11
Applying the Framework: Case Studies Using the Prototype Safety Monographs
The second phase of the Food and Drug Administration’s (FDA’s) request to the Institute of Medicine (IOM), after proposing a framework for the safety evaluation of dietary supplement ingredients and releasing it for comment, was to develop at least six prototype monographs for dietary supplement ingredients using the system outlined in the initial framework and, through this process, evaluate the proposed framework and revise it as necessary.
Based on this experience and on comments received by industry representatives and other stakeholders about the proposed process (see Appendix B for a summary of the comments received), the framework was revised to that described in Chapters 3. The prototype monographs are available on the Internet (www.iom.edu/fnb) and, for the sake of brevity, the summary and conclusions for each of the six ingredients are included in Appendixes D through I. This chapter contains a discussion of the process used to evaluate each dietary supplement ingredient, the principles derived from the process of evaluation, and a rationale for focused monographs, developed following review of two of the full prototype monographs (saw palmetto and chaparral).
PROTOTYPE MONOGRAPH DEVELOPMENT
The monographs developed to evaluate the proposed framework and process are referred to as “prototypes” for several reasons. Because the six monographs were simultaneously prepared within the timeline of the overall report, the information collected is not expected to be as complete as
what might be collected if qualified staff at FDA or another organization were specifically charged with undertaking only monograph generation. For example, the timeline of this project required that industry and other stakeholders be given only a few weeks to a month to review the information included in the draft prototype monographs to determine if there was a need to volunteer data in their possession prior to the public meeting. In addition, the sources of information and the process for systematically collecting information were evaluated during this process and thus continually modified as subsequent monographs were developed. Thus the monographs, as well as the monograph summaries and the focused monographs in this volume, are considered prototypes and as such are not authoritative statements of the National Academies or the IOM on the safety of the specific dietary ingredients, but are put forward as examples of what might be done.
The six supplement ingredients selected to serve as the subjects of the prototype monographs were chaparral, chromium picolinate, glucosamine, melatonin, saw palmetto, and shark cartilage. Development of the prototype monographs proved to be a useful tool to assess the practicality and ability of the Framework to guide a dietary supplement ingredient safety evaluation.
Criteria Used to Select Prototype Monograph Ingredients
The six ingredients were selected to fulfill several criteria and thus test the proposed framework from a variety of perspectives. One criterion, for example, was to review a variety of types of ingredients; thus the selection of ingredients for prototype monographs included at least one botanical, one well-characterized vitamin or mineral, one animal product, one hormonal product, and one “new” (post-October 1994) product. Another criterion was that the selected ingredients include substances for which a range in the types and quality of available information were anticipated. Finally, it was decided that selected ingredients should not be undergoing safety research by committee members. The range of ingredient types, available data sources, and resulting level of concern allowed the evaluation of various aspects of the proposed framework and provided an opportunity to improve the recommended approach.
Signal Detection
It is worth noting that the reasons substances were flagged initially as described in the proposed framework (the report previously released for comment) would have also brought them to FDA’s attention according to
the Framework presented in this final report. In the case studies that follow, additional reasons that might have generated a signal for FDA to examine the dietary supplement ingredient are also noted. Using the Framework, FDA would have considered the nature of the evidence readily available as a result of the signal detected and decided, based on initial review, that a level of concern existed. A decision whether to continue monitoring or to move into the integrative evaluation of the dietary supplement ingredient would then have been made.
Integrative Evaluation
An IOM staff monographer developed a search strategy for each ingredient based on recommendations from the committee and began collecting and collating the data available. In order to simulate the initial evaluation in which an internal scientific review would determine which aspects were relevant to include in a comprehensive evaluation, a working group was assembled that included some committee members plus consultants who were chosen based on specific expertise relevant to the dietary supplement ingredient. An initial meeting was held of each prototype working group with the monographer to give direction to the literature search and additional relevant data to be included; at this point, the main activity was to complete the monograph outline.
The working groups reviewed and revised the developing draft monographs as new information was provided and consulted with the monographer via conference calls. Following the procedures outlined in the proposed framework, draft prototype monographs without recommendations or conclusions were released for comment and additional input. Stakeholders were notified of their existence and how to provide comment and feedback.
External Advisory Committee
The role of the working group next changed to become a prototype of an external advisory committee in which participants reviewed and analyzed the data and developed statements regarding concerns about safety. A second meeting was held of each working group in which the group served in the capacity of an external advisory committee. At the second meeting, they received oral comments from stakeholders and reviewed additional information received. They subsequently drafted recommendations and conclusions (see summary analyses, Appendixes D through I).
USE OF THE FRAMEWORK IN EVALUATING PROTOTYPE INGREDIENTS
The goal of the monograph development phase was to test the proposed process and learn from it to improve the overall framework. Below, each prototype is described as a case study to illustrate how principles and concepts from the Framework were used to meet the objectives of each step. The triggering signals are indicated as presumed in this illustration as it is not possible to know whether FDA would have initially detected them as signals or not.
