1
Introduction
The outlook for women with breast cancer has improved inrecent years. Because of the combination of improved treatments and the benefits of mammography screening, breast cancer mortality has decreased steadily since 1989.2 And yet, closer scrutiny reveals a complicated picture. Some breast cancers are lethal, and each year about 40,000 women and 400 men die from breast cancer in the United States. Others are not fatal, and some women diagnosed with breast cancer will needlessly undergo mastectomies, chemotherapy, and radiation. But no one knows which breast cancers are destined to be lethal and which are not, because too little is known about the cellular processes that determine cancer progression, and the diagnostic tools used today cannot distinguish small preinvasive lesions that will progress from those that will not. For now, all breast cancers must be viewed as if they may be fatal. This means treatments for invasive breast cancer that range from uncomfortable (lumpectomy) to grueling (chemotherapy, radiation, and mastectomy), none of which guarantees a cure.
Although therapy for breast cancer has improved over the years, it is clear that there are no reliable ways of preventing this cancer. It is also clear that treatments are generally more effective when breast tumors are small and localized than when they are large and have already invaded other tissues. Even the drastic measure of undergoing a double mastectomy will substantially reduce the risk but will not completely eliminate the possibility of developing breast cancer. The limited methods for preventing and treating breast cancers leave early detection as the most promising approach for reducing morbidity and mortality from breast cancer.
A SAFETY NET
Mammography is a safety net that saves thousands of lives each year, yet thousands more slip through that net (see Box 1-1). Many women who would benefit from mammography do not undergo regular screening. Others who do undergo regular screening develop breast cancers that were not detected by their mammography exam. Additionally, then there are others whom screening mammography is unlikely to benefit, such as those who have no access to treatment or whose breast cancer is unresponsive to treatment even when detected early.
The goal of screening for breast cancer is not to detect all breast abnormalities; the goal is to prevent deaths from breast cancer. Thus the benefits of mammography depend on the availability of effective treatment. Despite the common misconception, screening mammography does not benefit women by reducing their risk of breast cancer, but rather by reducing mortality through detecting breast cancer at earlier and more treatable stages.
|
BOX 1-1 In 2002, approximately 60.5 percent of women aged 40 to 64 received mammograms in the United States. Based on U.S. Census Bureau data for 2002, this means that:
An estimated 15,300 women aged 40 to 64 died of breast cancer in 2003. The risk of breast cancer rises steeply with age, but the use of mammography screening increases much less with age. Approximately 63.8 percent of women 65 and over received mammograms in the United States in 2002. Based on Census Bureau data for 2002, this means that:
An estimated 23,000 women over 65 died of breast cancer in 2003. It is generally several years from the time a lethal breast cancer is first detected and the time of death. In most cases, women who die of breast cancer in a particular year are not the same women who receive a screening mammogram or even are first diagnosed that year. SOURCE: U.S. Census Data for July 2003; Cancer Prevention and Early Detection Facts and Figures, ACS 2004. |
Although mammography saves lives, it is not perfect. Depending on the study, the sensitivity of screening mammography ranges from 83 to 95 percent, which means that as many as 17 percent of cancer cases may go undetected by mammography. As many as three-quarters of the breast lesions that are biopsied in the United States as a result of a suspicious mammogram are benign.9,11 More effective approaches to the early detection and diagnosis of breast cancer would go a long way toward improving the care of women concerned about their risk of breast cancer—both by reducing the number of false alarms and unnecessary biopsies and by decreasing the number of cases that go undetected.
BEYOND MAMMOGRAPHY
There are several potential ways to improve detection of breast cancer: more widespread use of mammography, better quality mammography, or development of new technologies. Of these three, greater use of mammography, as it now exists, even though it remains an imperfect screening technology, would likely save the most lives in the short run.
