2
Why Study Gas Hydrate?
Gas hydrate is an ice-like substance that forms at low temperature and high pressure when adequate amounts of water and gases such as carbon dioxide or methane and higher-order hydrocarbon gases are present (Figure 2.1). Because 1 m3 of solid hydrate typically contains 160 m3 of gas at standard temperature and pressure (STP),2 large volumes of natural gas may be stored efficiently in this form (Sloan, 2003). Although the total amount of methane trapped in gas hydrate and the geological processes that lead to concentrated gas hydrate deposits are poorly understood, existing knowledge suggests that gas hydrate represents a potential fossil fuel resource for the future (e.g., Kvenvolden, 1993a; Kvenvolden and Lorenson, 2001; Milkov and Sassen, 2002). It is also likely that the presence and decomposition of gas hydrate has had an impact on global climate in the past (Kvenvolden, 1993a; Dickens et al., 1997; Kennett et al., 2003). The presence of offshore structures may induce gas hydrate decomposition causing catastrophic collapses (Hovland and Gudmestad, 2001). However, such effects on climate change and seafloor stability are the subjects of active research and involve considerable uncertainty in their quantification; further research would help to document these effects.
Quantitative evaluation of the resource potential of gas hydrate, and of its response to local and global environmental change, requires knowledge of how it forms in nature and of its in situ physical properties. This knowledge is difficult to obtain because hydrate is not generally stable at
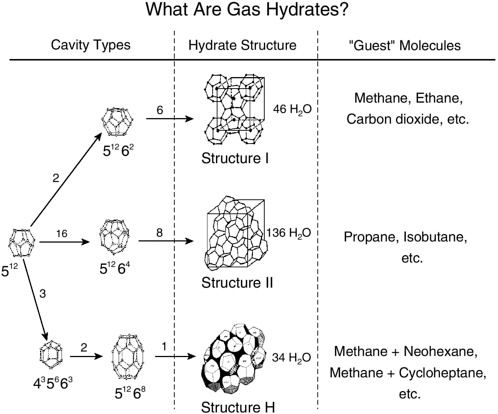
FIGURE 2.1 The hydrate crystal structures seen in the middle column are shown as their smallest repeating, or unit, structures. Given at the right of each crystal is the reported number of water molecules necessary to form the unit crystal. The “guest” molecules referred to in the right-hand column indicate the pure guests which will form in each structure under normal conditions, with methane the primary molecule of interest in this report. In the left-hand column, the cavity types are pictured with the number of pentagonal and hexagonal faces indicated as 5n6n where n refers to the number of faces. For example, 51264 indicates that the cavity has 12 pentagonal faces and 4 hexagonal faces. In the left-hand column, the n For example, the figure shows that pure propane will form in the structure II unit crystal, which has a host composed of 136 water molecules, with 16 of the 512 cavities and 8 of the 51264 cavities.
SOURCE: Reprinted with permission of Nature (Sloan, 2003) Macmillian Publishers, Ltd.
Earth’s surface. For example, methane hydrate is not stable at atmospheric pressure unless the temperature is below −60°C (Sloan, 2003). Natural gas hydrate recovered from the seafloor or from the subsurface decomposes rapidly when it is sampled, leading to the dramatic phenomenon of burning ice (Figure 2.2). Sophisticated laboratory and
field techniques that integrate results from a broad spectrum of scientific disciplines are needed to
-
quantify the resource potential of gas hydrate;
-
develop safe and effective methods for recovering and transporting this resource;
-
mitigate the effects of its development on the environment and as a geohazard; and
-
understand the role of gas hydrate in the global carbon cycle and its potential for impact on global climate change.
