4
Historical Context of Poison Control
At the beginning of the 20th century, there was no modern specialty of toxicology, no poison control centers, no oversight of pharmaceutical manufacturing or drug labeling, and little knowledge regarding the treatment of poisonings in the United States. Household and occupational toxic hazards were poorly understood. As public health concepts were in their formative stages, surveillance of toxic exposures and the morbidity and mortality associated with these exposures were virtually unknown. Similarly, emergency response personnel and systems of emergency care that could respond to poisonings in the home or workplace were virtually nonexistent.
This chapter summarizes some of the key historical determinants of poison control and management as a modern health care service. Through a review of these developments, we can better understand the origin of our current “system” of poison control and management. Understanding this history can enhance efforts to advance this vital health care service by identifying potential barriers to the evolution of an optimal poison control and management system.
BRIEF OVERVIEW
Issues of misbranding, mislabeling, and adulteration of food and drugs concerned those who were involved in public health as well as health care providers and led to the founding of the United States Pharmacopeia (1820), the American Medical Association (1847), the American
Pharmacists Association (1852), and other organizations. Concerns about food and drug safety, beginning in the 19th century, provided the impetus for the first human clinical food additive trials to demonstrate safety and efficacy in December 1902 (Hurt, 1985). These efforts led to the Pure Food and Drug Act of 1906 (Lewis, 2002). This legislation (“Wiley Act”) created the Food, Drug, and Insecticide Administration, which in 1930 became the Food and Drug Administration (FDA). The Act required approval for foods and drugs meant for human consumption. Subsequently, the federal government passed a number of laws, created regulations, and proposed other controls and management of poisoning. These efforts are summarized in Box 4-1.
In the 1930s, childhood poisoning was recognized as a significant component of pediatric practice and patient morbidity. Unfortunately, little information existed regarding the toxicity of household products and management recommendations. Jay Arena, M.D., a pediatrician at Duke University, began to systematically collect information regarding toxic hazards in the early 1930s and provided advice to physicians on poisoning cases in the surrounding area. He provided one of the first reports on the hazards of household products to children (Martin and Arena, 1939). Louis Gdalman, R.Ph., a pharmacist in Chicago, collected information during World War II. He developed a toxicological information system using index cards and eventually converted to microfiche. This system eventually covered more than 9,000 commercial and consumer products. Moreover, Gdalman established the precursor to the modern poison control center by personally taking telephone calls 24 hours per day (Botticelli and Pierpaoli, 1992; Burda and Burda, 1997).
Although recognized as a growing problem during this period, the magnitude of childhood poisoning was not appreciated until a 1949–1950 epidemiological study focusing on children under 5 years of age reported a significant number of poisoning deaths (Bain, 1954). In 1950, the American Academy of Pediatrics (AAP), which was founded in 1930, established its Accident Prevention Committee, chaired by George M. Wheatley, M.D. That committee surveyed the 3,000 members of AAP and found that 49 percent of reported “accidents” treated by AAP members involved poisoning (Wheatley, 1953).
In 1953, Edward Press, M.D., and Gdalman developed the first formal poison control center in Chicago. Their center provided professional telephone advice and included a standard data collection form (Botticelli and Pierpaoli, 1992; Burda and Burda, 1997). These centers rapidly developed, with as many as 265 by 1958 and 661 by 1978 (Scherz and Robertson, 1978).
Provision of timely information to physicians regarding drugs and the toxicity of other agents was the driving force for poison control center
|
BOX 4-1
|
operations. These early leaders also recognized that effective telephone triage could avert unneeded medical visits or lead to early treatment at home. Hence, centers soon began to provide advice directly to laypersons and nonphysician care providers. This feature distinguishes poison control centers in the United States from similar centers in other countries, where the task of giving advice remains largely restricted to physicians.
In 1957, the Surgeon General established the National Clearinghouse for Poison Control Centers (NCHPCC) within the FDA. At the time, the FDA and the U.S. Department of Agriculture represented the only federal agencies related to consumers with jurisdiction over drugs and chemicals. Product ingredient information was provided and poison exposures were tracked through NCHPCC. Funding was also provided to develop the text Clinical Toxicology of Commercial Products, authored by Robert Gosselin, M.D., Harold Hodge, M.D., and Marion Gleason at the University of Rochester.
At the 1958 AAP annual meeting, the American Association of Poison Control Centers (AAPCC) was founded (Mofenson, 1975) (see Box 4-2 for the AAPCC statement of objectives). AAPCC continues to serve as the voluntary association for poison control centers. As the lead professional organization regarding poison control and management, AAPCC—along with other toxicology groups—continues to host medical toxicology scientific presentations and continuing education sessions at its annual meeting in combination with several other societies. In 1968, both the American Academy of Clinical Toxicology and the American College of Emergency Physicians were founded. One impact of both organizations was to take the focus of poisoning beyond pediatric exposures. The American Board of Medical Toxicology gave its first examination for physician toxicologists in 1974 and fellowship training programs were instituted at about the same time. Emergency medicine was recognized as a specialty in the
|
BOX 4-2 To provide a forum for poison centers and interested individuals to promote the reduction of morbidity and mortality from poisonings through public and professional education and scientific research. To set voluntary standards for poison center operations. SOURCE: http://www.aapcc.org//aapcc.htm. |
United States in 1979 when it received conjoint status from the American Board of Medical Specialists (ABMS) and became a primary specialty 10 years later. Medical toxicology began administering examinations in 1974 and was recognized by the ABMS as a certificate of added qualification in 1994. Although AAP has remained active in the area of poisonings with its Section on Injury and Poison Prevention, founded in 1990, the organization no longer sponsors AAPCC meetings.
