G
STATISTICAL REPORT
ANALYSIS OF THE NYBC, NMDP, AND NHLBI CORD BLOOD DATA
Robert Gibbons
Director, Center for Health Statistics
Professor of Biostatistics and Psychiatry
University of Illinois at Chicago
OVERVIEW
This brief report outlines the results of an analysis of the combined New York Blood Center (NYBC), National Marrow Donor Program (NMDP), and the National Heart, Lung, and Blood Institute (NHLBI) Cord Blood Banking and Transplantation (COBLT) study cord blood datasets. The objective of the analysis was to examine the determinants of long-term survival of patients who received cord blood transplants during the period from 1993 to 2004. In doing so, we simultaneously examined the time to transplant failure (i.e., failure to engraft with donor cells) and mortality using a competing risk survival model. As such, we can estimate cumulative time-to-event curves for transplant failure, mortality, and survival. These curves can in turn be compared between subpopulations characterized by cell dose and human leukocyte antigen (HLA) mismatch, the two primary variables that are the focus of our analysis. These comparisons can be further adjusted for case-mix variability in terms of age, weight, sex, race, cytomegalovirus (CMV) infection, disease type, and disease severity. Based on the statistical model, we can draw inferences regarding the effects of cell dose and HLA mismatch on long-term survival, transplant failure, and death. These statistical inferences can then be used to derive policy statements regarding which combinations of cell dose and HLA mismatch are acceptable in order to optimize long-term survival.
METHODS
The median length of follow-up of these patients was 4 months, with a range of 1 to 129 months (time to death or length of living at follow-
up). The data were analyzed using a person-time competing risk survival model (see Gibbons et al., 2003), in which the competing risks were death or transplant failure. Transplant failure included failure to engraft or autologous reconstitution (i.e., engraftment with the recipients own cells). In those cases in which engraftment time was not recorded, it was set to time of autologous reconstitution, or time of backup transplant, or time of death. If none of these events occurred (n = 43 cases), engraftment time was set to 31 days, which is the value recommended by NYBC. The primary variables of interest were the degree of HLA match (low/intermediate at HLA-A and HLA-B and high resolution for HLA-DRB1), and the total nucleated cells per kilogram (TNC/kg), expressed as low (<2.5 × 107 cells/kg), medium (2.5 to 5.0 × 107 cells/kg), and high (>5.0 × 107 cells/kg). The primary hypotheses were that (1) an imperfect match decreases survival time by increasing transplant failure and mortality, and (2) an increase in cell dose increases survival time by decreasing transplant failure and decreasing the rate of mortality. To adjust for case mix, the covariates of time (in months), age, weight (kg), disease type (leukemia versus genetic or other), risk (0 to 3 on the International Bone Marrow Transplant Registry [IBMTR] scale), race, sex, and CMV infection were also included in the analysis. Site was added to the model to adjust for overall differences among the three sites in both transplant failure and mortality rates. The NYBC Blood Bank provided all transplants from 1993 through 1998 (n = 562). NMDP provided all transplants for their blood banks for which consent for outcomes research was obtained, in which presumed consent was assumed for patients who died before they could provide consent (n = 192). This occurred because for many cases consent was obtained retrospectively. NHLBI provided all on-study (COBLT study) protocol cases that received a COBLT study cord blood unit (n = 210). The final data set comprised 755 cases (NYBC, n = 384; NMDP, n = 165; NHLBI, n = 206) after exclusion of patients with prior transplants, HLA matches of less than 4/6, cases with missing data, and transplants that were not conducted in the United States.
Two model parameterizations were used. The first was a main-effects model, and the second included the interaction between cell dose and HLA mismatch. The second, more flexible model allows the effect of cell dose to vary depending on the degree of HLA mismatch. Both models included case-mix adjustment for the previously mentioned covariates. The main-effects model provides a more easily interpretable test of the two primary null hypotheses, whereas the model with cell dose-by-HLA interaction better reproduces the observed proportions of patients who survived, had transplant failures, and died.
One-tailed statistical tests were used to test HLA and TNC/kg effects; otherwise, two-sided tests were used to test the case-mix covariates.
