8
Genetic Factors in Ethnic Disparities in Health
Richard S. Cooper
I very early got the idea that what I was going to do was prove to the world the Negroes were just like other people.—W.E.B. DuBois
Biology is being transformed by the advent of technology that allows us to define the molecular basis of genetic variation. Having pushed physics off the pedestal reserved for “big science,” biologists have sequenced the genomes of half a dozen organisms, altered the sequence in even more, and cataloged millions of the DNA variants found in humans. The technological capacity to read and manipulate genes has in turn generated speculation that our ability to solve health problems will be transformed in a similarly dramatic fashion. Acknowledging that we are in the early stages of this new era, the practical accomplishments of genetic medicine to date are much more modest, however. Although great success has been achieved with the rare monogenic disorders, for the common chronic illnesses that account for most of the death and disability in our society, genomics has yet to elucidate the pathophysiology in important ways or improve treatment (Cooper and Psaty, 2003; Khoury, 2000; Lander, 1996; Report of the Advisory Committee on Health Research, 2002).
Describing the genetic underpinnings of common chronic diseases is a challenge of infinitely greater complexity than obtaining a sequence of nucleotides or finding single gene mutations. A quantum leap in biology will be required before the genes and the associated physiologic abnormalities that confer susceptibility to chronic disease can be understood. Given the intertwined effects of genes and environment on these conditions, the question remains as to whether or not important genetic causes can even be identified. Nonetheless, the exploration of the genome has accumulated unstoppable momentum and will profoundly alter our understanding of the
biological world, even if it does not transform the practice of medicine or public health.
Genomics is connected to public health science through population genetics and epidemiology, and to the everyday practice of public health through race. An important goal of this discussion will be to try to disentangle genomics from race, based on the argument that they are categorically different ways of framing the epidemiologic questions. This is more than an intellectual challenge, however, because deeply held beliefs about the relative influence of nature and nurture on variation in disease patterns between populations bind the two together. After centuries of reliance on race as a surrogate for genes, the impulse has been to merely incorporate molecular data as new details, leaving the accepted framework in place. However, this solution can only be temporary. Among its many consequences, molecular genetics has made the current model of race obsolete and, in the long run, untenable. As a result, through no initiative of its own, public health suddenly has been presented with the opportunity to rethink one of its most intractable problems. Perhaps, one might argue, that will be the most important contribution of genetics to public health: Given our complete inability to devise effective solutions to racial inequalities in health, discarding what now passes for theory could be a salutary development.
The two main dimensions of the race controversy can be discussed separately. First, the “ideological” concept of race informs popular discourse and shapes policy, with a parallel impact in public health. This version of race is defined by social and historical forces and is used to create and justify many of the divisions that exist among people of varying religious, ethnic, or geographic backgrounds. This concept assumes the existence of categories that have no scientific foundation—at least none based on molecular data. This concept has been challenged since Darwin (1981), yet it persists for ideological purposes (Cooper, 1984; Montagu, 1964; Root, 2001). Although everyone in public health needs to be reminded of the importance and illegitimacy of this notion, and those who have not yet heard the news need to be informed, there is little of substantive importance that is really new to add to this debate: We should begin by simply acknowledging that race in the world of politics, and all the nutritional, educational, and social influences it entrains, continues to be the determining influence on ethnic variation in health.
A second use of race has assumed new relevance. As a label for regional populations, race has a long history in population genetics, and in this arena, important opportunities exist to revisit old questions on interethnic variation in health. At stake is whether or not we can move beyond the indirect methods applied in epidemiology or the generalizations built on estimation of genetic distance that have preoccupied population geneticists and anthropologists (Cavalli-Sforza, Menozzi, and Piazza, 1996; Relethford,
1998). Specifically, it is now possible to ask a set of testable questions: Can the global variation in the human genome be aggregated into subunits, and do those units correspond to the categories we call race? Can we assess the relative magnitude of shared and nonshared genetic material among population groups? Is there variation in causal genetic polymorphisms that is associated with important differences in chronic disease risk? Is it possible to conceptualize the collective human genome as a whole, and express that concept in quantitative terms?
Of course, complete answers to these questions are still well beyond our grasp. Some of the questions, like the aggregation of variants within population groups, are likely to be answered in the near future, while others, like the relative frequencies of causal variants for chronic diseases, may never be fully answered. Yet molecular genetics is changing the way we think about human variation, and it is crucial that this change has a positive impact on medicine and public health. Even though the noxious effects of racism—the social and economic consequences of the ideology—will only be eliminated through a political process, it remains the obligation of biological scientists to contribute to this eventual outcome by providing a clear description of the natural phenomena as we understand them. With an eye to history, it will be necessary, first and foremost, to ensure that the mistakes surrounding racial comparisons in the past are not repeated using molecular data.
In its current usage among epidemiologists, who have provided most of what we know about interethnic variation in health, the “common sense” or popular meaning of race is accepted as a given, unsupported by biological evidence, and serves both as a construct that frames research questions and a premise on which explanations are based. This standard application of race has obvious limitations and has resulted in widespread misunderstanding about the potential of genes to influence health (Cooper, Kaufman, and Ward, 2003; Kaufman and Cooper, 1996; Krieger, Rowley, Herman, Avery, and Phillips, 1993). As in society at large, incorporation of these notions into the intellectual grammar of science can lead to racist practice (Cooper and Kaufman, 1998). Thus, one of the aims of this paper will be an attempt to explore the role of scientific racism within the discipline of public health, and examine how that shapes the discourse and the research agenda.
ETHNIC DISPARITIES IN HEALTH STATUS
The focus of this discussion will be on the broad medical syndromes that account for most of the disability and premature mortality in the U.S. population. The first problem that arises when examining the racial/ethnic health patterns is how best to organize the data. As is well known, the definitions used by government agencies are explicitly not based on biologi-
cal categories (Cooper, 1994; Hahn, 1992; Lott, 1993); instead this system was developed to meet the political obligations of the Census. The designation of “black,” “white,” “American Indian,” and “Asian” are considered races, while “Hispanic” is a language or cultural grouping, and the conglomerate category of “Asian/Pacific Islanders/American Indian” is often used to collapse data from many smaller race groups. There is no way to map these categories directly onto genetic subpopulations, although there is some broad correspondence between the racial/ethnic labels and the continent of origin of the ancestral populations.
With the availability of vital statistics on both Hispanics and Asian/ Pacific Islanders, we now have a reasonably clear description of the patterns of common disease in the U.S. racial/ethnic groups (Table 8-1). Health status will be discussed in more detail in other sections of this volume, the more limited purpose here is to frame the specific question that needs to be addressed by a genetic analysis. The first and most striking feature is the heterogeneity that exists among the groups. The most prevalent notion of minority health status in the United States is built on the “deficiency model,” that is, an expectation of poorer outcomes for groups other than whites. Dismissed in the past as artifactual, the relative advantage enjoyed by Hispanics, despite similar education and income to blacks, is now undeniable. Characterized as the “Hispanic paradox,” an active research agenda exists
TABLE 8-1 Health Status Measures in Racial/Ethnic Groups in the United States, 1998
|
Cause of Death |
Age-Adjusted Death Rates* |
|||
|
White |
Black |
Hispanic |
Asian |
|
|
All causes |
450.4 |
690.9 |
432.8 |
264.6 |
|
Heart disease |
121.9 |
183.3 |
84.2 |
67.4 |
|
Coronary heart disease |
79.2 |
92.5 |
54.7 |
42.9 |
|
Stroke |
23.3 |
41.4 |
19.0 |
22.7 |
|
Cancer |
121.0 |
161.2 |
76.1 |
74.8 |
|
COPD |
21.9 |
17.7 |
8.5 |
7.4 |
|
Pneumonia/influenza |
12.7 |
17.4 |
9.8 |
10.3 |
|
Liver disease/cirrhosis |
7.1 |
8.0 |
11.7 |
2.4 |
|
Diabetes mellitus |
12.0 |
28.8 |
18.4 |
8.7 |
|
HIV infection |
2.6 |
20.6 |
6.2 |
0.8 |
|
External causes |
46.7 |
68.8 |
44.7 |
24.4 |
|
Infant Mortality per 1,000 |
6.0 |
13.6 |
5.8 |
5.5 |
|
Life expectancy (years from birth) |
77.3 |
71.3 |
>80? |
>80? |
|
*Per 100,000. SOURCE: National Center for Health Statistics (2000). |
||||
in epidemiology to explain this counterintuitive finding (Markides and Coreil, 1986). Unreported “shoebox” burials were said to contribute to low infant mortality, while a healthy migrant effect and the return of sick elderly to their country of origin accounted for low adult mortality (James, 1993; Markides and Coreil, 1986). A number of cohort studies now document low age-specific death rates in Hispanics, primarily Mexican Americans, which cannot be ascribed to these biases (Wei et al., 1996). This relative advantage is not universal, however; in many Hispanic communities, obesity and diabetes occur at much greater frequencies than among whites (Diehl and Stern, 1989; Harris et al., 1998).
On the other hand, black Americans experience higher rates of all the major causes of death except chronic obstructive pulmonary disease and liver disease (Table 8-1). The excess rates of cardiovascular disease (CVD) have long been recognized as being secondary to the high prevalence of hypertension (Cooper, 1993). Despite high rates of hypertension, coronary heart disease mortality was lower among blacks than whites over the past half century, and it was once widely held that blacks were constitutionally resistant to atherosclerosis (Johnson and Payne, 1984). Rates of coronary heart disease in blacks now exceed whites (Cooper et al., 2001). Asian Americans experience remarkably lower death rates, particularly from CVD (Liao, McGee, and Cooper, 1999). Type II diabetes had been less common in blacks in the first half of the 20th century; it now occurs twice as often among blacks as among whites (Harris et al., 1998; Stamler et al., 1979).
Death rates from common malignant neoplasms are highest among black Americans (Table 8-2). The black excess is found in all the common forms of cancer except myeloma, and the differences are particularly marked in the younger age groups. Potential genetic influences are given considerable attention in studies of prostate cancer, where blacks have an incidence twice that of whites (National Center for Health Statistics [NCHS], 2000;
TABLE 8-2 Death Rates from Malignant Neoplasms in Racial/Ethnic Groups in the United States, 1998
|
Racial/Ethnic Group |
Total* |
Lung |
Breast |
|||
|
Men |
Women |
Men |
Women |
Women |
||
|
White |
146 |
106 |
49.4 |
27.4 |
19.0 |
|
|
Black |
208 |
129 |
70.8 |
27.2 |
26.2 |
|
|
American Indian/ Alaskan Native |
96 |
74 |
33.9 |
16.5 |
10.8 |
|
|
Asian/Pacific Islander |
91 |
63 |
24.6 |
11.2 |
9.3 |
|
|
Hispanic |
93 |
64 |
21.4 |
8.3 |
12.5 |
|
|
*Per 100,000. SOURCE: National Center for Health Statistics (2000). |
||||||
Robbins, Whittemore, and Thom, 2000). Lung cancer has attracted less speculation, despite a black to white mortality rate ratio of 1.0:1.4; overall, blacks smoke less than whites, so the etiologic forces at work are obscure (NCHS, 2000). Breast cancer mortality is higher in blacks than whites, and at a younger age the excess is twofold; the long-awaited downturn in mortality that began in 1992 has been observed only in whites. Known mutations at the BRCA loci account for a substantial proportion of breast cancer cases only among women of Jewish ancestry.
Deaths from diabetes and liver disease are higher among Hispanics than whites, although total mortality is lower and life expectancy among Hispanics is thought to exceed 80 (Table 8-1). All-cause mortality for Asians is remarkably low—only 38 percent of the rate among blacks. The risk of dying from HIV is 2.4 times higher in Hispanics than whites, but 8 times higher in blacks. Infant mortality is lower in Hispanics and Asians than whites, but more than twice as high in blacks; the persistently higher rates among blacks are driven in large measure by prematurity and low birthweight (Kleinman and Kessel, 1987). The prevalence of diabetes is currently 14 percent among Mexican Americans, 12 percent among blacks, and 7 percent among whites (Harris et al., 1998).
In general, other measures of health status are consistent with this overall picture. Self-reported health is rated lowest by blacks and Native Americans, followed in order by Hispanics, whites, and Asian/Pacific Islanders (McGee, Liao, Cao, and Cooper, 1999). These differences tend to be accentuated with increasing age (McGee, Liao, Cao, and Cooper, 1999). Similar patterns exist for disability (Liao, McGee, Cao, and Cooper, 1998). Higher incidence of Alzheimer’s disease has been reported among African Americans, independent of the prevalence of APOE-4 by some investigators (Tang et al., 1998), but not others (Bohnstedt, Fox, and Kohatsu, 1994).