During the late 1970s, systems of emergency care were developed following passage of the Emergency Medical Services (EMS) Systems Act of 1973 (Pub. L. No. 93–154). The application of technology and centralized public service (communication) access points produced the opportunity for integration of poison control centers within EMS systems. Concurrent steps to enhance home safety (e.g., product labeling, smaller quantities of over-the-counter medications per package, prescription drug safety caps, childproof cabinet locks) coincided with a shift in awareness of mortality risk to include adult poisoning as a major emphasis of care. Given a growing emphasis on adult poisoning management and poisoning response with EMS services, an increasing number of leaders in medical toxicology, including the poison control center medical directors, began to come from a background in emergency medicine followed by a fellowship in clinical toxicology (see Chapter 5). Professional activities by medical toxicologists, pharmacists, and nurses also have grown dramatically in both the management and operation of poison control centers. Centers no longer use clerical personnel or sanitarians to manage exposures.
Certain aspects of poisoning prevention in the past 30 years have been independent of poison control center clinical functions. For example, the introduction by the FDA of imprint codes on tablets and capsules provided a much improved method of tablet and capsule identification. Although the system has major drawbacks (e.g., use of logos that are difficult to categorize, describe, and list), there has been faster determination of potential medication exposures (Marder et al., 2001; Symonds and Robertson, 1967).
Similarly, the use of safety caps on medications and chemical compounds has reduced the number of exposures in children and may have been the single most important reason for reduction in morbidity and mortality since the early 1970s (Anonymous, 1982; Arena, 1959; Palmisano, 1981; Rodgers, 1996; Walton, 1982). Although there have been various public campaigns to use other child safety devices, the only data that exist are related to safety caps. Child-resistant packaging was required for various prescription drugs beginning in 1974. Looking at the period from 1974 to 1992 and comparing it with a previous period, estimates show a reduction of 460 child deaths and a mortality rate reduction of 45 percent as a result of this packaging (Rodgers, 1996). Figure 4-1 from that study
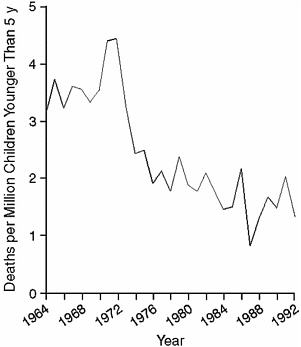
FIGURE 4-1 Child mortality rates due to the unintentional ingestion of oral prescription drugs, 1964–1992.
SOURCE: Rodgers (1996).
shows the drop in child mortality rates over the time period; Figure 4-2 shows the difference between the predicted rates with and without child-resistant packaging.
Events both before and after September 11, 2001, have heightened national concerns regarding homeland security and the threat of radiologic, biological, and chemical weapon exposure. The Health Resources and Services Administration (HRSA) and the Centers for Disease Control and Prevention (CDC) have recognized the importance of poison control centers as a component of an all-hazards emergency planning and response system that is integrated with state health departments and supports regional and hospital-based emergency service efforts. As discussed in Chapters 5 and 9, the incorporation of poison control centers in this manner has been variable and remains underdeveloped. This new potential role—combined with substantive changes in funding and federal oversight—clearly marks a break with the past and the beginning of a new period for poison control centers.
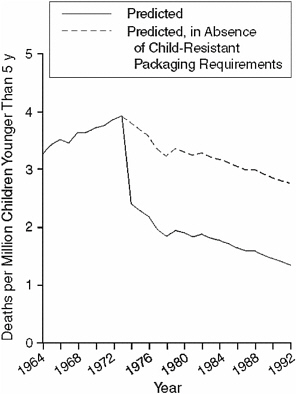
FIGURE 4-2 Fitted model for predicted child mortality rates due to unintentional ingestion of oral prescription drugs, 1964–1992.
SOURCE: Rodgers (1996).
POISON CONTROL CENTERS
Origins
Although the preceding overview provides a brief context for poison control centers, the detailed history of their development provides further insight into their current status and function.
Edward Press, with the support of the AAP Illinois Chapter, the Illinois Department of Public Health, seven hospitals, five Chicago medical schools, the American Medical Association, the FDA, and others, formed a committee on April 1, 1953, to begin development of the first poison control center (Botticelli and Pierpaoli, 1992; Burda and Burda, 1997). By 1954, 11 centers had been established in the city of Chicago alone, with the
objective of providing information to physicians for treatment of children exposed to toxic agents. During this time, visiting nurses from the board of health also visited the homes of poisoning victims in Chicago.
In 1961, the advisory committee of the poison control center in Chicago consolidated the 11 poison control centers into one information center at Presbyterian–St. Luke’s Hospital. In 1962, the Master Poison Control Center was established with Joseph R. Christian, M.D., as medical director, and Chicago pharmacist Gdalman as director responsible for operations. The advisory committee also resolved its concerns about professional liability exposure and agreed to allow direct calls to the poison control center from the public.