GAS HYDRATE AS A FOSSIL FUEL RESOURCE
There are several published estimates of the total amount of methane stored in gas hydrate worldwide (Figure 2.3). These estimates range over several orders of magnitude and are generally based on an estimate of the volume of continental margins and Arctic permafrost basins that fall within the gas hydrate stability zone (GHSZ) and their assumed gas hydrate content. A widely cited estimate suggests that gas hydrate may account for 1019 g of carbon (1.87× 1016m3), an amount approximately twice that of all other hydrocarbon resources combined and 100 times that of conventional gas resources (Kvenvolden and Lorenson, 2001). Milkov (2004) has shown that global estimates of the methane stored in gas hydrate have decreased over time, and suggests that the estimate of Kvenvolden (1988) may be too large by a factor of 10 (Figure 2.3). Milkov’s estimate, however, does not include focused deposits, which are of most interest to resource evaluation. The global distribution of such hydrate “sweet spots” is not well known because the high-resolution seafloor and subseafloor imaging studies needed to identify such deposits are rare. The possible magnitude of this resource, coupled with an ever-increasing demand for energy, has driven national research and development programs in the United States, Canada, Japan, Korea, and India to assess the potential resource value of methane hydrate and develop recovery techniques.
Consumption of natural gas in the United States has been increasing rapidly for the past several decades (Figure 2.4). Moreover, because natural
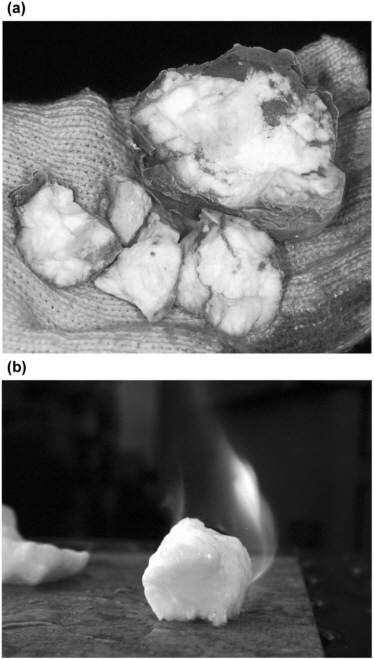
FIGURE 2.2 (a) Gas hydrate recovered from beneath the seafloor offshore Oregon. (b) Burning methane released from gas hydrate as it dissociates.
SOURCES: (a) Figure courtesy of the Ocean Drilling Project at Texas A&M University, and photographer John Beck; (b) figure reprinted with permission from Dr. Stephen Masutani, University of Hawaii, Hawaii Natural Energy Institute, Ocean Resources Applications Laboratory. Copyright © 2003 Ocean Resources Applications Laboratory. Photographer Liujuan Tang.
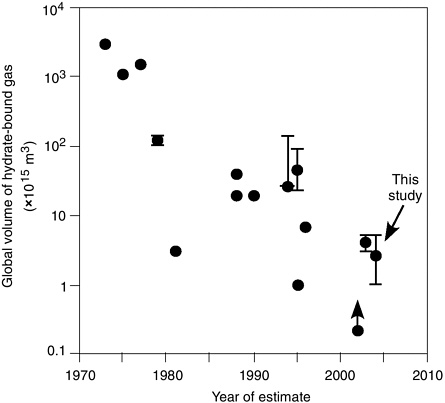
FIGURE 2.3 Global estimates of the volume of hydrate-bound gas in marine sediments versus the year in which the estimate was made.
SOURCE: Data from Milkov (2004). Reprinted from Milkov (2004), Figure 1A, and with permission of Elsevier Science B.V., Amsterdam.
gas is a cleaner-burning fuel, it is expected to be preferred for the next several decades even if alternative energy sources are developed to replace fossil fuel. A shortfall in natural gas supply from conventional and unconventional sources is expected to occur in about 2020 (Energy Information Administration, 2002). Methane hydrate from below the permafrost in the Arctic or beneath the seafloor in the U.S. exclusive economic zone (EEZ) may have the potential to alleviate this projected shortfall.
It is important to note that while global and national inventories of the total amount of methane stored in gas hydrate are useful for bringing attention to the importance of gas hydrate studies, these
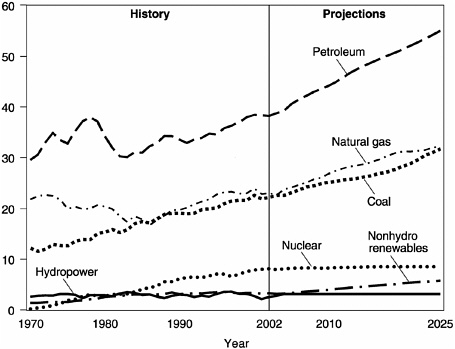
FIGURE 2.4 Consumption of natural gas in the United States has been increasing rapidly for the past several decades.
