Executive Summary
Understanding is a two-way street. —Eleanor Roosevelt
ABSTRACT
|
I have a very good doctor. He takes the time to explain things and break it down to me. Sometimes, though, I do get stuff that can be hard—like when I first came home from the hospital and I had all these forms and things I had to read. Some words I come across I just can’t quite understand (National Center for the Study of Adult Learning and Literacy, 2003).1 |
Nearly half of all American adults—90 million people—have difficulty understanding and acting upon health information. The examples below were selected from the many pieces of complex consumer health information used in America.
-
From a research consent form: “A comparison of the effectiveness of educational media in combination with a counseling method on smoking habits is being examined.” (Doak et al., 1996)
-
From a consumer privacy notice: “Examples of such mandatory disclosures include notifying state or local health authorities regarding particular communicable diseases.”
-
From a patient information sheet: “Therefore, patients should be monitored for extraocular CMV infections and retinitis in the opposite eye, if only one infected eye is being treated.”
Forty million Americans cannot read complex texts like these at all, and 90 million have difficulty understanding complex texts. Yet a great deal of health information, from insurance forms to advertising, contains complex text. Even people with strong literacy skills may have trouble obtaining, understanding, and using health information: a surgeon may have trouble helping a family member with Medicare forms, a science teacher may not understand information sent by a doctor about a brain function test, and an accountant may not know when to get a mammogram.
This report defines health literacy as “the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions” (Ratzan and Parker, 2000). However, health literacy goes beyond the individual obtaining information. Health literacy emerges when the expectations, preferences, and skills of individuals seeking health information and services meet the expectations, preferences, and skills of those providing information and services. Health literacy arises from a convergence of education, health services, and social and cultural factors. Although causal relationships between limited health literacy and health outcomes are not yet established, cumulative and consistent findings suggest such a causal connection.
Approaches to health literacy bring together research and practice from diverse fields. This report examines the body of knowledge in this emerging field, and recommends actions to promote a health-literate society. Increasing knowledge, awareness, and responsiveness to health literacy among health services providers as well as in the community would reduce problems of limited health literacy. This report identifies key roles for the Department of Health and Human Services as well as other public and private sector organizations to foster research, guide policy development, and stimulate the development of health literacy knowledge, measures, and approaches. These organizations have a unique and critical opportunity to ensure that health literacy is recognized as an essential component of high-quality health services and health communication.
INTRODUCTION
|
A two-year-old is diagnosed with an inner ear infection and prescribed an antibiotic. Her mother understands that her daughter should take the prescribed medication twice a day. After carefully studying the label on the bottle and deciding that it doesn’t tell how to take the medicine, she fills a teaspoon and pours the antibiotic into her daughter’s painful ear (Parker et al., 2003). |
Modern health systems make complex demands on the health consumer. As self-management of health care increases, individuals are asked to assume new roles in seeking information, understanding rights and responsibilities, and making health decisions for themselves and others. Underlying these demands are assumptions about people’s knowledge and skills.
National and international assessments of adults’ ability to use written information suggest that these assumptions may be faulty. Current evidence reveals a mismatch between people’s skills and the demands of health systems (Rudd et al., 2000a). Many people who deal effectively with other aspects of their lives may find health information difficult to obtain, understand, or use. While farmers may be able to use fertilizers effectively, they may not understand the safety information provided with the fertilizer. Chefs may create excellent dishes, but may not know how to create a healthy diet. Indeed, health literacy can be a hidden problem—because it is often not recognized by policy makers and health care providers, and because people with low literacy skills or who are confused about health care may be ashamed to speak up about problems they encounter with the increasingly complex health system (Baker et al., 1996; Parikh et al., 1996). Without improvements in health literacy, the promise of scientific advances for improving health outcomes will be diminished.
The Institute of Medicine (IOM) convened the Committee on Health Literacy, composed of experts from a wide range of academic disciplines and backgrounds, to assess the problem of limited health literacy and to consider the next steps in this field. The committee addressed the following charge:
-
Define the scope of the problem of health literacy. The intent is to clarify the root problems that underlie health illiteracy. This would include identifying the affected populations and estimating the costs for society. Develop a set of basic indicators of health literacy to allow assessment of the extent of the problem at the individual, community, and national levels.
-
Identify the obstacles to a creating a health-literate public. These are likely to include the complexity of the health care system, the many and often contradictory health messages, rapidly advancing technologies, limits within public education to promote literacy of adults as well as children, etc.