Chaparral
Presumed Signals
Concerns expressed about the safety of chaparral by several authoritative sources signaled that chaparral’s safety should be investigated, as did several SN/AEMS1 reports of serious adverse events associated with chaparral use (coma, severe hepatic injury, hepatitis, and kidney failure).2 Authoritative sources included FDA itself which, before the Dietary Supplement and Health Education Act, issued a press release warning of a potential relationship between chaparral use and liver toxicity (FDA, 1992), and the American Herbal Products Association’s Botanical Safety Handbook (McGuffin et al., 1997) notation that Health Canada did not allow chaparral as an orally administered, nonmedicinal ingredient. Possible liver problems were also mentioned in several other secondary sources of information (Foster and Tyler, 1999; NMCD, 2002).
While the use of chaparral may not be widespread, the seriousness of the adverse events and the several authoritative sources citing potential problems, especially liver problems, were considered to be a strong signal requiring the evaluation of the nature of the evidence regarding potential liver and other problems. This is consistent with Chapter 4 guidelines that reports of liver necrosis or acute liver failure be considered in an expedited manner due to their potential for mortality and morbidity.
Initial Review: Considering the Nature of the Evidence
The nature of the case studies and reports to FDA were considered first, as described in Chapter 4. Serious adverse events occurred in individuals
|
1 |
Special Nutritional Adverse Event Monitoring System, as available on FDA’s website. |
|
2 |
This chapter is presented as a review of the prototype monographs as case studies. The actual references for statements made are in the full prototype monographs available at www.iom.edu/fnb. |
who reported taking chaparral but no other supplements or drugs, suggesting that the adverse events were not due to concomitantly consumed substances. Reported liver problems were clinically documented. In the case report of jaundice and toxic liver disease, the individual elected to rechallenge and the symptoms recurred. Recurrence of symptoms after rechallenge is considered a convincing factor in assessing strength of association. Thus, the adverse event reports are most consistent with the description of higher concern in the right column in Table 4-1: “Describes a well-documented serious adverse event, with plasma levels at a relevant range (if available) and demonstrates dechallenge and rechallenge (if possible), temporality, and strong attribution.”
In addition to the human safety data, there were also indications that persons with pre-existing liver disease might consume chaparral.3 The fact that the very persons most susceptible to liver problems might be using chaparral should raise the concern level even higher, consistent with the discussion of particularly susceptible subpopulations in Chapter 9.
Given the high level of concern about the human data on liver problems and the additional concern about particularly susceptible subpopulations, the Framework directs FDA to proceed with an integrative evaluation of all available data about chaparral (i.e., other human data, animal data, in vitro data, and data about the safety of related substances). In this case, FDA could instead elect to do a focused monograph on the risk of liver adverse effects from chaparral consumption because the evidence available at this point indicates the greatest concern is for liver damage (see discussion of a focused monograph, later in this chapter).
Integrative Evaluation
Although FDA could have chosen to focus its efforts on liver problems, the prototype chaparral integrative evaluation was conducted with a more broad-based strategy. Studies focused on the safety of chaparral were limited in number and value, so the literature search included a search for information about safety problems associated with nordihydroguaiaretic acid (NDGA), a component of chaparral.
The human data considered included information about hepatic adverse events reported to FDA and published as case reports (as discussed in the previous section). Anecdotal information about the traditional use of chaparral as a tea was also considered. This information was only considered relevant to chaparral use as a tea, because as described in the mono-
|
3 |
For specifics, see discussion of conditions of use (Section B) in prototype monograph, available at www.iom.edu/fnb. |
graph and in Chapters 4 and 6, the similarity of a product form greatly impacts the chemical constituents consumed, and apparent safety associated with one form should not be extrapolated to another form. Specifically, in the case of chaparral, NDGA concentrations are likely to be higher in chaparral products that are not water-extracted teas. Only one of the human adverse events appeared to be associated with chaparral tea use. Thus the integrative evaluation distinguished between safety problems associated with chaparral consumption as a tea and safety problems associated with other chaparral products likely to contain more NDGA.
Animal data included studies about NDGA. They suggested that chaparral and NDGA administration result in anti-implantation activity or inhibition of ovulation and increase resorption of fetuses, respectively. As indicated in Chapter 5, the animal reproductive toxicity effects were deemed particularly important to consider because it is unlikely they would be detected by human use, even with long-term traditional use (see Chapter 4 discussion of “Considering the Relevance of Historical Use” ). Also important to the consideration of liver toxicity was the information uncovered about kidney damage, which in addition to indicating a potential risk for renal toxicity might also suggest harm at other sites of NDGA metabolism, such as the liver.
In vitro data relevant to the safety of chaparral were also considered. In vitro studies on NDGA provided mechanistic information, such as lipoxygenase and cyclooxygenase inhibition and inhibition of prostaglandin synthesis.
Information about the safety of related substances and the chemical structure of chaparral constituents raised two possible concerns. First, NDGA is functionally related (see Chapter 8) to prostanoid pathway inhibitors, which are contraindicated during the first and third trimesters of pregnancy. Second, the chemical structure of NDGA indicates that it is likely to be a substrate for cytochrome P450-dependent quinone formation. Finally, in vitro data suggest NDGA is functionally related to 5-α-reductase inhibitors, which are contraindicated in pregnancy due to effects on male development in utero.
