2
Hydrogen Chloride1
Acute Exposure Guideline Levels
SUMMARY
Hydrogen chloride (HCl) is a colorless gas with a pungent, suffocating odor. It is used in the manufacture of organic and inorganic chemicals, oil-well acidizing, steel pickling, food processing, and minerals and metals processing. A large amount of HCl is released from solid rocket fuel exhaust. It is an upper respiratory irritant at relatively low concentrations and may cause damage to the lower respiratory tract at higher concentrations. HCl is very soluble in water, and the aqueous solution is highly corrosive.
The lowest acute exposure guideline level (AEGL) values are based on a 45-minute (min) no-observed-adverse-effect level (NOAEL) of 1.8 parts per million (ppm) in exercising adult asthma patients (Stevens et al. 1992). No uncertainty factors (UFs) were applied for inter- or intraspecies variability because the study population consisted of sensitive humans. The same 1.8-ppm value was applied across the 10- and 30-min and 1-, 4-, and 8-hour (h) exposure times, because mild irritance generally does not vary greatly over time, and because it is not expected that prolonged exposure will result in an enhanced effect.
The AEGL-2 for the 30-min and 1-, 4-, and 8-h time points was based on severe nasal or pulmonary histopathology in rats exposed at 1,300 ppm for 30 min (Stavert et al. 1991). A modifying factor (MF) of 3 was applied to account for the relatively sparse database describing effects defined by AEGL 2. The AEGL-2 values were further adjusted by a total UF of 10–3 for intraspecies variability, supported by the steep concentration- response curve, which implies little individual variability; and 3 for interspecies variability. Using the default value of 10 for interspecies variability would bring the total adjustment to 100 (total UF×MF) instead of 30. That would generate AEGL-2 values that are not supported by the total data set, including data on exercising asthmatic subjects, an especially sensitive subpopulation, because exercise increases HCl uptake and exacerbates irritation; no effects were noted in exercising young adult asthmatic subjects exposed to HCl at 1.8 ppm for 45 min (Stevens et al. 1992). A total UF of 10, accompanied by the MF of 3, is most consistent with the total database (see Section 6.3 for detailed support of uncertainty factors). Thus, the total factor is 30. Time-scaling for the 1-h AEGL exposure period was accomplished using the Cn×t=k relationship (C=concentration, t=time, and k is a constant), where n=1 based on regression analysis of combined rat and mouse LC50 data (concentrations lethal to 50% of subjects) (1 min to 100 min) as reported by ten Berge et al. (1986). The 4- and 8-h AEGL-2 values were derived by applying an MF of 2 to the 1-h AEGL-2 value, because time-scaling would yield a 4-h AEGL-2 of 5.4 ppm and an 8-h AEGL-2 of 2.7 ppm, close to the 1.8 ppm tolerated by exercising asthmatic subjects without adverse health effects. The 10-min AEGL 2 was derived by dividing the mouse RD50 (concentration expected to cause a 50% decrease in respiratory rate) of 309 ppm by a factor of 3 to obtain a concentration causing irritation (Barrow 1977). It has been determined that human response to sensory irritants can be predicted on the basis of the mouse RD50. For example, Schaper (1993) has validated the correlation of 0.03×RD50=
TABLE 2–1 Summary of AEGLs Values for Hydrogen Chloride (ppm [mg/m3])
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
End Point (Reference) |
|
AEGL-1 (Nondisabling) |
1.8 (2.7) |
1.8 (2.7) |
1.8 (2.7) |
1.8 (2.7) |
1.8 (2.7) |
NOAEL in exercising asthmatic subjects (Stevens et al. 1992) |
|
AEGL-2 (Disabling) |
100 (156) |
43 (65) |
22 (33) |
11 (17) |
11 (17) |
Mouse RD50 (Barrow et al. 1977); histopathology in rats (Stavert et al. 1991) |
|
AEGL-3 (Lethal) |
620 (937) |
210 (313) |
100 (155) |
26 (39) |
26 (39) |
Estimated NOEL for death from 1-h rat LC50 (Wohlslagel et al. 1976; Vernot et al. 1977) |
|
Abbreviations: LC50, concentration lethal to 50% of subjects; mg/m3, milligrams per cubic meter; NOAEL, no-observed-adverse-effect level; NOEL, no-observed-effect level; ppm, parts per million; RD50, concentration expected to cause a 50% decrease in respiratory rate. |
||||||
Threshold Limit Value (TLV) as a value that will prevent sensory irritation in humans. The multiplier 0.03 represents the half-way point between 0.1 and 0.01 on a logarithmic scale, and Alarie (1981) has shown that the RD50 multiplied by 0.1 corresponds to “some sensory irritation,” while the RD50 value itself is considered “intolerable to humans.” Thus, it is reasonable that one third of the RD50, a value half-way between 0.1 and 1 on a logarithmic scale, may cause significant irritation to humans. Furthermore, one-third of the mouse RD50 for HCl corresponds to an approximate decrease in respiratory rate of 30%, and decreases in the range of 20–50% correspond to moderate irritation (ASTM 1991).
The AEGL-3 values were based on a 1-h rat LC50 study (Wohlslagel et al. 1976; Vernot et al. 1977). One-third of the 1-h LC50 of 3,124 ppm was
used to estimate a concentration causing no deaths. That estimate is inherently conservative (no deaths were observed in the same study at 1,813 ppm). A total UF of 10 will be applied—3 for intraspecies variation, because the steep concentration-response curve implies limited individual variability; and 3 to protect susceptible individuals. Using a full value of 10 for interspecies variability (total UF of 30) would yield AEGL-3 values that are inconsistent with the overall data set (see Section 7.3 for detailed support of UFs). Thus, the total UF is 10. The value was then time-scaled to the specified 10- and 30- min and 4-h AEGL exposure periods using the Cn×t=k relationship, where n=1 based on regression analysis of combined rat and mouse LC50 data (1 min to 100 min) as reported by ten Berge et al. (1986). The 4-h AEGL-3 value was also adopted as the 8-h AEGL-3 value because of the added uncertainty of time scaling to 8-h using a value of n derived for exposure durations up to 100 min.
1. INTRODUCTION
HCl is a colorless gas with a pungent, suffocating odor. It is hygroscopic and produces whitish fumes in moist air. HCl is produced as a by-product of chemical syntheses of chlorinated compounds and is used in the manufacture of organic and inorganic chemicals, oil-well acidizing, steel pickling, food processing, and the processing of minerals and metals. A large amount of HCl is released from solid rocket fuel exhaust. It is very soluble in water, and the aqueous hydrochloric acid is quite corrosive (EPA 1994). The physicochemical data for hydrogen chloride are shown in Table 2–2.
2. HUMAN TOXICITY DATA
2.1. Acute Lethality
2.1.1. Case Reports
No data concerning human lethality from HCl exposure were located in the available literature.
TABLE 2–2 Physicochemical Data for Hydrogen Chloride
|
Parameter |
Value |
Reference |
|
Synonyms |
Muriatic acid, hydrochloric acid |
AIHA 1989 |
|
Chemical formula |
HCl |
AIHA 1989 |
|
Molecular weight |
36.47 |
AIHA 1989 |
|
CAS registry no. |
7647–01–0 |
AIHA 1989 |
|
Physical state |
Colorless, fuming gas |
AIHA 1989 |
|
Relative density |
1.268 at 25°C |
AIHA 1989 |
|
Boiling/flash point |
−85°C/nonflammable |
AIHA 1989 |
|
Solubility in water |
Very soluble (82.3 g/100 ml) |
EPA 1994 |
|
Conversion factors in air |
1 mg/m3=0.67 ppm 1 ppm=1.49 mg/m3 |
AIHA 1989 |
2.2. Nonlethal Toxicity
2.2.1. Experimental Studies
Five male and five female adult asthmatic subjects (age 18 to 25 years [y]) were exposed to filtered air or HCl at 0.8 ppm or 1.8 ppm for 45 min (Stevens et al. 1992). Exposure levels were verified by an online filtering system during exposures and analyzed by ion exchange chromatography. Actual mean exposure concentrations were 0, 0.8±0.09, or 1.84±0.21 ppm. The subjects were healthy, except for having asthma, and wore half-face masks to allow for nasal and oral breathing and to control exposure of the eyes. The 45-min exposure sessions consisted of 15 min of exercise (treadmill walking at 2 miles per hour at an elevation grade of 10%) followed by 15 min of rest followed by another 15 min of exercise. Exposures to the test atmospheres were separated by at least 1 week (wk). Subjects rated severity of symptoms before, during, and after exposure on a scale of 1 to 5 (5 being most severe). Symptoms rated included upper respiratory (sore throat and nasal discharge), lower respiratory (cough, chest pain or burning, dyspnea, wheezing), and other (fatigue, headache, dizziness, unusual taste or smell). Pulmonary function measurements were performed while subjects were seated in a pressure-compensated volume-displacement body plethysmograph. The following parameters were measured: total respiratory resistance, thoracic gas volume at functional residual capacity,
forced expiratory volume, forced vital capacity, and maximal flow at 50% and 75% of expired vital capacity. Nasal work of breathing and oral ammonia levels were also measured. No adverse treatment-related effects were observed. There were no treatment-related increases in severity of upper respiratory, lower respiratory, or other symptoms reported by participants. No significant differences were reported between test and control exposures with regard to any of the pulmonary function tests. No treatment-related changes were observed in nasal work of breathing data. Oral ammonia levels showed a significant increase after exposure to both concentrations of HCl but not after exposure to air; the study authors conclude that this finding is counterintuitive and offer no explanation for the observation.
2.2.2. Case Reports
Reactive airways dysfunction syndrome (RADS) is an asthma-like condition that develops after a single exposure to high levels of a chemical irritant. Symptoms occur within minutes to hours after the initial exposure and may persist as nonspecific bronchial hyper-responsiveness for months to years (Bernstein 1993). It was given the name RADS by Brooks et al. (1985) in a retrospective analysis of 10 previously healthy people who had developed persistent airway hyper-reactivity after a single, high-level exposure to a chemical irritant. The acronym then gained acceptance in the medical community (Nemery 1996), because a name had finally been given to a clinical entity that physicians had encountered. Little or no published evidence had been previously available to verify the claim that asthma symptoms could be a consequence of a single inhalation exposure. This syndrome has been described after exposure to HCl. Promisloff et al. (1990) reported RADS in three male police officers (36–45 y old) who responded to a roadside chemical spill. The subjects were exposed to unquantified amounts of sodium hydroxide, silicon tetrachloride, and HCl as a by-product of trichlorosilane hydrolysis; due to the mixture if irritants involved in the release, it is likely that all compounds contributed to the RADS observed after this accident. In another report, Boulet (1988) described the case of a 41-y-old male nonsmoker who had a 6-y history of mild asthma. After cleaning a pool for 1 h with a solution containing hydrochloric acid, he developed a rapidly progressive and severe bronchospasm that was eventually diagnosed as RADS. No exposure concentration was reported. Turlo and Broder (1989) describe a retrospective review of occupational asthma records. A 57-y-old male, with a smoking history of
12 pack-years, had symptoms consistent with RADS after occupational exposure to hydrochloric acid and phosgene. No exposure concentrations were reported.
Other data concerning acute inhalation exposure to HCl in humans are qualitative and dated, making accurate exposure assessment difficult. A summary of those data is presented in Table 2–3.
