8
Malaria Control
INTRODUCTION
This chapter reviews malaria control over the last century, tracking malaria’s retreat from much of the world to its current lines of demarcation. It also describes individual control methods targeting both the mosquito vector and the human reservoir of infection and the current status of diagnosis and vaccine development. The chapter concludes with a discussion of malaria control strategies, including national and regional policies and programs operating today.
HISTORICAL OVERVIEW
Historically, malaria’s reach extended far beyond the tropics. Until the 19th century, transmission occurred in much of the temperate world, including parts of England, Holland, Germany, central and southeastern Europe, Asia, India, China, and the Americas (Shiff, 2002). In North America, the disease reached as far north as New York, and even Montreal (Barber, 1929). In the early 20th century, the Tennessee Valley Authority brought hydroelectric power to the southeastern United States, modernizing the region. As housing and lifestyles improved, and the human reservoir of infection decreased, malaria retreated (Desowitz, 1999). Malaria also disappeared during the first half of the 20th century from most of Europe following changes in land use, agricultural practices, house construction, and targeted vector control (Greenwood and Mutabingwa, 2002).
Then came the golden days of DDT, a highly effective insecticide first used as a delousing agent at the end of World War II. During the 1950s and 1960s, indoor residual spraying with DDT was the centerpiece of global malaria eradication efforts. DDT’s months-long ability to kill or deter adult female mosquitoes resting on treated walls after feeding led to further declines in malaria in India, Sri Lanka, the former Soviet Union, and other countries. By 1966, campaigns using DDT spraying, elimination of mosquito breeding sites, and mass treatment had freed more than 500 million people (roughly one-third of the population previously living in malarious areas) from the threat of disease (Shiff, 2002). Unfortunately, eradication was not sustained due to high program costs, community resistance to repeated house spraying, and the emergence of resistance to DDT. By the late 1960s, the hope of eradicating malaria through vector control was finally abandoned (Guerin et al., 2002). In many countries, the pendulum then swung to overreliance on chloroquine, a widely available antimalarial drug.
Sub-Saharan Africa was always a special case. With the exception of a few pilot programs, no sustained malaria control efforts were ever mounted there (Greenwood and Mutabingwa, 2002). The biggest obstacle was the widespread distribution of Anopheles gambiae, a long-lived and aggressive malaria vector. The entomological inoculation rate (EIR) (which measures the frequency with which a human is bitten by an infectious mosquito) rarely exceeds five per year in Asia or South America. In contrast, EIRs of over 1,000 have been recorded in several parts of sub-Saharan Africa (Greenwood and Mutabingwa, 2002).
Today, the global burden of malaria is concentrated in sub-Saharan Africa where stable, endemic disease is linked to poverty and highly efficient vectors. The insecticide-treated bednet (ITN)—first shown in The Gambia to reduce overall childhood mortality by 60 percent when combined with malaria chemoprophylaxis (Alonso et al., 1991)—is the vector control tool with the greatest promise for Africa. At the Africa Summit on Roll Back Malaria in Abuja, Nigeria in 2000, leaders from 44 African countries set a target of 60 percent ITN coverage of pregnant women and infants in Africa by 2005, an ambitious goal requiring roughly 160 million ITNs at an estimated cost of US$1.12 billion (Nahlen et al., 2003). Sadly, the goal is still far from being met. At the same time, insecticide resistance (involving pyrethroids and DDT) is a growing problem in Africa, along with environmental change brought by agriculture and other types of development that foster mosquito breeding. International sponsors also have withdrawn support for DDT due to environmental concerns.
With respect to malaria’s human reservoir, the overriding challenge facing Africa is the development of drug resistance by Plasmodium falciparum to cheap and effective treatments (chloroquine and sulfadoxine-
pyrimethamine [SP]), compounded by large and, in some cases, mobile infected populations.
BASIC PRINCIPLES OF MALARIA CONTROL
Successful malaria control programs traditionally use multiple interventions. In 1952, Paul Russell (a noted Rockefeller Foundation malariologist, and former head of the Allied antimalaria campaign in Italy) listed five approaches to malaria eradication (Russell, 1952):
-
Measures to prevent mosquitoes from feeding on humans (human-vector contact)
-
Measures to prevent or reduce the breeding of mosquitoes
-
Measures to destroy mosquito larvae
-
Measures to kill or reduce the lifespan of adult mosquitoes
-
Measures to eliminate malaria parasites from humans
Since Russell’s era, an increasing emphasis on the control of human disease has produced three additional strategies (Beales and Gilles, 2002):
-
Measures to prevent and reduce malaria mortality (especially in high-risk groups)
-
Measures to reduce malaria morbidity
-
Measures to reduce malaria transmission
Today’s control efforts mainly rely upon the interruption of human-vector contact and treatment of infected persons. Personal protection via ITNs or curtains is generally preferred in settings where vectors feed indoors during nighttime sleeping hours. ITNs also kill malaria vectors and reduce the local intensity of transmission (the “mass effect”). Indoor residual spraying (IRS) with DDT or a pyrethroid insecticide is another way to reduce bites by vectors whose feeding and resting habits render them susceptible, as long as the majority of houses in a targeted community are sprayed. IRS also is the preferred vector control method during malaria epidemics and in refugee camps since trained spray teams can rapidly cover likely areas of transmission.
Case management—which encompasses prompt access to health care, an accurate diagnosis, and effective treatment—is the other cornerstone of malaria control. The current failure to control malaria with drugs often starts with a failure to deliver appropriate case management to many malaria sufferers, particularly at the periphery of health systems.
Other control strategies outlined by the World Health Organization (WHO) in 1993 include early forecasting of malaria epidemics and the
development of epidemiological information systems; capacity-building in basic and applied research; and ongoing assessment of ecological, social, and economic determinants of disease within affected countries and regions (WHO, 1993). Effective field operations also require expertise and teamwork. Qualified personnel with the scientific knowledge, skills, and authority are perhaps the single most important resource needed for effective vector control (Roberts et al., 2000). The same need for knowledge applies to those rendering clinical care to malaria patients: from parents, village health care workers, drug sellers, and traditional healers to laboratory workers, nurses, doctors, and other health care professionals.
INSECTICIDES AND INSECTICIDE RESISTANCE
Insecticides in Public Health
Immediately after World War II, DDT and other chlorinated hydrocarbon insecticides formed the mainstay of malaria control. DDT was initially developed as a public health insecticide prior to its widespread agricultural use and recognition as an environmental pollutant (Curtis and Lines, 2000). Of note, when used indoors in limited quantities, DDT’s entry into the global food chain is minimal (Attaran et al., 2000). (For a full summary of DDT’s role in public health, readers are referred to a recent review [Taverne, 1999]).
Today, despite concerns over their environmental effects and possible inactivation by mosquito vectors, chemical insecticides remain key elements in malaria control.
A WHO-coordinated research program is now in place to develop new candidate insecticides and test their activity and safety(WHO, 1996a). The specifications for pesticides used in public health are part of the WHO Pesticide Evaluation Scheme (WHOPES).
Classified by chemical characteristics, the most common insecticides currently used in public health practice are:
-
Petroleum oils and their derivatives
-
Active constituents of flowers of pyrethrum (pyrethrins) or newer synthetic compounds of this group (pyrethroids)
-
Chlorinated hydrocarbons (e.g., dichloro-diphenyl-trichloroethane (DDT), hexachlorocyclohexane (HCH), and dieldrin)
-
Organophosphorous insecticides (e.g., malathion, and temephos)
-
Carbamates (e.g., propoxur, and carbaryl)
-
Insect growth regulators (e.g., diflubenzuron, methoprene, and pyriproxyfen)
Pyrethrum, an extract of dried chrysanthemum flowers, is the oldest effective insecticide known. Both pyrethrum and its natural and synthetic relatives (pyrethrins and pyrethroids) are nerve poisons that rapidly permeate and kill adult insects with high margins of mammalian safety. They also demonstrate rapid knock down (i.e., immobilizing) and repellant effects. The chief drawback of the class is its relatively short-lived action, although newer synthetic compounds such as permethrin and deltamethrin are more stable than naturally occurring products. The residues of DDT, in contrast, remain active for up to a year following application to impervious surfaces such as plastered walls (on mud brick, DDT loses its insecticidal effect faster). DDT’s long-term repellant, and contact irritant effects probably contribute as much or more than its direct insecticidal action in controlling malaria transmission (Roberts et al., 2000).
On a molecular level, all major classes of chemical insecticide exert their principal effects within the nerve tissue of targeted insects. DDT and pyrethroids cause persistent activation of sodium channels (Soderlund and Bloomquist, 1989), while pyrethroids also act on receptors that normally govern inhibitory neurotransmission, and organophosphates and carbamates target acetylcholinesterase.
Insecticide Resistance
Levels of resistance in insect populations reflect the amount and frequency of insecticide contact as well as inherent characteristics of the target species. Thus far, DDT resistance has not developed in long-lived disease vectors such as tsetse flies or triatomid bugs (definitive hosts of African sleeping sickness and Chagas’ disease, respectively). Mosquitoes, in contrast, have several characteristics suited to rapid development of resistance, including a short life cycle and abundant progeny.
In 1946, only two species of malaria vector were resistant to DDT. However, by 1966 the emergence of resistance was clear: 15 species were resistant to DDT, and 36 species were resistant to dieldrin (WHO Expert Committee on Insecticides, 1970). By 1991, 55 anopheline vectors demonstrated resistance to one or more insecticides. Of these, 53 were resistant to DDT, 27 to organophosphates, 17 to carbamates, and 10 to pyrethroids (WHO, 1992a,b). A decade later, some form of pyrethroid resistance (either decreased mortality, or decreased excito-repellancy of mosquitoes by pyrethroid-impregnated ITNs) had been reported from countries in Asia, Africa, and South America (Takken, 2002).
Three major groups of inactivating enzymes (glutathione S-transferases, esterases, and monooxygenases) are responsible for metabolic resistance to DDT, pyrethroids, organophosphates, and carbamates in Anopheles mos-
quitoes. Knock-down resistance (kdr) is a separate resistance phenotype linked to a point mutation in sodium channels targeted by both pyrethroids and DDT. Although prevalent in A. gambiae in West Africa, kdr has not impaired ITN efficacy in the region (Sina and Aultman, 2001; Hemingway and Bates, 2003). In southern Africa, in contrast, the local vector A. funestus has acquired metabolic resistance to pyrethroids, rendering ITNs ineffective (Chandre et al., 1999; Brooke et al., 2001). A looming concern for the future is that A. gambiae in equatorial Africa will acquire the same metabolic resistance to pyrethroids seen in A. funestus in southern Africa. Currently, metabolic resistance to pyrethroids in A. gambiae is limited to focal areas of West Africa and Kenya (Ranson et al., 2002).
In coming years, strategies to decrease insecticide resistance may include rotations, mosaics, and mixtures of agricultural and environmental insecticides guided by mathematical models (Tabashnik, 1989). Until now, little field-testing of models has been conducted; however, with new biochemical and molecular field tools, large-scale trials of resistance management are feasible. Treating ITNs with two insecticides with differing mechanisms of action is another approach that may be implemented in the near future. In West Africa, bi-treated nets pairing pyrethroids with carbosulfan (a carbamate insecticide), or chlorpyrifos-methyl (an organophosphate insecticide) are currently under evaluation (Muller et al., 2002).
INSECTICIDE-TREATED BEDNETS AND INDOOR RESIDUAL SPRAYING
History of ITNs
More than two thousand years before Ronald Ross and Giovanni Battista Grassi showed that mosquitoes transmit malaria, human beings used nets to fend off night-biting insects. Mosquito nets appear in historical records from the Middle East to West Africa to Papua New Guinea (Lindsay and Gibson, 1988). The Greek writer Herodotus (484 - ?425 BC) described how Egyptians living in marshy lowlands protected themselves with fishing nets.
|
Every man there has a net which he uses in the daytime for fishing, but at night he finds another use for it: he drapes it over the bed … and then crawls in under and goes to sleep. Mosquitoes can bite through any cover or linen blanket … but they do not even try to bite through the net. Herodotus, The Histories |
By the early 19th century, British colonists in India—most likely inspired by the example of Punjabi fishermen—also were sleeping under nets. However, it was not until World War II that textiles and insecticides were combined. In central Asia, the Soviet army applied juniper oil to bednets to repel mosquitoes, and sand flies bearing malaria and leishmaniasis (Blagoveschensky et al., 1945), while the American military in the Pacific theater impregnated bednets and jungle hammocks with 5 percent DDT to ward off malaria and filariasis (Harper et al., 1947).
Interest in insecticide-impregnated nets as a malaria control tool resurfaced in the late 1970s and early 1980s. By then, synthetic pyrethroids were the logical insecticide choice because of their low mammalian toxicity and known efficacy in killing and repelling a variety of nuisance and disease-bearing insects. Several governments including the Philippines, Solomon Islands, and Vanuatu began to include ITN promotion as one of their malaria control objectives (Chavasse et al., 1999). However the most successful government-financed ITN programs today are found in China and Vietnam, where the public sector’s chief contribution is to offer regular net re-treatment services. When re-treatment is provided free of charge (e.g., China and Vietnam), coverage is generally high (Curtis et al., 1992). Conversely, in Africa, where many nets and insecticides have been provided free or at subsidized prices through local projects and NGOs, less than 5 to 20 percent of nets are re-treated (Snow et al., 1999; Rowley et al., 1999; Guillet et al., 2001).
Individual and Community Effects of ITNs
Child Mortality
After a number of small-scale studies in the 1980s showed favorable effects, the first large-scale study of ITNs plus chemoprophylaxis reported a 60 percent reduction of all-cause child mortality (Alonso et al., 1991). These results prompted the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) to sponsor four randomized controlled trials in Africa to assess the effect of ITNs on all-cause mortality in African children in different epidemiologic settings. A cluster randomization design was used in all four trials. In The Gambia (D’Alessandro et al., 1995), a 25 percent reduction in all-cause mortality was seen in children less than 9 years old. In Kenya (Nevill et al., 1996) and Ghana (Binka et al., 1996), the introduction of ITNs was associated with 33 and 17 percent reductions in all-cause child mortality, respectively, in children under 5 years of age. Study populations in all three sites ranged from 60,000 to 120,000 (Table 8-1).
The fourth randomized controlled trial in Burkina Faso (Habluetzel et
TABLE 8-1 Protective Efficacy of ITNs: Reductions in Child Mortality in Five Randomized Controlled Trials
|
Country in Which Study Was Performed |
Percent Reduction in Child Mortality |
EIR |
|
The Gambia (D’Alessandro et al., 1995)a |
25% |
1-10 |
|
Kenya (Nevill et al., 1996) |
33% |
10-30 |
|
Ghana (Binka et al., 1996) |
17% |
100-300 |
|
Burkina Faso (Habluetzel et al., 1997) |
15% |
300-500 |
|
Kenya (Phillips-Howard et al., 2003) |
16% |
200-300 |
|
aThis study was considered an effectiveness, as opposed to an efficacy, study. |
||
al., 1997) examined insecticide-treated curtains (ITCs) rather than bednets in roughly 100,000 residents of a region with alternating high and low malaria seasons. Baseline mortality, approaching 45 per thousand, was the highest to date among the four African ITN trials (Diallo et al., 1999). After 2 years of tracking, the use of ITCs was associated with a 15 percent decrease in all-cause mortality in Burkina Faso, concentrated in the first year of use.
Viewed as a group, the four TDR-sponsored randomized controlled trials demonstrate decreasing ITN efficacy with increasing transmission pressure, since sites experiencing higher EIRs (100-500 infective bites per person per year, i.e., Ghana, and Burkina Faso) witnessed lower ITN benefits.
The most recent group-randomized controlled trial of permethrin-treated bednets conducted in western Kenya (Hawley et al., 2003a) was designed to assess ITN efficacy at an upper range of year-round transmission. This study yielded an overall protective efficacy of 16 percent in all-cause child mortality; thus, ITN benefits were validated in an area of very high transmission. Maximum effect was dependent on regular re-treatment of ITNs, however. For example, the protective efficacy of ITNs in children aged 1-11 months fell from 26 to 17 percent when re-treatment was delayed beyond 6 months. The Kenyan trials also demonstrated roughly 90 percent transmission reduction from a baseline EIR of 60-300 (Gimnig et al., 2003b). When ITNs are combined with highly effective therapy such as artemisinin combination therapies (ACTs)—even in highly endemic areas—it is possible that the EIR could decline even further, approaching 0 (Personal communication, N. White, Mahidol University, February, 2004).
Child and Maternal Morbidity
Acute and chronic consequences of childhood malaria include uncomplicated febrile episodes with parasitemia, and anemia. Data from a large meta-analysis suggest that ITN use under stable transmission conditions roughly halves mild malaria episodes in children under five (Lengeler, 2001). In a nonrandomized trial of ITNs in southwestern Tanzania, treated nets conferred protective efficacy of 62 and 63 percent, respectively, on parasitemia and anemia in children under 5 (Abdulla et al., 2001). In an area of high perennial transmission in western Kenya, ITNs delayed the time to first infection in infants from 4.5 to 10.7 months (ter Kuile et al., 2003a).
Repeated malaria infection also causes anemia and morbidity in pregnant women and newborns. Four randomized controlled trials of ITNs in pregnancy have shown variable benefits in different transmission settings. In Thailand and The Gambia (areas with lower, seasonal transmission), ITNs significantly reduced malaria parasitemia and maternal anemia (Dolan et al., 1993; D’Alessandro et al., 1996); in The Gambia, they also increased birth weight (D’Alessandro et al., 1996). However, similar benefits were not seen in areas with more intense transmission (coastal Kenya and Ghana) (Shulman et al., 1998; Browne et al., 2001), raising concern that ITNs might not protect pregnant women in areas with a very high EIR. This concern was allayed by the most recent findings.