Although a number of new technologies are poised to expand the suite of current options, most advances are likely to be incremental improvements in existing technologies. The most significant technology changes to be adopted in clinical practice since 2001, when the Institute of Medicine and the National Research Council’s Mammography and Beyond report was published, have been improvements in existing technology. Four new digital mammography systemsa and three new systems for computer-aided detectionb were approved by the Food and Drug Administration and are all on the market now. Thus far, the accuracy of digital mammography has been shown to be equivalent to, but not superior to, traditional film-screen mammography, although clinical studies are still under way.14
There have also been changes relevant to breast cancer detection that are only indirectly related to technology developments. First, the sense of crisis concerning the shortage of breast imagers has deepened, and mammography facilities continue to close. Second, the Health Insurance Portability and Accountability Act Privacy Rule took effect in April 2003 and is a source of great concern to those conducting certain types of clinical studies. Third, the extent to which ductal carcinoma in situ (DCIS) cases are overtreated remains unclear, but the most recent data suggest that until it is
possible to predict the outcome of individual cases of DCIS, the wisest course for mammographically detected DCIS is full diagnostic workup and treatment. Another change since the publication of Mammography and Beyond is that the storm over Goetzsche and Olsen’s criticism of the value of screening mammography has largely subsided. Every expert organization that has reviewed their critique together with other published data has concluded that the evidence indicates that screening mammography saves lives.
Other promising new technologies might improve the early detection and diagnosis of breast cancer, but none are ready for widespread clinical use. Some are based on advances in imaging technology, others on advances in molecular biology, and still others on a combination of both. Certain innovations might someday allow the stages in the traditional care pathway to be telescoped. For example, a blood test that identified cancer risk might also detect active cancer, if present, thereby creating a direct link between prescreening and diagnosis. Another blood test might determine that a woman’s risk of breast cancer is so low that yearly mammograms might not be necessary.
INVESTING IN RESEARCH
Fostering the invention and early stage development of medical technology is essential and depends on the nurturing of basic medical research. With the possible exception of AIDS, breast cancer research receives more funding than any other disease—due, in no small part, to the long-standing and tireless efforts of breast cancer activists. The National Cancer Institute (NCI) currently supports more research projects and clinical studies for breast cancer than for any other type of cancer.c In addition to the National Institutes of Health (NIH), breast cancer research is supported by private health charities and the Department of Defense (DoD), which together provide more than $300 million per year, for a total of roughly $800 million per year. By comparison, NCI spent $311 million on prostate cancer and the DoD’s Medical Research Program spent $85 million for a total of just under $400 million.
The committee believes that current priorities for basic research are appropriate. The investment in basic research over the past few decades has yielded a wealth of knowledge that supports the invention of a rich array of powerful new technologies—from imaging devices that can display the activity of individual cell types to assays that can simultaneously measure
|
c |
According to the NCI website, 2,932 breast cancer projects and 112 clinical trials are supported. In comparison, the average for all 56 types of cancer (or aspects of cancer) listed by NCI is only 8 clinical trials and 276 projects.17 |
the activity of thousands of genes or proteins, among others. But considerable time elapses between the development of a promising technology and determining whether its promise can be realized.
Inevitably, more exciting new technologies are announced than are proven useful in clinical practice. Although basic research enables the development of early stage technologies, different strategies are needed to identify which technologies are truly feasible and add clinical value by improving people’s health or the delivery of health care services. This involves large-scale, well-designed multicenter clinical trials. However, clinical trials have historically received substantially less support from NIH than basic research.
Even when technologies have been shown to offer clinical benefit, they add no value until they are adopted in clinical practice and used effectively. It is often many years from the time when early adopters, or leading-edge clinicians, adopt new technologies and when those technologies become widely and effectively used in the larger health care community. The likelihood that an innovation will be adopted into clinical practice depends not only on its performance characteristics, but also on the capacity of health care organizations to integrate it effectively into their practices. Indeed, effective application of new advances in medicine is a serious bottleneck in improving patient care and a source of concern to the medical community.12,24 Balas and Boren estimated that the interval between discovery and application for innovations has been an average of 17 years.3 The committee believes it is also important to analyze which new technologies are adoptable in clinical practice. This would entail a more comprehensive approach to technology assessment than is commonly practiced.