Growing sophistication in descriptive epidemiology, particularly related to CVD and diabetes, has made it possible to model the relationship between risk factor exposures and subsequent disability and disease rates. Measurement of smoking habits, blood pressure, and cholesterol in young adulthood has been shown to predict directly the quality of life and health care experience of persons over 65 (Daviglus et al., 1998). In broad strokes, therefore, health among the elderly can be linked to surveillance data on known exposures. Against this increasingly well-defined epidemiologic background, we are observing growing inequality by social class and geographic region, as well as race/ethnicity (Cooper et al., 2001; Pappas, Queen, Hadden, and Fisher, 1993). Thus, while coronary heart disease rates have been declining at a rate of about 3 percent a year among whites nationwide, CVD mortality has turned upward among blacks in Mississippi (Jones et al., 2000). Blacks in communities located in the center of large cities have also experienced declining health; life expectancy for black men in Atlanta,
Baltimore, St. Louis, Los Angeles, and several other cities was less than 60 years in 1992 (Good, 1998).
The contrasts in disease patterns among U.S. racial and ethnic groups are obviously much more complex than can be described in this brief overview. The relevant question for this discussion is how the influence of genetics on variation in health outcomes among U.S. racial/ethnic groups might be recognized. The syndromes that have attracted the most attention are hypertension, asthma, dementia, low birthweight, renal disease, obesity/diabetes, and prostate cancer among blacks and, to a lesser extent, diabetes in Hispanics and Native Americans; in each instance the markedly elevated incidence ratios, with whites as the reference group, have fueled speculation about potential genetic predisposition. The magnitude and consistency of the ethnic differentials, such as in relation to hypertension and prostate cancer, lends credence to these arguments, although the potential environmental contribution is universally acknowledged.
A focus on specific syndromes can be misleading, however. It is essential to remember that the health disadvantage extends across a range of key public health measures. Although the hypothesis of genetic predisposition may seem plausible taken one disease at a time, when faced with the pattern as a whole, the probability that the black disadvantage is primarily genetic becomes remote. Rather than postulating a genetic cause for each condition, a more parsimonious explanation would suggest a common-source exposure to a disease-promoting environment. Likewise, a universal characteristic of the syndromes that vary across ethnic groups, with the exception of prostate cancer, is a strong social class gradient. Most of these syndromes have also shown marked secular trends in recent decades, and the prevalence changes across generations among migrants (Collins, Wu, and David, 2002). Stated in its complementary form, the entire basis for the genetic predisposition hypothesis lies in the contrasts in disease rates between historically defined racial/ethnic populations living in the same country. Clearly a strong set of assumptions regarding equal levels of exposure to environmental factors is required to sustain this hypothesis.
Although it is subject to many of the same caveats, a different test of the racial predisposition hypothesis is provided by comparisons of genetically related populations in contrasting social settings. All forms of CVD, including hypertension, are low in West Africa, and the levels are equal to U.S. whites in the Caribbean (Cooper et al., 1997a). The evolution of hypertension risk occurs in parallel with changes in known risk factors (Figure 8-1) (Cooper et al., 1997a). The blood pressure gap between blacks and whites is narrow in Cuba (Ordunez-Garcia, Espinosa-Brito, Cooper, Kaufman, and Nieto, 1998) and between blacks and persons of Indian descent in Trinidad (Miller, Maude, and Beckles, 1996). Blacks in Brazil have more hypertension than whites, but the differential is also smaller than in the
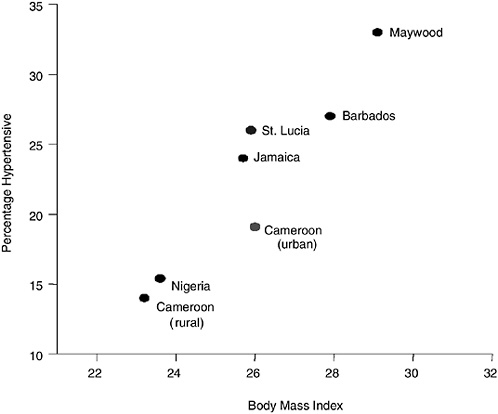
FIGURE 8-1 Hypertension prevalence and body mass index in populations of the African diaspora.
United States (Cooper and Rotimi, 1994; Sichieri, Oliveira, and Pereira, 2001). Obesity and diabetes are infrequent in Africa and among Native American groups not living in U.S. reservations (Cooper et al., 1997b; Esparza et al., 2000; King and Rewers, 1991). Diabetes is less common among blacks than whites in Brazil (Franco, 1992). Asthma and dementia are less common in Africa than among U.S. blacks (Hendrie et al., 1995; Litonjua, Carey, Weiss, and Gold, 1999). The rate of prostate cancer in blacks outside the United States is not yet reliably known, although high rates have been reported from Jamaica (Glover et al., 1986). Foreign-born women of African descent have children whose birthweight on average is close to whites (David and Collins, 1997; Friedman et al., 1993), and the disparity only emerges after a period of residence in the United States (Collins et al., 2002). Life expectancy in Jamaica and Barbados is longer than among U.S. blacks, where the estimated income is 1/30th to 1/5th as high. Although this pattern is still logically consistent with a predisposition inherent in blacks that is unmasked by environmental stimuli in the United States, it demonstrates that genes are not the determining factor in any of
these examples. To avoid misunderstanding on this point, however, it must be acknowledged that among members of populations that share a common environment, genetic susceptibility can play a crucial role in determining who develops a particular illness; the issue addressed here has been the variation in aggregate health status among groups across time and place.
In summary, the broad pattern of racial/ethnic variation in disease occurrence seen in the United States has formed the basis for strong arguments in favor of genetic predisposing factors among blacks and, to a lesser extent, Hispanics and Native Americans. However, the competing hypothesis that the root cause is embedded in the historical and social circumstances peculiar to each of these groups is more consistent with the data (Chaturverdi, 2001). Furthermore, the probability that genes account for the general pattern of health disadvantage is untenable and it must follow that the claims made, for example, by investigators studying diabetes, renal failure, hypertension, and prostate cancer regarding genetic predisposition cannot all be true based on this joint probability. Likewise, the presence of a strong environmental hypothesis, based on the overall pattern, creates a prior assumption against genetic predisposition for any given disease. But these arguments are simply logical inferences; the possibility that genes make an important contribution to interethnic variation of a major disease cannot be dismissed. The contemporary standard will require molecular evidence in order to resolve the question of the relative balance of genes versus environment. Nonetheless, as in all other branches of science, the rules require that the null hypothesis is the only legitimate starting point; the burden of proof should fall on those who claim the genetic, not social, content of race is causal.
THE CONTRIBUTION OF GENETIC EPIDEMIOLOGY TO UNDERSTANDING ETHNIC DISPARITIES
The search for nongenetic explanations of racial/ethnic variation has occupied epidemiologists for many years, and this experience has important implications for the study of genetic factors. Traditionally, epidemiology has placed emphasis on studies that use the individual as the unit of measurement. When group variation is of interest, it is modeled as the average of the individuals, rather than through any emergent or higher order properties. Alternatively, a second approach uses ecological analyses and attempts to analyze social and economic forces that impinge on groups, taking as the unit of analysis a community or population subgroup. Although risk factor epidemiology at the individual level has enjoyed enormous success, its limitations as a tool to understand variations in population health have also been recognized (Koopman, 1996). However, the conceptual framework for ecologic studies is less well established, and the proposition that economic inequality and institutionalized racism, for example, should be considered causes of
health status variation generally has not been embraced. However, just as the scope of biological problems can be defined at different levels—the molecule, the organism, or the population—the nature of the explanations at these levels must take different forms (Rose, 1998). Variation in health status across racial/ethnic groups uniquely requires consideration of social processes that are not individual traits, but group-level phenomena.
Genetic epidemiology must also confront this dilemma, albeit under different constraints, and fashion methods that are appropriate to understanding variation among both groups and individuals. Unfortunately, while traditional epidemiology makes unwarranted assumptions about the role of the individual in shaping his or her health status independent of social context, the conventional approach to studying genetic influences is guided by the sense that individuals are endowed with intrinsic qualities by virtue of membership in a particular race (Cooper and Kaufman, 1998; Kaufman and Cooper, 2001; Kaufman, Cooper, and McGee, 1997). In both instances, whether standard risk factors or genetic influences are being studied, the emphasis on inherent attributes creates a bias against considering social context (Lewontin, 1995; Rose, 1998). Examples of this effect are particularly apparent in the “pregenomic era” when inferences about genotype were based exclusively on data obtained from examination of the phenotype (Brancati, Kao, Folsom, Watson, and Szklo, 2000; Grim and Robinson, 1996; Robbins et al., 2000).
Indirect Methods of Assessing Genetic Factors as a Cause of Ethnic Disparities
A variety of indirect methods have been used to assess the contribution of genetics to interethnic differences in disease susceptibility (Table 8-3). In one approach the simple demonstration that a trait is heritable, and varies systematically between groups, leads directly to the inference that observed differences might be genetic in origin. Characterized as the “heritability hang-up” by geneticists (Feldman and Lewontin, 1975), these inferences have no logical basis. A trait can be highly heritable in each of two popula-
TABLE 8-3 Methods to Detect Genetic Effects on Interethnic Variation in Health
|
Approach |
Statistical Method |
|
Indirect |
Heritability |
|
|
The “subtraction method” |
|
Molecular |
Genome scan |
|
|
Candidate genes |
tions, and the mean difference between them could have nothing to do with genetic effects. For example, the proportion of the variance that is familial for height is approximately 80 percent, yet short stature among the Japanese compared to Europeans cannot be ascribed to differential frequencies of genetic variants. When the Japanese move to the United States, or adopt a Westernized diet, attained height increases rapidly with each generation.
The second common indirect method involves the attempt to partial out “environmental” factors by adjusting the trait for covariates and then arguing that “what is left over,” or the residual effect after subtracting external exposures, is likely to be genetic (Kaufman and Cooper, 1996). Because this “subtraction method” has become the standard approach in epidemiology and continues to be widely applied, it deserves consideration in some detail. A classic example of this procedure was demonstrated by the analysis of the difference in blood pressures among blacks and whites in the screening phase of the Hypertension Detection and Follow-up Program Cooperative Group (1977). Because a strong social class effect exists for blood pressure, and blacks and whites differ in socioeconomic status (SES), the data were stratified by educational level to examine whether the racial effect persisted (Figure 8-2). In fact, while an SES gradient is observed in both races, a significant gap exists at equivalent educational categories.
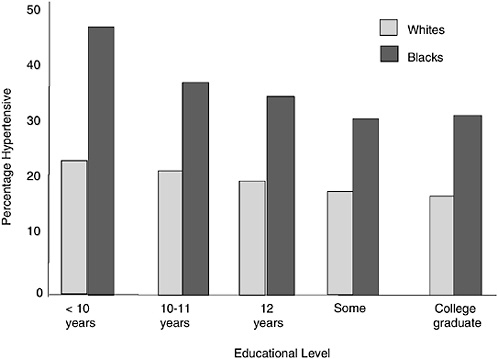
FIGURE 8-2 Hypertension prevalence by education, whites and blacks; Hypertension Detection and Follow-up Program.
The logical basis for the “subtraction method” is deceptively straightforward. An investigator begins with the recognition that an exposure, which, for example, might be correlated with risk of early mortality, occurs at a higher level in blacks than in whites. Because the relationship between this exposure and the outcome of interest is confounded, adjustment is necessary to make an unbiased comparison. The expectation is that if SES or other exposure variables fully explain the between-group difference, then equal rates of the outcome will be observed. A residual difference can then be attributed to intrinsic attributes of the two races, that is, genetics. For example, when a differential persisted after “controlling for SES,” it was concluded that “race-related biologic differences contribute to higher prostate cancer mortality in blacks” (Robbins et al., 2000, p. 493). Noting that “obesity is not a sufficient explanation” of racial differences in diabetes, it was inferred that “other factors must be involved, and most likely these are genetic” (Anonymous, 1989, p. 199). Based on similar reasoning, other investigators concurred that “the most straightforward interpretation of the observation that African American race has a strong independent association with diabetes mellitus is that African Americans are more susceptible … than their white counterparts” (Brancati, Whelton, Kuller, and Klag, 1996). Noting that blood pressures are higher in “Africans, American Caribbeans and other black populations … at the same level of salt intake,” the conclusion was reached that this “difference is likely to be genetically determined” (Law, Frost, and Wald, 1991). Similar analyses have been used to suggest that a genetic cause exists for differences in obesity, heart failure survival, birthweight, asthma, osteoporosis, and essentially all other traits that vary among racial/ethnic groups (Brancati et al., 1996; Carson, Ziesche, Johnson, and Cohn, 1999; Van den Oord and Rowe, 2000).
