Biomimetic Strategies in Vascular Tissue Engineering
JENNIFER L. WEST
Departments of Chemical Engineering and Bioengineering
Rice University
Houston, Texas
INTRODUCTION
Cardiovascular disease, the leading cause of death in the United States, claims more lives each year than the next five leading causes of death combined (American Heart Association, 2003). Coronary heart disease caused more than one in every five American deaths in 2000 and required approximately 500,000 coronary artery bypass graft surgeries (CABGs) that year. Bypass grafting is also used in the treatment of aneurysmal disease or trauma.
At present, surgeons use autologous tissue and synthetic biomaterials as vascular grafts. Transplantation of autologous tissue has the best outcome in applications such as CABG because synthetic grafts, such as those made from expanded polytetrafluoroethylene or polyethylene terepthalate, fail in small-diameter applications (ID < 6 mm) due to the formation of blood clots and scar tissue. However, the supply of autologous tissue is often limited, either because of prior procedures or because of peripheral vascular disease. Recent advances in tissue engineering have raised hope that substitutes for blood vessels may one day be fabricated for small-diameter applications, such as CABG, where treatment options are often severely limited.
TISSUE ENGINEERING
Tissue engineering is the application of engineering principles to the design of tissue replacements, usually formed from cells and biomolecules. Tissue-
engineered products are already commercially available for skin and cartilage. Typically, an engineered tissue is formed by harvesting a small sample of the patient’s cells, expanding them in culture, then seeding the cells onto a scaffold material. Scaffold materials, usually biodegradable synthetic polymers, are intended to define the size and shape of the new “tissue” and to provide mechanical support for the cells as they synthesize the new tissue. The cell-seeded scaffolds can then either be implanted into the patient, with tissue formation occurring in situ, or cultured further in vitro until their properties are more similar to those of normal tissue before implantation. This culture period is often carried out in a bioreactor to provide appropriate mechanical conditioning during tissue formation.
Most tissue-engineering strategies attempt to create small-caliber vascular grafts by closely mimicking the structure, function, and physiologic environment of native vessels. Normal arteries have three distinct tissue layers (Figure 1): the intima, the media, and the adventitia. The intima consists of a monolayer of endothelial cells that prevents platelet aggregation and regulates vessel permeability, vascular smooth muscle cell behavior, and homeostasis. In the medial layer, smooth muscle cells and elastin fibers aligned circumferentially provide most of the vessel’s mechanical strength (Wight, 1996). The adventitial layer contains fibroblasts, connective tissue, the microvascular supply, and a neural
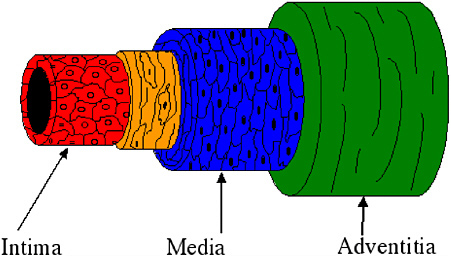
FIGURE 1 The arterial wall is composed of three distinct layers: the intima, the media, and the adventitia. The intima, composed of endothelial cells, provides a nonthrombogenic surface. In the medial layer, smooth muscle cells and elastin fibers align circumferentially to provide mechanical integrity and contractility. The outer layer, the adventitia, is a supportive connective tissue.
network that regulates the vasotone of the blood vessel. The re-creation of some or all of the vessel layers and their properties may result in the development of a patent, functional vascular graft. In all likelihood, a successful graft will require an intima and a media.
As described above, the creation of a tissue-engineered vascular graft (TEVG) usually involves the harvest of desired cells, cell expansion in culture, cell seeding onto a scaffold, culture of the construct in an environment that induces tissue formation, and implantation of the construct back into the patient. Many options must be considered for each step in this process.
Efforts to create TEVGs remain in an early developmental stage, but several potential problems have already been identified. The patency of the graft is threatened by thrombosis, in all likelihood due to the retention of endothelial cells after implantation or alterations in endothelial cell function after culture in vitro. Also, the possibility of bursting after implantation in the physiological flow environment would have catastrophic consequences. Mechanical properties of TEVGs are generally lower than those of native arteries. Thus, a number of approaches are being tried to improve them. All of the strategies discussed above—cell source, genetic modification, scaffold materials, and culture conditions—are likely to impact the fabrication of an optimal, clinically useful TEVG.
Cell Sources for Vascular Tissue Engineering
The development of a functional TEVG is likely to require the construction of an intima and media composed of endothelial and smooth muscle cells. Limitations imposed by immunogenicity will probably require that autologous cells be used, so the majority of studies to date have used differentiated smooth muscle and endothelial cells isolated from harvested blood vessels. But problems with donor-site morbidity and the performance of these cell types in engineered tissues have led to the consideration of alternative cell sources. Recent advances in stem cell biology may lead to suitable progenitors that can be effectively differentiated into endothelial and smooth muscle cells for use in vascular tissue engineering.
