6
Going Beyond Current Clinical Studies
ABSTRACT
Clinical studies are essential to ensure the safety of infant formulas and any systematic deviation from normal physical growth and development attributable to a new ingredient should be considered a safety threat. Growth studies, currently a centerpiece of clinical evaluation of infant formulas, should include precise and reliable measurements of weight and length velocity and head circumference. Appropriate measures of body composition also require assessment. Duration of follow-up measurements should at least cover the period when infant formula remains the sole source of nutrients in the diet of the infant. However the committee believes that growth studies are not sufficient on their own to assess ingredients new to infant formulas. Specific guidelines are needed to determine “normal” growth and to establish what represents a biologically meaningful difference among groups of infants consuming different formulas. Specific recommendations are needed to establish a level of difference that represents a safety concern.
Regulatory guidelines should ensure that infant outcomes encompass, as the Food and Drug Administration (FDA) has proposed, “all aspects of physical growth and normal maturational development.” Any systematic differences in clinical outcomes that can be attributed to an ingredient new to infant formulas should be considered a safety concern that requires careful evaluation and, if needed, further clinical study to identify the pathway through which the infant has been affected. The committee recommends that a hierarchy of two levels of clinical assessment be implemented with regard to growth and organ systems. Level 1 assessments should include checking for signs of all adverse laboratory indicators of the major organ systems. Level 2 assessments should include in-depth measures of organ systems or functions that would be performed to explain abnormalities found in level 1 assessments or specific theoretical concerns not typically addressed by level 1 tests.
There are a number of reasons why it is equally important to include developmental-behavioral outcomes in future studies of the safety of ingredients new to infant formulas: the measures are sensitive to exposure to toxic substances, they can have long-term predictive value, and bidirectional brain-behavior links exist. Therefore, assessment of clinical endpoints should include measurement of infant sensory-motor, cognitive, affectual, and neural function with instruments that follow recommended criteria. The committee recommends that a hierarchy of three levels of clinical assessment be developed and implemented to determine what levels are appropriate to apply with regard to developmental-behavioral-neural outcomes. The levels of assessment are: level 1 assessments, including developmental screening measures; level 2 assessments, including in-depth measures of infant functions in major developmental areas (single assessment for each area with one instrument); and level 3 assessments, including in-depth measures of infant functions in major developmental areas (repeated assessment with multiple instruments).
The instruments used for these assessments should satisfy the following criteria: be age appropriate, have predictive value for long-term consequences, be adequately sensitive, have documented brain-behavior links, have cross-species generalizability, assess specific function, and be easy to administer. In addition, the committee considers that certain design features (e.g., adequate statistical power) are essential in all clinical studies.
INTRODUCTION
This chapter provides an overview of clinical studies and a brief overview of the current regulatory requirements for them. The first part of the chapter includes a rationale for clinical assessment of growth, specific recommendations on what should be measured, and guidelines for interpretation of results. In the second part, the committee describes more specific clinical endpoints in each of the organ systems likely to be affected by ingredients new to infant formulas. In the last part of the chapter considerable attention is paid to behavioral and developmental endpoints because of the young infant’s heightened sensitivity to potentially toxic substances and the long-term consequences of such exposures.
THE IMPORTANCE OF CLINICAL STUDIES
While preclinical laboratory and animal studies have substantial value for identifying potential safety concerns, they are limited in their ability to predict what may happen in human infants. Clinical studies in human infants are needed for several reasons. First, extrapolation from animal studies may be limited by differences between animal and human structure, physiology, and development. Second, extrapolation from isolated tissue studies is limited by the inability of such models to assess functions in the context of whole organ systems where coordination and integration are the rule. For example, the digestion and absorption of nutrients requires coordination of numerous gastrointestinal functions. Third, there may be no available animal or tissue models to test specific functions. For example, it is not possible to use animal models to duplicate clinically relevant allergic reactions to foreign proteins, to determine the effects of a substance on acceptance or tolerance of an infant formula, or to test some of the higher cognitive functions found only in humans.
CURRENT REGULATORY GUIDELINES FOR CLINICAL STUDIES
Canada’s Food and Drug Regulations
There are no specific requirements for clinical testing of infant formulas set out under Canada’s Food and Drug Regulations in Division 16 (Food Additives), Division 25 (Infant Formula), or Division 28 (Novel Foods) (Canada, 2001). Division 25 of the Regulations requires that a premarket submission with respect to a new infant formula or an infant formula that has undergone a major change in composition, manufacturing, or packaging include the evidence relied on to establish that the infant formula is nutritionally adequate to promote acceptable growth and development in infants when consumed in accordance with the directions for use. Divisions 16 and 28 require that data be submitted to Health Canada that include information used to establish the safety of a food additive or a novel food, respectively. Health Canada refers manufacturers to internationally accepted guidelines for clinical testing or asks to be consulted because decisions are made on a case-by-case basis.
Sections 409 and 412 of the Federal Food, Drug and Cosmetic Act
There are no explicit requirements for clinical testing of infant formulas specified under Section 409 of the Food, Drug and Cosmetic (FD&C) Act. Section 409 stipulates that a petition to establish safety of a food additive shall contain “all relevant data bearing on the physical or other technical effect such additive is intended to produce …,” but it does not dictate a specific type of clinical study.
Current regulations for infant formulas under Section 412 of the FD&C Act do not define quality factor requirements, such as physical growth, but only describe required nutrient levels, without considering bioavailability. This gap is addressed in a proposed rule (FDA, 1996), where assessment of physical growth, using anthropometry, is proposed “as an integrative indicator of net overall nutritional quality of the formula.” The proposed rule further states, “as the science evolves, FDA anticipates being able to progress beyond generalized, nonspecific indicators of overall nutritional intakes (e.g., measures of physical growth) to more specific and sensitive measures of biochemical and functional nutritional status” (FDA, 1996, P. 36181). Thus neither the current nor the proposed rules identify specific requirements for other clinical studies.
FDA Redbook
FDA does not require petitioners to conduct human clinical studies to support the safety of food additives or color additives used in food, but, if deemed necessary, it recommends that the studies conform to guidelines presented in section VI.A. of the Redbook (OFAS, 2001, 2003). These guidelines are comprehensive and relevant for the clinical testing of ingredients new to infant formulas.
General guidance is provided to identify the scientific and ethical principles for clinical studies, including the need for presentation of a defensible rationale for human studies. The Redbook states that this rationale should be based on:
-
adequate preclinical investigations,
-
results of clinical studies conducted elsewhere,
-
consideration of the organs and organ systems that may be affected, and
-
careful attention to the qualifications of investigators and the safety and ethical treatment of subjects in clinical trials.
The Redbook suggests the sequence of and subjects for clinical studies. Early clinical studies are to determine the “metabolism and level of the food or food additive that gives an adverse or toxic response in man” (specifically physiological studies of the additive’s disposition, its potential to induce enzyme levels or increase activity, and its interactions with other nutrients) (OFAS, 2001, P. 183). In general children are to be excluded from these early (typically acute or shorter duration) clinical studies. However tolerance studies, which are to be included among early studies, need to be conducted in infants because of the special nature of infant formulas.
Infants are more likely to be included in what the Redbook describes as chronic intake studies, which are to be conducted once general safety in humans is established in the early adult studies. Here, the Redbook provides specific guidance on protocol design, study population, and statistical analyses, as well as on how reports of clinical studies should be presented. Box 6-1 lists questions that should be answered when conducting studies to determine the safety of a proposed additive.
GENERAL APPROACH TO CONDUCTING CLINICAL STUDIES
In the conceptualization of the range of infant health concerns, the committee was guided by the following: “FDA considers the concept of ‘healthy growth’ to be broad, encompassing all aspects of physical growth and normal maturational development, including maturation of organ systems and achievement of normal functional development of motor, neurocognitive, and immune systems. All of these growth and maturational developmental processes are major determinants of an infant’s ability to achieve his/her biological potential, and all can be affected by the nutritional status of an infant” (FDA, 1996, P. 36179).
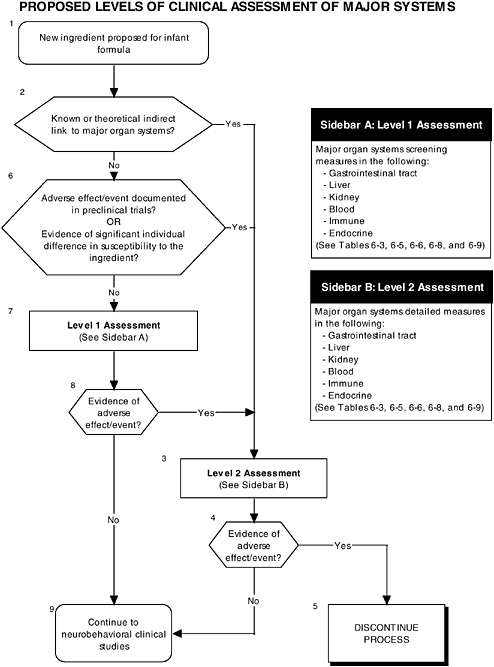
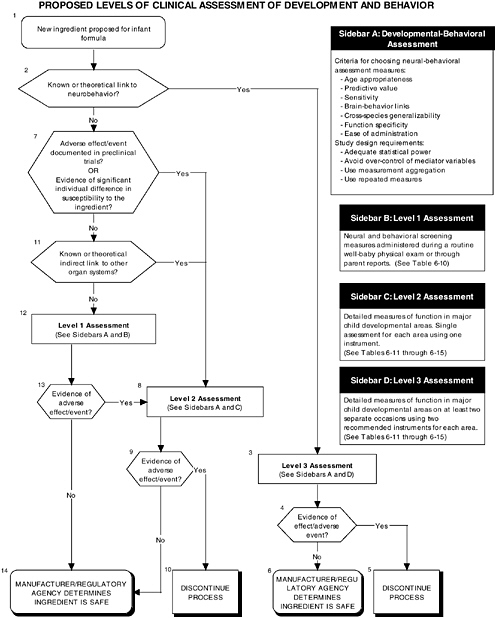
The committee proposes the use of a multilevel approach to establish more comprehensive guidelines to ensure that infant outcomes encompass “all aspects of physical growth and normal maturational development.” Figure 6-1 illustrates the three different types of clinical studies recommended by the committee, including assessment of growth, organ systems, and development and behavior. Figures 6-2 and 6-3 further explain the clinical studies through the proposed two-level approach to organ systems and the three-level approach to development-
|
BOX 6-1 Questions That Should Be Answered When Conducting Clinical Studies
SOURCE: OFAS (2001, 2003). |
behavior. There are decision-making points within each of these three types of clinical studies that will be discussed in detail in subsequent sections of this chapter. (In keeping with the charge to the committee, proposed guidelines focus on the health and well-being of term infants only.)
The committee recognizes that all clinical studies would need to be reviewed and approved by human-subject research review boards. Because the clinical studies to determine the safety of new ingredients will be carried out in healthy infants, the committee does not recommend the use of highly invasive tests, such as tissue biopsies or gastrointestinal incubations.
OVERVIEW OF RECOMMENDED LEVELS OF ASSESSMENT
RECOMMENDATION: Any adverse systematic differences in clinical outcomes that can be attributed to an ingredient new to infant formulas should be considered a safety concern that requires careful evaluation and, if needed, further clinical study to identify the pathway through which the infant has been affected.
A hierarchy of two levels of clinical assessment should be implemented for organ systems:
-
Level 1 assessments. Check of signs for all adverse laboratory indicators.
-
Level 2 assessments. In-depth measures of organ systems or functions that would be performed to explain abnormalities found in level 1 assessments or specific theoretical concerns not typically addressed by level 1 tests.
A hierarchy of three levels of clinical assessment should be implemented for developmental-behavioral measures:
-
Level 1 assessments. Developmental screening measures.
-
Level 2 assessments. In-depth measures of infant functions in major developmental areas (single assessment for each area with one instrument).
-
Level 3 assessments. In-depth measures of infant functions in major developmental areas (repeated assessment with multiple instruments).
GROWTH
Growth is well recognized as a sensitive, but nonspecific, indicator of the overall health and nutritional status of an infant. Monitoring infant growth has always been an integral part of pediatric care and is particularly important for young infants. Growth and nutrient requirements per kilogram of body weight are higher during the first few months of infancy than during any other period of life. Furthermore, the greatest percentage of dietary intake is devoted to supporting growth at this time, and thus nutritional imbalances are likely to be reflected in growth rates.
The committee believes that the inability of a formula to support normal growth represents a significant harm to infants and therefore growth is an essential endpoint for all safety assessments of an ingredient new to infant formulas. Any systematic deviation from normal physical growth attributable to a new ingredient should be considered a safety threat.
Under current regulations the core of the requirements focuses on meeting certain levels of specific nutrients. The concept of quality factors has not been defined, but proposed
regulations include a subsection on quality factors, with a focus on physical growth. Despite the absence of quality factors in current legislation, there appears to be a strong consensus that growth should be a quality factor for infant formulas. In the United States FDA recognized the need for clear guidelines on the assessment of growth and commissioned a report from the American Academy of Pediatrics’ (AAP) Committee on Nutrition Task Force on clinical testing of infant formulas with respect to nutritional suitability for term infants (AAP, 1988). The task force identified the following types of clinical studies as useful in the premarket evaluation of formulas: acceptance or tolerance studies, gains in weight and length, food intake, body composition, serum chemical indices, and metabolic balance studies. Most of the recommendations of the task force were incorporated into the proposed changes to the infant formula act (FDA, 1996).
Currently clinical studies tend to follow the proposed rule, the 120-day growth study being the main method used to assess the ability of an infant formula to sustain normal infant growth. The proposed rule would codify standards for clinical growth studies by specifying methods (controlled clinical trials), duration (4 months), measurements (weight, recumbent length, and head circumference), and ages at measurement (at 2 and 4 weeks, then at least monthly thereafter), with a further requirement that individual infant data be plotted against Centers for Disease Control and Prevention (CDC) reference curves for weight and length.1
The AAP task force concluded that “rate of gain in weight gain is the single most valuable component of the clinical evaluation of infant formula” (AAP, 1988, P. 7). Further, it judged that length assessment is unnecessary because significant differences in length gain would not occur in the absence of differences in weight gain, and that there is a higher potential for measurement error and thus misclassification of growth in length. While the committee concurs with the centrality of weight gain in clinical assessment, it also believes that length and head circumference should be measured in growth studies in order to evaluate the effects of substances on other aspects of growth, such as skeletal growth and body proportions.
Notably absent from existing and proposed requirements are specific guidelines on what constitutes “normal” growth, or what represents a biologically meaningful difference among groups of infants consuming different formulas. Recommendations are needed both to define the most relevant comparison groups for clinical studies and to establish a level of difference that represents a safety concern. These are challenging and critical questions that will be discussed in later sections.
In addition, the committee recommends that guidelines go beyond growth studies to assess the safety of ingredients new to infant formulas. Deficits in brain function and effects of specific micronutrients may occur in the absence of differences in physical growth. Furthermore, while a “decrease in the growth rate during infancy is the earliest indication of nutritional failure” (Fomon, 1993, P. 48), growth deficits are likely to appear only secondary to effects on specific organs or tissues, and they may not appear for some time after nutritional insult. Thus growth studies should be considered a necessary, but not sufficient, part of human clinical studies of the safety of ingredients new to infant formulas (see Figure 6-1, Box 3).
Measuring Growth
Ascertainment of growth status typically relies on anthropometric assessment, which is noninvasive and highly practical, requires relatively little training to achieve reliability, and is accomplished with low-cost, low-tech tools. Further, there are ample descriptions of standard anthropometric methods and reference data for the interpretation of measurements (Kuczmarski et al., 2000; Lohman et al., 1988). Although each has limitations and advantages (Table 6-1), the committee recommends the following measures of infant growth for clinical studies (see Figure 6-1, Box 3):
-
Weight is an overall measure of body size and is responsive to acute insults, such as infectious morbidity or changes in nutrient intakes. Attained weight is hard to interpret in the absence of length data since an underweight child could be well proportioned or thin, with different implications for morbidity risk.
-
Recumbent length is an overall indicator of linear or bone growth. Length reflects genetic factors and growth history. It is less responsive to acute insults, and the response of length to varying nutrition levels typically lags behind the response in weight.
-
Weight for length is an indicator of relative weight (thinness or overweight). These measures are typically expressed as a Z-score or a percentile based on comparison with national reference data.
-
Head circumference is often used in clinical settings as an overall, nonspecific indicator of brain growth. It has limited usefulness in screening for potential developmental or neurological disabilities, but it is useful in comparison with other anthropometrics to assess proportionality. The ratio of mid-arm to head circumference is a less commonly used index of proportionality.
-
Body composition is a more sensitive indicator of infant nutritional status than measures of size. Depending on the method used, measurements can provide the mass of lean tissue, fat tissue, total body water, and bone. Methods vary greatly in terms of invasiveness, feasibility, cost, technology, need for trained personnel, accuracy, reliability, and precision. The most feasible methods for assessing infant body composition include anthropometry (e.g., skinfold measurements), dual X-ray absorptiometry (DEXA), and isotope dilution. A recent review concluded that for intergroup comparisons, skinfold thicknesses were useful, but for individual infant assessments, DEXA was recommended (Koo, 2000). In the absence of reference data based on a large sample of infants, the interpretation of body
TABLE 6-1 Limitations and Advantages of Recommended Growth Assessments
|
Recommended Assessment |
Limitations |
Advantages |
|
Rate of weight gain |
Nonspecific |
Good global measure of infant growth and health, easy to measure reliably |
|
Rate of length gain |
Difficult to measure accurately, deficits less likely unless weight is also compromised |
Provides important additional information about linear/skeletal growth and proportionality |
|
Head circumference |
Nonspecific |
Easy to measure accurately, adequate global measure of head and brain growth and proportionality |
|
Body composition |
Difficult to measure accurately, best method requires expensive equipment (dual-energy X-ray absorptiometry) |
More precise information about possible metabolic effects of ingredients, possible better long-term predictor of health outcomes |
TABLE 6-2 Limitations and Advantages of Common Measurements of Body Composition
|
Method |
Relevant Papers/Measurement |
Limitations |
Advantages |
|
Skinfold |
Schmelzle and Fusch (2002); body fat in neonates and young infants: validation of skinfold thickness versus dual-energy X-ray absorptiometry |
Can be inaccurate |
Rapid, low cost |
|
Dual-energy X-ray absorptiometry |
Butte et al. (1999); fat mass in infants and toddlers: comparability of total body water, total body potassium, total body electrical conductivity, and dual-energy X-ray absorptiometry |
Requires expensive equipment |
Rapid, precisely estimates bone mineral content, fat mass, and lean body mass |
|
Isotope dilution |
|
Expensive and needs specialized equipment |
Noninvasive, safe |
-
composition outcomes should rest on the comparison of groups in randomized controlled trials. Additional information on the methods used to assess body composition is provided in Table 6-2.
RECOMMENDATION: Growth studies should include precise and reliable measurements of weight and length velocity and head circumference. Duration of measurements should cover at least the period when infant formula remains the sole source of nutrients in the infant diet. Appropriate measures of body composition also require assessment.
Defining Normal Growth
The purpose of growth assessment is to determine whether a child is growing “normally.” The definition of normal, inadequate, or excess growth rests largely on comparison of individual measurements with reference data that represent the distribution of sizes found in healthy infants of a given age and sex. While there is no clear cut point to define a size at which there is an abrupt elevation in risk of poor outcomes, measurements that fall above the 95th or below the 5th percentiles of an accepted reference are typically cause for concern. While short periods of abnormal growth rate may not be of concern, low or high rates over several months may be related to increased morbidity risk, both in the long and short term. Therefore a single measurement of attained size at a given age is not a sufficient measure of growth. Repeated, appropriately spaced measurements are needed to calculate growth rate. A clinical assessment of infant growth for the purpose of determining the safety of an ingredient new to infant formulas must therefore be based on a longitudinal study, with repeated measures at relatively frequent intervals during the period when growth is most rapid and during the time period when formula serves as the sole source of infant nutrition.