The “subtraction method” has been criticized (Kaufman and Cooper, 1996, 1999). Using the empirical measures of SES that are available, such as education, fails to eliminate the difference in exposure to the myriad factors that constitute the social influences on health for U.S. blacks and whites. These weak proxies cannot summarize the influence of lifestyle exposures. If one postulates that only modest residual confounding exists in these models, the results will be highly biased in favor of a “genetic” effect when none exists (Kaufman et al., 1997). Conceptually this approach violates the assumptions necessary in the counterfactual framework used in epidemiologic research (Greenland and Robins, 1986; Kaufman and Cooper, 1999).
From a technical perspective, the statistical models used in epidemiology are not designed to address the question being asked by the “subtraction method.” The primary goal of observational epidemiology is to generate point estimates of exposure-outcome relationships that can be
distinguished from the null, that is, to find effects that meet standard criteria for statistical significance. Although the actual size of this effect may be of some interest, in many cases it is difficult to estimate accurately, given imprecision in measurement of both the exposure and the outcome. Furthermore, simply knowing that an exposure has a noxious effect (e.g., tobacco smoke, environmental lead) can often be sufficient to motivate the policy to remove or curb this exposure. The “subtraction method,” on the other hand, presupposes that the variables used in the model capture all or the great majority of the variance attributable to factors in the environment. Clearly this assumption cannot be met, and it will never be possible to measure all of the lifetime contributions of known or unrecognized exposures. Furthermore, the measurement of many exposures is not comparable across groups; for example, earning power at similar levels of education is very different for blacks and whites, and purchasing power is different for similar incomes (Auerbach and Kringold, 2001). Contrariwise, race/ethnicity, as socially defined, is measured with precision and will absorb the information latent in the confounders, an outcome that would be apparent if sensitivity analyses were conducted (Kaufman et al., 1997). The point being made here is not that epidemiologists occasionally rely on fuzzy logic or fall back on loosely formulated generalizations. Instead, it is being argued that an inferential process that can offer no evidence for or against the hypothesis is central to the analysis strategy epidemiologists have used to study race.
In fact, the “subtraction method” has so many limitations and biases that the relevant question becomes, why has it been accepted as the standard approach? Whatever more general speculation might be invited by this question, it seems clear that a strong a priori assumption about the essentialist nature of race is required before the “subtraction method” makes sense as an analytic tool. The contribution of genetics to interethnic variation for most health traits remains completely unknown, making this a legitimate area of controversy. However, the current indirect methods are incapable of generating empirical evidence on this question, any more than alchemy can advance the field of chemistry. Faith in the potential of these methods, and the uncritical willingness to use them, demonstrates instead a belief that race, as currently operationalized in public health, codes for substantial information about genetic factors that influence disease susceptibility.
In addition to the methods that attempt to estimate genetic effects in a semiquantitative way, a substantial literature on racial/ethnic variation in health simply posits a genetic cause and argues that any observed differences could be the explanation. A group of investigators recently justified this approach by pointing out—correctly—that it represents accepted practice: “Because of observed group differences in the risk of hypertension …
research continues to treat these groups as distinct biological entities and investigates the genetic factors that might account for the differences …” (Brewster, Clark, and van Montfrans, 2000, p. 1541). Within this framework they proceeded to “hypothesize that the genetic factor increasing the ability of black people … to develop higher blood pressures … is greater total intracellular activity of the central regulatory enzyme of energy metabolism, creatine kinase …” (Brewster et al., 2000, p. 1541). Unfortunately for this argument, while racial differences in creatine kinase activity have been reported, no evidence links creatine kinase with hypertension and no genetic mechanisms are identified; why someone would propose this physiologic measure as a cause of black-white differences in hypertension is therefore obscure.
Postulating that any observed group differences could be a cause invites a serious risk of Type I error. For example, similar theories, based on intracellular calcium, telomere length, insulin resistance, renin secretion, kallikrein, beta-hydroxylase, growth factors, melanin, cation transport, and a host of other physiologic traits, all of which lack an established causal relationship to hypertension, have been proposed as “genetic causes” of the excess hypertension in blacks (Cooper and Kaufman, 1998; Cooper and Rotimi, 1994; Fekete et al., 1996; Fray and Douglas, 1993; Gillum, 1979; Saad et al., 1991). In the same manner, a difference in resting metabolic rate between blacks and whites has been advanced as a cause of the excess obesity among blacks, even though no causal link between resting metabolic rate and obesity has been established (Kimm et al., 2002; Morrison, Alfaro, Khoury, Thornton, and Daniels, 1996; Yanovski, Reynolds, Boyle, and Yanovski, 1997). It is hard to conceive of stronger evidence of a commitment to an essentialist notion of race than exemplified by this literature.
Molecular Approaches to the Study of Genes That Could Influence Interethnic Variation in Health
The current challenge in the study of interethnic variation in health is how to move from inferences based on the phenotype, which is a product of the interaction between genes and environment, to those based on genotype. Because this territory is almost entirely uncharted in studies of humans, assumptions must be made about the likely magnitude and distribution of the effects that should be sought (Clayton and McKeigue, 2001). For any given genotype-phenotype relationship, an inverse relationship exists between the size of the effect of a given variant and the number of variants involved. For diseases like hypertension, where many genetic variants are likely to be involved, it therefore follows that the effect of any one variant will be small. Contrariwise, in monogenic disorders a single mutation can have a devastating effect. Because single gene disorders generally
confer a selective disadvantage, these mutations are infrequent in number and are maintained at a low rate in the population. Unique among monogenic disorders, sickle cell disease and related red cell defects have attained high frequency in endemic malaria regions. Despite the attention they have received, in industrial societies like the United States these monogenic diseases make a negligible contribution to interethnic variation. Based on the calculation of excess deaths, for example, hematologic disorders account for only 0.3 percent of the black-white differential (Cooper, 1984).
One gene with a large effect is easier to find than many with small effects, and the great successes of molecular genetics to date have been in monogenic disorders. The contradiction between studying the rare genetic disorders and the public support required for “big science” has not been lost on geneticists, however. As noted in a recent editorial, with the Human Genome Project coming to an end, the challenge to biologists is to “make the genome relevant to public health. This relevance will not be found by identifying genes associated with rare hereditary diseases, but by … (tackling) … the common, complex diseases” (Anonymous, 2002, p. 199). Accordingly, over the past decade, genetic epidemiology has shifted its focus dramatically from monogenic to common chronic diseases, driven in large part by the new opportunities for molecular analysis (Collins, Lonjou, and Morton, 1999; Lander, 1996; Lander and Schork, 1994). Conditions that have public health impact, like hypertension, diabetes, atherosclerosis, obesity, and the common cancers, are now being subjected to intense scrutiny.
Although several variants are assumed to combine to create susceptibility to these traits, whether they are oligogenic (determined by moderate numbers of variants, each with moderate effects) or fully polygenic (many variants, each with small effects) is still unknown. No matter which model is adopted, the search for these variants has been challenging (Chagnon, Perusse, Weisnagel, Rankinen, and Bouchard, 2000; Doris, 2001; Levy et al., 2000; Risch, 2000) and the weak impact of individual variants may be the least of the problem. While most chronic conditions have substantial genetic components, as demonstrated by the familial aggregation, environmental exposures are likely to play the dominant role. Interactions of these environmental factors, many of which may still be unidentified, with the genetic substrate create complex patterns that are still difficult to conceptualize and even harder to measure.
Defining genetic factors that account for interethnic variation in health is dependent on the success of this “gene hunting” operation in a stepwise process. First, influential variants must be identified, and then their effect and frequency compared across groups. Two general approaches are currently being used in this first stage to search for the genetic variants. Anonymous markers that do not code protein sequences are genotyped in family
members, and the co-segregation of chromosomal segments with the phenotype is used to localize potential variants. This method, known as the “genome scan,” relies on linkage between the marker and the trait, and has been applied with great success to monogenic disorders. At the next stage, “positional cloning” is used to identify the variant itself. A second, complementary approach postulates candidate genes at the outset, from physiology or animal experimental research primarily, and tests specific variants in these genes. The candidate gene approach can use linkage analysis, based on related individuals, or the case-control design, known in genetic epidemiology as an “association study.”
Finding Genes: The Genome Scan Approach
A broad experience with genome scans now exists, involving traits that range from blood pressure and height to psychiatric disorders (Doris, 2001; Hirschhorn et al., 2001; Laitinen et al., 2001; Levy et al., 2000; Province et al., 2003; Wu et al., 2002). As already suggested, this method has met with only modest success when applied to complex traits (Altmuller, Palmer, Fischer, Scherb, and Wjst, 2001; Hugot et al., 2001; Risch, 2000; Tavtigian et al., 2001). The statistical power of this method—that is, the ability to find an influential locus if one exists—is limited, while the risk of a Type 1 error—false-positive results—is high. If, for example, the total genetic variance associated with a trait is 30 percent, and 10 genes are involved, then the method needs to have sufficient power to detect average effects of 3 percent. In general, power for most genome scan studies is only adequate for effects in the 10 to 20 percent range. On the other hand, these assumptions may not be entirely realistic because it is unlikely that the impacts of various susceptibility genes are evenly distributed, and there is reason for optimism that some loci influence a good deal more than 3 percent of the variance in some families.
Given the focus here on the potential contribution of genes to interethnic variation in health, a detailed summary of the accomplishments based on the genome scan as an aid to cloning susceptibility genes is not necessary. The application of the genome scan approach is still at the first stage for most conditions—attempting to find reproducible evidence of linkage to particular chromosomal regions (Chagnon, Perusse, Weisnagel, Rankinen, and Bouchard, 2000; Doris, 2001). However, some consistency has begun to emerge. Obesity, a continuously distributed trait with a polygenic inheritance pattern, can serve as an illustrative example. Evidence of linkage using the genome scan approach has emerged in several studies for regions on chromosomes 3 and 7 (Chagnon et al., 2000). In a meta-analysis of 6,800 individuals from four ethnic groups, a region on chromosome 3 was linked to obesity in more than one of the racial/ethnic populations and in
the combined sample at a high level of statistical significance (log odds = 3.6) (Wu et al., 2002). With independent replication in multiple population samples, some confidence is warranted in the linkage finding on chromosome 3; the second stage, pulling a causal variant out of a region spanning 10 to 20 million nucleotides, is nonetheless daunting. Hypertension appears to be more resistant to this approach, where even more inconsistency across racial groups has been observed (Doris, 2001; Province et al., 2003), and studies of psychiatric disorders also have been hard to replicate (Pato, Schindler, and Pato, 2000). On the other hand, the number of influential genes may vary across traits, making some easier to unravel than others, and enhanced analytic tools are being developed for both the laboratory and statistical methods that may reduce the technical obstacles.
A more general problem related to genes and ethnic disparities emerges from this literature. The interpretation of genome scans from various ethnic population samples immediately confronts a dilemma: Should we regard these populations as separate entities? In other words, having undertaken a genome scan involving whites and blacks, should we pool the results, or treat them as separate samples? If we find differences between the two samples, should we regard these as an indication that different genetic effects exist in the two groups, or should we conclude simply that they are the result of sampling variation, as might occur with repeated studies in the same ethnic group? Given our ignorance about the existence of subunits within the human species, or their correspondence to groups we label as races, there is no a priori basis for conducting analyses separately. Technical problems arise in linkage analysis when divergent populations are pooled because the allele frequencies of the “microsatellite” markers may vary across groups, but this limitation can be overcome. From this perspective, it is illuminating to observe that all analyses performed to date have carefully adhered to the principle of distinguishing among population groups as if they were genetic categories. Furthermore, some analysts have concluded that different results obtained in samples of blacks and whites provide evidence of different underlying genetic effects (Collaborative Study on the Genetics of Asthma, 1997), a conclusion that, even in the short interval that has passed since the work was conducted, can be recognized as wholly unjustified. As with indirect methods, the internal evidence from within the discipline on the conduct of this research demonstrates a commitment to a concept of race that has its provenance outside science.
