Appendix J
Recent Findings on Test Performance Brian P.Mulhall, M.D., M.P.H.
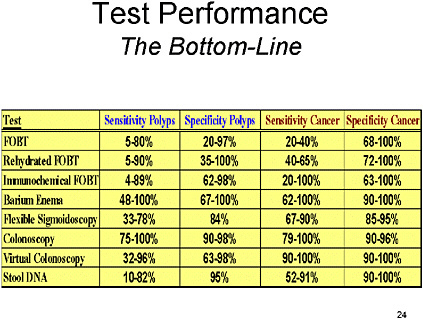
SLIDE 1

SLIDE 1 NOTES: I was asked to cover recent evidence on test performance for all the major colorectal cancer screening tests.
I tried to take a meta-analytic approach to every test I examined, but was frustrated by the quality, quantity and heterogeneity of the various studies examining each individual test. So, my review will be largely descriptive, followed by a summary of what, in my opinion, we know about the various tests.
SLIDE 2
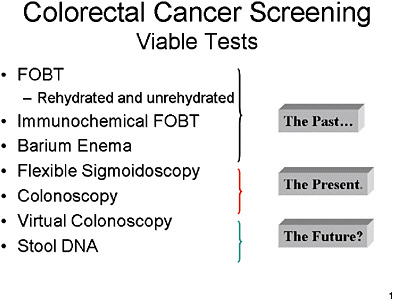
SLIDE 2 NOTES: Here is a list of what I consider tests that are viable for colorectal cancer screening.
They begin with FOBTs and barium enema. These are to some extent viewed by both clinicians and the public as part of the past.
Flexible sigmoidoscopy and, more importantly, colonoscopy, are where clinicians and the public currently see the state-of-the-art for colorectal cancer screening.
Virtual colonoscopy and Stool DNA tests are largely in the future, from an operational point of view, though they have recently received a good amount of attention in the press.
SLIDE 3
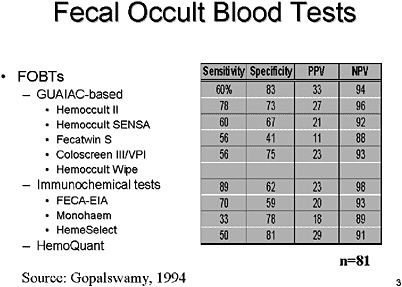
SLIDE 3 NOTES: Fecal Occult Blood Tests (FOBTs) have been in use for several decades and provide some of the best data supporting the indication for CRC screening in order to prevent colorectal cancer (Ederer et al., 1997; Mandel et al., 1993). There are over a half-dozen different tests that are GUAIAC-based and even more that have been developed using immunochemical assays to address some of the limitations of GUAIAC-based stool studies Fecal occult blood tests can also be immunological (e.g., HemoQuant).
Ideally, we would want to see a study that compares all of these various types of tests head-to-head. In the mid-1990’s Dr. Gopalswamy and colleagues attempted to do just that. (See this slide.) Unfortunately, that study suffers from a too-small sample size as well as uncertainty about the definition of the outcome measures used.
Consequently, we are left with a variety of studies of individual tests and must try to compare their outcomes.
SLIDE 4
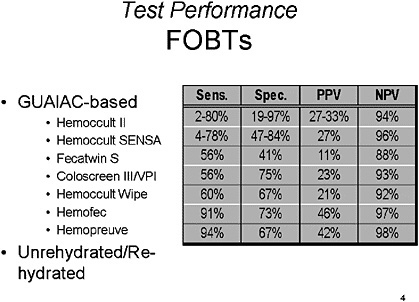
SLIDE NOTES 4: FOBTs—There is a range of results reported for the GUAIAC-based stool studies, with sensitivity for detection of polyps ranging from 5–98% and sensitivity for detection of cancer of 18–92 percent. Specificity for cancer and/or polyps ranges from 90–100 percent, but large cohort studies emphasize that a significant number of CRCs are missed by FOBTs (Bouvier et al., 2001; Lieberman et al., 2001). Results differ based on the design of the study, number of tests used, method of follow-up and the cut-off used for positive results, for example, all adenomas, adenomas > 1 cm or actual colorectal cancer (Brevinge et al., 1997; Favennec, 1992; Gopalswamy et al., 1994; Greenberg et al., 2000; Hope et al., 1996; Kewenter et al., 1988; Ko et al., 2003; Niv et al., 1995; Rockey et al., 1998; Rosen et all., 1997; Sung et al., 2003).
In general, the overall sensitivity of GUAIAC-based FOBTs is low and they are frequently positive as a result of upper gastrointestinal lesions (Lieberman et al., 2001; Rockey et al., 1998). There is no clearly superior test among the FOBT alternatives, but rehydration of FOBTs does appear to improve sensitivity in several studies but decreases the specificity (Kewenter et al., 1988; Walter et al., 1991).
SLIDE 5
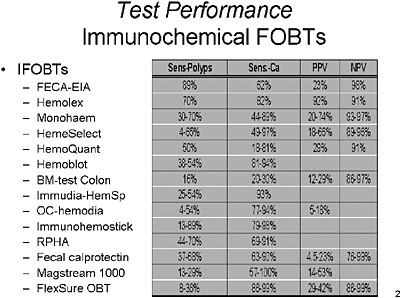
SLIDE 5 NOTES: IFOBTS—There is a broad range of results for Immunochemical FOBTs (IFOBTs) impacted by the number of studies used, the population screened, the ‘gold standard’ used and the lesion (and size) used to define a positive result (Gopalswamy et al., 1994; Greenberg et al, 2000; Ito et al, 1996; Jeanson et al, 1994; Ko et al., 2003; Miyoshi et al., 2000; Nakama et al., 1997; Nakama et al., 1999; Nakama et al., 2001; Rosen et al., 1997; Rosen et al., 2000; Rosen et al., 1995; Sieg et al., 1998; Wong et al., 2003). For IFOBTs, the range of sensitivities for detection of polyps ranges from 4–98 percent and for colorectal cancer ranges from 18–100 percent. Reported specificity for CRC is generally greater than 95 percent.
Based on the review of the literature, there is also no clearly superior IFOBT at present time, but it does appear to have improved performance characteristics compared to GUAIC-based FOBTs in a number of studies. A recent study has argued against the assertion that IFOBTs are superior to FOBTs (Ko, et al., 2003), and others have also not demonstrated a conclusive advantage (Rosen et al. 1997; Rosen et al., 2000).
SLIDE 6
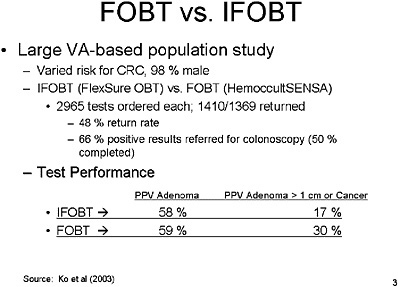
SLIDE 6 NOTES: A new article published by Ko and colleagues (Ko et al., 2003) looked at a population of veterans at varied risk for colorectal cancer, almost all male. The purpose of the study was to compare the overall performance of IFOBTs and FOBTs.
In this study, 3,000 of each test type were ordered, but only half of those in each group were returned by the patient. That equates to a 48 percent return rate. That rate is consistent with other studies in our population.
Of those who had a positive result on the test, only 66 percent were referred to colonoscopy, and of that number, only 50 percent actually received that colonoscopy. Dr. Ko’s study showed that the overall Positive Predictive Value (PPV) of IFOBT and FOBT for either adenomas or cancers was about the same. For large adenomas or cancer, IFOBT was not as good as FOBT, but the difference was not statistically significant.
