4
Cardiac Sensitization
In this chapter, the subcommittee presents background information on the development of cardiac sensitization as a toxic end point, the various cardiac-sensitization methods that may be used for determining the toxicity of halocarbons, and the pharmacokinetics of cardiac sensitization.
DEVELOPMENT OF CARDIAC-SENSITIZATION STUDIES
The identification of cardiac sensitization as a potential adverse reaction to an airborne chemical goes back almost 100 years. Cats lightly anesthetized with chloroform were unexpectedly sensitive to injected epinephrine (Levy and Lewis 1911). When the animals inhaled chloroform at 0.5% or 2.0% in air and then received a bolus intravenous injection of epinephrine (total dose, up to 65 micrograms [μg]), they had a “heterogenetic” electrocardiograph (ECG) pattern, that is, short pauses in heartbeat followed by tachycardia. Continued administration of chloroform ultimately resulted in ventricular fibrillation. Later studies showed that the variations in cardiac sensitivity depended on the duration and degree of anesthesia (Levy 1913). Light anesthesia with chloroform produced more cardiotoxic effects than deeper surgical anesthesia, possibly because of a decrease in central nervous system impulses to the heart. Levy found a number of published cases in which humans had been overcome by chloroform and medical treatment had consisted of injecting epinephrine (to stimulate the cardiovascular system). In many cases, the patients died after exhibiting tachycardia followed by ventricular fibrillation. The increased sensitivity of the heart to epinephrine brought about by exposure to a specific organic chemical was referred to as cardiac sensitization.
In 1937, Meek et al. (1937) refined the experimental protocol of Levy and used dogs as the experimental animal. They also demonstrated an increased sensitivity of the heart to hydrocarbons (cyclopropane) when inhalation was accompanied by intravenous injections of epinephrine. On the basis of those studies, the potential hazard associated with administering hydrocarbon anesthetic agents followed by epinephrine became clearly recognized.
As a result of those and later studies on the ability of anesthetic agents to produce cardiac arrhythmia in the presence of exogenous epinephrine, it became evident that hydrocarbons, both halogenated and nonhalogenated, alone or in combination with injected epinephrine could sensitize the myocardium to produce cardiac arrhythmia. The hydrocarbon concentrations required to produce such sensitization ranged from 0.5% to 90% in air.
Although cardiac arrhythmia presents a risk to anesthetized patients, it was not until the 1960s, when chlorofluorocarbons (CFCs) began to be used as aerosol propellants in consumer products, that cardiac sensitization received more toxicologic consideration. CFC propellants were sniffed to reach light anesthesia, that is, to get “high”; and there were 65 reported deaths from such abuse (Bass 1970; Reinhardt et al. 1971). Such deaths occurred during or shortly after inhalation of high concentrations of the aerosols and were generally accompanied by physical or other stress. The deaths were thought to be due to ventricular fibrillation resulting from cardiac sensitization caused by the combination of inhalation of high concentrations of aerosol propellants and high blood concentrations of endogenous epinephrine produced by excitement. At autopsy, there were no unusual pathologic findings, and no anatomic changes were seen in the heart, brain, or other organs. Cardiac sensitization as the cause of death was typically based on circumstantial evidence at the scene—the position of the body and empty aerosol cans and a lack of autopsy findings that might otherwise be responsible for the death.
Such abuse was of concern to the CFC manufacturers, who began to develop a toxicologic method that could determine the cardiac-sensitization potential of the chemicals. Reinhardt et al. (1971) and Clark and Tinston (1973) worked on identifying an appropriate animal model and determining appropriate doses of exogenous epinephrine to simulate circulating blood epinephrine.
As CFCs have been phased out over the last 2 decades in compliance with the Montreal Protocol, the search for effective alternatives has focused on using cardiac sensitization as a mechanism for ranking the human health risk posed by the alternative chemicals. In some applications, the exposures are very brief, lasting several seconds to a few minutes.
METHODS FOR STUDYING CARDIAC SENSITIZATION
Cardiac sensitization can be studied using exogenously administered (injected) epinephrine or by induction of high concentrations of epinephrine with external stimuli. This section briefly describes the differences between the study methods and discusses the results that may be obtained with each.
Exogenous-Epinephrine Studies
In response to reported deaths in humans, apparently associated with sniffing of aerosol propellants, Reinhardt et al. (1973) developed a systematic screening approach for determining the cardiac-sensitization potential of unsubstituted and halogenated hydrocarbons. The fixed-epinephrine-dose protocol has been modified recently, to what is referred to as the epinephrine-titration protocol, to account for individual test-animal variation in sensitivity to epinephrine. The protocols differ in the dose of epinephrine used and the procedure for its administration. Each method affects the risk assessments associated with human exposures.
