5
Preparing for the Next Disease Outbreak
OVERVIEW
Although it is possible that the future will bring a more contagious, deadly form of SARS, it is certain to bring influenza and other infectious diseases, some of which may be introduced intentionally. Recognizing that it would be impossible to address the vast array of potential microbial threats individually, public health policy makers are formulating strategies to evaluate and respond to outbreaks of all kinds. Lessons learned from the recent SARS epidemic regarding surveillance and containment were described in earlier chapters; this chapter will discuss additional strategic issues, including anticipating the confluent threats of SARS and influenza, understanding the epidemiological factors that are likely to shape future epidemics, and ensuring that public health institutions and legal frameworks are appropriately designed for responding to any new outbreaks.
Like SARS and influenza, many of the microbial pathogens to come are likely to be viral zoonoses. The paper by Richard Webby and Robert Webster in this chapter argues that the trends that ushered SARS into the human population are in fact similar to those seen over a century of influenza outbreaks. As with SARS, livestock and poultry markets provide a breeding ground for influenza outbreaks, and laboratory sources appear to have sparked at least one epidemic. Although recent severe outbreaks of avian influenza have not featured viral transmission between humans, it may be only a matter of time until a highly contagious flu, such as the strain that is estimated to have caused over 20 million and perhaps as many as 40 million deaths in 1918–1919, confronts the world.
In the case of influenza, in which the virus can be anticipated to some extent, vaccines and antiviral therapies can play a significant role in containing an epi-
demic. However, strategic actions recommended against influenza that could also inform efforts to better prepare for other viral disease outbreaks have yet to be implemented. These strategies include:1
-
stockpiling of broad-spectrum antiviral drugs,
-
advanced development of pandemic strain vaccines,
-
the establishment of surge capacity for rapid vaccine production, and
-
the development of models to determine the most effective means of delivering therapies during an outbreak.
It is evident from the experience of the late 2003 influenza season that our supply and effectiveness of antiviral drugs, capabilities to accurately predict the best viral strain for annual vaccine production, and mechanisms for surge capacity production remain inadequate (Treanor, 2004). Recognition of these vulnerabilities led numerous workshop participants to call for greater scientific and financial investments to strengthen our defenses against these certain future threats.
However, most emerging infections other than influenza will represent a truly novel threat for which the world is inadequately prepared. In these cases, models based on detailed observations from previous epidemics can be used to predict demands on hospital capacity during a hypothetical epidemic and to guide the timing and nature of quarantine measures. Two papers in this chapter (Amirfar et al. and Kimball et al.) examine the modeling strategies that have been used for analyzing public health responses to epidemics as well as the particular challenges that SARS presented for international disease surveillance and alert networks. As with other public health measures, these strategies are potentially applicable not just to SARS but to any future outbreaks in which appropriate actions to protect the public’s health must be taken swiftly (and possibly even before the complete clinical profile of the new disease and the etiological agent behind it are fully understood).
When containment measures such as quarantines must be put in place, establishing the trust of the public is crucial to their effectiveness. Social cohesion and compliance with SARS quarantine in Toronto, for example, have been attributed in part to a combination of clear communication and practical guidance by public health authorities. In the extreme case of mandatory quarantine, enforcement requires careful planning and a clear understanding of public health law. This is particularly true in the United States, where quarantine is likely to necessitate the coordination of federal, state, and local jurisdictions and legal authorities. As Gene Matthews’ paper elaborates, additional legal considerations include: due process, which requires proper notice; legal representation; court-reviewed decisions; and remote communications to permit a quarantined person to be heard in
court, as well as practical contingencies such as the need for law enforcement officials to serve notice of quarantine.
As the world becomes more conscious of microbial threats to health, countries are increasingly recognizing the necessity of reporting outbreaks promptly and cooperating fully in international efforts to contain them. Indeed, if there is one piece of good news to be noted from last year’s epidemic, it is the fact that—as David Heymann and Guenael Rodier observe in this chapter—an array of diagnostic and surveillance tools, coordinated strategies of containment, and international collaboration among scientists and public health authorities were in this case able to control the outbreak of SARS, even in the absence of curative drugs or vaccines. Nevertheless, last year’s experiences further reinforce the lessons that HIV/AIDS, influenza, Ebola, malaria, and a host of other persistent and emerging infectious diseases have already made clear—that the health of any one nation cannot be isolated from the health of its neighbors, and that public health challenges in any locality have the potential to reverberate swiftly around the globe. Karen Monaghan’s paper for the National Intelligence Council, which concludes this chapter, summarizes the continuing threat that SARS may still pose, as well as the challenges that lie ahead for attempting to contain any further deadly outbreaks of SARS or other infectious diseases in the future.
ARE WE READY FOR PANDEMIC INFLUENZA?
Richard J. Webby and Robert G. Webster2
Division of Virology, Department of Infectious Diseases, St. Jude Children’s Research Hospital
Reprinted with permission from Webby and Webster, 2003. Copyright 2003 AAAS.
During the past year, the public has become keenly aware of the threat of emerging infectious diseases with the global spread of severe acute respiratory syndrome (SARS), the continuing threat of bioterrorism, the proliferation of West Nile virus, and the discovery of human cases of monkeypox in the United States. At the same time, an old foe has again raised its head, reminding us that our worst nightmare may not be a new one. In 2003, highly pathogenic strains of avian influenza virus, including the H5N1 and H7N7 subtypes, again crossed from birds to humans and caused fatal disease. Direct avian-to-human influenza transmission was unknown before 1997. Have we responded to these threats by better preparing for emerging disease agents, or are we continuing to act only as crises arise? Here we consider progress to date in preparedness for an influenza pan-
demic and review what remains to be done. We conclude by prioritizing the remaining needs and exploring the reasons for our current lack of preparedness for an influenza pandemic.
In February 2003, during a family visit to mainland China, a young girl from Hong Kong died of an unidentified respiratory illness. After returning to Hong Kong, both her father and brother were hospitalized with severe respiratory disease, which proved fatal to the father. When H5N1 (avian) influenza virus was isolated from both patients, the World Health Organization (WHO) went to pandemic alert status (WHO, 2003a). At about the same time, there were rumors of rampant influenza-like disease in China. Influenza experts feared that H5N1 influenza virus had acquired the ominous capacity to pass from human to human. That outbreak is now known to have been SARS, caused by a novel coronavirus.
In March 2003, another alarming situation arose on the other side of the world. A highly pathogenic H7N7 avian influenza outbreak had recently erupted in the poultry industry of the Netherlands (Koopmans et al., 2003), and workers involved in the slaughter of infected flocks contracted viral conjunctivitis. The H7N7 virus isolated from these patients had several disquieting features: Not only could it replicate in the human conjunctiva, but there was also evidence of human-to-human spread. Nearby herds of swine (which are often implicated in the adaptation of influenza viruses to humans) also showed serologic evidence of exposure (Koopmans et al., 2003). When a veterinarian died of respiratory infection (Abbott, 2003; Koopmans et al., 2003; Sheldon, 2003; van Kolfschooten, 2003), WHO again acknowledged the presence of a severe threat (WHO, 2003b).
Luckily, the worst-case scenarios did not come about in either of the 2003 avian influenza virus scares. However, the year’s events eliminated any remaining doubts that global advance planning for pandemic influenza is necessary. They also highlighted how far, as a scientific community, we have come since the 1997 event: We are now much better equipped with technologies and reagents to rapidly identify and respond to pandemic influenza threats. On the other hand, the legislative and infrastructure changes needed to translate these advances into real public health benefits are alarmingly slow.
The Role of WHO in Influenza Surveillance and Control
In 2001, WHO initiated the development of a Global Agenda for Influenza Surveillance and Control. Its four main objectives are to strengthen influenza surveillance, improve knowledge of the disease burden, increase vaccine use, and accelerate pandemic preparedness (Stohr, 2003). In May 2002, this document was adopted after proposals and public comment were invited. The document advocates the development of methods and reagents that can be used to rapidly identify all influenza virus subtypes, thereby allowing integrated influenza surveillance in humans and in other animals. WHO, with its global influenza network of more than 100 laboratories and its distinguished record of planning for
yearly interpandemic influenza, is ideally situated to play a broader role in facilitating international cooperation for the rapid exchange of viruses, reagents, and information. Influenza continually evolves at the human–lower animal interface and thus can be unpredictable. As an example, within a brief period, the H7N7 virus events occurred in European poultry and humans, H5N1 viruses infected Asian poultry and humans, and novel, rapidly spreading reassortant viruses were isolated in swine in the United States (Olsen, 2002; Zhou et al., 1999). Therefore, the capacity to simultaneously manage multiple potential pandemic situations is important. The WHO global agenda document will help to prioritize areas of influenza research and facilitate national pandemic preparedness plans.
Prioritization of Viral Subtypes for Surveillance and Control
Influenza experts agree that another influenza pandemic is inevitable and may be imminent (Figure 5-1). A major challenge in controlling influenza is the sheer magnitude of the animal reservoirs. It is not logistically possible to prepare reagents and vaccines against all strains of influenza encountered in animal reservoirs, and therefore, virus subtypes must be prioritized for pandemic vaccine and reagent preparation. Preliminary findings have identified the H2, H5, H6, H7, and H9 subtypes of influenza A as those most likely to be transmitted to humans. (Influenza viruses are typed according to their hemagglutinin [H] and neuraminidase [N] surface glycoproteins.) The influenza A subtypes currently circulating in humans, H1 and H3, continue to experience antigenic drift. That is, their antigenic surface glycoproteins are continually modified, allowing them to escape the population’s immunity to the previous strain and thus to continue causing annual outbreaks. Although these continual modifications may lead to an increase in virulence, the mildness of the past three influenza seasons suggests that the dominance of the H1N1 and H3N2 viruses is waning as their ability to cause serious disease becomes increasingly attenuated. H2 influenza viruses are included in the high-risk category because they were the causative agent of the 1957 “Asian flu” pandemic and were the only influenza A subtype circulating in humans between 1957 and 1968. Counterparts of the 1957 H2N2 pandemic virus continue to circulate in wild and domestic duck reservoirs. Under the right conditions (which are still not completely understood), H2N2 viruses could again be transmitted to and spread among humans, none of whom under the age of 30 years now has immunity to this virus. Seroarchaeology data from the late 19th and early 20th centuries indicate that only the H1, H2, and H3 influenza virus subtypes have been successfully transmitted among humans. It is possible, but unlikely, that they are the only subtypes able to do so.
Not only are the H1, H2, and H3 influenza viruses of concern, but the H5 subtype has threatened to emerge as a human pandemic pathogen since 1997, when it killed 6 of 18 infected humans. Before that event, the receptor specificity of avian influenza viruses was thought to prevent their direct transmission to hu-
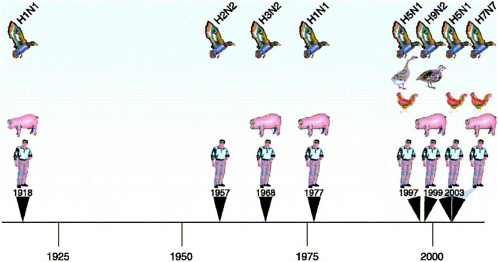
FIGURE 5-1 Timeline of human influenza over the past 100 years. The black triangles represent documented human influenza A infections characterized by multiple cases. In each instance the species of animals implicated in the emergence of disease is highlighted. Since 1997 there has been a disproportionate increase in the number of reports of novel subtypes in humans and in the number of animal and bird species involved, suggesting that the next influenza pandemic is imminent.
mans. Transmission from aquatic birds to humans was hypothesized to require infection of an intermediate host, such as the pig, that has both human-specific (α-6 sialic acid) and avian-specific 2-3 sialic acid) receptors on its respiratory epithelium. The 1997 H5N1 event demonstrated that domestic poultry species may also act as intermediate hosts. H5N1 viruses continue to emerge and evolve despite heroic measures taken to break their evolutionary cycle in the live poultry markets of Hong Kong: the elimination of live ducks and geese (the original source), the elimination of quail (the source of the internal genes of H5N1/97), and the institution of monthly “clean days,” when all 1,000-plus retail markets are emptied and cleaned.
Two things have become clear. Live poultry markets are potential breeding grounds for influenza and other emerging disease agents, and there is an Asian source of H5N1 influenza viruses outside of Hong Kong SAR. Between 1997 and 2003, H5N1 virus was isolated from duck meat imported from China into Korea (Tumpey et al., 2002) and Japan (ProMED-mail, 2003). These observations suggest that ducks and possibly other avian species in mainland China are a reservoir of H5N1, although there have been no official reports of H5N1 virus in China.
At the beginning of the SARS outbreak, China missed an opportunity to show the world its considerable intellectual and scientific potential (Enserink, 2003a). In the case of H5N1 influenza, a pandemic in waiting, it remains to be seen whether China will show leadership in proactively addressing the problem. Concerted national and international efforts are required to deal effectively with the threat.
The third virus subtype on the most wanted list is H7. The H7 and H5 viruses have a unique ability to evolve into a form highly virulent to chickens and turkeys by acquiring additional amino acids at the hemagglutinin (HA) cleavage site (HA cleavage is required for viral infectivity) (Steinhauer, 1999). The highly pathogenic H7N7 influenza viruses that were lethal to poultry infected the eyes of more than 80 humans and killed one person (Enserink, 2003b). In the case of this outbreak, the Netherlands’ policy of openness was important in reducing the potential threat and should serve as a model. When the virus was first detected at the end of February 2003, the European Community and international community, via the Office International des Epizooties, were notified so that surrounding countries, including Belgium and Germany, could immediately respond if the disease was detected. Culling of all poultry on infected farms and quarantine of surrounding farms succeeded in eradicating the virus once the etiologic agent was identified. After human infection was observed, an anti-influenza drug was given as prophylaxis, and vaccination with the current human influenza vaccine was done to reduce the likelihood that the avian virus would reassort with human H1N1 and H3N2 strains.
The remaining two viral subtypes on the priority list, H6 and H9, do not share the virulent phenotypes of the H5 and H7 viruses, but still pose a considerable threat. Both of these influenza viruses have spread from a wild aquatic
bird reservoir to domestic poultry over the past 10 years. H9N2 viruses have also been detected in humans and in pigs (Peiris et al., 1999, 2001) and have acquired human-like receptor specificity (Matrosovich et al., 2001). Neither of these viruses was able to infect chickens before the mid-1980s. Now, for unknown reasons, H9 viruses are endemic in chickens in Eurasia and H6 viruses are becoming endemic in both Eurasia and the Americas. These facts highlight the continuing adaptation of influenza viruses in the aquatic bird reservoirs to domestic chickens.
The Challenge of Developing Candidate Vaccines
If the next influenza pandemic were to begin tomorrow, inactivated vaccines would offer the only immediate means of mass prophylaxis, yet their supply is limited by inadequate production capabilities and suboptimal utilization of adjuvants (Fedson, 2003; IOM, 2003). The stocks of antiviral drugs are too low to cope with an epidemic and would be quickly depleted (IOM, 2003). Tissue culture–based and live attenuated vaccines are now licensed in some countries, and could supplement the supply of inactivated vaccine. Further development of these options is urgently needed to provide alternative substrates in the face of a pandemic.
Since the 1970s, influenza vaccines have been made by exploiting the tendency of the segmented influenza genome to reassort (Wood and Williams, 1998). This natural process has been used to produce vaccine strains that simultaneously contain gene segments that allow them to grow well in eggs and gene segments that produce the desired antigenicity. Natural reassortment is allowed to occur in embryonated chicken eggs, and reassortants with the desired characteristics are selected. These recombinant vaccine strains contain the hemagglutinin and neuraminidase genes of the target virus (encoding glycoproteins that induce neutralizing antibodies); their remaining six gene segments come from A/Puerto Rico/ 8/34 (H1N1), which replicates well in eggs and is safe for use in humans (Kilbourne, 1969). These “6+2” reassortants are then grown in large quantities in embryonated chicken eggs, inactivated, disrupted into subunits, and formulated for use as vaccines. Although this process creates an effective and safe influenza vaccine, it is too time-consuming and too dependent on a steady supply of eggs to be reliable in the face of a pandemic emergency. Even during interpandemic periods, 6 months is required to organize sufficient fertile chicken eggs for annual vaccine manufacture (Gerdil, 2003), and the preparation of the desired “6+2” recombinant vaccine strain can be a time-consuming process. Influenza vaccine preparation is seasonal and is a remarkable achievement, in that an essentially new vaccine is made every year. However, two of the viruses of greatest concern, those of the highly pathogenic H5 and H7 subtypes, cannot be successfully grown in eggs. Their unique ability to accumulate multiple basic amino acids at the site of hemagglutinin cleavage increases their ability to spread systemically in an in-
fected host and cause significant disease (Steinhauer, 1999). This feature also renders H5 and H7 viruses rapidly lethal to chicken embryos.
