Appendix J
Prototype Focused Monograph: Review of Liver-Related Risks for Chaparral1
I. DESCRIPTION OF THE INGREDIENT
A. Chaparral as a Dietary Supplement Ingredient
Chaparral is one name for an herbaceous woody shrub that grows in the southwestern region of the United States and the northern region of Mexico. It is also called creosote bush or greasewood. The common Spanish names are hediondilla, which means “little smelly one,” because the bush has a strong odor similar to the smell of creosote (a distillate of coal/ wood tar used as a wood preservative), and gobernadora, which means “governess,” because the bushes can dominate an area by creating an ad-
verse environment for the growth of other plants, resulting in a monoculture in some areas (Schultz and Floyd, 1999). One remarkable feature of the chaparral bush is the complex resinous coating on the leaves that serves as a chemical defense against grazing by herbivores and against attack by insects. The chemicals in the resin find their way into the desert soil surrounding the chaparral plant and discourage growth by other plant species, thus effectively reducing competition for water and nutrients (Mabry et al., 1977).
Chaparral is formally known as Larrea tridentata (Sessé and Moc. ex DC.) Coville (synonymous with Larrea mexicana Moric.) of Zygophyllaceae (McGuffin et al., 1997). Historically, the dry leaves, green stems, and fine twig tips of chaparral were used for various ailments. Since about 1969, these same plant components have been used as dietary supplements. Various forms have been available: dried plant material for making teas (water extracts), aqueous-alcoholic extracts or tinctures, and tablets or capsules containing ground, dried plant material. During the past 10 years, chaparral products have not been as readily available as in the past; however, each of these forms is currently available in the U.S. marketplace in varying degrees.
B. Individual Components
Table A contains a list of the components of primary interest in chaparral (i.e., present in leaves, stems, and twigs). Some components of chaparral are common in other plants and are widespread in the human diet. The major components of the resinous coating of chaparral are lignans (Mabry et al. 1977; Sakakibara et al. 1976) which can comprise up to 80 percent of some extracts of chaparral, such as methanol extracts of green leaves or green stems (Hyder, 2001). Lignans are low-molecular-weight plant products made up of phenylpropanoid dimers or trimers. Mature chaparral leaves contain lower amounts of lignans than new leaves (Gisvold and Thaker, 1974). The major lignan in chaparral is nordihydroguaiaretic acid (NDGA) (Downum et al., 1988), which is a derivative of guaiaretic acid and is a catechol having two hydroxyl groups on each of the two phenol rings. NDGA comprises approximately 10 percent of the dry leaf weight, but may be as much as 15 percent in some instances (Obermeyer et al., 1995). NDGA comprises approximately 50 percent of the phenolic resin extracted from the external surface of the leaves (Botkin and Duisberg, 1949; Mabry et al., 1977; Sakakibara et al., 1976). Chaparral also contains guaiaretic acid and other substituted guaiaretic acid derivatives (Table A). Other lignans in chaparral are classified as furanoid lignans and 1 aryl tetralin lignans. The latter are structurally related to podophyllotoxins.
Chaparral contains flavonoids as non-water-soluble aglycones, as wa-
ter-soluble glycosides, and as sulfated flavonoids (Mabry et al., 1977). Chaparral also contains triterpenes, including sapogenins (Mabry et al., 1977). The aglycone forms of the flavonoids and triterpenes are listed in Table A. Chaparral contains volatile oils, wax esters, sterols, and other hydrocarbons (Mabry et al., 1977; Waller and Gisvold, 1945).
Although a number of the known components of chaparral exhibit cytotoxic activity under various conditions, these effects are judged to be weak and require high concentrations of the substance, and thus would extrapolate to the ingestion of large amounts of chaparral in order to exhibit potential toxic activity in humans. Additionally, many of these components are present in the diet from other sources.
C. Description of Dietary Supplement Preparations and Amounts Ingested in Ordinary Use
Chaparral is sold in several forms, one of which is the dried, broken leaves, green stems, and fine twig tips that can be brewed as a tea (i.e., an aqueous extract). An example of the modern preparation of chaparral tea would be to steep 7 to 8 g of crumbled dried leaves, stems, and twigs in one quart of hot water. In ordinary use as a water extract, chaparral might be consumed in the amount of 1 to 3 cups of chaparral tea per day for a period of 2 to 3 weeks (Micromedex, 2002).
Another form of chaparral is a tincture or aqueous alcohol extract. The ordinary use of such an extract might be 20 to 30 drops per day for a period of 2 to 3 weeks (Micromedex, 2002).
Chaparral is also available as a dried leaf powder (frequently sold in capsule or tablet form). Typical suggested uses of such capsules or tablets would be one to two 500-mg capsules or tablets per day for 2 to 3 weeks.
Chaparral is also available as a component of various botanical mixtures sold as tinctures and as loose leaves, stems, and twigs for teas. Chaparral dried leaf capsules are also available in combination with silymarin (a flavanolignan complex from milk thistle), vitamin C, or other antioxidants.
II. INFORMATION RELEVANT TO LIVER CONCERNS
A. Human Use Information and Safety Data
1. Historical use
Chaparral has been used for many centuries for a variety of medicinal purposes (Heron and Yarnell, 2001). Native populations in the southwestern United States have used chaparral tea for decades without published evidence of toxicity. Most processing of chaparral used in American Indian
cultures involved aqueous extracts, such as hot water teas (Heron and Yarnell, 2001). A tea has very little NDGA (a constituent of concern, see below) compared with an alcoholic extract or the powdered dry leaf because NDGA is poorly soluble in water (Obermeyer et al., 1995). Pima Indians used the tea orally as a diuretic, emetic, or expectorant, and topically as an antiseptic or poultice (Mabry et al., 1977). In many American Indian cultures, chaparral tea has been used to mitigate colds, bronchitis, and other breathing problems; for menstrual cramps; and for numerous intestinal problems. It has also been applied topically for painful joints, skin infections, snakebites, burns, and allergies (Mabry et al., 1977; Moerman, 1998). The leaves have been used both as a decoction in a bath or as an external poultice for rheumatism and arthritis, as well as for scratches, wounds, and bruises (Moerman, 1998). There are a few reports of the use of chaparral extracts by southwest native healers in the management of type 2 diabetes (Gowri et al., 2000). In the medical literature there is a paucity of reports involving the ingestion of chaparral capsules or tablets, except for those resulting in adverse effects (described below).
2. Adverse effects
The clinical data suggest a pattern of hepatotoxicity. This pattern is discussed in more detail below. One difficulty in evaluating the clinical data on chaparral is that in most of the cases, the chaparral preparation ingested was not described in any detail. Additionally, the product purity and quality were not reported.
Clinical trial data: Table B provides a summary of a small clinical trial that was conducted among 59 terminal cancer patients to examine the effect of NDGA and chaparral tea on tumor growth. Thirty-six patients consumed chaparral tea (16–24 oz/d) while 23 patients consumed NDGA (250–3,000 mg/day). Selected blood tests and urinalysis were repeated at 2 to 4 week intervals. An analysis of the 45 patients who were treated for at least 4 weeks suggested that there were no hematological or chemical abnormalities that could be attributed to the treatment. Patients reported minor adverse effects as described in Table B. Of the 59 treated patients, no pattern of hepatotoxicity was reported following consumption of either chaparral tea or NDGA by the terminally ill cancer patients. The reasons why 14 subjects dropped out were not reported.
Clinical case reports: Table C-1 summarizes clinical case reports of patients who took chaparral without the added complication of additional ingredients. Careful inspection of Table C-1 reveals 9 cases of well-diag-
nosed hepatotoxicity (Cases #1–9) and 6 cases of suspected or probable hepatotoxicity (Cases #10–15). The cases are arranged in order of apparent severity with the most severe case, which required a liver transplant, presented as Case #1. Many of these patients ingested chaparral in capsule or tablet form (Cases #1–3, 5–11). Most of the chaparral products were unidentified as to whether they contained dried plant material or extracts. The listed amount of chaparral ingested ranged from 0.3 to 6 g/day; however, this information was not included in all case reports. The duration of chaparral use (which is not indicated in 2 of the cases) ranged from 20 days to “many years.” It is notable that Case #15 was the only patient known to use chaparral tea: 4 bags daily for 1.5 years. The product used by this patient was examined using microscopic and chromatographic analysis and was correctly identified as Larrea tridentata with no evidence of biochemical or biological contamination (Sheikh et al., 1997). The severity of the liver damage in these case reports does not seem to correlate directly with either the amount of chaparral consumed or the duration of use. There are five cases with documented recovery from liver damage after cessation of chaparral use (Cases #2, 5, 8, 9, 10). There is one case (#8) documenting a return of jaundice following resumption of chaparral ingestion.
Table C-2 summarizes the clinical case reports of patients who took chaparral in combination with other supplements or ingredients, primarily other botanicals. The six cases of hepatotoxicity found in Table C-2 are difficult to evaluate because of the confounding factor of possible adverse effects due to these other substances. These cases include well-documented hepatotoxicity (Cases #17–20) but the cause of the liver damage is difficult to interpret. Two cases returned to normal after cessation of chaparral (Cases #18, 22). There are also reports (Cases #25–29 and Series A) of subjects taking an aqueous alcoholic extract (90 percent ethanol) as 8 to 10 percent of a formula with other herbs, ingesting a total of 30 to 240 mL over a period of 40 days to 5 months, with no indication of liver damage according to liver function tests.
Adverse event reports to Special Nutrition/Adverse Event Monitoring System (SN/AEMS): Table D presents the available information on cases reported in the SN/AEMS. The 18 reports include 12 cases indicating varying degrees of liver damage. (These 12 cases are included among the patients in Table C-1.) It should be noted that in the SN/AEMS reports there is no indication of whether a causal relationship exists between the adverse event and chaparral ingestion.
3. Interactions
There are no known interactions with chaparral.
4. Consequences of unusually large intake or chronic cumulative use
There may be adverse effects associated with consumption of excessively large amounts of chaparral (Heron and Yarnell, 2001). This type of overuse is typically related to encapsulated chaparral products.
B. Animal Studies
Animal studies on chaparral: There were no animal studies identified that showed liver toxicity as the result of chaparral administration. In studies with rats, significant toxic effects were demonstrated following administration of chaparral; however, the nature of the toxicity was not documented (Nakazato et al., 1998; Ulreich et al., 1997). The ethanol:water tincture of chaparral administered to the rats was lethal in the relatively large amounts administered in these studies. In all, there is evidence of considerable toxic effects from four different animal models: using rats (Konno et al., 1987; Nakazato et al., 1998; Ulreich et al., 1997), hamsters (Granados and Cardenas, 1994), chickens (Zamora, 1984), and insects (Mabry et al., 1977) with relatively high exposures to chaparral. In considering all of the animal studies (Table E), the evidence evaluating any aspects of the safety of chaparral in animal studies is minimal.
Acute studies on NDGA in animals: The evidence evaluating the safety of NDGA, a major component of chaparral, is more substantial but is still incomplete (Table E). NDGA administered by gavage to rats and mice was reported to have an LD50 of > 4 g/kg body weight. NDGA was somewhat more toxic in guinea pigs, with an LD50 of 0.8 g/kg body weight. Thus, the LD50 is less than 100× a typical human intake.
Chronic studies on NDGA in animals: Chronic studies on the safety of NDGA are limited to toxicity studies conducted primarily in small rodents (Table E). Rats fed NDGA at 0.5 percent of their diet exhibited massive hemorrhages and multiple renal cysts in experiments reported only in abstract (Cranston et al., 1947) and reviews (Lehman et al., 1951). Strong evidence has been published that NDGA fed to rats at high doses (1 or 2 percent of the diet) clearly leads to various pathological changes. In various rat models, growth inhibition and structural changes in or near the kidney have been shown to develop within 2 to 6 months (Cranston et al., 1947; Gardner et al., 1986, 1987; Lehman et al., 1951). Renal and mesenteric cysts form within 6 to 12 months of NDGA feeding (Goodman et al., 1970; Grice et al., 1968; Lehman et al., 1951). By 18 months of feeding 1 percent NDGA (Grice et al., 1968) or 6 months of feeding 2 percent NDGA (Evan and Gardner, 1979), the development of renal and mesenteric cysts is profound. The renal cysts contained degenerating tubular cells and the renal
damage was predominantly in the proximal convoluted tubules. Biochemical analysis showed no free NDGA in the lymph nodes or kidney extracts; only the orthoquinone metabolite of NDGA could be detected (Grice et al., 1968).
In complementary studies, single dose administration of 250 mg of NDGA into the small intestine of rats revealed formation of the orthoquinone metabolite at the region of the ileocecal junction (Grice et al., 1968).
Studies using other species and/or other routes of administration verify the toxicity of NDGA (Giri and Hollinger, 1996; Hsu et al., 2001; Madrigal-Bujaidar et al., 1998; Mikuni et al., 1998; Telford et al., 1962).
Other observations: Pretreatment of rats with NDGA (50 mg/kg body weight, by gavage) significantly aggravated indomethacin-induced gastric ulcers (Cho and Ogle, 1987). Treatment of rats with NDGA (10 μg/kg body weight, by intravenous administration) worsened ischemia-reperfusion injury to liver (Okboy et al., 1992).
C. In Vitro Studies
Investigations on the in vitro effects of chaparral and NDGA on a variety of chemical and biological systems are summarized in Tables F-1 and F-2. While a few studies involved extracts of chaparral, most focused on the effects of NDGA, and a few considered the effects of other lignans with structural similarities to NDGA.
Some cytochrome P450 oxidations are inhibited by NDGA in vitro (Agarwal et al., 1991; Capdevila et al., 1988).
D. Liver-Related Information About Related Substances
Studies on taxonomically related substances: Five species of Larrea are recognized: the bifolate species, L. tridentata (native to the southwestern United States and northern Mexico), L. divaricata (native to northwestern Argentina and parts of Peru), and L. cuneifolia (native to Argentina), plus two multifolate species that grow at high altitudes, L. nitida (native to certain parts of South America, especially Argentina) and L. ameghinoi (native to a few parts of South America) (Brinker, 1993–1994). No useful safety data were found on L. cuneifolia, L. nitida, or L. ameghinoi. The toxicity of L. divaricata, a South American species that is taxonomically related to L. tridentata, has been studied to a very limited extent. A water extract of the dried leaves was injected into mice intraperitoneally and the LD50 was found to be 10 g/kg body weight for males and 4 g/kg for females (Anesini et al., 1997).
Substances related to the individual components of chaparral: Table G contains a list of substances that were considered as structurally, taxonomically, or functionally related to the components of chaparral (present in leaves, stems, and twigs). Known toxicities of these related substances were considered in evaluating the potential toxicity of chaparral. For comparison, Table A contains a listing of the known components of chaparral with the chemical structures of those that may be relevant to the safety of chaparral. In Table G it should be noted that larreantin is a potential hepatotoxin and is known to be present in the root of L. tridentata (Luo et al., 1988). Several mechanisms were considered whereby it might be possible that chaparral products could contain larreantin. First, chaparral root might be included with the other plant material (leaves, stems, and twigs). Second, under certain environmental conditions, a component of the root of a plant might physiologically be present in the leaves. Third, the presence of trace amounts of larreantin in the leaves, stems, or twigs could have been undetected. Although each of these mechanisms is possible, it seems unlikely that larreantin is present in chaparral preparations in significant amounts.
