8
STATE PROGRAMS IN SPINAL CORD INJURY
More than one-quarter of the states in the United States have passed legislation creating programs expressly devoted to spinal cord injury research. Most state programs, launched in the late 1990s, represent an important new trend in which state legislatures channel funds to a particular area of health research. However, there is nothing new about states investing in research.
For more than three decades, state governments have carved out a role for themselves in supporting research within their borders. States’ total research and development spending for all areas of science and health was approximately $88 million in the mid-1960s. By 1995 that spending had surged to $3 billion nationwide (Jankowski, 1999). The prime motivations behind state investments in research have been to propel economic growth and to improve the health of their citizenries (SSTI, 1997, 1999a; Jankowski, 1999).
This chapter examines state programs for spinal cord injury research to determine how they are structured and how states—as well as researchers—stand to benefit from their creation. It then looks in depth at three spinal cord injury programs that have successfully leveraged the funds received from their own states to draw in much larger sums in federal research funding. The goal is to set the stage for the chapter’s final section on New York State. That section examines the unique strengths of New York State’s institutions and researchers in neurological, basic, clinical, and translational research on spinal cord injuries and offers recommendations on what distinctive contributions New York’s spinal cord injury research program can make to accelerate the search for improving the outcome after a spinal
cord injury. Many of the chapter’s recommendations for New York State are also applicable to other states interested in setting or revising strategic directions for their spinal cord injury research programs. The states can learn much from one another to develop and strengthen their spinal cord injury research programs.
STATE PROGRAMS AND LEGISLATION
Since 1988, 14 states have passed legislation that has resulted in annual funding for spinal cord injury research of about $27 million (Table 8-1).
TABLE 8-1 State Legislation Relevant to Spinal Cord Injury Research
|
State |
Year Legislation Enacted |
Year Legislation Proposed but Not Enacted |
|
California |
2000a |
|
|
Colorado |
|
2004a |
|
Connecticut |
1999 |
|
|
Florida |
1988 |
|
|
Illinois |
2000a |
|
|
Indiana |
1998 |
|
|
Iowa |
|
2004a |
|
Kansas |
2001 |
|
|
Kentucky |
1994 |
|
|
Maryland |
2000 |
|
|
Massachusetts |
2004a |
|
|
Michigan |
|
1989 |
|
Minnesota |
2000a |
|
|
Missouri |
2001 |
|
|
New Jersey |
1999a |
|
|
New York |
1998a |
|
|
Ohio |
|
2000a |
|
Oregon |
1999a |
|
|
Pennsylvania |
|
2000 |
|
South Carolina |
2000 |
|
|
South Dakota |
|
2003 |
|
Texas |
|
1999a |
|
Virginia |
1997 |
|
|
Washington |
|
2004a |
|
aThe legislation specifically notes that research is conducted to cure spinal cord injuries. NOTE: The data were compiled in October 2004 and are based on a review of state legislature websites, searches on Lexis-Nexis, and telephone interviews. The table includes the year that the legislation was first enacted or considered (i.e., data on later years when the legislation was revised or considered are not included). Enacted legislation supersedes proposed legislation (e.g., legislation considered in 1996 but approved in 1998 is listed as enacted in 1998 and does not appear in the proposed legislation column). |
||
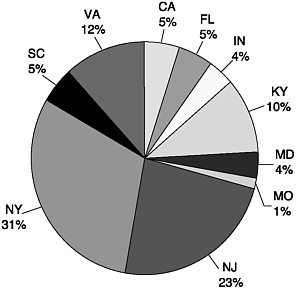
FIGURE 8-1 Percentage of total state spinal cord injury research funding for states with dedicated spinal cord injury research programs. Data for only 10 states are listed. Oregon, Illinois, and Connecticut do not have budgets for their programs. The Massachusetts program was approved in August 2004, and the budget amounts have not yet been specified.
Another 10 states have proposed but have not yet enacted similar legislation. The surge in state legislation, which occurred from the late 1990s to 2001, reflects growing acceptance and awareness that motor vehicle crashes are the leading cause of spinal cord injuries. The concept behind most state legislation can be traced back to a pioneering 1988 Florida law that designated a set percentage of revenues from fines for unsafe driving for spinal cord injury care and research. Today, the amounts and the percentages vary, but the majority of the 14 states each spend at least $1 million each year for spinal cord injury research. New York State supports the program with the largest amount of state funding, $8.5 million per year (Figure 8-1).
The structures and sources of funding vary among the state programs (see Appendix H). New York, for example, collects funds from a surcharge of $51 for traffic violations, whereas New Jersey adds an additional $1 to each motor vehicle or traffic violation fine. Some state programs, such as those in Florida, Indiana, and Kentucky, designate that the funds obtained
from surcharges go to support specific university programs. Other states diversify the types of awards that they grant and allow any university-based researcher in the state to apply. South Carolina, for example, provides an estimated $1 million every 2 years for individual and small pilot research grants, career development awards, and faculty recruitment initiatives. Maryland also distributes $1 million annually for spinal cord injury research through a tax on health insurers. Several states have developed or contribute funding to extensive research centers, including the Miami Project to Cure Paralysis and the Kentucky Spinal Cord Injury Research Center (see below). Some states use their funds for patient care, in addition to research.
The largest and most innovative state programs (see below) have used state funds as seed money to expand their programs’ sizes, scopes, and impacts by using their funds to support pilot projects that generate enough data to help them garner more state, federal, and private financing.
BENEFITS TO STATES FROM THEIR INVESTMENTS
In the aggregate, states invest billions of dollars each year on research and development across all fields of science and technology. Those state expenditures represent a consistent trend that began more than three decades ago. The earliest statistics, gathered in 1965, revealed that states collectively spent about $88 million annually on research and development. That amount rose to $3 billion in 1995 (Jankowski, 1999). These data were reported by the National Science Foundation, based on a 1995 survey of 1,000 state agencies and universities. The total is likely to be significantly higher today, almost a decade later, but no recent surveys have been conducted.
What is known about state spending for research and development in general and biomedical research in particular? What motivates this investment? And what is the return on the investment? These questions are addressed in the next section.
OVERVIEW OF STATE RESEARCH AND DEVELOPMENT SPENDING
The 1995 survey found that the vast majority of the $3 billion in state research and development spending (73 percent, or $2 billion) went to academic institutions in each state. Most of the remainder went to state agencies (14 percent, or $408 million). Of the 73 percent distributed to academic institutions, the majority (67 percent) went to public universities, while the remainder was directed to private universities within the state (Jankowski, 1999).
An overwhelming proportion of the $3 billion in state research and development spending was for research, as opposed to the physical plant infrastructure ($228 million). States typically financed their research and development expenditures from one of four sources: general revenues, lottery proceeds, revenue bonds, and specially designated tax funds. Another source, which accounted for about 9 percent of state spending, was from federal research dollars passed through state agencies (e.g., funding for state health department research from the Centers for Disease Control and Prevention). Revenue bonds floated by a state are commonly used to finance the research infrastructure, such as new construction and equipment (Personal communication, M. Skinner, State Science & Technology Institute [SSTI], November 11, 2004).
These amounts are likely to be relevant to biomedical research because biomedical research accounts for a large share of the spending on research. Although the 1995 survey did not compile actual amounts by field of research, it did report that biological and medical sciences received the highest proportion of state funds, regardless of whether they were directed to academic institutions or to state agencies (Figure 8-2). Engineering and environmental sciences were ranked second and third, respectively. Since 1995, when the survey was conducted, a huge infusion of state funds to life sciences research has been obtained from a new source: state tobacco settle-
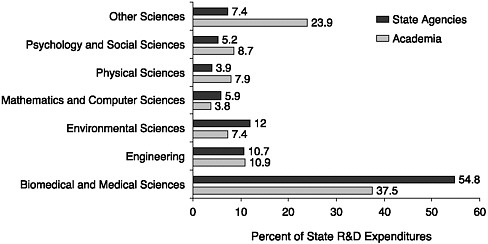
FIGURE 8-2 State government research and development expenditures, by performer of the research and field, 1995.
SOURCES: Battelle Memorial Institute/SSTI, 1998; Jankowski, 1999; National Science Foundation, 2004.
ments (SSTI, 1999b). The tobacco industry agreed to pay $250 billion over the next 25 years to resolve Medicaid lawsuits filed by the states to cover their tobacco-related health care expenditures (Center for Social Gerontology, 2004). Many state legislatures have allocated their settlements to fund life sciences research more generally rather than smoking-related research per se.