Finally, the totality of information summarized above is considered according to the scientific principles for evaluating and integrating data, as described in Chapter 10. As stated in Chapter 10, “In the absence of scientific studies designed specifically to test safety of a dietary supplement, concern for public safety may be raised by the presence of even a few reports of possible safety concerns, when viewed together and constituting the weight of available evidence.” No scientific studies designed specifically and adequately to test the safety of chaparral were found. The information suggesting safety is limited to the possible historical use of chaparral without documented adverse effects. The information suggesting risk of NDGA-
associated reproductive abnormalities is consistent and is biologically plausible. Liver risk is biologically plausible but less consistent given the negative animal data, but lack of observed effects is not considered as important as observation of effects (see Chapter 10). Overall, the weight of the evidence indicates higher concern with NDGA consumption—a concern that is then applied to chaparral consumption, with the possible exception of chaparral tea.
Chromium Picolinate
Presumed Signals
Chromium picolinate was flagged for review because secondary sources mentioned that its use has been reported in cases of renal toxicity, and because secondary sources discussed its purported effect on insulin regulation and theoretical risk when used with insulin by persons with diabetes (NMCD, 2002). Other signals could have brought this dietary supplement ingredient to the attention of FDA as well. For example, the SN/AEMS documented serious adverse events in individuals ingesting chromium picolinate, including severe seizure, ventricular tachyarrhythmia, and jaundice. These adverse events would have been considered a strong indication that chromium picolinate warranted attention. Chromium picolinate’s widespread prevalence of use, including its common inclusion in many combination dietary supplements, also suggests value in devoting attention to the risk of the use of chromium picolinate as a supplement ingredient.
Initial Review: Considering the Nature of the Evidence
The renal toxicity cases that signaled chromium picolinate as needing attention were evaluated following the description outlined in Chapter 4. As described in the chromium picolinate prototype monograph, confounders existed, such as concomitant drug consumption and pre-existing conditions, and there was no information about persons experiencing adverse effects ending and then resuming chromium picolinate intake (challenge/ rechallenge). Thus, using the criteria from Chapter 4, the concern level about the signal would be relatively low based on the available information.
More context was provided by reviewing additional adverse event reports from the SN/AEMS, which showed two deaths in individuals taking two or more supplements containing chromium. Again, these reports showed that the users consumed a multitude of supplements and did not include information that led to a strong association (see Chapter 4) with
chromium picolinate; therefore, the concern level remained lower even though the event is considered most serious.
In addition to the initial signal about renal toxicity, the initial signal of concern about diabetics ingesting chromium picolinate was also considered by reviewing results of a clinical trial. This concern was not deemed a higher concern given that a 10-day trial with 162 diabetic subjects did not reveal changes in clinical parameters associated with glucose regulation problems following insulin administration.
In the case of chromium picolinate, consideration of the nature of the evidence about renal toxicity and glucose regulation signals did not elevate concern to a higher level. Following the Framework, a lower to moderate concern suggests that FDA note what was learned about the signals detected, the serious adverse events, and the insulin regulation concern and then continue to monitor until new signals of concern suggest more consideration is warranted.
Integrative Evaluation
Although not suggested by the review of the two signals above, if FDA did choose to conduct an integrative evaluation of chromium picolinate because of the seriousness of these serious adverse event reports or other reasons, it would be appropriate to follow a broad-based strategy because the serious adverse events reported suggest potential damage to several organ systems. Because chromium picolinate contains a trace element that is poorly excreted, FDA would want to pay particular attention to the impact of dose on safety.
The chromium picolinate prototype monograph preparation process began with a relatively broad-based strategy for identifying risks that might be associated with chromium picolinate ingestion. Specifically, the strategy was to look for information relevant to the major possible toxicities evident from the human data and to look for animal and in vitro toxicity studies that revealed any other concerns regarding chromium picolinate. This strategy revealed information about carcinogenesis and oxidative stress—information that might not have been apparent in a reactive monitoring approach.
Glucosamine
Presumed Signal
Glucosamine was flagged because secondary sources raised concerns about its use by persons with diabetes (Medical Economics Co., 2001; NMCD, 2002). Animal and in vitro data suggested the potential for glu-
cosamine to cause insulin resistance. Use of this information as a signal illustrates that animal and/or in vitro data are appropriate signals to identify concern to humans.
In addition, while not a data signal per se, the high prevalence of glucosamine use among older individuals who are likely to be at risk for type 2 diabetes suggested that if risks are associated with glucosamine and biological activities that interfere with glucose control, they could have large impact on public health as this subpopulation would be particularly vulnerable to insulin problems. Thus this information about a potentially vulnerable subpopulation should encourage the evaluation of glucosamine risk sooner than some other ingredients.
