Appendix D
Source Technologies
This appendix provides supporting information for the discussions of technology alternatives in Chapter 2, the hybrid systems in Chapter 3, and the advanced concepts in Chapter 6. It describes background information on new and advanced power sources in the 2-W, 20-W, 100-W, and 1- to 5-kW regimes that was not included in Energy-Efficient Technologies (NRC, 1997). Table D-1 provides a comprehensive list of the source technologies discussed in both reports.
BATTERIES
Batteries are electrochemical devices that convert the chemical energy of active materials into electrical energy. A battery cell comprises a negative electrode (anode) and a positive electrode (cathode) having differing electrical potentials; these electrodes are electronically separated but are ionically connected with an electrolyte. Current collectors, packaging, and interconnects are needed to deliver the energy safely to a load. This ensemble is shown schematically in Figure D-1. The arrangement or geometry of the cell has a significant impact on the discharge properties of the cell. An excellent overview of battery chemistries, their definitions, design, and properties is available at http://voltaicpower.com.1 Most battery manufacturers also have detailed descriptions of their batteries’ chemistries and properties. Also see sources such as the Handbook of Batteries (Linden and Reddy, 2002).
Primary Batteries
Primary batteries can be discharged once and then must be discarded. Most primary battery technologies are very mature, but there are several systems that might be improved to the point where they could have a significant impact on the military. The R&D efforts for commercial batteries are concentrated on the design of new form factors for specific device applications and on the search for materials capable of high-energy/high-power performance. Research on less mature chemistries can still yield improvements in power and energy.
The properties of commercial Li/SO2, Li/MnO2, and Li/(CF)x batteries are summarized in Table D-2. The military uses Li/SO2 batteries for many applications, specifically in the BA 5590, which is the workhorse of soldier electronics. These have a theoretical voltage of 3.1, a working voltage of 2.8, and a practical energy density of 170 Wh/kg. D-cell configurations on Li/SO2 batteries have specific energies of 210 Wh/kg (e.g., SAFT LO26SX). The trend is to replace the Li/SO2 batteries with Li/MnO2 batteries, which have fewer safety constraints.
The Li/MnO2 battery is a commercially available primary system, and Li/MnO2 button cells (123A and 223A) are used for small device applications such as watches, calculators, cameras, and clocks. The theoretical voltage of the reaction is 3.5, but it has a practical voltage of 3.3. Its electrolyte is an organic solvent with a Li salt. The military is currently taking orders on Li/MnO2 batteries for use in SINCGARS radios and the like from SAFT2 and Brentronics (BA-5372/U, 5368/U, X567/U). The properties of commercial Li/MnO2 batteries are summarized in Table D-2.
The Li/(CF)x cell was first introduced in Japan by Matsushita (Panasonic3) in the early 1970s. Li/(CF)x coin cells and BR 2/3A cells are two popular commercial cells. Li/(CF)x coin cells are used mainly in low-drain devices such as electronic watches and calculators. BR 2/3A cells are used
|
1 |
Last accessed on January 28, 2004. |
|
2 |
Found at www.saftbatteries.com. Last accessed on January 28, 2004. |
|
3 |
Found at http://www.panasonic.com/industrial/battery/oem/chem/lithion/index.html. Last accessed on January 28, 2004. |
TABLE D-1 Overview of All Power Source Alternatives
|
Power System |
State of the Art, 1997a |
State of the Art, 2003 |
Item Considered |
Scaling Laws |
Impact on Soldier Power |
|
Primary battery (includes metal/air) |
Mature. Up to 800 Wh/kg in low-specific-power configurations |
Mature. SOA not significantly advanced beyond NRC (1997) report. |
Energy density. Safety. Power density. Environmental impact. |
Known |
Heavy, one-time use. Current battery of choice for combat missions. Potential for use in hybrids. |
|
Secondary battery |
Mature. Li ion: 100 Wh/kg in development. |
Mature in commercial applications. Li ion: 140 Wh/kg available; 200 Wh/kg in development. |
Energy density. Cycle life. Power density. Safety and cost. |
Known |
Stand-alone energy supply for many missions. Can be used in hybrid mode for high-energy missions. |
|
Fuel cell (hydrogen) |
Exploratory development. Many systems at laboratory scale. Power levels to 150 W considered. |
Beta prototypes with various hydrogen sources tested in field. Power to 150 W. |
Fuel reformers. Water management. Safety. |
Known |
New capability; potential for use in hybrid system. Less weight. Cost savings. Requires new battlefield fuel. |
|
Fuel cell (methanol) |
Emerging. Not considered. |
Beta prototypes developed at power levels of 20 to 50 W. 20% efficiency. |
Fuel and fuel crossover. Catalyst. Cost. |
Known |
New capability. Less weight. Cost savings. Requires new battlefield fuel. |
|
Fuel cell (solid oxide) |
Emerging. Not considered. |
Emphasis on small sizes. Laboratory prototypes in 20-W range. Research in high-capacity designs. |
High temperature. Materials. Integration and systems. |
Known |
New capability. Less weight. Easier to utilize battlefield fuels. More efficient. |
|
Internal combustion |
Some versions mature. Hobby application sizes coupled to generators. No commercial products on market. |
Commercial applications with motor-alternator combinations in 30 to 100 W/kg range. Efficiencies greater than 20%in 500-W sizes. Emerging modified hobby engines operate on diesel. |
Fuels. Vibrations. Life. |
Known |
Inexpensive technology. Potential for high-energy missions. Can probably be made to function with JP fuels. Current role as battery charger. |
|
External combustion (includes Stirling) |
Not considered. |
100 W/kg specific power demonstrated for motor-alternator with efficiency of 29%. System efficiencies projected to be >20%. Laboratory 35-to 50-W systems available for beta prototypes; 1- to 2-kW beta prototypes available with ~20% system efficiencies. System-specific power appears to be around 30 W/kg. |
Fuels. Specific power. System-specific energy. Signatures. |
Known |
New stealth capability. Inexpensive technology. Can be made to operate on JP fuels. Potential for high-energy missions. |
|
Microturbine |
Emerging. Considered promising. |
Not considered owing to lack of progress in producing workable systems. |
Fuels. Specific power. System-specific energy. Materials. Cost. |
Unknown |
|
|
Power System |
State of the Art, 1997a |
State of the Art, 2003 |
Item Considered |
Scaling Laws |
Impact on Soldier Power |
|
Thermoelectric |
Some versions mature. Low potential. Best system efficiency on order of 5%; converter efficiencies projected to 10%. |
Insufficient progress to consider for current applications. Progress in new high-ZT materials makes technology worth watching for long term. |
Efficiency. Materials-specific power. System-specific energy. |
Known |
Not applicable owing to low efficiency. Possible niche application in small sizes. |
|
Thermo-photovoltaic (TPV) |
20% TPV cells demonstrated. System projections to 20%. |
Not considered owing to lack of progress in systems. |
|
Known |
|
|
Nuclear isotope |
Limited consideration. Rejected owing to cost, safety, environmental considerations, and lack of infrastructure. |
Not considered. |
Safety. Environmental impact. Cost. Public acceptance. |
Known |
|
|
Alkali metal thermal-to-electric converter |
Speculative technology. Systems projection to 500 W/kg. |
Not considered owing to lack of progress. |
|
Known |
|
|
Energy harvesting; solar |
Some versions mature. |
Considered for low-capacity niche applications. |
|
Known |
Driver for reducing power demand. |
|
NOTE: SOA, state of the art; Li ion, lithium ion; JP, jet propellant; ZT, thermoelectric figure of merit. aNRC, 1997. |
|||||
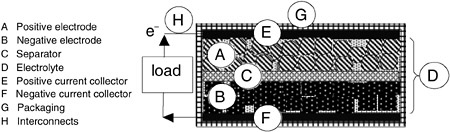
FIGURE D-1 Schematic cross section of a battery.
in cameras, though the Li/MnO2 2/3A cell is the more popular choice for cameras due to its lower cost. As summarized in Table D-2, Li/(CF)x has higher theoretical specific energy than Li/MnO2 cells (2120 Wh/kg vs. 900 Wh/kg) and an open circuit potential (OCV) of about 3.2 V. The theoretical OCV, based on free-energy calculations, is about 4.5 V. The difference between theoretical and practical OCV values has been discussed by Whittingham (1975). A comparison of the practical performance of Li/(CF)x vs. that of Li/MnO2 is shown in Table D-2.
In spite of the much higher theoretical specific energy in a Li/(CF)x cell, (CF)x is much lighter than MnO2 (2.5 g/cc vs. 4.5 g/cc) and gives comparable practical energy performance in commercial small cells. During the discharge of the cell, the carbon monofluoride in the positive electrode changes from a poor conductor to a more conductive amorphous carbon when discharged. Thus, the reaction efficiency increases with discharge. Li/(CF)x cells are known for their high-temperature performance (as high as 150°C according to Panasonic coin cells data), long shelf life (>10 years), and
TABLE D-2 Attributes of Advanced Primary Batteries
|
|
Chemistry |
||
|
Attribute |
Lithium Sulfur Dioxide (Li/SO2) |
Lithium Manganese Dioxide (Li/MnO2) |
Lithium Carbon Monofluoride (Li/(CF)x) |
|
Discharge reaction |
2Li + 2SO2 → Li2S2O4 |
xLi + MnIVO2 → LixMnIIIO2 |
xLi + (CF)x → xLiF + C |
|
Theoretical voltage (V) |
3.10 |
3.50 |
4.50 |
|
Working voltage (V) |
2.95 |
3.30 |
3.50 |
|
Energy density (Wh/L)a |
385 |
480-510 |
1,040 |
|
Specific energy (Wh/kg)a |
210 |
210-250 |
600 |
|
Power density (W/L) |
<180 |
<230 |
<23 |
|
Specific power (W/kg) |
<100 |
<100 |
<14 |
|
Shelf life |
|
5 yr |
>10 yr |
|
Reference |
SAFT LI26SX |
Duracell 2/3A |
Eagle-Picher LCF-112 |
|
Cell capacity (Ah) |
7.5 |
1.4 |
39.4 |
|
aThe energy density and specific energy values are based on density and specific power values, respectively. |
|||
TABLE D-3 Attributes of Leading Secondary Batteries
|
|
Chemistry |
||
|
Attribute |
Lithium Ion |
Nickel Metal Hydride (MH/NiOOH) |
Lithium/Sulfur |
|
Negative electric discharge |
LiC6 = Li+ + C6 + e− |
MH + OH− = M + H2O + e− |
Li = Li+ + e− |
|
Positive electric discharge |
Li1⁄2CoO2 + 1⁄2Li+ + 1⁄2e− = LiCoO2 |
NiOOH + H2O + e− = Ni(OH)2 + OH− |
Sx + 2e− = Sx= |
|
Overall reaction |
LiC6 + 2Li1⁄2CoO2 = C6 + 2LiCoO2 |
MH + NiOOH = Ni(OH)2 + M |
2Li + Sx = Li2Sx |
|
Theoretical voltage (V) |
~4.2 |
1.2 |
2.1 |
|
Working voltage (V) |
3.6 |
1.0 |
1.8 |
|
Cost (initial, $/Wh) |
~10 |
~3 |
~0.25 |
|
Energy density (Wh/L)a |
450-490 |
220 |
225 |
|
Specific energy (Wh/kg)a |
160-175 |
63-75 |
170 |
|
Power density (W/L) |
<570 |
850 |
50 |
|
Specific power (W/kg) |
<200 |
220 |
50 |
|
Life cycles |
300-1,000 |
600-12,000 |
300-650 |
|
Environment (°C) |
−20 to +60°C |
−30 to +65°C |
+25 to +60°C |
|
Reference |
Sanyo 18650 |
Linden and Reddy (2002) |
Polyplus 1 Ah cells |
|
aThe energy density and specific energy values are based on the power density and specific power values, respectively. |
|||
high specific energy at low to medium powers. In comparison with Li/MnO2, the main disadvantages of Li/(CF)x are low power capability and high cost.
Secondary Batteries
Secondary batteries can be recharged. There are numerous commercially available secondary batteries that are used commercially, such as lead-acid, silver-zinc, and metal-hydride systems. This appendix describes systems that have advanced technologically since 1997, including Li ion and Li polymer chemistries, nickel metal hydride, and lithium sulfur. Attributes of these batteries are summarized in Table D-3.