Embraced as a lifesaving idea by the pediatric community, the number of centers rapidly increased nationally from 1953 to 1958, when 265 poison control centers were reported to exist. This expansion occurred with no consistent funding or formal organizational structure (Arena, 1983). It was not until the 1970s that emergency medicine became a potent force creating professional demand for improved poison information. The result was the extension of standardized poison and drug information and consistent access to toxicologists. By that time, more than 660 poison control centers had developed (Scherz and Robertson, 1978). Recognizing the changing epidemiological trends, poison control centers began in the 1970s to expand their efforts beyond a primarily pediatric focus to serving the full population. However, the prevention education in centers has continued to emphasize pediatric poisoning prevention.
Evolution of Structure and Function
As noted previously, poison control centers were established to provide drug and chemical toxicity information and patient management guidance to physicians. These services were expanded to handle telephone calls from laypersons in the 1960s. Initially, most centers consisted simply of a telephone and a designated individual to answer that telephone. The individual responding to the calls at times was a clerical person, pediatric house officer (physician in training, pharmacist), or other interested (or designated) person. Neither training nor educational materials were standardized.
In 1957, the first efforts to standardize poison information were undertaken within the FDA by NCHPCC. These included (1) index cards containing information on drugs, chemicals, household products, and plants, and (2) a monthly newsletter summarizing the poisoning literature. NCHPCC also funded the publication of a book, The Clinical Toxicology of Commercial Products, with the first edition in 1957 (Food and Drug Administration).
In the 1970s, poison control centers began to offer clinical toxicology fellowship programs for physicians and other scientists. A medical subspecialty certificate of added qualification in medical toxicology became possible in 1994 for diplomates of the American Board of Emergency Medicine, the American Board of Pediatrics, or the American Board of Preventive Medicine through the American Board of Medical Specialties. Currently, candidates for this subspecialty certification must complete a residency in one of the sponsoring boards or other boards by petition and a medical toxicology fellowship affiliated with a poison control center.
Professional activities by pharmacists and nurses have grown dramatically in both the management and operation of poison control centers. Centers no longer use clerical personnel to manage exposures. Beginning in the early 1980s, AAPCC developed and promoted criteria enabling nonphysicians to become specialists in poison information. As an adjunct to their extensively trained personnel, nonphysician, pharmacist, and nursing personnel could serve as a supervised poison information provider.
Designations of poison control centers were made by each state health department, peaking in 1978 with 649 sites in the 50 states and 12 more in U.S. territories and the Virgin Islands (Scherz and Robertson, 1978) (Table 4-1). However, there were few large poison control centers and the number receiving more than 1,000 calls per year never exceeded 80. In 1970, less than 6 percent of poison control centers received more than 9 to 10 calls per day, or 3,285 to 3,650 per year (Manoguerra, 1976).
In the absence of a federal certifying body or federal poison control center regulations for staffing and operations, AAPCC developed certification systems for both the centers and their personnel (Lovejoy et al., 1994). As a result of an increasing expectation for center certification, tenuous financial support, and economically driven service cutbacks by hospitals and teaching institutions, the number of centers dropped rapidly over three decades.
In 1983, the number of centers had dropped to 395 and in 1994 to 87. By 2002, there were 64 centers reporting to the AAPCC Toxic Exposure Surveillance System (TESS) data collection system, with coverage of 99.8 percent of the U.S. population, a dramatic increase from the 52 percent of the population covered in 1993 (Lovejoy et al., 1994; Watson et al., 2003). Following introduction of the national toll-free number, 100 percent of the U.S. population is currently covered by 64 centers. The surviving poison control centers developed from the consolidation and expansion of the early centers (Rumack et al., 1978). Such centralization of information and treatment was shown early to reduce poisoning mortality; for example, in one hospital it declined from 8 to 4 percent (Teitelbaum, 1968). Reorganization of poison control centers largely has been driven by local economic mandates rather than by public health initiatives. Currently, several cen-
TABLE 4-1 Poison Information Centers: States, Territories, and Virgin Islands, 1978
|
Alabama |
8 |
New Jersey |
34 |
|
Alaska |
5 |
New Mexico |
6 |
|
Arizona |
17 |
New York |
21 |
|
Arkansas |
8 |
North Carolina |
8 |
|
California |
9 |
North Dakota |
7 |
|
Colorado |
9 |
Ohio |
13 |
|
Connecticut |
10 |
Oklahoma |
8 |
|
Delaware |
1 |
Oregon |
1 |
|
Florida |
32 |
Pennsylvania |
74 |
|
Georgia |
11 |
Rhode Island |
4 |
|
Hawaii |
1 |
South Carolina |
2 |
|
Idaho |
3 |
South Dakota |
2 |
|
Illinois |
102 |
Tennessee |
8 |
|
Indiana |
33 |
Texas |
21 |
|
Iowa |
4 |
Utah |
1 |
|
Kansas |
14 |
Vermont |
0 |
|
Kentucky |
10 |
Virginia |
19 |
|
Louisiana |
5 |
Washington |
11 |
|
Maine |
1 |
West Virginia |
16 |
|
Maryland |
6 |
Wisconsin |
5 |
|
Massachusetts |
6 |
Wyoming |
2 |
|
Michigan |
28 |
Canal Zone |
1 |
|
Minnesota |
27 |
District of Columbia |
1 |
|
Mississippi |
13 |
Guam |
1 |
|
Missouri |
15 |
Puerto Rico |
5 |
|
Montana |
3 |
Virgin Islands |
4 |
|
Nebraska |
2 |
Total 50 States and the |
|
|
Nevada |
2 |
District of Columbia = 650 |
|
|
New Hampshire |
1 |
Total = 661 |
|
|
SOURCE: Adapted from Scherz and Robertson (1978). |
|||
ters supply parts or all of the service needs of other states. Some centers serve areas at a great distance (e.g., the Oregon Poison Center serves Alaska and the Rocky Mountain Poison and Drug Center in Colorado serves Hawaii). States that do not have onsite centers provide services such as education and outreach through community organizations and state public health agencies.