In the western Kenya trial, complete data were available in nearly 3,000 pregnancies (ter Kuile et al., 2003b). Before the study began, up to one-third of all infants were born preterm, small for gestational age, or with low birth weight. ITN-using pregnant women (gravidae 1-4) experienced a 38 percent reduction in maternal parasitemia, a 47 percent reduction in malarial anemia, and a 35 percent reduction in placental malaria at the time of delivery, while their newborns demonstrated a 28 percent reduction in low birth weight.
Community and Population Effects
In addition to conferring benefits upon individual users, ITNs can protect nonusers within ITN households as well as nonusers in nearby houses. Such effects were first noted in early village-scale ITN trials in Burkina Faso (Robert and Carnevale, 1991), Tanzania (Magesa et al., 1991), Kenya (Beach et al., 1993), and Zaire (Karch et al., 1993). More recent ITN studies have confirmed community-wide reductions in vector populations (Hii et al., 1997; Binka et al., 1998; Hii et al., 2000; Howard et al., 2000; Maxwell et al., 2002). Some ITN trial data have even demonstrated spatial effects on health. In Ghana, child mortality increased by 6.7 percent for every 100 m away from an intervention compound (Binka et al., 1998),
while in western Kenya, mortality, parasitemia, and anemia decreased in unprotected children living within 300 m of households from ITN villages (Gimnig et al., 2003a). The minimum ITN coverage needed to achieve community benefits is 50 to 60 percent of households within a neighborhood with suitable indoor, night-biting vectors (Hawley et al., 2003b). In Asia, in contrast, ITNs have had mixed results because vectors often bite outdoors in the late evening (or sometimes in the early morning), and both children and adults are susceptible.
Long-Lasting Insecticidal Nets
At present a single insecticide treatment of a conventional cotton or nylon mosquito net lasts for 6 to 12 months. “Long-lasting insecticidal nets” (with insecticide incorporated directly in net fibers) would eliminate the need for regular re-treatment. Two prototypes (Olyset and Permanet) are now on the market while others are being developed (Moerman et al., 2003). One early problem with the Vestergaard-manufactured Permanet was inconsistency among batches; in a study of randomly-sampled new unwashed, traditionally washed, and up to 18 months field-used products, insecticide concentration was much reduced after two washes, and mosquito mortality reached unacceptably low levels after only 12 months (Muller et al., 2002). These problems have presumably been rectified since Permanets produced by Vestergaard are now approved by WHO and production is slated to increase to one million nets per month (Personal communication, B. Greenwood, London School of Hygiene and Tropical Medicine, March 2004).
Indoor Residual Insecticide Spraying
Sprayed insecticides to kill adult mosquitoes were introduced on a large scale in the mid-1930s. Pyrethrum was first used for indoor residual spraying (IRS) in southern Africa and India and later replaced by DDT after World War II. IRS is most effective in reducing mosquitoes that rest indoors following a blood meal. To be effective, IRS does not have to kill all Anopheles at once but simply prevent a large proportion from surviving 12 to 14 days (the time it takes for a malaria parasite to develop to the infective stage within the mosquito). Even with the hardiest vectors, this can be achieved with a daily mortality of 40 to 50 percent. In places with lower malaria endemicity, daily mosquito mortality of 20 to 25 percent generally is adequate (Beales and Gilles, 2002).
Just as ITNs extend benefits to nonusers in the community, IRS is especially effective when applied on a large scale, since this maximizes the reduction in mosquito lifespan, and overall transmission. This so-called
mass effect has been well documented in a number of IRS trials. Specific examples include three demonstration projects in Africa: an observational study in the Pare-Taveta Malaria Scheme in Tanzania where dieldrin reduced malaria transmission from an annual EIR of 10-50 to <1 (Pringle, 1969; Bradley, 1991); a trial in Kisumu, Kenya, where IRS with fenitrothion reduced malaria transmission by 96 percent compared to baseline over 2 years (Payne et al., 1976); and the Garki project in northern Nigeria where IRS with propoxur also substantially decreased transmission and improved infant and child mortality (Molineaux, 1985) (Box 8-1).
Over the last 30 years, DDT-based IRS has declined, in part, because of DDT resistance among malaria vectors. A lack of sustained government support and financing as well as general disapproval of DDT by the international community also have contributed to IRS’s restricted use in sub-Saharan Africa. In parts of Asia, Latin America, and southern and northeastern Africa where IRS is still used, it is typically organized and paid for by governments (for example, government-funded DDT house spraying was recently reinstated in KwaZulu Natal, South Africa, and the Madagascar highlands because of rising prevalence of pyrethroid-resistant A. funestus vectors and human malaria cases [Hargreaves et al., 2000]). IRS also is used in urban epidemics and refugee camps worldwide, and sometimes provided by foreign and multinational companies for the protection of employees and local communities in malaria-endemic areas (Sharp et al., 2002a).
The implementation of IRS is not trivial, and, if incorrectly performed, may be quite ineffective (Shiff, 2002). Houses and animal shelters within a target area should receive IRS before the start of the transmission season and at regular intervals thereafter. Before application of insecticide, all furniture, hanging clothing, cooking utensils, food and other items should be removed from human habitations, and left covered outside. Emulsions or solutions of insecticide are often preferred over suspensions of water-dispersible powders, which leave whitish deposits. Mud and porous plaster walls retain less IRS insecticide than wood or non-absorptive surfaces.
Barriers to ITN and IRS Use
ITNs and IRS both require user cooperation, albeit in different ways. Current-generation ITNs must be properly installed, faithfully used, and retreated with insecticide every 6 to 12 months in order to maintain extended efficacy. IRS, in comparison, is passive but intrusive. Some residents of endemic areas forfeit IRS benefits by painting or replastering sprayed walls, while other families evade IRS altogether by locking their houses during the spraying round (Mnzava et al., 2001; Goodman et al., 2001). In addition, some housing or shelter materials such as plastic sheeting are not amenable to residual spraying.
|
BOX 8-1 In the early 1970s, an ambitious malaria control experiment was undertaken to determine whether malaria transmission could be interrupted in a highly endemic area of Africa. The experiment was conducted in a group of villages near the town of Garki in northern Nigeria. Villages were allocated to three intervention groups.
The main findings of the Garki project were as follows:
The Garki project was planned at a time when eradication of malaria was still viewed as a serious option for highly endemic areas such as Nigeria. The experiment showed that interrupting transmission was not possible even when a full armamentarium of malaria control tools was applied. Its findings helped to redirect attention from eradication to controlling the clinical effect of malaria in Africa. Although the study did not focus on malaria-related morbidity and mortality to the same degree as would be likely today, its parasitemia data suggest that mortality and morbidity from malaria would have fallen dramatically in study villages during the 2-year period of integrated malaria control. Brian Greenwood, London School of Hygiene and Tropical Medicine |
Despite its proven benefits, current ITN use in sub-Saharan Africa also is low. Most households in malaria endemic areas do not possess any net, insecticide-treated or not. In nine African countries surveyed between 1997 and 2001, a median 13 percent of households had one or more nets of any kind; a median 1.3 percent of households in three countries owned at least one ITN; and across 28 countries, only 15 percent of children under age 5 were sleeping under any net (WHO/UNICEF, 2003). Not surprisingly, net ownership and use are lowest in poor households.
In addition to purchase cost and re-treatment, one additional barrier to ITN use is the common misconception that ITNs are meant to control mosquitoes as opposed to malaria. In urban areas with untreated wastewater and high year-round populations of “nuisance” culicine mosquitoes, this misconception favors ITN use. In rural areas, however, mosquito densities and mosquito nuisance are generally lower despite year-round biting by clandestine female anophelines. As a result, ITN use is rarely sustained night after night, especially during the dry season (Gyapong et al., 1996; Binka et al., 1996; Binka and Adongo, 1997).
In western Kenya, the use of ITNs was observed directly in nearly 800 households (Alaii et al., 2003a). About 30 percent of ITNs in homes were unused. Children less than 5 years of age were less likely to use ITNs than older individuals, and ITNs were more likely to be used in cooler weather. Neither mosquito numbers, relative wealth, number of house occupants, nor educational level of the head of the household influenced adherence. Excessive heat was often cited as a reason for not using a child’s ITN. Researchers also commented on the effort required of caregivers to store and rehang the ITN on a daily basis (Alaii et al., 2003a).
Finally, misunderstandings about malaria also lower incentives to use ITNs and/or IRS. In southern Ghana, the Adangbe people believe that asra, a local disease that resembles malaria, is the result of prolonged exposure to heat (Agyepong, 1992). In Bagamoyo District, Tanzania, degedege—a local term for fever and convulsions—is often blamed on a bird-spirit instead of cerebral malaria (Makemba et al., 1996). In western Kenya, many ITN trial participants believed that malaria was a multicausal disease and that ITNs were therefore only partly effective (Alaii et al., 2003b).
OTHER VECTOR CONTROL MEASURES
Household and Community Measures
In areas of high malaria transmission, prevalence of infection may vary significantly over relatively short distances. This has been observed not only in Africa (Greenwood, 1999) but in other countries, such as Papua New
Guinea (Graves et al., 1988), and Pakistan (Strickland et al., 1987). Local factors (in addition to IRS and insecticide-impregnated materials) that affect the microepidemiology of malaria are house siting, screening and construction, proximity of animals to human dwellings, and use of mosquito deterrents such as repellants, aerosols, and fumigants.
House Siting and Construction
Despite the fact that anopheline mosquitoes can fly substantial distances, the proximity of houses or villages to a breeding site strongly influences malaria risk, especially where breeding sites are restricted. In a suburb of Dakar, Senegal, malaria prevalence rose steeply from the center to the edge of town adjacent to marshy breeding sites of Anopheles arabiensis (Trape et al., 1992). In Sri Lanka, the risk of malaria was much higher among those who lived in poor quality houses within 2.5 km of a river where A. culicifacies bred (Gunawardena et al., 1998).
House design and construction also influence the risk of malaria (Schofield and White, 1984). Eaves—which allow interior ventilation, and the escape of smoke from cooking fires—are a common feature that facilitate mosquito access to sleeping areas in houses in the tropics. Using mud or plaster to fill in eaves (Lindsay and Snow, 1988), or hanging eaves curtains (Curtis et al., 1992) reduce human-vector contact. In Sri Lanka, Gunawardena et al. (1998) estimated that the cost of upgrading all low-quality housing (whose residents suffered a fourfold risk of malaria compared to families living in well-constructed houses in the same locale) would be balanced by savings in malaria treatment costs over a period of 7 years.
Animals
In some communities, animals live in or near houses. Zooprophylaxis is a term that suggests the possible diversion of mosquito bites from humans to nearby animals. However, this diversion depends entirely on the biting habits of the local vector and varies from species to species. In some cases, livestock may actually attract certain mosquitoes that would otherwise avoid human habitats, resulting in increased malaria exposure to household members (Hewitt et al., 1994; Bouma and Rowland, 1995; Mouchet, 1998). In Pakistani and Afghan refugee camps, malaria cases were concentrated in communities that kept cattle, presumably because the local vectors A. culicifacies and A. stephensi were preferentially attracted to these households (Bouma and Rowland, 1995).
Repellants, Aerosols, and Fumigants
Many communities use aromatic smokes to deter mosquitoes. In The Gambia, tree bark combined with synthetic perfumes (locally known as churai) reduced the number of mosquitoes entering a room but not the incidence of malaria (Snow et al., 1987). In contrast, traditional fumigants in Sri Lanka decreased malaria (van der Hoek et al., 1998). In Thailand, a mixture of DEET (N,N-diethyl-m toluamide) and a paste made from a local tree (wood apple) was an effective repellant when applied to the skin (Lindsay et al., 1998).
Commercially manufactured coils containing pyrethroids or DDT also repel mosquitoes (Charlwood and Jolley, 1984; Bockarie et al., 1994). Although coils are cheap, households may spend substantial sums of money on items of this kind. In Dar es Salaam, Tanzania, average household expenditure on antimosquito measures was in the region of US$2-3 per month (Chavasse et al., 1999).
Environmental and Biologic Management
Since A. gambiae can breed in virtually any puddle, larval control in sub-Saharan Africa has always been challenging. Where vector breeding sites are few in number and easily identified, however, environmental or biologic control of larval breeding sites is often feasible. Petroleum oil larvicides have played an important part in mosquito control since the beginning of the 20th century. Breeding sites also may be eliminated by draining or filling in pools, modifying the boundaries of rivers or their run-off systems, and creating impoundments (reservoirs behind dams). Intermittent drying of rice fields, stream sluicing or flushing, salination of coastal marshes or lagoons (for example, using tidegates), shading of stream banks, and clearing of vegetation are naturalistic manipulations that proved beneficial in controlling certain vectors, primarily in India and southeast Asia. Although eclipsed by residual insecticides for several decades, many of these environmental methods of vector control are now back in vogue with strong WHO endorsement.
Biological control strategies, including use of bacteria such as Bacillus thuringiensis subsp. israelensis (Bti), or larvivorous fish, also have been combined with other control measures with variable success (Romi et al., 1993; Karch et al., 1993; WHO, 1999; Kaneko et al., 2000). Bti spores produce a toxin that is poisonous to mosquitoes and other aquatic insects but harmless to plants, animals, and humans. Bacillus sphaericus (Bsph) multiplies in polluted waters, and produces a longer-acting toxin than Bti; however, resistance to Bsph toxin is present in some mosquito populations in India, Brazil, and France (WHO, 1999).
Genetic Control
Genetic control refers to any method that reduces an insect’s reproductive or disease-transmitting potential through alteration of its hereditary material. The oldest form of genetic control is sterile insect technology (SIT), a proven strategy in past campaigns against screw worm, tsetse flies, and Mediterranean fruitflies. Unfortunately, when mass hybrid sterility was tried against A. gambiae in Burkina Faso, West Africa, few matings actually took place between the sterile males and wild female anophelines. Producing large numbers of sterile yet competitive male mosquitoes, and successfully releasing them in the wild remain major operational hurdles.
First predicted decades ago by Curtis (Curtis, 1968), genetically modified mosquitoes are now another potential means of vector control. The reasoning is as follows. Since only some anopheline mosquitoes transmit malaria, genes encoding the nontransmitting phenotype, or genes that prevent malaria parasites from developing within mosquitoes altogether, could be inserted into vector genomes (Collins, 1994; James et al., 1999). The feasibility of this approach was recently shown when a synthetic gene inserted into A. stephensi almost fully prevented its ability to transmit a strain of rodent malaria (Ito et al., 2002). However, the same practical obstacle facing SIT—namely, the rapid replacement of native mosquito populations—affects genetically modified mosquitoes. Concerns also have been raised about the fitness of genetically modified mosquitoes, the negative consequences of unstable genetic modifications, and public reservations regarding deployment of genetically altered organisms (Clarke, 2002).
TREATMENT AND CHEMOPREVENTION
The primary aim of malaria treatment is saving lives. Prompt, effective treatment in the early stages of falciparum malaria reduces the risk of death as much as 50-fold, whereas effective treatment after progression to severe illness produces only a five-fold reduction in the risk of dying (White, 1999). However, malaria treatment also can reduce malaria transmission in endemic areas. This section reviews the role of treatment as a control measure capable of reducing malaria transmission, as well as past and present chemoprevention strategies in residents of malaria-endemic areas. (See Chapter 9 for more detailed information regarding antimalarial drugs, drug resistance, and treatment protocols.)
Antimalarials and Reduction of Malaria Transmission
A key objective of many early studies of widespread antimalarial distribution was interrupting malaria transmission (Greenwood, 2004). Two approaches were tried: treatment of symptomatic cases; and mass drug
administration, which included mass chemoprophylaxis, and the Pinotti method—the systematic addition of antimalarial drugs to salt.
Treatment of Symptomatic Cases
The notion that treating symptomatic individuals might indirectly protect an entire malaria-exposed population dates back to Robert Koch and the early years of the 20th century (Harrison, 1978). At that time, with this goal partly in mind, quinine was used extensively in Italy and elsewhere. Since quinine has little effect on gametocytes, however, it had little effect on transmission overall.
Today, in contrast, artemisinin-based drugs (which do kill early-stage gametocytes as well as asexual parasites) have helped to decrease transmission in selected areas of Asia and Africa. On the Thai-Burmese border, where field studies using ACTs were first undertaken in 1991, replacing mefloquine with mefloquine-artesunate as first-line treatment for symptomatic malaria substantially reduced the incidence of local P. falciparum infection (Nosten et al., 2000). Widespread use of artemisinins and ACTs—along with ITNs or IRS—also contributed to a marked decline in the overall incidence of falciparum malaria in Vietnam (Hung et al., 2002) and South Africa (Barnes et al., 2003) (Box 8-2)—both areas with relatively low EIRs. Whether widespread use of ACTs will bring about a similar outcome in areas of higher malaria transmission (EIR > 100) is still unknown.
Mass Drug Administration
Unlike Southeast Asia and South Africa, many malaria infections in sub-Saharan Africa—especially in older children and adults—are asymptomatic and untreated but have parasite densities sufficient for transmission (von Seidlein et al., 2002). Under these conditions, reducing malaria transmission by use of a gametocytocidal drug requires that asymptomatic as well as symptomatic carriers be treated. This led to the concept of mass drug administration (MDA). During MDA, the entire population of a community known to contain many asymptomatic infected subjects receives treatment without first determining who is actually parasitemic.
