Making Better Drugs for Children with Cancer
EXECUTIVE SUMMARY
The oncology world is justifiably proud of the fact that cancer during childhood and adolescence has been transformed from a death sentence into long-term survival for most of those affected, at least in the United States and other countries with access to good health care. Today, major improvements in survival from childhood cancers that still have a poor prognosis—and reducing the substantial short- and long-term adverse effects of current therapies—will come only with new treatments. The near absence of research in pediatric cancer drug discovery threatens to halt the progress in childhood cancer treatment achieved during the past four decades.
The achievements of the past 40 years are testament to the efforts of pediatric oncologists, radiation oncologists, and surgeons nationally and internationally who systematically evaluated the anticancer drugs developed during this period, largely for cancers in adults, for use in children. They built up effective regimens primarily by increasing the intensity of therapy in high-risk patients. Even now, adjustments in treatment regimens are continually being tested to enhance effectiveness and reduce unwanted effects, but further improvements through intensification of therapy with the same drugs are likely to be small in magnitude.
As devastating as cancer is among children in the United States (cancer remains the leading cause of death by disease in U.S. children between 1 and 15 years of age), the number of children affected is small. From the point of view of companies developing drugs and other agents to treat
cancer, the pediatric cancer drug market is often well below the radar screen, and typically it has not made business sense to invest in research and development for these cancers. Many drugs developed for adults have been found effective in children, in large part because most have a generalized affinity for cancer cells. The dark side of this characteristic is that noncancerous tissue may be damaged in the process, causing the well-known adverse effects of anticancer drugs. But in fact, the biological and clinical characteristics of nearly all childhood cancers differ substantially from adult cancers. Over the past few years, differences at the molecular level have been documented for all the major childhood cancers, and herein lies the promise: the molecular abnormalities represent a place to start searching for drug “targets.” Cutting-edge science notwithstanding, market forces are not sufficient to drive the process and bring to the bedside new drugs for children with cancer. Because so much of the technical capacity for drug discovery and development for pediatric cancers already exists—much of it supported by the National Institutes of Health (NIH) intramurally or extramurally, as well as in the private for-profit sector—it is possible that these drugs could emerge from an alternate pathway. Specifically, a “public– private partnership” could knit together the pieces in a virtual research and development (R&D) network. Networks such as this are relatively new, but are working well for cystic fibrosis, tuberculosis, malaria, and other neglected tropical diseases. The resources already in place for pediatric cancers are poised for this development.
Recommendation 1: A new public–private partnership, involving government, industry, academic and other research institutions, advocacy groups, philanthropies, and others, should be formed to lead pediatric cancer drug discovery and development.
R&D specifically for childhood cancers is not the only way to improve treatment. New drugs in development for adult cancers may prove useful for children, including some that, in the final analysis, fail to provide significant benefit to adults. This poses a dilemma for companies that would never be able to recoup development costs if a full-scale effort resulted only in a drug for children. At whatever stage a product is in development, if this is the case, the government should consider taking over the development process, either directly or through funding external work.
Recommendation 2: The National Cancer Institute should assume responsibility as the developer of last resort for agents that show promise only in children if companies decide not to proceed with full-scale development.
Even when an agent is shown effective in adults, historically, long delays have intervened between the time testing is begun in adults and in children. Because there are relatively few children with these cancers, clini-
cal trials can extend years longer than they do in adults. There are ways to shorten this period, mainly by beginning trials earlier. The National Cancer Institute (NCI) has begun to facilitate this by funding the first consolidated preclinical testing program for pediatric cancers, so that the necessary steps before the first use in children are completed as quickly as possible. Other steps must be taken to encourage companies to allow drugs to enter pediatric clinical trials earlier. Legislative initiatives over the past decade to encourage broader testing of all types of drugs in children could play a role in this, although because of issues specific to cancer, they have as yet had little impact on testing or labeling cancer drugs for children.
Recommendation 3: The pharmaceutical industry, National Cancer Institute, and Food and Drug Administration should act to reduce the delay in beginning pediatric clinical studies of agents in development for adult cancers.
INTRODUCTION
The successes that have been achieved in treating childhood cancers stand as beacons against the less dramatic improvements for adults with cancer. Progress began to accelerate in the 1960s and 1970s, as treatment regimens were built up, primarily by building combinations of chemotherapeutic drugs. But since that time, progress in most childhood cancers has come mainly from increasing the dose intensity of existing drugs in children at increased risk of disease recurrence, better understanding of the diseases, and infrequently from adopting new drugs. Acute lymphocytic leukemia (ALL), the most common childhood cancer, is a case in point: developments in the laboratory—discoveries about the biologic underpinnings of leukemia—have led to more precise subcategorization and risk stratification of patients. Better understanding of how to use the existing drugs in light of the biologic information was more important than the development of new drugs (including etoposide; introduced in 1983 and the last major addition to ALL regimens, it is featured only in treating a small minority of high-risk patients). Nonetheless, in the past 20 years, 5-year survival has improved from 50 percent to 80 percent (U.S. Cancer Statistics Working Group, 2004).
Nearly all drugs currently used to treat children with cancer, with the exception of a few antileukemia drugs developed in the 1950s and early 1960s, were developed for adult cancers and then found effective in children. The remarkable success of regimens built with these drugs might argue for continuing on this path. But it is also true that most common types of childhood cancer are distinct from adult cancers clinically, pathologically, cytologically, and in the molecular abnormalities that underlie them (Table 1). If the discovery and development of new agents for childhood cancers were to capitalize on today’s science—which in large measure it does not—there is every reason to believe that cure rates could be improved for all pediatric cancers, including those for which current long-term survival is very low, such as brain tumors. Moreover, advances based on more targeted agents could entail considerably fewer short- and long-term toxicities, which themselves become lifelong medical problems for many survivors (IOM, 2003).
This report identifies the major issues to be addressed in developing new agents for childhood cancers, the gaps in research and development, and the steps that have been suggested to move the process forward, and makes a new proposal to capitalize on today’s science to bring new treatments to children’s cancers. The Institute of Medicine (IOM) has previously issued reports related to the development and testing of drugs of all types for children (IOM, 2000) and the ethical conduct of clinical research in-
volving children (IOM, 2004), both of which include discussions relevant to the issues explored here.
A key point is that the financial market for childhood cancer drugs is very small—below the dollar value needed to interest profit-making drug and biotechnology companies. Even incentives such as orphan drug provisions and the recent “pediatric incentive” offered for testing products in children cannot tip the balance sufficiently. For-profit companies are unlikely to invest in the early discovery stages of R&D for drugs that, even if successful, have such a limited market (assuming the drug is useful only for children). At least in some cases, however, companies may find it worth-while to engage in late-stage development and manufacturing of low-volume drugs once their potential has been established (i.e., “de-risked” from a development perspective).