Fixed-Epinephrine-Dose Protocol
Table 4-1 lists the steps (and their durations) of the cardiac-sensitization screening method of Reinhardt et al. (1971). A conscious male beagle is fitted with a flow-through mask and exposed to various concentrations of the test chemical in air. The animal is given an intravenous epinephrine injection before exposure and a second injection during exposure. Its ECG
TABLE 4-1 General Protocol for Cardiac Sensitization in Dogs
|
Time, min |
Activity |
|
0 |
Start; control (air) administration |
|
2 |
Administer epinephrine intravenously |
|
5 |
|
|
7 |
Begin test-chemical administration |
|
10 |
|
|
12 |
Administer epinephrine challenge dose intravenously |
|
15 |
|
|
17 |
Stop test-chemical administration |
|
Source: Adapted from Reinhardt et al. 1971. |
|
response is continuously monitored. The dog breathes air alone for the first 7 min of the experiment. A control intravenous injection of epinephrine (8 g per kilogram [kg]) in 1 milliliter (mL) of saline is administered at 2 min into the experiment over a 9-sec interval, and exposure to air continues for an additional 5 min. Each dog serves as it own control. If the dog shows a cardiac arrhythmia in response to the control injection of epinephrine, he is not used for that chemical concentration, although he may be used for another concentration. Thus, any response that is seen in response to exposure to the test chemical occurs at a dose of epinephrine which does not otherwise cause cardiac sensitization. For 7-17 min, the dog inhales a given concentration of the chemical-air mixture. After 5 min of exposure to the chemical-air mixture (12 min into the study), a challenge injection of epinephrine (8 g/kg) is given. If the concentration of the chemical produces cardiac sensitization, an arrhythmia (potentially life-threatening) would be seen on the ECG. After the 10-min exposure to the chemical, the study is stopped (17-min point into the protocol).
The dose of epinephrine used to challenge the animal and the exposure duration are important variables in inducing cardiac sensitization. High doses of epinephrine produce ventricular fibrillation, so cardiac-sensitization tests must use smaller doses. Reinhardt et al. (1971) used an epinephrine dose of 8 g/kg, which resulted in a dose rate of about 50 g/kg per minute. That was inherently conservative in as much as the dose rate of epinephrine was about 10 times the dose calculated to occur in humans during times of stress (5 g/kg per minute) (Price et al. 1958; Mullin et al. 1972).
Reinhardt et al. (1971) investigated the length of exposure to dichlorodifluoromethane (CFC-12) required to induce cardiac sensitization. Groups of seven dogs received an epinephrine injection at 2 min and then 10 min later inhaled CFC-12 at 7.0% or 13.5% for 30 sec. None of the dogs at the lower concentration and two of seven dogs at the higher concentration exhibited cardiac sensitization, including one case of cardiac arrest. In Reinhardt’s standard 17-min protocol (1971), exposure to 2.5% CFC-12 for 5 min produced no cardiac sensitization, whereas exposure to 5.0% CFC-12 resulted in cardiac sensitization in five of 12 dogs. The absence of cardiac sensitization in dogs exposed to CFC-12 at 2.45-2.58% for 30 min or even 60 min before epinephrine challenge suggests that the threshold for cardiac sensitization may be independent of length of exposure (Reinhardt et al. 1971). The 5-min threshold for cardiac sensitization was demonstrated in later experiments (Reinhardt et al. 1971); 5-min exposures to CFC-113 at 0.5% (5,000 ppm) resulted in serious arrhythmias in 10 of 29 dogs, but none at 0.25% (2,500 ppm), and only one of 12 dogs exposed at 0.25% (2,500 ppm) for 6 h before epinephrine challenge developed an arrhythmia.
The results of the screening studies (Reinhardt et al. 1971) with various hydrocarbons showed that cardiac sensitization occurred generally at concentrations of 5-20%, although CFC-11 and CFC-113 induced arrhythmias at concentrations as low as 0.5%. Those screening studies, although not intended for quantitative risk assessments, can be used to rank fluorocarbons with regard to their cardiac-sensitization potential. CFC-11 is considered to be a “strong sensitizer”; CFC-12, which produced arrhythmias at 5.0% (2.5-7.5%), a “moderate sensitizer”; and CFC-115, which required concentrations of 15.0% or more to produce arrhythmias, a “weak sensitizer.” Since the Reinhardt et al. studies, nearly 100 halocarbons and hydrocarbons have been tested, and most have shown cardiac-sensitization potential with the dog model. Iodotrifluoromethane (CF3I), which produces arrhythmia at 0.4% in the dog (see next section), would rank as a strong sensitizer according to the above criteria.
The results seen with CF3I and other selected fluorocarbons have been reviewed by Brock et al. (2003). The studies that they reviewed strongly indicate that arrhythmias that occur after epinephrine challenge result from exposure to the test chemical and are potentially life-threatening. No serious arrhythmia followed any control epinephrine injection or followed a challenge injection in several control experiments that used air alone.
Epinephrine-Titration Protocol
With the recognition that CFCs were stratospheric ozone depleters, efforts increased to identify alternative chemicals. Prominent among the possible alternatives were hydrochlorofluorocarbons (HCFCs) and hydrofluorocarbons (HFC). Cardiac-sensitization studies of those compounds were undertaken, in large part, under the coordination of the international industry consortium Program for Alternative Fluorocarbon Toxicity Testing (PAFT). The protocol used for the studies was based on that of Reinhardt et al. (1971). However, instead of using the same dose of epinephrine in all dogs, the dose of epinephrine was titrated for each animal to help to control for individual dog variation in response to cardiac sensitizers (Brock et al. 2003). Each dog received an epinephrine dose that ranged from 1 to 12 μg/kg while exposed to air to determine the minimal arrhythmic dose. If an arrhythmia was observed, the dose was decreased; if no arrhythmia was observed, the dose was increased up to a maximum dose of 12 μg/kg. Once the minimal arrhythmic dose of epinephrine was established, it would be used when the dog was exposed to the test chemical in accordance with the Reinhardt et al. protocol.
