3
The Implementation of the Smallpox Vaccination Program
As described in Chapter 2, a long chain of events and policy decisions led to the revival of civilian smallpox vaccination in the United States. Plans to implement pre-event vaccination of a limited number of health care and public health personnel began to take shape in late 2002.
A detailed timeline of the vaccination program is provided at the end of this chapter (Table 3-2). The chapter does not address every event in the timeline, but in the first section highlights a short list of relevant events and program milestones. Each subsection begins with a description of an event and then moves on to a broader discussion of its significance. For example, the monkeypox outbreak that occurred several months into the implementation of the program is summarized, and then there is a discussion of the outbreak as a proxy event that tested public health preparedness in general and smallpox preparedness specifically.
Major markers on the civilian smallpox vaccination program timeline include the following (see Figure 3-1 for a graph of weekly vaccination numbers in January–September 2003, with several key events):
-
December 13, 2002—National smallpox vaccination policy is announced.
-
January 24, 2003—Civilian pre-event smallpox vaccination begins.
-
March 19, 2003—War with Iraq begins. Both the buildup to the war and the declared end of major combat appeared to have an effect on the smallpox vaccination program.
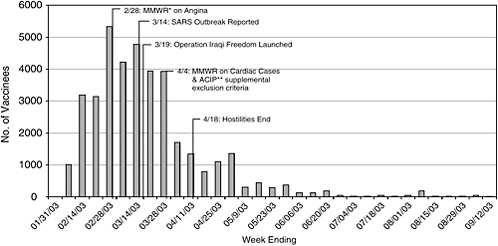
FIGURE 3-1 Timeline of smallpox vaccination program with number of weekly vaccinations and key events.
SOURCE: Henderson (2003b).
*MMWR = Morbidity and Mortality Weekly Report.
**ACIP = Advisory Committee on Immunization Practices.
-
Late March and early April 2003—Concerns about vaccine and program safety reach high point in response to reported fatal cardiac adverse events and cases of heart inflammation, known as myo/pericarditis.
-
April 2003—Smallpox vaccination compensation plan is enacted in response to widespread concern about vaccine-related injuries.
-
April 2003—General Accounting Office (GAO, now Government Accountability Office) report provides first systematic assessment of vaccination program progress and highlights challenges.
-
May 2003—Department of Health and Human Services (DHHS) makes supplemental funding available for smallpox vaccination program.
-
May and June 2003—Monkeypox outbreak tests public health (and smallpox) preparedness.
-
June 2003—Advisory Committee on Immunization Practices (ACIP) recommends ending smallpox vaccination after completing vaccination of response teams; vaccination continues, very slowly approaching 40,000 vaccinees.
The section on program milestones is followed by a discussion of two noteworthy features of the program (congressional interest and involvement, and the significance of the parallel military smallpox vaccination program). The chapter’s third section focuses on the committee’s findings
about challenges that arose in the course of the vaccination program and about which the committee has written in previous reports, included here as Appendixes B-G. The fourth and final section highlights some of the favorable outcomes of the smallpox vaccination program.
In this chapter and elsewhere in this report, the committee has cited multiple articles from the mass media on the smallpox vaccination policy and on the program implementation. Using news media references was necessary because of the limited scientific peer-reviewed and other formal literature available on the newly initiated and continuing program. Although newspaper articles may capture events in a manner that is incompletely documented, subjective, and even out of context, the committee found that some themes emerged consistently from diverse media sources and provided useful information about how the program was perceived in the public health and health care communities. More important, mass media coverage of the program was concordant with the information presented at committee meetings by state and local public health officials and health care administrators, with the congressional testimony of public health leaders, and with findings from qualitative surveys that became available later in the course of the program. Mass media reports reflected the perceptions of key constituencies and the public; their perceptions of CDC, the program, and the federal government’s role may provide insight into the lessons to be learned from this program.
MAJOR MILESTONES AND RELEVANT EVENTS
The Policy Is Announced
|
On December 13, 2002, President George W. Bush announced that smallpox vaccine would be administered to selected civilians and members of the military. The announcement was the culmination of planning and decision-making that spanned the latter half of 2002. The president explained that “government has no information that a smallpox release is imminent. Yet it is prudent to prepare for the possibility that terrorists would kill indiscriminately” with biologic weapons (White House, 2002b). |
To prepare the nation for the threat of smallpox, the military vaccination program would provide mandatory1 vaccination to selected members
of the military and offer vaccination to others who “serve America in high-risk parts of the world,” and a civilian smallpox vaccination program would make the vaccine “available on a voluntary basis to medical professionals and emergency personnel and response teams that would be the first on the scene in a smallpox emergency” (White House, 2002b). Although the president’s announcement acknowledged and reiterated that there was no imminent danger and that pre-event vaccination would therefore be limited to specified groups, he stated that vaccination would be offered to members of the general public “who insist on being vaccinated” (White House, 2002b).
The program’s general structure and timeline were only sparsely outlined in the president’s announcement and the White House news release. Additional details were conveyed in later telebriefings, program guidance, and other communications from the secretary of Health and Human Services and from Centers for Disease Control and Prevention (CDC) officials (CDC, 2002a, 2002b, 2002d; Connolly and Milbank, 2002; McGlinchey, 2003a). In the joint CDC–DHHS telebriefing on December 14, 2002, the number of prospective vaccinees was discussed in some detail (CDC, 2002d). DHHS Secretary Tommy Thompson said that state pre-event vaccination plans designated a total of 439,584 people to be offered the vaccine.2 State plans included 1,100 public health smallpox response teams, adding up to 20,000 personnel that would be vaccinated, and 4,500 health care teams, adding up to 400,000 personnel that would be vaccinated; and DHHS officials also gave the figure of 10 million as a secondary target to include all health care workers and other first responders who would volunteer to be vaccinated (CDC, 2002d; Connolly and Milbank, 2002; McGlinchey, 2003a).
The public health and health care communities came to understand that the program would progress in three stages or phases:
-
Phase I would involve the vaccination of designated members of health care and public health smallpox response teams with a goal of about 500,000 and a timeline of 30 days.
-
Phase II would involve the expansion of vaccination to up to 10 million health care personnel and other first responders, such as firefighters and police.
-
In Phase III, intended to begin in 2004, vaccination with a new, not yet approved, vaccine would be offered to members of the public who in the absence of a smallpox release insisted on being vaccinated.
The Program Begins
|
The program did not begin immediately after the president’s announcement, because government coverage of liability (of vaccine manufacturers, hospitals, and health departments that would operate vaccination clinics) in the provision of bioterrorism countermeasures (vaccine) would not go into effect until weeks later. On January 22, 2003, CDC began shipping smallpox vaccine from its vaccine stockpiles to the 11 states that had requested it. On January 24, 2003, the secretary of Health and Human Services declared that the smallpox vaccination program could begin under the authority of an amendment to the Public Health Service Act by Section 304 of the Homeland Security Act3 (DHHS, 2003a). The secretary’s declaration marked the true beginning of the smallpox vaccination program, in that states, territories, and municipalities chose to defer program implementation until the protections conferred by the Homeland Security Act went into effect (Kemper, 2003a). |
Vaccination programs in the 62 states, territories, and municipalities began gradually. Some jurisdictions ordered vaccine stocks as soon as CDC made them available and began vaccinating immediately after the program was authorized. Other jurisdictions delayed ordering vaccine and initiating vaccination in order to finalize their plans, or in expectation of CDC’s completion of program components (such as the safety system and informational materials), or to await the settlement of the unresolved vaccine injury compensation issue for people injured by the vaccine or the accidental, inadvertent transmission of vaccinia from a vaccination site.
The vaccination program was generally supported by the public health and health care communities in recognition of the need for biopreparedness (ANA, 2002; Hardy, 2002; Libbey 2003). A survey of state health officials in June 2002 found that a majority (77 percent of 44 respondents) favored smallpox vaccination of designated response teams (Banks and Hannan, 2002). Two surveys of physicians, nurses, and other health care personnel largely working in emergency departments, conducted in late 2002, found that that a majority of respondents (61 percent of 1,165 respondents in one survey, 73 percent of 1,701 respondents in the other) expressed a willingness to receive smallpox vaccination as part of a pre-event program (Everett et al., 2002; Yih et al., 2003). However, support of the program by the public health and health care communities was qualified because of questions and concerns about several aspects of the program; these contributed
to implementation delays and to other challenges. Subjects of concern ranged from the scientific—such as the reliability of historical measures for estimating transmission rates, and the relevance and accuracy of historical adverse events data—to the procedural and administrative, including the structure of the vaccination program and its effect on overall bioterrorism preparedness, the actual and opportunity costs of the program, the adequacy of measures to address compensation and liability, and the safety of the vaccine. Questions and concerns were communicated in open letters to DHHS and to the White House, in press releases and policy statements, at congressional hearings, and at meetings of the present committee (ANA, 2002; Baker, 2003; Libbey, 2003; NGA, 2003; Rosado, 2003).
This committee’s first report recognized that the smallpox vaccination program was an atypical vaccination campaign that was neither a research study nor an ideal public health program, but a public health component of bioterrorism preparedness (IOM, 2003a). The committee also emphasized the program’s voluntary nature and the need for a focus on safety, requiring active monitoring of side effects related to vaccination.
Unlike the views and input of national organizations, which were made public through press releases and other formal communications, the perspectives of individual hospitals and those of health care and public health workers were captured largely in mass media reports. In late January and early February 2003, people involved in the program were interviewed or cited in newspaper articles and news broadcasts; their comments reflected a wide array of feelings and opinions. They included commitment to doing what was needed to protect the public’s health, confusion and suspicion about the rationale for the program, confidence in the usefulness of vaccination, concern about the vaccine’s safety and about potential loss of wages because of non-life-threatening but important postvaccination symptoms, and criticism of or worry about the reluctance of many public health and health care workers to be vaccinated (Bavley and Dvorak, 2003; Kemper, 2003a; Marchione, 2003; McKenna, 2003; Meckler, 2003a). The net results of individual and institutional concerns were hesitation and low participation among public health and health care workers and great variability in hospital participation.
Hospitals differed greatly in their anticipated degree of participation in the smallpox vaccination program. Some hospitals planned to participate, at least by having a small number of staff members vaccinated to serve later as vaccinators, but others (DHHS Secretary Thompson estimated about one-third of hospitals) planned to opt out (Bavley and Dvorak, 2003; Ornstein and Richardson, 2003). Some of the hospitals that did not plan to participate in vaccination chose to hold training sessions and to educate their staff on smallpox disease and smallpox vaccination (Judson, 2003; Toomey, 2003). Hospitals that chose not to participate in pre-event vacci-
nation cited various reasons: the desire to avoid infecting immunosuppressed patients, concerns about strain on their workforce (due to sick time necessary because of complications or to prevent patient exposure), and questions about liability if nonstaff (families and patients) were inadvertently infected with the vaccinia virus (Associated Press, 2003; McCullough, 2003; McNeil, 2003; Ornstein and Bonilla, 2003; Pasternack, 2003). Some newspaper editorials described hospital decisions against implementing vaccination as “deplorable” and characterized workers who opted out as “refuseniks” and “vaccine-dodgers” (New York Times, 2002; Washington Post, 2002; Boston Herald, 2003; Washington Times, 2003). Physicians spoke of being criticized as “unpatriotic” for not being willing to receive the vaccine (Connolly, 2003a). Despite such tensions, CDC guidelines and the efforts of public health agencies sought to ensure that the voluntary nature of the program was preserved while the implementation of the biopreparedness policy continued.
Confusion About the Program Goals and Timeline
The program goals and timetable were not communicated clearly, and that created confusion and challenges throughout the implementation of the program (ACIP, 2002; Connolly and Milbank, 2002; Meckler, 2002). Later communications appeared to augment the confusion and created the perception (also discussed in Chapter 4) that the program was characterized by frequently shifting goals rather than by a clear purpose and effective implementation.
