2
Compositional Analysis
The keystone of compositional analysis of bullet lead (CABL) is the analytical method. Before bullet matching, statistical analysis, or legal interpretation, the concentrations of elements in the bullet lead must be measured correctly. Any good analytical method relies on correct sample preparation, fitness of the instrument for the purpose, proper use of the instrumentation, and reliability. Proper documentation and transparency of the method are also necessary. Those topics are discussed in greater detail in this chapter.
PREVIOUS INSTRUMENTAL METHODS
Historically, a number of instrumental methods have been used for the determination of elements in lead, including atomic absorption spectrometry (AAS),1 neutron activation analysis (NAA)2 spark source mass spectrometry (SSMS),3 wavelength dispersive x-ray fluorescence (WDXRF) spectroscopy,4
inductively coupled plasma-optical emission spectroscopy (ICP-OES),5 and inductively coupled plasma-mass spectrometry (ICP-MS).6 (The references cited in this paragraph are intended to document the historical progression of the analysis technique, and are not intended to represent the state of the art of current technology.)
Based on committee member’s own expertise and knowledge of these techniques and familiarity with the recent literature, each of those instrumental methods has advantages and disadvantages. AAS is a single-element technique (one element at a time can be measured) that is limited in the overall number of elements that can be determined, although the elements of current interest for CABL can be determined. It also suffers from limited dynamic (working) range and is prone to interferences due to the sample matrix. NAA requires ready access to a nuclear reactor. SSMS has an advantage in that it requires minimal sample preparation; however, reliable quantitative analysis with SSMS is difficult. SSMS instrumentation also is not widely available. WDXRF spectroscopy suffers from inadequate limits of detection and has been used primarily for qualitative or semi-quantitative analysis.
ICP-MS has a sensitivity advantage over optical techniques, such as AAS and ICP-OES, and has a greater dynamic range than AAS. The major drawback of ICP-MS is that the lead sample matrix can suppress the element signals and can deposit on the sampling cone; this reduces ion throughput and yields erratic results.7 That drawback can be avoided by precipitating the lead with sulfuric acid before ICP-MS analysis. However, the added precipitation step increases overall sample preparation time and lowers the precision and accuracy of the element measurements.
INDUCTIVELY COUPLED PLASMA-OPTICAL EMISSION SPECTROPHOTOMETRY
The analytical characteristics of ICP-OES make it a useful technique for metal determinations.8 A typical ICP-OES instrument has the following components:
-
Sample introduction system (nebulizer).
-
Torch assembly.
-
High-frequency generator.
-
Transfer optics and spectrometer.
-
Detector(s).
-
Computer interface.
For analysis, samples generally are dissolved to form an aqueous solution of known weight and dilution. The solution is aspirated into the nebulizer, which transforms it into an aerosol. The aerosol then proceeds into the plasma, it is transformed into atoms and ions in the discharge, and the atoms (elements) are excited and emit light at characteristic wavelengths. The intensity of the light at the wavelengths associated with each element is proportional to that element’s concentration.
The ICP-OES torch consists of three concentric tubes—known as the outer, middle, and inner tubes—usually made of fused silica. The torch is positioned in a coil of a radio-frequency generator. The support gas that flows through the middle annulus, argon, is seeded with free electrons that gain energy from the radio-frequency field. The energized electrons collide with the argon gas and form Ar+ ions. Continued interaction of the electrons and ions with the radio-frequency field increases the energy of the particles and forms and sustains a plasma, a gas in which some fraction of the atoms are present in an ionized state. At the same time, the sample is swept through the inner loop by the carrier gas, also argon, and is introduced into the plasma, allowing the sample to become ionized and subsequently emit light.
Temperatures in the plasma are typically 6,000–10,000 K.9 To prevent a possible short circuit and meltdown, the plasma must be insulated from the rest of the instrument. Insulation is achieved by the flow of the outer gas, typically argon or nitrogen, through the outer annulus of the torch. The outer gas sustains the plasma and stabilizes the plasma position.