Finding Genes: The Candidate Gene Approach
An even more extensive literature exists for candidate genes, given the less demanding technical requirements for this design compared to the genome scan. The approach to this enormous field, as above, must be to
focus on an illustrative example. Hypertension is the most common disease trait in our population, with a lifetime risk approaching 90 percent, and a population-attributable risk for mortality of about 10 percent (Vasan et al., 2002). It has been recognized since the 1930s that black Americans have a prevalence rate that is twice as high as found among whites, and hypertension is the largest single contributor to the black-white disparity (Cooper and Rotimi, 1997). Uncontrolled hypertension is a direct cause of stroke, coronary heart disease, renal failure, heart failure, and vascular dementia. The detailed understanding of the physiology of hypertension has made it possible to identify a large number of candidate genes. The renin-angiotensin system (RAS), which plays a key role in regulating salt and water metabolism and maintaining vascular tone, has been particularly well studied. The RAS has four principal components. Angiotensinogen (AGT), the protein substrate produced by the liver, circulates in excess in the plasma and is cleaved by renin to make angiotensin-I (Ang-I). Ang-I is rapidly cleaved by the angiotensin converting enzyme (ACE) to Ang-II, which then interacts with tissue receptors. Heralding a false spring early in the course of candidate gene research, investigators from Utah and France demonstrated that a highly significant association of variants in the AGT gene influenced both circulating AGT levels and risk of hypertension (Jeunemaitre et al., 1992). Identification of an easily typed marker in ACE—the so-called “insertion/ deletion” or “I/D” marker—provoked an outpouring of publications on this gene as well. Subsequent meta-analyses suggested modest effects for variants in these two genes (Soubrier, 1998; Staessen et al., 1999), although much of this literature was negative.
Because it has been well characterized, the RAS provides a useful summary of the current state of knowledge regarding candidate genes and their potential impact on interethnic variation in health. The ACE gene is composed of a sequence of 24,000 nucleotides, of which 75 are known to vary. The first problem confronting the genetic epidemiologist is how to summarize and manage this variation. The vast majority of research to date has used only one variant in a gene to capture all the genetic information, and it is generally not known whether this marker is causal, simply linked physically to (i.e., confounded by the presence of) other causal variants at a neighboring site, or uninformative about hidden causal variants.
Attempting to address these questions immediately provokes the more fundamental question posed earlier: What is the scope of human genetic variation and how do we measure it? Variation can be reasonably summarized in two ways. The most basic unit of genetic information is the base pair, known as the “single nucleotide polymorphism” (SNP) when it is found in more than one form. Given the nature of evolution, and the relative youth of our species, large segments of chromosomes are found in identical “blocks,” as one might expect in large families (Reich et al.,
2001). These blocks, sometimes referred to as “haplotypes,” span large distances in the genome, and the SNPs within these blocks are inherited in a fixed pattern (i.e., all SNPs in the blocks are in the same form). In many instances only two or three different blocks will be present in the population. In addition to the specific SNPs, the size and distribution of the haplotypes also characterize the diversity within a population, reflecting the combined effects of age and size.
Initial work on ACE linked the I/D variant to circulating levels of the enzyme (Rigat et al., 1990). However, this variant was found in an intron, or a noncoding segment of the gene, and was simply linked to the causal variants. The challenge then became one of dissecting the ACE gene into sufficiently small segments to isolate the causal SNPs. Analysis of the ACE gene in a large number of individuals confirmed previous findings of greater genetic diversity in persons of African origin (Zhu et al., 2000). A total of 28 haplotypes were identified among blacks, while only 3 occurred in moderate frequency among Europeans (Zhu et al., 2000). The high information content available in the African-origin samples in turn made it possible to conduct fine mapping studies, that is, to localize effects within the gene. Two areas were identified that appeared to influence ACE levels. Subsequently these techniques were applied to a sample of Nigerians and a moderately strong association with blood pressure was confirmed in the same manner (Zhu et al., 2001). The limited haplotype diversity present in the European samples at this particular locus would not have permitted similar analyses (Reich et al., 2001; Zhu et al., 2003a), although there is no reason to think that the effect varies between ethnic groups.
Ultimately molecular epidemiology requires identification of the variation in DNA sequence that causes variation in the phenotype. Hundreds of mutations have been found in many monogenic disorders, such as cystic fibrosis and retinitis pigmentosa (Cystic Fibrosis Genetic Analysis Consortium, 1994; RetNet1), although a few common variants account for most cases. The impact of these variants on the phenotype can in some instances be little influenced by the environment. In polygenic disorders, however, the effects of different variants within the same gene and variants in different genes are obviously more subtle and complex. Certain effects will be present only in certain environments, and interactions between sets of genetic variants are also likely to occur.
In effect, there is no abstract “genetic effect” because the actions of genes are only detectable by their influence on a phenotype, and therefore the product of a particular environment. Given that the mixture of environment and genetic background may vary across racial/ethnic populations, in many instances it may be difficult to isolate the causal genetic effect separately from the environmental effect. Certainly it will be difficult to summa-
rize all the possible genetic effects into an overall estimate of the relative degree of susceptibility of two populations.
Pharmacogenetics
In addition to the potential role that genetic variation might play in disease susceptibility, there is currently great interest in whether the response to therapeutic drugs can be predicted by genotype. The most important aim of pharmacogenetics is to sort patients into “responders” and “nonresponders,” thereby improving the efficiency of the medical encounter. The complementary aim is to identify persons who might be at high risk of severe side effects from a particular drug. A parallel interest exists in whether these responses vary by race. Given the widespread use of drugs for chronic conditions, particularly among the elderly, the public health implications of relative efficacy could be substantial.
The frequencies of polymorphisms affecting drug metabolism do vary among population groups (McLeod, 2001). The critical question, as in other applications of the race concept, is whether this classification scheme improves or harms our understanding of the variation we observe. Using molecular techniques, a direct test can be made of the question of whether or not geographic patterns of genetic variation structure interindividual variation in drug response (Wilson et al., 2001). To test this hypothesis, microsatellite markers, similar to those used in genome scan analyses, were typed on individuals from eight regional populations and the population structure—the degree of genetic relatedness of the groups—was inferred (Wilson et al., 2001). Genotyping was then conducted on polymorphisms in drug-metabolizing enzymes of the cytochrome p450 system. Finally, a comparison was made of the relative accuracy with which variants in the enzymes were predicted using standard ethnic labels versus the genetic clustering algorithm (Wilson et al., 2001). Two important results emerged. First, adding molecular data to the classification process improved prediction over ethnic labels for three of the four enzymes examined. However, both schemes were relatively ineffective at predicting individual responses, reminding us again that variation among individuals is orders of magnitude greater than variation among population groups. Pharmacogenetics confronts the same dilemma faced over the years by epidemiology; despite the perceived meaning of race, and its enormous currency in social life, the genetic content of these categories never meets our expectations.
Focused candidate gene studies have started to yield positive results, however. Hypertension presents an attractive opportunity for pharmacogenomics. Heterogeneity in drug response exists among individuals and across classes of agents and, because lifelong therapy is required, tailored prescribing could be more effective than “trial-and-error” methods. In a
sample of 387 patients, the blood pressure response to a therapeutic challenge with a diuretic was examined after stratifying on a marker associated with the “G protein” (Turner, Schwartz, Chapman, and Boerwinkle, 2001). Among persons homozygous for the T allele, the fall in systolic blood pressure was 16 mmHg, compared to 10 mmHg among persons with the opposite genotype (Turner et al., 2001). Although no differences were noted in the degree of response by genotype among blacks and whites, the prevalence of the T allele was much higher among blacks than whites (Turner et al., 2001). A recent report of a nutritional intervention also showed a markedly different response in blood pressure on the basis of a genotype in the AGT gene (Svetky et al., 2001). Again, the “at risk” allele was more frequent in blacks than whites (Svetky et al., 2001). Taken together, these studies suggest that provocative interventions may be more effective at unmasking genetic effects, at least for blood pressure, and open new opportunities to define the role of candidate genes. Likewise, they present the first reasonable evidence that factors conferring genetic susceptibility to hypertension may be differentially distributed among these population groups. However, only limited marker sets were examined in both of these studies and replication certainly will be required (Yancy, 2001).
A related controversy has arisen over the interpretation of differences among ethnic groups in drug response seen in treatment trials. In a post hoc analysis of a randomized trial of ACE inhibitors for heart failure, the drug was found to reduce the rate of hospitalization significantly more in whites than in blacks (Exner, 2001). Reanalysis of two other small heart failure trials also demonstrated nonsignificant racial differences in response to treatment with ACE inhibitors (Carson et al., 1999). Based on this evidence, a request was approved by the Food and Drug Administration to test a non-ACE inhibitor drug for heart failure as the first “race-specific” therapy. Attempts to drop inclusion of ACE inhibitor therapy for blacks as part of quality assurance requirements were also reported (Masoudi and Havranek, 2001). However, the evidence in support of this race-specific effect has been challenged. The original analysis was based on the rate of hospitalization, only one of the nonfatal end points from the large Studies of Left Ventricular Dysfunction (SOLVD) (Exner, 2001). When development of heart failure symptoms was examined in SOLVD, the relative efficacy of the ACE inhibitor was the same in blacks and whites (relative risk 0.58 versus 0.55, respectively) (Dries, Cooper, Strong, and Drazner, 2002). Most likely the original result obtained by Exner (2001) was due to a Type 1 error. Drug choice is increasingly dictated by the evidence from clinical trials; there is currently no basis for thinking that race should override trial evidence. A treatment that has been unequivocally established, like ACE inhibition, must be assumed to work in all subgroups of humans.
WHAT IS THIS THING CALLED RACE?
As evident from the preceding sections, it is being argued here that race is the organizing principle of the debate over genes and ethnic disparities. There is essentially no distinction, it is further argued, between the popular notions of race that are absorbed into consciousness as categories inherent in nature, like gender or species, and the concept of race as applied in biological research. But an external event is now impinging on the social process that creates and maintains this idea—molecular genetics has given us probes that make it possible to explore the biologic reality behind the accepted beliefs. The moment is not too far off when the race concept will acquire an empirical foundation, displacing, or at least challenging, its ideological claims. Can we predict what this concept might look like?
As with all branches of science, accretion of data around a new construct requires a symbolic or metaphorical image to represent the abstract notion that is the organizing principle. Revisiting earlier questions after the excursion through genetic epidemiology, we can now ask, what image should we use to describe the shape of the collective human genome? The sequencing phase of the Human Genome Project has spawned a variety of metaphors, usually related to the basic theme of the “letters in the alphabet of biology” or the “Book of Life.” Ignoring the inevitable letdown that followed the “genome hype,” the publication of the human sequence has not immediately opened new vistas on the biological world (Anonymous, 2002; Lewontin, 2000). This occurred in part, of course, because we do not know how to read this alphabet. Years of painstaking molecular biology will be required to decipher the individual signals encoded in the genome, most of which are shared across the biological world. On the other hand, current technology is reasonably well suited to the challenge of describing the overall pattern of variation within the species (Reich et al., 2001; Romualdi et al., 2002). This exploration, as argued previously, should inform our understanding of the concept of race.
The technical study of human genetic variation traditionally has fallen within population genetics. A major experimental tool has been the estimation of genetic distance as a means of reconstructing ancestral relationships and the history of geographic dispersion (Cavalli-Sforza et al., 1996). Typically this research program has relied on the metaphor of the tree, placing various modern populations on branches with ever-increasing degrees of separation. As a means of representation, the phylogenetic tree is more of a diagram than a symbolic image, and geneticists are appropriately skeptical of the broad implications of this classification scheme for phenotypic traits. Genetic distance by itself is not meant to imply that populations could not share genetic variants. Given their encounters with data, as well as the past association with eugenics, many geneticists question the value of racial
distinctions (McLeod, 2001; Wilson et al., 2001). Nonetheless, the image of the tree places emphasis on degrees of separation and subverts the question of similarity, or the relationship of the parts to the whole.
A different image is more widely used than the phylogenetic tree. In the popular mind, race is accepted as an important, albeit imprecise, proxy for heritable influences on traits ranging from IQ to obesity (Herrnstein and Murray, 1994; Kimm et al., 2002). The image employed is the normal distribution, with the mean of one population group shifted relative to the other. The genome is conceived of as a fixed, independent cause, projected directly onto the phenotype, without the filtering or conditioning effect of environment. By measuring the distribution of a trait, such as height or blood pressure, we can therefore infer the distribution of the underlying genetic determinants. Developed in its most complete form in relation to IQ, but borrowed wholesale by public health, this construct provides the foundation for the hypothesis of discrete “genes for intelligence” or “genes for hypertension” (Muntaner, Nieto, and O’Campo, 1996).
