Appendix C
Scaling Up Antiretroviral Therapy in Resource-Limited Settings: Treatment Guidelines for a Public Health Approach
2003 REVISION
The creation of the present guidelines would not have been possible without the participation of numerous experts.
The World Health Organization wishes to express special gratitude to the Writing Committee that developed this document. This Committee was chaired by Professor Scott Hammer of Columbia University (New York City, USA) and its other members were Diane Havlir (University of California at San Francisco, USA), Elise Klement (Médecins sans Frontières, France), Fabio Scano (WHO/ HTM/STB, Switzerland), Jean-Ellie Malkin (ESTHER, France), Jean-François Delfraissy (CHU BICETRE, ANRS, Paris, France), Joep Lange (International AIDS Society, Sweden), Lydia Mungherera (GNP+, Uganda), Lynne Mofenson (National Institute of Health, NICHD, USA), Mark Harrington (Treatment Action Group, New York, USA), Mauro Schechter (Universidade Federal do Rio de Janeiro, Brazil), N. Kumarasamy (YRG Centre for AIDS Research and Education, India), Nicolas Durier (Médecins sans Frontières, Thailand), Papa Salif Sow (University of Dakar, Senegal), Shabir Banoo (Medicines Control Council, South Africa) and Thomas Macharia (Nazareth Hospital, Kenya).
This document was developed through an expert consultation process in which account was taken of current scientific evidence and the state of the art in the treatment of HIV infection. The primary focus was the context of resource-limited settings. After the production of draft guide-
Reprinted with permission, World Health Organization. Copyright WHO, 2004.
lines by the Writing Committee in October 2003, the document was sent to more than 200 institutional and organizational partners worldwide and made available for public consultation from 28 October to 14 November 2003 on the WHO and ITAC websites. WHO wishes to acknowledge comments and contributions by Alexandra Calmy (Switzerland), Andrew Hill (USA), Annabel Kanabus (United Kingdom), Anthony Amoroso (USA), Anthony Harries (Malawi), Artur Kalichman (Brazil), Bernard Taverne (Senegal), Beverley Snell (Australia), Bess Miller (USA), Brian Eley (South Africa), Carrie Jeffries (USA), Charles Gilks (WHO, Switzerland), Chris Duncombe (Thailand), Chris Green (Indonesia), Clement Malau (Australia), David Cohn (USA), Diana Gibb (United Kingdom), Emanuele Pontali (Italy), Emilia Rivadeneira (USA), Eric Van Praag (USA), Fionuala Mcculagh (Cameroon), Francis Onyango (WHO, AFRO), François Dabis (France), Gray Sattler (Philippines), Guido Levi (Brazil), Heloisa Marques (Brazil), Herbert Peterson (WHO, Switzerland), Isabelle Girault (United Kingdom), Jaime Uhrig (Myanmar), Jeffrey Sturchio (USA), Joia Mukherjee (Haiti), Jonathan Cohn (USA), Jose Zuniga (USA), Karin Timmermans (Indonesia), Karyaija Barigye (USA), Keith Alcorn (United Kingdom), Kenji Tamura (WHO, Switzerland), Kulkanaya Chokephaibulkit (Thailand), Lali Khotenashvilli (WHO, EURO), Leon Levin (South Africa), Márcia Dal Fabbro (Brazil), Marcia Rachid (Brazil), Marga Vitgnes (South Africa), Maria Vigneau (WHO, Switzerland), Marinella de la Negra (Brazil), Marta Segu (Spain), Monica Beg (WHO, Switzerland), Mukadi Ya-Diul (USA), Olavo Munhoz (Brazil), Paul Jareg (Norway), Paula Fujiwara (IUATLD, France), Peter Anton (South Africa), Peter Godfrey-Faussett (United Kingdom), Pier Angelo Todo (Italy), Praphan Pranuphak (Thailand), Ricardo Marins (Brazil), Richard Laing (WHO, Switzerland), Robin Gray (WHO, Switzerland), Rosana Del Bianco (Brazil), Sailesh Upadhyay (Nepal), Stephen Spector (USA), Sudarshan Kumari (India), Taimor Nawaz (Bangladesh), Thurma Goldman (USA), Vincent Habiyambere (WHO, Switzerland), William Burman (Denver, USA) and Wladimir Queiroz (Brazil) during the public consultation process. Their contributions were discussed by the Writing Committee on 26 October 2003 and, where appropriate, the draft guidelines were amended to take their suggestions into account.
WHO also wishes to thank the Agence Nationale de Recherche contre le SIDA, Paris, for hosting the meeting of the Writing Committee on 15–17 October 2003.
This work was coordinated by Marco Vitória and Jos Perriëns of WHO/ HTM/HIV, Geneva, Switzerland.
ABBREVIATIONS
ABC
abacavir
ACTG
AIDS Clinical Trials Group
AIDS
acquired immunodeficiency syndrome
ALT
alanine aminotransferase
ART
antiretroviral therapy
ARV
antiretroviral
ATV
atazanavir
bid
twice daily
CD4
T-lymphocyte CD4+
CNS
central nervous system
d4T
stavudine
DART
development of antiretroviral therapy in Africa
ddI
didanosine
DOT
directly observed therapy
EFV
efavirenz
ENF (T-20)
enfuvirtide
FBC
full blood count
FDC
fixed-dose combination
FTC
emtricitabine
GI
gastrointestinal
HAART
highly active antiretroviral therapy
Hgb
haemoglobin
HIV
human immunodeficiency virus
HIVab
human immunodeficiency virus antibody
IDU
injecting drug user
IDV
indinavir
LPV
lopinavir
MTCT
mother-to-child transmission (of HIV)
NAM
nucleoside analogue mutation
NFV
nelfinavir
NGO
nongovernmental organization
NNRTI
non-nucleoside reverse transcriptase inhibitor
NsRTI
nucleoside analogue reverse transcriptase inhibitor
NtRTI
nucleotide analogue reverse transcriptase inhibitor
NVP
nevirapine
PCR
polymerase chain reaction
PI
protease inhibitor
qd
once daily
RT
reverse transcriptase
RTI
reverse transcriptase inhibitor
RTV
ritonavir
RTV-PI
ritonavir-boosted protease inhibitor
sgc
soft gel capsule
SQV
saquinavir
TB
tuberculosis
TDF
tenofovir disoproxil fumarate
TLC
total lymphocyte count
UN
United Nations
UNAIDS
Joint United Nations Programme on HIV/AIDS
WBC
white blood cell
WHO
World Health Organization
ZDV
zidovudine (also known as AZT)
/r
low dose ritonavir
INTRODUCTION
The advent of potent antiretroviral therapy (ART) in 1996 led to a revolution in the care of patients with HIV/AIDS in the developed world. Although the treatments are not a cure and present new challenges with respect to side-effects and drug resistance, they have dramatically reduced rates of mortality and morbidity, have improved the quality of life of people with HIV/ AIDS, and have revitalized communities. Moreover, HIV/AIDS is now perceived as a manageable chronic illness rather than as a plague (Palella et al., 2003).
Unfortunately, most of the 40 million people currently living with HIV/ AIDS reside in developing countries and do not share this vastly improved prognosis (Joint United Nations Programme on HIV/AIDS and World Health Organization, 2003). WHO conservatively estimated that, at the end of 2003, some 6 million people in developing countries were in immediate need of life-sustaining ART. However, only about 400 000 persons were being treated, over a third of them in Brazil. At the UN General Assembly High-Level Meeting on HIV/AIDS on 22 September 2003, WHO declared that the lack of access to HIV treatment was a global health emergency. WHO calls for unprecedented action to ensure that by the end of 2005 at least 3 million people in need of ART will have access to it.
In order to achieve this target, WHO will develop a strategic framework with the following pillars:
-
global leadership, strong partnership and advocacy;
-
urgent sustained country support;
-
simplified standardized tools for the delivery of ART;
-
an effective and reliable supply of medicines and diagnostics;
-
rapid identification and reapplication of new knowledge and success.
The present updated and simplified treatment guidelines are a cornerstone of the WHO 3-by-5 Plan and are more directive than its predecessor with respect to first-line and second-line therapies. They take into account not only the evidence generated by clinical trials and observational studies on the efficacy and side-effects of the treatment regimens discussed, but also the experience gained with ART by programmes in resource-limited settings and the cost and availability of drugs in those settings. By taking this approach, WHO seeks to assist countries and regions in providing effective antiretroviral therapy to the millions of individuals in immediate or imminent need of treatment. This document, dealing with recommendations for ARV treatment and monitoring, is intended to be a component of a comprehensive package of care at the country level, including the prevention and treatment of opportunistic infections, nutritional programmes and psychosocial support for infected persons. Treatment for HIV, facilitated by these guidelines, complements the full range of HIV prevention efforts for uninfected people at the country level.
The following recent advances in the ART field have been considered in the preparation of this revision:
-
clinical trial data, including those suggesting the inferior virological efficacy of the triple nucleoside combination, ZDV/3TC/abacavir (ABC) in comparison with a three-drug or four-drug efavirenz-based regimen;
-
the availability of the nucleotide analogue, tenofovir disoproxil fumarate (TDF);
-
toxicity concerns regarding the dual nucleoside component of stavudine (d4T)/didanosine (ddI);
-
increasing recognition of the extent of drug class cross-resistance among the nucleoside and nucleotide analogues;
-
the approval of a new nucleoside analogue, emtricitabine (FTC), a protease inhibitor, atazanavir (ATV), the fusion inhibitor, enfuvirtide (ENF, T-20) and increasing availability and clinical experience with generic ARV preparations, particularly in fixed-dose combinations and blister packs (ENF will not be considered further in this document because of the requirement for parenteral administration and the cost of the drug, making it impractical for use in resource-limited settings). These treatment guidelines are part of WHO’s commitment to the treatment of persons living with HIV/AIDS. The first edition of these recommendations, published in April 2002, reflected the best practices at that time on the basis of a review of evidence. In this rapidly evolving field, WHO recognized at the outset that the recommendations would have to be regularly updated. The present revision has been brought forward as a result of new scientific data and the increasing reality of ART scale-up in many countries.
DOCUMENT OBJECTIVES
Currently, fewer than 5% of people in developing countries who need ART can access the medicines in question. WHO believes that at least 3 million people needing care should be able to get the medicines by 2005. This represents almost a tenfold increase. These treatment guidelines are intended to support and facilitate the proper management and scale-up of ART in the years to come by proposing a public health approach to achieve the goals. The key tenets of this approach are as follows.
-
Scaling-up of antiretroviral treatment programmes with a view to universal access, i.e. all persons requiring treatment as indicated by medical criteria should have access to it.
-
Standardization and simplification of ARV regimens so as to support the efficient implementation of treatment programmes in resource-limited settings.
-
Ensuring that ARV treatment programmes are based on scientific evidence in order to avoid the use of substandard protocols that compromise the outcomes of individual patients and create a potential for the emergence of drug-resistant virus. However, it is also important to consider the realities with respect to the availability of human resources, health system infrastructures and socioeconomic contexts so that clear and realistic recommendations can be made.
While it is hoped that this document will be useful to clinicians in resource-limited settings, it is primarily intended for use by treatment advisory boards, national AIDS programme managers and other senior policy-makers who are involved in the planning of national and international HIV care strategies in developing countries. The treatment guidelines serve as a framework for selecting the most potent and feasible ARV regimens as components of expanded national responses for the care of HIV-infected individuals. The framework aims to standardize and simplify antiretroviral therapy, as with tuberculosis (TB) treatment in national TB control programmes, while acknowledging the relative complexity of HIV treatment. Accordingly, options for first-line and secondline regimens are presented, bearing in mind the need to strengthen health systems that often lack staffing power and monitoring facilities, with a view to maximizing the quality and outcomes of the treatments offered.
The guidelines consider when ART should begin, which ARV regimens should be introduced, the reasons for changing ART and the regimens that should be continued if treatment has to be changed. They also address how treatment should be monitored, with specific reference to the side-effects of ART and drug adherence, and make specific recommendations for certain subgroups of patients.
WHEN TO START ARV THERAPY IN ADULTS AND ADOLESCENTS
WHO recommends that, in resource-limited settings, HIV-infected adults and adolescents should start ARV therapy when the infection has been confirmed and one of the following conditions is present.
-
Clinically advanced HIV disease:
-
WHO Stage IV HIV disease, irrespective of the CD4 cell count;
-
WHO Stage III disease with consideration of using CD4 cell counts <350/mm3 to assist decision-making.
-
WHO Stage I or II HIV disease with CD4 cell counts <200/mm3 (Table A).
The rationale for these recommendations is as follows. The treatment of patients with WHO Stage IV disease (clinical AIDS) should not be dependent on a CD4 cell count determination. However, where available, this test can be helpful in categorizing patients with Stage III conditions with respect to their need for immediate therapy. For example, pulmonary TB can occur at any CD4 count level and, if the CD4 cell count level is well maintained (i.e. >350/mm3), it is reasonable to defer therapy and continue to monitor the patient. For Stage III conditions a threshold of 350/mm3 has been chosen as the level below which immune deficiency is clearly present such that patients are eligible for treatment when their clinical condition portends rapid clinical progression. A level of 350/mm3 is also in line with other consensus guideline documents (DHHS; Yeni et al., 2002). For patients with Stage I or Stage II HIV disease the presence of a CD4 cell count <200/mm3 is an indication for treatment. In cases where CD4 cell counts cannot be assessed the presence of a total lymphocyte count of 1200/mm3 or below can be used as a substitute indication for treatment in the presence of symptomatic HIV disease. While the total lymphocyte count correlates relatively poorly with the CD4 cell count in asymptomatic persons, in combination with clinical staging it is a useful marker of prognosis and survival (Badri and Wood, 2003; Beck et al., 1996; Brettle, 1997; Fournier and Sosenko, 1992; Kumarasamy et al., 2002; van der Ryst et al., 1998). An assessment of viral load (e.g. using plasma HIV-1 RNA levels) is not considered necessary before starting therapy. Because of the cost and complexity of viral load testing, WHO does not currently recommend its routine use in order to assist with decisions on when to start therapy in severely resource-constrained settings. It is hoped, however, that increasingly affordable methods of determining viral load will become available so that this adjunct to treatment monitoring can be more widely employed. It should be noted that the current WHO Staging System for HIV Infection and Disease for Adults and Adolescents was developed several years ago
TABLE A. Recommendations for Initiating Antiretroviraltherapy in Adults and Adolescents with Docummented HIV Infection
|
If CD4 testing available, it is recommended to document baseline CD4 counts and to offer ART to patients with: |
|
• WHO Stage IV disease, irrespective of CD4 cell count • WHO Stage III disease (including but not restricted to HIV wasting, chronic diarrhoea of unknown etiology, prolonged fever of unknown etiology, pulmonary TB, recurrent invasive bacterial infections or recurrent/persistent mucosal candidiasis), with consideration of using CD4 cell counts <350/mm3 to assist decision-makinga • WHO Stage I or II disease with CD4 cell counts 200/mm3b If CD4 testing unavailable, it is recommended to offer ART to patients with: • WHO Stage IV disease, irrespective of total lymphocyte count • WHO Stage III disease (including but not restricted to HIV wasting, chronic diarrhoea of unknown etiology, prolonged fever of unknown etiology, pulmonary TB, recurrent invasive bacterial infections or recurrent/persistent mucosal candidiasis), irrespective of the total lymphocyte countc • WHO Stage II disease with a total lymphocyte count 1200/mm3d |
|
aCD4 count advisable to assist with determining need for immediate therapy. For example, pulmonary TB may occur at any CD4 level and other conditions may be mimicked by non-HIV etiologies (e.g. chronic diarrhoea, prolonged fever). bThe precise CD4 level above 200/mm3 at which ARV treatment should start has not been established. cThe recommendation to start ART in all patients with stage III disease, without reference to total lymphocyte counts reflects consensus of expert opinion. It took into account the need of a practical recommendation that allows clinical services and TB programmes in severely resource constrained settings to offer access to ART to their patients. As some adults and adolescents with stage III disease will be presenting with CD4 counts above 200, some of them will receive antiretroviral treatment before the CD4 < 200 threshold is reached. However, if CD4 counts cannot be determined, starting ART earlier in these patients was not considered problematic. dA total lymphocyte count of 1200/mm3 can be substituted for the CD4 count when the latter is unavailable and HIV-related symptoms exist. It is not useful in the asymptomatic patient. Thus, in the absence of CD4 cell testing, asymptomatic HIV-infected patients (WHO Stage I) should not be treated because there is currently no other reliable marker available in severely resourceconstrained settings. |
and has consequent limitations. Adaptations at the level of national programmes may therefore be appropriate. Nevertheless, it remains a useful tool for assisting in defining parameters for initiating therapy in resource-limited settings and thus has continued to be applied in this revision.