Initial Review: Considering the Nature of the Evidence
The nature of the evidence in the animal studies producing the original signal was reviewed. These studies did indeed confirm that glucosamine can lead to insulin resistance in animals and the in vitro studies supported the biological plausibility of such an effect. However, the animal studies that described this effect were experimental investigations on the basic biology of glucosamine, not traditional toxicology studies, and the high blood concentrations of glucosamine (approaching 1 mM) were deemed highly unlikely to be achieved by the amounts ingested orally by humans. The insulin effects would probably be categorized as a Category B (Table 5-1) effect. It is not clear how millimolar blood glucosamine in animals compares with human glucosamine because of significant metabolism: assuming less than 1 percent of orally ingested glucosamine reaches the bloodstream would suggest lower concern, while a 1 to 10 percent assumption would suggest moderate concern.
Additional information was sought to put the animal data’s suggestion of insulin regulation problems into context. At this stage in the process, a full literature review was not conducted, but a focused search for human data relevant to insulin resistance was completed. Data from human clinical studies did not suggest an increased risk of insulin resistance, although most of the studies examined (especially older studies) were of relatively short duration and/or did not specifically report on blood or urine glucose levels.
At this point FDA could have decided that the nature of the evidence did not indicate a need to undertake an integrative evaluation, including the development of a monograph, because the level of concern remained lower to moderate. Therefore, instead of an integrative evaluation, this dietary supplement ingredient could have been designated for monitoring of both new signal-generating information and answers to particularly relevant questions. For example, a periodic search for human data suggesting problems of insulin regulation or reporting blood glucosamine levels following
ingestion could be put in place. In addition, FDA, working with the National Institutes of Health’s (NIH’s) Office of Dietary Supplements, could request that laboratory indicators of insulin resistance and diabetes, as well as blood glucosamine levels, be monitored in subsequent clinical studies of glucosamine.
Integrative Evaluation
If FDA chose to proceed with an integrative evaluation for glucosamine, it could follow either a broad-based or a more focused strategy. Glucosamine appears widely used by older individuals, whose baseline incidence rates for several health problems are relatively high. Thus a relatively small increase in risk of any of these conditions associated with glucosamine use might have substantial public health impact, and it might therefore be appropriate to conduct a broad-based evaluation and proactively evaluate all potential risks of harm.
Alternatively, because the signal identified a risk specifically for insulin resistance, FDA could focus its integrative evaluation on insulin resistance and diabetes. In this case, while the topics of a literature search would be fewer than with a more proactive evaluation of all possible risks, it would not be sufficient to look for mention of glucosamine and insulin resistance or glucosamine and diabetes in abstracts of publications. Instead, the literature search would need to identify a wider variety of studies to be read in detail to determine if relevant data on glucosamine and insulin resistance exist. Relevant data might be noted only as a minor point in clinical studies, or it might be revealed only by detailed reading of publications on nonhuman data. In summary, the literature search strategy would need to be sufficiently broad to identify all potentially relevant studies that would then be examined to decide which studies are of importance for the integrative evaluation.
Melatonin
Presumed Signals
Melatonin was flagged because of serious adverse event reports to SN/ AEMS. These events were cardiovascular and psychiatric/central nervous system related in nature. Another signal could have been melatonin’s regulation as a drug in other countries, a fact signaling that the ingredient’s potential risk should be examined.
Initial Review: Considering the Nature of the Evidence
The medical literature was searched to explore cardiovascular and psychiatric types of reports. Case reports indicated that melatonin might exacerbate psychiatric conditions, and it has been reported to cause seizures. Case reports included evidence that supported a causal relationship between melatonin and the occurrence of seizures, including challenge and rechallenge data. Animal data relevant to the issue were also examined. There was little animal data relevant to the behavioral effects, but data describing significant effects on the reproductive axis of animals added to the level of concern. Thus, at this step, multiple factors came together to suggest a higher level of concern regarding the use of melatonin, indicating the need for an integrative evaluation. In addition, there was concern that adverse events such as seizures and psychiatric problems worsened in persons particularly susceptible to these problems.
Integrative Evaluation
The integrative evaluation of melatonin began as a focused effort looking at the initial signal of seizure occurrence in an at-risk population, but the preliminary review to gather additional information yielded other areas of concern related to melatonin’s physiological role as a hormone. Once these data were identified, it did not seem appropriate to ignore them as they might also indicate that melatonin was a risk to public health.
Each of the various categories of data (human, animal, in vitro, and related substances data) was important to the development of the recommendations and conclusions of the working group. While there was a large amount of human data, it was collected following short-duration exposure to melatonin. Animal studies of some duration helped to put the human data in context. The in vitro data were useful for understanding the potential for melatonin to stimulate or inhibit the activity of other hormones. Evidence from human, animal, and in vitro studies in which doses far exceeded the usage of melatonin as a dietary supplement were also considered.
Saw Palmetto
Presumed Signals
Saw palmetto was selected for review because of two serious cardiac events reported to SN/AEMS. Other signals of concern could have been evidence that saw palmetto may have or has similar biological activity to a regulated drug (finasteride) and saw palmetto’s use as a drug in several
European countries. The finasteride example illustrates how functional relatedness can signal concern, as discussed below.