Just as the discovery of microorganisms transformed our understanding of infection, the ability to measure genotype challenges previous concepts of human variation. First and foremost is the question, do racial categories delimit human variation in a meaningful way? Or, as 19th century biologists would say, are we “carving nature at the joints?” Since the first scientific speculation on the genetic composition of our species, the discrete versus continuous nature of variation has been a central controversy. To support his general argument that evolution shaped the biological world and united it as a single whole, Darwin (1981, p. 194) resisted the notion of racial categories:
[T]he most weighty of all the arguments against treating the races of man as distinct species, is that they graduate into each other, independently in many cases, of their having intercrossed. Man has been studied more carefully than any other organic being, and yet there is the greatest possible diversity amongst capable judges whether he should be classed as a single species or race …. This diversity of judgment does not prove that the races ought not to be ranked as a species but it shows that it is hardly possible to discover clear distinctive characters between them. Every naturalist who has had the misfortune to undertake the description of a group of highly varying organisms, has encountered cases precisely like that of man; and if of a cautious disposition, he will end by uniting all the forms which graduate into each other as a single species; for he will say to himself that he has no right to give names to objects which he cannot define.
This question can now be reformulated in the technical language of molecular genetics. A contemporary version is summarized by Templeton (1999, p. 647):
Race is generally used as a synonym for subspecies, which traditionally is a geographically circumscribed, genetically differentiated population. Sometimes traits show independent patterns of geographic variation such that some combination will distinguish most populations from all others. To avoid making “race” the equivalent of a local population, minimal thresholds of differentiation are imposed. Human “races” are below the thresholds used in other species, so valid traditional subspecies do not exist in humans. A “subspecies” can also be defined as a distinct evolutionary lineage within a species. Genetic surveys and the analyses of DNA haplotype trees show that human “races” are not distinct lineages, and that this is not due to recent admixture; human “races” are not and never were “pure.” Instead, human evolution has been and is characterized by many locally differentiated populations coexisting at any given time, but with sufficient genetic contact to make all of humanity a single lineage sharing a common evolutionary fate.
The evidence for this common evolutionary lineage, with its origins in Africa, continues to emerge with increasing clarity as genomic science advances. Recent developments now demonstrate this phenomenon at the level of the organization of the genome. As described previously, DNA variants that are physically close to each other tend to be correlated, that is, two individuals who inherit a particular variant at a locus will tend to share the variants found at loci in close physical proximity on the same chromosome. This result occurs because “blocks” or pieces of chromosome are passed down over time through the population (Reich et al., 2001; Stephens et al., 2001). This pattern of sharing is dissipated as the distance between variants increases. If, however, race or ethnicity defines a natural unit within the species, then variants at physically unlinked loci (e.g., on different chromosomes) also should be shared more commonly among members of that race. A more general way to formulate this question is to ask, to what degree is there structure in the human genome? Massive genotyping experiments based on randomly selected segments of the human genome have made it possible to test this question directly (Gabriel et al., 2002; Reich et al., 2001; Stephens et al., 2001), revisiting with genetic data Lewontin’s analysis based on variation in proteins (Lewontin, 1972, 1974). These efforts are ongoing, but, initial results suggest that although the distribution of variants from representative continental populations cannot be considered entirely random, the degree of correlation among unlinked markers is negligible in quantitative terms (Romualdi et al., 2002). Thus, although variation among populations exists for some genetic variants, this variation is not aggregated or “packaged” in demographic units, but for the most part occurs piecemeal or random; it is continuous over space rather than occurring in discrete categories (Romualdi et al., 2002). One cannot
reliably predict the probability of unknown variants from the frequencies of known variants.
The Practical Implications of Race for Public Health Today
The question that race brings to public health is not whether random polymorphisms aggregate, but whether disease variants aggregate. While it is possible, although unproven, that some individual variants could be particularly influential, it is usually assumed that susceptibility to complex diseases involves the sum of many variants, and these variants occur at a relatively high frequency in the population—the so-called “common disease-common variant” hypothesis (Chakravarti, 1999; Lander, 1996). Distributed across the genome, each locus will have a weak effect, and a complementary set of these variants is necessary to create susceptibility. Alternatively, there could be many rare variants that in combination confer susceptibility (Pritchard, 2001). As noted earlier, the effects of these mutations are likely to be uniformly small, although the possibility does exist that some genes could have moderate to large effects.
These assumptions have considerable relevance for interethnic variation. Common genetic mutations are by necessity old, given the time required for them to become widely distributed, and they are usually found in all ethnic groups (Halushka et al., 1999). The “common disease-common variant” hypothesis would therefore lead to the conclusion that the important disease-susceptibility mutations are distributed in a global pattern. On the other hand, rare variants would have most likely arisen after dispersion from Africa. For example, the mutations underlying Tay-Sachs and cystic fibrosis, which occur frequently in European subpopulations, are rare in Africa. Just as the mutations for these monogenic disorders are unequally distributed in ethnic groups, rare variants with large effects on chronic diseases could be spread unevenly in modern populations. However, if the “common disease-common variant” hypothesis is correct, as seems most likely, multiple polymorphisms would have to be present in one population, but absent in others.
What mechanisms could therefore be involved in generating the clusters of susceptibility variants necessary to create excess risk in an ethnic population? Two plausible mechanisms can be invoked—the play of chance or evolutionary selection. Influential variants could be differentially distributed by chance in particular ethnic groups, and this must occur to some extent. Viewed across all conditions, however, one would expect a balanced pattern of advantage and disadvantage when comparing large populations. As argued earlier, the possibility that chance could have created the pattern of health differentials seen in the United States, particularly the
pervasive black disadvantage, is so remote as to be implausible. As the basis for variation in any single disease, chance cannot be ruled out, but it should be an explanation of last resort, particularly if one assumes a combination of several variants is required. In terms of a general principle, we are left with selection as the main organizing theory.
Could selection have created racial variation in susceptibility to the diseases of late adulthood that have emerged in industrialized society? Most of the diseases of public health concern were not subject to selective pressure in the past. Nonetheless, some of the underlying traits—such as blood pressure and body composition—probably were because survival value would be attached to adequate functioning of these systems. Variation in susceptibility to common disease among regional populations therefore could have resulted from differential selective pressure in varying environments. This model, of course, has been used successfully to explain the variation in sickle cell and thalassemia. Likewise, it must apply to skin pigmentation, although the mechanism has been difficult to establish (Robins, 1991). Several attempts have been made to transfer this model to chronic conditions, such as blood pressure and obesity that result from dysregulation of physiologic systems under the stress of the modern lifestyle. The “slavery hypothesis” was developed to explain excess hypertension in blacks based on the proposition that a bottleneck, or a severe restriction of the population diversity, occurred during the “middle passage.” This hypothesis states that selective mortality caused by salt-wasting disorders created genetic susceptibility to hypertension among the surviving African Americans who avidly retained salt (Grim and Robinson, 1996; Wilson, 1986). The “thrifty gene” hypothesis argues that the selection advantage against starvation conferred by an ability to store excess calories as fat is the cause of diabetes in Native Americans and Pacific Islanders (Hegele, 2001; Neel, 1999; Ravussin and Bogardus, 1990; Weiss, Ferrell, and Hanis, 1984).
In effect these hypotheses are an attempt to apply the race concept and are subject to the general criticisms outlined previously. In addition, more specific tests can be proposed. For example, the historical evidence does not support high mortality during the “middle passage” of slavery and there is no genetic evidence of a “bottleneck” that narrowed diversity among blacks in the Americas (Curtin, 1992; Gabriel et al., 2002; Zhu et al., 2003b). To the contrary, evidence exists for a demographic bottleneck during the formation of the European population (Reich et al., 2001). The epidemic of obesity and subsequent diabetes has now overwhelmed so many racial and ethnic groups that this particular “thrifty gene” must be a human gene; diabetes occurs at an equally high prevalence among such disparate groups as black Americans, Mexican Americans, Australian Aborigines, Polynesians, Chinese in Mauritius, and Saudi Arabians (Franco, 1992; Harris et al., 1998; King and Rewers, 1991; O’Dea, Spargo, and Nestel, 1982). Despite these shortcomings, the
“slavery hypothesis” and the “thrifty gene” struck a responsive chord and have achieved wide acceptance. Again the interesting question becomes, why are these “just so” stories embraced as fact? As with the other examples discussed here, the concept of race creates a belief structure that is receptive to explanations built on genetic determinism.
Race as a Diagnostic Test for Genetic Disorders
Race could serve as a surrogate marker for risk of specific single-gene mutations. In formal terms the precision of “race as a diagnostic test” can be evaluated using the standard clinical measures of sensitivity, specificity, and predictive power. The broadest public health experience with this application lies with postnatal screening for sickle cell (Centers for Disease Control and Prevention, 2003). After the demonstration that penicillin was effective in life-threatening infections in newborns with sickle cell, neonatal screening programs were initiated. In the initial phases only black infants were screened. However, it was soon recognized that sensitivity, or the case detection rate, was not optimal. Black race is not a reliable marker for the prevalence of sickle cell disease in the United States (Table 8-4). In many states universal screening has now been implemented, increasing the number of cases identified by as much as 30 percent (i.e., sensitivity before universal screening was only 70 percent, which is inadequate for a screening procedure).
In a contrary example, the high prevalence of Tay-Sachs among Ashkenazi Jews permits more focused screening for this condition (Khoury, 2000). It may also be possible that other genetic mutations could vary so markedly by race/ethnicity that targeted screening or interventions will be justified, but the nonassortative mating common in multiethnic societies will dilute this effect, and the example of sickle cell is probably more typical.
TABLE 8-4 Racial/Ethnic Variation in the Prevalence of Sickle Cell Disease Among Live Births, 1990
|
Racial/Ethnic Group |
Prevalence* |
|
White |
2 |
|
Black |
289 |
|
Hispanic, total |
5 |
|
Hispanic, eastern states |
90 |
|
Hispanic, western states |
3 |
|
Asian |
7 |
|
Native American |
36 |
|
*Per 100,000. SOURCE: CDC, Office of Genetics and Disease Prevention. |
|
It is still difficult, however, to define in practical terms the independent public health role of race as a marker for genetic susceptibility. If it is important to know whether a patient has a variant that confers susceptibility or an unusual drug response, it will have to be measured directly, although targeted screening may be justified in some instances. Community surveillance data on disease incidence, not inference from race, is likely to remain the only reliable measure on which to base public health interventions.
GENETICS AND THE HEALTH STATUS OF BLACK AMERICANS
No discussion of genetic factors and racial/ethnic disparities would be complete without consideration of the contrasts drawn between Africans and non-Africans. The first imperative derives from the undeniable centrality of distinctions between black and white as the basis of the meaning of race. Although the history of assimilation into U.S. society has many chapters, and they all include stories of hard times, discrimination, slavery, and institutionalized racism directed at persons of African descent have inflicted the deepest wounds. Secondly, the public health goal of “eliminating disparities” takes its meaning from the health disadvantage among blacks summarized in Table 8-1. Urgent health problems exist for many U.S. subpopulations, but a uniform pattern of disadvantage, stretching from perinatal health to dementia and reduction of life expectancy, is found only among black Americans. Finally, the impact of race on other aspects of social life, such as housing, education, and employment, is more significant among blacks.
Africa entered the modern world through a distinctive route. Having nurtured the physical development of our species and generated the foundational ideas of “Western culture” in Egypt (Bernal, 1987), Africa fell behind in the development of technologically based societies. Reunited with the mainstream of Western history through the slave trade and colonialism, Africans remain subject to domination by a persistently hostile culture wherever they live (Gilroy, 1993; Gossett, 1973; Oliver and Atmore, 1992). Consequently Africans on the continent have not shared the material benefits of the capitalist economy and the modern nation state, while their descendants abroad have been excluded from full citizenship in the countries that they did so much to build. This historical framework determines not only the current health status of black populations, but the way in which this health status is studied and explicated.
In response to the challenge posed by empirical notions of inheritance, Europeans excluded Africans from the “human race” (Gossett, 1973; Gould, 1981; Montagu, 1964). As the science of genetics matured in technical and theoretical capacity, this position has been subject to growing
challenge (Darwin, 1981; Gould, 1981; Lewontin, Rose, and Kamin, 1984; Montagu, 1964). Nonetheless, contemporary Western consciousness, in both the popular and scientific realm, continues to rely heavily on assumptions about the “otherness” and “inferiority” of Africans and their descendants (Gilroy, 2000; Gosset, 1973; Gould, 1981; Montagu, 1964). Without taking account of these two complementary forces—the history of the European encounter with Africa and the intellectual and ideological framework that is used to describe its consequences—an analysis of the health status of African Americans will continue to substitute justification for explanation.