RECOMMENDED FIRST-LINE ARV REGIMENS IN ADULTS AND ADOLESCENTS
Countries are encouraged to use a public health approach to facilitate the scale-up of ARV use in resource-limited settings as delineated in the WHO 3-by-5 Plan. This means that ART programmes should be developed which can reach as many people as possible who are in need of therapy and requires that ARV treatment be standardized. In particular, it is suggested that countries select a first-line regimen and a limited number of second-line regimens, recognizing that individuals who cannot tolerate or fail the first-line and second-line regimens will be referred for individualized care by specialist physicians. The use of standardized regimens is an essential component of the 3-by-5 Plan and will facilitate WHO’s efforts to assist Member States with achieving this goal. This is the approach to ARV regimen selection taken in the present document. Among the factors that should be considered in the selection of ART regimens at both the programme level and the level of the individual patient are:
-
potency;
-
side-effect profile;
-
laboratory monitoring requirements;
-
potential for maintenance of future treatment options;
-
anticipated patient adherence;
-
coexistent conditions (e.g. coinfections, metabolic abnormalities);
-
pregnancy or the risk thereof;
-
use of concomitant medications (i.e. potential drug interactions);
-
potential for infection with a virus strain with diminished susceptibility to one or more ARVs, including that resulting from prior exposure to ARVs given for prophylaxis or treatment;
-
very importantly, availability and cost.
The use of quality-assured1 antiretrovirals in fixed-dose combinations (FDCs)2 or as blister packs3 is another important consideration as this pro-
|
1 |
Quality-assured medicines assembled in fixed-dose combinations (FDCs), in the context of this document, include individual products which have been deemed to meet or exceed international standards for quality, safety and efficacy. In the case of drug combinations whose components are from different manufacturers the international standards include a requirement for clinical bioequivalence studies to establish therapeutic interchangeability of the components. For WHO’s work on prequalification of ARVs see: http://www.who.int/medicines/organization/qsm/activities/pilotproc/proc.shtml |
|
2 |
Fixed-dose combinations are based on the principle of inclusion of two or more active pharmacological products in the same pill, capsule, tablet or solution. |
|
3 |
A blister pack is a plastic or aluminum blister containing two or more pills, capsules or tablets. |
motes better adherence and, in turn, limits the emergence of drug resistance. It also facilitates ARV storage and distribution logistics. Additional considerations relevant to the developing world include access to a limited number of ARV drugs, limited health service infrastructures (including human resources), the need to deliver drugs to rural areas, high incidences of TB and hepatitis B and/or C in populations and the presence of varied HIV types, groups and subtypes. The previous (April 2002) version of these treatment guidelines recommended that countries should select a first-line treatment regimen and identified regimens composed of two nucleosides plus either a non-nucleoside, or abacavir, or a protease inhibitor as possible choices. Since that version was published, many countries have started ARV treatment programmes and have chosen their first-line treatment regimens, taking into account how the above factors would come into play in the different settings. The majority of treatment programmes in developing countries have opted for a regimen composed of two nucleosides and a nonnucleoside RT inhibitor. Triple nucleoside regimens including abacavir were almost never selected because of their cost and concerns over hypersensitivity reactions, and regimens containing a protease inhibitor became secondary options, mainly because of their cost, notwithstanding price decreases. However, high pill counts, their side-effect profile and more difficult logistics (some requiring a cold chain) were probably also considerations.
The Writing Committee examined non-nucleoside-based regimens and took account of clinical experience with the efficacy and toxicity of the nucleoside reverse transcriptase inhibitor (NRTI) and non-nucleoside reverse transcriptase inhibitor (NNRTI) components, the availability of fixed-dose combinations (Annex D), the lack of a requirement for a cold chain, and drug availability and cost. On this basis the Committee concluded that the four first-line ARV regimens listed in Table B were appropriate for adults and adolescents. These regimens consist of a thymidine analogue NRTI, i.e. stavudine (d4T) or zidovudine (ZDV), a thiacytidine NRTI, i.e. lamivudine (3TC), and an NNRTI, i.e. nevirapine (NVP) or efavirenz (EFV).
The choice between d4T and ZDV should be made at the country level on the basis of local considerations but it is recommended that both drugs be available. d4T is initially better tolerated than ZDV and does not require haemoglobin monitoring. However, among the NRTIs, it has been consistently most associated in developed countries with lipoatrophy and other metabolic abnormalities, including lactic acidosis, particularly when combined with didanosine (ddI). It can also cause peripheral neuropathy and pancreatitis. ZDV has also been implicated in metabolic complications of therapy but to a lesser extent than d4T. Initial drug-related side-effects (headache, nausea) are more frequent with ZDV and the drug can cause severe anaemia and neutropenia, which, at the very least, requires that haemoglobin should be monitored before and during treatment with ZDV. d4T can be substituted for ZDV in the event of intolerance to the latter and
vice versa (except in cases of suspected lactic acidosis, in which instance neither drug should be prescribed). However, the initial need for less laboratory monitoring might, at present, favour d4T as the nucleoside of choice for the majority of patients in ART programmes in settings with severe resource limitations where rapid scaling-up is intended.
3TC is a potent NRTI with an excellent record of efficacy, safety and tolerability. It can be given once or twice daily and has been incorporated into a number of fixed-dose combinations. Emtricitabine (FTC) is a recently approved nucleoside analogue that is structurally related to 3TC, shares its resistance profile and can be given once daily (Bang and Scott, 2003). It is currently being tested as a coformulated product with tenofovir disoproxil fumarate (TDF). Because of the relatively recent approval of FTC in a limited number of countries it is not included in WHO’s recommended first-line regimens but this may change in the light of future experience with the drug and its availability and cost.
The dual nucleoside component of d4T/ddI is no longer recommended as part of first-line regimens because of its toxicity profile, particularly in pregnant women (Boubaker et al., 2001). It is also worth emphasizing that ZDV and d4T should never be used together because of proven antagonism between them (Pollard et al., 2002).
TDF has a long intracellular half-life and can therefore be used as part of once-daily triple-drug regimens. It has been shown that TDF is an effective component of first-line regimens in combination with 3TC and efavirenz (EFV) (Gallant and Deresinski, 2003; Staszewski et al., 2003). It is generally well tolerated although there have been reports of renal insufficiency in patients receiving TDF (Karras et al., 2003; Schaaf et al., 2003; Verhelst et al., 2002). However, worldwide experience with the drug is still relatively limited. In addition, its limited availability and relatively high cost in developing countries continue to be significant factors. For the purposes of the present treatment guidelines, therefore, discussion of its use will be restricted to second-line therapy. As experience, availability and cost issues in resource-limited settings become clarified the inclusion of TDF in WHO-recommended first-line regimens should be reconsidered.
Globally, NNRTI-based regimens are now the most widely prescribed combinations for initial therapy. They are potent and relatively simple but are inactive in respect of HIV-2 and group O of HIV-1. EFV and NVP are both potent NNRTIs with demonstrated clinical efficacy when administered in appropriate combination regimens. However, differences in toxicity profile, a potential for interaction with other treatments, and cost, allow the formulation of both positive and negative recommendations on their use (Staszewski et al., 2003; Ena et al., 2003; Keiser et al., 2002; Law et al., 2003; Martin-Carbonero et al., 2003; Moyle, 2003; van Leth et al., 2003). NVP has a higher incidence of rash, which may be severe and life-threaten-
ing, and a greater risk of hepatotoxicity, which may also be life-threatening. This makes the drug less suitable for treating patients who use other hepatotoxic medications, or drugs that can cause rash, or both, such as rifampicin. The major toxicities associated with EFV are related to the central nervous system (CNS), teratogenicity and rash. (Rash is more frequent in children than adults, is generally mild, and usually does not require discontinuation of therapy.) The CNS symptoms typically abate after 10 to 14 days in most, but not all, patients. EFV should be avoided in persons with a history of severe psychiatric illness, when there is a potential for pregnancy, and during pregnancy. EFV may be considered to be the NNRTI of choice in patients with TB coinfection, and NVP may be the best choice in women of childbearing potential or who are pregnant. EFV should not be given to women of childbearing potential unless effective contraception can be assured. However, it is important to emphasize that EFV and NVP may interact with estrogen-based contraceptive pills. NVP is available as part of three-drug FDC which could be used when assured-quality formulations of proven bioequivalence are available.
The use of the five-drug formulary approach (d4T or ZDV) + 3TC + (NVP or EFV) translates practically into four possible regimens (Table B) and provides options for drug substitutions in respect of toxicity (Table C). Because each is considered an appropriately potent, standard-of-care regimen with respect to efficacy, other factors should determine what a country chooses as a lead regimen.
Table B lists some of the factors that should be taken into account in making this decision. ARVs in FDCs and blister packs have potential advantages over conventional drug packaging: they are helpful tools for simplifying treatment and promote adherence. Moreover, they can minimize prescription errors, improve adherence of health care workers to treatment standards, decrease errors in drug administration, improve drug management (because of fewer items and a single expiration date), simplify drug forecasting, procurement, distribution and stocking because fewer items and lower volumes are necessary, and reduce the risk of misuse of single drugs. FDCs also present challenges with respect to the individualization of dosing of individual components, the treatment of children and the differential half-lives of drugs when treatment is interrupted. Laboratory monitoring requirements should also be taken into account.
When d4T/3TC/NVP or ZDV/3TC/NVP is chosen as the first-line regimen the availability of the two-drug combination (d4T/3TC or ZDV/3TC) is also important for use with NVP lead-in dosing during the first two weeks of treatment and for managing some toxicities associated with NVP (Annex D). Additional drugs should be available in districts (level 2) or regional hospitals (level 3). This tiered approach to ARV regimen availability can be paralleled by a tiered monitoring strategy for health care systems.
TABLE B. First-line ARV Regimens in Adults and Adolescents and Characteristics That Can Influence Choice
|
ARV regimen |
Major potential toxicities |
Usage in women (of childbearing age or pregnant) |
|
d4T/3TC/NVP |
d4T-related neuropathy, pancreatitis and lipoatrophy; |
Yes |
|
|
NVP-related hepatotoxicity and severe rash |
|
|
ZDV/3TC/NVP |
ZDV-related GI intolerance, anaemia, and neutropenia; NVP-related hepatotoxicity and severe rash |
Yes |
|
d4T/3TC/EFV |
d4T-related neuropathy, pancreatitis and lipoatrophy; EFV-related CNS toxicity and potential for teratogenicity |
Nob |
|
ZDV/3TC/EFV |
ZDV-related GI intolerance, anaemia and neutropenia; EFV-related CNS toxicity and potential for teratogenicity |
Nob |
|
aPeople with TB disease and HIV coinfection. bWomen of childbearing potential or who are pregnant. cThese combinations have not been prequalified by WHO but could be used if assured-quality formulations of proven bioequivalence were available. dObtained from: Sources and prices of selected medicines and diagnostics for people living with HIV/AIDS, June 2003 (www.who.int/HIV_AIDS). |
||
Additional Considerations for First-line Therapy Including Treatment of HIV-2 and Group O HIV-1 Infections
PI-based Regimens
While PI-based regimens remain an accepted standard of care for initial regimens, their high cost relative to NNRTI-based regimens makes their use problematic in resource-limited countries seeking to achieve rapid scale-up of therapy. Advantages of PI-based regimens (e.g. PI plus two NRTIs), however, are proven clinical efficacy and well-described toxicities. Disadvantages are higher pill counts, food and water requirements in some cases,
|
Usage in TB coinfectiona |
Availability three-drug fixed-dose combination |
Laboratory monitoring requirements |
Price for leastdeveloped countries, June 2003 (US$/year)d |
|
Yes, in rifampicin-free continuation phase of TB treatment. Use with caution in rifampicin-based regimensa |
Yes |
No |
281-358 |
|
Yes, in rifampicin-free continuation phase of TB treatment. Use with caution in rifampicin-based regimensa |
Yesc |
Yes |
383-418 |
|
Yes, but EFV should not be given to pregnant women or women of childbearing potential, unless effective contraception can be assured |
No. EFV not available as part of FDC; however, partial FDC available for d4T/3TCc |
No |
350-1086 |
|
Yes, but EFV should not be given to pregnant women or women of childbearing potential unless effective contraception can be assured |
No. EFV not available as part of FDC; however, partial FDC available for ZDV/3TC |
Yes |
611-986 |
significant interactions with other drugs that preclude or complicate their use during TB treatment regimens using rifampicin, metabolic abnormalities and the need for a functioning cold chain for ritonavir-boosted regimens. Consequently, in these treatment guidelines, PI-based regimens are primarily reserved for second-line therapy. They should be considered as first-line regimens, however, in circumstances where there is concern for the presence of NNRTI resistance (e.g. prevalence in the community exceeding 5-10%) (Hirsch et al., 2003), where there are viral types with known insensitivity to NNRTIs (e.g. HIV-2 or HIV-1 group O) or where there is intolerance of the NNRTI class of agents. Considerations include (d4T or ZDV) + 3TC combined with either lopinavir/ritonavir (LPV/r), saquinavir/
ritonavir (SQV/r), indinavir/ritonavir (IDV/r), or nelfinavir (NFV), the choice(s) being dictated by national programme priorities. Ritonavirboosted PIs are becoming preferred because of their high potency (Walmsley et al., 2002) and relatively lower pill burden, but the requirement for a cold chain and the support of frequent laboratory monitoring present problems for many low-resource countries. LPV/r is administered as a twice-daily regimen and is relatively well tolerated, but frequently causes elevations in plasma lipid levels. SQV/r can be administered once daily is known to achieve adequate blood levels in pregnancy and is compatible with rifampicin coadministration. However the pill burden with currently available formulations is high and gastrointestinal side-effects are frequent. NFV, although considered less potent than LPV/r, is an acceptable alternative, has been used extensively in pregnancy and does not require cold chain facilities. However, it is less effective against HIV-2 infection than other PIs (Adje-Toure et al., 2003; van der Ende et al., 2003; Smith et al., 2001). IDV/r also can be considered an alternative but is associated with a moderate incidence of renal adverse effects, particularly nephrolithiasis, and requires vigorous hydration.