As the program progressed more slowly than expected, it became evident that the original goals and timeline would not be met. However, the 500,000 and 30-day figures had become de facto program goals, as the mass media seized on numerical figures as indicators of program progress both locally and nationally. In February 2003, many CDC officials cautioned the public and those involved in the program against focusing on numbers and acknowledged that CDC was moving away from the initial 30-day timeline, arguing that the variation in public health system structures across the country and in local needs and characteristics made it impossible and undesirable to require specific numbers and set a strict deadline (CDC, 2003d). In communication with members of the mass media in February 2003, DHHS and CDC personnel de-emphasized the focus on numbers of vaccinees and began to emphasize the importance of preparedness. One CDC spokesperson stated: “We’re trying to do a better job of clarifying what the purpose of this program is, and the purpose of this program is to better prepare our country to respond to a bioterrorism event involving smallpox” (McGlinchey, 2003a). Despite that, CDC continued to urge rapid implementation of the smallpox vaccination program without
guidance on how states could determine the pace and scope of their vaccination efforts (GAO, 2003).
Cardiac Adverse Events and Other Safety Concerns
|
In late March 2003, three vaccinees—two civilian women and one man in the military program—died from myocardial infarction (heart attack) within 5, 6, and 22 days of smallpox vaccination, respectively. All three had a history of heart disease or risk factors, including smoking and hypertension, so it was not immediately clear whether their deaths were related to vaccination. Later study showed that the deaths were consistent with what would have been expected in the population, and there was no evidence that smallpox vaccination created a higher-than-expected risk of heart attacks. But their deaths, combined with concern about a newly identified cardiac adverse event, had a substantial chilling effect on the willingness of volunteers to receive the vaccine. |
A total of 1,000 people were vaccinated in the first 2 weeks of the program (different jurisdictions began at different times). After that, the number of vaccinees grew at a relatively steady rate of roughly 3,000–5,000 every week. That changed with the appearance of cardiac adverse events at the end of March 2003, when the program slowed down to fewer than 1,500 per week. By the end of April 2003, only a few hundred volunteers were being vaccinated every week (Henderson, 2003b). The number of weekly vaccinations continued to decline and never recovered, reaching a handful of vaccinees weekly, then monthly. Between April 30, 2004, and July 31, 2004, 25 people received smallpox vaccination, and during August 2004, 5 people were vaccinated (CDC, 2004a, 2004b, 2004c).
Cardiac Adverse Events
The myocardial infarction cases were only some of the cardiac adverse events associated with the civilian and military vaccination programs. Several cases of myocarditis and pericarditis (types of heart inflammation collectively labeled myo/pericarditis) were identified in the civilian program after smallpox vaccination, beginning in February 2003. Although they made a more subtle impression than fatal heart attacks, their association with vaccination was more worrisome. A report in the March 28, 2003, issue of Morbidity and Mortality Weekly Report listed seven cardiac adverse events in the civilian program: three myocardial infarctions, including the two fatal civilian cases noted above; two cases of angina; and two cases
of myo/pericarditis (CDC, 2003f). By March 21, 2003, the military program had documented 10 cases of myo/pericarditis in addition to the heart attack noted above (CDC, 2003f; Connolly, 2003c). Recent and historical evidence supported an association between myo/pericarditis and smallpox vaccination (CDC, 2003k). The Department of Defense (DoD) identified a likely causal association between smallpox vaccination and myo/pericarditis (Halsell et al., 2003); the evidence of a causal association in the civilian population remained unclear (ACIP, 2003b).
Concern about cardiac complications associated with smallpox vaccine caused apprehension in people planning to be vaccinated and led many states to temporarily suspend vaccination and wait for CDC guidance (Connolly, 2003c; Kuhles and Ackman, 2003). The Advisory Committee on Immunization Practices (ACIP) and the ACIP Working Group on Smallpox Vaccination—created in February 2003 to monitor vaccination program communication, surveillance, and research activities (ACIP, 2003a)—held an emergency meeting on March 28, 2003, to make recommendations to CDC about medical screening of potential vaccinees and follow-up of persons with cardiovascular risk factors after vaccination (CDC, 2003h). The working group recommended the deferral of volunteers who had known cardiac disease, those who had three or more risk factors (for example, smoking, high blood pressure, and high blood cholesterol concentrations) for heart disease, and those over 50 years old (Neff, 2003a). ACIP accepted the first two recommendations but not the last, because of concerns that it would exclude a substantial proportion of potential civilian vaccinees likely to be older and previously vaccinated (and therefore considered less vulnerable to vaccine complications) (Neff, 2003b). ACIP also looked at historical evidence about myo/pericarditis, found largely in the context of military vaccination among young Finnish men (Helle et al., 1978; Kanjalainen et al., 1983). U.S. data from the 1960s were limited to pediatric vaccination, in which myo/pericarditis could have been missed (CDC, 2003h). Finally, ACIP recommended to CDC the following exclusion criteria: known underlying heart disease with or without symptoms and the presence of three or more of the known major cardiac risk factors (CDC, 2003a, 2003h). CDC accepted ACIP’s recommendations and moved rapidly to revise the vaccination information package, screening materials, and informed consent form.
Myo/pericarditis, the cardiac complication not previously recognized among expected adverse events of smallpox vaccination, constituted a major safety finding and was later identified in the context of clinical trials of a second-generation smallpox vaccine. The pharmaceutical company Acambis in April 2004 temporarily suspended volunteer recruitment for the ACAM2000 clinical trials of cell-culture smallpox vaccine because of the occurrence of at least three cases of myo/pericarditis (Roos, 2004a). In
September 2004, the Food and Drug Administration lifted the clinical hold on enrollment in the ACAM2000 trials and concurred with Acambis that enrollment could be closed and analysis of the data could begin. Acambis undertook 12-month followup of affected study subjects.
Both DoD and CDC conducted followup of vaccinees with myo/pericarditis (Mootrey, 2003). Research on myo/pericarditis is needed, and efforts are already in progress. In July 2004, the National Institutes of Health provided funding for research on the effect of smallpox vaccine on cardiac cells in mice (Roos, 2004b).
Vaccination Program Safety Profile
In the weeks surrounding the beginning of the program, health care and public health organizations described their unease regarding specific safety issues related to the vaccination program. Although the program was voluntary, the potential of inadvertent transmission of vaccinia virus meant that adults and children who had neither consented to vaccination nor been screened for contraindications could become infected and face the risk of severe adverse events or even death (AAP, 2003). Some organizations also feared that the pace of the vaccination program could make it difficult to arrange staff schedules to provide time for leave or furlough in order to ensure patient safety (Burstein, 2002; Peterson, 2002; Schulman, 2002; Baker, 2003).
Reintroducing the smallpox vaccine in the absence of the disease required special attention to safety, including screening for contraindications and preventing the inadvertent transmission of vaccinia virus to contacts because the risk-benefit ratio was less clear in the absence of naturally occurring smallpox. CDC and its state and local counterparts worked to ensure safety at every step before, during, and after vaccination. CDC made every effort to develop effective and efficient screening methods and guidelines for pre-event smallpox vaccination clinics. In response to the present committee’s recommendations, CDC developed an information sheet for contacts of vaccinees and modified the Pre-Event Vaccination System to document active surveillance of vaccine-related adverse events that required hospitalization or outpatient care, contraindications to vaccination among volunteers or their household contacts not identified before vaccination, and vaccinia-virus transmission to contacts (CDC, 2003e; IOM, 2003a). Volunteers were given multiple opportunities to learn about the vaccine and vaccination and to opt out if they determined that they were unable or unwilling to be vaccinated, and a thorough informed consent process was put into place.
The ACIP identified several contraindications to smallpox vaccination, and CDC included them in the screening process and in training and education materials. Prospective vaccinees would be excluded from vaccination for the following reasons: age (no one under 18 years old would be vaccinated in nonemergency situations), history of allergic reaction to vaccine or its components, breastfeeding, and moderate or acute illness. Prospective vaccinees would also be excluded if they or immediate household contacts had any of the following contraindications: pregnancy; disease, conditions, or treatments that cause immunosuppression or immune deficiency; and any acute, chronic, or exfoliative skin conditions, such as eczema and atopic dermatitis (CDC, 2003b). Furthermore, there are many people with compromised immune systems (because of HIV infection, immunosuppression for organ transplantation, or cancer therapy) for whom smallpox vaccine would hold a greater risk, and these conditions were included among the contraindications for smallpox vaccinations. Although there are many clinical data on reactions to smallpox vaccine, they predate contemporary immunosuppression. And very little information about fetal vaccinia and adverse events in inadvertently vaccinated pregnant women is available. After the vaccination of six civilians who later discovered that they were pregnant, ACIP established a pregnancy registry that would conduct follow-up of civilian and DoD pregnant women who were vaccinated (CDC, 2003g).
Concerns about program safety persisted. The present committee and the Association of State and Territorial Health Officials (ASTHO) recommended a pause between phases I and II to evaluate safety and ensure an adequate level of planning for expanded vaccination to a new population that required extensive communication and education for safety (for example, prevaccination screening and postvaccination site care). Phase III, intended to make the vaccine available to insistent members of the public, seemed even more problematic, in that it would pose public health threats, vast logistic challenges, and special and intensive communication requirements. Also, the final phase of the program would offer a potentially harmful vaccine in the context of an unknown risk, creating a philosophic conflict with health care and public health workers’ injunction to “do no harm” (AAP, 2003; Libbey, 2002).
In February 2004, the program’s safety profile reflected a small number of cases of inadvertent inoculation, indicating that vaccinees were probably effectively educated to prevent transmission. That and the fact that three of the four historically noted serious adverse events did not occur at all are also likely indicators of effective training and screening (see Table 3-1).
TABLE 3-1 Number of Reported Cases of Selected Adverse Events Associated with Smallpox Vaccination Among Civilians, by Type, United States, January 24-December 31, 2003
|
Adverse Events |
Number of Casesa |
||
|
Suspected |
Probable |
Confirmed |
|
|
Eczema vaccinatum |
— |
— |
— |
|
Fetal vaccinia |
— |
— |
— |
|
Generalized vaccinia |
2 |
— |
1 |
|
Inadvertent inoculation, nonocular |
11 |
— |
9 |
|
Ocular vaccinia |
1 |
— |
2 |
|
Progressive vaccinia |
— |
— |
— |
|
Erythema multiforme major (Stevens-Johnson syndrome) |
— |
— |
— |
|
Myo/pericarditis |
16 |
5 |
— |
|
Postvaccinial encephalitis or encephalomyelitis |
1 |
— |
— |
|
Pyogenic infection of vaccination site |
— |
— |
— |
|
SOURCE: CDC (2004d). a “Adverse events that have been associated with smallpox vaccination are classified on the basis of evidence supporting the reported diagnoses. Cases verified by virologic testing are classified as confirmed. Cases are classified as probable if possible alternative etiologies are investigated and excluded and supportive information for the diagnosis is found. Cases are classified as suspected if they have clinical features compatible with the diagnosis, but either further investigation is required or investigation of the case did not provide supporting evidence for the diagnosis” (CDC, 2003m). |
|||
The War in Iraq
|
Military action in Iraq began on March 19, 2003. Both the events leading up to the war and the period after the declared end of major combat may have influenced public opinion and attitudes about and participation in the smallpox vaccination program. President Bush declared an end to major hostilities in Iraq in April 2003 (although military action continued). |
As described in Chapter 2, the smallpox vaccination policy grew out of a series of discussions among top government officials about the possible existence of smallpox virus outside the two known repositories in Russia and the United States and about the threat of deliberate release of smallpox virus. The scope and content of intelligence considered in decision-making were not made known to the public until the publication of the Senate Intelligence Committee’s Report on the U.S. Intelligence Community’s Pre-
war Intelligence Assessments on Iraq (assuming that this evidence was used to make smallpox vaccination policy), which found the evidence of the presence of smallpox virus in Iraq to be weak (U.S. Senate Select Committee on Intelligence, 2004).