CHILDHOOD CANCERS: BACKGROUND
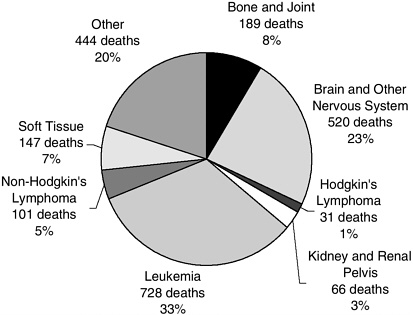
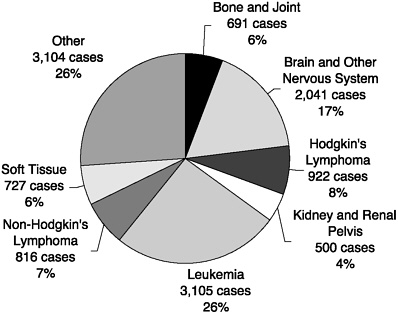
Childhood cancer is rare. In the United States, an estimated 11,900 children and adolescents under age 20 were diagnosed with cancer in the year 2001 and about 2,200 died (Figures 1 and 2). These cases represent an incidence rate of about 16 per 100,000 per year (roughly 1 per 6,400 children per year). Between birth and 20 years of age, about 1 in 333 Americans develops cancer. A few types of cancer predominate—leukemias, and tumors of the brain and nervous system, the lymphatic system, kidneys, bones, and muscles. For the most part, these cancers are distinct biologically and clinically from the cancers common in adults, even if the names are similar. Although most children and adolescents now survive their cancer, it is the third leading cause of death among children ages 1 to 4, and second only to accidents among children ages 5 to 14 (Minino and Smith, 2001) (Table 2). Genetic factors and certain prenatal exposures (e.g., ionizing radiation, diethylstilbestrol [DES]) and postnatal exposures (e.g., viruses, ionizing radiation) are known to increase the risk of developing some childhood cancers, but for most cases, the cause remains unknown, precluding strategies for prevention.
Cancer in the first half of the 20th century was nearly synonymous with death for children. Fewer than 10 percent survived. But beginning in the 1960s, discoveries of effective treatments, particularly for the leukemias, began to change that picture. Improvements since the 1960s have been steady and significant, dramatic in some cases. For some types, however—particularly brain tumors, acute nonlymphocytic leukemias, and metastatic solid tumors—treatment and survival are still poor (Table 3). (See Appendix A for a review of the epidemiology and treatment of childhood cancers.)
TABLE 1 Molecular Abnormalities That Are Common in Childhood Cancer but Rare or Nonexistent in Adult Cancer
|
Disease |
Translocation |
Fusion/Molecular Abnormality |
New Patients per Year in U.S. Children (0–19 y)* |
|
Alveolar rhabdomyosarcoma |
t(2;13)(q35;q14) t(1;13)(p36;q14) |
PAX3-FKHR PAX7-FKHR |
<200 |
|
Ewing’ s sarcoma family tumor |
t(11;22)(q24;q12) t(21;22)(q22;q12), t(2;11;22), t(12;22) t(7;22)(p22;q12) t(17;27)(q12;q12) t(2;21;22) |
EWS-FLI1 EWS-ERG EWS-ETV1 EWS-E1AF EWS-FEV |
<400 |
|
Malignant melanoma of soft parts |
t(12;22)(q13;q12) |
EWS-ATF1 |
|
|
Desmoplastic small round cell tumor |
t(11;22)(p13;q12) |
EWS-WT1 |
|
|
Extraskeletal myxoid chondrosarcoma |
t(9;22)(q22;q12) t(9;17)(q22;q11.2) |
EWS-CHN (TEC) RBP56 (hTAFII68)-CHN(TEC) |
|
|
Synovial cell sarcoma |
t(X;18)(p11;q11) |
SYT-SSX1 or SSX2 |
|
|
Myxoid liposarcoma |
t(12;16)(q13;p11) t(12;22)(q13;q12) t(12;22;20)(q13;q12;q11) |
TLS(FUS)-CHOP EWS-CHOP EWS-CHOP |
|
|
Congenital fibrosarcoma |
t(12;15)(p13;q25) |
ETV6-NTRK3 |
|
AGENTS IN USE AND IN TESTING FOR CHILDREN WITH CANCER
Most of the drugs and biologics used routinely in primary treatment regimens for pediatric cancers were approved before 1990, about half before the mid-1980s (Table 4). As for adults with cancer, treatment with multiple combinations of drugs over an extended period is the norm in children (see Appendix A). The drugs used are all primarily used to treat cancer in adults and were initially approved for that purpose. Some either have been subsequently approved by the Food and Drug Administration (FDA) for pediatric indications or provide some pediatric dosing information in the label. For the first time in more than a decade, initial approval of a new cancer drug was granted for a pediatric indication: clofarabine to treat refractory or relapsed acute lymphocytic leukemia in children was approved by the FDA in late December 2004 (U.S. Food and Drug Administration, 2004). Clofarabine has orphan status,1 which provides for 7 years of exclusive marketing once the drug is approved, and the FDA has granted an additional 6 months of exclusivity under the pediatric incentive provision of the Best Pharmaceuticals for Children Act (discussed later). An early clinical trial (Phase I) in adult patients with advanced solid tumors has been completed, but applications for adult indications are likely years away.
Medicines in Development for Children 2004 (PhRMA, 2004), a publication of the Pharmaceutical Research and Manufacturers of America (PhRMA), lists 32 products being tested for childhood cancers. These include drugs and biologics, about half of which are already approved for adult indications. Of those not yet approved, all are in more advanced testing in adults than in children. For 4 of the 32 agents, applications for pediatric labeling have been submitted, 2 of them are in Phase III trials, and another 3 are in multiple-phase trials that include Phase III. The others are in Phase I and II trials.
THE EXISTING CAPACITY FOR DEVELOPING NEW AGENTS SPECIFICALLY FOR CHILDHOOD CANCERS
Considerable basic research on childhood cancers has resulted in the identification of molecular abnormalities unique to those cancers (Table 1). If pursued, some of these could lead to new treatments, as has been the case
TABLE 2 Number of Deaths and Death Rates for the 10 Leading Causes of Death in Specified Age Groups, United States, 2000
|
|
Ages 1–4 Years |
||
|
Cause of Deatha |
Rankb |
Number |
Rate |
|
All causes |
— |
4,942 |
32.6 |
|
Accidents |
1 |
1,780 |
11.7 |
|
Motor vehicle accidents |
|
630 |
4.2 |
|
All other accidents |
|
1,150 |
7.6 |
|
Congenital malformations, deformations, and chromosomal abnormalities |
2 |
471 |
3.1 |
|
MALIGNANT NEOPLASMS |
3 |
393 |
2.6 |
|
Assault (homicide) |
4 |
318 |
2.1 |
|
Diseases of the heart |
5 |
169 |
1.1 |
|
Influenza and pneumonia |
6 |
96 |
0.6 |
|
Septicemia |
7 |
91 |
0.6 |
|
Certain conditions originating in the perinatal period |
8 |
84 |
0.6 |
|
In situ neoplasms, benign neoplasms, and neoplasms of uncertain or unknown behavior |
9 |
56 |
0.4 |
|
Cerebrovascular diseases |
10 |
45 |
0.3 |
|
Chronic lower respiratory diseases |
— |
— |
— |
|
Human immunodeficiency virus (HIV) disease |
— |
— |
— |
|
Intentional self-harm |
— |
— |
— |
|
All other causes |
— |
1,439 |
9.5 |
|
NOTE: Data are based on continuous file of records received from the states. Rates per 100,000 population in specified group. Figures are based on weighted data rounded to the nearest individual, so categories may not add to totals. |
|||
for targeted agents for adult cancers. However, there are no major R&D programs in either industry or the government devoted to developing new drugs for childhood cancers. As already explained, even with incentives such as orphan drug provisions, the market for pediatric cancer drugs is too small for pharmaceutical companies to recoup their investments in developing new products, and full-scale drug development is not considered a government function.
