4
Diagnostics, Therapeutics, and Other Technologies to Control SARS
OVERVIEW
The strong possibility that SARS will return is being addressed by multiple sectors, including public health planners preparing for a broad range of challenges and contingencies (see also Chapter 1); researchers developing clinical diagnostics and technologies for infection control, as well as antiviral drugs and vaccines; and epidemiologists searching for clues from the recent SARS epidemic that could prevent a future outbreak or reduce its impact. Each of these perspectives is discussed in this chapter.
The development of a diagnostic test to rapidly detect SARS in its early stages is a top research priority. Because researchers do not know which tissues contain the highest concentrations of virus in the presymptomatic stages of infection, this task is particularly challenging. Reverse-transcription polymerase chain reaction (RT-PCR), a method to detect viral nucleic acids, is considered to be a likely platform for early SARS testing due to its high analytical sensitivity and speed. An evaluation of two RT-PCR protocols presented in this chapter found them to be highly specific for the SARS coronavirus; however, the tests were determined to be insufficiently sensitive to reliably detect the virus in respiratory specimens. Without a clinical diagnostic test, suspected cases of SARS must be confirmed in the laboratory, using RT-PCR or slower methods of detection—involving serology or viral culture, isolation, and identification by electron microscopy—thereby causing a significant increase in the time required for an accurate diagnosis.
This chapter also includes a description of an alternative diagnostic platform—the mass spectroscopic identification of microbial nucleic acid signatures—that can be adapted to detect the SARS coronavirus. Using technology originally designed for the environmental surveillance of biowarfare agents, this platform could potentially identify the SARS virus directly from a patient sample, obviating the need for time-consuming viral culture. This method is designed to distinguish between SARS and other coronaviruses, and perhaps even between genetic variants of the SARS virus; however, direct comparisons of sensitivity between this and other SARS detection systems using patient samples have yet to be conducted.
Several workshop participants expressed concern about the limited capacity in health care systems—particularly related to workforce and facilities shortages—that present a significant barrier to preparations for SARS and other threats to public health. It was suggested at the workshop by Jerome Schentag that this situation might be mitigated in some degree through the use of flexible approaches to isolating SARS patients. One such approach, discussed in this chapter, is a mobile technology that destroys viral particles and droplets in the air. These mobile units, by isolating individual patients being transported to and within hospitals, potentially could be used to protect staff during high-risk procedures such as intubation or bronchoscopy, to decontaminate larger areas such as hospital waiting rooms or airplanes, and to create air exchange systems for isolation facilities or areas within hospitals. Importantly however, it was noted during the workshop that the technologies described here must be thoroughly evaluated to determine their suitability for containing SARS in a variety of clinical settings before they are recommended for use.
Research has proceeded rapidly to develop antiviral drugs and vaccines to combat SARS. Previous antiviral discovery efforts by researchers at Pfizer on the human rhinovirus protease 3C—a functional, genetic, and structural analog to a key SARS coronavirus protease that has therefore been named “3C-like” (3CL)—are recounted in this chapter. This knowledge has aided in a search for 3CL protease inhibitors, a project undertaken by Pfizer in collaboration with scientists at the National Institute of Allergy and Infectious Diseases and the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID). Several candidate inhibitors have been selected by bioassay and are currently being evaluated for clinical development, while others are being sought through alternative strategies such as structure-based design and combinatorial chemistry. A vaccine for SARS—even if steered along a highly streamlined route to development—might still postdate a return of SARS, perhaps by several years. Nevertheless, because the medical need for developing such a vaccine and/or effective antiviral drugs is perceived to be acute, several pharmaceutical and biotechnology companies have taken up this challenge.
EVALUATION OF REVERSE TRANSCRIPTION-PCR ASSAYS FOR RAPID DIAGNOSIS OF SEVERE ACUTE RESPIRATORY SYNDROME ASSOCIATED WITH A NOVEL CORONAVIRUS
W.C. Yam, K.H. Chan, L.L.M. Poon, Y. Guan, K.Y. Yuen, W.H. Seto, and J.S.M. Peiris
Department of Microbiology, Queen Mary Hospital, The University of Hong Kong, Hong Kong, People’s Republic of China
Reprinted with permission, American Society for Microbiology.
Copyright 2003, American Society for Microbiology. All Rights Reserved.
The reverse transcription (RT)-PCR protocols of two World Health Organization (WHO) severe acute respiratory syndrome (SARS) network laboratories (WHO SARS network laboratories at The University of Hong Kong [WHO-HKU] and at the Bernhard-Nocht Institute in Hamburg, Germany [WHO-Hamburg]) were evaluated for rapid diagnosis of a novel coronavirus (CoV) associated with SARS in Hong Kong. A total of 303 clinical specimens were collected from 163 patients suspected to have SARS. The end point of both WHO-HKU and WHO-Hamburg RT-PCR assays was determined to be 0.1 50 percent tissue culture infective dose. Using seroconversion to CoV as the “gold standard” for SARS CoV diagnosis, WHO-HKU and WHO-Hamburg RT-PCR assays exhibited diagnostic sensitivities of 61 and 68 percent (nasopharyngeal aspirate specimens), 65 and 72 percent (throat swab specimens), 50 and 54 percent (urine specimens), and 58 and 63 percent (stool specimens), respectively, with an overall specificity of 100 percent. For patients confirmed to have SARS CoV and from whom two or more respiratory specimens were collected, testing the second specimen increased the sensitivity from 64 and 71 percent to 75 and 79 percent for the WHO-HKU and WHO-Hamburg RT-PCR assays, respectively. Testing more than one respiratory specimen will maximize the sensitivity of PCR assays for SARS CoV.
A global outbreak of a new emerging illness, severe acute respiratory syndrome (SARS), was associated with a novel coronavirus, SARS CoV (Lee et al., 2003; Peiris et al., 2003a; Tsang et al., 2003). By the end of April 2003, more than 1,500 patients were diagnosed with SARS in Hong Kong. Transmission within hospitals was a major contributor to disease amplification. Rapid laboratory confirmation of SARS CoV infection was important for managing patient care and for preventing nosocomial transmission. While serological testing was reliable as a retrospective diagnostic method, diagnosis of the infection in the early phase of the illness was important for patient care. The identification of the etiological agent and its partial gene sequence data made it possible to develop molecular diagnostic methods for SARS CoV (Drosten et al., 2003; Peiris et al., 2003b). The protocols were made available through the WHO website (http://www.who.int/csr/sars/primers/en). This study evaluates two of the first-generation reverse transcription (RT)-PCR assays that were used during this outbreak.
Materials and Methods
Patients and Specimen Collection
Specimens were available for 163 patients who presented with clinically suspected SARS according to the WHO definition (WHO, 2003) and who were admitted to three acute regional hospitals in Hong Kong between 26 February and 17 April 2003. For each patient, paired acute- and convalescent-phase serum samples and at least one respiratory specimen were collected for study. A total of 303 specimens (124 nasopharyngeal aspirate specimens, 65 throat swab specimens, 95 urine specimens, and 19 stool specimens) were available for study. Respiratory specimens were collected between days 1 and 5 after admission, whereas urine and stool specimens were collected between days 5 and 10. The acute-phase sera were collected in the first week of illness, and the convalescent-phase sera were collected 21 days after the onset of clinical symptoms. Nasopharyngeal aspirate specimens were assessed by rapid direct immunofluorescent antigen detection for influenza virus A and B, para-influenza virus types 1, 2, and 3, respiratory syncytial virus (RSV), and adenovirus as described previously (Chan et al., 2002). Paired serum samples were assayed for increasing titer against CoV. Nasopharyngeal aspirate and stool specimens from patients suffering from unrelated diseases were collected as controls.
Extraction of CoV RNA
Nasopharyngeal aspirate and throat swab specimens were suspended in viral transport medium. Urine specimens were transported in sterile containers. Stool specimens were mixed in viral transport medium (diluted 1:10) and microcentrifuged at 10,000 × g for 1 min, and supernatant was collected. Viral RNA was extracted from 140 μl samples using a Qiagen viral RNA mini kit (Qiagen, Hilden, Germany). The initial processing of specimens was performed under biohazard level 2 containment conditions. After lysis of the sample by the lysing buffer, the mixture was applied to a spin column as described by the manufacturer. The extracted RNA was eluted in a total volume of 50 μl of RNase-free water before RT-PCR amplification.