Although concern has been expressed that states without poison control centers would be less well served than states with centers, there is no evidence to show decreased call rates in states without centers physically present, such as Alaska, Hawaii, Wyoming, Montana, North Dakota, and Idaho.
Evolution of Data Collection and Poisoning Surveillance
Collection of data by early poison control centers was fragmented and generally nonstandardized. In 1957, NCHPCC collected limited data from centers and published a yearly statistical report for the aggregate of reporting centers approximately 24 months after the end of the data collection year.
AAPCC developed the Toxic Exposure Surveillance System, a data collection system, in 1983. TESS is the data source for AAPCC annual reports (Watson et al., 2003). TESS was developed in part to supply marketable, comprehensive data for pharmaceutical companies and federal agencies (e.g., Consumer Product Safety Commission, or CPSC). TESS also provides poison control centers with a standardized poisoning exposure record. This system initially used “mark sense” forms (to be described later in this chapter), but has been advanced to digital format. Annual TESS summary data for reporting poison control centers are published annually in the American Journal of Emergency Medicine (Watson et al., 2003).
During the past several years, changes to TESS have been funded by CDC and its Agency for Toxic Substances and Disease Registry. Funding in excess of $6 million has largely supported enhancement of the proprietary software underlying TESS and has not subsidized data collection by poison control centers.
TESS provides useful exposure data, but captures only a fraction of the most seriously poisoned patients (Blanc et al., 1995; Hoppe-Roberts et al., 2000). From a historical perspective, a key shortcoming of TESS is that it did not develop as a public access database comparable to governmental sources of other vital statistics.
The TESS data collection program is proprietary to AAPCC and thus is not managed by any public health or government agency. Consistent with this fact, the underlying software for both data collection and analysis was developed and remains owned by a private company with ties to AAPCC. As new data fields have been added to TESS over time, individual poison control centers must provide the additional time and personnel required to acquire the data without full compensating revenue. However, approximately $250,000 from the sale of data is returned to individual centers annually based on the number of cases submitted as partial compensation. This compensation from the AAPCC central office ranges from $3,000 to $7,000 per center each year. Individual centers reporting to the Committee estimated the net cost of providing such data (i.e., beyond that compensated) ranges from $50,000 to $100,000 per year. Federal agencies such as CPSC and the FDA, as well as most state agencies, also purchase TESS data reports.
Technology
Poison Information
Drug information and poisoning management cards used by the early poison control centers were replaced by microfiche as the required database grew. The first major commercial product for this purpose was the Poisindex® (founded in 1973 by Barry H. Rumack). Although there were several other databases such as ToxiFile from Illinois and a compilation of NCHPCC cards from Detroit, they did not publish for more than a few years. Poisindex was published on microfiche in August 1974 and contained a compilation of consumer and commercial products coded to treatment algorithms (Rumack, 1975). These “managements” were written by an editorial board and covered care of exposed patients at home or in a health care facility. Poisindex was provided electronically for mainframe computers beginning in 1981. In 1985, Poisindex was published on CD-ROM for the first time and coupled with a personal computer. Although CD-ROM continues to be its major method of distribution, the software is also available through Internet subscription and over private intranets. Poisindex is used by all U.S. poison control centers and the majority of centers around the world. Validity of the Poisindex database has been independently verified (Wan et al., 1993). Data contained within Poisindex is provided voluntarily by consumer product, industrial, and other manufacturers and repackagers. A company called Micromedex employs an internal staff to obtain and code products, as well as to prepare the management documents for review by an outside editorial board.
Data Acquisition
Although NCHPCC summarized data from many poison control centers to provide estimates of poisoning in the United States, the process suffered from limited standardization of data collection and definition and from its voluntary submission nature. AAPCC began centralized collection of poison exposures with TESS in 1983 using “mark sense” paper forms that were scanned and converted to digital data. Current data collection occurs using a computerized program that allows data capture during a poison call. The embedded product codes in Poisindex were used by AAPCC to enable connection of each case with an appropriate product or products. Currently, four computer-based data collection products interface with TESS and are used for data collection (see Chapter 8).