Motivation for State Spending on Research and Development
State spending on research and development is largely driven by the quest for economic growth. The recognition of research and development as a growth engine became more apparent during the 1990s, when states increasingly began to incorporate expansion of their research and development capacities into their economic development plans (SSTI, 1997). An analysis of governors’ state of the state speeches, inaugural addresses, and budget speeches signaled a consistently high level of interest in expanding the state’s research and development capacity to promote economic development (Jankowski, 1999). In New York State, for example, Governor George Pataki spearheaded several research and development initiatives worth more than $500 million, including the formation of the New York State Office of Science, Technology, and Academic Research (NYSTAR). NYSTAR issued a report that attributes its creation as “reflect[ing] the recognition that New York’s world-class public and private research universities and academic centers are powerful economic development engines that can help create high-tech jobs and opportunity in New York” (NYSTAR, 2001). The following are some of the specific economic objectives that motivate most states to invest in research (SSTI, 1997, 1999a; Attorney General of California, 2004; Battelle Technology Partnership Practice/SSTI, 2004):
-
to propel a state’s economic growth by strengthening the capacity of the state’s public and private universities;
-
to attract additional investment from federal and private sources;
-
to attract or retain home-grown businesses, investment capital, and high-paying jobs;
-
to expand access to high-quality education and to cultivate an educated workforce;
-
to encourage academia-industry collaborations to commercialize the goods and services that result from research; and
-
to obtain revenues from patents, royalties, and licenses (Jankowski, 1999).
During the past 10 or more years, states have increasingly begun to
focus more specifically on biomedical research. In addition to the economic benefits listed above, states view the biosciences as a rapidly growing industry sector and as a means to improve the health of its citizens (Battelle Technology Partnership Practice/SSTI, 2004). In contrast to earlier efforts, which were more broad based, states are now targeting specific niches within biomedical research, such as spinal cord injury research. The motivation comes from several considerations:
-
to accelerate research to improve health care services and quality of life;
to reduce the high cost of care for recipients of assistance from state health programs (e.g., Medicaid) and state employees;
-
to prevent injuries and improve motor vehicle safety; and
-
to improve the health and productivity of the state’s entire workforce.
Returns on the Investment
Several high-profile studies have sought to quantify the economic and health benefits of state support for biomedical research and development in terms of job creation, the health care costs saved (e.g., the hospitalizations avoided), the value of an increased life span, and reduced morbidity and disability (Silverstein et al., 1995; Lasker Foundation, 2000). The overall cost savings derived from the economic and health benefits of support for biomedical research are estimated to be $69 billion annually (Silverstein et al., 1995). Although evaluation of these estimates is important and demanding, they are national in focus and do not specify the economic and health benefits to a given investor, such as a state or a local government.
Several smaller studies, cited by Silverstein and colleagues (1995), have been conducted to assess the economic returns to states that invest in biotechnology. A Bank of Boston study found that 25.5 jobs were created for every $1 million spent on biotechnology research (Bank of Boston Economics Department, 1991; Silverstein et al., 1995). Similar benefits were estimated in California and Maryland (California Health Care Institute, 1993; Maryland Department of Economic and Employment Development, 1994).
The most relevant study for the purposes of this report was conducted by a team of New York State-based university economists who, at the request of the New York Academy of Medicine, quantified the returns to New York State from investments in biomedical research (Sclar and Aries, 2000). The researchers surveyed 20 biomedical institutions within the state, covering 86 percent of the state’s population. They asked the institutions about the grants that they receive, grant-related expenditures, and institutional expenditures. They assessed the economic impact by applying an
|
BOX 8-1 For every $1 million invested, a state or local government can expect:
SOURCES: Aries and Sclar, 1998; Sclar and Aries, 2000. |
input-output economic model that can trace the effects of research spending on the economy (industries and households) of the region where the research took place. Apart from the direct effects of research funding (e.g., employee compensation and the purchase of goods and services), ripple effects came in two forms: the secondary expenditures of vendors whose businesses were stimulated by the institutional spending (i.e., indirect effects) and induced spending effects resulting from the increased household incomes that the cumulative chain of spending creates. The study found that research investment led to high payoffs in terms of well-paying jobs (an average compensation package of $115,000 per employee) and additional tax revenues from businesses, including excise taxes, property taxes, fees, licenses, and sales taxes, as well as income taxes to the state and federal governments. The magnitude of the effect is presented in Box 8-1.
Advantages of State Programs for Studying Spinal Cord Injuries
Testimony to the committee and interviews with scientific directors of state programs showed that state-sponsored spinal cord injury research programs offer several advantages to researchers: flexibility; the capacity to leverage more funding, especially for renovation or new construction; a steady form of financing (e.g., from motor vehicle surcharges); and a strong investment in the regional economy.
Flexibility
Flexibility is a major reason that researchers and institutions obtain funding from states. Grantees often use state funding for pilot studies that
give them an edge to compete successfully for grants from the National Institutes of Health (NIH), which are highly competitive, lucrative, and prestigious. In each model program described in this chapter, funds from state or local grants were parlayed into the receipt of NIH grants of double or triple the value of the state or local grant. State funds have also been used to fill the gaps left unfilled by NIH grants. Examples include endowed faculty chairs (see the description below of the Kentucky Spinal Cord Injury Research Center), lecture series, and special fellowships. Flexibility in state funding enables researchers to pursue high-risk research or to capitalize on new and unexpected research directions. In sum, state funds not only are used to establish a program but also can be used as a building block.
New Construction or Renovation
State funding has been used, directly or indirectly, for renovation and new construction. Physical infrastructure not only is important for research in its own right but also is key to attracting new talent. The Miami Project to Cure Paralysis was able to build a $36 million building with partial state funding. The project obtained $10 million from the state as a one-time line item in the state budget that matched the funds that the project had raised from a private donor. The receipt of state funds for construction helped the program secure even more private funding. In this instance, state funds for construction were separate from the state’s annual fund for research and treatment for spinal cord injuries.
States can finance construction by floating bonds. That was the preferred vehicle of financing listed in a survey reported by the National Science Foundation (see above). State-issued bonds will also be used by the state of California to finance the building of new facilities under its stem cell research initiative that was approved by voters in 2004.2
NIH rarely funds new construction through its extramural research program, although it does fund new construction for public health priorities, such as, most recently, biological defense. NIH construction grants also impose restrictions. They are normally capped at $4 million. In recent years the annual congressional appropriations for these grants, which represent funding for the Research and Facilities Improvement Program, has been approximately $110 million to $120 million (U.S. Senate Committee on Appropriations, 2003). A portion of NIH’s Centers of Biomedical Research Excellence3 grants ($500,000) can also be used for renovation. How-
ever, these grants are generally awarded only to those states that have historically been unsuccessful in competing for NIH grants. Furthermore, NIH infrastructure grants often require matching funds.
Reliability
State funding also provides a reliable and steady stream of resources if the funding comes from a dedicated revenue generator, such as fines for surcharges on motor vehicle violations. Stable funding enables multiyear planning, which is important for research continuity. Program directors in states that have yearly line-item appropriations rather than dedicated funding sources for spinal cord injury research emphasized the limitations of the year-to-year variability and the need to expend scarce resources to lobby state legislators. It is imperative that a certain level of funding be ensured each year for long-term organization and planning, the continuity of personnel, and more rapid progress in research.
Investment in Regional Impact
State governments, in contrast to the federal government, have a more direct and enduring investment in the success of their spinal cord injury research and development programs. As discussed earlier, states have increasingly come to view their research and development programs as part of their economic development plans or as a means to improve health care services for their populations. Even without a direct financial investment in a spinal cord injury research and development program, states can help to build a program by, for example, fostering linkages to local governments or to biopharmaceutical firms in the region. New York City, for example, set up an important program to help young biomedical investigators (Box 8-2). Furthermore, states can also help to steer patients to clinical trials for acute spinal cord injuries by virtue of their direct management of regional trauma systems.
