Executive Summary
Consumer interest in health and self-care has expanded the market for a wide range of products, including dietary supplements. Total sales of dietary supplements have grown to over $18 billion per year. As with conventional foods, when used as recommended, many dietary supplements are probably safe. However, increased use of supplements and the broad spectrum of products that qualify as dietary supplements as defined by the Dietary Supplement Health and Education Act of 1994 (DSHEA) make the determination of risk to the health of the consumer, a sizeable task. In addition, the limitations imposed by DSHEA—that the Food and Drug Administration (FDA) determine what is unsafe without requiring that specific information on safety be presented by manufacturers prior to marketing or that manufacturers submit to the FDA any reports they have received on serious adverse events associated with dietary supplement use—serve to make the safety regulation of dietary supplements a sizeable challenge.
THE COMMITTEE’S TASK
FDA must approach evaluating the safety of dietary supplement ingredients in a manner that is cost effective and science based within this regulatory environment. In order to assist in developing such an approach, FDA turned to the Institute of Medicine and the National Research Council of the National Academies to provide a framework for evaluating the safety of dietary supplement ingredients. FDA requested that a committee of experts (1) develop a proposed framework for categorizing and prioritizing
dietary supplement ingredients sold in the United States based on safety issues, (2) describe a process for developing a system of scientific reviews, (3) utilize the proposed framework to develop at least six scientific reviews or monographs as prototypes, and (4) revise the framework based on comments received.
The final Framework described in this report is the result of the committee’s deliberations over the last 30 months and comments received on the proposed framework issued in July 2002. This Framework includes guidance on considering the various categories of data, taking into consideration methods other expert bodies have used to categorize and review supplement safety issues.
REGULATORY BACKGROUND
Current regulatory approaches to the safety evaluation of dietary supplements in the United States are a product of several key pieces of legislation that span the twentieth century, culminating in the passage of DSHEA in 1994. Since the passage of the 1938 Federal Food, Drug, and Cosmetic Act (FDCA), FDA has wrestled with the most appropriate approach to regulating dietary supplements and several attempts have been met with resistance by industry as well as by segments of the public.
In 1958, the Food Additives Amendment to the FDCA defined food additives and provided that they must undergo a premarket approval process unless they were considered to be generally recognized as safe (GRAS) (Table ES-1). FDA subsequently attempted to regulate the botanical industry by alleging that individual botanical products were unapproved food additives; this approach was subsequently struck down by the courts, recognizing that the applicability of the provisions of the FDCA to products containing a vitamin, mineral, or botanical ingredient (whether it was considered a drug or a food, for example) depended on the product’s intended use, as determined usually by the labeling and advertising claims for the product.
Congress acted further to delineate FDA’s authority by passing DSHEA in 1994. DSHEA established the first comprehensive definition of dietary supplements as legally equivalent to foods (Box ES-1). Most importantly, DSHEA established a regulatory framework for dietary supplements that defined FDA’s authority over these products. FDA bears the burden of proof in determining that a dietary supplement ingredient presents a “significant or unreasonable risk of illness or injury” (see Box ES-2) rather than being authorized by statute to require the manufacturer to provide data supporting its safety, as is authorized for substances added to foods1 or for drugs.
For new dietary ingredients (those not marketed in the United States prior to passage of DSHEA in 1994), manufacturers or distributors must notify FDA at least 75 days before introducing a dietary supplement ingredient and must provide FDA with the information that is the basis upon which the manufacturer2 has concluded that dietary supplement or ingredient will reasonably be expected to be safe.
THE SAFETY FRAMEWORK FOR DIETARY SUPPLEMENTS
The definition developed for a “framework” was based on review of other existing frameworks. The Framework consists of two components: (1) a process for prioritizing, evaluating, and describing available information to establish risk of harm, and (2) a set of science-based principles that serve as guidelines for evaluating risk to human health.
For the Framework to be useful, FDA must have adequate resources for implementation. To be credible, it must be scientifically based and include guidelines for obtaining and integrating the totality of the information from many areas of science. Adequate staff with appropriate expertise must be available within FDA to administer the process and evaluate the information.