While acknowledging the potential of molecular genetics, the tone in this presentation generally has been one of skepticism or outright rejection of many aspects of research on the genetics of race and health. However, there is one crucial area where a convincing answer to an important question has emerged. As suggested previously in the analysis of candidate gene data, the scope of human genetic diversity can now be modeled with a high degree of sophistication. In analyses based on large numbers of SNPs as well as haplotype distributions, the distinction between African and all non-African populations stands out with unmistakable clarity. Primate evolution occurred over millions of years in Africa (Klein, 1989), yet modern humans migrated to other continents only 50,000 to 100,000 years ago, beginning from an original population of about 10,000 (Harpending et al., 1998; Stephens et al., 2001). These migrations either involved small numbers of individuals, or were subject to severe demographic bottlenecks, as in the case of Europe (Reich et al., 2001). As a result the repository of human diversity within modern African populations is greater than among those outside Africa (Harpending et al., 1998).
Within a pattern that completely overlaps, the haplotypes and genetic variants in populations outside of Africa are a subset of those found in Africa; that is, they are a sample of the African whole (Figure 8-3). Given the small size of the founder populations outside Africa, by chance the frequency of particular haplotypes has been exaggerated in some instances, and other haplotypes have been lost. The development of regional populations can therefore be thought of as small pseudopods or “outpouchings” that became detached, and then increased in size, magnifying the distinctive characteristics of the original sample.
In Figure 8-4 the RAS haplotypes illustrate the relatively even distribution among U.S. blacks, in comparison to the “lumpy” pattern among whites. Africans are therefore the trunk of the phylogenetic tree, the origin of our species, the center, not the outlier or the peculiar “other.” Of all of the stories told about race, so far this is the only one with an empirical basis. It is deeply ironic, therefore, that the regional population that is the source of all other populations and today contains the most complete reser-
FIGURE 8-3 Distribution and overlap of haplotype blocks in three major continental groups.
voir of human diversity has been repeatedly characterized as uniquely shaped by evolution and “genetically susceptible” to the broad disease syndromes that now occur worldwide.
Perhaps the story of genetic susceptibility should be rewritten. The humans who left Africa took with them a sample of the common genetic variants that had been collected through random mutation over our evolutionary history; a more complete repository of these mutations remained in Africa. The assortment of these variants in a given individual provides the sum of genetic risk for a specific chronic disease manifested in the modern industrialized environment. The distribution of SNPs and haplotypes among racial/ethnic groups does not demonstrate a generalized pattern of “lumping” or shared variance among contemporary regional populations. Although some important susceptibility alleles may be shared unequally, this
FIGURE 8-4 Frequency distribution of haplotypes in the RAS genes in whites and blacks.
has not been demonstrated yet. Africans share virtually all the common variants found in humanity and should represent the genetic “central tendency” of our species. The image of the genome that suggests itself based on this story is a time-lapse photograph of the sun—a globular whole, fringed with a corona that spurts outward and collapses to the center again.
Studies of population variation in health status will continue to be constrained if deterministic theories like the “thrifty gene” are applied whenever a disease emerges at a higher prevalence in a new ethnic group. The properties of the genome that make it a single unit shared across all regional populations would be a more appropriate premise for comparative analyses.
CONCLUSIONS
The study of genes and variation in the health of populations continues to struggle under two constraints imposed by social reality—one that influences how we frame the questions and the other that determines how people are arranged in the hierarchy. Our notion of race suggests certain answers, thereby prejudicing the questions, and the arrangement of people
by race and class structures the environmental exposures, and, by extension, many aspects of health status. Although both constructs—race and inequality—confront us as products of the natural world, they are in fact reflecting an image of what we have made. That image has become nearly impossible to decipher where race and inequality merge.
Stripping away its claims to a natural origin, what is race? A name we call ourselves, sein finn, as they say in Irish—we ourselves—or a name for the Other? Until very recently the study of race had never been a possibility for science, despite the depths of its penetration into biology. We now have reason to believe that era is about to end as empirical questions begin to dominate the research agenda. Does race have any meaning beyond geography and history? Does the genome of our species aggregate into subunits? Does it even branch into trees? If we see it as a whole, will it look like the sun? These questions, which can be answered only with molecular data, are now at least “askable.”
But scientific questions are not asked in a vacuum. Although it can legitimately be argued that in some instances the results of experimentation describe the shape of nature, questions are patently a human construct, made from materials already available to us in the social environment. Given the potency of race as a cultural idea and its influence on scientific thinking, there is reason for concern about where the attempts to discover the genetic determinants of health and disease will take us. An analogy to history may help frame this dilemma. In questioning the “memory-storage” model of history, attention has been drawn to the variable chance with which events are recorded and archived and how narratives about these events reflect the understanding of the present (Trouillot, 1995). Differential claims on the archive, and differential chances of being the hero of the narrative, are determined by differentials in power. A record of uneven quality, subjected to the filter of ideology, does not yield a description of the past, but rather a context within which we interpret the events of today. Although not a myth, because history makes a different set of claims on reality, the narratives of the past are never true or objective. In that sense, there is no “pastness,” only an understanding of what shaped the present.
Genetics, like history, queries the past, attempting to use the record left in the genome to understand how evolution and population dynamics created the current pattern of variation. Although the course of human history—wars, famine, migration, conquest, and slavery—biases and complicates the record, the ability to know all the sequence variation means that the geneticist’s archive is far more egalitarian and complete than is the historian’s. One need only consider the story of Thomas Jefferson’s family to appreciate the gap between what is recorded in history books and what is recorded in our genome (Ellis, 1998; Foster et al., 1998). If in fact the genetic history of our species is essentially complete and unbiased, the
opportunities for objective description are limitless, constrained only by the questions we are asking. We can, in a sense, no longer blame our sources, but only ourselves.
But of course, after a moment’s reflection, we know this is a false hope; the questions we ask are determined by the history of our consciousness, itself created from the narrative written by those in power today from an inconsistent record of the past. We are returned to the original problem—biological and social history can never be divorced. Biological scientists like to think that they work in an intellectual space uncontaminated by superstition and prejudice and therefore resist the rhetoric of context. But race is a true chimera—part geography, part history, part genes—and neither the idea nor the practice can be understood as anything other than the fantastic creation of the human mind. There is no use of race that isolates the biological from the social.
The study of genes and ethnic variation in health occupies contested territory, where genotype is hidden within phenotype and the biologic is molded by the social. Race attempts to distill and simplify this dialectical process into a linear pattern of cause and effect, obfuscating everything. We are looking to population genetics to give structure to this debate, using empirical observation as the scaffold. Can it relieve us entirely of the concept of race? Races exist only in distinction to each other; “whiteness” is defined by “blackness,” and each is endowed with a distinct essence. If in fact there is no “other,” then there is no essential difference, and the notion becomes empty of meaning. As on so many other occasions, however, these optimistic pronouncements will have no authority, or, at the very least, they will come too late in Western history. We live in a world where every construct is racialized—citizenship, personal identity, neighborhood, health—and it is unlikely that the evidence of genetics will be sufficient to counterbalance the enormous weight of social ideology. But within the narrow discipline of genetic epidemiology, is it too much to ask that essentialist notions, which molecular data are now demonstrating to be mere superstition, should no longer hold sway?
ENDNOTE
|
1. |
Refers to Rtinal Information Network. Information can be found at www.sph.uth.tmc.edu/Retnet/home.htm. |
REFERENCES
Altmuller, J., Palmer, L.J., Fischer, G., Scherb, H., and Wjst, M. (2001). Genomewide scans of complex human disease: True linkage is hard to find. American Journal of Human Genetics, 69, 936-950.
Anonymous. (1989). From the Health Resources and Services Administration. Journal of the American Medical Association, 261(2), 199.
Anonymous. (2002). The HGP: End of phase 1. Nature Genetics, 30(2), 125.
Auerbach, J.A., and Kringold, B.K. (Eds.). (2000). Income, socioeconomic status and health: Exploring the relationships. Washington, DC: National Policy Association.
Bernal, M. (1987). Black Athena: The Afroasiatic roots of classical civilization. New Brunswick, NJ: Rutgers University Press.
Bohnstedt, M., Fox, P.J., and Kohatsu, N.D. (1994). Correlates of mini-mental status examination scores among elderly demented patients: The influence of race-ethnicity. Journal of Clinical Epidemiology, 47, 1381-1387.
Brancati, F.L., Whelton, P.K., Kuller, L.H., and Klag, M.J. (1996). Diabetes mellitus, race and socioeconomic status: A population based study. Annals of Epidemiology, 6, 67-73.
Brancati, F.L., Kao, W.H., Folsom, A.R., Watson, R.L., and Szklo, M. (2000). Incident type 2 diabetes mellitus in African American and white adults: The Atherosclerosis Risk in Communities Study. Journal of the American Medical Association, 283, 2253-2259.
Brewster, L.M., Clark, J.F., and van Montfrans, G.A. (2000). Is greater tissue activity of creatine kinase the genetic factor increasing hypertension risk in black people of sub-Saharan African descent? Journal of Hypertension, 18, 1537-1544.
Carson, P., Ziesche, S., Johnson, G., and Cohn, J.N. (1999). Racial differences in response to therapy for heart failure: Analysis of the vasodilator-heart failure trials. Vasodilator-Heart Failure Trial Study Group. Journal of Cardiac Failure, 5, 178-187.
Cavalli-Sforza, L., Menozzi, P., and Piazza, A. (1996). The history and geography of human genes. Princeton, NJ: Princeton University Press.
Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/genomics/activities/newborn/sickle [accessed September 26, 2003].
Chagnon, Y.C., Perusse, L., Weisnagel, S.J., Rankinen, T., and Bouchard, C. (2000). The human obesity gene map: The 1999 update. Obesity Research, 8, 89-117.
Chakravarti, A. (1999). Population genetics—making sense out of sequence. Nature Genetics, 22, 239-247.
Chaturverdi, N. (2001). Ethnicity as an epidemiology determinant—crudely racist or crucially important? International Journal of Epidemiology, 30, 925-927.
Clayton, D., and McKeigue, P.M. (2001). Epidemiological methods for studying genes and environmental factors in complex diseases. Lancet, 358(9290), 1356-1360.
Collaborative Study on the Genetics of Asthma. (1997, April). A genome-wide search for asthma susceptibility loci in ethnically diverse populations. National Genetics, 15(4), 389-392.
Collins, A., Lonjou, C., and Morton, N.E. (1999). Genetic epidemiology of single-nucleotide polymorphisms. Proceedings of the National Academy of Sciences of the United States of America, 96, 15173-15177.
Collins, J.W., Jr., Wu, S.Y., and David, R.J. (2002). Differing intergenerational birthweights among descendants of U.S.-born and foreign-born whites and African Americans in Illinois. American Journal of Epidemiology, 155, 210-216.
Cooper, R. (1994). A case study in the use of race and ethnicity in public health surveillance. Public Health Reports, 109, 46-52.
Cooper, R., and Rotimi, C. (1997). Hypertension in blacks. American Journal of Hypertension, 10(7), 804-812.
Cooper, R., Rotimi, C., Ataman, S., McGee, D., Osotimehin, B., Kadiri, S., Muna, W., Kingue, S., Fraser, H., Forrester, T., Bennett, F., and Wilks, R. (1997a). Hypertension prevalence in seven populations of African origin. American Journal of Public Health, 87(2), 160-168.
Cooper, R., Rotimi, C., Kaufman, J.S., Owoaje, E.E., Fraser, H., Forrester, T., Wilks, R., Riste, L.K., and Cruickshank, J.K. (1997b). Prevalence of NIDDM among populations of the African diaspora. Diabetes Care, 20(3), 343-348.
Cooper, R., Cutler, J., Desvigne-Nickens, P., Fortmann, S.P., Friedman, L., Havlik, R., Hogeliln, G., Marler, J., McGovern, P., Morosco, G., Mosca, L., Pearson, T., Stamler, J., Stryer, D., and Thom, T. (2001). Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States: Findings of the National Conference on CVD Prevention. Circulation, 102, 3137-3147.
Cooper, R.S. (1984). A note on the biological concept of race and its application in epidemiological research. American Heart Journal, 108, 715-723.
Cooper, R.S. (1993). Health and the social status of blacks in the United States. Annals of Epidemiology, 3, 137-144.
Cooper, R.S., and Kaufman, J.S. (1998). Race and hypertension: Science or nescience? Hypertension, 32, 813-816.
Cooper, R.S., and Psati, B.M. (2003). Genomics and medicine. Annals of Internal Medicine, 137, 576-580.
Cooper, R.S., and Rotimi, C. (1994). Hypertension in populations of West African origin: Is there a genetic predisposition? Journal of Hypertension, 12, 215-227.
Cooper, R.S., Kaufman, J., and Ward, R. (2003). Race and genomics. New England Journal of Medicine, 348, 1166-1170.
Curtin, P.D. (1992). The slavery hypothesis for hypertension among African Americans: The historical evidence. American Journal of Public Health, 82, 1681-1686.
Cystic Fibrosis Genetic Analysis Consortium. (1994). Population variation of common cystic fibrosis mutations. Human Mutation, 4, 167-177.