2.2.3. Epidemiologic Studies
Epidemiologic studies regarding human exposure to HCl were not available.
2.3. Developmental and Reproductive Toxicity
No human developmental or reproductive toxicity data concerning HCl were identified in the available literature.
2.4. Genetic Toxicology
No data concerning the genotoxicity of HCl in humans were identified in the available literature.
2.5. Carcinogenicity
Data concerning carcinogenicity from exposure to HCl are equivocal. A study of U.S. steel-pickling workers showed an excess risk for lung cancer in individuals exposed primarily to hydrochloric acid for at least 6 months (mo) (Beaumont et al. 1987, as cited in IARC 1992). However, no exposure concentrations were available and the subjects had also been exposed to mists of other acids. In a follow-up of the same cohort, Steenland et al. (1988) observed an excess incidence of laryngeal cancer. Again, the data are confounded by possible exposure to other acid gases, including sulfuric acid. In three case-control studies, no association was observed between occupational exposure to HCl and lung (Bond et al. 1986, as cited in IARC 1992), brain (Bond et al. 1983), or kidney (Bond et al. 1985) cancer. In another report, Bond et al. (1991) examined the records of 308 workers who
TABLE 2–3 Inhalation Exposure of Humans to Hydrogen Chloride
|
Approximate Concentration |
Exposure Time |
Effect |
Reference |
|
0.77 ppm; 1–5 ppm; 10 ppm |
Unspecified |
Geometric mean of odor thresholds; odor threshold; odor threshold |
Amoore and Hautala 1983; Heyroth 1963; Leonard et al. 1969 |
|
0.8 ppm and 1.8 ppm |
45 min |
No effects in exercising asthmatic subjects |
Stevens et al. 1992 |
|
≥5 ppm |
Unspecified |
Immediately irritating |
Elkins 1959 |
|
>10ppm |
Occupational |
Highly irritating, although workers develop some tolerance |
Elkins 1959 |
|
10 ppm |
Prolonged |
Maximum tolerable |
Henderson and Hagard 1943 |
|
10–50 ppm |
A few hours |
Maximum tolerable |
Henderson and Hagard 1943 |
|
35 ppm |
Short |
Throat irritation |
Henderson and Hagard 1943 |
|
50–100 ppm |
1 h |
Maximum tolerable |
Henderson and Hagard 1943 |
|
1,000–2,000 ppm |
Short |
Dangerous |
Henderson and Hagard 1943 |
died of lung, bronchus, or trachea cancer. The workers were divided into groups for exposure duration as follows: <1 y, 1–4.9 y, or >5 y. Exposure concentrations were 0, 0.25, 1.5, or 3.75 ppm. No association was found between HCl exposure and cancer incidence. In a Canadian population-based case-control study, an increased risk for oat cell carcinoma was suggested in workers exposed to hydrochloric acid; however, no excess risk was observed for all types of lung cancer combined or for other histological types of lung cancer individually (Siemiatycki 1991, as cited in IARC 1992).
2.6. Summary
No treatment-related effects were observed in exercising, young adult asthmatic subjects exposed to HCl at 0.8 ppm or 1.8 ppm for 45 min. Reac-
tive airway dysfunction syndrome (RADS) has been described in people exposed to undetermined concentrations of HCl. Data concerning carcinogenicity from exposure to HCl are equivocal and are confounded by occupational exposure to other chemicals. No data concerning genetic toxicology or developmental or reproductive toxicity in humans from HCl exposure were located in the available literature.
3. ANIMAL TOXICITY DATA
3.1. Acute Lethality
3.1.1. Guinea Pigs
Malek and Alarie (1989) observed 100% mortality in exercising guinea pigs exposed to HCl at 586 ppm for approximately 3 min, although no deaths were observed in guinea pigs exposed at 162 ppm for 30 min. Burleigh- Flayer et al. (1985) exposed guinea pigs to HCl at 320, 680, 1,040, or 1,380 ppm for 30 min. Mortality was as follows: 2/8 during exposure at 1,380 ppm; 1/8 following exposure at 1,380 ppm; and 2/8 following exposure at 1,040 ppm. These studies describe both lethal and nonlethal effects and are described in detail in Section 3.2.2.
No mortality was observed in guinea pigs (unspecified strain) exposed to HCl at 3,667 ppm for 5 min; however, at 4,333 ppm for 30 min or 667 ppm for 2 to 6 h, 100% mortality was observed (Machle et al. 1942).
3.1.2. Rats and Mice
Darmer et al. (1974) examined the acute toxicity of HCl vapor or aerosol in groups of 10 male Sprague-Dawley rats exposed to HCl at 410–30,000 ppm and groups of 10 male ICR mice exposed to HCl at 2,100–57,000 ppm for 5 or 30 min. HCl concentrations were monitored continuously during exposures, and chloride ion specific electrode analysis was utilized to determine actual concentrations. Particle size distribution was analyzed for aerosol generation. Animals were observed for 7 d post-exposure. Examination of animals dying during exposure revealed moderate to severe gross changes in the lungs and upper respiratory tract. Badly damaged nasal and tracheal epithelium, moderate to severe alveolar emphysema, atelectasis, and spotting of the lung were noted at necropsy. Survivors at the higher concentrations exhibited a clicking breathing noise, difficulty
TABLE 2–4 LC50 Values for Hydrogen Chloride Vapor and Aerosol in Rats and Mice (ppm)
|
Species |
10-min LC50 Values |
30-min LC50 Values |
||
|
Vapor |
Aerosol |
Vapor |
Aerosol |
|
|
Rat |
41,000 |
31,000 |
4,700 |
5,600 |
|
Mouse |
13,700 |
11,200 |
2,600 |
2,100 |
|
Source: Darmer et al. 1974. |
||||
breathing, and bloody discharge from the nares. The authors conclude that there is no differential toxicity between the vapor and aerosol. Mice appear to be more sensitive than rats to the acute inhalation toxicity of HCl. LC50 values are presented in Table 2–4 (above).
Wohlslagel et al. (1976) also examined the acute toxicity of HCl in rats and mice (data are also reported in Vernot et al. 1977). Groups of 10 male CFE (Sprague-Dawley derived) rats and groups of 10 female CF-1 (ICR derived) mice were exposed to HCl vapor for 60 min. HCl concentrations were continuously monitored during exposures by specific ion analysis. Toxic signs observed during exposure included increased grooming and irritation of the eyes, mucous membranes, and exposed skin. By the end of the exposure period, rapid, shallow breathing and yellow-green fur discoloration were observed. Necropsy of animals that died during or after exposure revealed pulmonary congestion and intestinal hemorrhage in both species. Rats also showed thymic hemorrhages. Calculated LC50 values were 3,124 ppm for rats and 1,108 ppm for mice. As was also reported in the Darmer (1974) study, mice appear to be more sensitive than rats to the acute inhalation toxicity of HCl. Data are summarized in Table 2–5.
Higgins et al. (1972) also compared HCl toxicity in rats and mice. Groups of 10 Wistar rats and 15 ICR mice were exposed to various concentrations of HCl vapors for 5 min. HCl concentrations were monitored continuously during exposures via specific ion electrode analysis. Again, data suggest that mice are more sensitive to the lethal effects of HCl than are rats. Data are summarized in Table 2–6.
Buckley et al. (1984) exposed groups of 16–24 male Swiss-Webster mice (25–30 g) to HCl at 309 ppm (RD50) 6 h/day (d) for 3 d. HCl concentrations were analyzed at least once per hour during exposures using infrared spectrometry. All mice were moribund or had died after the three exposures. Exfoliation, erosion, ulceration, and necrosis of the respiratory epithelium were observed.
TABLE 2–5 Mortality in Rats and Mice Exposed to Hydrogen Chloride for 60 Min
|
Rats |
Mice |
||
|
Concentration (ppm) |
Mortality |
Concentration (ppm) |
Mortality |
|
1,813 |
0/10 |
557 |
2/10 |
|
2,585 |
2/10 |
985 |
3/10 |
|
3,274 |
6/10 |
1,387 |
6/10 |
|
3,941 |
8/10 |
1,902 |
8/10 |
|
4,455 |
10/10 |
2,476 |
10/10 |
|
LC50 (95% CI)=3,124 ppm (2,829–3,450) |
LC50 (95% CI)=1,108 ppm (874–1,404) |
||
|
Abbreviation: CI, confidence interval. Sources: Wohlslagel et al. 1976; Vernot et al. 1977. |
|||
In another study, Anderson and Alarie (1980) reported a 30-min LC50 value of 10,137 ppm for normal mice and a value of 1,095 ppm for trachea-cannulated mice.
3.1.3. Rabbits
No mortality was observed in rabbits (unspecified strain) exposed to HCl at 3,667 ppm for 5 min; however, at 4,333 ppm for 30 min or 667 ppm for 2 to 6 h, 100% mortality was observed (Machle et al. 1942).
3.2. Nonlethal Toxicity
3.2.1. Nonhuman Primates
Kaplan (1985) exposed juvenile male baboons (1 per concentration) to HCl at 190, 810, 2,780, 11,400, 16,570, or 17,290 ppm for 5 min. HCl exposure concentrations were continuously monitored using a “modified French standard test method.” This method is based on continuous titration of the chloride ion with silver nitrate. The animals had been trained to perform an escape test. Escape was observed at 11,400 ppm and 17,290 ppm, and avoidance was observed at all other concentrations. The author
TABLE 2–6 Mortality in Rats and Mice Exposed to Hydrogen Chloride for 5 Min
|
Rats |
Mice |
||
|
Concentration (ppm) |
Mortality (%) |
Concentration (ppm) |
Mortality (%) |
|
30,000 |
0 |
3,200 |
7 |
|
32,000 |
10 |
5,060 |
7 |
|
39,800 |
60 |
6,145 |
13 |
|
45,200 |
70 |
6,410 |
0 |
|
57,290 |
90 |
7,525 |
40 |
|
|
8,065 |
13 |
|
|
9,276 |
33 |
||
|
13,655 |
40 |
||
|
26,485 |
87 |
||
|
30,000 |
87 |
||
|
LC50 (95% CI)=40,989 ppm (34,803–48,272) |
LC50 (95% CI)=13,745 ppm (10,333–18,283) |
||
|
Abbreviation: CI, confidence interval. Source: Higgins et al. 1972. |
|||
attributed these responses to irritation from the HCl exposure. No effects were noted at 190 ppm. Coughing and frothing at the mouth were observed in the animal exposed at 810 ppm, and shaking of the head, rubbing of the eyes, profuse salivation, and blinking were observed in animals exposed at higher concentrations. The baboons exposed to HCl at 16,570 ppm and 17,290 ppm died from bacterial infections several weeks after exposure. Necropsy indicated pneumonia, pulmonary edema, and tracheitis accompanied by epithelial erosion. In another study, Kaplan et al. (1988) exposed groups of three ketamine-anesthetized male baboons to HCl at target concentrations of 0, 500, 5,000, or 10,000 ppm for 15 min and observed for 3 mo. Exposures were accomplished in head boxes. HCl exposure concentrations were continuously monitored using a “modified French standard test method.” Respiratory rates during exposure were increased approximately 30%, 50%, and 100% for the 500-, 5,000-, and 10,000-ppm groups, respectively. Arterial blood gas decreased 40% during the 15-min exposure at 5,000 ppm and 10,000 ppm, persisted for 10 min following exposure, and
returned to baseline values by 3 d post-exposure. The increased respiratory rates were attributed to a compensatory response to the decrease in arterial oxygen, which in turn was attributed to upper airway broncho-constriction. No difference in pulmonary function or CO2 challenge response tests was observed 3 d or 3 mo post-exposure when compared with baseline values. In a follow-up report (Kaplan et al. 1993a), no exposure-related differences in pulmonary function or CO2 challenge response tests were reported 6 or 12 mo post-exposure, with the exceptions of respiratory rate at 5,000 ppm and 10,000 ppm, tidal volume at 5,000 ppm, and minute volume at 5,000 ppm and 10,000 ppm. Histopathologic examination was performed 12 mo post-exposure on one control animal, three animals in the 5,000-ppm group, and three animals in the 10,000-ppm group. One high-concentration animal exhibited pulmonary hemorrhage, edema, fibrosis, and bronchiolitis in the median right lung lobe. In another animal in the 10,000-ppm group, zonal atelectasis and focal multiple hemorrhages were observed in the right lung lobe. Focal, patchy hemorrhages were also observed in the three animals in the 5,000-ppm group as well as in the control group.