Each element emits several specific wavelengths of light in the ultravioletvisible spectrum that can be used for analysis. The selection of the optimal wavelength for a sample depends on a number of factors, such as the other elements present in the sample matrix. The light emitted by the atoms of an element must be converted to an electric signal that can be measured quantitatively. That is achieved by resolving the light with a diffraction grating and then using a solid-state diode array or other photoelectric detector to measure wavelength-specific intensity for each element emission line. The concentration of the elements in the sample is determined by comparing the intensity of the emission signals from the sample with that from a solution of a known concentration of the element (standard).
TABLE 2.1 Summary of Elemental Analysis Techniques
|
Technique |
Advantages |
Disadvantages |
|
AAS |
Low detection limits |
Few elements, time-consuming, matrix effects |
|
NAA |
Low detection limits |
Few elements, requires access to reactor |
|
SSMS |
Low detection limits, multiple elements |
Difficult quantification, surface-sensitive |
|
WDXRF |
Multiple elements, solid and liquid samples |
Detection limits too high |
|
ICP-MS |
Low detection limits, multiple elements, isotope analysis |
Matrix effects |
|
ICP-OES |
Low detection limits, multiple elements, limited spectral interferences, good stability, low matrix effects |
Liquid samples only |
One of the main advantages of ICP-OES for elemental analysis is that it can be used to measure almost all the elements in the periodic table. The technique has a wide dynamic concentration range and can measure elements at trace to high concentrations. Detection limits for most elements are in the range of micrograms per liter to milligrams per liter. Another advantage of ICP-OES is that multielemental quantitative analysis can be carried out in a period as short as 1 min with a small amount of solution (0.5–1.0 mL). Those characteristics make ICP-OES a useful method for elemental analysis in forensic laboratories. ICP-OES is a technique that combines good quantitative multielement capability, wide linear dynamic ranges, good sensitivity, limited spectral and chemical interferences, low detection limits, and speed and ease of data handling and reporting with widespread (multiple-vendor) instrument availability and reasonable cost. Table 2.1 summarizes the advantages and disadvantages of ICP-OES and other elemental analysis techniques.
The Federal Bureau of Investigation (FBI) has been conducting bullet lead analysis for over 30 years. Initially, NAA was used to quantify three elements—antimony (Sb), copper (Cu), and arsenic (As)—in bullet lead. The FBI began to use ICP-OES in place of NAA in 1990, and over a period of several years expanded the list of elements to seven: arsenic, antimony, tin (Sn), copper, bismuth (Bi), silver (Ag), and cadmium (Cd).
CURRENT FBI PROTOCOL
The “Principle and Scope” section of the current FBI procedure, Comparative Elemental Analysis of Firearms Projectile Lead by ICP-OES,10 reads as follows:
The concentrations of selected elements in the lead portion of bullets, shot pellets, and similar firearms projectiles serve to chemically characterize the source of lead. Some chemical elements present in these leads are intentionally specified and/or added by the ammunition manufacturer (e.g., antimony and arsenic). Other chemical elements typically found in these leads are present as unspecified contaminants (e.g., copper, tin, bismuth, and silver). Distinct and subtle differences in the concentrations of manufacturer controlled elements and uncontrolled trace elements provide a means of differentiating among the leads of different manufacturers, among the leads in individual manufacturers’ product lines, and among specific batches of lead used in the same product line of a manufacturer.
This procedure [ICP-OES] provides a method for determining and comparing the concentrations of seven elements: antimony, copper, arsenic, silver, tin, bismuth, and cadmium in the lead component of projectiles. Quantitative analysis is performed by dissolving the specimen and using the method of ICP-OES for measurement of individual element concentrations. Quantitation is achieved by comparison of specimens with a certified bullet lead reference standard ([National Institute of Standards and Technology Standard Reference Material] C2416).
The current FBI procedure is not documented in a complete and detailed format that would allow other laboratories skilled in the art to practice or even fully evaluate it. The “Principle and Scope” section of the documented procedure should be expanded to define the precision and accuracy of the method and the concentration ranges of all seven elements for which the method is applicable. Some precision data on the ICP-OES analytical method were presented in two FBI publications from 1988 and 199111 and are shown below in Tables 2.2 and 2.3. The published precision data, precision data from crime-scene and suspect bullet samples, and other, newer precision data more reflective of the current FBI CABL procedure should be included in the written protocol. The protocol should also describe how precision differs in the low, middle, and high ranges of each element’s measurable concentrations.
