2
State of Scientific Understanding
In this chapter the state of understanding of radiative forcing from individual agents is reviewed. Over the past 15 years, the Intergovernmental Panel on Climate Change (IPCC) has produced assessments in which at least one chapter has been devoted to a thorough review of current understanding about radiative forcings. The discussions here summarize the findings of the IPCC’s Third Assessment Report (IPCC, 2001) and scientific advances since it was published.
The Third Assessment Report (IPCC, 2001) includes a summary figure of the global and annual mean radiative forcings from 1750 to 2000 due to a range of perturbations (Figure 2-1), including the well-mixed greenhouse gases, ozone, aerosols, aviation effects on clouds, land use, and the Sun. The largest positive forcing (warming) since 1750 is associated with the increase of the well-mixed greenhouse gases (carbon dioxide [CO2]; nitrous oxide [N2O]; methane [CH4]; and chlorofluorocarbons [CFCs]) and amounts to 2.4 W m−2. The greatest uncertainty in Figure 2-1 is associated with the direct and indirect radiative effects of aerosols. If the actual negative forcing from aerosols were at the high end (most negative) of the uncertainty range, then it would have offset essentially all of the positive forcing due to greenhouse gases (see also Boucher and Haywood, 2001).
According to the IPCC definition, applied to the data in Figure 2-1, “The radiative forcing of the surface-troposphere system due to the perturbation in or the introduction of an agent is the change in net irradiance at the tropopause after allowing for stratospheric temperatures to readjust to radiative equilibrium, but with the surface and tropospheric temperatures and state held fixed at the unperturbed values.” This definition of forcing is
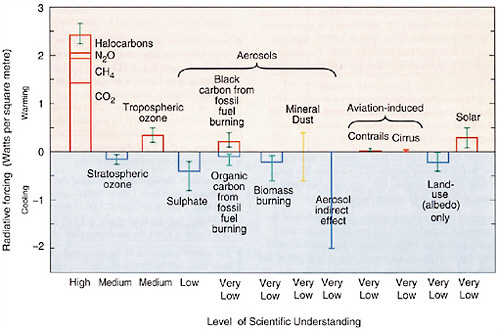
FIGURE 2-1 Estimated radiative forcings since preindustrial times for the Earth and troposphere system (TOA radiative forcing with adjusted stratospheric temperatures). The height of the rectangular bar denotes a central or best estimate of the forcing, while each vertical line is an estimate of the uncertainty range associated with the forcing, guided by the spread in the published record and physical understanding, and with no statistical connotation. Each forcing agent is associated with a level of scientific understanding, which is based on an assessment of the nature of assumptions involved, the uncertainties prevailing about the processes that govern the forcing, and the resulting confidence in the numerical values of the estimate. On the vertical axis, the direction of expected surface temperature change due to each radiative forcing is indicated by the labels “warming” and “cooling.” SOURCE: IPCC (2001).
restricted to changes in the radiation balance of the Earth-troposphere system imposed by external factors, with no changes in stratospheric dynamics, without any surface and tropospheric feedbacks in operation, and with no dynamically induced changes in the amount and distribution of atmospheric water. A somewhat broader perspective is applied in this chapter to include, in particular, volcanic aerosols, the effects of land-use changes and aerosols on precipitation, and the radiative forcing due to changes in ocean color.
Figure 2-1 has been an effective way to portray the relative magnitudes of different radiative forcings, the associated scientific uncertainties, and an assessment of the current level of understanding. It has been used widely in the scientific and policy communities. However, it has some important limitations, including the following:
-
The figure does not provide information about the timescales over which each of the forcings is active. For example, the greenhouse gases in the first bar (CO2, CH4, N2O, and halocarbons) remain in the atmosphere for decades or longer, whereas the various aerosols persist for days to weeks.
-
The figure shows globally averaged forcings and therefore does not provide information about regional variation in forcing or vertical partitioning of forcing.
-
The figure does not provide information about other climate effects of each forcing agent, such as impacts on the hydrological cycle.
-
The figure gives the impression that one can simply sum the bars to determine an overall or net radiative forcing; however, such a calculation does not give a reasonable description of the cumulative effect of all the forcings.
-
The uncertainty ranges are generally estimated from the range of published values and cannot be readily combined to determine a cumulative uncertainty.
-
The figure does not consistently indicate the forcing associated with specific sources (e.g., coal, gas, agricultural practices).
-
The figure omits nonradiative forcings as discussed in this report.
Although it would be unrealistic to expect a single figure to fully portray all of these aspects of radiative forcings, there are clearly opportunities to improve upon Figure 2-1 and to introduce new figures that address these limitations in the next IPCC report.
WELL-MIXED GREENHOUSE GASES
The radiative forcing due to CO2, CH4, N2O, and various halocarbons is due to absorption of infrared (IR) radiation. It is well characterized and well understood. These gases remain in the atmosphere long enough to be well mixed; thus, their abundances are well known and have little spatial variability. Their concentrations have increased substantially since preindustrial times (see Table 2-1), and they are the greatest contributors to total anthropogenic radiative forcing. As shown in Figure 2-1, the IPCC estimate of the radiative forcing due to well-mixed greenhouse gases is +2.43 W m−2 from 1750 to 1998 (present), comprising CO2 (1.46 W m−2),
TABLE 2-1 Well-Mixed Greenhouse Gases
|
|
CO2 |
CH4 |
N2O |
CFC-11 |
HFC-23 |
CF4 |
|
Preindustrial concentration |
~280 ppm |
~700 ppb |
~270 ppb |
0 |
0 |
40 ppt |
|
Concentration in 1998 |
365 ppm |
1745 ppb |
314 ppb |
268 ppt |
14 ppt |
80 ppt |
|
Rate of concentration changea |
1.5 ppm/yrb |
7.0 ppb/yrb |
0.8 ppb/yr |
−1.4 ppt/yr |
0.55 ppt/yr |
1 ppt/yr |
|
Atmospheric lifetime |
5 to 200 yrc |
12 yrd |
114 yrd |
45 yr |
260 yr |
>50,000 yr |
|
NOTE: CF4 = perfluoromethane; CFC-11 = chlorofluorocarbon-11; HFC-23 = hydrofluorocarbon-23; ppm = parts per million; ppb = parts per billion; ppt = parts per trillion. a Rate is calculated over the period 1990 to 1999. b Rate fluctuated between 0.9 and 2.8 ppm yr-1 for CO2 and between 0 and 13 ppb yr-1 for CH4 over the period 1990 to 1999. c No single lifetime can be defined for CO2 because of coupling with surface reservoirs. d This lifetime has been defined as an “adjustment time” that takes into account the indirect effect of the gas on its own residence time. SOURCE: IPCC (2001). |
||||||
CH4 (0.48 W m−2), N2O (0.15 W m−2), and halocarbons (0.34 W m−2) (IPCC, 2001). The estimated uncertainty associated with this forcing is 10 percent, with that for CO2 and N2O being less and that for the other gases being greater. The estimated uncertainty for halocarbons is 10-15 percent for those molecules that have been studied in detail and is not well characterized for other halocarbons. Recent research on well-mixed greenhouse gases focuses on refining the models used to do radiative transfer calculations (e.g., Evans and Puckrin, 1999), considering the small temporal and spatial variations in concentrations which can lead to errors up to about 5-10 percent (Forster et al., 1997; Myhre and Stordal, 1997; Freckleton et al., 1998), and accounting for the extent to which clouds reduce radiative forcing (e.g., Myhre and Stordal, 1997).
The IPCC estimate for CH4 forcing includes an observation-based estimate for both the direct forcing of CH4 and the indirect forcing due to changes in the hydroxyl radical (OH) and tropospheric ozone (O3) resulting from methane oxidation. The oxidation of CH4 leads to a net loss of OH in the atmosphere, thereby lengthening the CH4 lifetime. It is estimated that this indirect effect of CH4 increases its radiative forcing by 25-35 percent over the direct CH4 forcing (Lelieveld and Crutzen, 1992; Brühl, 1993; Lelieveld et al., 1993, 1998; Hauglustaine et al., 1994; Fuglestvedt et al., 1996). The oxidation of CH4 also leads to the formation of tropospheric ozone, indirectly increasing the CH4 forcing by 30-40 percent through the greenhouse effect of the additional tropospheric O3. In the stratosphere, oxidation of CH4 is a source of water vapor. In situ measurements of water vapor in the lower stratosphere indicate an increase of about 1 percent per year for 1954-2000 (Rosenlof et al., 2001), whereas satellite measurements of water vapor in the stratosphere in the 1990s showed no steady rate of change (Randel et al., 1996). A 1 percent annual increase in stratospheric water vapor would be associated with an estimated radiative forcing of 0.2 W m−2 since 1980 (Forster and Shine, 1999). The oxidation of CH4 can explain only a fraction of such a water vapor increase.