Identifying Appropriate Comparison Groups
As discussed in Chapter 3, there are challenges in selecting appropriate comparison groups for clinical studies to assess the safety of infant formulas. The gold standard design—the double-blind, randomized, controlled trial—randomly assigns comparable groups of
infants to receive either the formula containing the new ingredient or a previously approved formula. Implicit in this design is the assumption that infants fed the approved formula form the appropriate comparison group. However when testing for deviation from optimal infant growth, the appropriate comparison group should be one that demonstrates optimal growth. Since growth of healthy breastfed infants is considered optimal, then exclusively breastfed infants form the most appropriate comparison group. The committee recommends using dual control groups—breastfed infants and infants fed the previously approved formula without the new ingredient—in order to ensure a thorough analysis.
Breast versus formula feeding cannot ethically be randomly assigned, nor could these feeding conditions be blinded. Instead, reference data from healthy breastfed infants, measured at comparable intervals using identical methods, can be used for comparative purposes. This would allow multiple intergroup comparisons that would put differences between two infant formulas in perspective relative to formula-breastfeeding differences, as has been done in trials of formula containing long-chain polyunsaturated fatty acids (LC-PUFAs) (Auestad et al., 2001). The World Health Organization is currently working to create a growth reference for breastfed infants that should be suitable for such comparisons in the future (Garza and de Onis, 1999).
Estimating Intake
All clinical trials must include an estimation of daily formula intake in order to determine which effects are the result of different levels of intake and which are to the result of the specific ingredient. For example, if an ingredient alters taste or palatability, it may change the level of intake.
Specifying a Level of Difference in Growth That Represents a Safety Concern
This is important for interpreting average group differences, as well as deviations from normal growth in an infant that are attributable to being fed a different formula. There is very little scientific evidence to establish a level of difference associated with long- or short-term health consequences. The AAP task force (AAP, 1988) recommended that a weight gain difference greater than 3 g/day over 3 to 4 months should be considered nutritionally significant. Over 3.5 months, this would represent a difference of about 320 g. This is less than the difference between the 25th and 50th percentile and equivalent to the difference between the 90th and 95th percentile of weight at age 3.5 months for boys based on the CDC growth charts (Kuczmarski et al., 2002). No specific evidence to support this level of difference was provided. In a clinical setting, a diagnosis of failure to thrive is based on rate of weight gain and weight-for-length status interpreted in the context of growth history. Fomon (1993) defines failure to thrive in comparison with U.S. reference data for weight increments and weight-for-length status. He uses two standard deviations relative to 2-month increment data for infants under 6 months of age and a weight-for-length below the 5th percentile as cutpoints.
For perspective, it may be useful to compare reported differences in growth rates of healthy breastfed and formula-fed infants. Data from Fomon’s infant growth studies (Nelson et al., 1989) show differences of 2.4 g/day in boys and 1.3 g/day in girls who were breastfed versus formula fed from 8 to 112 days of life. This would result in a 250-g difference in boys and a 135-g difference in girls over the 104 days. Based on more recent data, Dewey and colleagues (1992) compared growth of infants in the DARLING study and found consistently higher weight velocities (g/mo) in formula-fed versus breastfed boys in the first 6
months of life, but no significant effects of feeding mode on weight velocity in girls. The cumulative effect of the differences in weight velocity among boys amounted to about 284 g over a period of 4 months. Furthermore, infants who were breastfed for 12 months or more were leaner, had smaller skinfolds, and had a lower percent body fat. These differences persisted into the second year of life (Dewey et al., 1993).
Kramer and colleagues (2002) recently reported results of a large study of Belarus infants and found that infants who were exclusively breastfed for at least 3 months had weight and length Z scores that were about 0.2 standard deviations above those of infants who were weaned in the first month of life. Using data from the Third National Health and Nutrition Examination Survey, Hediger and colleagues (2000) found no differences in weight status by feeding method in 4- to 7-month-old children, but between 8 and 11 months of age, infants who had been exclusively breastfed had weights that were about one-fifth of a standard deviation below the U.S. reference median. This would represent a 200-g difference in growth associated with breastfeeding among infants of average size.
In comparison with published growth velocity reference data from the Fels Longitudinal Study (Guo et al., 1991), 3 g/day roughly represents the differences between the major percentile lines in the 3-month increment data (e.g., for boys, the 25th, 50th, and 75th percentiles were 23, 27, and 31 g/day, respectively). The clinical or functional significance of such differences is not well established.
Body composition is not typically assessed as a part of normal well-child care in clinical settings. The interpretation of body composition measures has been particularly challenging because extensive reference data on infants is lacking, and few studies have attempted to identify specific health risks associated with levels of body fat or lean tissue. More than a decade ago, the AAP task force concluded that methods to determine body water, body fat, and bone mass had “not reached the stage of precision, noninvasiveness and convenience that would make them feasible as a part of routine clinical testing of infant formulas” (AAP, 1988). However the state of the art has changed dramatically since then, and it is now possible to assess body composition using a variety of minimally invasive and precise methods. Furthermore, Butte and colleagues (2000) recently published reference data for infant body composition using a four-compartment model to estimate fat and fat-free mass, a deuterium dilution to measure total body water, and DEXA to measure bone mineral content.
It is important to evaluate body composition in the context of safety. Ultimately the goal of assessment is to identify levels of difference in body composition that are associated with immediate or long-term disease risk. The relevant component of body composition to measure will depend on the nature of the added ingredient. For example, if an ingredient is likely to have metabolic effects, it will be important to assess the relative contribution of fat and fat-free mass since these components may differentially reflect underlying factors related to energy and protein balance. In contrast, for other ingredients, bone mineral content may be more relevant.
Interpreting Inadequacies and Excesses in Growth Outcomes
The health implications of inadequate growth increments are not well described except in the context of severe undernutrition, which is an event unlikely to occur in closely monitored infant-feeding trials. There is evidence, primarily from populations with high poverty levels, of an association between more severe length and weight deficits (stunting and wasting) and impaired immune function (Forse et al., 1994), increased risk of morbidity and mortality (Pelletier et al., 1993), and poor developmental outcomes (for review, see
Grantham-McGregor et al., 2000). Mild growth deficits tend not to be strongly related to specific health outcomes independent of other nutritional risk factors. In comparisons of breastfed and formula-fed infants, despite differences in growth patterns, Dewey and colleagues (1991) found no differences in behavior or activity levels of breastfed and formula-fed infants.
While the focus in the past has been on nutritional inadequacies or growth deficits associated with formula feeding, it is important to also assess the potential for a new ingredient to cause excess growth. Aside from the risks associated with macrosomia in newborns, there is a lack of information on the immediate consequences of excess weight or of differences in body composition during infancy. While not related to feeding, infants with macrosomia associated with maternal gestational diabetes are at increased risk of postnatal obesity. This would seem to be an effect of the mother’s diabetes on body composition, with macrosomic infants having a significantly higher percent body fat (Fee and Weil, 1960). Of greater concern is the long-term consequences of excess infant growth, particularly in light of the worldwide epidemic of child and adult obesity. There is inconsistent evidence that fatness or excess weight gain in infancy predicts later obesity. When associations between excess infant growth and later outcomes do exist, they could reflect genetic factors or common behaviors, such as a consistent tendency of parents to overfeed.
The best evidence supporting an association between rapid infant weight gain and later risk of overweight comes from a prospective cohort study of more than 19,000 participants in the National Perinatal Collaborative Study. Researchers assessed the relationship of weight gain in the first 4 months of life to overweight at age 7 years (defined as body mass index [BMI] > 95th percentile of the CDC growth charts). After adjusting for birthweight, gestational age, sex, race, firstborn status, maternal BMI, and maternal education, they found that for each 100-g weight gain increase per month, the risk for overweight at age 7 increased by about 30 percent (Stettler et al., 2002b). Furthermore, nearly one-fifth of overweight status at age 7 could be attributed to infancy weight gain above the highest quintile. Stettler and colleagues (2002a) also found that weight gain in the first year of life was strongly associated with overweight and obesity in the school years among children living in the Seychelles. In a large British cohort born in the 1990s, more rapid weight gain in the first 2 years of life, evidenced by an increase in a weight-for-age Z score of greater than 0.67, was associated with higher BMI, percent body fat, and total fat mass in later childhood (Ong et al., 2000). In Pima Indian children, a population with a very high prevalence of obesity and type 2 diabetes, Lindsay and colleagues (2002) found two periods characterized by excess weight gain relative to the CDC growth reference. These were from 1 to 6 months and 2 to 11 years of age. Although Dietz (1994) did not identify infancy as one of the three critical periods in childhood for the development of obesity, the more recent findings summarized above have led researchers to suggest that infancy represents another critical period for the development of obesity later in life.
In contrast, there are a number of studies that find no evidence that overweight babies are destined to become overweight adults, unless they have obese parents. For example, Whitaker and colleagues (1997) found that risk of obesity in young adults was not increased by obesity at age 1 to 2 years unless at least one parent was also obese. Infancy is characterized by a substantial capacity for compensatory growth following a period of failure to achieve growth potential or a period of excess growth, thus limiting the long-term consequences of relatively short periods of abnormal growth. Butte and colleagues (2000) compared multiple dimensions of body composition among breastfed and formula-fed infants. Despite significant differences in early infancy, they found no persistent difference by feeding method beyond age 12 months.
There remains controversy over the extent to which deficits or excesses in overall growth, growth of specific organs and tissues, or differences in fat versus lean tissue have long-term effects on physiological functioning and disease risk. Again, the evidence of long-term effects tends to focus on the extremes in child size. For example, stunting in childhood is associated with short stature in adults, which is in turn associated with lower work capacity among adults engaged in physically demanding jobs and increased risk of poor obstetric outcomes in women. However a recent study of a cohort of Finnish children was the first to show that infant obesity was significantly associated with later development of type 1 diabetes (Hypponen et al., 2000). The hypothesized mechanism is hyperinsulinemia and damage to beta cells associated with early excess body fat. Based on research among Indian infants, Yajnik (2001) has hypothesized that deficits in skeletal muscle in infancy may contribute to insulin insensitivity and risk of type 2 diabetes later in life.
There is increasing evidence that growth deficits in utero and in the early postnatal period have important long-term health consequences owing to “programming” of structure or metabolic functioning by nutritional inadequacies. The focus of most of the research has been on fetal programming (Godfrey and Barker, 2001), but there is also evidence of effects of postnatal growth deficits resulting in small size at 1 year of age (Vijayakumar et al., 1995). Furthermore, there is evidence to suggest that feeding mode during infancy has long-term effects on lipid profiles (Cowin and Emmett, 2000; Plancoulaine et al., 2000), risk of later obesity (Armstrong and Reilly, 2002; for a review of effects on obesity, see Butte, 2001), and risk of other diseases (Leeson et al., 2001). However there is no particular substance in milk to which these effects may be attributed.
Evaluating the 120-Day Growth Study
Although not currently a requirement, manufacturers often provide 120-day growth studies to demonstrate healthy growth.2 The committee was specifically asked to evaluate the adequacy of the currently used 120-day growth study. Conceptual issues on the measurement of growth are discussed above, so the following section is confined to a discussion of the duration of the study. The first 120 days of life is a period during which infant formula is most likely to be the sole source of nutrition for the infant and a period of high growth rates and, thus, a period of high susceptibility to dietary intake. From a practical perspective, it may be difficult to recruit infants whose parents are willing to forego the introduction of other foods until after 6 months of age, and there is no reason to think that an adverse effect of an ingredient new to formulas would be detected only between 4 and 6 months of age. However a study length of 120 days may be insufficient for several reasons. First, human milk is recommended as the sole nutrient source for infants for at least the first 4 months (AAP, 1997; IOM, 1991) and preferably for the first 6 months of life (ADA, 2001; IOM, 1991; WHO, 2002). When intended as a human-milk substitute, exclusive formula feeding should be recommended for the same period of time. Ideally formula should be tested for the entire period for which it is intended to be fed as the sole source of infant nutrition, consistent with breastfeeding guidelines. This is particularly true since intake of infants receiving only formula will be greater in the period from 4 to 6 months of age.
Second, serious limitations of the 120-day growth study are that it does not allow for the determination of delayed effects or for understanding longer-term effects of early perturbations in growth. Longer-term follow-up of participants in clinical studies should be recommended, with the duration to cover at least the period when infant formulas remain a substantial source of nutrients in the infant diet.
In summary, the committee recognizes that to establish levels of growth that indicate a safety concern is a difficult endeavor. However the committee concludes that any systematic and statistically significant difference in size or growth rate between infants fed a formula containing a new ingredient versus human milk or a previously approved formula should be a safety concern.
SPECIFIC ORGAN SYSTEMS
The Importance of Assessing Specific Organ Systems
As described in the previous section, the committee recommends that growth studies should remain the centerpiece of clinical testing of ingredients new to infant formulas. However growth deficits are likely to appear only secondary to effects on specific organs or tissues and may not appear for some time after nutritional insult. The major organ systems should also be studied when assessing the safety of an ingredient new to infant formulas (see Figure 6-1, Box 3 and Figure 6-2).
The gastrointestinal tract is the first organ that encounters ingested ingredients. It serves to protect the infant from environmental pathogens, antigens, toxins, and other noxious agents (Walker, 2002). In healthy infants, gastrointestinal tract functions assure that normal growth and development occur, provided the infant is offered the necessary nutrients. Any of these functions may be affected by the diet or ingredients in the diet, and impaired function can lead to inadequate nutrient availability.
The immune system is also important during infancy because of its regulatory effects of a substance on immune competence and the potential for an inflammatory or allergic response to a new ingredient. Food allergy and other adverse reactions to food are more common in infants than in any other age group. This is partly a reflection of the relative immaturity of the infant immune system. Compared with older children and adults, young infants have low immunoglobulin A concentrations and thus reduced binding of antigens in the gut. The infant immune system is not fully mature at birth, with deficits in the ability to prevent invasion of pathogens and to respond to antigens. Of particular concern in the context of ingredients new to infant formulas is the increased permeability of the gut mucosal barrier in the presence of inflammation or infection or if the integrity of the epithelial cell layer is disrupted. The increased permeability allows macromolecules to be absorbed and stimulates allergic responses to food proteins. Furthermore, immature lysosomal function in mucosal cells and limited intracellular proteolysis may result in further intestinal damage and increased permeability.
Finally, the endocrine system is not fully developed until after puberty has occurred. It is possible that an ingredient new to infant formulas could affect endocrine development or expression of endocrine function. An example of such a possibility is the phytoestrogen content of an infant formula having an effect on the development or expression of estrogen-responsive tissues. Therefore clinical studies should include additional assessments to ensure that infants:
-
grow and develop according to standards,
-
consistently display normal vital signs,
-
feed and stool normally,
-
do not vomit (aside from infantile reflux),
-
demonstrate consistently normal laboratory values (blood count, blood chemistries, liver function, renal function, and urine analysis),
-
do not present immunologically related injuries, and
-
do not present signs of endocrine disruption.
RECOMMENDATION: Assessment of clinical endpoints should include signs or adverse laboratory indicators of the major organ systems, including the gastrointestinal tract, kidneys, blood, and immunological and endocrinological systems.
Assessing the Gastrointestinal Tract
The gastrointestinal tract consists of the hollow organs (mouth, pharynx, esophagus, stomach, small bowel, and colon) and the solid organs (liver and pancreas). The functions of the hollow organs are as follows:
-
Motility. The propulsion of lumenal contents through the gastrointestinal tract occurs as the result of contractions of two layers of perpendicularly oriented smooth muscle. Beginning in the esophagus, the movement of the walls of the hollow tube mixes and propels lumenal contents. Nutrients and water are absorbed and waste is extruded during the passage of substances through the tubular gastrointestinal tract.
-
Digestion and absorption. Digestion begins in the mouth with salivary enzymes and continues through the colon, where some digestion of carbohydrate can occur, especially in infants. A relative fat malabsorption occurs in infants compared with adults (Fomon et al., 1970). Similarly, pancreatic secretion of amylase and starch digestion is less in infants than in adults. Absorption of nutrients occurs throughout the gastrointestinal tract, beginning in the small bowel. Absorption can be passive (diffusion), active, or carrier mediated.
-
Secretion. Secretion of substances, such as acid, pepsin, bile, and enzymes, is necessary to digest nutrients. In addition to the digestive material, the gastrointestinal tract secretes hormones and paracrine substances that modulate the function of other cells.
With the exception of pancreatic exocrine function and bile acid synthesis and composition, development of the gastrointestinal tract is essentially complete at birth for infants born after a 34-week gestation (Antonowicz and Lebenthal, 1977; Auricchio et al., 1965; Fredrikzon et al., 1978; Hadorn et al., 1968; Hamilton, 2000; Lindberg, 1966; Montgomery et al., 1999; Norman et al., 1972; Watkins, 1985; Watkins et al., 1973).
Clinically relevant tools are available to assess each function of the gastrointestinal tract and some tools are more specific than others. For example, normal growth and development occurs only when the gastrointestinal tract is functioning optimally. But slowed or inadequate growth, as the common denominator of impaired gastrointestinal function, does not identify the function that is impaired. Table 6-3 lists the functions of the gastrointestinal tract and general (level 1 assessments) and specific (level 2 assessments) clinical outcome measures that can be used to assess whether a specific function has been impaired. Table 6-4 lists the advantages and disadvantages of each of the outcome measures.
Motility can be assessed by measurement of esophageal, antroduodenal, small bowel, and rectal contractions (Scott, 2000); gastric emptying time (Di Lorenzo et al., 1987); and
TABLE 6-3 Gastrointestinal Tract Clinical Endpoints
|
Function |
Level 1 Assessments |
Level 2 Assessmentsa |
|
Absorption |
Growth velocity |
Stool fat, stool protein, stool carbohydrate, stool alpha-1-antitrypsin, stool pH, balance studies, blood levels of specific nutrients |
|
Allergic |
Vomiting, diarrhea, irritability, colitis |
Serum IgE, quantitate peripheral eosinophils, skin tests, RAST, challenge tests |
|
Barrier |
Not applicable |
Polyethylene glycol, lactulose/mannitol, 51Cr EDTA, proteins, stool cultures, serum antigens |
|
Biotransformation |
Serum liver enzyme levels |
Blood levels of bile acids or specific drugs and their metabolites, serum liver enzyme levels, isotope excretion scans |
|
Digestion |
Growth velocity |
Stool: fat, protein, sugars, pH |
|
Homeostasis |
Not applicable |
Balance studies, blood/tissue levels of specific nutrients |
|
Immunological |
Serum antibodies |
Response to oral vaccinations, stool cultures |
|
Motility |
Absence of vomiting, stool pattern, growth velocity |
Measurements of transit times (e.g., charcoal, hydrogen breath tests) |
|
Secretory |
Growth velocity |
Measure specific hormones, stool chymotrypsin, elastase, alpha-1-antitrypsin |
|
Metabolism of macronutrients Protein |
Growth velocity, serum liver enzyme levels, liver size by examination |
Urine and serum amino acid levels, serum glucose serum proteins (e.g., albumin, prealbumin, clotting factors, alpha-1-antitrypsin, transferrin), serum liver enzyme levels, liver size by examination and ultrasound |
|
Lipid |
Liver size by examination, serum liver enzyme levels |
Serum lipid levels (cholesterol, phospholipids, triglycerides), serum glucose, liver size, and fat content by examination and ultrasound, urine organic acids |
|
Carbohydrate |
Liver size by examination, serum liver enzyme levels |
Serum glucose levels, serum liver enzymes, liver size by examination and ultrasound |
|
NOTE: The petitioner (or manufacturer), in consultation with the expert panel, will determine which tests are required based on a thorough analysis of the potential effects of the new ingredient. apH = potential of hydrogen, IgE = immunoglobulin E, RAST = radioallergosorbent test, EDTA = ethylene-diaminetetraacetic acid. |
||
transit time (Scott, 2000). The muscle fibers, nervous tissue, and some neurotransmitters can be evaluated with biopsies. Generally, however, motility is functionally normal if there is no vomiting, if the stool pattern is normal, and if growth velocity is normal. If measurements of motility are needed they should be carried out by noninvasive techniques.