Functionally related substances: It was reported that NDGA is metabolized to an orthoquinone derivative (De Smet, 1993; Grice et al., 1968), which could be further metabolized by conjugation to glutathione. Because hepatic levels of glutathione are often limiting, drugs undergoing glutathione conjugation could interact negatively with the quinone derivative of NDGA by both substances drawing on glutathione reserves in the liver, leading to glutathione depletion (Slattery et al., 1987).
Knowledge about chemical structures of chaparral components: As stated above, NDGA is metabolized to an orthoquinone derivative (De Smet, 1993; Grice et al., 1968). Acetaminophen is also a quinone, but one that is understood to be cytotoxic and to cause substantial liver problems. Large doses of acetaminophen cause centrilobular hepatic necrosis (Hojo et al., 2000). The current understanding is that the hepatotoxicity of acetaminophen is due to cytochrome P450-dependent formation of N-acetyl para-(benzo)quinone imine (NAPQI) in the centrilobular region of the liver (Harman et al., 1991; Hojo et al., 2000; Holme et al., 1984).
By extrapolation, the site of chaparral toxicity might be expected to reflect the site of metabolism of NDGA to the quinone. NAPQI causes mitochondrial damage, including inhibition of oxidative phosphorylation (Andersson et al., 1990; Fujimura et al., 1995; Moore et al., 1985). Likewise, NDGA causes inhibition of the mitochondrial electron transport chain. Thus there are some similarities between NAPQI toxicity and NDGA toxicity that can be used to hypothesize a mechanism of hepatotoxicity based on the formation of a NDGA quinone. Acetaminophen-induced toxicity is also
seen in the kidney, another site of metabolism of the drug to NAPQI. Therefore, one might hypothesize that chaparral ingestion would lead to toxicity in the kidney also if chaparral toxicity is related to NDGA metabolism to a toxic quinone, which is purely theoretical. Indeed, renal toxicity of NDGA is evident in animal studies (Table E).
III. OTHER RELEVANT INFORMATION
A. Sources
Chaparral grows as a wild desert shrub. It is an evergreen bush that grows in arid regions and can reach a height of 9 feet (Brinker, 1993–1994). The identification of the plant used as L. tridentata is very important to the safety of the chaparral product.
Misnaming and species identification: There may be some instances of substitution of another plant product for L. tridentata. L. divaricata has been commonly confused with L. tridentata. The two species are very similar in appearance (Brinker, 1993-1994), but originate from distinct locations. The major source of confusion is the misnaming of the two species, even in published reports of clinical or experimental data (Gisvold, 1947; Smart et al., 1970).
Contaminants and adulterants: Adulteration has not been reported.
Processing issues: As described in the human use information section, most processing of chaparral used in American Indian cultures involved aqueous extracts, such as hot water teas (Heron and Yarnell, 2001). A tea has very little NDGA compared with an alcoholic extract or the powdered dry leaf because NDGA is poorly soluble in water (Obermeyer et al., 1995).
The extraction liquid generally used to make a tincture of chaparral has a high percent of ethanol (up to 95 percent, v/v) so that the extract will contain phenolic compounds, such as NDGA and flavonoids. The amount of solvent-extractable natural products does not change considerably regardless of whether fresh or dried leaves are used in processing (Mabry et al., 1977). The solvent used does make a considerable difference in the quantity of individual components. Diethyl ether gives a high yield, as compared with 85 percent aqueous methanol, which also extracts considerable chlorophyll (Mabry et al., 1977).
Analytic issues: Analytical methods have been published for the determination of a number of components of chaparral. These methods include gas liquid chromatography, high pressure liquid chromatography, and mass
spectrometric analysis of lignans (Gonzalez-Coloma et al., 1988; Obermeyer et al., 1995; Valentine et al., 1984), and the ammonium molybdate spectrophotometric assay for NDGA (Duisberg et al., 1949). Pharmacokinetic analysis of NDGA administered to mice used a method with a limit of detection of 0.5 μg/mL plasma or serum (Lambert et al., 2001).
B. Conditions of Use Suggested or Recommended in Labeling or Other Marketing Material
Common popular uses: Chaparral has had numerous ethnomedicinal, homeopathic, and folk medicine uses. Homeopathic medicine has used chaparral tea in the treatment of colds, cold sores, coughs, bronchitis, viral infections, urinary tract infections, indigestion, heartburn, abdominal cramps, enteritis, dysentery, parasites, dysmenorrhea, menstrual cramps, premenstrual syndrome, neuritis, and sciatica (Heron and Yarnell, 2001; Mabry et al., 1977). Chaparral has been used as an abortifacient and as a means to increase fertility (Heron and Yarnell, 2001). Chaparral products have been described as having a beneficial impact on liver metabolic functions (Heron and Yarnell, 2001).
In folk medicine, chaparral has been used for leukemia and many different types of cancers. It has been suggested that chaparral contains immune-stimulating polysaccharides and that NDGA may have some antitumor properties. From conventional medical sources there is anecdotal and in vitro evidence of cytotoxic activity with varying toxicity depending on the concentration of NDGA.
Currently chaparral is marketed to consumers for arthritis, rheumatism, and bursitis; as an antioxidant; for immune function; for various cancers, such as melanomas, leukemia, breast cancer, ovarian cancer, and Kaposi’s sarcoma; as a blood and liver cleanser; as a diuretic; for colds and the flu; for herpes family viruses including herpes simplex, herpes zoster, cytomegalovirus, and Epstein-Barr; and for acne and skin disorders.
C. Liver-Related Cautions Noted
Cautions provided in labeling or other marketing material: A review of chaparral product labels and Internet marketing materials indicates that many (but not all) provide cautions to consumers to seek advice from health care providers before using the product if they have a history of liver or kidney disease or currently have digestive problems and to avoid using if pregnant or nursing. One caution indicated that the chaparral product was not intended for long-term use. Two informational websites that did not sell chaparral products suggested that people consuming chaparral tea should drink 3 cups a day for a maximum of 2 weeks unless under the care
of a physician or health practitioner experienced in the use of botanical medicines. At least three informational websites that did not sell products cautioned against the use of chaparral capsules since several adverse event reports were associated with this form.
Cautions issued by manufacturing associations: In 1994 the American Herbal Products Association (AHPA) commissioned a review. Four case studies were examined and it was concluded that:
… since the patients were ingesting chaparral during the time each developed acute hepatitis, most likely of a hepatocellular nature, it is reasonable to conclude a relationship exists between the ingestion and the disease. However, no clinical data were found in the medical records to indicate that chaparral is inherently a hepatic toxin. Moreover, each patient had a medical history not incompatible with prior liver disease. A fair conclusion is [that] the disease in each patient was the result of an individual idiosyncratic reaction to the drug [botanical product], possibly the result of an autoimmunologic reaction, which given the quantity of chaparral ingested in this country, must be exceedingly rare (AHPA, 2002).
Following the Food and Drug Administration (FDA) warning issued in 1992 (see Section IIIE, below), many manufacturers voluntarily removed most products containing this botanical (FDA, 1993). In 1995 AHPA recommended that if member companies chose to sell chaparral, all consumer labeling contain the following informational language:
Seek advice from a health care practitioner before use if you have had, or may have had, liver disease. Discontinue use if nausea, fever, fatigue or jaundice (e.g., dark urine, yellow discoloration of the eyes) should occur (APHA, 2002).
D. Usage Patterns
Prevalence of use in the general population: According to a survey conducted by the Herb Research Foundation from 1973 to 1993, at least 200 tons of chaparral was sold in the U.S. market (Blumenthal, 1993). This would be equivalent to 500 million doses at 500 mg/dose. No current data are available; there has been no recent tracking of sales data.
Knowledge of use by particular groups: There are no published surveys in the literature that provide knowledge about the use of chaparral by specific groups. However, anecdotal reports suggest that indigenous American Indian groups in the southwestern United States and Hispanics may use chaparral, primarily as an aqueous extract (tea).
E. Information on Regulatory Actions
FDA actions: In late August and early September 1992, FDA and the Centers for Disease Control and Prevention (CDC) were informed of two cases in which individuals consuming chaparral over several weeks experienced severe jaundice and abdominal pain. These cases, and the potential link between acute nonviral hepatitis and chaparral, were discussed in an issue of Morbidity and Mortality Weekly Report (CDC, 1992).
In a press release issued in December 1992, the FDA Commissioner conveyed a public advisory against the purchase or consumption of chaparral because it was associated with acute toxic hepatitis (FDA, 1992). FDA advised chaparral users to stop taking chaparral immediately and to consult a physician if a user had a history of liver disease or was not feeling well (FDA, 1992). Subsequent warnings were issued (CFSAN, 1993).
The major lignan in chaparral is NDGA, a potent antioxidant. Beginning in 1943, NDGA (at 0.02 percent, w/w) was used as an antioxidant in many foods (Mabry et al., 1977). In 1968 NDGA lost its status as a generally recognized as safe ingredient. FDA then required removal of NDGA from most foods. The U.S. Department of Agriculture oversees the safety of meats and meat products; at this time, it allows use of NDGA as an antioxidant in lard, animal shortening, and other products that are susceptible to the development of rancidity.
Other relevant regulatory actions: Canadian regulations do not allow chaparral as a nonmedicinal ingredient for oral-use products (McGuffin et al., 1997). The current edition of the German Commission E monographs does not mention chaparral (Blumenthal, 1998).
F. Available Information on Physiological and Biochemical Aspects
Very little is known about the digestion, absorption, distribution, metabolism, and excretion of some chaparral components (triterpenes). Other components have been well characterized (i.e., fatty acids and other hydrocarbons).
There is one pharmacokinetic study on NDGA. In female mice, NDGA was administered i.v. at 50 mg/kg to yield a primary half-life of 30 min and a secondary half-life of 135 minutes, with the peak plasma concentration Cmax being 15 μg/mL (Lambert et al., 2001). It has been reported that a major metabolite of NDGA is the orthoquinone derivative (De Smet, 1993; Grice et al., 1968).
G. Supplementary Information
No information applicable to liver concerns.
IV. TABLES ON CHAPARRAL
*|
Chaparral: Individual Components |
|
|
Chaparral: Summary of Adverse Effects in a Clinical Trial |
|
|
Chaparral: Summary of Clinical Case Reports |
|
|
Summary of Clinical Case Reports and a Case Series Report with Chaparral Used in Combination |
|
|
Chaparral: Summary of Adverse Event Reports |
|
|
NDGA: Summary of Animal Studies |
|
|
Chaparral: Summary of In Vitro Studies |
|
|
NDGA: Summary of In Vitro Studies |
|
|
Chaparral: Related Substances that Might Suggest Risk |
V. SUMMARY AND CONCLUSIONS
A. Summary
Reports of chaparral toxicity are inconsistent. Reportedly native populations in the southwestern United States have used chaparral tea for decades without evidence of toxicity. In addition, a clinical study looking at chaparral tea and NDGA in advanced, incurable cancer patients showed no evidence of hepatotoxicity. Limitations of this study included a lack of detail on those who did not complete the trial (25 percent of the subjects). This evidence is somewhat inconsistent with other information on chaparral use, as follows.
There are nine reported cases of definite hepatotoxicity temporally related to chaparral use as a single known agent; there are an additional six cases of possible hepatotoxicity. Five of the cases exhibited documented recovery after cessation of chaparral use and one case exhibited abnormal liver function upon rechallenge. One patient required an orthotopic liver transplant but had major confounding variables, such as hepatitis C and prior drug and ethanol abuse. In all the other cases, liver function tests became significantly abnormal with clinically evident jaundice that reversed upon discontinuation of chaparral use. In at least three cases of chaparral-associated hepatotoxicity, the patient had prior history of alcohol abuse or underlying liver disease and may represent a vulnerable population.
In determining causation, one looks for a dose-response relationship.
The amount of chaparral ingested ranged from 0.3 to 6 g/day over periods ranging from 20 days to “many years.” The absence of pharmacokinetic data or even characterization of the formulations ingested made it difficult to determine actual dose in the various case reports. Thus there was no apparent dose-response relationship, although evidence of toxicity was clearly reflected in abnormal liver function tests.
Another important factor in determining causation is characterization of the product responsible for the adverse effect. In most of the reported cases, the product ingested by the subject was simply described as chaparral capsules or tablets. This description does not reveal whether the contents of the capsule or tablet were dried, ground plant material or dried extract. Further, without examination of the quality of the product, contamination or adulteration cannot be ruled out.
Only in 1 out of the 15 case reports of chaparral-associated hepatotoxicity was it reported that a chaparral tea had been ingested. This is important because chaparral tea contains very little NDGA or other lipophilic compounds as compared with other preparations, such as a dried extract prepared with an organic solvent. If NDGA is the causal agent, the content of NDGA in various preparations becomes an important variable in determining causality.
Animal studies evaluating chaparral did not show hepatotoxicity. Animal studies evaluating NDGA did not exhibit hepatotoxicity, but did exhibit renal proximal tubular damage and cyst formation. In other studies, rodents exhibited both renal and hepatic toxicity in response to the toxic quinone imine from acetaminophen; this involves proximal tubular damage, but not cyst formation. A plausible mechanism in both hepatotoxicity and nephrotoxicity is the cytochrome P450-dependent metabolism of NDGA to a toxic quinone with failure to remove this reactive metabolite by conjugation if glutathione is limiting. The link between the nephrotoxicity of NDGA in animals and the hepatotoxicity of chaparral in humans is not definite, but similar links have been shown with structurally related chemicals, such as the quinone of acetaminophen.
While the human data strongly suggest an association between chaparral consumption and hepatotoxicity, a number of confounding factors also require consideration. The temporal clustering of the majority of the hepatotoxicity cases (1992–1993) provides some suggestion of a localized contamination problem. Inadequate characterization of the preparations used by individual patients does not allow determination of possible product contamination during harvesting/processing or natural alterations in composition of chaparral plants due to environmental factors. If typical chaparral preparations contained hepatotoxic principles, it is possible that many more reports of human hepatotoxicity during the period of significant chaparral use (1970–1992) would have emerged. Pre-existing liver disease, in-
cluding excessive alcohol use, hepatitis, or chronic acetaminophen use, could possibly have predisposed some of the individuals to hepatotoxicity. Such possibilities are hypothetical, but the quality of the data provided in the case reports is inadequate to rule out such possibilities.
B. Conclusions and Recommendations About Liver Concerns
Conclusions (concerns and caveats): The available literature raises concern for hepatic toxicity. The reasons for concern about hepatotoxicity and possibly related nephrotoxicity can be summarized as case reports showing a pattern of hepatotoxicity, nephrotoxicity in rats given NDGA, and in vitro studies showing that NDGA exhibited cytotoxic activity. The consistency and biological plausibility of these observations is strengthened by knowledge of NDGA structure and knowledge about mechanisms of quinone toxicity.
While the human data strongly suggest an association between chaparral consumption and hepatotoxicity, a number of confounding factors also require consideration. There was a clinical study (published in 1970) in which serum glutamic-oxaloacetic transaminase (SGOT), a marker of liver damage, was evaluated; this was an uncontrolled, poorly designed study, yet no elevation in SGOT was reported. However, the subjects were critically ill cancer patients and 15 of the subjects (25 percent of the total) were removed from the study. At the time of this study, there was no awareness of a possible relationship between chaparral ingestion and hepatotoxicity; these individuals could have been removed from the study because elevations in SGOT were used to indicate a measure of general health and appropriateness, a possible criteria to remain in the study.