TROPOSPHERIC AND STRATOSPHERIC OZONE
Atmospheric ozone modifies the radiative budget of the Earth system by absorbing radiation both in the IR and in the ultraviolet (UV). It acts both as a radiative forcing agent and as a climate feedback. Ozone is produced and destroyed by solar UV radiation and by chemical reactions involving natural and anthropogenic gases. Changes in ozone driven by anthropogenic emissions represent a forcing. However, ozone concentrations also respond to changes in temperature and UV radiation, transport patterns, and natural emissions from lightning and vegetation; these responses represent climate feedbacks. In what follows, tropospheric and
stratospheric ozone are discussed separately because they are produced by different mechanisms and have very different radiative implications.
Tropospheric Ozone
Only 10 percent of atmospheric ozone resides in the troposphere, but this small fraction is of particular importance for climate forcing. Tropospheric ozone directly affects the radiative budget of the troposphere, and pressure broadening allows ozone absorption lines in the troposphere to extend into otherwise optically thin regions of the spectrum.
Ozone is produced in the troposphere by photochemical oxidation of volatile organic compounds (VOCs) and carbon monoxide (CO) in the presence of nitrogen oxides (NOx = NO + NO2). Anthropogenic emissions of these precursors have caused large increases in tropospheric ozone over the past century. The increase is estimated to be 50-100 percent globally according to current global three dimensional chemical transport models (CTMs), and the resulting radiative forcing is in the range 0.2-0.5 W m−2 (IPCC, 2001). An important caveat is that the CTMs are unable to reproduce the low ozone concentrations observed in the late nineteenth century and early twentieth century, suggesting (if the observations are correct) that they overestimate the natural source of ozone. Model calculations constrained with the historical observations indicate a larger forcing from anthropogenic tropospheric ozone, up to 0.8 W m−2 (Mickley et al., 2001; Shindell and Faluvegi, 2002).
Beyond this direct radiative forcing effect, tropospheric ozone also has an indirect effect as the primary precursor of the OH radical. Increasing ozone causes tropospheric OH to increase, thus decreasing the lifetime of methane and facilitating aerosol nucleation (both negative forcings). Assessing this indirect effect is complicated because the increase in ozone is driven by emissions of its precursors, which themselves have intrinsic effects on OH. Increasing NOx thus causes OH to increase, while increasing CO and VOCs cause OH to decrease. According to the current generation of CTMs, OH concentrations have decreased by about 10 percent over the past century (Wang and Jacob, 1998). Another indirect effect of tropospheric ozone is to cool the stratosphere (Joshi et al., 2003; Mickley et al., 2004), affecting stratospheric ozone and polar stratospheric cloud (PSC) levels.
The greatest uncertainty in quantifying the direct radiative forcing from tropospheric ozone lies in reconstructing its concentration field in the past and projecting it into the future. The inability of current models to reproduce ozone observations from the early twentieth century could reflect calibration problems in the observations, as well as model errors in the estimates of natural sources. CTMs also have problems in simulating the
well-calibrated ozone trends over the past 30 years (Fusco and Logan, 2003), implying that fundamental problems remain in our understanding of tropospheric ozone chemistry. Uncertainty in quantifying the indirect radiative effect of ozone is related mainly to the complexity of factors controlling OH concentrations (Lawrence et al., 2001). Uncertainties in predicting the climatic response to changes in tropospheric ozone are also large and require further investigation using general circulation models (GCMs).
Stratospheric Ozone
Depletion of stratospheric ozone over the past 30 years has caused both a positive radiative forcing at the Earth’s surface (due to increased UV penetration) and a negative forcing (due to reduced IR emission from the stratosphere to the troposphere). The consensus from current radiative models constrained by observed ozone trends is that the net forcing is negative and of magnitude −0.10 ± 0.05 W m−2. Forster and Tourpali (2001) argue that about half of this forcing is due to an increase in tropopause heights and thus should not be considered a forcing but rather a feedback. The main indirect radiative effects of stratospheric ozone depletion are (1) increased UV penetration to the troposphere, increasing tropospheric OH concentrations and hence decreasing the lifetime of methane (IPCC, 2001), and (2) changes in stratospheric water vapor.
Several GCM studies have examined the climate response to changes in stratospheric ozone. Shindell et al. (1999) finds that changes in the upper stratosphere elicit far greater surface climate response than changes in the lower stratosphere. Stuber et al. (2001) find that changes in stratospheric ozone have a greater effect per unit forcing than changes in CO2, largely because of feedbacks associated with stratospheric water vapor.
The greatest uncertainty in quantifying radiative forcing from past changes in stratospheric ozone is the vertical distribution of the ozone trend in relation to temperature, since the magnitude of the forcing depends crucially on temperature (IPCC, 2001). Another critical issue is to better quantify indirect radiative forcings, particularly the effect on stratospheric water vapor, which could double the effective forcing according to Stuber et al. (2001).
DIRECT EFFECT OF AEROSOLS
Aerosol particles both scatter and absorb radiation, representing a direct radiative forcing; scattering generally dominates (except for black carbon particles) so that the net effect is of cooling. Global models have demonstrated the important role of sulfate aerosols in providing the cooling effect missing in past models of the atmospheric radiation balance (Kiehl et
al., 1995). The average global mean aerosol direct forcing from fossil fuel combustion and biomass burning is in the range from −0.2 to −2.0 W m−2 (IPCC, 2001). This large range results from uncertainties in aerosol sources, composition, and properties used in different models. Recent advances in modeling and measurements have provided important constraints on th direct effect of aerosols on radiation (Ramanathan et al., 2001a; Russell et al., 1999; Conant et al., 2003). Critical gaps, discussed further below, relate to
-
spatial heterogeneity of the aerosol distribution, which results from the short lifetime (a few days to a week) against wet deposition;
-
chemical composition, especially the organic fraction;
-
mixing state and behavior (hygroscopicity, density, reactivity, and acidity); and
-
optical properties associated with mixing and morphology (refractive index, shape, solid inclusions).
The chemical composition of particles is in general not well known. The mixing state and relative humidity history of sulfate-nitrate-ammonium aerosols have important implications for their water content and hence their direct radiative effect (Martin et al., 2004). Uncertainties are particularly large for the 20 to 70 percent of particle mass that consists of organic compounds (NARSTO, 2003). Measurement of organic components is inherently difficult for three reasons: (1) small sample sizes and analytical difficulties, (2) the complexity of mixtures, and (3) artifacts in sampling procedures. The ideal approach for characterizing organic mass in aerosol particles would identify, molecule by molecule, the composition of each individual particle. Such instrumentation is unlikely to become available in the near future. In the meantime, it will be important to use partial information from traditional and new approaches, including evolved gas analysis techniques, gas chromatography, time-of-flight and chemical ionization mass spectrometry, Fourier transform infrared spectroscopy, and near-edge X-ray absorption fine structure (Morrical et al., 1998; Schauer et al., 1999; Huebert and Charlson, 2000; Russell et al., 2002; Bahreini et al., 2003; Russell, 2003).
Radiative and climate models generally assume that aerosols are “externally mixed,” that is, individual particles are made up of a single component (Koch, 2001; Cooke et al., 2002). Actual aerosol particles are multicomponent mixtures for which properties, such as water uptake, differ from those expected from the simple addition of components because of nonlinear interactions between components. The consequence for radiative forcing is that water uptake by particles is not predicted accurately. The presence of organic compounds has two competing effects on particle hy-
groscopicity: (1) it reduces the mass of water taken up, and (2) it initiates water uptake at a lower relative humidity (called the deliquescence relative humidity) (Ming and Russell, 2002). The reduction in water uptake results from the typically low solubility of organic compounds. Particles containing organic compounds will grow less as a function of relative humidity, meaning that models that use the properties of sulfate aerosol will overestimate the direct radiative forcing.
Many GCM aerosol schemes tend to omit organic particles or underestimate their size at typical boundary layer humidity (~80 percent). Chung and Seinfeld (2002) estimate the effect of organic carbon on radiative forcing of the climate to be between −0.09 and −0.21 W m−2, with the range of uncertainty driven by the role of water uptake by organic aerosols. The combination of the role of organic carbon and its water uptake with the externally or internally mixed states of other components results in a direct aerosol forcing range of −0.86 to −1.26 W m−2, or an uncertainty of ±50 percent (Chung and Seinfeld, 2002). In addition to water uptake, uncertainties in aerosol lifetime and optical properties contribute to the range of uncertainty.
In addition to these general difficulties in describing the direct radiative forcing from aerosols, specific uncertainties relate to (1) light-absorbing black carbon, and (2) categorization of aerosol types in modeling. These are discussed below.
Black Carbon
Individual aerosol particles may contain light-absorbing carbon-containing compounds referred to collectively as “black” carbon (BC) or equivalently as soot. In addition to elemental carbon, BC frequently includes low-volatility solid or liquid organic compounds, typically composed of long hydrocarbon chains with high molecular weights (Marley et al., 2001). The presence of trace amounts of BC (as little as 5 to 10 percent of the total mass in anthropogenic aerosols) can result in large atmospheric solar absorption. This absorption can be enhanced when BC is embedded in refractive particles (Chylek et al., 1996; Fuller et al., 1999). Current understanding of the global emission of BC is uncertain by factors of two or more (Cooke et al., 2002; Bond et al., 2004). Biomass burning and fuel combustion are the two main contributors. BC has been detected in remote oceanic regions, implying hemispheric-wide dispersal.