SOURCE: Figure courtesy of Ted McCallister, Energy Information Administration, U.S. Department of Energy; modified from Energy Information Administration (2002).
estimates are of limited value in evaluating the production potential of a gas hydrate deposit. To do this, a better understanding of the geologic factors that lead to highly concentrated hydrate deposits and their and their geophysical dynamics is needed. Gas hydrate equilibrates with gas, and a better knowledge of the gas-gas hydrate system is required to understand resources on the seafloor as well as the potential effects of hydrate on global climate and on seafloor stability.
GAS HYDRATE AND GLOBAL CLIMATE CHANGE
Methane is a powerful greenhouse gas with a Greenhouse Warming Potential (GWP) 23 times that of CO2 on a per-molecule basis. Because methane is much less abundant than CO2 in the atmosphere today, 1.7 parts per million (ppm) compared with 370 ppm, methane’s total anthropogenic warming impact is only about one-half that of CO2. Methane is also the primary gaseous constituent of naturally occurring
gas hydrate deposits. Sudden release of methane from gas hydrate therefore has the potential to affect global climate.
Dickens (2003a) has proposed that gas hydrate may act as a sort of capacitor in the global carbon and climate cycle, storing large amounts of methane until nature triggers a change in the system that results in destabilization, releasing it to the ocean and atmosphere. Several investtigators have postulated both negative and positive feedback effects from gas hydrate destabilization in response to global warming and/or sea level change (Paull et al., 1991; Kennett et al., 2003). One such scenario is that rising sea level in response to global warming and melting of the ice caps will flood the Arctic coastal plain, melting the permafrost and releasing methane from Arctic gas hydrate (Kvenvolden, 1993a). An alternative scenario, with a negative feedback, is that climate cooling will lower sea level, decreasing the pressure on the seafloor and destabilizing gas hydrate, thus releasing methane to the atmosphere and counteracting the cooling (Paull et al., 1991). Recently, isotopic evidence for the release of methane from gas hydrate during a dramatic warming period accompanied by mass extinctions in the Paleocene has been documented (Dickens et al., 1995). Several other such warming episodes have also been suggested and attributed to destabilization of gas hydrate (e.g., Nisbet, 1990; Haq, 1998).
The most recent hypothesized effect is the Quaternary “clathrate gun” hypothesis (Kennett et al., 2003), which speculates that sudden releases of methane from submarine gas hydrate are responsible for Quaternary fluctuations of Earth’s climate on orbital and millennial time scales. This hypothesis is controversial (Dickens, 2003b). Atmospheric methane concentrations (reconstructed from polar ice cores) varied rapidly between 450 and 700 parts per billion (ppb) during these millennial events. Alternative or complementary explanations for the rapid changes in methane concentration call on expansion and contraction of wetlands in response to the climatic change (e.g., Brook et al., 1999; Maslin and Burns, 2000). Alternative explanations for the millennial fluctuations in Earth’s climate cite changes in thermohaline circulation (e.g., Clark et al., 2002). In summary, there are some interesting but highly speculative hypotheses that attribute past climate variations to methane hydrate release. These hypotheses have yet to be confirmed, and even the most emphatic proposals suggest that much more research is needed.
Methane oxidation in the ocean is an important controlling factor in the atmospheric release of methane. Microbes are effective at oxidizing methane in sediment and the water column under both oxic and anoxic
conditions. Anaerobic oxidation is nearly total in diffusion-controlled anoxic sediments (Reeburgh, 1980; Alperin et al., 1988) and is a major sink term (almost 100 times larger than the next sink term, evasion at the air-sea interface) in the Black Sea water column budget (Reeburgh et al., 1991). Methane concentrations in oxic water columns are much lower than in anoxic sediments, and there have been direct oxidation rate measurements. For example, Valentine et al. (2001) made tracer measurements of methane oxidation rates in the Eel River basin, an area of active hydrate disassociation and seafloor venting. Their results are consistent with studies involving independently-dated ocean water masses by Scranton and Brewer (1978) and Rehder et al. (1999) that show a time scale for methane oxidation of about 50 years. The ocean would be a much larger source of atmospheric methane if not for this oxidizing effect (Reeburgh et al., 1993).