RT-PCR Amplification
The RT-PCR protocols of two WHO SARS network laboratories (Table 4-1) were evaluated in this study. The WHO SARS network laboratory at the University of Hong Kong (WHO-HKU) used a single RT step to synthesize cDNA, followed by subsequent PCR amplification with specific primers in another reaction tube (Peiris et al., 2003a). The WHO SARS network laboratory at the Bernhard-Nocht Institute in Hamburg, Germany (WHO-Hamburg) used a single
TABLE 4-1 RT-PCR Protocols for Rapid Diagnosis of CoV Associated with SARSa
|
Characterisitic or component of protocol |
RT |
WHO-HKU PCR |
RT-PVR |
Second PCR |
|
Primer sequences |
|
TACACACCIFCAGCGTTG CACGAACGTIGACGAAT |
ATGAATTACCAAGTCAATGGTTAC CATAACCAGTCGGTACAGCTAC |
GAAGCTATfCGTCACG CTGTAGAAAATCCTAGCTGGAG |
|
Sense |
|
|||
|
Antisense |
||||
|
Reagent formulation |
Superscript II RTA ( Invitrogen) (i) 4 μl of 5x first-strand buffer (ii) 10 mM DTT (iii) 500 μMdNTP (iv) 0.15 μg of random primer (v) 200 U of Superscript II (vi) 12 μl of RNA extract (vii) Make up total volume of 20 μl |
AmpliTaq Gold (Roche) (i) 5 μl of 10x reaction buffer (ii) 200 μM dlU (iii) 2.5 μM MgSO4 (iv) 250 nM (each) primer (v) 2 U of AmpliTaq Gold (vi) 2 μl of RT product (vii) Make up total volume of 50 μl |
Superscript II RT-PCR (Invitrogen) (i) 10 μl of 2x reaction buffer (ii) 2.45 mM MgSO4 (iii) 500 μM (each) primer (iv) 0.4 μl of RTA-Taq mixture (v) 2 μl of RNA extract (vi) Make up total volume of 20 μl |
AmpliTaq Gold (Roche) (i) 5 μl of 10x reaction buffer (ii) 200 μM dNTP (iii) 2.5 μM MgSo4 (iv) 200 nM (each) primer (v) 2 U of AmpliTaq Gold (vi) 1 μl of RT-PCR product (vii) Make up total volume of 50 μl |
|
Thermal cycling profile |
(i) 25°C, 10 min (ii) 42°C, 50 min (iii) 94°C, 3 min |
(i) 94°C, 10 min (ii) 40 cycles (a)94°C, 30 s (b) 50°C, 40 s (c) 72°C, 15 s (iii) 72°C, 10 min |
(i) 45°C, 30 min (ii) 95°C, 3 min (iii) 10 cycles (a) 95°C, 10 s (b)60°C, 10 s (decrease by 1°C/cycle) (c) 72°C, 20 s (iv) 40 cycles (a) 95°C, 10 s (b) 56°C, 10 s (decrease by 1°C/cycle) (c) 72°C, 20 s |
(i) 95°C, 5 min (ii) 10 cycles (a)95°C, 10 s (b) 60°C, 10 s (decrease by 1°C/cycle) (c) 72°C, 30 s (iii) 20 cycles (a) 95°C, 10 s (b) 56°C, 10 s (c) 72°C, 30 |
|
Expected PCR product size (bp) |
|
182 |
189 |
108 |
|
aThe RT-PCR protocols of two WHO SARS network laboratories. WHO-HKU (Peiris et al., 2003a) and WHO-Hamburg (Drosten et al., 2003) are also available online (http://www/who.int/esr/sars/primers/en). Abbreviations: RTA, reverse transcriptase; DDT, dithiotheritol; dNTP, deoxynucleoside triphosphate. |
||||
RT-PCR step, followed by transfer of the initial PCR products to the nested PCR amplification mixture (Drosten et al., 2003). Positive and negative controls were included in each run, and all precautions to prevent cross-contamination were observed. For nested PCR, RT-PCR amplicon tubes were spun (in pulses) before the tubes were opened using separate Eppendorf tube openers for transferring RT-PCR products to the nested PCR mix. Negative control was incorporated for every five nested PCRs to monitor cross-contamination. Amplified products were electrophoresed through a 2 percent agarose gel in Tris-borate buffer. Target bands were visualized by staining with ethidium bromide.
CoV Immunoglobulin G Serology
Smears of CoV-infected Vero cells were prepared, fixed in acetone for 10 min, and stored at –80°C before use (Peiris et al., 2003a). Each batch of SARS CoV-infected cell smears with 60 to 70 percent infected cells was prepared and tested with a high-titer, positive-control serum sample from a confirmed SARS patient as a standard to assess sensitivity and batch-to-batch variations. Serial twofold dilutions starting with a 1:10 dilution of each patient serum sample were added to the smears and incubated for 30 min at 37°C. After two 5-min washes in phosphate-buffered saline, fluorescein isothiocyanate-conjugated goat anti-human immunoglobulin G (INOVA Diagnostics, Inc., San Diego, California) was added to the smears, and the smears were incubated for 30 min at 37°C. Acute- and convalescent-phase serum samples from each patient were assayed for SARS CoV antibodies in the same experiment to minimize experimental variations. The titer was determined as the highest dilution of serum exhibiting fluorescence of the infected cells. A weakly positive patient serum sample was included as a control in each run. A sample was scored as a positive result if the fluorescent intensity was equal to or higher than that of the positive control.
Determination of the End Points of the RT-PCR Assays
A 96-well microtiter plate containing 0.1 ml of confluent Vero cells was used to determine the 50 percent tissue culture infective dose (TCID50) of SARS CoV under biohazard level 3 containment conditions. Tenfold serial dilutions of a cell-adapted SARS CoV strain from 10–1 to 10–8 were prepared. One hundred microliters of each dilution were added to each well of four replicate wells and incubated at 37°C for 2 to 3 days to observe cytopathic effect. TCID50s were determined by the Kärber method (Ballew, 1992). For the same serial dilutions of virus, 100-μl samples were subjected to RNA extraction, and the end points of the two RT-PCR assays were determined.
Results
Of 303 specimens from clinically suspected SARS cases (see Table 4-2), 145 were positive by one or both PCR assays and more than 87 percent of PCR-positive samples were identified by both PCR assays. Common respiratory viral pathogens, including influenza virus A and B, parainfluenza virus types 1, 2, and 3, RSV, and adenovirus, were not detected in the 124 nasopharyngeal aspirate specimens. The end point for both WHO-HKU and WHO-Hamburg RT-PCR methods was determined to be 0.1 TCID50. The acute-phase serum samples from all patients were seronegative for SARS CoV. Eighty-six patients were confirmed to have SARS CoV infections on the basis of seroconversion. Using seroconversion as the gold standard for SARS diagnosis, the sensitivities of the WHO-HKU and WHO-Hamburg RT-PCR assays were found to be 61 and 68 percent (nasopharyngeal aspirate specimens), 65 and 72 percent (throat swab specimens), 50 and 54 percent (urine specimens), and 58 and 63 percent (stool specimens). A specificity of 100 percent was exhibited by both RT-PCR assays, as none of the seronegative patient samples and control samples gave a positive PCR result. Among the 163 patients, two or more respiratory specimens (nasopharyngeal aspirate or throat swab specimens) were available from 41 patients. Of the 41 patients, 28 were subsequently confirmed to have SARS CoV on the basis of seroconversion. In these 28 patients, the numbers of first specimens positive for WHO-HKU and WHO-Hamburg RT-PCR were 18 and 20, respectively, but testing a second specimen increased the overall sensitivity from 64 and 71 percent to 75 and 79 percent, respectively.
Discussion
In Hong Kong, SARS is a serious respiratory illness that led to significant morbidity and mortality (Donnelly et al., 2003). The diagnosis depends mainly on the clinical findings of an atypical pneumonia not attributed to another cause and a history of exposure to a suspect or probable case of SARS or to the respiratory secretions and other bodily fluids of individuals with SARS. Definitive diagnosis of this novel CoV relies on classic tissue culture isolation, followed by electron microscopy studies to identify the virus on cell culture, which is technically very demanding. Serological testing for increasing titer against SARS-associated CoV was shown to be highly sensitive and specific (Peiris et al., 2003a) but was not suitable for rapid laboratory diagnosis. The rapid isolation and characterization of the novel CoV associated with SARS allowed for the timely development of diagnostic tests (Marra et al., 2003; Rota et al., 2003). RT-PCR protocols of two WHO SARS network laboratories were evaluated for rapid diagnosis of SARS-associated CoV in Hong Kong. The end point for the novel CoV by both RT-PCR assays was similar to the previous finding for human CoV (Vabret et al., 2001), yet sufficient diagnostic sensitivity was not achieved, despite attaining a
TABLE 4-2 Performance of RT-PCR Assays for Rapid Detection of CoV Associated with SARS
|
|
No. of specimens positive by RT-PCR assay |
||||
|
Specimens (no.) |
No. of specimens tested |
Seroconversiona |
WHO-HKU |
WHO-Hamburg |
Both WHO-HKU and WHO-Hamburg |
|
Clinically suspected SARS |
|
||||
|
Nasopharygneal aspirate specimens (124) |
72 |
+ |
44 |
49 |
43 |
|
|
52 |
– |
0 |
0 |
0 |
|
Throat swab specimens (65) |
54 |
+ |
35 |
39 |
33 |
|
Urine specimens (19) |
78 |
+ |
39 |
42 |
39 |
|
Stool specimens |
19 |
+ |
11 |
12 |
11 |
|
Controls |
|
||||
|
Nasopharygneal aspirate specimens |
22b |
ND |
0 |
0 |
0 |
|
Stool specimen |
21c |
ND |
0 |
0 |
0 |
|
aA fourfold rise of more in antibody titer against CoV was considered seroconversion (+). ND, not done. bSamples positive for other viral pathogens included nine samples positive for influenza virus A, one sample positive for influenxa virus B, six samples positive for adenovirus, and six samples positive for RSV by immunoflourescence (Chan et al., 2002). cNo intestinal pathogens detected. |
|||||
specificity of 100 percent. A recent study using real-time RT-PCR revealed that the viral load in nasopharyngeal aspirate specimens peaked in the second week of the illness (Peiris et al., 2003b). Results indicated a more sensitive RT-PCR assay is essential for rapid diagnosis of SARS CoV during the early stage of disease. Due to the nature of respiratory specimens with inconsistent pathogen loads at various sample times, testing of multiple specimens has been shown to increase the sensitivity of laboratory diagnosis for Mycobacterium tuberculosis (Nelson et al., 1998). Testing a second respiratory specimen by RT-PCR increased the sensitivity of diagnosis for SARS CoV.