The direct forcing due to carbonaceous aerosols can be separated into three components:
-
A portion of the direct solar beam is scattered back to space, which leads to a reduction in solar radiation reaching the surface. This reduction
-
manifests as increased reflection at the top of the atmosphere (TOA), i.e., a negative radiative forcing (cooling).
-
A portion of the direct solar beam is absorbed by the aerosol, and this atmospheric absorption leads to further reduction in solar radiation reaching the surface. As shown later, this shielding of the surface by BC is the dominant absorption term for anthropogenic aerosols with as little as 10 percent of BC. This absorption leads to a positive radiative forcing of the atmosphere and a negative radiative forcing of the surface.
-
The upward diffuse beam from scattered radiation is absorbed by the BC aerosol, reducing the solar radiation that escapes to space and resulting in a positive radiative forcing for the surface-atmosphere column. This effect could be large in cloudy skies if BC lies above low clouds (Haywood and Ramaswamy, 1998).
The TOA forcing reported in IPCC and other global warming studies is the sum of processes (1) and (2). Process (3), albeit the largest in terms of magnitude, does not contribute significantly to TOA forcing, since it adds solar radiation to the atmosphere and reduces surface solar heating by the same magnitude. The net effect of BC is to increase the radiative heating of the atmosphere and decrease the radiative heating of the surface. The TOA radiative forcing is the sum of the surface and the atmospheric forcing. At the TOA the BC effect opposes the cooling effect of sulfates and organics, while at the surface all aerosols lead to reduction of solar radiation. Thus, aerosol-induced changes at the surface can far exceed those at the TOA.
The direct effect of BC aerosol in the atmosphere has important implications for “global dimming.” Black carbon emissions may have increased by a factor of two to four during the last 50 years (Novakov et al., 2003). Given such large increases in BC emissions and the large impact of BC on reducing surface solar radiation, large decreases in surface solar radiation should be observed downwind of major sources of BC. Long-term negative trends in surface solar irradiances have been observed by surface radiometers worldwide (Ohmura et al., 1998; Stanhill and Cohen, 2001; Liepert, 2002). The reported trends in the annual mean irradiance vary from −5 percent (10 W m−2) between 1958 and 1985 for all land stations to about −1 to −3 percent per decade for the last four decades over many of the 1500 stations in the global datasets. The decreases are so large that there is skepticism about the measurements. Trends in surface radiometer all-sky observations are subject to large uncertainties due to difficulties in maintaining accurate calibration for routine surface observations in remote locations and measurement errors inherent in broadband radiometric measurements (Dutton et al., 2001).
This global dimming is thought to be caused by both absorbing aerosols and increases in cloud cover (Liepert, 2002). Most of the radiometer
observations are over land, and we need to understand whether they are affected by local urban haze. Anthropogenic absorbing aerosols, by themselves, can reduce land-averaged solar radiation by about 3 to 5 W m−2 (Ramanathan et al., 1995; Jacobson, 2002). Such large reductions in surface solar radiation have implications for the hydrological cycle, since roughly 70 percent of the absorbed solar radiation is balanced by the latent heat flux of evaporation.
This global dimming may also be related to changes in the ratio of direct and diffuse solar irradiance received by vegetation. As documented by Gu et al. (2003), the increase in diffuse irradiance for the two years following the eruption of Mount Pinatubo in 1991 resulted in a 23 percent increase of noontime photosynthesis of a deciduous forest in 1992 under cloudless conditions. The increased diffuse irradiance permits a greater penetration of photosynthetically active sunlight into the canopy. However, if there is a sufficient reduction of total solar irradiance received at the ground, vegetation growth could be stunted. Chameides et al. (1999), for example, reported on reductions in crop yield in China due to reduced total solar irradiance from pollution aerosols. Krakauer and Randerson (2003) concluded from tree ring data that with respect to volcanic eruptions, the beneficial effect of aerosol light scattering for high northern latitudes appears to be offset entirely by the deleterious effect of eruption-induced climate change. Using field observations, Niyogi et al. (2004) found that increased aerosol loading led to increases in carbon assimilation for forests and crops and decreases for grasslands. The effect on carbon assimilation was larger with aerosols than with clouds, since clouds reduced the total solar irradiance more than the aerosols did.
Deposition of BC aerosols over snow-covered areas can result in changes to the surface albedo (Chylek et al., 1983). Further reductions in albedo occur due to the enhanced melting that accompanies the heating of absorbing soot particles in snow. Chylek et al. (1983) estimate this enhancement to be up to a factor of ten in the rate of melting. Recent model results indicate radiative forcings of +0.3 W m−2 in the Northern Hemisphere associated with albedo effects of soot on snow and ice (Hansen and Nazarenko, 2004).
Model Discretization of Aerosols
Typically the behavior of aerosol particles in the atmosphere has been described in models by discretization of both size and chemical composition. The continuous particle size spectrum is described by a limited number of modes, moments, or sections (Seinfeld and Pandis, 1998). For many problems, such as the evolution of marine aerosol, the computational simplicity of a few modes is sufficient to characterize changes in the particle
distribution (Pandis et al., 1994). Other problems, including those involving aerosol-cloud interactions, require ~100 size categories to accurately represent the indirect effect of particles on cloud properties (Russell et al., 1999). While the simplified models are known to represent measured size distributions incompletely, the uncertainties associated with these simplifications can easily be quantified. GCMs employing this approach have not yet had the computational power to evaluate the sensitivity of aerosol radiative forcing to their simplified treatments of aerosol size distributions.
Initial models of particle evolution assumed that the composition of particles consists of a single internal mixture, both for computational simplicity and for lack of contradictory measurements (Warren and Seinfeld, 1985). In the last decade, chemical composition has been discretized in a fashion similar to the treatment of particle size, typically by “lumping” particles with similar chemical compositions into a few categories. This approach has been used to represent the externally mixed nature of aerosol particles, typically including categories such as “sulfate,” “sea salt,” “dust,” and “carbonaceous” (Jacobson et al., 1994; Pandis et al., 1994; Russell and Seinfeld, 1998; Jacobson, 2001; Koch, 2001; Garrett et al., 2003). The small number of categories has enabled their inclusion in GCM simulations. However, this does not reflect the variety of mixtures actually present in the troposphere (Murphy et al., 1997). The complexity of particle structures, their heterogeneities, and their mixing states (Russell et al., 2002) will have to be addressed to represent their hygroscopic and optical properties. For example, a small amount of absorbing organic compounds mixed in with sea salt aerosol can be sufficient to change the associated radiative forcing from negative to positive, especially over low albedo surfaces such as clouds, ice, and coastal areas (Randles et al., 2004).
INDIRECT EFFECT OF AEROSOLS
Aerosols interact with clouds in several ways that can affect the climate system, in particular by acting as cloud condensation nuclei (CCN) and ice nuclei. These interactions are generally referred to as the indirect effects of aerosols. Table 2-2 summarizes the various aerosol indirect effects. As shown in the table, aerosols can lead to both warming (positive forcing) and cooling (negative forcing), complicating the analysis of their net effect.
Aerosols have several indirect effects on warm stratiform clouds. The Twomey effect, also known as the first indirect aerosol effect, refers to the enhanced reflection of solar radiation due to more but smaller cloud droplets in a cloud whose liquid water content remains constant (Twomey, 1959). The IPCC Third Assessment Report concluded that the first indirect aerosol effect amounts to 0 to −2 W m−2 in the global mean (IPCC, 2001). In addition, more but smaller cloud droplets reduce the precipitation effi-
TABLE 2-2 Overview of the Different Aerosol Indirect Effects Associated with Clouds
|
Effect |
Cloud Type |
Description |
Sign of TOA Radiative Forcing |
|
First indirect aerosol effect (cloud albedo or Twomey effect) |
All clouds |
For the same cloud water or ice content, more but smaller cloud particles reflect more solar radiation |
Negative |
|
Second indirect aerosol effect (cloud lifetime or Albrecht effect) |
All clouds |
Smaller cloud particles decrease the precipitation efficiency, thereby prolonging cloud lifetime |
Negative |
|
Semidirect effect |
All clouds |
Absorption of solar radiation by soot leads to evaporation of cloud particles |
Positive |
|
Glaciation indirect effect |
Mixed-phase clouds |
An increase in ice nuclei increases the precipitation efficiency |
Positive |
|
Thermodynamic effect |
Mixed-phase clouds |
Smaller cloud droplets inhibit freezing, causing supercooled droplets to extend to colder temperatures |
Unknown |
|
Surface energy budget effect |
All clouds |
The aerosol-induced increase in cloud optical thickness decreases the amount of solar radiation reaching the surface, changing the surface energy budget |
Negative |
ciency and therefore enhance cloud lifetime and, hence, cloud reflectivity, which is referred to as the second indirect aerosol or cloud lifetime effect (Albrecht, 1989). Absorption of solar radiation by black carbon leads to heating of the air, which can result in an evaporation of cloud droplets. This is referred to as the semidirect effect (Hansen et al., 1997). The absorption of soot is 2 to 2.5 times greater if soot is present in the cloud droplets (Chylek et al., 1996); thus the magnitude of the semidirect effect depends crucially on the location of black carbon with respect to the cloud. This warming can partially offset cooling due to the indirect aerosol effect. Both
the cloud lifetime effect and the semidirect effect involve feedbacks because the cloud lifetime and cloud liquid water content change; therefore, they were not included in the radiative forcing bar chart of the IPCC (2001) assessment.
