6
AquaSentinelSM: Biosensors for Rapid Monitoring of Primary-Source Drinking Water
Elias Greenbaum1
Oak Ridge National Laboratory
INTRODUCTION
Most of the phytoplankton present in surface waters is composed of algae (Palmer, 1959). The diversity of responses or adaptations to changes in light intensity observed in microalgae suggests that these microorganisms are able to live in a variety of habitats. Seasonal changes in the available light habitats are related to seasonal algal succession patterns (Richardson et al., 1983).
Chlorophyll a fluorescence induction curves reflect the efficiency of energy conversion in Photosystem II (PS II) reaction centers (Krause and Weis, 1991). As a result, they can be used as indicators of the physiological state of algal cells. The ratio of variable to maximal fluorescence (Fv/Fmax), defined as the maximal fluorescence minus the minimal fluorescence divided by the maximal fluoroscence (Fmax − Fs)/Fmax), is equal to the quantum yield of Photosystem II. It can be calculated from fluorescence induction curves of photosynthetic tissue that is illuminated by a saturating flash of light (Schreiber et al., 1994; Falkowski and Raven, 1997).
Since the introduction of the pulse amplitude modulation (PAM) measuring technique and the saturation pulse method in 1986 (Schreiber et al., 1986, 2002), chlorophyll fluorescence parameters have been very useful in ecotoxicological studies. Very recently, a dual-channel chlorophyll fluorometer called the ToxY-PAM was introduced for the detection of toxic substances in water (Schreiber et al., 2002). The authors reported detection of the urea-based herbicide Diuron in very small concentrations. The sensitivity was below the 0.1-µg/L detection limit for a single toxic substance in water as specified by the European Commission drinking water regulation.
The purpose of this paper is to describe proof-of-principle experiments that led to AquaSentinel, a continuous water monitoring system in the field of homeland security. The system uses naturally occurring photosynthetic microorganisms as biosensors for the detection of chemical antagonists in primary-source drinking water. Among the toxic compounds tested were potassium cyanide (KCN), methyl parathion (MPt), Diuron (DCMU), and Paraquat. Hydrogen cyanide has an odor characteristic of bitter almonds and is completely miscible in water. In this study, the water-soluble salt KCN was used. The cyanide ion is an extremely toxic and fast-acting poison. MPt is an organophosphorus insecticide and a cholinesterase inhibitor that is structurally and functionally similar to the chemical warfare agents classified as nerve agents. Severe exposure of humans and animals can lead to convulsions, unconsciousness, cardiac arrest, and death. DCMU is a substituted urea-based herbicide. It is a nonionic compound with moderate water solubility. The U.S. Environmental Protection Agency has ranked DCMU fairly high (i.e., as a Priority B chemical) with respect to potential for groundwater contamination. Paraquat is a herbicide that is highly soluble in water. Death is usually due to progressive pulmonary fibrosis and epithelial proliferation in the lungs (Guidelines for Canadian Drinking Water Quality, 1996).
EXPERIMENTAL
Studies were performed using “as-is” freshwater samples and their naturally occurring populations of phytoplankton.
Fluorescence Measurements and Toxic Agents
Figure 6.1 illustrates the successive stages of data acquisition and analysis for fluorescence induction curves by
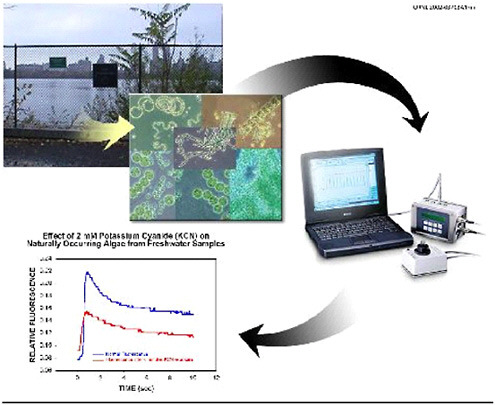
FIGURE 6.1 AquaSentinel: a continuous water monitoring system using naturally occurring algae as biosensors.