3.2.2. Guinea Pigs
Outbred English short-haired male guinea pigs (2–4 per group, weight 325–400 g) were exposed to HCl at 0, 107, 140, 162, or 586 ppm while exercising on a wheel (Malek and Alarie 1989). Actual HCl concentrations were determined by sampling from a port just above the head of the running animal. Impingers containing 0.1 N sodium hydroxide were utilized. Animals had been exercising on the wheel for 10 min prior to the start of HCl exposure. Animals were exposed for 30 min or until incapacitation occurred. The 107-ppm group showed signs of mild irritation, while the other groups exhibited coughing and gasping prior to incapacitation. Summary data are presented in Table 2–7.
These data are in apparent conflict with other guinea pig data. For example, no deaths were observed after exposure to HCl at 500 ppm for 15 min, and three of six guinea pigs died after exposure at 4,200 ppm for 15 min (Kaplan et al. 1993b).
In another study, Burleigh-Flayer et al. (1985) exposed groups of male English smooth-haired guinea pigs (4–8 per group, weight 330–450 g) to HCl at 320, 680, 1,040, or 1,380 ppm for 30 min. HCl concentrations were measured colorimetrically. Sensory irritation was defined as decreased respiratory rate and a prolonged expiratory phase, and respiratory irritation
TABLE 2–7 Summary Data for Guinea Pigs Exposed to Hydrogen Chloride During Exercise
|
Concentration (ppm) |
Number |
% Incapacitated |
Time to Incapacitation (min) |
% Mortality |
|
107±26 |
3 |
0 |
NA |
0 |
|
140±5 |
3 |
100 |
16.5±8.6 |
0 |
|
162±0 |
2 |
100 |
1.3±0.9 |
0 |
|
586±51 |
4 |
100 |
0.65±0.08 |
100a |
|
aTime to death was 2.8±0.8 min. Abbreviation: NA, not applicable. Source: Malek and Alarie 1989. |
||||
was defined as initial increased respiratory rate followed by a decrease due to a pause following each expiration. Sensory irritation was observed almost immediately in animals exposed at 680, 1,040, and 1,380 ppm, and appeared after 6 min of exposure in the 320-ppm group. Sensory irritation was replaced by pulmonary irritation in a concentration-related manner as sufficient HCl reached the deeper lung tissue. Time to onset of pulmonary irritation was approximately 18, 12, 7, and 3.5 min for the 320-, 680-, 1,040-, and 1,380-ppm groups, respectively. Corneal opacity was observed in one of four survivors from the 680-ppm group, five of five survivors from the 1,380-ppm group, and four of six from the 1,040-ppm group; no opacity was observed in the 320-ppm group. Histologic examination of the lungs was performed only on animals exposed at 1,040 ppm. Alveolitis accompanied by congestion and hemorrhage was observed 2 d post-exposure, and inflammation, hyperplasia, and mild bronchitis were observed 15 d post-exposure.
Kaplan et al. (1993b) exposed groups of six male English smooth-haired guinea pigs to HCl at 0, 500, or 4,200 ppm (nominal concentration) for 15 min. (Actual concentrations were 0, 520, or 3,940 ppm.) Exposure at 500 or 4,200 ppm caused a 20% decrease in respiratory frequency accompanied by a compensatory 2-fold increase in transpulmonary pressure. Exposure at 500 ppm had little effect on blood gases or arterial pH. Exposure at 4,200 ppm also had little effect on blood gases; however, a decrease in arterial pH was observed in exposed animals when compared with control animals. Necropsy performed 90 d post-exposure revealed lymphoid
hyperplasia of the nasal mucosa and lungs and hemorrhage of the lungs in both control animals and animals exposed at 500 ppm. Exposure at 4,200 ppm resulted in the deaths of three guinea pigs at 2 min, 2.5 min, and 27 d post-exposure, respectively. Necropsy of those decedents showed pulmonary congestion, severe congestion of the nasal turbinates, severe tracheitis, and desquamation of bronchiolar epithelia. In the three guinea pigs of the 4,200-ppm exposure group necropsied 90 d post-exposure, cloudy corneas, focal hemorrhage, focal pneumonia, esophageal hyperkeratosis, atelectasis, and pulmonary lymphoid hyperplasia were observed.
3.2.3. Rats
Groups of three adult male Sprague-Dawley rats were exposed head-only to HC1 at 200, 295, 784, 1,006, or 1,538 ppm for 30 min (Hartzell et al. 1985). Concentration-related decreases in respiratory frequency ranging from 35% to 67% were observed starting at approximately 2 min into the exposure period. Concentration-related decreases in minute-volume ranging from 30% to 69% were also observed.
Groups of eight male Fischer-344 rats were exposed to filtered air or HCl at 1,300 ppm for 30 min (Stavert et al. 1991). HCl concentrations were determined a minimum of three times per exposure using ion-specific electrodes. Each treatment had a nose-breathing group and a mouth-breathing group. Animals were sacrificed 24 h post-exposure. Nose-breathing rats exposed to HCl (actual concentration 1,293±36 ppm) exhibited severe, necrotizing rhinitis, turbinate necrosis, thrombosis of nasal submucosa vessels, and pseudomembrane formation in the anterior portion of the nasal cavity. No effects were observed in the lungs of nose-breathers. Nasal cavities of mouth-breathers exposed to HCl (actual concentration 1,295± 25 ppm) were essentially unaffected; however, severe, ulcerative tracheitis accompanied by necrosis and luminal ulceration was observed. Polymorphonuclear leukocytes were observed in the submucosa between tracheal rings, in connective tissue around the trachea, and in alveoli surrounding terminal bronchioles in the mouth-breathers.
Kaplan et al. (1993b) exposed groups of six female Sprague-Dawley rats to HCl at 0 or 4,200 ppm (nominal concentration) for 15 min. (Actual concentrations were 0 or 3,890 ppm.) Exposure at 3,890 ppm caused a 40% decrease in respiratory frequency accompanied by a compensatory 1.2-fold increase in arterial pressure. Exposure at 3,890 ppm also caused a decrease
in arterial pH, an increase in PaCO2 values, and a transient decrease in PaO2 values when compared with control animals. Necropsy performed 90 d post-exposure revealed microphthalmia in four rats and minimal focal atelectasis in one lobe of the lungs of two animals exposed at 3,890 ppm. Minimal focal atelectasis was also observed in two control animals.
3.2.4. Mice
Barrow et al. (1977) exposed groups of male Swiss-Webster mice (25–30 grams [g]) to HCl at concentrations ranging from 40 ppm to 943 ppm for 10 min. HCl concentrations were determined continuously using ion-specific electrodes. An RD50 of 309 ppm was defined.
In another study, Barrow et al. (1979) exposed groups of four male Swiss-Webster mice (25–30 g) to HCl at concentrations ranging from 20 ppm to 20,000 ppm for 10 min. Twenty-four hours after exposure, the mice were sacrificed by cervical dislocation. Precise individual group concentrations were not presented in the report. A decreased respiratory frequency, indicative of sensory irritation, was observed in animals exposed at >50 ppm. Deaths were observed in two of four mice exposed at 8,000 ppm and in four of four mice exposed at 19,300 ppm. Polymorphonuclear leukocyte infiltration of the conjunctiva was observed in animals exposed at 480 ppm, corneal necrosis was observed at 700 ppm, and ocular globe damage was observed at 3,000 ppm. Nasal epithelium ulceration was observed in the 120-ppm group, necrosis and damage to nasal bones in the 700-ppm group, and complete destruction of the nasal bones in the 7,000-ppm group.
Male Swiss-Webster mice were exposed to HCl at concentrations ranging from 17 ppm to 7,279 ppm for 10 min (Lucia et al. 1977). HCl concentrations were determined continuously using ion-specific electrodes. Animals were sacrificed 24 h post-exposure, and the upper respiratory tract was examined histologically. Small superficial ulcerations were observed in the respiratory epithelium at the junction with the squamous epithelium at 17 ppm. As HCl concentrations increased, the ulceration increased until it extended up the sides and into the nasal septum. The lower two-thirds of the upper respiratory tract was damaged at 723 ppm, and the entire mucosa was destroyed at 1,973 ppm. The squamous epithelium of the external nares was destroyed at 493 ppm, and the external support structures were destroyed at 1,088 ppm. Total destruction of mucosa and support structures as well as total destruction of the eyes was observed at 7,279 ppm.
Kaplan et al. (1993b) exposed groups of six male ICR mice to HCl at 0, 500, or 2,500 ppm (nominal concentration) for 15 min. (Actual concentrations were 0, 475, or 2,550 ppm.) Exposure to HCl at 475 ppm and 2,550 ppm caused 10% and 40% decreases in respiratory frequency, respectively, that were accompanied by compensatory increases in pressure. Four mice exposed at 475 ppm died within 90 d post-exposure, and all mice exposed at 2,550 ppm died by day 14 post-exposure. No histopathologic abnormalities were observed in controls or in mice exposed at 475 ppm. Moderate to severe lung congestion, necrosis of the tracheal mucosa, paranasal sinus exudate, and moderate lung edema were observed in animals exposed to HCl at 2,550 ppm. Results from this study are in apparent conflict with several other reported studies. For example, Darmer et al. (1974) reported a 5-min mouse LC50 of 13,700 ppm and a 30-min LC50 of 2,600 ppm, and Wholslagel et al. (1976) and Vernot et al. (1971) reported a 1-h LC50 of 1,108 ppm.
3.3. Developmental and Reproductive Toxicity
Female Wistar rats were exposed to HCl at 302 ppm for 1 h either 12 d prior to mating or on day 9 of gestation (Pavlova 1976). No information concerning test atmosphere concentration analysis was reported. One-third of the animals died, and dyspnea and cyanosis were observed. Congestion, edema, and hemorrhage were observed in the lungs of animals that died. Fetal mortality was higher (p<0.05) in rats exposed during pregnancy and was possibly secondary to severe maternal effects. When female Wistar and mixed-strain rats were exposed to HCl at 302 ppm for 1 h prior to mating, 30% of the Wistar rats and 20% of the mixed-strain rats died from the exposure. In animals surviving 6 d, decreased blood oxygen saturation was observed as well as kidney, liver, and spleen damage. Treatment also altered the estrous cycle. In rats mated 12–16 d post-exposure and sacrificed on day 21 of gestation, increased fetal mortality, decreased fetal weight, and increased relative fetal lung weight were observed.
