6
Health Systems
|
Mr. G. is a 64-year-old man with chronic hypertension, diabetes, a high cholesterol level, and gout. He saw his primary care doctor because his left leg was swollen and painful. His doctor diagnosed an early cellulitis and prescribes an antibiotic to be taken for 10 days. After 4 days, Mr. G. went to the emergency department, unable to walk because of intense pain and swelling of his entire left leg. His blood sugar and blood pressure were both very high, and he was admitted to the hospital to treat his infection and control his blood pressure. During his emergency department treatment and admission, he was examined by, and spoke to, four different doctors. The fifth doctor to take a history and examine Mr. G discovered that he had taken none of his seven chronic medications, nor the newly prescribed antibiotic given to him when his infection first appeared. Mr. G. explained “You see, I already take 19 pills a day, and when I got another one I got confused about my timing, and I was just so scared I might mess up. My daughter usually helps me with my medicines, but she’s been sick and I didn’t want to worry her.” |
THE CONTEXT OF HEALTH SYSTEMS
Navigating the U.S. health-careand public health delivery systems is a complex task with numerous layers of bureaucracy, procedures, and processes. Consequently, an adult’s ability or inability to navigate these systems may reflect systemic complexity as well as individual skill levels. Patients, clients, and their family members are typically unfamiliar with these systems and the associated jargon. Even highly educated individuals may find the systems too complicated to understand, especially when people are made more vulnerable by poor health. Official documents, including informed consent forms, social services forms, and public health and medical instructions, as well as health information materials often use jargon and technical language that make them unnecessarily difficult to use (Rudd et al., 2000).
|
When you have medical forms and stuff, I don’t think it should be complicated for a person to understand what its saying (Rudd and DeJong, 2000). |
Some of the complexity of the health-care system arises from the nature of health care and public health itself, the mix of public and private financing, and the health information and health-care delivery settings. Unlike many other countries, the United States does not have a single organized national health-care system. Furthermore, the United States has no national health surveillance system, and few common norms exist for basic preventive services such as immunizations. Threats of bioterrorism and new emerging diseases such as SARS continue to complicate the picture of health care.
In the past, health management was primarily the domain of the physician, but greater responsibility for health management has shifted to the patient as health care has evolved and cost pressures on care have increased. This has been called self-management, and was identified in the Institute of Medicine (IOM) Priority Areas for National Action report (IOM, 2003b) as one of two cross-cutting issues in improving health-care quality that present the opportunity to improve health across the lifespan, at all stages of health service. In order to make appropriate self-management decisions, health information consumers must locate health information, evaluate the information for credibility and quality, and analyze the risks and benefits, activities that rely on health literacy skills. Consumers must be able to express health concerns clearly by describing symptoms in ways the providers can understand. Both patients and health-care providers must be able to ask pertinent questions and fully understand the available medical information.
Improvement of health literacy was identified by the IOM report as an essential component of self-management that would affect nearly all aspects of health care. The IOM report further noted that system and policy changes to improve self-management would require involvement by most health-care organizations and providers to address all types of health conditions, providing a means to improve health care for all Americans. Figure 6-1 below is a depiction of the “ecology of health service organizations” that form the U.S. health system (Shortell and Kaluzny, 2000). This figure illustrates the complex relationships of organizations and programs that form the basis of the U.S. health-care system that adults are expected to navigate.
The organizations with the most direct impact on patients are in the inner circles and those with a more indirect influence are in the outer circles. Although this complex system could be simplified somewhat by consolidating some of the depicted organizations (such as hospitals and physician groups) into a variety of health networks and health systems, other factors deter such consolidation. For example, tightly integrated managed care systems have failed to grow in response to consumer demands for more choice. Accessing and using the systems effectively is further complicated by the mix of private and public financing mechanisms and interrelationships.
Individuals and families must learn how to interact with employers, supplemental private insurance companies, federal and state government
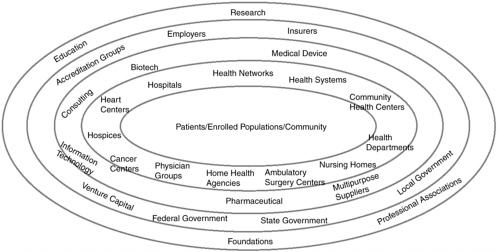
FIGURE 6-1 The concentric ecology of organizations in the health-care sector.
SOURCE: Health Care Management: Organization, Design, and Behavior, 4th Edition, by Shortell and Kaluzny. © 2000. Reprinted with permission of Delmar Learning, a division of Thomson Learning: www.thomsonrights.com. Fax 800 730-2215.
programs, and providers who are paid directly, “out-of-pocket.” To complicate matters further, individuals seldom interact with only one aspect of the health-care system. They make decisions about the severity of illness, the ease and cost of various treatment options, and move from self-care to seeking advice, from public to private options, and from primary to tertiary care in complex “patterns of resort” (Scrimshaw and Hurtado, 1987). Yet studies indicate that individuals and families lack the information needed for these activities. Chapter 2 provides evidence that health literacy affects the interaction of individuals with components of the health-care system, and may further affect health status and outcomes. Studies suggest that health information is often more difficult to comprehend than other types of information (Root and Stableford, 1999). Extensive research shows that communication of health information through printed material, multimedia, and interpersonal exchange is often not successful (Baker and Wilson, 1996; Berland et al., 2001; Davis et al., 1996; Graber et al., 1999; MacKinnon, 1984; Meade et al., 1989; Meeuwesen et al., 2002; Reid et al., 1995; Roter et al., 2001; Rudd, 2003; Rudd et al., 2000; Smith et al., 1998). Print and interactive materials have been consistently found to be less understandable by their audience than the authors intended (Kerka, 2000; Rudd et al., 2000). Complex materials frequently used by employers, insurance companies, government programs, and providers, such as consent forms and questionnaires, are difficult for many people to use appropriately (Gaba and Grossman, 2003; Hochhauser, 1997; Kaufer et al., 1983; Morales et al., 2001; Osborne and Hochhauser, 1999).
All but the most sophisticated health policy experts have difficulty understanding the many facets of health and health care. Most of us are confused by our hospital and medical bills, the choices we have to make regarding health plans and health coverage, and the often contradictory news accounts about the most effective treatments or preventive strategies. Imagine having to face this complexity if you are one of the 90 million American adults who this committee has found “lack the functional literacy skills in English to use the U.S. health care system.”1
Finding 6-1 Demands for reading, writing, and numeracy skills are intensified due to health-care systems’ complexities, advancements in scientific discoveries, and new technologies. These demands exceed the health literacy skills of most adults in the United States.
Emerging Issues in the Health System Context2
In addition to the general complexity of the current system, the committee has identified a number of emerging themes or issues that are important aspects of the health system context with respect to health literacy. These include
-
Chronic disease care and self-management
-
Patient–provider communication
-
Patient safety and health-care quality
-
Access to health care and preventive services
-
Provider time limitations
-
Health expenditures
-
Consumer-directed health care
Each of these topics is briefly discussed in the section that follows, with particular attention to the ways in which these issues may function as barriers to those with limited health literacy. Other issues that are currently salient in the health-care context include health disparities and the increasingly complex health information context; these topics are discussed in Chapter 5.
Chronic Disease Care and Self-Management
Chronic disease, and the problem of people having to cope with multiple, comorbid conditions, is a critical issue in health care, particularly as the health-care delivery system becomes more and more complex and patients must become the integrators of their own care. Chronic disease exemplifies the interaction of health literacy and health, as patients’ health is often dependent on their ability and willingness to carry out a set of health activities essential to the management and treatment of chronic diseases (IOM, 2002c). The last few decades have seen tremendous advances in the
|
2 |
The information in this section is drawn in part from the background paper “Improving Chronic Disease Care for Populations with Limited Health Literacy,” commissioned by the committee from Dean Schillinger, M.D. The committee appreciates his contribution. The full text of the paper can be found in Appendix B. |
care of chronic conditions, including an array of therapeutic options, risk factor modification for secondary prevention of comorbid conditions, the availability of home monitoring tools, and the growth of disease management programs (Bodenheimer, 1999; McCulloch et al., 2000). Despite these advances, health quality and clinical outcomes of patients with chronic diseases vary significantly across sociodemographic lines (Fiscella et al., 2000; Piette, 1999, 2000; Vinicor et al., 2000).
Care for those with chronic diseases is more difficult for patients, providers, and families when patients cannot understand or remember the given directions, and or when directions that are given are incomplete and unclear (IOM, 2003b). This is also true in other contexts, such as acute care and public health interventions. The collaboration between patients, providers, the system of care, and the community that is required to optimize health outcomes adds a significant layer of complexity to the delivery of health care to individuals with chronic disease. Effective disease management is predicated on systematic, interactive communication between a population of patients with the disease and the providers and health system with whom they interact (Norris et al., 2002a, b; Von Korff et al., 1997), all occurring in the context of a supportive community whose resources are aligned with patients’ needs (Wagner, 1995).
Chronic care involves an ongoing process of patient assessments, adjustments to treatment plans, and reassessments to measure change in patient health status. Without timely and reliable information about patients’ health status, symptoms, and self-care, the necessary health education, treatments, or behavioral adjustments may come late or not at all, compromising patients’ health and increasing the likelihood of poor outcomes. Self-management is essential to successful chronic disease care; patients must remember any self-care instructions they have received from their provider, be able to correctly interpret symptoms or results of self-monitoring, and appropriately problem-solve regarding adjustments to the treatment regimen. Patients also must know when and how to contact the provider should the need arise. A number of studies demonstrate that patients remember and understand as little as half of what they are told by their physicians (Bertakis, 1977; Cole and Bird, 2000; Crane, 1997; Rost and Roter, 1987; Roter, 2000). In addition, because they have knowledge deficits, patients with limited health literacy may be less equipped to overcome such gaps in understanding and memory once they are at home (Williams et al., 1998a, b) and difficulties reading or interpreting instructions (Crane, 1997; Williams et al., 1998b). Cross-sectional studies involving patients with diabetes suggest that traditional self-management education may not eliminate health literacy-related disparities in chronic disease outcomes (Schillinger et al., 2002; Williams et al., 1998b). Focus groups of patients with limited health literacy have identified “health system navigation” (such as knowing whom,
for what, and when to call for assistance with a problem) as a particularly daunting aspect of chronic disease management (Baker et al., 1996).
Building on the Chronic Care Model of Wagner et al. (2001), Schillinger has developed a framework that explores ways to improve chronic disease care and is based on health literacy and related research. This framework is discussed in greater detail in Appendix B, but briefly, the model represents the evidence base for chronic disease care and supports the importance of executive leadership and incentives to promote quality, systems to track and monitor patients’ progress and support timely provider decision-making (Piette, 2000), patient self-management training (Lorig et al., 1999; Von Korff et al., 1997), and community-oriented care. Since self-management practices and clinical outcomes in chronic disease care appear to vary by patients’ level of health literacy (Kalichman and Rompa, 2000; Schillinger et al., 2002; Williams et al., 1998b), the Chronic Care Model, and similar comprehensive, population-based disease management approaches, may offer insights into the ways in which limited health literacy affects chronic disease care and could identify points for potentially successful intervention.
Patient–Provider Communication
To Err Is Human reported that communication failure was the underlying cause of fully 10 percent of adverse drug events (IOM, 2000b). Management of complex drug therapies, especially in elderly patients, is extremely difficult and requires special attention to the ability of the patient to understand and remember the amount and timing of dose, as well as behavioral modifications required by the regimen (e.g., dietary restrictions) (IOM, 2000b). The patient’s health literacy level as it affects the communication process is therefore an important consideration in health outcomes.
There is some evidence of a failure of communication with patients with limited health literacy as currently measured. Patients with chronic diseases and limited health literacy have been shown to have poor knowledge of their condition and of its management (Williams et al., 1998a, b), often despite having received standard self-management education. Patients with limited health literacy have greater difficulties accurately reporting their medication regimens and describing the reasons for which their medications were prescribed (Schillinger et al., 2003a; Williams et al., 1995) and more frequently have explanatory models3 that may interfere with adher-
ence (Kalichman et al., 1999). Understanding these explanatory models is essential to good communication regarding care (Good and Good, 1981; Kleinman, 1980).
While, by definition, patients with limited health literacy have problems with literacy- and numeracy-related tasks in the health-care setting, a recent study among patients with diabetes demonstrated that these patients also experience difficulties with oral communication (Schillinger et al., 2004). Patients appear to have particular problems with both the decision-making and the explanatory, technical components of dialogue. Furthermore, patients with limited health literacy may be less likely to challenge or ask questions of the provider (Baker et al., 1996; Street, 1991) and may cope by being passive or appearing uninterested (Cooper and Roter, 2003; Roter, 2000; Roter and Hall, 1992; Roter et al., 1997).
Health literacy is one of a number of influences on the communication process. Communication between a health-care provider and patient during outpatient visits may also be hampered by several related factors. These include the relative infrequency and brevity of visits, language barriers, differences between providers’ and patients’ agendas and communication styles and other cultural barriers, lack of trust between the patient and provider, overriding or competing clinical problems, lack of timeliness of visit in relation to disease-specific problem, and the complexity and variability of patients’ reporting of symptoms and trends in their health status.
The complexities that arise from the interplay of cultural processes and variations in health literacy underlie communications and interactions in the health-care system. Typically this balance is part of a healing relationship governed by cultural beliefs and rituals that manifest as a cultural language. Health-care providers need to comprehend this cultural language in order to reduce misunderstanding. Reciprocal to this understanding are the skills of the people among the different cultural groups to understand what health-care providers are communicating regarding their diagnosis, risks, and treatment options.
Diverse cultural groups value distinct processes by which harmony and balance is maintained in the relationships of everyday life. Typically this balance is referred to as healing and along with the dynamics surrounding human relationships, these become “healing relationships” governed by cultural beliefs and rituals that manifest as culturally based language during health and illness. Cultural contexts dominate healing relationships. “Physicians and other health care providers need communication skills … to reduce misunderstanding and the risk of incorrect diagnosis and … to develop … treatment plans that are compatible with the patient’s reality” (Molina et al., 1994:35). Reciprocal to this communication are the skills of the people (individual, families, and communities among different cultural
groups) to understand what health-care providers are discussing about diagnoses, risks, and treatment options. In contrast, aboriginal peoples have a vision of healing that informs the “process of healing oneself, relationships with families” and “maintaining balance among their mental, physical, emotional and spiritual dimensions as human beings” (Ross, 1996:147). In Westernized health care, the concept of healing is typically used during experiences with illness or disruptions in health status. In contrast, some aboriginal cultural languages present healing as an aspect of health and everyday living. “Instead healing is seen as an everyday thing for everyone, something which, like sound nutrition, creates health. In short, the healing perspective must be built into the attitudes and process that shape every aspect of everyday” (Ross, 1996:147).
In another example, Native Hawaiians comprehend healing through a system for maintaining harmonious relationships and resolving conflicts within the extended family; this system is called Ho’oponopono, which means setting to right. The Ho’oponopono process is a conceptual framework informed by several cultural concepts that become evident as the steps in the cultural ritual. These include problem identification, discussion, seeking forgiveness; release of conflicts and hurts; and the closing phase. Ho’oponopono is especially useful as a mental health intervention (e.g., for alcohol or substance abuse, domestic violence, anger) but also complements interventions for coping with diseases.
|
“Then the ho’ola said Mom should confess to me and before God Jehovah. She did. She asked me to forgive her and I did. I wasn’t angry….And later Mom’s sickness left her. Of course, she still had diabetes, but the rest—being so confused and miserable—all that left her” (Shook, 1985:109). |
This broader humanistic perspective of healing is critical to comprehend aboriginal and Native Hawaiian health behaviors and to understand the importance of healing rituals for inclusion in treatment options and caring interventions during health and illness. These culturally based views of health suggest a trajectory for forming healing relationships in that they illuminate a fuller range of human experiences during health and illness. Understanding a greater diversity of healing relationships supports health literacy among different cultural groups. To this end, good health communications are a key pathway to building the continuous healing relationships that have been cited as an important goal for twenty-first century health-care system design (IOM, 2001). Poor communications and relationships translate into unfavorable outcomes, particularly delays in care seeking, refusal, lack of continuity of care, and disparities (IOM, 2003a).
Patient Safety and Health-Care Quality4
The IOM report To Err Is Human (IOM, 2000b) clarifies the links between miscommunication and medical and health errors and adverse events. Lack of cultural competence and inattention to health literacy can both compromise patient safety through a number of mechanisms. For example, a variety of problems can result if culture and language are not taken into account including
-
Failure to get accurate medical histories
-
Failure to obtain informed consent
-
Poor health knowledge and understanding of health conditions
-
Poor treatment adherence
-
Medication errors
-
Lower utilization of preventive and other health-care services
-
Poor patient satisfaction
To the extent that low health literacy may be more prevalent among some racial and ethnic minority groups, these individuals may be at higher risk for adverse events stemming from poor communication. There is a need to understand the independent contributions of cultural competence and health literacy to patient safety, as well as the interactions between cultural competence and health literacy.
Patients’ varied perspectives, values, beliefs, and behaviors regarding health and illness are consistently cited as integral to quality care in several IOM reports (2001, 2003a). To protect people from undue harm, eliminate errors and adverse events, prevent unnecessary human suffering, and be more accountable to quality and cost-effective care, cultural processes beckon attention, particularly to erode the culture of blame, reform teamwork, and redesign organizational dynamics within health-care systems. A principle of patient safety is to include patients in safety designs and the processes of care (IOM, 2000b). To do so, it is essential to understand cultural nuances of what patient safety means to different people and what beliefs, values, and actions inform people’s understanding of safe care come into play.