Li ion batteries encompass several different chemistries, including LiCoO2, LiNiO2, and LiMn2O4 positive electrodes. The Li ion cell was introduced commercially in the early 1990s by the Sony Corporation.4 It has the advantages of high cell voltage (~3.6 V), high specific energy (>100 Wh/ kg), and long cycle life (~1,000 deep cycles). Li ion batteries’ power and energy characteristics are summarized in Table D-3. Li ion batteries quickly captured the market for camcorders, cell phones, and notebook computers in spite of their high cost, and small cells of cylindrical and prismatic form are being manufactured at the rate of close to a billion cells per year.
The cells can be recharged because the active materials can accommodate the movement of Li atoms (and electrons)
|
4 |
Found at http://www.sanyo.com/industrial/batteries/. Last accessed on January 28, 2004. |
into and out of the structure, with a minimum of disruption to that structure. This structural integrity is important in maintaining a long cycle life. The negative electrode is made of various types of carbon and graphite (the original Sony cell used LiCoO2). The CoO2 has a layered structure that readily accommodates the Li without the formation of a new structure (or new phase).
Although Li ion cells have the best performance of any available rechargeable battery, they have a number of problems that are currently being addressed by the R&D community. Overcharge or overdischarge can lead to capacity loss and even cell failure in the form of thermal runaway and fire, so each cell has a protective microcircuit that controls the voltage limits of the cell and the recharge process. The solvents for the electrolyte are flammable organic liquids (such as ethylene carbonate and dimethyl carbonate), so there is research on flame-retardant additives. Also, because the cobalt oxide positive electrodes are expensive, alternative low-cost, high-capacity positive electrode materials are being explored, including LiNiO2-based, LiMnO2-based, Li(Mn,Ni,Co)O2-based, and LiFePO4-based materials. Some nickel-containing materials are close to commercialization. Performance can also degrade by spontaneous film formation on the electrodes, so there are efforts to find additives for the electrolyte that control film formation and film properties.
Li polymer cells are derivatives of the Li ion cells. They have the same electrochemistry, but the liquid electrolyte is gelled with a polymer such as polyvinylidene fluoride (PVdF) or polyethylene oxide so that it is immobilized and behaves like a polymer. The gel offers flexibility in the shape of the cell and eliminates any free-flowing liquid. Li polymer cells have performance similar to that of the Li ion cell, with specific energy values up to about 150 Wh/kg and 300 Wh/L for −20°C to +60°C, and have been recently introduced to the commercial market.5
The nickel metal hydride, or MH/NiOOH, cell has become very popular for many consumer applications, including portable electronics and power tools. It has largely replaced the Ni-Cd (Cd/NiOOH) cell in the consumer market, because of concern about the environmental impact of cadmium. The MH/NiOOH cell has an aqueous electrolyte of potassium hydroxide, which offers a much higher conductivity than the nonaqueous electrolytes used in lithium cells, so it can be discharged at high power. Both of the electrode reactions in Table D-3 are reversible and have rapid reaction rates, so high specific power values can be achieved, but their specific energy is less than 100 Wh/kg, which limits its usefulness. Other problems with this system include its low cell voltage (~1.2 V), limited temperature range for reasonable operation, and the need for charging at a relatively low temperature (<45°C).
Li/S cells offer the opportunity for very high specific energy (theoretical value = 2,600 Wh/kg) and low cost, using environmentally benign materials. Their characteristics are summarized in Table D-3. The drawback of this battery system is its short cycle-life, which is due to the sulfur electrode. During operation of the cell, polysulfides of several stoichiometries form and dissolve in liquid electrolytes, allowing them to migrate throughout the cell. This stability issue has been addressed by using gel and polymer electrolytes that prevent migration of the sulfur species. Sion Power Corporation6 is striving to introduce commercial lithium/sulfur batteries in 2004 with 1-Ah pouch-style cells.
Metal/Air Batteries
Metal/air cells comprise a cathode that uses oxygen in the air as an oxidant and a solid fuel as the anode. They are different from fuel cells and other batteries in that the anode is consumed during operation. Often, metal/air cells are described as semi-fuel cells. Metal/air cells are being studied because they have the advantage of using air as an inexhaustible cathode reactant, leading to compact, anode-limited cells with high energy density. Carbon/air batteries are grouped with this class of power sources even though they operate at elevated temperatures.
The properties of metal/air and carbon/air electrochemical couples are summarized in Table D-4. The total metal/air reaction is the sum of the reaction of the oxidation at the metal anode and the reduction of oxygen at the air cathode:
4M + nO2 + 2nH2O → 4M(OH)n
M + nO2 → MO2n
where M is the metal and n depends on the valence change for the oxidation of the metal. Most metal/air cells do not have a long shelf life once they are activated with electrolyte and exposed to air, because the metal anode tends to react with water in the aqueous electrolyte or moisture in the air to generate hydrogen:
M + nH2O → M(OH)n + n/2 H2
Moisture in the air is a big factor in the performance of metal/air cells. Too much moisture causes flooding of the air electrodes, while insufficient moisture causes water to evaporate from the cells and dries out the electrolyte. In addition, metal/air cells that use alkaline electrolyte also suffer from the buildup of carbonates in the electrolyte from the reaction with CO2 in the air. Finally, the slow gas-solid
|
5 |
Found online at http://www.ulbi.com/product-grid.asp. Last accessed on January 28, 2004. |
|
6 |
Found online at http://www.sionpower.com. Last accessed on January 28, 2004. |
TABLE D-4 Attributes of Metal/Air and Carbon/Air Batteries
|
Attribute |
Lithium/Air |
Aluminum/Air |
Magnesium/Air |
Zinc/Air |
Carbon/Air |
|
Discharge reaction |
2 Li + H2O + 1⁄2O2 → 2LiOH |
4Al + 3O2 + 6H2O → 4Al(OH)3 |
Mg + 1⁄2O2 → MgO |
Zn + 1⁄2O2 → ZnO |
C + O2 = CO2 |
|
Theoretical voltage (V) |
3.40 |
2.70 |
3.10 |
1.60 |
1.00 |
|
Working voltage (V) |
2.85 |
1.10-1.40 |
1.60 |
1.00-1.20 |
|
|
Theoretical specific energy of metal/fuel (Wh/kg) |
13,000 |
8,100 |
6,800 |
1,300 |
9,100 |
|
Specific energy (Wh/kg) |
2,600 (est.) |
1,620 (est.) |
700 |
260 |
2,400 (projected) |
diffusion of oxygen at the cathode makes most metal/air cells suitable only for low-to-moderate specific energy sources.
There is renewed interest by the Army in studying the feasibility of Li/air cells due to their theoretically high energy density and specific energy. The Li/air chemistry is attractive because it combines Li, the electronegative material with the highest capacity, with air. Li has a theoretical specific energy of 13,000 Wh/kg assuming a theoretical cell voltage of 3.4 V, though only 2.85 V is achieved in practice. The Li/air reaction is given in Table D-4. Due to the high reactivity of Li with water, the undesirable competing reaction is
Li + H2O → LiOH + 1/2 H2
Besides the undesirable high reactivity of Li with water, the kinetics of oxygen diffusion through the cathode also limits the Li/air cells, although recent efforts in Li/oxygen rechargeable cells and fuel cells should improve the kinetics of the air cathode in Li/air cells. Following the pioneering work of the EIC group on developing the Li/O2 battery, Read et al. (2003) found that the oxygen solubility in the electrolyte and the electrolyte viscosity had direct impact on the discharge rate of Li/O2 cells (Abraham and Jiang, 1996; Read et al., 2003).
Promising results on Li/air cells were obtained recently at PolyPlus Battery Company.7 Using a novel protective coating on the Li, researchers at PolyPlus were able to demonstrate complete discharge of a 50-μm-thick Li anode at 0.3 mA/cm2 in air. This preliminary result suggests that the corrosion and rate issues with the Li/air system can be resolved, but further research is needed to evaluate the feasibility of scaling up this technology and the stability of the coated Li in extended storage.
Aluminum also has a high specific energy, 8,100 Wh/kg, assuming a theoretical cell voltage of 2.7, though the voltage of Al/air cells is about 1.3 in practice. As with Li/air cells, Al reacts with water in the electrolyte to form Al(OH)3 and hydrogen gas. In practice, Al/air cells can use either neutral (saline) or alkaline electrolyte. The saline electrolytes have low corrosion rates and are used mainly for low-power applications. Al/air cells with alkaline electrolytes are high-rate cells owing to the high conductivity of the electrolyte, but they also exhibit high corrosion rates. Thus, alkaline Al/air batteries are often used as reserve batteries that are activated before use by adding the electrolyte. For portable military applications, saline Al/air cells might be useful in a hybrid configuration as an energy source. Work is needed to develop Al alloys that are less reactive with water and to develop electrolyte formulations in which Al(OH)3 is less soluble in order to minimize loss of electrolyte conductivity.
Magnesium has a specific energy of 6,800 Wh/kg, assuming a theoretical cell voltage of 3.1 in Mg/air cells, although the actual cell voltage is about 1.6 in practice. In alkaline electrolyte, the Mg is passivated by the formation of Mg(OH)2. The insoluble surface film of Mg(OH)2 protects the Mg from further reaction with water but causes a voltage delay, seen also in Li/SOCl2 batteries. Mg/air batteries were not commercialized in the past and were used mostly for undersea, low-rate applications, with 700 Wh/kg demonstrated. Mg/air cells were designed to deliver 3-4 W for one year or longer. Recently, attempts were made by Evionyx to commercialize Mg/air cells.
Zn/air batteries are being considered by the Army for hybrid systems; they are commercially available in button format for use in hearing aids. The theoretical specific energy is 1,300 Wh/kg, but in an operational cell 260 Wh/kg can be expected. The Zn/air system is subject to capacity loss due to leakage, electrolyte dry out, and carbonation, problems that have never been solved sufficiently for the battery to have a long life once it is activated. Those problems have not prevented Electric Fuel from making the 30/60 Ah BA-8180/U Zn/air battery for the Army.
Carbon/air batteries are in theory attractive, because carbon is an energy-dense fuel with a specific energy of 9,100 Wh/kg, and the batteries are safe to carry and non-toxic. Researchers have tried for decades to design devices for the electrochemical conversion of carbon to electricity, and progress has been made toward this goal in recent years. These systems are similar to other metal/air systems in that
|
7 |
Found online at http://www.polyplus.com/. Last accessed on January 28, 2004. |
they use a solid fuel and air as the oxidizer, but they operate at elevated temperatures (>650°C) to fully oxidize carbon to carbon dioxide. Because the final reaction product, CO2, is a gas, these systems do not suffer over time from the buildup of solid reactant products, as is the case with Al, Li, Mg, and Zn systems. The efficiency of the energy conversion process is calculated to be in excess of 80 percent due to the lower heating value (LHV) of carbon. The operating temperature and cell efficiency are a function of the activity of the cell electrodes and of the type of electrolyte, with most designs utilizing electrolytes of either molten carbonate or solid oxide.
Lawrence Livermore National Laboratory (LLNL) has been studying carbon/air batteries and fuel cells as power sources. LLNL has made innovations in its anode and carbon fuel to achieve cells with significant power densities of up to 500 mA/cm2 at 0.8 V, and they project energy values for their system in excess of 2,400 Wh/kg and 900 Wh/L. Because of these preliminary data, carbon-air batteries were identified as a top technology at the ARL/CECOM Energy and Power Workshop of October 2002. However, progress on carbon/air batteries is still at an early stage, and no system has ever been fully designed and integrated, even at the breadboard stage. Key challenges remain—for instance, in the thermal management of the cells, the methods to continuously feed the carbon to the cell anode, and the start-up time of the cells. It is too early (TRL = 2) to accurately predict the contribution of carbon/air systems to the Army, although basic research in this area is worthwhile.
ELECTROCHEMICAL CAPACITORS
There has been a surge of interest in electrochemical capacitors (supercapacitors or ultracapacitors, abbreviated EC), which produce one or two orders of magnitude more energy than traditional electrostatic capacitors. They are of particular interest for use in hybrid systems like those described in Chapter 3. In an electrostatic capacitor, the electrical energy is derived via charge accumulation and stored on the positive and negative plates, separated by a vacuum or a dielectric layer in a nonfaradaic process. In contrast, in a battery the electrical energy is derived from a change in the oxidation state of the active materials and is often accompanied by chemical changes to the structure via a faradaic process. The faradaic process is slow because it involves diffusion of ions into the bulk of active materials. Consequently, batteries usually are operated at lower power than ECs. However, ECs usually have less energy than batteries because most of the charge is stored near the surface layers of the electrodes and not in the bulk of the material. Finally, capacitors usually have a much longer cycle life than rechargeable batteries since the cycling process does not induce chemical or structural changes in the electrode materials. The failure of capacitors usually can be attributed to the breakdown of the dielectric layer or the electrolyte. The characteristics of ECs and batteries are compared in Table D-5.