Genetic Modification of Vascular Cells
Genetic engineering of vascular cells ex vivo may be an effective strategy for improving the properties of tissue-engineered grafts. The leading cause of failure in vascular grafts has been attributed to thrombosis, or the formation of blood clots. Seeding small-diameter vascular graft constructs with cells genetically engineered to secrete anti-thrombotic factors may improve graft patency rates.
A platelet aggregation inhibitor, nitric oxide (NO), for example, has shown promising results. Using an ex vivo approach, bovine smooth muscle cells
liposomally-transfected with NO synthase III (NOS III) and GTP cyclohydrolase, which produces a cofactor essential for NOS activity, were grown as monolayers on plastic slides or biomaterials of interest and then placed in a parallel plate flow chamber. Whole blood was introduced into the flow chamber to assess platelet adherence to the cell monolayers. The number of platelets that adhered to the NOS-transduced smooth muscle cells was significantly lower than the number that adhered to mock-transduced smooth muscle cells and similar to the number that adhered to cultured endothelial cells (Scott-Burden et al., 1996).
The genetic modification of cells used to seed the TEVG may prove beneficial to improving the mechanical properties of TEVGs, which are related in large part to the composition and structure of the extracellular matrix (ECM). ECM cross-linking, which can result from the enzymatic activity of lysyl oxidase (LO) (Aeschlimann and Paulsson, 1991), may be a means of improving mechanical properties of the TEVG. LO, a copper-dependent amine oxidase, forms lysine-derived cross-links in connective tissue, particularly in collagen and elastin (Rucker et al., 1998). A gene therapy strategy has demonstrated that the mechanical properties of tissue-engineered collagen constructs are enhanced by using vascular smooth muscle cells transfected with LO (Elbjeirami et al., 2003). The elastic modulus and ultimate tensile strength of collagen gels seeded with LO-transfected smooth muscle cells nearly doubled compared to gels seeded with mock-transfected smooth muscle cells. The improved mechanical properties resulted from increased ECM cross-linking rather than increased amounts of ECM, changes in ECM composition, or increased cellularity. This strategy may ultimately lead to the enhancement of the mechanical characteristics of TEVGs and minimize the time required for in vitro culture prior to implantation.
Scaffolds for Vascular Tissue Engineering
Tissue engineers have had to choose either natural (e.g., collagen; Weinberg and Bell, 1986) or synthetic (e.g., polyglycolic acid; Niklason et al., 1999) polymer scaffolds. Each has advantages and disadvantages. Ideally, there would be specific cell-material interactions (like the interactions between cells and collagen), as well as control over material properties and the ease of processing that synthetic polymers offer.
Therefore, biomimetic derivatives of polyethylene glycol (PEG) are being studied as scaffolds for vascular tissue engineering. PEG-based materials are hydrophilic, biocompatible, and intrinsically resistant to protein adsorption and cell adhesion (Gombotz et al., 1991; Merrill and Salzman, 1983). Thus, PEG essentially provides a “blank slate,” devoid of biological interactions, upon which the desired biofunctionality can be built. Because aqueous solutions of acrylated PEG can be rapidly photopolymerized in direct contact with cells and tissues (Hill-West et al., 1994; Sawhney et al., 1994), this is an easy method of cell seeding. Furthermore, PEG-based materials can be rendered bioactive by
including proteolytically degradable peptides in the polymer backbone (West and Hubbell, 1999) and by grafting adhesion peptides (Hern and Hubbell, 1998) or growth factors (Mann et al., 2001a) into the hydrogel network during the photopolymerization process. Recently, PEG hydrogels that largely mimic the properties of collagen have been developed (Gobin and West, 2002; Mann et al., 2001b).
The elastin-derived peptide VAPG has been shown to be specific for smooth muscle cell adhesion, and PEG hydrogels modified with this adhesive peptide, rather than RGDS, support adhesion and the growth of vascular smooth muscle cells but not fibroblasts or platelets (Gobin and West., 2003). Moreover, bioactive molecules like TGF-β may be covalently incorporated into scaffolds to induce protein synthesis by vascular smooth muscle cells.
TGF-β has been reported to stimulate the expression of several matrix components, including elastin, collagen, fibronectin, and proteoglycans (Amento et al., 1991; Lawrence et al., 1994). TGF-β covalently immobilized to PEG-based hydrogels significantly increased collagen production of vascular smooth muscle cells seeded within these scaffold materials (Mann et al., 2001a). Mechanical testing of these engineered tissues also determined that the elastic modulus was higher in TGF-β-tethered PEG scaffolds than in PEG scaffolds without TGF-β, indicating that material properties for TEVGs may be improved using this technology. A cell-seeded graft formed from this biomimetic hydrogel scaffold is shown in Figure 2.
