3
Nuclear-Explosive Materials
Chapter 2 examined the possibilities for applying monitoring and transparency measures to all categories of nuclear weapons and to their nuclear-explosive components. This chapter considers the further challenges of transparency and monitoring for military and civilian stocks of nuclear-explosive materials (NEM). These materials are readily convertible by nuclear weapon states—or other states or groups that have knowledge of nuclear weapons technology—into the nuclear-explosive components of actual weapons. And the size of the NEM stocks determines, to a reasonable approximation, how many weapons of particular types could be made. Moreover, the difficulty of producing such materials means that their acquisition is and will remain a limiting factor for states or sub-national groups aspiring to make such weapons.
Meaningful constraints on stocks of NEM require knowing how much NEM is possessed by whom and being able to monitor additions and subtractions. Achieving such constraints and the ability to monitor them is important not only for building confidence among nuclear weapon states about the current and potential future sizes of the arsenals of the other nuclear weapon states, but also for building international confidence in the durability of reductions in those arsenals and for limiting and monitoring the risks of proliferation of nuclear weapons to additional actors.
The importance of NEM stocks resides not just in their role in determining the breakout potential from agreed or unilaterally undertaken limits on the nuclear arsenals of the existing global and regional nuclear weapon states, but also in their role as a reservoir of proliferation potential to both other state and nonstate actors. Stocks of NEM held by non-nuclear weapon states confer the potential for these states to acquire nuclear weapons of the simplest types quite quickly once a decision to do so has been made. Moreover, all such NEM stocks represent nuclear weapon production potential for any state or nonstate actor that is able to steal these materials or to buy or otherwise acquire them from their legitimate or illegitimate possessors.
This chapter begins with an introduction to the characteristics of NEM, the means by which these materials are produced, and current stocks and flows of NEM in the military and civilian sectors. (This treatment is supplemented with more detail in Appendix A) The chapter then addresses the challenges of transparency and monitoring for NEM, first in conceptual terms and then in terms of the specific bilateral and multilateral measures that have been undertaken up until now in connection with cooperative efforts to account for, secure, and protect both military and civilian materials1
DEFINITION, CHARACTERISTICS, AND PRODUCTION OF NEM
All nuclear weapons rely on the energy released by an explosively growing fission chain reaction—a process in which heavy nuclei split into lighter ones following absorption of free neutrons and, in splitting, release more neutrons that in turn induce more fissions, and so on. Only a few nuclides2 of the hundreds that exist are capable of sustaining the explosive nuclear chain reaction needed for a nuclear weapon. Such nuclear-explosive nuclides include U-235, U-233, and all the isotopes of plutonium, among others. A nuclear-explosive material is one in which the proportions of nuclear-explosive nuclides and nonexplosive nuclides of the same elements are such as to permit an explosive chain reaction if the material is present in suitable quantity, density, chemical form and purity, and configuration.
In the simplest nuclear weapons, the fission chain reaction is the only source of the nuclear energy that is released. In more advanced nuclear weapons, such as “boosted” fission weapons and thermonuclear weapons, some of the energy is generated by fusion reactions that are ignited by energy from the fission explosion.
|
1 |
The arguments in this chapter build on those in National Academy of Sciences, Committee on International Security and Arms Control, Management and Disposition of Excess Weapon Plutonium, 2 vols. (Washington, DC: National Academy Press, 1994 and 1995); Steve Fetter, Verifying Nuclear Disarmament, Occasional Paper 29, Henry L. Stimson Center, Washington, DC, 1996; and Independent Bilateral Scientific Commission on Plutonium Disposition, Final Report, Washington, DC: President's Committee of Advisors on Science and Technology, The White House, and Russian Academy of Sciences, June 1997. |
|
2 |
“Nuclide” is the general term for a species of atom as characterized by both its atomic number (equal to the number of protons in the nucleus, which determines the element to which a nuclide belongs) and its mass number (equal to the number of protons and neutrons combined, which determines which isotope of the element it is). See Appendix A. |
(Fusion reactions merge light nuclides, most notably isotopes of hydrogen, to form heavier ones, accompanied by a large release of energy.) In boosted fission weapons, the energy directly added by the fusion reactions is very modest, but the high-energy neutrons emitted by these reactions lead to a large increase in the amount of fission that takes place; in thermonuclear weapons a significant fraction of the energy released comes from fusion reactions.
Countries aspiring to make boosted and thermonuclear weapons, however, cannot do so without first mastering simpler pure-fission weapons. Terrorists working without the support of a state would not be able to make the much more demanding boosted and thermonuclear weapons at all. Thus it is mastery of the explosive fission chain reaction—including possession of the quantities of NEM needed to achieve one—that governs who can make nuclear weapons.
Types of NEM
The most widely used definitions of the isotopic mixtures and concentrations constituting NEM are as follows: 3
-
Any mixture of uranium-235 (U-235) with the more abundant, non-nuclear-explosive isotope U-238 in which the U-235 concentration is 20 percent or more is considered NEM. This form of NEM is referred to as highly enriched uranium (HEU).4
-
Any mixture of U-233 with U-238 when the U-233 concentration is 12 percent or more is considered NEM.5
|
3 |
See IAEA, IAEA Safeguards Glossary, 2001 Edition (Vienna: International Atomic Energy Agency, 2002). Available as of January 2005, at: http://www-pub.iaea.org/MTCD/publications/PDF/nvs-3-cd/PDF/NVS3_prn.pdf and Nuclear Energy Research Advisory Committee (NERAC), Attributes of Proliferation Resistance for Civilian Nuclear Power Systems (Washington, DC: U.S. Department of Energy, October 2000). |
|
4 |
Nuclear explosives can in principle be made with material containing somewhat less than 20 percent U-235, but the amount of material required at enrichments below 20 percent is very large. |
|
5 |
At this percentage, the mass of material required for criticality is similar to that for a mixture of U-238 and U-235 containing 20 percent U-235. See, for example, C. W. Forsberg, C. M. Hopper, J. L. Richter and H. C. Vantine, Definition of Weapons-Usable Uranium-233, ORNL/TM-13517 (Oak Ridge, TN: Oak Ridge National Laboratory, March 1998). |
-
Any mixture of plutonium isotopes in which the concentration of plutonium-238 (Pu-238) is less than 80 percent is considered NEM.6
These materials are considered NEM irrespective of whether the uranium or plutonium are present in metallic form or as oxides, or nitrates, or fluorides, or some other compound. This is because, even if a particular uranium or plutonium compound will not itself support a nuclear explosion (and some will), transforming such compounds chemically into the metal is a straightforward operation that would be within the reach of any group with a modicum of competence in chemistry.
Mixtures of NEM with other elements, in compounds or otherwise, can differ greatly in the difficulty of separating out the NEM in a purity that would permit an explosion, however. In particular, the intense radiation field emitted by typical spent nuclear fuel from civil power reactors presents great technical difficulties (and hazards) in the separation of the contained NEM (a mix of plutonium isotopes amounting altogether to 1-2 percent of the mass of the spent fuel) from the accompanying fission products and low enriched uranium. Accordingly, the NEM in spent fuel is considered to be a smaller proliferation hazard than NEM in most other forms, and in international practice is subject to less stringent monitoring and security measures.
Fortunately, NEM does not exist in nature in any significant quantity, and all types of NEM are quite difficult to produce, creating an important constraint on access to nuclear weapons capabilities.
-
U-235, for example, constitutes only about 0.7 percent of naturally occurring uranium; achieving the higher U-235 concentration needed for a nuclear weapon (or for most types of nuclear reactors) requires “uranium-enrichment” technology that is difficult to master and costly, as discussed further below.
-
The isotopes of plutonium (most importantly Pu-239, but also Pu-238, Pu-240, Pu-241, and Pu-242) are practically nonexistent in nature; they can be obtained in
-
quantity only by bombarding naturally occurring “fertile” materials with neutrons in an accelerator or a reactor, then separating the plutonium from accompanying elements (also discussed further below).
-
U-233 is likewise essentially nonexistent in nature and producible in quantity only in a reactor or accelerator; relatively little U-233 appears to have been produced for weapon purposes to date, nor has this isotope been produced in significant quantities in civilian nuclear energy operations (although its use as the fissile component in a “thorium fuel cycle” has been much analyzed and discussed).
More obscure nuclides that could sustain an explosive nuclear chain reaction include neptunium-237 and several isotopes of americium, curium, and californium. These have been less important than plutonium, U-235, and U-233 because they have existed until now in much smaller amounts and because producing them in quantity is even more difficult.7
The fuels that generate energy from fusion in boosted and thermonuclear weapons—notably tritium, deuterium, and lithium—might also be argued to be nuclear explosives. But no means is yet known for releasing explosive nuclear energy from these fusion fuels alone, so their possession without the material required for an explosive fission chain reaction does not enable the manufacture of nuclear weapons. It is possible that the importance of tritium in advanced weapon design might nonetheless make it a focus for limits and monitoring similar to those for NEM in a more comprehensive nuclear arms limitation and transparency regime, but we do not treat the problem of accomplishing this in this report.8
|
7 |
A case can be made, however, that attention does need to be given to monitoring and protecting the growing stocks of at least some of these nuclides, most notably Np-237 and Am-241. See David Albright and Lauren Barbour, “Troubles Tomorrow? Separated Neptunium 237 and Americium,” in David Albright and Kevin O'Neill, eds., The Challenges of Fissile Material Control (Washington, DC: Institute for Science and International Security, 1999). |
|
8 |
But see Martin B. Kalinowski and Lars C. Colschen, “International Control of Tritium to Prevent Horizontal Proliferation and to Foster Nuclear Disarmament,” Science and Global Security 5 (1995), pp. 131-230. Available as of January 2005, at: http://www.princeton.edu/%7Eglobsec/publications/pdf/5_2kalinowski.pdf, which treats the benefits, challenges, and possibilities of international controls and verification for tritium in considerable detail. |
Key Characteristics of NEM
HEU can be used to make a nuclear weapon using either the relatively simple “gun type” design concept or the more complicated “implosion” design concept: plutonium isotopes, irrespective of the mixture, will work only in weapons of the implosion type.9 In either case, however, nuclear weapon design is easiest—and the mass of NEM involved is smallest—when the nuclear material is not just barely NEM but is “weapon grade.” This is generally taken to be greater than 90 percent U-235 in HEU and greater than 90 percent Pu-239 in plutonium.
Because the bare critical mass of weapon-grade HEU is about 60 kilograms, a hypothetical gun-type weapon could be made with this amount of material, while an implosion weapon could be made from considerably less of the same material. The International Atomic Energy Agency (IAEA) defines a “Significant Quantity” (SQ) relevant to construction of a nuclear weapon to be 25 kilograms of U-235 in HEU; the SQ value for plutonium is set at 8 kilograms, as is the SQ for U-233 (which like U-235 will work in either gun-type or implosion designs).10
Considerably less knowledge and manufacturing skill are needed to make a gun-type weapon than to make an implosion weapon, and a gun-type design is more likely to work without nuclear testing than an implosion weapon. In addition, because of the relative ease of handling HEU compared with plutonium, HEU is even a greater threat than plutonium as the potential object of theft for use by terrorists or proliferant nations with limited access to nuclear weapon expertise.
Pathways to Obtain NEM
The principal pathways exploited to date for the production of NEM have been (a) mining of uranium ore, followed by enrichment of the concentration of U-235 to nuclear-explosive levels,
|
9 |
These and many other aspects of the science and technology of NEM are elaborated in Appendix A. |
|
10 |
The IAEA definition of SQ reads: “the approximate amount of nuclear material for which the possibility of manufacturing a nuclear explosive device cannot be excluded. Significant quantities take into account unavoidable losses due to conversion and manufacturing processes and should not be confused with critical masses.” See International Atomic Energy Agency, IAEA Safeguards Glossary: 2001 Edition (Vienna: International Atomic Energy Agency, 2002), p. 23, as well as Appendix A. |
and (b) creation of plutonium by absorption of neutrons in U-238 in a reactor, followed by chemical separation of the plutonium from the accompanying fission products and uranium. The two approaches are described briefly here; additional detail is provided in Appendix A.
Uranium-235
Natural uranium, as mined, contains 0.72 percent of the nuclear-explosive nuclide U-235 and 99.27 percent U-238, which is not a nuclear explosive. (About 0.006 percent is U-234, which is also not a nuclear explosive.) Enrichment of the U-235 concentration to nuclear-explosive levels, that is, to 20 percent U-235 or more, is a sufficient technological challenge to have constituted one of the principal technical barriers to the spread of nuclear weapons capability over the past 60 years.
The currently practical processes for enriching the concentration of U-235 exploit the 1.3 percent difference in mass between U-235 and U-238 atoms. The uranium is first converted to uranium hexafluoride gas (UF6), which can then be processed to achieve a degree of separation of the slightly lighter uranium hexafluoride gas molecules containing U-235 from the slightly heavier uranium hexafluoride molecules containing U-238. The two most widely used means of doing this have been (a) gaseous diffusion plants, which exploit the difference in the diffusion rates of the lighter and heavier molecules through a “cascade” of thousands of porous barriers, and (b) centrifuge plants, which use stages of hundreds or thousands of sophisticated, ultra-high-speed, gas centrifuge machines to separate the molecules based on their differing inertial masses.
The gaseous diffusion and centrifuge plants currently in use around the world in connection with civilian nuclear power generation are operated to enrich uranium only to a U-235 concentration of 3 to 5 percent, which cannot produce a nuclear explosion. In terms of the “enrichment work” needed to separate isotopes, these concentrations are more than half way toward the 90+ percent enrichment levels desirable for nuclear weapons. In principle, commercial enrichment plants could be operated in a manner to do the remaining work needed to bring this low enriched reactor fuel up to weapon-usable levels.
Separated Plutonium
Plutonium-239 is produced when U-238 absorbs neutrons produced in a reactor or by an accelerator. Consequently, Pu-239 is
produced automatically in any nuclear reactor containing U-238 in its fuel. The Pu-239 itself then absorbs neutrons to produce higher isotopes of plutonium in quantities depending on the irradiation time. (See Table 3-1 for the isotopic composition of various grades of plutonium.)
TABLE 3-1 Compositions of Various Grades of Plutonium
|
Grade |
Pu-238 |
Pu-239 |
Pu-240 |
Pu-241 |
Pu-242 |
|
Super-grade |
--- |
0.98 |
0.02 |
--- |
--- |
|
Weapon-grade |
0.00012 |
0.938 |
0.058 |
0.0035 |
0.00022 |
|
Reactor-grade |
0.013 |
0.603 |
0.243 |
0.091 |
0.050 |
|
MOX-grade |
0.019 |
0.404 |
0.321 |
0.178 |
0.078 |
|
FBR blanket |
--- |
0.96 |
0.04 |
--- |
--- |
|
Pu-241 includes its Am-241 daughter. Reactor grade Pu is from 33 MWd/kg HM LEU fuel stored 10 years before reprocessing. MOX grade is from 33 MWd/kg HM 3.64 percent fissile Pu MOX stored 10 years before reprocessing. Adapted from: J. Carson Mark, “Explosive Properties of Reactor Grade Plutonium,” Science and Global Security 4 (1993), pp. 111-128. See Appendix A for elaboration of the relevant definitions and parameters. |
|||||
The plutonium produced in this way is, by the nature of the process, intimately mixed with fission products, as well as with uranium-238 that has not absorbed neutrons. In this form the plutonium cannot be used to make a nuclear weapon but must first be separated from the fission products and the U-238. This can be accomplished by chemical means, since Pu-239 and other isotopes of plutonium form distinct chemical compounds. The term “separated plutonium,” is used when the concentrations of accompanying fission products and uranium are reduced to levels such that the material, if present in sufficient quantity, would support a nuclear explosion.
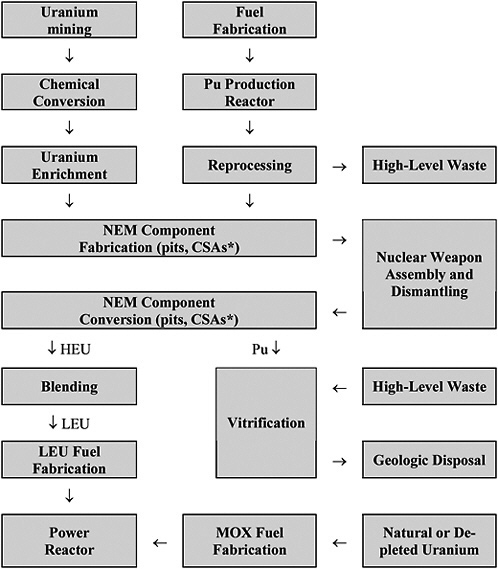
Figure 3-1 shows in schematic form the production, utilization, and disposition pathways for HEU and plutonium in the nuclear weapon and nuclear energy complexes.
STOCKS AND FLOWS OF NEM IN THE MILITARY AND CIVIL SECTORS
The quantity, character, and geographic distribution of stocks and flows of military and civil NEM worldwide are important dimensions of the challenge of achieving transparency and monitoring for these materials.11 More detail on these stocks and flows is provided in Appendix A.
World Military and Civilian NEM Stockpiles
The United States and Russia hold the largest stockpiles of NEM, but only limited information about them is available publicly. The United States keeps a computerized national plutonium and HEU inventory, including both Department of Energy (DOE) and nongovernment stockpiles, known as the Nuclear Materials Management and Safeguards System (NMMSS).12 What has been released publicly from this database up until now includes principally detailed data on U.S. warhead dismantlement rates; a detailed production history for U.S. plutonium, plus data on the stockpiles of this material; and official information on total U.S. production of HEU (but not the detailed production history or information on the current stockpile). Official information on the size, locations, and characteristics of Russia's stockpiles of warheads and NEM remains classified at this writing.