ECs have electrolytes separating the two electrodes instead of the vacuum or dielectric layer present in electrostatic capacitors. The electrolyte not only serves as an ionic conductor but is also the source of ion separation and accumulation at the electrode/electrolyte interface. The electrolyte can be either aqueous or nonaqueous, but because nonaqueous electrolytes can be used with higher operating voltages, they lead to higher energy densities than aqueous electrolytes. Electrochemical capacitors can also be subdivided into asymmetric and symmetric types. In symmetric ECs, energy storage is nonfaradaic in both electrodes, but in asymmetric ECs charge storage in one of the electrodes is faradaic (or like a battery).
The maximum specific energy and power density for various types of capacitors are listed in Table D-6. Recent advances entailed the use of nano-materials such as nano-Li4Ti5O12 to increase the power density of electrochemical capacitors (Amatucci, 2001). The energy density can be further increased by creating a battery + EC hybrid system with a mixture of activated carbon and a lithiated oxide (e.g., LiCoO2) for the positive electrode and nano-Li4Ti5O12 for the negative electrode (Amatucci, 2003). An energy density in excess of 30 Wh/kg at a power density of 3,000 W/kg can be obtained with such configuration. Such hybrid configurations help to bridge the gap between the energy and power characteristics of batteries and capacitors.
FUEL CELLS
Fuel cells are currently under intense research and development as power sources for a range of applications, including portable power, automobiles, and large-scale power plants. A fuel cell produces electrons via the electrocatalytic reduction and oxidation of an oxidizer and a fuel, respectively.8
For portable power sources, the proton exchange membrane fuel cell (PEMFC), the direct methanol fuel cell (DMFC), and the solid oxide fuel cell (SOFC) are the most attractive. The attributes of these three fuel cell systems (their operating temperatures, electrode reactions, and pros and cons) are given in Table D-7. The electrode reactions and operation of a PEMFC cell are shown schematically in Figure D-2: Hydrogen fuel is oxidized at the anode to protons that flow through a solid polymer electrolyte, and the protons
|
8 |
Numerous texts are dedicated to fuel cells. An excellent basic resource is James Larminie and Andrew Dicks’s Fuel Cell Systems Explained (John Wiley & Sons, Ltd, 2000). Web-based resources include http://www.fuelcells.org/fchandbook.pdf (last accessed on January 28, 2004), http://www.eere.energy.gov/hydrogenandfuelcells/education.html (last accessed on January 28, 2004) and http://voltaicpower.com/FuelCell/Frames.htm (last accessed on January 28, 2004). |
TABLE D-5 Overall Comparison of Electrochemical Capacitor and Battery Characteristics
|
Capacitor Characteristics |
Battery Characteristics |
|
Intrinsically sloping charge and discharge curve. |
Ideally, constant (thermodynamic) discharge or recharge potential, except for Li intercalation systems. |
|
Because of preceding characteristic, has good intrinsic stage-of-charge indication. |
Does not have good intrinsic state-of-charge indication except for Li intercalation systems. |
|
Relatively poor energy density. |
Moderate or good energy density, depending on equivalent weights and electrode potentials of active materials. |
|
Good power density. |
Relatively poorer power density, depending on kinetics. |
|
Excellent cyclability or cycle life due to simple addition or withdrawal of charges (in double-layer type). |
Less cycle life by a factor of 1/100 to 1/1,000 due to irreversibility of redox and phase-change processes in three dimensions. |
|
Internal infrared (IR) due to high-area matrix and electrolyte. |
Internal IR due to electrolyte and active materials. |
|
Little or no polarization, but capacitor may be temperature-dependent. |
Significant temperature-dependent activation polarization (faradaic resistance). |
|
Long lifetime except for corrosion of current collectors and so on. |
Poorer lifetime due to degradation or reconstruction of active materials. |
|
Electrolyte conductivity can diminish on charging due to ion adsorption. |
Electrolyte conductivity can decrease or increase on charging, depending on chemistry of cell reactions (e.g., with lead-acid). |
|
Can be constructed in bipolar configuration. |
Can be constructed in bipolar configuration. |
|
SOURCE: Conway, 1999. |
|
TABLE D-6 Attributes of Electrochemical Capacitors
|
Capacitor Type |
Operating Voltage (V) |
Maximum Specific Energy (Wh/kg) |
Maximum Power Density (W/kg) |
Cycle Life |
Examples |
|
Electrostatic |
Frequency-dependent |
0.01-0.05 |
107 |
>106 |
Mica, Mylar, paper |
|
Electrolytic |
Frequency-dependent |
0.05-0.10 |
106 |
>106 |
Ta2O5, Al2O3 |
|
Symmetric electrochemical capacitor (aqueous) |
0.9-1.2 |
7.16a |
104 |
>105 |
Carbon/carbon |
|
Symmetric electrochemical capacitor (nonaqueous) |
2.0-2.5 |
9.41a |
104 |
>105 |
Carbon/carbon |
|
Asymmetric electrochemical capacitor (aqueous) |
1.3-1.7 |
50.35a |
104 |
>105 |
Ni(OH)2/carbon |
|
Asymmetric electrochemical capacitor (nonaqueous) |
2.5-3.0 |
34.51a |
104 |
>105 |
Carbon/Li4Ti5O12 |
|
aCalculated data from Zheng, 2003. |
|||||
recombine at the cathode via the reduction of oxygen to form water. Because the electrolyte only conducts ions, the electrons are forced through an external circuit and bear the potential of the voltage difference between the electrocatalytic reactions at the cathode and anode, minus ohmic losses. The electrolyte/electrode ensembles are referred to as membrane electrode assemblies (MEAs), with the fabrication of these MEAs having a significant bearing on their efficiency. Each fuel cell operates nominally between 0.5 and 0.9 V, and the system voltage is increased by stacking multiple cells together.
The principle advantages of fuel cells over other energy converter technologies (e.g., internal combustion engines) are the promise of fewer moving parts, longer life expectancy with less maintenance, lower operating pressures and temperatures, elimination of noxious emissions, and higher overall thermodynamic conversion efficiencies of fuel to electricity. The by-product of fuel cells is water, so they will
TABLE D-7 Attributes of Fuel Cells for Portable Power
|
Fuel Cell Type |
Operating Temperature (°C) |
Anode (Fuel) Reaction |
Cathode (Oxygen) Reaction |
Pros |
Cons |
|
Proton exchange membrane fuel cell |
60-80 |
H2 = 2H+ + 2e− |
1⁄2 O2 + 2H+ + 2e− = H2O |
Prototype and commercially available units in a range of sizes (10 W to 1 MW). High power density. Amenable to rapid manufacturing. Rugged. High efficiency. Greatest government and commercial investment. |
Hydrogen storage. Sensitive to poisoning. High cost. Difficult to operate from logistics fuels. |
|
Direct methanol fuel cell |
40-60 |
CH3OH + H2O = CO2 + 6H+ + 6e− |
6H+ + 6e− + 3/2O2 = 3H2O |
Prototypes available. Liquid fuel with no reformer. Significant government and commercial investment. |
High cost. Low efficiency due to materials problems with catalysts and membrane. |
|
Solid oxide fuel cell |
700-1,000 |
On hydrogen: H2 + O2− = H2O + 2e− On logistics fuel: CxH2y + (2x + y)O2− = yH2O + xCO2 + (x + y/2)e− |
1⁄2 O2 + 2e− = O2− (x + y/2)O2 + (x + 2y)e− = (2x + y)O2− |
Large (>1 kW) prototypes available. Tolerant to poisons. Most compatible with logistics fuels. Significant government and commercial investment. |
High temperature management and corrosion. Start-up time for high temperature system. Fragility of ceramic system. |
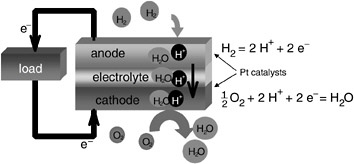
FIGURE D-2 Schematic of proton exchange membrane fuel cell. Hydrogen is catalytically oxidized by platinum at the anode to protons; the protons flow through the solid polymer membrane electrolyte to the cathode, where they reduce oxygen to water on platinum catalysts. The electrons from and to the oxidation and reduction reactions are forced through an external load. Additional components of the fuel cell are interconnects, current collectors, and sometimes gas diffusion layers. Multiple cells are combined to form a stack.
ideally be able to produce this valuable commodity for the soldier in the field. In spite of all of these advantages, however, the practical emergence of fuel cells has been delayed by material science challenges and by the lack of a mature technology and a supporting industrial base.
The selection of the fuel is critical to the building of high-energy portable power sources, given that the fuel is the source of the energy and the fuel cell is merely the conversion system. The specific energy and energy density of various fuels are listed in Table D-8. Hydrogen gas has the
TABLE D-8 Specific Energy and Energy Density of Various Fuels
|
Fuel |
Btu/cf |
Btu/lb |
Wh/L |
Wh/kg |
|
Methane (g) |
183,000 |
221,500 |
1,892 |
142,979 |
|
Methane (L) |
183,000 |
570,000 |
1,892 |
367,937 |
|
Propane (L)a |
724,000 |
19,937 |
7,485 |
12,869 |
|
Butane (L)a |
695,000 |
19,678 |
7,186 |
12,702 |
|
Methanol |
432,000 |
8,700 |
4,466 |
5,616 |
|
Ethanol |
570,000 |
11,600 |
5,893 |
7,488 |
|
Ammonia |
317,000 |
8,000 |
3,277 |
5,164 |
|
Hydrogen (g)b |
56,000 |
52,000 |
579 |
33,566 |
|
Hydrogen (L)c |
229,000 |
52,000 |
2,368 |
33,566 |
|
Gasoline |
876,000 |
19,100 |
9,057 |
12,329 |
|
JP-8 (logistics fuel) |
963,880 |
18,600 |
9,925 |
12,006 |
|
NOTE: Data are in terms of their lower heating value (LHV), or use at temperatures greater than the boiling point of water. For a DMFC operating at 60°C, the higher heating value (HHV) of methanol is 6,088 Wh/kg. The HHV of hydrogen gas is 39,504 Wh/kg at 15°C. aLiquid at 27°C. bGas at 27°C and 3,000 psi. cLiquid at cryogenic temperature (−253°C) and 1 atm. |
||||
highest specific energy of all fuels, but it also has the lowest energy density. The energy density of hydrogen gas improves if it is stored at high pressure (5,000 psi) or in metallic hydrogen storage alloys, as discussed below. Logistics fuels (such as JP-5 and JP-8) are the best choice for energy density, but the fuel must be reformed to hydrogen for portable proton exchange membrane (PEM) systems. There are pros and cons for each fuel cell that must be considered in the context of the particular fuel cell.
All the reactant, product, and thermal management functions of the fuel cell are accomplished with balance-of-plant (BOP) components/systems. Depending on the size and/or complexity of the fuel cell system, BOP components may be intimately integrated into the fuel cell stack or attached as distinct external components. BOP components can be energetically passive or require some parasitic power from the fuel cell stack to operate, so careful attention must go into fuel-cell design to achieve a high-efficiency system.
Proton Exchange Membrane Fuel Cells
Hydrogen PEMFCs are the simplest and most reliable type of fuel cell demonstrated to date. Hydrogen-fueled PEMFCs have been shown to be robust and reliable in real-world field tests, generating power at subzero temperatures up to normal operating temperatures of ~80°C. Work on hydrogen-fueled PEMFCs is currently receiving substantial government and commercial funding in the United States and abroad, as they have been identified as the best fuel cell for automobiles.
Stack development of PEMFCs is fairly mature as a result of large investments by the public and private sectors over the last 10 to 15 years. The electrolyte in PEMFCs is usually a perfluorosulfonic membrane (e.g., DuPont’s Nafion), and new, lower-cost membranes are emerging (e.g., Polyfuel and Gore). The anode and cathode reactions are typically catalyzed by platinum at loadings of 0.2 mg/cm2, but these loadings should decrease as research in this area progresses. The membranes and catalysts must be appropriately humidified, and their performance suffers when they become too dry or wet.