3.4. Genetic Toxicology
Genotoxicity results for HCl are equivocal. At a concentration of 25 µg/well, it was positive in a DNA repair assay in E. Coli, and it induced
chromosomal nondisjunction in D. melanogaster at 100 ppm for 24 h. However, negative results were obtained in a Syrian hamster embryo cell transformation assay and in an adenovirus SA7 assay (NTIS 2000).
3.5. Carcinogenicity
Rats exposed to HCl at 10 ppm for 6 h/d, 5 d/wk for life developed increased incidences of tracheal and laryngeal hyperplasia compared with controls; however, no increase in the incidence of cancerous lesions was observed over controls (Sellakumar et al. 1985). Male Sprague-Dawley rats exposed at 10.2 ppm for 6 h/d, 5 d/wk for 382 exposures over a 588-d period did not show an increased incidence of nasal tumors (Albert et al. 1982).
3.6. Summary
HCl is a sensory and respiratory irritant and causes changes in the upper respiratory tract at relatively low concentrations and short exposure times. As concentrations and exposure times increase, effects progress to the lower respiratory tract and may involve pulmonary edema and histopathologic changes. There appears to be no differential toxicity between HCl vapor and aerosol, and mice appear to be more sensitive than rats to the effects of HCl. Exposure at 190 ppm for 5 min was the no-effect level in baboons, while those exposed at 16,570 ppm and 17,290 ppm exhibited pulmonary edema, pneumonia, and died from bacterial infections weeks after exposure. Mild irritation was observed in guinea pigs exposed at 107 ppm for 30 min, and guinea pigs exposed at concentrations ranging from 140 ppm to 586 ppm exhibited gasping prior to incapacitation and/or death. Exercising guinea pigs exposed to HCl at 320–1,380 ppm for 30 min exhibited a continuum of effects from sensory irritation to pulmonary irritation to death. Nose-breathing rats exposed to HCl exhibited severe nasal pathology, and similarly exposed mouth-breathing rats showed tracheal pathology. Several studies in mice also confirm the progression of effects from sensory irritation to lower respiratory tract involvement.
Fetal mortality was higher in rats exposed to HCl during pregnancy and 12–16 d prior to mating than it was in unexposed rats; however, no validation of exposure concentrations was provided. No data concerning the genotoxicity of HCl were located. In two lifetime studies, there was no increase in the incidence of cancerous lesions in rats exposed to HCl.
4. SPECIAL CONSIDERATIONS
4.1. Metabolism and Disposition
Information concerning the metabolism and disposition of hydrogen chloride (HCl) is sparse. HCl is not metabolized; however, hydrogen and chloride ions adsorbed in the respiratory tract may be distributed throughout the body (NRC 2000).
4.2. Mechanism of Toxicity
HCl is a respiratory irritant and is corrosive. When inhaled, it solubilizes in mucous present in the nasal passages, and when the scrubbing mechanism of the upper respiratory tract is saturated, it may enter the lower respiratory tract (NRC 1991). At a molecular level, HCl dissociates in water to form hydronium and chloride ions. The hydronium ion is a proton donor. It could catalyze cleavage of organic molecules and be involved in hydroxylation of carbonyl groups and polymerization and depolymerization of organic molecules (EPA 1994).
4.3. Structure-Activity Relationships
Although the AEGL values for HCl are based on empirical toxicity data, it is important to consider the relative toxicities of HCl and other structurally similar chemicals. The compounds most closely related to HCl are other hydrogen halides, HF and HBr. It might be anticipated that relationships exist in this chemical class between structure and respective toxicities in animals and humans. However, because of differences in size and electron configuration in the various halogen atoms, substantial differences exist with respect to their chemical and physical properties, which in turn are responsible for their toxicologic properties. That is particularly true in the case of acutely toxic effects resulting from inhalation exposure.
For example, HCl has a considerably higher ionization constant than HF, and is therefore classified as a stronger acid than HF. Consequently, higher concentrations of proton-donor hydronium ions are generated from HCl in aqueous solutions under the same conditions. The protons readily react with cells and tissues, resulting in HCl’s irritant and corrosive properties. On the other hand, the fluoride ion from dissociated HF is a strong nucleophile or Lewis base that is highly reactive with various organic and
inorganic electrophiles (biologically important substances), also resulting in irritation and tissue damage.
In addition to differences in chemical properties, difference in water solubility might be a significant factor in the acute inhalation toxicity of these substances. HF and HBr are characterized as infinitely and freely soluble in water, respectively, but the solubility of HCl, although still high, is lower (67 g/100 g of water at 30 C) (Budavari et al. 1989). Thus, it is likely that HF is more effectively scrubbed in the nasal cavity than HCl, resulting in less penetration to the lungs and less severe toxicity. The effectiveness of the scrubbing mechanism was demonstrated in a study that addressed the acute toxicities of HF, HCl, and HBr and the deposition (scrubbing) of those chemicals in the nasal passages. Stavert et al. (1991) exposed male Fischer-344 rats to each of the hydrogen halides at 1,300 ppm for 30 min and assessed damage to the respiratory tract 24 h after the exposure. The nasal cavity was divided into four regions and examined microscopically. For all three hydrogen halides, tissue injury was confined to the nasal cavity. Tissue injury in the nasal cavity was similar following exposures to HF and HCl and involved moderate to severe flbrinonecrotic rhinitis in nasal region 1 (most anterior region). For HF and HCl, the lesions extended into region 2, but regions 3 and 4 were essentially normal in appearance, as was the trachea. Nasal cavity lesions following exposure to HBr were limited to region 1 and were similar in extent to those produced by HF and HCl, showing that all three chemicals are well scrubbed. No lung or tracheal injury was evident for any of the chemicals, although accumulations of inflammatory cells and exudates in the trachea and lungs following exposure to HCl indicated that HCl may not be as well scrubbed in the nasal passages as HF and HBr. However, that possibility is modified by the authors’ observation of lower minute-volumes in the HF- and HBr-exposed rats, so that greater amounts of HCl were inhaled. Morris and Smith (1982) also showed that at concentrations up to 226 ppm, >99.7% of inspired HF might be scrubbed in the upper respiratory tract of the rat.
In a series of experiments with HF and HCl that used guinea pigs and rabbits as the test species, Machle and coworkers (Machle and Kitzmiller 1935; Machle et al. 1934, 1942) concluded that the acute irritant effects of HF and HCl were similar, but the systemic effects of HF were more severe, presumably because chloride ion is a normal electrolyte in the body, and fluoride ion is not. However, the conclusions involving systemic effects followed repeated exposures.
Aside from lethality studies, no clear evidence is available to establish the relative toxicities of HCl and HF. At concentrations ranging from 100 ppm to 1,000 ppm for 30 min, Kusewitt et al. (1989) reported epithelial and
submucosal necrosis, accumulation of inflammatory cells and exudates, and extravasation of erythrocytes in the nasal region of rats exposed to HF, HCl, or HBr. The severity of injury increased with increasing concentration, and the relative toxicities of the hydrogen halides were reported as HF>HCl> HBr. However, in a later study by the same authors, Stavert et al. (1991) reported no difference in the toxicities to the nasal regions or the lung in nose-breathing or mouth-breathing rats exposed to HF, HCl, or HBr at 1,300 ppm for 30 min.
At the high concentrations necessary to cause lethality during exposure durations of 5 min to 1 h, HF is approximately twice (1.8–2.2 times) as toxic to the rat as HCl (Table 2–8). The relationship is similar for the mouse within this time period (2.2–3.2 times); however, for respiratory irritants such as HCl, the mouse “may not be a good model for extrapolation to humans,” because “mice appear to be much more susceptible to the lethal effects of HCl than other rodents or baboons…. To some extent, this increased susceptibility may be due to less effective scrubbing of HCl in the upper respiratory tract” (NRC 1991). Quantitative data for HBr were limited to one study, but that study also showed that HF was more toxic than either HCl or HBr.
On the basis of empirical lethality (LC50) data in rats, rabbits, and guinea pigs, the exposure time-LC50 relationship for HCl using the equation Cn×t=k results in an n value of 1. That is comparable to an n value of
TABLE 2–8 Relative Toxicities of HF, HCl, and HBr indicated by LC50 Values (ppm)
|
Species |
Exposure Duration |
HF |
HCl |
HBr |
Reference |
|
Rat |
5 min |
18,200 |
41,000 |
|
Higgins et al. 1972 |
|
Mouse |
|
6,247 |
13,750 |
|
|
|
Rat |
30 min |
2,042 |
4,700 |
Rosenholtz et al. 1963 (HF); MacEwan and Vernot 1972 (HCl) |
|
|
Mouse |
|
|
2,644 |
||
|
Rat |
1 h |
1,395 |
3,124 |
Wohlslagel et al. 1976 |
|
|
Mouse |
|
342 |
1,108 |
||
|
Rat |
1 h |
1,278 |
2,350 |
2,858 |
MacEwan and Vernot 1972 |
|
Mouse |
|
501 |
1,322 |
814 |
|
|
Abbreviations: HBr, hydrogen bromide; HCl, hydrogen chloride; HF, hydrogen fluoride; LC50, lethal concentration in 50% of subjects. |
|||||
2 empirically derived from rat and mouse lethality data for HF. Hence, although HF is more toxic than HCl at the higher concentrations and shorter exposure durations, the rate of decrease in the LC50 threshold is less (i.e., less slope in the curve derived from Cn×t=k) for HF than for HCl. As a result, the LC50 values, and therefore the lethal toxicities of HCl and HF, are comparable at 4 h and 8 h. This shift in relative lethal toxicity across time also is reflected in the AEGL-3 values developed for HCl and HF.
Considering the greater water solubility of HF compared with HCl, it is possible that the more effective scrubbing of HF in the nasal passages is responsible for the apparent decrease in the relative toxicities of HF and HCl at lower concentrations associated with longer exposure durations. Conversely, the greater toxicity of HF at higher concentrations associated with the shorter exposure durations might be due to saturation of the scrubbing mechanism and higher concentrations in the lower respiratory system.
4.4. Other Relevant Information
4.4.1. Species Variability
Differences in responses to HCl exposure have been observed between primates and rodents. Rodents exhibit sensory and respiratory irritation upon exposure to high concentrations of HCl. Dose-related decreases in respiratory frequency, indicative of a protective mechanism, are observed in rodents, while baboons inhaling HCl at 500, 5,000, or 10,000 ppm exhibited concentration-dependent increases in respiratory frequency, indicative of a compensatory response to hypoxia and a possible increase in the total dose delivered to the lung (NRC 1991).
Kaplan (1988) found that five of six mice died when exposed to HCl at 2,550 ppm for 15 min; however, baboons survived exposure at 10,000 ppm for 15 min. The LC50 values reported by Darmer et al. (1974), Wohlslagel et al. (1976), and Higgins et al. (1972) indicate that mice are approximately 3 times more sensitive than rats to the effects of HCl. That increased susceptibility might be due to less effective scrubbing of HCl in the upper respiratory tract of mice compared with rats (NRC 1991). Guinea pigs also appear to be more sensitive than rats; however, various guinea pig studies have provided conflicting results (Kaplan et al. 1993b).