The accuracy of the ICP-OES method was addressed by Schmitt et al.12 and in an FBI publication from 1991.13 Good statistical correlation was shown by Schmitt et al. between NAA and ICP-OES results for Cu and Sb.
The FBI’s analytical procedure calls for three 60-mg samples (named a, b, and c at random) to be taken from each lead specimen through cutting. Representatives of the FBI informed the committee that each set of samples includes two calibration standards prepared from Standard Reference Material (SRM) C2416. Control samples derived from SRM C2416 (bullet lead), SRM C2415
TABLE 2.2 Within-Bullet Variability Measurements Based on ICP-OES
|
Brand |
Variabilitya |
As |
Sb |
Sn |
Cu |
Bi |
Ag |
|
CCI |
RSD, % |
NA |
1.7 |
NA |
1.7 |
3.8 |
1.9 |
|
|
Range, ppm |
|
23,800–29,900 |
|
97–381 |
56–180 |
18–69 |
|
Federal |
RSD, % |
3.7 |
1.5 |
2.5 |
1.5 |
6.7 |
2.3 |
|
|
Range, ppm |
1,127–1,645 |
25,700–29,000 |
1,100–2,880 |
233–329 |
30–91 |
14–19 |
|
Remington |
RSD, % |
NA |
1.5 |
NA |
1.5 |
3.4 |
1.8 |
|
|
Range, ppm |
|
5,670–9,620 |
|
62–962 |
67–365 |
21–118 |
|
Winchester |
RSD, % |
NA |
1.9 |
NA |
2.1 |
4.4 |
1.9 |
|
|
Range, ppm |
|
2,360–6,650 |
|
54–470 |
35–208 |
14–61 |
|
Note: RSD is relative standard deviation. NA indicates the data are not available because concentrations are too low to be accurately determined. aMean relative standard deviations of triplicate measurements of each bullet and the range in concentrations for all bullets of each brand examined in Peele et al. 1991; 10 bullets per brand were analyzed in triplicate. Source: Table adapted from Peele et al., 1991. |
|||||||
TABLE 2.3 Precision of Analytical Results Based on ICP-OES
|
Variabilitya |
As |
Sb |
Sn |
Cu |
Bi |
Ag |
|
Range of concentrations of 50 bullets, µg/g |
1,000–1,900 |
2,500–6,800 |
1,400–2,600 |
71–483 |
53–221 |
14–56 |
|
Mean RSD, % of triplicates |
3.4 |
1.7 |
3.5 |
2.0 |
5.3 |
2.7 |
|
aMean relative standard deviations of triplicate measurements of 50 bullets. Source: Taken from Peters et al., 1988. |
||||||
(battery lead), and SRM C2417 (lead base alloy) are also included, as stated in the “Calibration and Control of Analytical Procedure” section of the FBI protocol.14 All SRMs are lead-based alloys. The calibration and control samples are also divided into three sub-samples randomly labeled “a,” “b,” and “c.”
The FBI’s “Calibration and Control of Analytical Procedure” section lacks much of the information that is normally present in well-documented analytical protocols throughout the chemical industry. For example, standard FBI practice states that “a” calibration standards, “a” control samples, and all “a” series bullet lead sub-samples are run first, then the “b” series, and then the “c” series. This sequence is not described in the protocol. Although seemingly a minor detail,
this is of great importance because decisions are based on measurement precision, and factors that affect measurement precision need to be carefully controlled and documented.
The FBI’s sample-digestion procedure for bullet lead evidence not only has evolved, but the committee learned, has not always been followed exactly. Once a single method is chosen, its viability should be ensured, and the procedure should be followed for every sample. It is most reliable if a universal procedure is used for all samples.
The “Decision Criteria” section of the FBI protocol describes the use of SRM C2416, SRM C2415, and SRM C2417 as quality check samples. Control values (limits) are given as means ± 2 standard deviations (SDs) for all seven elements. Most analytical laboratories use a formal control chart system. Such a system defines an average value of the measured variable, warning limits (means ± 2SD), and control limits (means ± 3SD), all based on historical data. If measured values are beyond the control limits, the process is considered to be out of control. Measured values outside the warning limits but within the control limits and values that are within the control limits but show trends (that is, movement in one direction or cyclical movement) are indicative of instrumental or procedural problems that should be fixed before the process becomes out of control.15 A formalized control chart system would allow the FBI Laboratory to detect analytical problems early and keep the rate of false-positive matches low. Such a system is easily implemented with a software routine that translates collected data into standardized control charts.
