2
HIV/AIDS Care in the Third Decade: Opportunities and Challenges in the Changing Epidemic
The year 2001 marked the beginning of the third decade of the AIDS epidemic. In just over 20 years, HIV/AIDS had changed the landscape of medicine, public health, and health care in the United States and the world. The rapid changes in treatments, however, threaten to out-pace the design of the health care delivery system for individuals with HIV and AIDS. In 1996, the introduction of effective new antiretroviral therapies changed the clinical course and outcome of this illness. Until the introduction of highly active antiretroviral therapy (HAART), AIDS was associated with an inevitable functional deterioration and death. Acute illness brought people into the care system, clinical care occurred in a hospital setting with intense outpatient follow-up, and prevention was focused on those at risk of becoming infected.
The third decade of the HIV/AIDS epidemic offers a remarkable opportunity to extend the productive years of life of people living with HIV/AIDS (PLWH/A). The challenge will be to restructure the public health care financing and delivery system so that uninsured and underinsured PLWH/A have access to appropriate treatments without disparity, to offer the comprehensive set of services required to promote adherence to their medications to individuals affected by co-morbid conditions, and to prevent new infections by making prevention a routine part of care.
In the United States, 816,149 AIDS cases and 467,910 AIDS-related deaths have been reported as of December 2001 (CDC, 2002a). It is estimated that approximately 40,000 people in this country are newly infected with HIV each year, and the disease remains an imminent and serious
threat to public health (Karon et al., 2001). Globally, the picture is starker. The Centers for Disease Control and Prevention (CDC) estimates that the number of people living with HIV/AIDS worldwide is 40 million, and that during 2001 the world’s death toll from AIDS reached 3 million (CDC, 2002a). Although the epidemic had slowed until recently in most of the developed world, the National Intelligence Council (2002) predicts that by 2010 the numbers of those infected with the virus could reach 75 million in Nigeria, Ethiopia, Russia, India, and China alone (NIC, 2002). In 2002, the United Nations Population Division lowered its estimate of the world’s population for 2050 by 400 million people, largely because of the effect of HIV/AIDS (United Nations, 2003).
If the epidemic has maintained a staggering pace, so too has the fight against it. Scientific discovery has resulted in a rapid gain in knowledge about the disease, dissemination of prevention and treatment information, and changes in expectation and outcomes. The first widely distributed reports of the disease occurred in 1981 and concerned homosexual men (CDC, 1981). Over the next two years, at-risk populations were further defined to include injection drug users, individuals with hemophilia and others who had received blood products, and Haitians; universal precautions for health care workers and other professionals whose work put them in contact with blood and other bodily fluids had been published; and the virus that caused the disease had been identified (CDC, 1982a,b,c,d,e, 1983; Barre-Sinoussi et al., 1983). Advances in knowledge and treatment options continued throughout the eighties and early nineties, and by 1996 combination antiretroviral therapy became (and remains) the standard of care for those infected with HIV. The impact of HAART was dramatic—the number of deaths from AIDS fell by 43 percent between 1995 and 1997 (Figure 2-1) (CDC, 2002a). In all, it took only 15 years from the first noted incidence of this new disease to the development of therapies that can be effective against it.
The rapid pace of the development of new technology to fight the disease continues. In January 2003 the Food and Drug Administration (FDA) announced the expansion of availability of a rapid HIV test, which returns results in a matter of minutes rather than days or weeks. The FDA approved the expansion in the hopes that combining administration and results of the test into one clinic visit would increase the numbers of people seeking the test and entering the care system if testing positive (FDA, 2003a). In March 2003, the first in a new class of drugs called fusion inhibitors was granted accelerated approval, expanding the options of those for whom other treatments have failed (FDA, 2003b).
This promising evolution of treatment does not, however, mean that the HIV epidemic is over or that it soon will be. The decrease in deaths brought about by new treatments, coupled with the steady number of new
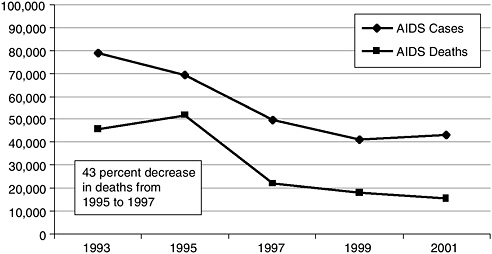
FIGURE 2-1 Numbers of AIDS cases diagnosed and AIDS deaths reported, 1987 through 2001.
SOURCE: CDC, 2002a.
infections, indicates that more people than ever are living with HIV and AIDS (CDC, 2002a). As a consequence, the population at risk for transmitting the disease—those already infected—continues to grow (Figure 2-2). In 2003, CDC released preliminary data showing an increase in the number of AIDS cases reported in 2002 over 2001 (CDC, 2003). Though the increase was small (2.2 percent), it was the first since 1993 and could be an early warning that the system is missing opportunities to prevent those with HIV infection from progressing to AIDS. The loss of these opportunities, both for treatment to prevent disease progression and for intervention to reduce risky behaviors and promote prevention, occurs when infected individuals remain outside the care system, and eventually results in a greater burden to the system. Furthermore, those newly infected with HIV are more likely than in the past to be poor, members of a racial/ethnic minority group, and uninsured or publicly insured (Levi and Hidalgo, 2001). Those groups that traditionally have been at high risk, such as men who have sex with men (MSMs) and injection drug users (IDUs), have been joined by the seriously mentally ill, women of color, and the homeless.
The third decade of the HIV/AIDS epidemic presents great opportunities and challenges for care providers and policy makers. Treating this deadly disease effectively is now possible for many individuals. But treatment regimens can be complex and expensive. The epidemic also continues its entrenchment in vulnerable populations suffering from co-morbid con-
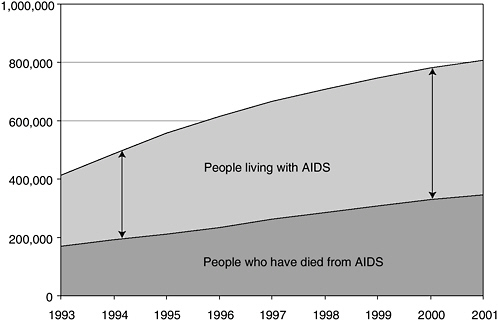
FIGURE 2-2 People living with AIDS as a proportion of cumulative AIDS cases.
SOURCE: CDC, 2002a.
ditions that can complicate their seeking and obtaining care. The ability of new therapies to slow the disease, both at an individual and a population level, is determined both by their availability to those who need them and the ability of recipients to follow complex regimens. Even then, it is possible that the treatment will fail and drug resistance will develop. Given the continuing shifts in the epidemic, the care system’s ability to provide treatment in a timely manner to save lives, avert disability, and prevent the spread of the disease is increasingly challenged. This, in turn, challenges policy makers at all levels to ensure that programs meant to support the care system do so in a way that facilitates the mission of that system.
The following sections discuss the changing elements of the epidemic that require consideration in a restructured HIV care delivery system: the effect of HAART on the course of the disease, as well as the risk of toxicities and resistance with HAART; the central role of adherence to therapy; the changing demographics of the epidemic and the challenges they present; and the increasing incidence of both medical and social co-morbid conditions among PLWH/A. To provide the background for these discussions, however, it is necessary to understand the natural history of the individual HIV infection.
THE NATURAL HISTORY OF INDIVIDUAL HIV INFECTION
HIV is believed to have entered human populations from chimpanzees in Africa in the first half of the 20th century. Comparisons with closely related viruses in chimpanzees show that this crossover was likely made on multiple occasions (Korber et al., 2000; Hahn et al., 2000). In the United States, there is evidence the virus was present as early as 1977, though its long latency period rendered it invisible (Gottlieb, 2001).
The course of HIV disease varies by individual and is not fully predictable, and the full effects of current antiretroviral management on the natural history of the disease remain uncertain. Still, the scientific and medical communities have learned a great deal in the past 20 years, and the natural history of HIV infection is now better understood (Polk et al., 1987; Mellors et al., 1996, 1997; Vlahov et al., 1998; Pezzotti et al., 1999). This understanding is important to the care system because it indicates when care will be needed and which services will be necessary and appropriate at each point in the disease process.
Initial/Primary HIV Infection
The majority of newly infected individuals develop what is known as primary HIV infection or acute retroviral syndrome. Acutely infected persons are symptomatic, often sufficiently so to seek medical care. These symptoms—fever, rash, fatigue, generalized lymphadenopathy, and nausea among others—are flu-like and appear within days to several weeks of the moment of infection. Primary HIV infection usually resolves in a matter of weeks and is not life threatening. Because the symptoms are characteristic of infection by less serious viruses, the opportunity to identify HIV infection is often missed at this stage (Quinn, 1997; Kahn and Walker, 1998).
During the symptomatic phase of acute infection, virus replication is unchecked by the immune system. Individuals in this disease stage are highly infectious. This is of great importance from a public health perspective because unsafe behavior in this phase may readily lead to transmission. It is estimated that more than half of all HIV infections may be transmitted during this stage of infection (Schacker et al., 1998). Therefore, increasing the identification of HIV during this silent phase of the disease and providing prevention counseling to infected individuals are key strategies for managing the progression of the epidemic.
Asymptomatic HIV Infection
After full antibody reaction to HIV infection is established (typically within three to six months), the infection is said to be in the “chronic” or
“established” stage (Fauci, 1993; Levy, 1993; Pantaleo et al., 1993). Symptoms of acute infection have resolved by this point. The term “asymptomatic HIV infection” applies to this phase when the person is unaware of any symptoms of infection. This phase may last for 1 to 10 or more years, even without antiretroviral therapy (Haynes et al., 1996). During this period, however, the virus is still actively replicating and the infected individual may unknowingly transmit the virus. Also, the infection is gradually changing the individual’s complex immune system, most notably by causing a reduction in the number of CD4+ T-lymphocytes (CD4 cells) in the peripheral circulation.1 Even though there are no clinical manifestations of the disease, the immune system begins to deteriorate (Pantaleo et al., 1993). As the CD4 cell count begins to decline, individuals who are asymptomatic, or have nonacute conditions such as chronic fatigue, may meet the established treatment guidelines criteria to receive HAART. The challenge with many HIV-infected individuals in this stage who are focused on more immediate needs, such as housing and employment, is to engage them in treatment and promote retention in care and adherence to therapy.