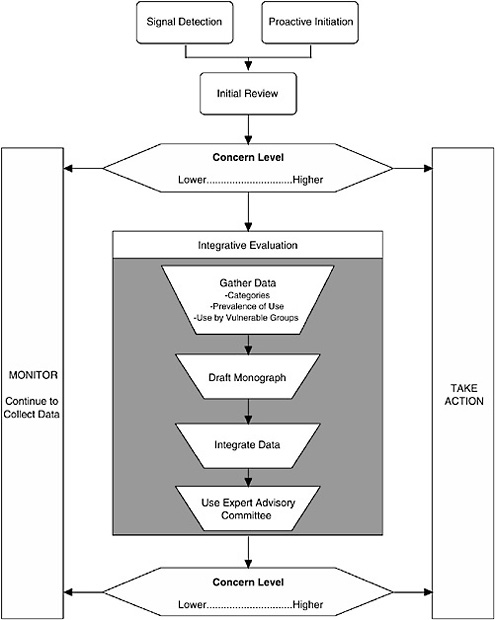
The Framework described here (see Figure ES-1) characterizes the nature of the scientific evidence that FDA is likely to encounter and describes a process for organizing this evidence to assess where a dietary supplement ingredient3 lies on a spectrum of concern.4 As the level of concern increases, so does the potential for a “significant or unreasonable risk,” the standard warranting regulation under the FDCA, as amended by DSHEA.
I. The Process
Three major components comprise the process:
-
Signal detection
-
Initial review of available information
-
Integrative evaluation
|
2 |
The term manufacturer is used for simplicity, but the statutes related to dietary supplements refer to both manufacturers and distributors, which may or may not be the same for a given dietary supplement ingredient or product. |
|
3 |
In order to be consistent with the FDA’s regulatory role, the definition of “dietary supplements” used is that of DSHEA (Box ES-1). |
|
4 |
The use of the term “concern” denotes a need for further investigation and inquiry by FDA based on a relative level of interest arising from initial information. |
TABLE ES-1 Current Status of Foods, Food Additives, Drugs, and Dietary Supplements under the Food and Drug Administration (FDA) Regulations
|
|
Dietary Supplements |
||
|
Status |
Containing Ingredients in Use prior to DSHEAa |
Containing “New” Ingredients Introduced after DSHEA (10/15/94) |
Conventional Foodsb |
|
Premarket approval required |
No |
No; FDA notification 75 days prior to sale required; FDA has 3 options: (1) respond with objection, (2) respond with no objection, (3) not respond |
Noe |
|
Postmarket reporting or surveillance by industry required |
No |
No |
No |
|
Burden of proof of safety |
FDA must demonstrate significant or unreasonable risk of harm to remove product from market |
FDA must demonstrate significant or unreasonable risk of harm to prevent product from being marketed |
FDA must demonstrate that food is injurious to health to remove product from market |
|
a DSHEA = Dietary Supplement and Health Education Act of 1994. b Here “conventional foods” refers to whole agricultural commodities. c This description applies to “new” drugs. Many over-the-counter drugs are regulated under FDA’s Over-the-Counter Drug Review procedures, which do not provide for postmarketing surveillance. d GRAS = generally recognized as safe (as defined by the 1958 Food Additives Amendment to Food, Drug, and Cosmetic Act). |
|||
|
|
Substances Added to Foods |
||
|
New Drugsc |
GRASd Pre-1958 |
GRAS Notice (previously, “Affirmation” Petition) |
Food Additive Petition |
|
Yes |
No |
No; manufacturer voluntarily may notify FDA of basis of selfdeclaration as GRASf; FDA will respond with letter of objection or no objection within 90 days |
Yes; with FDA approval becomes an approved food additive |
|
Yes |
No |
No |
Rarely |
|
Manufacturer provides risk/ benefit analysis acceptable to FDA |
FDA conducts risk assessment to determine if GRAS recognition should be withdrawn |
Manufacturer must demonstrate reasonable certainty of no harm for intended use through scientific procedure or history of use |
Manufacturer must present adequate risk assessment to demonstrate reasonable certainty of no harm for intended use |
|
e In 2001 FDA proposed in the Federal Register (66:4706) a rule requiring marketers of food developed through biotechnology to notify the agency at least 120 days before commercial distribution and to provide information to demonstrate that the product is as safe as its conventional counterpart. f While the final regulations for the notification procedure are not yet published, the interim policy outlined by FDA in the proposed regulations invites interested persons who determine that a substance is GRAS to notify FDA of such GRAS determinations as described in the proposed regulation 21 C.F.R. § 170.36 (b) and (c). |
|||
|
BOX ES-1 The term dietary supplement:
Dietary supplements are further defined as products that are labeled as dietary supplements and are not represented for use as a conventional food or as a sole item of a meal or the diet. Supplements can be marketed for ingestion in a variety of dosage forms including capsule, powder, softgel, gelcap, tablet, liquid, or, indeed, any other form so long as they are not represented as conventional foods or as sole items of a meal or of the diet (FDCA, as amended, § 402). |
Signal Detection
Given the large number of dietary supplement ingredients and that dietary supplements are assumed to be safe in general, it is unlikely that FDA will have the resources or need to evaluate each ingredient uniformly. Thus it is assumed that some “signal” will indicate that an ingredient’s safety may need to be reviewed. When a signal is detected and the credibility of the signal and its relationship to a serious adverse effect in humans is evaluated, it is up to FDA to decide to take the next step.