The most promising means of expediting the response to pandemic influenza is the use of plasmid-based reverse genetic systems to construct influenza virions and vaccines. These systems also offer a successful alternative means of producing H5 and H7 vaccine seed strains. Because viable viruses can be generated from individually cloned cDNA copies of each of the eight viral RNA segments, reassortment can be prospectively defined and directed, and the extra amino acids at the HA cleavage site (which are associated with high virulence) can be removed to allow rapid generation of a vaccine seed strain in eggs. Plasmids encoding the internal genes of the base vaccine are already available. A vaccine seed strain can be created by cloning the appropriate hemagglutinin and neuraminidase genes from the target virus, altering its HA connecting peptide if necessary, and transfecting an appropriate cell line (see Figure 5-2). This technology has been shown to be effective for the production of reassortants carrying several different surface glycoprotein combinations, including those considered to have a high pandemic potential (Hoffman et al., 2002; Liu et al., 2003; Schickli et al., 2003; Subbarao et al., 2003). The next step is to take these plasmid-derived influenza vaccines through clinical trials to address crucial questions such as number and quantity of doses and the role of adjuvants. Most of the vaccines derived after the 1997 H5N1 episode by various alternative strategies induced a disappointing immune response (Wood, 2001). The optimal pandemic vaccination regimens can be anticipated only by collecting the necessary data and experience through clinical trials of vaccines against different subtypes of influenza virus.
Although they are well suited to the manufacture of inactivated influenza vaccines, reverse genetic systems introduce new variables. One of the most limiting of these is the need to use cell lines. There are surprisingly few suitable accredited cell lines and cell banks available, and many of those are the property of pharmaceutical companies. The practical options are very few, in view of the technical and regulatory restrictions. Perhaps the only cell line that meets all criteria for international use at this time is the African green monkey kidney cell line, Vero. However, although Vero cell lines are in widespread laboratory use, only those that are derived from WHO-approved sources and have a detailed history are acceptable for manufacture of human pharmaceuticals. A second new variable is the use of a genetically modified virus seed strain. Because the traditional vaccine strains are made by natural reassortment, they have escaped being labeled “genetically modified.” This difference, although largely semantic, may affect the acceptance of the new vaccines. Before many of these traits can be tested, the virus must be amplified, inactivated, purified, and formulated for vaccine use (Gerdil, 2003).
In preparing for a pandemic threat, collaboration between government, industry, and academia is needed to overcome the obstacles and guarantee the most rapid production of a vaccine candidate. The recent SARS episode has shown that international collaboration in the face of a truly global threat is indeed possible.
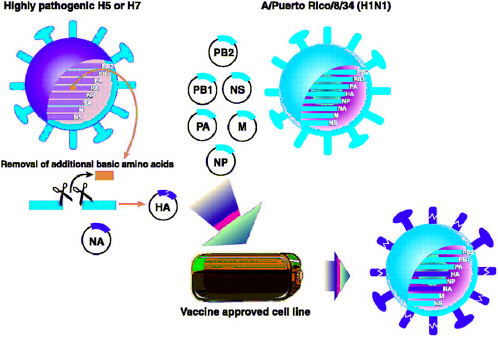
FIGURE 5-2 Proposed method of influenza vaccine seed virus production using the eight-plasmid reverse genetics system (Hoffman et al., 2002). The hemagglutinin (HA) and neuraminidase (NA) genes from the target strain are cloned into the bacterial plasmid vector pHW2000 in a process that allows for the alteration of the HA cleavage site when necessary (see text for explanation). These two plasmids, along with six others containing the remaining influenza A gene segments derived from the master vaccine strain A/Puerto Rico/8/34 (H1N1), are then introduced into a suitable cell line (e.g., Vero). After expression of positive- and negative-sense RNA and viral proteins from these plasmids, a productive replication cycle is initiated and viable virus particles are produced.
The Safety Testing of Candidate Pandemic Vaccines and Liability Issues
Unfortunately, there are only a few facilities available to carry out safety testing under the high-level biocontainment conditions required for handling highly pathogenic influenza viruses. Overcoming the technical hurdles to efficient vaccine production is only the start of a long, expensive process. Manufacturing scale-up presents its own problems, not least because plant workers will have no immunity to the pathogens they will be handling. Of prime importance is vaccine safety testing, but the need for safety testing will have to be balanced against the need for rapid mass production of a vaccine. In response to the 2003 H5N1 scare in Hong Kong, WHO has created an Interim Biosafety Risk Assessment (WHO Global Influenza Programme, in press) guideline for the safety testing of pandemic vaccines, particularly the H5 and H7 subtypes, signifying a substantial advance in preparedness for the production of a pandemic influenza vaccine.
A major risk for all vaccine manufacturers is the occurrence of adverse reactions in a percentage of recipients. These reactions may be attributable to the vaccine, to the host, or (most likely) to a unique combination of the vaccine and the host genetic factors. Guillain-Barré syndrome in human beings first became apparent during the U.S. swine influenza vaccination program (Roscelli et al., 1991; Safranek et al., 1991). The inevitability of adverse reactions underscores the product liability dilemma inherent in any vaccine program. The risk of devastating financial liability, and the unavailability or high cost of liability insurance, are increasingly discouraging vaccine manufacture, especially for universal use.
Legislative measures can be taken to reduce the impact of liability exposure. For example, the U.S. Congress passed the National Childhood Injury Compensation Act of 1986 (the “Vaccine Act”), which created a no-fault compensation program funded by an excise tax on vaccines. Plaintiffs need only establish that their injuries were caused by the vaccine. Claimants who are not satisfied with the administrative decision may still elect to sue the manufacturer, but the legal arguments available to the claimant are limited. Although the Vaccine Act represents progress in achieving a balance between consumer and manufacturer concerns, it would not apply to vaccines given to the general population, such as those for influenza or smallpox. Congress again attempted to address these concerns in a provision of the Homeland Security Act of 2002, and an Institute of Medicine panel is currently wrestling with the problem as well; however, drug manufacturers remain hesitant. The bottom line is that unless the government authorities of every country implement mechanisms that equitably limit vaccine liability, no prospective vaccine for H5N1, H7N7, or any other threatening influenza virus is likely to be produced for universal human use. It is hoped that governments will rise to the occasion after a crisis emerges, but logic suggests that the issue should be addressed now.
Antiviral Drugs
A global influenza strategy would call for the stockpiling of influenza antiviral drugs for use in the event of a pandemic until vaccines can be prepared. “But,” as noted by Albert Osterhaus (Abbott, 2003b), “no country has yet started to stockpile antiviral drugs.” The potential value of antivirals was demonstrated in the recent H7N7 outbreak in poultry and humans. Further, because epidemiological modeling has suggested that it is more infectious than SARS (Ferguson et al., 2003; Lipstitch et al., 2003; Riley et al., 2003), influenza is unlikely to be controllable by SARS-like quarantine measures. The estimated US$ 10 billion cost of SARS and the societal disruption it caused in China and Toronto make a compelling case for stockpiling of antiviral drugs.
Pandemic influenza has already threatened twice in 2003. The events associated with these outbreaks show that we are in a much better position to rapidly respond to an influenza threat than we were in 1997; however, much remains to be accomplished. Overall, our state of preparedness is far from optimal.
Priorities to Ensure Pandemic Preparedness
To conclude, let us revisit our concern that the next influenza pandemic alert may involve a virus that has acquired the capacity to spread from human to human. What are our most urgent needs?
-
A sufficiently large supply of anti-influenza drugs to reduce the severity and spread of infection. Specific efficacious drugs are available, but no country has yet invested in stockpiling.
-
A vaccine matching the subtype of the emerging pandemic influenza strain that has been tested in clinical trials and for which manufacturers are prepared to “scale up” production. Such a vaccine would probably not match the emerging strain antigenically and would not prevent infection, but it could reduce the severity of illness until a matching vaccine is produced. Such vaccines have been discussed for 20 years. None is available, but specific plans to produce such a vaccine are currently being formulated.
-
The preparation, testing (safety and clinical trials), and availability of a vaccine derived by reverse genetics. The scientific technology is in place to achieve this goal, but manufacturing, intellectual property, and liability issues remain unresolved. In the event of a pandemic, reverse genetics would be the most rapid means by which to produce an antigenically matched vaccine. To be truly prepared, such a vaccine needs to be produced and tested now to identify and resolve the issues, rather than doing so in direct response to an emergency.
-
An improvement in the global influenza vaccine manufacturing capacity. Without the use of adjuvants, the current capacity is inadequate and could not be quickly augmented. The country best prepared to meet this need is Canada; in Ontario, influenza vaccination is recommended and available at no charge to people of all ages during the influenza season (Schabas, 2001). This progressive strategy during interpandemic years will ensure the vaccine-manufacturing capacity of that region.
The conclusion of this analysis is inescapable: The world will be in deep trouble if the impending influenza pandemic strikes this week, this month, or even this year. It is now time to progress from talking about pandemic vaccines to taking action. Our hope is that the “Ontario experiment” will inspire other regions of the world to similarly promote the expansion of manufacturing capacity for influenza vaccines.
Although reverse genetics offers great advantages for the rapid preparation of influenza vaccine strains and for understanding pathogenesis (Hatta et al., 2001), the reverse side of this benefit is its potential for the development of bioterrorism agents (Krug, 2003). Regardless of human endeavors, nature’s ongoing experiments with H5N1 influenza in Asia and H7N7 in Europe may be the greatest bioterror threat of all. The time for talking is truly over. We must be prepared.
MODELING A RESPONSE STRATEGY
Sam Amirfar, M.D., Mary Koshy, M.P.A., and Nathaniel Hupert, M.D., M.P.H.
Department of Public Health, Weill Medical College of Cornell University
Containment of the 2002–2003 severe acute respiratory syndrome (SARS) epidemic posed unprecedented challenges to health care delivery and public health systems worldwide. In addition to the human costs of infection in medical workers, efforts to contain the spread of the virus led to widespread disruptions in the provision of routine medical care. Response strategies for potential recurrences of SARS will need to address treatment of infected individuals, quarantine of potential victims, and health system action plans that lead to containment of the outbreak without undue impact on the delivery of care for the wider populace. Since the outbreak of SARS, several computational models have been developed to investigate the transmission dynamics of the SARS coronavirus. Although these studies have identified certain parameters (e.g., maximum allowable delay in quarantining new cases) that may lead to more efficient management of new outbreaks, further research is needed to better define the practical steps required for such optimized response strategies. This chapter summarizes the current state of theoretical modeling for SARS and proposes a research agenda to improve forecasting of resource requirements at the hospital, health system, and regional levels for containment of future outbreaks.
Eight models of SARS transmission and control were published in the English and Chinese scientific literature in 2003 (Chen, 2003; Chowell et al., 2003; Lin et al., 2003; Lipsitch et al., 2003; Lloyd-Smith et al., 2003; Riley et al., 2003; Shi, 2003; Wang and Zhao, 2003). Seven of these utilize the standard SEIR (susceptible, exposed, infectious, recovered) dynamic mathematical model of disease transmission or variations on that model accounting for the use of quarantine (Table 5-1) (Chen, 2003; Chowell et al., 2003; Lipsitch et al., 2003; Lloyd-Smith et al., 2003; Riley et al., 2003; Shi, 2003; Wang and Zhao, 2003). SEIR models can provide estimates of critical parameters for a disease outbreak, such as the basic reproductive number R0 (that is, the number of new cases for every existing case) or maximal lag time for isolation of new cases (Dye and Gay, 2003).
Five of these studies consider outbreak response variables that reflect both public health activity (e.g., time to isolation of each new case) and hospital-based measures (e.g., efficacy of isolation and reduction in transmission rate of virus) (Chowell et al., 2003; Lipsitch et al., 2003; Lloyd-Smith et al., 2003; Riley et al., 2003; Shi, 2003). For example, Chowell and colleagues predicted that containment of the Canadian outbreak would require a time-to-isolation of 3 to 6 days and a 50 to 90 percent reduction in person-to-person transmission from identified cases (Chowell et al., 2003). In a similar fashion, most of these papers provide model-derived threshold values, but do not focus on the practical steps needed to attain them. Only one paper, by Lloyd-Smith and colleagues (2003), went into
TABLE 5-1 SARS Dynamic Transmission Models
|
Authors |
Disease Model Typea |
Data Sources |
Key Parameter |
Threshold Values |
|
Chen et al. |
SIR, deterministic |
Hong Kong, Beijing |
In-hospital transmission |
N/A |
|
Wang and Zhao |
SIR, deterministic |
Hong Kong, Beijing |
N/A |
N/A |
|
Chowell et al. |
SEIJR, deterministic |
Toronto, Hong Kong, Singapore |
R0=1.2 a. Time to diagnosis b. Isolation effectiveness |
a. 3-6 days to diagnosis b. 50-90% effectiveness of quarantine in stopping population-based spread |
|
Riley et al. |
SEIR, stochastic/deterministic |
Hong Kong |
R0=2.7 a. Time to isolation b. Infection control c. Population contact rate |
a. 50% reduction in hospital infection and population contact rate b. Complete cessation of pop ulation movement between regions |
|
Lipsitch et al. |
SEI(Q)R, stochastic/deterministic |
Singapore |
R0=1.2 a. “Public healthinterventions” b. Population contact rate |
Variable based on other modeled factors |
|
Lloyd-Smith et al. |
SEIR, deterministic with Monte Carlo simulation and heterogeneous stochastic effects |
Hong Kong, Singapore |
a. Time to isolation b. Isolation effectiveness (hospital-based contact precaution and case management measures) c. HCW-community contact |
If R0~3, then need: a. < 3 days to isolation of new cases b. 80% reduction in transmission |
|
Shi |
SIR, stochastic Monte Carlo |
Vietnam |
R0=1.8 Days to strict isolation |
≤ 7 days to strict is olation of new cases |
|
aS = susceptible, E = exposed, I = infective, J = diagnosed, Q = quarantined, R = recovered. |
||||
sufficient detail about methods of disease containment to provide practical guidance for public health and hospital managers in attaining these goals. For example, these authors found that efforts to interrupt health care worker-to-patient transmission would yield greater improvements in epidemic containment than reductions in population-based transmission. This finding provided the basis for a practical recommendation to initiate hospital-wide campaigns to increase contact precautions and strict case management of infected individuals. Additionally, this report alone—among the eight model-based papers—acknowledges that containment efforts would be carried out in an environment of limited hospital resources, where scarcity of items such as gowns, gloves, and masks would require prioritization of population-wide and hospital-based strategies.
These eight reports provide the beginnings of an evidence base on which to design effective response strategies for future SARS outbreaks. In parallel with these efforts, a number of researchers have developed prediction models for medical outcomes of SARS patients (Table 5-2) (Booth et al., 2003; Chan et al., 2003; Donnelly et al., 2003; Han et al., 2003; He et al., 2003). The current challenge is to use these findings from both theoretical modeling and patient care to assist health planners in practical ways. For example, hospital administrators may benefit from guidance on determining when in the course of an epidemic it is better to cease all admissions, isolate a specific ward, or simply isolate a number of patients in individual rooms. More complex response models may begin to weigh the relative benefits of drastic steps such as shuttering entire hospitals in order to contain the spread of SARS in light of the potential harms that may accrue to affected communities through the loss of routine medical care capacity. Such cost-benefit studies will highlight the difficult choices faced by health planners and hospital administrators in the real-world setting of financial and resource constraints. Finally, with the prospect of a SARS vaccine on the horizon, new models will be needed to quantify optimal pre- and post-detection vaccination rates for disease containment given the significant resource requirements of any mass vaccination campaign. Recent efforts to model mass antibiotic prophylaxis strategies for bioterrorism response may provide insight into the methods and data requirements for this type of logistical modeling as well as techniques (e.g., Internet-based platforms) for wide dissemination of modeling tools (Hupert and Cuomo, 2003; Hupert et al., 2002).
Publication of data on resources consumed in isolating and treating SARS patients as well as quarantine of potentially infected individuals will assist modelers in developing realistic forecasting models capable of leading public health and hospital planners through “what if” scenarios that may require difficult trade-offs of personnel, materials, and patient care arrangements. The more accurate the data underlying these models, the better they can serve planners and their communities. The goal of such efforts should be to give every decision maker the ability to understand, in relevant terms and for their particular institution or community, not just the knowledge that containment of SARS would require isolation
TABLE 5–2 Prediction Models for Medical Outcomes of SARS Patients
|
Authors |
Model Type |
Predictor Variable(s) |
Outcomes of Interest |
|
Booth et al. |
Multivariable regression |
Diabetes, comorbidity |
Death, intensive care admission |
|
Chan et al. |
Multivariable regression |
Age, diabetes, heart disease |
Death |
|
Han et al. |
Correlation |
Radiology information technology systems use |
Infection rate |
|
He et al. |
Multivariable regression |
Age, hypoxia, thrombocytopenia, hypernatremia, renal failure |
Death |
|
Donnelly et al. |
Gamma distribution |
Age, infection to onset, onset to admission |
Death |
of new cases within a certain number of days, but also an estimate of how to go about achieving that containment goal (i.e., how many staff, rooms, media campaigns, and other factors). Planning models that focus on critical resources in this manner can provide guidance for live exercises and may influence future investments in both infrastructure (e.g., installation of negative pressure isolation rooms) and disposable medical equipment (e.g., gowns and masks).
REPORTING, SURVEILLANCE, AND INFORMATION EXCHANGE: THE SARS IMPERATIVE FOR INNOVATION
Ann Marie Kimball,3 Bill Lober,4 John Kobayashi,5 Yuzo Arima,6 Louis Fox,7 Jacqueline Brown,8 and Nedra Floyd Pautler9
Asia Pacific Economic Cooperation, Emerging Infections Network (EINET)
The emergence and widespread transmission of severe acute respiratory syndrome (SARS) in the winter of 2003 severely tested national, regional, and global reporting and surveillance systems for emergent infectious diseases. It presented a three-pronged challenge: (1) alerting responsible authorities; (2) rapidly describing the geographically diverse outbreaks in a consistent and useful fashion; and (3) providing guidance for prevention and control strategies based on experience in varied locations. Given the persistent emergence of new infections in recent years in the Asia Pacific—accompanied by the continued increase in population size and the greater range and volume of trade and travel in the region—this scenario must be considered a harbinger for the future. The gaps brought to light in this experience should be used to guide the rapid deployment of laboratory and communications systems in the region. In this article, the informatics components of the response to SARS are described and characterized. Prospective areas for applications of new technologies are discussed.