The advantage of using a titrated epinephrine dose is that it allows for individual animal sensitivities. Although Reinhardt et al. (1971) determined that a dose of 8 μg/kg injected over 9 sec tended to be optimal in the fixed-epinephrine-dose protocol, Hardy et al. (1994) found that titration of the dose for each dog with epinephrine at doses of 2-12 μg/kg was more sensitive for detecting cardiac sensitization. In addition, there is less chance of choosing an epinephrine dose for an animal that might itself induce arrhythmia or, conversely, might not induce any change in heartbeat when the chemical being evaluated is a sensitizer at that level. However, differences in the epinephrine dose make it difficult to compare results of the two protocols. The epinephrine titration protocol might be expected to yield a different no-observed adverse-effect level (NOAEL) or lowest-observed-adverse-effect level (LOAEL) for a test chemical than would be found with the fixed-epinephrine protocol. That has been the case with a few chemicals, such as HCFC-141b: in one study, the cardiac-sensitization LOAEL with the epinephrine-titration method was 9,000 ppm (epinephrine at 10 μg/kg), but in another study with the Reinhardt et al. fixed-epinephrine dose protocol (8 μg/kg), the LOAEL was 5,000 ppm. In a third study of HCFC-141b also with the titration method, the LOAEL was 20,000 ppm (epinephrine at 10 μg/kg) (Brock et al. 2003). The concentration of CFC-11 required to induce cardiac sensitization in dogs ranged from 5,000 ppm in a fixed-epinephrine-dose study to about 10,000 ppm in a later epinephrine-titration protocol, although the reason for the variability is unclear. The selection of a LOAEL or NOAEL for a risk assessment based on those experiments is equally uncertain.
In the first titration study, the lowest concentration tested was 9,000 ppm; it caused arrhythmia in one of four dogs. However, in the second titration study conducted in the same laboratory, the NOAEL was 10,000 ppm and the LOAEL was 20,000 ppm, the next highest concentration tested. Given that none of four dogs responded at 10,000 ppm in the first study (Brock et al. 1995) and only one of two at 9,000 ppm, these results are all consistent with a threshold near 10,000 ppm.
Using the Reinhardt protocol with a titrated epinephrine dose, the potential for iodotrifluoromethane (CF3I) to induce cardiac sensitization in the dog was evaluated (Kenny et al. 1995; Dodd and Vinegar 1998). The study involved male beagles exposed at 0.1% (6 dogs), 0.2% (5 dogs), 0.4% (5 dogs), and 1.0% (1 dog) (1,000, 2,000, 4,000, and 10,000 ppm) CF3I with epinephrine doses of 1-8 μg/kg (see Table 4-2). Exposures at 0.1% and 0.2% did not result in any response, regardless of the epinephrine dose. One dog that received 0.1% CF3I (epinephrine at 8 μg/kg) had no response; but when it was exposed to 1.0% CF3I, fatal ventricular fibrillation (FVF)
TABLE 4-2 Cardiac-Sensitization Responses to CF3I
|
Dog |
Epinephrine Dose, μg/kg |
CF3I Concentration |
|||
|
0.1% |
0.2% |
0.4% |
1.0% |
||
|
1 |
8 |
Nega |
— |
— |
FVF |
|
2 |
8 |
Neg |
Neg |
FVF |
— |
|
3 |
8 |
Neg |
Neg |
— |
— |
|
4 |
1 |
Neg |
Neg |
— |
— |
|
5 |
4 |
Neg |
Neg |
— |
— |
|
6 |
1 |
Neg |
Neg |
— |
— |
|
aNeg, no response;—, not tested; FVF, fatal ventricular fibrillation. Source: Kenny et al. 1995. |
|||||
occurred. A second dog showed no effect at 0.1% or 0.2% but had FVF at 0.4% (epinephrine at 8 μg/kg), so no additional exposures were conducted. No blood concentrations of CF3I were measured in any dog. On the basis of these responses in dogs, the NOAEL for the 5-min exposure (before epinephrine challenge) to CF3I was therefore considered to be 0.2% (2,000 ppm), and the threshold for response 0.4% (4,000 ppm).
Endogenous-Epinephrine Studies
As noted earlier, the doses of exogenous epinephrine used in the fixed-epinephrine protocol of Reinhardt et al. (1971) are about 10 times the physiological concentrations that might occur in humans under stress conditions (Mullin et al. 1972). To determine whether halogenated hydrocarbons would induce sensitization without the administration of exogenous epinephrine, “endogenous-epinephrine” studies were conducted in dogs by Reinhardt et al. (1971) and Mullin et al. (1972). Reinhardt et al. (1971) exposed beagles to a mixture of 80.0% fluorocarbon and 20.0% oxygen for 30 sec while frightening the animals with a loud noise. In contrast with the exogenous-epinephrine studies, no other substances were given to the animals; the frightened dogs were expected to release endogenous epinephrine. Results from Reinhardt et al. (1971) are shown in Table 4-3.
Table 4-3 highlights the importance of increased epinephrine, whether exogenous or endogenous, in cardiac sensitization. It also shows that the concentration of epinephrine did not substantially change the number of dogs that had cardiac arrhythmias. For example, when dogs were exposed to 80.0% HCFC-142b, it was apparent that noise was a contributing factor:
TABLE 4-3 Cardiac Sensitization with Endogenousa or Exogenous Epinephrine
|
Compound with Noise or Epinephrine |
No. Dogs Exposed |
No. Marked Responses |
Exposure Without Epinephrine, ppm |
LOAEL, ppmb |
|
CFC-11 + noise |
12 |
2 |
800,000 |
|
|
CFC-11 + epinephrine |
12 |
1 |
— |
5,000 |
|
CFC-114 + noise |
12 |
1 |
800,000 |
— |
|
CFC-114 + epinephrine |
12 |
1 |
— |
25,000 |
|
CFC-12 + epinephrine or noise |
12 |
0 |
— |
50,000 |
|
HCFC-142b + epinephrine |
6 |
0 |
— |
25,000 (NOAEL) |
|
HCFC-142b + epinephrine |
12 |
5 |
— |
50,000 |
|
HCFC 142b + noise |
12 |
5 |
800,000 |
— |
|
HCFC-142b (no noise) |
12 |
1 |
— |
800,000 |
|
Noise only |
6 |
0 |
— |
— |
|
aDogs were exposed to 80.0% compound (20.0% O2) for 30 sec while being frightened with loud noise. bLOAEL determined in screening studies with intravenous exogenous epinephrine. Source: Adapted from Reinhardt et al. 1971. |
||||
five of 12 dogs exhibited cardiac sensitization compared with one of 12 dogs that received the chemical only. No cardiac sensitization was observed with noise alone during air exposures. Thus, without the stimulation of the epinephrine, the threshold increased 32-fold; with the noise, the threshold was 16 times as high as with the chemical alone.