SLIDE 7
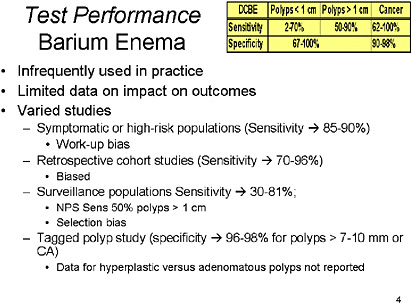
SLIDE 7 NOTES: Barium Enema—Though more widely used in the past, double-contrast barium enema (DCBE) has fallen out of favor with primary care providers, radiologists and gastroenterologists. The reason for this bias against BE is unclear, but may be related to published concerns about sensitivity (Rex et al., 1997).
Over 60 studies have looked at DCBE in a variety of settings. Studies that have examined symptomatic or high-risk patients, have determined that BE has sensitivity for polyps of 48–100 percent and for CRC of 62–100 percent (Glick et al., 1998; deZwart et al., 2001). Retrospective studies in cohorts of patients known to have CRC have shown that DCBEs done prior to the eventual diagnosis were 70–96 percent sensitive for CRC (Jensen et al., 1990; Johnson et al., 1983; Loose et al., 1974; Rex et al., 1997). Though no study has been done in a truly asymptomatic, average-risk screening population, the studies that have examined patients undergoing surveillance for polyps or in those with positive findings on previous studies have demonstrated a sensitivity of 30–81 percent, varying greatly depending on the size threshold used for detection of polyps (Steine et al., 1993; Saito et al., 1989; Brewster et al., 1994). Sensitivity for CRC was 80 percent in one study (Kewenter et al., 1995). There is limited data on the specificity of DCBE, but one study that evaluated tagged polyps determined the specificity for polyps > 10 mm to be 96 percent (Rex et al., 1986). Several additional studies showed the sensitivity to be 90–96 percent for polyps, depending on size thresholds, and for cancer or polyps the sensitivity was 98 percent in one study (Williams et al., 1974). In general, the gold standard in these studies was optical colonoscopy or flexible sigmoidoscopy (for left-sided lesions).
One study that compared DCBE to Flexible Sigmoidoscopy showed a clear superiority for Flexible Sigmoidoscopy (sensitivity for CRC 62 percent vs. 85 percent, whole bowel vs. rectosigmoid visualization, respectively) (Jensen et al., 1990). When used in combination, these studies had a sensitivity of 96 percent for all neoplasms > 1 cm and a specificity of 99 percent. Performance may be improved with multiple readings (Markus et al., 1990).
There are many biases inherent to the studies of DCBE for detection of polyps and cancer, most that would have biased estimates of sensitivity upwards (Glick et al., 1998). The most important deficit in the literature regarding this technology is the lack of studies on test performance in average-risk screening populations. Nonetheless, the studies cited suggest acceptable sensitivity and specificity that make the inadequate study of, and under-utilization of, this screening modality perplexing.
In summary, I would suggest that the sensitivity of the DCBE is somewhere in the 50–70 percent range for polyps, and perhaps higher than that for cancer. The specificity is 80 percent plus if you look at polyps and cancers combined.
SLIDE 8
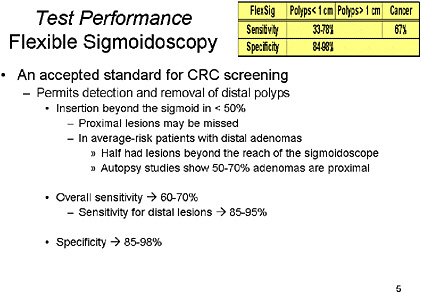
SLIDE 8 NOTES: Flexible Sigmoidoscopy: This test has long been used for CRC screening, but has obvious limitations based on the length of insertion of the traditional Flexible Sigmoidoscopy (FlexSig) endoscope. It limits examination to the rectosigmoid, as fewer than 50 percent of FlexSigs are able to examine beyond the sigmoid colon (Ott et al., 1982; Osgard et al., 1998; Painter et al., 1999; Stewart et al., 1999). A great number of well-designed studies have examined the performance of this test, and it has a proven ability to reduce the incidence of CRC and improve mortality (Brenner et al., 2001; Newcomb et al., 2003; Walsh et al. 2003; Wherry et al., 1994). As such, data from FlexSig studies has been extrapolated to support the efficacy of Colonoscopy (Khullar et al., 1997; Rex et al., 2002).
In summary, overall sensitivity is probably 60 to 70 percent, but sensitivity for distal lesions within the reach of the FlexSig, it is in the 85 to 95 percent range, and specificity is reasonably high at 85 to 98 percent.
SLIDE 9
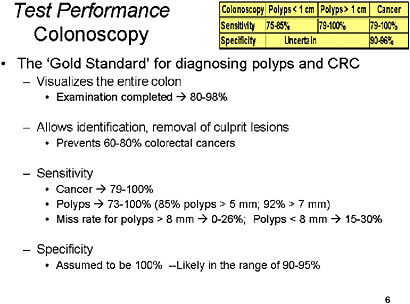
SLIDE 9 NOTES: Colonoscopy—Long considered the ‘gold standard’ for CRC screening, this test provides visualization of the entire colon and allows for removal of specimens for histopathologic assessment. Because it has been the intervention that has followed most of the aforementioned tests, the reduction in CRC and mortality from cancer has been attributed to colonoscopy.
Its sensitivity compared to radiographic studies and autopsy findings has been reported to range from 80–100 percent for CRC and from 70–100 percent for polyps (Brewster et al., 1994; Irvine et al., 1988; Pickhardt et al., 2003; Postic et al., 2002; Saito et al., 1989). Colonoscopy has a miss rate for colonic polyps ranges from 0 to 27 percent, but ranges according to size of the adenoma or cancer used as criteria and the endoscopic technique (Hixson et al., 1990; Hixson et al., 1991; Leinicke et al., 1977; Rex et al., 1997; Rex et al., 2000; Sheheda et al., 2002). The cecum cannot be reached in 0 to 18 percent of cases (Cirocco and Rusin, 1995; Lichtenstein et al., 1999; Nelson et al., 2002; Obrecht et al. 1984; Shumaker et al., 2002).
Because there has been no clear standard against which to compare colonoscopy, its true specificity has not been adequately described. However, using radiographic methods and segmentally unblinded colonoscopy, we can estimate that the specificity of colonoscopy is in the range of 90–98 percent. Use of chromoendoscopy may improve performance characteristics of colonoscopy (Eisen et al., 2002; Kiesslich et al., 2001; Lee et al., 2003; Tsuda et al., 2002).
To summarize, sensitivity for polyps is somewhat greater than 75 percent, and for cancer, closer to 100 percent. Specificity for polyps is unknown; for cancer it is in the 90–96 percent range.
SLIDE 10
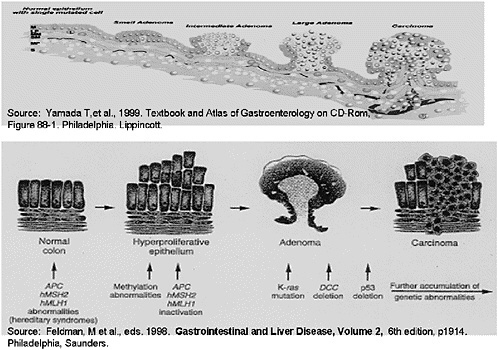
SLIDE 10 NOTES: STOOL DNA—Stool DNA is a provocative new technology. Its development was based on a number of observations about the natural history of colorectal cancer.
The progression of normal colonic mucosa through a series of mutational events that eventually leads to dysplastic and later neoplastic tissue is well-described. Dysplastic tissues exfoliate intact viable colonocytes. Those colonocytes have a representative genetic signature, and may manifest some of these abnormalities. The observation of this process has led to studies attempting to isolate either individual or combined sets of tumor markers from stool in order to define the risk of colorectal cancer in populations. Most studies to date have focused on groups with known colorectal cancer or those at higher risk.