Drug Recognition
One useful computerized drug identification database is Drugdex. It interfaces with Poisindex and is published by Micromedex (http://www.micromedex.com) as part of a suite of databases used by many poison control centers. Electronic matching between the computerized case reports filled out by center specialists and the product and ingredient indexes of Poisindex is instantaneous. This drug identification software works seamlessly with the four electronic data collection programs currently used by centers for uploading data to AAPCC as part of TESS.
Communications
The enhancement of satellite, microwave, and cable communications systems has improved the ability of laypersons and professionals to contact poison control centers. This development of a sophisticated communications network has allowed specialists in one center or region to provide backup support for another center or region. A nationwide telephone number, 1-800-222-1222, was introduced in 2002 and allows triage of poison control center exposure or information calls to a center anywhere in the United States. Before this telephone number was introduced, poison control centers used a combination of local telephone numbers and state or regionwide toll-free numbers.
Funding of Poison Control Centers
Initially, funding for work performed by the poison control centers was borne by the host institution and by the pediatricians and pharmacists who were involved. There is no indication that any of the indirect federal funding through Medicare supplements to teaching hospitals was used in centers. The sponsoring institution sometimes provided consulting personnel from the laboratory and other areas of the hospital without direct charge. There was no federal funding beyond the money used within NCHPCC to generate epidemiological poisoning summaries, treatment cards, and the book, The Clinical Toxicology of Commercial Products (Food and Drug Administration, 1957). It has been long held that poison control centers save money for the health care system by avoiding the need for emergency department visits as well as permitting treatment at home (Food and Drug Administration, 1973; Harrison et al., 1995, 1996; Krenzelok, 1998; Morton, 1998; Olson et al., 1999; Woolf et al., 1997; Zuvekas et al., 1997).
Poison control centers have not been included as specific requirements in the key public health block grants to state health departments
that were established in the early 1980s, so there has been no national strategic or tactical plan for their development or linkage with public health departments. This has resulted in center function variation over time, with each center evolving its own culture within its own institutional and community structure. The variability between the current poison control centers in terms of both geographic location and institutional support is largely a product of each center’s funding history and functional role for its sponsoring institution. For example, those centers located in a children’s hospital often have served as part of the institution’s community outreach program, while those located in a school of pharmacy often have served primarily as part of the school’s education and training environment. In both examples, the institutions have been willing to absorb some costs, although in one instance the poison control center provides community goodwill and raises the recognition of the institution, whereas in the other the center primarily enriches a training program.
One poison control center director wrote in a pharmacy journal in 1976: “The development of poison control centers over the past 20 years has been haphazard. Each state was allowed to organize its own system resulting in very little uniformity of services and quality control” (Manoguerra, 1976:p. 382). Historically and recently many mandates, such as an expectation to participate in emergency planning and response, have been without accompanying financial support. Until the early 1970s, most medical directors received salaries from other sources and not from the poison control center. Sources of center funding included hospitals (primarily children’s hospitals for community outreach), states, counties, cities, health insurance firms, universities, schools of pharmacy, public health agencies, and contracts with pharmaceutical firms, chemical companies, and others (see Chapter 5). Poison control centers have rarely been considered central to a community’s health care, and many have closed as institutions have faced financial difficulties. Hospital and other institutional administrators have not seen the centers as a revenue source, but instead as public relations, education, or training cost centers. Only a handful of the centers have developed affiliated clinics or inpatient services that create direct hospital revenue. Many of the centers that have closed did not meet the needs of their community. Regionalization resulted in consolidation and closure of some centers, producing a more focused use of resources. As Table 4-1 indicates, some states had a large number of centers, and their closure was more likely to have benefited the system than hurt it. There has not been a clear understanding about how many centers should exist. When the first centers were designated by various state health departments starting in 1958, there was no concept of regionalization comparable to what exists today.
FDA Report on Poison Control Management
A 1973 FDA report (Food and Drug Administration, 1973) evaluated a variety of central and regional models for providing poison control services (see Box 4-3). These proposals were intended to bring about economies of scope and scale. One proposal suggested that a single national poison control center “with a single national center utilizing inward Wide Area Telephone Service (inward WATS) to answer calls on a nationwide basis” (p. 77) be established to replace the existing collection of centers (at that time there were 597). It was estimated that “45 persons would be required for a presumed call volume of 320,000 using 13 phone lines, but if the call volume doubled, it would require 22 phone lines and 78 people. The cost of 13 lines was estimated at $296,000 per year and at an average cost of $15,000 it was estimated that 45 personnel would cost $675,000 per year.” It was suggested that expert staff were needed to “back-up para-medicals answering phones” and that they would be “pharmacists or physicians” (p. 88). The report noted possible drawbacks to establishing a national center, including (1) persuading the public to call a toll-free number, (2) following up with patients at long distances, and (3) the required increases in federal funding.
Several other recommendations in the report (see Box 4-3) were instituted over time by poison control centers, although the FDA, which commissioned the report, ceased to be involved in center activities in 1987.
Other key findings contained in the 1973 FDA report characterized the poisoning rates of the time:
-
The mortality rate from accidental poisoning combined across all ages was increasing 4.4 percent annually, while the mortality rate from accidental poisoning among children under age 5 was decreasing 4 percent annually. (Although exposures in children under the age of 5 are still considered “accidental” exposures, in older children and adults they are considered either “unintentional” or “intentional.”)