MODELS OF STATE-SPONSORED SPINAL CORD INJURY RESEARCH PROGRAMS
The following sections profile the efforts of three states to support state spinal cord injury research programs (Kentucky, Florida, and California). The material in this section was gathered by interviewing the director or the scientific director of the state’s major spinal cord injury research center. These programs offer three different models that have all been extremely successful in making a significant research contribution and in stimulating
|
BOX 8-2 What began as a municipal program to enhance the recruitment of young biomedical investigators to institutions in New York City turned out to be a case study of the high economic returns—in both human (scientific talent) and funding terms—that can be obtained by investing in biomedical research. The New York City Council launched the program in 1997 by allocating $15 million over 5 years to the New York Academy of Medicine. The program was confined to grant support for new assistant professors or postdoctoral fellows in eight research-intensive New York City institutions, six of which were academic medical centers. Each year, each institution was allowed to submit up to four proposals selected by the dean. The grants provided $100,000 annually for 3 years for each awardee, with renewal for the second and third years dependent on the results of a scientific review of progress reports. Funds could be used for the investigator’s salary and equipment and laboratory supplies, but the institutional overhead was limited to 8 percent. The program was intended to promote any type of research on diseases of importance to urban populations, whether it was clinical, translational, or basic research. A committee whose members were the deans or presidents of the eight institutions oversaw the program, and the president of the New York Academy of Medicine chaired the committee. Proposals mirrored the general format of R01 grants at the National Institutes of Health (NIH). Experts selected by the president of the Institute of Medicine of the National Academy of Sciences, which oversaw the review process, reviewed the proposals for scientific quality. Ten grants were awarded annually, usually for the proposals achieving the 10 highest review scores. Evaluation of the program revealed that the first 3 years of funding had enabled grantees to amass an additional $18 million in direct and indirect grant awards, largely from NIH. Additional funding was received by 70 percent of the grantees. This $18 million represented a return of about 200 percent on the $9 million in grants awarded to the first 30 young investigators that the program funded. A standard input-output economic model was used to calculate the return on the investment (Aries and Sclar, 1998) The inputs were $27 million (the original $9 million awarded during the first 3 years of the program plus the $18 million in additional extramural grants) plus the standard multiplier effects that incorporate direct, indirect, and induced spending effects. The model suggested that each million dollars of research funding to these research-intensive institutions generated approximately 20 full-time equivalent jobs. On this basis, the level of research funding in the program at that time could be expected to generate approximately 540 new full-time equivalent positions, with employee compensation totaling $32.1 million, as well as approximately $1.1 million in indirect business taxes and $9.7 million in state and federal taxes, primarily income taxes (Barondess, 2002). |
the state’s economy by creating high-paying jobs and increasing state and local tax revenues.
Kentucky
History and Role of State Funding
In 1994, Kentucky passed legislation creating the Kentucky Spinal Cord and Head Injury Trust, which directed traffic violation surcharges to spinal cord and head injury research. A state senator, Tim Shaughnessy, whose niece had a spinal cord injury, spearheaded the legislation. Christopher Shields of the University of Louisville was instrumental in establishing the Kentucky Spinal Cord Injury Research Center.
The enabling legislation targeted trust fund revenues to two Kentucky universities, the University of Louisville and the University of Kentucky (Box 8-3). From 1995 to 2004, the trust paid out $0.725 to $2.15 million annually for individual research grants (depending on the amount of surcharges collected). These funds were competitively awarded to researchers
|
BOX 8-3 Type of Program: Centralized program in two universities State Trust Fund Revenues: $1.5 million to $3 million per year from vehicle surcharges Trust Fund Use: Research, endowed chairs, graduate student and postdoctoral support, and a visiting lecture series Estimated Annual Research Budget: $3.5 million plus salaries from endowed chairs for faculty at the Kentucky Spinal Cord Injury Research Center (see Figure 8-3) Growth Indicators Since the Program’s Inception: NIH research grants: $0 to $2.5 million per year Principal investigators: 2 to 16 (8 in the center plus 8 affiliated faculty at the universities) Personnel: 6 to 60 FTEs (plus 30 full-time equivalents at affiliated laboratories) Space: 700 to 11,000 square feet |
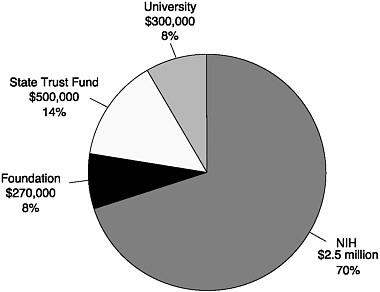
FIGURE 8-3 Kentucky Spinal Cord Injury Research Center funding at the University of Louisville: Estimated sources of yearly research budget.
SOURCE: Personal communication, Scott R. Whittemore, Kentucky Spinal Cord Injury Research Center, February 27, 2005.
from the two universities after review by an external study section. In the early years, the majority of these funds went to University of Kentucky researchers, but over the past few years they have been equally divided between the two institutions. The remainder of this section focuses on the University of Louisville because its program is exclusively devoted to spinal cord injury, whereas the University of Kentucky’s emphasis is on head injury research.
The University of Louisville’s program expanded over time to become the Kentucky Spinal Cord Injury Research Center. Today, the center has both a scientific and clinical director, attesting to its broad research focus. Its mission is to “develop successful spinal cord repair strategies in the laboratory that can be taken to the clinic in a timely and responsible fashion” (KSCIRC, 2004).
Kentucky Spinal Cord Injury Research Center
Over the past decade, the trust fund has been used to transform a small laboratory at the University of Louisville into a nationally recognized program of research in spinal cord injuries. The trust fund’s annual investment
of about $1.5 million has been strategically used as seed money to attract large amounts of private and public investments. Since 1998, the program gained momentum by accumulating more than $20 million in federal research funds. The university designated the program a center in 2002, and the center expects sustained growth.
The program is highly centralized around four senior and four junior investigators and state-of-the-art core facilities for animal surgery, electrophysiology, behavioral analysis, gene therapy, and microscopic analyses. Its clinical research focuses on the use of novel imaging methods to assess the acutely injured human spinal cord. The center has set its sights on expanding its faculty in the area of clinical rehabilitation research. Much of its operating research budget, around 67 percent, is from NIH grants; the second largest source of revenues is from the state trust fund (Figure 8-3). The remainder is split between university and foundation funds. The center uses trust fund revenues in several ways, most commonly to fund competitively awarded research proposals and to endow faculty chairs. Driven by a commitment to collaboration the center has a research agenda that encourages translational research and that seeks out collaborations to extend the impact of the center (see below).
The state’s trust fund revenues have been instrumental in building the center. The trust fund is a stable and flexible source of funding. A board of directors oversees the fund. The board of directors has seven members: two from each university and other members appointed by the state medical society or governor. Although the trust funds were largely steered toward individual research grants at first, the University of Louisville program shifted its expenditures to launch significant growth. Starting in 1997, trust funds began to be used as a nucleus for garnering private, state, and federal funds. The key step was the awarding of $500,000 to each university for faculty development. The University of Louisville used those funds for newly endowed faculty chairs that required matching funds. The $500,000 was matched by a private donation; and the total, $1 million, was matched again by a novel state program set up by the Kentucky governor, known as Bucks for Brains.4 Over time, $10 million has been raised and has been used to endow five chairs of $2 million each. The funds are invested, with salaries produced from the interest on the principal. All of the private donations thus far have come from Norton Healthcare, which supports the
|
BOX 8-4
|
Department of Neurological Surgery at the University of Louisville. The endowed chairs have been used to recruit four senior investigators to the Kentucky Spinal Cord Injury Research Center or to endow laboratories to which outstanding junior faculty are recruited. More recently, each university has received $150,000 per year for graduate student and postdoctoral fellow support.
Another key to growth has involved the efforts of the Kentucky Spinal Cord Injury Research Center faculty in generating new federal research dollars from NIH and the National Science Foundation (Box 8-4). In addition to individual research grants, several large infrastructure grants, as well as program project grants, have been awarded for core resources and program projects. Thus far, none of the funds has been used for new construction, but that is the goal of current fund-raising efforts being done in the community.
Florida
History and Role of State Funding
In 1988, the state of Florida allocated $250,000 to the Miami Project to Cure Paralysis (further detail below) as a result of the efforts of advocates for spinal cord research. Legislation was passed in 1992 to designate a percentage of state revenues from traffic violations for research at the University of Miami and the University of Florida. Distributions from the fund are approximately $500,000 annually for each university and support brain and spinal cord injury research. In some years these distributions are
supplemented by general revenues in the state’s annual budget directed to the Miami Project to Cure Paralysis. Because the amount of general revenues depends heavily on lobbying, the total amount of state funding varies from year to year. Over the last 4 years, state funding for the Miami Project to Cure Paralysis oscillated between a total of $750,000 and $1.4 million annually (Box 8-5). This uncertainty in state funding, however, affects its capacity for multiyear planning.
Although the state funds for the project vary from year to year, the programs that receive the funding have flexibility over its use. The Evelyn F. and William L. McKnight Brain Institute at the University of Florida, College of Medicine has used the moneys it receives for many different projects designed to improve programmatic development. State funds have been used as seed money to support preliminary research required to obtain NIH research project, program, and training grants. Individual researchers, including senior researchers, received funds ($400,000 over 2 years) to develop sustainable research programs allowing them to successfully compete for NIH grants. The University of Florida program also used some of the state allocations to help support a human clinical trial, which investigated the feasibility and safety of neural tissue transplantation in patients with syringomyelia.
