3
TOOLS FOR ASSESSING SPINAL CORD INJURY AND REPAIR
Because the spinal cord is encased in the protective armor of the vertebrae, investigation of the site of the injury or the effects of potential therapies has required the development of a diverse set of research tools. In the past 40 years the rapid progress in the technologies available to perform experiments has largely been responsible for the great strides that have been made in understanding the basic principles of neuroscience. Studies with animal models have been instrumental in the rapid development of neuroscience and understanding of the biology of the spinal cord. The advent of cell culture techniques has provided a means to isolate and grow cells. Researchers can now isolate specific molecules and proteins and examine their roles in neuronal injury and repair in laboratory animals that mimic human spinal cord injuries. Recent advances in imaging techniques and methods for investigation of the actions of genes have advanced the understanding of spinal cord injuries even further. They also provide researchers with the tools that they need to examine changes in the spinal cord at the molecular and structural levels, for example, improving knowledge of the inhibitory conditions that serve as barriers to neuronal regeneration.
This chapter describes the important genetic and in vitro tools that have been developed to advance spinal cord injury research; the key animal models that are used to mimic human spinal cord injuries and the major limitations of the existing animal models; and the outcome measures that have been developed to assess spinal cord injuries and the effectiveness of experimental therapies, including the development of imaging technologies.
MOLECULAR, GENETIC, AND IN VITRO TOOLS
Techniques have been developed that allow researchers to isolate and grow populations of neurons to investigate the effects of specific proteins and molecules on neuronal injury and repair. Neurons can be grown in isolation or with glial cells such as oligodendrocytes or Schwann cells to study the processes of axonal outgrowth and myelination. Investigators use molecular biology-based techniques, such as DNA or protein analysis, that can be used to easily visualize or analyze outcomes.
Demonstrating the power of a cell culture experiment, the simple growth-cone turning assay led to the discovery that altering various molecules inside the growing axon regulates protein and cyclic nucleotide activities, which, in turn, can convert an axon’s response to a growth-inhibiting molecule from one of repulsion to one of attraction (Song et al., 1998). When this application is applied to regenerating axons in the rat spinal cord, investigators showed that the regrowth of transected neurons has the potential to be enhanced considerably (Neumann et al., 2002; Qiu et al., 2002). Furthermore, the recent elucidation of the signaling pathways responsible for this switch in response may lead to the discovery of a strategy for enhancing axon regeneration (Wen et al., 2004).
Often, in vitro assays can be used in experiments with animal models, thus allowing researchers to verify and examine the effects detected in vitro to be evaluated in a more complex system. For example, chondroitin sulfate proteoglycans were found to inhibit neurite outgrowth in in vitro experiments (Snow et al., 1990). Analysis with animal models demonstrated that the levels of these proteoglycans are enhanced, or up-regulated, during central nervous system (CNS) injury (Snow et al., 1990) and led to the development of a strategy to break down these substances and promote the regrowth of axons in the intact rat spinal cord after an injury (Bradbury et al., 2002).
Animal Models for Molecular and Genetic Studies
Models consisting of multiple-transgenic animals have been developed to investigate molecular mechanisms and to identify the molecules critical for specific processes (Table 3-1). These models provide a better understanding of the genetic and molecular basis by which spinal cord circuits, specific neuronal subtypes, and synapses are formed (Shirasaki and Pfaff, 2002; Lanuza et al., 2004). For example, by studying the development of the nervous system of the fruit fly (Drosophila melanogaster), researchers have identified numerous molecules that can regulate the growth of the axon and the formation of neuronal connections (Vaessin et al., 1991; Kidd et al., 1998; Kraut et al., 2001; Jin, 2002). This information should provide
TABLE 3-1 Animal Models Commonly Used to Identify Genes Involved in Axon Growth and Circuit Formation
|
Animal |
Technique(s) Primary |
Utility |
|
Fruit fly |
Transgenic |
Identify and investigate molecular expression patterns; perform genetic experiments to identify the molecules involved in axon growth and guidance and the reformation of neuronal connections |
|
Worm |
Transgenic |
Identify and investigate molecular expression patterns and perform genetic experiments |
|
Fish |
Transgenic, transection, |
Examine motor control and the central pattern generator after transection of the spinal cord and investigate axonal regeneration models |
|
Mouse |
Transgenic, imaging |
Identify and investigate molecular expression patterns; perform genetic experiments to identify the molecules involved in axon growth and guidance and the reformation of neuronal connections; examine cellular and molecular basis of spinal cord circuits |
the insights needed to reconstruct effective circuits once axonal regeneration has been achieved.
ANIMAL MODELS OF SPINAL CORD INJURY
Animal models allow in-depth investigation of the anatomical and molecular changes that occur in response to a spinal cord injury at a level of detail that would not be possible or ethical in studies with humans. These insights are critical for the design and interpretation of the results of studies with humans. Without the knowledge gleaned from studies with animals, the spinal cord would remain the equivalent of a black box and therapies aimed at restoring function would be limited. For example, experiments with rodents demonstrated that the neurons in the spinal cord are able to regenerate after an injury (Richardson et al., 1980; Xu et al., 1995).
Researchers have developed a variety of animal models that mimic
different attributes associated with spinal cord injuries. Depending on the purpose of the study and the specific aspect of the injury to be investigated, researchers determine which animal model most closely replicates the injury in humans (Tables 3-2 and 3-3). In 2000, the International Spinal Research Trust published guidelines that describe four characteristics that are required for an optimal model of spinal cord injury (Ramer et al., 2000):
-
The nature and the extent of the lesion should be precisely defined. If there is doubt about the extent of a lesion or whether axons have been spared, then interpretations of regeneration can be misleading.
-
A histological method should be available to detect the growth of axons through the lesion.
-
A method should be available to analyze the functional synaptic transmission beyond the lesion by measuring the electrical activity that neurons use to communicate with one another.
-
A behavioral measure should be available that is capable of detecting restoration of known circuits.
It is important to examine therapies in a system that best mimics the condition of the individual with a spinal cord injury. For example, therapies designed for individuals with chronic conditions should not be tested in animal models immediately after the animal has received the injury but should be tested only after the animal is in the chronic stage of the injury (Kwon et al., 2002a; Houle and Tessler, 2003; Kleitman, 2004). Further-
TABLE 3-2 Value of Animal Models for Spinal Cord Injury Research
|
TABLE 3-3 Criteria for Choosing an Ideal Animal Model
|
|
SOURCE: Croft, 2002. |
more, each type of spinal cord injury (Chapter 2) is different and presents its own set of challenges; therefore, each requires its own standard animal model that reliably mimics the complications experienced by individuals with that type of spinal cord injury.
A number of animal models have been developed, including models that mimic compression, contusion, and transection (Table 3-4). Blunt contusion injuries account for 30 to 40 percent of all human spinal cord injuries (Hulsebosch, 2002); thus, the contusion model provides an important tool that researchers can use to examine the neuropathology of the injury and to test the efficacies of different therapeutic agents. In 1978, the clip compression technique was developed by researchers to simulate the continual pressure and displacement of the spinal cord common in spinal cord injuries, which is not reproduced in contusion injuries (Rivlin and Tator, 1978). This procedure has provided researchers with a great deal of information about the pathophysiology of the spinal cord during the acute stages of the injury; the timing, necessity, and effectiveness of releasing the pressure from the spinal cord; and potential therapies (Kwon et al., 2002b). To target and eliminate particular groups of neurons, methods that generate microlesions (Magavi et al., 2000) and that leave the vast majority of the nervous system intact have been developed. Using this strategy, the functional consequences that result from losing the nerve groups can be systematically examined. Researchers are determining the neuronal populations responsible for specific spinal cord injury deficits, including the root causes of chronic pain (Gorman et al., 2001).