AquaSentinel. In a similar laboratory setup with a Walz XE-PAM fluorometer (Heinz Walz GmbH, Effeltrich, Germany), the standard fluorescence cuvette in the fluorometer was replaced with a flow-through model (Hellma Cells, Inc., Model QS-131, Plainview, NY). The cuvette inlet was connected to a glass-bottle reservoir that contained the water samples, and the outlet drained to waste. The system is designed to mimic the flow of river or lake water through the fluorescence detection system. This experimental arrangement allowed continuous monitoring and replacement of water samples in a manner similar to that for the contemplated operation of a real-world biosensor system. Fluorescence induction curves were measured before and during exposure to toxic agents. Fluorescence excitation and emission wavelengths were 660 and 685 nm, respectively. A halogen lamp actinic light source illuminated the cuvette at an intensity of 500 µE-m−2·s−1 via a fiber-optic cable through direct connection to the cell chamber.
Fluorescence induction curves were recorded every 5 minutes, and data collection for each curve was completed within 10 seconds. Data extracted from the fluorescence induction curves were used to calculate Fs, Fmax, Fv (variable fluorescence = Fmax − Fs), and the efficiency of PSII photochemistry (Fv/Fmax). A 200-mL water sample was placed in a jacketed reservoir. The sample was stirred continuously and maintained in darkness with a black cloth. The reservoir was connected to the flow-through fluorescence cell with flexible tubing. To obtain a homogeneous sample before each recording, the volume in the fluorescence cell was replaced three times. After control data were collected, the volume in the reservoir was adjusted to 100 mL and the toxic agent was added. The toxic agents were prepared as stock solutions prior to addition to the reservoir and were injected directly into the top of the vessel and immediately mixed with the sample. Spent samples were drained into a waste bottle. Upon arrival in the laboratory from collection sites at the rivers, the water samples were kept under a fluorescent lamp at an illumination of 50 µE-m−2-s−1 until use.
RESULTS
Experiments were performed with field samples drawn from the Clinch River at Clark Center Recreation Park in Oak Ridge, Tennessee. The Clinch is the main source of drinking water supply for the city of Oak Ridge. Figures 6.2a through 6.2d show the effect of KCN, MPt, DCMU, and Paraquat, respectively, on naturally occurring algae from the Clinch River.
The results shown here demonstrate that naturally occurring freshwater algae can be used as biosensor material for the detection of toxic agents in sunlight-exposed primary drinking water supplies. These agents block electron transport, impair light energy transfer, or generate toxic secondary photoproducts, all of which provide signals that can trig-
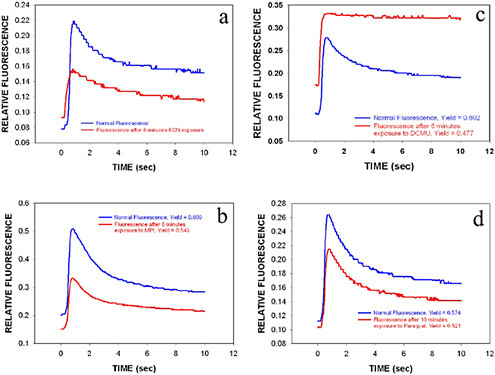
FIGURE 6.2a-d Effect of 2 mM KCN, 20 µM MPt, 10 µM DCMU, and 30 µM Paraquat, respectively, on time-resolved fluorescence signal of naturally occurring algae from the Clinch River. There is a six-minute delay between collection of the two curves in each plot shown. In plots a-c, the top curve shows is the normal fluorescence trace of the water sample, while for plot d it is the bottom curve. Time zero is the initiation of fluorescence excitation of the sample measured.
ger an alarm. Our results showed that the tissue-based biosensors experienced a decrease in photochemical yield when exposed to KCN, MPt, DCMU, and Paraquat. A detectable effect was observed in every freshwater sample tested.