The committee notes that separating the aerosol indirect forcing into “first” and “second” kinds is not necessarily a useful construct. Although the first kind accounts for a significant and distinguishable set of properties between polluted and unpolluted clouds, the lifetimes of clouds are such that it is never really observed. Clouds respond quickly to shifts in droplet sizes, so by the time observations are made, clouds have already progressed into the “second” mode. Alternatively, the indirect forcing could be calculated for clouds with fixed water amounts or for clouds with water amounts that are free to adjust to the changes in droplet sizes.
Surface-based and satellite data have provided evidence of these aerosol effects on warm clouds. Feingold et al. (2003) used various observations at the Department of Energy Atmospheric Radiation Measurement (ARM) site in Oklahoma to estimate the indirect aerosol effect from the partial derivative of the logarithm of cloud droplet radius with respect to the logarithm of the aerosol extinction. Defined in this way, the indirect aerosol effect can be compared to its estimates from satellite retrievals. They find that the indirect effect at the ARM site is larger than estimated from POLDER satellite data by Breon et al. (2002). However, Rosenfeld and Feingold (2003) pointed out that limitations of the POLDER satellite retrievals could explain the discrepancy. Penner et al. (2003) combined ARM data together with a Lagrangian parcel model at the ARM sites in Oklahoma and Alaska to provide observational evidence of a change in radiative forcing due to the indirect aerosol effect. Long-term observations with satellites over Europe and China show evidence for the semidirect effect (Krüger and Graßl, 2002, 2004). Kim et al. (2003) reported regional aerosol influences on TOA flux of up to 50 W m−2. Schwartz et al. (2002) reported albedo enhancements due to the Twomey effect of as much as 0.2. None of these techniques, however, enable estimates of the anthropogenic indirect aerosol effect globally.
Climate model estimates of the first and second indirect aerosol effects and the semidirect aerosol effect are still very uncertain. Kristjansson (2002) concluded that the Twomey effect is three times more important than the cloud lifetime effect, whereas Lohmann et al. (2000) simulated a cloud lifetime effect that is 40 percent larger than the Twomey effect. Estimated magnitudes of the Twomey effect and cloud lifetime effect are −0.5 to −1.9 W m−2 and −0.3 to −1.4 W m−2, respectively (Lohmann and Feichter, 2004). Based on model results by Penner et al. (2003) and Lohmann and Feichter (2001), the estimated magnitude of the semidirect effect is +0.1 to −0.5 W m−2. Whereas climate models predict an increase in liquid water due to the
cloud lifetime effect, observations of ship tracks show that polluted clouds have less liquid water, not more (Platnick et al., 2000; Coakley and Walsh, 2002). Thus, the enhancement of cloud water through the suppression of precipitation in polluted clouds is still not understood completely and therefore contributes to the significant uncertainty attributed to the aerosol indirect forcing.
Liu and Daum (2002) estimated that the magnitude of the first indirect aerosol effect can be reduced by 10 to 80 percent by including the influence that an increasing number of cloud droplets has on the shape of the cloud droplet spectrum. When this dispersion effect is taken into account in global climate models, the reduction is rather moderate and amounts to 15 to 35 percent (Peng and Lohmann, 2003; Rotstayn and Liu, 2003). Lower estimates of the indirect aerosol effect are in better agreement with inverse calculations based on historical climate record data of oceanic and atmospheric warming (Forest et al., 2002; Knutti et al., 2002; Anderson et al., 2003a).
The presence of ice in large-scale or convective clouds allows for further aerosol-cloud interactions. For large-scale, mixed-phase clouds,
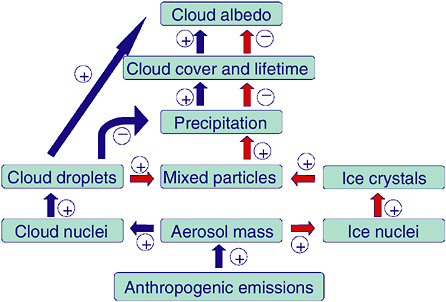
FIGURE 2-2 Schematic of the warm indirect aerosol effect (blue arrows) and glaciation indirect aerosol effect (red arrows). SOURCE: Adapted from Lohmann (2002).
Lohmann (2002) showed that if in addition to mineral dust, a fraction of the hydrophilic soot aerosol particles is assumed to act as contact ice nuclei at temperatures between 0° and −35°C, then a “glaciation indirect effect” results (Figure 2-2). Increases in contact ice nuclei in the present-day climate result in more frequent glaciation of clouds and increase the amount of precipitation via the ice phase. Observations in the presence of Saharan African dust indicate that mildly supercooled clouds at temperatures between −5 and −9°C are already glaciated (Sassen et al., 2003).
For convective mixed-phase clouds, Rosenfeld and Woodley (2000) analyzed aircraft data together with satellite data to show that pollution aerosols suppress precipitation. This hypothesis is supported by a modeling study with a cloud-resolving model by Khain et al. (2001). Taking these results to the global scale, Nober et al. (2003) evaluated the sensitivity of the general circulation to the suppression of precipitation by anthropogenic aerosols by implementing a simple warm cloud microphysics scheme into convective clouds. They found large instantaneous local aerosol forcings reducing the warm-phase precipitation (thermodynamic effects). Menon et al. (2002b) showed that absorbing aerosols over China change the atmospheric stability and vertical motion by heating the air and, thus, the large-scale circulation and the hydrological cycle.
The IPCC aviation report (Penner et al., 1999) identified the effects of aircraft on upper tropospheric cirrus clouds as a potentially important climate forcing. One aspect may be described as the direct effect due to the formation of condensation contrails as a result of supersaturated air from the aircraft. This effect can be nonnegligible as was found during the three-day grounding of all U.S. commercial aircraft following the September 11, 2001, terrorist attacks. An anomalous increase in the average diurnal temperature range over the United States was observed and partly attributed to the absence of contrails from jet aircraft (Travis et al., 2002). Aerosols emitted by the aircraft may also cause indirect effects associated with an increase in ice nuclei in the upper troposphere. Evidence of a climate effect of air traffic was first provided by Boucher (1999) who used ship-based measurements of cloud cover together with fossil fuel consumption data for aircraft to show that recent increases in air traffic fuel consumption are accompanied by an increase in cirrus cloudiness. Recent studies (Lohmann and Kärcher, 2002) suggest that the impact of aircraft sulfur emissions on cirrus properties via homogeneous freezing of sulfate aerosols is probably small. Hence the question has been raised whether aircraft-generated black carbon particles serving as heterogeneous ice nuclei, as found by Ström and Ohlsson (1998), may have a significant impact on cirrus cloudiness and cirrus microphysical properties.
Aerosols can also modify latent and sensible heat fluxes at the surface, thus exerting a nonradiative forcing on the hydrological cycle. Increasing
aerosol and cloud optical depth cause a reduction of solar radiation at the surface. For the surface energy balance to reach a new equilibrium state, the latent and sensible fluxes have to adjust. Because evaporation has to equal precipitation on the global scale, a reduction in the latent heat flux leads to a reduction in precipitation. As shown in model simulations by Liepert et al. (2004), despite an increase in greenhouse gases, increases in optical depth due to the direct and indirect anthropogenic aerosol effects can cause a reduction in evaporation and precipitation. This mechanism is consistent with observations of decreased evaporation from open pans of water over the last 50 years. Roderick and Farquhar (2002) characterized steadily decreasing pan evaporation due to decreases in solar irradiance resulting from increasing cloud coverage and aerosol concentration. Increasing aerosol optical depth associated with scattering aerosols alone in otherwise clear skies has been shown to produce a larger fraction of diffuse radiation at the surface, which results in greater carbon assimilation into vegetation (and therefore greater transpiration) without a substantial reduction in the total surface solar radiation (Niyogi et al., 2004).
LAND-COVER AND LAND-USE CHANGES
Land-use changes include irrigation, urbanization, deforestation, desertification, reforestation, grazing of domestic animals, and dryland farming. Each of these alterations in landscape produces significant changes in radiative forcing (e.g., Pitman, 2003; Kabat et al., 2004). Global maps of land-cover changes over the past 300 years are shown in Figure 2-3. In addition, changes in tropical forests have been reported by O’Brien (2001). There are historical land use datasets (e.g., Ramankutty and Foley, 1999) as well as satellite-based land-cover datasets. Satellite products include the DISCover dataset developed under the auspices of the International Geosphere-Biosphere Programme (Loveland et al., 2000) and land-cover datasets based on MODIS/Terra data (Strahler et al., 1999).
