15
A Neurovisceral Integration Model of Health Disparities in Aging
Julian F. Thayer and Bruce H. Friedman
Significant ethnic disparities exist in the health of elderly Americans that cover a broad range of disorders, from the psychological to the physiological, and that are manifested in both morbidity and mortality differences. The life expectancies of minority groups in the United States and other industrialized countries are often dramatically reduced (see Chapter 2, this volume; Williams, 1997). Moreover, morbidity is often greater in ethnic minorities. For example, hypertension rates in African Americans are among the highest in the world, with the age-adjusted rate being more than 50 percent higher than for white Americans. African Americans also develop hypertension earlier and have much higher average blood pressures and higher rates of stage 3 hypertension than whites. These factors combine to produce higher rates of stroke mortality (80 percent higher), heart disease mortality (50 percent higher), and hypertension-related end-stage renal disease (320 percent higher) than whites (National High Blood Pressure Education Program, 1997). The prevalence of diabetes also varies greatly by ethnic group. The prevalence of known diabetes in African Americans is more than 1.7 times the rate of whites, whereas for some Native American tribes, the rate is more than 5.2 times that of whites (Black, 2002).
A number of potential pathways, both institutional and individual, to these health disparities have been identified, and include differential access to care, differential treatment, differential exposure to environmental pathogens, and differential exposure to chronic stress (Krieger, 2000). In this latter category, discrimination and racism have been implicated. This stressor is believed to be able to elicit the perception of threat from the environ-
ment, which in turn leads to a plethora of negative affective states, such as fear, anxiety, anger and hostility, and depression. Both acutely and chronically, these affective states may lead to negative health outcomes (Kiecolt-Glaser, McGuire, Robles, and Glaser, 2002; Krantz and McCeney, 2002). That ethnic minorities might be differentially exposed to racism-related stress and negative affect has been proposed as a possible causal factor in the observed health disparities (Clark, Anderson, Clark, and Williams, 1999; Williams and Neighbors, 2001). However, any comprehensive model of emotions and health must account for the complex mix of cognitive, affective, behavioral, and physiological concomitants of normal and pathological affective states and dispositions, and how these might impact health. In addition, the concept of stress is often invoked to explain the impact of psychosocial factors on physiological processes and health. However, this concept is plagued by a lack of a precise and widely accepted definition, and by a lack of specificity in the organismic mechanisms by which stress produces its effects (Eriksen and Ursin, 2002). Despite recent attempts to reconceptualize stress effects in terms of allostatic load (McEwen, 1998), the situation has not substantially improved (Eriksen and Ursin, 2002; Kiecolt-Glaser et al., 2002).
The impact of multiple pathways on ethnic health disparities must be acknowledged, but this chapter focuses on psychosocial factors. Broadly defined in terms of stress, negative emotion, and perceived racism, the core question is how these psychosocial factors are instantiated in physiological processes that can lead to disease and death. With the added premise that ethnic minorities are differentially and excessively exposed to discrimination and racism, the underlying causes of the health disparities begin to be revealed.
Due to the scope of the issues involved, coverage of the literature will be more illustrative than comprehensive. However, wherever possible, references to more comprehensive reviews and key primary sources are provided. We begin in a broad context within which to view the observed health disparities in the elderly by presenting evidence for the role of autonomic imbalance in disease and negative affective states and dispositions. The notion of appropriate energy regulation as a factor in health and disease is emphasized. Next, a brief description of a neurovisceral model of emotion regulation and dysregulation is offered, in which heart rate variability (HRV) is used to index important aspects of autonomic, affective, and cognitive system regulation. This model may help to explicate the complex interrelationships that exist in the connection between psychosocial factors on the one hand and health and disease on the other. This model utilizes a dynamical systems approach, and stresses the role of inhibitory processes via parasympathetic mechanisms in maintaining optimal energy regulation. A discussion follows in which perseverative thinking is viewed
as the core cognitive toxic factor. Evidence is presented on the relationship among perseverative thinking, HRV, and poor health outcomes. The central concomitants of perseverative behavior and their links to hypervigilance also will be considered. Next, the relevance of this model to health disparities is shown. Relevant experimental and correlational data in support of elements of the model are supplied, including how they might relate to health disparities. Finally, we offer recommendations for future research that might flesh out the model and further guide the understanding of health disparities in the elderly.
AUTONOMIC IMBALANCE IN DISEASE AND NEGATIVE EMOTIONS
There is growing evidence for the role of the autonomic nervous system (ANS) in a wide range of diseases. The ANS is generally conceived to have two major branches—the sympathetic system, associated with energy mobilization, and the parasympathetic system, associated with vegetative and restorative functions. Normally, the activity of these branches is in dynamic balance. For example, there is a well-documented circadian rhythm such that sympathetic activity is higher during daytime hours and parasympathetic activity increases at night. Other periodicities are present, and the activity of the two branches can be rapidly modulated in response to changing environmental demands. More modern conceptions of organism function based on complexity theory hold that organism stability, adaptability, and health are maintained through variability in the dynamic relationship among system elements (Friedman and Thayer, 1998a, 1998b; Thayer and Friedman, 1997; Thayer and Lane, 2000). Thus, patterns of organized variability, rather than static levels, are preserved in the face of constantly changing environmental demands. This conception, in contrast to homeostasis, posits that the system has multiple points of stability, which necessitate a dynamic organization of resources to match specific situational demands. These demands can be conceived in terms of energy regulation such that the points of relative stability represent local energy minima required by the situation. For example, in healthy individuals, average heart rate (HR) is greater during the day, when energy demands are higher, than at night, when energy demands are lower. Thus, the system has a local energy minimum or attractor for daytime and another for nighttime. Because the system operates “far from equilibrium,” the system is always searching for local energy minima to minimize the energy requirements of the organism. Consequentially, optimal system functioning is achieved via lability and variability in its component processes, and rigid regularity is associated with mortality, morbidity, and ill health (Lipsitz and Goldberger, 1992; Peng et al., 1994).
Another corollary of this view is that autonomic imbalance, in which one branch of the ANS dominates over the other, is associated with a lack of dynamic flexibility and health. Empirically, there is a large body of evidence to suggest that autonomic imbalance, in which typically the sympathetic system is hyperactive and the parasympathetic system is hypoactive, is associated with various pathological conditions (Malliani, Pagani, and Lombardi, 1994). In particular, when the sympathetic branch dominates for long periods of time, the energy demands on the system become excessive and ultimately cannot be met, eventuating in death. The prolonged state of alarm associated with negative emotions likewise places an excessive energy demand on the system. On the way to death, however, premature aging and disease characterize a system dominated by negative affect and autonomic imbalance.
Like many organs in the body, the heart is dually innervated. Although a wide range of physiologic factors determines HR, the ANS is the most prominent. Importantly, when both cardiac vagal (the primary parasympathetic nerve) and sympathetic inputs are blocked pharmacologically (for example, with atropine plus propranolol, the so-called double blockade), intrinsic HR is higher than the normal resting HR (Jose and Collison, 1970). This fact supports the idea that the heart is under tonic inhibitory control by parasympathetic influences. Thus, resting cardiac autonomic balance favors energy conservation by way of parasympathetic dominance over sympathetic influences. In addition, the HR time series is characterized by beat-to-beat variability over a wide range, which also implicates vagal dominance. Lowered HRV is associated with increased risk of mortality, and HRV has been proposed as a marker for disease (Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology, 1996).
Resting HR can be used as a rough indicator of autonomic balance, and several large studies have shown a largely linear, positive dose-response relationship between resting HR and all-cause mortality (see Habib, 1999, for review). This association was independent of gender and ethnicity, and showed a threefold increase in mortality in persons with HR over 90 beats per minute (bpm) compared to those with HRs of less than 60 bpm. It was suggested that this relationship is due to the role of HR as a major determinant of myocardial oxygen demand and the direct link of HR to the rate of myocardial energy use.
Brook and Julius (2000) have recently detailed how autonomic imbalance in the sympathetic direction is associated with a range of metabolic, hemodynamic, trophic, and rheologic abnormalities that contribute to elevated cardiac morbidity and mortality. Although the relationship between HR and cardiovascular morbidity and mortality may be assumed, the fact that autonomic imbalance and HR are related to other diseases may not be as obvious. However, links do exist. For example, HRV has been shown to be associated with diabetes mellitus, and decreased HRV has been shown to
precede evidence of disease provided by standard clinical tests (Ziegler, Laude, Akila, and Elgwhozi, 2001). In addition, immune dysfunction and inflammation have been implicated in a wide range of conditions associated with aging including cardiovascular disease, diabetes, osteoporosis, arthritis, Alzheimer’s disease, periodontal disease, and certain types of cancers as well as declines in muscle strength and increased frailty and disability (Ershler and Keller, 2000; Kiecolt-Glaser et al., 2002). The common mechanism seems to involve excess proinflammatory cytokines such as interleuken 1 and 6 and tumor necrosis factor. Importantly, increased parasympathetic tone and acetylcholine (the primary parasympathetic neurotransmitter) have been shown to attenuate release of these proinflammatory cytokines, and sympathetic hyperactivity is associated with their increased production (Das, 2000; Maier and Watkins, 1998; Tracey, 2002). Thus, autonomic imbalance may be a final common pathway to increased morbidity and mortality from a host of conditions and diseases.
Although the idea is not new (Sternberg, 1997), several recent reviews have provided strong evidence linking negative affective states and dispositions to disease and ill health (Friedman and Thayer, 1998b; Kiecolt-Glaser et al., 2002; Krantz and McCeney, 2002; Musselman, Evans, and Nemeroff, 1998; Rozanski, Blumenthal, and Kaplan, 1999; Verrier and Mittleman, 2000). All of these reviews implicate altered ANS function and decreased parasympathetic activity as a possible mediator in this link.
An additional pathway between psychosocial stressors and ill health is an indirect one, in which psychosocial factors lead to poor lifestyle choices, including a lack of physical activity and the abuse of tobacco, alcohol, and drugs. Both sedentary lifestyle and substance abuse are associated with autonomic imbalance and decreased parasympathetic tone (Ingjaldsson, Laberg, and Thayer, 2003c; Nabors-Oberg, Sollers, Niaura, and Thayer, 2002; Reed, Porges, and Newlin, 1999; Rossy and Thayer, 1998; Weise, Krell, and Brinkhoff, 1986). In fact, the therapeutic effectiveness of smoking cessation, reduced alcohol consumption, and increased physical activity rest in part on their ability to restore autonomic balance and increase parasympathetic tone.
In sum, autonomic imbalance and decreased parasympathetic tone in particular may be the final common pathway linking negative affective states and dispositions, including the indirect effects via poor lifestyle, to numerous diseases and conditions associated with aging as well as increased morbidity and mortality.
THE MODEL IN A NUTSHELL
A comprehensive model of emotions and health must account for the complex mix of cognitive, affective, behavioral, and physiological
concomitants of normal and pathological affective states and dispositions, and how these might impact health. In this chapter, a model is outlined that integrates some of these components into a functional and structural network that may help to guide the understanding of emotion and health. Functionally, this network involves autonomic, affective, and cognitive regulation, and structurally it entails reciprocal inhibitory circuits between prefrontal cortex and subcortical evolutionarily primitive motivational structures, that can be indexed by rhythmic activity of the cardiovascular system. Research that relates functional aspects of this circuitry to psychophysiological regulation will be briefly reviewed. We emphasize the relationships among autonomic, affective, and cognitive regulation in organism health, and propose a group of underlying physiological systems that integrate these functions in the service of self-regulation and adaptability. This network is placed in the context of a dynamical systems model that involves feedback and feedforward circuits, with special attention to negative feedback mechanisms and inhibitory processes. It will be shown that the negative behavioral states and dispositions associated with a relative autonomic sympathetic imbalance reflect a disinhibition of positive feedback circuits that are normally under tonic inhibitory control.