Because most rodents are obligatory nose-breathers, whereas humans may be mouth-breathers, especially during exercise, Stavert et al. (1991)
studied the effects of inhalation of HCl via the nose and mouth in rats. HCl was delivered directly to the trachea by cannulation; concentrations that produced effects confined to the nasal passages in nose-breathing rats resulted in serious lower respiratory tract effects and/or deaths in orally cannulated rats. These results indicate that the site of injury and resultant toxicologic effects differ with mouth- and nose-breathing, the former mode resulting in more severe responses under similar exposure situations. Thus, species that breathe through their mouth (humans) may be more sensitive to the effects of HCl than those that are obligate nasal breathers (rodents).
4.4.2. Unique Physicochemical Properties
HCl gas is hygroscopic and dissolves in water to produce hydrochloric acid. Concentrated hydrochloric acid is 37% HCl. When HCl gas absorbs moisture, it is highly reactive with metals and releases hydrogen gas (EPA 1994).
4.4.3. Concurrent Exposure Issues
Gases such as carbon monoxide, carbon dioxide, hydrogen cyanide, nitrogen dioxide, and nitrogen are often present with HCl during fires. The temperature, moisture content, and particular mix of gases can influence toxicity. For example, carbon monoxide has been shown to weaken the irritant effects of HCl (Sakurai 1989). However, Higgins et al. (1972) found no effect on mortality of rats and mice exposed to HCl alone or in combination with carbon monoxide.
Wohlslagel et al. (1976) examined the hazard of simultaneous acute inhalation exposure to HCl, HF, and alumina dust, which are components of solid rocket motor exhaust. Sixty-minute LC50 values were determined for both rats and mice. Exposures were to HCl alone, HF alone, and combination exposures to HCl and HF. Data suggested that in both species exposure to both gases simultaneously resulted in physiologically additive mortality incidences. No synergism, potentiation, or antagonism were observed. Gross and histopathologic examinations of rats and mice exposed to combinations of the corrosive gases showed no sites of damage additional to those observed in animals exposed to a single gas. The addition of alumina dust to atmospheres containing HCl and HF vapors did not increase or decrease mortality in either species.
HCl in smoke generated by flaming thermodegradation polyvinyl chloride (30-min LC50 of 2,141 ppm) was slightly more lethal to rats than HCl in smoke generated by nonflaming thermodegradation (30-min LC50 of 2,924 ppm), which was slightly more lethal than pure HCl (30-min LC50 of 3,817 ppm) (Hartzell et al. 1987).
4.4.4. Subchronic Exposure
Groups of 31 male and 21 female rats or 31 male and 21 female mice were exposed to HCl at 0, 10, 20, or 50 ppm for 6 h/d, 5 d/wk for 90 d (Toxigenics 1984). After the fourth exposure, 15 males and 10 females per group per species were sacrificed. Decreased body weight was observed in both genders of both species exposed at 50 ppm for 4 d. Hematology and urinalysis were unremarkable for those animals. Rats exhibited minimal to mild rhinitis after exposure to the three HCl concentrations for 4–90 d. The rhinitis was concentration- and duration-related and occurred in the anterior portion of the nasal cavity. After 90 d, mice in the 50-ppm group developed varying degrees of cheilitis with accumulations of hemosiderin-laden macrophages in the perioral tissues. In addition, at all three concentrations, mice developed signs of minor, reversible degeneration (eosinophilic globules) in the epithelial cells lining the nasal turbinates.
Machle et al. (1942) exposed three rabbits, three guinea pigs, and one monkey to HCl at 34 ppm for 6 h/d, 5 d/wk for 4 wk. No histopathologic effects were noted at necropsy. Clinical signs during or after exposure and other experimental details were not reported.
Mice exposed to HCl at 310 ppm for 6 h/d for 5 d exhibited necrosis, exfoliation, erosion, and ulceration of the nasal respiratory epithelium; however, no histopathologic effects were noted in the lung (Buckley et al. 1984).
5. RATIONALE AND PROPOSED AEGL-1
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, nonsensory effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
5.1. Summary of Human Data Relevant to AEGL-1
Five male and five female exercising adult asthmatic subjects (ages 18–25 y) were exposed to filtered air or HCl at 0.8 ppm or 1.8 ppm for 45 min (Stevens et al. 1992). No treatment-related effects were observed.
5.2. Summary of Animal Data Relevant to AEGL-1
No animal data consistent with effects defined by AEGL-1 were available.
5.3. Derivation of AEGL-1
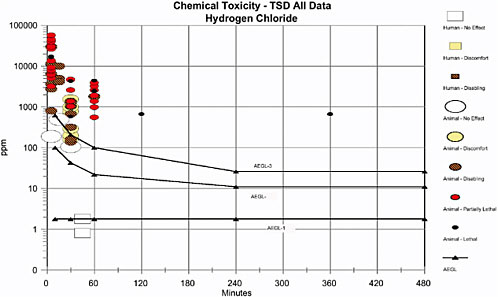
Because appropriate human data exist for exposure to HCl, they were used to identify AEGL-1 values. Exposure to HCl at 1.8 ppm for 45 min resulted in a no-observed-adverse-effect level (NOAEL) in 10 exercising young adult asthmatic subjects (Stevens et al. 1992). Because exercise will increase HCl uptake and exacerbate irritation, those asthmatic subjects are considered a sensitive subpopulation. Therefore, because the test subjects were a sensitive subpopulation and the end point was essentially a no-effect level, no uncertainty factory (UF) was applied to account for sensitive human subpopulations. Adequate human data were available, so no UF was applied for animal to human extrapolation. The no-effect level was held constant across the 10- and 30-min and 1-, 4-, and 8-h exposure time points. That approach was considered appropriate because mild irritant effects generally do not vary greatly over time, and the end point of a no-effect level in a sensitive population is inherently conservative. The values for AEGL-1 are given in Table 2–9. Figure 2–1 is a plot of the derived AEGLs and the human and animal data on HCl.
6. RATIONALE AND PROPOSED AEGL-2
AEGL 2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or serious, long lasting adverse health effects or an impaired ability to escape.
TABLE 2–9 AEGL-1 Values for Hydrogen Chloride (ppm [mg/m3])
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
1.8 (2.7) |
1.8 (2.7) |
1.8 (2.7) |
1.8 (2.7) |
1.8 (2.7) |
6.1. Summary of Human Data Relevant to AEGL-2
Human data consistent with effects defined by AEGL-2 from exposure to HCl are not appropriate for use in derivation because the descriptions of concentration, exposure time, and effects are subjective.
6.2. Summary of Animal Data Relevant to AEGL-2
Studies in baboons involving escape performance tests (Kaplan 1987) and pulmonary function (Kaplan et al. 1988, 1993a) resulted in effects consistent with those defined by AEGL-2. However, the baboon study descriptions suggest that individual baboons may have been used for more than one exposure. Responses of both guinea pigs (Malek and Alarie 1989; Burleigh-Flayer et al. 1985) and rats (Stavert et al. 1991) also are consistent with effects defined by AEGL-2. Other studies, although designed fairly well, produced effects more severe than that defined by AEGL-2 (Darmer et al. 1974; Buckley et al. 1984; Barrow et al. 1979; Hartzell et al. 1985).
6.3. Derivation of AEGL-2
The AEGL-2 for the 30-min and 1-, 4-, and 8-h time points was based on severe nasal or pulmonary histopathology in rats exposed to HCl at 1,300 ppm for 30 min (Stavert et al. 1991). A modifying factor (MF) of 3 was applied to account for the relatively sparse database describing effects defined by AEGL-2. The AEGL-2 values were further adjusted by a total UF of 10–3 for intraspecies variability, supported by the steep concentration-response curve, which implies little individual variability; and 3 for interspecies variability. Using the default value of 10 for interspecies variability would bring the total adjustment to 100 instead of 30. That would generate AEGL-2 values that are not supported by data on exercising asthmatic subjects, an especially sensitive subpopulation. Exercise increases HCl uptake and exacerbates irritation; no effects were noted in exercising
young adult asthmatic subjects exposed to HCl at 1.8 ppm for 45 min (Stevens et al. 1992). Using a total UF of 30 would yield 4- and 8-h values of 3.6 ppm (instead of 11 ppm). The prediction that humans would be disabled by exposure for 4 h or 8 h to 3.6 ppm cannot be supported when exercising asthmatic subjects exposed to one-half that concentration for 45 min exhibited no effects. The shorter time points would yield values 4 to 7 times the 1.8-ppm value; however, confidence in the time-scaling for HCl is good for times up to 100 min, because the value of n was derived from a regression analysis of rat and mouse mortality data with exposure durations ranging from 1 min to 100 min. The 30-min value of 43 ppm derived with a total UF of 10 is reasonable in light of the fact that baboons exposed at 500 ppm for 15 min experienced only a slightly increased respiratory rate. Therefore, a total UF of 10, accompanied by the MF of 3, is most consistent with the database. Thus, the total factor is 30. Time-scaling for the 1-h AEGL exposure period used the Cn×t=k relationship, where n= 1 based on regression analysis of combined rat and mouse LC50 data (1 min to 100 min) as reported by ten Berge et al. (1986). The 4- and 8-h AEGL-2 values were derived by applying an MF of 2 to the 1-h AEGL-2 value, because time-scaling would yield a 4-h AEGL-2 of 5.4 ppm and an 8-h AEGL-2 of 2.7 ppm, close to the 1.8 ppm tolerated by exercising asthmatic subjects without observed adverse health effects. Repeated-exposure rat data suggest that the 4- and 8-h values of 11 ppm are protective. Rats exposed to HCl at 10 ppm for 6 h/d, 5 d/wk for life exhibited only tracheal and laryngeal hyperplasia, and rats exposed to HCl at 50 ppm for 6 h/d, 5 d/wk for 90 d exhibited only mild rhinitis.
The 10-min AEGL-2 was derived by dividing the mouse RD50 of 309 ppm by a factor of 3 to obtain a concentration causing irritation (Barrow 1977). It has been determined that human response to sensory irritants can be predicted on the basis of the mouse RD50. For example, Schaper (1993) has validated the correlation of 0.03×RD50=TLV as a value that will prevent sensory irritation in humans. The 0.03 represents the half-way point between 0.1 and 0.01 on a logarithmic scale, and Alarie (1981) has shown that the RD50 multiplied by 0.1 corresponds to “some sensory irritation,” whereas the RD50 value itself is considered “intolerable to humans.” Thus, it is reasonable that one-third of the RD50, a value half-way between 0.1 and 1 on a logarithmic scale, might cause significant irritation to humans. Furthermore, one-third of the mouse RD50 for HCl corresponds to an approximate decrease in respiratory rate of 30%, and decreases in the range of 20–50% correspond to moderate irritation (ASTM 1991). The values for AEGL-2 are given in Table 2–10.
TABLE 2–10 AEGL-2 Values for Hydrogen Chloride (ppm [mg/m3])
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
100 (156) |
43 (65) |
22 (33) |
11 (17) |
11 (17) |
The 10-min value is supported by the baboon studies, where no effects were noted in a baboon exposed at 190 ppm for 5 min (Kaplan 1987), and no exposure-related effects on tidal volume or PaO2 were observed in anesthetized baboons exposed at 500 ppm for 15 min (Kaplan et al. 1988). Also, when the rat data of Stavert et al. (1991) are extrapolated back to 10 min, a value of 130 ppm is obtained, suggesting that the proposed 10-min value is protective.