Land-use and land-cover changes can affect the Earth’s radiative bal-
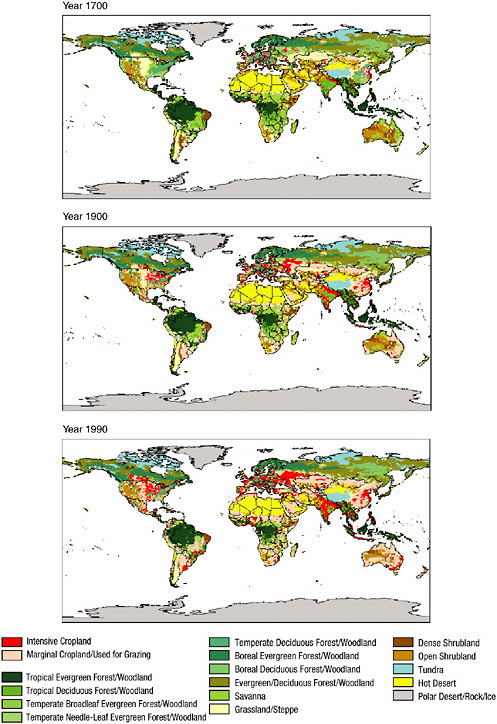
FIGURE 2-3 Global estimate of land use and land cover for (a) 1700, (b) 1900, and (c) 1990. The human-disturbed landscape includes intensive cropland (red) and marginal cropland used for grazing (pink). Other landscape includes, for example, tropical evergreen and deciduous forest (dark green), savannah (light green), grassland and steppe (yellow), open shrubland (maroon), temperate deciduous forest (blue), temperate needleleaf evergreen forest (light yellow), and hot desert (orange). SOURCE: Klein Goldewijk (2001).
ance both directly and indirectly. Direct effects include the change of albedo and emissivity resulting from the different types of land covers that modify the amount of shortwave radiation absorbed at the surface and of longwave radiation absorbed and emitted at the surface. For example, the development of agriculture in tropical regions typically results in an increase of albedo from a low value of forest canopies (0.05-0.15) to a higher value of agricultural fields, such as pasture (0.15-0.20). In contrast, irrigated fields in arid areas tend to have a lower albedo than the bare, typically bright soils they cover. The seasonal variation of albedo as a result of land-cover change can also have pronounced effects on the net radiation at the Earth’s surface.
As shown in Figure 2-1, the IPCC (2001) reports the global-averaged forcing due to albedo change alone as −0.25 ± 0.25 W m−2. The level of scientific understanding is listed as “very low.” The uncertainties in the albedo change reflect the complexity of the land surface (e.g., type of vegetation, phenology, density of coverage, soil color). When aggregating regional information about land surface up to the global scale, large global average uncertainty ranges result. A recent assessment of the albedo change estimates a range of −0.6 to 0.5 W m−2, with the negative values being more likely (Myhre and Myhre, 2003).
Indirect effects of land-cover change on the net radiation include a variety of processes related to (1) the ability of the land cover to use the radiation absorbed at the ground surface for evaporation, transpiration, and sensible heat fluxes (the impact on these heat fluxes caused by changes in land cover is sometimes referred to as thermodynamic forcing); (2) the exchange of greenhouse and other trace gases between the surface and the atmosphere; (3) the emission of aerosols (e.g., from dust); and (4) the distribution and melting of snow and ice. These effects are discussed below.
Indirect Effects of Land-Use and Land-Cover Change
Changes in soil wetness can significantly modify the energy balance of continental surfaces. When soil moisture is high, most of the radiative energy absorbed at the ground surface is used for physical evaporation and transpiration of water. The latent heat flux is large, the sensible heat flux is small (in arid areas, it can even be negative, a process known as the “oasis effect”), the land-surface temperature is relatively low (compared to conditions with more sensible heat flux with the same net radiation), and as a result, the longwave radiation emitted by the land surface is relatively low. As a result, the atmospheric boundary layer that develops above such land is typically thin and moist. In contrast, when the soil is dry, there is no latent heat flux, the sensible heat flux is large, the land surface temperature is higher, and as a result, the longwave radiation emitted by the land surface is relatively high. The planetary boundary layer developing above such land
is typically deep and dry. Note also that soil wetness is a function of precipitation, which itself can be affected by land cover, resulting in complex land-atmosphere feedbacks. For example, Figure 2-4 illustrates schematically the alteration of fluxes as a result of the conversion of forest to cropland. The conversion alters the transpiration of water vapor into the atmosphere. The surface albedo is usually higher when vegetation is reduced, exposing some of the soil.
Land-use and land-cover change can also have indirect effects by affecting fluxes of greenhouse gases from the land surface. Land cover influences the release into the atmosphere of water vapor, a greenhouse gas with obvious impacts on clouds and precipitation. The biological activity at the ground surface can be a sink or a source of carbon dioxide, methane, and other gases, but these processes are still not understood and are not represented well (if at all) in climate models.
Interactions between the land and atmosphere complicate the interpretation of CO2 trends. CO2 concentrations change in response to vegetation, ocean, and erosional feedbacks, as well as anthropogenic emissions; some of these fluxes are responses to variations of the climate system on multiple
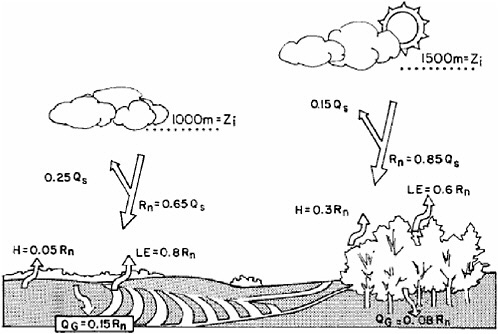
FIGURE 2-4 Schematic, based on observations in southwest France, of the influence on the surface energy budget of land-use change from forest to cropland. SOURCE: Kabat et al. (2004).
timescales. Biophysical feedbacks (such as transpiration) operate on time-scales of minutes, while biogeochemical feedbacks (such as plant growth) become significant only after several days and longer. Biogeographic changes (such as species composition) occur over years and longer.
Land cover affects the amount of aerosols that can be lifted by wind into the atmosphere. In general, the less dense the vegetation and the more intense the human activity on the land, the more aerosols can be lifted by dust storms and other updrafts. The impact of aerosols is discussed in detail in other sections of this report.
Snow has a very significant impact on the land-cover albedo and, as a result, on the radiation balance of the land cover. However, the same amount of snow at a specific location and time has a different synergistic effect with different land-cover types. For example, the albedo of an evergreen coniferous tree canopy is lower than that of an adjacent snow-covered clearing. Land type also affects the duration of snow on the ground.
Partitioning Between Latent and Sensible Heat Fluxes on a Regional Scale
Changes in the partitioning of net radiative fluxes into sensible and latent can substantially alter the atmospheric circulation. Using the results from Chase et al. (2000a), where a conservative estimate of land-cover change by humans was specified, Pielke et al. (2002) reported a globally averaged redistribution of sensible and latent turbulent heat fluxes on the order of 1 W m−2. Therefore, the spatial redistribution of the surface turbulent fluxes indicates that the net radiation received at the surface is changed in how the energy is inserted into the atmosphere. The surface forcing of climate, as a result, is altered.
The effect on local climate can be substantial. Marshall et al. (2004a,b) documented major alterations in summer rainfall and temperature, and in freeze occurrence, due to land conversion in the twentieth century in Florida. The observed land change between the pre-1900 period and 1993 in Florida is shown in Figure 2-5. Figure 2-6 shows model results for the change in net radiation at the surface, averaged over July-August, in response to that land-use change. Over the land area shown in Figure 2-5, the two-month, area-averaged reduction in rainfall was 10-12 percent. Freeze intensity and duration also changed as a result of land-use conversion. In the agricultural area just south of Lake Okeechobee, the draining of marshes and their replacement with orchards and other agricultural crops resulted in greater radiation loss to space with a resultant longer and more severe freeze.
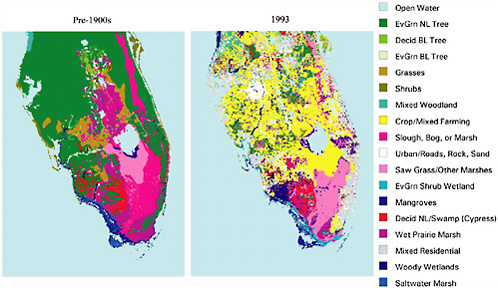
FIGURE 2-5 U.S. Geological Survey land-cover data in Florida for (left) pre-1900 and (right) 1993. SOURCE: Marshall et al. (2004b).