Darwin, C. (1981). The descent of man. Princeton, NJ: Princeton University Press.
David, R.J., and Collins, J.W., Jr. (1997). Differing birthweight among infants of U.S.-born blacks, African-born blacks and U.S.-born whites. New England Journal of Medicine, 337, 1209-1214.
Daviglus, M.L., Liu, K., Greenland, P., Dyer, A.R., Garside, D.B., Manheim, L., Lowe, L.P., Rodin, M., Lubitz, J., and Stamler, J. (1998). Benefit of a favorable cardiovascular risk-factor profile in middle age with respect to Medicare costs. New England Journal of Medicine, 339, 1122-1129.
Diehl, A.K., and Stern, M.P. (1989). Special health problems of Mexican-Americans: Obesity, gallbladder disease, diabetes mellitus, and cardiovascular disease. Advances in Internal Medicine, 34, 79-96.
Doris, P.A. (2001). Hypertension genetics, single nucleotide polymorphisms, and the common disease: Common variant hypothesis. Hypertension, 39, 323-331.
Dries, D.L., Cooper, R.S., Strong, M., and Drazner, M.H. (2002). Efficacy of ACE-inhibition in reducing progression of asymptomatic left ventricular dysfunction to symptomatic heart failure in black and white patients. Journal of the American College of Cardiology, 40, 311-317.
Ellis, J.J. (1998). American sphinx: The character of Thomas Jefferson. New York: Vintage Books.
Esparza, J., Fox, C., Harper, I.T., Bennett, P.H., Schulz, L.O., Valencia, M.E., and Ravussin, E. (2000). Daily energy expenditure in Mexican and USA Pima Indians: Low physical activity as a possible cause of obesity. International Journal of Obesity and Related Metabolic Disorders, 24, 55-59.
Exner, D.V. (2001). Lesser response to angiotensin-converting-enzyme inhibitor therapy in black as compared with white patients with left ventricular dysfunction . New England Journal of Medicine, 344, 1351-1357.
Fekete, Z., Kimura, M., Horiguchi, M., Gardner, J.P., Nash, F., and Aviv, A. (1996). Differences between store-dependent Ca2+ fluxes in lymphocytes from African Americans and whites. Journal of Hypertension, 14, 1293-1299.
Feldman, M.W., and Lewontin, R.C. (1975). The heritability hangup. Science, 190, 1163-1168.
Foster, E.A., Jobling, M.A., Taylor, P.G., Donnelly, P., de Knijff, P., Mieremet, R., Zerial, T., and Tyler-Smith, C. (1998). Jefferson fathered slave’s last child. Nature, 396, 27-28.
Franco, L.J. (1992). Diabetes in Brazil: A review of recent survey data. Ethnicity and Disease, 2, 158-165.
Fray, J.C.S., and Douglas, J.G. (1993). Pathophysiology of hypertension in blacks. New York: Oxford University Press.
Friedman, D.J., Cohen, B.B., Mahan, C.M., Lederman, R.I., Vezina, R.J., and Dunn, V.H. (1993). Maternal ethnicity and birthweight among blacks. Ethnicity and Disease, 3, 255-269.
Gabriel, S.B., Schaffner, S.F., Nguyen, H., Moore, J.M., Roy, J., Blumensteil, B., Higgins, J., DeFelice, M., Lochner, A., Faggart, M., Liu-Cordero, S.N., Adeyemo, A., Cooper, R.S., Rotimi, C., Ward, R., Lander, E.S., Daly, M.J., and Altshuler, D. (2002). The structure of haplotype blocks in the human genome. Science, 296, 2225-2229.
Gillum, R.F. (1979). Pathophysiology of hypertension in blacks and whites: A review of the basis of racial blood pressure differences. Hypertension, 1, 468-475.
Gilroy, P. (1993). The Black Atlantic: Modernity and double consciousness. Cambridge, MA: Harvard University Press.
Gilroy, P. (2000). Against race: Imagining political culture beyond the color line. Cambridge, MA: Harvard University Press.
Glover, F.E., Coffey, D.S., Douglas, L., Cadogan, M., Russell, H., Tulloch, T., Baker, T.D., Wan, R.L., and Walsh, P.C. (1986). The epidemiology of prostate cancer in Jamaica. Journal of Urology, 159, 1984-1986.
Good, G. (1998). Life expectancy in big cities of the United States, 1992. Chicago: City of Chicago Department of Public Health.
Gossett, T.F. (1973). Race: The history of an idea in America. New York: Schocken Books.
Gould, S.J. (1981). The mismeasure of man. New York: W.W. Norton.
Greenland, S., and Robins, J.M. (1986). Identifiability, exchangeability, and epidemiological confounding. International Journal of Epidemiology, 15(3), 413-419.
Grim, C.E., and Robinson, M. (1996). Blood pressure variation in blacks: Genetic factors. Seminars in Nephrology, 16, 83-93.
Hahn, R.A. (1992). The state of federal health statistics on racial and ethnic groups. Journal of the American Medical Association, 267, 268-271.
Halushka, M., Fan, J.B., Bentley, K., Hsie, L., Weder, A., Cooper, R.S., Lipshutz, R., and Chakravarti, A. (1999). Patterns of single-nucleotide polymorphisms in candidate genes for blood pressure homeostasis. Nature Genetics, 22, 239-247.
Harpending, H.C., Batzer, M.A., Gurven, M., Jorde, L.B., Rogers, A.R., and Sherry, S.T. (1998). Genetic traces of ancient demography. Proceedings of the National Academy of Sciences of the United States of America, 95, 1961-1967.
Harris, M.I., Flegal, K.M., Cowie, C.C., Eberhardt, M.S., Goldstein, D.E., Little, R.R., Wiedmeyer, H.M., and Byrd-Holt, D.D. (1998). Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults: The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care, 21, 518-524.
Hegele, R.A. (2001). Genes and environment in type 2 diabetes and atherosclerosis in Aboriginal Canadians. Current Atherosclerosis Reports, 3, 216-221.
Hendrie, H.C., Osuntoken, B.O., Hall, K.S., Ogunniyi, A.O., Hui, S.L., Unverzagt, F.W., Gureje, O., Rodenberg, C.A., Baiyewu, O., and Musick, B.S. (1995). Prevalence of Alzheimer’s disease and dementia in two communities: Nigerian Africans and African Americans. American Journal of Psychiatry, 152, 1485-1492.
Herrnstein, R., and Murray, C. (1994). The Bell Curve: Intelligence and class structure in American life. New York: Free Press.
Hirschhorn, J.H., Lindgren, C.M., Daly, M.J., Kirby, A., Schaffner, S.F., Burtt, N.P., Altshuler, D., Parker, A., Rioux, J.D., Platko, J., Gaudet, D., Hudson, T.J., Groon, L.C., and Lander, E.S. (2001). Genomewide linkage analysis of stature in multiple populations reveals several regions with evidence of linkage to adult height. American Journal of Human Genetics, 69, 106-116.
Hugot, J.P., Chamailard, M., Zouali, H., Lesage, S., Cezard, J.P., Belaiche, J., Almer, S., Tysk, C., O’Morain, C.A., Gassull, M., Binder, V., Finkel, Y.U., Cortot, A., Modigliani, R., Laurent-Puig, P., Gower-Rousseau, C., Macry, J., Colombel, J.F., Sahbatuou, M., and Thomas, G. (2001). Association of the NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature, 31, 599-603.
Hypertension Detection and Follow-up Program Cooperative Group. (1977). Race, education and prevalence of hypertension. American Journal of Epidemiology, 89, 2277-2281.
James, S.A. (1993). Racial and ethnic differences in infant mortality and low birthweight: A psychosocial critique. Annals of Epidemiology, 3, 130-136.
Jeunemaitre, X., Soubrier, F., Kotelevtsev, Y.V., Lifton, R.P., Williams, C.S., Charru, A., Hunt, S.C., Hopkins, P.N., Williams, R.P., Lalouel, J., and Corvol, P. (1992). Molecular basis of human hypertension: Role of angiotensinogen. Cell, 71, 169-180.
Johnson, K.W., and Payne, G.H. (1984). Report of a working conference on coronary heart disease in black populations. American Heart Journal, 108, 633-862.
Jones, D.W., Sempos, C.T., Thom, T.J., Harrington, A.M., Taylor, H.A., Jr., Fletcher, B.W., Mehrotra, B.D., Wyatt, S.B., and Davis, C.E. (2000). Rising levels of cardiovascular mortality in Mississippi, 1979-1995. American Journal of the Medical Sciences, 319, 131-137.
Kaufman, J.S., and Cooper, R.S. (1996). Descriptive studies of racial differences in disease: In search of the hypothesis. Public Health Reports, 110, 662-666.
Kaufman, J.S., and Cooper, R.S. (1999). Seeking causal explanations in social epidemiology. American Journal of Epidemiology, 150, 113-120.
Kaufman, J.S., and Cooper, R.S. (2001). Considerations for use of racial/ethnic classification in etiologic research. American Journal of Epidemiology, 154, 291-298.
Kaufman, J.S., Cooper, R.S., and McGee, D. (1997). Socioeconomic status and health in blacks and whites: The problem of residual confounding and the resiliency of race. Epidemiology, 6, 621-628.
Khoury, M.J. (2000). Genetics and public health in the 21st century. Oxford, England: Oxford University Press.
Kimm, S.Y.S., Glynn, N.W., Aston, C.E., Damcott, C.M., Poehlman, E.T., Daniles, S.R., and Ferrell, R.E. (2002). Racial differences in the relation between uncoupling protein genes and resting energy expenditure. American Journal of Clinical Nutrition, 75, 714-719.
King, H., and Rewers, M. (1991). Diabetes in adults is now a Third World problem. Bulletin of the World Health Organization, 69, 643-648.
Klein, R.G. (1989). The human career: Human biological and cultural origins. Chicago: University of Chicago Press.
Kleinman, J.C., and Kessel, S.S. (1987). Racial differences in low birthweight: Trends and risk factors. New England Journal of Medicine, 317, 749-753.
Koopman, J.S. (1996). Emerging objectives and methods in epidemiology. American Journal of Public Health, 86, 630-632.
Krieger, N., Rowley, D.L., Herman, A.A., Avery, B., and Phillips, M.T. (1993). Racism, sexism, and social class: Implications for studies of health, disease, and well-being. American Journal of Preventive Medicine, 9(Suppl. 6), 82-122.
Laitinen, T., Daly, M.J., Rioux, J.D., Kauppi, P., Laprise, C., Petays, T., Green, T., Cargill, M., Hahtela, T., Lander, E.S., Laitinen, L.A., Hudson, T.J., and Kere, J. (2001). A susceptibility locus for asthma-related traits on chromosome 7 revealed by genome-wide scan in a founder population. Nature Genetics, 28, 87-91.
Lander, E.S. (1996). The new genomics: Global views of biology. Science, 274, 536-539.
Lander, E.S., and Schork, N.J. (1994). Genetic dissection of complex traits. Science, 265, 2037-2048.
Law, M.R., Frost, C.D., and Wald, N.J. (1991). By how much does dietary salt reduction lower blood pressure? British Medical Journal, 302, 811-815.
Levy, D., DeStefano, A.L., Larson, M.G., O’Donnell, C.J., Lifton, R.P., Gavras, H., Cupples, L.A., and Myers, R.H. (2000). Evidence for a gene influencing blood pressure on chromosome 17: Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the Framingham Heart Study. Hypertension, 36, 477-483.
Lewontin, R.C. (1972). Apportionment of human diversity. Journal of Evolutionary Biology, 6, 381.
Lewontin, R.C. (1974). The genetic basis of evolutionary change. New York: Columbia University Press.
Lewontin, R.C. (1995). Human diversity. New York: Scientific American Library.
Lewontin, R.C. (2000). It ain’t necessarily so: The dream of the human genome and other illusions. New York: New York Review Books.
Lewontin, R.C., Rose, S., and Kamin, L. (1984). Not in our genes: Biology, ideology and human nature. New York: Pantheon Books.
Liao, Y., McGee, D.L., Cao, G., and Cooper, R.S. (1998). Black-white differences in disability and morbidity in the last years of life. American Journal of Epidemiology, 149, 1097-1103.
Liao, Y., McGee, D.L., and Cooper, R.S. (1999). Mortality among US adult Asians and Pacific Islanders: Findings from the National Health Interview Surveys and the National Longitudinal Mortality Study. Ethnicity and Disease, 9, 423-433.
Litonjua, A.A., Carey, V.J., Weiss, S.T., and Gold, D.R. (1999). Race, socioeconomic factors, and area of residence are associated with asthma prevalence. Pediatric Pulmonology, 28, 394-401.