The most advanced portable PEM/H2 systems use compressed hydrogen to simplify the fuel issue. But even when operated on pure hydrogen fuel, PEMFC systems require extensive control systems for optimum operation. Figure D-3 shows a mass flow diagram of a hydrogen-fueled, field-tested portable power system, the Ball Aerospace PPS-50 (TRL = 7). The fuel cell stack was obtained from H-Power, a now-defunct small company, although there are now other suppliers of 50-W fuel cells (e.g., Protonex and Neah). A complex BOP architecture is expressed for this system in order to provide greater versatility for end users wishing to operate the fuel cell system in as broad a range of environments as possible without retrofit. The PPS-50 fuel cell system is electronically controlled with a microcontroller utilizing various sensors for monitoring stack voltage, current, temperature, and so on. The system is designed to manage hydrogen delivery, oxidant air feed, cooling air for heat removal, and product water from the stack (Ball Aerospace, 2003).
The weight of the system is 2.9 kg, its volume is 4.26 L, and its demonstrated specific energy is 540 Wh/kg when running a 6 percent by weight hydrogen solution at 50 W for 72 hr (3,600 Wh at 6.6 kg). A 24-hour mission at 50 watts would have a specific energy of 286 Wh/kg (Ball Aerospace, 2004). Lower power (<20-W) hydrogen PEM
fuel cell systems have also been developed by Ball Aerospace and others, thereby demonstrating the scalability and versatility of the technology.
Hydrogen Sources
The successful operation of a PEM/H2 system is contingent on the identification and integration of a suitable hydrogen source. Whereas the PEMFC stack technology itself may be at TRL 7 to 8, the TRL levels of the hydrogen sources can be as low as 2, which drags down the readiness of the whole system. The integration issues, discussed below, keep some of the technologies from being implemented on a small scale for the soldier. The military must also consider any logistics or safety burdens that might be imposed by the use of the hydrogen source.
There are two types of hydrogen source: Type 1, hydrogen storage sources; and Type 2, hydrogen generation sources. Additionally, hydrogen sources are characterized by their need for fuel cell system resources such as electricity, heat, water, and so on. A Type 1 or Type 2 hydrogen source that is independent of the fuel cell system is termed a passive hydrogen source. Hydrogen sources requiring fuel cell resources (heat, electricity, water, etc.) are considered active and/or coupled. The characteristics of several active and passive hydrogen sources are detailed in Table D-9.
Hydrogen storage systems include pressurized tanks and metal hydrides. A pressure vessel is a passive hydrogen storage system where the hydrogen exists in its diatomic form (H2) and can be extracted via a pressure differential and without the application of heat. Pressurized hydrogen is convenient and follows dynamic fuel cell loads. State-of-the-art composite pressure vessels with valve and regulator assemblies routinely demonstrate better than 5 weight percent (of system weight) H2 gas storage when sized for large PEMFCs (e.g., ~50-kW systems for automobiles). Owing to the larger surface area:volume ratio in smaller systems and the relatively larger weight burden of the gas regulator, 5 weight percent hydrogen storage is not available for portable power sources, although it might be possible with development effort. Compressed hydrogen has been plagued by the perception that it is unsafe, but the Department of Energy is in the process of assessing safety issues, as recommended in a recent report (NRC, 2003).
Cryogenic hydrogen storage systems, such as the space shuttle’s supercritical hydrogen storage tanks, demonstrate 29 weight percent H2 and are active storage systems because heat must be applied to remove the hydrogen as boil-off gas.
TABLE D-9 Dependence of Select Hydrogen Sources on Fuel Cell Resources
|
|
Specific Energy Ideal (Wh/kg) |
|
Dependence on Fuel Cell Resources |
|||||
|
Energy Source |
Net Systema |
Theoreticalb |
Fuel Processor Efficiencyc |
H2O |
H2 |
Electric |
Heat |
Central |
|
Liquid H2 (PRSA at 29%) |
4,340 |
9,477 |
0.96 active |
|
|
x |
x |
x |
|
HP H2 gas (8,500 psi, 5.3%) |
826 |
1,732 |
1.00 passive |
|
|
|
|
|
|
Metal hydride (2%) |
312 |
654 |
1.00 active |
|
|
|
x |
|
|
LiAlH4 + xNH3 → yH2 (AHHG) |
2,071 |
4,343 |
1.00 passive |
|
|
|
|
|
|
AlH3 + ∇ → Al + 3/2H2 (thermal decomposition) |
1,677 |
3,662 |
0.98 active |
|
|
x |
|
x |
|
C(SiH3)4 + 6H2O → 12H2 + C(SiO3/2)4 (organosilane) |
||||||||
|
With own H2O |
1,464 |
3,233 |
0.95 active |
|
|
x |
|
|
|
With fuel cell H2O |
2,486 |
5,794 |
0.90 active |
x |
|
x |
|
x |
|
LiH + H2O → LiOH + H2 (LiH hydrolysis with own H2O) |
1,209 |
2,537 |
1.00 passive |
|
|
|
|
|
|
With fuel cell H2O |
3,949 |
8,283 |
0.90 active |
x |
|
x |
|
x |
|
LiAlH4 + 2H2O → LiAlO2 + 4H2 (LiAlH4 hydrolysis) |
||||||||
|
With own H2O |
1,665 |
3,564 |
0.98 active |
|
|
x |
|
x |
|
With fuel cell H2O |
2,979 |
6,943 |
0.90 active |
x |
|
x |
|
x |
|
2NH3 + ∇ → N2 + 3H2 (NH3 cracking) |
2,213 |
5,802 |
0.90 active |
|
x |
x |
|
x |
|
CH3OH + H2O + ∇ → CO2 + 3H2 |
||||||||
|
MeOH/H2O reforming |
1,600 |
3,948 |
0.85 active |
|
x |
x |
|
x |
|
With fuel cell H2O |
2,353 |
6,168 |
0.80 active |
x |
x |
x |
|
x |
|
|
2,317 |
6,088 |
0.90 active |
x |
|
x |
|
x |
|
NOTE: PRSA, power reactant storage assemblies; AHHG, ammonia hydride hydrogen generator; HP, high pressure; DMFC, direct methanol fuel cell. aWeight of containment, catalyst, coreagents, and converter/reactor plant not included in specific energy calculation. Net system energy yield assumes 100 percent chemical yield from reaction, with subsequent energy losses due to fuel cell voltage of 0.7 V, fuel cell system and generator balance-of-plant efficiency, and fuel utilization efficiency. bSame assumptions as in footnote a except that all converter efficiencies are 1.0 and fuel cell voltage is 1.229 V. cImpact on fuel cell balance-of-plant only. |
||||||||
Metal hydride storage systems are active because heat is required to desorb or release hydrogen from the hydride. Metal hydride systems operated at room temperature can release more than 2 percent by weight H2, and those used at higher temperatures can release more than 4 percent by weight H2. Rechargeable metal hydrides have limited cycle lifes of 100 to 1,000 charge/discharge cycles due to decrepitation of the metal, which causes packaging problems. Likewise, many high-performance composite pressure vessels and cryogenic storage systems also have limited cycle life and must be inspected and recertified regularly. As in the case of compressed hydrogen, the actual weight percent of hydrogen stored will decrease with the size of the hydrogen storage system.
Hydrogen generation sources include chemical hydride generators, fuel reformers, and electrolyzers, all of which produce hydrogen gas by a chemical reaction. Elements occupying the first three rows of the periodic table (before the transition elements) form hydrogen-rich storage compounds and are frequently considered practical fuels for hydrogen-generating systems. The choice of which hydrogen-bearing compound to employ when engineering a hydrogen source is based on the gravimetric and volumetric hydrogen density of the compound. Figure D-4 shows the gravimetric density of hydrogen in several light-element compounds, including compounds containing carbon, nitrogen, oxygen, silicon, and other light metal atoms, that are traditional candidates for hydrogen generators. The hydrogen-generating compound is selected not only on the basis of its energy content, but also on the basis of engineering considerations such as handling, storage, and mixing approaches and the releasability of hydrogen from these compounds with low energy penalty and low system complexity.
The reformation of oxygenated organic fuels such as methanol with water (a mildly endothermic process), the thermal decomposition of light metal hydrides such as AlH3, and the cracking of ammonia all require some energy from either the fuel cell or the combustion of hydrogen. These sources of hydrogen are generally controllable at the expense of additional system complexity, cost, and—often—reliability. The reforming could be carried out in conjunction with a hydrogen separation membrane to yield pure hydrogen with no nitrogen dilution. The advantage of methanol and ammonia is that tank pressures are low. Ammonia has the additional attribute of having only hydrogen and nitrogen products, the latter of which would be rejected by a membrane.
Reforming hydrocarbons—a source of hydrogen for PEMFCs—is even more challenging. Because these fuel cells operate at low temperatures (60-80°C) and temperatures above 400°C are needed for hydrocarbon reforming,
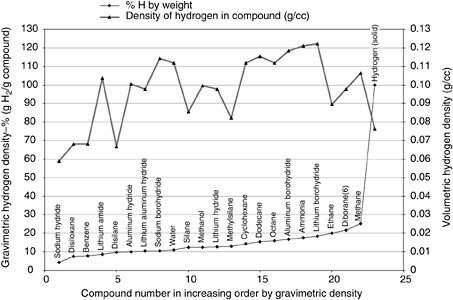
FIGURE D-4 Specific gravimetric hydrogen densities of select compounds.
an additional reformer is needed to convert hydrocarbon-based fuels to hydrogen. The additional reformer adds significantly to the weight and size of the small system. Water is required in reforming to shift carbon monoxide (CO) impurities to CO2, both for human safety and to prevent the Pt catalysts from being poisoned or deactivated by the CO. Sulfur impurities, which are also deleterious to the Pt catalysts, must also be removed with sorbents. The requirements for fuel reforming were covered in detail by JASON (2003). Overall, reforming decreases the energy content of the fuel and adds considerable size and weight to the fuel cell system, so much so that the PEMFCs operating on logistics fuel were not considered to be a viable technology for portable power (see Chapter 2).
In the future, reforming for PEMFCs might be solved with compact microchannel or MEMS reformers, particularly for methanol solutions. Microchannel reactors are at the heart of a compact methanol steam reformer that has been developed by researchers at PNNL for the Army. The PNNL team has worked on microchannel reformers for a variety of fuels (Hu et al., 2003). A fully packaged microchannel reformer system for methanol, including CO cleanup, heat exchangers, and integration with a 150-W PEMFC stack, is now being evaluated by CECOM.
Recent research also reports that ethanol may be reformed at high conversion rates (Deluga et al., 2004), although this technology is presently too immature to make recommendations. Flammability and safety are the key issues that must be addressed for the DOD to consider alcohol-based fuels.
Direct Methanol Fuel Cells
DMFC technology avoids problems with fuel storage, because the catalysts at the fuel cell anode can directly oxidize liquid methanol, eliminating the need for complicated fuel storage components. DMFCs are a derivative of PEMFC technology in that they both use solid electrolytes (typically Nafion) and have similar Pt catalysts at the cathode side. At the anode, they utilize platinum-ruthenium (Pt-Ru) catalysts, which work for the methanol oxidation reaction in Table D-7. However, because the catalysts suffer from efficiency losses, or overpotentials, DMFCs typically operate at 0.3 V less than hydrogen-fueled PEMFCs (0.4 V vs. 0.7 V), making the thermodynamic efficiency of the DMFC cell chemistry low (32 to 40 percent). The practical efficiency is even lower as a result of the technological issues discussed below, among them methanol crossover.
Key technological issues in DMFCs are their high catalyst loadings, methanol crossover, and water management. DMFC anodes have 10-fold higher catalyst loadings per unit area than hydrogen-fueled PEMFCs. The cost of catalyst reflects the price of precious metal futures and makes devices delivering more than 100 W prohibitive in cost. Promising research is under way on how to replace the Pt-Ru catalysts with catalysts having little or no precious metal content, but a practical solution has not yet been developed.
Methanol crossover refers to the leakage of fuel from the anode compartment to the cathode. This is a serious problem, because the methanol that reacts with oxygen at the cathode produces no electricity, decreases the activity of the catalysts at the cathode, and creates an increased thermal burden on the overall DMFC system. Methanol and other low-weight alcohols cross through polymeric membranes because methanol resembles proton-water complexes (or hydronium ions) at the molecular level, allowing it to be dragged along with the ions conducting through the membrane. Strategies have been developed to use a selective gas diffusion layer (or other means) to control the rate at which methanol is supplied to the anode, thereby reducing or eliminating the amount of excess methanol that can permeate to the cathode (Grubb, 1970; Ren et al., 2001). Such approaches have led to fuel utilization rates of better than 93 percent, but they also limit the power density of the membrane or stack.