SOLAR FORCING
Irradiance
The Sun’s electromagnetic radiation powers the Earth’s climate. The blackbody temperature of the solar surface is about 5800 K, and as a result, the spectrum of solar radiation peaks at visible wavelengths. Solar irradiance is the electromagnetic radiation from the Sun incident at the top of the Earth’s atmosphere at a distance of one astronomical unit (1 AU = 1.5 × 1011 m), corresponding to the mean Earth-Sun distance. Total solar irradiance (TSI), the integral of spectral irradiance, has a mean value of 1365 ± 1 W m−2 according to Active Cavity Radiometer Irradiance Monitor (ACRIM), Earth Radiation Budget Satellite (ERBS), and Solar and Heliospheric Observatory (SOHO) space-based radiometry. The recently launched Solar Radiation and Climate Experiment (SORCE) radiometer suggests a lower absolute value of 1358 W m-2. Approximately equal amounts of energy are radiated above and below 742 nm.
Solar irradiance forcing of climate arises from the change in electromagnetic radiation at 1 AU produced by variations in the Sun’s activity. This forcing is the result of changes generated within the Sun, not changes in the Earth’s orbit around the Sun. Calibrated radiometers on various
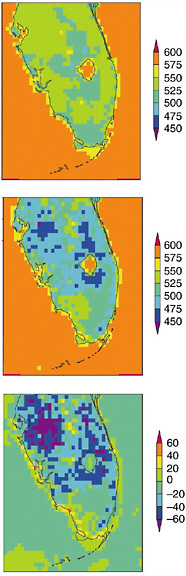
FIGURE 2-6 July-August average net daytime (12Z-23Z) radiation received at the surface for the pre-1900 landscape (top), 1989 large-scale meteorology with the 1993 landscape (middle), and the difference between the 1989 simulation and the pre-1900 case (bottom) in units of W m−2. The daytime land average for the pre-1900 case is 530 W m−2 and for the 1989 simulation is 503 W m−2. SOURCE: Adapted from Marshall et al. (2004b).
spacecraft have measured the total solar irradiance since the late 1970s. There is an 11-year cycle in total solar irradiance of peak-to-peak amplitude ~1 W m−2 (0.1 percent) in the past three cycles. Allowing for reflection of 30 percent of this incident energy (Earth’s albedo) and averaging over the globe, the corresponding climate forcing is of order 0.2 W m−2.
Multiple TSI datasets have been combined into a composite time series of daily total solar irradiance from 1979 to the present. This requires the cross-calibration of measurements made by overlapping datasets to adjust
for the different absolute scales of individual radiometers and the comparison of time-dependent trends among radiometers having different solar exposures and different design. Different assumptions about radiometer performance led to different reconstructions of TSI for the past two decades. In one reconstruction (Fröhlich and Lean, 2002), an 11-year total irradiance cycle of amplitude ~1 W m−2 (0.1 percent) has approximately equal values during successive solar minima (1986 and 1996), whereas in another reconstruction, the 1996 minimum is 0.5 W m−2 (0.05 percent) higher than in 1986. Willson and Mordvinov (2003) argue that this is evidence for a long-term secular trend in total irradiance underlying the activity cycle. If real, this increase in total solar irradiance would imply a secular climate forcing of 0.1 W m−2 over the past two decades. The actual increase, however, occurred during a two-year interval from 1990 to 1992 and coincides with apparent drifts in the ERBS radiometer on the Nimbus 7 spacecraft, used in the reconstructions to connect observations by ACRIM I and II radiometers. This argues against any detectable long-term trend in the observed irradiance to date. Likewise, models of total solar irradiance variability that account for the influences of solar activity features—dark sunspots and bright faculae—do not predict a secular change in the past two decades.
Knowledge of solar irradiance variations is rudimentary prior to the commencement of continuous space-based irradiance observations in 1979. Models of sunspot and facular influences developed from the contemporary database have been used to extrapolate daily variations during the 11-year cycle back to about 1950 using contemporary sunspot and facular proxies, and with less certainty annually to 1610. The reconstructions are based on records of the number of sunspots on the Sun’s surface, which commenced with the first telescopic observations of the Sun in the early seventeenth century. These remain the longest existing direct record of solar activity.
Most historical reconstructions of total solar irradiance have assumed that the 11-year activity cycle is superimposed on a longer-term varying background component. In these reconstructions, which have been widely used in climate change simulations, estimated TSI increases from the Maunder Minimum in the late seventeenth century to the present are in the range of 0.2 to 0.4 percent (Lean, 2000), which corresponds to climate forcing in the range of 0.4 to 0.6 W m−2. Circumstantial evidence from cosmogenic isotope proxies of solar activity (14C and 10Be) and plausible variations in Sun-like stars motivated the assumption of long-term secular irradiance trends, but recent work questions the evidence from both. Preliminary modeling of magnetic field transport on the solar surface suggests that cosmogenic isotopes (which are controlled by heliospheric magnetic fields) can vary in different ways and have larger secular trends than irradiance (which is controlled by magnetic fields confined to the solar atmosphere)
(Lean et al., 2002). Critical examination of a broader distribution of stars and examination of their “solar-likeness” appear to contradict the initial findings that noncycling Sun-like stars undergo Maunder-type episodes with reduced overall brightness. Likewise, an initial study of (uncalibrated) solar images failed to find evidence of a varying brightness component in the past century (Foukal and Milano, 2001). The most recent studies therefore raise the possibility that long-term solar irradiance variations may be limited to 11-year cycles.
Solar forcing estimates based on changes in total solar irradiance are only approximations of the actual forcing. This is because of the wavelength dependence of both the magnitude and the variability of the solar spectrum and of atmospheric absorption that differentially attenuates the spectrum. Forcing estimates based on total solar irradiance assume that energy at all wavelengths reaches the Earth’s surface (or at least the troposphere) and that radiation at all wavelengths changes by the same amount. However the solar spectrum has less flux, but varies more, at shorter wavelengths. Furthermore, the Earth’s stratosphere absorbs solar irradiance at wavelengths less than 310 nm. The energy in the solar spectrum at wavelengths from 200 to 300 nm (15.3 W m−2) changes by about 1 percent during the 11-year cycle and accounts for 13 percent of the corresponding total irradiance cycle (Lean et al., 1997). Variations in solar ultraviolet irradiance alter the production and destruction of ozone, thereby influencing stratospheric temperature, dynamics, and chemistry. The subsequent coupling of the stratosphere with the troposphere (via radiative and dynamical pathways) is considered to produce indirect climate forcing by solar irradiance.
Knowledge of variations in solar spectral irradiance is much poorer than for total solar irradiance. Observations have been made primarily in the ultraviolet spectrum, for only one decade, at wavelengths less than 400 nm. Estimates of variations in the visible and infrared regions have thus far relied on models of the wavelength dependence of the competing sunspot and faculae influences. Only the recently launched SORCE spacecraft has the capability to measure the solar spectral irradiance with the needed long-term precision. Preliminary data already raise questions about the modeled infrared spectrum variability (Fontenla et al., 2003). Whereas current understanding is that faculae are dark in the IR spectrum, SORCE observes increased IR irradiance when faculae are present on the Sun.
Ionization and Production of Cloud Condensation Nuclei
Galactic cosmic rays have one billionth of the total solar irradiance energy, but can reach the troposphere where they produce ions that may serve as nuclei for cloud condensation, with subsequent climatic impacts.
Because the heliosphere influences their transport, cosmic rays exhibit fluctuations that mirror solar activity. When solar activity is high, the more complex magnetic configuration of the heliosphere and the solar wind that flows through it from the Sun to the Earth reduce the cosmic ray flux. There is a close inverse correspondence with solar activity of products of the collision of cosmic ray particles with particles in the Earth’s atmosphere (smaller pions, muons), which ground-based neutron monitors have been measuring since the 1950s. The approximate 15 percent modulation of cosmic ray flux by solar activity produces an energy change less than one millionth of the energy change in the 0.1 percent total solar irradiance cycle.
Cosmic rays also interact with air nuclei to produce isotopes such as 14C in tree-rings and 10Be in ice cores. Fluctuations in the 14C and 10Be records (which are superimposed on the larger variations associated with changes in the Earth’s magnetic field) are believed to reflect primarily changes in long-term solar activity (Stuiver, 1965; Beer et al., 1990), although climate effects cannot be ruled out (Lal, 1988). During the last 100 years, the 10Be record suggests a 15 percent overall decline in cosmic ray flux.
By altering the population of cloud condensation nuclei and hence microphysical cloud properties (droplet number and concentration), cosmic rays may induce processes analogous to the indirect effect of tropospheric aerosols (Carslaw et al., 2002). Since the plasma produced by cosmic ray ionization in the troposphere is part of an electric circuit that extends from the Earth’s surface to the ionosphere, cosmic rays may also affect thunderstorm electrification (Carslaw et al., 2002).