The rationale for the vaccination program was not formally linked with the possible and later impending war with Iraq, a nation suspected of possessing weapons of mass destruction (perhaps including smallpox virus, on the basis of information from the 1990s). However, the public comments of three public health officials (CDC Director Julie Gerberding, DHHS Secretary Tommy Thompson, and National Immunization Program Director Walter Orenstein) and two legislators (Senators Judd Gregg and Bill Frist) alluded to the war in a way that could be construed as suggestive of a link or suggested that the war served as an impetus for the increased number of vaccinees and rapid program implementation (Connolly, 2003a; Hallow, 2002; Manning and Sternberg, 2002; Rath and Turcotte, 2003; Reuters, 2003). Multiple mass media reports indicate that some public health and health care workers believed that the vaccination program was linked with the war, and public opinion about the vaccination program was split, not unlike public opinion about the war (Russell, 2003). Evidence from the mass media and from an ASTHO survey suggests that the perceived association between the war and the vaccination program was one of several reasons for suspicion and concern among prospective vaccinees, as well as a barrier to vaccination (ASTHO, 2003). Although government officials neither updated nor reiterated the smallpox threat assessment, mass media reports showed a downward shift in public perception about the level of risk of smallpox release and therefore a decreased motivation to receive the vaccine (Fiorill, 2003; Manning, 2003; McKenna, 2003; Yee, 2003). That shift in public perception may have contributed to the decline in the vaccination rate in April and May 2003.
The Compensation Plan
|
In April 2003, a compensation plan for people who experienced a smallpox vaccine injury was signed into law, largely addressing concerns about the adequacy of provisions available to protect people injured by smallpox vaccination and resolving some concerns about institutional liability in the event of inadvertent transmission of vaccinia. |
Early in the implementation of the program, health care and public health organizations and labor unions expressed concern about the lack of adequate provisions for vaccine injury compensation and for some types of
personal and institutional liability (GAO, 2003). Some states chose to wait until these issues were resolved to begin vaccination, and many prospective volunteers expressed confusion about what protections were available to them and reluctance to assume risks without adequate assurance of protection (MacLeod, 2003; Roos, 2003a). In January 2003, the American College of Emergency Physicians (ACEP), the American Hospital Association (AHA), and the American Nurses Association (ANA), and others found that the narrow definition of liability coverage provided under Section 304 of the Homeland Security Act seemed to provide protection to the vaccine manufacturer, the vaccinator, and the institution operating a vaccination clinic but left other institutions and people without coverage (such as hospitals that do not have vaccination clinics although their personnel may receive vaccination elsewhere and vaccinated personnel in a noncovered institution who may be liable for inadvertently infecting a patient). Furthermore, ACEP, AHA, and ANA were concerned about an incomplete and confusing patchwork of compensation solutions (for example, worker compensation not applicable to volunteers and differences among states) and the lack of a no-fault compensation mechanism for volunteers who experience complications and for people inadvertently infected by vaccinees. Although some states and institutions provided coverage under worker compensation or other mechanisms, available coverage was fragmentary at best. The American Public Health Association, the American College of Occupational and Environmental Medicine, the Service Employees International Union (SEIU), the Association of Federal, State, County, and Municipal Employees, and many others called for the development of comprehensive compensation mechanisms to protect people injured by smallpox vaccination (APHA, 2002; SEIU, 2002; August, 2003; Russell, 2003). SEIU and other health professionals’ labor unions also called for a safer bifurcated needle (SEIU, 2002). The present committee urged CDC to clarify the status of compensation mechanisms as part of the informed consent process. As a result, CDC added information about compensation issues in the Vaccine Information Statement (Box 3-1).
On January 23, 2003, members of the Senate asked the White House to provide a plan for vaccine injury compensation (Daschle et al., 2003). Early proposals would provide coverage of vaccine injuries for those who would be vaccinated within 180 days of the program’s initiation; this created concern about inappropriate pressure to receive the vaccine (Meckler, 2003b). The comprehensive compensation plan was proposed by DHHS in early March 2003, 6 weeks after the expected start of the vaccination program. The Senate Committee on Health, Education, Labor, and Pensions passed the smallpox compensation bill on April 2, 2003, and the Smallpox Emergency Personnel Protection Act of 2003 (SEPPA, PL 108-20) was signed into law by President Bush on April 30, 2003. The SEPPA
|
BOX 3-1 January 2003 – Members of the Senate ask the White House for smallpox vaccine injury compensation. Smallpox vaccination program begins. March 6, 2003 – DHHS proposes a smallpox compensation plan. April 2, 2003 – Senate Committee on Health, Education, Labor and Pensions passes smallpox compensation bill. April 30, 2003 – President Bush signs into law the Smallpox Emergency Personnel Protection Act (SEPPA, PL 108-20) establishing a smallpox vaccine injury compensation program. August 27, 2003 – SEPPA interim final rule: Smallpox Vaccine Injury Table published in Federal Register. December 16, 2003 – SEPPA interim final rule: Administrative policies, procedures, and requirements guiding the program published in Federal Register. SOURCES: Daschle et al. (2003); DHHS (2003d); Federal Register (2003a, 2003b). |
interim final rule, with the smallpox vaccine injury table specifying the injuries, disabilities, conditions, and deaths that would be covered by the program (previously published in August 2003), was issued on December 16, 2003 (Federal Register, 2003b).
The General Accounting Office Report: An Early Assessment of Program Progress
|
On April 30, 2003, 3 months after the beginning of civilian vaccination, the General Accounting Office (GAO, now the Government Accountability Office) released its evaluation of the progress of the smallpox vaccination program. |
GAO was asked to examine the implementation of the smallpox vaccination program and to describe program challenges. In an April 2003 report, GAO found that the program progressed more slowly than anticipated owing in part to a demanding program schedule that did not allow sufficient time for preparation either at CDC or state levels. GAO noted that CDC responded to questions about the lower-than-expected numbers
of vaccinees by stating that even as few as 50,000 vaccinated people could mount an effective response (GAO, 2003). However, that number was not formally announced as the new target figure for the national pre-event vaccination program, and the report stated that CDC did not provide the evidence for the smaller number, nor did it outline what level of vaccination was necessary for smallpox preparedness (GAO, 2003; McGlinchey, 2003a).
As the smallpox vaccination program moved away from an emphasis on numbers to an emphasis on smallpox preparedness, public health agencies at the state and local levels reported that they lacked guidance about what preparedness meant and about how to assess whether they were prepared for a potential smallpox release (GAO, 2003; Selecky, 2003). The lack of clarity about program goals identified above remained a problem. The GAO report recommended that CDC provide guidance to its grantees for revising phase I vaccination targets and for expanding the program in the second phase.
Supplementary Funding for the Smallpox Vaccination Program
|
On May 5, 2003, DHHS notified the states that $100 million in supplemental funding would be made available to support smallpox vaccination efforts. The additional funding aimed to address state and local public health agency concerns about the costs imposed by the program. Although the overall effect of the funding has not been assessed, one local health official testified before a U.S. House of Representatives committee that the supplemental funding came too late for her public health agency, which had “cut other commitments” to implement the smallpox vaccination program (U.S. House of Representatives, 2004). |
The timing of the smallpox vaccination program coincided with a period of intense budgetary crises in most state governments (NGA, 2003). Soon after the beginning of the program, ASTHO, the National Association of County and City Health Officials (NACCHO), and various state and local health officials, some of whom considered the program an unfunded mandate, provided program cost estimates that far exceeded CDC’s estimate (Colacecchi and Jones, 2003; Libbey, 2003; Rosado, 2003). CDC’s testimony before the Senate Committee on Health, Education, Labor, and Pensions on January 30, 2003, gave an estimated cost of vaccination of $10-15 per person, compared with the estimates of NACCHO and state and local health officials, which ranged from $100 to $400 per vaccinee (Colacecchi and Jones, 2003; Libbey, 2003). Although vaccination kits
(vaccine, diluent, and needles) were provided to state and local public health agencies at no charge, public health agencies and their national representatives asserted that CDC’s calculations did not include the activities and capabilities required to plan and implement smallpox vaccination clinics, including training, education, and communication (NACCHO, 2003a, 2003b). The National Association of Counties (NACo) and the National Governors Association (NGA) also expressed concern about the financial consequences of the smallpox vaccination program for the public health infrastructure and in the context of state and county budget deficits (NGA, 2003; Rosado, 2003). NGA, NACo, ASTHO, and NACCHO called for additional federal funding to prevent the diversion of funds from general bioterrorism preparedness and public health activities to smallpox-specific efforts and argued that funding would be especially needed to support the expansion of smallpox vaccination to a second phase (Hardy, 2002; Libbey, 2002, 2003; NGA, 2003). One estimate of the cost of phase II was $600 million to $1 billion (Kuhles and Ackman, 2003). The present committee and others called for an assessment of the full costs of the smallpox vaccination program (APHA, 2002; IOM, 2003b).
The April 2003 GAO report found that state and local health officials experienced substantial financial and workforce burdens on state and local public health agencies (GAO, 2003). ASTHO and NACCHO conducted additional study of the cost of the first phase of smallpox vaccination and found that it ranged from $79 to $1,784 per vaccination, and they estimated an average cost per vaccination of $265 and $204, respectively (NACCHO, 2003b).
The Monkeypox Outbreak
|
In May and June 2003, a monkeypox outbreak was identified in Wisconsin; and cases were later found in Illinois, Indiana, Kansas, Missouri, and Ohio (CDC, 2003l). The disease, resembling smallpox, tested preparedness. Although familiarity with smallpox disease appeared to be an asset, at least one possibly systemic problem surfaced; CDC was not alerted about the outbreak for 13 days. |
The monkeypox outbreak did not mark the first time during the course of the smallpox vaccination program that a naturally emerging infectious disease threat surfaced. In late 2002 and early 2003, a novel coronavirus emerged in China and spread rapidly to other nations along travel routes, causing widespread alarm and economic damage by crippling the tourism and travel industries from Hong Kong to Toronto (IOM, 2004). By sum-
mer 2003, when it seemed to recede, severe acute respiratory syndrome (SARS) had sickened about 8,000 people across Asia, Europe, and North America and caused the deaths of nearly 800. SARS placed enormous strains on many public health agencies in the United States. The emergence of SARS and later monkeypox during the course of the smallpox vaccination program was a reminder of the importance of public health preparedness for a wide array of potential problems (the “all-hazards” approach used by other agencies). Naturally occurring diseases, from West Nile virus to monkeypox to SARS, require capabilities, resources, training, education, and communication channels similar to those needed to respond to deliberate attack with bioweapons and could therefore serve as proxy events. The committee has discussed the usefulness of proxy events in its sixth report (see Appendix G) and has recommended that CDC support a system to ensure the continuing collection, synthesis, and sharing of lessons learned and best practices public health response to proxy events.
A child in Wisconsin was identified as having the first case of monkeypox in the United States during this outbreak. The child had contracted the disease from a sick pet prairie dog. The disease was ultimately traced to a Gambian giant rat and other exotic rodents that infected a number of prairie dogs. Humans were infected by contact with pets; most of the patients had confirmed exposure to infected rodents and no cases of solely human-to-human transmission were reported. By the end of the outbreak, 71 cases in the six states had been reported to CDC; 35 cases were laboratory-confirmed, and 36 were suspect and probable. On June 12, 2003, CDC made a recommendation, on the basis of expert opinion and limited evidence that people exposed to monkeypox be given smallpox vaccine (CDC, 2003j). Thirty people received smallpox vaccine to prevent transmission of monkeypox; 7 were vaccinated before exposure and 23 after exposure, and no severe adverse events were reported among vaccinees (CDC, 2003l).