The examination of more than one respiratory specimen is necessary to maximize the sensitivity of RT-PCR assays for SARS CoV. As molecular characterization of this novel CoV is ongoing, targeting genomic segments of the virus for diagnostic application is still unclear. Amplification of a second genome region may further increase test specificity. In this study, the high specificity and concordance of both RT-PCR assays verified that the amplified genomic segments for both protocols are suitable for diagnostic application. Incorporation of internal probe hybridization will probably increase the sensitivity of the WHO-HKU RT-PCR assay. In this global outbreak of SARS, prompt communication and exchange of information among the WHO collaborating laboratories facilitate development of rapid diagnostic assays with shortened turnaround time. The availability of the protocols on the WHO website was helpful to diagnostic laboratories. The collaborative approach can be invaluable in our efforts to understand and control emerging pathogens in the future.
Acknowledgments
We thank Christian Drosten of the Bernhard-Nocht Institute (Hamburg, Germany) and TIB-MOLBIOL (Hamburg, Germany) for providing DNA primers used in the WHO-Hamburg RT-PCR protocol. We also thank the staff of the Department of Microbiology, Queen Mary Hospital, The University of Hong Kong for their technical assistance.
NOVEL BIOSENSOR FOR INFECTIOUS DISEASE DIAGNOSTICS
Rangarajan Sampath and David J. Ecker
Ibis Therapeutics, a division of Isis Pharmaceuticals
We describe a novel approach for surveillance of emerging infectious diseases that can be used for rapid and broad identification of infectious disease causative agents. The premise of our technology is that we can provide rapid, sensitive, and cost-effective detection of a broad range of “normal” pathogenic organisms and simultaneously also diagnose disease caused by a biological weapon or an unexpected emerging infectious organism. This broad-function
technology may be the only practical way to rapidly diagnose diseases caused by a bioterrorist attack or emerging infectious diseases that otherwise might be missed or mistaken for a more common infection.
According to a recent review (Taylor et al., 2001), more than 1,400 organisms are infectious to humans. These numbers do not include numerous strain variants of each organism, bioengineered versions, or pathogens that infect plants or animals. Paradoxically, most of the new technology being developed for detection of infectious agents incorporates a version of quantitative PCR, which is based on the use of highly specific primers and probes designed to selectively detect specific pathogenic organisms. This approach requires assumptions about the type and strain of bacteria or virus. Experience has shown that it is very difficult to anticipate where the next emerging infectious agent might come from, as was the case with the outbreak of SARS early in 2003. An alternative to single-agent tests is to do broad-range consensus priming of a gene target conserved across groups of organisms (Kroes et al., 1999; Oberste et al., 2000, 2001, 2003). The drawback of this approach for unknown agent detection and epidemiology is that analysis of the PCR products requires the cloning and sequencing of hundreds to thousands of colonies per sample, which is impractical to perform rapidly or on a large number of samples. New approaches to the parallel detection of multiple infectious agents include multiplexed PCR methods (Brito et al., 2003; Fout et al., 2003) and microarray strategies (Wang et al., 2002, 2003; Wilson et al., 2002). Microarray strategies are promising because undiscovered organisms might be detected by hybridization to probes on the array that were designed to bind conserved regions of previously known families of bacteria and viruses.
Here we present an alternative, a universal pathogen-sensing approach for high-throughput detection of infectious organisms that is capable of identifying previously undiscovered organisms (see Figure 4-1).
Our strategy is based on the principle that, despite the enormous diversity of microbes, all forms of life on earth share sets of essential common features in the biomolecules encoded in their genomes. Bacteria, for example, have highly conserved sequences in a variety of locations on their genomes. Most notable is the universally conserved region of the ribosome, but there are also conserved elements in other noncoding RNAs, including RNAse P and the signal recognition particle, among others. There are also conserved motifs in essential protein-encoding genes, in bacteria as well as viruses. Use of such broad-range priming targets across the broadest possible grouping of organisms for PCR, followed by electrospray ionization mass spectrometry for accurate mass measurement, enables us to determine the base composition (numbers of A, G, C, and T nucleotides) of the PCR amplicons. The measured base compositions from strategically selected locations of the genome are used as a signature to identify and distinguish the organisms present in the original sample. An important feature of the primer design strategy used in our approach is the positioning of propynylated
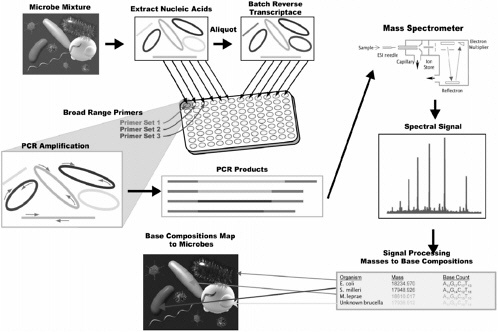
FIGURE 4-1 Overview of the universal pathogen sensor for the detection of a diverse mixture of microbial organisms present in a sample. Genomic DNA, or cDNA obtained by batch reverse-transcription of RNA, from each sample are amplified using broad range PCR primers to generate a complex mixture of PCR products. This mixture of DNA is directly sprayed into a mass spectrometer that essentially weighs each intact nucleic acid strand in the mixture at the same time, This measurement is done at high mass accuracy, which enables us to calculate the exact number of A’s, C’s, G’s, and T’s that make up the DNA in our sample. This count serves as a base-composition fingerprint that can be mapped back to specific organisms. Examination of multiple base-count fingerprints for each organism generated by multiple pairs of broad-range primers to conserved sites distributed across the microbial genome (not shown) allows discrimination of microbial species and subspecies with great accuracy.
nucleotides (5-propynyl deoxy-cytidine and deoxy-thymidine) at highly conserved sequence positions that enables priming of short consensus regions and significantly increases the extent to which broad groups of organisms can be amplified (Barnes and Turner, 2001a,b; Wagner et al., 1993). Furthermore, we use multiple target sites spread across different parts of the genome to add further resolution and lower the risk of missed detections.
A key to the development of a practical broad priming technology is the ability to characterize signals produced by infectious organisms in the milieu of the background that might have an excess of harmless organisms. While cloning and exhaustively sequencing many colonies can solve this, this cannot be done in a rapid diagnostic device. Our strategic breakthrough was the use of mass spectrometry to analyze the products of broad-range PCR. Mass spectrometry is remarkably sensitive and can measure the weight and determine the base composition from small quantities of nucleic acids in a complex mixture with a throughput of about a sample per minute. The ability to detect and determine the base composition of a large number of PCR amplicons in a mixed sample enables analysis and identification of broad-range PCR products essentially instantaneously. In contrast to cloning and sequencing, the information product of the mass spectrometer is base composition. While the base composition of a gene fragment is not as information rich as the sequence, a base-composition signature can be thought of as a unique index of a specific gene in a specific organism. Our detection algorithm searches a database to link each sequence for a particular organism to a composition signature so that the presence of the organism can be inferred from the presence of the signature.
During the SARS epidemic outbreak in early 2003, we demonstrated that the above-described paradigm of identification of microbial nucleic acid signatures by mass spectrometry could be adapted to identify the SARS virus. In the absence of a SARS genome sequence at the onset of the epidemic, pairs of broad primers that were designed to broadly target all other known coronaviruses were used to test clinical isolates obtained from the Centers for Disease Control and Prevention (CDC). We showed that the SARS virus potentially could be identified directly from a patient sample, obviating the need for time-consuming viral culture. We further showed that this method could distinguish between SARS and other known coronaviruses, including the human coronaviruses 229E and OC43. While direct comparisons of sensitivity, using actual patient samples, have yet to be conducted between this and other methods employed to detect SARS, we did show, using titred SARS virus spiked into human serum, that we could obtain PCR sensitivities of <1 PFU, which is consistent with our previous experience. The details of the above study will be published elsewhere (Sampath et al., under preparation).
One of the limitations of our approach is that base compositions, like sequences, vary slightly from isolate to isolate within species. We have shown that it is possible to manage this diversity by building probability “clouds”
around the composition constraints for each species. This permits identification of organisms in a fashion similar to sequence analysis, albeit with somewhat lower resolution. It is counterintuitive that base composition has sufficient resolving power to distinguish organisms (one might suspect that sequences from different organisms will degenerate to similar overlapping compositions). A rigorous mathematical analysis has shown, however, that base composition retains more than sufficient information to solve the problem, provided the target sequences are strategically selected. It is important to note that, in contrast to probe-based techniques, mass spectrometry determination of base composition does not require prior knowledge of the composition in order to make the measurement, only to interpret the results. In this regard, our strategy is like DNA sequencing and phylogenetic analysis, but at lower resolution. However, the resolution provided by this analysis is more than sufficient for most rapid diagnostic applications such as identification of any organism, or to classify organisms into known phylogenetic groupings (Sampath et al., under preparation).