-
Assess the approaches that have been attempted to increase health literacy both in the United States and abroad. Identify the gaps in research and programs that need to be addressed. The focus should be on public health interventions attempting to increase health literacy of the public rather than on improving health provider/primary care interactions.
-
Identify goals for health literacy efforts and suggest approaches to overcome the obstacles to health literacy in order to reach these goals. These might include research or policy initiatives, interventions, or collaborations that would promote health literacy.
WHAT IS HEALTH LITERACY?
In this report, the committee accepted the definition of health literacy presented by the National Library of Medicine (Selden et al., 2000) and used in Healthy People 2010 (HHS, 2000):
The degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions (Ratzan and Parker, 2000).
Health literacy is a shared function of social and individual factors. Individuals’ health literacy skills and capacities are mediated by their education, culture, and language. Equally important are the communication and assessment skills of the people with whom individuals interact regarding health, as well as the ability of the media, the marketplace, and government agencies to provide health information in a manner appropriate to the audience.
The committee developed a framework for health literacy which identifies three major areas of potential intervention and forms the organizational principle of this report (see Figure ES-1). This framework illustrates the potential influence on health literacy as individuals interact with educational systems, health systems, and cultural and social factors, and suggests that these factors may ultimately contribute to health outcomes and costs. The proposed framework is a model, because available research supports only limited conclusions about causality. However, the cumulative effect of a body of consistent evidence suggests that causal relationships may exist between health literacy and health outcomes. Research is needed to establish the nature of the causal relationships between and among the various factors portrayed in the framework.
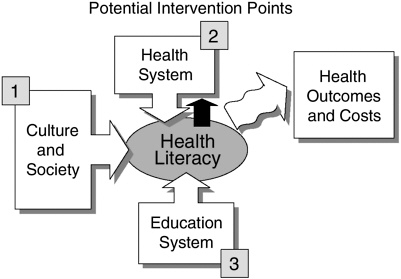
FIGURE ES-1 Potential points for intervention in the health literacy framework.
The committee reviewed the strengths and limitations of currently available measures of literacy and health literacy. Health literacy involves a range of social and individual factors, and includes cultural and conceptual knowledge, listening, speaking, arithmetical, writing, and reading skills. However, most of the tools currently available to measure health literacy primarily measure reading skills, and do not include other critical skills. Furthermore, adults’ reading abilities are often estimated with a “grade level” measure, an estimate that is imprecise at best. Advancement of the field of health literacy requires the development of new measures which can be used to establish baseline levels and monitor change over time.
Finding 2-1 Literature from a variety of disciplines is consistent in finding that there is strong support for the committee’s conclusion that health literacy, as defined in this report, is based on the interaction of individuals’ skills with health contexts, the health-care system, the education system, and broad social and cultural factors at home, at work, and in the community. The committee concurs that responsibility for health literacy improvement must be shared by these various sectors. The committee notes that the health system does carry significant but not sole opportunity and responsibility to improve health literacy.
Finding 2-2 The links between education and health outcomes are strongly established. The committee concludes that health literacy may be
one pathway explaining the well-established link between education and health, and warrants further exploration.
Finding 2-3 Health literacy, as defined in this report, includes a variety of components beyond reading and writing, including numeracy, listening, speaking, and relies on cultural and conceptual knowledge.
Finding 2-4 While health literacy measures in current use have spurred research initiatives and yield valuable insights, they are indicators of reading skills (word recognition or reading comprehension and numeracy), rather than measures of the full range of skills needed for health literacy (cultural and conceptual knowledge, listening, speaking, numeracy, writing, and reading). Current assessment tools and research findings cannot differentiate among (a) reading ability, (b) lack of background knowledge in health-related domains, such as biology, (c) lack of familiarity with language and types of materials, or (d) cultural differences in approaches to health and health care. In addition, no current measures of health literacy include oral communication skills or writing skills and none measure the health literacy demands on individuals within different health contexts.
THE EXTENT AND ASSOCIATIONS OF LIMITED HEALTH LITERACY
Studies of health literacy or of literacy in health contexts suggest that limited health literacy skills, as measured by current assessment tools, are common, with significant variations in prevalence depending on the population sampled (see Chapter 3). People of all literacy levels may be able to manage texts that they frequently encounter and use for everyday activities, but will often face problems with difficult and confusing types of text (Kirsch et al., 1993).