CONCLUSIONS
The data presented in this paper indicate that biosensors based on fluorescence induction curves of naturally occurring freshwater algae can be used to detect cyanide, methyl parathion, DCMU, and Paraquat in primary water supplies under appropriate experimental conditions. In the context of current state-of-the-art biosensor research, they are unique: in the case of sunlight-exposed drinking water, the biosensors occur naturally in the medium to be protected. When combined with encrypted data telecommunication and a database-lookup library containing pertinent data for healthy algae, this approach to the protection of sunlight-exposed primary drinking water supplies may be of practical value under real-world conditions.
ACKNOWLEDGMENTS
The authors thank Dr. K. Thomas Klasson for designing spreadsheets for data analysis and Ms. Beverly S. Mathis for secretarial support. This research was supported by the Tissue-Based Biosensors Program, Defense Advanced Research Projects Agency, under MIPR No. 99-H250 with Oak Ridge National Laboratory. It was also supported by the U.S. Department of Energy Office of Basic Energy Sciences. Oak Ridge National Laboratory is managed by UT-Battelle, LLC, for the U.S. Department of Energy under contract DE-AC05-00OR22725. The data contained in this paper were collected
as part of the studies published in Rodriguez, M., Jr., Sanders, C.A., and Greenbaum, E. 2002. Biosensors for rapid monitoring of primary-source drinking water using naturally-occurring photosynthesis. Biosens. Bioelectron. 17:843-849.
REFERENCES
Falkowski, P.G., and J.A. Raven, 1997. Aquatic Photosynthesis. Malden, MA: Blackwell Science Publications.
Guidelines for Canadian Drinking Water Quality, 6th ed. 1996. Ottawa, Canada: Ministry of Supply and Services.
Krause, G.H., and E. Weis, 1991. Chlorophyll fluorescence and photosynthesis: The basics. Ann. Rev. Plant Physiol. 42:313-349.
Palmer, C.M. 1959. Algae in Water Supplies. Public Health Service Publication No. 657. Cincinnati, OH: U.S. Department of Health, Education, and Welfare.
Richardson, K., J. Beardall, and J.A. Raven, 1983. Adaptation of unicellular algae to irradiance: An analysis of strategies. New Phytol. 93:157-191.
Schreiber, U., W. Bilger, and U. Schliwa, 1986. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 10:51-62.
Schreiber, U., W. Bilger, and C. Neubauer, 1994. Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. Ecol. Stud. 100:49-70.
Schreiber, U., J.F. Muller, A. Haugg, and R. Gademann, 2002. New type of dual-channel PAM chlorophyll fluorometer for highly sensitive water toxicity biotests. Photosynth. Res. 74:317-330.
DISCUSSION
Fertilizers and Data Interpretation
Parry Norling, of RAND (now with the Chemical Heritage Foundation), began the discussion with a question about fertilizers and whether they might interfere with the fluorescence signal. He thought that high concentrations of fertilizer might stimulate growth of algae and cause a false fluorescence reading.
Dr. Greenbaum responded that while a high concentration of fertilizer could stimulate a bloom of algae, it would be a benign effect. He said that with this technology it is possible to distinguish between changes in fluorescence emissions that are caused by a malicious act and changes caused by benign acts. Dr. Greenbaum said that when dealing with primary-source fresh drinking water, the quality of the water and the quality of the physiological states of the algae should be relatively constant over long periods of time.
Mr. Norling then asked an additional question regarding the fluorescence signal shown earlier for the herbicide Diuron. He noticed that in one chart Dr. Greenbaum showed that the fluorescence signal increased, while in another it decreased (data not shown here).