7. RATIONALE AND PROPOSED AEGL-3
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
7.1. Summary of Human Data Relevant to AEGL-3
No data concerning human lethality from HCl exposure were located in the available literature.
7.2. Summary of Animal Data Relevant to AEGL-3
Baboon exposure studies involving an escape performance test and pulmonary function tests resulted in effects consistent with those defined by AEGL-3 (Kaplan 1987; Kaplan et al. 1988). Other well-designed studies identified disabling effects and lethality in guinea pigs (Malek and Alarie 1989; Burleigh-Flayer et al. 1985), rats, and mice (Darmer et al. 1974; Wohlslagel et al. 1976; Vernot et al. 1977; Barrow et al. 1979; Buckley et al. 1984; Hartzell et al. 1985).
7.3. Derivation of AEGL-3
The AEGL-3 was based on a 1-h rat LC50 study (Wohlslagel et al. 1976; Vernot et al. 1977). One-third of the l-h LC50 value of 3,124 ppm was used as an estimated concentration causing no deaths. That estimate is inherently conservative (no deaths observed in the same study at 1,813 ppm). A total UF of 10 will be applied—3 for intraspecies variation, because the steep concentration-response curve implies limited individual variability; and 3 to protect susceptible individuals. Using a full value of 10 for interspecies variability (total UF of 30) would yield AEGL-3 values that are inconsistent with the overall data set.
A number of factors argue for the use of a UF of 10 instead of 30: (1) the steep concentration-response curve for lethality observed in the Wohlslagel et al. (1976) study in which the estimated LC0 (one-third of the LC50 of 3,124 ppm) is lower than the experimental LC0 of 1,813 ppm. The LC0 selection is conservative, and the steep concentration-response curve argues for little interindividual variability; (2) AEGL-3 values generated from a total UF of 30 would be close (within a factor of 2) to the AEGL-2 values generated from data on exercising asthmatic subjects; (3) Sellakumar et al. (1985) exposed rats to HCl at 10 ppm for 6 h/d, 5 d/wk for life and only observed increased trachael and laryngeal hyperplasia. The estimated 6-h AEGL-3 using an intraspecies UF of 3 is 17 ppm, close to the concentration inhaled in the lifetime study in which only mild effects were induced; and (4) rats exposed to HCl at 50 ppm for 6 h/d, 5 d/wk for 90 d (Toxigenics 1984) exhibited mild rhinitis. This level is already twice the AEGL-3 value, which is intended to protect against death.
Thus, the total UF was set at 10. It was then time-scaled to the specified 10- and 30-min and 4-h AEGL exposure periods using the Cn×t=k relationship, where n=1 based on regression analysis of combined rat and mouse LC50 data (1 min to 100 min) as reported by ten Berge et al. (1986). The 4-h AEGL-3 also was adopted as the 8-h AEGL-3 because of the uncertainty of time-scaling to 8 h with an n value derived from exposure durations of up to 100 min. The values for AEGL-3 are given in Table 2–11.
The 5-min rat LC0 of 30,000 ppm (Higgins et al. 1972) supports the 10-min AEGL-3 value. Extrapolating that value across time (n=1) to 10 min and applying a UF of 10 yields a value of 1,500 ppm, suggesting that the proposed AEGL-3 value is protective. Also, if the 5-min rat LC50 of 41,000 ppm for HCl vapor (Darmer et al. 1974) is divided by 3 to estimate a no-effect level for death, extrapolated to 10 min, and a UF of 10 is applied, a supporting value of 683 ppm is obtained.
TABLE 2–11 AEGL-3 Values for Hydrogen Chloride (ppm [mg/m3])
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
620 (937) |
210 (313) |
100 (155) |
26 (39) |
26 (39) |
8. SUMMARY OF PROPOSED AEGLS
8.1. AEGL Values and Toxicity End Points
The derived AEGLs for various levels of effects and durations of exposure are summarized in Table 2–12. A NOAEL for sensory irritation in exercising asthmatic subjects was used for AEGL-1. Severe nasal and pulmonary effects in rats and a modification of the mouse RD50 were used for AEGL-2. An estimated no-effect level for death in rats was used for AEGL-3.
8.2. Other Exposure Criteria
Standards set by other organizations appear in Table 2–13.
8.3. Data Quality and Research Needs
Human data are limited to one study showing no significant effects in asthmatic subjects and to dated anecdotal information. Furthermore, the
TABLE 2–12 Summary of AEGL Values for Hydrogen Chloride (ppm [mg/m3])
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
|
AEGL-1 (Nondisabling) |
1.8 (2.7) |
1.8 (2.7) |
1.8 (2.7) |
1.8 (2.7) |
1.8 (2.7) |
|
AEGL-2 (Disabling) |
100 (156) |
43 (65) |
22 (33) |
11 (17) |
11 (17) |
|
AEGL-3 (Lethal) |
620 (937) |
210 (313) |
100 (155) |
26 (39) |
26 (39) |
TABLE 2–13 Extant Standards and Guidelines for Hydrogen Chloride (ppm)
|
Guideline |
Exposure Duration |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
|
AEGL-1 |
1.8 |
1.8 |
1.8 |
1.8 |
1.8 |
|
AEGL-2 |
100 |
43 |
22 |
11 |
11 |
|
AEGL-3 |
620 |
210 |
100 |
26 |
26 |
|
ERPG-1a |
3 |
|
|||
|
ERPG-2a |
20 |
||||
|
ERPG-3a |
150 |
||||
|
NIOSH IDLHb |
50 |
||||
|
NIOSH RELc |
|
5 (ceiling) |
|||
|
OSHA PEL-TWAd |
5 (ceiling) |
||||
|
ACGIH TLV-STELe |
5 |
|
|||
|
NRC SPEGLf |
|
1 |
|||
|
NRC EEGLg |
|
20 |
|
||
|
NRC SMACh |
5 |
|
|||
|
German MAKi |
|
5 |
|||
|
Dutch MACj |
5 |
||||
|
aERPG (emergency response planning guidelines) of the American Industrial Hygiene Association (AIHA 2001). ERPG-1 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing symptoms other than mild, transient adverse health effects or without perceiving a clearly defined objectionable odor. The ERPG-1 for HCl is based on objectionable odor. The ERPG-2 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing irreversible or other serious health effects or symptoms that could impair an individual’s ability to take protection action. The ERPG-2 for HCl is based on animal studies suggesting serious eye and respiratory irritation above 20 ppm and below 100 ppm. The ERPG-3 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing life-threatening health effects. The ERPG-3 for HCl is based on animal data suggesting that concentrations exceeding 150 ppm for 1 h may produce severe, possibly life-threatening health effects, such as pulmonary edema, in a heterogeneous population. bIDLH (immediately dangerous to life and health standard of the National Institute of Occupational Safety and Health) (NIOSH 1994). The IDLH represents the maximum concentration from which one could escape within 30 min without any escape-impairing symptoms or irreversible health effects. The IDLH for HCl is based on acute inhalation toxicity data in humans. |
|||||
involvement of RADS in HCl toxicity is unclear. Many more data are available for animal exposures; however, many of those studies used compromised animals or very small experimental groups, resulting in limited data for many species but no in- depth database for a given species. Also, some studies involve very short exposures to high concentrations of HCl. Thus, confidence in the AEGL values is at best moderate.
9. REFERENCES
AIHA (American Industrial Hygiene Association). 2001. Emergency Response Planning Guidelines. Hydrogen Chloride. Fairfax, VA: AIHA.
Alarie, Y. 1981. Dose-response analysis in animal studies: Prediction of human responses. Environ. Health Perspect. 42:9–13.
Albert, R.E., A.R.Sellakumar, S.Laskin, M.Kuschner, N.Nelson, and C.A.Snyder. 1982. Gaseous formaldehyde and hydrogen chloride induction of nasal cancer in the rat. JNCI 68:597–603.
Amoore, J.E., and E.Hautala. 1983. Odor as an aid to chemical safety: Odor thresholds compared with threshold limit values and volatiles for 214 industrial chemicals in air and water dilution. J. Appl. Toxicol. 3:272–290.
Anderson, R.C., and Y.Alarie. 1980. Acute lethal effects of polyvinylchloride thermal decomposition products in normal and cannulated mice. The Toxicologist A3.
ASTM (American Society for Testing and Materials). 1991. Method E981. Pp. 610–619 in Standard Test Method For Estimating Sensory Irritancy of Airborne Chemicals, Volume 11.04. Philadelphia, PA: ASTM.
Barrow, C.S., H.Lucia, and Y.C.Alarie. 1979. A comparison of the acute inhalation toxicity of hydrogen chloride versus the thermal decomposition products of polyvinylchloride. J. Combust. Toxicol. 6:3–12.
Barrow, C.S., Y.Alarie, M.Warrick, and M.F.Stock. 1977. Comparison of the sensory irritation response in mice to chlorine and hydrogen chloride. Arch. Environ. Health 32:68–76.
Beaumont, J.J., J.Leveton, K.Knox, T.Bloom, T.McQuiston, M.Young, R.Goldsmith, N.K.Steenland, D.P.Brown, and W.E.Halperin. 1987. Lung cancer mortality in workers exposed to sulfuric acid mist and other acid mists. JNCI 79:911–921.
Bernstein, J.A. 1993. Reactive Airways Dysfunction Syndrome (RADS). DPICtions 12:1–3.
Bond, G.G., R.R.Cook, P.C.Wright, and G.H.Flores. 1983. A case-control study of brain tumor mortality at a Texas chemical plant. J. Occup. Med. 25:377–386.
Bond, G.G., R.J.Shellenburger, G.H.Flores, R.R.Cook, and W.A.Fiskbeck. 1985. A case-control study of renal cancer mortality at a Texas chemical plant. Am. J. Ind. Med. 7:123–139.
Bond, G.G., G.H.Flores, R.J.Shellenburger, J.B.Cartmill, W.A.Fiskbeck, and R.R.Cook. 1986. Nested case-control study of lung cancer among chemical workers. Am. J. Epidemiol. 124:53–66.
Bond, G.G., G.H.Flores, B.A.Stafford, and G.W.Olsen. 1991. Lung cancer and hydrogen chloride exposure: Results from a nested case-control study of chemical workers. J. Occup. Med. 33:958–961.
Boulet, L-P. 1988. Increases in airway responsiveness following acute exposure to respiratory irritants: Reactive airway dysfunction syndrome or occupational asthma? Chest 94:476–481.
Brooks, S.M., M.A.Weiss, and I.L.Bernstein. 1985. Reactive airways dysfunction syndrome (RADS): Persistent asthma syndrome after high level irritant exposures. Chest 88:376–384.
Buckley, L.A., X.Z.Jiang, R.A.James, K.T.Morgan, and C.S.Barrow. 1984. Respiratory tract lesions induced by sensory irritants at the RD50 concentration. Toxicol. Appl. Pharmacol. 74:417–429.
Burleigh-Flayer, H., K.L.Wong, and Y.Alarie. 1985. Evaluation of the pulmonary effects of HCl using CO2 challenges in guinea pigs. Fundam. Appl. Toxicol. 5:978–985.