Other models have also been proposed to account for the effects of emotions and stress on health and disease. The allostatic load model has garnered much recent attention, particularly in medical circles (McEwen, 1998). The recently proposed psychoneuroimmunology model highlights the increasing role that inflammation has been shown to play in a wide variety of diseases, including cardiovascular disease (Kiecolt-Glaser et al., 2002). The reactivity model has been the stalwart in psychophysiology and behavioral medicine (see Krantz and McCeney, 2002). The neurovisceral integration model (NIM) builds on these prior models, but has important differences from them as well. First, the NIM is a true multilevel model incorporating factors from the sociopolitical to the molecular. Second, the NIM specifies in detail the core cognitive toxic factor, that of perseverative thinking, and explicates its neural and physiological concomitants. Third, the NIM proposes the autonomic nervous system, with an emphasis on the parasympathetic nervous system, as the final common pathway linking psychological and physiological states and dispositions. Fourth, the NIM is based on a nonlinear dynamical systems perspective and highlights the role of feedback and feedforward networks and their interplay in the maintenance of the dynamic flexibility of the organism. Finally, the NIM emphasizes inhibitory, negative feedback processes and explicates their physiological substrates at both the central and peripheral levels. Thus the NIM incorporates many aspects of the prior models and is not contradictory to them, but expands on them in ways
that are needed to account for the extant data and to generate testable hypotheses for future research.
The Central Autonomic Network
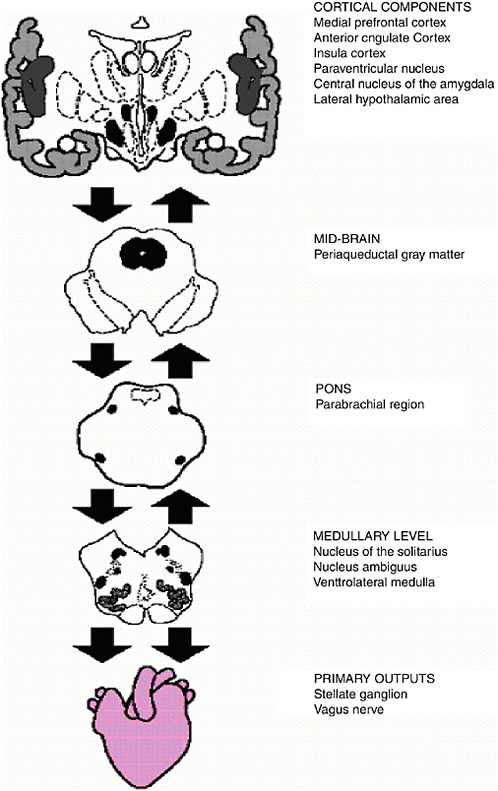
Investigators have identified functional units within the central nervous system (CNS) that support goal-directed behavior and adaptability. One such entity is the central autonomic network (CAN) (Benarroch, 1993, 1997). Functionally, this network is an integrated component of an internal regulation system through which the brain controls visceromotor, neuroendocrine, and behavioral responses that are critical for goal-directed behavior, adaptability, and health. Structurally, the CAN includes the anterior cingulate, insular, orbitofrontal, and ventromedial prefrontal cortices, the central nucleus of the amygdala, the paraventricular and related nuclei of the hypothalamus, the periaquaductal gray matter, the parabrachial nucleus, the nucleus of the solitary tract (NTS), the nucleus ambiguous, the ventrolateral medulla, the ventromedial medulla, and the medullary tegmental field (see Figure 15-1). These components are reciprocally interconnected such that information flows bidirectionally between lower and higher levels of the CNS. The primary output of the CAN is mediated through preganglionic sympathetic and parasympathetic neurons that innervate the heart via the stellate ganglia and vagus nerve, respectively. The interplay of these inputs to the cardiac sino-atrial node produces the complex variability that characterizes the HR time series (Saul, 1990). Thus, the output of the CAN is directly linked to HRV. Notably, vagal influences dominate cardiac chronotropic control (Levy, 1990). In addition, sensory information from peripheral end organs such as the heart and the immune system are fed back to the CAN. Thus, HRV is an indicator of central-peripheral neural feedback and CNS-ANS integration.
Moreover, the CAN has many features of a dynamical system. First, the components of the CAN are reciprocally interconnected, allowing for unbroken positive and negative feedback interactions and integration of autonomic responses. Second, the CAN consists of numerous parallel, distributed pathways, which permit multiple avenues to a given response. For example, a HR change of 72 to 90 bpm can be attained by various permutations of sympathetic and vagal input, including increased sympathetic or decreased vagal activity or some combination of the two, or by other processes such as circulating hormones. Moreover, within the CAN, direct and indirect paths can regulate output to preganglionic sympathetic and parasympathetic neurons. Third, CAN activity is state dependent and thus sensitive to initial conditions (see Glass and Mackey, 1988).
The CAN receives and integrates visceral, humoral, and environmental information; organizes autonomic, endocrine, and behavioral responses to
environmental challenges; and is under tonic inhibitory control. This inhibition is achieved by γ-aminobutyric acid (GABA), the main inhibitory CNS neurotransmitter, emanating from interneurons within the NTS. Disruption of this pathway may lead to hypertension and sinus tachycardia, and represents a disinhibition of sympathoexcitatory circuits in the CAN (Benarroch, 1993, 1997; Masterman and Cummings, 1997; Spyer, 1989).
Other functional units within the CNS serving executive, social, affective, attentional, and motivated behavior in humans and animals have been identified (Damasio, 1998; Devinsky, Morrell, and Vogt, 1995; Masterman and Cummings, 1997; Spyer, 1989). One such network has been termed the anterior executive region (AER; Devinsky et al., 1995). The AER and its projections regulate behavior by monitoring the motivational quality of internal and external stimuli. The AER network has been called the “rostral limbic system” and includes the anterior, insular, and orbitofrontal cortices, amygdala, periaquaductal gray, ventral striatum, and autonomic brainstem motor nuclei. Damasio (1998) has recognized a similar neural “emotion circuit” for which there is considerable structural overlap with the CAN and the AER (Thayer and Lane, 2000).
We propose that the CAN, the AER network, Damasio’s (1998) “emotion circuit,” and related systems (Masterman and Cummings, 1997; Spyer, 1989) represent a common central functional network recognized by different researchers from diverse approaches. This CNS network is associated with the processes of response organization and selection, and serves to control psychophysiological resources in attention and emotion (Friedman and Thayer, 1998a, 1998b; Thayer and Friedman, 1997). Additional structures are flexibly recruited to manage specific behavioral adaptations. This sparsely interconnected neural complex allows for maximal organism flexibility in accommodating rapidly changing environmental demands. When this network is either rigidly coupled or completely uncoupled, the ability to recruit and utilize appropriate neural support to meet a particular demand is hampered, and the organism is thus less adaptive.
Autonomic Regulation
Autonomically mediated HRV is useful as an index of neurovisceral integration and organismic self-regulation. The interaction of sympathetic and parasympathetic outputs of the CAN at the sino-atrial node produces the complex beat-to-beat variability that marks a healthy, adaptive organism. Vagal activity dominates HR control, and thus HR is under tonic inhibitory vagal control (Levy, 1990; Uijtdehaage and Thayer, 2000). HRV is also associated with prefrontal cortex activity (Lane, Reiman, Ahern, and Thayer, 2001), and the prefrontal cortex has been inversely related to
subcortical activity in structures such as the amygdala that have been implicated in primitive motivation systems (Davidson, 2000).
Several lines of research point to the significance of HRV in emotions and health. Decreased HRV is linked with a number of disease states, including cardiovascular disease, diabetes, obesity, and lack of physical exercise (Stein and Kleiger, 1999). Reduced vagally mediated HRV is also associated with a number of psychological disease states, such as anxiety, depression, and hostility. For example, low HRV is consistent with the cardiac symptoms of panic anxiety as well as with its psychological expressions in poor attentional control and emotion regulation, and behavioral inflexibility (Friedman and Thayer, 1998a, 1998b). Similar reductions in HRV have been found in depression (Thayer, Smith, Rossy, Sollers, and Friedman, 1998), generalized anxiety disorder (Thayer, Friedman, and Borkovec, 1996), and posttraumatic stress disorder (Cohen, Matar, Kaplan, and Kotler, 1999). Low levels of vagal cardiovascular influence serve to disinhibit sympathoexcitatory influences. Due to differences in the temporal kinetics of the autonomic neuroeffectors, sympathetic effects on cardiac control are relatively slow (order of magnitude seconds) compared to vagal effects (order of magnitude milliseconds; see Saul, 1990). Thus, when this rapid vagal cardiac control is low, HR cannot change as quickly in response to environmental changes. In this view, the prefrontal cortex modulates subcortical motivational circuits to serve goal-directed behavior. When the prefrontal cortex is taken “offline” for whatever reason, a relative sympathetic dominance associated with disinhibited defensive circuits is released.
Human evidence for the inhibitory role of the frontal cortex comes from a recent study of HR and HRV before and after right- and left-side intracarotid sodium amobarbital (ISA) injection (Ahern et al., 1994). HR changes were similar during each hemisphere’s pharmacological inactivation. During the 10-minute inactivations of either hemisphere, HR increased, peaked around the third minute, and gradually declined toward baseline values. These data indicate that the frontal cortex exerts tonic inhibition on brainstem sympathoexcitatory circuits. There were lateralized effects: larger and faster HR increases occurred during right-hemisphere inactivation. Moreover, vagally mediated HRV decreases were also greater in the right-hemisphere inactivations, mirroring the hemispheric effects on HR. These results support anatomical and physiological findings that right-hemispheric autonomic cardiac inputs are associated with greater chronotropic (rate) control.
The effects of the ISA test are largely restricted to anterior neural structures, which include the orbital and medial prefrontal cortices (Ahern et al., 1994; Hong et al., 2000). These areas are linked broadly with biopsychological functions such as affective, cognitive, and autonomic regulation (Thayer and Lane, 2000). Additionally, these structures are related to
inhibitory control of behavior in general (Roberts and Wallis, 2000) and cardiac behavior in particular (Verberne and Owens, 1998). It is noteworthy that direct and indirect pathways connect these areas with vagal motor output regions (Ter Horst, 1999). Many researchers have proposed inhibitory cortical-subcortical circuits (Benarroch, 1993, 1997; Masterman and Cummings, 1997; Mayberg et al., 1999; Spyer, 1989), but our group is the first to tie these circuits to HRV (Thayer and Friedman, 2002; Thayer and Lane, 2000). The ISA test results provide compelling evidence that cortical structures tonically inhibit sympathoexcitatory circuits by way of vagal mechanisms.
It has been proposed that the prefrontal cortex is taken “offline” during emotional stress to let automatic, prepotent processes regulate behavior (Arnsten and Goldman-Rakic, 1998). This selective prefrontal inactivation may be adaptive by facilitating predominantly nonvolitional behaviors associated with subcortical neural structures such as the amygdala to organize responses without delay from the more deliberative and consciously guided prefrontal cortex. In modern society, however, inhibition, delayed response, and cognitive flexibility are vital for successful adjustment and self-regulation, and prolonged prefrontal inactivity can lead to hypervigilance, defensiveness, and perseveration.
A common reciprocal inhibitory cortical-subcortical neural circuit may structurally link psychological processes such as emotion with health-related physiology, and this circuit can be indexed with HRV. This neural network permits the prefrontal cortex to inhibit subcortical structures associated with defensive behaviors, and thus promote flexible responsiveness to environmental changes. For example, when faced with threat, tonic inhibitory subcortical control can be withdrawn quickly, leading to sympathoexcitatory survival (“fight or flight”) responses. However, when this network is disrupted, a rigid, defensive pattern emerges with associated perseverations in cognitive, affective, and autonomic behavior. This protracted state of action readiness and associated sympathetic activity may be the pathogenic state underlying the increased morbidity and mortality found in chronic negative psychological states and dispositions.