We envision developing applications where human clinical samples can be analyzed for diagnostically relevant levels of disease-causing agents and biological weapons simultaneously. We envision that the technology will be used in reference labs, hospitals, and the laboratory response network (LRN) laboratories of the public health system in a coordinated fashion with the ability to report the results via a computer network to a common data-monitoring center in real time. Clonal propagation of specific infectious agents, as occurs in the epidemic outbreak of infectious disease, can be tracked with base composition signatures, analogous to the pulse field gel electrophoresis fingerprinting patterns used in tracking the spread of specific food pathogens in the CDC Pulse Net system (Swaminathan et al., 2001). Effectively, our technology provides a digital barcode in the form of a series of base composition signatures, the combination of which is unique for each organism. This capability enables real-time infectious disease monitoring across broad geographic locations, which may be essential in a simultaneous outbreak or attack in different cities.
Acknowledgments
This methodology described is being developed jointly by Ibis and Science Applications International Corporation (SAIC) under a Defense Advanced Research Projects Agency (DARPA) sponsored program known as TIGER. A detailed description of the technology will be published separately. More than 25 key participants who contributed significantly to the development and implementation of various aspects of the technology are not listed individually by name.
IN VITRO ANTIVIRAL ACTIVITY OF HUMAN RHINOVIRUS 3C PROTEASE INHIBITORS AGAINST THE SARS CORONAVIRUS
David A. Matthews,1Amy K. Patick,1Robert O. Baker,2Mary A. Brothers,1Peter S. Dragovich,1Chris J. Hartmann,2Theodore O. Johnson,1Eric M. Mucker,2Siegfried H. Reich,1Paul A. Rejto,1Peter W. Rose,1Susan H. Zwiers,2and John W. Huggins2,3
The construction of a homology model of the 3C-like (3CL) protease derived from the SARS coronavirus (SCoV) is described. This model is used to qualitatively evaluate the potential for several Michael acceptor-containing human rhinovirus 3C protease inhibitors to also disrupt the function of the SCoV 3CL enzyme. The antiviral activity of three such compounds (AG7088, AG7404, and AG7122) determined against SCoV in cell culture is reported (see Figure 4-2). The former two molecules fail to inhibit in vitro replication of SCoV up to the highest concentrations tested (100 μg/mL) while AG7122 exhibits measurable antiviral activity against SCoV that is distinguishable from cytotoxicity (EC50 = 14.1 μg/mL, CC50 >100 μg/mL).
Severe acute respiratory syndrome (SARS) is a potentially serious global health concern and the disease has been responsible for considerable negative economic impact in affected regions (Poutanen et al., 2003; Tsang et al., 2003). A newly discovered coronavirus (SCoV) has been strongly implicated as the causative agent of SARS by independent research conducted at several laboratories around the world (Drosten et al., 2003; Fouchier et al., 2003; Ksiazek et al., 2003; Lee et al., 2003; Peiris et al., 2003a). Recently, sequencing and analysis of the SARS virus genome has led to the identification of gene products that may be critical for viral replication (Marra et al., 2003; Rota et al., 2003). In particular, such analysis suggests that the SARS pathogen, like other known coronaviruses (Ziebuhr et al., 2000), encodes a critical enzyme that is required for C-proximal processing of two overlapping polyproteins produced by cellular translation of the viral RNA. This coronavirus enzyme has been termed a “3C-like” (3CL) protease due to numerous similarities with the well-known picornavirus 3C proteases including substrate preferences, particularly the requirement of a P1 glutamine residue, and the use of cysteine as an active site nucleophile during catalysis (Hegyi and Ziebuhr, 2002; Ziebuhr et al., 2000). In addition, the coronavirus 3CL and picornavirus 3C proteins share a similar polypeptide fold, as evidenced by comparison of the crystal structure of the 3CL protease derived from porcine transmissible gastroenteritis coronavirus (Anand et al., 2002) with
those of corresponding picornaviral 3C enzymes (e.g., human rhinovirus 3C protease [HRV 3CP]) (Matthews et al., 1999).
The structural and functional homologies noted above between the coronavirus 3CL and picornavirus 3C enzymes were apparent (Anand et al., 2002) before the relationship between SARS and its causative agent was disclosed. Coincident with the announcement that a new coronavirus causes SARS and prior to publication of the SARS virus genome, we initiated a computational study to explore whether Michael acceptor-containing HRV 3CP inhibitors (discovered during our previous efforts to identify antirhinoviral therapeutic agents [Matthews et al., 1999]) might also bind to coronavirus 3CL proteases. The availability of the SCoV genetic sequence (Fouchier et al., 2003; Marra et al., 2003; Rota et al., 2003) enabled us to further extend these studies using a proprietary homology model of the SARS 3CL enzyme. Recently, Anand et al. (2002) disclosed an independent computational evaluation of several known picornaviral 3C protease inhibitors against SARS 3CL and reported that, although such molecules were not necessarily optimized, they could serve as starting points for the design of new SARS antiviral agents. In this communication, we report an alternate computational assessment of the potential for several Pfizer compounds to inhibit SARS 3CL along with their experimentally determined antiviral activities against SCoV in cell culture.
Our homology model for SARS 3CL protease was created using the atomic coordinates of TGEV 3CL protease (PDB accession code 1LVO) as a template. BLAST was employed to identify the 3CL protease from the genomic RNA sequence of SARS (AY274119). Minor adjustment to the BLAST output resulted in an alignment with high percent identity and few gaps (see Figure 4-3), and this alignment was used to create a homology model with the MODELLER package in Insight2000 (Sali and Blundell, 1993). Twelve residues with high structural conservation (see Table 4-3) were identified by visual inspection of the human rhinovirus 3C (1CQQ) and TGEV 3CL protease (1LVO) structures, as well as the SARS 3CL protease homology model. The structures were superimposed in a common reference frame by minimizing the root mean square distance (r.m.s.d.) between the backbone atoms of these residues, with r.m.s.d. < 0.6 Å (Drosten et al., 2003; Fouchier et al., 2003; Ksiazek et al., 2003; Peiris et al., 2003a).
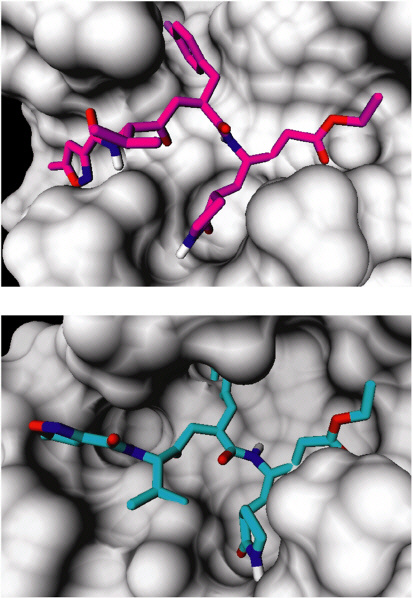
Putative Michael acceptor-containing SARS 3CL protease inhibitors from the Pfizer chemical archive were computationally evaluated by first creating three-dimensional structures of the compounds using CORINA version 2.6 (Sadowski and Gasteiger, 1993), then employing a constrained docking approach using AGDOCK (Gehlhaar et al., 1999) to determine their binding mode to the model. The geometry of the reaction product as observed in the co-crystal structure of one such Pfizer compound (AG7088) with human rhinovirus 3C protease (Matthews et al., 1999) (PDB accession code 1CQQ) was used as a template to model the covalent binding of Michael acceptors with the active site cysteine of SARS 3CL protease (see Figure 4-4). The Michael acceptor portions of the in-

FIGURE 4-3 Sequence alignment between the TGEV 3CL protease (PDB accession code 1LVO) and SARS 3CL protease used to create the homology model.
hibitors were forced onto the template structure and then constrained during docking. The resulting protein-ligand interactions were qualitatively evaluated by visual inspection. The outcome of computationally docking AG7088 into the SARS 3CL homology model is also depicted (see Figure 4-4).
Of the Michael acceptor-containing HRV 3CP inhibitors examined, only two have been the subject of human clinical trials: AG7088 (rupintrivir) (Matthews et al., 1999; Patick et al., 1999) as an intranasally administered agent and AG7404 (Dragovich et al., 2003) as an orally delivered compound. Unfortunately, our computational evaluation detected relatively poor complementarity between the compounds’ P3 and P4 substituents and SARS 3CL that resulted in numerous
TABLE 4-3 Residues Employed for the Superposition of Human Rhinovirus (HRV) 3C Protease and TGEV 3CL Protease Structures Along with Corresponding SARS 3CL Amino Acids
|
HRV (1CQQ) |
TGEV (1LVO) |
SARS |
|
PRO-38 |
PRO-39 |
PRO-39 |
|
THR-39 |
ARG-40 |
ARG-40 |
|
HIS-40 |
HIS-41 |
HIS-41 |
|
LYS-143 |
ILE-140 |
LEU-141 |
|
SER-144 |
ALA-141 |
ASN-142 |
|
GLY-145 |
GLY-142 |
GLY-143 |
|
TYR-146 |
THR-143 |
SER-144 |
|
CYS-147 |
CYS-144 |
CYS-145 |
|
ILE-160 |
MET-161 |
MET-162 |
|
HIS-161 |
HIS-162 |
HIS-163 |
|
VAL-162 |
HIS-163 |
HIS-164 |
|
GLY-163 |
LEU-164 |
MET-165 |
structural clashes and several unsatisfied hydrogen bonds.4 These findings are in partial contrast with those reported by Anand et al. which suggest “easy accommodation” of the AG7088 P4 substituent by SARS 3CL (Anand et al., 2003). Our evaluation also indicated that a truncated compound related to AG7088 which lacks both P3 and P4 substituents (AG7122) interacted more favorably with the SARS 3CL protein (Johnson et al., 2002). In order to help define the accuracy of our modeling efforts, the potential for all three molecules (AG7088, AG7404, and AG7122) to inhibit the SARS virus in cell culture was evaluated.