Although there has been little systematic study of the monkeypox experience, the anecdotal reports of federal, state, and local public health agencies suggest that smallpox preparedness activities had a favorable effect on the response to the monkeypox outbreak (McGlinchey, 2003b). Clinicians were familiarized with poxvirus diseases, and communication linkages between the health care and public health communities (for example, for reporting and surveillance) were strengthened. Trained vaccinators were available to vaccinate affected people with smallpox vaccine, and vaccine supplies were available regionally. Unfortunately, a dysfunction in the system was identified when the initial cases of monkeypox were not reported to CDC for 13 days; local experts apparently tried to identify the pathogen by using only their local and state resources (CDC, 2003i; Mitchell, 2003). In a smallpox outbreak, such a delay could be expensive and deadly.
June 2003 ACIP Recommendation to End the Smallpox Vaccination Program
|
At the June 2003 ACIP meeting, the ACIP recommended that for safety reasons the federal government not expand smallpox vaccination beyond the health care and public health response team members still being vaccinated (ACIP, 2003b). (In May 2003, the present committee had called for a pause in the vaccination program to assess safety and to plan carefully before extending vaccination to more people.) |
In June 2003, CDC reported that the number of vaccinees working in hospitals was small—only 40 percent of acute-care hospitals had at least one staff member vaccinated, and only 1 in 10 hospitals had two or more vaccinated staff members—a deficiency in numbers that could require a regional rather than a local response in some areas in the event of a smallpox-virus release (CDC, 2003a; Yee, 2003). The smallpox vaccination program did not come to an official stop in response to the ACIP recommendation, but the pace of vaccination continued to decline. By July 25, 2003, the total number of civilian vaccinees was 38,004—far short of the 500,000 that had been given as a program target and still short of the 50,000 that CDC had suggested in GAO’s assessment (CDC, 2003c; GAO, 2003). A year later, on July 31, 2004, civilian vaccinations had reached a cumulative total of 39,579 (CDC, 2004c). Nearly 2 years after the beginning of the program, smallpox vaccination has all but come to a halt, with a mere handful of vaccinations each month.
In the months after the vaccination rate began to fall, CDC did not formally urge states and other jurisdictions to increase their vaccinees to specified numbers, and it did not take any steps to formally reiterate the need for the vaccine. A Washington Post article in July 2003 noted the recent silence on the part of White House and federal officials who made the decision to vaccinate and legislators who had been vocal supporters of the vaccination program (Connolly, 2003b). The same article quoted CDC Director Gerberding: “ ‘Can we stand up clinics across the country tomorrow to immunize our nation in 10 days? No,’ she acknowledged. Still, we ‘have made enormous progress.’ ”
In July 2004, DHHS adviser D.A. Henderson stated that the smallpox vaccination of first responders was no longer needed, because enough vaccine was available to vaccinate the nation, if needed, and many cities had improved their capability to respond to a potential smallpox attack (Calabresi and August, 2004; Malenic, 2004).
At the time this report was written, the program was languishing, and
there was nearly complete silence on the part of the federal government about the status and future of this biopreparedness program; no official update of program progress or impact had been provided (see additional discussion in Chapter 4).
NOTEWORTHY FEATURES OF THE PROGRAM
Congressional Interest and Involvement
Members of Congress contributed to the smallpox vaccination program at various points in its evolution, from policy development to evaluation. Some policy-makers contributed to the early discussion of policy options. In the weeks and months before the smallpox vaccination program was announced, Senators Bill Frist and Judd Gregg and others publicly urged the government to consider making smallpox vaccine available to all Americans to facilitate individual choice (Frist, 2002; Gregg, 2002; McKenna, 2003). Several congressional committees and subcommittees held hearings on the subjects of smallpox vaccination and bioterrorism preparedness beginning soon after the September 11, 2001, attacks. Testimony before Congress as early as October 2001 (ASTHO, 2001) informed legislators about the need for a smallpox response plan, the need for additional resources, the need for a compensation mechanism for injuries associated with smallpox vaccination, and problems in the implementation of the program (NGA, 2003; U.S. House of Representatives, 2004). For example, at a July 2003 hearing of the Senate Committee on Health, Education, Labor and Pensions, members of the Senate expressed concerns about the slow progress of smallpox vaccination and questioned federal officials about possible causes, including delays in finalizing the table of vaccine-related injuries that could be compensated under the new federal compensation provisions (Heil, 2003). Congress also played an important role in moving the legislation to provide a comprehensive plan for compensation of people injured by smallpox vaccine (Rath and Turcotte, 2003).
Finally, members of Congress asked GAO to evaluate progress in the smallpox vaccination program; this led to the April 2003 report described above (GAO, 2003). In January 2004, a year after the beginning of smallpox vaccination, some members of Congress issued a report that critiqued the smallpox vaccination program and called for changes to ensure and strengthen smallpox preparedness (U.S. House of Representatives Select Committee, 2004).
The Relationship Between the Civilian and Military Vaccination Programs
The focus of this report is the implementation of CDC’s civilian smallpox vaccination program. However, past committee reports and activities reflect its continuing interest in the parallel program implemented by DoD. The civilian and military programs are inherently related, and the committee believes that there is much to be learned from the two programs taken individually and together. DoD staff made presentations at the committee’s meetings and responded to committee inquiries about the military program’s progress, its administrative and educational efforts, and its safety system and related research.
The military vaccination program began immediately after the president’s announcement on December 13, 2002, and has advanced at a steady and rapid pace, reaching and surpassing 600,000 vaccinees (DoD, 2004b). The smallpox vaccination program provided a unique opportunity for collaboration between CDC and DoD, and although the two programs involved very different circumstances and populations, there was much to be learned from both. The military population included a much higher percentage of young people never before vaccinated (and likely to be in very good health because of the nature of their job and their ages), whereas vaccination among the civilian population involved generally older people, most of whom had been vaccinated in the past. Also, the military program required vaccination for designated personnel, whereas the civilian program was voluntary. Unlike civilian vaccinees, who would pose a potential risk of inadvertent inoculation to spouses or household contacts, military personnel who were vaccinated were likely to live in settings and have duties that could expose a higher number of contacts to inoculation, not just spouses and intimate partners. For example, military activities and facilities are likely to require close physical interaction among personnel, several people may be required to use the same bedding consecutively, and laundry is processed in a communal fashion. Those factors had the potential to increase inadvertent exposure of nonvaccinated people to vaccination sites and secretions.
Both the military and civilian programs conducted follow-up of adverse events (CDC, 2003k), and the ACIP Working Group on Smallpox Vaccine Safety reviewed safety data generated by both programs. Although efforts were made to facilitate the flow of information between DoD and CDC, administrative difficulties and questions arose. For example, both CDC and DoD posted weekly updates of adverse events in their programs, but at times, information about adverse events in the military program was not communicated to the public or to the public health community in a
timely fashion. Also, if there were inadvertent inoculation of civilian contacts of military vaccinees, it was not immediately clear which program—the civilian or the military—would include these cases in its adverse events surveillance.
Studies of reported adverse events (such as myocardial infarctions and myo/pericarditis) (CDC, 2003b) benefited from having the larger combined civilian and military vaccinees as potential study populations. For example, the data from the military supported the finding in the civilian population that cases of myo/pericarditis were associated with smallpox vaccination. However, it was also more difficult to identify adverse events specifically caused by the smallpox vaccine in the military population, because members of the military often received multiple concurrent vaccinations.
DoD has also made some important findings in its smallpox vaccination program. For example, in November 2003, two independent panels examined four deaths potentially related to DoD’s vaccination program and found that one (the April 2003 death of a 22-year-old reservist) may have been triggered by several vaccinations she received, including smallpox vaccine (DoD, 2003, 2004a). In February 2004, DoD reported that an infant contracted tertiary vaccinia infection from breastfeeding (the mother was inadvertently inoculated by the father) (CDC, 2004e). In the August 25, 2004, Smallpox Vaccination Program Safety Summary, DoD reported that over 631,000 personnel had been vaccinated and that most adverse events had occurred at a rate lower than historical rates (DoD, 2004c).
Despite some early challenges, the collaboration between CDC and DoD gave the ACIP Working Group on Smallpox Vaccine Safety access to the substantial amount of data gathered by the much larger military program. The committee has previously expressed its hope that the Department of Defense Serum Repository and the Millennium Cohort Study will serve as resources for CDC as it follows up vaccinees and learns about the long-term sequelae of serious adverse events (IOM, 2003d).
PROGRAM CHALLENGES
A Push for Rapid Implementation Without Adequate Preparation
The committee has found that owing to the initial emphasis on rapid implementation of the smallpox vaccination program, CDC had little or no time to finalize or test many of the program components (such as the completeness or consistency of vaccine information and education materials) or to address identified barriers to implementation (IOM, 2003a, or refer to Appendix B in this report). That may explain many of the problems with the execution of the program, such as the financial and opportunity-
cost problems reported by many state and local public health agencies, the lack of an adverse event compensation plan and the many delays in developing and implementing it with needed clarification on liability issues, unfinalized informed consent materials, and the lack of an appropriate and complete data system in the first 3 weeks of the program.
Although rapid program implementation would have been warranted in the face of an impending crisis, government’s assurances that there was no imminent threat made the call for rapid implementation perplexing.
The Informed Consent Process
Like other aspects of smallpox vaccination program implementation, the informed consent process suffered from the program’s rapid start and ambitious timeline. The early weeks of the program appeared to be caught up in a whirlwind of enormous effort on the part of CDC (GAO, 2003). CDC staff developed dozens of educational training materials, provided technical assistance and held regular conference calls with state public health agency leadership, and worked on communication plans. The crucial importance of the informed consent information and forms was recognized from the beginning, but additional time was needed to make corrections and improvements in the materials. That meant that some of the items were not final at the time vaccination began. The committee’s concerns about the informed consent form were related to larger issues, such as the lack of adequate compensation provisions and the program’s unique nature as a public health program established for national security reasons, that at a practical level implied a public health intervention with known risk and unknown benefits. For those reasons, the committee expressed concern in its first report to CDC that the informed consent form did not include an explanation of the state of compensation mechanisms for volunteers who would be injured by the vaccine (IOM, 2003a, or refer to Appendix B). The committee believed that there were ethical reasons for including clear language about compensation on the informed consent form, and it noted that there was a tension between the desire to maximize participation of appropriate candidates in the program and the imperative to minimize participation of those with contraindications and to create conditions that would allow those unwilling to receive the vaccine to feel comfortable in declining. CDC delayed for several weeks updating the informed consent and vaccination information materials with information about injury compensation to avoid deluging jurisdictions with yet another in a series of changes that seemed to cause dismay and logistical difficulties.
The Data System
An effective data system was needed before the program started. Unfortunately, the Pre-event Vaccination System (PVS) was not operational until 3 weeks after the vaccination program began. The haste of implementation did not allow CDC to ensure that the system met the needs of state public health agencies, nor did it allow time for the creation of an active adverse event surveillance system. Some states reported difficulties in using the PVS, including the fact that PVS relied on a readily available Internet connection, which some of the implementing entities lacked (Pezzino, 2003). The PVS was also needed to place rates of adverse events in context to facilitate accurate understanding, an appropriate alternative to having single cases get mass media attention. The committee discussed and made specific recommendations pertaining to CDC’s data system in several of its earlier reports (IOM, 2003a, 2003b, 2003c) (see Appendixes B, C, and D). CDC later developed an active adverse events surveillance system.
The relatively slow progress of the program and the small numbers of vaccinees also complicated efforts to evaluate data for safety purposes (GAO, 2003). The committee is not aware of whether CDC has conducted a comprehensive assessment of the safety data system functioning, the completeness of the data gathered, and their relevance to the continuation of vaccination efforts.
Other Challenges
The smallpox vaccination program highlighted some of the challenges facing the health care delivery system and characterizing its relationship with public health. The program asked public health professionals and their health care colleagues to communicate and collaborate more intensively than usual. Mechanisms for communication (such as reporting by clinicians, and informing by public health authorities) between the health care and public health communities vary greatly (in both quantity and quality) across jurisdictions in all elements of concern to public health, including disease reporting in general (IOM, 2002a; Hirshon, 2003; Temte, 2003). The smallpox vaccination program and associated training and information activities required efforts that may have enhanced the channels available for communication. However, there was some concern in the clinician community that relying on the Internet as a main channel of communication posed a risk of bypassing clinicians who do not use the Internet or of getting lost in the midst of a barrage of other messages (Temte, 2003). Additional, redundant communication channels and extensive, regular training and education are needed to reach all clinicians and to facilitate the
rapid movement of information between the health care and public health communities and among local, state, and national public health agencies.
