GURTs: Fertile Fertile seeds; plants transgenes expressed only when induced Fertile seeds; on same GE crop variety: dead seeds on other plants FIGURE 3-1 Proposed transgenic bioconfinement methods in plants. V-GURT, variety genetic use restriction technology; T-GURT, trait genetic use restriction technology. controversial, especially in developing countries. The V-GURT approach induces seeds that grow into plants that produce nonviable offspring when they are cultivated in farmers' fields. Induction can occur by soaking the seed source in a solution that induces a promoter, setting the stage for late- acting lethality in ripened seeds (Figure 3-1, V-GURT example 1). In field- grown plants, a promoter that is expressed late in seed development acti- vates a lethal gene that renders the seeds unviable but still fully formed, which is important if the seed is to be sold for food, feed, or other uses. However, seeds in the original seed lot that are not induced properly can develop into fertile plants rather than sterile ones. Such incomplete sterility seems quite likely, based on the status of the technology (Daniell, 2002), and other V-GURTs are likely to be more effective. To avoid the problem of incomplete induction of sterility, plants could be engineered with sterility as the default condition, and breeders could use a stimulus to induce a pro- moter to render them fertile (Figure 3-1; adapted from FAO, 2002). Several related transgenic sterility methods are in development com- mercially and by independent researchers, but little has been published about them beyond general descriptions in patent applications (FAO, 2002). One exception is the research published by a group that developed a method called "recoverable block of function" (Kuvshinov et al., 2001), which consists of a DNA sequence element (a "blocker") that interrupts a specific molecular or physiological function in the host plant, leading to death of
74 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS the host plant or its seeds. A second DNA sequence element (for "recovery") restores the blocked function in the host plant. The blocker and the recovery sequences are physically linked to the transgene of interest in one construct so that they integrate into the genome together and remain united during sexual reproduction. The recovery function is designed to be activated by exogenous chemical or physical treatment. Thus, the dispersal of pollen or seeds with the recoverable block of function construct would result in progeny that would die or be unable to reproduce because the recovery function would be inactive. The work is still in the early stages, and it might or might not reach commercial development. Sterility systems for genetically engineered plants have been criticized because they would prevent growers from saving seed and having the option of using transgenes to improve local varieties. If implemented widely, V-GURTs such as the terminator technology would force growers to buy new seed each year to benefit from modern varieties. Many growers do buy new, certified seed each year, to save time and obtain a high-quality product that is free of contaminating pathogens and weed seeds. Many food crops and annual ornamental plants are sold as F1 hybrids, among them corn, sunflower, and petunias. Seeds from those plants can be saved but they do not "breed true," so new seeds must be purchased each year. The socio- economic issues surrounding V-GURTs and other sterility methods are discussed in Chapter 1. Environmental effects of the methods are discussed later in this chapter. V-GURT methods could be useful for bioconfinement of grasses, trees, and other horticultural species in which it is desirable to strongly limit gene flow. The social, political, and ethical issues attending the use of V-GURTs in food crops will need to be addressed. Strengths Reversible sterility methods could become very useful for bioconfine- ment because they could be used to block the dispersal of pollen and seeds that bear unwanted transgenes. Weaknesses The effectiveness of those novel methods has not been determined nor has their acceptability to consumers. The efficacy of reversible sterility could be diminished by gene silencing or recombination events that cause the sterility construct to become dissociated from the transgenes that require confinement. Research is needed to develop appropriate inducible pro- moters. Public access to data on the efficacy of transgenic reversible sterility, including long-term studies of transgene stability, will be essential. The technology should not be used in food crops for which growers need to save
PLANTS 75 seeds for future planting or breeding. Possible environmental concerns should be evaluated on a case-by-case basis and are discussed later in this chapter. V-GURTs will not prevent clonal propagation of many plants, such as some species of grasses, shrubs, and trees. Mortality of Vegetative Propagules Vegetative spread, both natural and human-mediated, is common in perennial species. Vegetative clones of semidomesticated and nondomesticated grasses, trees, and shrubs can spread over large areas and survive for decades as new ramets are produced and old ones die off. Some plants--especially species that occur along river margins and shorelines--also have vegetative parts that break off and disperse. Many perennial crops, horticultural plants, and woody species can be multiplied and distributed by rooting clonal segments of the plant and meristematic tissue. Depending on the plant's growth habit and ability to be cloned, strategies for minimizing vegetative propagation could be an essential component of bioconfinement. The ability to propagate plants vegetatively is often desirable for commercial produc- tion, but in wild species, this trait often is associated with enhanced com- petitive ability. Transgenic methods can be used to restrict the spread of vegetative propagules, such as tillers, rhizomes, and root suckers. Given that it will rarely be practical to breed plants that have lost this ability, one of the few options for bioconfinement of vegetative parts is to use a GURT that is induced to kill the plant at some point in its development before it is cloned or propagated (FAO, 2002). Many inducible promoters could be used, including those triggered by chemical applications or winter conditions. Programmed cell death (PCD) is a normal part of development, and, when it is better understood, that response to stress in plants as well as animals (Zhivotovsky, 2002) could be developed into a transgene bio- confinement method for vegetative propagules. Pontier and colleagues (1999) observed that a senescence-like process is triggered during the for- mation of necrotic lesions in disease-resistant plants. They suggested that cells committed to die in resistant plants during this hypersensitive response (HR) to pathogens might release a signal that induces senescence in neigh- boring cells. The signaling pathway responsible for PCD and HR involves changes in the antioxidant systems that are activated by nitric oxide and reactive oxygen species (De Pinto et al., 2002). AtMYB30, transcriptional regulation gene, has been identified as a positive regulator of the hyper- sensitive cell death program in plants in response to pathogen attack (Vailleau et al., 2002). Several lesion mimic mutants have been isolated in Arabidopsis and in other plants that display accelerated HR (Jambunathan et al., 2001). Lesion mimics also can be generated in plants by various
76 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS transgenes (Mittler and Rizhsky, 2000), such as the lethal leaf spot 1 (Lls1) gene, which is conserved between plant species and acts to suppress cell death (Spassieva and Hille, 2002). A virus-induced silencing of the Lls1 gene in tomato produces a phenotype that resembles the Lls1 mutant in maize, in which large necrotic lesions form in response to aging and envi- ronmental stresses, such as light, wounding, and pathogen attack. Those results suggest that, with additional research, it will be possible to environ- mentally trigger widespread cell death in escaped vegetative propagules of genetically engineered plants either by overexpressing the antioxidant- signaling-pathway genes or by regulating the upstream genes, such as AtMYB30 and LLt1, which control PCD. Strengths It is theoretically possible to develop transgenic suicide systems that can be induced to block vegetative reproduction. Weaknesses Designing plants that self-destruct reliably at a given time or stage is a formidable technical challenge that could require extensive research and development. Any bioconfinement method that relies on transgenic approaches and inducible promoters could fail because of gene silencing, recombina- tion, or incomplete induction of specific promoters in the transgene construct. Confining Pollen-Mediated Spread of Transgenes The discussion thus far has addressed biological methods for confining general reproductive capability in transgenic plants. Confinement of pollen- mediated gene flow can be used to reduce the need for physical isolation of transgenic plants, especially if seed dispersal and vegetative spread are of no concern. This section begins with a discussion of male sterility, which can be achieved through conventional and transgenic approaches. Nontransgenic Male Sterility Male sterility, the inability of a plant to produce fertile pollen, is a useful tool for hybrid breeding and hybrid seed production because self- pollination is prevented. Nontransgenic male sterility is used in sunflower, sorghum, and canola to produce hybrid crops; mechanical removal of pollen-bearing tassels is used more often to produce hybrid maize. Natu- rally occurring male sterility can be either "genic" or cytoplasmic. Genic male sterility results from mutations in nuclear genes. In most cases, cyto-
PLANTS 77 plasmic male sterility is based on mitochondrial genome rearrangements that lead to partial incompatibilities with the nuclear genome. Naturally occurring genic male sterility has been reported for many plant taxa. How- ever, this trait is difficult to use in hybrid seed production because it is usually a dominant genetic trait and therefore is difficult to achieve without elaborate crossing schemes. Recently, genic male sterility has been pro- duced by transgenic methods. Experience with various crop plants demonstrates that male sterility is seldom perfect. Cytoplasmic systems, for example, can be overcome by nuclear restorer genes, temperature shifts, and other environmental factors (Burns et al., 1991; Hanna, 1989; Kumari and Mahadevappa, 1998; Michalik, 1978). Even though reversion to fertility occurs infrequently, seed producers must routinely patrol their plots to cull the occasional fertile plant. Thus, the opportunity for reversion is a disadvantage if pollen- mediated gene flow must be kept to a minimum. For some species, the low frequency of reversion could be manageable, however, and the use of cyto- plasmic male sterility would improve the efficacy of physical containment (Pedersen et al., 2003). Patrolling and culling for revertants might not be appropriate for extensive, long-term field trials or for use with large plants such as trees that produce flowers at heights that are difficult to monitor. Genic male sterility might not be as susceptible to reversion. Reversions of nuclear male sterility genes would be expected only at the normal rate of background mutation. Thus, genic male sterility systems could be prefer- able to cytoplasmic systems if pollen-mediated gene flow must be kept to a minimum. There is substantial concern over transgene flow from GEOs to natural populations of related plants via pollen, so the use of male sterility is recommended whenever feasible. Transgenic Male Sterility Transgenic male sterility could allow for hybrid seed production to be introduced to crops for which natural genic or cytoplasmic systems do not exist. This could be a boon for productivity because hybrid seed crops often exhibit heterosis (hybrid vigor). Nuclear male sterility has been engineered in several species, including tobacco, rice, maize, alfalfa and Brassica, by using the Bacillus amyloliquefaciens barnase gene, which encodes a secreted ribonuclease that is cytotoxic. Zhan and colleagues (1996) fused the promoter of the rice-pollen-specific gene PS1 to the barnase gene. In transgenic tobacco plants, there was a range from reduced pollen fertility to complete sterility (Zhan et al., 1996). One technical challenge of using cell ablation to obtain male sterility is that, if expression of the toxin is leaky--if it occurs in cells other than the flower buds--the plant can be damaged. If it is not possible to achieve
78 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS sufficient specificity of expression to prevent secondary effects in non- targeted tissues, then ablation will not be a useful bioconfinement tech- nique. Burgess and colleagues (2002) addressed the issue, and they showed that targeting specificity can be enhanced by engineering the barnase gene into 2 complementary fragments expressed from 2 promoters that overlap in expression. When coexpressed the 2 barnase fragments complement each other to reconstitute barnase activity (cytotoxicity). Male sterility resulted when expression of the partial barnase genes was targeted to the tapetum in a genetically engineered tomato. All 13 tomato progeny that inherited both transgenes were male-sterile. This dual-component system also allows genetically engineered lines to be used in hybrid seed production, because the progeny that inherit only a single barnase gene fragment are male- fertile. Crossing 2 lines homozygous for 1 barnase gene fragment each will produce a male-sterile hybrid. Another approach to obtaining seed production in lines engineered for sterility is to introduce a "restorer" gene from a second line that can over- come the toxicity of the sterility gene. Restorer genes often are found in naturally occurring male-sterile plant populations. Jagannath and colleagues (2002) developed a transgenic line in Indian oilseed mustard (Brassica juncea) that was male-sterile by the action of the barnase gene but that was restored to fertility in the presence (expression) of a barstar gene, thus permitting both bioconfinement and the option for heterosis breeding. Strengths The biology of male sterility has been studied intensively by crop breeders. New transgenic methods could be more reliable than are other genetic mechanisms for inducing male sterility. Some nontransgenic methods also could be useful. When effective, male sterility can greatly reduce pollen- mediated crop-to-crop and crop-to-wild gene flow. Weaknesses Most types of male sterility are leaky, so it will be important to test the reliability of this trait in a representative range of environmental condi- tions. Also, transgenic methods could fail if gene silencing or recombination separates the confined gene from the sterility system. Another disadvantage is that male-sterile crops grown for seed will need sufficient incoming pollen to guarantee high seed set, and if transgenic male-sterile plants are pollinated by sexually compatible weeds, their progeny (if fertile) could establish weedy crop-wild populations that have undesirable transgenic traits. As with all methods of reducing pollen flow, the potential of seed dispersal and vegeta- tive propagation should be examined to ensure adequate confinement.