The FBI (and perhaps other law-enforcement laboratories) has multiple examiners performing CABL and has employed many examiners over the lifetime of the technique. To ensure the validity of the CABL results, each examiner should be tested regularly for proficiency in carrying out the test. This proficiency testing should ensure the ability of the analyst to distinguish bullet fragments that are compositionally indistinguishable from fragments with similar but distinguishable compositions. As part of this testing, Gage R&R studies16 should be carried out to assess the repeatability and reproducibility of the analysts involved in performing CABL. Proficiency testing is common in analytical laboratories and helps to ensure the overall quality of results. The proficiency tests are formalized and documented.17
FBI representatives stated that distribution of the FBI’s analytical protocol was tightly controlled until the document was requested by this committee. That controlled distribution was to ensure that only the newest version of the protocol was in use at any given time. But publication of the protocol and the research and data that support it in peer-reviewed journals or at a minimum publication of the protocol in other public venues would offer an opportunity for review and validation of the protocol. Publication options for the protocol include such a limited venue as Forensic Science Communications (on the FBI Web site), where the protocol could appear as a “Standards and Guidelines” article similar to “Standard Guide for Using Scanning Electron Microscopy/X-ray Spectrometry in Forensic Paint Examinations,”18 and the Federal Register, which has a much broader distribution. Once the protocol is officially documented in the public domain, each FBI analyst should follow it without deviation.
SELECTION OF COMPARISON ELEMENTS
The current FBI CABL method measures seven elements (As, Sb, Sn, Cu, Bi, Ag, and Cd). The selection of the elements has evolved, and it is unclear how their selection for comparison was made. The appropriateness of the elements selected depends on how discriminating the comparison of each element is in defining the composition of a volume of lead.
The FBI has published its assessment of the discriminating capabilities of individual elements in bullet lead comparisons.19 The relative importance of the elements for discrimination between lead sources decreases in this order: Cu and As > Sb > Bi and Ag. Sn was not included in the appraisal, because it was not observed in the brands of ammunition used for the studies. Measurement of Cd was not added to the FBI’s CABL procedure until 1995; therefore, Cd also was not included in the published studies.
A data set of elemental concentration measurements of bullet lead from 1,837 bullets compiled by the FBI was chosen as a basis for a statistical study of the discriminating ability of the seven elements. Information about the data set can be found in Chapter 3. Between-bullet standard deviations and correlations were calculated from the 1,837-bullet data set and demonstrated that correlation between the concentrations of some of the elements exist.
The variability in the 1,373-bullet subset can be characterized by using principal components analysis (PCA). PCA is a mathematical procedure that transforms a number of possibly correlated variables into a smaller number of non-correlated variables called principal components. The most common use of PCA is dimension reduction: often, a fewer number of variables (defined as
TABLE 2.4 Assessment of Elemental Discriminating Ability Via Principal Components Analysis
|
Elements |
Percentage of Total Variation |
|
Sb, Sn, Cd |
83.6 |
|
Sb, Sn, Cd, As |
96.1 |
|
Sb, Sn, Cd, As, Cu |
98.2 |
|
Sb, Sn, Cd, As, Cu, Bi |
99.6 |
|
Sb, Sn, Cd, As, Cu, Bi, Ag |
100 |
linear combinations of the original variables) contain a large proportion of variability of the entire data set. The first principal component accounts for as much of the variability in the data as possible, and each succeeding principal component accounts for as much of the remaining variability as possible. PCA was used here on the 1,373-bullet dataset (see Chapter 3, “Description of Data Sets”) to compare the variability of the 1373 bullets when all 7 elemental measurements are used with the variability when all possible 3-, 4-, 5-, and 6-element subsets are used. By choosing the elements that contain most of the variability, one can minimize the false match probability. For complete details on how PCA was conducted, see Appendix H.