Eventually, the CD4 cell count falls from above 500 cells/ml, the threshold of a normally functioning immune system, to 200 cells/ml, an indicator of severe immune suppression, and can fall even lower. This drop in the CD4 cell count is significant because it increases the risk of serious and potentially fatal opportunistic infections or cancers (Polk et al., 1987; Mellors et al., 1996, 1997; Vlahov et al., 1998; Pezzotti et al., 1999). Antiretroviral therapy applied during the asymptomatic phase of disease can raise CD4 cell counts predictably and durably, preventing or delaying the stage of life-threatening immune deficiency commonly referred to as AIDS (Detels et al., 1998).
Symptomatic HIV Disease/AIDS
Advances in treatment have changed the ways in which the clinical stages of HIV disease are viewed. The difference between asymptomatic and symptomatic HIV disease is less obvious than the terms imply. With progressive immune depletion—perhaps especially if the plasma viral load2
is very high—patients may begin to note fatigue and malaise. Minor infections such as oral candidiasis are seen frequently and the risk of more serious complications rises as the CD4 cell count falls below 200 cells/ml. Some of these infections, notably Pneumocystis carinii pneumonia, can be prevented with prophylactic antibiotics, while others cannot (CDC, 2002a). Along with antiretrovirals, these prophylactic antibiotics, as well as certain vaccines that can prevent complicating infections, are essential components of medications that must be provided to maintain the health of the individual infected with HIV (USPHS/IDSA, 2001).
Death from HIV Disease
Prior to the availability of current effective antiretroviral therapy, development of AIDS and death were predictable outcomes among HIV-infected patients (Polk et al., 1987; Mellors et al., 1997; Vlahov et al., 1998; Pezzotti et al., 1999). Death usually followed several months of progressive debilitation, wasting, and often, dementia. Many suffered blindness from cytomegalovirus retinitis or endured intractable diarrhea.
Today, the pattern is more complex and not as predictable (Pezzotti et al., 1999). Antiretroviral therapies can continue at least partially to suppress viral replication and to maintain some integrity of the immune system. Although HAART options can be exhausted, and some individuals still die of AIDS, it is increasingly likely that the cause of death for an HIV-infected person will be tuberculosis or hepatitis C virus (CDC, 1999, 2002b).
EFFECT OF HAART ON HIV PROGRESSION AND CARE
The impact of antiretroviral therapy on the outcome of HIV infection is one of the most dramatic developments in medical history. Therapies for treating HIV have come so far that it is possible to forget the bleak outlook of the early to mid-eighties, when the best that medical technology could offer was palliative care that only delayed death for a short time. Once a retrovirus was established as the cause of the disease, researchers were able to focus their efforts on blocking the replication of the virus in the body. In 1986, the National Institutes of Health (NIH) organized the AIDS Clinical Trials Group (ACTG), which has studied dozens of therapies and continues to do so today. The findings from this research group provide the foundation of the current guidelines for antiretroviral therapy (Sepkowitz, 2001).
|
|
individual—the higher the viral load, the sicker the patient. Measuring viral load is an important part of gauging a treatment regimen’s effectiveness (HIV/AIDS Treatment Information Service, 1999). |
In 1987, AZT became the first drug approved for the treatment of AIDS based on the interim results of a randomized clinical trial. Its introduction raised the hopes of the HIV-positive community. In 1989, however, the results from the completed ACTG trial showed that AZT could slow disease progression, but did not impact survival rates (Volberding et al., 1990; Sepkowitz, 2001; Bartlett et al, 2001). Subsequently, the results of the Concorde trial in Europe showed that long-term disease progression rates were also unaffected by AZT (Seligmann et al., 1994).
Seven years would pass before breakthrough research on viral load measurements and the efficacy of triple-drug therapy was first reported (Bartlett et al., 2001). A longitudinal study on viral load as an indicator of disease stage revealed that by monitoring the amount of the virus present in the plasma, therapeutic benefit of drug therapy could be assessed in days or weeks rather than the months required by monitoring CD4 cell counts (Mellors et al., 1996). The results of a trial of triple-drug therapy were equally exciting and some believed that eradication of the virus was close at hand (Bartlett et al., 2001).
Unfortunately, that was not the case, but morbidity and mortality did decrease sharply after the introduction of HAART therapy (Figure 2-3) (Palella et al., 1998; Detels et al., 1998; Chiasson et al., 1999). By 1998, the number of individuals receiving HAART therapy had risen dramatically,
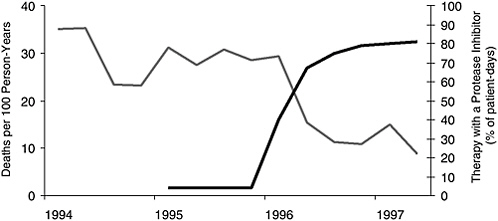
FIGURE 2-3 Mortality and frequency of use of combination antiretroviral therapy including a protease inhibitor among HIV-infected patients with fewer than 100 CD4 cells per cubic millimeter, according to calendar quarter, from January 1994 through June 1997.
SOURCE: Palella et al., 1998, Copyright 1998, Massachusetts Medical Society. All rights reserved.
and the number of deaths had fallen just as dramatically (Palella et al., 1998). Even those with severely weakened immune systems (indicated by a CD4 cell count of less than 50 cells/ml) had significantly fewer opportunistic illnesses when taking potent drug regimens (Miller et al., 2000).
The results to date in clinical practice remain impressive. In fact, it is no longer possible to give an evidence-based estimate of the median survival of those with HIV disease who are treated with appropriate drugs because not enough time has elapsed. Opportunistic infections, previously common, are now less so (USPHS/IDSA, 2001). Opportunistic malignancies—especially Kaposi’s sarcoma and non-Hodgkins lymphoma of the central nervous system—have all but disappeared in those receiving effective antiretroviral therapy (Jacobson et al., 1999; Pezzotti et al., 1999). The success of HAART is tempered by its challenges, however. All antiretroviral medications carry the risk of side effects and adverse reactions, ranging from transient nausea and headache to serious or even fatal metabolic disorders (Montessori et al., 2004; Nolan, 2003). These adverse reactions can force a change in drug regimen and limit future treatment options (Ledergerber et al., 1999; Lucas et al., 1999). In addition, chronic conditions that develop as a result of the medications, such as diabetes and heart disease, must be treated along with the HIV infection in the long term (Carr et al., 1999).
To promote the adoption of the new therapies as quickly as possible, NIH sponsored a Panel to Define Principles of HIV Therapy (Box 2-1) that released its first report in 1998. Among other things the principles stressed the importance of individualized care, adherence to treatment regimens, and continuous monitoring and contact with the care system (CDC, 1998a).
Current Treatment Guidelines
The U.S. Department of Health and Human Services’ (DHHS) Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents generally recommend HAART when the CD4 cell count falls below 350, although for individuals with CD4 cell counts of 200 to 349 other factors are considered. These include the individual’s willingness and ability to begin therapy, current state of immunodeficiency and risk of progression, and the potential for adverse reactions and side effects. Combination regimens of three drugs are most commonly used, and one-drug regimens (monotherapy) are never recommended (DHHS, 2004).
The goal of therapy is durable suppression of HIV replication so that measured levels in the peripheral blood fall below the sensitivity levels of assays, usually below 50 HIV copies/ml. Therapy is monitored to follow the expected rise in CD4 cell counts and the fall in plasma viral load. All patients also require laboratory and clinical assessment for disease or drug-related toxicity (DHHS, 2004). For individuals who do not respond as
|
BOX 2-1
SOURCE: CDC, 1998a. |
expected to HAART, assays of genetic mutations associated with antiretroviral resistance are also commonly used, and some studies suggest an improved outcome if drug doses are adjusted for measured serum drug concentrations (DHHS, 2004). In many patients, antiretroviral therapy fails to fully suppress the virus either immediately or after a period of success. In this event, altering some or all of the drugs in the prescribed regimen may be required (DHHS, 2004).
Adherence
The central challenge of HAART is adherence, or the ability of an individual to consistently follow the prescribed treatment regimen. Adherence is crucial in the treatment of any illness; however, its importance is magnified in the treatment of HIV for two reasons. First, fully successful suppression of the virus in the blood requires very high levels of adherence. Second, poor adherence can contribute to the development of drug-resistant strains of the virus, which can then be transmitted to others. This results in reduced treatment effectiveness and options in those individuals who have never been treated for HIV before and thus constitutes a public health threat. The factors that influence an individual’s ability to adhere to HAART include the regimen itself, an individual’s personal characteristics, and the social environment of the patient (Ickovics and Meisler, 1997; Catz et al., 2000; Stone, 2002; Gebo et al., 2003).
The ability to fully adhere to a treatment regimen for any illness is almost never complete; in general, 80 percent compliance is considered adherent (Piliero and Colagreco, 2003; Rabkin and Chesney, 1999). Although rates of compliance of those with HIV on HAART are generally higher than those of individuals with other chronic illnesses, HAART requires unprecedented adherence of more than 90 percent to receive optimal benefit (Harrigan et al., 2003; Garcia de Olalla et al., 2002; Bangsberg et al., 2001; McNabb et al., 2001; Paterson et al., 2000). While adherence levels in clinical trials have been high, results in the clinical setting have not been as successful (Escobar et al., 2003). A number of studies using multiple methods to measure adherence in various settings and populations have indicated that patients’ adherence to HAART averages 70 to 80 percent. Significantly, the studies show that few individuals are able to achieve the adherence levels required to receive the maximum benefit from the medication (Golin et al., 2002; Liu et al., 2001; Bangsberg et al., 2000). The inability of large numbers of patients to achieve the high levels of adherence required for complete viral suppression underscores the need to develop and provide appropriate adherence support as a routine part of HIV care.
The second factor that must be considered in any discussion of adherence is its role in the development of drug resistance. Drug resistance can
lead to treatment failure, and resistance can develop across an entire class of drugs, not just the one currently prescribed, limiting future treatment options. Moreover, the development of drug resistance carries consequences beyond immediate treatment failure. Drug-resistant strains of the virus can be transmitted, compromising effective control of the epidemic and presenting a serious threat to public health. Emerging evidence indicates that the number of newly infected individuals who exhibit drug resistance is growing (Wensing et al., 2003; Grant et al., 2002; Little et al., 2002). Complete viral suppression, obtained through greater than 90 percent adherence, leaves little room for drug resistance to develop. In circumstances of less than total viral suppression, however, the virus begins to select for drug resistance as it replicates and evolves. If a drug-resistant virus is then transmitted, HAART regimens will not be as effective in the newly infected individual. Thus, the best opportunity for delaying development of AIDS will have been lost (Little et al., 2002).
