D
Applying the Recommended Approaches
The special needs and vulnerabilities of infants require a clear and complete set of guidelines to assess the safety of infant formulas. Guidelines must also provide an appropriate level of flexibility to address the multitude and diversity of possible new ingredients. It is not realistic or desirable to provide specific recommendations for each potential new ingredient. Thus the committee recommends that the manufacturer or notifier and an expert review panel establish the relative importance of potential adverse effects for each new ingredient and determine the types of preclinical and clinical studies and in-market surveillance needed to accurately assess safety.
The committee was requested to apply the recommended tools and approaches to the specific situation of adding long-chain polyunsaturated fatty acids (LC-PUFAs) to infant formulas and to consider another example of a specific ingredient, if appropriate. Probiotics was selected as the second case study to demonstrate the flexibility of the algorithms when analyzing a “complex” ingredient comprised of a variety of different components that would be contained in a microorganism or microorganism mix. This appendix describes the steps in the committee’s recommended approaches that should be considered when assessing the safety of LC-PUFAs and probiotics.
As discussed in Chapter 1, algorithms diagram the safety assessment process into a step-by-step decision tree. The algorithms presented in Chapters 4 through 7 are provided to summarize the appropriate level of assessment by considering the harm and the potential adverse effects of a new ingredient. The approaches presented in this appendix are not meant to provide all of the information, events, or tests that need to be assessed to ensure safety, but rather to exemplify needed steps in the review process. The corresponding chapters provide more information on specific levels and tests.
The committee emphasizes that the purpose of these case studies is not to make conclusions about the completeness of the information or the safety of the new ingredient, but to point out assessment processes or steps that need to be considered. The algorithms that follow utilize an asterisk (*) with corresponding text underlined to indicate steps that the committee concluded could have been included if the notifier had used the committee’s
recommended algorithms to guide decisions about the type of safety assessments to apply. The asterisk with corresponding text underlined does not imply that the step was not performed or considered by the qualified experts, but that the information was not available for review by the committee. Note that under the proposed recommendation (Chapter 4), the expert panel can choose to include or not include certain tests in the submission.
LONG-CHAIN POLYUNSATURATED FATTY ACIDS
For the case of LC-PUFAs, the committee applied the information given to the Food and Drug Administration available through the Freedom of Information Act in Generally Recognized as Safe (GRAS) Notices 000041 and 000080 to its recommended algorithms, comparing the steps taken by the manufacturer, notifier, or expert panel to determine the safety of ARASCO (arachidonic acid-rich single-cell oil) and DHASCO (docosahexaenoic acid-rich single-cell oil) in infant formulas. Figure D-1 provides an overview of the proposed process. The sources of ARASCO and DHASCO have no prior use in foods in the United States, but they are used in infant formulas in Europe.
Preclinical Studies
As shown in Figure D-2, structure, stability, and solubility characterization studies are an important part of preclinical assessment. Figure D-3 illustrates that level 2 assessments would have been applied in determining the safety of the LC-PUFAs. These in-depth measures of the organ and neurological systems would further investigate abnormalities and/or are theoretically related to structure or function. In this case it appeared that there were some minor effects on organ systems. It is not obvious which of the proposed testing regimes in Chapter 5 were followed. The nonhuman primate studies were limited. These are considered an appropriate model to study changes in general behavior and speed of neural processing in response to the addition of LC-PUFAs. Chapter 5 provides more details on structure, stability, and solubility characterization, as well as the committee’s recommendations for level 2 assessments.
Clinical Studies
As seen in the overview of the proposed clinical guidelines (Figures D-4, D-5, and D-6), it is not clear whether assessments of body composition, immune response, auditory function, and temperament were conducted. Several of these tests (to be determined by expert panels), applied at level 2 or level 3, are especially important to determine the safety of LC-PUFAs because theoretical safety concerns exist. For example, LC-PUFAs affect immune response, and they have been linked to neural development. Chapter 6 provides the committee’s findings and recommendations on body composition and immune, auditory, and temperament assessment.