The temporal clustering of the majority of the hepatotoxicity cases (1992–1993) provides some suggestion of localized contamination or a variation in constituent concentration, perhaps due to inadequate characterization or lack of standardization. It is unfortunate that animal studies were not conducted at the time this cluster of hepatotoxic events was reported. During a period of 20 years (1973–1993), 200 tons of chaparral was sold on the U.S. market, equivalent to 500 million doses at 500 mg/ dose. If typical chaparral preparations contained hepatotoxic principles, it is possible that many more reports of human hepatotoxicity during the period of significant chaparral use (1970–1992) would have emerged. Traditional uses of chaparral tea by native populations have not revealed reports of hepatotoxicity. Pre-existing liver disease, including excessive alcohol use, hepatitis, or chronic acetaminophen use, may have predisposed some of the individuals to hepatotoxicity. Since the quality of the data provided in the case reports is inadequate to rule them out, such possibilities remain hypothetical.
The evidence for toxicity of chaparral in humans is supported by a similar toxicity observed in animal studies using NDGA. Classic toxicity studies with NDGA were conducted in several species, and toxicity of NDGA was demonstrated over a range of doses; this is a common finding in toxicity studies using different animal species (Ashby, 2002). Of the animal studies reported, only two identified hepatic effects following administration of NDGA to rats or mice; the one mouse study used intraperitoneal administration of NDGA and is confounded by coadministration of endotoxin, a known hepatotoxin. Thus only minimal hepatotoxicity was exhibited in animals treated with NDGA. However, if toxicity of a compound is related to the site of its metabolism, hepatotoxicity would be expected because liver is the major site of xenobiotic metabolism. Instead, nephrotoxicity was the major toxicity found in rats treated with NDGA; this nephrotoxicity is discussed in detail below (Kacew, 2001).
Of the 15 reported cases of chaparral-associated hepatotoxicity, only 1 was associated with ingestion of chaparral tea, whereas 11 cases were associated with ingestion of capsules or tablets containing chaparral. If NDGA contributes to the toxicity, it is important to note that NDGA and other nonpolar compounds, including lignans, appear to be minimal in a water extract/tea in contrast to an alcoholic extract (Obermeyer et al., 1995). This differential extraction of lignans by water versus alcohol extraction (Obermeyer et al., 1995) is explained by the lipophilic character of lignans. Therefore, alcoholic extracts of leaf or other aerial plant parts would contain larger amounts of NDGA and other lipophilic compounds than a water extract/tea.
NDGA can be expected to be a substrate for cytochrome P450-dependent quinone formation based on its chemical structure, as well as on evidence discussed by Obermeyer et al. (1995). A plausible mechanism of cytotoxicity of NDGA is the cytochrome P450-dependent metabolism to a toxic quinone and failure to remove this reactive metabolite by conjugation if glutathione is limiting. The link between the nephrotoxicity of NDGA in animals and hepatotoxicity of chaparral in humans is based on the fact that both the renal proximal tubules and the liver are major sites of xenobiotic metabolism. A parallel finding has also been demonstrated in rodents; both renal and hepatic toxicity develop in response to the toxic quinone imine from acetaminophen.
Summary of the conclusions: Although substantial limitations exist in the available information, concerns about the hepatotoxicity of chaparral remain based on the weight of the evidence discussed above. This is especially applicable for certain groups, including those with pre-existing hepatic conditions, those taking drugs that affect liver function, and those with current or prior alcohol abuse. There is more concern with ingestion of
chaparral preparations containing leaves/stems or alcoholic extracts than with the ingestion of aqueous extracts (i.e., teas) because of the higher content of NDGA and other lipophilic compounds in the former preparations.
C. Data Gaps and Future Research Recommended
Detailed toxicity studies in animals are needed to explore the possible dose-response relationship in the development of hepatotoxicity and nephrotoxicity as the result of chaparral ingestion. In animal studies, pair feeding should be included in the experimental protocol due to possible aversion to the chow if NDGA has been added (Goodman et al., 1970). Ideally, studies should compare the different preparations of chaparral (i.e., powdered leaf, alcoholic extract, and water extract).
The differences in the chemical composition of the various preparations of chaparral need to be explored. The literature shows that a preponderance of toxicities were associated with preparations other than tea; hepatotoxicity was not reported in a clinical trial of cancer patients drinking chaparral tea. This suggests there that there are differences in the bioavailability of the various components of chaparral that result from differences in the chemical composition of the preparations. These differences need to be explored in detail.
In all further research, it is important to carry out careful product characterization. A qualified taxonomist should identify the plant material, and a botanical sample should be retained in an herbarium for future reference. It is important to carefully describe the plant part utilized. As an example, newer leaves should be distinguished from older leaves because newer leaves contain a higher proportion of the NDGA-containing resin. Chaparral roots contain a quinone not reported to be present in the aerial parts of the plants and, thus, roots should be carefully excluded. The plant material should be chemically profiled, including a quantitative determination of NDGA and other lignans. As a quality measure, there should be an analysis of metals since chaparral plants concentrate metals from the soil (Gardea-Torresdey et al., 2001). Furthermore, when reporting human experience with ingesting chaparral, the formulation is important to note. The formulation can best be critically evaluated if the manufacturer, date, and lot number are reported.
VI. LITERATURE SEARCH STRATEGY
This prototype focused monograph was prepared by excluding information not possibly related to hepatotoxicity after conducting a literature search for the full prototype monograph. This was probably a more effec-
tive approach, although more time consuming, than initially limiting searches to liver information because information not about liver toxicity per se, but possibly related to liver toxicity, could be identified.
To prepare the chaparral monograph, the databases indicated below were searched using the terms [chaparral] OR [Larrea tridentata] (in any field). In the AGRICOLA database, it was necessary to limit the search to exclude other meanings of the word “chaparral.” These searches were conducted in April 2002 and yielded approximately 125 citations (excluding duplicate citations brought up by the various databases). The databases were independently searched for the entire genus Larrea, and articles pertaining to the North American plant were investigated to confirm that all articles that actually reported on L. tridentata were considered. Citations for many references that predate the electronic databases were collected from among the reference sections of the literature reviewed. Because the number of published articles on this topic are limited, an effort was made to collect abstracts representing research in this area. In August 2002, the databases were searched again for more recent articles and a few citations were added. A literature search on NDGA was also conducted in April 2002 and yielded approximately 325 citations (excluding duplicate citations brought up by the various databases). As NDGA is commonly used as a reagent, the search parameters were limited as follows: [nordihydroguaiaretic acid] OR [NDGA] (in title field) for most databases; [nordihydroguaiaretic acid] OR [NDGA] (in any field) for TOXLINE and AGRICOLA.
Electronic searches were conducted using the following databases: PubMed (1966–2002, TOXLINE Core inclusive), TOXLINE (TOXLINE Core and TOXLINE Special), EMBASE (1980–2002), and AGRICOLA (1979–2002). It should be noted that EMBASE contains a considerable amount of foreign literature and AGRICOLA contains a considerable amount of the veterinary literature. WorldCat, EMB/Cochrane Reviews, IBIDS, BEAST, and Dissertation Abstracts were used to a limited extent. NAPRALERT was used for natural products. Patents were accessed using the website of the U.S. Patent and Trademark Office. Information on the SN/AEMS and regulatory actions taken by FDA were obtained from the FDA website. Information on regulatory actions by the Federal Trade Commission (FTC) was obtained from the FTC website. It is highly recommended that Chemical Abstracts also be used, although this database was not used for the prototype monographs due to the limitations of time and resources. In the database search, no restriction was placed on language or type of publication. However, the ability to interpret non-English-language publications was limited. The foreign language literature was included when it was deemed important in order to be complete. The majority of the
literature cited is drawn from primary research sources, followed by secondary sources as appropriate.
VII. LITERATURE CITED
ACS (American Cancer Society). 1970. Unproven Methods of Cancer Treatment: Chaparral Tea. New York: ACS.
Adlercreutz H, Bannwart C, Wahala K, Makela T, Brunow G, Hase T, Arosemena PJ, Kellis JT Jr, Vickery LE. 1993. Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. J Steroid Biochem Mol Biol 44:147–153.
Afifi FU, Al-Khalil S, Abdul-Haq BK, Mahasneh A, Al-Eisawi DM, Sharaf M, Wong LK, Schiff PL Jr. 1991. Antifungal flavonoids from Varthemia iphionoides. Phytother Res 5:173–175.
Agarwal R, Wang ZY, Bik DP, Mukhtar H. 1991. Nordihydroguaiaretic acid, an inhibitor of lipoxygenase, also inhibits cytochrome P-450-mediated monooxygenase activity in rat epidermal and hepatic microsomes. Drug Metab Dispos 19:620–624.
AHPA (American Herbal Products Association). 2002. Code of Ethics and Business Conduct. Silver Spring, MD: AHPA.
Alderman S, Kailas S, Goldfarb S, Singaram C, Malone DG. 1994. Cholestatic hepatitis after ingestion of chaparral leaf: Confirmation by endoscopic retrograde cholangio-pancreatography and liver biopsy. J Clin Gastroenterol 19:242–247.
Andersson BS, Rundgren M, Nelson SD, Harder S. 1990. N-acetyl-p-benzoquinone imineinduced changes in the energy metabolism in hepatocytes. Chem Biol Interact 75:201–211.
Anesini C, Boccio J, Cremaschi G, Genaro A, Zubillaga M, Borda LS, Borda ES. 1997. In vivo antitumoural activity and acute toxicity study of Larrea divaricata Cav. extract. Phytother Res 11:521–523.
Ashby J. 2002. Scientific issues associated with the validation of in vitro and in vivo methods for assessing endocrine disrupting chemicals. Toxicology 181–182:389–397.
Banskota AH, Tezuka Y, Tran KQ, Tanaka K, Saiki I, Kadota S. 2000. Methyl quadrangularates A-D and related triterpenes from Combretum quadrangulare. Chem Pharm Bull (Tokyo) 48:496–504.
Batchelor WB, Heathcote J, Wanless IR. 1995. Chaparral-induced hepatic injury. Am J Gastroenterol 90:831–833.
Bernhard HO, Thiele K. 1981. Additional flavonoids from the leaves of Larrea tridentata. Planta Med 41:100–103.
Bhuvaneswaran C, Dakshinamurti K. 1972. Inhibition of electron and energy transfer in rat liver mitochondria by nordihydroguaiaretic acid. Biochemistry (Mosc) 11:85–91.
Biswal SS, Datta K, Shaw SD, Feng X, Robertson JD, Kehrer JP. 2000. Glutathione oxidation and mitochondrial depolarization as mechanisms of nordihydroguaiaretic acid-induced apoptosis in lipoxy-genase-deficient FL5.12 cells. Toxicol Sci 53:77–83.
Blalock JE, Archer DL, Johnson HM. 1981. Anticellular and immunosuppressive activities of food-borne phenolic compounds. Proc Soc Exp Biol Med 167:391–393.
Blumenthal M. 1993. Herb industry and FDA issue chaparral warning. Herbalgram 28:38–39.
Blumenthal M. ed. 1998. Therapeutic Guide to Herbal Medicines. The Complete German Commission E Monographs. Austin, TX: American Botanical Council.
Bohnstedt CFMTJ. 1979. The volatile constituents of the genus Larrea (Zygophyllaceae). Rev Latinoamer Quim 10:128–131.
Botkin C, Duisberg P. 1949. The Nordihydroguaiaretic Acid Content of the Creosote Bush. Bulletin 349. Las Cruces, NM: Agricultural Experiment Station, New Mexico College of Agriculture and Mechanic Arts.
Brinker F. 1993. The Chaparral Handbook. Portland, OR: Eclectic Medical Publications.
Brinker F. 1993–1994. Larrea tridentata (DC) Coville (chaparral or creosote bush). Br J Phytother 3:10–29.
Burba JV, Becking GC. 1969. Effect of the antioxidant nordihydroguaiaretic acid on the in vitro activity of catecholo-methyl transferase. Arch Int Pharmacodyn Ther 180:323–330.
Burk D, Woods M. 1963. Hydrogen peroxide, catalase, glutathione peroxidase, quinones, NDGA, and phosphopyridine nucleotides in relation to X-ray action on cancer cells. Rad Res Suppl 3:212–246.
Capdevila J, Gil L, Orellana M, Marnett LJ, Mason JI, Yadagiri P, Falck JR. 1988. Inhibitors of cytochrome P-450-dependent arachidonic acid metabolism. Arch Biochem Biophys 261:257–263.
CDC (Centers for Disease Control and Prevention). 1992. Chaparral-induced toxic hepatitis—California and Texas, 1992. Morb Mortal Wkly Rep 41:812–814.
CFSAN (Center for Food Safety and Applied Nutrition). 1993. Illnesses and Injuries Associated with the Use of Selected Dietary Supplements. Online. Available at http://www.cfsan.fda.gov/~dms/dsill.html. Accessed August 26, 2002.
Chae YH, Ho DK, Cassady JM, Cook VM, Marcus CB, Baird WM. 1992. Effects of synthetic and naturally occurring flavonoids on metabolic activation of benzo[a]pyrene in hamster embryo cell cultures. Chem Biol Interact 82:181–193.
Chang J, Skowronek MD, Cherney ML, Lewis AJ. 1984. Differential effects of putative lipoxygenase inhibitors on arachidonic acid metabolism in cell-free and intact cell preparations. Inflammation 8:143–155.
Chirikdjian JJ. 1973. Flavonoide von Larrea tridentata. Zeitschrift Für Naturforschung [C] 28C:32–35.
Chirikdjian JJ. 1974. Isolation of kumatakenine and 5,4'-dihydroxy-3,7,3'-trimethoxiflavone from Larrea tridentata. Pharmazie 29:292–293.
Cho CH, Ogle CW. 1987. Potentiation of indomethacinduced gastric ulcers in rats by nordihydroguaiaretic acid (NDGA), an inhibitor of lipoxygenase. Asia Pacif J Pharmacol 2:49–52.
Clark F, Reed R. 1992. Chaparral-induced toxic hepatitis—California and Texas, 1992. J Am Med Assoc 268:3295–3298.
Cranston E, Jensen MJ, Moren A, Brey T, Bell ET, Bieter RN. 1947. The acute and chronic toxicity of nordihydroguaiaretic acid. Fed Proc 6:318–319. Abstract.
Dahlgren C. 1991. Effect of different inhibitors on the intracellularly and extracellularly generated chemiluminescence induced by formylmethionyl-leucyl-phenylalanine in polymorphonuclear leukocytes. Cellular response in the presence of mannitol, benzoate, taurine, indomethacin and NDGA. J Biolumin Chemilumin 6:29–34.
Damayanthi Y, Lown JW. 1998. Podophyllotoxins: Current status and recent developments. Curr Med Chem 5:205–552.
De Smet PAGM. ed. 1993. Adverse Effects of Herbal Drugs. Vol. 2. Berlin: Springer-Verlag. Pp. 231–240.
Downum KR, Dole J, Rodriguez E. 1988. Nordihydroguaiaretic acid: Inter- and intrapopulational variation in the Sonoran Desert creosote bush (Larrea tridentata, Zygophyllaceae). Biochem Syst Ecol 16:551–555.
Duisberg PC, Shires LB, Botkin CW. 1949. Determination of NDGA in the leaf of Larrea divericata. Anal Chem 21:1393–1396.
Evan AP, Gardner KD Jr. 1979. Nephron obstruction in nordihydroguaiaretic acid-induced renal cystic disease. Kidney Int 15:7–19.
FDA (Food and Drug Administration). 1992. FDA Advice to Chaparral Users. Online. Available at http://www.fda.gov/bbs/topics/ANSWERS/ANS00456.html. Accessed July 1, 2004.