Understanding the dynamics of adherence is an important part of HIV/AIDS care. The DHHS Guidelines list a number of factors that affect an individual’s ability to adhere to a HAART regimen, including active alcohol and substance use and active mental illness (see Box 2-2). The transformation of HIV/AIDS to a chronic disease, which was brought about by the development of HAART, allows for useful comparisons to other chronic illnesses such as diabetes in terms of which factors influence adherence to treatment. The American Public Health Association highlights some of the lessons learned from the diabetes experience that may be useful in promoting adherence to HIV treatments in its Adherence to HIV Treatment Regimens: Recommendations for Best Practices (2002) (see Box 2-3). Among these lessons are that treatment is a collaborative process between patient and provider rather than a directive one from provider to patient, that
|
BOX 2-2
SOURCE: DHHS, 2004. |
|
BOX 2-3 Shared Factors Individuals can remain largely asymptomatic for long periods of time. Treatment can make the patient feel worse and can cause other serious health problems. Treatment is lifelong, and the primary objective is to prevent deterioration in health as opposed to eliminating the disease. Treatment is complex. Dose–response relationship between adherence and benefit to the patient is particularly important but not always clear. Patient must actively manage day-to-day treatment. Both conditions occur disproportionately in populations underserved by the health care system. Both conditions carry negative social stigma. Factors other than adherence influence the course of the illness, even those with excellent adherence may experience disease progression and poor outcomes. Differing Factors HIV generally considered to be more deadly than diabetes, possibly leading to a sense of futility about treatment. HIV generally associated with body wasting (being too thin) whereas diabetes is generally associated with obesity. Treatment goals for diabetes are more flexible than for HIV. The “window of opportunity” for beginning treatment and developing good adherence to achieve optimal outcomes for HIV is shorter than that for diabetes. Side effects of HIV treatment are generally more severe than those stemming from treatment for diabetes. SOURCE: APHA, 2002. |
fostering adherence takes sustained time and effort on the part of both patient and provider, and that there is no single solution or strategy for success in adherence (APHA, 2002). Although, as noted earlier, adherence to HIV treatments is generally higher than for other chronic illnesses, these lessons learned over decades of diabetes treatment are applicable because HAART is much less forgiving than any other treatment regimen for any illness.
Medication regimen is the most common factor cited for nonadherence to HAART because of side effects and complexity of the drug regimen, which can require dosing up to three times a day and have dietary restric-
tions (Chesney, 2003; Bartlett et al., 2001). Nonadherence due to regimen complexity may be relieved somewhat as more antiretroviral medications are approved for once-daily dosing (Piliero and Colagreco, 2003). Other issues in adherence stem from the patient and the patient/health care provider relationship. Studies have shown that depression, lack of belief in the efficacy of the medicine, and lack of confidence in ability to adhere to the regimen predict nonadherence to HAART (Catz et al., 2000; Singh et al., 1999). There is a great deal of evidence that active alcohol or drug use contributes to nonadherence, although good adherence can be achieved among this population (Chesney, 2003; Escobar et al., 2003; Tucker et al., 2003; Lucas et al., 2002; Chesney et al., 2000). Lack of HIV-related knowledge and low literacy levels in general are also associated with poor adherence (Kalichman et al., 1999). One study of individuals with excellent adherence to HAART found that generally those with high adherence rates believed that the medication was and would continue to work and had trust and confidence in their primary care provider. They also were motivated by a strong desire to stay healthy that made taking their medications a priority, even when they were actively using drugs and alcohol (Malcolm et al., 2003).
Although these factors do predict poor adherence in overall study populations, it has also been shown that it is difficult for clinicians to predict adherence levels in individuals. In one study, physicians and clinic nurses were able to predict an individual’s adherence less than half the time (Paterson et al., 2000). This is a significant issue when it concerns members of groups that are already highly stigmatized, such as those with a mental illness, because it could lead to the denial of therapy based on a presumption of the inability to adhere. Bogart and colleagues (2000) found in a 1998 survey that physicians relied on a variety of nonmedical factors in determining whether or not to prescribe HAART, including demographic and psychiatric factors such as homelessness, age, and history of psychiatric hospitalizations.
It is also important to note that many of the factors shown to inhibit an individual’s ability to adhere are not immutable, but can be influenced with appropriate interventions. As noted earlier, there is evidence that even in populations where it is generally thought adherence will be low, such as individuals who are homeless, a significant proportion can attain high enough levels of adherence to realize some (though less than optimal) therapeutic benefit from the medication. From this evidence, it appears that certain interventions, such as treatment for depression, may increase adherence levels, allowing individuals to gain greater treatment benefit (Bangsberg et al., 2000). Appropriate adherence support provided as a routine part of HIV care offers the opportunity to get the most out of therapy and helps to reduce the likelihood that drug resistance will develop.
CHANGE IN AFFECTED POPULATIONS AND THE IMPACT ON TREATMENT
In 1993, the National Research Council’s Panel on Monitoring the Social Impact of the AIDS Epidemic noted that “instead of spreading out to the broad American population, as was once feared, HIV is concentrating in pools of persons who are also caught in the ‘synergism of plagues’,” a situation in which “poverty, poor health and lack of health care, inadequate education, joblessness, hopelessness, and social disintegration converge” (NRC, 1993, p. 7). This trend has not reversed in the nearly 10 years since that report was released. HIV has continued its march into the most vulnerable populations in society: the uninsured, racial/ethnic minorities, those with substance use disorders and mental illness, homeless persons, and unsupervised youth (Karon et al., 2001; Levi and Hidalgo, 2001). These are the populations that publicly funded care is intended to help—individuals without financial, social, or personal resources upon which to draw in the event of a catastrophic illness such as HIV. In a move to offer these resources, in 1990 Congress enacted the CARE Act to provide safety net funds for health and supportive services for individuals living with AIDS and HIV infection who have either no or inadequate insurance.
The complex needs of the HIV population require provision of supportive services to overcome barriers to receiving primary care, including case management, housing, food, transportation, and mental health and substance abuse treatment. The greatest disparities in receiving care are manifest in these vulnerable populations, and providing access to HIV care clinicians is only part of what is needed (Shapiro et al., 1999).
This section discusses the demographic shifts of the epidemic and the co-morbidities faced by vulnerable populations that affect access to care, adherence to medications, and continuity of care. Mental health and drug dependence disorders and the effects of poverty disproportionately affect these groups and present challenges to them in meeting life’s basic necessities, such as adequate food, housing, and health care. These populations are characterized further by being in transition between settings, for example, from the community, to the criminal justice system, to substance abuse and mental health facilities, and at times to homeless shelters. This complicates ensuring continuity of care for these individuals and requires communication and coordination between these settings. Finally, while the primary care setting is a focus for care provision, the delay between the time of a vulnerable patient’s test results and entry into care can be long and retention in care can be difficult. Vulnerable patients need assistance that prepares them for entering and staying in primary care as well as assistance with navigating the health care system.
Demographics
In the United States, the epidemic grew rapidly through the mid-1980s before decreasing and then leveling off in 1998 through the present (CDC, 2002a). Whereas HIV was once considered a disease of white men who have sex with men, people of racial and ethnic minority groups now represent the majority of Americans in the categories of new AIDS cases, new HIV cases, people living with AIDS, and AIDS-related deaths (CDC, 2002a).
Black3 and Hispanic communities have been hit especially hard by this epidemic. Although blacks and Hispanics together accounted for 70 percent of all new AIDS cases in 2001, these groups made up only an estimated 26 percent of the total U.S. population (Figure 2-4) (CDC, 2002a; U.S. Census Bureau, 2000). In 2001, the AIDS case rate among blacks was nearly 10 times the rate among whites, while the AIDS case rate among Hispanics was nearly 4 times higher than for whites (CDC, 2002a). For the age group 25 to 44, AIDS remains the leading cause of death for blacks, the third leading cause of death for Hispanics, and the fifth leading cause of death for whites (NCHS, 2001). Blacks and Hispanics are also represented disproportionately compared with whites in the number of deaths related to AIDS. In 2001, out of 15,603 estimated total deaths of persons with AIDS, blacks accounted for 51.5 percent, Hispanics accounted for 18.4 percent, and whites accounted for 28.8 percent (CDC, 2002a).
The disease also increasingly affects women. The proportion of annual new AIDS cases represented by adult/adolescent women rose from 16 percent in 1993 to 25 percent in 2001 (CDC, 1994, 2002a). In 2001, women also accounted for 32 percent of new HIV cases. The growing number of HIV and AIDS cases among the general population of women follows a growing trend in the heterosexual transmission rate. The proportion of AIDS cases linked to heterosexual transmission accounted for 6.4 percent in 1993 and 16 percent in 2001 (CDC, 1994, 2002a).
The geographic distribution of the epidemic is also shifting (Figure 2-5). The estimated incidence of AIDS in the South appears to be rising.4 Seven of the 10 states with the highest AIDS case rates are located in this region. As in the United States as a whole, minority populations are disproportionately affected. Of people living with AIDS (PLWA) in the South, 53 percent are African American, although they make up only 19 percent of the total population of the region (Kaiser Family Foundation, 2002). HIV-infected
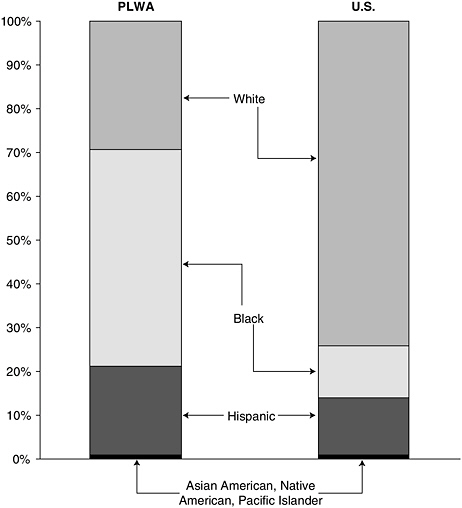
FIGURE 2-4 Proportion of people living with AIDS (PLWA) by race/ethnicity compared to total United States population.
individuals in the South are also more likely to be uninsured or publicly insured (Bozzette et al., 1998). These shifts both demographic and geographic represent the epidemic’s move into populations that are traditionally underserved by the health care system and for whom current prevention messages are either inaccessible or lack resonance.
Co-Morbid Conditions
The rising prevalence of HIV/AIDS among individuals with co-morbid conditions adds another layer of complexity to the current state of the epidemic. These conditions can be medical in nature, such as existing
hepatitis or tuberculosis infection, or may involve substance abuse. They can also be what the Committee terms “social co-morbidities,” that is, underlying social conditions that contribute to the complexities of care. Homelessness, for example, is not a disease process, but it is a definable state that affects the course of HIV infection. Often these co-morbidities occur in clusters as a mixture of existing, and preexisting, medical and social conditions that are both paths to infection and barriers to care. Comorbidities and social conditions affecting HIV-infected individuals are not mutually exclusive, nor do they reside in well-defined populations; they often overlap with one another. Additionally, the extent to which someone is affected by co-morbid conditions, such as substance use disorder or mental illness, is episodic and dependent on a number of variables. An episode of binge substance use or severe mental illness, for example, can also result in a period of incarceration or homelessness.