Alternatively, numerous researchers are investigating new membranes that are less permeable by methanol while retaining protonic conductivity. New polymers are being developed at Virginia Tech, PolyFuel, Inc., and the Gas Technology Institute,9 several of which have been shown to conduct three times less methanol than conventional Nafion membranes (Hickner et al., 2002; Cooper and Cox, 2003). The new materials are still being evaluated for their ability to withstand long-term exposure to variations in methanol concentration, temperature, and current flux without degradation in performance, so their practical impact is not yet known. Membranes that are stable in high methanol concentrations and exhibit lower crossover will also allow the system to be stored at lower temperature without concern for freezing of the fuel cell stack (a 5 molar solution of MeOH freezes at about −10°C, while an 8 molar solution freezes at −21°C).
Water management is a concern because of the large amount of water involved in the DMFC reaction (see Table D-7). Extensive BOP is required to condense and store the product water and to mix pure methanol with the stored water to obtain methanol concentrations that are optimized for the membrane and stack (see Figure D-5). Product water management is further complicated by a phenomenon known as the electro-osmotic drag (EOD) of water across the membrane from the anode to cathode. Approximately 3 water molecules are dragged across the membrane for each proton conducted, so as many as 18 water molecules can be dragged from the anode to the cathode for every molecule of methanol oxidized. The rapid depletion of water at the anode could result in flooding of the cathode if active water recovery
|
9 |
Found online at http://www.gastechnology.org. Last accessed on January 28, 2004. |
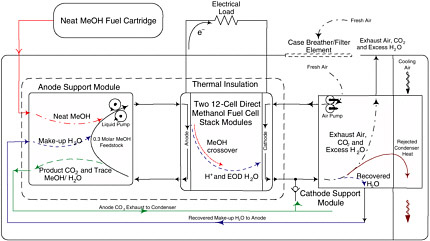
FIGURE D-5 Schematic of Ball Aerospace 20-W DMFC energy converter showing the active fuel and water management BOP subsystems used to maximize the specific energy yield of methanol above 2.0 Wh/g. SOURCE: Ball Aerospace.
from the cathode back to the anode is not practiced. Electroosmotic drag has also been attributed to methanol crossover, so the lower the EOD, the smaller the methanol crossover. PolyFuel has demonstrated EOD coefficients of less than one (one water per proton) for MEAs based on its proprietary Z1 membrane and a corresponding reduction in methanol crossover to one-third that of Nafion (Cooper and Cox, 2003). Recently, novel low power (<5 W) DMFC concepts demonstrated by Mechanical Technology Inc. (MTI) MicroFuel Cells have broken this paradigm by demonstrating a passive laboratory DMFC operating on 100 percent methanol at the anode.
A 20-W DMFC system, the DMFC-20, has been developed by Ball Aerospace in collaboration with Los Alamos National Laboratory for the DARPA Palm Power program. The fully packaged system (TRL = 6) is now being tested by the U.S. Army and others. The diagram for the system is shown in Figure D-5. The system utilizes recirculated mixtures of 0.250 to 1.00 molar solutions of methanol (MeOH) and water. A fresh feedstock fuel mixture is circulated through the anode, sweeping out product CO2 in the fuel cell anode exhaust. Careful systems design must consider mixed phases (gases and liquids) simultaneously managed by the system. Orientation-insensitive operation of the DMFC-20 is achieved, but it is complicated by the presence of liquids and gases in both the anode and cathode loops of the system. This DMFC-20 exhibits a specific energy yield approaching 2.0 Wh/g MeOH (0.33 (ηS) × 6.088 Wh/g), and improvements in the catalyst and membrane technology might increase the yield to perhaps 3.0 Wh/g MeOH.
The energy density of a fully integrated 20-W system is 540 Wh/kg when equipped with two fuel canisters, sufficient for a 72-hour mission. This operational configuration weighs 2.95 kg, has a volume of 2.93 L, and provides 1,600 Wh of energy. The energy converter alone weighs 1.75 kg and has a volume of 1.56 L. The DMFC-20 system with an 800-Wh fuel canister is shown in Figure D-6.
Additionally, Ball Aerospace has developed a 60-W DMFC, and Giner Electrochemical Systems has developed 50- and 150-W units. Smart Fuel Cells of Germany also reports the development of a portable DMFC.
Other DMFCs are being developed for operation at 0.5 to 2.5 W specifically as power modules for cell phones. In this power regime, both Polyfuel and MTI have demonstrated DMFC power source prototypes (TRL = 5-6) suitable for powering cell phones in the 0.5- to 2-W class. All of the 0.5- to 2-W systems in this power class are air-breathing and employ passive fuel management. Control electronics are typically employed to perform voltage boosting and internal battery recharging. The first Polyfuel prototype was fitted directly to the back of a Nokia cell phone and was able to power the phone in standby and receive mode.10 An

FIGURE D-6 A DARPA/Ball Aerospace and Technologies operational DMFC-20 (20-W direct methanol fuel cell with a hard-packaged 500-cc fuel canister). SOURCE: Ball Aerospace.
onboard secondary battery or ultracapacitor is used to provide peak power (2 to 3 W for transmit mode). Power systems of this type are not presently part of PolyFuel’s business model but serve to demonstrate the use of its alternative non-Nafion membrane for DMFC applications. MTI MicroFuel Cell,11 however, has adopted a business model for the commercialization of small 0.5- to 5-W DMFC power sources for cell phones and small personal electronics such as personal digital assistants (PDAs). Several of the company’s prototypes are displayed on its Web site at http://www.mtimicrofuelcells.com/technology/prototypes.cfm.12 MTI demonstrated several of these systems at the 2003 fuel cell seminar, held November 3-7, 2003, in Miami Beach, Florida. The demonstrated 0.5-W continuous and 2-W peak operational MTI prototype is 0.09 L and weighs ~0.09 kg (Acker, 2003). The purported energy yield from pure methanol is 1.0 Wh/cc MeOH (1.25 Wh/g MeOH); however, the current systems operate on a 50 percent water/methanol mixture stored in small 20-cc fuel cartridges. For a 72-hour mission, four of these cartridges would be required for 40 Wh of energy (36 Wh for the mission). The total 72-hour mission system weight is calculated to be 0.165 kg, yielding a specific system energy of 242 Wh/kg (also 242 Wh/L, as this system is about as dense as water). MTI has also demonstrated a larger 5-W DMFC configured in the form factor of a BA5590 size battery of 0.9 L (2 in. × 4.4 in. × 5 in.) (Acker, 2003). The system also includes a secondary battery able to provide 50-W peak power surges for an undisclosed duration. This system employs an active air mover (fan) for cathode air handling and cooling. The converter weight is estimated at 1 kg and includes an internal 100-cc, 50 percent water/methanol mix fuel cartridge. The energy yield from this unit is also purported to be 1.0 Wh/cc MeOH. The specific energy estimate for a 360-Wh mission (72-hour mission at 5 W average power) is 212 Wh/kg, requiring eight fuel cartridges (400Wh/1.88 kg). MTI announced in an October 2003 press release that it had achieved passive operation on 100 percent methanol at the anode (MTI, 2003). This allows neat methanol to be used in the fuel cartridges, thereby increasing the specific energies given above by as much as 50 percent.
The committee also obtained data from Motorola Labs on performance specifications for its 2.5-W DMFC.13 The
|
11 |
Found online at http://www.mtimicrofuelcells.com. Last accessed on January 28, 2004. |
|
12 |
Last accessed on January 28, 2004. |
|
13 |
Data provided to committee members Jeff Schmidt and Karen Swider Lyons by Jeanne Pavio, Manager, Fuel Cell Development, Motorola Labs. |
energy converter operates on pure methanol, yielding ~0.8 to 0.9 Wh/g MeOH, and weighs 350 g without fuel. Here, too, the energy converter density is about 1 g/cc, so the converter volume without fuel is ~350 cc. The calculated specific energy of this system for a 144-Wh mission (72 hr at 2 W) is 262 Wh/kg (144 Wh/0.55 kg).
The efficiency of the low-power (5 W or less) DMFCs is less than that of higher power (20 W and higher) systems, because the former typically use passive BOP components and the latter use active BOP management. The smaller DMFC systems usually have passive air cathodes and noncirculating liquid, or a small fan that passes air over the cathode to help evaporate product water and replenish oxygen. The fuel is a premixture of methanol and water supplied by a small cartridge mechanically pressurized by a spring or elastomer. These low-power systems operate at reduced temperatures, thereby lowering the operating voltage of the cell, usually by 0.3 V or less. The use of premixed fuels, the lower voltages of cell operation, and the need for voltage-boost electronics reduce the overall efficiency of the system. Hence the specific energy yield of the <5-W DMFCs is typically less than 1.2 Wh/g when pure methanol is the fuel and less than 0.6 Wh/g when premixed methanol is the fuel. Advantages of the low-power, passive DMFCs are their limited complexity (eventually translating to lower cost) and quietness.
By comparison, the energy yield from a fully active DMFC-20 is typically between 1.5 and 2.0 Wh/g on pure methanol. The two- to threefold improvement in specific energy yield in the Ball Aerospace DMFC-20 system is largely due to the system’s active management of reactants and products by BOP components. At larger sizes, BOP reactant and product management become more efficient overall, and they become smaller relative to the overall system size and weight. BOP components for >20-W systems are commercially available or could be developed within a relatively short time, making them immediately suitable for systems development and integration. In the future, it is possible that BOP components that can improve the specific energy yields of smaller systems may be realized by MEMS technologies or mesoscopic machines/devices.
Solid Oxide Fuel Cells
Solid oxide fuel cells (SOFCs) have been in development in the United States, with support from DOE, since the 1960s, predominantly for use as terrestrial power plants. SOFCs were not considered as a possible power source in Energy-Efficient Technologies (NRC, 1997). However, in the last few years, several R&D efforts, many of them under the DARPA Palm Power program, have focused on developing man-portable SOFCs for military and commercial applications (1 to 100 W). The successful operation of SOFCs is dependent on robust materials and cell designs. A general review of the findings can be found in: Ceramic Fuel Cells (Minh, 1993). DOE’s Fuel Cell Handbook also gives a thorough review of SOFCs (DOE, 2002).
The clear advantage of SOFCs as portable power sources for the military is their ability to operate on hydrocarbons with little or no reforming. Because they operate at high temperatures (600-800°C), one can take advantage of internal reforming, whereby the fuel is oxidized by the reactants at the fuel cell anode. This section focuses on issues faced by the operation of small SOFCs on logistics fuels (JP-8) or other hydrocarbons.
Hydrocarbon fuels can be oxidized to CO2 and H2O at the SOFC anode, as summarized in Table D-7. If there is insufficient oxygen for complete oxidation of the hydrocarbon, CO may form, which may also be used as a fuel:
CO + 1/2O2− = CO2 + e−
At high temperatures, particularly in the presence of metal catalysts, CO undergoes reversible disproportionation to coke (solid carbon) and carbon dioxide:
2CO = C(s) + CO2
Coke formation is possible either at the SOFC anode as hydrogen and fuel are consumed and the gas equilibrium changes, or in the fuel exit lines as an unreacted fuel in the exhaust cools. If coke is not controlled, it can adversely affect the performance of the cell by blocking catalyst surfaces and fuel passages. Coke formation is often prevented by adding water to shift the reaction to CO and H2:
C(s) + H2O = CO + H2
For military systems, it would be ideal to not have to carry additional water for operation of the fuel cell, which would significantly penalize the energy density of the system, so methods for the suppression of coke formation are critical. It may be possible to efficiently recover some water from the anode exhaust (see Table D-7), but steam:carbon ratios of 2 or 3 are typically required to prevent coking, necessitating an additional water source when heavy hydrocarbons are reformed. Industrial processes often use steam:carbon ratios of 5 to 8 to extend the lifetime of the reforming catalysts. A possible solution is to carry a small amount of excess water, which can be used for internal reforming and then recuperated. This approach is being exploited by Altex Technologies Corporation to produce an efficient reformer for JP-8 (Ball Aerospace, 2004).