Given the significant number of dietary supplement ingredients, FDA’s attention should focus on signals that indicate that a serious5 health problem may result due to ingestion of a dietary supplement ingredient.
|
BOX ES-2 Section 4. Safety of Dietary Supplements and Burden of Proof on FDA. DSHEA amends § 402 (21 U.S.C. 342) by adding the following: (f) (1) If it is a dietary supplement or contains a dietary ingredient that—
In any proceeding under this paragraph, the United States shall bear the burden of proof on each element to show that a dietary supplement is adulterated. The court shall decide any issue under this paragraph on a de novo basis. (2) Before the Secretary may report to a United States attorney a violation of the paragraph (1)(A) for a civil proceeding, the person against whom such proceeding would be initiated shall be given appropriate notice and the opportunity to present views, orally and in writing, at least 10 days before such notice, with regard to such proceeding. SOURCE: FDCA, P.L. 75-717 § 402, as amended 21 U.S.C. § 342(f) (2001). |
In contrast to reacting based on detecting a signal, FDA may decide to proactively initiate a review of a dietary supplement ingredient due to high prevalence of use in the general population, high level of use by a particularly vulnerable population, or other factors.
One of the requirements of the study was to develop a framework that would include criteria for how the review of safety of dietary supplements
and ingredients should be prioritized. However, given the wide variety of dietary supplement ingredients available, the multiple forms of an ingredient for sale (e.g., pills, concentrates, extracts), the voluntary and thus varying nature of the data available on an ingredient, and the wide variety of adverse effects that are possible for dietary supplements and the dependence of such effects on exposure levels, a simple scheme for priority setting is not feasible nor scientifically defensible.
Initial Review of Available Information
The second component of the Framework is to conduct an initial review of available information. First, the nature of the information generating the signal is examined to determine the appropriate level of concern regarding a risk to human health. This component is not envisioned as a detailed analysis of data, but rather as an assessment of the concern level warranted by the nature of the evidence (e.g., quality of the report, applicability to humans, route of exposure) and whether the information raises questions that require further examination.
Second, some effort may be made to gather easily available data to place the detected signal in context; such additional information may come from many sources, including other categories of data. Thus this initial review of the signal information need not be limited to reviewing only the information associated with the signal. If reviewing the signal results in a moderate level of concern, data from other categories should be considered as well.
Since it is assumed by DSHEA that dietary supplements are safe, there should be relatively few dietary supplement ingredients that will be categorized as of higher concern after the initial review and thus warrant further examination. This allows FDA to focus its efforts on the few dietary supplement ingredients that are strong candidates for regulation.
Integrative Evaluation
The third step of the Framework is conducting an integrative evaluation for those dietary supplement ingredients that are deemed to warrant further investigation based on the preliminary data reviewed in the second step. There are four aspects to the Integrative Evaluation component (see Figure ES-1): in-depth literature searching and reviewing, drafting of a safety monograph based on this information, integrating the available data into an analysis to complete the monograph, and possibly referring the draft monograph and accompanying information to an expert advisory committee for additional input prior to FDA determining whether to take regulatory action.
Focused Versus Broad-Based Evaluation. An integrative evaluation may be reactive to the signal and focused in nature in that it is being conducted to examine a specific moderate or high-level concern about an ingredient, or it may be more proactive and broad-based in that it looks for any risk associated with use of the dietary supplement ingredient. For example, a proactive integrative evaluation might be initiated simply because a large percentage of the population is using the ingredient, rather than as a reaction to a particular safety concern.