RESULTS
Table G-1 displays the summary statistics for all variables used in the analysis by outcome (alive, dead, and failure to engraft with donor cells). Table G-1 reveals that of the three blood banks, NHLBI had the highest survival rates (p < .021). This finding is consistent with the fact that the NHLBI COBLT study was a controlled trial with entrance criteria that may have excluded some patients with potentially poorer outcomes. Furthermore, the data from NYBC were for an earlier period in time (1993 to 1998), and may reflect higher mortality rates because of the use of the older technology available at that time. By contrast, the NMDP had the highest transplant failure rate. One must be careful about interpreting these results, given that the different sites sampled different populations during different time periods, and these different populations may not have been fully represented by the case-mix adjustment in the model. Nevertheless, the use of three different sites provides a result that is more generalizable to the entire U.S. population.
TABLE G-1 Summary Statistics Predictors by Outcome (n = 755 Transplants)
|
|
|
Outcome |
|||
|
Predictor |
|
Alive |
Dead |
Engraftment Failure |
Probability |
|
N |
|
323 |
214 |
218 |
|
|
Site |
NYBC |
0.41 |
0.32 |
0.28 |
|
|
|
NMDP |
0.39 |
0.24 |
0.36 |
|
|
|
NHLBI |
0.50 |
0.25 |
0.25 |
.021 |
|
Leukemia |
Yes |
0.64 |
0.67 |
0.73 |
.071 |
|
Sex |
Female |
0.41 |
0.42 |
0.44 |
.802 |
|
Race |
African American |
0.06 |
0.17 |
0.13 |
.001 |
|
CMV |
Positive |
0.40 |
0.48 |
0.45 |
.182 |
|
HLA |
6/6 |
0.61 |
0.23 |
0.15 |
|
|
|
5/6 |
0.48 |
0.25 |
0.28 |
|
|
|
4/6 |
0.35 |
0.33 |
0.32 |
.001 |
|
Age (yr) |
|
3.76 |
5.49 |
6.51 |
.006 |
|
Weight (kg) |
|
13.03 |
15.46 |
17.62 |
.065 |
|
Risk IBMTR |
|
1.18 |
1.41 |
1.60 |
.001 |
|
TNC/kg (107) |
|
7.01 |
6.33 |
5.43 |
.015 |
Table G-1 also reveals that for the surviving patients, blacks represented only 6 percent of all patients but represented 17 percent of the patients who engrafted and then died and 13 percent of the patients who failed to engraft (p < .001). HLA mismatch shows that patients with a perfect match had increased survival rates, decreased death rates, and higher engraftment rates than mismatched patients in general and patients matched at 4/6 antigent in particular (p < .001). Age was related to outcome, where surviving patients were younger (3.76 years) than patients who died (5.49 years) and patients who failed to engraft (6.51 years) (p < .006). Differences in weight paralleled those found for age (p < .065). Surviving patients had lower IBMTR risk scores (1.18) than patients who died (1.41) or patients who failed to engraft (1.60) (p < .001). Finally, higher TNC/kg ratios were found for surviving patients (7.01 × 107 TNC/kg) relative to those ratios for those who died (6.33 × 107 TNC/kg), and those in whom engraftment failed (5.43 × 107 TNC/kg) (p < .015).
Table G-2 contains the maximum-likelihood estimates, standard errors, and probability values for the main-effects model. In terms of death versus survival, mortality rates decreased over time (the month variable in Table G-2, which indicates the time to the event in months; p < .001); older age significantly increased the likelihood of death (p < .027), as did race other than Caucasian (p < .004). As previously noted, the NHLBI COBLT study had lower mortality rates than the NYBC site (p < .045). In terms of cell dose, both the medium (p < .003) and high (p < .046) cell doses significantly decreased the rate of mortality from that achieved with the low cell dose. The same was true for high versus low TNC/kg on engraftment (p < .002). Similarly, a 4/6 HLA match increased the rate of mortality relative to that of a 6/6 HLA match (p < .008), and both 4/6 and 5/6 HLA matches increased the engraftment failure rate relative to that achieved with a 6/6 match (p < .002 and p < .007 respectively).