MDA as a method of reducing malaria transmission gained momentum after the 8-aminoquinolones (of which primaquine is the leading prototype) were discovered. This class of drugs is highly effective at killing gametocytes of P. falciparum. One of the first trials to investigate 8-aminoquinolones as MDA agents took place in a Liberian rubber plantation in 1930. Mass treatment with plasmoquine led to a marked decrease in parasite prevalence and a reduction in infected mosquitoes in two treated camps (Barber et al., 1932). During the 1960s and 1970s, several more
|
BOX 8-2 Ingwavuma district in northern KwaZulu Natal carried the highest malaria burden in South Africa until 2000. This changed after improvements in vector control in KwaZulu Natal and neighboring southern Mozambique during 2000, and the implementation of an artemisinin combination therapy (ACT), artemether-lumefantrine (AL), in January 2001. These interventions were followed by a 78 percent decrease in malaria case notifications in the province within 1 year. There was a further 75 percent decrease in notifications in 2002, and this decrease was sustained throughout 2003 (Figure 8.2-1). 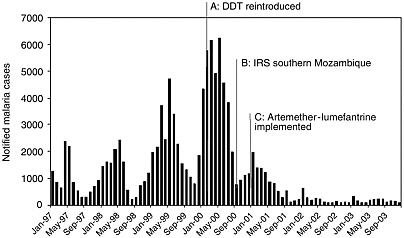
FIGURE 8.2-1 The synergistic effect of improved vector control and artemisinin combination therapy, artemether-lumefantrine, on malaria transmission in KwaZulu Natal, South Africa.
SOURCE: Muheki C, Barnes K, McIntyre D. 2003. Economic Evaluation of Recent Malaria Control Interventions in KwaZulu, South Africa: SEACAT Evaluation. SEACAT. |
|
The success of this dual intervention is further reflected by the 89 percent decrease in both malaria-related hospital admissions (range 81-92 percent), and deaths (range 78-93 percent) across the three rural district hospitals that serve the Ingwavuma community. Despite KwaZulu Natal also carrying South Africa’s highest burden of the HIV/AIDS epidemic, the total number of “all cause” hospital admissions also was reduced following improved malaria control. Dr Hervey Vaughan Williams, medical superintendent of Mosvold hospital, a rural district hospital in Ingwavuma, reports that “during the peak of the 2000 malaria epidemic, hospital admissions were averaging three patients per bed.” Following the reduction in the overall number of hospital admissions, it is expected that the standard of care for all patients could be improved. These improvements in malaria control also have been welcomed by the local communities. During focus group discussions on malaria, there was general enthusiasm for the change in malaria treatment. One female household head reported “Every year I used to have malaria. This year I heard that new pills were coming. I was very sick with malaria. They gave me the new pills … the (next) morning I was very fine. … I have cultivated and harvested and I’ve never had malaria again this year.” These comments are in contrast with the opinion of female household heads on SP, e.g., “Those tablets that were used before the present ones … most of us didn’t like taking them … because after taking them you would feel as if the malaria has become more severe than before.” The community perceptions that those with malaria should seek health care urgently at public-sector facilities, is likely to have contributed to the marked public health effect observed following the implementation of AL. These perceptions were described during focus group discussions: “There is no way to treat malaria besides taking him to the clinic”; “Once you waste time at the sangoma [traditional healer], you die.” The sustained benefits to public health following the implementation of artemether-lumefantrine in KwaZulu Natal are most encouraging. These reflect the benefits of ACT in significantly decreasing treatment failure and gametocyte carriage rates, within a context of effective vector control, and a relatively well developed rural primary health care infrastructure. Karen Barnes, University of Cape Town (2003) |
MDA trials took place in Africa and Asia with the primary aim of interrupting transmission (von Seidlein and Greenwood, 2003). By today’s standards, the studies were poorly designed and their results difficult to assess; however, in nearly all cases, MDA failed to interrupt transmission, although it did markedly reduce parasite prevalence (Greenwood, 2004) (Box 8-1, The Garki Project). In 1981, the largest-ever MDA program produced essentially the same outcome. After a single round of chloroquine plus primaquine was given to roughly eight million people in Nicaragua, there was an immediate, marked decrease in clinical P. vivax malaria, but transmission continued and the incidence of malaria soon returned to its previous level (Garfield and Vermund, 1983).
In most MDAs, tablets have been given, sometimes under supervision. Although this ensures effective dosing, it is onerous. As an alternative to tablets, Pinotti devised the concept of drug delivery using medicated salt (Pinotti, 1954). During the 1950s, a number of trials of medicated salt were conducted in malaria-endemic areas (Payne, 1988); they generally led to a reduced incidence of clinical episodes of malaria at the cost of rapid emergence of antimalarial resistance (Meuwissen, 1964; Giglioli et al., 1967). It is now known that exposing malaria parasites to suboptimal doses of antimalarial drugs over time is an ideal way to induce resistance. Thus, medicated salt has no place in malaria control today.
One MDA program that incorporated other control interventions was recently reported from the island of Aneityum, Vanuatu. In this case, the effort was successful. Eight rounds of MDA with chloroquine-SP-primaquine combined with ITNs and environmental control measures eliminated falciparum infection from the island (Kaneko et al., 2000).
Chemoprophylaxis
Antimalarial chemoprophylaxis is often used by short-term, nonimmune visitors to high-risk areas. Its direct benefits to individuals are obvious, while its indirect benefits include expanded international tourism, business travel, and economic development of malarious regions. However, the use of antimalarial drugs to protect the resident population of malaria-endemic areas has always been more controversial (Greenwood, 2004). The following two sections summarize past data and current trends regarding the use of drugs to protect high-risk individuals within P. falciparum endemic areas from acute infection.
Chemoprophylaxis in Children
In 1956, McGregor and others reported the results of a trial in The Gambia in which children received chloroquine weekly from birth until
age 2. Children who received chemoprophylaxis had fewer episodes of malaria, grew better, and had higher mean hemoglobin values than children in the control group (McGregor et al., 1956). These findings were later reproduced in studies in several other African countries (Prinsen Geerligs et al., 2003).
In the 1980s, a 5-year trial using weekly Maloprim (pyrimethamine plus dapsone) during the rainy season in children under 5 was conducted in The Gambia (Greenwood et al., 1988). Overall mortality was approximately 35 percent lower in children on prophylaxis. Protected children also had fewer clinical attacks of malaria, and a higher mean packed cell volume than control children. These results were sustained over several years (Allen et al., 1990), and are at least as impressive as results obtained with ITNs. A more recent study in Tanzanian infants using Maloprim also showed a marked reduction in clinical attacks of malaria, and the incidence of severe anemia (Menendez et al., 1997).
Chemoprophylaxis in Pregnant Women
Antimalarial chemoprophylaxis in pregnant women was first studied in a Nigerian mission hospital in 1964 (Morley et al., 1964). Chemoprophylaxis with pyrimethamine substantially increased birth weight overall, but particularly in infants born to primigravidae (women pregnant for the first time). Later studies in other African countries confirmed chemoprophylaxis’s positive effects on birth weight (Garner and Gulmezoglu, 2000), and maternal hemoglobin levels (Greenwood et al., 1989), particularly during first and second pregnancies (Greenwood, 2004). Drugs used for chemoprophylaxis in pregnancy include chloroquine, pyrimethamine, proguanil, Maloprim, and mefloquine. Although rarely implemented on any scale until recently, WHO recommended that all pregnant women in areas of moderate or high malaria transmission receive weekly chemoprophylaxis with chloroquine during their second and third trimesters. Because of drug resistance, this policy no longer offers much benefit.
Obstacles to Targeted Chemoprophylaxis
Despite impressive results in clinical trials, targeted chemoprophylaxis has never been recommended for children residing in malaria-endemic countries, and it has rarely been implemented in pregnant women. Concerns over cost, sustainability, and safety have all contributed to its poor uptake (Greenwood, 2004). In addition, some experts are concerned that young children routinely administered chemoprophylaxis may fail to develop natural immunity. In The Gambia, children who received chemoprophylaxis for 4-5 years did experience a statistically significant increase in clinical attacks
of malaria in the year after prophylaxis ended; however overall protection from death extended to age 10 following antimalarial prophylaxis during early years of life (Greenwood et al., 1995).
A final theoretical objection to chemoprophylaxis for high-risk individuals in malaria-endemic regions is its possible induction of drug resistance. Although medicated salt and the unrestricted use of chloroquine and pyrimethamine in the 1960s contributed to the initial emergence and spread of drug resistance (Payne, 1988), there is little evidence to suggest that targeted chemoprophylaxis produces the same effect to any meaningful degree. Chemoprophylaxis will inevitably lead to an increase in drug pressure, but this may be an acceptable price to pay if benefits are large. The minimal risk of drug resistance also is likely to be mitigated by the future use of combination therapy.
Intermittent Preventive Treatment
Intermittent preventive treatment (IPT) is a full therapeutic course of antimalarial treatment administered at specified times whether or not a recipient is infected. Unlike chemoprophylaxis (which aims to sustain blood levels above the mean inhibitory concentration for a prolonged period), IPT yields shorter bursts of protective drug levels separated by periods when drug levels are too low to inhibit parasite growth. In general, there are still many unknowns regarding IPT’s mechanisms of action. In the case of SP (the drug most widely used for IPT in children and pregnant women), for example, it is still not clear if IPT works primarily by eliminating existing parasites, or through a long-acting prophylactic effect of the drug (Greenwood, 2004).
IPT in Pregnant Women (IPTp)
In Malawi, Schultz and others found that a full course of SP given twice during pregnancy protected against low birth weight to a significantly greater degree than weekly chloroquine chemoprophylaxis (Schultz et al., 1994). In Kenya, a controlled trial with IPTp with SP given two or three times during pregnancy also reduced severe anemia in women pregnant for the first or second time (Shulman et al., 1999). In another area of Kenya, however, HIV-infected women required more than two or three doses of IPTp to prevent placental infection (Parise et al., 1998). Since all trials were conducted when P. falciparum was more sensitive than it is now, it is unlikely that IPT using SP would achieve the same effects today.
WHO now recommends that IPTp with SP be given at each antenatal clinic attendance after quickening (usually between the 16th and 18th week of pregnancy), replacing chemoprophylaxis as the preferred method for the
prevention of malaria in pregnancy (WHO/UNICEF, 2003). Benefits in HIV-infected women are unclear, however, as is the efficacy of IPTp in areas where malaria transmission is highly seasonal or endemicity is low (SP resistance is particularly widespread in low transmission areas). In addition, it is not yet clear what drug or combination of drugs will eventually replace SP for intermittent preventive treatment in pregnancy.
IPT in Infants (IPTi)
The IPT concept has recently been applied to the prevention of malaria in infants. In Tanzania, administration of a full dose of SP to infants concurrent with the second and third doses of diphtheria-pertussistetanus, and measles vaccines reduced the incidence of clinical malaria attacks and severe anemia during the first year of life by 59 and 50 percent, respectively (Schellenberg et al., 2001). Amodiaquine given in full therapeutic dose three times during the first year of life yielded similar results (Massaga et al., 2003). Also important, no rebound in malaria attacks or anemia occurred during the second year of life in children who received IPTi as infants. Trials of IPTi using SP are nearing completion in Ghana and Kenya, and further trials have recently started in Mozambique, Gabon, and Kenya. These trials will help to determine under what conditions IPTi will prove most effective, as well as the EIR threshold below which it will not be useful.
IPT in Children (IPTc)
In many areas of Africa, perhaps covering as much as 50 percent of the population at risk, the major burden of malaria is not in infants but in older children. This is especially true in countries of the Sahel and sub-Sahel where malaria transmission is intense but seasonal. A pilot study in Senegal employing SP plus artesunate at 1-month intervals throughout the transmission season is currently exploring whether the IPT principle also can be applied to older children (Greenwood, 2004).
DIAGNOSTIC METHODS
The correct and timely diagnosis of malaria is critically important to the individual patient in whom the disease may quickly progress to a life-threatening stage. Rapid diagnosis, both on an individual and a population level, also is an important tool in overall malaria control. This section reviews currently available methods of diagnosis, with emphasis on newer rapid tests that require relatively little technical expertise to perform. Such tests are now facilitating the delivery of effective treatment in a variety of
low-transmission settings, and will eventually find their place in malaria case management in high-transmission settings as well.
Clinical (Presumptive) Diagnosis
Although studies reveal that health providers cannot reliably identify malaria by clinical signs and symptoms alone (Weber et al., 1997; Perkins et al., 1997; Chandramohan et al., 2002), the use of clinical diagnosis is common in endemic areas. Ease, speed, and low cost are the advantages of presumptive diagnosis; disadvantages include overdiagnosis—which contributes in turn to wasted drugs, adverse drug effects, and accelerating drug resistance—as well as underdiagnosis of malaria. Since other common diseases—acute lower respiratory tract infection in children, in particular—may mimic the signs and symptoms of malaria, clinical diagnosis also can result in missed diagnoses of other treatable conditions (Redd et al., 1992; Rey et al., 1996). In fact, the difficulty of differentiating malaria from pneumonia was one of the major factors that led to the establishment of Integrated Management of Childhood Illnesses (IMCI)—an algorithmic approach to the treatment of sick children now used in many developing countries (Personal communication, B. Greenwood, London School of Hygiene and Tropical Medicine, March 2004). Whatever the theoretical pros and cons of presumptive diagnosis, however, it often is all that is available.
Light Microscopy
The current gold standard for the routine laboratory diagnosis of malaria and monitoring of cure is the microscopic examination of thin and thick blood films stained with Giemsa’s, Wright’s, or Field’s stain (Warhurst and Williams, 1996). In the thick film technique, a “thick” layer of blood, containing several layers of red blood cells (RBCs), is concentrated on a small area of a microscope slide. The red and white cell membranes are lysed, and the intracellular remnants examined for the presence of plasmodial forms. The thin film technique produces a monolayer of RBCs, facilitating species identification, and serial counting of parasites. Thin films are particularly helpful in severe malaria, both as a fast diagnostic test (White and Silamut, 1989), and a prognostic tool (Silamut and White, 1993; Phu and Day, 1995).
Although experienced microscopists may detect as few as 10-50 parasites/uL, and identify the species of 98 percent of parasites seen (Moody, 2002), in actuality, obtaining such results requires a high level of skill and time, as well as optimal maintenance of equipment and reagents. Such conditions almost never exist on the periphery of health care in Africa, and other poor, malaria-endemic locales worldwide.
In one African trial, malaria diagnosis by light microscopy was shown to reduce drug use (Jonkman et al., 1995). However, the current reality in many endemic settings is that clinicians question the reliability of light microscopy results, or frankly disregard them (Barat et al., 1999). Mixed infections (involving more than one plasmodial species) also are routinely underreported by light microscopy (Personal communication, N. White, Mahidol University, March 2004).
Fluorescent Microscopy
The quantitative buffy coat test (QBC, Becton Dickinson) is a modification of light microscopy which exploits the affinity of parasite nucleic acid for acridine orange, a fluorescent dye. This test was adapted for malaria diagnosis using patented microhematocrit tubes pre-coated with acridine orange and fitted with a plastic float that spreads the buffy coat (the white blood cell and parasite fraction of blood) against the edge of the tube. When centrifuged, malaria parasites and leukocytes take up dye and collect at a predictable location within the tube where they can then be seen under ultraviolet light.
In field trials, QBC was slightly more sensitive than conventional light microscopy (Rickman et al., 1989; Tharavanij, 1990). Disadvantages of QBC are its need for electricity, special equipment, and supplies; its increased cost relative to light microscopy; and its inability to differentiate P. falciparum from other human malaria species.
Rapid Diagnostic Tests (RDTs)
A third diagnostic approach involves “dipstick” tests, which eliminate the need for a microscope and detect parasite antigens in blood by rapid immunochromatography using various antibodies. More than 20 such tests are currently marketed, and several are made in malaria-endemic countries. The majority are based on histidine-rich protein 2 (HRP-2) found in P. falciparum. Compared with light microscopy and QBC, HRP-2 tests yield a rapid and highly sensitive diagnosis of falciparum infection (WHO, 1996b; Craig and Sharp, 1997). The main drawbacks of HRP-2 tests are their high per-test cost (lowest price circa 50 U.S. cents) and inability to quantify the intensity of infection. In addition, HRP-2 antigen may persist for days or weeks following successful treatment. As a result, HRP-2 tests do not distinguish cured from nonresolving (i.e., drug-resistant) infections (WHO, 1996b). Several tests using HRP-2 to detect P. falciparum also include a second antigen to distinguish P. falciparum from other malaria species, although sensitivity generally is lower for P. vivax, and other non-P. falciparum species (Murray et al., 2003).
The OptiMAL dipstick detects the parasite enzyme lactate dehydrogenase (pLDH), which is actively produced by all malaria parasites during growth in human red cells (Piper et al., 1999). Unlike HRP-2, pLDH disappears from the bloodstream along with malaria parasites following successful treatment. OptiMAL also distinguishes P. falciparum from the other malaria species without differing levels of sensitivity for other species of malaria. In general, performance of OptiMAL in field studies has been high, but sensitivity in some recent trials has been erratic, perhaps due to batch problems or poor test handling (Murray et al., 2003). Quality control procedures for all rapid test types need improvement.
Field Application of Rapid Diagnostic Tests
In selected tropical countries and resource-poor settings where microscopy is either unavailable or unreliable, RDTs have great potential to reduce inappropriate antimalarial chemotherapy. For one thing, it is much easier to train someone to perform an RDT reliably than to be a reliable microscopist. RDTs have been used successfully in remote tribal groups in forested areas of central India (Singh et al., 2000), in refugee camps on the Thai-Burma border (Bualombai et al., 2003), and in rural health centers in Cambodia (Rimon et al., 2003). However, none of these areas approach the transmission levels of certain highly endemic areas of sub-Saharan Africa.