In-Market Surveillance
Figure D-7 illustrates the levels of proposed in-market surveillance. Selection of an appropriate type of in-market surveillance should be based on theoretical concerns about the new ingredient and/or results from preclinical and clinical studies. As long as preclinical and clinical studies are properly conducted, adverse outcomes should be rare and it would take a considerable period of time to collect sufficient data in order to reaffirm the GRAS status
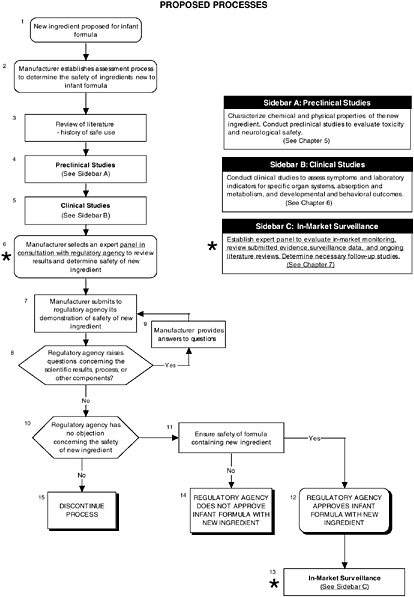
FIGURE D-1 Proposed process for evaluating the safety of ingredients new to infant formulas algorithm: Application by using the long-chain polyunsaturated fatty acid Generally Recognized as Safe (GRAS) Notifications 000041 and 000080 as a case study. An asterisk (*) along with the corresponding text underlined indicate steps that were either not apparent or not carried out within the GRAS notifications 000041 and 000080 provided to the committee. In-market assessment should be planned in conjunction with preclinical and clinical testing. This algorithm is modeled after the U.S. Generally Recognized as Safe Notification process; similar schemes can be adapted to other regulatory processes. ![]() = a state or condition,
= a state or condition, ![]() = a decision point,
= a decision point, ![]() = an action, sidebar = an elaboration of recommendation or statement.
= an action, sidebar = an elaboration of recommendation or statement.
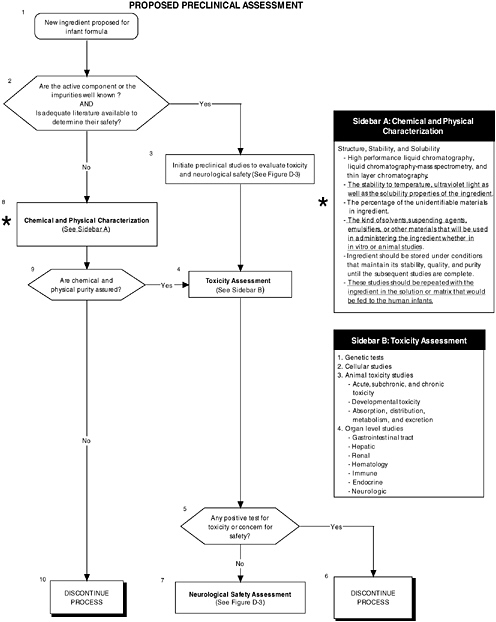
FIGURE D-2 Proposed preclinical assessment algorithm: Application by using the long-chain polyunsaturated fatty acid Generally Recognized as Safe (GRAS) Notifications 000041 and 000080 as a case study. An asterisk (*) along with the corresponding text underlined indicate steps that were either not apparent or not carried out within the GRAS notifications 000041 and 000080 provided to the committee. ![]() = a state or condition,
= a state or condition, ![]() = a decision point,
= a decision point, ![]() = an action, sidebar = an elaboration of recommendation or statement.
= an action, sidebar = an elaboration of recommendation or statement.