The smallpox vaccination program also highlighted the fact that the health care system is under great strain. Many hospitals made considerable efforts to participate in the vaccination program and to conduct training and other preparedness activities, but the added burden brought into relief the other challenges facing them (such as overcrowding and staff shortages) and the health care system in general (Grady and Altman, 2003; HealthLeaders, 2003). Smallpox preparedness and the broad concerns of all-hazards preparedness served as a reminder that surge capacity may be a challenge for many communities in the event of a crisis—hospitals have limited beds available, there are staffing shortages, and there are large populations of uninsured people that will need prophylaxis or care in the event of a smallpox-virus release or other bioterrorist attack (Anderson, 2003). Furthermore, the first responder communities included in smallpox vaccination and other preparedness activities (because they have health and safety responsibilities and interact with the health care system) have other responsibilities, and additional interaction and dialogue are needed to ensure realistic expectations and smooth functioning of a multidisciplinary response to public health disasters, such as a smallpox-virus release.
FAVORABLE OUTCOMES
Despite the considerable challenges that arose in the course of the smallpox vaccination program, its implementation has provided opportunities for learning about smallpox vaccination and the conduct of biopreparedness programs and has led to some favorable outcomes. (The committee wrote on this subject at length in its fourth report, included here in Appendix E.)
Opportunities for Learning
Programmatically, CDC accomplished much in the implementation of the smallpox vaccination program, working under great time pressures to develop and enhance the range of capabilities needed to respond to a potential smallpox-virus release. CDC provided training in smallpox vaccination and smallpox disease to public health and medical personnel, developed communication plans and tools, and regularly interacted with state and local public health agencies, answering questions related to implementation and providing technical assistance.
Chapter 4 discusses two major lessons learned from the smallpox vaccination program, but the committee asserts that there are many lessons yet
to be learned with respect to research and evaluation. There are many components of the smallpox vaccination program, both procedural issues and areas of scientific evidence, that could be mined for lessons that could be applied in future biopreparedness programs and in planning for other kinds of public health threats, such as pandemic influenza. For example, how did states select the people they initially considered to be potential smallpox vaccinees, and what has been learned about the composition and structure of response teams that might be useful in other types of public health emergencies? What has been learned from the compensation and liability quandary that would be useful in planning other biopreparedness activities? With regard to ethics, what can be learned from the evident tension between concern about the risks posed by the vaccine and altruism or between the imperative to have a voluntary program with truly informed consent and the need to implement a biopreparedness program to prepare the public health and health care workforce? What can be learned from the discussion about high-priority groups for vaccination and related complex decision-making processes? How well did the vaccine adverse events reporting systems (including active surveillance) work? CDC needs to complete, assess, and publish the results of its evaluative studies conducted during the program. Such findings are critical if the need arises to renew vaccination efforts, and they could be derived from a variety of studies described by CDC staff to the committee during open committee meetings. Important questions include these:
-
What was the general rate of work-limiting or recreation-limiting activities due to symptoms or illnesses in the first month after vaccination?
-
What are the current rates of serious adverse events related to smallpox vaccine in the civilian and military populations?
-
What are current findings from the pregnancy registry, including pregnancy outcomes?
The committee urges that a concerted effort be made to document and publish all information from the program that could facilitate future programs at the intersection of public health and national security.
Other Favorable Outcomes
CDC’s efforts and those of CDC’s state and local partners facilitated the forging of multiple partnerships between state and local public health agencies and the health care and first responder communities (Gursky, 2003). Multiple presenters at the committee’s meetings spoke of the partnerships among the health care and public health communities and between
public health agencies and first responders (Anderson, 2003; Bresnitz, 2003; Fischler, 2003; Nikolai, 2003; Toomey, 2003).
The smallpox vaccination program provided coincidental preparation for the monkeypox event. A great deal of training about smallpox and, to a lesser extent, orthopoxviruses was implemented, and the health care community was more prepared to identify unusual rashes and probably more attuned to any symptoms out of the ordinary. That meant that when monkeypox appeared in the United States, there was a greater awareness and even readiness among health care providers. Because of the smallpox vaccination program, vaccine was readily available in all the states that had monkeypox cases, and trained and experienced personnel were available to screen, vaccinate, and follow up (Yee, 2003).
The smallpox vaccination program is also reported to have had a favorable effect on the state and local public health response to SARS, which emerged late in 2002 and continued through spring 2003 (Staiti et al., 2003). Although there is little empirical evidence to pinpoint or quantify improved performance, public health agencies have reported improved communication with their health care counterparts and an improved surveillance system (Judson, 2003; Selecky, 2003; Skivington, 2003; Witt, 2003).
Finally, the vaccination program provided opportunities to learn more about adverse vaccine effects in adults. Adverse events surveillance during implementation led to the identification of a new serious adverse event. Cases of myo/pericarditis were confirmed in the military program, and probable cases were identified in the civilian program, necessitating followup and future study.
The vaccination program served as a case study of biopreparedness with relevance for future similar endeavors. The committee has previously urged CDC to take full advantage of the data collected and experience gained in the course of implementing the program (IOM, 2003b, 2003d). Evaluation and research activities could be undertaken in areas ranging from the administrative to the scientific, from determining the overall cost of the smallpox vaccination program and specific components to assessing the opportunity cost to public health agencies and identifying long-term effects of vaccine-related adverse events.
CONCLUDING OBSERVATIONS
Implementation of the smallpox vaccination program began in January 2003 and is continuing. The rate of vaccination rose gradually for the first several weeks but then began a steep decline from which it never recovered—monthly vaccination numbers dropped to the single digits during summer 2004.
This chapter provides a summary of key milestones in the course of the program and other major events that occurred during implementation and may have affected or been affected by the program. The program experienced considerable implementation challenges. However, it also provided opportunities to gain experience with a broad multisector and interdisciplinary effort of biopreparedness and led to novel findings about potential complications from smallpox vaccine.
The committee hopes that CDC and its partners at the state and local levels will ensure that what has been accomplished and learned through the great investment of effort and resources is sustained and is integrated into the full spectrum of public health preparedness.
TABLE 3-2 Smallpox Vaccination Program Timeline
|
Date |
Events (policy, program, and other developments) |
IOM Committee Meeting or Report |
|
September 2000 |
DHHS contracts with OraVax (now a part of Acambis, Inc.) for new smallpox vaccine to be delivered in 2004 (CIDRAP and IDSA, 2004). |
|
|
June 2001 |
ACIP recommendation on smallpox (vaccinia) vaccine: because of low risk of deliberate release and indeterminate risk to population, limit vaccination to laboratory or medical personnel working with non-highly-attenuated orthopox viruses (CDC, 2001). “Dark Winter,” a war game for senior-level officials, is conducted by Center for Strategic and International Studies in partnership with Johns Hopkins Center for Civilian Biodefense Studies and ANSER Institute for Homeland Security. Exercise included a smallpox outbreak spreading to 25 states and 15 countries (ANSER Institute for Homeland Security, 2003). |
|
|
September 2001 |
Terrorist attacks in New York, Arlington (Virginia), and Pennsylvania. DHHS placed an order for 40 million doses of smallpox vaccine with Acambis (20-year contract for cell-culture vaccine) (IOM, 2002b). |
|
|
October 2001 |
Letters containing anthrax spores delivered through U.S. mail. |
|
Date |
Events (policy, program, and other developments) |
IOM Committee Meeting or Report |
|
November 2001 |
DHHS awards $428 million contract to Acambis/Baxter to produce smallpox vaccine (DHHS, 2001). NIH-funded researchers began to examine efficacy of diluted Dryvax smallpox vaccine (NIH, 2001). |
|
|
February 2002 |
CDC asks ACIP to review its recommendations on smallpox vaccination. |
|
|
April 2002 |
NIAID study finds that Dryvax smallpox vaccine may be diluted to expand supply (Frey et al., 2002; NIH, 2002). Dilutions of 1:5 and 1:10 resulted in take rates approximately as high as undiluted vaccine. |
|
|
June 2002 |
ACIP meets and drafts supplemental recommendations on smallpox vaccination (vaccinate up to 20,000 health care and public health workers) (ACIP, 2002). Public Health Security and Bioterrorism Preparedness and Response Act of 2002 signed into law (FDA, 2002). |
|
|
October 2002 |
ACIP meets again and updates recommendations on smallpox vaccination. ACIP also recommends offering vaccine to up to 500,000 health care and public health personnel (CDC, 2002a). |
IOM Committee on Smallpox Vaccination Program Implementation convened at request of CDC |
|
November 2002 |
President signs Homeland Security Act (White House, 2002a). Designated CDC staff members receive smallpox vaccination (epidemiologic investigation teams) (Associated Press, 2001). Mass media report that Bush administration intelligence review has concluded that four nations (Iraq, North Korea, Russia, and France) may possess covert and illegal stocks of smallpox virus (Gellman, 2002). |
|
|
December 2002 |
States submit to CDC smallpox response plans and smallpox pre-event vaccination plans (CDC, 2002c). CDC completes initial review of state smallpox vaccination plans President announces smallpox vaccination program. |
First meeting of IOM Committee on Smallpox Vaccination Program Implementation |
|
Date |
Events (policy, program, and other developments) |
IOM Committee Meeting or Report |
|
|
HHS telebriefing on smallpox policy (White House, 2002b); initial goal: vaccinate 500,000 workers in 30 days. |
|
|
January 2003 |
Letter to White House issued by minority members of Senate calling for smallpox vaccine injury compensation. CDC begins shipping smallpox vaccine to states. Department of Homeland Security established. DHHS secretary authorizes civilian smallpox vaccinations. Civilian smallpox vaccination begins. |
First report of IOM committee |
|
February 2003 |
Media reports cite lack of a compensation plan as a barrier to smallpox vaccination (MacLeod, 2003; Meckler, 2003c). DHHS announces contracts to develop safer smallpox vaccines (DHHS, 2003b). DoD has vaccinated over 100,000 against smallpox. Morbidity and Mortality Weekly Report (MMWR) notifies of one case of angina 4 days after smallpox vaccination (CDC, 2003p). DoD reports first cases of myocarditis among personnel recently immunized with smallpox vaccine (CDC, 2003f). |
Second meeting of IOM committee |
|
March 2003 |