Estimates of global stocks of plutonium and HEU as of the end of 2003, compiled from publicly available information by the Institute for Science and International Security, are shown in Table 3-2. The totals are approximately 1,900 metric tons each of plutonium and HEU,13 amounting to more than 200,000 SQ of the former and about 75,000 SQ of the latter.
|
11 |
The most extensive unclassified compendium of such information is David Albright, Frans Berkhout, and William Walker, Plutonium and Highly Enriched Uranium 1996: World Inventories, Capabilities, and Policies (New York: Stockholm International Peace Research Institute and Oxford University Press, 1997). Albright and colleagues periodically post updates to this work at the Web site of the Institute for Science and International Security available as of January 2005, at: http://www.isis-online.org. |
|
12 |
See the NMMSS Web site, available as of January 2005, at: http://www.nmmss.com/. |
|
13 |
A metric ton is 1000 kilograms or 2204.6 pounds. The HEU estimates are expressed as “weapon-grade uranium equivalent,” in which inventories at a range of enrichment values above 20 percent have been converted, based on U-235 content, to equivalent tons of uranium enriched to 93 percent U-235. |
The HEU of military origin is mainly in intact weapons; in weapon components, ingots, oxides, and scrap; in naval fuel; and in fuel for military plutonium production and tritium production reactors. In the United States, as of the end of 2003, about 125 tons of HEU of military origin that had been declared excess to military needs was under civil control, prior to being blended down to low enriched uranium (LEU) for use in power reactors. In Russia, about 300 tons of HEU of military origin that had similarly been declared excess to military needs was likely still in military custody. The few tens of tons of HEU of civil origin, which are mainly in research reactors and the fresh or spent fuel for these, are not much compared with the major power military stockpiles, but at circa 2,000 SQ they represent a serious risk in terms of possible use in weapons by proliferant states or terrorist groups.
TABLE 3-2 ISIS Estimates of Global Inventories of Plutonium and HEU (Metric tons, end of 2003, rounded)
|
Material |
Military Origin |
Civil Origin |
Total |
|
HEU |
1840 |
60 |
1900 |
|
Pu |
260 |
1595 |
1855 |
|
of which irradiated |
-- |
1365 |
1365 |
|
of which unirradiated |
260 |
230 |
490 |
|
As indicated in Table 3-2, about 500 tons of the world's plutonium is in unirradiated form (often referred to as “separated” form, meaning that it has been separated from the intensely radioactive fission products that accompany it in irradiated nuclear fuel). This unirradiated or separated material requires at most straightforward chemical processing (for example, to convert it from plutonium nitrate or plutonium oxide to plutonium metal) before it can be used in a weapon. The 230 tons of this material of civil origin amounts by itself to something like 80,000 Significant Quantities. The further 1,400 tons of irradiated plutonium—mostly in the cores or spent fuel from power reactors—is considered to be a smaller proliferation hazard because of the need for technically demanding reprocessing to extract the plutonium in weapon-usable form. The actual difficulty and danger of that reprocessing operation vary considerably, however, with the degree of irradiation experienced by the fuel and the time that has passed since irradiation. Adapted from: David Albright and Kimberly Kramer, “Fissile Material Stockpiles Still Growing,” Bulletin of the Atomic Scientists, (November/December 2004), pp 14-16. See also the underlying analysis on the Web Site of the Institute for Science and International Security, available as of January 2005, at: http://www.isis-online.org. |
|||
Of the world's military stockpiles of HEU and plutonium, the United States and Russia possess more than 95 percent. The remainder is possessed by the United Kingdom, France, China, India, Pakistan, Israel, and North Korea. Civilian plutonium in power reactor fuel exists in all of the dozens of countries where power reactors exist. Separated civilian plutonium exists in significant
quantities in several of the nuclear weapon states as well as in Germany, Japan, Belgium, and Switzerland. At least kilogram quantities of civilian HEU for research reactors exist at approximately 135 operating HEU-fueled research reactors in more than 40 countries, ranging from the United States to Ghana.14 Most of these research reactors have only small amounts of HEU—but some, including a significant number outside the nuclear weapon states, have enough fresh HEU for a bomb. Even more have enough HEU for a bomb if irradiate fuel that is not radioactive enough to deter suicidal terrorists from taking it and using it in a bomb is taken into account.15
Flows of NEM
All of the five de jure nuclear weapon states have indicated they are not reprocessing plutonium or producing HEU for weapons. India, Pakistan, Israel, and North Korea continue production that is small on the scale of global stockpiles, but significant in the context of their modest existing stocks.
Overall, the global stockpile of HEU is declining by more than 30 tons each year, as only modest production continues; 30 tons are blended to LEU in the U.S.-Russian HEU purchase agreement every year; some U.S. excess HEU is blended each year; and additional amounts of HEU are consumed as fuel in research reactors, nuclear-powered naval vessels, nuclear-powered icebreakers, and the like.16 Numerous shipments of large quantities of HEU over thousands of kilometers take place in Russia every year (and to a much lesser extent in the United States), as HEU components are shipped from weapons dismantlement sites and HEU is processed and blended to LEU. International shipments of HEU, almost en-
|
14 |
Matthew Bunn and Anthony Wier, Securing the Bomb: An Agenda for Action (Washington, DC: Nuclear Threat Initiative and the Project on Managing the Atom, Harvard University, May 2004), pp. 58-59, and references cited therein. Available as of January 2005, at: http://www.nti.org/e_research/analysis_cnwmupdate_052404.pdf. |
|
15 |
See Edwin Lyman and Alan Kuperman, “A Re-Evaluation of Physical Protection Standards for Irradiated HEU Fuel” (paper presented at the 24th International Meeting on Reduced Enrichment for Research and Test Reactors, Bariloche, Argentina, November 5, 2002). It should be noted, however, that fresh or spent research reactor fuel could not be used to make a nuclear explosive until the uranium was separated from the aluminum or other inert matrix, since the small density of the uranium in the fuel greatly increases the critical mass. |
|
16 |
In many cases, the spent fuel from these systems remains HEU, but the total amount of HEU (in tons of 93 percent U-235 equivalent) is reduced as U-235 is fissioned. |
tirely as fuel for research reactors or targets for medical isotope production reactors, have declined to a low level since the 1992 Schumer Amendment placed strict limits on U.S. HEU exports.
Stocks of both separated and unseparated plutonium, by contrast, are increasing every year, and international flows are substantial. The operation of the world’s civilian power reactors leads to the discharge of about 80 tons per year of plutonium embedded in 8,000 tons of spent nuclear fuel.17 In recent years, roughly 20 tons of this material has been separated by reprocessing each year, and the rate of fabrication of separated plutonium into mixed oxide fuel for actual loading into power reactors has been about one half that amount, leading to a growing stockpile of civilian separated plutonium that will soon surpass the amount of separated plutonium in all the world’s military stockpiles combined.18 (In addition, roughly 1.2 tons of separated plutonium is reprocessed from the spent fuel of Russia’s three remaining military plutonium production reactors each year, which continue to operate because they provide essential heat and power to nearby communities, and whose fuel was not designed for long-term storage.19) Since the plutonium inventory in spent nuclear fuel has been growing at about 60 tons per year, the total plutonium inventory in spent plus active nuclear fuel has been growing at about 70 tons per year.
Large quantities of plutonium in spent fuel are routinely shipped to reprocessing plants, and large quantities of weapon-usable separated plutonium are shipped from reprocessing plants to fuel fabrication plants and, in the form of fabricated mixed oxide (MOX) fuel, from fabrication plants to reactor sites, each year. Such shipments of separated plutonium take place on a large scale
|
17 |
Unless otherwise noted, estimates in this discussion of plutonium flows are from David Albright, Frans Berkhout, and William Walker, Plutonium and Highly Enriched Uranium 1996: World Inventories, Capabilities, and Policies (New York: Stockholm International Peace Research Institute and Oxford University Press, 1997) and updates posted on the Web site of the Institute for Science and International Security, available as of January 2005, at: http://www.isis-online.org. |
|
18 |
For a recent tabulation of data on civilian plutonium stockpiles declared to the IAEA, see Matthew Bunn, “Unclassified Estimates of Russia’s Plutonium and HEU Stockpiles—And World Civilian Plutonium Stockpiles: A Summary and Update,” Revision 1, Managing the Atom Project, Belfer Center for Science and International Affairs, John F. Kennedy School of Government, Harvard University, July 23, 2003 (unpublished). |
|
19 |
For a discussion, see U.S. Congress, General Accounting Office, Nuclear Proliferation: DOE’s Effort to Close Russia’s Plutonium Production Reactors Faces Challenges, and Final Shutdown is Uncertain, GAO-04-662 (Washington, DC: Government Accountability Office, June 2004). Available as of January 2005, at: http://www.gao.gov/new.items/d04662.pdf. |
within France (which has the world’s most active plutonium recycling program), and on a more modest scale to and from Belgium (which has a modest-size MOX fabrication plant), and from France and the United Kingdom to customers in Germany, Japan, and elsewhere. Limited shipment of military plutonium from weapon dismantlement sites to storage sites presumably takes place, but all plutonium components from dismantled weapons in the United States, and most in Russia, are believed to be stored at the weapon dismantlement sites.
NEM TRANSPARENCY AND MONITORING: GENERAL ISSUES
In principle, transparency and monitoring arrangements for NEM could be analogous to the case of warheads discussed in Chapter 2; they could consist of making declarations of the stocks possessed at a given time, cooperating in the measures needed for others to confirm that the declarations are correct, and allowing and facilitating the monitoring of the stocks from that time forward (including the monitoring of additions and subtractions). The approaches and tools available for implementing these practices in the case of stocks of NEM are substantially similar to those treated in Chapter 2 for the case of intact weapons and components, notably:
-
providing comprehensive declarations of the locations, quantities, types, and physical, chemical, and isotopic forms of all NEM stocks;
-
allowing inspections of declared NEM facilities and sites to confirm and clarify the declarations;
-
maintaining and making available, for inspection and analysis, records of the locations, characteristics, and operating histories of facilities capable of producing, modifying, or destroying NEM;
-
applying and interrogating tags and seals on containers and storage rooms for NEM;
-
installing and operating monitored perimeter-portal systems that exploit radiation and other distinctive signatures to confirm that what enters and leaves any given facility is what it is supposed to be;
-
equipping storage, production, and processing areas with appropriate sensors and accountability systems to monitor declared activity and detect undeclared activity related to NEM at those sites, the recordings from
-
which can either be examined during periodic inspections or uploaded via the Internet or satellites for transmission to a monitoring center; and
-
allowing for on-site inspections of both declared and suspect sites in the event of detection of suspicious activity or unexplained discrepancies.
As in the case of nuclear weapons, such “cooperative transparency” for NEM could be supplemented by information gathered unilaterally by individual states (through National Technical Means, information obtained by clandestine operations, and information obtained from defectors and whistle blowers).
Comparing the Transparency Challenges of NEM and Nuclear Weapons
The rest of this chapter emphasizes aspects of transparency and monitoring for NEM that differ from what has already been presented in relation to intact weapons and their components in Chapter 2. Such differences are related, among other issues, to accounting uncertainties, secrecy issues, physical evidence of production, and the existing system of monitoring of civilian NEM in non-nuclear weapon states by the IAEA.
Accounting Uncertainties
In the case of intact nuclear weapons and their nuclear-explosive components, the numbers are at least precisely known by the countries that possess them. Their inventories are confined to a relatively limited number of sites (at least in peacetime), and both the incentives and the capabilities of the countries that own them to rigorously keep track of them are high. By contrast, NEM occur in a much wider variety of applications and locations (civil as well as military) than nuclear weapons. Many of the forms in which NEM exist also are not “item countable” but rather are bulk commodities that are inherently more difficult to keep track of. Indeed, NEM accounting even by those with unrestricted access to the relevant facilities is plagued by measurement uncertainties, including both those resulting from the inherent limits of available measuring equipment and those from the “holdup” of material in inaccessible
locations in the facilities that produce and process these materials.20
In the United States, for example, when the U.S. government prepared a detailed inventory of its plutonium holdings through 1994, including a comparison of the current inventories at its facilities with the records of production and use of plutonium, it reported total cumulative “inventory differences”—that is, unexplained differences between input to various facilities and the sum of output and present inventory—of 2.8 tons of plutonium, 2.5 percent of the 111.4 tons produced or acquired.21 (In addition, 3.4 tons of plutonium was estimated to have been lost to waste, though the uncertainties in assessing the specific amounts of plutonium in such wastes are large.) There is no evidence that any of this material was stolen (though that possibility cannot be entirely excluded). Rather, these inventory differences are generally the result of inaccurate measurement (particularly during the first decades of the nuclear age, when measurement technology was in its infancy and the premium was on production to support the arms competition, rather than accountancy), holdup of material within facilities (such as material plated onto the interior surfaces of pipes), and possibly overestimation of how much material was produced in the first place. Nevertheless, clearly such irreducible uncertainties, amounting to enough material for hundreds of nuclear weapons in the case of the United States and Russia, will have to be taken into
|
20 |
See, for example, U.S. Department of Energy, Deputy Assistant Secretary for Security Evaluations, Increasing Fissile Inventory Assurance Within the U.S. Department of Energy (Washington, DC: Department of Energy, January 1995). This study concluded that “an accurate inventory is necessary for continued assurance against theft or diversion,” and that inventories accurate enough to meet that goal or to “fully support international activities” such as permitting inspection of DOE sites were not yet in place – in part because “most of the holdup at DOE facilities has not been accurately measured, and some has not been measured at all,” and because some 10 tons of plutonium and 100 tons of HEU existed in scrap and other forms that were difficult to measure accurately. In response, DOE established a Fissile Material Assurance Working Group, which made a wide range of recommendations for improving accounting practices at DOE, many of which have since been implemented. (See Thomas P. Grumbly, memorandum to Victor H. Reis, Alvin L. Alm, Martha A. Krebs, and Terry R. Lash, “Fissile Material Assurance Working Group Recommendations,” February 11, 1997). The difficulties of achieving accurate measurements of material in waste, holdup, and scrap remain substantial. |
|
21 |
U.S. Department of Energy, Plutonium: The First 50 Years: United States Plutonium Production, Acquisition, and Utilization From 1944 Through 1994 (Washington, DC: Department of Energy, February 1996). Available as of January 2005, at: http://www.osti.gov/html/osti/opennet/document/pu50yrs/pu50y.html. Another useful treatment of this and related points is Steve Fetter, Verifying Nuclear Disarmament, Occasional Paper 29, Henry L. Stimson Center, Washington, DC, 1996. |
account in considering how accurate and effective any potential regime of declarations and the monitoring of these could be.
Practices that render material accounting programs ineffective as a means of confirming that enough material for a bomb has not been removed continue to be uncovered at U.S. sites, and similar practices presumably take place at sites in other states as well.22 Russia has not yet prepared an inventory comparable with the published U.S. plutonium inventory, though U.S. and Russian experts have discussed such an effort.23 Discussions with Russian experts concerning accountancy practices in the former Soviet Union suggest that the uncertainties there will be even higher, and the complications in matching current inventories to production histories even greater.24
Bookkeeping for HEU is also difficult, in part because the U-235 concentration varies so widely in both enriched material and in the depleted “tails” from enrichment. (Freshly enriched uranium can vary from 1 percent U-235 in very low enriched fuel for cer-
|
22 |
For the U.S. case, see Martha C. Williams and Dewey L. Whaley, “Observed Practices That Can Adversely Affect an MC&A Program,” in Proceedings of the 44th Annual Meeting of the Institute of Nuclear Materials Management, Phoenix, Arizona, July 14-17, 2003 (Northbrook, IL: Institute of Nuclear Materials Management, 2003). Williams and Whaley report, for example, cases where holdup in process was not measured but defined as the difference between input and output—a practice that makes it impossible to detect whether the difference is actually caused by unauthorized removal of material. For a case in a non-nuclear weapon state subject to IAEA safeguards, it is instructive to consider the case of Japan’s Tokai reprocessing plant, where IAEA estimates and Japanese estimates of material began to diverge as soon as the facility began operating in the 1970s, and it was not until decades later, after the difference had increased to some 200 kilograms of plutonium, that improved approaches to measuring the plutonium being sent to waste, which were then retroactively applied to estimate the amount of plutonium sent to waste over the facility’s lifetime, were finally agreed and implemented, bringing Japanese and IAEA estimates into line. See, for example, International Atomic Energy Agency, “New Measurement Techniques Correct Pu Inventory in Japanese Reprocessing Plant,” PR/2003/02, January 28, 2003. |
|
23 |
Gennadi M. Pshakin et al., “Russian-American Cooperation in Developing a Russian Plutonium Registry,” in Proceedings of the 43rd Annual Meeting of the Institute of Nuclear Materials Management (Northbrook, IL: Institute of Nuclear Materials Management, 2002). |
|
24 |
Russia does not yet have a complete national computerized inventory of its stockpiles, only a combination of computer-based and paper records. Many Russian facilities have not had the resources to perform complete measured inventories of their nuclear material holdings in recent years, and most Russian experts expect that such inventories would reveal substantial differences from paper records on the inventories. The chief engineer for one of Russia’s major plutonium production facilities, for example, reported that until U.S.-Russian cooperation began, the very concept of inventory differences or material unaccounted for did not exist at his facility: the difference between input and output was defined as losses to waste. Matthew Bunn, “The Threat in Russia and the Newly Independent States,” 2004. Available as of January 2005, at: http://www.nti.org/e_research/cnwm/threat/russia.asp. |
tain reactors up to 80-98 percent U-235 in HEU for use in weapons and some naval propulsion reactors. Depleted uranium typically contains 0.2-0.4 percent U-235, but sometimes is outside these bounds.) The bookkeeping problem is further complicated by the possibilities for recycling of uranium in a number of ways; for example, natural uranium might be irradiated in the reactor to produce plutonium, followed by use of the residual uranium recovered at the reprocessing plant as input to a uranium enrichment plant making HEU. In addition, uranium of specific U-235 concentrations needed for particular applications can be and has been produced by blending HEU with depleted uranium, natural uranium, or LEU. Record keeping of the quantities and concentrations of the input and output flows from such operations was not always complete, and the gaps make it very difficult to reconcile existing inventories exactly with records of past production, use, and reuse.
As with plutonium, moreover, significant amounts of uranium with varying degrees of enrichment are held up in the equipment and piping of enrichment and processing facilities (such as weapon component or fuel fabrication facilities); this is particularly the case for gaseous diffusion enrichment plants. In addition, in the United States at least, the quantities of HEU in scrap and other difficult-to-measure forms are far larger than the comparable quantities of plutonium. A U.S. declaration on its HEU production was completed in the late 1990s and declassified in 2001, but has not been made public. The unexplained inventory differences in that inventory are presumably substantial, and it should be expected that when Russia prepares a comparable inventory, the uncertainties will be even larger (though Russia long ago transitioned from gaseous diffusion to centrifuges for its enrichment operations, and centrifuge enrichment involves lower irreducible accounting uncertainties, because of the much lower quantity of in-process uranium at any given time).25
The military plutonium and HEU stockpiles that exist in other states are dramatically smaller than those in the United States and Russia. The stockpiles in Britain, France, and China each amount to a few percent of the U.S. or Russian stockpiles and the stockpiles in India, Pakistan, Israel, and North Korea each amount to far
less than 1 percent of the U.S. or Russian stockpiles. Thus the total magnitude of the accounting uncertainties for NEM in these cases should be expected to be dramatically smaller than that in the U.S. and Russian cases, even if the uncertainties are the same or worse in terms of the percentage of the total quantity of NEM produced.