In an effort to determine whether cardiac sensitization would occur without administration of exogenous epinephrine, Mullin et al. (1972) exposed beagles to various concentrations of CFCs while they ran on a treadmill. Exercise has been found to increase circulating epinephrine in dogs by a factor of 5 after 15 min at 500 ft/min (Ohukuzi 1966).
Animals were exposed to increasing concentrations of CFC-11, CFC-12, or CFC-114. If the test compound at a specific concentration were a sensitizing agent, an arrhythmia such as multiple ventricular beats, would be seen on the electrocardiogram. Results from the study are shown in Table 4-4.
TABLE 4-4 Treadmill Studies of Cardiac Sensitization
|
Compound |
Exposure Concentration, ppm |
No. Dogs Exposed |
No. Marked Responses |
LOAEL,a ppm |
LOAEL,b ppm |
|
CFC-11 |
5,000 |
8 |
0 |
5,000 |
10,000 |
|
|
7,500 |
8 |
0 |
|
|
|
|
10,000 |
7 |
0 |
|
|
|
CFC-114 |
25,000 |
6 |
0 |
25,000 |
50,000 |
|
|
50,000 |
7 |
1 |
|
|
|
|
100,000 |
7 |
1 |
|
|
|
CFC-12 |
50,000 |
6 |
0 |
50,000 |
100,000 |
|
|
75,000 |
6 |
0 |
|
|
|
|
100,000 |
6 |
1 |
|
|
|
aLOAEL determined in screening studies with intravenous epinephrine. bLOAEL based on endogenous epinephrine studies. Source: Adapted from Mullin et al. 1972. |
|||||
The inhaled concentrations of CFCs required to induce cardiac sensitization in exercising dogs were 2-4 times as high as those needed for animals receiving intravenous exogenous epinephrine. The study demonstrated that the LOAEL of a compound may depend on the circulating epinephrine concentrations. Brock et al. (2003) stated the following:
The importance of experiments involving endogenous adrenaline production is three-fold. First, these experiments confirmed the validity of the standard 5-minute screening study using injected epinephrine as a valid ranking tool. Secondly, these data (especially the ‘fright’ studies) provide more evidence that the phenomenon of cardiac sensitization is most likely the mechanism of death in aerosol ‘sniffing’ episodes. Finally, these data indicate that the cardiac-sensitization protocol is a conservative measure of toxicity relative to the circulating blood levels of epinephrine following intravenous injection.
No studies of CF3I with endogenous epinephrine were found in the publicly available literature.
BLOOD AND TISSUE PHARMACOKINETICS
The goal of the studies described above was to identify the lowest concentration of halocarbons or other agents that would induce cardiac
sensitization on the basis of a dose-response relationship. That information was used to rank the halocarbons in terms of cardiac-sensitization potency. To help to establish the dose-response relationship, investigators (Azar et al. 1973; Trochimowicz et al. 1974) attempted to correlate the concentration of the test chemical in air that would induce cardiac sensitization with the arterial and venous blood concentrations of the chemical at the time of sensitization.
One approach to estimating the blood concentrations of these chemicals after inhalation exposure is physiologically based pharmacokinetic (PBPK) modeling. This technique permits estimates of body burden that are correlated with the magnitude and duration of exposure. The use of PBPK models to estimate arterial blood concentrations rather than using the airborne exposure concentration as the measure of dose is discussed in Chapter 5.
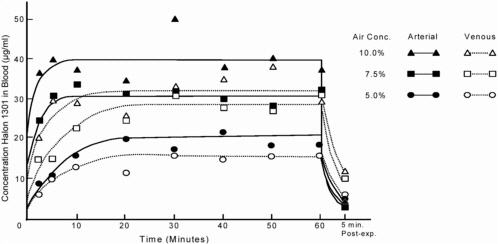
Mullin et al. (1979) showed that blood concentrations of Halon 1301 increase rapidly during the first 5 min of inhalation exposure and reach equilibrium after about 20 min (Figure 4-1). At 5 min of exposure at 5.0%, the arterial concentration was 10.7 μg/mL. By 20 min, arterial concentration had increased to 19.9 μg/mL, and it stayed there for the remainder of the hour-long exposure. At 7.5% and 10.0%, the arterial concentrations at 5 min were 30.9 and 40.0 μg/mL, and at 20 min were 30.9 and 35.4 μg/mL, respectively. Although the arterial concentrations values increased between 5 and 20 min with exposure to 5.0%, the venous concentrations were constant (10.3 and 11.3 μg/mL, respectively). Overall, the similarity of these values at each exposure concentration suggests that equilibrium between blood and air is reached rapidly. At the end of the 60-min exposure period, blood concentrations dropped rapidly in the first 5 min and then decreased more slowly (Azar et al. 1973; Mullin et al. 1979). Other CFCs, such as CFC-12 and CFC-113, have shown a similar pharmacokinetic pattern of uptake and elimination (Azar et al. 1973; Trochimowicz et al. 1974; Mullin et al. 1979).