Findings from the National Adult Literacy Survey (NALS) and International Adult Literacy Surveys (IALS) indicate that a large percentage of adults lack the literacy skills needed to meet the demands of twenty-first century society. More than 47 percent, or 90 million, of U.S. adults have difficulty locating, matching, and integrating information in written texts with accuracy and consistency. Of the 90 million with limited literacy skills, about 40 million can perform simple and routine tasks using uncomplicated materials. An additional 50 million adults can locate information in moderately complicated texts, make inferences using print materials, and integrate easily identifiable pieces of information. However, they find it difficult to perform these tasks when complicated by distracting information and complex texts (Kirsch, 2001; Kirsch et al., 1993).
These findings have serious implications for the health sector. Over 300
studies, conducted over three decades and assessing various health-related materials, such as informed consent forms and medication package inserts, have found that a mismatch exists between the reading levels of the materials and the reading skills of the intended audience. In fact, most of the assessed materials exceed the reading skills of the average high school graduate (Rudd et al., 2000a).
Studies suggest that while individuals with limited health literacy come from many walks of life, the problem of limited health literacy is often greater among older adults, people with limited education, and those with limited English proficiency (e.g., Beers et al., 2003; Gazmararian et al., 1999; Williams et al., 1995). For individuals whose native language is not English, issues of health literacy are compounded by issues of basic communication and the specialized vocabulary used to convey health information.
Associations with Health Knowledge, Behavior, and Outcomes
Research linking limited health literacy as it is currently measured to health knowledge, health behaviors, and health outcomes is accumulating. Patients with limited health literacy and chronic illness have less knowledge of illness management than those with higher health literacy (Kalichman et al., 2000; Schillinger et al., 2002; Williams et al., 1998a, b). Compared to those with adequate health literacy, patients with limited health literacy have decreased ability to share in decision-making about prostate cancer treatment (Kim et al., 2001), lower adherence to anticoagulation therapy (Lasater, 2003; Win and Schillinger, 2003), higher likelihood of poor glycemic control (Schillinger et al., 2002), and lower self-reported health status (Arnold et al., 2001; Baker et al., 2002; Kalichman and Rompa, 2000; Kalichman et al., 2000; Williams et al., 1998a, b).
Financial Associations of Limited Health Literacy
The limited amount of data available suggests that there is an association between health literacy, health-care utilization, and health-care costs. Baker and others (2002) found that public hospital patients with limited health literacy had higher rates of hospitalization than those with adequate health literacy. This increased hospitalization rate may be associated with greater resource use. Another analysis (Friedland, 1998) concluded that the additional health expenditure attributable to inadequate reading skills (as identified by the NALS) in 1996 was $29 billion. This estimate would increase to $69 billion if as few as half the individuals with marginal reading skills were also not health literate. Weiss and Palmer (2004) reported on a direct measure of cost in a small sample of Medicaid patients in
Arizona. Patients with reading levels at or below third grade had mean Medicaid charges $7,500 higher than those who read above the third grade level.
For this report, David Howard examined the expenditure data collected in association with the Baker and colleagues (2002) utilization study (see Appendix B). He found that predicted inpatient spending for a patient with inadequate health literacy was $993 higher than that of a patient with adequate reading skills. A difference of $450 remained after controlling for health status, although the causality of the associations between health status and health-care cost could not be determined. In both analyses, higher emergency care costs were incurred by individuals with limited health literacy compared to those with marginal or adequate health literacy as measured by the Test of Functional Health Literacy in Adults (TOFHLA), while pharmacy expenses were similar and outpatient expenditures lower.
Although a robust estimate for the effect of limited health literacy on health expenditures is lacking, the magnitudes suggested by the few studies that are available underscore the importance of addressing limited health literacy from a financial perspective.
Finding 3-1 About 90 million adults, an estimate based on the 1992 NALS, have literacy skills that test below high school level (NALS Level 1 and 2). Of these, about 40–44 million (NALS Level 1) have difficulty finding information in unfamiliar or complex texts such as newspaper articles, editorials, medicine labels, forms, or charts. Because the medical and public health literature indicates that health materials are complex and often far above high school level, the committee notes that approximately 90 million adults may lack the needed literacy skills to effectively use the U.S. health system. The majority of these adults are native-born English speakers. Literacy levels are lower among the elderly, those who have lower educational levels, those who are poor, minority populations, and groups with limited English proficiency such as recent immigrants.