By using these types of bioactive materials, scientists may be able to capture the advantages of a natural scaffold, such as specific cell-material interactions and proteolytic remodeling in response to tissue formation, and take advantage of the benefits of a synthetic material, namely the ease of processing and the ability to manipulate mechanical properties.

FIGURE 2 A PEG-based scaffold seeded with smooth muscle cells and endothelial cells ready for insertion into a bioreactor for in vitro culture of a TEVG (left, top view; right, side view). The cell-seeded scaffold is formed via photopolymerization, so the dimensions can be easily tailored for a given application, and cells are homogeneously seeded throughout the material. The scaffold is designed to degrade in response to cellular proteolytic activity during tissue formation.
Bioreactors for Mechanical Conditioning
In vivo, the pulsatile nature of blood flow puts radial pressure on the vessel wall, which subjects smooth muscle cells within the medial layer to cyclic strain. Thus, a great deal of research has focused on the response of smooth muscle cells to cyclic stretching. This research has demonstrated the importance of including cyclic strain during the fabrication of vascular tissue, particularly with respect to ECM synthesis and tissue organization. For example, smooth muscle cells seeded on purified elastin membranes and exposed to two days of cyclic stretching (10 percent beyond resting length) align perpendicular to the direction of applied strain; they also incorporate hydroxyproline into protein three to five times more rapidly than stationary controls, indicating increased collagen synthesis in response to strain (Leung et al., 1976).
Considering the profound effects of cyclic strain on the orientation of smooth muscle cells, ECM production, and tissue organization, preculture of vascular graft constructs in a pulsatile flow bioreactor system may help recreate the natural structure of native vessels and improve the mechanical properties of the construct. The mechanical stimuli from pulsatile flow could generate the cyclic strain necessary to alter ECM production, thereby creating a histologically organized, functional construct with satisfactory mechanical characteristics for implantation. Figure 3 is a schematic drawing of a typical pulsatile flow bioreactor system.
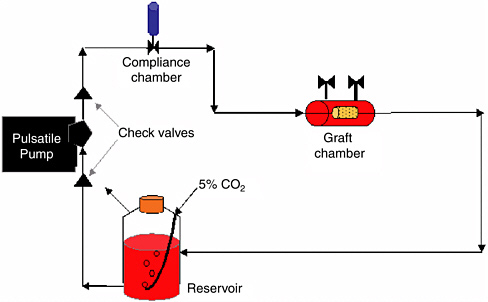
FIGURE 3 Diagram of a typical pulsatile-flow bioreactor for the culture of TEVGs. The pulse frequency and amplitude can be controlled via the pump, and the resultant strain environment can be controlled by altering the mechanical properties of the scaffold material.
To develop a blood vessel substitute, Niklason et al. (1999) cultured PGA constructs in a pulsatile blow bioreactor generating 165 beats per minute (bpm) and 5 percent radial strain. The pulse frequency of this system was chosen to mimic a fetal heart rate, which was believed to provide optimal conditions for the formation of new tissue. However, most investigations on mechanical conditioning have been conducted at 60 bpm, more representative of an adult heart rate, with promising outcomes. Therefore, the optimal bioreactor culture conditions for the development of a TEVG have not yet been determined. Nevertheless, this technology shows a great deal of promise for the production of a blood vessel substitute with the necessary mechanical and biochemical components.
CONCLUSION
Although a great deal of progress has been made on the creation of TEVGs, many challenges remain—particluarly preventing thrombosis and improving the mechanical properties of the graft. The development of a patent TEVG that grossly resembles native tissue now requires more than eight weeks of culture time. Thus, even with advances in the field, TEVGs are not likely to be used in emergency situations because of the time necessary for cell expansion, ECM production and organization, and the attainment of desired mechanical strength. Furthermore, unless advances in immune acceptance render the use of allogenic and xenogenic tissues feasible, autologous tissues will continue to be necessary to prevent an immunogenic response. TEVGs have not yet been subjected to clinical trials to determine their long-term efficacy. At that point, off-the-shelf availability and cost will be the biggest hurdles to the development of a feasible TEVG product.
Despite the many obstacles that must be overcome, the potential benefits of small-diameter TEVGs are exciting. In the near future, a non-thrombogenic TEVG with sufficient mechanical strength for clinical trials may be developed. Such a graft will have the minimum characteristics of biological tissue necessary to remain patent for a time comparable to current vein graft therapies.
As science and technology advance, TEVGs may evolve into complex blood vessel substitutes. They may become living grafts, capable of growing, remodeling, and responding to mechanical and biochemical stimuli in the surrounding environment. These blood vessel substitutes will closely resemble native vessels in their structure, composition, mechanical properties, and function. They will also have vasoactive properties, that is, they will be able to dilate and constrict in response to stimuli.
