5
Organic Contaminants in the Environment: Challenges for the Water/Environmental Engineering Community
Richard G. Luthy
Stanford University
INTRODUCTION
Hydrophobic organic compounds, such as polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs), bind strongly to sediments. They can thus serve as a long-term source of contaminants in water bodies and biota long after the original source has been removed. Advances in analytical techniques make it possible to measure even the smallest amount of anthropogenic contaminants present in sediment. Yet the ability to relate sediment concentrations to water quality, biological availability, and toxicological effects is hindered by inadequate understanding of the binding and release of the contaminants in sediment. The inherent heterogeneity of sediment makes it difficult to describe in terms of bulk sediment physicochemical parameters such as total organic carbon content, surface area, and particle size distribution. Management of sediments and the control of sediment contaminants are among the most challenging and complex problems faced by environmental engineers and scientists and will become increasingly more so as other organic contaminants enter the environment in greater quantities.
SEDIMENT CHEMISTRY AND BIOUPTAKE
Sediments typically consist of a few weight percent of organic matter and minerals. The organic carbon environment acts as an attractor or accumulator of hydrophobic compounds, such as PCBs. Organic carbon fraction is thus taken as a measure of the sorption capacity for regulatory purposes. This enables normalization of the aqueous equilibrium relationship for sediments containing different amounts of organic carbon. However, organic carbon in sediment comes in different forms that may have very different sorption capacities for hydrophobic organic compounds. In addition to natural sources such as vegetative debris, decayed remains of plants and animals, and humic matter, sediment organic carbon is also derived from particles of coal, coke, charcoal, and soot that are known to have extremely high sorption capacities. A comparison of reported organic carbon-normalized phenanthrene partition coefficients (Koc) for different sorbents is shown in Figure 5.1. These data indicate that hydrophobic organic compounds associated with soot- or coaltype carbon may be orders of magnitude less available in the aqueous phase than those associated with natural organic matter in soils and sediment.
As discussed previously (Ghosh et al., 2003), several groups have carried out work indicating the possible importance of soot carbon in sorption processes. For instance, it was shown that elevated PAH partitioning in sediment samples could be explained on the basis of the soot carbon content and known high PAH sorption capacity of soot. It was concluded that sorption of PAHs on soot-phase carbon in sediments may impact in situ bioavailability of PAHs. It is not clear though from any of the studies conducted whether natural organic matter or black carbon in sediments comprises the predominant repository of hydrophobic contaminants such as PCBs and PAHs. However, in earlier work (Ghosh et al., 2000), it was found from direct analysis of separated fractions and particle-scale microanalysis that the majority of PAHs in sediment are associated with coal-derived particles and that these PAHs are strongly bound, unavailable for biological treatment, and unavailable for uptake in earthworms.
More recently the particle-scale understanding of PCB and PAH distributions in sediments has been extended by comparing sediments from three different geographical locations and by illustrating the effect of PAH association with particle types on bioslurry treatment (Figure 5.2). When sediments were sampled from Hunters Point in San Francisco Bay, an area with high PCB concentrations, the sediment was carefully analyzed and compared with that from the other areas. Once again, coal, char, wood, charcoal, and other carbon-containing compounds were found. It was found that
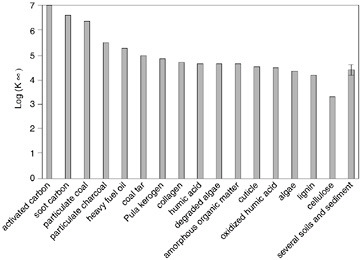
FIGURE 5.1 Organic carbon-normalized partition coefficient for phenanthrene for different types of organic carbon. SOURCE: Ghosh et al. (2003).
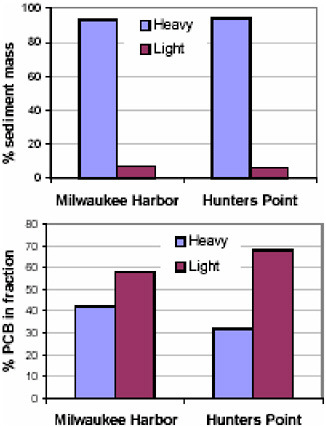
FIGURE 5.2 Distribution of PCBs in sediments.
such material in sediments from the field tended to bind or accumulate PAHs and PCBs (Ghosh et al., 2003).