The role of the recently approved protease inhibitor, atazanavir (ATV) in resource limited settings is currently unclear. The drug has the advantage of once-daily administration and does not induce hyperlipidaemia when administered without ritonavir boosting. It can also be given with low-dose ritonavir to enhance its potency (Haas et al., 2003; Piliero, 2002; Sanne et al., 2003). It is a reasonable alternative but much greater experience has been gained with the other PIs listed. Firmer recommendations will be made as the cost and availability of ATV, and experience with the drug, become clearer.
Triple NRTI-based Regimens
In the 2002 edition of these guidelines the ZDV/3TC/abacavir (ABC) regimen was considered the most user-friendly with respect to both patients and programmes (two pills per day and absence of significant drug interactions). The main disadvantages noted were uncertainty about its potency when the viral load was very high in patients with advanced disease, uncertainty as to whether the drugs, particularly ABC, would become available at an affordable cost, and the potential for fatal ABC hypersensitivity reactions. Recently released data from ACTG A5095 Study demonstrate that ZDV/3TC/ABC had a significantly higher virological failure rate than the other two study arms combined (ZDV/3TC/EFV or ZDV/3TC/ABC/EFV), 21% vs. 10% respectively, with a median follow-up of 32 weeks (Gulick et al., 2003). Importantly, significant differences in virological outcome were seen in persons with viral loads above and below 100 000 HIV RNA
copies/ml. The study remains blinded with respect to the two EFV-containing arms. The incorporation of these findings into clinical practice and guidelines policy presents challenges because of the perceived advantages of triple nucleoside regimens, especially their attractiveness in the setting of coinfection with TB. It is important to note that the efficacy of ZDV/3TC/ ABC in ACTG A5095 was comparable to that reported in previously reported studies of this regimen in the treatment of naive persons (Ibbotson and Perry, 2003; Staszewski et al., 2001). Moreover, in ACTG A5095 the CD4 cell responses were comparable to those of the combined EFV-containing arms. Thus, its virological inferiority to EFV-based regimens in a directly comparative trial moves this triple NRTI combination to a lower tier of consideration but does not, and should not, remove it from serious consideration. It may be useful, for example, when NNRTIs cannot be used because of intolerance or drug resistance and when PI-based regimens are not available. In particular, this regimen is a viable alternative for the management of patients coinfected with TB when antiretroviral and anti-TB therapy are coadministered. For the purposes of these guidelines it is considered to be a secondary alternative for initial therapy in specific situations (e.g. active TB coinfection, HIV-2 infection). It is also important to note that the ongoing DART trial will provide crucial additional information on the safety of ZDV/3TC/ABC in comparison with ZDV/3TC/TDF and ZDV/3TC/NVP in 3000 treatment-naïve patients in Africa (Kityo, 2003).
It should not be assumed that any triple NRTI regimen is comparable to any other: each triple NRTI combination needs to be evaluated on its own merits. Illustrative of this is the recently presented study of the combination of TDF/ 3TC/ABC administered once daily, in which there was a high virological failure rate (49%) and a high incidence of the K65R mutation, which confers crossresistance to non-ZDV nucleoside analogues (Gallant et al., 2003). This specific combination should be avoided in the light of these data. Similarly, in a 24-patient pilot study, TDF/ddI/3TC dosed once daily resulted in a 91% virological failure rate and a high incidence of the K65R mutation (Gilead, 2003). Another recent study reported low efficacy and a high frequency of adverse events with d4T/ddI/ABC (Gerstoft et al., 2003). These combinations should be avoided.
REASONS FOR CHANGING ART IN ADULTS AND ADOLESCENTS
It may be necessary to change ART because of either toxicity or treatment failure.
Toxicity
Toxicity is related to the inability to tolerate the side-effects of medication and to the significant organ dysfunction that may result. This can be monitored clinically on the basis of patient reporting and physical examination, and there may also be a limited number of laboratory tests, depending on the specific combination regimen that is utilized and the health care setting.
If a change in regimen is needed because of treatment failure, a new second-line regimen becomes necessary. When the toxicity is related to an identifiable drug in the regimen, the offending drug can be replaced with another drug that does not have the same side-effects, e.g. substitution of d4T for ZDV (for anaemia) or NVP for EFV (for CNS toxicity or pregnancy). Given the limited number of ARV combination options available in resource-limited settings, it is preferable to pursue drug substitutions where feasible so that premature switching to completely new alternative regimens is minimized. Table C lists the first-level medication switch options for toxicity for the four combination regimens listed in Table B. For life-threatening or more complex clinical situations, referral to district or regional hospital centres is recommended.
Treatment Failure
Treatment failure can be defined clinically as assessed by disease progression, immunologically using measurement of the CD4 counts, and/or virologically by measuring viral loads. Clinical disease progression should be differentiated from the immune reconstitution syndrome, an entity that can be seen early after ARV is introduced. This syndrome is characterized by the appearance of signs and symptoms of an opportunistic disease a few weeks after the start of potent ARV therapy in the setting of advanced immunodeficiency, as an inflammatory response to previously subclinical opportunistic infection. It is also possible that this immunological reconstitution may lead to the development of atypical presentations of some opportunistic infections.
Definitions of clinical and CD4-related treatment failure are listed in Table D. As viral loads are not normally available in resource-limited settings it is recommended that programmes primarily use clinical, and, where possible, CD4 count criteria, in order to define treatment failure. Similarly, drug resistance testing will not become a routine part of clinical care in resource-limited settings in the foreseeable future and so is not considered in these recommendations. However, it should be recognized that, in the developing world, treatment failure will be recognized later solely on the basis of clinical and/or CD4 criteria, thus providing a greater opportunity for drug resistance mutations to evolve before regimen change. This can
TABLE C. Major Potential Toxicities of First-line ARV Regimens and Recommended Substitutions
|
Regimen |
Toxicity |
Drug substitution |
|
d4T/3TC/NVP |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ZDV/3TC/NVP |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
d4T/3TC/EFV |
|
|
|
|
|
|
|
|
|
|
|
ZDV/3TC/EFV |
|
|
|
|
|
|
|
aSwitching off d4T typically does not reverse lipoatrophy but may slow its progression. TDF and ABC can be considered as alternatives but availability is currently limited in resource constrained settings. In the absence of TDF or ABC availability, ddI or ZDV are additional alternatives to consider. bPI can be LPV/r or SQV/r. IDV/r or NFV can be considered as alternatives (see text). |
||
compromise the NRTI component of the alternative regimen through drug class cross-resistance.
CLINICAL AND LABORATORY MONITORING
WHO recommends that in resource-limited settings the basic clinical assessment before the initiation of ART include documentation of past
TABLE D. Clinical and CD4+ Cell Count Definitions of Treatment Failure in HIV+ Adults and Adolescents
|
Clinical signs of treatment failure |
CD4 cell criteria for treatment failure |
|
|
|
|
|
|
|
|
|
|
aImmune reconstitution syndrome (IRS) is characterized by the appearance of signs and symptoms of an opportunistic disease a few weeks after the start of potent antiretroviral therapy in the setting of advanced immunodeficiency, as an inflammatory response to previously subclinical opportunistic infection. It is also possible that this immunological reconstitution may lead to the development of atypical presentations of some opportunistic infections. bRecurrence of TB may not represent HIV disease progression, as reinfection may occur. Clinical evaluation is necessary. cIf patient is asymptomatic and treatment failure is being defined by CD4 cell criteria alone, consideration should be given to performing a confirmatory CD4 cell count if resources permit. |
|
medical history, identification of current and past HIV-related illnesses, identification of coexisting medical conditions that may influence the timing of initiation and choice of ART (such as TB or pregnancy), and current symptoms and physical signs. Active TB should be managed in accordance with national TB control programmes.
In order to facilitate the scale-up of ARV use in resource-limited settings, WHO has tiered its monitoring recommendations to primary health
TABLE E. Recommended Tiered Laboratory Capabilities for ARV Monitoring in Limited-Resource Settingsa
|
Primary health care centres (level 1) |
District hospitals (level 2) |
Regional referral centres (level 3) |
|
Haemoglobin (if ZDV is being considered for use)b |
FBC and differential test by second serological method |
|
|
CD4+ cell countc |
||
|
Pregnancy testingd |
FBC and differential |
Full serum chemistries including (but not restricted to electrolytes, renal function, liver enzymes, lipids)d |
|
Referral for sputum smear for TB (if microscopy not available) |
CD4+ cell countc |
|
|
|
ALT |
Pregnancy testingd |
|
|
Pregnancy testingd |
Sputum smear for TB |
|
|
Sputum smear for TB |
Viral load testinge |
|
aThis table only considers testing that is desirable for proper monitoring of ARV toxicity, efficacy and two prominent concomitant conditions (pregnancy and TB). It is not meant to be comprehensive with respect to other diagnostic capabilities that are important in the comprehensive care of HIV-infected persons. Other resources are available for these considerations. bIn primary health care centres where laboratory facilities are not available or in the absence of laboratory-based haemoglobinometry, the WHO haemoglobin colour scale can be used together with clinical signs to evaluate anaemia (more details at www.who.int/bct/). cScale-up of ART under the 3-by-5 Plan does not require uniform CD4 testing availability but, because of the value of this test in patient monitoring, WHO will work with Member States to make this a reality. dEFV should not be given to women of childbearing potential unless adequate contraception is assured, not to women in the first trimester of pregnancy. eBecause of the cost and technical issues associated with viral load testing, this test is not currently recommended as part of the present treatment guidelines. However, it is hoped that more cost-effective technologies will allow regional referral centres to acquire this capability, given its utility in assessing treatment failure. |
||
care centres (level 1), district hospitals (level 2) and regional referral centres (level 3) (Table E). WHO recognizes the importance of laboratory monitoring for efficacy and safety but does not want restricted infrastructure for these tests to place undue limitations on the scale-up effort.
This section concentrates on the basic clinical and laboratory monitoring indicated for the WHO-recommended first-line regimens outlined in Table B. These recommendations are designed to be implemented at the
level of community health centres and/or that of district hospitals, working in concert, with backup from regional referral centres. National programme managers, working with WHO to implement the 3-by-5 Plan, should determine country-specific policies on how and where decisions about initiating therapy for individual patients are to be made. Similarly, the specific interactions of the health care delivery system levels for maximizing ART efficacy and safety require decisions to be made at the national programme level.
Clinical and laboratory assessments are considerations at baseline (pre-ART) and on treatment. Many studies conducted in developed and developing countries have demonstrated a reasonable correlation between TLC with CD4 levels in symptomatic patients (Badri and Wood, 2003; Beck et al., 1996; Brettle, 1997; Fournier and Sosenko, 1992; Kumarasamy et al., 2002; van der Ryst et al., 1998). This means that even if CD4 cell count testing is unavailable, simple tools such as haemoglobin measurement and TLC can be used as laboratory markers to initiate HAART in resource-poor settings. The baseline clinical assessment is the same for all four recommended first-line regimens. It should include:
-
staging of HIV disease;
-
determination of concomitant medical conditions (e.g. TB, pregnancy, major psychiatric illness);
-
detailing of concomitant medications, including traditional therapies;
-
assessment of patients’ readiness for therapy.
Once therapy has begun, clinical assessment should cover
-
signs/symptoms of potential drug toxicities (Table D);
-
adherence;
-
response to therapy;
-
weight;
-
basic laboratory monitoring considerations as listed in Table F.
Need for Scale-up of Laboratory Capacity
WHO recognizes the current limitations on laboratory capacity in resource limited settings. The 3-by-5 Plan is designed to move forward with current realities in place. WHO will work with Member countries and diagnostic manufacturers to scale up laboratory infrastructure at the country level so as to permit the uniform availability of CD4 testing, wider availability of automated haematology and chemistry testing, and regional availability of viral load testing. This will require choosing uniform, cost-
TABLE F. Basic Laboratory Monitoring for Recommended First-line ARV Regimens at Primary Health Care Centres (Level 1) and District Hospitals (Level 2)
|
Regimen |
Laboratory assessment at baseline (pretherapy) |
Laboratory assessment on therapy |
|
d4T/3TC/NVP |
Desirable but not required: CD4 |
Symptom-directed determination of ALT for toxicity CD4 q6-12 months, if available, for efficacy |
|
ZDV/3TC/NVP |
Recommended: Hgb Desirable but not required: FBC, CD4 |
Symptom-directed determination of Hgb, WBC, ALT for toxicity CD4 q6-12 months, if available, for efficacy |
|
d4T/3TC/EFV |
Pregnancy test (mandatory) Desirable but not required: CD4 |
Symptom-directed testing but none routinely required for toxicity CD4 q6-12 months, if available, for efficacy |
|
ZDV/3TC/EFV |
Pregnancy test (mandatory) Recommended: Hgb Desirable but not required: FBC, CD4 |
Symptom-directed determination of Hgb, WBC for toxicity CD4 q6-12 months, if available, for efficacy |
effective methodologies at the country level and ensuring supplies of reagents and the maintenance of equipment.
CHOICE OF ARV REGIMENS IN THE EVENT OF TREATMENT FAILURE OF FIRST-LINE COMBINATIONS IN ADULTS AND ADOLESCENTS
WHO recommends that the entire regimen be changed from a first-line to a second-line combination in the setting of treatment failure. The new second-line regimen should involve drugs that retain activity against the patient’s virus strain and should preferably include at least three new drugs, one or more of them from a new class, in order to increase the likelihood of treatment success and minimize the risk of cross-resistance.