Darmer, K.L., E.R.Kinkead, and L.C.DiPasquale. 1974. Acute toxicity in rats and mice exposed to hydrogen chloride gas and aerosols. Am. Ind. Hyg. Assoc. J. 35:623–631.
DFG (Deutsche Forschungsgemeinschaft). 2000. List of MAK and BAT Values, Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area, Report No. 35 [in German]. Weinheim, Federal Republic of Germany: Wiley VCH.
Elkins, H.B. 1959. The Chemistry of Industrial Toxicology, 2nd Ed. New York, NY: John Wiley & Sons. Pp. 79–80.
EPA (U.S. Environmental Protection Agency) 1994. Health Assessment Document for Chlorine and Hydrogen Chloride. External Review Draft. ECAO-R-065. Environmental Criteria and Assessment Office, Research Triangle Park, NC.
EPA (U.S. Environmental Protection Agency). 1995. Hydrogen Chloride. Integrated Risk Information System (IRIS) [Online]. Available: http://www.epa.gov/iris/subst/0396.htm.
Hartzell, G.E., H.W.Stacy, W.G.Swiztzer, D.N.Priest, and S.C.Packham. 1985. Modeling of toxicological effects of fire gases: IVV. Intoxication of rats by carbon monoxide in the presence of an irritant. J. Fire Sci. 3:263–279.
Hartzell, G.E., A.F.Grand, and W.G.Swiztzer. 1987. Modeling of toxicological effects of fire gases: VI. Further studies on the toxicity of smoke containing hydrogen chloride. J. Fire Sci. 5:368–391.
Henderson, Y., and H.W.Hagard. 1943. Noxious Gases. New York: Reinhold Publishing Corp. Pp. 126.
Higgins, E.A., V.Fiorca, A.A.Thomas, and H.V.Davis. 1972. Acute toxicity of brief exposures to HF, HCL, NO2, and HCN with and without CO. Fire Tech. 8:120–130.
HSDB (Hazardous Substances Data Bank). 1996. Hydrogen chloride [Online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/search/f?./temp/~LRWhf3:1 [July 22, 1996].
IARC (International Agency for Research on Cancer). 1992. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 54: Occupational Exposures to Mists and Vapors from Strong Inorganic Acids; and Other Industrial Chemicals. Lyon, France: IARC. Pp. 189–211.
Kaplan, H.L., et al. 1985. Effects of combustion gasses on escape performance in the baboon and rat. J. Fire Sci. 3:228–244.
Kaplan, H.L. 1987. Effects of irritant gases on the avoidance/escape performance and respiratory response of the baboon. Toxicology 47:165–179.
Kaplan, H.L., A.Anzeuto, W.G.Switzer, and R.K.Hinderer. 1988. Effects of hydrogen chloride on respiratory response and pulmonary function of the baboon. J. Toxicol. Environ. Health 23:473–493.
Kaplan, H.L., W.G.Switzer, R.K.Hinderer, and A.Anzeuto. 1993a. A study on the acute and long-term effects of hydrogen chloride on respiratory response and pulmonary function and morphology in the baboon. J. Fire Sci. 11:459–484.
Kaplan, H.L., W.G.Switzer, R.K.Hinderer, and A.Anzeuto. 1993b. Studies of the effects of hydrogen chloride and polyvinyl chloride (PVE) smoke in rodents. J. Fire Sci. 11:512–552.
Kusewitt, D.F., D.M.Stavert, G.Ripple, T.Mundie, and B.E.Lehnert. 1989. Relative acute toxicities in the respiratory tract of inhaled hydrogen fluoride, hydrogen bromide, and hydrogen chloride. Toxicologist 9:36.
Leonardos, G., Kendall, and N.J.Barnard. 1969. Odor threshold determinations of 53 odorant chemicals. J. Air Pollut. Control Assoc. 19:91–95.
Lucia, H.L., C.S.Barrow, M.F.Stock, and Y.Alarie. 1977. A semi-quantitative method for assessing anatomic damage sustained by the upper respiratory tract of the laboratory mouse, Mus musculis. J. Combust. Toxicol. 4:472–486.
Machle, W., K.V.Kitzmiller, E.W.Scott, and J.F.Treon. 1942. The effect of inhalation of hydrogen chloride. J. Ind. Hyg. Toxicol. 22:222–225.
Malek, D.E., and Y.Alarie. 1989. Ergometer within a whole-body plethysmograph to evaluate performance of guinea pigs under toxic atmospheres. Toxicol. Appl. Pharmacol. 101:340–355.
Nemery, B. 1996. Late consequences of accidental exposure to inhaled irritants: RADS and the Bhopal disaster. Eur. Respir. J. 9:1973–1976.
NIOSH (National Institute for Occupational Safety and Health). 1994. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLHs). U.S. Department of Health and Human Services, National Institute for Occupational Safety and Health, Cincinnati, OH.
NRC (National Research Council). 1987. Emergency and Continuous Exposure Guidance Levels for Selected Airborne Contaminants, Vol. 7. Washington DC: National Academy Press.
NRC (National Research Council). 1991. Permissible Exposure Levels and Emergency Exposure Guidance Levels for Selected Airborne Contaminants. Washington, DC: National Academy Press. Pp. 37–52.
NRC (National Research Council). 2000. Spacecraft Maximum Allowable Concentrations for Selected Airborne Contaminants, Vol. 4. Washington DC: National Academy Press.
NTIS (National Technical Information Service). 2000. Hydrogen Chloride. Registry of Toxic Effects of Chemical Substances (RTECS) [Online]. Available: http://www.ntis.gov/search/product.asp?ABBR=SUB5363&starDB=GRAHIST [October 1, 2000].
OSHA (Occupational Safety and Health Administration). 1999. CFR 29 Part 1910. Occupational Safety and Health Standards. Air Contaminants. U.S. Department of Labor, Washington, DC.
Pavlova, T.E. 1976. Disturbance of development of the progeny of rats exposed to hydrogen chloride. Bull. Exp. Biol. Med. 82:1078–1081.
Promisloff, R.A., G.S.Lenchner, and A.V.Cichelli. 1990. Reactive airway dysfunction syndrome in three police officers following a roadside chemical spill. Chest 98:928–929.
Sakurai, T. 1989. Toxic gas tests with several pure and mixed gases using mice. J. Fire. Sci. 7:22–77.
Schaper, M. 1993. Development of a database for sensory irritants and its use in establishing occupational exposure limits. Am. Ind. Hyg. Assoc. J. 54:488–544.
Ministry of Social Affairs and Employment (SDU Uitgevers). 2000. National MAC (Maximum Allowable Concentration) List, 2000. Ministry of Social Affairs and Employment, The Hague, The Netherlands.
Sellakumar, A.R., C.A.Snyder, J.J.Solomon, and R.E.Albert. 1985. Carcinogenicity of formaldehyde and hydrogen chloride in rats. Toxicol. Appl. Pharmacol. 81:401–406.
Siemiatycki, J., ed. 1991. Risk Factors for Cancer in the Workplace. Boca Raton, FL: CRC Press.
Stavert, D.M., D.C.Archuleta, M.J.Behr, and B.E.Lehnert. 1991. Relative acute toxicities of hydrogen fluoride, hydrogen chloride, and hydrogen bromide in nose- and pseudo-mouth-breathing rats. Fundam. Appl. Toxicol. 16:636–655.
Steenland, K., T.Schnorr, J.Beaumont, W.Halperin, and T.Bloom. 1988. Incidence of laryngeal cancer and exposure to acid mists. Br. J. Ind. Med. 45:766–776.
Stevens, B., J.Q.Koenig, V.Rebolledo, Q.S.Hanley, and D.S.Covert. 1992. Respiratory effects from the inhalation of hydrogen chloride in young adult asthmatics. JOM 34:923–929.
ten Berge, W.F., A.Zwart, and L.M.Appleman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapours and gases. J. Hazard. Mater. 13:301–309.
Toxigenics, Inc. 1984. 90-day Inhalation Toxicity Study of Hydrogen Chloride Gas in B6C3F1 Mice, Sprague-Dawley Rats, and Fischer-344 Rats, Revised. Decatur, IL: Toxigenics, Inc. Pp. 66.
Turlo, S.M., and I.Broder. 1989. Irritant-induced occupational asthma. Chest 96:297–300.
Vernot, E.H., J.D.MacEwen, C.C.Haun, and E.R.Kinkead. 1977. Acute toxicity and skin corrosion data for some organic and inorganic compounds and aqueous solutions. Toxicol. Appl. Pharmacol. 42:417–423.
Wohlslagel, J., L.C.DiPasquale, and E.H.Vernot. 1976. Toxicity of solid rocket motor exhaust: Effects of HCl, HF, and alumina on rodents. J. Combust. Toxicol. 3:61–70.