Stocks of tested compounds were made by dissolving them in DMSO to a concentration of 20 mg/mL. Compounds were then diluted to 400 mg/mL in cell culture medium [high glucose Dulbeco’s Modified Eagle Medium (DMEM) supplemented with 1% fetal calf serum, 10 U/mL penicillin-streptomycin, and 12.5 ng/mL fungizone], serially diluted threefold in medium, and 50 μL added to 96-well microtiter plates of confluent Vero 76 cells already containing 100 μL medium. At each compound concentration, three wells were infected with 2×102 pfu/well (MOI = 0.001) of SCoV (strain 200300592) in 50 μL medium, while three were left uninfected for cytotoxicity determination (50 μl medium added to each well). The plates were incubated at 37°C in a 5 percent CO2 atmosphere, examined daily, and were stained once virus-infected, untreated cells showed maximum cytopathic effect (about 3 days). Neutral red was added to the medium to give a final concentration of 0.22 mg/mL, and cells were returned to the incubator for 90 minutes. The medium containing neutral red was removed, the wells were rinsed twice with buffered saline solution, and plates were decontaminated by soaking in 10 percent buffered formalin followed by a water wash. Retained stain was solubilized by adding 100 μL of a 50 percent ethanol, 50% 0.01 M ammonium phosphate (NH4H2PO4) (pH 3.5) solution. The plates were incubated for 15 minutes at room temperature and the optical density (OD) of the wells at a wavelength of 450 nm was measured on a plate reader. The data were graphed and analyzed using the four parameter-logit curve fit option of the computer program SoftMax Pro (Molecular Devices, Menlo Park, CA) to determine the 50 percent inhibitory (EC50) and cytotoxic (CC50) compound concentrations.
As shown in Table 4-4, both AG7088 and AG7404 failed to inhibit in vitro replication of SCoV up to the highest concentrations tested (100 mg/mL). In contrast, AG7122 exhibited moderate but measurable inhibition of SCoV that was distinguishable from cytotoxicity (see Figure 4-5, Table 4-4). Although these antiviral data parallel our qualitative computational evaluation of the three molecules against the SARS 3CL protease, other factors such as differing cell permeability properties may also influence the results. We are therefore uncertain whether the poor complementarity noted in silico is responsible for the lack of
TABLE 4-4 In Vitro Antiviral Activity (EC50) and Cytotoxicity (CC50) of Pfizer Compounds Determined Against SCoV in Cell Culture
|
Compound |
Antiviral Activity (μg/ml) |
Cytotoxicity (μg/ml) |
|
AG7088 |
>100 |
>100 |
|
AG7404 |
>100 |
>100 |
|
AG7122 |
14.1 |
>100 |
observed activity of AG7088 and AG7404 against SCoV in cell culture. While this inactivity is disappointing, the antiviral effects displayed by AG7122 encouragingly suggest that proper optimization of such Michael acceptor-containing protease inhibitors may lead to agents with improved anti-SCoV properties.5 Since the majority of the Michael acceptors contained in the Pfizer chemical archive are optimized against HRV 3CP, we do not anticipate that their exhaustive screening against SCoV will afford ideal therapeutic agents. However, we
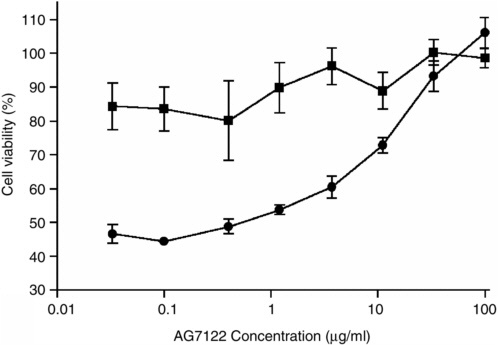
FIGURE 4-5 Antiviral activity of AG7122 against SCoV in Vero 76 cells. Cells were treated with the indicated compound concentrations and infected with virus (circles) or left uninfected (squares). Cell viability was measured by neutral red uptake, and is expressed as a percentage of the value in uninfected, untreated wells. Data shown are the mean of three replicate wells. Bars indicate the standard error of the mean.
are still continuing such in vitro evaluation and have identified several additional Pfizer compounds that display improved antiviral activity (EC50 = 1-2 μg/mL, CC50 >100 μg/mL) relative to that exhibited by AG7122. We are currently using these molecules to help refine our SARS 3CL computational model and will report the progress of our endeavors in due course.6
SARS: CLEARING THE AIR
Jerome J. Schentag, Pharm. D.,7,8,9Charles Akers, Ph.D.,8Pamela Campagna,8and Paul Chirayath8
The integrated technologies incorporated into the FailSafe Mobile Containment Systems have a wide range of applications, including homeland security, bioterrorism, disaster management, airborne infection control, sick-building syndrome, and facility environmental service applications. The specific objective of this overview is to focus on the use of FailSafe Mobile Containment Systems for isolation precautions in a medical environment. FailSafe has not used these devices directly in an outbreak of severe acute respiratory syndrome (SARS), and thus actual clinical experience will not be reported here. Given that a major component of the spread of SARS occurs via aerosolized droplets, the systems described for clearing the air may be applicable to the containment of this new viral pathogen in hospitals and health care systems.
The guidelines for isolation precautions for hospitals and health care facilities are outlined by the Centers for Disease Control and Prevention (2004) and American Institute of Architechts (2001). These guidelines outline the precautions that infection control personnel should take to mitigate the spread of infection within facilities and protect the health care worker. Precautions must be taken to prevent the spread of infection from direct contact with contaminated surfaces (contact contamination), from large droplets of infectious material that fall out of the air, or from small droplets that can be carried by the air stream throughout the hospital (airborne contamination).
The guidelines for the creation of an isolation room are based on the principle that the isolation room is maintained under negative pressure to minimize the ability of any airborne contamination from entering the hospital. To validate the design recommendations, the precautions listed in Box 4-1 must be taken.
|
BOX 4-1
|
Failsafe Air Safety Systems Approach
FailSafe Air Safety Systems (FASS) manufactures two medical isolation units the Model 77 and the Model 07 (see Figure 4-6)—that provide personalized isolation and infection control. These medical units employ a patented air safety process that was developed in response to the lack of market availability of portable containment systems. Both the Transport Isolation Unit and the Portable Isolation Unit are equivalent in technology to an isolation room, but have the ability to bring isolation to an infected patient.
The Model 77 can be moved to an area and set up in minutes. The main components are a prefilter; an industrial, high-capacity, micro-fiberglass HEPA filter; ultraviolet lamp(s); and a high-volume blower.
The Model 07 provides isolation on wheels. At 27 inches wide, a single attendant can handle and move the unit throughout hallways and corridors. The main components are a prefilter; an industrial, high-capacity, micro-fiberglass HEPA filter; ultraviolet lamp(s); and a high-volume blower. These units are also battery powered to provide for isolation during transport.
Both of the Medical Isolation Units can be rolled to the location of a suspected infected patient, where aerosols containing SARS viruses are drawn into the system while clean air is filtered and recirculated into the air. The flexibility of the FASS Medical Isolation Units allows for a wide variety of applications:
-
Immediate isolation of patients with SARS, tuberculosis, or unknown respiratory infection.
-
Dual-use flexibility to provide isolation containment (negative pressure enclosure) at any place at any time.
-
A system that does not alter the infrastructure within the enclosed protective area.
-
A cost-effective solution to emergency isolation.
-
Clean air for extended use.
FASS Applications
The FASS Medical Isolation Units are fume hoods on wheels that combine the proven HEPA filter capacity of 99.97 percent capture at 0.1 microns with ultraviolet light. This toxic microbial capture and containment system builds on years of proven studies specifically involving Bacillus anthracis (anthrax) and smallpox, and can readily be applied to infection control of SARS-related incidents. These units are approved by the Food and Drug Administration (FDA) and satisfy CDC guidelines for isolation. They are the only FDA-approved portable isolation units currently on the market.
SARS Response: Deployment Considerations
FailSafe Medical Isolation Units can be deployed in several ways as a response to a suspected SARS incident:
-
Immediate isolation and evacuation of a suspected SARS patient.
-
Transport of infected patients through crowded population (e.g., airports, train stations).
-
Transport to hospital or triage area.
-
Transport within hospital (from emergency room to SARS isolation floor).
Emergency workers can provide isolation and unrelated medical treatment to suspected SARS patients within the confines of the Medical Isolation Units while protecting caregivers and the healthy population. Bedridden patients showing symptoms of SARS can be quarantined immediately without having to be moved to another room or facility.
System Description
Both of these FASS Isolation Units combine HEPA filtration with UVGI irradiation. The units consist of a mobile platform that allows the patient to sit in a mobile chair or a bed that is surrounded by a plastic curtain. The outside air is drawn under the curtain, across the patient, and then up into the air-purifying system that consists of a HEPA filter and a UVGI lamp, thereby reducing infectious aerosols such as tuberculosis and SARS.