Drafting a Safety Monograph. In most cases, the integrative evaluation will be documented in a monograph that summarizes the categories of data available and their use in drawing conclusions about the potential risk associated with use of the ingredient; it should include the conclusions of the expert committee and/or FDA. The science-based guiding principles described in the following section of this summary, and explained in detail in Chapters 4 through 10, should be used to reach a decision regarding whether there is an unreasonable risk of illness or injury.
Integrating the Data to Determine Risk. When evidence on a dietary supplement ingredient presents a moderate or higher level of concern relative to this risk, biological plausibility and consistency should be evaluated, especially when independently convincing data are not available. Such an analysis can be represented by creating a causal model diagram—a tool to visualize how the different types of available data link together to establish risk (described in Chapter 10).
The principles described for considering the various categories of data (Chapters 4 through 8), as well as the principles describing how to integrate among and within categories of data (Chapter 10), are applied in the integrative evaluation.
It is expected that FDA may want further input from an advisory committee on many of the dietary supplement ingredients undergoing an integrative evaluation because only ingredients with significant potential for concern are likely to reach this stage.
Decision to Take Action. The results of the integrative evaluation should play a pivotal role in establishing that a supplement ingredient is unsafe. If an advisory committee is used, its findings and rationale should be posted with the monograph on FDA’s website. One of the important components of DSHEA was that the public should be educated about dietary supplements. FDA thus has a responsibility to educate consumers about the safety of supplement ingredients, and the public availability of the completed monographs can be an important aspect of the educational process.
Decision to Continue to Monitor. When review of information, either at the initial review step or as a result of an integrative evaluation, indicates a lower level of concern, FDA should continue to monitor information it receives relative to the dietary supplement ingredient. Monitoring consists of either passively watching for new signals of other concerns about the ingredient, as well as maintaining search strategies to routinely search the scientific literature for new data to address specific existing concerns or to identify new concerns. FDA relies on the industry to perform this function in the case of drugs as part of the required postmarketing surveillance; since there is no required postmarketing surveillance for dietary ingredients, ongoing assessment of relevant literature is thus FDA’s responsibility.
II. Applying Science-Based Principles to Establish Risk
Given the variety of types of information that are likely to be available, the Framework classifies scientific information into four broad categories for use in determining the potential for serious harm for a specific dietary supplement ingredient (see Box ES-3):
-
human data,
-
animal studies,
-
information on related substances, and
-
in vitro experiments.
Individual chapters describe the types of information that may be available in each of these data categories and considerations for using the different categories of data in evaluating the potential of a dietary supplement ingredient to cause harm (Chapters 4 through 7). Also described are how to consider the potential for dietary supplement interactions with drugs and other xenobiotics6 (Chapter 8), important considerations that should be factored into evaluations when vulnerable populations consume dietary supplements (Chapter 9), and considerations for integrating the available data from various sources to weave together the information to determine an overall level of concern (Chapter 10) using a causal model diagram.
Spectra of Concern
The Framework also includes a qualitative method to evaluate the nature of the evidence for a specific piece of information within a particular
|
BOX ES-3
|
data category (i.e., human, animal, in vitro, or information about related substances). Distinguishing characteristics determine where a piece of information falls on the continuum or spectrum of lower level to higher level of concern. This is summarized in diagrams (see the figures in Chapters 4 through 8) referred to as spectra of concern.
Evidence that results in a higher level of concern indicates a more immediate priority for investigating further to determine if an unreasonable risk to public health exists. In contrast, a single piece of information resulting in a lower level of concern may suggest continued routine monitoring for new evidence is warranted—monitoring for new evidence that might elevate the level of concern and thus its priority for increased scrutiny.
It is important to recognize that for most dietary supplement ingredients it will be difficult, if not impossible, to find optimal information from all data categories.
General Principles and Concepts When Considering Data
Concentration of Substances at the Sites of Action. A critical factor in determining toxicity of an ingredient is not necessarily the ingested amount, but the concentration of a dietary supplement’s active constituents at its sites of action.
Absence of Evidence. Absence of evidence of risk does not indicate that there is no risk. Even if a study showing lack of adverse effects is reported, if the study is not adequately designed to identify risk (e.g., not sufficiently powered, incompletely reported, does not include positive controls, or otherwise has inadequate mechanisms for detecting adverse events), it is not scientifically valid to use such information to mitigate suggested risk from other sources.
