10
Water Solutions and Strategies in the Chemical Industry
Carol R. Jensen*
The Dow Chemical Company
Large technology-driven companies have both responsibilities and opportunities to use water resources wisely. This involves careful use of water (conservation), as well as responsible control of contaminants in discharge streams (pollution control). In addition, there may be opportunities to leverage technology and knowledge to opportunities outside these companies as products or services. The Dow Chemical Company is an example of a company that actively participates in each of these water-related activities.
The purpose of this paper is to review examples of Dow’s activities in each of these areas. It will cover two aspects: (1) Dow as a water user and (2) Dow as a supplier of clean water technology. It is hoped that Dow’s experiences will enable and encourage other companies and organizations to enhance their water-related activities.
DOW—A WATER USER
Overall, Dow is a significant user of water. Globally it uses about 900 billion pounds of fresh water per year (~300 million gallons per day). Approximately 90 percent of this water is pre-treated before use, and 70 percent is post-treated and discharged after being used only once. From a financial standpoint, Dow invests about $100 million annually for water acquisition, pre-treatment, post-treatment, and disposal. Overall, about $1.2 billion of capital infrastructure is employed for water treatment and disposal. The water demand at its major sites is increasing by 6 percent each year. Over the next 10 years, Dow expects to make water-related capital investments of $1 billion and increase its annual water-related operating costs by $100 million. Water is clearly a significant economic issue for Dow. Management of water resources and assets across the company is recognized as a critical activity.
World-class companies, however, need to manage more than just resources or assets. They also need to manage public perception, particularly in the communities where they are located. In 1996 Dow announced 10-year public goals related to its environmental, health, and safety activities. These goals were voluntarily proposed. Dow’s annual progress toward these goals can be tracked on its public web site, www.dow.com. These goals include the following reductions in emissions to air and water for Dow’s global operations.
-
Reduce chemical emissions by 50 percent.
-
Reduce priority compounds by 75 percent.
-
Reduce the amount of waste and wastewater generated per pound of production by 50 percent.
-
Reduce dioxin emissions by 90 percent.
-
Reduce energy use per pound of production by 20 percent.
Figure 10.1 shows Dow’s progress toward its wastewater reduction goal. In 2002, Dow produced 2.85 pounds of wastewater for each pound of product. This reduction has occurred as Dow’s total production has increased.
Dow’s chemical processes also produce waste chemicals, some of which are released to the air or water in small quantities. Figure 10.2 shows Dow’s progress toward its global public goal for dioxin emission. In this figure, dioxin is defined as the totality of 7 dioxins and 10 furans, and “TEQ” denotes “toxic equivalent,” a quantitative measure of the combined toxicity of a mixture of dioxin-like chemicals. Dow released a total of 8.68 g of dioxin (TEQ) in 2002. It is on target to meet its public 2005 goal of 4.05 g. These small amounts are dispersed in literally trillions of gallons of water, resulting in concentrations on the order of a few parts per
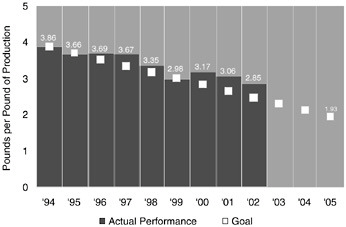
FIGURE 10.1 Dow’s progress toward its public 10-year wastewater reduction goal.
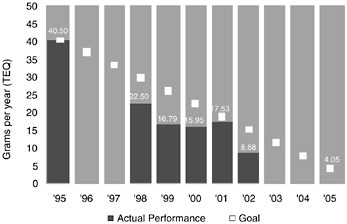
FIGURE 10.2 Dow’s progress toward its 10-year public dioxin emission goal.
quadrillion (1015) or less. Developing the sampling and analytical technology to detect these concentrations has been a formidable challenge for Dow.
The magnitude of cost and complexity of Dow’s water-related activities prompted it to create an Environmental Operations Business in 1998. One of the goals of this business was to integrate Dow’s best practices in water, manage resources and technology, and optimize supplier relationships globally across the entire company. Pragmatically, creating a “business” or “profit-and-loss” entity in a company elevates the importance of its activities by making it financially accountable.
The Environmental Operations Business is responsible for managing Dow’s water envelope globally. It also manages water rights, conveyance, quality, use, and treatment for discharge. It is responsible for $3 billion of water-related assets, and consists of about 700 employees. It has the goal of reducing costs of all environmental operations by $1 billion dollars over the next 50 years. During its first year of operation it achieved a 3-5 percent reduction in water use across the entire company. Several specific examples follow.