FDA. 1993. Dietary Supplements: Making Sure Hype Doesn’t Overwhelm Science. Online. Available at http://www.fda.gov/bbs/topics/CONSUMER/CON00259.html. Accessed August 26, 2002.
Fernandez S, Hurtado LM, Hernandez F. 1979. Fungicidal components of creosote bush resin. In: Geissbuhler H, ed. Advances in Pesticide Science. Vol. 2. New York: Pergamon Press. Pp. 315–355.
Ferrandiz ML, Ramachandran Nair AG, Alcaraz MJ. 1990. Inhibition of sheep platelet arachidonate metabolism by flavonoids from Spanish and Indian medicinal herbs. Pharmazie 45:206–208.
Fronczek FR, Caballero P, Fischer NH, Fernandez S, Hernandez E, Hurtado LM. 1987. The molecular structure of 3'-demethoxynorisoguaiacin triacetate from creosote bush (Larrea tridentata). J Natural Prod 50:497–499.
Fujii K, Jaffe H, Bishop Y, Arnold E, Mackintosh D, Epstein SS. 1970. Structure-activity relations for methylenedioxyphenyl and related compounds on hepatic microsomal enzyme function, as measured by prolongation of hexobarbital narcosis and zoxazolamine paralysis in mice. Toxicol Appl Pharmacol 16:482–494.
Fujimura H, Kawasaki N, Tanimoto T, Sasaki H, Suzuki T. 1995. Effects of acetaminophen on the ultra-structure of isolated rat hepatocytes. Exp Toxicol Pathol 47:345–351.
Gardea-Torresdey JL, Arteaga S, Tiemann KJ, Chianelli R, Pingitore N, Mackay W. 2001. Absorption of copper (II) by creosote bush (Larrea tridentata): Use of atomic and x-ray absorption spectroscopy. Environ Toxicol Chem 20:2572–2579.
Gardner KD Jr, Evan AP, Reed WP. 1986. Accelerated renal cyst development in deconditioned germ-free rats. Kidney Int 29:1116–1123.
Gardner KD Jr, Reed WP, Evan AP, Zedalis J, Hylarides MD, Leon AA. 1987. Endotoxin provocation of experimental renal cystic disease. Kidney Int 32:329–334.
Giri SN, Hollinger MA. 1996. Effect of nordihydroguaiaretic acid and ibuprofen on bleomycin and hyperoxia-induced changes in lung superoxide dismutase, prostaglandins and lethality. Arch Toxicol 70:271–276.
Gisvold O. 1947. A preliminary survey of the occurrence of nordihydroguaiaretic acid in Larrea divaricata. J Am Pharm Assoc 37:194–196.
Gisvold O, Thaker E. 1974. Lignans from Larrea divaricata. J Pharm Sci 63:P1905–P1907.
Gnabre J, Huang RCC, Bates RB, Burns JJ, Calderea S, Malcomson ME, McClure KJ. 1995. Characterization of anti-HIV lignans from Larrea tridentata. Tetrahedron 51:12203–12210.
Gonzalez-Coloma A, Wisdom CS, Undel PW. 1988. Ozone impact on the antioxidant nordihydroguaiaretic acid content in the external leaf resin of Larrea tridentata. Biochem Syst Ecol 16:59–64.
Goodman T, Grice HC, Becking GC, Salem FA. 1970. A cystic nephropathy induced by nordihydroguaiaretic acid in the rat. Light and electron microscopic investigations. Lab Invest 23:93–107.
Gordon DW, Rosenthal G, Hart J, Sirota R, Baker AL. 1995. Chaparral ingestion. The broadening spectrum of liver injury caused by herbal medications. J Am Med Assoc 273:489–490.
Gowri MS, Reaven GM, Azhar S. 1998. Effect of Masoprocol on glucose transport and lipolysis by isolated rat adipocytes. J Investig Med 46:144A. Abstract.
Gowri MS, Azhar RK, Kraemer FB, Reaven GM, Azhar S. 2000. Masoprocol decreases rat lipolytic activity by decreasing the phosphorylation of HSL. Am J Physiol 279:E593–E600.
Granados H, Cardenas R. 1994. Biliary calculi in the golden hamster. The prophylactic action of the creosote bush (Larrea tridentata) in pigmented cholelithiasis produced by vitamin A. Rev Gastroenterol México 59:31–35.
Grant KL, Boyer LV, Erdman BE. 1998. Chaparral-induced hepatotoxicity. Integr Med 1:83–87.
Grice HC, Becking G, Goodman T. 1968. Toxic properties of nordihydroguaiaretic acid. Food Cosmet Toxicol 6:155–161.
Habermehl G, Christ B. 1974. Die freien steroide in Larrea divaricata. Phytochemistry 13:1293–1294.
Habermehl G, Moeller H. 1974. Isolation and structure of larreagenin A. Justus Liebigs Ann Chem. 1-6:169–175.
Harman AW, Kyle ME, Serroni A, Farber JL. 1991. The killing of cultured hepatocytes by N-acetyl-p-benzoquinone imine (NAPQI) as a model of the cytotoxicity of acetaminophen. Biochem Pharmacol 41:1111–1117.
Heiser RW, Cheng CC, Pardini RS. 1977. Inhibition of mitochondrial electron transport by partially demethylated dihydroguaiaretic acid. Pharmacol Res Commun 9:917–926.
Heron S, Yarnell E. 2001. The safety of low-dose Larrea tridentata (DC) Coville (creosote bush or chaparral): A retrospective clinical study. J Altern Complement Med 7:175–185.
Hojo M, Hanioka K, Miyata M, Yamazoe Y. 2000. Hepatotoxicity of acetaminophen and N-acetyl-p-benzoquinone imine and enhancement by fructose. Xenobiotica 30:933–991.
Holme JA, Dahlin DC, Nelson SD, Dybing E. 1984. Cytotoxic effects of N-acetyl-p-benzo-quinone imine, a common arylating intermediate of paracetamol and N-hydroxyparacetamol. Biochem Pharmacol 33:401–406.
Hsu BR, Juang JH, Fu SH, Kuo CH, Lu WT. 2001. Reduction in primary nonfunction of syngeneic islet transplants with nordihydroguaiaretic acid, a lipoxygenase inhibitor. Cell Transplant 10:255–262.
Hyder PW. 2001. Total Phenolics, Condensed Tannins, and Nordihydroguaiaretic Acid (NDGA) as Potential Allelopathic Compounds in Creosote Bush and Tarbush in the Northern Chihuahuan Desert. Las Cruces, NM: New Mexico State University. Dissertation.
Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. 2002. Mechanisms of hepatotoxicity. Toxicol Sci 65:166–176.
Kacew S. 2001. Confounding factors in toxicity testing. Toxicology 160:87–96.
Kamitani H, Geller M, Eling T. 1998. Expression of 15-lipoxygenase by human colorectal carcinoma Caco-2 cells during apoptosis and cell differentiation. J Biol Chem 273:21569–21577.
Katz M, Saibil F. 1990. Herbal hepatitis: Subacute hepatic necrosis secondary to chaparral leaf. J Clin Gastroenterol 12:203–206.
Kellett GL, Barker ED, Beach NL, Dempster JA. 1993. Effect of nordihydroguaiaretic acid on glucose absorption, metabolism and (Na++K+)-ATPase activity in rat jejunum. Biochem Pharmacol 45:1932–1935.
Klaassen CD, ed. 1995. Casarett and Doull’s Toxicology. The Basic Science of Poisons. 5th ed. New York: McGraw-Hill.
Konno C, Martin A, Ma B-X, Lu Z-Z, Xue H-Z, Erdelmeier CAJ, Nuzzo NA, Soejarta DD, Cordell GA, Waller DP, Fong HHS. 1987. Search for fertility regulating agents from Larrea tridentata. Proceedings: The First Princess Chulabhorn Science Congress 1987, International Congress on Natural Products. 2:328–337.
Konno C, Xue HZ, Lu ZZ, Ma BX, Erdelmeier CAJ, Che CT, Cordell GA, Soejarto DD, Waller DP, Fong HHS. 1989. 1-Aryl tetralin lignans from Larrea tridentata. J Natural Prod 52:1113–1117.
Konno C, Lu ZZ, Xue HZ, Erdelmeier CA, Meksuriyen D, Che CT, Cordell GA, Soejarto DD, Waller DP, Fong HH. 1990. Furanoid lignans from Larrea tridentata. J Natural Prod 53:396–406.
Korn SJ, Horn R. 1990. Nordihydroguaiaretic acid inhibits voltage-activated Ca2+ currents independently of lipoxygenase inhibition. Mol Pharmacol 38:524–530.
Kozubik A, Hofmanova J, Hola J, Netikova J. 1993. The effect of nordihydroguaiaretic acid, an inhibitor of prostaglandin and leukotriene biosynthesis, on hematopoiesis of gamma-irradiated mice. Exp Hematol 21:138–142.
Lambert JD, Meyers RO, Timmermann BN, Dorr RT. 2001. Pharmacokinetic analysis by high-performance liquid chromatography of intravenous nordihydroguaiaretic acid in the mouse. J Chromatogr B Biomed Sci Appl 754:85–90.
Lehman AJ, Fitzhugh OG, Nelson AA, Woodard G. 1951. The pharmacological evaluation of antioxidants. Adv Food Res 3:197–208.
Lopez-Lazaro M, Martin-Cordero C, Ayuso MJ. 1999. Flavonoids of Retama sphaerocarpa. Planta Med 65:777–778.
Luo Z, Meksuriyen D, Erdelmeier CAJ, Fong HHS, Cordell GA. 1988. Larreantin, a novel, cytotoxic naphthoquinone from Larrea tridentata. J Org Chem 53:2183–2185.
Luther H, Jordanov D, Ludwig P, Schewe T. 1991. Inhibition of rabbit erythroid 15-lipoxygenase and sheep vesicular gland prostaglandin H synthase by gallic esters. Pharmazie 46:134–136.
Mabry TJ, Hunkiker JH, DiFeo DR. 1977. Creosote Bush: Biology and Chemistry of Larrea in New World Deserts. Stroudsburg, PA: Dowden, Hutchinson & Ross.
Madrigal-Bujaidar E, Diaz Barriga S, Cassani M, Molina D, Ponce G. 1998. In vivo and in vitro induction of sister-chromatid exchanges by nordihydroguaiaretic acid. Mutat Res 412:139–144.
Maldonado Z, Hoeneisen M, Silva M. 1993. Constituents of Haplopappus bezanillanus and H. hirtellus. Bol Soc Chil Quim 38: 43–48.
McGuffin M, Hobbs C, Upton R, Goldberg A, eds. 1997. American Herbal Products Association’s Botanical Safety Handbook. Boca Raton, FL: CRC Press.
Micromedex (Micromedex Thomson Healthcare). 2002. Chaparral. Online. Available at http://www.cooperfitness.com/content/Support/Pharmaceutical/AltMed/Detail.asp?DocID=1489. Accessed August 26, 2002.
Mikuni M, Yoshida M, Hellberg P, Peterson CA, Edwin SS, Brännström M, Peterson CM. 1998. The lipoxygenase inhibitor, nordihydroguaiaretic acid, inhibits ovulation and reduces leukotriene and prostaglandin levels in the rat ovary. Biol Reprod 58:1211–1216.
Miles DH, Chittawong V, Hedin PA, Kokpol U. 1993. Potential agrochemicals from leaves of Wedelia biflora. Phytochemistry 32:1427–1429.
Moerman DE. 1998. Native American Ethnobotany. Portland, OR: Timber Press.
Moore M, Thor H, Moore G, Nelson S, Moldeus P, Orrenius S. 1985. The toxicity of acetaminophen and N-acetyl-p-benzoquinone imine in isolated hepatocytes is associated with thiol depletion and increased cytosolic Ca2+. J Biol Chem 260:13035–13040.
Moridani MY, Galati G, O’Brien PJ. 2002. Comparative quantitative structure toxicity relationships for flavonoids evaluated in isolated rat hepatocytes and HeLa tumor cells. Chem Biol Interact 139:251–264.
Mufti NA, Shuler ML. 1996. Possible role of arachidonic acid in stress-induced cytochrome P450IA1 activity. Biotechnol Prog 12:847–854.
Nagano N, Imaizumi Y, Hirano M, Watanabe M. 1996. Opening of Ca(2+)-dependent K+ channels by nordihydroguaiaretic acid in porcine coronary arterial smooth muscle cells. Jpn J Pharmacol 70:281–284.
Nakayama T. 1994. Suppression of hydroperoxide-induced cytotoxicity by polyphenols. Cancer Res 54:1991s–1993s.
Nakazato PZ, Ulreich JB, Boles JL, Patel MR, Rorie CJ, Fisher RL, Brendel K. 1998. Nephrotoxicity and hepatotoxicity of chaparral (Larrea tridentata) in rats and humans. Toxicologist 37:287. Abstract.
Njoku CJ, Hopp DC, Alali F, Asuzu IU, McLaughlin JL. 1997. Dihydroguaiaretic acid: A bioactive component of the stem bark of Pycnanthus angolensis. Planta Med 63:580–581.
Noro T, Oda Y, Miyase T, Ueno A, Fukushima S. 1983. Inhibitors of xanthine oxidase from the flowers and buds of Daphne genkwa. Chem Pharm Bull (Tokyo) 31(11):3984-3987.
Obermeyer WR, Musser SM, Betz JM, Casey RE, Pohland AE, Page SW. 1995. Chemical studies of phytoestrogens and related compounds in dietary supplements: Flax and chaparral. Proc Soc Exp Biol Med 208:6–12.
Okboy N, Yegen C, Aktan AO, Dosluoglu HH, Sav A, Yalin R, Ercan S. 1992. The effect of iloprost and NDGA in ischemia reperfusion injury in rat liver. Prostaglandins Leukot Essent Fatty Acids 47:291–295.
Palacios P, Gutkind G, Rondina RV, de Torres R, Coussio JD. 1983. Genus Baccharis. II. Antimicrobial activity of B. crispa and B. notosergila. Planta Med 49:128.
Pardini RS, Heidker JC, Fletcher DC. 1970. Inhibition of mitochondrial electron transport by nordihydroguaiaretic acid (NDGA). Biochem Pharmacol 19:2695–2699.
Pardini RS, Kim CH, Biagini R, Morris RJ, Fletcher DC. 1973. Inhibition of mitochondrial electron transport systems by norisoguaiacin. Biochem Pharmacol 22:1921–1925.
Parry EW. 1993. Cycloheximide or nordihydroguaiaretic acid protects mice against the lethal and hepatocytolytic effects of a combined challenge with D-galactosamine and bacterial endotoxin. J Comp Pathol 108:185–190.
Pavani M, Fones E, Oksenberg D, Garcia M, Hernandez C, Cordano G, Munoz S, Mancilla J, Guerrero A, Ferreira J. 1994. Inhibition of tumoral cell respiration and growth by nordihydroguaiaretic acid. Biochem Pharmacol 48:1935–1942.
Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett J, Reaven GM. 1998. Metabolic effects of masoprocol in a rodent model of non-insulin-dependent diabetes mellitus. J Investig Med 46:144A. Abstract.
Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, Brignetti D, Luo J, Khandwala A, Reaven GM. 1999. Effect of masoprocol on carbohydrate and lipid metabolism in a rat model of type II diabetes. Diabetologia 42:102–106.