PLANTS 79 Transgenes in Chloroplast DNA A potentially powerful strategy to reduce or prevent the flow of transgenes through pollen grains of most flowering crops is to incorporate the transgenes into the plant chloroplast or plastid genome instead of incorporating them into the plant nuclear genome (Gray and Raybould, 1998; Maliga, 2001, 2002, 2003). In most flowering plants, chloroplast genes are maternally inherited and are not carried by pollen (Maliga, 2002). Plastid genetic transformation was first developed for tobacco (Svab et al., 1990), but the technology also has been used to transfer genes into Arabidopsis (Sikdar et al., 1998), rice (Khan and Maliga, 1999), tomato, (Ruf et al., 2001), and potato (Sidorov et al., 1999). Eventually, the technology could provide a useful bioconfinement measure in other species, including turfgrasses. In addition to its use for bioconfinement, chloroplast gene transfer technology could offer commercial advantages over nuclear gene transfer methods. The production of a transgene product in chloroplasts is much higher than that for nuclear transgenes. Nuclear transgenes typically result in 23% total soluble proteins (reviewed by Kusnadi et al., 1997), whereas concentrations from chloroplast transgenes can be as high as 18% (Khan and Maliga, 1999). The production of chloroplast transgene products has been regarded to be 10- to 300-fold higher than that for genes transferred to nuclear genomes (Heifetz, 2000; Staub et al., 2000)--a production rate that could be especially useful for pharmaceuticals and industrial com- pounds. High concentrations of transgene-produced insecticides (Bacillus thuringiensis [Bt] toxins) could be needed for high-dose strategies to delay the evolution of insects that are resistant to plant-produced pesticides (Briggs, 1999). The greater production is possible because chloroplast transgenes are present as multiple gene copies per cell, and they are little affected by pre- or post-transcriptional gene silencing (Heifetz, 2000). A plastid genome could be transformed by homologous recombination, which allows the integration of transgenes at a specific site. That amount of precision could reduce the unintended phenotypic effects of transgenes, although it is not yet feasible for nuclear transformation. The very high level of expression of a transgene or of stacking multiple transgenes in the chloroplast could disturb the function of normal plant physiology and therefore could hamper performance of the genetically engi- neered crop. Other limitations of chloroplast-based bioconfinement relate to questions about whether plastid DNA can be inherited paternally (via pollen). In gymnosperms, such as conifers, plastid genomes are transmitted primarily paternally; most flowering plants transfer plastid genomes mater- nally. However, approximately one-third of the flowering plants investi- gated (Mogensen and Rusche, 2000) exhibited some degree of paternal or biparental plastid inheritance. For example, rye (Mogensen and Rusche,
80 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS 2000), chaparrel (Yang et al., 2000), kiwi (Tustolini and Cipriani, 1997), and the medicinal herb Damiana (Cipriani et al., 1995) are flowering plants with plastid genomes that are transmitted paternally. Some species exhibit polymorphism, with paternal inheritance accompanied by biparental and maternal variants (Rusche et al., 1995; Schumann and Hancock, 1991). In alfalfa, plastid genome inheritance is paternal (Keys et al., 1995) or bipa- rental (Losoff et al., 1995; Zhu et al., 1993). In some flowering plants, the inheritance of plastid DNA can be even more complicated. For example, interspecific hybrids of calla lilies exhibited maternal chloroplast transmis- sion in the first hybrid generation and either maternal or paternal inherit- ance in backcrosses (Yao and Cohen, 2000). Leakage can occur even in flowering plants for which paternal inheritance predominates (Avri and Edelman, 1991). Chloroplast genes also can "jump" into the nuclear genome, although the chances of this happening seem remote. This has occurred over long periods of evolution and investigators are attempting to document gene exchange between cell plastids and the nucleus. A recent study documented a 0.0006 chance for such transfer, and such transplanted chloroplast genes were not expressed in the nucleus (Huang et al., 2003). Should the chloroplast- targeted gene construct contain chloroplast-specific transcriptional control systems, the targeted genes could integrate in the plant nuclear genome at a very low percentage, but they will not be functional and should not be a major concern (Daniell and Parkinson, 2003). Despite the environmental and economic advantages, chloroplast transgene technology has not come into routine use, largely because the two gene transfer methods known for this technology have not been successfully applied to most crops. Biolistic bombardment of totipotent cells (cells that can produce whole fertile plants) and polyethylene-glycol-mediated naked DNA transfer into chloroplasts, followed by regeneration of whole selected transgenic plants, are still in development. More research is needed in this promising area. Strengths Chloroplast-specific transgenes would not be spread in the pollen of most cultivated plants. This approach could prevent transgene dispersal in pollen while preventing some of the disadvantages of male sterility, such as loss of pollen for cross-pollination. Weaknesses Technical difficulties have prevented this bioconfinement method from being feasible, and many types of desirable traits cannot be produced by
PLANTS 81 proteins that are confined to chloroplasts. Also, the leakiness of the system will need to be demonstrated empirically on a case-by-case basis. Like all methods in this section, seed-mediated dispersal of the transgene is not prevented. In addition, wild-to-crop pollination could occur in some situa- tions, resulting in hybrid progeny that are transgenic and potentially weedy. Cleistogamy (Closed Flowers) Cleistogamous flowers are those that never open; such flowers neces- sarily fertilize themselves (Lord, 1981). Therefore, creating plants with obligate cleistogamy has been mentioned as a possible bioconfinement method (e.g., Lu, 2003), although the committee is not aware of research on this topic. Theoretically, closed-flower varieties could be developed by selecting for sepals and other flower parts that encase the anthers and stigma. Obligate cleistogams would not be able to fertilize other plants, nor would they be able to be fertilized by other plants. With repeated self- pollination, obligate cleistogams that are derived from previous outcrossing would be subject to the genetic load uncovered by repeated inbreeding. Indeed, apparently no wild plant species produce flowers that are all cleistogamous; those species that produce them produce open "chasmogamous" flowers as well (Lord, 1981). Strengths At best use of obligate cleistogams would be an effective method of preventing gene escape by pollen. Weaknesses The method is not being developed, and perpetual self-fertilization could result in inbreeding depression. Transgene escape by seed or vegeta- tive reproduction could still occur for plants with obligate cleistogamy. Apomixis (Asexually Produced Seeds) Apomictic plants reproduce asexually by clonally produced seed (Grant, 1981; Richards, 1997). Progeny produced by apomixis (agamopermy) are usually genetically identical to the parent, and therefore uniform within and between generations. Many plant species can reproduce sexually and asexu- ally, but those that have dispensed with sex altogether are rare. Some breeders and genetic engineers have sought to introduce apomixis to the final products of plant improvement to fix and propagate superior hybrid genotypes. Highly productive hybrid plants thus are produced easily,
82 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS and there is no need to maintain inbred lines and cross them to create hybrids (Bock, 2002). Because obligately apomictic organisms do not require the fusion of female and male gametes to produce progeny and because they cannot be fertilized by gametes from another individual to create hybrid progeny, apomixis has been suggested as a bioconfinement method (Bock, 2002; Daniell, 2002; Gressel, 1999). If an organism is fully asexual and fully male-sterile, as for certain potato cultivars, then it cannot cross with other organisms. Unfortunately, obligate apomixis is extremely rare; many apomictic plant species retain low to moderate sexual seed production (Grant, 1981; Richards, 1997). Furthermore, moderate to high pollen fertility is common in apomictic plants (Grant, 1981; Richards, 1997), and many apomictic species require pollination to stimulate seed formation, even though gamete fusion does not occur (this is called "pseudogamy" or "semigamy" [Richards, 1997]). If fertile pollen introduces an allele for apomixis into a natural sexual population, it could spread quickly through the natural population (van Dijk and van Damme, 2000). Sexual reproduction has short-term disadvantages that are attributable to the "cost of sex." A parent passes on 100% of its genes to asexually-produced progeny, but only 50% of their genes to outcrossed, sexually-produced progeny. Therefore apomyctic organisms have an automatic two-fold fitness advantage over sexual organisms. Population genetic models have shown repeatedly that apomictic organisms always replace outcrossing sexual organisms when all else is equal (e.g., Charlesworth, 1989; Marshall and Brown, 1981; Will- iams 1975; Maynard Smith, 1978). If the apomictic allele is linked to the transgene, the resulting "selective sweep" could spread the transgene much more quickly and effectively than if the transgenic organisms were nonapomicts. The replacement of a sexual, genetically variable population with hybrid apomicts can lead to extinction by swamping (e.g., Ellstrand and Elam, 1993; Levin et al., 1996). This short-term advantage of asexuality is thought to account for the fact that if a species complex includes both sexual and apomict genotypes, the apomicts typically have a much wider distribution (e.g., Bierzychudek, 1985); for example, in the case of dandelions, the apomicts are widespread, and the sexual populations are mostly restricted to narrow refugia. Strengths Obligate apomixis with full male sterility will be an effective bio- confinement method only if the confinement goal is to prevent the formation of hybrid progeny.
PLANTS 83 Weaknesses Studies of naturally occurring apomixis suggest that this bioconfinement method could be leaky. Given that even obligate apomicts still produce seeds, the method cannot be used for bioconfinement if the goal is to prevent the production of progeny that might disperse. Also, it will be important to confirm that apomictic GEOs cannot establish invasive populations. Transgenes Absent from Seeds and Pollen Nontransgenic Scions on Transgenic Rootstock Many woody perennial crops, such as cultivars of grape, citrus, and avocado, are grown as grafted composites of two genotypes. The lower, root-bearing portion is the rootstock; the upper portion that bears flowers, fruits, and seeds is the scion. Lev-Yadun and Sederoff (2001) suggested that it is possible to graft nontransgenic scions of woody species onto transgenic rootstocks that have not yet reached reproductive age and that have had their branches pruned. Then, only nontransgenic reproductive structures are formed on those plants. In some species, vegetative propagules pro- duced by the rootstock or other adventitious rootstock growth could still result in the production of transgenic flowers. Recognizing this, Lev-Yadun and Sederoff suggested double grafting those species that have adventitious growth from the roots, starting with a transgenic shoot grafted (an "interstock") to a wild-type rootstock and then grafting again with a nontransgenic scion. The result would be a transgenic section sandwiched between the nontransgenic material. This technique would be appropriate for trees tested and deployed on limited scales and those sold as grafts, such as fruit trees and ornamental trees. Strengths As long as the transgenic rootstock or interstock cannot produce branches that bear flowers or vegetative propagules--and as long as that can be tested and demonstrated in appropriate environments--this could prove to be a simple and effective method of bioconfinement. Weaknesses The technique could not be used for nonwoody species, and it can be applied only for transgenic traits that are expressed in the rootstock or
84 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS interstock. This method would not be appropriate for large scale tests with forest trees. Excision of Transgenes before Reproduction Other researchers have proposed strategies for excising transgenes from plants before they begin sexual reproduction (Keenan and Stemmer, 2002) so a transgenic trait, such as herbicide resistance, could be expressed in the early stages of plant growth but the transgene would not spread in pollen or seeds. Unlike other V-GURTs, this method would not result in sterility, and growers could use saved seed (minus the transgene). A chemically induced or flower-specific promoter could be used to drive a recombinase enzyme that excised the transgene cassette for herbicide resistance; that transgene is located between two specific recombinase sites --Cre and loxP for example. This is an extension of other methods proposed for removing selectable marker transgenes (e.g., Zuo et al., 2001). However, it is much simpler to remove a marker gene in a few specimens before seed multiplication and commercial release than it is to remove genes from vast numbers of field- grown plants. Coaxing a promoter to work reliably in every flowering structure of every plant before pollen or seeds are formed is a major hurdle. Perhaps the strategy will be applicable in some crops at some time in the future, after technical problems with the method have been overcome. If the transgenic trait is not needed in the fruits or seeds, the approach could be useful. Strengths Transgene excision could be used to block the dispersal of transgenes in pollen and seeds, without requiring seed suicide. Weaknesses It could be extremely difficult to guarantee the reliability of the system. And it cannot be used for transgenic traits that must to be expressed in seeds or other reproductive structures. Artificially Induced Transgene Expression A promising method for reducing the effects of unwanted transgenes is to use a system in which the transgenic trait is activated by an artificial stimulus, such as a chemical spray (Figure 3-1; Daniell, 2002; FAO, 2002). Salicylic acid, for example, could be used to induce plants to produce pest- fighting compounds when pest populations reach a given threshold. With-
PLANTS 85 out the spray, the inducible promoter would not be activated and the plants would lack the trait for insect or disease resistance. Likewise, seed produced by the crop or plants that the crop crosses with would not express the trait, although they would carry the transgene construct. In another example, a chemical could be applied before the crop is sprayed with herbicide to induce herbicide resistance (Gressel, 2002). Because the trait expression is restricted by an inducible promoter, this approach is considered a T-GURT. The extent to which the system prevents the unauthorized acquisition of the trait will depend on the specificity of the stimulus, such as the type of chemical spray that is used. Strengths Use of artificially induced transgene expression could restrict transgene expression to limited periods. T-GURT constructs would not be expressed in other generations or in crop-wild hybrids unless a particular stimulus was applied. Thus, the spread of transgenes would not be prevented, but bioconfinement of particular traits would be possible. Weaknesses T-GURTs are still in the early stages of development and might not prove practical. Although they could give access to some transgenic traits that are useful on a transitory basis, other traits could require constant expression of the transgene to achieve a desired result (e.g., enhanced latex production in slow-growing guayule shrubs). Reducing Gene Flow to Crop Relatives Several approaches could be used to restrict the spread of transgenes to sexually compatible wild relatives and cultivars of a crop. None of those methods is in use, and they are not likely to achieve complete containment of transgenes. Nonetheless, they could come into use in the future, espe- cially in combination with other biological and nonbiological confinement methods. Repressible Seed Lethal Confinement A group in Canada recently proposed a strategy for blocking gene flow to nontransgenic crops and wild relatives (Figures 3-1 and 3-2; Schernthaner et al., 2003). The approach involves inserting a "seed lethality" transgene into the crop plant's DNA. The transgene is tightly linked to a transgene that codes for a novel trait such as disease resistance. The plant is crossed
86 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS with another plant that has a transgene that codes for repression of the seed-lethal gene. Then, selected F1 offspring from the cross are used for seed multiplication and production for sale. With this system, field-grown commercial plants produce viable seeds when they naturally self-pollinate or cross with each other in the field (Figure 3-2; 25% of the seeds are expected to be inviable because they would be homozygous for the seed-lethal transgene and would lack the repressor transgene). However, if a transgenic plant spontaneously crosses with a different crop variety, a wild conspecific, or a different species, all progeny with the seed-lethalnovel-trait construct will lack the repressor transgene, and all seed will be inviable. This occurs because the seed-lethal novel-trait construct and the repressor transgene segregate independently. Ideally, the repressor transgene would be inserted at the same location on the homologous chromosome as the seed-lethalnovel-trait construct, so that the repressor and seed-lethal transgenes would not segregate together, FIGURE 3-2 Repressible seedlethal bioconfinement. Adapted from Schernthaner et al., 2003. SL, seed-lethal gene; R, repressor gene.
PLANTS 87 except as a result of rare recombination events (chromosome crossovers). During meiosis and gamete formation, the haploid pollen and ovules inherit one transgene construct or the other, but not both. Thus, outgoing crop pollen and incoming noncrop pollen produce inviable seeds with this system. Presumably, all seeds would complete normal development up to the point of maturation, and germination would be the only step affected by the seed- lethal transgene. The repressible seed-lethal method is similar to V-GURTs, such as the terminator technology, but it differs in that farmers can obtain viable seed from a transgenic crop. Farmers in developing countries would be able to save and distribute seeds for future use, although as much as 25% of a seed crop could be inviable, which is unlikely to be acceptable. Farmers would not be able to cross a transgenic crop with local varieties, so in this sense their use of agronomically valuable crop genes is still restricted, as it is with other GURTs. Strengths The extent of pollen-mediated transgene dispersal would be greatly reduced or eliminated because offspring that inherit both the new transgenic trait and the tightly linked seed-lethal transgene would not inherit the repressor transgene and thus would not pass the novel transgene to their offspring. At the same time, unauthorized use of the transgenic crop for further breeding would be difficult or impossible, which is an advantage. Weaknesses This method is still in the early stages of development, and several technical hurdles must be overcome before it can be used as a bioconfine- ment method. For example, site-specific insertion of transgenes has yet to be achieved in plants. Partial confinement is possible, though, as long as the transgenic constructs are located on homologous chromosomes. Concerns about the consequences for nearby relatives of producing dead seeds from the crop are similar to those for terminator and related transgenic sterility methods. Continued use of this system in a single locale could lead to the introgression of the repressor gene into nearby natural populations. When it reaches a high enough frequency, the presence of that allele would render the method ineffective in preventing introgression into that population. Finally, the method does not prevent seed-mediated dispersal of the transgene, for example by natural seed dispersal, spillage during harvest and transport, seed mixing, or international distribution of food aid.