Example 1
To meet expansion needs and to meet Dow’s long-term environmental objectives, the Terneuzen site in Netherlands completely restructured its water infrastructure. The site now recycles 80 percent of its treated fresh wastewater and has reduced energy consumption for producing boiler feedwater by 90 percent. This is equivalent to reduced production of 55,000 tons of CO2 per year (or saving 930,000 million Btu per year).
Other significant results of this project include the following:
-
annual savings of U.S. $1.2 million for the cooling tower water supply as a result of recycling treated fresh wastewater, instead of importing fresh water;
-
by replacing multistage flash evaporation of seawater with reverse osmosis technology, 50 tons of low pressure steam is made available per hour for alternative uses on-site; and
-
long-term availability of fresh water in the region made possible by the significant increase in recycling.
The Terneuzen case demonstrated clearly that conservation and growth are compatible. In fact, in many cases, it makes excellent business sense to pursue water conservation and recycling.
Example 2
In Freeport, Texas, home of Dow’s largest site, an initial goal of 30 percent reduction in freshwater use was set. By simply looking closely at water use, Dow found that it was losing 13,000 gallons per minute, merely through the inefficiency of water flow control within the system. This problem was remedied through simple enhancements to water management protocols, resulting in an annual savings of $135,000 per year in water and electricity.
This example demonstrated that saving water often comes not from new technologies but from better management of water transmission from one point to another. This success was the result of a Six Sigma project. Six Sigma methodology is playing a major role at Dow in providing a consistent, thorough methodology for evaluating water envelope management issues.
Example 3
A cooling tower optimization project that is under way at Dow’s operations site in Midland will reduce freshwater

FIGURE 10.3 Dow’s recently developed internal slogan to encourage wise use of water resources.
consumption by 37 million gallons a year. What was learned from this project is being replicated at four other Dow sites. One of the clear advantages of a global Environmental Operations Business (EOB) approach is that successes at one location can be effectively leveraged across the entire global corporation.
Dow’s Environmental Operations Business is also responsible for creating a mind-set and culture for good water management. Figure 10.3 shows Dow’s recently developed slogan to keep a cultural awareness of the importance of good water management prominent.
DOW—A SUPPLIER OF CLEAN WATER TECHNOLOGY
The second half of this overview of Dow’s water-related activities deals with extensions of Dow’s technologies to external applications. Dow has a rich and extensive history of developing and supplying water purification and handling technologies. Figure 10.4 highlights some of the major categories of Dow’s participation. These include a variety of chemical treatment approaches: (1) ion exchange; (2) specific technologies for the electronics, pharmaceutical, and biotechnology industries; and (3) separation membranes, among others. In many cases, technology developed for Dow internal needs is now being supplied to satisfy external needs and applications.
Separation membranes, in particular, are a class of technologies with diverse and rapidly growing uses. Dow began research on membrane technology in the mid-1950s. Early research focused on developing microporous hollow-fiber technology that led to kidney dialysis membranes, reverse osmosis (RO) membranes, and gas separation membranes.
This overview highlights Dow’s activities in reverse osmosis and nanofiltration. In 1986, Dow acquired the FilmTec Corporation, a small company located in Minnesota. This accelerated Dow’s participation in the RO and nanofiltration market.
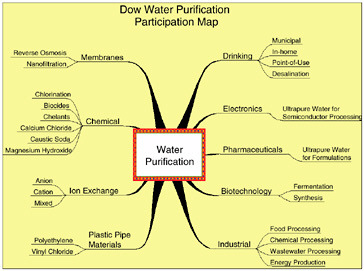
FIGURE 10.4 Examples of Dow’s activities in water purification technology.
FilmTec’s standard membrane family is based on an interfacial reaction that forms an aromatic polyamide thin film at the surface of a microporous support layer. This ultrathin film is a cross-linked polymer network that serves as the discriminating layer that does the separation. Depending on cross-link density and other factors, this layer can selectively reject various salt ions, organics, or other contaminants while allowing essentially pure water to pass through. This membrane structure is “wound” into a spiral configuration, forming a modular element that is easily installed into a cylindrical pressure vessel.
The total cost of operation of a typical RO membrane plant has been estimated to include 5 percent as membrane replacement cost and 44 percent as electrical power. One of the contributions to society that large corporations such as Dow make is to continually lower technology costs, thus enabling more widespread use of those technologies. Figure 10.5 shows the historical decrease in feed pressure required to yield a constant water production rate from Dow’s brackish water elements. This reduction in pressure has significantly decreased the cost of power for reverse osmosis plants.