Civilian plutonium and HEU stockpiles in non-nuclear weapon states are already monitored by the IAEA (see below). Here, too, accounting uncertainties pose significant issues, at least for those types of facilities where NEM is bulk processed in large quantities (such as plutonium reprocessing plants, or facilities that fabricate fuels containing plutonium or HEU). International standards have been developed for the expected accuracy of material measurement in different processes, and are regularly updated.26 Currently, the standard deviation of safeguards measurements at a large reprocessing plant are expected to be in the range of 1 percent of throughput and the uncertainties at a centrifuge enrichment plant only in the range of 0.2 percent of throughput (no large gaseous diffusion enrichment plants are under IAEA safeguards at present). But the uncertainties at a waste store are expected to be in the range of 20 percent of the stored material.27
Secrecy Issues
A difference that makes transparency for NEM easier to implement than transparency for weapons is that the characteristics of many forms of NEM are less sensitive and accordingly less highly classified than the characteristics of actual weapons. While nuclear weapons are unambiguously military,28 large quantities of NEM
|
26 |
For the most recent update for particular kinds of measurements, see H. Aigner et al, “International Target Values 2000 for Measurement Uncertainties in Safeguarding Nuclear Materials,” Journal of Nuclear Materials Management 30 (Winter 2002). Available as of January 2005, at: http://www.inmm.org/topics/contents/JNMMPaperITV.pdf. |
|
27 |
See the presentations in International Atomic Energy Agency, International Course on Agency Safeguards 44, Vienna, Austria, October-December 2000. |
|
28 |
Non-weapon uses of nuclear explosives for major construction projects, stimulation of natural gas deposits, and the like were explored experimentally by the United States and the Soviet Union in the 1960s and 1970s and also attracted some interest subsequently in China. Potential applications of nuclear explosives for space propulsion and for defending the earth from wayward comets and asteroids have been proposed but never pursued beyond the conceptual stage. None of these possibilities is currently attracting much attention: U.S. and Russian experiments with “Peaceful Nuclear Explosives” (PNEs) showed little promise of economic and environmentally acceptable use. Although the Chinese initially proposed language to permit monitored tests for peaceful purposes during the negotiations for the Comprehensive Nuclear Test Ban Treaty (CTBT), it was generally recognized that this would greatly weaken the treaty, perhaps fatally, since it would provide a convenient cover for weapon development. In return for China’s withdrawal of its proposal, a provision was incorporated |
were produced and are being used for civilian purposes, and the characteristics of these are not classified. (Information on specific locations where enough material for a bomb exists may be sensitive, however, particularly if these locations are not well secured.)
The secrecy situation with respect to military NEM is more complex. The United States has declassified and published detailed information on its plutonium inventory and past production, and information on at least its total production of HEU; the United Kingdom has also declassified detailed information on its plutonium production and current inventory, and its current HEU inventory. But Russia and other states with military NEM stockpiles continue to regard both the size of their current inventories and the production histories of these inventories as secret information. Similarly, while the United States now regards most of the general characteristics of weapons plutonium as unclassified, Russia still counts both the isotopic and chemical composition of weapons plutonium as secrets,29 and it is likely that other nuclear weapon states currently have similar policies. The specific isotopic compositions of HEU used for military purposes are classified in both the United States and elsewhere, as are the chemical and physical forms of HEU used as naval fuel, the amounts of such fuel used each year, and the amounts of such material present at particular locations.
In short, substantial quantities of NEM around the world are not classified, and thus pose fewer monitoring challenges than warheads do, but there are also substantial quantities of NEM that are not in assembled nuclear weapons or weapon components but that are nonetheless subject to very significant secrecy constraints.
Physical Evidence of Production
Another important difference between transparency and monitoring for warheads and for NEM is that production of NEM, in some cases, leaves behind physical evidence that can be compared
|
|
in Article VIII that at a review conference 10 years after the treaty entered into force any party could request consideration of the possibility of conducting tests for peaceful purposes. If the Conference decided by consensus (without objection) that such tests would be permitted, an appropriate treaty amendment would be submitted to a special amendment conference (Article VIII), which could adopt the amendment by a majority vote provided no state vetoed the action. In short, a double veto essentially precluded a future amendment permitting tests for peaceful purposes. |
with declarations of past production, to check that the evidence is consistent with the declarations. In the case of nuclear warheads, production records for warhead assembly and disassembly plants can be exchanged, as discussed in Chapter 2, but there is nothing about the physical state of these plants that would help confirm the number of nuclear weapons that had been assembled or disassembled there. In the case of plutonium, the moderator or structural materials in plutonium production reactors absorb neutrons as irradiation of nuclear material to produce plutonium proceeds. In a process known as “nuclear archaeology,” these structural materials can be examined to estimate how much plutonium was produced in that reactor, and this evidence can be compared with declarations and other information.
Similar physical evidence of total production is not available for uranium enrichment, but examinations of depleted uranium from enrichment operations can provide some information on how much material was processed when, with what levels of U-235 in the waste. When such information is combined from declarations and other sources, it can help to build confidence that a declaration of production is accurate, or highlight discrepancies that suggest it may not be. These subjects are addressed in more detail in Chapter 4.
IAEA Monitoring of Civilian NEM Stocks in Non-Nuclear Weapons States
The differences described above between intact warheads and NEM have negative and positive implications for transparency and monitoring. The negative aspect of this difference is that monitoring of NEM is more difficult, given the size and dispersal of the stocks and flows and facilities associated with the nonweapon uses of these materials, than it would be if NEM were confined to the nuclear weapon sector. The positive aspect is that the civil-military “dual use” character of NEM makes monitoring of some NEM much less sensitive. As a result, the world community has been able to establish under the Nuclear Non-Proliferation Treaty (NPT) of 1970 a system under which all civilian NEM in non-nuclear-weapon state parties to that treaty are declared to, and inspected by the IAEA—as are all facilities in those states capable of producing NEM—under detailed terms negotiated between the agency and the individual states. All states except India, Israel, Pakistan, and with its recent withdrawal, North Korea, are parties to the NPT. Similarly, civilian NEM within the EURATOM states of the European Union are under EURATOM safeguards, even if the states
concerned are nuclear weapon states (Britain and France).30 Thus, the only NEM stockpiles not generally already subject to monitoring even more intrusive than most of the measures assessed in this report are military NEM stockpiles, and the civilian NEM stockpiles of nuclear-weapon states other than Britain and France.31
As discussed further below, the IAEA safeguards system has provided invaluable experience with procedures and technologies for monitoring civil nuclear materials stocks and facilities while respecting the sensitivities of the possessor countries, but at the same time has demonstrated the limitations of existing procedures. The system, which has been under more or less constant expansion and improvement since its establishment in 1970, provides an extensive experience base for measures to monitor NEM, and could be extended to cover civil NEM in nuclear weapon states, and at least the portion of military NEM stocks that these states deem surplus to their military needs.
TRANSPARENCY AND MONITORING FOR NEM: HISTORY, STATUS, AND THE ROAD AHEAD
The main efforts to date on developing elements of transparency and monitoring for military NEM have occurred in the context of U.S.-Russian relations since the end of the Cold War.32 Some multilateral efforts in this domain have also taken place, most importantly under the auspices of the IAEA. Transparency and monitoring for civil NEM, on the other hand, have been driven largely by the international safeguards responsibilities and practices of the IAEA pursuant to the NPT. In this section we augment
|
30 |
Like the IAEA, EURATOM relies on material accounting supplemented with containment and surveillance for its safeguards system, though the specific standards, approaches, and purposes of the EURATOM system are somewhat different. See Commission of the European Communities, “Operation of Euratom Safeguards in 2002” (Brussels: European Commission, 2003). Available as of January 2005, at: http://europa.eu.int/comm/energy/nuclear/safeguards/doc/com_2003_0764_en.pdf. |
|
31 |
Some of the civilian material in nuclear-weapon states or in non-parties to the NPT is under IAEA safeguards, under voluntary agreements between the weapon states and the Agency, or at the insistence of countries that supplied particular facilities or materials. A Chinese enrichment plant supplied by Russia, for example, is under Agency safeguards, and an Indian reprocessing plant has safeguards during those periods when it is processing nuclear material provided by the United States or other suppliers that insist on such safeguards. |
|
32 |
A number of U.S.-Russian efforts at transparency for NEM from dismantled nuclear weapons were already mentioned in Chapter 2 in connection with the discussion there of the post-Cold War U.S.-Russian nuclear-weapons initiatives. |
the discussion in Chapter 2 of U.S.-Russian initiatives relating to military NEM, then turn to the multilateral dimension of efforts toward transparency and monitoring for this material, and finally treat the IAEA-centered efforts relating to civilian NEM. Under each of these headings, there is a brief review of the recent history, current status, and relevant transparency issues, together with consideration of options to improve these capabilities.
U.S.-Russian Transparency and Monitoring Efforts for Military NEM
The treatment in Chapter 2 of the linked transparency initiatives for nuclear weapons and military NEM is augmented in this section under six subheadings: transparency for NEM from dismantled weapons; exchange and confirmation of declarations on total stocks of NEM; transparency at Nunn-Lugar sites; monitoring issues in plutonium production and disposition; unilateral openness initiatives and informal cooperation; and lab-to-lab cooperation on transparency technologies, followed by a concluding discussion of considerations and options looking ahead.33
Transparency for NEM from Dismantled Weapons
Even prior to the September 1994 Clinton-Yeltsin summit agreement mentioned in Chapter 2, U.S. Secretary of Energy Hazel O’Leary and Russian Minister of Atomic Energy Victor Mikhailov agreed to establish a regime of mutual inspections to confirm the inventories of plutonium and HEU removed from dismantled nuclear weapons. This initiative eventually came to be called, somewhat redundantly, “Mutual Reciprocal Inspections” (MRI). In 1994 and 1995 U.S. and Russian experts carried out a number of joint experiments and came close to agreeing on the specific types of measurements that would be used to confirm that an inspected canister contained a plutonium weapon component; a less intrusive
|
33 |
Key sources for additional detail on each of these topics are Matthew Bunn, Anthony Wier, and John P. Holdren, Controlling Nuclear Warheads and Materials: A Report Card and Action Plan (Washington, DC: Nuclear Threat Initiative and the Project on Managing the Atom, Harvard University, March 2003) and the companion Nuclear Threat Initiative Web site available as of January 2005, at: http://www.nti.org/cnwm. See also James Goodby, “Transparency and Irreversibility in Nuclear Warhead Dismantlement,” in Harold A. Feiveson, ed., The Nuclear Turning Point: A Blueprint for Deep Cuts and De-Alerting of Nuclear Weapons (Washington, DC: The Brookings Institution, 1999), and Oleg Bukharin and Kenneth Luongo, U.S.-Russian Warhead Dismantlement Transparency: The Status, Problems, and Proposals, PU/CEES Report No. 314 (Princeton, NJ: Center for Energy and Environmental Studies, Princeton University, April 1999). Available as of January 2005, at: http://www.ransac.org/new-web-site/pub/reports/transparency.html. |
regime was proposed for inspections of HEU components. The proposed agreement was never completed, however, in part because the two sides failed to negotiate a cooperative agreement to provide the legal basis for exchanging limited types of classified nuclear information.34
Exchange and Confirmation of Declarations on Total Stocks of NEM
As noted in Chapter 2, Presidents Clinton and Yeltsin agreed at their September 1994 summit and again at the summit of May 1995 that their governments would exchange detailed information on stocks of NEM as well as on inventories of nuclear weapons themselves. The May 1995 summit statement also called for an agreement on “other cooperative measures, as necessary to enhance confidence in the reciprocal declarations of fissile material stockpiles.” And the March 1997 Clinton-Yeltsin summit statement mentioned yet again the desirability of exploring transparency measures for nuclear materials. The bilateral measures to increase transparency contemplated in these statements did not materialize by the end of the Clinton Administration, in part because there was no cooperative agreement to lift the secrecy restraints on the relevant information.35 The Bush Administration has not pursued either warhead dismantlement transparency or comprehensive data exchanges relating to stockpiles of nuclear warheads and NEM, but in the context of the Moscow Treaty, has established a joint U.S.-Russian working group on transparency in offensive nuclear forces. As of early 2005, there had been no public statement that this group had agreed to pursue any particular transparency measures.
Transparency at Nunn-Lugar Storage Sites
For those Nunn-Lugar projects related to carrying out dismantlement required by arms control agreements—where Russia had already taken the decision to allow inspection as part of the nego-
|
34 |
Both the U.S. and Russian legal systems impose stringent requirements for protecting classified information related to nuclear weapons. In 1994 Congress amended the Atomic Energy Act to provide legal authority to negotiate an “Agreement for Cooperation” with Russia that would provide the legal basis for exchanging classified nuclear information (known under the act as “restricted data”) for nonproliferation and arms control purposes. The two sides began negotiating such an agreement in 1995 and had it nearly completed by late 1995, but at that time the Russian government called off further talks pending a “policy review,” and the talks have never resumed. |
|
35 |
See the Department of State's annual report on Moscow Treaty implementation, available as of January 2005, at: http://www.state.gov/t/ac/rls/or/25474.htm. |
tiation of the initial agreement—problems of secrecy and access to the sites have generally not been unduly burdensome. This has not been the case, however, where no previous arms control requirement to allow access to the relevant sites or information exists, as is the case with most of the projects related to NEM.
In particular, the United States and Russia agreed in principle, early in the implementation of the Nunn-Lugar Cooperative Threat Reduction program, that transparency measures would be applied at the storage facility for surplus Russian weapon NEM that was then proposed to be built at Mayak with U.S. assistance. The negotiation of the details of these measures, however, has proven difficult. The overlap of three different proposed transparency regimes at this one facility—unilateral Nunn-Lugar transparency, bilateral MRI measures, and international IAEA verification—complicated negotiations considerably.
In recent years, although the focus has narrowed to just the unilateral Nunn-Lugar transparency, disagreements have continued, as Russia judged that a number of the measures the United States proposed would reveal information that is secret in the Russian system. As of January 2005, the Mayak facility has been completed, but the bilateral transparency arrangements are still not agreed. In the approach currently under discussion, the surplus plutonium will arrive in the form of spherical metal ingots contained in cans (prepared without U.S. assistance), on which external measurements will be made to verify that at least a threshold quantity of plutonium is inside each container, the fact that it is roughly weapon grade, and perhaps also the fact that it is in metallic form. Agreement has not been reached in part because Russian negotiators assert that the mass of plutonium stored at the facility is itself secret under Russian secrecy rules.36 Earlier proposals by the United States for measurements of a larger number of attributes, and for monitoring of the “upstream” steps leading to the fabrication of the metal ingots were rejected by the Russian side—in part because the U.S. side offered no parallel monitoring of similar steps in the United States.
Monitoring Issues in Plutonium Production and Disposition
The 1997 U.S.-Russian agreement on ending production of weapons plutonium includes a requirement for monitoring meas-
|
36 |
Nuclear Threat Initiative, “Mayak Storage Facility Transparency,” Monitoring Stockpiles. Available as of January 2005, at: http://www.nti.org/e_research/cnwm/monitoring/mayak.asp. |
ures to confirm the shutdown status of those plutonium production reactors that are already shut down (all of the U.S. plutonium production reactors, and all Russian plutonium production reactors except for three that were also the principal regional sources of heat and electricity) and to confirm that plutonium produced in Russia’s three remaining plutonium production reactors after 1994 would not be used in weapons.37 To confirm that the plutonium offered for monitoring was in fact the plutonium produced in these reactors, the agreement specified that U.S. monitors would be able to take measurements to confirm that the ratio of Pu-240 to total plutonium and the ratio of Am-241 to Pu-241 were below certain thresholds (the former to confirm that the material submitted for monitoring was weapon-grade plutonium, and the latter to confirm that it had been recently separated). For years after the entry into force of the agreement, however, Russia and the United States could not agree on the specific monitoring measures for the stored plutonium that U.S. monitors should be allowed to implement, largely because of Russian concerns that more specific details of the isotopic characteristics of the plutonium than the ratios covered in the agreement, still considered classified in Russia, would be revealed. Since 2002, U.S. monitors have been allowed to conduct monitoring visits to the facilities where the plutonium is stored, but U.S. and Russian experts are still jointly developing measurement equipment that will allow appropriate measurements to be taken while addressing Russian concerns.38
Similarly, the 2000 U.S.-Russian Plutonium Management and Disposition Agreement specified that a variety of monitoring measures would be put in place to confirm that the material subject to disposition was weapon grade, that disposition actually took place, and that the material was not returned to weapons. Virtually no progress has been made in negotiating specifics of such monitoring arrangements. U.S. officials believe that these talks will not move forward until larger issues affecting the viability of the plutonium disposition effort are resolved, including international fi-
|
37 |
The text of the agreement is available as of January 2005, at: http://www.ransac.org/new-website/related/agree/bilat/core-conv.html. |
|
38 |
See, for example, the brief discussion on the official Web site of the Department of Energy’s Warhead and Fissile Material Transparency Program, available as of January 2005, at: http://www.nnsa.doe.gov/na-20/wfmt.shtml. |
nancing and management arrangements and a resolution of the U.S.-Russian dispute over liability in the event of an accident.39
Unilateral Openness Initiatives and Informal Cooperation
Somewhat offsetting the slow pace of negotiated bilateral increases in transparency, the United States has taken some unilateral initiatives to increase the openness of its nuclear activities, including those related to NEM. Information that has been declassified covers a broad range, from details of past radiation experiments on humans to data on the number of U.S. nuclear tests. In addition to the release of this information, visits to a wide range of nuclear facilities by the public and by Russian representatives have been permitted. As indicated earlier, however, the information the United States has declassified so far about its military stocks of NEM has been less than complete, and what has been made public up until now by Russia about its military NEM stocks is even less complete.