The communication process plays a central role in this understanding. For example, a recent study in California of pediatric outpatient visits that used Spanish-language interpreters found an average of 31 interpretation errors per pediatric clinic visit (Flores et al., 2003), two-thirds of which had clinical consequences. The errors included errors in the dose and duration
of prescribed drugs and missed information on patient allergies. Errors were most common with untrained interpreters. Amoxicillin for an ear infection was translated as one teaspoon three times a day in the ears, rather than by mouth. Steroid ointment for a baby’s face was translated as “rub on the body” and twice a day for three or four days was translated as “in four days.”
In building a safer health-care system, strategies are needed to include culture and communication, inclusive of patients and families, as an integral component of interventions to reduce medical and health errors and adverse events like these.
The interaction of health literacy, culture, language, and safety is not limited to patient safety. Worker safety is also influenced by health literacy. Workers must employ safeguards against hazardous materials and procedures. They are expected to read warning labels and right-to-know postings and take important precautionary measures. Yet, for some, the information may not accessible. The potential of low literacy and limited English proficiency to affect worker safety has been noted by health and safety educators and unions (Wallerstein, 1992). A recent National Academies workshop on Latino worker safety (NRC, 2003) noted that Spanish-speaking workers are four times more likely to be injured or killed on the job than English-speaking workers. The need to improve and properly translate signage, safety and use instructions, and training procedures is noted in that report (NRC, 2003). Strategies suggested to improve worker safety training include participatory education and popular education approaches (e.g., Wallerstein, 1992). Further research in this area is critical to the development of successful safety programs.
|
I prepared all my material, cut all my pieces of Formica, and I opened the can and I didn’t really read the label. There was a red label on the can and even if I would have looked at it, I didn’t know what it was. I couldn’t even read it. Come to find out my lung was poisoned from that material I was using (Rudd and DeJong, 2000). |
Crossing the Quality Chasm: A New Health System for the 21st Century (IOM, 2001) proposed that health-care processes are to be redesigned according to 10 rules, 6 of which allude to cultural context and 5 of which relate to health literacy via information and communications. These rules are shown in Box 6-1. Twenty-first century health care will unravel the complexities that arise from the interplay of cultural processes and health literacy. Proposed changes include customizing care based on patient needs and values and accommodating differences in patient preferences and an-
|
BOX 6-1
SOURCE: IOM (2001). |
ticipating patient needs. Customizing (to match individual specifications) and tailoring (to adapt for special needs or purpose) approaches to patient care services are based on the assumption that understanding of people’s needs, characteristics, and respect for beliefs and values are operating. Such understanding and respect is rooted in patient-centered care (Gerteis et al., 1993). Customized and tailored information, communication, and education are integral to individualized care (IOM, 2001). To customize and tailor care means that health literacy is subsumed within a cultural context
during clinical encounters. This report also recommended that a “patient-centered” approach be implemented to ensure that patients have full understanding of all of their options (IOM, 2001).
Access to Health Care and Preventive Services
Literacy and disparities are two sides common to a health phenomenon rather than two separate problems. Data continue to emerge to support the idea that the different needs of particular individuals and groups who have been historically marginalized or disenfranchised due to their ethnic or racial heritage, or social group identity or membership, continue to be unmet by the health-care system (IOM, 2003a). Inattention to patient preferences, lack of patient-centered care, and insufficient English proficiency (written or verbal) are contextual qualities that fuel health disparities among particular groups and individuals in the United States. In this way, limited health literacy may be a precursor to and condition of health disparities. Interventions to increase communications, improve access to health information, promote the understanding of meaning from facts, and transfer knowledge to actions are likely to reduce the negative impact of low literacy on patients’ access and use of health services.
Health services access involves many factors. These include financial ability to use services, lack of appropriate services located where people can reach them, times when services are available, health-care staff’s ability to appropriately navigate language and culture with patients, and respectful treatment of patients. Ethnic background and minority status also influence access to care (IOM, 2003a). Across all populations, the individuals most likely to be dissatisfied with seeking care are members of minority groups. These minority groups indicated they felt their race, ethnicity, and ability to pay for services directly affected their level of care (IOM, 2002b). An IOM report found differences in access and in treatment of patients who are poor, African American, Latino, and American Indian, among others (IOM, 2003a). Health literacy is an additional factor that should be considered when examining access to care and use of preventive services. Preliminary research supports such a link. This research is reviewed in Chapter 3. Briefly, individuals with limited health literacy as determined by the available measures are less likely to use preventive services such as mammograms and pap smears (Scott et al., 2002), and may come to the attention of the health-care system at a more advanced stage of disease (Bennett et al., 1998).
A lack of health insurance and lack of access to affordable services may lead people to postpone or not participate in care, particularly preventative care such as screenings, but also including recommended medical tests, treatments, and prescribed medications (Kaiser Commission on Medicaid
and the Uninsured, 2003a). Rates of uninsurance in the United States vary by race and ethnicity at 12 percent of Caucasians, 20 percent of Asian Americans, 22 percent of African Americans, more than 25 percent of Native Americans, and more than 33 percent of Hispanics (Kaiser Commission on Medicaid and the Uninsured, 2003b). Uninsured patients are more likely to have poorer health than they would if insured (IOM, 2002a). Many African-American women who are uninsured or underinsured put their families’ welfare ahead of their own, especially when financial resources are limited. They seek treatment too late or not at all (IOM, 1999). Further, people who are uninsured or underinsured are more likely to rely on emergency departments for care (IOM, 2002b). An example of this can be seen in asthma. If someone cannot afford the appropriate workup, consultation, and medications to properly control asthma, they (or their children in the case of childhood asthma) will be less likely to prevent attacks that may lead to emergency department use (IOM, 2002b).
The problems created by the financial inability to use services may be exacerbated by health literacy issues, including limited knowledge and information surrounding early symptoms of serious illness and the value of prevention. Preliminary evidence discussed in Chapter 3 suggests that these socioeconomic, cultural, and health literacy factors may be associated with higher costs in the long run, when expensive tertiary care and emergency department services become necessary.
Limited local health resources and services can also impede access to care. Grocery stores and heath services are among the many resources that are limited in low-income and remote areas. Transportation may be a major obstacle; if services are difficult to get to or far away, people may do without, or postpone the use of the service. For some individuals often only limited primary care treatment services are available conveniently. So, for example, women needing mammograms may have to travel to a facility where this can be done, which can be a deterrent to obtaining that service. Limitations on hours of availability can also reduce access. Some clinics operate only or primarily during business hours. This may create problems for many people seeking health services. In particular, low-income patients may have a harder time getting time off from work, or finding child care, in order to seek care or take a family member to get care. Health services that have weekend and evening hours could potentially help prevent the unnecessary use of emergency departments.
Limitations on Provider Time
A major impediment to appropriate communication about health is the limitation on provider time that is often required by HMOs, public clinics, and health-care reimbursement plans. For example, most plans do not
reimburse for time spent in instructing patients on how to manage diabetes. It is nearly impossible to deal with literacy, language, and cultural issues within the context of a 10–15-minute patient visit. Ironically, the result of poor communication and abbreviated or no patient education is higher use of emergency services, greater severity of illnesses, failure to follow instructions and use medications properly, and other “errors” which ultimately result in increased health-care costs.
In this regard, Linzer and colleagues (2000) have established that time pressure is related to lower levels of physician career satisfaction, especially in the managed care setting. Federman and others (2001) have demonstrated that failure of physicians to acknowledge patient concerns, provide explanations of care, and spend sufficient time with patients may contribute to patients’ decisions to discontinue care at their usual site of care. Discussing these papers in an editorial Warde (2001) states that “it seems that a physicians ability to engage the patient without distraction from other activities is an important determinant in the quality of an encounter with a patient … true access means being psychologically available to conduct interviews that center on the physical, social, and psychological needs of each patient.” She goes on to suggest that “through its effect on the doctor–patient relationship, time pressure may diminish the very outcomes that health plans strive to achieve: high quality of care, access, cost-effective resource utilization, and patient satisfaction” (Warde, 2001). Encounters with limited-literacy patients can only require more time to overcome these barriers.
Health Expenditures
Health expenditures are of concern to decision makers in both the public and private sectors, and particularly to private employers and small businesses. Health spending has increased as a percentage of gross domestic product (GDP) as growth in health expenditures has outpaced growth in the economy as a whole. The Department of Health and Human Services (HHS)5 projects that the increase in health expenditures will fall to about 7 percent in the 2003–2007 period, and to 6.6 percent during 2008–2011 (Heffler et al., 2002). During this period health spending will continue to
outpace the overall economy by 2.5 percent per year, resulting in growth from 13.2 percent of GDP in 2000 to 17 percent in 2011. There is no reason to believe that these increases will moderate in the near term, and policy attention from both public and private sectors will continue. This attention has led to a renewed discussion of health-care policy as a national- and state-level issue that has included fundamental questions such as whether health insurance should be tied to employment, the relative roles of the public and private sectors, and whether consumers themselves should assume more responsibility for health-care costs and quality.
The most recent national estimate of expenditures for 2001 is $1.4 trillion or $5,035 per capita, based on a growth rate of 8.7 percent in 2001 (Levit et al., 2003). These expenditures are the highest in the world. The reasons for these high rates are complex. Although greater volume is often cited as the primary reason for higher costs in the United States as compared to other countries, it has been found that prices of services, rather than volume, are responsible for higher costs in the United States. Administrative costs in the private sector are also much higher than in other countries or in Medicare. While some combination of new technology and increased demand has led to increases in both prices and utilization, these vary across the nation. A recent study indicates that variation in expenditures between states is in part due to underlying demographics like age and wage rates, and in part due to the supply of hospitals and physicians (Martin et al., 2002). In addition, states with higher managed care penetration have lower expenditures. This is thought to be attributable to “both lower premium rates charged by HMOs and spillover effects of competition on non HMO premiums” (Martin et al., 2002).
Preliminary evidence on the cost implications of limited health literacy (presented in Chapter 3), while not conclusive, give some idea of the magnitude of impact of health literacy on national medical expenditures. Efforts to improve health literacy, or to limit its detrimental effects, may provide an important contribution to health-care policy addressing rising health expenditures (Heffler et al., 2002; Levit et al., 2002; Martin et al., 2002).
Consumer-Directed Health Care
A major emerging issue in the health system is increased consumer involvement in health-care choices. This movement has evolved primarily from self-insured employers who have developed a series of “consumer-driven” or “consumer-directed” health plans. These plans require consumers to make decisions about how they want to spend their health-care dollars, including how much co-insurance and out-of-pocket expenses to budget, which providers to see, and what services are really necessary
(Edlin, 2002). Consumer-directed health care appears to have developed because of the failure of both regulation and market forces to produce satisfactory cost containment results, and a belief that providing consumers with incentives can produce a more efficient and responsive system. The Foundation for Accountability, with support from the Agency for Healthcare Research and Quality (AHRQ), produced in June 2002 a “how-to guide” called “Who’s in the Driver’s Seat? Increasing Consumer Involvement in Health Care” (FACCT, 2002). This guide describes 48 different strategies currently being tried, which encompass employers implementing disease management programs, purchasing coalitions teaching enrollees about quality, health plans improving doctor–patient communications, consumer organizations relying on members’ expertise to develop communication materials, and researchers studying how people acquire and use information to improve their health. More narrowly, much attention is being given to health insurance vehicles which run the gamut from flexible spending accounts, medical savings accounts and defined contribution plans to new Internal Revenue Service-approved “health reimbursement arrangements” (Scandlen, 2003). Several new companies, such as Definity and Lumenos, have formed to market new consumer-driven vehicles (Elswick, 2003). These specific insurance products are still too new to assess their impact and effectiveness and some are skeptical that they will grow dramatically (Elswick, 2003).
In a broader sense, many in both the public and private sectors see increased consumer involvement in coverage and care decisions as a major force to improve the cost and quality issues that other approaches have not been able to achieve. If this is the case, the burden for persons with limited health literacy—who already face the challenges outlined in this report—will surely increase significantly.
Finding 6-2 Health literacy is fundamental to quality care, and relates to three of the six aims of quality improvement described in the IOM Quality Chasm Report: safety, patient-centered care, and equitable treatment. Self-management and health literacy have been identified by IOM as cross-cutting priorities for health-care quality and disease prevention.
Health Law and Health Literacy6
Legislatures and courts are beginning to respond to the issues raised by limited health literacy in the context of health care. Current laws require
health-care providers to furnish translators for patients who do not speak English, and interpreters to patients who have seeing or hearing disabilities.7 Current laws do not address the problem of patients with limited literacy. We found only a few cases in which literacy itself was pivotal in resolving the tort8 claim or lawsuit, two of which are discussed briefly below.
Cases in which literacy is pivotal to the tort claim are likely to be more common in the near future. The identification of health literacy as a cross-cutting contributor to health services quality (IOM, 2003b) indicates a need for legislative policies supporting health literacy as a contributor to good health care. These policies would affect the development of common law, which is driven by policy considerations. In this context, these policy considerations are likely to be defined on the basis of what is good health care. Each state has the power to develop its own common law or statutory law, which, in the absence of a single national policy, may contribute to variations between states in the recognition of health literacy as a contributor to good health care.
Two areas of health law and health care that are particularly central to health literacy are the standard of reasonable care and the informed consent process. It is around these areas that policy likely to affect common law could be developed.
The Standard of Reasonable Care
When a patient suffers an injury that was either directly caused by the health care rendered, or which could have been avoided by appropriate health care, the injured patient (or his or her family) may consult an attorney to determine whether the health-care provider is legally responsible for the injury. In making that assessment, the attorney will examine federal and state statutes, administrative regulations, and court decisions to determine what the legal standard is for the particular service provided. The court decisions, referred to as the common law, will in most instances include a reference to the standard of reasonable care. In most circumstances, reasonable care means care rendered in accordance with the standards of the profession. Courts seek to determine what a prudent health-care worker would do in similar circumstances on the evidence of professional standards. Practice standards developed by professional groups provide part of
this evidence; other evidence may be found in federal and state statutes and their implementing regulations, accreditation standards of professional associations, and by-laws of hospitals.
The treating physician or nurse is held liable for an injury only where ordinary care in accordance with the standards of the profession would have avoided the accident, rather than as a guarantor of a good outcome. Therefore, in most instances the professions control the standards by which their members are judged. There are two important qualifications to the profession’s control of the standards. First, the provider may be found negligent if he or she has special knowledge indicating that following the ordinary standard in the particular circumstances involved will expose the patient to an unreasonable risk of harm. Second, in rare instances, a court may declare that the whole profession may be found negligent for following a practice that new information or alternatives have shown to be unduly dangerous.
The concept of reasonable care can be applied to considerations of health literacy in the context of health care. The case of Incollingo v. Ewing9 provided a court opinion reflecting both the rule that a profession (or a large percentage of it) may be found negligent, and the rule that each physician must use his or her own personal knowledge. This case involved a child who died as a result of suffering aplastic anemia due to the consumption of a wide-spectrum antibiotic (Chloromycetin) prescribed at first by the child’s pediatrician for a throat infection and abdominal pains, and then renewed by a second physician after a telephone request by the child’s mother.
The doctor who initially prescribed Choloromycetin sought to justify his conduct on the ground that he was aware of the risk that the drug could cause aplastic anemia and therefore made sure not to allow for a renewal of the prescription. In his view, the child first presented to him with a throat infection that he regarded as a major ailment. He argued that even the plaintiff’s expert agreed that in prescribing this antibiotic he acted in the same manner as 95 percent of the doctors in Philadelphia where he practiced. The court noted that the drug’s package insert warned that the drug should not be prescribed for minor ailments, and the mother produced expert testimony at trial that the child indeed had a minor ailment when the drug was first prescribed. Consequently, the court found that the jury was allowed to find the first doctor negligent for not using his personal knowledge appropriately.
The doctor who renewed the prescription sought to defend his action
on the ground that most doctors in Philadelphia would have prescribed the same drug for a minor ailment at that time, based on a belief that the drug was effective and posed a very small risk of serious harm. He acknowledged that the package insert warned against such prescriptions of the drug, but contended that the medical community often prescribes the drug because the manufacturer had minimized its risks in communications directly to the prescribing doctors. Rejecting this argument, the court emphasized that medical custom is not always controlling, reflecting the rule that a profession (or a large percentage of it) may be found negligent. The health-care provider must exercise reasonable care, “giving due regard to the advanced state the profession at the time.”10
A similar view was expressed in Helling v. Carey11 (1974), in which the appellate court declared as a matter of law that a prevailing medical custom was negligent. A patient suffered blindness as a result of undiagnosed and untreated glaucoma. The patient had been under the care of the physicians for a number of years, and had never received a test of intraocular pressure. The prevailing custom of ophthalmologists was to perform routine pressure tests for early glaucoma only on patients who were over 40 years of age, because the risk of glaucoma was, in their view, too small in persons under 40 to justify routine pressure tests. The court ruled that the doctors were negligent as a matter of law, notwithstanding the evidence that they followed the custom of their specialty at the time. Noting that a low-risk, inexpensive test could have prevented a serious illness such as blindness in a long-term patient who was experiencing loss of vision, the court declared that reasonable care dictated the performance of the test.
This suggests that if future health literacy research supports the existence of associations between low-risk, inexpensive approaches to limited health literacy and reduced morbidity or mortality, the rule that a profession (or a large percentage of it) may be found negligent could apply to the failure to use health literacy interventions in clinical settings, including programmatic changes in health information provision. Providers could also be responsible when their knowledge indicated that a lack of patient understanding would expose the patient to an unreasonable risk of harm.
The Doctrine of Informed Consent
The doctrine of informed consent, which obligates the physician to inform the patient of the risks, benefits, and alternatives to undergoing or
refusing to undergo the treatment recommended by the physician, has extensive legal and research implications when addressing health literacy.