Robison TW, Sevanian A, Forman HJ. 1990. Inhibition of arachidonic acid release by nordihydroguaiaretic acid and its antioxidant action in rat alveolar macrophages and Chinese hamster lung fibroblasts. Toxicol Appl Pharmacol 105:113–122.
Sakakibara M, Mabry TJ. 1975. A new 8-hydroxyflavonol from Larrea tridentata. Phytochemistry 14:2097–2098.
Sakakibara M, Timmermann BN, Nakatani N, Waldrum H, Mabry TJ. 1975. New 8-hydroxyflavonols from Larrea tridentata. Phytochemistry 14:849–851.
Sakakibara M, DiFeo D Jr, Nakatani N, Timmerman B, Mabry TJ. 1976. Flavonoid methyl ethers on the external leaf surface of Larrea tridentata and L. divaricata. Phytochemistry 15:727–731.
Sapienza PP, Ikeda GJ, Obermeyer WR. 1997. In vitro effect of chaparral on xenobiotic metabolizing enzymes in rat liver. Online, FDA 1997 Science Forum (Poster Abstract):J9. Abstract.
Schegg KM, Welch W Jr. 1984. The effect of nordihydroguaiaretic acid and related lignans on formyltetrahydrofolate synthetase and carboxylesterase. Biochim Biophys Acta 788:167–180.
Schultz JC, Floyd T. 1999. An immigrant to the Southwest, the creosote bush is a desert survivor that dominates the landscape and engineers biodiversity. Natural History 108:24–29.
Seufferlein T, Seckl MJ, Schwarz E, Beil M, Wichert G, Baust H, Luhrs H, Schmid RM, Adler G. 2002. Mechanisms of nordihydroguaiaretic acid-induced growth inhibition and apoptosis in human cancer cells. Br J Cancer 86:1188–1196.
Shad JA, Chinn CG, Brann OS. 1999. Acute hepatitis after ingestion of herbs. South Med J 92:1095–1097.
Sheikh NM, Philen RM, Love LA. 1997. Chaparral-associated hepatotoxicity. Arch Intern Med 157:913–919.
Shen YC, Chen CY, Chen YJ, Kuo YH, Chie CTLYM. 1997. Bioactive lignans and taxoids from the roots of formosan Taxus mairei. Chin Pharm J (Taipei) 49:285–296.
Shi L, Pardini RS. 1995. Effect of NDGA on beef heart mitochondria and EMT6 mouse mammary carcinoma cells. Res Commun Mol Pathol Pharmacol 90:235–254.
Slattery JT, Wilson JM, Kalhorn TF, Nelson SD. 1987. Dose-dependent pharmacokinetics of acetaminophen: Evidence of glutathione depletion in humans. Clin Pharmacol Ther 41:413–418.
Smart CR, Hogle HH, Vogel H, Broom AD, Bartholomew D. 1970. Clinical experience with nordihydroguaiaretic acid—“Chaparrel tea” in the treatment of cancer. Rocky Mt Med J 67:39–43.
Smith AY, Feddersen RM, Gardner KD Jr, Davis CJ Jr. 1994. Cystic renal cell carcinoma and acquired renal cystic disease associated with consumption of chaparral tea: A case report. J Urol 152:2089–2091.
Smith BC, Desmond PV. 1993. Acute hepatitis induced by ingestion of the herbal medication chaparral. Aust NZ J Med 23:526.
Stetler-Stevenson WG, Krutzsch HC, Liotta LA. 1992. TIMP-2: Identification and characterization of a new member of the metalloproteinase inhibitor family. Matrix Suppl 1:299–306.
Su W, Tseng LL, Lin MC, Chang HJ, Lee KC, Chou KJ, Lo YK, Cheng JS, Chang HT, Wang JL, Liu CP, Chen WC, Jan CR. 2002. Effect of nordihydroguaiaretic acid on intracellular Ca2+ concentrations in C6 glioma cells. Neurochem Int 40:249–254.
Tang DG, Honn KV. 1997. Apoptosis of W256 carcinosarcoma cells of the monocytoid origin induced by NDGA involves lipid peroxidation and depletion of GSH: Role of 12-lipoxygenase in regulating tumor cell survival. J Cell Physiol 172:155–170.
Telford IR, Woodruff CS, Linford RH. 1962. Fetal resorption in the rat as influenced by certain antioxidants. Am J Anat 110:29–36.
Ulreich JB, Okafor JM, Chavez RC, Le T, Maveddat M, Boles JL, Zavala JL, Stringer SK, Young CJ, Nakazato PZ. 1997. Hepatotoxicity of chaparral in Fischer 344 rats. Toxicologist 36:228. Abstract.
Valentine JL, McKenzie L, Kovarik F. 1984. Gas chromatographic determination of nordihydroguaiaretic acid in Larrea divaricata. Anal Lett 17:1617–1626.
Van der Merwe MJ, Jenkins K, Theron E, van der Walt BJ. 1993. Interaction of the dicatechols rooperol and nordihydroguaiaretic acid with oxidative systems in the human blood. A structure-activity relationship. Biochem Pharmacol 45:303–311.
Vedovato M, Salvatorelli G, Daniele C, De Paoli Vitali E. 1994. Changes in human epithelial kidney cells induced by nordihydroguaiaretic acid in vitro. Cytobios 78:19–22.
von Ardenne M, Chaplain R, Reitnauer P. 1969. In vitro measurements of damage to cancer with a good substrate supply, following combined attack by NDGA and 40’C hypothermia, with and without irradiation at a dose of 1000 r. Arch Geschwulstforsch 34:1–12.
Wagenknecht B, Schulz JB, Gulbins E, Weller M. 1998. Crm-A, bcl-2 and NDGA inhibit CD95L-induced apoptosis of malignant glioma cells at the level of caspase 8 processing. Cell Death Differ 5:894–900.
Waller CW, Gisvold O. 1945. A phytochemical investigation of Larrea divaricata Cav. J Am Pharm Assoc Sci Ed 34:78–81.
Weidenborner M, Hindorf H, Jha HC, Tsotsonos P, Egge H. 1989. Antifungal activity of isoflavonoids against storage fungi of the genus Aspergillus. Phytochemistry 28:3317–3319.
Whitman S, Gezginci M, Timmermann BN, Holman TR. 2002. Structure-activity relationship studies of nordihydroguaiaretic acid inhibitors toward soybean, 12-human, and 15-human lipoxygenase. J Med Chem 45:2659–2661.
Xue HZ, Lu ZZ, Konno C, Soejarto DD, Cordell GA, Fong HHS, Hodgson W. 1988. 3-Beta-(3,4-dihydroxycinnamoyl)-erythrodiol and 3-beta-(4-hydroxycinnamoyl)-erythrodiol from Larrea tridentata. Phytochemistry 27:233–235.
Yamamoto S, Nakadate T, Nakaki T, Ishii K, Kato R. 1982. Prevention of glucose-induced insulin secretion by lipoxygenase inhibitor. Eur J Pharmacol 78:225–227.
Yamamura H, Sakamoto K, Ohya S, Muraki K, Imaizumi Y. 2002. Mechanisms underlying the activation of large conductance Ca2+-activated K+ channels by nordihydroguaiaretic acid. Jpn J Pharmacol 89:53–63.
Zamora JM. 1984. Cytotoxic, Antimicrobial and Phytochemical Properties of Larrea tridentata Cav. Auburn, AL: Auburn University. Dissertation.
Zamora JM, Mora EC, Parish EJ. 1992. A comparison of the cytotoxicity of nordihydroguaiaretic acid and its derivatives. J Tenn Acad Sci 67:77–80.
TABLE A Chaparral: Individual Components
|
Substance |
Structure |
Safety Issues |
|
Lignans, nordihydroguaiaretic acid (NDGA), and other substituted guaiaretic acid derivatives |
||
|
NDGA (Duisberg et al., 1949; Waller and Gisvold, 1945) Present in all parts of Larrea tridentata, including leaves, stems and twigs at 5–15% of the dry leaf weight (Mabry et al., 1977) |
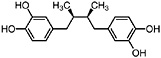 |
Animal studies: see Table E for safety issues from animal studies with NDGA In vitro study: see Table F-2 for safety issues from in vitro studies with NDGA |
|
Dihydroguaiaretic acid (Obermeyer et al., 1995) Partially demethylated dihydroguaiaretic acid is also present (Gisvold and Thaker, 1974) |
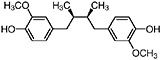 |
In vitro study: weak cytotoxic activity (IC50 1–3 μg/mL) (Njoku et al., 1997) In vitro study: weak cytotoxic activity (10 μg/mL) and (Gisvold and Thaker, 1974) |
|
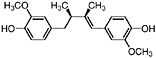
Guaiaretic acid (Obermeyer et al., 1995) |
 |
No data suggestive of toxicity are available |
|
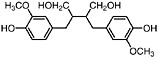
Secoisolariciresinol (Obermeyer et al., 1995) Present in the stems of L. tridentata (Konno et al., 1990) |
 |
In vitro study: weak cytotoxic activity (IC50 0.6–8.3 μg/mL) (Shen et al., 1997); see Table F-2 |
|
Lignans, furanoid |
||
|
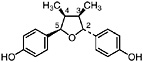
Larreatricin (Konno et al., 1987) Present in the stems of L. tridentata (Konno et al., 1990) in trace amounts (0.001% of dry leaf weight) |
 |
No data suggestive of toxicity are available |
|
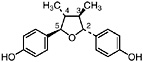
4-epi-Larreatricin (Konno et al., 1987) Present in the leaves, twigs and stems of L. tridentata (Konno et al., 1990) in trace amounts (up to 0.0003% of dry leaf weight) |
 |
No data suggestive of toxicity are available |
|
Substance |
Structure |
Safety Issues |
|
3′′-Hydroxy-4-epilarreatricin (Konno et al., 1987) Present in the leaves and twigs of L. tridentata (Konno et al., 1990) in trace amounts (0.0008% of dry leaf weight) |
 |
No data suggestive of toxicity are available |
|
3′, 3′′-Dimethoxylarreatricin (Konno et al., 1987) Present in the stems of L. tridentata (Konno et al., 1990) in trace amounts (0.0002% of dry leaf weight) |
 |
No data suggestive of toxicity are available |
|
3,4-Dehydrolarreatricin (Konno et al., 1987) Present in the stems of L. tridentata (Konno et al., 1990) in trace amounts (up to 0.0002% of dry leaf weight) |
 |
No data suggestive of toxicity are available |
|
Larreatridenticin (Konno et al., 1987) Present in the stems of L. tridentata in trace amounts (0.00008% of dry leaf weight) |
 |
No data suggestive of toxicity are available (Konno et al., 1987) |
|
Lignans, 1-aryl tetralin |
||
|
All of the 1-aryl tetralin lignans are structurally related to podophyllotoxins (Damayanthi and Lown, 1998) |
||
|
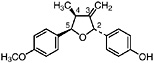
Isoguaiacin (Konno et al., 1987) Present in the stems of L. tridentata (Konno et al., 1990) in trace amounts (0.00002% of dry leaf weight) |
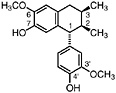 |
No data suggestive of toxicity are available |
|
Substance |
Structure |
Safety Issues |
|
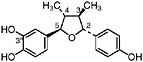
6-O-Demethylisoguaiacin Norisoguaiacin, R1=OCH3, R2=OH (Gisvold and Thaker, 1974) Present in the stems of L. tridentata (Konno et al., 1990) at 0.003% of the dry leaf weight |
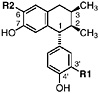 |
In vitro study: inhibited electron transport (0.2 μM, rat liver mitochondria) (63 μM, bovine heart mitochondria) (Pardini et al., 1973) In vitro study: weak cytotoxicity (100 μg/mL) (Gisvold and Thaker, 1974) In vitro study: inhibited carboxylesterase (30 μM) and formyltetrahydrofolate synthetase (350 μM) (Schegg and Welch, 1984) |
|
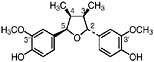
3′ Demethoxyisoguaiacin R1=H, R2=OCH3 (Gisvold and Thaker, 1974) 3′ Demethoxy-6-O-demethylisoguaiacin 3′ Demethoxynorisoguaiacin, nor-3′-demethoxyisoguaiacin, NDI, R1=H, R2=OH (Fronczek et al., 1987) Present in the twigs (Konno et al., 1990) and leaves (Fronczek et al., 1987) of L. tridentata (Konno et al., 1989) in trace amounts (up to 0.005%) |
|
No data suggestive of toxicity are available No data suggestive of hepatotoxicity are available |
|
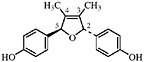
6-3′-Di-O-demethylisoguaiacin 3′-Hydroxynorisoguaiacin, R1=OH, R2=OH (Konno et al., 1987) Present in leaves and twigs of L. tridentata (Konno et al., 1989) at 0.004% of the dry leaf weight |
|
No data suggestive of toxicity are available |
|
Flavonoids (present as aglycones and glycosides) |
||
|
Flavonoids are widespread in the human diet and no toxicities have been associated with them |
||
|
Apigenin (Sakakibara et al., 1976) |
 |
In vitro study: weak cytotoxic activity (0.8 mg/disc) (Palacios et al., 1983) |
|
Substance |
Structure |
Safety Issues |
|
|
|
In vitro study: weak cytotoxic activity (25 μg/mL) (Chae et al., 1992) In vitro study: inhibitor of CYP 1A1 (IC50 16 μg/mL) (Chae et al., 1992) |
|
Apigenin 7-methyl ether Genkwanin (Sakakibara et al., 1976) |
 |
In vitro study: weak cytotoxicity (0.4 mg/disc) (Palacios et al., 1983) In vitro study: inhibitor of MAO (Noro et al., 1983) and CYP 1A1 (IC50 > 50 μg/mL) (Chae et al., 1992) |
|
Gossypetin 3,7-dimethyl ether R1=CH3, R2=CH3, R3=H, R4=H (Sakakibara and Mabry, 1975) Present in leaves of L. tridentata (Sakakibara et al., 1976) |
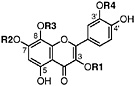 |
No data suggestive of toxicity are available |
|
Gossypetin 3,7,3′-trimethylether 5,8,4′-Trihydroxy-3,7,3′-trimethoxyflavone, R1=CH3, R2=CH3, R3=H, R4=CH3(Sakakibara et al., 1975) Present in leaves of L. tridentata (Sakakibara et al., 1976) |
|
No data suggestive of toxicity are available |
|
Gossypetin 3,7,8,3′-tetramethyl ether Ternatin, R1=CH3, R2=CH3, R3=CH3, R4=CH3 Present in leaves of L. tridentata (Bernhard and Thiele, 1981) |
|
No data suggestive of toxicity are available |
|
Substance |
Structure |
Safety Issues |
|
Herbacetin 3,7-dimethyl ether 8-Hydroxy-kaempferol, R1=CH3, R2=CH3, R3=H, R4=H (Sakakibara et al., 1975) Present in leaves of L. tridentata (Sakakibara et al., 1976) |
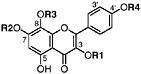 |
No data suggestive of toxicity are available |
|
Herbacetin 3,7,8-trimethyl ether R1=CH3, R2=CH3, R3=CH3, R4=H Present in leaves of L. tridentata (Bernhard and Thiele, 1981) |
|
No data suggestive of toxicity are available |
|
Herbacetin 3,7,4′-trimethyl ether R1=CH3, R2=CH3, R3=H, R4=CH3 (Fernandez et al., 1979) |
|
No data suggestive of toxicity are available |
|
Kaempferol (Chirikdjian, 1973; Sakakibara et al., 1976) |
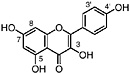 |
In vitro study: weak cytotoxic activity (18 μg/mL) (Chae et al., 1992) In vitro study: inhibitor of CYP 1A1 (IC50 14 μg/mL) (Chae et al., 1992) |
|
Kaempferol 3-methyl ether Isokaempferide, R1=CH3, R2=H, R3=H (Chirikdjian, 1973; Sakakibara et al., 1976) |
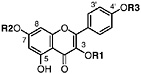 |
In vitro study: cytotoxicity (IC50 5 μM (Banskota et al., 2000) |
|
Kaempferol 7-methyl ether Rhamnocitrin, R1=H, R2=CH3, R3=H (Sakakibara et al., 1976) |
|
No data suggestive of toxicity are available |
|
Kaempferol 3,7-dimethyl ether Kumatakenin, R1=CH3, R2=CH3, R3=H (Sakakibara et al., 1976) |
|
In vitro study: weak cytotoxicity (300 μg/agar plate) (Afifi et al., 1991) |
|
Substance |
Structure |
Safety Issues |
|
Kaempferol 3,4′-dimethyl ether R1=CH3, R2=H, R3=CH3 (Mabry et al., 1977) |
|
No data suggestive of toxicity are available |
|
Luteolin 3′-methyl ether Chrysoeriol, R1=H, R2=CH3 (Sakakibara et al., 1976) Luteolin 7,3′-dimethyl ether Velutin, R1=CH3, R2=CH3 (Sakakibara et al., 1976) |