Affective Regulation
Affect regulation is a valuable skill that has clear implications for health. Emotions represent a distillation of an individual’s perception of personally relevant environmental interactions, including not only challenges and threats but also the ability to respond to them (Frijda, 1988). Viewed as such, emotions reflect the integrity of one’s ongoing adjustment to constantly changing environmental demands. Emotions have also been characterized as an organism’s response to the environment that allows for rapid
mobilization of multiple action subsystems (Levenson, 1988). In this context, emotions are the uninterrupted output of continuous sequential behavior. These lawful behavioral sequences are organized around biologically important functions and have been termed “behavioral systems” (Timberlake, 1994). Put another way, emotions act to coordinate efficient responses for goal-directed behavior. For example, when faced with danger, a defensive behavioral system might be activated. The first stage of this sequence might involve the experience of anxiety, increased HR and a general shift toward sympathetic autonomic activity, and vigilant scanning of the environment for signs of danger. If a threat is identified, the next stage might involve fear and mobilization for “fight or flight.” However, in contemporary life, fully aggressive or escape responses are rarely appropriate. As Frijda (1988) noted, specific emotions imply specific eliciting stimuli, specific action tendencies including selective attention to relevant stimuli, and specific reinforcers. When this system works properly, it promotes flexible adaptation to shifting environmental demands. In another sense, an emotional response represents a selection of an optimal response and the inhibition of less functional ones from a broad behavioral repertoire, with the goal of matching energy use to fit situational requirements.
Several psychophysiological measures have proven to be useful indices of affect regulation. One is the reflexive startle blink, the magnitude of which can be affected by emotional state. The emotion-modulated startle is a robust phenomenon that has been demonstrated in a wide range of experimental situations, and has been broadly linked to affective and motivational phenomena (Lang, 1995). Similarly, HRV has been associated with a diverse range of processes, including affective and attentional regulation (Porges, 1992; Porges, Doussard-Roosevelt, and Maita, 1994). The relationship between these two important measures of affective regulation was recently investigated (Ruiz-Padial, Sollers, Vila, and Thayer, 2003). Ninety female participants viewed pleasant, neutral, and unpleasant pictures while exposed to acoustic startle stimuli. Eyeblink strength to startle probes was recorded both during affective foregrounds and intertrial intervals, and the relationship between resting HRV and startle magnitudes was examined. Resting HRV was found to be inversely related to both intertrial interval and emotion-modulated startle magnitude. In addition, subjects with the highest HRV showed the most differentiated emotion-modulated startle effects, whereas those with the lowest HRV showed significant augmentation of startle to neutral foregrounds and marginally potentiated startle to pleasant foregrounds. Thus, individuals with low HRV reacted to neutral, harmless stimuli as if they were aversive and threatening, and also had a tendency to react similarly to positive stimuli. In addition, individuals with high HRV were able to best match their response to situational demands and thus respond most appropriately to the energy requirements of the
situation. The findings are consistent with our model that posits that prefrontal cortical activity modulates subcortical motivation circuits in the service of goal-directed behavior and appropriate energy regulation. Moreover, persons with low HRV showed evidence of hypervigilance and the activation of a defensive behavioral system in response to nonthreatening stimuli.
Numerous studies in both animals and humans suggest that an intact amygdala is associated with larger startle magnitude and emotion-potentiated startle during an unpleasant foreground (Aggleton and Young, 2000; Angrilli et al., 1996). A related structure, the bed nucleus of the stria terminalis, has been implicated in the general startle sensitivity associated with anxiety in rats and negative affect in humans (Bradley and Lang, 2000). Evidence for a role of medial prefrontal activity in startle response regulation can be found in its inverse association with amygdaloid activity (Davidson, 2000). The reported relationship between startle modulation and HRV provides further support for the notion that prefrontal activity is inversely related to structural functions associated with defensiveness, and thereby moderates interactions with the environment (Thayer and Lane, 2000). These results also further suggest that HRV can be used to index activity in this network of neural structures associated with emotional regulation.
Attentional Regulation and Executive Function
Attentional regulation and the ability to inhibit prepotent but inappropriate responses are also important for health in a complex environment. Many tasks important for survival in today’s world involve cognitive functions such as working memory, sustained attention, behavioral inhibition, and general mental flexibility. These tasks are all associated with prefrontal cortical activity (Arnsten and Goldman-Rakic, 1998). Deficits in these cognitive functions tend to accompany aging, and are also present in negative affective states and dispositions such as depression and anxiety. Stress can also impair cognitive function and may contribute to the cognitive deficits observed in various mental disorders. It is also possible that autonomic dysregulation contributes to decline in attention and cognitive performance. A series of experiments in our lab have been conducted to examine this issue, and will be described.
In a recent experiment, Johnsen et al. (2003) examined inhibitory responses in an emotional Stroop paradigm. Dental phobics were first exposed to recorded scenes of dental procedures and then administered the emotional Stroop test. In addition to the traditional color-congruent and color-incongruent words, phobic subjects also were asked to respond to neutral words and dental-related words (e.g., “drill” and “cavity”) that
were threatening to them. All subjects exhibited longer reaction times to the color-incongruent words and the dental-related threat words, and thus displayed a difficulty in inhibiting prepotent responses. However, greater HRV was associated with faster reaction times to these words, consistent with the link among vagally mediated HRV, inhibitory ability, and frontal lobe function. These results support the idea that vagally mediated HRV is associated with efficient attentional regulation and greater ability to inhibit prepotent but inappropriate responses.
Subsequent studies further examined executive function and working memory in healthy individuals. In the first experiment, subjects performed a number of tasks involving continuous performance, including a simple reaction time task, a choice reaction time task, and three tasks that involved delayed responding and working memory (Hansen, Johnsen, and Thayer, 2003; Johnsen, Hansen, Murison, and Thayer, 2001). These latter tasks involved the presentation of a sequence of digits that required a response when a digit was identical to one that appeared either one or two back in the series, and have been shown to be associated with prefrontal activity (Goldman-Rakic, 1998). HRV and cortisol responses were recorded, and subjects were grouped into low and high HRV groups. Performance on tasks involving simple and choice reaction times did not differ between these groups. However, on tasks associated with prefrontal activity, subjects in the low HRV group performed more poorly in terms of reaction time, number of errors, and number of correct responses than those in the high HRV group. In addition, the groups did not differ in baseline, morning, or evening cortisol, but the low HRV group showed larger cortisol responses to cognitive tasks that lasted into the posttask recovery period. Stress is associated with an increased cortisol release, and cortisol plays a major role in immune function through its association with proinflammatory cytokines (Kiecolt-Glaser et al., 2002). Cortisol is also known to impair function on cognitive tasks associated with prefrontal cortex (Lupien, Gillin, and Hauger, 1999). Thus, the low HRV group was less stress tolerant as indexed by cortisol responses and more impaired cognitively than the high HRV group.
In another study in the series, subjects performed the same tasks as described earlier, but half did so under threat of electric shock (Hansen, Johnsen, Sollers, and Thayer, 2002). Again, subjects were divided into two groups based on resting HRV levels. In the shock threat condition, task performance involving delayed responding and prefrontal activity was significantly impaired in the low HRV group. Thus, persons with high HRV were more stress tolerant and less affected by the threat compared to those with low HRV. In yet another study, HRV was manipulated by having half of the subjects in a physically active group undergo mild detraining for 4 weeks. Aerobic capacity and HRV were significantly reduced in this group
compared to those who maintained their fitness and HRV levels. All subjects again performed the above cognitive tasks: once before the 4-week detraining period, and once after. The detrained, lower HRV group failed to show the expected learning effect associated with repeated performance of the cognitive tasks, and thus did not reap the typical benefit of previous task exposure.
Taken together, these results support the usage of HRV to index efficient allocation of attentional and cognitive resources needed for efficient functioning in a challenging environment in which delayed responding and behavioral inhibition are key. In addition, these data show that low HRV marks increased risk to stress exposure. Significantly, these results provide a connection among stress-related cognitive deficits, high negative affect, and negative health consequences via the common mechanism of autonomic imbalance and low parasympathetic activity.
Summary of the Model
Autonomic, cognitive, and affective regulation assist an organism in facing the challenge of an environment in constant flux. However, the importance of inhibitory processes in self-regulatory behavior has not yet made its way into the dominant thinking in this area. From a systems perspective, inhibitory processes can be viewed as negative feedback circuits that permit the interruption of ongoing behavior and redeployment of resources to other tasks. When these negative feedback mechanisms are compromised, positive feedback loops may develop as a result of disinhibition. These positive circuits can have disastrous consequences by promoting hypervigilance, perseveration, and continued system activation, thereby limiting resource availability for other processes. This state of affairs can provide a chronic pathogenic substrate for psychological processes and emotions to negatively impact health. For example, at the synaptic level, “… substances which interfere with inhibitory synaptic action would cause unfettered excitatory action of neuron onto neuron and so lead to convulsions” (McGeer, Eccles, and McGeer, 1978, p. 134).
Healthy systems involve both positive and negative feedback circuits (Glass and Mackey, 1988; Goldberger, 1992). That inhibitory circuits may be indexed by vagally mediated HRV has several implications. First, vagal influences on the cardiovascular system represent negative chronotropic and dromotropic (conduction) mechanisms that are associated with system flexibility, responsivity, and stability (Levy, 1990; Porges, 1992). Second, as an index of central-peripheral neural feedback mechanisms, vagally mediated HRV represents a psychophysiological resource that the organism can bring to bear on environmental challenges (Friedman and Thayer, 1998b). Third, framing the diverse self-regulatory functions and dysfunctions observed in
terms of vagal as opposed to sympathetic processes may be a more parsimonious representation of the data (Friedman and Thayer, 1998b; Thayer and Friedman, 1997). From this perspective, the relative sympathetic activation and autonomic imbalance seen in psychological and physiological disorders may represent disinhibition due to faulty inhibitory mechanisms. One key additional factor makes this conceptualization in terms of autonomic imbalance particularly relevant to the health disparities in aging. Whereas the traditional risk factors such as smoking, total cholesterol, obesity, and even hypertension, many of which are used to index allostatic load (Seeman, Singer, Rowe, Horwitz, and McEwen, 1997), lose their predictive value in old age, autonomic imbalance continues to be predictive of morbidity and mortality (Kiecolt-Glaser et al., 2002; Palatini and Julius, 1999).
Perseverative Thinking as the Core Cognitive Toxic Factor
Perseverative thinking, common to a number of negative affective states and dispositions, including depression, anxiety disorders, posttraumatic stress disorder, and perhaps many medically unexplained syndromes, is repetitive, abstract, and involuntary and represents a failure of inhibitory neural processes (Thayer and Lane, 2000). This cognitive mode is thought to serve several different functions. The most straightforward function attributed to worry and perseverative thinking is an attempt, albeit thwarted, at constructive mental problem solving (Davey, 1994). In support of this role, Davey (1994) and coworkers found positive correlations between worry and problem-focused coping, but only after partialing out the effect of trait anxiety. Thus, worry appeared to be associated with a habitual tendency for active problem solving combined with low confidence in succeeding in it. This is similar to the concept of “John Henryism” that has been related to exaggerated stress responses and poor health (James, 1994). Tallis and Eysenck (1994) proposed a tripartite function of worry. First, worry serves an alarm function, acting to interrupt ongoing behavior and directing awareness toward an issue demanding immediate solution. Second, worry has a prompt function, continuously representing unresolved threatening situations to awareness. Third, worry is proposed to have a preparation function, anticipating threat and readying the organism for a situation in which intense motor activation is needed. Obviously, such a situation is rare relative to typical levels of worry and perseverative thinking. Thus, perseverative thinking theoretically engenders a protracted state of psychophysiological “action preparation” without resolution.
Thus, perseverative thought reflects a disinhibition of a potentially adaptive frontal lobe mechanism in higher organisms. The frontal lobes have reciprocal neural connections with more evolutionarily primitive subcortical structures that are partially responsible for basic approach and
avoidance behavior. When these structures are disinhibited, a number of processes associated with threat response are unleashed, including hypervigilance and fear, as well as changes such as increased HR and blood pressure associated with autonomic imbalance. These processes could be viewed as sensitization-like as well as disinhibitory, and their behavioral “hallmarks” of hypervigilance and fear might pertain to any threat, including that associated with discrimination and racism.