88 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS Cross-Incompatibility Crosses between species, both plant and animal, often fail because of events that prevent fertilization or embryo development (Futuyma, 1998). Such barriers to hybridization are called "cross-incompatibility." Crosses between cross-incompatible species either fail entirely or fail most of the time. In plants, cross-incompatibility can be expressed as the inability of pollen to germinate, as abnormal pollen tube growth, as failure of pollen tubes to penetrate the ovary, or as the spontaneous abortion of seeds and fruits after fertilization (Levin, 1978). It has been suggested that alleles for cross-incompatibility could be identified and moved into transgenic varieties to prevent them from mating with other varieties (Evans and Kermicle, 2001). Research on "lock and key" approaches to prevent pollen from fertilizing ovules on other varieties of sexually compatible plants is still very preliminary, as described at a recent workshop (Arcand, 2003). Strengths If absolute, bilateral incompatibility is created between different lineages, species, subspecies, and so on, natural hybridization can be pre- vented. Weaknesses Such bioconfinement methods have not yet been created and tested. Because some cross-incompatibility barriers can be breached by environ- mental factors, such as high temperature or the presence of pollen from a compatible relative (e.g., Richards, 1997), it is possible that engineered incompatibilities might also be environmentally labile. Obviously, cross- incompatibility will not prevent the movement of transgenes in seed or vegetative propagules created by the transgenic plant. Chromosomal Location in Allopolyploids This technique involves placing transgenes in chromosomes that would be preferentially excluded in crop-wild progeny because of problems with chromosomal pairing at meiosis. Many crops--bread wheat, peanuts, and coffee--are allopolyploids that house multiple genomes derived from dif- ferent sources. For example, bread wheat is a hexapolyploid with three paired sets of homologous chromosomes (2n = 6x = AABBDD). Because there is an even number of matching sets of chromosomes, bread wheat plants undergo normal meiosis and gamete formation--as though they were diploids. Often, only one genome of the crop is homologous with that of a
PLANTS 89 related weed. Alleles on those chromosomes will be transmitted to the weed by backcrossing with hybrids. But it has been thought that alleles on the other genomes would face a considerable barrier to transmission. For example, bread wheat, Triticum aestivum, and jointed goatgrass, Aegilops cylindrical, which is a weed, share the same D set of chromosomes. Bread wheat's A and B sets are not homologous with the chromosomes of jointed goatgrass. If a novel allele is incorporated into either the A or the B chromo- somes of bread wheat, they should be preferentially excluded over the generations of backcrossing to the wild parent and have considerable diffi- culty introgressing into wild populations (Gressel, 1999). In fact, Lin (2001) noted that a transgene that confers herbicide resistance was inherited by jointed goatgrass when it occurred on the D genome, which is shared by both taxa, but not when it occurred on the unshared B genome. This is encouraging, but it will still be important to measure the frequency of rare episodes of recombination that could allow transgenes to move into non- homologous sets of chromosomes. Apparently, other experimental work has revealed that chromosomal location is not a sure safeguard in allo- polyploids of wheat (Zemetra, unpublished data). Some data are available for a similar cytogenetic situation involving another allopolyploid crop. Oilseed rape, Brassica napus (2n = 4x = AACC), shares one set of chromosomes (the A set) with the weed B. campestris (= B. rapa) (2n = AA). Therefore, it might be expected that if alleles of concern were placed on rape's other set of chromosomes (the C set) they would be preferentially excluded in the wild (Gressel, 1999). When Metz and colleagues (1997) observed a strong decrease in the frequency of a transgene in progeny resulting from a backcross of a B. napus × B. campestris hybrid to B. campestris, they explained that decrease as the result of such preferential exclusion. Tomiuk and colleagues (2000) examined the situa- tion further and reached a different conclusion. First, they found that other cytogenetic data (Fantes and Mackay, 1978) showed no preferential exclu- sion of the C genome in backcrosses. Second, they created a model to examine the data of Metz and colleagues (1997) more closely. They found that an alternative, equally parsimonious, hypothesis could not be excluded: that the "decrease in the frequency of transgenic plants within the first backcross generation can also easily be explained by selection against transgenic A-chromosomes of B. napus" (Tomiuk et al., 2000). They con- clude, "without more detailed genetic information...no decision can be made in favor of the A- or C-genome as the safer candidate with respect to the introgression of transgenes into wild populations" (Tomiuk et al., 2000). Clearly, there is a need for more experimental work of this type. As with some of the other proposed bioconfinement methods, this one would work primarily for preventing or reducing introgression from the transgenic organism to wild populations.
90 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS Strengths If this method were fully effective, it would prevent backcrossing to the wild parent, limiting introgression of transgenes into populations of non- transgenic wild relatives. Weaknesses The technique would not necessarily limit transmission of transgenes into the F2 progeny of crop-wild hybrids. Fitness Reduction in Transgenic Crop-Wild Progeny Gressel (1999) proposed "tandem constructs" for "transgenic mitiga- tion (TM)" in crops. His idea is to link to both sides of a transgene alleles that would confer a substantial disadvantage to a weed or volunteer. Here is the overall scheme: (1) Tandem constructs of genes genetically act as tightly-linked genes, and their segregation from each other is exceedingly rare, (2) There are traits that are either neutral or positive for a crop that would be deleterious to a typical or volunteer weed, or to a wild species; and (3) Because weeds are strongly competitive amongst themselves and have large seed outputs, individuals bearing even mildly harmful traits are quickly eliminated from populations. Even if one of the TM genes mutates, is deleted, or crosses over, the other flanking TM gene will remain providing mitigation (Gressel, 2002). Traits that would be beneficial under cultivation, but detrimental to plants in the wild, are common in modern agronomic crops: lack of second- ary seed dormancy, uniform ripening, lack of shattering, dwarfing, or susceptibility to a specific herbicide (Gressel, 1999, 2002). For tandem constructs to be effective, the traits conferred by the flanking alleles must be dominant relative to their counterparts in wild plants. Although the genetic basis and chromosomal location for such traits are unknown for most crops, data are beginning to accumulate (e.g., Burke et al., 2002; Gepts, 2001; Gressel, 2002). To illustrate how a tandem construct might work, Al- Ahmad and Gressel (2002) created an experimental model system. They transformed tobacco with an herbicide resistance gene linked to a dwarfing gene. The dwarfed plants proved competitively inferior to the wild-type segregants, and only at the lowest density treatment did the dwarfs form flowers.
PLANTS 91 Strengths If effective in a wide variety of environments, TM would limit the introgression of transgenes in the wild. Although the tandem-construct concept was designed specifically to prevent crop alleles from establishing in free-living populations, it is clear that it can be extended to many bioconfinement situations as long as the transgenic mitigator confers a substantial disadvantage to organisms that bear it in an environment for which the primary transgene was not intended. Weaknesses This method depends on using linked alleles that are harmful to wild relatives but neutral or beneficial for crops . Although they will frustrate the evolution of weediness, if introgression of the biocontrol alleles into a very small population did occur by pollen or seed swamping, then depressed fitness of future generations of that population could result, increasing the risk of extirpation of that population, which would be a concern if an endangered wild relative is involved. Phenotypic and Fitness Handicaps Many cultivated plants are highly domesticated, requiring special con- ditions, such as amended soil; irrigation; and protection from weeds, pests, and pathogens, to survive and reproduce. Maize, soybean, tomato, and many other food crops fall into this category because they rarely, if ever, become naturalized. In contrast, other species (Bermuda grass, raspberry, poplar, spruce) are more similar to their wild progenitors and are therefore more likely to establish feral populations. Conventional and transgenic methods can be used to select cultivated genotypes in both groups of plants that are increasingly "domesticated," in the sense that they need specific human intervention to be able to survive and reproduce. Handicap methods could be most useful in species that are relatively undomesticated, but even species like maize could become easier to confine by adding handicaps that restrict growth under standard agricultural conditions. Handicap strategies are similar to the tandem construct method for lowering the fitness of any feral or wild plants that carry unwanted transgenes, but they are more general in that the crop plants have new features that make them more dependent on specialized growing conditions. One of many possible approaches to establishing a biological handicap is to select for plants that are "chemically dependent." Auxotrophs are a class of mutants that depend on the exogenous supply of a nutrient that arises from a mutation in a biosynthetic pathway. Such fitness-reducing
92 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS mutations could be selected after mutagenesis or created by transforma- tion. Numerous examples of plant auxotrophs created by exposure to mutagens have been reported in the literature (Blonstein et al., 1988; El Malki and Jacobs, 2001; Fracheboud and King, 1988, 1991; Meinke, 1991; Wright et al., 1991). Few genetically engineered auxotrophs have been reported, but they are likely to become plentiful as more developmen- tally important genes are identified. One possible strategy is to use knock- out mutants that delete a vital biological process, such that the plant requires artificial growing conditions for survival. In another approach, Baroux and colleagues (2001) reported developmental mutations in Arabidopsis that were created by transactivation of barnase and that af- fected the embryos and growing shoots of adult plants. When the barnase protein was expressed during embryogenesis it destroyed most of the cellu- lar RNA, leading to the production of sterile seed. To maintain genetically engineered lines that carry auxotrophic mutations (for survival during field tests and production), it also will be necessary to develop transgenic methods to overcome the mutation. Thus, the creation of or inclusion of auxotrophic or developmental mutations could be one way to prevent transgene escape into the environment. Another possibility would be to select for life history traits and mor- phological variants of fast-growing trees, so they have greatly restricted branching and short stature at maturity (Mann and Plummer, 2002). If miniature poplar trees were selected for rapid growth and commercially important traits, such as low lignin content, those genotypes and their progeny might be unfit for survival other than in intensively managed settings. By adding a back-up confinement method, such as male sterility, gene flow and persistence of genetically engineered traits in other poplar populations could be so low as to become negligible. Strengths Auxotrophy and other handicap strategies might contribute eventually to integrated confinement methods. Weaknesses The methods described above are still in the early stages of develop- ment. To be effective, it will be important to ensure that bioconfined transgenes remain tightly linked to handicap traits and that they do not segregate with weedier traits after episodes of sexual reproduction and gene flow. Although they will frustrate the evolution of weediness, if introgres- sion of the biocontrol alleles into a very small population did occur by pollen or seed swamping, then depressed fitness of future generations of
PLANTS 93 that population could result, increasing the risk of extirpation of that popu- lation, which would be a concern if an endangered wild relative is involved. As with all types of bioconfinement, the leakiness of the methods should be determined empirically under realistic field conditions before they are used to prevent the spread of transgenes. Reducing Exposure to Transgenic Traits In some cases, the reason for choosing bioconfinement of a transgene could be to reduce human or environmental exposure to transgene prod- ucts. This sometimes can be accomplished using special promoters, such that the transgene is expressed in some parts of a plant but not in others. Plant tissue and organ-specific gene expression can be used to produce a heterologous protein (a protein conferred by a transgene) that occurs only or mainly in specific tissues or organs. Most transgenic crops have constitu- tive promoters that allow the transgene to be expressed at all times through- out the plant. In the future, many more options will be available, including chemically induced, tissue-specific promoters (e.g., Mett et al., 1996). Here, we review a few examples from the large body of research findings on tissue-specific promoters. Some of them also could be useful in bioconfine- ment methods, such as inducible lethality in seeds, which involve targeted blocking of plant growth and development. Green-Specific (Chloroplast-Targeting) Gene Expression The photosynthesis-specific promoter of the ribulose 1-5, bisphosphate carboxylase (rubisco) gene of tomato has been used to express a gus gene (a marker for transgene expression) in green tissues of apple trees (Gittins et al., 2000). That promoter also has been used to regulate other genes in Arabidopsis and maize (Poirier et al., 1992; Zhong et al., 2003). Building on this research, it should be possible to keep transgenic seeds, pollen, and roots free from specific transgene products. Because pollen does not contain chloroplasts, photosynthesis-targeted gene expression could be an ideal method for reducing exposure to transgenic products in pollen. A better but more difficult method for achieving green-specific gene expression is to transfer genes directly into the chloroplast genome rather than to the nuclear genome. (See above section on chloroplast transformation.) Roots and Tuber-Specific Gene Expression Promoters specific to roots have been used to produce heterologous proteins that are not produced in other parts of the plant (Sakuta and Satoh, 2000; Yamamoto et al., 1991). The roots of carrots and a few other
94 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS species contain carotenoids, so the genes of interest could be expressed using a carotenoid-specific gene promoter at the same time the presence of the heterologous gene products is minimized in other parts of the plant (Fraser et al., 1994). Tuber-specific promoters have been used in potatoes and other crops. For example, human interleukin genes under the control of a patatin promoter have been expressed in vitro in potato microtubers. In that experiment, the microtubers functioned as bioreactors to produce large amounts of interleukin (Park and Cheong, 2002). Potato tubers also have been used as bioreactors for production of a sucroselike compound using a gene from Erwinia rhapontici (Boernke et al., 2002). Another root-specific location for transgene expression in legumes is the root nodule, within which symbiotic bacteria fix nitrogen. A complete soybean leghemoglobin gene was exclusively expressed in root nodules of transgenic Lotus corniculatus. In this case, gene expression was observed after transgenic roots were infected with a nitrogen-fixing bacterium (Stougaard et al., 1987). Vascular-Tissue-Specific Gene Expression Some insects, such as aphids and hoppers, that suck plant sap by feed- ing on plant vascular tissues, could be controlled by biopesticides that are expressed only in the plant vascular systems. Several strategies for vascular- tissue-specific gene expression have been identified in experimental systems. When a marker gene (gus) was expressed with the maize streak virus coat protein promoter in transgenic rice, the GUS protein was produced only in the vascular tissues, particularly in phloem-associated tissues (Mazithulela et al., 2000). Also, when the Commelina yellow mottle virus promoter was used to express the gus gene in transgenic oat, vascular-specific production of the GUS protein was observed in shoots, leaves, floral bracts, roots, and vegetative parts of ovaries but not in reproductive cells (Tolbert et al., 1998). Phloem-specific gene expression also was produced in transgenic rice plants using the RTBV (rice tungro bacilliform virus) promoter (Yin et al., 1997). Flower- and Fruit-Specific Gene Expression To control insects and pathogens that attack young flowers, transgenes can be expressed in the sepals and not in anthers, seeds, or other plant parts. For example, experiments on Forsythia X intermedia cv. Spring Glory using the ans gene showed that ans is exclusively expressed in sepals at the early stages of flower development (Rosati et al., 1999). A similar method of tissue specificity of gene expression was used to express the genes of interest in carotenoid-rich parts of plants, such as tomato fruits, while
PLANTS 95 avoiding the presence of the heterologous gene products in other plant parts (Fraser et al., 1994). Pollen-Specific Gene Expression The need for pollen-specific transgene expression may be relatively uncommon, unless the transgenic trait is needed specifically in pollen. In some applications, the desired trait might be reduced allergenicity. For example, antisense technology can be used to reduce or eliminate the harm- ful expression of a naturally occurring gene in pollen. The allergic asthma effect of ryegrass pollen was reduced by inserting a pollen-specific promoter to drive an antisense gene that silenced an allergen gene (Bahalla et al., 1999). Seed-Specific Gene Expression Seed-specific gene expression can be used to produce the gene product only in the seed parts such as the embryo or the aleuron. The method could be useful for transgenes that confer improved seed quality or protection from insects during seed storage. Two barley aleuron-specific promoters from genes that encode lipid transfer protein (Ltp1) and chitinase (Chi26) were used to express the gus marker gene in grains of transgenic rice (Hwang et al., 2001). Similar experiments to demonstrate the efficacy of other seed- specific promoters have been carried out in soybean, tobacco, and bean (Baeumlein et al., 1987; Cho et al., 1999; Ellis et al., 1988; Iida et al., 1995). Late-acting, seed-specific promoters also can be used to kill the seeds just before they are fully ripe, as in the terminator and related applications described above. Strengths Tissue- and organ-specific promoters could be useful for reducing the amount of novel protein that a plant produces or for targeting specific organs, such as anthers, in order to interfere with their development. Weaknesses Some of the currently available tissue- and organ-specific promoters are not as precise or effective as would be required to avoid transgene expres- sion in other parts of the plant. Basic research is still needed on the regula- tion of gene expression, including studies of genes that could be used for tissue- and organ-specific gene expression and for genes that could be turned
96 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS on or off by chemical intervention or by other methods, such as exposure to extreme temperatures. In many examples described above, bioconfinement would not be improved, although exposure to unwanted transgene prod- ucts could be reduced. Choice of Alternative Organisms or "Abstinence" In many cases confinement can be obtained from specific aspects of the biology of an organism. Some GEOs, such as vaccine-producing microalgae, can be grown and harvested indoors, thereby obviating the need to develop bioconfinement for field conditions. If field releases are essential for a given genetically engineered application, some characteristics of the transgene "host" species, such as whether it is traded as a commodity crop, like corn and soybean, could greatly influence whether bioconfinement is needed. Likewise, if all possible plant species that host a given genetically engi- neered application require strict containment rather than confinement, plans to produce field-released plants should be abandoned. Choosing which GEOs to develop and which to abandon is effectively a form of bio- confinement. The biosafety reasons for choosing a particular organism and the place of its deployment--in the field or indoors, for example--are varied. The choice of an appropriate plant for producing an industrial compound must consider whether it could cause harm to humans if consumed. A plant that typically is grown to produce a common food product would be a poor choice for engineering to produce that compound, unless the plant were to be grown under stringent conditions of confinement (Ellstrand, 2003b). This is an important issue for any novel compound or GEO for which zero tolerance is given for bioconfinement failure. Increased security in bioconfinement can be obtained in three ways that involve the choice of the system: choosing a different organism to engineer, choosing not to grow genetically engineered plants outdoors, or choosing to abandon the project. Each is examined below, using the example of a novel industrial compound that must not enter the human food supply: Organism choice. Choosing an organism that is not used for food or feed could prevent that compound from entering the human food chain. Many nonfood plants have been successfully transformed, including tobacco, petunia, and duckweed. Likewise, plants, such as belladonna, are known to be toxic, and they can be used because they already are avoided as a food source. Field release choice. In many cases, valuable industrial compounds can be grown in high concentrations in plants. It is much easier to monitor plants that are grown and processed indoors and to control their reproduc-
PLANTS 97 tive processes. Likewise, alternative organisms that can be grown indoors, such as microbes, could be used to produce some compounds. For example, genetically engineered insect larvae could be grown in vats under strict biosafety procedures and processed before reaching adulthood. Choice not to proceed. Growing organisms that produce extraordinary amounts of a toxic compound might require such stringent bioconfinement that a project would not be cost-effective. Considered broadly, the decision not to develop a given GEO is a form of bioconfinement. The committee recognizes that some biotechnology companies already have decided not to proceed with projects because of intractable biosafety issues. Also, some reasons for applying genetic engineering in the first place can be addressed using alternative approaches, such as improving integrated pest manage- ment, obviating the need to develop genetically engineered pest-protected plants. Strengths The careful choice of which organism to develop, whether to proceed with field release, or whether to abandon an idea or project altogether constitute bioconfinement. By making the decision early, expensive and difficult confinement options are rendered unnecessary. Weaknesses Choosing a new organism can set a project back in time and cost, and there is always the chance that the organism will not prove commercially viable. Baseline information and optimal breeding and cultivation tech- niques for the new organism might need to be developed. The choice of not growing a GEO outdoors can limit profitability, especially if techniques for indoor cultivation must be developed. Choosing not to proceed with a project is an even more difficult decision economically, especially if there has been substantial early investment. Moreover, abandonment of a project could prevent some benefits from being realized. The following sections include a discussion of bioconfinement options for genetically engineered trees, and short overviews of related topics in grasses and algae. Trees and grasses have reached the field-testing stage of development. Seaweed and other macroalgae are just beginning to be inves- tigated and produced. Some features of those organisms pose unique chal- lenges for effective bioconfinement, whereas other issues are common to many types of GEOs. In addition, although some species have a long history of cultivation and genetic improvement, others are essentially undomesti- cated, so there is little baseline knowledge of relevant biological information.