Mental Illness5
Individuals with both mental illness and HIV represent a large and vulnerable segment of the HIV-infected population. The prevalence of mental illness among those infected with HIV has been estimated to be quite high: about 50 percent of those in HIV care have some form of a comorbid mental illness (Bing et al., 2001). In addition, people with mental illness are at higher risk for HIV than those without it (Cournos and McKinnon, 1997; Stoskopf et al., 2001). Among people with serious mental illness, the seroprevalence of HIV ranges from 4 to 23 percent, with an average of 7 percent, compared with prevalence of less than 1 percent in the United States population as a whole (Carey et al., 1997; Cournos and McKinnon, 1997).
Individuals who have a mental illness have been considered at increased risk of acquiring or transmitting HIV because they have a greater likelihood of high-risk sexual behavior or substance abuse (Cournos and McKinnon, 1997; Johnson, 1997; Carey et al., 1997; Sullivan et al., 1999). Underlying these concerns are the behavioral and cognitive manifestations of the disorders themselves—such as impaired decision making and perception of risks, low motivation, impulsivity, and vulnerability to sexual victimization. Recent studies that empirically examine whether the presence of mental illness can increase the risk of transmission of HIV give a more nuanced and complex portrait of the problem, however, suggesting variation in risk depending on the psychiatric diagnosis or nature of symptoms.
The evidence reveals that serious mental illness—but not depression
|
5 |
For an expanded discussion of HIV and mental illness, see Appendix C. |
and anxiety—are associated with risky behavior, and that youth also increases risk. A meta-analysis of 34 studies investigated the impact of depression or anxiety on high-risk sexual behaviors, defined as having multiple partners and/or unprotected sex. The samples included individuals with mental illness alone, as well as those with mental illness and HIV. The study found little evidence that depression and anxiety are associated with more risky behavior (Crepaz and Marks, 2001).
Findings suggest that serious mental illness, without co-morbid HIV, does increase the likelihood of engaging in high-risk sexual behaviors (Carey et al., 1997; Cournos and McKinnon, 1997; Sullivan et al., 1999). Two relatively small studies have addressed the question of the impact on sexual behavior of having co-morbid mental illness and HIV. These studies have found an increased likelihood of engaging in high-risk sexual behaviors for those with more psychotic symptoms, those with problem drinking, and those not receiving HIV counseling (Tucker et al., 2003). Patients with serious mental illness were also found to have high rates of risky behavior, including sex with a known injection drug user, prostitution, and male–male sexual contact (Meyer et al., 1995).
Researchers at RAND, interviewing 159 treatment providers at 72 mental health and HIV treatment programs in New York City and Los Angeles, found that screening for HIV and risk behaviors in mental health agencies occurs haphazardly, given the range of clients’ nonpsychiatric and other medical needs that compete for the attention of providers. In contrast, HIV treatment agencies tend to place high priority on screening and care for mental illness, as clinicians generally perceive the mental health of clients to be central to successful HIV treatment and adherence (Personal communication, P. Mendel, RAND Corporation, 2002). Nevertheless, because research has long established that depression is missed in 40 to 60 percent of patients in primary care (Hirschfeld et al., 1997; DHHS, 1999), it would not be surprising if depression often went undetected in HIV care. One of the few other studies of this problem found that community mental health clinicians in New Hampshire reported lack of specific knowledge about comorbid mental illness and HIV and reported interest in receiving training (Brunette et al., 2000).
Another study, which focused directly on the barriers to receiving HIV care for individuals whose co-morbid serious mental illness and HIV infection are already known, compared nearly 300 seriously mentally ill and HIV-positive patients in Los Angeles and New York City to patients from the HIV Cost and Services Utilization Study (HCSUS)6 from the same geographic region and with HIV alone. It found that people with serious
mental illness and HIV were more likely to experience barriers to care than those with HIV alone (Personal communication, A. Fremont, RAND Corporation, 2002). Barriers to care were measured by a 3-item index—not getting needed medical care, going without care because of lack of money, or going without food in order to pay for care.
The relationship between mental illness and adherence to HAART has been investigated in several studies, most of which relied on measures of depression or anxiety symptoms or distress rather than psychiatric diagnoses per se. Although not all studies have found a relationship between adherence and psychological well-being, a number of studies have found depressive symptoms, hopelessness, psychological distress, and overall stress to be associated with lower antiretroviral adherence.
Paterson and colleagues (2000) studied 81 HIV patients, and tracked adherence with a microelectronic monitoring system. The study found that active psychiatric illness, primarily depression, was an independent risk factor for nonadherence, and that nonadherence was significantly associated with treatment failure. Catz and colleagues (2000) also found that depression was a risk factor for self-reported nonadherence in a sample of 72 patients at a teaching hospital. A study in Spain by Gordillo and colleagues (1999) of 366 patients also found that depression was a risk factor for poor adherence. Chesney and colleagues (2000), studying 75 patients at 10 United States sites, determined that nonadherent patients reported higher levels of perceived stress. Singh and colleagues (1999), using the Beck Hopelessness Scale and other measures, found that hopelessness and loss of motivation were associated with nonadherence.
One study of serious mental illness and adherence to HAART conducted by investigators at RAND found that about 40 percent of subjects were adherent (more than 90 percent adherence), while 31 percent had very poor adherence (less than 50 percent) (Personal communication, D. Kanouse, RAND Corporation, 2002). The 47 participants in this study had bipolar depression (n=24), schizophrenia (n=12), schizoaffective disorder (n=5), or psychotic depression (n=6). The overall average adherence rate was 66 percent of prescribed doses, a rate similar to general clinic or community populations. The finding that a large percentage of participants were adherent to their drug defied conventional wisdom that individuals with serious mental illness lack the capacity to adhere to a complex dosing schedule. Still, a third of the sample had very poor adherence, a finding that prompted the investigators to suggest further research to identify barriers and inform the development of tailored interventions for those with serious mental illness to achieve greater adherence, and thus greater treatment benefits.
In summary, the research on mental illness and adherence to HAART indicates that symptoms of depression and psychological distress are associ-
ated with lower adherence. There is little research on the relationship between adherence and actual diagnoses of depression or anxiety. One study of serious mental illness finds, contrary to expectations, that people with one of the more serious diagnoses are not necessarily more likely to be nonadherent. Further research on the relationship between mental illness and adherence to HAART would help identify the specific causes of nonadherence in this population and the interventions and approaches that can help promote adherence.
Substance Use Disorders
Another serious challenge for HIV care involves individuals with substance use disorders; treating this population requires a range of services, including substance abuse treatment, linked to primary care. Injection drug use in particular was identified early in the epidemic as a route of transmission, and CDC has conducted public health surveillance on the population of injection drug users as a result. Of the 40,000 new HIV infections each year, an estimated 25 percent are directly attributable to injection drug use. In the eastern seaboard cities, injection drug use accounts for at least half of the AIDS cases. Injection drug use is associated with 26 percent of all AIDS cases among African Americans, 31 percent among Hispanics, and 19 percent among whites. Overall, CDC estimates that injection drug use, directly and indirectly, accounts for 36 percent of AIDS cases in men and 57 percent of cases in women (CDC, 2002a).
Because CDC surveillance only identifies the risk category for infection, it is difficult to determine current rates of substance abuse in HIV-infected populations. Federal data sources such as the National Household Survey on Drug Abuse or Monitoring the Future each estimate the prevalence of substance use, but do not collect information about HIV infection; therefore, the overall estimate of those infected with HIV who also have substance use problems is difficult to determine. From a summary of the published literature, Holmberg (1996) earlier estimated that there were 1.3 million injection drug users in the United States and that 30 percent were HIV infected; broad population-based estimates of HIV infection by different drug and different routes of administration (i.e., other than injection) are not available. One study found that nearly 40 percent of HIV-infected individuals in care reported using an illicit drug other than marijuana and that 12 percent screened positive for drug dependence (Bing et al., 2001). This same study also found that nearly 50 percent of the sample screened positive for mental health disorders and that screening positive for a psychiatric disorder was independently associated with screening positive for drug dependence.
Other data also suggest that substance abuse is common in HIV-infected or at-risk populations. Sullivan et al. (1998) found a high rate of drug use in
a facility-based study of nearly 1,000 MSMs from 12 states and metropolitan areas—51 percent had used marijuana, 31 percent used noninjected cocaine, and 16 percent injected crack cocaine in the five years preceding the interviews. In a study of HIV-positive women, 19.7 percent reported using crack, 15.4 percent reported using cocaine, and 8.8 percent reported injecting drugs within the past six months (Wilson et al., 1999). These data suggest that substance use is a frequent issue in dealing with HIV-infected patient populations.
Despite the need for integrated care for HIV-infected individuals with comorbid substance abuse, there is little intersection, and often conflict, between the two treatment systems. Adding further complexity is the conflict between abstinence-based and harm-reduction treatment approaches within the substance abuse treatment system. The HIV care system, concerned principally with keeping the HIV-infected individual in contact with the system, can be at odds with the abstinence approach to substance abuse treatment, which expels anyone who does not meet the strict standards of a program (Hsu, 2001).
There are many barriers to accessing HIV care for an individual with active substance abuse. The greatest barrier—but certainly not the only one—is active substance abuse itself. Active substance abuse has a demonstrated association with delayed HIV care seeking, making it likely that substance users are overrepresented in the HIV-infected population that is outside of the care system. One study of outpatient visits in two urban hospitals found that 39 percent of patients entering HIV care for the first time had delayed care seeking for one year, 32 percent for more than two years, and 18 percent for more than five years (Samet et al., 1998). In this study, injection drug use was associated with delayed care seeking. In a study of IDUs, Celentano and colleagues (2001) found the delay in initiating HAART was less than one year for active versus former drug users. One study of HIV-positive crack cocaine smokers found that one-third of the study population had not seen a provider for HIV-related care in the past year.
In this context, access to substance abuse treatment is revealed as a priority for this population. There are significant barriers here as well, and it has been estimated that nearly three-quarters of those who need substance abuse treatment do not receive it (Amaro, 1999). One of the greatest barriers to substance abuse treatment is lack of capacity to provide services to all individuals seeking care due to inadequate funding. For PLWH/A seeking substance abuse treatment—a population with especially complex needs—this problem is particularly acute. Co-location of substance abuse treatment and HIV care is difficult because of financing and bureaucratic issues that occur at the institutional and governmental policy levels. Lack of health insurance coverage presents another barrier, particularly for minorities,
recent immigrants, and people who are homeless (Hsu, 2001). Low-income women of color face multiple barriers in that they, too, are more likely to lack health insurance coverage and have dependent children. It is estimated that nearly two-thirds of women of color with HIV infection have at least one child under the age of 20, adding further complexity to their lives and their care (Hsu, 2001; HRSA, 1999).