First civilian instances of myo/pericarditis identified, later classified as suspected and probable (CDC, 2003s). DHHS proposes smallpox vaccination compensation plan. Homeland security threat level changed to orange (high) (White House, 2003a). Surgeon general, CDC director, and others are vaccinated against smallpox (Kemper, 2003b). War with Iraq begins on March 19, 2003 (White House, 2003b). Maryland woman and Florida woman die from heart attack 5 and 22 days, respectively, after smallpox vaccination. Man dies from myocardial infarction 6 days after smallpox vaccination. ACIP recommends additional cardiac exclusion criteria for smallpox vaccination (CDC, 2003f; Kemper, 2003c). CDC issues Health Alert Network health advisory to avoid vaccinating people with |
Second report of IOM committee |
|
Date |
Events (policy, program, and other developments) |
IOM Committee Meeting or Report |
|
|
cardiac risk factors and recommends temporary deferral for heart patients who volunteer for vaccination (CDC, 2003q). Multiple states temporarily postpone all smallpox vaccination clinics. CDC accepts ACIP’s exclusion criteria and revises fact sheets, screening materials, and informed consent form. |
|
|
April 2003 |
More states suspend smallpox vaccination programs indefinitely, others for a limited length of time (Kemper, 2003c). Virginia defers initiation of smallpox vaccination. Media reports death of female reservist after receiving smallpox and anthrax vaccines (Mendieta, 2003; Roos, 2003c). GAO report Smallpox Vaccination: Implementation of National Program Faces Challenges finds that 6% of target population has been vaccinated by week 10 of program; data are insufficient to assess safety (GAO, 2003). On April 30, 2003, president signs into law Smallpox Emergency Personnel Protection Act of 2003, which establishes no-fault Smallpox Vaccine Injury Compensation Program (CDC, 2003r). |
|
|
May 2003 |
President declares end of major combat operations in Iraq (White House, 2003c). DHHS makes $100 million in supplemental funding available for the smallpox vaccination program (DHHS, 2003c). Monkeypox outbreak reported in several states including Wisconsin and Texas (CDC, 2003l). Media reports that in April and May, some states have begun offering smallpox vaccine to first responders (ABC 13 News, 2003; Murphy, 2003). |
Third meeting of IOM committee Third report of IOM committee |
|
June 2003 |
CDC Recommends smallpox vaccine to protect persons exposed to monkeypox (CDC, 2003j). ACIP recommends against expansion of smallpox vaccination program beyond “first phase” (ACIP, 2003c). |
|
|
Date |
Events (policy, program, and other developments) |
IOM Committee Meeting or Report |
|
August 2003 |
Oregon Health Sciences University researchers find that smallpox immunity may persist for decades, but there is disagreement about the meaning of the findings (Roos, 2003b). |
Fourth report of IOM committee |
|
October 2003 |
Ohio decides against offering smallpox vaccination to first responders (Shockman, 2003). MMWR reports that a review of death records shows that 1947 NYC smallpox vaccination campaign did not lead to increase in cardiac deaths (CDC, 2003o). |
|
|
November 2003 |
Two independent panels examine four deaths potentially related to DoD’s smallpox vaccination program and found that one (April death of 22 year-old reservist) may have been triggered by vaccinations including vaccinia (DoD, 2003). |
Fourth meeting of IOM committee |
|
December 2003 |
Federal government issues interim final rule for Smallpox Emergency Personnel Protection Act of 2003 (SEPPA), plan for smallpox vaccine injury compensation. CDC updates smallpox case definitions. |
Fifth report of the IOM committee |
|
January 2004 |
HHS secretary’s declaration regarding administration of smallpox countermeasures extended until and including January 23, 2005 (keeping SEPPA in place). |
|
|
February 2004 |
Military infant contracts vaccinia from breastfeeding (CIDRAP, 2004). DoD reports that 581,183 service members received smallpox shots from December 13, 2002, to February 11, 2004. Seventy-two vaccinees, or about 1 in 8,072, suffered myopericarditis, and there were 30 cases of vaccinia infection in contacts of vaccinees. Other complications included 36 cases of generalized vaccinia, most of which required only outpatient treatment, and one case of encephalitis (DoD, 2004d). |
|
|
March 2004 |
|
Fifth meeting of the IOM committee |
|
Date |
Events (policy, program, and other developments) |
IOM Committee Meeting or Report |
|
April 2004 |
Acambis temporarily suspends volunteer recruitment for its clinical trials of cell-culture smallpox vaccine because of occurrence of at least three cases of myo/pericarditis in one trial (Roos, 2004a). DoD reports that 10 HIV-positive members of military who received smallpox vaccination did not experience adverse events (Tasker et al., 2004). |
|
|
May 2004 |
A review of New York City death certificates from 1946, 1947, and 1948 shows that the 1947 mass smallpox vaccination campaign in New York did not show an increase in cardiac deaths postvaccination (Thorpe et al., 2004). |
|
|
June 2004 |
DoD announces anthrax and smallpox vaccinations for all personnel deployed by Central Command and, for first time, select units in Pacific Command. Since December 2002, 625,000 troops have been vaccinated against smallpox (Malenic, 2004). |
|
|
July 2004 |
The Senate Select Committee on Intelligence issues report on US intelligence community’s prewar intelligence assessments on Iraq; among other findings, report describes evidence on Iraq’s possession of smallpox as weak (U.S. Senate Select Committee on Intelligence, 2004). |
Sixth report of the IOM committee Sixth meeting of the IOM committee |
|
September 2004 |
Aventis Pasteur vaccine produced in the 1950s shown to be effective, even in dilutions of 1:5 and 1:10 (Talbot et al., 2004). |
|
|
October 2004 |
Acambis and Bavarian Nordic A/S win $177 million U.S. government contract to produce safer smallpox vaccine (DHHS, 2004). CIA issues Comprehensive Report of the Special Advisor to the Director of Central Intelligence on Iraq’s WMD. Report concludes that, although Iraq had capability to work with smallpox virus, there is “no direct evidence that Iraq either retained or acquired smallpox virus isolates or proceeded with any follow up smallpox related research” (CIA, 2004). |
REFERENCES
AAP (American Academy of Pediatrics). 2003. Testimony of Jon Abramson, Chair, Committee on Infectious Diseases of the American Academy of Pediatrics. Senate Health, Education, Labor, and Pensions Committee. Heading on the Smallpox Vaccination Plan: Challenges and Next Steps.
ABC 13 News. 2003. Virginia’s First Responders Get Smallpox Vaccinations. WSET/ABC 13 News.
ACIP (Advisory Committee on Immunization Practices). 2002. Record of the Meeting of the Advisory Committee on Immunization Practices (Centers for Disease Control and Prevention, National Immunization Program). Atlanta, GA.
ACIP. 2003a. Record of the meeting of the Advisory Committee on Immunization Practices, February 26-27, 2003.
ACIP. 2003b. Record of the meeting of the Advisory Committee on Immunization Practices, June 18-19, 2003.
ACIP. 2003c. Advisory Committee on Immunization Practices (ACIP) Statement on Smallpox Preparedness and Vaccination. [Online] Available at http://www.bt.cdc.gov/agent/smallpox/vaccination/pdf/acipjun2003.pdf. Accessed July 17, 2003.
ANA (American Nurses Association). 2002. Statement to the IOM’s Committee on Smallpox Vaccination Program Implementation.
Anderson R. 2003. Presentation Before the IOM Committee on Smallpox Vaccination Program Implementation. Transcript of Meeting Three on May 1, 2003, Washington, DC.
ANSER Institute for Homeland Security. 2003. Dark Winter. [Online] Available at http://www.homelandsecurity.org/darkwinter/index.cfm. Accessed September 14, 2004.
APHA (American Public Health Association). 2002. APHA Policy Statement on Smallpox Vaccination. [Online] Available at http://www.apha.org/legislative/policy/smallpox.pdf. Accessed June 17, 2003.
Associated Press. 2001, November 5. CDC Workers Get Smallpox Vaccination.
Associated Press. 2003, February 3. St. Vincent Won’t Give Smallpox Vaccinations. KARK.Com.
ASTHO (Association of State and Territorial Health Officials). 2001. Testimony Before the Senate Committee on Appropriations, Subcommittee on Labor, Health and Human Services, and Education Appropriations, October 3, 2001. Bioterrorism Preparedness. [Online] Available at http://www.astho.org/templates/display_pub.php?u=JnB1Yl9pZD0zMTQ=. Accessed December 12, 2004.
ASTHO. 2003. ASTHO Smallpox Preparedness Survey Results. [Online] Available at http://www.astho.org/pubs/spxsummaryb2.pdf. Accessed May 21, 2003.
August J (Assistant Director for Health and Safety, Department of Research and Collective Bargaining Services). 2003. Testimony for the American Federation of State, County and Municipal Employees (AFSCME). Senate Appropriations Committee: Labor, Health and Human Services and Education Subcommittee , January 29, 2003.
Baker M. 2003. Testimony on Behalf of the Service Employees International Union, AFL-CIO Before the U.S. Senate Committee on Health, Education, Labor, and Pensions on the Administration’s Smallpox Vaccination Plan: Challenges and Next Steps. January 30, 2003. [Online] Available at www.SEIU.org.
Banks S, Hannan C. 2002. ASTHO Ensures State Health Officials Are Heard in Smallpox Debate. [Online] Available at http://astho.org/templates/displau_pub.php?pub_id=336. Accessed September 15, 2004.
Bavley A, Dvorak J. 2003, February 8. Safety Concerns Hinder Participation in Smallpox Vaccination Program. Kansas City Star.
Boston Herald. 2003, January 23. Editorial: Vaccine Dodgers Grow.
Bresnitz E. 2003. Presentation. Transcript from the IOM’s Committee on Smallpox Vaccination Program Implementation Meeting Two on February 13, 2003, Washington, DC: 62-85.
Burstein J. 2002. Statement of the American College of Emergency Physicians. Transcript from the IOM’s Committee on Smallpox Vaccination Program Implementation Meeting One on December 19, 2002, Washington, DC:267-271.
Calabresi M, August M. 2004, July 26. Was Smallpox Overhyped? (The Smallpox Scare: Was the Germ-Warfare Risk Overblown?). TIME 16.
CDC (Centers for Disease Control and Prevention). 2001. Vaccinia (smallpox) vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2001. Morbidity and Mortality Weekly Report 50(RR-10):1-25.
CDC. 2002a. CDC Telebriefing Transcript: ACIP Smallpox Vaccine Meeting Briefing, October 17, 2002. [Online] Available at http://www.cdc.gov/od/oc/media/transcripts/t021017.htm. Accessed August 1, 2004.
CDC. 2002b. Supplemental Guidance for Planning and Implementing the National Smallpox Vaccination Program (NSVP).
CDC. 2002c. Press Release, December 12, 2002. CDC Initial Review of State Smallpox Vaccination Plans Complete. [Online] Available at http://www.cdc.gov/od/oc/media/pressrel/r021212.htm. Accessed August 1, 2004.
CDC. 2002d. CDC Telebriefing Transcript: HHS Teleconference on Smallpox Policy. [Online] Available at http://www.cdc.gov/od/oc/media/transcripts/t021214.htm. Accessed January 10, 2003.
CDC. 2003a. Interim Smallpox Fact Sheet: Smallpox Vaccine and Heart Problems. [Online] Available at http://www.bt.cdc.gov/agent/smallpox/vaccination/heartproblems.asp. Accessed January 15, 2001.
CDC. 2003b. Smallpox Fact Sheet—Information for Clinicians: Smallpox (Vaccinia) Vaccine Contraindications.
CDC. 2003c. Smallpox Vaccination Program Status by State, July 25, 2003.
CDC. 2003d. CDC Telebriefing Transcript: Smallpox Vaccine Adverse Events Monitoring and Response System. [Online] Available at http://www.cdc.gov/od/oc/media/transcripts/t030206.htm. Accessed March 11, 2003.
CDC. 2003e. Notice to readers: smallpox vaccine adverse events monitoring and response system for the first stage of the smallpox vaccination program. Morbidity and Mortality Weekly Report 52(05):88-89, 99.
CDC. 2003f. Cardiac adverse events following smallpox vaccination—United States, 2003. Mortality and Morbidity Weekly Report 52(12):248-250.
CDC. 2003g. Notice to readers: national smallpox vaccine in pregnancy registry. Mortality and Morbidity Weekly Report 52(12):256.
CDC. 2003h. Notice to readers: supplemental recommendations on adverse events following smallpox vaccine in the pre-event vaccination program: recommendations of the Advisory Committee on Immunization Practices. Mortality and Morbidity Weekly Report 52(13):282-284.
CDC. 2003i. CDC Telebriefing Transcript: Update: Monkeypox Investigation in the U.S.
CDC. 2003j. Updated Interim CDC Guidance for Use of Smallpox Vaccine, Cidofovir, and Vaccinia Immune Globulin (VIG) for Prevention and Treatment in the Setting of an Outbreak of Monkeypox Infections. CDC Guidelines and Resources. [Online] Available at http://www.cdc.gov/ncidod/monkeypox/treatmentguidelines.htm. Accessed December 15, 2004.