To quantify the role of gas hydrate in global warming, research is needed in the following areas:
-
quantification of the distribution of gas hydrate in marine sediments and polar land areas;
-
determination of the geologic time scales over which gas hydrate forms;
-
determination of methane flux, including oxidation effects, into the ocean from vents associated with focused methane hydrate deposits; and
-
knowledge of the historical latitudinal distributions and geometric configurations of land masses and ocean basins.
GAS HYDRATE AND SEAFLOOR STABILITY
The impact of gas hydrate on seafloor stability is important for evaluating the safety of offshore structures as well as for understanding its role in climate change. Depressurized gas hydrate is metastable (Figure 2.5). If temperature increases at a fixed pressure or if pressure decreases at a fixed temperature, hydrate may pass out of the stability zone and dissociate causing a geohazard. A geohazard is defined as a constraint imposed by a particular geological feature or process that may have an adverse effect on the natural environment or any man-made operation. For example, slope instability associated with the dissociation of a hydrate is a geohazard. Since hydrate encases methane at high concentrations, when
it is destabilized, the sediment may be converted to a gassy, water-rich fluid, thereby triggering seafloor subsidence and massive landslides.
There is circumstantial evidence that gas hydrate may contribute to slope instability along continental margins, especially during periods of lower sea level (e.g., Nisbet and Piper, 1998). Since landslides can lead to tsunamis that threaten coastal communities, a better understanding of this correlation is needed to ensure the safety of offshore structures and cables. This in turn requires a better understanding of the distribution of gas hydrate in marine sediments and the development of a means to estimate its distribution through the appropriate combination of remote sensing and ground-truthed data. Efforts to understand this phenomenon and to develop a predictive model through detailed analysis of in situ conditions are ongoing—for example, in the region of the Storrega slide, off the coast of Norway (Bouriak et al., 2000; Bryn et al., 2003).
Soil instability induced by offshore drilling and production operations represents a potential geohazard adjacent to offshore structures, where hydrate occurrence may result in foundation problems. Commercial oil and gas drilling operations and research drilling associated with the Integrated Ocean Drilling Program (IODP) are moving into regions where gas hydrate may present a safety issue. Numerous drilling and production problems are attributed to the presence of hydrate: for example, uncontrolled gas release during drilling operations, well-casing collapse, and gas leakage to the surface. Gas hydrate may dissociate (subsurface) due to heating by warm drilling fluids or from the production of hot hydrocarbons from depth during conventional production. Moreover, efforts to extract oil or gas from beneath hydrate-bearing sediment may decrease the pressure at the base of the gas hydrate stability field. Any of these processes can result in hydrate dissociation and a dramatic change in the geotechnical properties of the sediment, leading to borehole instability, release of gas, and potential structural and safety concerns. Although there is anecdotal evidence of structural collapse due to gas hydrate dissociation, industry generally avoids areas where hydrate deposits are suspected to be, thereby limiting accessibility to hydrocarbon resources.
DISTRIBUTION AND DYNAMICS OF GAS HYDRATE IN NATURE
Regardless of whether the objective is to understand the impact of hydrate on long-term climate change or to predict the stability of an offshore structure, it is clear that a better understanding of the factors that
control gas hydrate distribution within sediments, validation of remote sensing estimates of hydrate distribution, and predictive models of the response of gas hydrate to environmental perturbation are needed. In this section the current state of knowledge of these issues is summarized briefly.
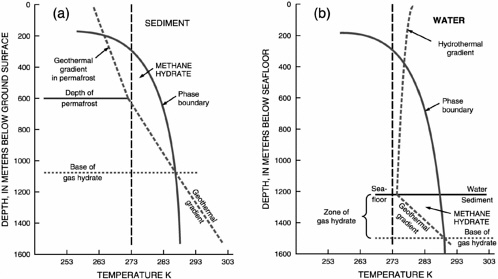
Figure 2.5 shows schematically the physical setting that determines gas hydrate stability in Arctic and marine environments. Whether gas hydrate can form is determined by pressure and temperature in the subsurface, as well as by the availability of enough gas and water to form hydrate. Beneath Earth’s surface, pressure is approximately proportional to depth, although the detailed relationship between depth and pressure requires knowledge of the density and porosity of the sediments and knowledge of whether the pressure is hydrostatic or lithostatic3 (or in between). The temperature is dependent on the local geothermal gradient, which is determined by the regional geologic setting and by local subsurface hydrology.