SLIDE 11
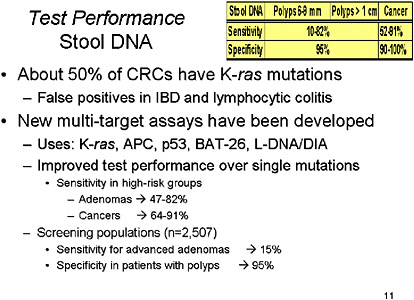
SLIDE 11 NOTES: It has been determined from patients with proven CRC that a number of mutations can be detected in cells obtained from stool specimens that correspond to mutations in the neoplasm in those affected individuals. Unfortunately, no individual mutation is found in all colorectal neoplasms. Consequently, researchers have assessed the utility of individual markers or multi-target assays. Some of the most common mutations in current assays include K-ras, APC, p53, BAT-26 and L-DNA (or DIA). An additional marker that has been studied is the D-Gal-B (1à3)D-GalNAc tumor marker (Sakamoto et al., 1993).
One recent study used a multi-target assay in order to assess test characteristics in patients with advanced neoplasia, and showed this assay had sensitivities of 64 percent and 57 percent for cancers and advanced adenomas, respectively (Tagore et al., 2003). Another study using similar multi-target assay also showed a sensitivity ranging from 73–82 percent for adenomas and 91 percent for cancers (Ahlquist et al., 2000). In a feasibility study, BAT-26 alone showed a sensitivity of 37 percent and a specificity of 100 percent (Traverso et al., 2002). K-ras has been assessed in a number of studies and its sensitivity as a sole marker ranges from 18–80 percent, but there are still some problems with non-neoplastic tissues manifesting stool positive for K-ras (specificity of 76 percent for organic disease in the colon) (Ahlquist et al., 2002; Villa et al., 1996).
Although sensitivity for adenomas of multi-target marketer tests has been on the order of 50 to 80 percent in high-risk groups, and even higher than that for cancers, a recent industry-sponsored study presented to the American College of Gastroenterology found sensitivity for advanced adenomas of only 15 percent with specificity of 95 percent.
To summarize, this is an exciting area that deserves further investigation, but is not yet ready for widespread screening of the population for CRC (Ahlquist, 2000). Definitions: DIA=DNA Integrity Assay. L-DNA=“Long” DNA or High Molecular Weight DNA.
SLIDE 12
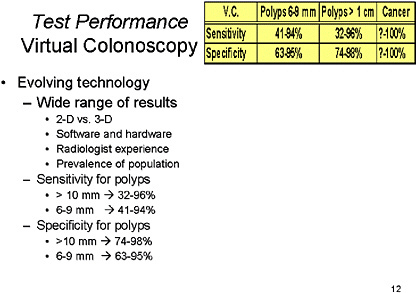
SLIDE 12 NOTES: Virtual colonoscopy is the latest technology to receive high visibility in the press. It is important to recognize, though, that this is still an evolving technology. As such, there has been a broad range of results (Gluecker et al., 2002; Johnson et al., 2003a; Laghi et al., 2002; Pescatore et al., 2000; Pickhardt et al., 2003; Pineau et al., 2003; Spinzi et al., 2001; Wong et al., 2002; Yee et al., 2001). That may be because of the range of technologies used, focusing on 2-D imaging, a combination of 2-D and 3-D, or 3-D imaging only, and a variety of those modalities at that.
The most recent publication provided the most impressive results to date, but these results have yet to be replicated (Pickhardt et al., 2003). The by-patient sensitivity for patients with polyps 10 mm and larger ranges from 75 percent to 100 percent and the by-polyp sensitivity for polyps 10 mm and larger ranges from 50 percent to 100 percent, with specificity ranging from 90–100 percent (Dachman, 2002). The inter-observer reliability appears to range from poor to very good, with kappa statistics ranging from—
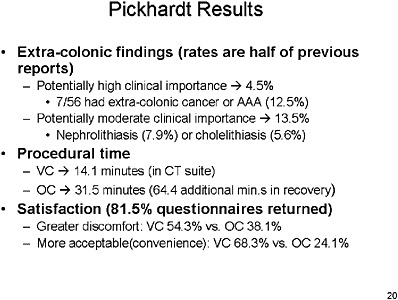
0.56 to 0.89. ((Gluecker et al., 2002; Laghi et al., 2002; Pickhardt et al., 2003; Pescatore et al., 2000; Johnson et al., 2003b). Also important, the incidence of significant extra-colonic findings is 4.5–13 percent, which will impact the cost and cost-effectiveness of this test (Dachman, 2002; Kiesslich et al., 2001; Pickhardt et al., 2003).
In summary, for polyps, there is a broad range of sensitivity, and a narrower range for specificity. However, the sensitivity and specificity for cancer is approaching 100 percent if all of the studies are taken into account.
SLIDE 13
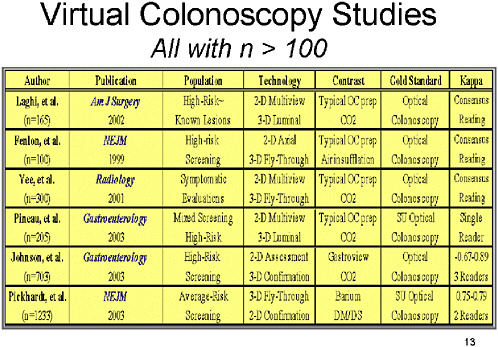
SLIDE 13 NOTES: Here is a list of the six studies with a sample size of 100 or greater. All have been reported in the recent past, before the conduct of this workshop.
Note that the populations studied are varied. Most of these studies have been in high-risk individuals with known lesions, or represent screening in high-risk populations. The only study that examined the use of virtual colonoscopy in average-risk individuals is the study reported recently in the New England Journal of Medicine by Pickhardt et al.
Several factors affect the results. The first is the use (or non-use) of contrast materials to enhance the image. Most of the studies reported on VC procedures using typical optical colonoscopy preparations of either air or CO2 insufflation. Two studies used added contrast to help subtract stool material from the image.
Second, the majority of studies used general optical colonoscopy as the gold standard for a true positive result. A more unbiased assessment of overall performance, however, is achieved by the use of segmental unblinding, which requires colonoscopists to re-examine patients for lesions found only on VC.
Third, measurement of inter-rater reliability is important in understanding the performance of VC. Most of the studies did not examine this aspect, because they had either a single reader or consensus readings.
SLIDE 14
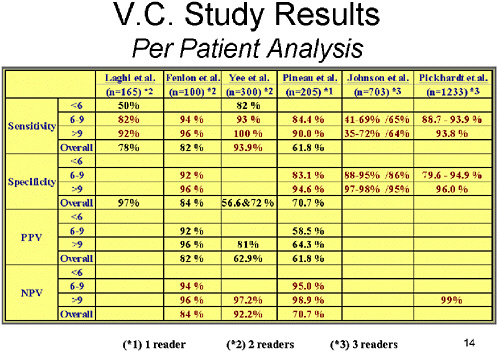
SLIDE 14 NOTES: Looking at the per-patient data gives the possibility of determining what will happen to patients who are subjected to screening by this modality.
Across all six studies, the sensitivity for small polyps has not been well studied. However, for intermediate polyps (between 6 and 9 mm), the sensitivity is probably 85 percent or more. (The Johnson study is an outlier for a variety of reasons.) Sensitivity for large polyps is generally 85 to 90 percent, but it is over 90 percent in most of the studies.
Specificity for intermediate-sized polyps has been 85–95 percent, and specificity for large polyps has been about 95 percent. The positive predictive value ranged from 60 to 90 percent, and the negative predictive value in general has been between 95 and 99 percent.
