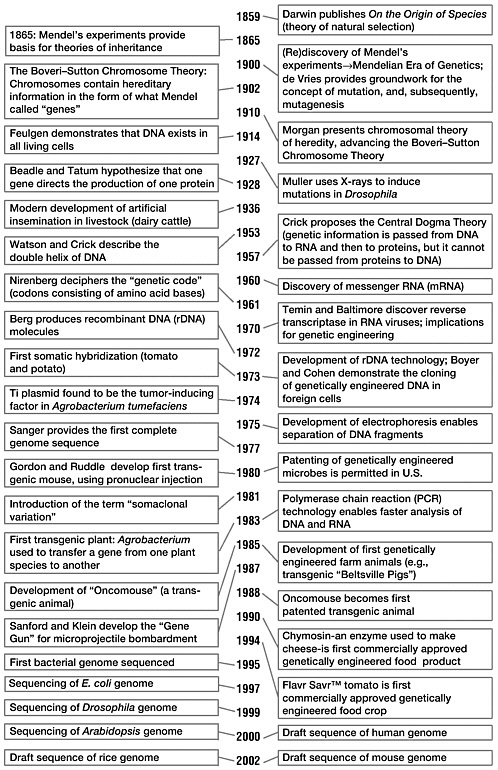
1
Introduction
HISTORICAL BACKGROUND
New techniques, collectively referred to as biotechnology, have been developed to improve the shelf life, nutritional content, flavor, color, and texture of foods, as well as their agronomic and processing characteristics. One specific biotechnology method is genetic engineering, a type of genetic modification that is the basis for many recent advances in breeding technology (see Appendix A: Glossary, for more comprehensive definitions of key terms used throughout this report). Like any new technology, genetic engineering carries with it some level of uncertainty and requires ways to predict and assess potential unintended effects, whether adverse or beneficial.
Throughout history humans have bred plants, animals, and microbes to achieve traits desirable for different uses. This was done mainly through simple selection and crossbreeding based on the most desired qualities, such as plant vigor, appearance, and taste. It was not until the mid-1800s that scientific understanding of trait inheritance began to emerge. During the 1860s, through his experiments that hybridized different varieties of peas, Gregor Mendel demonstrated the process of heredity (Mendel, 1866). His revolutionary experiments paved the way for modern agriculture by showing that, through controlled pollination crosses, genetic characteristics are inherited in a logical and predictable manner. Since that time many plants have been bred to include desirable traits, such as pest and disease resistance and the ability to overcome environmental stresses. Major gains in crop yields have been attributed partially to advances in these classical plant breeding techniques. Undoubtedly, conventional breeding will continue to play an essential role in improving agricultural crops, domestic animals, and microorganisms used in food production.
GENETIC MODIFICATION OF FOOD
Operational Definitions
The terminology used to describe various methods of genetic modification can have different meanings to different readers and can be interpreted in many ways. For the purposes of this report, the committee agreed upon a set of operational definitions for specific terms used to describe methods of genetic modification.
Although in popular parlance the term genetically modified (GM) often is used interchangeably with genetically engineered (GE) and biotechnology, in this report genetic modification refers to a range of methods used to alter the genetic composition of a plant or animal, including traditional hybridization and breeding. Genetic engineering is one type of genetic modification that involves making an intentional targeted change in a plant or animal gene sequence to effect a specific result (see Figure 1-1) through the use of recombinant deoxyribonucleic acid (rDNA) technology. Biotechnology refers to methods (including genetic engineering) other than conventional breeding used to produce new plants, animals, and microbes. Conventional breeding is used to describe traditional methods of breeding, or crossing, plants, animals, or microbes with certain desired characteristics for the purpose of generating offspring that express those characteristics.
Overview of Methods to Genetically Modify Plants and Animals
As exemplified by Mendel’s research, conventional breeding by crossing has been conducted for centuries to produce genetic modifications in crop plants and farm animals. Even his early experiments, while relatively simple from today’s perspective, yielded unexpected results (Mendel, 1866). The concept of dominant inheritance stems from Mendel’s unexpected finding that in a cross of white- and red-flowered plants in which the parents were homozygous, the first generation was uniform (F1) but none of the offspring showed an intermediate color, and the second generation (F2) produced three times more red- than white-flowered offspring. This result helped illustrate the distinction between phenotype (physical characteristics) and genotype (genetic pattern).
Plants and animals can be genetically modified in a variety of ways, each requiring some level of human intervention. Traditional methods include selection and crossbreeding, while more contemporary techniques include embryo rescue, cell fusion, somaclonal variation, mutation breeding, and cell selection. Genetic engineering, or rDNA modification, is achieved through different techniques leading to specifically designated genetic changes. There also are methods of genetic manipulation, different from rDNA technology, that use viral vectors to introduce foreign DNA into host cells.
All methods of genetic modification hold the potential, either intentionally or unintentionally, to alter levels of primary metabolites (such as proteins, lipids,
and carbohydrates) and a wide variety of secondary metabolites with either beneficial or adverse results. For example, introducing new proteins or increasing the levels of endogenous proteins in a food product may increase its potential for allergenicity, but genetic engineering may also reduce the allergenicity of a plant used for food or reduce its levels of known toxins.