The Miami Project to Cure Paralysis has historically used state funding
|
BOX 8-5 Type of Program: Centralized in one facility State Funding: Variable, but ranges from $750,000 to $1.4 million each year (2000 to 2004) Use of State Funds: Primarily salaries and equipment for new faculty to perform the pilot research needed to obtain federal grants Estimated 2005 Budget: $15.8 million (see Figure 8-4) Growth Indicators Since the Program’s Inception: NIH grants and contracts: $100,000 (1987 and 1988) to $5.9 million (2005) Principal investigators: 7 to 26 Personnel: 15 to 212 (including fundraisers and administrators) Space: 800 square feet (1986) to 118,000 square feet in the new building (new building cost, $36 million) |
|
BOX 8-6
|
to attract young investigators and give them the tools that they need to successfully compete for NIH grants. With input from a senior advisory committee, the project’s scientific director allocates the state funding after each new faculty member submits a research proposal. The funds are spent on salary and equipment for individual projects; but core facilities, which are funded by NIH program project grants and other sources, are available in the same building. All of the young faculty recruited in the past 7 years have later successfully competed for NIH grants.
For its new $36 million building, the Miami Project to Cure Paralysis received a large, one-time infusion of $10 million in state matching funds, after it had raised $10 million from a private benefactor, Lois Pope, after whom the building is named. The remaining funds were collected from a variety of private sources. Thus, in addition to the yearly appropriation, the state made a sizable contribution to new construction (Box 8-6).
The Miami Project to Cure Paralysis
The Miami Project to Cure Paralysis was founded in 1985, and is the nation’s largest single program devoted to spinal cord injury research. It traces its origins to the combined vision of a neurosurgeon and three families with firsthand exposure to spinal cord injuries. What began as a $300,000 private gift in 1985 has blossomed into a broad-based research center at the University of Miami School of Medicine, with 26 researchers and annual funding of about $16 million.
The stunning growth of the project is driven by the philosophy of its founding neurosurgeon, Barth Green, who wrote about “gather[ing] a critical mass of scientists … in one center under one roof with a mutual committed goal of curing paralysis” (Kleitman, 2001). Significant segments of the funding for the Miami Project to Cure Paralysis come from private philanthropy and active fundraising and from NIH grants (Figure 8-4). The funds from the state of Florida are mainly directed to new faculty to help
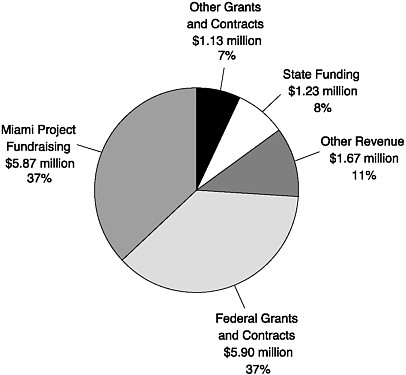
FIGURE 8-4 Fiscal year 2005 budgeted income for the Miami Project to Cure Paralysis.
SOURCE: Adapted from a presentation by Mary Bunge to the Institute of Medicine Committee on Spinal Cord Injury, September 27, 2004.
them generate results from pilot studies, which are highly advantageous in gaining NIH grant funding. The state funding for new faculty has been the engine behind garnering more NIH grants.
The project’s scope is comprehensive and multidisciplinary. Although the majority of research resources (70 percent) are spent on basic research, the project’s overall perspective is that basic research should be geared so that the research findings can be translated to clinical research. Consequently, the project has core resources for animal models, including the establishment of spinal cord lesions, postsurgery care, behavioral testing, image analysis, histology, electron microscopy, and viral vector preparation. Those core resources allow the rapid translation of the basic research, particularly because the uniformity of the lesions and analysis of their effects are paramount. The project also receives funding from NIH contracts for studies to replicate important findings from other laboratories, training of students and fellows, and support for university scholars (see the
description of the Facilities of Research Excellence in Spinal Cord Injury [FOR-SCI] in Chapter 6). Replication studies are necessary to translate the findings from basic research into clinical practice, but they are rarely done because researchers in all scientific fields are rewarded for innovation, not replication.
The project also sponsors or participates in clinical trials, including clinical trials of drugs and rehabilitation devices. The project maintains a database with information on more than 2,000 chronically injured individuals who wish to participate in clinical research. This is easily accomplished because the university’s hospital is a regional trauma center that treats approximately 100 cases of acute spinal cord injury per year.
The Miami Project to Cure Paralysis also has a fertility program that has helped nearly 75 men with spinal cord injuries father children. It is one of two institutions in North America that collects spinal cord tissue from individuals with spinal cord injuries. It has amassed a bank with more than 200 samples from individuals with spinal cord injuries that researchers and clinicians use to investigate the etiology, mechanisms of complications, and potential avenues of treatment.
Recently, the project has begun to work with the Food and Drug Administration (FDA) to explore the process of obtaining approval for combination therapeutic interventions involving several drugs or other modes of therapeutic intervention. Investigators have found that the drug rolipram, a type IV-specific phosphodiesterase inhibitor that has FDA approval for use for another indication, is neuroprotective and growth promoting because it prevents cyclic AMP hydrolysis. When rolipram and additional cyclic AMP are combined with Schwann cell grafts, they promote axonal growth in an animal model of spinal cord injury (Pearse et al., 2004). Combination therapies, particularly those that combine cell-based therapies with drugs, will have far more complex regulatory requirements than single therapeutic approaches.
An external advisory committee evaluates the Miami Project to Cure Paralysis every 3 to 4 years. This form of evaluation is supplemented by the standard peer-review process involved in obtaining NIH grants.
California
History and Role of State Funding
In 2000, California Governor Gray Davis signed The Roman Reed Spinal Cord Injury Research Act of 1999, which is devoted to finding treatments for spinal cord injuries. The Act is named for Roman Reed, who sustained a spinal cord injury while playing college football. Reed’s family, along with many others, pressed for the legislation under the auspices of
Californians for a Cure. Their efforts galvanized the legislature to authorize up to $2 million annually, which is appropriated as a line item in the state budget. The state funds provided by the legislature are allocated to the University of California Office of the President, which in turn allocates the funds to the Reeve-Irvine Research Center to administer. After the first 5 years, the Roman Reed Research Program has grown to a consortium of 150 researchers across 10 California universities and institutions. Its decentralized structure is similar to that of the center to receive the first grant from the New York State Spinal Cord Injury Research Program (see below).
Reeve-Irvine Research Center
The Reeve-Irvine Research Center named for actor Christopher Reeve and philanthropist Joan Irvine Smith, was established in 1998 through a lead gift from Joan Irvine Smith that endowed a chair in spinal cord injury research at the University of California at Irvine and established a research endowment. The mission of the center is to carry out research on injuries to and diseases of the spinal cord that result in paralysis or other loss of neurological function, with the goal of finding treatments.
In addition to the center’s three core faculty whose labs are physically located in the Reeve-Irvine Research Center, scientists and physician scientists at the University of California at Irvine have been recruited to participate in the center’s research and training activities. Currently, there are 15 “center associates” carrying out research on nervous system injury, stroke, and neurodegenerative disorders as well as on basic processes that underlie nervous system development, regeneration, and plasticity. Several center associates are active clinicians as well as scientists, and bring a unique clinical perspective to the Reeve-Irvine Research Center.
Roman Reed Research Grants Program
The Roman Reed Research Program consists of a state-of-the-art core laboratory facility (with a budget of $400,000 per year) and a research grants program, both of which are open to any California-based researcher. The core laboratory, situated in the Reeve-Irvine Research Center, is equipped with animal facilities, dedicated laboratory space, and trained technical personnel who can readily produce uniform and standardized types of spinal cord injuries in rodent models. Most research conducted by the program is basic, but to be funded, it must lend itself to the legislation’s intent: ready translation to finding treatments for spinal cord injuries.