It was further found that for Milwaukee Harbor and Hunters Point, the lighter-weight coal, charcoal, wood, and particulate organic carbon comprised a small percentage of the sediment; about 5-6 percent (or 7 percent by weight). It was in this small percentage of organic carbon that two-thirds of the PCBs analyzed for were found. It appears that over a period of time perhaps decades, PCBs naturally undergo a repartitioning process to the material in sediment. There is no indication that the PCBs and that material were co-disposed; rather over a long time, PCBs tend to accumulate with this material, strongly bond, and become less bioavailable.
These results suggest a new way of dealing with these contaminants and sediments, taking advantage of an ability to change the chemistry. Perhaps, the bioavailability of these hydrophobic compounds depends on the particle type to which they are bound, and the bioavailability can be changed by adding sorbent carbonaceous particles. Thus, the new strategy is to manage contaminated sediments using in situ stabilization.
ORGANIC SEQUESTRATION AND BIOAVAILABILITY CONTROL
This greater understanding of the interactions of organic contaminant with sediments may enable improved methods of sequestering and controlling the bioavailability of those contaminants. Removal of these contaminants can pose significant problems because such efforts often lead to additional release of contaminants into the aqueous environment. An excellent example of this is Lauritzen Channel, a Superfund site in Richmond Harbor, San Francisco Bay, that was contaminated with DDT (dichlorodiphenyltrichloroethane) over many years. Although the site was dredged as part of a Superfund site cleanup, postremediation monitoring found that unacceptably high levels of pesticides remain in Lauritzen Channel.
One possible approach to more effectively address the presence of organic contaminants in sediments is to change the chemistry of the sediment—not to destroy the compounds in situ, or dig them up and haul them off somewhere else—in order to change the bioavailability of the contaminants. This approach involves adding activated charcoal to sediments to prevent organic contaminants from entering the aqueous environment.
Absorption Efficiency Tests
Activated carbon is used in water treatment, but never before was it thought that adding activated carbon to sediments would achieve any benefit. The effect of activated carbon on sequestration of organic contaminants in sediments was tested here using benthic organisms indigenous to the San Francisco Bay (Figure 5.3). Clams (Macoma), worms (Neanthes), and arthropods (Leptocheirus) were each added to separate, activated, carbon-treated sediment samples from Hunters Point.
The results of the clam tests show that for sediment treated
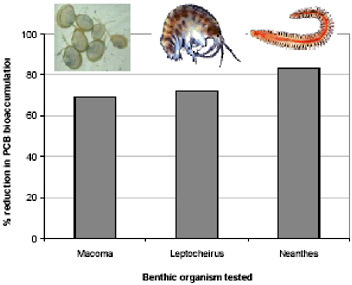
FIGURE 5.3 Species comparison of PCB bioaccumulation reduction.
for one month there was a 69 percent reduction in bioaccumulation of PCBs. This result is quite dramatic and demonstrates that by adding carbon to sediment, PCBs are repartitioned in such a way that they are no longer bioavailable. Similar results were obtained for Leptocheirus and Neanthes.
In a set of complementary tests, particles with varying amounts of particulate organic carbon were spiked with either benzo[a]pyrene or tetrachloro-PCB and then fed to clams (Figure 5.4). These were short feeding studies in which clams were fed individual particles and then watched to see what was ingested and where the PCB went. That is, the amount of PCB retained versus the amount that passes through the clam is measured. These are called absorption efficiency tests with a PCB or benzo[a]pyrene spiked onto the particle and fed to the clams for eight hours.