Finding 3-2 On the basis of limited studies, public testimony, and committee members’ experience, the committee concludes that the shame and stigma associated with limited literacy skills are major barriers to improving health literacy.
Finding 3-3 Adults with limited health literacy, as measured by reading and numeracy skills, have less knowledge of disease management and of health-promoting behaviors, report poorer health status, and are less likely to use preventive services.
Finding 3-4 Two recent studies demonstrate a higher rate of hospitalization and use of emergency services among patients with limited literacy. This higher utilization has been associated with higher health-care costs.
THE CONTEXTS OF HEALTH LITERACY AND OPPORTUNITIES FOR INTERVENTION
Culture and Society
|
The ho’ola said Mom should confess to me and before God Jehovah. She did. She asked me to forgive her and I did. I wasn’t angry.... And later Mom’s sickness left her. Of course, she still had diabetes, but the rest—being so confused and miserable—all that left her (Shook, 1985:109). |
Culture is the shared ideas, meanings, and values that are acquired by individuals as members of a society. Culture is socially learned, continually evolves, and often influences us unconsciously. We learn culture through interactions with others, as well as through the tangible products of culture such as books and television (IOM, 2002). Culture gives significance to health information and messages, and can shape perceptions and definitions of health and illness, preferences, language and cultural barriers, care process barriers, and stereotypes. These culturally influenced perceptions, definitions, and barriers can affect how people interact with the health care system and help to determine the adequacy of health literacy skills in different settings.
The fluid nature of culture means that health-care encounters are rich with differences that are continuously evolving. Differing cultural and educational backgrounds between patients and providers, as well as between those who create health information and those who use it, may contribute to problems in health literacy. Culture, cultural processes, and cross-cultural interventions have been discussed in depth in several recent IOM reports and represent possible nexuses of culture and health literacy (IOM, 2002, 2003a).
It is important to understand how people obtain and use health information in order to understand the potential impact of health literacy. Information about health is produced by many sources, including the government and the food and drug industries, and is distributed by the popular media. Commercial and social marketing of health information, products, and services is a multibillion dollar industry. People are frequently and repeatedly exposed to quick, often contradictory bits of information. This
inundation with information has increased as the Internet has become an increasingly important source of health information. Socioeconomic status, education level, and primary language all affect whether consumers will seek out health information, where they will look for the information, what type of information they prefer, and how they will interpret that information. Limited health literacy decreases the likelihood that health-related information will be accessible to all (Houston and Allison, 2002).
Finding 4-1 Culture gives meaning to health communication. Health literacy must be understood and addressed in the context of culture and language.
Finding 4-2 More than 300 studies indicate that health-related materials far exceed the average reading ability of U.S. adults.
Finding 4-3 Competing sources of health information (including the national media, the Internet, product marketing, health education, and consumer protection) intensify the need for improved health literacy.
Finding 4-4 Health literacy efforts have not yet fully benefited from research findings in social and commercial marketing.
The Educational System
Adult education is an important resource for individuals with limited literacy or limited English proficiency. A major source of support for American adult education programs in literacy is the U.S. adult basic education and literacy (ABEL) system. ABEL programs provide classes in topics that support health literacy including basic literacy and math skills, English language, and high school equivalence, and predominantly serve students with literacy and math skills in NALS Levels 1, 2, or the low end of NALS Level 3. Sadly, these programs serve far fewer than the millions of Americans who could benefit.
Both childhood literacy education and childhood health education can provide a basis for health literacy in adulthood. Although most elementary, middle, and high schools require students to take health education, the sequence of coursework is not coordinated. The percentage of schools that require health education increases from 33 percent in kindergarten to 44 percent in grade 5, but then falls to 10 percent in grade 9, and 2 percent in grade 12. The absence of a coordinated health education program across grade levels may impede student learning of needed health literacy skills. Furthermore, only 9.6 percent of health education classes have a teacher
who majored in health education or in combined health and physical education (Kann et al., 2001).
In 1995, the Joint Committee on National Health Standards published the National Health Education Standards with the subtitle Achieving Health Literacy. These standards describe the knowledge and skills essential for health literacy, and detail what students should know and be able to do in health education by the end of grades 4, 8, and 11. They provide a framework for curricula development and student assessment. Unfortunately, these standards have not been widely met.