An immediate challenge lies in ensuring that incremental advances deliver the greatest possible value to patients. This will require the development of systems to permit evidence-based choices among suites of evolving options. The key to improving the detection and diagnosis of breast cancer is, therefore, not necessarily to focus on the single emerging technology that is the most deserving of support, but rather on the systems that provide the best possible health outcomes for breast cancer patients.
In many cases, some approaches or technologies are better than others under certain circumstances, but they are rarely better in all circumstances. The challenge lies in knowing what to use when. Most clinical studies for cancer detection are designed to evaluate a single new technology and do not provide the data that permit evidence-based choices among different options or how to sequence those options to maximize overall efficiency and effectiveness.d Rather than evaluating each technology only on its own,
it must be considered in the overall context of clinical practice. There is a critical need for evidence that will support rational integration of different approaches to breast cancer detection and that will consider the organizational issues.
SCOPE OF THIS STUDY
This study is a sequel to Mammography and Beyond, released by the Institute of Medicine (IOM) and the National Research Council (NRC) in 2001. The committee that produced that study was asked to (1) review existing technologies and (2) identify promising new technologies for breast cancer detection technologies. They were also asked to analyze the steps in medical technology development relevant to breast cancer detection technologies, including the policies that influence technology adoption.
The previous committee thus provided an overview of current and near-term technologies for breast cancer detection and outlined the long and arduous path from invention to adoption of new technologies (Table 1-1). The present committee reviewed the conclusions and recommendations in Mammography and Beyond, endorses them, and agrees that they should be supported. Indeed, several of them already have been implemented. Because the present committee decided not to repeat the previous committee’s excellent work, this report does not attempt a comprehensive review of current and emerging breast cancer detection technologies, nor does it provide an in-depth analysis of medical technology development.
TABLE 1-1 Imaging Technologies for Breast Cancer Reviewed in Mammography and Beyond
|
Technology |
Description |
FDA approved* |
|
Film-screen mammography |
The standard x-ray technique |
Yes |
|
Full-field digital mammography |
Digital version of x-ray technique |
Yes |
|
Ultrasound |
Forms images by reflection of megahertz frequency |
Yes |
|
Magnetic resonance imaging (MRI) |
Forms images using radio emissions from nuclear spins |
Yes |
Both were covered in the previous report. However, an overview of current technologies that are under development for breast cancer detection is important background for this report and is provided in Appendix A. This report also builds on the work of the previous committee by analyzing what improvements or innovations in breast cancer detection and diagnosis will have the greatest impact on reducing the toll of breast cancer, and what can be done to foster those improvements (see the statement of task in Box 1-2). Where there is overlap between the two reports, this committee relied most heavily on information published after the Mammography and Beyond report was written. Roughly 80 percent of all references were published in 2000 or later. (Although Mammography and Beyond was published in 2001, the final draft was submitted in 2000.)
Attention is focused on near-term improvements that could be accomplished within 5 to 15 years. A single breakthrough technology that will revolutionize the early detection of breast cancer is not likely in the next few years, and in any case, like other technologies that might change the landscape further in the future, it is unpredictable. The committee did not identify specific devices, protocols, or procedures that should be most encouraged, because that should be determined by evidence based on carefully designed studies. In the meantime, it is important to apply current knowledge more effectively to develop systems that will ensure access to new technologies as soon as they are ready—that is, when they have been demonstrated to be safe, effective, and to add value to the tools already available to improve breast cancer outcomes.