The FailSafe Mobile Containment System is a patented process (U.S. Patent No. 6,162,118 [18 December 2000] entitled “Portable Isolation Device and Method”) that integrates the technologies of filtration, ultraviolet germicidal irradiation, and ozone oxidation. The FailSafe process primary technology is based on high-efficiency filtration using a glass fiber HEPA filtration media that collects and traps particles greater than 0.1 micron with an efficiency greater than 99.97 percent. The filtration will collect most biological pathogens, including fungi, bacteria, and encapsulated viruses. To ensure that the pathogens collected and trapped on the HEPA filter are neutralized, the HEPA filter media surface face is illuminated with ultraviolet germicidal irradiation. Another advantage of illuminating both faces of the HEPA filter is that viruses smaller than 0.1 micron will be neutralized by irradiation.
FailSafe Mobile Containment Systems (NOT the medical Model 77 or 07 units) also incorporate ozone generation capability as a third technology. Ozone is generated with the use of ultraviolet (UV) lamps that will convert atmospheric oxygen into ozone. At concentrations below NIOSH limits, the ozone will chemically react with volatile organic compounds or odor. The FailSafe Mobile Containment Systems also have the capability of generating very high ozone levels that can be used for neutralizing pathogens on surfaces such as walls, ceilings, and floors.
Setup and Operation
The Medical Isolation Units for health care are designed with operational simplicity to make it a “turnkey” operation and to allow health providers to focus on the individual patient and the biological contamination itself. The units are designed for easy use with three switches, and the controls are simple, as follows:
-
Power up the system. Check to see that the system is working properly and that the operation light is on. Turn the FASS system ON and select the appropriate fan speed to begin air scrubbing, treatment, and capture.
-
Identify suspected infected patient.
-
Place patient in Model 07 chair, or encompass sickbed under Model 77 unit. Place plastic curtains around patient.
Preliminary Efficacy Testing
Laboratory testing: FDA 510k application. The HEPA filtration and UVGI irradiation components used in the FASS units are incorporated in Model 07 and Model 77 to protect medical personnel transporting TB and other infectious patients. Preliminary laboratory testing was performed on these units by an independent laboratory for FDA Class II certification.
Discussion of Biological Efficacy
Filtration
HEPA filters. The safety and health protection offered by HEPA (High-Efficiency Particulate Air) filtered fume hoods has long been established by the FDA, CDC, Environmental Protection Agency (EPA), NIOSH, ASTM, and JCAHO. HEPA Filtration is the “Best Available Control Technology” at 99.99 percent at 0.3-micron efficiency level and is “Generally Accepted Control Technology” at 99.97 percent at 0.1-micron efficiency level. The added feature of the new 0.1-micron advanced filters is the “gel” seal and micro fiberglass construction that allows combining these filters with UV light disinfection. HEPA filters combined with charcoal and prefilters are the highest approved filters available for NIOSH-certified respirators. There are no adverse safety, health, or environmental aspects to HEPA filters. HEPA filters are now the primary filtration media for electronic clean room assembly, hospital surgery rooms, bioengineering, pharmaceutical processes, and any applications where maximum reduction or removal of submicron particulates is required. Air from HEPA filters is free of 99.99 percent of all particles larger than 0.3 microns (including bacterial, fungal, and other opportunistic microbiological organisms) according to the size exclusion as described in Table 4-5.
Generally, HEPA filters belong to the “interception” family of filters and are variously referred to as “absolute” or “super interception.” Such filters have a deep bed of randomly positioned fibers in which the total bed depth is very large in comparison to the average fiber diameter and effective pore or free-path cross-sectional area. Even though the media may be only 1/16 thick, this is an enormous distance compared to the 0.3- to 1.0-micron fiber diameter. The passage through which air must flow is not straight, but full of twists and turns. As particulates impact on the fibers, they adhere. Thus the pore size becomes increasingly smaller, resulting in the filter efficacy increasing. New HEPA filters, used by FailSafe in Models 77 and 07, provide efficiency down to 0.1-micron particles at a removal efficiency of 99.97 percent.
HEPA filter bed media manufactured from glass fibers are reflective to ultraviolet irradiation, allowing the UVGI irradiation to partially penetrate the filter bed. The result of the combination of UVGI with ozone generation and the HEPA
TABLE 4-5 Relative Size of Fungus, Bacteria, and Viruses
|
Microbe |
Size Range (diameter–micron) |
|
Fungus |
0.2–80 |
|
Bacteria |
0.2–2.0 |
|
Viruses |
0.02–0.3 |
|
CDC guideline cutoff |
0.3 |
|
FASS unit cutoff |
0.1 |
filter is that the bacteria, fungi, and viruses that are trapped in the filter media will be exposed to sufficient irradiation and ozone concentration to disinfect the filter. The advantage of this antimicrobial treatment combination is that the air stream is inhibited from becoming recontaminated from any growth on the filter media resulting in particle breakthrough.
Ultraviolet
UV irradiation can cause eye damage and surface burns on unshielded human skin, eyes, and other organs. Therefore the UV lights used in the FASS units are sealed inside and not visible to the operator or other personnel.
Ultraviolet radiation, in the wavelength range of 2,250 to 3,020 angstroms as used for air/surface disinfection and sterilization, is referred to as ultraviolet germicidal irradiation or UVGI. Ultraviolet germicidal radiation was first applied to disinfect water systems in 1909. Its use in air purification was first evaluated in the laboratory in the 1920s, in an operating room in the 1930s to sterilize the air in an operating room (Sharp, 1939), and in a school ventilation system to reduce measles infection (Riley, 1972). It is also common practice to use to disinfect medical equipment.
UVGI is currently being employed to control bacteria, fungus, and algae growth on surfaces. European breweries have been using UVGI to control microbial growth on cooling coils since 1975. The use of UVGI can control microbial growth on filter surfaces that are subject to moisture or high humidity that will allow for natural fungal growth. Figure 4-7 illustrates a filter with natural fungal growth and a filter that was irradiated with UVGI at a rated intensity of 100 micro/cm at a distance of 1m from the midpoint of the filter (Kowalski and Bahnfleth, 2000). This surface disinfection protects the air stream from being recontaminated due to bacterial, fungus, or viruses that are collected by the filter media.
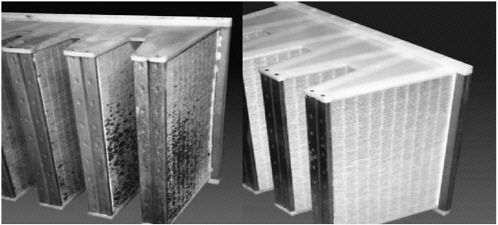
FIGURE 4-7 (left) Microbial growth on nonirradiated filters. (right) Microbe-free UV irradiated filters (Kowalski and Bahnfleth, 2000).
Microbial Response to Ultraviolet Radiation
The FASS system is an integration of room recirculation to rid the air of biological threats and surface disinfection to kill the biothreat that is collected on the HEPA filters. The primary target of UV radiation is the microorganism DNA molecule with the predominant injury of strand breakage and the formation of photo-induced byproducts such as thymine diamers. This damaged DNA cannot be used for cell reproduction or for proper mRNA templates that is required for the formation of all cellular toxic products. Viruses are especially susceptible to UVGI, more so than bacteria, and are also difficult to filter because of their size. However, viruses are more susceptible to ultraviolet radiation at wavelengths slightly above the normal UVGI broadband wavelength of 253.7 nm.
Microorganisms, when exposed to UVGI irradiation, will be killed or decreased in population at a rate according to a first order equation:
S(t) = e–kIt
where k = standard decay-rate constant, cm2/microW-s
I = Intensity of UVGI irradiation, microW/cm2
t = time of exposure (sec)
The rate constant [k] is unique to each microorganism and defines its sensitivity of each microorganism to UVGI intensity.
The dose of ultraviolet radiation that an airborne microbe receives depends on the amount of time the microbe is being irradiated and the UV intensity. The upper limit of kill rate is obtained by mixing the air within the UVGI exposure chamber. This mixed airflow will have an average velocity that will determine the exposure time required for all microbes in the air stream. If the air is not mixed, then the flow will be partial laminar resulting in the microbes receiving different dosages of UV radiation. Microbes nearest the UV lamp will get the highest dosages and those near the wall of the chamber will have significantly less exposure to the UV radiation. Laboratory experiments can be used to determine the upper limit of Kill Rate Constant (mixed air) and lower limit of Kill Rate Constant (unmixed air).
Ozone
Ozone, an allotropic form of oxygen, possesses unique properties when it oxidizes or interacts with chemical and biological systems. Ozone, best known for its protective role in the earth’s ecological environment and its interaction with industrial pollutants, has bactericidal, virucidal, and fungicidal actions that have been used in water treatment, odor control, and medicinal applications. Ozone [O3], a powerful oxidant reacting with organic molecules containing double or triple bonds, yields many complex byproducts. It is this property of ozone that has been applied as a disinfectant and sterilant against bacteria, viruses, and fungi.