While Russia has not yet matched all of the U.S. openness initiatives, a significant increase in openness since the collapse of the Soviet Union is apparent. Particularly in the contexts of lab-to-lab cooperation on scientific projects and U.S.-Russian cooperation in securing and accounting for nuclear warheads and materials, Russia has allowed visits to a broad range of formerly secret nuclear sites. These visits and discussions, along with Russian visits to many U.S. nuclear sites, have created an unprecedented window to improve each nation’s understanding of the other’s nuclear complex and activities. Russia has also declassified full information on past Russian nuclear testing, paralleling the information the United States released earlier on its own nuclear testing program. Information on the size, locations, and characteristics of Russia’s stockpiles of warheads and fissile materials remains classified at this writing.
The road to greater openness has by no means been a smooth one. In both Russia and the United States, high-level support for increased openness has often been countered by intense opposition from nuclear security bureaucracies. In Russia, a remarkable period of openness immediately following the collapse of the Soviet Union was followed by a struggle that continues to this day, as Russia’s security services push to reassert control and limit access
|
39 |
Matthew Bunn, “Russian Plutonium Disposition,” 2004, Reducing Excess Stockpiles. Available as of January 2005, at: http://www.nti.org/e_research/cnwm/reducing/rpdispose.asp. |
to potentially sensitive sites and information, while U.S.-Russian cooperation continues to establish its value and expand. In the United States, the openness initiatives of the 1990s have given way, in the aftermath of the September 11th attacks, to substantial retrenchment, including attempts to reclassify material that was previously in the public domain.
Lab-to-lab Cooperation on Transparency Technologies
Building on the model of the material protection, control, and accounting (MPC&A) program—in which laboratory experts working directly together succeeded in demonstrating technology, building trust, and establishing a constituency to expand similar programs, eventually leading to new government-to-government agreements—U.S. and Russian laboratories began in 1994 a modest program to jointly develop and demonstrate transparency technologies. The first initiative in this effort was a demonstration of “remote monitoring,” using video cameras and similar technologies to monitor material in storage, without requiring on-site inspectors. Equipment was hooked up to monitor HEU in storage at the Kurchatov Institute in Moscow, and at Argonne National Laboratory-West in Idaho, with the images and data uplinked via satellite.40
In subsequent years, the two sides have jointly developed and experimented with a range of technologies that could be applicable to confirming warhead dismantlement without revealing sensitive information, and to other transparency and monitoring tasks. These have included, for example, approaches to the use of templates and attributes to confirm the presence of nuclear warheads or of particular types of NEM in containers. Since the September 11th attacks, the focus of this lab-to-lab work has shifted to include detection of explosives and of nuclear materials for counterterrorism purposes.41
|
40 |
Robert L. Martinez, Dennis Croessmann, Vladimir Sukhoruchkin, Alexander Grigoriev, and Mark Sazhnev, “American-Russian Remote Monitoring Transparency Program: Accomplishments During the Past Year,” in Proceedings of the 38th Annual Meeting of the Institute of Nuclear Materials Management (Northbrook, IL: Institute of Nuclear Materials Management, 1997). |
|
41 |
The Department of Energy maintains an official Web site describing this work, available as of January 2005, at: http://www.nnsa.doe.gov/na-20/wfmt.shtml. For other descriptions, see, for example, U.S. Department of Energy, Office of Nonproliferation and Nuclear Security, Warhead and Fissile Material Transparency Program Strategic Plan (Washington, DC: Department of Energy, May 1999) and the papers available at the Web site of the Applied Monitoring and Transparency Laboratory, available as of January 2005, at: http://amtl.iwapps.com. |
Considerations and Options Looking Ahead
Limitations on NEM transparency between the United States and Russia constitute, in our judgment, the greatest current obstacle to strengthening U.S.-Russian cooperation on MPC&A and hence one of the principal barriers to reducing the danger that NEM will fall into the hands of terrorists, agents of proliferant states, or black marketeers who would sell to either.42 The U.S. and Russian governments need to reach studied conclusions about the appropriate balance between secrecy and openness in the service of their national security interests, and then arrive at a common understanding that both can enforce within their national security establishments. The United States should decide what access to its own facilities it is willing to accept by the Russians, in exchange for the benefits of U.S. access to corresponding facilities in Russia.
In this connection, the U.S. government could update the detailed declaration released in the mid-1990s on the history of production and utilization of military Pu, leading to the current stockpile, and fulfill its promise to release similarly detailed information relating to U.S. military HEU. Correspondingly, the United States could encourage Russia to complete its own national inventories and histories for military Pu and HEU and to share this information with the United States, and preferably more widely. The United States and Russia could then proceed to demonstrate jointly and to deploy measures for helping to confirm the accuracy of these declarations, including exchanges and analysis of production records, the use of “nuclear archaeology” techniques, and spot checks of declared amounts at particular sites under conditions designed to protect information that remains sensitive. Here as elsewhere, some information considered particularly sensitive could be exchanged in encrypted or message digest form to be made available at a later date or on selective demand as discussed in Chapter 2 for sensitive weapons information.
Reciprocity in these activities could have a beneficial effect on the programs. It could, for example, be important to accelerate im-
|
42 |
See Matthew Bunn, Anthony Wier, and John P. Holdren, Controlling Nuclear Warheads and Materials: A Report Card and Action Plan (Washington, DC: Nuclear Threat Initiative and the Project on Managing the Atom, Harvard University, March 2003) and John Holdren and Nikolai Laverov, Letter Report from the Co-Chairs of the Joint Committee on U.S.-Russian Cooperation on Nuclear Non-Proliferation. The U.S. National Academies and the Russian Academy of Sciences, February 2003. Available as of January 2005, at: http://www4.nationalacademies.org/news.nsf/isbn/s02052003?OpenDocument. |
plementation of existing material protection projects across the Russian nuclear complex, a goal to which the United States is committed. It could also be vital for ensuring progress at certain highly sensitive sites, such as Russia’s two main warhead production and maintenance facilities. If the United States and Russia agree to proceed with reciprocity in this arena, then a clear and agreed definition of the measures, and a description of the rationale for them, would increase the probability of success.
In pursuing the appropriate kinds and degrees of transparency for these purposes, it will be important to be attentive to the advantages and disadvantages of public versus classified exchanges between the two governments. Indeed, it will be necessary to think carefully about what kinds of information would be shared only between the United States and Russia, what kinds shared only with the NPT-authorized nuclear weapon states as a group, what kinds shared with all governments in good standing under the NPT, and which kinds made public.43
International Monitoring of Excess Military NEM
Some progress toward placing excess U.S., Russian, and possibly other NEM under international monitoring to verify for the world that it is never again returned to weapons—a step recommended reports from both The National Academies and Independent Bilateral Scientific Commission44—was made in the years after those reports were published, but that progress has now essentially ground to a halt.
Declarations of Excess Material
In 1995 President Clinton declared that some 225 tons of U.S. NEM was excess to U.S. military needs, and would no longer be available for military use. As the details were provided subse-
quently, this figure increased somewhat: total U.S. excess declarations include 52.5 tons of plutonium and 174 tons of HEU. This represented slightly more than half of the plutonium stockpile that the U.S. departments of Energy and Defense possessed, but a much smaller fraction of the HEU stockpile – because the U.S. Navy reserved nearly all HEU that met its quality standards for future use as naval fuel. In 1993 Russia had agreed to sell the United States LEU blended from 500 tons of its weapons-grade HEU—effectively declaring this HEU excess to its military needs—and in 1996-1997 Russia responded to Clinton’s declaration by declaring that “up to” 50 tons of plutonium, along with 500 tons of HEU, was excess to its military needs. The Russian declaration represented a smaller fraction of Russia’s total stockpile of separated plutonium, but a larger fraction of Russia’s stockpile of HEU, compared with the U.S. declaration. (It is notable that from the beginning there was no effort to negotiate how much NEM should be declared excess and how much should remain in each country’s military stockpile; this was left entirely as a matter for unilateral determinations and declarations.) Years later, the United Kingdom followed suit, declaring that 4.4 tons of its plutonium stockpile (including 0.3 ton of weapons-grade plutonium) was excess to its military needs, along with large quantities of uranium (though no HEU).45
Initiatives, Agreements, and Obstacles
In September 1993 President Clinton announced that the United States would make its excess fissile material eligible for IAEA safeguards in order to assure the world that these materials were not being used for nuclear weapons. Classification issues, budget constraints, and safety concerns related to monitoring material in radioactive facilities, however, have slowed progress on this front. As of early 2003, 12 tons of the U.S. excess military NEM was under IAEA safeguards (10 tons of HEU and 2 tons of plutonium), and the IAEA had verified the down blending of more than 20 additional tons of U.S. HEU. IAEA monitoring is in place for the continuing blend-down of excess U.S. HEU at BWX Technologies in Lynchburg, Virginia.46
|
45 |
See Matthew Bunn, “IAEA Monitoring of Excess Nuclear Material,” Monitoring Stockpiles. Available as of January 2005, at: http://www.nti.org/e_research/cnwm/monitoring/trilateral.asp. |
|
46 |
Ibid. |
At the Moscow Nuclear Safety and Security Summit in 1996, the assembled leaders agreed that excess fissile material should be placed under international safeguards as soon as it is practicable to do so. On that occasion, Russian President Yeltsin made a commitment to place the storage facility being built at Mayak, which was then expected to hold an estimated 50 tons of plutonium and a much larger amount of HEU, under IAEA safeguards,47 but this pledge has not yet come to fruition. The United Kingdom has made its excess nuclear material eligible for international safeguards; while the IAEA has not had the resources to apply safeguards to this material, it is under EURATOM safeguards.
The Trilateral Initiative
There is consensus among the IAEA, the United States, and Russia that putting excess military NEM under IAEA oversight represents a fundamentally new mission for that international agency: namely verifying nuclear disarmament in nuclear weapon states possessing many weapons, rather than verifying nonproliferation in non-nuclear weapon states. Consequently, new terminology (“verification” rather than “safeguards”) and new approaches should be used. In September 1996, following up on the Clinton and Yeltsin pledges to allow the IAEA to verify excess NEM, the United States, Russia, and the IAEA established a “Trilateral Initiative” to discuss the broad range of issues related to placing excess military NEM under IAEA verification.
During several years of work, U.S. and Russian scientists developed and tested approaches to allow the IAEA to confirm that plutonium objects in containers had certain attributes of weapons components (e.g., at least a threshold mass of plutonium, at least a threshold ratio of Pu-239 to total plutonium, plutonium in metallic form, a generally symmetric shape), without revealing classified information. Legal experts worked out an approach to a new agreement that would no longer allow the United States and Russia to remove the material from verification at any time, as their vol-
|
47 |
Because excess HEU from dismantled weapons is being processed to LEU for sale to the United States, Russia does not plan to store HEU at Mayak. Moreover, Russia currently plans to store only 25 tons of plutonium at Mayak: the 34 tons of excess weapons plutonium covered by the U.S.-Russian Plutonium Management and Disposition Agreement, minus the roughly 9 tons of that material that is plutonium oxide produced since 1994, is in storage at Seversk and Zheleznogorsk. See Matthew Bunn, “Mayak Fissile Materials Storage Facility,” Securing Nuclear Warheads and Materials. Available as of January 2005, at: http://www.nti.org/e_research/cnwm/securing/mayak.asp and the discussion above in the section on “Monitoring Issues in Plutonium Production and Disposition.” |
untary safeguards agreements with the IAEA do. A number of options for financing such IAEA verification activities were explored.
At the IAEA General Conference in September 2002, the parties issued a statement in which they “declared victory” on the initiative, contending that they had successfully developed a regime, and demonstrated techniques that would make it possible to put plutonium in classified forms under international verification. In reality, however, the effort came nearly to a halt without coming to fruition, as (a) neither the United States nor Russia has yet agreed to put any nuclear material under this type of verification;48 (b) agreement remained elusive on whether IAEA monitoring would continue on the material until it met standard IAEA “safeguards termination” criteria (i.e. the point at which the IAEA no longer monitors material, such as NEM in nonrecoverable waste);49 and (c) there was no agreement on who would pay for such verification.
The 2000 U.S.-Russian Plutonium Management and Disposition Agreement includes a provision requiring each party to begin consultations with the IAEA “at an early date,” and to conclude agreements with the IAEA to allow it to conduct verification beginning “not later in the disposition process” than when the plutonium has been processed to an unclassified form and is placed in storage at a conversion or conversion/blending facility, or when it is received at a fuel fabrication or immobilization facility (whichever is sooner). This is now expected to be the future focus of activities related to IAEA monitoring of U.S. and Russian excess plutonium, but neither the United States nor Russia has begun serious discussions with the IAEA concerning such verification. (As noted earlier, even U.S.-Russian discussions of bilateral transparency for the disposition process have not yet gotten seriously underway.)
Considerations and Options Looking Ahead
We believe that world confidence in the reality and irreversibility of U.S. and Russian nuclear arms reductions would benefit from multilateral rather than merely bilateral monitoring of the excess stocks of NEM. If nuclear arms reductions proceeded to the point that nuclear weapon states other than the United States and Russia see fit to declare some of their military NEM as surplus to military needs, having an established international monitoring operation for such materials already in place would be very advantageous. We believe that both for monitoring the current U.S. and Russian surpluses and for potential extension to surplus military NEM in other states, using the stature, experience, and capabilities of the IAEA is a better option than trying to construct a new multilateral verification institution.
Establishing the capacity of the IAEA to discharge this function for the U.S. and Russian surplus NEM now, and for other surpluses later, would require strengthening of the agency to deal with a set of responsibilities that has been expanding in relation to civil NEM and the detection of clandestine nuclear weapon programs. The costs of the IAEA safeguarding the excess NEM in the United States and Russia would be small by security standards but large in the context of the IAEA budget. The United States and Russia could take responsibility for IAEA's costs for this mission; seek to mobilize a wider coalition of the IAEA member states most interested in verified disarmament to share the costs; seek agreement on mandatory contributions to a special fund for this purpose from all IAEA member states; or propose other financing mechanisms. This funding should be considered a separate matter from the needed increases in the IAEA budget for its expanded safeguards functions for civil NEM.
Transparency and Monitoring for Civil NEM
The measures currently in place for implementing international transparency with respect to civil NEM are principally those negotiated by the IAEA under Safeguards Agreements with individual non-nuclear weapon states, pursuant to the requirement of the NPT. The public transparency thus generated is limited, inasmuch as IAEA rules do not permit sharing most of the information it develops in its safeguards programs with the world community. Moreover, the 1957 EURATOM Agreement, which predates the NPT and the IAEA, provides for safeguarding of civil NEM in the
countries of the European Community, but EURATOM, like the IAEA, does not reveal the information developed in its safeguards activities. The issue of public openness of transparency deserves careful review, as the release of some information could be helpful in building confidence and laying a foundation for further steps in the control of NEM, while the release of other information could aid potential proliferators and terrorists or create counterproductive political problems
Traditional IAEA Responsibilities and Methods
Traditional IAEA safeguards are designed to detect Significant Quantities of nuclear material with “high confidence” and in a “timely manner.”50 As noted above, the IAEA defines a Significant Quantity as 8 kilograms of plutonium or U-233, or 25 kilograms of U-235 in HEU. “High confidence” is usually taken to mean 90 percent or more probability of detecting diversion of the defined Significant Quantity. The definition of “timely” is based on, but not necessarily identical to, the IAEA’s estimate of the time a proliferant state would need to convert the diverted material into a finished weapon component. For HEU or separated plutonium metal, the IAEA sets this time at 7-10 days; for NEM in forms such as pure oxides or other compounds, mixed compounds, or scrap, the estimate is 1-3 weeks. In reality the “conversion time” could be less or more depending on the amount of advance preparation for weaponization.51
The IAEA audits each country’s records of nuclear material inventories and the changes in these inventories that occur in each relevant facility, and collects data to verify the accuracy of those records. IAEA inspectors measure and estimate amounts of nuclear material, count discrete items such as fuel rods, affix tags and seals to track whether items have been moved or tampered with, and install and monitor video cameras and radiation detectors to track activity around and movement of the relevant items and materials.
|
50 |
See IAEA, IAEA Safeguards Glossary, 2001 Edition (Vienna: International Atomic Energy Agency, 2002). Available as of January 2005, at: http://www-pub.iaea.org/MTCD/publications/PDF/nvs-3-cd/Start.pdf.; Steve Fetter, Verifying Nuclear Disarmament, Occasional Paper 29, Henry L. Stimson Center, Washington, DC, 1996; and Office of Technology Assessment, United States Congress, Nuclear Safeguards and the International Atomic Energy Agency, OTA-ISS-615 (Washington, DC: U.S. Government Printing Office, June 1995). |
|
51 |
See IAEA, IAEA Safeguards Glossary, 2001 Edition (Vienna: International Atomic Energy Agency, 2002). Available as of January 2005, at: http://www-pub.iaea.org/MTCD/publications/PDF/nvs-3-cd/Start.pdf. |
Inspectors are also supposed to compare the design of proliferation-relevant facilities (such as uranium enrichment plants, fuel fabrication plants, and spent-fuel reprocessing plants) with the actual construction in order to verify their capacities and the flows of materials within them, and to evaluate the measurement systems used by the operators. The frequency of inspections depends on the quantity and weapon relevance of the nuclear material.
The traditional approach to IAEA safeguards was negotiated in the early 1970s, when most states believed that nuclear energy would be fundamental to their energy economies, and the non-nuclear weapon states were quite concerned that the requirement to accept IAEA safeguards should not put them at a commercial disadvantage in this key technology, in competition with the weapon states, who faced no such inspection requirement. As a result, the traditional safeguards regime was intentionally designed to be focused almost exclusively on declared nuclear sites, and within those on agreed “strategic points” for monitoring the nuclear material. In traditional safeguards agreements, the IAEA is explicitly required to collect only the minimum information needed to carry out its responsibilities under the agreement. In practice, the pressure from member states that IAEA inspections not be unduly intrusive created a situation in which the IAEA virtually never attempted to use the vague authority it was given in traditional agreements to request special inspections at undeclared sites.