Figure 1 lists the second-line regimens that might be considered in
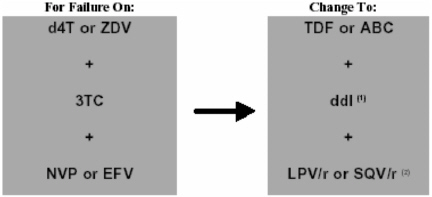
FIGURE 1. Recommended second-line regimens in adults and adolescents in the event of treatment failure of first-line ARV regimens.
adults and adolescents for the first-line regimens identified in Table B. When (d4T or ZDV) + 3TC are used as part of the first-line regimen, nucleoside cross-resistance may compromise the potency of alternative dual nucleoside components in the second-line regimen, especially in the presence of long-standing virological failure. In this situation it is necessary to make empirical alternative choices with a view to providing as much antiviral activity as possible. Given the cross-resistance that exists between d4T and ZDV, second-line regimens that might offer more activity include TDF/ ddI or ABC/ddI. The issues of cost and drug hypersensitivity with ABC remain. Furthermore, high-level ZDV/3TC coresistance confers diminished susceptibility to ABC. TDF can be compromised by multiple nucleoside analogue mutations (NAMs) but often retains activity against nucleoside-resistant viral strains. It is attractive in that, like ddI, it is administered once daily. TDF raises the level of ddI and the dose of the latter should therefore be reduced when the two drugs are given together, in order to reduce the chance of ddI-associated toxicity (e.g. neuropathy and pancreatitis).
Because of the diminished potential of almost any second-line nucleoside component, a ritonavir-enhanced PI (RTV-PI) component, i.e. lopinavir (LPV)/r, saquinavir (SQV)/r or indinavir (IDV)/r, is preferable to nelfinavir (NFV) in second-line regimens, given their potency (Walmsley et al., 2002). NFV can be considered as an alternative for the PI component if a ritonavir-enhanced PI is not available, if a cold chain is not secure or if there is a clinical contraindication to the use of another PI.
Despite being considered a potent option, IDV/r is associated with substantial renal side-effects and should also be considered as an alterna-
tive. As noted above, the role and availability of ATV/r in the developing world cannot be fully specified at present.
For treatment failure with a first-line PI-based regimen, the choice of an alternative regimen depends on the reason for the initial choice of a PI-based, rather than an NNRTI-based, regimen. If the reason was suspected NNRTI resistance or HIV-2 infection the choice of the alternative regimen is not straightforward. In these situations the options depend on the constraints imposed by the circumstances of individual patients, the capabilities of individual managements to test for resistance to drugs, and the limited ARV formulary that may exist in particular country programmes.
Treatment failure on a triple NRTI regimen is more easily managed because two important drug classes (NNRTIs and PIs) will have been spared. Thus a RTV-PI + NNRTI +/- alternative NRTIs (e.g. ddI and/or TDF) can be considered if drug availability permits.
CONSIDERATIONS FOR SPECIFIC CATEGORIES OF PATIENTS
Women of Childbearing Potential or Pregnant Women
The guiding principle for the treatment of women of childbearing potential or pregnant women is that therapeutic decisions should be based solely on their need and eligibility for ART as outlined in Section III. The special circumstances of pregnancy or breast-feeding raise additional issues concerning toxicity to mothers and children, the choice of ARV drugs, and the prevention of HIV transmission from mothers to infants. These matters should be dealt with in the context of assuring optimal treatment to preserve the health of the mothers. Consequently, the recommended WHO first-line regimen for this patient subgroup is:
(d4T or ZDV) + 3TC + NVP.
The choice of ART for women with the potential to become pregnant must involve a consideration of the possibility that the ARV drugs may be received early in the first trimester, before the recognition of pregnancy and during the primary period of fetal organ development. EFV should be avoided in such women because of its potential for teratogenicity. Women who are receiving ART and do not wish to become pregnant should have effective and appropriate contraceptive methods available to them in order to reduce the likelihood of unintended pregnancy. In those women for whom effective contraception can be assured, EFV remains a viable option for the NNRTI component of the regimen. Women who are receiving ART and become pregnant should continue their treatment unless they are in the
first trimester of pregnancy and EFV has been part of the regimen, in which circumstances EFV should be discontinued and replaced by NVP.
For pregnant women it may be desirable to initiate ART after the first trimester, although for such women who are severely ill the benefit of early therapy clearly outweighs any potential fetal risks, and therapy should be initiated in these cases. Additionally, the dual NRTI combination of d4T/ ddI should be avoided in pregnancy and only used when no other alternatives exist, because of the potential increased risk of lactic acidosis with this combination in pregnant women.
Symptomatic NVP-associated hepatic or serious rash toxicity, although uncommon, is more frequent in women than in men and is more likely to be seen in women with comparatively elevated CD4 cell counts (>250/mm3) (Boehringer-Ingleheim Pharmaceuticals, 2003; Imperiale et al., 2002; Stern et al., 2002, 2003). It is not known if pregnancy further predisposes women to such toxicities but cases have been reported in pregnant women (Langlet et al., 2000; Lyons et al., 2003).
An important issue is the potential impact of NVP prophylaxis for the prevention of MTCT on the subsequent treatment of mothers and their infected infants. This question has arisen in the past two years because a single point mutation is associated with resistance for NVP. Mutations associated with NNRTI drug resistance have been detected in plasma virus in approximately 20% of women following single-dose NVP prophylaxis at six weeks postpartum; higher rates of mutant virus (67%) have been detected at six weeks postpartum where women have received two doses instead of a single intrapartum dose of NVP for the prevention of transmission (Eshleman et al., 2001; Sullivan, 2002). Additionally, NVP resistance can develop even among women receiving additional antiretroviral drugs if they have detectable viral replication at the time of administration of single-dose NVP; genotypic NVP resistance was detected at six weeks postpartum in 15% of women who received single-dose NVP and who had received ZDV alone or combination antiretroviral drugs during pregnancy and intrapartum (Chaowanachan et al., 2003; Cunningham et al., 2002). Resistance to 3TC is also associated with a single mutation. In a study in which 3TC was added to ZDV therapy at 32 weeks of gestation in pregnant women in France, the 3TC resistance mutation M184V was observed at six weeks postpartum in 39% of women (Mandelbrot et al., 2001); 3TC resistance was also detected at one week postpartum in 12% of women receiving ZDV/3TC for four weeks for the prevention of MTCT in the PETRA study (Giuliano et al., 2003). No ZDV or 3TC resistance was observed with intrapartum/oneweek-postpartum ZDV/3TC in the SAINT study in South Africa (Giuliano et al., 2003; Sullivan, 2002).
There is no information about the clinical consequences of the selection of these resistance mutations for responses to future antiretroviral therapy
in women or infected infants. The mutations fade with time but doubtless remain archived in minor viral subpopulations and have the potential to reemerge when a subsequent regimen containing NNRTI or 3TC is introduced. Studies are in progress and others are planned with a view to determining whether single-dose NVP prophylaxis compromises subsequent HAART with NNRTI-based regimens. This is one of the most pressing operational research questions in the field.
Until definitive data are available on this matter, women who have received single-dose NVP prophylaxis or 3TC prophylaxis for the prevention of MTCT should be considered eligible for NNRTI-based regimens and should not be denied access to life-sustaining therapy.
Several country programmes are already considering the use of short-course triple combination therapy for the prevention of MTCT in women who are not yet in need of treatment for their own HIV infection, and the cessation of therapy postpartum if the women do not require its continuation for their own health. The use of highly active combination therapy in such situations should prevent the emergence of resistance to the drugs and should also be highly effective in reducing perinatal HIV transmission to infants. However, this intervention also exposes both mother and fetus to potential drug toxicities in situations where therapy is not required for maternal health. Studies are in progress with a view to assessing the safety and efficacy of this approach for women and their infants, particularly for the prevention of MTCT in breast-feeding women.
When a PI-based option is preferred to an NNRTI-based regimen during pregnancy, SQV/r or NFV are reasonable choices, given the safety experience in pregnancy.
It is important to note that ARV drugs have the potential to either decrease or increase the bioavailability of steroid hormones in hormonal contraceptives. The limited data available suggest that potential drug interactions between many ARVs (particularly some NNRTIs and PIs) and hormonal contraceptives may alter safety and effectiveness of both the hormonal contraceptives and the ARVs. It is not known whether the contraceptive effectiveness of progestogen-only injectable contraceptives (such as depot medroxyprogesterone acetate and norethisterone enantate) would be compromised, as these methods provide higher blood hormone levels than other progestogen-only hormonal contraceptives, as well as than combined oral contraceptives. Studies are underway to evaluate potential interactions between depot medroxyprogesterone acetate and selected PI and NNRTI drugs. Thus, if a woman on ARV treatment decides to initiate or continue hormonal contraceptive use, the consistent use of condoms must be recommended for preventing HIV transmission and may also compensate for any possible reduction in the effectiveness of the hormonal contraceptive.
Children
When to Start ARV Therapy in Infants and Children
The laboratory diagnosis of HIV infection in infants aged under 18 months is difficult because of the persistence of maternal antibody. Virological tests are required in order to make definitive diagnoses of HIV infection in this age group. WHO recommendations for the initiation of ARV therapy in children are therefore divided into categories related to age and the availability of virological diagnostic tests (Table G). When CD4 cell assays are available the use of the CD4 cell percentage is recommended for decision-making on ARV treatment rather than of the absolute CD4 cell count, because the former varies less with age (Annex B) (Embree et al., 2001; Shearer et al., 2003; Wade and Ades, 1994). WHO strongly encourages the development of tests applicable to resource-limited settings which would allow early diagnosis of HIV infection in infants. The availability of such tests is critical to the development of improved recommendations for the initiation of therapy in infants aged under 18 months.
-
For HIV-seropositive infants aged under 18 months, WHO recommends the initiation of ARV therapy in the following circumstances:
-
The infant has virologically proven infection (using either HIV DNA PCR, HIV RNA assay, or immune-complex dissociated p24 antigen) and has:
-
WHO Paediatric Stage III HIV disease (i.e. clinical AIDS) (Annex E), irrespective of CD4%; or
-
WHO Paediatric Stage II disease (Annex E), with consideration of using CD4 <20% to assist in decision-making; or
-
WHO Paediatric Stage I (i.e. asymptomatic) (Annex E) and CD4 <20% (asymptomatic children, i.e. WHO Stage I, should only be treated when there is access to CD4 assays).
-
-
If virological tests to confirm HIV infection status are not available but CD4 cell assays are available, WHO recommends that ARV therapy can be initiated in HIV-seropositive infants who have WHO Stage II or III disease and a CD4 percentage below 20%. In such cases, HIV antibody testing must be repeated at the age of 18 months in order to definitively confirm that the children are HIV-infected; ARV therapy should only be continued in infants with confirmed infection.
-
For HIV-seropositive children aged 18 months or over, WHO recommends initiation of ARV therapy in the following circumstances:
-
WHO Paediatric Stage III HIV disease (i.e. clinical AIDS) (Annex E), irrespective of CD4 %; or
-
-
WHO Paediatric Stage II disease (Annex E), with consideration of using CD4 <15% to assist decision-making; or
-
WHO Paediatric Stage I (i.e. asymptomatic) (Annex E) and CD4 <15%.
It should be noted that breast-feeding infants are at risk of HIV infection during the entire period of breast-feeding, and that a negative virological or antibody test at one age does not exclude the possibility of infection occurring subsequently if breast-feeding continues.
As in HIV-infected adults, the total lymphocyte count significantly correlates with the risk of mortality in HIV-infected children (European Collaborative Study, 2004; Mofenson et al., 2003). The 12-month risk of mortality is >20% for children aged under 18 months with a total lymphocyte count of <2500/mm3 and for children aged 18 months or more with a total lymphocyte count of <1500/mm3. In cases where the CD4 cell count cannot be assessed, therefore, the total lymphocyte count may be used as a substitute indication for the treatment of infants or children with documented HIV infection in the presence of symptomatic disease (WHO Paediatric Stage II or III). It is preferable that an abnormal total lymphocyte count or CD4 cell count/percentage be confirmed with a second test before therapeutic decisions are made but it is recognized that this may not always be possible.
WHO recognizes that the current staging system for HIV infection in children was developed several years ago and that many of the clinical symptoms in Paediatric Stage II and III are not specific for HIV infection and may significantly overlap with those seen in children without HIV infection in resource-limited settings. Recognizing this limitation, WHO is planning a consultation with paediatric experts in order to revise the classification system in 2004. In the interim, however, the use of this WHO disease classification (Annex F) can be of value in assisting to define parameters for the initiation of therapy in resource-limited settings, although individual adaptation at the country programme level may be appropriate.
The penetration of ARVs into human breast milk in lactating women has not been quantified for most ARVs. Although some ARVs, such as nevirapine, are known to be present in breast milk, the concentration and quantity of drug ingested by infants would be less than those needed to achieve therapeutic levels. Consequently, if a breast-feeding infant is ill enough to require ARV treatment (Table G), the administration of ARVs at standard paediatric doses should be initiated regardless of whether the mother is receiving ARV therapy. Infected breast-feeding infants whose mothers are receiving ARV therapy may ingest subtherapeutic levels of some ARVs, and this could lead to the development of drug resistance in the infant’s virus. It is not known whether ARVs should be administered
TABLE G. Recommendations for Initiating ART in Infants and Children
|
CD4 testing |
Age |
HIV diagnostic testing |
Treatment recommendation |
|
If CD4 testing is available |
< 18 months |
HIV virological testing not available but infant is HIV antibody-seropositive (Note: HIV antibody test must be repeated at age 18 months to obtain definitive diagnosis of HIV infection) |
WHO Paediatric Stages II and III disease with CD4 < 20%a |
|
|
|
Positive HIV virological testb |
WHO Paediatric Stage III (i.e. AIDS) (Annex F) irrespective of CD4% WHO Paediatric Stage II disease (Annex F), with consideration of using CD4 <20% to assist in decision-makinga,c WHO Paediatric Stage I disease (i.e. asymptomatic) (Annex F), CD4 <20%a,d |
|
|
18 months |
HIV antibody-seropositive |
WHO Paediatric Stage III disease, irrespective of CD4% WHO Paediatric Stage II disease, with consideration of using CD4 <15% to assist in decisionmakinga, c WHO Paediatric Stage I disease with CD4 < 15%a,d |
|
If CD4 testing is not available |
< 18 months |
HIV virological testing not available but infant HIV antibody-seropositive |
WHO Paediatric Stage III, irrespective of total lymphocyte count WHO Paediatric Stage II disease, with consideration of using total lymphocyte count <2500/mm3 to assist in decisionmakingf |
|
|
18 months |
HIV antibody-seropositive |
WHO Paediatric Stage III irrespective of total lymphocyte count WHO Paediatric Stage II disease, with consideration of using total lymphocyte count <1500/mm3 to assist in decision-makingf |
|
aA CD4 cell percentage <20% corresponds to an absolute CD4 count of approximately <1000/mm3for children aged <12 months and <750/mm3 for children aged 12-18 months; CD4 <15% corresponds to <500/mm3 for children aged 1-5 years and to <200/mm1 for children aged > 6 years. bHIV DNA PCR or HIV RNA amplification assays or immune complex dissociated p24 antigen assays. cCD4 cell percentage is advisable to assist with determining the need for immediate therapy. dIf a child is asymptomatic and treatment is being initiated on basis of CD4 criteria, consideration should be given to performing a confirmatory CD4 assay if resources permit. eMany of the clinical symptoms in the WHO Paediatric Stage II and III disease classification are not specific for HIV infection and significantly overlap those seen in children without HIV infection in resource-limited settings; thus, in the absence virological testing and CD4 cell assay availability, symptomatic HIV-seropositive infants <18 months of age should only be considered for ARV therapy in exceptional circumstances (e.g. a child with a classic AIDS-defining opportunistic infection such as Kaposi’s sarcoma, Pneumocystis carinii pneumonia or cryptococcal meningitis). If ARVs are given to a symptomatic HIV-seropositive infant in the absence of a definitive virological diagnosis, HIV antibody testing should be repeated at the of age 18 months to confirm infection status; ARV therapy should only be continued in infants with confirmed HIV infection. fA total lymphocyte count of <2500/mm3 for children aged <18 months or of <1500/mm3 for children aged ≥ 18 months can be substituted for CD4% when the latter is unavailable and HIV-related symptoms exist. Its utility in asymptomatic children is unknown. In the absence of CD4 cell testing, therefore, asymptomatic HIV-infected children (WHO Paediatric Stage I) should not be treated because no other reliable marker is currently available in severely resource-constrained settings. |
|||
during the breast-feeding period to infants with documented HIV infection who do not require ARV therapy themselves but whose mothers are receiving ARV treatment, and further research is needed on this matter.