Although the inhibitory and lethal effects of ozone on pathogenic organisms have been observed since the latter part of the 19th century, the mechanisms for these actions have not yet been satisfactorily highlighted. The most often cited explanation for ozone’s bactericidal effects centers on disruption of envelope integrity through peroxidation of phospholipids. There is also evidence for interaction with proteins (Mudd et al., 1969). In one study (Ishizaki et al., 1987) exploring the effect of ozone on E. coli, investigators found cell membrane penetration with ozone, subsequent reaction with cytoplasmic substances, and conversion of the closed circular plasmid DNA to open circular DNA. It is notable that higher organisms have enzymatic mechanisms to stabilize disrupted DNA and RNA, which could provide a partial explanation for why, in clinical treatment, ozone appears to be toxic to infecting organisms and not to the patient (Cech, 1986).
Ozone possesses fungicidal effects, although the mechanism is poorly understood. In one study, Candida utilis cell growth inhibition with ozone was greatly dependent on phases of their growth, budding cells exhibiting the most sensitivity to its presence (Matus et al., 1981). Interestingly, low doses of ozone stimulated the growth and development of Monilia fructagen and Phytophtora infestans, while higher doses were inhibitory (Matus et al., 1982). Thus, high concentrations of ozone are required for effective antimicrobial activity.
Viruses have been studied during their interaction with ozone (Roy et al., 1981). After 30 seconds of exposure to ozone, 99 percent of the viruses were inactivated and demonstrated damage to their envelope proteins, which could result in failure of attachment to normal cells and breakage of the single-stranded RNA.
The Occupational Safety and Health Administration (OSHA) has set Public Health Air Standards of 0.1 ppm for 8 hours or 0.3 ppm for 15 minutes as the limit of the amount of ozone to which people can be safely exposed. Air cleaners based on ozone must not generate ozone levels above the Public Health Standards, which are far below any antimicrobial activity or effective odor control. Low ozone concentrations, below the EPA-acceptable indoor limit, have been used as air cleaners, but their effectiveness has been questioned by many studies (Dyas et al., 1983; Foard et al., 1997). At high ozone concentration, ozone has been used to decontaminate unoccupied spaces of some chemical and biological contaminants and odors such as smoke.
Air Flow
The Center for Disease Control and Prevention’s guidelines for air flow into an isolation room state that there shall be greater than 12 air changes per hour (ACH). However, a higher ACH means more efficiency in removing any airborne infectious materials. There are two settings on the air flow volumes. The number of ACH obtained is a function of room volume, as illustrated in Table 4-6, which is color coded based on obtaining 12 ACH as the minimal level required for meeting CDC guidelines for isolation precautions.
TABLE 4-6 ACH as a Function of Isolation Room Volume and FASS Capabilities (calculated on a 10 percent reduction in air flow capability)
|
Room Size L × W × H |
Room Volume (cu ft) |
FASS 700 (ACH) |
FASS 1000 (ACH) |
FASS 2000 (ACH) |
|
9' × 12' × 8' |
864 |
43.8 |
62.5 |
125.0 |
|
12' × 12' × 8' |
1,152 |
32.8 |
46.9 |
93.8 |
|
15' × 12' × 8' |
1,440 |
26.3 |
37.5 |
75.0 |
|
15' × 20' × 8' |
2,400 |
15.8 |
22.5 |
45.0 |
|
20' × 20' × 8' |
3,200 |
11.8 |
16.9 |
33.8 |
|
20' × 30' × 8' |
4,800 |
7.9 |
11.3 |
22.5 |
|
30' × 30' × 8' |
7,200 |
5.3 |
7.5 |
15.0 |
Summary
The described FASS Medical Isolation Units are available in the United States, Canada, and Asia from FailSafe Air Safety Systems Corporation of Tonawanda, NY. They may offer the best opportunity to increase the numbers of isolation rooms in hospitals and especially in emergency rooms. By doing this, they provide a cost-effective solution to the challenge of new viral pathogen outbreaks. It must be emphasized that these units will only control respiratory transmissions, and are not a substitute for contact precautions or for treatment of the infection itself. Traditional measures still must be instituted to deal with surface contamination. For cleanup of biological contamination, the FASS Mobile Containment Systems also generate ozone to eradicate pathogens from surfaces. These units should be used in conjunction with the Models 77 and 07 for additional remediation of the hospital or emergency room environment.
REFERENCES
American Institute of Architects. 2001. Guidelines for Design and Construction of Hospital and Health Care Facilities, AIA.
Anand K, Palm GJ, Mesters JR, Siddell SG, Ziebuhr J, Hilgenfeld R. 2002. Structure of coronavirus main proteinase reveals combination of a chymotrypsin fold with an extra alpha-helical domain. EMBO Journal 21(13):3213-24.
Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R. 2003. Coronavirus main proteinase (3clpro) structure: basis for design of anti-SARS drugs. Science 300(5626):1763-7.
Ballew HC. 1992. Neutralization. In: Specter S, Lancz G, eds. Clinical Virology Manual. New York: Elsevier. Pp. 229-41.
Barnes TW 3rd, Turner DH. 2001a. C5-(1-Propynyl)-2'-deoxy-pyrimidines enhance mismatch penalties of DNA:RNA duplex formation. Biochemistry 40(42):12738-45.
Barnes TW 3rd, Turner DH. 2001b. Long-range cooperativity due to C5-propynylation of oligopyrimidines enhances specific recognition by uridine of ribo-adenosine over ribo-guanosine. Journal of the American Chemical Society 123(37):9186-7.
Brito DA, Ramirez M, de Lencastre H. 2003. Serotyping streptococcus pneumoniae by multiplex PCR. Journal of Clinical Microbiology 41(6):2378-84.
Centers for Disease Control and Prevention. 1994. Guidelines for preventing the transmission of mycobacterium tuberculosis in health-care facilities, 1994. Morbidity & Mortality Weekly Report Recommendations & Reports 43(RR-13):1-132.
Cech T. 1986. RNA as an enzyme. Scientific American 255(5):64-76.
Chan KH, Maldeis N, Pope W, Yup A, Ozinskas A, Gill J, Seto WH, Shortridge KF, Peiris JSM. 2002. Evaluation of the directigen FluA+B test for rapid diagnosis of influenza virus type A and B infections. Journal of Clinical Microbiology 40(5):1675-80.
Donnelly CA, Ghani AC, Leung GM, Hedley AJ, Fraser C, Riley S, Abu-Raddad LJ, Ho LM, Thach TQ, Chau P, Chan KP, Lam TH, Tse LY, Tsang T, Liu SH, Kong JH, Lau EM, Ferguson NM, Anderson RM. 2003. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. [Erratum Appears in Lancet. May 24, 2003. 361(9371):1832]. Lancet 361(9371):1761-6.
Dragovich PS, Prins TJ, Zhou R, Johnson TO, Hua Y, Luu HT, Sakata SK, Brown EL, Maldonado FC, Tuntland T, Lee CA, Fuhrman SA, Zalman LS, Patick AK, Matthews DA, Wu EY, Guo M, Borer BC, Nayyar NK, Moran T, Chen L, Rejto PA, Rose PW, Guzman MC, Dovalsantos EZ, Lee S, McGee K, Mohajeri M, Liese A, Tao J, Kosa MB, Liu B, Batugo MR, Gleeson JP, Wu ZP, Liu J, Meador JW 3rd, Ferre RA. 2003. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3c protease inhibitors. 8. Pharmacological optimization of orally bioavailable 2-pyridone-containing peptidomimetics. Journal of Medicinal Chemistry 46(21):4572-85.
Drosten C, Gunther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguiere AM, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC, Muller S, Rickerts V, Sturmer M, Vieth S, Klenk HD, Osterhaus AD, Schmitz H, Doerr HW. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. New England Journal of Medicine 348(20):1967-76.
Dyas A, Boughton BJ, Das BC. 1983. Ozone killing action against bacterial and fungal species: microbiological testing of a domestic ozone generator. Journal of Clinical Pathology 36:1102-4.
Foard K, van Osdell D, Steiber R. 1997. Investigation of gas-phase ozone as a potential biocide. Applied Occupational Environmental Hygiene 12:535-42.
Fouchier RA, Kuiken T, Schutten M, van Amerongen G, van Doornum GJ, van den Hoogen BG, Peiris M, Lim W, Stohr K, Osterhaus AD. 2003. Aetiology: Koch’s postulates fulfilled for Sars virus. Nature 423(6937):240.
Fout GS, Martinson BC, Moyer MW, Dahling DR. 2003. A multiplex reverse transcription-Pcr method for detection of human enteric viruses in groundwater. Applied & Environmental Microbiology 69(6):3158-64.
Gehlhaar DK, Bouzida D, Rejto PA. 1999. Rational drug design: novel methodology and practical applications. Parrill L, Rami Reddy M, eds. Washington, DC: American Chemical Society, Pp. 292-311. ACS symposium series 719.
Hegyi A, Ziebuhr J. 2002. Conservation of substrate specificities among coronavirus main proteases. Journal of General Virology 83(Pt 3):595-9.
Ishizaki K, Sawadaishi D, Miura K, Shinriki N. 1987. Effect of ozone on plasmid DNA of E. coli in situ. Water Research 21(7):823-8.
Johnson TO, Hua Y, Luu HT, Brown EL, Chan F, Chu SS, Dragovich PS, Eastman BW, Ferre RA, Fuhrman SA, Hendrickson TF, Maldonado FC, Matthews DA, Meador JM, Patick AK, Reich SR, Skalitzky DJ, Worland ST, Yang M, Zalman LS. 2002. Structure-based design of a parallel synthetic array directed toward the discovery of irreversible inhibitors of human rhinovirus 3C protease. Journal of Medicinal Chemistry 45:2016-23.