The concept behind the core laboratory is to attract new researchers to the field by making it relatively easy for them to turn their ideas into
|
BOX 8-7 Type of Program: Decentralized in multiple universities and institutions State Funding: Up to $2 million annually under the Roman Reed Spinal Cord Injury Research Act; funding varies from year to year but ranges from $1 million to $2 million (2000 to 2004) Use of State Funds: $400,000 per year for an open core laboratory; the remainder is used for grants to California-based researchers in public or private institutions Growth Indicators Since the Program’s Inception: Roman Reed grants: 57 new research projects funded throughout the state (2000 to 2004); 9 are for investigators new to the spinal cord injury research field NIH and foundation grants: $3.7 million in Roman Reed Research Program funding to state researchers (the total over the first 3 years) has led to $18 million in other grants and 24 new jobs in California Researchers: between 2001 and 2004, more than 180 California researchers have participated in 57 Roman Reed Research Program research projects |
experiments that can be performed with animals. In fact, about one-quarter of the projects from the program’s inception have been conducted by researchers who are new to the field (Box 8-7).
The grant program, which funds about 20 projects each year (about $100,000 each), encourages use of the funds as seed money to obtain larger amounts of federal and private funding. That goal has been realized, considering that $3.7 million in Roman Reed Research Program funding to state researchers (over the first 3 years) has led to $18 million in other grants and 24 new jobs in California.
The program’s concept and structure were established in 2001 by a town meeting of interested researchers from the University of California system, NIH specialists, and members of the External Advisory Board representing leaders from public, private, and non-profit entities. Attendees refined and elaborated a plan suggested by the Scientific Steering Committee. The final plan calls for the allocation of approximately $400,000 of the state’s yearly appropriation to run the core laboratory; the remainder is directed to research grants, a small number of fellowships, and other training opportunities. The appropriation varies from year to year but has a ceiling of $2 million, as authorized under the Act.
Grant proposals undergo a two-tier review process. An external advisory committee first reviews the proposals and assigns priority scores on the basis of the merit and the appropriateness of the proposal to the program’s goals. Using those rankings, the Scientific Steering Committee decides on the distribution of funds. Grants are administered through the Reeve-Irvine Research Center at the University of California at Irvine. Except for its core facility, which serves the Roman Reed Research Program, the Reeve-Irvine Research Center is funded separately. The 3 principal investigators located at the Reeve-Irvine Research Center and the 15 research associates on the campus apply for Roman Reed Research Program funds just as other California-based researchers do. The center also receives NIH contract funding, totaling $2.6 million for 2003 through 2008, for training and research facilities for spinal cord injury research. This center also holds an NIH FOR-SCI contract to replicate the findings from other laboratories.
A key goal of the program is to foster collaboration and communication, both for scientists and for the lay public. Beginning in 2002 the program has sponsored an annual Roman Reed Research Meeting, which includes presentations by grant recipients, a poster session for graduate students and postdoctoral fellows that allows sharing of preliminary research findings, and a Meet the Scientists Forum for scientists and the lay public. The purpose of the meeting is to bring investigators together as they launch projects to promote collaborations and devise experiments that take advantage of economies of scale (Box 8-8).
Impact of New Stem Cell Research Initiative in California
In November 2004 California voters approved Proposition 71, which provides a fresh infusion of about $295 million annually for stem cell research in California (approximately $3 billion over 10 years).5 The financing for the research comes from state-issued long-term bonds. A substantial portion of the funds allocated during the first years of the program will go to the establishment of research facilities. The goal of Proposition 71 is to circumvent specific restrictions on NIH funding of stem cell research projects involving human embryos. Although it gives priority to embryonic stem cell research, the proposition broadly covers stem cells of all types, whether they come from an embryo, a fetus, or an adult or from humans or animals. The proposition explicitly prohibits human reproductive cloning research.
Proposition 71 is likely to benefit spinal cord injury research, one of the commonly named conditions identified to benefit from stem cell research.
|
BOX 8-8
|
Some 10 percent of current applications for funding under the Roman Reed Research Program propose the use of stem cells. The sheer magnitude of new funds is likely to stimulate more research on spinal cord injuries in California. Additionally, Proposition 71 requires the establishment of a 29-member Independent Citizen’s Oversight Committee and stipulates that this committee must include an advocate for spinal cord injury research who is to be appointed by the governor.
The impact of Proposition 71 is likely to extend beyond California’s borders. Other states may find that some of their finest researchers, lured by the availability of new resources, may move their laboratories to California. Some may be spinal cord injury researchers. Other state governments, including those in New Jersey, Wisconsin, Illinois, and Minnesota, are responding by enacting or debating stem cell research legislation to help to retain senior investigators and attract young researchers (Garvey, 2004).
NEW YORK STATE SPINAL CORD INJURY RESEARCH PROGRAM
In 1998, New York State passed legislation to establish a new program whose ambitious mission is to support research “towards a cure for [spinal
cord] injuries and their effects.”6 Funding for the program comes from a surcharge on fines for traffic violations, which is directed to the newly created Spinal Cord Injury Research Trust Fund. With a ceiling of $8.5 million in annual appropriations, the funding level is the largest of any of those for state programs (Figure 8-1). Its size and scope give the program the potential to become a major force in spinal cord injury research.
The program possesses several strong features, all of which are discussed here:
-
a sophisticated grant review structure (two tiered) and scientific board;
-
a strong translational component through its legislative mission and through its first center grant award;
-
multiple types of grants; and
-
an expansion capacity obtained by drawing on the unique strengths of New York’s biomedical research and clinical research programs.
Legislation to establish the spinal cord injury research program was originally proposed by Senator Vincent Leibell and Assemblyman Edward Griffith and was signed into law by Governor George Pataki on July 14, 1998.6 This legislation is often referred to as the Paul Richter Bill, after a New York state trooper who was shot while on duty in 1973. Christopher Reeve was also a strong advocate.
The legislation mandated the formation of a 13-member panel, the Spinal Cord Injury Research Board, to solicit, receive, and review research proposals and to make recommendations to the Commissioner of Health for approval of funding. The individuals chosen to serve on the Spinal Cord Injury Research Board have expertise in neuroscience, neurology, neurosurgery, neuropharmacology, rehabilitative medicine, and advocacy and are appointed by the governor and senior legislative officers, as shown in Table 8-2.7 Board members serve for 4 years and may serve no more than two consecutive terms. The governor appoints one board member to serve as the chair.
In addition to reviewing grant applications, the Spinal Cord Injury Research Board is required to submit progress reports to the governor and the legislature on January 31 of each year to describe the previous year’s funded research and the board’s accomplishments. Unlike the Miami Project to Cure Paralysis, beyond the yearly submission of progress reports and the
TABLE 8-2 Spinal Cord Injury Research Board Appointments
|
Person Appointing Board Member |
Number of Appointees |
|
Governor |
7 |
|
Senate President |
2 |
|
Assembly Speaker |
2 |
|
Senate Minority Leader |
1 |
|
Assembly Minority Leader |
1 |
|
Total number of board members |
13 |
sponsorship of this Institute of Medicine report, the New York State Spinal Cord Injury program has no periodic mechanism for the independent evaluation of its overall performance in meeting its strategic objectives.
Research proposals are reviewed by using a two-tiered process: an initial review for scientific merit and a review for programmatic relevance. Initially, a scientific advisory committee reviews the research proposals. The state of New York contracts with a company to select members of the Scientific Advisory Committee and to manage the peer review process. Members of the Scientific Advisory Committee are researchers who are not affiliated with New York State research institutions and who are experts in fields relevant to spinal cord injuries.
The Scientific Advisory Committee reviews the proposals for their scientific merit and then ranks them. Using the Scientific Advisory Committee’s recommendations as a starting point, board members review the proposals and rank the applications according to their consistency with the Spinal Cord Injury Research Board mission, potential impact, and scientific feasibility. Applications that meet these criteria are forwarded to the commissioner of health for review and approval.
Types of Grants
Although New York State has not specified research topics for funding, it has targeted projects that involve tissue repair, regeneration, and restored function.8 It uses several types of granting mechanisms to support these research efforts and has three different funding mechanisms: the Collaborations to Accelerate Research Translation; the Innovative, Developmental, or Exploratory Activities; and the Center of Research Excellence grants.
The lead recipient institution for each of these grants must be located in New York State, but collaborative institutions may be located outside the state. A brief description of each type of funding mechanism follows.
-
Collaborations to Accelerate Research Translation (CART). CART comprises a 4-year grant that provides a maximum of $300,000 each year to direct costs for cross-disciplinary, translational research. This grant does not support “program projects, research centers, or large scale clinical trials” (New York State Spinal Cord Injury Research Board, 2002). Principal investigators are required to commit more than 10 percent full-time equivalent time toward research.
-
Innovative, Developmental, or Exploratory Activities (IDEA). IDEA is a 2-year grant that provides a total of $300,000 in program costs. According to the request for proposals, these grants support research projects that “hold out significant likelihood of leading to breakthroughs or new avenues of investigation” (New York State Spinal Cord Injury Research Board, 2002). The grant supports preliminary research with the expectation that principal investigators will later pursue additional, larger-scale funding elsewhere.