The classical finding of a large orienting response followed by habituation is an example of sensitization (Ursin, 1998). Similarly, the phenomenon of long-term potentiation, so important for memory, can be viewed as a type of sensitization (Thayer and Friedman, 2002). The tuning of the organism to novel stimuli followed by habituation to innocuous stimuli is characteristic of healthy and adaptive functioning. In contrast, failure to habituate to innocuous stimuli leads to vigilance and defensiveness that is the hallmark of pathologies such as anxiety disorders. This maladaptive mode exemplifies perseverative behavior that can be seen as a positive feedback loop. Interruption of this ongoing state is associated with inhibition and negative feedback. In the context of our model of neurovisceral integration, vagal control of cardiovascular function (as well as activity of the prefrontal cortex) is associated with these inhibitory processes.
This phenomenon was examined in a study that compared generalized anxiety disorder (GAD) patients to a matched control group who were exposed to threat and nonthreat words in an S1—S2 paradigm (Thayer, Friedman, Borkovec, Johnsen, and Molina, 2000). Briefly, the S1—S2 paradigm involves the presentation of a series of paired stimuli in which an initial cue stimulus (S1) is followed after a fixed interstimulus interval (ISI) by a second stimulus (S2). A robust triphasic HR response has been described during the ISI (Bohlin and Kjellberg, 1979). An initial HR deceleration following S1 is followed by HR acceleration over the next several cardiac beats. Finally, just prior to S2, a second HR deceleration occurs. The initial deceleration has been interpreted as an orienting response to novelty (Sokolov, 1963). Phasic cardiac changes found in the S1—S2 paradigm have been shown to be vagally mediated (Porges, 1992). It was reasoned that GAD entails excess vigilance to environmental threat and low disengagement from unimportant events. This attentional style would produce a failure to habituate to novel innocuous stimuli in the GAD group, whereas nonanxious controls would show habituation. Because HR response magnitude is positively related to vagally mediated HRV (Porges, 1992), it was predicted that relative to persons with GAD, nonanxious controls initially would show larger orienting responses that would habituate rapidly.
These predictions were supported by data that showed the GAD group to have smaller cardiac orienting responses and impaired habituation to neutral words, relative to the nonanxious controls. Diminished orienting is consistent with findings of low HRV in GAD, and the impaired habituation
suggests excess vigilance to a perceived perpetually threatening environment. The inability to inhibit attention to harmless stimuli leads to a positive feedback loop that spirals out of control. Thus, worry becomes the preferred response to an ever-widening range of situations, maintaining anxiety in the face of disconfirming data. However, the chronic perception of threat may lead to a restriction of behavior such that the individual is paradoxically exposed to less novel, disconfirming information. Particularly in the elderly, this limitation can lead to social isolation and physical inactivity. Again, positive feedback perpetuates the existing dysfunctional state.
These mechanisms could operate outside of conscious awareness at a precognitive or preconscious level. One of us recently found similar phenomena in alcoholics exposed to briefly presented (Ingjaldsson, Thayer, and Laberg, 2003a) and nonconsciously presented alcohol stimuli (Ingjaldsson, Thayer, and Laberg, 2003b). These findings are consistent with classic work on perceptual defense (see Mackinnon and Dukes, 1962, for review) and the psychophysiology of attention (e.g., Graham and Clifton, 1966; Sokolov, 1963), as well as more contemporary notions of preattentive discrimination and selective processing of threat in anxiety (Mathews, 1990). Furthermore, the rapid mobilization of resources for action fits the dynamical systems view of emotion as an emergent response or attractor driven by motivational factors (see Globus and Arpaia, 1994; Thayer and Lane, 2000).
Several recent studies further highlight the fact that perseverative thinking can have effects outside of conscious awareness. In one study, healthy individuals were brought into a sleep laboratory and randomly divided into two groups (Hall et al., 2004). One group was told that in the morning they would be asked to give a speech that would be evaluated (stress group). The other participants were told that they would be allowed to read popular magazines upon awakening (control group). The results indicated that relative to the control group, the stress group had decreased HRV during both rapid eye movement (REM) and non-REM sleep. They also had poorer sleep maintenance and lower automated delta counts assessed via electroencephalography. Another recent study found that daytime worry and daily hassles were associated with decreased HRV and increased HR on the succeeding night (Brosschot, van Dijk, and Thayer, 2003). Taken together, these studies suggest that the effects of perseverative cognition are not restricted to periods when such activity is consciously perceived, but can have effects that extend to periods in which perseverative cognition is not accessible to conscious awareness.
Perseverative Thinking and Poor Health
Perseverative thinking is a central feature of many psychological disorders that also have been associated with poor physical health outcomes. For
example, chronic and transient episodes of anxiety, depression, and anger all have been associated with cardiovascular disease (Musselman et al., 1998; Rozanski et al., 1999; Verrier and Mittleman, 2000). A core characteristic of these perseverative states is the perception that control over a stressor is threatened. Only when a threat to control is perceived is the stressor’s full potential for activating the organism manifested, because there is no apparent way to cope with the stressor. Perceived uncontrollability of stress (or related concepts like hopelessness) has been documented as a chief characteristic of both stressors and individuals that accounts for potentially pathogenic physiological states and health problems (Brosschot et al., 1998; Everson et al., 1996; Frankenhäuser, 1980; Lundberg and Frankenhäuser, 1978; Steptoe and Appels, 1989; Ursin, 1987; Ursin and Hytten, 1992).
From this perspective, perseverative thinking might be viewed as the cognitive manifestation and source of nourishment of the deeper underlying experience of perceived uncontrollability. The concept of perseverative thinking thus may help to explain the health effects of perceived uncontrollability by accounting for the prolongation of its physiological effects. Specifically, perseverative thinking sustains the physiological response to a stressor by prolonging uncertainty over stressor control in the coping process. A theoretical implication of this concept is an emphasis on the time dimension in stress research. Associations between perseverative thinking on the one hand and physiological and health consequences on the other are implicit in view of the crucial role of uncertainty and uncontrollability in the stress-disease link.
Indeed, preliminary evidence for a positive association between dispositional worry and general health exists (Brosschot et al., unpublished data). In this exploratory study of more than 250 first-year psychology students, the disposition to worry (Borkovec, 1985) was found to be correlated with subjective health complaints (r = 0.64, p < 0.001). The association was lower when the effect of trait anxiety was controlled (partial r = 0.18, p < 0.05), which suggests that the association was at least partly due to “pure” worry tendencies and not entirely to the disposition to express negative affect. In a related study from the same lab in older, part-time students, further evidence accrued for a causal role for worry. Seven students were instructed to limit worry every day to a 30-minute designated “worry period” late in the day, for one week. Compared to 3 days prior to initiating this procedure, the worry “postponement” group had fewer health complaints during the last 3 days of the intervention week, as opposed to 10 control students who only registered their worry periods ( p < 0.05). Effects were stronger for common cold or flu-like complaints and coughing than for more conventional psychosomatic complaints like headache or dizziness. Although sample sizes were too small for strong conclusions,
these results collectively imply that time spent worrying and health complaints are positively related. A psychoneuroimmunological path from perseverative thinking to infectious and perhaps other immune-related diseases may be inferred. As noted earlier, autonomic imbalance and decreased parasympathetic activity have been shown to be associated with immune function via excess proinflammatory cytokines (Das, 2000; Tracey, 2002).
Another way in which perseverative thinking may be causally related to autonomic imbalance and disease is by decreased vagally mediated HRV. Diminished tonic HRV and the associated reduction of vagally mediated cardiovascular control has been associated with a variety of pathological states and dispositions, including diabetes, myocardial infarction, congestive heart failure, and hypertension (for review see Friedman and Thayer, 1998b; Malliani et al., 1994; Stein, Bosner, Kleiger, and Conger, 1994; Stein and Kleiger, 1999). As an index of vagally mediated cardiovascular activity, HRV reflects a negative feedback mechanism that is crucial for the self-regulation of behavior. Vagal activity has negative cardiac chronotropic and dromotropic effects that promote efficient cardiovascular function by restraining cardiac rate and electrical conduction speed, which is vital to attain cardiac stability, responsiveness, and flexibility (Levy, 1990; Verrier, 1987).
Chronic worry was recently shown to be related to increased risk of coronary heart disease (Kubzansky et al., 1997). Thus, the perseverative thinking characteristic of worry not only can lead to increased anxiety, but is also associated with an increased cardiovascular disease risk. Transition worry is associated with decreased vagal activity (Thayer et al., 1996). Thus, one mechanism that might link worry to elevated disease risk is low vagal activity. Similar models of decreased vagal activity have been proposed to describe the relationship between other psychological factors and physiological health. For example, Brosschot and Thayer (1998) relate diminished vagal activity to hostility and cardiovascular disease risk, and vagal depression has been suggested as the link between psychological factors and myocardial ischemia (Kop et al., 2001; Sroka, Peimann, and Seevers, 1997).
A possible key role in this process is played by decreased medial prefrontal cortex activity. The frontal cortex may tonically inhibit limbic (amygdala) activity (Skinner, 1985), and this limbic activity has been associated with autonomically mediated defensive behavior, including increased HR and blood pressure. More recently, direct and indirect pathways by which the frontal cortex modulates limbic activity, especially via parasympathetic activity, have been identified (Ter Horst, 1999; Ter Horst and Postema, 1997). Ter Horst relates these connections to increased risk of reinfarction and death in postmyocardial infarction depression. The thrust of this line of thought is that when faced with threat, tonic inhibitory limbic control can be rapidly decreased, leading to sympathoexcitatory fight or flight survival responses. Disruption of this inhibitory control allows a
rigid, defensive behavioral pattern to emerge with associated hypervigilance and perseverative behavior, manifested in cognitive, affective, and autonomic inflexibility.
Perseverative thinking and low HRV may, in fact, reflect the breakdown of a common reciprocal inhibitory cortical-subcortical neural circuit. This network of reciprocally interconnected structures allows the prefrontal cortex to inhibit subcortical activity associated with defensive behavior, and thus foster flexible control of behavior in response to changing environmental demands. As noted earlier, disruption of this network might lead to disinhibition of defensive perseverative behaviors, including hypervigilance.
Perseverative Thinking and HRV
In a study of the autonomic characteristics of GAD and its cardinal feature, worry, spectral analysis of HRV was used to investigate the effects of a 10-minute relaxation period and a 10-minute worry period in persons with GAD and nonanxious controls (Thayer et al., 1996). Results highlighted two main effects. First, persons with GAD had lower vagally mediated HRV compared to controls across all experimental conditions, including baseline. The second main effect indicated that worry in both GAD and nonanxious control groups was associated with reduced HRV. A similar effect was observed in an HRV study in which an analogue sample of GADs and a nonanxious control group engaged in periods of imagery and worry (Lyonfields, Borkovec, and Thayer, 1995). Resting HRV was recorded both at the beginning and at the end of the experimental session. GAD subjects showed reduced HRV across all recording periods, with little change from one period to the next. Although the nonanxious controls did show differences among experimental conditions, the worry condition was associated with the greatest reduction in HRV. This reduction during worry was greater than the reduction during imagery of the same topic.
Tonic low HRV in GAD, as well as the phasic HRV reduction during worry in nonanxious subjects, represents a breakdown of inhibitory processes that assist efficient self-regulation, including the interruption of ongoing behavior. Thus, an excitatory positive feedback loop emerges, leading to, or perhaps reflected in, perseverative thinking. Thus, the normally fine-tuned ability to adjust to change becomes a rigid, inflexible response disposition. In behavioral terms, the defensive attentional style that characterizes GAD is ultimately detrimental to functioning because it impairs adaptive, versatile responding. Such defensiveness constrains one’s behavioral repertoire by limiting the range of appropriate responses through the compromised ability to inhibit inappropriate responses. In other words, behavioral options become restricted to the more automatic and prepotent anxious cognitive and behavioral tendencies. More ex-
treme examples of this perseverative behavior have been associated with frontal lobe dysfunction.