Lott, J.T. (1993). Policy purposes of race and ethnicity: An assessment of federal racial and ethnic categories. Ethnicity and Disease, 3, 221-228.
Markides, K.S., and Coreil, J. (1986). The health of Hispanics in the Southwestern United States: An epidemiologic paradox. Public Health Reports, 101, 253-265.
Masoudi, F.A., and Havranek, E.P. (2001). Race and responsiveness to drugs for heart failure. New England Journal of Medicine, 345, 767.
McGee, D.L., Liao, Y., Cao, G., and Cooper, R.S. (1999). Self-reported health status and mortality in a multi-ethnic cohort. American Journal of Epidemiology, 149, 41-46.
McLeod, H.L. (2001). Pharmacogenetics: More than skin deep. Nature Genetics, 29, 247-248.
Miller, G.J., Maude, G.H., and Beckles, G.L. (1996). Incidence of hypertension and non-insulin dependent diabetes mellitus and associated risk factors in a rapidly developing Caribbean community: The St. James survey, Trinidad. Journal of Epidemiology and Community Health, 50, 497-505.
Montagu, A. (Ed.). (1964). The concept of race. London: Collier-Macmillan.
Morrison, J.A., Alfaro, M.P., Khoury, P., Thornton, B.B., and Daniels, S.F. (1996). Determinants of resting energy expenditure in young black girls and young white girls. Journal of Pediatrics, 129, 637-642.
Muntaner, C., Nieto, F.J., and O’Campo, P. (1996). The bell curve: On race, social class, and epidemiologic research. American Journal of Epidemiology, 144, 531-536.
National Center for Health Statistics. (2000). Health, United States, 2000, with adolescent health chartbook. Hyattsville, MD: Author.
Neel, J.V. (1999). The “thrifty genotype” in 1998. Nutrition Review, 57, S2-9.
O’Dea, K., Spargo, R.M., and Nestel, P.J. (1982). Impact of westernization on carbohydrate and lipid metabolism in Australian Aborigines. Diabetologia, 22, 148-153.
Oliver, R., and Atmore, A. (1992). Africa since 1800. New York: Cambridge University Press.
Ordunez-Garcia, P., Espinosa-Brito, A.D., Cooper, R.S., Kaufman, J., and Nieto, F.J. (1998). Hypertension in Cuba: Evidence of a narrow black-white difference. Journal of Human Hypertension, 12, 111-116.
Pappas, G., Queen, S., Hadden, W., and Fisher, G. (1993). The increasing disparity in mortality between socioeconomic groups in the United States, 1960 and 1986. New England Journal of Medicine, 329, 103-109.
Pato, C.N., Schindler, K.M., and Pato, M.T. (2000). Genetic analyses of schizophrenia. Current Psychiatry Reports, 2, 137-142.
Pritchard, J.K. (2001). Are rare variants responsible for susceptibility to complex diseases? American Journal of Human Genetics, 69, 124-137.
Province, M.A., Kardia, S.L.R., Ranade, K., Rao, D.C., Theil, B.A., Cooper, R.S., Risch, N., Turner, S.T., Cox, D.R., Hunt, S.C., Weder, A.B., and Boerwinkle, E. (2003). Meta-analysis combining genome wide linkage scans from four multicenter networks searching for human hypertension genes: The Family Blood Pressure Program (FBPP). American Journal of Hypertension, 16, 144-147.
Ravussin, E., and Bogardus, C. (1990). Energy expenditure in the obese: Is there a thrifty gene? Infusionstherapie, 17, 108-112.
Reich, D.E., Cargill, M., Bolk, S., Ireland, J., Sabeti, P.C., Richter, D.J., Lavery, T., Kouyoumjian, R., Farhadian, S.F., Ward, R., and Lander, E.S. (2001). Linkage disequilibrium in the human genome. Nature, 444, 199-204.
Relethford, J.H. (1998). Genetics of modern human origins and diversity. Annual Review of Anthropology, 27, 1-23.
Report of the Advisory Committee on Health Research. (2002). Genomics and world health. Geneva, Switzerland: World Health Organization.
Rigat, B., Huyber, C., Alhenc-Gelas, F., Cabmien, F., Corvol, P., and Soubrier, F. (1990). An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. Journal of Clinical Investigation, 86, 1343-1346.
Risch, N. (2000). Searching for the genetic determinants in a new millennium. Nature, 405, 847-856.
Robbins, A.S., Whittemore, A.S., and Thom, D.H. (2000). Differences in socioeconomic status and survival among white and black men with prostate cancer [Letter]. American Journal of Epidemiology, 152, 493.
Robins, A.H. (1991). Biological perspectives on human pigmentation. Cambridge, England: Cambridge University Press.
Romualdi, C., Balding, D., Nasidze, I.S., Risch, G., Robichaux, M., Sherry, S.T., Stoneking, M., Batzer, M.A., and Barbujani, G. (2002). Patterns of human diversity, within and among continents, inferred from biallelic DNA polymorphisms. Genome Research, 12, 602-612.
Root, M. (2001). The problem of race in medicine. Philosophy of the Social Sciences, 31, 20-39.
Rose, S. (1998). Lifelines: Biology beyond determinism. Oxford, England: Oxford University Press.
Saad, M.F., Lillioja, S., Nyomba, B.L., Castillo, C., Ferraro, R., De Gregorio, M., Ravussin, E., Knowler, W.C., Bennett, P.H., and Howard, B.V. (1991). Racial differences in the relation between blood pressure and insulin resistance. New England Journal of Medicine, 324, 733-739.
Sichieri, R., Oliveira, M.C., and Pereira, R.A. (2001). High prevalence of hypertension among black and mulatto women in a Brazilian survey. Ethnicity and Disease, 11, 412-418.
Soubrier, F. (1998). Blood pressure gene at the angiotensin I-converting enzyme locus. Chronicle of a gene foretold. Circulation, 97, 1763-1765.
Staessen, J.A., Kuznetsova, T., Wang, J.G., Emelianov, D., Vlietinck, R., and Fagard, R. (1999). M235T angiotensinogen gene polymorphism and cardiovascular renal risk. Journal of Hypertension, 17, 9-17.
Stamler, R., Stamler, J., Dyer, A., Garside, R., Cooper, R., Berkson, D., Lindberg, H.A., Stevens, F., Schoenberger, J.A., Shekelle, S., Paul, O., Leeper, M., Garside, D., Tokich, T., and Hoeksema, R. (1979). Asymptomatic hyperglycemia and cardiovascular diseases in three Chicago epidemiologic studies. Diabetes Care, 2, 131-141.
Stephens, J.C., Schneider, J.A., Tanguay, D.A., Choi, J., Acharya, T., Stanley, S.E., Jiang, R., Messer, C.J., Chew, A., Han, J.-H., Duan, J., Carr, J.L., Lee, M.S., Koshy, B., Kumar, A.M., Zhang, G., Newell, W.R., Windemuth, A., Zu, C., Kalbfleisch, T.S., Shaner, S.L., Arnold, K., Schulz, V., Drysdale, C.M., Nandablan, K., Judson, R.S., Runao, G., and Vovis, G.F. (2001). Haplotype variation and linkage disequilibrium in 313 human genes. Science, 293, 489-493.
Svetky, L.P., Moore, T.J., Simons-Morton, D.G., Appel, L.J., Bray, G.A., Sacks, F.M., Ard, J.D., Mortensen, R.M., Mitchell, S.R., Conlin, P.R., and Desari, M., for the DASH collaborative research group. (2001). Angiotensinogen genotype and blood pressure response in the Dietary Approaches to Stop Hypertension (DASH) study. Journal of Hypertension, 19, 1949-1956.
Tang, M.-X., Stern, Y., Marder, K., Bell, K., Gurland, B., Lantigua, R., Andrews, H., Fen, L., Tycko, B., and Mayeux, R. (1998). The APOE-E4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. Journal of the American Medical Association, 279, 751-755.
Tavtigian, S.V., Simard, J., Teng, D.H., Abtin, V., Baumgard, M., Beck, A., Camp, N.J., Carillo, A.P., Chen, Y., Dayananth, P., Desrochers, M., Dumont, M., Farnham, J.M., Frank, D., Frye, C., Ghaffari, S., Gupte, J.S., Hu, R., Illiey, D., Janeck, T., Kort, E.N., Laity, K.E., Leavitt, A., Leblanc, G., McArthur-Morrison, J., Pederson, A., Penn, B., Peterson, K.T., Reid, J.E., Richards, S., Schroeder, M., Smith, R., Snyder, S.C., Swedlund, B., Swensen, J., Thomas, A., Tranchant, M., Woodland, A.M., Labrie, F., Skolnick, M.H., Neuhausen, S., Rommens, J., and Cannon-Albright, L.A. (2001). A candidate prostate cancer susceptibility gene at chromosome 17p. Nature Genetics, 27, 172-180.
Templeton, A.R. (1999). Human races: A genetic and evolutionary perspective. American Anthropologist, 100, 632-650.
Trouillot, M.R. (1995). Silencing the past: Power and the production of history. Boston, MA: Beacon Press.
Turner, S.T., Schwartz, G.L., Chapman, A.B., and Boerwinkle, E. (2001). C825T polymorphism of the G protein beta(3)-subunit and antihypertensive response to a thiazide diuretic. Hypertension, 37, 739-743.
Van den Oord, E.J.C.G., and Rowe, D.C. (2000). Racial differences in birth health risk: A quantitative genetic approach. Demography, 37, 285-298.
Vasan, R.S., Beiser, A., Seshadri, S., Larson, M.G., Kannel, W.B., D’Agostino, R.B., and Levy, D. (2002). Residual lifetime risk for developing hypertension in middle-aged women and men. The Framingham Heart Study. Journal of the American Medical Association, 287, 1003-1010.
Wei, M., Valdez, R.A., Mitchell, B.D., Haffner, S.M., Stern, M.P., and Hazuda, H.P. (1996). Migration status, socioeconomic status, and mortality rates in Mexican Americans and non-Hispanic whites. The San Antonio Heart Study. Annals of Epidemiology, 6, 307-313.
Weiss, K.M., Ferrell, R.E., and Hanis, C.L. (1984). A New World syndrome of metabolic diseases with a genetic and evolutionary basis. Yearbook of Physical Anthropology, 27, 153-178.
Wilson, J.F., Weale, M.E., Smith, A.C., Gratrix, F., Fletcher, B., Thomas, M.G., Bradman, N., and Goldstein, D.B. (2001). Population genetic structure of variable drug response. Nature Genetics, 29, 265-269.
Wilson, T.W. (1986). History of salt supplies in West Africa and blood pressures today. Lancet, 1, 784-786.
Wu, X., Cooper, R.S., Borecki, I., Hanis, C., Bray, M., Lewis, C.E., Zhu, X., Kan, D., Luke, A., and Curb, D. (2002). A combined analysis of genome-wide linkage scans for BMI from the NHLBI Family Blood Pressure Program. American Journal of Human Genetics, 70, 1247-1256.
Yancy, C.W. (2001). Race and response to adrenergic blockade with carvedilol in patients with chronic heart failure. New England Journal of Medicine, 344, 1358-1365.
Yanovski, S.Z., Reynolds, J.C., Boyle, A.J., and Yanovski, J.A. (1997). Resting metabolic rate in African-American and Caucasian girls. Obesity Research, 5, 321-325.
Zhu, X., McKenzie, C., Forrester, T., Nickerson, D.A., Cooper, R.S., and Rieder, M.J. (2000). Localization of a small genomic region associated with elevated ACE. American Journal of Human Genetics, 67, 1144-1153.
Zhu, X., Bouzekri, N., Southam, L., Cooper, R.S., Adeyemo, A., McKenzie, C.A., Luke, A., Chen, G., Elston, R.C., and Ward, R. (2001). Linkage and association analysis of angiotensin I-converting enzyme (ACE) gene polymorphisms with ACE concentration and blood pressure. American Journal of Human Genetics, 68, 1139-1148.
Zhu, X., Yan, D., Cooper, R.S., Luke, A., Weder, A., and Chakravarti, A. (2003a). Linkage disequilibrium and haplotype diversity in the genes of the renin-angiotensin system: Findings from the Family Blood Pressure Program . Genomic Research, 13, 173-181.
Zhu, X., Yen-Pei, C., Chang, D., Yan, D., Weder, A., Cooper, R., Luke, A., Kan, D., and Chakravarti, A. (2003b). Associations between hypertension and genes in the renin-angiotensin system. Hypertension, 41(5), 1027-1034.











































