10
Research and Development for New Antimalarial Drugs
INTRODUCTION
As long as malaria persists as a global health problem, new drugs to treat and prevent it will be needed to replace the old ones as they lose effectiveness. Just a few years ago, there were few new drugs in the pipeline and antimalarial resistance was rising. The situation was desperate. It is now healthier than it has been for many decades, with several new combinations and entirely new classes of drug under development. The continued investment in basic science by agencies such as the U.S. National Institutes of Health, the European Commission, and the Wellcome Trust has provided the scientific underpinnings for these developments. But more significantly, genuine progress has resulted from the creation of the Medicines for Malaria Venture (MMV, a public-private partnership devoted to malaria drug development), the continued activities of the Walter Reed Army Institute of Research (WRAIR) in the United States, and the drug development efforts of the World Health Organization (WHO) Special Programme on Research and Training for Tropical Diseases (TDR). These organizations and their partners form what is basically a single international network of collaborators in malaria drug development (in contrast to the competitive character of most profit-driven drug development). This is cause for optimism, but current funding is still very modest and inadequate to complete development as products move downstream.
The crisis addressed by the IOM committee in this report relates to the economics of drugs to replace chloroquine as first-line treatment for uncomplicated malaria. However, the broader needs that must be ad-
dressed by global R&D also include drugs for patients with severe disease, drugs for children (and others) in coma who are unable to take oral drugs, and safe prophylaxis for pregnant women and possibly infants.
This chapter briefly reviews the history and current landscape of R&D for antimalarial drugs.
ANTIMALARIAL DRUG RESEARCH AND DEVELOPMENT
Between 1975 and 1999, only four of almost 1,400 new drugs developed worldwide were antimalarials, and all were at least in part the products of publicly funded research. The pharmaceutical industry largely disengaged from research and development of new drugs for malaria (and other diseases of the poor) in the 1970s because such drugs offered little potential return on investment (Veeken and Pecoul, 2000). This timing was particularly unfortunate because pharmaceutical science was on the brink of major advances and malaria drug development, in large part, missed out on the application of these advances. On the positive side, a sense of opportunity has been heightened by recent decoding of the Plasmodium and Anopheles genomes.
Malaria and other “neglected diseases” (including tuberculosis, filariasis, trypanosomiasis, leishmaniasis, dengue, to mention but a few)—unlike AIDS—are rarities in high-income countries. The world’s poor cannot providentially benefit from new drugs developed for wealthier markets. In the case of antimalarials, the only real commercial market is travelers (whose needs differ greatly from those living in malaria-endemic areas). That industrialized market totals only US$200-300 million per year, well below the radar screen for industry.
The market for innovative new drugs for neglected diseases is further depressed by poor regulatory infrastructure in many countries, and by competing counterfeit drugs. The net effect is that about 10 percent of global drug R&D resources is directed at diseases accounting for 90 percent of the global disease burden (Global Forum for Health Research, 2002). Malaria is among the most poorly resourced diseases.
The UNICEF/UNDP/World Bank/WHO Special Programme for Training and Research in Tropical Diseases (WHO/TDR)
WHO/TDR’s engagement in antimalarial drug development (as well as drugs for other target diseases) began more than 20 years ago. Its funding for malaria drug development historically ranges around US$2-4 million per year. WHO/TDR hosted, matured, and developed an initial portfolio for the Medicines for Malaria Venture (MMV) through 1998-1999 until MMV was established as an independent entity in November 1999.
Early on, WHO/TDR worked with WRAIR and pharmaceutical companies to develop mefloquine and halofantrine. More recently, it has collaborated with pharmaceutical partners to develop injectable artemether and injectable arteether for the treatment of severe malaria. WHO/TDR also sponsored the application for U.S. Food and Drug Administration approval of rectal artesunate in severe malaria (application pending). Other recent accomplishments and ongoing work include:
-
Gaining regulatory approval of chlorproguanil-dapsone in collaboration with GlaxoSmithKline (GSK) in 2003
-
Developing a fixed-dose combination of chlorproguanil-dapsone plus artesunate with GSK and MMV
-
Working with Novartis to extend Coartem use in children down to 5 kg and to develop appropriate, user-friendly, and informative packaging
Public-Private Partnerships and the Medicines for Malaria Venture (MMV)
Public-private partnerships for drug development are relatively new. While they are still few, and not exclusively focused on neglected diseases of poor countries (some have formed around rare, or orphan, diseases), such collaborations are becoming very important, reviving R&D in areas that have lain fallow too long. A number of administrative arrangements are possible, but the key is tapping into a variety of skills and resources from institutions in the public and private sectors, often in a “virtual” organization. One of the most successful public-private partnerships involved in neglected diseases is the Medicines for Malaria Venture (MMV), begun in 1999, and profiled below. A new public-private partnership, the Drugs for Neglected Diseases Initiative (DNDi) has taken on late-stage development of two artemisinin coformulations as pilot projects (although the long-term focus of the DNDi will be on diseases other than malaria, e.g., African sleeping sickness).