|
Routine use |
Infrequent use |
Clinical data suggests a role for… |
Clinical data not yet available |
|
Screening and Diagnosis |
|
|
|
|
Screening and Diagnosis |
|
|
|
|
Diagnosis |
|
Screening |
|
|
|
Diagnosis |
Screening |
|
|
Technology |
Description |
FDA approved* |
|
|
Scintimammography |
Sense tumors from gamma-ray emission of radioactive pharmaceutical |
Yes |
|
|
Thermography |
Seeks tumors by infrared signature |
Yes |
|
|
Electrical impedance imaging |
Maps the breast’s impedance with low-voltage signal |
Yes |
|
|
Optical imaging |
Localizes tumors by measuring scattered near-infrared light |
|
|
|
Electrical potential measurement |
Identifies tumors by measuring potentials at array of detectors on skin |
|
|
|
Positron emission tomography |
Forms images using emission from annihilation of positrons from radioactive pharmaceuticals |
Yes |
|
|
Novel ultrasound techniques |
Includes compound imaging, which improves resolution; 3D and Doppler imaging |
|
|
|
Elastography |
Uses ultrasound or MRI to infer the mechanical properties of tissue |
|
|
|
Magnetic resonance spectroscopy |
Analyzes tissue’s chemical makeup using radio emissions |
|
|
|
Thermoacoustic computed tomography |
Generates short sound pulses within breast using RF energy and constructs a 3D image from them |
|
|
|
Microwave imaging |
Views breast using scattered microwaves |
|
|
|
Hall-effect imaging |
Picks up sonic vibrations of charged particles exposed to a magnetic field |
|
|
|
Magnetomammography |
Senses magnetic contrast agents collected in tumors |
|
|
|
*Most of these technologies are approved for uses other than screening. Also, strictly speaking some devices are “approved” and others are “cleared” (see Chapter 6 for discussion of FDA device approval). |
|||
|
Routine use |
Infrequent use |
Clinical data suggests a role for… |
Clinical data not yet available |
|
|
|
Diagnosis |
|
|
|
|
Diagnosis |
|
|
|
|
Diagnosis |
|
|
|
|
Diagnosis |
|
|
|
|
Diagnosis |
|
|
|
|
|
Screening and diagnosis |
|
|
|
|
Screening and diagnosis |
|
|
|
|
Screening and diagnosis |
|
|
|
|
Screening and diagnosis |
|
|
|
|
Screening and diagnosis |
|
|
|
|
Screening and diagnosis |
|
|
|
|
Screening and diagnosis |
|
|
|
|
Screening and diagnosis |
|
Adapted from table in IEEE Spectrum. http://www.spectrum.ieee.org/pubs/spectrum/0501/cancert1.html [Accessed August 28, 2003]. |
|||
|
BOX 1-2 The committee will (a) consider which of the existing and evolving approaches hold the greatest promise for improving the early detection and diagnosis of breast cancer and (b) analyze the degree to which different stages in the development of innovative medical technologies might act as bottlenecks, particularly for those technologies that promise to improve the early detection of breast cancer. Strategies to improve the efficiency of those steps will be identified and evaluated, with the goal of accelerating the flow of the most promising new approaches from the conceptual stage to clinical practice. In addition to recommending strategies to enhance the discovery, development, and dissemination of approaches to the early detection and diagnosis of breast cancer, a fundamental and overarching goal of this study will be to improve the understanding of the media and the general public about the public health issues underlying the development of new approaches. Specific tasks include:
|
The committee believes the lives of many women could be saved by adopting several key strategies, including:
-
improving the organization of breast cancer screening and the interpretation of mammograms,
-
developing more individually tailored approaches to the early detection of breast cancer, and
-
focusing on how different technologies can be optimally integrated into clinical practices, instead of evaluating only the performance characteristics of individual technologies.
All of these steps should be supported by evidence, which unfortunately is not always the case in the adoption of new technologies into clinical practice.
The challenge of developing and integrating the “right” medical technologies so that patients receive the best possible treatment applies to all fields of medicine—especially where early detection is as important to health and survival as it is in breast cancer. Thus this report should be of interest to all those who are concerned with the development and application of medical technologies. Its primary audiences, however, are those working to reduce the toll of breast cancer through early detection, including breast cancer activists and women’s health organizations; breast cancer researchers; technology developers; clinicians such as breast imagers, primary care physicians, and oncologists; federal, state, and private research sponsors; and those who regulate medical technologies through the FDA, Centers for Medicare and Medicaid Services (CMS), private payers, and Congress. (CMS manages the Medicare program, which is historically the largest health care payer in the United States.)