Initial Review: Considering the Nature of the Evidence
The cardiac events reported to FDA were placed in perspective by reviewing whether a pattern of cardiac events existed in clinical trials or published case reports. There was no consistent pattern of serious adverse events reported, and they were largely nonserious or their relevance to saw palmetto was unconvincing. The concern level from the original signal was thus lower and would be unlikely to prompt an integrative evaluation according to the Framework process.
If saw palmetto’s drug status and possible mechanistic similarity to the regulated drug finasteride was the original signal, the initial review may have prompted an integrative evaluation. Investigation of this signal would have revealed in vitro evidence and animal evidence suggesting antiandrogenic effects, such as 5-α-reductase inhibition. Male human reproductive developmental anomalies are a feature of the congenital deficiency of 5-α-reductase, and the prescription 5-α-reductase inhibitor, finasteride, has been found to cause similar anomalies in male rats. Consequently, labeling of finasteride notes that it is contraindicated in women and children. In summary, the information about saw palmetto’s similarity to finasteride and similar drugs should raise concern, consistent with the following guiding principle (see “Constituents Functionally Related to Known Classes of Toxic Compounds” in Chapter 6): “When data (i.e., in vitro or animal data) suggest that a dietary supplement constituent targets a receptor, enzyme, or other biological target in a manner similar to a compound known to be toxic, concern is warranted, especially if the dietary supplement constituent is known to reach the biological target in a relevant concentration.”
Finally, the high prevalence of saw palmetto use is a modifying factor, suggesting an evaluation of the safety of this dietary supplement ingredient might be of value even if initial review did not result in higher concern if resources are available for such a proactive integrative evaluation. Benign prostatic hyperplasia is a very common condition among older men, and the prevalence of saw palmetto use among individuals with this disease is relatively high, suggesting that if saw palmetto were unsafe, the public health impact might be substantial. On the other hand, available evidence does not suggest this dietary supplement ingredient is frequently used in women or male children, the individuals to which the concerns apply.
Integrative Evaluation
As with all six ingredients selected as prototypes, data on saw palmetto was reviewed in a manner analogous to a proactive integrative evaluation, despite the lower level of concern about the original signal that prompted its selection. This integrative evaluation was relatively broad based in that all information deemed to possibly have anything to do with toxicity was collected in the initial data search.
The impact of a data collection strategy is illustrated in the saw palmetto example. If the data collection had been limited in scope to concerns raised by human data, and the consideration of animal and in vitro data had been limited to data from traditional animal toxicity studies and validated in vitro studies, testosterone pathway concerns would probably have been overlooked. This is because the information that raised concerns was from mechanistic types of in vitro studies and from animal studies that were not classical toxicity studies. In summary, considering data from studies other than animal toxicology and validated in vitro studies was useful because the available animal studies were too limited in scope (lacking reproductive toxicology) to detect safety issues.
Shark Cartilage
Presumed Signals
Shark cartilage was flagged because of a case report of hepatitis following ingestion. Another signal of concern could have been information suggesting that shark cartilage had antiangiogenic activity, a mechanism that raises concerns with drugs.
Initial Review: Considering the Nature of the Evidence
Five clinical case reports of adverse liver effects were considered. One SN/AEMS report was confounded by concomitant ingestion of a supplement containing known hepatotoxins. There was not a consistent pattern in the other reports sufficient to overcome the lack of adequate information regarding confounders. Consideration of the limited animal data—summaries of unpublished animal toxicology available in the published literature—did not suggest reason for specific concerns given that no overt signs of toxicity were reported. In summary, a lower level of concern would have resulted from an initial review of shark cartilage’s liver effects.
As described above, shark cartilage’s purported antiangiogenic mechanism could also have triggered an initial review. An initial review of data relevant to this signal would have uncovered reports of antiangiogenic activity in animal models (non-oral administration) and in vitro models
suggesting a mechanism by which shark cartilage might possibly lead to serious adverse events, such as teratogenicity. Establishing an appropriate level of concern from these data would have required additional information to place the antiangiogenic activity in perspective. Specifically, information about functionally related antiangiogenic substances would suggest that these agents could potentially cause serious effects, including teratogenic effects. This information indicates that the effect observed in animal data is a Category A effect (severe developmental effects, Table 5-1). Dose comparisons between animals and humans are difficult because of the wide variability in products referred to as shark cartilage, and the fact that the shark cartilage was not orally administered in the reports of antiangiogenic effects in animals. Because the constituent responsible for this purported activity is unknown, its concentration in blood cannot be compared between animals and humans.
In addition to the information presented above, use by a potentially vulnerable subpopulation raises concern. Shark cartilage appears to be used largely and to a significant degree by people with significant and often life-threatening diseases, such as cancer and rheumatoid arthritis, who, as a result of their disease or medical treatments, may be at increased risk for supplement-drug interactions and certain other adverse events, such as liver-related problems. Thus even without signals of higher concern to suggest an integrative evaluation in reaction to signals, a proactive integrative evaluation might be justified if resources are available to proactively conduct them.