Expanding Responsibilities and Reducing the Mismatch Between Authority and Resources
Whether the tools and authority of the IAEA and its inspectors are adequate in practice to meet its stated obligations has long been questioned by outside analysts and indeed by IAEA officials them-selves.52 New attention was focused on this question when inspections in the aftermath of the 1991 Gulf War confirmed that Iraq had pursued an extensive nuclear weapon program while a non-nuclear weapon state member of the NPT subject to IAEA safeguards. Deficiencies in the IAEA’s operating capabilities were highlighted by the agency’s focus on declared NEM facilities, its failure to exploit even its weak authority to conduct inspections of suspect undeclared facilities, and its inability (under the agency’s
current operating rules) to focus inspection efforts preferentially on states of particular proliferation concern.
As a consequence, the IAEA developed new safeguards measures in its 93+2 program. These measures were incorporated in the voluntary “Additional Protocol” to the agency’s safeguards agreements with individual states to provide added confidence that nuclear material has not been diverted from openly declared civilian activities, and that there are no undeclared hidden nuclear weapons activities. For each state that agrees to the Additional Protocol, these new measures include:53
-
An expanded declaration covering a detailed description of the state’s entire nuclear program (not just the activities involving nuclear materials, which already must be declared under existing IAEA agreements set forth in INFCIRC/153); declarations, including blue-prints, of new facilities before construction begins; information on the import-export of certain equipment and material; and an outline of nuclear fuel cycle plans for the coming 10 years;
-
broader physical access to declared locations and facilities, not restricted to agreed “strategic points” as in INFCIRC/153, and improved explicit access to suspicious undeclared locations, including environmental monitoring for detection of proscribed activities;
-
improved procedures for getting inspectors in and information out, including restrictions on a state’s ability to reject particular inspectors, a requirement that states issue multi-entry visas to inspectors, reductions in the advance notice of inspections that must be provided to the host state, and allowance for direct communication by inspectors to IAEA headquarters or regional offices and for direct transmission of information from surveillance and measurement devices.
For those states that implement the Additional Protocol, the IAEA prepares an overall assessment of the nuclear activities of the state, and attempts to draw conclusions not only as to whether there has been any diversion of NEM from declared facilities—the traditional focus of IAEA safeguards—but also as to whether there
|
53 |
See IAEA, INFCIRC/540 - Model Protocol Additional To The Agreement(S) Between State(S) And The International Atomic Energy Agency For The Application Of Safeguards (Vienna: International Atomic Energy Agency, September 1997). Available as of January 2005, at: http://www.iaea.org/Publications/Documents/Infcircs/. |
are any significant undeclared nuclear activities. Once the IAEA has had time to examine all the relevant information and draw the conclusion that there is no evidence of undeclared activities, it then begins to implement “integrated safeguards,” an approach combining the traditional and new measures with the openness resulting from the new measures, making it possible to reduce the intensity of the older measures (such as the frequency of inspections at particular types of facilities). In the negotiation of the Additional Protocol, a tacit understanding was reached that after an initial pulse of increased expenditure, the new measures should be cost neutral, that is, that in general every dollar spent on the new measures should be matched by a dollar cut from traditional measures. Whether this is wise, and how much reduction in the intensity of traditional measures is justified, remain the subjects of controversy—though the specific integrated safeguards inspection approaches for most types of nuclear facilities are now agreed.
A substantial driver behind this controversy has been the budget picture. From 1986 to 2003, the IAEA was not permitted any real growth in its budget (as part of a more general effort to restrain cost growth of agencies within the U.N. system), even though the number of parties to the NPT increased substantially, the number of Significant Quantities of material under safeguards increased more than threefold, and new expenditures were required to implement the new safeguards approach. The IAEA’s budget for safeguards worldwide, including both its regular budget and extrabudgetary contributions, amounts to roughly $100 million. This amount, which was insufficient for the agency’s original mission, is plainly entirely inadequate now and will become increasingly so in the future. In 2003 the IAEA Board of Governors and General Conference approved a $19.4 million increase in the IAEA’s safeguards budget, to be phased in over four years.54 Nevertheless, in virtually every part of the IAEA’s safeguards and security operations, limited resources remain an important constraint.
Access to information is as important as access to resources. With the advent of the Additional Protocol and the requirement that the IAEA prepare integrated assessments of the nuclear activities of each state, it has been widely accepted that the IAEA will seek information from open sources (such as newspaper accounts) and from member states, including their intelligence agencies. The
amount of information available to the IAEA from intelligence sources remains very limited, however, and increased sharing of information could significantly increase the IAEA’s effectiveness. Similarly, we judge that it would be extremely valuable for the nuclear supplier states to provide information to the IAEA on all approved nuclear or dual-use transfers, all denials of such transfers, and all cases of illegal transfers or attempted transfers, to give the IAEA a complete picture of the procurement activities of NPT parties.
A gap in the IAEA’s authority is the fact that the five nuclear weapon states as defined under the NPT have no obligation to place either their military or their civilian NEM under IAEA safeguards, although they may do so voluntarily. As noted above, voluntary commitments by the United States and Russia to place NEM under IAEA safeguards have so far been very limited and slow to be put into effect. Although the United States has offered to place all of its civilian nuclear energy facilities under IAEA safeguards, the IAEA has understandably chosen not to spend its limited resources safeguarding these U.S. facilities on the grounds that it was highly unlikely a country with so many nuclear weapons and no prohibition on producing additional military NEM would divert civil NEM to make more weapons. British and French civil nuclear facilities are covered by safeguards agreements implemented by EURATOM, and a few civil facilities in these countries are also inspected by the IAEA. As of the end of 2001, the IAEA was applying safeguards to one nuclear power plant and one uranium enrichment plant in China, and one civil NEM storage facility in Russia.55
Considerations and Options Looking Ahead
We believe that broadening and strengthening the IAEA’s safeguards activities is the most urgent and important agenda item in the category of enhancing multilateral transparency. Such an effort could include, as a start, full implementation of the innova-
|
55 |
IAEA, “Annual Report” 2001 (Vienna: International Atomic Energy Agency, 2002). Available as of January 2005, at: http://www.iaea.org/Publications/Reports/Anrep2001/. The IAEA’s voluntary offer agreements with the UK, the United States, France, Russia, and China are described in IAEA INFCIRC document numbers 263, 288, 290, 327, and 369, respectively, available as of January 2005, at: http://www.iaea.org/Publications/Documents/Infcircs/ |
tions and extensions in safeguards practices developed in the IAEA’s 93+2 Program and embodied in the Additional Protocol.56
At present, acceptance of the Additional Protocol is strictly voluntary. Nevertheless, in early 2005 there were 91 signatories to the Additional Protocol and 68 countries in which it was actually in force or being provisionally applied.57 In February 2004 President Bush proposed that the Nuclear Suppliers Group (NSG), whose members have long required that states accept full-scope IAEA safeguards as a condition of supply, also require states to adopt the Additional Protocol as a condition of supply. At the summit of the Group of Eight (G8) industrialized democracies at Sea Island, Georgia, in June 2004, the G8 leaders endorsed this approach. The NSG has not yet reached consensus on such a requirement, but it is to be hoped that they will do so soon.
A further step could be a decision to make the Additional Protocol a mandatory requirement for all states party to the NPT. This would greatly strengthen the IAEA’s effectiveness. Such a decision would probably require action by the U.N. Security Council (which has the power to make law binding on all states to deal with threats to international peace and security), or an agreed interpretation of the NPT, which might be made at the 2005 Review Conference or a subsequent conference. Making the Additional Protocol mandatory could be more politically acceptable to some states concerned with the discriminatory nature of the NPT if it were also mandatory on the nuclear weapon state parties to the NPT, and if it were coupled with mandatory verification of those states’ civil nuclear activities.
Further, the United States and other states that make substantial investments in collection of nuclear-related intelligence could substantially increase the fraction of the information available to them that they provide to the IAEA, putting the information in a form
|
56 |
IAEA, INFCIRC/540 - Model Protocol Additional To The Agreement(S) Between State(S) And The International Atomic Energy Agency For The Application Of Safeguards (Vienna: International Atomic Energy Agency, September 1997). Available as of January 2005, at: http://www.iaea.org/Publications/Documents/Infcircs/. |
|
57 |
See IAEA, “Strengthened Safeguards System: Status of Additional Protocols” (Vienna: International Atomic Energy Agency, June 16, 2004). Available as of January 2005, at: http://www.iaea.org/OurWork/SV/Safeguards/sg_protocol.html. Ninety states have signed the protocol, as has Taiwan, which in IAEA practice does not count as a state. EURATOM is also an institutional party, and all the EURATOM states are now parties. The figure of 68 includes 65 states where the protocol is in force; Taiwan, where it is also in force; and Libya and Iran, where it is being provisionally applied. |
that will not compromise sources and methods, and is designed to support the IAEA’s efforts to develop an integrated picture of the nuclear activities of each non-nuclear weapon state party to the NPT and the Additional Protocol. As noted above, we believe that states should also make a commitment to provide the IAEA information on all nuclear and dual-use exports, denials of exports, and cases of illegal exports or attempted exports.
There is also more to be done to provide the IAEA the resources it needs, including both the need to finance IAEA verification activities in nuclear weapon states, and the IAEA’s ongoing safeguards activities in non-nuclear weapon states. A substantially bigger budget increase will be required if the IAEA is to fulfill the dramatic increase in verification activities and obligations it is now being called upon to implement and do so effectively. At the same time, additional resources would also be needed for the IAEA’s Nuclear Security Fund, including both greater voluntary contributions and consideration of including at least a portion of the IAEA’s nuclear security activities in the regular budget, paid for through mandatory assessments from member states.
The adequacy of the IAEA’s values for “Significant Quantities” of NEM and for what constitutes “timely detection” could be reexamined periodically in light of the probable spread of sophisticated knowledge of nuclear weapon design concepts and fabrication techniques. Regardless of the outcome of such reviews, confidence in monitoring and providing “timely detection” could be increased by expanded application of near-real-time accountancy in the most sensitive facilities, notably enrichment and fuel-reprocessing plants, which could be achieved by using the Internet or satellite uplinks to relay information from sensors inside the plants directly to IAEA headquarters.58
REDUCING NEM STOCKS, FLOWS, AND SITES
The preceding sections have treated the approaches, obstacles, and possibilities for NEM transparency and monitoring in a largely qualitative way, that is, without specific reference to the quantitative measures (total stocks of materials, rates of production and
disposition, numbers of sites) that bear on both the motivation for and the difficulty of transparency and monitoring for these materials. The real and perceived risks of breakout, diversion, and theft at the core of concerns about NEM tend to grow, all else being equal, in various proportions to these quantitative factors. Breakout and diversion concerns rise with total stocks, flows, and potential flows in the form of production capacity; theft concerns rise mainly with number of sites (on the supposition that more sites are more difficult to guard, given limited resources) and with flows that provide opportunities for thieves. In addition, transparency and monitoring tend to be more easily accomplished—again, all else being equal—the lower the number of sites and the smaller the sizes of the stocks and flows. All this motivates interest in reductions in sites, stocks, and flows as one way to reduce real and perceived risks and to ease the tasks of transparency and monitoring. But the processes of reduction in stocks and sites can themselves lead to increased requirements for transparency and monitoring (in response to international demand for reassurance that the reductions are real).
Total stocks of NEM, whether military or civilian, can be reduced from what they would otherwise be by reducing the rate at which these materials are produced and/or by increasing the rate at which they undergo final disposition (blenddown to LEU in the case of HEU and irradiation/burnup or immobilization/isolation in the case of plutonium). The flows of NEM, meaning the frequency and quantity of transfers from one location to another, depend on rates of production, use, and disposal, as well as on choices about technology and about approaches to materials management.
The remainder of this section focuses on the principal processes for reducing NEM stocks, flows, and sites, namely, the conversion of research reactors to run on LEU rather than HEU; choice and management of civil nuclear energy technologies to minimize NEM stocks, flows, and sites; actions to consolidate NEM at fewer sites; cutoff of production of NEM for weapons; and final disposition of HEU and plutonium. In each case, issues relating to transparency and monitoring are identified, the current status discussed, and consideration is given to options looking forward.
Conversion of Research Reactors from HEU
An important form of dispersed HEU stocks could be eliminated altogether if the use of HEU in research reactors were phased out worldwide. As seen in Table 3-2 above, these facilities contain
altogether about 20 metric tons of HEU, much of it only lightly irradiated (hence relatively easy to process to remove the fission products) and some not irradiated at all. Most research reactors that use HEU are capable of operating with redesigned fuel that uses LEU instead. Alternatively, research reactors that are obsolete or unneeded could be shut down—and their HEU removed—as a way to complete the elimination of this “target of opportunity” for diversion to terrorists and proliferant states.
History, Status, and Transparency Issues
Recent estimates indicate that around 135 research reactors worldwide continue to operate with HEU. In addition, a number of HEU-fueled research reactors that have been shut down still have HEU stored at the reactor site.59 The majority of these research reactors were originally supplied by the United States, nearly all of the rest by the Soviet Union/Russia.
For the past 25 years, the U.S. Reduced Enrichment for Research and Test Reactors (RERTR) program has been developing proliferation-resistant low enriched fuels to replace HEU fuel in research reactors and helping U.S. and U.S.-supplied reactors convert. Scores of reactors have successfully converted, many more HEU-fueled reactors have ceased operation, and some tons of (mostly irradiated) HEU fuel have been shipped back to the United States. A new LEU-molybdenum fuel compatible with almost all of the research reactors in the world today has recently been developed by Argonne National Laboratory and is expected to be licensed and available for purchase by around 2010.60 At the same time, however, one new research reactor using HEU has recently been constructed in Germany.
In recent years, the United States has been pursuing several separate programs to reduce security threats posed by HEU at research reactors and related or similar facilities. As just noted, there is a quarter-century-old effort to help U.S.-supplied research reactors convert to LEU fuels, and to develop and implement LEU targets for medical isotope production. This program has begun coop-
|
59 |
See Matthew Bunn, Anthony Wier, and John P. Holdren, Controlling Nuclear Warheads and Materials: A Report Card and Action Plan (Washington, DC: Nuclear Threat Initiative and the Project on Managing the Atom, Harvard University, March 2003), pp. 131-32 and references therein. |
|
60 |
See Matthew Bunn and Anthony Wier, “Converting Research Reactors,” on the Controlling Nuclear Weapons and Materials section of the Nuclear Threat Initiative Web site. Available as of January 2005, at: http://www.nti.org/e_research/cnwm/securing/convert.asp. |
eration with Russia to develop fuels so that Soviet-supplied research reactors can be converted to LEU as well.61 At the same time, the United States is seeking to take back HEU fuel that it supplied from sites around the world, an effort that was restarted in 1996. This is an essential element of the RERTR program, inasmuch as the offer to take the spent fuel off reactor operators’ hands if they would agree to convert to LEU is a key incentive for the operators to convert. Both the conversion effort and the U.S. take-back effort have typically focused on reactors with one megawatt of thermal power or more, which need regular supplies of fresh HEU fuel, but lower-power facilities such as critical assemblies and pulsed power facilities also often have substantial quantities of HEU on-site.
The United States, Russia, and the IAEA have launched a tripartite initiative to take back Soviet-supplied HEU located at vulnerable sites around the world to secure facilities in Russia, where, in most cases, it is to be blended to LEU.62 Separate efforts, sometimes pursued bilaterally and sometimes through the IAEA, have been pursued to upgrade security for HEU at such sites without removing it. In addition, over the years several high-profile removals of HEU from potentially vulnerable facilities have been organized as separate efforts, not directly part of any of these initiatives, including Project Sapphire (the removal of almost 600 kilograms of HEU from Kazakhstan in 1994), Operation Auburn Endeavor (the removal of a few kilograms of fresh and irradiated HEU from the former Soviet republic of Georgia in 1998), and Project Vinca (the removal of 48 kilograms of 80 percent enriched HEU from a facility near Belgrade in the former Yugoslavia in 2002). Following the Vinca effort, in the U.S.-Russian-IAEA initiative, HEU has been removed from Romania, Bulgaria, and Libya.
This approach of addressing the security dangers posed by vulnerable HEU in many separate programs with different manage-
|
61 |
A Soviet RERTR program had started in the 1970s, but was terminated in the course of the collapse of the Soviet Union. See, e.g., Oleg Bukharin, Christopher Ficek, and Michael Roston, “U.S.-Russian Enhanced Research and Test Reactor (RERTR) Cooperation,” Russian-American Nuclear Security Advisory Council, Princeton University, 2003; Alexander Vatulin et al., “Progress of Russian RERTR Program: Development of a new type of fuel element for Russian-built research reactors,” paper presented at the International Conference on Research Reactor Fuel Management (RRFM 2002) Ghent, Belgium, March 17-20, 2002. |
|
62 |
See Matthew Bunn and Anthony Wier, “Converting Research Reactors,” Securing Nuclear Warheads and Materials. Available as of January 2005, at: http://www.nti.org/e_research/cnwm/securing/convert.asp. |
ment and approaches unfortunately left a number of important gaps. As just one of several examples, two-thirds of U.S.-supplied HEU abroad was not covered by the U.S. HEU take-back program as originally structured, and the incentives offered for facilities to send their HEU back to the United States were sufficiently limited that, as of early 2004, half of the material that was covered in the program was not expected to return.63 Recognizing this problem, in May 2004 U.S. Secretary of Energy Spencer Abraham announced the Global Threat Reduction Initiative (GTRI), which consolidates all of these efforts, and is intended to provide a comprehensive approach to securing and removing NEM (and related equipment, as well as radiological material) from small, potentially vulnerable sites around the world.64 The new Department of Energy Office of Global Threat Reduction is in the process of fleshing out the details of the new initiative.
Considerations and Options Looking Ahead
We judge that the problems posed by HEU-fueled research reactors for monitoring related to nuclear arms control, nuclear nonproliferation, and protection against nuclear terrorism would be most effectively addressed by completing as expeditiously as possible the conversion to LEU of all such reactors that are convertible and still worth operating and shutting down the rest. A number of specific steps could be taken in this direction.