The utility of antigen detection in areas of Africa hyperendemic for malaria is less clear, because the tests are not quantitative, and the mere finding of parasites may be insufficient grounds for a diagnosis of malaria. One study in Zimbabwe indicated that mistreatment was reduced by up to 81 percent when ParaSight-F was used compared to presumptive diagnosis (Mharakurwa et al., 1997). However, the improvement in diagnostic accuracy was most marked in the hypodendemic part of the study region.
In summary, when to introduce RDTs into areas of sub-Saharan Africa hyperendemic for falciparum malaria remains an open question. One perspective on the use of RDTs comes from Asia, where providing the means to diagnose and treat malaria on the village level has coincided with a dramatic fall in morbidity and mortality. The fact that drug pressure is a major contributor to transmission of resistant parasites is another reason why universal treatment without RDTs should not be permitted indefinitely anywhere in the world. However, in high prevalence areas of sub-Saharan Africa, universal treatment of clinically suspected cases with ACTs is the safest near-term strategy, given the desperate need for rapid, in- or near-home treatment in order to save lives. Eventually, combining RDTs and ACTs in areas of Africa of low and medium malaria prevalence could
reduce the number of illnesses misdiagnosed as malaria and yield major savings in terms of drug cost and delayed onset of drug resistance. In the meantime, following the introduction of ACTs in Africa, pilot programs and operational research combining ACTs and RDTs in a variety of epidemiologic settings and at-risk populations should be encouraged.
Molecular Methods
When malaria patients are parasitemic, their blood also contains malaria DNA and RNA. Applying a DNA probe to a filter paper with a small spot of human blood can identify malaria genetic material, including mutations or gene amplifications conferring drug resistance (Plowe and Wellems, 1995). Today, such methods are increasingly being used to detect molecular markers of drug resistance to chloroquine and SP. As long as the filter papers are properly handled, DNA can be recovered from them months after obtaining the samples (Farnert et al., 1999).
DNA and RNA techniques for rapid malaria diagnosis are currently impractical in most endemic regions, but their role may expand in selected populations as the tests become simpler and cheaper. Sensitive PCR techniques can detect parasites at a density as low as 1 per microliter, in contrast to 10-50 parasites per microliter with expert light microscopy (Greenwood, 2002).
MALARIA VACCINES
No malaria vaccine has yet entered routine use, and a safe and effective vaccine is at least another 10 years away (Greenwood and Alonso, 2002). This fact alone supports the central argument of this report, namely that a subsidy for effective treatment is an urgent priority that cannot wait until another control measure such as a vaccine is implemented. Nonetheless, the last 2 decades have seen significant progress in malaria vaccine research (Greenwood and Alonso, 2002). Highlights are summarized below.
The malaria vaccine era launched in the 1970s when, building on earlier work in rodent models, investigators at the University of Maryland demonstrated that irradiated sporozoites of P. falciparum and P. vivax protected naïve volunteers against challenge by infective mosquitoes (Clyde, 1975). For the next 2 decades, advances came mainly in experimental models rather than in human trials (Kwiatkowski and Marsh, 1997). In 1990, the first report of a vaccine field trial in an endemic area was published: a study of pre-erythrocytic vaccine in Burkina Faso (Guiguemde et al., 1990). A recent meta-analysis of malaria vaccine trials documented 18 trials with 10,971 participants using pre-erythrocytic and blood-stage vaccines (Graves and Gelband, 2003). Table 8-2 lists vaccines in field trials as
TABLE 8-2 Candidate Malaria Vaccines in Clinical Trials
|
Group (field collaboration) |
Vaccines (type) |
Target |
|
Apovia, USA; New York University, USA |
ICC-1132 (protein) |
Pre-erythrocytic |
|
GlazoSmithKline Biologicals, Belgium; and WRAIR, USA (Medical Research Council [MRC] Laboratories, The Gambia; Centro de investigacao em Suade de Manhica [CISM], Mozambique) |
RTS,S (protein) |
Pre-erythrocytic |
|
Malaria Vaccine Development Unit, National Institutes of Health, USA |
Pvs25, AMA-1 (protein) |
Transmission blocking, blood stage |
|
Naval Medical Research Center (NMRC), USA; Vical USA |
Pf-CS, Pf-SSP2/TRAP, Pf-LSA-1, Pf-EXP-1, Pf-LSA-3 (DNA vaccines) |
Pre-erythrocytic |
|
New York University, USA |
CS (synthetic polymers, MAPs, polyoximes) |
Pre-erythrocytic |
|
Oxford University, UK (MRC Laboratories, The Gambia; Wellcome-Kenya Medical MVA Research Institute [KEMRI] collaboration, Kilifi, Kenya) |
DNA ME-TRAP, ME-TRAP, FP9 ME-TRAP, MVA-CS (DNA and recombinant viral) |
Pre-erythrocytic |
|
Statens Serum Institut (SSI), Copenhagen; Institut Pasteur; Institute of Lausanne, Switzerland |
GLURP, MSP-3 (synthetic peptide) |
Pre-erythrocytic, blood stage |
|
Walter and Eliza Hall Institute of Medical Research, Melbourne; Queensland Institute of Medical Research (QIMR), Brisbane; Swiss Tropical Institute; Biotech Australia Pty (Papua New Guinea Institute of Medical Research) |
MSP-1, MSP-2, RESA (protein) |
Blood stage |
|
Walter Reed Army Institute of Research (WRAIR), USA (KEMRI, Kisumu, Kenya) |
FMP-1 (protein) |
Blood stage |
|
EXP = exported protein. LSA = liver stage antigen. MAP = multiple antigen peptide. Pvs = Plasmodium vivax surface protein. AMA-1 = apical membrane antigen-1. RESA = ring infected erythrocyte surface antigen. SSP2 and TRAP are synonyms: sporozoite surface protein 2 and thrombospondin-related adhesion protein. Only vaccines being tested in clinical trials as of May 2003 are listed. Field collaborations are listed only if field trials of the candidate had begun as of May 2003. SOURCE: Moorthy et al. (2004). |
||
of May 2003 (Moorthy et al., 2004). Additional single and combination antigens are currently in various stages of pre-clinical assessment.
A comprehensive discussion of all malaria vaccines is beyond the scope of this report; however, certain basic concepts and prototype vaccines are covered below. For further details of specific vaccine antigens and strategies, readers are directed to several excellent published reviews (Moore et al., 2002; Carvalho et al., 2002; Mahanty et al., 2003; Moorthy et al., 2004).
Natural Immunity to Malaria
In malaria-endemic areas, natural human infection with P. falciparum induces some degree of immunity which first protects against severe, then mild malaria (McGregor, 1974). Maintaining functional immunity requires repeated infective mosquito bites, however (Cohen et al., 1961; Marsh and Howard, 1986). Of the 5,300 antigens encoded by P. falciparum, possibly 20 trigger key protective immune responses of both major types—antibody, and T-cell dependent—that follow natural exposure (Moorthy et al., 2004).
Vaccine Strategies Based on Parasite Life Cycle Stages
Unlike natural infection, experimental vaccines have traditionally targeted one of malaria’s three biologic stages in its human or mosquito host: sporozoite, merozoite, or gametocyte. The goal of pre-erythrocytic (or sporozoite) vaccines is to block the initial entry of sporozoites into human liver cells. A current candidate of this class is RTS,S—a recombinant protein vaccine which pairs hepatitis B surface antigen DNA with DNA encoding a large portion of P. falciparum circumsporozoite protein. In a randomized controlled trial of three-dose RTS,S in Gambian adults, vaccine efficacy was 34 percent over a 15-week surveillance period (falling from 71 percent efficacy in the first 9 weeks to 0 percent in the next 6 weeks) (Church et al., 1997). Although short-lived, this vaccine-induced defense constituted the first protection against natural P. falciparum infection by a pre-erythrocytic vaccine. Phase I trials of RTS,S in children aged 1-11 years in The Gambia and Mozambique have now been completed, and a trial in children 1-4 years in Mozambique is currently under way. However, it is likely to be 10 years at least before RTS,S finds a place in routine immunization programs, even if the current pediatric trials are successful (Personal communication, B. Greenwood, London School of Hygiene and Tropical Medicine, March 2004).
In contrast to the all-or-none protective action of pre-erythrocytic vaccines, merozoite (or blood stage) vaccines have two potential effects: limiting RBC invasion, and reducing disease complications. Merozoite surface
protein-1 (MSP-1) is the best characterized antigen mediating RBC invasion; it forms the basis of several candidate vaccines. Although some data suggest that vaccine-induced antibodies to MSP-1 may block natural malaria-protective antibodies (Holder et al., 1999), an anti-invasion vaccine based on MSP-1 has now moved to an adult phase I study in western Kenya.
Intravascular attachment of schizonts to endothelial cells in the brain, kidneys, and placenta is the most serious consequence of falciparum malaria. PfEMP-1 antigen is the main ligand for this sequestration event. Unfortunately, this antigen’s high degree of variability, rapid rate of antigenic variation, and high copy number within individual parasites complicates vaccine development. Some investigators remain hopeful that conserved regions of PfEMP-1 may yield protective responses in humans (Moorthy et al., 2004).
Sexual-stage vaccines, rather than protecting vaccinated individuals, are meant to reduce malaria transmission, especially in combination with other control methods. Their principle is as follows. Anti-gametocyte antibodies induced in vaccinated humans enter female anophelines via blood meals. These antibodies interfere with parasite development in the vector’s midgut, preventing further transmission. One major drawback to sexual stage vaccine candidates is their lack of commercial appeal to major pharmaceutical companies. Unlike sporozoite vaccines in particular (which could find a sizable market in tourists, migrants, and military personnel entering malarious zones) (Hoffman, 2003), sexual-stage vaccines are unlikely to be marketed outside malaria-endemic countries. An NIH-sponsored clinical trial of a recombinant protein (Pfs25) P. falciparum gametocyte vaccine is currently in development.
What Is Really Needed from a Malaria Vaccine?
Modern malaria vaccine development has followed a somewhat chance course thus far, guided by discoveries of particular antigens or enthusiasms of individual investigators (Greenwood and Alonso, 2002). However, as resources increase and more potential vaccine antigens are identified, defining the primary characteristics needed in a malaria vaccine becomes essential.
A perfect malaria vaccine would induce lifelong sterilizing immunity, provide cross-species protection, protect the very young, and be compatible with routine EPI (Expanded Programme on Immunization) vaccines. Since none of these goals will be achieved quickly, vaccine researchers are faced with a choice: either to work on a safe, highly efficacious and commercially viable vaccine (such as a pre-erythrocytic vaccine) for the short-term traveler or to work on a vaccine that offers more long-lasting protection, aug-
menting (as opposed to replacing) natural immunity in residents of malaria-endemic areas. For example, among semi-immune recipients, a blood-stage vaccine that lowered parasite density might prove beneficial even if it had little effect on the incidence of infection. Similarly, a partially effective preerythrocytic vaccine might protect against death from malaria just as ITNs do. Whether or not an imperfect malaria vaccine justifies use in a particular setting will depend upon its efficacy relative to other available control tools, its acceptability, and its cost.
Pregnant women in malaria-endemic areas, especially primigravidae, have a special need for enhanced malaria protection because of malaria’s damaging effects in pregnancy. In some areas, it is becoming difficult to provide this protection through any other means (e.g., chemoprophylaxis, or intermittent treatment). A vaccine that prevented sequestration of P. falciparum parasites in the placenta might lessen the chance of a pregnant woman giving birth to a low-birth weight baby, but would not protect her from anemia.
Single versus Combination Candidates
For various reasons, including malaria’s antigenic variation and protein polymorphisms, it is unlikely that any single malaria antigen will be found that meets all of the criteria for a perfect vaccine. Thus, the most effective malaria vaccines are likely to contain a “cocktail” of antigens from the same stage of the parasite’s life cycle, or different antigens from different stages. The most extensively tested combination vaccine thus far is SPf 66, which includes several erythrocytic-stage antigens originally found protective in Colombia (Pattarroyo and Armador, 1999). However, trials using SPf66 in holoendemic areas of Africa showed marginal, if any, protection (Alonso et al., 1994; D’Alessandro et al., 1995), nor did the vaccine confer protection upon Tanzanian infants when administered as part of their initial EPI vaccine package (Acosta et al., 1999). The vaccine also failed to protect Karen children in a malarious region of northwestern Thailand (Nosten et al., 1996).
The first multistage, multiantigen P. falciparum vaccine candidate was NYVAC-Pf7, an attenuated vaccinia virus genetically engineered to include seven P. falciparum genes encoding the pre-erythrocytic antigens CSP and LSA1; the asexual blood stage antigens MSP1, AMA1 and SERA; and the transmission-blocking antigen PFs25 (Tine et al., 1996). Initial studies did not demonstrate protection against P. falciparum in human volunteers, although detectable immune responses were elicited (Ockenhouse et al., 1998). MuStDO (Multi-Stage Malaria DNA Vaccine Operation) is an ongoing collaboration involving several scientific institutions worldwide, including the Naval Medical Research Center (NMRC), the U.S. Agency for
International Development (USAID), the Noguchi Memorial Institute of Medical Research in Accra, Ghana, and the Navrongo Health Research Centre in Navrongo, Ghana. Experimental development of the MuStDO vaccine is currently focused on 15 P. falciparum antigens (5 pre-erythrocytic and 10 erythrocytic proteins) (Kumar et al., 2002), although future versions also may incorporate transmission-blocking antigens.
Practical Realities of Vaccine Testing and Use
Selecting which malaria antigens should enter clinical trials was not a major problem when there were relatively few candidates to choose from. However, characterization of the P. falciparum genome has now identified hundreds of parasite proteins that could potentially be tested as individual vaccine components. Selecting future vaccine components should rest upon: 1) evidence that the antigen plays an important role in the survival or pathogenicity of the parasite; 2) evidence from animal experiments that the antigen induces protective immunity in vivo; and 3) evidence that immune responses to the antigen are associated with protection (Greenwood and Alonso, 2002). If a vaccine candidate passes Phase I and Phase II testing, further considerations in designing phase III trials include study site, study population, study size, and appropriate clinical end points (Table 8-3). Another important factor that will influence whether a partially effective malaria vaccine is introduced into routine use will be its cost effectiveness relative to other malaria control measures, such as ITNs (Graves, 1998; Goodman et al., 1999).
TABLE 8-3 Possible End Points for Phase-III Malaria Vaccine Trials
|
End Point |
Advantages |
Disadvantages |
|
Death |
Most important public health end point |
Requires large trial |
|
Malaria mortality rate |
|
Difficult to measure |
|
Severe clinical malaria |
Important end point |
Requires high hospital admission rate |
|
Mild, clinical malaria |
Requires relatively small trial |
May miss an effect on severe disease |
|
Parasitemia |
Easy to measure |
May miss an important clinical effect |
|
SOURCE: Greenwood and Alonso (2002). |
||
No matter when and where vaccination enters widescale use, it is unlikely to supplant the need for effective treatment of drug-resistant falciparum malaria for several decades (Personal communication, B. Greenwood, London School of Hygiene and Tropical Medicine, March 2004).
INTEGRATED CONTROL PROGRAMS AND SPECIAL SETTINGS
Past and Present Models
Over recent years, several historical examples have been cited as evidence that malaria control could be achieved in endemic areas by combining environmental and human interventions. In the 1930s and 1940s, successful multipronged approaches to control transmission by A. gambiae operated in Brazil, Egypt, and Zambia (Utzinger et al., 2001; Killeen et al., 2002). In the Zambian copper mines, clearing vegetation, modifying river boundaries, draining swamps, and applying oil to open bodies of water coupled with house screening, mosquito nets, and quinine treatment reduced the local incidence of malaria by 70 to 95 percent for over 30 years (Utzinger et al., 2001). Today, contemporary versions of such programs are run by Exxon-Mobil in Cameroon and Chad, British Petroleum in Angola, and Konkola copper mine in Zambia.
In addition to commercial operations with a financial incentive to protect workers or residents from malaria using various combinations of environmental and vector control measures plus chemotherapy, local health authorities in endemic and epidemic settings have sometimes mounted integrated malaria programs within the normal health structure incorporating some or all of these elements (Shiff, 2002). Needless to say, such programs require coordination and planning, cooperation of local communities, and sustainable financing. A campaign that combined IRS plus artemisinin-containing combination therapy in KwaZulu Natal province, South Africa (in response to a local epidemic of multidrug resistant Plasmodium falciparum) is the most recent example of a successful government-sponsored integrated control program (Muheki et al, 2003). In recent years, research studies in Sierra Leone (Marbiah et al., 1998) and The Gambia (Alonso et al., 1993) have also supported the packaging of ITNs plus targeted chemoprophylaxis as a successful control package. On the other hand, the systematic deployment of ACTs in refugee camps on the northwest border of Thailand (population 120,000) produced a sustained reduction of more than 90 percent in the incidence of falciparum malaria without any additional control intervention (Nosten et al., 2000).
Urban Malaria
Integrated approaches are especially suited to malaria control programs based in urban areas. Although urban centers traditionally experience lower rates of malaria transmission than rural areas, the rapid increase in the world’s urban population has major implications for the epidemiology of malaria (Robert et al., 2003). This is particularly true in sub-Saharan Africa, the most rapidly urbanizing continent (United Nations, 1999). In 1900, 10 percent of Africans lived in an urban area, whereas today almost half of the population in sub-Saharan Africa lives in urban or suburban areas. This proportion is expected to continue to rise over the next 25 years.