Central Concomitants of Perseverative Behavior
Perseverative behavior is associated with depression, anxiety, and hostility among other negative affective states and dispositions. The current discussion will be restricted to anxiety and hostility, based on a recent review of the physiological concomitants of rumination and depression (Siegle and Thayer, 2003). As mentioned earlier, the main inhibitory CNS neurotransmitter is GABA. Animal and human work often converge to suggest that anxiety and its associated perseverative activity are related to decreased GABA receptor binding in the medial prefrontal and orbital frontal cortices. For example, in a murine model of anxiety, decreased GABAA-receptor clustering was associated with harm avoidance behavior and an explicit memory bias for threat cues (Crestani et al., 1999). Mice with reduced GABAA-receptor clustering showed enhanced reactivity to threat stimuli (an effect that was reversed by diazepam), a facilitation of trace conditioning in a fear conditioning paradigm, and a deficit in ambiguous cue discrimination. These findings are remarkably similar to the HR acceleration to and explicit memory bias for threat words, and failure to habituate to neutral words, found in generalized anxiety disorder patients in a conditioning paradigm (Friedman, Thayer, and Borkovec, 2000; Thayer et al., 2000).
Positron emission tomography (PET) has been used to examine benzodiazepine GABAA-receptor kinetics in humans with and without panic disorder (Malizia et al., 1998). Compared to nonanxious controls, panic disorder patients showed a global decrease in benzodiazepine site binding, with the largest decreases in the orbitofrontal and insular cortices. Decreased blood flow in the right medial frontal cortex also has been reported in self-induced anxiety (Kimbrell et al., 1999). These cortical areas have been implicated in anxiety and are also associated with HRV (Lane et al., 2001). Similar altered orbitofrontal chemistry has been found in anxious humans (Grachev and Apkarian, 2000). Relative to low anxious subjects, high anxious subjects showed reduced levels of a number of orbitofrontal neurochemicals, including GABA.
Recent neuroimaging studies have also examined patterns of cerebral blood flow associated with anger and aggressive behavior. Using single photon emission computed tomography, decreased prefrontal activity was found in 40 psychiatric patients who exhibited aggressive behavior within the 6-month period prior to scanning compared to psychiatric patients without a history of aggression (Amen, Stubblefield, Carmichael, and Thisted, 1996). In nonaggressive individuals, it is clear that anger inhibition is the most common response to provocation, mainly due to social norms
(Brosschot and Thayer, 1998). Consistent with this notion, PET data showed lateral orbitofrontal cortex (LOFC) activation during imagery-driven anger in men (Dougherty et al., 1999). These researchers noted that the LOFC and the associated “prefrontal” circuit are considered pivotal to response inhibition and the mediation of social behavior. Therefore, the reported LOFC activation was hypothesized to represent inhibition of an aggressive response during the anger provocation. Similar right prefrontal activation during self-induced anger, as compared to self-induced anxiety, has been reported (Kimbrell et al., 1999). However, in this study both self-induced anxiety and self-induced anger were associated with decreased frontal activity relative to a neutral condition, suggesting that inhibition pertains in general to emotional behavior.
The amygdala is often held to be the key limbic structure in emotional behavior. For example, electrical stimulation of the amygdala has been associated with a range of defensive behaviors, including increased cortisol, heart rate, and blood pressure (see Davis and Whalen, 2001, for review). Brain imaging data show that both depression severity and dispositional negative affect are correlated with amygdala activity (Davidson, 2002; Drevets, 1999). Although there is evidence that the amygdala responds to both appetitive and aversive stimuli, recent conceptualizations suggest that vigilance regulation and the detection of biologically relevant stimuli is the basic function of the amygdala (Davis and Whalen, 2001). Prolonged amygdaloid activation can lead to excess threat awareness and may form the foundation of psychiatric disorders such as anxiety and depression. This overalert state also has been linked to decreased cardiac output and increased peripheral vascular resistance (Winters, McCabe, Green, and Schneiderman, 2000). Interestingly, this cardiovascular pattern is relatively more prevalent in African Americans and may be a factor in the increased levels of hypertension and related morbidity and mortality in this group (Anderson, McNeilly, and Myers, 1991; Brosschot and Thayer, 1998, 1999). Furthermore, variation in alpha-adrenergically mediated vascular tone may be associated with insulin resistance and thus provide a link to the increased diabetes risk found in African Americans (Brook and Julius, 2000).
In sum, decreased prefrontal activity as well as increased amygdala activity have been associated with anxiety, depression, and related pathological states and dispositions. These structures are both part of the CAN and form the core of an emotion regulation system that may become dysfunctional as reflected in perseverative behavior.
Perseverative Thinking as a Balance Between Inhibition and Excitation
In a normal, relaxed, and safe environment, the prefrontal cortex tonically inhibits limbic activity while it is responsively monitoring internal and
external stimuli and guiding goal-directed behavior. The prefrontal cortex is receptive to a broad range of information, but is not specifically directed to potential concerns (e.g., fears such as racism and discrimination) of the organism. Such is the case in situations in which the organism does not expect threat or immediate danger. However, when the situation becomes ambiguous or threat becomes more imminent, or is ever present as it might be for minorities under the constant threat of potential discrimination, prefrontal inhibition of the limbic brain is partially released, potentially generating hypervigilance and perseverative thinking. This partial disinhibition is associated with a hyperalert state of action readiness. In this situation, the organism is obviously not switched completely into a defensive, fight-flight excitatory state. Instead, under limbic influence, the nature of prefrontal function is converted from flexible and open to experience into a state of anticipatory rehearsal of feared scenarios, and vigilant scanning of available information. Under immediate threat, however, the prefrontal cortex is disengaged nearly completely, albeit temporarily, in normal or healthy subjects, resulting in the full and sometimes explosive manifestation of the emotion (anger attack, actual flight, shouting, panicking).
Thus, perseverative thinking can be viewed as a demonstration of the reciprocal nature of prefrontal-amygdala communication. The amygdala sends signals of threat warning to the prefrontal cortex, leading to hypervigilance and rehearsal of feared scenarios, but it is able to do this because of prefrontal disinhibition itself. The disinhibition is in turn due to the rapid and rough perception of immediate threat, which emerges from the integration of ongoing environmental perception with memory associations (conditioned responses), the storage and activation of which is, again, largely under amygdala influence (LeDoux, 2000). In other words, in a bottom-up manner, lower brain centers, in cooperation with memory, demand the prefrontal cortex to occupy itself with rehearsing feared scenarios and stimulate vigilant and biased scanning of internal and external information. At the same time, in a top-down mode, the prefrontal inhibition of lower brain centers is maintained. Perseverative thinking may in fact lead to solutions to the threat, resulting in diminished activation and restoration of the relaxed state dominated by prefrontal inhibition of subcortical sympathoexcitatory circuits.
Finally, it is clear from these findings and theoretical considerations that hypervigilance and perseveration have physiological consequences, that is, signs of stress and limbic-induced autonomic imbalance such as low HRV, elevated HR and cortisol, and particularly elevated blood pressure and peripheral resistance (the so-called “vigilance reaction”; see Winters et al., 2000). Due to partially maintained limbic inhibition by the prefrontal cortex, this activation is often moderate and may appear blunted (cf. Young, Nesse, Weder, and Julius, 1998). But prolonged vagal withdrawal renders
the ANS inflexible and unresponsive to changing environmental demands. Therefore autonomic activation is still high enough (but sustained) to cause damage (e.g., hypertension and cardiovascular disease) (Brosschot and Thayer, 1998), but also low enough, for example, for anxious subjects to learn to worry to prevent full manifestation of fear responses (Borkovec and Hu, 1999). Thus, the autonomic imbalance associated with anticipatory coping and delayed physiological recovery from discrete stressors may be associated with hypervigilance and perseveration due to partial release of prefrontal inhibition of the amygdala. That these anticipatory and recovery responses have been associated with increased blood pressure and peripheral resistance (Gregg, James, Matyas, and Thorsteinsson, 1999) has special importance because this pattern has been linked to increased hypertension and related morbidity and mortality in African Americans.
We have written extensively about perseverative thinking and its physiological and psychological concomitants (Brosschot and Thayer, in press; Brosschot et al., 2003; Friedman and Thayer, 1998a; Ingjaldsson et al., 2003c; Siegle and Thayer, 2003; Thayer and Friedman, 2002; Thayer and Lane, 2002; Thayer and Ruiz-Padial, 2002; Thayer et al., 1996). Physiological concomitants include autonomic imbalance as indexed by decreased HRV, decreased prefrontal cortex activity, increased amygdala activity, excess and prolonged cortisol responsivity, altered immune function, increased blood pressure and peripheral resistance responses in anticipatory coping, increased blood pressure and peripheral resistance during recovery from stress, sustained and prolonged pupil dilation, and poor tolerance to and delayed recovery from stress in general. All of these physiological responses are associated with poor health outcomes.
In addition, a psychological profile associated with perseverative thinking has emerged. This profile is marked by hypervigilance to threat and failure to habituate to innocuous stimuli, impaired cognitive function on tasks demanding delayed responding and executive functions, a lack of inhibitory behavior, denial and an avoidant coping style, increased neuroticism, decreased conscientiousness and impulse control, thought intrusions, lack of perceived control, and greater levels of depression, anxiety, and hostility. Again, all of these psychological responses are linked to poor health outcomes.
RELEVANCE TO HEALTH DISPARITIES
Discrimination and racism have been strongly implicated in the health disparities between ethnic minorities and majority group members (Clark et al., 1999; Gee, 2002; Karlsen and Nazroo, 2002; Krieger, 2000; Williams and Neighbors, 2001). Among the various institutional and individual paths by which discrimination and racism may to lead health disparities, one at
the individual level posits that minority group members are exposed to chronic excess stress. The NIM model details how such stress might “get under one’s skin,” as it were, and lead to both pathophysiology and psychopathology. The final common path of these conditions may be autonomic imbalance, but the question remains as to whether minority group members show evidence of chronic and excessive levels of stress exposure that might lead to an autonomic imbalance that favors poor health outcomes.
The answer to this question appears to be an unqualified yes. Supportive evidence has been provided in the cases of African Americans (Clark et al., 1999), Chinese Americans (Gee, 2002), and of minority groups in England and Wales (Karlsen and Nazroo, 2002). In the United States, for example, perceived interpersonal discrimination has been associated with a number of factors associated with autonomic imbalance, such as depression and psychological stress, high blood pressure, low birthweight, poor self-rated health, smoking, and physical inactivity (Karlsen and Nazroo, 2002).
The literature paints a vivid picture of persons exposed to discrimination and racism. These people tend to show signs of anger, frustration, anxiety, depression, helplessness, hopelessness, resentment, fear, and paranoia (Clark et al., 1999). In addition, such individuals tend to be hypervigilant, distrustful, and wary; often find themselves in ambiguous situations with respect to unfair treatment; interpret ambiguous and harmless situations as threatening; are prone to rumination about the causes and consequences of perceived unfair treatment; engage in anticipatory coping in response to the potential discrimination; and engage in denial and avoidant coping with respect to discrimination (Williams and Neighbors, 2001). It is important to note the similarity between the psychological profiles associated with perseverative thinking and that of persons exposed to discrimination. Moreover, minority status is associated with numerous diseases and increased morbidity and mortality, as discussed at the beginning of this chapter.