A summary of the results of PCA is given in Table 2.4. About 96% of the total variation was found with four elements (Sb, Sn, Cd, and As). The elements that contributed the least variation were Bi and Ag. The latter finding is consistent with the findings of the FBI and Randich.20
The results of PCA of the 1,373-bullet data set suggest that the FBI is obtaining the greatest amount of information and discrimination by measuring Sb, Sn, Cd, As, and Cu. Although little power to detect matches would be lost if Ag or Bi were dropped from the analytical procedure, using ICP-OES, no time or effort would be saved by measuring five rather than seven elements.
The committee considered whether analyzing additional elements would improve the predictive or matching power of CABL. Te and Se were focused on as the most promising candidates. Te in bullet lead has been quantified using ICP-MS.21 However, Te, Se, and other elements that might be considered occur at ppm or sub-ppm levels, at or near the detection limit of the analytical technique. The precision of the measurement decreases quickly as measurements are taken near the detection limits of the instrument. As a result, the committee does not see analysis of additional elements as offering a significant improvement to the FBI’s procedure.
INSTRUMENTAL METHODS FOR FURTHER STUDY
Some instrumental methods seem to hold promise for CABL. The most noteworthy are described below.
Measurement of Lead Isotopic Compositions
The relative amounts of lead isotopes (206Pb, 207Pb, and 208Pb) in different geographic regions can differ from 17% to 36%.22 The reason for the variation in lead isotopic composition is the radioactive decay of thorium and uranium to lead by the following paths.23
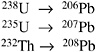
If sufficient precision and mass resolution is available, ICP-MS may be able to distinguish the origins of lead on the basis of isotopic ratios. One early study used ICP-MS to distinguish lead sources (for example, paint, foundry ash, and soil) in pollution studies.24 Although this technique does not appear to be particularly effective with domestically produced bullets that are made of lead from secondary smelters and thus may have a homogenized lead isotopic signature, some foreign bullets are made of lead from primary sources and could have characteristic lead isotopic signatures. The FBI may want to pursue research on this technique in the future.
High Resolution Mass Spectrometry and Inductively Coupled Plasma-Mass Spectrometry
Initially, ICP-MS was dominated by low-resolution quadrupole-based instruments.25 Although these instruments were sensitive and had lower limits of detection than ICP-OES, they were prone to interference problems, which limited their utility in lead isotopic analysis. The development of higher-resolution ICP-MS instruments—the first double-focusing ICP-MS commercial instruments appeared in the early 1990s26—may offer an improvement in the isotopic analysis of lead in bullets.
One high-resolution MS approach for use in examining lead isotope ratios was reported by Andrasko et al.,27 whose work demonstrated the ability of thermal ionization mass spectrometry (TIMS) to provide high-precision lead isotopic ratios for differentiating bullet samples. TIMS is the “standard” accepted method of isotopic ratio determination because of its potential precision. However, TIMS requires that the lead be separated from other elements before analysis because various mass-bias effects are generated during the ionization of lead from different matrices. This would be necessary whether the isotopic ratio determination was performed for lead or for any of the other trace elements in the bullet sample. The authors stated that this approach would be extremely difficult to implement on a routine basis.
More recently, a study was carried out with high-resolution ICP-MS based on a multi-collector (MC) system.28 The use of multi-collectors is a key feature of TIMS that allows for simultaneous high-precision measurement of the isotopes of interest. The MC-ICP-MS instrument allows for the simultaneous measurement of the relevant lead isotopes, with the advantages of TIMS and the advantages of ICP-MS because it does not require the isolation of lead from other elements before analysis. The results showed that the MC-ICP-MS instrument had precision and accuracy that were about ten times better than those in a similar study of quadrupole ICP-MS.29 Differences were observed with bullets obtained from economically isolated regions of the world, such as the former Soviet Union and South Africa. Although the study illustrated the possibility of differentiating between projectile lead in countries where a large amount of lead is recycled (such as the United States), the researchers were unable to utilize these analyses for determination of the lead deposit or source in such countries. Such a result would be expected whether the technique was used to measure the isotope ratio of the lead or of any of the trace elements in U.S.-manufactured bullets.