Informed consent in health care and research. In most cases, consent forms involve the use of structured and technical language to disclose subjects’ rights, roles, and responsibilities. They contain complex descriptions of institutional practices, financial and insurance considerations, legal concerns, advanced medical technologies, and potential risk/benefit considerations. Cumulative research over the past two decades and across three continents shows that consent forms for treatment and research are written at a level beyond the skills of most patients involved in research (Criscione et al., 2003; Freda et al., 1998; Goldstein et al., 1996; Gribble, 1999; Grossman et al., 1994; Grundner, 1980; Hammerschmidt and Keane, 1992; Hopper et al., 1995, 1998; Jubelirer, 1991; Lawson and Adamson, 1995; LoVerde et al., 1989; Mader and Playe, 1997; Mathew and McGrath, 2002; McManus and Wheatley, 2003; Meade and Byrd, 1989; Ogloff and Otto, 1991; Ordovas et al., 1999; Osuna et al., 1998; Rivera et al., 1992; Tait et al., 2003; Tarnowski et al., 1990). Examples of consent form text at different reading levels are provided in Table 6-1.
The readability of consent forms is often at a scientific level that contributes to information overload, poor understanding, and misinformed consent (Benson and Forman, 2002; Davis et al., 1994; Hopper et al., 1995; Meade and Howser, 1992; Philipson et al., 1995; Raich et al., 2001; Reicken and Rovich, 1982; Sugarman et al., 1998, 2002). In 2003, Paasch-Orlow et al. (2003) reported results of a cross-sectional study of 114 web sites from U.S. medical schools regarding their Institutional Review Board (IRB) readability standards and informed consent templates. Specific readability standards were found on 61 web sites (54 percent) and were found to range from a fifth-grade reading level to a tenth-grade reading level, while other sites contained descriptive guidelines such as “simple lay language.” Results revealed that informed consent text often falls short of the institutions’ own readability standards and suggest that federal oversight is associated with better readability. Figure 6-2 shows the difference between the readability of informed consent forms and the readability required by the IRBs.
Furthermore, while patients who sign consent documents often report their understanding of the research or treatment and satisfaction with the consent process, they may not fully understand the consent given (Horng et al., 2002; Pope et al., 2003; Vohra et al., 2003; Williams et al., 2003).
As pointed out in Chapter 1 of this report, all people (not just those with low educational levels) are at risk for low health literacy. The informed consent process brings with it particular challenges that may further impede understanding. This is in part attributable to the inherent complex-
TABLE 6-1 Examples of Informed Consent Text Provided by Institutional Review Boards at U.S. Medical Schools*
|
Readability Level |
Voluntary Participation |
No Direct Benefits |
|
4th Grade† |
“You don’t have to be in this research study. You can agree to be in the study now and change your mind later. Your decision will not affect your regular care. Your doctor’s attitude toward you will not change.” |
“There is no benefit to you from being in the study. Your taking part may help patients in the future.”‡ |
|
8th Grade† |
“Participation in this study is entirely voluntary. You have the right to leave the study at any time. Leaving the study will not result in any penalty or loss of benefits to which you are entitled.” |
“There is no direct benefit to you from being in this study. However, your participation may help others in the future as a result of knowledge gained from the research.” |
|
12th Grade§ |
“Your participation in this study is strictly voluntary. You have the right to choose not to participate or to withdraw your participation at any point in this study without prejudice to your future health care or other services to which you are otherwise entitled.” |
“There may be no direct benefit to me, however, information from this study may benefit other patients with similar medical problems in the future.” |
|
*All the examples are taken directly from medical-school Web sites unless otherwise noted. †The readability level is based on the Flesch-Kincaid readability scale. ‡The passage was modified to present key concepts at a fourth-grade reading level. §The readability level is based on the Fry readability formula. SOURCE: Excerpted from Table 1 in Paasche-Orlow et al. (2003). Copyright © 2003 Massachusetts Medical Society. All rights reserved. Reprinted with permission. |
||
ity and nature of informed consent information. But, it also relates to the multitude of psychosocial, ethical, and situational factors that may surround the clinical need for informed consent, such as hospitalization, emergency heart surgery, participation in Phase I cancer clinical trial, genetic testing, new vaccine for HIV, use of surgical placebos for Parkinson’s disease, or separation of conjoined twins.
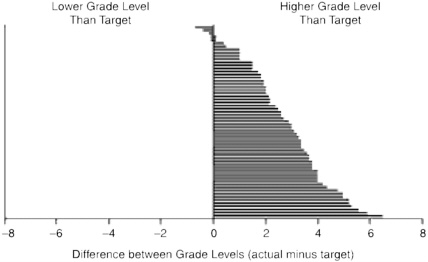
FIGURE 6-2 Difference between actual readability and target readability of informed consent documents. Each bar represents 1 of the 61 institutional review boards that indicated a specific grade-level target as a readability standard.
SOURCE: Paasche-Orlow et al. (2003). Copyright © 2003. Massachusetts Medical Society. All rights reserved.
A signature on a consent form is not adequate evidence that informed consent has been obtained. In providing informed consent, a research participant faces significant challenges, which are not adequately addressed through standard policy procedures (Triantafyllou et al., 2002). In many cases, patients and providers may disagree about the need for and adequacy of consent. Patients tend to see consent as necessary more frequently than providers, hold different views on whether true informed consent was obtained, and may be less than satisfied with the amount of information exchanged (Bray and Yentis, 2002; Cox, 2002; Gardner and Jones, 2002; King, 2001; Mathew and McGrath, 2002; McManus and Wheatley, 2003; Osuna et al., 2001; Schopp et al., 2003). These differences in views and biases in information may not be recognized by the provider or patient (Hewlett, 1996), and affect the patient’s right to self-determination12 and self-decision,13that is, the right to make any informed decision. Appelbaum (1997) notes that in communicating with patients, clinicians and researchers often underplay the risks associated with the randomized trials, and the benefits associated with standard care.
|
“I was in my early 30s and having problems with my girl parts. I was bulging in the vaginal area and I knew this was not normal. The doctor told me that it would be an easy repair and could be done. The surgery was set up. On the night before surgery, I remember having lots of papers pushed toward me to sign. I signed them because I needed to do this. I had surgery the next day and my recovery went very well. I had a large scar on my lower abdomen. I went for my six-week follow-up visit and was asked by the nurse how I was doing since my hysterectomy. No one had ever used those words before, but I knew what they meant. I had never asked any questions. I made the assumption that all doctors knew exactly what they were doing and had better intelligence than me. I was too humiliated to reveal to the doctor and nurse that I did not know what had been done to me. Communication had broken down and failed me.” “No one knew that I could not read well. Actually, I could read … but only one word at a time. By the time I got to the end of a sentence—I had no comprehension of what I had just read. I struggled to read. All that I read I would read three times. I kept books in front of me so others would not find out. I thought that if others found out, that they would think I was stupid. To check on the spelling of a word, I would call the library (that way no one could see me). There are many ways to hide poor reading from others.” Personal story graciously provided by Toni Cordell, Adult Learner and Literacy Advocate, as told to C.D. Meade, September 2003. |
Legal precedents. While no case directly addresses health literacy, cases exist in which theories of negligence, informed consent, and literacy have been brought to bear. A case that illustrates the potential issues that must be considered when examining literacy and informed consent is Hidding v. Williams14 (1991). The patient underwent a laminectomy that resulted in complete loss of control of his bowel and bladder. Prior to surgery he signed a consent form that stated that a risk of the surgery was “loss of bodily function.” Since the patient died prior to trial his wife prosecuted his personal injury claim and offered the only testimony from the patient’s view regarding the consent process. She testified that although her husband signed the form, he had only a fifth-grade education and minimal reading skills. She read the consent form for him and did the best she could to explain it. However, she thought “loss of function of bodily organs” meant that he might not be able to get up and walk around right away after the
surgery. She had no idea that it meant he faced a risk of loss of control of his bladder, and thus she did not include that information when she attempted to explain the risks he was confronting. In light of this testimony the judge, sitting without a jury, ruled that informed consent was not obtained. The court explained:
The physician is required to disclose material risks in such terms as a reasonable doctor would believe a reasonable patient would understand. In order for a reasonable patient to have awareness of a risk he should be told in lay language the nature and severity of the risk and the likelihood of its occurrence. A bland statement as to a risk of ‘loss of function of bodily organs’ when not accompanied by any estimate of its frequency does not amount to understandable communication of any specific real risk.15
The case supports the concept that a written document of a patient’s consent is evidence that an informed consent was obtained, but is not conclusive and can be rebutted by other evidence. Thus, while it is important to have documentary evidence of advising a patient of risks, benefits, and alternatives, the existence of that document does not prevent the court from considering whether the information deemed critical to making a meaningful expression of consent to the treatment was conveyed to the patient by a means that was likely to enable patient comprehension. Signed consent documents are treated in this manner in most states. Evidence that the patient could not read or comprehend the form leaves the issue of whether an informed consent was given to evidence regarding the communication process, such as verbal conversations, picture displays, and videos. The same is true with respect to instructions given to the patient or the patient’s family as to monitoring physical condition, administering medication, and so forth.
Finding 6-3 The readability levels of informed consent documents (for research and clinical practice) exceed the documented average reading levels of the majority of adults in the United States. This has important ethical and legal implications that have not been fully explored.
Governmental and Agency Roles
Roles of the Federal Government
Many of the federal health agencies have programs and activities for documenting and improving the health literacy of our nation. These agen-
cies can influence the health-care and public health systems to develop and support integrated strategies addressing health literacy and can increase the scientific knowledge base about health literacy by fostering research and collaboration.
In addition, the federal government plays a central role in the production and dissemination of health-related information and the regulation of such information from other sources. The involvement of specific agencies such as the Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) in these activities is described below. One widely known regulatory action in this regard is the Health Insurance Portability and Accountability Act of 1996. On April 14, 2003, as mandated by this act and in accordance with the Office of Civil Rights National Standards to Protect the Privacy of Personal Health Information, a notice of privacy practices was disseminated to all consumers entering the health-care system across the country (in hospitals, dental offices, pharmacies, and other health service locations).16 The law requires that this information be “read and understood” by consumers. Printed information was distributed in a variety of formats and languages to convey how medical information may be used and disclosed including information relating to treatment, payment and health-care operations, business associates, fundraising, research, appointment reminders, treatment alternatives, benefits and services, and persons involved in a patient’s care. However, the committee observed that the text disseminated to convey this important information varied widely in its nature, scope, and complexity. It is very likely that the privacy documents were written above the reading level of many Americans. Until formal evaluations are conducted, it remains unknown how well consumers fully understand the federal regulations that must be “read and understood” before care is provided.
We summarize here the activities of those agencies with important roles relating to health literacy in the health system context. This is not an exhaustive list of all federal agencies with related work, but is intended to highlight those that either have ongoing activity in this area or show particular promise to influence the issue. Chapters 4 and 5 highlight some of the health literacy-related activities of federal agencies in the contexts of the educational system and culture and society, including those related to language issues and interpretation in health care.
Office of the Secretary, Office of Disease Prevention and Health Promotion As the lead agency for the health literacy objective of Healthy People 2010
|
16 |
Health Insurance Portability and Accountability Act of 1996. http://www.hhs.gov/ocr/hipaa/. |
(HHS, 2000), the Office of Disease Prevention and Health Promotion (ODPHP) has been actively working to raise awareness about health literacy, to identify and coordinate health literacy activities across HHS, to convene HHS agencies to work collaboratively on health literacy, and to identify external partners. In 2000, ODPHP established a partnership with the National Center for Education Statistics of the U.S. Department of Education to develop the health literacy measures that are included in the 2003 National Assessment of Adult Literacy. These data represent the first national measures on health literacy, and will be used to assess the Healthy People 2010 objective. ODPHP has also collaborated with outside organizations on health literacy by including health literacy in its Memoranda of Understanding with several organizations, including the American Medical Association (AMA) and the Academy of General Dentistry.
Centers for Medicare & Medicaid Services (CMS) Individuals considered at highest risk for limited education and low health literacy are the elderly and those with low incomes. Many are enrolled in the Medicare and Medicaid programs administered by the Centers for Medicare & Medicaid Services (CMS). Medicare is a federally run health insurance plan covering nearly 40 million people in the United States who are 65 years of age and older, disabled, or have permanent kidney failure. Medicaid provides health assistance to certain individuals and families with low incomes or resources, and, in contrast to Medicare, is a state-administered program. See below for further information on Medicaid. Both Medicare and Medicaid are complicated and confusing programs, with health literacy issues in enrollment, making choices, patient rights, and terminology (Hudman, 2003; Scala, 2002).
Because CMS runs the Medicare program, it is directly responsible for communication with people covered under Medicare about health insurance coverage, their rights and protections in Medicare, and their health plan options. Communication about the Medicaid program is a function of each state, and CMS works with the states to ensure that people in Medicaid receive the information they need (see below for further information on Medicaid). In both cases, CMS works to ensure that these communications are accurate, reliable, relevant, understandable, and, to the extent possible, culturally appropriate. One way it does so is through the provision of agency-wide communication guidelines and training materials such as “Writing and Designing Print Materials for Beneficiaries: A Guide for State Medicaid Agencies.” CMS also uses consumer research and training, consultation with literacy experts, and communication guidelines to develop materials for consumers that are intended to be easy to navigate and understand through format, design, and wording modifications. Consumer research and testing includes target audience members with lower education
levels, from a variety of ethnic, racial, income, and health experience backgrounds.
The Food and Drug Administration The FDA regulates and provides information about drugs, biological products, medical and radiological devices, the food supply, and cosmetics. Three general areas of FDA activity related to health literacy are advertising, outreach, and labeling.
-
Advertising. Advertising for prescription drugs is regulated by the Code of Federal Regulations (21 CFR 202) and is enforced by the FDA. Criteria indicate that both print and broadcast advertisements must not be misleading, must provide balanced information about risks and benefits, must state the major risks, and, for print advertisements, must contain a brief summary statement of effectiveness. Broadcast advertisements also are required to include “adequate provision” for methods to obtain more detailed information, such as through a print ad, a toll-free telephone number, or by asking a health-care provider.
-
Outreach. Outreach to consumers and patients is a central activity for the FDA. The FDA develops public service campaigns and announcements, maintains web information for consumers, and carries out educational programs on specific topics. Challenges to successful consumer outreach at the FDA include: getting information to a wide variety of consumers with different needs, abilities, and desires; encouraging consumers to use the information; simplifying information without losing meaning or becoming too lengthy; and ensuring balance between risks and benefits (Lechter, 2002).
-
Labeling. The FDA approves and has legal jurisdiction over the content of labels for prescription and over-the-counter medications as well as biologics and medical devices. Aspects of medication labeling overseen by the FDA include medication guides, patient package inserts, and the standardized over-the-counter Drug Facts format. The FDA also performs research on label comprehension and the actual use of labels by consumers, monitors the prescription information provided to consumers by the private sector, carries out consumer outreach, and monitors prescription drug advertising.
An FDA regulation requires that over-the-counter medication labels be written: “… in such terms as to render them likely to be read and understood by the ordinary individual, including individuals of low comprehension, under customary conditions of purchase and use.”17 The sponsor or
manufacturer of a medication is responsible for producing labels that comply with this requirement, and may conduct label comprehension studies that require the participants to apply the label information in hypothetical situations. The FDA reviews the results of these studies in order to strengthen the label, and to determine whether the medication can safely and effectively be used without professional guidance. Participants in the studies include individuals with “low comprehension” as required by the regulation mentioned above. Low comprehension is typically defined as having an eighth-grade reading level or below, and the Rapid Estimate of Adult Literacy in Medicine (REALM) is frequently used to make this determination.
The Centers for Disease Control and Prevention As the lead public health agency of the United States, the CDC has a central role in successfully communicating information on health and illness to all members of the public. The CDC identifies “Providing credible information to enhance health decisions” (CDC, 2003) as one of the central goals of its mission. Related to health literacy, the CDC’s focus has encompassed efforts around plain language including training, testing and pre-testing materials, surveys, and the provision of health information to TV shows, networks, writers, and producers. The CDC has addressed issues of culture in several of its programs. For example, the National Institute for Occupational Safety and Health added a Spanish-language section to its web site in 2001, and the National Immunization Program developed educational material for American Indians and Native Alaskans in 2003. Currently, CDC is redesigning its web site based on a CDC web evaluation completed in 2002. The evaluation showed that consumers looking for basic health information regarding disease and disease prevention are the largest segment of visitors to the CDC web site.
The National Institutes of Health The National Institutes of Health (NIH) play the crucial role of determining federal funding for health literacy research, and thus in large part set the research agenda on the topic in the United States. Figure 6-3 shows NIH funding of health literacy over the past 6 years.18 These data were derived from a search of the NIH CRISP database from 1993 to 2002 using the following operands: “health literacy,” “health and literacy,” “health and readability,” and “literacy and readability.” The grants retrieved were examined for relevance to the field
|
18 |
The committee thanks Patrick Weld and K. Visnawath, Ph.D., of the National Cancer Institute for their contributions to this section of the report. Mr. Weld and Dr. Viswanath performed this search and analysis of the CRISP database. An expanded description of the methods of their work can be found in Appendix A. |
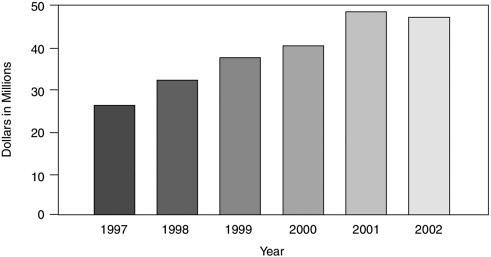
FIGURE 6-3 National Institutes of Health grant funding over the 1997–2002 period.
of health literacy. A total of 906 grants for a 10-year period were identified, but 229 of the grants from 1993–1996 contain missing financial data. The figure shows the funding totals from the 565 grants that remained after eliminating grants that were not funded, and grants for which data were missing.