Table 4-5 shows the cardiac-sensitization results and mean arterial and venous concentrations of various halocarbons at the 5-min sampling time according to the Reinhardt et al. protocol. Although the inspired concentrations of halocarbons required to produce cardiac sensitization ranged from 0.5% to 15.0%, the arterial and venous concentrations at 5 min were similar for the various types of halocarbons. That is, two-carbon halocarbons (CFC-113, CFC-114, and CFC-115) tended to result in lower blood concentrations at induction of cardiac sensitization than did one-carbon halocarbons (CFC-11 and CFC-12). The difference may be related to the water solubility of the halocarbon.
TABLE 4-5 Blood Concentrations of Halocarbons Associated with Cardiac Sensitization
|
Compound |
Exposure Concentration, ppm |
No. Dogs Sensitized/ Exposed |
5-Min Blood Concentration, μg/mL |
|
|
Arterial |
Venous |
|||
|
Halon 1301 |
50,000 |
0/62 |
10.7 |
10.3 |
|
|
75,000a |
1/18 |
30.9 |
14.8 |
|
|
100,000 |
7/69 |
40.0 |
29.8 |
|
CFC-11 |
1,000 |
0/12 |
10.9 |
6.6 |
|
|
5,000a |
1/12 |
28.6 |
19.7 |
|
|
10,000 |
5/12 |
53.2 |
37.2 |
|
CFC12 |
1,000 |
ND |
1.0 |
0.9 |
|
|
25,000 |
0/12 |
ND |
ND |
|
|
50,000a |
5/12 |
35.3 |
22.8 |
|
|
100,000 |
46.3 |
ND |
39.8 |
|
CFC-113 |
1,000 |
ND |
2.6 |
1.5 |
|
|
2,500 |
0/12 |
ND |
ND |
|
|
5,000a |
10/29 |
12.5 |
4.9 |
|
|
10,000 |
3/4 |
18.0 |
12.1 |
|
CFC-114 |
1,000 |
ND |
0.4 |
0.3 |
|
|
25,000a |
1/12 |
13.8 |
7.2 |
|
|
50,000 |
7/12 |
23.6 |
10.0 |
|
CFC-115 |
100,000 |
ND |
2.8 |
1.9 |
|
|
150,000a |
1/13 |
5.8 |
3.9 |
|
|
250,000 |
4/12 |
11.4 |
5.9 |
|
aLOAEL based on Reinhardt et al. 1971. Abbreviation: ND, not determined. Sources: Azar et al. 1973; Trochimowicz et al. 1974; Mullin et al. 1979. |
||||
In addition to solubility, the blood:air partition coefficient also influences the uptake and elimination of halocarbons in the body. For example, short-chain CFC-12 is only slightly soluble in blood. It is readily absorbed from the lungs into the bloodstream, where it rapidly equilibrates with blood as a function of the blood:air partition coefficient and essentially reaches steady state within minutes (Azar et al.1973). As shown in Figure 4-1 for Halon 1301, blood concentrations rapidly decrease once exposure ends. The observed increase in blood concentrations for these and other halocarbons clearly indicates a multiplicative relationship between concentration and length of exposure: steady-state blood concentrations occur within about 5 min of exposure.
Longer exposures do not substantially change the plateau blood concentrations of most halocarbons. That was demonstrated by Beck et al. (1973), who showed that dogs exposed to Halon 1211 at 8.0%, 5.0%, or 2.0% for 1, 2, or 5 min, respectively, had blood concentrations of 21-24 μg/mL when cardiac sensitization was induced. They found that cardiac sensitization was independent of whether the blood concentration was achieved rapidly by exposure at high concentrations or more slowly at lower concentrations. As noted earlier, Reinhardt et al. (1971) found that cardiac sensitization occurred with a 5-min exposure to 5.0%, but not 2.5% CFC-12. Furthermore, longer exposures—up to an hour—at 2.5% still produced no cardiac arrhythmias. Induction of cardiac sensitization appears to correlate with the peak blood concentration of the halocarbon before epinephrine challenge.
Determining peak blood concentrations requires knowledge of the arterial and venous concentrations of the test chemical. As shown in Figure 4-1, arterial blood concentrations of halocarbons are greater than venous concentrations during exposure, but this reverses when exposure ceases. That suggests that halocarbons are taken up by body tissues (Azar et al. 1973). In another series of experiments with CFC-11 and CFC-12 (Trochimowicz et al. 1974), dogs were exposed to various concentrations for 5 min and then immediately sacrificed, and halocarbon concentrations were measured in about 10 tissues. Although they are not detailed here, tissue concentrations of halocarbon were directly correlated with the blood and inhaled concentrations associated with cardiac sensitization. In addition, there was no evidence of retention of halocarbon in tissues after acute inhalation. Together, those studies suggest that steady-state blood concentrations of the halocarbons are reached within about 5 min of exposure, at least in the dog, and the peak blood concentrations depend on the exposure concentrations. It is the peak blood concentration that is related to the induction of cardiac sensitization. Prolonged exposure to an airborne concentration of halocarbon that does not achieve the critical blood concentration does not appear to increase the risk of cardiac sensitization.