Long-chain hydrocarbons, in either the feed or the exit gas, are also prone to decomposition into coke owing to thermal degradation, and it is not clear if JP-8 can be successfully fed directly into the hot fuel cell. JP-8 can be converted to lighter hydrocarbons, which are less prone to thermal decomposition into coke, either by steam reforming or in a catalytic partial oxidation (CPOX) reforming unit. CPOX
reforming units typically have Rh-based catalysts and operate at ~700°C. The CPOX units are compact and lightweight, but the overall system suffers from a drop in system efficiency because part of the fuel is oxidized in the CPOX unit before it enters the SOFC. As a result, it is diluted with nitrogen from the air and has less energy content.
Fuel processing might also be accomplished for future portable SOFCs with MEMS-based microchemical systems. Army-funded MURI programs on high-temperature microchemical systems for fuel reforming are being carried out at the Massachusetts Institute of Technology and the University of Illinois at Urbana-Champaign (UIUC). At MIT, novel microreactors and heat exchangers are being created for fuel combustion and reforming. Researchers recently reported on a microchemical reactor for butane and ammonia processing (Arana et al., 2003). The UIUC program has developed robust alumina microburners (Raimondeau et al., 2003) and penny-sized reactors that can produce the equivalent of 40 W of H2 from NH3 (Paur, 2003). If such systems can be proven reliable, efficient, and inexpensive, they may be useful as lightweight reforming components. Work by the University of Pennsylvania (Park et al., 2000) has shown direct oxidation, or internal reforming, of various long-chain hydrocarbons on the laboratory scale using new ceria/copper catalysts. The hydrocarbons are fed directly into the SOFC anode, where they are oxidized, eliminating the need for a CPOX unit to break down the hydrocarbons and water for internal reforming. The development of these systems is still preliminary, and the catalysts may face stability problems at temperatures over 700°C. A CPOX unit may still be needed with JP-8 fuel, as it may not be possible to feed the heavy hydrocarbons into the SOFC without thermal decomposition. More research is needed to determine whether the laboratory observations can be scaled into a practical military system. A system operating via direct oxidation on logistics fuels would have a clear military advantage as it would have no need for a prereformer or reformer.
Metal dusting of stainless steels, or corrosion of Fe- and Ni-containing materials due to the formation of metal carbides, may also be a failure mechanism in compact SOFCs operating on logistics fuels, as it can occur in gas streams rich in carbon monoxide and hydrogen between 425°C and 815°C.
Lastly, the sulfur in logistics fuels may react deleteriously with the catalysts in the SOFC anode and decrease performance. Although SOFC anode catalysts can tolerate up to 50 ppm of sulfur in the gas stream, logistics fuels typically contain more than 10,000 ppm sulfur. There are several options for solving the sulfur problem: The sulfur tolerance of the anodes can be improved, the sulfur can be removed by adsorption or scrubbing methods, or the SOFCs may be designed to operate on prepackaged, sulfur-free fuels. Sulfur-tolerant anodes would be ideal but have remained elusive to date. Adding a sulfur sorbent to the system is practical but increases the weight and complexity. The removal of sulfur with sorbents is being explored by researchers at Penn State, in collaboration with Altex Technologies Corporation. Use of a prepackaged fuel may be ideal for SOFCs but introduces logistics issues similar to those for primary batteries, albeit less severe, since there is a 10-fold higher energy density in the fuel compared with batteries.
Small SOFC systems do not enjoy the high efficiencies (>50 percent) reported for large SOFC systems, because the heat produced from the hydrogen oxidation and oxygen reduction reactions is insufficient to maintain the heating temperature of the fuel cells, necessitating the burning of fuel to keep the fuel cell stack hot. For a 20-W system, for example, 50 W of thermal energy might be needed to maintain the SOFC stack at 800°C. However, the heat generated by the electrochemical reactions might be only 25 W, leaving a shortfall of 25 W. This might be met by burning unutilized fuel, but it might also require the burning of additional fuel. Therefore, it is best to assume practical efficiencies on the order of 30 percent for small SOFCs operating on a liquid hydrocarbon fuel. If a CPOX unit can be avoided, for instance with direct oxidation, the system efficiency might be higher (~35 percent).
SOFCs targeted for operation at 2, 20, and 100 W and 1 to 3 kW are likely to have vastly different designs. Devices to produce 2 W cannot be fabricated except—possibly—using a MEMS-type design. Such an approach is the subject of an Army-funded multiuniversity research initiative (MURI) program at MIT (TRL 1 to 2) and is being pursued by Lilliputian Systems of Woburn, Massachusetts. The development of MEMS-based SOFCs is very high risk, because issues such as high-temperature seals (for managing the thermal mismatch between the silicon-based MEMS structure and SOFC materials) and the thermal engineering are critical. It is also not clear if long-term SOFC operation can be achieved, given the possibility of failure of seals and/ or poisoning of the YSZ electrolyte by silicon from the MEMS fuel-cell frame and traces of silicon from the fuel reformer.
Several 20-W SOFC designs are under development for the DARPA Palm Power program, and it is apparent that the designs and beliefs that are upheld for large terrestrial systems apply differently to these mesoscale systems. Note that portable SOFCs are still in the early demonstration stage (TRL 2 to 4), so much of the discussion below is based on projections.
The 20-W SOFC discussed in Chapter 2 is the microtubular-based system by Adaptive Materials, Inc. (AMI) of Ann Arbor, Michigan. Attributes of the system are summarized in Table D-10. The fuel cell stack comprises microtubules about 1.7 mm in diameter and 12-14 cm in length. The active SOFC part of each tube is 6 cm long, with several millimeters of the tube being dedicated to an integrated CPOX unit for breaking down the fuel (butane) to light hydrocarbons. The gas inlets for the tubes are at 200°C, enabling low temperature seals. The microtubular design is
TABLE D-10 Characteristics of Butane-Fueled 20-W Solid Oxide Fuel Cell System by Adaptive Materials, Inc.: Breadboard Versus Projected Attributes
|
Characteristic |
Laboratory Achieved, Breadboard |
3-Day Mission, Projected |
|
Specific energy |
340 Wh/kg (10 days) |
1,000 Wh/kg |
|
Power density |
1.8 W/cm2 of tube |
|
|
Specific power |
4.7 W/kg (wet); 200 W/kg (stack) |
13.9 W/kg (wet) |
|
Fuel |
Butane, 2.0 kg |
Butane, 0.4 kg |
|
Signature |
|
Thermal exhaust plume less than soldier’s breath |
|
Cost (initial) per Wh |
Not known |
Not known |
|
Fuel consumption |
27.8 g/hr |
6.25 g/hr |
|
System weight, dry |
2.3 kg |
1.0 kg |
|
System weight, wet |
4.3 kg |
1.45 kg |
|
Efficiency at rated power |
6% (20-W continuous load) |
29% |
|
Altitude impact |
85% at 20,000 ft (demonstrated) |
>90% at 15,000 ft |
|
Form factor |
|
4 × 4 × 8 in. (approximately) |
|
Life/cycles |
Demonstrated multiple start/stop cycles |
Minimum 250 start/stop cycles |
|
Maintenance |
|
Minimal, simple module replacement |
|
Environment |
No underwater |
No underwater |
|
Orientation dependence |
|
None |
|
Shock/vibration |
|
100% survival after 10 ft drop onto concrete |
|
Technology readiness level |
4 |
|
|
Start-up time |
<3 min |
|
|
Fuel utilization |
85% |
|
also resistant to thermal shock, allowing the AMI system to be started in 1 to 3 min. As of February 2004, AMI had demonstrated a packaged 20-W SOFC prototype system that is thermally self-sustaining on butane fuel. The demo systems comprise a fuel cell stack, insulation, recuperator, electronic controls, and a battery (TRL = 4). The exhaust temperature of this packaged system is 40 to 50°C, and the package has survived 40 G drop tests (Crumm, 2004). It can also be stopped and started multiple times with no detrimental effects on performance. The system still suffers from inadequate thermal insulation and heat recuperation, so the power efficiency is presently about 13 percent and the energy density is 510 Wh/kg. With improvements in the BOP and systems engineering, AMI expects to build stacks with a rated specific energy of 1,000 Wh/kg for a 72-hr mission and 2,500 Wh/kg for a 240-hr mission.
SOFC systems of 75 to 200 W would be attractive as battery chargers if they could be run directly on logistics fuels. For these intermediate-sized systems, planar designs might be more practical than microtubular designs, as the active area of the SOFC plates can be somewhat enlarged while minimizing the resistance from interconnects. MSRI of Salt Lake City, Utah, has successfully demonstrated a breadboard ~100-W planar SOFC that operates on hydrogen, methanol, and ammonia. As developed, it was too heavy to be used as a portable power source, but it is now being integrated into a lightweight 75-W system by Mesoscopic Devices of Broomfield, Colorado. Operation on a sulfur-containing logistics fuel has not yet been demonstrated. Like the AMI system above, the MSRI planar stack can also be stopped and started with little decrease in the fuel cell performance; unlike larger SOFCs, small fuel cells can withstand thermal stresses.
SOFCs may also be developed as 1- to 3-kW systems. ITN Energy Systems estimates that a 5-kW system would weigh 15.5 kg dry and 118 kg with enough fuel for 72 hr (based on 40 percent net system efficiency). The dry weight of the system is competitive with PEMFC technology, as shown in Chapter 2, and steps could be taken to further reduce the weight of the stack. A hydrocarbon-fueled 2-kW SOFC is commercially available from Acumentrics,14 but it has not been optimized for compactness.
SMALL ENGINES
Energy-Efficient Technologies for the Dismounted Soldier (NRC, 1997) considered small engines as a potential energy source for the dismounted soldier but did not consider them for specific applications. In general, the conclusions
|
14 |
Found online at http://www.acumentrics.com. Last accessed on January 28, 2004. |
drawn in that report are still valid, and the following is intended to upgrade the material in that study.
Small Internal Combustion Engines
Numerous small engines in the commercial sector may be adaptable to military needs. These range from hobby engines such as those used by model airplane enthusiasts to the more common types used in gardening tools such as leaf blowers and string trimmers. There are also a variety of advanced engine concepts, such as the micro internal combustion swing engine being developed at the University of Michigan (Crumm, 2004).
A survey of manufacturers’ technical data for hobby engines (see, for example, OSengines.com and hobbyhobby.com) shows that the best engines have a specific power of 2 to 3 kW/kg for both two- and four-cycle engines, with the four-cycle engines being slightly heavier. In general, fuel consumption is 0.3 to 0.4 kg/kWh. Some manufacturers are introducing electronic fuel injection to produce an engine with lower fuel consumption. There is no data in the technical literature on the reliability of such engines; in general, hobbyists use hobby nitro fuel with 10-15 weight percent oil.
In the small engines developed for the consumer market, both two- and four-cycle engines are available. In general, two-cycle engines are about 10-15 percent efficient. Fourcycle engines are up to 25 percent efficient for mechanical energy. Both routinely use regular unleaded gasoline and some have been converted to run on other fuels. The specific power of these engines is about 0.45 kW/kg, with specific fuel consumption 0.2-0.3 kg/kWh, depending on operating parameters, among other things. Considerable data on reliability exist for these engines, and thousands of hours of life are possible with routine maintenance.
A variety of fuels can be used, ranging from methanol to diesel. Several companies, such as D-Star, Eagle Development,15 and Foster-Miller, Inc.,16 are taking hobby motors and motors designed for consumer tools, both two- and four-cycle versions, and converting them to run on the diesel cycle. In general, the fuel is a mixture of ether and kerosene. However, D-Star is using an atomizer to precondition the fuel and has successfully run converted engines on JP-8 for several hours. The Naval Surface Warfare Center Carderrock Division has run a Davis engine for longer than 20 hours on JP-8, with little or no engine fouling. There are insufficient data from these tests to estimate reliability.
To be usable for soldier power, suitable electrical generators must be used. In general, small permanent-magnet alternators have specific power of about 140 W/kg. Lightweight aircraft alternators have specific power of about 200 W/kg for units in the 500-W class. Both types can be over 80 percent efficient in converting kinetic energy to electricity. Taking the converter and engine efficiencies together, it is possible to achieve a maximum system efficiency of 20 percent or more from fuel to electricity.
Small engines typically have problems with durability and reliability. Use of battlefield logistics fuels presents problems for all small engines. In many cases heavily loaded bearings and sliding surfaces require lubrication. Logistics fuels typically have low lubricity, and this requires the use of lubricating additives. These additives seldom burn completely, so they contaminate the engine exhaust with noxious products. Deposits are likely to form on internal engine parts and on surfaces near the engine exhaust.