Finding 5-1 Significant obstacles and barriers to successful health literacy education exist in K-12 education programs.
Finding 5-2 Opportunities for measuring literacy skill levels required for health knowledge and skills, and for the implementation of programs to increase learner’s skill levels, currently exist in adult education programs and provide promising models for expanding programs. Studies indicate a desire on the part of adult learners and adult education programs to form partnerships with health communities.
Finding 5-3 Health professionals and staff have limited education, training, continuing education, and practice opportunities to develop skills for improving health literacy.
Health Systems
Health systems in the United States are complex and often confusing. Their complexity derives from the nature of health care and public health itself, the mix of public and private financing, and the variations across states and between types of delivery settings. An adult’s ability to navigate these systems may reflect this systemic complexity in addition to individual skill levels. Even highly skilled individuals may find the systems too complicated to understand, especially when these individuals are made more vulnerable by poor health. Directions, signs, and official documents, including informed consent forms, social services forms, public health information, medical instructions, and health education materials, often use jargon and technical language that make them unnecessarily difficult to use (Rudd et al., 2000b). In addition, cultural differences may affect perceptions of health, illness, prevention, and health care. Lack of mutual understanding of health, illness and treatments, and risks and benefits has implications for behavior for both providers and consumers, and legal implications for providers and health systems. Imagine having to face this complexity if you
are one of the 90 million American adults who lack the functional literacy skills in English to use the U.S. health care system.2
Health literacy permeates all areas of the provider–consumer information exchange, and provides a common pathway for the successful transfer of information. A number of emerging areas are likely to increase the burden of limited health literacy on those entering and using the health-care system. These include demands inherent in chronic disease management, increased use of new technologies, decreased time for patient/provider discussions, and legal and regulatory requirements.
Many different interventions and approaches that may hold promise for addressing limited health literacy are being attempted across health-care systems, professional organizations, federal and state agencies, educational institutions, and community and advocacy groups across the United States and in other countries. Those profiled in the report are indicators of the creativity and promise for future improvements in countering the effects of limited health literacy. However, few of these approaches have been formally evaluated, and most are fragmented single approaches rather than part of a systematic approach to health literacy. In order for progress to be made, many more systematic demonstrations must be funded and rigorously evaluated.
Finding 6-1 Demands for reading, writing, and numeracy skills are intensified due to health-care systems’ complexities, advancements in scientific discoveries, and new technologies. These demands exceed the health-literacy skills of most adults in the United States.
Finding 6-2 Health literacy is fundamental to quality care, and relates to three of the six aims of quality improvement described in the IOM Quality Chasm Report: safety, patient-centered care, and equitable treatment. Self-management and health literacy have been identified by IOM as cross-cutting priorities for health-care quality and disease prevention.
Finding 6-3 The readability levels of informed consent documents (for research and clinical practice) exceed the documented average reading levels of the majority of adults in the United States. This has important ethical and legal implications that have not been fully explored.
VISION FOR A HEALTH-LITERATE AMERICA
The evidence and judgment presented in this report indicate that heath literacy is important to improving the health of individuals and popula-
tions. This is supported by the conclusions and statements of others. Health literacy was one of two cross-cutting factors that affect health care identified by the IOM in its recent report Priority Areas for National Action in Quality Improvement (IOM, 2003b). The Surgeon General recently stated that “health literacy can save lives, save money, and improve the health and well being of millions of Americans … health literacy is the currency of success for everything I am doing as Surgeon General” (Carmona, 2003).
More needs to be known about the causal pathways between education and health, the role of literacy, and the discrete contribution of health literacy to health. With this knowledge we will be able to understand which interventions and approaches are the most appropriate and effective. This Committee believes that a health-literate America is an achievable goal. We envision a society within which people have the skills they need to obtain, interpret, and use health information appropriately and in meaningful ways. We envision a society in which a variety of health systems structures and institutions take responsibility for providing clear communication and adequate support to facilitate health-promoting actions based on understanding. We believe a health-literate America would be a society in which:
-
Everyone has the opportunity to improve their health literacy.
-
Everyone has the opportunity to use reliable, understandable information that could make a difference in their overall well-being, including everyday behaviors such as how they eat, whether they exercise, and whether they get checkups.
-
Health and science content would be basic parts of K-12 curricula.
-
People are able to accurately assess the credibility of health information presented by health advocate, commercial, and new media sources.