As part of wider efforts to consolidate NEM at fewer sites (see below), the HEU from all converted and shut-down research reactors could be removed to centralized sites where its storage can be
|
63 |
See DOE, Office of Inspector General, Audit Report: Recovery of Highly Enriched Uranium Provided to Foreign Countries, DOE/IG-O638 (Washington, DC: Department of Energy, February 2004). Available as of January 2005, at: http://www.fas.org/irp/agency/doe/ig-heu.pdf. For more on the gaps that existed in U.S. efforts as of early 2004 see Matthew Bunn and Anthony Wier, Securing the Bomb: An Agenda for Action (Washington, DC: Nuclear Threat Initiative and the Project on Managing the Atom, Harvard University, May 2004), pp. 58-59. |
|
64 |
For accounts of the GTRI, see Spencer Abraham, remarks to the IAEA, Vienna: May 26, 2004. Available as of January 2005, at: http://www.energy.gov/engine/content.do?PUBLIC_ID=15949&BT_CODE=PR_SPEECHES&TT_CODE=PRESSSPEECH; Spencer Abraham, remarks to the Eisenhower Institute, Washington, DC, June 14, 2004. Available as of January 2005, at: http://www.energy.gov/engine/content.do?PUBLIC_ID=16020&BT_CODE=PR_SPEECHES&TT_CODE=PRESSSPEECH; and U.S. Department of Energy, “Global Threat Reduction Initiative High-lights,” March 26, 2004. Available as of January 2005, at: http://www.energy.gov/engine/doe/files/dynamic/264200491138_Vienna_GTR_Fact%20Sheet_FINAL1_052604%20.pdf. |
more secure and its monitoring (preferably international) can be more reliable than at the dispersed research reactor sites. In this connection, it would be important to ensure that the new GTRI is pursued rapidly, comprehensively, and flexibly, in particular with a focus on providing adequate incentives, targeted to the needs of each facility, for facilities to give up the HEU at their sites. Funding could be increased to speed the availability of the advanced replacement LEU fuel expected to make it possible to convert all remaining research reactors that it makes sense to continue to operate. Take-back efforts for HEU fuel could be accelerated, with new attention to provision of incentives for participation by facilities that do not require fresh fuel or spent fuel management. And the tripartite U.S.-Russia-IAEA initiative for take-back of fuel from Russian-supplied research reactors could be pushed ahead. There is little monitoring difficulty and no security trade-off because the facilities involved are not sensitive from a military standpoint.
In addition to research and medical isotope production reactors, HEU-fueled reactors in submarines, surface warships, and icebreakers could be looked at more closely with respect to the problems their fueling systems and their spent fuel could pose for a more comprehensive regime of controls and monitoring for NEM.65 The case of naval reactors is clearly far more sensitive and difficult than that of research reactors from the standpoint of both performance trade-offs and protection of classified information during monitoring but appears to be manageable on a cooperative basis.
Minimizing NEM Stocks, Flows, and Sites in Civil Nuclear Energy Generation
NEM can play a role in the normal operation of civil nuclear energy systems in two ways: reactor designs that require (or “prefer”) the use of fresh fuel made from NEM and the choice of reprocessing of spent fuel (from a reactor of any design) in order to separate NEM from fission products and other diluents in the spent fuel. The first is a choice about the “front end” of the fuel cycle; the second is a choice about the “back end,” although it usually
entails the recycling of the NEM separated at the back end into fresh fuel for use at the front end.
Today, the use of NEM in the front end of the fuel cycle, other than the NEM recycled from reprocessing, is not a major issue. Only one commercial power reactor in operation uses HEU as its fuel: the BN-600 fast-neutron breeder reactor in Russia. (A small number of smaller, experimental fast-neutron reactors also use HEU in their fuel.) The 20-30 percent enriched uranium fuel required by some breeder reactor designs is a considerably smaller proliferation risk than the 90 percent or 80 percent enriched material often used in research reactors, because of the large amount of HEU needed to constitute a critical mass at these lower enrichments.66 Future fast-neutron reactors, if they are built and deployed, will likely use plutonium or U-233 recovered from spent fuel as their primary fuel, possibly mixed with other actinides and some of the fission products from spent fuel. (Such recycling is discussed in more detail below.) While there continue to be a few advocates for versions of the high-temperature gas-cooled reactor (HTGR) intended to breed U-233 from thorium using fuel enriched to weapon-grade or above),67 it presently appears unlikely that major deployments of power reactors with material posing such a high
|
66 |
Such material would provide useful source material for further enrichment, reducing the amount of separative work required to produce bomb material. Very roughly, ten times less enrichment work is required, if the assay of U-235 in the tails is the same. Specifically, 19-21 SWU/Kg is required to produce 93 percent product using 20 percent feed, compared with 180 to 240 SWU/Kg for natural feed, depending on tails assay (0.4 to 0.2 percent). But the difference is much larger if those enriching 20 percent feed allow significant amounts of the U-235 to be lost to the tails: only 6.98 SWU/kg are required to produce 93 percent product using 20 percent feed with a tails assay of 15 percent. Much the same is true of typical LEU for a light-water reactor, however: 62 SWU/kg are required to produce 93 percent product using 4.5 percent feed, with a tails assay of 3 percent. |
|
67 |
In some concepts a reactor intended to make U-233 from thorium would contain fuel with very little U-238, so as to avoid making plutonium in the fuel. This is not essential, however. In an HTGR with 20 percent enriched uranium fuel mixed with thorium, plutonium production would be reduced to 30 kilograms per year for 1 gigawatt-electric of capacity operating at 90 percent capacity factor, and the U-233 produced would be isotopically denatured by the U-238 in the fuel. See, for example, Harold A. Feiveson, Frank von Hippel, and Robert H. Williams, “Fission Power: An Evolutionary Strategy,” Science 203 (1979), pp. 330-337. Similarly, a variety of thorium-fuel designs are now being pursued (including for light-water reactors) in which thorium, U-238, and U-235 are all present in the fuel; the result, in some of these designs, is a modest extension of the amount of energy that can be generated from available uranium resources, combined with substantially lower production of substantially lower-quality plutonium in the spent fuel, than is typical for the spent fuel from light-water reactors operating with LEU fuel. A fast-reactor core is one that can sustain a chain reaction driven by fast neutrons, for which the enrichment requirement is the same as the minimum for a weapon, that is, around 20 percent U-235 or 12 percent U-233. (See Appendix A.) |
proliferation hazard in their fresh fuel will occur in the foreseeable future.
The more substantial proliferation liability associated with the “front end” of the nuclear fuel cycle is not the direct use of NEM but the use of facilities that could be used to produce NEM. The same enrichment technologies and facilities used to make LEU for light-water reactors—the dominant reactor type in the world today—can be used to produce HEU for weapons. Indeed, an enrichment facility using gas centrifuges—the most cost-effective of the enrichment technologies commercially deployed today—can be reconfigured from LEU production to HEU production very quickly. The case of Iran has focused international attention on the possibility that a country could build such a facility while remaining within the NPT, then withdraw from the NPT and quickly begin producing HEU for nuclear weapons. Moreover, centrifuge enrichment plants large enough to produce a bomb’s worth of HEU each year could potentially be small and difficult for either intelligence systems or inspectors to find. The recent revelation that a global black-market network centered on Pakistan’s A.Q. Khan was peddling centrifuge technology to Iran, North Korea, Libya, and possibly others, and had initially acquired centrifuge designs and expertise illegally from Europe, highlights the proliferation danger posed by the spread of centrifuge technologies.
The use of NEM is a major issue at the “back end” of the nuclear fuel cycle. With currently available reprocessing and recycling approaches, plutonium or U-233 is completely separated from accompanying fission products before being incorporated into fresh fuel. As indicated above, the limited use of plutonium recycle in a small fraction of the world’s current nuclear electricity generation has already led to significant plutonium stocks and flows in the civilian sector. If breeding and recycling were more heavily used, the associated stocks and flows of separated, directly weapon-usable material could become truly immense.68 This poses challenges for international efforts to ensure against diversion and theft of any of this material, and as with enrichment plants, the possession of a reprocessing plant built while a party to the NPT would allow a state to withdraw from the NPT and rapidly begin separating NEM for weapons use.
Three general approaches could be used to avoid or reduce the proliferation liabilities and monitoring challenges associated with the use of NEM recycling and breeder reactors:
-
postponing, minimizing, or altogether avoiding the use of breeding and recycling;69
-
attempting to develop advanced breeder-reactor fuel cycles in which the recycled plutonium or U-233 would never be completely separated from fission products (meaning that these materials would only become weapon usable if they underwent an additional reprocessing step); and
-
utilizing the concept of carefully guarded and internationally owned, managed, and monitored nuclear energy complexes, in which enrichment, fuel fabrication, power generation, and fuel reprocessing would all take place within a single site from which no NEM would ever emerge.
Clearly, combinations of these approaches can be envisioned, including postponing the use of breeding and recycling until they are economically beneficial and further development makes these technologies more proliferation resistant and/or until the internationalized nuclear-energy-complex approach is accepted as a requirement for their use.
History, Status, and Transparency Issues
After an extended internal and external debate about the benefits versus the liabilities of plutonium recycle and breeder reactors, the Ford Administration announced in 1976—and the Carter Administration subsequently strongly reiterated—that it was U.S. policy to refrain indefinitely from fuel reprocessing for commercial recycle of plutonium and from deployment of breeder reactors for electric power production and, further, that the United States would try to persuade other countries to refrain from commercial
|
69 |
Postponing or avoiding recycling has the liability of making the requirements for uranium and for uranium enrichment services larger than they would be if recycling were used. How soon and to what extent the liability of increased uranium needs would become a significant one depends both on the rate of growth of nuclear electricity generation worldwide and on the extent to which relatively low-cost uranium resources prove extendable by some combination of new discoveries and advanced technologies for exploiting low-grade uranium ores, including ordinary granite (10-20 parts per million U) and sea water (3 parts per billion U). The increased enrichment requirement associated with postponing or avoiding plutonium recycle could aggravate the proliferation risks associated with enrichment, unless it is successfully internationalized. See, e.g., Richard L. Garwin and Georges Charpak, Megawatts and Megatons: The Future of Nuclear Power and Nuclear Weapons (Chicago: The University of Chicago Press, 2002). and John Deutch, Ernie Moniz, et al., The Future of Nuclear Power: An Interdisciplinary MIT Study (Cambridge, MA: Massachusetts Institute of Technology, 2003). Available as of January 2005, at: http://web.mit.edu/nuclearpower/. |
use of these technologies. The rationale for this position was precisely the desire to minimize commercial stocks and flows of plutonium, on nonproliferation grounds; furthermore, analyses indicated that, given the low cost availability of uranium, there would be no economic benefit for the foreseeable future from recycling or breeding in comparison with once-through use of LEU fuel in existing commercial reactor types.70
This policy was controversial both within the United States, where many in the nuclear energy industry felt that moving to plutonium recycle and breeding was the technically logical progression for nuclear energy technology and should be pursued, and elsewhere, particularly in France, Belgium, Germany, Switzerland, the United Kingdom, Japan, and the Soviet Union, which either were starting to practice breeding and/or recycling or were committed to doing so in the near future. A concerted effort by the Carter Administration to develop international support for a moratorium on commercial recycling of plutonium and breeder reactors failed to rally hoped-for support. Early in the Reagan Administration, the Carter policy was reversed, although this had essentially no practical effect because U.S. electric utilities had by then concluded that these technologies were indeed uneconomic given prevailing and foreseeable uranium prices. The continuing de facto U.S. moratorium on reprocessing and commercialization of breeder reactors initially had no discernible effect on the enthusiasm for these technologies, particularly in France, Japan, and the Soviet Union.
In the 1990s the Clinton Administration restored restraint on reprocessing and breeding in nuclear energy systems as stated U.S. policy, but retreated from the Carter version by saying that the United States would not actively discourage its allies from using these technologies. The May 2001 national energy policy document of the George W. Bush Administration called for the United States to continue to “discourage the accumulation of separated plutonium” in civil nuclear fuel cycles, while proposing pursuit of “fuel conditioning” technologies such as pyroprocessing that could reduce waste streams and increase proliferation resistance;71 the
Bush document also proposed collaborative research with other countries on approaches to reprocessing superior to those available today.72
At this writing, the United Kingdom and France are continuing commercial reprocessing of plutonium not only from their own spent fuel but also under commercial contracts with Japan, Germany, Belgium, and Switzerland, among others. Few additional foreign reprocessing contracts are available, however. The United Kingdom has announced that its Thermal Oxide Reprocessing Plant (THORP) will close in 2012; its Magnox reprocessing plant will shut down when the aging Magnox reactors are closed.73 French reprocessing is currently planned to continue, focusing on reprocessing domestic spent fuel. Serious technical problems, however, are plaguing the French breeder program.
While reprocessing also continues in Russia, with the collapse of the Soviet Union, Russia drastically slowed its formerly ambitious breeder reactor program. And at this writing, the one potential medium-size “breeder,” the BN-600, is actually operating as a “burner” reactor and work on a larger one is proceeding very slowly. In addition, soaring cost estimates are beginning to generate internal debate in Japan about the wisdom of that country’s previous strong commitment to reprocessing and breeding and Germany has lost interest in both breeding and recycling, en route to questioning the future of nuclear energy in Germany altogether.
New research and development initiatives such as the U.S.-led “Generation IV” effort have focused renewed attention on advanced breeder designs, but most of these focus on systems where the recycled plutonium or U-233 always remains mixed with other actinides and/or some fission products, to increase proliferation resistance. Still, no international consensus has emerged on whether avoiding or minimizing commercial traffic in separated plutonium would be an essential element of any suitably compre-
|
|
National Research Council Electrometallurgical Techniques for DOE Spent Fuel Treatment: Final Report (Washington, DC: National Academies Press, 2000), p. 18. |
hensive approach to nuclear arms control, nuclear nonproliferation, and nuclear counterterrorism.
Considerations and Options Looking Ahead
There is no question that the use of NEM and technologies for producing NEM in civil nuclear energy systems greatly increases the problems associated with the control and monitoring of NEM and thereby increases the risk of nuclear proliferation and nuclear terrorism, as well as the potential for future breakout in any future regime that entails much smaller nuclear arsenals. Indeed, the challenges posed by unrestrained use of NEM in civil nuclear energy to adequate defenses against nuclear proliferation and nuclear terrorism and to adequate verification of a more stringent and comprehensive arms control regime, were one desired, have been asserted by some to be virtually insurmountable.74
We believe that it is therefore important to revisit the question of whether and how the use of NEM in civil nuclear energy should be restrained, both for immediate purposes of nuclear nonproliferation and nuclear terrorism prevention and for the longer-term possibility of a more comprehensive nuclear arms limitation regime. Since the Ford and Carter administrations’ announcements in the mid-1970s that the United States would refrain from commercial reprocessing of spent fuel, plutonium recycle, and deployment of breeder reactors, the proliferation and terrorism dangers from reprocessing and recycle of NEM have become even clearer than they were then. It has also become clearer that pushing forward with breeding and/or reprocessing/recycle any time soon will incur significant economic penalties, and greatly increased public controversy over the future of nuclear energy generation, thus reducing rather than enhancing the prospects for an expanded contribution from nuclear energy to meeting society’s pressing energy needs.75
In these circumstances, we conclude that the United States should consider what steps could be taken to implement the stated Bush Administration policy of continuing to discourage the accumulation of separated plutonium in civil fuel cycles. One approach
was envisioned by President Bush in his February 2004 speech on nuclear nonproliferation, which called for ceasing the export of enrichment and reprocessing technologies to states that did not already possess them, and offering credible guarantees of fuel cycle supply to any state that agreed not to have enrichment and reprocessing facilities of its own.76
Measures that could be considered that would go beyond those called for by President Bush would include the U.S. government refraining from, and prohibiting U.S. firms from engaging in, any form of multilateral nuclear-energy cooperation or assistance likely to contribute to advancing commercial breeding or commercial reprocessing in any country. The U.S. effort could also press, and where practicable offer incentives to, Russia, France, and Japan to declare moratoria on civil reprocessing, with some of the plutonium fuel fabrication capacity that would otherwise be made surplus by this step then being turned to the task of fabricating fuel from surplus civilian and weapon plutonium (see the discussion of “disposition” below).
Specifically, an agreement with Russia on a joint 20-year moratorium on further civil plutonium separation coupled with a joint R&D program on more proliferation-resistant approaches to reprocessing and recycling could be a major contribution in buying time to resolve this problem. Such an agreement was almost completed at the end of the Clinton Administration. Under such an approach, research on advanced approaches to breeding and reprocessing that might be able to reduce the vulnerability of such operations to breakout (of nuclear weapon states from agreed limits) and to proliferation and theft—and that might be able to lower costs, improve safety, or bring waste management advantages—could be continued. For at least the next two decades, however, such research might be constrained not to progress to large-scale development (which would entail processing and handling significant quantities of NEM), and it might be confined as far as possible to nuclear weapon states, so as to minimize diffusion of exper-
|
76 |
President George W. Bush, remarks to the National Defense University, February 11, 2004, available as of January 2005, at: http://www.whitehouse.gov/news/releases/2004/02/20040211-4.html. For a useful discussion of how such fuel cycle guarantees might be implemented, from some one with experience both at the IAEA and in leading major commercial fuel cycle activities, see Pierre Goldschmidt, “The Proliferation Challenge of the Nuclear Fuel Cycle in Non-Nuclear Weapon States,” remarks to the Institut Français des Relations Internationales, April 26, 2004, available as of January 2005, at: http://www.iaea.org/NewsCenter/Statements/DDGs/2004/goldschmidt26042004.html. |
tise and experience with reprocessing and plutonium-handling technologies that would be of value to potential proliferators.77 The rationale for not proceeding to large-scale development for at least two decades includes both the high desirability of minimizing reprocessing-linked proliferation and terrorism hazards in this critical time frame and the near certainty that the availability of relatively low-cost uranium would prove sufficient to support any plausible rate of growth of nuclear energy worldwide through at least 2050.
As for full-scale development and deployment of civilian nuclear reactor types that require the use of HEU in their fresh fuel, this too could be postponed unless and until there is a strong case that such reactors and their fuel cycles can offer a combination of economic, safety, waste management, and back-end-of-fuel-cycle nonproliferation advantages sufficient to offset the nuclear proliferation, nuclear terrorism, arms limitation breakout, and NEM-monitoring liabilities of the “front end” use of HEU.
Serious consideration should also be given to a more farreaching solution proposed in the past and recently advanced again by IAEA Director General Mohamed ElBaradei to place all production of NEM under direct international control.78 This would place all uranium enrichment as well as all plutonium separation and plutonium fuel fabrication (whether this uses reprocessed civil plutonium or surplus military plutonium) under multilateral management and control, not just under IAEA safeguards. This would ensure not only full transparency and accountability but also that physical protection meets a high and internationally agreed standard, and that individual states could not convert their facilities to military production by withdrawing from the NPT. Under this arrangement participants would be guaranteed access to fuel containing LEU, which would be returned to the international entity after irradiation.