Close mimicry of native blood vessels may ultimately also be important in the engineering of other tissues that depend on vasculature to sustain function. As our understanding of the factors involved in cardiovascular development and function improves, we may one day develop TEVGs that will greatly improve the lives of people with vascular disease and other life-threatening conditions.
REFERENCES
Aeschlimann, D., and M. Paulsson. 1991. Cross-linking of laminin-nidogen complexes by tissue transglutaminase: a novel mechanism for basement membrane stabilization. Journal of Biological Chemistry 266(23): 15308–17.
Amento, E.P., N. Ehsani, H. Palmer, and P. Libby. 1991. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arteriosclerosis and Thrombosis 11(5): 1223–1230.
American Heart Association. 2003. Heart Disease and Stroke Statistics: 2003 Update. Available online at: <http://www.americanheart.org/presenter.jhtml?identifier=4439>.
Elbjeirami, W.M., E.O. Yonter, B.C. Starcher, and J.L. West. 2003. Enhancing mechanical properties of tissue engineered constructs via lysyl oxidase crosslinking activity. Journal of Biomedical Materials Research 66A: 513–521.
Gobin, A.S., and J.L. West. 2002. Cell migration through defined, synthetic extracellular matrix analogues. The Federation of American Societies for Experimental Biology Journal 16(7): 751–753.
Gobin, A.S., and J.L.West. 2003. Val-ala-pro-gly, an elastin-derived non-integrin ligand: smooth muscle cell adhesion and specificity. Journal of Biomedical Materials Research 67A(1): 255–259.
Gombotz, W.R., W. Guanghui, T.A. Horbett, and A.S. Hoffman. 1991. Protein adsorption to poly(ethylene oxide) surfaces. Journal of Biomedical Materials Research 25(12): 1547–1562.
Hern, D.L., and J.A. Hubbell. 1998. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. Journal of Biomedical Materials Research 39(2): 266–276.
Hill-West, J.L., S.M. Chowdhury, A.S. Sawhney, C.P. Pathak, R.C. Dunn, and J.A. Hubbell. 1994. Prevention of postoperative adhesions in the rat by in situ photopolymerization of bioresorbable hydrogel barriers. Obstetrics and Gynecology 83: 59–64.
Lawrence, R., D.J. Hartmann, and G.E. Sonenshein. 1994. Transforming growth factor β1 stimulates type V collagen expression in bovine vascular smooth muscle cells. Journal of Biological Chemistry 269(13): 9603–9609.
Leung, D.Y.M., S. Glagov, and M.B. Mathews. 1976. Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science 191(4226): 475–477.
Mann, B.K., R.H. Schmedlen, and J.L. West. 2001a. Tethered-TGF-β increases extracellular matrix production of vascular smooth muscle cells. Biomaterials 22 (5): 439–444.
Mann, B.K., A.S. Gobin, A.T. Tsai, R.H. Schmedlen, and J.L. West. 2001b. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: synthetic ECM analogs for tissue engineering. Biomaterials 22(22): 3045–3051.
Merrill, E.A., and E.W. Salzman. 1983. Polyethylene oxide as a biomaterial. American Society of Artificial Internal Organs Journal 6(2): 60–64.
Niklason, L.E., J. Gao, W.M. Abbott, K.K. Hirschi, S. Houser, R. Marini, and R. Langer. 1999. Functional arteries grown in vitro. Science 284(5413): 489–493.
Rucker, R.B., T. Kosonen, M.S. Clegg, A.E. Mitchell, B.R. Rucker, J.Y. Uriu-Hare, and C.L. Keen. 1998. Copper, lysyl oxidase and extracellular matrix protein cross-linking. The American Journal of Clinical Nutrition 67(5)(Suppl): 996S–1002S.
Sawhney, A.S., C.P. Pathak, and J.A. Hubbell. 1993. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(a-hydroxy acid) diacrylate macromers. Macromolecules 26: 581–587.
Scott-Burden, T., C.L. Tock, J.J. Schwarz, S.W. Casscells, and D.A. Engler. 1996. Genetically engineered smooth muscle cells as linings to improve the biocompatibility of cardiovascular prostheses. Circulation 94(Suppl II): II235–II238.
Weinberg, C.B., and E. Bell. 1986. A blood vessel model constructed from collagen and cultured vascular cells. Science 231(4736): 397–400.
West, J.L., and J.A. Hubbell. 1999. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules 32(1): 241–244.
Wight, T.N. 1996. Arterial Wall. Pp. 175–202 in Extracellular Matrix, Vol.1, edited by W.D. Comper. Amsterdam, Netherlands: Harwood Academic Publishers.