In these tests, carbon was ground down to about 20 mm, and clean diatoms were added to mark the start of eating. The clams were fed for eight hours on four separate days. Then a mass balance was calculated to determine how much PCB was retained in the clam and how much appeared in the feces of the clam. When you do that and normalize the data relative to absorption efficiency by diatoms you obtain a relative bioavailability or relevant absorption efficiency. For clams the absolute number was about 90 percent, but diatoms were also measured on every test for consistency from test to test. The results of the benzo[a]pyrene or the tetrachloro-PCB on activated carbon show that when the clam ingested an activated carbon particle with PAH or PCB on it, it passed right through. Only 1-2 percent of the contaminant was retained. This indicates that the bioavailability of the contaminant is negligible.
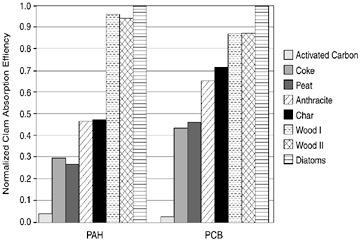
FIGURE 5.4 Clam absorption efficiency.
Aqueous Equilibrium Tests
Figure 5.5 shows the aqueous equilibrium concentrations of PCBs in the presence of sediment from a shipyard in San Francisco that has been either untreated or treated with activated carbon. The results of the study show that after treatment for one month there was about 90 percent reduction in PCB concentration. Thus, the effect seems to be apparent relatively quickly.
A biomimetic device for uptake of contaminants in organism lipids was created from a polyethylene bag filled with fish lipid immersed in a vial containing sediment. The bag was allowed to be in contact with the sediment a period of time. Together with others like it, this is an interesting screening tool and has proven very useful for characterizing
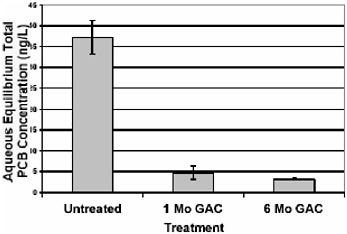
FIGURE 5.5 Aqueous equilibrium tests of PCBs in sediments treated with activated carbon.
TABLE 5.1 Sum of 16 U.S. Environmental Protection Agency Priority Pollutant PAHS, Extractable Oily Matter (Oil and Grease), and Total Organic Carbon Content of Study Lampblack-Impacted Soils and Fisher Lampblack
|
|
Total PAHs (mg/kg)a |
Oily Matter (wt %) |
TOC (wt %) |
Lampblack Fraction (wt %) |
% of Total PAHs on Lampblack Fraction |
|
Fisher LB |
700 |
0.1 |
|
100 |
100 |
|
CA-5 |
1,410 |
1.5 |
14 |
22 |
96 |
|
CA-2 |
8,350 |
4.9 |
71 |
65 |
100 |
|
CA-17 |
12,700 |
3.9 |
47b |
53 |
95 |
|
CA-18 |
17,600 |
4.9 |
50 |
62 |
81 |
|
CA-10 |
35,200 |
9.1 |
94 |
84 |
99 |
|
amg/kg soil for the five field soil samples and mg/kg lampblack for the Fisher lampblack. bReported by Steven B. Hawthorne, University of North Dakota. SOURCE: Hong et al. (2003). |
|||||
sediments and natural waters. This study showed that there was a 73-77 percent reduction in the uptake of PCBs with treatment with carbon.
The combined results of all these tests (including some not described here) such as bioaccumulation in three different organisms, aqueous concentration, and absorption efficiency by clams, suggest that it is possible to change the bioavailability of a compound. The next steps are to look at chemistry and bioavailability and the linkages between the microscale (particle) and the macroscale (food web) processes.
LAMPBLACK-IMPACTED SOILS
Another example of contaminant-solid interactions involves recent work (Hong et al., 2003) with California Oil Gas site samples. Before natural gas was distributed in California, gas was made by gasifying oil, and there are many old gas plant sites around the state. The oil would be cracked to obtain methane and a solid residue would remain. Sometimes the residue could be used as fuel and sometimes it was buried on-site.
There are a number of these plants in California, and samples from five different sites were investigated. Large amounts of PAHs were found in this material ranging from thousands to tens of thousands of milligrams per kilogram PAH (Table 5.1).