98 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS GENETICALLY ENGINEERED TREES Two fundamentally different technologies are used to transfer genes into trees: Agrobacterium-mediated gene transfer and microprojectile bom- bardment (or biolistics). The naturally evolved plant transformation system of Agrobacterium is considered more reliable for producing stable trans- formants, so it is the system of choice for most species. However, not all trees are susceptible to Agrobacterium. Conifers, for example, are espe- cially resistant, so biolistics has been the method of choice (Klein et al., 1988). An advantage of biolistics is the relative ease of cotransforming genes on separate plasmid vector DNA (Bishop-Hurley et al., 2001), and cotransformation lends itself well to multiple-component systems for bio- confinement that prevent dispersal of transgenes (Table 3-2). Although particle bombardment is easy, the approach tends to deliver several transgene copies into each recipient cell, and they often integrate as tandem repeats. This arrangement frequently leads to gene silencing or excision (loss) of the transgene (see Box 3-1). Agrobacterium-mediated transformation is more likely to result in the integration of a stable, single, full-length copy of the transgene in a region of the chromosome where genes are actively expressed. Agrobacterium-mediated gene transfer is effective for use in many fruit and nut trees (reviewed by Trifonova and Atanassov, 1996) and in hardwood timber species. Conifer species are beginning to yield to Agrobacterium-mediated gene transfer as well, through the use of multiple copies of virulence genes (Wenck et al., 1999). Bioconfinement of Trees In many cases, confinement of genetically engineered trees is not neces- sary. In fact, the transfer of resistance genes could be used to restore popu- lations of trees that are threatened by exotic pests and insects (Adams et al., 2002). Examples include American chestnut and American elm, which were lost in the past century; Fraser fir and eastern hemlock, which have declined recently across much of their native ranges in the eastern United States; and oak trees, which are experiencing sudden death in western states. Genetic engineering could help restore those species within a manageable period and without the genetic dilution that occurs with sexual hybridization. If the introduction of genetically engineered trees is restricted to areas they once inhabited, growth and eventual spread should remain restricted to their natural ranges. Thus, beyond limiting spread to the sites of introduc- tion, confinement techniques might not be necessary in restoration projects. Another trait that might not always require confinement is lignin modi- fication. Lignin is important to the structural integrity and adaptive strate- gies of vascular plants, but it is problematic for agroindustrial use of crops
PLANTS 99 TABLE 3-2 Genetically Engineered Woody Plants, Permits Approved by APHIS for Field Tests in the United States, 19892003 Organism Phenotype (Number of Submissions) Gene Apple AP - Flowering time altered (1) LFY BR - Fire blight resistant (7) Cec-B or Att-E FR - Apple scab resistant (3) CHT IR - Oblique banded leafroller (1) CHT; CHI IR - Coleopteran resistant (1) CryIA(b) and CryIA(c) IR - Lepidopteran resistant (7) CryIA(c) PQ - Brown spot resistant (2) PPO PQ - Ethylene synthesis reduced (1) ACCS antisense PQ - Fruit ripening altered (4) SAMT or ES; ACCS antisense PQ - Sugar alcohol levels increased (5) SPDH or SDH Avocado FR - Fungus resistance (1) Def Citrus sinensis BR Xanthomonas campestris LYZ X Poncirus resistant (1) trifoliate Coffee PQ - Caffeine concentration reduced (1) XMT antisense PQ - Ethylene production reduced (2) ACO or ACS Cranberry IR - Lepidopteran resistant (1) CryIA(a) Eucalyptus HT; MG (1) CBI; GUS grandis Grape BR - crown gall resistant (4) CBI FR - Botrytis resistant (3) CBI IR - Lepidopteran, Criconemella, CryIA(c); GNA Meloidogyne (1) FR - Powdery mildew resistant (10) PGUS; LGB; PGLC; CHT; PGL MG, SM (4) AHAS variant or ALS; CBI, NPTII PQ - Improved fruit quality (1) ALS; CBI VR - Closterovirus resistant (3) CBI VR - Nepovirus resistant (2) CBI VR - Nepovirus resistant; CBI B - Closterovirus resistant (1) VR - CBI (2) CBI Grapefruit BR - Citrus canker resistant (1) SBP IR - Aphid resistant (1) GNA MG (1) GUS; NptII VR - Closterovirus resistant (2) CTV-CP continued
100 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS TABLE 3-2 Continued Organism Phenotype (Number of Submissions) Gene Papaya FR - Fruit rot, powdery mildew, CHT Phytophthora (1) IR - Leafhopper resistant (1) GNA PQ - Ethylene production reduced (2) ACSp; Cre VR PRSV resistant (15) PRSV-CP Pear AP (1) Rol BR - Fire blight resistant (1) Cec-B PQ - Fruit ripening altered (3) SAMT Persimmon AP - Drought and cold tolerant (1) COX; SORS; GUS FR (1) PGIP; GUS IR - Lepidopteran resistant (1) CryIA(c) MG (1) GUS Pine MG (17) GUS; NptII; CBI PQ - Decreased lignin (1) CBI Plum PQ - Ethylene production reduced (1) ACOp VR - PPV resistant (2) PPV-CP Poplar AP - Altered lignin biosynthesis (4) 4CL, OMT, C4H, COMT BR - Crown gall resistant (1) IAAm FR - Septoria and others (2) PGL; OXA FR - General (Venturia, etc.) (2) BAC HT - CBI (8) CBI HT - Glyphosate tolerant (19) CBI; EPSPS; or GOX HT - Glyphosate; phosphinothricin; Barnase; Barstar; PAT PQ (1) MS1; CBI HT Phosphinothricin; MG (1) PAT IR - Coleopteran resistant (13) CBI or CryIIIA IR - Lepidopteran resistant ( 1) CryIA(c) IR - Leaf beetle resistant (1) CryIIIA MG and SM (1) NptII; CAT MG only (4) GUS; CBI OO - Cell wall altered (1) CBD OO - Flowering time altered (1) CBI OO - Sterility (3) Barnase; DTA; LFY PR (2) P450; MIR PN (1) GS Populus HT (7) CBI deltoides MG (2) GUS; NptII Rhododendron FR - Phytophthora resistant (2) Magainin MG (1) GFP; NptII
PLANTS 101 TABLE 3-2 Continued Organism Phenotype (Number of Submissions) Gene Raspberry FR (1) PGIP FR - Fruit rot resistant (1) PGIP VR - RBDV resistant (3) RBDV-MP VR - ToRSV resistant (1) ToRSV-CP PQ - Fruit ripening altered (3) PGIP; PGIP and SAMH Service berry IR - Lepidopteran resistant (1) CryIA(c) Spruce IR - Lepidopteran resistant (1) CryIA(c) Sweetgum AP - Altered plant development (1) CBI AP - Fertility altered (2) CBI HT (4) CBI HT - 2,4-D tolerant (2) Tfd HT - Glyphosate tolerant (3) CBI HT - Phosphinothricin (1) CBI MG only (4) GUS Walnut AP - Adventitious root formation (2) rol and CBI AP - Cutting rootability increased (1) Rol AP - Flowering altered (1) LFY BR - Bacterial leaf blight resistant (1) TMK FR; IR; VR (1) LRV-CP; LEC; SAR; rol IR Lepidopteran resistant (5) CryIA or CryIA(c) NR - Pratylenchus vulnus resistant (1) GNA NOTE: Field test data downloaded from Information Systems for Biotechnology, http:// www.nbiap.vt.edu/cfdocs/fieldtests2.cfm Feb. 19, 2003; updated May 23, 2003; does not include submissions denied or withdrawn. Phenotype Key: AP, agronomic properties; BR, bacterial resistance; FR, fungal resistance; HT, herbicide tolerant; IR, insect resistant; MG, marker gene; NR, nematode resistance; OO, other; PN, plant nutrition; PR, bioremediation; PQ, product quality; SM, selectable marker; VR, virus resistance. Gene Key: 4CL, 4-Coumarate:CoA ligase antisense gene from poplar; ACO, ACC oxidase antisense from coffee; ACOp, ACC oxidase antisense from Prunus; ACS, ACC synthase antisense from coffee; ACSp, ACC synthase antisense from papaya; AHAS, acetohydroxyacid synthase; ALS, acetolactate synthase; Att-E, attacin gene from Hyalophora cecropia; BAC, bacteropsin gene from Halobacterium halobium; BARNASE, barnase gene; barstar, barstar gene from Bacillus amyloliquefaciens; C4H, Cinnamate 4-hydroxylase gene from Populus tremuloides; CAT, chloramphenicol acetyltransferase gene from E. coli; CBD, cellulose binding protein gene from Clostridium cellulovorans; CBI, confidential business information; Cec-B, cecropin gene from Hyalophora cecropia; CHI, chitobiosidase probably of fungal origin; CHT, chitinase probably of fungal origin; CLRV-CP, coat protein gene from CLRV; COMT, caffeate O-methyltransferase gene from Populus tremuloides; COX, choline oxidase; Cre, recombinase from Bacteriophage P1; CryIA(c), CryIA(c) crystal toxin gene from Btk; CryIIIA, CryIIIA crystal toxin gene from Bt; CrylA, crystal toxin gene A from Bt; CTV-CP, coat protein gene from CTV; Def, defensin from Arabidopsis thaliana; DTA, diptheria toxin continued
102 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS TABLE 3-2 Continued A gene from Corynebacterium diptheriae; EPSPS, 5-enolpyruvylshikimate-3-phosphate syn- thase; ES, ethylene forming enzyme from apple; GFP, green fluorescent protein from Aequorea Victoria; GNA, lectin gene from snowdrop (Galanthus nivalis agglutinin); GOX, glyphosate oxidoreductase gene; GS, glutamine synthase gene; GUS: E. coli -glucuronidase gene; HYR, hygromycin phosphotransferase gene; IAAm, IAA monooxygenase gene; LEC, lectin genes from barley, rubber tree, and/or stinging nettle; LFY, leafy homeotic regulatory gene from Arabidopsis thaliana; LGB, lignan biosynthesis protein gene from pea; LYZ, lysozyme gene from cow; magainin, magainin gene from Xeanopus laevis; MIR, mercuric ion reductase from E. coli ; MS1, male sterility protein gene from Populus trichocarpa; NPTII, neomycin phospho- transferase gene from E. coli; OMT, O-methyltransferase gene from Populus tremuloides; OXA, oxalate oxidase gene from E. coli; P450, cytochrome P450 gene from man; PAT, phosphinothricin acetyl transferase gene from Strep. hygroscopicus; PGIP, polygalacturonase inhibitor protein from bean; PGL, anitmicrobial peptide gene from wheat; PGUS, -glucuronidase from pea; PGLC, B-1,3-glucanase antisense from pea; PPO, Polyphenol oxidase from Apple; PPV-CP, Coat protein gene from PPV; PRSV-CP, Coat protein gene from PRSV; RBDV-MP, nonfunctional RBDV movement protein; rol, rol hormone gene from Agrobacterium rhizogenes; SAMH, S-adenosylmethione hydrolase from E. coli; SAMT, S-adenosylmethionine transferase from E. coli; SAR, systemic acquired resistance gene from tobacco; SBP, synthetic binding peptide to Xanthomonas; SORS, sorbitol synthase from apple; SDH, sorbitol dehydrogenase from apple; SPDH, sorbitol 6-phosphodehydrogenase from apple; Tfd, monooxygenase gene from Alcaligenes eutrophus; TMK, receptor kinase gene from rice; ToRSV-CP, ToRSV coat protein gene; XMT, xanthosine-N7-methyltransferase antisense from coffee. BOX 3-1 Stability of Transgenic Confinement Stable gene expression is a necessity for bioconfinement that is based on transgenic approaches. Expression must be stable throughout the lifespan of the organism, and it must be adequate to accomplish confinement. For inducible or regulated genes, there must be confidence that expression will reach needed levels at the appropriate times, year after year for perennials. Just as transfor- mants are selected for strong, stable expression of agronomic transgenes during crop development, so too should sufficient evaluation be given to the stability of the engineered bioconfinement method. In the context of using transgenic methods for bioconfinement, instability that is not detected before field releases is clearly undesirable. The question of stable integration and expression of foreign genes is important for long-lived species, such as trees and turfgrasses (Pena and Seguin, 2001). There are two ways that transgene instability can occur--through the loss of the gene from the host or through the shutdown of expression of the gene in the host plant (gene silencing). Transgene loss. Not all transformation leads to stable integration and inheritance of the transgene. A first step in plant transformation is to identify and discard those cells or plants in which the transgenes have been lost. Stable transformants are considered those that pass the transgene on to subsequent generations through meiosis or, in perennial plants, to be present continuously for several years (dor- mancy cycles). Transgenes can still be lost after several generations, however continued
PLANTS 103 BOX 3-1 Continued (e.g. Srivastava et al., 1996). Of the two technologies--Agrobacterium-mediated gene transfer and microprojectile bombardment--the latter can deliver many copies of the transgene into each recipient cell. Those multiple copies often integrate as tandem repeats, which can lead to excision (loss) of the transgenes or to gene silencing. Although Agrobacterium-mediated gene transfer tends to provide more stable integration, complex integration patterns can occur, including truncation of parts of the T-DNA (e.g., McCabe et al., 1999). Gene silencing. After transformants are selected in which the transgene has stably integrated, loss of the phenotype can occur subsequently, because of the loss of expression of the transgene--by gene silencing. In the Arabidopsis thaliana model system, transgene inactivation has been correlated with multiple copies of the transgene, with the presence of vector backbone sequences, with DNA methylation, and with transgene position in a genome (De Buck et al., 2001; De Wilde et al., 2001; Meza et al., 2002). In Arabidopsis thaliana lines that con- tained single copies of antibody genes, De Wilde and colleagues (2001) found that silencing of transgenes can result from gene dosage effects. Homozygous lines exhibited gene silencing, and hemizygous plants showed high transgene expres- sion. Meza and colleagues (2002), however, reported that the known mechanisms of gene silencing are not always sufficient or necessary for the induction of trans- gene silencing in T-DNA-transformed Arabidopsis lines. De Buck and colleagues (2001) showed that convergent transcription of transgenes that occur, as in an inverted repeat orientation, can trigger gene silencing in Arabidopsis. Highly transcribed transgenes or transgene loci that produce double-stranded RNA because of the presence of inverted repeats can result in gene silencing. Based on their observations with transgenic Arabidopsis, Beclin and colleagues (2002) proposed a complex pathway for RNA silencing in plants in which transgene methylation would result from production or action of dsRNA. Post-transcriptional gene silencing induced by double-stranded RNA--termed RNAi (for RNA-interference)--occurs naturally in plants as part of a defense mech- anism against virus infection. Tenllado and colleagues (2003) showed that expres- sion of transgene constructs encoding hairpin RNA homologues can interfere with virus multiplication in a sequence-dependent manner. Double-stranded RNA was identified as the triggering structure for the induction of a specific and highly effi- cient RNA silencing system. The enzyme complexes facilitate the processing of dsRNA into characteristic small RNA species, known as small interfering RNAs (siRNA) that promote degradation of cognate RNAs. Tang and colleagues (2003) conducted a biochemical analysis of RNA silencing and reported that endonuclease complexes guided by small RNAs (endogenous microRNA) are a common feature of RNA silencing in animals and plants. Metzlaff (2002) showed that one component of a signal that transmits RNA silencing rapidly from silenced to nonsilenced cells by short- and long-distance signaling involves a specific, degradation-resistant RNA. Transgene expression instability is an active area of research, with important implications for the long-term efficacy of deregulated transgenes. Some causes of transgene silencing, such as multiple copy number, can be detected easily during the early stages of development of new genetically engineered varieties. Progress is being made on new methods for detecting and reducing other causes of gene silencing. More research is needed to explain the causes of transgene instability so that researchers can develop more sophisticated techniques to minimize the problem.