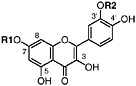 |
No data relative to safety are available No data suggestive of toxicity are available |
|
Quercetin (Chirikdjian, 1973) Some investigators have not been able to identify quercetin as a component of L. tridentata (Mabry et al., 1977) |
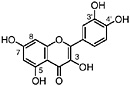 |
No data suggestive of hepatotoxicity are available |
|
Quercetin 3-methyl ether R1=CH3, R2=H, R3=H, R4=H (Chirikdjian, 1973) Some investigators have not been able to identify quercetin 3-methyl ether as a component of L. tridentata (Mabry et al., 1977) |
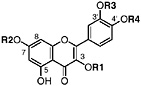 |
No data suggestive of toxicity are available |
|
Quercetin 3′-methyl ether Isorhamnetin, R1=H, R2=H, R3=CH3, R4=H (Chirikdjian, 1973; Sakakibara et al., 1976) |
|
No data suggestive of toxicity are available |
|
Quercetin 3,7-dimethyl ether Kumatakenine, R1=CH3, R2=CH3, R3=H, R4=H (Chirikdjian, 1974; Sakakibara et al., 1976) |
|
No data suggestive of toxicity are available |
|
Quercetin 3,3′-dimethyl ether R1=CH3, R2=H, R3=CH3, R4=H (Sakakibara et al., 1976) |
|
No data suggestive of toxicity are available |
|
Substance |
Structure |
Safety Issues |
|
Quercetin 7,3′-dimethyl ether Rhamnazin, R1=H, R2=CH3, R3=CH3, R4=H (Sakakibara et al., 1976) |
|
In vitro study: weak cytotoxicity (50% growth inhibition at 4–30 μg/mL) (Lopez-Lazaro et al., 1999; Miles et al., 1993) |
|
Quercetin 3,7,3′-trimethyl ether R1=CH3, R2=CH3, R3=CH3, R4=H (Sakakibara et al., 1976) |
|
No data suggestive of toxicity are available |
|
Quercetin 3,7,4′-trimethyl ether Ayanin, R1=CH3, R2=CH3, R3=H, R4=CH3(Gnabre et al., 1995) |
|
No data suggestive of toxicity are available |
|
Quercetin 7,3′,4′-trimethyl ether R1=H, R2=CH3, R3=CH3, R4=CH3 (Korn and Horn, 1990) |
|
No data suggestive of toxicity are available |
|
Quercetin 3,7,3′,4′tetramethyl ether Retusine, R1=CH3, R2=CH3, R3=CH3, R4=CH3 (Sakakibara et al., 1976) |
|
In vitro study: weak cytotoxicity (50–200 μM) (Weidenborner et al., 1989) |
|
Dihydromyricetin 3′,5′dimethyl ether Dihydrosyringetin (Sakakibara et al., 1976) |
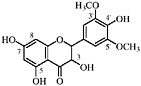 |
No data suggestive of toxicity are available |
|
5,4′-Dihydroxy-3,7,3′trimethoxyflavone (Chirikdjian, 1974) |
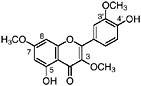 |
In vitro study: cytotoxicity (IC50 5 μM) (Banskota et al., 2000; Maldonado et al., 1993) |
|
Substance |
Structure |
Safety Issues |
|
Triterpenes (present as aglycones and glycosides, which are also called saponins) |
||
|
3β-(4-Hydroxycinnamoyl)-erythrodiol (Konno et al., 1987) Present in the stems of L. tridentata (Xue et al., 1988) |
 |
No data suggestive of toxicity are available |
|
3β-(3,4-Dihydroxycinnamoyl)-erythrodiol (Konno et al., 1987) Present in the stems of L. tridentata (Xue et al., 1988) |
 |
No data suggestive of toxicity are available |
|
Triterpenes: sapogenins |
||
|
Larreagenin A Present in the leaves of L. tridentata (Habermehl and Moeller, 1974) |
 |
No data suggestive of toxicity are available |
|
Larreic acid Present in the leaves of L. tridentata (Habermehl and Moeller, 1974) |
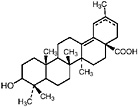 |
No data suggestive of toxicity are available |
|
Other components |
||
|
Volatile oils (0.1–0.2% of fresh leaf weight by steam distillation) (Mabry et al., 1977) |
||
|
Monoterpene hydrocarbons: camphene, ∆-3-carene, limonene, α-pinene; traces of α-fenchene, β-ocimene, β-pinene (Bohnstedt, 1979; Mabry et al., 1977) |
||
|
Oxygenated monoterpene: borneol, bornyl acetate, camphor, p-cymene, linalool; traces of copaene, 2-rossalene (Bohnstedt, 1979; Mabry et al., 1977) |
||
|
Sesquiterpene hydrocarbons: α-bergamotene, calamenene, cuparene, α-curcumene, edulane, β-santalene (Bohnstedt, 1979) |
||
|
Oxygenated sesquiterpenes: α-agarofuran, β-eudesmol, γ-eudesmol, farnesol (Bohnstedt, 1979) |
||
|
Aromatic hydrocarbons: benzaldehyde, benzyl acetate, benzyl butanate, 1,2-dihydro-1,5,8-trimethyl-naphthalene, ethyl benzoate, o-methyl anisate, methyl naphthalene; traces of acetophenone (Bohnstedt, 1979) |
||
TABLE B Chaparral: Summary of Adverse Effects in a Clinical Trial
|
Study Design |
Subjects |
Parameters Monitored |
Supplement |
Adverse Effects and Related Findings |
|
Smart et al., 1970a 36 subjects in chaparral tea group; 23 subjects in NDGA group; no control group |
Condition: advanced incurable malignancy Age: unknown Male/Female: unknown Concurrent medications: at least one subject continued treatment with 5-fluorouracil Pre-existing medical conditions: none mentioned beyond malignancy |
Parameters monitored: Regression vs. nonregression Hemoglobin, white blood cell count, BUN, SGOT, and urinalysis (before trial and at 2–4 wk intervals in the majority of subjects) Subjective improvement Duration was not stated but most subjects (≥ 45 out of the total 59 subjects) were treated for ≥ 4 wk |
Chaparral tea group: Number of subjects: 36 Dose: chaparral tea, 16–24 oz/d Route: oral Frequency: 1×/d Duration: unknown NDGA group: Number of subjects: 23 Dose: NDGA, 250–3,000 mg/d Route: oral Frequency: 1×/d Duration: unknown |
Combined groups: Adverse effects: fever (1 subject) In a significant number of subjects, there appeared to be stimulation of tumor growth No clinical laboratory abnormalities were observed that could be attributed to chaparral tea or NDGA Total of 14 subjects (25%) withdrew before 4 wk and were not evaluated |
|
NOTE: NDGA = nordihydroguaiaretic acid, BUN = blood urea nitrogen, SGOT = serum glutamic-oxaloacetic transaminase. a This study was evaluated as published with additional information and quotes from one of the authors published by the American Cancer Society (ACS, 1970). |
||||
TABLE C-1 Chaparral: Summary of Clinical Case Reports
|
Category/Case/Referencea |
Number of Subjectsb |
Date |
|
Case #1 Sheikh et al., 1997 |
1* |
|
|
Case #2 Clark and Reed, 1992; Sheikh et al., 1997 |
1* |
7/92 |
|
Case #3 Sheikh et al., 1997 |
1* |
10/92 |
|
Case #4 Sheikh et al., 1997 |
1* |
1/93 |
|
Case #5 Clark and Reed, 1992; Sheikh et al., 1997 |
1* |
7/92 |
|
Case #6 Sheikh et al., 1997 |
1* |
3/93 |
|
Subject/Supplement |
Adverse Effectsc and Related Findings |
|
38-year-old female (Sheikh’s patient no. 10) Chaparral capsules, 400 mg/d taken as a nutritional supplement for many Subject history: drug abuse, previous alcohol use and previous hepatitis C infection |
Diagnosis: chaparral-induced toxic liver damage or “chaparral may have potentiated or exacerbated the underlying liver disease in this individual” (Sheikh et al., 1997) Subject had elevated aminotransferase levels and joint stiffness of the right hand; eventually required a liver transplant |
|
41-year-old female (Clark’s patient no. 2 and Sheikh’s patient no. 2) Chaparral (259 mg/d as tablets for 11 wk) taken for a skin condition; estimated cumulative dose of 68–96 g (Sheikh et al., 1997) Concurrent drugs or herbals: none |
Diagnosis: chaparral-induced toxic liver damage (Clark and Reed, 1992; Sheikh et al., 1997) Subject presented with jaundice and abdominal pain of 4 wk duration; discontinued chaparral use and returned to normal within several months |
|
44-year-old female (Sheikh’s patient no. 5) Chaparral capsules, 2,400 mg/d for 10 d then 800 mg/d for 10 d, taken for allergies and fatigue; estimated cumulative dose of 24.8 g (per Sheikh et al., 1997; actual estimated cumulative dose would be 32 g) |
Diagnosis: chaparral-induced toxic liver damage (Sheikh et al., 1997) Subject had fatigue, jaundice, dark urine, nausea, abdominal pain, and diarrhea; recovered within 7 wk |
|
60-year-old female (Sheikh’s patient no. 6) Single-ingredient chaparral product |
Diagnosis: chaparral-induced toxic liver damage (Sheikh et al., 1997) Subject had symptoms of cholecystitis and elevated aminotransferase levels; recovered within 7 wk |
|
42-year-old male (Clark’s patient no. 1 and Sheikh’s patient no. 1) Chaparral (3× 500 mg capsules/d for 6 wk); supplement promoted as “free radical scavenger”; estimated cumulative dose of 96 g (Sheikh et al., 1997) |
Diagnosis: hepatic dysfunction secondary to chaparral ingestion (Clark and Reed, 1992; Sheikh et al., 1997) Subject presented with scleral icterus and diffuse jaundice; returned to normal within 1 mo; history of alcohol use |
|
25-year-old male (Sheikh’s patient no. 8) Chaparral, 3,840 mg/d for 2–3 wk, then 5,760 mg/d for 10 wk, capsules; taken for asthma; estimated cumulative dose of 70–114 g (Sheikh et al., 1997) |
Diagnosis: chaparral-induced toxic liver damage (Sheikh et al., 1997) Subject had fatigue, jaundice, and dark urine; recovered within 2 wk |
|
Subject/Supplement |
Adverse Effectsc and Related Findings |
|
57-year-old female (Sheikh’s patient no. 7) Chaparral, 480 mg/d for 8 wk, capsule; estimated cumulative dose of 24 g (Sheikh et al., 1997) Patient history: past use of conjugated estrogen (possible hepatotoxin with persistent effects but deemed unlikely in this case) |
Diagnosis: chaparral-induced toxic liver damage confirmed by liver biopsy (Sheikh et al., 1997) Subject had fatigue, jaundice, abdominal pain, light stools, and pruritus; recovered within 1 wk |
|
71-year-old male (Batchelor’s patient no. 1) Chaparral leaf as tablets Concomitant medication: none Patient history: concomitant and long-term alcohol use (14 oz wine/d) Subject-elected rechallenge: symptoms recurred upon restarting chaparral without alcohol |
Diagnosis: jaundice and possible toxic liver damage (Batchelor et al., 1995) Subject presented with a 2-wk history of flu-like illness, ascites, and jaundice Symptoms resolved within 2 mo after cessation of chaparral and alcohol intake; restarted chaparral without alcohol and within 1 mo symptoms of liver damage recurred Subject was readmitted with jaundice, ascites, scleral icterus, and nausea; liver biopsy showed diffuse necrosis with inflammation, portal tract expansion, mild cholestasis, and mild fibrous septation; after 3 mo, symptoms again resolved and a repeat biopsy indicated marked improvement |
|
33-year-old female “Chaparral leaf” (15 tablets/d for 3–4 mo, then various amounts), symptoms waxed and waned with variation in dose |
Diagnosis: drug-induced hepatotoxicity due to ingestion of chaparral leaf (Katz and Saibil, 1990) Subject presented with jaundice, ascites, abdominal pain, fatigue, scleral icterus, anorexia, and pedal edema due to subacute hepatic necrosis; responded to diuretic therapy Subject workup was negative except for use of chaparral; the most common causes of hepatitis were ruled out, including an autoimmune response Subject recovered with cessation of chaparral intake |
|
43-year-old female (Batchelor’s patient no. 2) Chaparral leaf (≥ 3 tablets/d) for 6 wk, taken for relief from chronic tension headaches |
Diagnosis: subacute chaparral-induced liver damage (Batchelor et al., 1995) Subject presented with a 2-wk history of flu-like illness and jaundice; recovered within 4 mo |
|
Category/Case/Referencea |
Number of Subjectsb |
Date |
|
Case #11 Sheikh et al., 1997 |
1* |
5/93 |
|
Case #12 Sheikh et al., 1997 |
1* |
1/93 |
|
Case #13 Sheikh et al., 1997 |
1* |
|
|
Case #14 Sheikh et al., 1997 |
1* |
|
|
Case #15 Sheikh et al., 1997 |
1* |
|
|
a The numbering of cases is consistent with that used in the full prototype monograph for chaparral, which included a Case #16 and a Case #24. These two cases are not included here because they do not address hepatotoxicity, the subject of the focused monograph. b Many of the subjects included in Tables C-1 and C-2 as case reports are also included in Table D as Special Nutrition Adverse Event Monitoring System reports and are identified with an asterisk. Some cases that do not have an asterisk may also be included in more than one table. However, verification of duplicate cases was not possible given the available data. |
||
|
Subject/Supplement |
Adverse Effectsc and Related Findings |
|
Concomitant medication: aspirin Patient history: infrequent alcohol use |
|
|
36-year-old female (Sheikh’s patient no. 16) Chaparral as capsules, taken for 1 y; taken to gain energy Concurrent medication: naproxen sodium and ketorolac trimethamine |
Diagnosis: possible subacute liver damage (Sheikh et al., 1997) Subject experienced nausea and abdominal pain with elevated bilirubin, but other clinical labs were within normal limits; recovered within 3 wk |
|
63-year-old male (Sheikh’s patient no. 14) Chaparral taken for 8 wk for degenerative arthritis of the knees Patient history: alcohol use |
Diagnosis: subacute liver damage (Sheikh et al., 1997) Subject was asymptomatic, with transient (< 1 wk) elevated bilirubin and aminotransferase levels |
|
57-year-old female (Sheikh’s patient no. 11) Chaparral taken for 4 wk |
Diagnosis: hepatotoxicity (Sheikh et al., 1997) Subject had jaundice and abnormal liver enzyme tests for 1 wk but refused follow-up; information is incomplete |
|
54-year-old female (Sheikh’s patient no. 12) Chaparral, 1,600 mg/day for 2.8 wk taken as a “cleanser”; estimated cumulative dose of 38 g (Sheikh et al., 1997) |
Diagnosis: consistent with chaparral-associated liver damage (Sheikh et al., 1997) Subject had jaundice and abdominal pain; recovered within 6 wk; information is incomplete |
|
39-year-old female (Sheikh’s patient no. 13) Chaparral tea, 4 bags of single-ingredient chaparral tea daily for approximately 1.5 y (78 wk) taken for weight loss |
Diagnosis: consistent with chaparral-associated liver damage (Sheikh et al., 1997) Subject had jaundice and abdominal pain; recovered within 1 wk; information is incomplete |
|