SLIDE 15
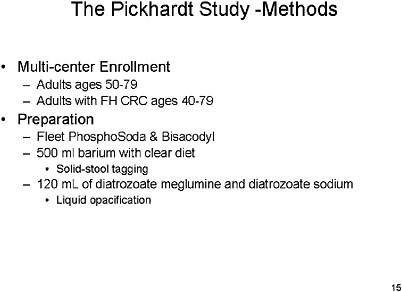
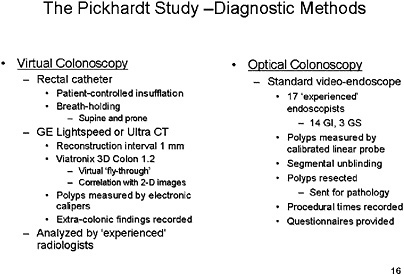
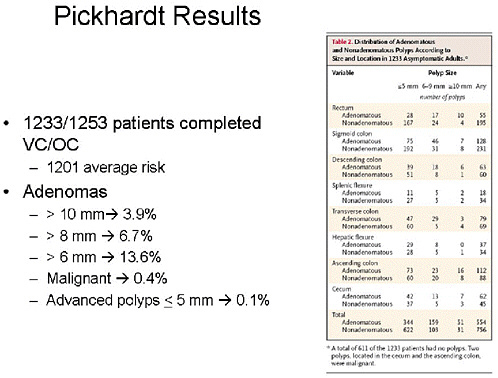
SLIDE 15 NOTES: The Pickhardt Study—In my opinion, The Pickhardt study (Pickhardt et al., 2003) is the one against which many others will be assessed in the future. This was a multi-center enrollment study. Average-risk individuals, mostly men, and a few high-risk individuals as defined by family history. (Of 1,233 patients, 1,201 were average-risk.)
The preparation was typical of optical colonoscopy, but barium was added for solid stool tagging, and diatrozoate meglumine and diatrozoate sodium were used to subtract liquids from the image. Diagnostic studies were performed in a typical fashion, but they used a rectal catheter with patient-controlled insufflation, looked at supine and prone images, and used high-speed GE technology. The reconstruction interval was small. Proprietary software was used to generate the 3D fly-though image, and 2D images were used only for confirmation.
Polyps were measured with electronic calipers. Extra-colonic findings were examined. And, all of the readers had read between 25 and 50 VC procedures via this technology before entering the study.
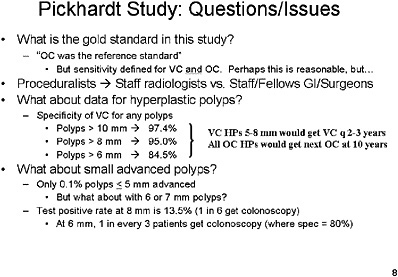
Optical colonoscopy was performed in a standard manner by 17 endoscopists, but as one of the endoscopists in the trial I am aware that the skill level varied among the 17. This study also had a stringent set of criteria for exclusion of individuals from randomization, most notably if they had a history of previous colonic studies or of any signs of cancer or polyps.
SLIDE 17
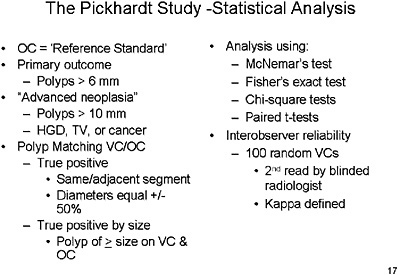
SLIDE 17 NOTES: The Pickhardt study employed segmental unblinding, which means that as the colonoscope passes through a segment, any indication from the VC results that a lesion may have been missed would lead to re-examination of that segment of the colon to attempt to verify its existence.
Polyps were matched between VC and OC by a finding of polyp in the same or adjacent segment with diameters that were within 50 percent of one another.
All polyps found on optical colonoscopy were resected and sent for pathology.
Procedure times were recorded, and questionnaires were provided to patients. The reference standard for a true positive was the segmentally unblinded optical colonoscopy result.
SLIDE 19
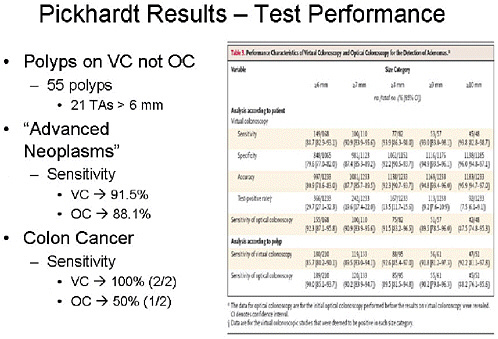
SLIDE 19 NOTES: To summarize comparative test performance, VC found 55 polyps not seen on OC, and 21 of those were tubular adenomas greater than 6 mm. The sensitivity for advanced neoplasia was 91.5 percent for VC and 88 percent for colonoscopy. The difference was not statistically significant.
SLIDE 21
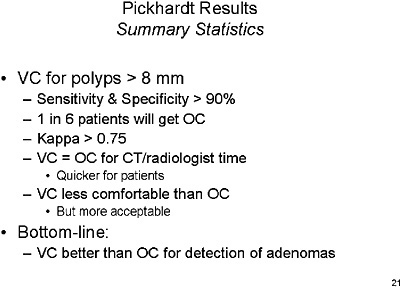
SLIDE 21 NOTES: Overall summary statistics for VC performance—with a cutoff range of 8 mm or greater, sensitivity and specificity of VC are greater than 90 percent. However, test positivity rates are high, which implies that 1 in 6 patients would be referred for optical colonoscopy.
The bottom line from this study is that VC is equivalent or better than OC for finding adenomas.
SLIDE 23

SLIDE 23 NOTES: Here is a brief description of some provocative technologies ‘not (yet) ready for prime time:’
Flurodioxyglucose Positron Emission Tomography (FDG-PET) FDGs take up metabolically active cells. In a recently published study (Drenth, et al., 2001)., this technology achieved a sensitivity of 74 percent and specificity of 84 percent for polyps greater than 3 mm or cancers throughout the colonic tract.
Dark lumen MR colography (Lubolt et al., 1998; Morrin et al., 2001) probably has characteristics similar to VC based on CT, but that technology has been less well studied. One preliminary study showed very high sensitivity for large polyps and lower sensitivity for polyps in the intermediate range (Lubolt et al., 1998).
Chromoendoscopy (Kiesslich et al., 2001; Lee et al., 2003)—the application of indigocarmine dye through a colonoscope—may actually improve the ability to analyze tissue characteristics during the endoscopic procedure. However, that capability may come at the expense of increased procedure cost and time.
SLIDE 26

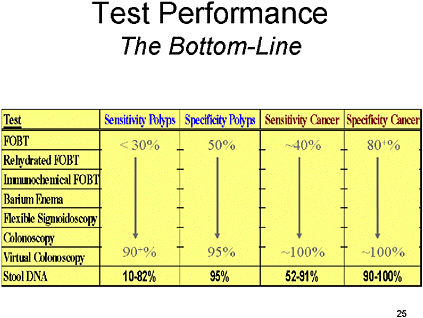
SLIDE 26 NOTES: To summarize the entire literature, I would say that at present we have no clear gold standard for overall analysis. However, the segmentally unblinded colonoscopy using complementary studies is probably the best approach to defining the gold standard.
FOBTs and IFOBTs have a wide range of sensitivity, but even at low sensitivity, they have demonstrated a benefit in reducing colorectal cancer.
Although barium enema has acceptable sensitivity and specificity, it is in my opinion a technology on the wane, with fewer people trained to perform the procedure.