TABLE 3-4 Commonly Used Animal Models of Spinal Cord Injury
Issues Regarding Animal Models
Mimicking Transection and Compression Injuries
To make certain that the results from transection experiments are correctly interpreted and to minimize the variability in results, it is important that transection methods be standardized and that control animals be prepared at the same time that the experimental animals are treated. For example, to ensure that the recovery of function is due to axonal regeneration and not spared spinal cord circuitry, researchers must precisely perform transections of the spinal cord and must be sure that the axons projecting from the neurons are completely severed. If not all of the axons are severed, sparing and sprouting from uninjured axons become issues. It is important to note that damage to the dura mater as a result of a penetrating injury (including experimental transection) provides a route for the invasion of fibroblasts into the injury site (Zhang et al., 1996, 2004). Furthermore, in mice, there is extensive invasion of fibroblasts even without damage to the dura and the fibroblasts participate in the formation of a tissue matrix that is supportive for regeneration of at least some types of CNS axons. Following penetrating injuries, the potential contribution of fibroblasts (positive or negative) must be considered in evaluating experimental interventions to promote repair and functional recovery
By virtue of the means by which compression injuries occur, there is a large amount of variability in the severities of spinal cord injuries. However, when initial compression studies are performed, it is important to be able to study a large population of animals that have the exact same initial injury characteristics before the experimental therapeutic intervention. Protocols have been developed to help minimize the variability in injury from animal to animal. Three impactors are widely accepted as standard methods for the delivery of contusion injuries to rodents: the Ohio State University (OSU) impactor, the Infinite Horizons device, and the Multicenter Animal Spinal Cord Injury Study (MASCIS) impactor (Bresnahan et al., 1987; Noyes, 1987; Kwo et al., 1989; Gruner, 1992; Young, 2002).
Genetic Variability Between and Among Species
Although it is important to test therapeutic interventions in animals before they can become established treatments in the clinic, genetic differences between animal species can potentially result in different responses to spinal cord injuries or treatments. For example, in response to injury, humans and rats develop a cavity in the spinal cord, but this does not occur in mice (although the precise cellular and molecular bases for this are not yet
|
BOX 3-1 Three groups of investigators recently used the gene-knockout strategy to examine whether Nogo, a potential inhibitor of axon growth (see Chapter 2), was responsible for preventing neuronal regeneration after an injury (Steward et al., 2003). Researchers coordinated their research efforts and published their findings in papers published in the same issue of the journal. Each group removed a specific part of a mouse’s chromosome that is responsible for Nogo, with the hypothesis that if Nogo is responsible for inhibiting neurons from growing, then its removal would facilitate regeneration after a spinal cord injury. However, the experiments found contradictory results. One study reported that the loss of Nogo increased the extent of neuronal regeneration, as predicted (but only in young mice), and the second study reported a more modest enhancement; however, the third group did not find any significant difference (Kim et al., 2003; Simonen et al., 2003; Zheng et al., 2003). The various results could have been due to differences in the ages and the genetic backgrounds of the mice, the strategy used to delete the Nogo gene, and the compensatory changes in other genes. In order to better understand the differences in these results, two of the groups have set up a collaboration to share their mice and perform their own analyses. This example demonstrates the value of genetic techniques, the importance of consistency in experimental design, the need to replicate experimental results, and the value of collaborative and collegial interactions between research groups. |
well understood). In amphibians, regeneration readily occurs directly through the glial scar.
Different strains of the same animal species may respond differently to spinal cord trauma. For example, the nature and the extent of the secondary injury and wound healing vary in different strains of mice (Inman et al., 2002). Although these differences in responses between strains and species complicate comparison of the results of studies with different animal species, they may provide important insights about the specific genes that affect postinjury signaling cascades (Inman et al., 2002). Furthermore, the differences observed in experiments with the Nogo gene (Box 3-1) provide important lessons about the necessity to replicate experiments.
Scale
The human spinal cord is more than four times as long as the rat’s entire CNS (brain and spinal cord). Figure 3-1 demonstrates the difference in size between the entire CNS of a rat and the caudal end of a human spinal cord. A contusion or transection trauma in humans can affect upwards of 2 to 3 centimeters of the spinal cord, which is approximately 10
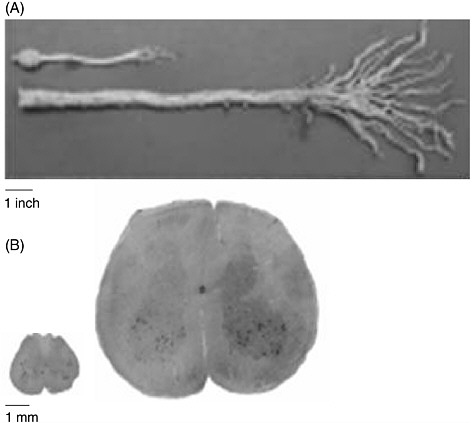
FIGURE 3-1 Size discrepancy between the rat and the human spinal cords.
The human spinal cord is more than four times as long as the entire CNS of the rat. (A) A caudal segment of the human spinal cord, including the cauda equina. The human cauda equina is approximately the same length as the entire CNS of a rat, which includes its brain. (B) The diameter of the human spinal cord is also much larger than that of the rat spinal cord. Twenty slices of a rat spinal cord can fit inside one slice of a human cord.
SOURCE: Reprinted with permission, from Dobkin and Havton, 2004. Copyright 2004 from Annual Reviews.
times the length of the 1 to 3 millimeters often affected by contusion injuries in rats (Metz et al., 2000). Consequently, regeneration of nerve fibers over a few vertebral segments in a rat—which can result in the restoration of function—is equivalent to only a fraction of the distance that is needed to restore function in humans (Dobkin and Havton, 2004). Furthermore, because neurons from both species demonstrate the same degree of spontaneous sprouting of their axons, approximately 2 millimeters (von Meyenburg et al., 1998), there are added complexities in promoting sufficient axon
growth in humans (Dobkin and Havton, 2004). Although parts of the white matter of the human spinal cord are almost as large as the entire diameter of the rat spinal cord (Figure 3-1), there is no significant difference in the capacity for oligodendrocyte precursor cells to migrate to remyelinate axons in rats and humans.
One of the issues regarding the differences in scale between smaller laboratory animals and humans that has been discussed is the extent to which testing is needed in primate models. Depending on the treatment, it may be advisable to examine the efficacies of some cell therapies in primates. However, there are also limitations in the use of non-human primates for mimicking human responses. For example, some types of monkeys have specific antibodies that can attack and inhibit the survival of human cells. Additionally, the bioavailability and metabolism of anti-rejection drugs in non-human primates and humans differ significantly. Therefore, rodents have frequently been used as the preferred model to study the efficacies of new immunosuppressive agents because of similarities in metabolism between rodents and humans. In addition, experiments are sometimes performed in rabbits and cats, which have larger spinal cords and are also less expensive and easier to maintain than primates. Furthermore, few tests have been developed to assess changes in spinal cord recovery in nonhuman primates. The committee believes that every therapy need not necessarily be tested in primates before clinical trials are performed with humans and that tests with primates be limited to those that will answer questions that are best explored only with non-human primate models.
Next Steps
The promise accorded by the methodical testing of therapies with animal models is beginning to pay off. Scientists have identified numerous inhibitory molecules and receptors that prevent the regeneration of neurons in the spinal cord and have clarified the pathways by which the inhibitory response can be modulated.