This study also seeks to improve the understanding of the media and the public about the public health issues underlying the development of new approaches. The committee tackled this goal in two ways: first, by explaining commonly misunderstood concepts in cancer screening—particularly the standards of evidence for screening technologies and the analysis of risk—and second, by reviewing some of the problems and consequences of media coverage of medical advances, especially those related to breast cancer.
PUBLIC EXPECTATIONS ARE HIGH
Public expectations for reducing the toll of breast cancer are high, and the public has a seemingly insatiable appetite for news about breast cancer, as indicated by the frequency of that topic in magazines with large female readerships.7,25 Undoubtedly this need for news is partially because breast cancer is the disease that women fear most.16
The mass media, both news and advertising, are the main sources of health information for many Americans.18 Unfortunately, there is considerable evidence that the media portray breast cancer unrealistically, and that women’s perceptions of their risk of having breast cancer and dying from it—and, not coincidentally, of the benefits of various tests and treatments for breast cancer, including mammography—are similarly skewed.5,7,22
New treatments or technologies are often presented in the media as “breakthroughs” that promise unqualified advances.5 Even though breakthrough technologies are rare and unpredictable, media reports encourage the hope that such a technology for breast cancer detection is lurking in the shadows. In fact, virtually all medical technology development has its roots in basic research, and the world of research is so well-lit by the pressure to publish that there are few, if any, dark corners in which important technology advances lie hidden. Moreover, the technical backgrounds of members of the committee responsible for this report including surgery, radiology, molecular biology, imaging, physics, information technology, and epidemiology should ensure sufficient access to events in the research and development community to assure readers that the committee is not overlooking truly promising new developments.
Risk in the Media
Research indicates that women tend to overestimate their lifetime risk of developing and dying from breast cancer, and particularly the likelihood of that happening before age 50 (reviewed by Burke and colleagues).1,4,6,7,13,15,19 Many women mistakenly believe that their short-term risk of breast cancer diminishes with age.8,10,20 Investigating the media as a possible source of such misperceptions, Burke and his colleagues examined 172 vignettes illustrating women’s experiences with breast cancer that appeared in a broad sample of popular U.S. magazines over a four-year period.7 The age distribution of women in the vignettes was almost the reverse of the actual age distribution of breast cancer (Figure 1-1). Nearly half of the vignettes featured women diagnosed with breast cancer before age 40; such women account for only about 5 percent of breast cancer cases. Yet, the vignettes rarely referred to women age 60 or above with breast cancer, which is when the majority of cases occur.4,10
Gripping stories that generate fear of breast cancer in young women may also increase demand for tests and treatments perceived to improve the chances of surviving this disease. Hundreds of articles and television stories in the early 1990s portrayed high-dose chemotherapy (which included bone marrow transplantation) as the only hope for patients with advanced breast cancer, despite a lack of evidence that this risky and expensive procedure actually extended survival.5 In the mid-1980s, about 100 women per year received high-dose chemotherapy; in 1994, more than 4,000 of these procedures were performed. Similarly, deceptive marketing of MRI for breast cancer screening played on women’s desire for an “accurate” test (Box 1-3).