It was also found that the PAHs vary in their bioavailability. When partitioning studies were done, in looking at what PAHs will be solubilized in water when in contact with some of that material, the lampblack residuals seem to fall into two distinct levels, one high and one low. This is quite interesting because these samples at the low level will pass the California Environmental Protection Agency risk base screening levels for drinking water sources, while the higher level will not.
To determine what was happening, nuclear magnetic resonance (NMR) was used to characterize the lampblack material. NMR data indicated that the material is 100 percent aromatic. It thus will tend to bond PAHs very strongly as long as there is no residual oil. Aromatic oil also comes from this material as long as the concentration is not too high. There thus seems to be a competition between adsorption on the lampblack (high) and absorption on the aromatic oil (low) (Figure 5.6).
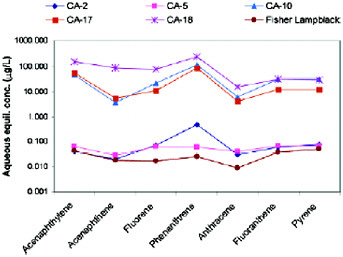
FIGURE 5.6 Aqueous equilibrium concentrations of PAHs for the six study materials as measured by the air-bridge technique.
SOURCE: Hong et al. (2003).
BIOAVAILABILITY OF CONTAMINANTS IN SOILS AND SEDIMENTS
To address this bioavailability issue, a committee commissioned by the National Research Council’s (NRC’s) Water Science and Technology Board undertook a two-year study to understand the bioavailability of contaminants in soils and sediments and released its report entitled Bioavailability of Contaminants in Soils and Sediments: Processes, Tools, and Applications (NRC, 2003).
The committee looked at definitions of bioavailability and current use of soil sediment risk management; addressed the physical, chemical, and biological processes comprising the science of bioavailability; assessed measurement tools; and determined how to move forward in regulation.
In terms of the definition of bioavailability, it is thought of as the physical, chemical, and biological processes that affect exposure of organisms to contaminants and uptake of contaminants. Bioavailability processes in soil or sediment include release of a solid-bound contaminant and subsequent transport, direct contact of a bound contaminant, uptake by passage through a membrane, and incorporation into a living system.
One finding from this study was that bioavailability processes are embedded within human health and ecological risk frameworks, but they are often unclear and hidden. These biological processes are physical and chemical and therefore, in principle, can be measured, described, and modeled. This is important because such information leads to mechanistic understanding, and in order to manipulate bioavailability, mechanistic understanding is essential.
Ecological Risk
In order to move the kinds of technologies talked about here for managing sediments into the field, it will be necessary to obtain better mechanistic knowledge at the field scale. This requires expertise of ecologists who know about feeding processes, particle ingestion, and so forth. Quantitative models of fish are also needed to predict the impact of cleaning up at places such as Hunters Point in Alameda, Moffet Field, and Mare Island.
Bioavailability Tools
There are many sophisticated physical, chemical, and biological tools available for the critical analysis of bioavailability. Analytical tools such as X-ray absorption spectroscopy or microscale double laser mass spectrometry have been used to locate and identify PAHs in sediment material. Biological tools are used to evaluate the entry of a contaminant into living organisms without measuring processes A-C. However, the NRC committee’s view of this was that there is little consensus about optimal approaches for measuring bioavailability. The absorption efficiency test described earlier is mechanistic and is a controlled experiment. It provides useful information, but it still must be validated with field materials. Thus, each kind of test will have its limitations. In the committee’s report (NRC, 2003), a table is provided that specifies generic strengths and limitations of many of the currently available tools. The criteria used to evaluate the tools reflect the committee’s opinion that mechanistic approaches (which determine the form and associations of a contaminant) have the greatest potential for ultimately defining bioavailability processes and narrowing uncertainties.
The regulators and industry, on the other hand, tend to prefer simplified operational tests such as extractions which are shortcuts that study mechanistic processes (e.g., equilibrium partitioning) or estimate bioavailability indirectly (e.g., toxicity bioassays). However, these tests lack explanatory capability and have limited applicability. What is needed are mechanistic approaches that will elucidate contaminant form and association.