VALIDITY OF THE CARDIAC-SENSITIZATION PROTOCOL
The mechanism for cardiac sensitization in humans is unknown, but results of cardiac-sensitization studies with epinephrine using the method of Reinhardt et al. (1971) may be used to establish human exposure limits for halocarbons. For over 30 years, when exposures to halocarbons have been maintained below the NOAEL defined by the studies, there have been
no reported deaths. When exposures exceeded the NOAEL, however, as may occur in such confined spaces as military tanks, airplane wings, large degreasers, or large leaking refrigeration units, incidents of cardiac problems, some fatal, have been reported (NIOSH 1989). Furthermore, cardiac sensitization can be demonstrated in animals exposed to halocarbons with noise, shock, or exercise as the only stimulus for epinephrine (endogenous epinephrine), albeit at halocarbon concentrations greater than those which produce cardiac arrhythmias in the presence of exogenous epinephrine (Reinhardt et al. 1971; Mullin et al. 1972).
Cardiac-sensitization potential may also be evaluated from studies that do not employ an epinephrine challenge. In these studies, induction of cardiac arrhythmia typically does not occur or it occurs at much higher exposure concentrations than with epinephrine challenge studies (Reinhardt et al. 1971). A study conducted at Huntingdon Life Sciences (2000) to determine the arterial and venous blood concentrations of CF3I in dogs during and after 10-min nose-only exposures confirmed this reduced cardiac-sensitization potential. Six male beagles were exposed to CF3I at 0.3% or 0.4%, five dogs were exposed to 0.5% or 2.5%, and one dog was exposed to 5.0%. Blood samples were taken during exposure and for 1 h after exposure for use in the development of a PBPK model (see Chapter 6). Except for the lack of exogenous epinephrine administration, the protocol used was that of Reinhardt et al. No cardiac sensitization was seen at any CF3I concentration even though the exposures at 2.5% were 6.25 times higher than the concentration that induced a cardiac arrhythmia when the dogs were given an injection of epinephrine. The exposure to 5.0%, the highest concentration tested, was stopped after 4 minutes due to excessive adverse clinical signs. Even at this concentration, coupled with the observations of severe stress, the only cardiac sign reported was marked tachycardia in the one dog tested. Adverse clinical signs seen during exposure at 0.3% CF3I included agitation (3/6 dogs), deep breathing (1/6 dogs), and vomiting 1 h after exposure (1/6 dogs). At 0.4% CF3I, only one dog exhibited deep breathing during exposure and another dog vomited 48 min after exposure ceased. At 0.5%, no adverse clinical signs were observed during or after exposure in the five dogs; at 2.5%, adverse clinical signs during exposure included rigid legs (3/5 dogs), arched back (2/5), excessive swallowing (2/5), shallow breathing (1/5), and moderate salivation (1/5).
For an experimental evaluation, studies with multiple or different catecholamines could provide insight into the mechanism of action of halocarbon-induced cardiac sensitization. The subcommittee notes that the same cardiac-sensitization protocol is used for developing hazard informa-
tion for traditional risk assessments and PBPK-modeled risk assessments. The difference is that the “modeled” risk assessment goes one step further than a traditional risk assessment and predicts the time that it will take to achieve the maximal “safe” blood concentration at different exposure concentrations (see Chapter 5).
In spite of the considerable dataset available from cardiac-sensitization tests with halocarbons, critical questions remain. Are cardiac-sensitization studies conducted in beagles satisfactory for predicting the potential of a halocarbon or halocarbon substitute to produce arrhythmia in humans? If so, do the tests reliably predict the concentration at which humans will develop an arrhythmia?
Answering those two questions touches a number of issues. It is well known that halocarbons may be antiarrhythmic at some concentrations and proarrhythmic at others (Muir et al. 1959; Purchase 1966). It has also been suggested that halocarbons increase the likelihood that stress will produce arrhythmias in humans and that the arrhythmias result from sensitization of the heart to epinephrine; that is, the arrhythmias are merely an exaggeration of the proarrhythmic effects of epinephrine. One important concern is that in humans under stress, norepinephrine, epinephrine, and dopamine—all potentially proarrhythmic catecholamines—increase in the order norepinephrine >>> epinephrine > dopamine. It is unlikely that the pathogenesis of a ventricular arrhythmia precipitated by the interactions of a halocarbon with epinephrine may mimic production of arrhythmias by halocarbons and stress in humans, in as much as epinephrine is only one of the catecholamines produced in humans by stress. The issue could be addressed by studies conducted in dogs exposed to increasing concentrations of halocarbons and given a mixture of all catecholamines at concentrations that mimic those observed in humans under stress.
The mechanism of sudden death in humans exposed to halocarbons is not known. Sudden death may be caused by ventricular fibrillation, asystole hypotension, respiratory arrest, acute heart failure (rarely), or a combination thereof. It is dangerous to presume that all sudden deaths result from ventricular fibrillation even if fibrillation can be produced in combination with halocarbons in dogs. If the mechanism of death is not fibrillation, studies conducted on dogs exposed to both epinephrine and halocarbons, although perhaps interesting, are not relevant to human deaths.
It is well known that ventricular arrhythmias may be produced by the actions of various compounds on the heart, on the brain (particularly the area postrema), or both. The knowledge of a mechanism of action may be extremely important in understanding hazardous concentrations. For example, if the mechanism involves increased automaticity of Purkinje or
“M” fibers, blockade of the delayed rectifier currents of ventricular depolarizations, increased temporal dispersion of repolarization, altered Ca++-calmodulin interaction, or altered balance in parasympathetic and sympathetic efferent activity, then different conclusions can be drawn about how to identify impending arrhythmia and how it might be prevented. This issue could be addressed with a comprehensive panel of in vitro studies (for example, patch clamp, Purkinje fiber, Langendorff) and in vivo studies (for example, close exposures of the brain and heart at relatively low concentrations and intravenous exposures).