Digestion and absorption can be monitored by quantifying stool fat (Fomon et al., 1970; van de Kamer et al., 1949); protein, such as alpha-1-antitrypsin (Dinari et al., 1984); carbohydrate content (Grant et al., 1989); and breath tests (Fernandes et al., 1978; Maffei et al., 1977; Perman et al., 1978; Robb and Davidson, 1981; Thomas et al., 1981). The amount of specific nutrients can be quantified in blood. However if growth velocity remains normal, it is unlikely that digestion is adversely affected by dietary intake.
Some secretory functions of the gastrointestinal tract can be assessed by quantifying levels of specific hormones or enzymes (e.g., gastrin, cholecystokinin, trypsin, lipase, or motolin) in the blood or stool. Growth velocity falters if the secretory functions are impaired.
For some nutrients the gastrointestinal tract regulates absorption based on nutrient
TABLE 6-4 Advantages and Disadvantages of Various Organ Clinical Endpoints
|
Outcome Measure |
Advantages |
Disadvantages |
|
Balance studies |
Accurate assessment of specific nutrients |
Requires admission to a clinical research center |
|
Bleeding time |
Easy, safe, accurate |
Painful, requires a small incision, does not identify specific clotting abnormality |
|
Blood pressure |
Noninvasive, used in routine health assessment, standards available for children |
Requires personnel with some training |
|
Dual-energy X-ray absorptometry scan (bone mineralization) |
Accurate for bone mineralization, fat mass, and lean body mass |
Normative data not available for infants, may require sedation, requires expensive equipment |
|
Fecal fat–72 hour |
Noninvasive |
Collection starts and ends with marker, in-home collection may be difficult, collection in a clinical research center is more accurate |
|
Growth velocity |
Established normal values, noninvasive, inexpensive, readily available technology |
Does not identify specific function that is deficient or impaired |
|
Motility |
Specific, can identify area of the gastrointestinal tract where an abnormality occurs |
Invasive, limited to centers, time intensive in infants who cannot cooperate, but can measure some aspects by noninvasive techniques (e.g., hydrogen breath tests) |
|
Permeability studies (polyethylene glycol, sugars, antigens) |
Easy to use probes |
Requires urine or blood collection |
|
Serum levels of nutrients, hepatic enzymes, bile acids, chemistries, blood gas, blood counts, specific proteins |
Accurate measure of circulating nutrient levels, liver function, hematological function |
Requires blood draw, some assays available only in centers |
|
Stool components (fat, enzymes, protein, pH, reducing substances, cultures) |
Noninvasive, relatively easy to collect in infancy |
Depending on the test, varying specificity and sensitivity |
|
Urine analysis, quantitation of nutrients, estimate of glomular filtration |
Noninvasive, accurate |
Collection may be difficult in infants |
|
Ultrasound |
Noninvasive, gold standard to size abdominal organs and assess cirrhosis, fatty infiltration in liver, and inflammation in bowel |
Relatively expensive |
|
Vaccine response |
Accurate measure of B-cell function |
Requires injection of vaccine, blood draw |
levels. This regulation is often complex and involves other organs, such as the liver and kidney for calcium homeostasis (IOM, 1997), and the liver, spleen, and bone marrow for iron (IOM, 2001). Balance studies, stable isotopes, levels of specific nutrients in blood or tissue, and storage forms of specific nutrients can be assessed.
The gastrointestinal tract is the site at which interaction with a food allergen occurs.
Different factors predispose for the development of food allergy, such as family history, immune deficiency, or early exposure to antigens. Food allergy can consist of type I, III, or IV reactions. Allergic reactions of this organ include enteropathy, colitis, and nonspecific reactions, such as recurrent vomiting, bowel edema, obstruction, constipation, occult bleeding, and colic. The manifestations of food allergy vary with age and site of food antigen exposure. In infancy food allergy can be assessed by evaluating the infant for vomiting, diarrhea, malabsorption, gastrointestinal loss of blood or protein, and constipation, and by performing challenge tests.
It is unlikely that the human term-infant gastrointestinal tract is more permeable than that of older infants and children (Sanderson and Walker, 1993). One study using human α-lactalbumin as a marker of permeability showed that serum concentration of this protein was increased in term breastfed infants for the first several months of life (Jakobsson et al., 1986). However others, using bovine β-lactoglobulin in formula-fed infants, did not show a change in gastrointestinal permeability over the first several months of life (Roberton et al., 1982). In healthy term infants, gastrointestinal permeability may be increased by allergy (Boehm et al., 1992; Dupont et al., 1989; Falth-Magnusson et al., 1986; Heyman et al., 1988; Juvonen et al., 1990; Schrander et al., 1990), infection (Holm et al., 1992), and perhaps colic (Lothe et al., 1990). Permeability can be assessed by using the inert carbohydrates (e.g., lactulose and mannitol), polyethelene glycol 4000, 51Cr ethylenediaminetetra-acetate, and heterologous proteins (e.g., bovine β-lactoglobulin) or homologous proteins (e.g., human α-lactalbumin) (Bjarnason et al., 1995; Sanderson and Walker, 1993).
The pancreas serves as a secretory- (exocrine) and hormone- (endocrine) producing organ. Exocrine functions are difficult to assess in clinical studies in healthy infants but, as noted above, can be assessed by directly quantifying lumenal concentrations of enzymes and bicarbonate before and after a stimulus. It is only when pancreatic exocrine secretion is dramatically decreased that a deceleration of growth velocity occurs (Huynh and Couper, 2000). Severe pancreatic insufficiency can be monitored by measuring fat or certain enzymes (e.g., trypsin) in stools. Pancreatic endocrine dysfunction is most often manifested as diabetes, which can be assessed by obtaining serum insulin concentrations, blood, and urine glucose (Huynh and Couper, 2000).
The liver plays a central role in the metabolic adaptation of the fetus to extrauterine life through glucogenolysis, gluconeogenesis, and the regulation of amino acid and fat metabolism (Karpen and Suchy, 2001). These functions of the liver can be assessed by quantifying urine and blood amino acid levels; urine organic acid levels; blood proteins, lipids, ammonia, and bicarbonate; liver fat; and ultrasound. However if the liver is unable to function normally with respect to carbohydrate, protein, or lipid metabolism, normal growth velocity will not be maintained.
In addition, the liver synthesizes and excretes bile acids (Setchell and O’Connell, 2001). Bile acid synthesis, the bile acid pool, and intralumenal bile acid concentrations gradually increase during the first year of life. Bile acid secretion is maximal at birth and cannot be further stimulated. This function of the liver can be assessed by quantifying blood levels of liver-derived enzymes as a marker of hepatocyte integrity, by quantifying blood levels of bile acids and isotope excretion scans as a marker of hepatic excretory function, and by measuring serum levels of specific drugs and their metabolites (Batres and Maller, 2001).
Assessing the Kidneys
The kidneys perform vital functions, including filtration of plasma, reabsorption of water and electrolytes, excretion of wastes, and the production of hormones that control
TABLE 6-5 Kidney Clinical Endpoints
blood pressure, calcium homeostasis, and red cell production (Binley et al., 2002; Gleim, 2000; McMurray and Hackney, 2000). Specific tests (level 2 assessments) can be performed to identify each of these functions, but general assessments (level 1 assessments) of blood pressure, urinary analysis, growth velocity, serum creatinine, blood urea nitrogen, calcium, bicarbonate, and a complete blood count will establish if renal function is abnormal or adversely affected by a component of the diet (Table 6-5).
Specific functions of the kidney that can be assessed include glomerular filtration rate (GFR), which can be measured by quantifying the clearance of a substance that is freely filtered across the capillary wall and is neither reabsorbed nor secreted by the tubules. The optimal measurement of GFR is insulin clearance (Arant et al., 1972). Clinically, however, GFR can be estimated by the clearance of endogenous creatinine. At serum levels of creatinine exceeding 2.0 mg/dL, changes in renal function can be monitored by the serum creatinine concentration. GFR is adequate for healthy term infants, but it does not approximate adult rates until about 3 years of age. Renal tubular reabsorption and urine acidification is less at birth and for several months thereafter than it is for adults. This function is adequate for healthy infants, but contributes to fluid and electrolyte abnormalities in infants who are ill or are fed an inappropriate diet (Goldsmith and Novello, 1992).
The kidney also serves as an endocrine organ, synthesizing and degrading prostaglandins, kallikrein-kinin, and renin-angiotensin, which control blood pressure. Hydroxylation of vitamin D creates the hormone that controls calcium homeostasis, which occurs in the kidney. Erythropoetin, the glycoprotein that regulates both steady-state and accelerated red blood cell production, is governed by oxygen availability to the kidney. Blood levels of these hormones and the substances they regulate can be quantified.
Assessing the Blood
The hematological system consists of red blood cells, white blood cells, platelets, and proteins. The function of the red blood cell is to transport oxygen to tissues. This function is performed by hemoglobin, which combines reversibly with oxygen, allowing the red blood cells to transport oxygen from the lungs and deliver it to tissues. Hemoglobin accounts for more than 95 percent of the total protein and about 90 percent of the dry weight of the red blood cell. Red blood cell function can be assessed by quantifying the number of cells, the hemoglobin concentration, and the hematocrit. Membrane lipid analysis, fragility studies, observation of the blood smear, and a reticulocyte count can also be performed (Brugnara and Platt, 1998).
The capacity of white blood cells to produce antibodies to antigens is intact at birth. In general, white blood cell function can be assessed by quantifying the total white cell count, the absolute count of specific cells, skin tests, and immunoglobulin levels. The specific function of phagocytic cells, such as chemotaxis, ingestion, and oxidative metabolism, can be assessed in isolated cells. Some products of these functions, such as myeloperoxidase, can be quantified in blood or stool. Specific lymphocyte function can also be assessed in isolated cells and by quantifying inflammatory mediators in blood. Abnormalities in white blood cell function can be suspected clinically by occurrence of frequent infections or infections caused by low-virulence pathogens.
Platelets are important in homeostasis. Platelet activity is assessed by quantifying the number and morphology of platelets in a blood sample and by assessing platelet aggregation and specific platelet functions. Platelet function can also be assessed by performing a bleeding time (Handin, 1998).
Several clotting factor concentrations in blood are lower during the neonatal period than in adulthood (Esmon, 1998). The lower level of clotting factors is associated with prolonged prothrombin and partial thromblastin time. After the neonatal period, coagulation is the same as that of adults. Coagulation function can be assessed by quantifying each of the following factors: I through XII, plasminogen, antithrombin III, prekallikrein, and high molecular weight kininogen. Coagulation can also be assessed by performing a thrombin time and a partial thromboplastin time or by noting if abnormal bleeding is present (see Table 6-6).
Assessing Immunological and Allergic Activity
A newborn’s digestive system is fairly mature, but may only incompletely break down food proteins. Thus infants are especially susceptible to allergic sensitization. The immune response of newborn infants is predominantly associated with the Th2-type of the helper T-cell population, possibly because of in utero priming of fetal T cells by transplacental passage of common environmental allergens and dietary antigens. In general normal infants exhibit a low-grade immunological response to subsequent exposure to such environmental
TABLE 6-6 Blood Clinical Endpoints
agents after birth, which is limited to immunoglobulin (Ig) G and IgM isotypes and to the Th1-type of the helper T-cell population (Holt et al., 1999). During further development the neonatal immune system continues to shift towards Th1-type response. It has been proposed that alterations in the neonatal mucosal environment (e.g., a change in microflora), the use of formulas (and lack of breastfeeding), antibiotics, mucosal infections, and a highly hygienic environment in early infancy may lead to further increase in the Th2-type of helper T cells that were primed in utero (Holt et al., 1999). Th2-type helper T-cell expression is currently considered the hallmark of allergic immunopathology (Kay, 2001).
Many of the proteins added to infant formulas are functional proteins (i.e., proteins that are added for their function—not as a source of amino acids) and to maintain their function, they must be resistant to digestion, a property shared with allergens. The addition to infant formulas of novel proteins (including glycoproteins or lipoproteins), which by their nature can induce allergic or other adverse reactions, requires clinical testing. Human-milk proteins are not expected to be allergenic in humans since they are produced by the human mammary gland. Possible exceptions are the proteins that originate from maternal dietary components, such as cow-milk proteins. Also, as has been recognized in the production of biotech crops (Kok and Kuiper, 2003), the commercial production of milk proteins using recombinant technologies may produce unintended and unexpected side effects. For instance, one milk protein produced in recombinant microorganisms may differ from native proteins in level of glycosylation, posttranslational modifications, or minor amino acid sequences, which may change the allergenicity potential. Furthermore, there is potential for contamination with compounds deriving from the genetically modified organism used as the protein source.
The central aspect of clinical testing in infants should include the evaluation of a diverse spectrum of immune functions in response to an added substance. To develop the appropriate tests for assessing the safety of the immunological responses to new substances, it is useful to first identify the target tissues affected by the interaction of ingested substances with the host immune system (Table 6-7).
TABLE 6-7 Target Tissues and Signs Derived from Interactions of a New Ingredient with a Host Immune System Target Tissue
|
Target Tissue |
Immunoglobulin E-Mediated |
Mediated by Other Immunological Mechanisms |
|
Gastrointestinal |
Infantile colic |
Food-induced enterocolitis and proctocolitis |
|
Eosinophilic gastroenteritis |
Allergic gastroenteritis, eosinophilic (postprandial nausea, weight loss) |
|
|
Oral allergy (angioedema) |
Celiac-like disease |
|
|
Gastrointestinal tract anaphylaxis (nausea, chronic diarrhea) |
|
|
|
Celiac disease |
|
|
|
Airway |
Rhinitis-conjunctivitis |
Heiner syndrome (pulmonary hemosiderosis) |
|
Laryngeal edema-obstruction |
|
|
|
Acute bronchospasm |
|
|
|
Skin, joint, blood vessels |
Urticaria |
Dermatitis herpetiforms |
|
Atopic dermatitis |
Contact sensitivity |
|
|
|
Contact irritation (acidic fruits and vegetables) |
|
|
|
Migraine |
|
|
|
Arthritis |
|
|
SOURCE: Sampson (1996, 2002). |
||
When measuring immunocompetence, clinical assessment of allergic response should include evaluation of specific signs, laboratory testing for evidence of specific immune responses, inflammatory cytokines, and allergen-specific response to challenges. IgE isotype is present at birth and, therefore, specific signs of IgE-mediated allergic reactions, such as urticaria, vomiting, diarrhea, respiratory signs, and anaphylaxis, can occur in the neonate. Clinical signs of atopic dermatitis, including erythema, edema, crusts, excoriations, lichenification, dryness, degree of itch, and loss of sleep, should be evaluated. The latter are evaluated using a scoring system for the extent and intensity of dermatitis (European Task Force on Atopic Dermatitis, 1993). Gastrointestinal responses, particularly the chronic type, are generally presumed to be T-cell mediated, but may also be associated with specific IgE-mediated immunological interactions. These include enterocolitis, proctocolitis, enteropathy, and a subset of allergic eosinophilic esophagitis/enteropathy (e.g., vomiting and diarrhea) with eosinophil infiltration of the affected portion of the gastrointestinal tract (Sicherer et al., 2001).
Laboratory testing for evidence of specific immune responses should include the determination of serum and mucosal antibody profile, including IgE, and measurement of T-cell-mediated immune responses and of specific proinflammatory and immunoregulatory cytokines synthesized after exposure to the new ingredient. Specific IgE responses to allergens can be assessed by measuring serum IgE concentrations by the radioallergosorbent test for binding of IgE. These tests can be used as level 1 and level 2 assessments, as indicated in Table 6-8, based on the criteria specified by the committee. Specific level 2 assessments should be performed when there is evidence of adverse effects from the more general level 1 assessments.
However because allergic sensitization is a rare event, level 1 assessments in unselected infants may not have the power to detect such responses. The evaluation of subpopulations of infants selected for pre-existing allergies will also not be helpful because they will not be sensitized to the novel protein under consideration. Thus the potential allergenicity of such ingredients must be carefully evaluated in the preclinical studies described in Chapter 5.
TABLE 6-8 Available and Potential Tools for Assessment of Immnological and Allergic Outcomes
|
Tool |
Assessment Level |
Current Use in Assessment |
Potential Value for Safety Endpoint Testing |
|
|
Advantage |
Disadvantage |
|||
|
Potential for antigenicity and immunogenicity |
1 |
Yes |
Screening to demonstrate the ability to induce an immune response |
Nonspecific, does not predict potential for disease |
|
Serologic evidence of prior exposure to ingredient |
|
|||
|
Serum antibody |
1 |
Yes |
Provides specific evidence of prior exposure to specific ingredient; can differentiate between recent and past exposure |
Does not predict potential for disease; requires peripheral blood samples |
|
Cellular immunity |
2 |
Yes |
Sensitive marker for immunoregulatory vs proinflammatory immune response |
Difficult to perform routinely; requires cellular or tissue sample; not standardized |
Sensitized cells release specific cytokine and chemokine mediators that can be quantified. These include substances such as histamine and tryptase, as well as markers of inflammation in the gastrointestinal tract. The latter may be of value in the evaluation of allergic response since inflammation is a risk factor for increased sensitization. Markers of intestinal inflammation include eosinophil cationic protein in serum and feces, α-1 antitrypsin, and tumor necrosis factor alpha (Majamaa et al., 1996). Skin prick and patch tests have good negative predictive value, but poor positive predictive value, and therefore are of more limited use in clinical testing.
As with other clinical studies, the most definitive clinical assessments are accomplished by double-blind, controlled trials. However investigators should consider that oral provocation of sensitive subjects could result in severe reactions and therefore study conditions should be carefully designed and controlled.
Assessing Endocrinological Activity
The endocrine system consists of multiple organs that secrete a wide variety of hormones that are responsible for maintaining the proper biochemical milieu of the body. Hormones are biochemicals that are secreted by the various glands (e.g., pituitary, thyroid, parathyroid, pancreas, adrenal, testes, and ovary) and act at other sites within the body. These hormones usually act by signaling biochemical reactions at cell membranes or intracellularly. Changes in endocrine function may either be intrinsic (e.g., a decrease in thyroid function because iodine is missing from the diet) or extrinsic because of chemicals (including nutrients) in the formula that may act as endocrine effectors or disruptors. Changes in hormone function may first be evident in growth changes. Most changes may take months or years to become evident; for example, sex hormone disruption may not be obvious until puberty.
Breastfeeding does not eliminate the concern for infant exposure to hormones. Oral contraceptive hormones are excreted in milk, and cohorts have been followed long term to ensure that the concentrations seen do not change the onset or course of puberty. Any substance added to an infant formula that may change secretion or function of growth or sex hormones may require follow-up through adolescence.
Exposure to endocrine disruptors in the environment may be brief in relation to the life span. Changes may thus be transient or not measurable. Some parameters that may show an immediate effect upon disruption of the endocrine function are thyroid-stimulating hormone (TSH), triiodothyronine (T3), thyroxine blood glucose (T4), blood calcium, phosphorus, and urine-specific gravity. Measurement of possible long-term effects include ovarian or testicular function, obesity, and pituitary function. Most endocrine measurements can be conducted by collecting either the blood or the urine. It is important to consider the age, sex, dietary status, body size, and medications during the interpretation of the numerical value of any clinical test for the endocrine system, especially during the gestational age. Level 1 screening should contain at least one test from all of the major substances, organs, and outcomes of the endocrine system. They include growth (e.g., insulin-like growth factor-1), the thyroid (TSH, T3, T4), the adrenals (cortisol, adrenocorticotropic hormone), the parathyroid (calcium, parathyroid hormone), antidiuretic hormone (urine osmolality), and glucose/insulin (Sperling, 1996). If there are any adverse events detected, this leads to a level 2 assessment, which includes immunoassays, binding proteins, and imaging techniques (e.g., body imaging, radionuclide imaging) (Sperling, 1996). A summary of the clinical endpoints for the endocrine system is provided in Table 6-9.