If the principle could be established that there would be no new facilities capable of producing NEM in any state, including the nuclear weapon states, except under international control, this would
|
77 |
See John Deutch, Ernie Moniz, et al., The Future of Nuclear Power: An Interdisciplinary MIT Study (Cambridge, MA: Massachusetts Institute of Technology, 2003). Available as of January 2005, at: http://web.mit.edu/nuclearpower/. The authors argue that a re-evaluation in 2020 would come in ample time to allow for adequate development and deployment of breeding and reprocessing options by 2050, if that seemed appropriate. |
|
78 |
Mohamed ElBaradei, “Toward a Safer World,” The Economist (October 16, 2003), p. 47. |
be a major step toward eliminating a large weakness in the nonproliferation regime, which currently allows participants to come right to the edge of a nuclear weapon capability while legally remaining within the regime.
In short, constraints could be introduced on utilization of NEM in the fuel cycle at no economic sacrifice for some time to come while efforts are pursued to deal with the threats these developments entail. If agreement cannot be reached on implementing these constraints, MPC&A procedures could be introduced to ameliorate the threat—at significantly increased costs and uncertainty as to effectiveness.
Other Actions to Consolidate NEM at Fewer Sites
Reducing the number of, or eliminating entirely, research reactors that use HEU and postponing, minimizing, or avoiding nuclear energy technologies that entail the use of HEU and/or separated plutonium at dispersed sites are two ways to reduce the number of locations where these materials must be monitored and guarded, as well as to reduce the amount of transportation of the materials among sites. Reducing the number of R&D facilities, civil and military, that work with appreciable quantities of either HEU or plutonium is another way to do this.
Some of these actions would reduce not only the number of sites but the total quantity of NEM as well. But it is worth emphasizing that actions that merely consolidate NEM in fewer sites without reducing the total quantity (as nuclear energy complexes with large numbers of reactors would do) are also worthwhile. Consolidating the national inventories of civil and military NEM in fewer (and better protected and monitored) facilities is the most straightforward example of this. Consolidating civil NEM across countries, into multinationally monitored NEM “banks,” would be a more ambitious and highly worthwhile approach.
Consolidation brings benefits for NEM security as well as for NEM monitoring because it is technically easier and more economical to thoroughly protect and monitor a few facilities, each with a lot of material, than to protect and monitor many facilities, each with much smaller amounts. In addition, insofar as most corrupt guards or officials and criminal groups probably would not aspire to steal more than a few tens or at most a few hundreds of kilograms of NEM in one attempt (because the difficulty of transportation would complicate the task and increase the chance of be-
ing apprehended), the theft risk does not increase in proportion to the amount of material stored in one place.79
History, Status, and Transparency Issues
Both in the United States and in Russia, the post-Cold War downsizing of the two countries’ nuclear weapon production complexes has led to a reduction of the number of facilities processing and storing military NEM, though much remains to be done in both cases. In Russia, for example, scores of former nuclear weapon storage sites have been closed down, two of the four weapon assembly and disassembly facilities have been closed, one of the two facilities for processing NEM into weapon components has been closed, most of the facilities that once stored fresh HEU naval fuel have been closed (and the others equipped with effective security and accounting systems), the enrichment plants no longer produce HEU, and 10 of the 13 plutonium production reactors (all of which also used 90 percent enriched HEU “spike” fuel) have been closed. Currently, plutonium from the dismantling of surplus nuclear weapons (which is taking place at four sites) is to be shipped to the new storage facility at Mayak, which at this writing is completed but not yet in operation. HEU from dismantled Russian nuclear weapons is being stored at the four dismantling sites, and is then transported thousands of kilometers for processing to LEU as part of the U.S.-Russian HEU Purchase Agreement. HEU for fueling nuclear-powered icebreakers and naval vessels continues to be fabricated, shipped over thousands of kilometers, and used in quantities comparable to or larger than all HEU used by research reactors worldwide.
In the United States, scores of buildings that once held NEM have had all of the NEM removed, as has the Rocky Flats site, where plutonium weapons components were fabricated (resulting in substantial savings in annual security costs). Currently, all dismantling takes place at the Pantex facility near Amarillo, Texas, and the resulting plutonium is stored in bunkers at the same site. The HEU from dismantled U.S. nuclear weapons is stored at Oak Ridge. Excess HEU from there and from other sites is being shipped to processing facilities for blending to LEU. Very large quantities of HEU exist, however, which have been reserved for
use as naval fuel, rather than being declared excess. As in Russia, this material is processed, fabricated, shipped, and used on a very large scale every year.
Elsewhere in the world, the pace of consolidating NEM sites has been quite slow. With the successes in removing Soviet-supplied HEU from the former Yugoslavia, Romania, Bulgaria, and Libya in 2002-2004, and the announcement of the Global Threat Reduction Initiative (GTRI) in 2004, it is to be hoped that progress will accelerate. It will be important for the implementers of the GTRI to ensure that all nuclear materials that pose substantial proliferation threats are covered, and not just HEU research reactor fuel. As just one example, a research center at Kharkov, in Ukraine, holds a substantial quantity of 90 percent enriched HEU in the form of oxide powder, though it has no research reactor.80
Considerations and Options Looking Ahead
We believe that substantial further consolidation of NEM in the nuclear weapon states would be desirable, particularly in Russia. Many Russian facilities still have NEM in many different buildings on-site, though in the context of U.S.-Russian MPC&A cooperation, work is underway to consolidate the materials at these sites into central storage facilities. Only very modest progress is being made in removing materials entirely from sites within Russia. To accelerate that effort, the United States could work with the Russian government to:
-
convince the Russian government to draw up a plan for consolidation of the number of buildings and sites where NEM and nuclear weapons exist, offering assistance in preparing and implementing such a plan (as has been discussed between U.S. and Russian experts);
-
change the incentives that currently make most Russian sites eager to retain their NEM, and to structure a set of incentives to encourage facilities to give up their NEM;
-
begin converting HEU-fueled research reactors within Russia (not only Soviet-supplied facilities outside of Russia) to use LEU fuels, including critical assemblies and pulse power facilities with significant quantities of HEU; and
-
develop and implement LEU fuels for Russia’s nuclear-powered icebreakers and ultimately for naval vessels as well.
The partial measures that have been taken to date under this heading mainly reflect a post-Cold War interest in reducing the vulnerability of NEM stocks to theft by criminals, terrorists, and agents of proliferant states. If a more comprehensive approach to nuclear arms control were adopted encompassing NEM, an expanded set of measures for reducing vulnerable stocks and flows of those materials would become increasingly important.
Removing NEM entirely from the most vulnerable sites is generally a superior strategy to trying to upgrade security for it, in place, at its current locations. It is germane here that some sites have little prospect of the continuing revenue streams that would be needed to maintain adequate security over the long run, even assuming that initial assistance is provided to put a suitable security system in place. Some vulnerable facilities are in locations that are inherently difficult to secure, either because the environment does not allow for an adequate security infrastructure or because the location entails the possibility of threats too big for any plausible security system to handle, such as civil war.
Cutoff of Production of NEM for Weapons
In a world awash in NEM, we believe that a permanent ban on the production of NEM for weapons could only be a benefit for nuclear theft prevention, nonproliferation, and arms control. It could also ultimately ease the overall problem of monitoring a more comprehensive nuclear arms limitation regime, if one materialized, even as it created some specific new challenges in connection with monitoring the production halt itself.
Decisions to halt production of NEM for weapons have been made unilaterally by some states in the past and may be made by others in this way in the future; such decisions may also be part of a multilateral or international agreement to refrain from such production. At present, all non-nuclear weapon states party to the NPT are subject to a verified ban on the production of NEM for weapons. The five de jure nuclear weapon states party to the NPT (the United States, Russia, the United Kingdom, France, and China) are not covered by this ban, but they are no longer producing NEM for weapons. There is, however, no formal commitment by the de jure nuclear weapon states to make these halts permanent. Thus the utility of such an agreement depends on the willingness of the
nonmember states of the NPT to participate without having to join the NPT. In sum, all states are currently either covered by a ban on producing NEM for weapons or are voluntarily abiding by it except the de facto nuclear weapon states (India, Israel, and Pakistan), which are not members of the NPT, and North Korea since its recent withdrawal from the NPT.
Unilateral Initiatives
To the extent that production halts of NEM for weapons are unilateral and not subject to verification through bilateral, multilateral, or IAEA inspections, confirmation that declared cutoffs are in fact being honored would depend primarily on information gathered by National Technical Means, which are discussed in Chapter 4. In general, large scale plutonium-production reactors and fuel reprocessing plants for separating plutonium from the fuel of such reactors are relatively conspicuous undertakings. Their existence would be difficult to conceal over any extended period of time. Without inspections, however, it would be difficult if not impossible, to determine whether any of the plutonium from reprocessing plants was destined for weapons. Similarly, gaseous diffusion uranium enrichment plants would be very difficult to conceal for an extended period of time, but the existence and operation of small gas centrifuge or laser enrichment plants would be more difficult to detect—and once detected, it would be difficult to confirm without inspection whether such plants were operating or not. Without inspections, moreover, determination of whether the output of an operating enrichment plant was LEU or HEU would also be difficult if not impossible, as would be the determination of whether any HEU produced was destined for weapons, tritium production reactors, naval reactors, research reactors, or civil power reactors of types that use HEU.
U.S.-funded efforts to end the production of separated plutonium from the three remaining Russian plutonium producing reactors in Siberia began with attempts to arrange alternate sources of heat and power for their regions so that the reactors could be shut down, then shifted to a focus on modifying the fuel for the reactors so that it would produce little plutonium and would not need to be reprocessed for technical reasons as is currently the case, and now have shifted back to replacing the reactors with other sources of heat and electricity. Although the United States and Russia originally agreed in 1994 that the reactors would be shut down by 2000, they are now expected to operate until some time between 2008 and 2011, with the separated plutonium subject to U S. monitoring
to assure that it has not been diverted from storage to weapons.81 During 2003, the United States let contracts to the U.S. firms that are to oversee construction of the replacement power supplies, and reached agreement with Russia on access and other implementation matters.82
Fissile Material Cutoff Treaty
Since 1978 the U.N. General Assembly has supported resolutions calling for a stand-alone international convention calling for a cutoff of production of NEM for weapons by all states. But precisely what would be prohibited and what would be permitted under such a cutoff, and what would be monitored in order to verify it, as well as whether such a pact should be linked to other arms control issues, remain contentious questions.
The prospects for an international Fissile Material Cutoff Treaty (FMCT) formally prohibiting the production of NEM for weapons by all states appeared to improve when in 1993 the Clinton Administration reversed the U.S. position from opposition to active support for a comprehensive international cutoff agreement, and a resolution adopted by consensus in the U.N. General Assembly called for negotiation of a “non-discriminatory multilateral and internationally and effectively verifiable treaty banning the production of fissile material for nuclear weapons or other nuclear explosive devices.”83 In 1995, following consultations with states participating in the Geneva-based Conference on Disarmament (CD), the conference agreed to begin negotiations on an FMCT. But that mandate for negotiation expired with the end of that year's conference session and has been renewed only once since then, for three weeks in 1998. Despite repeated calls from the UN General Assembly and NPT review conferences to pursue a cutoff treaty, negotiations have not resumed because the CD operates on the basis of consensus and a few states have been able to block any further negotiations on a cutoff because of disagreements about its scope
|
81 |
U.S. Department of Energy, FY2004 Detailed Budget Justification–Defense Nuclear Nonproliferation (Washington, DC: Department of Energy, February 2003), p. 713. |
|
82 |
See, for example, the brief discussion on the official Web site of the Department of Energy's Eliminating Weapon-Grade Plutonium Production (EWGP) program, available as of January 2005, at: http://www.nnsa.doe.gov/na-20/ewgpp.shtml. |
|
83 |
United Nations General Assembly, Prohibition of the Production of Fissile Materials for Weapons or Other Nuclear Explosive Devices, UNGA 48/75L, December 16, 1993. Available as of January 2005, at: http://www.acronym.org.uk/fissban/fmctdesc.htm#one. |
and purpose and a desire to link it to consideration of other issues, such as a ban on the weaponization of space.84
In 2003 China softened its position linking the start of negotiations to parallel discussions on preventing an arms race in outer space, but at the same time the Bush Administration launched an extended review of U.S. policy supporting a fissile material cutoff. In July 2004 Jackie Sanders, the U.S. Ambassador to the CD, announced that the review “raised serious concerns that realistic, effective verification of an FMCT is not achievable.”85 A State Department policy paper explained the change in the U.S. position further: “Effective verification of an FMCT would require an inspection regime so extensive that it could compromise key signatories’ core national security interests and so costly that many countries will be hesitant to accept it. Moreover, we have concluded that, even with extensive verification measures, we will not have high confidence in our ability to monitor compliance with an FMCT.”86
Given the fact that there is now a de jure cutoff of NEM production for weapons for all non-nuclear weapons states party to the NPT and a de facto moratorium on such production by the five nuclear weapon states party to that treaty, the immediate impact of a comprehensive FMCT would be on the four nonmembers of the NPT (India, Israel, Pakistan, and North Korea), which are still, in fact, producing NEM for weapons. The issue of resumption of the FMCT negotiations and in particular the problem of inclusion of these four states is not a technical but a political problem beyond the scope of this study.
Considerations and Options Looking Ahead
We judge that immediately available steps that the United States might take toward a more complete and durable halt to production of NEM for weapons—and toward transparency and verification measures adequate to support this—include the following:
|
84 |
See, for example, U.S. Department of State, Fissile Material Cutoff Treaty (Washington, DC: Department of State, June 29, 1999). Available as of January 2005, at: http://usinfo.state.gov/topical/pol/arms/stories/fsmbckgr.htm and Hui Zhang, “A Chinese View on a Fissile Material Cut-off Treaty,” Journal of Nuclear Materials Management 30 (4) (2002). |
|
85 |
“U.S. Proposals to the Conference on Disarmament,” Jackie W. Sanders; Permanent Representative to the Conference on Disarmament and Special Representative of the President for the Nonproliferation of Nuclear Weapons. Remarks to Conference on Disarmament Geneva, Switzerland July 29, 2004. Available as of January 2005, at: http://www.state.gov/t/ac/rls/rm/2004/34929.htm. |
|
86 |
“Fissile Material Cut-off Treaty Policy,” Department of State Press Release, July 29, 2004. |
-
Completion of the program to replace the heat and power from Russia’s three remaining plutonium production reactors so that they can be shut down;
-
Agreement and implementation of monitoring measures to confirm U.S. and Russian statements that these two countries are no longer producing HEU;
-
Conduct of joint U.S.-Russian demonstrations to verify that older reprocessing plants are not separating Pu for weapons; and
-
Pursuit a truly international moratorium on the production of NEM for weapons as a precursor to a verifiable international treaty banning such production permanently.
The verification measures for a FMCT would clearly need to include declarations for all reprocessing and enrichment facilities, whether currently operational or not, and for all other facilities that store or process NEM subject to the treaty. There would presumably also be a need for declarations of all facilities that store or handle preexisting NEM not addressed by the cutoff, in order to deal with the problem of discriminating preexisting material from new production.87
It would seem simplest for the monitoring procedures under an FMCT to be coincident, in the case of non-nuclear weapon states, with those currently applied by the IAEA under the Additional Protocol, and for new or amended agreements with nuclear weapon states to adhere as closely as possible to the same approaches, with only such modifications as required to address special circumstances of those states such as dual-purpose facilities and facilities not designed to accommodate standard IAEA procedures.88
Technical issues that will need to be addressed in a monitoring system for an FMCT include how to monitor older, nuclear-weapon-state reprocessing and enrichment plants and how to verify quantities and composition of naval fuel using HEU produced after an FMCT is concluded (assuming that such production has not been banned) without compromising classified information on fuel composition and design. These are manageable problems on which the United States and Russia could begin working together, and with the IAEA, on how best to solve them. By far the most difficult issue, however, will be the political problem of including the four non-NPT members (India, Israel, Pakistan, and North Korea) in such an agreement, since it would primarily affect them at this time.
Final Disposition of NEM
Final disposition covers reducing surplus stocks of NEM by putting them in locations from which they would be very difficult or impossible to recover, or by mixing them with contaminants that make them unusable for weaponry and from which they can only be separated again with great difficulty, or by a combination of these means. Final disposition reduces NEM stocks over time to below what they would otherwise be and eases the task of monitoring what remains. But the transport and processing of NEM associated with accomplishing final disposition will in themselves create opportunities for theft or diversion and difficulties for monitoring that may be more serious during the period when disposition is taking place than those associated with simple guarded storage of the NEM, and they would require intense attention to ensuring that effective security and monitoring measures are in place throughout the process.
Concepts and Technologies for Final Disposition
A study conducted by the Committee on International Security and Arms Control (CISAC) from 1992 to 1995 at the request of the U.S. government addressed the possibilities for disposition of sur-
|
|
INFCIRC/540 - Model Protocol Additional To The Agreement(S) Between State(S) And The International Atomic Energy Agency For The Application Of Safeguards (Vienna: International Atomic Energy Agency, October 1993); all available as of January 2005, at: http://www.iaea.org/Publications/Documents/Infcircs/. |
plus military NEM in considerable detail.89 The CISAC reports recommended that the United States and Russia pursue long-term disposition options that (a) minimize the time during which this material is stored in forms readily usable for nuclear weapons; (b) preserve material safeguards and security during the disposition process, seeking to maintain the same high standards of security and accounting applied to stored nuclear weapons; (c) result in a form from which the HEU would be as difficult to recover for weapons use as from commercial LEU, and the plutonium would be as difficult to recover for weapons use as the much larger and growing quantity of plutonium in commercial spent fuel (the “spent fuel standard”); and (d) meet high standards of protection for public and worker health and the environment.
Disposition of HEU
In the case of HEU, achieving these goals is technically straightforward. Highly enriched uranium can be blended with natural uranium—or with the depleted-in-U-235 “tails” from previous uranium enrichment or very low enriched uranium for technical reasons—to produce proliferation-resistant LEU, which is a valuable commercial fuel. This was the basis of the “HEU deal” concluded between the United States and Russia in the early 1990s, as well as the U.S. decision to undertake a similar blending process for most of its own stockpile of excess HEU.