In the Arctic, temperatures and pressures appropriate for gas hydrate formation are encountered several hundred meters beneath the surface within the permafrost zone. The GHSZ, however, extends for several hundred meters beneath this region, until the effect of increasing temperature overtakes the effect of increasing pressure. In the oceans, the top of the GHSZ is encountered within the water column at a depth that depends on ocean temperature and ranges from about 300 to 500 m. The seafloor at greater than 500 m water depth is everywhere within the GHSZ. In the subsurface, the thickness of the stability zone depends on water depth and on the geothermal gradient. With a constant geothermal gradient, the thickness increases as water depth increases. As the geothermal gradient increases, the thickness of the stability zone decreases. Subsurface hydrology can locally perturb the geothermal gradient by transporting warm fluids from greater depth into the gas hydrate stability field.
It is important to realize that gas hydrate is not present at every location with favorable conditions as specified in Figure 2.5. Hydrate will be present only if the concentration of hydrate-forming gas is high enough that porewaters are supersaturated, and the degree of super-saturation depends on the salinity of the porewater and on the type of gas present to form gas hydrate. The phase boundary shown in both panels of
Figure 2.5 applies to pure methane hydrate. Porewater in sediments can be either fresher than seawater or several times more saline, depending on local geologic conditions or the rate of in situ gas hydrate formation, thus changing the local GHSZ by hundreds of meters. The presence of higher-order hydrocarbon gases (ethane, propane, etc.) will also change the phase boundary significantly. Finally, kinetic factors affect the rate of hydrate formation, where gas hydrate will form in the sediments, and the effect that gas hydrate has on the physical properties of the sediment. These properties depend on sediment grain size (e.g., Clennell et al., 1999) and probably other parameters such as effective stress (e.g., Torres et al., 2004). Understanding this complexity in nature requires inter-disciplinary studies that include laboratory experiments to monitor gas hydrate formation under controlled conditions, numerical modeling experiments to extend laboratory results, and field observations. Field observations are essential to ground-truth laboratory measurements and improve modeling experiments.
Origin of the Gas in Gas Hydrate
Two sources have been hypothesized to explain the origin of the free and seeping gas associated with hydrate. The first hypothesized source is thermogenic gas from deep beneath the GHSZ.4 The second is biogenic methane generated in situ by microbial methanogenesis (e.g., Claypool and Kvenvolden, 1983; Cragg et al., 1996; Boetius et al., 2000). These sources can be distinguished on the basis of isotopic ratios, and the relative contribution of each source varies from site to site. Numerical models that incorporate one or both types of sources have been proposed and used to predict the amount of gas hydrate present and the time scales over which gas hydrate deposits develop (e.g., Rempel and Buffett, 1998; Egeberg and Dickens, 1999; Xu and Ruppel, 1999; Davie and Buffett, 2001; Chen and Cathles, 2003; Torres et al., 2004). Measured porewater profiles of methane and sulfate (e.g., Ruppel, 2000; Valentine et al., 2001) have been used to estimate the relative rates of hydrate
replenishment via upward methane diffusion from the sediments below and hydrate decomposition due to interactions with warmer water above.
New data will improve estimates of the balance between these two sources at different sites. When combined with modeling to constrain rates of gas hydrate formation and maintenance, these data can be used to assess the amount and concentration of methane ultimately recoverable from any particular ocean-floor hydrate deposit. Measurements of methane flux (the amount of methane per unit time flowing through and equilibrating with a particular gas hydrate deposit) will be necessary to help constrain these models and address the following issues:
-
How much, how fast, and where is methane venting from bottom seeps into the water column?
-
How much methane is venting through the water column and in sediment porewaters?