Analysis of cloud cover data reveals decadal variations apparently related to solar-modulated galactic cosmic ray fluxes (Svensmark and Friis-Christensen, 1997), but because solar activity modulates both cosmic ray fluxes and solar irradiance, it is difficult to distinguish which forcing mechanism is responsible for such empirical evidence. The evidence can readily be reinterpreted as association of solar irradiance and cloud cover (Udelhofen and Cess, 2001; Kristjánsson et al., 2002). Using 16.5 years of cloud cover data from the International Satellite Cloud Climatology Project (ISCCP), Kristjánsson et al. (2002) found that low cloud cover correlates better and more consistently with total solar irradiance than with galactic cosmic rays. The data suggest that solar irradiance variations are amplified by interactions with sea surface temperature, which in turn interacts with low cloud cover. In another study, Udelhofen and Cess (2001) found a high coherence between cloud cover inferred from ground-based observations and solar variability over the United States from 1900 to 1987 (but of opposite phase to that found in the ISCCP low clouds). Using cloud coverage simulated by a climate model, they found cloud cover variations in phase with solar
variability but not with the galactic cosmic ray flux. They suggest that the cloud variability is affected by a modulation of the atmospheric circulation resulting from variations in the solar-UV-ozone-induced heating of the atmosphere.
Orbital Variation
Earth’s distance from the Sun does not remain constant at 1 AU. Rather, the eccentricity of Earth’s orbit (currently 0.0167) and the tilt of its axis relative to the orbital plane result in continual changes in the amount and distribution of solar electromagnetic radiation that the Earth receives. In modern times this variation is ±3.5 percent during the year, with maximum energy and minimum distance in January.
Indirect Effects Through the Stratosphere
Of the Sun’s mean total radiative output of 1365 W m−2, 15 W m−2 (~1 percent) of energy is in the ultraviolet spectrum and does not reach the Earth’s surface (e.g., Lean et al., 1997). This energy is deposited in the stratosphere, where it drives ozone formation (and also destruction). Although unavailable for direct forcing of climate, it may induce indirect climate effects as a result of radiative and dynamical coupling of the stratosphere and troposphere. The regional pattern of such indirect climate forcing is likely quite different from the effects of direct surface heating by solar radiation.
The effect of solar cycle UV irradiance changes on stratospheric ozone are now relatively well established as a result of extensive space-based datasets that span more than two solar activity cycles (McCormack et al., 1997). As Figure 2-7 illustrates, the 11-year cycle of ~1 percent peak-to-peak amplitude in middle UV radiation is associated with a 2 to 3 percent modulation of global total atmospheric ozone. The solar UV-induced ozone effects vary with geographic location and altitude, and appear to induce a significant tropopause response (Hood, 2003).
As with tropospheric climate, solar-induced ozone changes occur simultaneously with other natural and anthropogenic effects that must be understood and quantified in order to isolate the solar component (Jackman et al., 1996; Geller and Smyshlyaev, 2002). Most evident is a long-term downward trend in total ozone concentrations associated with increasing concentrations of CFCs. The 11-year solar cycle is superimposed on this trend (Figure 2-7), as are the influences of volcanic aerosols (which warm the stratosphere while cooling the surface), greenhouse gas increases (which
cool the stratosphere while warming the surface), and internal variability modes (in particular the quasi-biennial oscillation).
Energetic particles (1 to 100 MeV) produced during eruptive solar events can also produce significant episodic ozone depletion, primarily at higher latitudes (where the particles preferentially enter the Earth’s atmosphere) and for relatively short periods (days). Ozone depletion arises from the odd nitrogen chemical destruction cycle that the particles initiate (Jackman et al., 2001). These depletion events, whose frequency and strength vary with solar activity, are superimposed on the more sustained solar UV radiation-induced ozone changes that occur during the 11-year solar cycle.
The extent to which solar UV radiation and energetic particle effects
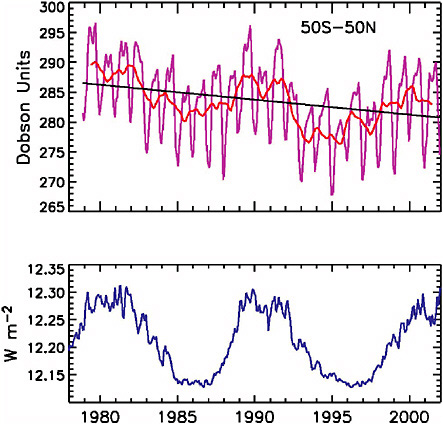
FIGURE 2-7 Top panel: global total atmospheric ozone observed by the TOMS satellite (McCormack et al., 1997). Bottom panel: solar ultraviolet irradiance observed at 200-295 nm (Lean et al., 1997).
have indirect climatic impacts depends on the coupling of the stratosphere with the troposphere. Both radiative and dynamical couplings are surmised (Figure 2-8). Since ozone absorbs electromagnetic radiation in the UV, visible, and IR spectral regions, changes in ozone concentration can affect Earth’s radiative balance by altering both incoming solar radiation and outgoing terrestrial radiation. Simulations of this effect (Lacis et al., 1990) show (Figure 2-8) that the net change of surface temperature depends on
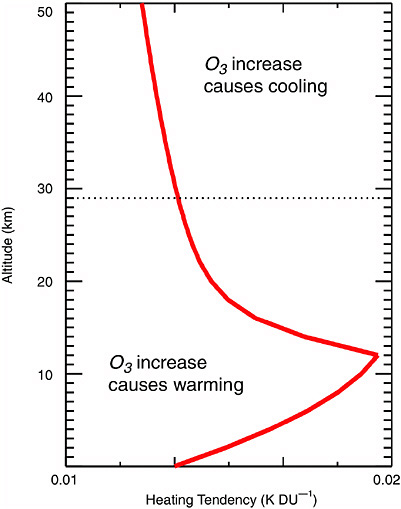
FIGURE 2-8 Change in surface temperature resulting from a change in ozone concentration as a function of altitude. SOURCE: adapted from Lacis et al. (1990).
the altitude of the ozone change; increases below 29 km produce surface warming, and increases above 29 km produce surface cooling. Model simulations suggest that such radiative coupling effects can alter the strength of the Hadley cell circulation, with attendant effects on, for example, Atlantic hurricane flows (Haigh, 2003).
Solar-induced indirect effects on climate may also involve altered modes of variability. Model simulations and analyses of patterns of variability suggest that the Arctic Oscillation (AO), or Northern Annular Mode (NAM), and its subset the North Atlantic Oscillation (NAO) propagate from the stratosphere to the troposphere (Baldwin and Dunkerton, 1999). Radiative forcings that impact the stratosphere could alter this coupling. Contemporary observations suggest that the NAM manifests itself primarily in the North Atlantic sector, as the NAO, during solar cycle minima, and extends more uniformly over all longitudes, as the AO, during solar maxima (Kodera, 2002). The effect of the Sun on the NAM may further depend on the phase of the quasi-biennial oscillation (QBO) in stratospheric equatorial winds (Ruzmaikin and Feynman, 2002). Reduced solar activity in the Maunder Minimum may have produced a negative NAO phase (compared with the current positive phase), based on empirical analysis of historical surface temperature fields and model simulations (Shindell et al., 2001b). Additional evidence that the phase of the QBO changes with the solar cycle (Salby and Callaghan, 2000) underscores the complicated, multifaceted nature of indirect solar effects on climate.
VOLCANIC ERUPTIONS
Emissions from volcanic eruptions have multiple effects on climate as listed in Table 2-3 (Robock, 2002). A number of studies have evaluated the role of volcanic forcing in climate change during the twentieth and earlier centuries (Free and Robock, 1999; Crowley, 2000; Bertrand et al., 2002; Bauer et al., 2003). These studies suggest that volcanic forcing is the dominant source of natural global radiative forcing over the past millennium. The greater prevalence of explosive volcanic activity during both the early and the late twentieth century and the dearth of eruptions over the interval from 1915 to 1960 represents a significant natural radiative forcing of twentieth century climate (e.g., Crowley, 2000). Similarly, the longer-term volcanic radiative forcing has been associated with a significant long-term forced cooling from A.D. 1000 to A.D. 1900 resulting from a general increase in explosive volcanic activity in later centuries (Crowley, 2000; Bertrand et al., 2002; Bauer et al., 2003; Crowley et al., 2003; Hegerl et al., 2003). Some spatially resolved simulations of volcanic forcing indicate a large continental summer cooling but a tendency for a dynamically induced, offsetting winter warming (Stenchikov et al., 2002; Shindell et al.,
TABLE 2-3 Effects of Large Explosive Volcanoes on Weather and Climate
|
Effect and mechanism |
Begins |
Duration |
|
Reduction of diurnal cycle |
Immediately |
1-4 days |
|
Blockage of shortwave and emission of longwave radiation |
|
|
|
Reduced tropical precipitation |
1-3 months |
3-6 months |
|
Blockage of shortwave radiation, reduced evaporation |
|
|
|
Summer cooling of Northern Hemisphere tropics and subtropics |
1-3 months |
1-2 years |
|
Blockage of shortwave radiation |
|
|
|
Reduced Sahel precipitation |
1-3 months |
1-2 years |
|
Blockage of shortwave radiation, reduced land temperature, reduced evaporation |
|
|
|
Stratospheric warming |
1-3 months |
1-2 years |
|
Stratospheric absorption of shortwave and longwave radiation |
|
|
|
Winter warming of Northern Hemisphere continents |
6-18 months |
1 or 2 winters |
|
Stratospheric absorption of shortwave and longwave radiation, dynamics |
|
|
|
Global cooling |
Immediately |
1-3 years |
|
Blockage of shortwave radiation |
|
|
|
Global cooling from multiple eruptions |
||
|
Blockage of shortwave radiation |
Immediately |
Up to decades |
|
Ozone depletion, enhanced UV radiation |
|
|
|
Dilution, heterogeneous chemistry on aerosols |
1 day |
1-2 years |
|
SOURCE: Robock (2000). |
||
2003). This result contrasts with the response to solar forcing, for which the dynamical and radiative responses appear to reinforce constructively.