TABLE 6-9 Endocrine Clinical Endpoints
|
Function |
Level 1a |
Level 2b |
|
Growth |
IGF-1 |
IGFBP-3 |
|
Thyroid |
TSH immunoassays, T3, T4 |
Binding proteins, radionuclide imaging |
|
Adrenal |
ACTH, cortisol |
CRH, plasma rennin, plasma aldosterone, body imaging |
|
Parathyroid |
Calcium, PTH |
Phosphorus |
|
Antidiuretic hormone |
Urine osmolality |
Urine sodium excretion |
|
Glucose/insulin |
Plasma insulin, glucose concentration |
|
|
NOTE: The petitioner (or manufacturer), in consultation with the expert panel, will determine which tests are required based on a thorough analysis of the potential effects of the new ingredient. aIGF-1 = insulin growth factor-1, TSH = thyroid-stimulating hormone, T3 = triiodothyronine, T4 = thyroxine, ACTH = adrenocorticotropic hormone, PTH = parathyroid hormone. bIGFBP-3 = insulin growth factor-binding protein-3, CRH = corticotrophin-releasing hormone. SOURCE: Sperling (1996). |
||
DEVELOPMENTAL-BEHAVIORAL OUTCOMES
“… subtle behavioral effects can appear well in advance of clear neurological dysfunction.”
The Importance of Assessing Developmental-Behavioral Outcomes
There are a number of reasons why it is essential to include developmental-behavioral outcomes in future studies of the safety of ingredients new to infant formulas (see Figure 6-1, Box 6 and Figure 6-3). First, behavioral outcome measures are sensitive to exposure to toxic substances, particularly at low exposure levels. There are potential consequences to the infant of deviations from normal developmental pathways. Therefore when evaluating the addition of ingredients new to infant formulas, avoidance of type II errors (failure to detect a real effect) may be more critical then avoidance of type I errors (accepting a spurious finding as significant). In order to minimize the likelihood of failing to detect developmentally meaningful consequences associated with the addition of ingredients new to infant formulas, investigators must go beyond traditional toxicological and morphological assessments. As Weiss (1995) has argued, focusing only on easily observed physical malformations may seriously underestimate the actual impact of toxic exposure at either an individual or a population level. Supporting this position, evidence from a number of studies indicates that following exposure to toxins, subtle behavioral effects can appear well in advance of clear neurological dysfunction (Evangelista de Duffard and Duffard, 1996; Sobotka et al., 1996).
Inclusion of developmental-behavioral deficits may be particularly critical when investigating low-level exposure to toxins (Gaylor et al., 1998). It has been hypothesized that when multiple outcomes can be affected by exposure to toxic substances, behavioral outcomes may be among the most sensitive to toxic effects. When expressed in terms of level of exposure required to produce an effect, going from highest to lowest required level, the order of outcome sensitivity would be: mortality > malformations > physical growth > behavior (Vorhees, 1986). This hypothesized hierarchical ordering has been documented with regard to exposure in utero to vitamin A, salicylates, mercury, and alcohol (Adams, 1993; Jacobson and Jacobson, 2000). While the structural or functional domains affected by exposure to toxins will vary depending on the developmental course of different organ and functional systems (Shaheen, 1984), in the first year of life there is rapid development of these systems. What this means is that during the time period of maximum human exposure
to infant formulas, subtle, but important, developmental consequences may not be detected in nonhuman-based preclinical studies that primarily focus on toxicity or morphological changes and that may not include potentially more sensitive developmental-behavioral endpoints that are comparable with those assessed at the human level.
Second, developmental-behavioral measures can have long-term predictive value. Little direct evidence is available comparing the relative long-term consequences of outcomes such as physical malformations versus behavioral deviations. However it has been hypothesized that the long-term consequences of alterations in some components of behavioral development may be more critical than physical consequences in terms of affecting the individual’s ability to adapt to environmental demands (Russell, 1992). For example, both facial changes and cognitive deficits are associated with fetal alcohol syndrome. The most likely candidate to influence the individual’s ability to succeed in school would be the cognitive consequences of fetal alcohol syndrome (Vorhees, 1986).
A third reason to include developmental-behavioral outcomes in studies of the safety of ingredients new to infant formulas is that bidirectional brain-behavior links exist (e.g., brain development mediates changes in behavioral competence, but the child’s interactions with his or her environment also can influence brain development). Although this report discusses neural and behavioral development separately from each other, these two areas of development are closely interlinked. Obviously changes in central nervous system (CNS) structure and function act as critical mediators for infant developmental-behavioral changes (Johnson, 2001; Lozoff et al., 1998; Nelson, 1994, 1995). However the converse is also true. There is increasing evidence showing that brain development can reflect changes in the child’s environment (Greenough and Black, 1992; Nelson and Bloom, 1997; Schore, 1994). For example, systematic differences in infant brain electrical activity have been related to differences in rearing styles between depressed and nondepressed mothers (Dawson and Ashman, 2000). Environmental changes can be driven by changes in the child’s behavioral patterns, as seen in studies showing systematic changes in maternal independence- and dependence-fostering behaviors as their infant shows increased levels of functional competence (Kindermann, 1993). This means that exposure to toxic substances that initially impact upon brain development will result in synergistic bidirectional influences upon brain and behavior.
Criteria for Including Developmental-Behavioral Outcome Measures
Outcome Domains
When developmental-behavioral outcomes have been used, much of the research in behavioral teratology and behavioral toxicology was designed to investigate whether there were cognitive deficits associated with exposure to potentially toxic substances, such as lead (e.g., Bellinger, 1995; Cory-Slechta, 1990). However it is important to recognize that development in the early years of life is characterized by changes across multiple domains (Masten and Coatsworth, 1998; Wachs, 1999). Because infant development is multifaceted, it is important to go beyond a relatively narrow focus on cognitive outcomes in order to fully evaluate the potential adverse effects of the addition of ingredients new to infant formulas (Struthers and Hansen, 1992; Vorhees, 1986). Normality of development in one developmental domain does not necessarily mean that there will be normality across all domains (Lester et al., 1995). At a minimum, assessment of the potential developmental-behavioral consequences of the addition of ingredients new to infant formulas should encompass outcomes in the domains of: (1) sensory and motor development, (2) cognitive development, (3) affect and temperament, and (4) neural integrity (see Table 6-10).
RECOMMENDATION: Assessment of clinical endpoints should include measurement of infant sensory-motor, cognitive, affectual, and neural function with instruments that follow recommended criteria.
Level of Assessment Required
Ideally the level and type of assessment utilized to evaluate the safety of an ingredient new to infant formulas would be driven by known links between the class of the added ingredient and the specific aspects of brain and behavioral development. Within this framework, knowledge of which and to what degree specific neural structures, processes, or behavioral functions were influenced by a given ingredient would tell the researcher which brain areas or processes and which behavioral functions should be tested to determine possible adverse consequences associated with the new ingredient. There is a solid body of knowledge on nutrient-brain-behavior links for a few substances, such as iron, and a growing body of knowledge on such links for polyunsaturated fatty acids (Rao and Georgieff, 2000). Unfortunately, for the most part, the knowledge is insufficient to base the choice of neural-behavioral measures on the empirical evidence alone. For the majority of new ingredients added to infant formulas, choice of the level of assessment and the domains assessed will require balancing the empirical evidence on substance-brain-behavior links with integration of results from the preclinical studies on a given substance, with knowledge of the long-term relevance of different outcome measures. To this end, the committee proposes a hierarchical set of criteria to determine the level of assessment that is needed in future studies of the potential developmental-behavioral-neural consequences of new ingredients added to infant formulas (see Figure 6-3).
Level 1 assessments are based on using behavioral and neural screening measures that can be easily administered using parental report or during a routine well-baby physical exam, which can be carried out as part of a clinical trial using a standardized examination protocol (Table 6-10). Because these measures are lower in sensitivity than the measures sufficient for use in the other levels described here, what appears to reflect normal development on these measures does not necessarily mean that an ingredient added to infant formulas is safe. Because of the lower sensitivity of level 1 assessment measures, more stringent criteria must be met to justify use of such measures. Hence level 1 assessments are recommended only if all of the following decision criteria occur:
-
There is no existing evidence indicating a direct link between the new ingredient, metabolites, secondary effectors, or source and impairments in either neural or behavioral functions in infancy. In the absence of empirical evidence, there is no accepted theory that would suggest a plausible link between the new ingredient or new ingredient source and either neural or behavioral functions in infancy.
-
There is no existing evidence for significant individual differences in susceptibility to the ingredient, metabolites, secondary effectors, or source (e.g., there is little evidence supporting a link between intake of food coloring or sugar and attention deficit disorder in children, but there is evidence that a small proportion of children with attention deficit disorder may have adverse reactions to food coloring or sugar that can accentuate their hyperactive-inattentive behavior [Christensen, 1996]).
-
There are neither empirical nor theoretical links between the ingredient, metabolites, secondary effectors, or source and the functioning of other organ systems that might indirectly influence brain or behavioral development (e.g., given existing evidence on relations between nutrition and brain development [Rao and Georgieff, 2000], a new ingredient, metabolite,
-
secondary effector, or source that had an adverse influence on the digestive system could well result in subtle nutritional deficits that could in turn influence brain development).
-
There is no evidence of adverse effects in preclinical studies, including adverse effects with potentially plausible alternative explanations (e.g., the effects are viewed as the result of random chance or the reviewers believe that there may be methodological or statistical problems in the studies). This means that even if potentially plausible alternative explanations are offered to explain adverse findings, level 1 assessments would not be warranted since adverse effects were found.
If all of the above criteria are met, then level 1 assessment instruments can be used in clinical studies of the safety of new ingredients added to infant formulas. Recommended screening instruments appropriate for level 1 neural and behavioral assessments in the first year of life are shown in Table 6-10. The screening instruments are low cost and, therefore, the committee recommends studying behavior and development from each of the domains listed in the table (visual development, audition, motor development, cognition, temperament, and neural integrity).
Level 2 and 3 assessments involve detailed measures of child function in major developmental areas. For level 2 assessments, one instrument is used for each area of function, and only a single assessment occasion is required. Three of the criteria discussed above are also involved in decisions as to whether level 2 assessments should be required. They are required when there is not a plausible direct empirical or theoretical link between the new ingredient or new ingredient source and impairments in either neural or behavioral functions in infancy, but one or more of the following criteria occur:
-
Based on either existing evidence or accepted theory, there are plausible links between the ingredient and other organ systems that might indirectly influence neural or behavioral development (e.g., recombinant proteins that had an adverse influence on the digestive system or pre- or probiotics that changed the nature of intestinal flora/fauna, which could also influence digestive processes; in either case, one result could be subtle nutritional deficits that in turn could act to influence brain development).
-
There is evidence of adverse effects in preclinical studies for organ systems that could influence neural or behavioral development. This means that even if potentially plausible alternative explanations are offered to explain adverse findings, level 2 assessments would be required since adverse effects did occur. For example, given current knowledge linking iron deficiency in infancy to adverse cognitive and neural development (Rao and Georgieff, 2000), recent evidence showing lower-than-predicted iron retention by young infants (Fomon et al., 2000) would be a preclinical adverse effect that would require use of at least level 2 assessment procedures in clinical trials involving new ingredients that either included iron or could influence iron absorption.
-
There is existing evidence for significant individual differences in susceptibility to the ingredient.
Level 3 assessments also require detailed measures of child function in major developmental areas. In addition, if more than one recommended instrument is available for a given function, then a second recommended instrument that assesses either converging or complementary functions should be used on each assessment occasion. Assessment on at least two separate occasions is required within the first year. Level 3 assessments are required when the criteria for level 2 assessments are met and/or the following additional criterion occurs:
TABLE 6-10 Screening Measures Used in Level 1 Assessment of Infants Exposed to Formulas Containing New Ingredients
|
Function |
Measure |
Assessment Source |
Nature of Deviation from Expected Development |
|
Visual development |
Visual attention to stimuli (Lester and Tronick, 2001) |
Standardized medical examination carried out as part of a clinical trial |
Infant not tracking or responding to visual stimuli (e.g., direction if gaze not follow moving visual stimuli) |
|
Audition |
1. Observation of infant orientation to sounds (Cobo-Lewis and Eilers, 2001) 2. Given documented links between hearing acuity and early language development (Cobo-Lewis and Eilers, 2001), an alternative measurement would be to assess the infant’s acquisition of language landmarks in the first year, such as appearance of syllabic contours at around 3 mo, onset of babbling between 6–9 mo, and recognizing familiar words between 7–8 mo (Bloom, 1998; Posner, 2001) |
Parent report or standardized medical examination carried out as part of a clinical trial |
Infant not responding to auditory stimulation or showing language development that is below what would be expected for a infant at a given chronological age |
|
Motor development |
Age at which infant achieves motor landmarks like postural control, eye-hand coordination, sitting, crawling |
Standardized medical examination carried out as part of a clinical trial |
Degree of deviation from expected development can be assessed using standard norms such as those found in the Revised Bayley motor scales (1993) |
|
Cognition |
Norm-referenced parent report measures of developmental progress on adaptive behavior scales, such as the survey form of the Vineland Adaptive Behavior Scales (Sparrow et al., 1984) |
Parent report or standardized medical examination carried out as part of a clinical trial |
Level of developmental competence two or more standard deviations below the norm |
|
Temperament |
Short but validated parent report measure of infant temperament, such as the 24-item Infant Characteristics Questionnaire (Bates and Bayles, 1984) |
Parent report or standardized medical examination carried out as part of a clinical trial |
Infant rated as consistently expressing a high intense negative mood |
-
Based on either existing evidence or accepted theory, there is a plausible direct link between the new ingredient or new ingredient source and impairments in either neural or behavioral functions in infancy.
RECOMMENDATION: A hierarchy of three levels of clinical assessment should be implemented:
-
Level 1 assessments. Developmental measures that are easily administered but low in sensitivity.
-
Level 2 assessments. In-depth measures of infant functions in major developmental areas (single assessment for each area with one instrument).
-
Level 3 assessments. In-depth measures of infant functions in major developmental areas (repeated assessments with multiple instruments).
Identifying Appropriate Measurement Instruments
There are a large number of potential instruments available to assess outcomes in the multiple domains in which development occurs during the first year of life. Therefore it is important to specify criteria that would allow the identification of measurement instruments that are more appropriate for use in level 2 and level 3 assessments of the potential developmental-behavioral consequences of the addition of new ingredients to infant formulas. Based on a review of the literature, the committee has identified seven criteria to rank the utility of instruments used to assess infant sensory-motor, cognitive, affectual, and neural function in level 2 and level 3 studies. The criteria are:
-
Age appropriateness. Because infant intake of formulas is at a maximum during the first year of life, the committee recommends concurrent measures that can be administered during this time period.
-
Potential long-term consequences. It is essential that assessment measures are not only reliable, but also that they either have predictive value for functional competence beyond the first year of life or are precursors for critical mediators of later functional competence. An example of the latter type of measure would involve the assessment of infant behaviors that interfere with the establishment of adequate caregiver-infant relations (Chasnoff et al., 1987).
-
Sensitivity. An instrument may be appropriate for the first year of life and may have long-term predictive value, but still be relatively insensitive to exposure to toxic substances. Given the consequences to the developing infant, avoidance of type II errors (failure to detect a real effect) may be more critical then avoidance of type I errors when evaluating addition of new ingredients to infant formulas. To maximize the ability to detect adverse consequences, measures should demonstrate sensitivity to exposure to toxic substances during infancy. In recommending a sensitivity criterion, the committee fully recognizes that new ingredients for infant formulas will have been extensively evaluated during the preclinical trials, making it highly unlikely that substances that are clearly toxic will be tested in clinical trials. Thus the committee does not expect the assessment instruments described below to be used in clinical trials involving known toxic substances. However if an assessment instrument has not been shown to be sensitive to known toxins in previous research studies, it is unlikely that it will be sensitive to potentially subtle adverse effects of those new ingredients that are tested in clinical trials. As a result, the committee requires, where possible, that the
-
instruments used must have previously demonstrated the ability to detect adverse consequences associated with children’s exposure to known toxins.
-
Brain-behavior links. To maximize the ability to understand the mechanisms that underlie deleterious effects associated with the addition of ingredients new to infant formulas, outcome measures at the human level should have documented links to CNS structural and functional development. Knowledge of the brain-behavior link allows investigators to select which behavioral outcomes are most likely to be affected when toxins are known to impact on a given set of neural functions and which neural functions to select when toxins are known to impact on a specific set of behaviors (Jacobson and Jacobson, 2000).
-
Cross-species generalizability. To maximize generalizability from preclinical nonhuman research to human clinical research, outcome measures used in human studies should have behavioral analogues at the nonhuman level. (See Table 5-11 for information on integrated tests across species.)
-
Function specificity. When there is a choice of instruments it is desirable to avoid using global outcome measures that combine multiple outcome dimensions into a single score. The effects on a specific outcome dimension of exposure to a toxin may be lost when the score on this dimension is combined with scores on other nonaffected dimensions (Grandjean et al., 1996; McCall and Appelbaum, 1991). Alternatively, children exposed to different toxins may end up with the same global score, but may arrive at this score via different developmental paths, thus masking the differential impact of different toxins (Jacobson, 1998).
-
Ease of administration. Developmental-behavioral measures used in the infancy period should be noninvasive and, all other criteria being satisfied, they should be relatively easy to administer.
Utilizing Appropriate Study Design
In addition to the criteria for instrument selection, a review of the research literature also indicated that certain requirements should be an essential part of any human study that examines the potential consequences of the addition of new ingredients to infant formulas. The committee recommends that the following four requirements be met.
-
Establish adequate statistical power. As noted earlier, there is a critical need to avoid type II errors when evaluating safety. Investigators should document that there is sufficient statistical power to detect adverse effects for all studies in this area. The importance of establishing the adequacy of statistical power is illustrated in studies investigating the developmental-behavioral consequences of the addition of LC-PUFAs to infant formulas. In a recent review of this question, Wroble and colleagues (2002) cited seven studies involving term infants. The committee assessed the statistical power of these studies plus two additional studies with term infants that were not cited in this review. In computing statistical power, it was assumed that studies should be able to detect at least moderate effect sizes. The analysis indicated that the average level of statistical power across these nine studies was 0.56 (median power = 0.48, range across studies = 0.34–0.97). Not surprisingly, in their review Wroble and colleagues (2002) concluded that the evidence for developmental-behavioral benefits for term infants from adding LC-PUFAs to infant formulas was inconsistent at best. While adverse consequences of such additions were reported in two of the nine studies on which the committee did power calculations (Jensen et al., 1997; Scott et al., 1998), for the majority of studies reviewed statistical power was inadequate to detect either beneficial
-
or adverse consequences of the addition of LC-PUFAs to infant formulas. Demonstration that statistical power is sufficient to detect at least moderate-effect sizes is strongly recommended for all future clinical studies on the safety of the addition of new ingredients to infant formulas. Documentation of sufficient power is the responsibility of those seeking to add a new ingredient.
-
Avoid control of mediator variables. A typical design strategy is to statistically control for confounding variables that may covary with the predictor (toxin) or the outcome variable (e.g., statistically control for the influence of sociodemographic covariates of lead exposure in infants). However it is critical to distinguish between a confounder and a mediator variable. Confounder variables are independent risk factors that occur at a higher level among those exposed to a toxic substance than those not exposed. In contrast, mediator variables are developmental risk factors whose occurrence is caused by exposure to a toxic substance (Neuspiel, 1995). Mediator variables covary with the predictor and the outcome variable because they are the mechanism through which the predictor influences the outcome. If mediating variables are statistically controlled, overcorrection may occur and a real risk effect may be missed (Jacobson and Jacobson, 1996). For example, prenatal alcohol exposure has been shown to influence infant temperament, which influences mother-child interaction, which influences child cognitive performance (O’Connor et al., 1993). In this case it would be a mistake to covary out either infant temperament or mother-infant interaction since these are mediators of the effects of prenatal alcohol exposure (for a recent review of design and statistical procedures used to assess the impact of mediator variables, see Shrout and Bolger, 2002).
-
Use measurement aggregation. In developmental-behavioral studies not all measures of a construct have equal sensitivity. Therefore it is important to use converging multiple measures of the construct so a real link is not missed due to reliance on a single, less-sensitive measure.