Feed pressure reductions, element cost decreases, increased salt rejection (selectivity), and other performance enhancements have significantly lowered the historical RO processing cost. Figure 10.6 depicts this decline graphically for both brackish water and seawater desalination. The steady decline in power requirements and capital investment has enabled larger and larger RO plants to be built. Figure 10.7 shows the historical progression of increased capacity for the world’s largest reverse osmosis membrane plants, including future plants that are expected to be built. Over a period of 15 years the world’s largest plants have grown from 1 million or 2 million gallons a day to more than 100 million gallons a day.
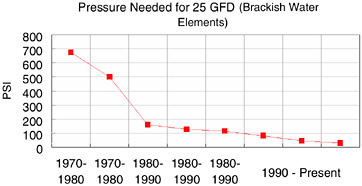
FIGURE 10.5 Historical reduction in operating pressure for Dow’s reverse osmosis membranes.
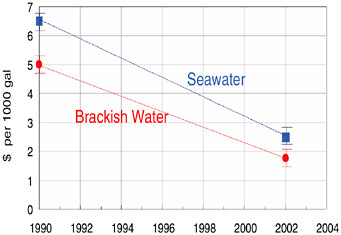
FIGURE 10.6 Historical cost reduction trends for seawater and brackish water reverse osmosis processes.
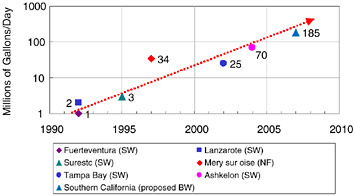
FIGURE 10.7 Reverse osmosis membrane facilities—largest plants as a function of time, with nanofiltration (NF), seawater (SW), and brackish water (BW).
The Mery sur Oise nanofiltration plant in a suburb of Paris, France, illustrates some of the unique capabilities of Dow’s membrane science. This plant supplies water for 500,000 residents. Water from the Oise River, over time, has experienced increasing amounts of agricultural runoff, particularly pesticides and herbicides. The local water authority wanted to remove these agricultural contaminants while maintaining the naturally occurring calcium and magnesium ions. Water quality, particularly taste, was a critical driver for this technology.
Dow designed a specific membrane chemistry and structure to meet these requirements. A plant containing 9,100 nanofiltration modules was built based on this technology. It has been in successful operation since 1999.
Figure 10.8 illustrates another application of nanofiltration membrane technology. Dow and Marathon Oil of Houston, Texas, developed a nanofiltration membrane process to remove sulfate from the seawater used for injection into offshore oil field reservoirs. On an offshore oil platform, the only water available for injection is seawater. Barium sulfate scale results when sulfate ions in seawater come in contact with barium in the reservoir rock commonly found in the North Sea and West African oil fields. This scale plugs the pores in the reservoir rock, preventing the flow of oil and thus jeopardizing subsequent oil production. In the past, the problem of sulfate scale formation was tackled by chemical dosing. Nanofiltration enables production of offshore reservoirs where chemical dosing is not adequate or feasible. This example again illustrates the link between energy and water purification. A cost-effective reduced-energy process for sulfate removal actually enables the production of oil in offshore reservoirs that could not normally be produced.
Jurong Island, Singapore, provides a final example of Dow’s membrane separation capabilities. This island is one of seven southern islands off Singapore. A modern, integrated petrochemical complex is located there that is a key element of Singapore’s strategy to develop Jurong Island into a world-class chemicals hub in the Asia-Pacific Region.
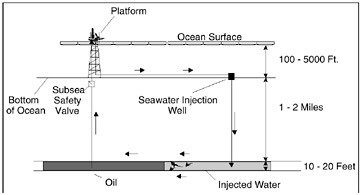
FIGURE 10.8 Nanofiltration membranes remove sulfate ions that could precipitate from water injected into oil reservoirs from offshore platforms.
About half of Singapore’s potable water comes from neighboring Malaysia under supply agreements scheduled to expire in 2016 and 2036. Jurong Island’s booming petrochemical industry represents a rapidly increasing demand for high-grade industrial water.
In January 2000, SembCorp Utilities & Terminal started a high-capacity reverse osmosis plant designed to treat 6 million gallons per day. This plant will recover up to 86 percent of the tertiary treated sewage water and turn it into reusable high-grade industrial water. The inlet water is biologically active such that conventional membranes would rapidly lose productivity through fouling. Dow installed recently developed, fouling-resistant membrane elements to meet the challenge. This example illustrates how technology developments such as fouling-resistant membrane elements are expanding the role of reverse osmosis technology to applications where it formerly could not be used.