In fact, many African cities have unique features that still favor the transmission of malaria. Unlike Western cities with their distinct patterns of land use, housing construction, public utilities, and social services, many African cities have no such infrastructure, and feature larval habitats such as rice fields and garden wells adjacent to residential, market, and commercial areas (Robert et al., 2003), as well as suburban slums and shantytowns. Limited data also suggest that certain anopheline vectors may be adapting to urban ecology. In Accra, Ghana, A. gambiae has adapted to water-filled domestic containers and polluted water habitats (Chinery, 1990). In a newly urbanized area of Kenya, A. gambiae also bred in temporary man-made sites during the rainy season (Khaemba et al., 1994).
Excluding large private operations, it is currently believed that reducing malaria in a sustained fashion by vector control alone is near impossible in areas of high and moderate transmission (Trape et al., 2002). On the other hand, areas with naturally low or unstable transmission—such as highlands, mountain areas, semi-arid regions and urbanized areas—are far more amenable to vector control. As urbanization progresses, anopheline breeding sites in African cities should decrease and localize, thus facilitating control by classical methods such as drainage, larviciding, and indoor spraying (Trape and Zoulani, 1987; Trape et al., 1992). In the future, the upside of sub-Saharan urbanization may be the creation of malaria-free zones harboring a significant proportion of a country’s population. This success could, in turn, stimulate expanding eradication efforts using vector control and other integrated interventions.
Epidemic Malaria
Malaria epidemics generally occur in regions where transmission is low or absent most of the time and populations lack protective immunity, although epidemics also can strike higher-transmission areas when there is a breakdown in health or environmental services, increasing drug resistance, or recent immigration of nonimmune individuals (e.g., laborers coming to
work in a construction or other development project). Climate change also contributes to the development of epidemics. In terms of population effect, two common features of nearly all malaria epidemics are: 1) an equal risk of clinical disease and death among children and adults, and 2) a clinical burden that outweighs and overwhelms available health services (Snow and Gilles, 2002).
Epidemics and Climate Change
On the fringes of endemic areas, climate often restricts malaria transmission—i.e., normal conditions are either too dry, too wet, or too cold for propagation of vectors or parasites. In such sites, small environmental changes can trigger an epidemic. The El Niño Southern Oscillation (a weather event that originates in the Pacific Ocean but has wide-ranging global consequences, in particular droughts and floods) has been particularly well studied in this regard (Kovats et al., 2003). For example, El Niño-related droughts have been associated with malaria outbreaks in Sri Lanka (Bouma and van der Kaay, 1996), Colombia (Poveda et al., 2001), and Irian Jaya (Bangs and Subianto, 1999). Conversely, in highland regions, elevated temperatures due to El Niño may increase malaria transmission, as occurred in Northern Pakistan in 1981-1991(Bouma et al., 1996). Higher than usual temperatures, and heavy rainfall also have contributed to short-term increases in highland malaria in Rwanda (Loevinsohn, 1994) and Uganda (Lindblade et al., 1999).
Despite the effect of climate change on highland malaria, emerging evidence suggests that drug resistance has influenced highland epidemics in east Africa to a far greater degree than any other environmental factor (Hay et al., 2002). For example, chloroquine resistance has been identified as a key factor in the resurgence of malaria on the Kericho tea estates in Kenya where climate, environment, human population, health care provision, and malaria control measures remained stable (Shanks et al., 2000). Similarly, the malaria resurgence in the Usambara mountains of Tanzania has now been linked to the rise in antimalarial drug resistance (Bodker et al., 2000) as opposed to previously postulated climate change (Matola et al., 1987).
Mass Population Movements
There are many reasons for mass migration: war and civil strife, economic resettlement, environmental and natural disasters. Under appropriate conditions, this mobility can affect malaria transmission. Among 20 countries with a high risk of malaria transmission in the Americas, 16 identified human mobility as a major cause of persistence of transmission (PAHO, 1995). Migration also has been associated with the spread of drug-
resistant malaria in Africa and Southeast Asia and with a dramatic change in the local epidemiology of malaria in Pakistan (Verdrager, 1986; Thimasarn et al., 1995; Kazmi and Pandit, 2001). In Kenya, the seasonal movement of many workers from lowlands to highland tea plantations has triggered epidemics (Malakooti et al., 1998; Shanks et al., 2000). Seasonal influx of migrant farm workers from Central and South America has produced local outbreaks of malaria in the United States (Zucker, 1996).
Similar data link malaria outbreaks with political upheaval and migration. In Southeast Asia, malaria was the leading cause of morbidity and mortality among Cambodian refugees upon arrival in eastern Thailand (Glass et al., 1980) and the Karen refugees in western Thailand (Luxemburger et al., 1996). In Central Asia, civil war and the collapse of the health care infrastructure led to a reemergence of malaria in Tajikistan (Pitt et al., 1998). In East Timor and Afghanistan, recent relief efforts have been complicated by malaria (Ezard, 2001; Sharp et al., 2002b). Africa’s examples are most numerous. To name just a few: malaria was the leading cause of death among Mozambican refuges in Malawi and Ethiopian refugees in eastern Sudan, whereas fever due to malaria was second only diarrheal disease as the leading cause of morbidity and mortality among Rwandan refugees entering eastern Zaire (now Democratic Republic of Congo) (Bloland and Williams, 2002).
Following a mass migration into a malarious region, added risk factors for malaria outbreaks include: substandard housing and environmental protection from nighttime anopheline bites, deliberate relocation near open water, overcrowding, proximity of livestock, low socioeconomic status, poor nutritional status, a lack of immunity to malaria, destruction or over-burdening of existing infrastructure, and scarce to non-existent health care services (Bloland and Williams, 2002).
Roll Back Malaria (RBM) Goals
One pillar of Roll Back Malaria’s efforts to reduce Africa’s malaria burden is the application of malaria early warning systems (MEWS) to speed responses to epidemics (WHO, 2000; WHO, 2001). Specifically, the Abuja target aims to identify, and respond to 60 percent of malaria epidemics in Africa within 2 weeks of onset and detection (WHO, 2001). The main tools of MEWS are:
-
Forecasting (usually refers to seasonal climate forecasts)
-
Early warning (monitoring meteorological conditions such as rainfall and temperature)
-
Early detection (based on routine clinical surveillance)
Although RBM reports that MEWS generally are performing well in southern Africa and programs have started in Ethiopia, Kenya, Uganda, Tanzania, and Sudan (WHO/UNICEF, 2003), a recent failure to detect epidemics in Kenya is revealing. In summer 2002, early warning based on district-level rainfall estimates had the potential to detect two epidemics in four highland districts in southwestern Kenya. District-specific warnings could have given 4 weeks notice of possible emergency conditions. In this case, not only did the early warning system fail, early detection based on outpatient surveillance also was delayed because reporting was only being performed monthly. The authors of a retrospective analysis of the 2002 Kenya experience advocate better planning based on known seasonal epidemiologic risks (Hay et al., 2003).
Antimalarial Treatment and Other Control Measures During Epidemics
A timely and effective response to a possible malaria epidemic typically requires the deployment of additional drug stocks, use of highly effective treatments, and vector control, in particular, widespread IRS (Luxemburger et al., 1998; Rowland and Nosten, 2001). Early diagnosis also is important during rapid refugee influx; rapid diagnostic tests have proved very useful in such situations (Nosten et al., 1998). In addition, drug treatment policies that take into account the immune status of victims are needed. Artemisinin-based combinations are particularly attractive during epidemics because of their clinical efficacy and independent effects on gametocyte carriage (which, in turn, reduces transmission). Since epidemics frequently involve a high proportion of the population for a relatively short period, one or more rounds of mass drug administration (MDA) along with other control measures is another option for rapidly terminating an outbreak (von Seidlein and Greenwood, 2003). If MDA is chosen, the drug selected needs to kill gametocytes. It also should be given as promptly as possible, which poses a major logistical challenge.
MALARIA CONTROL PROGRAMS AND DRUG POLICIES
|
Malaria control cannot be a campaign; it should be a policy, a long-term programme. It cannot be accomplished by spasmodic effort. It requires the adoption of a practical programme … that will be sustained for a long time. (Boyd, 1949) |
Malaria Control Programs—Recent History and Lessons Learned
The strategy for malaria control approved by the WHO in 1979 stressed the need for program flexibility—namely, the adaptation of any and all available methods to realistic aims set by national health authorities, and feasible within the limitations of human and material resources (WHO, 1979). Subsequently, Article VIII of the World Declaration on Malaria Control (WHO, 1992b) made at the WHO Ministerial Conference on Malaria in October 1992 issued this landmark avowal: “We commit ourselves and our countries to control malaria and will plan for malaria control as an essential component of national development.”
In reality, however, the final decades of the twentieth century witnessed a steady decline in malaria control as countries moved from large vertically run organizations focused on IRS and malaria eradication (admittedly, many such programs were flawed) to a new common denominator: “a village health worker with a jar of chloroquine tablets” (Personal communication, B. Greenwood, London School of Hygiene and Tropical Medicine, March 2004). A few countries such as India kept their central control programs after the eradication era ended, but most African countries did not. Today, many African countries are painstakingly rebuilding their national programs in order to access new resources for malaria control available through the Global Fund and other donor agencies.
Although recommending an ideal template for country-level malaria control is not the mission of this report, some general observations are in order. A modern malaria control program should possess at least five components: a public health surveillance system, curative services, preventive interventions, a program for community involvement, and a capacity to perform special studies (operational research) as needed (Bloland and Williams, 2002). Special functions that should also be undertaken at the national level include drug resistance monitoring and pharmacovigilance to survey for adverse or unexpected effects of new antimalarial treatments such as ACTs. Rather than returning to the massive yet narrowly focused organizations of the past, resurrected programs should occupy a middle ground between a centrally directed structure and one that allows full participation at all levels of the health care system. In addition, national malaria programs should consider creative partnerships with the private sector. A few examples of modern public-private synergies might include training private practitioners in malaria treatment; government-sponsored social marketing campaigns to complement the sale (or subsidized distribution) of ITNs; or government licensing of pharmacies with personnel certified to dispense antimalarials, and other essential medicines on the periphery of health care.
Antimalarial Drug Policy—Recent Examples and Lessons Learned
Today, prompt and effective treatment is the key to reducing drug-resistant malaria’s increasing morbidity and mortality. Rational antimalarial drug policies are therefore essential elements of any national malaria control program. Because many countries with growing resistance to first-, second-, and even third-line antimalarial drugs are burdened with debt and poorly financed health budgets, a change in antimalarial drug policy is economically daunting. The process of policy change can also be time consuming and involved.
A detailed analysis of Kenya’s change from chloroquine to SP emphasizes the complexity of the decision-making and implementation process even when two drugs are similar in cost (Shretta et al., 2000). Chloroquine resistance was first acknowledged at a meeting organized by the Kenya Medical Research Institute in January 1989. In 1991, the Ministry of Health requested a review of the scientific evidence in support of a revision. In October 1997, draft guidelines were finally issued, reflecting more than 20 independent studies over 14 years documenting chloroquine failure.
Even after consensus has been reached, multiple factors can delay country-level implementation for another 18 months or more: political and financial support, training of health care providers, and sensitization of the general population, which is crucial for ultimate success of the antimalarial changeover (WHO/UNICEF, 2003). In the future, a culture in which changes in malaria treatment, based on sound evidence, can occur on a fairly regular basis will be crucial. This is happening to some extent now (e.g., in Kenya, and Tanzania) but far more flexibility, and rapid response is needed in most malaria endemic countries.
Current Status of National Malaria Drug Policy in Africa, Asia, and South America
In 1993, Malawi was the first sub-Saharan country to switch from chloroquine to SP as first-line therapy for P. falciparum. Between 1998 and 2001, Kenya, Uganda, Tanzania, Zanzibar, Rwanda, and Burundi followed suit (like Malawi, they either chose SP monotherapy or a combination of SP plus chloroquine or amodiaquine). Countries that have now adopted and (more recently) implemented ACTs include Tanzania (Zanzibar) (2001), Zambia (2001), and Burundi (2002). In South Africa, the provinces where ACTs have been fully implemented are KwaZulu Natal (Coartem), and Mpumalanga (artesunate+SP) (WHO/UNICEF, 2003). First-line antimalarial drug policies for selected countries in Africa, Asia, and South America, as of March 2003, are listed in Table 8-4.
TABLE 8-4 Countries/Regions Using ACTs as First-Line Therapy for Uncomplicated Malaria
|
Region/Country |
First-Line Treatment |
|
Africa Region |
|
|
Burundi |
AS+AQ |
|
Comoros |
AL |
|
South Africa (KwaZulu Natal) |
AL |
|
Zambia |
AL |
|
Zanzibar |
AS+AQ |
|
Western Pacific Region |
|
|
Cambodia |
AS(3d)+MQ |
|
China, Yunnan, Hainan |
ATM/AS(5d) |
|
Lao PDR |
AS+MFQ |
|
Viet Nam, north |
ATS/AS(5d)+PQ |
|
Viet Nam other |
AS(3d)+MQ25 |
|
South-East Asia Region |
|
|
Bhutan |
MQ+AS(3d) |
|
Myanmar |
AS(3d)+MQ |
|
Thailand |
MQ+AS(3d) |
|
America Region |
|
|
Bolivia |
MQ15+AS(3d)CQ+PQ |
|
Guyana |
AL |
|
Peru (Amazon area) |
AS(3d)+MQ AS(3d)+SP |
|
Suriname |
AL |
|
AL=Artemether-lumefantrine AQ=Amodiaquine AS=Artesunate ATM=Artemether ATS=Artemisinin CQ=Chloroquine MQ=Mefloquine MQ15=Mefloquine 15mg/kg MQ25=Mefloquine 25mg/kg PQ=Primaquine Q=Quinine SP=Sulfadoxine-pyrimethamine T=Tetracycline SOURCE (as of March, 2003): http://rbm.who.int/amdp/amdp_amro.htm, http://rbm.who.int/amdp/amdp_afro, http://rbm.who.int/amdp/amdp_emro.htm, http://rbm.who.int/amdp/amdp_searo |
|
Regional Networks and Future Policy Implications
At present, most control policies are still organized at a national or subnational level (Kaul and Faust, 2001), but interest in regional approaches is increasing. For one thing, individual countries can no longer make drug policy decisions in isolation. Given the fluid nature of borders, they now realize that their and their neighbors’ policies affect each other. There are increasing calls for information exchanges among neighboring countries about their respective malaria situations, malaria treatment policies, and approaches used to combat drug-resistant malaria.
Recognizing that a regional approach offers a better platform for addressing malaria control problems that influence policy, several networks and initiatives have been established, most notably, the East African Network for Monitoring Antimalarial Treatments (EANMAT), the West African Networks for Monitoring Antimalarial Treatments (WANMAT I and II), the South East African Combination Antimalarial Therapy (SEACAT) Evaluation, a Central African network (Réseau d’Afrique Centrale des Thérapeutiques Antipaludiques, or RACTAP), the Amazon Malaria Initiative (AMI), and the Asian Collaborative Training Network for Malaria (ACTMalaria). These networks focus on partnership and shared goals in malaria control with a particular focus on training, research, and operational issues.
Pending full implementation of ACTs in Africa and elsewhere, one current dilemma that many malaria-endemic countries face is the practical question of when—in the face of growing antimalarial drug resistance—a change in drug policy should be implemented. Should it be initiated early, when only a small fraction of the population is at risk, or at a later stage, when risk affects a higher proportion of the susceptible population? Until recently, WHO recommended a change to more effective treatment when clinical failure rates exceeded 25 percent 14 days following treatment; now it has lowered the ceiling to >15 percent clinical and >25 percent total failure (i.e., clinical + parasitologic [WHO/HTM/RBM/2003.50]). No matter what standard threshold is selected, uniform change points fail to take into account districts where malaria is seasonal or epidemic, and local populations are therefore more vulnerable to adverse clinical outcomes from drug resistant malaria.
In addition, many authors have noted that the original WHO categories of response (grace, alert, action, and change) were developed in response to the relatively slow rate at which chloroquine resistance emerges. In contrast to chloroquine and amodiaquine, SP resistance emerges and spreads quickly in areas of high transmission (Talisuna et al., 2002). Finally, there are technical questions regarding the WHO test itself. Since it does not provide information on treatment failure beyond 14 days, many
experts believe that estimates of resistance based on these tests are significantly lower than the real rates of treatment failure, which malaria patients in east Africa are actually suffering (EANMAT, 2003). A recent analysis of a sample comprising 23 percent of all randomized trials published since 1965 suggests that the 14-day test could miss as many as 63 to 100 percent of treatment failures (Stepniewska et al., in press).
CONCLUSION
In summary, as of 2004, malaria control can be viewed either as a cup half-full or half-empty. On the positive side: more money has become available for malaria control (although not nearly enough), long-lasting ITNs are finally becoming a reality, combination therapy with artemisinins is more widely available than before (although a radical solution is still needed for ACTs to reach the vast majority of malaria sufferers, especially in sub-Saharan Africa), and vaccine research is progressing. Vector control and ITNs will never have a major effect in many areas of Asia, on the other hand, because of local vector behavior; there, the main strategy must be effective treatment. And despite many earnest hopes for a vaccine solution to the world’s malaria problem, vaccines are far from ready and will never control malaria by themselves—in Africa especially, there will always be a role for vector control and drugs. Improved drug delivery (including home management of severe malaria with ACTs) is another practical issue that is currently under consideration but will require even more attention after effective first-line treatments reach Africa.
REFERENCES
Abdulla S, Schellenberg JA, Nathan R, Mukasa O, Marchant T, Smith T, Tanner M, Lengeler C. 2001. Impact on malaria morbidity of a programme supplying insecticide treated nets in children aged under 2 years in Tanzania: Community cross sectional study. British Medical Journal 322(7281):270-273.