-
Center of Research Excellence (CORE). CORE is a 5-year grant that provides up to $3 million in total costs each year for multi-institutional, collaborative research projects. Funds are used for three to six interrelated research projects, one of which must be a treatment study. Awardees must partner with a patient care facility, in this case, the Helen Hayes Hospital, the New York State Department of Health’s rehabilitation facility, which also conducts clinical trials. Those projects most likely to be funded are at institutions that have already demonstrated a high level of expertise. This is a one-time-only grant (New York State Spinal Cord Injury Research Board, 2003). The first CORE grant was awarded for an integrated translational program that linked together 11 research institutions within New York and other states (see Box 8-9).
Funding History and Focus
The legislation sets a ceiling of allocating $8.5 million annually for research. During the program’s first year of awarding grants, $3.6 million was awarded. In the next year, 2001, nearly $7 million was distributed to nine research projects (CRPF, 2000; Times Newsweekly, 2001). Table 8-3 lists the number of awards and the total amounts distributed since 2000.
The grants awarded in 2000 focused largely on basic research, including axonal guidance, plasticity, growth factors, regeneration, calcium channels, and adhesion molecules (New York State Spinal Cord Injury Research
|
BOX 8-9 Spinal Cord Injury Center of Research Excellence (CORE) The New York State Spinal Cord Injury Research Program’s first center grant, which was for $15 million over 5 years, was awarded to a unique network of researchers from 11 institutions. The major goal is to translate basic science into safe and effective treatments for the acute or chronic phase of a spinal cord injury. Through a decentralized yet highly coordinated effort, the research team has set as its primary objective the development of drugs in combination with other therapies (e.g., cell replacement therapies) that can be moved safely and rapidly to human clinical trials at the seven medical schools within its network, among other clinical sites. The centerpiece of the grant is an in vitro screening program that uses eight cell-based or organelle-based assays to test 2,000 drugs previously approved by the Food and Drug Administration and marketed for other conditions. Restriction of the screening to Food and Drug Administration-approved drugs has the advantage that the drugs have already been shown to be safe. “Toxicity often defeats new compounds,” says Rajiv Ratan of Burke Medical Institute, one of the principal investigators funded by the grant. The screening program is designed to find drugs with the capacity to overcome three major complications of spinal cord injuries: glial cell inhibition, cell death, and demyelination. A drug found to overcome all three complications then progresses to detailed physiological assessment with animal models, including real-time imaging, to ensure penetration into the central nervous system and to establish therapeutic efficacy. Some of the physiological testing will be done with grant funds at a nearby pharmaceutical firm. If a compound is successful in studies with the animal model, it will be tested in studies with humans through the center’s evolving clinical trials network, which will have a centralized data management and analysis structure. Grant funds and the findings that they generate will be key to building the program by obtaining grants from the National Institutes of Health and partnerships with drug or biotechnology companies. |
Board, 2002). The year 2001 grants were also awarded for basic research, including research on neuron regulation, axonal guidance, myelination, repair and protection, and glial cells (New York State Spinal Cord Injury Research Board, 2002). Year 2002 grants focused on axon regeneration, cellular mechanisms after an injury, injury diagnostics, and treatment (New York State Spinal Cord Injury Research Program, 2003). The large center award, made in 2003, was for translational research.
TABLE 8-3 New York State Spinal Cord Injury Research Program’s Grant Award History
|
Year |
Number of Awards |
Total Funds Awarded (in millions) |
|
2000 |
10a |
$ 3.6 |
|
2001 |
9 |
$ 7 |
|
2002 |
15b |
$ 8.4 |
|
2003 |
1 |
$15 |
|
aOf 45 applications submitted (22 percent success rate). bFive researchers received CART awards, and 10 received IDEA awards. SOURCES: CRPF, 2000; Times Newsweekly, 2001; New York State Spinal Cord Injury Research Board, 2004. |
||
Challenges Facing New York State’s Program
Expanding the Number of Spinal Cord Injury Researchers in New York
In 2003, only six principal investigators in New York State received NIH grants (R01 grants) for projects specifically designated to be related to research on spinal cord injuries (Table 8-4), and there were no program project grants or center grant recipients in the state in this specifically designated research area. For the years 1998 to 2003, New York ranked
TABLE 8-4 NIH R01 Grants for Spinal Cord Injury Research in New York State, 1998 to 2003
|
Year |
Number of R01 Grants |
|
1998 |
5 |
|
1999 |
6 |
|
2000 |
4 |
|
2001 |
3 |
|
2002 |
5 |
|
2003 |
6 |
|
NOTE: The number of R01 grants for each year was derived by searching the NIH CRISP database (see Appendix A for more details). The search was restricted to the term “spinal cord injury,” and the activity was restricted to research projects, specifically those supported by R01 grants. SOURCE: NIH, 1999. |
|
third in the nation in grants for spinal cord injury-related research (Table 8-5) as well as third in the nation for overall NIH grants (NIH, 2004a) (Figure 8-5).
These data suggest that New York State has too few spinal cord injury researchers to accomplish its legislatively mandated mission: to cure spinal cord injuries or their effects. The greatest challenge for the New York State program will be to attract new researchers to the spinal cord injury research field, either by collaboration with or recruitment from researchers in related fields of neuroscience or neurology and bioengineering. It is hoped that over the next 3 years the number of researchers in New York focused on fundamental and translational studies related to spinal cord injuries will at least double.
TABLE 8-5 NIH Research Grants Related to Spinal Cord Injuries in States with State-Funded Spinal Cord Injury Research Programs, 1998 to 2003
|
State |
Fellowshipsa |
Training Grants |
Career Development Awards |
R01 Awards |
|
California |
5 |
1 |
2 |
31 |
|
Connecticut |
1 |
0 |
0 |
4 |
|
Florida |
0 |
1 |
2 |
13 |
|
Illinois |
3 |
0 |
0 |
7 |
|
Indiana |
0 |
0 |
0 |
1 |
|
Kentucky |
2 |
0 |
1 |
7 |
|
Maryland |
0 |
1 |
1 |
5 |
|
Massachusetts |
0 |
0 |
0 |
6 |
|
Missouri |
1 |
0 |
2 |
6 |
|
New Jersey |
0 |
0 |
0 |
0 |
|
New York |
3 |
1 |
4 |
11 |
|
Oregon |
0 |
0 |
0 |
0 |
|
South Carolina |
0 |
0 |
0 |
2 |
|
Virginia |
0 |
0 |
1 |
4 |
|
Total for 14 states |
15 |
4 |
13 |
97 |
|
Total for 50 states |
27 |
4 |
21 |
194 |
|
aFellowships include predoctoral, postdoctoral, and senior fellowships. NOTE: Duplicate awards were removed. The number of awards was based on narrow searches of the NIH CRISP database. The database was searched with the term “spinal cord injury” for each of the types of grants and awards. SOURCE: NIH, 1999. |
||||
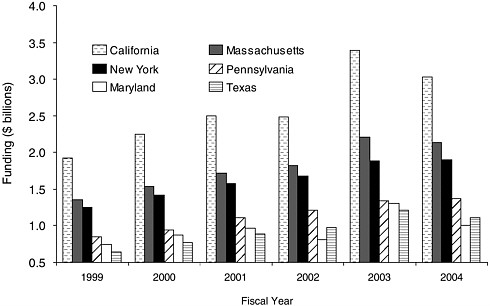
FIGURE 8-5 Overall NIH support for research in top six states, 1999 to 2004.
NOTE: NIH support for 1999 through 2004 was based on reviews of the levels of funding for extramural awards (by state).
SOURCE: NIH, 2004a.
Funding and Administrative Issues
There are several indications that an overly burdensome bureaucracy has resulted in funding delays for research grants. Several individuals testified to the committee that the New York State program has been slow to allocate and deliver grant funds after a project’s approval, with the lag time generally being almost 1 year from the grant award announcement to the receipt of funding (see Appendix A; IOM Committee on Spinal Cord Injury Workshop, September 27, 2004). The program’s centerpiece, its CORE grant for translational research involving 11 institutions, has experienced a 1-year funding delay. Delays are highly disruptive and discourage investigators from getting involved in spinal cord injury research with the New York State program, which is exactly the opposite of the program’s intent.
Furthermore, issues regarding board members’ appointments and attendance remain. The legislation behind the New York State program specifies a 13-member board; 7 of these members must be appointed by the governor, and the others are appointed by leaders of the state legislature. Several slots on the board have been unfilled, which has presented difficulty in fulfilling the work of the board.