Hypothesis
The SARS experience represents a precursor to future scenario planning for the Asia Pacific. Descriptive data suggest both successes and gaps in timeliness,
laboratory diagnostic tools, and useful, practical transparent communications among sectors within nations, and between nations partnered in trade and travel. This report will focus on gaps in the latter two areas: practical transparent communications among sectors and between nations.
Methods
We conducted a focused and systematic review of the 2003 SARS epidemic based on (1) our EINET experience operating an electronic, multisectoral communications network in the region, in collaboration with (2) a literature review for the identification of potential applications of informatics technology based on the 2003 experience (including response management, collaboration, capacity development, tabletops/training, and other factors).
Findings/Conclusions
SARS presented the confluence of three urgent requirements of the global public health informatics response: (1) expansion of knowledge about the disease in a rapid, systematic manner, particularly in microbiology and epidemiology through collaborative discovery; (2) communication of appropriate aspects of that knowledge base to guide implementation of isolation, quarantine, and prevention measures by public health workers and other policy makers; and (3) mitigation of adverse societal response through broader social communication. However, with concurrent outbreaks in numerous locations, each of these requirements rapidly increased in complexity. Working relationships in the Asia Pacific public health community have been formed in the course of the outbreak response that can be reinforced in the present “inter SARS” period. Specific computing and telecommunications tools can be expanded to assist more fully in the public health response. We propose the use of a virtual tabletop (scenario) tool to proactively implement improved communications and collaboration strategies in the region.
Background
The SARS outbreaks of 2003 have been described in numerous scientific reports (CDC, 2003a). In fact, the unprecedented volume and speed of scientific discovery and the dissemination of that knowledge has been the subject of a report (Drazen and Campion, 2003). This report focuses on (1) how informatics and telecommunications strategies assisted in the timeliness of this effort; and (2) what technologies or strategies could be tested and applied in the current “inter SARS” period to assure public health readiness for the future.
The factors related to the emergence of new infectious diseases have been described for more than a decade (IOM, 1992, 2003). The role of anthropogenic factors of emergence related to microbial pathogens in humans, while generally
understood to be important, has become the object of systematic biomedical and interdisciplinary research. The overlay of globalization in manufacturing, commerce, travel, and trade on an uneven public health and sanitary infrastructure has put some populations at risk of new infections. These risks become reality in epidemics that increasingly challenge our ability to respond effectively.
The Asia Pacific has witnessed the emergence of numerous new human pathogens, including Nipah virus, enterovirus 71, E. coli 0157H:7, and Cyclospora Cayetanensis. The reemergence of “old” pathogens such as cholera and multidrug-resistant tuberculosis has also affected the region. This may reflect the pace of change that countries bordering the Pacific Ocean have experienced in their demographics, migration, and rapid shifts in economic activity. In addition, these nations are among the most trade dependent in the world. The Asia Pacific dwarfs other regions of the globe in the volume and dollar value of trade and travel revenues.
Asia has had sustained growth of Internet connectivity over the past decade, despite economic crises in the region (Kimball et al., 1999). In a recent report, the International Telecommunications Union (ITU, 2003) noted that the number of broadband subscribers rose 72 percent in 2002, with Korea (21 subscribers per 100 inhabitants), Hong Kong (15 per 100), and Canada (11 per 100) showing the highest rates of broadband use. In Korea, “Disweb,” an electronic surveillance system, has been in place since 1999 using web-based reporting over the Internet. Many other economies are increasingly integrating Internet-based reporting into their disease alert and surveillance systems.
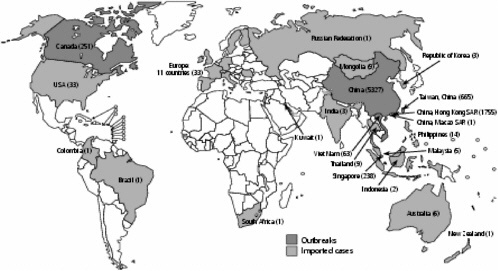
While numerous electronic disease surveillance and alert networks are operating in the region, the Asia Pacific Emerging Infections Network (APEC-EINET) is unique in that it includes membership from trade and commerce (see Figure 5-3) as well as health. Now in its eighth year of operation, the network spans the entire Asia Pacific community. The network consists of a user group of more than 500 in 19 of the 21 APEC economies. Providing a biweekly bulletin and enriched website, the APEC-EINET is supported by APEC, the U.S. government, and the University of Washington.
Methods
Of the 1,150 articles entered into the Medline index with “SARS” in their text, 60 include the word “information” and 2 include “information technology” (Eysenbach, 2003). The 60 information-related articles were scanned for discussion on informatics or information technology employed during the outbreak by scientists or public health workers. In addition, informal discussions were held in person and through electronic communications with World Health Organization/Geneva (WHO/Geneva) and regional academic institutions and public health organizations to augment the information available for review in this report. Because the SARS experience is still being understood, the data obtained through personal communications may be incomplete.
After compiling this information, we segregated our conclusions into the informatics domains of (1) generation of new biomedical knowledge about the SARS agent; and (2) generation of new knowledge about the epidemiology of SARS disease prevention for purposes of predicting and monitoring success in control. We provide our assessment based on this analysis of the need for specific new communications and collaboration strategies.
Results
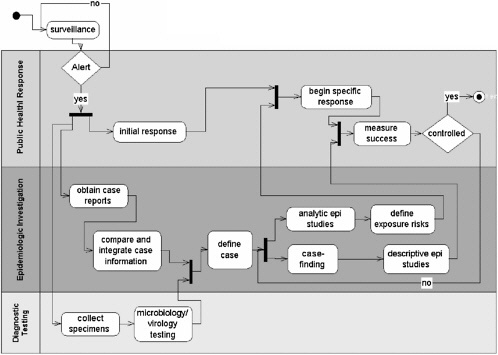
If the basic systems model of an outbreak alert, investigation, and response resembles the work model in Figure 5-4, then numerous frontiers for information technology application and evaluation exist. This diagram integrates business processes and the information flow that supports these processes in the course of work done to investigate and respond to an outbreak (Kitch and Yashoff, 2002). The focus of international information technology application during SARS centered on three aspects, which are shown in the figure: alert, diagnosis (biomedical discovery), and epidemiologic investigation.
Alert
According to WHO, the earliest alerts about an unknown pneumonia in Guandong were discovered by Global Public Health Information Network
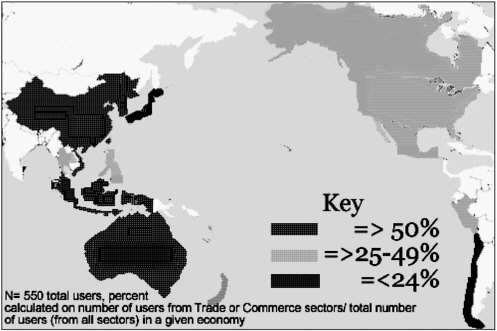
FIGURE 5-4 Percentage of APEC EINet users from trade and commerce by economy.
(GPHIN) (Heymann et al., 2001). Essentially a webcrawler, text-mining tool, this application was developed with Canadian government funding and implemented through an agreement with WHO in 2000. The “hits” generated daily are reviewed manually in Canada, and about 200 reports are forwarded to WHO per day. However, despite such alerts, all reports from GPHIN require independent verification from reliable sources on site (Grein et al., 2000; Hsueh et al., 2003).. In the absence of such confirmation, an international alert cannot be issued.
Biomedical Discovery
Response depends on diagnosis of what an outbreak is or is not. In the case of SARS, new scientific discovery was probably the largest beneficiary of new information technology, and this was in line with its priority in enabling effective public health response. Bioinformatics software tools were used extensively to identify the genome of SARS (Li et al., 2003), calculate the likelihood of frequencies in the annotation process (Ruan et al., 2003), and model the virus for prospective drug design among other uses. These tools, employed by teams of scientists across international boundaries, allowed bench scientists to rapidly generate new information about the SARS agent.
Interlaboratory communication was a second area in which the Internet and communications technologies added value. Stohr and colleagues report on the multicenter collaboration convened by WHO to “identify the causal agent and to develop a diagnostic test” (WHO Multicentre Collaborative Network, 2003). The 11 laboratories were located in nine countries. Countries both affected and not affected by SARS figured among the nine.
The electronic tools implemented included: (1) a secure, password-protected website where primer sequences and other information were posted for researchers; (2) electronic mail communications using the Internet; and (3) the telephone for daily teleconferences. Probably as important, the ethical framework for collaboration was established through an agreed protocol for sharing results and information. This protocol protected the work of scientists involved and fostered information sharing for advancement of the mutual collaboration. This networked activity of distributive efforts was efficient, resulting in the discovery and initial description of the coronavirus of SARS over the period of one month.
Epidemiologic Knowledge
Disease investigation was carried out in earnest at each of the outbreak sites. Case counts and mortality counts were reported through PROMED, WHO, EINET, and the media. However, in our experience with EINET, the need for practical guidance for the Asia Pacific outstripped the available information in
the first weeks of the epidemic. We received numerous queries about hospital isolation procedures, quarantine, airport measures, treatment, and other issues. While recommendations addressing these eventually were posted by international authorities, practitioners in closely linked but unaffected economies desired more specific and detailed information in a more timely manner.
WHO has convened the Global Outbreak Alert and Response Network partners over the past 8 years to begin to address exactly the kind of crisis presented by SARS. This activity proved to be a major asset to WHO in coping with SARS. However, the secure network and website approach that was implemented was less able to cope with the volume and diversity of information required. Specifically, the need for detailed information by public health authorities in unaffected areas was not optimally met (Kimball and Pautler, 2003).
The ability to monitor the impact of interventions is important to modulating the public health response. The key epidemiologic parameter to be followed is the reproductive rate of the epidemic in progress. If this rate is above 1.0, the epidemic will continue to expand as it infects new susceptibles at a greater rate than infected individuals recover (Lipsitch et al., 2003). This rate relies on modeling, and parameters that are difficult to collect through field investigation. In retrospect, only some of the affected localities were able to collect quality data in adequate amounts to enable such modeling to be reliably applied (Donnelly et al., 2003). As noted by one group, “Limited data and inconclusive epidemiologic information place severe restrictions on efforts to model the global spread of the SARS etiological agent” (Chowell et al., 2003).
Because our own user group includes trade and commerce officials from a number of APEC economies (Figure 5-4), our network was one of the few that provided updates on the epidemic situation in the region systematically to individuals not employed in the health sector. Although we have no quantitative information to document this, anecdotally we have been told this was useful in decision-making during the epidemic period.
Discussion: “Inter-SARS” Preparedness
SARS presented a challenge on both the research and response fronts. However, a similar challenge would be faced with any acute, severe viral respiratory infection for which diagnostic, treatment, and containment recommendations had not been well established. Influenza is an agent that could produce a similar picture and create similar chaos in the region. Thus, the overall concept of “preparedness” for such a natural disaster can serve to inform our actions in preparing for the “next wave.” In fact, in the midst of SARS, this genre of concept surfaced in the literature (Augustine, 2003).
The processes to address two major domain needs of the SARS response—laboratory research and epidemic investigation—were not truly ad hoc during the outbreak period. The basic structures of the two collaborative
groups—the linked laboratories and the outbreak alert and response partners (including the implementation of GPHIN)—had been created over the years prior to the outbreak. However, the implementation of emergency response was ad hoc, as was the area of epidemiologic investigation. Response encountered obstacles in communications, which can be partially addressed through preparedness exercises.
Tabletop or scenario exercises have been a centerpiece in preparation for emergency response in the United States. In Japan and Korea, exercises of alert and syndromic surveillance systems have been conducted to prepare for events such as the World Cup (Suzuki et al., 2003). The scenario “Dark Winter” convened high-level policy makers to discuss smallpox preparedness planning in the United States, and the more recent “Global Mercury” exercise carried out by the Global Health Security Action Group demonstrated the utility of this approach internationally (U.S. Department of State, 2003).
The tabletop as envisioned will: (1) bring together research universities and their public health counterparts in a collaborative process to tailor a scenario for their location in response to the threat of a travel-related, highly infectious disease; (2) create automated access to pertinent information sources at multiple sites that will add value to actual response efforts should these be needed; (3) promote international communications and collaboration using newer communications strategies among partners, thus ensuring the availability of these new tools to the public health community; and (4) create a flexible scenario for use in preparedness domestically and potentially by multiple APEC economies in training efforts. We believe the use of access node communications (see Box 5-1) for collaborative conferencing will demonstrate added value in the collaborative design process and in the debriefing on generic lessons learned in the exercises.
Beyond the virtual tabletop exercise, systematic analysis of the integrated workflow diagram suggests many other potential application sites for new information technologies. One apparent area would be the development of a software tool that could allow individual outbreak sites to assess their own data and calculate their own rate of reproduction for the outbreak they are experiencing (Chowell et al., 2003; Donnelly et al., 2003; Lipsitch et al., 2003). Such a tool could enable local public health officials to step up or step down response as success is or is not achieved. However, such a tool would rely heavily on the generation of reliable field investigation data in a timely way. The generation, compilation, and analysis of these data during the course of an outbreak remain the cornerstone of successful outbreak curtailment. Innovations in information technology need to be evaluated for their ability to support the key function of effective public health outbreak response.
Electronic networking and promoting intersectoral collaboration figure among the five strategies adopted by APEC to respond to emergent infections (Asia-Pacific Economic Corporation, 2001). The virtual tabletop will begin
|
BOX 5-1 “The Access Grid™ is one example of advanced communications resources now accessible within the Asia Pacific. An ensemble of resources including multimedia large-format displays, presentation and interactive environments, and interfaces to Grid middleware and to visualization environments, access grid nodes are used to support group-to-group interactions across the Grid. The Access Grid (AG) is used for large-scale distributed meetings, collaborative work sessions, seminars, lectures, tutorials, and training. The Access Grid thus differs from desktop-to-desktop tools that focus on individual communication. The Access Grid is now used at over 150 institutions worldwide (including institutions in Japan, Taiwan, Korea, Singapore, Canada, US, China, Hong Kong, Thailand). Each institution has one or more AG nodes, or “designed spaces,” that contain the high-end audio and visual technology needed to provide a high-quality compelling user experience. The nodes are also used as a research environment for the development of distributed data and visualization corridors and for the study of issues relating to collaborative work in distributed environments” (www.accessgrid.org). |
to leverage the sophistication already in place in communications and computing in the Asia Pacific in the service of the public good. Specifically, (1) communications technologies and middleware capacities of Asia Pacific research and education telecommunication networks are in place to be tested and adapted within the EINET community to support reporting, surveillance, and information exchange, particularly through the use of the Access Grid; and (2) a network of Pacific Rim research universities are being brought into the effort to serve as primary points of access for these advanced networks and technologies and as hubs of a broader communications network with the capacity to engage public health as well as other professionals throughout the APEC community.
PUBLIC HEALTH LAW PREPAREDNESS
Gene Matthews, J.D.
Legal Advisor, Centers for Disease Control and Prevention
The Central Intelligence Agency’s (CIA’s) unclassified report on severe acute respiratory syndrome (SARS) sets the tone for our current status on legal pre-
paredness for the next outbreak. “The effective application and efficacy of quarantine and isolation [during the SARS epidemic] proved a pleasant surprise to the public health community,” the CIA reports. “Equally unexpected was the widespread acceptance of the need for these measures by the general public.”
Another perspective on legal preparedness for an outbreak of infectious disease in the United States can be gained by considering a pair of paradoxes. The first paradox is that, in the same year (1954), the need for community-wide public health control measures was greatly reduced through the development of the Salk polio vaccine, and the U.S. Supreme Court initiated a trend toward increased procedural protections of individual liberties with its ruling that in Brown vs. Board of Education. Prior to 1954, the United States had regularly used community-wide quarantine in our legal system as a public health control measure. During the past 50 years, however, the judicial, legislative, and executive branches have each established ways to increase the protection of individual rights from government infringement. So, the public reaction to SARS was indeed surprising, as the CIA report says, because it marked the first true meeting in the United States of historical public health quarantine and modern civil liberties.
The second paradox could be referred to as “the paradox of the silos.” As the U.S. government has evolved during the past 50 years, we have developed more governance, but we have partitioned various responsibilities and authorities into different jurisdictional silos. We now have a public health silo stratified at the federal, state, and local levels, and it is separated from the silos of law enforcement, emergency management, agriculture, animal control, medical services, courts, transportation, and others. Reports of the SARS outbreak in China described silos of health care in that country as well. Health care in military hospitals, for example, was totally separate from health care in hospitals run by the railway system. When this silo effect occurs in complex governments, the legal structure is limited in its ability to arch over and effectively connect all the jurisdictions that need to respond to the problem. This is the challenge we face in the “paradox of the silos.”