“Low-literacy” components were included in studies in cancer, childhood development and reading, arthritis, asthma, diabetes, HIV, mental health, Alzheimer’s, health disparities, and studies in Spanish-speaking populations. In addition, “low literacy” was included in seven Requests for Applications since 2000, in studies such as Environmental Justice (sponsored by the National Institute of Environmental Health Sciences), Adult and Family Literacy (sponsored by the National Institute of Child Health and Human Development), Native American Research Centers for Health, oral health disparities, diet and physical activity assessment, and cancer communications.
Although the increase in funding over the past decade clearly represents a positive trend, the amount funded by NIH for programs and projects that examine health literacy is a small segment of the NIH budget. This amount, which is equivalent to $20 to $50 million per year, has consistently been less than half of 1 percent of the annual grant funding by NIH.
The Health Resources and Services Administration The Health Resources and Services Administration (HRSA) of HHS provides both service and
educational programs intended to improve access to health care, the quality of health care, and health outcomes. HRSA’s programs serve millions of diverse people from multiple racial and ethnic groups with differing educational levels who are frequently of low-income socioeconomic status. Educational programs provide training in interpretation, cultural competence, and communication for health-care providers, while HRSA’s service programs provide funding and direction for health-care delivery sites across the nation. Health-care delivery sites focused on various populations and types of care are administered by the various bureaus of HRSA, including the HIV/AIDS Bureau, the Bureau of Primary Health Care, and the Maternal and Child Health Bureau. The service programs utilize community health workers (also known as promotoras, outreach workers, or lay health workers) who are lay members of the community and work in association with the local health-care system, providing health education, interpreting health information, and assisting in obtaining access to services.
Agency for Healthcare Research and Quality The AHRQ is the part of HHS that sponsors, carries out, and disseminates research on health-care quality, medical errors and patient safety, health-care cost, and health disparities. AHRQ recently sponsored an evidence-based review of health literacy research to answer the following questions:
-
What is the relationship between literacy and health outcomes, use of health services, and resources?
-
What is the relationship between health literacy and racial disparities in health and health care?
-
What are effective interventions to reduce the impact of health literacy?
-
What are effective literacy-related interventions for reducing racial disparities in health and health care?
Although the results of this project were not available at the time of publication of this report, AHRQ worked with the IOM to provide preliminary information that was valuable in informing the work of the Committee.
The Department of Veterans Affairs The Department of Veterans Affairs (VA) is responsible for providing federal benefits, including health care, to the nation’s veterans and their dependents. The VA’s National Center for Health Promotion and Disease Prevention (NCP) uses patient education and health promotion techniques in order to increase quality of life and reduce health-care costs. The VA patient population is a vast heterogeneous group—6 million “enrolled” veterans, and over 25 million “eligible” vets.
They span all races, numerous cultures, all levels of education, all socioeconomic strata, and both genders, with approximately 85 percent males. The VA patient population also shows a wide age range of 18 years and up, with two basic spikes—20- to 30-year-old veterans with short military stints, and those over 50 years, representing retired military. The heterogeneity of the population points to the need for awareness of differences in attitudes, perceptions, and level of technological adeptness. Disabilities in the veteran population (including blindness, deafness, and mental disorders) also present barriers to access and communication. The NCP uses various techniques to ensure that educational and health promotional materials respond to all of these issues and needs.
Roles of State Governments
State governments have also identified health literacy as a critical issue. In 2002, the Council of State Governments undertook a national research project with the goals of (1) gathering data from the latest findings on health literacy, (2) determining what states are doing to make it easier for someone with low health literacy to navigate the health-care system, and (3) preparing a report providing information and tools necessary for state leaders to determine what appropriate action they might take. They sent a National Survey on Health Literacy Initiatives to state governor’s offices, departments of heath, Medicaid and State Children’s Health Insurance Program offices, departments of education, and offices of heath literacy. This resulted in a 2002 publication, The State Officials Guide to Health Literacy (Matthews and Sewell, 2002).
The most important finding from this survey is that health literacy is an emerging issue that few states have addressed specifically and directly. They report that while no state is addressing health literacy in a comprehensive, multifaceted manner, individual agencies in a handful of states—including Georgia, Illinois, Massachusetts, and Virginia—have established programs, hired staff, or created task forces to respond to low health literacy and its effects on health-care delivery. A number of states are involved in activities that make it easier for someone with low health literacy to navigate public assistance programs—such as simplifying enrollment materials and procedures—or to increase health literacy by setting health education standards in both K-12 and adult literacy classes. Examples of some of these efforts are discussed further below in the section on approaches to health literacy.
As discussed briefly above, Medicaid, the health-care assistance program for low-income individuals and families, is a state-run program. Each state sets its own guidelines for eligibility and services for Medicaid, under the general guidance of CMS. As reviewed in Chapter 3, individuals with lower income or resources are more likely than those with higher incomes
or resources to have limited literacy skills. Those enrolled in Medicaid, therefore, may be more likely to encounter barriers to care related to health literacy. Box 6-2 discusses the differences across states in dealing with health literacy and related issues in contracts with managed care providers of health care to those enrolled in Medicaid.
Roles of Regulatory Agencies
There are two main private organizations in the United States for accreditation and review of health-care facilities and providers: the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) and the National Committee for Quality Assurance (NCQA).
Joint Commission on Accreditation of Healthcare Organizations The private, nonprofit JCAHO is the oldest and largest health accreditation organization in the United States, providing accreditation to over 17,000 organizations including hospitals, health-care networks, home care agencies, and nursing homes. Its mission is “to continuously improve the safety and quality of care provided to the public through the provision of health care accreditation and related services that support improvement in health care organizations” (JCAHO, 2003). JCAHO approaches health literacy through its standards on patients’ rights and on patient and family education and responsibilities. These standards include assessing patient and family involvement in care and care decisions, the informed consent process, and hospital patient education tailored to patients’ assessed needs, abilities, learning preferences, and readiness to learn. Compliance with these standards is assessed through document review, staff interviews, and review of patient complaints. However, it is not clear how effective these standards are in improving organizational performance regarding health literacy issues, and significant changes in this process are planned for 2004.
National Committee for Quality Assurance NCQA is a private, not-for-profit organization dedicated to improving health-care quality, best known for its work in assessing and reporting on the quality of the nation’s managed care plans through their accreditation and performance programs. NCQA identifies health literacy as a critical step in ensuring patient participation in their health care. Testimony to the Committee from NCQA indicates that thus far it has been unable to create a valid and reliable measure that is feasible to apply at either the health plan or provider level. Such a measure is critical in trying to hold health-care providers and insurers accountable for both initial health literacy and improvement in health literacy (personal communication, L. Gregory Pawlson, M.D., M.P.H., NCQA, June 13, 2003). Regardless, there are a few provisions related to
|
BOX 6-2 Information from the Center for Health Services Research and Policy’s database on Medicaid managed care contracts (Rosenbaum et al., 2001) indicates that guidelines and requirements for Medicaid managed care providers vary across the states. Language in medicaid managed care contracts, valid as of 2001, shows that although a number of states’ contracts do address some literacy- and language-related issues, there are no specific requirements related to health literacy in any state, and many states do not address these issues at all or do so inconsistently. Among the 42 states with risk contracts that are captured in the Medicaid Managed Care database, 33 states and the District of Columbia included in their Medicaid managed care contracts some type of requirement pertaining to the provision of easily understood information. There is a great deal of variability across the states in how this is defined, and the method of determining if the requirement is being met. Notably, in most states, the requirement refers to the understanding of written material only. An exception is California, which requires that both verbal and written information be provided in a manner that can be easily understood. In some cases, the Medicaid managed care contract includes general language about patient comprehension such as the statement in the Massachusetts Medicaid Contract that requires that “written information…[be provided]…in a format and manner that is easily readable, comprehensible to its intended audience…” (Rosenbaum et al., 2001). Frequently, states indicate that written information must be readable at a specific reading grade level (see Chapter 2 for a discussion of reading grade levels). The reading grade level indicated varies significantly across states, ranging from as high as grade 8 in Montana and North Dakota, to grade 4 in a number of states, to a less-specific “suitable reading comprehension level” in Maryland. Still other states require that “plain language” be used. Overall, a sixth- |
health literacy issues that are currently part of the NCQA standards for managed care organizations, such as:
-
Quality Improvement Standard 4, which determines whether the organization assesses the cultural, ethnic, racial, and linguistic needs of its members and adjusts the availability of practitioners within its network, if necessary.
-
Patient Rights and Responsibilities 4, which assesses whether the organization provides translation services within its member services telephone function based on the linguistic needs of its members.
In addition, there is an indirect assessment of clinical function in the Consumer Assessment of Health Plan Survey. This survey includes a query
|
grade reading level is the most frequently cited requirement among the states that mention a reading level. A related issue is that some of the information provided to patients must be provided “as written” in federal law, and Medicaid managed contracts may specifically exclude such language from requirements to be easily understood. For example, in Texas, patient information about advance directives is excluded from its general readability requirement since certain language is required by federal law. Methods to determine readability level or whether information is easily understood also vary across states. Some states name specific programs, indices, or tests that must be used to determine reading grade level; these include methods such as the Fry Readability Index, Flesch Scale Analysis, or the McLaughlin Simplified Measure of Gobbledygook Index. Other states’ contracts simply state the desired reading grade level. Some states require that the managed care organization itself determine if its patient information meets the requirements, while in other states this task is reserved for the state or its designee. Clearly, much of this language such as that referring to the intended audience implies that attention should be given to individuals who do not speak English or who speak English as a second language, but less frequently is this explicitly stated. A few states do, however, require that information be provided in the other languages when the minority-language group makes up greater than 10 percent of the population. However, contracts more frequently include provisions for translation services and cultural competence. The Medicaid managed care contracts of 12 states do not include a requirement for services to be provided to persons whose primary language is not English. Although 25 states include a requirement that Medicaid managed care providers must insure cultural competence, only 10 of these states explicitly define what is meant by cultural competence. NOTE: The information presented in this section was obtained from Rosenbaum et al. (2001). |
which asks whether the member looked for any information about how a health plan works in written materials or on the Internet, and asks how much of a problem, if any, it was to find or understand this information.
OPPORTUNITIES IN HEALTH SYSTEMS
The understanding that no one size fits all is fundamental to the understanding of health literacy. There is not a simple solution to a complex problem. Determinants and approaches for health literacy are complex, dynamic, and require unique know-how, strategic thinking, and understanding of the audience. While we have stressed the importance of contributions of three contexts (the educational system, culture and society, and the health system) for improving health literacy, in Finding 2-1 we highlight
that the health-care system “carries significant opportunity and responsibility to improve health literacy.” This section explores options for how the health-care system can approach this responsibility to limit the negative impact that limited health literacy has on health care and health outcomes.
The strategies discussed in this section are based on current knowledge of interventions for limited literate individuals in health-care settings and on the small but growing evidence about how health literacy might be affected by various interventions. There are a few examples of interventions specifically designed to incorporate health literacy into health systems, and some information is available from the literature regarding interventions in other related fields such as health communication and health education. However, scientific investigation of interventions to minimize the impact of limited health literacy and promote the development of health literacy skills in the context of the health-care system is in its infancy. Conclusions about the success of health literacy programs based on these areas should be made cautiously. Research to evaluate the efficacy of these interventions is needed, and the committee’s discussions represent the present consensus of experts in the field.
Overview of Current Efforts
There are limited systematic approaches to health literacy and some of the organizations addressing health literacy are not based in academic institutions. It is important to be aware of approaches not yet evaluated or published in the peer-reviewed literature. Therefore, the committee commissioned an overview of approaches to health literacy currently being used in the United States that have not appeared in the peer-reviewed literature.19
The commissioned authors conducted a survey of organizations that “could make a difference” with health literacy. The types of organizations were identified first, and then the individuals most likely to be aware of health literacy issues and activities in that organization were contacted. The survey was a 5-minute, 15-question, web-based survey based on a previous survey to assess health literacy activities at the state level (Matthews and Sewell, 2002).
Information collected included: whether health literacy is considered in program development and service activities; the degree to which organiza-
|
19 |
The committee thanks Terry Davis, Ph.D.; Julie Gazmararian, M.P.H., Ph.D.; and Estela Marin, M.A., for their contributions to this section of the report. Drs. Davis and Gazmararian and Ms. Marin designed, carried out, and analyzed the survey. An expanded description of the methods of their work can be found in Appendix A. |
tions follow health literacy principles in their programs; target audience(s) for activities; whether organizations pilot test materials for comprehension or cultural competence; evaluation of materials; which activities people associate with health literacy and lessons learned. Of the 101 individuals e-mailed, completed surveys were received from 95 individuals from a variety of government agencies, public and private interest groups, as well as related business groups.
Eighty-one percent of respondents indicated that they consider health literacy in their program development and service activities. Almost all of the respondents associated health literacy with “comprehension” (94 percent) or “clear language” (92 percent). The majority associated health literacy with “cultural competence” (80 percent), “no jargon” (72 percent), or “readability testing” (71 percent) and “self-management” (67 percent). In addition to the pre-coded categories, respondents had the opportunity to provide other areas they associated health literacy with. A few other topics identified included issues of autonomy, empowerment, decision-making; patient safety and error reduction; numeracy and computation; provider responsibility for patient understanding; and quality of care. Table 6-2
TABLE 6-2 Frequency of Doing Health Literacy-Related Activities
summarizes the type of health literacy activities that respondents reported they or their organization were involved in. Almost 95 percent of respondents indicated that they simplified language and checked readability, and 93 percent indicated that they reformatted materials to make them more user-friendly either regularly or sometimes. Minority (e.g., Hispanic, African American, etc.) and low-income populations were the primary target group for these health literacy activities.
It is important to view the information gained from this survey with caution. The survey was sent to a limited number of organizations and individuals. The respondents are not generally representative of their respective organizations. Some of the nonrespondents may have wished to avoid confusion between personal opinion and institutional goals and policy, avoiding the possibility that their response would be interpreted as an institutional statement of policy rather than a personal view.
Approaches to Health Literacy in the Health System
Given the newness of the field, it is not surprising that little empirical work exists at this time. Much more needs to be done to provide solid guidance to all stakeholders for the most effective interventions in a variety of settings. However, as the survey above indicates, many different approaches that show promise are currently being tried; such innovations need to be encouraged and evaluated. In this section, we review different categories of approaches, look at some of the studies that have evaluated such approaches, consider what is already known about that approach from other fields, and showcase a number of promising efforts that are examples of the kind of work being done in this field.
For heuristic purposes, we have structured the following discussion around six categories of interventions under which most efforts appear to fall. These categories are as follows:
-
Provision of Simplified/More Attractive Written Materials
-
Technology-Based Communication Techniques
-
Personal Communication and Education
-
Combined Approaches
-
Tailored Approaches
-
Partnerships
In addition, it is important to note that training of educators and providers is one of the most important areas of current activity in the health literacy field. Because training cuts across nearly all of the intervention
categories above, we have chosen not to break it out as a separate area, but will mention particularly notable efforts in this area where appropriate.
Table 6-3 summarizes results from a sample of studies that have examined interventions taking place in, or sponsored by, health-care organizations. They are organized into the categories listed above, although we acknowledge that there is necessarily some overlap among categories with some types of interventions. These studies were gleaned from a review of the database on health literacy performed by Michael Pignone and colleagues as a commissioned project for AHRQ,20 as well as from recently published abstracts and testimony. This selection of studies was chosen to represent an array of various types of interventions in different populations and is not intended to be comprehensive. Studies included here did not have to measure the literacy level of participants. Overall, the studies displayed in Table 6-3 reflect the early state of the field. Pignone and colleagues evaluated the strength of the available evidence for health literacy interventions as part of the above-mentioned project for AHRQ (Pignone, 2003). The four criteria used to determine reliability were (1) the study was randomized, (2) the study design minimized confounding, (3) literacy was measured, and (4) the result of the intervention was reported by literacy level.
Five of the studies in Table 6-3 have been identified by Pignone (2003) as the most methodologically reliable of those that have been published (Davis et al., 1998b; Meade et al., 1994; Michielutte et al., 1992; Murphy et al., 2000; Wydra, 2001). Wydra and colleagues (2001) found that their interactive videodisc intervention increased reported self-care ability among cancer patients, but that the literacy level of the patients had no effect on this increase. Meade and colleagues (1994) examined the effectiveness of conveying information about colon cancer via videotape versus a printed booklet. Compared to controls, participants receiving any information demonstrated increased knowledge, but the difference between the two modes of communication was not significant. Davis and colleagues (1998b) found that those with the lowest literacy levels, after receiving a simplified and more appealing brochure, did not show a similar increase in comprehension similar to those with higher literacy skills. In contrast, Michielutte and colleagues (1992) also provided two different brochures—one with text and one illustrated—and found that even while two brochures had no influence on comprehension in the total research sample, those in the lowest literacy levels showed increased comprehension with the illustrated format. It is possible that a larger sample would show a different effect.