Unique Strengths of New York State’s Research Infrastructure
New York State has an unquestionably strong biomedical research infrastructure that could be drawn upon to build a strong program of research on spinal cord injuries. As mentioned above, New York State ranks third among the states in terms of total NIH grant funding, based on an analysis of data published by NIH. Key indicators of New York State’s strengths are summarized in Table 8-6.
This section highlights the strengths of the biomedical research infrastructure and sets the context for understanding current state efforts in spinal cord injury research.
Unique Concentration of Researchers and Institutions
New York State stands out by its confluence of researchers, medical schools, universities, and numbers of individuals with spinal cord injuries.
TABLE 8-6 Indicators of New York State’s Biomedical and Neuroscience Research Infrastructure
|
Indicator |
Number or Amount |
Year(s) |
Rank Among 50 States |
|
Total NIH grant funds, all types |
$1.9 billion |
2004 |
3 |
|
Total NIH grants to medical schools |
$943 million |
2002 |
2 |
|
Total NIH grants (R01 grants) specific to spinal cord injury research |
11 |
1998–2003 |
6 |
|
Society for Neuroscience members |
1,854 |
2004 |
2 |
|
Number of medical schools in the state |
12 |
2004 |
1 |
|
State funds for spinal cord injury research |
$8.5 million |
Annual |
1a |
|
Nearby states with spinal cord injury research programs |
4b |
NAc |
NA |
|
aRank among 14 other states with spinal cord injury research programs. bNew Jersey, Connecticut, Maryland, Massachusetts. cNA = not applicable. SOURCES: NIH, 1999; New York State Spinal Cord Injury Research Board, 2002; AAMC, 2004; NIH 2004a,b; Personal communication, F. Johnson, Society for Neuroscience, November 19, 2004. |
|||
No other state has, in such a close geographic proximity, as many resources vital to building a formidable research capacity in spinal cord injuries. In 2004, the state received nearly $2 billion from NIH (Figure 8-5), ranking only behind California and Massachusetts. New York has sustained its third-place ranking over the past 5 years (Figure 8-5), but the state faces increasingly stiff competition from other states. Although New York was the leading recipient of NIH funds in the 1980s, from 1981 to 1995 New York’s share of total NIH research funding gradually eroded, from a high of 15 percent in 1981 to 11 percent in 1995 (Sturman et al., 1997, 2000).
Apart from NIH, grant funding for biomedical research comes from a variety of other sources (e.g., the U.S. Department of Veterans Affairs, state and local governments, and private sources). In a 1998 survey of the 20 largest New York State-based biomedical research institutions, NIH funding accounted for about 51 percent of research revenues from all sources, defined as federal, state, and local governments; industry; and foundations (Sclar and Aries, 2000). Thus, the total biomedical research funding from all sources in New York State is estimated to be nearly $4 billion.
Capacity for Clinical Research and Clinical Trials
New York has more medical schools than any other state. Of the nation’s 125 medical schools, New York has 12; the state with the next highest number, California, has 8 (AAMC, 2004). In addition to hospital and outpatient facilities, New York is also home to several centers of rehabilitation medicine, including the Mount Sinai Spinal Cord Injury Model System center, Burke Rehabilitation Hospital, Rusk Institute of Rehabilitation, Helen Hayes Hospital, the Bronx Veterans Affairs Medical Center, and several other U.S. Department of Veterans Affairs hospitals. The most research-intensive areas within the state are the New York City metropolitan area9 and the Buffalo-Rochester area (Sclar and Aries, 2000).
Beyond its borders, New York is strategically situated in a populous region with a high density of major medical centers and medical schools. Its neighboring states—New Jersey, Connecticut, Pennsylvania, and Maryland—also have strong research and clinical capacities (Brookings Institution, 2002). These states rank high in terms of NIH research dollars (Table 8-5). All of the states except Pennsylvania have state spinal cord injury programs (Table 8-1). More than 4,700 life scientists work in the New York City metropolitan area, which includes New York City, Long Island, and northern New Jersey. This region has 20 institutions that grant Ph.D.’s in the life sciences (Brookings Institution, 2002).
These features make New York State situated to forge regional networks of clinical, basic, and translational research on spinal cord injuries. A regional clinical trials center could facilitate efforts to link the many resources in the New York region. The proximity of multiple trauma centers in and near New York City offers the opportunity to coordinate efforts on acute phase clinical trials. Further, pilot studies examining the impact of health care delivery such as the immediate triage and rapid transport of spinal cord injured patients to specialized centers could be conducted.
Concentration of Expertise in Neuroscience, Neurosurgery, and Neurology
New York also has a rich concentration of researchers with expertise in neuroscience, including several Nobel Prize winners. Of the 36,000 basic scientists and clinicians who are members of the Society for Neuroscience, 1,854 (about 5 percent) are from New York State (Personal communication, F. Johnson, Society for Neuroscience, November 19, 2004). New York’s neuroscience or neurology research programs at Columbia University, New York University, Cornell University, Rockefeller University, and the State University system are world renowned. The abundance of researchers in fields that overlap the spinal cord injury research field makes it possible for the New York State Spinal Cord Injury Research Program to expand by cultivating collaborations and by sharing core facilities, equipment, and other resources. Opportunities to increase the focus on spinal cord injury research and draw talented researchers to New York could be enhanced through the funding of two to four endowed chairs in spinal cord injury research at New York universities.
Potential for Private and Public Linkages
Another major strength of New York is its rich potential for institutional linkages with pharmaceutical firms, foundations, and patient advocacy organizations. Helping to forge such linkages is the Biomedical Research Alliance of New York, a for-profit alliance of 138 affiliates, including large New York State-based university medical centers. This alliance largely focuses on aiding the organization and start-up of clinical research, including the preparation of regulatory documents and submissions to institutional review boards. Public and private research organizations stand to gain by creating local and regional consortia for clinical trials and other collaborations that are key to greater access by individuals with spinal cord injuries and research efficiency.
Apart from clinical trials, core facilities for animal research are an important shared resource. Just as the Miami Project to Cure Paralysis and
the Reeve-Irvine Research Center in California have created core facilities for production and analysis of the findings from studies with animal models, a New York-based institution could also create the same types of facilities through various cost-sharing agreements between payers. The Christopher Reeve Paralysis Foundation, which has distributed more than $40 million in research grants over the past two decades, is headquartered in nearby New Jersey.
New York and neighboring states are headquarters to numerous pharmaceutical companies. A new economic analysis by the pharmaceutical industry attempted to measure the relative intensities of their activities by state by formulating a new measure, the biopharmaceutical innovation pipeline index. By this new index, New York State ranked 10th in the nation; most of its neighboring states ranked higher. The index captures measures in four areas: biopharmaceutical research funding, biopharmaceutical risk capital funding, biopharmaceutical industry human capital and workforce, and biopharmaceutical innovation output (DeVol et al., 2004). A separate analysis found that since 1995 biopharmaceutical firms in the New York City metropolitan area have attracted more than $639 million in venture capital (Brookings Institution, 2002).
Finally, the state of New York is another potential source of help in forming public and private partnerships. In 1999, the New York State legislature passed a comprehensive law to fund a $522 million economic development stimulus by providing support for science and academic research. The New York State Office of Science, Technology, and Academic Research is the administrative locus. This 1999 law enhanced what was already a strong state investment. A survey in 1995 found that New York ranks fourth in the nation in total state investment in research and development (Jankowski, 1999).
Strengthening the Research Infrastructure in New York State
As demonstrated in this chapter, state spinal cord injury research programs can make a significant contribution to the research endeavor to find a cure for spinal cord injuries. Several model spinal cord injury research programs, working with fewer resources than those available in New York State, used funding from their states as seed money to build programs of far greater magnitude and scientific impact than those in New York State. New York State, with the highest level of state funding for spinal cord injury research of any state program, has the potential to assume a leadership role in spinal cord injury research. However, for it to do so, its program must have a sustainable research infrastructure. New York has an impressive concentration of researchers who could be working on spinal cord injury research. Continued efforts to support and strengthen the program will
attract these scientists into the field. To maximize its efforts and build its program, the New York State program should continue to enter into collaborations that draw on the unique strengths of New York’s biomedical expertise, clinical caseload, and infrastructure, as well as the strengths of the region beyond New York’s borders.
RECOMMENDATIONS
Further development of the New York State Spinal Cord Injury Research Board should build on the recommendations presented in this report, with additional focus on the following recommendations.