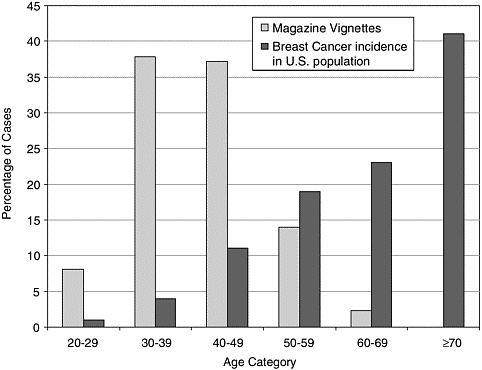
FIGURE 1-1 Personal stories in women’s magazines overrepresent the incidence of breast cancer in younger women and underrepresent it among older women.7
Reports of “Breakthroughs” Are More Prevalent Than Actual Breakthroughs
The sense of exaggerated risk surrounding breast cancer provides fertile ground for the marketing of tests and treatments promising to reduce that risk, such as MRI screening.21 Blood tests, imaging technology, even cancer care and surgical products are increasingly marketed directly to consumers. Many of these direct-to-consumer advertisements encourage readers’ fear so as to promote the advertised product or service.
Another problematic form of marketing occurs indirectly through media reports of medical developments while they are still “works in progress,”5 such as press releases discussing the content of articles at the time of their publication in medical journals26 and research abstracts from scientific meetings. A scientific meeting is a forum for scientists to present works in progress, and nearly half of that work remains unpublished23—typically, because the results could not be replicated, or they were too inconclusive to pass peer review. Lisa Schwartz and her colleagues tracked
|
BOX 1-3 Aggressive marketing of MRI for breast cancer screening by AmeriScan™ incensed breast imagers for years because the manufacturer exaggerated the accuracy of the technology in a widely distributed series of Internet, television, radio, and newspaper ads. The original ad claimed 100 percent accuracy, although—with pressure from the FDA—that was revised to “almost 100 percent.” Even that is an overstatement because most people presume that accuracy includes both specificity and sensitivity. Although the sensitivity of MRI is very high, specificity is relatively low and MRI does not reliably detect microcalcifications. Although some breast MRI applications are supported by the literature, screening is not one of them. MRI has been proven effective for uses such as evaluating women with breast implants that may have ruptured. It is also used for women known to have breast cancer to evaluate the extent of tumors prior to surgery, or after surgery to monitor response to treatment. After several years of outcry among breast imagers, the Medical Board of California and the San Francisco District Attorney’s Office jointly filed a lawsuit in San Francisco Superior Court on October 23, 2003, against the founder and medical director of AmeriScan.™ Less than two weeks later, AmeriScan™ announced its closure. |
the outcome of presentations at high-profile scientific meetings that received mass media coverage and found that in as many as one in four of these presentations the findings were never published—which means that they were never subjected to peer review, or they were and failed. Even press releases of published material can be unreliable. A study of press releases issued by leading medical journals found that the releases routinely failed to mention study limitations or industry funding, and often presented data in formats that exaggerated findings.26
STUDY PROCESS
This study was carried out by a committee on which all major areas of breast cancer detection were represented, including breast cancer screening, diagnosis, and treatment; clinical trials expertise; cancer and molecular biology; medical technology development and evaluation; health care administration; and technology innovation and adoption. The committee supplemented its expertise through several workshops, which were organized as information-gathering, brainstorming sessions (see Appendix B for
Workshop Agendas). Committee members heard from technology developers representing small and large companies and from researchers developing some of the most innovative systems for early detection of breast cancer, including a variety of imaging, biological, and computer technologies. The committee also heard from the leaders of several of the most important clinical studies aimed at improving systems for the early detection of breast cancer. Finally, the committee heard from senior staff at the federal agencies that regulate the availability of new medical technologies, as well as representatives of private insurance, who also serve as gatekeepers. The committee has made extensive use of relevant published review papers, and referred back to the original papers only if the review failed to include all of the pertinent data.
A series of reports on breast cancer have been produced by, or under the aegis of, the IOM and NRC’s National Cancer Policy Board. These reports include Mammography and Beyond: Developing Technologies for the Early Detection of Breast Cancer (2001), Meeting Psychosocial Needs of Women with Breast Cancer (2004), and Improving Mammography Quality Standards (in progress, 2004). As part of that series, this report explores ways of improving early detection and diagnosis of breast cancer in women through developing better technologies and advancing their introduction and application, and educating women and other interested stakeholders about mammography and other detection modalities. The ultimate objective of this and all the other studies is better care and better outcomes for women with breast cancer and their families.