Suggested studies using the MC-ICP-MS approach would involve combining elemental analysis with the lead isotopic analysis in an attempt to increase the number of independent variables and improve the overall distinguishing ability of bullet lead analysis. The FBI should consider this for future study if foreign sources of bullet lead increase in the United States.
Laser Ablation Inductively Coupled Plasma-Mass Spectrometry
Laser ablation (LA) coupled with ICP-MS has been increasingly studied over the last 5 years for the determination of elements in solid samples.30 LA-
ICP-MS has a number of advantages for the analysis of solid samples, including minimal sample preparation, no loss of volatile elements, reduced contamination from reagents, and high sample throughput.
The main disadvantage of LA-ICP-MS is that its precision and accuracy are worse than those of ICP-MS with conventional pneumatic nebulization. Recently, several internal standard approaches were reported to improve overall accuracy and precision.31 It may be advantageous to monitor future advancements of this method.
High Performance Inductively Coupled Plasma-Optical Emission Spectroscopy
A method to improve measurement precision of ICP-OES by an order of magnitude or more was published in 1998; additional papers were published in 2000 and 2001.32 The method is a ratio-based procedure that relies on the cancellation of correlated high-frequency noise in the instrument combined with a new way to reduce the effects of low-frequency signal drift. The drift-correction procedure models low-frequency drift in repeated measurements and corrects the data to a “drift-free” condition. Although the published method is quite involved, development of a simplified adaptation that could substantially improve the analytical precision of ICP-OES for bullet lead analysis might be possible. That could help to provide better discrimination between bullet compositions. The reliance on improved instrumental precision to improve discrimination assumes that this precision is a significant source of error in the overall measurement and evaluation procedure.
FINDINGS AND RECOMMENDATIONS
Finding: The current analytical technology used by the FBI—inductively coupled plasma-optical emission spectroscopy (ICP-OES)—is appropriate and is currently the best available technology for the application.
Recommendation: The FBI Laboratory’s analytical protocol should be revised to contain all details of the inductively coupled plasma-optical emission spec
|
|
1995, 11, 967; Leach, J. J. Allen, L. A.; Aeschliman, D. B.; and Houk, R. S. Anal. Chem. 1990, 71, 440; Gunther, D.; Hattendorf, B.; and Audetat, A. J. Anal. At. Spectrom. 2001, 16, 1085; Mason, P. R. D. and Mank, A. J. G. J. Anal. At. Spectrom. 2001, 16, 1381. |
troscopy (ICP-OES) procedure and to provide a better basis for the statistics of bullet comparison. Revisions should include:
-
Determining and documenting the precision and accuracy of the ICP-OES method and the concentration range of all seven elements to which the method is applicable.
-
Adding data on the correlation of older neutron activation analysis and more recent ICP-OES results and any additional data that address the accuracy or precision of the method.
-
Writing and documenting the unwritten standard practice for the order of sample analysis.
-
Modifying and validating the digestion procedure to assure that all of the alloying elements and impurities in all samples (soft lead and hard lead) are dissolved without loss.
-
Using a more formal control-chart system to track trends in the procedure’s variability.
-
Defining a mechanism for validation and documentation of future changes.
Recommendation: The FBI should continue to measure the seven elements As, Sb, Sn, Cu, Bi, Ag, and Cd as stated in the current analytical protocol.
Recommendation: A formal and documented comprehensive proficiency test of each examiner needs to be developed by the FBI. This proficiency testing should ensure the ability of the analyst to distinguish bullet fragments that are compositionally indistinguishable from fragments with similar but analytically distinguishable composition. Testing could be internal or external (for example, conducted by the National Institute of Standards and Technology), and test results should be maintained and provided as appropriate. Proficiency should be tested regularly.
Recommendation: The FBI should publish the details of its CABL procedure and the research and data that support it in a peer-reviewed journal or at a minimum make its analytical protocol available through some other public venue.
Recommendation: Because an important source of measurement variation in quality-assurance environments may be the analyst who makes the actual measurements, measurement repeatability (consistency of measurements made by the same analyst) and reproducibility (consistency of measurements made by different analysts) need to be quantified through Gage R & R studies. Such studies should be conducted for the FBI comparison procedures.
Recommendation: The FBI’s documented analytical protocol should be applied to all samples and should be followed by all examiners for every case.