Even though no comprehensive R&D program exists to develop drugs for childhood cancers, the components that would constitute such a pipeline do exist—in universities, in academic medical centers, within pharmaceutical and biotechnology companies, and to a surprising degree, within NCI (and other parts of NIH). NCI’s role in supporting basic research is well known, but many of its activities in later stages of drug discovery and development are not.
TABLE 3 Five-Year Relative Survival from Childhood Cancer (Age 0–19 at Diagnosis) by Period of Diagnosis, 1975–1979 and 1995–2000
|
|
5-Year Relative Survival (%) |
|||
|
|
1975–1979 |
1995–2000 |
||
|
Type of Cancer |
Male |
Female |
Male |
Female |
|
Bone and joint |
43 |
57 |
71 |
64 |
|
Brain and other nervous system |
57 |
60 |
72 |
75 |
|
Hodgkin’s lymphoma |
86 |
88 |
96 |
96 |
|
Leukemia |
44 |
53 |
75 |
78 |
|
Acute lymphocytic |
52 |
64 |
82 |
84 |
|
Acute myeloid |
23 |
21 |
46 |
54 |
|
Neuroblastoma |
52 |
57 |
66 |
66 |
|
Non-Hodgkin’s lymphoma |
42 |
58 |
79 |
82 |
|
Soft tissue |
62 |
70 |
73 |
71 |
|
Wilms’ tumor |
73 |
76 |
92 |
92 |
|
All sites |
58 |
68 |
77 |
81 |
|
SOURCE: Jemal et al. (2004). |
||||
NCI has built a great deal of flexibility into the way its programs can interact with researchers wanting to engage their services. For example, screening of compounds can be done through agreements that allow intellectual property to be vested with the compound’s originator and assure confidentiality of results. Late development of agents can occur through clinical trial agreements with industry, in which industry supplies the compound and receives data from NCI-sponsored trials. Cooperative Research and Development Agreements allow companies to partner with NCI in various development steps, including clinical trials.
The NCI Developmental Therapeutics Program (DTP) supports the range of services, from compound screening to development of new agents, that constitute the steps needed for an Investigational New Drug (IND) filing with the FDA (i.e., permission to begin clinical testing). These services are available to academic researchers and to industry. The Cancer Therapy Evaluation Program (CTEP) encompasses the next steps, including filing IND applications with the FDA and funding and conducting all phases of clinical trials. DTP and CTEP both have in-house activities as well as grants and contracts with outside organizations. The activities relevant to developing pediatric cancer drugs are mentioned here.
Developmental Therapeutics Program
DTP has developed a remarkable range of resources and services for drug discovery and development. In the area of drug discovery, DTP maintains repositories of synthetic and natural compounds, a variety of biological molecules (e.g., mouse and human cytokines, monoclonal antibodies, growth factors, interferons, interleukins), human and animal tumor cell lines, tumor specimens, and laboratory animal models of various types. Programs that allow researchers to gain access to both drug discovery and development services have also been developed.
Drug Discovery Resources
More than 140,000 synthetic and natural compounds are available for screening for anticancer activity, held in various NCI repositories. The Natural Products Repository holds more than 50,000 plant samples collected from Africa, Central and South America, and Southeast Asia, and more than 10,000 marine vertebrates and marine algae, mainly from the Indo-Pacific region. Investigators may request samples of various types to test (e.g., against a specific receptor on which the researcher is working).
Other repositories have been established to supply radio-labeled compounds, biologics, reference standards and reagents, tumor material, and materials for research on angiogenesis (cell cultures and regulatory chemicals).
NCI can supply a wide variety of rats, mice, and guinea pigs with particular genetic and other characteristics needed for different types of drug development research.
Drug Discovery Screening Services
The In Vitro Cell Line Screening Project accepts both natural and synthetic compounds from scientists in the academic and industrial communities worldwide, and tests them for anticancer activity against 59 human tumor cell lines. Compounds for testing are selected by the NCI Drug Synthesis and Chemistry Branch, based on the degree of novelty of the structure of the molecule and any associated biological information provided by the supplier. The service is provided free of charge. Suppliers retain intellectual property rights to compounds, and are given the results of completed testing.
Up to 3,000 compounds can be screened per year against the tumor cell lines, which include leukemia, melanoma, and cancers of the lung, colon, brain, ovary, breast, prostate, and kidney. An advantage of the screening program is that the results of testing against the 59 cell lines (including dose
TABLE 4 Commonly Used Agents for Primary Treatment of Childhood Cancers
|
Drug |
Original Approval Date of NCE1 |
Approved Use2 |
Pediatric Dosage3 |
Pediatric Uses and Administration, or Indication4 |
|
arsenic trioxide |
2000 |
APL |
NHL, APL |
Yes (but limited) |
|
L-asparaginase |
1983 or earlier |
ALL |
ALL, LL |
Yes |
|
bleomycin |
7/31/73 |
Testicular cancer, lymphomas, squamous cell carcinomas (various), malignant pleural effusions |
HL, germ cell tumors |
No |
|
carboplatin |
3/3/89 |
Ovarian cancer |
Osteosarcoma, neuroblastoma, retinoblastoma |
No |
|
cisplatin |
7/16/99 |
Testicular/ovarian tumors, bladder cancer |
Osteosarcoma, neuroblastoma, CNS tumors |
No |
|
cyclophosphamide |
7/3/86 |
Many types of cancer |
Osteosarcoma, NHL, ALL, neuroblastoma, HL |
Yes |
|
cytarabine |
Before 1/1/82 |
Lymphomatous meningitis |
AML, ALL, NHL |
No |
|
dactinomycin |
Before 1/1/82 |
Many childhood cancers and some cancers of adulthood |
Wilms’ tumor, Ewing’s sarcoma, rhabdomyosarcoma, testicular carcinoma, osteosarcoma, neuroblastoma, germ cell neoplasms, melanoma |
Yes |
|
daunorubicin HCl |
6/23/98 |
ALL |
NHL, AML, LL, ALL, HL |
Yes |
|
doxorubicin |
Before 1/1/82 |
Many types of cancer |
NHL, Wilms’ tumor, neuroblastoma, osteosarcoma, Ewing’s sarcoma, germ cell neoplasms, carcinomas, hepatoblastomas, hepatocellular carcinomas |
No dosage |
response information) produce a response pattern that can be added to the database of compounds already tested, to assign a putative mechanism of action to a test compound, or to determine that the response pattern is unique and not similar to any of the standard prototype compounds in the database. The response pattern may point to specific molecular targets with which the compound is likely to interact.
Drug Discovery Databases and Analytical Tools
Vast quantities of data and tools for analysis are available publicly on the NCI web site. The databases include the results of all chemicals screened against the Human Tumor Cell Line (about 43,000 compounds), tens of thousands of compounds screened in anticancer yeast assays, chemical structures for more than 200,000 compounds, and other smaller data collections. Tools for analyzing the results of assays and other investigations are also publicly accessible through the NCI web site.
Access to Discovery Resources: The RAND Program
The Rapid Access to NCI Discovery Resources (RAND) program assists academic and nonprofit investigators with the tasks that constitute the discovery stage of anticancer drug research. RAND can assist in the discovery of small molecules, biologics, or natural products using NCI intramural or contracted resources. Some activities supported include the development of high-throughput screening assays, computer modeling, recombinant target protein production and characterization, and chemical analog generation.