-
The average number of poisoning incidents reported to NCHPCC per poison control center reporting between 1965 and 1971 increased for all ages combined, as well as for each age group separately, except for children under 5 years of age. For the latter group, the number of incidents decreased.
In addition, other events were believed to have a favorable impact on the incidence of poisoning at that time:
-
Enactment of and initial regulations created under the Poison Prevention Packaging Act (the Act was being administered by the FDA at that time prior to the formation of CPSC).
|
BOX 4-3
|
NOTE: The report addresses the federal system and then individual centers. SOURCE: Food and Drug Administration (1973, pp. 107–131). |
-
Enactment and initial regulations created under the Hazardous Substances Labeling Act.
-
Establishment of Poison Prevention Week.
-
Legislation passed in 1967 limiting the number of aspirin tablets in baby aspirin bottles.
-
Growth in the number of poison control centers and to a lesser extent the establishment of NCHPCC and publication of The Clinical Toxicology of Commercial Products (released during the period 1957–1959); periodically updated.
The report estimated that in 1972 consumers saved $4 million and avoided 400,000 emergency department visits by receiving free treatment information by telephone from poison control centers.
PUBLIC OVERSIGHT
FDA
In 1979, the FDA’s NCHPCC reached its zenith with 45 staff members. The center provided support services but no direct funding to poison control centers. Participation in NCHPCC statistical reports was a voluntary center activity. The development of a taxonomy of poisoning by NCHPCC for standardized reporting was incomplete and no standardization of center organization, service delivery, or clinical outcomes assessment was achieved. As NCHPCC’s emphasis evolved into a monthly compilation of toxicology literature, the FDA became less committed to management of NCHPCC and finally ceased to work in this area in 1987.
HRSA and Other Organizations
Poison control centers do not operate within any single federal mandate having regulatory or reporting authority. Furthermore, there is no requirement that emergency departments, critical care units, or any other care facilities or providers contact or report poisoning cases to any center. Although some states, such as New York, have requirements for reporting of poisoning cases as well as food poisoning cases, the discrepancies among various databases suggest that capture of all cases does not occur. This may be due to definitional issues addressed in one of the Committee’s recommendations in Chapter 10. The center’s role is generally unclear to most health care providers; a center is generally regarded as a place that the public and professionals can call about poison exposures.
Since 2000, more than $60 million have been infused into the AAPCC and poison control centers through the Poison Control Center Enhancement and Awareness Act (Pub. L. No. 106–174), which was enacted in 2000 and reauthorized in 2003 (see Appendix 4-A for the Reauthorization). HRSA and CDC were given responsibility by the Secretary of Health and Human Services for implementing the Act. HRSA administers stabilization and incentive grants to the poison control centers. These grants are reviewed each year for goals, progress, and financial accounting. Interestingly, the related HRSA grant guidelines have discouraged the use of such funds to support delivery of existing services (i.e., to provide fiscal stabilization); instead, they have been earmarked for “enhancement of services.” Thus, the Act has done little to stabilize poison control centers in dire financial need of support for basic service delivery.
CDC administers funds to the AAPCC under the Act for upgrading TESS, developing a national poison control telephone number, and developing new nationwide media campaigns. AAPCC has received up to
$5 million per year to support projects such as revisions of a proprietary data collection instrument and development of a real-time surveillance tool that collects data from poison control centers. To date, no real-time data feedback are provided by AAPCC to the centers nor do the centers have real-time access to the electronic database; rather, they are notified by telephone about suspicious activities identified through this real-time tool. Furthermore, centers have only limited access to their own electronic and national data upon request. AAPCC also receives federal income from the Environmental Protection Agency and CPSC for access to TESS surveillance data.
PUBLIC HEALTH LINKAGES
As noted previously, public health agencies, for the most part, had little involvement with poison control centers until 2001, when bioterrorism and related activities created interest in poison control center activities. Few public health leaders were involved during the formative years of poison control centers. This may have been related in part to NCHPCC being located within a regulatory agency. A few centers have developed relationships with public health agencies, but only rarely have they received significant funding or other support. Furthermore, a few states have provided Maternal and Child Health Bureau funding to poison control centers (see Chapter 9).
An Institute of Medicine report on the role of the public and private sectors in injury prevention mentions poisoning only briefly, despite listing it as the third leading cause of injury-related death. Furthermore, the report does not include AAPCC data collection and TESS in its list of more than 30 databases (Bonnie et al., 1999).
Some public health agencies, particularly at the state level, have maintained close ties with their state’s poison control centers, but lack of funding has limited such participation. Public health authorities have indicated interest in drugs of abuse and other issues under their purview but, for example, have rarely been interested in unintentional drug ingestion in the home. The lack of collaboration with public health agencies also may be related to the observation that poison control centers have owned their data and most have required compensation from other agencies to share those data.
State activities in poison control centers have been quite variable. New York had a particularly focused development in this area in coordination with the larger picture of injury control (Fisher, 1986; Fisher et al., 1986). A review of poison control statutes that existed by the early 1980s was conducted and some early strategic planning was suggested at about that time (Fisher, 1981; Russell and Czajka, 1984).