Additional resources and tools are still needed in some areas, however. Animal models need to be developed for solid spinal cord injuries, as they account for a significant portion of human spinal cord injuries (Hulsebosch, 2002). Primate models of contusion injury are particularly needed, as well as standard animal models for cervical spinal cord injuries. Furthermore, there is no standard laboratory animal model that spinal cord injury researchers can use to examine fine motor control of the upper extremities or the loss of the sensory modality proprioception, which is responsible for limb position and immediately varying the degree of muscle contraction in response to external stimuli. When individuals with spinal cord injuries lose their proprioception, they are unable to move freely and interact comfort-
ably with the external environment (see Box 5-1). Therefore, the development of a standard animal model that mimics the loss of proprioception will facilitate the development of therapies in a timely fashion.
It is important that researchers use standardized animal models and that they use them consistently. The National Institute of Neurological Disorders and Stroke (NINDS), in recognition of the need to train researchers who work on spinal cord injuries, collaborated with Ohio State University to design a course that emphasizes competency in the technical approaches required for standard animal care and treatment and experimental design (Ohio State University, 2004). In addition, the University of California at Irvine has developed a similar course. These courses provide researchers with the opportunity to be trained to use the same standards for animal research. By training multiple researchers to use standard techniques, consistent animal injury models can be implemented. These models will increase the extent to which research results can be compared and improve the extent to which animal models can be used to predict clinical outcomes in humans.
OUTCOME MEASURES USED TO ASSESS INJURY AND RECOVERY
Because of the variations in the severity and the nature of the outcomes that individuals with spinal cord injuries experience, it is often difficult for health care professionals and researchers to assess the success of a particular intervention. Similarly, it is difficult for preclinical researchers to consistently assess progress in laboratory animal experiments and to determine the amount of progress, if any, that results from natural recovery, drug therapy, surgical intervention, or rehabilitation.
Outcome Measures Used to Assess Spinal Cord Injury in Animal Models
Tests developed to examine the recovery of function in laboratory animals have been designed primarily to examine motor function (Table 3-5; Appendix D). However, to accelerate the translation of research in other areas, including sexual function, bladder and bowel control, and chronic pain relief, standard tests need to be developed to assess experimental therapies for each of these major complications (Widerstrom-Noga and Turk, 2003).
Researchers use a standard scale, the Basso, Beattie, and Bresnahan (BBB) scale, to assess the recovery of motor function in rats (Basso et al., 1995). The foundation of the BBB scale is the assessment of hind-limb movements in rats with spinal cord injuries. The 21-point BBB scale is sensitive enough that small gains in motor function are reflected in changes
TABLE 3-5 Tools Used to Assess Spinal Cord Injuries in Laboratory Animals
|
Functional recovery |
||
|
|
Basso, Beattie, and Bresnahan (BBB) scale, an open-field locomotor test for rats |
|
|
|
• |
Is based on 5-point Tarlov scale |
|
|
• |
Analyzes hind-limb movements of a rat in an open field |
|
|
• |
Is a 21-point scale used to assess locomotor coordination |
|
|
• |
Rates parameters such as joint movements, the ability for weight support, limb coordination, foot placement, and gait stability |
|
|
• |
Small changes in tissue correlate to large changes on the scale |
|
|
• |
Assesses walking, not other movements requiring coordinated spinal cord activity |
|
|
• |
Does not assess pain, bowel, bladder, or sexual function |
|
|
Basso Mouse Scale (BMS), an open-field locomotor test for mice |
|
|
|
• |
Is an adaptation of rat BBB scale to examine the recovery of hind-limb locomotor function |
|
|
• |
Assesses walking, not other movements requiring coordinated spinal cord activity |
|
|
• |
Does not assess pain, bowel, bladder, or sexual function |
|
|
Neuronal activity assessment by electrophysiology |
|
|
|
• |
Assesses MEPs or SSEP |
|
|
• |
Stimulates corresponding cortical areas of the brain and records response in target nerves to see if connections are still functional |
|
|
• |
Correlates to impairment of locomotor activity |
|
|
• |
Is noninvasive |
|
|
• |
Neuronal activity may not correlate with functional changes |
|
|
• |
Hard to assess subtle but critical improvements to circuitry |
|
|
• |
Does not directly assess pain, bowel, bladder, or sexual function |
|
|
Forepaw withdrawal |
|
|
|
• |
Investigates recovery of heat perception |
|
|
• |
The forepaw is placed on a heat block and the time that it takes for the animal to withdraw it is measured |
|
|
• |
Forepaw withdrawal requires motor function |
|
|
• |
Does not assess pain, bowel, bladder, or sexual function |
|
|
Directed forepaw reaching |
|
|
|
• |
Looks at coordinated limb and muscle movement |
|
|
• |
Requires rats to reach under a barrier and pick up food with forepaws |
|
|
• |
Limited scale for assessment |
|
|
• |
Does not assess pain, bowel, bladder, or sexual function |
|
Morphological assessment of recovery |
||
|
|
Histology |
|
|
|
• |
Is used to look at the morphology of axons and assess the degree of tissue sparing, injury, and recovery |
in the outcome score. However, the scale has several limitations as it assesses only the functional recovery of the hind limbs and not other elements of fine motor control that are required for coordinated activity regulated by the spinal cord; does not examine the recovery of sensory modalities, including pain and temperature sensations; does not assess other complications that arise as a result of spinal cord injuries, including bowel and bladder function, pain, or sexual capacity; and is not linear.
Outcome Measures Used to Assess Spinal Cord Injury in Humans
Clinicians have available more than 30 assessment tests and surveys that they can use to examine individuals with spinal cord injuries (see Appendix D), including the American Spinal Injury Association (ASIA) scale and measures that assess all the major complications associated with spinal cord injuries. As discussed in further detail in Chapter 5, each of these measures assesses a specific aspect of recovery from spinal cord injury or evaluates the individual’s quality of life and is not designed to examine all the major complications that arise because of a spinal cord injury.
MONITORING REAL-TIME PROGRESSION OF SPINAL CORD INJURIES
Biomarkers
It is hoped that in the near future biomarkers will be available for diagnosis or prediction of the clinical course of an individual after a spinal cord injury; however, no biomarkers are currently available to identify the changes occurring in the cells in the living spinal cord, such as neurite outgrowth, cell death, or changes in gene expression. Researchers have identified a large number of potential biomarkers (Table 3-6) and are developing practical methods to assess changes to those markers that could be used in the clinical setting. Once biomarkers are available and validated, they could be used to aid researchers and clinicians with making a diagnosis and establishing a prognosis, monitoring changes over time, and evaluating therapeutic interventions.
Trauma to the spinal cord affects a large number of biochemical cascades and reactions, but specific details about the genes involved in these processes are not well understood. Most of these changes are reflected by changes in mRNA and protein levels (Table 3-7). Since mRNA is copied, or transcribed, from DNA and provides the transcript that the cell uses to synthesize new proteins, analysis of mRNA or protein levels could reveal information about changes in cellular events. Advances in microarray technologies over the last decade have made it possible for researchers to examine the expression patterns of hundreds, if not thousands, of genes at the same time by comparing changes in gene activity in spinal cord samples from healthy and injured individuals. Using biomarkers, microarrays, and other tools, investigators have started to assess the complexity of the biological response to spinal cord injury. The full potential uses of biomarkers for spinal cord injury research include the following:
-
Diagnosis and prognosis. The expression profile of a biomarker, especially proteins, could provide clinicians with information that aids in establishment of a diagnosis and a prognosis of a patient’s injury. For instance, the progression of multiple sclerosis (MS) can be determined by examining the levels of a major myelin component, myelin basic protein, whose concentration increases in the cerebrospinal fluid in response to a demyelinating episode. Experiments with laboratory animals have identified similar gene expression fluctuations in response to spinal cord injuries. For example, the onset of the acute immune response is characterized by increases in the levels of the interleukin-6 protein (Segal et al., 1997; Carmel et al., 2001; Song et al., 2001; Nesic et al., 2002), whereas apoptosis, or the controlled death of cells that begins in the secondary stage of the injury, is regulated, in part, by changes in the levels of the Fas protein (Li et al., 2000;
TABLE 3-6 Criteria for Determining and Validating a Biomarker Used to Monitor Spinal Cord Injury Progression and Recovery of Function
-
Casha et al., 2001). Thus, identification of specific fluctuations in the levels of proteins like interleukin-6 and Fas could inform clinicians about changes in an individual’s level of injury.