APPENDIX A Time-Scaling Calculations for Hydrogen Chloride
Derivation of AEGL-1
|
Key study: |
Stevens et al. 1992 |
|
Toxicity end point: |
No-observed-adverse-effect level in exercising asthmatic subjects. |
|
Time-scaling: |
C1×t=k (ten Berge 1986); (1.8 ppm)1×0.75 h=1.35 ppm·h |
|
Uncertainty factor: |
None |
|
10-min, 30-min, 1-h, 4-h, and 8-h AEGL-1: |
1.8 ppm |
Derivation of AEGL-2
|
10-min AEGL-2 |
|
|
Key study: |
Barrow et al. 1977 |
|
Toxicity end point: |
Mouse RD50 of 309 ppm to obtain a concentration causing irritation. |
|
10-min AEGL-2: |
309 ppm÷3=100 ppm |
|
30-min, 1-, 4-, and 8-h AEGL-2 |
|
|
Key Study: |
Stavert et al. 1991 |
|
Toxicity end point: |
Severe nasal (nose-breathers) or pulmonary (mouth-breathers) effects in rats exposed at 1,300 ppm for 30 min. |
|
Time-scaling: |
C1×t=k (ten Berge 1986); (1,300 ppm)1×0.5 h=650 ppm·h |
|
Uncertainty factors: |
3 for intraspecies variability 3 for interspecies variability |
|
Modifying factor: |
3 for sparse database |
|
30-min AEGL-2: |
C1×0.5 h=650 ppm·h C=1,300 ppm 30 min AEGL-2=1,300 ppm÷30=43 ppm |
|
1-h AEGL-2: |
C1×1 h=650 ppm·h C=650 ppm 1-h AEGL-2=650 ppm÷30=21.6 ppm |
|
4-h AEGL-2: |
1-h AEGL-2÷2=1 ppm |
|
8-h AEGL-2: |
1-h AEGL-2÷2=11 ppm |
Derivation of AEGL-3
|
Key Study: |
Wholslagel et al. 1976; Vernot et al. 1977 |
|
Toxicity end point: |
One-third of the rat 1-h LC50 as an estimate of a no-effect level for death (3,124 ppm÷3=1,041 ppm) |
|
Time-scaling: |
C1×t=k (ten Berge 1986) (1,041 ppm)1×1 h=1,041 ppm·h |
|
Uncertainty factors: |
3 for intraspecies variability 3 for interspecies variability |
|
10-min AEGL-3: |
C1×0.167 h=1,041 ppm·h C=6,234 ppm 10-min AEGL-3=6,234 ppm÷10=623.4 ppm |
|
30-min AEGL-3: |
C1×0.5 h=1,041 ppm·h C=2,082 ppm 30-min AEGL-3=2,082 ppm÷10=208 ppm |
|
1-h AEGL-3: |
C1×1 h=1,041 ppm·h C=1,041 ppm 1-h AEGL-3=1,041 ppm÷10=104.1 ppm |
|
4-h AEGL-3: |
C1×4 h=1,041 ppm·h C=260.25 ppm 4-h AEGL-3=260.25 ppm÷10=26 ppm |
|
8-h AEGL-3: |
8-h AEGL-3=4-h AEGL-3=26 ppm |
APPENDIX B ACUTE EXPOSURE GUIDELINE LEVELS FOR HYDROGEN CHLORIDE (CAS Reg. No. 7647–01–0)
DERIVATION SUMMARY
|
AEGL-1 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
1.8 ppm |
1.8 ppm |
1.8 ppm |
1.8 ppm |
1.8 ppm |
|
Key reference: |
Stevens, B. et al. 1992. Respiratory effects from the inhalation if hydrogen chloride in young adult asthmatics. JOM. 34:923–929. |
|||
|
Test species/strain/number: human/adult asthmatic subjects/10 |
||||
|
Exposure route/concentrations/durations: inhalation at 0, 0.8, or 1.8 ppm for 45 min while exercising (1.8 ppm was determinant for AEGL-1) |
||||
|
Effects: No treatment-related effects were observed in any of the individuals tested |
||||
|
End point/concentration/rationale: The highest concentration tested was a no-effect level for irritation in a sensitive human population (10 asthmatic individuals tested) and was selected as the basis for AEGL-1. Effects assessed included sore throat, nasal discharge, cough, chest pain or burning, dyspnea, wheezing, fatigue, headache, unusual taste or smell, total respiratory resistance, thoracic gas volume at functional residual capacity, forced expiratory volume, and forced vital capacity. All subjects continued the requisite exercise routine for the duration of the test period. |
||||
|
Uncertainty factors/rationale: |
||||
|
Interspecies: 1, test subjects were human |
||||
|
Intraspecies: 1, test subjects were sensitive population (exercising asthmatic subjects) |
||||
|
Modifying factor: Not applicable |
||||
|
Animal to human dosimetric adjustment: Insufficient data |
||||
|
Time-scaling: The AEGL-1 values for a sensory irritant were held constant across time because it is a threshold effect and prolonged exposure will not result in an enhanced effect. In fact one may become desensitized to the respiratory tract irritant over time. Also, this approach was considered valid since the end point (no treatment-related effects at the highest concentration tested in exercising asthmatics) is inherently conservative |
||||
|
Data quality and research needs: The key study was well conducted in a sensitive human population and is based on no treatment-related effects. In addition, the direct-acting irritation response is not expected to vary greatly among individuals. Therefore, confidence in the AEGL values derived is high. |
|
AEGL-2 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
100 ppm |
43 ppm |
22 ppm |
11 ppm |
11 ppm |
|
Key references: |
Stavert et al. 1991. Relative acute toxicities of hydrogen chloride, hydrogen fluoride, and hydrogen bromide in nose-and pseudo-mouth-breathing rats. Fundam. Appl. Toxicol. 16:636–655. (30-min, 1-, 4-, and 8-h) Barrow, C.S., Alarie, Y., Warrick, M., and Stock, M.F. 1977. Comparison of the sensory irritation response in mice to chlorine and hydrogen chloride. Arch. Environ. Health. 32:68–76. (10-min) |
|||
|
Test species/strain/number: F-344 rats, 8 males/concentration (30-min, 1-, 4-, and 8-h); Male Swiss Webster mice (10-min) |
||||
|
Exposure route/concentrations/durations: inhalation at 0 or 1,300 ppm for 30 min (1,300 ppm was determinant for 30-min, 1-, 4-, and 8-h AEGL-2) |
||||
|
Effects (30-min, 1-, 4-, and 8-h): 0 ppm, no effects; 1,300 ppm, severe necrotizing rhinitis, turbinate necrosis, thrombosis of nasal submucosa vessels in nose-breathers; 1,300 ppm, severe ulcerative tracheitis accompanied by necrosis and luminal ulceration in mouth-breathers (determinant for AEGL-2); RD50=309 ppm (determinant for 10-min AEGL-2) |
||||
|
End point/concentration/rationale: 1,300 ppm for 30 min, severe lung effects (ulcerative tracheitis accompanied by necrosis and luminal ulceration) or nasal effects (necrotizing rhinitis, turbinate necrosis, thrombosis of nasal submucosa vessels histopathology) in pseudo-mouth-breathing male F-344 rats (30-min, 1-, 4-, and 8-hr); RD50 of 309 ppm÷3 to estimate irritation (10-min) |
||||
|
Uncertainty Factors/Rationale (30-min, 1-, 4-, and 8-hr): |
||||
|
Total uncertainty factor: 10 |
||||
|
Intraspecies: 3, steep concentration-response curve implies limited individual variability |
||||
|
Interspecies: 3, the use of an intraspecies uncertainty factor of 10 would bring the total uncertainty/modifying factor to 100 instead of 30. That would generate AEGL-2 values that are not supported by data on exercising asthmatic subjects, an especially sensitive subpopulation because exercise increases hydrogen chloride uptake and exacerbates irritation. No effects were noted in exercising young adult asthmatic subjects exposed to HCl at 1.8 ppm for 45 min (Stevens et al. 1992). Using a total UF of 30 would yield 4- and 8-h values of 3.6 ppm (instead of 11 ppm). It is not supportable to predict that humans would be disabled by exposure at 3.6 ppm for 4- or 8-h when exercising asthmatic subjects exposed to one-half that level for 45 min had no effects. The |
||||
|
shorter time points would yield values 4–7 times above 1.8 ppm; however, the confidence in the time scaling for hydrogen chloride is good for times up to 100-min because the n value was derived from a regression analysis of rat and mouse mortality data with exposure durations ranging from 1 min to 100 minutes. The 30-min value of 43 ppm derived with the total UF of 10 is reasonable in light of the fact that baboons exposed to 500 ppm for 15 min experienced only a slightly increased respiratory rate |
||||
|
Modifying factor: 30-min, 1-, 4-, and 8-h: 3, based on sparse database for AEGL-2 effects and the fact that the effects observed at the concentration used as the basis for AEGL-2 were somewhat severe 10-min: the 10-min AEGL-2 was derived by dividing the mouse RD50 of 309 ppm by a factor of 3 to obtain a concentration causing irritation (Barrow et al. 1977). One-third of the mouse RD50 for hydrogen chloride corresponds to an approximate decrease in respiratory rate of 30%, and decreases in the range of 20–50% correspond to moderate irritation (ASTM 1991). |
||||
|
Animal to human dosimetric adjustment: Insufficient data |
||||
|
Time-scaling: Cn×t=k where n=1, based on regression analysis of combined rat and mouse LC50 data (1 min to 100 min) reported by ten Berge et al. (1986). Data point used to derive AEGL-2 was 30 min. AEGL-2 values for 1-h exposure period was based on extrapolation from the 30-min value. The 4- and 8-h AEGL-2 values were derived by applying a modifying factor of 2 to the 1-h AEGL-2 value because time scaling would yield a 4-h AEGL-2 value of 5.4 ppm and an 8-h AEGL-2 of 2.7 ppm, close to the 1.8 ppm tolerated by exercising asthmatic subjects without adverse health effects. |
||||
|
Data quality and research needs: Confidence is moderate since the species used is more sensitive than primates to the effects of hydrogen chloride, the chemical is a direct-acting irritant, and a modifying factor was included to account for the relative severity of effects and sparse database. |
|
AEGL-3 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
620 ppm |
210 ppm |
100 ppm |
26 ppm |
26 ppm |
|
Key references: |
Vernot, E.H., MacEwen, J.D., Haun, C.C., Kinkead, E.R. 1977. Acute toxicity and skin corrosion data for some organic and inorganic compounds and aqueous solutions. Toxicol. Appl. Pharmacol. 42:417–423. Wohlslagel, J., DiPasquale, L..C., Vernot, E.H. 1976. Toxicity of solid rocket motor exhaust: Effects of HCl, HF, and alumina on rodents. J. Combustion Toxicol. 3:61–70. |
|||
|
Test species/strain/gender/number: Sprague-Dawley rats, 10 males/concentration |
||||
|
Exposure route/concentrations/durations: inhalation at 0, 1,813, 2,585, 3,274, 3,941, or 4,455 ppm for 1 h |
||||
|
Effects: |
Concentration |
Mortality |
|
|
|
|
0 ppm |
0/10 |
||
|
1,813 ppm |
0/10 |
|||
|
2,585 ppm |
2/10 |
|||
|
3,274 ppm |
6/10 |
|||
|
3,941 ppm |
8/10 |
|||
|
4,455 ppm |
10/10 |
|||
|
LC50 reported as 3,124 ppm (determinant for AEGL-3) |
||||
|
End point/concentration/rationale: one-third of the 1-h LC50 (1,041 ppm) was the estimated concentration causing no deaths. |
||||
|
Uncertainty Factors/Rationale: |
||||
|
Total uncertainty factor: 10 |
||||
|
Intraspecies: 3, steep concentration-response curve implies limited individual variability |
||||
|
Interspecies: 3, because (1) the steep concentration-response curve for lethality observed in the Wohlslagel et al. (1976) study in which 1,041 ppm (one-third of the LC50 of 3124 ppm) was lower than the LC0 of 1,813 ppm. This is a conservative selection of the LC0 and the steep concentration-response curve argues for little interindividual variability; (2) AEGL-3 values generated from a total uncertainty factor of 30 would be close to the AEGL-2 values (within a factor of 2) generated above which are reasonable when compared with data on exercising asthmatics; (3) Sellakumar et al. (1985) exposed rats to HCl at 10 ppm for 6 h/d, 5 d/wk for life and only observed increased trachael and laryngeal hyperplasia. The estimated 6-h AEGL-3 using an intraspecies uncertainty factor of 3 is 17 ppm, close to the level used in the lifetime |
||||
|
study in which only mild effects were induced; (4) rats exposed at 50 ppm for 6 h/d, 5 d/wk for 90 d (Toxigenics 1984) exhibited mild rhinitis. This level is already 2 times that of the AEGL-3 value for death. Thus, the total uncertainty factor is 10. |
||||
|
Modifying factor: Not applicable |
||||
|
Animal to human dosimetric adjustment: Insufficient data |
||||
|
Time scaling: Cn×t=k where n=1, based on regression analysis of rat and mouse mortality data (1 min to 100 min) reported by ten Berge et al. (1986). Reported 1-h data point was used to derive AEGL-3 values. AEGL-3 values for 10-min, 30-min, and 4-h were based on extrapolation from the 1-h value. The 4-h value was adopted as the 8-h value. |
||||
|
Data quality and research needs: Study is considered appropriate for AEGL-3 derivation because exposures are over a wide range of HCl concentrations and utilize a sufficient number of animals. Data were insufficient to derive a no-effect level for death. One-third of the LC50 has been utilized previously for chemicals with steep concentration-response curves. Also, in the key study, no deaths were observed in rats exposed at 1,813 ppm. |