-
Use repeated measures. It is important to look at performance on outcome measures across time. This is partly because the impact of exposure to a toxin may vary depending on age level (neural maturation) of the infant (Shaheen, 1984). In addition, the sensitivity of various measures of the same dimension may vary depending on the age of the child (e.g., intramodal visual recognition memory is a sensitive predictor at 7 months of age but not at 12 months; crossmodal transfer, which taps the same cognitive dimension, but at a more complex level, is not a sensitive predictor at 7 months of age but is at 12 months (Rose et al., 1989).
Assessing Sensory and Motor Functions
Visual and Auditory Function
Instruments that could be used to evaluate infant sensory competencies in studies on the developmental effects of adding new ingredients to infant formulas are shown in Table 6-11. Even though the instruments described in the table have not been specifically linked to CNS development, sensory functions tapped by these instruments clearly have specific associations with brain structure and function. While preferential-looking procedures to test visual function and visual reinforcement audiometry to test auditory function have not been used in studies with infants exposed to toxins, they have been extensively used in clinical studies. These two procedures are thus recommended as being “state of the art” in the behavioral assessment of visual and auditory function. They could be easily combined with the func-
tional neurological measures described later in this section, such as brain stem-evoked response, to obtain converging measurements of infant sensory competence.
Motor Function
In contrast to the areas of visual and auditory assessment, where there are relatively few behavioral measures available in the first year of life, there are far more choices available for the measurement of early motor function. Also in contrast to assessment of sensory function, none of the motor measures can be described as being the “gold standard.” A list of available measures for the assessment of infant motor function are shown in Table 6-12. Even though specific instruments described in this table have not been linked to CNS development, links between motor and brain development have been well established. Abnormal motor behavior has been reported as characteristic of infants exposed to toxins (Schneider et al., 1989), but studies linking such exposure to performance on the instruments described in Table 6-12 have been relatively rare. In the absence of more satisfactory measures, two instruments are provisionally recommended to assess adequate motor development in studies involving the safety of addition of new ingredients to infant formulas: the Alberta Infant Motor Scale and the Movement Assessment of Infants Scale. While the long-term predictive validity of these specific scales has yet to be established, early motor competence per se is an important precursor for later critical developmental functions, such as exploration, goal-directed behavior, and spatial learning (Bertenthal et al., 1984; Bushnell and Boudreau, 1993; Smitsman, 2001). In addition, evidence from several studies has indicated that these instruments are sensitive to early exposure of human infants to toxic substances.
Assessing Cognitive Development
The majority of behavioral teratology and behavioral toxicology research studies that assessed infant cognitive development as an endpoint utilized the Bayley Scales of Infant Development as an outcome measure (Jacobson and Jacobson, 2000; Kaltenbach and Finnegan, 1989). There are a variety of reasons to question the heavy reliance on the Bayley scales, including poor predictive validity of performance when administered to infants less than 2 years of age (Bendersky and Lewis, 2001; Colombo, 1993), and the fact that even in the revised version of the scale, the first-year mental scale is still heavily weighted with motor items. These problems are particularly critical given the fact that it is during the first year when infant formulas are most likely to be consumed. In addition, the earlier (Bayley, 1969) version of the Bayley scale, used in the majority of previous research on this topic, yields a global mental development score. One consequence of using a global score is that the significant impact of developmental risk factors on a specific cognitive dimension may be lost when the depressed score on the affected dimension is combined with scores on other nonaffected cognitive dimensions (Grandjean et al., 1996; Jacobson, 1998; McCall and Appelbaum, 1991). While the most recent revision of the Bayley scales does allow scoring of dimensional subscale scores, the heterogeneous factor structure of these subscales still increases the likelihood that a specific effect on a single cognitive dimension could well be attenuated (Bendersky and Lewis, 2001). Thus the global score problem has not been completely solved, even in the revised edition of this scale (Bendersky and Lewis, 2001).
The importance of going beyond global cognitive assessments in studies on the impact of potential developmental risk factors has been strengthened by both conceptual and empiri-
TABLE 6-11 Behavioral Measures Available to Test Sensory Functions in Clinical Studies on the Safety of New Additions to Infant Formulas
|
Description |
Selection Criteria Met |
Rating |
Comments |
|
Tests of visual function |
|
||
|
Preferential looking procedures under controlled conditions using stimuli with different levels of visual contrast to assess visual acuity and contrast sensitivity (Posner, 2001) |
Can be administered during the first year (Mayer and Arendt, 2001) Documented links to central nervous system (CNS) structure or function (Slater, 2001) Analogous measures available at the nonhuman level (Banks and Salapatek, 1983; Jacobs and Blakemore, 1988) Assesses specific functions (Posner, 2001) Relative ease of administration (Mayer and Arendt, 2001) |
Recommended for use in either level 2 or level 3 assessments |
Current standard measure of preferential looking is the Teller Acuity Card Procedure (Teller et al., 1986) Early acuity deficits can have long-term consequences (Posner, 2001), but little evidence on the specific predictive validity of this procedure A lack of response may be due to infant fatigue or reduced attention and examiner experience essential for younger infants (Mayer and Arendt, 2001) |
|
Tests of auditory function |
|
||
|
Visual reinforcement audiometry, where the child’s head turning toward different sounds at different frequency levels are reinforced by an interesting visual stimulus (Moore et al., 1977) |
Can be administered during the first year (Cobo-Lewis and Eilers, 2001) Documented links to CNS structure or function (Fernald, 2001) Assesses specific functions (Cobo-Lewis and Eilers, 2001) Relative ease of administration (Cobo-Lewis and Eilers, 2001) |
Recommended for use in either level 2 or level 3 assessments |
Can be taught quickly since the behavioral response is very clear and computers can be used to control the recording and reinforcements Early hearing problems have long-term consequences, but little evidence on the predictive value of this procedure per se Except under highly controlled laboratory conditions, best used with infants at or above 5 mo of age (Cobo-Lewis and Eilers, 2001) |
|
Observer-based psychoacoustic procedures, based on the same principle as visual reinforcement audiometry except that observers utilize multiple, often subtle, cues to judge if an infant is orienting towards a sound stimulus (Olsho et al., 1987) |
Can be administered during the first year (Cobo-Lewis and Eilers, 2001) Documented links to CNS structure or function (Fernald, 2001) Assesses specific functions (Cobo-Lewis and Eilers, 2001) |
Not recommended for level 2 assessments, but can be used as the alternate instrument for level 3 assessments |
Can be used with infants as young as 2 mo of age, but requires specialized and extensive examiner training (Cobo-Lewis and Eilers, 2001) |
TABLE 6-12 Behavioral Measures Available to Test Motor Functions in Clinical Studies on the Safety of New Additions to Infant Formulas
|
Description |
Selection Criteria Met |
Rating |
Comments |
|
Alberta Infant Motor Scale Norm-referenced, observational-based assessment of gross motor and postural development (Piper and Darrah, 1994) |
Can be administered during the first year (Piper and Darrah, 1994) Has shown sensitivity to exposure to toxic substances during the first year (Fetters and Tronick, 1996) Documented links to central nervous system (CNS) structure or function (Tanner, 1970) Assesses specific functions (Piper and Darrah, 1994) |
In the absence of more adequate measures, recommended as the best option available for use in either level 2 or level 3 studies |
Range from birth–18 mo (Piper and Darrah, 1994); has satisfactory test-retest and interobserver reliability and discriminative validity; little assessment of fine motor skills Background in infant motor development important for valid observations |
|
Movement Assessment of Infants Scale Assesses muscle tone, reflexes, and functional movement; 65 items with risk points for each item (Harris et al., 1984) |
Can be administered during the first year (Case-Smith and Bigsby, 2001) Has shown sensitivity to exposure to toxic substances during the first year (Arendt et al., 1998; Fetters and Tronick, 1996) Documented links to CNS structure or function (Tanner, 1970) Assesses specific functions (Harris et al., 1984) |
In the absence of more adequate measures, recommended as the best option available for level 2 or level 3 assessments |
Range from birth–12 mo; inconsistent evidence on predictive validity (Case-Smith and Bigsby, 2001) Experience with infant motor development essential for valid administration (Chandler et al., 1980) |
|
Revised Bayley Psychomotor Scale Revised version of earlier Bayley motor scale that assesses gross and fine motor skills (Bayley, 1993) |
Can be administered during the first year (Bayley, 1993) Documented links to CNS structure or function (Tanner, 1970) |
Meets few selection criteria; use only under limited or special circumstances |
84% item overlap from 1969 version; better predictive validity for clinical than nonclinical populations (Bendersky and Lewis, 2001) Data inconsistent in regard to sensitivity of Bayley motor scale to exposure to toxic substances (e.g., Mayes and Cicchetti, 1995; Singer et al., 1997 vs. Hurt et. al., 1995; Jacobson et al., 1996) |
cal advances in the study of early cognitive development. Specifically, there is increasing agreement among developmental researchers that early intellectual development can be characterized by performance on three specific dimensions, which the committee recommends as the preferred dimensions of early cognitive performance for future clinical research studies. These are:
-
Speed of processing, as assessed on tasks like habituation and visual expectancy performance (Colombo, 1993; Dougherty and Haith, 1997). Tasks of this type may be sensitive to nutritional intakes related to neural developmental processes involving myelination, synaptogenesis, and pruning (Rao and Georgieff, 2000).
-
Recognition memory, as assessed on tasks like novelty preference and conjugate reinforcement (Fagen and Ohr, 2001; Rovee-Collier and Barr, 2001). Tasks of this type may be sensitive to intake of energy-glucose, iron, and zinc, which may relate to hippocampal development (Nelson et al., 2000; Pinero et al., 2001; Takeda, 2001).
-
Behavioral inhibition, as assessed on measures like the A-not-B task (Fagen and Ohr, 2001; McCall, 1994). Tasks of this type may be sensitive to nutrients involved in monoamine synthesis (Lozoff et al., 1998).
Table 6-13 summarizes the available evidence that the committee reviewed in order to recommend these specific measures of cognitive function during the first year of life. While the table does not include an exhaustive list, it clearly illustrates the utility of assessment procedures that tap the three dimensions in studies on the potential cognitive consequences associated with the addition of new ingredients to infant formulas. Evidence suggests that toxic substances may differentially impact on different dimensions of cognitive performance (e.g., prenatal alcohol exposure is related to slower processing speed in infancy, but not to memory performance, and prenatal exposure to polychlorinated biphenyls adversely affects infant memory, but not processing speed [Jacobson, 1998]). This pattern of specificity may reflect differential sensitivity of the exposure of various brain areas to toxic substances, underlying different dimensions of early information processing (Jacobson and Jacobson, 2000; Mayes and Bornstein, 1995; Rao and Georgieff, 2000). The likely possibility of specificity of effects due to different potential toxins underlies the recommendation to assess at least two different dimensions of infant cognitive performance in level 3 studies on the cognitive impact of the addition of new ingredients to infant formulas.
Assessing Infant Temperament
Over the past 15 years there has been an increasing focus on noncognitive aspects of early development, with specific reference to the domain of infant temperament (Kohnstamm et al., 1989). Temperament refers to early-appearing, biologically rooted individual differences in behavioral tendencies that are relatively stable across contexts and time (Bates, 1989). The biological roots of individual differences in temperament include genetic influences (Goldsmith et al., 1994), exposure to toxic substances (Alessandri et al., 1995), and nutritional deficiencies (Wachs, 2000). While researchers have identified a number of different domains of infant temperament, recent formulations have indicated that these different domains converge on two major dimensions: reactivity and self-regulation (Rothbart and Bates, 1998). Reactivity refers to the speed, quality, and strength of the infant’s reaction to stimulation (e.g., stimulus sensitivity, negative affect). Self-regulation refers to the quality and level of the infant’s ability to control emotional responses (e.g., soothability, attentional allocation). Individual differences in temperament-driven behavioral patterns, like tenden-
cies towards approach and inhibition, are based on the balance between individual self-regulation and reactivity.
There have been two major approaches used to assess infant temperament. The first involves parent report measures using standardized scales. While parent report measures have been criticized for assessing parental emotional qualities rather than child temperament characteristics, reviews indicate that such measures are predictive in ways that would not occur if they were just measuring parental characteristics (Wachs and Bates, 2001). The second approach utilizes structured laboratory-based assessments of infant temperament. These assessments involve presenting a series of structured situations to an infant, videotaping the infant’s behavior during the situations, and then coding the observed behaviors into temperament-related dimensions. Laboratory-based assessment procedures have been criticized for only sampling a restricted set of child behaviors. However the strengths of this approach include high intercoder reliability when using trained coders and the fact that these procedures do predict later developmental outcomes (Wachs and Bates, 2001).
At present, few of the standard parent report or laboratory-based assessment approaches for assessing infant temperament have been utilized in behavioral teratology or behavioral toxicology studies. However there are a number of reasons that support the need for use of such measures in future studies on questions involving the safety of new ingredients added to infant formulas. Specifically, evidence indicates that measures of temperament in the first year of life predict later personality (Rothbart et al., 2000), as well as attention and behavioral problems in preschool and school-age children (Bates, 2001; Posner, 2001; Robson and Pederson, 1997). The predictive function of early temperament problems may be partly due to an increased likelihood of problems in parent-child reactivity or with impairments in children’s ability to self-regulate (Bendersky et al., 1996; O’Connor et al., 1993; Rossetti Ferreira, 1978; Rothbart and Bates, 1998; Schneider et al., 1989).
In addition, individual differences in early temperament have been linked to structural and functional development of the CNS (Nelson, 1994; Posner, 2001; Rothbart et al., 1994), and nonhuman analogues to human temperament measures have been reported (Higley and Suomi, 1989; Laughlin et al., 1991; Spear, 1995). Finally, and perhaps most critically, although standard temperament instruments have rarely been used in studies of human infants exposed to toxic substances, a number of studies from the behavioral toxicology and teratology literature have reported that such infants do show behavioral impairments that are clearly temperament driven, including problems in reactivity (e.g., increased irritability, hypo- or hyper-reactivity to stimuli [Hill et al., 1989; Lester et al., 1995]), and in self-regulation (e.g., poor state control, low consolability, and poor impulse control [Chasnoff et al., 1987; Mayes et al., 1995]). One consequence of toxin-driven differences in infant temperament is that infants exposed to toxic substances are harder to test and are less likely to complete testing due to temperament characteristics, such as high activity, high distractibility, high negative emotionality, hypersensitivity, or low consolability (Fagen and Ohr, 2001; Mayes et al., 1995; Struthers and Hansen, 1992). Infants who fail to complete testing due to temperament characteristics may be at increased risk for later developmental problems (Bathurst and Gottfried, 1987; Sebris et al., 1984).
Table 6-14 lists recommended indices of infant temperament for future studies of the safety of the addition of new ingredients to infant formulas. Based on available evidence, the committee strongly recommends using converging laboratory and parent report measures of temperament rather than just laboratory or just parent report measures. In addition, the committee recommends obtaining both mother and father reports for parent report measures (Wachs and Bates, 2001). Recommended parent report measures include the Infant Behavior Questionnaire or the Infant Characteristics Questionnaire. Either the LAB-TAB
TABLE 6-13 Measures Available to Test First-Year Cognitive Function in Clinical Studies on the Safety of New Additions to Infant Formulas
|
Description |
Selection Criteria Met |
Rating |
Comments |
|
Habituation Repeated presentation of a visual or auditory stimulus to an infant; observers code length of fixation to the stimulus and decline in attentional behavior with repeated presentations (Colombo, 1993) |
Can be administered during the first year (Colombo, 1993). Has predictive value beyond the first year of life (McCall and Carriger, 1993; Ruff and Rothbart, 1996; Slater, 1997) Has shown sensitivity to exposure to toxic substances during the first year (Hill et al., 1989; Jacobson and Jacobson, 2000) Documented links to central nervous system (CNS) structure (Nelson, 1995) Assesses specific functions (Colombo, 1993) Relative ease of administration (Fagen and Ohr, 2001) |
Recommended for use in either level 2 or level 3 assessments |
The most sensitive predictors of later cognitive function are total looking time and duration of longest fixation, both of which decline with age (Fagen and Ohr, 2001); infants whose fixation times do not decline with age are showing slower processing speed and would be considered as being at risk (Colombo, 1993) Sensitivity of habituation procedure to toxic exposure may be higher in first several months of life (Fagen and Ohr, 2001) |
|
Recognition memory Based on the preference of infants for novel stimuli; infants are familiarized with a stimulus and then the familiar stimulus is paired with a novel stimulus; over repeated trials with different stimuli, observers code whether the infant orients toward the familiar or the novel stimulus (Rose and Orlian, 2001). In intramodal tasks the familiar and novel stimuli are in the same modality; cross-modal transfer is a more complex task in that it involves the child being familiarized with the stimulus in one sensory modality (e.g., tactual) and tested in a different |
Can be administered during the first year (Colombo, 1993) Has predictive value beyond the first year of life (Rose and Feldman, 1995; Rose et al., 1989, 1991; Slater, 1997) Has shown sensitivity to exposure to toxic substances during the first year (Jacobson et al., 1996; Rose and Orlian, 2001); Struthers and Hansen, 1992) Documented links to CNS structure (Johnson, 2001; Nelson, 1995) Analogous measures available at the nonhuman primate level (Grant-Webster et al., 1990; Rose and Orlian, 2001) |
Recommended for use in either level 2 or level 3 assessments |
Less than 53% preference for novel stimuli is used as the cut-off to distinguish at-risk from not-at-risk infants (Fagan et al., 1986; Jacobson et al., 1996) Intramodal memory tasks are a stronger predictor of later cognitive performance below 12 mo; crossmodal tasks are a stronger predictor at 12 mo (Rose and Feldman, 1995) Crossmodal transfer can be seen in infants as young as 6 mo, but is much more characteristic of infants who are closer to 12 mo (Rose and Orlian, 2001) |
|
modality for novelty preference (e.g., visual) with the same familiar stimulus and a novel stimulus (Rose and Feldman, 1990) |
Assesses specific functions (Colombo, 1993) Relative ease of administration (Rose and Orlian, 2001) |
|
|
|
Conjugate reinforcement learning tasks Infant actions (e.g., leg kicking, arm waving, hitting a lever) activate an interesting audio-visual stimulus (e.g., mobile moves, clown face lights up, toy train moves); by repeating this procedure across sessions, measures of speed of acquisition, level of retention, and speed of extinction can be obtained (Rovee-Collier and Barr, 2001) |
Can be administered during the first year (Rovee-Collier and Barr, 2001) Has predictive value beyond the first year of life (Colombo, 1993; Fagen and Ohr, 2001) Has shown sensitivity to exposure to toxic substances during the first year (Alessandri et al., 1993) Documented links to CNS structure (Nelson, 1995) Assesses specific functions (Rovee-Collier and Barr, 2001) Relative ease of administration (Fagen and Ohr, 2001) |
Not recommended for level 2 assessments; can be used as the alternate instrument for level 3 assessments |
Speed of acquisition is not predictive of later intelligence, but retention of what has been learned is predictive (Fagen and Ohr, 2001) Tasks can be computer driven, which increases cost, but then requires little examiner training; other tasks are nonautomated but require a moderate amount of examiner training (Fagen and Ohr, 2001) |
|
A-not-B task Infant sees an interesting object hidden at a specific location; after infant retrieves the object, infant then sees the object hidden at a different location; does the infant go to the location where he/she previously found the object hidden or the location where he/she last saw the object hidden; the task can be made more difficult by increasing the time between when the infant saw the object hidden and when he/she is allowed to search for it (Ruff and Rothbart, 1996) |
Can be administered during the first year (Ruff and Rothbart, 1996) Documented links to CNS structure (Nelson, 1995; Posner, 2001) Analogous measures available at the nonhuman primate level (Diamond, 1990) Assesses specific functions (Ruff and Rothbart, 1996) Relative ease of administration (Ruff and Rothbart, 1996) |
Not recommended for level 2 assessments; can be used as the alternate instrument for level 3 assessments |
Past 6 mo of age, as infants get older, there is an increase in the amount of delay time infants can tolerate and still search successfully; older infants who search where the object was hidden only with no delay or who do not show increased tolerance for delay over time would be considered as being at risk (Ruff and Rothbart, 1996) |
|
Description |
Selection Criteria Met |
Rating |
Comments |
|
Visual expectation Infant seated before a stimulus panel that has lights that flash on and off in a regular sequence; assesses whether child orients to the next light in the sequence before it comes on; can obtain measures of reaction time and number of correct anticipations (Colombo, 1993) |
Can be administered during the first year (Colombo, 1993) Has predictive value beyond the first year of life (Dougherty and Haith, 1997) Assesses specific functions (Colombo, 1993) |
Not recommended for level 2 assessments; can be used as the alternate instrument for level 3 assessments |
Stimulus anticipation is a less-strong predictor of later cognitive performance than reaction time (Colombo, 1993) Visual expectation performance either does not distinguish between infants who were or were not exposed to toxic substances (Fagen and Ohr, 2001), or findings are inconsistent with regard to the question of whether exposed infants show slower or faster reaction times (Jacobson, 1998) Requires both sophisticated equipment and a high level of examiner training (Fagen and Ohr, 2001) |
|
Focused attention Usually assessed during free play; using standardized rating criteria, observers code the amount of time the infant is attending to properties of the object they are playing with or exploring the object to see what can be done with it (Lawson and Ruff, 2001) |
Can be administered during the first year (Ruff and Rothbart, 1996) Has predictive value beyond the first year of life (Lawson and Ruff, 2001) Documented links to CNS structure (Lawson and Ruff, 2001) Assesses specific functions (Ruff and Rothbart, 1996) |
Not recommended for level 2 assessments; can be used as the alternate instrument for level 3 assessments |
Infants showing less than 2% of time spent in focused attention during free-play task at 7 mo may be at developmental risk (Lawson and Ruff, 2001) Requires substantial training to learn to recognize focused attention and to maintain examiner calibration over time (Lawson and Ruff, 2001) |
|
Bayley Mental Development Scale: 2nd ed. (Bayley, 1993) |
Can be administered during the first year (Bayley, 1993) |
Meets few selection criteria; use only under limited or special circumstances |
Consistent evidence indicating low predictive validity of Bayley scale performance when administered below 2 y (Bendersky and Lewis, 2001; Colombo, 1993); inconsistency of findings when comparing Bayley mental development scores of infants |
or the Louisville Temperament Assessment Battery are recommended for laboratory-based measures.