-
There is monitoring and accountability for health literacy policies and practices.
-
Public health alerts, vital to the health of the nation, are presented in everyday terms so that people can take needed action.
-
The cultural contexts of diverse peoples, including those from various cultural groups and non-English-speaking peoples, are integrated in to all health information.
-
Health practitioners communicate clearly during all interactions with their patients, using everyday vocabulary.
-
There is ample time for discussions between patients and healthcare providers.
-
Patients feel free and comfortable to ask questions as part of the healing relationship.
-
Rights and responsibilities in relation to health and health care are presented or written in clear, everyday terms so that people can take needed action.
-
Informed consent documents used in health care are developed so that all people can give or withhold consent based on information they need and understand.
While achieving this vision is a profound challenge, we believe that significant progress can and must be made over the coming years, so that the potential for optimal health can benefit all individuals and populations in our society.
|
Recommendation 2-1 The Department of Health and Human Services and other government and private funders should support research leading to the development of causal models explaining the relationships among health literacy, the education system, the health system, and relevant social and cultural systems. Recommendation 2-2 The Department of Health and Human Services and public and private funders should support the development, testing, and use of culturally appropriate new measures of health literacy. Such measures should be developed for large ongoing population surveys, such as the National Assessment of Adult Literacy Survey, Medical Expenditure Panel Survey, and Behavioral Risk Factor Surveillance System, and the Medicare Beneficiaries Survey, as well as for institutional accreditation and quality assessment activities such as those carried out by the Joint Commission on Accreditation of Healthcare Organizations and the National Committee for Quality Assurance. Initially, the National Institutes of Health should convene a national consensus conference to initiate the development of operational measures of health literacy which would include contextual measures. Recommendation 3-1 Given the compelling evidence noted above, funding for health literacy research is urgently needed. The Department of Health and Human Services, especially the National Institutes of Health, Agency for Healthcare Research and Quality, Health Resources and Services Administration, the Centers for Disease Control and Prevention, Department of Defense, Veterans Administration, and other public and private funding agencies should support multidisciplinary research on the extent, associations, and consequences of limited health literacy, including studies on health service utilization and expenditures. Recommendation 4-1 Federal agencies responsible for addressing disparities should support the development of conceptual frameworks on the intersection of culture and health literacy to direct in-depth theoretical explorations and formulate the conceptual underpinnings that can guide interventions. 4-1.a The National Institutes of Health should convene a consensus conference, including stakeholders, to develop methodology for the incorporation of health literacy improvement into approaches to health disparities. 4-1.b The Office of Minority Health and Agency for Healthcare Research and Quality should develop measures of the relationships between culture, language, cultural competency, and health literacy to be used in studies of the relationship between health literacy and health outcomes. |
|
Recommendation 4-2 The Agency for Healthcare Research and Quality, the Centers for Disease Control and Prevention, the Indian Health Service, the Health Resources and Services Administration, and the Substance Abuse and Mental Health Services Administration should develop and test approaches to improve health communication that foster healing relationships across culturally diverse populations. This includes investigations that explore the effect of existing and innovative communication approaches on health behaviors, and studies that examine the impact of participatory action and empowerment research strategies for effective penetration of health information at the community level. Recommendation 5-1 Accreditation requirements for all public and private educational institutions should require the implementation of the National Health Education Standards. Recommendation 5-2 Educators should take advantage of the opportunity provided by existing reading, writing, reading, oral language skills, and mathematics curricula to incorporate health-related tasks, materials, and examples into existing lesson plans. Recommendation 5-3 The Health Resources and Services Administration and the Centers for Disease Control and Prevention, in collaboration with the Department of Education, should fund demonstration projects in each state to attain the National Health Education Standards and to meet basic literacy requirements as they apply to health literacy. Recommendation 5-4 The Department of Education in association with the Department of Health and Human Services should convene task forces comprised of appropriate education, health, and public policy experts