However, mechanistic approaches are less applicable at present. The way to build consensus on methods to measure reduction of bioavailability, as demonstrated earlier, is to follow multiple lines of evidence that will help us move forward and eventually lead to pilot studies (i.e., adaptive management). The NRC (2003) committee on bioavailability is a proponent of adaptive management, experimenting with tools and models. The committee did not recommend a national policy, but rather recommended that various experiments be tried in the field and those results used to develop a common systematic approach. Investment is necessary to gain this kind of mechanistic understanding and models.
ROLE OF REGULATORS
During the work carried out and presented in this paper, it was very important to work closely with the EPA, the California Department of Toxic Substance Control, and the Regional Water Quality Control Board from the beginning. On the sediment work at Hunters Point, all of these groups are advised on the studies taking place. It is important to ask regulatory agencies what they think about the tests being conducted. Is something being overlooked? Some things might appear interesting to a professor, but one might be missing something that would be a “show stopper.”
CONCLUSION
In the end it all comes back to chemistry. Chemistry plays a central role in all that has been described here. Chemistry has to go along with engineering, geosciences, biology, and toxicology. In order to reckon with and make progress on these complex problems such as PCBs in San Francisco Bay, this convergence of disciplines is essential.
ACKNOWLEDGMENT
Stanford University is fortunate to have support from the Department of Defense (DOD) and others who have contributed to this work.
REFERENCES
Ghosh, U.; R.G. Luthy; J.S. Gillette; and R.N. Zare, 2000 Environ. Sci. Technol 34:1729-1736.
Ghosh, U.; J.R. Zimmerman,; and R.G. Luthy, 2003 Environ. Sci. Technol. 37:2209-2217.
Hong, L.; U. Ghosh,; T. Mahajan,; R.N. Zare,; and Luthy, R.G. 2003 Environ. Sci. Technol. 37:3625-3634.
NRC. 2003. Bioavailability of Contaminants in Soils and Sediments: Processes, Tools, and Applications. Washington, DC: The National Academies Press.
DISCUSSION
Hudson River Cleanup
Parry Norling, of RAND (now at the Chemical Heritage Foundation), asked if Dr. Luthy’s research can be used to reverse the decision or to modify what will be done for cleanup of the Hudson River.
Dr. Luthy thinks that some experiments could certainly be carried out. There will be some hot spots in the Hudson that should be removed, and there is also a lot of sediment with low concentration of PCBs, where his research will probably play a big role. The approach will be to supplement what is done now, remove the hot spots, and then use his strategy afterwards or in conjunction with some capping. He thinks the strategy to dig up the Hudson and put it on the shore nearby communities such as Troy, New York, that do not want it will not achieve a desirable result.
Physical Transport Processes
Tim Shaw, of the University of South Carolina, congratulated Dr. Luthy on his nice work. Mr. Shaw liked the idea of adding the mediating compounds but wondered if physical transport processes have been examined. One of the big potential problems with this would be if the mediating compound was transported separately from the fine-grain sediments that contain the contaminants.
Dr. Luthy agreed and noted that the question is along the lines of what the hydrodynamics are and how one would get the compounds down there and make them work in the field. In a place like Hunters Point, people think that a certain size particle would be needed that would have some hydrodynamic characteristics similar to the material that is present there. It seems plausible that if hydrophobic organic compounds were put in an inch or two of sediment, they would not move. If there was clearly a high-energy site; however, these particles would be flushed away. There are some exceptions such as Seaplain in Alameda, where it is quiescent, in which the compounds could probably just be thrown in there. The behavior of the particles is very important, however, in most cases.
At a sediment conference in San Diego in April, some people from EPA were interested in adding to caps, putting clean sand down on top of the contaminated sediments. Along this line, activated carbon could be added to the sand and then there would not only be a diffusion barrier, but an active trap as well.
Removal of PCBs
Tom Troyer, of King Lee Technologies, said that PCBs are very hard to destroy. Now that it is better known where they are sequestered, he wondered if this leads to any possibility of using in situ destruction.