Neurological Function
Knowledge of the associations among toxin, brain, and behavior increases the ability to detect the neural precursors of developmental-behavioral consequences of early exposure to toxic substances; knowledge of toxin-affected neural precursors helps to select which behavioral outcomes are most likely to be affected by exposure to toxins; and knowledge of brain-behavior relations can aid the researcher in selecting which areas of the brain to investigate in children who display specific developmental deficits as a result of exposure to toxic substances (Jacobson and Jacobson, 2000). With the explosion in neuroimaging techniques, there have been major advances in the study of brain metabolism and electrical activity as a window to CNS structure and function in children (Nelson and Bloom, 1997; Posner, 2001). Neuroimaging techniques can provide a more objective assessment of brain activity than can neurobehavioral measures. Further, many of these new techniques offer the promise of allowing researchers to detect relatively subtle neural deficits that may result from exposure to toxic substances.
Table 6-15 lists measures of CNS structure and function that may be useful in the study of developmental consequences of exposure to new ingredients in infant formulas. Electroencephalographic assessment and measures based on assessment of event-related potential are specifically recommended. While the instruments listed in Table 6-15 fit most of the selection criteria presented earlier, it is important to recognize that almost all of these instruments are expensive and require highly trained staff to operate them and to interpret their results. The committee has noted in this table which instruments are relatively less costly or require relatively lower staff expertise. It should also be noted that not all existing measures of CNS structure and function are listed in the table. Certain measures, such as positron emission tomography (Bookheimer, 2000), X-ray computed tomography (Singer, 2001) and assessment of homovanillic acid levels as a marker for dopamine status (Needlman et al., 1995), are invasive procedures and thus contraindicated for normal infants. Other potentially promising measures, such as magnetic diffusion tensor imaging (Posner, 2001), magnetic encephalography, and functional near-infrared spectroscopy (Nelson et al., 2002), have only recently been developed and more needs to be known before their utility in studies on neural consequences of the addition of new ingredients to infant formulas can be determined.
SUMMARY
Infancy is a particularly vulnerable period of development. A difference in growth is likely to signal some alteration in underlying biological or physiological processes. The committee took the conservative position that any systematic difference in growth that could be attributed to a new formula ingredient rather than to chance alone should represent a safety concern and should require explanation and further study. The major organ systems should be studied because growth deficits are likely to appear only secondary to effects on specific organs or tissues and may not appear for some time after nutritional insult. The committee therefore recommends implementing a hierarchy of two levels of clinical assessments for organ evaluations.
It is essential to include developmental-behavioral outcomes in future studies of the safety of ingredients new to infant formulas because such measures are sensitive to exposure
TABLE 6-14 Measures Available to Test First-Year Temperament in Clinical Studies on the Safety of New Additions to Infant Formulas
|
Description |
Selection Criteria Met |
Rating |
Comments |
|
Parent report measures |
|
||
|
Infant Behavior Questionnaire 87-item, parent report measure; can be administered starting at age 3 mo; assesses 5 dimensions of temperament (Rothbart, 1986) |
Can be administered during the first year (Rothbart, 1986) Has shown sensitivity to exposure to toxic substances during the first year (Alessandri et al., 1995) Documented links to CNS structure (Goldsmith et al., 2000) Assesses specific functions (Rothbart and Bates, 1998) |
Recommended for use in either level 2 or level 3 assessments |
Infants rated as higher on negative affect, inhibition, and intensity (especially when linked to negative affect) and low on sociability, approach, positive affect, or soothability would be considered as being at greater risk for developmental problems. Relatively long measure and parents need to be literate in English |
|
Infant Characteristics Questionnaire 24-item, parent report measure; can be administered starting at age 4 mo; assesses level of high-intense negative mood (Bates and Bayles, 1984) |
Can be administered during the first year (Bates, 2001; Bates and Bayles, 1984) Assesses specific functions (Bates and Bayles, 1984) Relative ease of administration (Bates and Bayles, 1984) |
Recommended for use in either level 2 or level 3 assessments |
Infants rated as high on intense negative mood would be at greater risk for later developmental problems (Bates, 2001) |
|
Revised Infant Temperament Questionnaire 95-item, parent report measure; can be administered starting at age 4 mo; assesses 9 dimensions of temperament (Carey and McDevitt, 1978) |
Can be administered during the first year (Carey and McDevitt, 1978) Has predictive value beyond the first year of life (Sanson et al., 1996) Assesses specific functions (Martin et al., 1994) |
Not recommended for level 2 assessments, but can be used as the alternate instrument for level 3 assessments |
Infants rated as higher on negative affect, inhibition, and intensity (especially when linked to negative affect) and low on sociability, approach, positive affect, or soothability would be considered as being at greater risk for developmental problems Relatively long measure and parents need to be literate in English |
TABLE 6-15 Measures Available to Test First-Year Neural Function in Clinical Studies on the Safety of the Addition of Ingredients New to Infant Formulas
|
Description |
Selection Criteria Met |
Rating |
Comments |
|
Event-related potential Assessment of latency and amplitude of electrical changes in different regions in the brain in response to specific sensory stimulation (Nelson and Bloom, 1997) |
Can be administered during the first year (Posner, 2001) Has predictive value beyond the first year of life (Molfese and Molfese, 2001) Documented links to central nervous system (CNS) structure or function (Posner, 2001) Assesses specific functions (Nelson and Bloom, 1997) |
Recommended for use in either level 2 or level 3 assessments |
Noninvasive measure that is less sensitive to movement artifact then other neural measurements Requires substantial investment in equipment and examiner training (Posner, 2001) Spatial resolution is less adequate than temporal resolution, but still cannot assume 1 to 1 correspondence between brain electrical activity and what is recorded (Nelson et al., 2002) In contrast to evoked potentials, which assess the integrity of primary sensory pathways, measures appear to be assessing aspects of higher order cognitive processing such as attention |
|
Electroencephalograph (EEG) Based on computerized analysis of electrical activity in different areas of the brain; of particular relevance are studies of relative electrical activity in different hemispheres of the brain (Marshall and Fox, 2001) |
Can be administered during the first year (Marshall and Fox, 2001) Has predictive value beyond the first year of life (Fox et al., 2001) Has shown sensitivity to exposure to toxic substances during the first year (Kaneko et al., 1996; Needlman et al., 1995) Documented links to CNS structure or function (Posner, 2001) Analogous measures available at the nonhuman level (Needlman et al., 1995) Assesses specific functions (Marshall and Fox, 2001) |
Recommended for use in either level 2 or level 3 assessments |
Measures are noninvasive and are relatively inexpensive compared with other functional measures of the brain, but extensive training is required to avoid artifact and to interpret results correctly (Marshall Cannot assume a 1 to 1 correspondence between brain electrical activity and what is recorded (Nelson et al., 2002) and Fox, 2001) |
|
Description |
Selection Criteria Met |
Rating |
Comments |
|
Cardiac variability-vagal tone Changes in heart rate as a function of stimulation are related to changes in respiratory sinus arrhythmia that reflect changes in the parasympathetic nervous system (Posner, 2001) |
Can be administered during the first year (Porter, 2001) Has predictive value beyond the first year of life (Doussard-Roosevelt, et al., 2001; Porges et al., 1996) Has shown sensitivity to exposure to toxic substances during the first year (DiPietro et al., 1995) Documented links to CNS structure or function (Porter, 2001) |
Not recommended for level 2 assessments, but can be used as the alternate instrument for level 3 assessments |
Individual differences in vagal tone have been linked to differences in attention and temperament, but this measure may primarily reflect general reactivity (Posner, 2001). Vagal tone has been shown to distinguish between breast- and formula-fed infants (DiPietro et al., 1987). Can be used in the first year, but lower stability early in the first year suggests this measure may be more useful after 6 mo (Porter, 2001) Requires extensive instrumentation and extensive training for valid interpretation of results (Porter, 2001) |
|
Cortisol A hormonal measure based on the functioning of the hypothalamic-pituitary-adrenocortical axis; cortisol level can be viewed as the level of reactivity of the organism to stress (Gunnar, 2000) |
Can be administered during the first year (Gunnar, 2000) Has shown sensitivity to exposure to toxic substances during the first year (Gunnar and White, 2001) Documented links to CNS structure or function (Gunnar, 2000) Analogous measures available at the nonhuman level (Needlman et al., 1995) Relative ease of administration (Gunnar and White, 2001) |
Not recommended for level 2 assessments, but can be used as the alternate instrument for level 3 assessments |
Can be easily obtained from infant saliva, but since cortisol production follows a circadian rhythm and level of salivary cortisol can be influenced by recent intake of milk or milk products, assessment requires controls for time of day and milk exposure (Posner, 2001) Infants with consistently under- or overproduction of cortisol are considered as being at risk, but there are no specific norms as to what constitutes under- or over-reaction (Gunnar and White, 2001); might be useful for assessing impact of new ingredients affecting sterol synthesis (including estrogen/ phytoestrogen), along with its role as a stress marker |
to toxic substances and can have long-term predictive value. These measures also are important because bidirectional brain-behavior links exist. In the case of neurological and behavioral assessment, the committee recommends that a hierarchy of three levels of clinical assessment be applied.
REFERENCES
AAP (American Academy of Pediatrics). 1988. Clinical Testing of Infant Formulas with Respect to Nutritional Suitability for Term Infants. Report to the FDA. Committee on Nutrition. Elk Grove Village, IL: AAP.
AAP. 1997. Breast feeding and the use of human milk. Pediatrics 100:1035–1039.
ADA (American Dietetic Association). 2001. Position of the American Dietetic Association: Breaking the barriers to breastfeeding. J Am Diet Assoc 101:1213–1220.
Adams J. 1993. Structure-activity and dose-response relationships in the neural and behavioral teratogenesis of retinoids. Neurotoxicol Teratol 15:193–202.
Alessandri SM, Sullivan MW, Imaizumi S, Lewis M. 1993. Learning and emotional responsivity in cocaine-exposed infants. Dev Psychol 29:989–997.
Alessandri SM, Sullivan MW, Bendersky M, Lewis M. 1995. Temperament in cocaine-exposed infants. In: Lewis M, Bendersky M, eds. Mothers, Babies, and Cocaine: The Role of Toxins in Development. Hillsdale, NJ: Lawrence Erlbaum Associates. Pp. 273–286.
Antonowicz I, Lebenthal E. 1977. Developmental pattern of small intestinal enterokinase and disaccharidase activities in the human fetus. Gastroenterology 72:1299–1303.
Arant BS Jr, Edelmann CM Jr, Spitzer A. 1972. The congruence of creatinine and inulin clearances in children: Use of Technicon Autoanalyzer. J Pediatr 81:559–561.
Arendt R, Singer L, Angelopoulos J, Bass-Busdiecker O, Mascia J. 1998. Sensorimotor development in cocaine-exposed infants. Infant Behav Dev 21:627–640.
Armstrong J, Reilly JJ. 2002. Child Health Information Team. Breastfeeding and lowering the risk of childhood obesity. Lancet 359:2003–2004.
Auestad N, Halter R, Hall RT, Blatter M, Bogle ML, Burks W, Erickson JR, Fitzgerald KM, Dobson V, Innis SM, Singer LT, Montalto MB, Jacobs JR, Qiu W, Bornstein MH. 2001. Growth and development in term infants fed long-chain polyunsaturated fatty acids: A double-masked, randomized, parallel, prospective, multivariate study. Pediatrics 108:372–381.
Auricchio S, Rubino A, Murset G. 1965. Intestinal glycosidase activities in the human embryo, fetus and newborn. Pediatrics 35:944–965.
Banks MS, Salapatek P. 1983. Infant visual perception. In: Mussen PH, ed. Handbook of Child Psychology. Formerly Carmichael’s Manual of Child Psychology. 4th ed, vol 2. New York: John Wiley & Sons. Pp. 435–571.
Bates JE. 1989. Concepts and measures of temperament. In: Kohnstamm GA, Bates JE, Rothbart MK, eds. Temperament in Childhood. Chichester, Eng: John Wiley & Sons. Pp. 3–26.
Bates JE. 2001. Adjustment style in childhood as a product of parenting and temperament. In: Wachs TD, Kohnstamm GA, eds. Temperament in Context. Mahwah, NJ: Lawrence Erlbaum Associates. Pp. 173–200.
Bates JE, Bayles K. 1984. Objective and subjective components in mothers’ perceptions of their children from age 6 months to 3 years. Merrill Palmer Q 30:111–130.
Bathurst K, Gottfried AW. 1987. Untestable subjects in child development research: Developmental implications. Child Dev 58:1135–1144.
Batres LA, Maller ES. 2001. Laboratory assessment of liver function and injury in children. In: Suchy FJ, Sokol RJ, Balistreri WF, eds. Liver Disease in Children. 2nd ed. Philadelphia: Lippincott Williams & Wilkins. Pp. 155–169.
Bayley N. 1969. Manual for the Bayley Scales of Infant Development. New York: Psychological Corporation.
Bayley N. 1993. The Bayley Scales of Infant Development. 2nd ed. New York: Psychological Corporation.
Bellinger DC. 1995. Interpreting the literature on lead and child development: The neglected role of the “experimental system.” Neurotoxicol Teratol 3:201–212.
Bendersky M, Lewis M. 2001. The Bayley scales of infant development. Is there a role in biobehavioral assessment? In: Singer LT Zeskind P, eds. Biobehavioral Assessment of the Infant. New York: Guilford Press. Pp. 443–459.
Bendersky M, Alessandri SM, Lewis M. 1996. Emotions in cocaine-exposed infants. In: Lewis M, Sullivan MW, eds. Emotional Development in Atypical Children. Mahwah, NJ: Lawrence Erlbaum Associates. Pp. 89–108.
Berenthal BI, Campos JJ, Barrett KC. 1984. Self-produced locomotion. In: Emde RN, Harmon RJ, eds. Continuities and Discontinuities in Development. New York: Plenum Press. Pp. 175–210.
Binley K, Askham Z, Iqball S, Spearman H, Martin L, de Alwis M, Thrasher AJ, Ali RR, Maxwell PH, Kingsman S, Naylor S. 2002. Long-term reversal of chronic anemia using a hypoxia-regulated erythropoietin gene therapy. Blood 100:2406–2413.
Bjarnason I, Macpherson A, Hollander D. 1995. Intestinal permeability: An overview. Gastroenterology 108:1566–1581.
Bloom L. 1998. Language acquisition in its developmental context. In: Kuhn D, Siegler RS, eds. Handbook of Child Psychology. 5th ed, vol 2. New York: John Wiley & Sons. Pp. 309–370.
Boehm G, Jakobsson I, Raiha N. 1992. Macromolecular absorption in small-for-gestational-age infants. Acta Paediatr 81:864–867.
Bookheimer SY. 2000. Methodological issues in pediatric neuroimaging. Ment Retard Dev Disabil Res Rev 6:161–165.
Brugnara C, Platt OS. 1998. The neonatal erythrocyte and its disorders. In: Nathan DG, Orkin SH, eds. Nathan and Oski’s Hematology of Infancy and Childhood. 5th ed. Philadelphia: WB Saunders. Pp. 19–52.
Bushnell EW. 1985. The decline of visually guided reaching during infancy. Infant Behav Dev 8:139–155.
Bushnell EW, Boudreau JP. 1993. Motor development and the mind: The potential role of motor abilities as a determinant of aspects of perceptual development. Child Dev 64:1005–1021.
Butte NF. 2001. The role of breastfeeding in obesity. Pediatr Clin North Am 48:189–198.
Butte N, Heinz C, Hopkinhson J, Wong W, Shypailo R, Ellis K. 1999. Fat mass in infants and toddlers: Comparability of total body water, total body potassium, total body electrical conductivity, and dual-energy x-ray absorptiometry. J Pediatr Gastroentrol Nutr 29:184–189.
Butte NF, Wong WW, Hopkinson JM, O’Brian Smith E, Ellis KJ. 2000. Infant feeding mode affects early growth and body composition. Pediatrics 106:1355–1366.
Campbell SK, Osten ET, Kolobe THA, Fisher AG. 1993. Development of the test of infant motor performance. Phys Med Rehabil Clin N Am 4:541–550.
Canada. 2001. Departmental Consolidation of the Food and Drugs Act and the Food and Drug Regulations (Food and Drugs Act). Ottawa: Minister of Public Works and Government Services Canada.
Carey WB, McDevitt SC. 1978. Revision of the infant temperament questionnaire. Pediatrics 61:735–739
Case-Smith J, Bigsby R. 2001. Motor assessment. In: Singer LT, Zeskind P, eds. Biobehavioral Assessment of the Infant. New York: Guilford Press. Pp. 423–441
Chandler LS, Andrews MS, Swanson MW. 1980. Movement Assessment of Infants. A Manual. Rolling Bay, WA: Chandler, Andrews, and Swanson.
Chasnoff IJ, Burns KA, Burns WJ. 1987. Cocaine use in pregnancy: Perinatal morbidity and mortality. Neurotoxicol Teratol 9:291–293.
Christensen L. 1996. Diet-Behavior Relationships: Focus on Depression. Washington, DC: American Psychological Association.
Cobo-Lewis AB, Eilers R. 2001. Auditory assessment in infancy. In: Singer LT, Zeskind P, eds. Biobehavioral Assessment of the Infant. New York: Guilford Press. Pp. 95–106.
Colombo J. 1993. Infant Cognition: Predicting Later Intellectual Functioning. Vol. 5. Newbury Park, CA: Sage Publications.
Cory-Slechta DA. 1990. Bridging experimental animal and human behavioral toxicology studies. In: Russell RW, Flattau PE, Pope AM, eds. Behavioral Measures of Neurotoxicity. Report of a Symposium. Washington, DC: National Academy Press. Pp. 137–158.
Cowin I, Emmett P. 2000. Cholesterol and triglyceride concentrations, birthweight and central obesity in pre-school children. ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. Int J Obes Relat Metab Disord 24:330–339.