104 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS and woody species because it reduces forage digestibility and is difficult to extract during pulp and paper making. The characterization of lignification genes and their exploitation for modulation of lignin profiles in transgenic plants has already been documented (Baumberger et al., 2002; Merkle and Dean, 2000; Sederoff, 1999). Boudet and colleagues (1998) reviewed the features essential to the use of transgenic approaches to lignin modification, including potential unwanted side effects and stability of transgene expres- sion. In general, it is expected that modifications to lignin content in geneti- cally engineered trees will either fall within the range of natural variation, which can include null mutations in key genes (MacKay et al., 1997), or will reduce fitness. The escape of those trees or their genes into wild popu- lations would thus provide new alleles that confer no particular selective advantage and that under many circumstances would be selected against in highly competitive situations. To confirm that lignin modifications, or epigenetic changes associated with the tissue culture process, have not inadvertently improved fitness in genetically engineered tree lines, data could be gathered on growth rates and other fitness traits during the initial, limited field trials that are typically mandated for evaluation of new geneti- cally engineered trees (McLean and Charest, 2000) before they are released. Such data on GE trees should be compared with data for nonregulated genotypes already in cultivation. In other situations, confinement of trees could be warranted by envi- ronmental concerns. For example, it would be prudent to confine traits that disrupt the attack of plantation-grown trees by indigenous organisms that normally coexist with the tree species in natural settings and that are part of larger food chains. This would include native herbivores that rely on tree species as a primary food source and microbes that are required for mycorrhizal symbioses or nutrient recycling. It also could include resistance to exotic pests that is accomplished by transgenes that also confer resistance to nontarget native species. Genetic engineering for resistance does not in itself create risk that is much different from that attributable to resistance genes that are incorporated through multigenerational backcross breeding. However, breeding for resistance traits has not been widely attempted for forest trees in the past because of the inherent difficulties in protracted multigenerational breeding. Thus, traditional knowledge does not exist to evaluate whether gene flow from resistant genotypes in managed stands to wild stands will disrupt existing food chains or have other significant non- target effects in subsequent generations. After field studies are done (e.g., during short-term trials, for example) for pest resistance genes bioconfinement could be considered less necessary. Finally, when the risks associated with environmental disruption after even very limited transgene escape are too great, the release of genetically engineered trees in some locations could be deemed inappropriate, even if
PLANTS 105 bioconfinement and physical confinement strategies are used in context. Hypothetical examples include the secretion of powerful allelopathic or antibiotic chemicals from the roots of those trees or the release of toxic, volatile compounds from their leaves. If the transgene were to provide a substantial competitive advantage, even severe restriction of transgene flow might be insufficient to prevent colonization after escape. Such extreme cases are trait and species specific and should be identified during risk analysis in the planning or development phases. For further discussion of the necessity of the bioconfinement of trees, see Box 3-2. BOX 3-2 When Will Bioconfinement be Necessary for Trees? In the past, trees were regarded as unfavorable organisms for research because of their mating systems, long life cycles, distinct juvenile-mature phases, and the fact that trees usually grow in natural settings in which genetic control of complex traits is obscured by environmental effects. However, molecular genetics, genomics and genetic engineering have opened new opportunities for research with trees. The first transgenic tree was an herbicide-resistant hybrid poplar (Fillatti et al., 1987). Genetic transformation has subsequently been applied to a variety of commercial and environmental objectives in forest trees, fruit and nut trees, and other woody perennials. Traits of interest for genetic engineering in trees include lignin (pulp) modification, increased growth and productivity, enhanced utilization of resources, pest and disease resistance, stress tolerance, herbicide resistance, optimization of mycorrhizal symbioses, phytoremediation of contaminated soils, and even production of anticancer drugs (Han et al., 1994). Genetically engineered trees are appearing with increasing frequency: Since 1989, more than 230 permits have been approved by the Animal and Plant Health Inspection Service in the United States and at least 65 have been granted in other countries for field trials on trees and other woody plants (Table 3-2). GE tree species in field tests range from pines to persimmons and poplar to papaya. Future applications of genetic engi- neering are likely to include restoration of species to native habitat, adaptation of trees to plantation management (domestication), enhanced fiber and biofuels pro- duction, and agroforestry. Concomitant with the many possibilities for improvement of trees through genetic engineering are questions about the efficacy and safety of the technology in trees and other perennial plant species. The concerns raised with GE trees include the long-term stability of expression of foreign genes, the long-term effects of trans- genes on nontarget species, and the long distance dispersal of transgenes through seed and pollen to wild tree stands. GE with trees presents a special challenge in that trees are often dominant species in their ecosystem and support a large web of organisms that either directly or indirectly rely on them as the ultimate source of nutrients. Many of the concerns over deployment of GE trees could be addressed with the use of appropriate bioconfinement techniques. If successful, bioconfine- ment could both protect the investments made in the development of genetically engineered trees and safeguard the environments in which they are grown. continued
106 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS BOX 3-2 Continued Initial tests of bioconfinement techniques could be conducted in fast-growing trees, such as hybrid poplar, in which testing over a full crop rotation could be as little as 6 years. In slower growing tree species, and for initial large scale tests, the testing of new bioconfinement techniques could be piggy-backed on releases of GE trees that carry transgenes of commercial importance but that are considered to pose little or no environmental or health risk. Combining bioconfinement tests with GE tree releases would provide commercial benefit upon harvest while gener- ating important data on the effectiveness of new confinement techniques, prior to their use in situations in which successful confinement is essential. Due to the length of time required to develop and test GE trees, decisions on whether or not to include bioconfinement methods should be made at the onset, rather than after the fact. Approaches to evaluating the need for bioconfinement are discussed in Chapters 2 and 6. The following questions could serve as a guide in the decision making process on when to incorporate bioconfinement with GE trees: · Has it been determined previously that bioconfinement was required for similar products (the same class of genes, gene products, and vectors in similar tree species)? · Has new empirical data been gathered to indicate that bioconfinement is still warranted? · If this class of genes has not been evaluated in trees before, what specific, novel risk(s) might the GE tree pose to the environment, assuming certain levels of gene flow? · Will there be closely related tree species within a distance of the site where the GE trees are grown to pose a risk of gene flow? · If risk to the environment is considered significant, then what degree of confinement will be necessary to render the risk acceptable? · Which currently available bioconfinement technique, if any, can provide sufficient dilution or exclusion of unwanted transgenes in other populations? · How will the success of bioconfinement efforts be evaluated? Risks of Most Concern with Trees Risks Associated with Gene Flow into Natural Populations One concern about genetically engineered trees is the consequences of gene flow from managed stands of those trees to wild populations (Slavov et al., 2002). Sexual hybridization can occur naturally between plant species that are within the same genus or occasionally, between related species in different genera. Transgene flow could be substantial if genetically engi- neered trees are permitted to reach sexual maturity and flower within the natural geographic range of wild relatives. The extent of hybridization
PLANTS 107 between transgenic plants and their wild relatives and the scale of geneti- cally engineered tree deployment (relative to the size of natural populations) will determine both the rate of the incorporation of transgenes into wild populations and the feasibility of confinement (Wilkinson et al., 2000). Some genetically engineered trees also could disperse seeds and establish naturalized populations. The first step in defining appropriate confinement strategies for geneti- cally engineered trees should be the acquisition of data on wild or natural- ized tree populations (location, species mixes, distances from anticipated GEO release sites). The next step would be to assess the extent to which gene flow in pollen or seeds occurs between managed and unmanaged populations. In commercial poplar plantations, studies involving non- transgenic DNA markers have shown unexpectedly low levels of gene flow to nearby wild populations, despite the potential for extensive gene flow (Slavov et al., 2002). Slavov and colleagues (2002) hypothesize that the low gene flow observed could be attributable to the DNA marker systems used. They developed a spatial simulation model that incorporates a variety of ecological and genetic parameters to estimate the levels of future transgene flow from poplar plantations. The model permits virtual experiments to investigate how genetics, ecology, and management might influence the magnitude and variance of gene flow over 50 or 100 years. Similar models should be developed for other species and other planting scenarios, so that confinement and monitoring programs can be evaluated and designed as necessary. Case-by-case analysis will be required for useful predictions of transgene flow. Gene flow is not expected to be a problem, in and of itself, unless it leads to undesirable consequences (Box 3-1). Effects on Nontarget Organisms One category of objectives with the genetic engineering of trees is to prevent or reduce damage from specific pests and pathogens in tree planta- tions. Genes that produce pesticides with activity against groups of organ- isms--such as Bt toxin genes that protect against lepidopterans--could require confinement or restriction in expression to a much greater degree than would species-specific toxins, especially if significant gene flow is possible. Not all transgenes will affect commensal organisms, even those that have marked effects on growth. Hampp and colleagues (1996) docu- mented that the in vitro synthesis of ectomycorrhiza between roots of transgenic aspen (Populus tremula × P. tremuloides) and Amanita muscaria were not affected by transformation and expression of indoleacetic acid (IAA) biosynthetic genes in roots. In contrast, Puterka and colleagues (2002) demonstrated that genetically engineering a clone of Bartlett pear, Pyrus communis L., (with a synthetic antimicrobial gene, D5C1) to control bacte-
108 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS rial fireblight disease, Erwinia amylovora conferred unintended activity against a nontarget pest organism, the pear psylla, Cacopsylla pyricola Foerster. In agriculture, such cross-reactivities can be beneficial. Within natural forest ecosystems, however, they might not be. Secondary Phenotypic Effects of Transgenesis As in nontransgenic methods of genetic modification, the process of inserting transgenes into plant cells and regenerating whole plants from those cells can result in different types of unintended phenotypic effects. Those "secondary" phenotypes can be caused by the transgene's location in the genome, by effects of the transgene on other traits (pleiotropy), by interactions among the transgene and native genes (epistasis), and by somaclonal mutations that occur during tissue culture. Carefully designed control lines can identify specific causes of unintended phenotypes, but such detailed analyses often are lacking. Several examples of unintended phenotypes in trees are described below. It is important to note that their range is expected to be much smaller in deregulated plants that have been extensively evaluated in field trials than in experimental lines used in research. It is also important to note that unintended effects are not neces- sarily undesirable. Ralph and colleagues (2001) reported on the production of unantici- pated benzodioxane structures in lignins of transgenic poplar plants defi- cient in COMT, an O-methyltransferase required to produce lignin syringyl units. This demonstrates the ability of plants to accommodate mutations in gene expression that might not be predicted based on current knowledge of biochemical pathways. Holefors and colleagues (2000) constructed transgenic apple rootstock clones that carried 18 copies of the Arabidopsis phyB gene. Multiple effects of phyB overexpression were observed, including reduction in stem length in 9 of 13 clones and reduction in shoot, root, and plant dry weights in all transformed clones compared with untransformed control plants. Atkinson and colleagues (2002) produced transgenic apple (Malus domestica Borkh. cv Royal Gala) trees that contained additional copies of a fruit-specific apple polygalacturonase gene (PG) under a constitu- tive promoter. In previous studies in transgenic tobacco (Nicotiana tabacum), PG overexpression had no effect on the plant, but in the transgenic apple it led to a range of phenotypes, including silvery colored leaves and premature leaf shedding. Mature leaves had malformed and malfunctioning stomata that perturbed water relations and contributed to a brittle leaf phenotype. O'Connell and colleagues (2002) produced transgenic tobacco plants that severely suppressed the activity of cinnamoyl-CoA reductase (CCR) as a model for altering lignin content in trees. Although transgenic lines had the
PLANTS 109 desired changes in lignin structure, some showed a range of aberrant phenotypes, including reduced growth. Other pleiotropic effects can help a plant. Hu and colleagues (1999) observed that transgenic aspen (Populus tremuloides Michx.) with down- regulated expression of the gene that encodes 4-coumarate:coenzyme A ligase (4CL) exhibited a 45% reduction in lignin and a 15% increase in cellulose. The total lignincellulose mass in the genetically engineered trees was essentially unchanged. Furthermore, leaf, root, and stem growth were substantially enhanced, and structural integrity was maintained both in the cells and in whole plants in the transgenic lines. Those results indicate that metabolic flexibility can sustain the long-term structural integrity required of woody perennials in transgenics. El Euch and colleagues (1998) trans- formed walnut (Juglans nigra × Juglans regia) with an antisense chalcone synthase (chs) gene that not only reduced flavonoid content in the stems of the plants but also enhanced adventitious root formation. Some studies have been designed to generate pleiotropic effects inten- tionally, such as the introduction of oncogenes (rolC) from Agrobacterium. Grunwald and colleagues (1999, 2001) compared the morphology, wood structure, and cell wall composition in rolC transgenic hybrid aspen (P. tremula × P. tremuloides) with nontransformed control trees. The transgenic trees had stunted growth, altered physiological parameters, and light green leaves that were smaller than normal. Numerous alterations also were observed in the formation and differentiation of xylem cells. In con- trast, when Tzfira and colleagues (1999) expressed rolC transgene in aspen (Populus tremula) they observed both accelerated growth and improved stem production index in the transgenic plants. Eriksson and colleagues (2000) produced transgenic hybrid aspen (Populus tremula × P. tremuloides) overexpressing a key regulatory gene in the biosynthesis of gibberellin (GA). The transgenic trees had improved growth rate and biomass, as expected, but they also had more numerous and longer xylem fibers than did wild- type plants. Not all engineered mutations in trees produce such secondary effects, of course. Bhatnagar and colleagues (2001) altered polyamine metabolism in cells of transgenic poplar (Populus nigra × P. maximowiczii) by express- ing a mouse Orn decarboxylase (odc) cDNA. The transgenic cells showed the expected effects on polyamines, but the overall arginine pathway was not affected and assimilation of nitrogen into glutamine kept pace with the increased demand for putrescine. Transgenic citrus seedlings that con- stitutively expressed the LEAFY (LFY) or APETALA1 (AP1) genes from Arabidopsis showed dramatically precocious flowering and fruit produc- tion, as desired, without exhibiting any other developmental abnormalities (Pena et al., 2001).