Recommended First-line ARV Regimens in Infants and Children
Studies of HAART in children demonstrate that similar improvements are seen in morbidity, mortality and surrogate markers with many different potent ARV regimens (De Martino et al., 2000; Gortmaker et al., 2001). Drug doses must be adjusted as a child grows in order to avoid the risk of underdosage and the development of resistance; dosing in children is therefore based on either body surface area or weight. Standardization is important so that non-expert personnel can safely dispense correct doses, and consequently it is desirable to provide health care workers with a table of drug doses that can be administered according to weight bands. Such tables may vary between localities in accordance with the availability of ARV drugs and formulations in the country concerned. In order to improve adherence, regimens chosen for children should take account of those that may be used by their parents in order to avoid different timings, and, if possible, to permit the use of the same drugs. WHO recognizes the need to provide assistance to countries in the development of such tables for training manuals so that ARV programmes can be implemented. Pending the development of a consensus on such tables in the course of 2004, samples of tables used by some paediatricians will be made available on request.
Some ARVs available for adults are also available in formulations specifically designed for children. However, formulations appropriate for use by young children who cannot swallow whole tablets or capsules are not widely available in resource-limited settings. For some ARVs, capsules and tablets are available in sufficiently low doses to enable accurate dosing for children (e.g. d4T capsules of 15, 20 and 30 mg, or NFV scored tablets that can be halved and crushed), and the pharmacokinetics of crushed tablets or sprinkled capsule contents in children have been evaluated. However, many drugs do not have solid formulations in doses appropriate for paediatric use and some solid formulations do not have all drug components evenly distributed in the tablets (e.g. fixed-dose ZDV/3TC). The use of tablets that require cutting up, particularly unscored tablets, can result in the underdosing or overdosing of children, which can lead to an increased risk of resistance or toxicity. Moreover, the doses cannot easily be adjusted as the children grow. However, WHO recognizes that until appropriate formulations can be made more widely available the splitting of adult-dose solid formulation ARVs, while suboptimal, may be the only way a severely ill child can receive therapy, and should be considered when no alternatives are available. Health care providers should be aware that current fixed-
dose combination formulations may not contain the appropriate doses of each of the component drugs for children on a weight basis. This is a specific problem for the NVP component of the fixed-dose formulation of ZDV/3TC/NVP, for which additional NVP may be necessary if tablets are used to treat younger children (Annex F). WHO strongly encourages the development of formulations appropriate for paediatric use, particularly solid formulations in doses that can be used by paediatric patients (e.g. crushable tablets or openable capsules), as liquid formulations may have a more limited shelf-life than solid formulations, they may be more expensive, they may be difficult to store and they may require the use of syringes for accurate administration.
The preferred first-line treatment option for children includes (d4T or ZDV) + 3TC plus an NNRTI (NVP or EFV) (Table H), for the same reasons as discussed for adult initial ARV regimens. A caveat is that EFV cannot be used currently in children under 3 years of age because of a lack of appropriate formulation and dosing information, although these matters are under study. Consequently, for children aged under 3 years or weighing under 10 kg, NVP should be the NNRTI of choice. The use of ZDV/3TC/ ABC as first-line therapy is now considered a secondary alternative because of the results obtained with ACTG A5095 in adults; further data are awaited.
EFV would be the NNRTI of choice for children who require ARV therapy but need or are receiving anti-TB therapy containing rifampicin. For children under 3 years of age who require ARV therapy while receiving anti-TB therapy, the use of ZDV/3TC/ABC should be considered while the TB therapy is being administered, as SQV/r is not available in a formulation that is appropriate for children of this age. Monitoring for possible ABC hypersensitivity should be assured. SQV/r may also be considered for older children who can receive adult doses of the drugs (i.e. children weighing 25 kg).
TABLE H. Recommended First-line ARV Regimens for Infants and Children
|
First-line regimen |
Comment |
|
d4T or ZDV |
|
|
plus 3TC |
|
|
plus N VP or EF V |
NNRTI choice:
|
If a mother has received ARV during pregnancy, either to reduce MTCT or for her own disease, there is a possibility that the baby may become infected with drug-resistant virus. Additionally, resistance could be induced de novo in an infected infant who is exposed to an ARV drug being used for prophylaxis before the infection status of the infant is known. This is a particular problem if NVP or 3TC has been used, either alone or as a component of a two-drug regimen, for prophylaxis of MTCT, because a single point mutation is associated with resistance to these two drugs (Eshleman et al., 2001; Mandelbrot et al., 2001). Following single-dose NVP, 46% of infants have NNRTI-associated mutations (primarily the Y181C mutation, which may not always be associated with cross-resistance to EFV). As has been observed in mothers, these mutations fade with time but probably remain as minor viral subpopulations (Eshleman et al., 2001). It is not known whether ARV choices should be modified for infants who have been exposed to ARVs used for the prevention of MTCT. Studies in children are in progress or are planned, as they are in mothers, to investigate whether single-dose NVP prophylaxis compromises subsequent HAART with NNRTI-based regimens. WHO recognizes the urgency of such research. However, until there are data allowing these questions to be definitively answered, children who require ARV therapy and who have previously received single-dose NVP or 3TC as part of MCTC prophylaxis should be considered eligible for NNRTI-based regimens and should not be denied access to life-sustaining therapy.
Clinical Assessment of Infants and Children Receiving ARV Therapy
Important clinical signs of response to ARV therapy in children include: improvement in growth in children who have been failing to grow; improvement in neurological symptoms and development in children who have been demonstrating delay in the achievement of developmental milestones or encephalopathy; and/or decreased frequency of infections (bacterial infections, oral thrush, and/or other opportunistic infections).
Laboratory assessments for children on ARV therapy are the same as those recommended for adults (Table G). In addition to the clinical assessments recommended for adults, the clinical monitoring of ARV treatment in children should cover:
-
nutrition and nutritional status;
-
weight and height growth;
-
developmental milestones;
-
neurological symptoms.
Reasons for Changing ARV Therapy in Infants and Children
The principles on which to base changes in therapy for children are similar to those applied for adults, and the management of drug toxicity is the same. If toxicity is related to an identifiable drug in the regimen, the offending drug can be replaced with one that does not have the same side-effects. In children, important clinical signs of drug failure include: a lack of growth in children who show an initial response to treatment, or a decline in growth among children who show an initial growth response to therapy; a loss of neurodevelopment milestones or the development of encephalopathy; and the recurrence of infections, such as oral candidiasis that is refractory to treatment (Lindsey et al., 2000; Verweel et al., 2002; Saulsbury, 2001; McCoig et al., 2002) (Table I). It should not be concluded, on the basis of clinical criteria, that an ARV regimen is failing until the child in question has had a reasonable trial on the therapy (e.g. the child should have received the regimen for at least 24 weeks).
TABLE I. Clinical and CD4 Count Definitions of Treatment Failure in Infants and Children
|
Clinical signs of treatment failure |
CD4 cell criteria for treatment failurea |
|
|
|
|
|
|
|
|
|
|
|
|
|
aIf a child is asymptomatic and treatment failure is being defined by CD4 cell criteria alone, consideration should be given to performing a confirmatory CD4 count if resources permit. bThis must be distinguished from immune reconstitution syndrome, which can occur in the first three months following the initiation of HAART and does not signify treatment failure. |
|
Because of age-related declines in CD4 absolute cell counts until the age of 6 years, when near-adult levels are reached, it is difficult to use such counts for assessing therapy failure in younger children. However, for children aged 6 years or more, similar CD4 cell count criteria to those used for adults are appropriate (Table E). Because the CD4 cell percentage varies less with age it can be used to gauge treatment response regardless of age. No data are available on the use of total lymphocyte counts for the evaluation of response to ARV therapy.
Recommended Second-line ARV Therapy for Infants and Children
Second-line therapy for children in the event of failure of a first-line regimen includes a change in the nucleoside backbone, in accordance with the same principles as are applied for adults (e.g. from ZDV + 3TC to ABC + ddI), plus a protease inhibitor (Table J). The use of PIs other than LPV/r and NFV is more problematic in children because of a lack of suitable paediatric drug formulations for IDV and SQV and a lack of appropriate dosing information for ritonavir-boosted PIs other than LPV/r. However, the use of SQV/r can be considered as an alternative for children who are able to swallow capsules and who weigh 25 kg or more, and can therefore receive the adult dosage. TDF cannot be recommended for paediatric treatment at present because of limited data on appropriate dosing for children, particularly those aged under 8 years, and because of questions about bone toxicity, which may be of more concern and/or more frequent in growing children than in adults.
TABLE J. Recommended ARV Regimens for Infants and Children with Treatment Failure
|
First-line regimen |
Second-line regimen |
|
d4T or ZDV |
ABC |
|
plus |
plus |
|
3TC |
ddI |
|
plus |
plus |
|
NNRTI: |
Protease inhibitor: |
|
NVP or EFV |
LPV/r or NFV, or SQV/r if weight 25 kg |
People with Tuberculosis Disease and HIV Coinfection
Tuberculosis is an entry point for a significant proportion of patients eligible for ART. ART is recommended for all patients with TB who have CD4 counts below 200 cells/mm3, and should be considered for patients with CD4 counts below 350 cells/mm3. In the absence of CD4 cell counts, ART is recommended for all patients with TB. It is acknowledged that this will result in the treatment of individuals with CD4 cell counts over 350 who otherwise would not receive ART. The treatment of TB remains a central priority for patient management and should not be compromised by ART (Santoro-Lopes et al., 2002; Giarardi et al., 2000; Badri et al., 2002; Harvard University, 2001).
Patients with TB merit special consideration because comanagement of HIV and TB is complicated by rifampicin drug interactions with NNRTIs and PIs, pill burden, adherence and drug toxicity. Data supporting specific treatment recommendations are incomplete and research is urgently needed in this area (Burman and Jones, 2001; Wagner and Bishai, 2001; Havlir and Barnes, 1999; Dean et al., 2002). Taking the available data into account, the first-line treatment recommendation for patients with TB and HIV coinfection is (ZDV or d4T) + 3TC + EFV (600 or 800 mg/day). The 800mg dose of EFV achieves higher drug levels than those seen in the absence of rifampicin and thus may reduce the chance of HIV drug resistance. However, it can also increase the toxicity risk. SQV/RTV 400/400 mg bid, SQV/r 1600/200 mg qd (in soft gel formulation-sgc) or LPV/ RTV 400/400 mg bid in combination with the NRTI backbone are alternatives to EFV, although tolerability, clinical monitoring and risk of resistance may be problematic. Endorsement of these PI-based regimens requires further data. ABC is another alternative to EFV with the advantages of low pill burden, no interaction with rifampicin, and suitability for administration to children weighing 25 kg or less, for whom appropriate EFV dosing information is not yet available. Concerns about this regimen include ones relating to monitoring for hypersensitivity syndrome and virological potency. Data on the use of NVP + rifampicin are limited and conflicting. NVP levels are reduced in the presence of rifampicin, and higher NVP doses have not been evaluated. Although some clinical experience reports adequate viral and immunological response and acceptable toxicity, this regimen should only be considered when no other options are available. For women of childbearing age (without effective contraception), pregnant women, and children with TB, either SQV/r or ABC + (d4T or ZDV) + 3TC is recommended. For children weighing 25 kg or less, (d4T or ZDV)/3TC/ABC is recommended as an alternative (Lopez-Cortes et al., 2002; Patel et al., 2003; Pedral-Samapio et al., 2003; Dean et al., 1999; Ribera et al., 2001, 2002; Oliva et al., 2003; la Porte et al., 2002).
TABLE L. ART Recommendations for Individuals with TB Disease and HIV Coinfection
|
CD4 cell count |
Recommended regimen |
Comments |
|
CD4 <200 mm3 |
Start TB treatment. Start ART as soon as TB treatment is tolerated (between 2 weeks and 2 months):a |
Recommend ART. EFV is contraindicated in pregnant women or women of childbearing potential without effective contraception. |
|
|
||
|
CD4 200-350/mm3 |
Start TB treatment. Start one of the regimens below after the initiation phase (start earlier if severely compromised): |
Consider ART. |
|
|
EFV-containing regimensb or NVP-containing regimens in case of rifampicin-free continuation phase TB treatment regimen. |
|
|
CD4 >350 mm3 |
Start TB treatment. |
Defer ART.e |
|
CD4 not available |
Start TB treatment. |
|
|
aTiming of ART initiation should be based on clinical judgement in relation to other signs of immunodeficiency (Table A). For extrapulmonary TB, ART should be started as soon as TB treatment is tolerated, irrespective of CD4 cell count bAlternatives to the EFV portion of the regimen include: SQV/RTV (400/400 mg bid), SQV/r (1600/200 mg qd in sgc), LPV/RTV (400/400 mg bid) and ABC. cNVP (200 mg qd for two weeks followed by 200 mg bid) may be used in place of EFV in absence of other options. NVP-containing regimens include: d4T/3TC/NVP or ZDV/3TC/ NVP. dEFV-containing regimens include d4T/3TC/EFV and ZDV/3TC/EFV. eUnless non-TB Stage IV conditions are present (Table A). Otherwise start ART upon completion of TB treatment. fIf no other signs of immunodeficiency are present and patient is improving on TB treatment, ART should be started upon completion of TB treatment. |
||
The optimal time to initiate ART in patients with TB is not known. Case-fatality rates in many patients with TB during the first two months of TB treatment are high, particularly when they present with advanced HIV disease, and ART in this setting might be life-saving. On the other hand, pill burden, drug-to-drug interaction, potential toxicity and immune reconstitution syndrome should be kept in mind when deciding on the best time to
begin treatment (Burman and Jones, 2001; Wagner and Bishai, 2001; Narita et al., 1998; Harries et al., 2001). The management of patients with HIV and TB poses many challenges, including that of achieving patient acceptance of both diagnoses. Pending current studies, WHO recommends that ART in patients with CD4 cell counts below 200/mm3 be started between two weeks and two months after the start of TB therapy, when the patient has stabilized on this therapy. This provisional recommendation is meant to encourage rapid initiation of therapy in patients among whom there may be a high mortality rate. However, deferring the start of ART may be reasonable in a variety of clinical scenarios. For example, in patients with higher CD4 cell counts the commencement of ART may be delayed until after the induction phase of TB therapy is completed in order to simplify the management of treatment.