Several recent investigations further support this psychological profile of minority group members as distrustful, engaging in anticipatory coping in response to potential discrimination, and hypervigilant. For example, Hunt (2000) recently reported that African Americans in Southern California, when compared to whites and Latinos, have the weakest “belief in a just world.” This concept measures the perceived fairness of a person’s interactions with the world and suggests that African Americans have greater mistrust. That this mistrust may be adaptive and appropriate in a society characterized by discrimination does not lessen its potential deleterious effects via hypervigilance. Similarly, Blascovich, Spencer, Quinn, and Steele (2001) have shown that African Americans exposed to stereotype threat have elevated mean arterial pressure (MAP) responses during a mild
cognitive challenge. Stereotype threat occurs when members of stereotyped groups find themselves in situations in which others may view them stereotypically such that the pressure to perform well is increased. Importantly, they also found that MAP was elevated during an intervening rest period when performance was not expected, but rumination or perseverative thinking may have occurred. Finally, Chen and Matthews (2001) have recently reported that low socioeconomic status (SES) and African-American children interpret ambiguous scenarios as conveying more hostile intent and inducing greater feelings of anger. In addition, these appraisals were associated with increased vascular responses as measured by impedance cardiography. Importantly, for low-SES African-American children, these appraisals and feelings were associated with vascular responses 3 years later. Thus these appraisal biases grew stronger over time in the African-American children and appeared to sensitize them to interpret ambiguous situations as more and more threatening over time. The authors suggest that this may be the result of the greater exposure of the African American children to discrimination and racism. Similar results have been reported in adult African Americans. Merritt, Bennett, and Williams (2002) found that in response to a story about a negative social interaction in which the actors were ambiguous with respect to ethnicity, the African-American males showed elevated MAP and diastolic blood pressure responses when they reported greater perceptions of racism evident in the story. Again, these elevated responses persisted into recovery periods when the actual stressor was no longer present, but when rumination and perseverative thinking were likely to occur. Taken together, these studies provide empirical support for the psychological profile described earlier and its deleterious effects in African Americns.
Due to the excess energy demand that autonomic imbalance places on the organism, individuals exposed to discrimination and racism may have a kind of premature aging that speeds their way to death and disability. The fact that autonomic imbalance continues to predict morbidity and mortality into old age when other risk factors have lost their predictive power adds further to the utility of the neurovisceral integration model in the understanding of health disparities in the elderly.
Preliminary Data in Support of the Model
We recently completed a pilot study that sought to examine in an elderly African-American sample some of the aspects of the model we have outlined. The total sample included 445 African Americans residing in east Baltimore. Psychophysiological assessment was completed on a subsample of 106 participants (50 males, 56 females) as part of the Healthy Aging in Nationally Diverse Samples (HANDLS) study that has been initiated at the
Intramural Research Program of the National Institute on Aging. This study was designed to examine the nature of health disparities using a multilevel approach ranging from the molecular to the social. Briefly, blood pressure and HR were continuously monitored during administration of two subtasks of the Perception of Affect Test (PAT) in this African-American sample. The PAT involved asking subjects to evaluate emotional expressions (and their intensity) in faces and sentences. Those who were able to correctly identify emotions in these stimuli showed lower blood pressure and total peripheral resistance responses as well as decreased depression scores. Older persons (age 52 and higher) performed more poorly than younger ones at correctly identifying emotions, and older men specifically had more difficulty in correctly identifying disgust and fear than their female counterparts. Older African-American males, therefore, showed the greatest deficits in emotional processing and the largest total peripheral resistance responses to the tasks.
A more thorough examination of the physiological responses indicated that HR and blood pressure increased from baseline in response to completion of the PAT tasks. Notably, blood pressure remained elevated during the subsequent recovery period. Indices of blood pressure, cardiac output, and total peripheral resistance revealed signs of elevated peripheral resistance during recovery. In addition, sympathetic (vascular) measures of HRV (reduced high-frequency and increased low-frequency power) and blood pressure variability (reduced high-frequency power) increased during recovery. Previous research showing vascular hyperreactivity among African Americans supports the present results (Brosschot and Thayer, 1999; Jones, Andrawis, and Abernethy, 1999).
The present data further suggest that high vascular reactivity may be associated with psychosocial factors. In this sample, higher levels of depressive symptoms were related to larger total peripheral resistance during task and recovery. In older women, self-reported loneliness was positively correlated with total peripheral resistance at baseline and recovery. These results underscore the multiple levels of system function that may contribute to health disparities in cardiovascular disease, especially hypertension.
A series of carefully selected genetic polymorphisms implicated in cardiovascular disease will be studied in future work to assess genetic contributions to autonomic balance. These are (1) angiotensin-converting enzyme (ACE) insertion/deletion mutation, (2) endothelial nitric oxide synthase (eNOS) G894T mutation, (3) eNOS T786C mutation, (4), angiotensinogen M235T mutation, (5) apolipoprotein E polymorphisms, and (6) serotonin transporter 5-HTTLPR polymorphism. Common features of these mutations are allelic frequency in populations studied greater than 0.2, association with cardiovascular disease (congestive heart failure, hypertension, myocardial infarction) and/or specific personality traits, and relation to a
measurable physiological difference among genotypes (e.g., decreased nitric oxide production, impaired angiotensin II formation, altered lipoprotein pattern, decreased serotonin transporter transcription efficiency). The study population will be genotyped for this array, and genotype patterns of the six genes as well as single polymorphisms will be linked to variability subgroups in ANS function using quartile analyses.
Preliminary observations indicate that high-frequency HRV is decreased in the basal state in the DD genotype of the ACE insertion/deletion polymorphism (Thayer et al., 2003), and that the allelic frequency of the eNOS G894T allele is markedly decreased in the HANDLS study population as compared to a white control group. This finding is intriguing because the T allele has been associated with increased likelihood of cardiovascular disease in other populations. If confirmed, this observation would exclude this source of cardiovascular risk in this African-American population. The DD phenotype of the ACE insertion/deletion gene has been associated with increased levels of circulating ACE and increased risk of sudden cardiac death in some populations. Our finding of decreased HRV and decreased baroreflex sensitivity in the DD phenotype is consistent with the literature linking this phenotype with increased cardiovascular disease risk. These preliminary findings attest to the promise of the candidate gene approach to better understand sources of variability in ANS function.
These preliminary data also suggest that psychosocial factors are related to physiological processes that have major implications for the health disparities observed in the United States. For example, depression, which has been suggested as a primary factor in the greater rates of hypertension in African Americans, was associated with both poor affective regulation and increased peripheral resistance. As detailed earlier, increased peripheral resistance is associated with autonomic imbalance via sustained activity in the amygdala and with hypervigilance and perseverative thinking. Increased peripheral resistance is also related to established hypertension and insulin resistance, and thus plays a role in both cardiovascular disease and diabetes (Brook and Julius, 2000).
Genetic factors also have been implicated in the health disparities, but our data suggest that at least one genetic polymorphism associated with increased risk in whites may not be a factor in African Americans (see Chapter 8, this volume). Another polymorphism, however, was found to have a relationship with HRV, and thus may interact with psychosocial factors to produce individual differences in disease risk. It is notable in this context that merely having a particular polymorphism is not sufficient to produce risk—the polymorphism must be expressed. Gene expression is a result of a complex process that includes exposure to environmental factors that can determine the expression of a cascade of genetic influences, the outcome of which is difficult to predict.
FUTURE DIRECTIONS AND RECOMMENDATIONS FOR RESEARCH
There are a number of important directions for future research. First and foremost, this research must take into account the requisite multiple levels of analysis to explicate the complex factors that contribute to the health disparities (Anderson, 1999; Brosschot and Thayer, 1998). The interaction of factors across these levels will be the source of many insights. This type of multidisciplinary research must face the current grant review system that often establishes narrowly focused study sections, which may miss the value of multilevel initiatives.
In addition, longitudinal, prospective studies will be necessary to untangle the causal factors that lead to the observed health disparities. These studies will require a major commitment on the part of funding agencies. It will also be necessary to examine factors across generations, and this will also tax the current review and funding structure.
More specific recommendations for research are also justified, such as the need to examine within-ethnic group variability. That is, individual differences exist within each ethnic group. For example, preliminary results of the ACE insertion/deletion polymorphism reported earlier suggest an intraethnic group source of variability that may modify the impact of other genetic as well as psychosocial influences. Such factors need to be identified and explored. Related to this need is the call for examination of intraindividual variability as distinct from interindividual variability. Relationships found among variables between individuals may not generalize to the relationships found among the same variables within individuals (Thayer and Lane, 2000). Thus, the repeated measurement of individuals over time is necessary to explicate those relationships at the individual level (Friedman, 2003).
Finally, studies must address the interplay of physiological, behavioral, affective, cognitive, and social processes and their impact on health, health behaviors, and disease. The neurovisceral integration model offers a framework to integrate such work across levels of analysis. It is key that the central nervous system be included, which entails neuroimaging and neuropsychological research to tap brain processes that support and accompany the many complex factors involved in health and disease in aging.
REFERENCES
Aggleton, J.P., and Young, A. (2000). The enigma of the amygdala: On its contribution to human emotion. In R.D. Lane and L. Nadel (Eds.), Cognitive neuroscience of emotion (pp. 106-128). New York: Oxford University Press.
Ahern, G.L., Labiner, D.M., Hutzler, R., Osburn, C., Talwar, D., Herring, A.M., et al. (1994). Quantitative analysis of the EEG in the intracarotid amobarbital test: I. Amplitude analysis. Electroencephalography and Clinical Neurophysiology, 91, 21-32.
Amen, D.G., Stubblefield, M., Carmichael, B., and Thisted, R. (1996). Brain SPECT findings and aggressiveness. Annals of Clinical Psychiatry, 8, 129-137.
Anderson, N.B. (1999). Solving the puzzle of socioeconomic status and health: The need for integrated, multilevel, interdisciplinary research. Annals of the New York Academy of Sciences, 896, 302-312.
Anderson, N.B., McNeilly, M., and Myers, H. (1991). Autonomic reactivity and hypertension in blacks: A review and proposed model. Ethnicity and Disease, 1, 154-170.
Angrilli, A., Mauri, A., Palomba, D., Flor, H., Birbaumer, N., Sartori, G., and di Paola, F. (1996). Startle reflex and emotion modulation impairment after a right amygdala lesion. Brain, 119, 1991-2000.
Arnsten, A.F.T., and Goldman-Rakic, P.S. (1998). Noise stress impairs prefrontal cortical cognitive function in monkeys: Evidence for a hyperdopaminergic mechanism. Archives of General Psychiatry, 55, 362-368.
Benarroch, E.E. (1993). The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clinic Proceedings, 68, 988-1001.
Benarroch, E.E. (1997). The central autonomic network. In P.A. Low (Ed.), Clinical autonomic disorders (2nd ed., pp. 17-23). Philadelphia: Lippincott-Raven.
Black, S.A. (2002). Diabetes, diversity, and disparity: What do we do with the evidence? American Journal of Public Health, 92, 543-548.
Blascovich, J., Spencer, S.J., Quinn, D., and Steele, C. (2001). African Americans and high blood pressure: The role of stereotype threat. Psychological Science, 12, 225-229.
Bohlin, G., and Kjellberg, A. (1979). Orienting activity in two-stimulus paradigms as reflected in heart rate. In H.D. Kimmel, E.H. van Olst, and J.F. Orlebeke (Eds.), The orienting reflex in humans (pp. 169-197). Hillsdale, NJ: Lawrence Erlbaum Associates.
Borkovec, T.D. (1985) Worry: A potentially valuable concept. Behaviour Research and Therapy, 23(4), 481-482.
Borkovec, T.D., and Hu, S. (1999). The effect of worry on cardiovascular response to phobic imagery. Behavioral Research Therapy, 28(1), 69-73.
Bradley, M.M., and Lang, P.J. (2000). Measuring emotion: Behavior, feeling, and psysiology. In R.D. Lane and L. Nadel (Eds.), Cognitive neuroscience of emotion (pp. 106-128). New York: Oxford University Press.
Brook, R.D., and Julius, S. (2000). Autonomic imbalance, hypertension, and cardiovascular risk. American Journal of Hypertension, 13, 112S-122S.