Although cardiac sensitization is a well-documented adverse effect of exposure to halocarbons, research on the mechanism by which it occurs is not extensive and has focused primarily on prediction of cardiac-sensitization potential on the basis of development of an arrhythmia. Among the various cardiac end points studied, a change in heart rate appears to be the best indicator of a prearrhythmogenic event (E. Kimmel, Wright-Patterson Air Force Base, personal commun., 2001). A shift in cardiac conductance and destruction of the sinus node was also seen by Hashimoto and Hashimoto (1972), although the halocarbon-epinephrine-induced arrhythmia could be corrected by increasing the heart rate with electric stimulation. Intraventricular pressure increases will also produce ventricular arrhythmia (Reynolds 1983). Thus, halogenated hydrocarbons in combination with epinephrine can result in disrupted heart rhythm. Possible mechanisms of cardiac sensitization are discussed in Box 4-1.
The sensitivity and specificity of the beagle cardiac-sensitization test for predicting arrhythmogenicity or magnitude of exposure in humans are unknown, and this lack of knowledge can be attributed to the difficulty in ascertaining whether death due to halocarbon exposure necessarily results from true sensitization to epinephrine. The lack of information makes it important to understand the mechanism of sensitization. For example, if patch-clamp studies show that halocarbons or halocarbons with catecholamines alter conductance over the delayed rectifier currents, animal surrogates that might possess polymorphisms similar to those of humans should be used.
If an arrhythmia in the presence of a halocarbon and a concentration of epinephrine that by itself does not produce an arrhythmia is a positive signal in the dog and it is presumed that the halocarbon concentration at which the arrhythmia just occurs is the LOAEL, the test might appear to have great sensitivity if the result occurs with all halocarbons known to produce arrhythmia at that concentration. However, it will still be unknown whether that concentration will produce an arrhythmia in humans. Thus, some halocarbons and some concentrations may be indicted incorrectly as
|
BOX 4-1 The mechanisms whereby some halocarbons sensitize the myocardium to the action of epinephrine is not well understood. One mechanism, “cardiac sensitization,” has been proposed for the interaction of halocarbons with catecholamines to produce ventricular arrhythmias. Dudley (2003) proposed that abnormal calcium cycling from the sarcoplasmic reticulum is the prime factor in cardiac sensitization. That is based on the observation that the initial ventricular premature depolarization results from a delayed after-depolarization, and it is thought that delayed after-depolarizations—as occur with ouabain toxicity—depend on abnormal calcium cycling. Abnormal calcium cycling between cytosol and sarcoplasmic reticulum depends on the balance between calcium exit from the sarcoplasmic reticulum over ryanodine channels and calcium entry into the sarcoplasmic reticulum through channels activated by energy from sarcoplasmic-endoplasmic reticulum calcium-ATPase (SERCA2a) via phosphorylation of phospholamban. Sympathetic activity—manifested by increased concentration of sympathetic neurohormones (norepinephrine, epinephrine, and dopamine)—is known to activate protein kinase A, which phosphorylates and activates ryanodine channels, renders them “leaky” to calcium (predominantly in diastole), and increases their sensitivity to calcium release from the sarcoplasmic reticulum. Increased sympathetic activity—and putatively halocarbons—may activate protein kinase A, which hyperphosphorylates the ryanodine receptor and results in depletion of the important regulatory protein FKBP12.6. Thus, one explanation of cardiac sensitization may be synergism between halocarbons and sympathetic activity on calcium kinetics; and an avenue to prevent cardiac sensitization—for example, with beta adrenergic blockade—may be to minimize the synergism at the ryanodine receptors, possibly by preventing hyperphosphorylation or depletion of FKBP12.6. |
cardiac sensitizers, and it will still be unknown whether halocarbons or particular halocarbon concentrations that are arrhythmogenic in the dog are also arrhythmogenic in humans.
As discussed previously in this chapter, it is known that CFC-11 and CFC-113 produce cardiac arrhythmias in dogs at 5,000 ppm and in humans at estimated concentrations of over 20,000 ppm without the use of exogenous epinephrine. HCFC-22, HCFC-141b, and Halon 1301 have been shown to cause death in humans, which has been attributed to cardiac arrhythmia. Several other halocarbons have been shown to react with the dog model, but overexposures in humans have not been reported.
Nevertheless, it is almost impossible to determine the sensitivity and specificity of a cardiac-sensitization test without knowing how many halocarbons (and at which concentrations) produce arrhythmias in both humans and the test animal (sensitivity) and how many halocarbons and concentrations that do not produce arrhythmias in humans also do not produce them in the test animal (specificity). It is impossible to answer the question whether the cardiac-sensitization test identifies halocarbons and their concentrations that might produce arrhythmias in humans unless the above issues are addressed. Box 4-2 identifies some considerations that should be included in refining cardiac-sensitization testing.
No uncertainty factors are necessary for extrapolation from dogs to humans, because the doses of exogenous epinephrine achieve plasma concentrations that are 10 times greater than those achieved during physiologic stress, such as exercise or loud noise. Therefore, the dog cardiac-sensitization test procedures are likely to be conservative enough to account for any uncertainty in dog-to-human extrapolation.
It is reasonable to conclude that the NOAEL for CF3I in humans for a 5-min exposure is 0.2% if epinephrine mimics the conditions of stress in humans, sudden death from halocarbons is caused by ventricular fibrillation, and all halocarbons identified as arrhythmogenic in the epinephrine-challenged dog are potentially arrhythmogenic in humans and halocarbons that do not produce arrhythmias in dogs also do not produce them in humans.