Injecting Drug Users
The clinical and immunological criteria for initiating HAART in substance dependent patients do not differ from those in the general recommendations. Injecting drug users who are eligible for ART should therefore be guaranteed access to this life-saving therapy. Special considerations for this population include dealing prospectively with lifestyle instability that challenges drug adherence and accounting for the potential drug interactions of ARVs with agents such as methadone. The development of programmes which integrate care of drug dependence (including drug substitution therapy) and HIV is encouraged. In such settings, approaches such as directly observed therapy can be implemented. Once-daily ARV regimens are being intensively explored in this arena and lend themselves to such approaches. The number of ARVs approved or being investigated for once-daily use is steadily increasing. They include 3TC, FTC, ddI, d4T, TDF, ABC, EFV, SQV/r, LPV/r and ATV.
The coadministration of methadone with EFV, NVP or RTV in HIVinfected individuals with a history of injecting drug use resulted in decreased plasma levels of methadone and signs of opiate withdrawal. Patients should be monitored for signs of withdrawal and their methadone dose should be increased in appropriate increments over time so as to alleviate withdrawal symptoms. An important option can thus be provided for treatment programmes directed at this vulnerable population.
ADHERENCE TO ANTIRETROVIRAL THERAPY
Adherence to ART is well recognized to be an essential component of individual and programmatic treatment success (Bang and Scott, 2003; Staszewski et al., 2003; Schaaf et al., 2003; Martin-Carbonero et al., 2003;
Adje-Toure et al., 2003; Piliero, 2002; Eshleman et al., 2001; Sullivan, 2002; Mandelbrot et al., 2001; Mofenson et al., 2003; Lindsey et al., 2000; Giarardi et al., 2000; Havlir et al., 2002). Studies of drug adherence in the developed world have suggested that higher levels of drug adherence are associated with improved virological and clinical outcomes and that rates exceeding 95% are desirable in order to maximize the benefits of ART. It is difficult to achieve rates this high over a long period of time. Numerous approaches to improving adherence have been investigated in the developed world and have begun to be explored in the developing world. Viral load testing will not be widely introduced in the developing world in the near future because of cost and technical considerations. Consequently, it is particularly important to focus on maximizing adherence in order to try to avoid drug resistance and ensure the durability of effect of ARV regimens.
The proper education of patients before the initiation of therapy is vital for the success of adherence strategies. Such education should cover basic information about HIV and its manifestations, the benefits and side-effects of ARV medications, how the medications should be taken and the importance of not missing any doses. Peer counsellors and visual materials can be particularly useful in this process. Keys to success once treatment has begun include trying to minimize the number of pills (in part through the use of FDCs), the packaging of pills (coblister packs when available), the frequency of dosing (no more than twice-daily regimens), avoidance of food precautions, fitting the ARVs into the patient’s lifestyle, and the involvement of relatives, friends and/or community members in support of the patient’s adherence.
After the initiation of therapy it is essential to maintain support for adherence. This should involve adherence assessments whenever there is a visit to a health centre, reinforcement of adherence principles to the patient by treatment supporters, and the continuous involvement of relatives, friends and/or community support personnel. Although the penetration of ART in the developing world has been low in relation to the burden of disease, important lessons have been learnt which can be incorporated into newly developing or expanding programmes. These lessons relate to the following measures.
-
Provision of medications free of charge through subsidization or other financing strategies for people who can least afford treatment. It has been suggested that cost-sharing may assist adherence, although experiences can be expected to vary between countries. Recent data from Senegal and other African countries indicates that cost-sharing is detrimental to long-term adherence. These issues need further exploration (Desclaux et al., 2003; Laniece et al., 2003).
-
Engagement of family or community members in adherence educa-
-
tion and maintenance programmes. Home visits can be particularly useful. Minimizing stigma through psychosocial support is essential.
-
Family-based care when more than one family member is HIVinfected. This is particularly important when both mother and child are infected.
-
Use of pillboxes or blister packs.
-
Directly observed therapy (DOT) or modified DOT programmes. This approach is resource-intensive and difficult to introduce on a large scale and for the lifelong duration of ART. However, it may be helpful for certain groups and for early patient training.
-
Use of mobile vans to reach rural communities.
-
At the programmatic level it is essential to ensure proper stock and storage of ARVs and the provision of necessary resources for culturally appropriate adherence programmes.
Adherence may be more difficult in pregnant women and immediately postpartum women than in non-pregnant individuals. Pregnancy-associated morning sickness and gastrointestinal upset may complicate ART and the situation may be exacerbated by ARV-associated side-effects or concern about the potential effects of drugs on the fetus. In the postpartum period, physical changes and the demands of caring for a neonate may compromise maternal drug adherence. Specific, culturally appropriate adherence supports should be developed at the country level in order to address the special problems associated with pregnant and postpartum women.
Adherence in children is a special challenge, particularly if the family unit is disrupted as a consequence of adverse health or economic conditions. Family-based HIV care programmes are one of the best approaches to ensuring children’s health. Moreover, it is imperative that paediatric formulations be improved and made widely available. Where possible they should match the adult regimens so that that family-based care can be pursued effectively and so that children can be properly dosed.
DRUG RESISTANCE SURVEILLANCE
ARV drug resistance is a major challenge to treatment programmes for both developed and developing countries. Currently, approximately 10% of new HIV-1 infections in the USA and Europe involve viral strains exhibiting resistance to at least one drug. Scale-up programmes in the developing world can take advantage of the lessons learnt in developed countries through proper initiation of potent regimens, incorporation of culturally appropriate adherence training and maintenance programmes, and synchronization with drug resistance surveillance and monitoring initiatives.
Drug resistance genotyping is not on the near-term or mid-term hori-
zon for individual patient management in resource-limited settings but country programmes are encouraged to develop or participate in drug resistance surveillance and monitoring programmes to assist with planning at the population level. This may involve developing or expanding genotypic capabilities at regional or national centres of excellence. Such capabilities can be considered an important public health tool that can be used to inform national, regional and global ARV scale-up programmes concerning trends in the prevalence of drug resistance so that decisions can be made to minimize its impact.
WHO recommends that countries planning to implement ART programmes should concurrently introduce HIV drug resistance sentinel surveillance systems. This will allow countries to detect potential drug resistance at the population level and to modify recommended treatment regimens accordingly. Initially, treatment-naive persons should be surveyed in order to establish prevalence rates of drug resistance in the infected population, and treatment-experienced persons should be monitored, particularly those diagnosed with their first episode of treatment failure. A Global HIV Drug Resistance Surveillance and Monitoring Network is being established by WHO in collaboration with partner organizations with a view to assisting Member States in this arena (Havlir et al., 2002).
CONCLUSIONS
Member States of WHO face both a great challenge and a great opportunity. The world community can confront the AIDS pandemic in developing countries with ART, the most effective life-sustaining tool in the HIV care package. The current nexus of political commitment, new sources of funding, ARV availability and lower drug prices have created this opportunity. WHO is committed to assisting resource-limited countries with the scale-up of ART through its comprehensive 3-by-5 Plan. The present updated ARV treatment guidelines are intended to help national programmes to provide ARV access for all infected adults and children in need of treatment.
ANNEX A. Dosages of Antiretroviral Drugs for Adults and Adolescents
|
Drug class/drug |
Dosea |
|
Nucleoside RTIs |
|
|
Abacavir (ABC) |
300 mg twice daily |
|
Didanosine (ddI) |
400 mg once daily (250 mg once daily if <60 kg) (250 mg once daily if administered with TDF) |
|
Lamivudine (3TC) |
150 mg twice daily or 300 mg once daily |
|
Stavudine (d4T) |
40 mg twice daily (30 mg twice daily if <60 kg) |
|
Zidovudine (ZDV) |
300 mg twice daily |
|
Nucleotide RTI |
|
|
Tenofovir (TDF) |
300 mg once daily (Note: drug interaction with ddI necessitates dose reduction of latter) |
|
Non-nucleoside RTIs |
|
|
Efavirenz (EFV) |
600 mg once dailyb |
|
Nevirapine (NVP) |
200 mg once daily for 14 days, then 200 mg twice daily |
|
Protease inhibitors |
|
|
Indinavir/ritonavir (IDV/r) |
|
|
Lopinavir/ritonavir (LPV/r) |
400 mg/100 mg twice dailyb (533 mg/133 mg twice daily when combined with EFV or NVP) |
|
Nelfinavir (NFV) |
1250 mg twice daily |
|
Saquinavir/ritonavir (SQV/r) |
1000 mg/100 mg twice daily or 1600 mg/200 mg once dailyb,d,e |
|
aThese dosages are in common clinical use. The dosages featured in this table were selected on the basis of the best available clinical evidence. Dosages that can be given once daily or twice daily were preferred in order to enhance adherence to therapy. The doses listed are those for individuals with normal renal and hepatic function. Product-specific information should be consulted for dose adjustments that may be indicated with renal or hepatic dysfunction or for potential drug interactions with other HIV and non-HIV medications. bSee TB section for other specific TB dosing. cThis dosage regimen is in common clinical use. Other IDV/r dosage regimens that range from 800 mg/200 mg bid to 400 mg/100 mg bid are also in clinical use. dDosage adjustment when combined with an NNRTI is indicated but a formal recommendation cannot be made at this time. One consideration is to increase the RTV component to 200 mg bid when EFV or NVP is used concomitantly. More drug interaction data are needed. eBoth the hard-gel and soft-gel capsule formulations can be used when SQV is combined with RTV. |
|
ANNEX B. Human Immunodeficiency Virus Paediatric Immune Category Classification System Based on Age-specific CD4+ T Cell Count and Percentage
|
|
<12 months |
|
1–5 years |
|
6–12 years |
|
|
Immune category |
No./mm3 |
% |
No./mm3 |
% |
No./mm3 |
% |
|
Category 1: No suppression |
≥1500 |
≥25% |
≥1000 |
≥25% |
≥500 |
≥25% |
|
Category 2: Moderate suppression |
750–1499 |
15%–24% |
500–999 |
15%–24% |
200–499 |
15%–24% |
|
Category 3: Severe suppression |
<750 |
<15% |
<500 |
<15% |
<200 |
<15% |
|
Modified from: Centers for Disease Control and Prevention. 1994 revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR 1994;43(No. RR-12):1-10. |
||||||
ANNEX C. Summary of Paediatric Drug Formulations and Doses
|
Name of drug |
Formulations |
Pharmaco-kinetic data available |
Age (weight), dose and dose frequency |
Other comments |
|
Nucleoside analogue reverse transcriptase inhibitors |
||||
|
Zidovudine (ZDV) |
Syrup: 10 mg/ml Capsules: 100 mg; 250 mg Tablet: 300 mg |
All ages |
<4 weeks: 4 mg/kg/dose twice daily 4 weeks to 13 years: 180 mg/m2/dose twice dailya Maximum dose: ≥ 13 years: 300 mg/dose twice daily |
Large volume of syrup not well tolerated in older children Syrup needs storage in glass jars and is light-sensitive Can be given with food Doses of 600 mg/m2/dose per day required for HIV encephalopathy Capsule can be opened and contents dispersed or tablet crushed and contents mixed with small amount of water or food and immediately taken (solution is stable at room temperature) Do not use with d4T (antagonistic antiretroviral effect) |
|
Name of drug |
Formulations |
Pharmaco-kinetic data available |
Age (weight), dose and dose frequency |
Other comments |
|
Lamivudine (3TC) |
Oral solution: 10 mg/ml Tablet: 150 mg |
All ages |
<30 days: 2mg/kg/dose twice daily ≥30 days or <60 kg: 4 mg/kg/dose twice daily Maximum dose: >60 kg: 150 mg/dose twice daily |
Well tolerated Can be given with food Store solution at room temperature (use within one month of opening) Tablet can be crushed and contents mixed with small amount water or food and immediately taken |
|
Fixed-dose combination of ZDV plus 3TC |
No liquid available Tablet: 300 mg ZDV plus 150 mg 3TC |
Adolescents and adults |
Maximum dose: >13 years or >60 kg: 1 tablet/dose twice daily (should not be given if weight <30 kg) |
Preferably, tablet should not be split Tablet can be crushed and contents mixed with small amount of water or food and immediately taken At weight <30 kg, ZDV and 3TC cannot be dosed accurately in tablet form |
|
Stavudine (d4T) |
Oral solution: 1 mg/ml Capsules: 15 mg, 20 mg, 30 mg, 40 mg |
All ages |
<30 kg: 1 mg/kg/dose twice daily 30 to 60 kg: 30 mg/dose twice daily Maximum dose: >60 kg: 40 mg/dose twice daily |
Large volume of solution Keep solution refrigerated; stable for 30 days; must be well shaken and stored in glass bottles Capsules can be opened and mixed with small amount of |