-
How much methane is coming from an underlying gas hydrate deposit versus an underlying gas reservoir versus in situ sediment (biogenic) sources?
-
How fast is the methane moving, and what is its rate of venting?
-
How quickly is the methane biodegraded both in sediments and in the water column?
-
Where does gas venting occur, and is it widespread or localized?
Observations of Gas Hydrate Mounds at the Seafloor
Mounds of gas hydrate have been observed directly at the seafloor during submersible dives and by using deep-towed cameras and remotely operated vehicles (ROVs). In some cases, most notably in the Gulf of Mexico, these mounds are not associated with well-defined regional bottom simulating reflectors (BSRs),5 indicating that the source of the gas is narrowly focused not diffuse (Milkov and Sassen, 2001). The rough topography of many of these seafloor gas hydrate deposits (Roberts and Carney, 1997) also suggests a structurally controlled, deep-
seated source. Additional occurrences of seafloor gas hydrate mounds have been inferred from high-resolution images of seafloor reflectivity (Johnson et al., 2003) and from methane anomalies in the water column (e.g., Merewether et al., 1985; Paull et al., 1995; Salyuk et al., 2002; Heeschen et al., 2003). In addition, in some areas in the Gulf of Mexico, there is geophysical evidence that the upward movement of gas can cause disruption of the sediment layers (Hovland, 2000). However, remote sensing of these focused deposits is an imprecise science. Only a few inferred sites have been ground-truthed, and many seafloor mounds have been found by chance, including the recent discovery by fishermen of massive hydrate mounds offshore Vancouver Island (Spence et al., 2001)(Plate 3a,b).
Some research suggests that these seafloor gas hydrate deposits may represent only a small percentage of the total amount of gas hydrate present in marine sediments (Milkov, 2004); other research suggests that episodic focused flow probably represents a much larger flux than more dispersed deposits (e.g., Clennell et al., 2000; Judd, 2003). Seafloor deposits are the most accessible and best-studied deposits and are accompanied in most cases by complicated and poorly understood faunal assemblages. In all cases that have been studied in detail, these deposits contain a mixture of biogenic and thermogenic gases and result from geological structures that focus gas from a deep-seated source to the seafloor (e.g., Roberts, 2001; Sassen et al., 2001; Tréhu et al., 2003, 2004; Milkov et al., 2004a). In some continental margin gas hydrate provinces, such as the Gulf of Mexico, this type of deposit represents the primary gas hydrate occurrence studied to date. However, dispersed gas hydrate not associated with hydrocarbon deposits have also been encountered in the Gulf of Mexico’s Orca basin (Pflaum et al., 1986). In other areas, such as along the tectonically active continental margin offshore of the U.S. Pacific Northwest, several seafloor deposits have been found along with widespread subsurface deposits inferred from remote sensing data. New hydrate-gas seep deposits of both types continue to be found at a very rapid rate in all kinds of environments in many continental margins worldwide.
The vigorous expulsion of gas bubbles in the water column above seafloor hydrate mounds, even when the mound is well within the nominal gas hydrate stability field, testifies to the dynamic nature of these seafloor deposits and the large amounts of gas often associated with them. The time scales over which these mounds form, evolve, and are destroyed (either by dissociation or by mechanical removal from the seafloor due to a spontaneous buoyancy instability and/or earthquakes) are poorly known. A
better understanding of these dynamic parameters leading to hydrate formation and destruction is necessary to evaluate whether these seafloor hydrate mounds represent a usable resource, whether they are an important factor in transferring methane from the ocean to the atmosphere, and where there may be enough free gas present to destabilize the slope.
Uncertainties of Estimating Gas Hydrate Volume in the Subsurface
Although gas hydrate could exist anywhere in the ocean basins at depths greater than 300-500 m, it is generally believed that there is not enough gas present to form gas hydrate throughout most of this region. Indications that gas hydrate is abundant along the margins of continents and in the Arctic have come primarily from seismic studies ground-truthed by very limited data from drilling deep boreholes. Some of the first indications of gas hydrate in marine sediments were based on recognition of a seismic reflection that cut across geologic reflections in continental margin sediments. The reflection occurred at depth beneath the seafloor and was consistent with the predicted depth of the base of the GHSZ (Tucholke et al., 1977; Shipley et al., 1979). The negative polarity of this reflection (known as BSR because it follows the seafloor rather than geologic features where the seafloor is approximately flat) indicates that it results from a decrease in velocity with depth. It is thought that this decrease in velocity with depth occurs because gas hydrate-bearing sediments with relatively high seismic velocity overlie free gas containing sediments with lower velocity.