At two of the three Russian facilities where the material is blended down under the HEU Purchase Agreement, the United States conducts continuous automated monitoring of the three pipes in the Y joint where the blending occurs; one carrying 90 percent enriched uranium hexafluoride, one carrying 1.5 percent enriched uranium hexafluoride used to blend down the HEU, and the pipe carrying the merged blend, with about 4 percent enrichment. (Slightly enriched material rather than natural or depleted uranium is being used for the blending to further dilute undesirable isotopes in the HEU, such as U-234 and U-236.) Installation of monitoring at the third facility was scheduled for late 2004.
In order to establish some level of confidence that the HEU indeed comes from dismantled weapons as required and not reserve stocks of HEU, the United States is allowed several visits each year to the facilities where HEU metal weapon components are cut into metal shavings and converted to oxide. During these visits, the United States has the opportunity to observe rough measurements of the U-235 enrichment of the weapons components in containers and of the resulting metal shavings and oxide in containers, to tag and seal containers being readied for shipment to the blending facility, and to review records of these activities that take place when U.S. inspectors are not present. Similarly, Russian inspectors have the right to conduct monitoring at the U.S. enrichment facility where the LEU is received and processed and at the U.S. fabrication facilities where the material is fabricated into reactor fuel.90
Disposition of Plutonium
Disposition of plutonium poses much more difficult technical challenges. Because all isotopes of plutonium are weapons usable, plutonium cannot be blended isotopically to an adequately proliferation resistant form in the way that HEU can. Given the current worldwide supply of cheap uranium and the high cost of fabricating reactor fuel that contains plutonium, the use of even “free” plutonium as fuel in reactors is uneconomic now and likely to remain so for at least the next few decades. Thus, all of the options for disposition of surplus weapon plutonium, including those that use the plutonium as fuel in civilian reactors, will require substantial investments. There is no disposition option that will “make money.”
The 1992-1995 CISAC study examined all plausible identified options for plutonium disposition, including placing the plutonium at the bottom of deep (several kilometers) boreholes in solid rock, burying it in special zones on the deep ocean floor, and launching it into the sun or out of the solar system on rockets. The study concluded that while all plutonium disposition options have draw-backs, the two least problematic options for achieving the four aims listed above for disposition of NEM are:
-
fabrication of the plutonium into mixed oxide fuel (a mixture of plutonium dioxide and uranium dioxide, termed
|
90 |
See, for example, Matthew Bunn, “Highly Enriched Uranium Transparency,” Monitoring Stockpiles. Available as of January 2005, at: http://www.nti.org/e_research/cnwm/monitoring/uranium.asp and references cited therein. The official Web Site of the HEU Transparency Implementation program is available as of January 2005, at: http://www.nnsa.doe.gov/na-20/heu_trans.shtml. |
-
“MOX”) for use on a “once through” basis in a limited number of civilian power reactors of currently operating types (albeit possibly with some modifications to increase the achievable plutonium loading per reactor in order to speed up the process or reduce the number of reactors needed); or
-
vitrification in combination with high-level radioactive waste, achieved by mixing the plutonium with fission products from previous military or civilian nuclear energy activities into molten glass to produce glass logs with mass, bulk, radioactivity, and resistance to chemical separation of the plutonium comparable with these properties for spent fuel bundles from civilian power reactors.
The residual (unfissioned) plutonium in the MOX approach would be part of the radioactive wastes similar to what would have been produced in any case from energy generation in the civilian power reactors chosen for the plutonium disposition mission, and the plutonium would remain a part of these wastes through whatever intermediate and final storage steps society choose for them.91 In the vitrification approach, the plutonium-bearing logs would likewise become part of a radioactive waste management burden that would exist in any case in the form of glass logs serving to immobilize previously generated fission products. Either of these options or a combination of them would be appropriate to achieve final disposition of plutonium. The 1995 CISAC report recommended that both options be developed expeditiously in parallel.
History, Status, and TransparencyIissues
The implementation of the U.S.-Russian HEU deal described in Chapter 1 was slowed and ultimately even imperiled by a number of management decisions, most importantly the decision in the mid-1990s to privatize the theretofore government-operated uranium-enrichment industry in the United States. Once the U.S. Enrichment Corporation (later renamed USEC), which had been the “executive agent” for the HEU deal on the U.S. side from the beginning, became private the resulting tension between profit mo-
tive and national security goals—the former favoring implementation of the deal on terms that would maximize returns to USEC’s stockholders and the latter favoring implementation on terms that would maximize the rate of down blending and transfer of Russian HEU—led to a series of delays, disagreements, and renegotiations.92
Despite these problems, by January 2005 Russia had blended down more than 230 tons of HEU to LEU under this program. The process is now proceeding at an annual rate of about 30 tons of HEU. The program is scheduled to end in 2013, when the 500 tons of HEU covered by the original deal will have all been blended down to LEU. This figure, which represents about half of the total Russian stockpile of HEU inside and outside of weapons, is equivalent to as many as 20,000 to 25,000 nuclear weapons, depending on design.93
Progress toward final disposition of excess weapons plutonium has been much slower. Following the CISAC recommendations and reviews by U.S. governmental and bilateral U.S.-Russian panels, the two options were embraced by the official U.S. announcement of the dual-track approach for plutonium disposition in December 1996. These options had been endorsed earlier at the international level at the U.S.-Russian summit in Moscow in April 1996, and at a subsequent international experts meeting in the fall of 1996. Currently, however, the immobilization option has been largely abandoned and the pursuit of the MOX option has been seriously slowed by legal and economic problems. So far, none of the weapons plutonium declared excess has been disposed of.
Both the United States and Russia have some but not all of the facilities they would need to undertake plutonium disposition. For the reactor option, new plutonium fuel fabrication facilities and plants for converting plutonium pits to oxide would be needed, and this would be the limiting requirement in both time and cost for
|
92 |
See, e.g., Thomas L. Neff, “Decision Time for the HEU Deal: U.S. Security vs. Private Interests,” Arms Control Today 31 (June 2001), pp. 12-17. Available as of January 2005, at: http://www.armscontrol.org/act/2001_06/nefjun01.asp. Most recently, USEC succeeded in forcing Russia to accept a new pricing structure that reduces payments to Russia by several tens of millions of dollars a year, compared with the previous agreement. This has generated considerable resentment among some Russian nuclear officials, but for now deliveries of the blended-down material are stabilized and USEC has an economic incentive to carry out the deal as rapidly as possible because the Russian material is now USEC’s lowest-cost source of supply. |
|
93 |
USEC Fact Sheet, “US-Russian Megatons to Megawatts Program: Recycling Nuclear Warheads into Electricity,” USEC Inc., December 31, 2004. Available as of January 2005, at: http://www.usec.com/v2001_02/HTML/megatons_fact.asp. |
beginning a large-scale plutonium disposition campaign in reactors. Use of existing European fuel fabrication facilities, at least for fabrication of initial fuel assemblies and perhaps the fuel for the first reactor loads, could significantly accelerate the schedule on which the reactor option could begin.
In the United States, far more reactors than needed have sufficient licensed lifetimes remaining to carry out the plutonium disposition mission, although identifying reactors willing to participate (even given substantial financial incentives) has proved to be a struggle. In Russia, only the eight 950 MWe VVER-1000 light-water reactors (LWRs) and the one BN-600 fast-neutron reactor fall into this category. Depending on the final conclusion about how much plutonium can be safely loaded into these reactors, and depending also on the desired pace of disposition under the MOX option, use of the eleven VVER-1000 reactors in Ukraine (whose fuel has been provided by Russia under long-term agreements) might be considered. Another possibility, proposed by Canada, is to use both U.S. and Russian plutonium in fuel for existing Canadian deuterium-uranium (CANDU) reactors.94
Both the United States and Russia have some but not all of the facilities that would be needed to immobilize plutonium with high-level wastes. In the United States, a major effort to vitrify high-level wastes from past reprocessing is just beginning at Savannah River and is planned at Hanford. Plutonium could be added to such waste glasses, but this would require either substantial modifications of existing facilities or the construction of new ones.95 Russia is already vitrifying high-level wastes at Chelyabinsk.
Under the September 2000 U.S.-Russian Plutonium Management and Disposition Agreement (PMDA),96 Russia is supposed to
|
94 |
For a summary of a range of potential approaches to accelerating the rate of plutonium disposition, see Matthew Bunn, Anthony Wier, and John P. Holdren, Controlling Nuclear Warheads and Materials: A Report Card and Action Plan (Washington, DC: Nuclear Threat Initiative and the Project on Managing the Atom, Harvard University, March 2003), pp. 156-161. |
|
95 |
An alternative method developed by DOE, known as "can-in-canister," also has promise. In this approach, the plutonium would be immobilized in small cans of glass or ceramic without high-level wastes (allowing existing glove-box facilities to be used), and these small cans would be arrayed inside the large canisters into which the high-level waste glass is being poured at the existing vitrification plant. |
|
96 |
Agreement Between the Government of the United States of America and the Government of the Russian Federation Concerning the Management and Disposition of Plutonium Designated as No Longer Required for Defense Purposes and Related Cooperation. Available as of January 2005, at: http://www.nti.org/db/nisprofs/russia/fulltext/plutdisp/pudispft.pdf. |
begin loading 34 tons of excess military plutonium97 in MOX fuel into civilian reactors around 2008, at an initial rate of 2 tons of Pu per year, increasing thereafter to 4 tons per year. Rosatom (formerly the Ministry of Atomic Energy) has always considered all separated plutonium to be a valuable energy resource irrespective of cost calculations showing that even using “free” military plutonium in fuel is more expensive than making fuel with the same energy value from freshly mined and enriched uranium. Consequently, the PMDA does not commit any Russian plutonium to disposition by immobilization with wastes.
The transparency and monitoring provisions in the PMDA are extensive and are the most informative available “official” wording for purposes of illustrating the complexity and sensitivity of transparency and monitoring procedures for NEM as seen by the two leading nuclear weapon states. Of particular note in these passages are (a) the extensive attention given to how monitoring can be accomplished while protecting information about the composition of the plutonium from the two countries’ weapons, which remains classified to varying degrees on both sides and (b) the delicate and ambiguous interplay of bilateral versus multilateral (IAEA) responsibilities and privileges in the verification process, leaving unresolved the question of what the IAEA role actually will be.
The United States agreed under the PMDA to dispose of 34 tons of excess weapon plutonium, as well, and agreed further that at least 25 tons of this would be loaded into civilian reactors in MOX fuel.98 It had been supposed by many that the United States would choose to use immobilization with wastes for disposition of the maximum amount allowed by agreement—that is, the remaining 9 tons of the declared weapon plutonium surplus, but the Department of Energy announced in February 2002 that the immobilization option in the U.S. disposition program was being set aside as an economy measure, leaving only the MOX option.99 Aside
|
97 |
The 34 tons of military plutonium will be blended with 4 tons of Russian reactor-grade plutonium in order to preserve the confidentiality of the isotopic mix in the weapon-grade material. |
|
98 |
The United States has declared a larger amount of military plutonium (52.5 tons) surplus to its needs, but 18.5 tons of this total are either reactor grade or highly contaminated and were therefore not credited by the Russians as something they needed to match. |
|
99 |
National Nuclear Security Administration, Office of Fissile Materials Disposition, Report to Congress: Disposition of Surplus Defense Plutonium at Savannah River Site (Washington, DC: National Nuclear Security Administration, February 15, 2002). Available as of January 2005, at: http://www.nci.org/pdf/doe-pu-2152002.pdf. |
from the liabilities of this “all eggs in one basket” approach, this decision poses the problem that 1-2 tons of the 34 consists of material too badly contaminated with other elements and compounds to be purifiable for use in MOX.
Even the slow U.S. and Russian timetables specified in the PMDA are no longer achievable. Discussions of an international financing and management approach for disposition of Russian excess plutonium have dragged on far longer than anticipated in the PDMA, and despite the inclusion of plutonium disposition as a priority item in the $20 billion G8 Global Partnership Against the Spread of Weapons and Materials of Mass Destruction, which raised hopes that sufficient financing would soon be pledged, no conclusion of these talks is yet in sight. Moreover, as a result of a dispute over liability in the event of an accident, the U.S. government was unwilling to extend the agreement that had provided the legal framework for the technical cooperation on plutonium disposition now underway, and that agreement expired in mid-2003. As a result, technical cooperation in preparation for building a MOX plant in Russia has been drastically slowed, and construction of the facility has been delayed by at least a year, and possibly more. Because both the administration and the Congress have linked the start of construction of a U.S. MOX facility to the start of construction of a Russian MOX facility, the U.S. facility has also been delayed by at least a year.100
Considerations and Options Looking Ahead
We judge that achieving appropriate transparency and adequate monitoring for final disposition of surplus military NEM pose entangled political and technical challenges that will require further effort to resolve. Notable among these are (a) monitoring the transformation from item-countable objects (pits) to bulk material (e.g., plutonium oxide or mixed oxide powders) in a situation where nearly all of the characteristics of the initial objects and some of the characteristics of the bulk material are classified and so cannot be revealed to the inspectors;101 (b) coping with processes in which
the composition of the material being monitored is continuously changing, in which radiation barriers complicate access, and in which losses to waste products in ways difficult to measure may complicate material accounting; and (c) assaying accurately the plutonium content of spent reactor fuel (in the MOX disposition option), which by the nature of reactor physics and technology will be variable across different fuel elements and even within them. The difficulty of meeting these challenges should not be under-rated, but neither should one suppose that they cannot be surmounted.102 We believe that they are fertile ground for increased U.S.-Russian technical cooperation and joint demonstrations, as well as for trilateral (US-Russia-IAEA) efforts.
Final disposition is a long-term project no matter what its priority and no matter what its pace; it cannot alleviate the need for rapid improvements in MPC&A. As with most long-term projects that are badly needed, however, the difficulty and duration of the disposition project make it all the more important to start early and come up to speed quickly. Large stocks of HEU and separated plutonium, no matter how well accounted for and protected against theft, represent a risk of breakout from nuclear arms limitation agreements by the states that own the material and control the territory on where it is stored, as well as a risk of the material falling into other hands as a result of a major societal disruption.
The longer the wait before the NEM is finally disposed of in ways that make its use in nuclear explosives very unlikely, the greater the chance that currently unforeseen developments could turn it into a major menace. Certainly there are significant transparency and monitoring challenges associated with the processes of final disposition that exceed the challenges of simple guarded storage on a continuing basis. Like the other challenges of disposition, however, we believe that those of transparency and monitoring will likely yield to concerted and cooperative effort if the political will exists to get it done.
|
|
would not permit multilateral involvement in monitoring except through the use of innovative approaches to protect the classified information. |
CONCLUSIONS
This chapter has examined the record of and potential for applying monitoring and transparency measures to military and civilian stocks of NEM. In doing so it has addressed, among other issues, the benefits of transparency and monitoring that would be associated with reductions in NEM stocks and flows and in the number of sites where NEM are stored—steps of obvious value in reducing opportunities for NEM theft and diversion—as well as the challenges that transparency and monitoring for the reductions processes themselves would pose. The United States and Russia have acquired substantial experience through their cooperation to improve the security of Russian stocks of NEM, including joint work on technologies and methods for enhanced transparency and monitoring. The work of the IAEA has provided extensive multilateral experience with monitoring and transparency for civilian NEM and some limited experience with military NEM as well.
Accounting, management control, and protection for NEM—the measures collectively referred to as MPC&A, which are pursued by nations for both economic and security reasons and by the international community as part of the nuclear nonproliferation regime—interact with transparency and monitoring in important and multifaceted ways. Transparency and monitoring are of limited value without competent MPC&A. Thus implementing and strengthening MPC&A, in addition to its direct benefits for security, is therefore sometimes the most important step that can be taken toward improved transparency and monitoring. At the same time, improved transparency and monitoring conversely can lead to identification and thus remedy weaknesses in MPC&A. Increases in transparency and monitoring of NEM, if accepted, could also accelerate efforts to strengthen MPC&A through cooperative measures. On the other hand, increased transparency can also complicate the task of MPC&A by providing information useful to those who would steal NEM.
We conclude that:
-
Transparency and monitoring measures for declared stocks of NEM at declared sites, comparable to those for nuclear weapons, could include:
-
comprehensive declarations describing the quantities and locations of all existing inventories of NEM, to-
-
-
gether with information on chemical forms and isotopic composition on the material;
-
declarations of inventories of NEM surplus to military and civilian needs; and
-
provisions for inspections of all declared facilities as well as of any undeclared suspicious activities.
-
A number of additional measures could help to reduce the stocks and flows of NEM, as well as to reduce the number of sites at which NEM are stored. Immediate efforts related to HEU are especially important given its greater utility for terrorists or states seeking simple nuclear weapons. These measures include:
-
accelerated disposition of excess HEU inventories through down blending and eventual use in reactor fuel;
-
replacement of HEU fuels in research reactors with high-density LEU fuels, where feasible, and decommissioning of nuclear reactors using HEU fuels when replacement is not possible;
-
disposition of excess separated plutonium either by conversion to MOX fuel for use in civil reactors or by mixing with fission products and immobilization;
-
a comprehensive cutoff of production of NEM for weapons;
-
a serious international effort to develop nuclear fuel cycles for civil reactors that minimize or eliminate the exposure of NEM; and
-
possible centralization under multinational control of all facilities capable of enriching uranium or reprocessing plutonium.
-
-
Two efforts that would provide great benefits for international efforts to increase transparency and monitoring for NEM are:
-
continued substantial improvements in national management, protection, control and accounting of all national holdings of NEM so that individual countries, in particular Russia and the United States, are fully aware of the quantity and status of all of their holdings of NEM and have provided effective protection against theft or diversion for all stocks of NEM; and
-
continued strengthening of the safeguards regime administered both bilaterally and by the IAEA, including universal applicability of the Additional Protocol, with
-
-
increased manpower and funding to carry out the expanded mandate.
Important efforts to support both these goals are underway, but they should be enhanced and accelerated.
-
Greatly improved management and decreased inventories of NEM, which are priorities on their own account, would be critical if limits on total numbers of warheads were contemplated. The lower such limits became, moreover, the greater would be the need for reduction of NEM stockpiles and high confidence in monitoring the stocks that remained.
-
While the technologies exist to achieve monitoring of NEM quantities with considerable accuracy and confidence under a cooperative framework, a new strengthened international consensus on the value of doing this would be necessary to solve cooperatively the many difficult problems involved.