TABLE 6-3 Samples of Published Studies of Interventions in Health-Care Settings
|
Citation |
Setting |
Study Design |
Population |
|
Provision of Simplified/More Attractive Written Materials |
|||
|
Davis et al., 1998c |
Private and university oncology clinics, and a low-income housing complex |
Nonrandomized trial |
n = 183 53 patients with cancer or another medical condition and 130 apparently healthy participants |
|
Davis et al., 1998b |
Three pediatric care facilities in Louisiana |
Randomized controlled trial |
n = 610 Parents bringing children to pediatric care facility |
|
Eaton and Holloway, 1980 |
Outpatient clinic at Veterans Administration hospital in Minneapolis, MN |
Randomized controlled trial |
n = 108 Outpatients who could read English, see normal size type, and were not receiving warfarin therapy |
|
Hussey, 1994 |
Geriatric outpatient clinic in a county hospital in the southwestern U.S. |
Controlled trial (nonrandomized) |
n = 80 Patients of clinic 65 years or older with at least one chronic health problem, and either of low socioeconomic status or indigent |
|
Intervention |
Outcome |
|
Simplified informed consent form versus standard form. 69 participants given Standard Southwestern Oncology Group (SWOG) consent form (16th grade level) and 114 participants given simplified form (7th grade level) developed for study. |
Participants preferred simplified form over the SWOG form, and 97% thought the simplified form was easier to read than the SWOG form (p < 0.0001). However, there was no significant difference in degree of understanding of the two forms. |
|
Polio vaccine information brochures: 304 parents received simplified LSU version, 306 received CDC version |
Overall, readers of LSU brochure showed significantly higher comprehension (65% vs. 60%, p < 0.01); however, comprehension for most items did not achieve clinical significance and parents in the two lowest reading levels did not demonstrate increased comprehension with the LSU brochure. LSU brochure was preferred over CDC version. |
|
Patient drug information guide about warfarin written at either 5th- or 10th-grade reading levels. |
Comprehension was greater for 5th-grade-level information compared to 10th-grade-level information (p < 0.001), and for patients with a higher reading ability compared to those with a lower reading ability (p < 0.001). Reading ability explained 24% of variance and grade level of materials explained 8% of variance. Participants’ perception of level of difficulty, understandability, and clarity of the material was more favorable for the group receiving the 5th-grade material as compared with those receiving the 10th-grade material. |
|
Color-coded method to increase medication compliance. Group 1 received verbal teaching about medications only; Group 2 received verbal teaching and color-coded medication schedule. |
Significant increase in knowledge in both Groups 1 and 2 (p < 0.001), with no difference between the groups in knowledge increase. Significant increase in compliance in both Groups 1 and 2 (p = 0.007), with a greater increase in compliance for Group 2 (color-coded schedule) among those with low compliance scores at baseline. |
|
Citation |
Setting |
Study Design |
Population |
|
Jacobson et al., 1999 |
Ambulatory clinic at Grady Memorial Hospital, Atlanta, GA |
Randomized controlled trial |
n = 433 Primary care patients that had not previously been vaccinated, and had one of the following: diabetes, heart failure, other chronic illness, 65 years or older |
|
Michielutte et al., 1992 |
A private family three public health clinics—OB/GYN, family planning, and sexually transmitted diseases |
Randomized trial |
n = 217 Women age 18 or older Women who reported no ability to read or who reported “serious illness” practice and were excluded |
|
Powell et al., 2000 |
Pediatric clinic at Northwestern University Medical Center, Chicago, IL |
Prospective cohort |
n = 66 families Parents of children 6 years or younger who obtained primary care from the clinic |
|
Technology-Based Communication Techniques |
|||
|
Kim et al., 2001 |
Urology clinics in two VA hospitals in Chicago |
Uncontrolled trial |
n = 30 100% male |
|
Meade et al., 1994 |
Cancer clinic |
Randomized trial |
n = 1100 |
|
Intervention |
Outcome |
|
Low-literacy (below 5th-grade) handout to increase patient– physician dialogue about pneumococcal vaccination and increases rates of immunization. Intervention group received handout encouraging patients to “ask your doctor about the pneumonia shot” and control group received a low-literacy handout about nutrition. |
Intervention group significantly more likely than control group (p < 0.001 for all variables) to discuss vaccine with clinician, to receive vaccine, to show brochure to clinician, and for the clinician to recommend vaccine. When adjusted for race, sex, age, education, health status, insurance status, clinician training, and vaccine indication, intervention group significantly more likely than control group (p < 0.001 for all variables) to discuss vaccine with clinician and to receive vaccine. |
|
Cervical cancer information brochures: 112 women received illustrated brochure, 105 received nonillustrated version. |
No difference overall in comprehension with the two brochures. When results were analyzed by reading level, the illustrated brochure was better comprehended by lower literacy patients. |
|
Injury prevention information provided by pictorial anticipatory guidance (PAG) sheet requiring limited reading skills or TIPP (The Injury Prevention Program). |
No significant differences in caretaker recall of injury prevention information assessed by telephone 2–4 weeks later. |
|
Evaluate the knowledge, satisfaction level, and treatment preferences of men newly diagnosed with prostate cancer after intervention of CD-ROM about prostate cancer. |
Satisfaction with information and likelihood of following treatment preference not significantly different by literacy or educational background. |
|
Colon cancer information presented in printed and videotaped formats. Subjects received either a booklet, viewed a videotape, or received no intervention. |
Participants receiving any intervention showed increased knowledge compared with controls (booklet = 23% and videotape = 26%, no intervention = 3%). |
|
Citation |
Setting |
Study Design |
Population |
|
Murphy et al., 2000 |
Sleep clinic at Louisiana State University Health Sciences Center |
Controlled trial (nonrandomized) |
n = 192 (20 of which were caregivers) Patients at sleep clinic 18 years or older If younger than 18, caregiver participated |
|
Pepe and Chodzko-Zajko, 1997 |
Urban health department in the Midwest |
Uncontrolled trial |
n = 20 Low-income, inner-city adults aged 60–80 |
|
Wydra, 2001 |
Four comprehensive cancer centers |
Randomized trial |
86 intervention patients, 88 controls Patients over age 18 who were receiving outpatient cancer treatment Those with less than 5th-grade reading level or brain or visual dysfunction were excluded |
|
Personal Communication and Education |
|||
|
Mulrow et al., 1987 |
Diabetes clinic in London |
Randomized trial |
n = 120 Patients with diabetes who were overweight and not taking insulin Patients with a history of diabetic ketoacidosis, diabetes onset prior to age 29, or over the age of 70 where excluded. |
|
Paasche-Orlow, 2003 |
Two urban medical centers |
Uncontrolled |
n = 80 Adults hospitalized for asthma exacerbation |
|
Intervention |
Outcome |
|
Information about sleep apnea from an instructional videotape or a simple brochure. |
Short-term knowledge about sleep apnea was less accurate for those with reading levels less than 8th grade than for those with reading levels at or above 9th grade. Video intervention significantly improved only two areas of knowledge for readers below grade 8 (p < 0.05). |
|
Cholesterol information videotape shown on follow-up visit. |
Increase in cholesterol knowledge score from baseline (62%) to two-week follow-up after videotape (77%). 6 week follow-up scores at 72%. Change in time in cholesterol knowledge was not significantly different between reading groups. |
|
Interactive videodisc program designed to improve self-care with respect to fatigue symptoms for patients with cancer. |
Intervention patients reported greater self-care ability after the intervention (p < 0.0001). Literacy level did not affect the amount of self-care ability gained (p = 0.31). |
|
Education program for patients with non-insulin-dependent diabetes to improve and sustain glucose and weight control. Subjects were assigned to (1) monthly group sessions with videotapes for diabetic persons with low literacy skills; (2) monthly group sessions without videotapes; or (3) no monthly sessions after first introductory section like Group 2. |
No statistically significant differences in change in HbA1C levels within or between groups at either 7 or 11 months. Differences in weight change were significant (p < 0.05) at 7 months but no change at 11 months. No significant change in knowledge score with intervention. |
|
One-on-one oral and written intervention at discharge covering medication schedule and metered dose inhaler technique |
After instruction, about 1 in 3 high-risk adults required additional instruction about medication regimen. Higher acute care utilization, not low health literacy, predicted the need for additional instruction. |
|
Citation |
Setting |
Study Design |
Population |
|
Rothman et al., 2003 |
University-based general internal medicine practice |
Randomized trial |
n = 206 Patients with diabetes and poor glucose control |
|
Schillinger et al., 2003b |
Two primary care clinics in San Francisco |
Uncontrolled trial |
n = 74 Patients with type 2– diabetes mellitus |
|
Combined Approaches |
|||
|
Davis et al., 1998a |
Public hospital in Shreveport, LA |
Randomized controlled trial |
n = 445 Women 40 years old or over, attending ambulatory or eye clinic, and no mammogram in the past year |
|
Tailored Approaches |
|||
|
Hayes, 1998 |
Emergency departments, Midwestern rural area |
Randomized trial |
n = 60 Elderly emergency room patients |
|
Intervention |
Outcome |
|
Comprehensive disease management intervention. |
Literacy levels predicted improvement in glucose control among a control group with usual care, but not among the intervention group in the disease management program. |
|
A measure of the extent to which physicians assess patient recall and comprehension in a public hospital setting. |
Higher health literacy and physician application of interactive communication strategy were associated with good glycemic control (p < 0.01). |
|
3 different interventions to increase mammography usage. Group 1: personal recommendation from one of the investigators. Group 2: recommendation and easy-to-read National Cancer Institute brochure. Group 3: recommendation, brochure, and and motivational program, 12-minute interactive educational including video based on focus groups from the target population. |
At 6 months after intervention, Group 3 showed significant increase (p = 0.05) in mammography utilization as compared to Groups 1 or 2. This increase was no longer significant at 2 years. In a multivariate analysis with age, race, literacy and mammography knowledge at baseline, the only significant predictor of mammography use at 6 months was the Group 3 intervention. |
|
Comparison of the level of reading knowledge from either preprinted discharge instructions (control) or individualized computer generated discharge instructions (intervention). Contacted by telephone for follow-up interview. |
Intervention group did better on Knowledge of Medication Subtest (p = 0.05). |
Provision of Simplified/More Attractive Written Materials
Most of the approaches to improving health literacy that the committee has encountered involve producing patient information materials that are written with simplified language, have improved format (for example, more white space and friendlier layout), or use pictograms or other graphic devices (see information from survey above). It should be recognized that although creating patient information at readability levels that match patient and family reading levels may achieve limited positive patient outcomes, it may not achieve improved comprehension in all situations. For example, while simplification of informed consent information resulted in less intimidation (Davis et al., 1998c) and lower consent anxiety and higher satisfaction (Coyne et al., 2003), it did not improve patient comprehension. An example of an effort in this category is a project of the Geriatrics Section at the University of California–San Francisco that is seeking to increase the accessibility of advance directives to individuals with limited literacy skills. An advance directive form has been developed that incorporates culturally appropriate text-enhancing graphics appropriate for individuals with limited literacy skills. The form, available in both Spanish and English, not only describes what an advance directive is, but also walks patients through the process of filling out the form. It is written at a fifth-grade reading level and is currently being pilot tested and compared to other standard advance directive forms.
Graphics and other visual devices (also referred to as pictograms) are often used to replace or supplement text in health information communications. Pharmacies or patient educators may use pictograms as part of patient education handouts, or as stickers to be placed on prescription medication packaging. These types of uses are not considered part of the official packaging, and thus are not regulated by the FDA. Concerns have been expressed that some of the pictograms currently in use do not accurately convey the intended information (Rother et al., 2002). They may be open to interpretation, and interpretation may vary by an individual’s background and experiences. Pictograms are thought to be particularly beneficial for communicating information to consumers who speak English as a second language and to those with lower reading ability levels (USP, 2003). However, research suggests that individuals with limited health literacy may have particular difficulties correctly interpreting pictograms (Price et al., 2003). A research projected funded by Pfizer, Inc. entitled “Pictograms: A Tool for Enhancing Health Care Outcomes Among Underserved Patients in Primary Care?” is currently examining whether pictograms affixed to medication bottles enhance long-term recall of medication knowledge in patients identified as having difficulty with their medications; however, no data are as yet available from this 200-patient study. The nonprofit organization
FIGURE 6-4 Examples of pictograms for patient education.
SOURCE: United States Pharmacopeial Convention, Inc. Reprinted with permission. Copyright © 2004 United States Pharmacopeial Convention, Inc. All rights reserved.
U.S. Pharmacopeial Convention, Inc. (USP) maintains a free library of 81 pictograms that may be downloaded from the Internet. Figure 6-4 shows several examples of pictograms from the USP pictogram library.
Guidelines, templates, and tools are available that inform investigators and clinicians about ways to rewrite consent documents and other communications so that they are easier to understand. For example, Philipson and others (1999) evaluated a writing improvement intervention program implemented at Hartford Hospital to help the research writer produce more comprehensible informed consent documents and determined that the program increased the percentages of consent documents that were readable and at the target reading grade level. The National Cancer Institute (1998) has prepared a set of templates and recommendations for the development of easy-to-understand informed consent documents for cancer clinical trials that are available online and were distributed to IRBs, hospitals, cancer centers, patient groups, and researchers. These templates are currently in use among cooperative cancer clinical trial groups across the United States. While such guidelines and tools are helpful aids, solutions must also support changes in policies at the institutional and national level and become part of ongoing federal regulatory and monitoring activities of local compliance and IRBs. Box 6-3 displays two versions of an informed consent document: the original and simplified versions.
Technology-Based Communication Techniques
A number of the interventions displayed in Table 6-3 tested the effectiveness of technology-based communication techniques such as videos,
|
BOX 6-3 Original Consent Document Women are being invited to participate in a research investigation to determine the efficacy of two methods of assisting pregnant women in their smoking cessation attempts. A comparison of the effectiveness of educational media in combination with a counseling method on smoking habits is being examined. It cannot be guaranteed that women may personally benefit from this investigation, but in some instances, the knowledge gained might be beneficial to others. It is important to recognize that participation in this study is entirely voluntary. Individuals may withdraw from the study at any time without penalty or loss of benefits to which they are otherwise entitled. Such withdrawal will not compromise any individual’s ability to receive medical care at this institution. Revised Simplified Consent Document Introduction You are being asked to take part in a study that looks at ways to help pregnant women stop smoking. We want to know if booklets can help you to stop smoking. We also want to find out how nurses can help you to stop smoking. We cannot be sure that this study will help you, but it may help others. Taking part in this study is up to you. You can stop taking part in it at any time. It will not get in the way of your care at this clinic. SOURCE: Doak et al. (1996). Reprinted with permission of the Oncology Nursing Society. |
CD-ROMs, and interactive multimedia programs. Findings from these studies are equivocal. Some show positive outcomes such as increased knowledge or self-care ability, while others show no effect. Two of these studies found no interaction between the technology-based intervention and the literacy level of the individuals in the studies (Kim et al., 2001; Wydra, 2001). Simply transforming text versions of disease-specific education to more visually oriented media (for example, CD-ROM), while associated with improvements in satisfaction, does not appear to increase knowledge among patients with limited health literacy (Kim et al., 2001).
Although there is not yet clear evidence about the effectiveness of these types of technology-based communication techniques for improving health literacy or mitigating the effects of limited health literacy, such techniques are supported by research in the fields of commercial and social marketing, health education, and health communication. Speaking of Health (IOM, 2002c) provides an overview of this evidence base. In addition, evaluation of interventions to improve the informed consent process provide support for technology-based communication techniques such as computerized decision aids (Rostom et al., 2002), videotape plus physician communication (Agre et al., 1997), telephone-based nursing interventions (Aaronson et al., 1996), and audiovisual documentation of oral consent (video + audiotape + photography) (Benitez et al., 2002).
The Foundation for Informed Medical Decision Making has produced education videos intended to aid in patient decision-making as part of shared decision-making programs in clinics, managed care organizations, and other settings (Kasper et al., 1992). Based on studies indicating which patient preferences are most likely to affect treatment choices, these products incorporate testimonials about patient experiences and describe the consequences of different choices. A systematic review of this approach indicated that such decision aids improve knowledge, reduce decisional conflict, and stimulate patients to be more active in decision-making without increasing anxiety, while having variable effects on decisions and outcomes of decisions (O’Connor et al., 1999). Because health literacy is important to the shared decision-making process, this type of approach is important to consider but, unfortunately, has not yet been evaluated with patients with limited health literacy or limited literacy.
One widely used type of technology-based communication technique is telephone-delivered interventions (TDIs) in which health-related counseling and reminders are delivered using the telephone. Speaking of Health (IOM, 2002c) reports that the efficacy of such interventions is strongly supported by the evidence base but that they are underused by diverse populations. TDIs can vary by the type of service provider, the extent to which the call is scripted and varies based on characteristics and responses of the individual, and the extent to which subsequent calls take into account information
|
BOX 6-4
|
from other encounters with the individual (IOM, 2002c). In addition, TDIs can be initiated by the individual through calls to helplines or services, or by the health system, through outbound calls (IOM, 2002c). The effect of these variable characteristics is yet to be evaluated among individuals with low literacy and low health literacy.
Testimony to the committee has revealed a wide array of activities in the category of technology-based communication techniques that are currently being used. Some of these are summarized in Box 6-4.
Personal Communication and Education
Approaches in the category of personal communication and education include efforts such as classes or health education sessions for patients or other individuals, communication techniques used by health-care providers, and patient navigator programs. Although a review of the field of health education programs is beyond the scope of this report, several studies in Table 6-3 evaluated the effectiveness of education programs or one-on-one communication for individuals with low literacy skills. The study by Paasche-Orlow and colleagues (2003) found that among a groups of adults hospitalized for asthma exacerbation, all of the patients benefited from the one-on-one instruction regarding medications and inhaler technique, and that low health literacy as determined by the Short Test of Functional Health Literacy in Adults (S-TOFHLA) was not related to the need for additional instruction before understanding was achieved (see Table 6-3). In contrast, a study of patients with diabetes by Rothman and colleagues (2003) found that among the control group receiving no intervention, those with higher health literacy as measured by the REALM were more likely to achieve glucose control after 6 months than patients with low literacy, and among the intervention group, there was no difference in glucose control improvement between those with higher and lower health literacy (see Table 6-3). These findings suggest that the intervention was successful at mitigating the adverse effects of limited health literacy. The evaluation by Schillinger and others (2003b) of physician’s use of an interactive communication strategy in which the physician assessed patient recall and comprehension is another promising approach for individuals with limited health literacy. They found that the use of this strategy was associated with better glycemic control, although higher health literacy as measured by the S-TOFHLA was also associated with good glycemic control.