|
|
|
|
|
food or water (stable in solution for 24 hours if kept refrigerated) Do not use with AZT (antagonistic antiretroviral effect) |
|
Fixed-dose combination of d4T plus 3TC |
No liquid available Tablet: d4T 30 mg plus 3TC 150 mg; d4T 40 mg plus 3TC 150 mg |
Adolescents and adults |
Maximum dose: 30-60 kg: one 30-mg d4T-based tablet twice daily >60 kg: one 40-mg d4T-based tablet twice daily |
Preferaby, tablet should not be split See comments under individual drug components |
|
Didanosine (ddI, dideoxyinosine) |
Oral suspension paediatric powder/ water: 10 mg/ml; in many countries needs to be made up with additional antacid Chewable tablets: 25 mg; 50 mg; 100 mg; 150 mg; 200 mg Enteric-coated beadlets in capsules: 125 mg; 200 mg; 250 mg; 400 mg |
All ages |
<3 months: 50 mg/m2/ dose twice dailya 3 months to <13 years: 90-120 mg/m2/dose twice daily or 240 mg/m2/dose once daily Maximum dose: ≥ 13 years or >60 kg: 200 mg/dose twice daily or 400 mg once daily |
Keep suspension refrigerated; stable for 30 days; must be well shaken Administer on empty stomach, at least 30 minutes before or 2 hours after eating If tablets dispersed in water, at least 2 tablets of appropriate strength should be dissolved for adequate buffering Enteric-coated beadlets in capsules can be opened and sprinkled on small amount of food |
|
Name of drug |
Formulations |
Pharmaco-kinetic data available |
Age (weight), dose and dose frequency |
Other comments |
|
Abacavir (ABC) |
Oral solution: 20 mg/ml Tablet: 300 mg |
Over age of 3 months |
<16 years or <37.5kg: 8 mg/ kg/ dose twice daily Maximum dose: >16 years or ≥ 37.5 kg: 300 mg/ dose twice daily |
Can be given with food Tablet can be crushed and contents mixed with small amount water or food and immediately ingested PARENTS MUST BE WARNED ABOUT HYPERSENSITIVITY REACTION ABC should be stopped permanently if hypersensitivity reaction occurs |
|
Fixed-dose combination of ZDV plus 3TC plus ABC |
No liquid available Tablet: ZDV 300 mg plus 3TC 150 mg plus ABC 300 mg |
Adolescents and adults |
Maximum dose: >40 kg: 1 tablet/ dose twice daily |
Preferably, tablet should not be split At weight <30 kg, ZDV/ 3TC/ ABC cannot be dosed accurately in tablet form MUST WARN PARENTS ABOUT HYPERSENSITIVITY REACTION. ZDV/3TC/ABC should be stopped permanently if hypersensitivity reaction occurs |
|
Non-nucleoside reverse transcriptase inhibitors |
||||
|
Nevirapine (NVP) |
Oral suspension: 10 mg/ ml Tablet: 200 mg |
All ages |
15 to 30 days: 5 mg/kg/dose once daily × 2 weeks, then 120 mg/m2/dose twice daily × 2 weeks, then 200 mg/m2/dose twice dailya >30 days to 13 years: 120 mg/m2/ dose once daily for 2 weeks, then 120-200 mg/m2/dose twice dailya Maximum dose: >13 yrs: 200 mg/ dose once daily for first 2 weeks, then 200 mg/ dose twice daily |
If rifampicin coadministration, avoid use (see TB section) Store suspension at room temperature; must be well shaken Can be given with food Tablets are scored and can be divided into two equal parts to give a 100 mg dose; can be crushed and combined with a small amount of water or food and immediately administered PARENTS MUST BE WARNED ABOUT RASH. Do not escalate dose if rash occurs (if mild/moderate rash, hold drug; when rash has cleared, restart dosing from beginning of dose escalation; if severe rash, discontinue drug) Drug interactions |
|
Name of drug |
Formulations |
Pharmaco-kinetic data available |
Age (weight), dose and dose frequency |
Other comments |
|
Efavirenz (EFV) |
Syrup: 30 mg/ ml (note: syrup requires higher doses than capsules; see dosing chart) Capsules: 50 mg, 100 mg, 200 mg |
Only for children over 3 years of age |
Capsule (liquid) dose for >3 years: 10 to 15 kg: 200 mg (270 mg = 9 ml) once daily 15 to <20 kg: 250 mg (300 mg = 10 ml) once daily 20 to <25 kg: 300 mg (360 mg = 12 ml) once daily 25 to <33 kg: 350 mg (450 mg = 15 ml) once daily 33 to <40 kg: 400 mg (510 mg = 17 ml) once daily Maximum dose: ≥ 40 kg: 600 mg once daily |
Capsules may be opened and added to food but have very peppery taste; however, can be mixed with sweet foods or jam to disguise taste Can be given with food (but avoid after high- fat meals, which increase absorption by 50%); best given at bedtime, especially first 2 weeks, to reduce CNS side-effects. Drug interactions |
|
Fixed-dose combination of d4T plus 3TC plus NVP |
No liquid available Tablet: 30 mg d4T/150 mg 3TC/200 mg NVP; 40 mg d4T/150 mg 3TC/ 200 mg NVP |
Adults and adolescents |
Maximum dose: 30-60 kg: one 30 mg d4Tbased tablet twice daily ≥ 60 kg: one 40 mg d4Tbased tablet twice daily |
Preferably, tablet should not be split At weight <30 kg, d4T/3TC/ NVP cannot be dosed accurately in tablet form; if tablets are split, NVP dose requirements will be inadequate for very young children and additional NVP is needed to give total of 200 mg/m2/dose twice daily |
|
|
|
|
|
Contains NVP, therefore dose escalation required (see NVP dosing recommendations) See comments under individual drug components |
|
Protease inhibitors |
||||
|
Nelfinavir (NFV) |
Powder for oral suspension (mix with liquid): 200 mg per level teaspoon (50 mg per 1.25 ml scoop): 5 ml Tablet: 250 mg (tablets can be halved; can be crushed and added to food or dissolved in water) |
All ages However, extensive pharmacokinetic variability in infants, with requirement for very high doses in infants <1 year |
<1 year: 50 mg/kg/dose three times daily or 7 5 mg/kg/dose twice daily >1 year to <13 years: 55 to 65 mg/kg/dose twice daily Maximum dose: ≥ 13 years: 1250 mg/dose twice daily |
Powder is sweet, faintly bitter but gritty and hard to dissolve; must be reconstituted immediately before administration in water, milk, formula, pudding, etc.; do not use acidic food or juice (which increase bitter taste); solution stable for 6 hours Because of difficulties with use of powder, use of crushed tablets preferred (even for infants) if appropriate dose can be given Powder and tablets can be stored at room temperature Take with food Drug interactions (less than ritonavir-containing protease inhibitors) |
|
Name of drug |
Formulations |
Pharmaco-kinetic data available |
Age (weight), dose and dose frequency |
Other comments |
|
Lopinavir/ritonavir, (LPV/r) |
Oral solution: 80mg/ml lopinavir plus 20 mg/ml ritonavir Capsules: 133.3 mg lopinavir plus 33.3 mg ritonavir |
6 months of age or older |
>6 months to 13 years: 225 mg/m2 LPV/57.5 mg/m2 ritonavir twice dailya or weight- based dosing: 7-15 kg: 12mg/kg LPV/ 3 mg/ kg ritonavir/ dose twice daily 15-40 kg: 10 mg/ kg lopinavir/2.5 mg/kg ritonavir twice daily Maximum dose: >40 kg: 400 mg LPV/ 100 mg ritonavir (3 capsules or 5 ml) twice daily |
Preferably, oral solution and capsules should be refrigerated; however, can be stored at room temperature up to 25° C (77° F) for 2 months; at temperature >25° C (77° F) the drug degrades more rapidly Liquid formulation has low volume but bitter taste Capsules large Capsules should not be crushed or opened but must be swallowed whole Should be taken with food Drug interactions |
|
aMetre 2 (m2) body surface area calculation: square root of (height in centimetres times weight in kilograms divided by 3600). |
||||
ANNEX D. Fixed-dose Combinations of ARVS Available on 1 December 2003
|
Three-drug |
d4T (40 mg) + 3TC (150 mg) + NVP (200 mg) |
|
fixed-dose |
d4T (30 mg) + 3TC (150 mg) + NVP (200 mg) |
|
combinations |
ZDV (300 mg) + 3TC (150 mg) + ABC (300 mg) |
|
ZDV |
(300 mg) + 3TC (150 mg) + NVP (200 mg) |
|
Two-drug |
d4T (30 mg) + 3TC (150 mg) |
|
fixed-dose |
d4T (40 mg) + 3TC (150 mg) |
|
combinations |
ZDV (300 mg) + 3TC (150 mg) |
|
NOTE: WHO encourages the use of fixed-dose combinations when formulations of assured quality and proven bioequivalence are available and offer operational advantages. Not all the FDCs in this table have been evaluated for prequalification by WHO. WHO operates a voluntary prequalification system, in which, as of 1 December 2003, three manufacturers prequalified ZDV/3TC combinations, two prequalified d4T/3TC/NVP combinations, and one prequalified ZDV/3TC/ABC. The list of WHO-prequalified manufacturers is continuously updated and is available at: http://www.who.int/medicines |
|
ANNEX E. WHO Staging System for HIV Infection and Disease and Disease in Adults and Adolescents
|
Clinical Stage I: |
|
4. Asymptomatic |
|
5. Generalized lymphadenopathy |
|
Performance scale 1: asymptomatic, normal activity |
|
Clinical Stage II: |
|
6. Weight loss <10% of body weight |
|
7. Minor mucocutaneous manifestations (seborrhoeic dermatitis, prurigo, fungal nail infections, recurrent oral ulcerations, angular cheilitis) |
|
8 Herpes zoster within the last five years |
|
9. Recurrent upper respiratory tract infections (i.e. bacterial sinusitis) |
|
And/or performance scale 2: symptomatic, normal activity |
|
Clinical Stage III: |
|
10. Weight loss >10% of body weight |
|
11. Unexplained chronic diarrhoea, >1 month |
|
12. Unexplained prolonged fever (intermittent or constant), >1 month |
|
13. Oral candidiasis (thrush) |
|
14. Oral hairy leucoplakia |
|
15. Pulmonary tuberculosis |
|
16. Severe bacterial infections (i.e. pneumonia, pyomyositis) |
|
And/or performance scale 3: bedridden <50% of the day during last month |
|
Clinical Stage IV: |
|
17. HIV wasting syndromea |
|
18. Pneumocystic carinii pneumonia |
|
19. Toxoplasmosis of the brain |
|
20. Cryptosporidiosis with diarrhoea >1 month |
|
21. Cryptococcosis, extrapulmonary |
|
22. Cytomegalovirus disease of an organ other than liver, spleen or lymph node (e.g. retinitis) |
|
23. Herpes simplex virus infection, mucocutaneous (>1month) or visceral |
|
24. Progressive multifocal leucoencephalopathy |
|
25. Any disseminated endemic mycosis |
|
26. Candidiasis of oesophagus, trachea, bronchi |
|
27. Atypical mycobacteriosis, disseminated or pulmonary |
|
28. Non-typhoid Salmonella septicaemia |
|
29. Extrapulmonary tuberculosis |
|
30. Lymphoma |
|
31. Kaposi’s sarcoma |
|
32. HIV encephalopathyb |
|
And/or performance scale 4: bedridden >50% of the day during last month |
|
aHIV wasting syndrome: weight loss of >10% of body weight, plus either unexplained chronic diarrhoea (>1 month) or chronic weakness and unexplained prolonged fever (>1 month). bHIV encephalopathy: clinical findings of disabling cognitive and/or motor dysfunction interfering with activities of daily living, progressing over weeks to months, in the absence of a concurrent illness or condition, other than HIV infection, which could explain the findings. |
ANNEX F. WHO Staging System for HIV Infection and Disease in Children
|
Clinical Stage I: |
|
1. Asymptomatic |
|
2. Generalized lymphadenopathy |
|
Clinical Stage II: |
|
3. Chronic diarrhoea >30 days duration in absence of known etiology |
|
4. Severe persistent or recurrent candidiasis outside the neonatal period |
|
5. Weight loss or failure to thrive in the absence of known etiology |
|
6. Persistent fever >30 days duration in the absence of known etiology |
|
7. Recurrent severe bacterial infections other than septicaemia or meningitis (e.g. osteomye litis, bacterial (non-TB) pneumonia, abscesses) |
|
Clinical Stage III: |
|
8. AIDS-defining opportunistic infections |
|
9. Severe failure to thrive (wasting) in the absence of known etiologya |
|
10. Progressive encephalopathy |
|
11. Malignancy |
|
12. Recurrent septicaemia or meningitis |
|
aPersistent weight loss >10% of baseline or less than 5th percentile on weight for height chart on 2 consecutive measurements more than 1 month apart in the absence of another etiology or concurrent illness. |
REFERENCES
Adje-Toure CA, Cheingsong R, Garcia- Lerma JG, et al. Antiretroviral therapy in HIV- 2-infected patients: changes in plasma viral load, CD4+ cell counts, and drug resistance profiles of patients treated in Abidjan, Côte d’Ivoire. AIDS 2003; 17(Suppl 3):S49-S54.
Badri M, Wood R. Usefulness of total lymphocyte count in monitoring highly active antiretroviral therapy in resource-limited settings. AIDS 2003;17(4):541-545.
Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet 2002;359:2059-2064.
Bang LM, Scott LJ. Emtricitabine: an antiretroviral agent for HIV infection. Drugs 2003;63(22):2413-2424; discussion 2425-2426.
Beck EJ, Kupek EJ, Gompels MM, et al. Correlation between total and CD4 lymphocyte counts in HIV infection: not making the good an enemy of the not so perfect. Int J STD AIDS 1996;7(6):422-428.
Boehringer-Ingelheim Pharmaceuticals, Inc. Viramune drug label. Revised 20 June 2003.
Boubaker K, Flepp M, Sudre P, et al. Hyperlactatemia and antiretroviral therapy: the Swiss HIV Cohort Study. Clin Infect Dis 2001;33(11):1931-1937.
Brettle RP. Correlation between total and CD4 lymphocyte counts in HIV infection. Int J STD AIDS. 1997;8(9):597.
Burman WJ, Jones BE. Treatment of HIVrelated tuberculosis in the era of effective antiretroviral therapy. Am J Respir Crit Care Med 2001; 164(1):7-12.
Chaowanachan T, Chotpitayasunondh T, Vanprapar N, et al. Resistance mutations following a single-dose intrapartum administration of nevirapine to HIV-infected Thai women and their infants receiving short-course zidovudine. 10th Conference on Retroviruses and Opportunistic Infections; 2003 Feb 10-14; Boston, Massachusetts (Abstract 855).
Cunningham CK, Chaix ML, Rackacewicz C, et al. Development of resistance mutations in women receiving standard antiretroviral therapy who received intrapartum nevirapine to prevent perinatal human immunodeficiency virus type 1 transmission: a substudy of Pediatric AIDS Clinical Trials Group protocol 316. J Infect Dis 2002;186:181-188.
De Martino M, Tovo P-A, Balducci M, et al. Reduction in mortality with availability of antiretroviral therapy for children with perinatal HIV-1 infection. JAMA 2000;284:190-197.
Dean G, Back D, de Ruiter A. Effect of tuberculosis therapy on nevirapine trough plasma concentration. AIDS 1999;13:2489-2490.
Dean GL, Edwards SG, Ives NJ, et al. Treatment of tuberculosis in HIV-infected persons in the era of highly active antiretroviral therapy. AIDS 2002; 16(1):75-83.
Desclaux A, Ciss M, Taverne B, et al. Access to antiretroviral drugs and AIDS management in Senegal. AIDS 2003;17(Suppl 3):S95-S101.