Quarantine and Public Health Law
The quarantine issue during the SARS outbreaks illustrates the sort of bridging of silos that has to occur in public health law. Most emergency public health measures imposed within a state are subject to state law and regulations, which can vary. Some state laws concerning quarantine are procedurally outdated, and some local laws may be very useful. The federal government also has authority concerning quarantine. The U.S. Department of Health and Human Services (HHS) has concurrent federal power to apprehend, detain, and conditionally release individuals to prevent interstate spread or international importation of certain diseases.
These federally quarantinable diseases are specified by executive order of
the President. On April 4, 2003, President Bush signed an executive order adding SARS to the list of conditions that warrant quarantines; other conditions include cholera, diphtheria, infectious tuberculosis, plague, smallpox, yellow fever, and suspected viral hemorrhagic fevers (e.g., Lassa, Marburg, Ebola, Congo-Crimean, and others not yet isolated or named). Violation of quarantine authority in these cases is a criminal misdemeanor under federal law.
How would federal, state, and local laws interact to address an infectious outbreak? State and local governments have primary responsibility for isolation, quarantine and most of the emergency public health powers. The federal government has the authority to prevent interstate spread and international importation, but it can accept state and local assistance in enforcing the federal quarantine regulations. Conversely, HHS can assist state and local officials in their control of communicable diseases.
Because SARS has the potential to spread rapidly into different states, the federal quarantine authority could be applied to a single SARS case inside a state or local jurisdiction as necessary. In other words, it would not be necessary to wait for an interstate spread of SARS actually to take place before the Centers for Disease Control and Prevention (CDC), part of HHS, used this federal authority. However, any CDC action on SARS using this authority would be carefully coordinated with the appropriate state or local officials. The CDC did not use this authority during the 2003 SARS outbreak.
However, a situation may arise in which all three concurrent jurisdictions come into play. For example, if a disease outbreak occurred in a New York airport, federal, state, and local authority—all with overlapping police power—could be used. Since such activities would require coordination with the “law enforcement silo,” the CDC is intensively pursuing joint training between law enforcement and public health officials.
Lessons from Toronto
Toronto exemplifies the surprising level of public acceptance of quarantine described in the aforementioned CIA report. Dr. Barbara Yaffee and attorney Jane Speakman report that of the 13,000 persons “voluntarily” quarantined in Toronto, only 27 needed to be served with a formal quarantine order, and only one person sought to appeal (and this person later withdrew the appeal after he was told how he was exposed to SARS and could potentially transmit the disease). Few legal or public health professionals would have expected that level of public cooperation. One possible explanation is that the intense media coverage rapidly demystified the concept of quarantine. Additional analysis of this social phenomenon is urgently needed.
Toronto also exemplified social cohesion in a public health emergency; residents showed responsibility and cooperation, rather than the divisiveness and panic that some public health, media, or legal experts might have predicted. The
experience seems to indicate that when the public is presented with clear communication and practical guidance in a public health emergency, they can behave quite responsibly. Interestingly, there are abundant examples in the literature of such temporary, cohesive community behavior in an emergency. Of course, it is difficult to speculate how much the Toronto (or Canadian) experience would resemble that of the United States in a similar situation. Yet despite the differences between the U.S. and Canadian legal systems, it does seem that the recent history of quarantine in Toronto will influence how the United States would handle a similar situation.
Another key lesson from Toronto, as well as from several Asian countries, is the broad range of situations encompassed under quarantine. These included “work quarantine,” a concept discussed by Martin Cetron (see Chapter 1). Through “work quarantine,” needed public service employees can go to work and be isolated there or at home, and continue to maintain essential services. This is an important new tool to have available to compliment “snow day” and “shelter-in-place” community emergency strategies
Finally, as CDC director Julie Gerberding said in a press conference during the SARS epidemic, the public health community must be prepared to act boldly and swiftly, yet treat individuals with dignity and fairness. That is a good description of what happened during the SARS outbreak in Toronto: People were treated fairly, they received clear messages about their situation, and quarantine proceeded smoothly.
Practical Steps for Legal Preparedness
Some practical steps to prepare for a possible resurgence of SARS or an outbreak of another infectious disease. A more detailed treatment is available at www.cdc.gov.
-
Know the relevant legislation. All states, and most cities and municipalities, have quarantine laws. Some of these laws have not been used on a community-wide basis in 50 or 80 years. All such laws need to be examined on a case-by-case basis to determine whether they could be applied appropriately if SARS returned.
-
Plan due process. Be able to take the necessary steps, even if the laws are old, to give a quarantined person notice, a way to be heard, legal representation, and a final decision that a court can review. On the other hand, recognize that due process should not interfere with isolation if lives are threatened.
-
Draft documents in advance. Examples of quarantine orders that were used in Texas, as well as orders that have been drafted in North Carolina and other areas, are available on the CDC Public Health Law website at www.phppo.cdc.gov/od/phlp. These documents are accompanied by affidavits, descriptions of due process mechanisms, and other contingent material.
-
Contact other jurisdictions. Put law enforcement, emergency management, and health care in touch with each other. Create legal mechanisms to bridge these “silos” horizontally and vertically, and to connect federal, state, and local jurisdictions as well as geographical clusters. Personal networking is vital before an event takes place.
-
Engage the courts in advance. Toronto judges were somewhat surprised when health agency lawyers introduced quarantine orders. If the judiciary is engaged in advance of an outbreak, it can manage due process or habeas corpus proceedings efficiently.
-
Anticipate practical problems. In Toronto, for example, contract civil process servers refused to serve quarantine orders during the SARS epidemic. Law enforcement agents were therefore required to serve these quarantine orders.
-
Plan electronic communications for quarantined persons. They should be able to participate in hearings via video feed, cell phone, or other mechanism, rather than risk transmitting disease in the courtroom.
-
Emphasize communication, communication, communication. Two key reasons explain why quarantine worked so smoothly in Toronto: it was perceived as being fair and everyone was generally aware of what was happening. Communication among the “silos” is critical to success or failure of governance in a public health emergency.
SARS: LESSONS FROM A NEW DISEASE
David L. Heymann and Guenael Rodier
World Health Organization
Reprinted with permission from WHO, 2003.
© Copyright World Health Organization, 2004. All Rights Reserved.
New diseases have been emerging at the unprecedented rate of one a year for the last two decades, and this trend is certain to continue. The sudden and deadly arrival of SARS on the global health stage early in 2003 was in some ways perhaps the most dramatic of all. Its rapid containment is one of the biggest success stories in public health in recent years. But how much of that success was a result of good fortune as well as good science? How narrow was the escape from an international health disaster? What tipped the scales? The international response to SARS will shape future strategies against infectious epidemics.
The day-by-day struggle to control the outbreak of severe acute respiratory syndrome (SARS) represents a major victory for public health collaboration. Key lessons emerge that will be invaluable in shaping the future of infectious disease control—and being ready for the day when the next new disease arrives without warning. First and most important is the need to report, promptly and openly, cases of any disease with the potential for international spread in a closely interconnected and highly mobile world. Second, timely global alerts can prevent im-
ported cases from igniting big outbreaks in new areas. Third, travel recommendations, including screening measures at airports, help to contain the international spread of an emerging infection. Fourth, the world’s best scientists, clinicians and public health experts, aided by electronic communications, can collaborate to generate rapidly the scientific basis for control measures. Fifth, weaknesses in health systems play a key role in permitting emerging infections to spread. Sixth, an outbreak can be contained even without a curative drug or a vaccine if existing interventions are tailored to the circumstances and backed by political commitment. Finally, risk communication about new and emerging infections is a great challenge, and it is vital to ensure that the most accurate information is successfully and unambiguously communicated to the public. WHO is applying these lessons across the Organization as it scales up its response to the HIV/AIDS emergency.
The First Cases
On March 12, 2003, WHO alerted the world to the appearance of a severe respiratory illness of undetermined cause that was rapidly spreading among hospital staff in Hong Kong Special Administrative Region (China) and Viet Nam. Within two days, it was clear that the illness was also spreading internationally along major airline routes when hospitals in Singapore and Toronto, Canada, reported seeing patients with similar signs and symptoms. The potential for further international spread by air travel was vividly illustrated on March 15. In the early hours of the morning, the head of WHO’s outbreak alert and response operations was woken by a call from health authorities in Singapore. A doctor who had treated the first cases of atypical pneumonia there had reported having similar symptoms shortly before boarding an international flight returning to Singapore from New York. Asked to intervene, WHO alerted the airline and health authorities in Germany, where the flight was scheduled for a stopover. The doctor and his wife disembarked in Frankfurt and were immediately hospitalized in isolation, becoming the first two cases in Europe. Because of these events, WHO issued a second, stronger alert later in the day. It set out a case definition, provided advice to international travellers should they develop similar symptoms, and gave the new disease its name: severe acute respiratory syndrome (SARS). The global outbreak of SARS became the focus of intense international concern, and it remained so for almost four months.
Origins and International Spread
SARS is a newly identified human infection caused by a coronavirus unlike any other known human or animal virus in its family. Analysis of epidemiological information from the various outbreak sites is still under way, but the overall case fatality ratio, with the fate of most cases now known, approaches 11 percent,
but with much higher rates among elderly people. Transmission occurs mainly from person to person during face-to-face exposure to infected respiratory droplets expelled during coughing or sneezing, or following contact with body fluids during certain medical interventions. Contamination of the environment, arising from fecal shedding of the virus, is thought to play a small role in disease transmission, illustrated by the almost simultaneous infection in late March of more than 300 residents of a housing estate in Hong Kong where faulty sewage disposal was identified. At present, the disease has no vaccine, no curative treatment, and no reliable point-of-care diagnostic test, though antibody tests have been developed that can reliably confirm previous infection using acute and convalescent sera. Management of SARS is supportive, and control strategies rely on standard epidemiological interventions: identification of those fitting the case definition, isolation, infection control, contact tracing, active surveillance of contacts, and evidence-based recommendations for international travellers. Though demanding and socially disruptive, particularly when large numbers of people were placed in quarantine, these standard interventions, supported by high-level political commitment, proved sufficiently powerful to contain the global outbreak less than four months after the initial alert.
The earliest cases of SARS are now thought to have emerged in mid-November 2002 in the southern Chinese province of Guangdong. Retrospective analysis of patient records, to date incomplete, has identified small clusters of cases, each traced to a different initial case, that occurred independently in at least seven municipalities, with the first case recorded on November 16, 2002, in Foshan City and the largest number of cases concentrated in Guangzhou City. Analysis has uncovered no links among the various initial cases in the clusters. Some cases with no previous known history of exposure also occurred (WHO, 2003c; Breiman et al., 2003). Early collaborative studies conducted in Guangdong have detected a virus almost identical to the SARS coronavirus in domesticated game animals—the masked palm civet cat and the raccoon dog—sold in Guangdong live markets, suggesting that these animals might play a role in transmission of the virus to humans.
The initial phase of the Guangdong outbreak, characterized by small, independent clusters and sporadic cases, was subsequently followed by a sharp rise in cases during the first week of February 2003, thought to result from amplification during care in hospitals. Cases gradually declined thereafter. Altogether, some 1,512 clinically confirmed cases occurred in the Guangdong outbreak, with health care workers in urban hospitals accounting for up to 27 percent of cases (WHO, 2003c; Chinese Center for Disease Control and Prevention, 2003). This pattern—occurrence in urban areas, with most cases concentrated in hospitals, and amplification during care—was repeated as the disease began to spread outside Guangdong Province to other areas in China and then internationally.
The first recorded case of SARS outside China occurred on February 21, 2003, when a medical doctor who had treated patients in Guangzhou City and
was himself suffering from respiratory symptoms spent a single night in a hotel in Hong Kong. Through presumed contact, the mechanism of which is not fully understood, he transmitted SARS to at least 16 other guests and visitors, all linked to the same hotel floor. They carried the virus with them as they entered local hospitals or traveled on to Singapore, Toronto, and Viet Nam. An international outbreak that eventually spread to 30 countries had thus been seeded. Figure 5-5 maps the distribution of 8422 cases and 916 deaths that had occurred by August 7, 2003.
Detection and Response
On March 15, 2003, when the second alert was made, the cause of SARS had not yet been identified. Cases were concentrated in hospital workers and did not respond to medicines known to be effective against a number of different lung infections. Many patients were rapidly progressing to severe pneumonia. The situation was alarming: no patients, including young and previously healthy health workers, had recovered. Many of the patients were in a critical condition, several required mechanical ventilatory support, and two had died. The spread to major cities around the world meant that any city with an international airport was at potential risk of imported cases. From the outset, WHO’s objective was clear: to halt further international spread and interrupt human-to-human transmission through a global containment effort, and by so doing to minimize opportunities for the disease to establish endemicity (see Box 5-2).
The global response to SARS was in reality the roll out of a way of detecting and responding to outbreaks that had been developed over the preceding seven years by WHO and its partners, partly as a result of major weaknesses that came to light during the 1995 Ebola outbreak in the Democratic Republic of the Congo and during previous outbreaks of plague in India and cholera in Latin America. The SARS response depended on collaboration of the world’s top public health and laboratory experts, and took advantage of up-to-date communication technologies, including the Internet and video and telephone conferencing.
Two principal partners of the WHO Global Outbreak Alert and Response Network (GOARN), an electronically interconnected network of experts and institutes formally set up in early 2000, contributed to the detection of the SARS outbreak. One was the Canadian Global Public Health Intelligence Network (GPHIN), a worldwide web-crawling computer application, used by WHO since 1997, that systematically searches for keywords in seven different languages to identify reports of what could be disease outbreaks. Throughout the outbreak, GPHIN provided the raw intelligence that helped WHO maintain up-to-date and high-quality information on indications that the disease might be spreading to new areas. The second partner was the WHO Influenza Laboratory Network of 110 laboratories in 84 countries that constantly keeps the world in general and
|
BOX 5-2 More than 95 percent of SARS cases occurred in the Western Pacific Region. As an immediate response, a SARS outbreak response and preparedness team—including international experts—was established in the Regional Office. The main objectives were to:
Teams of epidemiologists and infection control experts were immediately sent to China, including Hong Kong Special Administrative Region, as well as to the Philippines, Singapore and Viet Nam and across the southern Pacific, training health care workers in infection control procedures and preparing them for the possible arrival of the disease. Practical infection control and preparedness guidelines and training tools were developed, and the first version of preparedness guidelines was issued at the beginning of April. Logistic support and supplies (personal protective equipment, including masks, collection materials for blood and respiratory samples, and internationally approved containers for shipment of samples) were sent to both affected and unaffected countries, supported by a US$ 3 million grant from the Government of Japan. Countries were classified according to three levels of risk and three levels of capability to respond to SARS cases, in order for WHO to prioritize its support to countries. WHO worked closely with countries to ensure that enhanced surveillance was put in place to enable early detection of cases and contact tracing. Guidelines were drawn up on enhanced surveillance, hospital and community infection control, international travel, laboratory procedures and public awareness. To improve public awareness, close contact was established with national media focal points, and the web site of the Western Pacific Regional Office was regularly updated. A regional laboratory network was established to ensure that necessary testing for SARS could be done for countries with limited laboratory capacities. National and regional reference laboratories were identified and shipping of specimens was arranged between the laboratories. WHO’s efforts were paralleled by the contribution of Member States. Viet Nam was the first to interrupt local transmission of the virus. Other countries introduced a wide range of measures, including isolation, home quarantine and comprehensive contact tracing. The willingness of governments in the Western Pacific Region to put public health considerations ahead of economic concerns about the impact of SARS was crucial to the success of the collaborative effort. |
vaccine manufacturers in particular informed of which strains of influenza are circulating, so that an effective influenza vaccine can be produced each year.
On February 10, 2003, GPHIN and other partners of GOARN identified reports of an outbreak associated with health worker mortality and the closing of hospitals in Guangdong. One day later the Chinese government officially reported to WHO an outbreak of respiratory illness, beginning in mid-November, involving 300 cases and five deaths in Guangdong Province. Just over a week later, on 19 February, an outbreak of avian influenza was reported to the WHO Influenza Laboratory Network by the collaborating laboratory in Hong Kong. This outbreak first came to light when a 33-year-old man died of an unknown cause after returning from a family trip to Fujian Province, China. His 8-year-old daughter had died of a similar disease while in Fujian Province and his 9-year-old son was hospitalized in Hong Kong with the same symptoms. It was from this son that avian influenza virus was isolated and reported to the Influenza Laboratory Network. The same influenza virus had been identified in Hong Kong in 1997. Control efforts at that time required the slaughter and incineration of all chickens in the many live markets there; human-to-human transmission was never established.
This heightened level of alert led to the identification of an early SARS case in Viet Nam on February 28, 2003. At the same time as GOARN collected information about this outbreak in real time, it sent an international team of partners to work with the Viet Nam authorities to better understand the disease, and by March 12 GOARN had accumulated the initial information necessary to issue the first global alert. It was through the continued instant sharing of information by governments, public health experts, clinicians and laboratory scientists that evidence-based decisions could progressively be made, culminating in the successful containment of SARS.
Under GOARN, a virtual collaborative network of 11 leading laboratories, linked by a secure website and daily teleconferences, identified the SARS causative agent and developed early diagnostic tests. The network, in turn, served as a model for similar electronically linked groups of clinical and epidemiological experts who pooled clinical knowledge and compiled the epidemiological data needed to chart the outbreak’s evolution and assess the effectiveness of control interventions.