Drug Development Resources
Drug discovery produces promising compounds that still require “development” into a drug that can be used by patients. This involves both work on the product itself and testing, first in the laboratory, then in experimental animals, and finally, in human beings. NCI programs have been developed to carry out all of these tasks.
The Biological Resources Branch (BRB) is one of the extramural arms of DTP. The BRB supports preclinical and early (Phase I) clinical studies of biological response modifiers through a program of grants and contracts. These studies assess the effects of novel biological agents and explore relationships of biological responses with antitumor activity. An NCI Preclinical Repository distributes selected agents for peer-reviewed preclinical studies performed by both extramural and intramural investigators. Other
contracts support the production and in vivo evaluation of monoclonal antibodies, immunoconjugates, and other biologicals.
The Pharmaceutical Resources Branch (PRB) evaluates methods for synthesizing candidate molecules or isolating them from natural products, and eventually produces small batches for initial testing. Larger (but still relatively small) amounts are produced for clinical trials after scaling up. This entails preparation under FDA-prescribed “Good Manufacturing Practices” (GMPs) to ensure a high level of purity, which is checked by extensive testing. Production and testing is continually refined as molecules progress toward filing an IND application with the FDA to begin testing in human beings.
The development of suitable drug formulations for patients is central to PRB’s mission. This is a complex process that takes in an array of physical and chemical properties such as pH, solubility, light and oxygen sensitivities, and stability in various solvents, among many others. Enough of the product is subsequently manufactured by NCI for use in clinical trials. The necessary paperwork and filings are also handled by NCI.
The NCI Toxicology and Pharmacology Branch carries out the studies needed to develop drug formulations for eventual use by patients. This involves the range of laboratory and animal studies required by the FDA, including those required to establish starting doses for clinical trials.
Access to Drug Development Resources: RAID
Rapid Access to Intervention Development (RAID) is a program to bridge the gap between drug discovery in the university laboratory and a new drug for use in the clinic. It does this by making available to the academic research community, on a competitive basis, NCI resources for the preclinical development of drugs and biologics. RAID is intended to remove the most common barriers between laboratory discoveries and clinical trials of new molecular entities. The goal of RAID is clinical “proof of principle” that a new molecule or approach is a viable candidate for expanded clinical evaluation.
The tasks required vary from project to project. Some require only one or two key missing steps, and in other cases, the entire portfolio of development tasks may be needed to file an IND. Some typical tasks that can be supported by RAID include:
-
Definition or optimization of dose and schedule for in vivo activity
-
Development of pharmacology assays
-
Conduct of pharmacology studies with a predetermined assay
-
Acquisition of bulk substance (GMP and non-GMP)
-
Scale-up production from lab scale to clinical-trials lot scale
-
Development of suitable formulations
-
Development of analytical methods for bulk substances
-
Production of dosage forms
-
Stability assurance of dosage forms
-
Range-finding initial toxicology
-
IND-directed toxicology, with correlative pharmacology and histopathology
-
Planning of clinical trials
-
Regulatory affairs, so that FDA requirements are likely to be satisfied by participating investigators seeking to test new molecular entities in the clinic
-
IND filing advice
RAID began operation in 1998 and, as of July 2004, had supported 99 projects split about equally between small molecules (synthesized drugs) and biologics. About half have been completed as of early 2005 (median completion time is 27 months), including about 30 compounds that have reached the stage of IND filing.
Access to Drug Development Resources: NCI Drug Development Group
The NCI Drug Development Group (DDG) considers drug development opportunities from the NCI intramural, extramural corporate, or extramural academic communities where the originators are certain at the outset that NCI will hold the resulting IND and manage the clinical trials (in contrast to RAID, in which those tasks are turned back to the originating academic investigator for clinical trials). The heads of major units within NCI constitute the DDG membership, which advises the director of the Division of Cancer Treatment and Diagnosis. Preclinical activities fall under DTP and clinical activities fall under the Cancer Therapy Evaluation Program (and, to some extent, the Biological Resources Branch Oversight Committee).
Clinical Trials: The Children’s Oncology Group
The Children’s Oncology Group (COG) is part of NCI’s Cancer Therapy Evaluation Program, one of about a dozen cooperative groups that together cover all types of cancer and all therapeutic interventions. COG is the unified children’s cancer cooperative group formed by the merger of four cooperative groups that had been independently conducting pediatric cancer trials. More than 230 institutions, covering every U.S. state and across Canada, form the core, with additional centers in Europe, Australia,
and New Zealand. About 100 COG Phase II and Phase III trials are usually open for enrollment, and about 5,000 patients enroll in them each year. The Cooperative Group program supports the administrative and central scientific and statistical functions of each group, including COG, and maintains some centralized resources that groups can draw on within NCI.
COG and its antecedents are known for their inclusiveness of the pediatric oncology community, which is acknowledged to be tight knit, collaborative, and cooperative. Children with cancer are very likely to be treated in specialized centers by trained pediatric oncologists on state-of-the-art clinical regimens or in formal clinical trials. In fact, the clinical trial apparatus for children is the envy of adult clinical oncology researchers. Known mainly for multicenter Phase II and III trials, COG is also involved in early-stage trials through a COG Phase I/Pilot Consortium, the Pediatric Brain Tumor Consortium, and New Approaches to Neuroblastoma Therapy, a consortium to study promising treatments in neuroblastoma. The developing Pediatric Preclinical Testing Program (described below) will be run under CTEP’s auspices as well. In each case, NCI supports study development and monitoring, and pharmacokinetics and biology studies.
PUTTING THE PIECES TOGETHER
A great deal of relevant research exists toward the development of new treatments for childhood cancers in basic science, drug discovery, and drug development.
Basic Science
NCI and private funders of research (e.g., Saint Jude Children’s Research Hospital, Howard Hughes Medical Institute) have made substantial investments in the basic science of childhood cancers, with the work being carried out mainly in academic laboratories. Through this basic research, the molecular abnormalities of many pediatric cancers have been identified, at least some of which may represent valid drug targets. For most of these abnormalities, more work on validating them as drug targets—developing an understanding of their specific role in the process of carcinogenesis—will be needed before major programs focusing on them would be worth undertaking. There are, however, many promising starting points.
Drug Discovery
The weakest link in research for childhood cancer drugs, and the greatest threat to continued improvement in outcome for children with cancer, is pediatric drug discovery. Although NCI has developed capabilities in this
area (described above), it is still largely the province of the pharmaceutical and biotechnology industries. Companies maintain state-of-the-art, high-throughput screening technology and large “libraries” of compounds—major companies have libraries that number in the millions—that could become lead compounds to be developed into drugs, if they show activity when screened against relevant molecular targets. There may well be useful agents for pediatric cancers in these libraries, including some in development for adult cancers, but by and large, compounds are not screened against known pediatric cancer targets (Personal communication, D. Parkinson, Amgen, January 9, 2003). The optimization of compounds—maximizing their activity against targets and turning them into agents that can be used by human beings—is also a specialty of industry.