Dr. Greenbaum clarified that what Mr. Norling noticed was actually a general shift in the line rather than an increase or decrease. The curvature and shape of lines is measured to provide a “fingerprint” by overlaying the data. He said that there are subtle changes that sometimes come from natural variations in the algae. These must be determined by spending time in the river, collecting the data, building a database, and getting a good feel for the natural variance of the algal population in the water. Dr. Greenbaum said that it would then be possible to determine the change in the fingerprint associated with a particular toxin and then determine how large a variance there is between the standard data, the standard curve, and the one that is being collected. He said that how much variance to tolerate before raising a red flag must be determined. Whether changes in the photochemical efficiency are calculated by computing the Fv/Fmax photochemical efficiency or a point-by-point comparison of the curves, he said that it is possible to determine how large a variance the algae can tolerate. Once that variance exceeds the point of tolerance, the water can be analyzed to determine the cause of the variance.
Measuring Algal Metabolites
Dave Layton, of Lawrence Livermore National Laboratory, brought up how there are often problems associated with algal metabolites (taste and odor) in drinking water. He wondered if this technology could be used to determine the presence of contaminating algae in the water supply
Dr. Greenbaum responded that this is possible.
Applications for Manufacturing or Industrial Processes
Dave Rea, of DuPont (retired), asked whether this technology could be applied to looking at variations in effluents from manufacturing and industrial processes.
Dr. Greenbaum said that depending on the chemical composition or nature of the effluents, they should cause the same types of changes he has demonstrated for the deliberate toxins that might be introduced into water and thus could possibly be used for monitoring industrial waste.
Oligotrophic Systems
Tim Shaw, of the University of South Carolina, commented that a similar technology is used to identify nutrient limitation in coastal marine systems. He wondered whether an oligotrophic system that is already in a state of reduced Fv/Fmax photochemical efficiency might influence the signal obtained with this technology.
Dr. Greenbaum said that as long as algae are present it does not matter whether they are growing in nutrient-poor or nutrient-rich conditions to obtain the signature fluorescence response. The signal depends on the presence of the chlorophyll and the ability of the algae to undergo photosynthesis. He said that if the algae are growing in the wild, they are undergoing photosynthesis, and if they undergo photosynthesis, there will be a fluorescence signal to measure.
Effects of Sudden Changes
Mark Matsumoto, of the University of California at Riverside, wondered whether sudden nonhazardous changes, such as a spike in salt concentration or temperature, might influence detection of a hazardous substance.
Dr. Greenbaum replied that for most primary-source drinking waters, there would never be any spike in salt concentration or temperature. There are usually only slow diurnal variations. These natural variances are important to investigate in order to utilize naturally occurring biosensors such as the algae. He said that in the data collected so far, the natural diurnal variations occurred with a constant characteristic time that was much slower than the chemical interactions of the toxins with the algae in the water.
Mr. Matsumoto said that he wondered whether such a situation would give a false positive.
Dr. Greenbaum said that the photochemical efficiencies did not change in the measurements obtained. He said that the AquaSentinel technology measures diurnal variation which can affect photochemical efficiency when the sun is very strong, but this is a predictable natural diurnal variation. By looking at the second differential, which departs from the natural diurnal variations, a red flag goes up to indicate that the system has to be investigated further.
Effects of Water Turbidity
David Krabbenhoft, of the U.S. Geological Survey, noted that the rivers discussed in this presentation looked very clear and wondered if more humic or turbid waters would lead to a loss in signal sensitivity.
Dr. Greenbaum said that the water studied was quite clear but that it does have some sediment, including bacteria and other microorganisms. He said that a feature of this technology, however, is that nothing in the water fluoresces at 685 nm or has the characteristic time course. To the extent that particulate matter exists in the water, the signal decreases simply because of scattering. This means that the absolute amount of light would go down but the characteristic signal will not be affected.
Interference from Herbicides
Jay Means, of Western Michigan University, then asked if a background presence of herbicides such as triazines might decrease photosynthetic efficiency and raise the detection limits for the target compounds.
Dr. Greenbaum responded that if a body of water demonstrated a photochemical efficiency of 0.4 for the PS II reaction center, it would be necessary to question whether it should be used as a source of drinking water in the first place. He continued to say that when water is clean, there should be nothing in it that depresses the photochemical efficiency. If herbicides such as triazines are present, then the water should not be used as a primary source of drinking water.