The model with cell dose-by-HLA mismatch interactions improved the fit of the model to the data to a degree that approached significance (p < .096). For mortality, significant interactions for high cell dose versus low cell dose by 4/6 HLA match (p < .035) and 5/6 HLA match (p < .011) were found, which indicated that the differences between a perfect match (6/6) and imperfect matches (4/6 and 5/6) significantly decreased with increasing cell dose. No significant interactions were found for treatment failure. To illustrate these effects, the parameter estimates for the model with interactions were used to obtain case-mix-adjusted estimated proportions (see Table G-3). Table G-3 reveals that the model does an excellent job of tracking the observed data, despite the relatively small sample sizes, particularly for 6/6 of HLA matches (i.e., the predicted proportions are close to the observed proportions). In terms of survival, dramatic differences are observed between imperfect matches (4/6 and 5/6) and a perfect match (6/
TABLE G-2 Maximum Likelihood Estimates, Standard Errors, and Probabilities Main Effects Model
|
Variable |
Estimate |
Standard Error |
Z |
p value |
|
Dead vs. Alive |
||||
|
Intercept |
−2.73475 |
0.37533 |
−7.28628 |
.00000 |
|
Month |
−0.13208 |
0.00906 |
−14.57568 |
.00000 |
|
Age |
0.02265 |
0.01021 |
2.21911 |
.02648 |
|
Weight (kg) |
−0.00519 |
0.00620 |
−0.83705 |
.40257 |
|
Leukemia |
−0.35251 |
0.26778 |
−1.31640 |
.18804 |
|
Risk |
0.18298 |
0.10609 |
1.72487 |
.08455 |
|
Sex (female) |
−0.00246 |
0.15148 |
−0.01622 |
.98706 |
|
Race (black) |
0.57549 |
0.19849 |
2.89933 |
.00374 |
|
CMV |
0.18812 |
0.15245 |
1.23396 |
.21722 |
|
TNC/kg medium vs. low |
0.62626 |
0.22513 |
−2.78181 |
.00271 |
|
TNC/kg high vs. low |
0.44457 |
0.26332 |
−1.68832 |
.04568 |
|
HLA 5/6 vs. 6/6 |
0.36007 |
0.28361 |
1.26957 |
.10212 |
|
HLA 4/6 vs. 6/6 |
0.67548 |
0.28213 |
2.39424 |
.00833 |
|
NMDP vs. NYBC |
0.00482 |
0.20431 |
−0.02358 |
.98119 |
|
NHLBI vs. NYBC |
0.36697 |
0.18294 |
−2.00598 |
.04486 |
|
Engraftment Failure vs. Alive |
||||
|
Intercept |
−1.39919 |
0.45306 |
−3.08834 |
.00201 |
|
Month |
−0.79460 |
0.12055 |
−6.59149 |
.00000 |
|
Age |
0.01562 |
0.00954 |
1.63613 |
.10181 |
|
Weight (kg) |
−0.00834 |
0.00571 |
−1.46062 |
.14412 |
|
Leukemia |
−0.27092 |
0.26109 |
−1.03764 |
.29944 |
|
Risk |
0.25370 |
0.10328 |
2.45647 |
.01403 |
|
Sex (female) |
0.14352 |
0.15087 |
0.95132 |
.34144 |
|
Race (black) |
0.15866 |
0.21846 |
0.72629 |
.46766 |
|
CMV |
−0.03539 |
0.15221 |
−0.23253 |
.81613 |
|
TNC/kg, medium vs. low |
−0.23414 |
0.21963 |
−1.06607 |
.14320 |
|
TNC/kg, high vs. low |
0.80524 |
0.27153 |
−2.96561 |
.00151 |
|
HLA 5/6 vs. 6/6 |
0.81424 |
0.32836 |
2.47969 |
.00658 |
|
HLA 4/6 vs. 6/6 |
0.99567 |
0.33598 |
2.96350 |
.00152 |
|
NMDP vs. NYBC |
0.48969 |
0.19458 |
2.51661 |
.01185 |
|
NHLBI vs. NYBC |
−0.02394 |
0.19630 |
−0.12194 |
.90294 |
6) for low cell doses. The difference is approximately 40 percent in 2-year survival (see Table G-3 and Figure G-1). As cell dose increases, the HLA effect decreases. Although this finding is biologically questionable, the highest cell dose for a 6/6 match yielded a lower survival rate than the low and medium cell doses. This effect may simply be due to the small number of 6/6 HLA matches in the combined database (n = 34, see Table G-3). In terms of mortality, large differences between imperfect and imperfect matches
TABLE G-3 Estimated (Observed) Cumulative Competing Risk Survival Functions Proportion Experiencing the Event
were seen for the lowest cell dose, but these differences decreased with the medium cell dose, and then increased with the highest cell dose (relative to the medium cell dose). The effect of the highest cell dose on mortality should be the subject of further investigation, since there is no apparent biological explanation. In terms of treatment failure, patients with 6/6 matches exhibited dramatically decreased rates of engraftment failure relative to those for patients with 4/6 and 5/6 HLA matches for all cell doses; however, the HLA effect decreased with increasing cell dose.