Drug Testing
Preclinical Testing
The need for a preclinical testing program to identify promising drug candidates for pediatric cancers was recognized in the Best Pharmaceuticals for Children Act of 2002. It states (Section 15[c]) that the NCI Director “shall expand, intensify, and coordinate the activities of the Institute with respect to research on the development of preclinical models to evaluate which therapies are likely to be effective for treating pediatric cancer.” Although more agents in the development pipeline means more opportunity, it also means a greater need to select agents with the greatest likelihood of success for clinical trials, because the number of trials that can be completed is limited by the number of children potentially eligible. In vitro and in vivo models of the main forms of childhood cancer, predictive of responses in children, would be an enormous aid. That is the aim of the new NCI-sponsored program, which has been established for an initial 5-year period at St. Jude Children’s Research Hospital after a competitive proposal process. This organization will coordinate the program, but testing will take place in various laboratories that have developed or are using relevant models.
The contract was awarded in October 2004, so there are no results yet, but the program structure is spelled out in the Concept Proposal. Each year, 10 to 15 agents or combinations of agents will be tested against a panel of preclinical models representing about six relatively common types of childhood cancer. The plan calls for initial testing in 6 to 10 different xenografts in mice for each tumor type. If appropriate cell lines are available, in vitro tests could be carried out at the same time. Agents with known molecular targets would not necessarily be tested against all six cancer types, but cytotoxic agents would. Positive results would trigger testing in other mod-
els, such as transgenic and orthotopic mouse models, where they exist. Dose-response testing would be completed for all agents with continued positive results.
Clinical Trials
Clinical trials are the final phase of drug development. The machinery for clinical trials in childhood cancer is nearly optimal, given the constraints of small numbers of patients. Clinical trials are the norm in pediatric cancer. Translational research is strong, interest is high, and researchers and families are highly motivated.
What Is Missing?
This brief review of discovery, development, approval, and bringing to clinical care new cancer drugs for children identifies some strengths, some constructive new activities, some weaknesses, and some gaps. Scientific opportunity and technical capabilities are not lacking, but they have not been put into service for this purpose. What appears to be missing in order to realize the potential for new childhood cancer drugs is an organized focus on childhood cancers to coordinate the pieces and drive a process toward shortening the developmental time line and multiplying the numbers of possible new agents. Neither industry nor government can be expected to play this role alone. Not industry, because the market is too small and risky, even with orphan product incentives. Furthermore, although the federal government supports many of the components, and could potentially play a larger role, the government does not take the responsibility for the full stream of R&D and production of drugs for any condition (with some exceptions in military medicine). At the same time, both government and industry capacities are needed at different stages in the process, and cannot, in any practical sense, be duplicated. What is needed is a mechanism that would allow the relevant parties to contribute their expertise and research capacities at the appropriate points in the process. A model that has been pursued for other conditions (e.g., cystic fibrosis, malaria, tuberculosis) is an independent, not-for-profit consortium, foundation, or other entity that could enter into agreements with government and industry to develop a “virtual” R&D pipeline (see Box A). These efforts are all relatively young, so their success in bringing new products to market is just beginning.
The challenges involved in starting an enterprise to focus exclusively on drugs for pediatric cancer should not be underestimated. In addition to the obvious need for funding, a number of issues will need careful consideration and negotiation if government, industry, and academia are to collabo-
|
BOX A Many health conditions—from pediatric cancers to cystic fibrosis, to parasitic diseases of the tropics—suffer from a lack of research and development (R&D) for effective agents to treat them. Within the past decade, concern about some of these health conditions has led to the creation of a number of so-called “Public– Private Partnerships” that bring together the public, for-profit, and not-for-profit sectors. Most often, the core group catalyzing the organization is a not-for-profit interest group with a focus on the target health condition. In traditional health collaborations, the roles and way of working of the institutions involved follow the same patterns as when they act independently: They maintain autonomous decision making to fulfill their individual objectives. In the emerging models of collaboration between public and private sectors, participants depart from their traditional, independent modes of working and take on new types of roles. Additionally, they often provide resources “in-kind” such as special skills that the other institutions lack. The public–private partnerships that have been formed use a variety of organizational models, including foundations and not-for-profit corporations. Some (e.g., Global Alliance for TB Drug Development) have a large number of founding partners, and others (e.g., Cystic Fibrosis Foundation Therapeutics Inc.) are much more under the control of the founding organization. They are governed by various types of boards and advised by a range of scientific advisory groups. Certain functions are common to all, however, including the ability to enter into legal agreements with government and other private-sector for-profit and not-for-profit organizations, the ability to raise funds, and other functions. Two thriving public–private partnerships—Cystic Fibrosis Foundation Therapeutics Inc. and the Medicines for Malaria Venture—are described briefly below. The Cystic Fibrosis Foundation: Cystic Fibrosis Foundation Therapeutics Inc. The Cystic Fibrosis Foundation was established in 1955 and has been a strong advocate for people with cystic fibrosis (CF), providing various services, informing the public about the condition, and engaging in a variety of other activities. In the 1990s, the CF Foundation determined that opportunities for new drugs were not being pursued by industry because of the small market—about 30,000 people living with CF in the United States. In response, in 1997, the Foundation started the Therapeutics Development Program to provide funding to biopharmaceutical companies for CF drug discovery and development. Through its already-established clinical trials network, the Therapeutics Development Program provides the partner companies with the infrastructure for carrying out the necessary clinical trials. The CF Foundation’s nonprofit affiliate, which was established in 2000—Cystic Fibrosis Foundation Therapeutics Inc.—oversees these efforts. Its Board of Directors is made up of researchers, CF parents, and CF Foundation Trustees. Initially, the emphasis was on helping pharmaceutical and biotech companies fund Phase I and Phase II clinical trials. Despite incentives, such as the Orphan Drug Tax Credit, there is only minimal investment in diseases like CF that affect small |
|
numbers of individuals. It is expected that once promising Phase I and Phase II trials are completed, companies will be able to secure sufficient funds to conduct Phase III trials and take new products to market. Because of the need to increase the identification of promising new agents, the Therapeutics Development Program expanded to include a Therapeutics Discovery Component, which supports combinatorial chemistry and high-throughput screening, filling the gap between basic research and clinical trials. Once lead compounds are identified, companies and academic organizations are eligible for continued support through the Clinical Evaluation Component of the Therapeutics Development Program. Phase III clinical trials are conducted through the national network of CF Foundation care centers. A trial in 1993, of Pulmozyme®, is a good example: This large study enrolled patients for a period of 3 months, and was completed in less than a year. In contrast, the Phase I and Phase II trials can be somewhat challenging to initiate. Before the formation of the Therapeutics Development Network, investigators and pharmaceutical companies generally conducted such studies on an ad hoc basis. Companies would contact the centers individually, design the trials, and instruct the staff on how to perform specialized research using sophisticated techniques. The Therapeutics Development Network streamlines the process and ensures uniform data are collected. The Therapeutics Development Network will most often conduct these studies with major drug companies, but may also evaluate “nonsponsored” agents. For example, a steroid study, an ibuprofen study, and the first aerosolized tobramycin study were nonsponsored, so the CF Foundation provided support. Most CF Foundation funding comes directly from individual donors, with additional support from corporations and others. The Therapeutics Development Program represents a major new investment stream, however, which led the Foundation to establish a special fundraising program for it, in addition to drawing funds from existing sources of support. The Foundation’s largest commitment—a 5-year, up to $46.9-million investment with Aurora Biosciences for high-throughput screening—has yielded promising results. A number of other drug-specific projects are in various stages of development. The Medicines for Malaria Venture The Medicines for Malaria Venture (MMV) was established because the pharmaceutical industry has largely disengaged from antimalarial drug discovery and development, for economic reasons. The public sector recognizes the medical need for antimalarial drug R&D; it funds basic research and has well-developed clinical capabilities to support this. However, the drug R&D process needs more than funds and this type of scientific input. It requires considerable coordination and management, coupled with areas of specific scientific and technical expertise that are not generally found in the public sector. |
|
MMV began operations in 1999, when it became an independent foundation under Swiss law. The organization was conceived and developed by a strategic planning group with representation from the Swiss government, the World Health Organization, the International Federation of Pharmaceutical Manufacturers Associations, the World Bank, philanthropic organizations, and individual pharmaceutical companies. The budget, currently about $10 million per year, comes from some of these organizations, and other governments, corporations, and foundations. MMV is an agent of “virtual” drug discovery and “virtual” drug development, with all processes being outsourced, managed by a central unit. It is a model that is gaining in importance in the pharmaceutical industry. The management paradigm is not only to utilize cutting-edge science, but to engage cutting-edge managerial approaches to achieve its goals. An element attractive to potential donors and stakeholders is the competitive process by which projects are selected. By developing a portfolio of projects, which are assessed by competitive criteria and partnered by industry, MMV provides a greater chance of achieving success than investment in a single project or single institution or company. The business model followed by MMV allows it to be flexible in its approaches to forming agreements both at the research stage and the downstream commercialization stage for specific projects and products. Although still new, MMV already has developed a stronger pipeline for new malaria drugs than has ever existed before. |
rate effectively. These include issues of confidentiality, intellectual property, liability, rights to commercialization for nonpediatric cancer indications, organizational recognition, and others. These do not appear to be insurmountable problems, but they will have to be faced by any group taking up this cause.