Programs using community health workers and patient navigators use one-on-one interaction to assist patients in navigating the health-care system. Such programs show promise for helping to mitigate the effects of limited health literacy since health system navigation has been noted as a particular problem for individuals with limited health literacy. Community health workers are known by various names (including lay health workers, outreach workers, and promotoras) and are lay members of the community who work in association with the local health-care system to provide health education, interpret health information, and assist in obtaining access to services. HRSA’s Bureau of Primary Health Care plays a large role in training and providing support to community health workers in a number of settings, including farm worker promotoras at its Migrant Health Centers.
Patient navigator programs are often run by hospitals or other health-
care systems, and many historically have been designed for patients with cancer. One of the first patient navigator programs was initiated by Harold P. Freeman in 1990 at what was then Harlem Hospital Center in New York City. The impetus for the program came from findings by the American Cancer Society that poor people face specific barriers when attempting to seek diagnosis and treatment of cancer and that these barriers result in failure to seek care, or diagnosis at a later—and less treatable—stage of disease. This program seeks to alleviate financial barriers, communication and information barriers, medical system barriers (such as missed appointments and lost results), and fear and emotional barriers by providing a navigator to patients with suspicious results from initial screening tests. The navigator acts as the patient’s advocate in the interval between the screening and further diagnosis or treatment, assisting with such practical issues as paperwork for financial support, childcare, or transportation problems. The navigator also may translate medical jargon into understandable language, provide education about the disease and its treatment, assist the patient in communicating with and asking questions of his or her doctor, and be available to listen to fears and concerns (Health Care Association of New York, 2002). Follow-up from the first two years of the program showed that 87.5 percent of patients with navigators completed recommended breast biopsies, while the rate in patients without a navigator was 56.6 percent. Patients with navigators also completed the biopsy in significantly less time than those without navigators (Freeman et al., 1995). The Patient Navigator Program continues at the newly established Ralph Lauren Center for Cancer Care and Prevention in Harlem, and similar programs have since been established in other settings such as Solano County, California, where a Patient Navigator Program was founded in 1999 by the Solano Coalition for Better Health–Community Cancer Task Force (CancerActionNOW.org, 2003) and at the Washington Cancer Institute in Washington, DC.
Combined Approaches
Some approaches to health literacy have incorporated a number of the different types of approaches discussed above into one intervention. A study by Davis and colleagues (1998a) at a public hospital in Louisiana (shown in Table 6-3 above) sought to increase mammography usage in women attending ambulatory and eye clinics. One of the interventions provided a personal recommendation from one of the investigators, an easy-to-read brochure, and a 12-minute education program using a video based on focus groups. Results of this randomized controlled trial showed that this combined approach intervention was the only significant predictor of increased mammography usage at 6 months after controlling for literacy,
age, race, and baseline knowledge; patients who received only a recommendation or only a brochure did not show increased mammography usage. Similarly, some promising interventions that have sought to improve understanding for informed consent involve the combined use of simplified written and verbal presentation to help patients better understand and concentrate on the information (Chan et al., 2002; Langdon et al., 2002; Muss et al., 1979; Sorrell, 1991; Tindall et al., 1994; Young et al., 1990). Research about the effectiveness of combining tailored print communications with TDIs remains equivocal, with some studies supporting increased effectiveness while others showed no effect. This research is reviewed in Speaking of Health (IOM, 2002c). Molina Healthcare, a managed care organization that focuses on low-income and Medicaid populations, conducted an evaluation of a low-literacy program of this combined type. Families with a child younger than six years of age were randomly assigned to either the control group (n = 13,737 and 5,614 at 2 years) or an intervention group (n = 28,366 and 12,079 at 2 years) that received a self-help book in the mail, plus follow-up reminders. The self-help book was appropriate for low-literacy populations and was intended to supplement information provided by advice nurses that are available by phone to all plan members. Results after two years showed decreases among the intervention group in parents taking their child to the emergency department for fever, rash, and diarrhea/vomiting and increases in parental self-efficacy for the same symptoms. The intervention group also demonstrated a decrease in calls to the advice nurse, and were more likely to still be members of the plan after two years than those in the control group (Ryan, 2003).
Box 6-5 describes a promising combined approach to health literacy that uses written materials, patient educators, provider communication techniques, and follow-up telephone calls.
Tailored Materials
As Chapter 4 emphasizes, culture gives significance to health information and messages and thus approaches to health literacy should consider the background and experiences of people. Patient viewpoints can provide important insights about what, how, when, where, and in what manner information can best be presented. Research on the informed consent process that has looked at the experience from the perspective of the patient has found that this can aid in untangling the complex dynamics associated with the process (Albrecht et al., 2003; Cox, 2002; Ruckdeschel et al., 1996). As Ratzan (2001) points out, the challenge in designing effective and understandable health communications is to determine the optimal context, channels, and content which reflect the realities of people’s everyday lives, situations, and communication practices. In this way, messages are
|
BOX 6-5 Researchers and practitioners at the University of North Carolina have developed several chronic disease management programs that are designed to identify and overcome literacy-related barriers to care. The programs, which include interventions for diabetes, heart failure, chronic pain, and anticoagulation, are led by mid-level providers, mainly clinical pharmacist practitioners, who use evidence-based algorithms, a computerized patient registry, and literacy-independent teaching techniques to facilitate effective self-care and assure receipt of effective services and medications. The teaching techniques are used by clinical pharmacists and trained health educators in a one-on-one interaction with the patient during clinic visits, and feature:
In each area, the program organizers have systematically measured literacy as well as relevant health outcomes. For diabetes and anticoagulation, completed studies have found that these programs can mitigate the adverse effects of low literacy. Studies in heart failure and chronic pain are ongoing. Their success in improving outcomes has led to stable funding for these programs from the hospital’s quality improvement department. |
grounded within the sociopolitical and environmental structures of the community and can better reflect the everyday lives of people. Furthermore, health information that is developed from an interdisciplinary approach is more likely to be effective, adopted, and successfully diffused within individual communities (Allen, 2001; Manderson, 1999; Watters, 2003). Project Toolbox is one example of an approach that used the viewpoints and experiences of the intended population to design the program; it is described in Box 6-6.
Similarly, characteristics of the individual patient or the target population can be used to tailor existing materials to fit specific situations. This is frequently done using computer-based algorithms that take various patient characteristics into account. These characteristics might include language, age, gender, ethnicity, reading ability, health literacy level, and the specific
|
BOX 6-6 Project Toolbox, funded by the State of Florida in 1999, represented a communication initiative of the H. Lee Moffitt Cancer Center & Research Institute. This project aimed to develop culturally, linguistically, and literacy relevant educational tools for community health-care providers and lay health educators for reaching medically underserved African-American and Hispanic migrant and seasonal farm workers with breast, cervical, and prostate cancer early detection and screening information. Four English and Spanish language toolboxes were created using social marketing and community-participatory research processes (Meade et al., 2002; Meade et al., 2003). Each toolbox contained a videotape, flipchart, educational brochures, and facilitator guide. Materials were written at a grade 3–4 reading level. Concepts were communicated using visuals, testimonials, and pictures that fit the everyday realities of the intended audiences. |
questions, needs, and goals of the patient at that time. Research on tailored print communications has typically shown that they can improve health outcomes (for review, see Revere and Dunbar, 2001) but research also suggests that they are less effective at influencing individuals who are not thinking about making a behavior change (IOM, 2002c). Hayes (1998) found that patients who received easily readable tailored discharge instructions subsequently performed better on a test of medication knowledge than patients given the standard preprinted instructions (see Table 6-3). Some current approaches that have come to the attention of the committee also use this strategy. For example, the Veterans Administration designed and distributes a “Flu Toolkit” that can be tailored to the educational and cultural needs of specific locales. Another example is currently being investigated by researchers at the University of California–San Francisco. They have developed a simple new communication tool that allows clinicians to print for their patients an individualized, visual medicine schedule (VMS). The VMS is a computer-generated, single page of paper that contains the names and digital images of the prescribed doses of medication, with symbols signifying the daily dosing schedule. The VMS displays the doses on a weekly calendar to accommodate participants with medication doses that differ from day to day. It can be updated and printed by the provider using a color printer and can include instructions in Spanish and Chinese in addition to English.
However, such approaches may not meet the needs of those with limited health literacy. Investigators from the Department of Obstetrics and Gynecology at the University of California–San Francisco have developed and evaluated a computerized, prenatal-testing, decision-assisting tool (“PT
Tool”), which is designed to inform pregnant women (and their partners) about prenatal testing options, and to assist them in making informed choices regarding prenatal testing for chromosomal disorders. The PT Tool provides background information about chromosomal abnormalities and other birth defects, highlighting the personal nature of prenatal testing decisions and the important role of individual preferences and values. The information is tailored for the individual patient, providing individualized risk estimates and allowing women to assess their own preferences and select testing options and strategies about which they would like more information. In a recently completed randomized controlled trial, it was found that women who viewed PT Tool had significantly greater knowledge, more satisfaction, and less decisional conflict than women who viewed a computerized version of the educational pamphlets distributed to pregnant women by the State of California. Furthermore, women viewing PT Tool differed in their testing inclination toward and utilization of prenatal testing. That these effects were particularly strong in college-educated women, raises questions about whether the tool would be effective for individuals with limited health literacy. The investigators are now developing a new, lower-literacy version of PT Tool that will be more engaging and accessible through the use of narrated animations and visual displays.
Partnerships
The increasing complexity of health systems—including new definitions of health, evolution of new media and the discovery of innovative biologic and genomic interventions, as well as the needs of diverse audiences—demands broad, interdisciplinary, multisectoral approaches. An IOM report, Bridging Disciplines in the Brain: Behavioral and Clinical Sciences, observes that “Solutions to existing and future health problems will likely require drawing on a variety of disciplines and approaches in which interdisciplinary efforts characterize not only the cutting edge of research but also the utilization of knowledge” (IOM, 2000a: 1).
To advance health literacy, it will be necessary to develop coordinated activities with local programs and institutions that improve quality and access to services, strengthen systems, and formulate effective policies. This includes fostering broad, interdisciplinary approaches to health literacy and supporting the design and management processes necessary to make them successful. Additionally, this requires involvement beyond the traditional health-care system with universities, training institutions, private- and public-sector media, and governmental social service departments.
A number of promising approaches brought to the committee’s attention have featured interdisciplinary partnerships. Among the longest run-
ning and most well-known of these is the National Literacy and Health Program in Canada which currently involves a partnership among 27 national organizations in Canada and is coordinated by the Canadian Public Health Association. In existence for more than a decade, its objectives are to (1) raise awareness among health professionals about the links between literacy and health; (2) build commitment among participating national health associations to promote literacy and health; and (3) establish links between national health associations and the federal government, literacy organizations, and provincial health associations in order to coordinate action on literacy and health. The program has undertaken a number of major projects such as producing a multimedia training resource for health professionals, providing a literacy and health curriculum for youth, and producing guidelines for prescription medication manufacturers. Although the National Literacy and Health Program has not as yet been evaluated formally (such an evaluation is currently under way), it appears to have had a major impact on both the health professional and literacy community in Canada.
In the United States, federal-level collaborative efforts have revolved around Healthy People 2010 (HHS, 2000). Healthy People 2010, as previously discussed, includes an objective on health literacy which is “to improve the health literacy of persons with marginal or inadequate literacy skills.” The Office of the Secretary, ODPHP is the lead agency for this objective, and has been working to identify and coordinate health literacy activities across HHS, to convene HHS agencies to work collaboratively on health literacy, and to identify external partners. In 2000, ODPHP established a partnership with the National Center for Education Statistics, U.S. Department of Education, to develop health literacy measures that are included in the 2003 National Assessment of Adult Literacy. This assessment will provide the first national measures of health literacy. ODPHP has also collaborated with outside organizations on health literacy including the AMA and the Academy of General Dentistry.
Some state- and local-level partnerships also currently exist. For example, the Santa Clara (California) Medical Center, Santa Clara County Library, and Plane Tree Health Library have partnered since 2001 to operate a center for health literacy on the Medical Center campus. The community learning center provides information on a variety of medical topics and conditions in English, Spanish, and Vietnamese in a variety of formats (print, audio, and video) with a focus on easy-to-read materials. The Medical Center provides space readily accessible to patients, the Plane Tree Library provides supervision and expertise in resource development, and the Santa Clara Library recruits adult literacy students to visit the Center and provide literacy support to patrons referred by health-care providers. Since May 2001, more than 2,000 clients have been served.
Summary of Approaches
It can be seen that, as in the survey, most of the examples and studies cited above fall into the category of “simplifying materials,” which includes issues of multiple languages and translation, and can be used for enhanced provider–patient communication as well as in other settings. Several of them include the dimension of testing materials with the intended users for understanding and subsequently making appropriate modifications, which the committee has noted is very important. Some work is now in progress to develop nonprint approaches, such as visual aids, computer instruction, and entertainment programs. Many of the federal and some state efforts are in the training area, most notably many of the programs in the multiple bureaus of the HRSA. The emerging partnerships in both the public and private sectors will be critical if sustained and systematic progress is to be made.
When respondents to the survey discussed above were asked an open-ended question about lessons learned from their health literacy activities, three fairly consistent themes about lessons learned emerged:
-
Organizational support and policies are lacking
-
Quality standards are needed
-
A variety of health literacy modalities are needed to be effective
Some comments from survey responders that correspond to each of these themes appear in Box 6-7 to illustrate current sentiment in the field.
This glimpse of ongoing activities from the literature, from the survey, and from testimony and Committee knowledge indicates that concerned organizations and individuals have recognized the complexity of the health literacy problem and have begun to simplify and pilot test materials, and to acknowledge the need to test a wide variety of health literacy approaches. This is a substantial improvement from the 1980s and early 1990s. However, it also highlights the lack of national- and organizational-level policy. Health literacy activities seemed to be based on the interest and determination of a few people within organizations who frequently are not policy makers. Increased awareness and activities related to health literacy appear to spread anecdotally rather than as a result of policy. This type of approach to the problem of limited health literacy is not sufficient to ensure continued interest and improvement in health literacy interventions. The next decade must see a significant increase in the number of programs and approaches that are developed and evaluated, as well as more systematic approaches and partnerships within and across both the public and private sectors.
|
BOX 6-7
|
|
Finding 6-1 Demands for reading, writing, and numeracy skills are intensified because of health-care systems’ complexities, advancements in scientific discoveries, and new technologies. These demands exceed the health-literacy skills of most adults in the United States. Finding 6-2 Health literacy is fundamental to quality care, and relates to three of the six aims of quality improvement described in the IOM Crossing the Quality Chasm report: safety, patient-centered care, and equitable treatment. Self-management and health literacy have been identified by IOM as cross-cutting priorities for health-care quality and disease prevention. Finding 6-3 The readability levels of informed consent documents (for research and clinical practice) exceed the documented average reading levels of the majority of adults in the United States. This has important ethical and legal implications that have not been fully explored. Recommendation 6-1 Health-care systems, including private systems, Medicare, Medicaid, the Department of Defense, and the Veterans Administration should develop and support demonstration programs to establish the most effective approaches to reducing the negative effects of limited health literacy. To accomplish this, these organizations should:
Recommendation 6-2 HHS should fund research to define the needed health-literacy tasks and skills for each of the priority areas for improvement in health-care quality. Funding priorities should include participatory research that engages the intended populations. Recommendation 6-3 Health literacy assessment should be a part of healthcare information systems and quality data collection. Public and private accreditation bodies, including Medicare, NCQA, and JCAHO, should clearly incorporate health literacy into their accreditation standards. Recommendation 6-4 HHS should take the lead in developing uniform standards for addressing health literacy in research, service, and training applications. This includes addressing the appropriateness of research design and methods and the match among the readability of instruments, the literacy level, and the cultural and linguistic needs of study participants. In order to achieve meaningful research outcomes in all fields: |
|
REFERENCES
Aaronson NK, Visser-Pol E, Leenhouts GH, Muller MJ, van der Schot AC, van Dam FS, Keus RB, Koning CC, ten Bokkel Huinink WW, van Dongen JA, Dubbelman R. 1996. Telephone-based nursing intervention improves the effectiveness of the informed consent process in cancer clinical trials. Journal of Clinical Oncology. 14(3): 984–996.
Agre P, McKee K, Gargon N, Kurtz RC. 1997. Patient satisfaction with an informed consent process. Cancer Practice. 5(3): 162–167.
Albrecht TL, Ruckdeschel JC, Riddle DL, Blanchard CG, Penner LA, Coovert MD, Quinn G. 2003. Communication and consumer decision making about cancer clinical trials. Patient Education and Counseling. 50(1): 39–42.
Allen CE. 2001. 2000 presidential address: Eliminating health disparities. American Journal of Public Health. 91(7): 1142–1143.
Appelbaum PS. 1997. Rethinking the conduct of psychiatric research. Archives of General Psychiatry. 54(2): 117–120.
Baker LM, Wilson FL. 1996. Consumer health materials recommended for public libraries: Too tough to read? Public Libraries. 35: 124–130.
Baker DW, Parker RM, Williams MV, Pitkin K, Parikh NS, Coates W, Imara M. 1996. The health care experience of patients with low literacy. Archives of Family Medicine. 5(6): 329–334.
Benitez O, Devaux D, Dausset J. 2002. Audiovisual documentation of oral consent: A new method of informed consent for illiterate populations. Lancet. 359(9315): 1406–1407.