Integrative Evaluation
Of the three items mentioned above (liver effects, antiangiogenic potential, and use by vulnerable subpopulations), the antiangiogenic potential raised sufficient concern to warrant a reactive integrative evaluation. The integrative evaluation could therefore be focused on the very limited amount of data addressing this potential concern. The limited amount of data resulted in significant data gaps in the prototype shark cartilage monograph. Conclusions of higher concern were not reached, but the limited amount of data adequately addressing the issue left questions about shark cartilage’s antiangiogenic potential in pregnant women. There is no information to suggest that women of child-bearing age are particularly likely to use the ingredient, which appears to be used largely in an effort to treat cancer and age-related conditions.
The shark cartilage prototype monograph also illustrates the wide difference in preparations of some dietary supplement ingredients. Powders could widely differ from extract preparations. Consistent with the guiding principles, safety concerns raised with shark cartilage powder use had to be
assumed to occur in extracts as well, given the paucity of data about which constituents might cause effects and whether these constituents would be present in the extracts.
PRINCIPLES IDENTIFIED FROM THE PROTOTYPES
Developing the draft prototype monographs as illustrations of the Framework’s integrative evaluation process served to test the process and the scientific principles included in it. The Framework presented in this report was shaped by what was learned. Some of what was identified is important to understanding how to apply each component of the Framework.
Signal Detection
The Framework takes into consideration that a myriad of signals (See Box 11-1) could suggest a potential for harm due to the consumption of a given dietary supplement ingredient. The importance of considering different types of signals is illustrated by the variety of presumed signals that were detected for the prototype supplement ingredients. Some of these signals are scientific information, such as animal data. Others are possible associations between an ingredient and serious adverse events in humans. Some signals of possible risk are not pieces of scientific information per se but information that indicates other knowledgeable organizations have expressed concerns about the safety of the ingredient—governments in other countries may control the use of the ingredient or secondary sources may describe potential risks. In summary, a wide variety of signals needs to be considered to some degree to determine which warrant the initiation of a more substantive review (i.e., an integrative evaluation).
|
BOX 11-1
|
Ingredients for the six prototype monographs were selected based on limited knowledge, that is, a signal that a risk might exist. In going through the process, it became apparent that it is important initially to evaluate the signal of concern before investing effort and resources to do an exhaustive integrative evaluation. Thus the initial review step in the process was developed to use the guiding principles and spectrum of concern guidelines in Chapters 4 through 8 to efficiently determine if further investigation is warranted (i.e., to set priorities).
Initial Review
The initial review step in the process describes how a relatively cursory review of information about a particular signal will allow FDA to determine a preliminary level of concern as lower, moderate, or higher. The six ingredients illustrate that considering how the information fits into the spectra of concern described at the end of Chapters 4 through 9 will provide enough context to preliminarily determine how much concern the original signal warrants. The prototype ingredients also illustrate that additional information related to the original signal is also often needed to determine how much concern is warranted. If the signal resulted in a moderate concern level, considering other types of data helped determine whether other signals would also suggest value in conducting an integrative evaluation.
Finally, from a public health as well as a limited resources perspective, it is also appropriate to consider the prevalence of use and use by particularly susceptible populations at this stage in the process. An ingredient such as chaparral would not be as likely to affect as many persons, even if harmful, because of its limited current use. Saw palmetto, on the other hand, is used by a large number of U.S. men, warranting its consideration even if the initial review does not characterize concerns as a higher level.
In summary, no decisions regarding the potential for harm are being made in this step; the evaluation at this point is to decide if sufficient concern has been raised by the signal (and/or consideration of other evidence in the case of an initial moderate concern) to suggest an integrative evaluation is warranted. This step in the process allows FDA to set priorities for determining which dietary supplement ingredients require integrative evaluations and when continued monitoring might be a better use of limited resources.
Role of Monitoring
Among the six prototypes developed for this report, only two remained at a higher level of concern after the initial review (chaparral and melato-
nin).4 Thus for the other four dietary supplement ingredients, a decision to flag the ingredient for continued monitoring might have been made based on the original presumed signal. Monitoring consists of either passively watching for new signals of other concerns about the ingredient or routinely searching the scientific literature for new data to address a specific existing concern. For example, if the concerns about glucosamine and insulin regulation were assigned only a lower or moderate concern level because glucosamine’s bioavailability in animals and humans is unclear in the data, the scientific literature should be regularly searched to determine if new evidence addresses glucosamine bioavailability in animals or humans. Monitoring might also include working with the National Toxicology Program at the National Institute of Environmental Health Sciences or the Office of Dietary Supplements at NIH to initiate research addressing unanswered questions relative to some of the signals detected.
Integrative Evaluation
Broad-Based Versus Focused Approaches to Integrative Evaluation
If the concern level is moderate or higher, a decision may be made to undertake an integrative evaluation. The integrative evaluations developed into the prototype monographs for this report were comprehensive and hence labor-intensive. This approach did accomplish the objective of gaining enough information to improve and refine the scientific principles included in the Framework, but it also clearly demonstrated that a broad-based and comprehensive collection and consideration of all information relevant to the safety of the ingredient can be a resource-intensive undertaking. This is especially true if the ingredient itself or its constituents have been extensively studied, but not in studies particularly designed to address safety (i.e., if studies are designed specifically to evaluate the safety, consideration of these studies may be conclusive and preclude the necessity of collecting related, but less directly relevant, non-safety-focused information).