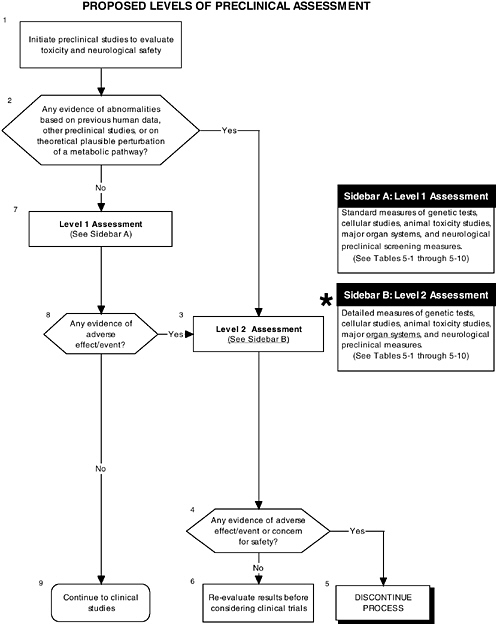
FIGURE D-3 Proposed levels of preclinical assessment algorithm: Application by using the long-chain polyunsaturated fatty acid Generally Recognized as Safe (GRAS) Notifications 000041 and 000080 as a case study. An asterisk (*) along with the corresponding text underlined indicate steps that were either not apparent or not carried out within the GRAS notifications 000041 and 000080 provided to the committee. ![]() = a state or condition,
= a state or condition, ![]() = a decision point,
= a decision point, ![]() = an action, sidebar = an elaboration of recommendation or statement.
= an action, sidebar = an elaboration of recommendation or statement.
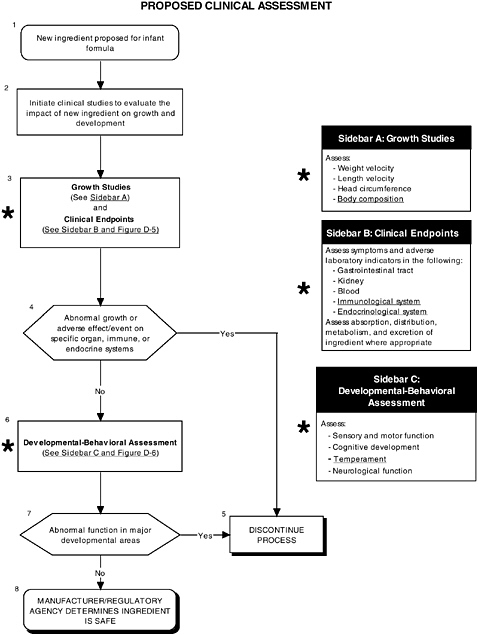
FIGURE D-4 Proposed clinical assessment algorithm: Application by using the long-chain polyunsaturated fatty acid Generally Recognized as Safe (GRAS) Notifications 000041 and 000080 as a case study. An asterisk (*) along with the corresponding text underlined indicate steps that were either not apparent or not carried out within the GRAS notifications 000041 and 000080 provided to the committee. ![]() = a state or condition,
= a state or condition, ![]() = a decision point,
= a decision point, ![]() = an action, sidebar = an elaboration of recommendation or statement.
= an action, sidebar = an elaboration of recommendation or statement.
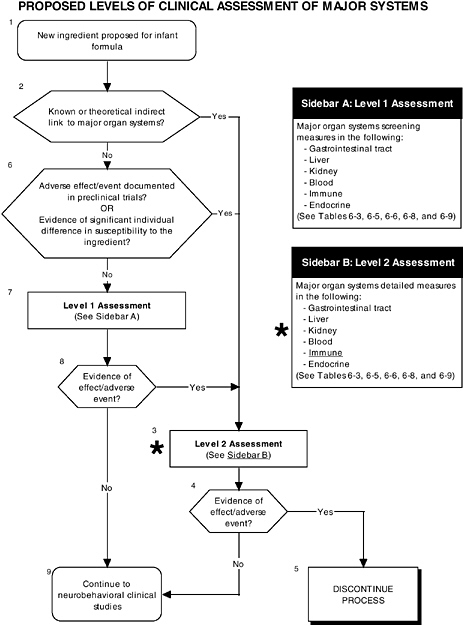
FIGURE D-5 Proposed levels of clinical assessment of major organ, immune, and endocrine systems algorithm: Application by using the long-chain polyunsaturated fatty acid LC-PUFA Generally Recognized as Safe (GRAS) Notifications 000041 and 000080 as a case study. An asterisk (*) along with the corresponding text underlined indicate steps that were either not apparent or not carried out within the GRAS notifications 000041 and 000080 provided to the committee. ![]() = a state or condition,
= a state or condition, ![]() = a decision point,
= a decision point, ![]() = an action, sidebar = an elaboration of recommendation or statement.
= an action, sidebar = an elaboration of recommendation or statement.