To further illustrate the magnitudes of these effects, the cumulative survival distributions for each HLA group and TNC/kg group are displayed in Figures G-1 to G-3. The figures provide estimated cumulative survival distributions out to 5 years. The most striking effect is displayed in Figure G-1 for the low cell dose, in which patients treated with units with 4/6 and 5/6 matches have virtually identical survival distributions that are approximately one-third of that seen for a perfect 6/6 match. This finding makes us question whether anything less than a perfect match should be considered for use in transplantation with a cell dose of 2.5 × 107 TNC/kg or less.
Finally, we tested the assumption underlying the use of the ratio TNC/kg. By taking the ratio, we must assume that the effects of TNC and weight
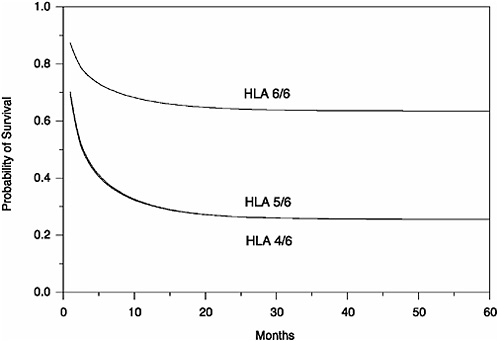
FIGURE G-1 Cumulative proportion alive (engrafted) (TNC/kg < 2.5 × 107).
are equally weighted in the ability to predict death and engraftment failure. Mathematically, the regression model is y = [a × log(TNC)] + [b × log(kg)] and the basic ratio assumption is that a is equal to −b (since increased TNC/kg is associated with a decreased risk of death and/or a decreased rate of failure to engraft). Adjusting for HLA mismatch, we found that for mortality, a was equal to −0.19 and b was equal to 0.36, whereas for engraftment failure a was equal to −0.32 and b was equal to 0.44. These findings support the use of the ratio TNC/kg for the modeling of cord blood transplant outcomes.
DISCUSSION
The results of this analysis reveal that if we take the competing risks of mortality and engraftment failure into consideration and adjust for disease type, risk, age, weight, CMV infection, sex, race, and time, the cumulative survival benefits for 4/6 and 5/6 HLA matches are grossly inferior to a perfect 6/6 HLA match for small cell doses (<2.5 × 107 TNC/kg). As cell
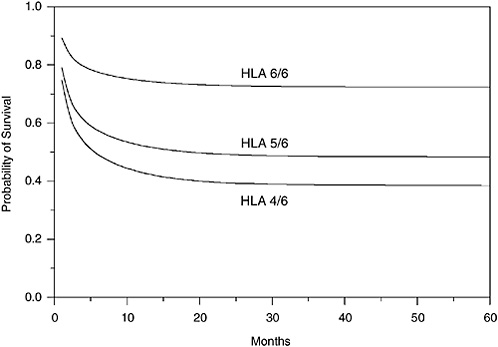
FIGURE G-2 Cumulative proportion alive (engrafted) (TNC/kg = 2.5 − 5.0 × 107).