Once in care, HIV-infected substance abusers face obstacles to staying in care and receiving quality care. Studies have shown that substance abusers are more likely to receive sporadic care in emergency departments. Chronic drug users are less likely to have a regular source of health care and are more likely than nondrug users to utilize emergency room and inpatient care (Markson et al., 1998; Laine et al., 2001; Welch and Morse, 2001). Other studies have shown that substance abusers are less likely to receive HAART than nonusers (Soloman et al., 1998; Celentano et al., 2001; Metsch et al., 2001; Turner et al., 2001). This is partly because of provider beliefs that substance abusers are less likely to adhere to treatment regimens and because of concerns surrounding interactions between HAART drugs and illicit drugs, psychotropic medications, and methadone.
Though active substance abuse is considered a predictor of poor adherence, the evidence indicates that the link is not always clear. Some studies have found an association between active substance use (particularly crack cocaine use) or heavy alcohol abuse and lower adherence (Cook et al., 2001; Hinkin et al., 2002; Mannheimer et al., 2002). Substance abuse, however, may also be associated with depression or other affective disorders that can affect adherence (Ekstrand et al., 2002; Mannheimer et al., 2002; Perry et al., 2002). This association may in turn further complicate adherence, while substance abuse symptoms may mask symptoms of depression or vice versa.
PLWH/A with co-morbid substance abuse and/or mental illness encounter many obstacles to accessing treatment, remaining in care, and adhering to treatment regimens. However, there are interventions that can improve utilization, retention, and adherence rates for these populations. Substance abuse and mental illness often co-occur with one another as well as HIV, and can be the underlying cause of other conditions that complicate HIV care, such as homelessness. Effective management of the HIV epidemic requires that the issues of substance abuse and mental illness be confronted by providing appropriate treatment to those who need it in care settings that are also equipped to provide HIV care.
Co-Morbid Infections
The most common medical co-morbidities associated with HIV are sexually transmitted diseases (STDs), hepatitis C virus (HCV) infection,
and tuberculosis (TB). The presence of an STD as a preexisting condition can lead to heightened risk of HIV infection in three ways: (1) STDs increase the infectivity of HIV, (2) STDs increase susceptibility to HIV infection, and (3) behaviors that lead to increased risk are highly related for HIV and STDs (IOM, 1997). Epidemiological studies suggest that people may be two to five times more likely to become HIV infected when other STDs are present (Levine et al., 1998; Patterson et al., 1998; IOM, 1997). A study of eight United States STD clinics in the early to mid-1990s found an overall prevalence of STDs among HIV-positive individuals of 32.6 percent. Among HIV-infected females, STD prevalence was 25 percent; among males, 35.1 percent; among blacks, 35.1 percent; among whites, 37.8 percent; among Latinos, 20.3 percent; among individuals under age 30, 35.1 percent; and among those older than 30, 31.5 percent (Rothenberg et al., 2000).
Nationally, about 25 percent of all HIV-infected individuals are estimated to have co-morbid HCV infection (CDC, 2002b). The risk is particularly high for injection drug use, the most common means of HCV transmission in the United States (Estrada, 2002). In a study of six drug treatment sites located throughout the United States, rates of hepatitis B and C viruses were consistently about 90 percent for older injection drug users (Murrill et al., 2002). CDC estimates that the rate of coinfection with HIV and HCV among injection drug users ranges from 50 to 90 percent. Comorbid HIV infection is associated with a more swift progression of HCV-related liver disease and cirrhosis, which may lead to limited tolerance for antiretroviral therapy due to hepatic side effects (Sulkowski et al., 2002; Ostrow, 1999; Greenberg, 1999; CDC, 2002b).
Individuals with HIV are especially vulnerable to tuberculosis. Because HIV infection suppresses the body’s immune system, HIV-infected persons are at increased risk of developing TB and, if infected, are 100 times more likely to progress to active TB than those not infected with HIV. CDC estimates that about 15 percent of all TB cases, and 30 percent of cases among individuals ages 25 to 44, occur among HIV-infected individuals (CDC, 1998b). Among injection drug users, CDC estimates that the TB incidence rate for those who are HIV positive is more than seven times that of those who are not (CDC, 1999). This is an area of particular concern because there is evidence that some common HIV and TB treatments may be incompatible and substitutions are cost prohibitive, thus complicating care (Spradling, 2000).
Social Co-Morbidities
As explained earlier in the chapter, the Committee uses the term social co-morbidity to describe an underlying social condition that affects the course of HIV disease in an individual. Social co-morbidities can be one
cause of circumstances that lead to disruption of care or inability to maintain adherence to a treatment regimen.
Homelessness
Homelessness creates challenges to access, adherence, and continuity of care for individuals infected with HIV. Inability to store or access medications, lack of routine medical care, poor nutrition, even the stress of being unstably housed can affect the course of HIV disease.
The experience of 1,445 HIV-infected Medicaid recipients in New York state, where 6 percent were homeless; 24.5 percent were “doubled up,” that is, staying with friends or relatives; and 69.5 percent were stably housed provides an example. The homeless and doubled up were less likely than the stably housed to have a regular source of care, a recent visit to a physician, and HIV-related medications. They were also more likely to use the emergency room for care (Smith et al., 2000). Once administrative databases for homeless shelters and AIDS case reporting were merged in Philadelphia, the incidence of AIDS was observed to be nine times higher in the shelters than the general population. The most predictive factors for AIDS within this homeless population were being male, being a minority (black), having a substance use disorder or mental illness, and lacking insurance (Culhane et al., 2001). In the same study, persons who were newly diagnosed with AIDS were three times more likely than the general population to become homeless during the follow-up period.
The overlap between homeless populations and populations with substance use disorders or mental illness that was demonstrated in the Philadelphia study also has been observed in multiple other studies (D’Amore et al., 2001; Martens, 2001; Rosenblum et al., 2001; Cheung et al., 2002; Kilbourne et al., 2002). Where it was measured, individuals in these studies were also more likely to have HIV, TB, and/or HCV (D’Amore et al., 2001; Rosenblum et al., 2001; Cheung et al., 2002).
Unsupervised Youth
Another group that faces challenges in receiving adequate care is HIV-infected unsupervised youth, usually homeless or runaways. In a Minnesota study of 201 street youth, 37 percent reported having 15 or more alcoholic drinks per week, 37 percent used marijuana three or more times a week, and 15 percent reported having used injection drugs at least once, including 6 percent who used injection drugs within the previous month (Lifson and Halcon, 2001). Underscoring the theme that there is considerable overlap across vulnerable populations, in a sample of HIV-infected adolescents in Washington, D.C., 53 percent had received psychiatric diagnoses prior to
their treatment at the clinic, 50 percent had a documented history of sexual abuse, and 82 percent had a history of substance use (Pao et al., 2000).
Undocumented and Legal Immigrants
Undocumented workers and other immigrants also face barriers to accessing and maintaining continuity of care for HIV. These individuals are especially vulnerable to the barriers presented by language difference, lack of cultural competency, and lack of insurance or other means to pay for care. In a pilot study of undocumented immigrants in southern California, less than 8 percent of the sample accessed nonemergency health care, and high-risk behavior or HIV (although no HIV testing was done) suggested that this population requires closer attention for HIV-related services (Loue and Oppenheim, 1994). Although considerable attention has been paid to immigrants with tuberculosis in the previous decade, HIV infection identification and treatment have not received an equal amount of attention (Weis et al., 2001). A summary of a needs assessment of recent migrants into a Texas county indicated that migration was associated with knowledge barriers for all types of services. Results also showed that recent immigration was a significant predictor of failure to receive government-administered basic services such as food services, but was not a significant predictor of failure to receive community-based organization-administered “specialized” services targeted specifically to HIV-positive individuals (Montoya et al., 1998). This indicates that there are programs and interventions that can reach this population.
Incarcerated Populations
A study conducted by Hammett et al. (2002) used data from 1997 to estimate that up to one-fourth of the people living with HIV in this country pass through a correctional facility each year. A recent assessment of voluntary counseling, testing, and referral (VCTR) in 48 correctional facilities throughout the country resulted in an HIV prevalence of 3.4 percent (Sabin et al., 2001). In one study, 85 percent of HIV infection in prison was associated with injection drug use prior to incarceration (Vlahov et al., 1989). Transmission of HIV infection in prison is rare (Brewer et al., 1988; Horsburgh et al., 1990). Although screening and prevention is not the focus, VCTR illustrates that contact with the correctional health care system can give public health professionals an opportunity to diagnose HIV and provide therapy to a population that might prove difficult to reach otherwise (Hammett et al., 2002; Sabin et al., 2001; Rich et al., 2001).
Because the majority of persons who enter a correctional facility will eventually return to their communities, the manner in which correctional
health services deal with the HIV-infected individual has important implications to the overall care of the HIV-infected population. Routine HIV testing is well accepted as a procedure offered to incoming prison inmates. Combination antiretroviral therapy has been associated with a reduction in mortality in prisons. A link between community HIV specialists and correctional health care providers is an important partnership for ensuring that HIV-infected patients have optimal care both inside prison and after release (Spaulding et al., 2002).
CONCLUSION
The current environment of HIV care is both more hopeful and more complex than it was 20 years ago. The early HIV care system was designed—consciously or not—to manage patients who entered with symptomatic, advanced disease and who died after several years of increasingly untreatable opportunistic diseases. A substantial portion of this time was spent in acute care hospitals and involved use of cumbersome, expensive, and invasive therapies. This model of care no longer applies. The changes in the treated natural history of HIV infection from an acute to a chronic disease model and the shift in populations most affected must be considered when crafting policies for the public financing and delivery of HIV care. The public care system must take advantage of the opportunities offered by effective treatments such as HAART while working to meet the challenges of the new epidemic.
|
Findings:
|
REFERENCES
Amaro H. 1999. An expensive policy: the impact of inadequate funding for substance abuse treatment. American Journal of Public Health 89(5):657–659.
APHA (American Public Health Association). 2002. Adherence to HIV Treatment Regimens: Recommendations for Best Practices, Pp. 126–130. [Online]. Available: http://www.apha.org/PPP/HIV/Best_Practices.pdf [accessed February 18, 2004].
Bangsberg DR, Hecht FM, Charlesbois ED, Zolopa AR, Holodniy M, Sheiner L, Bamberger JD, Chesney MA, Moss A. 2000. Adherence to protease inhibitors, HIV-1 viral load and development of drug resistance in an indigent population. AIDS 14(4):357–366.