However, Dr. Greenbaum said that this technology is not meant to be used to monitor already-contaminated sources of water. He said that the technology should be used where the assumption is that the water is clean from the start. If a terrorist decides to dump potassium cyanide or methyl parathion into that water, then there will be a point of reference by which to compare the original and current state of the water. He said that in most cases people are not drinking water that is so heavily contaminated that the photochemical efficiencies of the algae that live in the water are already suppressed. If the water is really bad, algae would not even be able to grow.
Mr. Means followed up with a comment that two-thirds of the surface water and probably a third of the groundwater supplies in the United States are contaminated with triazines.
Dr. Greenbaum responded that if that was true, the rivers studied would have indicated this. However, at the concentrations present in the rivers, he did not see any adverse effects of such compounds on the algae.
Application as an Air Sensor
Don Phipps, of Orange County Water District, asked if this technology might be used in the form of a biofilm of algae. He said that it should be possible to improve the signal-to-noise ratio because of the higher concentration of algae. He also said that this would be much simpler and would make it possible to utilize the technology in both air and water.
Dr. Greenbaum replied that there is already a company that makes a kit for culturing algae to produce biofilms. He did not agree that it would be simpler, however, because making a biofilm requires more labor. Using algae that occur naturally in the water renders the technology discussed in this paper non-labor intensive and reagentless. He noted that conventional biosensor technology is typically single use, but with this technology the biosensors are already present in the water and are a part of the environment being studied. He added that the important aspects of this technology are that it is non-labor intensive, can easily be automated, and monitors continuously in real time.
With respect to making a film for use as an air sensor, Dr. Greenbaum agreed that it would be very useful. He said that algae can be entrapped on a filter paper and then used to measure airborne gases such as mustard gases, tabun, or sarin. The algae in this form can then be used for measuring toxic agents in air and water. Work done in measuring airborne contaminants with entrapped algae on filter paper was published two years ago in the journal Biosensors and Bioelectronics. In fact, he said that this was the original application for the algal biosensor technology discussed here, but
that during the course of that program water protection became a high-priority issue so the technology was applied to water and not air.
System Cost
Tom Dillion, of Science Applications International Corporation, asked about the cost of the AquaSentinel system.
Dr. Greenbaum responded that the system could be built for less than $20,000 per unit, perhaps even less than $10,000, based on the price of the components of the system. A fluorometer is also needed but the one used here was more sophisticated than necessary for these special measurements. He said that the entire system could actually be put on a chip. Estimating that 500 to 1,000 of these units would sell around the world, the price would probably be less than $10,000.
System Location
Mr. Dillion followed up by saying that it would be good to utilize this technology in individual buildings. He said that in terms of protecting a water supply, dilution phenomena make it unlikely that attacks will happen at a water supply area.
Dr. Greenbaum replied that this technology only works for primary-source drinking waters because once that water has been filtered and disinfected, the algae are removed. Without algae, the fluorescence signal one would want to monitor would not exist.
Purposeful Nutrient Enhancement
Vasilios Manousiouthakis, of the University of California at Los Angeles, wondered if there might be compounds that could be mixed with the maliciously placed toxin so that it would help the algae grow, and therefore have some kind of compensatory effect on the sensors and prevent the needed detection level being reached.
Dr. Greenbaum responded that it would not be possible to do this because the presence of the nutrients needed to grow the algae would not change the sensing signal of toxins such as cyanide, methyl parathion, or DCMU. He said that the chemistry and specific action of these toxins are independent of the nutrients present in the system in this case. However, he said that the kinetics of fluorescence decay is the difference between algae grown in a nutrient-rich lab environment versus the more nutrient-poor field conditions. He said that in the lab the kinetics of decay are slower because the algae are much healthier than those living in the river, but that even when they are very healthy the algae are still susceptible to the harmful effects of the toxins.