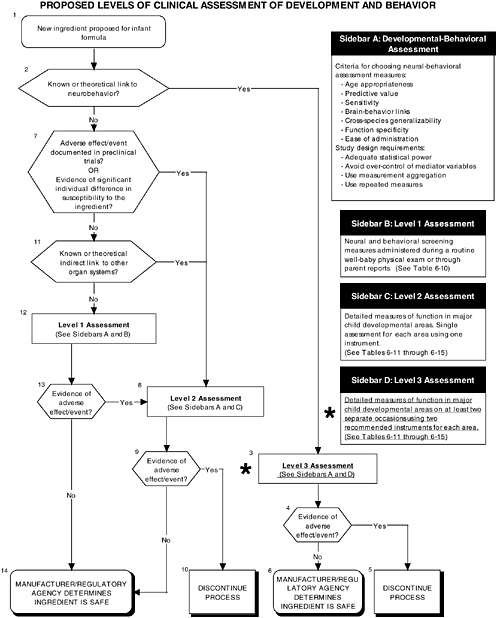
FIGURE D-6 Proposed levels of clinical assessment of development and behavior algorithm: Application by using the long-chain polyunsaturated fatty acid Generally Recognized as Safe (GRAS) Notifications 000041 and 000080 as a case study. NOTE: An asterisk (*) along with the corresponding text underlined indicate steps that were either not apparent or not carried out within the GRAS notifications 000041 and 000080 provided to the committee. ![]() = a state or condition,
= a state or condition, ![]() = a decision point,
= a decision point, ![]() = an action, sidebar = an elaboration of recommendation or statement.
= an action, sidebar = an elaboration of recommendation or statement.
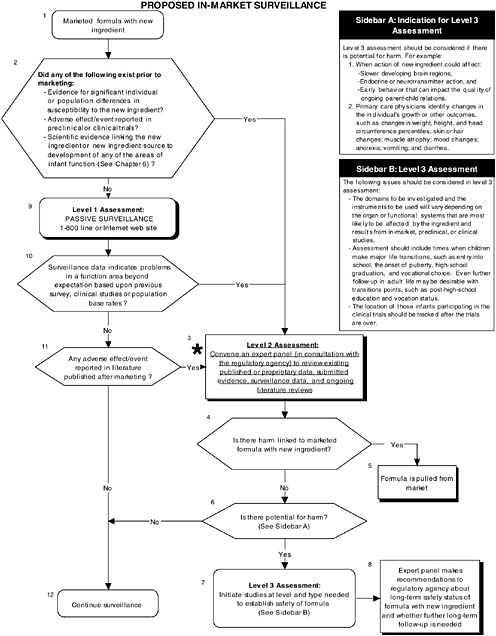
FIGURE D-7 Proposed in-market surveillance algorithm: Application by using the long-chain polyunsaturated fatty acid Generally Recognized as Safe (GRAS) Notifications 000041 and 000080 as a case study. NOTE: An asterisk (*) along with the corresponding text underlined indicate steps that were either not apparent or not carried out within the GRAS notifications 000041 and 000080 provided to the committee. ![]() = a state or condition,
= a state or condition, ![]() = a decision point,
= a decision point, ![]() = an action, sidebar = an elaboration of recommendation or statement.
= an action, sidebar = an elaboration of recommendation or statement.
of the ingredient. Chapter 7 provides more details on the committee’s recommendations for in-market surveillance. If needed, a selection of a qualified and unbiased expert panel is important to evaluate surveillance data and ongoing literature reviews to determine if follow-up studies are necessary.
PROBIOTICS
For the case of probiotics, the committee reviewed GRAS Notice 000049 to analyze the addition of probiotics to infant formula using its recommended algorithms. Figure D-8 provides an overview of the proposed process. Probiotics have a history of safe use in infant formulas in other countries, and a review of the scientific literature showed no significant adverse events linked to these ingredients.
Preclinical Studies
A probiotic is a complex ingredient (e.g., a microorganism) and thus, stability and solubility studies should be performed with the ingredient in solution, as well as in the matrix that would be fed to human infants (Figure D-9). Each probiotic should be tested separately, as well as in the combination that will be used in the infant formula. This is important because different probiotics may possess different chemical characteristics, nutritional contributions, and pharmacological and physiological activities, and they may be derived from novel sources or processes.