SUMMARY
Large chemical companies such as the Dow Chemical Company are playing critical roles as both users and suppliers of water technology. Dow, in particular, has proactively formed a global Environmental Operations Business with a mission to elevate the importance of water management and standardize best practices worldwide. Using tools such as Six Sigma, this business has established a track record of successes in water conservation and economic savings.
Dow has also embarked on a strategy of public accountability. It has voluntarily created long-term goals related to water use and dioxin-related releases to the environment. These goals were announced publicly. Progress toward achieving these goals is reported annually to the public.
Finally, Dow has a rich portfolio of water-related product technologies. Reverse osmosis and nanofiltration membrane technologies are highlighted in this paper because of their growing use in a broad spectrum of new water purification applications.
The authors hope that these examples of Dow Chemical’s water-related activities will enhance the industry’s awareness and commitment to proactive and intelligent management of water. There is immense value in the exchange of information between users and producers of water-related technologies. Chemical companies are uniquely positioned to impact the water industry because of their dual roles as water technology users and providers.
ACKNOWLEDGMENT
The authors thank the following Dow colleagues for contributing much of the information and perspective contained in this paper: Ian Barbour, Marvin Bourelle, Roy Davis, Paul Dean II, Peter Dulcamara, Tammy Falardeau, Karl Fennessey, Matt Hallan, Chuck Martz, Bill Mickols, John Osborne, George Quarderer, Lanny Robbins, Kristina Schnepf, Tracy Taylor, and Kelli Walsh.
DISCUSSION
Tertiary Wastewater of Singapore
Dick Carlson, of San Diego County, stated that he had understood that Singapore was moving toward performing reverse osmosis on tertiary wastewater for drinking water rather than just for industrial purposes.
Dr. Jensen responded that it may very well be. Although she has no direct knowledge of the reverse osmosis project, she has met a couple of times with the Economic Development Board, and she knows that it is focused heavily on desalination processes for drinking water. If people think about the industrial consumption there, they will realize how extensive it is.
Nanofiltration Technology
Vasilios Manousiouthakis, of the University of California at Los Angeles, asked if Dr. Jensen could provide some information on the nanofiltration technology and how it compares in terms of salt removal.
Dr. Jensen said that the nanofiltration technology is actually a derivative of Dow’s reverse osmosis technology. Nanofiltration is essentially designed on a case-by-case basis to selectively pass, so it is actually designed with different levels of cross-linking and different levels of free amine and free carboxylic acid. The technology can be designed so that it has different porosities and different affinities for separate ions. In terms of salt removal, it depends on the particular application, but generally there is less salt removal and higher water permeability for nanofiltration than for reverse osmosis.
Dow’s Environmental Operations
Frankie Wood-Black, of ConocoPhillips, was interested in how well Dow’s establishment of its environmental operations is working.
Dr. Jensen responded that it is working well. In the first couple of years, there has actually been some tremendous success at being able to identify some fairly large opportunities for cost reduction just by getting control of the envelope. There has also been the opportunity to have a strategic goaltargeted approach rather than a reactive approach. It has been around for five years, and has had some good success, and time will tell as to whether the success can be sustained.
Dennis Hjeresen, of the Green Chemistry Institute, asked for an idea as to how far that envelope extends. From the discussion it sounds as though it extends to the company, but what about the impact of Dow products on the
environment? How far does the thinking extend that there are a lot of developments in terms of pesticides, herbicides, and other products?
In her response, Dr. Jensen said the thinking does extend beyond the company. There is clearly a very focused effort on the subject of sustainable development. As new products are being developed, there is a tremendous sensitivity to what the ultimate outcome of these products will be.
Debbie Elcock, of Argonne National Laboratory, commented that waste and water reduction goals were set prior to the establishment of the EOB and asked what the driver was at that point, because it did not sound as though it was necessarily tied to profits.
Dr. Jensen replied that the goals were set in the early to mid-1990s before the establishment of the EOB and that they were not tied to profits, but to a sense of social responsibility. Once a company gets to be fairly large such as Dow, a lot of attention can be drawn to its environmentally related activities. It was originally set up to be sensitive to important issues and drive the organization to take action. The opportunity to look at someplace where one can actually save money and do something smart is a very high priority.