Acosta CJ, Galindo CM, Schellenberg D, Aponte JJ, Kahigwa E, Urassa H, Schellenberg JR, Masanja H, Hayes R, Kitua AY, Lwilla F, Mshinda H, Menendez C, Tanner M, Alonso PL. 1999. Evaluation of the SPf66 vaccine for malaria control when delivered through the EPI scheme in Tanzania. Tropical Medicine and International Health 4(5):368-376.
Agyepong IA. 1992. Malaria: Ethnomedical perceptions and practice in an Adangbe farming community and implications for control. Social Science and Medicine 35(2):131-137.
Alaii JA, Hawley WA, Kolczak MS, ter Kuile FO, Gimnig JE, Vulule JM, Odhacha A, Oloo AJ, Nahlen BL, Phillips-Howard PA. 2003a. Factors affecting use of permethrin-treated bed nets during a randomized controlled trial in western Kenya. American Journal of Tropical Medicine and Hygiene 68(4 Suppl):137-141.
Alaii JA, van den Borne HW, Kachur SP, Mwenesi H, Vulule JM, Hawley WA, Meltzer MI, Nahlen BL, Phillips-Howard PA. 2003b. Perceptions of bed nets and malaria prevention before and after a randomized controlled trial of permethrin-treated bed nets in western Kenya. American Journal of Tropical Medicine and Hygiene 68(4 Suppl):142-148.
Allen SJ, Snow RW, Menon A, Greenwood BM. 1990. Compliance with malaria chemoprophylaxis over a five-year period among children in a rural area of the Gambia. Journal of Tropical Medicine and Hygiene 93(5):313-322.
Alonso PL, Lindsay SW, Armstrong JR, Conteh M, Hill AG, David PH, Fegan G, de Francisco A, Hall AJ, Shenton FC. 1991. The effect of insecticide-treated bed nets on mortality of Gambian children. Lancet 337(8756):1499-1502.
Alonso PL, Lindsay SW, Armstrong Schellenberg JR, Konteh M, Keita K, Marshall C, Phillips A, Cham K, Greenwood BM. 1993. A malaria control trial using insecticide-treated bed nets and targeted chemoprophylaxis in a rural area of the Gambia, West Africa. 5: Design and implementation of the trial. Transactions of the Royal Society of Tropical Medicine and Hygiene 87(Suppl 2):31-36.
Alonso PL, Smith T, Armstrong-Schellenberg JR, Masanja H, Mwankusye S, Urassa H, Bastos de Azevedo I, Chongela J, Kobero S, Menendez C. 1994. Randomised trial of efficacy of SPf66 vaccine against Plasmodium falciparum malaria in children in southern Tanzania. Lancet 344(8931):1175-1181.
Attaran A, Roberts DR, Curtis CF, Kilama WL. 2000. Balancing risks on the backs of the poor. Nature Medicine 6(7):729-731.
Bangs M, Subianto DB. 1999. El Niño and associated outbreaks of severe malaria in highland populations in Irian Jaya, Indonesia: A review and epidemiologic perspective. Southeast Asian Journal of Tropical Medicine and Public Health 30:608-616.
Barat L, Chipipa J, Kolczak M, Sukwa T. 1999. Does the availability of blood slide microscopy for malaria at health centers improve the management of persons with fever in Zambia? American Journal of Tropical Medicine and Hygiene 60(6):1024-1030.
Barber MA. 1929. The history of malaria in the United States. USA Public Health Report 44:2575-2587.
Barber MA, Rice JB, Brown JY. 1932. Malaria studies on the Firestone rubber plantation in Liberia, West Africa. American Journal of Tropical Medicine and Hygiene 15:601-623.
Barnes KI, Durrheim DN, Jackson A. 2003. Epidemiology of malaria following implementation of artemether-lumefantrine as first-line treatment of uncomplicated disease in Kwa Zulu-Natal, South Africa. Antibiotics and Chemotherapy 7:8.
Beach RF, Ruebush TK II, Sexton JD, Bright PL, Hightower AW, Breman JG, Mount DL, Oloo AJ. 1993. Effectiveness of permethrin-impregnated bed nets and curtains for malaria control in a holoendemic area of western Kenya. American Journal of Tropical Medicine and Hygiene 49(3):290-300.
Beales PF, Gilles HM. 2002. Rationale and technique of malaria control. In: Warrell DA, Gilles HM, eds. Essential Malariology. 4th ed. London: Arnold Publishing.
Binka FN, Adongo P. 1997. Acceptability and use of insecticide impregnated bednets in northern Ghana. Tropical Medicine and International Health 2(5):499-507.
Binka FN, Kubaje A, Adjuik M, Williams LA, Lengeler C, Maude GH, Armah GE, Kajihara B, Adiamah JH, Smith PG. 1996. Impact of permethrin impregnated bed nets on child mortality in Kassena-Nankana district, Ghana: A randomized controlled trial. Tropical Medicine and International Health 1(2):147-154.
Binka FN, Indome F, Smith T. 1998. Impact of spatial distribution of permethrin-impregnated bed nets on child mortality in rural northern Ghana. American Journal of Tropical Medicine and Hygiene 59(1):80-85.
Blagoveschensky D, Bregetova N, Monchadsky A. 1945. An investigation of new repellants for the protection of man against mosquito attacks. Transactions of the Royal Society of Tropical Medicine and Hygiene 34:147-150.
Bloland PB, Williams H. 2002. Malaria Control During Mass Population Movements and Disasters. Committee on Population. National Research Council of the National Academies, Roundtable on the Demography of Forced Migration. Washington, DC: The National Academies Press.
Bockarie MJ, Service MW, Barnish G, Momoh W, Salia F. 1994. The effect of woodsmoke on the feeding and resting behaviour of Anopheles gambiae s.s. Acta Tropica 57(4):337-340.
Bodker R, Kisinza W, Malima R, Msangeni H, Lindsay S. 2000. Resurgence of malaria in the Usambara Mountains, Tanzania, an epidemic of drug-resistant parasites. Global Change and Human Health 1(2):134-153.
Bouma M, Rowland M. 1995. Failure of passive zooprophylaxis: Cattle ownership in Pakistan is associated with a higher prevalence of malaria. [See Comment]. Transactions of the Royal Society of Tropical Medicine and Hygiene 89(4):351-353.
Bouma MJ, Dye C, van der Kaay HJ. 1996a. Falciparum malaria and climate change in the northwest frontier province of Pakistan. American Journal of Tropical Medicine and Hygiene 55(2):131-137.
Bouma MJ, van der Kaay HJ. 1996b. The El Niño southern oscillation and the historic malaria epidemics on the Indian subcontinent and Sri Lanka: An early warning system for future epidemics? Tropical Medicine and International Health 1(1):86-96.
Boyd MF. 1949. Malariology. Philadelphia: Saunders.
Bradley DJ. 1991. Morbidity and mortality at Pare-Taveta, Kenya and Tanzania, 1954-66: The effects of a period of malaria control. In: Feachem RG, Jamison D, eds. Disease and Mortality in Sub-Saharan Africa. Oxford: Oxford University Press.
Brooke BD, Kloke G, Hunt RH, Koekemoer LL, Temu EA, Taylor ME, Small G, Hemingway J, Coetzee M. 2001. Bioassay and biochemical analyses of insecticide resistance in southern African Anopheles funestus (Diptera: Culicidae). Bulletin of Entomological Research 91(4):265-272.
Browne EN, Maude GH, Binka FN. 2001. The impact of insecticide-treated bednets on malaria and anaemia in pregnancy in Kassena-Nankana district, Ghana: A randomized controlled trial . Tropical Medicine and International Health 6(9):667-676.
Bualombai P, Prajakwong S, Aussawatheerakul N, Congpoung K, Sudathip S, Thimasarn K, Sirichaisinthop J, Indaratna K, Kidson C, Srisuphanand M. 2003. Determining cost-effectiveness and cost component of three malaria diagnostic models being used in remote non-microscope areas. Southeast Asian Journal of Tropical Medicine and Public Health 34(2):322-333.
Carvalho LJM, Daniel-Ribeiro CT, Goto H. 2002. Malaria vaccine: Candidate antigens, mechanisms, constraints and prospects. Scandinavian Journal of Immunology 56(4):327-343.
Chandramohan D, Jaffar S, Greenwood B. 2002. Use of clinical algorithms for diagnosing malaria. Tropical Medicine and International Health 7(1):45-52.
Chandre F, Darrier F, Manga L, Akogbeto M, Faye O, Mouchet J, Guillet P. 1999. Status of pyrethroid resistance in Anopheles gambiae sensu lato. Bulletin of the World Health Organization 77(3):230-234.
Charlwood JD, Jolley D. 1984. The coil works (against mosquitoes in Papua New Guinea). Transactions of the Royal Society of Tropical Medicine and Hygiene 78(5):678.
Chavasse D, Reed C, Attawall K. 1999. Insecticide Treated Net Projects: A Handbook for Managers. London and Liverpool: Malaria Consortium.
Chinery WA. 1990. Variation in frequency in breeding of Anopheles gambiae s.l. and its relationship with in-door adult mosquito density in various localities in Accra, Ghana. East African Medical Journal 67(5):328-335.
Church LW, Le TP, Bryan JP, Gordon DM, Edelman R, Fries L, Davis JR, Herrington DA, Clyde DF, Shmuklarsky MJ, Schneider I, McGovern TW, Chulay JD, Ballou WR, Hoffman SL. 1997. Clinical manifestations of Plasmodium falciparum malaria experimentally induced by mosquito challenge. Journal of Infectious Diseases 175(4):915-920.
Clarke T. 2002. Mosquitoes minus malaria. Nature 419(6906):429-430.
Clyde DF. 1975. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. American Journal of Tropical Medicine and Hygiene 24(3):397-401.
Cohen S, McGregor IA, Carrington S. 1961. Gamma-globulin and acquired immunity to human malaria. Nauchni trudove na Visshiia meditsinski institut, Sofiia 192:733-737.
Collins FH. 1994. Prospects for malaria control through the genetic manipulation of its vectors. Parasitology Today 10(10):370-371.
Craig MH, Sharp BL. 1997. Comparative evaluation of four techniques for the diagnosis of Plasmodium falciparum infections. Transactions of the Royal Society of Tropical Medicine and Hygiene 91(3):279-282.
Curtis CF. 1968. Possible use of translocations to fix desirable genes in insect pest populations. Nature 218(139):368-369.
Curtis CF, Lines JD. 2000. Should DDT be banned by international treaty? Parasitology Today 16(3):119-121.
Curtis CF, Myamba J, Wilkes TJ. 1992. Various pyrethroids on bednets and curtains. Memorias do Instituto Oswaldo Cruz 87(Suppl 3):363-370.
D’Alessandro U, Leach A, Drakeley CJ, Bennett S, Olaleye BO, Fegan GW, Jawara M, Langerock P, George MO, Targett GA. 1995. Efficacy trial of malaria vaccine SPf66 in Gambian infants. Lancet 346(8973):462-467.
D’Alessandro U, Langerock P, Bennett S, Francis N, Cham K, Greenwood BM. 1996. The impact of a national impregnated bed net programme on the outcome of pregnancy in primigravidae in the Gambia. Transactions of the Royal Society of Tropical Medicine and Hygiene 90(5):487-492.
Desowitz R. 1999. Milestones in the history of malaria. In: Wahlgren M, Perlmann P, eds. Malaria. Amsterdam: Harwood Academic.
Diallo DA, Habluetzel A, Cuzin-Ouattara N, Nebie I, Sanogo E, Cousens SN, Esposito F. 1999. Widespread distribution of insecticide-impregnated curtains reduces child mortality, prevalence and intensity of malaria infection, and malaria transmission in rural Burkina Faso. Parassitologia 41(1-3):377-381.
Dolan G, ter Kuile FO, Jacoutot V, White NJ, Luxemburger C, Malankirii L, Chongsuphajaisiddhi T, Nosten F. 1993. Bed nets for the prevention of malaria and anaemia in pregnancy. Transactions of the Royal Society of Tropical Medicine and Hygiene 87(6):620-626.
East African Network for Monitoring Antimalarial Treatment (EANMAT). 2003. The efficacy of antimalarial monotherapies, sulphadoxine-pyrimethamine and amodiaquine in east Africa: Implications for sub-regional policy. Tropical Medicine and International Health 8(10):860-867.
Ezard N. 2001. Research in complex emergencies. Medical emergency relief international. Lancet 357(9250):149.
Farnert A, Arez AP, Correia AT, Bjorkman A, Snounou G, do Rosario V. 1999. Sampling and storage of blood and the detection of malaria parasites by polymerase chain reaction. Transactions of the Royal Society of Tropical Medicine and Hygiene 93(1):50-53.
Garfield RM, Vermund SH. 1983. Changes in malaria incidence after mass drug administration in Nicaragua. Lancet 2(8348):500-503.
Garner P, Gulmezoglu AM. 2000. Prevention versus treatment for malaria in pregnant women. Cochrane Database of Systematic Reviews (2):CD000169.
Giglioli G, Rutten FJ, Ramjattan S. 1967. Interruption of malaria transmission by chloroquinized salt in Guyana, with observations on a chloroquine-resistant strain of Plasmodium falciparum. Bulletin of the World Health Organization 36(2):283-301.
Gimnig JE, Kolczak MS, Hightower AW, Vulule JM, Schoute E, Kamau L, Phillips-Howard PA, ter Kuile FO, Nahlen BL, Hawley WA. 2003a. Effect of permethrin-treated bed nets on the spatial distribution of malaria vectors in western Kenya. American Journal of Tropical Medicine and Hygiene 68(4 Suppl):115-120.
Gimnig JE, Vulule JM, Lo TQ, Kamau L, Kolczak MS, Phillips-Howard PA, Mathenge EM, ter Kuile FO, Nahlen BL, Hightower AW, Hawley WA. 2003b. Impact of permethrin-treated bed nets on entomologic indices in an area of intense year-round malaria transmission. American Journal of Tropical Medicine and Hygiene 68(4 Suppl):16-22.
Glass RI, Cates W Jr, Nieburg P, Davis C, Russbach R, Nothdurft H, Peel S, Turnbull R. 1980. Rapid assessment of health status and preventive-medicine needs of newly arrived Kampuchean refugees, Sa Kaeo, Thailand. Lancet 1(8173):868-872.
Goodman CA, Coleman PG, Mills AJ. 1999. Cost-effectiveness of malaria control in sub-Saharan Africa. Lancet 354(9176):378-385.
Goodman CA, Mnzava AE, Dlamini SS, Sharp BL, Mthembu DJ, Gumede JK. 2001. Comparison of the cost and cost-effectiveness of insecticide-treated bednets and residual house-spraying in Kwazulu-Natal, South Africa. Tropical Medicine and International Health 6(4):280-295.
Graves P, Gelband H. 2003. Vaccines for preventing malaria. Cochrane Database of Systematic Reviews (1):CD000129.
Graves PM. 1998. Comparison of the cost-effectiveness of vaccines and insecticide impregnation of mosquito nets for the prevention of malaria. Annals of Tropical Medicine and Parasitology 92(4):399-410.
Graves PM, Burkot TR, Carter R, Cattani JA, Lagog M, Parker J, Brabin BJ, Gibson FD, Bradley DJ, Alpers MP. 1988. Measurement of malarial infectivity of human populations to mosquitoes in the Madang area, Papua, New Guinea. Parasitology 96(Pt 2):251-263.
Greenwood B. 1999. What can the residents of malaria endemic countries do to protect themselves against malaria? Parassitologia 41(1-3):295-299.
Greenwood B. 2002. The molecular epidemiology of malaria. Tropical Medicine and International Health 7(12):1012-1021.
Greenwood B. 2004. The use of anti-malarial drugs to prevent malaria in the population of malaria-endemic areas. American Journal of Tropical Medicine and Hygiene 70:1-7.
Greenwood B, Alonso P. 2002. Malaria vaccine trials. Chemical Immunology 80:366-395.
Greenwood B, Mutabingwa T. 2002. Malaria in 2002. Nature 415(6872):670-672.
Greenwood BM, Greenwood AM, Bradley AK, Snow RW, Byass P, Hayes RJ, N’Jie AB. 1988. Comparison of two strategies for control of malaria within a primary health care programme in the Gambia. Lancet 1(8595):1121-1127.
Greenwood BM, Greenwood AM, Snow RW, Byass P, Bennett S, Hatib-N’Jie AB. 1989. The effects of malaria chemoprophylaxis given by traditional birth attendants on the course and outcome of pregnancy. Transactions of the Royal Society of Tropical Medicine and Hygiene 83(5):589-594.
Greenwood BM, David PH, Otoo-Forbes LN, Allen SJ, Alonso PL, Armstrong Schellenberg JR, Byass P, Hurwitz M, Menon A, Snow RW. 1995. Mortality and morbidity from malaria after stopping malaria chemoprophylaxis. Transactions of the Royal Society of Tropical Medicine and Hygiene 89(6):629-633.
Guerin PJ, Olliaro P, Nosten F, Druilhe P, Laxminarayan R, Binka F, Kilama WL, Ford N, White NJ. 2002. Malaria: Current status of control, diagnosis, treatment, and a proposed agenda for research and development. The Lancet Infectious Diseases 2(9):564-573.
Guiguemde TR, Sturchler D, Ouedraogo JB, Drabo M, Etlinger H, Douchet C, Gbary AR, Haller L, Kambou S, Fernex M. 1990. [Vaccination against malaria: Initial trial with an ant-sporozoite vaccine, (Nanp)3-Tt (Ro 40-2361) in Africa (Bobo-Dioulasso, Burkina Faso)]. [French]. Bulletin de la Societe de Pathologie Exotique 83(2):217-227.