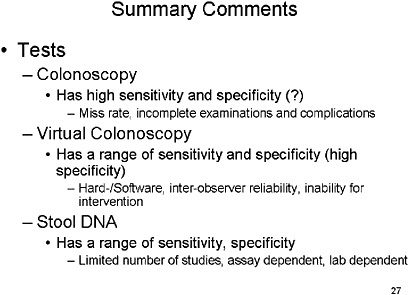
Colonoscopy has high sensitivity and specificity, but it is impacted by a significant miss rate, incomplete examinations and complications.
Virtual colonoscopy still has a broad range of sensitivity and specificity, and practical issues such as the hardware and software used, the inter-observer reliability, and radiologist training has to be addressed before it is ready for prime time.
SLIDE 27
SLIDE 27 NOTES: No notes.
REFERENCES
Adamsen, S. and O.Kronborg, Acceptability and compliance in screening for colorectal cancer with fecal occult blood test. Scand J Gastroenterol, 1984. 19(4): p. 531–4.
Ahlquist, D.A. and A.P.Shuber, Stool screening for colorectal cancer: evolution from occult blood to molecular markers. Clinica Chimica Acta, 2002. 315(1–2): p. 157–68.
Ahlquist, D.A., J.E.Skoletsky, K.A.Boynton, J.J.Harrington, D.W.Mahoney, W.E.Pierceall, S.N.Thibodeau, and A.P.Shuber, Colorectal cancer screening by detection of altered human DNA in stool: feasibility of a multitarget assay panel. Gastroenterol, 2000. 119(5): p. 1219–27.
Ahlquist, D.A., Molecular stool screening for colorectal cancer. Using DNA markers may be beneficial, but large scale evaluation is needed. BMJ, 2000. 321(7256): p. 254–5.
Beeker, C., J.M.Kraft, B.G.Southwell, et al., Colorectal cancer screening in older men and women: qualitative research findings and implications for intervention. J Community Health, 2000. 25(3): p. 263–78.
Bond, J.H., Colorectal Cancer Update. Prevention, Screening, Treatment and Surveillance for High-Risk Groups. Med Clin N Am, 2000. 84: p. 1163–82.
Bouvier, V., C.Herbert, H.Lefevre, and G.Launoy, Stage of extension and treatment for colorectal cancer after a negative test and among non-responders in mass screening with guaiac faecal occult blood test: a French experience. Eur J Cancer Prev, 2001. 10(4): p. 323–326.
Boyle, P., D.G.Zaridze, and M.Smans, Descriptive epidemiology of colorectal cancer. Int J Cancer, 1985. 36: p. 9–15.
Brenes, G.A. and E.D.Paskett, Predictors of stage of adoption for colorectal cancer screening. Prev Med, 2000. 31(4): p. 410–6.
Brenner, H., V.Arndt, T.Sturmer, C.Stegmaier, H.Ziegler, and G.Dhom, Long-lasting reduction of risk of colorectal cancer following screening endoscopy. Br J Cancer, 2001. 85(7): p. 972–6.
Brevinge, H., E.Lindholm, S.Buntzen, and J.Kewenter, Screening for colorectal neoplasia with faecal occult blood testing compared with flexible sigmoidoscopy directly in a 55–56 years’ old population. Int J Colorectal Dis, 1997. 12(5): p. 291–5.
Brewster, N.T., D.C.Grieve, and J.H.Saunders, Double-contrast barium enema and flexible sigmoidoscopy for routine colonic investigation. Br J Surg, 1994. 81(3): p. 445–7.
Byrne, M.F., Primary Screening With Colonoscopy for Colorectal Cancer: A Targeted Algorithm? Am J Gastroenterol, 2003. 98(12): p. 2587–8.
Chu, K.C., R.E.Tarone, W.H.Chow, B.F.Hankey, and L.A.Ries, Temporal patterns in colorectal cancer incidence, survival, and mortality from 1950 through 1990. [see comment]. J Natl Cancer Inst, 1994. 86(13): p. 997–1006.
Cirocco, W.C. and L.C.Rusin, Factors that predict incomplete colonoscopy. Dis Colon Rect, 1995. 38(9): p. 964–8.
Cooper, G.S., Z.Yuan, A.Chak, and A.A.Rimm, Patterns of endoscopic follow-up after surgery for nonmetastatic colorectal cancer. Gastrointest Endosc, 2000. 52(1): p. 33–8.
Dachman, A.H., Diagnostic performance of virtual colonoscopy. Abdom Imaging, 2002. 27(3): p. 260–7.
de Zwart, I.M., G.Griffioen, M.P.Shaw, C.B.Lamers, and A.de Roos, Barium enema and endoscopy for the detection of colorectal neoplasia: sensitivity, specificity, complications and its determinants. Clin Radiol, 2001. 56(5): p. 401–9.
Drenth, J.P., F.M.Nagengast, and W.J.Oyen, Evaluation of (pre-)malignant colonic abnormalities: endoscopic validation of FDG-PET findings. Eur J Nucl Med, 2001. 28(12): p. 1766–9.
Ederer, F., T.R.Church, and J.S.Mandel, Fecal occult blood screening in the Minnesota study: role of chance detection of lesions. [see comment]. J Natl Cancer Inst, 1997. 89(19): p. 1423–8.
Eisen, G.M., C.Y.Kim, D.E.Fleischer, R.A.Kozarek, D.L.Carr-Locke, T.C.Li, C.J.Gostout, S.J.Heller, E.A.Montgomery, F.H.Al-Kawas, J.H.Lewis, and S.B.Benjamin, High-resolution chromoendoscopy for classifying colonic polyps: a multicenter study. Gastrointest Endosc, 2002. 55(6): p. 687–94.
Erban, S., J.Zapka, E.Puleo, and M.Vickers-Lahti, Colorectal cancer screening in Massachusetts: measuring compliance with current guidelines. Eff Clin Pract, 2001. 4(1): p. 10–7.
Favennec, L., N.Kapel, D.Meillet, C.Chochillon, and J.G.Gobert, Detection of occult blood in stools: comparison of three gaiac tests and a latex agglutination test. [erratum appears in Ann Biol Clin (Paris) 1992. 50(8):618]. Ann Biol Clin (Paris), 1992. 50(5): p. 311–3.
Gann, P.H., J.E.Manson, R.J.Glynn, et al., Low-dose aspirin and incidence of colorectal tumors in a randomized trial. J Natl Cancer Inst, 1993. 85(15): p. 1220–24.
Giovanucci, E., E.B.Rimm, M.J.Stampfer, et al., Aspirin use and the risk of colorectal cancer and adenoma in male health professionals. Ann Intern Med, 1994. 121: p. 241–8.
Glick, S., J.L.Wagner, and C.D.Johnson, Cost-effectiveness of double-contrast barium enema in screening for colorectal cancer. AJR. Am J Roengenol, 1998. 170(3): p. 629–36.
Gluecker, T., G.Dorta, W.Keller, P.Jornod, R.Meuli, and P.Schnyder, Performance of multidetector computed tomography colonography compared with conventional colonoscopy. Gut, 2002. 51(2): p. 207–11.
Gopalswamy, N., H.P.Stelling, R.J.Markert, H.N.Maimon, S.D.Wahlen, and R.I.Haddy, A comparative study of eight fecal occult blood tests and HemoQuant in patients in whom colonoscopy is indicated. Arch Fam Med, 1994. 3(12): p. 1043–8.
Greenberg, P.D., L.Bertario, R.Gnauck, O.Kronborg, J.D.Hardcastle, M.S.Epstein, D. Sadowski, R.Sudduth, G.R.Zuckerman, and D.C.Rockey, A prospective multicenter evaluation of new fecal occult blood tests in patients undergoing colonoscopy. Am J Gastroenterol, 2000. 95(5): p. 1331–8.