CONCLUSIONS
Numerous reports on laboratory animals and humans indicate that cardiac sensitization can occur as a result of exposure to halocarbons and that most halocarbons with fluorine substitution are capable of sensitizing the heart to epinephrine. That endogenous concentrations of epinephrine, such as those achieved through exercise or by frightening an animal, can result in fatal cardiac arrhythmia is of particular concern for human exposures.
The studies reviewed in this chapter generally were conducted to rank halocarbons with regard to their cardiac-sensitization potential. The subcommittee cautions that the studies were not conducted with the goal of quantitative risk assessment of the compounds, and it notes that the results of the studies are nonetheless often used for PBPK modeling. The doses used in some of the procedures increase severalfold, and this makes it difficult to determine a precise NOAEL or LOAEL from a particular study.
|
BOX 4-2 Cardiac sensitization refers to the increased likelihood of ventricular arrhythmias—sometimes leading to death—caused by exposure to halocarbons. Cardiac sensitization has been explored by challenging animals, which have been exposed to graded doses of halocarbons, with incremental concentrations of epinephrine and comparing the arrhythmic dose of epinephrine required to produce ventricular ectopia during halocarbon exposure with that required before exposure. There are no strong data to support the extrapolation of this method performed on laboratory animals to humans, and the stress profile of arrhythmogenic catecholamines includes a balance among epinephrine, norepinephrine, and dopamine. There is no way of dealing with extrapolation without testing for the sensitivity and specificity of the method, but it is feasible to simulate a more physiologic catecholamine stress profile that might mimic more closely that observed in humans.1 The following is one possible scheme for exploring cardiac sensitization with dogs as surrogates for humans. Select dogs of varied sizes, ages, and sexes2 that might correspond to the humans at risk.3 Train dogs to stand quietly with a catheter in a peripheral vein for infusion of catecholamines and a head bubble for exposure to various concentrations of halocarbons. Challenge the dogs with continuous intravenous infusions4 of increasing doses of epinephrine, norepinephrine, and permutations of epinephrine and norepinephrine5 to determine the arrhythmogenic threshold of each. There may be a number of thresholds (such as, first premature ventricular depolarization, first monomorphic paroxysmal ventricular tachycardia, sustained monomorphic ventricular tachycardia, sustained pleomorphic ventricular tachycardia, and ventricular fibrillation).6 Determine the arrhythmic dose of catecholamines for each concentration of each halocarbon, to rank halocarbons according to potential to sensitize to ventricular arrhythmia. Another variation might be to expose dogs to any of the various pharmacologic agents that might be used recreationally (such as, cannibis, amphetamine, or cocaine) or medically (such as, beta blockers, ACE inhibitors, calcium-channel blockers,7 or drugs for erectile dysfunction) to mimic more closely the spectrum of humans at risk of halocarbon exposure. Suggesting these studies is not intended to imply that studies already conducted exposing beagles to increasing concentrations of epinephrine by bolus injections might not possess high sensitivity and specificity for predicting cardiac sensitization in humans. Rather, it is a supplement to consider factors (such as, polymorphisms, catecholamine profiles of stress, concomitant diseases, and concomitant pharmacologic interventions) that might confound results already published. Any or all of these proposed studies must be done carefully in a laboratory with good-laboratory-practice methods and experience in inhalation toxicology. |
Furthermore, various protocols have been used to assess cardiac sensitization with differing times between exposure onset and epinephrine challenge. As noted by Brock et al. (2003), “the key factor for inducing sensitization has been the arterial blood concentration of the agent at the time of epinephrine challenge. Therefore, understanding the pharmacokinetics of the agent helps interpret results of these studies within the context of risk assessment. It also allows better interpretation of potential exposure scenarios and the likelihood of cardiac-sensitizing concentrations being reached by individuals under various exposure conditions.”
Is the dog model, with injections of epinephrine, an appropriate model of human cardiac-sensitization potential? The model was developed by Reinhardt et al. (1971) after Bass (1970) and others reported on deaths resulting from deep breathing of aerosol propellants. Other catecholamines may be responsible, either in part or completely, for the development of cardiac arrhythmias in humans after overexposure to halocarbons, but in the dog model, administration of exogenous epinephrine gives rise to a cardiac response (ventricular premature depolarizations) that would be seen only at much higher concentrations of a hydrocarbon without the injection (Reinhardt et al. 1971; Brock et al. 2003). The most difficult assumption that one must make is that the dose, determined as a measured blood
concentration that does not produce an arrhythmia in the dog, will also not cause one in a human. As has been shown in studies with HCFC-142b and CFC-113 (NRC 1996), administration of epinephrine results in a highly sensitive model; this supports the assumption.
ARMY CONCERNS
Although they were not included in the statement of work for the subcommittee, the Army posed several questions with regard to the cardiac-sensitization potential of CF3I, and the subcommittee strove to address them. What follows is the first question and the subcommittee’s response. The other questions are discussed at the end of Chapter 5.
Is the information from cardiac-sensitization tests in dogs appropriate for developing safe exposure levels in humans? If these studies are valid to serve as a basis for human exposure levels, should the data be extrapolated to humans directly without using uncertainty factors? The subcommittee found that although it is difficult to ascertain absolutely that cardiac-sensitization studies in the dog are appropriate for developing safe exposures for humans, a substantial body of evidence nevertheless indicates that many halocarbons that produce cardiac arrhythmias in the dog also cause them in humans. No uncertainty factors are necessary for extrapolation from dogs to humans, because the doses of exogenous epinephrine achieve plasma concentrations that are 10 times greater than those achieved during physiologic stress. Therefore, the dog cardiac-sensitization test procedures are conservative enough to account for any uncertainty in dog-to-human extrapolations.