DELAYS IN TESTING APPROVED AGENTS IN CHILDREN
Even if a pipeline for new pediatric cancer drugs is established, drugs developed for adult cancers will likely continue to be a source of new treatments for children. For a number of reasons, the process of moving drugs from testing in adults to beginning testing in children has been very slow. It is accepted that, in general, Phase I clinical trials in children do not begin as early as in adults. Dose-finding and toxicity information from adults is used to set initial doses in pediatric trials. Few children with late-stage cancer are appropriate for such trials, so sequencing adults first is the usual practice.
There are two other reasons for the long period before information
relevant to treating children becomes available. First, there is the problem of finding enough eligible children to enroll in the trial (although for most pediatric cancers, this may not be not a significant limitation; the number of patients seeking new therapies exceeds the number of clinical trials that make such investigational therapies potentially available). Second, and more amenable to a solution, is that the lag between beginning adult trials and pediatric trials is longer than it has to be: there is strong interest on the part of the pediatric cancer research community in initiating the pediatric trials earlier than they have been.
Irinotecan, a relatively new cytotoxic drug used primarily for colorectal cancer, is an example of a drug developed for adult malignancies that was tested against a variety of pediatric cancers in children who had not responded to other drugs, only after the drug was approved by the FDA for use in adults. The first Phase I trial results in adults were published in Japan in 1991, followed by Phase I trial results in the United States published in 1993. An accelerated approval was granted by the FDA in 1996, based on Phase II trial results in adults. It was only then, in 1996, that Phase I trials in children were initiated. The first were completed in 1998, when Phase II trials began for irinotecan, in combination with other agents for various solid and central nervous system tumors that had not responded to available treatments and untreated metastatic rhabdomyosarcoma. The drug was not deemed effective in these pediatric trials, which were completed in 2003, 10 years after publication of the first Phase I trial results in adults. Having carried out and reported the studies to the FDA, the sponsor fulfilled its agreement for pediatric information and will get an additional 6 months of exclusivity for irinotecan, under the pediatric incentive provision of the Best Pharmaceuticals for Children Act (discussed in more detail later). Because the drug was not effective in children in the doses and schedules studied, there will be no change in the product label. However, it still appears promising in certain pediatric cancers and continues to be studied (Personal communication, M.P. Link, Stanford University School of Medicine, January 25, 2005).
Starting Pediatric Trials Earlier in the Drug Development Process
It is important to understand why pediatric trials are not started earlier as a routine matter. Although other factors can be important, the key decisions on timing of trials are made by a drug’s developer, which is usually (especially by the time a drug is in clinical trials) a for-profit pharmaceutical or biotechnology company. This requires that the sponsor be willing to carry out the trials or allow others to do so. Even if the manufacturer (and patent holder) is not the trial sponsor (e.g., if NCI sponsors the trial), the fact that pediatric trials are being conducted could imply a com-
mitment to proceed with the drug development process for as long as results warrant. For a drug that has not yet proven effective for an adult indication (i.e., trials are in early stages), a hypothetical risk is that the drug will prove useful only for a pediatric indication, and not the adult indication for which it was actually being developed. A pediatric-only market would not be big enough to attract a large pharmaceutical company economically in investing in further development or maintaining a drug in production, in the absence of an astronomical per-course price. A second hypothetical concern for companies is the possibility that approval of a new drug in adults could be delayed if a serious, unexpected adverse event occurred in a pediatric trial.
For these reasons many companies are reluctant to begin pediatric testing until effectiveness in adults is more certain, often only occurring following completion of adult Phase III trials. In a conservative situation, the pediatric trial may not commence until the company has obtained FDA approval for the new agent in adults. It should be appreciated that even adult cancer markets are relatively small compared to the market for drugs for cardiovascular conditions, for example. Despite the prevalence of the above concerns, there has been no documented instance of either a cancer drug being found effective exclusively for children, or an unexpected severe toxicity arising in a pediatric trial affecting approval for adults (although it should also be recognized that the opportunity for either concern to manifest itself has been very limited). The line of reasoning by companies described here, however, was affirmed generally by industry participants in a workshop held for this project (January 9, 2003, IOM Pediatric Oncology workshop). Even in the absence of data supporting the existence of such risk, companies may not see it in their interest to test earlier in children.
Completing Pediatric Clinical Trials More Quickly
There are limited ways to shorten the time it takes to conduct clinical trials to determine whether a drug is of benefit to patients. In the future, there may be validated surrogate end points that could cut the time to assessing clinical benefit, but these do not yet exist for pediatric cancers. Another way to improve timeliness is for patients to be entered over a shorter time span. The potential for this is also limited because a large proportion of children with cancer—about 5,000 per year—are already routinely enrolled in clinical trials, and enrollment in Phase III trials typically takes several years. Faster recruitment could be accomplished if there were fewer trials going on for each type of childhood cancer, or for rare pediatric tumors, if greater international collaboration could be arranged. Both of these avenues suggest a centralized process for deciding which new
agents should be given priority for clinical trials, and for organizing the trials. The latter exists through the Children’s Oncology Group, but the former has received less attention.
The threshold for starting a drug through pediatric clinical trials is generally higher than it is for beginning trials in adult cancers for two main reasons (both of which result in few patients who could enroll in such trials). First is the relative rarity of cancer in children, and second is the relatively high success rate of first-line treatment for those with the most common forms of pediatric cancers. The price of this success—including the short- and long-term adverse effects—is to limit the number of patients eligible to enroll in a clinical trial of a new agent. If trials are to be completed in a timely manner, there can be only a limited number open for any type of cancer. Whether agents are prioritized deliberately among the pool of possibles, or less systematically (by decisions not to go ahead with a new trial because others are already ongoing, regardless of the promise of the agent), the development of new agents in the future ultimately will be “rationed” by the supply of patients.