to delineate specific, feasible, and effective actions relevant agencies could take to improve health literacy through the nation’s K-12 schools, 2-year and 4-year colleges and universities, and adult and vocational education. Recommendation 5-5 The National Science Foundation, the Department of Education, and the National Institute of Child Health and Human Development should fund research designed to assess the effectiveness of different models of combining health literacy with basic literacy and instruction. The Interagency Education Research Initiative, a federal partnership of these three agencies, should lead this effort to the fullest extent possible. Recommendation 5-6 Professional schools and professional continuing education programs in health and related fields, including medicine, dentistry, pharmacy, social work, anthropology, nursing, public health, and journalism, should incorporate health literacy into their curricula and areas of competence. Recommendation 6-1 Health care systems, including private systems, Medicare, Medicaid, the Department of Defense, and the Veterans Administration should develop and support demonstration programs to establish the most effec- |
|
tive approaches to reducing the negative effects of limited health literacy. To accomplish this, these organizations should:
Recommendation 6-2 The Department of Health and Human Services should fund research to define the needed health literacy tasks and skills for each of the priority areas for improvement in health care quality. Funding priorities should include participatory research which engages the intended populations. Recommendation 6-3 Health iteracy assessment should be a part of healthcare information systems and quality data collection. Public and private accreditation bodies, including Medicare, the National Committee for Quality Assurance, and the Joint Commission on Accreditation of Healthcare Organizations should clearly incorporate health literacy into their accreditation standards. Recommendation 6-4 The Department of Health and Human Services should take the lead in developing uniform standards for addressing health literacy in research applications. This includes addressing the appropriateness of research design and methods and the match among the readability of instruments, the literacy level, and the cultural and linguistic needs of study participants. In order to achieve meaningful research outcomes in all fields:
|
REFERENCES
Arnold CL, Davis TC, Berkel HJ, Jackson RH, Nandy I, London S. 2001. Smoking status, reading level, and knowledge of tobacco effects among low-income pregnant women. Preventive Medicine. 32(4): 313–320.
Baker DW, Parker RM, Williams MV, Pitkin K, Parikh NS, Coates W, Imara M. 1996. The health care experience of patients with low literacy. Archives of Family Medicine. 5(6): 329–334.
Baker DW, Gazmararian JA, Williams MV, Scott T, Parker RM, Green D, Ren J, Peel J. 2002. Functional health literacy and the risk of hospital admission among Medicare managed care enrollees. American Journal of Public Health. 92(8): 1278–1283.
Beers BB, McDonald VJ, Quistberg DA, Ravenell KL, Asch DA, Shea JA. 2003. Disparities in health literacy between African American and non-African American primary care patients. Abstract. Journal of General Internal Medicine. 18(Supplement 1): 169.
Carmona RH. 2003. Health Literacy in America: The Role of Health Care Professionals. Prepared Remarks given at the American Medical Association House of Delegates Meeting. Saturday, June 14, 2003. [Online]. Available: http://www.surgeongeneral.gov/news/speeches/ama061403.htm [accessed: August, 2003].
Doak LG, Doak CC, Meade CD. 1996. Strategies to improve cancer education materials. Oncology Nursing Forum. 23(8): 1305–1312.
Friedland R. 1998. New estimates of the high costs of inadequate health literacy. In: Proceedings of Pfizer Conference “Promoting Health Literacy: A Call to Action.” October 7–8, 1998, Washington, DC: Pfizer, Inc. Pp. 6–10.
Gazmararian JA, Baker DW, Williams MV, Parker RM, Scott T, Greemn DCFSN, Ren J, Koplan JP. 1999. Health literacy among Medicare enrollees in a managed care organization. Journal of the American Medical Association. 281(6): 545–551.
HHS (U.S. Department of Health and Human Services). 2000. Healthy People 2010: Understanding and Improving Health. Washington, DC: U.S. Department of Health and Human Services.
Houston TK, Allison JJ. 2002. Users of Internet health information: Differences by health status. Journal of Medical Internet Research. 4(2): E7.
IOM (Institute of Medicine). 2002. Speaking of Health: Assessing Health Communication Strategies for Diverse Populations. Washington, DC: The National Academies Press.
IOM (Institute of Medicine). 2003a. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Smedley BD, Stith AY, Nelson AR, Editors. Washington, DC: The National Academies Press.
IOM (Institute of Medicine). 2003b. Priority Areas for National Action: Transforming Healthcare Quality. Adams K, Corrigan JM, Editors. Washington, DC: The National Academies Press.
Kalichman SC, Rompa D. 2000. Functional health literacy is associated with health status and health-related knowledge in people living with HIV-AIDS. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 25(4): 337–344.
Kalichman SC, Benotsch E, Suarez T, Catz S, Miller J, Rompa D. 2000. Health literacy and health-related knowledge among persons living with HIV/AIDS. American Journal of Preventive Medicine. 18(4): 325–331.