110 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS Instability of Transgene Expression Pena and Seguin (2001) pointed out that stable integration and expression of foreign genes is particularly important for long-lived species such as trees. Instability that is not detected prior to field release is clearly undesirable. A better understanding of the causes of transgene expression instability can help researchers develop more sophisticated techniques to minimize the problem. To investigate transgene silencing in a tree system, Kumar and Fladung (2001) analyzed aspen (Populus tremula L.) and aspen hybrid (P. tremula L. × P. tremuloides Michx.) lines transformed with the rolC phenotypic marker system and grown in vitro, in greenhouses and in the field. Their molecular analyses showed that, in the hybrid aspen genetically engineered lines, the inactivations were always a consequence of transgene repeats (multiple incomplete or complete copies). In wild nonhybrid aspen, however, instability in some of the transgenic lines was the result of a position effect. This indicates that the tree host genome has some control over expression of transgenes. Other studies have shown that expression of transgenes in poplar can be stable under field conditions in many cases (e.g. Meilan et al., 2002). Dominguez and colleagues (2002) studied transgene silencing in Mexican lime (Citrus aurantifolia [Christm.] Swing.) transformed with the Citrus tristeza virus coat protein gene. More than 30% of the transgenic limes that had regenerated under nonselective conditions exhibited silencing of the transgenes. They observed that inverted repeats as well as direct repeats and even single integrations triggered gene silencing. In contrast, Cervera and colleagues (2000) studied 70 transgenic citrus plants in a screenhouse over 45 years. They observed only 4 phenotypic off-type plants, all of which were the result of tetraploidy in the tissues used for transformation. Gene- silencing or pleiotropic effects were not to blame. Options and Constraints Sterility The creation of sterile trees has attracted wide support and interest as a method of bioconfinement. Strauss and colleagues (1995) discussed the regulatory and ecological rationales for engineering sterility in trees, the strategies for creating sterility-inducing transgenes, and the problems pecu- liar to engineering sterility in forest trees. Two primary options for geneti- cally engineering sterility in trees are being pursued: ablating floral tissues through floral-specific promotercytotoxin fusions and disrupting expres- sion of essential floral genes by gene suppression (Strauss et al., 1995). Both
PLANTS 111 options should be thoroughly tested, as each has advantages and disadvan- tages. There are other approaches for generating sterility that do not require genetic engineering. Cytoplasmic male sterility can be created through nuclear-cytoplasmic (mitochondrial) incompatibilities resulting from the crossing (sexual hybridization) of specific genotypes or related species (e.g. Shi and Hebard, 1997). Sterility has been observed to occur naturally in some tree species (e.g. Linares, 1985; Soylu, 1992) and has been selected in ornamental trees for which fruits are not desirable or when seedless (parthenocarpic) fruits are preferred (Rapoport and Rallo, 1990; Talon et al., 1992). As in so many aspects of designing safe genetically engineered trees (Pena and Seguin, 2001), long life span can create problems for the use of sterility as the sole bioconfinement tool. The systems developed for sterility in trees must be highly stable if they are to be trusted in cases where rotation lasts for 40 years or more. Stable suppression of fertility could require targeting of multiple floral genes or the combined use of several genetic mechanisms for inducing sterility and other bioconfinement methods. Engineering of complete sterility or male sterile lines could help to achieve gene confinement, and it could stimulate faster wood production, reduce the production of allergenic pollen, and (in the case of male sterility) facilitate hybrid breeding in trees. Triploidy Results of controlled crosses have shown that triploid clones used in genetic engineering experiments with poplar have a high level of innate sterility and are less competitive in mixed stands than are their wild rela- tives (Strauss and Meilan, 1997). Such chromosomal abnormalities could provide natural systems for bioconfinement. The stability of sterility in such lines must be evaluated, and an expected frequency of somatic reversions to fertility should be determined, or at least estimated, over the expected rotation for each tree crop before incorporation into confinement strategies. Gene Silencing RNA silencing can be induced in plants by several mechanisms includ- ing induction of effects associated with transcriptional, posttranscriptional, genomic DNA methylation, and gene dosage events. In trees and other perennial plants, gene silencing occurs naturally (Fraga et al., 2002) and transgenically (Dominguez et al., 2002; Kumar and Fladung, 2001). Ini- tially, posttranscriptional silencing was accomplished with antisense or cosuppression. These constructs usually result in only a modest proportion of silenced individuals, however, which is not useful for long-term field
112 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS trials of trees. The steps involved in gene silencing in plants are becoming better understood and should lead to the development of improved transgene bioconfinement tools for trees. If successful and durable under field conditions, gene silencing could find broad application in the improve- ment of transgenic trees and rootstocks. Fitness Handicaps Bioconfinement by auxotrophy involves the use of natural or transgenic mutant genotypes that lack a necessary biosynthetic component. Tree mutants would be rendered much less competitive than their wild relatives in unmanaged forest environments, or they would simply not survive, assuming that the missing biosynthetic component was not available to escaped plants and that the mutations could not be complemented (recovered) in hybrids with wild-type trees. The novel recoverable block of function (RBF) technique of Kuvshinov and colleagues (2001) is an engi- neered form of auxotrophy that could reduce gene flow from transgenic trees to wild relatives. The RBF system is superior to single-gene-mutation auxotrophs because hybrids between the transgenic plants carrying the RBF and the wild relatives would die or be unable to reproduce because of the blocking construct. In practice, most forms of auxotrophy would be diffi- cult to apply given the recessive nature of most auxotrophic mutations, the slow nature of the process to create homozygous individuals, and the high degree of inbreeding depression shown by trees. The large-scale chemical applications that are required for some auxotrophs could lead to ecological harm. However, use of the RBF technique for engineering conditional auxotrophy could be an effective way to confine transgenes and should be investigated more thoroughly for trees. Tissue-specific Expression It is often desirable to restrict the expression of transgenes in genetically engineered plants to the tissues that require the encoded activity. Promoters from genes in the lignin pathway have been demonstrated to impart tissue- and development-specific expression of marker genes in transgenic plants. The development of such "regulated" promoters opens the possibility for restricting expression of economically important transgenes in trees to those situations in which their gene products are required. In the case of resis- tance genes, such specificity could lessen the selection pressure on the dis- ease and pest populations and help avoid breakdown of resistance over the many years that a transgenic tree could grow. Regulated expression of transgenes for biotic resistance also will help lessen effects on nontarget organisms.
PLANTS 113 The transformation of poplar is often used to examine the roles of upstream and downstream regulatory elements from other tree genes. Two loblolly pine genes that were developed for xylem cDNA libraries exhibited tissue specificity effects in leaves of transgenic poplar that were promoter specific (No et al., 2000). Gray-Mitsumune and colleagues (1999) demon- strated the specificity of expression of the poplar PAL promoter to vascula- ture in both transgenic poplar and spruce. Genome-sequencing projects, such as the poplar genome sequencing project (http://www.ornl.gov/ipgc/), coupled with global analysis of gene expression studies, will unveil thou- sands of regulated promoters that will be tested for use in transgenic constructs in studies of functional genomics. Plastid Engineering As an alternative to nuclear transformation, transgene expression from the chloroplast genome offers several advantages, including high-level foreign protein expression and lack of pollen transmission for improved transgene confinement in angiosperms (reviewed by Bock, 2001). Daniell and colleagues (1998) first reported genetic engineering by stable integra- tion of a foreign gene into the tobacco chloroplast genome. Improved chloroplast-based expression systems now include vectors, expression cas- settes, and site-specific recombinases for the selective elimination of marker genes (Maliga, 2002). Because chloroplasts are transmitted through pollen in gymnosperms, the chloroplast transformation approach to bioconfine- ment is limited to hardwood tree species. An analogous system for bio- confinement of foreign genes in conifers would be mitochondrial transfor- mation, although stable, efficient mitochondrial transformation has not yet been reported. Given the extensive dispersal rates of genes through tree pollen, more effort should be placed on chloroplast transformation for bioconfinement in hardwood trees than has been reported to date, especially if sterility is not an alternative. Seed transmission of transgenes is also of more concern for tree species than it is for annual crops. Thus, chloroplast transformation would need to be combined with other methods to achieve strict bioconfinement in trees. Outlook for Bioconfinement of Transgenes in Trees Safe and effective bioconfinement methods should lead to greater acceptance of transgenic trees by the general public and to increased oppor- tunities for the creation and deployment of genetically engineered trees by researchers in the public and private sectors. Given the number of options available and the frequency with which new approaches are being reported, there is every reason to believe that effective bioconfinement methods will
114 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS soon be available for trees. Whether the full development, testing, and deployment of bioconfinement methods, as well as the application of genetically engineering itself, will be realized for trees is still open to ques- tion, however. This will only occur if there is acknowledgment by industry that bioconfinement of transgenes is necessary and beneficial; if the public accepts that there can be an acceptable risk; and if more public funding becomes available for the discovery, development, and appropriate testing of bioconfinement methods in trees. The likelihood of public acceptance of nominal risk associated with the growth of trees under bio- and physical confinement could be improved by the use of transgenes derived from the same or similar tree species and by the development and adoption of methods for monitoring wild populations for entry of transgenes from genetically engineered tree plantations. Those steps are already being pursued to a limited extent. For example, Marcus and colleagues (1997) isolated the gene for an antimicrobial peptide (MiAMP1) from the nut kernels of Macadamia integrifolia that inhibits the growth of fungal, oomycete, and gram-positive bacterial phytopathogens in vitro, but that is nontoxic to plant and mammalian cells. Such genes could prove as useful in genetic manipulations to increase disease resistance in transgenic trees as would be genes derived from bacteria. Connors and colleagues (2002) are studying whether modification of a cystatin gene isolated from American chestnut (Castanea dentata) could confer chestnut blight resistance. Diagnostic tests for transgenes can be applied readily to genetically engineered trees for determining the location of transgenes, as described above. However, because natural forests often cover such large areas it would be difficult to monitor them effectively on a random basis for transgene movement. Wilkinson and colleagues (2000) used remote sensing to identify sites of sympatry between Brassica napus and its progenitor species across 15,000 km2 of southeastern England before the release of transgenic B. napus plants. This work allowed the researchers to focus their activities in areas where transgene escape was most likely. The same approach could be taken to identify the best sites for release of genetically engineered trees and to determine where monitoring should occur. Efforts are under way to develop remote sensing that detects expression of transgenes based on the unique profile of volatile compounds that can serve as signatures for genetically engineered plants (www.aginfo.psu.edu/News/ march03/sentinel.html). Ghorbel and colleagues (1999) have shown that green fluorescent protein (GFP) can be used as a visible marker for selection of transgenic woody plants, as an alternative to antibiotic and herbicide selection. GFP might thus serve as a marker for monitoring trees for genetic escape through the use of remote or handheld ultraviolet (UV) light sources or fluorescence detectors.