Guillet P, Alnwick D, Cham MK, Neira M, Zaim M, Heymann D, Mukelabai K. 2001. Long-lasting treated mosquito nets: A breakthrough in malaria prevention. Bulletin of the World Health Organization 79(10):998.
Gunawardena DM, Wickremasinghe AR, Muthuwatta L, Weerasingha S, Rajakaruna J, Senanayaka T, Kotta PK, Attanayake N, Carter R, Mendis KN. 1998. Malaria risk factors in an endemic region of Sri Lanka, and the impact and cost implications of risk factor-based interventions. American Journal of Tropical Medicine and Hygiene 58(5): 533-542.
Gyapong M, Gyapong JO, Amankwa J, Asedem J, Sory E. 1996. Introducing insecticide impregnated bednets in an area of low bednet usage: An exploratory study in North-east Ghana. Tropical Medicine and International Health 1(3):328-333.
Habluetzel A, Diallo DA, Esposito F, Lamizana L, Pagnoni F, Lengeler C, Traore C, Cousens SN. 1997. Do insecticide-treated curtains reduce all-cause child mortality in Burkina Faso? Tropical Medicine and International Health 2(9):855-862.
Hargreaves K, Koekemoer LL, Brooke BD, Hunt RH, Mthembu J, Coetzee M. 2000. Anopheles funestus resistant to pyrethroid insecticides in South Africa. Medical and Veterinary Entomology 14(2):181-189.
Harper PA, Lisansky ET, Sasse BE. 1947. Malaria and other insect-borne diseases in the South Pacific campaign. American Journal of Tropical Medicine and Hygiene 27(1 Suppl):1942-1945.
Harrison, G. 1978. Mosquitoes, Malaria and Man: A History of the Hostilities Since 1880. New York: Dutton.
Hawley WA, Phillips-Howard PA, ter Kuile FO, Terlouw DJ, Vulule JM, Ombok M, Nahlen BL, Gimnig JE, Kariuki SK, Kolczak MS, Hightower AW. 2003a. Community-wide effects of permethrin-treated bed nets on child mortality and malaria morbidity in Western Kenya. American Journal of Tropical Medicine and Hygiene 68(4 Suppl):121-127.
Hawley WA, ter Kuile FO, Steketee RS, Nahlen BL, Terlouw DJ, Gimnig JE, Shi YP, Vulule JM, Alaii JA, Hightower AW, Kolczak MS, Kariuki SK, Phillips-Howard PA. 2003b. Implications of the Western Kenya permethrin-treated bed net study for policy, program implementation, and future research. American Journal of Tropical Medicine and Hygiene 68(4 Suppl):168-173.
Hay S, Renshaw M, Ochola SA, Noor AM, Snow RW. 2003. Performance of forecasting, warning and detection of malaria epidemics in the highlands of Western Kenya. Trends in Parasitology 19(9):394-399.
Hay SI, Rogers DJ, Randolph SE, Stern DI, Cox J, Shanks GD, Snow RW. 2002. Hot topic or hot air? Climate change and malaria resurgence in East African Highlands. Trends in Parasitology 18(12):530-534.
Hemingway J, Bates I. 2003. Malaria: Past problems and future prospects. After more than a decade of neglect, malaria is finally back on the agenda for both biomedical research and public health politics. EMBO Reports 4(SPEC. ISS.):S29-S31.
Hewitt S, Kamal M, Muhammad N, Rowland M. 1994. An entomological investigation of the likely impact of cattle ownership on malaria in an Afghan refugee camp in the North West Frontier Province of Pakistan. Medical and Veterinary Entomology 8(2):160-164.
Hii JL, Smith T, Mai A, Mellor S, Lewis D, Alexander N, Alpers MP. 1997. Spatial and temporal variation in abundance of anopheles (Diptera: Culicidae) in a malaria endemic area in Papua New Guinea. Journal of Medical Entomology 34(2):193-205.
Hii JL, Smith T, Mai A, Ibam E, Alpers MP. 2000. Comparison between anopheline mosquitoes (Diptera: Culicidae) caught using different methods in a malaria endemic area of Papua New Guinea. Bulletin of Entomological Research 90(3):211-219.
Hoffman SL. 2003. Current status of malaria vaccine development efforts. Paper commissioned by the Institute of Medicine, Washington, DC.
Holder AA, Guevara Patino JA, Uthaipibull C, Syed SE, Ling IT, Scott-Finnigan T, Blackman MJ. 1999. Merozoite surface protein 1, immune evasion, and vaccines against asexual blood stage malaria. Parassitologia 41(1-3):409-414.
Howard SC, Omumbo J, Nevill C, Some ES, Donnelly CA, Snow RW. 2000. Evidence for a mass community effect of insecticide-treated bednets on the incidence of malaria on the Kenyan coast. Transactions of the Royal Society of Tropical Medicine and Hygiene 94(4):357-360.
Hung LQ, De Vries PJ, Giao PT, Nam NV, Binh TQ, Chong MT, Quoc NTTA, Thanh TN, Hung LN, Kager PA. 2002. Control of malaria: A successful experience from Viet Nam. Bulletin of the World Health Organization 80(8):660-666.
Ito J, Ghosh A, Moreira LA, Wimmer EA, Jacobs-Lorena M. 2002. Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature 417(6887):452-455.
James AA, Beerntsen BT, Capurro Mde L, Coates CJ, Coleman J, Jasinskiene N, Krettli AU. 1999. Controlling malaria transmission with genetically-engineered, plasmodium-resistant mosquitoes: Milestones in a model system. Parassitologia 41(1-3):461-471.
Jonkman A, Chibwe RA, Khoromana CO, Liabunya UL, Chaponda ME, Kandiero GE, Molyneux ME, Taylor TE. 1995. Cost-saving through microscopy-based versus presumptive diagnosis of malaria in adult outpatients in Malawi. Bulletin of the World Health Organization 73(2):223-227.
Kaneko A, Taleo G, Kalkoa M, Yamar S, Kobayakawa T, Bjorkman A. 2000. Malaria Eradication on Islands. [See Comment]. Lancet 356(9241):1560-1564.
Karch S, Asidi N, Manzambi ZM, Salaun JJ. 1992. Efficacy of Bacillus sphaericus against the malaria vector Anopheles gambiae and other mosquitoes in swamps and rice fields in Zaire. Journal of the American Mosquito Control Association 8(4):376-380.
Karch S, Garin B, Asidi N, Manzambi Z, Salaun JJ, Mouchet J. 1993. [Mosquito nets impregnated against malaria in Zaire]. [French]. Annales de la Societe Belge de Medecine Tropicale 73(1):37-53.
Kaul I, Faust M. 2001. Global public goods and health: Taking the agenda forward. Bulletin of the World Health Organization 79(9):869-874.
Kazmi JH, Pandit K. 2001. Disease and dislocation: The impact of refugee movements on the geography of malaria in NWFP, Pakistan. Social Science and Medicine 52(7):1043-1055.
Khaemba BM, Mutani A, Bett MK. 1994. Studies of anopheline mosquitoes transmitting malaria in a newly developed highland urban area: A case study of Moi University and its environs. East African Medical Journal 71(3):159-164.
Killeen GF, Fillinger U, Kiche I, Gouagna LC, Knols BG. 2002. Eradication of Anopheles gambiae from Brazil: Lessons for malaria control in Africa? The Lancet Infectious Diseases 2(10):618-627.
Kovats RS, Bouma MJ, Hajat S, Worrall E, Haines A. 2003. El Niño and health. Lancet 362(9394):1481-1489.
Kumar S, Epstein JE, Richie TL, Nkrumah FK, Soisson L, Carucci DJ, Hoffman SL. 2002. A multilateral effort to develop DNA vaccines against falciparum malaria. Trends in Parasitology 18(3):129-135.
Kwiatkowski D, Marsh K. 1997. Development of a malaria vaccine. Lancet 350(9092):1696-1701.
Lengeler C. 2001. Comparison of malaria control interventions. Bulletin of the World Health Organization 79(1):77.
Lindblade KA, Walker ED, Onapa AW, Katungu J, Wilson ML. 1999. Highland malaria in Uganda: Prospective analysis of an epidemic associated with El Niño. Transactions of the Royal Society of Tropical Medicine and Hygiene 93(5):480-487.
Lindsay SW, Gibson ME. 1988. Bednets revisited—old idea, new angle. Parasitology Today 4(10):270-272.
Lindsay SW, Snow RW. 1988. The trouble with eaves: House entry by vectors of malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 82(4):645-646.
Lindsay SW, Ewald JA, Samung Y, Apiwathnasorn C, Nosten F. 1998. Thanaka (Limonia acidissima) and deet (di-methyl benzamide) mixture as a mosquito repellent for use by Karen women. Medical and Veterinary Entomology 12(3):295-301.
Loevinsohn ME. 1994. Climatic warming and increased malaria incidence in Rwanda. Lancet 343(8899):714-718.
Luxemburger C, Thwai KL, White NJ, Webster HK, Kyle DE, Maelankirri L, Chongsuphajaisiddhi T, Nosten F. 1996. The epidemiology of malaria in a Karen population on the western border of Thailand. Transactions of the Royal Society of Tropical Medicine and Hygiene 90(2):105-111.
Luxemburger C, Rigal J, Nosten F. 1998. Health care in refugee camps. Transactions of the Royal Society of Tropical Medicine and Hygiene 92(2):129-130.
Magesa SM, Wilkes TJ, Mnzava AE, Njunwa KJ, Myamba J, Kivuyo MD, Hill N, Lines JD, Curtis CF. 1991. Trial of pyrethroid impregnated bed nets in an area of Tanzania holoendemic for malaria. Part 2. Effects on the malaria vector population. Acta Tropica 49(2):97-108.
Mahanty S, Saul A, Miller LH. 2003. Progress in the development of recombinant and synthetic blood-stage malaria vaccines. Journal of Experimental Biology 206:3781-3788.
Makemba AM, Winch PJ, Makame VM, Mehl GL, Premji Z, Minjas JN, Shiff CJ. 1996. Treatment practices for degedege, a locally recognized febrile illness, and implications for strategies to decrease mortality from severe malaria in Bagamoyo district, Tanzania. Tropical Medicine and International Health 1(3):305-313.
Malakooti MA, Biomndo K, Shanks GD. 1998. Reemergence of epidemic malaria in the highlands of western Kenya. Emerging Infectious Diseases 4(4):671-676.
Marbiah NT, Petersen E, David K, Magbity E, Lines J, Bradley DJ. 1998. A controlled trial of lambda-cyhalothrin-impregnated bed nets and/or dapsone/pyrimethamine for malaria control in Sierra Leone. American Journal of Tropical Medicine and Hygiene 58(1):1-6.
Marsh K, Howard RJ. 1986. Antigens induced on erythrocytes by P. falciparum: Expression of diverse and conserved determinants. Science 231(4734):150-153.
Massaga JJ, Kitua AY, Lemnge MM, Akida JA, Malle LN, Ronn AM, Theander TG, Bygbjerg IC. 2003. Effect of intermittent treatment with amodiaquine on anaemia and malarial fevers in infants in Tanzania: a randomised placebo-controlled trial. Lancet 361(9372): 1853-1860.
Matola YG, White GB, Magayuka SA. 1987. The changed pattern of malaria endemicity and transmission at Amani in the eastern Usambara mountains, north-eastern Tanzania. American Journal of Tropical Medicine and Hygiene 90(3):127-134.
Maxwell CA, Msuya E, Sudi M, Njunwa KJ, Carneiro IA, Curtis CF. 2002. Effect of community-wide use of insecticide-treated nets for 3-4 years on malarial morbidity in Tanzania. Tropical Medicine and International Health 7(12):1003-1008.
McGregor IA. 1974. Mechanisms of acquired immunity and epidemiological patterns of antibody responses in malaria in man. Bulletin of the World Health Organization 50(3-4):259-266.
McGregor IA, Gilles HM, Walter JH, Davies AH, Pearson FA. 1956. Effects of heavy and repeated malaria infection on Gambian infants and children. Effects of erythrocytic parasitization. British Medical Journal ii:686-692.
Menendez C, Kahigwa E, Hirt R, Vounatsou P, Aponte JJ, Font F, Acosta CJ, Schellenberg DM, Galindo CM, Kimario J, Urassa H, Brabin B, Smith TA, Kitua AY, Tanner M, Alonso PL. 1997. Randomised placebo-controlled trial of iron supplementation and malaria chemoprophylaxis for prevention of severe anaemia and malaria in Tanzanian infants. Lancet 350(9081):844-850.
Meuwissen JHET. 1964. The use of medicated salt in an antimalaria campaign in west New Guinea. Tropical and Geographical Medicine 16:245-255.
Mharakurwa S, Manyame B, Shiff CJ. 1997. Trial of the ParaSight-F test for malaria diagnosis in the primary health care system, Zimbabwe. Tropical Medicine and International Health 2(6):544-550.
Mnzava AE, Sharp BL, Mthembu DJ, le Sueur D, Dlamini SS, Gumede JK, Kleinschmidt I. 2001. Malaria control—two years’ use of insecticide-treated bednets compared with insecticide house spraying in Kwazulu-Natal. South African Medical Journal 91(11):978-983.
Moerman F, Lengeler C, Chimumbwa J, Talisuna A, Erhart A, Coosemans M, D’Alessandro U. 2003. The contribution of health-care services to a sound and sustainable malaria-control policy. The Lancet Infectious Diseases 3(2):99-102.
Molineaux L. 1985. The impact of parasitic diseases and their control, with an emphasis on malaria and Africa. In: Vallin J, Lopez AD, eds. Health Policy, Social Policy and Mortality Prospects. Paris: IUSSP.
Moody A. 2002. Rapid diagnostic tests for malaria parasites. Clinical Microbiology Reviews 15(1):66-78.
Moore SA, Surgey EG, Cadwgan AM. 2002. Malaria vaccines: Where are we and where are we going? The Lancet Infectious Diseases 2(12):737-743.
Moorthy VS, Good MF, Hill AVS. 2004. Malaria vaccine developments. Lancet 363(9403): 150-156.
Morley D, Woodland M, Cuthbertson WF. 1964. Controlled trial of pyrimethamine in pregnant women in an African village. British Medical Journal i(5384):667-668.
Mouchet J. 1998. [Origin of malaria epidemics on the plateaus of Madagascar and the mountains of east and south Africa]. [French]. Bulletin de la Societe de Pathologie Exotique 91(1):64-66.
Muheki C, Barnes K, McIntyre D. 2003. Economic Evaluation of Recent Malaria Control Interventions in KwaZulu, Natal, South Africa: SEACAT Evaluation. Cape Town: SEACAT.
Muller O, Ido K, Traore C. 2002. Evaluation of a prototype long-lasting insecticide-treated mosquito net under field conditions in rural Burkina Faso. Transactions of the Royal Society of Tropical Medicine and Hygiene 96(5):483-484.
Murray CK, Bell D, Gasser RA, Wongsrichanalai C. 2003. Rapid diagnostic testing for malaria. Tropical Medicine and International Health 8(10):876-883.
Nahlen BL, Clark JP, Alnwick D. 2003. Insecticide-treated bed nets. American Journal of Tropical Medicine and Hygiene 68(4 Suppl):1-2.
Nevill CG, Some ES, Mung’ala VO, Mutemi W, New L, Marsh K, Lengeler C, Snow RW. 1996. Insecticide-treated bednets reduce mortality and severe morbidity from malaria among children on the Kenyan coast. Tropical Medicine and International Health 1(2): 139-146.
Nosten F, Luxemburger C, Kyle DE, Ballou WR, Wittes J, Wah E, Chongsuphajaisiddhi T, Gordon DM, White NJ, Sadoff JC, Heppner DG, Bathe K, Blood J, Brockman A, Cobley UT, Hacking D, Hogg D, Kyaw HU, Maelankiri L. 1996. Randomised double-blind placebo-controlled trial of SPf66 malaria vaccine in children in northwestern Thailand. Lancet 348(9029):701-707.
Nosten F, Hien TT, White NJ. 1998. Use of artemisinin derivatives for the control of malaria. [Erratum appears in Medecine Tropicale (Mars 1998) 58(4):368]. Medecine Tropicale 58(3 Suppl):45-49.
Nosten F, van Vugt M, Price R, Luxemburger C, Thway KL, Brockman A, McGready R, ter Kuile F, Looareesuwan S, White NJ. 2000. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: A prospective study. Lancet 356(9226):297-302.
Ockenhouse CF, Sun PF, Lanar DE, Wellde BT, Hall BT, Kester K, Stoute JA, Magill A, Krzych U, Farley L, Wirtz RA, Sadoff JC, Kaslow DC, Kumar S, Church LW, Crutcher JM, Wizel B, Hoffman S, Lalvani A, Hill AV, Tine JA, Guito KP, de Taisne C, Anders R, Ballou WR. 1998. Phase I/IIa safety, immunogenicity, and efficacy trial of NYVAC-Pf7, a pox-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. Journal of Infectious Diseases 177(6):1664-1673.
PAHO. 1995. Regional status of malaria in the Americas. Epidemiologic Bulletin 16:10-14.
Parise ME, Ayisi JG, Nahlen BL, Schultz LJ, Roberts JM, Misore A, Muga R, Oloo AJ, Steketee RW. 1998. Efficacy of sulfadoxine-pyrimethamine for prevention of placental malaria in an area of Kenya with a high prevalence of malaria and human immunodeficiency virus infection. American Journal of Tropical Medicine and Hygiene 59(5):813-822.
Patarroyo ME, Armador R. 1999. The first and toward the second generation of malaria vaccines. In: Wahlgren M, Perlmann P, eds. Malaria. Amsterdam: Harwood Academic Publishers.