Brosschot, J.F., and Thayer, J.F. (1998). Anger inhibition, cardiovascular recovery, and vagal function: A model of the link between hostility and cardiovascular disease. Annals of Behavioral Medicine, 20(4), 1-8.
Brosschot, J.F., and Thayer, J.F. (1999). Cardiovascular recovery after harassment with anger expression or inhibition. Gedrag & Gezondheid, 27(1/2), 8-14.
Brosschot, J.F., and Thayer, J.F. (in press). Worry, perseverative thinking and health. In L. Temoshok (Ed.), The expression and non-expression of emotion in health and disease. Hillsdale, NJ: Lawrence Erlbaum Associates.
Brosschot, J.F., Godaert, G.L.R., Benschop, R.J., Olff, M., Ballieux, R.E., and Heijnen, C.J. (1998). Experimental stress and immunological reactivity: A closer look at perceived uncontrollability. Psychosomatic Medicine, 60(3), 359-361.
Brosschot, J.F., van Dijk, E., and Thayer, J.F. (2003). Daily worrying and stressors increase daytime-and night-time cardiac activity. Psychosomatic Medicine, 65, A-4.
Chen, E., and Matthews, K.A. (2001). Cognitive appraisal biases: An approach to understanding the relation between socioeconomic status and cardiovascular reactivity in children. Annals of Behavioral Medicine, 23, 101-111.
Clark, R., Anderson, N.B., Clark, V.R., and Williams, D.R. (1999). Racism as a stressor for African Americans: A biopsychosocial model. American Psychologist, 54, 805-816.
Cohen, H., Matar, M.A., Kaplan, Z., and Kotler, M. (1999). Power spectral analysis of heart rate variability in psychiatry. Psychotherapy and Psychosomatics, 68, 59-66.
Crestani, F., et al. (1999). Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nature Neuroscience, 2, 833-839.
Damasio, A.R. (1998). Emotion in the perspective of an integrated nervous system. Brain Research Reviews, 26, 83-86.
Das, U.N. (2000). Beneficial effect(s) of n-3 fatty acids in cardiovascular disease: But, why and how? Prostaglandins, Leukotrienes, and Essential Fatty Acids, 63, 351-362.
Davey, G.C.L. (1994). Pathological worrying as exacerbated problem-solving. In G.C.L. Davey and F. Tallis (Eds.), Worrying: Perspectives on theory, assessment and treatment (pp. 35-60). New York: Wiley.
Davidson, R.J. (2000). The functional neuroanatomy of affective style. In R.D. Lane and L. Nadel (Eds.), Cognitive neuroscience of emotion (pp. 106-128). New York: Oxford University Press.
Davidson, R.J. (2002). Anxiety and affective style: Role of prefrontal cortex and amygdala. Biological Psychiatry, 51, 68-80.
Davis, M., and Whalen, P.J. (2001). The amygdala: Vigilance and emotion. Molecular Psychiatry, 6, 13-34.
Devinsky, O., Morrell, M.J., and Vogt, B.A. (1995). Contributions of anterior cingulate cortex to behavior. Brain, 118, 279-306.
Dougherty, D.D., Shin, L.M., Alpert, N.M., Pitman, R.K., Orr, S.P., Lasko, M., Macklin, M.L., Fischman, A.J., and Rauch, S.L. (1999). Anger in healthy men: A PET study using script-driven imagery. Biological Psychiatry, 46, 466-472.
Drevets, W.C. (1999) . Prefrontal cortical-amygdalar metabolism in major depression. Annals of the New York Academy of Sciences, 877, 614-637.
Eriksen, H.R., and Ursin, H. (2002). Sensitization and subjective health complaints. Scandinavian Journal of Psychology, 4(2), 189-196.
Ershler, W., and Keller, E. (2000). Age-associated increased interleukin-6 gene expression, late life diseases, and frailty. Annual Review of Medicine, 51, 245-270.
Everson, S.A., Goldberg, D.E., Kaplan, G.A., Cohen, R.D., et al. (1996). Hopelessness and risk of mortality and incidence of myocardial infarction and cancer. Psychosomatic Medicine, 58(2), 113-121.
Frankenhäuser, M. (1980). Psychobiologic aspects of life stress. In S. Levine and H. Ursin (Eds.), Coping and health (Series III, Vol. 12). NATO Conference Series. New York: Plenum.
Friedman, B.H. (2003). Idiodynamics vis-a-vis psychophysiology: An idiodynamic portrayal of cardiovascular activity. Journal of Applied Psychoanalytic Studies, 5, 425-441.
Friedman, B.H., and Thayer, J.F. (1998a). Anxiety and autonomic flexibility: A cardiovascular approach. Biological Psychology, 49, 303-323.
Friedman, B.H., and Thayer, J.F. (1998b). Autonomic balance revisited: Panic anxiety and heart rate variability . Journal of Psychosomatic Research, 44, 133-151.
Friedman, B.H., Thayer, J.T., and Borkovec, T.D. (2000). Explicit memory bias for threat words in generalized anxiety disorder. Behavior Therapy, 31, 745-756.
Frijda, N.H. (1988). The laws of emotion. American Psychologist, 43, 349-358.
Gee, G.C. (2002). Multilevel analysis of the relationship between institutional and individual racial discrimination and health status. American Journal of Public Health, 92, 615-623.
Glass, L., and Mackey, M.C. (1988). From clocks to chaos. Princeton, NJ: Princeton University Press.
Globus, G.G., and Arpaia, J.P. (1994). Psychiatry and the new dynamics. Biological Psychiatry, 35, 352-364.
Goldberger, A.L. (1992). Applications of chaos to physiology and medicine. In J.H. Kim and J. Stringer (Eds.), Applied chaos (pp. 321-331). New York: Wiley.
Goldman-Rakic, P.S. (1998). The prefrontal landscape: Implications of the functional architecture for understanding human mentation and the central executive. In A.C. Roberts, T.W. Robbins, and L. Weiskrantz (Eds.), The prefrontal cortex: Executive and cognitive functions (pp. 87-102). Oxford, England: Oxford University Press.
Grachev, I.D., and Apkarian, A.V. (2000). Anxiety in healthy humans is associated with orbital frontal chemistry. Molecular Psychiatry, 5, 482-488.
Graham, F.K., and Clifton, R.K. (1966). Heart-rate change as a component of the orienting response. Psychological Bulletin, 65, 305-320.
Gregg, M.E., James, J.E., Matyas, T.A., and Thorsteinsson, E.B. (1999). Hemodynamic profile of stress-induced anticipation and recovery. International Journal of Psychophysiology, 34, 147-162.
Habib, G.B. (1999). Reappraisal of heart rate as a risk factor in the general population. European Heart Journal Supplements, 1(H), H2-H10.
Hall, M., Vasko, R., Buysse, D., Thayer, J.F., Ombao, H., Chen, Q., Cashmere, J.D., and Kupfer, D. (2004). Acute stress affects autonomic tone during sleep. Psychosomatic Medicine, 66, 56-62.
Hansen, A.L., Johnsen, B.H., Sollers, J.J., and Thayer, J.F. (2002). Neural control of the heart modulates cognitive processing during stress. Clinical Autonomic Research, 12, 167 (abstract).
Hansen, A.L., Johnsen, B.H., and Thayer, J.F. (2003). Vagal influence in the regulation of attention and working memory. International Journal of Psychophysiology, 48, 263-274.
Hong, S.B., Kim, K.W., Seo, D.W., Kim, S.E., Na, D.G., and Byun, Y.S. (2000). Contralateral EEG slowing and amobarbital distribution in Wada test: An intracarotid SPECT study. Epilepsia, 41, 207-212.
Hunt, M.O. (2000). Status, religion, and the “belief in a just world”: Comparing African Americans, Latinos, and whites. Social Science Quarterly, 81, 325-343.
Ingjaldsson, J.T., Thayer, J.F., and Laberg, J.C. (2003a). Preattentive processing of alcohol stimuli. Scandinavian Journal of Psychology, 44, 161-165.
Ingjaldsson, J.T., Thayer, J.F., and Laberg, J.C. (2003b). Craving for alcohol and preattentive processing of alcohol stimuli. International Journal of Psychophysiology, 49, 29-39.
Ingjaldsson, J.T., Laberg, J.C., and Thayer, J.F. (2003c). Reduced heart rate variability in chronic alcohol abuse: Relationship with negative mood, chronic thought suppression, and compulsive drinking. Biological Psychiatry, 54, 1427-1436.
James, S.A. (1994). John Henryism and the health of African-Americans. Culture Medicine and Psychiatry, 18(2), 163-182.
Johnsen, B.H., Hansen, A.L., Sollers, J.J., Murison, R., and Thayer, J.F. (2001). Heart rate variability is inversely related to cortisol reactivity during cognitive stress. Psychosomatic Medicine, 64, 148 (abstract).
Johnsen, B.H., Thayer, J.F., Laberg, J.C., Wormnes, B., Raadal, M., Skaret, E., Kvale, G., and Berg, E. (2003). Attentional and physiological characteristics of patients with dental anxiety. Journal of Anxiety Disorders, 17, 75-87.
Jones, D.S., Andrawis, N.S., and Abernethy, D.R. (1999). Impaired endothelial-dependent forearm vascular relaxation in black Americans. Clinical Pharmacology Therapy, 65(4), 408-412.
Jose, A.D., and Collison, D. (1970). The normal range and determinants of the intrinsic heart rate in man. Cardiovascular Research, 4, 160-167.
Karlsen, S., and Nazroo, J.Y. (2002). Relation between discrimination, social class, and health among ethnic minority groups. American Journal of Public Health, 92, 624-631.
Kiecolt-Glaser, J.K., McGuire, L., Robles, T.F., and Glaser, R. (2002). Emotions, morbidity, and mortality: New perspectives from psychoneuroimmunology. Annual Review of Psychology, 53, 83-107.
Kimbrell, T.S., George, M.S., Parekh, P.I., Ketter, T.A., Podell, D.M., Danielson, A.L., Repella, J.D., Benson, B.E., Willis, M.W., Herscovitch, P., and Post, R.M. (1999). Regional brain activity during transient self-induced anxiety and anger in healthy adults. Biological Psychiatry, 46(4), 454-465.
Kop, W.J., Verdino, R.J., Gottdiener, J.S., O’Leary, S.T., Merz, C.N.B., and Krantz, D.S. (2001). Changes in heart rate and heart rate variability before ambulatory ischemic events. Journal of the American College of Cardiology, 38, 742-749.
Krantz, D.S., and McCeney, M.K. (2002). Effects of psychological and social factors on organic disease: A critical assessment of research on coronary heart disease. Annual Review of Psychology, 53, 341-369.
Krieger, N. (2000). Discrimination and health. In L. Berkman and I. Kawachi (Eds.), Social epidemiology (pp. 36-75). New York: Oxford University Press.
Kubzansky, L.D., Kawachi, I., Spiro, A., Weiss, S.T., Vokonas, P.S., and Sparrow, D. (1997). Is worrying bad for your heart? A prospective study of worry and coronary heart disease in the Normative Aging Study. Circulation, 95, 818-824.
Lane, R.D., Reiman, E.M., Ahern, G.L., and Thayer, J.F. (2001). Activity in medial prefrontal cortex correlates with vagal component of heart rate variability during emotion. Brain and Cognition, 47, 97-100.
Lang, P.J. (1995). The emotion probe—studies of motivation and attention. American Psychologist, 50, 372-385.
LeDoux, J.E. (2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23, 155-184.
Levenson, R.W. (1988). Emotion and the autonomic nervous system: A prospectus for research on autonomic specificity. In H.L. Wagner (Ed.), Social psychophysiology and emotion: Theory and clinical applications (pp. 17-42). Chichester, England: Wiley.
Levy, M.N. (1990). Autonomic interactions in cardiac control. Annals of the New York Academy of Sciences, 601, 209-221.
Lipsitz, L.A., and Goldberger, A.L. (1992). Loss of complexity and aging: Potential applications of fractals and chaos theory to senescence. Journal of the American Medical Association, 267(13), 1806-1809.