Kowalski WJ, Bahnfleth WP. 2000. UVGI design basics for air and surface disinfection. HPAC Engineering 72(January):100-10.
Kroes I, Lepp PW, Relman DA. 1999. Bacterial diversity within the human subgingival crevice. Proceedings of the National Academy of Sciences of the United States of America 96(25):14547-52.
Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, Rollin PE, Dowell SF, Ling AE, Humphrey CD, Shieh WJ, Guarner J, Paddock CD, Rota P, Fields B, DeRisi J, Yang JY, Cox N, Hughes JM, LeDuc JW, Bellini WJ, Anderson LJ, SARS Working Group. 2003. A novel coronavirus associated with severe acute respiratory syndrome. New England Journal of Medicine 348(20):1953-66.
Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, Ahuja A, Yung MY, Leung CB, To KF, Lui SF, Szeto CC, Chung S, Sung JJ. 2003. A major outbreak of severe acute respiratory syndrome in Hong Kong. New England Journal of Medicine 348(20):1986-94.
Marra MA, Jones SJ, Astell CR, Holt RA, Brooks-Wilson A, Butterfield YS, Khattra J, Asano JK, Barber SA, Chan SY, Cloutier A, Coughlin SM, Freeman D, Girn N, Griffith OL, Leach SR, Mayo M, McDonald H, Montgomery SB, Pandoh PK, Petrescu AS, Robertson AG, Schein JE, Siddiqui A, Smailus DE, Stott JM, Yang GS, Plummer F, Andonov A, Artsob H, Bastien N, Bernard K, Booth TF, Bowness D, Czub M, Drebot M, Fernando L, Flick R, Garbutt M, Gray M, Grolla A, Jones S, Feldmann H, Meyers A, Kabani A, Li Y, Normand S, Stroher U, Tipples GA, Tyler S, Vogrig R, Ward D, Watson B, Brunham RC, Krajden M, Petric M, Skowronski DM, Upton C, Roper RL. 2003. The genome sequence of the SARS-associated coronavirus. Science 300(5624):1399-404.
Matthews DA, Dragovich PS, Webber SE, Fuhrman SA, Patick AK, Zalman LS, Hendrickson TF, Love RA, Prins TJ, Marakovits JT, Zhou R, Tikhe J, Ford CE, Meador JW, Ferre RA, Brown EL, Binford SL, Brothers MA, DeLisle DM, Worland ST. 1999. Structure-assisted design of mechanism-based irreversible inhibitors of human rhinovirus 3c protease with potent antiviral activity against multiple rhinovirus serotypes. Proceedings of the National Academy of Sciences of the United States of America 96(20):11000-7.
Matus V, Lyskova T, Sergienko I, Kustova A, Grigortsevich T, Konev V. 1982. Fungi: growth and sporulation after a single treatment of spores with ozone. Mikol Fitopatot 16(5):420-23.
Matus V, Nikava A, Prakopava Z, Konyew S. 1981. Effect of ozone on the survivability of Candida utilis cells. Vyestsi AkadNauuk Bssr Syer Biyal Navuk 0(3):49-52.
Mudd JB, Leavitt R, Ongun A, McManus T. 1969. Reaction of ozone with amino acids and proteins. Atmospheric Environment 3:669-82.
Nelson SM, Deike MA, Cartwright CP. 1998. Value of examining multiple sputum specimens in the diagnosis of pulmonary tuberculosis. Journal of Clinical Microbiology 36(2):467-9.
Oberste M, Schnurr D, Maher K, al-Busaidy S, Pallansch M. 2001. Molecular identification of new picornaviruses and characterization of a proposed enterovirus 73 serotype. Journal of General Virology 82(Pt 2):409-16.
Oberste MS, Maher K, Flemister MR, Marchetti G, Kilpatrick DR, Pallansch MA. 2000. Comparison of classic and molecular approaches for the identification of untypeable enteroviruses. Journal of Clinical Microbiology 38(3):1170-4.
Oberste MS, Nix WA, Kilpatrick DR, Flemister MR, Pallansch MA. 2003. Molecular epidemiology and type-specific detection of echovirus 11 isolates from the Americas, Europe, Africa, Australia, Southern Asia and the Middle East. Virus Research 91(2):241-8.
Patick AK, Binford SL, Brothers MA, Jackson RL, Ford CE, Diem MD, Maldonado F, Dragovich PS, Zhou R, Prins TJ, Fuhrman SA, Meador JW, Zalman LS, Matthews DA, Worland ST. 1999. In vitro antiviral activity of AG7088, a potent inhibitor of human rhinovirus 3C protease. Antimicrobial Agents & Chemotherapy 43(10):2444-50.
Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, Nicholls J, Yee WK, Yan WW, Cheung MT, Cheng VC, Chan KH, Tsang DN, Yung RW, Ng TK, Yuen KY, SARS study group. 2003a. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361(9366):1319-25.
Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, Chan KH, Ng JS, Zheng BJ, Ng WL, Lai RW, Guan Y, Yuen KY, HKU/UCH SARS Study Group. 2003b. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 361(9371):1767-72.
Poutanen SM, Low DE, Henry B, Finkelstein S, Rose D, Green K, Tellier R, Draker R, Adachi D, Ayers M, Chan AK, Skowronski DM, Salit I, Simor AE, Slutsky AS, Doyle PW, Krajden M, Petric M, Brunham RC, McGeer AJ, National Microbiology Laboratory Canada, Canadian Severe Acute Respiratory Syndrome Study Team. 2003. Identification of severe acute respiratory syndrome in Canada. New England Journal of Medicine 348(20):1995-2005.
Riley RL. 1972. Airborne Infections. New York: Macmillan.
Rota PA, Oberste MS, Monroe SS, Nix WA, Campagnoli R, Icenogle JP, Penaranda S, Bankamp B, Maher K, Chen MH, Tong S, Tamin A, Lowe L, Frace M, DeRisi JL, Chen Q, Wang D, Erdman DD, Peret TC, Burns C, Ksiazek TG, Rollin PE, Sanchez A, Liffick S, Holloway B, Limor J, McCaustland K, Olsen-Rasmussen M, Fouchier R, Gunther S, Osterhaus AD, Drosten C, Pallansch MA, Anderson LJ, Bellini WJ. 2003. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 300(5624):1394-9.
Roy D, Wong PK, Engelbrecht RS, Chian ES. 1981. Mechanism of enteroviral inactivation by ozone. Applied Environmental Microbiology 41:718-23.
Sadowski A, Gasteiger J. 1993. From atoms and bonds to three-dimensional atomic coordinates: automatic model builders. Chemical Reviews 93:2567-81.
Sali A, Blundell TL. 1993. Comparative protein modelling by satisfaction of spatial restraints. Journal of Molecular Biology 234(3):779-815.
Sharp G. 1939. The lethal action of short ultraviolet rays on several common pathogenic bacteria. Journal of Bacteriology 37:447-59.
Swaminathan B, Barrett TJ, Hunter SB, Tauxe RV, CDC PulseNet Task Force. 2001. Pulsenet: the molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerging Infectious Diseases 7(3):382-9.
Taylor LH, Latham SM, Woolhouse ME. 2001. Risk factors for human disease emergence. Philosophical Transactions of the Royal Society of London—Series B: Biological Sciences 356(1411):983-9.
Tsang KW, Ho PL, Ooi GC, Yee WK, Wang T, Chan-Yeung M, Lam WK, Seto WH, Yam LY, Cheung TM, Wong PC, Lam B, Ip MS, Chan J, Yuen KY, Lai KN. 2003. A cluster of cases of severe acute respiratory syndrome in Hong Kong. New England Journal of Medicine 348(20):1977-85.
Vabret A, Mouthon F, Mourez T, Gouarin S, Petitjean J, Freymuth F. 2001. Direct diagnosis of human respiratory coronaviruses 229e and Oc43 by the polymerase chain reaction. Journal of Virological Methods 97(1-2):59-66.
Wagner RW, Matteucci MD, Lewis JG, Gutierrez AJ, Moulds C, Froehler BC. 1993. Antisense gene inhibition by oligonucleotides containing C-5 propyne pyrimidines. Science 260(5113):1510-3.
Wang D, Coscoy L, Zylberberg M, Avila PC, Boushey HA, Ganem D, DeRisi JL. Microarray-based detection and genotyping of viral pathogens. Proceedings of the National Academy of Sciences of the United States of America 99(24):15687-92.
Wang D, Urisman A, Liu YT, Springer M, Ksiazek TG, Erdman DD, Mardis ER, Hickenbotham M, Magrini V, Eldred J, Latreille JP, Wilson RK, Ganem D, DeRisi JL. 2003. Viral discovery and sequence recovery using DNA microarrays. PLOS Biology 1(2):257.
Wilson KH, Wilson WJ, Radosevich JL, DeSantis TZ, Viswanathan VS, Kuczmarski TA, Andersen GL. 2002. High-density microarray of small-subunit ribosomal DNA probes. Applied & Environmental Microbiology 68(5):2535-41.
WHO. 2003. Severe acute respiratory syndrome (SARS). Weekly Epidemiological Record 78(12):81-3.
Ziebuhr J, Snijder EJ, Gorbalenya AE. 2000. Virus-encoded proteinases and proteolytic processing in the Nidovirales. Journal of General Virology 81(Pt 4):853-79.