WHO issued daily updates about the outbreaks on its website to keep the general public—especially travellers—informed and, as far as possible, to counter rumours with reliable information. Equally important, the website was used to issue a range of evidence-based technical and practical guidelines for control as knowledge and information about the disease progressed and became available through the virtual groups of experts.
As more and more evidence accumulated through real time collaboration of public health experts, a range of additional evidence-based control measures became possible. It was soon evident, for example, that people with SARS continued to travel internationally by air after March 15, and that some of them had infected passengers sitting nearby. At the same time it was also apparent that contacts of
SARS patients likewise continued to travel, becoming ill once they arrived at their destination. Recommendations were therefore made that countries with major outbreaks should screen departing passengers to make sure that they did not have fever and other signs of SARS, or known contact with SARS patients.
As the outbreak continued in Hong Kong, contact tracing there further demonstrated that transmission of SARS was occurring outside the confined environment of the health care setting, and later suggested that it was also occurring following exposure to some factor in the environment, thus creating further opportunities for exposure in the general population. Additional evidence-based guidance was therefore made for sites where contact tracing could not link all cases to a chain of transmission, on the understanding that if the disease were spreading in the wider community it would greatly increase the risk to travellers and the likelihood that cases would be exported to other countries. This guidance was aimed at international travelers, and recommended that they postpone all but essential travel to designated areas in order to minimize their risk of becoming infected. Such guidance was also needed in view of the confusion created by several different national recommendations, many of which were based on criteria other than epidemiological data.
Authorities in areas where outbreaks were occurring responded to SARS with mass public education campaigns and encouraged populations to conduct daily fever checks. Hotlines and websites answered questions. Screening measures were set up at international airports and border crossings, and procedures of infection control were reinforced in hospitals. Singapore drew on its military forces to conduct contact tracing, while Hong Kong adapted a tracing system that had been developed for use in criminal investigations and electronically mapped the location of all residences of cases. Chinese authorities opened hundreds of fever clinics throughout the country where suspected SARS cases were triaged. Heads of state and ministers of health of of countries of the Association of Southeast Asian Nations (ASEAN) and the Asia–Pacific Economic Cooperation (APEC) met and resolved to establish closer collaborative mechanisms for disease surveillance and response. Health staff everywhere worked with dedication, and many, including WHO staff member Dr. Carlo Urbani, lost their lives.
On July 5, 2003, WHO announced that Taiwan, China, where the last known probable case of SARS had been isolated 20 days earlier, had broken the chains of human-to-human transmission. A recurrence of SARS cannot, however, be ruled out. Further research on many unresolved questions is needed. In the meantime, systems are now in place to detect a re-emergence should it occur (WHO, 2003d).
The Impact of SARS
The economic impact of the SARS outbreak has been considerable and illustrates the importance that a severe new disease can assume in a closely interde-
pendent and highly mobile world. Apart from the direct costs of intensive medical care and control interventions, SARS caused widespread social disruption and economic losses. Schools, hospitals, and some borders were closed and thousands of people were placed in quarantine. International travel to affected areas fell sharply by 50 to 70 percent. Hotel occupancy dropped by more than 60 percent. Businesses, particularly in tourism-related areas, failed, while some large production facilities were forced to suspend operations when cases appeared among workers.
A second impact is more positive: SARS stimulated an emergency response—and a level of media attention—on a scale that has very likely changed public and political perceptions of the risks associated with emerging and epidemic-prone diseases. It also raised the profile of public health to new heights by demonstrating the severity of adverse effects that a health problem can also have on economies and social stability. The resulting high level of political commitment was decisive in the containment of SARS and has much to say about the ability of nations to achieve public health results even when drugs and vaccines are not available to cure or prevent the infection.
Lessons Learnt
Although much about SARS—including its potential to reoccur—remains to be learnt through systematic analysis of existing data, and focused research activities in China, several important lessons are already apparent. WHO is applying these lessons across the entire Organization as it responds to the HIV/AIDS emergency.
The first and most compelling lesson concerns the need to report, promptly and openly, cases of any disease with the potential for international spread. Attempts to conceal cases of an infectious disease, for fear of social and economic consequences, must be recognized as a short-term stop-gap measure that carries a very high price: the potential for high levels of human suffering and death, loss of credibility in the eyes of the international community, escalating negative domestic economic impact, damage to the health and economies of neighboring countries, and a very real risk that outbreaks within the country’s own territory will spiral out of control. Following the adoption during the World Health Assembly in May 2003 of a resolution on the International Health Regulations, WHO has been confirmed in its responsibility to take on a strong coordinating role in leading the fight against any infectious disease that threatens international public health (WHO, 2003e). In a second resolution specific to SARS, all countries are urged to report cases promptly and transparently, and to provide information requested by WHO that could help prevent international spread. It was explicitly acknowledged that across-the-board strengthening of systems for outbreak alert and response was the only rational way to defend public health security against not only SARS but also against all future infectious disease threats, including those that might be deliberately caused (WHO, 2003f).
The second lesson is closely related: timely global alerts, especially when widely supported by a responsible press and amplified by electronic communications, worked well to raise awareness and vigilance to levels that can prevent imported cases of an emerging and transmissible infection from causing significant outbreaks. The global alerts issued by WHO on March 12 and 15 provide a clear line of demarcation between areas with severe SARS outbreaks and those with none or only a few secondary cases. Following the SARS alerts, all areas experiencing imported cases, with the exception of Taiwan, China, either prevented any further transmission or kept the number of locally transmitted cases very low. Figure 5-6 shows the weekly onset of 5,910 cases. A climate of increased awareness also helps to explain the speed with which developing countries readied their health services with preparedness plans and launched SARS campaigns, often with WHO support, to guard against imported cases.
The third lesson is that travel recommendations, including screening measures at airports, appear to be effective in helping to contain the international spread of an emerging infection. Initial analysis of data on in-flight transmission of SARS has implicated four flights in the exposure of 27 probable cases, of which 22 occurred on a single flight from Hong Kong to Beijing, China, on March 15. Some of these cases may also have been exposed elsewhere because of being in the same tour group. Following the recommendation of airport screening measures on March 27, no cases associated with in-flight exposure were reported; and initial information reveals that two probable SARS cases were identified by airport screening procedures in Hong Kong and immediately hospitalized. Travel recommendations based on the epidemiological evidence also gave areas where outbreaks were occurring a benchmark for quickly containing SARS, and then regaining world confidence that the area was safe from the risk of SARS transmission. In fact, passenger movement figures provided by Hong Kong International Airport show a rapid rebound from the lowest number of passengers, 14,670 (recorded just before May 23 when the travel recommendations were removed) to 54,195 on July 12, a little over a month later.
The fourth lesson concerns international collaboration: the world’s scientists, clinicians and public health experts are willing to set aside academic competition and work together for the public health good when the situation so requires. International collaboration greatly advanced understanding of the science of SARS. One month after the laboratory network was established, participating scientists collectively announced conclusive identification of the SARS virus; complete sequencing of its RNA followed shortly afterwards. The network of clinical experts provided a platform for comparison of patient management strategies to indicate to the world which treatments and strategies were effective. In addition, the epidemiology network confirmed the modes of transmission of SARS and began the long-term collaboration needed to understand clearly the clinical spectrum of disease, including its case fatality ratio, while also providing the information needed to regularly reassess and adjust the case definition.
Lesson five is that weaknesses in health systems can permit emerging infections to amplify and spread, and can compromise patient care. The strengthening of health systems thus deserves high priority. The people at greatest risk for SARS were health workers who either became infected by close face-to-face contact with patients or by procedures that brought them into contact with respiratory secretions. Women predominate among the lower ranks of health personnel in many countries; available data reveal that infected health care workers were 2.7 times more likely to be women than men, while infection was roughly equal between the sexes in the general population. The surge of SARS patients placed an enormous burden on health services, requiring facilities for isolation, long periods of intensive and expensive care, and the use of demanding and socially disruptive measures such as mass screening, contact tracing, active surveillance of contacts and—at some outbreak sites—enforced quarantine. Even in areas with highly developed social services, the burden of coping with SARS, including the large number of hospitals with patients and the high number of health workers who became infected, often required closing some hospitals and sections of others. As a result of SARS outbreaks, many long-standing and seemingly intractable problems that have traditionally weakened health systems are being corrected in fundamental and often permanent ways. New surveillance and reporting systems, methods of data management, mechanisms for collaborative research, hospital policies, procedures for infection control, and channels for informing and educating the public are part of the initial positive legacy of SARS that will shape the capacity to respond to future outbreaks of new or re-emerging infections.
Lesson six is that in the absence of a curative drug and a preventive vaccine, existing interventions, tailored to the epidemiological data and supported by political commitment and public concern, can be effectively used to contain an outbreak. The virtual laboratory, and clinical and epidemiological collaborating networks regularly provided information that was used by WHO and its partners to update guidance for containment. Initial guidance was provided for containing outbreaks nationally—as additional evidence was obtained, guidance to limit international spread was also provided. Areas where outbreaks were occurring, and countries which considered themselves at risk of imported cases from these areas, adapted WHO guidance for their use. Some countries introduced active surveillance of suspected contacts using surveillance cameras or military personnel. Others relied on self-surveillance by contacts who voluntarily isolated themselves in their homes and regularly checked for fever. Measures introduced at airports ranged from passive screening of passengers, involving optional completion of questionnaires, to the use of interviews conducted by health workers and sophisticated infrared equipment to screen all passengers for fever and indications of possible exposure. In addition to maximizing the impact of surveillance and screening, these measures were also considered by governments to be reassuring for national citizens as well as international travelers.
The seventh lesson highlights one of the major difficulties faced during the containment activities for SARS: risk communication about new and emerging infec-
tious diseases is a great challenge. Work along these lines is currently under way in conjunction with the risk that a biological agent might be used in an act of terrorism.
SARS will not be the last new disease to take advantage of modern global conditions. In the last two decades of the 20th century, new diseases emerged at the rate of one per year, and this trend is certain to continue (Woolhouse and Dye, 2001). Not all of these emerging infections will transmit easily from person to person as does SARS. Some will emerge, cause illness in humans and then disappear, perhaps to recur at some time in the future. Others will emerge, cause human illness and transmit for a few generations, become attenuated, and likewise disappear. And still others will emerge, become endemic, and remain important parts of our human infectious disease ecology.
The rapid containment of SARS is a success in public health, but also a warning. It is proof of the power of international collaboration supported at the highest political level. It is also proof of the effectiveness of GOARN in detecting and responding to emerging infections of international public health importance. At the same time, containment of SARS was aided by good fortune. The most severely affected areas in the SARS outbreak had well-developed health care systems. Had SARS established a foothold in countries where health systems are less well developed cases might still be occurring, with global containment much more difficult, if not impossible.
Although control measures were effective, they were extremely disruptive and consumed enormous resources resources that might not have been sustainable over time. If SARS reoccurs during an influenza season, health systems worldwide will be put under extreme pressure as they seek to isolate all those who fit the clinical case definition until diagnosis can be ascertained. Continued vigilance is vital.
SARS: DOWN BUT STILL A THREAT
Karen J. Monaghan
National Intelligence Council10
Reprinted with permission from National Intelligence Council, 2003.
Copyright 2003 National Intelligence Council.
Scope Note
This Intelligence Community Assessment (ICA) was requested by Secretary of Health and Human Services Tommy Thompson and Ambassador Jack Chow, Deputy Assistant Secretary of State for International Health Affairs. It highlights the evolution of severe acute respiratory syndrome (SARS) and the potential im-
|
10 |
Prepared under the auspices of Karen Monaghan, Acting National Intelligence Officer for Economics and Global Issues. Additional copies of this assessment can be downloaded from the NIC public website at www.odci.gov/nic or obtained from Karen Monaghan, Acting National Intelligence Officer for Economics and Global Issues, at (703) 482-4128. |
plications of the disease for the United States under several scenarios; this paper does not attempt to provide a scientific assessment of the epidemiology of SARS. Even though SARS has infected and killed far fewer people than other common infectious diseases such as influenza, malaria, tuberculosis, and HIV/AIDS, it has had a disproportionately large economic and political impact because it spread in areas with broad international commercial links and received intense media attention as a mysterious new illness that seemed able to go anywhere and hit anyone.
As the first infectious disease to emerge as a new cause of human illness in the 21st century, SARS underscores the growing importance of health issues in a globalized world. The December 1999 unclassified National Intelligence Estimate, The Global Infectious Disease Threat and Its Implications for the United States, warned that new and reemerging diseases would pose increasing challenges to the United States and the rest of the world. The 1999 estimate highlighted several key health trends that track with the emergence of SARS:
-
The forces of globalization, which are speeding the spread of infectious diseases and amplifying the impact, also are giving us better tools to protect human health.
-
Major infectious disease threats to the United States and the world, like HIV/AIDS, will continue to emerge, challenging our ability to diagnose, treat, and control them.
-
Infectious diseases will loom larger in global interstate relations as related embargoes and boycotts to prevent their spread create trade frictions and controversy over culpability.
In addition to coordinating the draft within the intelligence community, the National Intelligence Council asked several health experts to review the paper as part of its effort to capitalize on expertise inside and outside the government. The experts included Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health; Dr. Steve Ostroff, deputy director, National Center for Infectious Diseases, Centers for Disease Control and Prevention (CDC); and Dr. Joshua Lederberg, professor emeritus at Rockefeller University and Nobel laureate. The NIC also shared the draft with counterparts in Canada at the Privy Council Office, Intelligence Assessment Secretariat.
DISCUSSION
The Global Health Challenge
The emergence of SARS illustrates the challenge of battling infectious diseases in an increasingly globalized world. SARS is the latest of more than 35 new or reemerged infectious diseases over the last 30 years. Infectious diseases have long raged through human communities, but forces of globalization—including rapid growth in international trade and travel and increasing urbanization—have
amplified their spread and impact. These same forces of globalization, however, also have led to significant advances in communication, travel, and technology, which have aided in the fight against infectious diseases.
-
On balance, infectious disease pathogens have the upper hand because they constantly evolve new mechanisms that can exploit weak links in human defenses.
-
SARS has subsided for now, but many health experts warn that it is likely to come back when cooler weather returns to temperate areas, bringing a resurgence of respiratory infections.
Downsides of Globalization
Population growth and development are bringing more people into contact with non-domesticated animals, introducing new diseases more frequently into the human population. The transmission of pathogens from animals to humans is a process called zoonosis. Some researchers believe that SARS may have originated in China in animals such as wildcat species that were trapped and sold as food in exotic markets. In mid-August 2003, China lifted the ban on the sale and consumption of exotic animals imposed during the SARS epidemic.
-
HIV/AIDS, monkeypox, and hantavirus are other infectious diseases believed to have originated in animals.
Modern travel and labor migration patterns played a key role in spreading SARS after it emerged in November 2002 in Guangdong Province, China (see Figure 5-7). From Guangdong, the disease made its way to Hong Kong and then to Vietnam, Singapore, and Taiwan as well as Europe and North America.
-
Within China, as many as 180 million people are considered migrant labor, moving between rural areas, cities, and manufacturing centers in search of employment.
-
Asia has become a major hub for business and tourist travel, putting millions of passengers within 24 hours of almost every major city in the world, providing little time to identify and isolate people infected with diseases that may take several days to show symptoms.
-
More people also are migrating overseas to find jobs, and travel by workers and their families can spread diseases. For example, a Filipino nurse working in Toronto contracted SARS and transmitted it to family members on a visit to the Philippines.
In addition to spreading the disease geographically, global links also have amplified the economic and political impact of the disease. Even though SARS has killed far fewer people up to now—around 815—than those who die each
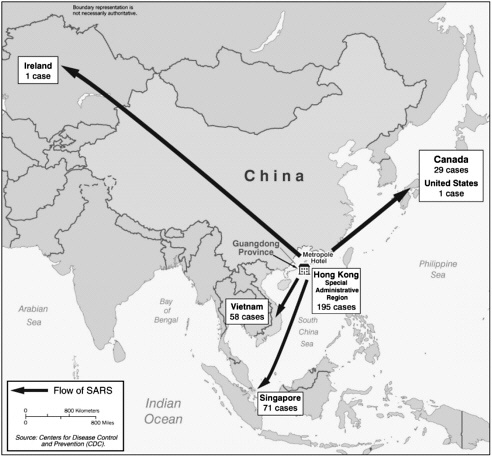
FIGURE 5-7 Portrait of a superspreader: spread of SARS from the Metropole Hotel in Hong Kong as of March 28, 2003.
year from more common maladies such as pneumonia, influenza, malaria, and tuberculosis, as a new disease it was more disruptive and generated more attention (see Figure 5-8). The disease exhibited some characteristics of a potentially explosive epidemic in the early stages, and SARS hit countries that have extensive commercial links with other parts of the world, generating widespread economic disruptions and media attention.
-
The outbreak of SARS in Asia and Canada disrupted a wide-ranging global network of businesses increasingly dependent on international trade and travel. Airlines were the highest profile economic victims, but service industries like tourism and supply chains in industries as diverse as seafood and microchips also were affected.
-
Intense media attention and uncertainty about the disease fueled widespread fear, even in some areas without any cases, exacerbating economic disruptions.
FIGURE 5-8 Comparative worldwide mortality of infectious diseases.a
-
The suspicion of Asians as carriers of the disease reduced patronage of Asian businesses and communities in the United States and sparked travel bans against Asian tourist groups and conference participants worldwide.