-
Treatment guidance. Analysis of gene expression during the course of the injury and recovery could provide clinicians with detailed informa-
-
tion about the molecular events that are responsible for changes in spinal cord reorganization that occur over time. With this knowledge, physicians might be able to avoid preventable complications and specifically target ongoing events when they treat spinal cord injuries.
-
Outcomes assessment. Biological expression data that are correlated to functional improvement, such as increased locomotion, improved bowel function, or reduced spasticity, may provide helpful means of assessing beneficial or harmful changes to the spinal cord that may be missed when the primary clinical end points are behavioral. The development of biomarkers that are specific for neuronal cell death, myelination, or nerve regeneration would be beneficial to both basic researchers and clinicians.
-
Potential therapeutic targets. The analysis of changes in specific gene products that are up- and down-regulated in response to a spinal cord injury could also provide researchers with a tool to identify specific targets that could be used for future drug development. Understanding of the molecular and cellular mechanisms involved in spinal cord injuries may permit identification of specific targets for therapeutic benefit.
Traditionally, biomarkers were identified by examining candidate genes involved in cellular events that occur as a result of a spinal cord injury and looking for other genes that were associated with the function of the candidate gene. This strategy led to the identification of many candidate genes, such as the Nogo gene and several of the interleukin genes, which have helped define the biological processes affected by a spinal cord injury. Although the individual process of identifying genes involved in a spinal cord injury has been critical for advancing the research, the process is also intrinsically biased and limited in its scope because of its dependence on previous detailed knowledge about the biological system under study. Another limitation is that changes in individual biomarkers may be induced by events other than spinal cord injuries. For example, the activities of the immediate-early genes c-fos and c-jun have been correlated to neurite outgrowth, but they are also involved in many other processes, including cancer metastasis. Therefore, for a single biomarker to provide sufficient predictive value, it must be specific to spinal cord injuries and provide a sensitive measure for the assessment of the process being examined. Consequently, changes in multiple genes will need to be assessed to understand gene responses specifically related to spinal cord injuries as is true in assessing breast cancer (Hollon, 2002).
Protein Expression Profiles of Spinal Cord Injury
Because the body contains more than 1 million proteins that regulate metabolism and disease (Watkins, 2001), proteomic techniques that ana-
TABLE 3-7 Changes in Gene Expression After Spinal Cord Injury, by Stage of Injury
|
Gene Function |
Primary Stage |
Secondary Stage |
Chronic Stage |
|
Apoptosis |
Caspases, c-jun, p53, Fas, FasL, CD95, rho |
Caspases, c-jun, NF-κB, HSP70 |
None |
|
Growth and differentiation |
Vimentin, TGFβ, ANIA-6 |
Vimentin, TGF, VGF, BDNF, TrkB (–) |
Vimentin, TrkB, BMPs |
|
Inflammation |
IL-6, IL-1β, IGFs, SOCS, MCP-1 (IESR-JE), ICAM-1, iNOS, GFAP, IL-4r, COX-2, IL-2Rα, HSP27 |
IL-6, IL-1, IGFs, SOCS, MCP-1 (IESR-JE), ICAM-1, iNOS, GFAP, TNF receptor, COX-3 (–), HSP27 |
IL-6, IL-1β, IGFs, HSP27 |
|
Regulation of ion transport |
Ca2+ ATPase (–) |
Ca2+ ATPase (–), K+ channels, Na+ channels (–), Na+/K+ ATPase (–) |
Ca2+ ATPase (–) |
|
Protection of neurons |
None |
Metallothionein I and II, survival motoneuron |
Metallothionein I and II |
|
Communication between neurons |
SNAP-25 (–), syntaxins (–), glutamate receptors |
SNAP-25 (–), syntaxins (–), synapsins (–), somatostatin (–), GABA transporters (–), glutamate receptors, GABA receptors (–), glutamate transporter |
GABA receptors |
lyze changes to individual or multiple proteins have the potential to provide investigators with information about cellular responses to spinal cord injuries. For example, Western blotting and immunohistochemistry allow investigators to examine modifications to a protein’s structure that may change its activity and cellular distribution.
Protein arrays, like DNA arrays, allow researchers to screen simultaneously many proteins for changes in expression levels that result from the onset of a disease or a therapeutic approach. However, protein arrays are not as encompassing as DNA arrays. Current protein array technology only allows about 10 percent of a cell’s total proteome to be represented on an array (2,000 to 3,000 proteins can be represented on a protein array,
whereas 47,000 genes can be represented on a DNA array). Such arrays could be tailored to the specific aspect of spinal cord injury being studied. In addition, advances in mass spectrometry now make it possible to characterize the levels and even the phosphorylation state of many hundreds of proteins, allowing greater insights into the specific activities of proteins.
Issues in Developing Biomarkers for Spinal Cord Injury
It is extremely difficult to obtain samples of mRNA or protein directly from spinal cord tissue without inducing further complications. The most practical sources of mRNA and protein are serum and cerebrospinal fluid.
However, a spinal tap—an invasive procedure, which requires the insertion of a special needle through the lumbar vertebral spine into the fluid space that surrounds the spinal cord—must be performed to obtain cerebrospinal fluid. Although serum is easier to collect by drawing blood samples, its analysis is complicated by the high concentration of several proteins (e.g., albumin, immunoglobulin G, and transferrin) that constitute approximately 80 percent of total serum proteins. These high background levels make it difficult to sieve through and detect changes in the levels of proteins that are present at low concentrations. Once a sample is obtained, issues about the usefulness of the contents remain. The mRNA derived from neurons and glia is not very abundant and degrades rapidly. Also, because serum and cerebrospinal fluid are indirect sources of spinal cord mRNA and proteins, the overall numbers of genes that are associated with spinal cord injuries are not well represented. Furthermore, the proteins that are present are typically restricted to those found on the exteriors of cells and the small intracellular concentrations of mRNA and proteins that are released when a cell dies, which further limits the pool of biomarkers that can be analyzed. Efforts are thus needed to improve the processes for detecting potential biomarkers.
Next Steps in Biomarker Development
Experimental therapies developed in the laboratory take as long as 7 to 15 years to enter into the clinic (Lakhani and Ashworth, 2001). To expedite this transition, spinal cord injury researchers should use strategies developed in other fields, including MS, Alzheimer’s disease, and cancer biology. For example, clinical studies for MS and brain metastasis have been established to analyze changes in protein levels in the serum and cerebrospinal fluid. These trials could provide the framework for biomarker studies involving individuals with spinal cord injuries.
In 2000, the National Cancer Institute established the Early Detection Research Network (EDRN) to guide the process of biomarker discovery in an effort to produce a useful population-screening tool (Kutkat and Srivastava, 2001). This network consists of three laboratory components: biomarker discovery laboratories, biomarker validation laboratories, and clinical epidemiological centers. EDRN also helped to establish standards for the development and evaluation of biomarkers and guide the process of biomarker discovery related to cancer biology. Using EDRN as a model, the spinal cord injury community can transfer many of the recommendations and strategies developed to facilitate progress on cancer research for spinal cord injury research. In 2001 and 2004 NINDS issued two program announcements that focused on advancing proteome arrays and identifying clinical biomarkers (NINDS, 2001a, 2004); these are not specifically fo-
cused on spinal cord injuries but do offer potential for advances in this area. Additionally, NINDS put out a request for proposals (NINDS, 2001b) for studies designed to define gene expression profiles following traumatic spinal cord injuries.