Past histories of radiative forcing by explosive volcanic activity are typically constructed from sulfate aerosols contained in annual ice core layers (e.g., Robock, 2000). Spikes of sulfate in the ice core records reflect volcanic injection to the lower stratosphere, where the lifetime is a year or longer, allowing transport to polar regions and eventual deposition after subsidence to the troposphere (Robock and Free, 1995). The longer the residence time of the aerosol in the lower stratosphere, the greater is the associated negative shortwave radiative forcing of the surface through the
reflection of radiation back to space. Assumptions must be made regarding the relationship between the sulfate aerosol deposited at the surface and the extent and duration of a significant stratospheric dust veil. These assumptions are highly uncertain and can be only partially tested for a few recent eruptions (e.g., Stenchikov et al., 1998). Greater concentrations of trapped sulfates are typically indicative of larger eruptions, although the proximity of the source region to the ice core may be a complicating factor. Explosive tropical eruptions are more likely to impart a significant global radiative forcing because they provide an opportunity for the aerosol to spread throughout the global lower stratosphere. An eruption is assumed to have occurred in the tropics if its aerosols are recorded in ice cores at both poles.
Other indices of past volcanic activity have also been developed in past work. These include the Volcanic Explosivity Index, or VEI, which is based on qualitative volcanological information and should therefore be used with caution in studies seeking quantitative estimates of climate response (Robock and Free, 1995), and the Dust Veil Index (DVI; see, e.g., Robock, 2000). Some authors argue that the use of climate information in some DVI estimates leads to a potential circularity in using this index to diagnose climate response. Tree-ring reconstructions of continental summer temperature variations have also been used to estimate past volcanic forcing histories (Briffa et al., 1998), although a similar circularity obviously exists if the associated volcanic histories are used to diagnose the climate response to volcanic forcing. Zielinski (2000) and Robock (2000) provide excellent reviews and critiques of various indices of past explosive volcanic activity.
Ice core volcanic radiative forcing estimates have been developed for the past century to the past couple of millennia by numerous researchers (Robock and Free, 1995, 1996; Robertson et al., 1998; Robock, 2000; Crowley, 2000; Ammann et al., 2003; Crowley et al., 2003). The choice of ice cores used to define the volcanic forcing chronology leads to some significant differences among these different estimates. Some of the estimates assume that tropical eruptions dominate the annual global mean radiative forcing (e.g., Free and Robock, 1999; Crowley, 2000), whereas other reconstructions seek to take into account the influence of the latitudinal and seasonal characteristics of the eruptions (Robertson et al., 1998; Ammann et al., 2003).
OCEAN COLOR
Recognized biologically related surface forcings associated with the oceans include ocean color as well as biogenic aerosol emissions (e.g., dimethyl sulfide). Ocean color refers to the radiance backscattered at the air-sea interface. It is determined by water molecules (the blue wavelengths in particular), phytoplankton and detrital particles, and nonbiogenic sedi-
ments in coastal waters (Yoder et al., 2001). Bacteria, viruses, colloids, and small bubbles are also possible contributors.
Shell et al. (2003) used a global climate model to assess the role of ocean color in the sea surface temperature and other aspects of the climate system. They found that phytoplankton warm the surface by about 0.05°C on a global average basis. They also found that the large-scale atmospheric circulation is significantly affected by regional alterations of ocean color. These results suggest that the radiative effects of phytoplankton should not be overlooked in studies of climate change.
Frouin and Iacobellis (2002) also determined that absorption of sunlight by phytoplankton must be included in the global radiation budget. They estimated that, compared to pure seawater, the globally and annually averaged outgoing radiative flux is decreased by 0.25 W m−2 due to ocean phytoplankton. In coastal and high-latitude regions, the forcing can reach around 1.5 W m−2. They also found that the amount absorbed was species dependent.
TELECONNECTIONS AND RADIATIVE FORCING
Linkages between weather or climate changes occurring in widely separated regions of the globe are referred to as teleconnections. The extent to which regionally concentrated radiative forcing can affect climate via teleconnections is a matter of current research. Determining the importance of regional forcings, such as those from aerosols or land-use change, requires an understanding of the role of teleconnections that can lead forcings in one region to have effects on other regions far away. Teleconnections are most commonly thought of with respect to the transport of energy by atmospheric waves (Tsonis, 2001). For example, regional and global weather patterns have been associated with sea surface temperature anomalies (e.g., Hoerling and Kumar, 2003). Radiative and nonradiative forcing due to regional land-use change can also result in large differences in atmospheric circulation patterns at large distances from the landscape disturbance. For example, land-use change can alter deep cumulonimbus patterns, which affect atmospheric circulation in distant regions (Chase et al., 2000a).
Avissar and Werth (2005) found that deforestation of tropical regions, through teleconnections similar to those produced during El Niño events, has a significant impact on the rainfall of other regions. In particular, they found that the U.S. Midwest is the continental region the most negatively affected by the deforestation of Amazonia and Central Africa during spring and summer, when rainfall decrease could severely damage agricultural productivity in that region. These results are summarized in Figure 2-9. Avissar and Werth (2005) conclude that tropical deforestation considerably
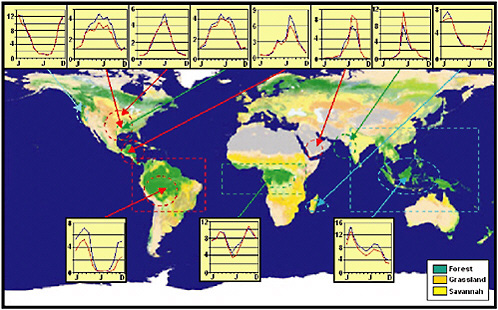
FIGURE 2-9 Annual cycle of precipitation (mm day−1) in continental regions particularly affected by the deforestation of Amazonia (red), Central Africa (green), and Southeast Asia (blue). The blue curves represent the mean monthly precipitation before massive deforestation started in tropical regions (i.e., the “control” case). The red curves indicate the corresponding precipitation following tropical deforestation. The size and location of the color-coded areas corresponding to the deforested regions are at scale. Color-coded ellipses indicate the regions in which tropical forest (in green on the 1-km resolution land-cover map used for the background) was replaced with a mixture of shrubs and grassland. SOURCE: Avissar and Werth (2005).
alters the sensible and latent heat released into the atmosphere and the associated change of pressure distribution modifies the zones of atmospheric convergence and divergence, which shift the typical pattern of the Polar Jet Stream and the precipitation that it engenders.
Radiative forcing by aerosols has also been associated with teleconnected responses in distant locations. For example, a GCM simulation by Chung and Ramanathan (2003) shows that absorbing aerosols over South Asia and the North Indian Ocean can cause subsidence motions over most of the tropics, which would have a drying effect (Figure 2-10).
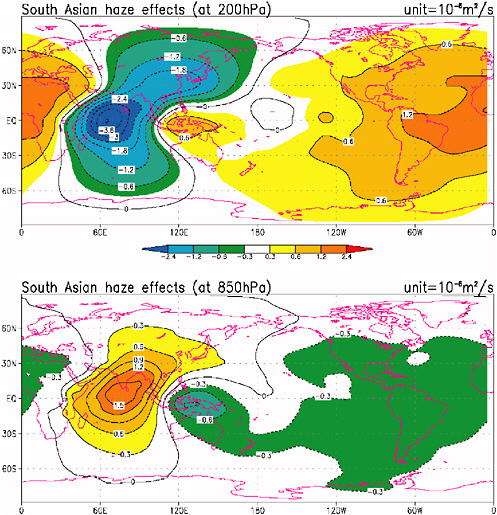
FIGURE 2-10 Velocity potential for the lower troposphere (850 hPa or about 1.5 km) in the lower panel and for the upper troposphere (200 hPa) in the upper panel. The solar heating by absorbing aerosols, mainly due to black carbon, is concentrated over South Asia and the North Indian Ocean (i.e., over the red shaded regions in the lower panel). The red region in the lower panel shows areas of convergence of air or alternately rising motions in response to the solar heating of the lower atmosphere by black carbon. The convergence at the lower levels is followed by divergence (air flowing out of the region) at the upper levels (the blue shaded region in the upper troposphere) over the source region. SOURCE: Adapted from Chung and Ramanathan (2003).



