A comprehensive preclinical level 1 assessment also should be conducted. As shown in Figures D-9 and D-10, preclinical studies are important in assessing the safety of probiotics since changing intestinal flora may lead to production of atypical components in the intestines. Chapter 5 provides more details on the committee’s recommendations for preclinical studies.
Clinical Studies
As seen in the overview of the proposed clinical guidelines (Figures D-11 and D-12), it is important to include appropriate measures of body composition and hepatic and endocrine function. The addition of probiotics could lead to formation of certain molecules at high levels not commonly present in the intestines. This could theoretically affect hepatic and endocrine function and other systems. Finally, clinical studies should include a comprehensive level 1 assessment of behavioral and neural screening measures (see Figures D-11, D-12, and D-13). Chapter 6 provides more information about these assessments.
In-Market Surveillance
Figure D-14 illustrates proposed in-market surveillance guidelines. Assuming that the recommended preclinical and clinical tests using probiotics detected no adverse effects, passive surveillance for in-market monitoring and level 2 long-term follow-up strategies are recommended. Chapter 7 provides more information about the committee’s recommendations on in-market surveillance.
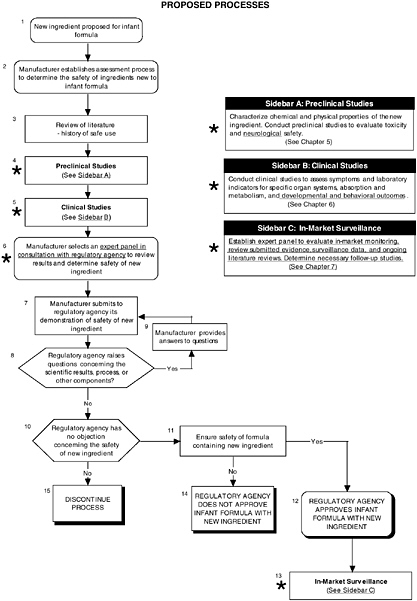
FIGURE D-8 Proposed process for evaluating the safety of ingredients new to infant formulas algorithm: Application by using the probiotics Generally Recognized as Safe (GRAS) Notification 000049 as a case study. An asterisk (*) along with the corresponding text underlined indicate steps that were either not apparent or not carried out within the GRAS notification 000049 provided to the committee. In-market assessment should be planned in conjunction with preclinical and clinical testing. This algorithm is modeled after the U.S. Generally Recognized as Safe Notification process; similar schemes can be adapted to other regulatory processes. ![]() = a state or condition,
= a state or condition, ![]() = a decision point,
= a decision point, ![]() = an action, sidebar = an elaboration of recommendation or statement.
= an action, sidebar = an elaboration of recommendation or statement.
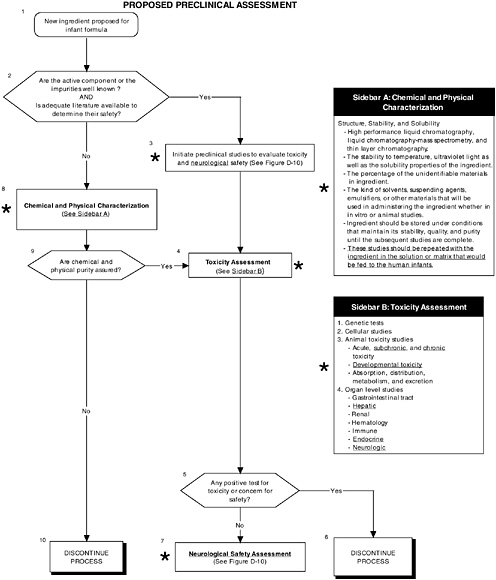
FIGURE D-9 Proposed preclinical assessment algorithm: Application by using the probiotics Generally Recognized as Safe (GRAS) Notification 000049 as a case study. An asterisk (*) along with the corresponding text underlined indicate steps that were either not apparent or not carried out within the GRAS notification 000049 provided to the committee. Many of the steps of chemical and physical characterization cannot be applied to probiotics. ![]() = a state or condition,
= a state or condition, ![]() = a decision point,
= a decision point, ![]() = an action, sidebar = an elaboration of recommendation or statement.