dose increases, the HLA effects diminish. The residual differences are largely due to the significantly reduced engraftment rates for 4/6 and 5/6 HLA matches relative to that for a perfect 6/6 HLA match. These results suggest that units with lower cell dose can be given to recipients with a perfect match, whereas the highest cell doses should be given to recipients with 4/6 or 5/6 HLA matches, assuming that two potential recipients are in competition for the same unit. Table G-4 presents a breakdown of cell dose by HLA matches. Table G-4 reveals that 20 percent of all transplants were conducted with low cell doses in patients with 4/6 and 5/6 HLA matches. This amounts to 154 patients for whom the survival rate could have been doubled by use of a medium cell dose, or almost tripled by use of a high cell dose. In addition, 7 percent of all transplants were done using medium or high cell doses in perfectly matched (6/6) patient-donor pairs. These larger cell doses could have potentially been used to increase survival for 4/6 or 5/6 mismatched patients, without compromise to the 6/6 patient had a similar unit with smaller cell dose been available. These two results indicate that over 25 percent of the actual transplants were inefficiently allocated, leading to either unnecessarily poor survival or the use of an unnecessarily high cell dose for a patient-donor pair with a 6/6 HLA match. Both cell dose and HLA match should be considered in the final allocation system.
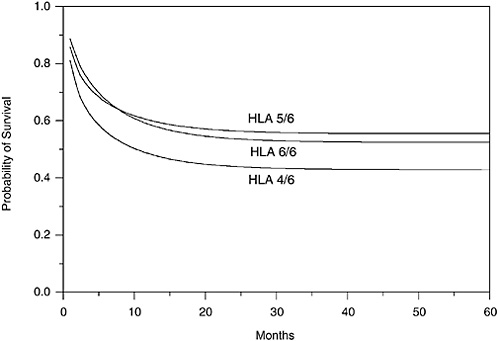
FIGURE G-3 Cumulative proportion alive (engrafted) (TNC/kg > 5.0 × 107).
TABLE G-4 Summary Statistics Cell Dose, Age, and HLA Mismatch, Number (Percent) of Patients (n = 755 Transplants)
|
|
Age (yr) |
HLA |
||||
|
TNC/kg (107) |
<5 |
5–16 |
>16 |
4/6 |
5/6 |
6/6 |
|
<2.5 |
118 |
21 |
35 |
95 |
59 |
20 |
|
|
(68) |
(13) |
(20) |
(54) |
(34) |
(12) |
|
2.5–5.0 |
157 |
70 |
20 |
132 |
94 |
21 |
|
|
(64) |
(28) |
(8) |
(54) |
(38) |
(9) |
|
>5.0 |
265 |
63 |
6 |
144 |
156 |
34 |
|
|
(79) |
(19) |
(2) |
(43) |
(47) |
(10) |
There are several limitations of this analysis. First, despite the pooling of data from all three major cord blood banks, the sample size is still small, given the large number of effects estimated from these data and the separate set of coefficients for the two competing risks. Second, there are only a small proportion of patients with a perfect 6/6 HLA match. Third, age and
cell dose are confounded in that only young children are capable of receiving high cell doses. For example, Table G-4 reveals that only 2 percent of the high cell doses were transplanted into patients over 16 years of age. Since cell dose is expressed as TNC/kg, presumably there are few units that have sufficient numbers of cells to yield ratios above 5 × 107 TNC/kg for adults. Research into the viability of combining multiple units for a single transplant should be investigated. Finally, analysis of larger datasets with larger cell volumes and greater numbers of perfectly matched recipients is needed to confirm these preliminary results.
REFERENCE
Gibbons RD, Duan N, Meltzer D, Pope A, Pehoet ED, Dubler NN, Francis CK, Gill B, Guinan E, Henderson M, Ildstad ST, King PA, Martinez-Maldonado M, Mclain GW, Murray JE, Nelkin D, Spellman MW, and Pitluck S. 2003. Waiting for organ transplantation: Results of an analysis of an Institute of Medicine committee. Biostatistics 42:207–222.