For many situations, the concern is very specific and can be addressed with an integrative evaluation focused on those concerns (documented in a
focused monograph). When concerns center around one type of adverse effect or potential toxicity, it will often be more efficient to focus data collection and analysis efforts on those particular concerns rather than including in the monograph all data about the ingredient. When the focused monograph is prepared, the focus should be clearly described so that the monograph is not interpreted as a complete summary of all risks associated with the ingredient.
Chaparral and saw palmetto are examples of when it may be more practical to focus the integrative evaluation on key issues of concern. Concerns about the safety of chaparral stemmed from a signal of reported hepatotoxic adverse events in humans. Thus all efforts could have focused on collecting and evaluating human, animal, in vitro, and related substances data that might have shed light on the relationship between chaparral and liver effects. Similarly, efforts for saw palmetto could have been focused on antiandrogenic concerns. To demonstrate the differences between focused and broad-based integrative evaluations, the chaparral and saw palmetto prototype monographs are presented both ways. The broader-based versions are available for review at http://www.iom.edu/fnb, along with the other full prototype monographs; the focused versions are included in Appendixes J (chaparral) and K (saw palmetto).
Data Gathering for the Integrative Evaluation in the Form of a Monograph
The first step in the integrative evaluation is collecting data about the ingredient, usually collated in the form of a monograph without summary and conclusion statements. The most valuable information is studies specifically designed to evaluate toxicity and detect adverse events. Sufficient data of this type rarely exist for dietary supplement ingredients,5 so relevant information found in studies not focused on safety also needs to be collected. For example, clinical studies on glucosamine and osteoarthritis had to be reviewed to determine if any information was presented regarding the development of insulin resistance in individuals taking glucosamine.
Strategies for collecting the needed information on the prototype dietary supplement ingredients were refined through the development of the six prototype evaluations. Specifically, for chaparral and shark cartilage, the first two ingredients to which the original proposed framework was applied, almost all information was collected and included. The second two ingredients, saw palmetto and glucosamine, were prepared by collecting almost all information that the scientists involved thought could provide
|
5 |
Except for some vitamins or minerals; see Chapter 3. |
any information about likely toxicities associated with these ingredients. Finally, melatonin and chromium picolinate were prepared by collecting only information relevant to the major possible toxicities in evidence from the human data and animal and in vitro toxicity studies. All human data were included in their prototype monographs, but only the animal, in vitro, and related substances data that were likely to reveal any targeted toxicities were included in their prototype monographs.
Given that resources are indeed limited, it is more important to efficiently collect data for integrative evaluations than to produce an exhaustive data collection document. The best approach from the experience with the six prototypes seems to be one between the second and third approaches used. That is, human data that are reasonably available should be collected. Data from animal and in vitro studies that are specifically designed to address safety and toxicity should be collected, as should relevant information about related toxic substances. Data relevant to the concerns raised by this information should then be collected. Finally, abstracts or other summaries of in vitro and animal data that are not from toxicity studies, or validated in vitro studies, per se, should be reviewed and used to judge which full reports need to be considered.
Integrating: Considering Consistency and Plausibility and Evaluating Heterogeneous or Seemingly Inconsistent Data
All data relevant to a particular concern often do not agree or point to the same conclusion. In preparing the prototype monographs, it became clear that articulating how to appropriately weave the different types of information together would be helpful. Thus Chapter 10 provides guidance through the difficult and often imprecise process of “weighing the evidence” to reach a scientifically appropriate conclusion about risk. It describes how biological plausibility and consistency are important, as is discounting “negative” data or studies that would not be expected to detect an effect even if it did occur.
Chaparral provides an illustrative example of how concerns raised by knowledge of the substance NDGA found in chaparral and its chemical structure provides biological plausibility to the human adverse events observed and a possible link to the nephrotoxicity observed in animals. Hypothesizing that lipid-soluble NDGA is the problematic substance is also consistent with the fewer number of adverse effects reported with ingestion of chaparral tea (an aqueous extract) as compared with capsules or tablets. As for reproductive effects of chaparral, there is consistency between the in vitro effects of NDGA on prostanoid synthesis and the animal effects observed. The reproductive effects, if attributable to NDGA, are also consistent with any claimed safe historical use of tea since it seems unlikely they
would be detected with historical use. Similarly, carcinogenic effects are not inconsistent with the historical use information since they would not be expected to be detected if they did occur.