The presence of a BSR is an imperfect proxy for the presence or quantity of gas hydrate because sediments containing gas hydrate have been recovered from sites beneath which there is no BSR (Mathews and von Huene, 1985), and because it does not provide information on focused gas hydrate deposits near the seafloor. Moreover, models of gas hydrate concentration based on BSR amplitude (e.g., Hyndman and Spence, 1992; Bangs et al., 1993; Lee et al., 1993; Singh et al., 1993; Katzman et al., 1994; Pecher et al., 1996; Korenaga et al., 1997; Tinivella and Accaino, 2000; Chand and Minshull, 2003) depend on a number of parameters, several of which are poorly constrained. For example, the effect of gas hydrate on seismic velocity depends strongly on whether the gas hydrate forms part of the sediment matrix or whether it forms as detached grains in the porespace (e.g., Helgerud et al., 1999).
The baseline seismic velocity in the absence of gas hydrate, needed to calibrate models, is also generally not known. Opportunities to test seismic models are limited because only a few sites have been drilled to date (e.g., MacKay et al., 1994; Holbrook et al., 1996; Guerin et al., 1999). Moreover, even when geophysical estimates of gas hydrate content have been compared to drilling results, in situ estimates have uncertainties due to limitations of the tools available to estimate the amount of gas hydrate in boreholes and recovered cores.
The imperfect knowledge of the distribution of gas hydrate will be much improved with the widespread and routine use of pressure coring techniques that enable direct measurement of the concentration of hydrate in sediments. For example, the Maurer/Anadarko project (discussed in Chapter 3), funded by the Department of Energy (DOE) Methane Hydrate R&D (Research and Development) Program, focused on recovering and testing hydrate deposits and serves as an example of how such drilling and sampling efforts could be set up and implemented. In addition, Leg 204 of the Ocean Drilling Program was also successful in recovering hydrate cores and serves as an example of how to core, retrieve, and handle cores containing hydrate (Tréhu et al., 2003, 2004).
Quantifying the Amount of Gas Hydrate in Boreholes
The only means for ground-truthing in situ estimates of gas hydrate is through analysis of geological, geophysical, and geochemical data from deep boreholes. Comprehensive studies of boreholes in gas hydrate-bearing regions include Ocean Drilling Program (ODP) Leg 164 to the Blake Ridge gas hydrate province of the Atlantic continental margin, ODP Legs 146 and 204 to the gas hydrate province of the Cascadia accretionary complex off the U.S. Pacific Northwest, the Mallik I and II projects in the Canadian Arctic, and drilling programs in the Nankai accretionary complex offshore Japan. All of these projects have been large multidisciplinary international efforts. DOE’s participation in Mallik II (2002) and ODP Leg 204 is discussed in Chapter 3.
To estimate the amount of in situ gas hydrate accurately, several types of data must be integrated, because different tools for quantifying the gas hydrate content of sediments penetrated and sampled by a borehole have different sensitivity and spatial resolution (e.g., Tréhu et al., 2004). Traditional tools for determining the gas hydrate content of recovered cores include measuring the total amount of gas released and recovered under pressure (e.g., Dickens et al., 2000; Milkov et al., 2003)
and the dilution of porewater chloride concentration due to release of fresh water when gas hydrate in the core dissociates after core recovery (e.g., Hesse and Harrison, 1981; Ussler and Paull, 2001). For logistical reasons, these techniques sample only a small percent of the total core and provide measurements that are spaced meters to tens of meters apart. Recently, these robust but spatially incomplete measurements have been supplemented by infrared core scans, which can image all cores. This technique is based on the endothermic property of gas hydrate dissociation, which results in cold spots where gas hydrate is currently or has recently dissociated (Ford et al., 2003; Tréhu et al., 2003, 2004).