Recommendation 8.1: Build and Strengthen New York State’s Research Infrastructure
The New York State Spinal Cord Injury Research Board should increase its research infrastructure to meet the program’s mission. The Board should
-
develop and sustain a vigorous recruitment and training effort for fundamental and translational research; the number of investigators should be increased progressively over the next 3 years with the goal of at least doubling the number of researchers focused on fundamental and translational studies of spinal cord injuries;
-
establish a coordinated statewide research network that encourages collaborations among individual investigators and interinstitutional research efforts; the Board should convene a statewide meeting of investigators and relevant stakeholders to plan a research strategy and coordinate research efforts;
-
cultivate formal linkages with researchers, programs, and biopharmaceutical companies in the region to forge partnerships for basic, translational, and clinical research; and
-
establish regional core laboratory facilities.
Recommendation 8.2: Develop a Regional Clinical Trials Center
The state of New York should use its unique strengths to establish a regional clinical trials center. This center should
-
develop and coordinate multicenter clinical trials to examine therapies for the treatment of spinal cord injuries;
-
sponsor a clinical trial of decompression as an early intervention and clinical trials of other therapies to be used during the acute phase of a spinal cord injury by using the special opportunities offered by New York City’s geographic location and the unique resources of its trauma centers; and
-
manage a clinical trials clearinghouse.
Recommendation 8.3: Restructure Research Funding and Oversight Processes
The New York State Spinal Cord Injury Research Board should work with the state of New York to reduce administrative burdens, improve the approval and grant distribution processes, and establish a rapid-response funding mechanism to capitalize on new research ideas.
Recommendation 8.4: Ensure Independent Evaluation
The New York State Spinal Cord Injury Research Board should establish an independent external review panel that meets periodically to rigorously assess the program’s efforts toward its stated mission to cure spinal cord injuries.
REFERENCES
AAMC (Association of American Medical Colleges). 2004. Medical Schools of the U.S. and Canada—Alphabetical Listing. [Online]. Available: http://www.aamc.org/members/listings/msalphaae.htm [accessed November 11, 2004].
Aries NR, Sclar ED. 1998. The economic impact of biomedical research: A case study of voluntary institutions in the New York metropolitan region. Journal of Health Politics, Policy & Law 23(1): 175-193.
Attorney General of California. 2004. Proposition 71—Stem Cell Research. [Online]. Available: http://www.voterguide.ss.ca.gov/propositions/prop71-title.htm [accessed November 11, 2004].
Bank of Boston Economics Department. 1991. The Job-Related Impacts of the Biotechnology Industry in Massachusetts. Boston: Bank of Boston.
Barondess JA. 2002. Municipal support of biomedical research. Academic Medicine 77(1): 31-33.
Battelle Memorial Institute/SSTI. 1998. Survey of State Research and Development Expenditures: Fiscal Year 1995. Westerville, OH: Battelle Memorial Institute.
Battelle Technology Partnership Practice/SSTI. 2004. Laboratories of Innovation: State Bioscience Initiatives 2004. Washington, DC: Biotechnology Industry Organization.
Brookings Institution. 2002. Profile of Biomedical Research and Biotechnology Commercialization: New York–Northern New Jersey–Long Island Consolidated Metropolitan Statistical Area. [Online]. Available: http://www.brookings.edu/dybdocroot/es/urban/publications/biotechnewyork.pdf [accessed November 4, 2004].
California Health Care Institute. 1993. The Health Care Technology Industry: What’s Growing in California. La Jolla, CA: California Health Care Institute.
Center for Social Gerontology. 2004. Tobacco Settlement Funds Daily Updates. [Online]. Available: http://www.tcsg.org/tobacco/settlement/updates.htm [accessed November 11, 2004].
CRPF (Christopher Reeve Paralysis Foundation). 2000. New York State Spinal Cord Research Trust Fund Awards $3.6 Million in Grants. [Online]. Available: http://www.charitywire.com/charity44/01180.html [accessed October 3, 2003].
DeVol R, Wong P, Bedroussian A, Wallace L, Ki J, Murphy D, Koepp R. 2004. Biopharmaceutical Industry Contributions to State and U.S. Economies. Santa Monica, CA: Milken Institute.
Garvey M. 2004, November 22. California stem cell project energizes other states to act. Los Angeles Times. p. A1.
Jankowski JE. 1999. What Is the State Government Role in the R&D Enterprise? NSF Report 99-348. Arlington, VA: National Science Foundation.
Kleitman N. 2001. Under one roof: The Miami Project to Cure Paralysis model for spinal cord injury research. Neuroscientist 7(3): 192-201.
KSCIRC (Kentucky Spinal Cord Injury Research Center). 2004. Overview. [Online]. Available: http://www.kscirc.org/overview.htm [accessed December 29, 2004].
Lasker Foundation. 2000. Exceptional Returns: The Economic Value of America’s Investment in Medical Research. New York: Lasker Foundation.
Maryland Department of Economic and Employment Development. 1994. The Impact of the National Institutes of Health in Maryland and the U.S. Baltimore, MD: Maryland Office of Research.
National Science Foundation. 2004. Science and Engineering Indicators 2004. Arlington, VA: National Science Foundation.
New York State Spinal Cord Injury Research Board. 2002. Request for Proposal: Guidelines and Instructions for CART and IDEA Grants (FY2002). [Online]. Available: http://www.wadsworth.org/new/rfp/scirb/finalrfp.pdf [accessed June 6, 2003].
New York State Spinal Cord Injury Research Board. 2003a. Center of Research Excellence. [Online]. Available: http://www.wadsworth.org/new/rfa/spinal/rfa.doc [accessed June 6, 2003].
New York State Spinal Cord Injury Research Board. 2003b. Program Profile. Albany: New York Department of Health.
New York State Spinal Cord Injury Research Board. 2004. $15 Million Award for Research to Find Treatment and Cure for Spinal Cord Injury and Paralysis Announced. [Online]. Available: http://www.wadsworth.org/new/rfa/spinalaward.htm [accessed January 7, 2005].
NIH (National Institutes of Health). 1999. Computer Retrieval of Information on Scientific Projects. [Online]. Available: http://crisp.cit.nih.gov/ [accessed January 8, 2004].
NIH. 2004a. NIH Extramural Awards by State and Foreign Site. [Online]. Available: http://grants.nih.gov/grants/award/state/state.htm [accessed January 7, 2005].
NIH. 2004b. NIH Extramural Awards Ranking Tables Medical Schools Long-Term Trends and Historical Data. [Online]. Available: http://grants1.nih.gov/grants/award/trends/medschl.htm [accessed January 7, 2005].
NYSTAR (New York State Office of Science, Technology, and Academic Research). 2001. Discovery Drives Progress in New York. [Online]. Available: http://www.nystar.state.ny.us/pa/papers120501.htm [accessed November 3, 2004].
Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. 2004. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nature Medicine 10(6): 610-616.
Sclar E, Aries N. 2000. Biomedical Research and the New York State Economy. New York: Council on Biomedical Research and Development, New York Academy of Medicine.
Silverstein SC, Garrison HH, Heinig SJ. 1995. A few basic economic facts about research in the medical and related life sciences. FASEB Journal 9(10): 833-840.
SSTI (State Science & Technology Institute). 1997. State Science and Technology Strategic Planning: Creating Economic Opportunity. Westerville, OH: State Science & Technology Institute.
SSTI. 1999a. Governors Talk Technology. [Online]. Available: http://www.ssti.org/Digest/1999/042399.htm [accessed November 11, 2004].
SSTI. 1999b. S&T Programs Funded through Tobacco Settlements. [Online]. Available: http://www.ssti.org/Digest/1999/111299.htm [accessed November 11, 2004].
Sturman LS, Sorin MD, Larkins E, Cavanagh KA, DeBuono BA. 1997. Losing ground: NIH funding to New York State researchers. Bulletin of the New York Academy of Medicine 74(1): 6-19.
Sturman LS, Sorin MD, Hannum RJ. 2000. Opportunities lost: NIH research funding to New York’s medical schools. Journal of Urban Health 77(1): 86-95.
Times Newsweekly. 2001. Research of Spinal Cord Injury Gets State Funds. [Online]. Available: http://www.timesnewsweekly.com/OldSite/092001/NewFiles/SPINAL.html [accessed October 3, 2003].
U.S. Congress, Senate Committee on Appropriations. 2003. Departments of Labor, Health and Human Services, and Education, and Related Agencies Appropriation Bill, 2004. 108th Cong., 1st Sess. June 23, 2003.






