Consistency and Biological Plausibility. Data will frequently need to be collated within the same category or across several categories to determine the appropriate overall level of concern. In integrating observations across categories of data, consistency and evidence of biological plausibility should raise the level of concern. This weaving together of available information can be facilitated, and conceptually illustrated, by the use of causal evidence models.
APPLICATION OF THE FRAMEWORK
In order to evaluate the initial framework proposed, prototype monographs were developed for a variety of dietary supplement ingredients.
Significant changes made to the initial framework resulted from this opportunity to test it, as well as from comments received after its initial release for comment (see Appendix B). Summaries of the six prototype7 monographs are included in Appendixes D through I. The full prototype monographs are available for viewing at www.iom.edu/fnb. Appendixes J and K contain examples of two focused prototype monographs to show how the FDA could focus on determining a level of concern related to one specific adverse effect.
FACTORS INFLUENCING USE OF THE FRAMEWORK
By definition, this Framework cannot be used to consider the possible benefits of consuming dietary supplements. The Framework also focuses on ingredients rather than products available in the marketplace. Another limitation is that, as with any evaluation of dietary supplement ingredients under the current regulatory scheme, the determination of what is unsafe depends on publicly available data or data made available voluntarily by industry.
FINDINGS
Ability to Determine Unreasonable Risk
Because of the limited and variable amount and types of data available, definitive statements judging safety may be difficult to completely substantiate scientifically. However, the principles used by the scientific community to determine the risk associated with the consumption or use of various substances, some of which are medical products, should also apply to dietary supplement ingredients, bearing in mind that dietary supplements, by virtue of DSHEA, have been assumed to be safe, but have not been required to be proven safe. Thus, the appropriate scientific standard to be used to overturn this basic assumption of safety is to demonstrate significant or unreasonable risk, not prove that an ingredient is unsafe.
|
7 |
The monographs were developed as a test of the processes and framework and are thus considered prototypes because it was not possible to duplicate the access and information available to FDA within the committee process, and because of time constraints (discussed in Chapter 11). The monographs should not be considered as representing authoritative findings related to these six dietary supplement ingredients. |
What Constitutes a Scientific Assessment of Unreasonable Risk?
Approaches taken by diverse organizations and governmental bodies, both within and outside the United States, which evaluate the safety and, at times, efficacy of dietary supplement ingredients vary in their relevance to the protection of the American public from risks associated with consumption of dietary supplement ingredients.
A number of these resources were reviewed to identify criteria for evaluating the relevance of other approaches. The purpose of such efforts varies substantially from organization to organization, focusing on quality, efficacy, safety, or a combination of these. Criteria outlined in Chapter 2 include importance of reliance on scientific data, consideration of all categories of such data (including animal data, in vitro data, data about the safety of related substances, and data on human use), use of appropriate expertise, and objectivity. Often the approaches were not sufficiently detailed or transparent to give a complete picture of the data considered, how sparse data were weighed and considered, the rationale behind the conclusions, or other questions regarding safety.
RECOMMENDATIONS
The following recommendations, while not part of the Framework itself, are designed to enhance the utility of the Framework and enhance the ability of FDA to protect consumers from unreasonable risk of illness or injury resulting from use of dietary supplements.
-
A prospective, systematic monitoring and tracking mechanism for dietary supplement ingredients should be maintained and refined.
A prospective, systematic method for recording and monitoring the history of safety issues of specific dietary supplements is necessary to implement the Framework so that FDA can evaluate the safety of dietary supplement ingredients. During the period of this study, FDA developed a new method of monitoring and tracking dietary supplement adverse event reports. However, a prospective system is required that enables tracking of information leading to all levels of concern.
The system should be open, transparent, and useful for establishing varying levels of concern related to dietary supplements as outlined in the Framework. Resources to support these activities should be provided to FDA.
-
Adequate resources to protect the consumer under DSHEA must be provided.
While the committee did not conduct an analysis of the cost of implementing this Framework, implementation of any framework for com-
prehensive safety evaluation will generate an additional workload for the responsible staff at FDA. For the Framework to be effective, adequate resources must be available to FDA to collect and analyze available information.