Greenlee, R.T., M.B.Hill-Harmon, T.Murray, et al., Cancer Statistics, 2001. CA Cancer J Clin, 2001(51): p. 15–36.
Hixson, L.J., M.B.Fennerty, R.E.Sampliner, and H.S.Garewal, Prospective blinded trial of the colonoscopic miss-rate of large colorectal polyps. Gastrointest Endosc, 1991. 37(2): p. 125–7.
Hixson, L.J., M.B.Fennerty, R.E.Sampliner, D.McGee, and H.Garewal, Prospective study of the frequency and size distribution of polyps missed by colonoscopy. J Natl Cancer Inst, 1990. 82(22): p. 1769–72.
Hope, R.L., G.Chu, A.H.Hope, R.G.Newcombe, P.E.Gillespie, and S.J.Williams, Comparison of three faecal occult blood tests in the detection of colorectal neoplasia. Gut, 1996. 39(5): p. 722–5.
Irvine, E.J., J.O’Connor, R.A.Frost, P.Shorvon, S.Somers, G.W.Stevenson, and R.H.Hunt, Prospective comparison of double contrast barium enema plus flexible sigmoidoscopy v colonoscopy in rectal bleeding: barium enema v colonoscopy in rectal bleeding. [comment]. Gut, 1988. 29(9): p. 1188–93.
Itoh, M., K.Takahashi, H.Nishida, K.Sakagami, and T.Okubo, Estimation of the optimal cut off point in a new immunological faecal occult blood test in a corporate colorectal cancer screening programme. J Med Screen, 1996. 3(2): p. 66–71.
Jeanson, A., J.Jamart, J.M.Maisin, R.Vanheuverzwyn, P.Gohy, J.C.Debongnie, and B. Rentier, Assessment of the new immunological test Hemoblot for detecting occult blood in faeces. Eur J Cancer Prev, 1994. 3(5): p. 407–12.
Jensen, J., J.Kewenter, M.Asztely, G.Lycke, and J.Wojciechowski, Double contrast barium enema and flexible rectosigmoidoscopy: a reliable diagnostic combination for detection of colorectal neoplasm. Br J Surg, 1990. 77(3): p. 270–2.
Johnson, C.D., A.Y.Toledano, B.A.Herman, A.H.Dachman, E.G.McFarland, M.A.Barish, J.A.Brink, R.D.Ernst, J.G.Fletcher, R.A.Halvorsen, Jr., A.K.Hara, K.D.Hopper, R.E. Koehler, D.S.Lu, M.Macari, R.L.Maccarty, F.H.Miller, M.Morrin, E.K.Paulson, J. Yee, M.Zalis, and American College of Radiology Imaging Network, Computerized tomographic colonography: performance evaluation in a retrospective multicenter setting. Gastroenterol, 2003. 125(3): p. 688–95.
Johnson, C.D., H.C.Carlson, W.F.Taylor, and L.P.Weiland, Barium enemas of carcinoma of the colon: sensitivity of double- and single-contrast studies. AJR Am J Roentgenol, 1983. 140(6): p. 1143–9.
Johnson, C.D., W.S.Harmsen, L.A.Wilson, R.L.Maccarty, T.J.Welch, D.M.Ilstrup, and D.A.Ahlquist, Prospective blinded evaluation of computed tomographic colonography for screen detection of colorectal polyps, [comment]. Gastroenterol, 2003. 125(2): p. 311–9.
Kewenter, J., H.Brevinge, B.Engaras, and E.Haglind, The yield of flexible sigmoidoscopy and double-contrast barium enema in the diagnosis of neoplasms in the large bowel in patients with a positive Hemoccult test, [see comment]. Endoscopy, 1995. 27(2): p. 159–63.
Kewenter, J., S.Bjork, E.Haglind, L.Smith, J.Svanvik, and C.Ahren, Screening and rescreening for colorectal cancer. A controlled trial of fecal occult blood testing in 27,700 subjects. Cancer, 1988. 62(3): p. 645–51.
Khullar, S.K. and J.A.DiSario, Colon cancer screening. Sigmoidoscopy or colonoscopy. Gastrointest Endosc Clin N Am, 1997. 7(3): p. 365–86.
Kiesslich, R., et al., Chromoendoscopy with indigocarmine improves the detection of adenomatous and nonadenomatous lesions in the colon, [see comment]. Endoscopy, 2001. 33(12): p. 1001–6.
Ko, C.W., J.A.Dominitz, and T.D.Nguyen, Fecal occult blood testing in a general medical clinic: comparison between guaiac-based and immunochemical-based tests. Am J Med, 2003. 115(2): p. 111–4.
Kronborg, O., Colonic screening and surveillance. Best Practice & Research in Clinical Gastroenterology, 2001. 15(2): p. 301–16.
Laghi, A., R.Iannaccone, I.Carbone, C.Catalano, V.Panebianco, E.Di Giulio, A.Schillaci, and R.Passariello, Computed tomographic colonography (virtual colonoscopy): blinded prospective comparison with conventional colonoscopy for the detection of colorectal neoplasia. Endoscopy, 2002. 34(6): p. 441–6.
Landis, S.H., T.Murray, S.Bolden, et al., Cancer Statistics. CA Cancer J Clin, 1999. 49: p. 8–31.
Lee, J.H., et al., Detection of colorectal adenomas by routine chromoendoscopy with indigocarmine. Am J Gastroenterol, 2003. 98(6): p. 1284–8.
Lee, J.H., J.W.Kim, Y.K.Cho, C.I.Sohn, W.K.Jeon, B.I.Kim, and E.Y.Cho, Detection of colorectal adenomas by routine chromoendoscopy with indigocarmine. Am J Gastroenterol, 2003. 98(6): p. 1284–8.
Leinicke, J.L., W.J.Dodds, W.J.Hogan, and E.T.Stewart, A comparison of colonoscopy and roentgenography for detecting polypoid lesions of the colon. Gastrointest Radiol, 1977. 2(2): p. 125–8.
Lichtenstein, G.R., P.D.Park, W.B.Long, G.G.Ginsberg, and M.L.Kochman, Use of a push enteroscope improves ability to perform total colonoscopy in previously unsuccessful attempts at colonoscopy in adult patients. Am J Gastroenterol, 1999. 94(1): p. 187–90.
Lieberman, D., Cost-effectiveness of screening colorectal cancer in the general population. Gastrointest Endosc, 2001. 54(4): p. 537–8.
Lieberman, D.A., D.G.Weiss; Veterans Affairs Cooperative Study, One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon, [see comment][summary for patients in Gastrointest Endosc, 2002 May; 55(6): p. 760–1]. N Engl J Med, 2001. 345(8): p. 555–60.
Loose, H.W. and C.B.Williams, Barium enema versus colonoscopy. Proc R Soc Med, 1974. 67(10): p. 1033–6.
Luboldt, W., P.Steiner, P.Bauerfeind, P.Peklonen, and J.F.Debatin, Detection of mass lesions with MR colonography: preliminary report. Radiology, 1998. 207: p. 59–65.
Mandel, J.S., J.H.Bond, T.R.Church, et al., Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med, 1993. 328(19): p. 1365–74.
Markus, J.B., S.Somers, B.P.O’Malley, and G.W.Stevenson, Double-contrast barium enema studies: effect of multiple reading on perception error. Radiology, 1990. 175(1): p. 155–6.
Miyoshi, H., M.Oka, K.Sugi, O.Saitoh, K.Katsu, and K.Uchida, Accuracy of detection of colorectal neoplasia using an immunochemical occult blood test in symptomatic referred patients: comparison of retrospective and prospective studies. Intern Med, 2000. 39(9): p. 701–6.
Morrin, M.M., et al., MR colonography using colonic distention with air as the contrast material: work in progress. AJR Am J Roentgenol, 2001. 176(1): p. 144–6.