THE NEED TO PRIORITIZE AGENTS FOR TESTING IN CHILDREN WITH CANCER
The case is made that, in absolute numbers, childhood cancer is rare (11,900 per year under age 20 in the United States). Even rarer are children for whom current treatments are ultimately unsuccessful—2,200 died each year at the beginning of the 21st century (U.S. Cancer Statistics Working Group, 2004). Compare this with the situation for adults: More than 200,000 women are diagnosed with breast cancer each year and a similar number of men are diagnosed with prostate cancer. Forty thousand women and 30,000 men die from these cancers each year. Similarly large numbers of people develop lung and colorectal cancer (American Cancer Society, 2004). (There are, of course, adult cancers that are as rare as childhood cancers, and the problems of developing and testing new agents for them may be equally daunting.)
A rational approach to prioritizing drugs for testing in children with cancer ideally should depend on a consensus of all constituents in the pediatric oncology community (academic researchers, the FDA, NCI, companies, and advocates) that the best scientific data should guide which new agents go into the pediatric clinic. Of course, profit potential and marketing strategies drive many industry decisions about which new drugs are developed for adults, whether it is between cancer and other indications or within cancer. However, other variables unrelated to consensus about the science also play an influential role, including:
-
The FDA’s decision to issue a written request for pediatric studies under the provisions of the Best Pharmaceuticals for Children Act (BPCA);
-
A company’s willingness to respond to the FDA’s written request for pediatric studies;
-
Recommendations for trials by the pediatric oncologists in the Children’s Oncology Group and pediatric oncology therapeutic consortia; and
-
Whether companies choose to continue to develop a drug for adults beyond Phase I.
The current difficulties in determining which agents are most promising stem, in large part, from the absence of industry-conducted laboratory research on drugs in their development pipeline that focus on pediatric tumor targets. This may, in part, be remedied in the coming years as the pediatric preclinical testing program moves ahead.
TESTING AND LABELING OF DRUGS FOR PEDIATRIC USE
Relatively few pharmaceutical labels contain information about their use in children because most products have not been tested in children, or the information is only in the published medical literature. Although many drugs are used safely and effectively even in the absence of this information in the product label, serious adverse events that might have been prevented with direct testing in children have occurred at times. The well known examples do not involve cancer drugs, but the lack of labeling applies across all drug categories. Deaths caused by the antibiotic chloramphenicol (Powell and Nahata, 1982); pediatric kernicterus (a yellow staining in the brain that causes a form of cerebral palsy) from sulfa drugs; seizures and cardiac arrest caused by bupivacaine, a commonly used anesthetic; and hazardous interactions between erythromycin and midazolam, a sedative, all might have been avoided if the drugs had been tested in children before being prescribed widely.2
Drugs are nearly always approved by the FDA to treat disease in adults, with dosages and other labeling elements based on the studies conducted to gain approval. Once marketed, the drugs are prescribed for children in adjusted dosages at the discretion of the prescribing physician, based on experience, consultation with colleagues, published papers, and other information. Until recently, companies have had no legal requirement, and little
incentive, to test drugs in children, with its attendant liabilities and operational complexities. But the medical community and the government perceived a need for better guidance on the use of products by children. Over the past decade, the FDA and Congress have taken two broad approaches to increasing the available information, referred to as (1) “the pediatric rule,” which has evolved into a federal law requiring that particular types of studies be performed in the course of the new drug approval process for drugs likely to be widely used in children, and (2) “the pediatric incentive,” which offers 6 months of added sales exclusivity for products already on the market that are still under patent or otherwise have market exclusivity (e.g., based on orphan drug status). The history of each approach is described in the following section.
These initiatives have thus far had only a limited effect on drugs for childhood cancer, but are potentially important precedents that may play a more important role in the future, as certain issues are resolved. Where labeling is concerned, the issue that sets cancer drugs apart from drugs for other conditions is that the cancers for which they are used in children are different biologically and clinically from cancers affecting adults, as explained earlier in this report. Clinical experience in adults is of limited relevance to use in children, on whom the drugs will be used for different types of cancer, in regimens with different drugs than used in adults. But the decision to test a drug for cancer in children becomes part of the larger issue of prioritizing clinical trials in pediatric cancers, which is critical because of the small numbers of children eligible to participate. Testing one agent often preempts testing another one because of the small numbers of patients available for clinical trials.
The Pediatric Rule
The Short-Lived 1994 Pediatric Rule
In 1994, the FDA assembled a list of the 10 most widely prescribed drugs in outpatient pediatrics (albuterol, Phenergan, ampicillin injections, Auralgan, Lotrisone, Prozac, Intal-aerosol, Zoloft, Ritalin, and Alupent). The approved labeling information for all 10 drugs—none of which is used to treat cancer—was inadequate or nonexistent for use in children. To remedy the situation, the FDA developed a “Final Pediatric Rule,” which it issued as a regulation in 1994.3 The rule “allowed efficacy data from adult
studies to be extrapolated to a pediatric population, if the disease under study existed in both the pediatric and adult populations and the response to therapy was substantially the same”—a condition that does not hold for cancer. Drug manufacturers were required to examine the data they already had and determine whether the information was sufficient to support pediatric labeling information. Full-scale clinical trials in children were not required: Clinical data derived from trials in adults could be coupled with pediatric-specific pharmacokinetic and adverse reaction data. If existing data were insufficient, manufacturers were not required to conduct new studies. They only had to include this wording on the label: “Safety and effectiveness in pediatric patients have not been established.” Furthermore, the rule only applied to drugs that were already on the market. The 1994 Rule had little effect on drugs for childhood cancer. Many companies promised to conduct studies in the future, but it was clear that a voluntary approach was not substantially increasing the number of products with pediatric labeling information. Under the next version of the rule, testing was mandatory.
The 1998 Pediatric Final Rule and the Pediatric Research Equity Act of 2003
The 1998 Pediatric Final Rule,4 which took effect in April 1999, “man-dates pediatric studies if an application for a claim is under review and the proposed indication is for a disease that exists in both adults and children.” This gave the FDA discretion to mandate specific pediatric studies and stated further that the “applicant also may be required to develop a pediatric formulation for a drug product that represents a meaningful therapeutic benefit to such patients over existing therapies.” Companies could request waivers if the mandated studies were highly impractical or if there was evidence that the product would be ineffective or unsafe for children. The 1998 Rule also had provisions for testing drugs already on the market, with the FDA bearing responsibility to demonstrate a need for testing.
The 1998 Rule, similar to the earlier version, applies only to diseases that are the same in adults and children. This has limited its application to cancer drugs because the prevalent cancers of children and adults are different (Pervan et al., 2001). No labeling changes have yet been reported for cancer drugs as a result of the rule.
In 1999, the Association of American Physicians and Surgeons, the
Competitive Enterprise Institute, and Consumer Alert filed a citizen petition with the FDA challenging the 1998 Rule. In 2000, the FDA denied the petition, and the same group brought suit against the FDA in U.S. District Court, asserting that the “Pediatric Rule exceeds the FDA’s statutory authority and that the Rule’s promulgation was arbitrary and capricious” (U.S. District Court for the District of Columbia, 2002). In October 2002, the District Court of the District of Columbia agreed, striking down the Pediatric Rule (U.S. District Court for the District of Columbia, 2002). The American Academy of Pediatrics and the Elizabeth Glaser Pediatric AIDS Foundation appealed (Pediatric AIDS Foundation, 2002), but the appeal was preempted by passage of the Pediatric Research Equity Act of 2003 (Pub. L. No. 108–155) in December 2003, enacting the provisions of the Pediatric Rule into law for 5 years.