Dawson G, Ashman SB. 2000. On the origins of a vulnerability to depression: The influence of the early social environment on the development of psychobiological systems related to risk for affective disorder. In: Nelson CA, ed. The Minnesota Symposia on Child Psychology. Vol 31: The Effects of Early Adversity on Neurobehavioral Development. Mahwah, NJ: Lawrence Erlbaum Associates. Pp. 245–279.
Dewey KG, Heinig MJ, Nommsen LA, Lonnerdal B. 1991. Adequacy of energy intake among breast-fed infants in the DARLING study: Relationships to growth velocity, morbidity, and activity levels. Davis Area Research on Lactation, Infant Nutrition and Growth. J Pediatr 119:538–547.
Dewey KG, Heinig MJ, Nommsen LA, Peerson JM, Lonnerdal B. 1992. Growth of breast-fed and formula-fed infants from 0 to 18 months: The DARLING Study. Pediatrics 89:1035–1041.
Dewey KG, Heinig MJ, Nommsen LA, Peerson JM, Lonnerdal B. 1993. Breast fed infants are leaner than formula fed infants at 1 y of age: The DARLING study. Am J Clin Nutr 57:140–145.
Diamond A. 1990. The development and neural bases of memory functions as indexed by the AB and delayed response tasks in human infants and infant monkeys. Ann N Y Acad Sci 608:267–309.
Dietz WH. 1994. Critical periods in childhood for the development of obesity. Am J Clin Nutr 59:955–959.
Di Lorenzo C, Piepsz A, Ham H, Cadranel S. 1987. Gastric emptying with gastroesophageal reflux. Arch Dis Child 62:449–453.
Dinari G, Rosenbach Y, Zahavi I, Sivan Y, Nitzan M. 1984. Random fecal β1-antitrypsin excretion in children with intestinal disorders. Am J Dis Child 138:971–973.
DiPietro JA, Larson SK, Porges SW. 1987. Behavioral and heart rate pattern differences between breast-fed and bottle-fed neonates. Dev Psychol 4:467–474.
DiPietro JA, Suess PE, Wheeler JS, Smouse PH, Newlin DB. 1995. Reactivity and regulation in cocaine-exposed neonates. Infant Behav Dev 18:407–414.
Dougherty TM, Haith MM. 1997. Infant expectations and reaction times as predictors of childhood speed and processing and IQ. Dev Psychol 33:146–155.
Doussard-Roosevelt JA, McClenny BD, Porges SW. 2001. Neonatal cardiac vagal tone and school-age developmental outcome in very low birth weight infants. Dev Psychobiol 38:56–66.
Dupont C, Barau E, Molkhou P, Raynaud F, Barbet JP, Dehennin L. 1989. Food-induced alterations of intestinal permeability in children with cow’s milk-sensitive enteropathy and atopic dermatitis. J Pediatr Gastroenterol Nutr 8:459–465.
Ellison PH. 1994. The INFANIB. A Reliable Method for the Neuromotor Assessment of Infants. Tucson, AZ: Therapy Skill Builders.
Ellison PH, Horn JL, Browning CA. 1985. Construction of an Infant Neurological International Battery (INFANIB) for the assessment of neurological integrity in infancy. Phys Ther 65:1326–1331.
Esmon CT. 1998. Blood coagulation. In: Nathan DG, Orkin SH, eds. Nathan and Oski’s Hematology of Infancy and Childhood. Philadelphia: WB Saunders. Pp. 1531–1556.
European Task Force on Atopic Dermatitis. 1993. Severity scoring of atopic dermatitis: The SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 186:23–31.
Evangelista de Duffard AM, Duffard R. 1996. Behavioral toxicology, risk assessment, and chlorinated hydrocarbons. Environ Health Perspect 104:353–360.
Fagan JF 3rd, Singer LT, Montie JE, Shepherd PA. 1986. Selective screening device for the early detection of normal or delayed cognitive development in infants at risk for later mental retardation. Pediatrics 78:1021–1026.
Fagen JW, Ohr PS. 2001. Learning and memory in infancy. Habituation, instrumental conditioning, and expectancy formation. In: Singer L, Zeskind P, eds. Biobehavioral Assessment of the Infant. New York: Guilford Press. Pp. 233–273.
Falth-Magnusson K, Kjellman N-IM, Odelram H, Sundqvist T, Magnusson K-E. 1986. Gastrointestinal permeability in children with cow’s milk allergy: Effect of milk challenge and sodium-cromoglycate as assessed with polyethyleneglycols (PEG 400 and 1000). Clin Allergy 16:543–551.
FDA (Food and Drug Administration). 1996. Current good manufacturing practice, quality control procedures, quality factors, notification requirements, and records and reports, for the production of infant formula; Proposed rule. Fed Regist 61:36153–36219.
Fee B, Weil WM. 1960. Body composition of a diabetic offspring by direct analysis. Am J Dis Child 100:718.
Fernald A. 2001. Hearing, listening, and understanding: Auditory development in infancy. In: Bremner G, Fogel A, eds. Blackwell Handbook of Infant Development. Malden, MA: Blackwell Publishers. Pp. 35–70.
Fernandes J, Vos CE, Douwes AC, Slotema E, Degenhart HJ. 1978. Respiratory hydrogen excretion as a parameter for lactose malabsorption in children. Am J Clin Nutr 31:597–602.
Fetters L, Tronick EZ. 1996. Neuromotor development of cocaine-exposed and control infants from birth through 15 months: Poor and poorer performance. Pediatrics 98:938–943.
Fomon SJ. 1993. Nutrition of Normal Infants. St. Louis: Mosby.
Fomon SJ, Ziegler EE, Thomas LN, Jensen RL, Filer LJ Jr. 1970. Excretion of fat by normal full-term infants fed various milks and formulas. Am J Clin Nutr 23:1299–1313.
Fomon SJ, Nelson SE, Ziegler EE. 2000. Retention of iron by infants. Ann Rev Nutr 1:273–290.
Forse RA, Bell SJ, Blackburn GL, Kabbash LG, eds. 1994. Diet, Nutrition, and Immunity. Boca Raton, FL: CRC Press.
Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. 2001. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Dev 72:1–21.
Fredrikzon B, Hernell O, Blackberg L, Olivecrona T. 1978. Bile salt-stimulated lipase in human milk: Evidence of activity in vivo and of a role in the digestion of milk retinal esters. Pediatr Res 12:1048–1052.
Garza C, de Onis M. 1999. A new international growth reference for young children. Am J Clin Nutr 70:169S– 172S.
Gaylor DW, Bolger PM, Schwetz BA. 1998. U.S. Food and Drug Administration perspective of the inclusion of effects of low-level exposures in safety and risk assessment. Environ Health Perspect 106:391–394.
Gleim GW. 2000. Renal responses to exercise and training. In: Garrett WE, Krikendall DT, eds. Exercise and Sport Science. Philadelphia: Lippincott Williams & Wilkins. Pp. 217–225.
Godfrey KM, Barker DJ. 2001. Fetal programming and adult health. Public Health Nutr 4:611–624.
Goldsmith DI, Novello AC. 1992. Clinical laboratory evaluation of renal function. In: Edelmann CM, ed. Pediatric Kidney Disease. Boston: Little, Brown. P. 471.
Goldsmith HH, Rothbart MK. 1991. Contemporary instruments for assessing early temperament by questionnaire and in the laboratory. In: Strelau J, Angleitner A, eds. Explorations in Temperament. International Perspectives on Theory and Measurement. New York: Plenum Press. Pp. 249–272.
Goldsmith HH, Losoya SH, Bradshaw DL, Campos JJ. 1994. Genetics of personality: A twin study of the five-factor model and parent-offspring analysis. In: Halverson CF, Kohnstamm GA, Martin RP, eds. The Developing Structure of Temperament and Personality from Infancy to Adulthood. Hillsdale, NJ: Lawrence Erlbaum Associates. Pp. 241–265.
Goldsmith HH, Lemery KS, Aksan N, Buss KA. 2000. Temperamental substrates of personality development. In: Molfese VJ, Molfese DL, eds. Temperament and Personality Development Across the Lifespan. Mahwah, NJ: Lawrence Erlbaum Associates. Pp. 1–32.
Grandjean P, White RF, Weihe P. 1996. Neurobehavioral epidemiology: Application in risk assessment. Environ Health Perspect 104:397–400.
Grant JD, Bezerra JA, Thompson SH, Lemen RJ, Koldovsky O, Udall JN. 1989. Assessment of lactose absorption by measurement of urinary galactose. Gastroenterology 97:895–899.
Grantham-McGregor SM, Walker SP, Chang S. 2000. Nutritional deficiencies and later behavioural development. Proc Nutr Soc 59:47–54.
Grant-Webster KS, Gunderson VM, Brubacher TM. 1990. Behavioral assessment of young nonhuman primates: Perceptual-cognitive development. Neurotoxicol Teratol 12:543–546.
Greenough WT, Black JE. 1992. Induction of brain structure by experience: Substrates for cognitive development. In: Gunnar MR, Nelson CA, eds. Developmental Behavioral Neuroscience. The Minnesota Symposia on Child Psychology. Vol. 24. Hillsdale, NJ: Lawrence Erlbaum Associates. Pp. 155–200.
Gunnar MR. 2000. Early adversity and the development of stress reactivity and regulation. In: Nelson CA, ed. The Effects of Early Adversity on Neurobehavioral Development. The Minnesota Symposia on Child Psychology. Vol. 31. Mahwah, NJ: Lawrence Erlbaum Associates, Publishers. Pp. 163–200.
Gunnar MR, White BP. 2001. Salivary cortisol measures in infant and child assessment. In: Singer LT, Zeskind PS, eds. Biobehavioral Assessment of the Infant. New York: Guilford Press. Pp. 167–189.
Guo SM, Roche AF, Fomon SJ, Nelson SE, Chumlea WC, Rogers RR, Baumgartner RN, Ziegler EE, Siervogel RM. 1991. Reference data on gains in weight and length during the first two years of life. J Pediatr 119:355–362.
Hadorn B, Zoppi G, Shmerling DH, Prader A, McIntyre I, Anderson CM. 1968. Quantitative assessment of exocrine pancreatic function in infants and children. J Pediatr 73:39–50.
Hamilton J. 2000. The pediatric patient: Early development and the digestive system. In: Walker WA, Durie PR, Hamilton JR, Walker-Smith JA, Watkins JB, eds. Pediatric Gastrointestinal Disease. 3rd ed. Hamilton, Ont: BC Decker. Pp. 1–11.
Handin RI. 1998. Blood platelets and the vessel wall. In: Nathan DG, Orkin SH, eds. Nathan and Oski’s Hematology of Infancy and Childhood. Philadelphia: WB Saunders. Pp 1511–1529.
Harris SR, Brady DK. 1986. Infant neuromotor assessment instruments: A review. Phys Occup Ther Pediatr 6:121–153.
Harris SR, Swanson MW, Andrews MS, Sells CJ, Robinson NM, Bennett FC, Chandler LS. 1984. Predictive validity of the “movement assessment of infants.” J Dev Behav Pediatr 5:336–342.
Hediger ML, Overpeck MD, Ruan WJ, Troendle JF. 2000. Early infant feeding and growth status of US-born infants and children aged 4–71 mo: Analyses from the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr 72:159–167.
Herskowitz J, Rosman NP. 1985. Pseudoseizure in a child with epilepsy. Am J Psychiatry 142:390–391.
Heyman M, Grasset E, Ducroc R, Desjeux JF. 1988. Antigen absorption by the jejunal epithelium of children with cow’s milk allergy. Pediatr Res 24:197–202.
Higley JD, Suomi SJ. 1989. Temperamental reactivity in non-human primates. In: Kohnstamm GA, Bates JE, Rothbart MK, eds. Temperament in Childhood. Chichester, Eng: John Wiley & Sons. Pp. 153–167.
Hill RM, Hegemier S, Tennyson LM. 1989. The fetal alcohol syndrome: A multihandicapped child. Neurotoxicology 10:585–596.
Holm S, Andersson Y, Gothefors L, Lindbert T. 1992. Increased protein absorption after gastroenteritis in children. Acta Paediatr 81:585–588.
Holt PG, Macaubas C, Stumbles PA, Sly PD. 1999. The role of allergy in the development of asthma. Nature 402:B12–B17.
Hopkins WD, Rilling JK. 2000. A comparative MRI study of the relationship between neuroanatomical asymmetry and interhemispheric connectivity in primates: Implication for the evolution of functional asymmetries. Behav Neurosci 4:739–748.
Hurt H, Brodsky NL, Betancourt L, Braitman LE, Malmud E, Giannetta J. 1995. Cocaine-exposed children: Follow-up through 30 months. J Dev Behav Pediatr 16:29–35.
Huynh H, Couper R. 2000. Pancreatic function tests. In: Walker WA, Durie PR, Hamilton JR, Walker-Smith JA, Watkins JB, eds. Pediatric Gastrointestinal Disease. 3rd ed. Hamilton, Ont: BC Decker. Pp. 1515–1528.
Hypponen E, Virtanen SM, Kenward MG, Knip M, Akerblom HK. 2000. Obesity, increased linear growth, and risk of type 1 diabetes in children. Diabetes Care 23:1755–1760.
IOM (Institute of Medicine). 1991. Nutrition During Lactation. Washington, DC: National Academy Press.
IOM. 1997. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academy Press.
IOM. 2001. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press.
Jacobs DS, Blakemore C. 1988. Factors limiting the postnatal development of visual acuity in the monkey. Vision Res 28:947–958.
Jacobson JL, Jacobson SW. 1996. Prospective, longitudinal assessment of developmental neurotoxicity. Environ Health Perspect 104:275–283.
Jacobson SW. 1998. Specificity of neurobehavioral outcomes associated with prenatal alcohol exposure. Alcohol Clin Exp Res 22:313–320.
Jacobson SW, Jacobson JL. 2000. Teratogenic insult and neurobehavioral function in infancy and childhood. In: Nelson CA, ed. The Minnesota Symposia on Child Psychology. Vol 31: The Effects of Early Adversity on Neurobehavioral Development. Mahwah, NJ: Lawrence Erlbaum Associates. Pp. 61–112.
Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Chiodo LM. 1996. New evidence for neurobehavioral effects of in utero cocaine exposure. J Pediatr 129:581–590.
Jakobsson I, Lindberg T, Lothe L, Axelsson I, Benediktsson B. 1986. Human alpha-lactalbumin as a marker of macromolecular absorption. Gut 27:1029–1034.
Jensen C, Prager T, Fraley J, Chen H, Anderson R, Heird W. 1997. Effects of dietary linoleic/alpha-linolenic acid ratio or growth and visual function of term infants. J Pediatr 131:200–209.
Johnson MH. 2001. Functional brain development in humans. Nat Rev Neurosci 2:475–483.
Jusczyk PW. 1985. The high-amplitude sucking technique as a methodological tool in speech perception research. In: Gottlieb G, Krasnegor NA, eds. Measurement of Audition and Vision in the First Year of Postnatal Life: A Methodological Overview. Norwood, NJ: Ablex Publishing. Pp. 195–222.
Juvonen P, Jakobsson I, Lindberg T. 1990. Macromolecular absorption and cows milk allergy. Arch Dis Child 65:300–303.
Kaltenbach KA, Finnegan LP. 1989. Prenatal narcotic exposure: Perinatal and developmental effects. Neurotoxicology 10:597–604.
Kaneko WM, Phillips EL, Riley EP, Ehlers CL. 1996. EEG findings in Fetal Alcohol Syndrome and Down Syndrome children. Electroencephalogr Clin Neurophysiol 98:20–28.
Karpen SJ, Suchy FJ. 2001. Structural and functional development of the liver. In: Suchy FJ, Sokol RJ, Balistreri WF, eds. Liver Disease in Children. Philadelphia: Lippincott Williams & Wilkins. Pp. 3–21.
Kay AB. 2001. Allergy and allergic diseases. First of two parts. N Engl J Med 344:30–37.
Kindermann TA. 1993. Fostering independence in mother-child interactions: Longitudinal changes in contingency patterns as children grow competent in developmental tasks. Int J Behav Dev 16:513–535.
Kohnstamm GA, Bates JE, Rothbart MK, eds. 1989. Temperament in Childhood. Chichester, Eng: John Wiley & Sons.
Kok EJ, Kuiper HA. 2003. Comparative safety assessment for biotech crops. Trends Biotechnol 21:439–444.
Koo WW. 2000. Body composition measurements during infancy. Ann N Y Acad Sci 904:383–392.
Kramer MS, Guo T, Platt RW, Shapiro S, Collet JP, Chalmers B, Hodnett E, Sevkovskaya Z, Dzikovich I, Vanilovich I. 2002. Breastfeeding and infant growth: Biology or bias? Pediatrics 110:343–347.
Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. 2000. CDC growth charts: United States. Adv Data 314:1–27.
Laughlin NK, Bushnell PJ, Bowman RE. 1991. Lead exposure and diet: Differential effects on social development in the rhesus monkey. Neurotoxicol Teratol 13:429–440.
Lawson KR, Ruff HA. 2001. Focused attention: Assessing a fundamental cognitive process in infancy. In: Singer LT, Zeskind PS, eds. Biobehavioral Assessment of the Infant. New York: Guilford Press. Pp. 3–17.
Leeson CP, Kattenhorn M, Deanfield JE, Lucas A. 2001. Duration of breast feeding and arterial distensibility in early adult life: Population based study. Br Med J 322:643–647.
Lester BM, Tronick EZ. 2001. Behavioral assessment scales. The NICU Network Neurobehavioral Scale, the Neonatal Behavioral Assessment Scale, and the Assessment of the Preterm Infant’s Behavior. In: Singer LT, Zeskind PS, eds. Biobehavioral Assessment of the Infant. New York: Guilford Press. Pp. 363–380.
Lester BM, Freier K, LaGasse L. 1995. Prenatal cocaine exposure and child outcome: What do we really know? In: Lewis M, Bendersky M, eds. Mothers, Babies, and Cocaine: The Role of Toxins in Development. Hillsdale, NJ: Lawrence Erlbaum Associates. Pp. 19–39.
Lindberg T. 1966. Intestinal dipeptidases: Characterization, development and distribution of intestinal dipeptidases of the human foetus. Clin Sci 30:505–515.
Lindsay RS, Cook V, Hanson RL, Salbe AD, Tataranni A, Knowler WC. 2002. Early excess weight gain of children in the Pima Indian population. Pediatrics 109:E33.
Lohman TG, Roche AF, Martorell R. 1988. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Books.
Lothe L, Ivarsson SA, Ekman R, Lindberg T. 1990. Motilin and infantile colic. A prospective study. Acta Paediatr Scand 7:410–416.
Lozoff B, Klein NK, Nelson EC, McClish DK, Manuel M, Chacon ME. 1998. Behavior of infants with iron-deficiency anemia. Child Dev 69:24–36.
Maffei HV, Metz G, Bampoe V, Shiner M, Herman S, Brook CG. 1977. Lactose intolerance, detected by the hydrogen breath test, in infants and children with chronic diarrhoea. Arch Dis Child 52:766–767.
Majamaa H, Miettinen A, Laine S, Isolauri E. 1996. Intestinal inflammation in children with atopic eczema: Faecal eosinophil cationic protein and tumour necrosis factor-alpha as non-invasive indicators of food allergy. Clin Exp Allergy 26:181–187.
Marshall DH, Fox NA. 2001. Electroencephalographic assessment and human brain maturation. A window into emotional and cognitive development in infancy. In: Singer LT, Zeskind PS, eds. Biobehavioral Assessment of the Infant. New York: Guilford Press. Pp. 341–356.
Martin RP, Wisenbaker J, Huttunen M. 1994. Review of factor analytic studies of temperament measures based on the Thomas-Chess structural model: Implications for the big five. In: Halverson CF Jr, Kohnstamm GA, eds. The Developing Structure of Temperament and Personality from Infancy to Adulthood. Hillsdale, NJ: Lawrence Erlbaum Associates. Pp. 157–172.
Masten AS, Coatsworth JD. 1998. The development of competence in favorable and unfavorable environments. Lessons from research on successful children. Am Psychol 53:205–220.