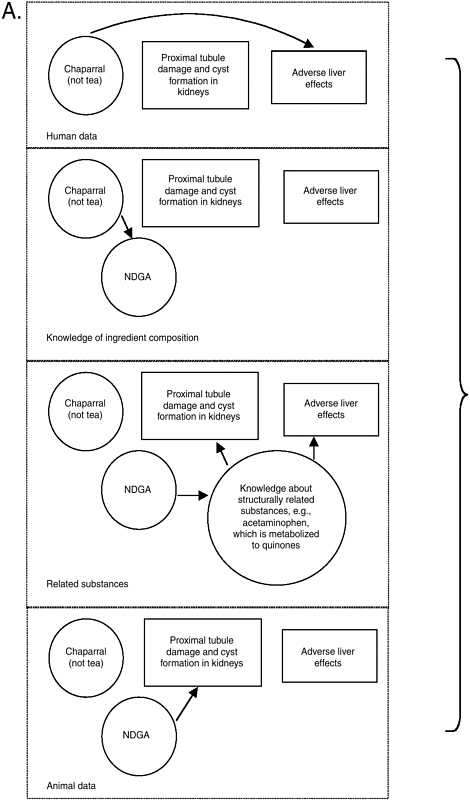
The prototype integrative evaluation process showed that with so many different types of information to process and consider, it is useful to note what is certain and focus on uncertainties. As explained in Chapter 10, causal model diagrams may be useful to visually illustrate what is known and where data gaps exist. In doing so, they can help focus thinking and information searching on remaining questions (as made more obvious by missing or weak linkage arrows). A causal model on the relationship between chaparral and liver effects is presented in Figure 11-1 as an example of the diagram’s value. A solid arched arrow illustrates a relationship between chaparral ingestion and adverse liver effects in humans, independent of knowledge about hazards that may be associated with NDGA. Another pathway between chaparral and liver effects is based on the relationship between chaparral and NDGA and knowledge of how chemical structures like NDGA can be metabolized into quinones that subsequently result in adverse liver effects. A related substance with this effect is acetaminophen.
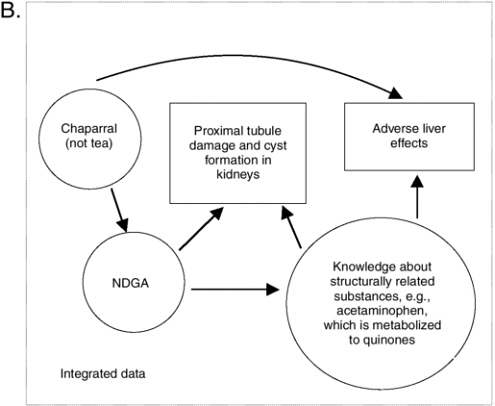
As described in Appendix J, the relationship of chaparral tea to adverse effects is less clear (hence there would be no solid line path between the tea and the adverse livers effect box). It is apparent from Figure 11-1 that if NDGA was found to be a component in chaparral tea, greater concern would be appropriate. Finally, this diagram illustrates how there may be some consistency between the proximal tubule damage observed in rodents following NDGA ingestion and the metabolism of NDGA to an orthoquinone derivative. In summary, it is the pattern of consistency and biological plausibility, illustrated by more than one path from chaparral to adverse liver effects, that raises even more concern about the safety of chaparral, especially when not prepared as a tea and when ingested by subpopulations particularly vulnerable because they have pre-existing liver conditions.
Use of External Advisory Committee
In the prototype development process, external experts were used to simulate the prescribed process of bringing in expertise particular to the ingredient’s safety concerns. Consultation with experts on the specific dietary supplement ingredient, the adverse effects of concern, the physiological system of concern, and the categories of data in need of review were all important to the development of relevant conclusions and recommendations, especially when data were sparse, inconsistent, or difficult to interpret. For example, it was helpful to have physicians and scientists very familiar with insulin and glucose regulation to consider glucosamine, experts in melatonin biology and endocrinology for melatonin consideration, experts familiar with metal toxicology and carcinogenicity to help with chromium picolinate, and experts in plant chemistry as chaparral and NDGA were considered. The Framework process outlined in Chapter 11 suggests that FDA may also want to involve external experts when data are not clear-cut, when expertise in particular aspects of human physiology is necessary and when the data to be considered require additional expertise to interpret.
Notably, in the process used to test and improve the Framework, working groups were organized to guide data collection into monographs, but it is not envisioned that FDA will use such working groups to prepare monographs. Instead, FDA will probably choose to collate data into a monograph form in-house or by using outside contractors. Where FDA may choose to involve external experts, if internal expertise needs to be supplemented is in conducting the integrative evaluation (i.e., the actual analysis of how to interpret the data and develop a conclusion).
Review and Updating of Monographs
Once a monograph has been developed, it can serve as a tool for effective monitoring of safety issues in that the monograph can be updated with new information as it becomes available. Thus developing monographs is consistent with developing a proactive, ongoing evaluation system for evaluating risk.
REFERENCES
FDA (Food and Drug Administration). 1992. Chaparral Warning. P92-38. December 10. Press Release.
Foster S, Tyler VE. 1999. Tyler’s Honest Herbal. A Sensible Guide to the Use of Herbs and Related Remedies. 4th ed. New York: Haworth Herbal Press.
McGuffin M, Hobbs C, Upton R, Goldberg A. 1997. American Herbal Product Association’s Botanical Safety Handbook . Boca Raton, FL: CRC Press.
Medical Economics Co. 2001. PDR for Nutritional Supplements. Montvale, NJ: Thomson Medical Economics Company.
NMCD (Natural Medicines Comprehensive Database). 2002. Natural Medicines Comprehensive Database. Online. Available at http://www.naturaldatabase.com/. Accessed January 18, 2002.