Bennett CL, Ferreira MR, Davis TC, Kaplan J, Weinberger M, Kuzel T, Seday MA, Sartor O. 1998. Relation between literacy, race, and stage of presentation among low-income patients with prostate cancer. Journal of Clinical Oncology. 16(9): 3101–3104.
Benson JG, Forman WB. 2002. Comprehension of written health care information in an affluent geriatric retirement community: Use of the test of functional health literacy. Gerontology. 48(2): 93–97.
Berland GK, Elliott MN, Morales LS, Algazy JI, Kravitz RL, Broder MS, Kanouse DE, Munoz JA, Puyol JA, Lara M, Watkins KE, Yang H, McGlynn EA. 2001. Health information on the Internet: Accessibility, quality, and readability in English and Spanish. Journal of the American Medical Association. 285(20): 2612–2621.
Bertakis KD. 1977. The communication of information from physician to patient: A method for increasing patient retention and satisfaction. Journal of Family Practice. 5(2): 217–222.
Bodenheimer T. 1999. Disease management—Promises and pitfalls. New England Journal of Medicine. 340(15): 1202–1205.
Bray JK, Yentis SM. 2002. Attitudes of patients and anaesthetists to informed consent for specialist airway techniques. Anaesthesia. 57(10): 1012–1015.
CancerActionNOW.org. 2003. CancerActionNOW.org: Compassionate Use for Cancer Patients. [Online]. Available: http://canceractionnow.org/ [accessed: September, 2003].
CDC (Centers for Disease Control and Prevention). 2003. About CDC. [Online]. Available: http://www.cdc.gov/aboutcdc.htm [accessed: October, 2003].
Chan Y, Irish JC, Wood SJ, Rotstein LE, Brown DH, Gullane PJ, Lockwood GA. 2002. Patient education and informed consent in head and neck surgery. Archives of Otolaryngology–Head and Neck Surgery. 128(11): 1269–1274.
Cole SA, Bird J. 2000. The Medical Interview: The Three-Function Approach. 2nd edition. St Louis: Mosby.
Cooper LA, Roter DL. 2003. Patient-provider communication: The effect of race and ethnicity on process and outcomes in health care. In: Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Smedley BD, Stith AY, Nelson AR, Editors. Washington, DC: The National Academies Press. Pp. 336–354.
Cox K. 2002. Informed consent and decision-making: Patients’ experiences of the process of recruitment to phases I and II anti-cancer drug trials. Patient Education and Counseling. 46(1): 31–38.
Coyne CA, Xu R, Raich P, Plomer K, Dignan M, Wenzel LB, Fairclough D, Habermann T, Schnell L, Quella S, Cella D, Eastern Cooperative Oncology Group. 2003. Randomized, controlled trial of an easy-to-read informed consent statement for clinical trial participation: A study of the Eastern Cooperative Oncology Group. Journal of Clinical Oncology. 21(5): 836–842.
Crane JA. 1997. Patient comprehension of doctor-patient communication on discharge from the emergency department. Journal of Emergency Medicine. 15(1): 1–7.
Criscione LG, Sugarman J, Sanders L, Pisetsky DS, St Clair EW. 2003. Informed consent in a clinical trial of a novel treatment for rheumatoid arthritis. Arthritis and Rheumatism. 49(3): 361–367.
Davis TC, Mayeaux EJ, Fredrickson D, Bocchini JA Jr, Jackson RH, Murphy PW. 1994. Reading ability of parents compared with reading level of pediatric patient education materials. Pediatrics. 93(3): 460–468.
Davis TC, Bocchini JA Jr, Fredrickson D, Arnold C, Mayeaux EJ, Murphy PW, Jackson RH, Hanna N, Paterson M. 1996. Parent comprehension of polio vaccine information pamphlets. Pediatrics. 97(6 Pt 1): 804–810.
Davis TC, Fredrickson DD, Arnold C, Murphy PW, Herbst M, Bocchini JA. 1998a. A polio immunization pamphlet with increased appeal and simplified language does not improve comprehension to an acceptable level. Patient Education and Counseling. 33(1): 25–37.
Davis TC, Holcombe RF, Berkel HJ, Pramanik S, Divers SG. 1998b. Informed consent for clinical trials: A comparative study of standard versus simplified forms. Journal of the National Cancer Institute. 90(9): 668–674.
Davis TC, Berkel HJ, Arnold CL, Nandy I, Jackson RH, Murphy PW. 1998c. Intervention to increase mammography utilization in a public hospital. Journal of General Internal Medicine. 13(4): 230–233.
Doak LG, Doak CC, Meade CD. 1996. Strategies to improve cancer education materials. Oncology Nursing Forum. 23(8): 1305–1312.
Eaton ML, Holloway RL. 1980. Patient comprehension of written drug information. American Journal of Hospital Pharmacy. 37(2): 240–243.
Edlin M. 2002. Consumer-Directed Health Care—The goals: More choice, more control. Healthplan Magazine. 43(2): 12–17.
Elswick, J (BenefitNews.com). 2003. Consumer-Driven Health on a Roll. [Online]. Available: http://www.benefitnews.com/detail.cfm?id=4734&terms=|elswick| [accessed: August, 2003].
FACCT (Foundation for Accountability). 2002. Who’s in the Drivers Seat? Increasing Consumer Involvement in Health Care. [Online]. Available: http://www.facct.org/facct/doclibFiles/documentFile_528.pdf [accessed: August, 2003].
Federman AD, Cook EF, Phillips RS, Puopolo AL, Haas JS, Brennan TA, Burstin HR. 2001. Intention to discontinue care among primary care patients: Influence of physician behavior and process of care. Journal of General Internal Medicine. 16(10): 668–674.
Fiscella K, Franks P, Gold MR, Clancy CM. 2000. Inequality in quality: Addressing socioeconomic, racial, and ethnic disparities in health care. Journal of the American Medical Association. 283(19): 2579–2584.
Flores G, Laws MB, Mayo SJ, Zuckerman B, Abreu M, Medina L, Hardt EJ. 2003. Errors in medical interpretation and their potential clinical consequences in pediatric encounters. Pediatrics. 111(1): 6–14.
Freda MC, DeVore N, Valentine-Adams N, Bombard A, Merkatz IR. 1998. Informed consent for maternal serum alpha-fetoprotein screening in an inner city population: How informed is it? Journal of Obstetric, Gynecologic, and Neonatal Nursing. 27(1): 99–106.
Freeman HP, Muth BJ, Kerner JF. 1995. Expanding access to cancer screening and clinical follow-up among the medically underserved. Cancer Practice. 3(1): 19–30.
Gaba AG, Grossman SA. 2003. Braine Readability of informed consent forms at Johns Hopkins Hospital clinical oncology research protocols (1991–1999). Proceedings of the American Society for Clinical Oncology. 22: 524.
Gardner AW, Jones JW. 2002. An audit of the current consent practices of consultant orthodontists in the UK. Journal of Orthodontics. 29(4): 330–334.
Gerteis M, Edgman-Levitan S, Walker JD, Stoke DM, Cleary PD, Delbanco TL. 1993. What patients really want. Health Management Quarterly. 15(3): 2–6.
Goldstein AO, Frasier P, Curtis P, Reid A, Kreher NE. 1996. Consent form readability in university-sponsored research. Journal of Family Practice. 42(6): 606–611.
Good BJ, Good MJD. 1981. The meaning of symptoms: A cultural hermeneutic model for clinical practice. In: The Relevance of Social Science for Medicine. Eisenberg L, Kleinman A, Editors. Dordecht, Holland: Reidel. Pp. 165–196.
Graber MA, Roller CM, Kaeble B. 1999. Readability levels of patient education material on the World Wide Web. The Journal of Family Practice. 48(1): 58–61.
Gribble JN. 1999. Informed consent documents for BRCA1 and BRCA2 screening: How large is the readability gap? Patient Education and Counseling. 38(3): 175–183.
Grossman SA, Piantadosi S, Covahey C. 1994. Are informed consent forms that describe clinical oncology research protocols readable by most patients and their families? Journal of Clinical Oncology. 12(10): 2211–2215.
Grundner TM. 1980. On the readability of surgical consent forms. New England Journal of Medicine. 302(16): 900–902.
Hammerschmidt DE, Keane MA. 1992. Institutional Review Board (IRB) review lacks impact on the readability of consent forms for research. The American Journal of the Medical Sciences. 304(6): 348–351.
Hayes KS. 1998. Randomized trial of geragogy-based medication instruction in the emergency department. Nursing Research. 47(4): 211–218.
Health Care Association of New York. 2002. HANYS Breast Cancer Demonstration Project. [Online]. Available: www.hanys.org/quality_index/Breast_Cancer_Project/pnresourcekit.htm [accessed: September, 2003].
Heffler S, Smith S, Won G, Clemens MK, Keehan S, Zezza M. 2002. Health spending projections for 2001–2011: The latest outlook. Faster health spending growth and a slowing economy drive the health spending projection for 2001 up sharply. Health Affairs. 21(2): 207–218.
Hewlett S. 1996. Consent to clinical research—Adequately voluntary or substantially influenced? Journal of Medical Ethics. 22(4): 232–237.
HHS (U.S. Department of Health and Human Services). 2000. Healthy People 2010: Understanding and Improving Health. Washington, DC: U.S. Department of Health and Human Services. [Online]. Available: http://www.health.gov/healthypeople [accessed: January 15, 2003].
Hochhauser M. 1997. Can your HMO’s documents pass the readability test? Managed Care. 6(9): 60A, 60G–60H.
Hopper KD, TenHave TR, Hartzel J. 1995. Informed consent forms for clinical and research imaging procedures: How much do patients understand? American Journal of Roentgenology. 164(2): 493–496.
Hopper KD, TenHave TR, Tully DA, Hall TE. 1998. The readability of currently used surgical/procedure consent forms in the United States. Surgery. 123(5): 496–503.
Horng S, Emanuel EJ, Wilfond B, Rackoff J, Martz K, Grady C. 2002. Descriptions of benefits and risks in consent forms for phase 1 oncology trials. New England Journal of Medicine. 347(26): 2134–2140.
Hudman J. 2003. Understanding the Medicaid and Low-Income Populations: Implications for Health Literacy. Presentation given at a workshop of the Institute of Medicine Committee on Health Literacy. April 30, 2003, Washington, DC.
Hussey LC. 1994. Minimizing effects of low literacy on medication knowledge and compliance among the elderly. Clinical Nursing Research. 3(2): 132–145.
IOM (Institute of Medicine). 1999. The Unequal Burden of Cancer: An Assessment of NIH Research and Programs for Ethnic Minorities and the Medically Underserved. Haynes MA, Smedley BD, Editors. Washington, DC: National Academy Press.
IOM. 2000a. Bridging Disciplines in the Brain, Behavioral, and Clinical Sciences. Pellmar TC, Eisenberg L, Editors. Washington, DC: National Academy Press.
IOM. 2000b. To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press.
IOM. 2001. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press.
IOM. 2002a. Care Without Coverage: Too Little, Too Late. Washington, DC: National Academy Press.
IOM. 2002b. Health Insurance Is a Family Matter. Washington, DC: The National Academies Press.
IOM. 2002c. Speaking of Health: Assessing Health Communication Strategies for Diverse Populations. Washington, DC: The National Academies Press.
IOM. 2003a. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Smedley BD, Stith AY, Nelson AR, Editors. Washington, DC: The National Academies Press.
IOM. 2003b. Priority Areas for National Action: Transforming Healthcare Quality. Adams K, Corrigan JM, Editors. Washington, DC: The National Academies Press.
Jacobson TA, Thomas DM, Morton FJ, Offutt G, Shevlin J, Ray S. 1999. Use of a low-literacy patient education tool to enhance pneumococcal vaccination rates: A randomized controlled trial. Journal of the American Medical Association. 282(7): 646–650.
JCAHO (Joint Commission on Accreditation of Healthcare Organizations). 2003. Facts about the Joint Commission on Accreditation of Healthcare Organizations. [Online]. Available: http://www.jcaho.org/about+us/index.htm [accessed: October, 2003].
Jubelirer SJ. 1991. Level of reading difficulty in educational pamphlets and informed consent documents for cancer patients. The West Virginia Medical Journal. 87(12): 554–557.
Kaiser Commission on Medicaid and the Uninsured. 2003a. Access to Care for the Uninsured: An Update. Pub. No. 4142. Washington, DC: The Henry J. Kaiser Family Foundation.
Kaiser Commission on Medicaid and the Uninsured. 2003b. The Uninsured: A Primer. Key Facts about Americans Without Health Insurance. Washington, DC: The Henry J. Kaiser Family Foundation.
Kalichman SC, Rompa D. 2000. Functional health literacy is associated with health status and health-related knowledge in people living with HIV-AIDS. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 25(4): 337–344.
Kalichman SCP, Ramachandran BB, Catz SP. 1999. Adherence to combination antiretroviral therapies in HIV patients of low health literacy. Journal of General Internal Medicine. 14(5): 267–273.
Kasper JF, Mulley AG Jr, Wennberg JE. 1992. Developing shared decision-making programs to improve the quality of health care. Quality Review Bulletin. 18(6): 183–190.
Kaufer DS, Steinberg ER, Toney SD. 1983. Revising medical consent forms: An empirical model and test. Law, Medicine, and Health Care. 11(4): 155–162.
Kerka S. 2000. Health and Adult Literacy. Practice Application Brief No. 7. Columbus, OH: ERIC Clearinghouse on Adult, Career, and Vocational Education.
Kim SP, Knight SJ, Tomori C, Colella KM, Schoor RA, Shih L, Kuzel TM, Nadler RB, Bennett CL. 2001. Health literacy and shared decision making for prostate cancer patients with low socioeconomic status. Cancer Investigation. 19(7): 684–691.
King J. 2001. Consent: The patients’ view—A summary of findings from a study of patients’ perceptions of their consent to dental care. British Dental Journal. 191(1): 36–40.
Kleinman A. 1980. Patients and Healers in the Context of Culture: An Exploration of the Borderland Between Anthropology, Medicine, and Psychiatry. Berkeley, CA: University of California Press.
Langdon IJ, Hardin R, Learmonth ID. 2002. Informed consent for total hip arthroplasty: Does a written information sheet improve recall by patients? Annals of the Royal College of Surgeons of England. 84(6): 404–408.
Lawson SL, Adamson HM. 1995. Informed consent readability: Subject understanding of 15 common consent form phrases. IRB; A Review of Human Subjects Research. 17(5–6): 16–19.
Lechter K. 2002. Patient Information Activities at the FDA. Presentation given at a workshop of the Institute of Medicine Committee on Health Literacy. December 10, 2002, Washington, DC.
Levit K, Smith C, Cowan C, Lazenby H, Martin A. 2002. Inflation spurs health spending in 2000. Health Affairs. 21(1): 172–181.
Levit K, Smith C, Cowan C, Lazenby H, Sensenig A, Catlin A. 2003. Trends in U.S. health care spending, 2001. Health Affairs. 22(1): 154–64.
Linzer M, Konrad TR, Douglas J, McMurray JE, Pathman DE, Williams ES, Schwartz MD, Gerrity M, Scheckler W, Bigby JA, Rhodes E. 2000. Managed care, time pressure, and physician job satisfaction: Results from the physician worklife study. Journal of General Internal Medicine. 15(7): 441–450.
Lorig KR, Sobel DS, Stewart AL, Brown BW Jr, Bandura A, Ritter P, Gonzalez VM, Laurent DD, Holman HR. 1999. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: A randomized trial. Medical Care. 37(1): 5–14.
LoVerde ME, Prochazka AV, Byyny RL. 1989. Research consent forms: Continued unreadability and increasing length. Journal of General Internal Medicine. 4(5): 410–412.
MacKinnon JR. 1984. Health professionals’ patterns of communication: Cross-purpose or problem-solving? Journal of Allied Health. 13(1): 3–12.
Mader TJ, Playe SJ. 1997. Emergency medicine research consent form readability assessment. Annals of Emergency Medicine. 29(4): 534–539.
Manderson L. 1999. New perspectives in anthropology and cancer control, disease and palliative care. Anthropology and Medicine. 6(3): 317–322.
Martin A, Whittle L, Levit K, Won G, Hinman L. 2002. Health care spending during 1991–1998: A fifty-state review. Health Affairs. 21(4): 112–126.
Mathew J, McGrath J. 2002. Readability of consent forms in schizophrenia research. Australian and New Zealand Journal of Psychiatry. 36(4): 564–565.
Matthews TL, Sewell JC. 2002. State Official’s Guide to Health Literacy. Lexington, KY: The Council of State Governments.
McCulloch DK, Price MJD, Hindmarsh M, Wagner EH. 2000. Improvement in diabetes care using an integrated population-based approach in a primary care setting. Disease Management. 3(2): 75–82.
McManus PL, Wheatley KE. 2003. Consent and complications: Risk disclosure varies widely between individual surgeons. Annals of the Royal College of Surgeons of England. 85(2): 79–82.
Meade CD, Byrd JC. 1989. Patient literacy and the readability of smoking education literature. American Journal of Public Health. 79(2): 204–206.
Meade CD, Howser DM. 1992. Consent forms: How to determine and improve their readability. Oncology Nursing Forum. 19(10): 1523–1528.
Meade CD, Byrd JC, Lee M. 1989. Improving patient comprehension of literature on smoking. American Journal of Public Health. 79(10): 1411–1412.
Meade CD, McKinney WP, Barnas GP. 1994. Educating patients with limited literacy skills: The effectiveness of printed and videotaped materials about colon cancer. American Journal of Public Health. 84(1): 119–121.