Integration of poison control centers and drug information centers was addressed at a number of sites in the late 1970s and early 1980s as pharmacists became more involved with their development (Czajka et al., 1979; Troutman and Wanke, 1983; Wanke et al., 1988).
SUMMARY
The following key messages can be drawn from the discussion provided in this chapter:
-
Poison control centers and the work of dedicated poison specialists have had a significant impact on U.S. health care. Key achievements include:
-
Development and implementation of medication safety caps.
-
Establishment of limits on the number of children’s aspirin tablets and subsequently other over-the-counter medications in a bottle.
-
Development of imprint code regulations to help speed identification of medications.
-
Use of TESS data to encourage passage of federal regulations in 1997 to reduce the number of iron tablets in a container.
-
Demonstration that nearly 80 percent of human exposures can be managed in the home using poison control center personnel guidance, thus reducing the burden on the health care system and providing reassurance to parents (Watson et al., 2003).
-
Demonstration of the ability to provide an immediate response to public health exposure concerns, such as anthrax, and subsequent participation in bioterrorism responses.
-
-
The current structure of poison control centers is quite variable and developed as a result of historical factors that may be irrelevant to current functional needs.
-
Poison prevention efforts have historically focused on children, despite more recent recognition of greater risk for morbidity and mortality in adults.
-
More emphasis has been placed on treating patients with drug abuse and alcohol problems as the role of poison control centers has broadened to include adults. Although medical toxicologists see such patients regularly as part of their management of critically ill patients, further integration of these aspects into poison control center services is warranted as part of the spectrum of poisoning treatment.
-
Attention to the special problems of the elderly, along with the important contributions of pharmacists in reducing adverse reactions in this population, deserves attention as an aspect of development of poison control centers.
-
Funding for poison control centers is piecemeal, and centers often receive unfunded mandates for data provision and other services. A current example is the expectation of active participation in regional emergency planning and response and the provision of additional data for all-hazards emergency preparedness and response surveillance without dedicated resources. Furthermore, federal grants earmarked for poison control center enhancement have done little to stabilize centers in need of financial support for basic service delivery.
-
There is considerable opportunity for coordination and cooperation between poison control centers and public health agencies at federal, state, and county levels. However, without federal or state points of accountability, many poison control center oversight roles have been assumed by the American Association of Poison Control Centers. These factors have led to a lack of integration of center data with the public health system.
117 STAT. 2888 PUBLIC LAW 108–194—DEC. 19, 2003
Public Law 108–194
108th Congress
|
|
An Act |
|
Dec. 19, 2003 / [S. 686] |
To provide assistance for poison prevention and to stabilize the funding of regional poison control centers. |
|
Poison Control Center Enhancement and Awareness Act Amendments of 2003. 42 USC 201 note. 42 USC 300d–71 note. |
Be it enacted by the Senate and House of Representatives of the United States of America in Congress assembled, SECTION 1. SHORT TITLE. This Act may be cited as the “Poison Control Center Enhancement and Awareness Act Amendments of 2003”. SEC. 2. FINDINGS. The Congress finds the following: |
|
|
(1) Poison control centers are our Nation’s primary defense against injury and deaths from poisoning. Twenty-four hours a day, the general public as well as health care practitioners contact their local poison centers for help in diagnosing and treating victims of poisoning and other toxic exposures. (2) Poisoning is the third most common form of unintentional death in the United States. In any given year, there will be between 2,000,000 and 4,000,000 poison exposures. More than 50 percent of these exposures will involve children under the age of 6 who are exposed to toxic substances in their home. Poisoning accounts for 285,000 hospitalizations, 1,200,000 days of acute hospital care, and 13,000 fatalities annually. (3) Stabilizing the funding structure and increasing accessibility to poison control centers will promote the utilization of poison control centers, and reduce the inappropriate use of emergency medical services and other more costly health care services. (4) The tragic events of September 11, 2001, and the anthrax cases of October 2001, have dramatically changed our Nation. During this time period, poison centers in many areas of the country were answering thousands of additional calls from concerned residents. Many poison centers were relied upon as a source for accurate medical information about the disease and the complications resulting from prophylactic antibiotic therapy. (5) The 2001 Presidential Task Force on Citizen Preparedness in the War on Terrorism recommended that the Poison Control Centers be used as a source of public information and public education regarding potential biological, chemical, and nuclear domestic terrorism. (6) The increased demand placed upon poison centers to provide emergency information in the event of a terrorist event |
|
involving a biological, chemical, or nuclear toxin will dramatically increase call volume. |
|
|
SEC. 3. AMENDMENT TO PUBLIC HEALTH SERVICE ACT. |
|
|
Title XII of the Public Health Service Act (42 U.S.C. 300d et seq.) is amended by adding at the end the following: |
|
|
“PART G—POISON CONTROL |
|
|
“SEC. 1271. MAINTENANCE OF A NATIONAL TOLL-FREE NUMBER. |
42 USC 300d–71. |
|