Because the technologies used to identify biological markers can detect small but significant changes in gene expression, they are sensitive to slight variations in protocol. In fact, the gene profiles obtained from experimental studies are affected by differences in the instruments used to analyze the samples and by small changes in the ways in which samples are collected (e.g., the relative time after injury that tissue is collected, the location of the injury, and the quantity of the specimen) (Bareyre and Schwab, 2003). A standard set of methods is needed to minimize variability and maximize reproducibility (Bareyre and Schwab, 2003).
Visualizing the Living Spinal Cord
The spinal cord is embedded in bone and is surrounded by cerebrospinal fluid, which precludes direct visualization. The advent of neuroimaging techniques has allowed investigators to visualize the spinal cord so that they can begin to study the progression of spinal cord injuries. Magnetic resonance imaging (MRI) and computed tomography (CT) provide real-time information about the state of the injury and recovery. Moreover, imaging is noninvasive and the same region of the spinal cord can be repeatedly visualized to identify changes occurring over time. Imaging technologies, biomarkers, and molecular genetic technologies are being combined to provide researchers with powerful tools to monitor the progression of the injury and recovery through the visualization of specific molecular markers that define cellular events and functional changes.
MRI is a safe and noninvasive method of evaluating the spinal cord that provides detailed pictures of hard-to-view areas of the spine, including the spinal canal, vertebra, and soft tissue (Levitski et al., 1999). Clinicians use MRI after an individual has an acute spinal cord trauma to visualize the location and the extent of the spinal cord trauma and compressive lesions (e.g., blood clots) (AANS/CNS, 2002). It is superior to positron emission tomography (PET), CT, and other imaging technologies for the detection of abscesses or other masses near the spinal cord and is used to monitor patients with chronic compression injuries. However, imaging technologies have practical limits in the setting of acute spinal cord traumas, as a patient may not be stable enough to enter an MRI machine or may have other medical priorities that take precedence over receiving a detailed image of the spinal cord.
Functional MRI (fMRI) can provide second-by-second images of the brain to reveal changes in neuronal activity in response to different sensory
stimuli and mental tasks. It allows researchers and clinicians to study the changes in injured neuronal circuits. However, fMRI relies on the metabolic changes that occur in response to neural activity and the images obtained by fMRI are not a direct measure of neural activity. Therefore, caution should be placed on interpretation of the accuracies of the spatial maps generated by fMRI (Ugurbil et al., 2003). The National Institutes of Health has recommended that fMRI techniques be developed to assess the degree of loss and recovery of sensation in rodents with contusion injuries to their spinal cords (Hofstetter et al., 2003; NINDS, 2004).
Radiologists use CT scans as a standard procedure to clarify areas of clinical concern (Youmans, 1996; AANS/CNS, 2002). Although MRI is better suited for analyzing the soft tissue of the spinal cord, the strength of using CT scans is in investigating the bone structure and detecting fractures of the vertebrae (Figure 3-2). Helical CT scans offer advantages over traditional radiology X-rays due to their speed in accruing the images and increased accuracy (4.5 minutes and 98.5 percent, respectively, for helical CT compared with 25 minutes and 43 percent, respectively, for X-rays). Therefore, in conjunction with MRI, CT scans provide useful tools for emergency clinicians (Nunez et al., 1994).
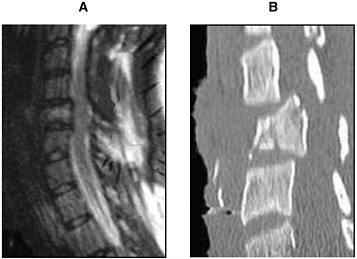
FIGURE 3-2 MRI (A) and CT (B) of an injured spinal cord. Imaging of a spinal cord contusion injury by MRI and CT helps to reveal different aspects of the injury. The MRI image on the left reveals the soft spinal cord and bone, whereas the CT scan image on the right clearly delineates bone structures.
SOURCE: Reprinted with permission, from AANS, 1999. Copyright 1999 from AANS.
Unlike MRI, fMRI, and CT scans, PET scans detect and localize specific naturally occurring proteins; molecules, such as sugars and water; and other substances, such as neurotransmitters, which have been modified to emit radioactive energy.
At present, PET scans are not commonly used in the clinic to assess spinal cord injuries. However, as discussed below, the technology has much potential to provide researchers and clinicians with a means by which to visualize changes in gene expression in the spinal cord.
Next Steps: Future Imaging Technologies
Imaging technologies provide clinicians with important tools to gauge the responses of patients to different therapies (Jacobs et al., 2003). The creation of sensitive assays that merge image-based technologies with biomarker research will allow investigators and clinicians to use specific tracers to localize molecular, genetic, and cellular processes in real time, thus providing further insight into the biological processes that affect the progression of the injury (Blasberg and Gelovani, 2002).
As of January 2005, no clinical studies in the United States were specifically examining the use of imaging marker technologies for the study of spinal cord injuries. In comparison, markers are used to assess the state of MS and Alzheimer’s disease and imaging techniques are used to monitor the effects of different treatments for these conditions. For example, imaging assays are being developed to visualize specific neurotransmitter levels and to determine if they are involved in memory loss (Brown et al., 2003).
The Future of Magnetic Resonance Technology
In animals with syringomyelia, diffusion-weighted MRI, which is sensitive to the diffusion or random motion of water molecules in tissue, can detect cystic lesions in the gray matter of the spinal cord (Schwartz et al., 1999). The increased sensitivity offered by diffusion-weighted MRI will enable physicians to detect specific complications of spinal cord injuries sooner, thus increasing the potential for treatment.
Magnetic resonance technology can be adapted to provide more than diagnostic information about the structural changes occurring in response to a spinal cord injury. In 2001, Bulte and colleagues used magnetic resonance to track oligodendrocyte stem cells that were prelabeled with super paramagnetic iron oxide nanocomposites, which are small beads invisible to the naked eye that can be detected by MR technology (Bulte et al., 1999, 2001). Using this approach, the investigators were able to track the real-time migration and integration of these oligodendrocyte stem cells for up to 6 weeks in the same animal, which is important for distinguishing the
efficacies of endogenous cells versus those of the exogenous transplanted stem cells.
The Future of PET Scans
PET scan technology is being developed to inform clinicians about whether drugs can bind to the appropriate targets. For example, clinicians are using PET scans to determine if treatments are effective by looking at the uptake of glucose, which tumors need to nourish their growth (Van den Abbeele and Badawi, 2002; Pollack, 2004). These effects can be observed before structural changes in the tumor can be detected.
Two caveats about the use of PET scans must be kept in mind. First, current technology does not have enough resolution to allow complete visualization through the entire diameter of the spinal cord. Furthermore, the current spatial resolution of commercial PET scanners is 4 mm but 2.5 mm resolution has been achieved in research instruments that use motion compensation. Second, information obtained from PET scans is based on metabolic events that correlate to neural activity and may not directly correspond to the location where the changes in activity are occurring. Therefore, the images generated by PET scans could be misleading because they may not accurately represent the spatial specificities of the changes (Ugurbil et al., 2003). However, refinements to PET scans could provide important information about the cellular states of the injury, such as gene activation or suppression in response to the injury; this would provide physicians with the ability to quantify responses to different spinal cord injury treatments (Brooks et al., 2003) and to identify functional changes before the onset of structural changes identifiable by MRI (National PET Scan Management, LLC, 2004). PET ligands have been developed that can detect glucose metabolism, inflammation, and receptor abundance, including agents that track the N-methyl-D-aspartate (NMDA) receptor activity and proteases. PET measures very different process than does MRI whose spatial resolution is superior. However, PET contrast resolution for identification of proteins can be hundreds of times greater than MRI depending on the target. The potentials of PET for assessing the severity of injury and the responses to therapy await application of high resolution systems with recently developed radiopharmaceuticals.