-
Adverse Event Reporting:
-
DSHEA should be amended to require that a manufacturer or distributor report to the FDA, in a timely manner, any serious adverse event associated with use of its marketed product of which the manufacturer or distributor is aware.
-
FDA should continue to work with the Poison Control Centers as a source of adverse event reports, and sufficient resources to support this activity should be provided.
-
FDA should increase efforts to inform health care professionals and consumers that they should use the MedWatch adverse event reporting program to report adverse events associated with the use of dietary supplement ingredients.
-
FDA MedWatch toll-free telephone number should be provided on product labels to facilitate reporting of adverse events.
-
Reports of adverse events are an important source of information by which FDA becomes aware of potential risks to public health from exposure to dietary supplement ingredients. It has been estimated that FDA receives reports of less than 1 percent of all adverse events associated with dietary supplements. While spontaneous adverse event reports have recognized limitations, they have considerable strength as potential warning signals of problems requiring attention, making monitoring by FDA crucial.
-
To initiate the 75-day premarketing review period, both the distributor and manufacturer should be required to provide FDA with all available data, both favorable and unfavorable, regarding the safety of the product.
-
When the formulation or processing of a dietary supplement ingredient is changed, it should be considered a new dietary ingredient and subject to regulatory oversight as such.
Many dietary supplement ingredients on the market today have new formulations and are produced through very different processes than related dietary supplement ingredients in traditional usage, or even other dietary supplement ingredients bearing the same name. This may result in markedly different bioactive substances of potential harm and very different kinetics (e.g., absorption, distribution in the body, metabolism, and excretion).
-
The FDA initiative to establish current Good Manufacturing Practices for dietary supplement ingredients is supported and additional efforts to develop standards for content uniformity should be undertaken. Sufficient resources to support these efforts should be provided by Congress.
While the focus of this report is on developing a framework and not on safety issues related to good manufacturing practices, these are inseparable because variability in content hampers the evaluation of safety.
-
Adoption of the labeling changes recommended in the report Inspector General Report: Dietary Supplement Labels: Key Elements is urged.
Required labeling information that would be of use to the consumer in making informed decisions about safety is limited. Current regulations related to source of a product only require the name and place of business of the manufacturer, packer, or distributor to be on the label. There are usually few manufacturers of a product, but many distributors or packers. Thus both sources need to be on the label.
-
Additional Research on the Potential to Cause Harm:
-
The continued development of effective working relationships and partnerships between FDA and the National Institutes of Health is encouraged.
-
FDA should ensure that its own National Center for Toxicological Research and the overall Department of Health and Human Services National Toxicology Program are optimally utilized when research is needed to further evaluate concerns.
-
All federally supported research on dietary supplements conducted to assess efficacy should be required to include the collection and reporting of all data related to safety of the ingredient under study.
-
There is no legal or regulatory requirement that dietary supplement ingredient manufacturers conduct toxicology or safety pharmacology studies on their products or ingredients. Thus experiments and studies to address safety issues will, in most cases, be initiated by FDA or other federal agencies.
BARRIERS TO EVALUATING THE SAFETY OF DIETARY SUPPLEMENTS
Through the process of developing the Framework to evaluate the safety of dietary supplement ingredients, a number of legal and regulatory barriers were identified that hamper FDA’s ability to protect the public
health. New drugs are subject to premarket approval, yet DSHEA excludes all dietary supplements from this requirement despite the fact that they may possess biological activities similar to those found in medications, and survey data demonstrate that dietary supplements are used by consumers for medicinal purposes. Further, under the provisions of DSHEA, FDA has no authority to require the collection or reporting of specific safety data from dietary supplement manufacturers or distributors after their products are made available for sale to the public.
It is very challenging to carry out the mandate of DSHEA given the limitations it imposes on the quantity and quality of the currently available scientific data related to the safety of dietary supplement ingredients. One of the key premises of DSHEA is that history of use is evidence of safety when applied to dietary supplements; as indicated in Chapters 4 and 6, there are significant scientific problems with this assumption.
In line with these findings, members of the scientific and medical community have strongly advised that the regulatory mechanisms for monitoring the safety of dietary supplements, as currently defined by DSHEA, be revised. The constraints imposed on FDA with regard to ensuring the absence of unreasonable risk associated with the use of dietary supplements make it difficult for the health of the American public to be adequately protected.