Benefits of Globalization
Intense international media coverage facilitated by global communication networks increased pressure on governments to respond effectively to SARS and
prompted many citizens and healthcare workers to be vigilant in taking precautions, monitoring symptoms, and seeking early treatment.
-
China initially tried to cover up SARS as it did with other diseases in the past, but international media scrutiny and leaks to the press led Beijing to publicly acknowledge and respond to the disease.
-
The public has been able to track the evolution of the disease more closely with everything from text messaging on cell phones to publicly and privately run websites; Singaporeans could even watch a special public service television channel devoted to SARS.
Modern communications and medical technologies provided key tools to combat SARS.
-
Health workers utilized the World Health Organization’s (WHO) global network of research facilities to share data and speed the identification of the virus causing SARS.
-
International medical journals took the rare step of promptly publishing research on SARS on the Internet prior to hard copy publication.
-
Thermal imaging equipment was acquired in numerous countries in an effort to screen large numbers of people for high fevers. Hong Kong employed software to track the spread of the disease in urban areas, and some countries employed cameras and electronic bracelets to help security officials enforce home quarantines.
Economic and Political Fallout of SARS
Government and private sector economists have had difficulty calculating the costs of the SARS epidemic. Early on, forecasters estimated that the macroeconomic impact would be negligible but hastily cut growth estimates for several economies, including China, as the disease spread, cases mounted, and the situation appeared to be out of control (see Table 5-3). Service industries, particularly airlines and tourism, were affected immediately. SARS began to threaten the retail and manufacturing sectors, particularly in China, when business trips and trade fairs were canceled, new orders were placed on hold, and investors delayed new expansion and constructions plans.
-
In late April, the World Bank cut its growth forecast for East Asia to 5.0 percent—from 5.8 percent in 2002—due in part to SARS.
-
In early May, the Asian Development Bank warned that East Asia could lose $US 28 billion in income and output if the disease continued until September.
-
Several investment banks shaved up to one percentage point off China’s growth forecasts and cautioned that a more serious slowdown could occur if SARS were not brought under control by July.
TABLE 5-3 Economic Impact of SARS
|
Economy |
Gross Domestic Product |
Employment |
Sectoral Impact |
Stimulus Packages |
Cumulative SARS cases (deaths)* |
|
China |
Early predictions of severe impact revised, forecasts suggest strong growth of 7 to 8 percent for 2003. |
Major impact on jobless rate, especially among migrant workforce; unemployment nearly doubles to over 8 million. |
Retail sales and restaurant sector stall, particularly in urban areas. Export and manufacturing have proven more resilient. |
No comprehen sive package, but some ad hoc measures for service sector, including tempo rary tax cuts. |
5,327(348) |
|
Hong Kong |
Official growth estimate cut to 1.5 percent from March-to-May earlier forecasts of 3 percent. |
Unemployment hits record high period, could swell to 10 percent by year end. 8.3 percent over |
Tourism and retail sector ravaged, but few signs SARS has hurt trade. Air traffic fell 80 percent in May. |
A US $1.8 billion relief package, including rent reductions and tax rebates, especially for hardest hit businesses. |
1755 (298) |
|
Taiwan |
Official growth estimate cut to 2.7 percent from earlier forecasts of 3.7 percent. |
Minimal impact as employers cut pay and grant unpaid leave but miss target of reducing unemployment to 4.5 percent in 2003. |
Tourism and retail sectors hardest hit. |
Emergency relief and economic stimulus packages worth $3.7 billion, and a 3-year $8.6 billion public works program. |
671 (84) |
-
North Korea imposed tight border restrictions and quarantines, slowing trade flows and temporarily closing a lucrative new tourist resort.
Recent data suggest that growth in most countries plummeted in April and May but started to recover as the disease was brought under control, reports of new cases dwindled, and the WHO removed countries from its travel advisory list. Most notably, no major disruptions in trade and investment flows occurred. Moreover, most factories in China, including those in Guangdong where the disease originated, continued to operate even during the height of the epidemic. In some countries, monetary and fiscal stimulus packages also helped to cushion the blow.
Certain locales, notably Hong Kong, Beijing, and Toronto, were hurt more than others. Moreover, additional indirect costs—the so-called SARS tax—probably will be incurred by businesses consumers, governments, and nongovernment agencies.
-
Collectively, the ASEAN countries—Brunei, Cambodia, Indonesia, Laos, Malaysia, Myanmar, the Philippines, Singapore, Thailand, and Vietnam—are estimated to have lost $US 25 billion to $US 30 billion, mostly in the tourism, service, aviation, and restaurant sectors.
-
Although China is forecast to achieve growth of 7 to 8 percent this year, the economies of China and Hong Kong will take longer to recover because the tourism, transport, communication, food, and entertainment industries suffered substantial losses.
-
Most analysts forecast that SARS would shave a minimal amount off Canada’s 2003 growth but cut 1 percent off Toronto’s $200 billion economy.
SARS dealt a body blow to the travel and tourism industries, already facing a slowdown from post-9/11 terrorism concerns. They will be slow to recover. Business travel has resumed more rapidly as firms catch up on a backlog of deals, but tourist travel is far below last year’s levels. Hotels in Asia are cutting room rates in a bid to attract customers.
An industry trade group estimates the tourist sector in China, Hong Kong, Singapore, and Vietnam will lose up to $US 10 billion and 3 million jobs this year because of SARS.
Airlines have restored most canceled flights, but carriers will have difficulty recouping lost revenues, and some may be forced into bankruptcy. The airline industry’s slow recovery will be a further drag on the aviation industry. Asian airlines were to account for one-quarter of Airbus deliveries and 30 percent of Boeing’s deliveries in 2003. Several Asia-Pacific carriers asked Airbus and Boeing to postpone deliveries of new aircraft. Both manufacturers have been counting on robust growth in the Asian travel market to boost revenues.
Anecdotal evidence suggests that some export-oriented industries, particu-
larly clothing manufacturers, temporarily shifted some orders to Bangladesh, Turkey, India, and Pakistan. Foreign electronics manufacturers, including a large Japanese electronics firm, shifted some production to plants in Philippines and Malaysia with highly specialized sectors and relatively low costs. There is no evidence to suggest that foreign manufacturers pulled out investments or permanently shifted production outside China or East Asian production plants. Some multinationals probably have begun to rethink the costs and benefits of concentrating investment in one country or region, however.
-
Over the last decade, China has attracted massive amounts of foreign direct investment (FDI)—$53 billion in 2002—thanks to its reputation as a low-cost and relatively low-risk manufacturing locale with a rapidly growing domestic market.
SARS has had minimal impact on global semiconductor production, even though nearly 80 percent of production in this $US 8 billion industry is located in Asia, largely in Taiwan and China.
None of the semiconductor operations was forced to curtail production, although SARS disrupted some visits by foreign equipment suppliers and prompted the temporary closing of some Hong Kong sales and marketing offices.
Political Impact
SARS seriously tested the leadership skills of politicians and civil servants in every country affected. The public was quick to criticize leaders in China, Canada, Hong Kong, and Taiwan for failing to grasp the seriousness of the situation, to act quickly to contain the spread, and to accept responsibility for missteps. In some countries, public confidence in the ability of government leaders and state institutions to protect them may be permanently damaged.
-
In China, SARS intensified behind-the-scenes jockeying between President Hu Jintao and his predecessor, Jiang Zemin, who initially downplayed the disease. Hu publicly acknowledged the threat of SARS, allowed greater media coverage of the crisis, and sacked one of Jiang’s loyalists as Minister of Health.
-
In Canada the Prime Minister, Premier of Ontario, and Mayor of Toronto drew fire from media and opposition party critics accusing them of failing to respond effectively and address public fears.
In contrast, the WHO and CDC lauded the Vietnamese government’s swift action and willingness to accept outside assistance, noting these factors were key to its success in containing the spread of SARS. In Singapore, the public expressed confidence and support for the government’s rigorous efforts to identify
and isolate suspected SARS patients. An early April poll showed three out of four Singaporeans were confident that the government could stop SARS.
Tracking the Downturn in SARS
Since WHO first issued a global alert about SARS in March 2003, almost 8,500 probable cases have been reported from 29 countries around the world, with most cases (over 7,000) occurring in China. At one point in May, over 180 new infections were being reported daily, mostly in China.
The number of SARS cases peaked in May and steadily declined worldwide with the WHO declaring on July 5 that all transmission chains of the disease had been broken. The decline may reflect a seasonal retreat of the disease in warmer months, which is common for respiratory illnesses in temperate climates. Nonetheless, the downturn clearly illustrates that, even in a globalized world, the old-fashioned work of identifying and isolating suspected cases, tracing and quarantining others who might be exposed, and issuing travel advisories can control an emerging disease.
-
Most countries hit by SARS had not used traditional public health tools such as quarantine and isolation on such a large scale for decades, which slowed the containment.
-
Governments also had to mobilize enormous resources to implement large-scale quarantine operations.
Surveillance
The first line of defense in arresting the spread of SARS has been the success in identifying possible cases—despite the lack of a proven screening test and symptoms common to many respiratory ailments. Taking people’s temperature generally has been the simplest, most cost-effective means of initial screening for possible SARS cases, followed by clinical examination for respiratory symptoms in those with fevers.11
-
Singapore issued over a million SARS toolkits with thermometers and facemasks to every residence in the country. Residents were regularly stopped at office buildings, schools, and other public places for temperature checks.
-
China mobilized local party and government officials, including 85 million family planning workers, to try to monitor citizens for symptoms. China also mobilized its large militia to provide the rural public with instructions on SARS prevention. The government distributed tens of thousands of thermometers to the provinces.
|
BOX 5-3 Origins. The SARS epidemic spread rapidly because people had little immunity to the newly emerged coronavirus that causes the disease. Close contact with sick individuals appears to be the primary means of virus transmission, although research indicates that SARS does not transmit as easily from person-to-person as more common diseases like the cold or flu. The disease spread most rapidly among healthcare workers and family members of infected individuals. Evidence indicates that the virus also is spread through contact with inanimate objects contaminated with virus-containing secretions. Recent detection of a related coronavirus in wildcat species in China raises concerns that SARS may continue to have an animal reservoir, which would complicate control efforts. Symptoms. SARS can progress rapidly from fever and cough to serious pneumonia after an average four-to-six-day incubation period, with up to 20 percent of patients needing mechanical ventilation to survive. In some patients, progression to pneumonia may be delayed. Death may occur several weeks to months after initial symptoms. Diagnosis. Accurate, rapid screening diagnostic tests for SARS are being developed but are not yet licensed in the United States. During the epidemic healthcare workers generally relied on clinical symptoms for detection. WHO defines a suspected SARS case as someone with a temperature over 38° Celsius, a cough or difficulty breathing, and one or more of the following exposures: close contact with a person who is a suspect or probable SARS case, or someone who has lived in or visited a region with SARS transmissions. A “probable case” is a suspected case with radiographic evidence of pneumonia or positive laboratory tests that may take days to weeks to complete. Treatment. No proven therapy is available for severe SARS pneumonia cases. Most clinicians employ respiratory support, antibiotics, fever reduction, and hydration. Some Chinese doctors have used steroids and the antiviral drug ribavirin with varying degrees of success. Fatalities. Although the overall lethality of SARS is higher than initially believed, most deaths continue to be among older patients and those with underlying health problems, such as diabetes or hepatitis B. The WHO reported in May 2003 that death rates vary substantially by age:
|
|
Preliminary reports on nonfatal cases showed SARS patients required longer hospital stays—an average of three weeks for those under 60 years of age—than patients with other typical respiratory viruses, raising the economic costs of the SARS outbreak. Moreover, preliminary evidence suggests that some people who survive SARS could suffer long-term respiratory damage that increases health complications and costs. |
-
After WHO confirmed that SARS could be transmitted on airline flights, including 22 infections traced to a single flight in March, airlines have become more stringent at keeping people who might be infected off airplanes.
Even though checks of passengers at airports were relatively effective at keeping infected people off airplanes, some lapses did occur.
-
Japan installed infrared thermometers to monitor passengers at Tokyo’s international airport after voluntary testing proved ineffective, but press reports indicate that the machines cannot keep up with all travelers at peak times.
-
An Asian man suspected of having SARS boarded a flight to the United States in May because his flight left before lab results were received and he had no other symptoms.
Quarantines and Isolation12
As SARS spread and political and economic stakes rose, countries took tougher measures to contain it. Some countries resorted to strong steps, such as closing schools despite the low number of cases among children, probably to compensate for weaknesses in their health-care infrastructure. Open societies seemed to have trouble enforcing quarantine orders.
-
Some Chinese citizens fled cities and industrial hubs in response to early government efforts to isolate suspected cases and quarantine their contacts. Subsequently, Beijing forcibly locked both patients and healthcare workers in hospitals during the peak of infections, and the government instituted fines for people violating isolation orders and employed citizens to keep outsiders out of various villages. Shanghai officials announced in late May they had quarantined nearly 29,000 people in the previous 2 months.
|
BOX 5-4 The World Health Organization (WHO) issued an international health warning on SARS in March 2003 and travel advisories regarding particular regions hit by the disease. The WHO, in collaboration with the U.S. Centers for Disease Control and Prevention (CDC) and other organizations, worked to identify the cause of the disease, assisted local investigators, and provided guidance on control measures. The SARS experience highlights the bureaucratic and technical limitations WHO faces in trying to identify and control the international spread of infectious diseases. Under existing international health regulations, countries are only required to report to WHO outbreaks of yellow fever, cholera, and plague. With these diseases, WHO, the United Nations, and domestic officials have the authority to intervene and prevent the movement of people and goods to avert cross-border transmission. With other diseases, WHO plays an advisory role, including issuing travel advisories and offering advice to member governments on screening procedures. Unless a country invites in WHO investigators, WHO has a limited ability to respond to outbreaks. Moreover, WHO has limited capability to investigate suspicious outbreaks before a country officially reports them.
|
|
BOX 5-5 Several factors appeared to facilitate a faster international reaction to SARS in comparison to other diseases in recent decades. Fear and Uncertainty. The rapid geographic spread of the mysterious illness created a sense of urgency to respond to a disease that seemed able to “go anywhere and hit anyone.” Stronger Leadership. The World Health Organization took a more public, activist stance in sounding the alarm and mobilizing the global response. Scientific Advances. New tools and techniques allowed researchers better and faster ways to study everything from patterns of lung damage to the genetic sequence of the coronavirus. Heightened Awareness of BW Threat. Concerns about the threat posed by biological weapons enhanced the ability and speed of many countries to identify new infectious diseases. Concern About Missing “Another” AIDS. Some health officials acknowledge they reacted more quickly to SARS partly due to fears that the world’s slow response in the 1980s to the emergence of HIV/AIDS allowed the disease to build up devastating momentum. |
-
Canada threatened those who violated quarantines with fines or court-ordered isolation after some people defied voluntary measures, but news reports indicate that some people violated quarantines when the SARS threat appeared to be fading.
-
Singapore’s strict quarantines proved particularly effective in bringing the disease under control.
Sometimes the most effective isolation and quarantine policies raised concerns about political freedom and human rights. For example, India and Thailand at one point isolated foreign visitors from countries that had SARS outbreaks, even though they did not have symptoms or known exposures.
-
North Korea, which has quarantined entire areas to deal with epidemics in the past, imposed such tight restrictions for SARS that it constrained some international aid flows.
Political Leadership
A key variable in managing the SARS epidemic was the willingness of political leaders to raise public awareness of the disease, focus resources, and speed the government response. As noted above, Vietnamese leaders promptly acknowl-
edged the SARS threat at an early stage in the outbreak and sought international help. In contrast, China’s political leaders clearly exacerbated the situation by initially suppressing news of the disease.
Reasons to Stay on Guard
Despite the downturn in cases, SARS has not been eradicated and remains a significant potential threat. Senior WHO officials and many other noted medical experts believe it highly likely that SARS will return. SARS, like other respiratory diseases such as influenza, may have subsided in the northern hemisphere as summer temperatures rise, only to come back in the fall.
-
Most infectious diseases follow a similar epidemiological curve, emerging, peaking, and declining over time to a steady state, but the number of infections, the lethality, and length of time can vary enormously.
-
Even as WHO officials removed the last of its travel advisories for SARS early this summer, officers repeatedly emphasized the risk that the disease would be back.
-
Some experts caution that SARS might even lay low for several years before reappearing, as diseases such as Ebola and Marburg have done.
-
The apparent reservoir of the coronavirus in animals, Beijing’s decision to lift the ban on sales of exotic animals, and lack of a reliable diagnostic kit, vaccine, or antiviral drug are factors that preclude eradication.
No Reliable Screening Tests
Diagnosis remains almost as much an art as a science as long as no proven screening test has been developed. Diagnostic kits currently under development can catch only about 70 percent of SARS cases, and their utility for widespread deployment is not yet known. SARS is difficult to detect, particularly in the early stages, even for countries with the most modern medical capabilities, raising the risk that healthcare workers will miss mild cases. Moreover, there is little prospect of a vaccine in the short-term.
Various countries have different definitions of suspected and probable cases and have changed the definitions over time.
SARS Could Mutate
Natural mutations in the coronavirus which causes SARS could alter basic characteristics of the disease, but whether a mutation would make SARS more or less dangerous is impossible to predict. A significant increase in the transmissibility or lethality of SARS obviously would pose greater health risks and raise fears around the globe.
-
Mutations could be particularly problematic if they alter the symptoms associated with SARS, making it harder to identify suspected cases.