“(a) IN GENERAL.—The Secretary shall provide coordination and assistance to regional poison control centers for the establishment of a nationwide toll-free phone number to be used to access such centers. “(b) RULE OF CONSTRUCTION.—Nothing in this section shall be construed as prohibiting the establishment or continued operation of any privately funded nationwide toll-free phone number used to provide advice and other assistance for poisonings or accidental exposures. “(c) AUTHORIZATION OF APPROPRIATIONS.—There is authorized to be appropriated to carry out this section $2,000,000 for each of the fiscal years 2000 through 2009. Funds appropriated under this subsection shall not be used to fund any toll-free phone number described in subsection (b). |
|
|
“SEC. 1272. NATIONWIDE MEDIA CAMPAIGN TO PROMOTE POISON CONTROL CENTER UTILIZATION. |
42 USC 300d–72. |
|
“(a) IN GENERAL.—The Secretary shall establish a national media campaign to educate the public and health care providers about poison prevention and the availability of poison control resources in local communities and to conduct advertising campaigns concerning the nationwide toll-free number established under section 1271. “(b) CONTRACT WITH ENTITY.—The Secretary may carry out subsection (a) by entering into contracts with one or more nationally recognized media firms for the development and distribution of monthly television, radio, and newspaper public service announcements. “(c) EVALUATION.—The Secretary shall— “(1) establish baseline measures and benchmarks to quantitatively evaluate the impact of the nationwide media campaign established under this section; and “(2) prepare and submit to the appropriate congressional committees an evaluation of the nationwide media campaign on an annual basis. “(d) AUTHORIZATION OF APPROPRIATIONS.—There are authorized to be appropriated to carry out this section $600,000 for each of fiscal years 2000 through 2005 and such sums as may be necessary for each of fiscal years 2006 through 2009. |
|
|
“SEC. 1273. MAINTENANCE OF THE POISON CONTROL CENTER GRANT PROGRAM. |
42 USC 300d–73. |
|
“(a) REGIONAL POISON CONTROL CENTERS.—The Secretary shall award grants to certified regional poison control centers for the purposes of achieving the financial stability of such centers, and for preventing and providing treatment recommendations for poisonings. |
|
|
|
“(b) OTHER IMPROVEMENTS.—The Secretary shall also use amounts received under this section to— “(1) develop standardized poison prevention and poison control promotion programs; “(2) develop standard patient management guidelines for commonly encountered toxic exposures; “(3) improve and expand the poison control data collection systems, including, at the Secretary’s discretion, by assisting the poison control centers to improve data collection activities; “(4) improve national toxic exposure surveillance by enhancing activities at the Centers for Disease Control and Prevention and the Agency for Toxic Substances and Disease Registry; “(5) expand the toxicologic expertise within poison control centers; and “(6) improve the capacity of poison control centers to answer high volumes of calls during times of national crisis. “(c) CERTIFICATION.—Except as provided in subsection (d), the Secretary may make a grant to a center under subsection (a) only if— “(1) the center has been certified by a professional organization in the field of poison control, and the Secretary has approved the organization as having in effect standards for certification that reasonably provide for the protection of the public health with respect to poisoning; or “(2) the center has been certified by a State government, and the Secretary has approved the State government as having in effect standards for certification that reasonably provide for the protection of the public health with respect to poisoning. “(d) WAIVER OF CERTIFICATION REQUIREMENTS.— “(1) IN GENERAL.—The Secretary may grant a waiver of the certification requirement of subsection (c) with respect to a noncertified poison control center or a newly established center that applies for a grant under this section if such center can reasonably demonstrate that the center will obtain such a certification within a reasonable period of time as determined appropriate by the Secretary. “(2) RENEWAL.—The Secretary may renew a waiver under paragraph (1). |
|
Effective date. |
“(3) LIMITATION.—In no instance may the sum of the number of years for a waiver under paragraph (1) and a renewal under paragraph (2) exceed 5 years. The preceding sentence shall take effect as if enacted on February 25, 2000. “(e) SUPPLEMENT NOT SUPPLANT.—Amounts made available to a poison control center under this section shall be used to supplement and not supplant other Federal, State, or local funds provided for such center. “(f) MAINTENANCE OF EFFORT.—A poison control center, in utilizing the proceeds of a grant under this section, shall maintain the expenditures of the center for activities of the center at a level that is not less than the level of such expenditures maintained by the center for the fiscal year preceding the fiscal year for which the grant is received. “(g) MATCHING REQUIREMENT.—The Secretary may impose a matching requirement with respect to amounts provided under a grant under this section if the Secretary determines appropriate. |
|
“(h) AUTHORIZATION OF APPROPRIATIONS.—There are authorized to be appropriated to carry out this section $25,000,000 for each of the fiscal years 2000 through 2004 and $27,500,000 for each of fiscal years 2005 through 2009. |
|
|
“SEC. 1274. RULE OF CONSTRUCTION. |
42 USC 300d–74. |
|
“Nothing in this part may be construed to ease any restriction in Federal law applicable to the amount or percentage of funds appropriated to carry out this part that may be used to prepare or submit a report.’’. |
|
|
SEC. 4. CONFORMING AMENDMENT. |
42 USC 14801 note. |
|
The Poison Control Center Enhancement and Awareness Act (42 U.S.C. 14801 et seq.) is hereby repealed. |
|
|
Approved December 19, 2003. |
|


