Lundberg, U., and Frankenhäuser, M. (1978). Psychophysiological reactions to noise as modified by personal control over noise intensity. Biological Psychology, 6, 51-59.
Lupien, S.J., Gillin, C.J., and Hauger, R.L. (1999). Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: A dose-response study in humans . Behavioral Neuroscience, 113(3), 420-430.
Lyonfields, J.D., Borkovec, T.D., and Thayer, J.F. (1995). Vagal tone in generalized anxiety disorder and the effects of aversive imagery and worrisome thinking. Behavioral Therapy, 26, 457-466.
Mackinnon, D.W., and Dukes, W. (1962). Repression. In L. Postman (Ed.), Psychology in the making: Histories of selected research problems (pp. 662-744). New York: Alfred A. Knopf.
Maier, S.F., and Watkins, L.R. (1998). Cytokines for psychologists: Implications of bi-directional immune-to-brain communication for understanding behavior, mood, and cognition. Psychological Review, 105, 83-107.
Malizia, A.L., Cunningham, V.J., Bell, C.J., Liddle, P.F., Jones, T., and Nutt, D.J. (1998). Decreased brain GABAA-benzodiazepine receptor binding in panic disorder. Archives of General Psychiatry, 55, 715-720.
Malliani, A., Pagani, M., and Lombardi, F. (1994). Methods for assessment of sympathovagal balance: Power spectral analysis. In M.N. Levy and P.J. Schwartz (Eds.), Vagal control of the heart: Experimental basis and clinical implications (pp. 433-454). Armonk, NY: Futura.
Masterman, D.L., and Cummings, J.L. (1997). Frontal-subcortical circuits: The anatomical basis of executive, social and motivated behaviors. Journal of Psychopharmacology, 11, 107-114.
Mathews, A. (1990). Why worry? The cognitive function of anxiety. Behaviour Research and Therapy, 28, 455-468.
Mayberg, H.S., et al. (1999). Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. American Journal of Psychiatry, 156, 675-682.
McEwen, B.S. (1998). Protective and damaging effects of stress mediators. New England Journal of Medicine, 338, 171-179.
McGeer, P.L., Eccles, J.C., and McGeer, E.G. (1978). Molecular neurobiology of the mammalian brain. New York: Plenum.
Merritt, M.M., Bennett, G.G., and Williams, R.B. (2002). Subtle racism and elevated cardiovascular reactivity among black males. Annals of Behavioral Medicine, 24, S18 (abstract).
Musselman, D.L., Evans, D.L., and Nemeroff, C.B. (1998). The relationship of depression to cardiovascular disease. Archives of General Psychiatry, 55, 580-592.
Nabors-Oberg, R., Sollers, J.J., Niaura, R., and Thayer, J.F. (2002). The effects of controlled smoking on heart period variability. IEEE Engineering in Medicine and Biology Magazine, 21, 65-70.
National High Blood Pressure Education Program. (1997). The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. NIH Publication no. 98-4080. Bethesda, MD: U.S. Department of Health and Human Services, National Heart, Lung, and Blood Institute.
Palatini, P., and Julius, S. (1999). The physiological determinants and risk correlations of elevated heart rate. American Journal of Hypertension, 12, 3S-8S.
Peng, C.K., Buldyrev, S.V., Hausdorff, J.M., Havlin, S., Mietus, J.E., Simons, M., Stanley, H.E., and Goldberger, A.L. (1994). Non-equilibrium dynamics as an indispensable characteristic of a healthy biological system. Integrated Physiologic Behavioral Science, 3, 283-293.
Porges, S.W. (1992). Autonomic regulation and attention. In B.A. Campbell, H. Hayne, and R. Richardson (Eds.), Attention and information processing in infants and adults (pp. 201-223). Hillsdale, NJ: Lawrence Erlbaum Associates.
Porges, S.W., Doussard-Roosevelt, J.A., and Maita, A.K. (1994). Vagal tone and the physiological regulation of emotion. Monographs of the Society for Research in Child Development, 59(2-3), 167-186, 250-283.
Reed, S.W., Porges, S.W., and Newlin, D.B. (1999). Effect of alcohol on vagal regulation of cardiovascular function: Contributions of the polyvagal theory to the psychophysiology of alcohol. Experimental and Clinical Psychopharmacology, 7(4), 484-492.
Roberts, A.C., and Wallis, J.D. (2000). Inhibitory control and affective processing in the prefrontal cortex: Neuropsychological studies in the common marmoset. Cerebral Cortex, 10, 252-262.
Rossy, L.A., and Thayer, J.F. (1998). Fitness and gender-related differences in heart period variability. Psychosomatic Medicine, 60, 773-781.
Rozanski, A., Blumenthal, J.A., and Kaplan, J. (1999). Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation, 99, 2192-2217.
Ruiz-Padial, E., Sollers, J.J., III, Vila, J., and Thayer, J.F. (2003). The rhythm of the heart in the blink of an eye: Emotion-modulated startle magnitude covaries with heart rate variability. Psychophysiology, 40, 306-313.
Saul, J.P. (1990). Beat-to-beat variations of heart rate reflect modulation of cardiac autonomic outflow. News in Physiological Science, 5, 32-37.
Seeman, T.E., Singer, B.H., Rowe, J.W., Horwitz, R.I., and McEwen, B.S. (1997). Price of adaptation—allostatic load and its health consequences: McArthur studies of successful aging. Archives of Internal Medicine, 157, 2259-2268.
Siegle, G.J., and Thayer, J.F. (2003). Physiological aspects of depressive rumination (pp. 79-104). In C. Papageorgiou and A. Wells (Eds.), Depressive rumination: Nature, theory, and treatment. New York: Wiley.
Skinner, J.E. (1985). Regulation of cardiac vulnerability by the cerebral defense system. Journal of the American College of Cardiology, 5, 88B-94B.
Sokolov, E.N. (1963). Perception and the orienting response. New York: MacMillan.
Spyer, K.M. (1989). Neural mechanisms involved in cardiovascular control during affective behavior. Trends in Neuroscience, 12, 506-513.
Sroka, K., Peimann, C.J., and Seevers, H. (1997). Heart rate variability in myocardial ischemia during daily life. Journal of Electrocardiology, 30, 45-56.
Stein, P.K., and Kleiger, R.E. (1999). Insights from the study of heart rate variability. Annual Review of Medicine, 50, 249-261.
Stein, P.K., Bosner, M.S., Kleiger, R.E., and Conger, B.M. (1994). Heart rate variability: A measure of cardiac autonomic tone. American Heart Journal, 127, 1376-1381.
Steptoe, A., and Appels, A. (1989). Stress, personal control and health. Chicester, England: Wiley.
Sternberg, E.M. (1997). Emotions and disease: From balance of humors to balance of molecules. Nature Medicine, 3, 264-267.
Tallis, F., and Eysenck, M.W. (1994). Worry: Mechanisms and modulating influences. Behavioural and Cognitive Psychotherapy, 22(1), 37-56.
Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology. (1996). Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation, 93, 1043-1065.
Ter Horst, G.J. (1999). Central autonomic control of the heart, angina, and pathogenic mechanisms of post-myocardial infarction depression. European Journal of Morphology, 37, 257-266.
Ter Horst, G.J., and Postema, F. (1997). Forebrain parasympathetic control of heart activity: Retrograde transneuronal viral labeling in rats. American Journal of Physiology, 273, H2926-H2930.
Thayer, J.F., and Friedman, B.H. (1997). The heart of anxiety: A dynamical systems approach. In A. Vingerhoets (Ed.), The (non) expression of emotions in health and disease. Amsterdam: Springer Verlag.
Thayer, J.F., and Friedman, B.H. (2002). Stop that! Inhibition, sensitization, and their neurovisceral concomitants. Scandinavian Journal of Psychology, 43, 123-130.
Thayer, J.F., and Lane, R.D. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61, 201-216.
Thayer, J.F., and Lane, R.D. (2002). Perseverative thinking and health: Neurovisceral concomitants. Psychology and Health, 17, 685-695.
Thayer, J.F., and Ruiz-Padial, E. (2002). Neurovisceral integration in emotion and health. International Congress Series: Proceedings of the 16th World Congress on Psychosomatic Medicine, 1241, 321-327.
Thayer, J.F., Friedman, B.H., and Borkovec, T.D. (1996). Autonomic characteristics of generalized anxiety disorder and worry. Biological Psychiatry, 39, 255-266.
Thayer, J.F., Smith, M., Rossy, L.A., Sollers, J.J., and Friedman, B.H. (1998). Heart period variability and depressive symptoms: Gender differences. Biological Psychiatry, 44, 304-306.
Thayer, J.F., Friedman, B.H., Borkovec, T.D., Johnsen, B.H., and Molina, S. (2000). Phasic heart period to cued threat and non-threat stimuli in generalized anxiety disorder. Psychophysiology, 37, 361-368.
Thayer, J.F., Merritt, M.M., Sollers, J.J., III, Zonderman, A.B., Evans, M.K., Yie, S., and Abernethy, D.R. (2003). Effect of angiotensin-converting enzyme insertion/deletion polymorphophism DD genotype on high-frequency heart rate variability in African Americans. American Journal of Cardiology, 92, 1487-1490.
Timberlake, W. (1994). Behavior systems, associationism, and Pavlovian conditioning. Psychonomic Bulletin and Review, 1, 405-420.
Tracey, K.J. (2002). The inflammatory reflex. Nature, 420, 853-859.
Uijtdehaage, S.B.H., and Thayer, J.F. (2000). Accentuated antagonism in the control of human heart rate. Clinical Autonomic Research, 10, 107-110.
Ursin, H. (1987). Personality, activation, and psychosomatic disease. In D. Magnusson, A. Oehman et al. (Eds.), Psychopathology: An interactional perspective. Personality, psychopathology, and psychotherapy (pp. 273-287). Orlando, FL: Academic Press.
Ursin, H. (1998). The psychology in psychoneuroendocrinology. Psychoneuroendocrinology, 23, 555-570.
Ursin, H., and Hytten, K. (1992). Outcome expectancies and psychosomatic consequences. In B.N. Carpenter et al. (Eds.), Personal coping: Theory, research, and application (pp. 171-184). Westport, CT: Praeger/Greenwood.
Verberne, A.J.M., and Owens, N.C. (1998). Cortical modulation of the cardiovascular system. Progress in Neurobiology, 54, 149-168.
Verrier, R.L. (1987). Mechanisms of behaviorally induced arrhythmias. Circulation, 76(Suppl. I), I48-I56.
Verrier, R.L., and Mittleman, M.A. (2000). The impact of emotions on the heart. Progress in Brain Research, 122, 369-380.
Weise, F., Krell, D., and Brinkhoff, N. (1986). Acute alcohol ingestion reduces heart rate variability. Drug and Alcohol Dependence, 17, 89-91.
Williams, D.R. (1997). Race and health: Basic questions, emerging directions. Annals of Epidemiology, 7, 322-333.
Williams, D.R., and Neighbors, H. (2001). Racism, discrimination and hypertension: Evidence and needed research. Ethnicity and Disease, 11, 800-816.
Winters, R.W., McCabe, P.M., Green, E.J., and Schneiderman, N. (2000). Stress responses, coping, and cardiovascular neurobiology: Central nervous system circuitry underlying learned and unlearned affective responses to stressful stimuli. In P.M. McCabe, N. Schneiderman, T. Field, and A.R. Wellens (Eds.), Stress, coping, and cardiovascular disease. Hillsdale, NJ: Lawrence Erlbaum Associates.
Young, E.A., Neese, R.M., Weder, A., and Julius, S. (1998). Anxiety and cardiovascular reactivity in the Tecumseh population. Journal of Hypertension, 16(12), 1727-1733.
Ziegler, D., Laude, D., Akila, F., and Elghozi, J.L. (2001). Time and frequency domain estimation of early diabetic cardiovascular autonomic neuropathy. Clinical Autonomic Research, 11(6), 369-376.