Pediatric Subcommittee of the FDA Oncologic Drugs Advisory Committee
Since 2000, the FDA has held public meetings of a new Pediatric Subcommittee of the Oncologic Drugs Advisory Committee to discuss broad principles that would underlie the agency’s application of the Pediatric Rule to oncology drugs, including the question of equivalence of adult and pediatric diseases. Congress subsequently established this Subcommittee in law in the Best Pharmaceuticals for Children Act in January 2002, broadening membership to include representatives of NCI, patient advocates, and the pharmaceutical industry. The Subcommittee’s mission stated in the law was to ensure that prioritization of agents for children with cancer, for the purposes of clinical trials, was consensus driven. The Subcommittee is advisory, with the FDA controlling its agenda, outcomes, and implementation of recommendations.
The Pediatric Incentive
While the FDA was forming and revising the 1994 and 1998 Pediatric Rules, Congress was working on a complementary strategy of offering companies an economic incentive to conduct studies in children in response to formal requests from the FDA. The incentive is a 6-month extension of marketing exclusivity for the company’s products containing the active ingredient studied (the “active moiety”), and applies both to approved and investigational drugs, but not to biologics. The pediatric exclusivity provision was written into Section 111 of the Food and Drug Administration Modernization Act (FDAMA) of 1997. Depending on the drug, studies requested might focus on one or more of the following: safety, efficacy, pharmacokinetics, pharmacodynamics, or other topics.
FDAMA set out tasks to establish the exclusivity provision. Among the first was publication by the Secretary of Health and Human Services of a list of then-approved drugs for which additional pediatric information was likely to produce health benefits for children. The list, which is updated annually, consists of all drugs approved for use in adults that could have indications for use in children. A company may choose to carry out studies, mutually agreed upon with the FDA, on any of its drugs on the list. The pediatric incentive has been extended through 2007 by incorporation into the BPCA.
The Pediatric Incentive has had a modest effect on pediatric cancer drugs. Over the life span of the pediatric incentive, in its various forms, the FDA reports issuing 31 written requests for studies of oncology products (U.S. Food and Drug Administration, 2005). Sixteen are readily identifiable as cancer related in the enumerated list of products (Table 5). Ten of these requests resulted in extensions of exclusivity, and in six of these cases, labels were changed, most adding information about dosing and side effects.
The incentive has not had an effect of moving forward the initiation of pediatric clinical trials. A company that conducts requested studies any time during the patent life of a drug is eligible for the 6 months of exclusivity, so there is no incentive for them to do so early in the life of the drug.
TABLE 5 Approved Oncology Drugs for Which the FDA Has Issued Written Requests for Studies Under the Best Pharmaceuticals for Children Acta
|
Drug Name |
Pediatric Exclusivity Granted by FDAb |
Pediatric Label Changesc |
Summary of Medical and Clinical Pharmacology Reviews Postedd |
|
Busulfan |
x |
x |
|
|
Carboplatin |
x |
|
x |
|
Cytarabine |
|
|
|
|
Epirubicin |
|
|
|
|
Fentanyl |
x |
x |
x |
|
Fludarabine |
x |
x |
x |
|
Gemcitabine |
x |
|
|
|
Gemtuzumab |
|
|
|
|
Hydroxyurea |
|
|
|
|
Imatinib |
|
|
|
|
Irinotecan |
x |
x |
x |
|
Oxaliplatin |
|
|
|
|
Tamoxifene |
x |
x |
|
|
Temozolomide |
x |
x |
x |
|
Topotecan |
x |
|
x |
|
Vinorelbine |
x |
|
|
|
ahttp://www.fda.gov/cder/pediatric/wrlist.htm. Last updated February 7, 2005. The text introducing this table states that these are requests made as of June 1998, so the coverage is not entirely clear. bhttp://www.fda.gov/cder/pediatric/exgrant.htm. Last updated February 14, 2005. chttp://www.fda.gov/cder/pediatric/labelchange.htm. Last updated January 7, 2005. dhttp://www.fda.gov/cder/pediatric/Summaryreview.htm. Summaries are posted for reports received in response to BPCA requests, but presumably not those submitted earlier, under the pre-BPCA incentive rules. Last updated January 28, 2005. eTamoxifen has no pediatric cancer indication. It was studied in female patients aged 2 to 10 years with McCune-Albright Syndrome and precocious puberty. |
|||
RECOMMENDATIONS
-
A new public–private partnership, involving government, industry, academic and other research institutions, advocacy groups, philanthropies, and others, should be formed to lead pediatric cancer drug discovery and development.
Advocacy groups, government, academic institutions, and the pharmaceutical and biotechnology industries should collaborate to establish a not-for-profit “public–private partnership” to fund and direct drug development for agents uniquely targeting pediatric cancers. Arrangements could be made, for example, for industry to relinquish intellectual property rights (or otherwise reduce barriers) associated with pediatric use of candidate compounds identified by screening their libraries against pediatric targets (while retaining rights for any other uses); for academic researchers and their institutions to act similarly regarding potential drug targets (also a contribution of intellectual property); for NCI to make available its drug development and clinical trial resources on a preferential basis; and for advocacy groups to contribute input from the patient community, fundraising, and leadership. A first step would be for each partner (industry, government, academia, research institutions, advocacy) to identify and support an individual to work for a period of time on a feasibility study, business plan (in consultation with professional business development experts), and plans for pilot projects, which would be seen as a means to prove the viability of the concept. The components of R&D for pediatric cancer drugs are, in many ways, better developed than for most other rare conditions, and money is already flowing to many of them. This entity could take advantage of those resources in a more focused way than currently occurs for pediatric cancer drugs.
-
The National Cancer Institute should assume responsibility as the developer of last resort for agents that show promise only in children if companies decide not to proceed with full-scale development.
Because of the small market potential, companies may decide against completing the development of agents that show promise largely for treating childhood cancers. If the public–private partnership described in the first recommendation is formed, NCI could carry out this work in conjunction with that organization. In addition, NCI resources should be made available to assist in development and production of pediatric dosage forms. NCI already has the programs in place to carry out these tasks, but they have not been focused specifically on pediatric drugs.
-
The pharmaceutical industry, National Cancer Institute, and Food and Drug Administration should act to reduce the delay in beginning pediatric clinical studies of agents in development for adult cancers.
NCI, pediatric oncology researchers, and other interested parties should continue to explore ways to test compounds in development for adult cancers in preclinical and early clinical trials as soon, and as quickly, as possible. Specifically:
-
The evolving NCI preclinical testing program should be fully supported and eventually expanded because it will play a key role in providing the evidence needed to begin early-stage clinical trials of new agents in children.
-
NCI should encourage and facilitate international consortia of pediatric cancer centers and clinical trials groups to provide a larger base of available patients for testing new anticancer agents.
-
The FDA should (with assistance from NCI and others) assemble a packet of information on resources for preclinical pediatric drug testing, which would be provided to every company submitting an IND for a new molecular entity for cancer.