Tracking Recovery with PET and Magnetic Resonance
Improvements to PET and MR technologies enable investigators to visualize the molecular signatures of damage and repair to the CNS. In an attempt to examine the activities of specific neuronal circuits, imaging markers that mimic neurotransmitters and receptors that are nonradioactive are
being created, including the iron analog annexin V (Schellenberger et al., 2002), the fluorescent marker Cy5.5 (Petrovsky et al., 2003), and markers that do not become active until they reach their target. Future modification and adaptation of these technologies could be used to examine specific stages of regeneration, including those designed to detect neurite outgrowth, astrocyte scarring, oligodendrocyte myelination, and immunological response.
Transgenic Animals: Following the Labeled Cell
At present it is difficult to follow the path of cell transplants (such as stem cells, Schwann cells, and olfactory ensheathing cells) in the living spinal cord; therefore, it is difficult to draw conclusions about the efficacy of an experiment with such cells. Continued advancement of imaging techniques will provide a mechanism by which investigators and clinicians can assess the integration of grafted tissue or cells into the preexisting neuronal network or monitor the response to gene therapy by tracking the transgene location. Transgenic animal models have thus been developed. Specific populations of cells in these animals are genetically engineered to be fluorescent or to emit a fluorescent signal when they are functionally activated. Such approaches, which use two-photon confocal imaging to detect the signal, can be directly applied to spinal cord preparations in vitro and administered to intact mice and rats. With improvements in the current technology, the use and improvement of near-infrared markers might also provide researchers with a means to monitor the progression of a spinal cord injury and recovery in laboratory animals.
Multidisciplinary Research and Bringing Molecular Imaging to the Clinic
The promise of molecular imaging technologies can be realized only if the technologies can be successfully transferred to the clinical setting. The transfer of these technologies will require cross-disciplinary collaborations and multidisciplinary research efforts among molecular and cellular biologists, imaging scientists, nanotechnologists, and clinicians. A review article by Massoud and Gambhir (2003) identified the following goals for the transfer of molecular imaging technologies from the research laboratory to the clinic:
-
develop noninvasive in vivo imaging methods that detect specific cellular and molecular processes, such as gene expression and protein-protein interactions;
-
monitor multiple molecular events in concert;
-
monitor the trafficking and targeting of cells;
-
optimize drug and gene therapies;
-
image drug effects at the molecular and cellular levels; and
-
assess the molecular pathology of disease progression.
Achieving these goals and translating those achievements into reliable clinical technologies will be critical steps toward the treatment and diagnosis of spinal cord injuries at the molecular level. To achieve these objectives, continued advances need to be made to overcome the challenges of biocompatibility, probe delivery, and high-resolution signal detection (Mahmood and Weissleder, 2002).
Cross-disciplinary collaboration and multidisciplinary research is needed to bring together molecular and cellular biologists, imaging scientists, nanotechnologists, and clinicians to reach these goals (Blasberg and Gelovani, 2002). Many of the imaging techniques used to examine the CNS were designed to visualize brain tumors or to assess Alzheimer’s disease, Parkinson’s disease, and MS. These resources and technologies can be applied or can provide models for spinal cord injury research. For instance, investigators are examining the utility of using multiphoton imaging techniques to monitor the progression of senile plaques in mice that model Alzheimer’s disease (Christie et al., 2001). This technology could also be modified to assess and monitor the progression of the glial scar formation that results from spinal cord injuries.
The cancer research field not only has led the way in developing technologies but also has helped to establish research centers that have been critical in creating a means for translating imaging technologies into the clinic. In particular, the National Cancer Institute has developed two programs: the Small Animal Imaging Resources Program (SAIRP) and the In Vivo Cellular and Molecular Imaging Centers (ICMIC) Program. These programs, along with support mechanisms sponsored by the National Institute of Biomedical Imaging and Engineering, provide mechanisms and model systems that can be used to promote the cooperative development of new imaging systems for spinal cord injury research and treatment.
RECOMMENDATIONS
Recommendation 3.1: Increase Training Efforts on Standardized Research Tools and Techniques
Spinal cord injury researchers should receive training in the use of standardized animal models and evaluation techniques. Pre- and postdoctoral fellowship training programs focused on spinal cord injury research should require participation in courses designed to train investigators on the appropriate use of the available tools and techniques.
Recommendation 3.2: Improve and Standardize Research Tools and Assessment Techniques
Preclinical research tools and animal models should be developed and refined to examine spinal cord injury progression and repair and assess the effectiveness of therapeutic interventions. These preclinical tools and assessment protocols should be standardized for each type and each stage of spinal cord injury. Particular emphasis should be placed on:
-
improving imaging technologies to allow real-time assessment of the current state and progression of the injury;
-
identifying biomarkers that can be used to monitor the progression of the injury and recovery;
-
developing additional animal models to explore the progression of spinal cord injury and repair;
-
establishing standardized sets of functional outcome measures for the evaluation of experimental therapies for each type and each stage of spinal cord injury in animal models; and
-
enhancing functional assessment techniques to examine motor function as well as secondary complications, including pain and depression of the immune system.
REFERENCES
AANS (American Association of Neurological Surgeons). 1999. Spinal Cord. [Online]. Available: http://www.neurosurgerytoday.org/what/patient_e/spinal.asp [accessed January 25, 2005].
AANS/CNS (American Association of Neurological Surgeons/Congress of Neurological Surgeons). 2002. Radiographic assessment of the cervical spine in symptomatic trauma patients. Neurosurgery 50(3 Suppl): S36-43.
Bareyre FM, Schwab ME. 2003. Inflammation, degeneration and regeneration in the injured spinal cord: Insights from DNA microarrays. Trends in Neurosciences 26(10): 555-563.
Bareyre FM, Haudenschild B, Schwab ME. 2002. Long-lasting sprouting and gene expression changes induced by the monoclonal antibody IN-1 in the adult spinal cord. Journal of Neuroscience 22(16): 7097-7110.
Basso DM, Beattie MS, Bresnahan JC. 1995. A sensitive and reliable locomotor rating scale for open field testing in rats. Journal of Neurotrauma 12(1): 1-21.
Blasberg RG, Gelovani J. 2002. Molecular-genetic imaging: A nuclear medicine-based perspective. Molecular Imaging: Official Journal of the Society for Molecular Imaging 1(3): 280-300.
Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. 2002. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416(6881): 636-640.
Bregman BS, McAtee M, Dai HN, Kuhn PL. 1997. Neurotrophic factors increase axonal growth after spinal cord injury and transplantation in the adult rat. Experimental Neurology 148(2): 475-494.
Bresnahan JC, Beattie MS, Todd FD III, Noyes DH. 1987. A behavioral and anatomical analysis of spinal cord injury produced by a feedback-controlled impaction device. Experimental Neurology 95(3): 548-570.
Brooks DJ, Frey KA, Marek KL, Oakes D, Paty D, Prentice R, Shults CW, Stoessl AJ. 2003. Assessment of neuroimaging techniques as biomarkers of the progression of Parkinson’s disease. Experimental Neurology 184(Suppl 1): S68-79.