Payne D. 1988. Did medicated salt hasten the spread of chloroquine resistance in Plasmodium falciparum? Parasitology Today 4(4):112-115.
Payne D, Grab B, Fontaine RE, Hempel JH. 1976. Impact of control measures on malaria transmission and general mortality. Bulletin of the World Health Organization 54(4): 369-377.
Perkins BA, Zucker JR, Otieno J, Jafari HS, Paxton L, Redd SC, Nahlen BL, Schwartz B, Oloo AJ, Olango C, Gove S, Campbell CC. 1997. Evaluation of an algorithm for integrated management of childhood illness in an area of Kenya with high malaria transmission. Bulletin of the World Health Organization 75(1 Suppl):33-42.
Phillips-Howard PA, Nahlen BL, Kolczak MS, Hightower AW, Ter Kuile FO, Alaii JA, Gimnig JE, Arudo J, Vulule JM, Odhacha A, Kachur SP, Schoute E, Rosen DH, Sexton JD, Oloo AJ, Hawley WA. 2003. Efficacy of permethrin-treated bed nets in the prevention of mortality in young children in an area of high perennial malaria transmission in western Kenya. American Journal of Tropical Medicine and Hygiene 68(4 Suppl):23-29.
Phu NH, Day NPJ. 1995. Intraleukocytic malaria pigment and prognosis in severe malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 89:197-199.
Pinotti M. 1954. Chemoprophylaxis of malaria by the association of an antimalarial drug to the sodium chloride used daily in the preparation of meals. In: Fifth International Congress of Tropical Medicine and Malaria, 1953, Vol. 2. Istanbul, Turkey.
Piper R, Lebras J, Wentworth L, Hunt-Cooke A, Houze S, Chiodini P, Makler M. 1999. Immunocapture diagnostic assays for malaria using plasmodium lactate dehydrogenase (pLDH). American Journal of Tropical Medicine and Hygiene 60(1):109-118.
Pitt S, Pearcy BE, Stevens RH, Sharipov A, Satarov K, Banatvala N. 1998. War in Tajikistan and re-emergence of Plasmodium falciparum. Lancet 352(9136):1279.
Plowe CV, Wellems TE. 1995. Molecular approaches to the spreading problem of drug resistant malaria. Advances in Experimental Medicine and Biology 390:197-209.
Poveda G, Rojas W, Quinones ML, Velez ID, Mantilla RI, Ruiz D, Zuluaga JS, Rua GL. 2001. Coupling between annual and ENSO timescales in the malaria-climate association in Colombia. Environmental Health Perspectives 109(5):489-493.
Pringle G. 1969. Experimental malaria control and demography in a rural East African community: A retrospect. Transactions of the Royal Society of Tropical Medicine and Hygiene 63:2-18.
Prinsen Geerligs PD, Brabin BJ, Eggelte TA. 2003. Analysis of the effects of malaria chemoprophylaxis in children on haematological responses, morbidity and mortality. Bulletin of the World Health Organization 81(3):205-216.
Ranson H, Claudianos C, Ortelli F, Abgrall C, Hemingway J, Sharakhova MV, Unger MF, Collins FH, Feyereisen R. 2002. Evolution of supergene families associated with insecticide resistance. Science 298(5591):179-181.
Redd SC, Bloland PB, Kazembe PN, Patrick E, Tembenu R, Campbell CC. 1992. Usefulness of clinical case-definitions in guiding therapy for African children with malaria or pneumonia . Lancet 340(8828):1140-1143.
Rey JL, Cavallo JD, Milleliri JM, L’Hoest S, Soares JL, Piny N, Coue JC, Jouan A. 1996. [Fever of unknown origin (FUO) in the camps of Rwandan refugees in the Goma region of in Zaire (September 1994)]. [French]. Bulletin de la Societe de Pathologie Exotique 89(3):204-208.
Rickman LS, Long GW, Oberst R, Cabanban A, Sangalang R, Smith JI, Chulay JD, Hoffman SL. 1989. Rapid diagnosis of malaria by acridine orange staining of centrifuged parasites. Lancet 1(8629):68-71.
Rimon MM, Kheng S, Hoyer S, Thach V, Ly S, Permin AE, Pieche S. 2003. Malaria dipsticks beneficial for IMCI in Cambodia. Tropical Medicine and International Health 8(6):536-543.
Robert V, Carnevale P. 1991. Influence of deltamethrin treatment of bed nets on malaria transmission in the Kou Valley, Burkina Faso. Bulletin of the World Health Organization 69(6):735-740.
Robert V, Macintyre K, Keating J, Trape JF, Duchemin JB, Warren M, Beier JC. 2003. Malaria transmission in urban sub-Saharan Africa. American Journal of Tropical Medicine and Hygiene 68(2):169-176.
Roberts DR, Manguin S, Mouchet J. 2000. DDT house spraying and re-emerging malaria. Lancet 356(9226):330-332.
Rogerson SJ, Chaluluka E, Kanjala M, Mkundika P, Mhango C, Molyneux ME. 2000. Intermittent sulfadoxine-pyrimethamine in pregnancy: Effectiveness against malaria morbidity in Blantyre, Malawi, in 1997-99. Transactions of the Royal Society of Tropical Medicine and Hygiene 94(5):549-553.
Romi R, Ravoniharimelina B, Ramiakajato M, Majori G. 1993. Field trials of Bacillus thuringiensis H-14 and Bacillus sphaericus (Strain 2362) formulations against Anopheles arabiensis in the central highlands of Madagascar. Journal of the American Mosquito Control Association 9(3):325-329.
Rowland M, Nosten F. 2001. Malaria epidemiology and control in refugee camps and complex emergencies. Annals of Tropical Medicine and Parasitology 95(8):741-754.
Rowley J, Cham B, Pinder M. 1999. Availability and affordability of insecticide of treating bednets in The Gambia. Presentation at the meeting of the 48th Annual Meeting of the American Society of Tropical Medicine and Hygiene. Washington, DC: American Society of Tropical Medicine and Hygiene.
Russell PF. 1952. Nation-wide malaria eradication projects. Anais do Instituto de Higiene e Medicina Tropical (Lisbon) 9(2):331-338.
Schellenberg D, Menendez C, Kahigwa E, Aponte J, Vidal J, Tanner M, Mshinda H, Alonso P. 2001. Intermittent treatment for malaria and anaemia control at time of routine vaccinations in Tanzanian infants: A randomised, placebo-controlled trial. Lancet 357(9267):1471-1477.
Schofield CJ, White GB. 1984. House design and domestic vectors of disease. Transactions of the Royal Society of Tropical Medicine and Hygiene 78(3):285-292.
Schultz LJ, Steketee RW, Macheso A, Kazembe P, Chitsulo L, Wirima JJ. 1994. The efficacy of antimalarial regimens containing sulfadoxine-pyrimethamine and/or chloroquine in preventing peripheral and placental Plasmodium falciparum infection among pregnant women in Malawi . American Journal of Tropical Medicine and Hygiene 51(5):515-522.
Shanks GD, Biomndo K, Hay SI, Snow RW. 2000. Changing patterns of clinical malaria since 1965 among a tea estate population located in the Kenyan highlands. Transactions of the Royal Society of Tropical Medicine and Hygiene 94(3):253-255.
Sharp B, van Wyk P, Sikasote JB, Banda P, Kleinschmidt I. 2002a. Malaria control by residual insecticide spraying in Chingola and Chililabombwe, Copperbelt Province, Zambia. Tropical Medicine and International Health 7(9):732-736.
Sharp TW, Burkle FM Jr, Vaughn AF, Chotani R, Brennan RJ. 2002b. Challenges and opportunities for humanitarian relief in Afghanistan. Clinical Infectious Diseases 34(Suppl 5):S215-S228.
Shiff C. 2002. Integrated approach to malaria control. Clinical Microbiology Reviews 15(2):278-293.
Shretta R, Omumbo J, Rapuoda B, Snow RW. 2000. Using evidence to change antimalarial drug policy in Kenya. Tropical Medicine and International Health 5(11):755-764.
Shulman CE, Dorman EK, Talisuna AO, Lowe BS, Nevill C, Snow RW, Jilo H, Peshu N, Bulmer JN, Graham S, Marsh K. 1998. A community randomized controlled trial of insecticide-treated bed nets for the prevention of malaria and anaemia among primigravid women on the Kenyan coast. Tropical Medicine and International Health 3(3):197-204.
Shulman CE, Dorman EK, Cutts F, Kawuondo K, Bulmer JN, Peshu N, Marsh K. 1999. Intermittent sulphadoxine-pyrimethamine to prevent severe anaemia secondary to malaria in pregnancy: A randomised placebo-controlled trial. Lancet 353(9153):632-636.
Silamut K, White NJ. 1993. Relation of the stage of parasite development in the peripheral blood to prognosis in severe falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 87(4):436-443.
Sina BJ, Aultman K. 2001. Resisting resistance. Trends in Parasitology 17(7):305-306.
Singh N, Saxena A, Valecha N. 2000. Field evaluation of the ICT Malaria P.f/P.v immunochromatographic test for diagnosis of Plasmodium falciparum and P. vivax infection in forest villages of Chhindwara, central India . Tropical Medicine and International Health 5(11):765-770.
Snow RW, Gilles HM. 2002. The epidemiology of malaria. In: Warrell DA, Gilles HM, eds. Essential Malariology. 4th ed. London: Arnold Publishing.
Snow RW, Bradley AK, Hayes R, Byass P, Greenwood BM. 1987. Does woodsmoke protect against malaria? Annals of Tropical Medicine and Parasitology 81(4):449-451.
Snow RW, McCabe E, Mbogo CN, Molyneux CS, Some ES, Mung’ala VO, Nevill CG. 1999. The effect of delivery mechanisms on the uptake of bed net re-impregnation in Kilifi district, Kenya. Health Policy and Planning 14(1):18-25.
Soderlund DM, Bloomquist JR. 1989. Neurotoxic actions of pyrethroid insecticides. Annual Review of Entomology 34:77-96.
Stepniewska K, Taylor WRJ, Mayxay M, Smithuis F, Guthmann J-P, Barnes K, Myint H, Price R, Olliaro P, Pukrittayakamee S, Hien TT, Farrar J, Nosten F, Day NPJ, White NJ. In press. The in vivo assessment of antimalarial drug efficacy in falciparum malaria; the duration of follow-up. Antimicrobial Agents and Chemotherapy.
Strickland GT, Zafar-Latif A, Fox E, Khaliq AA, Chowdhry MA. 1987. Endemic malaria in four villages of the Pakistani province of Punjab. Transactions of the Royal Society of Tropical Medicine and Hygiene 81(1):36-41.
Tabashnik BE. 1989. Managing resistance with multiple pesticide tactics: Theory, evidence, and recommendations. Journal of Economic Entomology 82(5):1263-1269.
Takken W. 2002. Do insecticide-treated bed nets have an effect on malaria vectors? Tropical Medicine and International Health 7(12):1022-1030.
Talisuna AO, Langi P, Bakyaita N, Egwang T, Mutabingwa TK, Watkins W, Van Marck E, D’Alessandro U. 2002. Intensity of malaria transmission, antimalarial-drug use and resistance in Uganda: What is the relationship between these three factors? Transactions of the Royal Society of Tropical Medicine and Hygiene 96(3):310-317.
Taverne J. 1999. DDT—to ban or not to ban? Parasitology Today 15(5):180-181.
ter Kuile FO, Terlouw DJ, Kariuki SK, Phillips-Howard PA, Mirel LB, Hawley WA, Friedman JF, Shi YP, Kolczak MS, Lal AA, Vulule JM, Nahlen BL. 2003a. Impact of permethrin-treated bed nets on malaria, anemia, and growth in infants in an area of intense perennial malaria transmission in western Kenya. American Journal of Tropical Medicine and Hygiene 68(4 Suppl):68-77.
ter Kuile FO, Terlouw DJ, Phillips-Howard PA, Hawley WA, Friedman JF, Kariuki SK, Shi YP, Kolczak MS, Lal AA, Vulule JM, Nahlen BL. 2003b. Reduction of malaria during pregnancy by permethrin-treated bed nets in an area of intense perennial malaria transmission in Western Kenya. American Journal of Tropical Medicine and Hygiene 68(4 Suppl):50-60.
Tharavanij S. 1990. New developments in malaria diagnostic techniques. Southeast Asian Journal of Tropical Medicine and Public Health 21(1):3-16.
Thimasarn K, Sirichaisinthop J, Vijaykadga S, Tansophalaks S, Yamokgul P, Laomiphol A, Palananth C, Thamewat U, Thaithong S, Rooney W. 1995. In vivo study of the response of Plasmodium falciparum to standard mefloquine/sulfadoxine/ pyrimethamine (MSP) treatment among gem miners returning from Cambodia. Southeast Asian Journal of Tropical Medicine and Public Health 26(2):204-212.
Tine JA, Lanar DE, Smith DM, Wellde BT, Schultheiss P, Ware LA, Kauffman EB, Wirtz RA, De Taisne C, Hui GS, Chang SP, Church P, Hollingdale MR, Kaslow DC, Hoffman S, Guito KP, Ballou WR, Sadoff JC, Paoletti E. 1996. NYVAC-Pf7: A poxvirus-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. Infection and Immunity 64(9):3833-3844.
Trape JF, Zoulani A. 1987. Malaria and urbanization in central Africa: The example of Brazzaville. Part III: Relationships between urbanization and the intensity of malaria transmission. Transactions of the Royal Society of Tropical Medicine and Hygiene 81(Suppl 2):19-25.
Trape JF, Lefebvre-Zante E, Legros F, Ndiaye G, Bouganali H, Druilhe P, Salem G. 1992. Vector density gradients and the epidemiology of urban malaria in Dakar, Senegal. American Journal of Tropical Medicine and Hygiene 47(2):181-189.
Trape JF, Pison G, Spiegel A, Enel C, Rogier C. 2002. Combating malaria in Africa. Trends in Parasitology 18(5):224-230.
United Nations. 1999. World Urbanization Prospects: The 1999 Revision, Key Findings. New York: United Nations Population Division.
Utzinger J, Tozan Y, Singer BH. 2001. Efficacy and cost-effectiveness of environmental management for malaria control. Tropical Medicine and International Health 6(9):677-687.
Van der Hoek W, Konradsen F, Dijkstra DS, Amerasinghe PH, Amerasinghe FP. 1998. Risk factors for malaria: A microepidemiological study in a village in Sri Lanka. Transactions of the Royal Society of Tropical Medicine and Hygiene 92(3):265-269.
Verdrager J. 1986. Epidemiology of the emergence and spread of drug-resistant falciparum malaria in south-east Asia and Australasia. Journal of Tropical Medicine and Hygiene 89(6):277-289.
Von Seidlein L, Greenwood BM. 2003. Mass administrations of antimalarial drugs. Trends in Parasitology 19(10):452-460.
Von Seidlein L, Clarke S, Alexander N, Manneh F, Doherty T, Pinder M, Walraven G, Greenwood B. 2002. Treatment uptake by individuals infected with Plasmodium falciparum in rural Gambia, West Africa. Bulletin of the World Health Organization 80(10):790-796.
Warhurst DC, Williams JE. 1996. Laboratory diagnosis of malaria. Journal of Clinical Pathology 49(7):533-538.
Weber MW, Mulholland EK, Jaffar S, Troedsson H, Gove S, Greenwood BM. 1997. Evaluation of an algorithm for the Integrated Management of Childhood Illness in an area with seasonal malaria in the Gambia. Bulletin of the World Health Organization 75(Suppl 1):25-32.
White NJ. 1999. Delaying antimalarial drug resistance with combination chemotherapy. Parassitologia 41(1-3):301-308.
White NJ, Silamut K. 1989. Rapid diagnosis of malaria. Lancet 1(8635):435.
WHO. 1979. Seventeenth Report of the Expert Committee on Malaria. Geneva: World Health Organization.
WHO. 1992a. Fifteenth Report of the Expert Committee on Vector Biology and Control. Technical Report Series No. 818. Vector Resistance to Pesticides. Geneva: World Health Organization.
WHO. 1992b. Presentation at the meeting of the World Declaration on the Control of Malaria, Ministerial Conference on Malaria. Geneva: World Health Organization.
WHO. 1993. WHO Technical Report Series. Plan of Action for Malaria Control 1993-2000. Geneva: World Health Organization.
WHO. 1996a. Report of the WHO Informal Consultation WHO/HQ, Geneva 7-11 October 1996. Evaluation and Testing of Insecticides. Geneva: World Health Organization.
WHO. 1996b. A rapid dipstick antigen capture assay for the diagnosis of falciparum malaria. Bulletin of the World Health Organization 74(1):47-54.
WHO. 1999. Report of the WHO Informal Cosultation, April 28-30, 1999. Draft Guideline Specifications for Bacterial Larvicides for Public Health Use. Geneva: World Health Organization.
WHO. 2000. WHO Expert Committee Report on Malaria: 20th Report. World Health Organization Technical Report Series 892.
WHO. 2001. Malaria Early Warning System, Concepts, Indicators, and Partners, A Framework for Field Research in Africa. WHO/CDS/RBM.
WHO Expert Committee on Insecticides. 1970. Insecticide Resistance and Vector Control. Geneva: World Health Organization.
WHO/UNICEF. 2003. The Africa Malaria Report 2003. Geneva: World Health Organization.
Zucker JR. 1996. Changing patterns of autochthonous malaria transmission in the United States: A review of recent outbreaks. Emerging Infectious Diseases 2(1):37-43.























