Creating good high-pressure gas sealing is also a problem for these small engines. Cylindrical surfaces appear to work best for sealing small engines.
Engines that have special lubrication systems have demonstrated the best durability and reliability. Minimal progress has been made in addressing these problems over the years, and there appears to be little hope of ever solving them.
MEMS-based Combustion Engines
Microturbines, or MEMS-based, micro gas turbine engines, were identified in Energy-Efficient Technologies (NRC, 1997) as a promising source of portable power for the Army of the future. The idea for such microturbines was conceived at the Massachusetts Institute of Technology, and that institution remains the leader in research in this area (Epstein et al., 1999). The development of MEMS microturbine systems was funded by the Army under a MURI program and is now funded at MIT under the Army Collaborative Technology Alliance.17 Similar efforts to realize centimeter-sized gas turbine generators in the 10- to 200-W range have been started by Honda (Japan), IHI (Japan), the University of Tokyo (Japan), ONERA (France), the Singapore Institute of Manufacturing Technology (Singapore), and the Katholieke Universiteit at Leuven (Belgium). These groups are using a variety of fabrication approaches ranging from silicon/silicon carbide micromachining (MIT and Singapore), to ceramic injection molding/sintering (Honda and IHI), to conventional metal machining (University of Tokyo, KUL). The growth of research in this area is evidenced by two recent international symposia: Power-MEMS 2003 (December 4-5, 2003, Chiba, Japan, www.getinet.org) and ISMME2003, the International Symposium on Micro-Mechanical
|
15 |
Found online at www.davisdieseldevelopment.com. Last accessed on January 28, 2004. |
|
16 |
Found online at www.Foster-miller.com. Last accessed on January 28, 2004. |
|
17 |
Found online at https://www.ctapower.org. Last accessed on January 28, 2004. |
Engineering (December 1-3, 2003, Tsukuba, Japan, http://shourai.hitachi.co.jp/ismme2003/ISMME.html#English).
Numerous technical issues must be addressed before an actual MEMS microturbine system can be realized. There are materials issues, because the MEMS microturbine operates at 800°C, which is well in excess of a practical temperature for the long-term use of Si. Emerging MEMS fabrication methods must therefore be applied to achieve more rugged materials, such as SiC or SiN. In this new size realm, the dimensions of every aspect of the microengines must be modeled and tested. A new magnetic generator must be developed to convert the engine’s motion to electricity. There are also critical issues with tribology and identifying bearings and a bearing design that can withstand the 1 to 1.4 million rpm of the microengine blades in the engine and the generator (Ehrich and Jacobsen, 2003). Near-term plans are for testing the engines with hydrogen, and much work is needed to run them on a hydrocarbon-based fuel.
Energy-Efficient Technologies (NRC, 1997) estimated the specific energy of a MEMS microturbine system to be 4,000 Wh/kg, but the MIT team has since changed its focus and is working on a 5 percent efficient system with a goal of 700 Wh/kg (Epstein et al., 2003). According to the formulas at the end of Appendix C and Figure C-4, any 5 percent efficient system will have a specific energy of less than 600 Wh/kg for a 1,440-W mission (72 hr at 20 W). Operating for 72 hr, a system weighing 250 g (excluding fuel) that operates at 5 percent efficiency would have a specific energy of 540 Wh/kg, and a 500-g system would be at 496 Wh/kg when operated for 3 days. To meet aggressive long-term goals of an order of magnitude increase in the energy of a battery, or approximately 2,000 Wh/kg, the system would have to run at >20 percent efficiency (see Figure C-4).
In addition to the challenges associated with system efficiency, the success of the MIT and other microengine programs hinges on the critical demonstration of self-sustainability for a gas turbine operation. Such a demonstration would indicate that a power plant was viable provided that problems with materials, bearings, and component efficiencies are solved. Until the projects are able to demonstrate the capability of a free-running micro gas turbine engine or a net positive engine output, they will remain at the TRL 1-2 stage in their development.
Refinement of components needed for efficient operation of the microengines also face critical challenges. Several components needed in a gas powered microturbine have been successfully demonstrated including a micro-scale high-speed compressor impeller and high-speed gas bearings (Johnston, et.al., 2003; Ehrich and Jacobson, 2003).
In the absence of a MEMS device that can operate in a self-sustained mode, fluid mechanics simulations would be required to evaluate the real performance potential and to estimate from the aggregation of components in the MIT system whether sufficient progress has been made toward achieving required component efficiencies. Such simulations are routinely made on larger sized turbo machinery to improve the component efficiencies by running system components on test rigs with very capable diagnostic flow measurement tools and thereby ascertain the real flow conditions within the machine. The diagnostic process is important because three-dimensional fluid flow analysis often fails to predict subtle but important effects within the machines, such as flow separations from the constraining walls. Such deviations from desired flows often greatly degrade component performance.
Detailed simulations, combined with three-dimensional fluid flow analysis, have allowed the optimization of larger machines to near-theoretical efficiency levels; however, the application of such simulations to microturbine components appears to be a very difficult challenge. The small size of the components makes application of existing diagnostic tools difficult if not impossible. If tools for this combination of analysis and design modification are not created, the process of component improvement may not proceed at a reasonable pace. The development of such tools, to be used in concert with existing analytical capabilities, will be time consuming and may be very expensive. Thus, the continuing improvements in component efficiencies that are required to make the microturbine generator a viable power source are likely to be very expensive and to have a limited chance of success. Because of the invention still required to achieve a coherent system design, the committee feels that the TRL of the MEMS microturbine technology is no higher than 2, and it did not consider the technology in Chapter 2.
Stirling Engines
External combustion engines such as steam engines and Stirling cycle engines were used in practical applications as long ago as 1800 but have since been largely relegated to history, except in a few embodiments, because of more efficient alternatives—namely, efficient internal combustion engines and electrical power from an ever-expanding grid. The primary advantage of the Stirling cycle is that the thermal process is steady state, which allows combustion optimization and energy recuperation. Further, steady-state combustion inherently has a lower acoustic signature than internal, impulsive combustion engines. For free-piston versions of Stirling engines, it is possible to operate two separate engines such that all vibration is canceled, resulting in an extremely quiet system. Early versions of Stirling engines employed exotic materials and had low specific power even though they were efficient converters of thermal energy to electricity. In recent years, however, advances in materials have resulted in components with high-temperature properties favorable enough to provoke interest in Stirling technology as a viable energy converter for some applications. It is currently a viable candidate for deep space exploration (http://www.grc.nasa.gov) and shows promise for battlefield and commercial applications (U.S. Army, 1993).
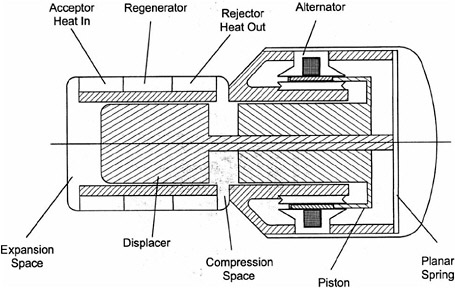
FIGURE D-7 Free piston Stirling engine showing component parts. SOURCE: SunPower Corporation.
Even though the technology has important desirable attributes, Energy-Efficient Technologies (NRC,1997) did not consider external combustion engines as viable candidates for soldier power owing to their low specific power, and to date, their primary commercial and military application is for cryogenic coolers.
The engine works because it is possible to alternately heat and cool an enclosed working fluid from a continuous flow external burner. The engine has five primary components: two pistons (or a piston and displacer), a regenerator, and two voids, or closed volumes, into which the gas and pistons expand (see Figure D-7). The regenerator section is a heat exchanger that alternately absorbs and releases heat. One of the volumes is maintained at a low temperature and is the compression space. The pistons are used to change the cylinder volume and to shuttle the working fluid back and forth. Work in the Stirling engine is generated by compressing and expanding the working fluid at different temperatures. The choice of working fluid is critical for the efficiency of the Stirling engine. While hydrogen would be the best working gas, it is difficult to contain in a sealed volume, so the most widely used gas is helium. There are several geometrical arrangements for Stirling engines. The free-piston version uses fluid forces to move the components, which results in no mechanical linkages to the piston or displacer. When coupled to a linear alternator, the device may use flex bearings or gas bearings, both of which allow the engine to be hermetically sealed into one compact unit with the fewest possible moving parts, none of which are in physical contact.
The most advanced Stirling engine that is likely to be applied for soldier power is the engine being developed by Sunpower, Inc. (Wood and Lane, 2003). The unit is currently being developed in a NASA Phase I SBIR for deep space applications. A recent visit to Sunpower confirmed the status of the system, which appears to be on the road to meeting or exceeding all specifications. The initial tests of the unit were at 31 W and a conversion efficiency of 29 pecent from thermal input to the head to electricity out.
As a general rule of thumb, one must at least double the mass of engine and converter for the remainder of the ancillaries needed to produce a working engine. Based on that figure, one could expect to produce a 35-W system with a dry weight of less than 1 kg. Figure D-8 is a conceptual drawing of a 20-W engine powered by a liquid propane energy source. It shows the relationship of the burner, heater head, and engine/alternator. It has a finned cooling system and would probably need forced air for cooling. This was
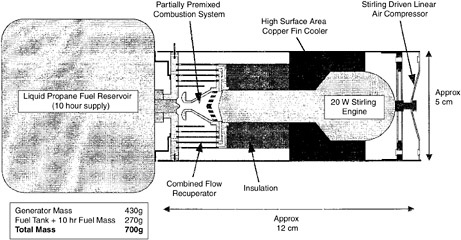
FIGURE D-8 Conceptual layout for a 20-W Stirling power system for soldier applications. Note that the unit is liquid-propane powered.
SOURCE: SunPower Corporation.
used as the basis for the system projections reflected in Chapter 2 (see Tables 2-3 and 2-4). The unit has been resized for 1,440 Wh and the use of JP fuels.
Figure D-9 shows a 1-kW Stirling engine recently purchased by Auburn University as part of its hybrid electric program for silent watch applications. It was made for the cogeneration market in Europe and has been tested for thousands of start-stop cycles.
It is unclear whether the advances made in specific power for small engines will also translate to larger sizes; however, if they do, the pressure vessel and linear alternator mass could be reduced significantly to raise the specific power from approximately 44 W/kg to approximately 100 W/kg. Reliability is one of the most desirable characteristics of Stirling engine technology. In various embodiments, key components and full systems have been tested for thousands of hours (Schreiber, 2001). The longest trial cited by Schreiber—a testbed for the key flexbearing in the unit—ran for 12.6 years.
ADVANCES IN OTHER AREAS
Advances have been made in the areas of thermoelectric energy, thermophotovoltaics, and energy harvesting that make these areas candidates for soldier energy sources.
Thermoelectric Energy
Energy-Efficient Technologies (NRC,1997) considered thermoelectric (TE) power systems not viable for soldier power applications owing to their inherently low conversion efficiency and low specific power. TE devices utilize specialty materials that are able to convert a thermal gradient to electricity. The systems typically require a large heat gradient for maximum efficiency. The maximum specific power for converters mentioned in the 1997 study was from 15-20 W/ kg, with conversion efficiencies less than 10 percent. Such efficiencies are typical of what is available commercially through firms such as Global Thermoelectrics,18 whose generators range from 15 to 500 W and in general have system specific power in the range 0.7 to 0.9 W/kg. The fuel is natural gas or propane and consumption is about 0.2 kWh/kg. Total system efficiency is about 2 percent. If a recuperated burner is used, the total efficiency could be improved to approximately 8 percent. The low efficiency (<10 percent) of these systems makes them inherently difficult to integrate into high-energy-density power sources, because they must be very lightweight, as noted in Figure C-4.
The efficiency of TE systems is directly related to the properties of the TE materials, so there has been consider-
|
18 |
Found online at www.globalte.com. Last accessed on January 28, 2004. |

FIGURE D-9 1-kW Stirling engine recently purchased by Auburn University as part of its hybrid electric program for silent watch applications. SOURCE: Auburn University.
able research on the materials. The Research Triangle Institute has achieved high figures of merit (ZT ~ 2.4), as reported by Nature, and is developing a small thermoelectric system that uses JP-8 as a fuel under the DARPA Palm Power program (Venkatasubramanian et al., 2001). The efficiency of its near-term projected system is 5 percent. As discussed in Appendix C (see Figures C-4 and C-5) and in the section on MEMS microturbines, low-efficiency systems must be very lightweight if they are to be implemented. Although the thermoelectric conversion devices may be light weight (~50 g), the fuel tank, insulation, and combustion components will increase the weight of the system. Therefore such devices may be useful for 24- and 72-hr 20-W missions only if the efficiency of the full system can be increased more than 10 to 15 percent, which will be difficult to achieve. The low TRL of the systems (2) prevents their consideration in the technology assessments of Chapter 2.