Dr. Luthy said that PCBs would be attracted to the carbon, and if iron were present it could reduce PCBs through reductive dehalogenation, and perhaps convert them to biphenyl, which would be degraded. He was not sure if the process would work in situ or if other things would compete with the zerovalent iron. He also wondered whether this might affect any really slow process of biological dehalogenation through microbes.
Tom Dillion, of Science Applications International Corporation, stated that he was involved about 25 years ago in a government program to remediate very low level radioactively contaminated sites. The program’s issue was removal or stabilization. Typically, the stabilization would cut the health effects by a thousand compared to removal and was somewhere between a hundred thousand and a million times more cost-effective. However, removal was always chosen.
Dr. Luthy responded that this is why it is important to get people like Ned Black and Fred Hetzel from the regulatory agencies engaged early on. Ned Black of the biological technical assistance group at EPA Region 9 has told him that nobody wants the PCBs there and yet they do not want them removed either. Dr. Luthy said that once dredging is started, it can create a big mess and become impossible to deal with.
DDT-Contaminated Sites
Vasilios Manousiouthakis, of the University of California at Los Angeles, asked Dr. Luthy to comment on the effectiveness of similar approaches for DDT-contaminated sites, such as the one in Palos Verdes.
Dr. Luthy did not know about Palos Verdes but stated that his group is now working on the Richmond channel. Richmond is a place where there was dredged DDT-contaminated sediment, new sand, and then new contaminated material on top of that. The chemistries of PCBs and DDT are not very different. DDT will undergo a slight transformation, but basically it is a hydrophobic compound and will probably be affected like PCBs.
Kinetics
Don Phipps, of the Orange County Water District, asked if the kinetics of absorption were relatively rapid and also wondered if one could consider building or using this material in a pillow-type format that could be put in contact with the sediments. The material would take up PCBs slowly and after a period of time could be replaced and recycled.
Dr. Luthy responded that mats can be put down but he has not investigated this area. In the work that his group has done, modeling shows that there are kinetics of uptake by carbon and diffusion through pore water. When things are well mixed, the release from the particles is limiting. There is a rapid release from the mineral fraction, but there is also coal and other material there. It will transfer to the carbon, like a solid-phase diffusion process.
Mr. Phipps pointed out that it may not be really practical to have this diffusion over a large area, but in a fairly small region the contamination is by diffusion into the water. If contamination is over a large area it may be possible to build a renewable cap and then slowly recover it that way. It would be like digging up the sediments without actually digging them up.
Carbon Treatment Concentration
Mark Matsumoto, of the University of California at Riverside, asked why only one concentration of carbon was used and if there is any way to determine the differences in the way the sediment and the activated carbon capture the contaminants..
Dr. Luthy said he started this work with support from DOD and some from Forbes, Stanford, and the Bioex Program. The first part of this work was to show that it is not harmful and that it is effective. For the starting dose of carbon, his group decided to double the total organic content (TOC) of the sediment.
In current work, they are halving and even going to a tenth of what they previously used. What the modeling shows is that if half this dose of carbon is set equal to the TOC, essentially the same results are obtained. When about a tenth of this dose is used with this sediment, higher concentrations result, approximately 1 percent by weight.
Steve Cabaniss, of the University of New Mexico, asked why the modeling shows that going to one-tenth will result in higher aqueous concentration.
Dr. Luthy replied that a higher concentration is being put on the carbon per unit weight. If it is sent to an absorption isotherm, higher concentrations of carbon yield higher aqueous concentrations. The capacity of carbon has not been exceeded, but more is put on the carbon per unit weight.
Fouling
Jay Means, of Western Michigan University, asked if Dr. Luthy had looked at any change in the efficiency of transfer as a function of fouling with biological films.
Dr. Luthy responded that his group has done studies for as long as six months and found that fouling improved with time. There would be other issues if other materials in the sediment could absorb and displace PCBs. This carbon will probably pick up some humic molecules from the sediment and become sticky or gummy, slowing any further uptake as seen in water treatment. He said it takes a long time for this to appear in water treatment.