Bangsberg DR, Perry S, Charlesbois ED, Clark RA, Robertson M, Zolopa AR, Moss A. 2001. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS 15(9):1181–1183.
Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. 1983. Isolation of a T-lymphocyte retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220(4599):868–871.
Bartlett JA, DeMasi R, Quinn J, Moxham C, Rousseau F. 2001. Overview of the effectiveness of triple combination therapy in antiretroviral-naïve HIV-1 infected adults. AIDS 15(11):1369–1377.
Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Turner BJ, Eggan F, Beckman R, Vitiello B, Morton SC, Orlando M, Bozzette SA, Ortiz-Barron L, Shapiro M. 2001. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Archives of General Psychiatry 58(8):721–728.
Bogart LM, Kelly JA, Catz SL, Sosman JM. 2000. Impact of medical and nonmedical factors on physician decision making for HIV/AIDS antiretroviral treatment. Journal of Acquired Immune Deficiency Syndromes 23(5):396–404.
Bozzette SA, Berry SH, Duan N, Frankel MR, Leibowitz AA, Lefkowitz D, Emmons CA, Senterfitt JW, Berk ML, Morton SC, Shapiro MF. 1998. The care of HIV-infected adults in the United States. HIV Cost and Services Utilization Study Consortium. New England Journal of Medicine 339(26):1897–1904.
Brewer TF, Vlahov D, Taylor E, Hall D, Munoz A, Polk BF. 1988. Transmission of HIV-1 within a statewide prison system. AIDS 2(5):363–367.
Brunette MF, Mercer CC, Carlson CL, Rosenberg SD, Lewis BF. 2000. HIV-related services for persons with severe mental illness: policy and practice in New Hampshire community mental health. Journal of Behavioral Health Services & Research 27(3):347–353.
Carey MP, Carey KB, Kalichman SC. 1997. Risk for human immunodeficiency virus (HIV) infection among persons with severe mental illnesses. Clinical Psychology Review 17(3):271–291.
Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. 1999. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidemia, and diabetes mellitus: a cohort study. The Lancet 353:2093–2099.
Catz SL, Kelly JA, Bogart LM, Benotsch EG, McAuliffe TL. 2000. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychology 19(2):124–333.
CDC (Centers for Disease Control and Prevention). 1981. Pneumocystis pneumonia—Los Angeles. MMWR 30(21):1–3.
CDC. 1982a. Epidemiologic notes and reports update on Kaposi’s sarcoma and opportunistic infections in previously healthy persons—United States. MMWR 31(22):294, 300–301. Atlanta, GA: CDC
CDC. 1982b. Opportunistic infections and Kaposi’s sarcoma among Haitians in the United States. MMWR 31(26):353–354, 360–361. Atlanta, GA: CDC
CDC. 1982c. Current trends update on acquired immune deficiency syndrome (AIDS)—United States. MMWR 31(37):507–508, 513–514. Atlanta, GA: CDC.
CDC. 1982d. Current trends acquired immune deficiency syndrome (AIDS): precautions for clinical and laboratory staffs. MMWR 31(43):577–580. Atlanta, GA: CDC.
CDC. 1982e. Update on acquired immune deficiency syndrome (AIDS) among patients with hemophilia A. MMWR 31(48):644–646, 652. Atlanta, GA: CDC.
CDC. 1983. Acquired immunodeficiency syndrome (AIDS): precautions for health-care workers and allied professionals. MMWR 32(34):450–451. Atlanta, GA: CDC.
CDC. 1994. HIV/AIDS Surveillance Report, 1993 5(4):1–33. Atlanta, GA: CDC.
CDC. 1998a. Report of the NIH panel to define principles of therapy of HIV infection. MMWR 47(RR–5):1–41. Atlanta, GA: CDC.
CDC. 1998b. Prevention and treatment of tuberculosis among patients infected with human immunodeficiency virus: principles of therapy and revised recommendations. MMWR 47(RR20):1–51. Atlanta, GA: CDC.
CDC. 1999. The Deadly Intersection Between TB and HIV. [Online]. Available: http://www.cdc.gov/hiv/pubs/facts/hivtb.htm [accessed November 1, 2003].
CDC. 2002a. HIV/AIDS Surveillance Report, 2001 Year-End Edition 13(2). Atlanta, GA: CDC.
CDC. 2002b. Frequently Asked Questions and Answers About Coinfection with HIV and Hepatitis C Virus. [Online]. Available: http://www.cdc.gov/hiv/pubs/facts/HIV-HCV_Coinfection.htm [accessed November 1, 2003].
CDC. July 28, 2003. HIV Diagnoses Climbing Among Gay and Bisexual Men. Press release. CDC Office of Communications. Atlanta, GA: CDC.
CDC. 2004. Cumulative AIDS Cases–Dot Maps. [Online]. Available at: http://www.cdc.gov/hiv/graphics/dotmaps.htm [accessed July 21, 2005].
Celentano D, Galai N, Sethi AK, Shah NG, Strathdee SA, Vlahov D, Gallant JE. 2001. Time to initiating highly active antiretroviral therapy among HIV-infected injection drug users. AIDS 15(13):1707–1715.
Chesney MA. 2003. Adherence to HAART regimens. AIDS Patient Care and Standards 17(4):169–177.
Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, Wu AW. 2000. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. Patient Care Committee & Adherence Working Group (AACTG). AIDS Care 12(3):255–266.
Cheung RC, Hanson AK, Maganti K, Keeffe EB, Matsui SM. 2002. Viral hepatitis and other infectious diseases in a homeless population. Journal of Clinical Gastroenterology 34(4):476–480.
Chiasson MA, Berenson L, Li W, Schwartz S, Singh T, Forlenza S, Mojica BA, Hamburg MA. 1999. Declining HIV/AIDS mortality in New York City. Journal of Acquired Immune Deficiency Syndromes 21(1):59–64.
Cook RL, Sereika SM, Hunt SC, Woodward WC, Erlen JA, Conigliaro J. 2001. Problem drinking and medication adherence among persons with HIV infection. Journal of General Internal Medicine 16(2):83–88.
Cournos F, McKinnon K. 1997. HIV seroprevalence among people with severe mental illness in the United States: a critical review. Clinical Psychology Review 17(3):259–269.
Crepaz N, Marks G. 2001. Are negative affective states associated with HIV sexual risk behaviors? A meta-analytic review. Health Psychology 20(4):291–299.
Culhane DP, Gollub E, Kuhn R, Shpaner M. 2001. The co-occurrence of AIDS and homelessness: results from the integration of administrative databases for AIDS surveillance and public shelter utilization in Philadelphia. Journal of Epidemiology and Community Health 55(7):515–520.
D’Amore J, Hung O, Chiang W, Goldfrank L. 2001. The epidemiology of the homeless population and its impact on an urban emergency department. Academic Emergency Medicine 8(11):1051–1055.
Detels R, Munoz A, McFarlane G, Kingsley LA, Margolick JB, Giorgi J, Schrager LK, Phair JP. 1998. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection durations. Journal of the American Medical Association 280(17):1497–1503.
DHHS (U.S. Department of Health and Human Services). 1999. Mental Health: A Report of the Surgeon General. Rockville, MD: DHHS, Substance Abuse and Mental Health Services Administration, Center for Mental Health Services; National Institutes of Health, National Institute of Mental Health.
DHHS. 2004. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. [Online]. Available: http://aidsinfo.nih.gov/guidelines/default_db2.asp?id=50 [accessed April 15, 2004].
Ekstrand M, Crosby GG, Paul J, Martin K, Bangsberg D, Stall RD. 2002 (July 7-12). Psychological Distress, Not Alcohol or Drug Use, Associated With ART Non-Adherence. Paper presented at the XIV International AIDS Conference, Barcelona, Spain.
Escobar I, Campo M, Martin J, Fernandez-Shaw C, Pulido F, Rubio R. 2003. Factors affecting patient adherence to highly active antiretroviral therapy. Annals of Pharmacotherapy 37(6):775–781.
Estrada AL. 2002. Epidemiology of HIV/AIDS, hepatitis B, hepatitis C, and tuberculosis among minority injection drug users. Public Health Reports 117(Suppl. 1):S126–S134.
Fauci AS. 1993. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science 262:1011–1018.
FDA (Food and Drug Administration). 2003a, January 31. HHS Extends Use of Rapid HIV Test to New Site Nationwide. News release. FDA Press Office. Washington, DC.
FDA. 2003b, March 13. FDA Approves First Drug in New Class of HIV Treatments for HIV Infected Adults and Children With Advanced Disease. News Release. FDA Press Office. Washington, DC.
Garcia de Olalla P, Knobel H, Carmona A, Guelar A, Lopez-Colomes J, Cayla JA. 2002. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. Journal of Acquired Immune Deficiency Syndromes 30(1):105–110.
Gebo KA, Keruly J, Moore RD. 2003. Association of social stress, illicit drug use, and health benefits with nonadherence to antiretroviral therapy. Journal of General Internal Medicine 18(2):104–111.
Golin CE, Liu H, Hays RD, Miller LG, Beck CK, Ickovics J, Kaplan AH, Wenger NS. 2002. A prospective study of predictors of adherence to combination antiretroviral medication. Journal of General Internal Medicine 17(10):756–765.
Gordillo V, del Amo J, Soriano V, Gonzalez-Lahoz J. 1999. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. AIDS 13(13):1763–1769.
Gottlieb MS. 2001. AIDS—past and future. New England Journal of Medicine 344(23):1788–1791.
Grant RM, Hecht FM, Warmerdam M, Liu L, Leigler T, Petropoulos CJ, Hellmann NS, Chesney M, Busch MP, Kahn JO. 2002. Time trends in primary HIV-1 drug resistance among recently infected persons. Journal of the American Medical Association 288(2):181–188.
Greenberg B, Berkman A, Thomas R, Hoos D, Finkelstein R, Astemborski J, Vlahov D. 1999. Evaluating supervised HAART in late-stage HIV among drug users: a preliminary report. Journal of Urban Health 76(4):468–480.
Hahn BH, Shaw GM, De Cock KM, Sharp PM. 2000. AIDS as a zoonosis: scientific and public health implications. Science 287(5453):607–614.
Hammett TM, Harmon MP, Rhodes W. 2002. The burden of infectious disease among inmates of and releasees from U.S. correctional facilities, 1997. American Journal of Public Health 92(11):1789–1794.