Kann L, Brener ND, Allensworth DD. 2001. Health education: Results from the School Health Policies and Programs Study 2000. Journal of School Health. 71(7): 266–278.
Kim SP, Knight SJ, Tomori C, Colella KM, Schoor RA, Shih L, Kuzel TM, Nadler RB, Bennett CL. 2001. Health literacy and shared decision making for prostate cancer patients with low socioeconomic status. Cancer Investigation. 19(7): 684–691.
Kirsch IS. 2001. The International Adult Literacy Survey (IALS): Understanding What Was Measured. Princeton, NJ: Educational Testing Service.
Kirsch IS, Jungeblut A, Jenkins L, Kolstad A. 1993. Adult Literacy in America: A First Look at the Results of the National Adult Literacy Survey (NALS). Washington, DC: National Center for Education Statistics, U.S. Department of Education.
Lasater L. 2003. Patient literacy, adherence, and anticoagulation therapy outcomes: A preliminary report. Abstract. Journal of General Internal Medicine. 18(Supplement 1): 179.
National Center for the Study of Adult Learning and Literacy. (Harvard School of Public Health, Department of Society, Human Development and Health). 2003. Voices from Experience. [Online]. Available: http://www.hsph.harvard.edu/healthliteracy/voices.html [accessed: December 4, 2003].
Parikh NS, Parker RM, Nurss JR, Baker DW, Williams MV. 1996. Shame and health literacy: The unspoken connection. Patient Education and Counseling. 27(1): 33–39.
Parker RM, Ratzan SC, Lurie N. 2003. Health literacy: A policy challenge for advancing high-quality health care. Health Affairs. 22(4): 147.
Ratzan SC, Parker RM. 2000. Introduction. In: National Library of Medicine Current Bibliographies in Medicine: Health Literacy. NLM Pub. No. CBM 2000-1. Selden CR, Zorn M, Ratzan SC, Parker RM, Editors. Bethesda, MD: National Institutes of Health, U.S. Department of Health and Human Services.
Rudd R, Moeykens BA, Colton TC. 2000a. Health and literacy. A review of medical and public health literature. In: Annual Review of Adult Learning and Literacy. Comings J, Garners B, Smith C, Editors. New York: Jossey-Bass.
Rudd RE, Colton T, Schacht R. 2000b. An Overview of Medical and Public Health Literature Addressing Literacy Issues: An Annotated Bibliography. Report #14. Cambridge, MA: National Center for the Study of Adult Learning and Literacy.
Schillinger D, Grumbach K, Piette J, Wang F, Osmond D, Daher C, Palacios J, Sullivan GaD, Bindman AB. 2002. Association of health literacy with diabetes outcomes. Journal of the American Medical Association. 288(4): 475–482.
Selden CR, Zorn M, Ratzan SC, Parker RM. 2000. National Library of Medicine Current Bibliographies in Medicine: Health Literacy. NLM Pub. No. CBM 2000-1. Bethesda, MD: National Institutes of Health, U.S. Department of Health and Human Services.
Shook EV. 1985. Ho’oponopono: Contemporary Uses of a Hawaiian Problem-Solving Process. Honolulu, HI: East-West Center, University of Hawaii Press.
Weiss BD, Palmer R. 2004. Relationship between health care costs and very low literacy skills in a medically needy and indigent Medicaid population. Journal of the American Board of Family Practice. 17(1): 44–47.
Williams MV, Parker RM, Baker DW, Parikh NS, Pitkin K, Coates WC, Nurss JR. 1995. Inadequate functional health literacy among patients at two public hospitals. Journal of the American Medical Association. 274(21): 1677–1682.
Williams MV, Baker DW, Honig EG, Lee TM, Nowlan A. 1998a. Inadequate literacy is a barrier to asthma knowledge and self-care. Chest. 114(4): 1008–1015.
Williams MV, Baker DW, Parker RM, Nurss JR. 1998b. Relationship of functional health literacy to patients’ knowledge of their chronic disease. A study of patients with hypertension and diabetes. Archives of Internal Medicine. 158(2): 166–172.
Win K, Schillinger D. 2003. Understanding of warfarin therapy and stroke among ethnically diverse anticoagulation patients at a public hospital. Abstract. Journal of General Internal Medicine. 18(Supplement 1): 278.


