ORGANIZATION OF THIS REPORT
Chapter 2 covers the basic principles of effective screening. These principles are well established, but they are confused so often in the media and even in some of the scientific literature that it is important to review them here. Many controversies over mammography have been entwined with debates over the appropriate interpretation of data from screening studies. Often what the public remembers most is only that “mammography is debatable,” even after the overwhelming majority of experts have concluded that the preponderance of data clearly supports the value of mammography. In addition, because there has been so much discussion about the importance of weighing the benefits and harms of screening mammography, and yet so little discussion about the actual harms, this chapter reviews the reported harms of mammography. Finally, because the increased frequency of DCIS diagnoses and treatment are cited so often as a negative consequence of widespread mammography screening, the DCIS dilemma is reviewed.
Chapter 3 explores strategies for improving mammography screening. Many of these strategies involve new approaches to organizing mammography services, with an emphasis on improving the quality of mammographic interpretation and the critical need for breast imaging specialists. Existing technologies that can compensate for the technical limitations of mammography are also discussed.
Chapter 4 discusses what is known about breast cancer risk factors, how risk perception is often confused, and how improved knowledge about breast cancer risk factors could be used to develop individualized strategies for breast cancer detection. The committee believes that better tools for understanding breast cancer risk will be invaluable for reducing breast cancer mortality.
Chapter 5 reviews the status and challenges of biologically based technologies for the detection and diagnosis of breast cancer. Undoubtedly such technologies hold the key to improving our understanding of breast cancer biology and should be pursued, but for the most part, these technologies have not yet been validated for the early detection of breast cancer and are still in their infancy.
Chapter 6 tackles the challenges inherent in translating research results and early stage inventions into clinically useful applications. For much of technology development, this is the weak link where so many technologies fail to realize their initial promise. This process is not merely bureaucratic although it may seem so to the public. Rather, it is a dynamic, researchdriven transition between the “possibly useful” and the “truly useful.” Methodological issues and federal agencies and programs that support this process are also described. Countless technologies that were believed initially to be major advances turn out to offer no benefit and may even harm patients. This stage represents the difference between belief-based medicine and evidence-based medicine.
Chapter 7 deals with the final, and possibly most neglected, phase in the development and application of new technologies—namely, that of ensuring that the technologies improve patients’ health. Without consideration of this phase, which includes how technologies are best used to complement each other, new technologies will fall short in saving women’s lives.
Finally, Chapter 8 summarizes the committee’s main findings and recommendations for developing new strategies for breast cancer detection and diagnosis.
REFERENCES
1. Alexander NE, Ross J, Sumner W, Nease RF Jr, Littenberg B. 1996. The effect of an educational intervention on the perceived risk of breast cancer. J Gen Intern Med 11(2):92-97.
2. American Cancer Society. 2001. Breast Cancer Facts and Figures 2001-2002. Atlanta, GA: American Cancer Society.
3. Balas E, Boren SA. 2000. Managing Clinical Knowledge for Health Care Improvement. Bemmel J, McCray AT, Editors. Yearbook of Medical Informatics: Patient-Centered Systems. Stuttgart, Germany: Schattauer Verlagsgesellschaft mbH. Pp. 65-70.
4. Black WC, Nease RF Jr, Tosteson AN. 1995. Perceptions of breast cancer risk and screening effectiveness in women younger than 50 years of age. J Natl Cancer Inst 87(10):720-731.
5. Brownlee S. 2003, August 3. Health, Hope and Hype: why the media oversells medical “breakthroughs.” The Washington Post.
6. Bunker JP, Houghton J, Baum M. 1998. Putting the risk of breast cancer in perspective. BMJ 317(7168):1307-1309.
7. Burke W, Olsen AH, Pinsky LE, Reynolds SE, Press NA. 2001. Misleading presentation of breast cancer in popular magazines. Eff Clin Pract 4(2):58-64.