Harrigan PR, Dong WY, Alexander C, Yip B, Ting L, Wynhoven B, Woodward J, Mo T, Hogg R, Montaner J. 2003. The association between drug resistance and adherence determined by two independent methods in a large cohort of drug naïve individuals starting triple therapy. The 2nd International AIDS Society Conference on HIV Pathogenesis and Treatment. Abstract no. LB12. [Online]. Available: http://www.ias.se/abstract/show.asp?abstract_id=11066 [accessed September 28, 2003].
Haynes BF, Pantaleo G, Fauci AS. 1996. Toward an understanding of the correlates of protective immunity to HIV infection. Science. 271(5247):324–328.
Hinkin C, Castellon S, Lam MN, Hardy DJ, Stefaniak M, Farchione T, Ropacki M, Thrasher D, Mason K, Schug R, Durvasula RS. 2002 (July 7–12). Predictors of Medication Adherence in HIV/AIDS. Paper presented at the XIV International AIDS Conference, Barcelona, Spain. [Online]. Available at: http://www.aegis.com/conferences/14wac/WePeB5828.html [accessed March 16, 2003].
Hirschfeld RM, Keller MB, Panico S, Arons BS, Barlow D, Davidoff F, Endicott J, Froom J, Goldstein M, Gorman JM, Marek RG, Maurer TA, Meyer R, Phillips K, Ross J, Schwenk TL, Sharfstein SS, Thase ME, Wyatt RJ. 1997. The National Depressive and Manic–Depressive Association consensus statement on the undertreatment of depression. Journal of the American Medical Association 277(4):333–340.
HIV/AIDS Treatment Information Service. 1999. Glossary of HIV/AIDS Related Terms. Third Edition. [Online]. Available: http://www.thebody.com/hivatis/glossary/contents.html [accessed March 11, 2003].
Holmberg SD. 1996. The estimated prevalence and incidence of HIV in 96 large U.S. metropolitan areas. American Journal of Public Health 86(5):642–654.
Horsburgh CR Jr, Jarvis JQ, McArthur T, Ignacio T, Stock P. 1990. Seroconversion to human immunodeficiency virus in prison inmates. American Journal of Public Health 80(2):209–210.
HRSA (Health Resources and Services Administration). 1999. HRSA Care Action: HIV Disease in Women of Color. Rockville, MD: HRSA.
Hsu LC, Vittinghoff E, Katz MH, Schwarcz SK. 2001. Predictors of use of highly active antiretroviral therapy (HAART) among persons with AIDS in San Francisco, 1996–1999. Journal of Acquired Immune Deficiency Syndromes 28(4):345–350.
Ickovics JR, Meisler AW. 1997. Adherence in AIDS clinical trials: a framework for clinical research and clinical care. Journal of Clinical Epidemiology 50(4):385–391.
IOM (Institute of Medicine). 1997. The Hidden Epidemic: Confronting Sexually Transmitted Diseases. Eng T, Butler W, eds. Washington, DC: National Academy Press.
Jacobson LP, Yamashita TE, Detels R, Margolick JB, Chmiel JS, Kingsley LA, Melnick S, Munoz A, for the Multicenter AIDS Cohort Study. 1999. Impact of potent antiretroviral therapy on the incidence of Kaposi’s sarcoma and non-Hodgkin’s lymphoma among HIV-1-infected individuals. Journal of Acquired Immune Deficiency Syndromes 21(Suppl 1):S34–S41.
Johnson DL. 1997. Overview of severe mental illness. Clinical Psychology Review 17(3): 247–257.
Kahn JO, Walker BD. 1998. Acute human immunodeficiency virus type 1 infection. New England Journal of Medicine 339(1):33–39.
Kaiser Family Foundation. 2002 (November 13–15). HIV/AIDS and Other Sexually Transmitted Diseases (STDs) in the Southern Region of the United States: Epidemiological Overview. Paper presented at the Southern States Summit on HIV/AIDS and STDs: A Call to Action, Charlotte, NC.
Kalichman SC, Ramachandran B, Catz S. 1999. Adherence to combination antiretroviral therapies in HIV patients of low health literacy. Journal of General Internal Medicine 14:276–273.
Karon JM, Fleming PL, Steketee RW, De Cock KM. 2001. HIV in the United States at the turn of the century: an epidemic in transition. American Journal of Public Health 91(7):1060–1068.
Kilbourne AM, Herndon B, Andersen RM, Wenzel SL, Gelberg L. 2002. Psychiatric symptoms, health services, and HIV risk factors among homeless women. Journal of Health Care for the Poor and Underserved 13(1):49–65.
Korber B, Muldoon M, Theiler J, Gao F, Gupta R, Lapedes A, Hahn BH, Wolinsky S, Bhattacharya T. 2000. Timing the ancestor of the HIV-1 pandemic strains. Science 288(5472):1789–1796.
Laine C, Hauck WW, Gourevitch MN, Rothman J, Cohen A, Turner BJ. 2001. Regular outpatient medical drug abuse care and subsequent hospitalization of persons who use illicit drugs. Journal of the American Medical Association 285(18):2355–2362.
Ledergerber B, Egger M, Opravil M, Telenti A, Hirschel B, Battegay M, Vernazza P, Sudre P, Flepp M, Furrer H, Francioli P, Weber R. 1999. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. The Lancet 353:863–868.
Levi J., Hidalgo J. 2001. Financing and delivery of HIV care. Minority Health Today: Mobilizing to Fight HIV/AIDS in the African-American Community. April 2001 Supplement: 33–43.
Levine WC, Pope V, Bhoomkar A, Tambe P, Lewis JS, Zaidi AA, Farshy CE, Mitchell S, Talkington DF. 1998. Increase in endocervical CD4 lymphocytes among women with nonulcerative sexually transmitted diseases. Journal of Infectious Diseases 177(1):167–174.
Levy JA. 1993. Pathogenesis of human immunodeficiency virus infection. Microbiological Reviews 57(1):183–289.
Lifson AR, Halcon LL. 2001. Substance abuse and high-risk needle-related behaviors among homeless youth in Minneapolis: implications for prevention. Journal of Urban Health 78(4):690–698.
Little SJ, Holte S, Routy JP, Daar ES, Markowitz M, Collier AC, Koup RA, Mellors JW, Connick E, Conway B, Kilby M, Wang L, Whitcomb JM, Hellmann NS, Richman DD. 2002. Antiretroviral-drug resistance among patients recently infected with HIV. New England Journal of Medicine 347(6):385–394.
Liu H, Golin CE, Miller LG, Hays RD, Beck K, Sanandaji S, Christian J, Maldonado T, Duran D, Kaplan AH, Wenger NS. 2001. A comparison study of multiple measures of adherence to HIV protease inhibitors. Annals of Internal Medicine 134(10):968–977.
Loue S, Oppenheim S. 1994. Immigration and HIV infection: a pilot study. AIDS Education and Prevention 6(1):74–80.
Lucas GM, Gebo KA, Chaisson RE, Moore RD. 2002. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS 16(5):767–774.
Malcolm SE, Ng JJ, Rosen RK, Stone VE. 2003. An examination of HIV/AIDS patients who have excellent adherence to HAART. AIDS Care 15(2):251–261.
Mannheimer S, Perkins M, Diamond B, Ford J, Holson N, Findley S, Colson P, El-Sadr W. 2002. (July 7-12). Predictors of Adherence to Antiretroviral Therapy in an Inner City Population. Paper presented at the XIV International AIDS Conference, Barcelona, Spain.
Markson LE, Houchens R, Fanning TR, Turner BJ. 1998. Repeated emergency department use by HIV infected persons: effect of clinic accessibility and expertise in HIV care. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 17(1):35–41.
Martens WH. 2001. A review of physical and mental health in homeless persons. Public Health Reviews 29(1):13–33.
McNabb J, Ross JW, Abriola K, Turley C, Nightingale CH, Nicolau DP. 2001. Adherence to highly active antiretroviral therapy predicts virologic outcome at an inner-city human immunodeficiency virus clinic. Clinical Infectious Diseases 33(5):700–705.
Mellors JW, Rinaldo CR Jr, Gupta P, White RM, Todd JA, Kingsley LA. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272(5265):1167–1170.
Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, Kingsley LA, Todd JA, Saah AJ, Detels R, Phair JP, Rinaldo CR Jr. 1997. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Annals of Internal Medicine 126(12):946–954.
Metsch L, Pereyra M, Brewer TH. 2001. Use of HIV health care in HIV-seropositive crack cocaine smokers and other active drug users. Journal of Substance Abuse Treatment 13(1–2):155–167.
Meyer I, Empfield M, Engel D, Cournos F. 1995. Characteristics of HIV-positive chronically mentally ill inpatients. The Psychiatric Quarterly 66(3):201–207.
Miller V, Sabin CA, Phillips AN, Rottmann C, Rabenau H, Weidmann E, Rickerts V, Findhammer S, Helm EB, Staszewski S. 2000. The impact of protease inhibitor-containing highly active antiretroviral therapy on progression of HIV disease and its relationship to CD4 and viral load. AIDS 14(14):2129–2136.
Montessori V, Press N, Harris M, Akagi L, Montaner JSG. 2004. Adverse effects of antiretroviral therapy for HIV infection. Canadian Medical Association Journal 170(2): 229–238.
Montoya ID, Bell DC, Richard AJ, Goodpastor WA, Carlson J. 1998. Barriers to social services for HIV-infected urban migrators. AIDS Education and Prevention 10(4): 366–379.
Murrill CS, Weeks H, Castrucci BC, Weinstock HS, Bell BP, Spruill C, Gwinn M. 2002. Age-specific seroprevalence of HIV, hepatitis B virus, and hepatitis C virus infection among injection drug users admitted to drug treatment in 6 US cities. American Journal of Public Health 92(3):385–387.
NCHS (National Center for Health Statistics). 2001. National Vital Statistics Report 49(11).
NIC (National Intelligence Council). 2002. Next Wave of HIV/AIDS: Nigeria, Ethiopia, Russia, India, and China. Washington, DC: National Intelligence Council.
Nolan D. 2003. Metabolic complications associated with HIV protease inhibitor therapy. Drugs 63(23):2555–2574.
NRC (National Research Council). 1993. The Social Impact of AIDS in the United States. Jonsen AR, Stryker J, eds. Washington, DC: National Academy Press.
Ostrow D. 1999. Practical prevention issues. In: Ostrow D, Kalichman S, eds. Psychosocial and Public Health Impacts of New HIV Therapies. New York: Kluwer Academic/Plenum.
Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigator. New England Journal of Medicine 338(13):853–860.
Pantaleo G, Graziosi C, Demarest JF, Butini L, Montroni M, Fox CH, Orenstein JM, Kotler DP, Fauci AS. 1993. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature 362(3418):355–358.