Nakama, H., B.Zhang, and X.Zhang, Evaluation of the optimum cut-off point in immunochemical occult blood testing in screening for colorectal cancer. Eur J Cancer, 2001. 37(3): p. 398–401.
Nakama, H., M.Yamamoto, N.Kamijo, T.Li, N.Wei, A.S.Fattah, and B.Zhang, Colonoscopic evaluation of immunochemical fecal occult blood test for detection of colorectal neoplasia. Hepatogastroenterology, 1999. 46(25): p. 228–31.
Nakama, H., N.Kamijo, K.Fujimori, A.S.Fattah, and B.Zhang, Relationship between fecal sampling times and sensitivity and specificity of immunochemical fecal occult blood tests for colorectal cancer: a comparative study. Dis Colon Rectum, 1997. 40(7): p. 781–4.
National Cancer Institute, http://www.cancer.org.
Nelson, D.B., K.R.McQuaid, J.H.Bond, D.A.Lieberman, D.G.Weiss, and T.K.Johnston, Procedural success and complications of large-scale screening colonoscopy. Gastrointest Endosc, 2002. 55(3): p. 307–14.
Newcomb, P.A., B.E.Storer, L.M.Morimoto, A.Templeton, and J.D.Potter, Long-term efficacy of sigmoidoscopy in the reduction of colorectal cancer incidence, [see comment]. J Natl Cancer Inst, 2003. 95(8): p. 622–5.
Newcomb, P.A., R.G.Norfleet, B.E.Storer, T.S.Surawicz, and P.M.Marcus, Screening sigmoidoscopy and colorectal cancer mortality. [see comment]. J Natl Cancer Inst, 1992. 84(20): p. 1572–5.
Niv, Y. and A.D.Sperber, Sensitivity, specificity, and predictive value of fecal occult blood testing (Hemoccult II) for colorectal neoplasia in symptomatic patients: a prospective study with total colonoscopy. Am J Gastroenterol, 1995. 90(11): p. 1974–7.
Obrecht, W.F., Jr., W.C.Wu, D.W.Gelfand, and D.J.Ott, The extent of successful colonoscopy: a second assessment using modern equipment. Gastrointest Radiol, 1984. 9(2): p. 161–2.
Osgard, E., J.L.Jackson, and J.Strong, A randomized trial comparing three methods of bowel preparation for flexible sigmoidoscopy. Am J Gastroenterol, 1998. 93(7): p. 1126–30.
Ott, D.J., W.C.Wu, and D.W.Gelfand, Extent of colonic visualization with the fiberoptic sigmoidoscope. J Clin Gastroenterol, 1982. 4(4): p. 337–41.
Painter, J., D.B.Saunders, G.D.Bell, C.B.Williams, R.Pitt, and J.Bladen, Depth of insertion at flexible sigmoidoscopy: implications for colorectal cancer screening and instrument design. [see comment]. Endoscopy, 1999. 31(3): p. 227–31.
Parikh, A., R.Ramamoorthy, K.H.Kim, B.K.Holland, and J.Houghton, Fecal occult blood testing in a noncompliant inner city minority population: increased compliance and adherence to screening procedures without loss of test sensitivity using stool obtained at the time of in-office rectal examination. Am J Gastroenterol, 2001. 96(6): p. 1908–13.
Parker, S.L., T.Tong, S.Bolden, et al., Cancer statistics, 1996. CA Cance J Clin, 1996. 46(1): p. 5–27.
Pescatore, P., T.Glucker, J.Delarive, R.Meuli, D.Pantoflickova, B.Duvoisin, P.Schnyder, A.L.Blum, and G.Dorta, Diagnostic accuracy and interobserver agreement of CT colonography (virtual colonoscopy). Gut, 2000. 47(1): p. 126–30.
Pickhardt, P.J., J.R.Choi, I.Hwang, J.A.Butler, M.L.Puckett, H.A.Hildebrandt, R.K.Wong, P.A.Nugent, P.A.Mysliwiec, and W.R.Schindler, Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. [see comment]. N Engl J Med, 2003. 349(23): p. 2191–2200.
Pignone, M., S.Saha, T.Hoerger, and J.Mandelblatt, Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the U.S. Preventive Services Task Force. [comment][summary for patients in Ann Intern Med, 2002. 137(2): p. 138]. Ann Intern Med, 2002. 137(2): p. 96–104.
Pineau, B.C., E.D.Paskett, G.J.Chen, M.A.Espeland, K.Phillips, J.P.Han, C.Mikulaninec, and D.J.Vining, Virtual colonoscopy using oral contrast compared with colonoscopy for the detection of patients with colorectal polyps, [comment]. Gastroenterol, 2003. 125(2): p. 304–10.
Postic, G., D.Lewin, C.Bickerstaff, and M.B.Wallace, Colonoscopic miss rates determined by direct comparison of colonoscopy with colon resection specimens. Am J Gastroenterol, 2002. 97(12): p. 3182–5.
Provenzale, D., Cost-effectiveness of screening the average-risk population for colorectal cancer. Gastrointest Endosc Clin N Am, 2002. 12(1): p. 93–109.
Rex, D.K., C.S.Cutler, G.T.Lemmel, E.Y.Rahmani, D.W.Clark, D.J.Helper, G.A.Lehman, and D.G.Mark, Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. [see comment]. Gastroenterol, 1997. 112(1): p. 24–8.
Rex, D.K., Colonoscopic withdrawal technique is associated with adenoma miss rates. Gastrointest Endosc, 2000. 51(1): p. 33–6.
Rex, D.K., Colonoscopy: a review of its yield for cancers and adenomas by indication. Am J Gastroenterol, 1995. 90(3): p. 353–65.
Rex, D.K., E.Y.Rahmani, J.H.Haseman, G.T.Lemmel, S.Kaster, and J.S.Buckley, Relative sensitivity of colonoscopy and barium enema for detection of colorectal cancer in clinical practice, [comment]. Gastroenterol, 1997. 112(1): p. 17–23.
Rex, D.K., G.A.Lehman, J.C.Lappas, and R.E.Miller, Sensitivity of double-contrast barium study for left-colon polyps. Radiology, 1986. 158(1): p. 69–72.
Rex, D.K., Rationale for colonoscopy screening and estimated effectiveness in clinical practice. Gastrointest Endosc Clin N Am, 2002. 12(1): p. 65–75.
Rockey, D.C., J.Koch, J.P.Cello, L.L.Sanders, and K.McQuaid, Relative frequency of upper gastrointestinal and colonic lesions in patients with positive fecal occult-blood tests. N Engl J Med, 1998. 339(3): p. 153–9.
Rozen, P., J.Knaani, and N.Papo, Evaluation and comparison of an immunochemical and a guaiac faecal occult blood screening test for colorectal neoplasia. Eur J Cancer Prev, 1995. 4(6): p. 475–81.
Rozen, P., J.Knaani, and Z.Samuel, Comparative screening with a sensitive guaiac and specific immunochemical occult blood test in an endoscopic study. Cancer, 2000. 89(1): p. 46–52.
Rozen, P., J.Knaani, and Z.Samuel, Performance characteristics and comparison of two immunochemical and two guaiac fecal occult blood screening tests for colorectal neoplasia. Dig Dis Sci, 1997. 42(10): p. 2064–71.
Rozen, P., Prospects for the worldwide control of colorectal cancer through screening. Gastrointest Endosc, 2002. 55(6): p. 755–9.
Saito, Y., P.Slezak, and C.Rubio, The diagnostic value of combining flexible sigmoidoscopy and double-contrast barium enema as a one-stage procedure. Gastrointest Radiol, 1989. 14(4): p. 357–9.