MMV is among the first public-private partnership established to tackle a major global disease. The initiative arose from discussions between WHO (primarily through WHO/TDR), a number of other partners, and the International Federation of Pharmaceutical Manufacturers Associations (IFPMA). Early partners in these exploratory discussions were the Global Forum for Health Research, the Rockefeller Foundation, the World Bank, the Swiss Agency for Development and Cooperation, the Association of the British Pharmaceutical Industry, and the Wellcome Trust. The idea was to combine the expertise of the pharmaceutical industry in drug discovery and development, and the public sector, with its depth of expertise in basic biology, clinical medicine, field experience, and above all, its responsibility
TABLE 10-1 Contributions to MMV, 2000-2002 ($US)
|
Sources |
2000 |
2001 |
2002 |
|
Foundation Capital3 |
1,197,619 |
2,309,741 |
——4 |
|
Bill & Melinda Gates Foundation |
5,000,000 |
5,000,000 |
5,000,000 |
|
Rockefeller Foundation |
2,300,000 |
1,000,000 |
1,000,000 |
|
The Wellcome Trust |
|
|
518,400 |
|
Swiss Government (DEZA/SDC) |
612,015 |
|
604,2301 |
|
U.K. Government (DFID) |
|
2,827,850 |
1,561,700 |
|
Dutch Government (NMDC) |
|
|
——2 |
|
World Bank via Global Forum |
500,000 |
750,000 |
500,000 |
|
WHO Roll Back Malaria |
|
2,500,000 |
|
|
Exxon Mobil |
100,000 |
100,000 |
100,000 |
|
Total |
9,709,634 |
14,487,591 |
9,284,3302 |
|
1A second amount of US$607,700 for 2003 from SDC, received in November 2002, was taken to Deferred Income. 2An additional amount of US$954,664 for 2002 from the Netherlands Minister for Development Cooperation was still to be transferred by December 31, 2002, through WHO UNDP/ WorldBank/WHO Special Programme for Research and Training in Tropical Diseases (TDR). This explains total Donations Received of US$10,238,994 as taken to income and Expenditure. 3The founding capital referenced in the statutes amounted in US$4,000,000 from WHO-Roll Back Malaria, and TDR. The TDR funds represent earmarked funding from the UK Department for International Development, the World Bank, and the Netherlands Minster for Development Cooperation prior to the foundation of MMV. 4At December 31, 2001, the amount of US$492,640 was still to be transferred from the World Health Organization. At December 31, 2002, the capital fund showed an outstanding balance of US$53,341. SOURCE: MMV, 2002. |
|||
to the public. MMV was officially launched in November 1999 as a Swiss foundation.
The level of financial contributions to MMV has grown annually, as have expenditures. Donors include the Bill & Melinda Gates Foundation, Rockefeller Foundation, The Wellcome Trust, Swiss Government, U.K. Government, Dutch Government, World Bank via the Global Forum for Health Research, and Exxon Mobil (Table 10-1). As well as direct financial support, contributions in kind have been made by all of the corporate partners in specific projects. Total annual expenditures (not including inkind support) have been:
-
US$3,216,472 in 2000
-
US$8,274,147 in 2001
-
US$12,575,256 in 2002
The 2002 forecasts for future MMV project needs are US$15 to 18 million in 2003, rising to US$20-25 million in 2004.