= an action, sidebar = an elaboration of recommendation or statement.
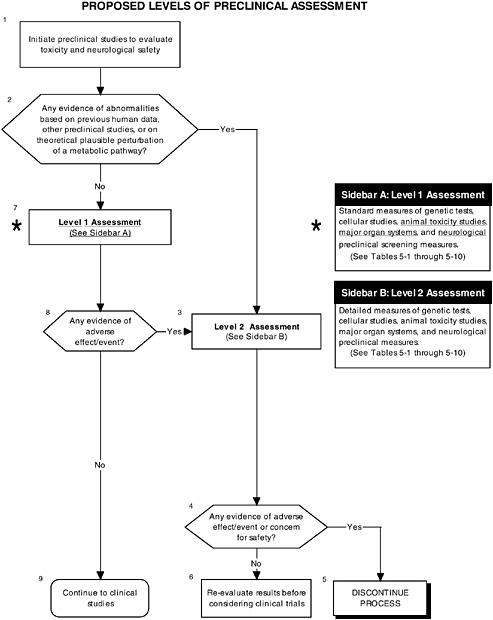
FIGURE D-10 Proposed levels of preclinical assessment algorithm: Application by using the probiotics Generally Recognized as Safe (GRAS) Notification 000049 as a case study. An asterisk (*) along with the corresponding text underlined indicate steps that were either not apparent or not carried out within the GRAS notification 000049 provided to the committee. ![]() = a state or condition,
= a state or condition, ![]() = a decision point,
= a decision point, ![]() = an action, sidebar = an elaboration of recommendation or statement.
= an action, sidebar = an elaboration of recommendation or statement.
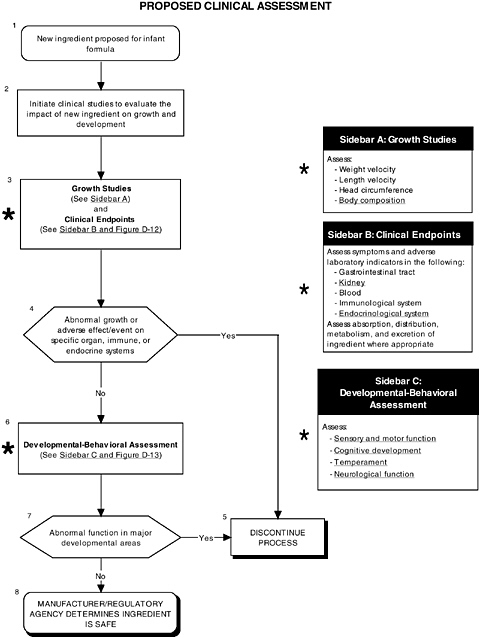
FIGURE D-11 Proposed clinical assessment algorithm: Application by using the probiotics Generally Recognized as Safe (GRAS) Notification 000049 as a case study. An asterisk (*) along with the corresponding text underlined indicate steps that were either not apparent or not carried out within the GRAS notification 000049 provided to the committee. ![]() = a state or condition,
= a state or condition, ![]() = a decision point,
= a decision point, ![]() = an action, sidebar = an elaboration of recommendation or statement.
= an action, sidebar = an elaboration of recommendation or statement.
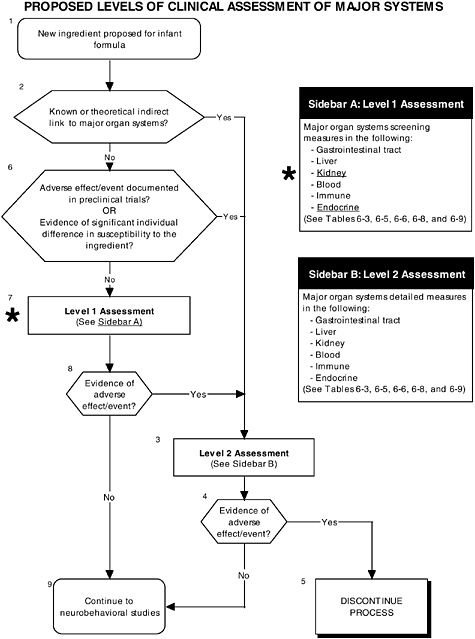
FIGURE D-12 Proposed levels of clinical assessment of major organ, immune, and endocrine systems algorithm: Application by using the probiotics Generally Recognized as Safe (GRAS) Notification 000049 as a case study. An asterisk (*) along with the corresponding text underlined indicate steps that were either not apparent or not carried out within the GRAS notification 000049 provided to the committee. ![]() = a state or condition,
= a state or condition, ![]() = a decision point,
= a decision point, ![]() = an action, sidebar = an elaboration of recommendation or statement.