DHHS. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Available from: URL: http://AIDSInfo.nih.gov/guidelines
Embree J, Bwayo J, Nagelkerke N, et al. Lymphocyte subsets in human immunodeficiency virus type 1-infected and uninfected children in Nairobi. Pediatr Infect Dis J 2001;20:397-403.
Ena J, Amador C, Benito C, et al. Risk and determinants of developing severe liver toxicity during therapy with nevirapine- and efavirenz-containing regimens in HIV-infected patients. Int J STD AIDS 2003;14(11):776-781.
Eshleman SH, Mracna M, Guay LA, et al. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012). AIDS 2001;184:914-917.
European Collaborative Study. Gender and race do not alter early-life determinants of clinical disease progression in HIV-1 vertically infected children. AIDS 2004 (in press).
Fournier AM, Sosenko JM. The relationship of total lymphocyte count to CD4 lymphocyte counts in patients infected with human immunodeficiency virus. Am J Med Sci. 1992;304(2):79-82.
Gallant JE, Deresinski S. Tenofovir disoproxil fumarate. Clin Infect Dis 2003;37(7):944-950.
Gallant JERA, Weinberg W, Young B, et al. Early non-response to tenofovir DF and abacavir and lamivudine in a randomized trial compared to efavirenz + ABC and 3TC: ESS30009 unplanned interim analysis. 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy; 2003 Sep 14-17; Chicago, Illinois.
Gerstoft J, Kirk O, Obel N, et al. Low efficacy and high frequency of adverse events in a randomized trial of the triple nucleoside regimen abacavir, stavudine and didanosine. AIDS 2003;17(14):2045-2052.
Giarardi E, Antonucci G, Vanacore P, et al. Impact of combination antireteroviral therapy on the risk of tuberculosis among persons with HIV infection. AIDS 2000;14:1985-1991.
Gilead. High rate of virologic failure in patients with HIV infection treated with once daily triple NRTI regimen containing didanosine, lamivudine, and tenofovir; 2003 (Letter).
Giuliano M, Palmisano L, Galluzzo CM, et al. Selection of resistance mutations in pregnant women receiving zidovudine and lamivudine to prevent HIV perinatal transmission . AIDS 2003;17:1570-1571.
Gortmaker SL, Hughes M, Cervia J, et al. Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med 2001;345:1522-1528.
Gulick RMRH, Shikuma CM, Lustgarten S, et al. ACTG 5095: a comparative study of 3 protease inhibitor-sparing antiretroviral regimens for the initial treatment of HIV infection. 2nd IAS Conference on HIV Pathogenesis and Treatment; 2003 Jul 13-16; Paris.
Haas DW, Zala C, Schrader S, et al. Therapy with atazanavir plus saquinavir in patients failing highly active antiretroviral therapy: a randomized comparative pilot trial. AIDS 2003;17(9):1339-1349.
Harries AD, Hargreaves NJ, Kemp J, et al. Deaths from tuberculosis in sub-Saharan African countries with a high prevalence of HIV-1. Lancet 2001;357(9267):1519-1523.
Harvard University. Consensus statement on antiretroviral treatment for AIDS in poor countries. Boston: Harvard University; 2001.
Havlir D, Vella S, Hammer S. The Global HIV Drug Resistance Surveillance Program: a partnership between WHO and IAS. AIDS 2002;16(10):7-9.
Havlir DV, Barnes PF. Tuberculosis in patients with human immunodeficiency virus infection. N Eng J Med 1999;340(5):367-373.
Hirsch MS, Brun-Vezinet F, Clotet B, et al. Antiretroviral drug resistance testing in adults infected with human immunodeficiency virus type 1:2003 recommendations of an International AIDS Society-USA Panel. Clin Infect Dis 2003;37(1):113-128.
Ibbotson T, Perry CM. Lamivudine/ zidovudine/abacavir: triple combination tablet. Drugs 2003; 63(11):1089-1098; discussion 1099-1100.
Imperiale SM, Stern JO, Love JT, et al. The VIRAMUNE (nevirapine) hepatic safety project: analysis of symptomatic hepatic events. 4th International Workshop on Adverse Events and Lipodystrophy in HIV; 2002 Sep 22-25; San Diego, California (Abstract 87).
Joint United Nations Programme on HIV/ AIDS (UNAIDS) and World Health Organization (WHO). AIDS epidemic update: 2003. Geneva.UNAIDS. Available from: URL: http://www.who.int/hiv/pub/epidemiology/epi2003/en/
Karras A, Lafaurie M, Furco A, et al. Tenofovir-related nephrotoxicity in human immunodeficiency virus-infected patients: three cases of renal failure, Fanconi syndrome, and nephrogenic diabetes insipidus. Clin Infect Dis 2003;36(8):1070-1073.
Keiser P, Nassar N, White C, et al. Comparison of nevirapine- and efavirenz-containing antiretroviral regimens in antiretroviral-naive patients: a cohort study. HIV Clin Trials 2002;3(4):296-303.
Keiser P, Nassar N, Yazdani B, et al. Comparison of efficacy of efavirenz and nevirapine: lessons learned for cohort analysis in light of the 2NN Study. HIV Clin Trials 2003;4(5):358-360.
Kityo C. A randomised trial of monitoring practice and structured treatment interruptions in the management of antiretroviral therapy in adults with HIV infection in Africa: the DART trial. 13th International Conference on AIDS and STIs in Africa (ICASA); 2003; Nairobi (Abstract 1098933).
Kumarasamy N, Mahajan AP, Flanigan TP, et al. Total lymphocyte count (TLC) is a useful tool for the timing of opportunistic infection prophylaxis in India and other resource constrained countries. J Acquir Immune Defic Syndr 2002;31(4):378-383.
la Porte C, Colbers E, Bertz R, et al. Pharmacokinetics of two adjusted dose regimens of lopinavir/ritonavir in combination with rifampin in healthy volunteers. 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy; 2002; San Diego, California (Abstract A-1823).
Laniece I, Ciss M, Desclaux A, et al.Adherence to HAART and its principal determinants in a cohort of Senegalese adults. AIDS 2003;17(Suppl 3):S103-S108.
Langlet P, Guillaume M-P, Devriendt J, et al. Fatal liver failure associated with nevirapine in a pregnant HIV patient: the first reported case. Gastroenterol 2000;118(Suppl 2):Ab-stract 6623 (101st Annual Meeting of the American Gastroenterological Association; 2000 May 21-24; San Diego, California).
Law WP, Dore GJ, Duncombe CJ, et al. Risk of severe hepatotoxicity associated with antiretroviral therapy in the HIV-NAT Cohort, Thailand, 1996-2001. AIDS 2003; 17(15):2191-2199.
Lindsey JC, Hughes MD, McKinney RE, et al. Treatment mediated changes in human immunodeficiency virus (HIV) type 1 RNA and CD4 cell counts as predictors of weight growth failure, cognitive decline, and survival in HIVinfected children. J Infect Dis 2000;182:1385-1393.
Lopez-Cortes L, Ruiz-Valderas R, Viciana P, et al. Pharmacokinetic interactions between efavirenz and rifampin in HIV-infected patients with tuberculosis. Clin Pharmacokinet 2002;41:681-690.
Lyons F, Hopkins S, McGeary A, et al. Nevirapine tolerability in HIV infected women in pregnancy – A word of caution (late breaker). 2nd IAS Conference on HIV Pathogenesis and Treatment; 2003 Jul 13-16; Paris.
Mandelbrot L, Landreau-Mascaro A, Rekacewicz C, et al. Lamivudine-zidovudine combination for prevention of maternal-infant transmission of HIV-1. JAMA 2001;285:2083-2093.
Martin-Carbonero L, Nunez M, Gonzalez-Lahoz J, et al. Incidence of liver injury after beginning antiretroviral therapy with efavirenz or nevirapine. HIV Clin Trials 2003;4(2):115-120.
McCoig C, Castrejon MM, Castano E, et al. Effect of combination antiretroviral therapy on cerebrospinal fluid HIV RNA, HIV resistance, and clinical manifestations of encephalopathy. J Pediatr 2002;141:36-44.
Mofenson LM, Harris DR, Moye J, et al. Alternatives to HIV-1 RNA concentration and CD4 count to predict mortality in HIV-1-infected children in resource-poor settings. Lancet 2003; 362 (9396):1625-1627.
Moyle GJ. NNRTI choice: has 2NN changed our practice? AIDS Read 2003;13(7):325-328.
Narita M, Ashkin D, Hollander E, et al. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med 1998;158:157-161.
Olivia J, Moreno S, Sanz J, et al. Coadministration of rifampin and nevirapine in HIVinfected patients with tuberculosis. AIDS 2003;17:637-642.
Palella FJ, Jr., Deloria-Knoll M, Chmiel JS, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med 2003; 138(8):620-626.
Patel A, Patel K, Patel J, et al. To study the safety and antiretroviral efficacy of rifampicin and efavirenz in antiretroviral-naïve tuberculosis coinfected HIV-1 patients in India. X Conference on Retroviruses and Opportunistic Infections; 2003; Boston, Massachusetts (Abstract 138).
Pedral-Samapio D, Alves C, Netto E, et al. Efficacy of efavirenz 600 mg dose in the ARV therapy regimen for HIV patients receiving rifampicin in the treatment of tuberculosis. 10th Conference on Retroviruses and Opportunistic Infections; 2003 Feb 10-14; Boston, Massachusetts (Abstract 784).
Piliero PJ. Atazanavir: a novel HIV-1 protease inhibitor. Expert Opin Investig Drugs 2002; 11(9):1295-1301.
Pollard RB, Tierney C, Havlir D, et al. A phase II randomized study of the virologic and immunologic effect of zidovudine + stavudine versus stavudine alone and zidovudine + lamivudine in patients with >300 CD4 cells who were antiretroviral naive (ACTG 298). AIDS Res Hum Retroviruses 2002;18(10):699-704.
Ribera E, Pou L, Lopez RM, et al. Pharmacokinetic interaction between nevaripine and rifampicin in HIV-infected patients with tuberculosis . J Acquir Immune Defic Syndr 2001;28:450-453.
Ribera E, Azuaje C, Montero F. Saquinavir, ritonavir, didanosine, and lamivudine in a once daily regimen for HIV infection in patients with rifampin-containing antituberculosis treatment. 14th International Conference on AIDS; 2002 Jul 7-12; Barcelona (Abstract ThPeB7280).
Sanne I, Piliero P, Squires K, et al. Results of a phase 2 clinical trial at 48 weeks (AI424-007): a dose-ranging, safety, and efficacy comparative trial of atazanavir at three doses in combination with didanosine and stavudine in antiretroviralnaive subjects. J Acquir Immune Defic Syndr 2003;32(1):18-29.
Santoro-Lopes G, de Pinho AM, Harrison LH, et al. Reduced risk of tuberculosis among Brazilian patients with advanced human immunodeficiency virus infection treated with highly active antiretroviral therapy. Clin Infect Dis 2002;34(4):543-546.
Saulsbury FT. Resolution of organ-specific complications of human immunodeficiency virus infection in children with use of highly active antiretroviral therapy. Clin Infect Dis 2001;32:464-468.
Schaaf B, Aries SP, Kramme E, et al. Acute renal failure associated with tenofovir treatment in a patient with acquired immunodeficiency syndrome. Clin Infect Dis 2003;37(3):41-43.
Shearer WT, Rosenblatt HM, Gelman RS, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 Study . J Allergy Clin Immunol 2003; 112(5):973-980.
Smith NA, Shaw T, Berry N, et al. Antiretroviral therapy for HIV-2-infected patients. J Infect Dis 2001;42:126-133.
Staszewski S, Keiser P, Montaner J, et al. Abacavir-lamivudine-zidovudine vs indinavirlamivudine-zidovudine in antiretroviral-naïve HIV-infected adults: A randomized equivalence trial. JAMA. 2001;285 (9):1155-1163.
Staszewski SGJ, Pozniak AL, Suleiman JMAH, et al. Efficacy and safety of tenofovir DF versus stavudine when used in combination with lamivudine and efavirenz in antiretroviral naive patients: 96-week preliminary interim results. 10th Conference on Retroviruses and Opportunistic Infections; 2003 Feb 10-14; Boston, Massachusetts.
Stern JO, Love JT, Robinson, PA, et al. Hepatic safety of nevirapine: Results of the Boehringer Ingelheim Viramune Hepatic Safety Project. 14th International Conference on AIDS; 2002 Jul 7-12; Barcelona (Abstract LBOr15).
Stern JO, Robinson PA, Love JT, et al. A comprehensive hepatic safety analysis of nevirapine in different populations of HIV infected patients. J Acquired Immune Defic Syndr 2003;34, Supl 1:S21-S33.
Sullivan J. South African Intrapartum Nevirapine Trial: selection of resistance mutations. 14th International Conference on AIDS; 2002 Jul 7-12; Barcelona (Abstract LbPeB9024).
Van der Ende ME, Prins JM, Brinkman K, et al. Clinical, immunological and virological response to different antiretroviral regimens in a cohort of HIV-2-infected patients. AIDS 2003;17(Suppl 3):S55-S61.
van der Ryst E, Kotze M, Joubert G, et al. Correlation among total lymphocyte count, absolute CD4+ count, and CD4+ percentage in a group of HIV-1-infected South African patients. J Acquir Immune Defic Syndr Hum Retrovirol 1998;19(3):238-244.
van Leth FHE, Phanuphak P, Miller S, et al. Results of the 2NN study: a randomized comparative trial of first-line antiretroviral therapy with regimens containing either nevirapine alone, efavirenz alone, or both drugs combined, together with stavudine and lamivudine. 10th Conference on Retroviruses and Opportunistic Infections; 2003 Feb 10-14; Boston, Massachusetts.
Verhelst D, Monge M, Meynard JL, et al. Fanconi syndrome and renal failure induced by tenofovir: a first case report. Am J Kidney Dis 2002;40(6):1331-1333.
Verweel G, van Rossum AMC, Hartwig NG, et al. Treatment with highly active antiretroviral therapy in human immunodeficiency virus type 1-infected children is associated with a sustained effect on growth. Pediatrics 2002;109(2):E25 Available from: URL: http://www.pediatrics.org/cgi/content/full/109/2/e25
Wade AM, Ades AE. Age-related reference ranges: significance tests for models and confidence intervals for centiles. Stat Med 1994;13:2359-2367.
Wagner KR, Bishai WR. Issues in the treatment of Mycobacterium tuberculosis in patients with human immunodeficiency virus infection. AIDS 2001;15(Suppl 5):S203-S212.
Walmsley S, Bernstein B, King M, et al. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med 2002;346(26):2039-2046.
Yeni PG, Hammer SM, Carpenter CC, et al. Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society-USA Panel. JAMA 2002;288(2):222-235.



























