c The clinical cases are arranged in decreasing order of apparent severity of the adverse effects. |
|
|
Subject/Supplement |
Adverse Effectsc and Related Findings |
|
59/60-year-old-female (Gordon’s patient and Sheikh’s patient no. 4 ) Chaparral (1.8 g/d as capsules) over previous 10–12 mo; 3 wk before admission, increased capsules to 3–5 g/d; estimated cumulative dose of 108 g (Sheikh et al., 1997) Concurrent medications: diltiazem HCl, atenolol, enteric-coated aspirin, nitroglycerin patch, and occasional acetaminophen Concurrent botanicals: garlic powder, nettle, chickweed tea |
Diagnosis: liver failure due to chaparral-induced toxic liver damage (Gordon et al., 1995; Sheikh et al., 1997) Subject presented with severe jaundice due to liver damage (including ascites, collapsed liver nodules with some regeneration, acute hepatitis, hepatocyte ballooning, portal inflammation, marked proliferation of bile ducts) Subject required a liver transplant and also experienced secondary renal failure requiring kidney transplant |
|
36-year-old female Chaparral (2 capsules/d for 8 wk prior to onset of symptoms) Patient history: hepatitis A at age 7 Concurrent herbals: Numerous, but not specified |
Diagnosis: severe acute toxic liver damage following ingestion of chaparral confirmed by liver biopsy (Smith and Desmond, 1993) Subject presented with a 5-wk history of anorexia, nausea, and malaise, and a 3-wk history of dark urine, icterus, and pruritus Subject discontinued chaparral use and recovered within 4 mo This is the only report from Australia |
|
27-year-old male Chaparral “leaf” (capsules—Arizona Natural; 1,500–2,500 mg/d for 10–12 mo), taken to “prevent illness,” label recommended 1,000 mg/d Patient history: occasional binge alcohol use in past few years; ceased smoking 4 yr earlier; denied illegal drug use; no prior transfusions Concurrent medication: none Concurrent supplement use: numerous, including Echinacea purpurea root plus another form of Echinacea (in 2 different preparations), goldenseal root, slippery elm bark (in 2 different preparations), mullein leaf, yerba santa leaf, ginger root, Capsicum annum (in 2 different preparations) |
Diagnosis: toxic liver damage confirmed by liver biopsy (Grant et al., 1998) Subject presented with nausea, vomiting, diarrhea, and abdominal pain; subject had jaundice; liver enzyme tests were elevated and continued to increase over 3 wk until admitted to hospital and chaparral intake ceased Infectious hepatitis was ruled out (tested for A, B, C, and E) Biopsy showed hepatocellular injury with necrosis and periportal inflammation Liver transplant was considered until subject began to improve 3 wk later; condition stabilized |
|
Subject/Supplement |
Adverse Effectsc and Related Findings |
|
45-year-old female (Sheikh’s patient no. 3 and probably Alderman’s patient) Chaparral, 1,440 mg/d as capsules for 2 wk then 1,920 mg/d for 8 wk; took chaparral as “general cleansing tonic”; estimated cumulative dose of 102 g (Sheikh et al., 1997) Patient history: concurrent alcohol use, obese, hypertension Concurrent medications: clonidine HCl, lovastatin (possible hepatotoxin, but unlikely in this case) Concurrent supplements: niacinamide, multivitamin tablets, magnesium, lecithin, passionflower, valerian, hops, and dandelion extract |
Diagnosis: chaparral-induced toxic liver damage confirmed by liver biopsy (Sheikh et al., 1997) (See Alderman et al., 1994) Subject presented with jaundice, fatigue, nausea, vomiting, abdominal pain, bouts of diarrhea, and constipation |
|
45-year-old female (probably Sheikh’s patient no. 3) Chaparral tablets, 160 mg/d for previous 2–3 mo, taken to “relieve the craving for alcohol” Patient history: prior alcohol abuse until 2–3 mo prior to diagnosis Concurrent prescription drugs: clonidine, also lovastatin during part of the time Concurrent herbals: passionflower, valerium, hops; dandelion extract for a short period |
Diagnosis: cholestatic hepatitis, possibly chaparral induced Alderman et al., 1994) (See Sheikh et al., 1997) Subject presented with jaundice without other symptoms; biopsy suggested cholangiolitic hepatitis due to drug Subject recovered after treatment with prednisone treatment |
|
33-year-old female (Sheikh’s patient no. 9) Chaparral, multi-ingredient product for 5–6 mo Concurrent medication: off and on use of acetaminophen (a possible hepatotoxin, but this effect is unlikely in this case) Concurrent supplements: liver oil, 1×/wk |
Diagnosis: chaparral-induced toxic liver damage (Sheikh et al., 1997) Subject presented with fatigue, jaundice, dark urine, nausea, vomiting, and abdominal pain Subject recovered within 4 wk after discontinuation of chaparral-containing product |
|
69-year-old male Chaparral, multi-ingredient herbal product (21 ingredients) (14 tablets/d, 6 wk) |
Diagnosis: possible chaparral-induced toxic liver damage (Shad et al., 1999) Subject presented with jaundice Subject recovered within 8 wk after discontinuation of the chaparral-containing product |
|
Subject/Supplement |
Adverse Effectsc and Related Findings |
|
49-year-old female (Heron’s patient no. 1) Multiherb tincture containing 8% chaparral,d ≤ 32 mL taken over 3.5 mo; proposed as a treatment for allergies Concurrent drug use: estrogens, progesterone, fluticasone nasal spray Concurrent herbals use: numerous |
Subject reported dizziness when 10 mL was taken at one time General clinical history and physical examination findings were unremarkable; clinical lab tests were virtually unchanged, including serum liver enzymes, BUN, creatinine, glucose, electrolytes, bilirubin, iron ferritin, lipoproteins, and CBC; no elevations in liver function tests that would have indicated liver damage (Heron and Yarnell, 2001) |
|
52-year-old female (Heron’s patient no. 2) Multiherb tincture containing 8% chaparral, ≤ 240 mL taken over 5 mo; proposed as a treatment for respiratory symptoms of allergies Concurrent medications: loratidine, clonazepam, zolpidem, valproic acid, thyroid hormone Concomitant botanicals use: numerous |
No elevations in liver function tests that would have indicated liver damage; changes in clinical laboratory values were likely related to concurrent medications; subject had chronic low WBC and platelet counts and low HDL cholesterol; TSH was high necessitating change in medication dose (Heron and Yarnell, 2001) |
|
53-year-old male (Heron’s patient no. 3) Multiherb tincture containing 7% chaparral, ≤ 34 mL taken over 40 d; proposed as treatment for painful axillary lymphadenopathy, which was later diagnosed as malignant melanoma, 5 mL, 1×/d Recurrence: 5 combined mL tincture, 3×/d plus topical chaparral in castor oil |
Clinical lab tests included serum liver enzymes, BUN, creatinine, glucose, electrolytes, bilirubin, iron ferritin, lipoproteins, and CBC No elevations in blood levels of hepatic enzymes that would indicate liver damage Subject’s clinical laboratory values did change in a manner consistent with the eventual diagnosis of malignant melanoma; the disease had metastasized (Heron and Yarnell, 2001) |
|
51-year-old female (Heron’s patient no. 4) Multiherb tinctures containing 10% chaparral, 5 mL, 3×/d, ≤ 138 mL taken over 3–4 mo; proposed as aid in weight-loss program |
General clinical history and physical examination findings were unremarkable; clinical lab tests were virtually unchanged, including serum liver enzymes, BUN, creatinine, glucose, electrolytes, bilirubin, iron ferritin, lipoproteins, and CBC |
|
Category/Case/Referencea |
Number of Subjectsb |
Date |
|
Case #29 Heron and Yarnell, 2001 |
1 |
4/97 |
|
Case Series A Heron and Yarnell, 2001 |
8 |
|
|
NOTE: Numbering of cases is continued from Table C-1. BUN = blood urea nitrogen, CBC = complete blood cell count, WBC = white blood cell count, HDL = high-density lipoprotein, TSH = thyroid-stimulating hormone. a The numbering of cases is consistent with that used in the full prototype monograph for chaparral, which included a Case #16 and a Case #24. These two cases are not included here because they do not address hepatotoxicity, the subject of the focused monograph. b Many of the subjects included in Tables C-1 and C-2 as case reports are also included in Table D as Special Nutrition Adverse Event Monitoring System reports and are identified with an asterisk. It was apparent that Case #16 was reported in two publications. Cases #18 and #19 appear to be the same subject, but this could not be confirmed based on the information reported. Some cases that do not have an asterisk may also be included in more than one table. However, verification of duplicate cases was not possible given the available data. |
||
|
Subject/Supplement |
Adverse Effectsc and Related Findings |
|
Concomitant use of botanicals: numerous |
No elevations in liver function tests that would have indicated liver damage and (Heron and Yarnell, 2001) |
|
Female (Heron’s patient no. 5) Multiherb tincture containing 10% chaparral, ≤ 215 mL taken intermittently over 14 mo; proposed as treatment for recurrent dental infections |
No elevations in liver function tests that would have indicated liver damage and (Heron and Yarnell, 2001) |
|
Multiherb tincture containing chaparral, ≤ 30 mL ingested |
No elevations in liver function tests that would have indicated liver damage and Heron and Yarnell, 2001) |
|
c The clinical cases are arranged in decreasing order of apparent severity of the adverse effects. d In the initial extraction, fresh (not dried) leaves and flowers of Larrea tridentata were lightly ground in ethanol:water (90:10) at 1:2.5 (w/v). (Heron and Yarnell, 2001). e One other case report involving cancer exists (Smith et al., 1994), but it was omitted from this table for the following reasons. The cause and effect relationship between the subject’s intake of chaparral and the development of cancer was not well documented. The subject (a 56-year-old female) used chaparral tea (3–4 cups/d) for 3 mo during the 1.5-y period prior to the diagnosis of cystic renal cell carcinoma. The subject also used taheebo tea (5–6 cups/d) for 6 mo almost 20 y earlier. Taheebo tea is reported to contain quinones. The date of the onset of the malignancy is unknown. In this report the correlation between the subject’s cancer and the consumption of chaparral tea seems to have been made on the basis of the known effects of nordihydroguaiaretic acid (the major lignan in chaparral) in causing multiple renal cysts in rats. Thus there is no evidence that the subject’s renal cancer was the result of consumption of chaparral. |
|
TABLE D Chaparral: Summary of Adverse Event Reports from the Special Nutritionals Adverse Event Monitoring System (SN/AEMS)a
TABLE E Nordihydroguaiaretic Acid (NDGA): Summary of Animal Studies
|
Species/Study Design |
Results and Conclusions |
||
|
Acute toxicity studies, gavage or oral administration |
|||
|
Toxicity Data |
Species |
LD50 |
Route |
|
|
Rat |
5.5 g/kg |
Gavage (Lehman et al., 1951) |
|
|
Mouse |
4 g/kg |
Gavage (Lehman et al., 1951) |
|
|
Mouse |
0.1–0.8 g/kg |
i.p. (Fujii et al., 1970; Kozubik et al., 1993; Madrigal-Bujaidar et al., 1998) |
|
|
Guinea pig 0.8 g/kg |
Gavage (Lehman et al., 1951) |
|
|
Rats (male, S/D), NDGA (single dose at 25 or 50 mg/kg body weight, gavage), ± indomethacin mucosa (20 mg/kg body weight, gavage), animals were sacrificed 4 hr later |
NDGA enhanced indomethacin-induced mast cell degranulation in gastric NDGA increased prostanoid activity in gastric glandular mucosa With NDGA pretreatment, indomethacin-induced lesions in the gastric mucosa were more severe; this is a possible cytotoxic effect (Cho and Ogle, 1987) |
||
|
Acute toxicity studies, other routes of administration |
|||
|
Rats (Wistar Albino), NDGA (1 dose at 10 μg/kg body weight, i.v.) |
In a model of ischemia reperfusion injury to the liver, rats were given NDGA 5 min before reperfusion (± iloprost, 25 μg/kg i.v., given just before warm ischemia) By histopathologic examination, liver damage was more extensive in the rats treated with NDGA compared with the control (saline-injected) animals (Okboy et al., 1992) |
||
|
Mice (female, CD1), NDGA (50 mg/kg body weight, i.v.) |
Pharmacokinetic analysis (using M4NDGA as a standard): Peak plasma concentration = 14 μg/mL Exposure (AUC) = 248 μg/mL/min Clearance = 202 mL/(min/kg) Volume of distribution = 3.4 L/kg Half-life in 1st compartment = 30 min Half-life in 2nd compartment = 135 min NDGA appears to follow a 2-compartment pharmacokinetic model (Lambert et al., 2001) |
||
|
Mice (male, CBA), NDGA (2 doses, 2-h apart, 50 mg/kg body weight, i.p.) |
At 2 wk after treatments (NDGA, galactosamine, and endotoxin), histological examination of liver showed small foci of necrosis adjacent to areas of infiltration of inflammatory cells; some of this hepatic damage was due to endotoxin administration (Parry, 1993) |
||
|
Species/Study Design |
Results and Conclusions |
|
Chronic toxicity studies, gavage or oral administration |
|
|
Rats (male, S/D), fed NDGA (2% of the diet, 0.4 g NDGA/d), up to 6 wk |
With NDGA treatment, renal lesions (interstitial and tubular, but not glomerular) were observed as follows: infiltration of inflammatory cells, tubular cell proliferation or necrosis, cyst formation. The timeline for the appearance of the renal lesions and the severity varied with the germ-free/conventional (i.e., contaminated) state of the animal. Specifically, after deconditioning (removal from germ-free environment), lesions appeared more rapidly (within 1 wk) and cyst formation was more severe than in rats housed conventionally from birth (Gardner et al., 1986) |