-
Researchers are studying a group of Canadians who tested positive for the SARS virus last spring but never got sick in order to see if they still might have infected others.
-
Mutations also would complicate the development of a treatment or vaccine, which already probably is several years away.
Difficult to Maintain Vigilance
The willingness of healthcare workers to serve in the face of significant infection risks has been a key variable in the battle against SARS and other emerging diseases. Most healthcare workers in countries hit by SARS toiled long hours under dangerous conditions. The rate of infection among hospital workers was much higher than among the general public, underscoring the difficulty even professionals had in maintaining stringent infection control procedures.
-
At one point 20 percent of those infected in Hong Kong were nurses, and over 300 healthcare workers were infected within a 17-day period in China during April.
Some health workers refused to work in SARS wards. This problem is likely to grow in both rich and poor countries if the disease resurges.
-
In Taiwan, where over 90 percent of SARS infections occurred in hospitals, over 160 health workers quit or refused to work on SARS wards. The government threatened to revoke their professional licenses.
-
The Chinese government fired at least six doctors who refused to treat SARS patients and barred them from practicing for life. China also tried to encourage healthcare workers by launching public relations campaigns hailing the work of the Angels in White, and Beijing offered bonus pay and staffed SARS hospitals with Army medical staff.
-
Press reports in Canada indicate that some nurses refused to work in SARS wards in Toronto despite a doubling of their wages and lobbied for an official government inquiry on the handling of the epidemic. Shortages in trained healthcare personnel were exacerbated when many healthcare workers fell ill to SARS and were replaced by workers with less training.
-
Taiwan appeared so eager to declare victory over SARS that it relaxed its standards before the disease was brought under control. Press reports suggest that some health-care workers were so fatigued from the crisis that they cut corners.
-
Canadian officials acknowledge that the second outbreak in Toronto resulted from hospitals relaxing infection control regimes too quickly.
SARS Scenarios
Faced with these uncertainties, we have constructed three scenarios to consider potential trajectories for the disease and the implications for the United States. We have not attempted to identify a most likely scenario because the future course of SARS will depend on a host of complex variables, including the scope of present infections, mutations in the virus, the vulnerability of host populations, how individuals and governments respond, and chance.
Scenario One: SARS Simmers
SARS could resurface this fall but be limited to random outbreaks in a few countries. Rapid activation of local and international surveillance systems and isolation procedures would be key to identifying suspect cases and containing the spread. Initially, some cases might elude detection by hospital workers and airport personnel, who have relaxed screening procedures since the disease ebbed. Smaller, poorly funded transit facilities would remain vulnerable because they lacked trained staff and equipment to effectively monitor all passengers.
-
In most affected countries, the small number of cases and transmission would render SARS more of a public health nuisance than a crisis.
Some countries would be tempted to hide a resurgence. China’s experience demonstrated that hiding an outbreak is increasingly difficult and costly in a globalized world, but some governments still probably calculate that transparency also has drawbacks. Indeed, the economic repercussions of WHO travel advisories for SARS probably reinforce the incentives countries have to hide or under-report cases.
-
The WHO had to lean on Beijing throughout the crisis to share data.
-
Some countries over the past decade have not acknowledged HIV/AIDS cases in the military for security reasons, suggesting they would withhold information on other diseases that might affect readiness.
Even if new SARS outbreaks were sporadic and small-scale, economic, political, and psychological ripples would occur. China faces the biggest risks. Although foreign investors are unlikely to withdraw substantial amounts of FDI, firms with considerable exposure to China might redirect a percentage of new investment to other locations to diversify their manufacturing operations. Companies that already have temporarily shifted some production outside China probably would establish more permanent arrangements.
-
Companies and governments outside China probably would attempt to exploit these concerns by more aggressively trying to turn temporary production into longer-term investments.
Multinationals also are likely to become more concerned about the “SARS tax” on their businesses, including increased healthcare expenditures for expatriate employees and expanded insurance to cover the risk to operations and personnel from infectious diseases. Some firms probably would calculate that the risks of frequent business travel outweighed the costs and switch to teleconferencing, telecommuting, and e-commerce.
-
SARS has alerted companies to the potential operational disruptions caused by a contagious disease, risks that are rarely priced into business costs or considered in contingency planning.
-
Whereas previous business continuity plans focused on data protection and recovery, businesses probably will begin to consider plans that involve protection of human resources, backup teams, and alternate locations for operation.
Paradoxically, keeping SARS out of the United States might become more difficult as fewer cases are seen, because health, transportation, and security workers are more likely to drop their guard in monitoring for infected people if only a few cases pop up now and then.
-
The U.S. status as a major hub for international travel increases the statistical risk that lapses in surveillance abroad could facilitate the spread of SARS to American cities.
-
It is difficult for many visitors to acquire visas for travel to the United States; thus they probably would be inclined to withhold information that could complicate their visit.
Scenario Two: SARS Spreads to Poor Countries, Regions
SARS could gain a foothold in one or more poor countries, potentially generating more infections and deaths than before but with relatively little international economic impact. Few poor countries have had SARS appear on their doorstep up to now because most have relatively few links to the affected regions, but the longer the disease persists the more likely it is that SARS will spread more widely.
-
Impoverished areas of Africa, Asia, and Latin America remain at potential risk for SARS because of weak healthcare systems and vulnerable populations. Even a small number of cases in large, underdeveloped cities such as Dhaka, Kinshasa, or Lagos could generate a large number of victims in a short period.
-
No evidence thus far suggests that people with malaria or HIV/AIDS are
-
more susceptible to becoming infected by SARS, but experience indicates that diseases are more lethal among sick and malnourished populations. Sub-Saharan Africa has the highest concentration of HIV-infected people in the world, and those with full-blown AIDS have severely deficient immune response.
Most poor countries would have trouble organizing control measures against SARS, especially if the disease gained momentum before it was identified by healthcare workers. Most countries have inadequate hospital facilities to effectively isolate large numbers of patients, and most hospitals even lack the resources to provide food and care to patients.
-
Voluntary home quarantine might not be viable in crowded urban slums, where large families might share small dwellings and people might have to go out each day for food or work.
-
Identifying and tracking down people who might have been exposed probably would be substantially more difficult in countries with poor infrastructure and underfunded local security services.
-
Repressive countries, fearful that the disease could spark political upheaval, probably would quarantine entire towns or villages with military force or incarcerate quarantine violators. Outside countries and international organizations providing assistance are likely to split over how much to condemn or withhold aid over apparent human rights violations.
The spread of SARS into various poor countries is likely to significantly disrupt local economies while having relatively little impact on broader international markets.
-
The local impact could be worse than in places like Taiwan and Canada, because people in poor countries are living closer to the margin and governments have less resources for emergencies. In countries with a much smaller pool of skilled workers, the loss of key personnel can have a relatively large effect on society—as HIV/AIDS has illustrated in Africa.
-
Even poor countries like Bangladesh have at least some global trade and business links that could be disrupted if they were hit by SARS, but the more isolated the country, the smaller the global economic impact probably would be.
The spread of SARS to poor countries also would complicate international efforts to control the disease.
-
Diagnosing SARS is likely to be more difficult among populations with many preexisting health problems.
-
Even if SARS claimed hundreds of victims in poor countries, their gov
-
ernments probably would not be inclined to devote substantial resources to the fight when other diseases—such as malaria, tuberculosis, and HIV/AIDS—were claiming many more lives.
The spread of SARS to countries with weak healthcare systems and vulnerable populations also is likely to make the disease appear more transmissible and lethal, heightening public fears in other parts of the world:
-
Poor, isolated regions of Russia and China would have trouble containing an outbreak, although their governments probably could mobilize more resources to respond once infections began to climb.
-
Even if SARS outbreaks were limited to poor countries, the persistence of the disease probably would fuel some unease around the world about a broader resurgence. The impact probably would marginally decrease demand for travel and increase demand for medical products.
An outbreak of SARS in poor countries would pose particular challenges for the United States and other governments and multilateral organizations providing assistance. WHO and CDC probably would come under pressure to provide money and technical assistance to compensate for weak healthcare systems. The higher the number of infected people, the more the international community would be called on to do something.
-
Neighboring countries are likely to press for help with disease monitoring to prevent SARS from spreading into their countries, especially if panic began generating refugee flows.
-
Repressive regimes like North Korea might accept material assistance but block outside experts from visiting, even at the risk of putting more of their own citizens at risk. North Korea in previous years has been accused of diverting NGO assistance to the military and not allowing outsiders to monitor how it is used.
Scenario Three: SARS Resurges in Major Trade Centers
SARS could stage a comeback this fall in the main places it hit before—such as China, Hong Kong, Taiwan, and Canada—or gain a foothold in other places with extensive international travel and trade links like the United States, Japan, Europe, India, or Brazil.
-
An outbreak almost certainly would spark another wave of WHO health warnings and travel advisories; Japan already has canceled an international conference on HIV/AIDS planned for this winter due to fears it would coincide with a resurgence of SARS.
Even if the number of infected persons were not greater in a second wave, an outbreak of SARS in major trade centers again would be likely to have significant economic and political implications. The resurgence of SARS in Asia probably would cause less disruption as citizens, companies, and governments learn to live with it, as they do with other diseases, unless the transmissibility or lethality rose substantially. Nonetheless, a second wave of SARS in Asia probably would prompt some multinationals to modestly reduce their exposure to the region if they concluded that SARS posed a long-term health challenge.
-
Given the size of the Asian market and low wage-rates, few companies are likely to yank existing production out of China unless SARS debilitates or kills large numbers of workers. Firms probably would divert some future investments to other regions to diversify their supply chains.
-
Disruptions due to SARS are likely to persuade some companies to loosen just-in-time production chains by creating some cushion in key inventories, increasing costs but not productivity.
-
Global trade and investment flows could seize up if quarantines shut down factories and shipments.
A substantial decline in China’s manu-facturing sector would reverberate in Southeast Asian economies that provide critical manufactured inputs, raw materials, and energy and disrupts production chains throughout East Asia.
Bigger outbreaks in places such as Europe and the United States would affect new sets of business and government players. The level of public fear almost certainly would be higher in places that had not been affected by the first wave of SARS, driving up social disruption and economic costs.
-
The economic cost of SARS probably would skyrocket if fears grew about the transmission of the disease in planes or on objects.
-
Some buyers this spring demanded that Asian manufacturers irradiate their export goods after research indicated that SARS could survive for several days on inanimate objects.
Even the health systems of rich countries could be overwhelmed if the resurgence of SARS cases coincided with the annual influenza epidemic this winter. As long as no quick and reliable test to diagnose SARS exists, people with fevers and a cough could overwhelm hospitals and clinics as healthcare workers struggled to distinguish patients with SARS and isolate them from others.
-
A pneumonia-like illness erupted in western Canada in mid-August, raising questions among health experts about whether a milder version of SARS had returned.
-
Surges of people seeking medical care almost certainly would increase the odds of healthcare workers missing some cases.
-
Some SARS patients have not displayed classic respiratory symptoms, suggesting some “silent” spreaders may not even know they have the disease, and some travelers with mild symptoms might lie about contact with infected persons to avoid quarantine.
Given the high economic and political stakes already seen in the SARS epidemic, some jurisdictions probably would try to fudge health data in an effort to avoid official health warnings or get them lifted more quickly.
-
Some governments might narrow the definition of “probable” SARS cases to reduce crowding in hospitals, yet such moves could spark tensions with WHO and other countries over the accuracy of data.
Building Better Defenses Against Disease
The emergence of SARS has sparked widespread calls for greater international surveillance and cooperation against such diseases. SARS has demonstrated to even skeptical government leaders that health matters in profound social, economic, and political ways.
|
BOX 5-6 Influenza is an ideal virus for worldwide spread (a pandemic) and many epidemilogists argue that the world is “overdue” for a major influenza pandemic. When a new type of flu virus emerges from a reassortment of animal and human viruses to which humans have no prior immunity, a pandemic may ensue. Scientists believe the past two influenza pandemics originated in China where people live in close contact with birds and swine, the major sources of animal flu viruses. Influenza spreads even more quickly than SARS because flu can be transmitted efficiently through the air. As a result, close contact is not required for people to become infected, making it almost impossible to trace and isolate ill people who are spreading the disease. Three major flu epidemics stand out in modern U.S. history:
|
-
The experience with SARS probably will help countries prepare for future disease outbreaks.
This intense focus on SARS has opened a window of opportunity to pursue bilateral and international cooperation against infectious diseases. The United States and WHO may be able to develop new institutional channels to foster long-term cooperation on health issues.
-
Momentum is likely to flag if SARS continues to subside and political leaders lose interest.
-
Budget constraints and turf battles almost certainly will retard progress and agreements may fail to be implemented at the provincial, state and local levels if added responsibilities are not accompanied by additional funding.
Areas of Need
Several countries already are seeking assistance from the WHO and the U.S. CDC in an effort to strengthen their health systems. Some even are moving to commit more resources.
-
Both China and Taiwan have held technical discussions with US officials exploring ways to improve their health system, and Beijing publicly has committed $1.3 billion in new funds.
Surveillance
Despite substantial progress in recent decades in building networks to monitor disease, the surveillance systems in most countries remain weak. Many surveillance systems have been built over the years to detect specific diseases, such as polio and guinea worm. The WHO also has created a global network of over 100 centers in 83 countries to track influenza. The longer-term challenge is to build networks throughout countries and regions and the means to issue warnings to national and international authorities.
-
Systems focusing on specific diseases generally have been more cost effective than trying to increase surveillance for all diseases, but either approach leaves holes.
-
International surveillance networks also must work out differences between countries over what health patterns are “normal” and which should set off alarm bells. The death of working-age pneumonia patients in the United States would be so unusual it would trigger closer examination, but this phenomenon probably was not considered abnormal in China in the early stage of SARS.
|
BOX 5-7 The SARS outbreak illustrates the difficulty in distinguishing the emergence of new infectious disease from the release of a BW agent. Ongoing efforts to improve global health surveillance, however, probably will aid international monitoring for detecting the possible release of biological warfare agents, especially traditional types. As baselines for natural diseases are established in the coming years, a deliberate release of traditional BW agents could be more readily recognized. Unfortunately, many developing countries probably will not acquire domestic detection capabilities, such as tools to identify genetic sequences in disease organisms. Moreover, history suggests that some countries will not support internal disease surveillance efforts for political or economic reasons, leaving significant gaps in a global surveillance system. |
-
Even if local health workers identify worrisome developments, many medical facilities in developing countries lack communications equipment and vehicles to alert national officials and transport samples or patients.
-
Although rapid online journal publication aided in sharing information on the new SARS virus, outbreak responders need to share data even earlier.
Epidemiological Expertise
Many countries lacked trained experts to map the trajectory of SARS. Such expertise was critical to understanding the transmissibility, lethality, and scope of the disease.
-
Press reports indicate that Chinese officials have had trouble processing and sharing research information within China and with outsiders, such as WHO.
Laboratory Facilities
Few countries have the sophisticated laboratories or trained personnel to do the hard science of cracking mysterious new illnesses. As a result, regional or mobile labs may be the most viable prospect for speeding up diagnoses and research.
-
WHO reports that staff in over 90 percent of developing country laboratories are not familiar with quality assurance principles, and 60 percent of the lab equipment is inoperable or outdated.
Equipment
The cost of basic diagnostic and protective equipment is relatively modest yet still unaffordable for many countries. SARS highlighted a widespread shortage of ventilators to support patients with pneumonia. The lack of adequate sterilization equipment raises the risk of spreading disease when medical instruments are reused.
-
The highest priority for many countries is likely to be diagnostic tests to determine which patients need to be isolated; the need for such tests would be all the more pressing if research indicates SARS can be transmitted through the blood supply.
|
BOX 5-8 SARS has focused greater international attention on the importance of health, but the new disease probably will not lead to a significant boost in the fight against HIV/AIDS in the coming years. Indeed, many countries are likely to view spending on diseases like SARS and HIV/AIDS as a zero-sum game in the short term.
China’s new health minister has said she plans to focus on HIV/AIDS now that SARS has subsided, according to press reports. Some AIDS activists and NGOs within China also have expressed hope that the government response to SARS will translate into more action on HIV/AIDS. A resurgence of SARS this winter could delay activity on AIDS, and some AIDS activists in China fear the government might believe the stringent controls used to fight SARS should be used against HIV/AIDS as well. |
-
Many countries need more ventilators to support patients with pneumonia. In addition, negative pressure rooms to isolate infected patients are in shorter supply; even many hospitals in affluent countries are not likely to have enough rooms to handle a serious outbreak.
Developing Countermeasures
Progress in developing diagnostic tests, treatments, and vaccines would fundamentally improve prospects for combating SARS. This will take time, however, and first-generation products often are not completely effective without further research and improvement.
-
Tracking down infected and exposed persons on airline flights also could be improved significantly if airlines retained electronic records of passenger lists.
Political Hurdles
Almost all countries will express support for improving international healthcare capabilities, but negotiations are likely to be contentious, and many players will see this as an opportunity to win concessions or score points with Washington. Some areas of possible contention are:
-
Money. Many developing countries will say they cannot improve their surveillance systems and healthcare infrastructure without significant outside assistance, in the form of training, equipment, or grants.
-
“Rich” vs. “poor” Diseases. Some developing countries may argue that they will work to improve surveillance for diseases like SARS if the United States and the international community do more to help them fight diseases which claim more lives in their countries, such as malaria and tuberculosis.