CDC. 2003k. Update: cardiac and other adverse events following civilian smallpox vaccination—United States, 2003. Mortality and Morbidity Weekly Report 52(27):639-642.
CDC. 2003l. Update: multistate outbreak of monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. Mortality and Morbidity Weekly Report 52(27):642-646.
CDC. 2003m. Update: adverse events following smallpox vaccination—United States, 2003. Mortality and Morbidity Weekly Report 52(13):278-282.
CDC. 2003n. Advisory Committee on Immunization Practices (ACIP) Statement on Smallpox Preparedness and Vaccination. [Online] Available at http://www.bt.cdc.gov/agent/smallpox/vaccination/pdf/acipjun2003.pdf. Accessed July 17, 2003.
CDC. 2003o. Cardiac deaths after a mass smallpox vaccination campaign—New York City, 1947. Morbidity and Mortality Weekly Report 25(39):933-936.
CDC. 2003p. Smallpox Vaccination Report Status and Adverse Events. [Online] Available at http://www.cdc.gov/od/oc/media/smpxrpt.htm. Accessed March 7, 2003.
CDC. 2003q. CDC Health Advisory (via Health Alert Network): Smallpox: People with Known Cardiac Disease Should Not Be Vaccinated. [Online] Available at http://www.phppo.cdc.gov/han/Documents/AlertDocs/OldAlerts/129.asp. Accessed January 26, 2005.
CDC. 2003r. Smallpox Emergency Personnel Protection Act of 2003: Benefits and Compensation for Smallpox Vaccine Injuries. [Online] Available at http://www.bt.cdc.gov/agent/smallpox/vaccination/bene-comp.asp. Accessed June 16, 2003.
CDC. 2003s. Smallpox vaccine adverse events among civilians—United States, March 4-10, 2003. Mortality and Morbidity Weekly Report 52(10):201-203.
CDC. 2004a. Smallpox Vaccination Program Status by State, April 30, 2004.
CDC. 2004b. Smallpox Vaccination Program Status by State, August 31, 2004.
CDC. 2004c. Smallpox Vaccination Program Status by State, July 31, 2004.
CDC. 2004d. Update: adverse events following civilian smallpox vaccination—United States, 2003. Mortality and Morbidity Weekly Report 53(05):106-107.
CDC. 2004e. Secondary and tertiary transfer of vaccinia virus among U.S. military personnel—United States and worldwide, 2002-2004. Morbidity and Mortality Weekly Report 53(05):103-105.
CIA (Central Intelligence Agency). 2004. Comprehensive Report of the Special Advisor to the Director of Central Intelligence on Iraq’s WMD. [Online] Available at http://www.cia.gov/cia/reports/iraq_wmd_2004/. Accessed January 26, 2005.
CIDRAP (Center for Infectious Disease Research and Policy). 2004, February 12. Contact Vaccinia in Soldier’s Baby Linked to Breast-feeding. CIDRAP News.
CIDRAP and IDSA (Infectious Diseases Society of America). 2004. Smallpox: Current, Comprehensive Information on Pathogenesis, Microbiology, Epidemiology, Diagnosis, Treatment, and Prophylaxis. [Online] Available at http://www.cidrap.umn.edu/cidrap/content/bt/smallpox/biofacts/smllpx-summary.html. Accessed August 23, 2004.
Colacecchi J, Jones M. 2003. Testimony Before the Senate Appropriations Committee: Senate Labor, Health and Human Services and Education Appropriations Subcommittee, January 29, 2003. State Smallpox Pre-event Phase 1 Vaccination Program Estimated Costs. [Online] Available at http://appropriations.senate.gov/releases/record.cfm?id=190233. Accessed January 21, 2005.
Connolly C. 2003a, February 24. Bush Smallpox Inoculation Plan Near Standstill; Medical Professionals Cite Possible Side Effects, Uncertainty of Threat. Washington Post, A6.
Connolly C. 2003b, July 17. Focus on Smallpox Threat Revived. Washington Post, A3.
Connolly C. 2003c, March 29. 2 States Halt Smallpox Shots: Pentagon Reports First Post-Inoculation Fatal Heart Attack. Washington Post.
Connolly C, Milbank D. 2002, December 14. U.S. Revives Smallpox Shot. Bush Says He Will Receive Vaccine with Military, Emergency Workers. Washington Post, A1.
Daschle T, and 21 members of Senate (Democrat). 2003, January 22. Letter to President George W. Bush.
DHHS (Department of Health and Human Services). 2001, November 28. News Release: HHS Awards $428 Million Contract to Produce Smallpox Vaccine. Acambis/Baxter Will Produce 155 Million Doses by End of 2002.
DHHS. 2003a, January 24. News Release: HHS Secretary Authorizes Smallpox Vaccination Program. [Online] Available at www.hhs.gov/news/press/2003pres/20030124e.html. Accessed January 25, 2003.
DHHS. 2003b, February 25. News Release: HHS Announces Contracts to Develop Safer Smallpox Vaccines.
DHHS. 2003c, May 5. News Release: Secretary Thompson to Release $100 Million to Assist States with Smallpox Vaccination Programs.
DHHS. 2003d, March 5. News Release: HHS Proposes Smallpox Vaccination Compensation Plan. [Online] Available at http://www.hhs.gov/news/press/2003pres/20030305.html. Accessed March 7, 2003.
DHHS. 2004, October 7. News Release: HHS Awards $232 Million in Biodefense Contracts for Vaccine Development.
DoD (Department of Defense). 2003. Panels Issue Reports on Vaccine Adverse Event Cases: Vaccination Possibly Related to Soldier’s Illness and Death. Press release, November 19, 2003.
DoD. 2004a. Vaccination Possible Cause of Soldier’s Illness and Death. [Online] Available at http://www.vaccines.mil/documents/337safetypanelQA.pdf.
DoD. 2004b, June 30. News Release: Anthrax, Smallpox Protection Policies Updated. News Release No. 624-04. [Online] Available at http://www.defenselink.mil/releases/2004/nr20040630-0955.html. Accessed January 30, 2005.
DoD. 2004c. DoD Smallpox Vaccination Program Safety Summary. [Online] Available at http://www.smallpox.army.mil/event/SPSafetySum.asp.
DoD. 2004d. Smallpox Vaccination Program Summary: DoD Smallpox Vaccination Program as of February 11, 2004.
Everett W, Coffin S, Zaoutis T, Halpern S, Kim J, Strom B. 2002. Smallpox Willingness: A National Study of Emergency Department Personnel. Presented to the IOM Committee on Smallpox Vaccination Program Implementation on December 19, 2002 .
FDA (Food and Drug Administration). 2002. The Bioterrorism Act of 2002. [Online] Available at http://www.fda.gov/oc/bioterrorism/bioact.html. Accessed January 26, 2005.
Federal Register. 2003a. Smallpox Vaccine Injury Compensation Program: Administrative Implementation. August 27, 2003, Washington, DC, 51492-51499. [Online] Available at http://www.gpoaccess.gov/fr/index.html.
Federal Register. 2003b. Smallpox Vaccine Injury Compensation Program: Administrative Implementation. December 16, 2003, Washington, DC, 70080-70106. [Online] Available at http://www.gpoaccess.gov/fr/index.html.
Fiorill J. 2003, October 24. U.S. Smallpox Official Acknowledges Vaccination “Targets Were Not Achieved”. Global Security Newswire.
Fischler D. 2003. Presentation Before the IOM Committee on Smallpox Vaccination Program Implementation. Transcript of Meeting Four on November 6, 2003, Washington, DC.
Frey SE, Newman F, Cruz J, Shelton WB, Tennant JM, Polach T, Rothman AL, Kennedy JS, Wolff M, Belshe RB, Ennis FA. 2002. Dose-related effects of smallpox vaccine. New England Journal of Medicine 346(17):1275-1280.
Frist B. 2002, August 9. Op-Ed: Deciding Who Is Protected Against Smallpox. New York Times.
GAO (General Accounting Office). 2003. Smallpox Vaccination: Implementation of National Program Faces Challenges. Washington, DC: General Accounting Office, GAO-03-578.
Gellman B. 2002, November 5. Findings Spur New Smallpox Concerns. Albany (NY) Times Union.
Grady D, Altman L. 2003, March 25. Experts See Gains and Gaps in Planning for Terror Attack. New York Times.
Gregg J. 2002, October 6. Commentary: Let the Public Choose on Smallpox Vaccine. Washington Post.
Gursky E. 2003. Progress and Peril: Bioterrorism Preparedness Dollars and Public Health. Century Foundation. [Online] Available at http://www.tcf.org/Publications/HomelandSecurity/Gursky_Progress_Peril.pdf. Accessed January 30, 2005.
Hallow R. 2002, November 24. Governors Seek Access to Smallpox Vaccine. Washington Times, A4.
Halsell JS, Riddle JR, Atwood JE, Gardner P, Shope R, Poland GA, Gray GC, Ostroff S, Eckart RE, Hospenthal DR, Gibson RL, Grabenstein JD, Arness MK, Tornberg DN. 2003. Myopericarditis following smallpox vaccination among vaccinia-naive U.S. military personnel. Journal of the American Medical Association 289(24):3283-3289.
Hardy G. 2002. Statement of the Association of State and Territorial Health Officials. Presentation Before the IOM Committee on Smallpox Vaccination Program Implementation. Transcript of Meeting One on December 19, 2002, Washington, DC.
HealthLeaders. 2003. Smallpox Scare: Hospitals May Incur Costs, Downtime from Vaccinations. Green Sheet, February 2003. [Online] Available at http://www.healthleaders.com/magazine/2003/feb/greensheet.php. Accessed July 7, 2004.
Heil E. 2003, July 24. Panel Questions HHS on Low Smallpox Vaccinations. GovExec.com. [Online] Available at http://www.govexec.com/dailyfed/0703/072409cd2.htm. Accessed September 29, 2003.
Helle EP, Koskenvuo K, Heikkila J, Pikkarainen J, Weckstrom P. 1978. Myocardial complications of immunisations. Annals of Clinical Research 10(5):280-287.
Henderson J. 2003a. National Smallpox Vaccination Program Update. Presentation Before the IOM Committee on Smallpox Vaccination Program Implementation on May 1, 2003.
Henderson J. 2003b. Update on National Smallpox Preparedness: Smallpox Preparedness Indicators. Presentation Before the IOM Committee on Smallpox Vaccination Program Implementation on November 6, 2003.
Hirshon J. 2003. Presentation to the IOM Committee on Smallpox Vaccination Program Implementation. Transcript of Meeting Four on November 6, 2003, Washington, DC.
IOM (Institute of Medicine). 2002a. The Future of the Public’s Health in the 21st Century. Washington, DC: The National Academies Press.
IOM. 2002b. Scientific and Policy Considerations in Developing Smallpox Vaccination Options: A Workshop Report. Washington, DC: The National Academies Press.
IOM. 2003a. Review of the Centers for Disease Control and Prevention’s Smallpox Vaccination Program Implementation: Letter Report #1. Washington, DC: The National Academies Press.
IOM. 2003b. Review of the Centers for Disease Control and Prevention’s Smallpox Vaccination Program Implementation: Letter Report #2. Washington, DC: The National Academies Press.
IOM. 2003c. Review of the Centers for Disease Control and Prevention’s Smallpox Vaccination Program Implementation: Letter Report #3. Washington, DC: The National Academies Press.
IOM. 2003d. Review of the Centers for Disease Control and Prevention’s Smallpox Vaccination Program Implementation: Letter Report #4. Washington, DC: The National Academies Press.
IOM. 2004. Learning from SARS: Preparing for the Next Disease Outbreak. Washington, DC: The National Academies Press.
Judson F. 2003. Presentation Before the IOM Committee on Smallpox Vaccination Program Implementation. Transcript of Meeting Three on May 1, 2003, Washington, DC.
Karjalainen J, Heikkila J, Nieminen MS, Jalanko H, Kleemola M, Lapinleimu K, Sahi T. 1983. Etiology of mild acute infectious myocarditis. Relation to clinical features. Acta Medica Scandinavica 213:65-73.