|
Rats (male, S/D), fed NDGA (2% of the diet), 3 wk |
With NDGA treatment loss of body weight and development of renal lesions were striking, but only when animals we also fed endotoxin-containing bacteria or were injected with endotoxin; renal lesions were not characteristic of classic endotoxin treatment Animals treated with endotoxin alone (without NDGA in the diet) did not develop renal lesions (Gardner et al., 1987) |
|
Rats (male, Wistar), fed NDGA (2% of the diet), ≤ 99 d |
Widespread lesions in the kidney: hydropic changes in tubular epithelial cells, tubular necrosis, proliferation of lysosomes in number and size, invasion by macrophages (Goodman et al., 1970) |
|
Rats (male, S/D), fed NDGA (2% of the diet), 1–24 wk |
With NDGA treatment, glomerular filtration rate was decreased as compared with controls Early during NDGA exposure, tiny polyps developed along the outer medullary segments of the collecting tubules in the kidney; this likely leads to partial nephron obstruction At 2 mo of NDGA exposure, kidneys were infiltrated by polymorphonuclear leukocytes and macrophages; basement membrane thickening, fibrosis, tubular atrophy, and eventually proximal tubular cell necrosis characterized adjacent to these infiltrate areas By 6 mo of NDGA exposure, cysts were found throughout the kidneys (Evan and Gardner, 1979) |
|
Rats (male and female, Wistar), fed NDGA (0.5% or 1% of the diet), 74 wk |
In 32 out of 33 rats given NDGA, there were cysts of the mesenteric lymph nodes at the ileocecal junction; in one rat the cystic nodes were invaded by a malignant reticulum cell sarcoma Mean body weight was lower in NDGA group vs. control group (Grice et al., 1968) |
TABLE F-1 Chaparral: Summary of In Vitro Studies
|
Substance |
Study Design |
Results and Conclusions |
|
Inhibition of enzymes |
||
|
Chaparral, methanol extract |
Enzyme assays using rat (male and female, Sprague-Dawley) liver microsomes incubated with 0.1–100 μg chaparral extract/mL |
At 10 μg/mL the chaparral extract inhibited glutathione S-transferase At 100 μg/mL the chaparral extract also inhibited aminopyrine N-demethylase (various cytochrome P450 forms), aniline hydroxylase (cytochrome P450 2E1), and UDP-glucuronyl transferase The activity of NADPH-cytochrome c reductase was increased by the larger amounts of the extract (Sapienza et al., 1997) |
TABLE F-2 Nordihydroguaiaretic Acid (NDGA): Summary of In Vitro Studies
|
Substance |
Study Design |
Results and Conclusions |
|
Apoptosis |
||
|
NDGA, 25 μM |
Cells in culture: SW 850 (human pancreatic cancer cell line) C4-I (human cervical cancer cell line) |
NDGA induced apoptosis within 3 hr reaching maximum in 12–16 hr Seufferlein et al., 2002) |
|
NDGA, 10 μM, 4–18 h |
Cells in culture: FL5.12 cells (mouse hematopoietic/ lymphocytic cell line, lipoxygenase-deficient) |
NDGA induced apoptosis and greatly increased caspase-3-like activity (Biswal et al., 2000) |
|
NDGA, IC50 5 μM |
Cells in culture: Walker-256 cells (rat epithelial carcinoma cell line, LLC-WRC 256 cells) |
NDGA induced apoptosis (Tang and Honn, 1997) |
|
NDGA, 25 μM |
Cells in culture: MTLN-3 cells (rat tumor cell line) RBL cells (rat tumor cell line) |
NDGA induced apoptosis (Tang and Honn, 1997) |
|
NDGA, 25 μM |
Cells in culture: A431 cells (human epithelial carcinoma cell line) HEH cells HL-60 cells (human myeloblastic cell line) U937 cells (human monocytes cell line) |
NDGA induced apoptosis in some human cell lines |
|
NDGA |
Cells in culture: PC-3 cells (human cell line) 1-IL cells DU145 cells (human cell line) WB35 cells (human cell line) WM983A cells (human cell line) neoT cells (human cell line) MCF-7 cells (human epithelial adenocarcinoma cell line) MCF-10A cells (human mammary epithelial cell line) HT-1080 cells (human epithelial fibrosarcoma cell line) |
NDGA (25–35 μM) did not induce apoptosis in other (human cell line) human cells (Tang and Honn, 1997) |
|
NDGA, 30 μM |
Cells in culture: LN-18 cells (human malignant glioma cell line) |
NDGA inhibited mediated by CD95 receptor apoptosis (Wagenknecht et al., 1998) |
|
Substance |
Study Design |
Results and Conclusions |
|
Cytotoxicity |
||
|
NDGA, LC50 200 μM |
Cells in culture: EMT6 cells (mouse mammary carcinoma cell line) |
NDGA caused slight cytotoxic activity in EMT6 tumor cells, likely related to depletion of sulfhydryl groups (Shi and Pardini, 1995) |
|
NDGA, LD50 9–20 μg/mL |
Cells in culture: Vero cells (African green monkey kidney epithelial cell line) Hep-2 cells (HeLa epithelial cell line) |
NDGA had weak cytotoxic activity (Zamora et al., 1992) |
|
NDGA, 25–250 μM, ≤ 72 hr |
Cells in culture: 786A cells (sarcoma cell line), IC50 0.24 mM TA3 cells (mammary cell line), IC50 0.21 mM |
NDGA had weak cytotoxic activity Addition of NDGA (250 μM) decreased cellular respiration and ATP concentration within 1 hr (Pavani et al., 1994) |
|
NDGA |
In cell suspensions: NDGA had cytotoxic anaerobic glycolysis and respiration |
NDGA inhibited aerobic and activity (Burk and Woods, 1963) |
|
NDGA |
Cells in culture: WISH cells (human HeLa cell line) |
NDGA decreased viability (ID50 100 μg/mL) Blalock et al., 1981) |
|
NDGA |
Cells in culture: Mouse L cells |
NDGA decreased viability (ID50 100 μg/mL) (Blalock et al., 1981) |
|
NDGA |
Cells in culture: Ehrlich ascites cells |
NDGA sensitized cells to X-ray irradiation (1,000 r) (von Ardenne et al., 1969) These data were published in German. Summary is based on the English abstract |
|
NDGA, 150–600 mM |
Tissue slices: Rat liver slices (male, Fisher 344) |
During incubation of precision-cut rat liver slices with NDGA, cell viability decreased by several indicators (decreased content of potassium, LDH, and glycogen) Ethanol was also cytotoxic and the total effect was additive (Ulreich et al., 1997) |
|
Substance |
Study Design |
Results and Conclusions |
|
NDGA, 150–600 mM |
Tissue slices: Rat kidney slices |
During incubation of precision-cut rat kidney slices with NDGA, cell viability decreased by two indicators (decreased content of potassium and LDH) (Nakazato et al., 1998) |
|
NDGA, 150–600 mM |
Tissue slices: Human liver slices Human kidney slices |
During incubation of precision-cut human liver or kidney slices with NDGA, cell viability decreased by two indicators (decreased content of potassium and LDH) Cytotoxicity was dose-dependent (Nakazato et al., 1998) |
|
NDGA, LD50 150 μM |
Isolated cells: Rat hepatocytes |
During a 2-hr incubation with NDGA or 21 different flavonoids and polyphenols, NDGA was one of the most cytotoxic, behind galangin and chrysin (Moridani et al., 2002) |
|
Inhibition of cellular processes |
||
|
NDGA, Ki 140 μM |
Isolated jejunal loops from rats (female Wistar), using luminal perfusion |
NDGA inhibited intestinal glucose absorption, glucose utilization, and lactate production (Kellett et al., 1993) |
|
NDGA, 30 μM |
Cells in culture: 3T3-4 cells |
NDGA enhanced glucose transport and metabolism (± insulin) (Reed et al., 1998, 1999). |
|
NDGA, 30 μM |
Isolated rat adipocytes |
NDGA enhanced glucose transport 2-fold (± 100 pM insulin) (Reed et al., 1998, 1999) |
|
NDGA |
Isolated rat pancreatic islets |
NDGA inhibited insulin secretion induced by glucose (Yamamoto et al., 1982) |
|
Substance |
Study Design |
Results and Conclusions |
|
NDGA, 100 μM |
Tissue culture: Isolated mouse pancreatic islets in culture for 1–2 wk |
NDGA in the culture media reduced insulin secretion induced by glucose (20 mM) even though the total insulin content of islets was equivalent in control and NDGA-treated cultures (Hsu et al., 2001) |
|
NDGA, 30 μM |
Isolated rat adipocytes |
NDGA inhibited lipolysis in response to isoproterenol or 8-chlorophenyltheo cAMP (Gowri et al., 1998) |
|
NDGA, 50 μM |
Isolated rat adipocytes |
NDGA reduced lipolytic activity induced by isoproterenol and decreased the phosphorylated form of hormone-sensitive lipase (Gowri et al., 1998) |
|
NDGA |
Cells in culture: SW 850 (human pancreatic cancer cell line) C4-I (human cervical cancer cell line) |
NDGA inhibited anchorage-dependent proliferation (data not shown) After incubation with NDGA for 8 hr, cells began to detach from tissue culture dish Incubation of cells with NDGA (25 μM) inhibited expression of cyclin D1 (while expression of cyclin E was unchanged) Incubation of cells with NDGA (25 μM) resulted in disruption of the cytoskeleton (actin stress fibers but not the circumferential actin filament network) Incubation of cells with NDGA (25 μM) activated stress-activated MAP kinases (JNK1/2 and p38mapk but not ERK1/2) (Seufferlein et al., 2002) |
|
Substance |
Study Design |
Results and Conclusions |
|
NDGA, 15–30 μM |
Cells in culture in soft agar: SW 850 (human pancreatic cancer cell line) C4-I (human cervical cancer cell line) |
NDGA inhibited colony formation in response to 0.5% or 10% fetal bovine serum, thought to represent anchorage-independent growth (Seufferlein et al., 2002) NDGA inhibited anchorage-dependent proliferation (data not shown) (Seufferlein et al., 2002) After incubation with NDGA for 8 hr, cells began to detach from tissue culture dish |
|
NDGA, 10 μM |
Cells in culture: HEK293 cells (human embryonic cell line) Porcine coronary arterial smooth muscle cells) |
NDGA at 10 μM activates the Ca2+-dependent K+ channel, releasing Ca2+ (Yamamura et al., 2002) |
|
NDGA, > 10 μM |
Cells in culture: HEK293 cells (human embryonic cell line) Porcine coronary arterial smooth muscle cells) |
NDGA at > 10 μM quickly causes a large increase the intracellular concentration of Ca2+ (Yamamura et al., 2002) |
|
NDGA, 5–100 μM |
Cells in culture: Rat C6 glioma cells |
NDGA increased the concentration of intracellular Ca2+ (Su et al., 2002) |
|
NDGA, 1–100 μM |
Isolated porcine coronary artery smooth muscle cells (inside-out and outside-in patches) |
NDGA opens the Ca2+-dependent K+ channel, except in the presence of very low cytosolic Ca2+ concentrations (Nagano et al., 1996) |
|
NDGA, 100 μM |
Bovine heart mitochondria |
NDGA inhibited mitochondrial electron transport (by inhibition of NADH-coenzyme Q reductase and succinate coenzyme Q reductase) (Pardini et al., 1970) |
|
NDGA |
Beef heart mitochondria |
NDGA reduced microsomal electron transport by inhibiting succinate cytochrome c reductase (Shi and Pardini, 1995) |
|
Substance |
Study Design |
Results and Conclusions |
|
NDGA, IC50 15 ηmoles/mg mitochondrial |
Rat liver mitochondria |
NDGA inhibited mitochondrial electron transport (Bhuvaneswaran and Dakshinamurti, 1972) |
|
Inhibition of enzymes |
||
|
NDGA |
Rat epidermal and hepatic microsomal cytochrome P-450 |
NDGA inhibited aryl hydrocarbon hydroxylase (CYP 1A and 1B) and inhibited 7-ethoxy-resorufin O-demethylase (CYP 1A) activities (Agarwal et al., 1991) |
|
NDGA, 100 μM |
Cells in culture: Hep-G-2 cells |
NDGA inhibited cytochrome 1A1 induction (hydrodynamic stress-induced) (Mufti and Shuler, 1996) |
|
NDGA |
Rat liver homogenate (IC50 6 μM) Human liver homogenate Human placenta homogenate |
NDGA inhibited catechol O-methyl transferase and (Burba and Becking, 1969) |
|
NDGA, Ki 125 μM |
Isolated jejunal loops from rats (female Wistar), luminal perfusion |
NDGA inhibited (Mg2+/Na+/ K+)-ATPase and (Na+/K+)- ATPase in jejunum (Kellett et al., 1993) |
|
NDGA |
Various enzyme sources |
NDGA inhibited carboxylesterase (2 μM) and inhibited formyltetrahydrofolate synthetase (ED50 100 μM) (Schegg and Welch, 1984) |
|
NDGA, 100 μM |
Microsomes |
NDGA inhibited cyclooxygenase (Van der Merwe et al., 1993) |
|
NDGA, IC50 1 μM |
Rat platelets |
NDGA inhibited platelet cyclooxygenase (Ferrandiz et al., 1990) |
|
NDGA, IC50 1–42 μM |
Intact cells and cell-free preparations |
NDGA inhibited 5-lipoxygenase activity (peritoneal neutrophils from female Wistar rats, IC50 2–4 μM); inhibited 15-lipoxygenase activity (isolated from soybean, IC50 4 μM); and inhibited |
|
Substance |
Study Design |
Results and Conclusions |
|
|
|
cyclooxygenase activity (peritoneal macrophages from male CD-1 mice, IC50 1–42 μM) (Chang et al., 1984). |
|
NDGA, IC50 0.2 μM |
Soybean lipoxygenase |
NDGA inhibited lipoxygenase (Whitman et al., 2002) |
|
NDGA, IC50 5 μM |
Human platelet 12-lipoxygenase |
NDGA inhibited human 12-lipoxygenase (Whitman et al., 2002). |
|
NDGA, IC50 0.1 μM |
Human reticulocyte 15-lipoxygenase |
NDGA inhibited human 15-lipoxygenase (Whitman et al., 2002) |
|
NDGA, IC50 10 μM |
Cells in culture: Caco-2 (human colon epithelial cell line) |
NDGA (10 μM) inhibited 15-lipoxygenase activity without inhibiting cyclooxygenase activity (data not shown) (Kamitani et al., 1998) |
|
NDGA, IC50 0.3 μM |
Rabbit erythroid 15-lipoxygenase |
NDGA inhibited 15-lipoxygenase (Luther et al., 1991) |
|
NDGA, IC50 180 μM |
Sheep vesicular gland prostaglandin H synthase |
NDGA inhibited prostaglandin H synthase (Luther et al., 1991) |
|
NDGA, 10 μM |
Rat alveolar macrophages and Chinese hamster lung fibroblasts phospholipase |
NDGA inhibited A2(Robison et al., 1990) |
|
NDGA, IC50 11 μM |
Human aromatase: Placental microsomes Choriocarcinoma cell line JEG-3 al., |
NDGA inhibited human aromatase (estrogen synthetase) (Adlercreutz et 1993) |
|
NDGA, Ki 94 μM |
Rabbit skeletal muscle enzyme |
NDGA inhibited phosphofructokinase (Kellett et al., 1993) |
|
NDGA, IC50 41 μM |
Rat liver microsomes |
NDGA inhibited aryl hydrocarbon hydroxylase (Agarwal et al., 1991) |
|
Other |
||
|
NDGA, 10 ηM |
Cells in culture: Human renal tubular cells (epithelial cells) |
NDGA increased the incorporation of hydroxyproline, a component of basement membrane (Vedovato et al., 1994) |
TABLE G Chaparral: Related Substances That Might Suggest Risk