THE WALTER REED ARMY INSTITUTE OF RESEARCH
The Military Infectious Disease Research Program1
The Walter Reed Army Institute of Research (WRAIR), through the United States Army Medical Research and Materiel Command (USAMRMC), is the leader in the United States for the development of new antimalarial drugs. Drug resistant malaria is considered a major military threat as well as an American public health issue. Military personnel, tourists, consultants, Peace Corps volunteers, and State Department employees traveling to, or residing in, malaria-endemic areas are at risk of illness and death from malaria.
When drug resistant malaria was first encountered by U.S. troops during the Vietnam conflict, the U.S. Army established a malaria research program to develop new prophylactic and therapeutic drugs for military use, coordinated through the Division of Experimental Therapeutics at the Walter Reed Army Institute of Research in Washington, D.C. The program was expected to maintain the expertise and laboratory capability to manage an experimental compound from the chemist’s bench, through clinical trials, and on to approval by the Food and Drug Administration. WRAIR’s accomplishments are summarized in Table 10-2. Funding for the program reached a peak of more than US$12 million in 1969 (equivalent to about US$70 million in 2003, adjusted for inflation) (Figure 10-1), but dropped off precipitously once the Vietnam conflict ended.
The WRAIR malaria drug development program never closed down completely, but government funding began to increase again only in the early 1990s (from about US$2 million in 1990 to about US$14 million in 2004, in constant 2004 dollars), partly in response to an IOM report on malaria research (IOM, 1991), and other favorable reports. Currently, WRAIR has Department of Defense directives to develop a new malaria prophylactic agent, and an intravenous artesunate formulation for severe and complicated malaria.
Perhaps more importantly, WRAIR has become an integral component of the global malaria drug development network, having established strategic alliances with MMV, and the National Institute of Allergy and Infectious Diseases Challenge Grant Program, as well as the pharmaceutical industry. Through MMV projects, WRAIR is supported by a wide range of
TABLE 10-2 Accomplishments of the WRAIR Malaria Drug Development Program
|
Drug |
WRAIR Role |
Collaborators |
|
Chloroquine-primaquine combination tablets |
Clinical trials and FDA approval for prophylaxis |
Sterling Winthrop |
|
Sulfadoxine/pyrimethamine (Fansidar) |
Clinical trials and FDA approval for prophylaxis |
Hoffman-LaRoche |
|
Mefloquine (Lariam) |
Invented and developed |
WHO Hoffman-LaRoche |
|
Halofantrine (Halfan) |
Invented and developed |
GlaxoSmithKline |
|
Arteether (Artemotil) |
Co-developed, clinical trials (Injectable, limited use because of potential neurotoxicity, discovered by WRAIR scientists in animal models) |
WHO, Artecef Approved in the Netherlands only |
|
Doxycycline |
Phase II challenge and clinical trials, FDA approval for prophylaxis |
Pfizer, FDA |
|
Azithromycin |
Efficacy trials for prophylaxis Ongoing research on combinations |
NIAID, Pfizer |
|
Atovaquone-proguanil (Malarone) |
Dose-ranging studies and efficacy trials |
GlaxoSmithKline |
|
Primaquine |
Investigation for prophylaxis (intended to lead to application for added FDA-approved indication to label, which now includes only treatment) |
Sterling Winthrop Naval Medical Research Institute |
|
Tafenoquine |
Discovered and in Phase III for prophylaxis |
GlaxoSmithKline NIAID |
|
SOURCE WRAIR. |
||
donors. This has allowed the research to benefit both military (drugs mainly for nonimmune adults entering malarious areas for short periods or moderate-length stays) and broader global applications that focus on residents of malaria-endemic countries.
An advantage of the WRAIR program is its in-house capabilities to take a compound from early discovery through to early clinical testing, much like a for-profit pharmaceutical company (although on a small scale).
Final drug development requires teaming with a commercial partner who contributes to financing in the late stages, before or at the time of clinical trials.
Antimalarials in Development2
MMV is the main driver of antimalarial drug projects, with many public- and private-sector collaborators. Projects closest to completion are, not surprisingly, several fixed-dose artemisinin combinations. Their likely registration dates, if successful, will be 2006-2007. The large number of fixed-dose combinations in development is currently justified, but as they progress, some may begin to appear more relevant than others. Factors that will influence this are: efficacy, safety, cost, stability, speed of development, and availability. Combinations in development include:
-
Chlorproguanil-dapsone-artesunate (Lapdap-artesunate) by GSK with WHO/TDR, and MMV. Phase 2 studies initiated.