Matheny AP Jr. 1989. Temperament and cognition: Relations between temperament and mental test scores. In: Kohnstamm GA, Bates JE, Rothbart MK, eds. Temperament in Childhood. Chichester, Eng: John Wiley & Sons. Pp. 263–282.
Matheny AP Jr. 1991. Play assessment of infant temperament. In: Schaefer CE, Gitlin K, Sandgrund A, eds. Play Diagnosis and Assessment. New York: John Wiley & Sons. Pp. 39–63.
Matheny AP Jr, Phillips K. 2001. Temperament and context: Correlates of home environment with temperament continuity and change, new born to 30 months. In: Wachs TD, Kohnstamm GA, eds. Temperament in Context. Mahwah, NJ: Lawrence Erlbaum Associates. Pp. 81–101.
Mattson SM, Riley EP. 1995. Prenatal exposure to alcohol. What the images reveal. Alcohol Health Res World 19:273–278.
Mayer DL, Arendt RE. 2001. Visual acuity assessment in infancy. In: Singer LT, Zeskind PS, eds. Biobehavioral Assessment of the Infant. New York: Guilford Press. Pp. 81–94.
Mayes LC, Bornstein MH. 1995. Developmental dilemmas for cocaine-abusing parents and their children. In: Lewis M, Bendersky M, eds. Mothers, Babies, and Cocaine: The Role of Toxins in Development. Hillsdale, NJ: Lawrence Erlbaum Associates. Pp. 251–272.
Mayes LC, Cicchetti DV. 1995. Prenatal cocaine exposure and neurobehavioral development: How subjects lost to follow-up bias study results. Child Neuropsychol 1:128–139.
Mayes LC, Bornstein MH, Chawarska K, Granger RH. 1995. Information processing and developmental assessments in 3-month-old infants exposed prenatally to cocaine. Pediatrics 95:539–545.
McCall RB. 1994. What process mediates predictions of childhood IQ from infant habituation and recognition memory? Speculations on the roles of inhibition and rate of information processing. Intelligence 18:107–125.
McCall RB, Appelbaum MI. 1991. Some issues of conducting secondary analyses. Dev Psychol 27:911–917.
McCall RB, Carriger MS. 1993. A meta-analysis of infant habituation and recognition memory performance as predictors of later IQ. Child Dev 64:57–79.
McMurray RG, Hackney AC. 2000. Endocrine responses to exercise and training. In: Garrett WE, Krikendall DT, eds. Exercise and Sport Science. Philadelphia: Lippincott Williams & Wilkins. Pp. 135–161.
Milani-Comparetti A, Gidoni EA. 1967. Routine developmental examination in normal and retarded children. Dev Med Child Neurol 9:631–638.
Miller LJ, Roid GH. 1994. The T.I.M.E. Toddler and Infant Motor Evaluation. A Standardized Assessment. San Antonio: Therapy Skill Builders.
Molfese DL, Molfese VJ. 2001. Cortical electrophysiology and language processes in infancy. In: Singer LT, Zeskind PS, eds. Biobehavioral Assessment of the Infant. New York: Guilford Press. Pp. 323–338.
Montgomery RK, Mulberg AE, Grand RJ. 1999. Development of the human gastrointestinal tract: Twenty years of progress. Gastroenterology 116:702–731.
Moore JM, Wilson WR, Thompson G. 1977. Visual reinforcement of head-turn responses in infants under 12 months of age. J Speech Hear Disord 42:328–334.
Nakahara K, Hayashi T, Konishi S, Miyashita Y. 2002. Functional MRI of macaque monkeys performing a cognitive set-shifting task. Science 295:1532–1536.
Needlman R, Frank DA, Augustyn M, Zuckerman BS. 1995. Neurophysiological effects of prenatal cocaine exposure: Comparison of human and animal investigations. In: Lewis M, Bendersky M, eds. Mothers, Babies, and Cocaine: The Role of Toxins in Development. Hillsdale, NJ: Lawrence Erlbaum Associates. Pp. 229–250.
Nelson CA. 1994. Neural bases of infant temperament. In: Bates JE, Wachs TD, eds. Temperament: Individual Differences at the Interface of Biology and Behavior. Washington, DC: American Psychological Association. Pp. 47–82.
Nelson CA. 1995. The ontogeny of human memory: A cognitive neuroscience perspective. Dev Psychol 31:723–738.
Nelson CA, Bloom FE. 1997. Child development and neuroscience. Child Dev 5:970–987.
Nelson CA, Wewerka S, Thomas KM, Tribby-Walbridge S, deRegnier R, Georgieff M. 2000. Neurocognitive sequelae of infants of diabetic mothers. Behav Neurosci 114:950–956.
Nelson CA, Bloom FE, Cameron JL, Amaral D, Dahl RE, Pine D. 2002. An integrative, multidisciplinary approach to the study of brain-behavior relations in the context of typical and atypical development. Dev Psychopathol 14:499–520.
Nelson SE, Rogers RR, Ziegler EE, Fomon SJ. 1989. Gain in weight and length during early infancy. Early Hum Dev 19:223–239.
Neuspiel DR. 1995. The problem of confounding in research on prenatal cocaine effects on behavior and development. In: Lewis M, Bendersky M, eds. Mothers, Babies, and Cocaine: The Role of Toxins in Development. Hillsdale, NJ: Lawrence Erlbaum Associates. Pp. 95–109.
Norman A, Strandvik B, Ojamae O. 1972. Bile acids and pancreatic enzymes during absorption in the newborn. Acta Paediat Scand 61:571–576.
O’Connor MJ, Sigman M, Kasari C. 1993. Interactional model for the association among maternal alcohol use, mother-infant interaction, and infant cognitive development. Infant Behav Dev 16:177–192.
OFAS (Office of Food Additive Safety). 2001. Toxicological Principles for the Safety Assessment of Direct Food Additives and Color Additives Used in Food. Redbook II-Draft. Washington, DC: OFAS, Center for Food Safety and Applied Nutrition, Food and Drug Administration.
OFAS. 2003. Redbook 2000. Toxicological Principles for the Safety of Food Ingredients. Online. Center for Food Safety and Applied Nutrition, Food and Drug Administration. Available at http://www.cfsan.fda.gov/~redbook/red-toca.html. Accessed November 19, 2003.
Olsho LW, Koch EG, Halpin CF, Carter EA. 1987. An observer-based psychoacoustic procedure for use with young infants. Dev Psychol 23:627–640.
Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. 2000. Association between postnatal catch-up growth and obesity in childhood: Prospective cohort study. Br Med J 320:967–971.
Pelletier DL, Frongillo EA Jr, Habicht JP. 1993. Epidemiologic evidence for a potentiating effect of malnutrition on child mortality. Am J Public Health 83:1130–1133.
Perman JA, Barr RG, Watkins JB. 1978. Sucrose malabsorption in children: Noninvasive diagnosis by interval breath hydrogen determination. J Pediatr 93:17–22.
Pinero D, Jones B, Beard J. 2001. Variations in dietary iron alter behavior in developing rats. J Nutr 131:311–318.
Piper MC, Darrah J. 1994. Alberta Infant Motor Scale: Construction of a Motor Assessment Tool for the Developing Infant. Philadelphia: WB Saunders Company. Pp. 25–35.
Plancoulaine S, Charles MA, Lafay L, Tauber M, Thibult N, Borys JM, Eschwege E, Laventie Ville Sante Study Group. 2000. Infant-feeding patterns are related to blood cholesterol concentration in prepubertal children aged 5–11 y: The Fleurbaix-Laventie Ville Santé Study. Eur J Clin Nutr 54:114–119.
Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. 1996. Infant regulation of the vagal ‘brake’ predicts child behavior problems: A psychobiological model of social behavior. Devel Psychobiol 29:697–712.
Porter FL. 2001. Vagal tone. In: Singer LT, Zeskind PS, eds. Biobehavioral Assessment of the Infant. New York: Guilford Press. Pp. 109–124.
Posner MI. 2001. The developing human brain. Dev Sci 4:253–387.
Rao R, Georgieff MK. 2000. Early nutrition and brain development. In: Nelson C, ed. The Minnesota Symposia on Child Psychology. The Effects of Adversity on Neurobehavioral Development. Vol 31. Mahway, NJ: Lawrence Erlbaum Associates. Pp. 1–30.
Robb TA, Davidson GP. 1981. Advances in breath hydrogen quantitation in paediatrics: Sample collection and normalization to constant oxygen and nitrogen levels. Clin Chim Acta 111:281–285.
Roberton DM, Paganelli R, Dinwiddie R, Levinsky RJ. 1982. Milk antigen absorption in the preterm and term neonate. Arch Dis Child 57:369–372.
Robson AL, Pederson DR. 1997. Predictors of individual differences in attention among low birth weight children. J Dev Behav Pediatr 18:13–21.
Roncagliolo M, Garrido M, Walter T, Peirano P, Lozoff B. 1998. Evidence of altered central nervous system development in infants with iron deficiency anemia at 6 mo: Delayed maturation of auditory brainstem responses. Am J Clin Nutr 68:683–690.
Rose SA, Feldman JF. 1990. Infant cognition: Individual differences and developmental continuities. In: Colombo J, Fagen J, eds. Individual Differences in Infancy: Reliability, Stability, Prediction. Hillsdale, NJ: Lawrence Erlbaum Associates. Pp. 229–245.
Rose SA, Feldman JF. 1995. Prediction of IQ and specific cognitive abilities at 11 years from infancy measures. Dev Psychol 31:685–696.
Rose SA, Orlian EK. 2001. Visual information processing. In: Singer LT, Zeskind PS, eds. Biobehavioral Assessment of the Infant. New York: Guilford Press. Pp. 274–292.
Rose SA, Feldman JF, Wallace IF, McCarton C. 1989. Infant visual attention: Relation to birth status and developmental outcome during the first 5 years. Dev Psychol 25:560–576.
Rose SA, Feldman JF, Wallace IF, McCarton C. 1991. Information processing at 1 year: Relation to birth status and developmental outcome during the first 5 years. Dev Psychol 5:723–737.
Rossetti Ferreira MC. 1978. Malnutrition and mother-infant asynchrony: Slow mental development. Int J Behav Dev 1:207–219.
Rothbart MK. 1986. Longitudinal observation of infant temperament. Dev Psychol 22:356–365.
Rothbart MK, Bates JE. 1998. Temperament. In: Eisenberg N, ed. Handbook of Child Psychology. 5th ed, vol 3. New York: John Wiley & Sons. Pp. 105–176.
Rothbart MK, Derryberry D, Posner MI. 1994. A psychobiological approach to the development of temperament. In: Bates JE, Wachs TD, eds. Temperament: Individual Differences at the Interface of Biology and Behavior. Washington, DC: American Psychological Association. Pp. 83–116.
Rothbart MK, Derryberry D, Hershey K. 2000. Stability of temperament in childhood: Laboratory infant assessment to parent report at seven years. In: Molfese VJ, Molfese DL, eds. Temperament and Personality Development Across the Life Span. Mahwah, NJ: Lawrence Erlbaum Associates. Pp. 85–118.
Rovee-Collier C, Barr R. 2001. Infant learning and memory. In: Bremner G, Fogel A, eds. Blackwell Handbook of Infant Development. Malden, MA: Blackwell Publishers. Pp. 139–168.
Ruff HA, Rothbart MK. 1996. Attention in Early Development. Themes and Variations. New York: Oxford University Press.
Russell RW. 1992. “Malnutrition” and the vulnerable brain. What a difference a molecule makes. In: Isaacson RL, Jensen KF, eds. The Vulnerable Brain and Environmental Risks. Vol 1. Malnutrition and Hazard Assessment. New York: Plenum Press. Pp. 109–126.
Sampson HA. 1996. Epidemiology of food allergy. Pediatr Allergy Immunol 7:42–50.
Sampson HA. 2000. Peanut allergy. N Engl J Med 346:1294–1299.
Sanderson IR, Walker WA. 1993. Uptake and transport of macromolecules by the intestine: Possible role in clinical disorders (an update). Gastroenterology 104:622–639.
Sanson A, Pedlow R, Cann W, Prior M, Oberklaid F. 1996. Shyness ratings: Stability and correlates in early childhood. Int J Behav Dev 19:705–724.
Schmelzle HR, Fusch C. 2002. Body fat neonates and young infants: Validation of skinfold thickness versus dual-energy x-ray absorptimetry. Am J Clin Nutr 76:1096–1100.
Schneider JW, Griffith DR, Chasnoff IJ. 1989. Infants exposed to cocaine in utero: Implications for developmental assessment and intervention. Infants Young Child 2:25–36.
Schore AN. 1994. Affect Regulation and the Origin of the Self. The Neurobiology of Emotional Development. Hillsdale, NJ: Lawrence Erlbaum Associates.
Schrander JJP, Unsalan-Hooyen RWM, Forget PP, Jansen J. 1990. 51Cr EDTA intestinal permeability in children with cow’s milk intolerance. J Pediatr Gastroenterol Nutr 10:189–192.
Scott DT, Janowsky JS, Carroll RE, Taylor JA, Auestad N, Montalto MB. 1998. Formula supplementation with long-chain polyunsaturated fatty acids: Are there developmental benefits? Pediatrics 102:1203–1204.
Scott RB. 2000. Motility disorders. In: Walker WA, Durie PR, Hamilton JR, Walker-Smith JA, Watkins JB, eds. Pediatric Gastrointestinal Disease. 3rd ed. Hamilton, Ont: BC Decker. Pp. 103–115.
Sebris SL, Dobson V, Hartmann EE. 1984. Assessment and prediction of visual acuity in 3- to 4-year-old children born prior to term. Hum Neurobiol 3:87–92.
Sereno MI. 1998. Brain mapping in animals and humans. Curr Opin Neurobiol 8:188–194.
Setchell KDR, O’Connell NC. 2001. Disorders of bile acid synthesis and metabolism: A metabolic basis for liver disease. In: Suchy FJ, Sokol RJ, Balistreri WF, eds. Liver Disease in Children. Philadelphia: Lippincott Williams & Wilkins. Pp. 701–733.
Shaheen SJ. 1984. Neuromaturation and behavior development: The case of childhood lead poisoning. Develop Psychol 20:542–550.
Shrout PE, Bolger N. 2002. Mediation in experimental and nonexperimental studies. New procedures and recommendations. Psychol Methods 7:422–445.
Sicherer SH, Noone SA, Koerner CB, Christie L, Burks, AW, Sampson HA. 2001. Hypoallergenicity and efficacy of an amino acid based formula in children with cow’s milk and multiple food hypersensitivities. J Pediatr 138:688–693.
Singer LT. 2001. General issues in infant assessment and development. In: Singer LT, Zeskind PS, eds. Biobehavioral Assessment of the Infant. New York: Guilford Press. Pp. 3–17.
Singer L, Arendt R, Farkas K, Minnes S, Huang J, Yamashita T. 1997. Relationship of prenatal cocaine exposure and maternal postpartum psychological distress to child developmental outcome. Dev Psychopathol 9:473–489.
Slater A. 1997. Can measures of infant habituation predict later intellectual ability? Arch Dis Child 77:474–476.
Slater A. 2001. Visual perception. In: Bremner G, Fogel A, eds. Blackwell Handbook of Infant Development. Malden, MA: Blackwell Publishers. Pp. 5–34.
Slota MC. 1983. Neurological assessment of the infant and toddler. Crit Care Nurse 3:87–92.
Smitsman MW. 2001. Action in infancy—Perspectives, concepts, and challenges: The development of reaching and grasping. In: Bremner G, Fogel A, eds. Blackwell Handbook of Infant Development. Malden, MA: Blackwell Publishers. Pp. 71–98.
Sobotka TJ, Ekelman KB, Slikker W Jr, Raffaele K, Hattan DG. 1996. Food and Drug Administration proposed guidelines for neurotoxicological testing of food chemicals. Neurotoxicology 17:825–836.
Sparrow SS, Balla DA, Cicchetti DV. 1984. Vineland Adaptive Behavioral Scales. Circle Pines, MN: American Guidance Service.
Spear LP. 1995. Alterations in cognitive function following prenatal cocaine exposure: Studies in an animal model. In: Lewis M, Bendersky M, eds. Mothers, Babies, and Cocaine: The Role of Toxins in Development. Hillsdale, NJ: Lawrence Erlbaum Associates. Pp. 207–227.
Sperling MA. 1996. Pediatric Endocrinology. Philadelphia: WB Saunders. Pp. 549–584.
Stettler N, Bovert P, Shamlaye H, Zemel BS, Stallings VA, Paccaud F. 2002a. Prevalence and risk factors for overweight and obesity in children from Seycheles, a country in rapid transition: The importance of early growth. Int J Obes Relat Metab Disord 26:214–219.
Stettler N, Zemel BS, Kumanyika S, Stallings VA. 2002b. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics 109:194–199.
Struthers JM, Hansen RL. 1992. Visual recognition memory in drug-exposed infants. J Dev Behav Pediatr 13:108–111.
Takeda A. 2001. Zinc homeostasis and functions of zinc in the brain. Biometals 14:343–351.
Tanner JM. 1970. Physical growth. In: Mussen PH, ed. Carmichael’s Manual of Child Psychology. 3rd ed, vol 1. New York: John Wiley & Sons. Pp. 77–155.
Teller DY, McDonald MA, Preston K, Sebris SL, Dobson V. 1986. Assessment of visual acuity in infants and children: The acuity card procedure. Dev Med Child Neurol 28:779–789.
Thomas DW, Sinatra FR, Merritt RJ. 1981. Random fecal alpha-1-antitrypsin concentration in children with gastrointestinal disease. Gastroenterology 80:776–782.
van de Kamer JH, ten Bokkel Huinink H, Weyers HA. 1949. Rapid method for the determination of fat in feces. J Biol Chem 177:347–355.
Vijayakumar M, Fall CH, Osmond C, Barker DJ. 1995. Birth weight, weight at one year, and left ventricular mass in adult life. Br Heart J 73:363–367.
von Hofsten C. 1991. Structuring of early reaching movements: A longitudinal study. J Mot Behav 23:280–292.
von Hofsten C, Fazel-Zandy S. 1984. Development of visually guided hand orientation in reaching. J Exp Child Psychol 38:208–219.
Vorhees CV. 1986. Principles of behavioral teratology. In: Riley EP, Vorhees CV, eds. Handbook of Behavioral Teratology. New York: Plenum Press. Pp. 23–48.
Wachs TD. 1999. The nature and nurture of child development. Food Nutr Bull 20:7–22.
Wachs TD. 2000. Linking nutrition and temperament. In: Molfese VJ, Molfese DL, eds. Temperament and Personality Development Across the Life Span. Mahwah, NJ: Lawrence Erlbaum Associates. Pp. 57–84.
Wachs TD, Bates JE. 2001. Temperament. In: Bremner G, Fogel A, eds. Blackwell Handbook of Infant Development. Malden, MA: Blackwell Publishers. Pp. 465–501.
Walker WA. 2002. Development of the intestinal mucosal barrier. J Pediatr Gastroenterol Nutr 34:S33–S39.
Watkins JB. 1985. Lipid digestion and absorption. Pediatrics 75:151–156.
Watkins JB, Ingall D, Szczepanik P, Klein PD, Lester R. 1973. Bile-salt metabolism in the newborn. Measurement of pool size and synthesis by stable isotope technique. New Engl J Med 288:431–434.
Weiss B. 1995. Incipient hazards of cocaine: Lessons from environmental toxicology. In: Lewis M, Bendersky M, eds. Mothers, Babies, and Cocaine: The Role of Toxins in Development. Hillsdale, NJ: Lawrence Erlbaum Associates. Pp. 41–55.
Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. 1997. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 337:869–873.
WHO (World Health Organization). 2002. The Optimal Duration of Exclusive Breastfeeding. Report of an Expert Consultation. Geneva: WHO.
Wroble M, Mash C, Williams L, McCall R. 2002. Should long chain polyunsaturated fatty acids be added to infant formula to promote development? J App Dev Psychol 23:99–112.
Yajnik CS. 2001. The insulin resistance epidemic in India: Fetal origins, later lifestyle, or both? Nutr Rev 59:1–9.