PLANTS 115 TRANSGENIC GRASSES Many types of grasses have been considered for genetic engineering, including forage grasses for rangeland and native grasses for biomass pro- duction or bioremediation (NRC, 2002a; Wipff and Fricker, 2001). The most advanced and most profitable species, however, are turfgrasses, and are used widely in landscaping and on golf courses, for example. There are more than 14,000 golf courses, 40,000 athletic fields, and 40 million parks and home lawns in the United States (Edminster, 2000). And the U.S. turfgrass seed market is second only to the hybrid corn seed market, with annual sales of $580 million to $1.2 billion (Wipff and Fricker, 2001). There is considerable interest and investment in turfgrass science, much of it supported by the United States Golf Association (Kenna, 2000). Genetically Engineered Turfgrasses Since 1993, the Animal and Plant Health Inspection Service has issued more than 200 permits for small field tests of transgenic turfgrass species in the United States (Table 3-3; Wipff and Fricker, 2001), although none has yet been deregulated by the U.S. Department of Agriculture. Monsanto's glyphosate-resistant creeping bentgrass (Agrostis palustris; http:// gophisb.biochem.vt.edu) was the first transgenic grass to be considered for approval. Monsanto also is developing a new lawn grass that requires less mowing than does its nontransgenic counterpart. Although the development of transgenic grasses has fallen behind the research being done on major crop plants, a great deal of basic research has addressed transgenic methods for improving turfgrass cultivars. Biolistic bombardment, protoplast DNA uptake (either through electroporation or mediated by polyethylene glycol), and recently Agrobacterium-mediated transfer of genes have been used for genetic transformation of turfgrasses. Techniques for in vitro regeneration also have been developed (e.g., Chai and Sticklen, 1998; Lee, 1996). Biolistic gene bombardment of creeping bentgrass (Agrostis palustris Huds.) with the reporter gus was developed, and a GUS enzyme histochemical assay was used to identify nonchimerically transformed plants (Zhong et al., 1993). Biolistic bombardment of turfgrass callus or suspension cells, and electroporation-mediated or polyethylene- glycol-mediated protoplasts have been used to transfer hygromycin, phosphinothricin, biolophos, NPTII, or G148 resistance selectable marker genes in stolonate bentgrass (Agrostis stolonifera var. palustris) (Sugiura et al., 1997, 1998), creeping bentgrass (Agrostis palustris Huds.) (Hartman et al., 1994; Lee et al., 1996; Liu, 1996, Zhong et al., 1993, 1998), red top (Agrostis alba L.) (Asano and Ugaki, 1994), orchardgrass (Dactylis glomerata L.) (Horn et al., 1998), tall fescue (Festuca arundinaceae Schreb)
116 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS TABLE 3-3 Genetically Engineered Turfgrasses, Permits Approved by APHIS for Field Tests in the United States, 19932003 Organism Phenotypes (Number of Permits) Gene Bermudagrass AP - Drought and salt tolerance LEA or Lsd or BADH increased (9) HT - Phosphinothricin tolerant (1) PAT Creeping AP - Aluminum tolerant (2) CISY bentgrass AP - Drought tolerant (3) LEA & OSTL & PAT; or THIL AP - Drought and salt tolerance LEA or Lsd or BADH increased (9) AP - Growth rate altered (3) CBI AP - Growth rate altered & CBI and/or EPSPS HT - Glyphosate tolerant (4) AP - Heat tolerant (2) THIL AP - Salt tolerance increased (7) BADH or LEA FR - Brown spot and dollar spot PAT; PI-II resistant (1) FR - Dollar spot resistant (21) TMK or AVP or GOX or BAC FR - Fusarium resistant (1) Bcl-xl FR - Rhizoctonia solani resistant (4) CBI FR - Rhizoctonia solani & AVP; BAC;FAD; GOX; Sclerotinia resistant (1) IMT; TMK FR - Sclerotinia resistant & CBI HT - Glyphosate tolerant (4) HT - Glyphosate tolerant (50) CBI or EPSPS HT - Phosphinothricin tolerant (25) PAT or CBI IR - Sod web worm resistant (2) CBI MG; SM - Hygromycin tolerant (4) GUS; HYR MG; SM - Spectromycin resistant (1) CBI Festuca FR - Rhizoctonia resistant (2) AGLC; Npr1; HYR; arundinacea CHT HT - Phosphinothricin tolerant (2) PAT MG; SM - Hygromycin tolerant (3) HYR; GUS PQ - Lignin decreased (3) CAD and/or COMT, COMT antisense Kentucky AP - Drought tolerant (1) BADH bluegrass AP - Drought and salt tolerance Lsd or BADH or LEA increased (8) AP - Growth rate altered (1) CBI AP - Growth rate altered & CBI HT - Glyphosate tolerant (6) FR - Rhizoctonia solani resistant (2) CBI HT - Glyphosate tolerant (6) CBI
PLANTS 117 TABLE 3-3 Continued Organism Phenotypes (Number of Permits) Gene Paspalum PQ - Lignin decreased (1) COMT; PAT notatum Perennial AP - Drought and salt tolerance BADH; HYR ryegrass increased (3) Poa pratensis AP - Growth rate altered & CBI × Poa HT - Glyphosate tolerant (1) arachnifera HT - Glyphosate tolerant (2) CBI Russian MG; SM - Hygromycin tolerant (3) GUSi; HYR wildrye St. Augustine AP - Growth rate altered & CBI grass HT - Glyphosate tolerant (6) HT - Glyphosate tolerant (6) CBI HT - Phosphinothricin tolerant (1) CBI Velvet HT - Phosphinothricin tolerant (1) PAT bentgrass NOTE: APHIS; Animal and Plant Health Inspection Service. Field test data downloaded from Information Systems for Biotechnology, http://www.nbiap.vt.edu/cfdocs/fieldtests2.cfm, May 23, 2003 does not include submissions denied or withdrawn. Phenotype Key: AP, agronomic properties; FR, fungal resistance; HT, herbicide tolerant; IR, insect resistant; MG, marker gene; PQ, product quality; SM, selectable marker. Gene Key: AVP, antiviral protein from pokeweed ; BAC, bacteropsin gene from Halobacterium halobium; BADH, Betaine aldehyde dehydrogenase from garden orach (Atriplex hortensis); Bcl-xl, B-cell lymphoma related gene from chicken; CAD, cinnamyl alcohol dehydrogenase from tall fescue; CBI, confidential business information; CHT, chitinase from rice; CISY, COMT, caffeate O-methyltransferase gene from tall fescue; EPSPS, 5-enolpyruvylshikimate- 3-phosphate Synthase; FAD, delta-9 desaturase from Saccharomyces cerevisiae ; GOX, glucose oxidase from Aspergillus niger; GUS: E. coli -glucuronidase gene; HYR, hygromycin phosphotransferase gene; IMT, inositol methyl transferase; LEA, late embryogenesis abundant protein gene from barley; Lsd (Sac), levansucrase gene from Bt; Npr1, nonexpressor of pathogenesis-related gene from Arabidopsis thaliana; NPTII, neomycin phosphotransferase gene from E. coli; OSTL, thaumatin-related protein from rice; PAT, phosphinothricin acetyl transferase gene from Strep. hygroscopicus; AGLC, -1,3-glucanase antisense from alfalfa; PI- II, proteinase inhibitor II from potato; SAR, systemic acquired resistance gene from Arabidopsis thaliana; THIL, thiamine biosynthetic enzyme from corn; TMK, receptor kinase gene from Arabidopsis thaliana.
118 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS (Dalton et al., 1995; Ha et al., 1992; Spangenberg et al., 1994; Wang et al., 1992), red fescue (Festuca rubra L.) (Spangenberg et al., 1994), perennial ryegrass (Lolium perenne L.) (Alpeter et al., 2000; Spangenberg et al., 1995), Italian ryegrass (Lolium multiflorum) (Potrykus et al., 1985), Japanese lawngrass (Zoysia japonica Steud.) (Inokuma et al., 1997, 1998), and European turf type red fescue (Festuca rubra L.) (Alpeter et al., 2000). More recently, the Agrobacterium-mediated genetic transformation system was used to transfer the gus reporter gene and the hygromycin phospho- transferase (HTP) genes in Korean lawngrass (Chai et al., 2000) and the GFP and HTP genes in creeping bentgrass (Agrostis palustris L.) (Chai et al., 2000). Potential for Gene Flow Grasses that are cultivated for turf, forage, and ornamental uses pose several challenges for bioconfinement because of their capacity for out- crossing, hybridization, and vegetative propagation. Also, many cultivated grasses are closely related to noxious weeds. Turfgrasses are open-pollinated plants that often cross-pollinate with weedy species. For example, the bentgrass group (Agrostis) has more than 100 species, many of them weeds that can hybridize with one another (Wipff and Fricker, 2001). Bermuda- grass (Cynodon dactylon. L. var. Prsoon) is an important perennial forage and turfgrass that is considered to be a weed in many regions of the United States. Should bermudagrass be genetically engineered to improve hardi- ness, the escape of transgenes has the potential to cause ecological and economic damage (Ellstrand and Hoffman, 1990). Giddings and colleagues (1997a) noted that, in the United Kingdom, forage grasses including Lolium perenne cultivars are sexually compatible with wild and feral species of the same genus and with fescue species (Festuca spp.; Figure 3-3). Agrostis species can hybridize with Polypogon species, and it is believed that Agrostis parlatore and A. moldavica are derived from past hybridization between Agrostis casstellana and Polypogon viridis (Wipff and Fricker, 2001). Some commercially important grass species can hybridize with nearby congeners and then switch to asexual seed production (apomixis), allowing crop genes to spread widely even when F1 hybrids are sexually sterile (Wipff and Fricker, 2001). Because turfgrasses are perennial, the longevity of unintended perennial hybrids between transgenic and wild plants will increase the opportunities for further backcrossing with other wild or domestic grasses. The rate of introgression of some turfgrasses is actually higher for interspecific and intergeneric hybrids than it is among intraspecies crosses (Wipff and Fricker, 2001). For example, creeping bentgrass is self-incompatible (self-sterile) but highly cross-compatible with other species (Bjorkman, 1960). An early report detected, through paternity analysis, more than 1%
PLANTS 119 FIGURE 3-3 A wild hybrid, F. arundinacea and L. multiflorum Lam. cross-pollination of nontransgenic creeping bentgrass plants at a distance of 8,000 m (Ellstrand and Hoffman, 1990). Turfgrasses have small pollen that can blow great distances. Normally, the two factors of distance and wind direction are considered to predict the distance that pollens can travel (Giddings, 2000; Giddings et al., 1997b). However, other factors, such as speed and wind turbulence--especially if "whirl winds" are present--are important in the unintended deposition of pollen in other fields. Other factors include relative humidity and temperature (Wipff and Fricker, 2001). Because there are no models to predict those factors, an old method of exponential power function (Bateman, 1947) can be used to predict turf- grass pollen disposition (Wipff and Fricker, 2001). Wipff and Fricker (2001) measured gene flow from herbicide-resistant transgenic creeping bentgrass into wild relatives. The primary objectives of the study were to investigate intra- and interspecific gene flow of transgenic creeping bentgrass in the Willamette Valley of Oregon, where nearly all U.S. bentgrass seed is produced. Pollen movement was determined by placing transects of nontransgenic creeping bentgrass around a nursery of 286 plants genetically engineered for tolerance to the herbicide glufosinate. In 1998, transgenic turfgrass pollen grains were observed to travel 1,066.8 m along southwest transects and 1,309.4 m along northeast transects from the
120 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS nursery. In 1999, transgenic pollen traveled 331.5 m to the southwest, 575.1 m to northeast, 262.4 m to the northwest, and 331.5 m to the southeast from the nursery. The experiments resulted in the introgression of the bar gene from creeping bentgrass into A. canina, A. capillaris, A. castellana, A. gigantea, and A. pallens species. Turfgrasses can vegetatively multiply easily and effectively by rhizomes and stolons. Those underground parts often are translocated by machinery. Birds and mammals also facilitate the dispersal of turfgrass because they feed and forage in and around turfgrass stands for seeds and insects. Grass seeds are ingested and excreted or carried on fur or feathers for deposition elsewhere. For all of the reasons discussed above, transgenic turfgrasses, perhaps especially creeping bentgrass, can be considered potentially difficult to con- fine (Box 3-3). It also must be recognized that bentgrass is a commercially important turfgrass because of its extensive use in golf courses: More than 65% of the transgenic field test permits issued have been for bentgrass (Table 3-3). Bioconfinement Methods for Transgenic Turfgrasses Each bioconfinement technique discussed above could be used in future transgenic turfgrass products. The possibilities include chloroplast trans- BOX 3-3 Turfgrass Might be Difficult to Confine Transgenic turfgrasses carry a particularly high risk of escape for two reasons: Turfgrasses are perennial, so they have many seasons in which to spread through pollen and seeds, and they form unintended hybrids (which themselves would be long-lived) easily. Turfgrasses are open-pollinated plants with a very high cross- ability, primarily with species that are aggressive weeds. Most turfgrasses have many species that outcross heavily among themselves (Giddings et al., 1997a) and even among different turfgrass genera. For example, in nature, Agrostis spp. (bentgrass) cross-breeds with members of the Polypogon genus; and it is believed that Agrostis parlatorei Breistr and A. moldavica Dobrescu and A. moldavica Beldie are derived from multiple cross-hybridization between A. casstellana and P. veridis (Wipff and Fricker, 2001). Also, there are several examples of anthropogenic hybrids between ryegrass (Lolium spp.) and Fescue (Festuca spp.) genera. Figure 3-3 shows a wild hybrid between tall fescue (F. arundinacea) and annual ryegrass (L. multiflorum Lam) developed by Tim Phillip at the University of Kentucky. More intensive bioconfinement methods, such as the use of plastid transgenesis and male sterility are needed in genetically engineered turfgrass production.
PLANTS 121 genesis, tissue- and organ-specific gene expression, male sterility, apomixis, terminator gene technology, gene silencing, suicide genes, ablation, exci- sion, and inducible promoters. However, few bioconfinement techniques have been reported for turfgrasses, in part because little funding has been available for basic research. A significant increase in support will be needed to promote development of an adequate arsenal of bioconfinement tech- niques for the safe use of transgenic turfgrasses. It should be noted that some transgenes could have beneficial effects, should they transfer to other grasses through pollen flow or by other means. Many people suffer from ryegrass pollen allergies, and ryegrass was recently genetically engineered with an antisense-mediated silencing of the gene (lot p5) that encodes the rye pollen allergen. The lot p5 gene antisense construct was expressed in ryegrass under regulation of a pollen-specific promoter. The pollen from those transgenic plants showed low IgE antibody-binding capacity of pollen extract as compared with control pollen, meaning that the pollen of the genetically modified ryegrass could contain minimal amounts of allergen or none at all (Bahalla et al., 1999). This could be of great benefit to allergy sufferers. TRANSGENIC ALGAE Microscopic and macroscopic algae are a diverse group of organisms that are taxonomically distinct from plants. Microalgae are discussed along with bacteria and other microbes in Chapter 5. Commercial production of macroalgae is an important sector of aquaculture, especially in Asia. Seaweeds, such as Laminaria, Porphyra, Undaria, and Graciliaria, are grown for food and food additives, including polysaccharides such as carageenan (Renn, 1997). Commercial transgenic macroalgae have not been developed, in part because of technical obstacles, but there is increasing interest in using them to enhance fuel, polysaccharide, fish feed, and phar- maceutical production and in environmental bioremediation (Minocha 2003; Stevens and Purton, 1997). As with grasses and trees, some commer- cially grown algae have tremendous potential to disperse and persist in natural habitats. Some algae are considered invasive because they out-compete native species and dominate marine ecosystems when introduced to new areas (Occhipinti-Ambrogi and Savini, 2003). Because algae often are cultured outside their native ranges, some nontransgenic species have been managed using bioconfinement methods. For example, a "biological design" method has been used in Maine to confine nonengineered nori (Porphyra spp.). An introduced species of nori (P. umbilicalis) is cultivated commercially on rafts that float in coastal waters where a closely related native species of nori also occurs. Concerns were raised that the introduced species would
122 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS become invasive and harm native populations by hybridization or competi- tion. However, extensive field studies documented that, under ambient conditions, the introduced species was not invasive and did not reproduce, most likely because of its poor survival in winter (Levine et al., 2001). Thus, this nonnative nori appears to be biologically confined, as long as its repro- ductive capacity continues to be inhibited by local conditions. Other bioconfinement methods would be needed for genetically engi- neered algae that can survive and spread in natural habitats near aquacul- ture facilities. There is no feasible method of inducing sterility in algae, and the lack of basic understanding of the biology of reproduction in most algae is a major obstacle to developing a feasible method in the near future. Macroalgae are plastic in growth form. They often have complex life histo- ries that involve multiple reproductive pathways, including parthenogenesis and vegetatively dispersed propagules. Researchers do not fully understand sex determination, reproduction, or other aspects of the life history of many species; in some cases, they have not even identified which life stage is reproductive. Therefore, any efforts to study and then biologically confine transgenic algae will have to proceed on a case-by-case basis. EFFECTIVENESS AT DIFFERENT SPATIAL AND TEMPORAL SCALES Most of the bioconfinement methods discussed here are equivalent to natural mechanisms of reproductive isolation that act to maintain species barriers. In plants, the leakiness of those species boundaries is well known (Arnold, 1997; Grant, 1981; Levin, 1978). Within species, distinctive breed- ing systems such as dioecy (male or female plants) and self-incompatibility also are known to be leaky (e.g., Lloyd, 2000; Poppendieck and Petersen, 1999). Moreover, experience suggests that sterility is rarely absolute. Thus, in most circumstances, single-method efforts at bioconfinement are likely to be less than 100% effective in preventing the escape of transgenes, espe- cially if large numbers of plants are involved. The same could be true of multiple-method bioconfinement efforts if there is a chance that individual methods could fail. Unless a bioconfinement method is 100% effective in preventing the movement of seed, pollen, spores, and vegetative propagules, its efficacy generally would vary considerably over different spatial and temporal scales. Spatial Scale Bioconfinement generally will work best for small numbers of plants that are physically isolated (on the order of kilometers at least) from other
PLANTS 123 populations of the same species or from compatible relatives. Relatively small plant populations tend to be gene flow sinks rather than gene flow sources. All other things being equal, when population sizes vary, gene flow tends to be asymmetric: There is more flow from large populations into small ones than the other way around (Handel, 1983; Levin and Kerster, 1975). Thus, if a bioconfined crop were planted in the midst of other varieties of the same species (e.g., maize grown in Iowa), the percentage of efficacy of less-than-perfect bioconfinement would be expected to drop radically as the number of bioconfined plants increased from dozens to thousands. First, the chance of genetic changes that "disarm" confinement traits, such as mutations that silence transgenic sterility systems, increases with population size. Second, larger populations are more likely to disperse pollen, seeds, or vegetative propagules than are small populations (e.g., Handel, 1983; Levin and Kerster, 1975), and this could compromise back- up strategies such as physical isolation of the bioconfined crop. Although most of the data that associate population size and gene flow come from the literature on pollen flow, there is every reason to assume that similar rela- tionships would occur for the dispersal of seed and vegetative propagules. Small populations could be common for a few types of transgenic crops--such as pharmaceutical-producing plants--that are grown commer- cially. The high economic value of those crops and the requirement to segregate them from related crops or wild species will mandate their culti- vation in small or isolated populations. However, most plants grown for other uses are likely to be cultivated on a much larger scale. If, for example, bioconfinement is desired for corn or tobacco varieties that produce indus- trial chemicals, some of those crops could be grown on thousands of acres with millions of plants at each site and millions of other, nontransgenic, plants growing nearby. Another aspect of spatial scale is the number of populations that will be cultivated and the number of regions in which the crop can be grown. Local varieties of corn and soybean are grown over vast areas in the United States; fruit orchards and vineyards tend to be smaller and more regional. Major commodity crops that constitute the basis of industrialized agriculture could pose the greatest challenges for bioconfinement because they are grown on an enormous scale. Likewise, forage crops planted on rangeland occupy vast geographic areas, especially in the western states. Even highly managed tree plantations and golf courses represent large populations, each of which consists of thousands or millions of individual plants. When bioconfined plants are grown in many regions, there is a greater chance that they will be planted in the proximity of sexually compatible cultivars or wild relatives. This magnifies the chances of unwanted effects should bioconfinement break down.