-
Multilateral Channels. European countries are likely to use the focus on health issues to renew pressure on the United States to work through multilateral organizations such as the Global Fund for AIDS, Tuberculosis, and Malaria.
-
Pharmaceutical Access. Any forum to discuss international health cooperation almost certainly will include some criticism of U.S. positions in the WTO on pharmaceutical sales. Research to develop tests, treatments, and vaccines is underway, but drug companies will have little incentive to bring such products to market without public sector support if SARS appears to fade away.
-
WHO Authority. Some countries probably will argue for strengthening the authority of the WHO to sanction states that do not share health data or bar outside health experts from visiting. Other countries, such as China and Malaysia, are likely to resist any moves they see as infringing on sovereignty. Taiwan almost certainly will continue trying to use health issues to win recognition from WHO and other multilateral organizations.
REFERENCES
Abbott A. 2003a. Human fatality adds fresh impetus to fight against bird flu. Nature 423(6935):5.
Abbott A. 2003b. Nature 423:5.
Asia-Pacific Economic Corporation. 2001. Infectious Diseases in the Asia Pacific Region: A Reason to Act and Acting with Reason. 22nd Industrial Science and Technology Working Group Strategy Paper. Singapore: Asia-Pacific Economic Corporation.
Augustine JJ. 2003. Developing a highly contagious disease readiness plan: the SARS experience. Emergency Medical Services 32(7):77-83.
Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, Walmsley SL, Mazzulli T, Avendano M, Derkach P, Ephtimios IE, Kitai I, Mederski BD, Shadowitz SB, Gold WL, Hawryluck LA, Rea E, Chenkin JS, Cescon DW, Poutanen SM, Detsky AS. 2003. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 289(21):2801-9.
Breiman RF, Evans MR, Preiser W, Maguire J, Schnur A, Li A, Bekedam H, MacKenzie JS. 2003. Role of China in the quest to define and control severe acute respiratory syndrome. [Review] [16 Refs]. Emerging Infectious Diseases 9(9):1037-41.
CDC. 2003a. Update: severe acute respiratory syndrome—worldwide and United States, 2003. MMWR–Morbidity & Mortality Weekly Report 52(28):664-5.
Chan JW, Ng CK, Chan YH, Mok TY, Lee S, Chu SY, Law WL, Lee MP, Li PC. 2003. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS). Thorax 58(8):686-9.
Chen Q. 2003. [Application of SIR model in forecasting and analyzing for SARS]. Beijing Da Xue Xue Bao 35(Suppl.):75-80.
Chinese Center for Disease Control and Prevention. 2003. Overview of the epidemics and repsonses to the severe acute respiratory syndrome (SARS) in the People’s Republic of China, Beijing.
Chowell G, Fenimore PW, Castillo-Garsow MA, Castillo-Chavez C. 2003. SARS outbreaks in Ontario, Hong Kong and Singapore: the role of diagnosis and isolation as a control mechanism. Journal of Theoretical Biology 224(1):1-8.
Department of State. 2003. Global mercury: an international bioterrorism exercise. Media Note. Available: http://www.state.gov/pa/prs/ps/2003/23878.htm.
Donnelly CA, Ghani AC, Leung GM, Hedley AJ, Fraser C, Riley S, Abu-Raddad LJ, Ho LM, Thach TQ, Chau P, Chan KP, Lam TH, Tse LY, Tsang T, Liu SH, Kong JH, Lau EM, Ferguson NM, Anderson RM. 2003. Epidemiological Determinants of Spread of Causal Agent of Severe Acute Respiratory Syndrome in Hong Kong. May 24, 2003. [Erratum Appears in Lancet 361(9371):1832]. Lancet 361(9371):1761-6.
Drazen JM, Campion EW. 2003. SARS, the Internet, and the journal. [See Comment]. New England Journal of Medicine 348(20):2029.
Dye C, Gay N. 2003. Epidemiology: modeling the SARS epidemic. Science 300(5627):1884-5.
Enserink M. 2003a. SARS in China. China’s missed chance. Science 301(5631):294-6.
Enserink M. 2003b. Infectious diseases: Avian flu outbreak sets off alarm bells. Science 300(5620):718.
Eysenbach G. 2003. SARS and population health technology. Journal of Medical Internet Research 5(2):e14.
Fedson DS. 2003. Pandemic influenza and the global vaccine supply. Clinical Infectious Diseases 36(12):1552-61.
Ferguson NM, Mallett S, Jackson H, Roberts N, Ward P. 2003. A population-dynamic model for evaluating the potential spread of drug-resistant influenza virus infections during community-based use of antivirals. Journal of Antimicrobial Chemotherapy 51(4):977-90.
Gerdil C. 2003. The annual production cycle for influenza vaccine. Vaccine 21(16):1776-9.
Grein TW, Kamara K-BO, Rodier G, Plant AJ, Bovier P, Ryan MJ, Ohyama T, Heymann DL. 2000. Rumors of disease in the global village: outbreak verification. Emerging Infectious Diseases 6(2):97-102.
Han H, Li X, Qu W, Shen T, Xu F, Gao D. 2003. [The regression analysis of the in hospital medical staffs infection rate and the application of the isolation measures including the emergency-isolation radiology information system]. Beijing Da Xue Xue Bao 35(Suppl.):89-91.
Hatta M, Gao P, Halfmann P, Kawaoka Y. 2001. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 293(5536):1840-2.
He WQ, Chen SB, Liu XQ, Li YM, Xiao ZL, Zhong NS. 2003. Death risk factors of severe acute respiratory syndrome with acute respiratory distress syndrome. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 15(6):336-7.
Heymann DL, Rodier GR, WHO Operational Support Team to the Global Outbreak Alert and Response, Network. 2001. Hot spots in a wired world: who surveillance of emerging and re-emerging infectious diseases. [Review] [44 Refs]. The Lancet Infectious Diseases 1(5):345-53.
Hoffmann E, Krauss S, Perez D, Webby R, Webster RG. 2002. Eight-plasmid system for rapid generation of influenza virus vaccines. Vaccine 20(25-26):3165-70.
Hsueh PR, Hsiao CH, Yeh SH, Wang WK, Chen PJ, Wang JT, Chang SC, Kao CL, Yang PC, SARS Research Group of National Taiwan University College of Medicine and, National Taiwan University Hospital. 2003. Microbiologic characteristics, serologic responses, and clinical manifestations in severe acute respiratory syndrome, Taiwan. Emerging Infectious Diseases 9(9):1163-7.
Hupert N, Cuomo J. 2003. BERM: The Weill/Cornell Bioterrorism and Epidemic Outbreak Response Model. [Online] Available: http://www.ahrq.gov/research/biomodel.htm [accessed September 17, 2003].
Hupert N, Mushlin AI, Callahan MA. 2002. Modeling the public health response to bioterrorism: using discrete event simulation to design antibiotic distribution centers. Medical Decision Making 22(5 Suppl.):S17-S25.
IOM (Institute of Medicine). 1992. Emerging infections: microbial threats to health in the United States. Washington, DC: National Academy Press.
IOM. 2003. Microbial threats to health: emergence, detection, and response. Washington, DC: The National Academies Press.
ITU Internet Report. Birth of broadband. [Online] Available: http://www.itu.net [accessed September 17, 2003].
Kilbourne ED. 1969. Future influenza vaccines and the use of genetic recombinants. Bulletin of the World Health Organization 141(3):643-5.
Kimball AM, Horwitch CA, O’Carroll PW, Arjoso S, Kunanusont C, Lin YS, Meyer CM, Schubert LE, Dunham PL. 1999. The Asian Pacific Economic Cooperation emerging infections network. American Journal of Preventive Medicine 17(2):156-8.
Kimball AM, Pautler NF. 2003. Lessons of SARS, The APEC-EINET experience. [Online] Available: http://apec.org/infectious/SARS_Lessons.pdf.
Kitch P, Yasnoff WA. 2002. Assessing the value of information systems. In: Gotham I, Smith P, Birkhead S, Davisson MC. Policy issues in developing information systems for public health surveillance systems of communicable diseases. In: O’Carroll P, Yasnoff WA, Ward ME, Ripp LH, Martin EL, eds., Public Health Informatics and Information Systems. New York: Springer Verlag.
Koopmans M, Fouchier R, Wilbrink B, Meijer A, Natrop G, Osterhaus ADME, van Steenbergen JE, du Ry van Beest Holle M, Conyn van Spaendonck MAE, Bosman A. 2003. Update on human infections with highly pathogenic avian influenza virus A/H7N7 during an outbreak in poultry in the Netherlands. Eurosurveillance Weekly 7(18).
Krug RM. 2003. The potential use of influenza virus as an agent for bioterrorism. Antiviral Research 57(1-2):147-50.
Li L, Wang Z, Lu Y, Bao Q, Chen S, Wu N, Cheng S, Weng J, Zhang Y, Yan J, Mei L, Wang X, Zhu H, Yu Y, Zhang M, Li M, Yao J, Lu Q, Yao P, Bo X, Wo J, Wang S, Hu S. 2003. Severe acute respiratory syndrome-associated coronavirus genotype and its characterization. Chinese Medical Journal 116(9):1288-92.
Lin G, Jia X, Ouyang Q. 2003. [Predict SARS infection with the small world network model]. Beijing Da Xue Xue Bao 35(Suppl.):66-9.
Lipsitch M, Cohen T, Cooper B, Robins JM, Ma S, James L, Gopalakrishna G, Chew SK, Tan CC, Samore MH, Fisman D, Murray M. 2003. Transmission dynamics and control of severe acute respiratory syndrome. Science 300(5627):1966-70.
Liu M. et al. 2003. Virology 314:589.
Lloyd-Smith JO, Galvani AP, Getz WM. 2003. Curtailing transmission of severe acute respiratory syndrome within a community and its hospital. Proceedings of the Royal Society of London. Series B, Biological Sciences 270(1528):1979-89.
Matrosovich MN, Krauss S, Webster RG. 2001. H9N2 influenza A viruses from poultry in Asia have human virus-like receptor specificity. Virology 281(2):156-62.
Olsen CW. 2002. The emergence of novel swine influenza viruses in North America. Virus Research 85(2):199-210.
Pearson H, Clarke T, Abbott A, Knight J, Cyranoski D. 2003. SARS: what have we learned? Nature 424(6945):121-6.
Peiris JS, Guan Y, Markwell D, Ghose P, Webster RG, Shortridge KF. 2001. Cocirculation of avian H9n2 and contemporary human H3n2 influenza a viruses in pigs in Southeastern China: potential for genetic reassortment? Journal of Virology 75(20):9679-86.
Peiris M, Yuen KY, Leung CW, Chan KH, Ip PL, Lai RW, Orr WK, Shortridge KF. 1999. Human infection with influenza H9N2. Lancet 354(9182):916-7.
ProMED-Mail. May 14, 2003. Influenza H5N1, avian-China: suspected. ProMED-mail. [Online] Available: http://www.promedmail.org [accessed January 17, 2004].
Riley S, Fraser C, Donnelly CA, Ghani AC, Abu-Raddad LJ, Hedley AJ, Leung GM, Ho LM, Lam TH, Thach TQ, Chau P, Chan KP, Lo SV, Leung PY, Tsang T, Ho W, Lee KH, Lau EM, Ferguson NM, Anderson RM. 2003. Transmission dynamics of the etiological agent of SARS in Hong Kong: impact of public health interventions. Science 300(5627):1961-6.
Riley S, Fraser C, Donnelly CA, Ghani AC, Abu-Raddad LJ, Hedley AJ, Leung GM, Ho LM, Lam TH, Thach TQ, Chau P, Chan KP, Lo SV, Leung PY, Tsang T, Ho W, Lee KH, Lau EM, Ferguson NM, Anderson RM. 2003. Transmission dynamics of the etiological agent of SARS in Hong Kong: impact of public health interventions. [See Comment]. Science 300(5627):1961-6.
Roscelli JD, Bass JW, Pang L. 1991. Guillain-Barré syndrome and influenza vaccination in the U.S. Army, 1980-1988. [See Comment]. American Journal of Epidemiology 133(9):952-5.
Ruan YJ, Wei CL, Ee AL, Vega VB, Thoreau H, Su ST, Chia JM, Ng P, Chiu KP, Lim L, Zhang T, Peng CK, Lin EO, Lee NM, Yee SL, Ng LF, Chee RE, Stanton LW, Long PM, Liu ET. 2003. Comparative full-length genome sequence analysis of 14 SARS coronavirus isolates and common mutations associated with putative origins of infection. Lancet 361(9371):1779-85.
Safranek TJ, Lawrence DN, Kurland LT, Culver DH, Wiederholt WC, Hayner NS, Osterholm MT, O’Brien P, Hughes JM. 1991. Reassessment of the association between Guillain-Barré syndrome and receipt of swine influenza vaccine in 1976-1977: results of a two-state study. Expert Neurology Group. American Journal of Epidemiology 133(9):940-51.
Schabas RE. 2001. Mass influenza vaccination in Ontario: a sensible move. CMAJ 164(1):36-7.
Schickli JH, Flandorfer A, Nakaya T, Martinez-Sobrido L, Garcia-Sastre A, Palese P. 2001. Plasmid-only rescue of influenza A virus vaccine candidates. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 356(1416):1965-73.
Sheldon T. 2003. Vet dies from pneumonia in avian flu case. British Medical Journal 326(7396):952.
Shi Y. 2003. Stochastic dynamic model of SARS spreading. Chinese Science Bulletin 48(13):1287-92.
Steinhauer DA. 1999. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology 258(1):1-20.
Stohr K. 2003. The global agenda on influenza surveillance and control. Vaccine 21(16):1744-8.
Subbarao K, Chen H, Swayne D, Mingay L, Fodor E, Brownlee G, Xu X, Lu X, Katz J, Cox N, Matsuoka Y. 2003. Evaluation of a genetically modified reassortant H5N1 influenza A virus vaccine candidate generated by plasmid-based reverse genetics. Virology 305(1):192-200.
Suzuki S, Ohyama T, Taniguchi K, Kimura M, Kobayashi J, Okabe N, Sano T, Kuwasaki T, Nakatani H. 2003. Web-based Japanese syndromic surveillance for FIFA World Cup 2002. Section II: Event-Based Syndromic Surveillance Systems, Journal of Urban Health: Bulletin of the New York Academy of Medicine. 80(2)(Suppl.) 1:i123.
Treanor J. 2004. Influenza vaccine—outmaneuvering antigenic shift and drift. New England Journal of Medicine 350(3):218-20.
Tumpey TM, Suarez DL, Perkins LE, Senne DA, Lee JG, Lee YJ, Mo IP, Sung HW, Swayne DE. 2002. Characterization of a highly pathogenic H5N1 avian influenza A virus isolated from duck meat. Journal of Virology 76(12):6344-55.
van Kolfschooten F. 2003. Dutch veterinarian becomes first victim of avian influenza. Lancet 361(9367):1444.
Wang D, Zhao X. 2003. [Empirical analysis and forecasting for SARS epidemic situation]. Beijing Da Xue Xue Bao 35(Suppl.):72-4.
Webby RJ, Webster RG. 2003. Are we ready for pandemic influenza? [Review] [37 Refs]. Science 302(5650):1519-22.
WHO Global Influenza Programme. In press.
Wood JM. 2001. Developing vaccines against pandemic influenza. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 356(1416):1953-60.
Wood JM, Williams MS. 1998. In: Nicholson KG, Webster RG, Hay AJ, eds., Textbook of Influenza. Oxford, UK: Blackwell Science. Pp. 317-23.
Woolhouse MEJ, Dye CE. 2001. Population biology of emerging and re-emerging pathogens. Philosophical Transactions of the Royal Society for Biological Sciences (356):981-2.
WHO (World Health Organization). 2003a. Influenza A(H5N1) in Hong Kong Special Administrative Region of China. [Online] Available: http://www.who.int/csr/don/2003_2_19/en/ [accessed January 17, 2004].
WHO. 2003b. Avian influenza in the Netherlands. [Online] Available: http://www.who.int/csr/don/2003_04_24/en/ [accessed January 17, 2004].
WHO. 2003c. Visit of WHO Team to Review the Outbreak of Atypical Pneumonia in Guandong Province, March 24-April 9, 2003, Final Report, April 30, 2003.
WHO. 2003d. Alert, verification and public health management of SARS in the post-outbreak period. [Online] Available: http://www.who.int/csr/sars/postoutbreak/en/ [accessed January 15, 2004].
WHO. 2003e. Revision of the International Health Regulations. [Online] Available: http://www.who.int/gb/EB_WHA/PDF/WHA56/ea56r28.pdf [accessed January 18, 2004].
WHO. 2003f. Severe Acute Respiratory Syndrome. [Online] Available: http://www.who.int/gb/EB_WHA/PDF/WHA56/ea56r29.pdf [accessed January 18, 2004].
WHO Multicentre Collaborative Network for Severe, Acute Respiratory Syndrome Diagnosis. 2003. A Multicentre Collaboration to Investigate the Cause of Severe Acute Respiratory Syndrome. Lancet 361(9370):1730-3.
Zhou NN, Senne DA, Landgraf JS, Swenson SL, Erickson G, Rossow K, Liu L, Yoon K, Krauss S, Webster RG. 1999. Genetic reassortment of avian, swine, and human influenza A viruses in American pigs. Journal of Virology 73(10):8851-6.