Thermophotovoltaics
Thermophotovoltaics, TPV, was considered a potential energy conversion mechanism for the soldier in Energy-Efficient Technologies (NRC, 1997). Advances in the art since that report have been steady, but no major breakthroughs have been reported. In a recent paper, R.R. Siergiej et al. (2002) reported the 20 percent conversion of thermal energy incident on cells to electric energy. The main programs in place are classified Navy programs and NASA programs aimed at deep space applications. Both the Navy and NASA programs use heat generated by nuclear sources.
There has been little progress in the development of fueled TPV systems, and, no system has yet been built with an end-to-end efficiency of more than a few percent. TPV is still not a viable candidate for soldier power due to the low efficiency of the technology.
Energy Harvesting
Energy-harvesting technologies were covered in detail in Appendix C of Energy-Efficient Technologies (NRC, 1997) and in a recent report from Pacific Northwest National Laboratory (De Steese et al., 2000). While energy-harvesting technologies are compelling because the energy source is inexhaustible, their specific power/power density capabilities must be realistically assessed. In general, energy-harvesting methods have low power and/or energy, making them inappropriate for standard Army soldier needs. The weight of the power conversion device can also make the specific power of the system poor.
For example, a heel strike system capable of generating 2 W (1 W per boot) would yield 16 Wh of energy if the soldier walked for 8 hours. This corresponds to 10 percent of the capacity of the BA-5590 primary battery or an equivalent weight savings of 100 per 8 hours of walking. The heel strike mechanism for extracting mechanical energy from walking might increase fatigue. This and other negative impacts on soldier performance must be taken into account. Obviously, heel strikes would be inappropriate for missions that do not include walking. However, they might find specialized functions—for instance, as the power source for sensors on a soldier’s boot that provide information about chemical, nuclear, and other hazards while the soldier is on patrol.
Another option is hand-cranking, several minutes of which can generate 10 to 100 W, enough to power a radio for a limited time. The requirement for hand cranking might have an impact on a stealth mission—or be an inconvenient exercise in the heat of battle or in the fatigue that ensues. Such devices are not lightweight and could be burdensome.
Other proven energy-harvesting methods involve thermoelectric and solar energy. Clearly, a soldier with thermoelectric devices might be able to take advantage of gradients between his/her body temperature and the ambient. Or, photovoltaic devices on the uniform could capture energy from the Sun.
It should be noted that many of the energy harvesting systems discussed above are at high TRLs: Heat/thermoelectric powered watches (from Citizen among others), hand-cranked radios, phones, and flashlights (from Freeplay among others), and solar cells are all commercially available. Even rudimentary piezoelectric heel strike devices have been demonstrated and are likely to further improve (Pelrine et al., 2001). Unfortunately, the weight, cost, and reliability of such devices do not make them viable for applications requiring 20 W or more power. Also, their power and energy can be variable and intermittent and might not be attractive for soldiers facing life-or-death situations.
For all these reasons, energy harvesting methods were not considered in Chapter 2 for technologies that require average power of 20 W or more. However, energy harvesting and human powered systems will become much more attractive in the overall future if the demand for soldier systems can be reduced to 2 W or less, as discussed in Chapter 7.
REFERENCES
Published
Abraham, K.M., and Z. Jiang. 1996. A polymer electrolyte-based rechargeable lithium/oxygen battery. J. Electrochemical Soc. 143: 1.
Acker, W. 2003. Military communications applications for fuel cells. Presented at the NDIA Tri-Services Power Expo, July 15-17, Norfolk, Va.
Amatucci, G., inventor. Telcordia Technologies, Inc., assignee. June 26, 2001. Rechargeable Hybrid Battery/Supercapacitor System. U.S. Patent 6,252,762.
Amatucci, G., inventor. Telcordia Technologies, Inc., assignee. February 11, 2003. High Energy Density Hybrid Battery/Supercapacitor System. U.S. Patent 6,517,972.
Arana, L.R., S.B. Schaevitz, A.J. Franz, M.A. Schmidt, K.F. Jensen. 2003. A microfabricated suspended-tube chemical reactor for thermally efficient fuel processing. J. Microelectromechanical Sys. 12(5): 600-612.
Ball Aerospace. 1998. PPS-50 Operator’s Manual. Boulder, Colo.
Ball Aerospace. 2003. Portable Fuel Cells and Power Management. Available at www.ball.com/aerospace/pdf/pps.pdf. Last accessed on January 28, 2004.
Ball Aerospace. 2004. Palm Power DMFC 20-watt system development: DMFC 20-watt system performance and delivery. Presentation by Jeffrey Schmidt to the DARPA Palm Power 2004 Program Review Meeting. Orlando, Fla. February 9-11.
Bar-Cohen, Yosef. 2001. Electroactive polymer actuators and devices. Smart Structures and Materials 2001, Yoseph Bar-Cohen, ed. Pp. 148-156.
Conway, B.E. 1999. Electrochemical Supercapacitors. Kluwer Academic/ Plenum Publishers.
Cooper, R., and P. Cox. 2003. PolyFuel—Power for the Wireless World. July 2003.
Crumm, A. 2004. Microtubule solid oxide fuel cells. Presented by Aaron Crumm, Adaptive Materials, to the DARPA Palm Power 2004 Program Review Meeting. Orlando, Fla. February 9-11.
Deluga, G.A., J.R. Salge, L.D. Schmidt, and X.E. Verykios. 2004. Renewable hydrogen from ethanol by autothermal reforming. Science 303(5660): 993-997.
De Steese, John G., Donald J. Hammerstrom, and Lawrence A. Schienbein. 2000. Electric power from ambient energy sources. PNNL Report 13336, September. Available from the National Technical Information Service, U.S. Department of Commerce.
DOE (Department of Energy). 2002. Fuel Cells Handbook. 6th ed. Available online at http://www.seca.doe.gov/refshelf.html. Accessed August 2004.
Ehrich, F.F., and S. A. Jacobson. 2003. Development of high-speed gas bearings for high-power density microdevices. Journal of Engineering for Gas Turbines and Power-Transactions of the ASME 125(1): 141-148.
Epstein, A.H., S.D. Senturia, I.A. Waitz;, J.H. Lang, S.A. Jacobson, F.F. Ehrich, M.A. Schmidt, G.K. Ananthasuresh, M.S. Spearing, K.S. Breuer, and S.F. Nagle. 1999. Massachusetts Institute of Technology, assignee. August 3, 1999. Microturbomachinery. U.S. Patent 5,932,940.
Grubb, William T., inventor. General Electirc Company, assignee. May 26, 1970. Process and Apparatus for Electrochemically Oxidizing Alcohol to Generate Electrical Energy. U.S. Patent 3,514,335.
Hickner, M., B. Pivovar, Y. Kim, J. McGrath, and P. Zelenay. 2002. The effects of sulfonated poly(arylene ether sulfone) copolymer proton exchange membrane post treatment on DMFC performance and properties. Proceedings of the Third International Symposium on Proton Conducting Fuel Cells. Salt Lake City, Utah, October 20-24.
Hu, J.L., Y. Wang, D. VanderWiel, C. Chin, D. Palo, R. Rozmiarek, R. Dagle, J. Cao, J. Holladay, and E. Baker. 2003. Fuel processing for portable power applications. Chem. Eng. J. 93(1): 55-60.
JASON. 2003. Portable Energy for the Dismounted Soldier. Study leader: Nathan S. Lewis, JSR-02-135. McLean, Va.: The MITRE Corporation, June 15.
Johnston, J.P., S. Kang, T. Arima, M. Matsunaga, J. Tsuru, and F.B. Prinz. 2003. Performance of a micro-scale radial-flow compressor impeller made of silicon nitride. Proceedings of the International Gas Turbine Congress (IGTC), Tokyo, pp 1-7.
Linden, D., and T.B. Reddy, eds. 2002. Handbook of Batteries, 3rd ed. New York: McGraw-Hill.
Minh, N.A. 1993. Ceramic fuel cells. Journal of the American Ceramics Society 76: 563-588.
MTI (MTI MicroFuel Cell, Inc.). 2003. MTI MicroFuel Cell’s chief technology officer to speak at 2003 fuel cell seminar. Press release. Available online at http://biz.yahoo.com/prnews/031030/nyth174_1.html. Last accessed on January 28, 2004.
NRC (National Research Council). 1997. Energy-Efficient Technologies for the Dismounted Soldier. Washington, D.C.: National Academy Press.
NRC. 2003. Future Hydrogen Production and Use. Letter Report. Washington, D.C.: The National Academies Press.
Park, S.D., J.M. Vohs, and R.J. Gorte, 2000. Direct oxidation of hydrocarbons in a solid-oxide fuel cell. Nature 404: 265-267.
Pelrine, Ron, Roy D. Kornbluh, Joseph Eckerle, Philip Jeuck, Seajin Oh, Qibing Pei, and Scott Stanford. 2001. Dielectric elastomers: Generator-mode fundamentals and applications. Proceedings of SPIE 4329.
Raimondeau S, D. Norton , D.G. Vlachos, and R.I. Masel. 2003. Modeling of high temperature microburners. Part 1. Proc. Combustion Inst. 29: 901-907.
Read, J., K. Mutolo, M. Ervin, W. Behl, J. Solfenstine, A. Driedger, and D. Foster. 2003. Oxygen transport properties of organic electrolytes and performance of lithium/oxygen battery. J. Electrochem. Soc. 150: A1351.
Ren, Xiaoming, and Shimson Gottesfeld, inventors. The Regents of the University of California, assignee. October 2, 2001. Enhanced Methanol Utilization in Direct Methanol Fuel Cell. U.S. Patent 6,296,964.
Schmidt, Jeffrey, and Tim Quakenbush. 2002. Fuel for Portable Power Systems Program. Final report. Prepared for the U.S. Army Research Office by Ball Aerospace and Technologies.
Schreiber, J.G. 2001. Assessment of the Free-Piston Stirling Converter as a Long Life Power Converter for Space. NASA/TM−2001-210604, AIAA−2000-3021.
Siergiej, R.R., B. Wernsman, S.A. Derry, R.G. Mahorter, R.J. Wehrer, S.D. Link, M.N. Palmisiano, R L. Messham, S. Murray, C.S. Murray, F. Newman, J. Hills, and D. Taylor. 2002. 20% Efficient InGaAs/InPAs thermophotovoltaic cells. Proceedings of the Fifth Conference on Thermophotovoltaic Generation of Electricity 653(1): 414-423.
U.S. Army. 1993. Front End Analysis of Soldier Individual Power Systems, USA-BRDC-TR//2541. E. Raskovich, ed. Ft. Belvoir, Va.: Ft. Belvoir Research, Development, and Engineering Center.
Venkatasubramanian, R., E. Siivola, T. Colpitts, and B.C. O’Quinn. 2001. Thin-film thermoelectric devices with high room-temperature figure-of-merit. Nature 413: 597-602.
Whittingham, M.S. 1975. Mechanism of reduction of fluorographite cathode. Journal of the Electrochemical Society 122: 526.
Wood, J.G, and N. Lane. 2003. Advanced 35 W free piston Stirling engine for space applications. Proc. Space Technology and Applications International Forum (STAIF) 2003. Albuquerque, N.Mex.: February 2-5.
Zheng, J.P. 2003.The limitations of energy density of battery/double-layer capacitor asymmetric cells. Journal of the Electrochemical Society 150 (4): A484-A492.
Unpublished
Epstein, A.H., S.A. Jacobson, F. Ehrich, Y. Gong, R. Khanna, J. Lang, H. Li, L. Liu, C. Livermore, H.S. Moon, J. Protz, N. Savoulides, M. Schmidt, C. Spadaccini, Z. Spakovszky, M. Spearing, C.J. Teo, I. Waitz, and D. Ward. Centimeter-diameter gas turbine generators for compact power. Poster presentation to Army Collaborative Technology Alliances Conference. May 2003.
Paur, R. Army research initiatives. Presentation by Richard Paur to the committee on August 20, 2003.




