-
Pyronaridine-artesunate by Shin Poong, Korea, in collaboration with WHO/TDR and MMV (MMV funded). Phase 1 planned for early 2004.
-
Piperaquine-dihydroartemisinin (Artekin II) by Holleykin, China. Already marketed in Asia, but not registered in Europe or Africa. Following
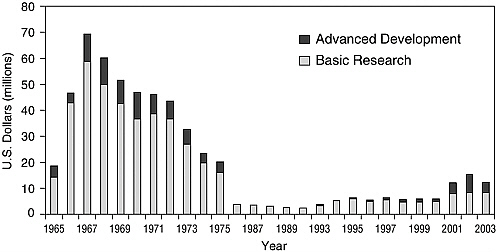
FIGURE 10-1 WRAIR malaria drug program budget from 1965 to 2003 in US$ millions adjusted for inflation.
-
assistance from WHO/TDR, and WHO-RBM an agreement has now been established between Hollekin and MMV for development to international standards. Clinical studies in progress, but further GLP preclinical work still required.
-
Mefloquine-artesunate, a proven combination as a non-fixed treatment regimen, is being developed as a fixed-dose combination. A consortium involving the Drugs for Neglected Diseases Initiative (DNDi), TROPIVAL (Bordeaux), and Far Manginhos of Brazil are involved with EU funding, and some WHO/TDR technical support. Phase 1 clinical studies in progress.
-
Amodiaquine-artesunate, another proven combination as a non-fixed treatment regimen, is in development by the same consortium as above. A commercial partner is being sought for manufacture and production. Phase 1 clinical studies in progress.
In addition to the combinations cited above, there are several new single agent drugs in development that also may ultimately be used in combination. Some of these agents may be dropped as studies progress and problems appear. Projects include:
-
Artemisone, a new artemisinin derivative being developed by Bayer with MMV support. Its potential advantage over existing artemisinins is lower neurotoxicity, and enhanced efficacy. This compound may be registered by 2007.
-
A new quinoline antimalarial, similar to amodiaquine, being developed by GSK, and the University of Liverpool with support from MMV, and some technical assistance from WHO/TDR. This compound resembles amodiaquine but potentially has an improved safety profile, and is highly active against chloroquine-resistant strains. This compound may be registered by 2008.
-
Fosmidomycin, a new class of antimalarial, is under development by Jomaa Pharmaceuticals in Germany. This also may be used in combination with other agents. The major issue is whether it can be given as a 3-day regimen.
-
A new class of synthetic endoperoxide, invented at the University of Nebraska, and initially supported by WHO/TDR, is now being developed by a consortium funded and managed by MMV and is licensed to Ranbaxy of India. A compound entered into full preclinical development late in 2003. The advantage of these compounds is that they have longer half-lives than the artemisinins, and so could serve as better partners than artemisinins for longer half-life drugs. A compound in this category could be registered by 2008.
Several earlier phase drug discovery activities funded by MMV also are under way. Thus, entirely new classes of antimalarials may start to come on to the market soon after 2010.
Several enhanced formulations of existing drugs also are under development.
-
Paediatric Coartem formulation by Novartis in collaboration with MMV, and TDR (MMV funded).
-
Rectal artesunate, developed by WHO/TDR, for use as a single administration for malaria patients unable to take antimalarial medication by mouth, before they are referred for hospital treatment.
-
Artesunate i.v. formulation for severe malaria; a collaboration between WRAIR, and MMV.
WRAIR has its own suite of new antimalarial drugs. Several families of compounds have progressed into preclinical development, including:
-
Pyrroloquinazolines: new analogs that show none of the toxicity common to the parent drug, and yet retain oral potency against highly resistant malaria in animal studies. Should enter clinical studies in 2005.
-
Third generation anti-folates: these show no cross-resistance in studies against multidrug resistant P. falciparum. They are under development in collaboration with Jacobus Pharmaceuticals.
-
Imidazolinedione derivatives: these compounds are unique in that they specifically kill liver stages of malaria but have no activity against blood stages. Because they are not in the 8-aminoquinolone drug class, they are not expected to create hemolysis in G6PD-deficient people. May enter clinical studies in 2005.