Kemper V. 2003a, January 17. States Lag at Start of Smallpox Program. Los Angeles Times.
Kemper V. 2003b, March 14. Few Volunteer for Smallpox Vaccination. Los Angeles Times.
Kemper V. 2003c, March 29. Guardsman Dies After Receiving Smallpox Vaccine; California Suspends All Inoculations and Wants New Federal Guidelines for Adverse Reactions. Los Angeles Times.
Kuhles D, Ackman D. 2003. The federal smallpox vaccination program: where do we go from here? Health Affairs (Web Exclusive) W3:503-510.
Libbey P. 2002. Presentation Before the IOM Committee on Smallpox Vaccination Program Implementation. Transcript of Meeting One on December 19, 2002, Washington, DC.
Libbey P. 2003. Testimony on Behalf of NACCHO (National Association of County and City Health Officials) Before the Subcommittee on Labor, Health, and Human Services, Education and Related Agencies, Committee on Appropriations, U.S. Senate. Hearing on Smallpox Vaccination, January 29, 2003.
MacLeod M. 2003, February 2. State Puts Smallpox Program on Hold Until Feds Resolve Liability Issue. Macomb Daily [Michigan].
Malenic M. 2004. U.S. to Expand Military Vaccinations. Global Security Newswire.
Manning A. 2003, April 22. Second Round of Smallpox Vaccinations Begins. USA Today.
Manning A, Sternberg S. 2002, October 7. Officials Ponder Timing, Sequence of Immunizations. USA Today.
Marchione M. 2003, February 7. Health Workers Enlist in Smallpox Fight; State Giving Hospitals Time to Educate Workers About Risk of Shot. Milwaukee Journal Sentinel, 15A.
McCullough M. 2003, February 7. Americans Aren’t Rolling Up Their Sleeves for Smallpox Inoculation. The Salt Lake Tribune.
McGlinchey D. 2003a, February 26. CDC Says It Never Aimed for 500,000 Smallpox Vaccinations. Global Security Newswire.
McGlinchey D. 2003b, June 12. Monkeypox Outbreak Tests Bioterrorism Response System. Global Security Newswire.
McKenna M. 2003, February 15. No Vaccination for Nation’s Fear; Unease Hinders Smallpox Program. Atlanta Journal-Constitution.
McNeil D. 2003, February 7. Many Balking at Vaccination for Smallpox. New York Times.
Mendieta A. 2003. A Young Soldier from the South Suburbs Died of a Mysterious Illness Before She Could Fulfill Her Dream of Serving Her Country on the Front Lines. Chicago Sun-Times, 6.
Meckler L. 2002, October 5. US Divided on Smallpox Policy. Associated Press.
Meckler L. 2003a, February 22. Smallpox Vaccinations Off to Slow Start. Associated Press.
Meckler L. 2003b, April 2. Lawmakers Split on Smallpox Compensation. Associated Press.
Meckler L. 2003c, February 5. Concerns About Compensation for People Injured by the Smallpox Vaccine Are Hampering Inoculation Program . Associated Press.
Mitchell S. 2003, June 12. Monkeypox Shows Gap in Bioterror Readiness. United Press International.
Mootrey G. 2003. Presentation Before the IOM Committee on Smallpox Vaccination Program Implementation. Transcript of Meeting Three on May 1, 2003, Washington, DC.
Murphy W. 2003, April 14. FDNY Sets Terms for Smallpox Vaccinees. Newsday.
NACCHO (National Association of County and City Health Officials). 2003a. Impact of Smallpox Vaccination Program on Local Public Health Services. Research Brief No. 9.
NACCHO. 2003b. Costs of Smallpox Vaccination Program: Summary of Preliminary Data. Research Brief No. 10.
Neff J. 2003a. Advisory Committee on Immunization Practices Smallpox Vaccine Safety Working Group: Summary of Vaccination Program Safety Monitoring, April 2003.
Neff J. 2003b. Presentation Before the IOM Committee on Smallpox Vaccination Program Implementation. Transcript of Meeting Three on May 1, 2003, Washington, DC.
New York Times. 2002, December 22. Editorial: Ducking Smallpox Vaccinations.
NGA (National Governors Association). 2003. Statement of the National Governors Association for the Senate Labor, Health and Human Services and Education Appropriations Subcommittee on Smallpox Vaccination Funding. Senate Appropriations Commitee: Senate Labor, Health and Human Services and Education Appropriations Subcommittee.
NIH (National Institutes of Health). 2001. Fact Sheet: Study Seeks to Determine Effectiveness of Diluted Smallpox Vaccine. [Online] Available at http://www.vrc.nih.gov/factsheets/btsmallpox.htm. Accessed January 26, 2005.
NIH. 2002. Press Release: NIAID Study Results Support Diluting Smallpox Vaccine Stockpile to Stretch Supply. [Online] Available at http://www.nih.gov/news/pr/mar2002/hhs-28.htm. Accessed January 26, 2005.
Nikolai K. 2003. Presentation Before the IOM Committee on Smallpox Vaccination Program Implementation. Transcript of Meeting Three on May 1, 2003, Washington, DC.
Ornstein C, Bonilla D. 2003, February 6. Smallpox Plan Divides Hospitals: Fifteen in the County Refuse Vaccinations for Employees. Nine Accept; Most Are Undecided. Los Angeles Times.
Ornstein C, Richardson L. 2003, February 8. U.S. Far Behind Schedule for Smallpox Vaccinations. Los Angeles Times.
Pasternack N. 2003, February 5. State to Give Vaccine: First Shots Will Be for Health Workers. Olympia (WA) Olympian.
Peterson C. 2002. Presentation Before the IOM Committee on Smallpox Vaccination Program Implementation. Transcript of Meeting One on December 18, 2002, Washington, DC.
Pezzino G. 2003. Presentation Before the IOM Committee on Smallpox Vaccination Program Implementation. Transcript of Meeting Three on May 1, 2003, Washington, DC.
Rath E, Turcotte J. 2003, March 6. Press Release: Senator Gregg to Move Smallpox Compensation Legislation Through Senate. Office of Judd Gregg, U.S. Senator for New Hampshire.
Reuters. 2003, March 27. Experts Urge Caution in US Smallpox Plan.
Roos R. 2003a, February 5. Many Doses of Smallpox Vaccine Shipped, But Few Shots Given so Far. CIDRAP News.
Roos R. 2003b, August 21. D.A. Henderson Critiques Report on Duration of Smallpox Immunity. CIDRAP News.
Roos R. 2003c, November 19. Vaccines Might Have Contributed to Death of Army Reservist. CIDRAP News.
Roos R. 2004a, April 13. Myopericarditis Cases Complicate Acambis Smallpox Vaccine Trials. CIDRAP News.
Roos R. 2004b, June 16. Pilot Study to Probe Cardiac Effects of Smallpox Vaccine. CIDRAP News.
Rosado E. 2003. Fact Sheet: Smallpox. National Association of Counties.
Russell S. 2003, January 18. Smallpox Vaccine Plan Starts Tuesday, Despite Concerns; Some Health Workers May Opt Out Over Risks, Compensation Issues. San Francisco Chronicle.
Schulman R. 2002. Presentation Before the IOM Committee on Smallpox Vaccination Program Implementation. Transcript of Meeting One on December 18, 2002, Washington, DC.
SEIU (Service Employees International Union). 2002. As Bush Plan for Mass Smallpox Stirs Controversy, Nation’s Largest Health Care Organization Says Hospital Workers, Patients Will Face Unnecessary Risk. [Online] Available at http://www.seiu.org/media/press_releases/press.cfm?ID=1096. Accessed January 8, 2003.
Selecky M. 2003. Presentation Before the IOM Committee on Smallpox Vaccination Program Implementation. Transcript of Meeting Three on May 1, 2003, Washington, DC.
Shockman L. 2003, March 28. Nationwide Smallpox Protection Program for Health Workers Is Shy of Goal. Toledo [Ohio] Blade.
Skivington S. 2003. Presentation Before the IOM Committee on Smallpox Vaccination Program Implementation. Transcript of Meeting Three on May 1, 2003, Washington, DC.
Staiti A, Katz A, Hoadley J. 2003. Has Bioterrorism Preparedness Improved Public Health? Center for Studying Health System Change Issue Brief No. 65.
Talbot T, Stapleton J, Bready R, Winokur P, Bernstein D, Germanson T, Yoder S, Rock M, Crowe J, Edwards K. 2004. Vaccination success rate and reaction profile with diluted and undiluted smallpox vaccine: a randomized controlled trial. Journal of the American Medical Association 292(10):1205-1212.
Tasker S, Schnepf G, Lim M, Caraviello H, Armstrong A, Bavaro M, Agan B, Delmar J, Aronson N, Wallace M, Grabenstein J. 2004. Unintended smallpox vaccination of HIV-1 infected. Clinical Infectious Diseases 38(9):1320-1322.
Temte J. 2003. Presentation Before the IOM Committee on Smallpox Vaccination Program Implementation. Transcript of Meeting Four on November 6, 2003, Washington, DC.
Thorpe L, Schwartz S, Manning S, Marx M, Frieden T. Mass smallpox vaccination and cardiac deaths, New York City, 1947. Emerging Infectious Diseases 10(5):917-920.
Toomey K. 2003. Presentation Before the IOM Committee on Smallpox Vaccination Program Implementation. Transcript of Meeting Two on February 13, 2003, Washington, DC.
U.S. House of Representatives. 2004. U.S. Representative Thomas M. Davis (R-VA) Holds Hearing on Health System Preparedness. House Government Reform Committee.
U.S. House of Representatives Select Committee on Homeland Security (Democratic Members). 2004. A Biodefense Failure: the Smallpox Vaccination Program One Year Later.
U.S. Senate Select Committee on Intelligence. 2004. Report on the U.S. Intelligence Community’s Prewar Intelligence Assessments on Iraq. [Online] Available at http://intelligence.senate.gov/iraqreport2.pdf. Accessed July 8, 2004.
Washington Times. 2003, January 23. Editorial: Smallpox refuseniks.
Washington Post. 2002, December 19. Editorial: Doctors’ Orders.
White House. 2002a. President Bush Signs Homeland Security Act. Remarks by the President at the Signing of H.R. 5005 the Homeland Security Act of 2002. [Online] Available at http://www.whitehouse.gov/news/releases/2002/11/20021125-6.html. Accessed January 26, 2005.
White House. 2002b. President Delivers Remarks on Smallpox. [Online] Available at http://www.whitehouse.gov/news/releases/2002/12/20021213-7.html. Accessed January 8, 2003.
White House. 2003a. National Threat Level Raised. Statement by Homeland Security Secretary Tom Ridge. [Online] Available at http://www.whitehouse.gov/news/releases/2003/03/20030317-8.html. Accessed January 26, 2005.
White House. 2003b. President Bush Addresses the Nation. [Online] Available at http://www.whitehouse.gov/news/releases/2003/03/20030319-17.html. Accessed January 26, 2005.
White House. 2003c. President Bush Announces Major Combat Operations in Iraq Have Ended. Remarks by the President from the USS Abraham Lincoln at Sea off the Coast of San Diego, California. [Online] Available at http://www.whitehouse.gov/news/releases/2003/05/iraq/20030501-15.html. Accessed January 26, 2005.
Witt D. 2003. Presentation Before the IOM Committee on Smallpox Vaccination Program Implementation. Transcript of Meeting Three on May 1, 2003, Washington, DC.
Yee D. 2003, June 19. CDC: Not Enough Health Workers Have Smallpox Shots. Associated Press.
Yih W, Lieu T, Rego V, O’Brien M, Shay D, Yokoe D, Platt R. 2003. Attitudes of healthcare workers in U.S. hospitals regarding smallpox vaccination. BMC Public Health 3:20.










