Brown D, Chisholm JA, Owens J, Pimlott S, Patterson J, Wyper D. 2003. Acetylcholine muscarinic receptors and response to anti-cholinesterase therapy in patients with Alzheimer’s disease. European Journal of Nuclear Medicine & Molecular Imaging 30(2): 296-300.
Bulte JW, Zhang S, van Gelderen P, Herynek V, Jordan EK, Duncan ID, Frank JA. 1999. Neurotransplantation of magnetically labeled oligodendrocyte progenitors: Magnetic resonance tracking of cell migration and myelination. Proceedings of the National Academy of Sciences (U.S.A.) 96(26): 15256-15261.
Bulte JW, Douglas T, Witwer B, Zhang SC, Strable E, Lewis BK, Zywicke H, Miller B, van Gelderen P, Moskowitz BM, Duncan ID, Frank JA. 2001. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nature Biotechnology 19(12): 1141-1147.
Carmel JB, Galante A, Soteropoulos P, Tolias P, Recce M, Young W, Hart RP. 2001. Gene expression profiling of acute spinal cord injury reveals spreading inflammatory signals and neuron loss. Physiological Genomics 7(2): 201-213.
Casha S, Yu WR, Fehlings MG. 2001. Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuroscience 103(1): 203-218.
Christie RH, Bacskai BJ, Zipfel WR, Williams RM, Kajdasz ST, Webb WW, Hyman BT. 2001. Growth arrest of individual senile plaques in a model of Alzheimer’s disease observed by in vivo multiphoton microscopy. Journal of Neuroscience 21(3): 858-864.
Croft BY. 2002. Animal models for imaging. Disease Markers 18(5-6): 365-374.
Di Giovanni S, Knoblach SM, Brandoli C, Aden SA, Hoffman EP, Faden AI. 2003. Gene profiling in spinal cord injury shows role of cell cycle in neuronal death. Annals of Neurology 53(4): 454-468.
Dobkin BH, Havton LA. 2004. Basic advances and new avenues in therapy of spinal cord injury. Annual Review of Medicine 55: 255-282.
Dubreuil CI, Winton MJ, McKerracher L. 2003. Rho activation patterns after spinal cord injury and the role of activated Rho in apoptosis in the central nervous system. Journal of Cell Biology 162(2): 233-243.
Fan M, Mi R, Yew DT, Chan WY. 2001. Analysis of gene expression following sciatic nerve crush and spinal cord hemisection in the mouse by microarray expression profiling. Cellular and Molecular Neurobiology 21(5): 497-508.
Gorman AL, Yu CG, Ruenes GR, Daniels L, Yezierski RP. 2001. Conditions affecting the onset, severity, and progression of a spontaneous pain-like behavior after excitotoxic spinal cord injury. Journal of Pain 2(4): 229-240.
Gruner JA. 1992. A monitored contusion model of spinal cord injury in the rat. Journal of Neurotrauma 9(2): 123-126.
Haberkorn U, Altmann A, Mier W, Eisenhut M. 2004. Impact of functional genomics and proteomics on radionuclide imaging. Seminars in Nuclear Medicine 34(1): 4-22.
Hofstetter CP, Schweinhardt P, Klason T, Olson L, Spenger C. 2003. Numb rats walk—A behavioural and fMRI comparison of mild and moderate spinal cord injury. European Journal of Neuroscience 18(11): 3061-3068.
Hollon T. 2002. Classifying breast cancer models. The Scientist 16(17): 20-24.
Houle JD, Tessler A. 2003. Repair of chronic spinal cord injury. Experimental Neurology 182(2): 247-260.
Hulsebosch CE. 2002. Recent advances in pathophysiology and treatment of spinal cord injury. Advances in Physiology Education 26(1-4): 238-255.
Inman D, Guth L, Steward O. 2002. Genetic influences on secondary degeneration and wound healing following spinal cord injury in various strains of mice. Journal of Comparative Neurology 451(3): 225-235.
Jacobs AH, Li H, Winkeler A, Hilker R, Knoess C, Ruger A, Galldiks N, Schaller B, Sobesky J, Kracht L, Monfared P, Klein M, Vollmar S, Bauer B, Wagner R, Graf R, Wienhard K, Herholz K, Heiss WD. 2003. PET-based molecular imaging in neuroscience. European Journal of Nuclear Medicine & Molecular Imaging 30(7): 1051-1065.
Jin Y. 2002. Synaptogenesis: Insights from worm and fly. Current Opinion in Neurobiology 12(1): 71-79.
Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS, Tear G. 1998. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell 92(2): 205-215.
Kim JE, Li S, GrandPre T, Qiu D, Strittmatter SM. 2003. Axon regeneration in young adult mice lacking Nogo-a/B. Neuron 38(2): 187-199.
Kleitman N. 2004. Keeping promises: Translating basic research into new spinal cord injury therapies. Journal of Spinal Cord Medicine 27(4): 311-318.
Kraut R, Menon K, Zinn K. 2001. A gain-of-function screen for genes controlling motor axon guidance and synaptogenesis in Drosophila. Current Biology 11(6): 417-430.
Kutkat L, Srivastava S. 2001. The early detection research network: A platform for communication and collaboration. Disease Markers 17(1): 3-4.
Kwo S, Young W, Decrescito V. 1989. Spinal cord sodium, potassium, calcium, and water concentration changes in rats after graded contusion injury. Journal of Neurotrauma 6(1): 13-24.
Kwon BK, Liu J, Messerer C, Kobayashi NR, McGraw J, Oschipok L, Tetzlaff W. 2002a. Survival and regeneration of rubrospinal neurons 1 year after spinal cord injury. Proceedings of the National Academy of Sciences (U.S.A.) 99(5): 3246-3251.
Kwon BK, Oxland TR, Tetzlaff W. 2002b. Animal models used in spinal cord regeneration research. Spine 27(14): 1504-1510.
Lakhani SR, Ashworth A. 2001. Microarray and histopathological analysis of tumours: The future and the past?. Nature Reviews—Cancer 1(2): 151-157.
Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M. 2004. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron 42(3): 375-386.
Levitski RE, Lipsitz D, Chauvet AE. 1999. Magnetic resonance imaging of the cervical spine in 27 dogs. Veterinary Radiology & Ultrasound 40(4): 332-341.
Li GL, Farooque M, Olsson Y. 2000. Changes of Fas and Fas ligand immunoreactivity after compression trauma to rat spinal cord. Acta Neuropathologica 100(1): 75-81.
Liu CL, Jin AM, Tong BH. 2003. Detection of gene expression pattern in the early stage after spinal cord injury by gene chip. Chinese Journal of Traumatology 6(1): 18-22.
Magavi SS, Leavitt BR, Macklis JD. 2000. Induction of neurogenesis in the neocortex of adult mice. Nature 405(6789): 951-955.
Mahmood U, Weissleder R. 2002. Some tools for molecular imaging. Academic Radiology 9(6): 629-631.
Massoud TF, Gambhir SS. 2003. Molecular imaging in living subjects: Seeing fundamental biological processes in a new light. Genes & Development 17(5): 545-580.
Metz GA, Curt A, van de Meent H, Klusman I, Schwab ME, Dietz V. 2000. Validation of the weight-drop contusion model in rats: A comparative study of human spinal cord injury. Journal of Neurotrauma 17(1): 1-17.
National PET Scan Management, LLC. 2004. How Does PET Compare with Other Imaging Modalities? [Online]. Available: http://www.nationalpetscan.com/petovew.htm [accessed November 11, 2004].
Nesic O, Svrakic NM, Xu GY, McAdoo D, Westlund KN, Hulsebosch CE, Ye Z, Galante A, Soteropoulos P, Tolias P, Young W, Hart RP, Perez-Polo JR. 2002. DNA microarray analysis of the contused spinal cord: Effect of NMDA receptor inhibition. Journal of Neuroscience Research 68(4): 406-423.
Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI. 2002. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron 34(6): 885-893.
NINDS (National Institute of Neurological Disorders and Stroke). 2001a. Biomarkers and Clinical Endpoints in Pediatric Clinical Trials. [Online]. Available: http://grants.nih.gov/grants/guide/pa-files/PA-01-043.html [accessed November 11, 2004].
NINDS. 2001b. Gene Expression Profiling in the Nervous System Following Traumatic Spinal Cord Injury. [Online]. Available: http://www.ninds.nih.gov/funding/2rfp_01_03.pdf [accessed October 4, 2004].
NINDS. 2004. Functional and Dysfunctional Spinal Circuitry: Role for Rehabilitation and Neural Prostheses. [Online]. Available: http://www.ninds.nih.gov/news_and_events/proceedings/spinalcircuitrywkshp_pr.htm [accessed November 11, 2004].
Noyes DH. 1987. Correlation between parameters of spinal cord impact and resultant injury. Experimental Neurology 95(3): 535-557.
Nunez DB, Ahmed AA, Coin C, et al. 1994. Clearing the cervical spine in multiple trauma victims: A time-effective protocol using helical computed tomography. Emergency Radiology 1(6): 273-278.
Ohio State University. 2004. Spinal Cord Injury: A Comprehensive Course. [Online]. Available: http://medicine.osu.edu/sci/Main.htm [accessed November 11, 2004].
Petrovsky A, Schellenberger E, Josephson L, Weissleder R, Bogdanov A Jr. 2003. Near-infrared fluorescent imaging of tumor apoptosis. Cancer Research 63(8): 1936-1942.
Pollack A. 2004, August 4. In drug research, some guinea pigs are now human. The New York Times. p. A2.
Qiu J, Cai D, Filbin MT. 2002. A role for cAMP in regeneration during development and after injury. Progress in Brain Research 137: 381-387.
Ramer MS, Harper GP, Bradbury EJ. 2000. Progress in spinal cord research: A refined strategy for the International Spinal Research Trust. Spinal Cord 38(8): 449-472.
Richardson PM, McGuinness UM, Aguayo AJ. 1980. Axons from CNS neurons regenerate into PNS grafts. Nature 284(5753): 264-265.
Rivlin AS, Tator CH. 1978. Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surgical Neurology 10(1): 38-43.
Saito N, Yamamoto T, Watanabe T, Abe Y, Kumagai T. 2000. Implications of p53 protein expression in experimental spinal cord injury. Journal of Neurotrauma 17(2): 173-182.
Schellenberger EA, Hogemann D, Josephson L, Weissleder R. 2002. Annexin V-Clio: A nanoparticle for detecting apoptosis by MRI. Academic Radiology 9(Suppl 2): S310-311.
Schwartz ED, Yezierski RP, Pattany PM, Quencer RM, Weaver RG. 1999. Diffusion-weighted MR imaging in a rat model of syringomyelia after excitotoxic spinal cord injury. American Journal of Neuroradiology 20(8): 1422-1428.
Segal JL, Gonzales E, Yousefi S, Jamshidipour L, Brunnemann SR. 1997. Circulating levels of IL-2R, ICAM-1, and IL-6 in spinal cord injuries. Archives of Physical Medicine and Rehabilitation 78(1): 44-47.
Shibuya S, Miyamoto O, Auer RN, Itano T, Mori S, Norimatsu H. 2002. Embryonic intermediate filament, nestin, expression following traumatic spinal cord injury in adult rats. Neuroscience 114(4): 905-916.
Shirasaki R, Pfaff SL. 2002. Transcriptional codes and the control of neuronal identity. Annual Review of Neuroscience 25: 251-281.
Simonen M, Pedersen V, Weinmann O, Schnell L, Buss A, Ledermann B, Christ F, Sansig G, van der Putten H, Schwab ME. 2003. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron 38(2): 201-211.
Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. 1990. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Experimental Neurology 109(1): 111-130.
Song G, Cechvala C, Resnick DK, Dempsey RJ, Rao VL. 2001. GeneChip analysis after acute spinal cord injury in rat. Journal of Neurochemistry 79(4): 804-815.
Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M. 1998. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science 281(5382): 1515-1518.
Steward O, Zheng B, Tessier-Lavigne M. 2003. False resurrections: Distinguishing regenerated from spared axons in the injured central nervous system. Journal of Comparative Neurology 459(1): 1-8.
Tachibana T, Noguchi K, Ruda MA. 2002. Analysis of gene expression following spinal cord injury in rat using complementary DNA microarray. Neuroscience Letters 327(2): 133-137.
Ugurbil K, Toth L, Kim DS. 2003. How accurate is magnetic resonance imaging of brain function? Trends in Neurosciences 26(2): 108-114.
Vaessin H, Grell E, Wolff E, Bier E, Jan LY, Jan YN. 1991. Prospero is expressed in neuronal precursors and encodes a nuclear protein that is involved in the control of axonal outgrowth in Drosophila. Cell 67(5): 941-953.
Van den Abbeele AD, Badawi RD. 2002. Use of positron emission tomography in oncology and its potential role to assess response to imatinib mesylate therapy in gastrointestinal stromal tumors (GISTs). European Journal of Cancer 38(Suppl 5): S60-65.
von Meyenburg J, Brosamle C, Metz GA, Schwab ME. 1998. Regeneration and sprouting of chronically injured corticospinal tract fibers in adult rats promoted by NT-3 and the mAb IN-1, which neutralizes myelin-associated neurite growth inhibitors. Experimental Neurology 154(2): 583-594.
Watkins K. 2001. Making sense of information mined from the human genome is a massive undertaking for the fledgling industry. Chemical & Engineering News 79(8): 26-45.
Wen Z, Guirland C, Ming GL, Zheng JQ. 2004. A CaMKII/calcineurin switch controls the direction of Ca2+-dependent growth cone guidance. Neuron 43(6): 835-846.
Widerstrom-Noga EG, Turk DC. 2003. Types and effectiveness of treatments used by people with chronic pain associated with spinal cord injuries: Influence of pain and psychosocial characteristics. Spinal Cord 41(11): 600-609.
Xu XM, Guenard V, Kleitman N, Bunge MB. 1995. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. Journal of Comparative Neurology 351(1): 145-160.
Youmans JR, ed. 1996. Neurological Surgery: A Comprehensive Reference Guide to the Diagnosis and Management of Neurological Problems. Philadelphia: W. B. Saunders.
Young W. 2002. Spinal cord contusion models. Progress in Brain Research 137: 231-255.
Zhang YP, Iannotti C, Shields LB, Han Y, Burke DA, Xu XM, Shields CB. 2004. Dural closure, cord approximation, and clot removal: Enhancement of tissue sparing in a novel laceration spinal cord injury model. Journal of Neurosurgery: Spine 100(4): 343-352.
Zhang Z, Fujiki M, Guth L, Steward O. 1996. Genetic influences on cellular reactions to spinal cord injury: A wound-healing response present in normal mice is impaired in mice carrying a mutation (Wld(s)) that causes delayed Wallerian degeneration. Journal of Comparative Neurology 371(3): 485-495.
Zheng B, Ho C, Li S, Keirstead H, Steward O, Tessier-Lavigne M. 2003. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron 38(2): 213-224.
Zurita M, Vaquero J, Zurita I. 2001. Presence and significance of CD-95 (Fas/APO1) expression after spinal cord injury. Journal of Neurosurgery 94(2 Suppl): 257-264.