Given the diverse assortment of techniques used to genetically modify plants and animals, it is clear that unintended adverse health effects potentially associated with these techniques do not fit a simple dichotomy of comparing genetic engineering with traditional breeding.
An important step for determining the likelihood of such unintended adverse health effects is assessing the compositional similarity between a conventional plant or animal and its genetically modified counterpart. Attributes of genetically engineered organisms (GEOs) typically compared with those of their traditionally bred counterparts include gene sources, phenotypic characteristics (such as size, shape, and color), composition (such as nutrients, antinutrients, allergens), and consumption patterns. Additional safety studies may be conducted, focusing on areas of greatest potential concern.
Comparing a GE plant or animal with its conventional counterpart alone is not sufficient for assessing the likelihood of unintended effects of genetic engineering and conventional breeding practices. It also is necessary to determine the frequency and nature of the associated unintended effects and to evaluate the methods that are potentially useful in assessing the safety of food products that result from use of these methods.
The Scope of This Report
While using biotechnology or conventional breeding techniques to enhance specific characteristics or increase the yield of food introduces the possibility of unintended deleterious effects on both human health and the environment, the focus of this report is health—including an examination of whether the likelihood of unintended adverse health effects from compositional changes is greater for foods that are genetically engineered than for those genetically modified using other methods (such as conventionally bred plants). Furthermore, this report evaluates currently used and newly developed methods for detecting unintended changes in genetically modified foods and also assesses and recommends techniques for predicting their potential health effects. However, it does not directly evaluate the potential health effects of specific engineered genes or proteins, nor does it assess the regulation of GE food.
THE CHARGE TO THE COMMITTEE
Three federal government agencies—the U.S. Department of Agriculture, the U.S. Department of Health and Human Services’ Food and Drug Administra-
tion, and the U.S. Environmental Protection Agency—asked the National Academies to convene a committee that would outline science-based approaches to assess or predict the unintended health effects of GE foods to aid in evaluating these products before they are sold to the public. The committee was charged with identifying appropriate scientific questions and methods for determining unintended changes in the levels of nutrients, toxins, toxicants, allergens, or other compounds in food from GEOs and outlining methods to assess the potential short- and long-term human health consequences of such changes.
The agencies also asked the committee to compare GE food with food derived from other genetic modification methods, such as crossbreeding, with respect to the frequency of compositional changes and the frequency and severity of the effects of these changes on consumer health. Finally, the committee was asked to discuss whether certain safety issues are specific to GE food and, if so, to recommend approaches for addressing these issues.
The committee’s charge did not include evaluating or making recommendations about policy issues, such as labeling GE foods, segregating foods in commerce, or preventing cross-contamination of foods.
Approach to the Task
The committee approached its task by gathering information from existing literature and from public workshop presentations by recognized experts (see Appendix B for the workshop agendas) and then deliberating on issues relevant to their charge.
From these discussions, the committee developed a theoretical framework for identifying appropriate comparators for GE and other GM foods, increasing scientific understanding of the determinants of compositional variability among foods, increasing understanding of the biological effects of secondary metabolites in food, developing more sensitive techniques for assessing potential unintended effects from food modification, and improving methods for tracking and tracing exposure in genetically modified food.
The committee’s deliberations about identifying appropriate comparators for GE food clarified that while such comparisons are necessary, they alone are not sufficient for determining the likelihood of producing an unintended adverse health effect. Consequently, this report focuses on an array of complementary science-based approaches for predicting and assessing unintended health effects of GE food and for evaluating the mechanisms by which unintended effects occur as a result of genetic modification.
Organization of the Report
This report is organized into seven chapters and an accompanying subreport on animal genetic manipulation and cloning. Chapter 2 describes the molecular
biological and biochemical methods of genetic manipulation of plants, animals, and microorganisms. Chapter 3 discusses the potential for unintended compositional changes from different methods of breeding. Chapter 4 outlines new approaches for identifying unintended changes in food composition. Chapter 5 details diverse ways that adverse health effects can occur from food, while Chapter 6 suggests methods for predicting and assessing those effects that result from intended and unintended compositional changes resulting from genetic modification. Chapter 7 presents the committee’s conclusions and recommendations. The subreport on animal genetic manipulation and cloning reviews the current literature and makes recommendations for methodologies that could be used to assess cloned animal products.
REFERENCES
The Arabidopsis Initiative. 2000. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408:796–815.
Mendel G. 1866. Experiments in plant-hybridization. Verh Naturforsch Ver Brunn Abh 4. Translated by Bateson W, reprinted in Peters J. 1959. Classic Papers in Genetics. Englewood Cliffs, NJ: Prentice-Hall. Pp. 2–20.
Moore G. 2003. Timeline of Plant Tissue Culture and Selected Molecular Biology Events. Online. University of Florida Institute for Food and Agricultural Sciences. Available at http://www.hos.ufl.edu/mooreweb/TissueCulture/class2/Timeline%20of%20Plant%20Tissue%20Culture%20and%20Selected%20Molecular%20Biology%20Events.doc. Accessed August 29, 2003.
University of Illinois. 1999. The Economics and Politics of GMOs in Agriculture. Online. College of Agricultural, Consumer, and Environmental Science, Bulletin 809. Available at http://web.aces.uiuc.edu/wf/GMOs.htm. Accessed August 29, 2003.