Because core recovery is often incomplete, geophysical logging methods are needed to sample the entire borehole. Borehole tools to identify gas hydrate through high-resolution geophysical measurements include resistivity imaging of the borehole wall (Collett and Ladd, 2000), determining elastic wave velocities and attenuation (e.g., Guerin et al., 1999), and nuclear magnetic resonance (NMR) imaging of the borehole. Because a core is not needed to obtain these estimates, geophysical borehole estimates can be made less expensively and in portions of the borehole where recovery is problematic. It is essential that geophysical techniques be ground-truthed by direct measurement and by employing geochemical methods.
Insights from Laboratory Studies of Gas Hydrate
Laboratory studies are an essential component of the effort to understand how gas hydrate behaves in nature. For example, knowledge of the hydrate molecular structure will enable a better analysis of the question of whether energy can be recovered economically from Arctic or oceanic gas hydrate. Similarly, a macroscopic field experiment often indicates the need for more laboratory-based science to address issues such as production efficiency. A typical progression of science involves experiments at the bench or laboratory level, followed by progression to the pilot scale, leading eventually to full-scale field-testing. The targeted projects funded by the DOE Methane Hydrate R&D Program and discussed in Chapter 3 all follow this model to some degree. Below are some of the insights and milestones determined from laboratory studies, since Humphrey Davy first discovered hydrate in 1811, almost two centuries ago:
-
The molecules that form guests in the hydrate structure are smaller than 0.9 nm—the size of normal pentane (Chen et al., 2001).
-
The types of hydrate crystals that contain hydrocarbons form three different structures (I, II, and H), each of which has been observed in nature. Most oceanic and permafrost hydrates are structure I (Sloan, 2003).
-
The pressures, temperatures, and compositions of hydrate have been measured under laboratory conditions. Accurate hydrate thermodynamic models, tested against this field and laboratory data, have provided an extremely cost-effective alternative to field experiments for the assessment of gas hydrate production techniques (Ballard and Sloan, 2002).
-
The pressure and temperature effects on hydrate stability of other components found in nature (e.g., salts, water, ice, sediments) have been measured and/or determined theoretically. These parameters are important for determining hydrate stability in the permafrost or the ocean (Ballard and Sloan, 2002).
-
The conditions for heat and mass transport through hydrate have been measured. Such measurements allow predictions of dissociation in natural hydrate reservoirs (Mori and Mochizuki, 2000).
-
The geological, geochemical, and geophysical settings and properties of hydrate have been measured. These types of measurement are frequently obtained on large projects in the field and then reanalyzed in smaller-scale laboratory experiments using both natural and artificial samples (Ripmeester, 2000).
-
Although all of the above results are for the steady state, it is clear that the natural environment is dynamic. Inroads are being made in measurement and prediction of the time-dependent, kinetic nature of hydrate. Such measurements will enable us to determine how rapidly hydrate will dissociate once a pulse of heat reaches the hydrate front (Clarke and Bishnoi, 2001).
Even with the laboratory-based insights summarized above, many uncertainties remain about how gas hydrate will respond to production attempts in the field. It must be noted that laboratory experiments rarely mimic nature unless they are very carefully designed and based on reliable in situ measurements. An interactive relationship between labor-
atory, numerical, and field experiments is critical for developing an economically viable plan for extracting energy from gas hydrate.
FEASIBILITY OF PRODUCING METHANE FROM GAS HYDRATE
Although the total amount of gas hydrate in the marine environment is estimated to be much greater than the amount of hydrate in the permafrost, permafrost hydrate is more accessible. Therefore, the first major effort to evaluate the feasibility of producing methane from gas hydrate was conducted by an international consortium in the northern Canadian permafrost. This project, carried out in 2002, was known as the Mallik II well (Takahashi et al., 2003) and followed a 1998 effort to characterize the hydrate at this site (Dallimore et al., 1999). The DOE was a participant in this effort, which is described in more detail in Chapter 3.
Given sufficient in-place reserves, there are no obvious technical or engineering roadblocks to prevent commercial production of gas from hydrate in the future. However, there are some technical and engineering challenges that have to be solved before commercial production can begin.