Pao M, Lyon M, D’Angelo LJ, Schuman WB, Tipnis T, Mrazek DA. 2000. Psychiatric diagnoses in adolescents seropositive for the human immunodeficiency virus. Archives of Pediatric & Adolescent Medicine 154(3):240–244.
Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, Wagener MM, Singh N. 2000. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine 133(1):21–30.
Patterson BK, Landay A, Andersson J, Brown C, Behbahani H, Jiyamapa D, Burki Z, Stanislawski D, Czerniewski MA, Garcia P. 1998. Repertoire of chemokine receptor expression in the female genital tract: Implications for human immunodeficiency virus transmission. American Journal of Cardiovascular Pathology 153(2):481–490.
Perry S, Bangsberg DB, Charlebois ED, Clark R, Karasic D, Dilley J, Sorensen J, Moss AR. 2002 (July 7–12). Depressive Symptoms Predict Adherence, Treatment Duration and Survival in an Urban Poor Cohort. Paper presented at the XIV International AIDS Conference, Barcelona, Spain. Abstract no. TuPeC4724.
Pezzotti P, Galai N, Vlahov D, Rezza G, Lyles CM, Astemborski J. 1999. Direct comparison of time to AIDS and infectious disease death between HIV seroconverter injection drug users in Italy and the United States: results from the ALIVE and ISS studies. AIDS Link to Intravenous Experiences. Italian Seroconversion Study. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 20(3):275–282.
Piliero PJ, Colagreco JP. 2003. Simplified regimens for treating HIV infection and AIDS. Journal of the American Academy of Nurse Practitioners 15(7):305–312.
Polk BF, Fox R, Brookmeyer R, Kanchanaraksa S, Kaslow R, Visscher B, Rinaldo C, Phair J. 1987. Predictors of the acquired immunodeficiency syndrome developing in a cohort of seropositive homosexual men. New England Journal of Medicine 316(2):61–66.
Quinn TC. 1997. Acute primary HIV infection. Journal of the American Medical Association 278(1):58–62.
Rabkin JG, Chesney M. 1999. Treatment adherence to HIV medications: the Achilles heel of the new therapeutics. In: Ostrow DG, Kalichman SC, eds. Psychosocial and Public Health Impacts of New HIV Therapies. New York: Kluwer Academic/Plenum.
Rich JD, Holmes L, Salas C, Macalino G, Davis D, Ryczek J, Flanigan T. 2001. Successful linkage of medical care and community services for HIV-positive offenders being released from prison. Journal of Urban Health 78(2):279–289.
Rosenblum A, Nuttbrock L, McQuistion HL, Magura S, Joseph H. 2001. Hepatitis C and substance use in a sample of homeless people in New York City. Journal of Addictive Diseases 20(4):15–25.
Rothenberg RB, Wasserheit JN, St. Louis ME, Douglas JM. 2000. The effect of treating sexually transmitted diseases on the transmission of HIV in dually infected persons: a clinic-based estimate. Ad Hoc STD/HIV Transmission Group. Sexually Transmitted Diseases 27(7):411–416.
Sabin KM, Frey RL, Horsley R, Greby SM. 2001. Characteristics and trends of newly identified HIV infections among incarcerated populations: CDC HIV voluntary counseling, testing, and referral system, 1992–1998. Journal of Urban Health 78(2):241–255.
Samet JH, Freedberg KA, Stein MD, Lewis R, Savetsky J, Sullivan L, Levenson SM, Hingson R. 1998. Trillion virion delay: time from testing positive for HIV to presentation for primary care. Archives of Internal Medicine 158(7):734–740.
Schacker TW, Hughes JP, Shea T, Coombs RW, Corey L. 1998. Biological and virologic characteristics of primary HIV infection. Annals of Internal Medicine 128(8):613–620.
Seligmann M, Warrell DA, Aboulker JP, Carbon C, Darbyshire JH, Dormont J, Eschwege E, Girling DJ, James DR, Levy JP, Peto TEA, Schwarz D, Stone AB, Weller IVD, Withnall R, Gelmon K, Lafon E, Swart AM, Aber VR. 1994. Concorde: MRC/ANRS randomized double-blind controlled trial of immediate and deferred zidovudine in symptom-free HIV infection. The Lancet 343:871–881.
Sepkowitz KA. 2001. AIDS—the first 20 years. New England Journal of Medicine 344(23):1764–1772.
Shapiro MF, Morton SC, McCaffrey DF, Senterfitt JW, Fleishman JA, Perlman JF, Athey LA, Keesey JW, Goldman DP, Berry SH, Bozzette SA. 1999. Variations in the care of HIV-infected adults in the United States: results from the HIV Cost and Services Utilization Study. Journal of the American Medical Association 281(24):2305–2315.
Singh N, Berman SM, Swindells S, Justis JC, Mohr JA, Squier C, Wagener MM. 1999. Adherence of human immunodeficiency virus-infected patients to antiretroviral therapy. Clinical Infectious Diseases 29(4):824–830.
Smith MY, Rapkin BD, Winkel G, Springer C, Chhabra R, Feldman IS. 2000. Housing status and health care service utilization among low-income persons with HIV/AIDS. Journal of General Internal Medicine 15(10):731–738.
Solomon L, Stein M, Flynn C, Schuman P, Schoenbaum E, Moore J, Holmberg S, Graham NM. 1998. Health services use by urban women with or at risk for HIV-1 infection: the HIV Epidemiology Research Study (HERS). Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 17(3):253–261.
Spaulding A, Stephenson B, Macalino G, Ruby W, Clarke JG, Flanigan TP. 2002. Human immunodeficiency virus in correctional facilities: a review. Clinical Infectious Diseases 35(3):305–312.
Spradling P. 2000 (July 9–14). Concurrent Use of Rifabutin and HAART: Evidence for Reduced Efficacy? Abstract TUOR B8277. XIII International AIDS Conference, Durban, South Africa.
Stone VE. 2002. Enhancing adherence to antiretrovirals: strategies and regimens. Medscape General Medicine 4(3). [Online]. Available: http://www.medscape.com/viewarticle/438193 [accessed June 21, 2003].
Stoskopf CH, Richter DL, Kim YK. 2001. Factors affecting health status in African Americans living with HIV/AIDS. AIDS Patient Care and STDS 15(6):331–338.
Sulkowski MS, Moore RD, Mehta SH, Chaisson RE, Thomas DL. 2002. Hepatitis C and progression of HIV disease. Journal of the American Medical Association 288(2):199–206.
Sullivan P, Nakashima A, Purcell DW, Ward JW. 1998. Geographic differences in noninjection and injection substance use among HIV-seropositive men who have sex with men: western United States versus other regions. Supplement to HIV/AIDS Surveillance Study Group. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 19(3):266–273.
Sullivan G, Koegel P, Kanouse DE, Cournos F, McKinnon K, Young AS, Bean D. 1999. HIV and people with serious mental illness: the public sector’s role in reducing HIV risk and improving care. Psychiatric Services 50(5):648–652.
Tucker JS, Kanouse DE, Miu A, Kogel P, Sullivan G. 2003. HIV risk behaviors and their correlates among HIV-positive adults with serious mental illness. AIDS and Behavior 7(1):29–40.
Turner BJ, Fleishman JA, Wenger N, London AS, Burnam MA, Shapiro MF, Bing EG, Stein MD, Longshore D, Bozzette SA. 2001. Effects of drug abuse and mental disorders on use and type of antiretroviral therapy in HIV-infected persons. Journal of General Internal Medicine 16(9):625–633.
United Nations. 2003. World Population Prospects: The 2002 Revision. New York: Population Division, Department of Economic and Social Affairs, United Nations.
U.S. Census Bureau. 2000. U.S. Census 2000. Washington, DC: U.S. Bureau of the Census.
USPHS/IDSA (U.S. Public Health Service/Infectious Diseases Society of America) Prevention of Opportunistic Infections Working Group. 2001. 2001 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. HIV Clinical Trials 2(6):493–554.
Vlahov D, Brewer F, Munoz A, Hall D, Taylor E, Polk BF. 1989. Temporal trends of human immunodeficiency virus type 1 (HIV-1) infection among inmates entering a statewide prison system, 1985–1987. Journal of Acquired Immune Deficiency Syndromes 2(3):283–290.
Vlahov D, Graham N, Hoover D, Flynn C, Bartlett JG, Margolick JB, Lyles CM, Nelson KE, Smith D, Holmberg S, Farzadegan H. 1998. Prognostic indicators for AIDS and infectious disease death in HIV-infected injection drug users: plasma viral load and CD4+ cell count. Journal of the American Medical Association 279(1):35–40.
Volberding PA, Lagakos SW, Koch MA, Pettinelli C, Myers MW, Booth DK, Balfour HH Jr, Reichman RC, Bartlett JA, Hirsch MS. 1990. Zidovudine in asymptomatic human immunodeficiency virus infection. A controlled trial in persons with fewer that 500 CD4-positive cells per cubic millimeter. The AIDS Clinical Trials Group of the National Institute of Allergy and Infectious Diseases. New England Journal of Medicine 322(14):941–949.
Weis SE, Moonan PK, Pogoda JM, Turk L, King B, Freeman-Thompson S, Burgess G. 2001. Tuberculosis in the foreign-born population of Tarrant county, Texas by immigration status. American Journal of Respiratory and Critical Care Medicine 164(6):953–957.
Welch K, Morse A. 2001. Survival patterns among HIV+ individuals based on health care utilization. Journal of the National Medical Association 93(6):214–219.
Wensing AMJ, van de Viver DAMC, Asjo B, Balotta C, Camacho R, de Luca A, de Mendoza C, Deroo S, Derdelinckx I, Grossman Z, Hamouda O, Hatzakis A, Hoepelman A, Horban A, Korn K, Kuecherer C, Leitner T, Nielsen C, Ormasson V, Perrin L, Paraskevix D, Puchhammer E, Roman F, Ruiz L, Salminen M, Schmit JCC, Soriano V, Stanczak G, Stanojevic M, Vandamme AM, Van Laethem K, Violin M, Yerly S, Zazzi M, Boucher CAB. 2003. Analysis from more than 1600 newly diagnosed patients with HIV from 17 European countries shows that 10% of the patients carry primary drug resistance: The CATCH-Study. The 2nd International AIDS Society Conference on HIV Pathogenesis and Treatment. Abstract no. LB01. [Online]. Available: http://www.ias.se/abstract/show.asp?abstract_id=111122 [accessed September 28, 2003].
Wilson TE, Massad LS, Riester KA, Barkan S, Richardson J, Young M, Gurtman A, Greenblatt R. 1999. Sexual, contraceptive, and drug use behaviors of women with HIV and those at high risk for infection: results from the Women’s Intergency HIV Study. AIDS 13(5):591–598.