124 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS Temporal Scale In the same vein, the efficacy of bioconfinement should decrease as temporal scale increases. The longer a population is in place, the greater the chance that bioconfinement will erode, and the more opportunities the population will have to disperse pollen, seed, and vegetative propagules. Perennials are long-lived by definition, but even annual plants can occur in long-lasting populations. Indeed, if some small amount of viable seed is released undetected into the soil, that seed bank can grow considerably over a series of years. Environmental conditions also vary from one year to the next, and the efficacy of bioconfinement varies under different environ- mental conditions; opportunities for failure increase over time. Perennials such as turfgrasses and trees can behave very differently from annual crops. Where annuals grow, flower, set seed, and die within a single year, perennials are heterogeneous. Depending on the species, they might or might not flower within a year of germinating. Some species do not flower for many years. Some perennial species live a few years; others (including some grasses and trees) can live for hundreds or even thousands of years. Many perennials (especially grasses) reproduce vegetatively, many do not. Each combination of species-specific temporal patterns will have a different influence on bioconfinement strategies. A perennial in which flowering is delayed for many years and in which vegetative reproduction does not occur will be relatively easy to confine, especially if plants are harvested thoroughly before they flower. At the other extreme, a perennial that creates vegetative propagules regularly, flowers at an early age, and continues to flower every year could be structured to produce so many progeny by seed, pollen, and propagule that finding an effective bioconfine- ment strategy could be a significant challenge. MONITORING AND MANAGING CONFINEMENT FAILURE The degree to which failed confinement can be monitored and managed depends on whether the GEOs are easily detected, the scale at which they are released into the environment, and their subsequent population dynamics and the degree to which they can hybridize with related species. Early detection of failed methods is important, especially if the confined transgenes are likely to spread, but this might be possible only for small- scale plantings of some crops. If a failed bioconfinement method can be recognized by distinctive phenotypic traits, such as the presence of flowers in otherwise sterile plant varieties, it might be possible to cull abnormal plants in small fields. That practice is used in certified seed production programs, where inspectors go through the fields to remove or cut off any "off-type" plants that do not conform to desired phenotypic standards.
PLANTS 125 However, failures of many bioconfinement methods will be much more difficult to detect. Elaborate experiments would be needed to identify the proper functioning of a repressible seed-lethal transgene. And most bio- confined plants will be grown on such large areas of land that repeated, comprehensive inspections would be impractical. For large-scale releases, it is important to have easily recognized diag- nostic features that allow the detection of failed confinement. In some cases, genetically based color traits, such as red kernels in corn, could be used to identify a particular transgene, assuming that the color trait stays tightly linked to the confined transgene. Distinctive phenotypes have been bred into some conventional crops, such as oilseed and "confectionary" sunflower, which have black seeds instead of striped seeds, respectively. Experimental lines of transgenic rice that have vitamin A precursors pro- duce recognizable yellow grains, hence the name "golden rice" (Ye et al., 2000). An advantage of visually distinctive traits is that they are easy to identify with minimal expertise. However, a disadvantage is that they could be unreliable because of phenotypic plasticity, variable gene expression, or recombination that separates the genetic marker from the bioconfined transgene. Transgenic methods could be used to introduce general or specific markers for the purpose of monitoring bioconfined transgenes. A general method could be to add a gene that expresses GFP, although that requires examining the plants in the dark with ultraviolet light--a technique with obvious limitations (Leffel et al., 1997). Another option is to assay for specific novel proteins in leaves or seeds using rapid enzyme-linked immuno- sorbent assays (ELISAs) that are similar to those at work in home test kits for pregnancy. Several companies market kits for detecting commonly used transgenes, such as antibiotic resistance proteins, that are often used as markers in genetically engineered plants. The kits are simple to use on leaf samples in the field, but false-negative results are common (Ilardi and Barba, 2001), and the cost of large-scale testing can be prohibitive. In some cases, transgenic resistance to a particular herbicide could be inserted in the same construct as a bioconfined transgene to monitor for possible failure. Seed lots could be sampled and screened for the presence of rare, unexpected transgenes by applying the herbicide to large numbers of plants grown in field experiments (e.g., Scheffler et al., 1993). Herbicide- resistant survivors could be analyzed further to confirm the presence of the unwanted transgene. This method could be used on a case-by-case basis, but if the bioconfinement method failed it might lead to the unwanted spread of herbicide resistance as well as to the spread of the bioconfined transgene. However, in short-term, small-scale experiments, herbicide resis- tance could be a useful marker for testing the efficacy of new bioconfinement methods before they are used on a commercial scale.
126 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS In the future, unique DNA fingerprints could be linked to bioconfined transgenes to function as "bio-barcodes" TM (Gressel, 2002). Those markers also could be useful for identifying nonconfined transgenes for labeling, but they require more elaborate and expensive laboratory techniques than are needed for the phenotypic traits mentioned above. Broothaerts and col- leagues (2001) described a multiplex polymerase chain reaction (PCR) tech- nique that simultaneously demonstrates the presence of a transgene sequence and an endogenous gene using a single reaction. Common transgene-specific primers were used in combination with conserved primers for polymorphic endogenous genes. The polymorphisms detected for the endogenous genes permitted the host plant's genotype to be determined, and they confirmed that the PCR had worked properly. The authors proposed the technology for use in protection against mislabeling of cultivars during subculturing and other laboratory and greenhouse operations, as well as for screening for transformants in the production of new transgene lines. The approach also would be useful in identifying cases of transgene escape into other culti- vars or genotypes of the same species and their sexually compatible wild relatives. Greater attention to the need for monitoring could lead to new and more effective approaches. For example, there is much interest in develop- ing a "synthetic nose" remote sensing system that could identify portions of an agricultural field that are under attack by insects. This method would detect and profile volatile emissions from the plants (www.aginfo.psu.edu/ News/march03/sentinel.html). Such devices are being developed for national defense and agronomic uses. Expression of transgenes for insect resistance also gives the genetically engineered plants a profile of volatile emissions that is different from that of wild-type plants of the same genotype, so it is possible that such transgene constructs could be detectable. Remote detec- tion systems could be used to survey large natural areas for transgene or plant escapes at some point in the future, but that possibility is still quite speculative. Given enough resources for statistically meaningful sampling efforts, it might be possible to detect failed bioconfinement, but there is still the problem of detecting failure early enough to mitigate or eradicate unwanted plants. If those plants reproduce and spread, either by further cultivation or by naturally occurring gene flow, subsequent efforts to stop the process could be futile. Therefore, plants that are judged to be serious enough risks should not be released because bioconfinement is always expected to be imperfect. Population, Community, and Ecosystem Effects Bioconfinement has rarely been used for cultivated plants, yet several
PLANTS 127 new methods could become available within the next 510 years. Given the diversity of methods that are under development (Table 3-1), it is difficult to project environmental effects. Here, a few examples can be used to illustrate possible direct and indirect consequences of future bioconfinement strategies. Two types of effects are discussed: those in which the confinement method functions as intended, and those that result from an unintended breakdown. For bioconfinement methods that rely on complete sterility, unwanted ecological or evolutionary effects are likely to be negligible if the method functions properly. For example, when a fully sterile crop or crop-wild hybrid produces no pollen, no viable seeds, and does not reproduce vegeta- tively, the transgene will not spread. Under what conditions could this pose a problem? A possible source of food for insects or wildlife could disappear if seed crops are eliminated through bioconfinement, although the ramifica- tions could be relatively unimportant in some circumstances. For example, if vast tracts of planted, seed-producing trees, such as Douglas fir, were replaced with sterile trees, animal populations that depend on the seed source could be harmed. Whether that would threaten ecologically, eco- nomically, or socially important species would require further, case-by-case investigation. Another hypothetical effect of transgenic sterility might occur if pollen from a crop with seed-specific sterility inundates small populations of wild relatives growing nearby. With extensive immigration of sterility-causing genes, the wild plants' seed production could be reduced (seeds sired by the transgenic pollen would be dead). Under some circumstances, this effect of "usurping" ovules and interfering with seed production might cause the native populations to shrink. However, few examples involving endangered wild relatives of crops have been identified (Hancock, 2003). Sexually com- patible taxa that occur near crops often are weedy or colonizing species for which small population size is not a concern. If bioconfinement were indi- rectly responsible for greater contact between the crop and the wild rela- tive, a possible case of unintended consequence could be argued. Moreover, if a crop's wild relatives are an important source of germplasm for further breeding, as is the case for perennial wild rice (Oryza rufipogon) in South- east Asia (Lu et al., 2003), extra precautions might be needed to ensure that gene flow from a V-GURT does not exacerbate the erosion of valuable genetic diversity. A more far-reaching fear among some members of the public is that sterility genes could spread throughout natural populations of wild rela- tives in a silenced (inactive) condition and later be reactivated, leading to massive die-off in populations of sexually compatible crop relatives. It is difficult to conceive of specific mechanisms that would support this
128 BIOCONFINEMENT OF GENETICALLY ENGINEERED ORGANISMS hypothesis, but further study should be considered for transgenic sterility methods. Other bioconfinement methods are intended to reduce the fitness of offspring from the crop or its crop-wild hybrids. Multiple scenarios for the fate of such fitness-decreasing transgenes should be considered to evaluate the effects of this process. First, if gene flow is extensive enough or the recipient population is small enough, deleterious transgenes could become fixed in feral or hybrid populations, perhaps leading to reduced popula- tions. This type of "demographic swamping" could occur along contact zones between the crop and its wild relatives (e.g., Haygood et al., 2003). Lower fitness that is shared by all members of small populations along the contact zone could cause the population to shrink and perhaps disappear. A second and perhaps more likely scenario is that fitness-reducing transgenes would be purged by natural selection, a process that is likely to occur with many types of "domestication" crop genes that enter wild or weedy popu- lations. Purging is expected to occur in populations for which gene flow is relatively low and the effective population size of wild relatives is larger than about 100 individuals. Large population size is common for most wild relatives of crop species. Male sterility is a bioconfinement method that sometimes is misunder- stood to be a danger to wild populations. Nontransgenic cytoplasmic male sterility has been used for decades to obtain hybrid seed in crops such as sunflower, canola, and sorghum (but not corn, for which mechanical de- tasseling is the commonly used method). Male sterility generally does not "breed true" or persist because of the large numbers of fertility-restoring genes that are found in cultivated and wild relatives of the crop (Besnard, 2000; Jan, 2000; Ohkawa, 1984; Yamagishi, 1998). In the future, new types of transgenic male sterility could come into common use for hybrid seed production in a wider variety of crops. Thus, male-sterile plants could be grown on much larger lands than at present, and it is possible that sterility would be passed on to plant offspring. If so, it is not expected that wild relatives of a crop would be harmed because fitness-reducing traits are quickly purged from large, interbreeding populations. It is also important to consider the possible indirect effects of various bioconfinement methods. For example, how would a bioconfinement method affect populations of nontarget organisms, such as pollinators and other beneficial insects? Could the method harm animals at higher trophic levels in food webs because their prey are adversely affected? Could a novel trait like apomixis allow a vigorous cultivar to establish feral populations that invade natural areas? Also, would the method facilitate the cultivation of novel crops that produce unhealthy residues or facilitate environmentally damaging agricultural practices? How would those effects compare with existing problems caused by conventional agriculture? There is no reason to
PLANTS 129 expect unwanted effects as a general feature of bioconfinement, but any large-scale release of novel GEOs should be accompanied by careful risk assessment. To thoroughly evaluate new methods it is necessary to examine anticipated benefits as well as possible risks of specific cases. It also is useful to consider possible consequences when bioconfinement methods do not function properly, for example because of gene silencing or recombination that disconnects linked transgenes (Box 3-1). The ecological and evolutionary consequences of failed methods will depend on the char- acteristics of the transgenic plant, the environment in which it occurs, and the effectiveness of physical confinement. Failure of confinement methods-- biological and otherwise--that are used to prevent pharmaceutical proteins in a commodity crop like maize from entering the food supply could lead to huge socioeconomic damage and unwanted effects on human health and nontarget organisms. Likewise, if bioconfinement fails to prevent the spread of an invasive horticultural variety, economic and environmental damage could be extensive. If bioconfinement is used with low-risk GEOs, however, the consequences of failure should be negligible. In general, the reason for investing in bioconfinement in the first place is usually strong enough to indicate the potential seriousness of the consequences of failure. Specific consequences of bioconfinement failure will depend on the type and the scale of the damage, as is discussed in Chapter 2, reflecting the "hazard × exposure" equation used in academic discussions of risk assess- ment (see also Figure 2-1). In some cases exposure could be very small (e.g., Slavov et al., 2002, model on gene flow from poplar). However, in complex and constantly evolving ecological systems, the probability of exposure and the risk of harm from such exposure can be difficult to quantify empirically. Also, public perception of risk often is based on other, less tangible criteria. A basic tenet of this report is that bioconfinement is likely to fail to some extent, even when multiple methods are used to safeguard against failure.