Meade CD, Calvo A, Cuthbertson D. 2002. Impact of culturally, linguistically and literacy relevant cancer information among Hispanic migrant and seasonal farmworkers. Journal of Cancer Education. 17: 50–54.
Meade CD, Calvo A, Rivera M, Baer R. 2003. Focus groups in the design of prostate cancer screening information for Hispanic farmworker and African American men. Oncology Nursing Forum. 30(6): 967–975.
Meeuwesen L, Bensing J, van den Brink-Muinen A. 2002. Communicating fatigue in general practice and the role of gender. Patient Education and Counseling. 48(3): 233–242.
Michielutte R, Bahnson J, Dignan MB, Schroeder EM. 1992. The use of illustrations and narrative text style to improve readability of a health education brochure. Journal of Cancer Education. 7(3): 251–260.
Molina C, Zambrana RE, Aguirre-Molina M. 1994. The influence of culture, class, and environment on health care. In: Latino Health in the U.S.: A Growing Challenge. Molina C, Aguirre-Molina M, Editors. Washington, DC: American Public Health Association. Pp. 23–43.
Morales LS, Weidmer BO, Hays RD. 2001. Readability of CAHPS® 2.0 Child and Adult Core Surveys. In: Seventh Conference on Health Survey Research Methods. Lynamon ML, Kulka RA, Editors. Pub. No. (PHS) 01-1013. Hyattsville, MD: Department of Health and Human Services.
Mulrow C, Bailey S, Sonksen PH, Slavin B. 1987. Evaluation of an Audiovisual Diabetes Education Program: Negative results of a randomized trial of patients with non-insulin-dependent diabetes mellitus. Journal of General Internal Medicine. 2(4): 215–219.
Murphy PW, Chesson AL, Walker L, Arnold CL, Chesson LM. 2000. Comparing the effectiveness of video and written material for improving knowledge among sleep disorders clinic patients with limited literacy skills. Southern Medical Journal. 93(3): 297–304.
Muss HB, White DR, Michielutte R, Richards F 2nd, Cooper MR, Williams S, Stuart JJ, Spurr CL. 1979. Written informed consent in patients with breast cancer. Cancer. 43(4): 1549–1556.
NCI (National Cancer Institute). 1998. Simplification of Informed Consent Documents. U.S. Department of Health and Human Services. [Online]. Available: http://www.cancer.gov/clinicaltrials/understanding/simplification-of-informed-consent-docs/ [accessed: September 30, 2003].
Norris SL, Nichols PJ, Caspersen CJ, Glasgow RE, Engelgau MM, Jack L, Isham G, Snyder SR, Carande-Kulis VG, Garfield S, Briss P, McCulloch D. 2002a. The effectiveness of disease and case management for people with diabetes. A systematic review. American Journal of Preventive Medicine. 22(4 Supplement): 15–38.
Norris SL, Nichols PJ, Caspersen CJ, Glasgow RE, Engelgau MM, Jack L, Snyder SR, Carande-Kulis VG, Isham G, Garfield S, Briss P, McCulloch D. 2002b. Increasing diabetes self-management education in community settings. A systematic review. American Journal of Preventive Medicine. 22(4 Supplement): 39–66.
NRC (National Research Council). 2003. Safety Is Seguridad: A Workshop Summary. Washington, DC: The National Academies Press.
O’Connor AM, Rostom A, Fiset V, Tetroe J, Entwistle V, Llewellyn-Thomas H, Holmes-Rovner M, Barry M, Jones J. 1999. Decision aids for patients facing health treatment or screening decisions: Systematic review. British Medical Journal. 319(7212): 731–734.
Ogloff JR, Otto RK. 1991. Are research participants truly informed? Readability of informed consent forms used in research. Ethics and Behavior. 1(4): 239–252.
Ordovas BJP, Lopez BE, Urbieta SE, Torregrossa S.R., Jimenez TNV. 1999. An analysis of patient information sheets for obtaining informed consent in clinical trials. Medicina Clinica. 112(3): 90–94.
Osborne H, Hochhauser M. 1999. Readability and comprehension of the introduction to the Massachusetts Health Care Proxy. Hospital Topics. 77(4): 4–6.
Osuna E, Perez-Carceles MD, Perez-Moreno JA, Luna A. 1998. Informed consent. Evaluation of the information provided to patients before anaesthesia and surgery. Medicine and Law. 17(4): 511–518.
Osuna E, Lorenzo MD, Perez-Carceles MD, Luna A. 2001. Informed consent: Evaluation of the information provided to elderly patients. Medicine and Law. 20(3): 379–384.
Paasche-Orlow M. 2003. Education of patients with asthma and low literacy. Abstract. Journal of General Internal Medicine. 18(Supplement 1).
Paasche-Orlow MK, Taylor HA, Brancati FL. 2003. Readability standards for informed-consent forms as compared with actual readability. New England Journal of Medicine. 348(8): 721–726.
Pepe MV, Chodzko-Zajko WJ. 1997. Impact of older adults’ reading ability on the comprehension and recall of cholesterol information. Journal of Health Education. 28(1): 21–27.
Philipson SJ, Doyle MA, Gabram SG, Nightingale C, Philipson EH. 1995. Informed consent for research: A study to evaluate readability and processability to effect change. Journal of Investigative Medicine. 43(5): 459–467.
Philipson SJ, Doyle MA, Nightingale C, Bow L, Mather J, Philipson EH. 1999. Effectiveness of a writing improvement intervention program on the readability of the research informed consent document. Journal of Investigative Medicine. 47(9): 468–476.
Piette JD. 1999. Satisfaction with care among patients with diabetes in two public health care systems. Medical Care. 37(6): 538–546.
Piette JD. 2000. Interactive voice response systems in the diagnosis and management of chronic disease. The American Journal of Managed Care. 6(7): 817–827.
Pignone M. 2003. Literacy and Health: Findings from the Systematic Review for AHRQ. Presentation at the Pfizer Health Literacy Initiative 6th National Conference. Key Advances in Health Literacy: Models for Action. September 18–19, 2003, Washington, DC.
Pope JE, Tingey DP, Arnold JM, Hong P, Ouimet JM, Krizova A. 2003. Are subjects satisfied with the informed consent process? A survey of research participants. [Comment]. Journal of Rheumatology. 30(4): 815–824.
Powell EC, Tanz RR, Uyeda A, Gaffney MB, Sheehan KM. 2000. Injury prevention education using pictorial information. Pediatrics. 105(1): e16.
Price S, Raynor DK, Knapp P. 2003. Developing Effective Medicine Pictograms for the UK. Abstract. Presentation at the Health Services Research and Pharmacy Practice Conference, Belfast. [Online]. Available: http://hsrpp.org.uk/abstracts/2003_41.shtml [accessed: December 15, 2003].
Raich PC, Plomer KD, Coyne CA. 2001. Literacy, comprehension, and informed consent in clinical research. Cancer Investigation. 19(4): 437–445.
Ratzan SC. 2001. Health literacy: Communication for the public good. Health Promotion International. 16(2): 207–214.
Reicken HW, Ravich R. 1982. Informed consent to biomedical research in Veterans Administration Hospitals. Journal of the American Medical Association. 248: 344–348.
Reid JC, Klachko DM, Kardash CAM, Robinson RD, Scholes R, Howard D. 1995. Why people don’t learn from diabetes literature: Influence of text and reader characteristics. Patient Education and Counseling. 25(1): 31–38.
Revere D, Dunbar PJ. 2001. Review of computer-generated outpatient health behavior interventions: Clinical encounters “in Absentia.” Journal of the American Medical Informatics Association . 8: 62–79.
Rivera R, Reed JS, Menius D. 1992. Evaluating the readability of informed consent forms used in contraceptive clinical trials. International Journal of Gynecology and Obstetrics. 38(3): 227–230.
Root J, Stableford S. 1999. Easy to read consumer communication: A missing link in medicaid managed care. Journal of Health Politics, Policy and Law. 24(1): 1–26.
Rosenbaum S, Sonosky CA, Shaw K, Zakheim MH. 2001. Negotiating the New Health System: A Nationwide Study of Medicaid Managed Care Contracts. Shin P, Repasch L, Managing Editors. Center for Health Services Research and Policy, George Washington University.
Ross R. 1996. Returning to the Teachings: Exploring Aboriginal Justice. Toronto: Penguin Books.
Rost K, Roter D. 1987. Predictors of recall of medication regimens and recommendations for lifestyle change in elderly patients. Gerontologist. 27(4): 510–515.
Rostom A, O’Connor A, Tugwell P, Wells G. 2002. A randomized trial of a computerized versus an audio-booklet decision aid for women considering post-menopausal hormone replacement therapy. Patient Education and Counseling. 46(1): 67–74.
Roter DL. 2000. The outpatient medical encounter and elderly patients. Clinics in Geriatric Medicine. 16(1): 95–107.
Roter DL, Hall JA. 1992. Doctors Talking with Patients/Patients Talking with Doctors: Improving Communication in Medical Visits. Westport, CT: Auburn House.
Roter DL, Stewart M, Putnam SM, Lipkin M Jr, Stiles W, Inui TS. 1997. Communication patterns of primary care physicians. Journal of the American Medical Association. 277(4): 350–356.
Roter DL, Stashefsky-Margalit R, Rudd R. 2001. Current perspectives on patient education in the US. Patient Education and Counseling. 44(1): 79–86.
Rother HA, London L, Maruping M, Miller S. 2002. Hazard Communication for Pesticide Safety in Developing Countries: When is the Message Adequate? Bethesda, MD: Society for Occupational and Environmental Health Conference: Pesticide Exposure and Health.
Rothman RL, Pignone M, Malone R, Bryant B, DeWalt DA, Crigler B. 2003. A longitudinal analysis of the relationship between literacy and metabolic control in patients with diabetes. Abstract. Journal of General Internal Medicine. 18(Supplement 1).
Ruckdeschel JC, Albrecht TL, Blanchard C, Hemmick RM. 1996. Communication, accrual to clinical trials, and the physician-patient relationship: implications for training programs. Journal of Cancer Education. 11(2): 73–79.
Rudd RE. 2003. Objective 11-2: Improvement of Health Literacy. In: Communicating Health: Priorities and Strategies for Progress. Washington, DC: Office of Disease Prevention and Health Promotion, U.S. Department of Health and Human Services.
Rudd RE, DeJong W. 2000. In Plain Language: The Need for Effective Communication in Medicine and Public Health [Video]. Cambridge, MA: Harvard University.
Rudd RE, Colton T, Schacht R. 2000. An Overview of Medical and Public Health Literature Addressing Literacy Issues: An Annotated Bibliography. Report #14. Cambridge, MA: National Center for the Study of Adult Learning and Literacy
Ryan M. 2003. Demand Management. Presentation for Molina Healthcare.
Scala M. 2002. Linking Health Literacy and Medicare Education. Presentation given at a workshop of the Institute of Medicine Committee on Health Literacy. December 10, 2002, Washington, DC.
Scandlen, G. (Galen Institute). 2003. Consumer Driven Health Care: New Tools for a New Paradigm . [Online]. Available: http://www.galen.org/news/New_Tools.pdf [accessed: August, 2003].
Schillinger D, Grumbach K, Piette J, Wang F, Osmond D, Daher C, Palacios J, Sullivan GaD, Bindman AB. 2002. Association of health literacy with diabetes outcomes. Journal of the American Medical Association. 288(4): 475–482.
Schillinger D, Machtinger E, Win K, Wang F, Chan L-L, Rodriguez ME. 2003a. Are pictures worth a thousand words? Communication regarding medications in a public hospital anticoagulation clinic. Abstract. Journal of General Internal Medicine. 18(Supplement 1): 187.
Schillinger D, Piette J, Grumbach K, Wang F, Wilson C, Daher C, Leong-Grotz K, Castro C, Bindman AB. 2003b. Closing the loop: Physician communication with diabetic patients who have low health literacy. Archives of Internal Medicine. 163(1): 83–90.
Schillinger D, Bindman A, Stewart A, Wang F, Piette J. 2004. Functional health literacy and the quality of physician-patient communication among diabetes patients. Patient Education and Counseling. 52(3): 315–323.
Schopp A, Valimaki M, Leino-Kilpi H, Dassen T, Gasull M, Lemonidou C, Scott PA, Arndt M, Kaljonen A. 2003. Perceptions of informed consent in the care of elderly people in five European countries. Nursing Ethics: An International Journal for Health Care Professionals. 10(1): 48–57.
Scott TL, Gazmararian JA, Williams MV, Baker DW. 2002. Health literacy and preventive health care use among Medicare enrollees in a managed care organization. Medical Care. 40(5): 395–404.
Scrimshaw SC, Hurtado E. 1987. Rapid Assessment Procedures for Nutrition and Primary Health Care: Anthropological Approaches to Improving Program Effectiveness. Los Angeles, CA: University of California at Los Angeles, Latin American Center.
Shook EV. 1985. Ho’oponopono: Contemporary Uses of a Hawaiian Problem-solving Process. Honolulu, HI: East-West Center, University of Hawaii Press.
Shortell SM, Kaluzny AD, Editors. 2000. Health Care Management: Organization, Design, and Behavior. 4th edition. Albany, NY: Delmar Publishers.
Smith H, Gooding S, Brown R, Frew A. 1998. Evaluation of readability and accuracy of information leaflets in general practice for patients with asthma. British Medical Journal. 317(7153): 264–265.
Sorrell JM. 1991. Effects of writing/speaking on comprehension of information for informed consent. Western Journal of Nursing Research. 13(1): 110–122.
Street RL Jr. 1991. Information-giving in medical consultations: The influence of patients’ communicative styles and personal characteristics. Social Science and Medicine. 32(5): 541–548.
Sugarman J, McCrory DC, Hubal RC. 1998. Getting meaningful informed consent from older adults: A structured literature review of empirical research. Journal of the American Geriatrics Society. 46(4): 517–524.
Sugarman J, Kurtzberg J, Box TL, Horner RD. 2002. Optimization of informed consent for umbilical cord blood banking. American Journal of Obstetrics and Gynecology. 187(6): 1642–1646.
Tait AR, Voepel-Lewis T, Malviya S. 2003. Do they understand? (Part II): Assent of children participating in clinical anesthesia and surgery research. Anesthesiology. 98(3): 609–614.
Tarnowski KJ, Allen DM, Mayhall C, Kelly PA. 1990. Readability of pediatric biomedical research informed consent forms. Pediatrics. 85(1): 58–62.
Tindall B, Forde S, Ross MW, Goldstein D, Barker S, Cooper DA. 1994. Effects of two formats of informed consent on knowledge amongst persons with advanced HIV disease in a clinical trial of didanosine. Patient Education and Counseling. 24(3): 261–266.
Triantafyllou K, Stanciu C, Kruse A, Malfertheiner P, Axon A, Ladas SD, European Society of Gastrointestinal Endoscopy. 2002. Informed consent for gastrointestinal endoscopy: A 2002 ESGE survey. Digestive Diseases. 20(3–4): 280–3.
USP. 2003. Drug Information: USP Pictograms. [Online]. Available: http://www.usp.org/drugInformation/pictograms/. [accessed: June, 2003].
Vinicor F, Burton B, Foster B, Eastman R. 2000. Healthy people 2010: Diabetes. Diabetes Care. 23(6): 853–855.
Vohra HA, Ledsham J, Vohra H, Patel RL. 2003. Issues concerning consent in patients undergoing cardiac surgery—The need for patient-directed improvements: A UK perspective. Cardiovascular Surgery. 11(1): 64–69.
Von Korff M, Gruman J, Schaefer J, Curry SJ, Wagner EH. 1997. Collaborative management of chronic illness. Annals of Internal Medicine. 127(12): 1097–1102.
Wagner EH. 1995. Population-based management of diabetes care. Patient Education and Counseling. 26(1–3): 225–230.
Wagner EH, Glasgow RE, Davis C, Bonomi AE, Provost L, McCulloch D, Carver P, Sixta C. 2001. Quality improvement in chronic illness care: A collaborative approach. The Joint Commission Journal on Quality Improvement. 27(2): 63–80.
Wallerstein N. 1992. Health and safety education for workers with low-literacy or limited-English skills. American Journal of Industrial Medicine. 22(5): 751–65.
Warde C. 2001. Time is of the essence. Journal of General Internal Medicine. 16(10): 712–713.
Watters EK. 2003. Literacy for health: An interdisciplinary model. Journal of Transcultural Nursing. 14(1): 48–54.
Williams BF, French JK, White HD, HERO-2 consent substudy investigators. 2003. Informed consent during the clinical emergency of acute myocardial infarction (HERO-2 consent substudy): A prospective observational study. Lancet. 361(9361): 918–922.
Williams MV, Parker RM, Baker DW, Parikh NS, Pitkin K, Coates WC, Nurss JR. 1995. Inadequate functional health literacy among patients at two public hospitals. Journal of the American Medical Association. 274(21): 1677–1682.
Williams MV, Baker DW, Honig EG, Lee TM, Nowlan A. 1998a. Inadequate literacy is a barrier to asthma knowledge and self-care. Chest. 114(4): 1008–1015.
Williams MV, Baker DW, Parker RM, Nurss JR. 1998b. Relationship of functional health literacy to patients’ knowledge of their chronic disease. A study of patients with hypertension and diabetes . Archives of Internal Medicine. 158(2): 166–172.
Wydra EW. 2001. The effectiveness of a self-care management interactive multimedia module. Oncology Nursing Forum. 28(9): 1399–1407.
Young DR, Hooker DT, Freeberg FE. 1990. Informed consent documents: increasing comprehension by reducing reading level. IRB: A Review of Human Subjects Research. 12(3): 1–5.









































