Sakamoto, K., M.Muratani, T.Ogawa, and Y.Nagamachi, Evaluation of a new test for colorectal neoplasms: a prospective study of asymptomatic population. Cancer Biother, 1993. 8(1): p. 49–55.
Shehadeh, I., S.Rebala, R.Kumar, R.J.Markert, C.Barde, and N.Gopalswamy, Retrospective analysis of missed advanced adenomas on surveillance colonoscopy. Am J Gastroenterol, 2002. 97(5): p. 1143–7.
Shumaker, D.A., A.Zaman, and R.M.Katon, Use of a variable-stiffness colonoscope allows completion of colonoscopy after failure with the standard adult colonoscope. Endoscopy, 2002. 34(9): p. 711–4.
Sieg, A., A.Hertel, M.R.John, K.Luthgens, and H.Schmidt-Gayk, Screening for colorectal neoplasms with a new immunological human faecal haemoglobin and albumin test. Eur J Cancer Prev , 1998. 7(4): p. 279–85.
Sieg, A., C.Thoms, K.Luthgens, M.R.John, and H.Schmidt-Gayk, Detection of colorectal neoplasms by the highly sensitive hemoglobin-haptoglobin complex in feces. Int J Colorectal Dis, 1999. 14(6): p. 267–71.
Silverman, M.A., Cancer screening in the elderly population. Hematol Oncol Clin North Am, 2000. 14(1): p. 89–112.
Slattery, M.L., S.L.Edwards, K.M.Boucher, et al., Lifestyle and colon cancer: An assessment of factors associated with risk. Am J Epidemiol, 1999. 150: p. 869–77.
Sonnenberg, A., Cost-effectiveness in the prevention of colorectal cancer. Gastroenterol Clin North Am, 2002. 31(4): p. 1069–91.
Spinzi, G., G.Belloni, A.Martegani, A.Sangiovanni, C.Del Favero, and G.Minoli, Computed tomographic colonography and conventional colonoscopy for colon diseases: a prospective, blinded study. Am J Gastroenterol, 2001. 96(2): p. 394–400.
Steine, S., A.Stordahl, O.C.Lunde, K.Loken, and E.Laerum, Double-contrast barium enema versus colonoscopy in the diagnosis of neoplastic disorders: aspects of decision-making in general practice. Fam Pract, 1993. 10(3): p. 288–91.
Stewart, B.T., J.O.Keck, A.V.Duncan, N.M.Santamaria, and P.Allen, Difficult or incomplete flexible sigmoidoscopy: implications for a screening programme, [see comment]. Aust N Z J Surg, 1999. 69(1): p. 19–21.
Sung, J.J., F.K.Chan, W.K.Leung, J.C.Wu, J.Y.Lau, J.Ching, K.F.To, Y.T.Lee, Y.W.Luk, N.N.Kung, S.P.Kwok, M.K.Li, and S.C.Chung, Screening for colorectal cancer in Chinese: comparison of fecal occult blood test, flexible sigmoidoscopy, and colonoscopy. Gastroenterol, 2003. 124(3): p. 608–14.
Tagore, K.S., M.J.Lawson, J.A.Yucaitis, R.Gage, T.Orr, A.P.Shuber, and M.E.Ross, Sensitivity and specificity of a stool DNA multitarget assay panel for the detection of advanced colorectal neoplasia. Clinical Colorectal Cancer, 2003. 3(1): p. 47–53.
Traverso, G., A.Shuber, L.Olsson, B.Levin, C.Johnson, S.R.Hamilton, K.Boynton, K.W. Kinzler, and B.Vogelstein, Detection of proximal colorectal cancers through analysis of faecal DNA. Lancet, 2002. 359(9304): p. 403–4.
Trends in screening for colorectal cancer-United States, 1997 and 1999. MMWR Mortal Wkly Rep, 2001. 50: p. 162–66.
Tsuda, S., B.Veress, E.Toth, and F.T.Fork, Flat and depressed colorectal tumours in a southern Swedish population: a prospective chromoendoscopic and histopathological study. Gut, 2002. 51(4): p. 550–5.
Verne, J.E., R.Aubrey, S.B.Love, I.C.Talbot, and J.M.Northover, Population based randomized study of uptake and yield of screening by flexible sigmoidoscopy compared with screening by faecal occult blood testing. BMJ, 1998. 317(7152): p. 182–5.
Villa, E., A.Dugani, A.M.Rebecchi, A.Vignoli, A.Grottola, P.Buttafoco, L.Losi, M.Perini, P.Trande, A.Merighi, R.Lerose, and F.Manenti, Identification of subjects at risk for colorectal carcinoma through a test based on K-ras determination in the stool, [comment]. Gastroenterol, 1996. 110(5): p. 1346–53.
Walsh, J.M. and J.P.Terdiman, Colorectal cancer screening: scientific review, [see comment]. JAMA, 2003. 289(10): p. 1288–96.
Walter, S.D., D.J.Frommer, and R.J.Cook, The estimation of sensitivity and specificity in colorectal cancer screening methods. Cancer Detect Prev, 1991. 15(6): p. 465–9.
Weaver, P., B.Harrison, G.Eskander, et al., Colon cancer in blacks. A disease with a worsening prognosis. J Natl Med Assoc, 1991. 83: p. 133–38.
Wherry, D.C. and W.M.Thomas, The yield of flexible fiberoptic sigmoidoscopy in the detection of asymptomatic colorectal neoplasia. [see comment]. Surg Endosc, 1994. 8(5): p. 393–5.
Williams, A.R., B.A.Balasooriya, and D.W.Day, Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut, 1982. 23(10): p. 835–42.
Williams, C.B., R.H.Hunt, H.Loose, R.H.Riddell, Y.Sakai, and E.T.Swarbrick, Colonoscopy in the management of colon polyps. Br J Surg, 1974. 61(9): p. 673–82.
Winawer, S.J. and M.Shike, Prevention and control of colorectal cancer, in Cancer Prevention and Control, P.Greenwald, B.S.Kramer, and D.L.Weed, Editors. 1995, Marcel Dekker: New York. p. 537–539.
Winawer, S.J., A.G.Zauber, M.N.Ho, et al., Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med, 1993. 329: p. 1977–81.
Winawer, S.J., R.H.Fletcher, L.Miller, et al., Colorectal cancer screening: clinical guidelines and rationale. Gastroenterol, 1997. 112: p. 594–62.
Wong, B.C., W.M.Wong, J.K.Chan, K.C.Lai, W.H.Hu, C.K.Chan, S.K.Lam, and D.L. Carr-Locke, Virtual colonoscopy for the detection of colorectal polyps and cancers in a Chinese population. J Gastroenterol Hepatol, 2002. 17(12): p. 1323–7.
Wong, W.M., S.K.Lam, K.L.Cheung, T.S.Tong, P.Rozen, G.P.Young, K.W.Chu, J.Ho, W.L.Law, H.M.Tung, H.K.Choi, Y.M.Lee, K.C.Lai, W.H.Hu, C.K.Chan, M.F. Yuen, and B.C.Wong, Evaluation of an automated immunochemical fecal occult blood test for colorectal neoplasia detection in a Chinese population. Cancer, 2003. 97(10): p. 2420–4.
Yee, J., G.A.Akerkar, R.K.Hung, A.M.Steinauer-Gebauer, S.D.Wall, and K.R.McQuaid, Colorectal neoplasia: performance characteristics of CT colonography for detection in 300 patients. Radiology, 2001. 219(3): p. 685–92.
Ziegler, R.G., S.S.DeVesa, and J.Fraumeni, Epidemiologic patterns of colorectal cancer, in Important Advances in Oncology, V.T.Devita, V.T., S.Hellman, and S.A.Rosenberg, Editors. 1986, Philadelphia: J.B.Lippincott.