8. Dolan NC, Lee AM, McDermott MM. 1997. Age-related differences in breast carcinoma knowledge, beliefs, and perceived risk among women visiting an academic general medicine practice. Cancer 80(3):413-420.
9. Elmore JG, Nakano CY, Koepsell TD, Desnick LM, D’Orsi CJ, Ransohoff DF. 2003. International variation in screening mammography interpretations in community-based programs. J Natl Cancer Inst 95(18):1384-1393.
10. Fulton JP, Rakowski W, Jones AC. 1995. Determinants of breast cancer screening among inner-city Hispanic women in comparison with other inner-city women. Public Health Rep 110(4):476-482.
11. Institute of Medicine and National Research Council. 2001. Mammography and Beyond: Developing Technologies for the Early Detection of Breast Cancer. Washington, DC: National Academy Press.
12. Institute of Medicine. 2003. Exploring Challenges, Progress, and New Models for Engaging the Public in the Clinical Research Enterprise: Clinical Research Roundtable Workshop Summary. Washington, DC: The National Academies Press.
13. Lavelle K, Charlton A. 1998. Women’s perception of risk of cancer. BMJ 317(7157):542.
14. Lewin JM, Hendrick RE, D’Orsi CJ, Isaacs PK, Moss LJ, Karellas A, Sisney GA, Kuni CC, Cutter GR. 2001. Comparison of full-field digital mammography with screen-film mammography for cancer detection: results of 4,945 paired examinations. Radiology 218(3):873-880.
15. McCaul KD, Branstetter AD, O’Donnell SM, Jacobson K, Quinlan KB. 1998. A descriptive study of breast cancer worry. J Behav Med 21(6):565-579.
16. MORI Medicine & Science Research. 2002, October 17. Women See Family History Not Old Age as Greatest Breast Cancer Risk [MORI is an approved survey firm for the British National Health Service]. Accessed May 13, 2003. Web Page. Available at: http://www.mori.com/polls/2002/breakthrough.shtml.
17. National Cancer Institute. Cancer Research Portfolio. 2003. Accessed June 1, 2004. Web Page. Available at: http://researchportfolio.cancer.gov/.
18. Phillips KP, Kanter EJ, Bednarczyk B, Tastad PL. 1991. Importance of the lay press in the transmission of medical knowledge to the scientific community. N Engl J Med (325):1180-1183.
19. Pilote L, Hlatky MA. 1995. Attitudes of women toward hormone therapy and prevention of heart disease. Am Heart J 129(6):1237-1238.
20. Press N. 1995. Survey, Orange County Region 10 California State Breast Cancer Early Detection Partnership Program (BCEDP). Sacramento, CA: California Department of Health.
21. Schwartz LM, Woloshin S. 2002. Marketing medicine to the public: a reader’s guide. JAMA 287(6):774-775.
22. Schwartz LM, Woloshin S. 2002. News media coverage of screening mammography for women in their 40s and tamoxifen for primary prevention of breast cancer. JAMA 287(23):3136-3142.
23. Schwartz LM, Woloshin S, Baczek L. 2002. Media coverage of scientific meetings: too much, too soon? JAMA 287(21):2859-2863.
24. Sung NS, Crowley WF Jr, Genel M, Salber P, Sandy L, Sherwood LM, Johnson SB, Catanese V, Tilson H, Getz K, Larson EL, Scheinberg D, Reece EA, Slavkin H, Dobs A, Grebb J, Martinez RA, Korn A, Rimoin D. 2003. Central challenges facing the national clinical research enterprise. JAMA 289(10):1278-1287.
25. Wells J, Marshall P, Crawley B, Dickersin K. 2001. Newspaper reporting of screening mammography. Ann Intern Med 135(12):1029-1037.
26. Woloshin S, Schwartz LM. 2002. Press releases: translating research into news. JAMA 287(21):2856-1858.


