= an action, sidebar = an elaboration of recommendation or statement.
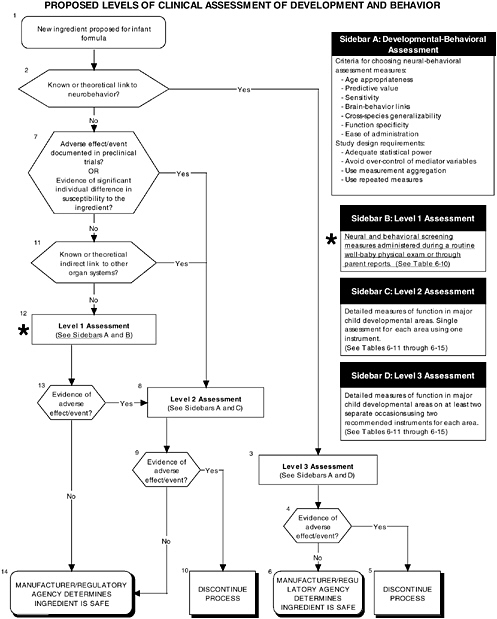
FIGURE D-13 Proposed levels of clinical assessment of development and behavior algorithm: Application by using the probiotics Generally Recognized as Safe (GRAS) Notification 000049 as a case study. An asterisk (*) along with the corresponding text underlined indicate steps that were either not apparent or not carried out within the GRAS notification 000049 provided to the committee. ![]() = a state or condition,
= a state or condition, ![]() = a decision point,
= a decision point, ![]() = an action, sidebar = an elaboration of recommendation or statement.
= an action, sidebar = an elaboration of recommendation or statement.
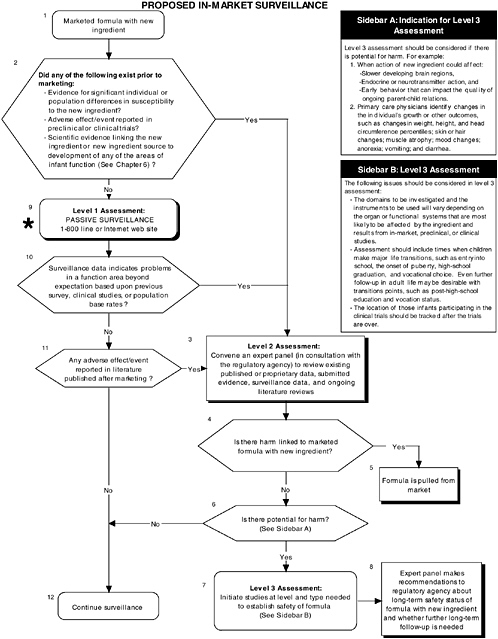
FIGURE D-14 Proposed in-market surveillance algorithm: Application by using the probiotics Generally Recognized as Safe (GRAS) Notification 000049 as a case study. An asterisk (*) along with the corresponding text underlined indicate steps that were either not apparent or not carried out within the GRAS notification 000049 provided to the committee. ![]() = a state or condition,
= a state or condition, ![]() = a decision point,
= a decision point, ![]() = an action, sidebar = an elaboration of recommendation or statement.
= an action, sidebar = an elaboration of recommendation or statement.
SUMMARY
These two case studies demonstrate the flexibility and utility of the proposed processes. They also indicate an important potential role for expert panels in determining the types and levels of assessment to ensure the safety of two very different ingredients. Their application also shows the importance of standardized elements and frameworks when considering the safety of new ingredients added to infant formulas.


