-
Tryptanthrins: a well-known, extremely potent class of chemicals with in vitro activity against trypanosomiasis as well as malaria. Difficulties with oral bioavailability have historically impaired progress with this drug class.
-
New macrolides: azithromycin nearly meets criteria as an effective malaria prophylactic drug. Many analogs have been identified with superior in vitro potency against multidrug-resistant malaria. PLIVA Pharmaceuticals is collaborating with WRAIR to develop these new analogs.
-
Chalcones: WRAIR is just beginning assessments of this drug class in collaboration with LICA Pharmaceuticals.
-
Methylene blue: this compound very rapidly kills malaria in animal studies, and appears to be nontoxic at effective doses. It may be a good partner for a combination antimalarial regimen. Its potential utility in malaria prophylaxis still being explored.
-
Mefloquine analogs: an in vitro assay to predict neurotoxicity has been established, in order to identify several potent mefloquine analogs which do not have in vitro neurotoxic effects.
-
Tafenoquine: Phase 2 studies have been completed in partnership with GlaxoSmithKline Pharmaceuticals. This product has a very long half-life, and may be effective for prophylaxis when taken monthly.
-
Azithromycin/chloroquine and azithromycin/quinine: these combinations are known to be safe and effective in children and in pregnant women. Pfizer Pharmaceuticals is collaborating with WRAIR in clinical trials in Thailand and Kenya.
CONCLUSIONS
Given the remarkably limited resources, the progress in antimalarial drug development over the past 5 years or so has been impressive. Direct expenditures total less than US$50 million in 2004, even including a large proportion of in-kind expenditures. For most of the products in development, however, the costliest phases—clinical trials—are yet to come. The global community must plan to increase funding resources for the core groups, MMV and WRAIR (mainly the responsibility of the U.S. government). As more projects mature beyond early development, the pharmaceutical industry also will need to step up in-kind contributions in the developmental areas it does best.
It is not a useful exercise to compare expenditures on antimalarial R&D to commercial drug development (which totals some hundreds of millions of dollars per successful product), but something of the scale should be kept in mind when global investments in antimalarials are considered. During the course of this study, the committee did not independently calculate an “appropriate” amount of investment in antimalarial R&D, but has concluded that current levels are less than optimal, and that increased funds will definitely be required merely to maintain the momentum of existing development activities. MMV, which manages about half the antimalarial R&D worldwide, has estimated its needs at US$30 million per year for development and early clinical studies (Personal communication, R. Ridley, WHO, March 2004). As compounds progress through to advanced clinical and field trials, at least an additional US$10 million per year will be needed (this also assumes continuing contributions in kind from industrial partners not included in these figures). The recently established European and Developing Countries Clinical Trials Partnership (whose funding goal over the next five years is 600 million Euros, including 200 million from the founding European Commission (European Union, 2004) could play a key role in this phase. Equal amounts totaling US$30 million per year plus extra funding for clinical trials and industry investments will be needed for the WRAIR program to remain productive.
REFERENCES
European Union. 2004. EDCTP at a Glance. [Online]. Available: http://europa.eu.int/comm/research/info/conferences/edctp/pdf/edctp-at-a-glance_en.pdf [accessed April 12, 2004].
Global Forum for Health Research. 2002. The 10/90 report of research 2001-2002. Geneva: Global Forum for Health Research.
IOM. 1991. Malaria: Obstacles and Opportunities. Oakes SC, Mitchell VS, Pearson GW, Carpenter CCJ, eds. Washington, DC: National Academy Press.
Medicines for Malaria Venture. 2002. Annual Report, 2002. Geneva: MMV.
Milhous W, Skillman DR. 2004. (unpublished manuscript). The military infectious disease research program: Strategic plan for antimalarial prophylaxis. Washington, DC: Walter Reed Army Institute of Medical Research.
Ridley RG. 2003. Overview of malaria drug development. Background paper prepared for World Bank/IOM expert consultation on the procurement and financing of antimalarial drugs, September 15-16, 2003, Washington, DC.
Veeken H, Pecoul B. 2000. Drugs for ‘neglected diseases’: A bitter pill. Tropical Medicine and International Health 5(5):309-311.













